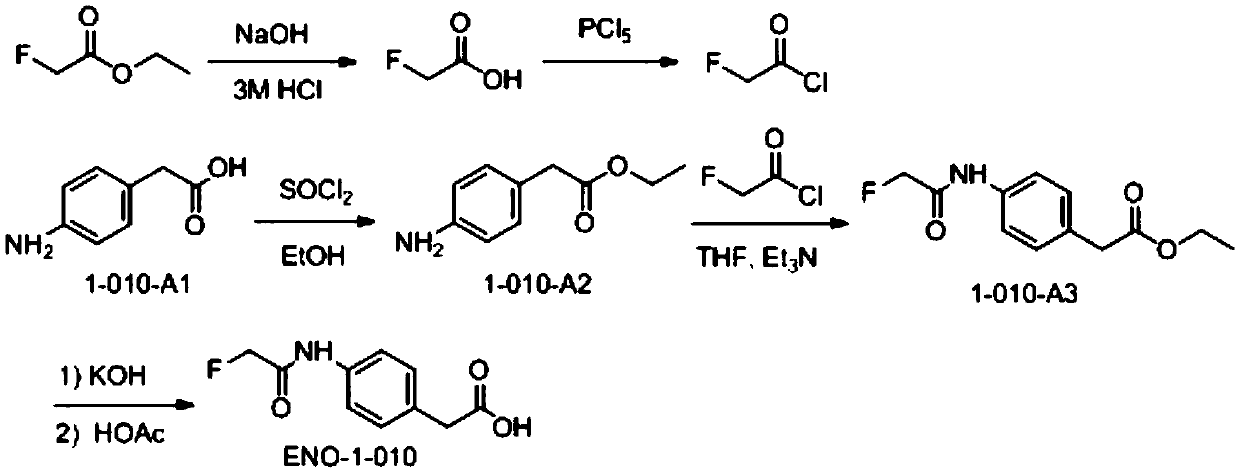
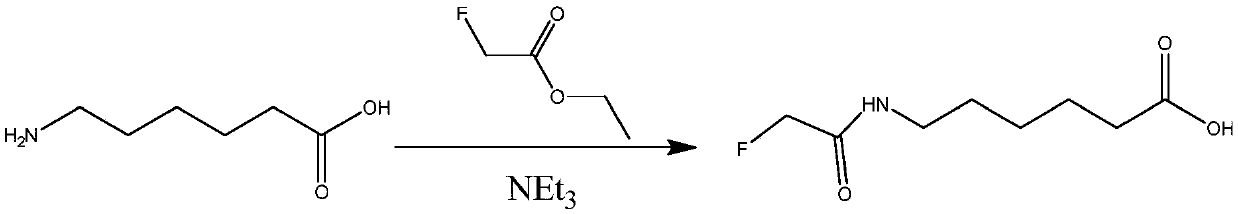
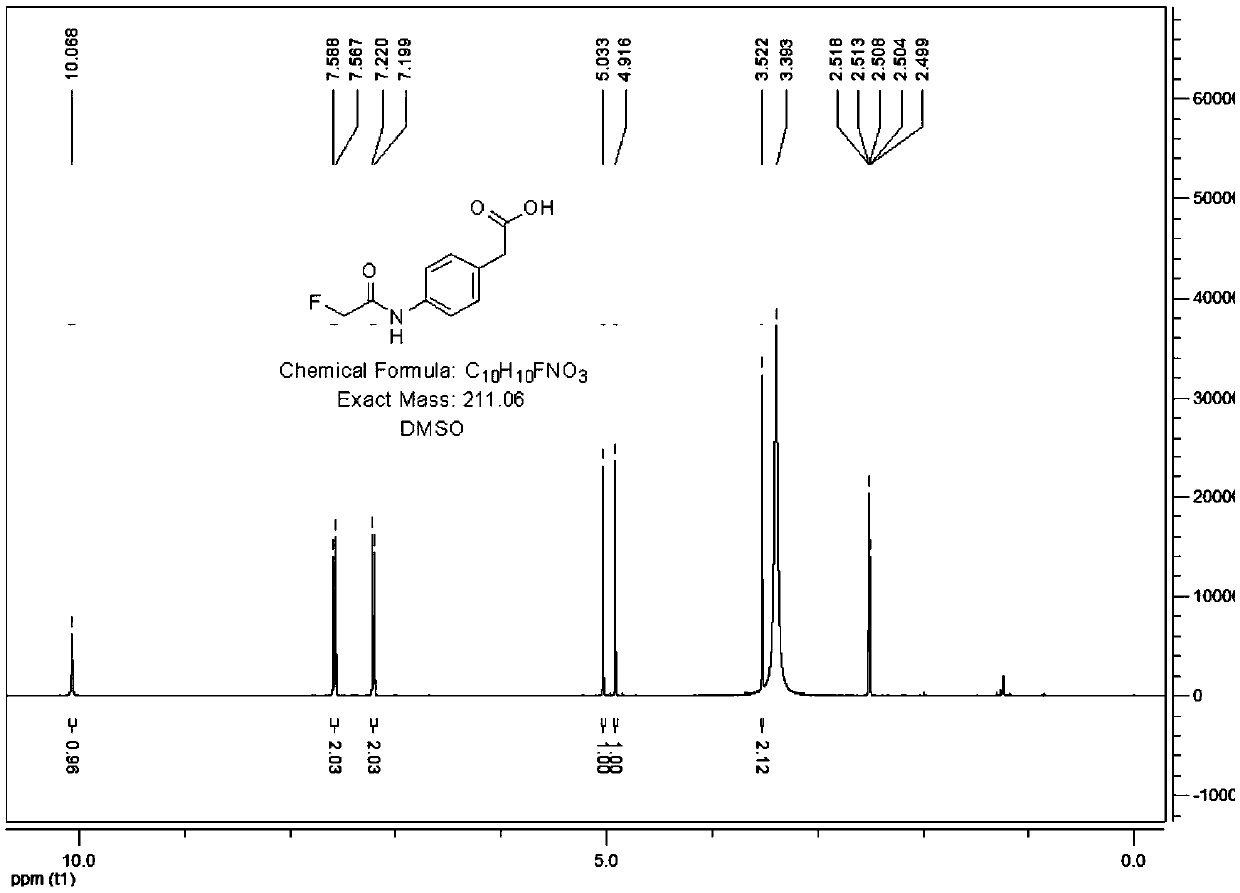
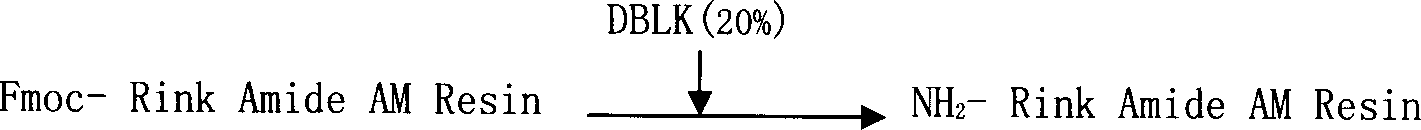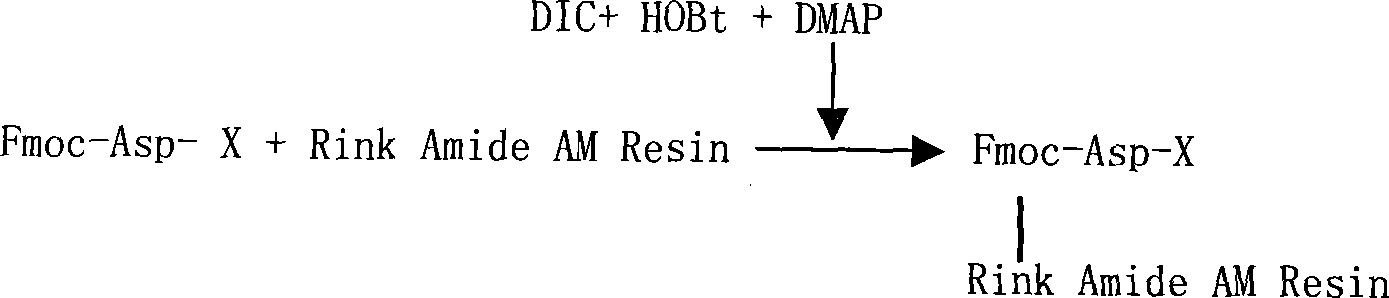Patents
Literature
240 results about "Fluoroacetic acid" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Fluoroacetic acid is a chemical compound with formula CH₂FCOOH. The sodium salt, sodium fluoroacetate, is used as a pesticide. It inhibits the aconitase step of the citric acid cycle.
Method for synthesizing Arbekacin and intermediate dibekacin thereof
InactiveCN101575354AHigh yieldReduce generationSugar derivativesSugar derivatives preparationFluoroacetic acidSynthesis methods
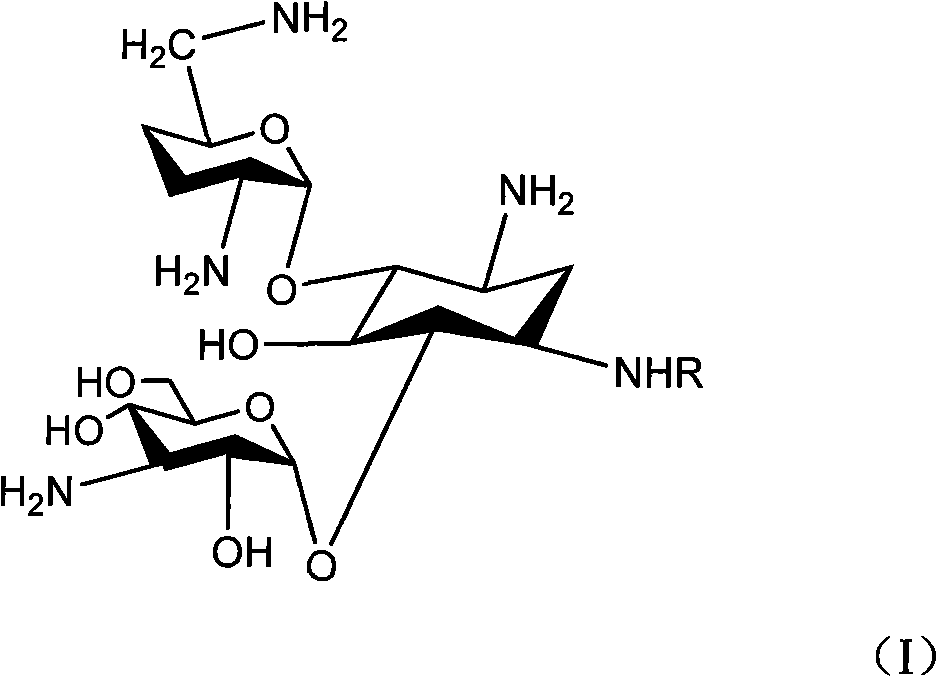
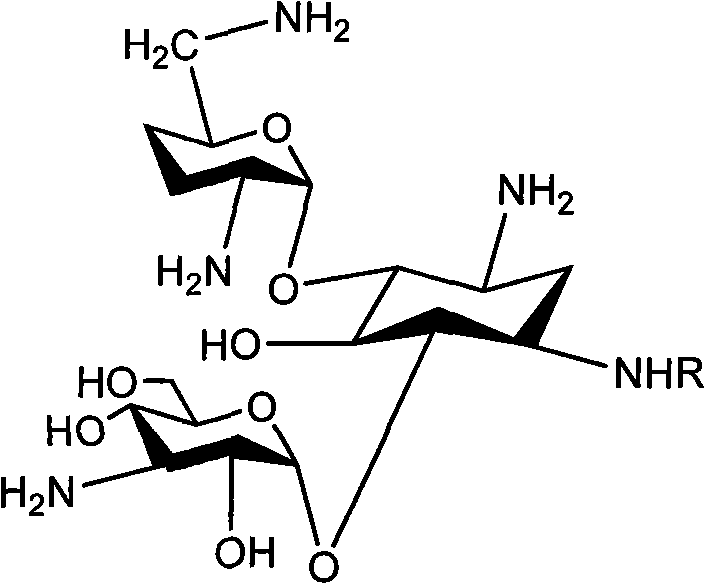
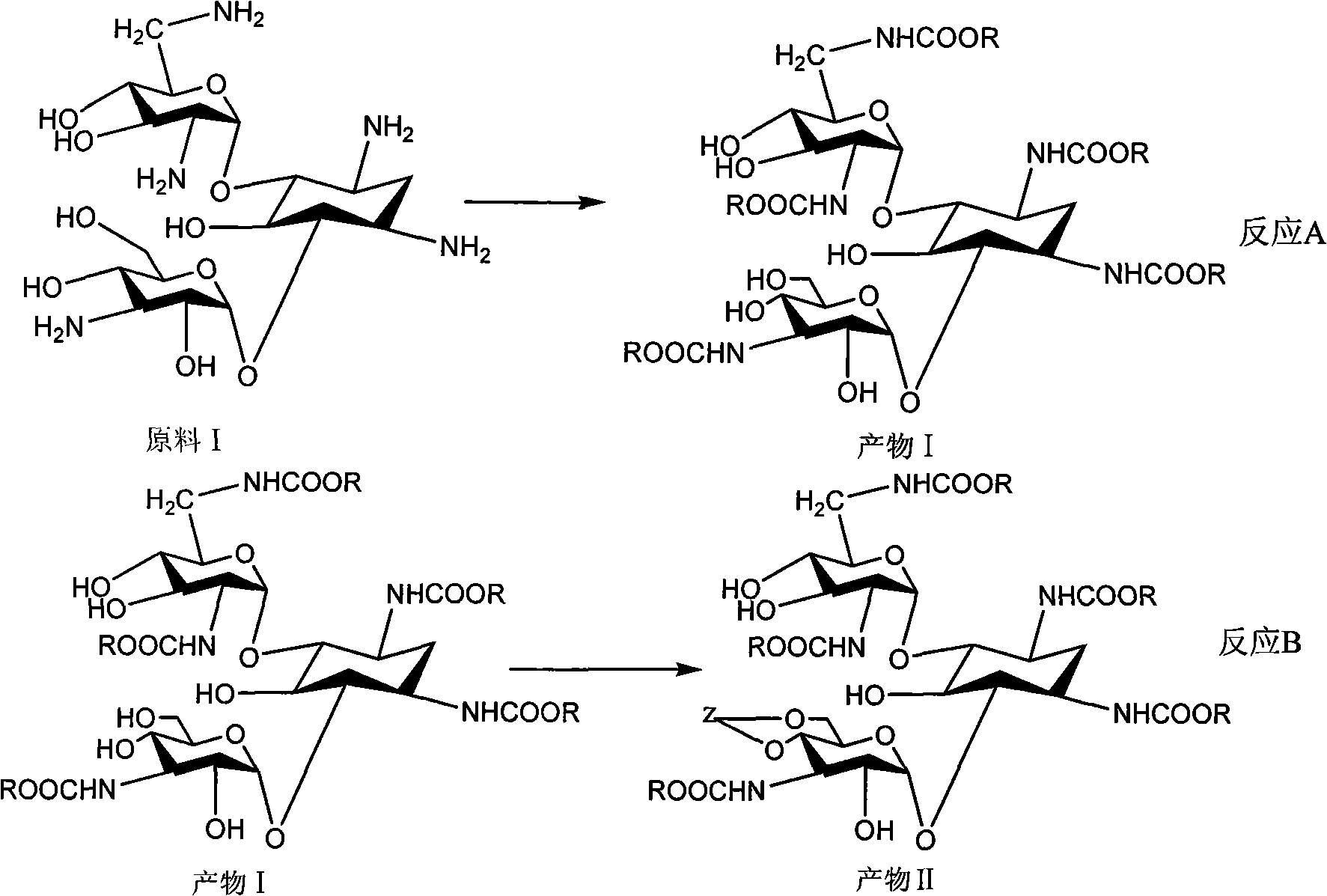
The invention relates to an organic synthesis method, in particular to a method for synthesizing an Arbekacin and an intermediate dibekacin thereof. The method comprises the following steps of: takinga kanamycin B as initial raw material, carrying out the following processes of aldol condensation, sulfonylation, sodium iodide replacement and elimination to form double bond, de-protection under acidic condition, amino-electron reduction and final hydrogenation, thus obtaining the dibekacin; taking 3',4'-dideoxy -3',4'-didehydro-kanamycin B as raw material, using a di-tert-butyl dicarbonate toselectively protect the amidogen of 3, 2', 6', 3'' sites; subsequently using the synthesized active ester to protect the 1-site amidogen; subsequently using tri-fluoroacetic acid to remove BOC; and carrying out hydrazinolysis and catalysis and hydrogenation of platinum oxide, thus obtaining the Arbekacin. The synthesis method has the advantages of simple operation, high outcome yield, reducing thecost of raw material, optimizing the reaction route, lowering the requirements to the reaction conditions and being beneficial to industrial production.
Owner:BEIJING UNIV OF CHEM TECH +1
Solid phase synthetic technique for thymosin alpha1
ActiveCN101104638AAdvantages of solid phase synthesis processEasy to purifyThymopoietinsPeptide preparation methodsFluoroacetic acidAcetic anhydride
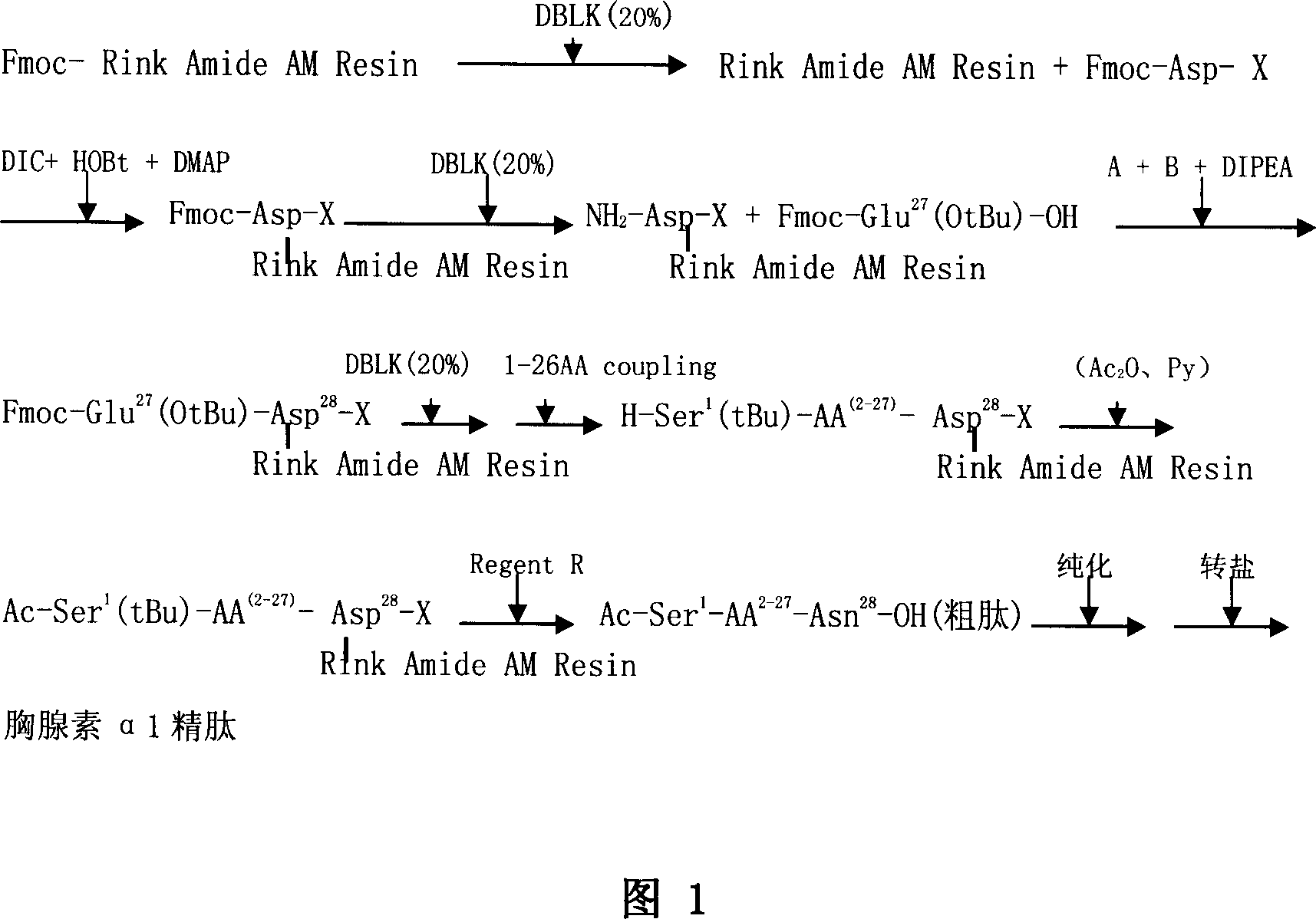
The invention relates to a solid-phase synthesis process of a thymosin alpha 1, belonging to the polypeptide solid-phase synthesis technical field. The invention comprises the following steps: a. a Fmoc-Rink Amide AM resin or a Fmoc-Rink Amide MBHA resin is used as carrier, an H2N-Rink Amide AM resin or an H2N-Rink Amide MBHA resin is obtained after deprotection of the Fmoc; b. side chain carboxyl group of Fmoc-Asp-X is connected with resin amino by the method of solid-phase synthesis to obtain the Fmoc-Asp (resin)-X; c. the left amino acid in the sequence is synthesized in solid-phase with the Fmoc strategy; d. after the amino protection group Fmoc of N terminal amino acid is removed, the N terminal amino acid is acetylated by acetic anhydride and pyridine; e. then the acetylated N terminal amino acid is cut by a cracking agent (tri fluoroacetic acid / benzoylate sulfide / 1, 2- dithioglycol / Anisole) to obtain the thymosin alpha 1; f. crude product of the thymosin alpha 1 is prepared and separated by HPLC to obtain the pure thymosin alpha 1. The invention can increase significantly the yield of the thymosin alpha 1 and decrease the production cost, which is helpful for scale production and has better industrialization prospect.
Owner:苏州天马医药集团天吉生物制药有限公司
Preparation method and application of 3-(4-chlorobutyl)-5-cyano-1H-indole
The invention relates to a preparation method and application of 3-(4-chlorobutyl)-5-cyano-1H-indole. The preparation method comprises the following steps: after dissolving 3-(4-chlorobutyryl)-5-cyano-1H-indole in a solvent, adding trifluoroacetic acid, adding sodium borohydride in batches, and treating the reaction liquid to obtain the 3-(4-chlorobutyl)-5-cyano-1H-indole. The method overcomes the defects in the existing preparation method of an important intermediate 3-(4-chlorobutyl)-5-cyano-1H-indole of an antidepressant vilazodone hydrochloride, and has obvious creativity and practical application value. The reaction formula is disclosed in the specification.
Owner:HANGZHOU HEZE PHARMA TECH
Ionic liquid capable of absorbing sulfur dioxide as well as preparation method and application of ionic liquid
InactiveCN105194982AImprove stabilityAvoid inactivationDispersed particle separationOXALIC ACID DIHYDRATEHigh absorption
The invention discloses ionic liquid capable of absorbing sulfur dioxide as well as a preparation method and application of the ionic liquid. Cations of the ionic liquid are nitrogen-containing organic matters and anions of the ionic liquid are organic acid groups. The nitrogen-containing organic matters are selected from one or ore of guanidine salts, alcamines, imidazoles, pyridines, tetrazoles, quaternary ammonium salts, thiazoles ad dicyandiamides matters; and the organic acid groups are selected from one or more of lactic acid, tartaric acid, citric acid, methanesulfonic acid, trifluoroacetic acid, malic acid, oxalic acid and acetic acid. The ionic liquid disclosed by the invention has a relatively good absorption effect on SO2 and relatively high selectivity. The preparation method is simple, reaction conditions are moderate, the raw materials are cheap and easily available, and the product has stable performances. An absorbent prepared from the ionic liquid has high absorption efficiency, can be cyclically utilized and has good stability, and can be used for absorbing the SO2 in flue gas at a high temperature; and the inactivation of the absorbent, caused by the fact that the ionic liquid is decomposed, is avoided.
Owner:黄锐
Polypeptide synthesis method for octreotide acetate
ActiveCN103351426AConvenient sourceReduce usagePeptide preparation methodsBulk chemical productionFluoroacetic acidAceric acid
The invention relates to a polypeptide synthesis method for octreotide acetate. The method comprises the following steps of: taking chloromethyl resin as a starting raw material, preparing a cesium salt from Boc-Thr(tBu)-OH, sequentially connecting amino acids with protecting groups according to a solid-phase synthesis method so as to obtain protected octapeptide resin, meanwhile, removing Boc protecting groups by sequentially using HCl / isopropyl alcohol, carrying out peptide connecting reaction in a manner of taking DIC and HOBT as condensing agents, carrying out reduction by using palladium carbon / hydrogen gas, meanwhile, cutting off peptide chains so as to obtain reduced octreotide, introducing air at the Ph of 7.8-9 so as to cyclize disulfide linkages, then, obtaining a crude octreotide product, and carrying out separation and purification through a C18 column, thereby preparing a fine octreotide acetate product. The method disclosed by the invention has the advantages that threoninol and Fmoc-threoninol are not adopted, the production cost is very low, the method has large-scale production capacity, the process is stable, the raw and auxiliary materials are convenient to obtain, the production cycle is short, the yield of connected peptide is high, the quality is stable, the use of highly-toxic reagents, such as hydrogen fluoride, trifluoroacetic acid and the like, is avoided, and the pollution caused by waste gas, waste water and waste residues is little.
Owner:SHANGHAI SOHO YIMING PHARMA
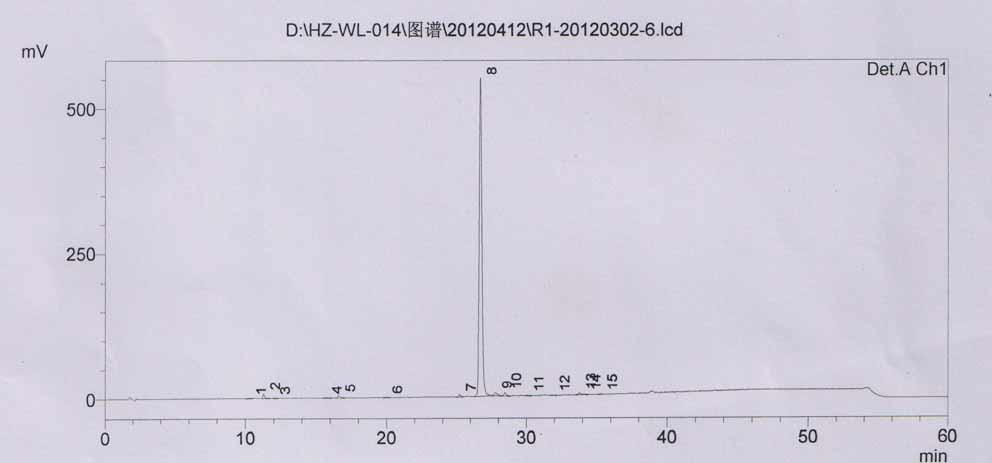
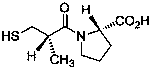
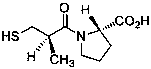
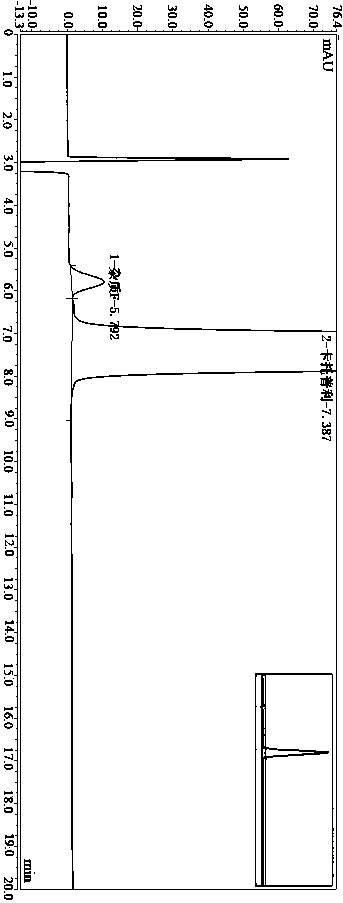
Method for determining impurity F in captopril tablets through high performance liquid chromatography
The invention discloses a method for determining an impurity F in captopril tablets through high performance liquid chromatography and belongs to the technical field of pharmaceutical analysis. Detection is performed under the conditions as follows: an amylase-tris(5-chloro-2-methyl phenyl carbamate) coated chromatographic column is used, normal hexane-absolute ethyl alcohol-trifluoroacetic acid serves as a mobile phase, a volume ratio of the normal hexane to absolute ethyl alcohol to trifluoroacetic acid is 80:20:0.1, a detection wavelength is 215nm, flow velocity is 1ml / min, a column temperature is 35 DEG C and a sample amount is 20[mu]l. A structural formula of the impurity F is as shown in the description. According to the method disclosed by the invention, the content of the impurityF in the captopril tablets can be quantitatively determined, so that the quality of the captopril tablets is effectively controlled. According to the method provided by the invention, the captopril and the impurity F can be proved to be effectively separated in a system suitability solution, and the method has high precision and high separation degree. A signal to noise ratio of a self-contrast solution is more than 10, and if the sample contains the impurity F, the impurity F can be detected.
Owner:ZHENGZHOU TAIFENG PHARMA CO LTD
Method for the preparation of ceftiofur sodium and its intermediates
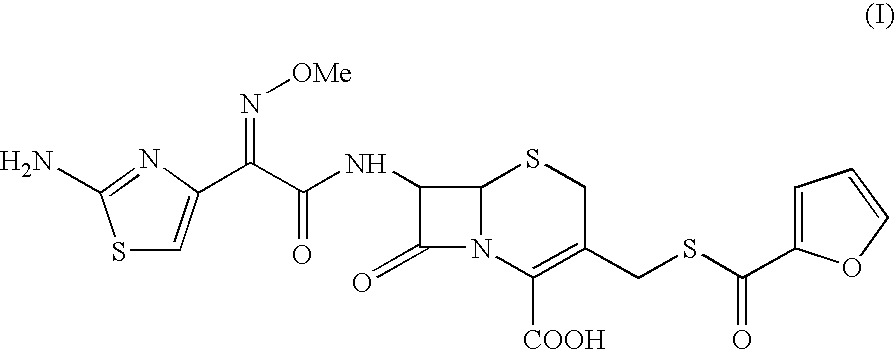
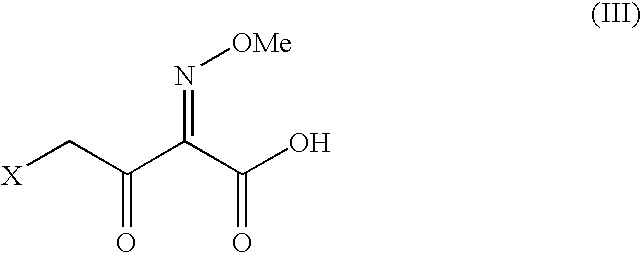
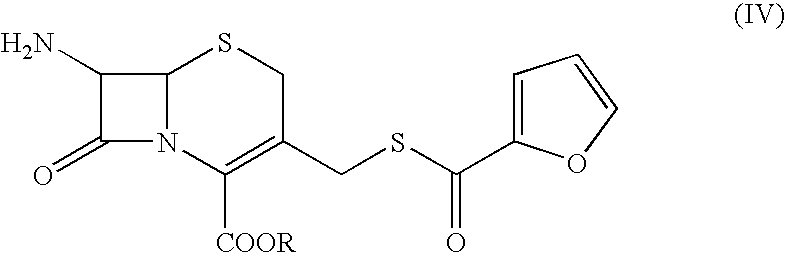
The present invention relates to preparation of Ceftiofur acid of formula (I),and its pharmaceutically acceptable salts. The process includes the steps of(i) condensing an activated derivative ofwherein the activated derivative is selected from acid halides, mixed anhydrides and active amides, and wherein X represents halogen atom selected from chlorine and bromine, with silylated derivative ofwherein R represents p-methoxybenzyl, p-nitrobenzyl or diphenylmethyl in the presence of a solvent at -40° C. to 0° C. to produce(ii) cyclising (V) with thiourea in the presence of water miscible solvent and sodium acetate at room temperature to produce cephalosporin(iii) deesterifying (VI) to produce (I) using anisole / trifluoroacetic acid, phenol / trifluoroacetic acid or formic acid at 0° C. to 10° C. and, if desired,(iv) converting (I), to its pharmaceutically acceptable salt. The invention also relates to intermediates (V) and (VI)
Owner:ORCHID CHEM & PHARM LTD
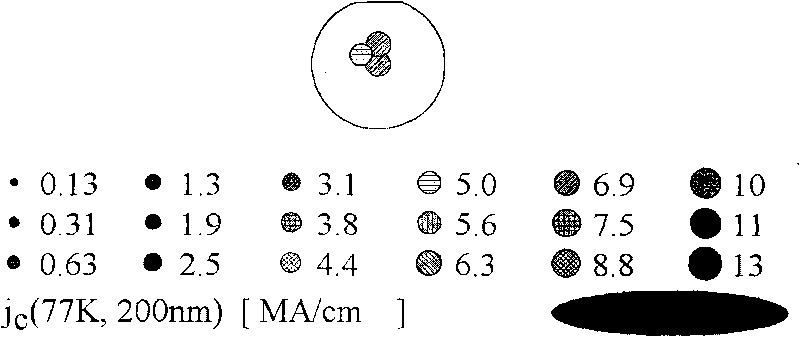
Method for preparing high temperature superconducting thin film by chemical process
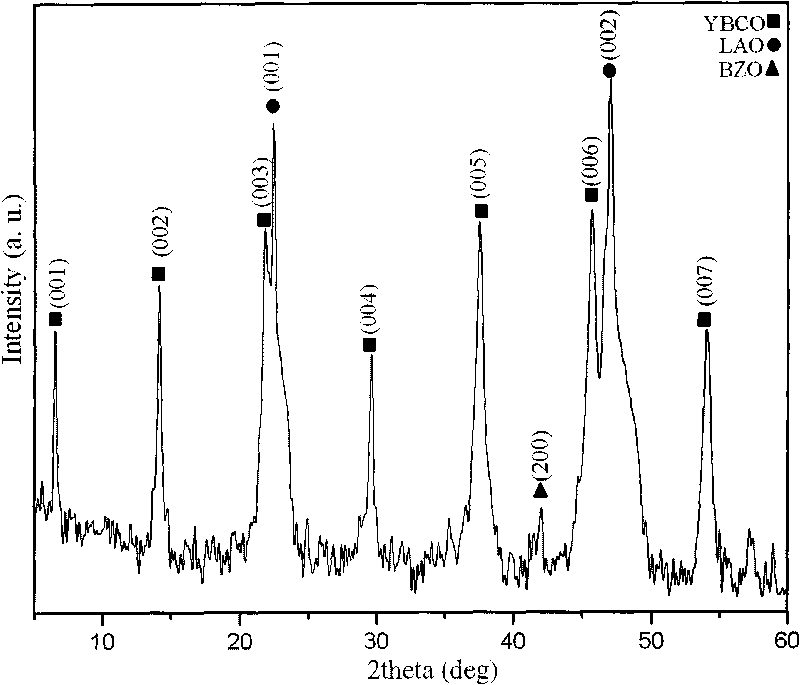
ActiveCN101752035AIncrease the critical current densitySuperconductors/hyperconductorsSuperconductor devicesRare-earth elementEvaporation
The invention provides a method for preparing high temperature superconducting thin film by chemical process. The method comprises the following steps: firstly preparing a precursor solution, by mixing Y(CH3COO)3, RE(CH3COO)3, Ba(CH3COO)2 and Cu(CH3COO)2 with ratio of (Y+RE) to Ba to Cu equaling to 1:2:3 (wherein RE is Nd, Sm, Eu, Gd and Dy) and dissolving in 20-30 mol percent aqueous trifluoroacetic acid solution; stirring to be uniform, performing vacuum drying by evaporation to the solvent to obtain gel; then adding methanol, stirring to be uniform, performing drying by evaporation to the solvent to obtain gel; then adding proper amount of methanol to prepare the precursor solution with the total concentration of metal ions of Y, RE, Ba and Cu being 0.8-3.0mol / L; then coating the precursor solution on a substrate; performing low temperature heat treatment at 400-410 DEG C to the coated thin film to decompose trifluoroacetic acid salt; and finally performing high temperature heat treatment at 800-850 DEG C and annealing process at 490-510 DEG C to form YBCO thin film doped with rare-earth element.
Owner:GRIMAT ENG INST CO LTD
Method for preparing 5-fluorouracil
The invention belongs to the field of organic chemistry synthesis and particularly relates to a method for preparing 5-fluorouracil. The method comprises the following steps: (1) after nitrogen displacement, adding sodium methoxide into toluene, then dripping part of ethyl formate at first, then dripping methyl fluoroacetate and the rest ethyl formate, and stirring after dripping for reaction; (2) adding methyl alcohol and sodium methoxide, stirring, reducing the temperature to 15-25 DEG C, adding urea for reaction, removing a solvent after reaction, re-adding water, cooling, stirring, regulating the pH to 3-4, and filtering to obtain the product. According to the invention, as ethyl formate and methyl fluoroacetate are mixed and dripped, and the dripping temperature is reduced, loss of ethyl formate is reduced, accordingly, reaction of ethyl formate and methyl fluoroacetate is promoted, and the yield is improved to be more than 74.5%; addition of the solvent is reduced, so that the solvent reclamation amount can be remarkably reduced and the manufacturing cost is greatly lowered.
Owner:SHANDONG JINCHENG PHARMA & CHEM
High-temperature superconducting nanometer composite film and method for preparing same
ActiveCN101747031AIncrease the critical current densitySuperconductors/hyperconductorsSuperconductor devicesFluoroacetic acidComposite film
The invention provides a method for preparing a high-temperature superconducting nanometer composite film, which comprises the following steps: preparing a precursor liquid, mixing Y(CH3COO)3, Ba(CH3COO) and Cu(CH3COO) in a molar ratio of 1:2:3 and dissolving the mixture in 20 to 30 mol percent aqueous solution of trifluoroacetic acid; uniformly stirring the solution and drying a solvent by distillation to obtain gel; then adding methanol in the gel, uniformly stirring the mixture, drying the solvent by distillation to obtain gel; adding methanol and zirconium(IV) acetylacetonat in an amount of 5 to 8 mol percent based on the total ion concentration of three metals to prepare precursor liquid, and the coating the precursor liquid on a monocrystal oxidate substrate with a two-inch diameter by the method of rotatable coating or lifting and pulling. The coated film is subjected to low temperature thermal treatment at 400 and 410 DEG C first to decompose trifluoroacetate, and finally the YBCO film containing nanometer barium zirconate is formed through the thermal treatment at the high temperature of between 800 and 850 DEG C and the annealing process at the temperature of between 490 and 510 DEG C.
Owner:GRIMAT ENG INST CO LTD
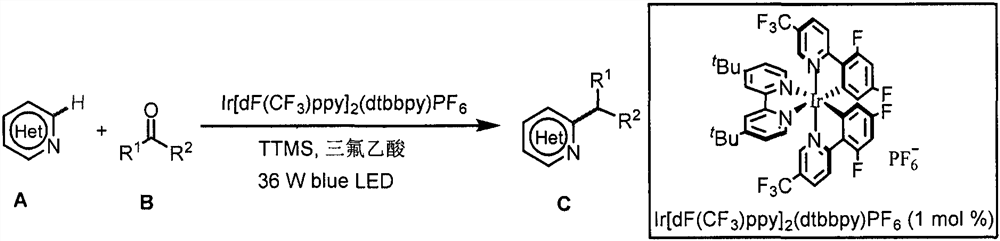
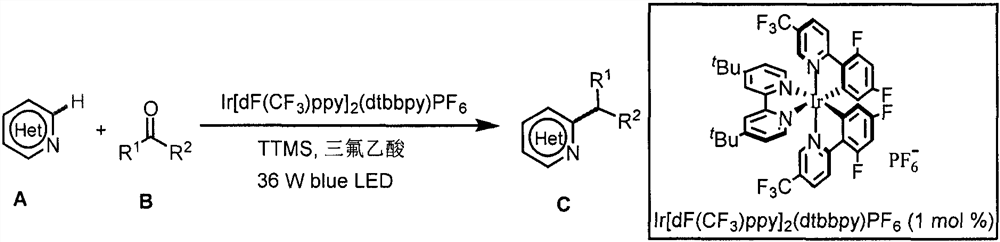
Application of light-promoted Minissci C-H alkylation reaction in preparation of alkyl-substituted azacycle
The invention belongs to the technical field of fine chemicals, and particularly relates to a method for preparing alkyl-substituted azacycle through a Minissci CH alkylation reaction which is promoted by light and takes aldehyde or ketone without an oxidizing agent as an alkyl radical source. A nitrogen-containing aromatic ring is mixed with a photocatalyst [Ir (dF (CF3) ppy) 2 (dtbbpy)] PF6, tris (trimethylsilyl) silane (TTMS), trifluoroacetic acid (TFA), aldehyde or ketone and an organic solvent, argon is blown into a reaction bottle, and a reaction is carried out under irradiation of 36W 490nm blue light. A sodium bicarbonate solution is added, liquid is separated, an organic layer is dried, a solvent is removed by spinning, and column chromatography is carried out to obtain a pure product.
Owner:NANKAI UNIV
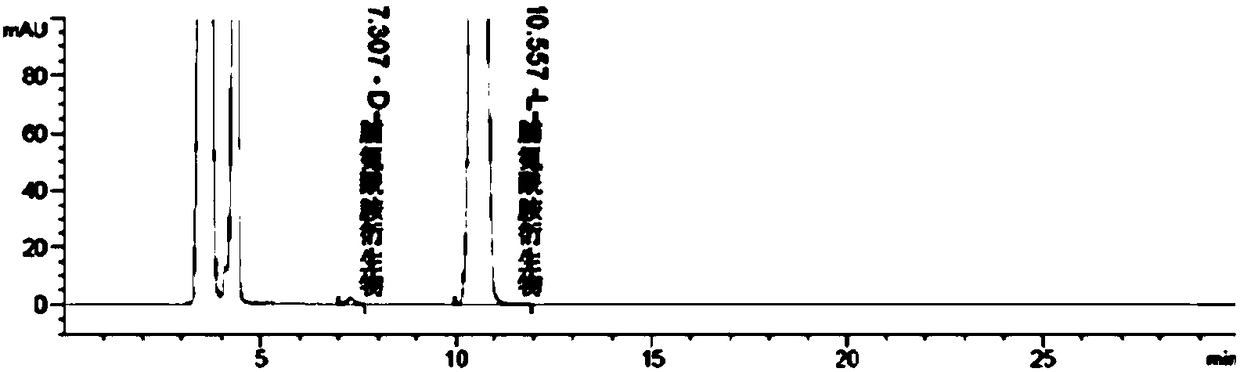
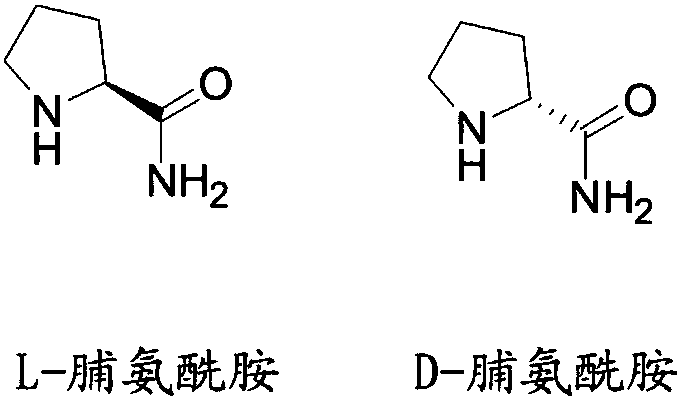
Liquid chromatographic analysis method of L-prolinamide
InactiveCN108226329ACompliant with derivatization requirementsHigh UV absorptionOrganic chemistryComponent separationTrifluoroacetic acidDerivatization
The invention provides a liquid chromatographic analysis method of L-prolinamide. The liquid chromatographic analysis method comprises the following steps: precisely measuring an L-prolinamide solution, a triethylamine ethanol solution and a derivatization reagent solution respectively, and uniformly mixing through vortex; reacting for a period of time; then adding a diethylamine ethanol solutionand uniformly mixing through the vortex; reacting for a period of time; then adding a trifluoroacetic acid ethanol solution and uniformly mixing through the vortex to obtain an L-prolinamide derivative solution; carrying out chromatographic analysis on the L-prolinamide derivative solution. The liquid chromatographic analysis method provided by the invention can be used for accurately and sensitively detecting the L-prolinamide and D-prolinamide.
Owner:ZHEJIANG TIANYU PHARMA
Synthetic method of silodosin
The invention provides a synthetic method of silodosin, which is high in yield, simple to operate and safe. The method comprises the following steps: (1) performing acidolysis on a compound shown in a structural formula 4 to obtain a compound shown in a structural formula 2 under the condition that acid used in acidolysis reaction is methylsulfonic acid, trifluoroacetic acid / sulfuric acid, acetic acid / sulfuric acid, polyphosphoric acid or orthophosphoric acid; (2) reacting the compound shown in the structural formula 2 with a compound shown in a structural formula 5 to obtain a compound shown in a structural formula 3; and (3) hydrolyzing the compound shown in the structural formula 3 or salts of the compound to obtain silodosin shown in the structural formula 1, wherein R1 in the structural formulas 2, 3 and 4 is ester groups such as butyryl, valeryl, isobutyryl, benzoyl, p-fluoro benzoyl, p-methyl benzoyl and m-methyl benzoyl; and R2 in the structural formula 5 is good leaving groups such as halogen or sulphonate such as bromine, chlorine, methanesulfonate, benzenesulfonate, p-methyl benzenesulfonate and the like. The method provided by the invention has the advantages that the method is easily-available in raw material and stable and safe in reaction, is convenient to operate, can avoid the reaction conditions which can cause material spraying, and is easy in process control and industrial amplification production.
Owner:KUNMING JIDA PHARMA
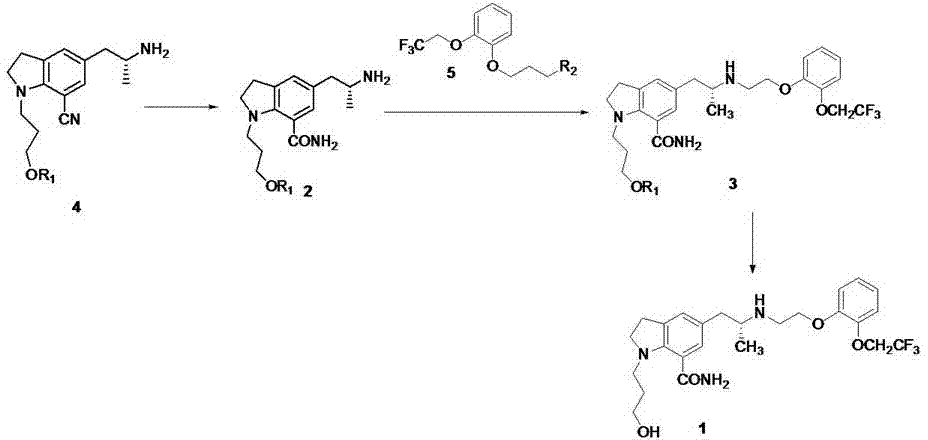
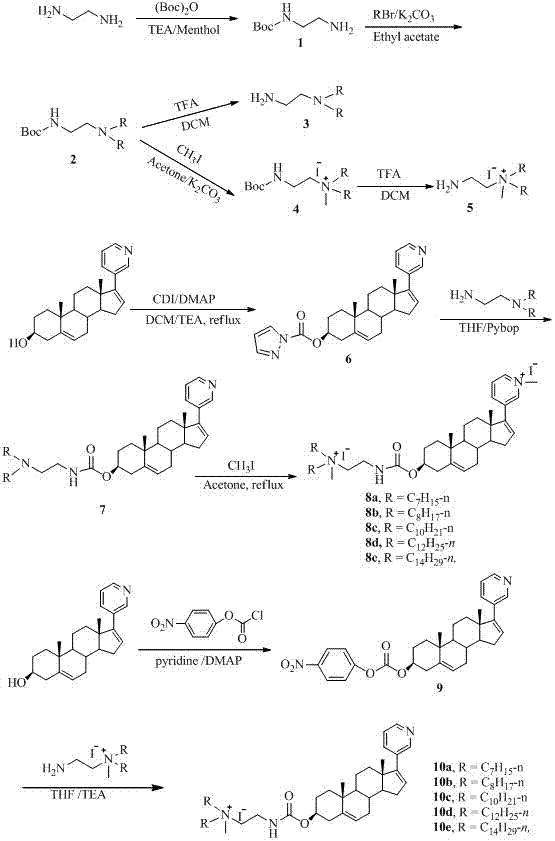
Synthesis of two types of abiraterone derivatives
The invention discloses preparation methods of two types of abiraterone derivatives. The preparation method of a first type of abiraterone derivatives comprises the following steps: by taking ethanediamine as a raw material, carrying out monoprotection on amino by using (Boc)2O, carrying out tertiary amination, and carrying out deprotection of trifluoroacetic acid to obtain N1,N1-di-n-alkyl-1,2-diamine; reacting abiraterone with CDI, then carrying out condensation reaction with the N1,N1-di-n-alkyl-1,2-diamine, and finally carrying out quaternary ammonium salinization reaction to prepare beta-N-methyl-N1,N1-di-n-alkyl carbamic acid-Py-N-methyl -abiraterone ester with different alkane chains. The preparation method of a second type of abiraterone derivatives comprises the following steps: by taking ethanediamine as a raw material, carrying out monoprotection on amino by using (Boc)2O, carrying out tertiary amination, carrying out quaternary ammonium salinization and carrying out deprotection of trifluoroacetic acid to obtain N-methyl-N1,N1-dialkyl ethanediamine; reacting abiraterone with nitrophenyl chloroformate, then carrying out condensation reaction with N-methyl-N1,N1-dialkyl ethanediamine to prepare beta-N-methyl-N1,N1-di-n-alkyl carbamic acid abiraterone ester with different alkane chains. The prepared abiraterone derivatives contain hydrophilic quaternary ammonium salt groups; compared with the abiraterone, the home-made two types of abiraterone derivatives are expected to have high water solubility.
Owner:HUNAN NORMAL UNIVERSITY
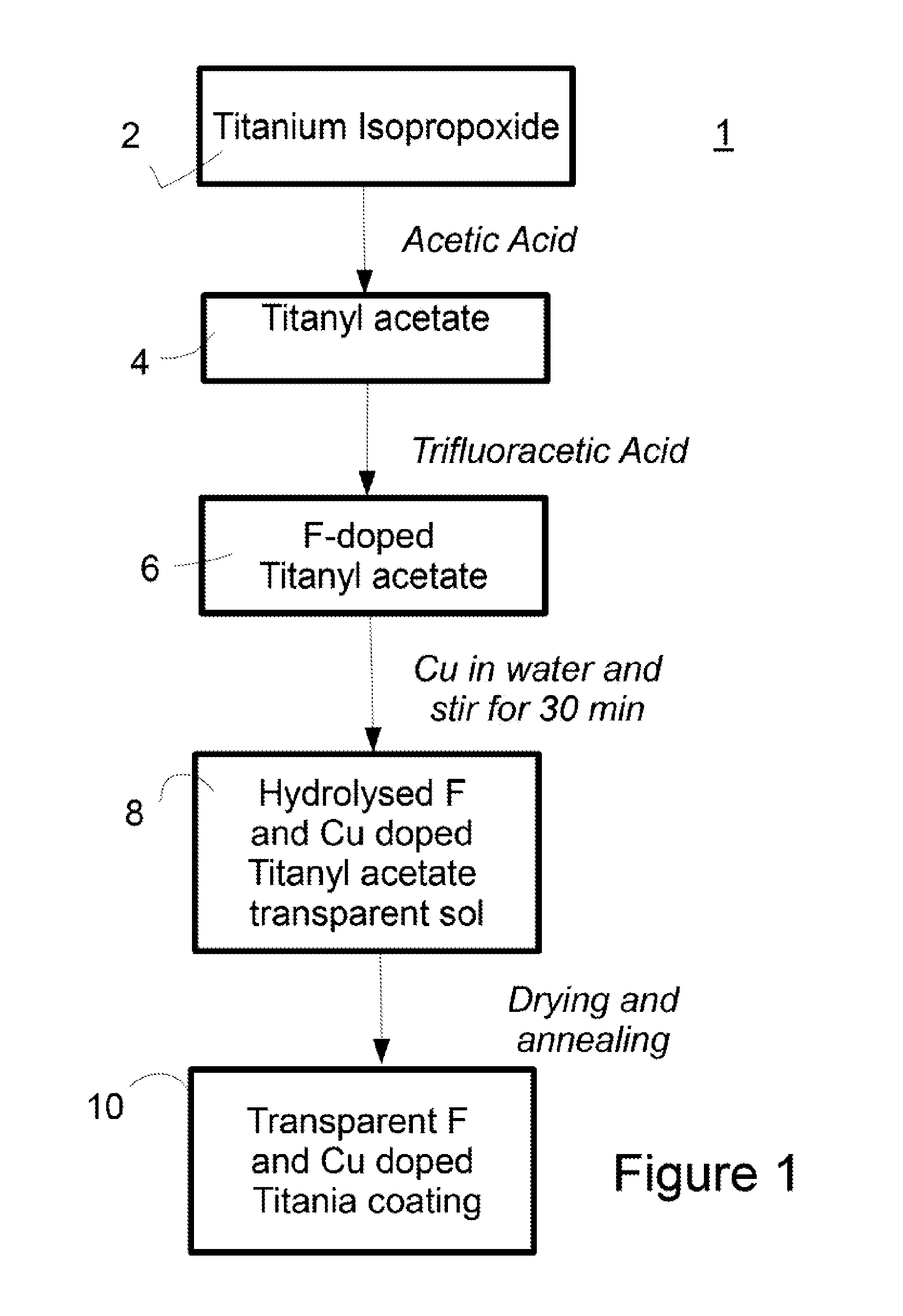
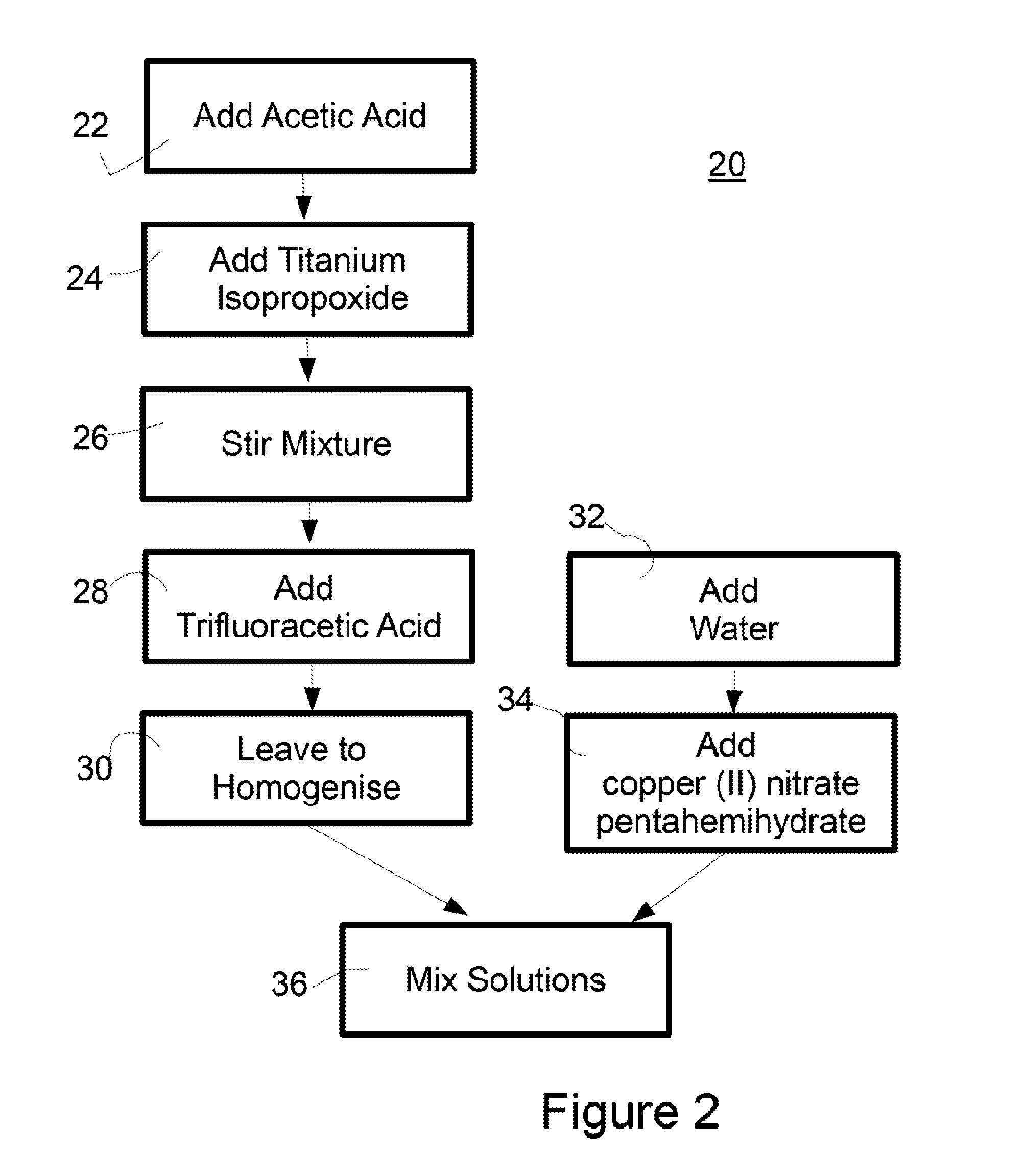
Surface Coating
ActiveUS20150164081A1Reduce packageReduce shipping costsBiocidePretreated surfacesAcetic acidFluoroacetic acid
A process for the preparation of an antimicrobial coating solution is described. The process comprises the steps of: (i) mixing a chelating agent with titanium alkoxide and fluoroacetic acid; and (ii) adding an aqueous solution to the mixture from step (i). The antimicrobial coating described is visible light activated. The coating is applied to surfaces and then heat treated to form a transparent layer on the surface. This is particularly advantageous where the surface is glass.
Owner:KASTUS TECHNOLOGIES DESIGNATED ACTIVITY COMPANY
Compound with hemicyanine-naphthalimide structure, and preparation method and application thereof
The invention relates to a compound with a hemicyanine-naphthalimide structure, and a preparation method and an application thereof. The structural formula of the compound is shown in the specification. The dissolvability, the maximum absorption, the optimum fluorescence emission wavelength and the quantum yield of the compound are tested in the spectrum property experiment of the compound; a solvent effect experiment of the fluorescence emission of the compound shows that the fluorescence emission of the compound has a substantial solvent effect; experiments of response of the compound to ions show that the absorption peak nearby 600nm gradually enhances with increase of the trifluoroacetic acid concentration, and the absorption peak at 490nm decreases with the increase of the trifluoroacetic acid concentration until disappearance, so the structure of the compound changes after trifluoroacetic acid is added; and fluorescence spectrum detection finds that the maximum emission spectrum intensity enhances with the addition of trifluoroacetic acid, so the compound can be used as an acidic fluorescence probe. The preparation method of the compound has the characteristics of simple operation, mild conditions and high reaction yield.
Owner:HENAN UNIVERSITY
Extraction process of small-molecular fucoidan and application of small-molecular fucoidan in cosmetics
InactiveCN108997446AEasy to makeEasy to synthesizeCosmetic preparationsSugar derivativesFreeze thawingFucoidan
The invention provides an extraction process of small-molecular fucoidan and an application of the small-molecular fucoidan in cosmetics. After kelp is made into kelp wall-broken slurry, a chitosan acetic acid solution is firstly used and is supplemented with sodium perborate to precipitate polysaccharides, and the precipitate is dispersed in distilled water and then is degraded by combining lysozyme with trifluoroacetic acid; and finally, the small-molecular fucoidan is separated by repeated freeze-thaw treatment. The obtained product has low molecular weight (200-300 Da), has high purity, has proper and good fluidity, and can be used for preparing the cosmetics.
Owner:北京康塑生物科技有限公司
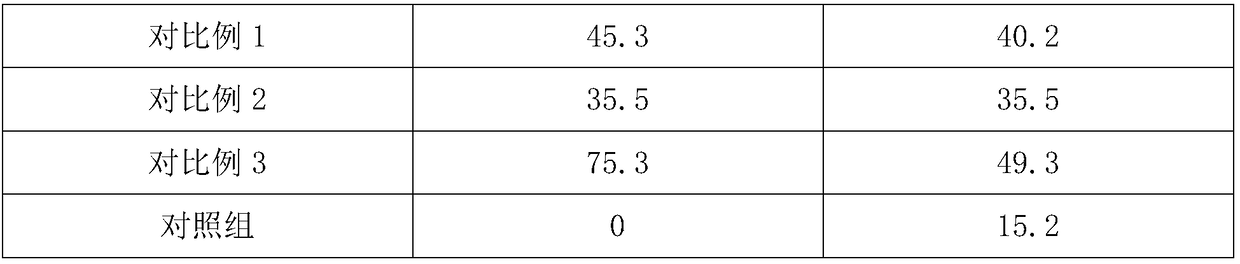
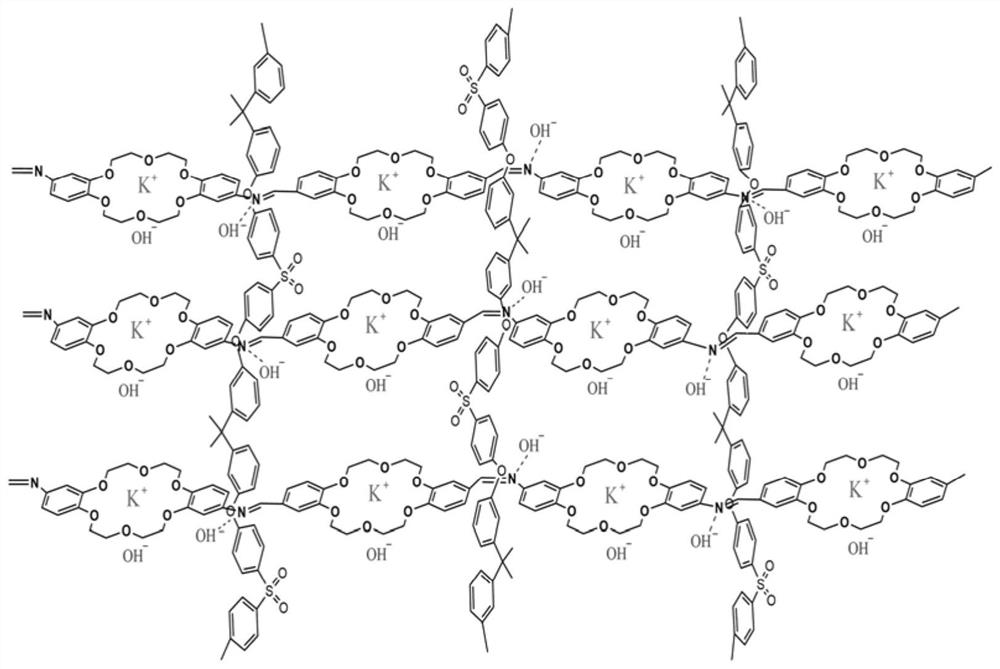
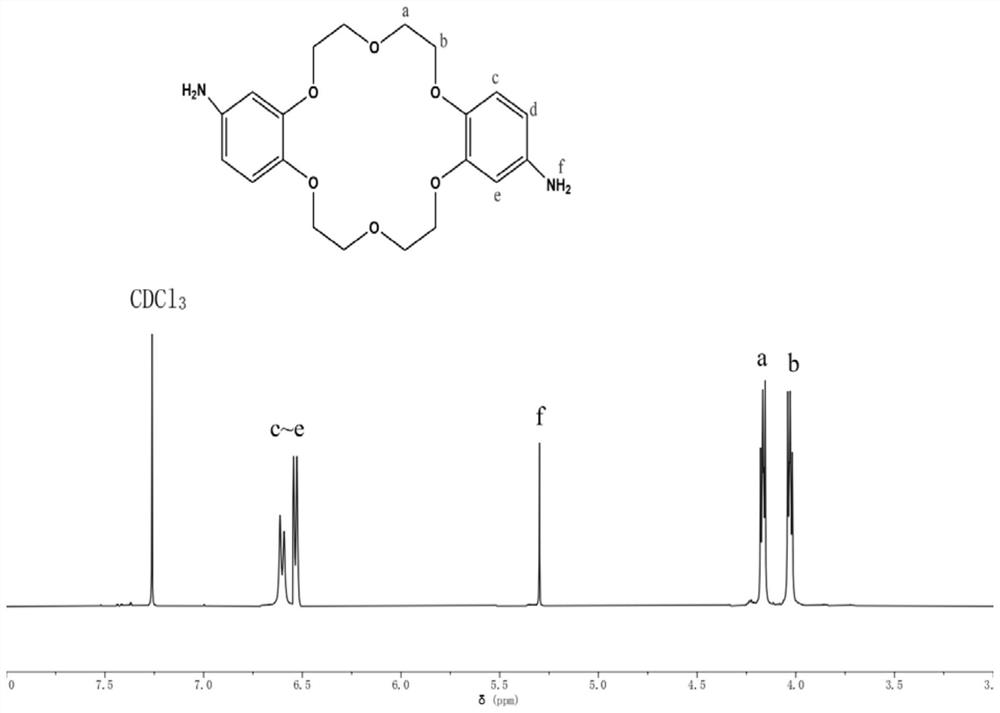
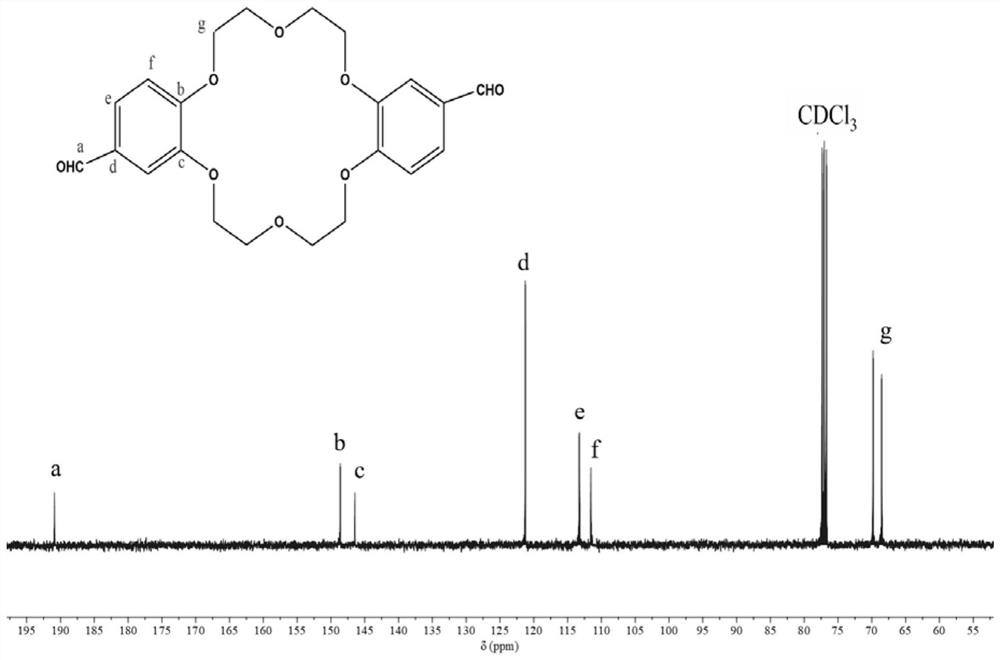
Preparation method of Schiff base crown ether polymer and application of Schiff base crown ether polymer in anion exchange membrane
The invention belongs to the technical field of fuel cells, and particularly relates to a preparation method of a Schiff base crown ether polymer and application of the Schiff base crown ether polymer in a modified polysulfone anion exchange membrane for a fuel cell. The preparation method comprises the following specific steps: adding trifluoroacetic acid into dibenzo-18-crown-6 and hexamethylenetetramine, so as to obtain dialdehyde dibenzo-18-crown-6; the preparation method comprises the following steps: adding dichloromethane and glacial acetic acid into dibenzo-18-crown-6, and dropwise adding a mixed acid solution of glacial acetic acid and concentrated nitric acid to obtain dinitrodibenzo-18-crown-6; the preparation method comprises the following steps: adding ethanol and an aqueous solution into dinitrodibenzo-18-crown-6 and sodium hydroxide, and then adding thiourea dioxide to obtain diaminodibenzo-18-crown-6; the preparation method comprises the following steps: dissolving dialdehyde dibenzene-18-crown-6 and diaminodibenzene-18-crown-6 into dichloromethane, so as to obtain a target product. The anion exchange membrane prepared by the invention has good alkali-resistant stability and mechanical properties.
Owner:LIAONING UNIVERSITY OF PETROLEUM AND CHEMICAL TECHNOLOGY
Method for determining content and associated substances of sorafenib tosylate in high-performance liquid phase chromatography
InactiveCN105181844AEffective controlStrict and effective controlComponent separationTosylic acidFluoroacetic acid
The invention discloses a method for determining content and associated substances of sorafenib tosylate. The method comprises the following steps: taking a reverse high-performance liquid phase chromatography, preparing a test solution and a reference solution, respectively measuring 10 micro liters of the test solution and 10 micro liters of reference solution, injecting the solution into a chromatograph, recording a chromatogram, calculating at a peak area according to an external standard method, and drying to complete the test on the content of the sorafenib tosylate and other substances. The method has the advantages that the blank that no method for testing and analyzing the content and associated substances of the sorafenib tosylate is provided at present is filled, the sorafenib tosylate and impurities can be separated by virtue of an acetonitrile-trifluoroacetic acid aqueous solution, the research development and production requirement can be met, and the associated substances in a sorafenib tosylate active ingredient can be more strictly and effectively controlled.
Owner:JIANGSU SINOBIOPHARMA
Synthesis method of tetracarboxylic dianhydride with fluorinated rigid structure
InactiveCN111303183AEfficient synthesis methodThe synthesis method is safeOrganic chemistryPolymer scienceFluoroacetic acid
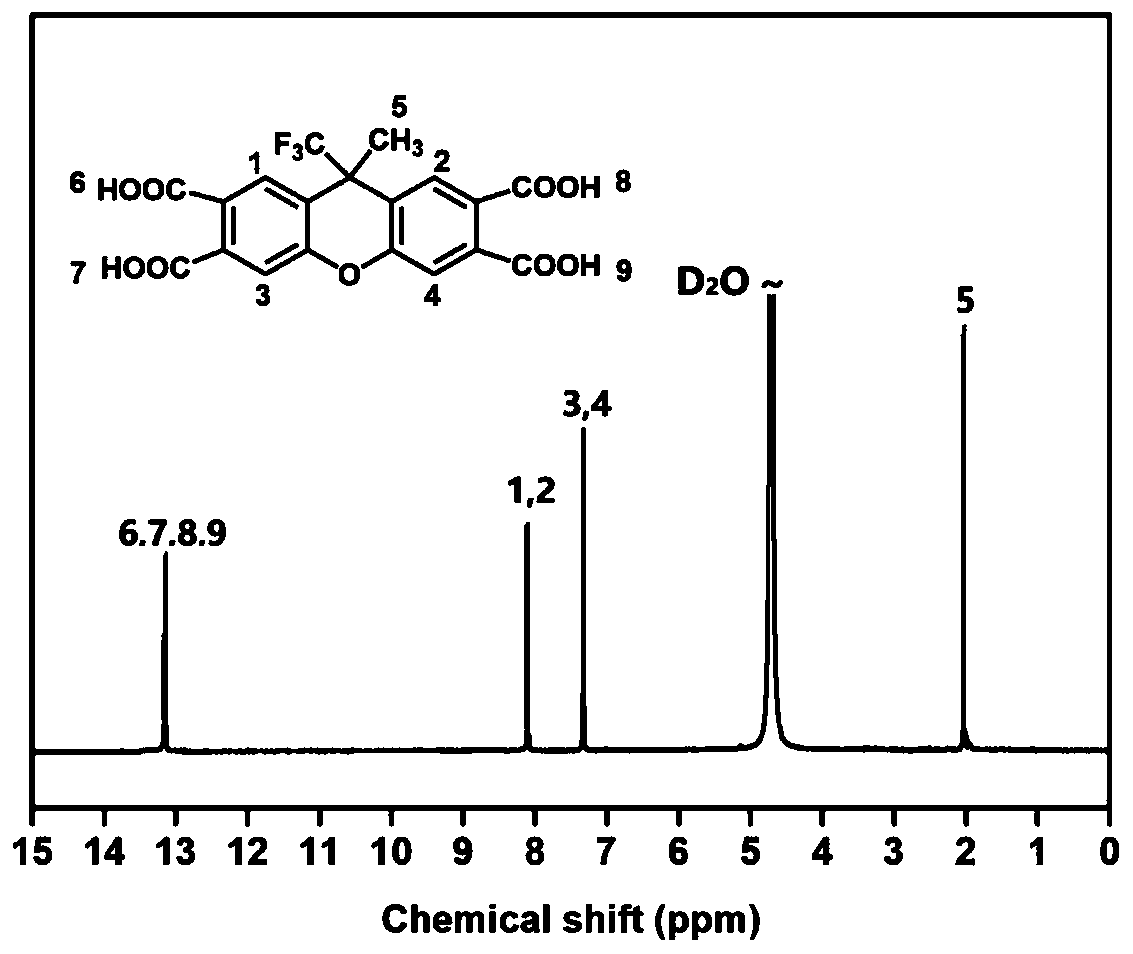
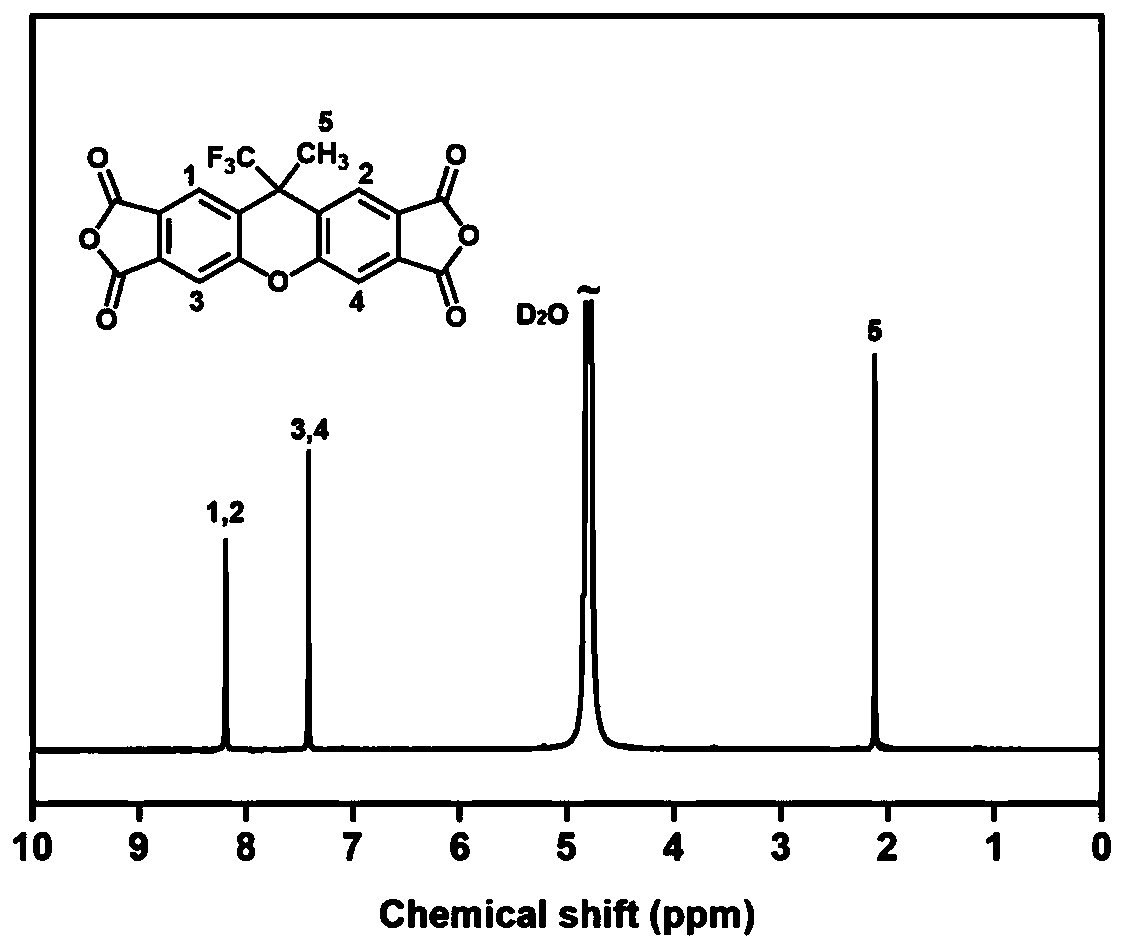
The invention provides a synthesis method of tetracarboxylic dianhydride with a fluorinated rigid structure. According to the method, trifluoromethanesulfonic acid, trifluoroacetic acid and / or sulfuric acid is adopted to replace hydrofluoric acid to serve as a catalyst to achieve conversion from 3,4-dimethylphenol or 3,3',4,4'-tetramethyldiphenyl ether to xanthene tetracarboxylic dianhydride, so that the whole synthesis method has the advantages of being efficient, safe and controllable.
Owner:浙江中科玖源新材料有限公司
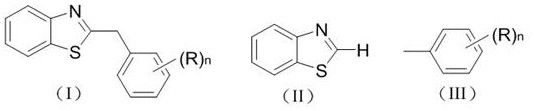
Photocatalytic synthesis method of C2 substituted 2H-benzothiazole benzylated derivative
The invention discloses a photocatalytic synthesis method of a C2 substituted 2H-benzothiazole benzylated derivative. The photocatalytic synthesis method comprises the following steps of: mixing 2H-benzothiazole with substituted methyl benzene; adding an oxidizing agent Selectfluor, an additive trifluoroacetic acid and a solvent acetonitrile, carrying out a normal temperature stirring reaction under the protection of nitrogen and the irradiation of an LED blue light lamp, carrying out TLC monitoring until the reaction is finished, and carrying out separation and purification on the reaction liquid to obtain the C2 substituted 2H-benzothiazole benzylated derivative. The new method for synthesizing the C2 substituted 2H-benzothiazole benzylated derivative through visible light induction by taking Selectfluor as an oxidizing agent, trifluoroacetic acid as an additive and acetonitrile as a solvent is high in atom economy, simple in catalytic system, good in product yield, wide in substraterange and suitable for popularization and application.
Owner:ZHEJIANG UNIV OF TECH
Energetic crystal material obtained through self-assembly of melamine nitrogen oxide and oxidant and preparing method thereof
ActiveCN110117212AImprove adjustabilityHigh densityOrganic chemistryExplosive ingredient compoundingDetonationHigh energy
The invention discloses a preparing method of an energetic crystal material obtained through self-assembly of a melamine nitrogen oxide and an oxidant. The preparing method comprises the steps of 1, preparing the melamine nitrogen oxide, wherein a sufficient amount of melamine is added to an appropriate amount of trifluoroacetic acid to be dissolved, an aqueous hydrogen peroxide solution is addeddropwise, reaction and filtering are conducted to obtain a white precipitate, the white precipitate is dissolved in an appropriate amount of water, alkaline is added to neutralize the solution to keepthe pH value at 7, and filtering is conducted to obtain the melamine nitrogen oxide; 2, preparing the energetic crystal material, wherein the melamine nitrogen oxide is dissolved in the aqueous hydrogen peroxide solution, nitric acid or perchloric acid separately, and through a series of operation, the energetic crystal material obtained through self-assembly of the melamine nitrogen oxide and the oxidant is obtained. The energetic crystal material obtained through self-assembly of the melamine nitrogen oxide with hydrogen peroxide, nitric acid and perchloric acid has the advantages of beingsimple in preparing technology, low in raw material cost, high in density and detonation performance and proper in sensitivity, and is a high-energy explosive with high potential application value.
Owner:INST OF CHEM MATERIAL CHINA ACADEMY OF ENG PHYSICS
Preparation method of fluoroacetamide hapten and application of monoclonal antibody
ActiveCN109575123AEasy to makeImprove economyOrganic compound preparationSerum albuminCarrier proteinKeyhole-limpet haemocyanin
The invention relates to the technical field of biological chemistry, and particularly discloses a preparation method of fluoroacetamide hapten and application of a monoclonal antibody. The fluoroacetamide hapten is synthesized by taking ethyl fluoroacetate and p-aminophenylacetic acid as main raw materials; the fluoroacetamide hapten and carrier protein bovine serum albumin (BSA) or keyhole limpet hemocyanin (KLH) are coupled through an active lipid method, and thus a conjugate is prepared. Through experimental verification, it shows that the prepared conjugate enables a living body to generate the antibody against fluoroacetamide, that is to say, the prepared conjugate is fluoroacetamide artificial antigen. The prepared fluoroacetamide artificial antigen is suitable for enzyme-linked immunological analysis of fluoroacetamide.
Owner:CHINA AGRI UNIV
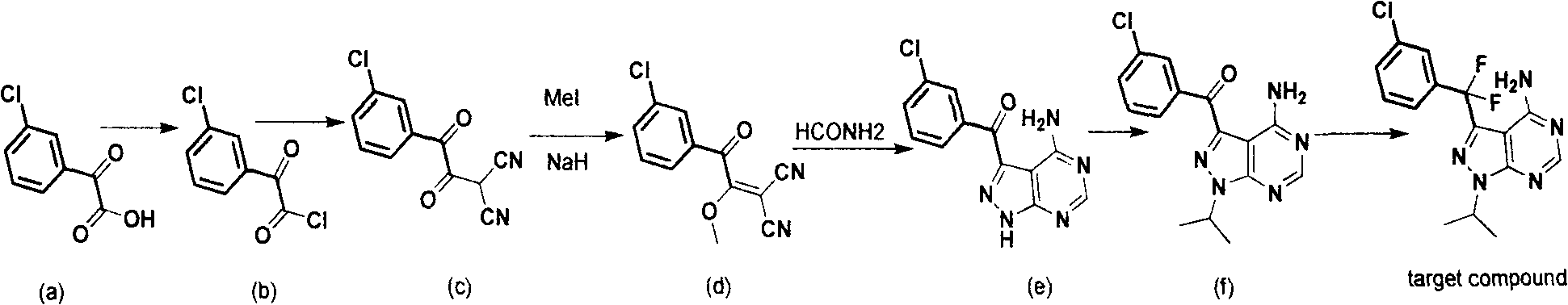
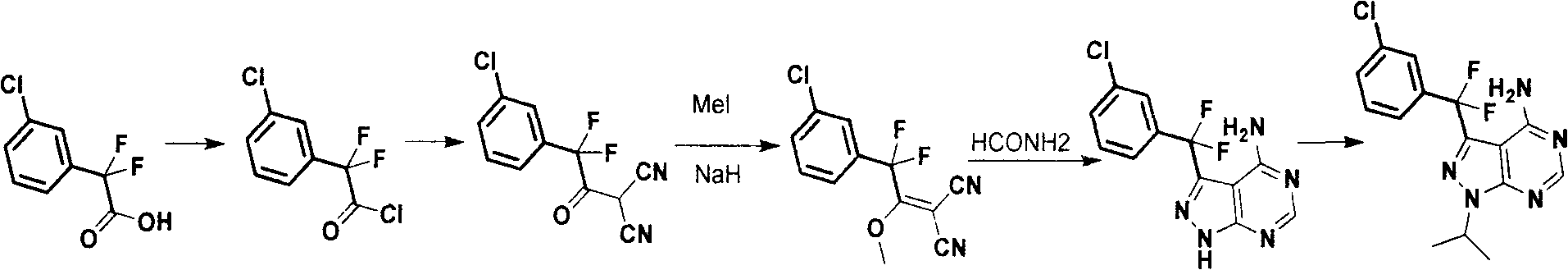
Synthesis process for anti-tumor new medicine 3-{(3-chlorphenyl)difluoromethyl-1-isopropyl-monohydro-pyrazolo(3,4-D)pyrimidine-4-amine compound
The invention discloses a synthesis method for an anti-tumor new medicine 3-{(3-chlorphenyl)difluoromethyl-1-isopropyl-monohydro-pyrazolo(3,4-D)pyrimidine-4-amine compound. The synthesis method comprises the following steps of: acylating 3-chlorphenyl-2 oxalic acid serving as an initiative raw material; reacting the acylated material with dipropionitrile; performing methylation; performing ring closure with hydrazine hydrate to obtain 5-amino-1-(3-chlorophenyl)-pyrazole-4-carbonitrile, crystallizing and purifying, heating and performing ring closure with formamide to obtain a target mother ring; performing fluorination on carbonyl by using DAST; introducing difluorine into an active group so as to improve the bioactivity of the compound; performing process optimization on the basis of the compound; and performing the similar steps by using 2-(3-chlorphenyl)-2,2-difluoroacetic acid as a raw material to obtain the target product.
Owner:CGENETECH (SUZHOU CHINA) CO LTD
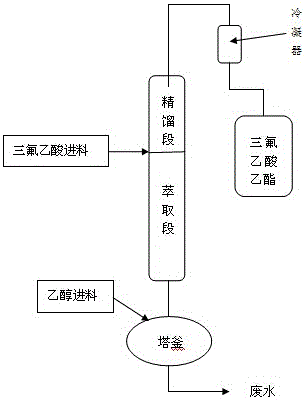
Ethyl trifluoroacetate preparation method
ActiveCN106431908ALow cost and readily availableEasy to operateOrganic compound preparationCarboxylic acid esters preparationAlcoholTrifluoroacetic acid
The invention discloses an ethyl trifluoroacetate preparation method which includes the steps: pre-adding an appropriate amount of trifluoroacetic acid, ethyl alcohol and concentrated sulfuric acid into a reaction kettle with a rectifying tower according to prescribed proportion, or directly adding a certain amount of ethyl trifluoroacetate; rising temperature, performing backflow, and adding the ethyl alcohol and the trifluoroacetic acid of predetermined proportion from the top ends of a tower kettle and an extraction section of the rectifying tower respectively after proportion of various components in the rectifying tower is balanced and stabilized; directly collecting products at the top end of the rectifying tower after the products are purified to obtain the ethyl trifluoroacetate. According to the preparation method, solid super-acid or concentrated sulfuric acid serves as a catalyst, the trifluoroacetic acid and the ethyl alcohol are continuously fed, water in the tower kettle is regularly and quantitatively removed, continuous generation is achieved, production capacity is improved, reaction yield can reach 99%, and product purity can reach 99.9%.
Owner:NANTONG BAOKAI CHEM
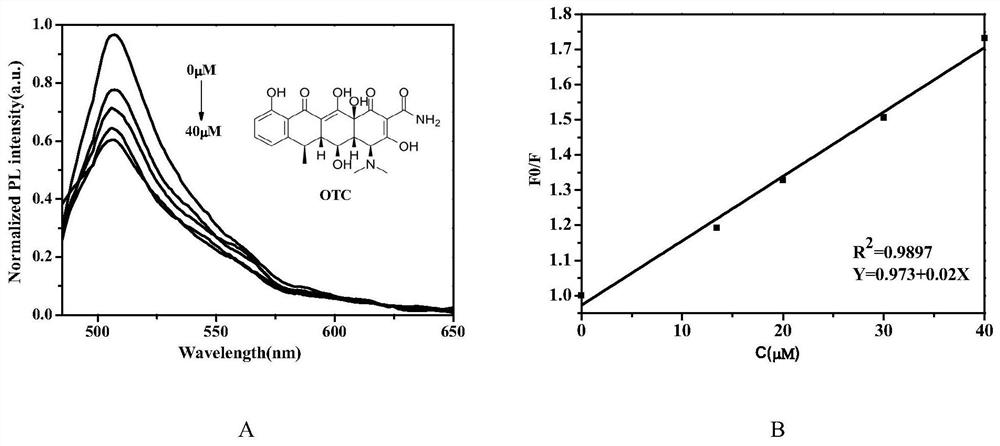
Synthesis method of water-soluble fluorescent probe and application of the water-soluble fluorescent probe to detection of antibiotics
ActiveCN112724166AMild reaction conditionsHigh sensitivityGroup 3/13 element organic compoundsFluorescence/phosphorescenceBiotechnologyBenzoic acid
The invention belongs to the technical field of chemical analysis and detection, and particularly relates to a synthesis method of a water-soluble fluorescent probe and application of the water-soluble fluorescent probe to detection of antibiotics. The fluorescent probe is prepared from raw materials such as 2,4-dimethylpyrrole, methyl p-formylbenzoate, trifluoroacetic acid, DDQ, triethylamine and a boron trifluoride diethyl etherate complex under mild reaction conditions, and is used for detecting the content of antibiotics in food, environment and other samples, such as roxithromycin, tetracycline and oxytetracycline. The detection method has the advantages of high sensitivity, good selectivity, short detection time and the like.
Owner:JIANGSU UNIV
Synthesis method of beta-trifluoromethyl substituted alcohol organic molecule
ActiveCN111155142ASimple ingredientsReduce usageElectrolysis componentsElectrolytic organic productionFluoroacetic acidPtru catalyst
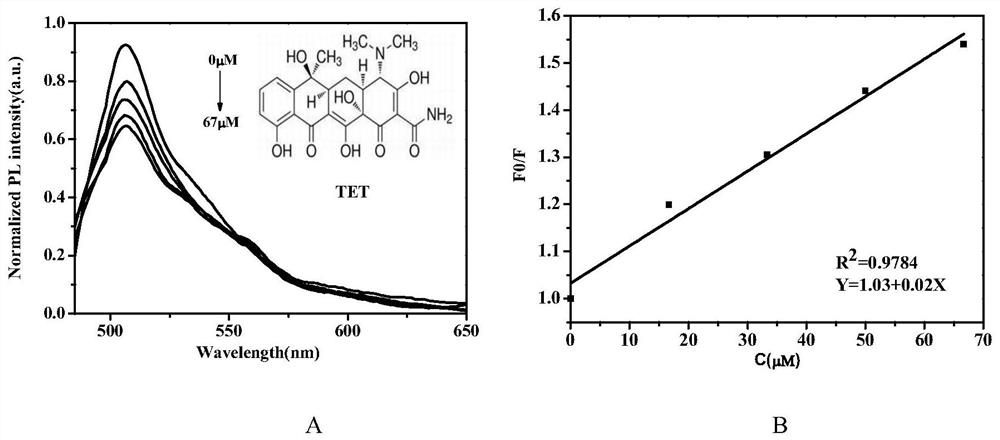
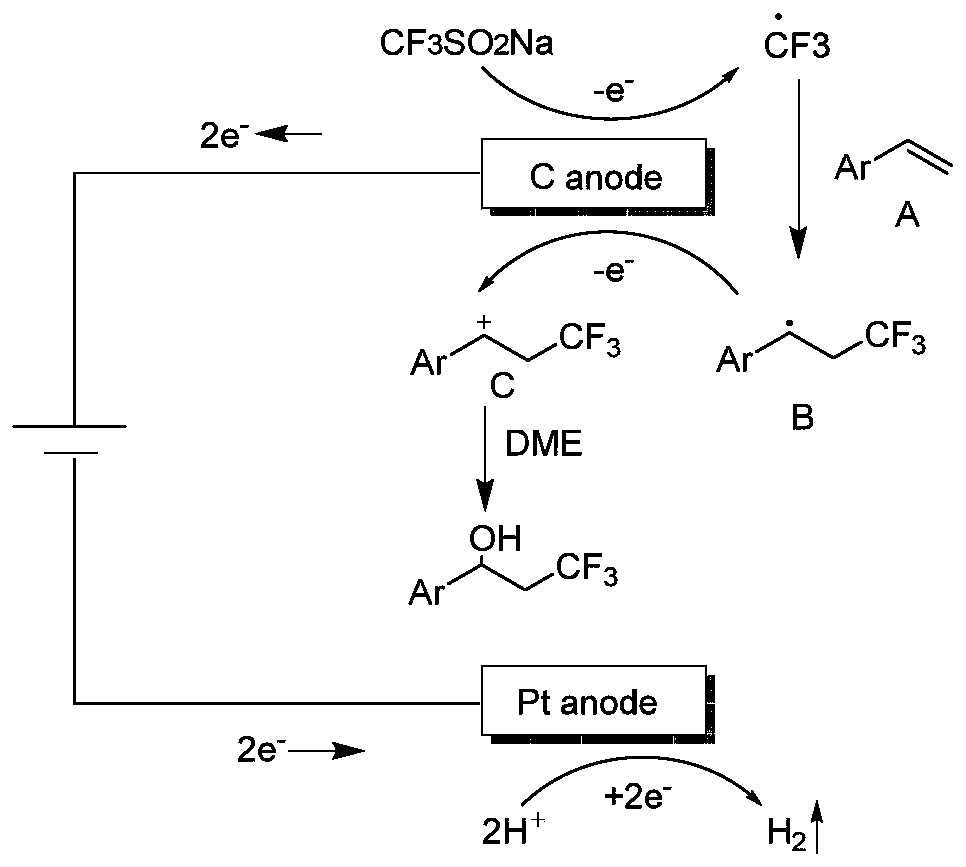
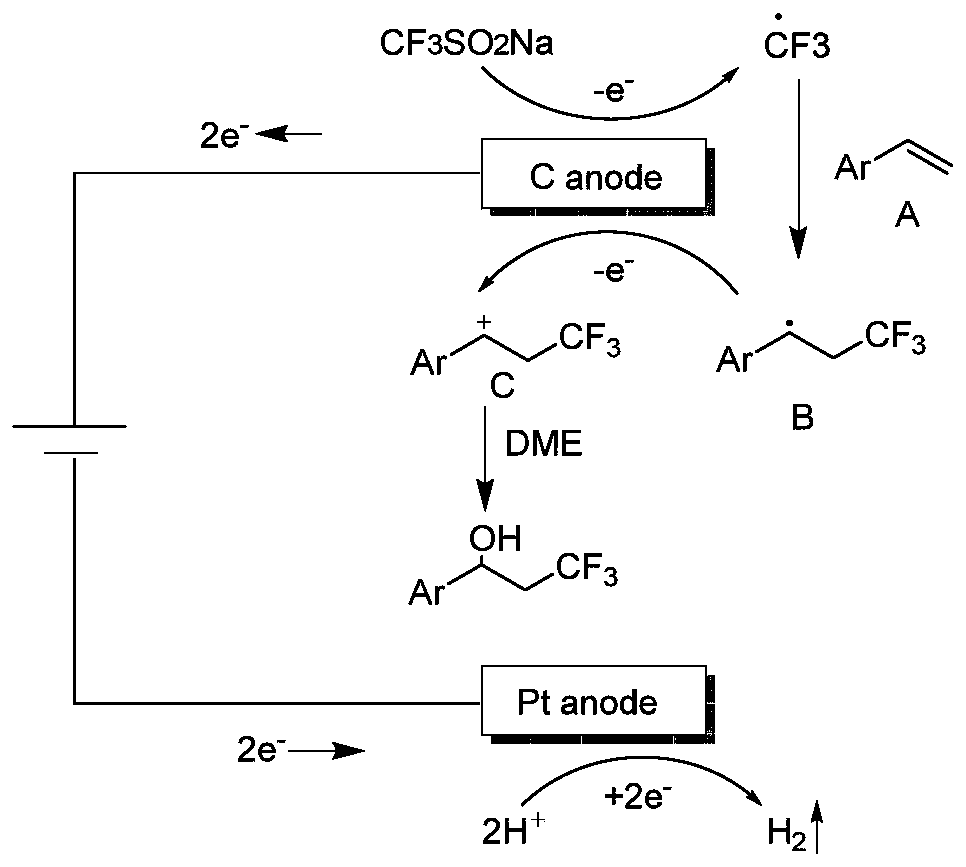
The invention discloses a synthesis method of a beta-trifluoromethyl substituted alcohol organic molecule. The method comprises the following steps: firstly, mixing a styrene substrate, sodium trifluoromethanesulfinate and an electrolyte salt lithium perchlorate; adding a solvent ethylene glycol dimethyl ether and a strong acid trifluoroacetic acid while stirring, then inserting a counter electrode below the liquid level of the solvent, and carrying out an electrocatalytic reaction under the conditions of constant current of 15 + / -3 mA and stirring to obtain the beta-trifluoromethyl substituted alcohol organic molecule corresponding to the substrate. The beta-trifluoromethyl substituted alcohol organic molecule is synthesized by using an electro-catalysis means, and an active intermediateis generated through direct initiation of single electron transfer, so the use of a metal catalyst and a peroxidant is avoided, the reaction system is green and efficient, and a practical and effective path is provided for the synthesis of the beta-trifluoromethyl substituted alcohol organic molecule.
Owner:NANJING UNIV OF SCI & TECH
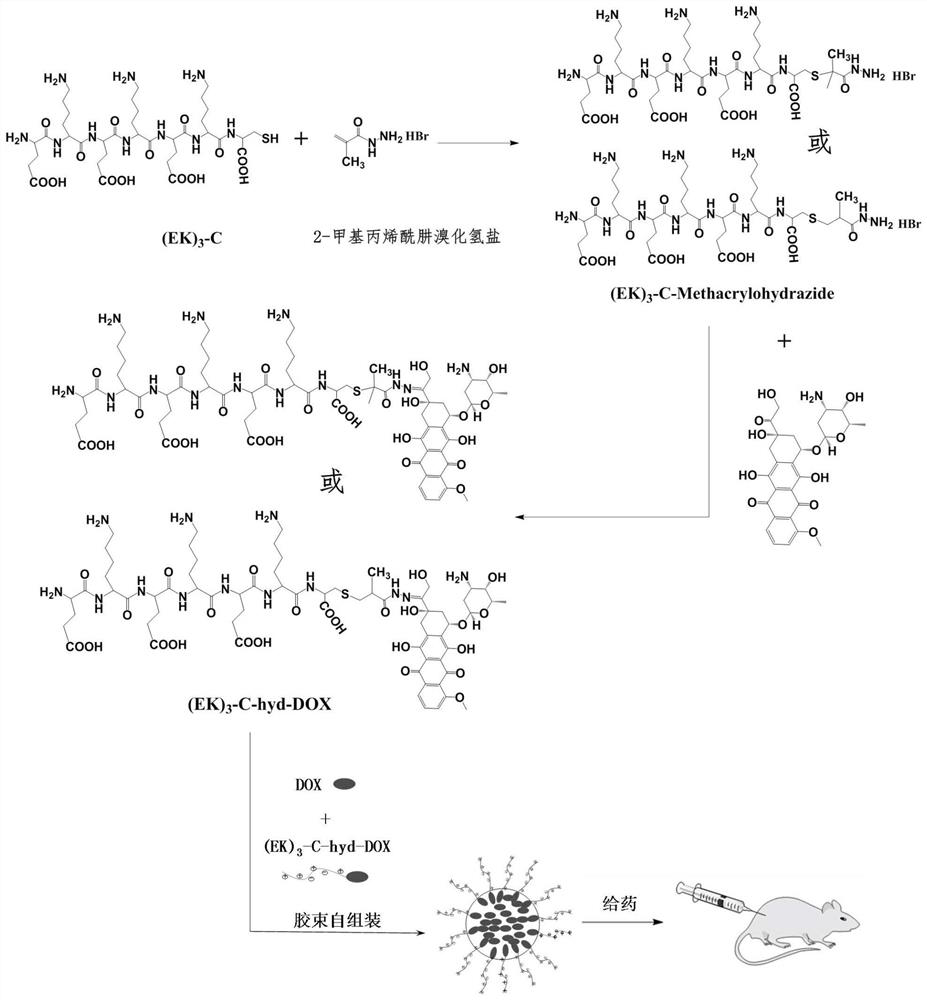
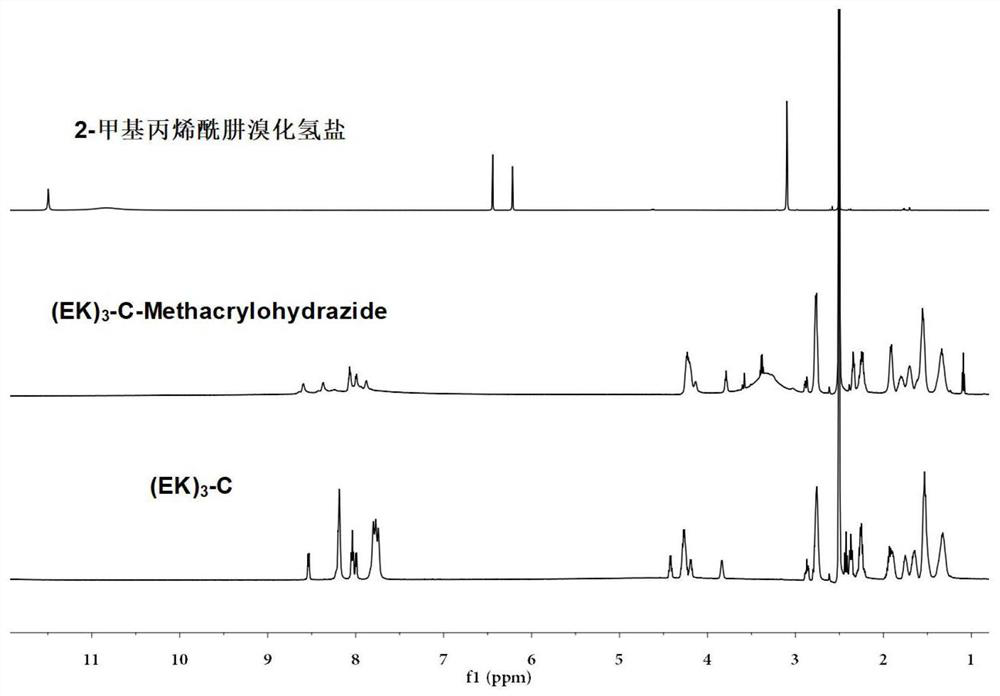
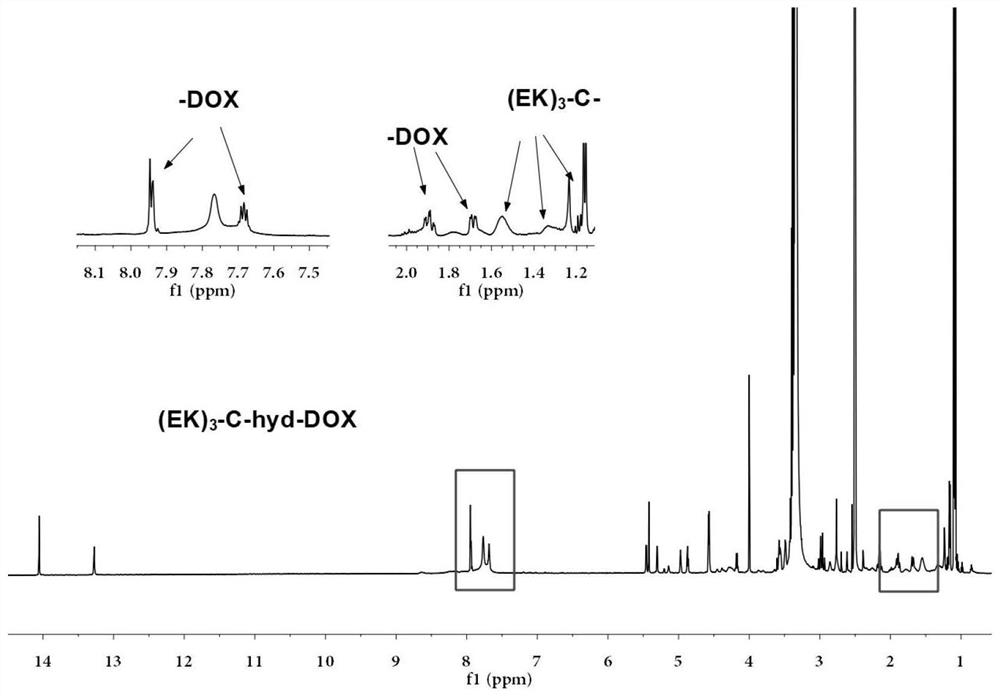
Sulfydryl-containing zwitterionic polypeptide modified doxorubicin derivative, nano-micelle and preparation methods of doxorubicin derivative and nano-micelle
ActiveCN111643678AQuick releaseGood pH responseOrganic active ingredientsNanomedicineFluoroacetic acidTrifluoroacetic acid
The invention provides a sulfydryl-containing zwitterionic polypeptide modified doxorubicin derivative, a nano-micelle and a preparation method of the nano-micelle, and belongs to the field of biological medicines. The doxorubicin derivative has a structure as shown in a general formula (I). The preparation method of the doxorubicin derivative comprises the following steps of in a polar solvent, mixing sulfydryl-containing zwitterionic polypeptide with acryloylhydrazine to react, and performing crystallizing to obtain a polypeptide derivative; and dissolving the polypeptide derivative and doxorubicin hydrochloride in the polar solvent according to a molar ratio of 1: (0.2-5), adding a trifluoroacetic acid catalyst, and carrying out a stirring reaction to obtain the doxorubicin derivative.The nano-micelle prepared from the doxorubicin derivative is long in in-vivo blood circulation time, high in drug loading capacity, small in toxic and side effects, good in pH value response and goodin tumor inhibition effect.
Owner:YANSHAN UNIV
Oxidation of hydrocarbons to acids in the presence of fluoro compounds
This invention relates to methods of oxidizing hydrocarbons, such as cyclohexane, o-xylene, m-xylene, and p-xylene, to form a respective acid, such as adipic acid, phthalic acid, isophthalic acid, and terephthalic acid, for example, by introducing into the reaction mixture small critical amounts of fluorocompounds. Preferable fluorocompounds are perfluoroacids, such as for example perfluoroacetic acid, perfluorobutyric acid, and perfluorooctanoic acid. By introducing the critical amounts of the fluorocompounds, the reactivity increases without sacrificing yield and / or selectivity.
Owner:RPC INC
New synthesis method of methyl fluoroacetate and ethyl fluoroacetate
ActiveCN112920050AImprove conversion efficiencySimple post-processingOrganic compound preparationCarboxylic acid esters preparationFluoroacetic acidPtru catalyst
The invention discloses a novel synthesis method of methyl fluoroacetate and ethyl fluoroacetate. The method comprises the following specific steps: adding methyl chloroacetate or ethyl chloroacetate into a reaction kettle, and starting stirring; after uniformly stirring, sequentially adding potassium fluoride, a catalyst A and a catalyst B, and closing a charging hole; heating to 120 DEG C by using heat-conducting oil, starting reaction, stopping heating by the heat-conducting oil and starting heat preservation when the kettle temperature reaches 180 DEG C and the kettle pressure reaches 0.8 MPa after 1 hour; after keeping heat preservation for 3 hours and the kettle temperature reaches 220 DEG C and the kettle pressure reaches 1.5 MPa, continuously preserving heat for 5 hours; and cooling to 80 DEG C, discharging, carrying out filter pressing, rinsing, rectifying filtrate through a rectifying tower, and recovering chloroacetate to obtain the product methyl fluoroacetate or ethyl fluoroacetate, the product quality can reach 99.5% or above, and the molar yield (metered by potassium fluoride) reaches 96% or above. The method has the advantages of mild reaction conditions, simple operation, easy control, high fluoro conversion rate, high product yield, simple post-treatment process, realization of solvent-free reaction, and low energy consumption.
Owner:宁夏森萱药业有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com