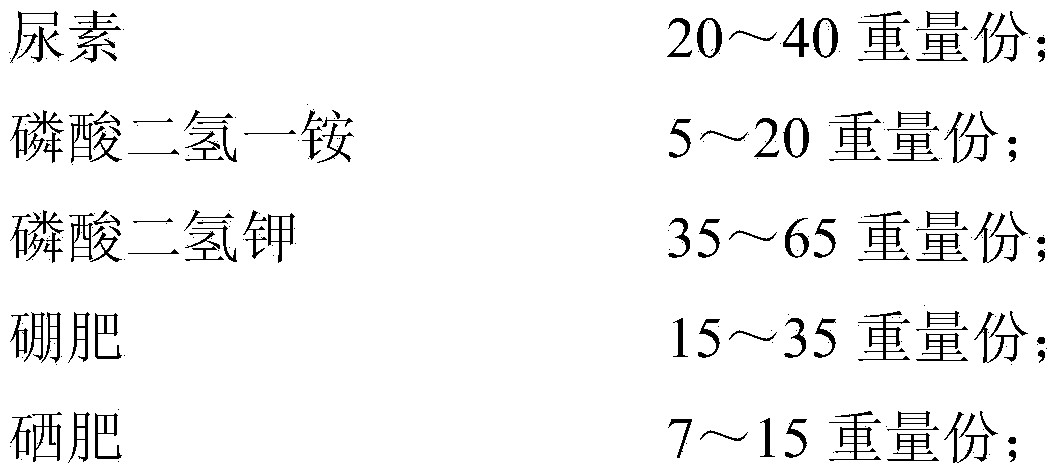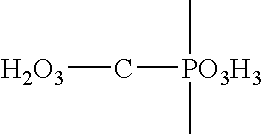Patents
Literature
126 results about "Captopril" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Captopril is used to treat high blood pressure (hypertension).
A kind of preparation method of jellyfish antihypertensive peptide
ActiveCN102286590AReduce manufacturing costActive preservationPeptide preparation methodsFermentationBiotechnologyCaptopril
The invention provides a preparation method for jelly fish neurotensin, which comprises the following steps of: carrying out metallic ion removal treatment on jelly fish; sequentially carrying out steps of homogenating, enzymolysis, enzyme deactivating, concentration, ultrafiltration, freeze drying and the like; and finally obtaining purified jelly fish neurotensin powder. The method combines the modern enzyme engineering technology, provides a novel efficient production path for the jelly fish neurotensin, improves the utilization efficiency of jelly fish resources and has the characteristics that the operation is simple, the extraction rate is high, and the product activity preservation is good. The production cost of the jelly fish neurotensin is reduced. The rich protein resources of the jelly fish are sufficiently utilized, and various metallic ions in jelly fish bodies are basically and clearly removed through the metallic ion removal treatment, so the subsequent enzymolysis and application are safer. Results of jelly fish neurotensin antihypertensive activity experiments adopting spontaneously hypertensive rat (SHR) models show that the group of the preparation method obviously differs from the blank control group. The jelly fish neurotensin prepared by the preparation method has the antihypertensive activity similar to positive control captopril.
Owner:吴川市天然食品加工有限公司
Disease prevention and frost resistance fruit selenium-enriching nutritional agent for fruit trees and preparation method of disease prevention and frost resistance fruit selenium-enriching nutritional agent
InactiveCN103449931AGood for balanced growthPromote growthFertilizer mixturesNutritional statusPhosphate
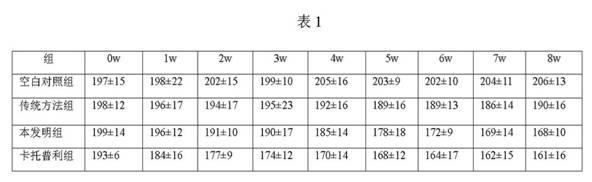
The invention discloses a disease prevention and frost resistance fruit selenium-enriching nutritional agent for fruit trees and a preparation method of the disease prevention and frost resistance fruit selenium-enriching nutritional agent. The nutritional agent consists of the following components in part by weight: 20-40 parts of urea, 5-20 parts of ammonium dihydrogen phosphate, 35-65 parts of potassium dihydrogen phosphate, 15-35 parts of a boron fertilizer, 7-15 parts of a selenium fertilizer, 3-8 parts of salicylic acid, 1-3 parts of captopril, 4-10 parts of humic acid, 3-5 parts of pyrazinamide, 2-6 parts of metronidazole, 10-20 parts of paclobutrazol, 0.4-1 part of methylglutarate and 0.5-2 parts of disopyramide phosphate. The selenium-enriching nutritional agent disclosed by the invention has strong pertinence, can be used for solving multiple prominent problems existing in the planting of the fruit trees simultaneously, and has multiple effects such as disease prevention, rotten fruit prevention, frost resistance, dehiscent fruit prevention, fruit dropping prevention, fruit plumpness promotion, nutrition, selenium enrichment and the like.
Owner:新疆久业富硒农业科技开发有限公司
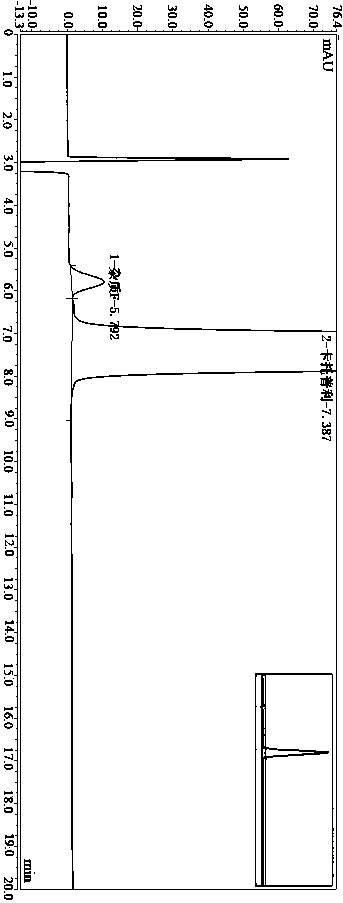
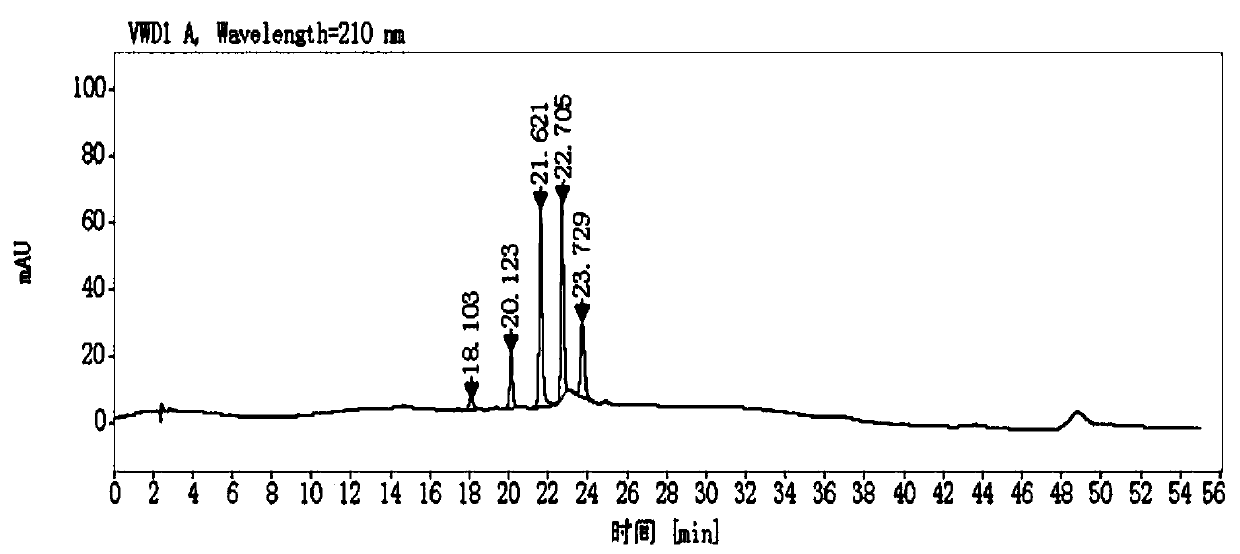
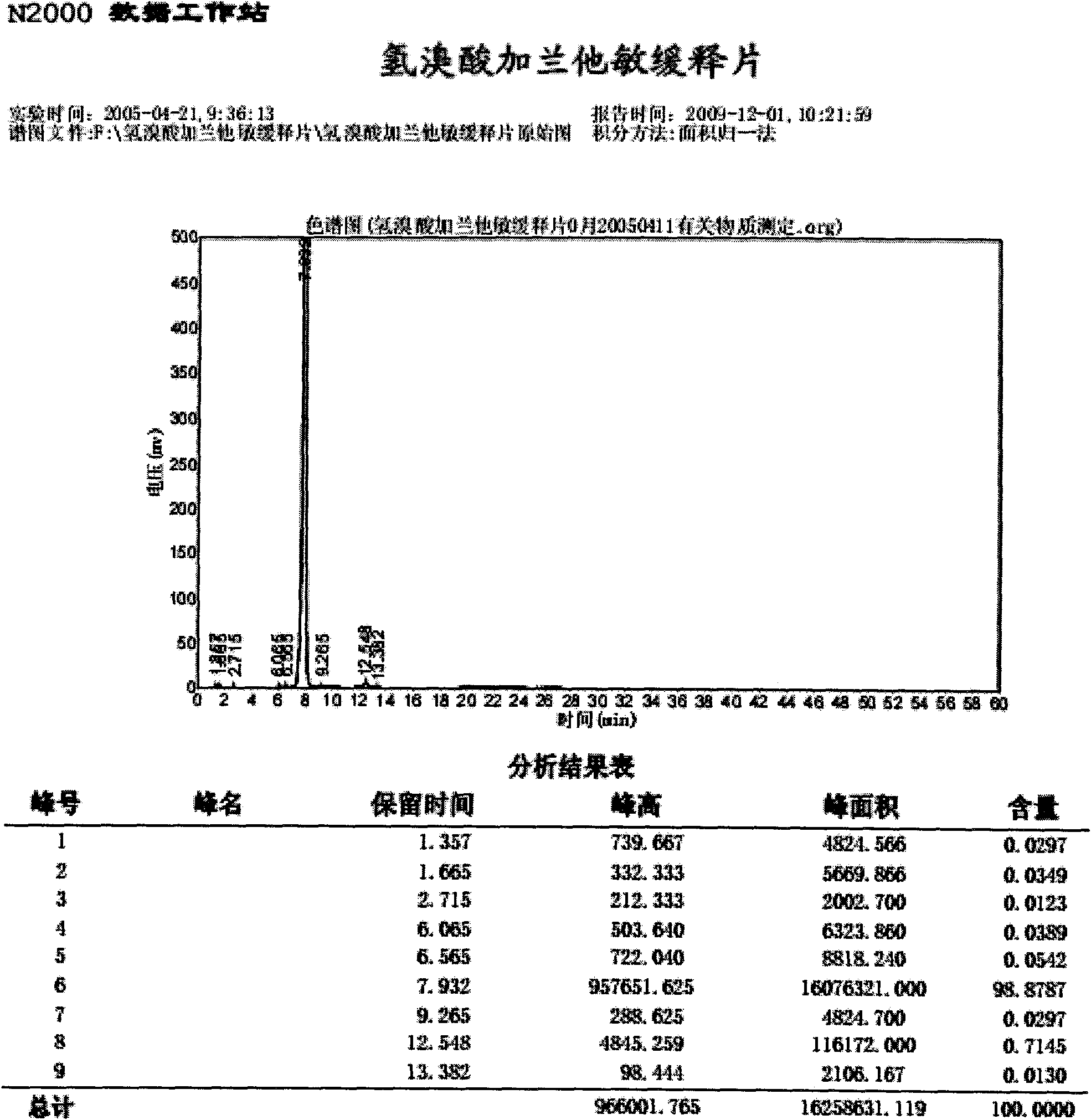
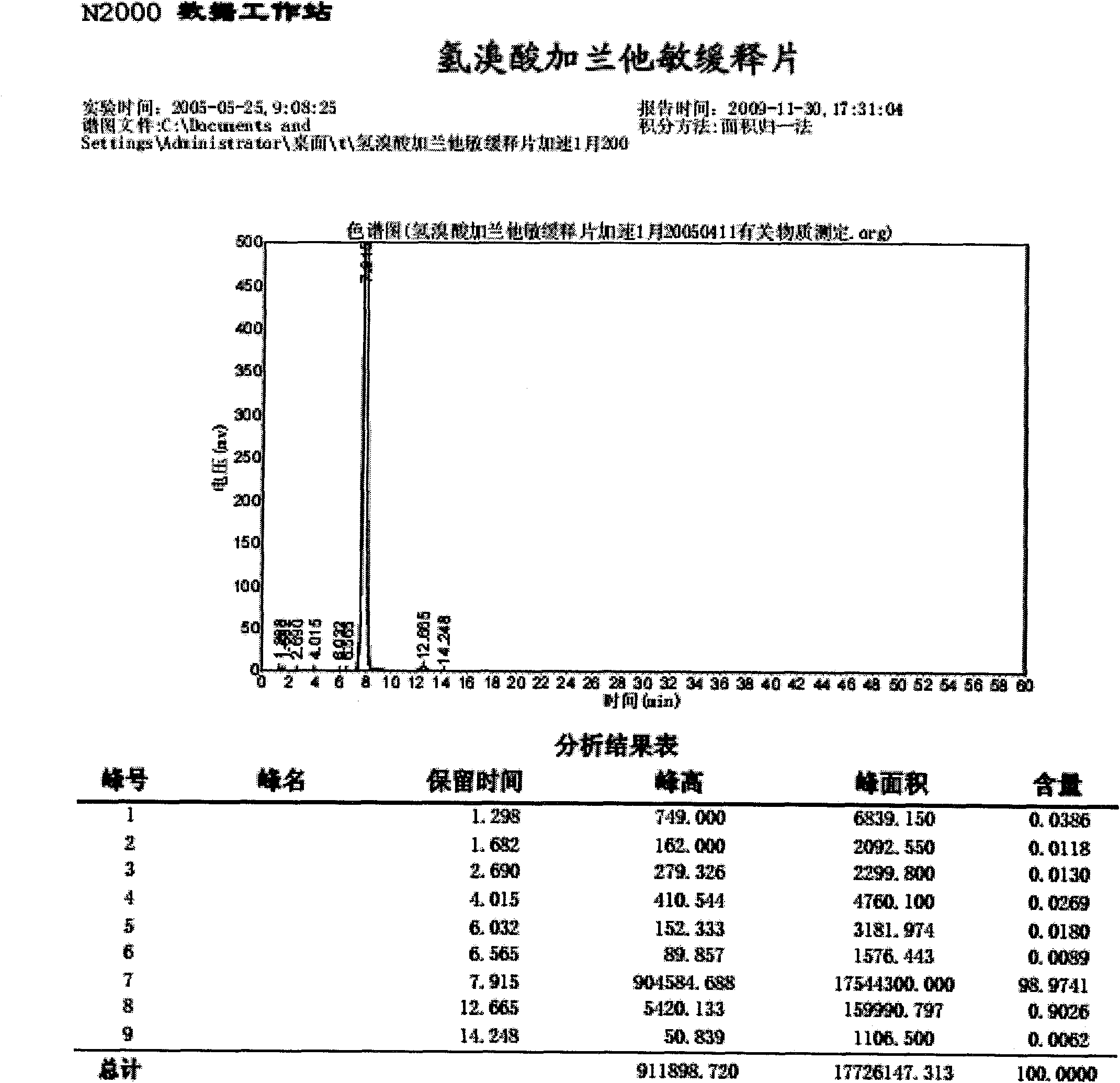
Method for determining impurity F in captopril tablets through high performance liquid chromatography
The invention discloses a method for determining an impurity F in captopril tablets through high performance liquid chromatography and belongs to the technical field of pharmaceutical analysis. Detection is performed under the conditions as follows: an amylase-tris(5-chloro-2-methyl phenyl carbamate) coated chromatographic column is used, normal hexane-absolute ethyl alcohol-trifluoroacetic acid serves as a mobile phase, a volume ratio of the normal hexane to absolute ethyl alcohol to trifluoroacetic acid is 80:20:0.1, a detection wavelength is 215nm, flow velocity is 1ml / min, a column temperature is 35 DEG C and a sample amount is 20[mu]l. A structural formula of the impurity F is as shown in the description. According to the method disclosed by the invention, the content of the impurityF in the captopril tablets can be quantitatively determined, so that the quality of the captopril tablets is effectively controlled. According to the method provided by the invention, the captopril and the impurity F can be proved to be effectively separated in a system suitability solution, and the method has high precision and high separation degree. A signal to noise ratio of a self-contrast solution is more than 10, and if the sample contains the impurity F, the impurity F can be detected.
Owner:ZHENGZHOU TAIFENG PHARMA CO LTD
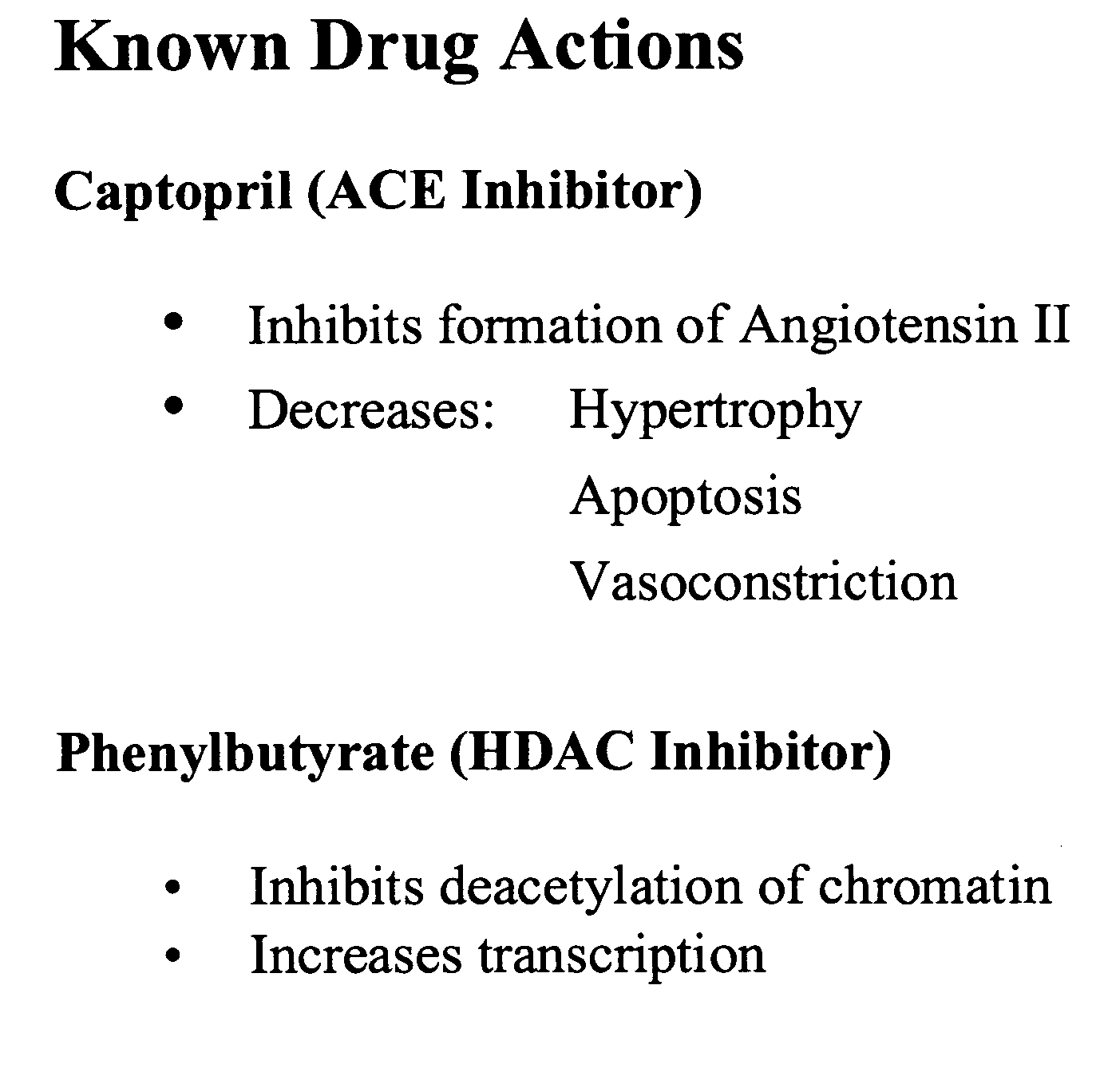
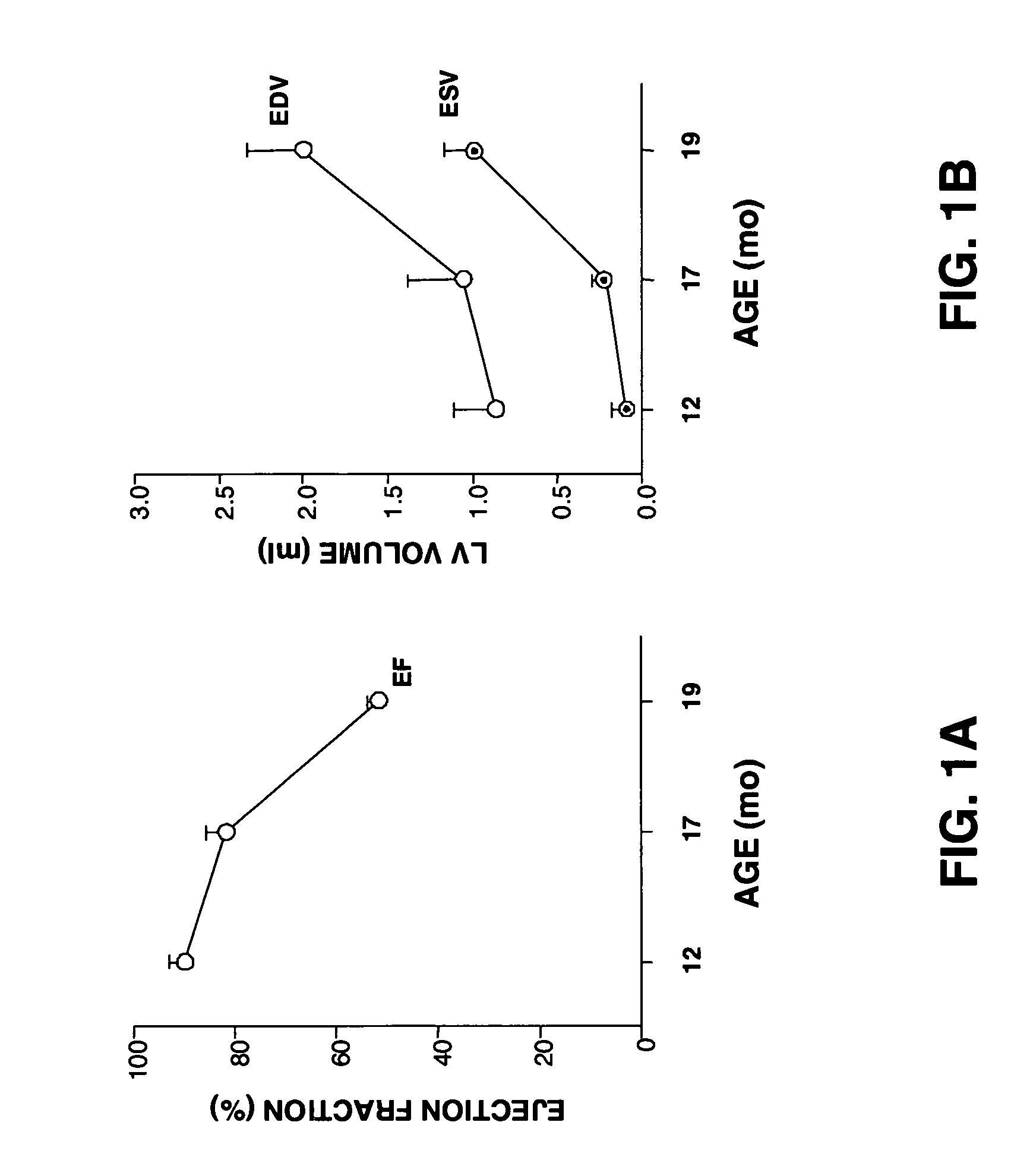
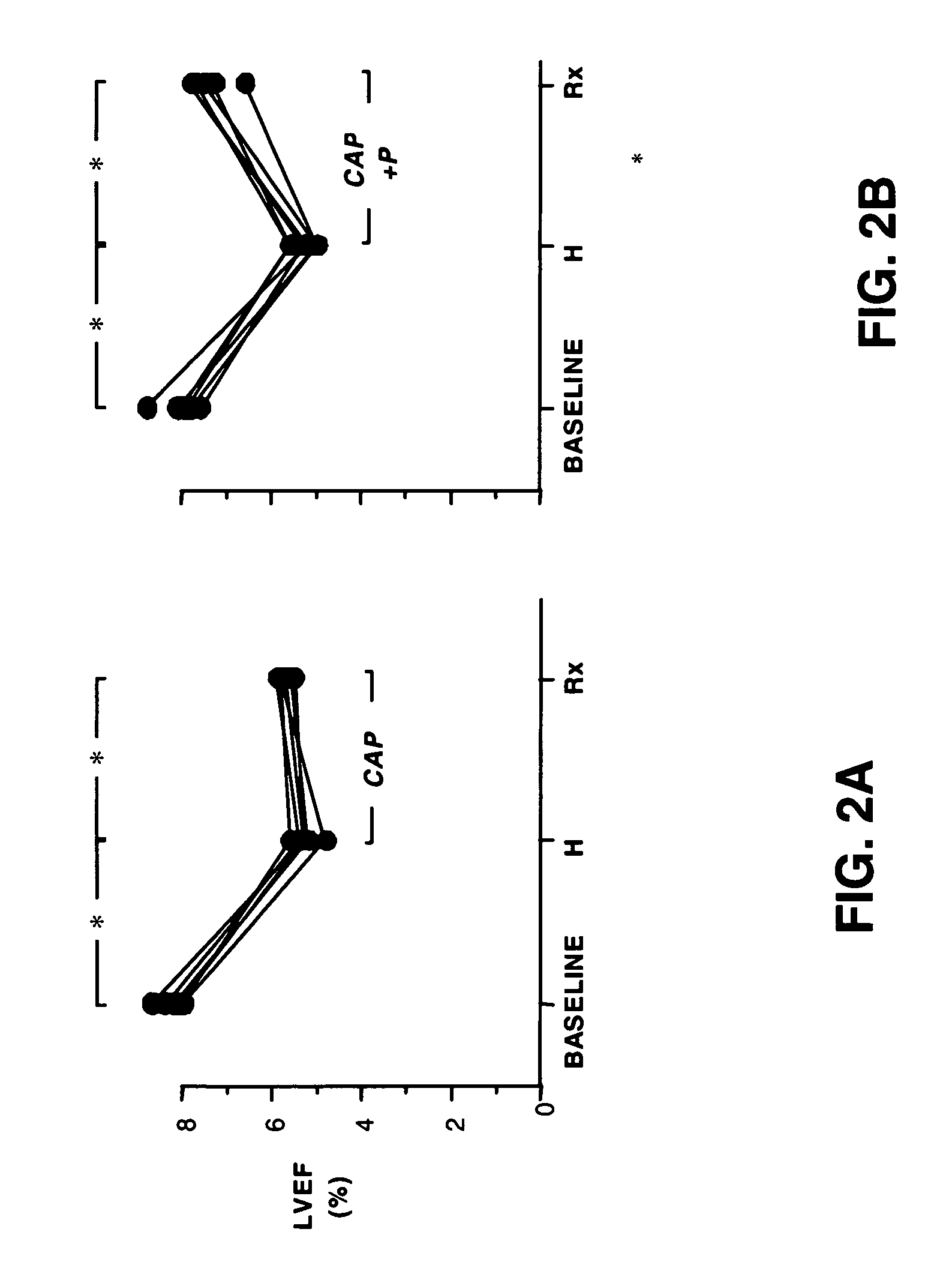
Method and composition for treating heart failure
A method and composition for treating, preventing or ameliorating heart failure, cardiac hypertrophy, and / or myocardial dysfunction includes administering a therapeutically effective amount of a HDAC inhibitor, such as phenylbutyrate, in combination with an ACE inhibitor, such as captopril.
Owner:THE GOVERNMENT OF THE UNITED STATES OF AMERICA AS REPRESENTED BY THE DEPT OF VETERANS AFFAIRS
Use of organic compounds
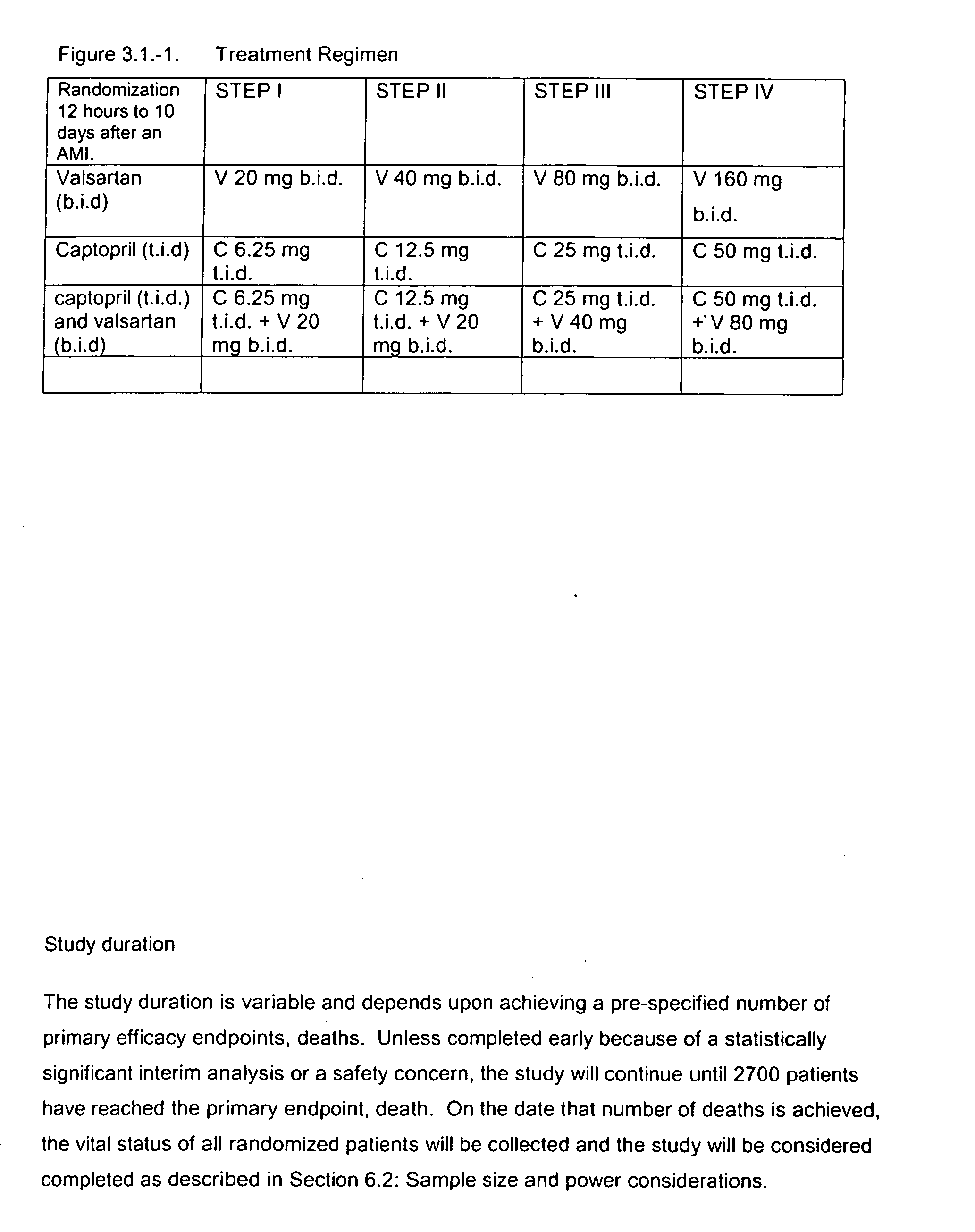
The invention relates to a method of treating cardiovascular disease and inducing cardiovascular remodeling and thereby reducing the risk of morbidity, especially stroke, and mortality following a MI, especially MI complicated with left ventricular dysfunction or heart failure, through the administration of an ARB, particularly valsartan and an ACE inhibitor, particularly captopril. The present invention also discloses dosing regimens and dosage packs for the above treatments.
Owner:GRAVES KURT CHUM
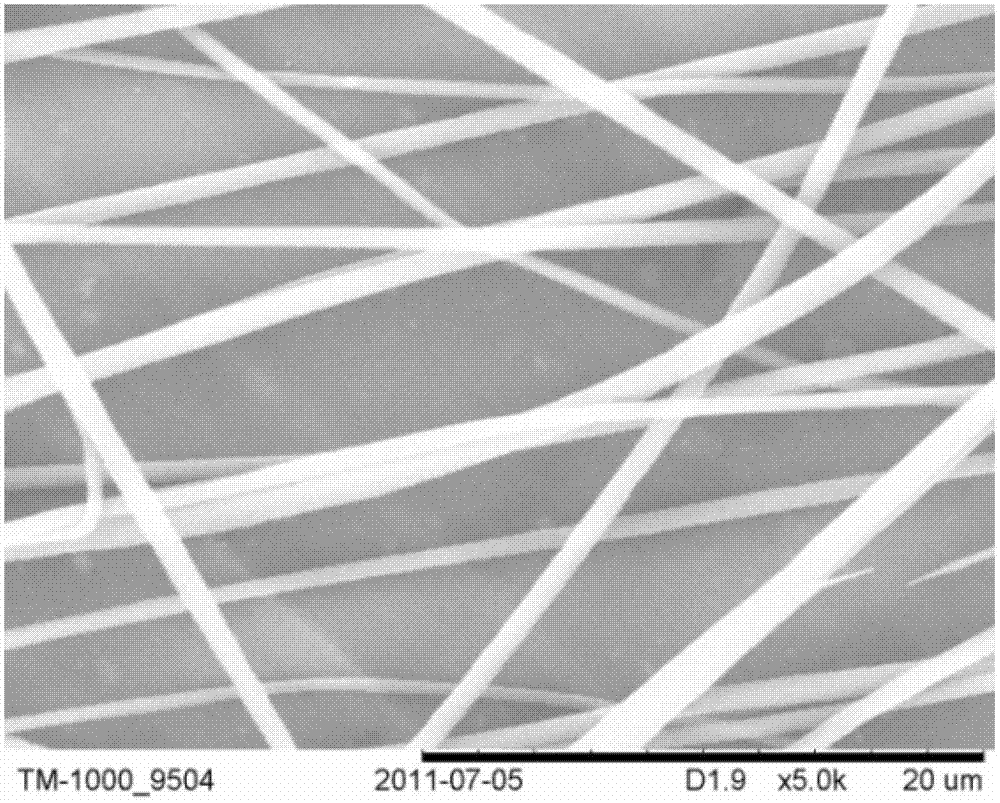
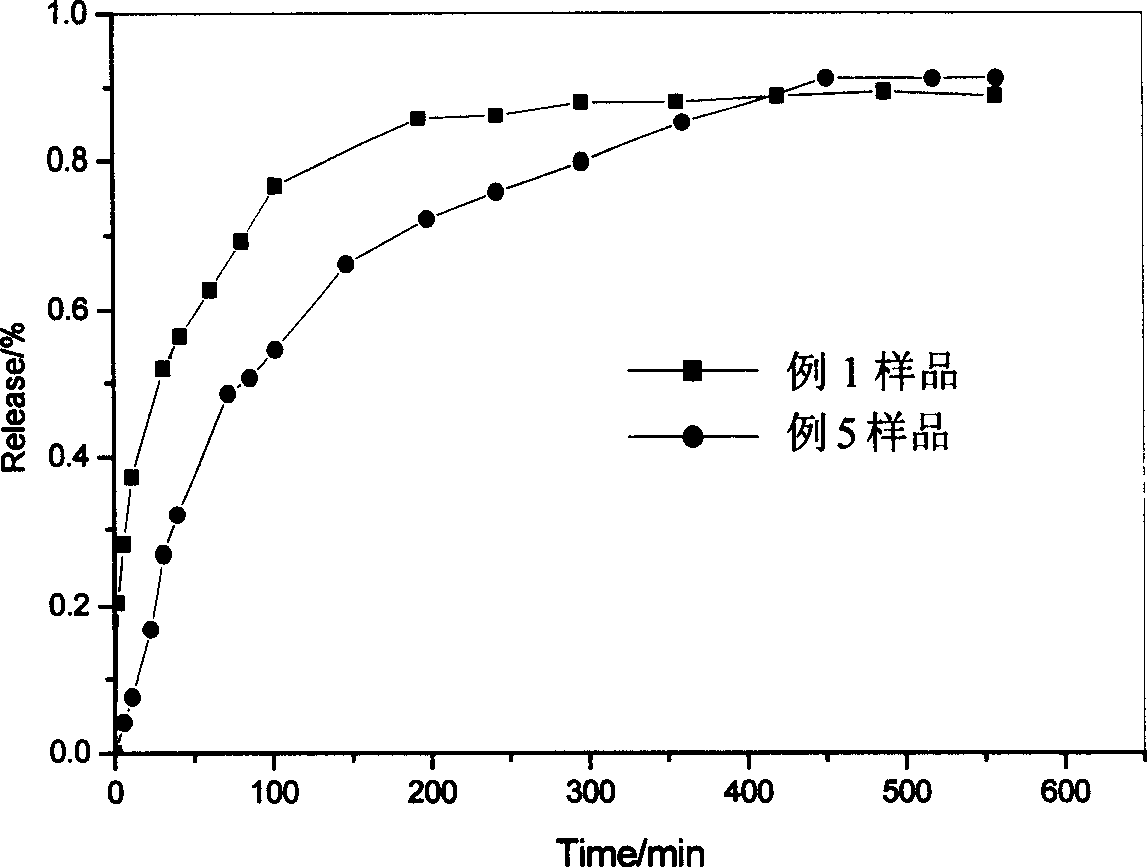
Captopril-carrying nano-grade fiber sustained-release system and preparation method thereof
InactiveCN102727441AControl releaseExtended release timePowder deliveryOrganic active ingredientsDrug availabilityFiber
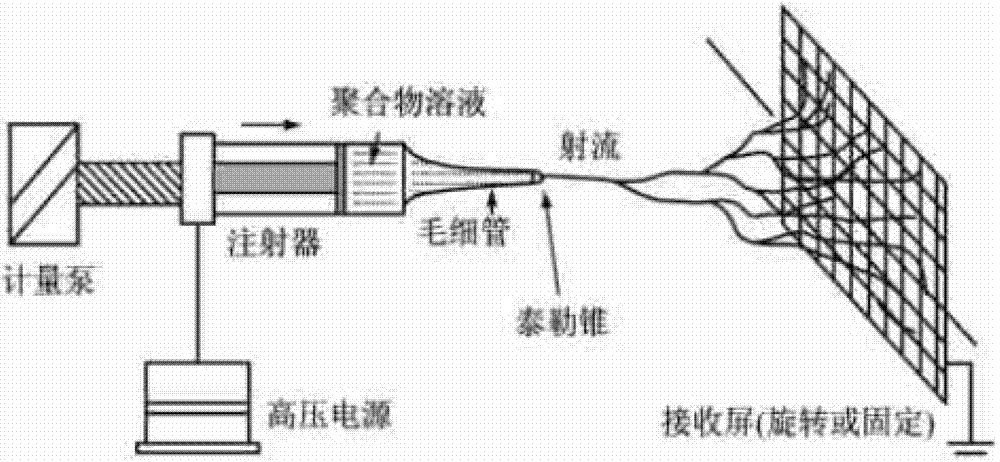
The invention relates to a captopril-carrying nano-grade fiber sustained-release system and a preparation method thereof. The sustained-release system comprises the components of: a biodegradable high-molecular material and captopril. The drug-loading rate of the system is 8-80wt%, and the diameter of the nano-grade fiber is 80nm-1.20mum. The preparation method comprises the steps that: (1) the biodegradable high-molecular material is dissolved in an organic solvent; the mixture is stirred until the material is completely dissolved; captopril is added and the mixture is stirred; the mixture is subjected to ultrasonic deaeration, such that a spinning liquid is obtained; (2) the spinning liquid is added into an injector, and electrostatic spinning is carried out, such that the captopril-carrying nano-grade fiber sustained-release system is obtained. According to the invention, with the nano-grade sustained-release system composed of the drug-carrying nano-grade fiber, drug release is effectively controlled. The biodegradable high-molecular material can be automatically degraded with human metabolism, such that an effect of sustained-release is achieved. The process is simple, an equipment cost is low, the conditions are easy to control, and drug availability is improved.
Owner:DONGHUA UNIV
Application of captopril to inhibition of scar hyperplasia
InactiveCN102335167ALow priceMature technologyOrganic active ingredientsDermatological disorderDiseaseCaptopril
The invention relates to application of captopril to the preparation of medicaments for treating and / or inhibiting scar hyperplastic diseases. Particularly, the hyperplastic diseases comprise hyperplastic scar, cheloid, urethral scar stricture and silicon gel breast augmentation prosthesis postoperative capsular contracture. When the diseases are treated, the captopril is used as an active ingredient, clinically-used emulsifiable paste, gel, injection and the like in different formulations are prepared by the conventional preparation process. The study indicates that the captopril can reduce the capacity of synthesizing pathologic scar fibroblast collagen and secreting the collagen, weaken the multiplication capacity of fibroblasts and reduce the expression of transforming growth factor-beta 1 (TGF-beta 1), and has a good treatment or prevention effect on pathologic scars. Simultaneously, the captopril acts on scar tissue directly, has the advantages of small dose, high curative effect, small side effect, mature raw material process, readily available materials, low cost and the like, and is expected to become a main medicament for treating and preventing the scars clinically.
Owner:PLASTIC SURGERY HOSPITAL CHINESE ACAD OF MEDICAL SCI
Compound blood pressure reducing prepn containing angiotonin converzyme inhibitor, calcium ion agonist and Estazolam
InactiveCN1526398AGood curative effectLittle side effectsOrganic active ingredientsPill deliveryCaptoprilSide effect
The present invention provides one new kind of compound blood pressure reducing preparation containing angiotonin converzyme inhibitor, calcium ion agonist, Estazolam and pharmaceutically acceptable carrier. The angiotonin converzyme inhibitor is selected from Enalapril, Ramipril, Benalapril, Lisinopril, Acertil, etc. as well as their mixture; and the calcium ion agonist is selected from Nitrendpine, Amlodipine Besylate, Nifedipine, Felodipine, etc. as well as their mixture. The present invention utilizes the synergistic effect between different medicines to raise the blood pressure lowering effect, reduce side effect and improve the compliance of patient.
Owner:杜晓锋
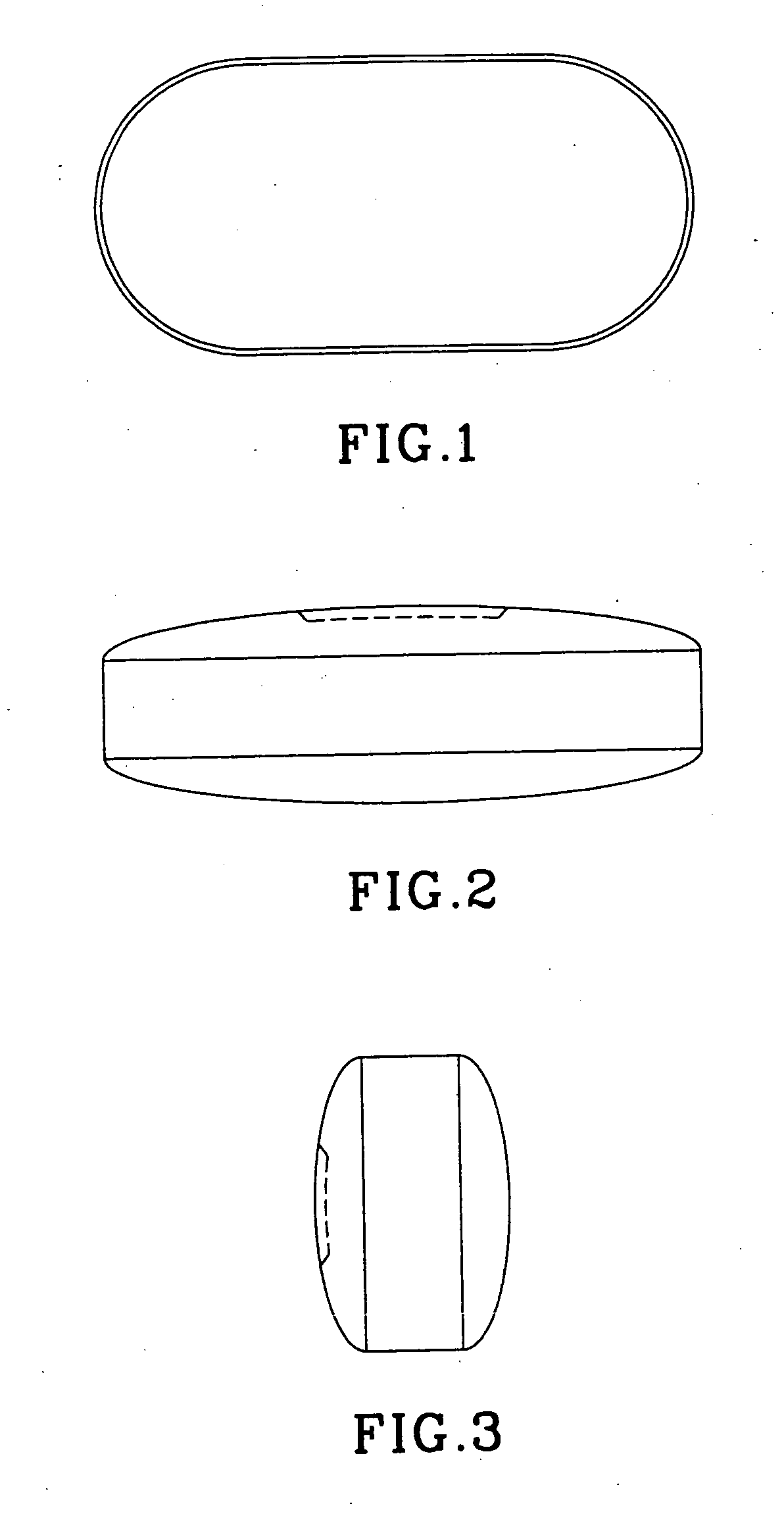
Film coated tablet for improved upper gastrointestinal tract safety
InactiveUS20070071822A1Protection is in progressInhibition releaseBiocideTetracycline active ingredientsIrritationUpper gastrointestinal
A novel oral dosage to be delivered to the stomach comprising a safe and effective amount of an active ingredient selected from the group consisting of emepronium bromidebromide, doxycycline, and other tetracyclines / antibiotics, iron preparations, quinidine, nonsteroidal anti-inflammatory drugs, alprenolol, ascorbic acid, captopril, theophylline, zidovoudine (AZT), bisphosphonates and mixtures thereof and pharmaceutically-acceptable excipients, wherein said oral dosage form is a generally oval form and film coated to facilitate rapid esophageal transit and avoid irritation in the mouth, buccal cavity, pharynx, and esophagus.
Owner:THE PROCTER & GAMBLE COMPANY
Application of Plukenetia volubilis husk extract in preparation of blood pressure lowering medicines
ActiveCN106038672AThe effect is stable and peacefulGood blood pressure effectCardiovascular disorderPlant ingredientsCaptoprilSide effect
The invention discloses an application of a Plukenetia volubilis husk extract in preparation of blood pressure lowering medicines. An extraction method for the Plukenetia volubilis husk extract comprises the following steps of crushing Plukenetia volubilis husks, adding a solvent into the crushed Plukenetia volubilis husks, carrying out decocting extraction, filtering out the husks, filtrating an extracting solution, and carrying out concentration, thereby preparing the Plukenetia volubilis husk extract. According to the application, the natural plant extract is adopted, so that toxic or side effects on human bodies caused by Western medicines such as captopril are avoided. The extraction method is easy and feasible and is flexible in manner. Through carrying out an intragastric administration test on rats by using the Plukenetia volubilis husk extract, a research result shows that the Plukenetia volubilis husk extract has a remarkable blood pressure lowering effect, so that the condition that the Plukenetia volubilis husks contain blood pressure lowering functional components is deduced. The Plukenetia volubilis husk extract belongs to plant extracts and is stable and mild in effect, no obvious toxic or side effects is discovered at present, and the abuse of the traditional Western medicines that sequelae of diabetes are aggravated is avoided while the Plukenetia volubilis husk extract plays a role in lowering blood pressure.
Owner:杜冰 +1
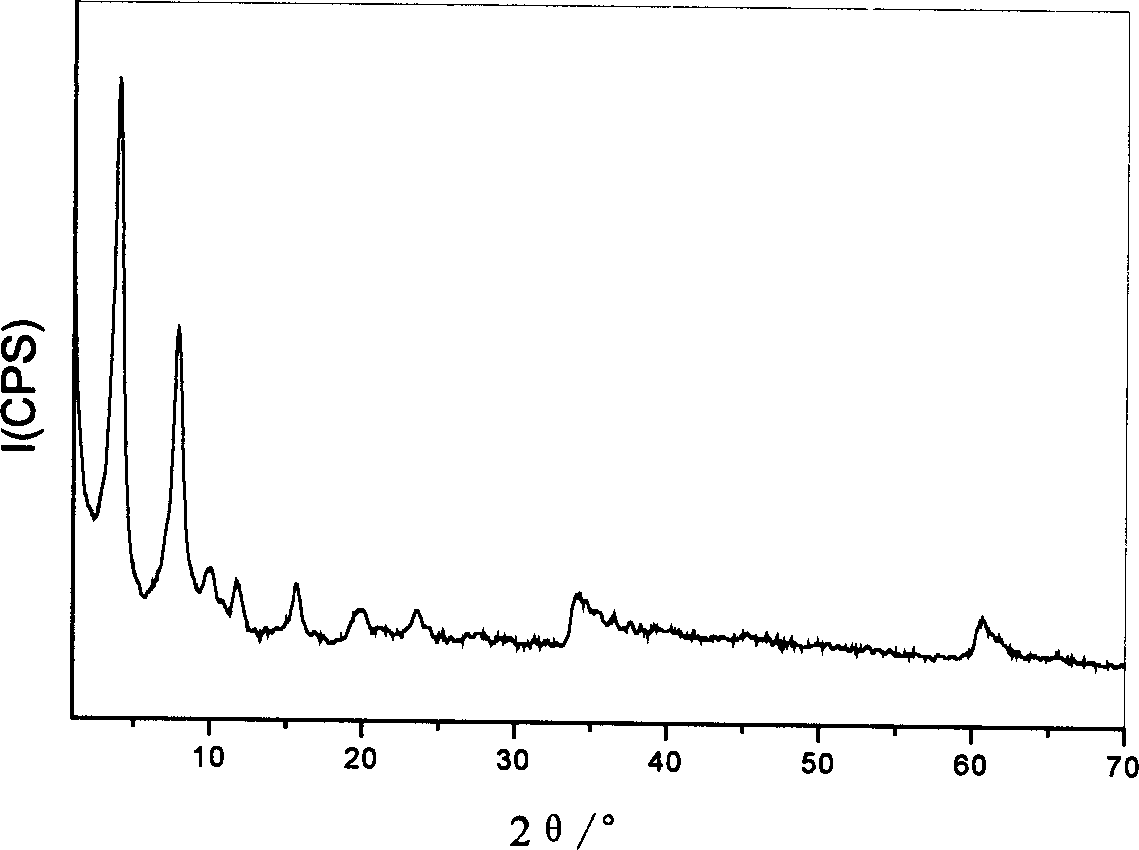
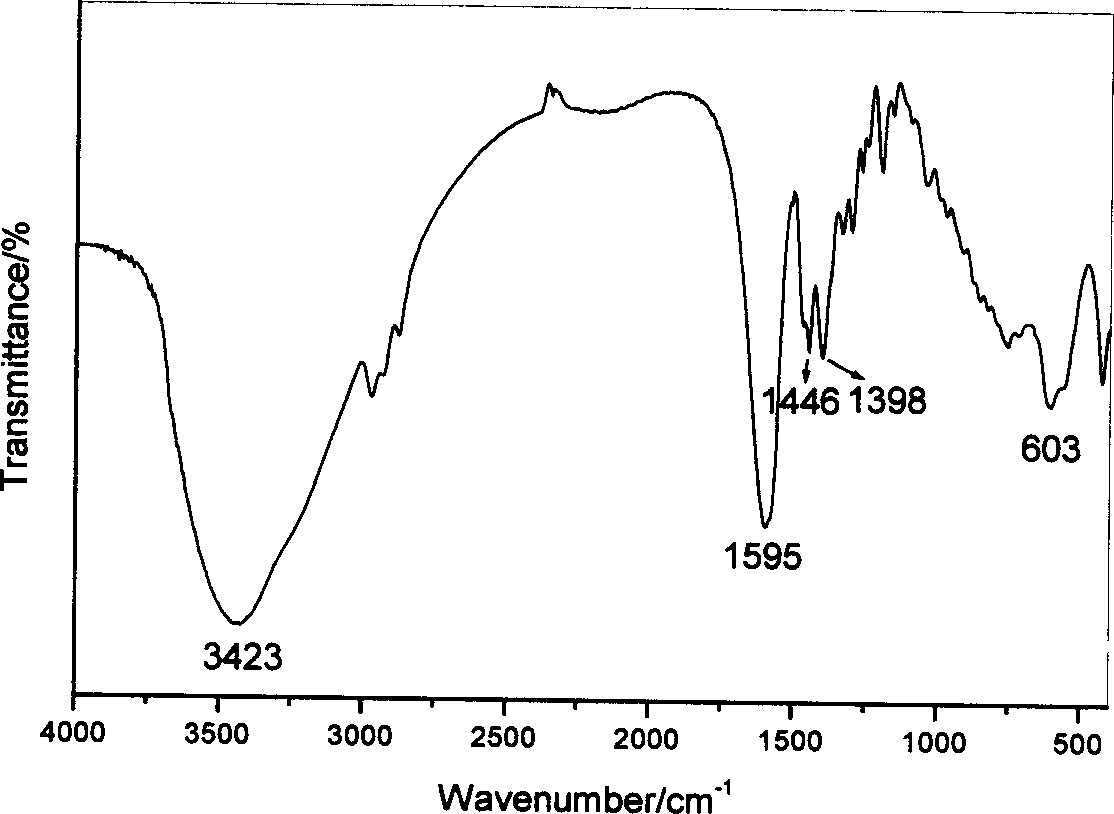
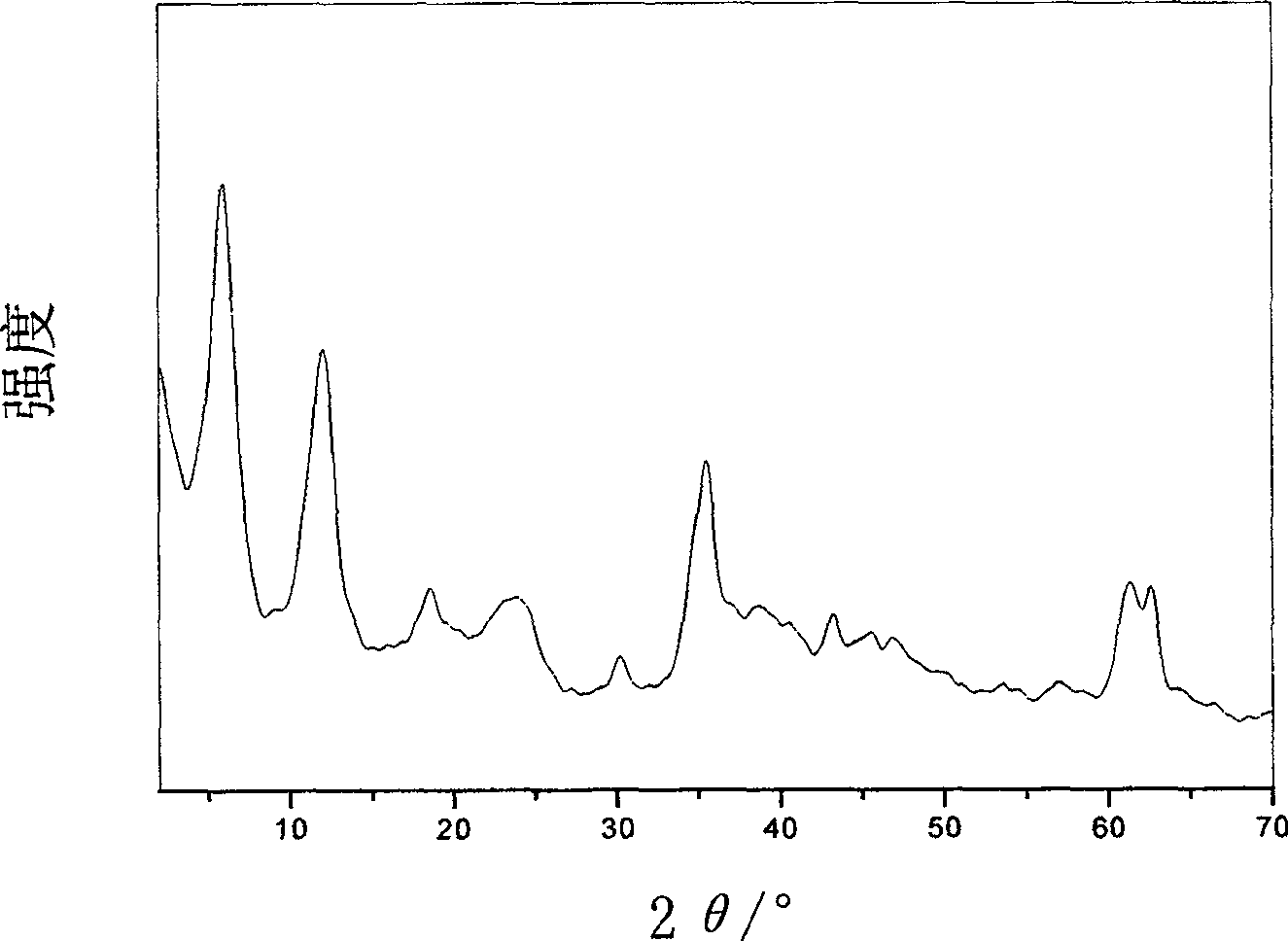
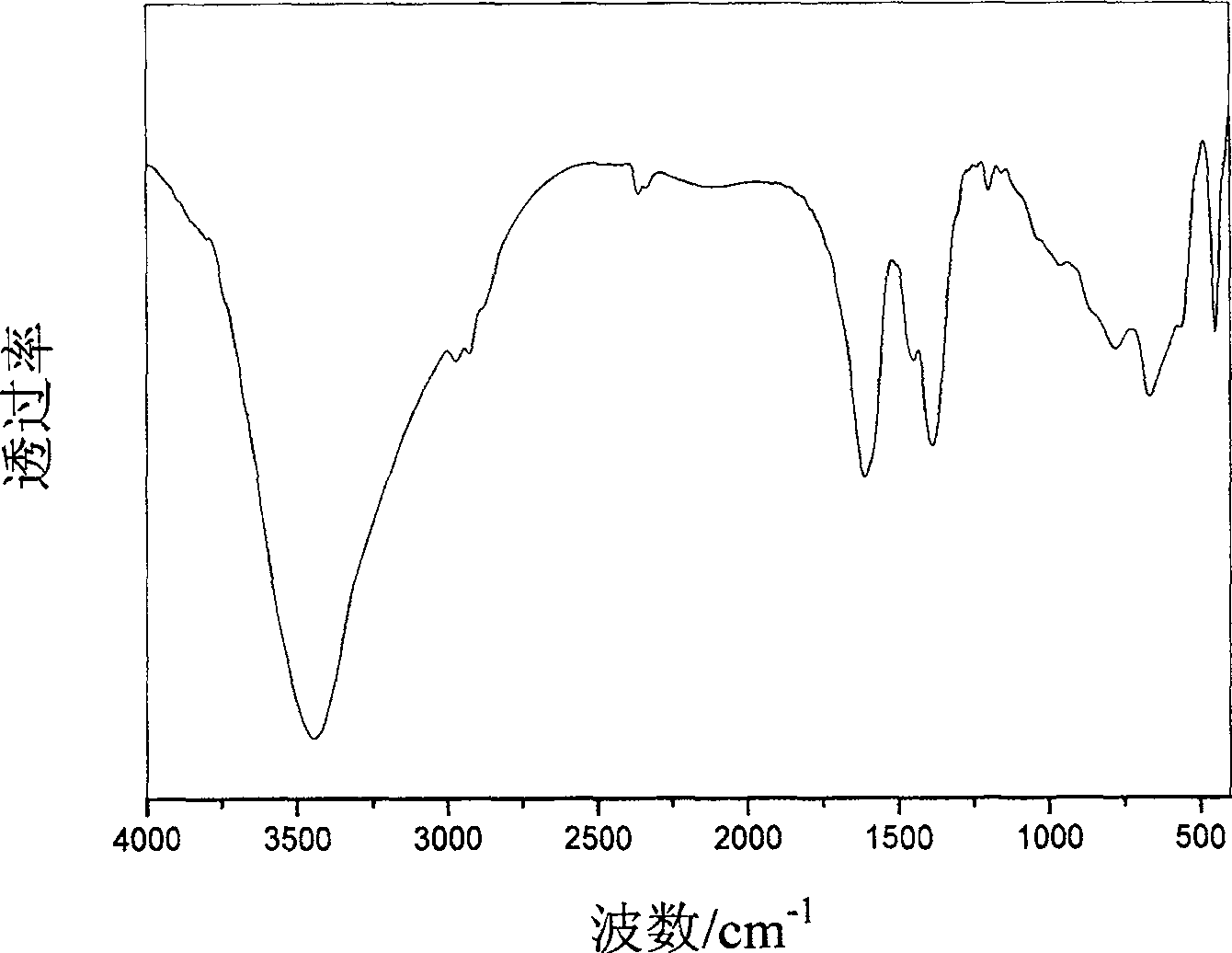
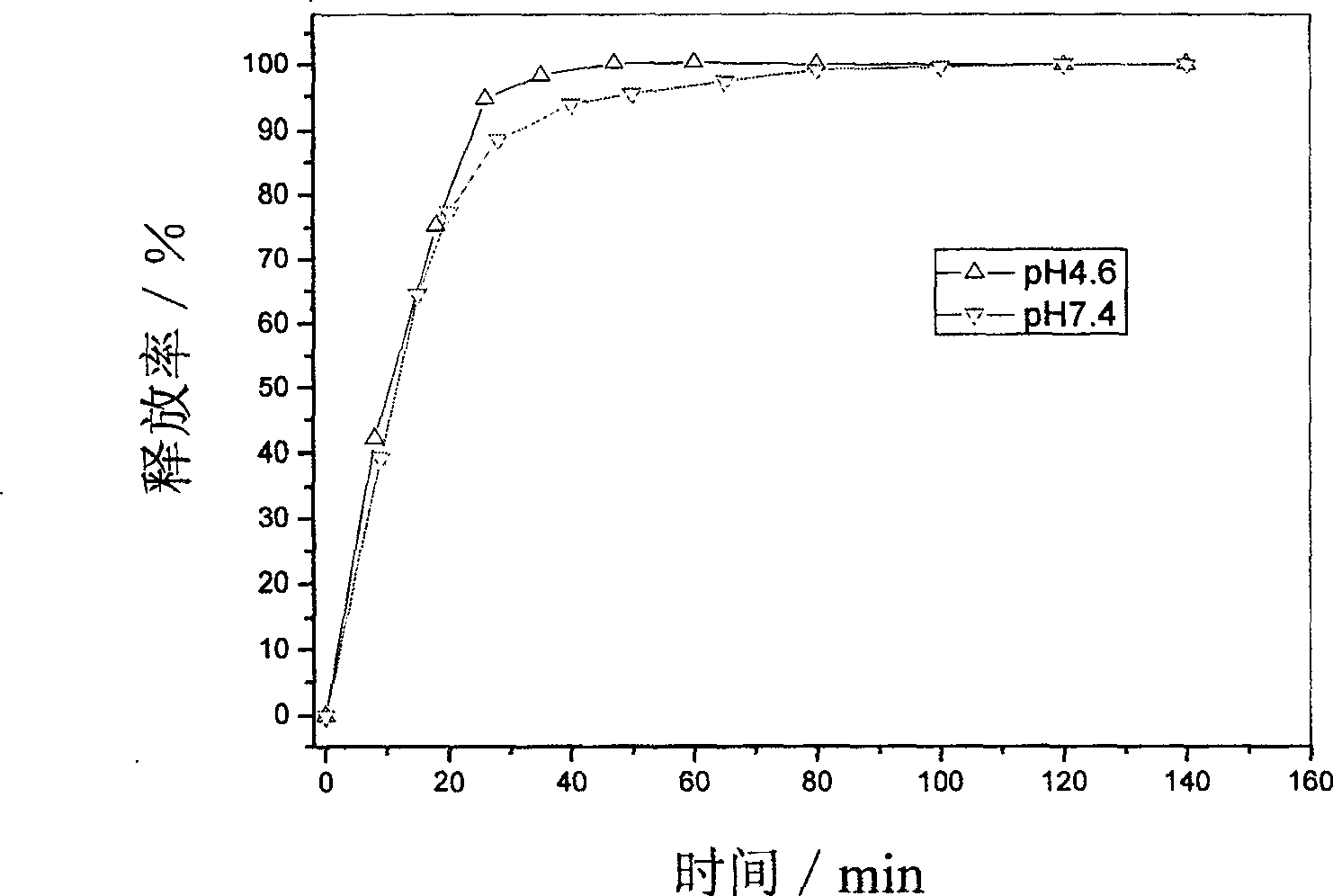
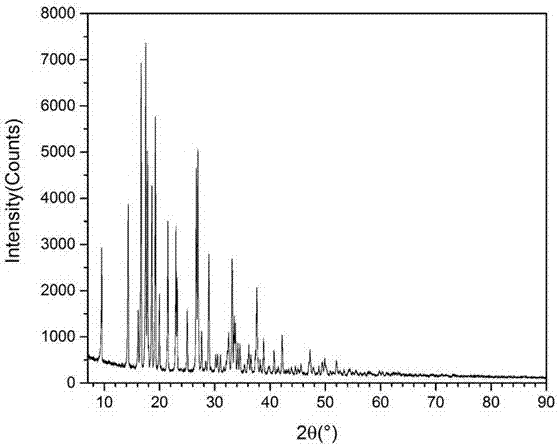
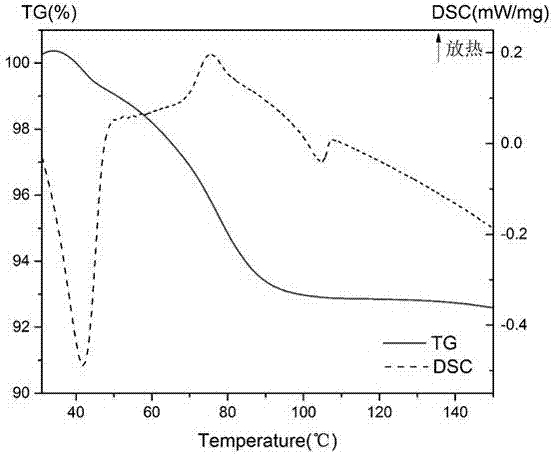
Supermolecular intercalation-structure slow-release captopril and its preparing method
InactiveCN1640395AStructure controlComposition controlOrganic active ingredientsSkeletal disorderCaptoprilSalt solution
The present invention relates to a supermolecular intercalation structure slow-released captopril and its preparation method. Said invention uses anionic lamellar material LDH as main body, uses captopril as intercalation guest, and utilizes two soluble metal salts to make them into a mixed salt solution, then makes said mixed salt solution and alkali solution of captopril undergo the process of intercalation assembling treatment so as to obtain supermolecular structure CpI-LDHs. Said invention also provides its chemical formula. In said slow-released captopril preparation the mass percentage content of captopril is 20-50% and the mass percentage content of water is 5-20%, and its slow-released efficiency duration can be up to 0.5 hr-12hr.
Owner:BEIJING UNIV OF CHEM TECH
Water soluble medicament sustained-release tablets and preparation method thereof
InactiveCN101829068ASmall toxicityImprove complianceNervous disorderPharmaceutical delivery mechanismCaptoprilDuodenal juice
The invention discloses water soluble medicament sustained-release tablets, which comprise the following components in part by weight: water soluble medicament 1-30, octadecanol 5-70, Eudragite L 100-55 2-50, talcpowder 2-30 and lactose 2-30. The water soluble medicament may be galanthamine hydrobromide, captopril, metoprolol tartaric acid or pseudoephedrine hydrochloride. In the invention, the octadecanol serving as a hydrophobic auxiliary material and the Eudragite L 100-55 sreving as a water soluble polymer auxiliary are adopted, so the release speed of the medicament can be controlled properly. The Eudragite L 100-55 is insoluble in gastric juice, but dissolves in duodenal juice to make high-viscosity sticky liquid, and thus the release and diffusion speed of the medicament can be reduced. The talcpowder which is a hydrophilic matter insoluble in water plays a porogen role in the sustained-release tablets. In the invention, the release speed of the medicament is regulated by regulating the mixing ratio of the medicament to the auxiliary material. Thus, the release speed of an active medicament is controlled.
Owner:XUZHOU PHOTOSYNTHETIC BIOLOGICAL NUTRIMENT
Application of two halophenol compounds in preparation of medicines of resisting type II diabetic nephropathy
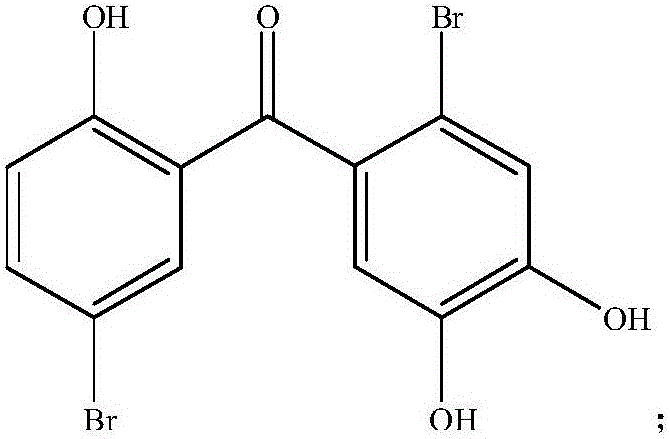
ActiveCN105816446AImprove protectionImprove kidney functionMetabolism disorderKetone active ingredientsCaptoprilMorpholine
The invention discloses a medical new application of two halophenol compounds 4,5,2'-trihydroxy-2,5'-dibromobenzophenone and 4,5,2'-tri(4-morpholine methanoyl)-2,5'-dibromobenzophenone, in particular an application in preparation of medicines of resisting type II diabetic nephropathy. Meanwhile, the invention also discloses a medicine of resisting type II diabetic nephropathy employing the two halophenol compounds as active ingredients. By the two compounds, the renal function of the type II diabetic nephropathy can be effectively improved; the main treatment indexes, namely uric acid, urine protein, microalbuminuria, transforming growth factor TGF-beta1, blood sugar, low-density lipoprotein and the like are superior to those of an existing clinic first-line medicine captopril; and the important application prospect in treatment of the diabetic nephropathy is displayed.
Owner:SHANXI MEDICAL UNIV
Compound medicine preparation for treating refractory hypertension
InactiveCN107308181AAvoid careful counting of multiple drug quantitiesAvoid the hassle of dosingOrganic active ingredientsCardiovascular disorderCaptoprilSide effect
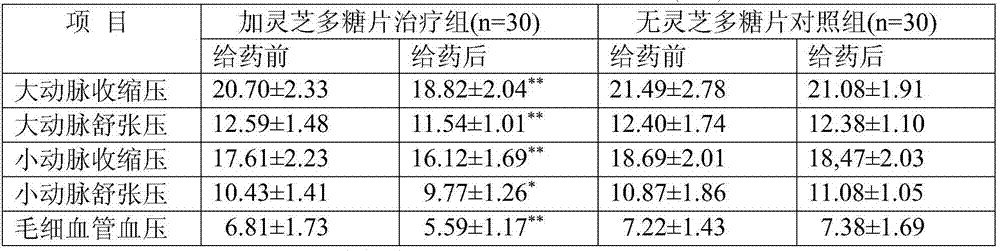
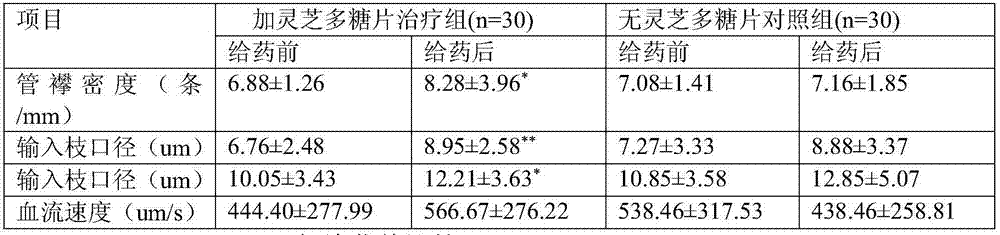
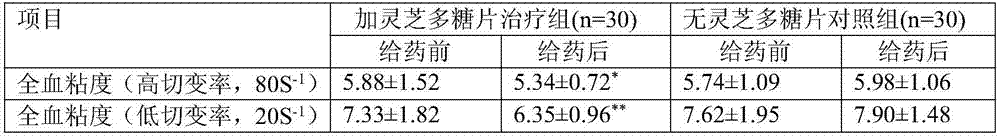
The invention discloses a compound medicine preparation for treating refractory hypertension, which is mainly prepared from the following components in parts by weight: 50 to 500 parts of ganoderan, 5 to 10 parts of nifedipine, 12.5 to 50 parts of captopril, 10 to 50 parts of hydrochlorothiazide and 12.5 to 100 parts of atenolol. According to the compound medicine preparation provided by the invention, the ganoderan is compounded with multiple antihypertensive drugs of conventional multiple-treatment to prepare a convenient-to-take compound preparation; the beneficial effects of synergetic antihypertensive effect and mutual side effect relieving / offsetting effect of the ganoderan and the four medicines are realized, the compliance of standardized medicine-taking of refractory hypertension patients is obviously improved, the therapeutic effect of obviously reducing the blood pressure level of the refractory hypertension or even realizing up-to-standard blood pressure control on part of the patients are achieved, and a novel convenient-to-take compound antihypertensive drug is provided for simplifying the drug use of the refractory hypertension patients and effectively reducing the blood pressure level.
Owner:卢广荣
Medicinal composition contg. katopril, and its prepn. method
InactiveCN1618426AWell mixedStable contentOrganic active ingredientsGranular deliveryCaptoprilLactose
A composite medicine containing captopril is prepared from captopril, microcrystalline cellulose, lactose, starch, hydroxypropyl cellulose, pregelatinized starch, magnesium stearate and polyvidone K30 through respectively granulating and mixing.
Owner:SHANGHAI XUDONG HAIPU PHARMA
Scar treatment external application western medicine composition preparation and application thereof
InactiveCN103432145AAnti-inflammatoryAntipruriticOrganic active ingredientsAntipyreticCaptoprilTreatment effect
The present invention relates to an external application western medicine composition preparation with functions of scar treatment, anti-inflammation, itching relieving and pain relieving, and an application thereof. The external application western medicine composition preparation comprises a medicine active ingredient and a pharmaceutically acceptable auxiliary material, wherein the medicine active ingredient mainly comprises 3-10% of acetylsalicylic acid and 8-15% of captopril. According to the present invention, the conventional preparation process is adopted to prepare different external application formulations in the clinic; pharmacodynamics experiment results show that: skin wound fibroblast proliferation is significantly inhibited, collagen fiber content is reduced, skin growth is promoted, and effects of anti-inflammation, itching relieving and pain relieving are provided; and the external application western medicine composition preparation has characteristics of synergetic effect of the compound medicine, significant treatment effect, low price and simple preparation, is a safe and effective skin scar treatment medicine, and has broad application prospects.
Owner:NINGXIA MEDICAL UNIV
Medication for treating high blood pressure
InactiveCN1958001ALow priceGood curative effectOrganic active ingredientsAnthropod material medical ingredientsCaptoprilBeta blocker
A medicine for treating hypertension is prepared from cinnamon bark, wild jujube, and beta receptor paralyzer, or diuretic, or calcium antagon, angiotonin converzyme depressant and angiotonin II receptor antagon.
Owner:陈绮年
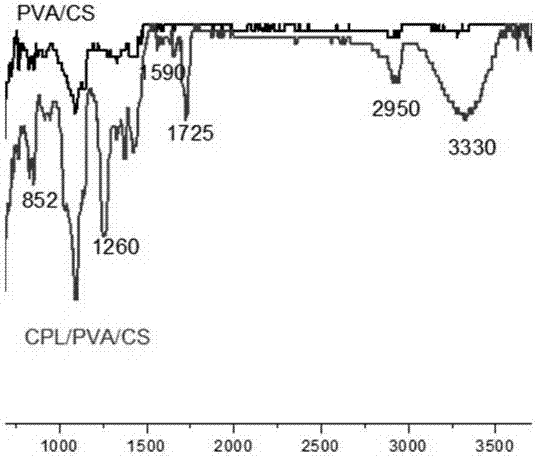
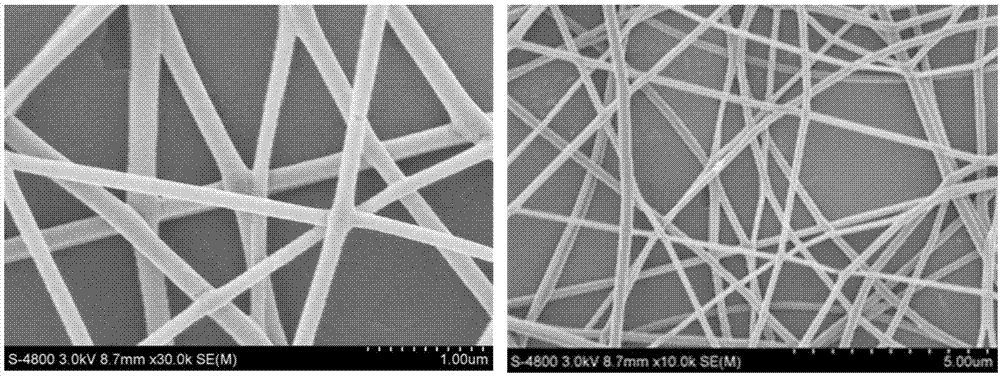
Preparation method of captopril-loading polyvinyl alcohol-chitosan nanometer fiber
InactiveCN107334749ASimple structureEasy to operateOrganic active ingredientsFilament/thread formingCaptoprilFiber
The invention discloses a preparation method of a captopril-loading polyvinyl alcohol-chitosan nanometer fiber. The preparation method comprises: S1, adding polyvinyl alcohol into deionized water to obtain a polyvinyl alcohol solution; S2, weighing chitosan, dissolving in deionized water, and adding 3 drops of acetic acid to obtain a clarified chitosan solution; S3, taking the chitosan solution obtained in the step S2, and adding to the polyvinyl alcohol solution obtained in the step S1 in a dropwise manner to prepare a PVA-CS precursor solution; S4, taking a captopril tablet, dissolving in deionized water, taking the supernatant, adding to the PVA-CS precursor solution obtained in the step S3 in a dropwise manner, and stirring to prepare a spinning solution; and S5, carrying out electrospinning on the spinning solution obtained in the step S4 to prepare the captopril-loading polyvinyl alcohol-chitosan nanometer fiber. According to the present invention, the optimal raw material ratio and the optimal preparation condition are provided, the captopril-loading polyvinyl alcohol-chitosan nanometer fiber is prepared by using the electrospinning technology, the advantages of simple structure, convenient operation, easy control and short process are provided, and the prepared captopril-loading polyvinyl alcohol-chitosan nanometer fiber can stably and slowly release and has good physiological compatibility.
Owner:SUZHOU UNIV
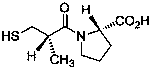
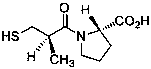
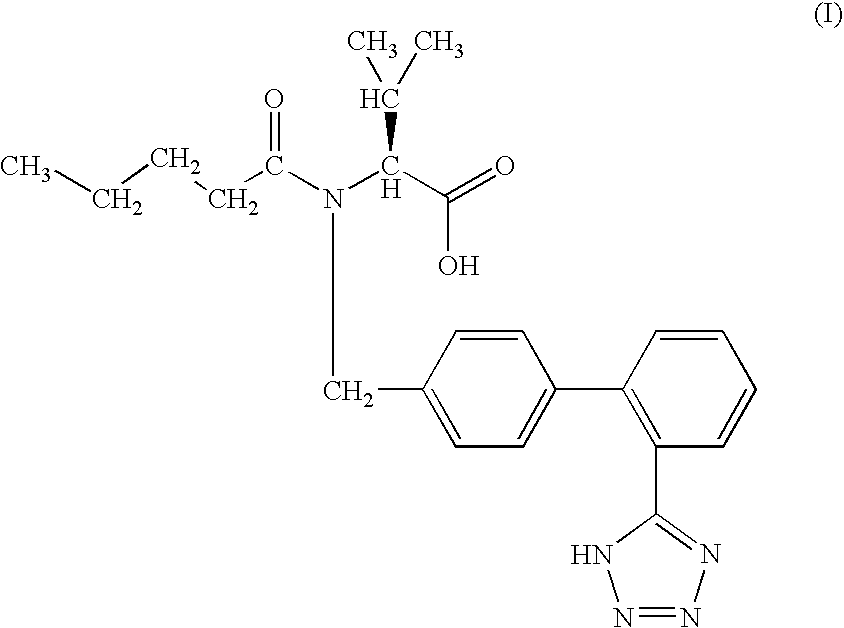
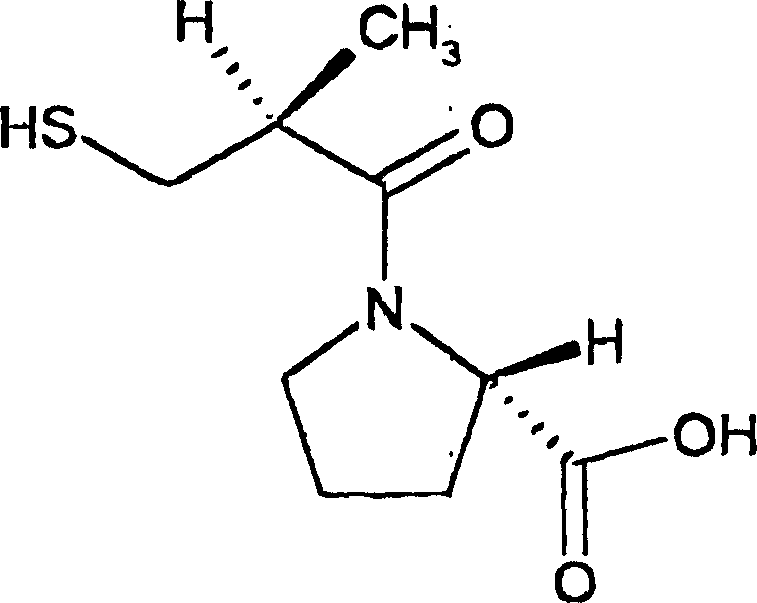
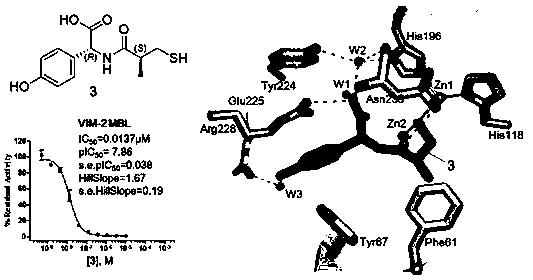
Preparation and use of 2-substituted-(S)-(3-mercapto-2-methylpropanoyl)-glycine derivatives
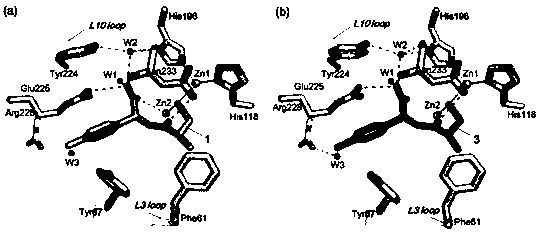
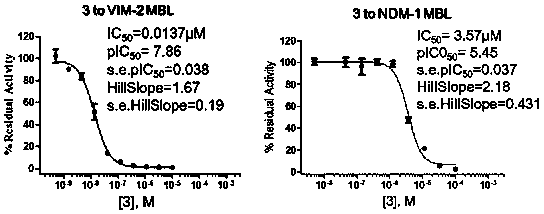
InactiveCN108117502AEasy to prepareMild reaction conditionsAntibacterial agentsOrganic active ingredientsCaptoprilX-ray
The invention provides a 2-substituted-(S)-(3-mercapto-2-methylpropanoyl)-glycine derivative with a structural formula represented by a formula I (shown in the description). The invention further provides a preparation process and use of the 2-substituted-(S)-(3-mercapto-2-methylpropanoyl)-glycine derivative. Pharmacodynamic tests prove that compounds has relatively good inhibitory activity to various metal beta lactamases (MBL), and particularly, a compound 3 has the optimal inhibitory activity and is remarkably superior to certain MBL small-molecule inhibitors such as captopril reported at present. X-ray crystal structure researches of compounds prove that the action mechanism between the compounds of the invention and MBL is characterized in that mercapto groups of the compounds are chelated with zinc ions at MBL active sites. Besides, antimicrobial activity tests prove that the compounds of the invention have very good bacteriostatic effect to multiple clinically separated superbacteria strains when being combined with beta-lactam antibiotic for use. The 2-substituted-(S)-(3-mercapto-2-methylpropanoyl)-glycine derivative has a clear and definite action mechanism and a remarkable pesticide effect, and new drug selection is likely to be provided for clinic.
Owner:SICHUAN UNIV
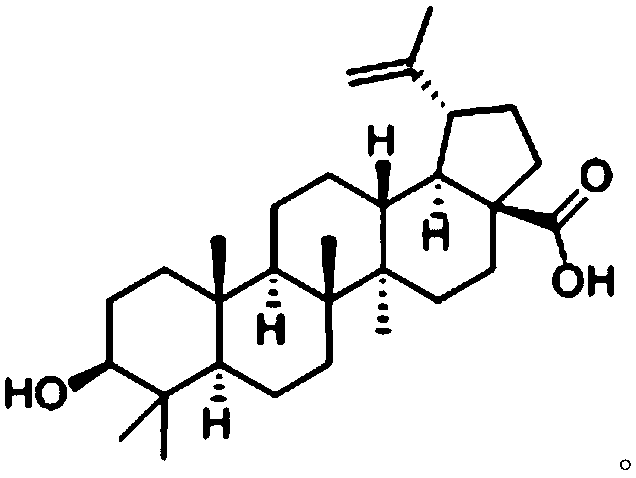
Application of fructus chaenomelis betulinic acid in preparing medicine for resisting hypertensive myocardial fibrosis
InactiveCN108159054AReduce contentImprove antioxidant capacityNatural extract food ingredientsAnhydride/acid/halide active ingredientsInflammatory factorsCaptopril
The invention discloses application of fructus chaenomelis betulinic acid in preparing a medicine for resisting hypertensive myocardial fibrosis, and belongs to the technical field of biological medicines. The fructus chaenomelis betulinic acid is applied to treat treating spontaneously hypertensive rats (SHR), and the result shows that after drug administration treatment for 12 weeks, blood pressure of each group of SHR is reduced, and serum endothelin (ET) contents of each dosage group of fructus chaenomelis betulinic acid and a captopril CAP group are remarkably reduced; the nitric oxide (NO) content is remarkably improved; according to each dosage group of fructus chaenomelis betulinic acid, the contents of MCP-1, TNF-alpha, IL-1, IL-6, CRP and MDA can be reduced, so that the result shows that the fructus chaenomelis betulinic acid can be beneficial to reducing of the content of cardiac muscle tissue inflammatory factors, reducing of damage of oxidative stress on tissues, improvingof tissue oxidation resistance, and improving of myocardial fibrosis through multiple target points.
Owner:SOUTHERN MEDICAL UNIVERSITY
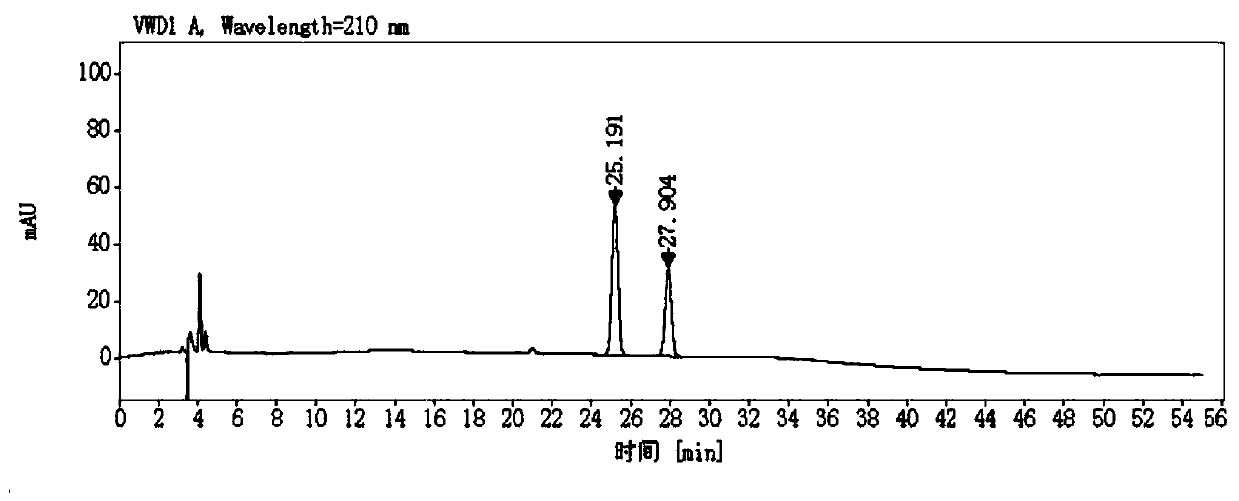
Ion chromatography-ultraviolet detection method for measuring content of captopril in captopril medicine
InactiveCN102520093AAccurate determination of contentEasy to separateComponent separationCaptoprilIon chromatography
The invention provides a novel method for detecting captopril. An ion chromatography cation exchange system and an ultraviolet detector are combined, appropriate separation conditions are selected according to the difference of the retention behavior of a substance on a chromatographic column, and the optimal ultraviolet detection wavelength is selected, so that the captopril can be separated and detected at relatively high sensitivity, and the method can be used for the detection of the content of the captopril in a captopril medicine, human body fluid pharmacokinetic study and the like.
Owner:ZHEJIANG UNIV
Application of NO donor compound in preparation of drugs for inhibiting invasion and metastasis capabilities of tumor cells rich in sulfhydryl molecules
The invention relates to application of an NO donor compound in preparation of drugs for inhibiting invasion and metastasis capabilities of tumor cells rich in sulfhydryl molecules. The NO donor compound is prepared from one or more of sulfhydryl-nitroso glutathione, nitric oxide, sodium nitrite, nitroglycerin, the sulfhydryl-nitroso glutathione, sulfhydryl-nitroso cysteine, sulfhydryl-nitroso captopril, sulfhydryl-nitroso-N-acetylpenicillamine, monosaccharide-sulfhydryl-nitroso-N-acetylpenicillamine and a conjugate of the monosaccharide-sulfhydryl-nitroso-N-acetylpenicillamine. The NO donor compound and the sulfydryl molecules in the tumor cells undergo sulfhydryl-nitrosylation reaction, so that sulfydryl is transformed into sulfhydryl nitroso, and further the invasion and metastasis capabilities of the tumor cells rich in the sulfhydryl molecules can be inhibited. The method for inhibiting the invasion and metastasis capabilities of the tumor cells rich in the sulfhydryl molecules, provided by the invention, provides a new direction for treating tumor metastasis and has obvious social benefits.
Owner:FUZHOU UNIV
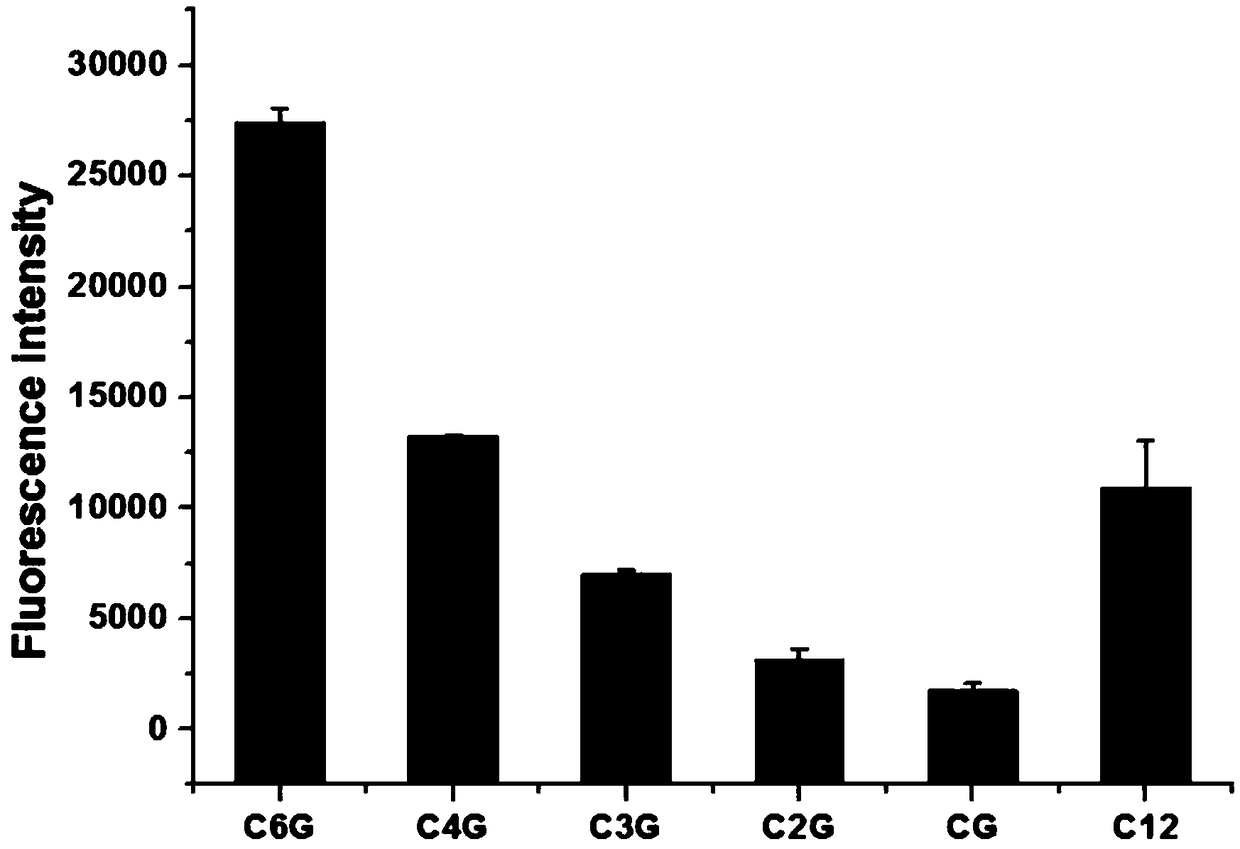
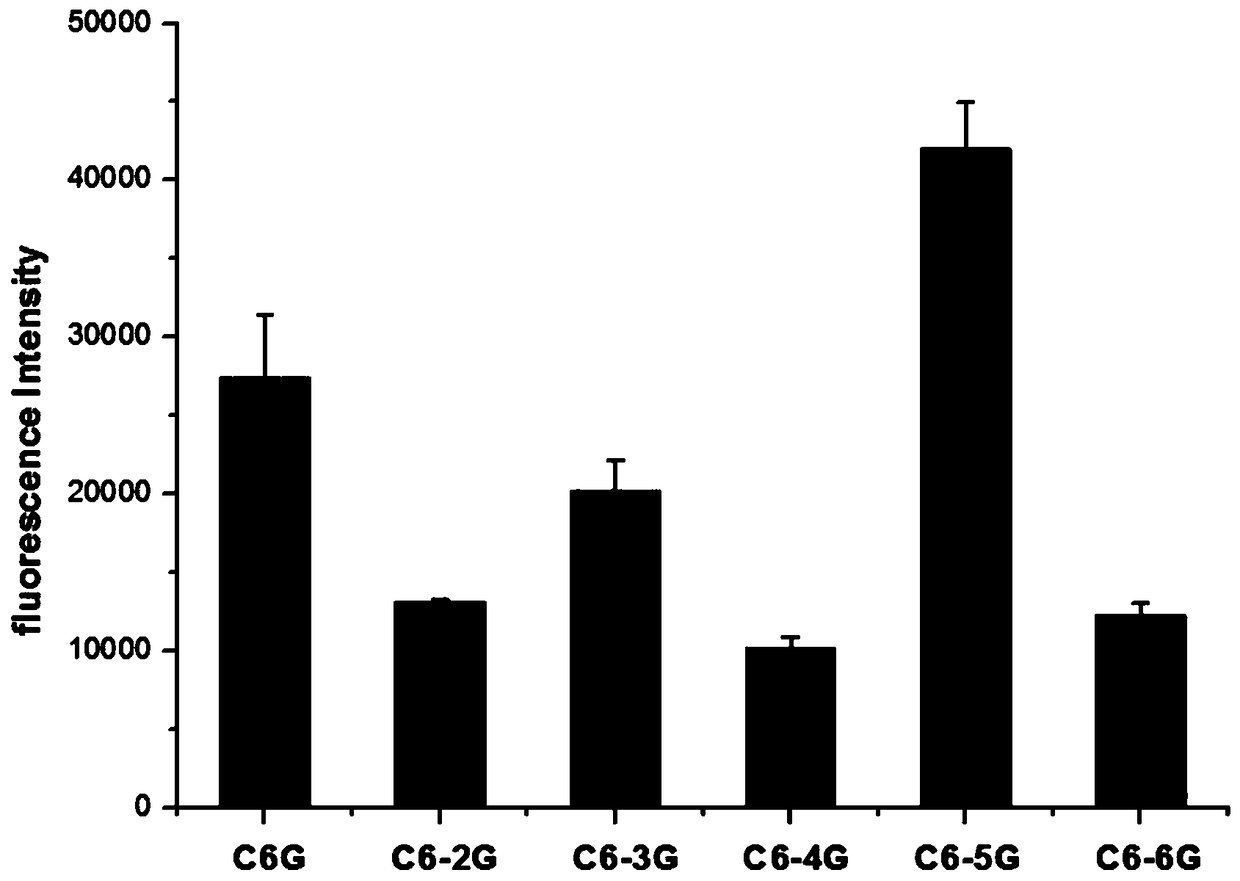
Detecting method for captopril through light emitting silver clusters synthesized by G insertion sequences
The invention discloses a detecting method for captopril through light emitting silver clusters synthesized by G insertion sequences, and belongs to the fields of food and medicine detection. A seriesof regular C-base-enriched DNA sequences are deigned for synthesizing nanometer silver clusters, the G insertion sequence with the best fluorescent brightness is selected for synthesizing the silverclusters, and a method for detecting captopril is built; a sample to be tested possibly containing captopril is added into a silver nanometer cluster solution, after a mixing reaction, and detection of captropril in the sample to be detected is achieved by observing the fluorescent strength of the formed mixed reaction system under the maximum excitation wavelength. The silver nanometer cluster synthesized by the DNA sequences is applied for detecting captopril, the detection linear range is 0.05-5 microgram / mL, the sensitivity can reach 0.05 microgram / mL, and the method has the advantages ofbeing easy, convenient and rapid to use, low in cost, high in sensitivity and the like, and can be applied to detection of captopril in healthy products of healthcare products, food, medicine productsand other healthy products.
Owner:JIANGNAN UNIV
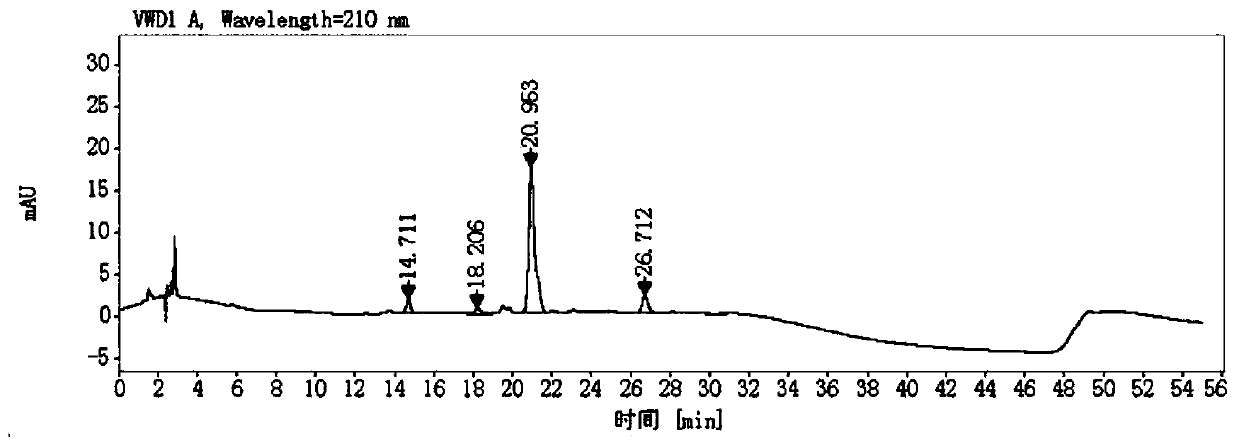
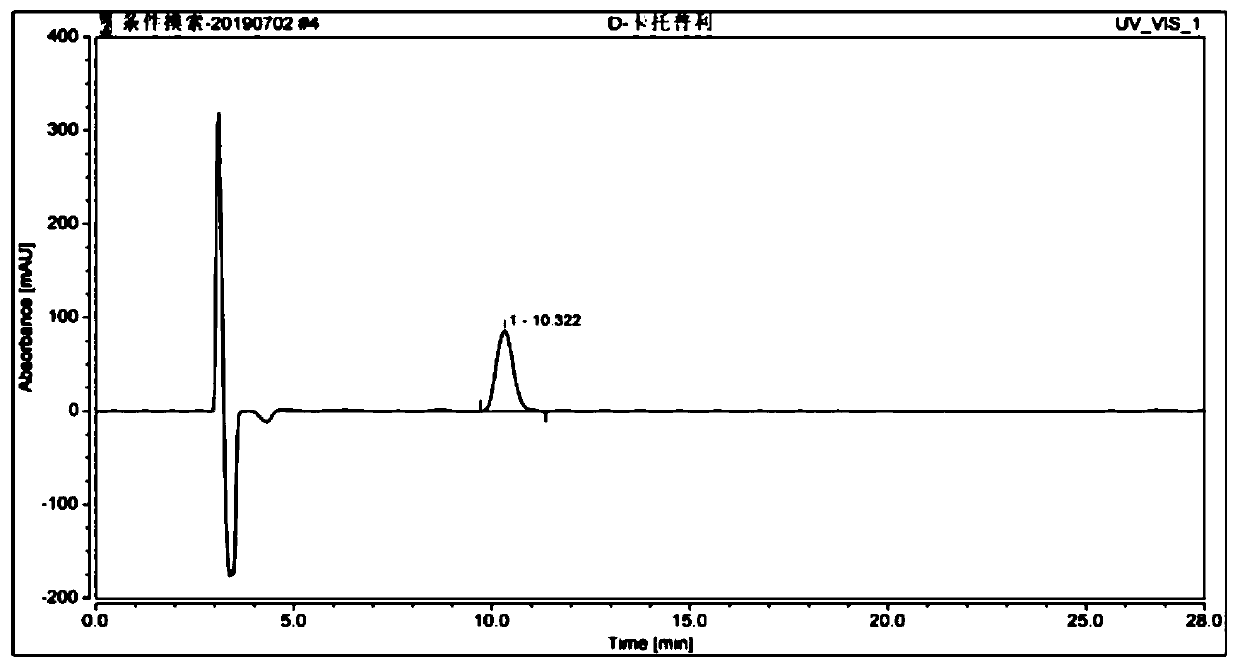
Method for determining D-captopril and captopril-related substances 8 in captopril tablets by adopting high performance liquid chromatography
The invention belongs to the technical field of pharmaceutical analysis, and particularly relates to a method for determining D-captopril and captopril related substances 8 in captopril tablets by adopting high performance liquid chromatography. The method comprises the steps that a test solution containing captopril is detected through adoption of high performance liquid chromatography, captopril, D-captopril and captopril related substances 8 are obtained, wherein the conditions of high performance liquid chromatography detection are as follows: a chromatographic column is an amylose-tri(3,5-xylylcarbamate) coating type chiral chromatographic column, the mobile phase is a mixed solution of n-hexane, isopropanol, methanol and trifluoroacetic acid, the elution mode is isocratic elution. According to the invention, the contents of D-captopril and captopril-related substances 8 in the captopril tablets can be quantitatively determined, so that the quality of the captopril tablets is effectively controlled.
Owner:SHANGHAI PUKANG PHARMA
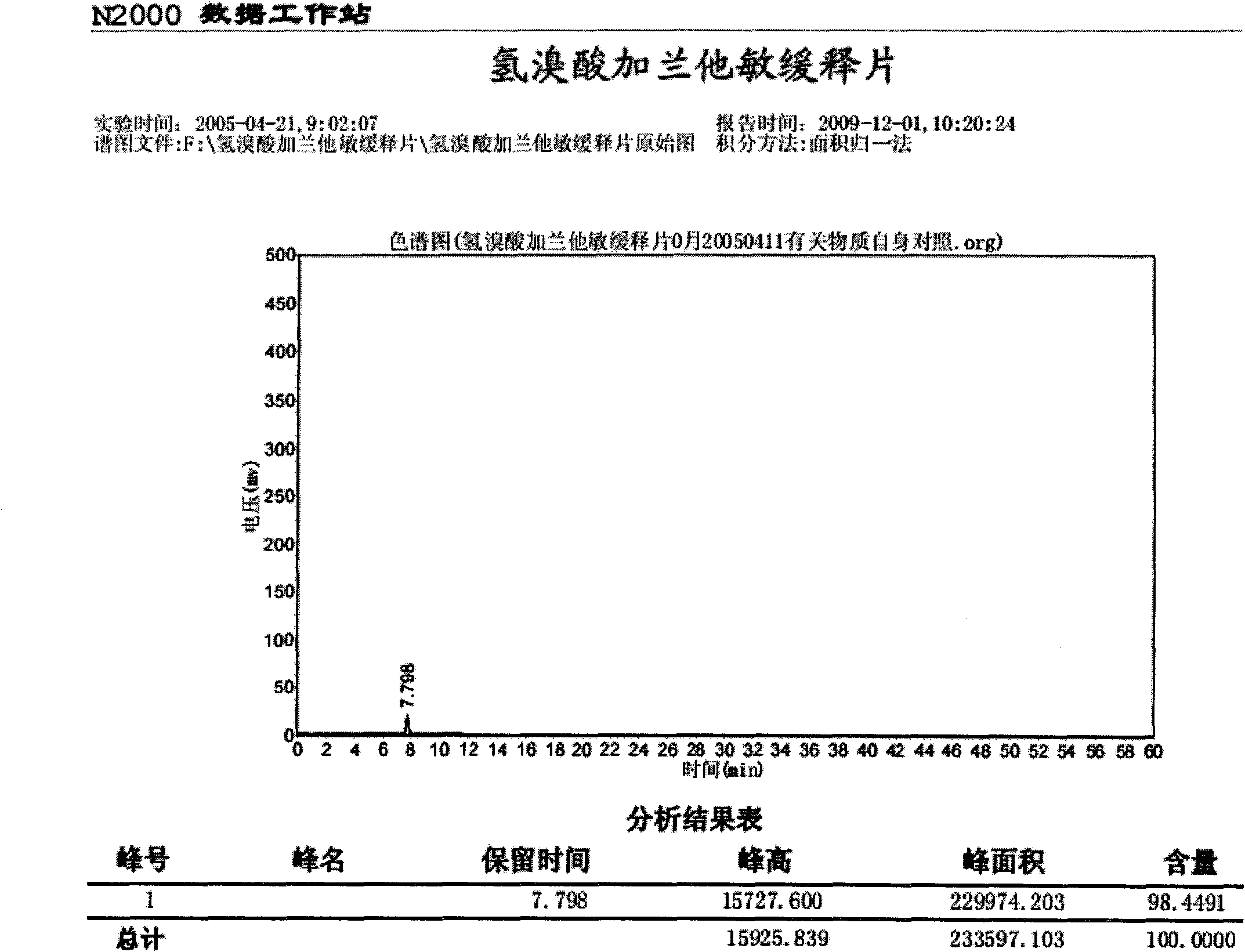
Controlled release preparation of captopril and its preparation process
InactiveCN1546018AOvercome the inconvenience of taking medicineOrganic active ingredientsPharmaceutical delivery mechanismCaptoprilRelease time
The invention relates to a controlled release formulation for Captopril, a hypotensive agent, and its preparing process, wherein the retardation release time of the slow release preparation can be as long as 4-6 hours, the effect duration of the preparation can be over 18 hours.
Owner:HANGZHOU MINSHENG PHARM CO LTD
Magnetic target slow-release catopril and its preparing method
InactiveCN100496613CGood curative effectSmall toxicityOrganic active ingredientsPharmaceutical non-active ingredientsCaptoprilPrill
The present invention relates to a magnetic target slowly-released captopril and its preparation method. Said invention uses spinel type ferrite and tri-iron tetroxide as magnetic material seed, uses anionic lamellar material LDHs as main body and uses captopril as intercalation guest, and utilizes self-assembling method to implement structure design of Cpl-LDHs / MP. Said magnetic captopril slow-release agent is shell-kernel structure, namely, the exterior of magnetic nano granule is covered with lamellar material Cpl-LDHs, specific saturated magnetization is 1.0-6.0 emu / g, its granular grain distribution is 20-200 nm, its captopril content in said slow-release preparation is 10-50%, and its slowly-released effective time can be up to 0.4h-13h.
Owner:BEIJING UNIV OF CHEM TECH
Sustained-release pellets containing captopril serving as active ingredient and preparation method thereof
InactiveCN102058541AAvoid burst phenomenonImprove securityOrganic active ingredientsGranular deliveryCaptoprilSustained release pellets
The invention discloses sustained-release pellets containing captopril serving as an active ingredient, which consist of medicament-containing pellets and sustained-release layers. The preparation method of the sustained-release pellets comprises: preparing the medicament-containing pellets by coating captopril on blank pellet cores, or by mixing an auxiliary material and the medicament and forming the mixture into pellets; and preparing coating liquid from a retarder, a pore forming agent and a plasticizer or an antisticking agent, spraying the coating liquid onto the outer layers of the medicament-containing pellets in a coating pan or fluidized bed to obtain sustained-release pellets. The sustained-release pellets prepared by the invention can effectively control the medicament to release in a certain period to maintain stable effective medicament concentration in blood and can avoid sudden release.
Owner:TIANJIN TIANCHENG PHARMA
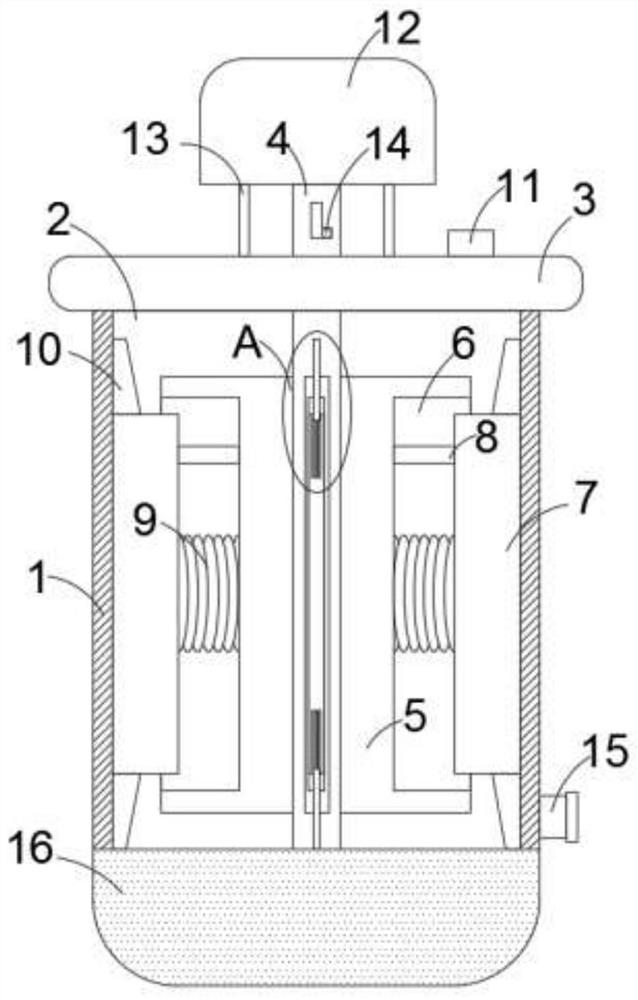
Cleaning equipment for captopril processing
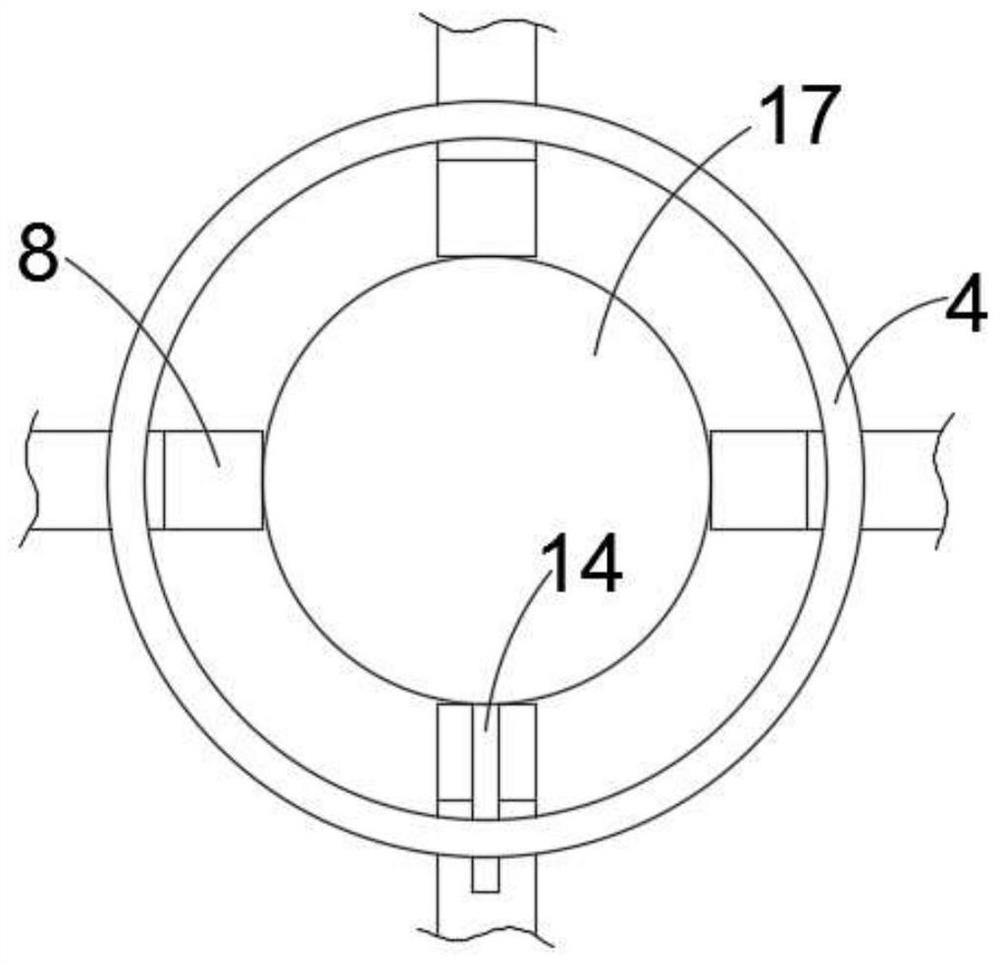
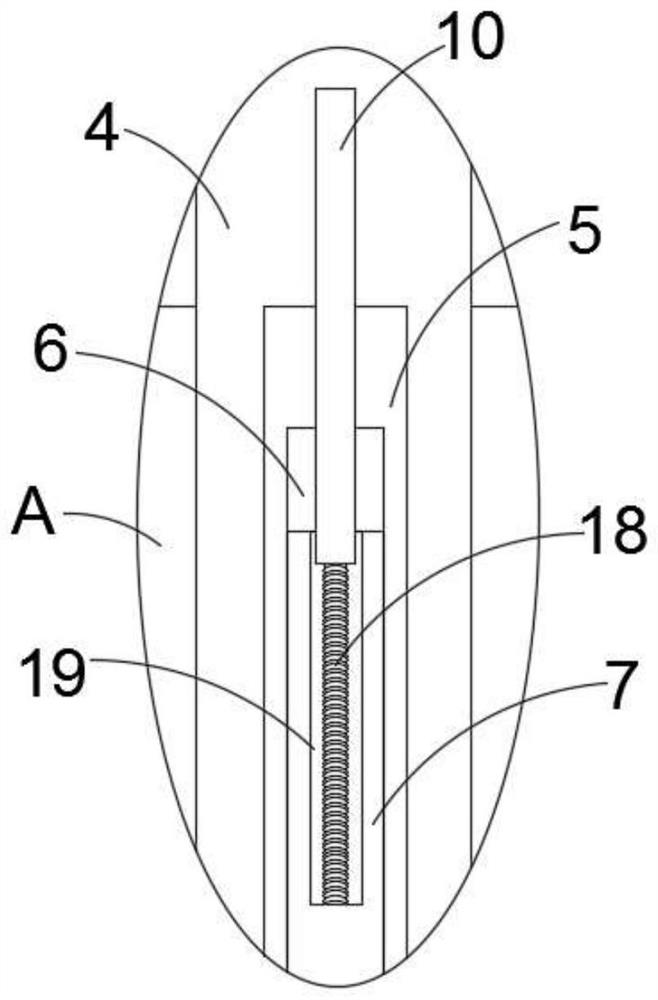
InactiveCN113510122AEasy to controlAchieve improvementHollow article cleaningCaptoprilElectric machine
The invention discloses cleaning equipment for captopril processing, which comprises a shell; a machining chamber is arranged in the shell; a rotating rod is rotatably arranged in the machining chamber; a top plate is fixedly arranged on the shell; a motor is fixedly arranged on the upper wall of the top plate; the motor is fixedly arranged on the top plate through two supporting rods; the rotating rod rotatably penetrates through the top plate and is fixedly connected with an output end of the motor; a plurality of stirring blades are fixedly arranged on the rotating rod in the circumferential direction; a scraping mechanism is arranged on each stirring blade; a heating furnace is further fixedly arranged on the lower side of the shell; a feeding opening is fixedly arranged on the top plate; a discharging port is fixedly arranged on the side wall of the lower end of the shell; and a sealing cover is further arranged on the discharging port in a covering mode. According to the cleaning equipment for the captopril processing provided by the invention, the inner wall can be cleaned and scraped through original machining equipment after captopril is machined, the problem that materials are attached to the inner wall and are difficult to clean is effectively solved, and the cleaning effect of the device is greatly improved.
Owner:JINXI SPRING PHARMA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com