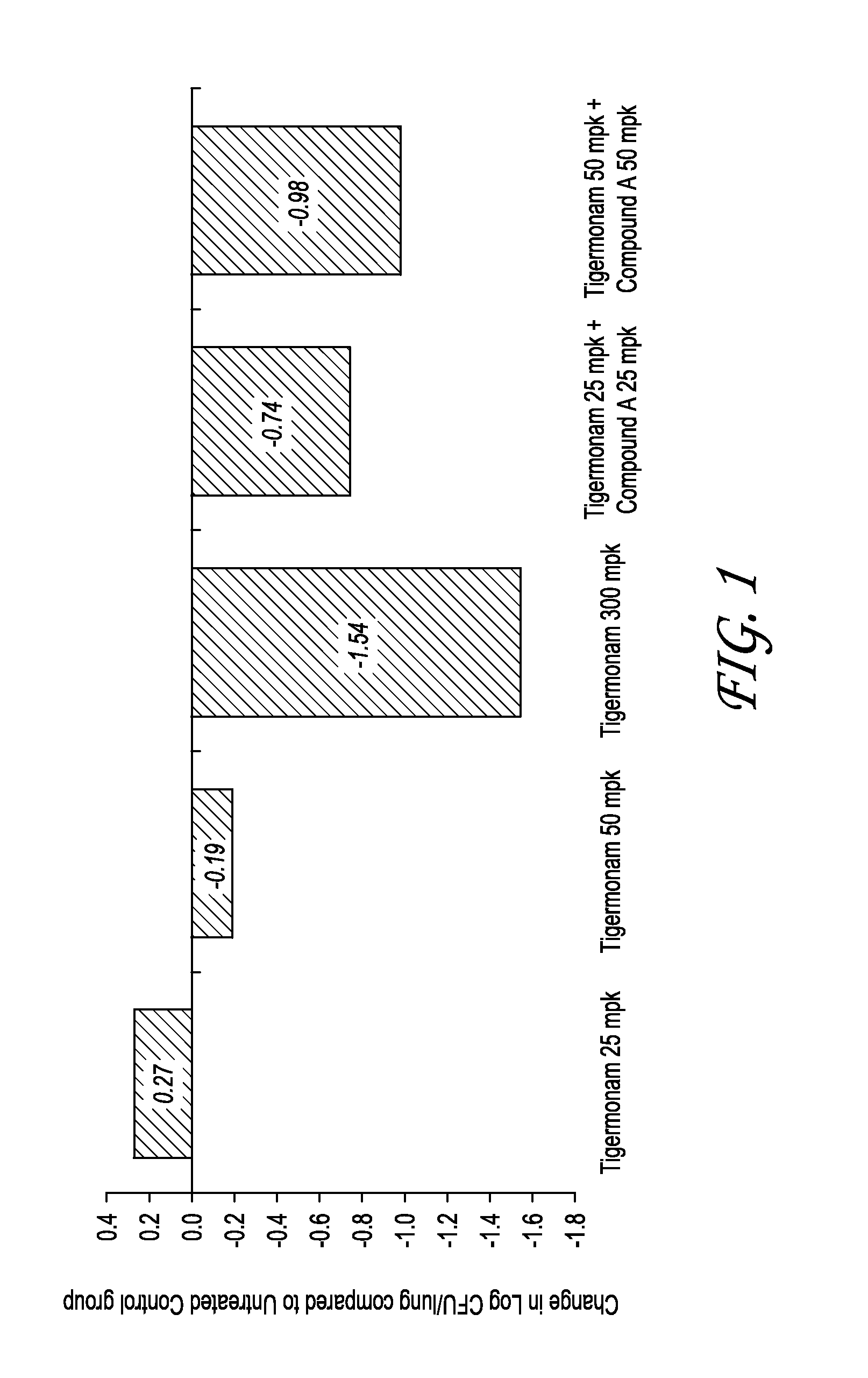
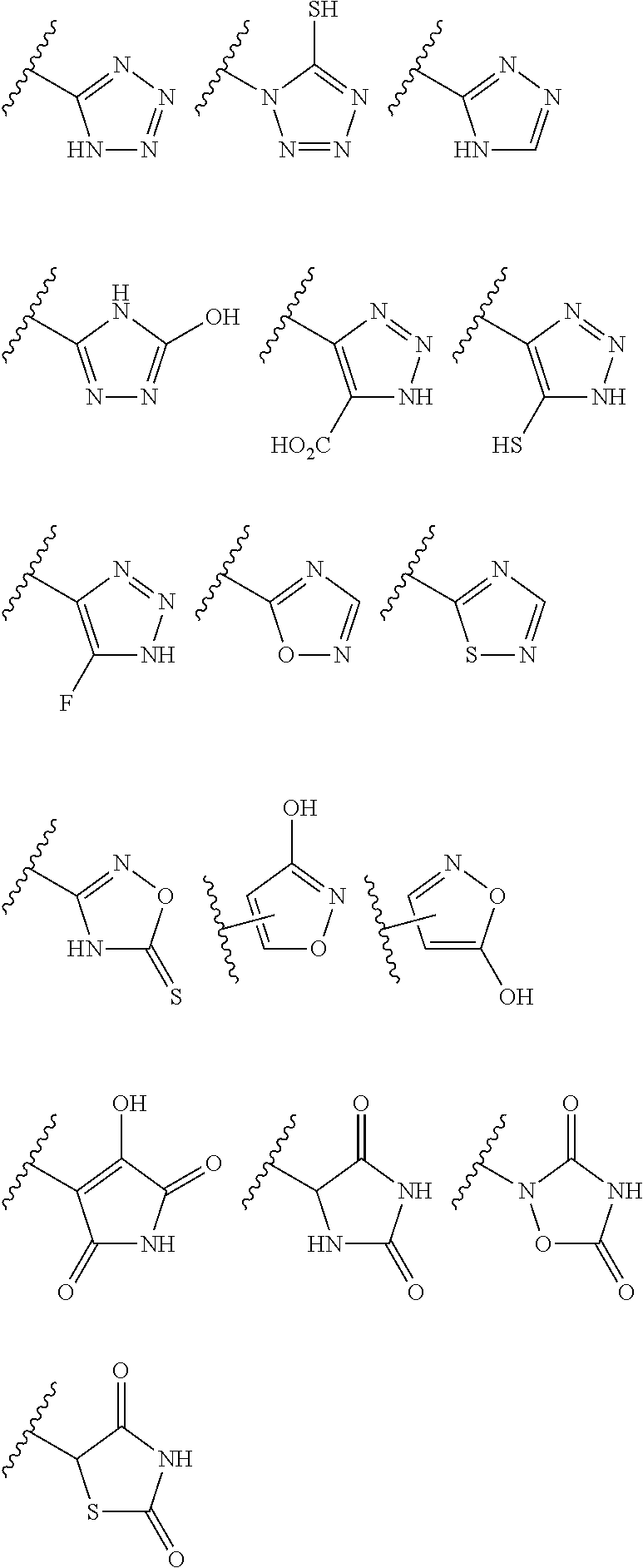
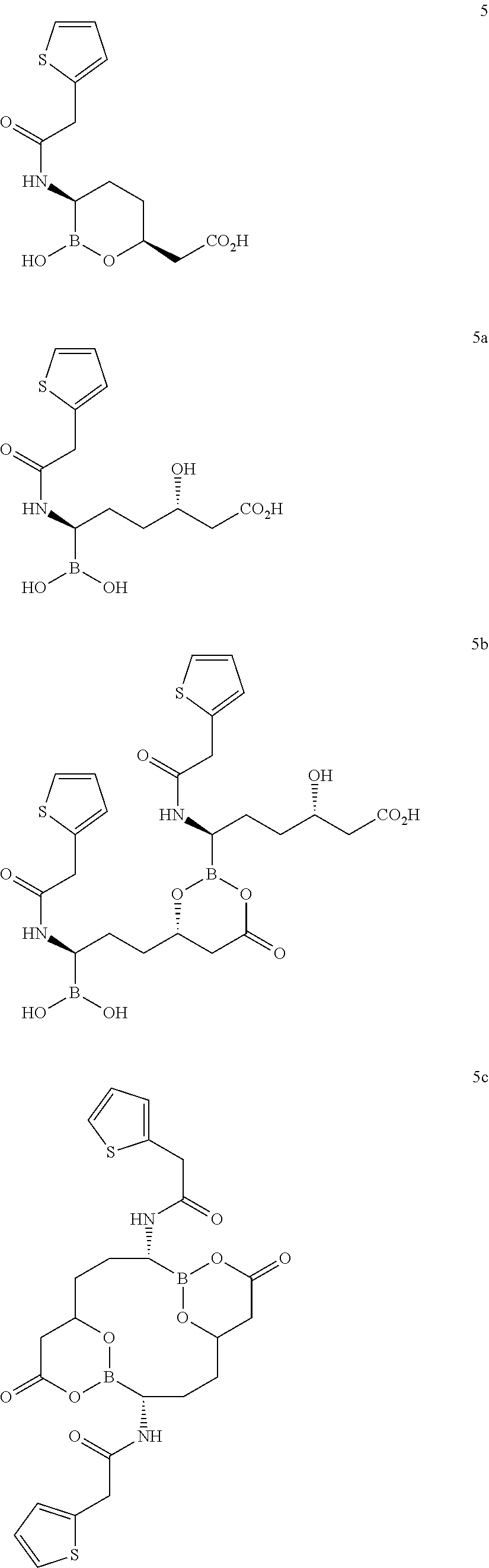
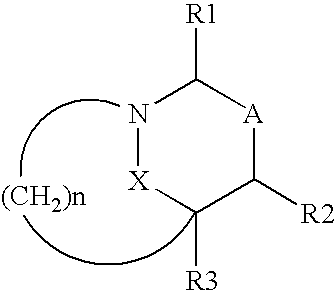
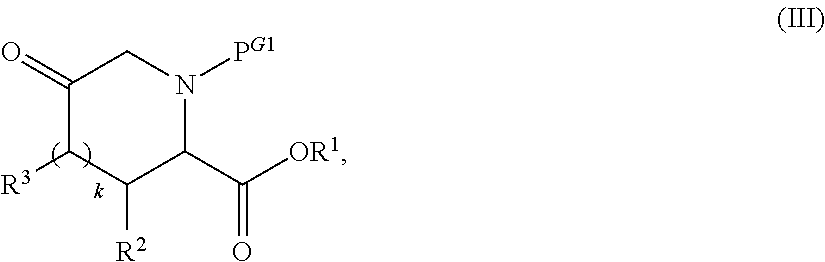Patents
Literature
95 results about "Β lactamases" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
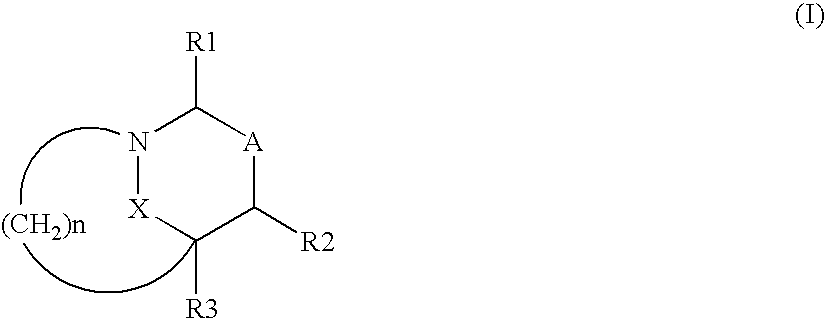
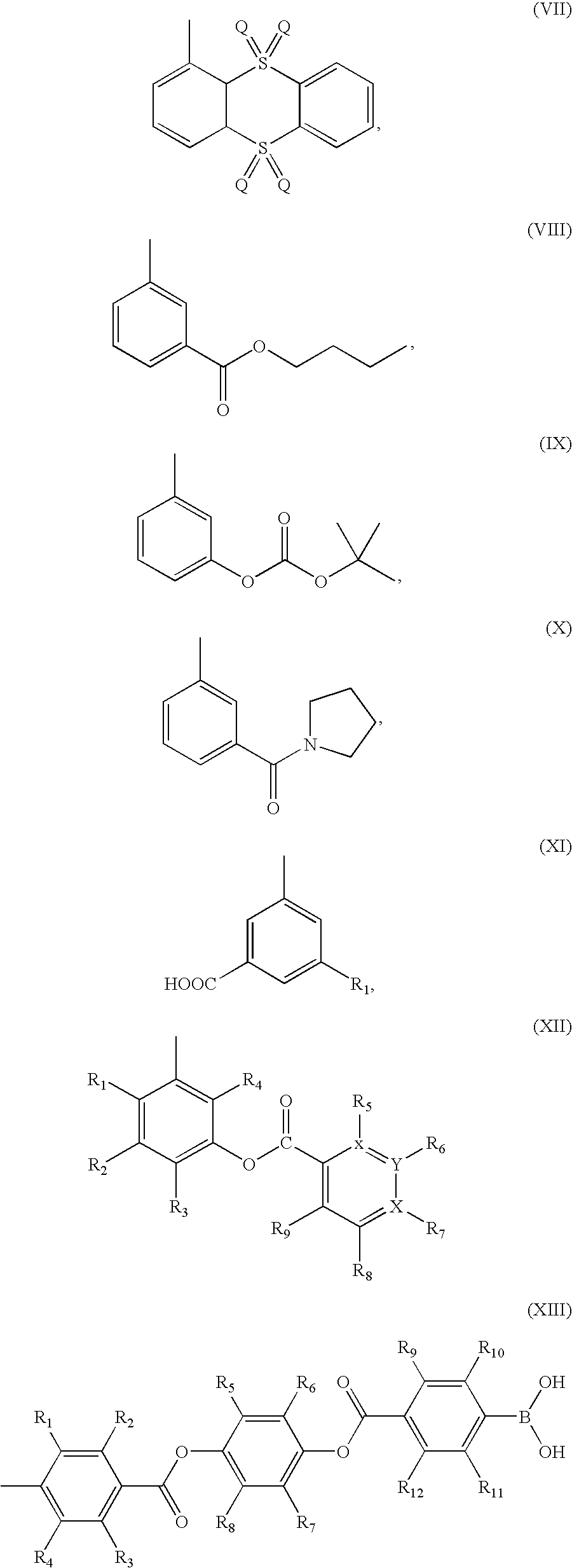
Heterocyclic compounds as inhibitors of beta-lactamases
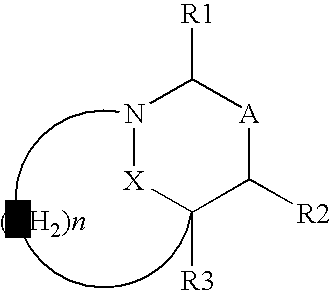
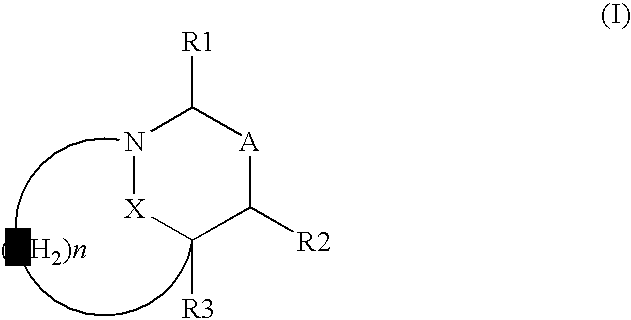
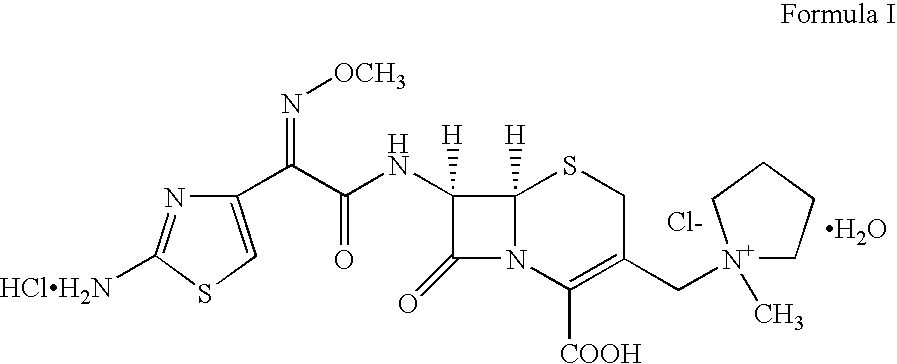
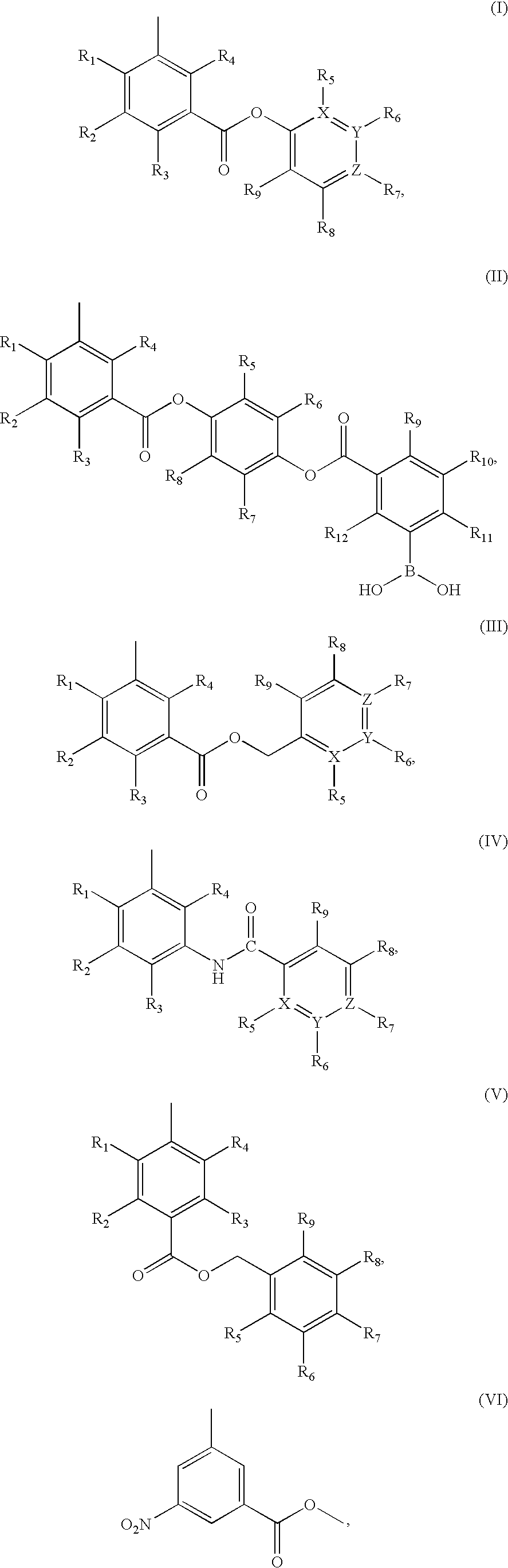
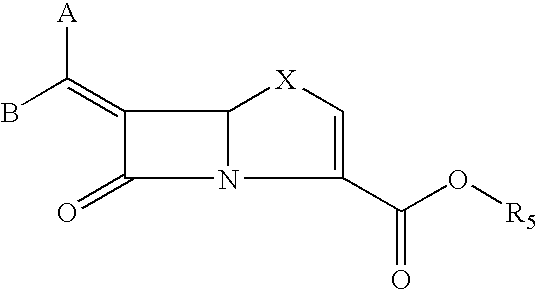
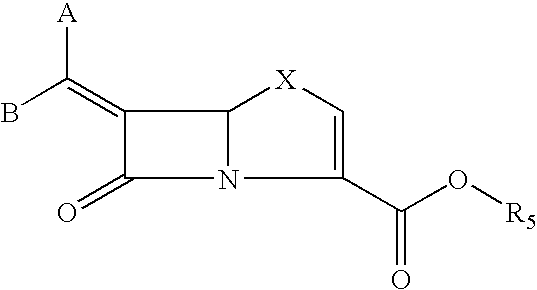
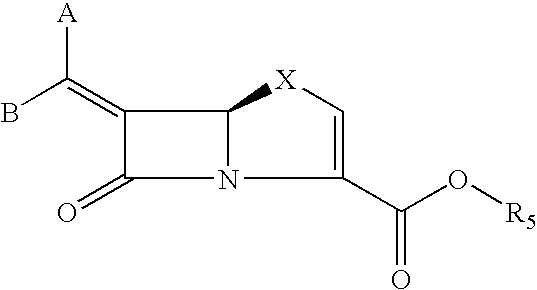
This invention discloses and claims methods for inhibiting bacterial β-lactamases and treating bacterial infections by inhibiting bacterial β-lactamases in man or an animal comprising administering a therapeutically effective amount to said man or said animal of a compound, or pharmaceutically acceptable salt thereof, of formula (I) either alone or in combination with a β-lactamine antibiotic wherein said combination can be administered separately, together or spaced out over time. Pharmaceutical compositions comprising a compound of formula (I), or a combination of a compound of formula (I) and a therapeutically effective amount of a β-lactamine antibiotic, and a pharmaceutically acceptable carrier are also disclosed and claimed.
Owner:APTALIS PHARMA
Beta-lactamase inhibitors
Substituted bicyclic beta-lactams of Formula I: (I), are β-lactamase inhibitors, wherein a, X, R1 and R2 are defined herein. The compounds and pharmaceutically acceptable salts thereof are useful in the treatment of bacterial infections in combination with β-lactam antibiotics. In particular, the compounds can be employed with a β-lactam antibiotics (e.g., imipenem, piperacillin, or ceftazidime) against microorganisms resistant to β-lactam antibiotics due to the presence of the β-lactamases.
Owner:MERCK SHARP & DOHME LLC
1,2,4-oxadiazole and 1,2,4-thiadiazole beta-lactamase inhibitors
β-Lactamase inhibitor compounds (BLIs) are disclosed, including compounds that have activity against class A, class C or class D β-lactamases. Methods of manufacturing the BLIs, and uses of the compounds in the preparation of pharmaceutical compositions and antibacterial applications are also disclosed.
Owner:MERCK SHARP & DOHME LLC
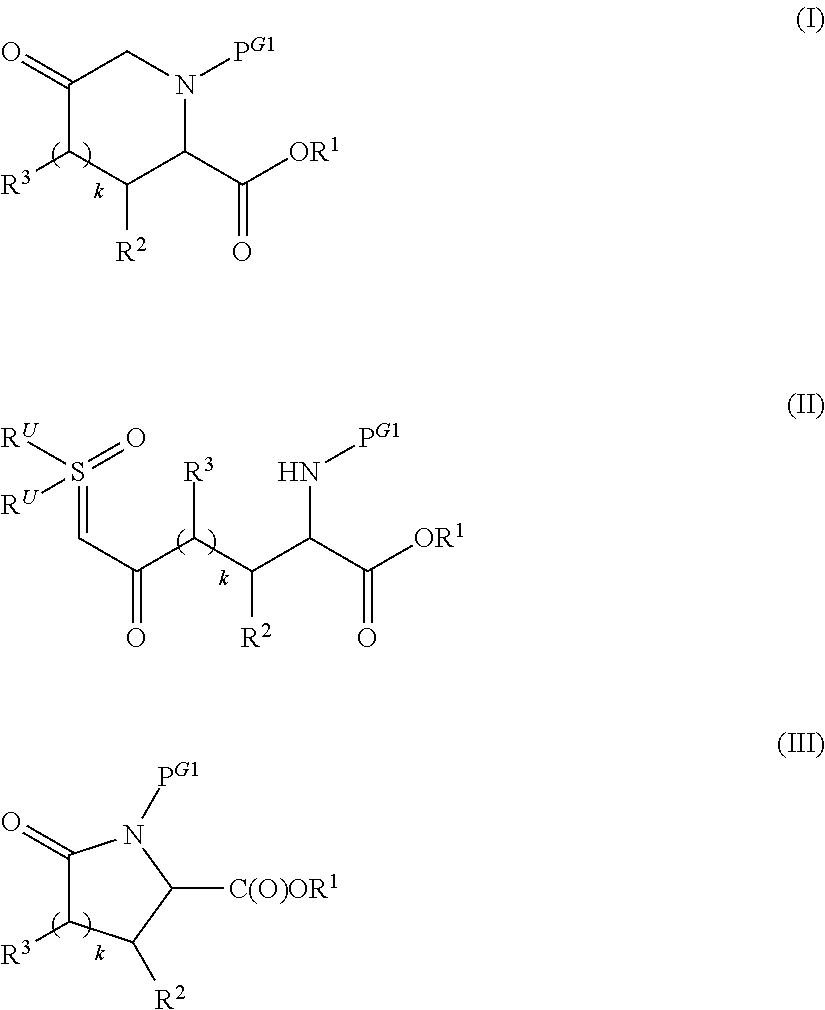
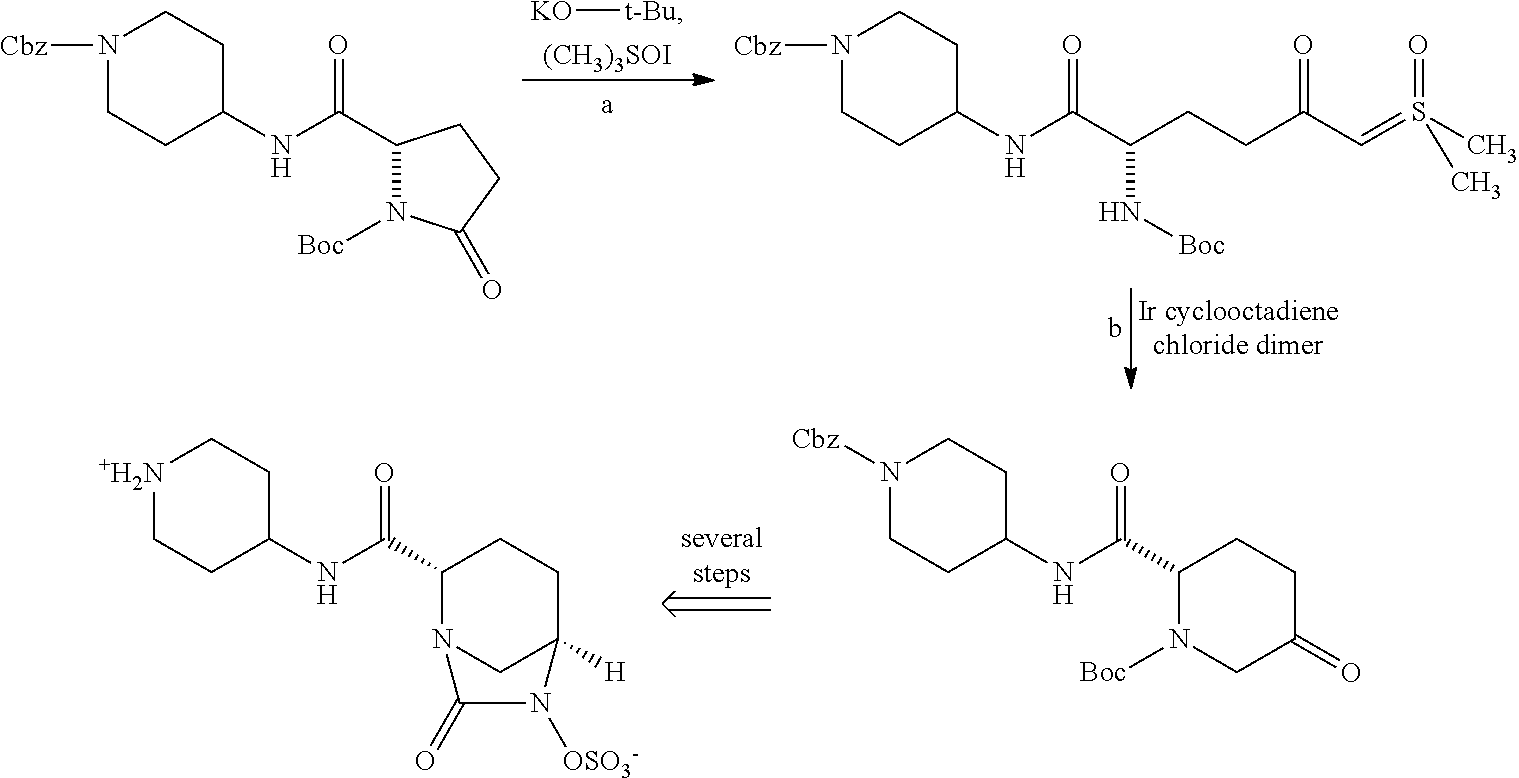
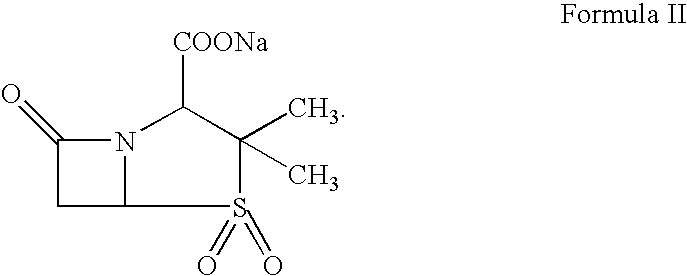
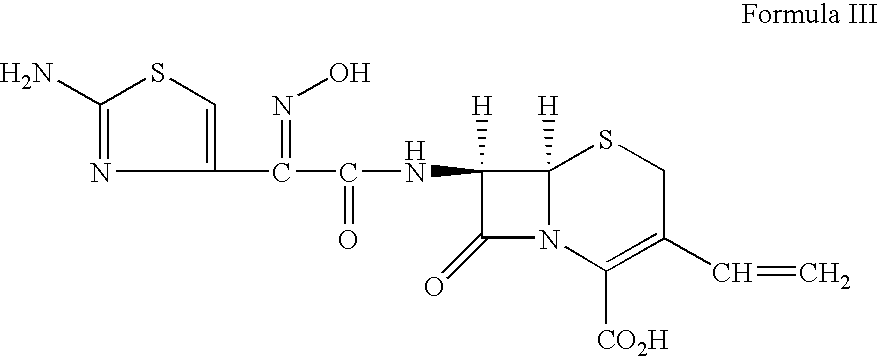
Preparation of alkyl esters of n-protected oxo-azacycloalkylcarboxylic acids
InactiveUS20120053350A1Small material requirementAvoid less flexibilityOrganic chemistryBulk chemical productionΒ lactamasesIridium
A process for the preparation of alkyl esters of N-protected oxo-azacycloalkylcarboxylic acids of Formula III: comprises contacting a ketosulfoxonium ylide of Formula II: with an iridium catalyst to obtain Compound III, wherein PG1 is an amine protective group; k is 0, 1, or 2; and RU, R1, R2, and R3 are defined herein. An embodiment of the process further com rises contacting a compound of Formula I: with a sulfoxonium halide of formula (RU)3S(O)Z, wherein Z is halide, in the presence of a strong base to obtain Compound II. Additional embodiments add a series of process steps leading to the synthesis of 7-oxo-1,6-diazabicyclo[3.2.1]octanes suitable for use as β-lactamase inhibitors.
Owner:MERCK SHARP & DOHME CORP
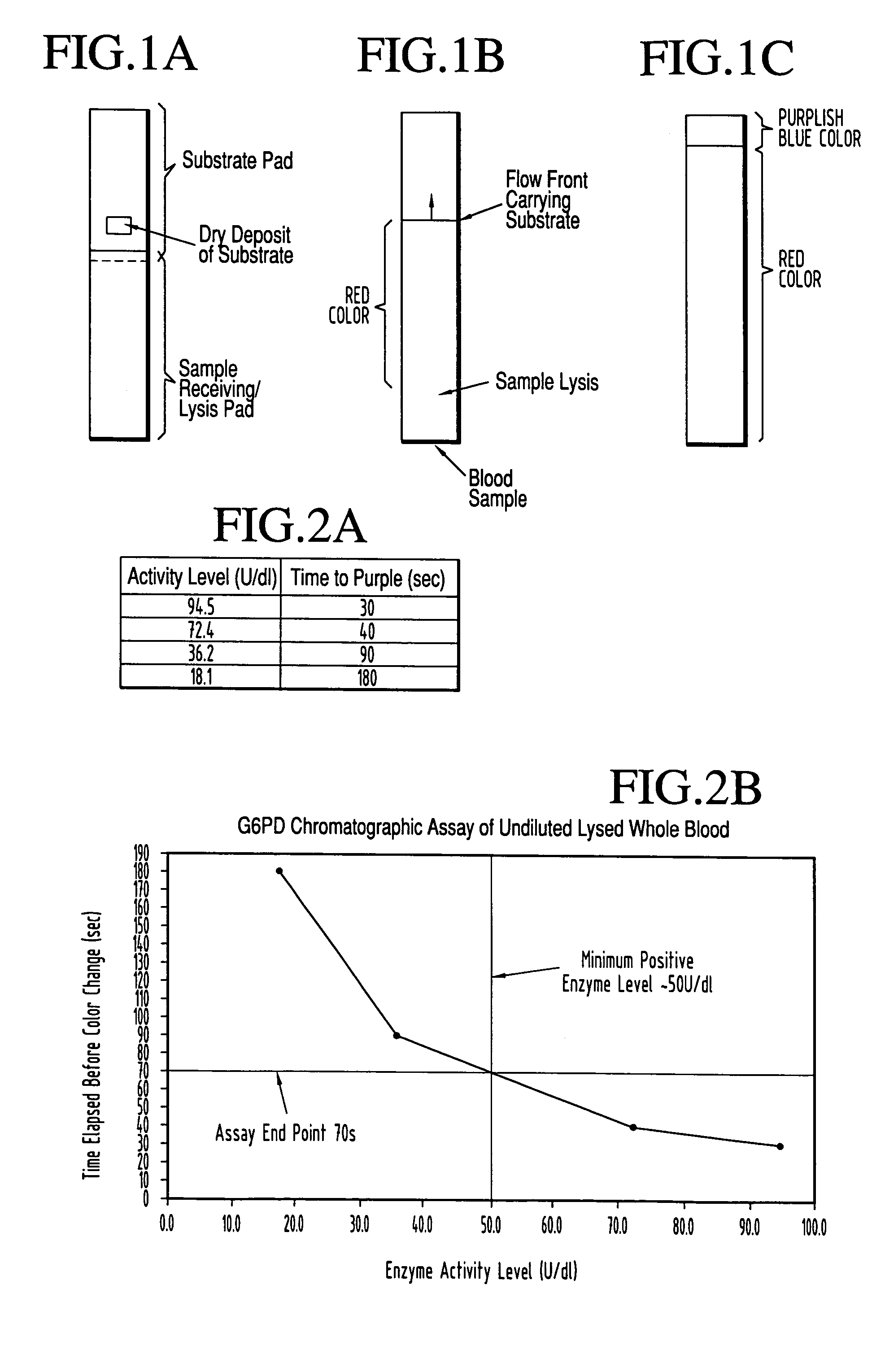
Dry chemistry, lateral flow-reconstituted chromatographic enzyme-driven assays
InactiveUS7425302B2Easy to observeHigh detection sensitivityBioreactor/fermenter combinationsBiological substance pretreatmentsAdditive ingredientPeroxidase
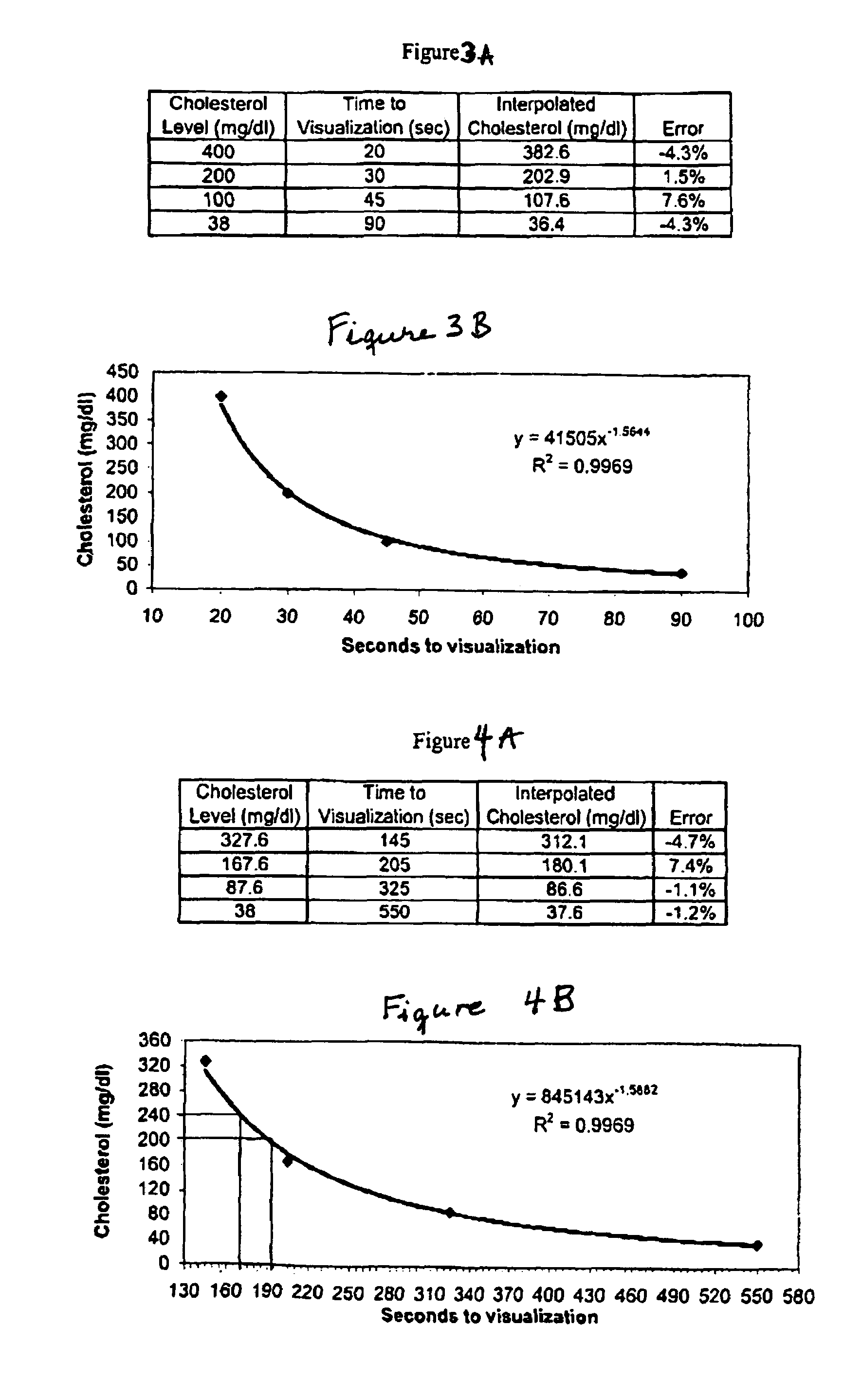
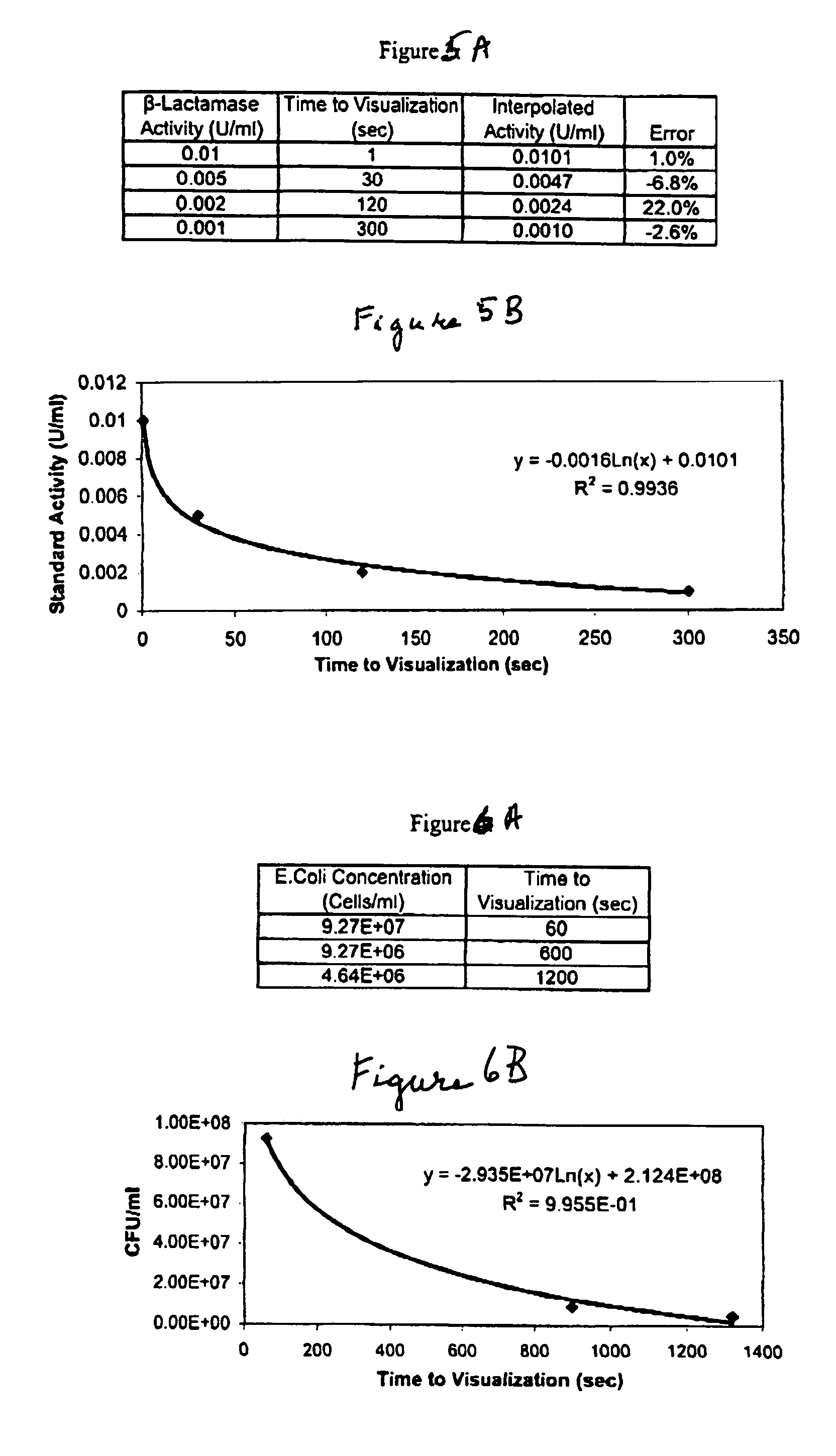
A lateral flow chromatographic assay format for the performance of rapid enzyme-driven assays is described. A combination of components necessary to elicit a specific enzyme reaction, which are either absent from the intended sample or insufficiently present therein to permit completion of the desired reaction, are predeposited as substrate in dry form together with ingredients necessary to produce a desired color upon occurrence of the desired reaction. The strip is equipped with a sample pad placed ahead of the substrate deposit in the flowstream, to which liquid sample is applied. The sample flows from the sample pad into the substrate zone where it immediately reconstitutes the dried ingredients while also intimately mixing with them and reacting with them at the fluid front. The fluid front moves rapidly into the final “read zone” wherein the color developed is read against predetermined color standards for the desired reaction. Pretreatment pads for the sample, as needed, (e.g. a lysing pad for lysing red blood cells in whole blood) are placed in front of the sample pad in the flow path as appropriate. The assay in the format of the invention is faster and easier to perform than analogous wet chemistry assays.Specific assays for glucose-6-phosphate dehydrogenase (“G-6PD”), total serum cholesterol, β-lactamase activity and peroxidase activity are disclosed.
Owner:ABBOTT DIAGNOSTICS SCARBOROUGH INC
Β-lactamase inhibitors
Substituted bicyclic beta-lactams of Formula I: (I), are β-lactamase inhibitors, wherein a, X, R1 and R2 are defined herein. The compounds and pharmaceutically acceptable salts thereof are useful in the treatment of bacterial infections in combination with β-lactam antibiotics. In particular, the compounds can be employed with a β-lactam antibiotics (e.g., imipenem, piperacillin, or ceftazidime) against microorganisms resistant to β-lactam antibiotics due to the presence of the β-lactamases.
Owner:MERCK SHARP & DOHME LLC
Therapy for Treating Resistant Bacterial Infections
The invention relates to an improved therapy for treating resistant bacterial infections caused by extended-spectrum β-lactamase (ESBLs)-producing strains in a warm-blooded animal, adjuvant step down therapy, and pharmaceutical compositions for such therapies. The invention also relates to a method for inhibiting bacterial resistance in ESBLs-producing strains so as to have better control over the therapy; achieve reduced hospital stay and adjuvant step down therapy so as to avoid recrudescence. In particular, the therapy includes antibacterial combination of cefepime with sulbactam via parenteral route, followed by oral third generation cephalosporin with a suitable β lactamase inhibitor.
Owner:PATEL MAHESH VITHALBHAI +5
Device and method for detecting antibiotic-inactivating enzymes
ActiveUS20050089947A1Facilitated releaseRobust in vitroMicrobiological testing/measurementBiological material analysisΒ lactamasesMicroorganism
A method for determining whether a microorganism produces an AmpC β-lactamase is disclosed in which a culture of a microorganism suspected of producing a β-lactamase that inactivates a β-lactam-containing antibiotic is admixed with an effective amount of each of i) a β-lactam-containing antibiotic, ii) a β-lactamase inhibitor to which AmpC β-lactamase is resistant, and iii) a permeabilizing agent for the microorganism present in a non-growth-inhibiting microorganism-permeabilizing amount to form an assay culture. That assay culture in maintained under appropriate culture conditions and for a time period sufficient to determine the interaction of the microorganism with the AmpC β-lactamase resistant inhibitor and antibacterial compound, and thereby determine the presence of an AmpC β-lactamase, wherein a positive test indicates the presence of an AmpC β-lactamase.
Owner:CREIGHTON UNIVERSITY
Isoxazole beta-lactamase inhibitors
β-Lactamase inhibitor compounds (BLIs) are disclosed, including compounds that have activity against class A, class C or class D β-lactamases. Methods of manufacturing the BLIs, and uses of the compounds in the preparation of pharmaceutical compositions and antibacterial applications are also disclosed.
Owner:MERCK SHARP & DOHME LLC
Beta-lactamase detecting reagent composition, detection kit and detection method
InactiveUS20060014230A1Quick checkOrganic chemistryMicrobiological testing/measurementΒ lactamasesNitrostyrol
The present invention provides a reagent composition for detecting β-lactamase including as a β-lactamase detection substrate 3-[2,4-dinitrostyryl]-7-(2-thienylacetamido]-3-cephem-4-carboxylic acid, or 7-[2-(2-aminothiazol-4-yl)-2-(1-carboxy-1-methylethoxy-imino)acetamido]-3-(2,4-dinitrostyryl)-3-cephem-4-carboxylic acid, and at least one β-lactamase inhibitor selected from the group consisting of clavulanic acid, aztreonam, ethylenediaminetetraacetic acid, and cloxacillin, which composition can detect β-lactamases rapidly and easily with high sensitivity. The present invention also provides a detection kit including the detecting reagent composition. Further, the present invention provides a β-lactamase detection method where a liquid specimen containing a target substance to be analyzed is brought into contact with the composition.
Owner:SHOWA YAKUHIN KAKO +1
Methods of treating bacterial infections
The present invention relates to compounds, compositions and methods for treating bacterial infections. Embodiments of the present invention include antibiotics and β-lactamase inhibitors to treat resistant infections.
Owner:REMPEX PHARM INC
Heterocyclic compounds as inhibitors of beta-lactamases
This invention discloses and claims methods for inhibiting bacterial β-lactamases and treating bacterial infections by inhibiting bacterial β-lactamases in man or an animal comprising administering a therapeutically effective amount to said man or said animal of a compound, or pharmaceutically acceptable salt thereof, of formula (I) either alone or in combination with a β-lactamine antibiotic wherein said combination can be administered separately, together or spaced out over time. Pharmaceutical compositions comprising a compound of formula (I), or a combination of a compound of formula (I) and a therapeutically effective amount of a β-lactamine antibiotic, and a pharmaceutically acceptable carrier are also disclosed and claimed.
Owner:FOREST LAB HLDG LTD
Boronic acid derivatives and therapeutic uses thereof
ActiveUS20180002351A1Antibacterial agentsBoron compound active ingredientsΒ lactamasesAcid derivative
Disclosed herein are antimicrobial compounds compositions, pharmaceutical compositions, the method of use and preparation thereof. Some embodiments relate to boronic acid derivatives and their use as therapeutic agents, for example, β-lactamase inhibitors (BLIs).
Owner:QPEX BIOPHARMA INC
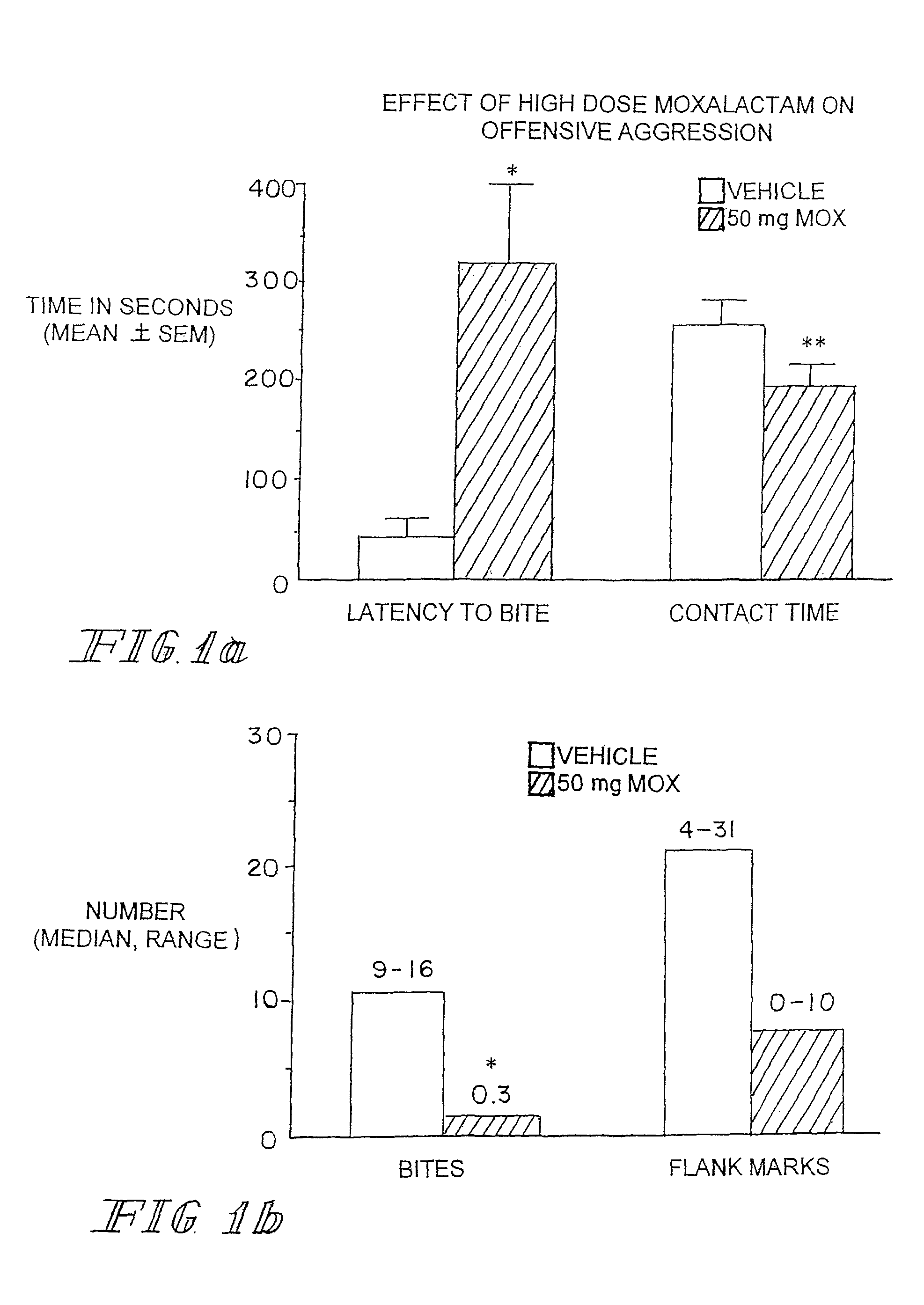
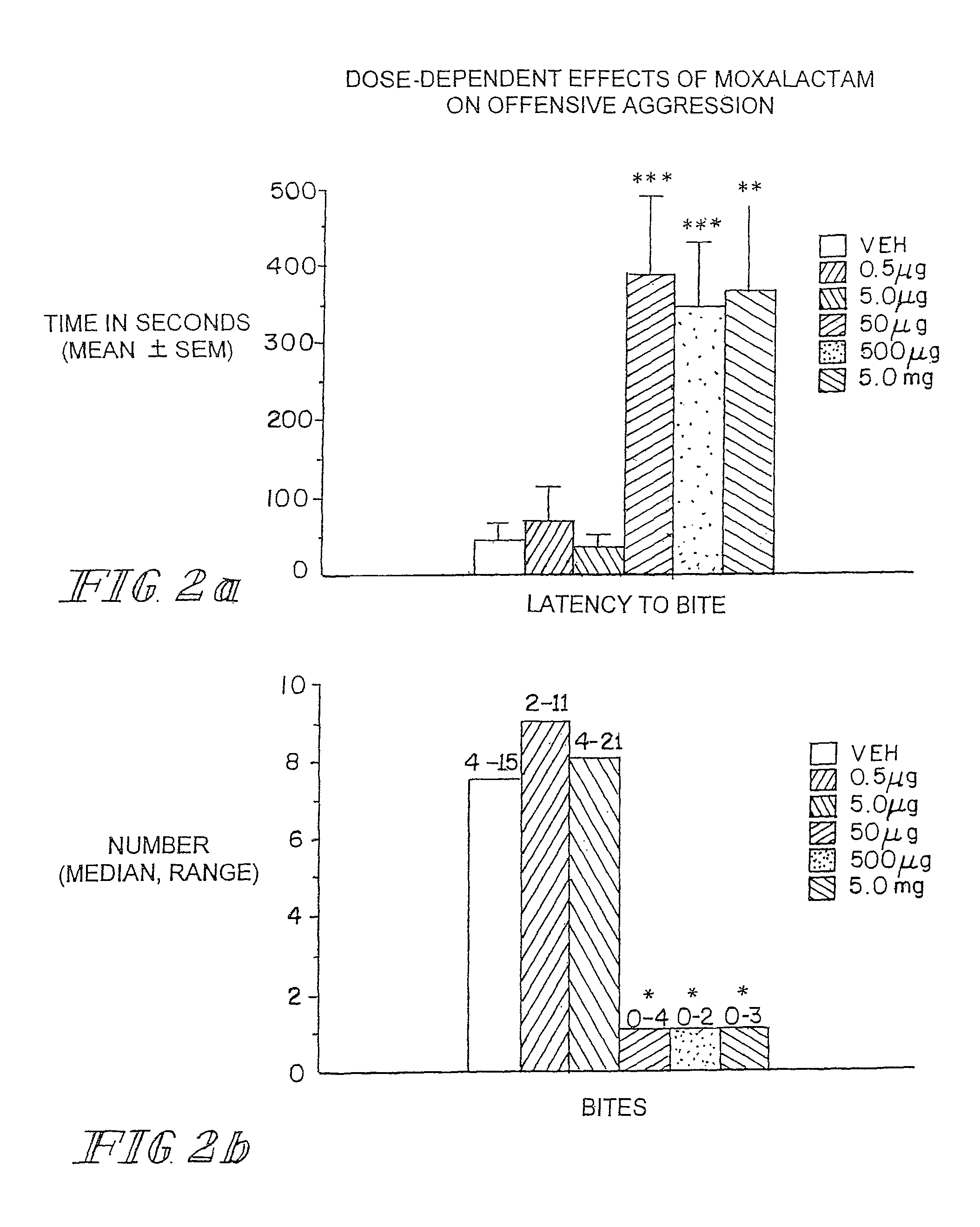
Neurotherapeutic Compositions and Method
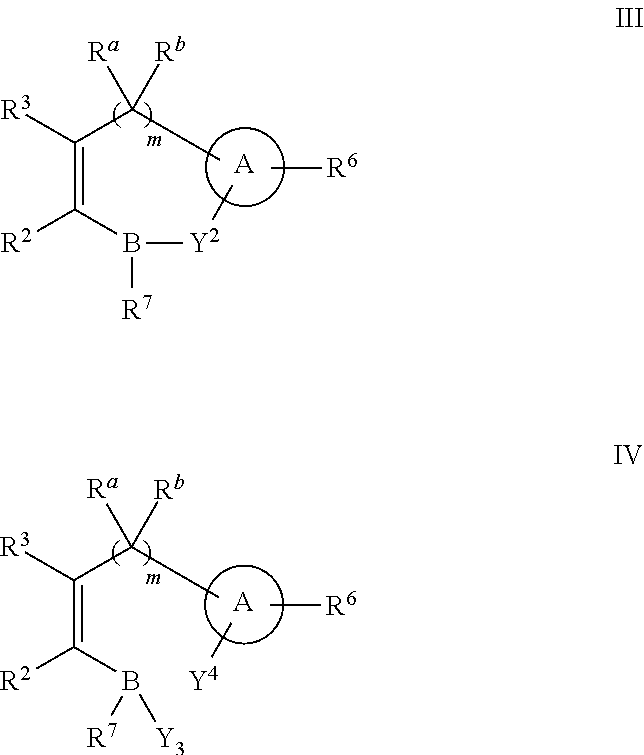
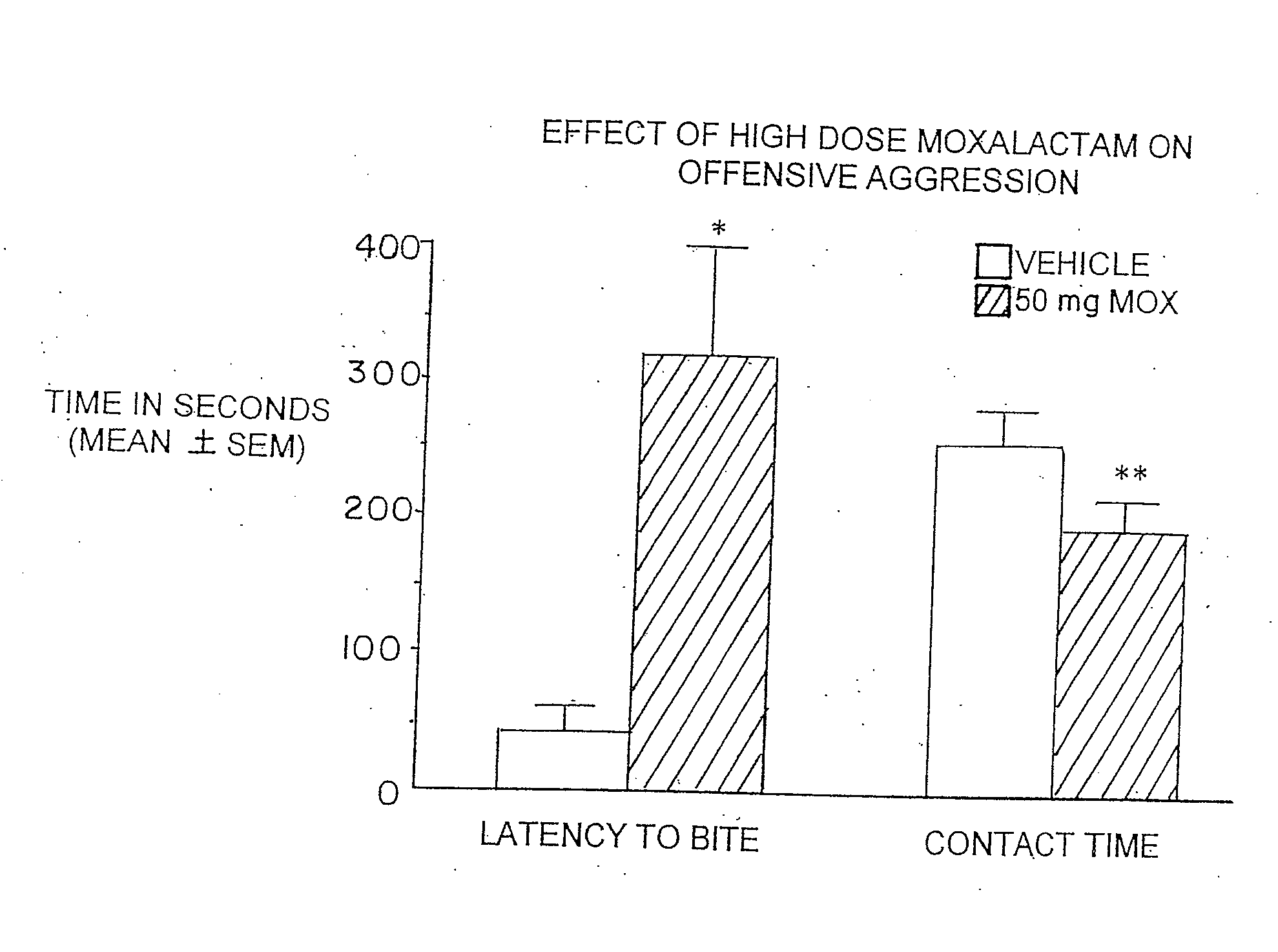
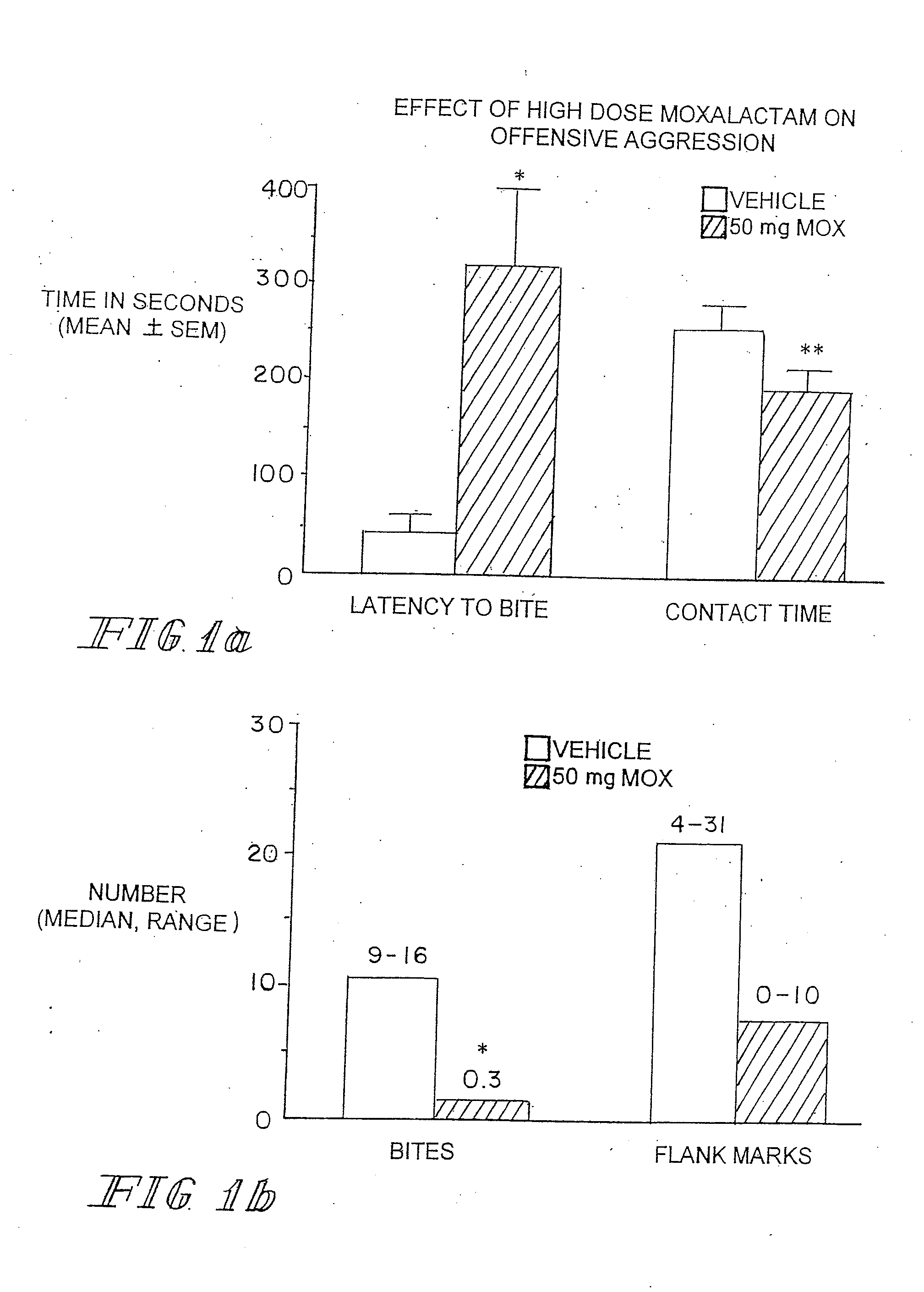
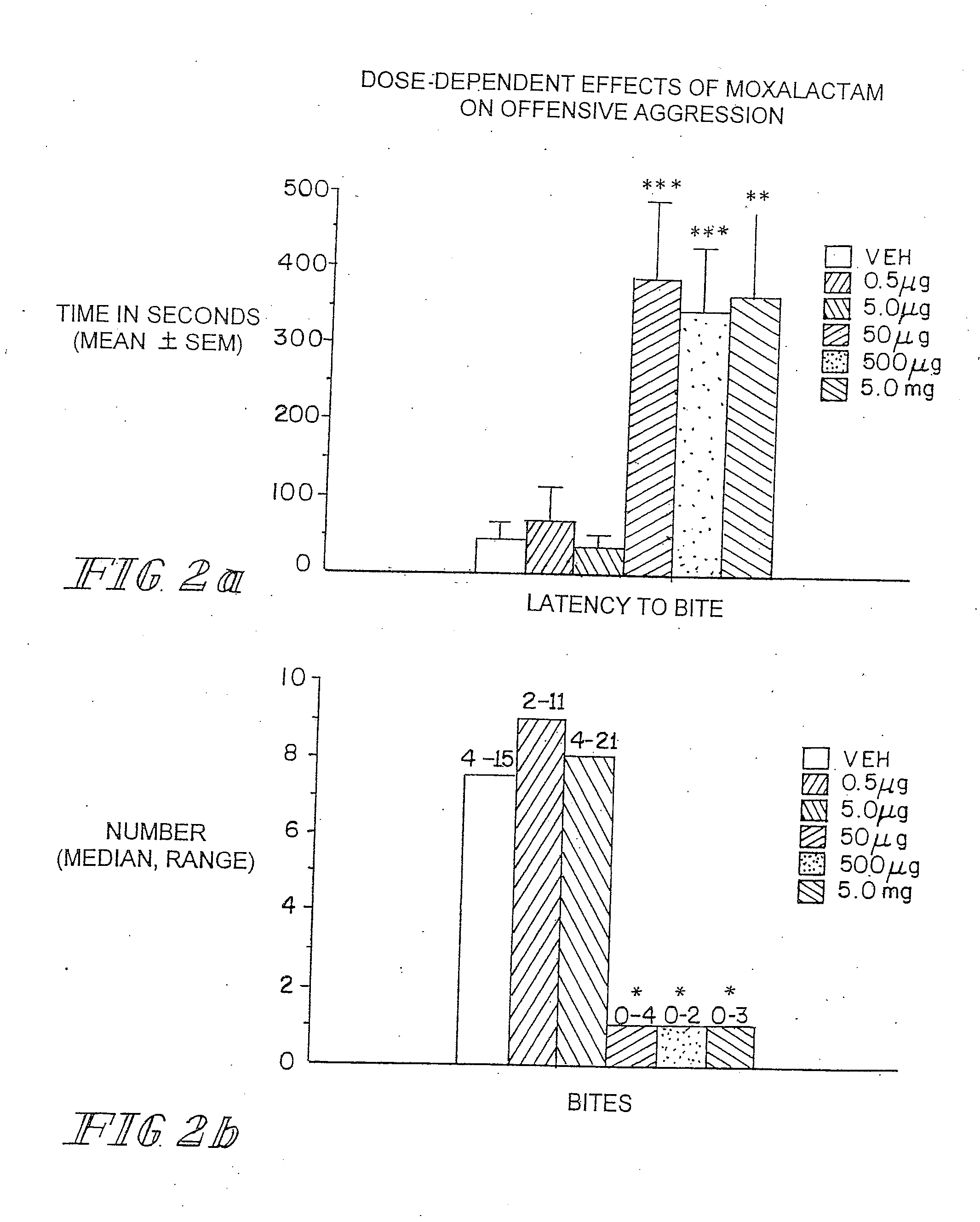
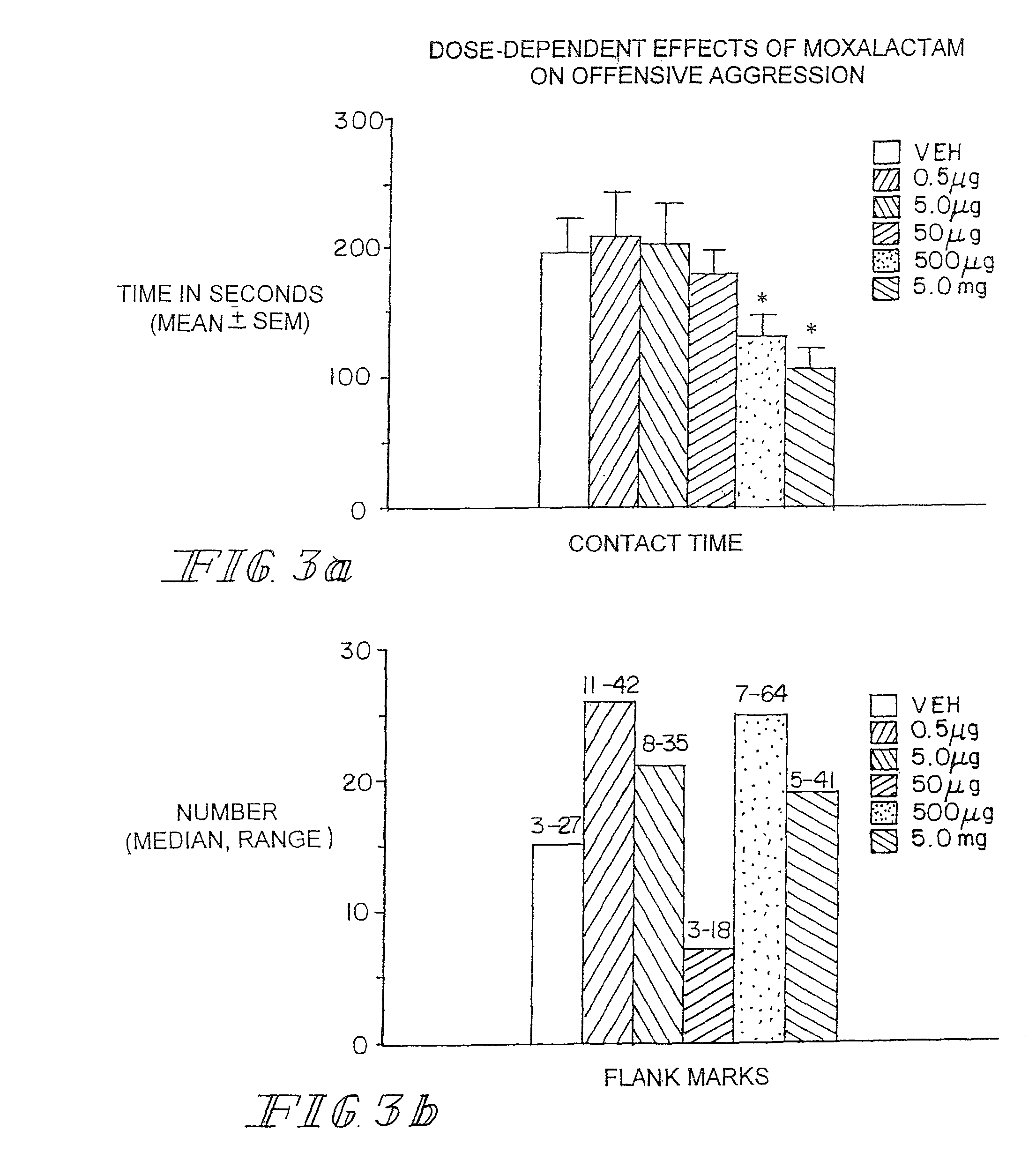
Administration of β-Lactam compounds, including β-lactam antibiotics and β-lactamase inhibitors provides significant neurotropic effects in warm-blooded vertebrates evidence inter alia by anxiolytic and anti-aggressive behavior modification and enhanced cognition. Therapeutic methods for using such compounds and their pharmaceutical formulations are described.
Owner:REVAAX PHARMA LLC
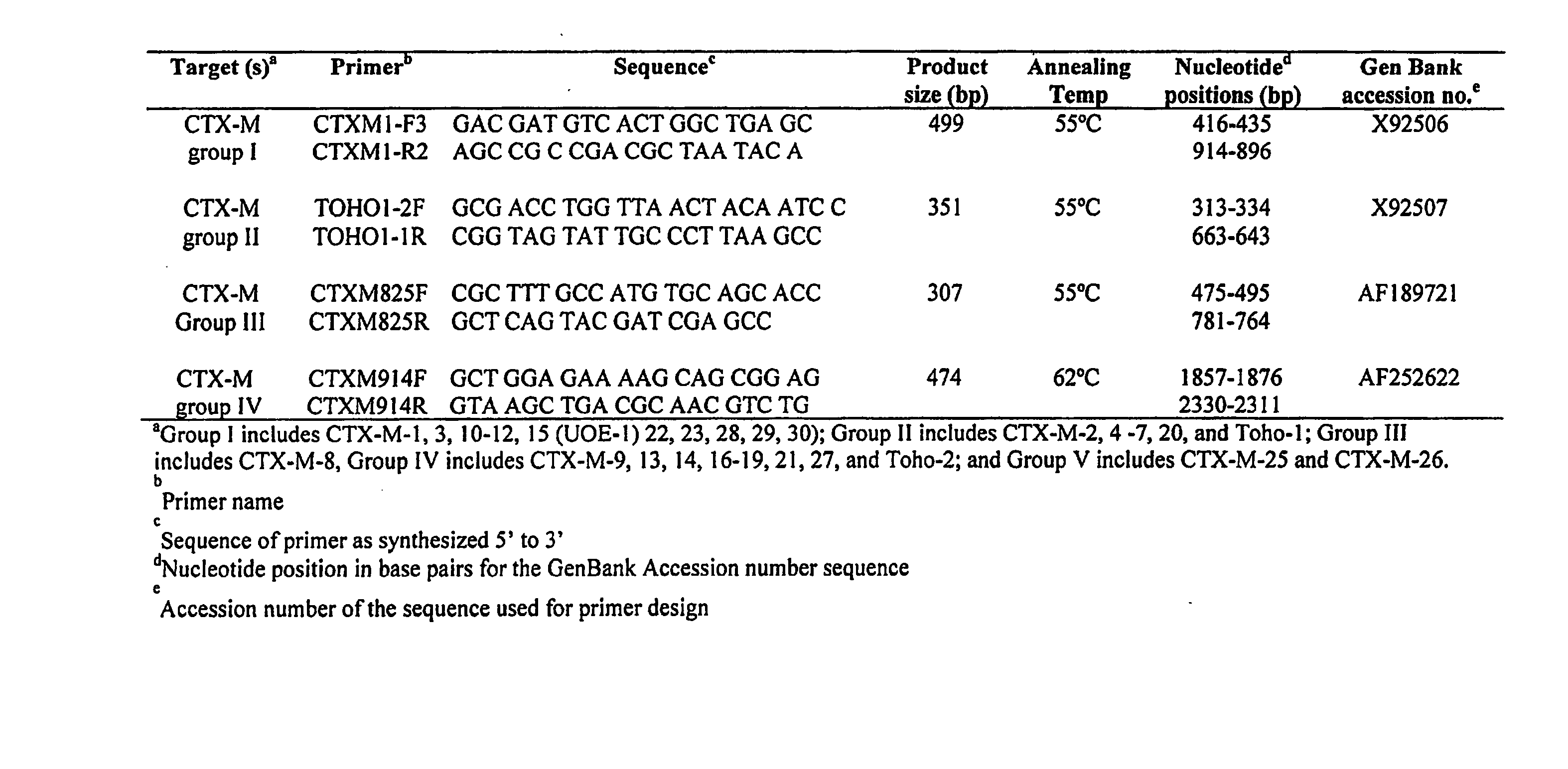
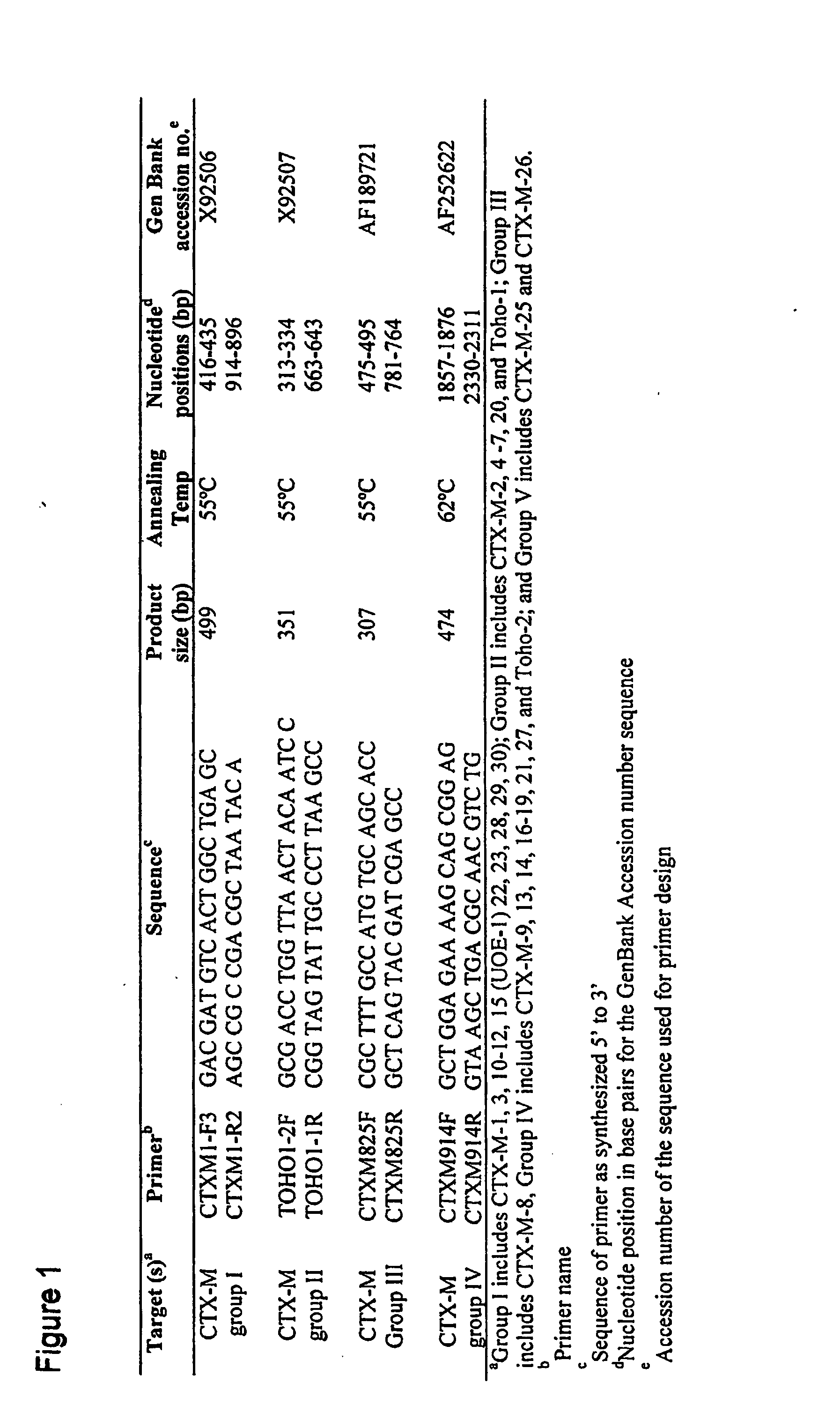
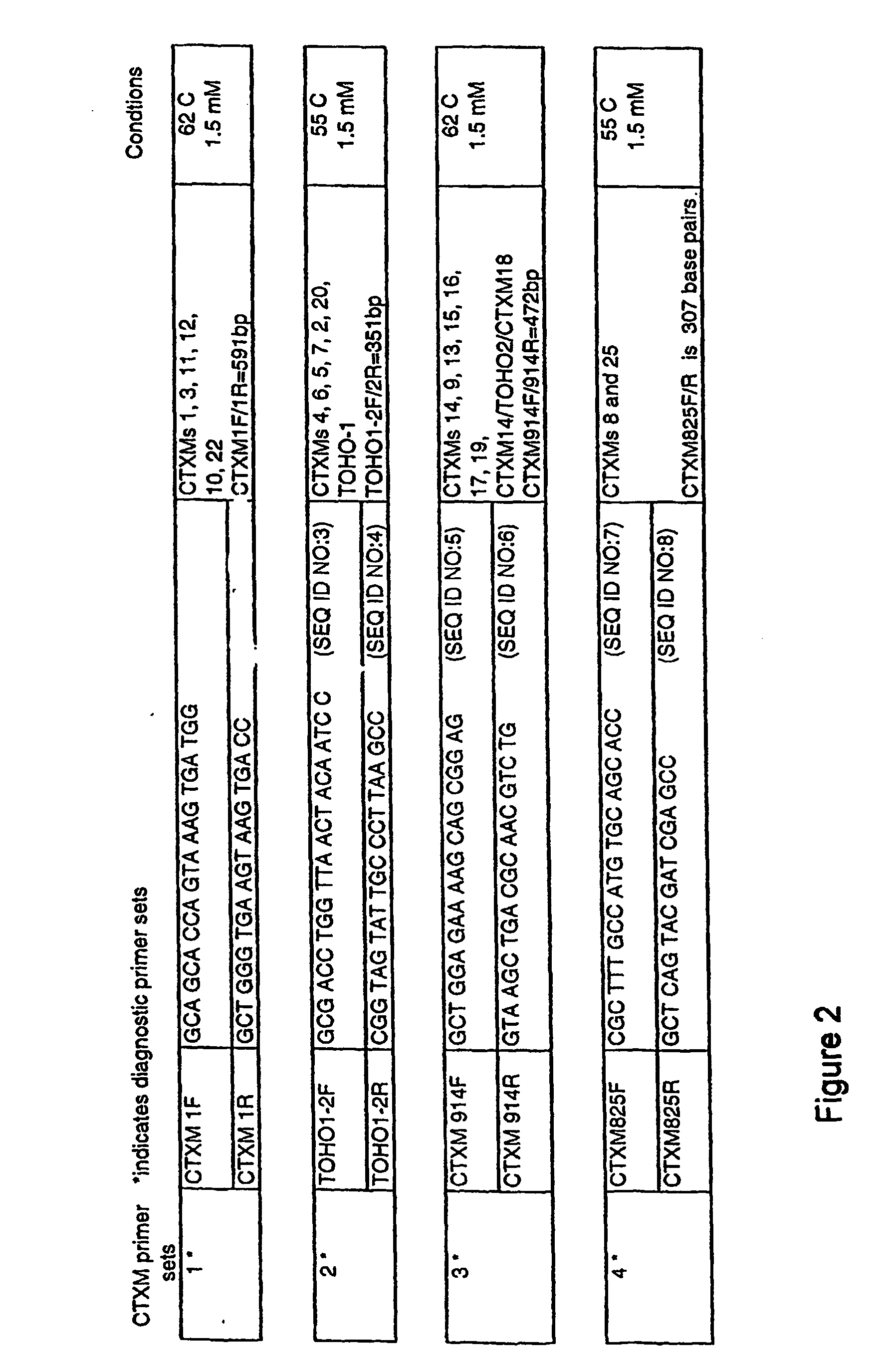
Primers for Use in Detecting Beta-Lactamases
InactiveUS20070248954A1Sugar derivativesMicrobiological testing/measurementΒ lactamasesGreek letter beta
Oliognucleotide primers are provided that are specific for nucleic acid characteristic of certain β-lactamase genes. The primers can be employed in methods to identify nucleic acid characteristic of family-specific (and even group-specific) β-lactamase enzymes in samples, and particularly, in clinical isolates of Gram-negative bacteria.
Owner:CREIGHTON UNIVERSITY
Beta-lactamase inhibitors and methods of use thereof
The invention provides novel non-β-lactam inhibitors of β-lactamases. In particular, the invention provides boronic acid-based compounds set forth in the specification. These compounds may be used with β-lactam antibiotics to bacterial infection, particularly, β-lactam-antibiotic-resistant bacterial infections. These compounds are also antibacterial agents by themselves. The invention further provides methods of using such compounds. Finally, the invention provides a pharmaceutical composition comprising these compounds.
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE +1
Neurotherapeutic compositions and method
Administration of β-Lactam compounds, including β-lactam antibiotics and β-lactamase inhibitors provides significant neurotropic effects in warm-blooded vertebrates evidence inter alia by anxiolytic and anti-aggressive behavior modification and enhanced cognition. Therapeutic methods for using such compounds and their pharmaceutical formulations are described.
Owner:REVAAX PHARMA LLC
Galenic pectinate formulation for colon-targeted delivery of antibiotic-inactivating enzymes and method of use thereof
Forms of colonic delivery suited to be used orally and designed for colonic delivery of active ingredients selected from the group comprising enzymes capable of inactivating macrolide antibiotics and related compounds, enzymes capable of inactivating quinolones, and β-lactamases.
Owner:CENT NAT DE LA RECHERCHE SCI +1
Device and method for detecting antibiotic-inactivating enzymes
ActiveUS7291480B2Facilitated releaseGrowth inhibitionMicrobiological testing/measurementBiological material analysisMicroorganismΒ lactamases
A method for determining whether a microorganism produces an AmpC β-lactamase is disclosed in which a culture of a microorganism suspected of producing a β-lactamase that inactivates a β-lactam-containing antibiotic is admixed with an effective amount of each of i) a β-lactam-containing antibiotic, ii) a β-lactamase inhibitor to which AmpC β-lactamase is resistant, and iii) a permeabilizing agent for the microorganism present in a non-growth-inhibiting microorganism-permeabilizing amount to form an assay culture. That assay culture in maintained under appropriate culture conditions and for a time period sufficient to determine the interaction of the microorganism with the AmpC β-lactamase resistant inhibitor and antibacterial compound, and thereby determine the presence of an AmpC β-lactamase, wherein a positive test indicates the presence of an AmpC β-lactamase.
Owner:CREIGHTON UNIVERSITY
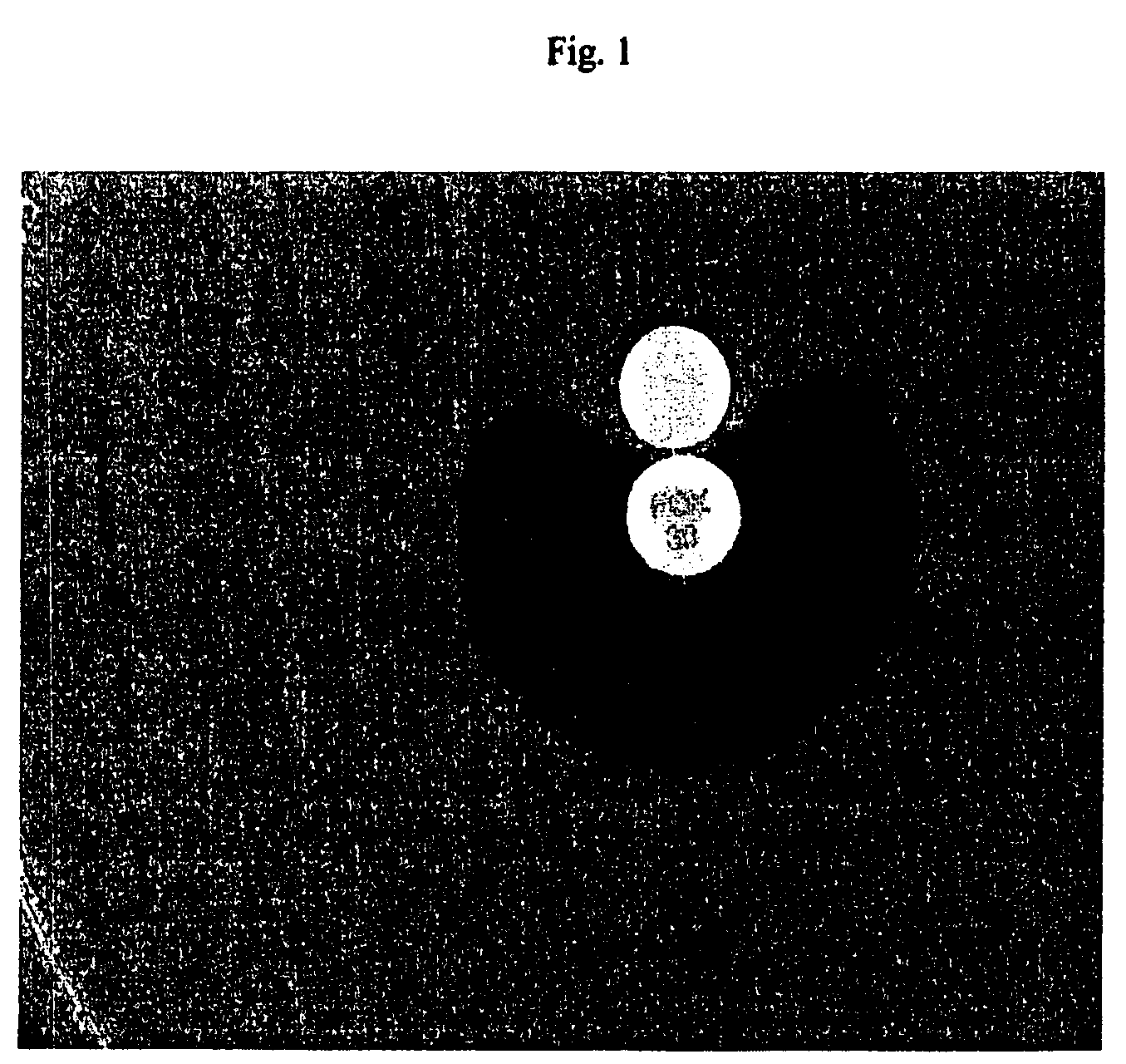
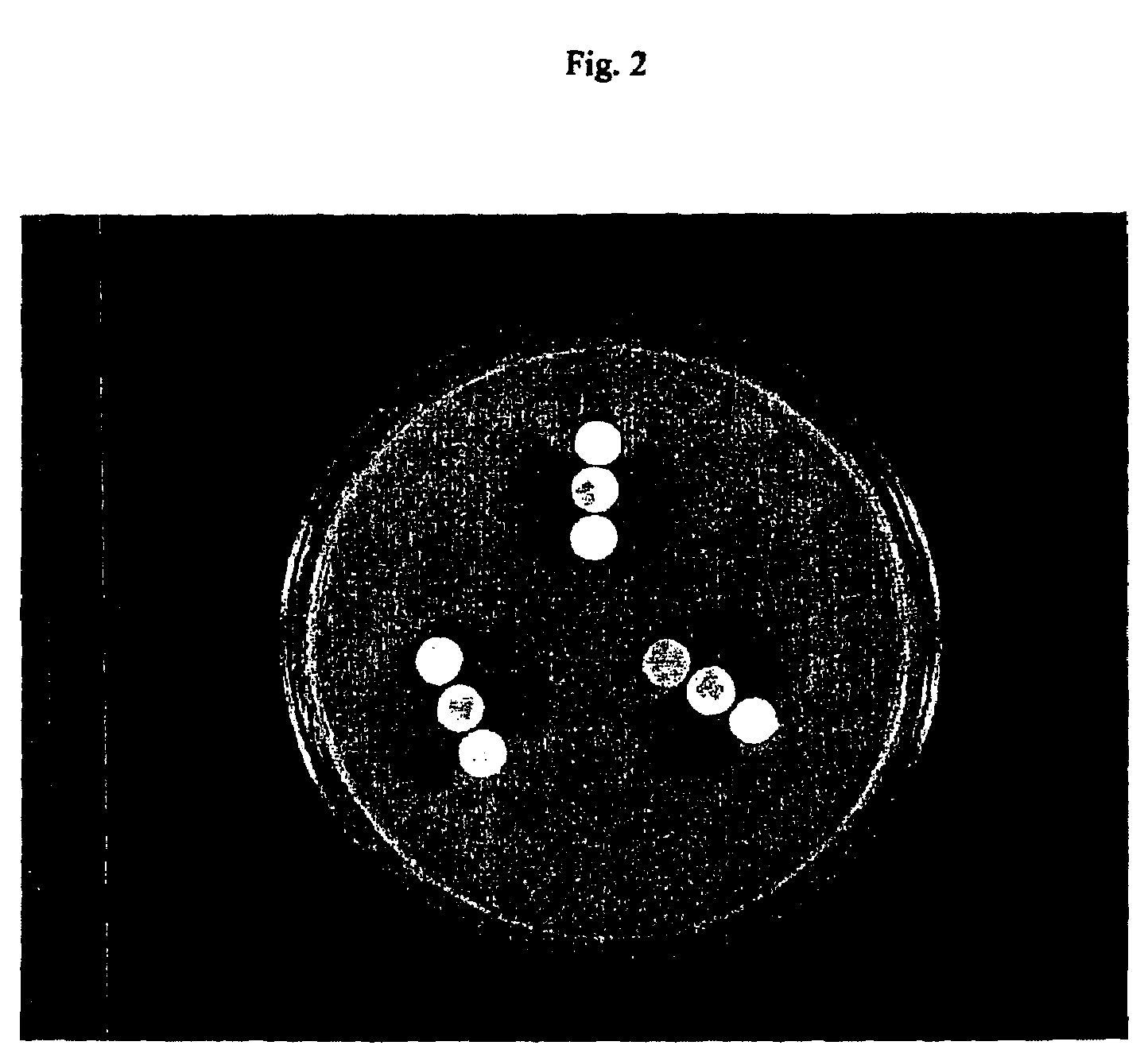
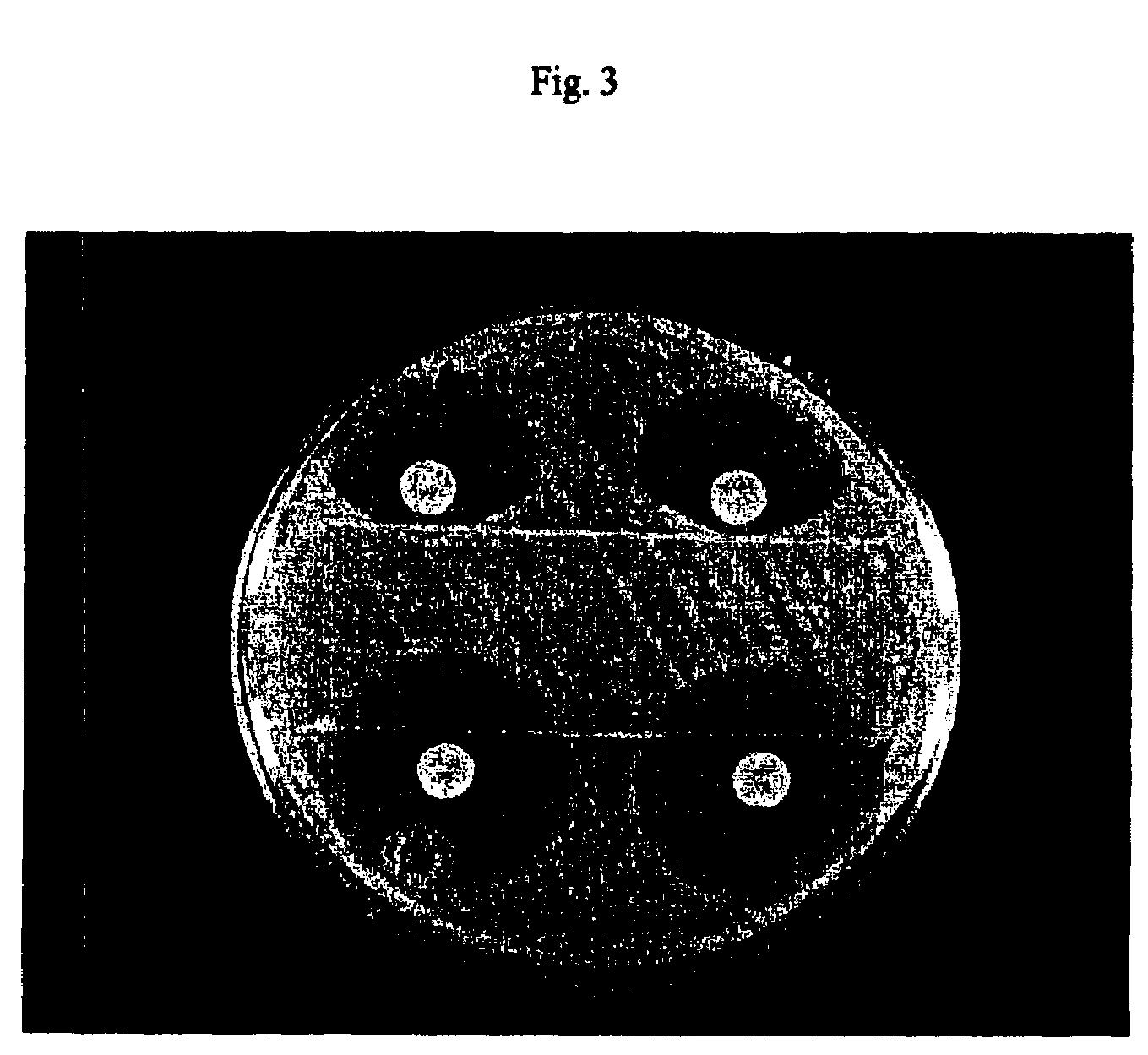
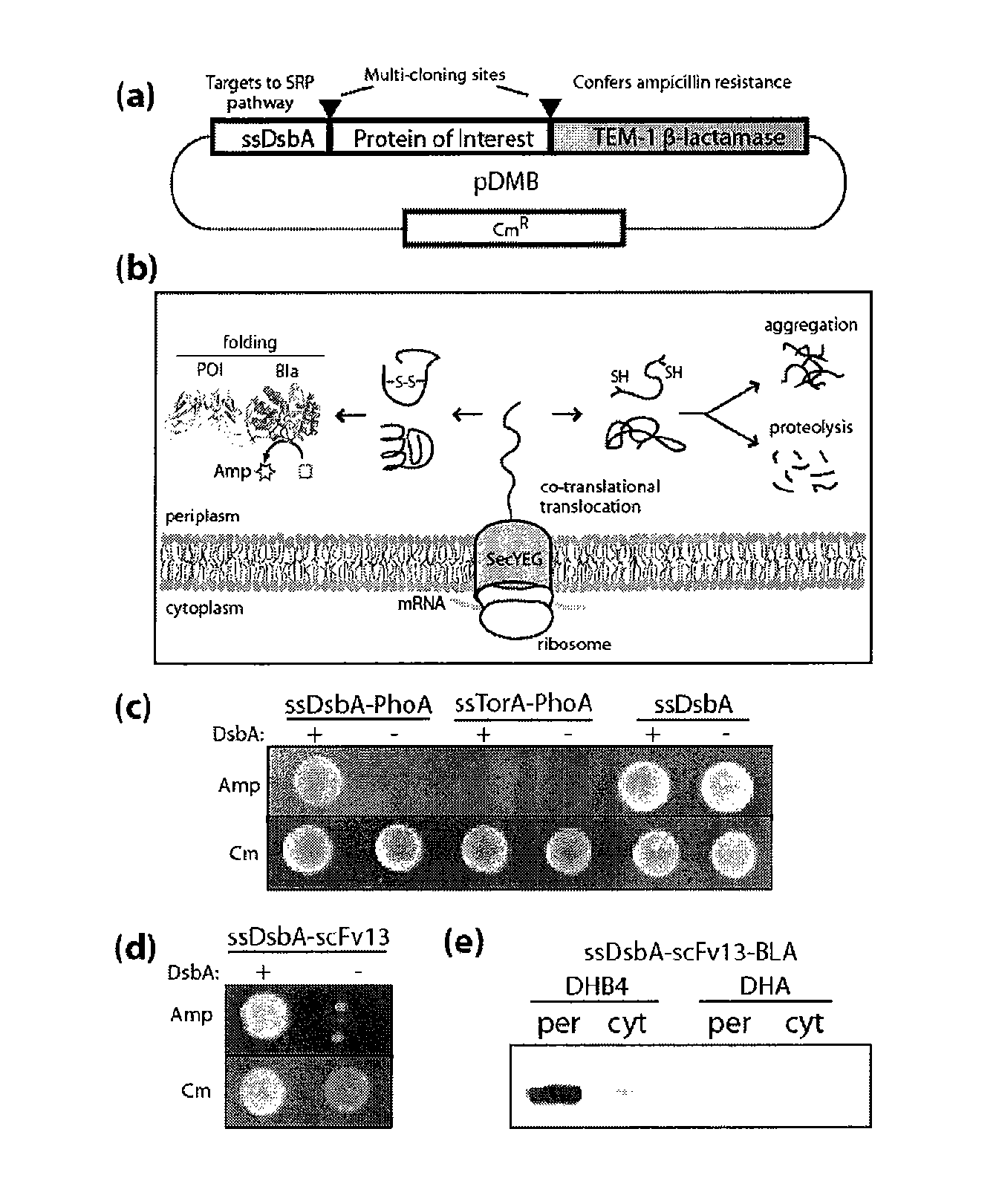
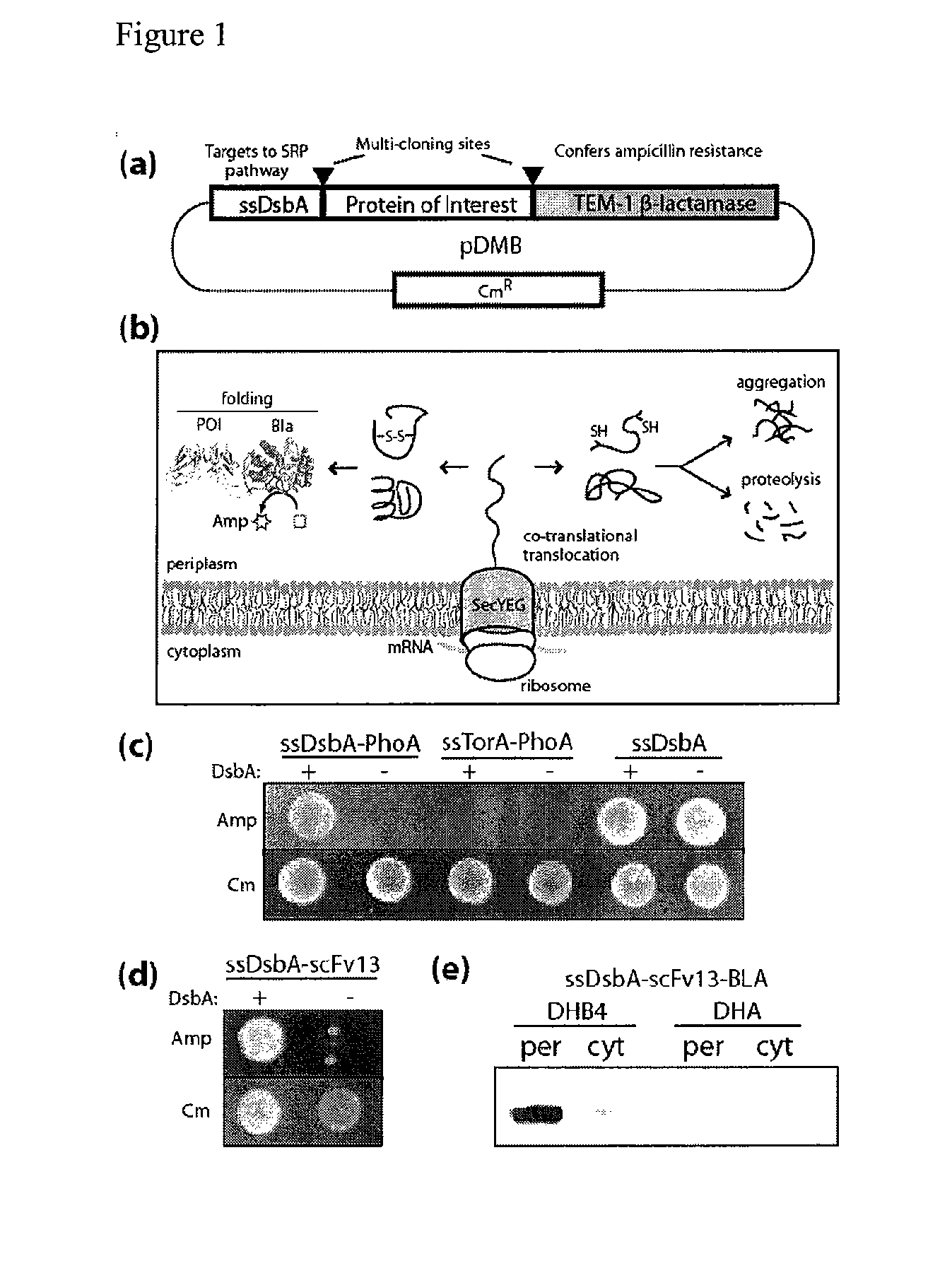
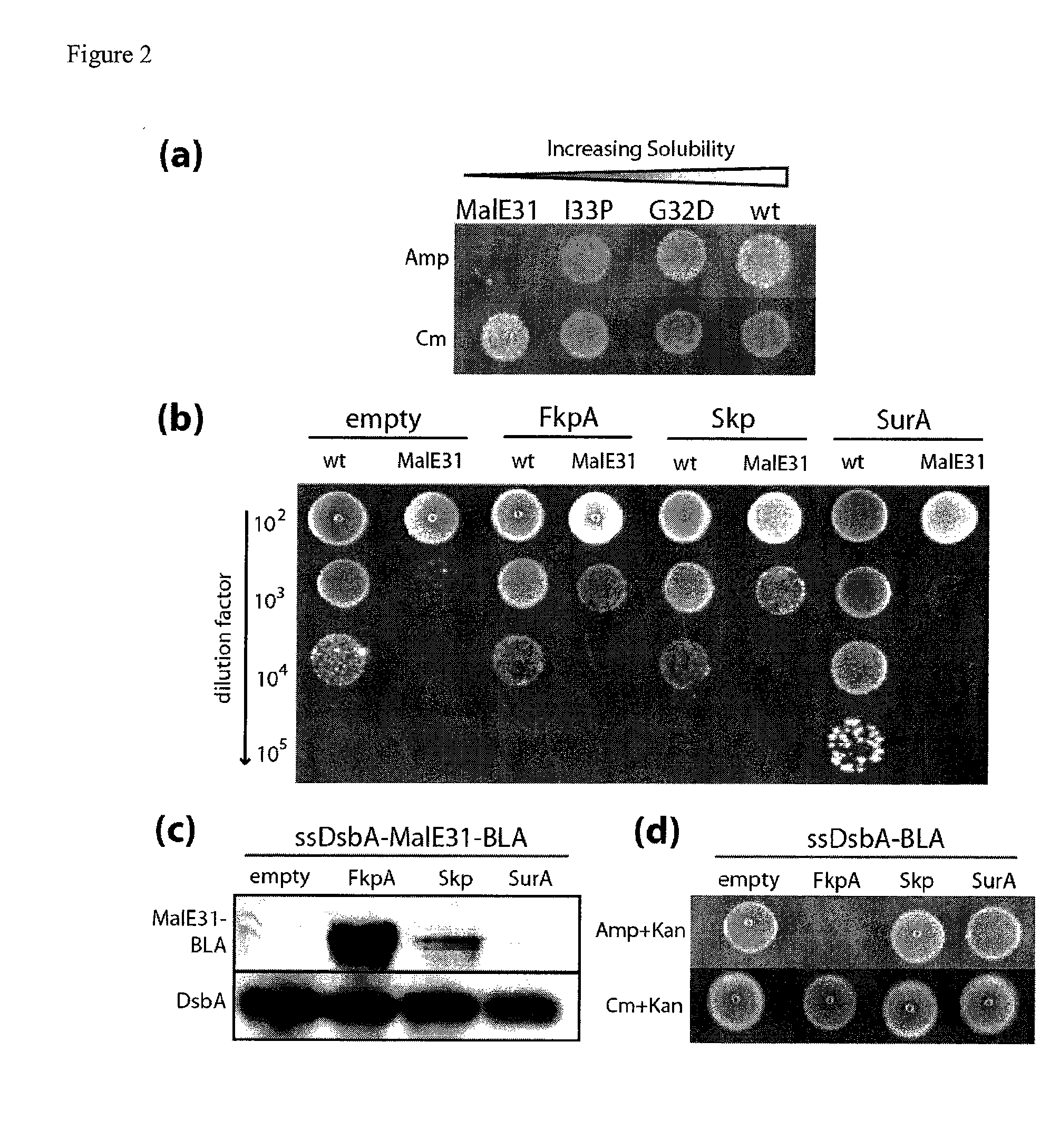
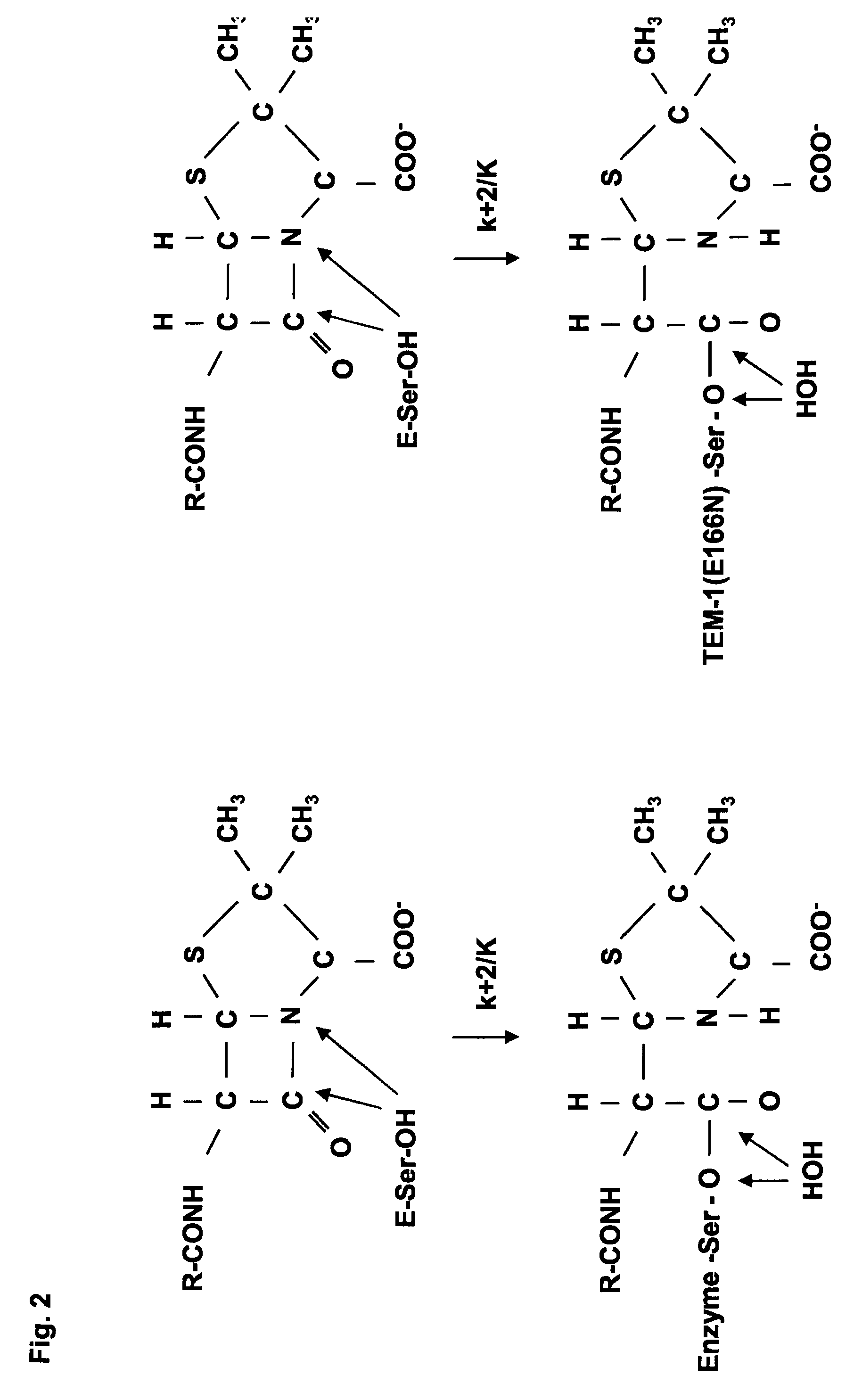
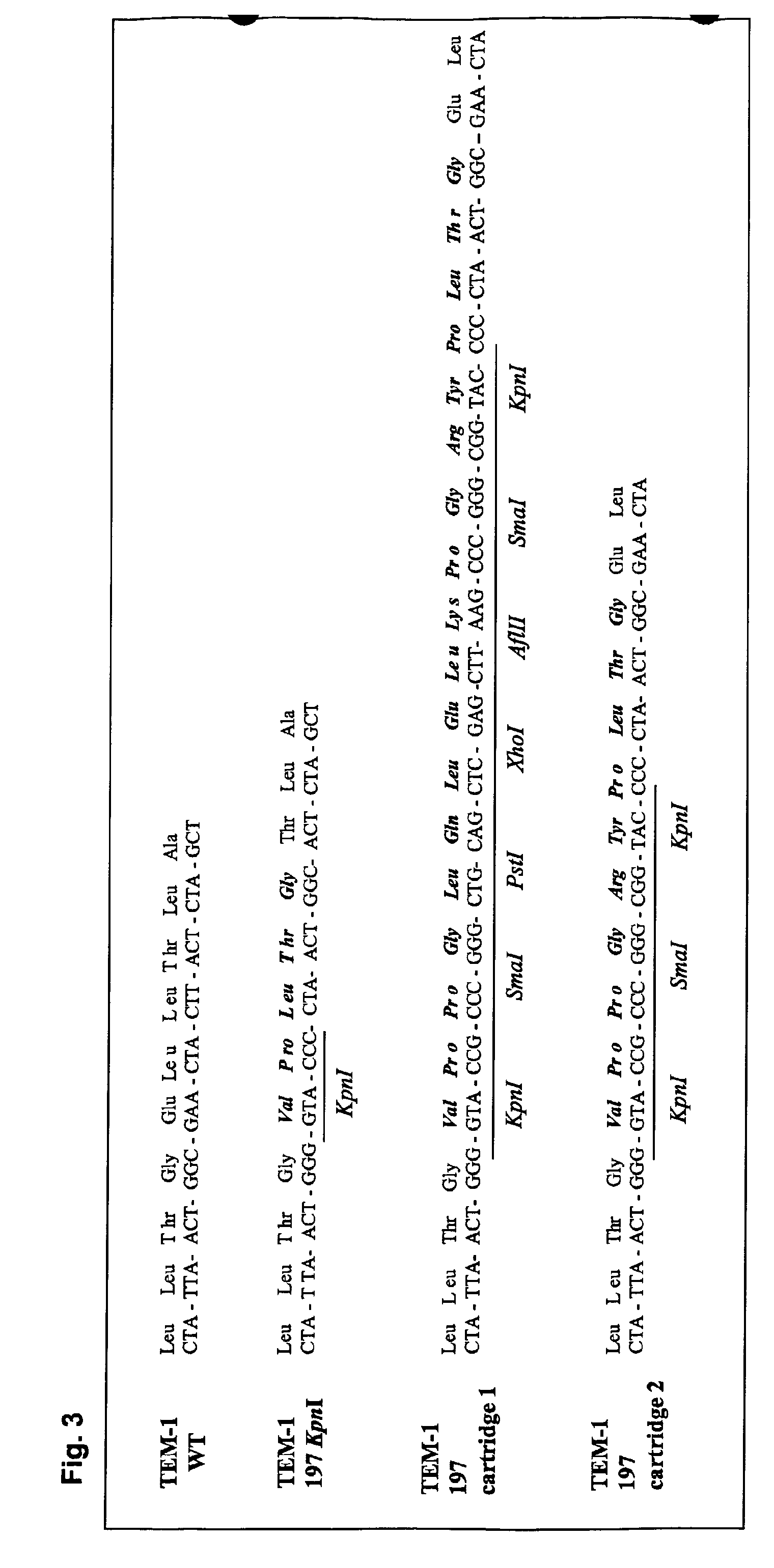
Genetic selection for protein folding and solubility in the bacterial periplasm
ActiveUS20100144546A1Sugar derivativesAntibody mimetics/scaffoldsSignal recognition particleSolubility
The present invention relates to the fields of microbiology, molecular biology and protein biochemistry. More particularly, it relates to compositions and methods for analyzing and altering (e.g., enhancing or inhibiting) protein folding and solubility (e.g., within periplasm). The present invention provides an engineered assay for protein folding and solubility in the E. coli periplasm based on co-translational translocation of a chimera comprising a protein of interest fused to TEM-I β-lactamase that is targeted for export via the signal recognition particle (SRP)-dependent pathway. Using an array of native and heterologous proteins, it is demonstrated that periplasmic folding behavior of proteins is intimately coupled to in vivo β-lactamase activity. As a result of this coupling, the reporter is useful for (1) facile discovery of extrinsic periplasmic factors that affect protein folding and solubility; and (2) genetic selection of solubility-enhanced proteins.
Owner:CORNELL UNIVERSITY
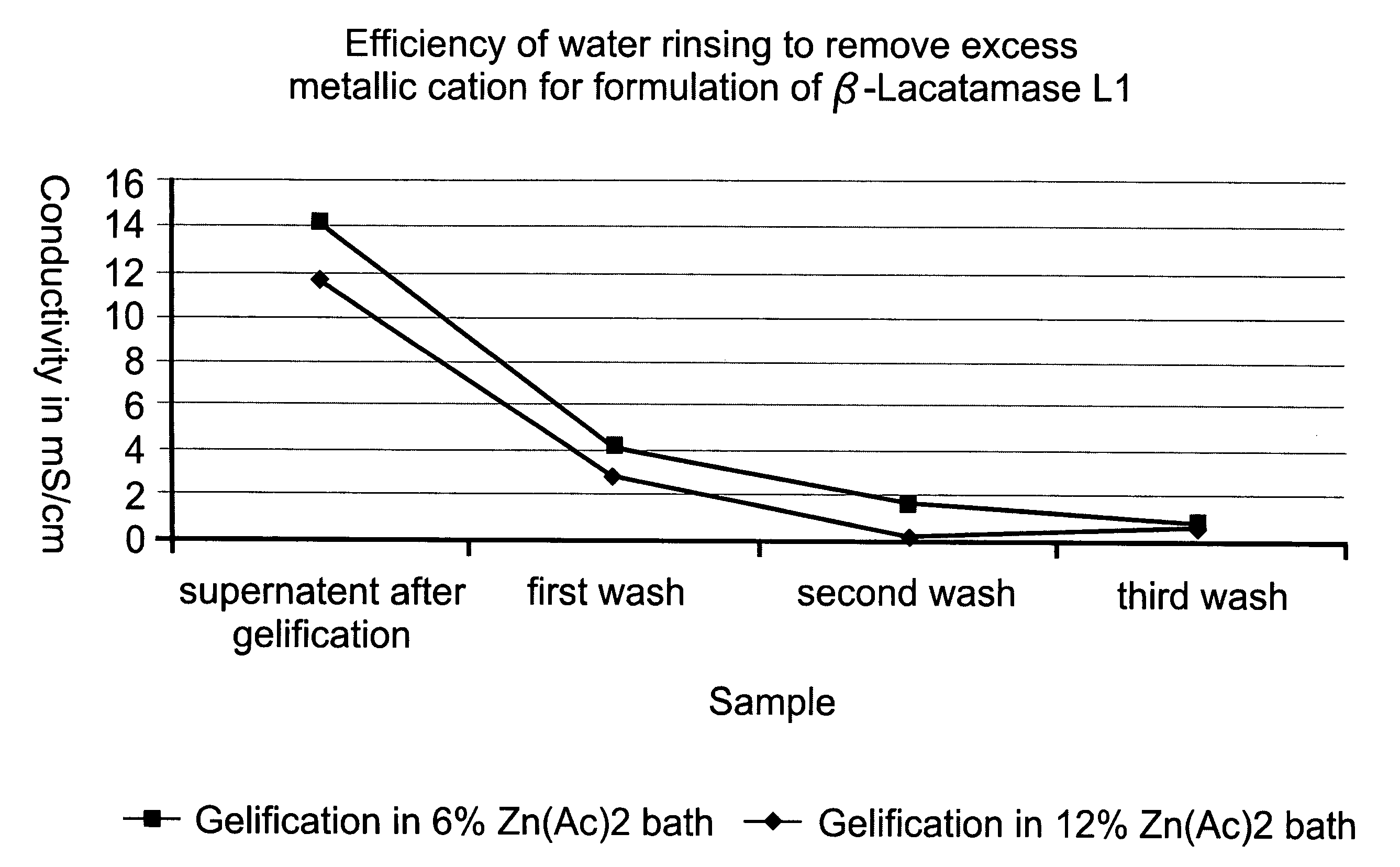
Colonic delivery of metallo-dependent enzymes
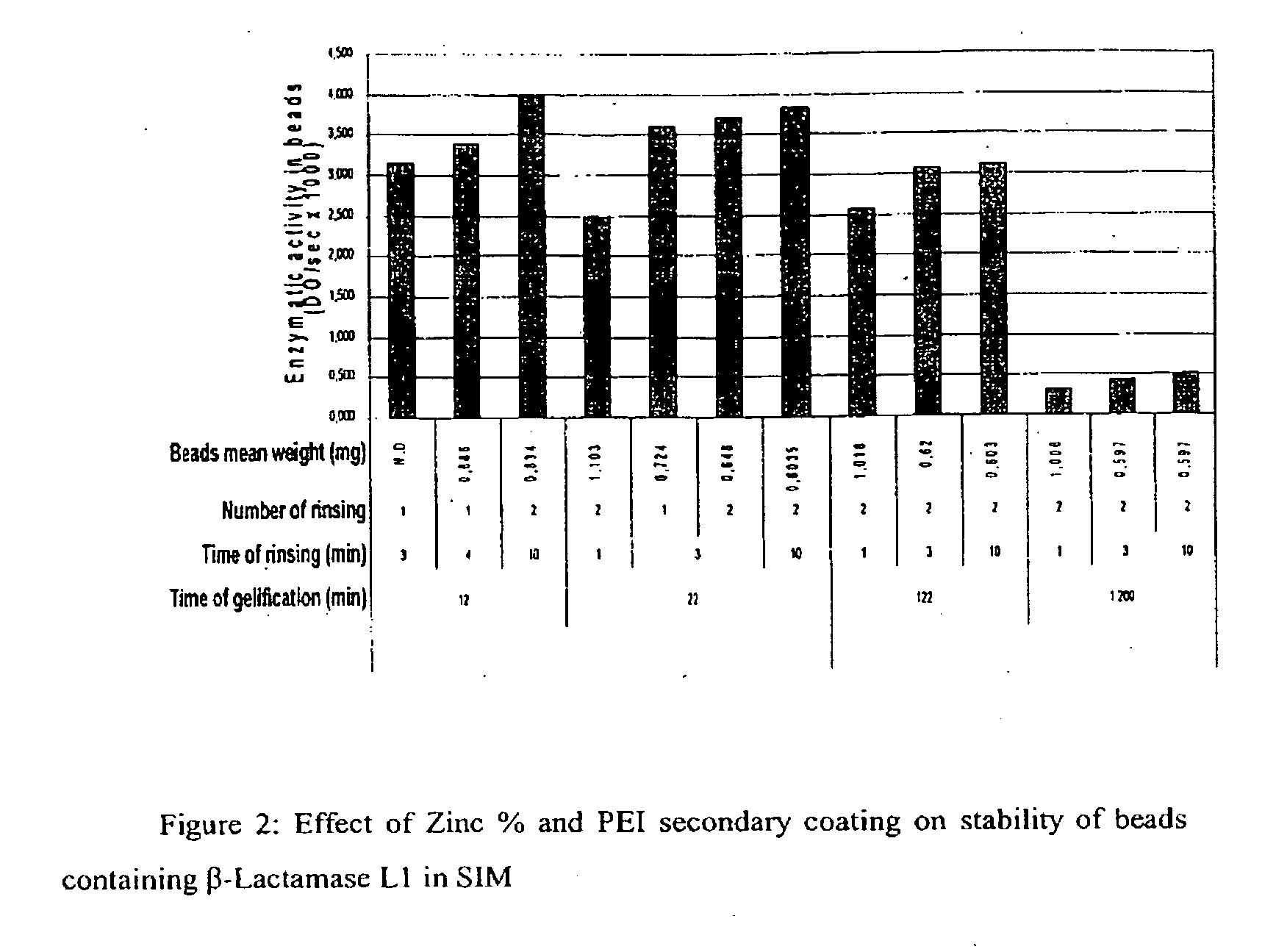
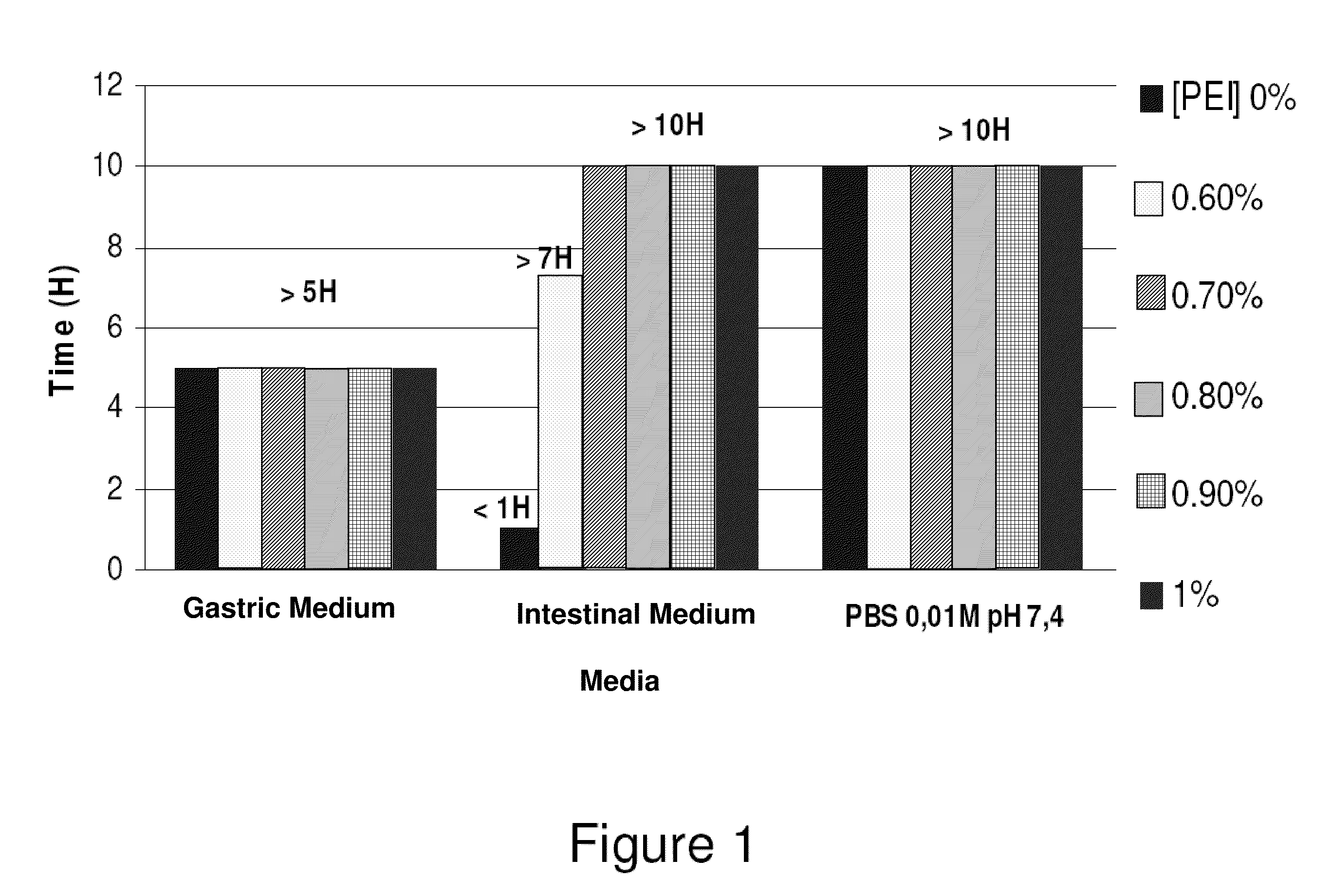
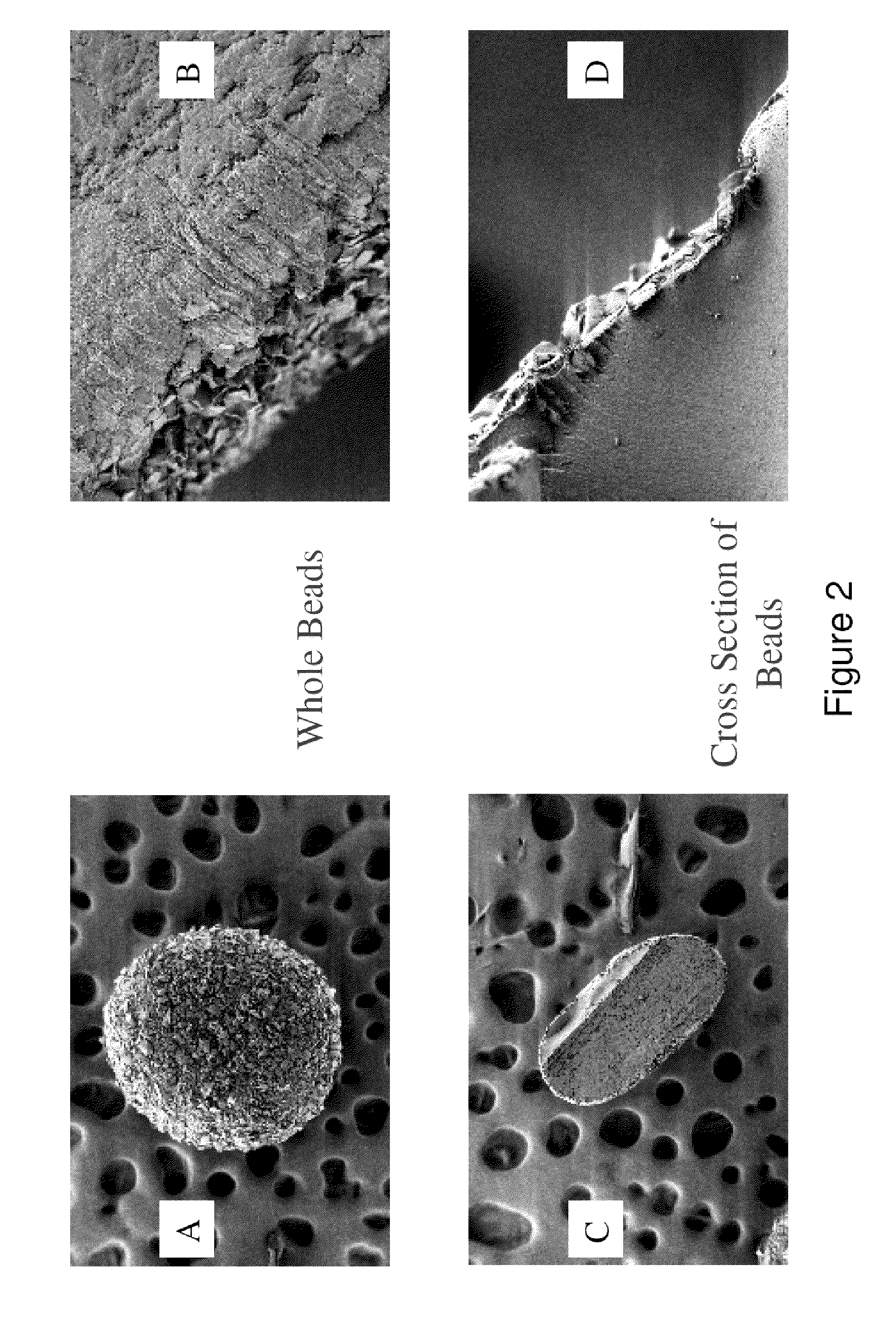
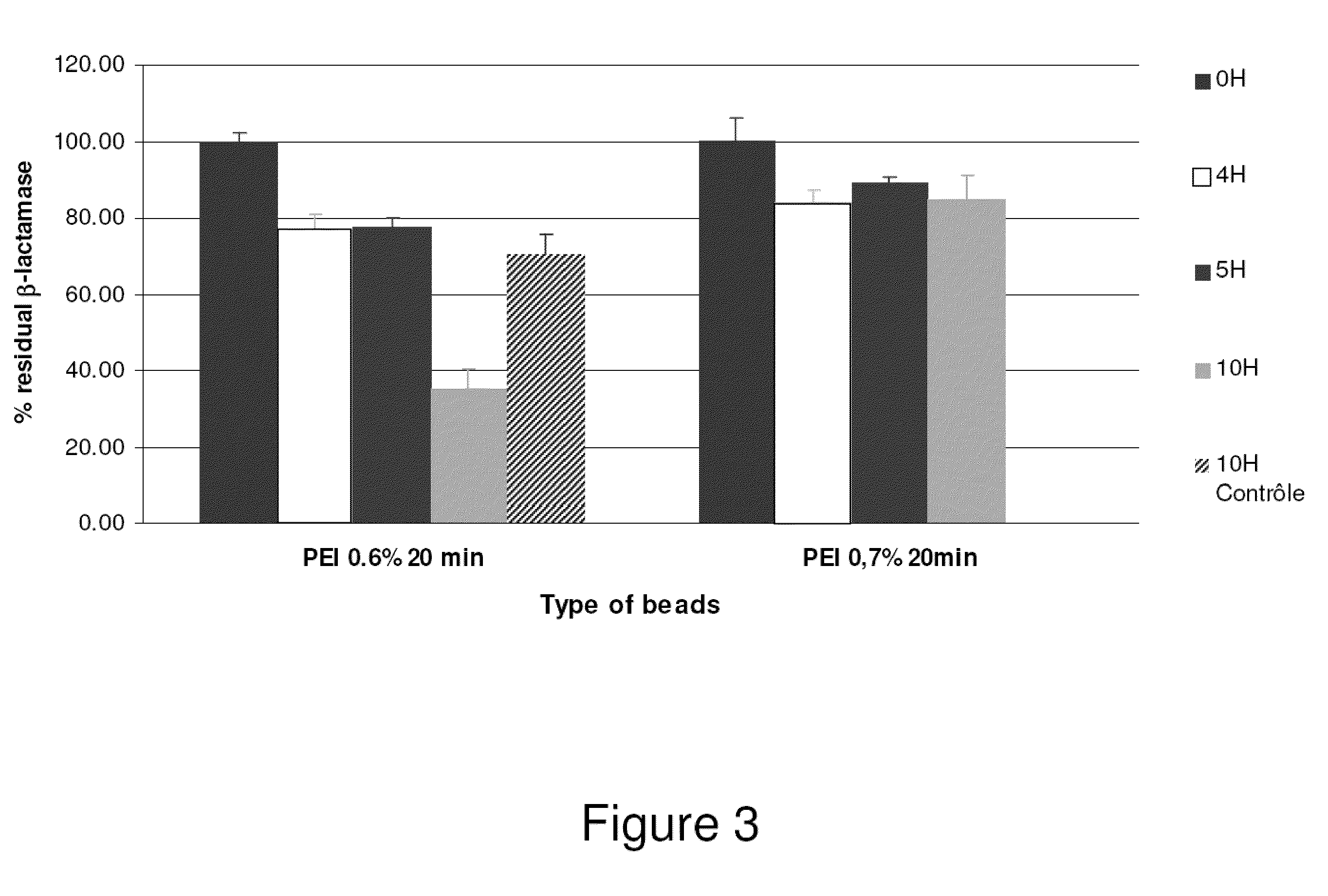
Drug delivery systems for delivering agents capable of reducing the quantity of residual antibiotics reaching the colon following oral or parenteral antibiotic therapy, and for delivering metallo-dependent enzymes, and methods of using the drug delivery systems, are disclosed. The drug delivery systems include pectin beads that encapsulate the active agent (which can be a metallo-dependent enzyme), where the pectin is crosslinked with zinc or any divalent cation of interest and the pectin beads are coated with Eudragit®-type polymers. The drug delivery systems are orally administrable, but can deliver the active agents to the colon. In some embodiments, they can administer the agents to various positions in the gastro-intestinal tract, including the colon. One metallo-dependent enzyme is the β-lactamase L1 from Stenotrophomonas maltophilia, and agents that inactivate macrolide, quinolone, fluoroquinolone or glycopeptide antibiotics can also be used. The delivery of the active agent can be modulated to occur at various pre-selected sites of delivery within the intestinal tract by gelling / crosslinking a mixture of the active agent, such as a metallo-dependent enzyme, and pectin, with divalent metallic cations such as Ca+2 or Zn+2. A stable metallo-dependent enzyme formulation can be delivered to the lower intestine or colon. The use of zinc cations to crosslink the pectin is particularly preferred when specific metallo-dependent enzymes, which are Zn2+ dependent, could interact with other cationic species if they were used to gel the pectin beads and thus adversely affect the activity of such metallo-dependent enzymes.
Owner:ASSISTANCE PUBLIQUE HOPITAUX DE PARIS +2
Galenic formulation for colon-targeted delivery of active ingredients
The invention concerns multiparticulate galenic formulations for oral administration and designed for colon targeted delivery of active principles selected from the group comprising enzymes capable of inactivating macrolides and the like, enzymes capable of inactivating quinolones and β-lactamases.
Owner:ASSISTANCE PUBLIQUE HOPITAUX DE PARIS +4
Peptide inhibitors of beta lactamases
Peptide inhibitors of β-lactamases have been identified by the synthesis of peptide arrays using synthesis SPOT technology. These peptide inhibitors of β-lactamase have activity against a broad spectrum of β-lactamases and are useful in a variety of applications.
Owner:BAYLOR COLLEGE OF MEDICINE
Shuttle vector for Bifidobacterium and Escherichia coli
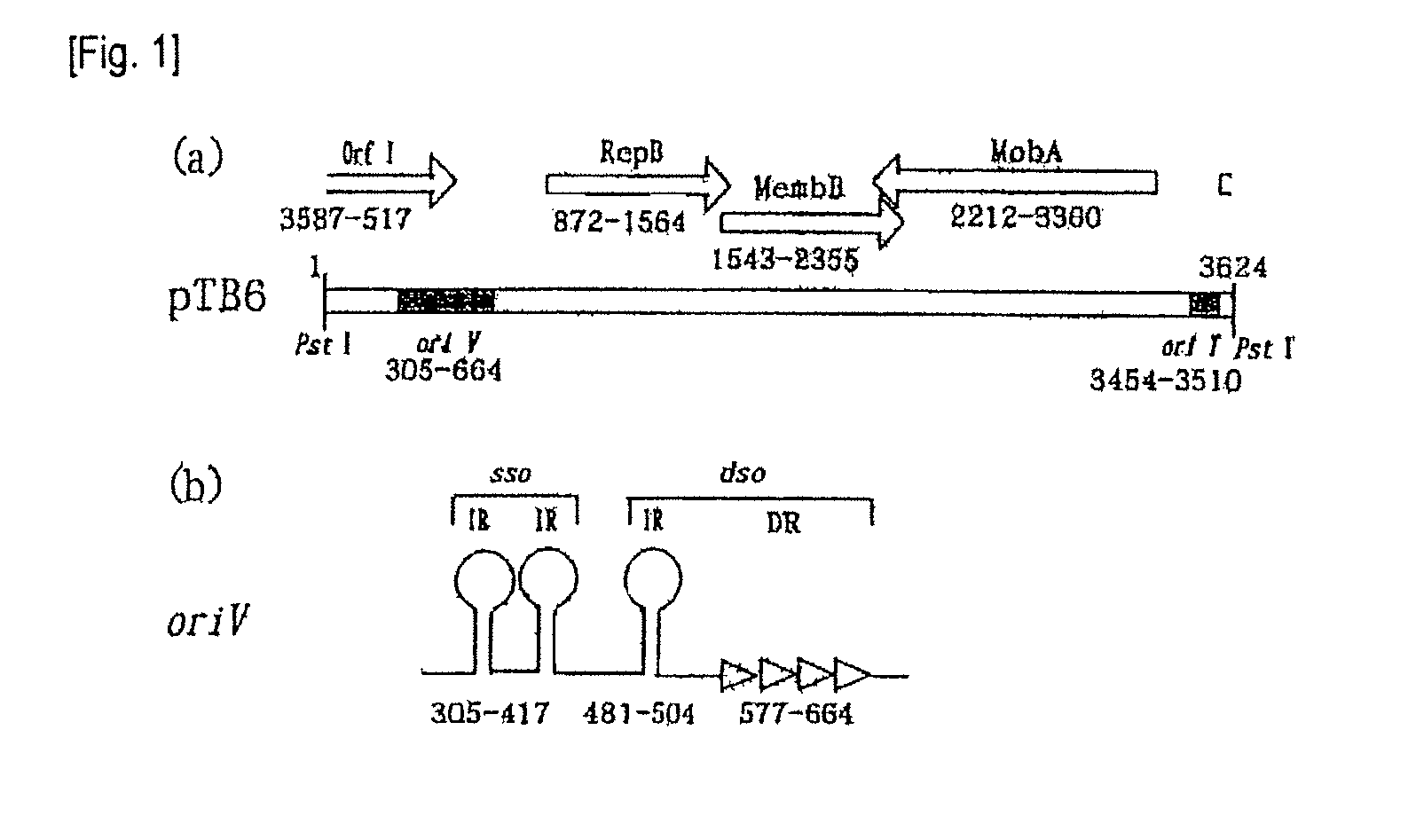
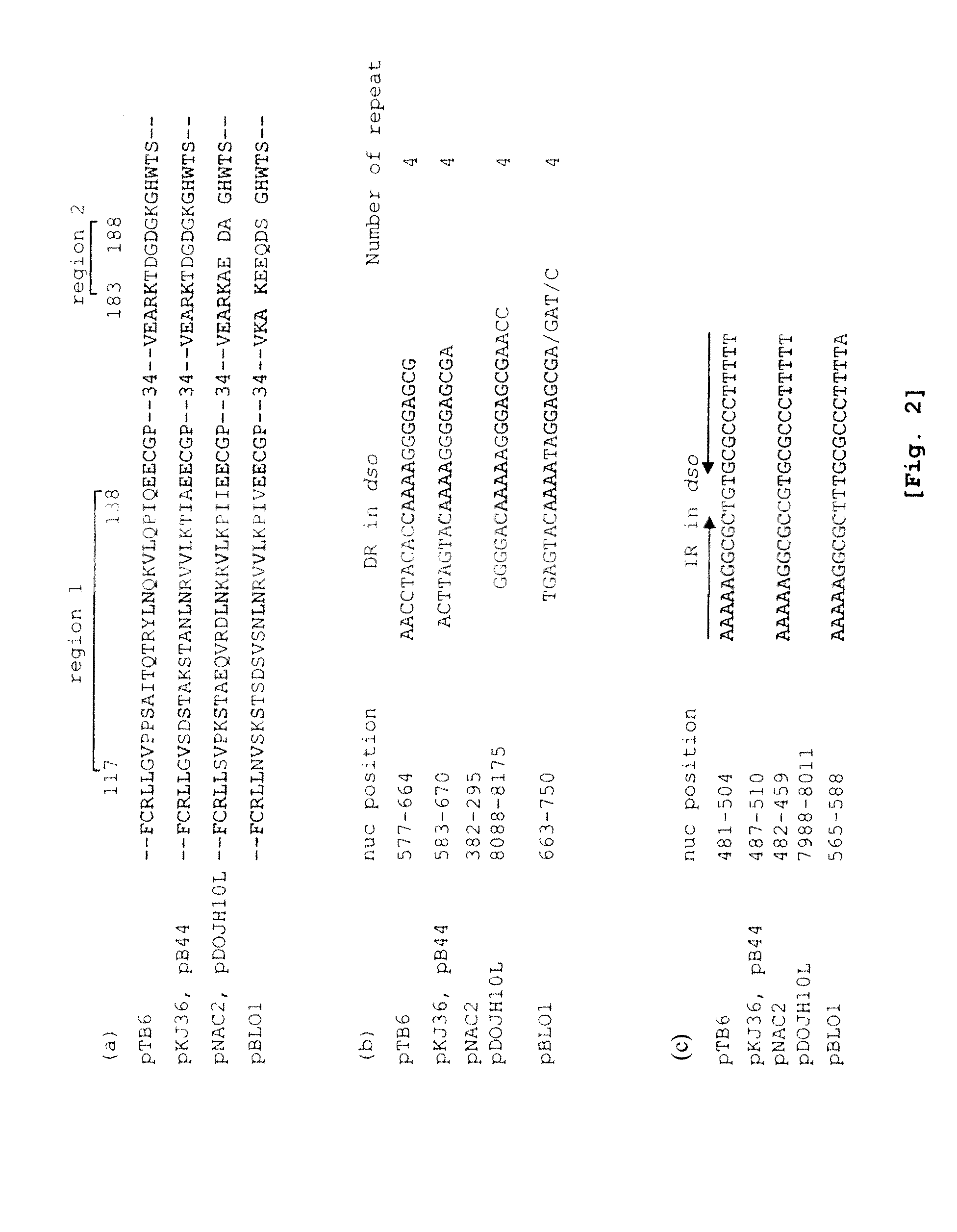
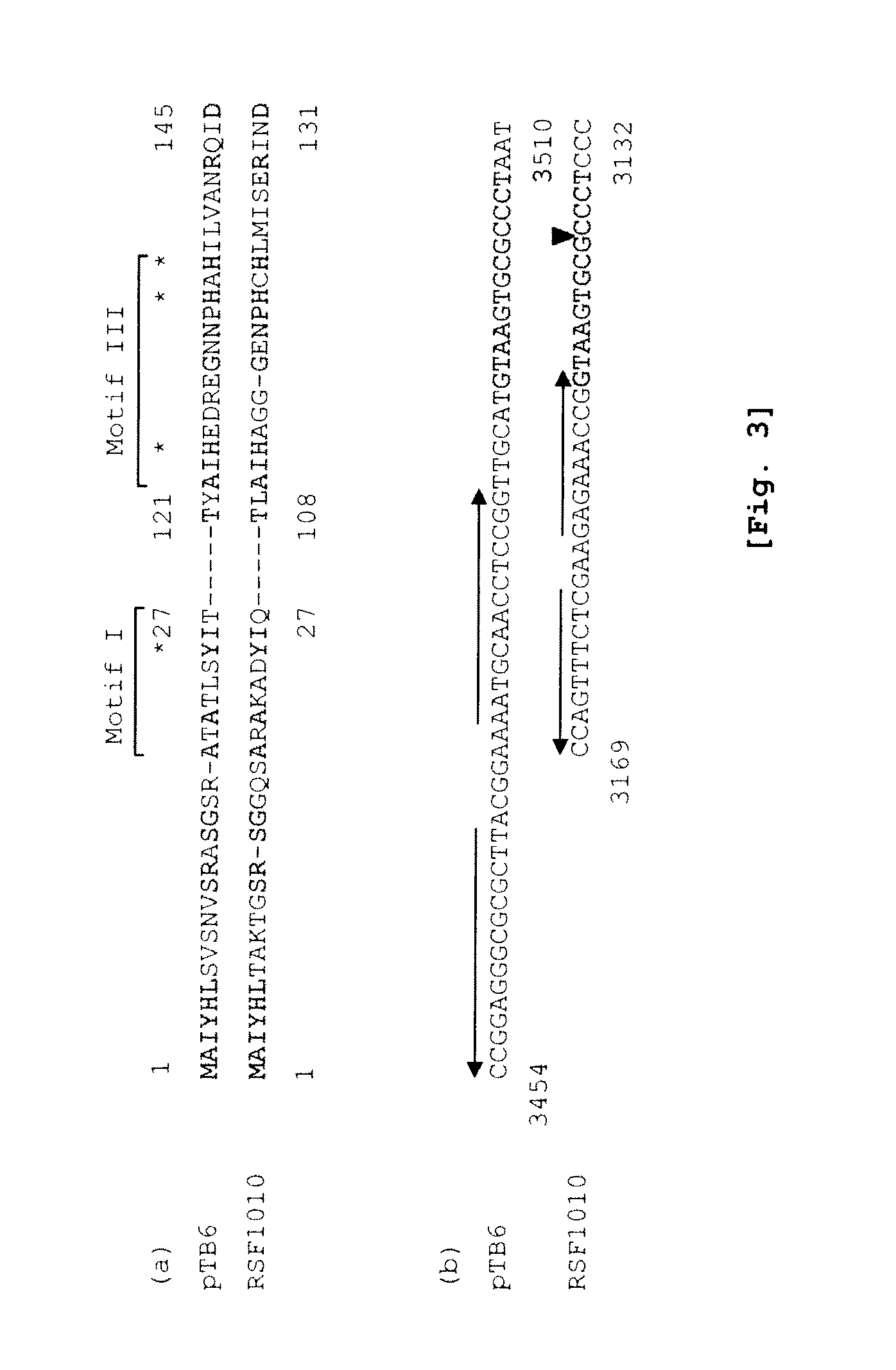
The present invention provides a shuttle vector for a microorganism of the genus Bifidobacterium (BM) and Escherichia coli having a wide host range and a large copy number in BM and capable of highly expressing a desired protein when used as an expression vector; an expression vector capable of expressing a desired gene in BM by use of the shuttle vector; BM transformed with the expression vector; and an antitumor agent comprising the BM as an active ingredient. It comprises a pTB6-derived region portion comprising a replication origin (oriV)-repB region of pTB6 but not comprising MembB, MobA, OrfI, and oriT regions of pTB6 and an Escherichia coli-derived plasmid portion comprising a replication origin (Puc Ori) region of Escherichia coli but having deleted DNA encoding an N-terminal region of an ampicillin resistance gene (ampR) expression product β-lactamase.
Owner:ANAEROPHARMA SCI
Bate-lactamase inhibitors
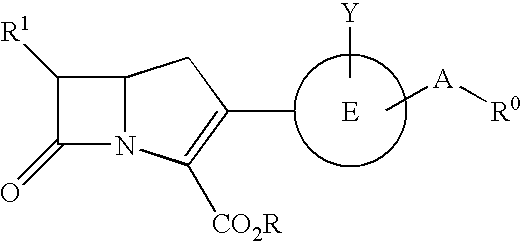
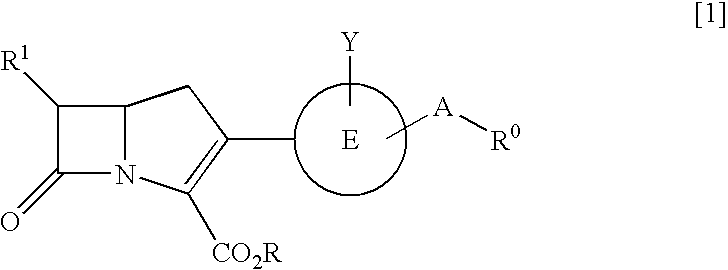
The present invention relates to broad spectrum β-lactamase inhibitors. More particularly, the invention relates to inhibitors of Class B metallo (MBL) and Class D (OXA) β-lactamases. A method of treating a bacterial infection is provided, wherein the method comprises administering to a mammalian patient in need of such treatment a compound of formula (I)whereinR1 is selected fromR2 is selected fromwith certain provisos as herein defined;in combination with a pharmaceutically acceptable β-lactam antibiotic in an amount which is effective for treating the bacterial infection.
Owner:DMITRIENKO GARY I +4
Compositions comprising carbacephem beta-lactam antibiotics and beta-lactamase inhibitors
The present invention relates to pharmaceutical compositions of carbocephem antibiotics with β-lactamase inhibitors useful for the treatment of bacterial infections, in particular infections caused by bacteria that express β-lactamases as a mechanism of resistance to β-lactam antibiotics.
Owner:BLANCA PHARMA
Tricyclic 6-alkylidene-penems as class-D beta-lactamases inhibitors
This invention relates to certain tricyclic 6-alkylidene penems which act as a inhibitor of class-D enzymes. β-Lactamases hydrolyze β-lactam antibiotics, and as such serve as the primary cause of bacterial resistance. The compounds of the present invention when combined with β-lactam antibiotics will provide an effective treatment against life threatening bacterial infections. In accordance with the present invention there are provided compounds of formula I which are useful for treatment of bacterial infections having class-D enzymes associated therewith: wherein: One of A and B denotes hydrogen and the other an optionally substituted fused tricyclic heteroaryl group; and X is S or O.
Owner:WYETH
Hybrid proteins of active-site serine β-lactamase
The present invention refers to a recombinant nucleotide sequence which codes upon expression for at least a part of a bifunctional hybrid active-site serine β-lactamase protein, wherein the β-lactamase protein is bearing at least one heterologous sequence, wherein in that the hybrid protein is having two functions, the first function is associated with the β-lactamase portion and the second function is associated with the heterologous sequence having a biological function which is different from the first function.
Owner:UNIV LIEGE
Carbapenem compounds
A compound or its pharmaceutically acceptable salt represented by the following formula:The invention is a carbapenem compound which has a potent antibacterial activity over a broad range of Gram negative and Gram positive bacteria, especially penicillin-resistant Streptococcus pneumoniae (PRSP) which has been isolated at an elevated frequency in recent years and thus causes a serious clinical problem, and Haemophilus influenzae which has acquired resistance against the existing β-lactam antibiotics over a wide scope due to penicillin-binding protein (PBP) mutations such as β-lactamase non-producing ampicillin-resistant (BLNAR) Haemophilus influenzae, and has excellent oral absorbability.
Owner:SUMITOMO DAINIPPON PHARMA CO LTD
Neurotherapeutic compositions
InactiveUS20080044471A1Effective neuroprotectiveAvoid problemsBiocideNervous disorderΒ lactamasesMedicine
Owner:KOPPEL GAY A
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com