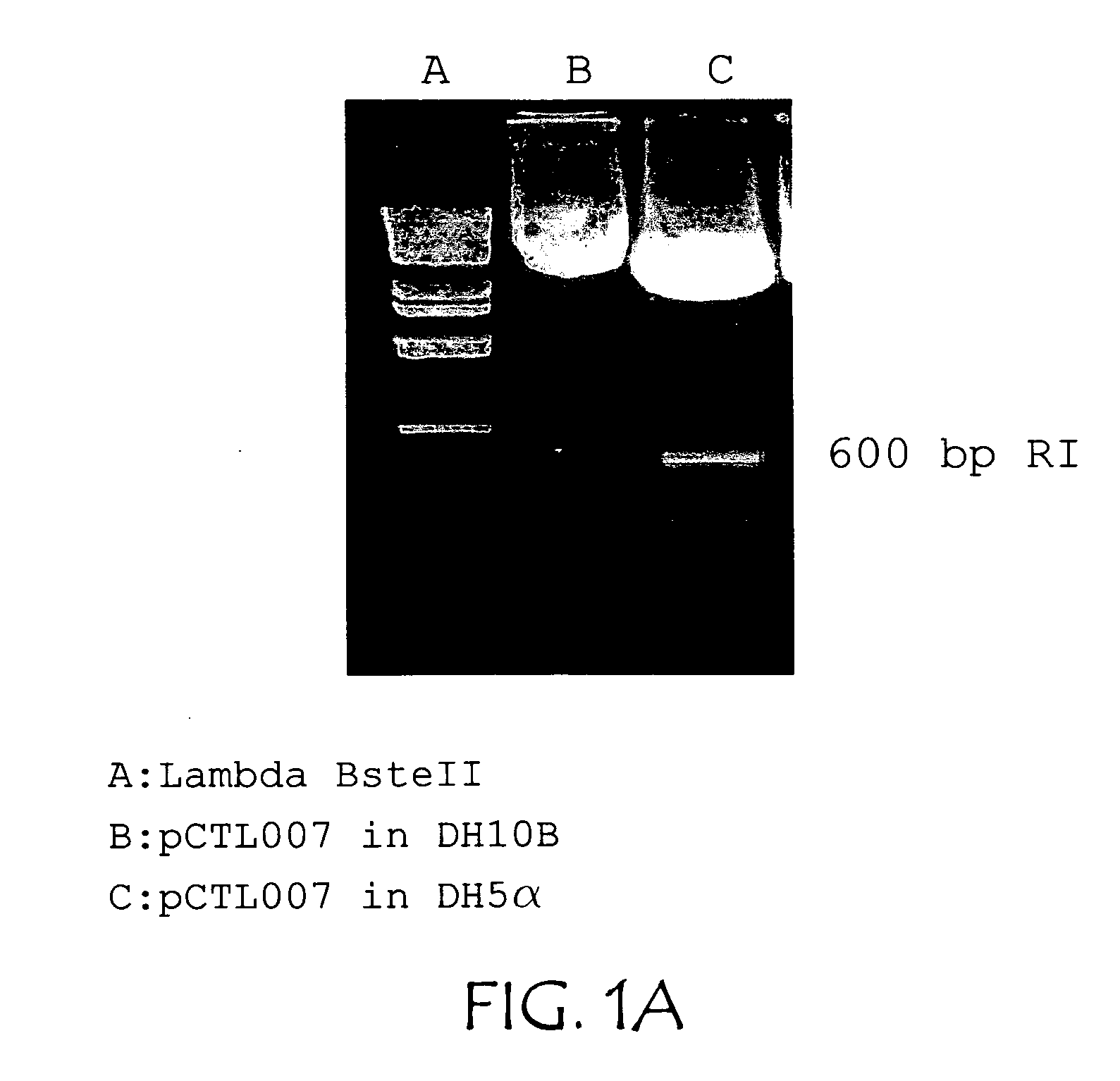
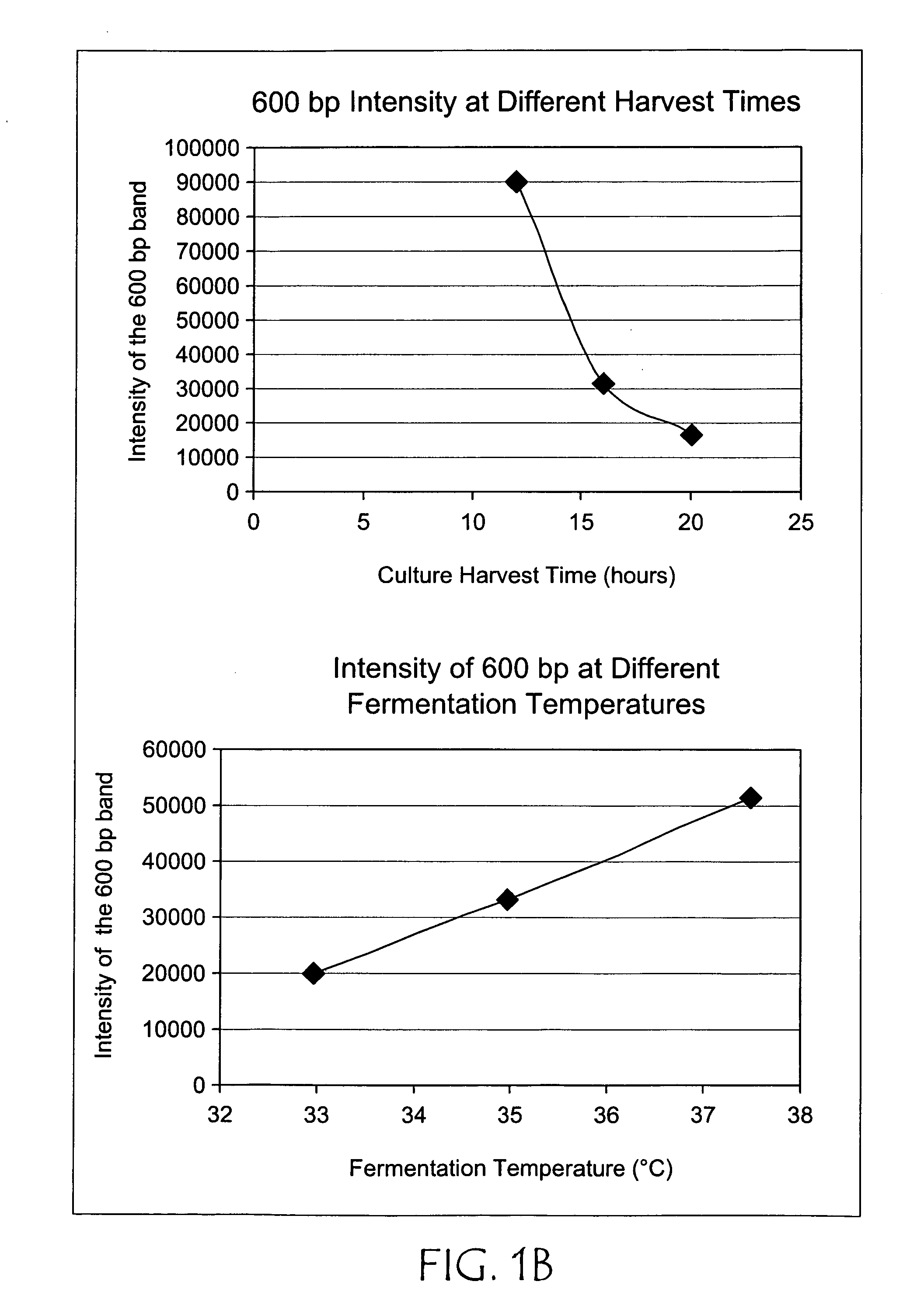
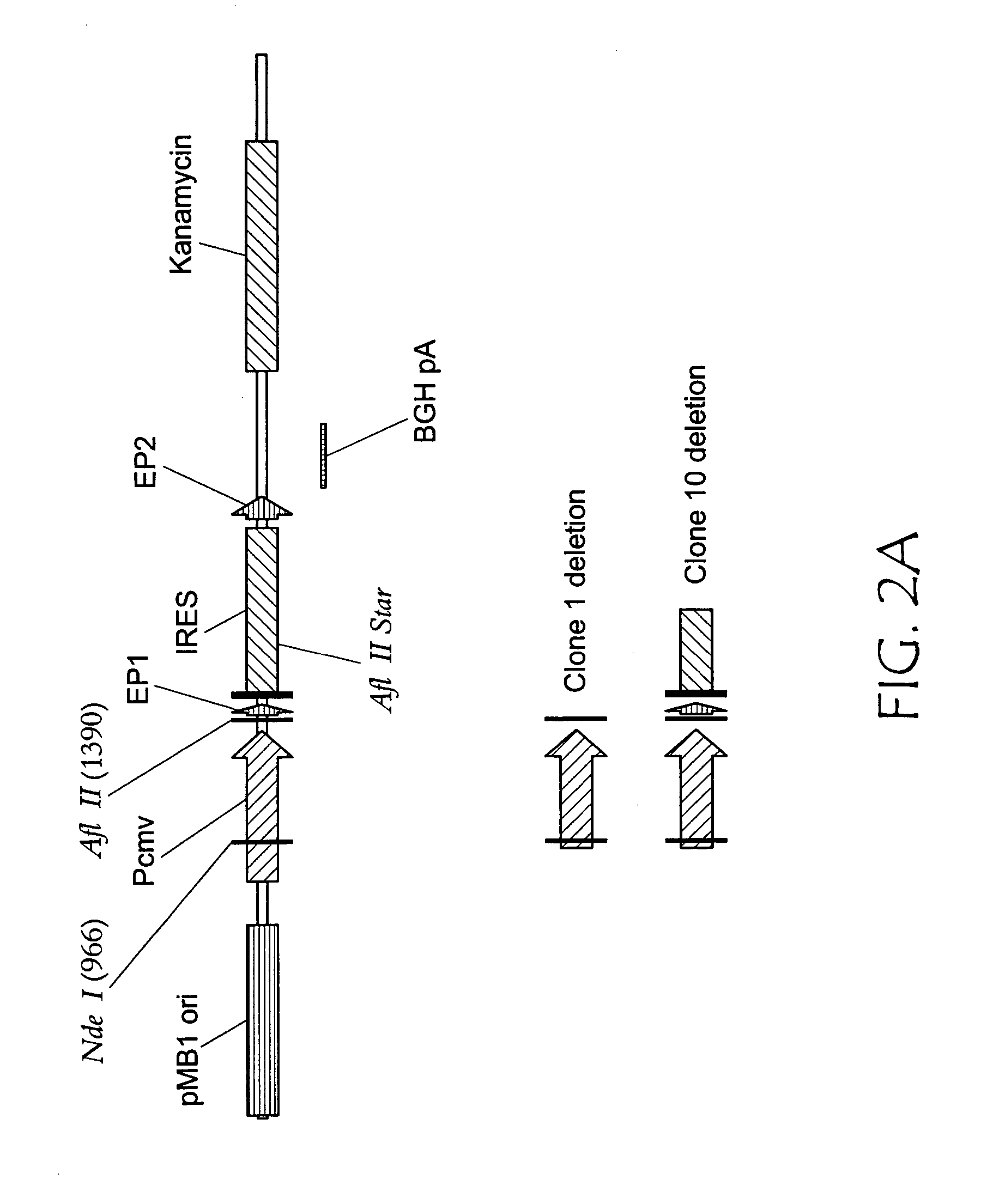
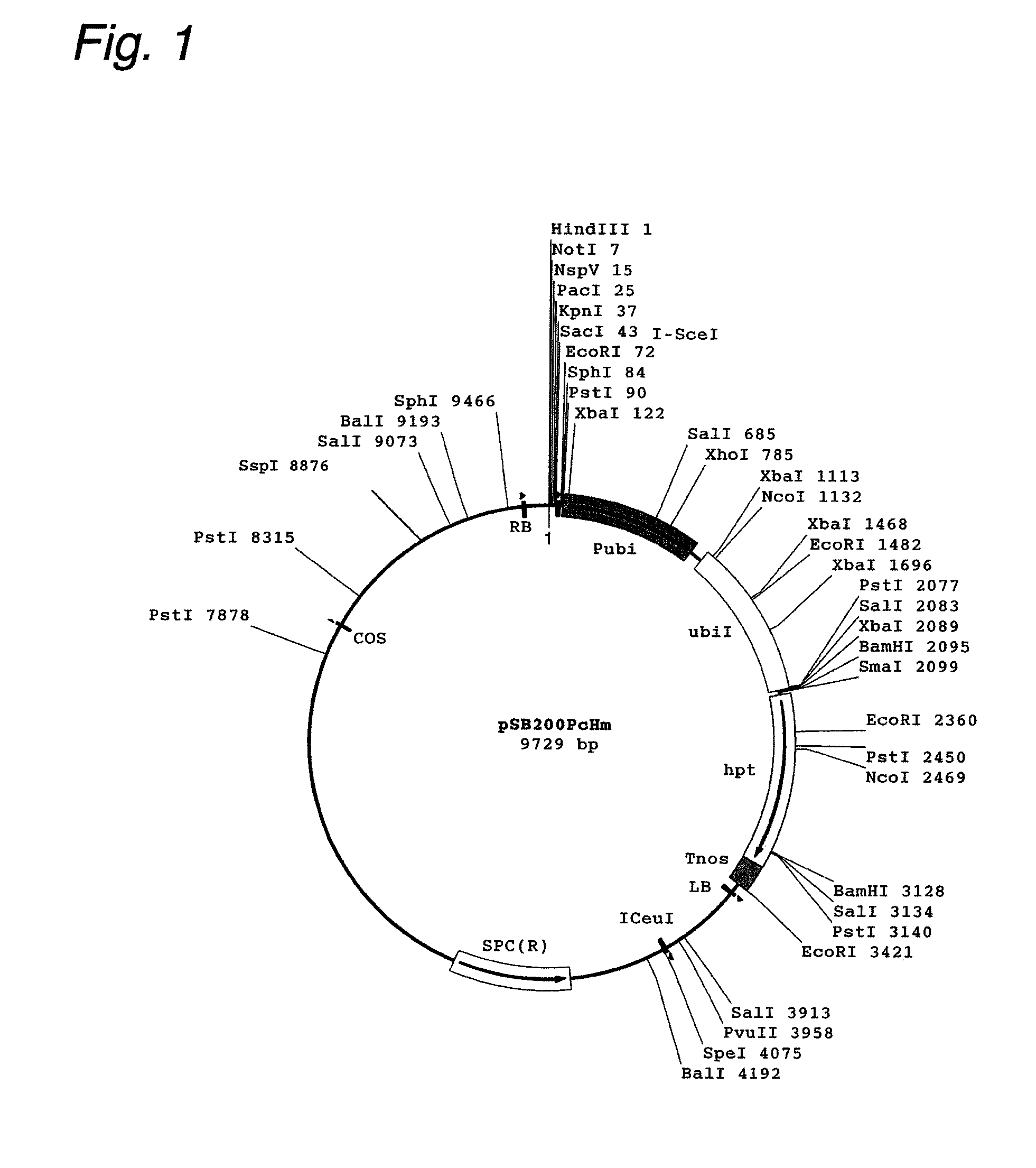
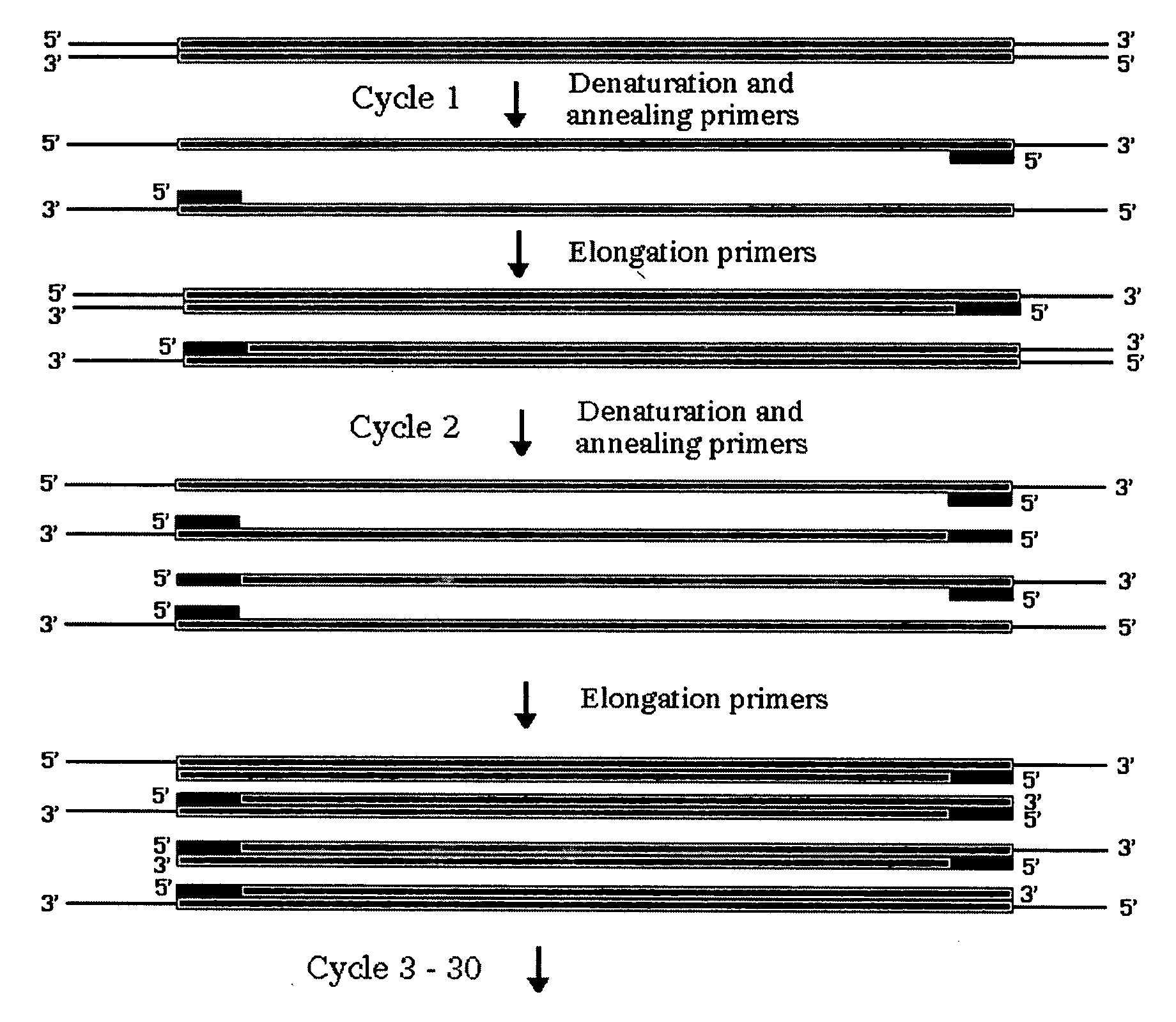
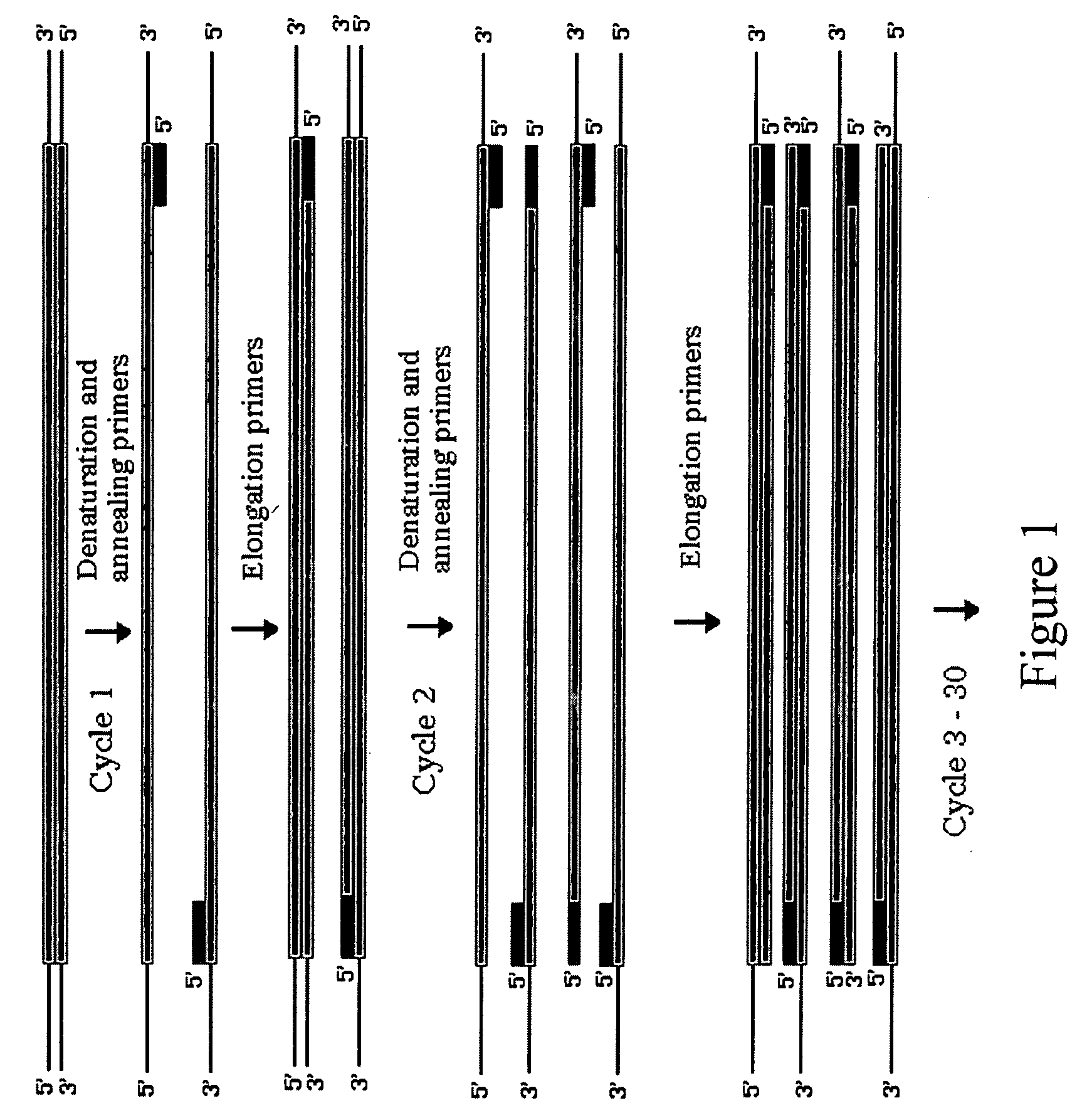
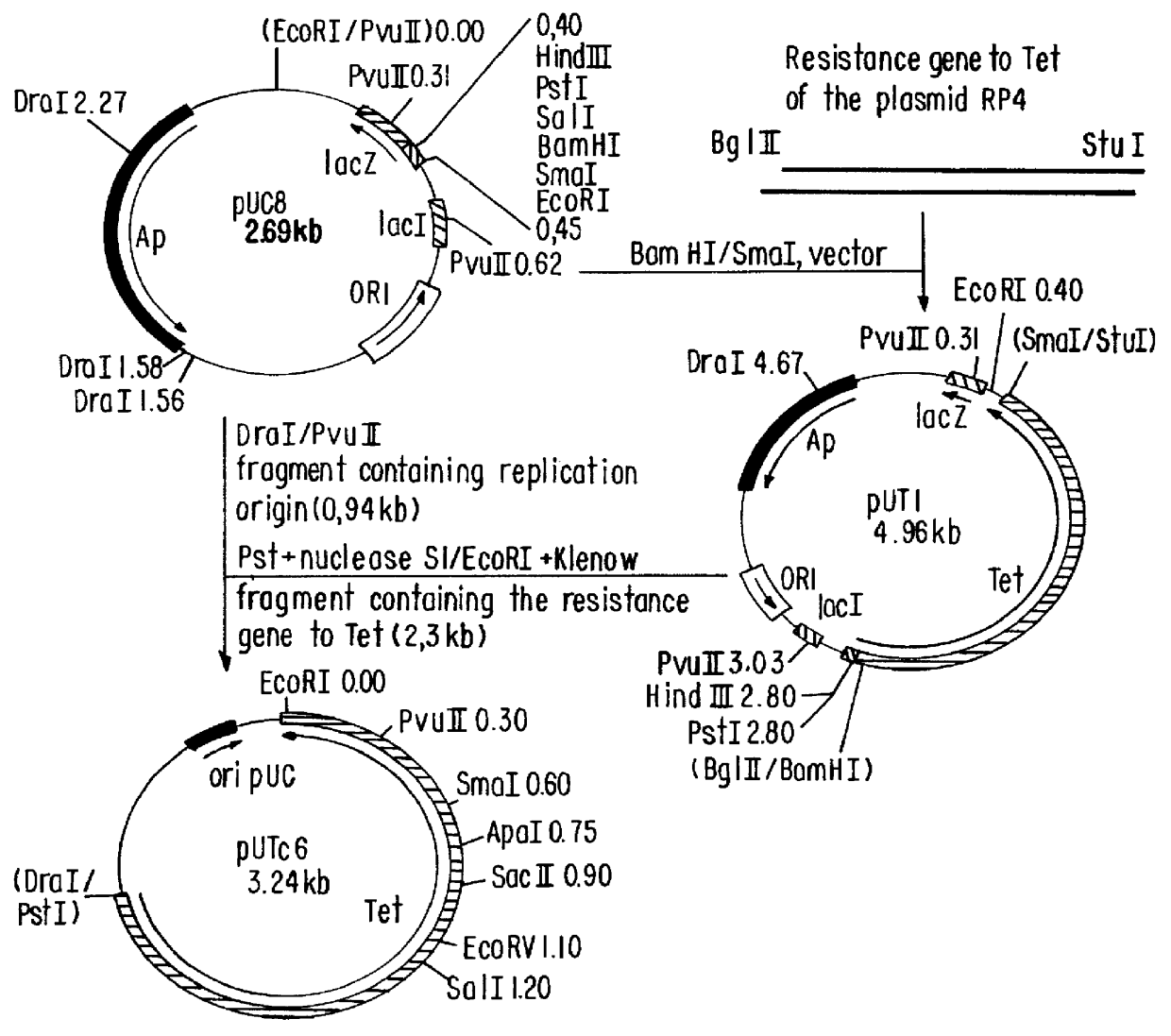
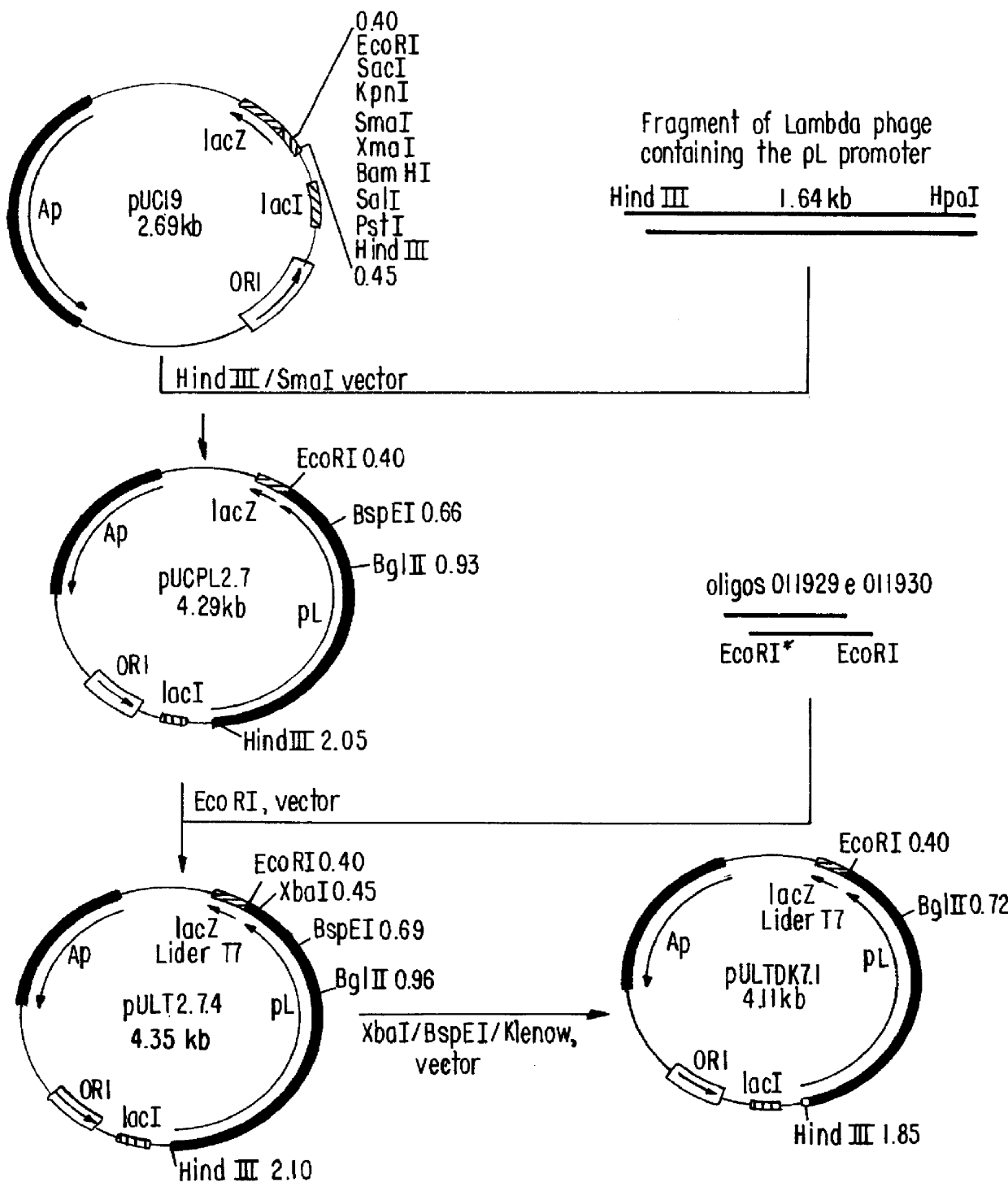
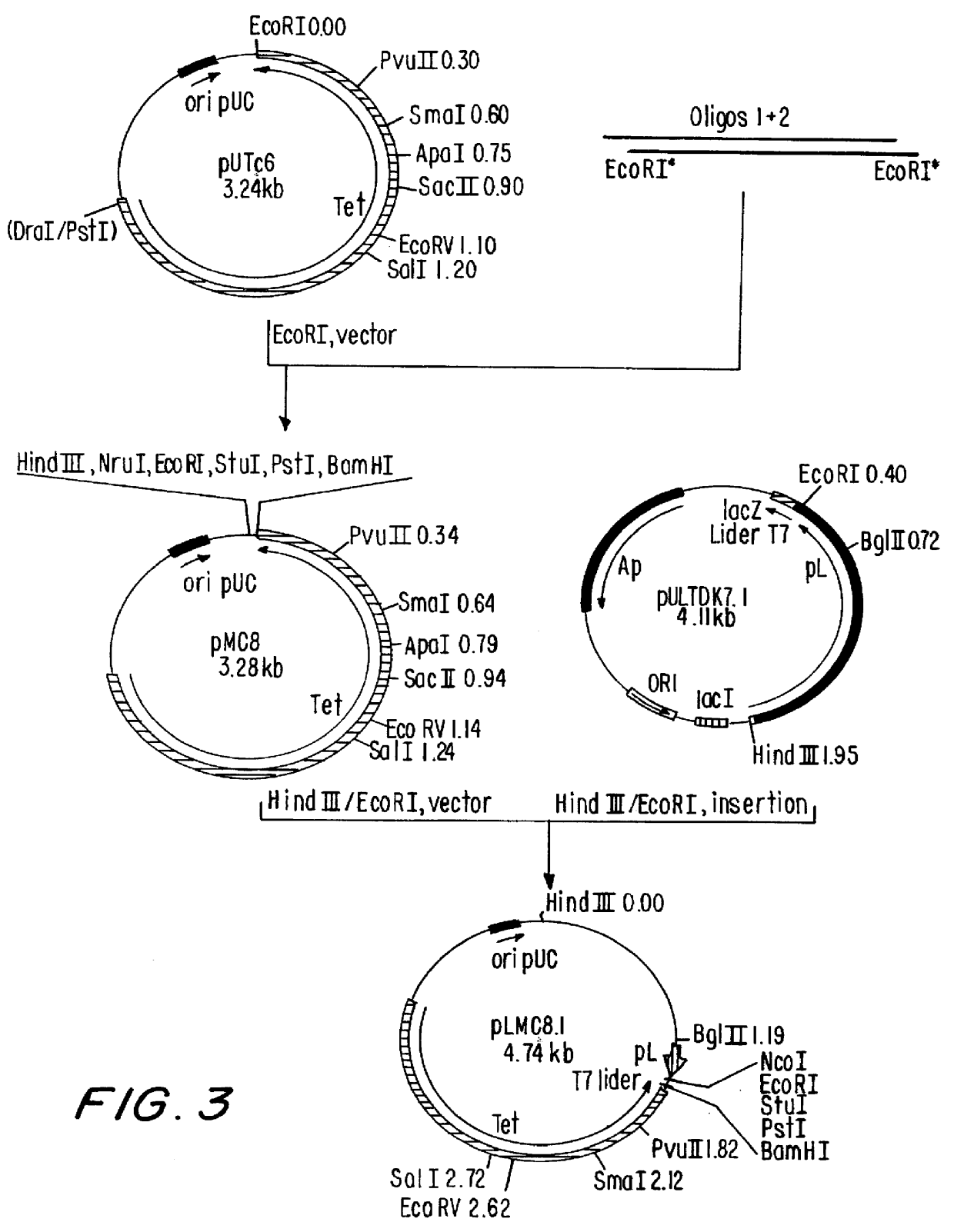
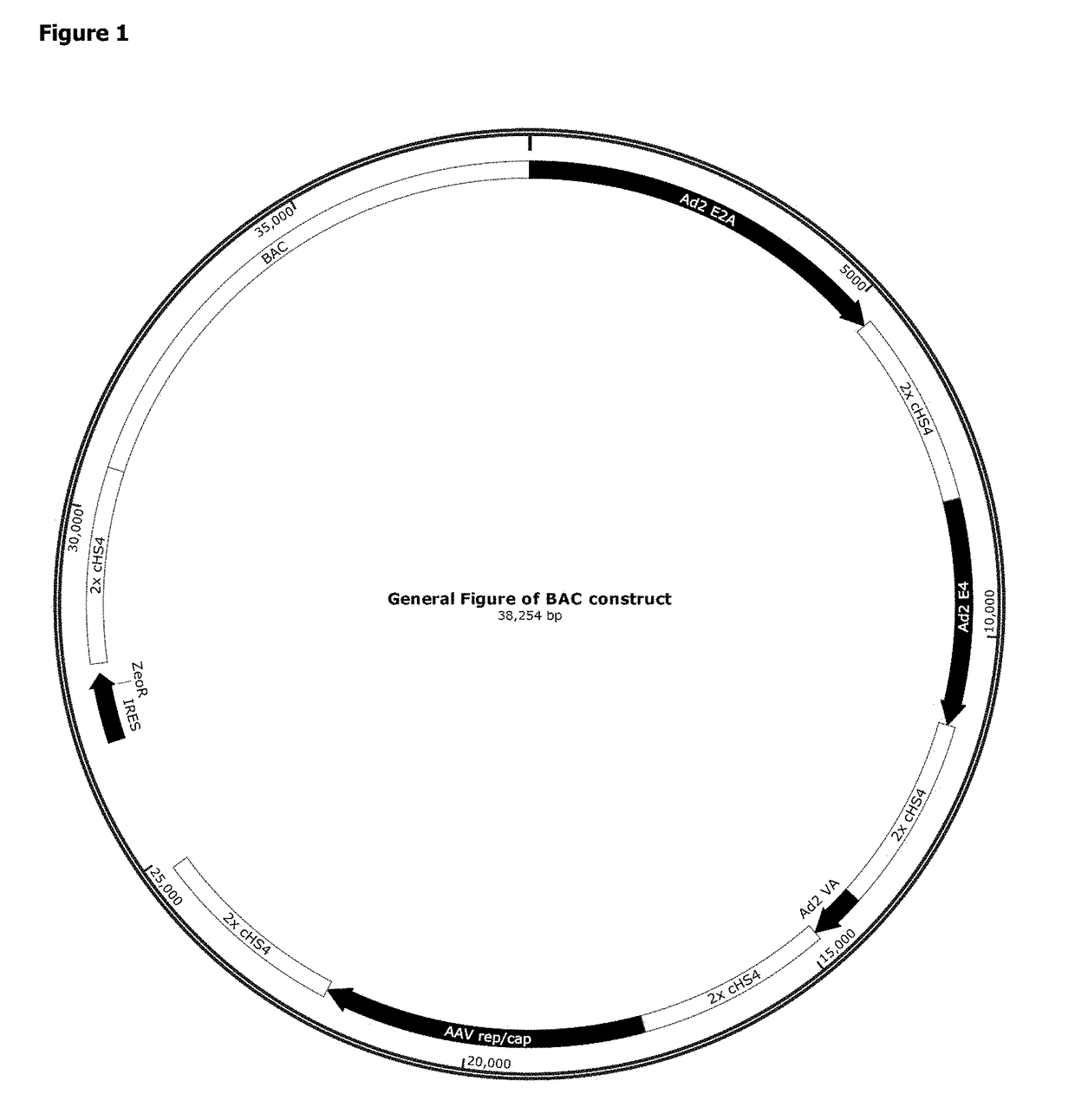
Patents
Literature
158 results about "Origin of replication" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
The origin of replication (also called the replication origin) is a particular sequence in a genome at which replication is initiated. Propagation of the genetic material between generations requires timely and accurate duplication of DNA by semiconservative replication prior to cell division to ensure each daughter cell receives the full complement of chromosomes. This can either involve the replication of DNA in living organisms such as prokaryotes and eukaryotes, or that of DNA or RNA in viruses, such as double-stranded RNA viruses. Synthesis of daughter strands starts at discrete sites, termed replication origins, and proceeds in a bidirectional manner until all genomic DNA is replicated. Despite the fundamental nature of these events, organisms have evolved surprisingly divergent strategies that control replication onset. Although the specific replication origin organization structure and recognition varies from species to species, some common characteristics are shared.
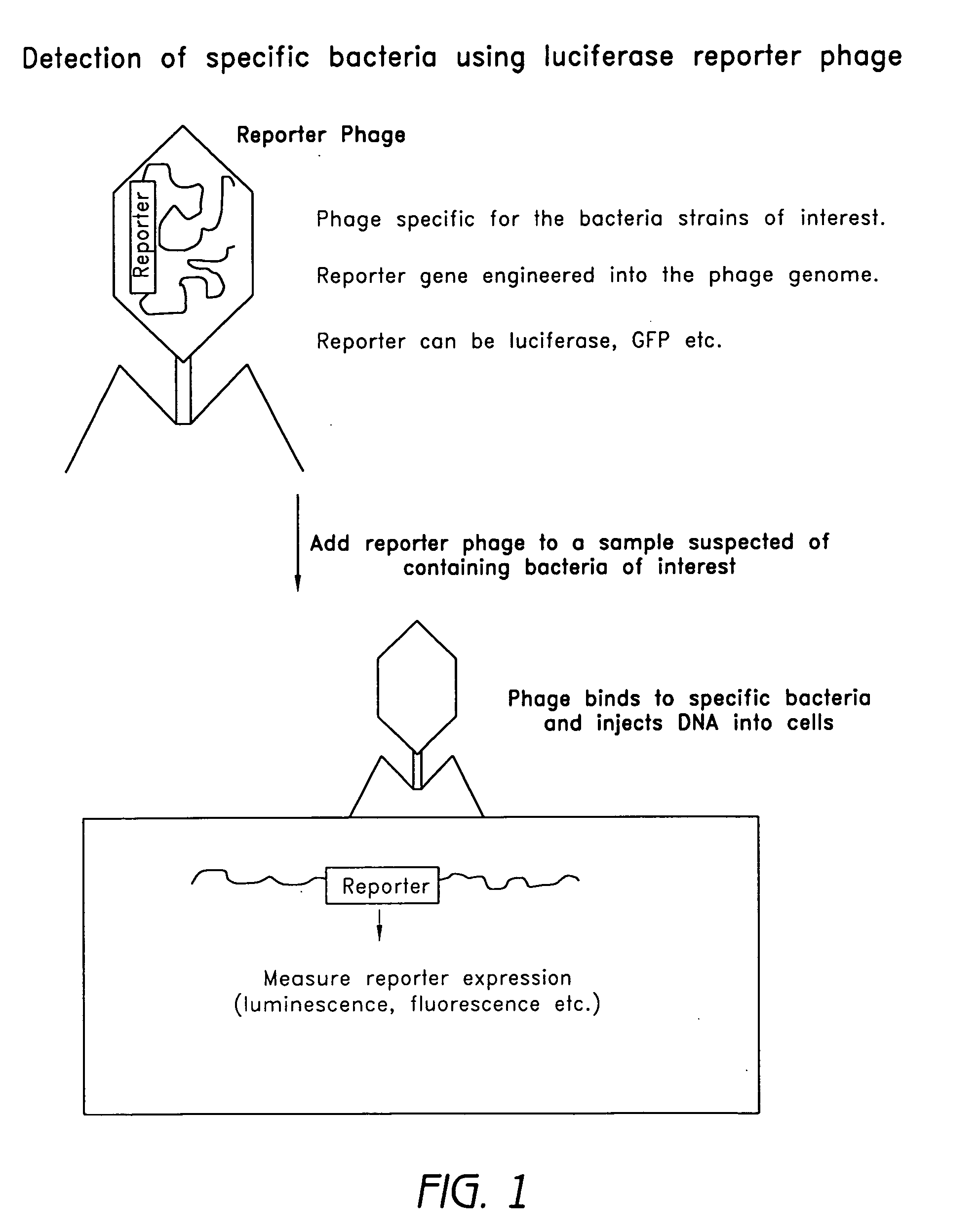
Reporter plasmid phage packaging system for detection of bacteria
InactiveUS20090155768A1Microbiological testing/measurementOther foreign material introduction processesBacteroidesOrigin of replication
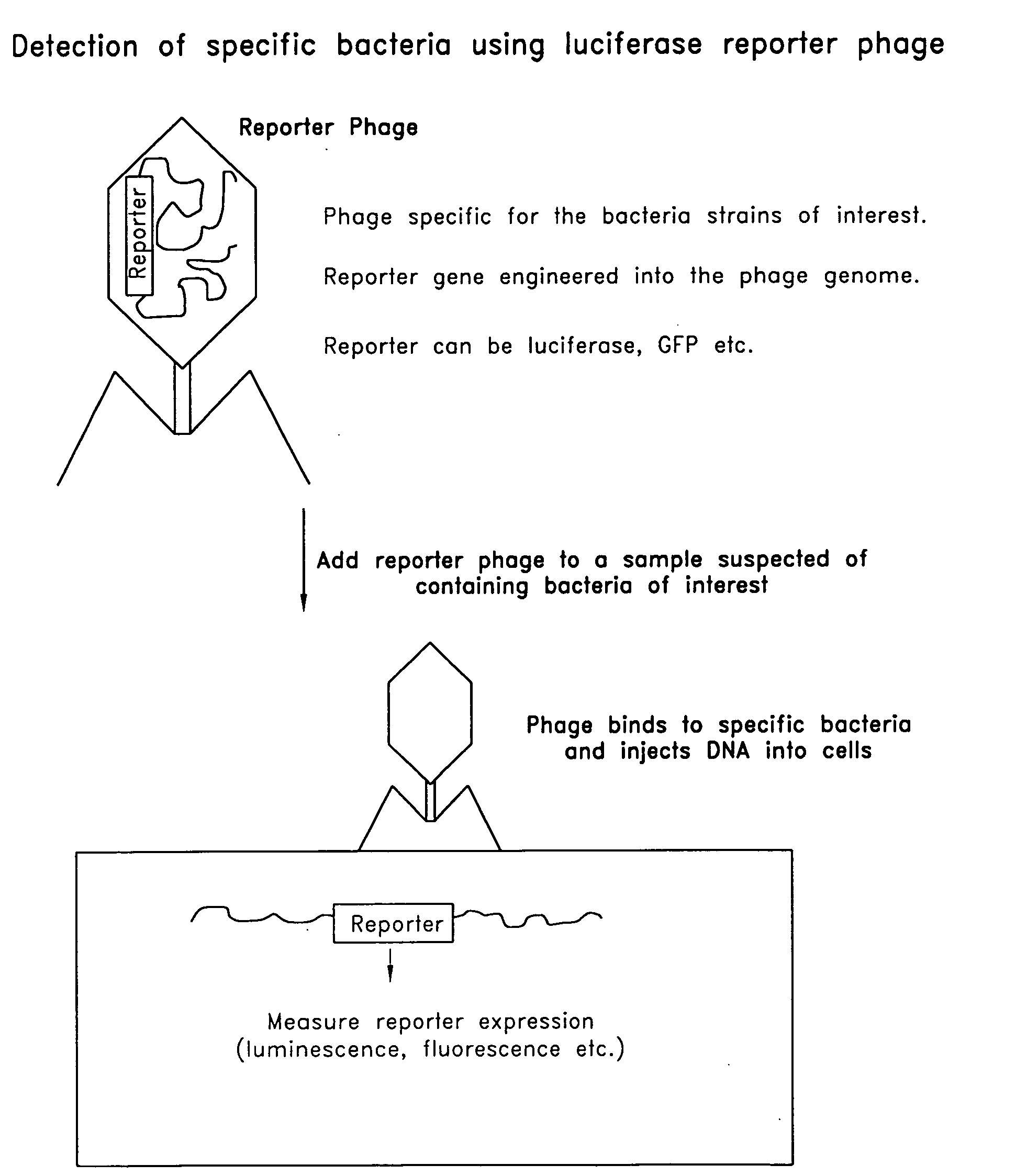
The invention is related to a transducing particle that comprises a bacteriophage coat and a DNA core that comprises plasmid DNA comprising: a) a host-specific bacteriophage packaging site wherein the packaging site is substantially in isolation from sequences naturally occurring adjacent thereto in the bacteriophage genome, b) a reporter gene, c) a bacteria-specific promoter operably linked to said reporter gene, d) a bacteria-specific origin of replication, and optionally e) an antibiotic resistance gene. The invention includes phage transducing particles, methods of making transducing particles, and methods of using the transducing particles in bacterial detection.
Owner:UNITED STATES OF AMERICA
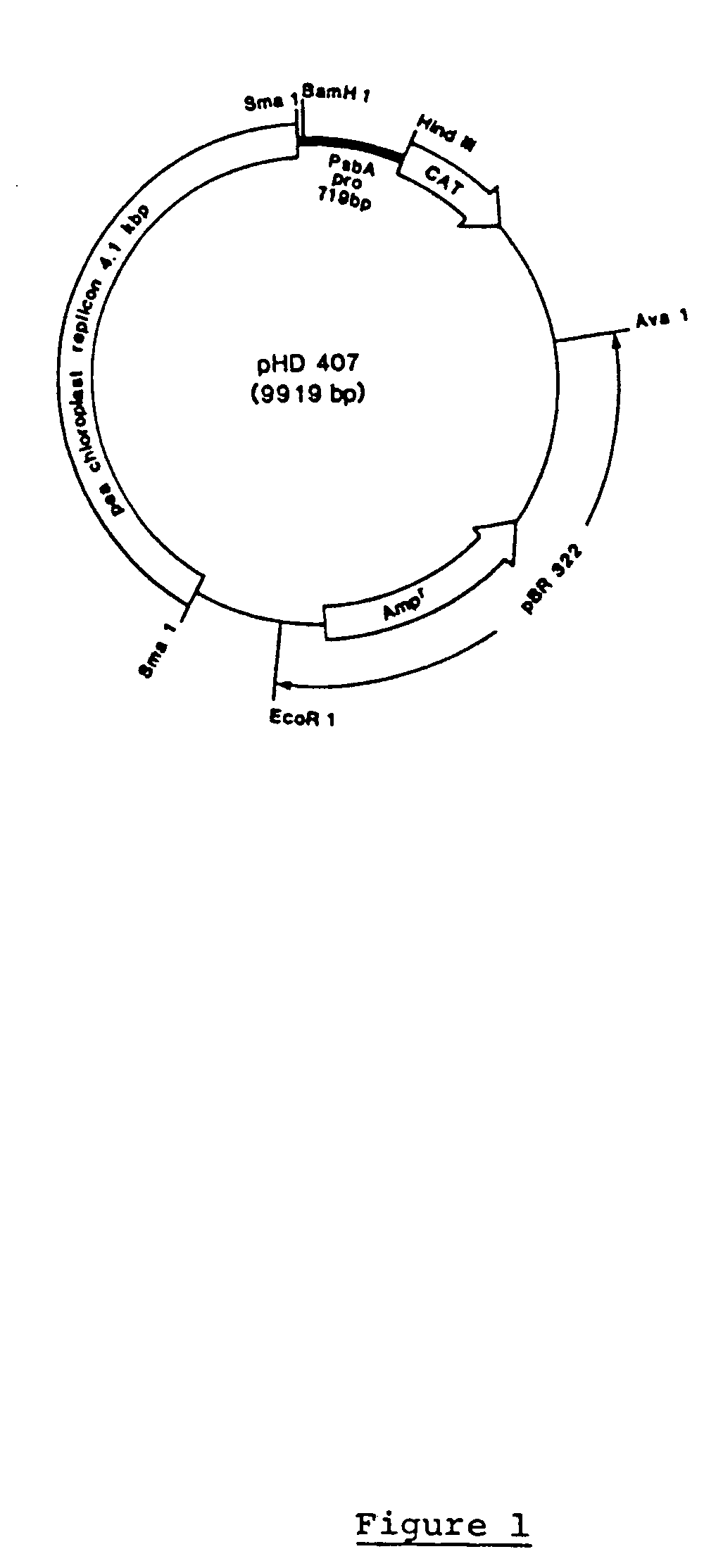
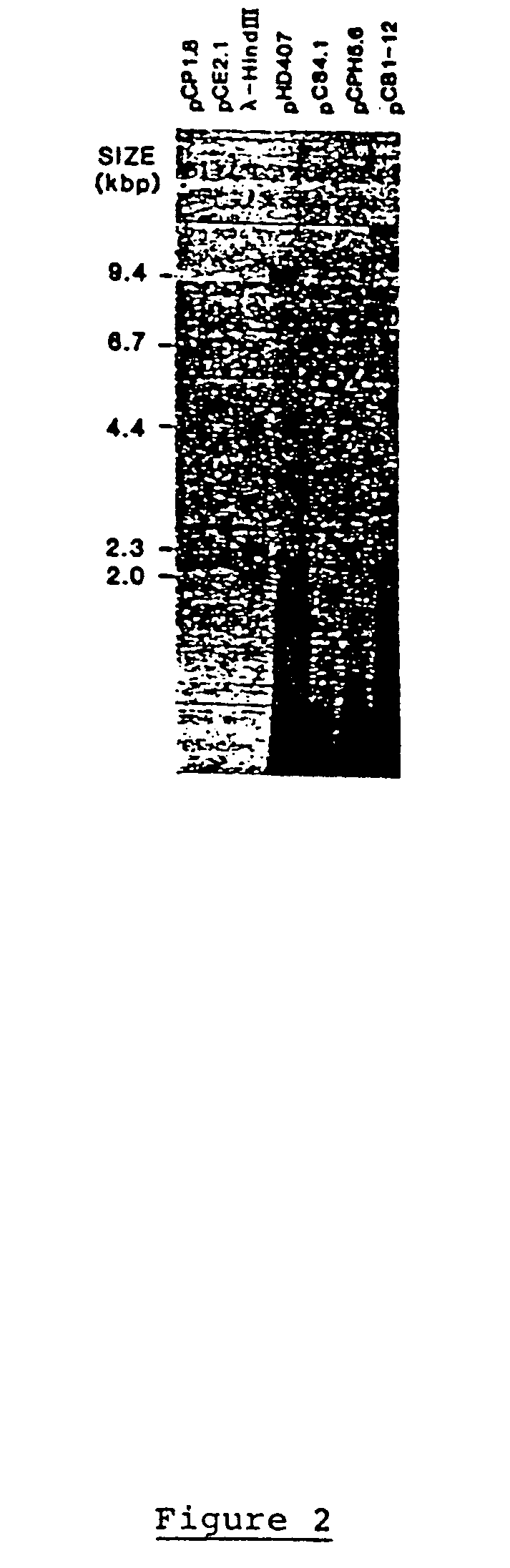
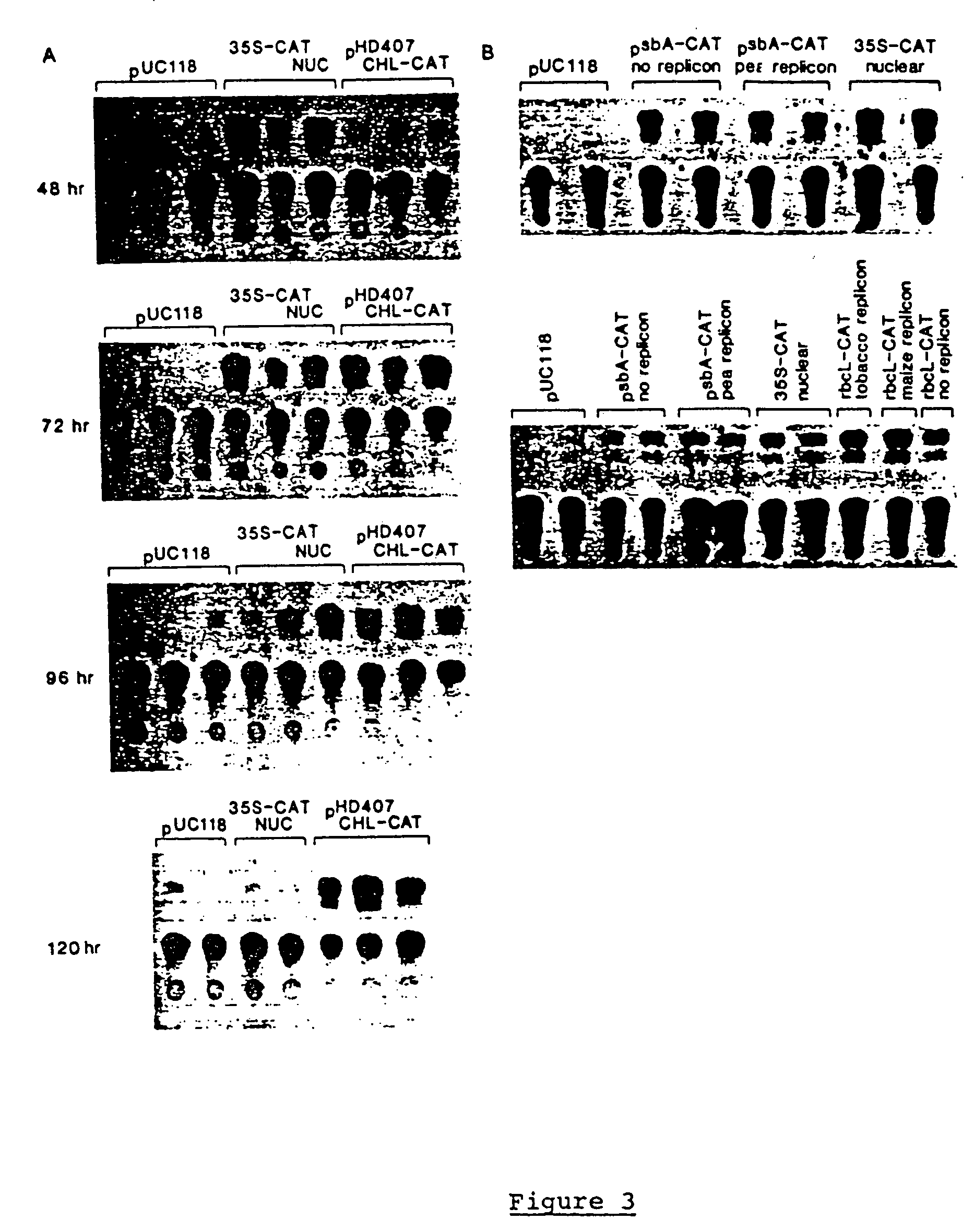
Genetic engineering of plant chloroplasts
Novel chimeric constructions and methods for their use are provided for expression of exogenous genes in a plant chloroplast. Particularly, expression is achieved by the use of a chloroplast or bacterial 5′ untranslated region in the expression cassette. The expression cassette may be integrated into the chloroplast genome by the use of chloroplast DNA flanking sequences, or may replicate autonomously if provided with a chloroplast origin of replication. Plants and cells containing the transformed chloroplasts are also provided. The constructs may be used with both monocotyledenous and dicotyledenous chloroplasts.
Owner:AUBURN UNIV
Methods and compositions for amplifying DNA clone copy number
A method for retrofitting DNA in a single-copy or high-copy vector, such as a fosmid or BAC, whereby an artificial transposon is used to introduce a conditional multi-copy origin of replication (“ori”) into the DNA in said vector. Following random in vitro or in vivo transposition of the ori-containing transposon into DNA in the single-copy or low-copy vector, the resulting insertion clones are introduced into a special host strain that contains a gene which encodes a polypeptide required for replication from the multi-copy ori. However, since the gene for this polypeptide is expressed from a tightly-regulated inducible promoter, the polypeptide is not expressed in the absence of inducer. On addition of inducer to the culture medium, the host cell synthesizes the polypeptide, which in turn activates replication from the multi-copy ori, thereby increasing the amount of clone DNA synthesized by the cell.
Owner:EPICENT TECH CORP
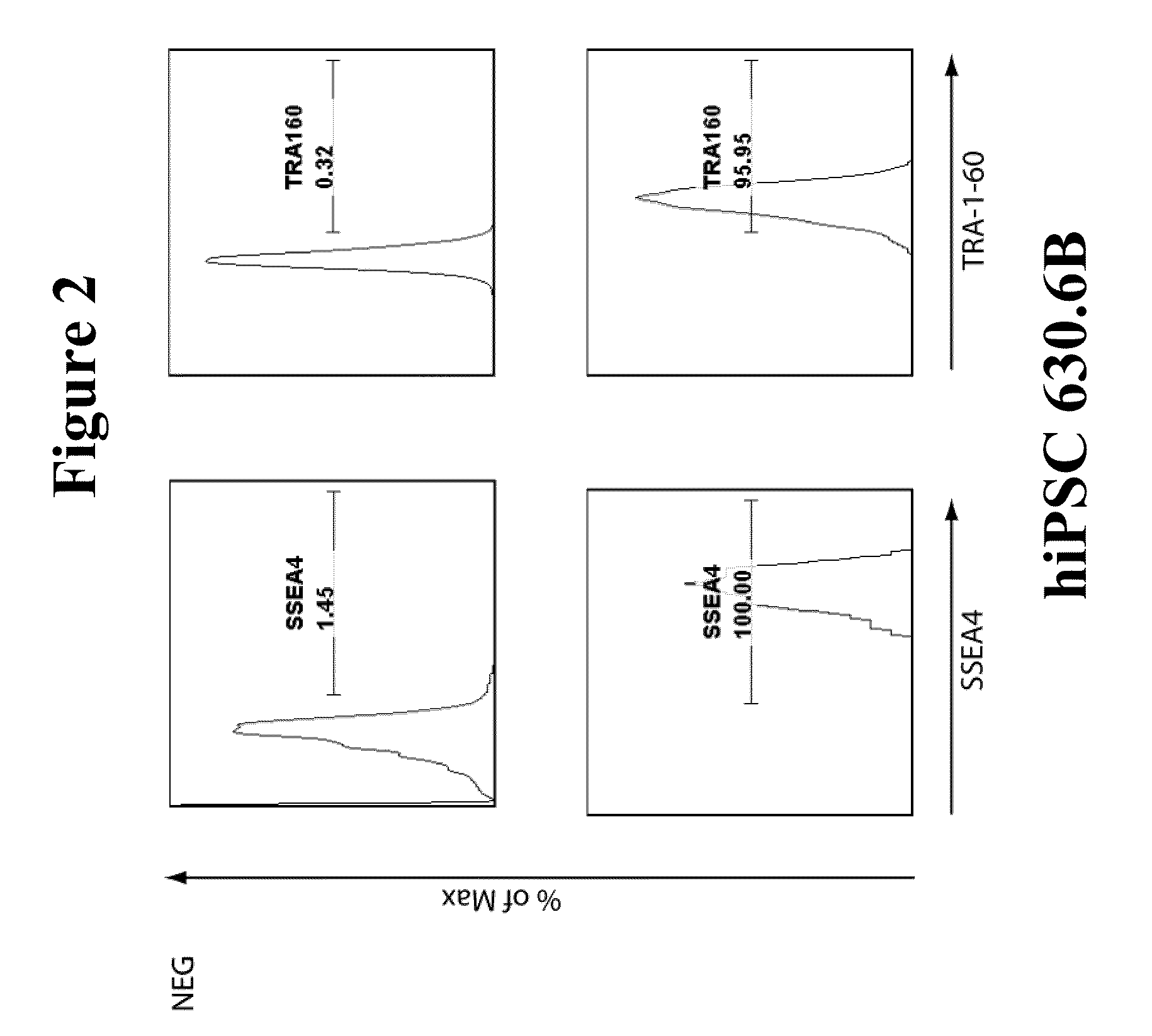
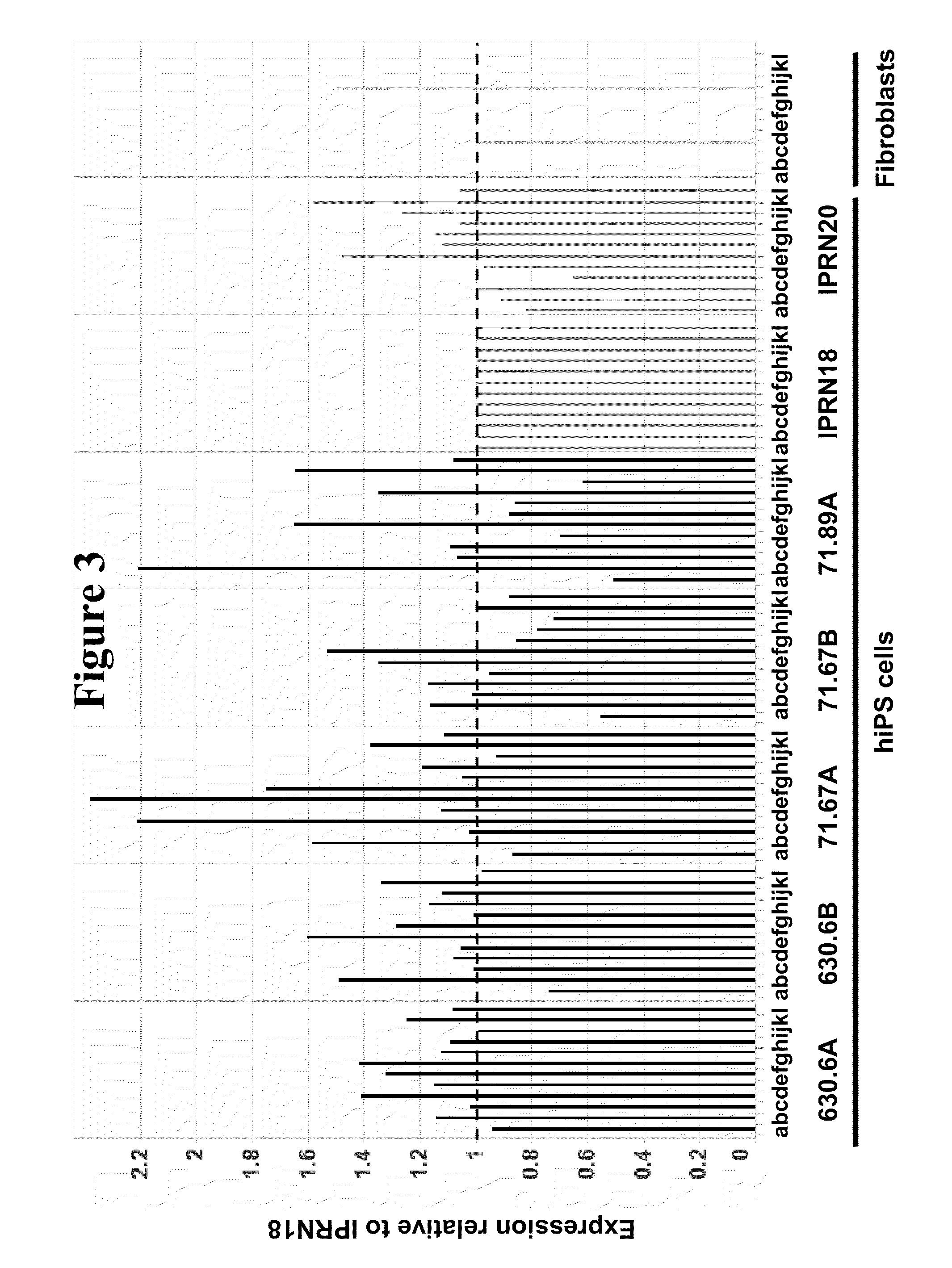
Integration-free human induced pluripotent stem cells from blood
InactiveUS8048675B1Genetically modified cellsArtificial cell constructsPluripotential stem cellOrigin of replication
Provided herein are methods for generating human induced pluripotent stem cells free from genomic integration of exogenous transgenes by transfecting into nucleated blood cells one or more DNA expression vectors (e.g., plasmid vectors) that do not contain a mammalian origin of replication, and encode and permit expression of one or more reprogramming factors (e.g., Oct4, Sox2, Klf4, and c-Myc). Also provided herein are the integration-free human induced pluripotent stem cells obtained by the methods described herein.
Owner:TRUE NORTH THERAPEUTICS
Method for assembling PCR fragments of DNA
InactiveUS7629120B2Simple stepsSugar derivativesMicrobiological testing/measurementOrigin of replicationSite-specific recombination
A process for assembling a series of DNA fragments generated by PCR into an ordered circular arrangement for replication and genetic work in cells. The PCR fragments are made with a modified nucleotide in the primers that can be removed with a DNA excision repair enzyme to generate a 3′ overhang. The 3′ overhangs are designed to allow directional annealing and thus sequential PCR fragments can be assembled by annealing the overhangs and subsequent ligation. Sequential addition of PCR fragments is facilitated by growing the chain on a solid support, and the assembled chain can be removed with a site specific recombinase if the first and last primers contain the recombinase site. The circularized assembled fragment can be directly used for cell transformation if the appropriate sequences are included, such as an origin of replication and a selectable marker.
Owner:RICE UNIV
Regulated antigen delivery system (RADS)
InactiveUS7341860B2Increase heightImprove the level ofBacteriaMicroorganism based processesOrigin of replicationGene delivery
Owner:WASHINGTON UNIV IN SAINT LOUIS
Compositions and methods for generating expression vectors through site-specific recombination
InactiveUS6551828B1Rapid and efficient generationImprove throughputOther foreign material introduction processesBiological testingOrigin of replicationSite-specific recombination
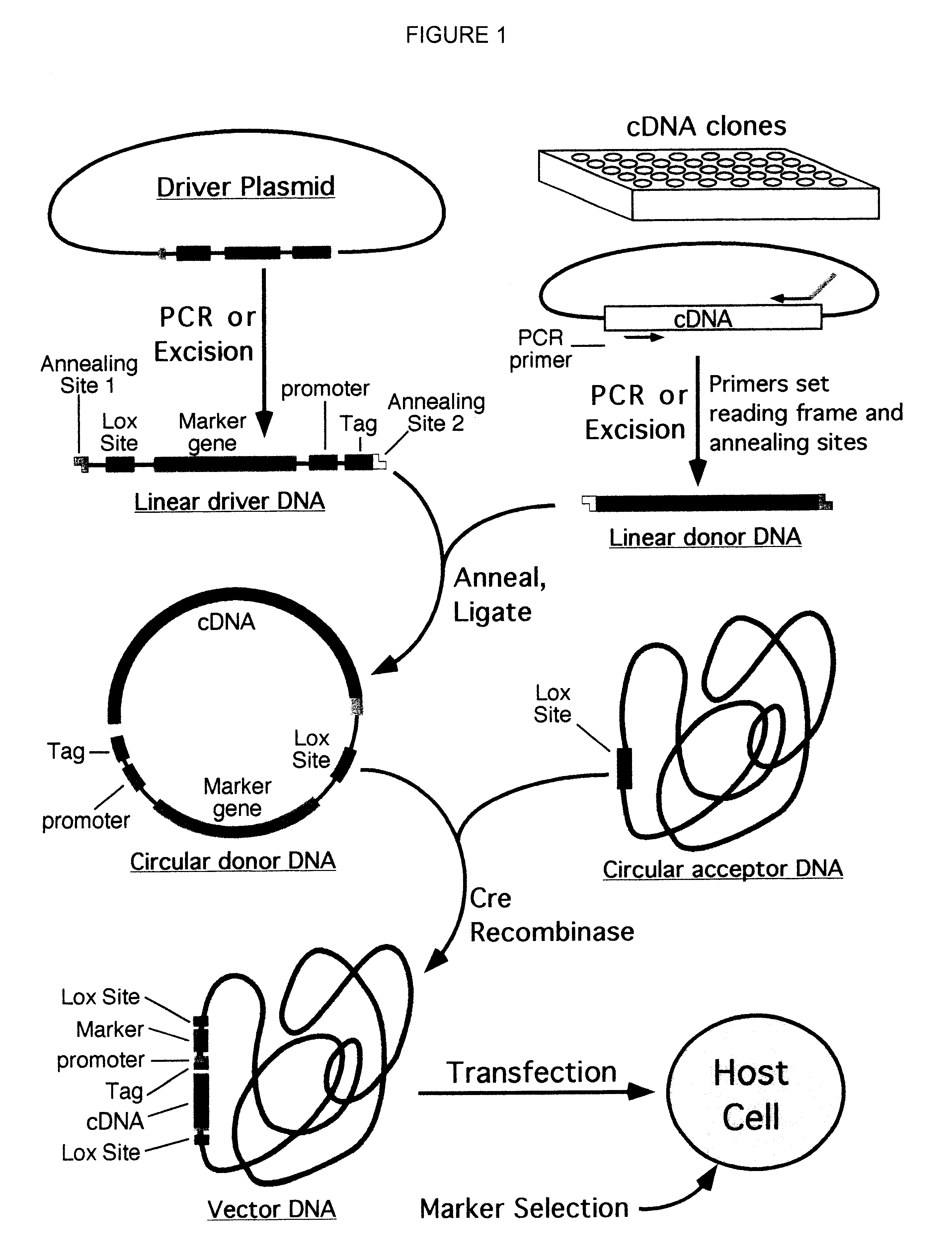
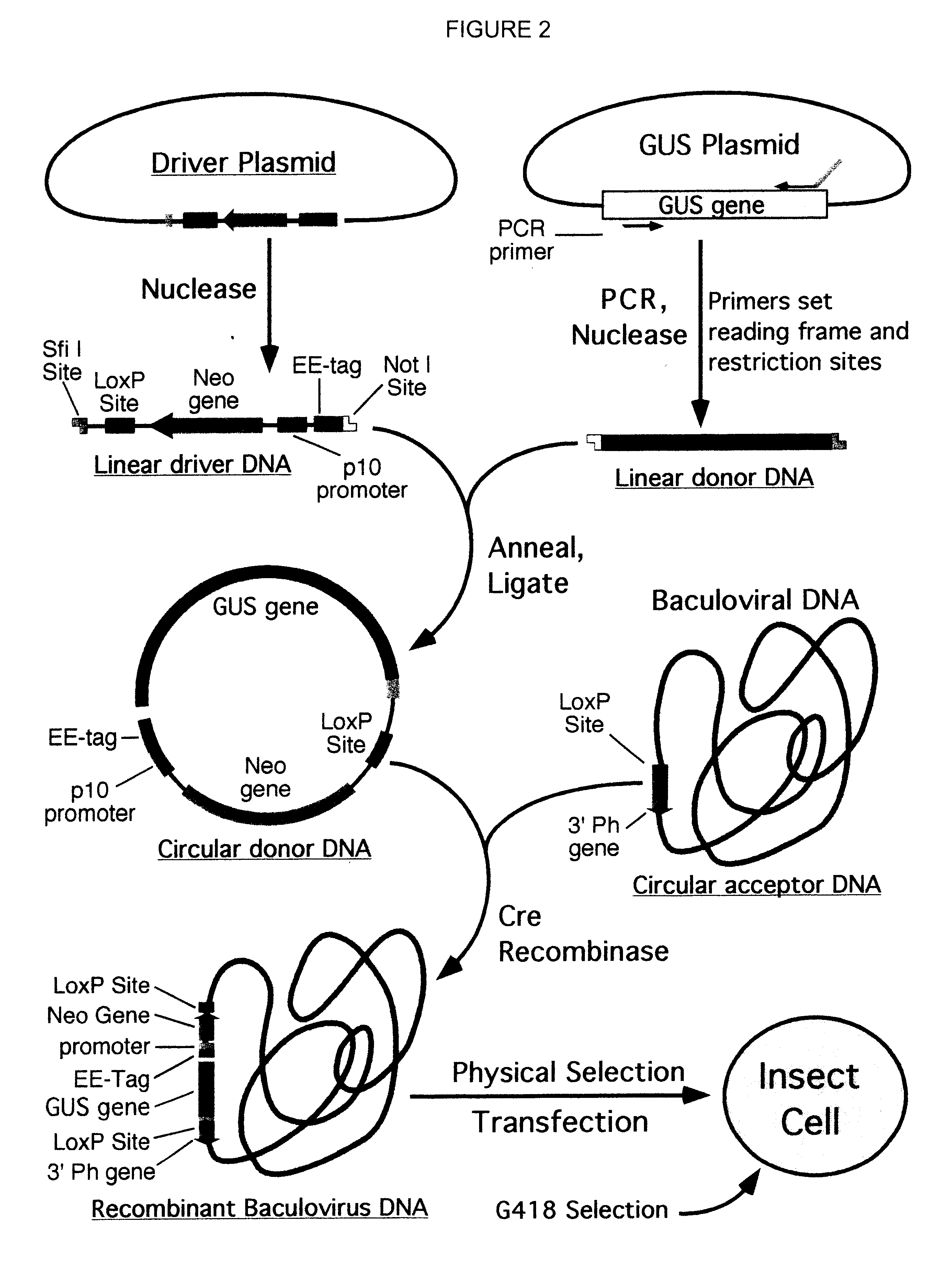
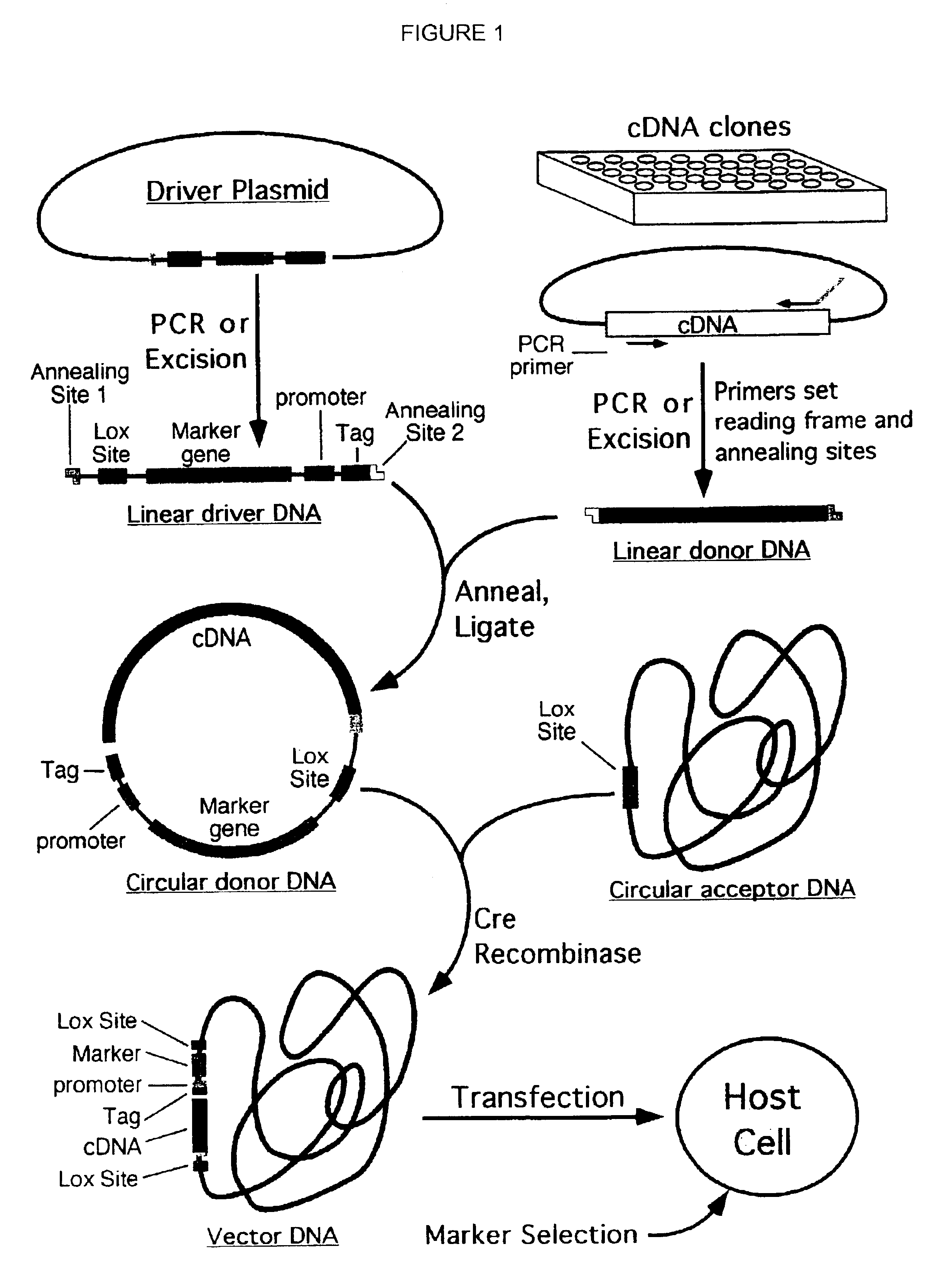
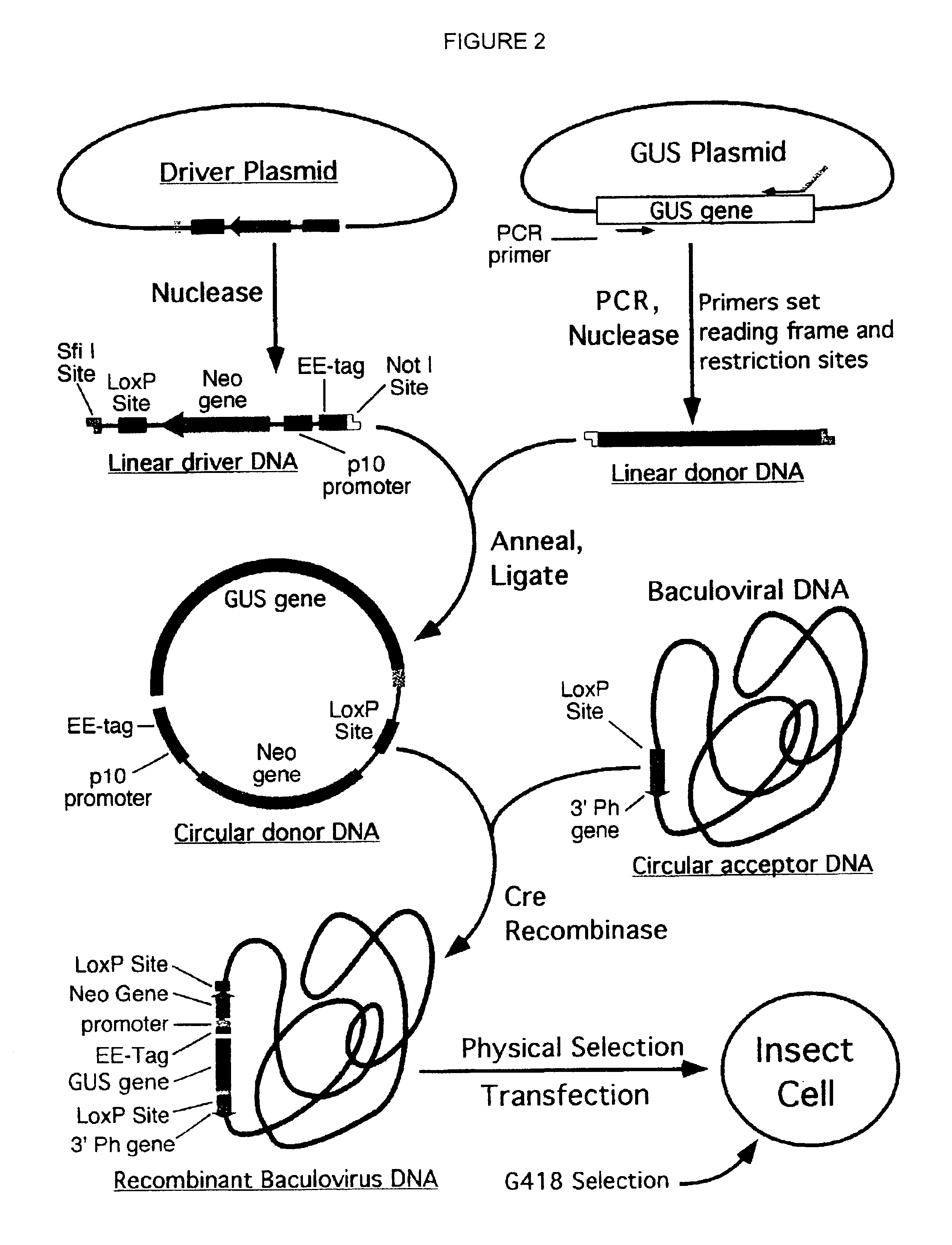
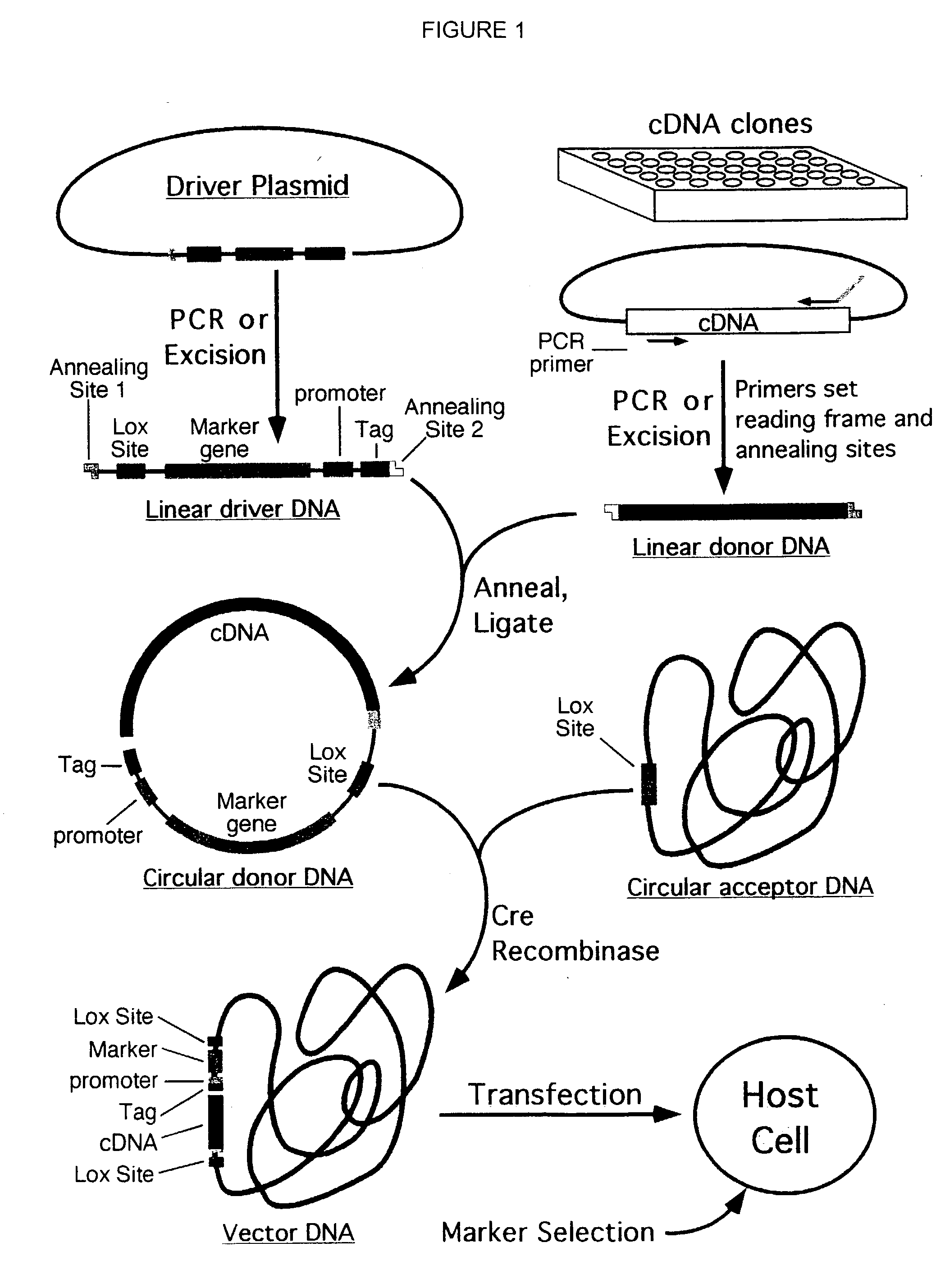
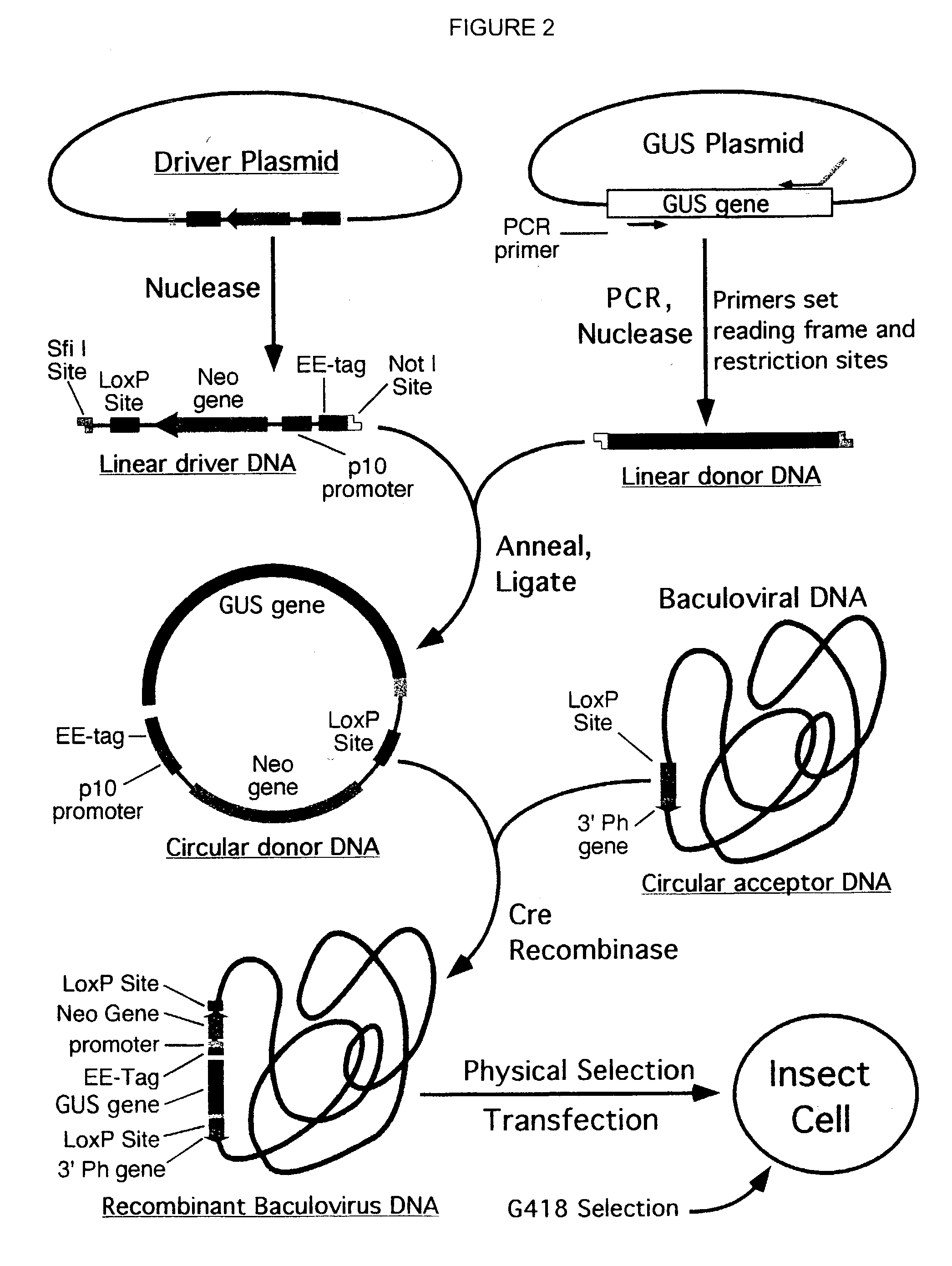
Compositions, kits, and methods are provided for use in a recombinational cloning or subcloning methods for constructing expression vectors which comprise: ligating a library of double-stranded linear donor DNAs, where each member of the library includes a donor DNA sequence, with a double-stranded linear driver DNA which includes a promoter sequence and a donor recombination site to form a single circular donor DNA, the single circular donor DNA not including an origin of replication, where the donor DNA sequence is under the transcriptional control of the promoter; and contacting the circular donor DNA and a circular acceptor acceptor vector in the presence of a recombinase to form a single fused circular vector, the circular acceptor vector comprising an origin of replication and an acceptor recombination site capable of recombining with the circular donor DNA.
Owner:PROTEMATION
Regulated antigen delivery system (RADS)
InactiveUS20050106176A1Effective exposureIncrease productionBacterial antigen ingredientsBacteriaGene deliveryOrigin of replication
We describe a regulated antigen delivery system (RADS) that has (a) a vector that includes (1) a gene encoding a desired gene product operably linked to a control sequence, (2) an origin of replication conferring vector replication using DNA polymerase III, and (3) an origin of replication conferring vector replication using DNA polymerase I, where the second origin of replication is operably linked to a control sequence that is repressible by a repressor. The RADS microorganism also has a gene encoding a repressor, operably linked to an activatible control sequence. The RADS described provide high levels of the desired gene product after repression of the high copy number origin of replication is lifted. The RADS are particularly useful as live bacterial vaccines. Also described is a delayed RADS system, in which there is a delay before the high copy number origin is expressed after the repression is lifted. The delayed RADS is also particularly useful for live bacterial vaccines. Also described are several control elements useful for these systems, as well as methods for providing immunity to a pathogen in a vertebrate immunized with the RADS microorganisms.
Owner:WASHINGTON UNIV IN SAINT LOUIS
Cloning system for construction of recombinant expression vectors
InactiveUS6953689B2Rapid and efficient generationImprove throughputSugar derivativesMicrobiological testing/measurementOrigin of replicationDNA
Cloning systems are provided for constructing expression vectors. In one aspect of the invention, a kit is provided for constructing one or more recombinant expression vectors. The kit comprises: a linear driver DNA comprising a promoter sequence, a donor recombination site, and at least one selectable marker, the linear driver DNA being capable of being ligated with one or more linear donor DNA comprising a donor DNA sequence to form one or more circular donor DNA; and a circular acceptor vector comprising an origin of replication and an acceptor recombination site capable of recombining with the circular donor DNA to form the recombinant expression vector for expressing the donor DNA sequence.
Owner:PROTEMATION
Gene expression system
InactiveUS7371542B2Reduce the amount of solutionHigh rateSugar derivativesAntibody mimetics/scaffoldsOrigin of replicationHigh level expression
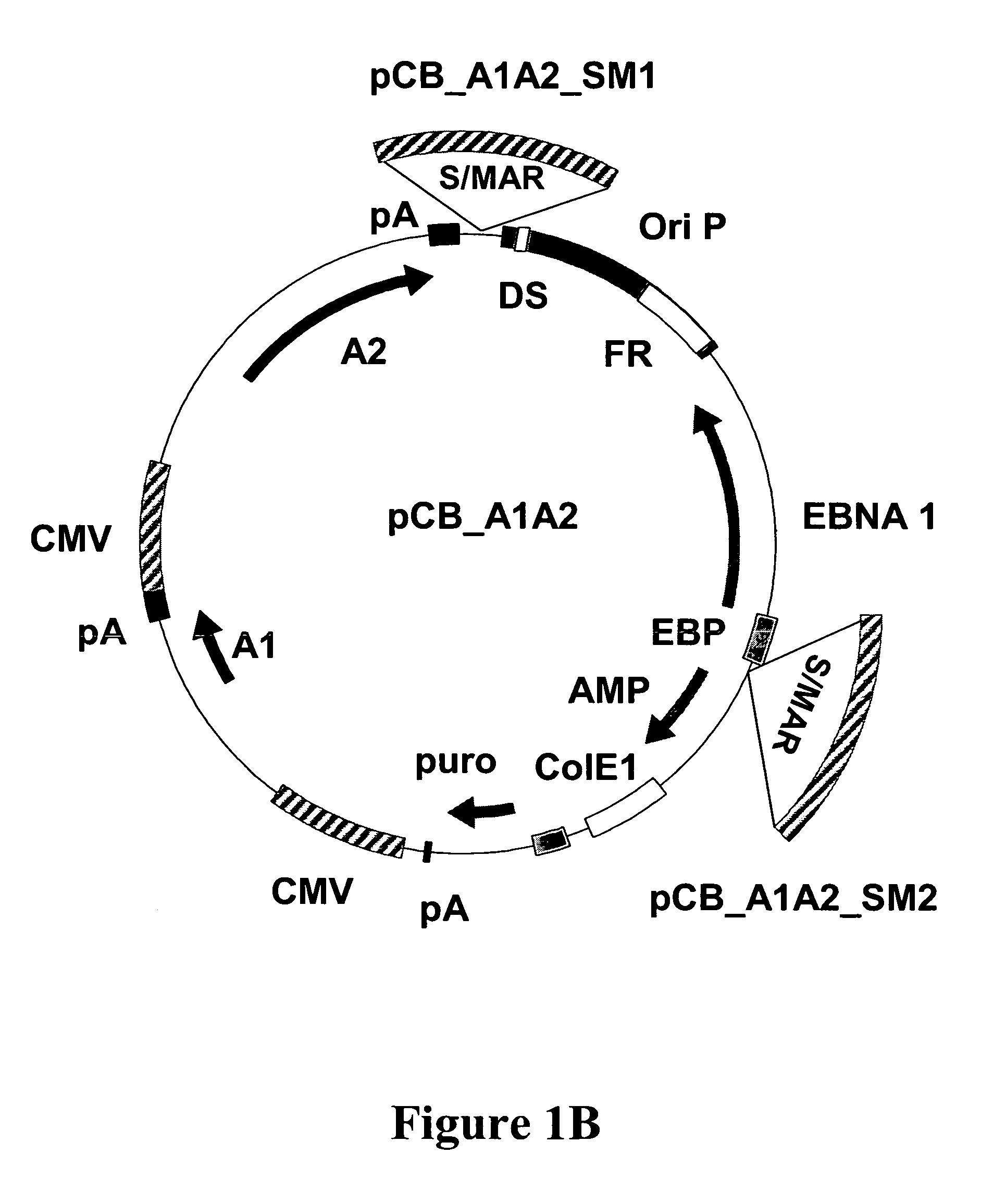
The present invention relates to nucleic acid molecules and in particular to vectors, comprising at least one gene of interest, at least one scaffold / matrix attached region (S / MAR), at least one origin of replication, and at least one replication initiation factor, cells comprising these, processes for their propagation, and their use, in particular for the high level expression of proteins which can be used as medicaments.
Owner:CYTOS BIOTECHNOLOGY AG
Methods and compositions of improved plant transformation
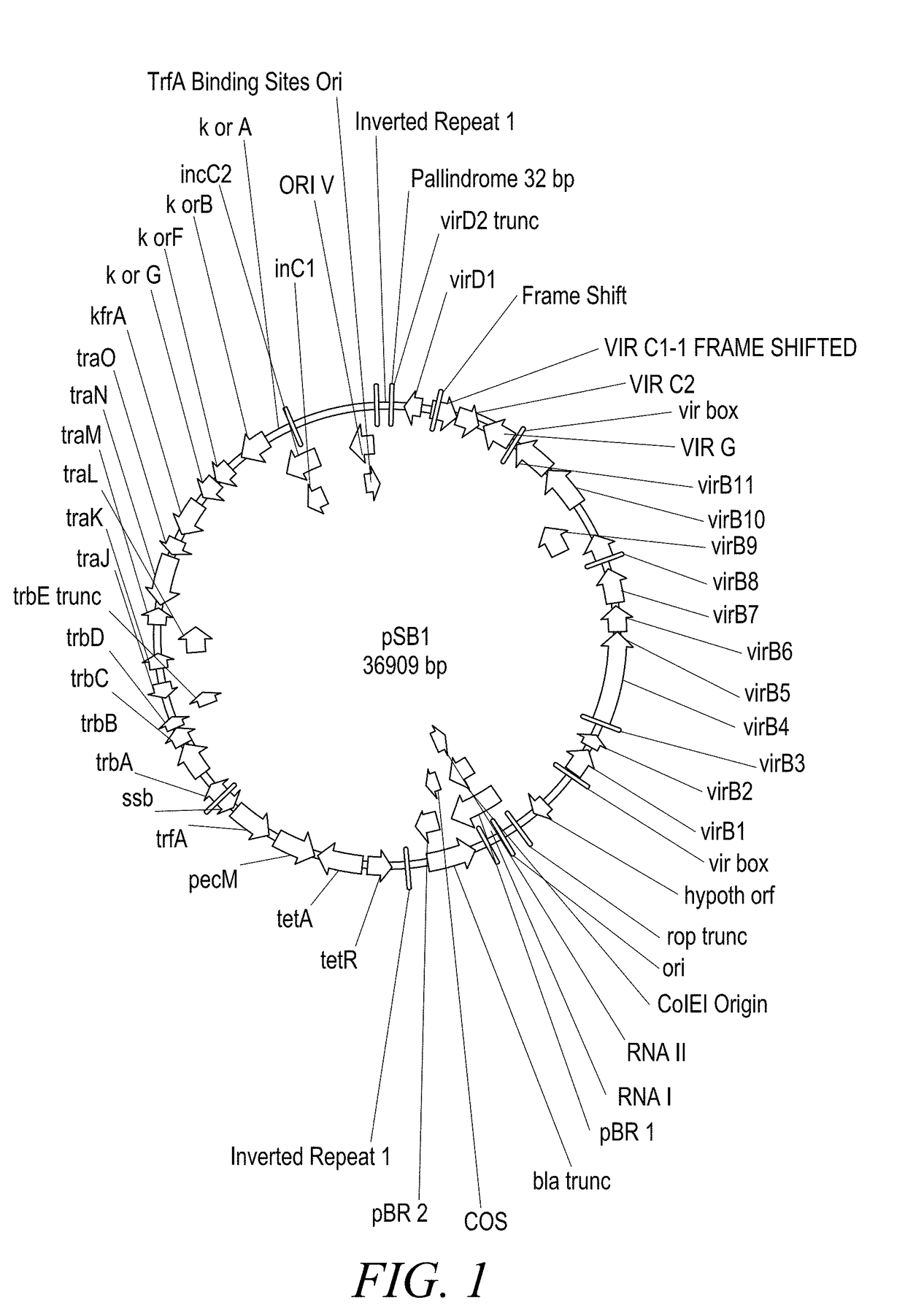
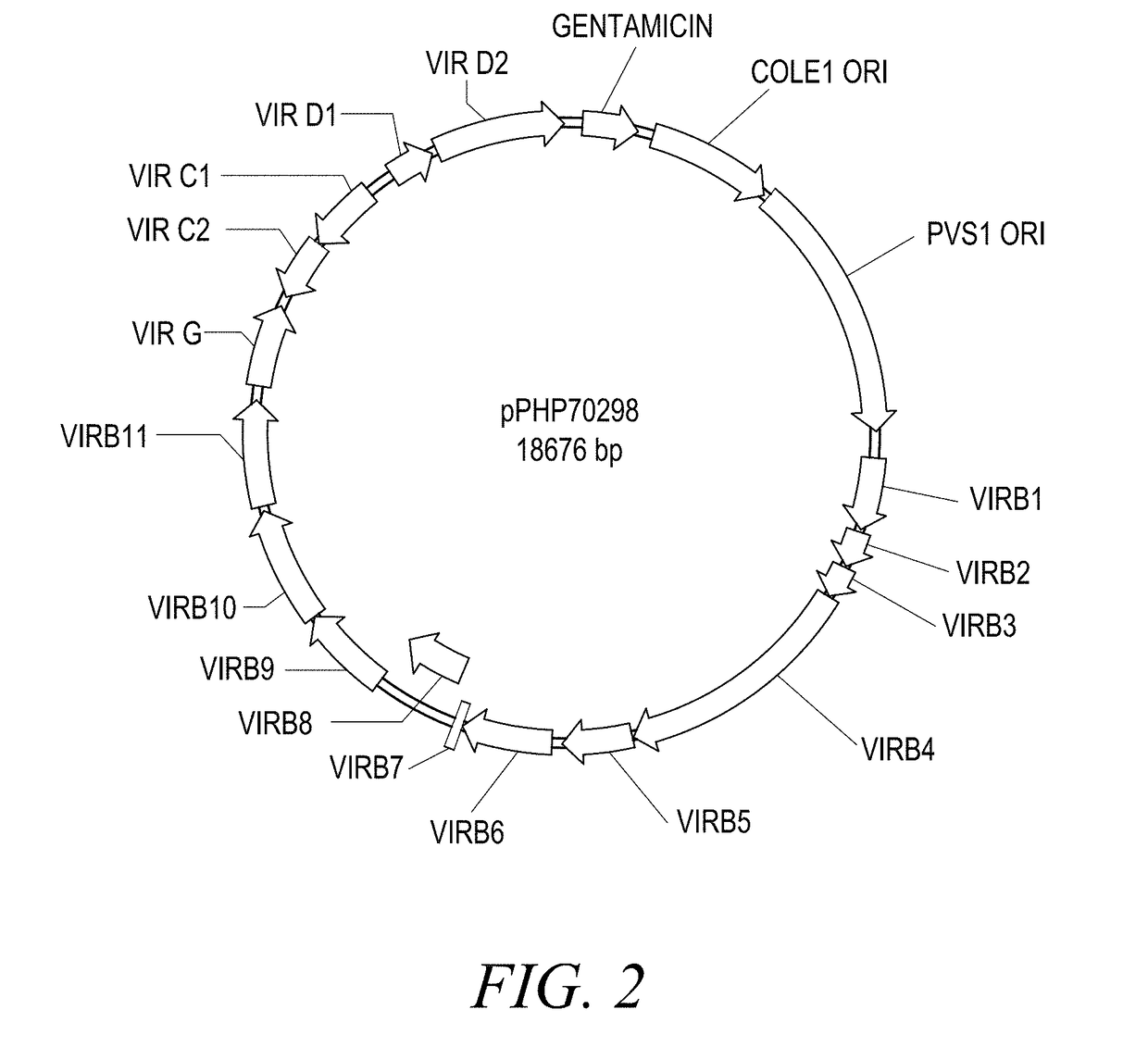
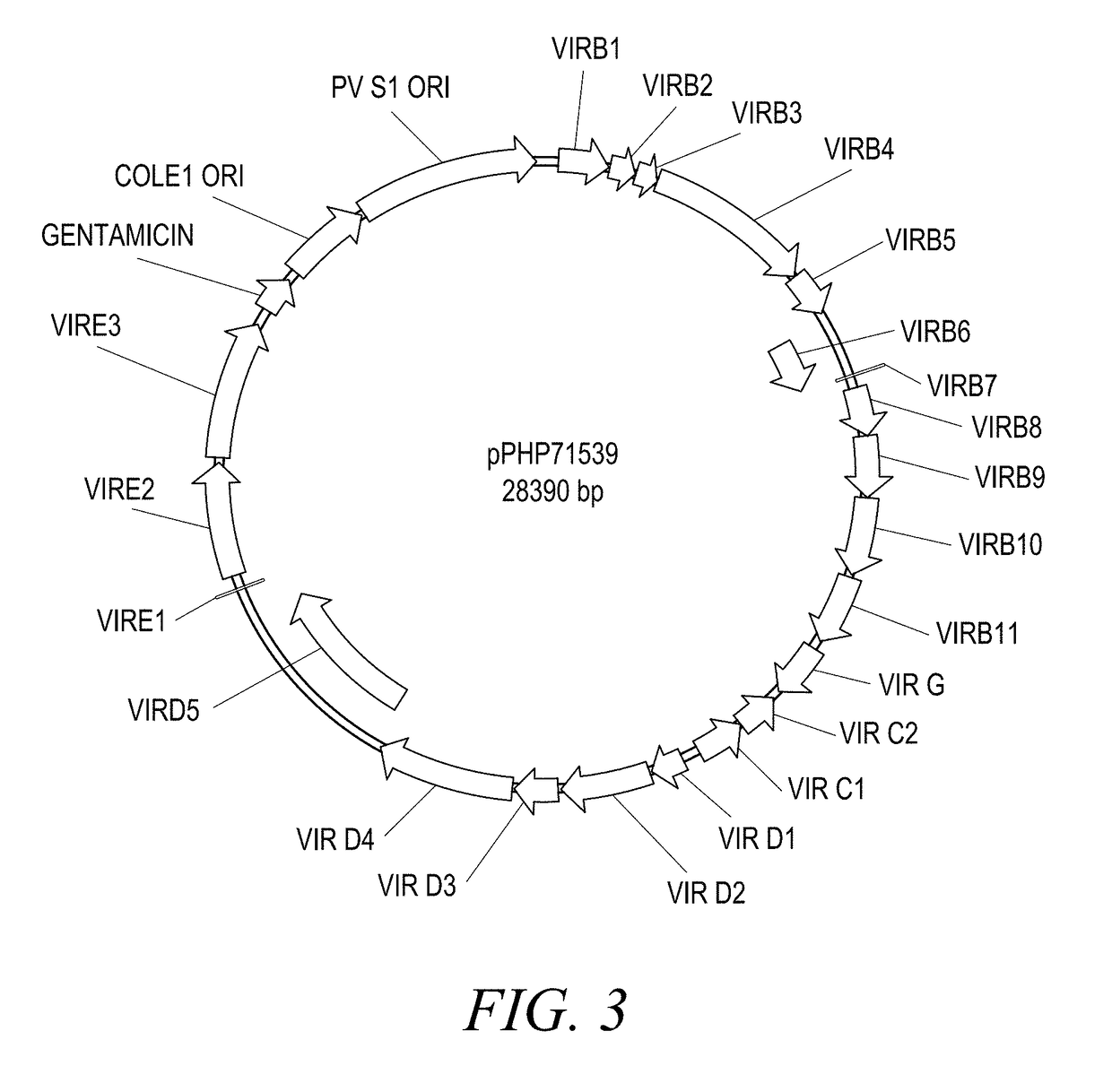
The present disclosure provides methods and compositions for the vectors comprising vir genes. The present disclosure further provides a vector comprising: (a) an origin of replication for propagation and stable maintenance in Escherichia coli; (b) an origin of replication for propagation and stable maintenance in Agrobacterium spp.; (c) a selectable marker gene; and (d) Rhizobiaceae virulence genes virB1-B11 or r-virB1-B11, virC1-C2 or r-virC1-C2, virD1-D2 or r-virD1-D2, and virG or r-virG, or variants and derivatives thereof, wherein the vector comprising the virulence genes r-virB1-B11, r-virC1-C2, r-virD1-D2, and r-virG further comprises a r-galls virulence gene, or variants and derivatives thereof. This abstract is intended as a scanning tool for purposes of searching in the particular art and is not intended to be limiting of the present disclosure.
Owner:PIONEER HI BRED INT INC
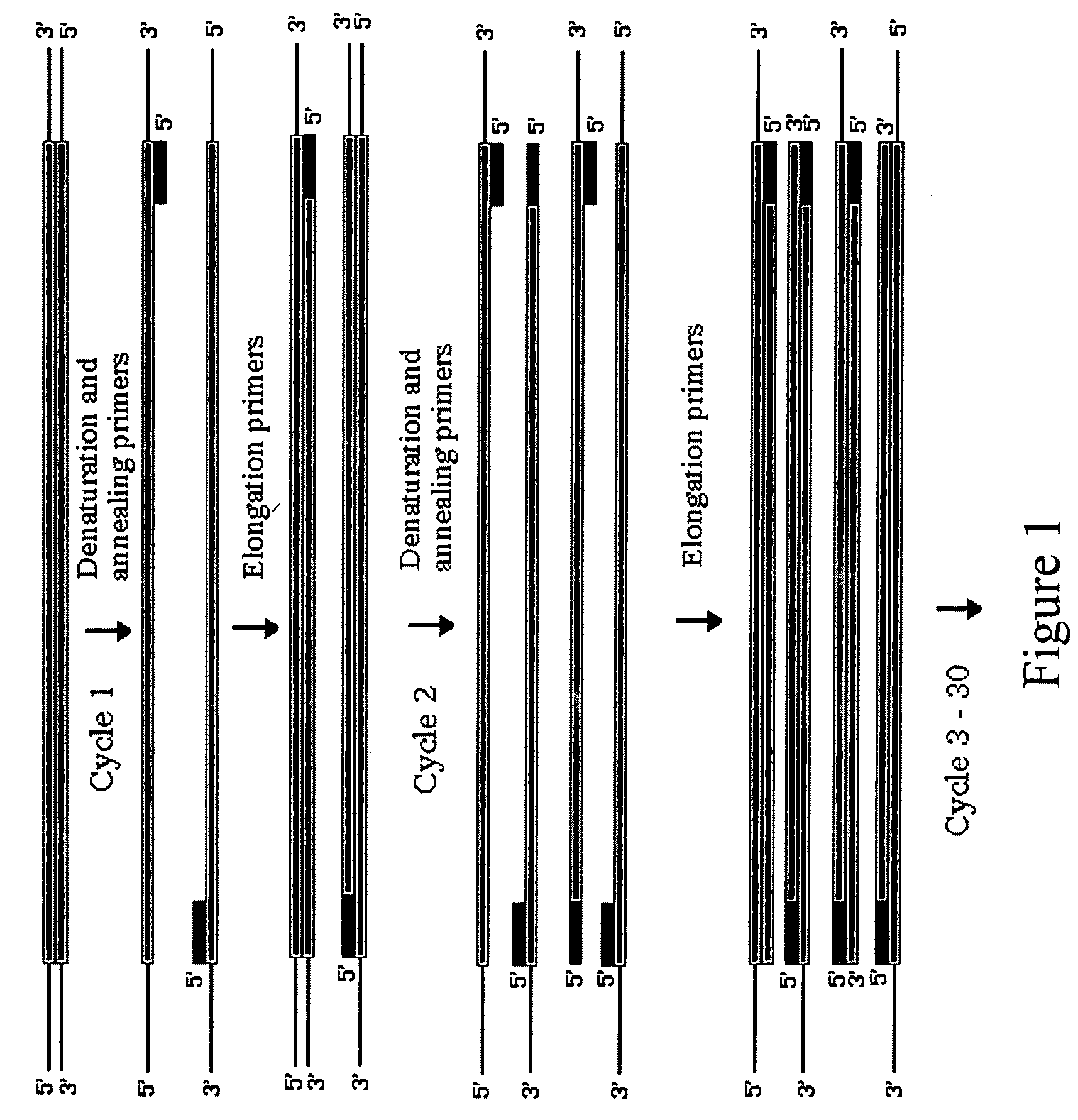
Nucleic Acid Amplification Method
InactiveUS20100015617A1Novel and effective nucleic acid amplification methodSimple and rapid detectionAnalysis using chemical indicatorsSugar derivativesOrigin of replicationNucleotide
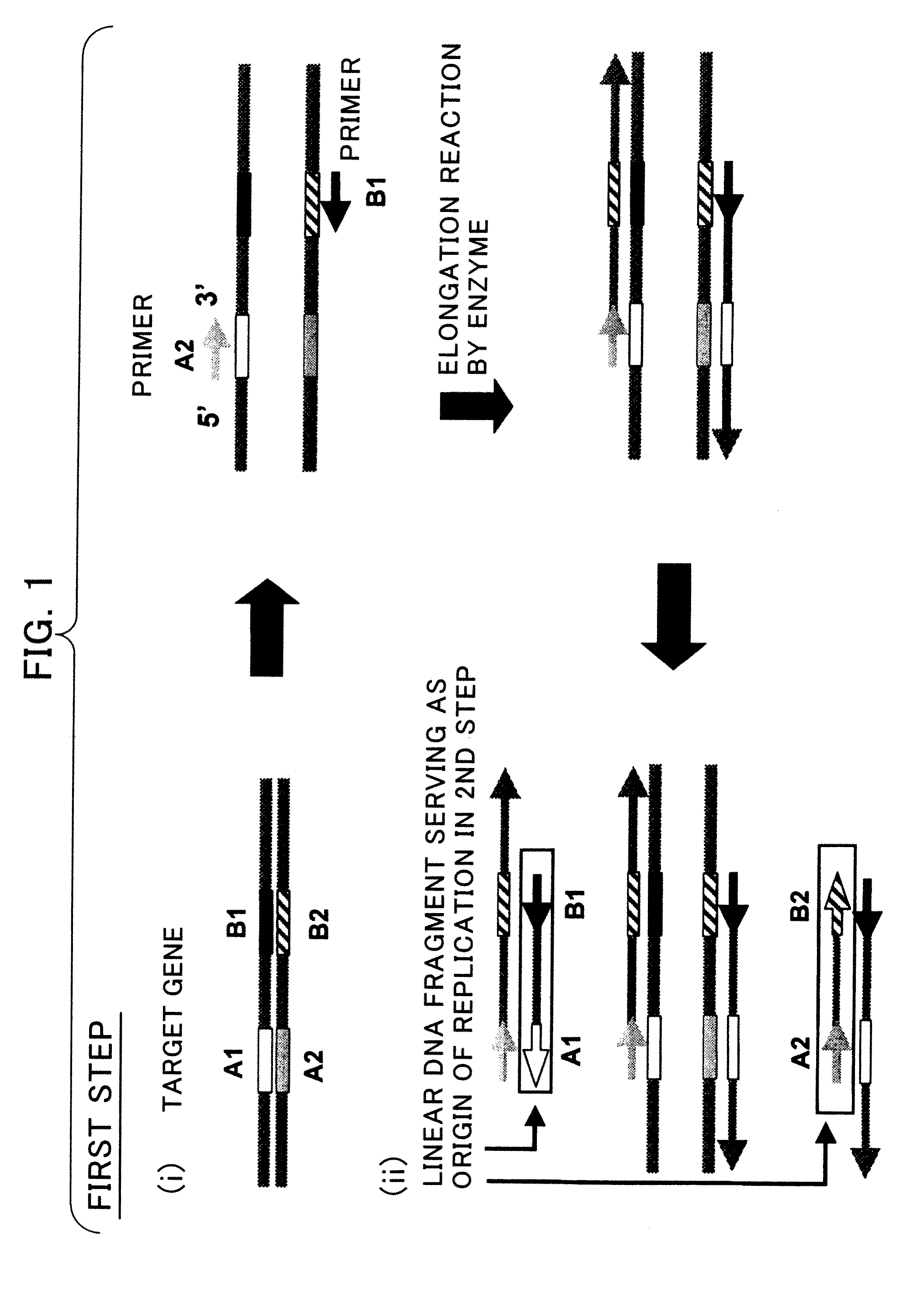
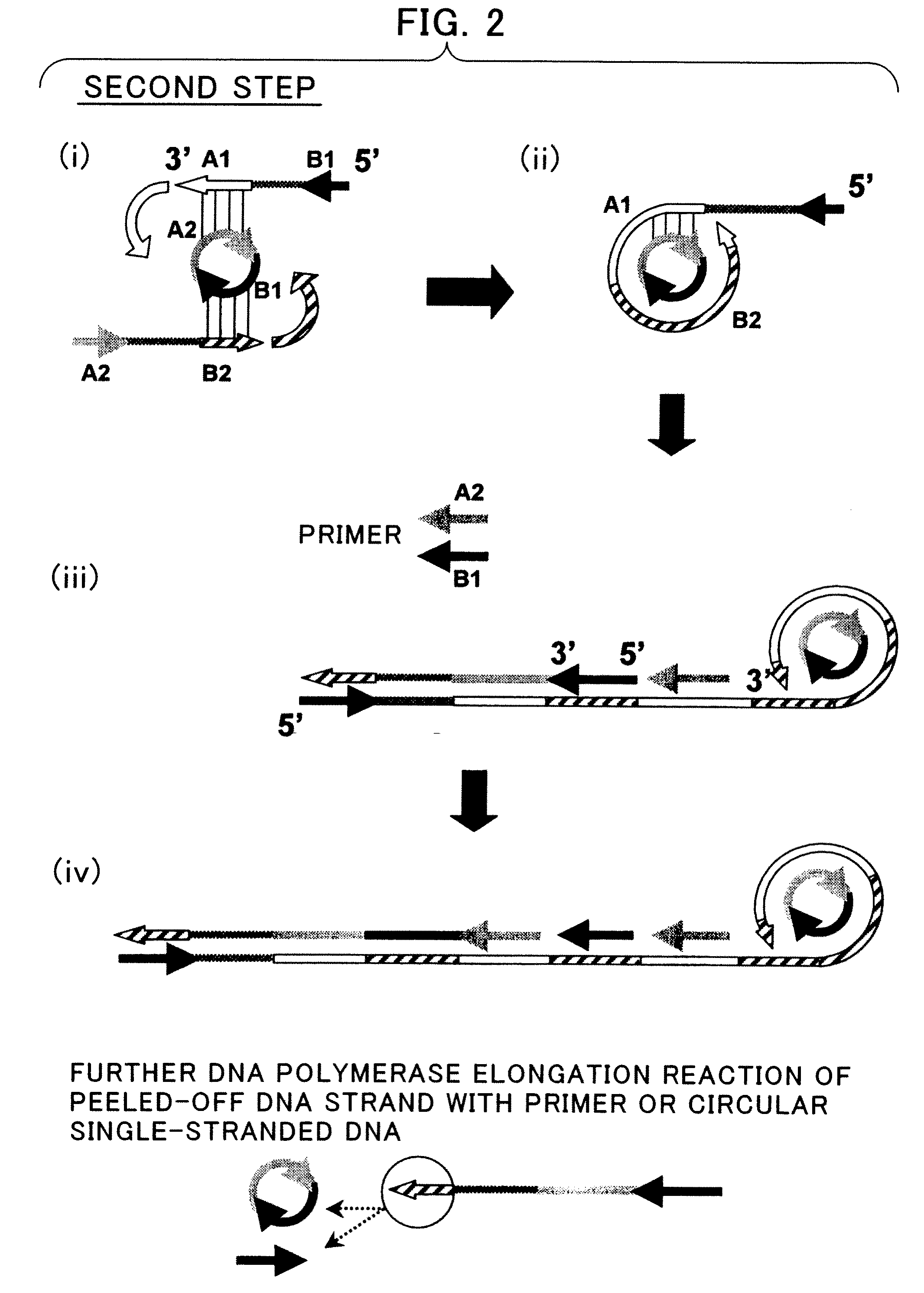
Disclosed is a nucleic acid amplification method which is based on a new principle and enables to amplify a nucleic acid having a specific nucleotide sequence in a simple manner, within a short time and with efficiency. The nucleic acid amplification method comprises the steps of: (a) conducting a DNA polymerase chain reaction by using, as a template, DNA comprising a nucleotide sequence to be amplified and using a primer pair having a nucleotide sequence complementary to the nucleotide sequence to be amplified, thereby producing a linear DNA fragment; and (b) conducting a chain-substituting DNA polymerase chain reaction in a chaining manner by using cyclic single-stranded DNA comprising the same nucleotide sequence as that of at least one of the primer pair as a template and employing the 3′-terminus of the linear DNA fragment produced in step (a) as the replication origin.
Owner:TOYO SEIKAN KAISHA LTD
Avoidance of undesirable replication intermediates in plasmid propagation
InactiveUS7390654B2Increase distanceEliminate productionVectorsSugar derivativesOrigin of replicationShuttle vector
Disclosed herein are improved plasmid shuttle vectors, vaccines based on them, and methods related to their construction and use. Particular arrangements of functional elements of such plasmids, namely origins of replication and eukaryotic transcription / translation control elements, which give rise to generally undesirable side-products upon propagation of the plasmids in bacterial culture are disclosed. These side-products apparently arise as terminated replication intermediates. Strategies both to eliminate accumulation of these side-products, and to make them useful as a vaccine adjuvant, are described.
Owner:MANNKIND CORP
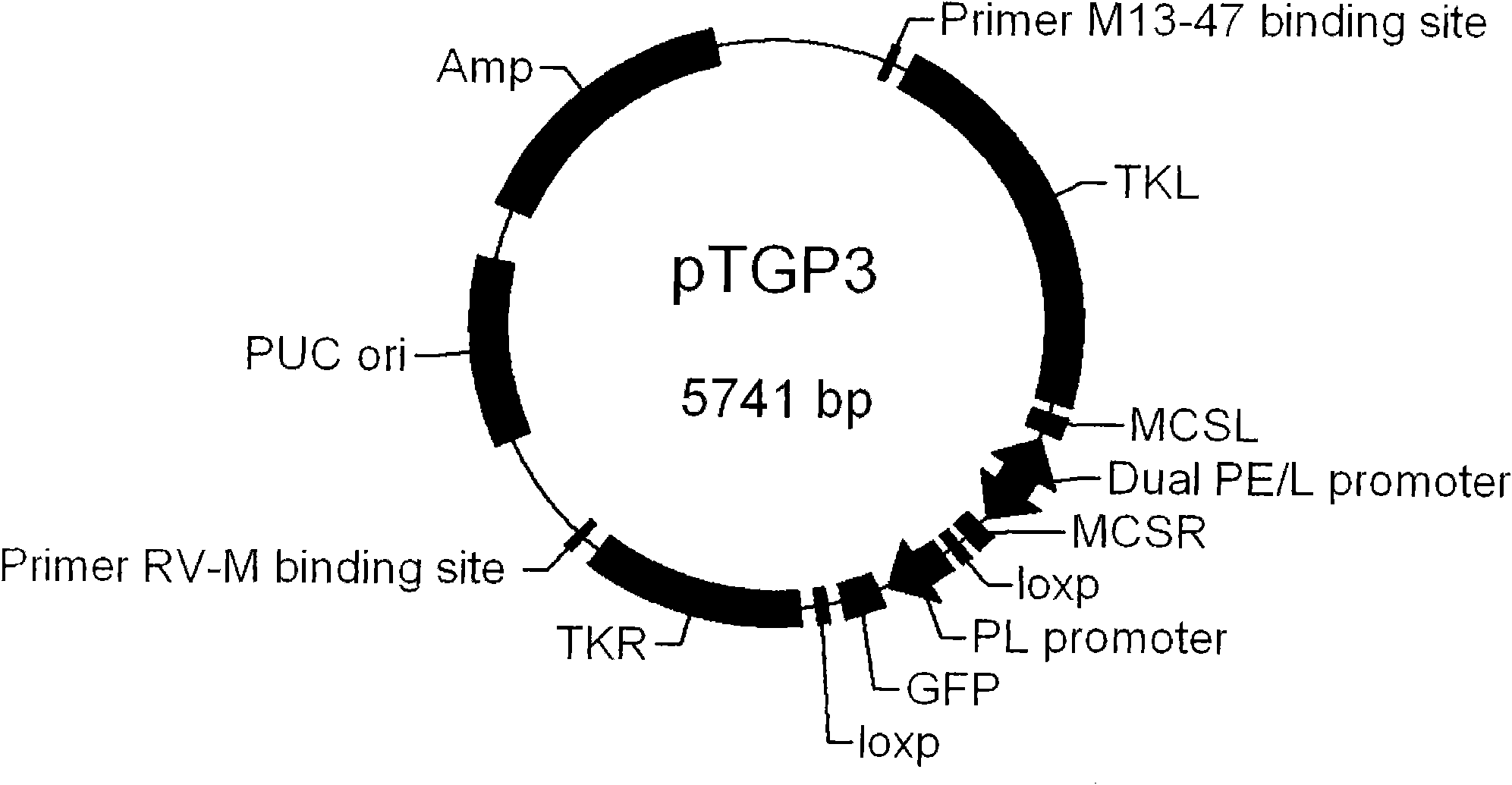
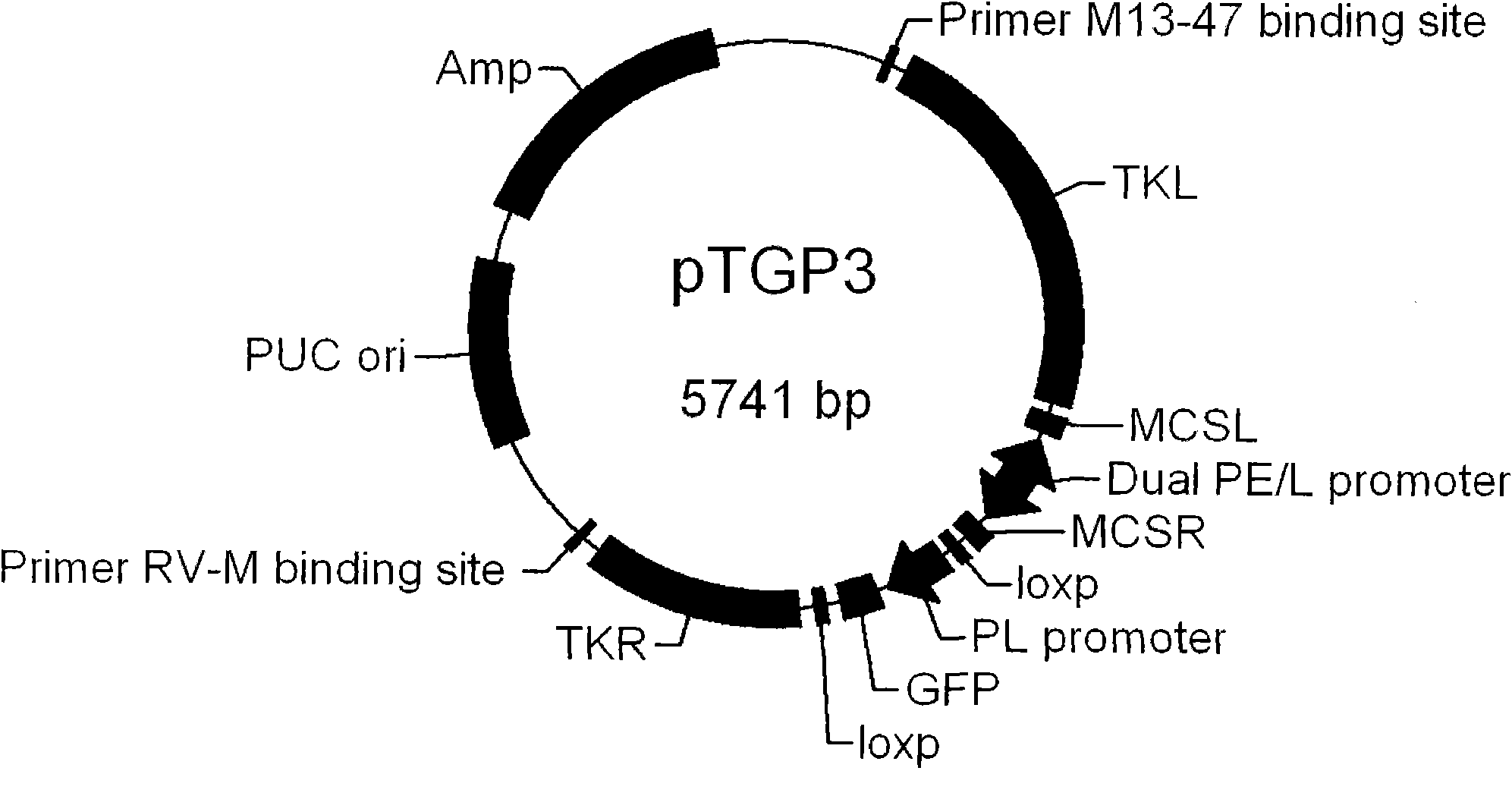
Fowlpox virus vector shuttle plasmid and application thereof
InactiveCN101775410APreserve immune efficiencyRetain the ability to replicateGenetic material ingredientsGenetic engineeringShuttle plasmidFowlpox virus
The invention provides fowlpox virus vector shuttle plasmid pTGP3 which comprises recombinant arms TKL and TKR, a bidirectional promoter PE / L, a fluorescent protein expression cassette, and a resistant marker gene and replication origin ori; the upstream and the downstream of the bidirectional promoter PE / L are respectively provided with cloning sites MCSL and MCSR; and both ends of the fluorescent protein expression cassette are provided with loxp sequences. The plasmid of the invention has two different screening markers, and the recombinant fowlpox virus prepared with the plasmid can express 1 to 3 types of gene with different meshes in the whole processes of the early and the later periods; the strong composite promoter with expression activity in the early and the later periods is applied so as to realize the all-process high-efficiency expression of a target gene; and the loxp sequences are introduced into both ends of the fluorescent protein expression cassette, so as to knock out the exogenous recombinant fowlpox virus screening markers. The invention lays foundation for the series and the scale application of the recombinant fowlpox virus in vaccine and biological drug research and development fields.
Owner:MILITARY VETERINARY RES INST PLA MILITARY MEDICAL ACAD OF SCI
Actinobacillus succinogenes shuttle vector and methods of use
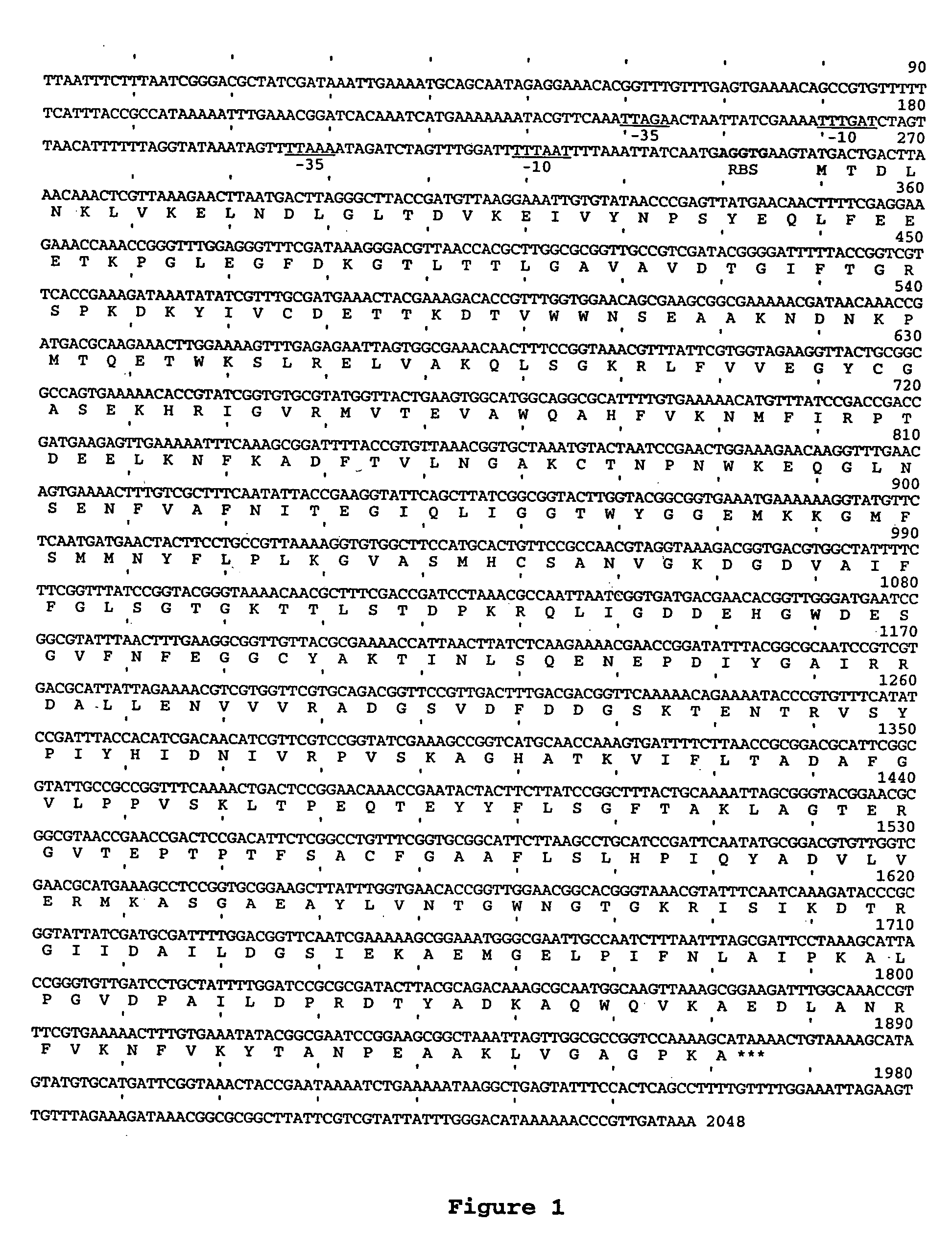
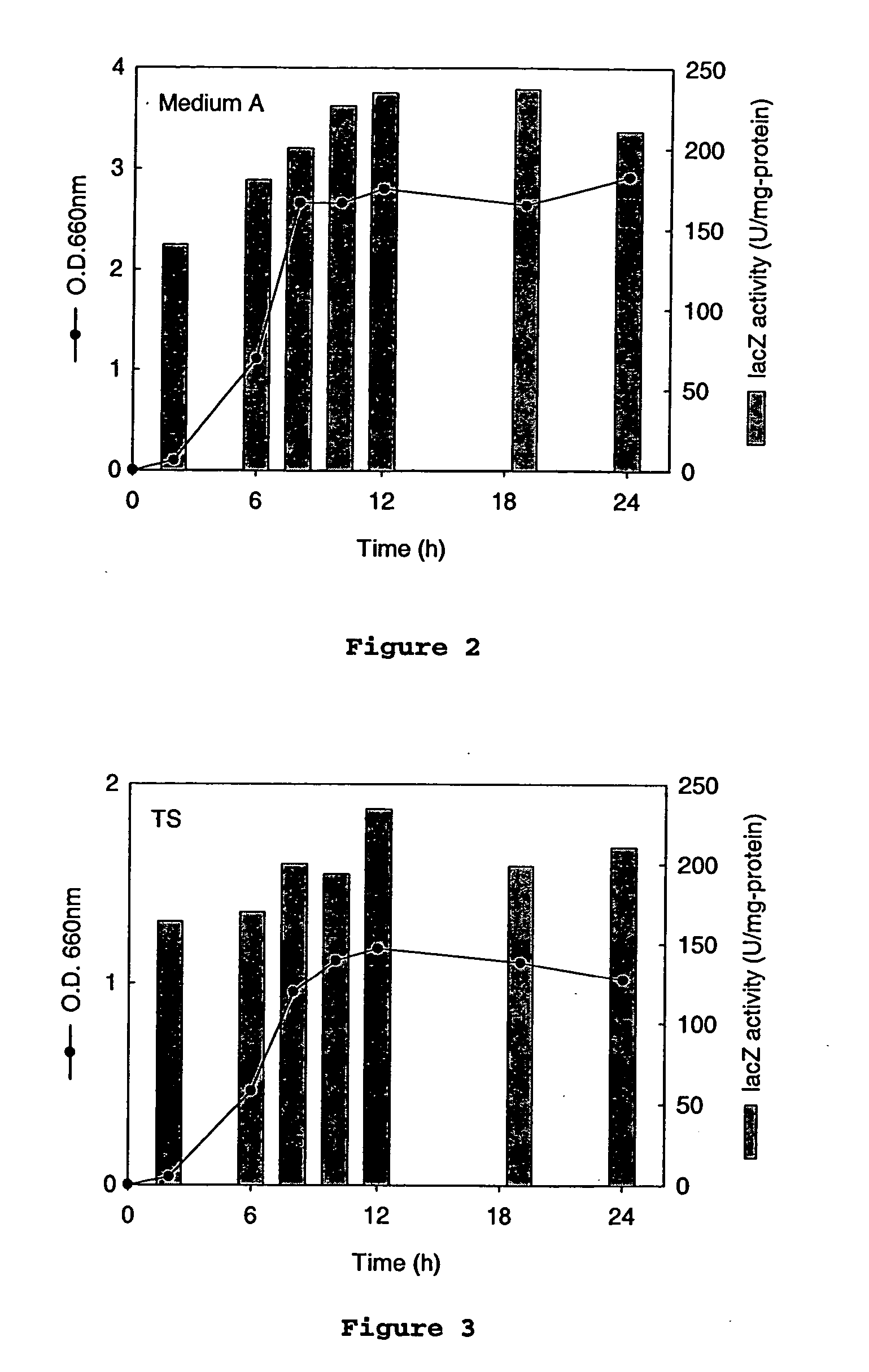
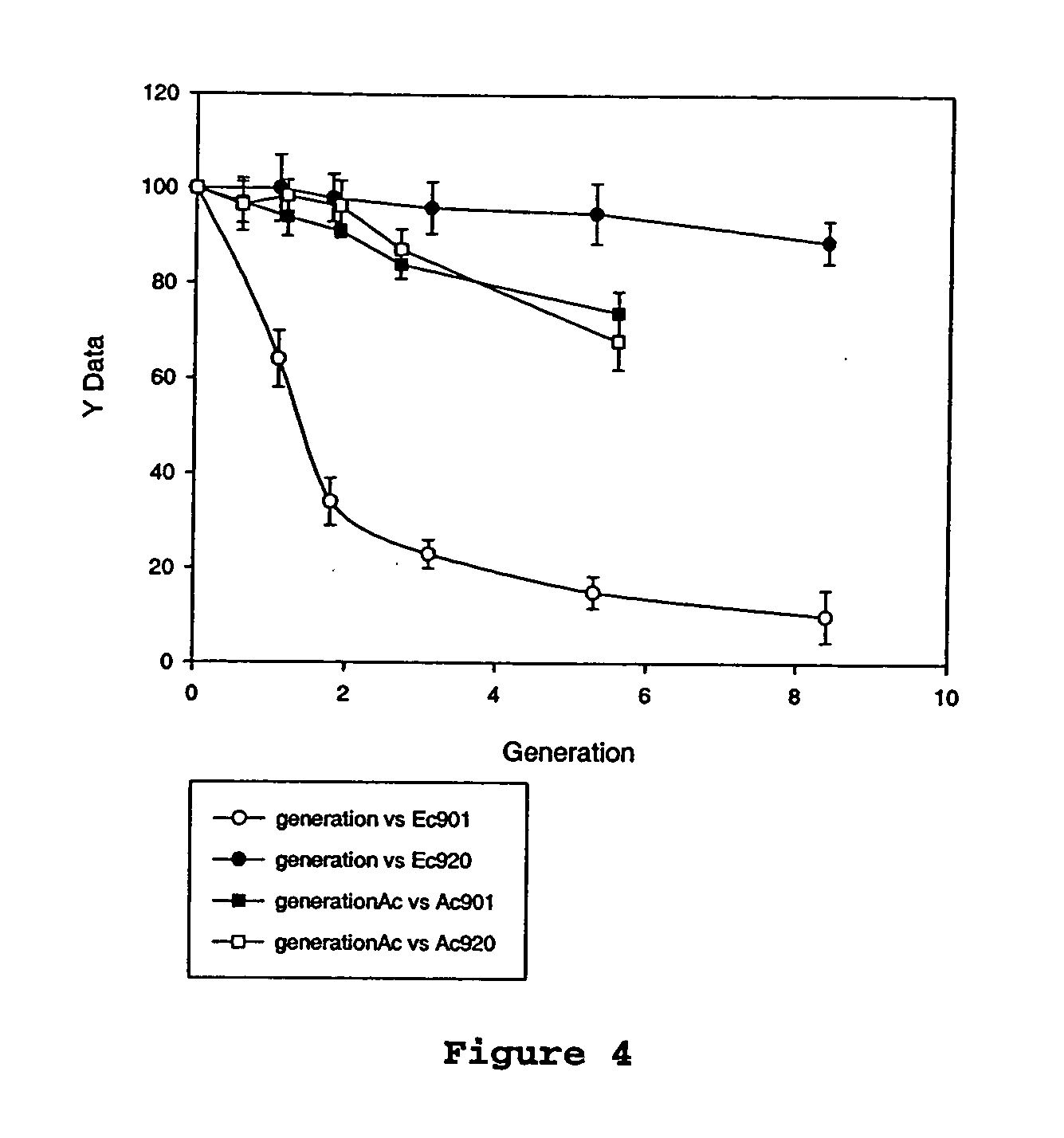
An Actinobacillus succinogenes plasmid vector which provides a means to overexpress proteins in A. succinogenes. The plasmid can be transformed efficiently by electroporation, and replicates in a stable manner in A. succinogenes. The plasmid comprises at least one marker gene, operably linked to a first promoter functional in Actinobacillus succinogenes, an origin of replication functional in Actinobacillus succinogenes, a second promoter isolated from Actinobacillus succinogenes, and a cloning site downstream from the second promoter. Plasmids pLGZ901, pLGZ920, pLGZ921, and pLGZ922 are disclosed. The pckA gene polypeptide sequence and nucleic acid sequence of Actinobacillus succinogenes, including the promoter and ribosome binding site, is disclosed. Furthermore, a method for producing a recombinant Actinobacillus succinogenes is described, including a method of transformation. Additionally, a recombinant Actinobacillus succinogenes is disclosed and a method for producing succinate utilizing this recombinant Actinobacillus succinogenes is described.
Owner:BOARD OF TRUSTEES OPERATING MICHIGAN STATE UNIV
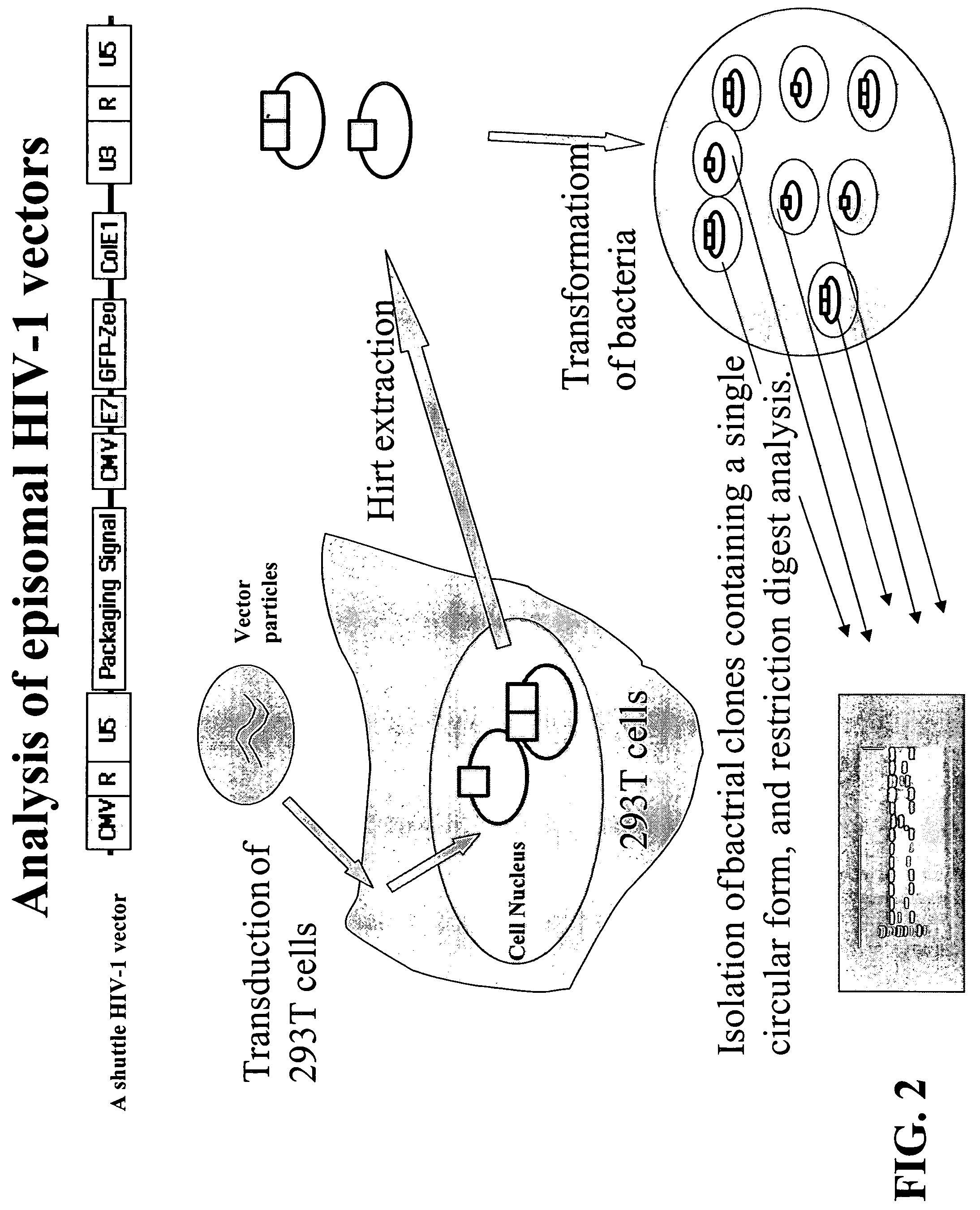
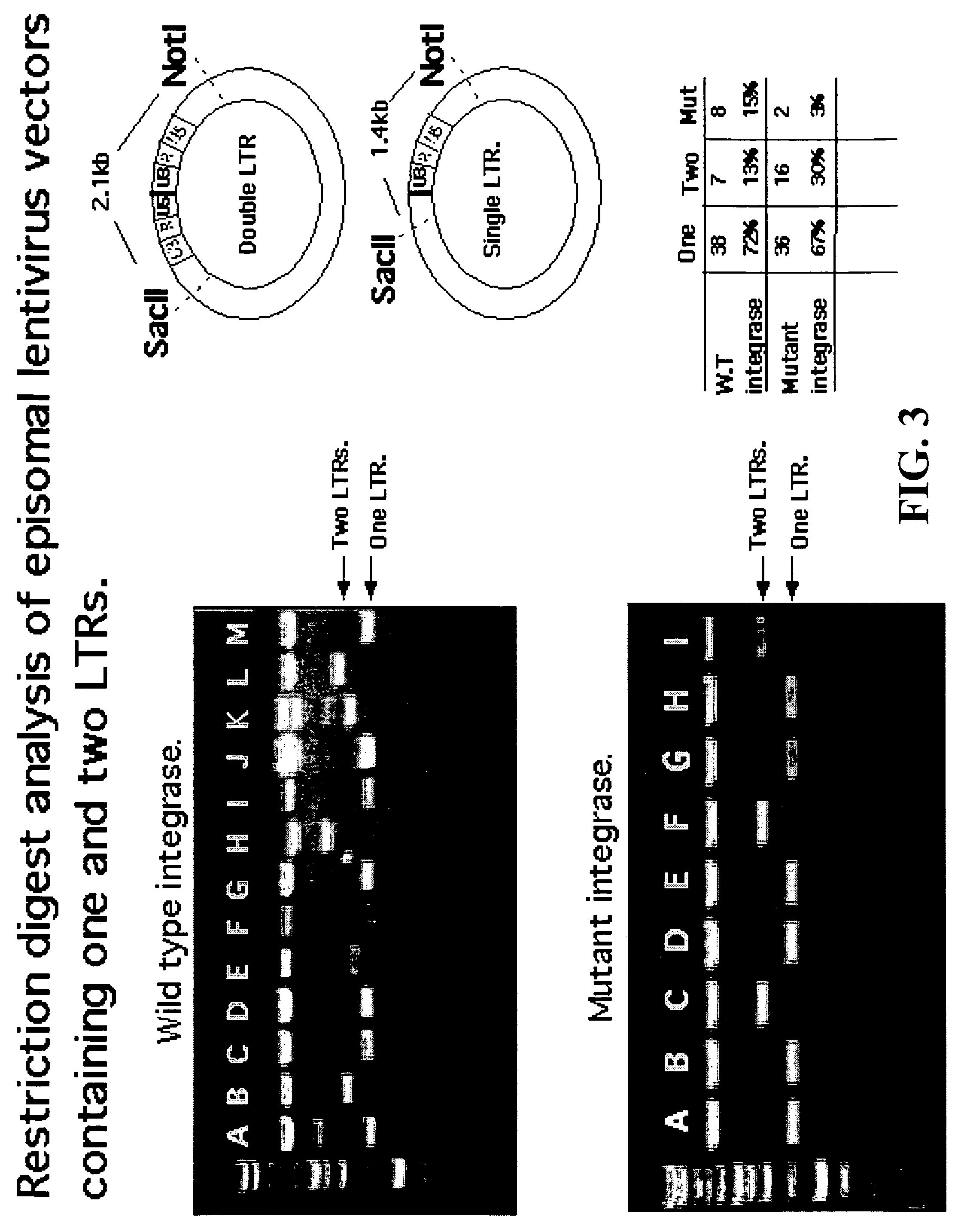
Single LTR lentivirus vector
The present invention provides an isolated nucleic acid comprising a single retroviral LTR, a polypurine tract, a packaging signal, a primer binding site and a rev responsive element. Further provided is an isolated nucleic acid comprising a heterologous nucleotide sequence, a single retroviral long terminal repeat (LTR), a packaging signal, a rev responsive element, a polypurine tract, a eukaryotic promoter, a primer binding site, a bacterial origin of replication and a bacterial selection marker. In addition, the present invention provides an isolated nucleic acid comprising a 5′ retroviral LTR and a 3′ retroviral LTR, a heterologous nucleotide sequence, a packaging signal, a rev responsive element, a polypurine tract, a eukaryotic promoter, a primer binding site, a bacterial origin of replication and a bacterial selection marker cassette, wherein the bacterial origin of replication and bacterial selection marker are located between the two LTRs.
Owner:KAFRI TAL +1
Lac shuttle vectors
The invention discloses a Lac shuttle vector, comprising at least (a) a region which regulates a plasmid copy number; (b) an eukaryotic gene expression cassette, which comprises at least an eukaryotic gene transcriptional promoter sequence, a multiple cloning site and a transcriptional terminator sequence; (c) a lactic acid bacteria plasmid sequence, which comprises a plus origin of replication, and a nucleic acid sequence encoding for a protein which relates to the lactic acid bacteria plasmid replication; and (d) a non-antibiotic resistance selection gene and the promoter sequence thereof. The Lac shuttle vector features a non-antibiotic resistance gene as a selection marker, which is useful in pharmaceuticals and foods.
Owner:ANAWRAHTA BIOTECH
Novel expression vectors and uses thereof
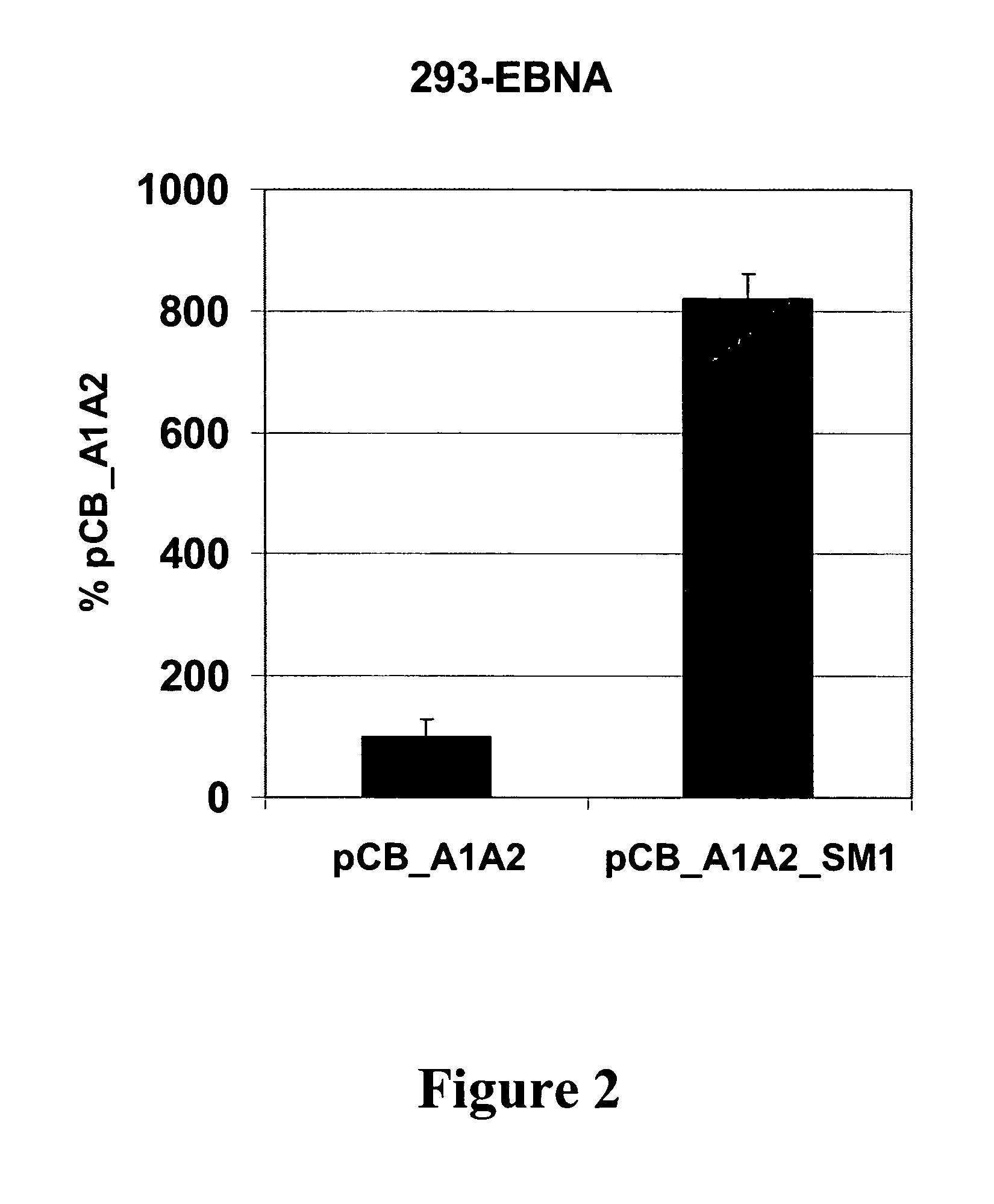
InactiveUS20050026137A1Increase the number ofAvoiding a severe drawbackGenetic material ingredientsVirus peptidesEpstein-Barr Virus Nuclear AntigensOrigin of replication
The present invention relates to novel vectors, to DNA vaccines and gene therapeutics containing said vectors, to methods for the preparation of the vectors and DNA vaccines and gene therapeutics containing the vectors, and to therapeutic uses of said vectors. More specifically, the present invention relates to novel vectors comprising (a) an expression cassette of a gene of a nuclear-anchoring protein, which contains (i) a DNA binding domain capable of binding to a specific DNA sequence and (ii) a functional domain capable of binding to a nuclear component and (b) a multimerized DNA sequence forming a binding site for the anchoring protein, and optionally (c) one or more expression cassettes of a DNA sequence of interest. In particular the invention relates to vectors that lack a papilloma virus origin of replication. The nuclear-anchoring protein might be the E2 protein of Bovine Papilloma Virus type 1 or Epstein-Barr Virus Nuclear Antigen 1. The invention also relates to vectors that lack an origin of replication functional in a mammalian cell. The invention further relates to methods for expressing a DNA sequence of interest in a subject.
Owner:FIT BIOTECH OY PLC
Avoidance of undesirable replication intermediates in plasmid propagation
InactiveUS20060269521A1Increase distanceEliminate productionBiocideVectorsOrigin of replicationShuttle vector
Disclosed herein are improved plasmid shuttle vectors, vaccines based on them, and methods related to their construction and use. Particular arrangements of functional elements of such plasmids, namely origins of replication and eukaryotic transcription / translation control elements, which give rise to generally undesirable side-products upon propagation of the plasmids in bacterial culture are disclosed. These side-products apparently arise as terminated replication intermediates. Strategies both to eliminate accumulation of these side-products, and to make them useful as a vaccine adjuvant, are described.
Owner:MANNKIND CORP
Cosmid vector for plant transformation and use thereof
ActiveUS8298819B2Efficient cloningStable maintenanceSugar derivativesOther foreign material introduction processesBiotechnologyEscherichia coli
The present invention aims to provide novel vectors for plant transformation.The vectors of the present invention are cosmid vectors having a full length of 15 kb or less characterized in that:1) they contain an origin of replication of an IncP plasmid, but do not contain any origin of replication of other plasmid groups;2) they contain the trfA1 gene of an IncP plasmid;3) they contain an oriT of an IncP plasmid;4) they contain the incC1 gene of an IncP plasmid;5) they contain a cos site of lambda phage and the cos site is located outside the T-DNA;6) they contain a drug resistance gene expressed in E. coli and a bacterium of the genus Agrobacterium; 7) they contain a T-DNA right border sequence of a bacterium of the genus Agrobacterium; 8) they contain a T-DNA left border sequence of a bacterium of the genus Agrobacterium; 9) they contain a selectable marker gene for plant transformation located between 7) and 8) and expressed in a plant; and10) they contain restriction endonuclease recognition site(s) located between 7) and 8) for cloning a foreign gene.
Owner:KANEKA CORP
Plasmid vector and method for building plant population by using plasmid vector
ActiveCN108034671AIncrease positive rateReduce the probability of false positive plantsHydrolasesVector-based foreign material introductionOrigin of replicationPlant population
The invention relates to a plasmid vector and a method for building a plant population by using the plasmid vector. The plasmid vector contains, at a replication origin, a gene expression box for expressing Cas9 protein, a first promoter, nucleotide sequences containing suicide gene sequences, a gRNA scaffold element and a termination signal, and at least one first enzyme cutting site located on the nucleotide sequence 5' end containing the suicide gene sequence and at least one second enzyme cutting site located at the nucleotide sequence 3' end containing the suicide gene sequence; furthermore, no first enzyme cutting site and no second enzyme cutting site exist in other positions of the plasmid vector. The first promoter is located at the upstream of the termination signal; the nucleotide sequences containing suicide gene sequences and the gRNA scaffold element are all located between the first promoter and the termination signal.
Owner:INST OF PLANT PROTECTION CHINESE ACAD OF AGRI SCI
Shuttle vector for Bifidobacterium and Escherichia coli
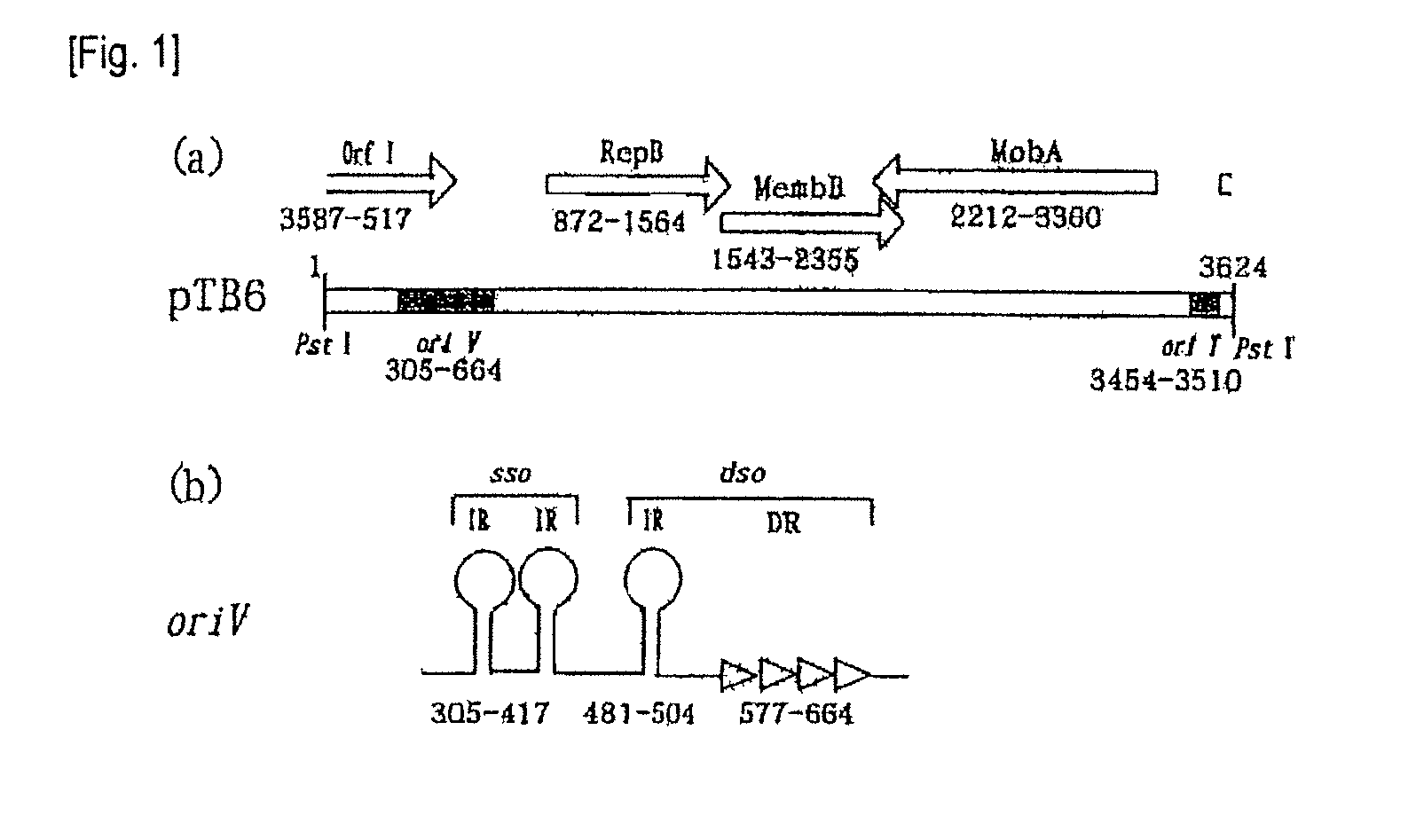
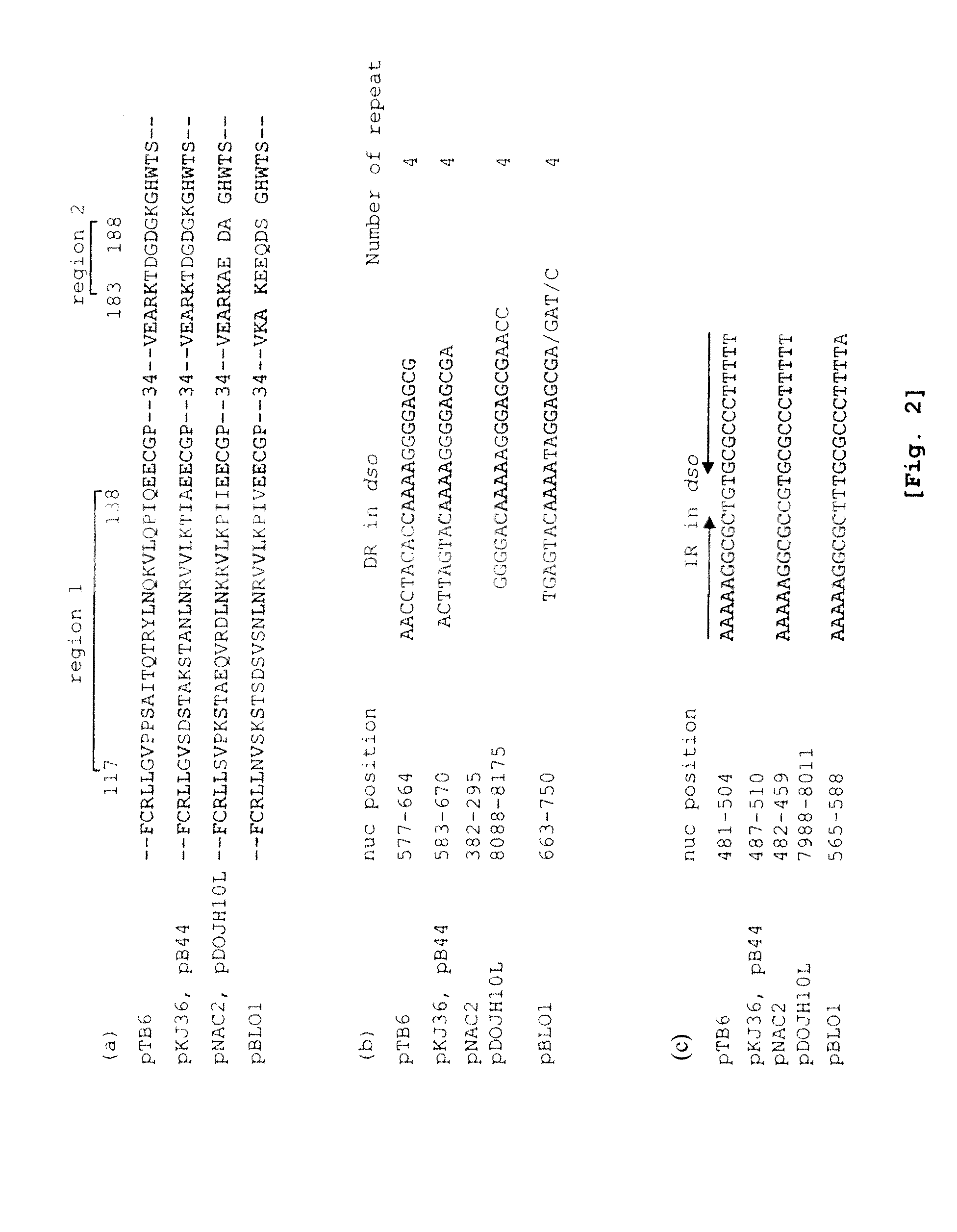
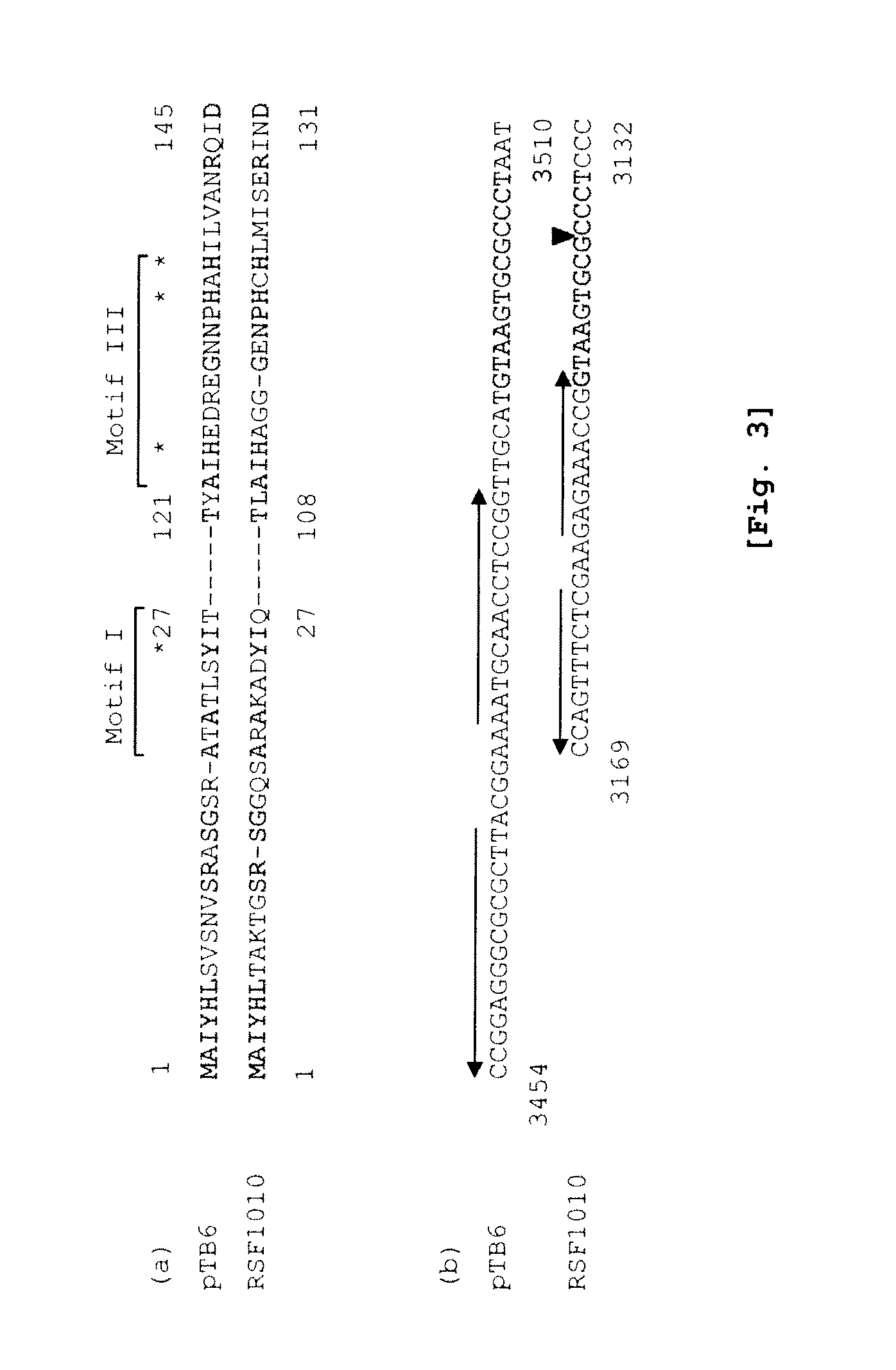
The present invention provides a shuttle vector for a microorganism of the genus Bifidobacterium (BM) and Escherichia coli having a wide host range and a large copy number in BM and capable of highly expressing a desired protein when used as an expression vector; an expression vector capable of expressing a desired gene in BM by use of the shuttle vector; BM transformed with the expression vector; and an antitumor agent comprising the BM as an active ingredient. It comprises a pTB6-derived region portion comprising a replication origin (oriV)-repB region of pTB6 but not comprising MembB, MobA, OrfI, and oriT regions of pTB6 and an Escherichia coli-derived plasmid portion comprising a replication origin (Puc Ori) region of Escherichia coli but having deleted DNA encoding an N-terminal region of an ampicillin resistance gene (ampR) expression product β-lactamase.
Owner:ANAEROPHARMA SCI
Integration-free human induced pluripotent stem cells from blood
InactiveUS20110281281A1Genetically modified cellsArtificial cell constructsOrigin of replicationReprogramming
Provided herein are methods for generating human induced pluripotent stem cells free from genomic integration of exogenous transgenes by transfecting into nucleated blood cells one or more DNA expression vectors (e.g., plasmid vectors) that do not contain a mammalian origin of replication, and encode and permit expression of one or more reprogramming factors (e.g., Oct4, Sox2, Klf4, and c-Myc). Also provided herein are the integration-free human induced pluripotent stem cells obtained by the methods described herein.
Owner:TRUE NORTH THERAPEUTICS
Cloning system for construction of recombinant expression vectors
InactiveUS20040005591A1Rapid and efficient generationImprove throughputSugar derivativesMicrobiological testing/measurementOrigin of replicationDNA
Cloning systems are provided for constructing expression vectors. In one aspect of the invention, a kit is provided for constructing one or more recombinant expression vectors. The kit comprises: a linear driver DNA comprising a promoter sequence, a donor recombination site, and at least one selectable marker, the linear driver DNA being capable of being ligated with one or more linear donor DNA comprising a donor DNA sequence to form one or more circular donor DNA; and a circular acceptor vector comprising an origin of replication and an acceptor recombination site capable of recombining with the circular donor DNA to form the recombinant expression vector for expressing the donor DNA sequence.
Owner:PROTEMATION
Method for assembling PCR fragments of DNA
InactiveUS20050048514A1Simple stepsSugar derivativesMicrobiological testing/measurementOrigin of replicationSite-specific recombination
A process for assembling a series of DNA fragments generated by PCR into an ordered circular arrangement for replication and genetic work in cells. The PCR fragments are made with a modified nucleotide in the primers that can be removed with a DNA excision repair enzyme to generate a 3′ overhang. The 3′ overhangs are designed to allow directional annealing and thus sequential PCR fragments can be assembled by annealing the overhangs and subsequent ligation. Sequential addition of PCR fragments is facilitated by growing the chain on a solid support, and the assembled chain can be removed with a site specific recombinase if the first and last primers contain the recombinase site. The circularized assembled fragment can be directly used for cell transformation if the appropriate sequences are included, such as an origin of replication and a selectable marker.
Owner:RICE UNIV
Vector for expression of heterologous protein and methods for extracting recombinant protein and for purifying isolated recombinant insulin
InactiveUS6068993AFast degenerationOvercome problemsBacteriaInsulinsBacteroidesOrigin of replication
The present invention relates to a vector for expression of a heterologous protein by a Gram negative bacteria, wherein the vector includes a nucleic acid such as DNA encoding the following: an origin of replication region; optionally and preferably a selection marker; a promoter; an initiation region such as translation initiation region and / or a ribosome binding site, at least one restriction site for insertion of heterologous nucleic acid, e.g. DNA, encoding the heterologous protein, and a transcription terminator. The inventive vector may contain DNA encoding the heterologous protein, e.g., pro-insulin such as pro-insulin with a His tag. Additionally, the invention provides a method for extracting a recombinant protein from within a recombinant Gram negative bacteria having a cell membrane, without lysing the bacteria, as well as a method for purifying an isolated recombinant human insulin, wherein the isolated recombinant human pro-insulin is subjected to sulfitolysis, Ni-chelation chromatography, renaturation, limited proteolysis and chromatography separation to provide purified, isolated, recombinant human insulin.
Owner:BIOMM +1
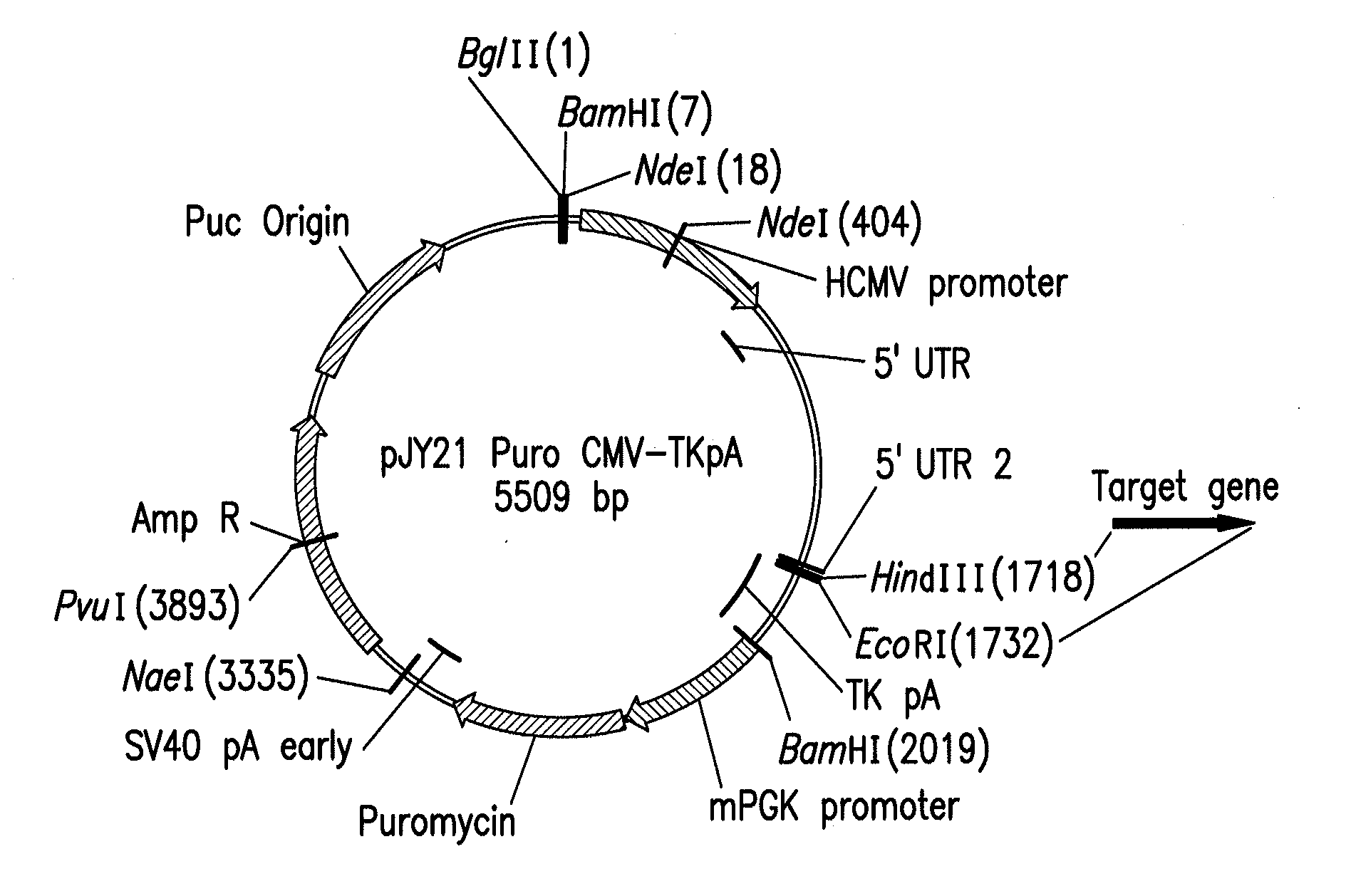
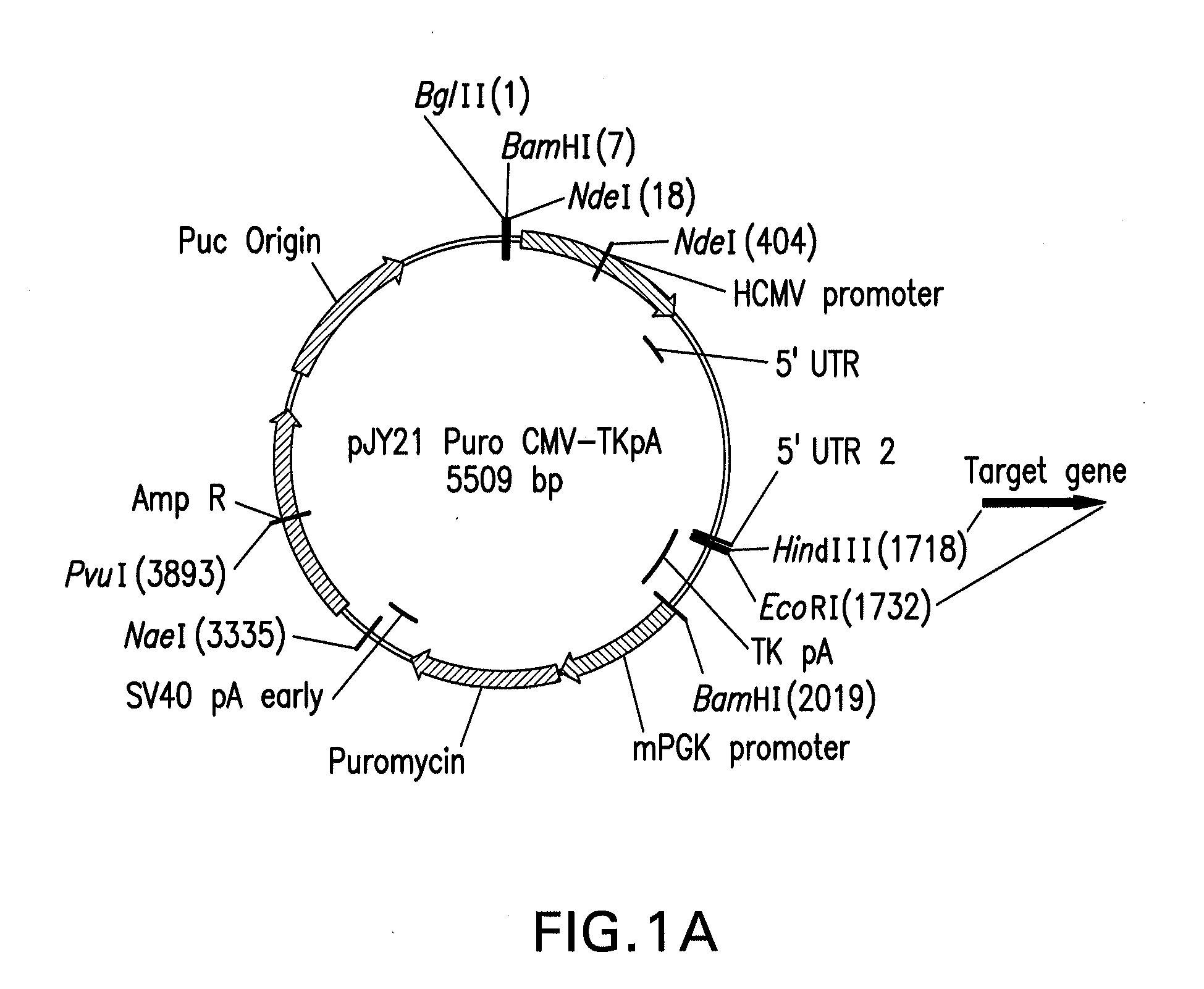
Mammalian expression vector pUHAB
The present invention relates to the construction and utilization of a new mammalian expression vector that contains a unique multiple cloning site (MCS), designated pUHAB. The pUHAB vector comprises a high copy replication origin (ColE1), a drug resistance gene (TK-Hygromycin), and a human cytomegalovirus promoter operably associated with a unique intron (hCMV / intron). Further, pUHAB comprises a selectable marker conferring resistance to kanamycin in bacterial cells, and a phage f1(+) region. pUHAB can be used to transiently or stably express cloned genes when transfected into mammalian cells. The invention also encompasses kits and host cells and cell lines comprising pUHAB, and methods of producing a recombinant protein using pUHAB.
Owner:MERCK SHARP & DOHME CORP
Methods for Adeno-Associated Viral Vector Production
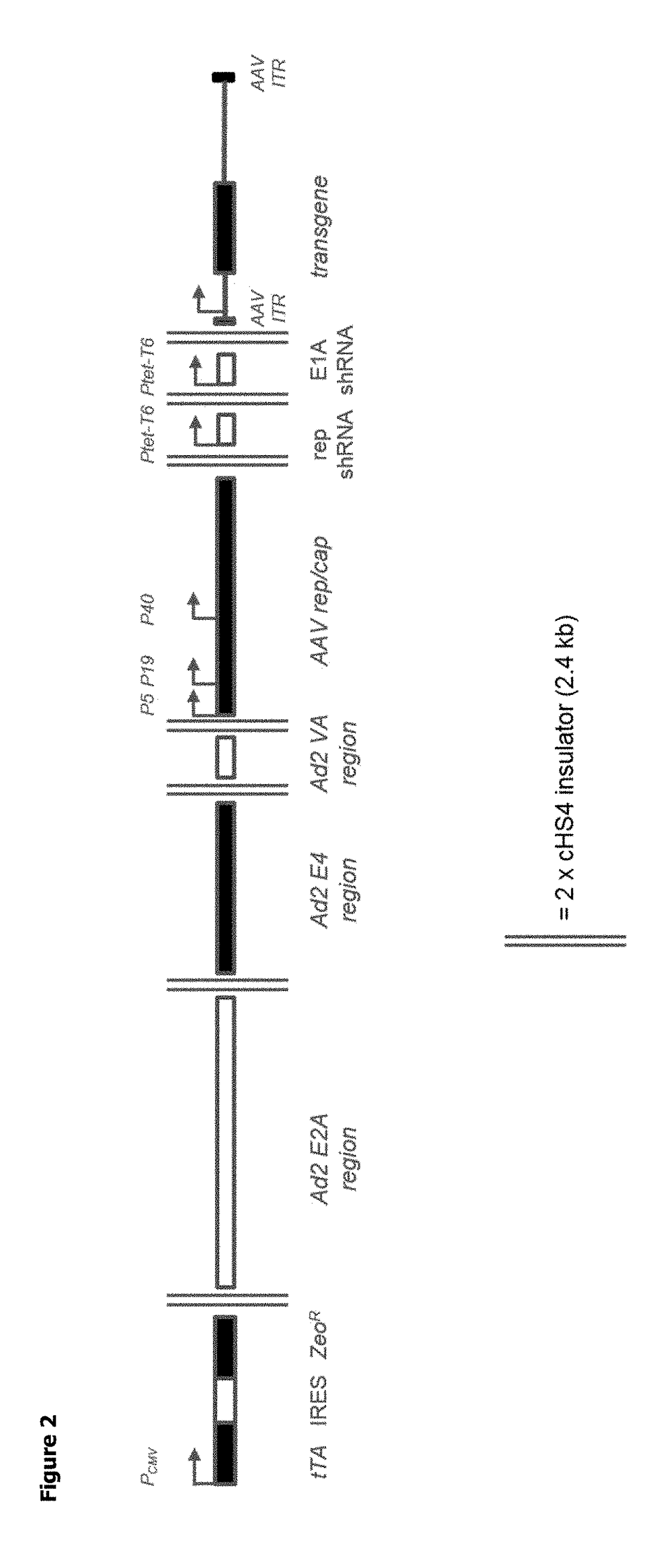
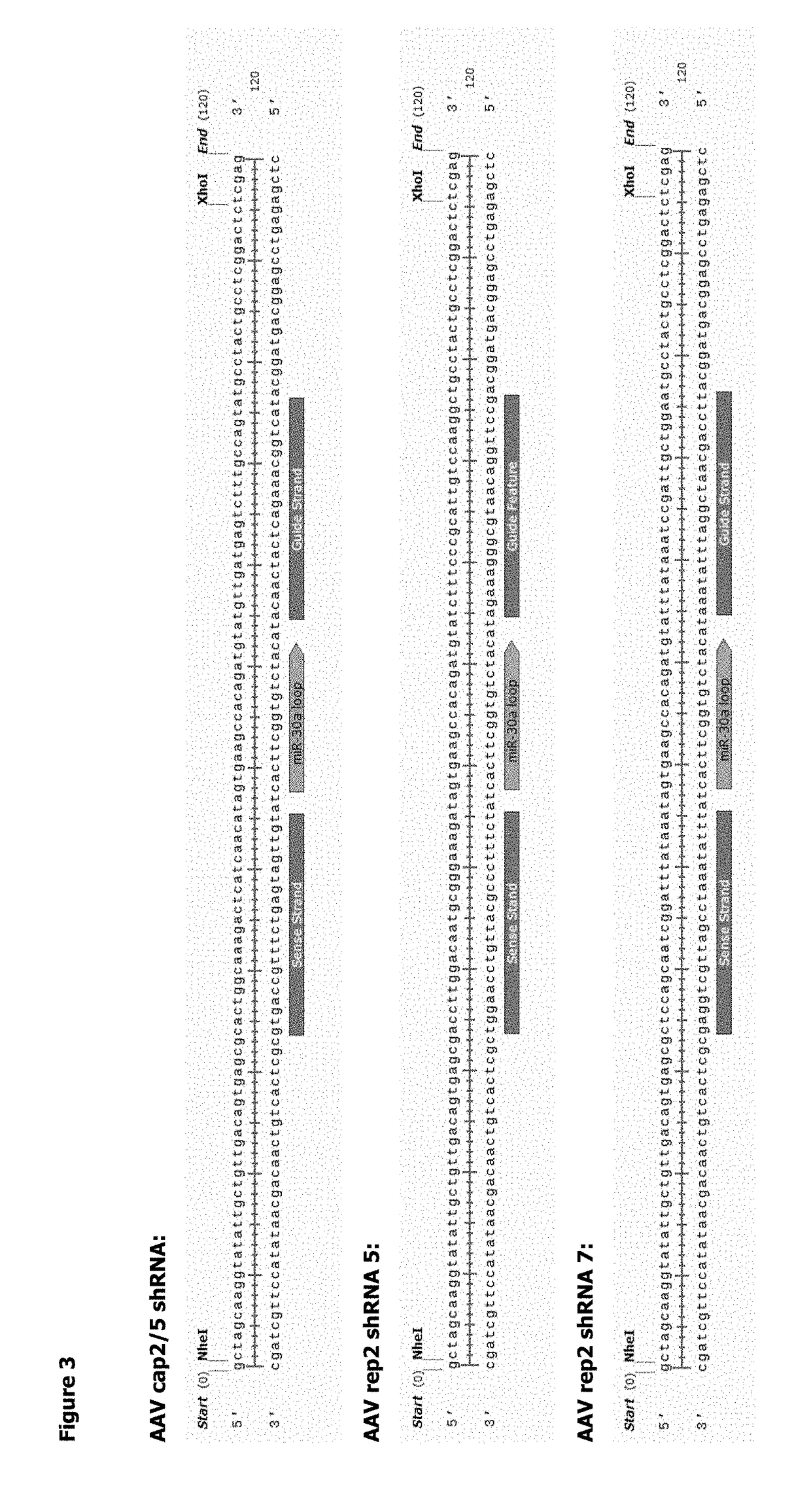
ActiveUS20180327722A1Easy to optimizeSimple working processVirus peptidesDsDNA virusesOrigin of replicationAdeno associate virus
The invention relates to an adeno-associated virus (AAV) producer cell comprising nucleic acid sequences encoding: rep / cap gene; helper virus genes; and the DNA genome of the AAV vector particle, wherein the nucleic acid sequences are all integrated together at a single locus within the AAV producer cell genome. The invention also relates to nucleic acid vectors comprising a non-mammalian origin of replication and the ability to hold at least 25 kilobases (kb) of DNA, characterized in that the nucleic acid vectors comprise nucleic acid sequences encoding: rep / cap gene, and helper virus genes. The invention also relates to uses and methods using the nucleic acid vectors in order to produce stable AAV packaging and producer cell lines.
Owner:GLAXOSMITHKLINE INTPROP DEV LTD
Expression vectors for recombinant protein production in mammalian cells
The invention provides expression vectors that support high levels of polypeptide expression in mammalian cells. The vectors contain at least one expression cassette for a target polypeptide; an expression cassette for a eukaryotic selectable marker protein; an expression cassette for a bacterial selectable marker protein, and a bacterial plasmid origin of replication.
Owner:MERCK SHARP & DOHME CORP
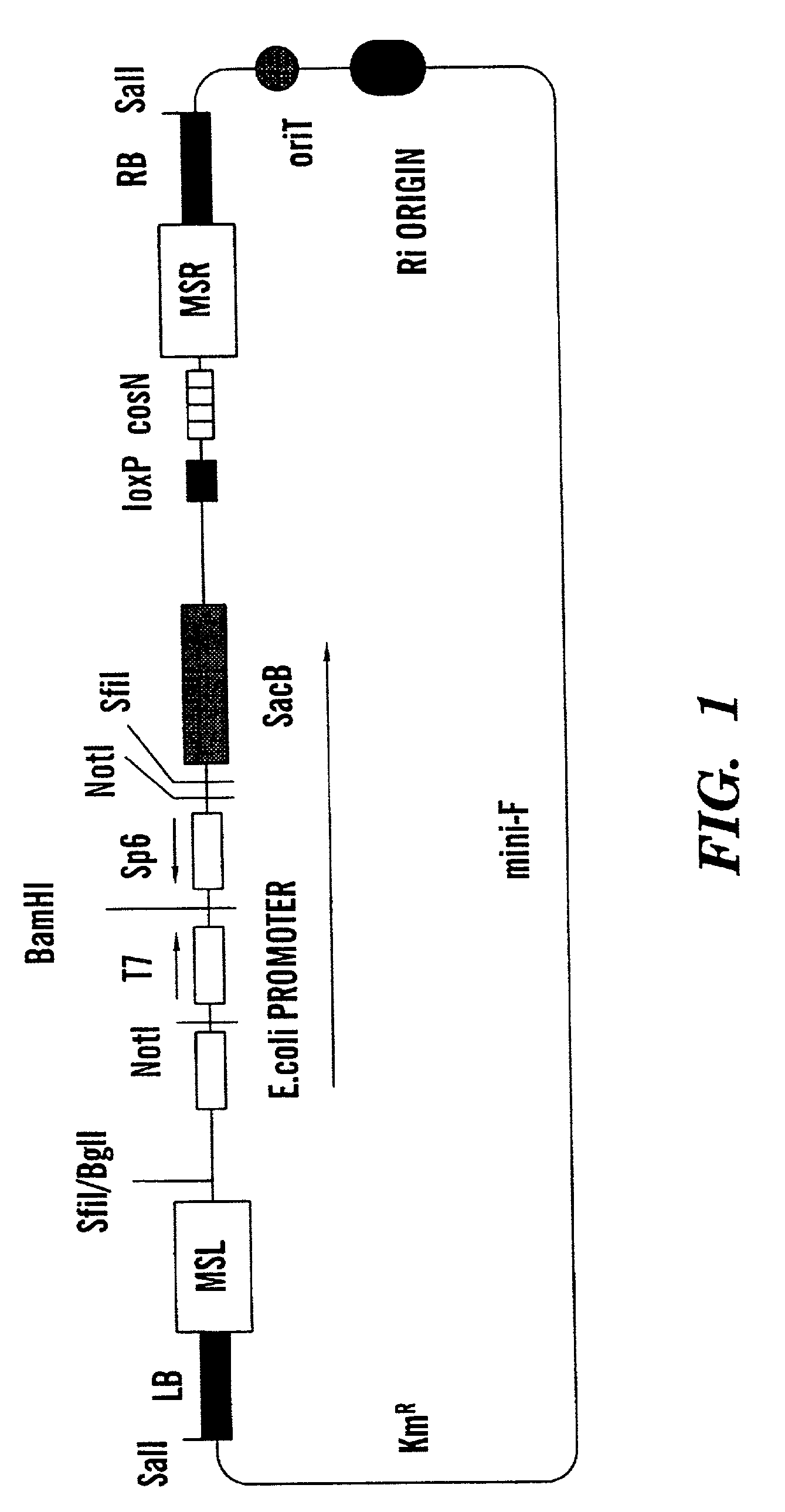
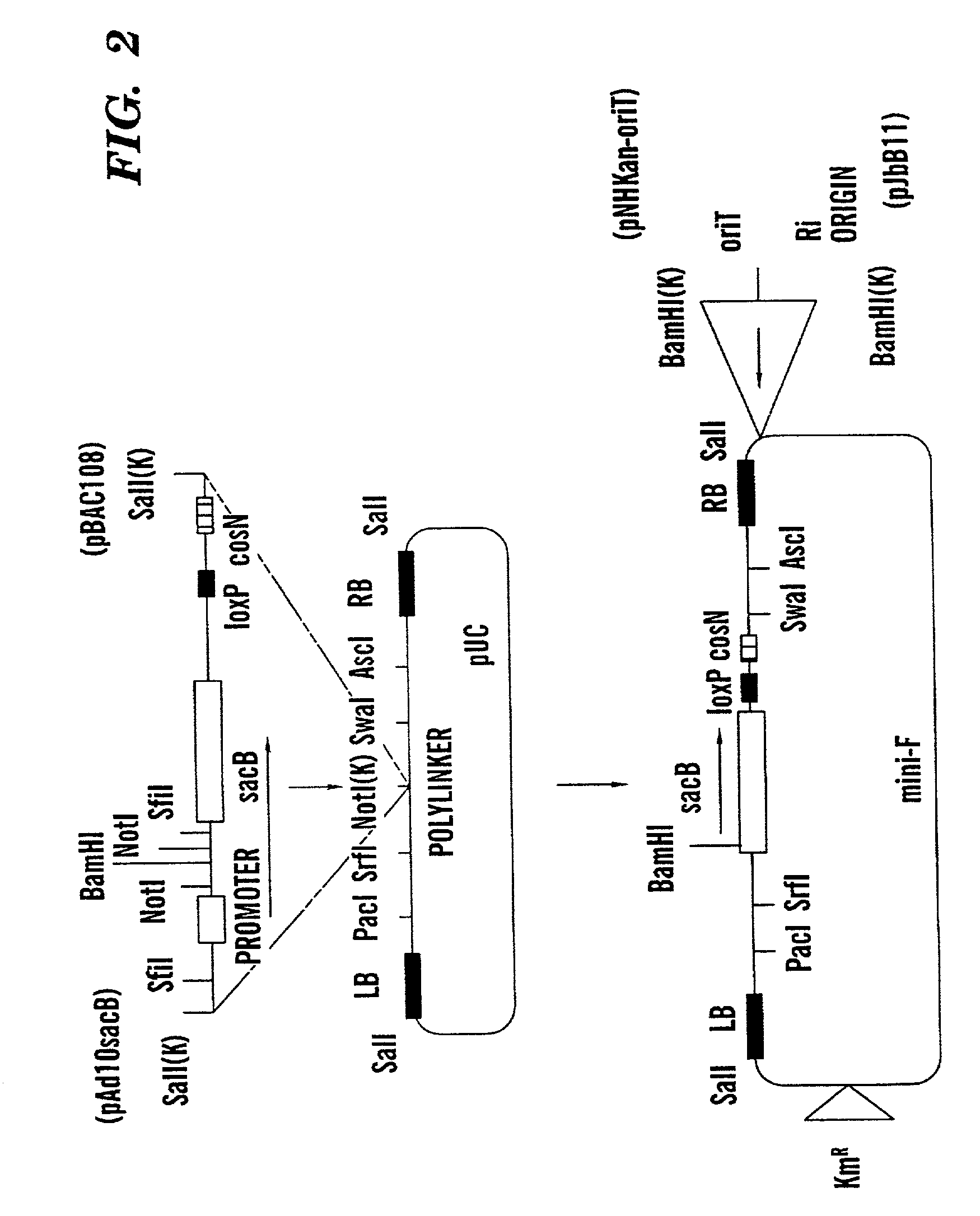
Binary BAC vector and uses thereof
InactiveUS20020123100A1Raise the possibilityStable maintenanceFungiBacteriaOrigin of replicationEscherichia coli
The present invention provides a method for transferring and expressing heterologous DNA in a non-plant host cell. The vector used in this method includes a backbone having a first origin of replication capable of maintaining heterologous DNA as a single copy in an Escherichia coli host cell. The vector further includes a unique restriction endonuclease cleavage site for insertion of heterologous DNA, and left and right Agrobacterium T-DNA border sequences flanking the unique restriction endonuclease cleavage site. In certain host cells, the T-DNA border sequences allow introduction of heterologous DNA located between the left and right T-DNA border sequences into a host cell. In preferred embodiments, the vector includes a second origin of replication capable of maintaining heterologous DNA as a single copy in a host cell such as Agrobacterium species or other prokaryotic cells.
Owner:CORNELL RES FOUNDATION INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com