Avoidance of undesirable replication intermediates in plasmid propagation
a technology of undesirable replication intermediates and plasmids, applied in the direction of dna/rna vaccination, biocide, antibody medical ingredients, etc., can solve the problems of reducing and reducing the yield of desired products. , to achieve the effect of increasing the distance between the origin of replication
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
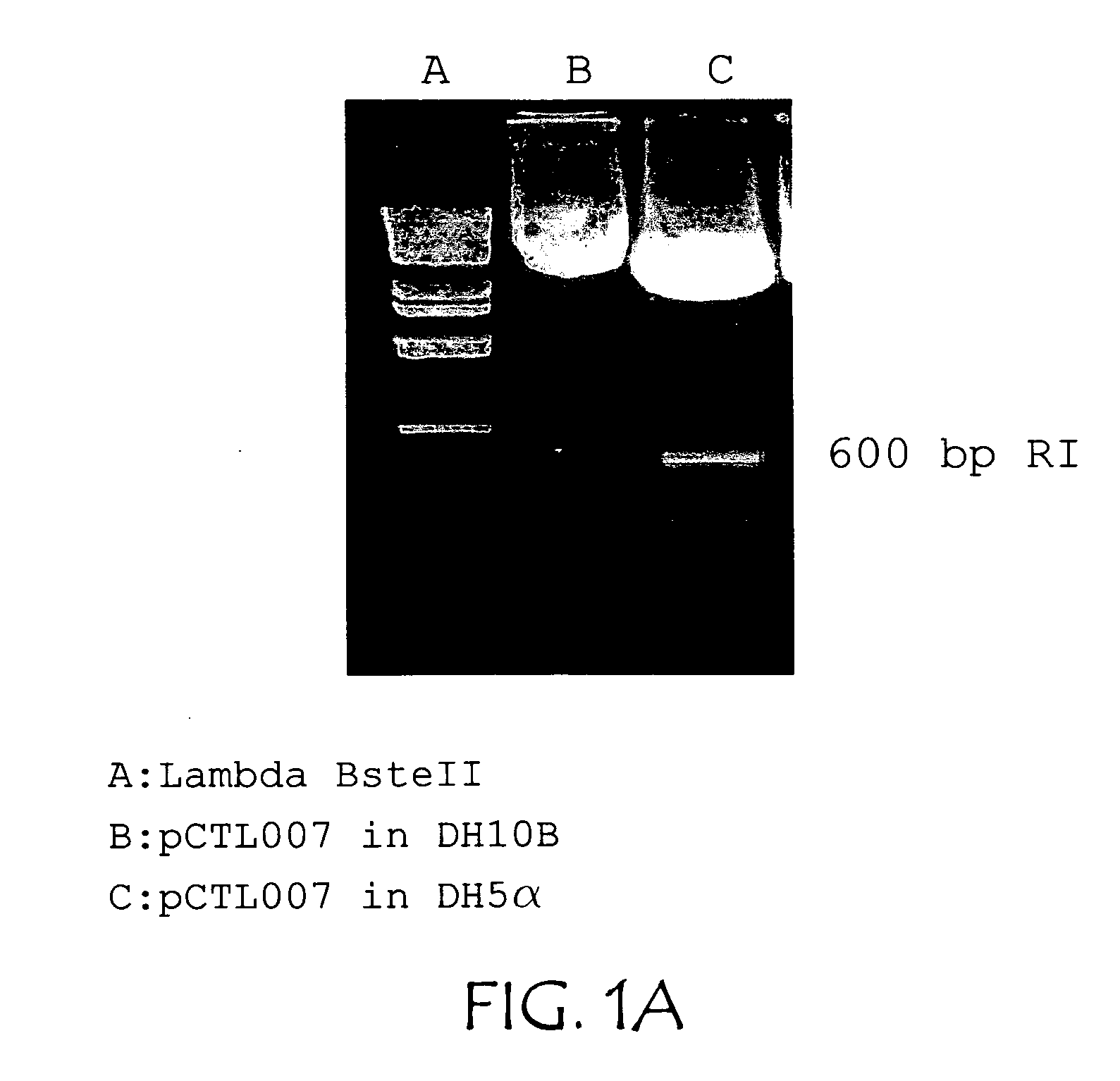
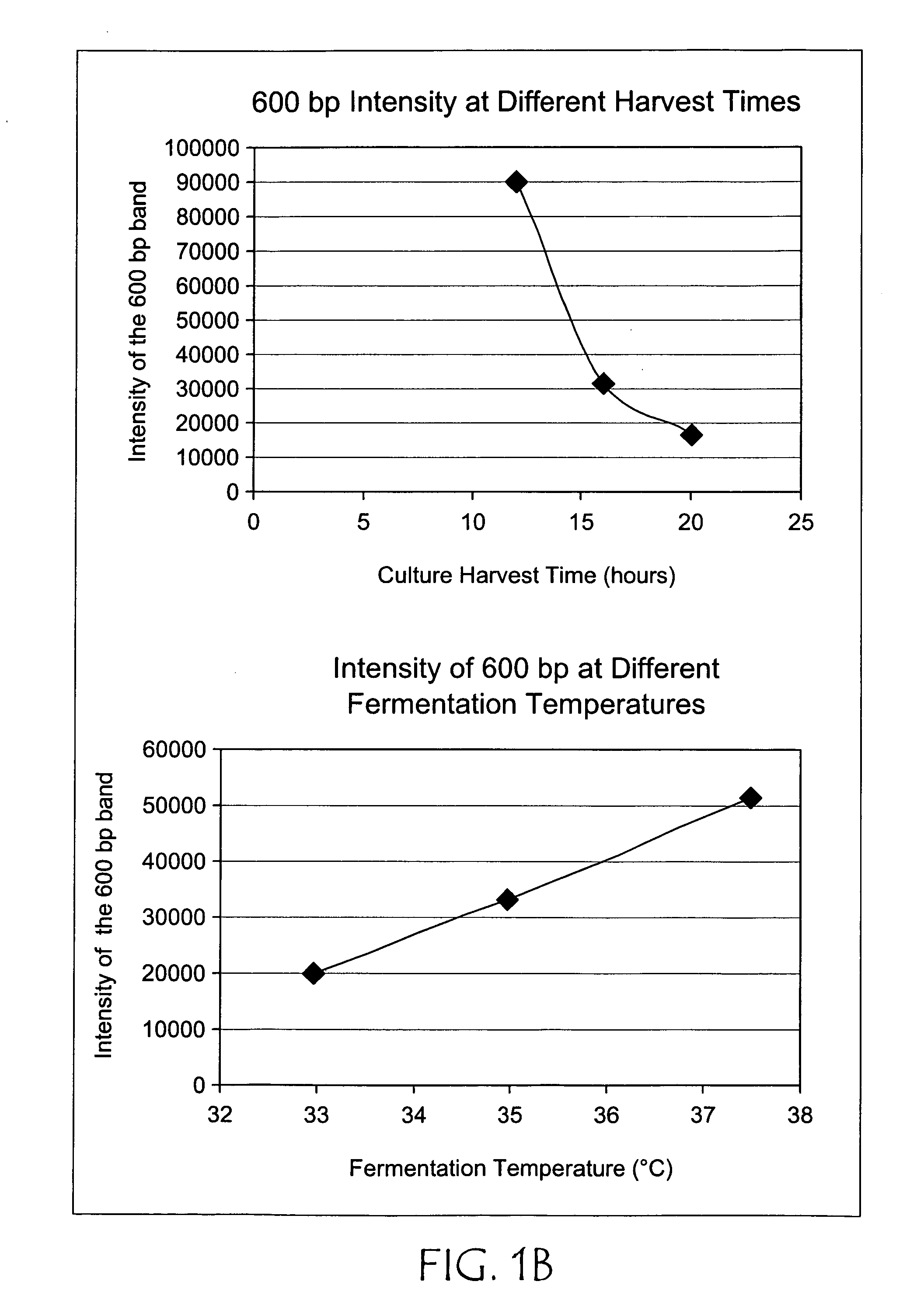
[0034] The plasmid pCTL002, a pVAX1 derivative, was propagated using in two different bacterial host strains, DH5α (sold by Life Technologies, Rockville, Md.) and DH10B (also known as Top10 as sold by Invitrogen Corporation, Carlsbad, Calif.) in 1.5 liter cultures using LB+kanamycin at 37° C. for 16 hrs. at 250 rpm in a rotary shaking incubator. Approximately two-thirds less replication intermediate accumulated in DH10B compared to DH5α (see FIG. 1A). Using the above conditions and DH10B the effect of temperature was examined by comparing cultures grown at 33, 35, and 37° C. and it was found that the ratio of plasmid to replication intermediate could be improved approximately 2.5-fold (see FIG. 1B). Twelve, sixteen, and twenty hour cultures were also compared, indicating that longer cultures resulted in less accumulation of replication intermediate relative to plasmid (FIG. 1B). In other experiments no effect of speed of shaking was noted.
example 2
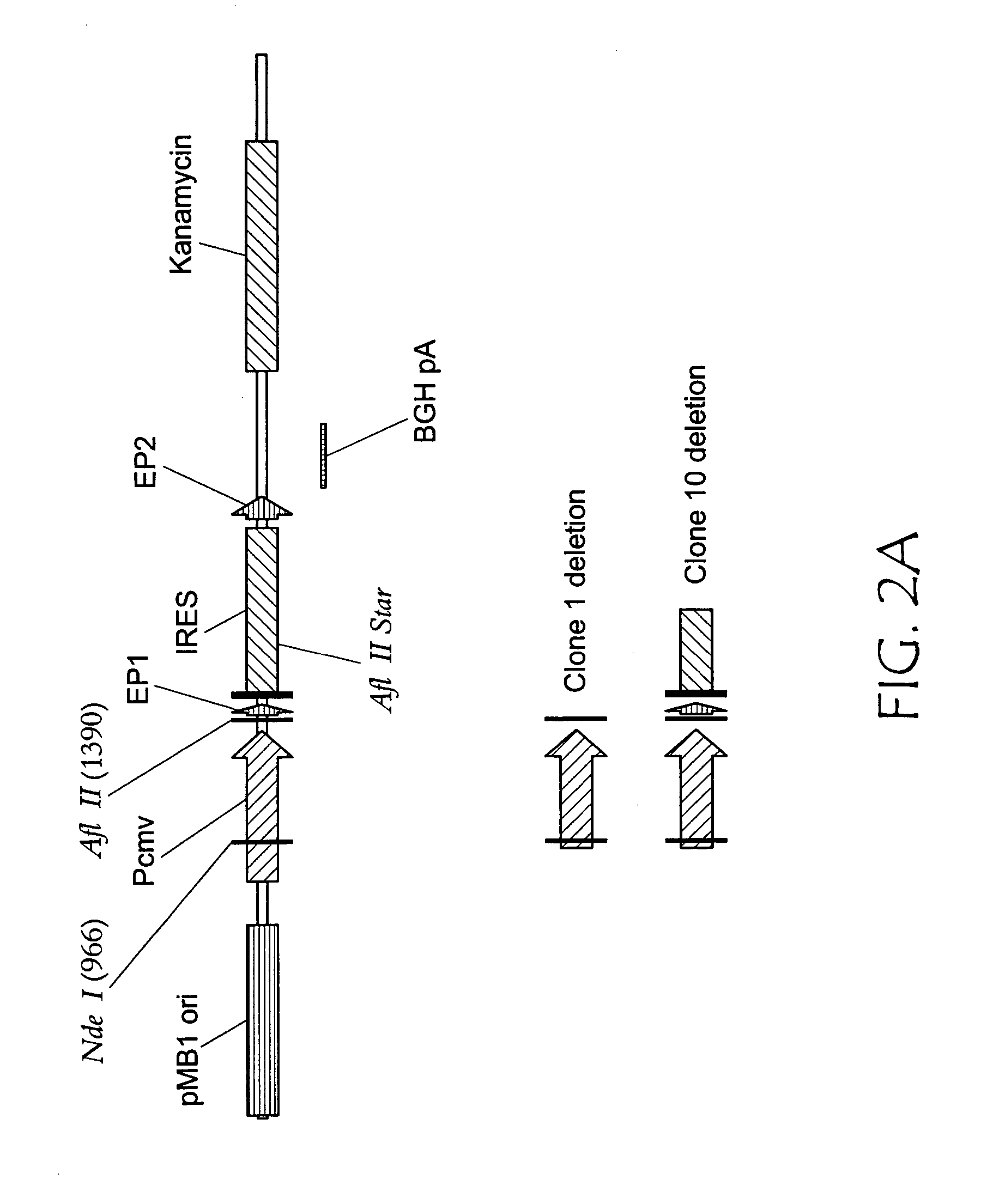
[0035] Two deletions were prepared in pCTL007 beginning at the NdeI site located midway through the CMV enhancer and continuing to either 1) the AflII site 5′ of start codon; or 2) an AflII* site ˜100 bases into the IRES (see FIG. 2A). Deletion 1) eliminated the ˜600 bp intermediate as well as an ˜360 bp intermediate (see FIG. 2B, clone 1), A ˜300 bp intermediate was unaffected. Deletion 2) eliminated all of the replication intermediates (see FIG. 2B, clone 10).
[0036] More concentrated material and gels providing greater resolution reveal a total of five replication intermediates in pCTL007 preparations (see FIG. 2C): The ˜300 bp band above is actually a doublet of 280 and 310 bp bands. Additionally there is a faint band at 490 bp. The ˜600 bp band can also be seen to be closer to 575 bp. All of these sizes are based on relative mobility of the bands versus commercial size standards in agarose gel electrophoresis and assessed by eye, and are thus still approximate. The cloning proc...
example 3
[0037] The 411 bp NdeI-NheI fragment of pCTL007, corresponding to most of the CMV promoter, but excluding the 18- and 16-base pair repeats at −427 to −410 and −413 to −398 of the CMV sequence, was replaced with an RSV promoter plus variable amounts of the removed CMV sequences. One construct, effectively deleting −209 onward of the CMV sequence and thus the termination site giving rise to the 580 bp fragment, nonetheless still gives rise to a fragment of similar size (see FIG. 3, clone 3). The termination site for this fragment is within the RSV promoter sequences, in the general vicinity of the transcriptional start site. Binding sites for several transcription factors are present although similarity to the transcription factor binding sites represented by the CMV repeat sequences is not readily discerned.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Digital information | aaaaa | aaaaa |
| Digital information | aaaaa | aaaaa |
| Distance | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com