Patents
Literature
15405 results about "Catheter device" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
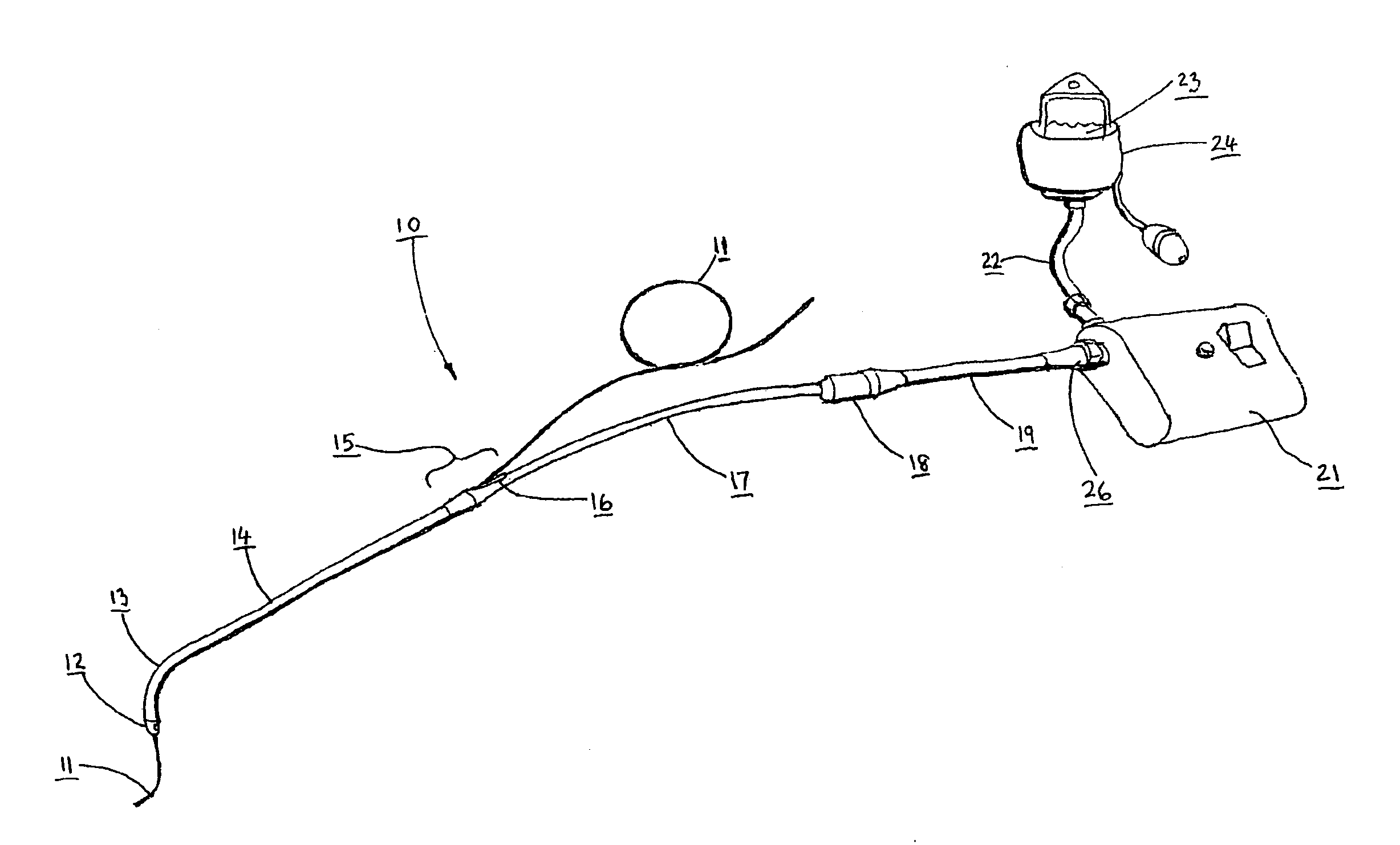
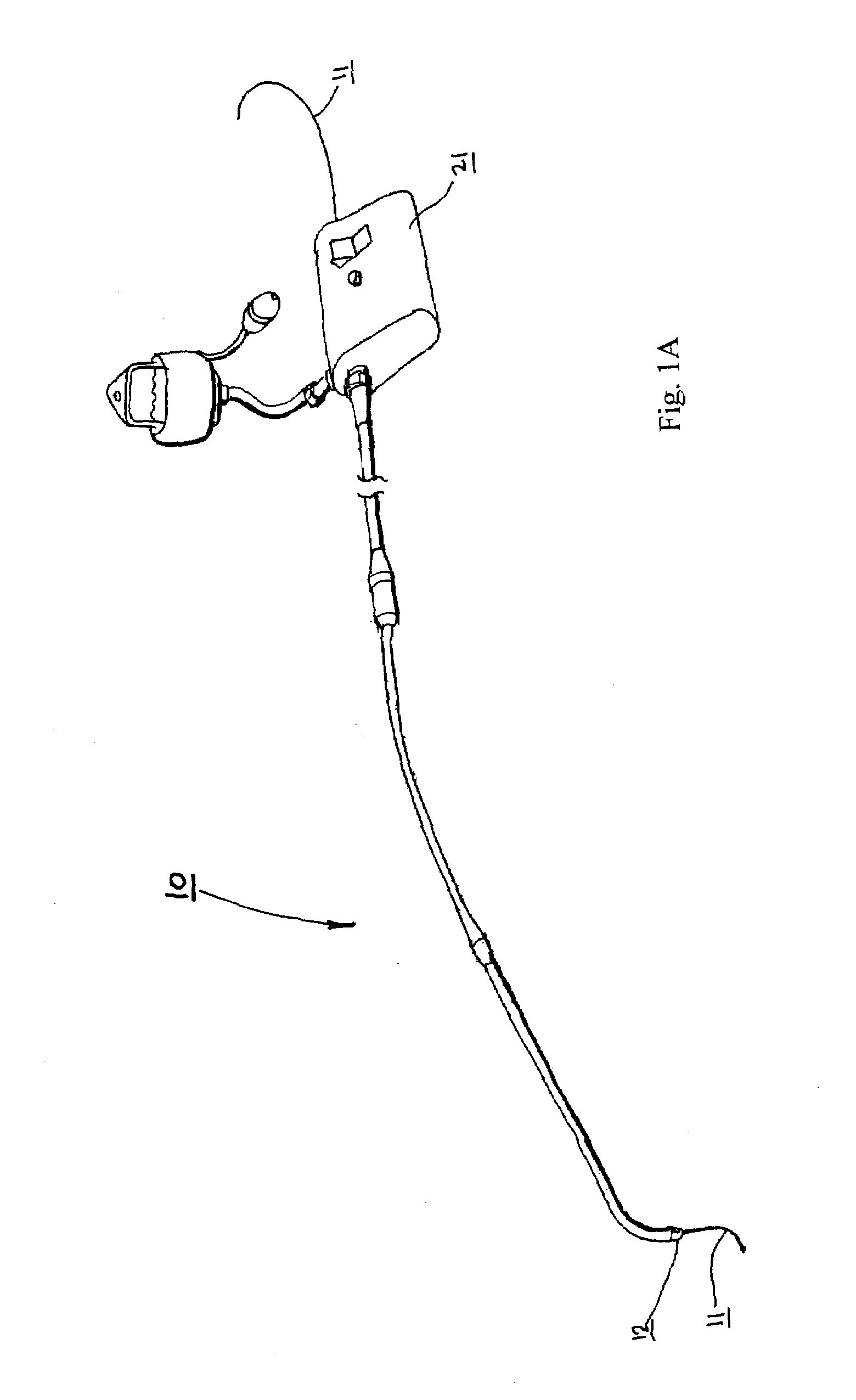
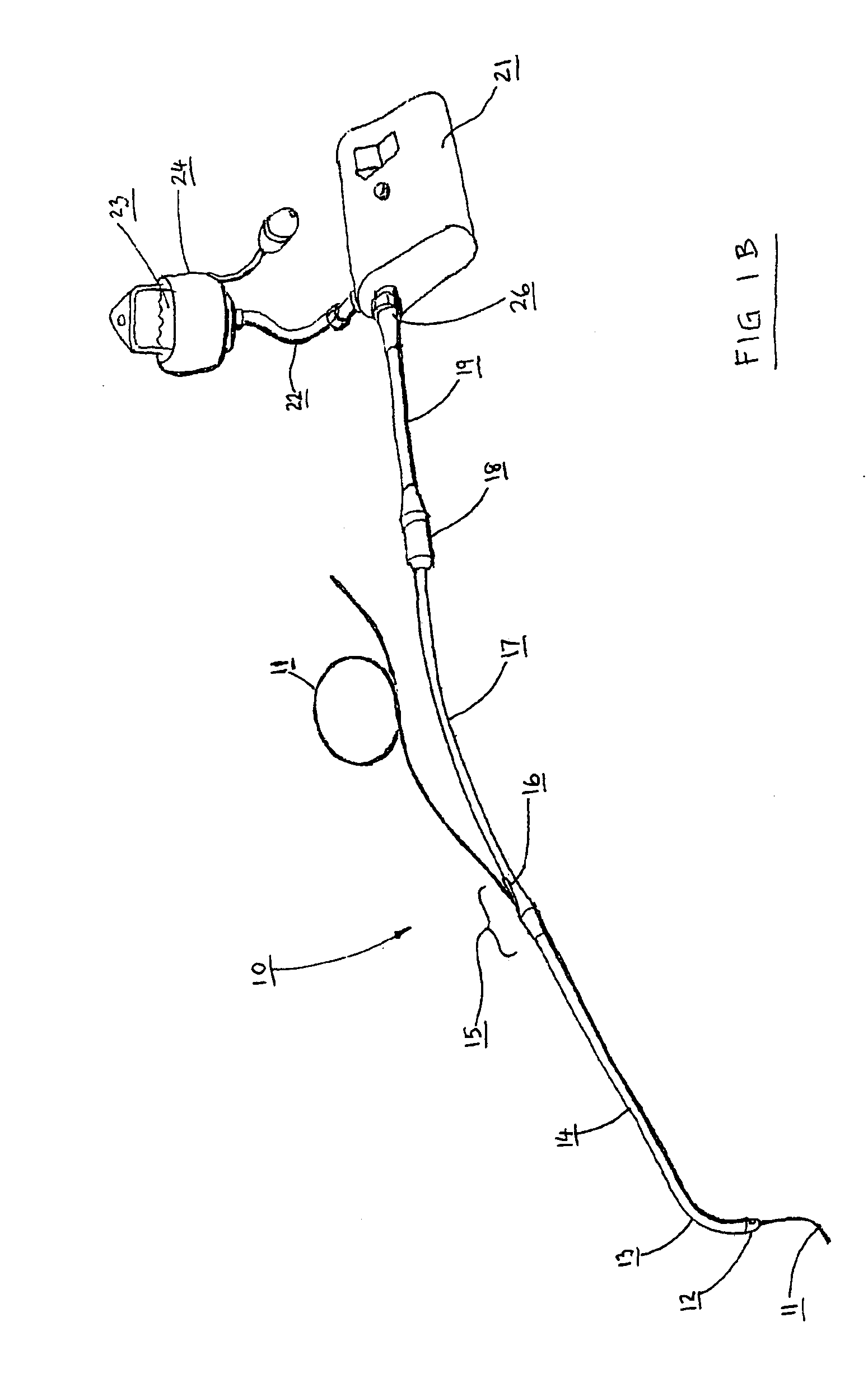
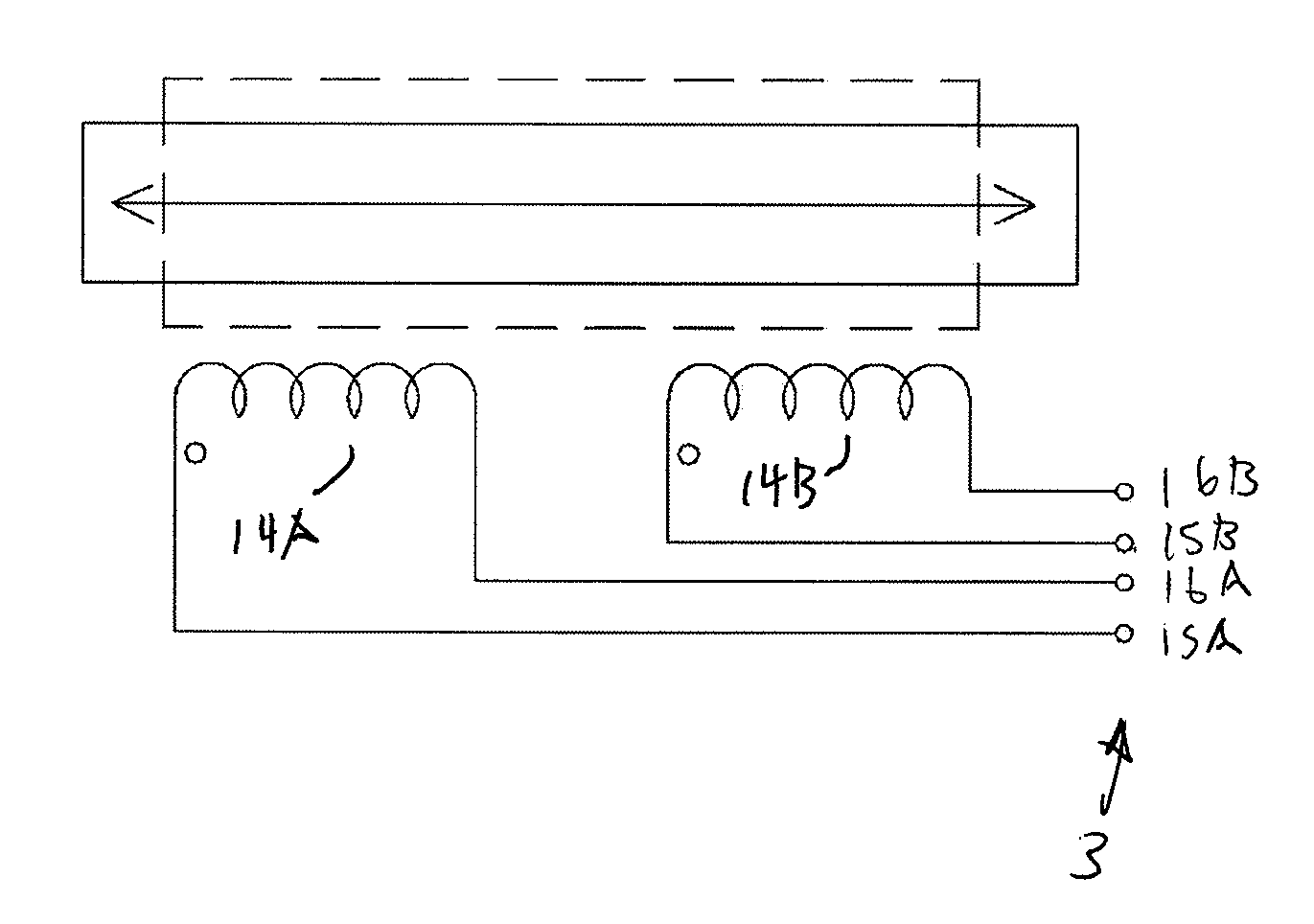
Catheters are medical devices that can be inserted in the body to treat diseases or perform a surgical procedure. By modifying the material or adjusting the way catheters are manufactured, it is possible to tailor catheters for cardiovascular, urological, gastrointestinal, neurovascular, and ophthalmic applications.
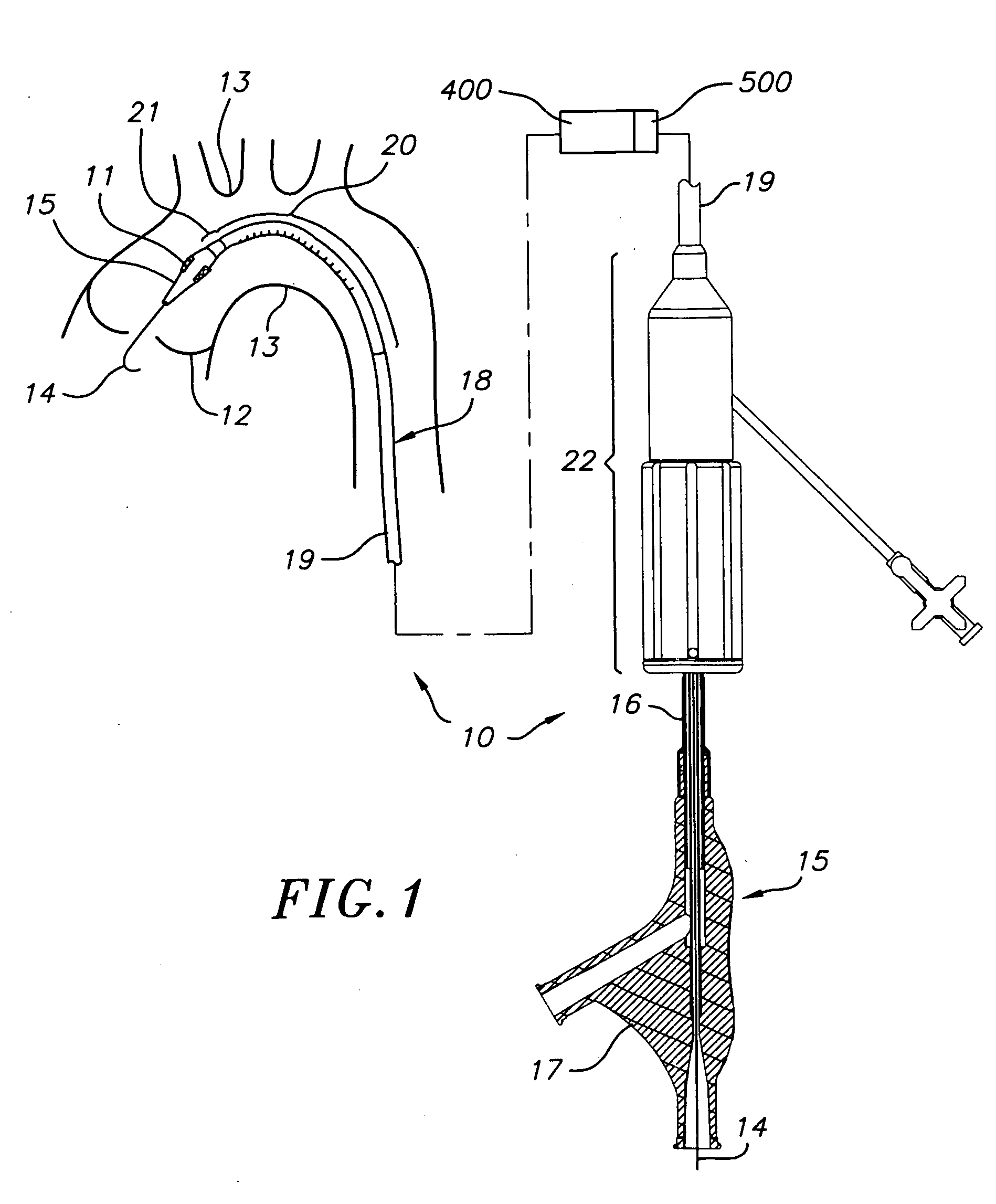
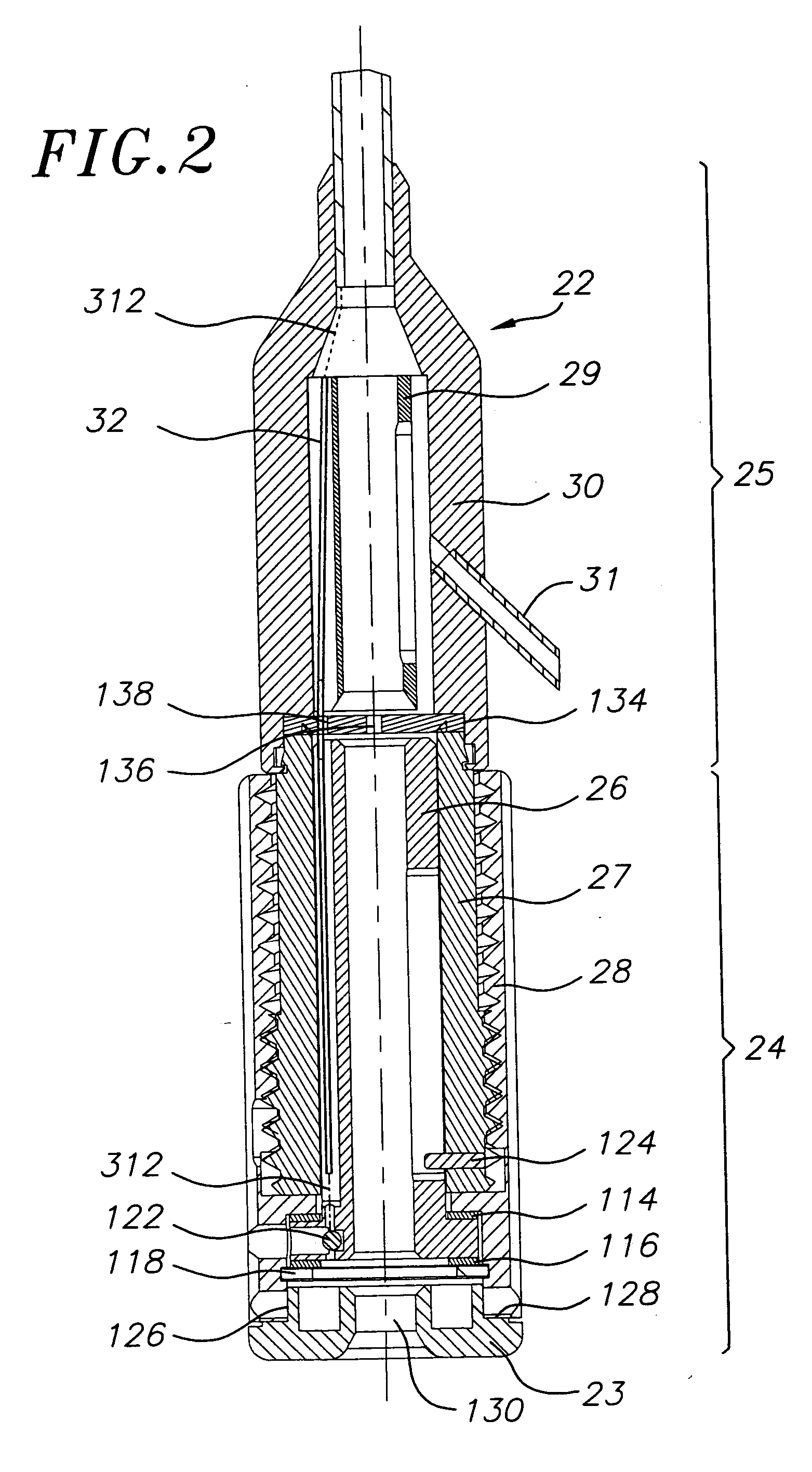
Prosthetic valve for transluminal delivery
InactiveUS7018406B2Preventing substantial migrationEliminate the problemBalloon catheterHeart valvesProsthesisCommissure
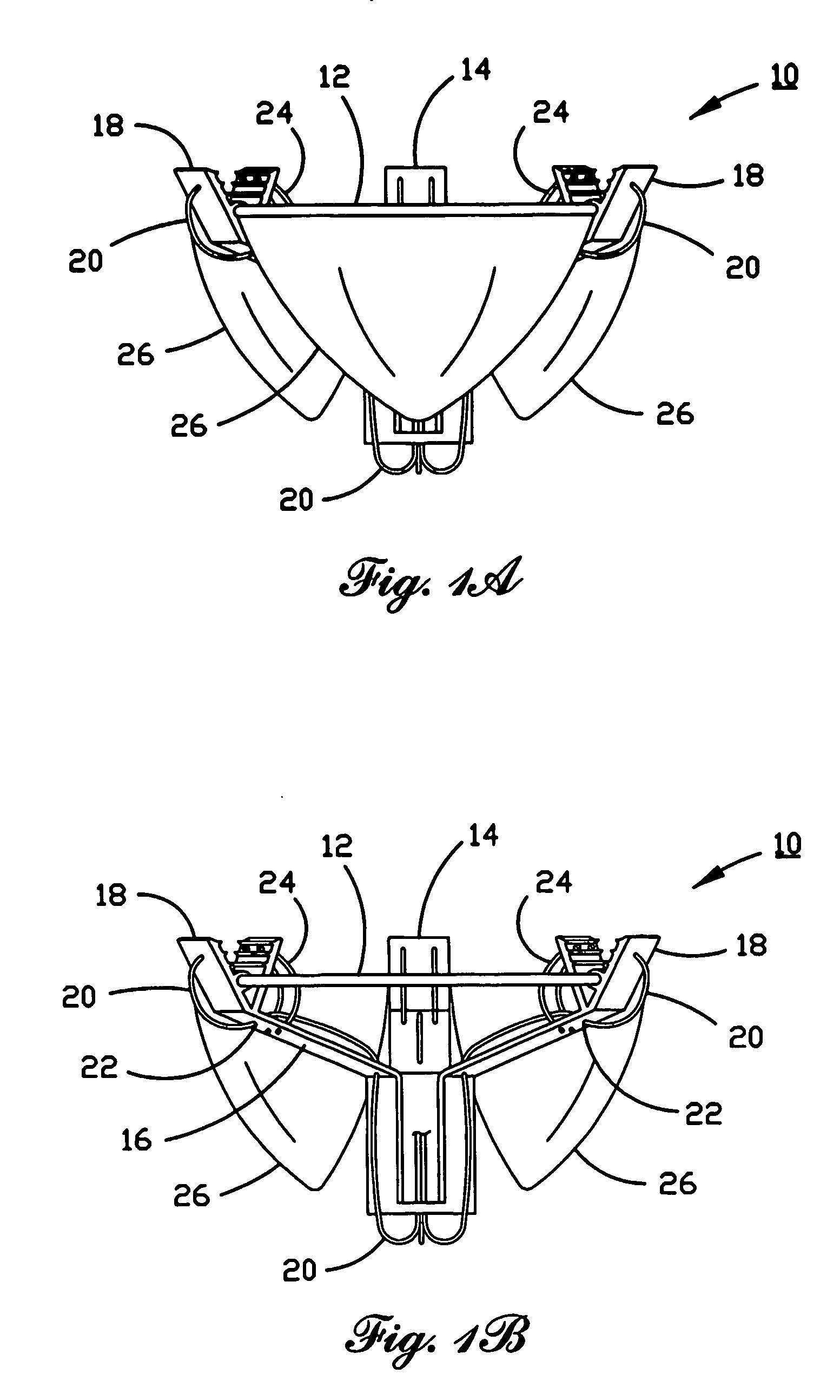
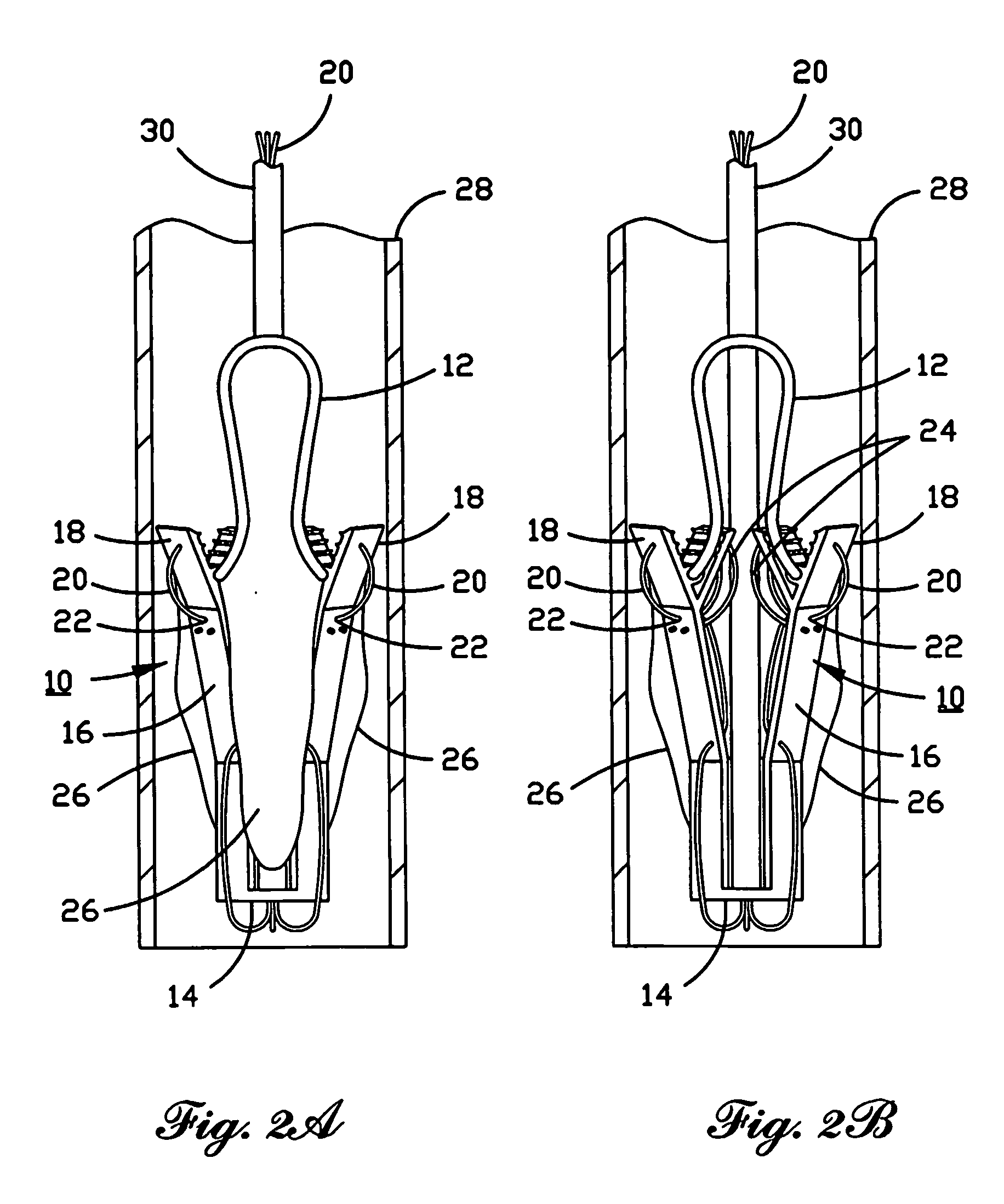
A prosthetic valve assembly for use in replacing a deficient native valve comprises a replacement valve supported on an expandable valve support. If desired, one or more anchor may be used. The valve support, which entirely supports the valve annulus, valve leaflets, and valve commissure points, is configured to be collapsible for transluminal delivery and expandable to contact the anatomical annulus of the native valve when the assembly is properly positioned. The anchor engages the lumen wall when expanded and prevents substantial migration of the valve assembly when positioned in place. The prosthetic valve assembly is compressible about a catheter, and restrained from expanding by an outer sheath. The catheter may be inserted inside a lumen within the body, such as the femoral artery, and delivered to a desired location, such as the heart. When the outer sheath is retracted, the prosthetic valve assembly expands to an expanded position such that the valve and valve support expand within the deficient native valve, and the anchor engages the lumen wall.
Owner:MEDTRONIC COREVALVE
Anastomosis apparatus for use in intraluminally directed vascular anastomosis
InactiveUS6248117B1Surgical needlesSurgical staplesVascular anastomosisMinimally invasive procedures
The present invention relates to new and useful apparatus for use with an intraluminally directed anvil apparatus for intraluminally directed vascular anastomosis of an end of a graft vessel to the wall of a receiving blood vessel that is performed according to a minimally invasive procedure. The intraluminally directed vascular anastomosis does not require the interruption of blood flow in the receiving blood vessel and it is versatile enough to suitably combine a variety of cutting, welding, soldering, sealing, and joining techniques such as stapling. The intraluminally directed anvil apparatus comprises an anvil and a wire used for signaling the optimal anastomosis site; this signaling can be performed when the initial exploration is performed. The intraluminally directed anvil apparatus is typically used with a catheter.
Owner:VITAL ACCESS CORP
Implantable prosthetic valve
InactiveUS6893460B2Prevent blood flowImprove sealingStentsBalloon catheterGuide tubeBiomedical engineering
A valve prosthesis device is disclosed suitable for implantation in body ducts. The device comprises support stent, comprised of a deployable construction adapted to be initially crimped in a narrow configuration suitable for catheterization through the body duct to a target location and adapted to be deployed by exerting substantially radial forces from within by means of a deployment device to a deployed state in the target location, the support stent provided with a plurality of longitudinally rigid support beams of fixed length; valve assembly comprising a flexible conduit having an inlet end and an outlet, made of pliant material attached to the support beams providing collapsible slack portions of the conduit at the outlet. When flow is allowed to pass through the valve prosthesis device from the inlet to the outlet the valve assembly is kept in an open position, whereas a reverse flow is prevented as the collapsible slack portions of the valve assembly collapse inwardly providing blockage to the reverse flow.
Owner:EDWARDS LIFESCI PVT
Automated annular plication for mitral valve repair
InactiveUS20060020336A1Reducing mitral regurgitationImprove efficiencyAnnuloplasty ringsSurgical staplesCoronary sinusMitral valve leaflet
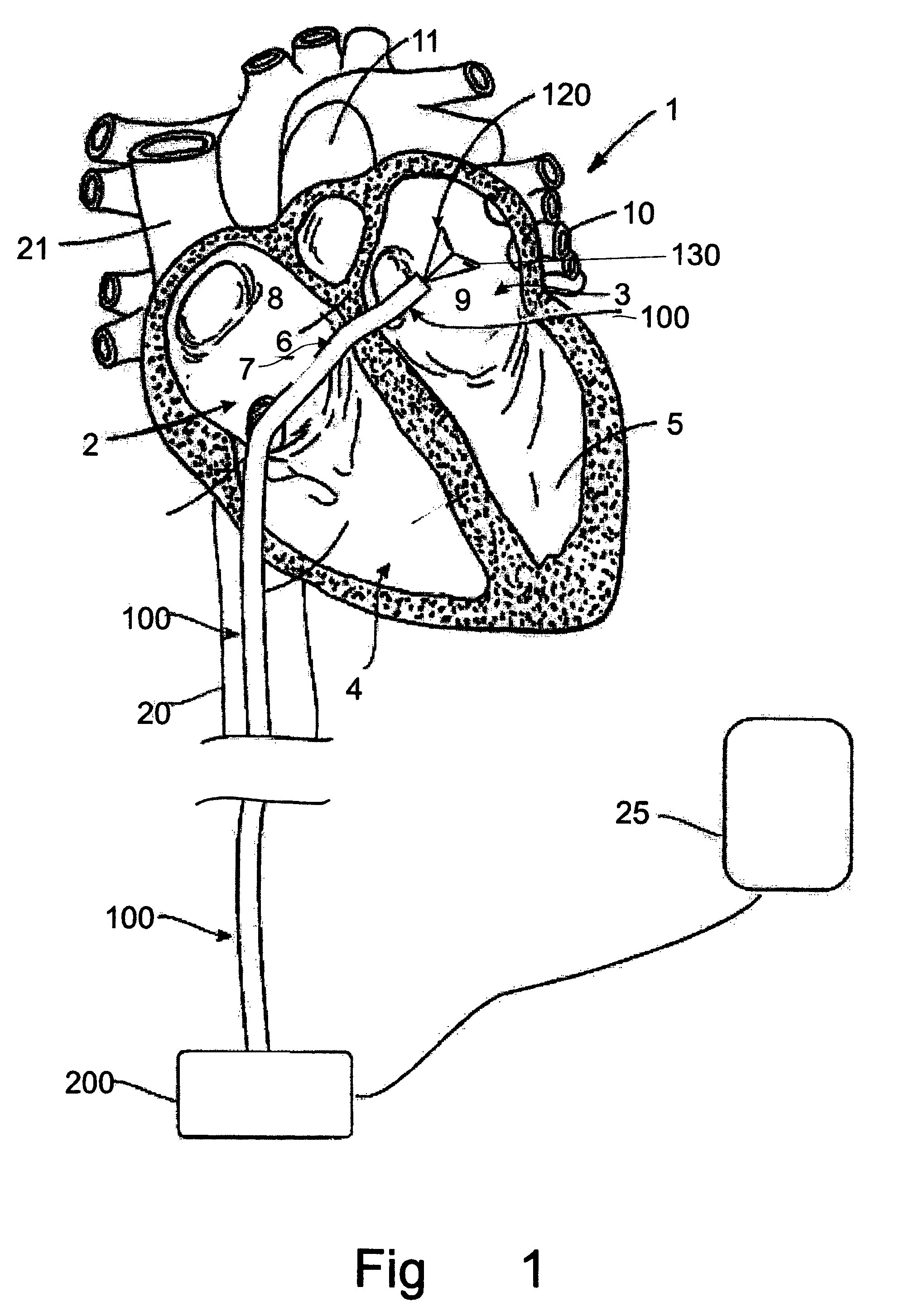
A method for reducing mitral regurgitation comprising: providing a plication assembly comprising a first anchoring element, a second anchoring element, and a linkage construct connecting the first anchoring element to the second anchoring element; positioning the first anchoring element in the coronary sinus adjacent to the mitral annulus, and positioning the second anchoring element in another area of the mitral annulus so that the linkage construct extends across the opening of the mitral valve and holds the mitral valve in a reconfigured configuration so as to reduce mitral regurgitation. An apparatus for reducing mitral regurgitation comprising: a plication assembly comprising a first anchoring element, a second anchoring element, and a linkage construct connecting the first anchoring element to the second anchoring element; and a catheter adapted to deliver the first anchoring element to the coronary sinus.
Owner:VIACOR INC
Surgical clip
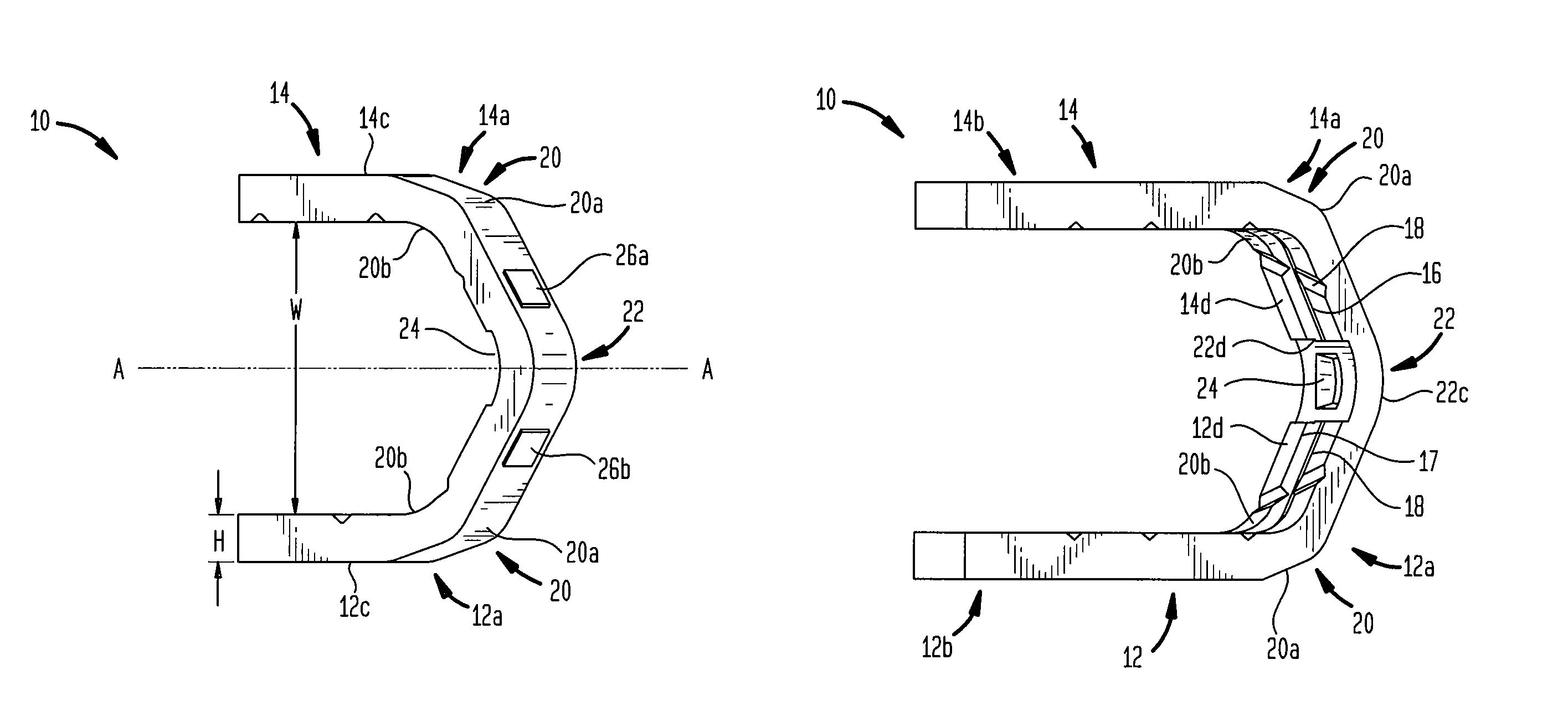
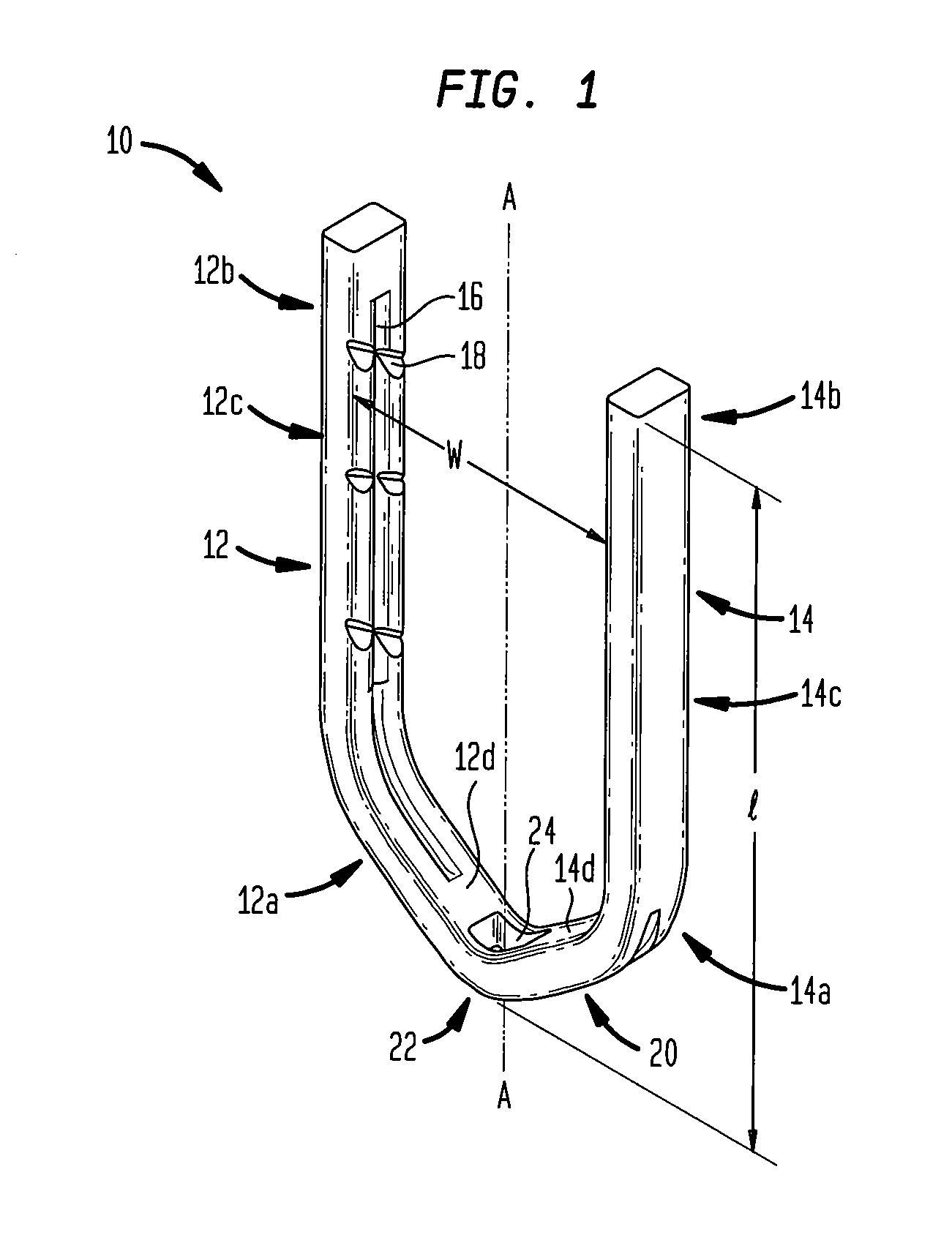
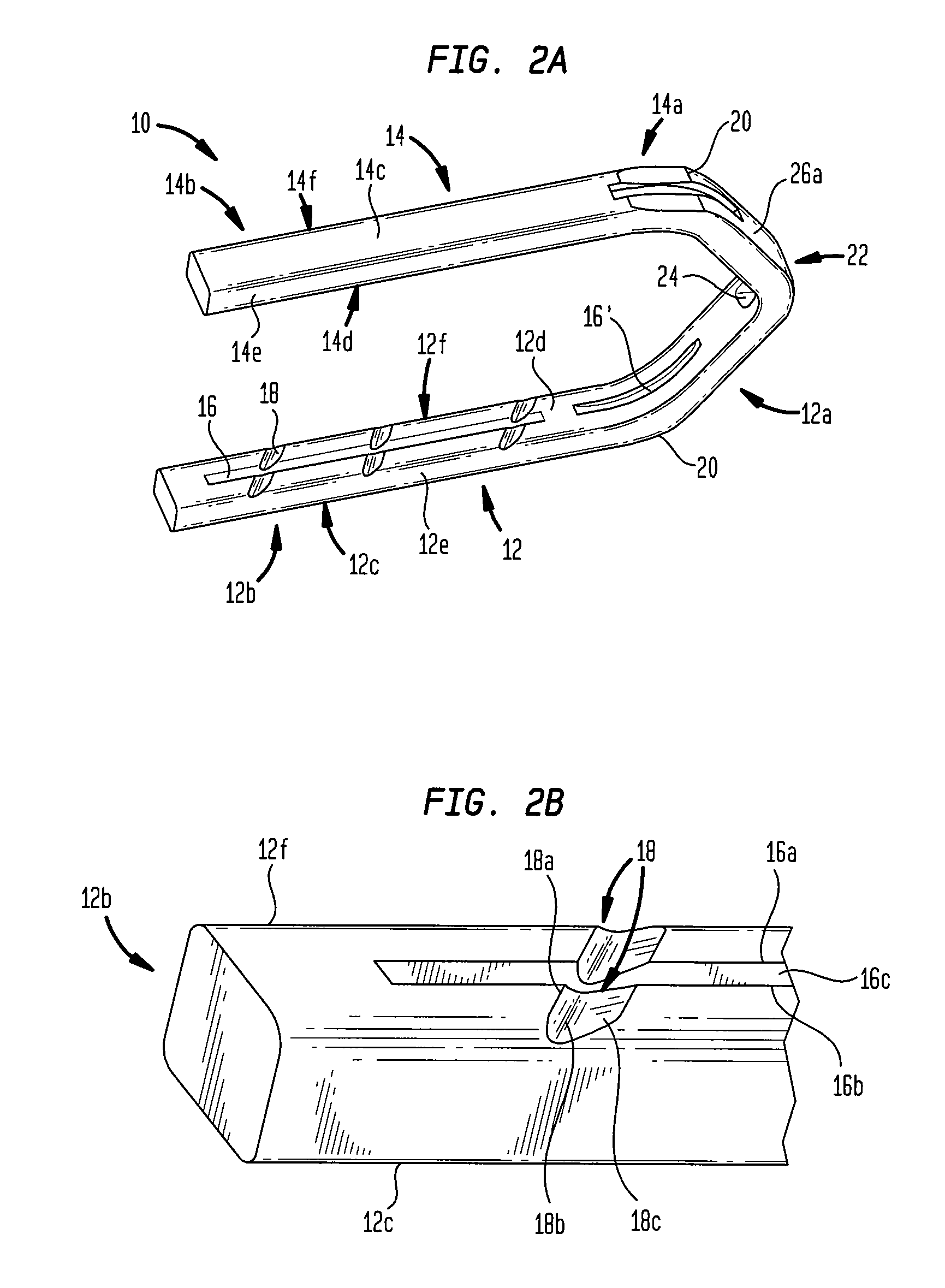
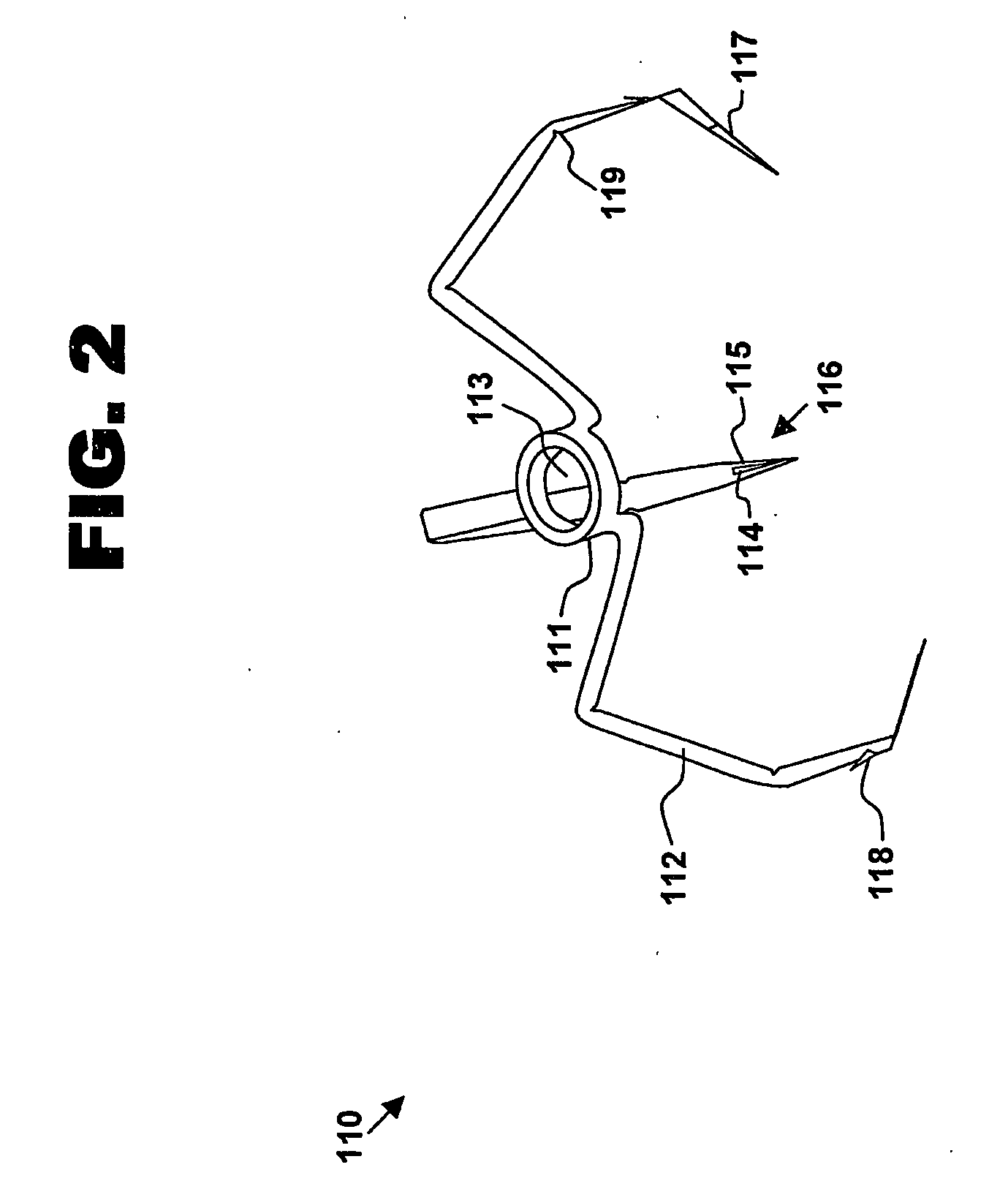
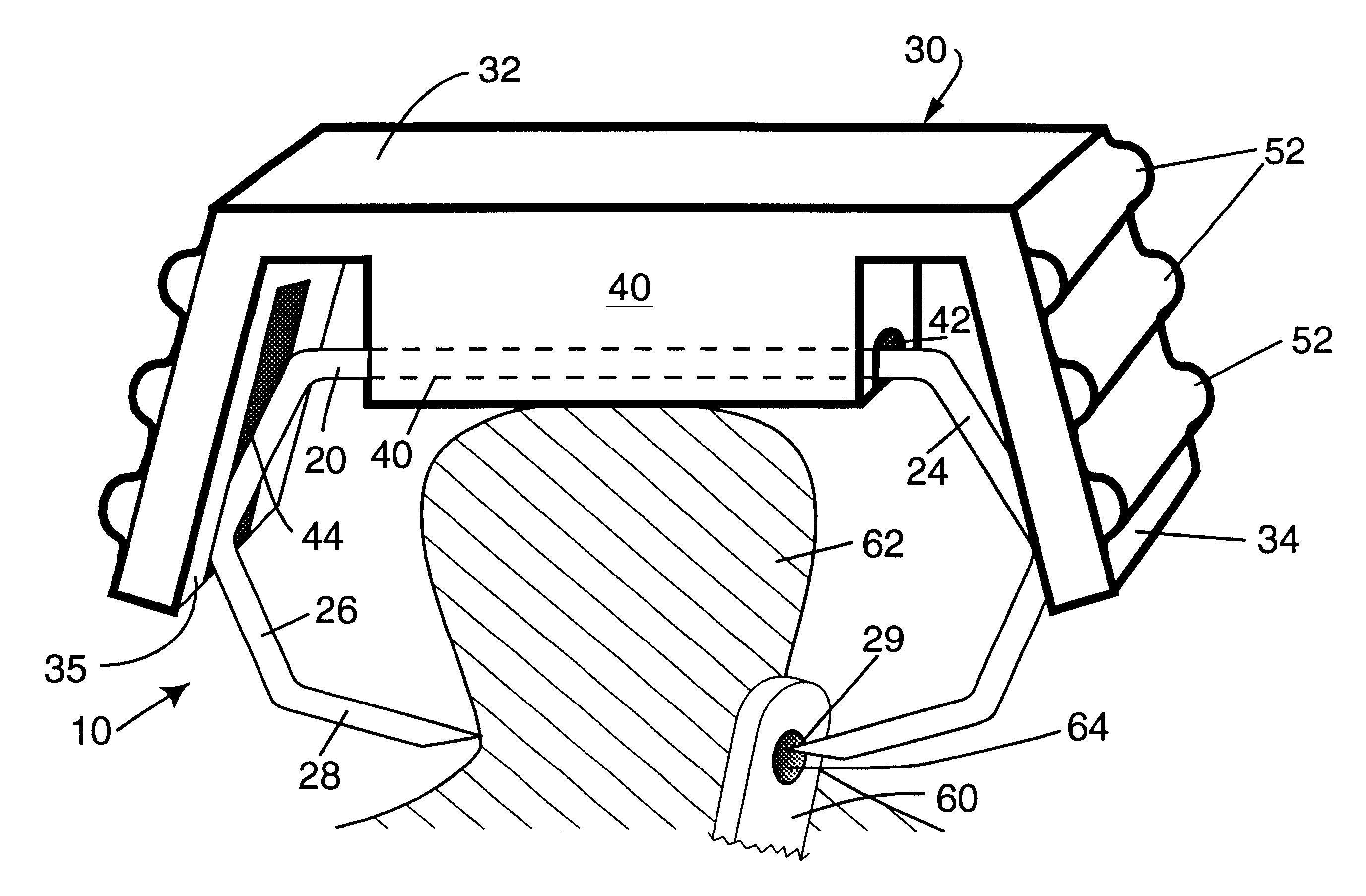
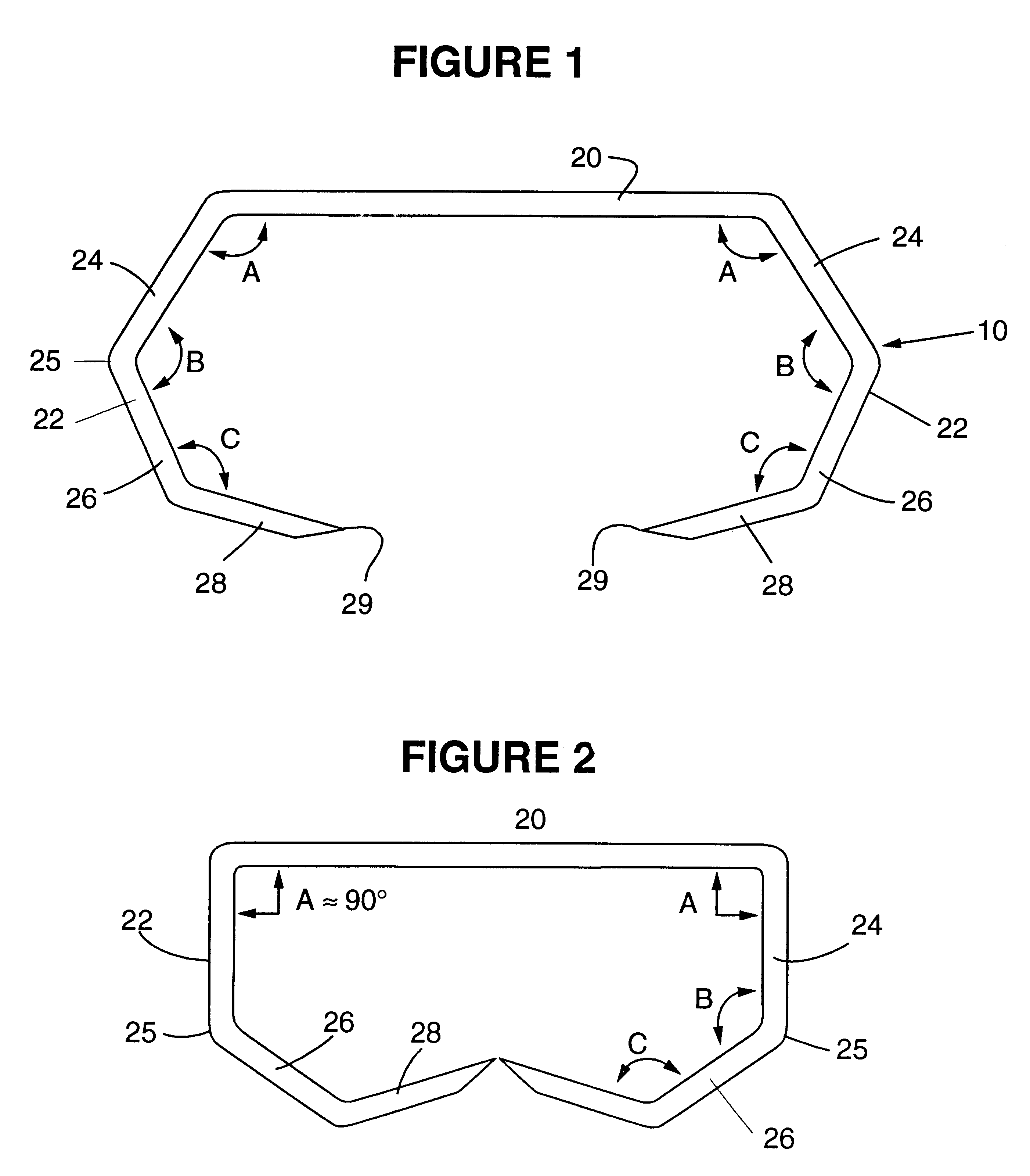
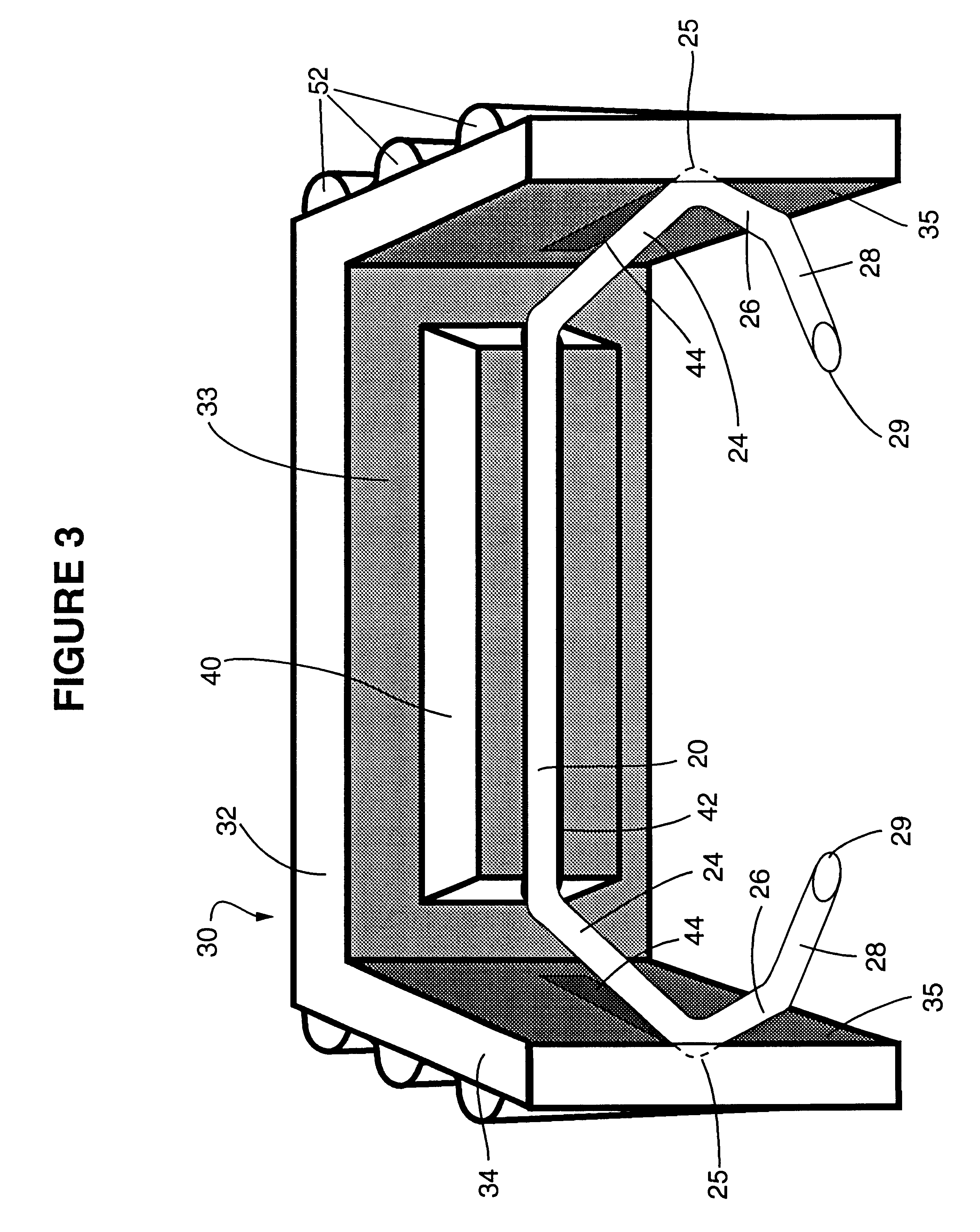
ActiveUS7699860B2Less deformationElasticitySuture equipmentsSurgical veterinarySurgical ClipsBlood vessel
A clip is provided that can be used for ligating tissue, such as vessels, other tubular ducts, and the like. The clip has opposed first and second leg members having proximal and distal ends. The proximal end of each leg member is connected by an apex having a notch formed therein. Moreover, each leg member has an inner tissue-contacting surface and an outer compression-receiving surface, both of which include features to provide a more secure ligation of the vessel or duct. A method of ligating vessels is also provided.
Owner:ETHICON ENDO SURGERY INC
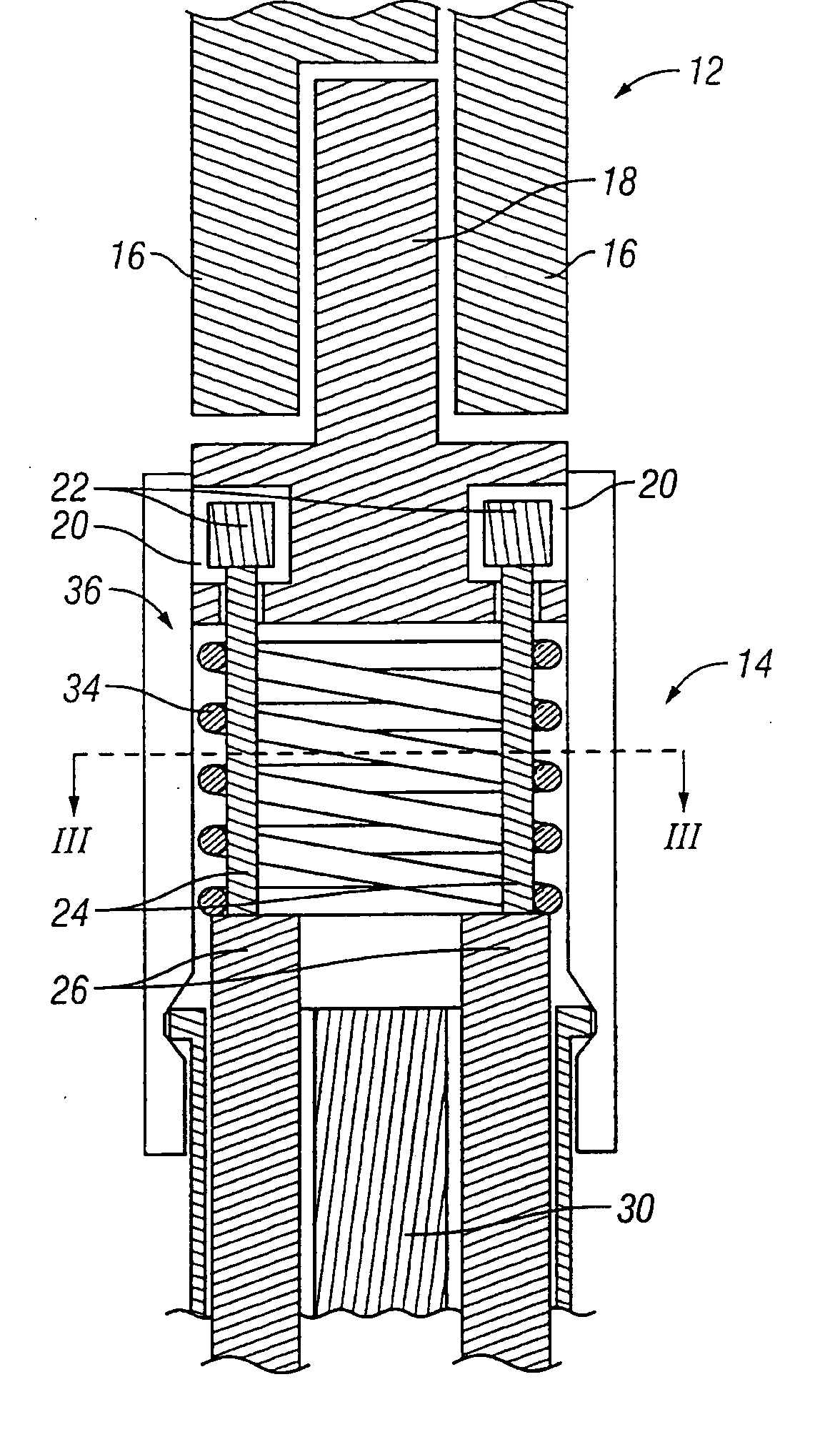
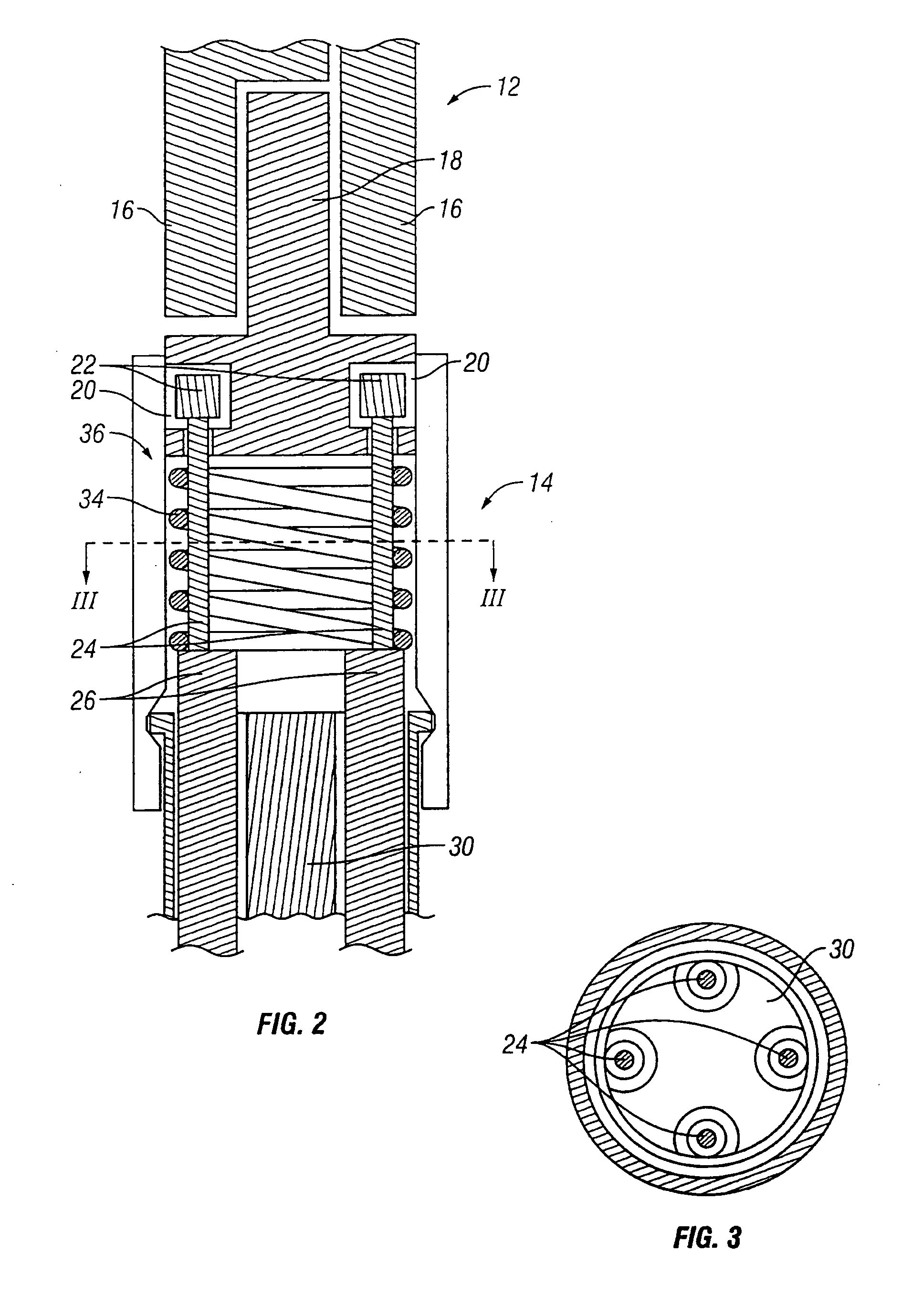
Electrophysiology/ablation catheter having lariat configuration of variable radius
InactiveUS7081114B2Easy to curlReduced radiusElectrotherapyDiagnostic recording/measuringDistal portionElectrophysiology
A remotely deflectable electrophysiology / ablation catheter of the type intended for placing into an interior passage of the heart is disclosed. The distal end of this elongated tubular catheter has a pair of tension / compression members each with a flattened end portion connected to the distal electrode and extending through the catheter casing and attached to a user moveable actuator for effecting the tension / compression thereon for remotely curling the distal end of the catheter. Spaced ring electrodes are provided adjacent the distal electrode. A permanent bend is pre-formed in the casing and tension / compression members adjacent the ring electrodes about an axis perpendicular to the elongated tension / compression members. Movement of the remote actuator causes the distal portion of the catheter to curl into a lariat in a plane perpendicular to the axis along the elongated catheter casing, thus permitting electrical mapping or ablation with the distal and / or ring electrodes about the inner surface of the heart passage into which the lariat is formed and situated. The lariat can achieve a curvature greater than 360 degrees and at a significantly reduced radius to allow insertion of the catheter distal end into passages of reduced dimension.
Owner:ST JUDE MEDICAL ATRIAL FIBRILLATION DIV
Device, system, and method for contracting tissue in a mammalian body
A device, a system and a method for contracting tissue in a mammalian body. The contracting device comprises a body and a plurality of legs radially splayed there from. Each leg includes a snap-acting spring tip. When a force is applied to the device, the tips operate to transform the device from a deployment state into a treatment state. Each leg may also be bent at one or more deformation elements in response to the application of force. The system comprises a contracting device slidably received within a delivery catheter. The method of contracting tissue in a mammalian body comprises delivering a contracting device in a lumen of a catheter proximate a treatment area, releasing the contracting device from the catheter, positioning legs of the contracting device on tissue to be contracted, exerting a force on the contracting device, transforming the device into a treatment state, and reducing a compass of the tissue in response to the attainment of the treatment state.
Owner:MEDTRONIC VASCULAR INC
Staple and staple applicator for use in skin fixation of catheters
An inexpensive surgical stapler, such as for use in securing vascular catheters, has a plastic applicator made for use with a single staple. The applicator has a backbone and two identical arms. The inside face of the backbone has a retaining channel that secures the crown portion of the staple against movement. The inside faces of the arms have guidance grooves that direct the movement of the staple as the applicator arms are squeezed with finger pressure. The outside faces of the arms are configured to permit gripping by the operator's fingers. The stapler can be used in lieu of suturing. Other staple and applicator assemblies can include two or more of such assemblies.
Owner:DEAN ALLGEYER M D
Cardiac tissue ablation instrument with flexible wrist
The present invention is directed to an articulate minimally invasive surgical instrument with a flexible wrist to facilitate the safe placement and provide visual verification of the ablation catheter or other devices in Cardiac Tissue Ablation (CTA) treatments. In one embodiment, the instrument is an endoscope which has an elongate shaft, a flexible wrist at the working end of the shaft, and a vision scope lens at the tip of the flexible wrist. The flexible wrist has at least one degree of freedom to provide the desired articulation. It is actuated and controlled by a drive mechanism located in the housing at the distal end of the shaft. The articulation of the endoscope allows images of hard-to-see places to be taken for use in assisting the placement of the ablation catheter on the desired cardiac tissue. The endoscope may further include couplings to releasably attach an ablation device / catheter or a catheter guide to the endoscope thereby further utilizing the endoscope articulation to facilitate placement of the ablation catheter on hard-to-reach cardiac tissues. In another embodiment, the articulate instrument is a grasper or any other instrument with a flexible wrist and a built-in lumen to allow an endoscope to insert and be guided to the distal end of the instrument.
Owner:INTUITIVE SURGICAL
Medical device introducer and obturator
An introducer having an elongate tubular member and a device connector releasably attached to a proximal end of the tubular member. The introducer allows exchange of medical instruments, such as a blood filter and cardioplegia catheter, through a single lumen. An obturator having a retractable blade for making incision on a tissue is insertable through the lumen of the introducer. Methods of using the obturator and the introducer for introducing medical device(s) into body cavity, such as a vessel or cardiac tissue, are also disclosed.
Owner:EDWARDS LIFESCIENCES CORP
Implantation system for annuloplasty rings
InactiveUS7485142B2Good coaptation of leafletImprove hemodynamic functionSuture equipmentsSurgical needlesEffective lengthShape-memory alloy
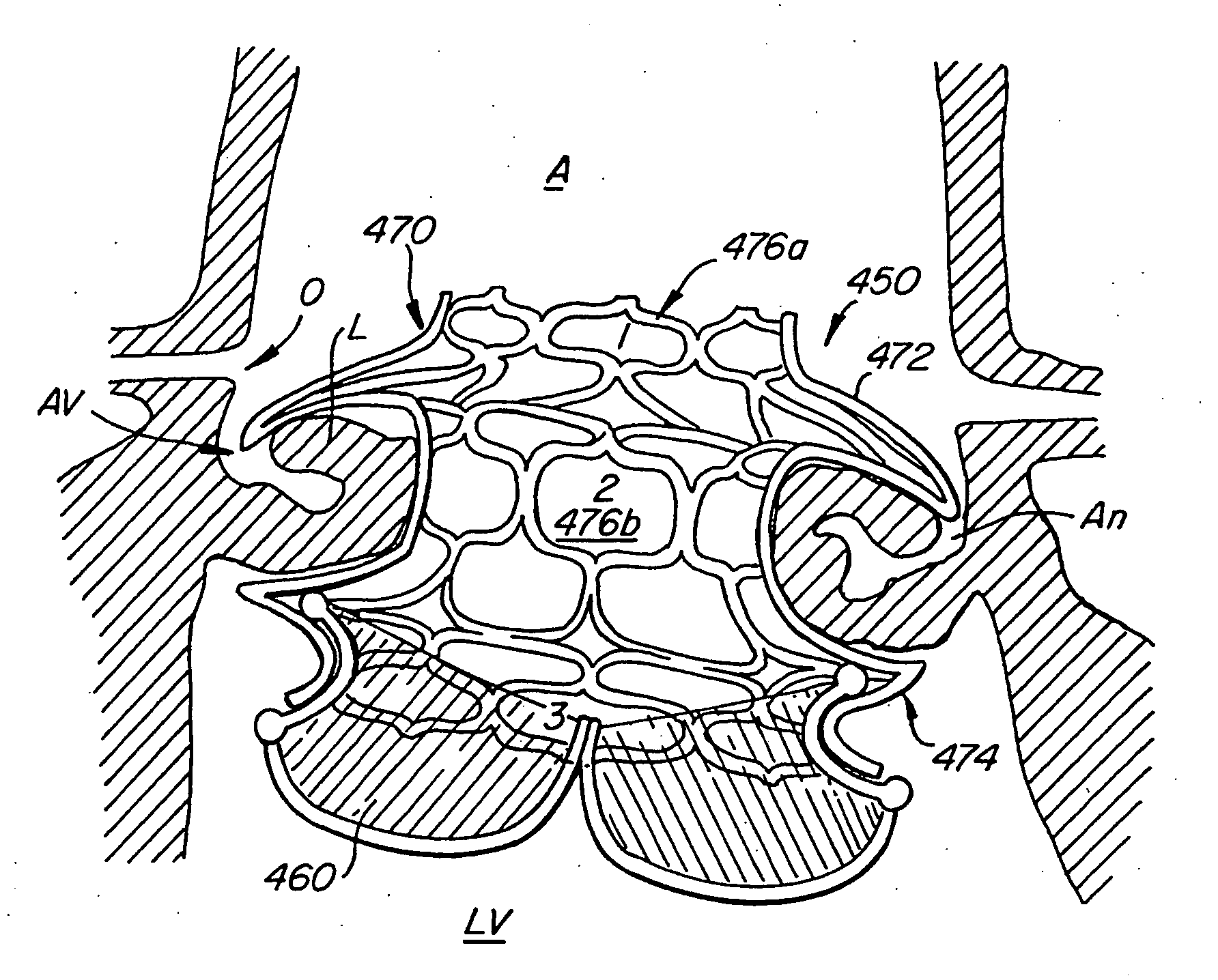
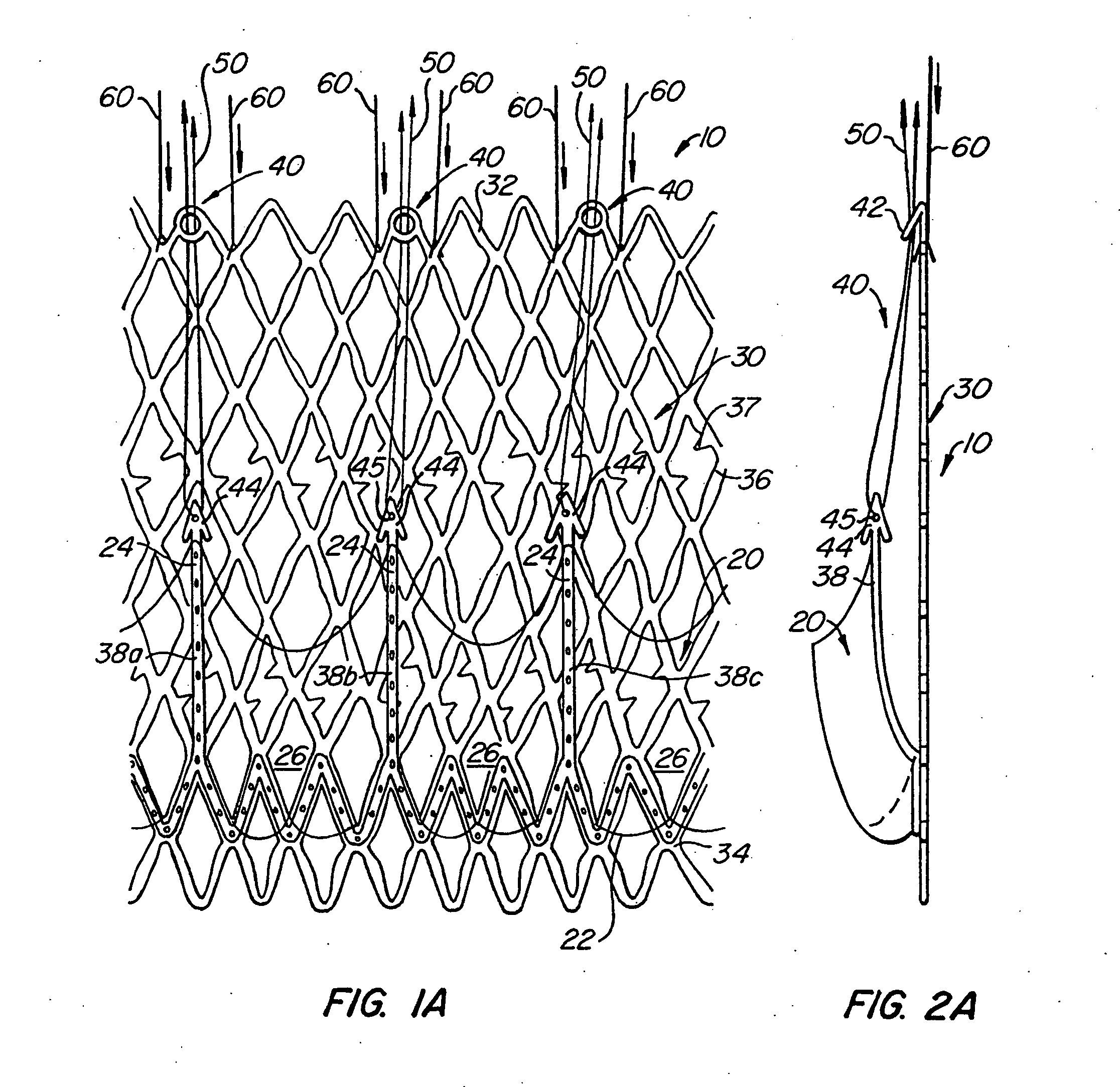
Methods for reconfiguring an atrioventricular heart valve that may use systems comprising a partial or complete annuloplasty rings proportioned to reconfigure a heart valve that has become in some way incompetent, a pair of trigonal sutures or implantable anchors, and a plurality of staples which may have pairs of legs that are sized and shaped for association with the ring at spaced locations along its length. These systems permit relative axial movement between the staples and the ring, whereby a patient's heart valve can be reconfigured in a manner that does not deter subtle shifting of the native valve components. Shape-memory alloy material staples may have legs with free ends that interlock following implantation. Annuloplasty rings may be complete or partial and may be fenestrated. One alternative method routes a flexible wire, preferably of shape-memory material, through the bights of pre-implanted staples. Other alternative systems use linkers of shape-memory material having hooked ends to interengage with staples or other implanted supports which, following implantation, decrease in effective length and pull the staples or other supports toward one another so as to create desired curvature of the reconfigured valve. These linkers may be separate from the supports or may be integral with them and may have a variety of shapes and forms. Various of these systems may be implanted non-invasively using a delivery catheter.
Owner:QUICKRING MEDICAL TECH LTD
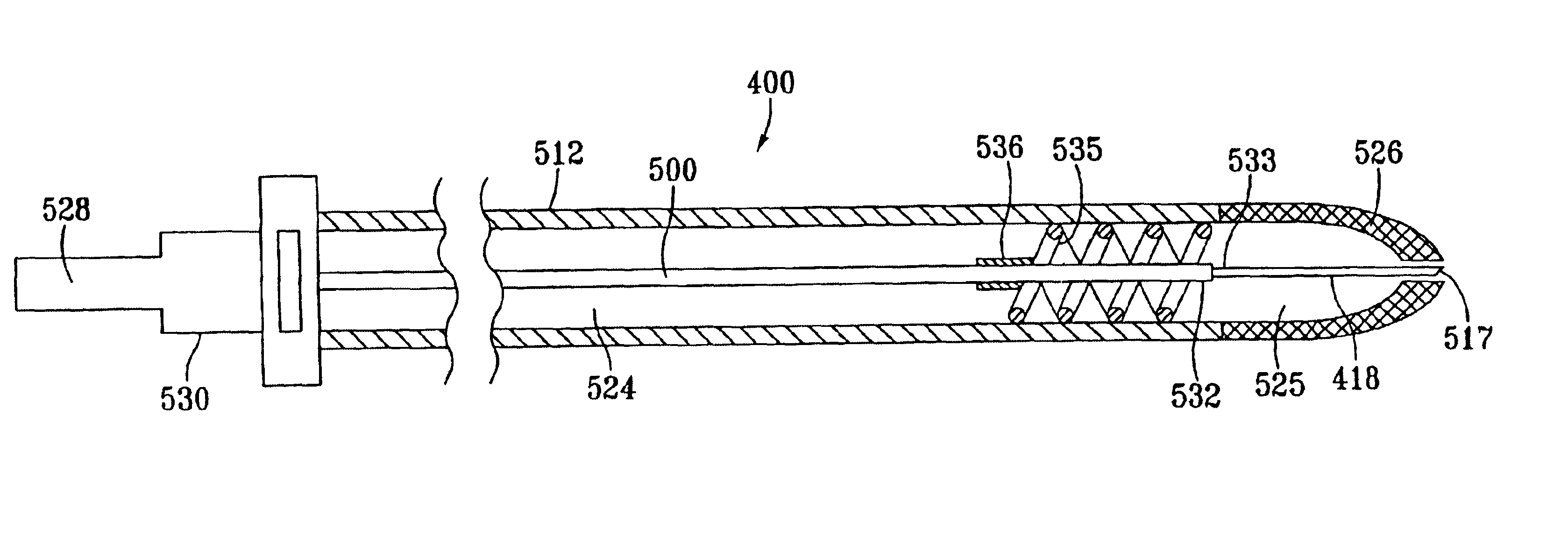
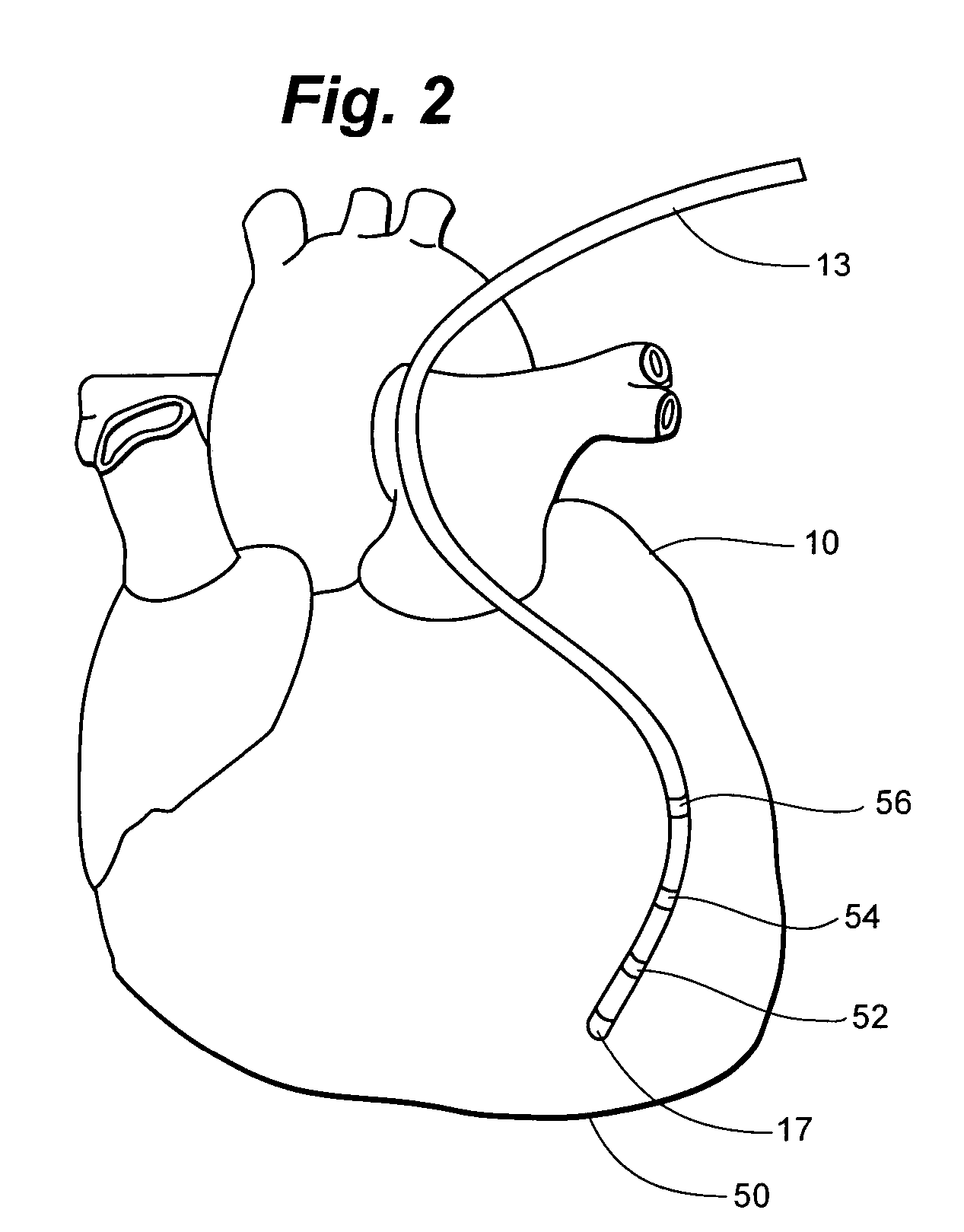
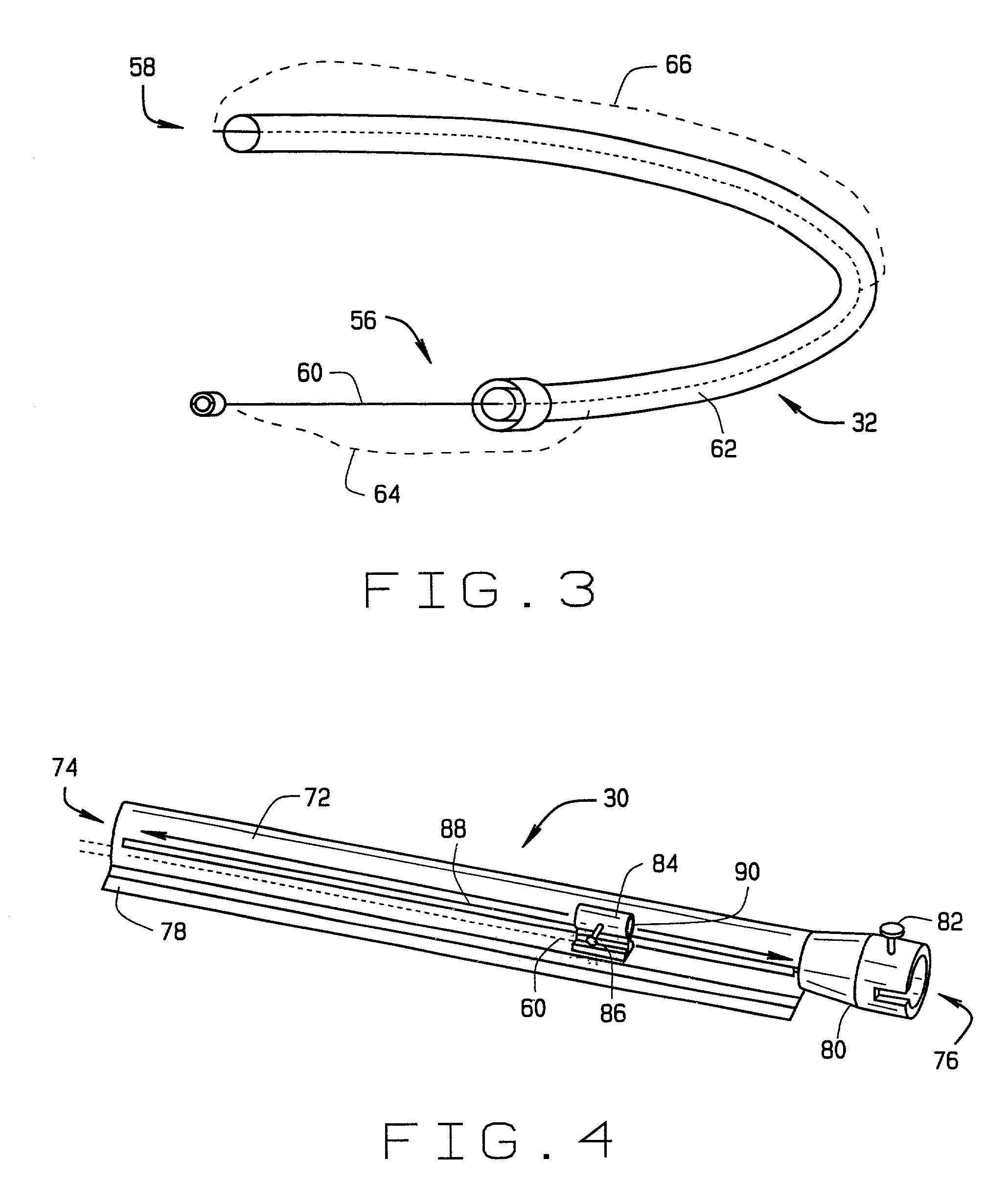
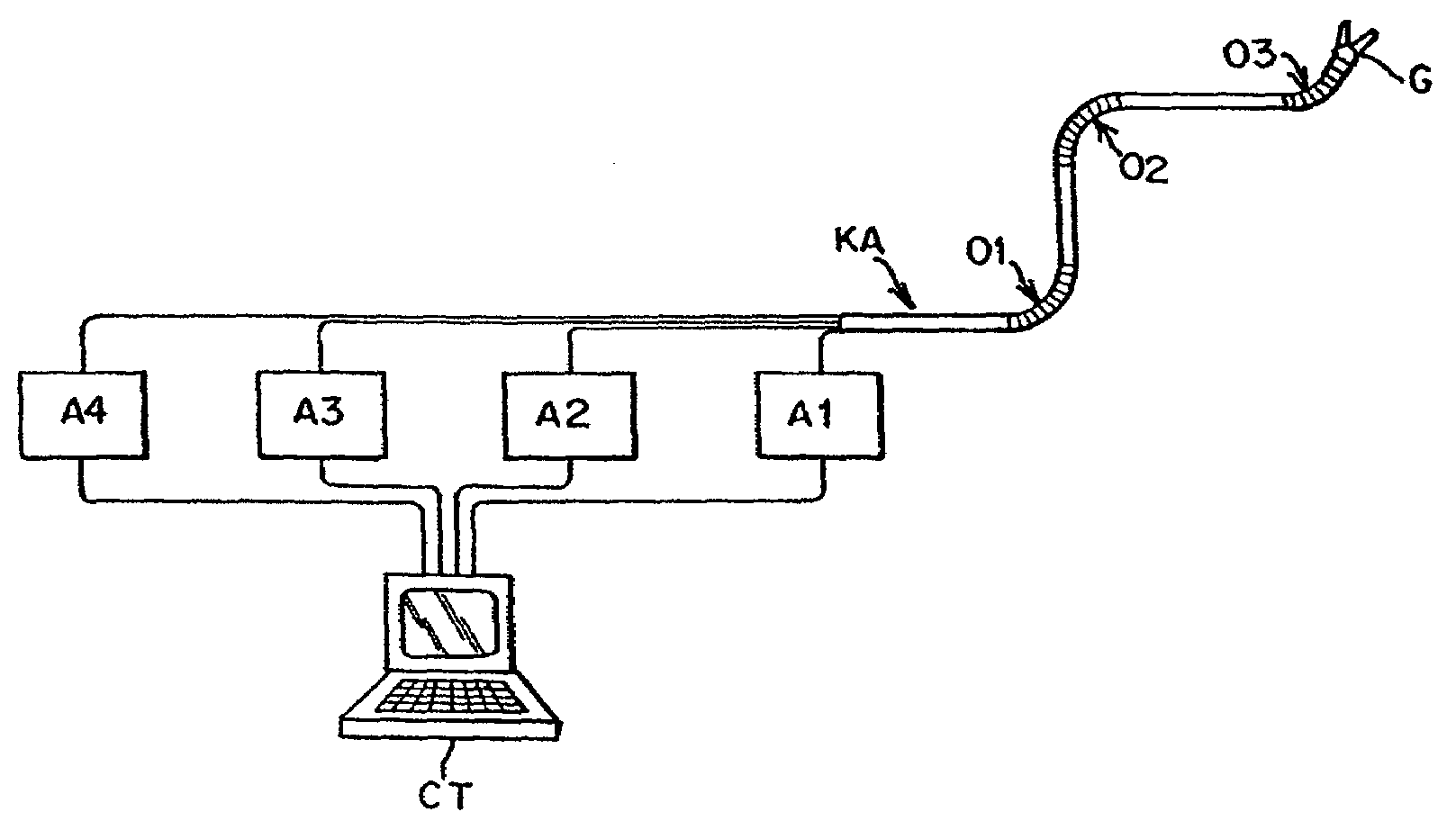
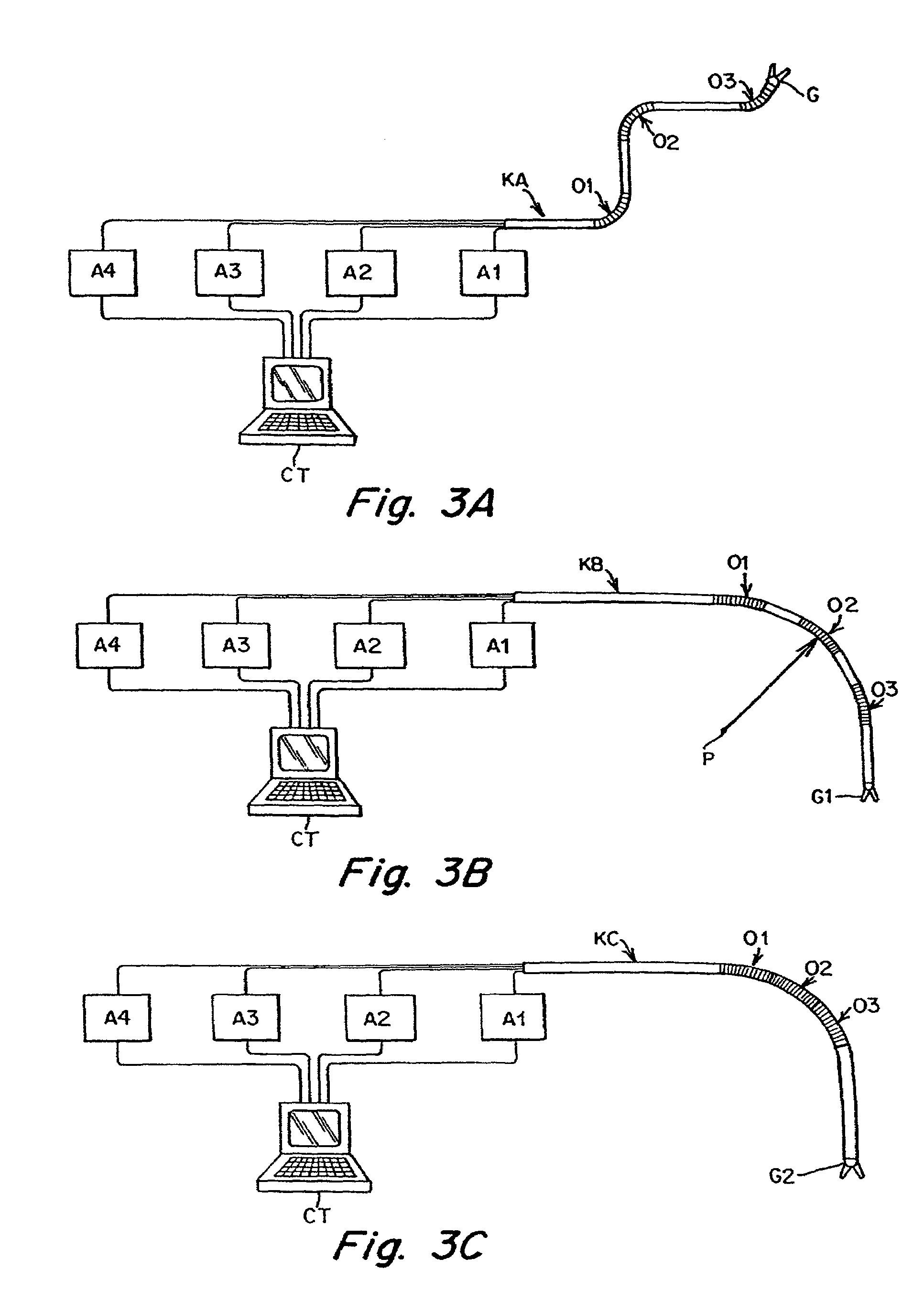
Catheter for conducting a procedure within a lumen, duct or organ of a living being
In an embodiment, the invention provides a catheter suitable for use in performing a procedure within a vessel, lumen or organ of a living having a distal end which is steerable, such as upon the application of compression. The catheter may be of the over the wire type, or alternatively may be a rapid exchange catheter. The catheter may provide for a rotating element which may be used to open a clogged vessel, or alternatively to provide information about adjacent tissues, such as may be generated by imaging or guiding arrangements using tissue detection systems known in the art, e.g., ultrasound, optical coherence reflectometry, etc. For rapid exchange catheters having a rotating element, there is provided an offset drive assembly to allow the rotary force to be directed from alongside the guidewire to a location coaxial to and over the guidewire.
Owner:KENSEY NASH CORP
Magnetic linear actuator for deployable catheter tools
InactiveUS20080297287A1Easy to controlGood precisionDiagnostic recording/measuringSensorsDiagnostic Radiology ModalityLinear motion
Using the linear forces that are provided by an electromagnetic solenoid applied near the distal end of a medical catheter, various surgical instruments can be actuated or deployed for use in interventional medicine. The linear actuator uses the principles of magnetic repulsion and attraction as a means for moving a bobbin that can be attached to various types of moving components that translate linear movements into the actuation of a tool that is attached to the linear actuator. Using independent solenoid coils, movement modality is increased from two possible positions to three.
Owner:NEURO KINESIS CORP
Method and apparatus for catheter navigation and location and mapping in the heart
InactiveUS7263397B2Enhance system functionsAccurate locationElectrocardiographyCatheterHeart chamberBiological activation
A medical system for finding and displaying the location of electrodes within the body. The electrodes may be used to measure the voltage on the heart wall and display this as an activation map on a geometry representing the heart chamber.
Owner:ST JUDE MEDICAL ATRIAL FIBRILLATION DIV
System and method for advancing, orienting, and immobilizing on internal body tissue a catheter or other therapeutic device
This invention provides a system and method that allows a therapeutic device, such as an atrial fibrillation microwave ablation catheter or ablation tip to be guided to a remote location within a body cavity and then accurately immobilized on the tissue, including that of a moving organ, such as the heart. In various embodiments, the system and method also enables accurate movement and steering along the tissue, while in engagement therewith. Such movement and engagement entails the use of vacuum or microneedle structures on at least two interconnected and articulated immobilizers that selectively engage to and release from the tissue to allow a crawling or traversing walking across the organ as the therapeutic catheter / tool tip applies treatment (AGE devices). In further embodiments that lack a movement capability (AID devices), the immobilizers allow a predetermined position for the introduced device to be maintained against the tissue while a treatment is applied to the location adjacent thereto. In the exemplary AID devices, variety of steering mechanisms and mechanisms for exposing and anchoring a catheter against the underlying tissue can be employed. In the exemplary AGE devices, a variety of articulation and steering mechanisms, including those based upon pneumatic / hydraulic bellows, lead screws and electromagnetic actuators can be employed.
Owner:ELECTROPHYSIOLOGY FRONTIERS SPA
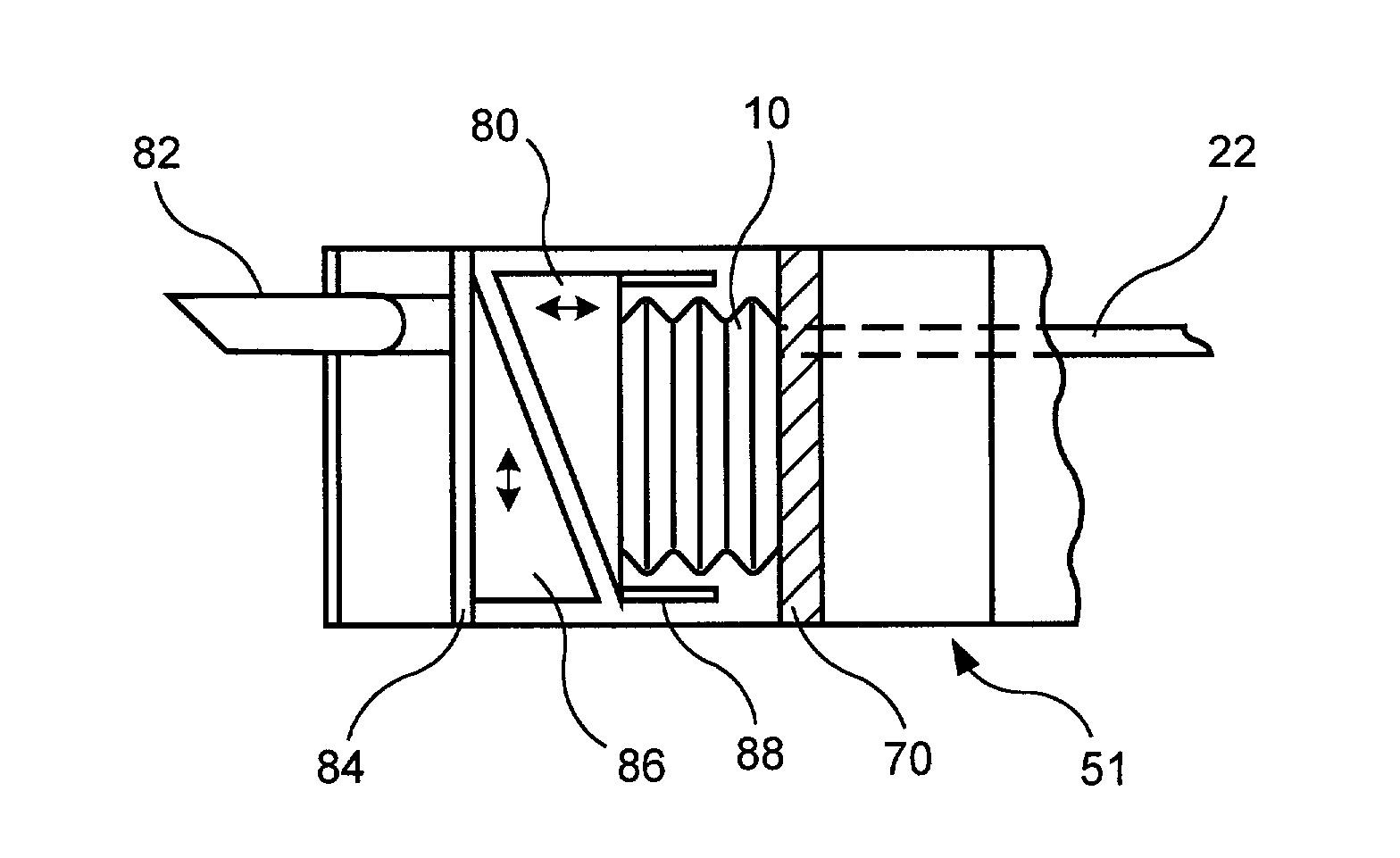
Balloon-type actuator for surgical applications
An actuator for surgical applications includes an inflation fluid conduit extending from a proximal end which, when the actuator is in an operative configuration, remains outside a patient's body, to a distal end and an inflatable member coupled to the distal end of the inflation fluid conduit so that, when inflation fluid is supplied to the inflatable member via the inflation fluid conduit, the inflatable member is expanded from a collapsed configuration to an expanded configuration, wherein, when in the collapsed configuration, the inflatable member includes a fold extending substantially transverse to a longitudinal axis thereof so that, when inflation fluid is supplied thereto, the inflatable member expands substantially along the longitudinal axis.
Owner:BOSTON SCI SCIMED INC
Cardiac tissue ablation instrument with flexible wrist
InactiveUS20060199999A1Promotes convenient, simplified manufacturing and assembly processesShorten the counting processEndoscopesSurgical manipulatorsWristInstrumentation
An articulate minimally invasive surgical instrument with a flexible wrist to facilitate the safe placement and provide visual verification of the ablation catheter or other devices in Cardiac Tissue Ablation (CTA) treatments is described. In one embodiment, the instrument is an endoscope which has an elongate shaft, a flexible wrist at the working end of the shaft, and a vision scope lens at the tip of the flexible wrist. The flexible wrist has at least one degree of freedom to provide the desired articulation. It is actuated and controlled by a drive mechanism located in the housing at the distal end of the shaft. The articulation of the endoscope allows images of hard-to-see places to be taken for use in assisting the placement of the ablation catheter on the desired cardiac tissue. The endoscope may further include couplings to releasably attach an ablation device / catheter or a catheter guide to the endoscope thereby further utilizing the endoscope articulation to facilitate placement of the ablation catheter on hard-to-reach cardiac tissues. In another embodiment, the articulate instrument is a grasper or any other instrument with a flexible wrist and a built-in lumen to allow an endoscope to insert and be guided to the distal end of the instrument.
Owner:INTUITIVE SURGICAL OPERATIONS INC
System and methods for advancing a catheter
InactiveUS7276044B2Reduce repeated x-ray exposureReduce X-ray exposureEar treatmentSurgical needlesControl systemEngineering
An advancer system is described for moving an elongate medical device within a body. The system includes a drive unit having a motor. The drive unit is configured to translate movement of the motor to the device so as to alternately advance and retract the device relative to the body. The advancer system also includes a user-operable control system configured to control the drive unit. The control system can interface with a magnetic navigation system. The above-described system allows an operating physician to control catheter advancement and retraction while remaining outside an x-ray imaging field. Thus the physician is freed from repeated x-ray exposure.
Owner:STEREOTAXIS
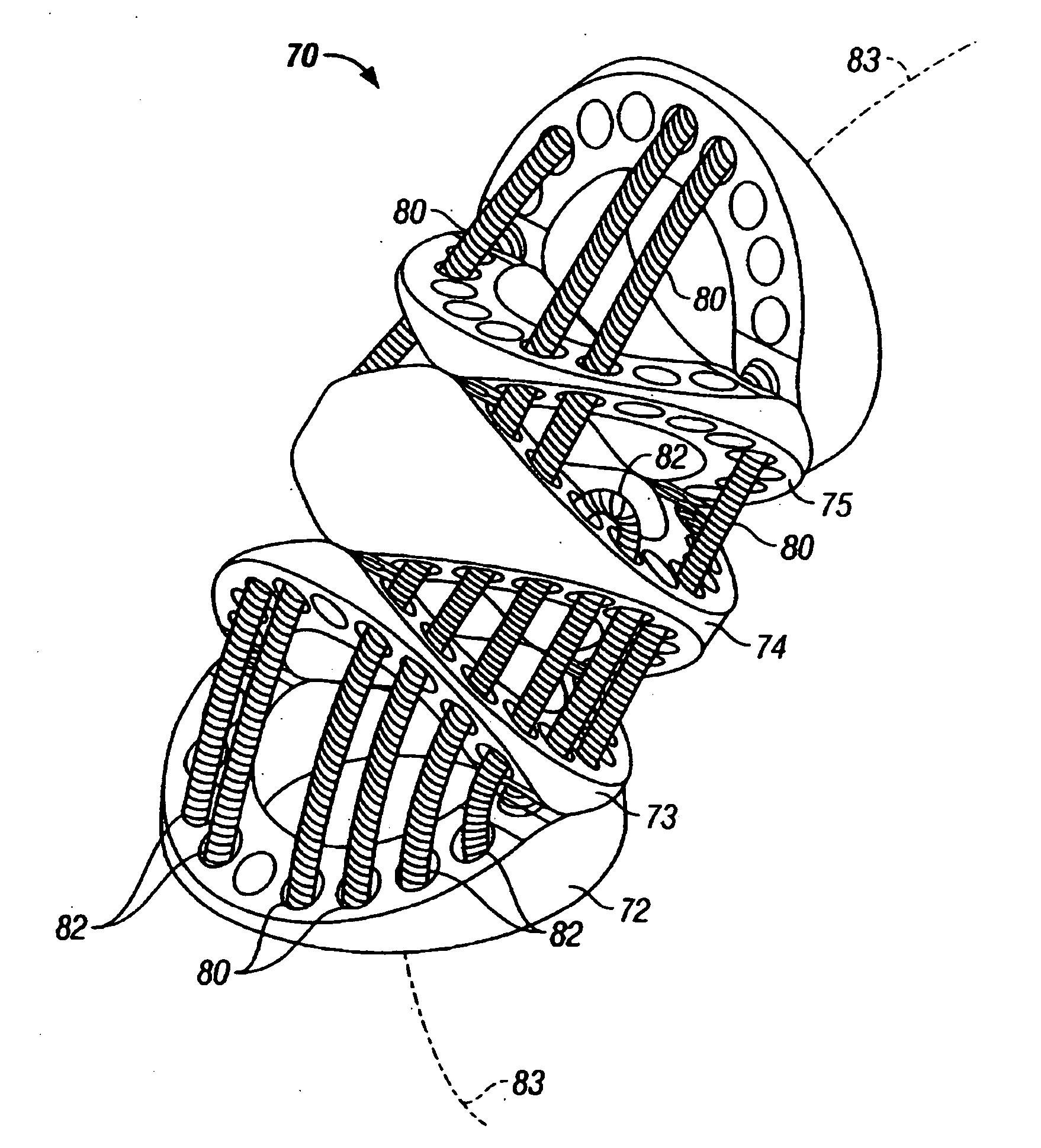
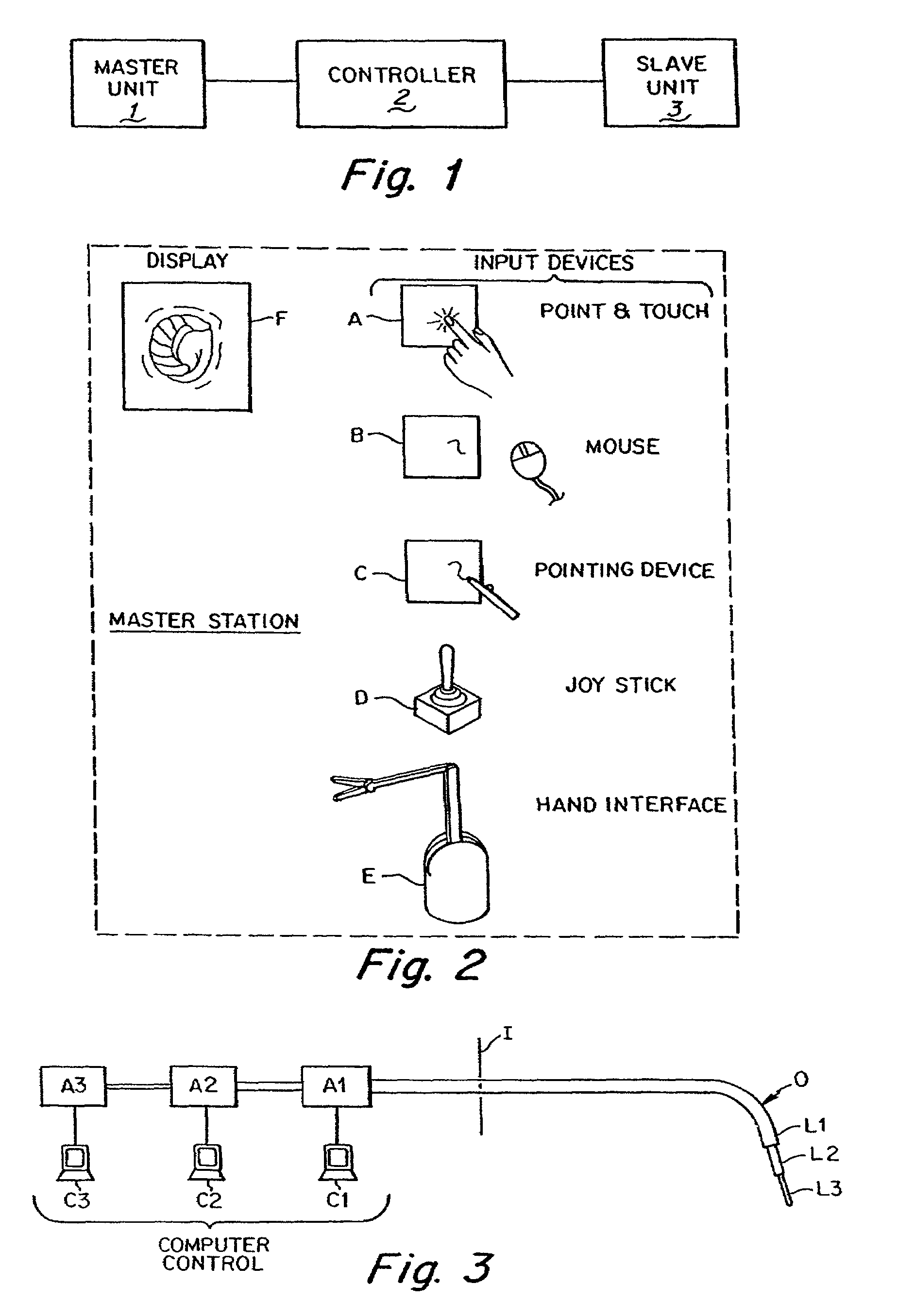
Interface assembly for controlling orientation of robotically controlled medical instrument
Interface assemblies for controlling an orientation of a working instrument of a robotic medical instrument system. A base member is coupled to a distal end of an instrument such as a robotically controllable catheter. A spacer element is retained between the base member and a platform member, which is movable relative to the base member about the spacer element. One or more control elements extending through a base member aperture can be used to control an orientation of the platform member and working instrument.
Owner:HANSEN MEDICAL INC
Non-cylindrical prosthetic valve system for transluminal delivery
InactiveUS20070043435A1Preventing substantial migrationEliminate the problemBalloon catheterHeart valvesCoronary arteriesProsthesis
A prosthetic valve assembly for use in replacing a deficient native valve comprises a replacement valve supported on an expandable prosthesis frame. If desired, one or more expandable anchors may be used. The prosthesis frame, which entirely supports the valve annulus, valve leaflets, and valve commissure points, is configured to be collapsible for transluminal delivery and expandable to contact the anatomical annulus of the native valve when the assembly is properly positioned. Portions of the prosthesis frame may expand to a preset diameter to maintain coaptivity of the replacement valve and to prevent occlusion of the coronary ostia. The prosthesis frame is compressible about a catheter, and restrained from expanding by an outer sheath. The catheter may be inserted inside a lumen within the body, such as the femoral artery, and delivered to a desired location, such as the heart. When the outer sheath is retracted, the prosthesis frame expands to an expanded position such that the valve and prosthesis frame expand at the implantation site and the anchor engages the lumen wall. The prosthesis frame has a non-cylindrical configuration with a preset maximum expansion diameter region about the valve opening to maintain the preferred valve geometry. The prosthesis frame may also have other regions having a preset maximum expansion diameter to avoid blockage of adjacent structures such as the coronary ostia.
Owner:MEDTRONIC COREVALVE
Percutaneous heart valve
ActiveUS20070016286A1Avoid flowStability and functioning of the heart valve are satisfactoryHeart valvesBlood flowValvular prosthesis
A percutaneously inserted bistable heart valve prosthesis is folded inside a catheter for transseptal delivery to the patient's heart for implantation. The heart valve has an annular ring, a body member having a plurality of legs, each leg connecting at one end to the annular ring, claws that are adjustable from a first position to a second position by application of external force so as to allow ingress of surrounding heart tissue into the claws in the second position, and leaflet membranes connected to the annular ring, the body member and / or the legs, the leaflet membranes having a first position for blocking blood flow therethrough and a second position for allowing blood flow therethrough. The heart valve is designed such that upon removal of the external force the claws elastically revert to the first position so as to grip the heart tissue positioned within the claws, thereby holding the heart valve in place. The body member and claws may be integrated into a one-piece design. The heart valve may be used as a prosthesis for the mitral valve, aortic valve, pulmonary valve, or tricuspid valve by adapting the annular ring to fit in a respective mitral, aortic, pulmonary, or tricuspid valve opening of the heart.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
Low profile heart valve and delivery system
Apparatus for endovascularly replacing a patient's heart valve, including: a delivery catheter having a diameter of 21 french or less; an expandable anchor disposed within the delivery catheter; and a replacement valve disposed within the delivery catheter. The invention also includes a method for endovascularly replacing a heart valve of a patient. In some embodiments the method includes the steps of: inserting a catheter having a diameter no more than 21 french into the patient; endovascularly delivering a replacement valve and an expandable anchor to a vicinity of the heart valve through the catheter; and deploying the anchor and the replacement valve.
Owner:BOSTON SCI SCIMED INC
Apparatus for imparting physician-determined shapes to implantable tubular devices
A tubular sleeve is provided for enabling a physician to impart a preselected shape in an implantable tubular device, such as a cardiac lead, a catheter, or some other tubular structure. The sleeve may be deformed by the surgeon before or at the time of implantation to customize the shape of the tubular device to the particular anatomical structures to be encountered by the device. To retain the deformation imparted by the physician, the sleeve may be composed of a heat-sensitive shape-memory material or an elastomeric material provided with a plastically deformable rib.
Owner:INTERMEDICS
User interface for tissue ablation system
ActiveUS8657814B2Simplified viewing and modifyingElectrocardiographyEndoscopesDisplay deviceTissue ablation
Devices, systems and methods are disclosed for the ablation of tissue. Embodiments include an ablation catheter that has an array of ablation elements attached to a deployable carrier assembly. The carrier assembly can be constrained within the lumen of a catheter, and deployed to take on an expanded condition. The carrier assembly includes multiple electrodes that are configured to ablate tissue at low power. Systems include an interface unit with a visual display that provides a visual representation of the geometry of the ablation elements and / or provides selection means for selecting an icon provided on the display.
Owner:MEDTRONIC ABLATION FRONTIERS
Heart valve delivery system
ActiveUS20070005131A1Easy to navigateIncrease thrustBalloon catheterHeart valvesProsthetic heartBalloon catheter
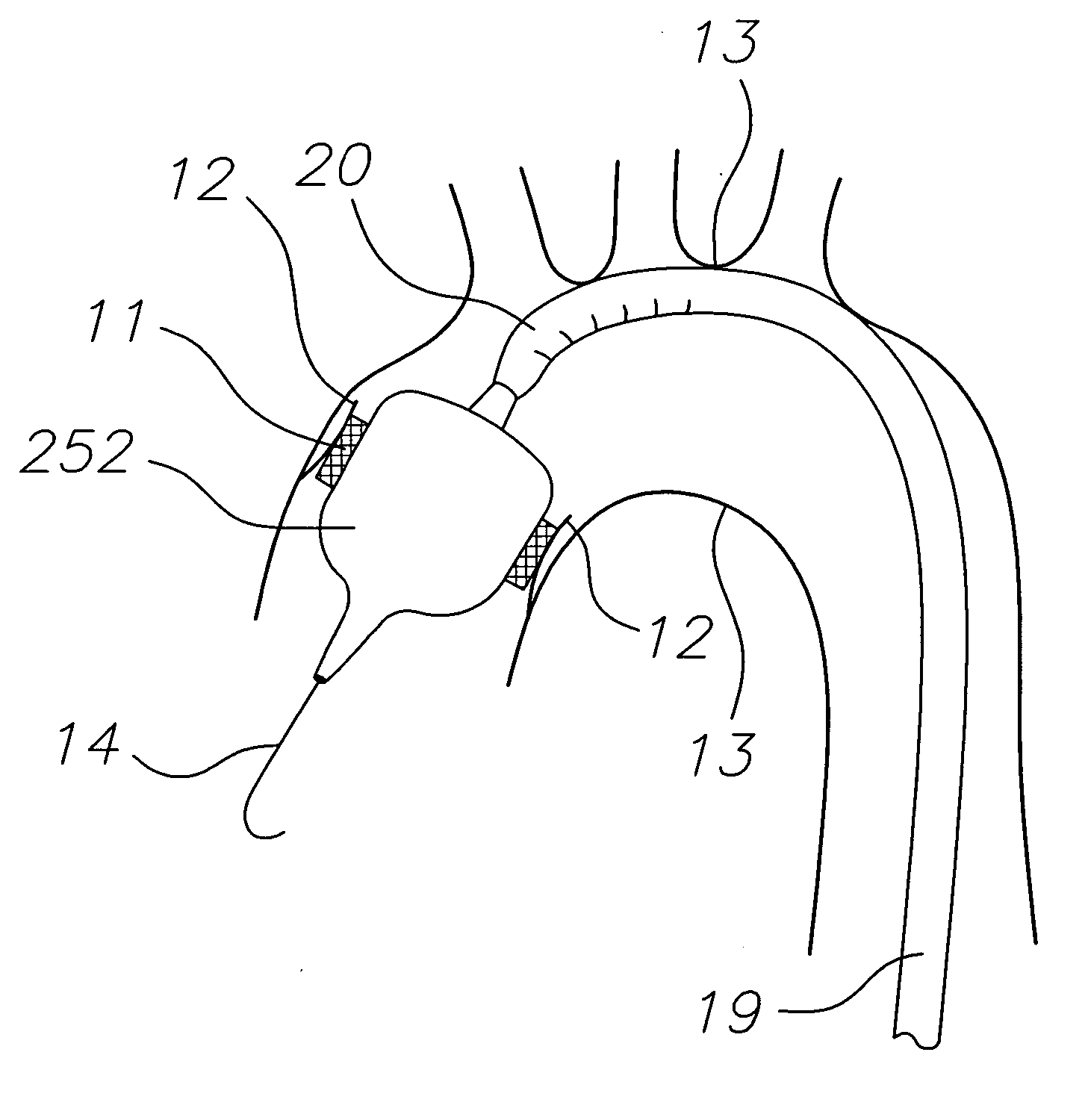
A delivery system for delivering a prosthetic heart valve to a native valve site within the human vasculature. The prosthetic valve is disposed on a balloon at the end of a balloon catheter. The balloon catheter passes through a delivery sleeve assembly and handle. A pull wire travels from the handle to a distal end of the delivery sleeve assembly. Actuation of the handle pulls on the pull wire, which causes openings in a slotted tube of the delivery sleeve assembly to close, thus causing the delivery sleeve assembly to bend. A stretchable cover is placed over the slotted tube for biasing the steerable catheter toward a straight position. Once advanced to the native valve site, the prosthetic valve is deployed by inflating the balloon.
Owner:EDWARDS LIFESCIENCES CORP
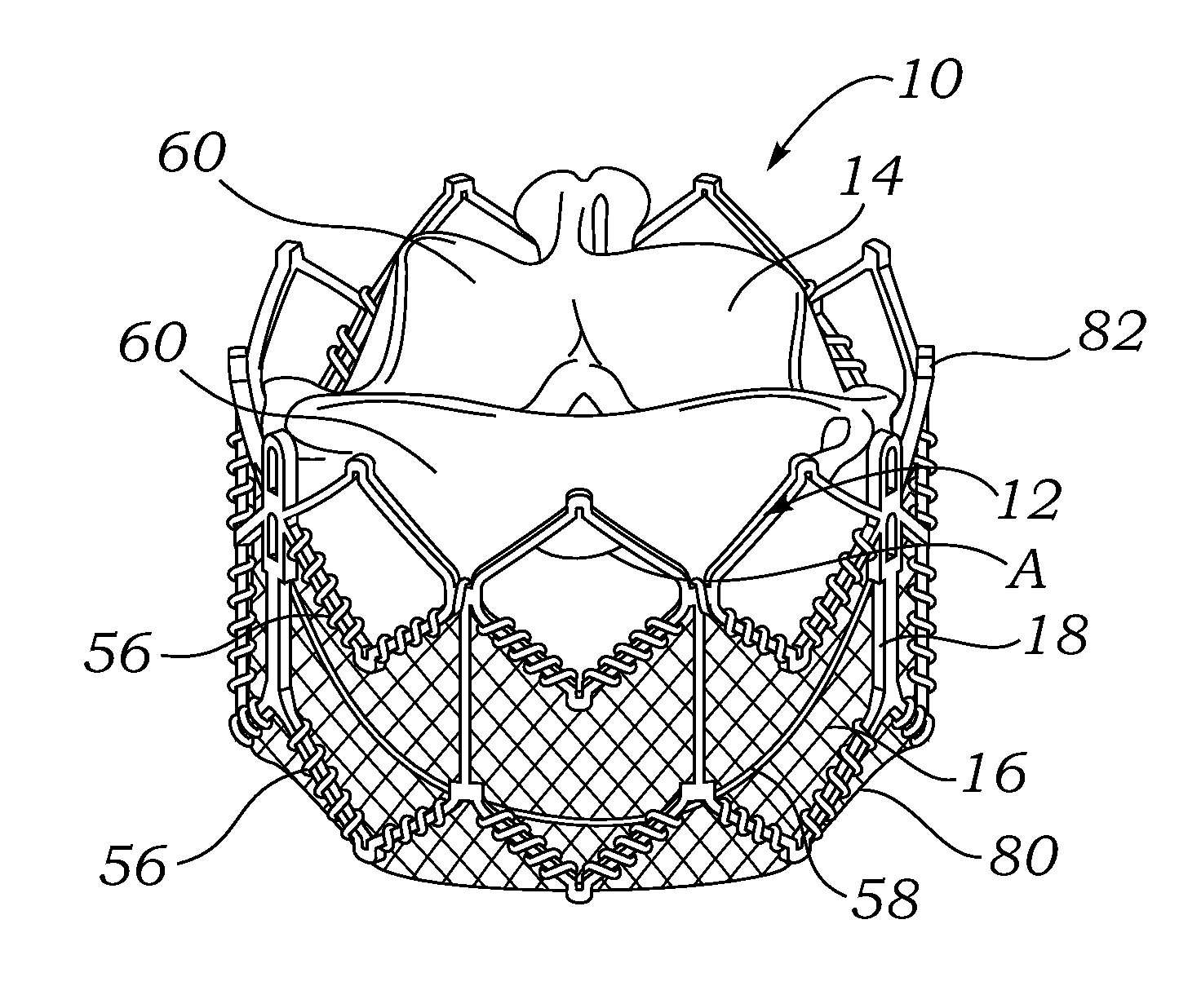
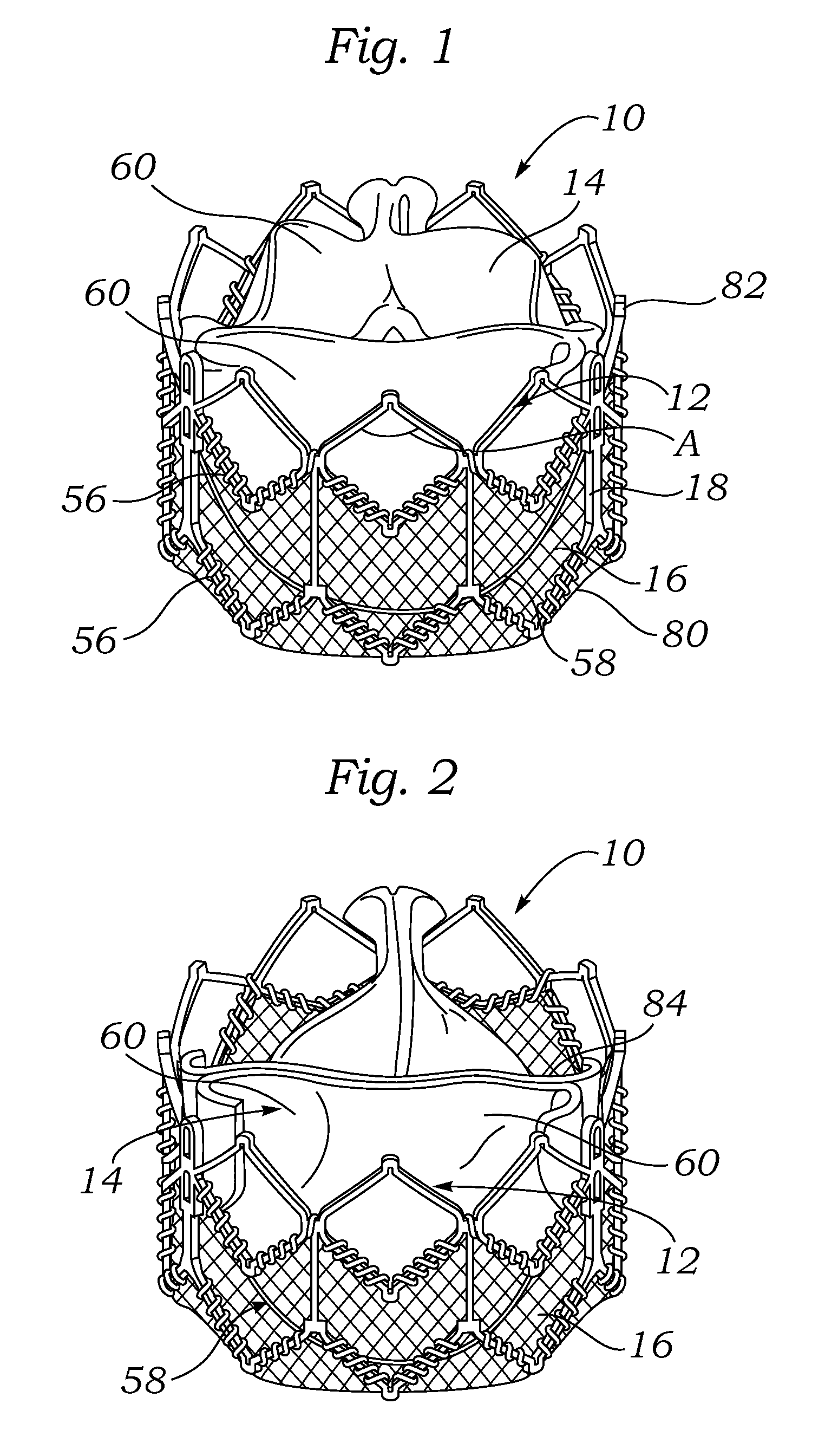
Low profile transcatheter heart valve
An implantable prosthetic valve, according to one embodiment, comprises a frame, a leaflet structure, and a skirt member. The frame can have a plurality of axial struts interconnected by a plurality of circumferential struts. The leaflet structure comprises a plurality of leaflets (e.g., three leaflets arrange to form a tricuspid valve). The leaflet structure has a scalloped lower edge portion secured to the frame. The skirt member can be disposed between the leaflet structure and the frame.
Owner:EDWARDS LIFESCIENCES CORP
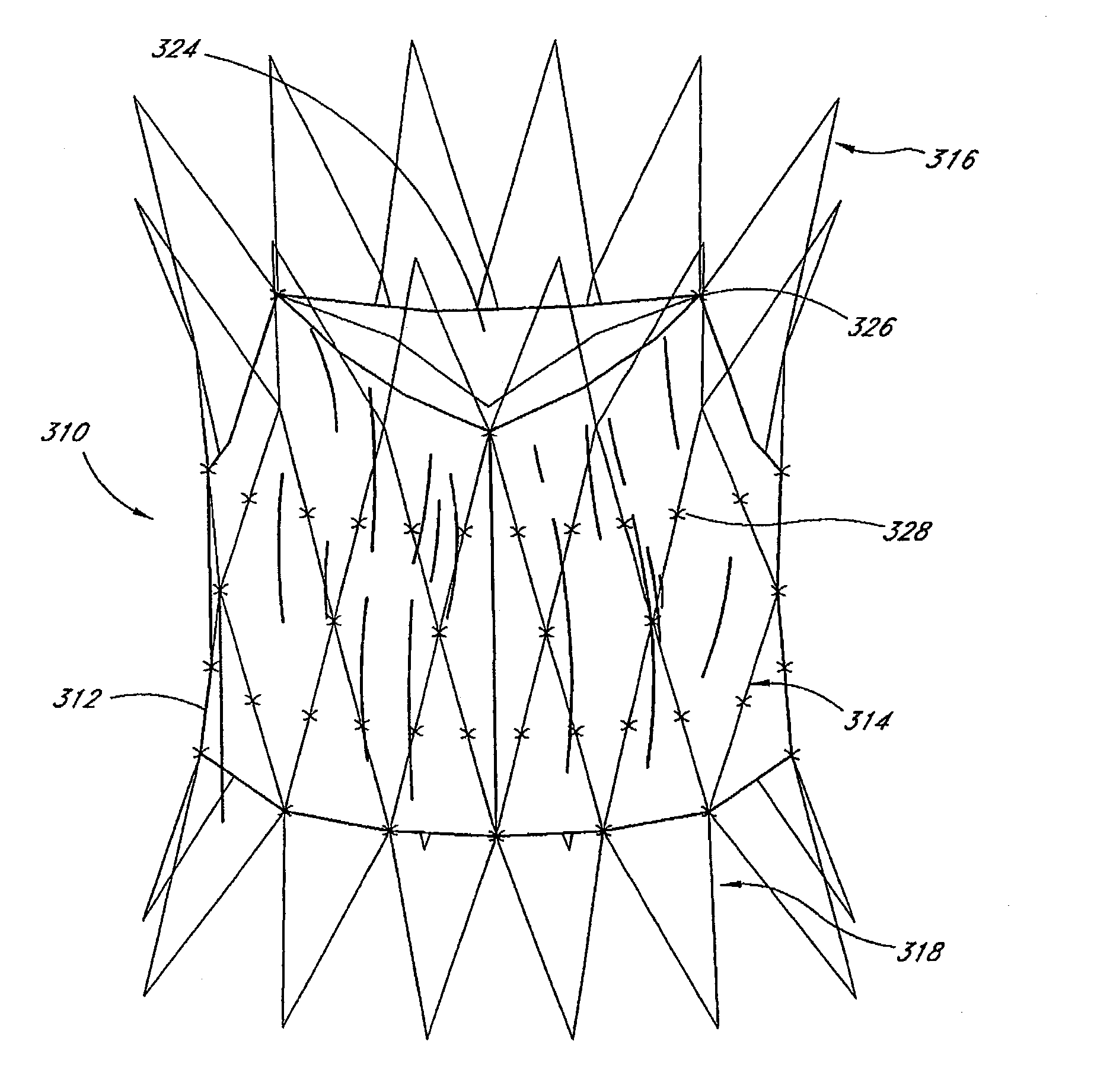
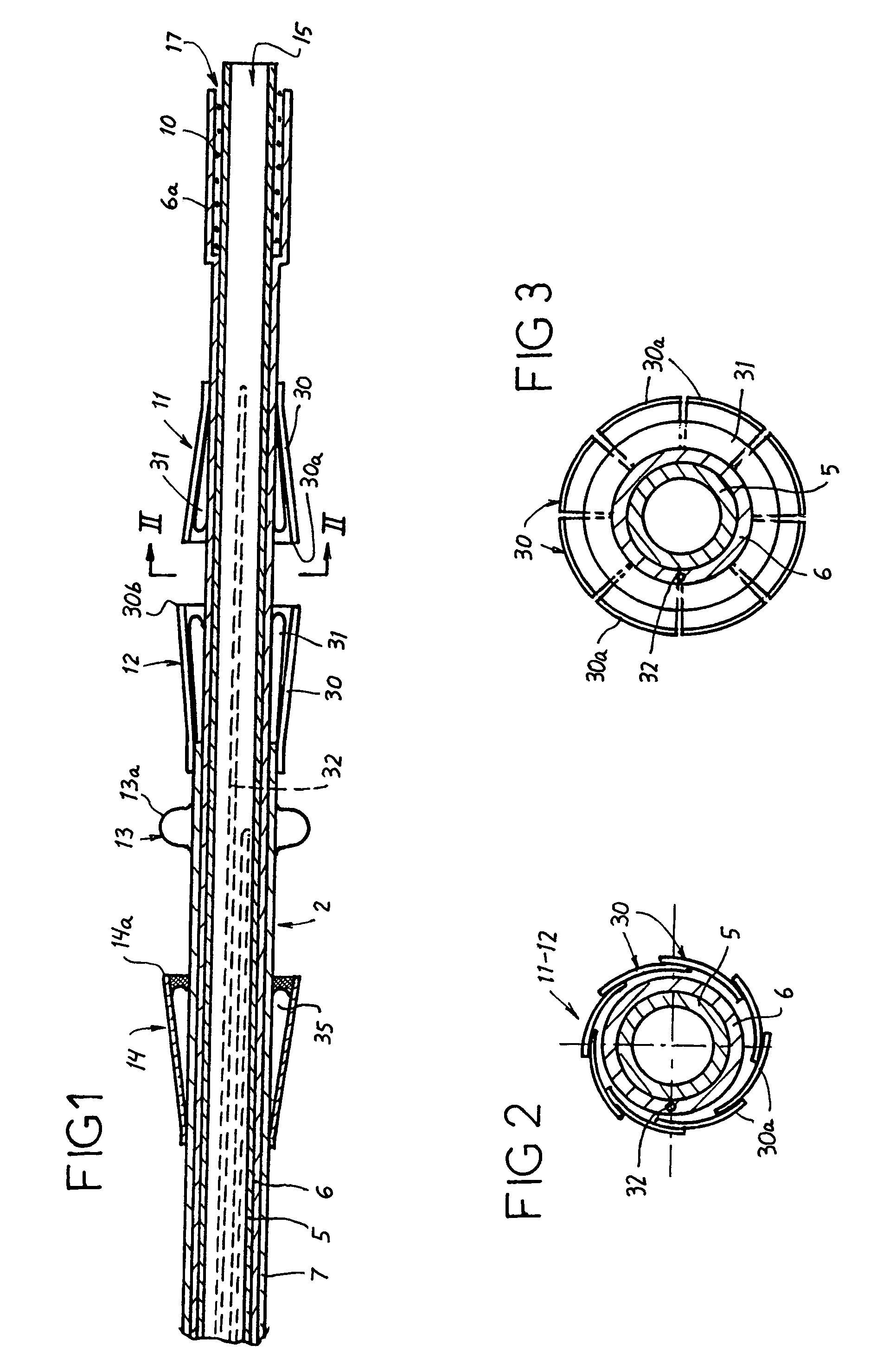
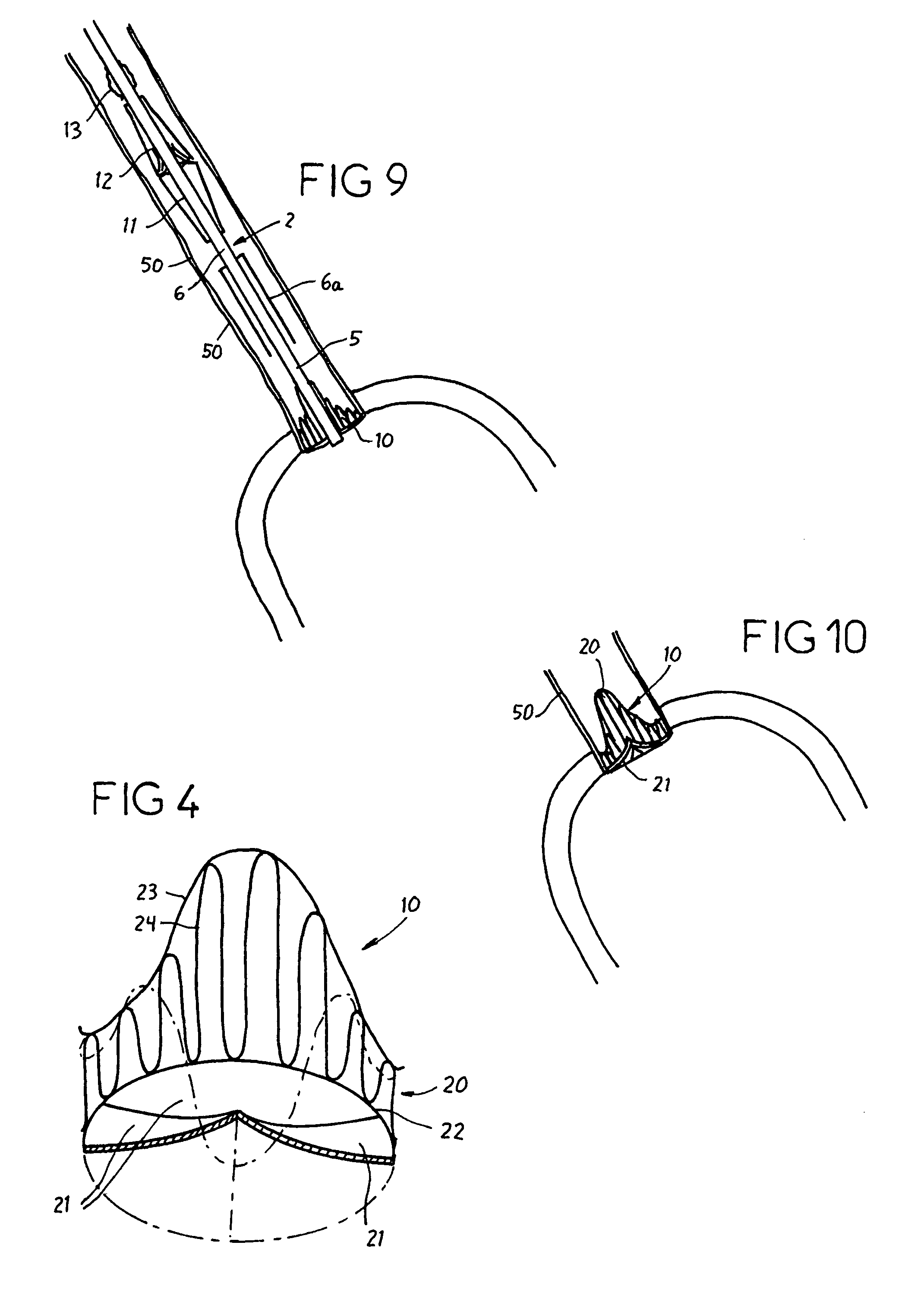
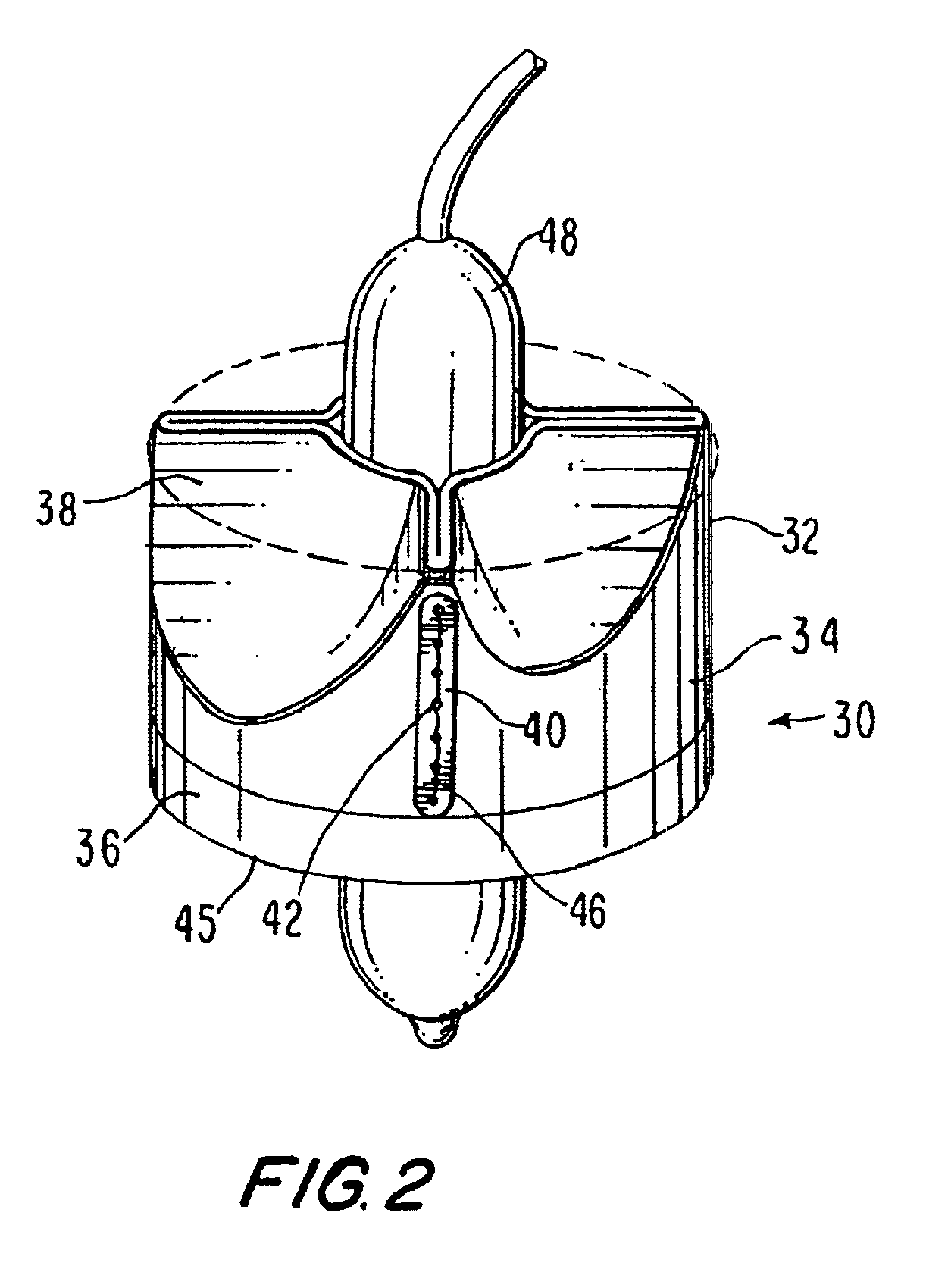
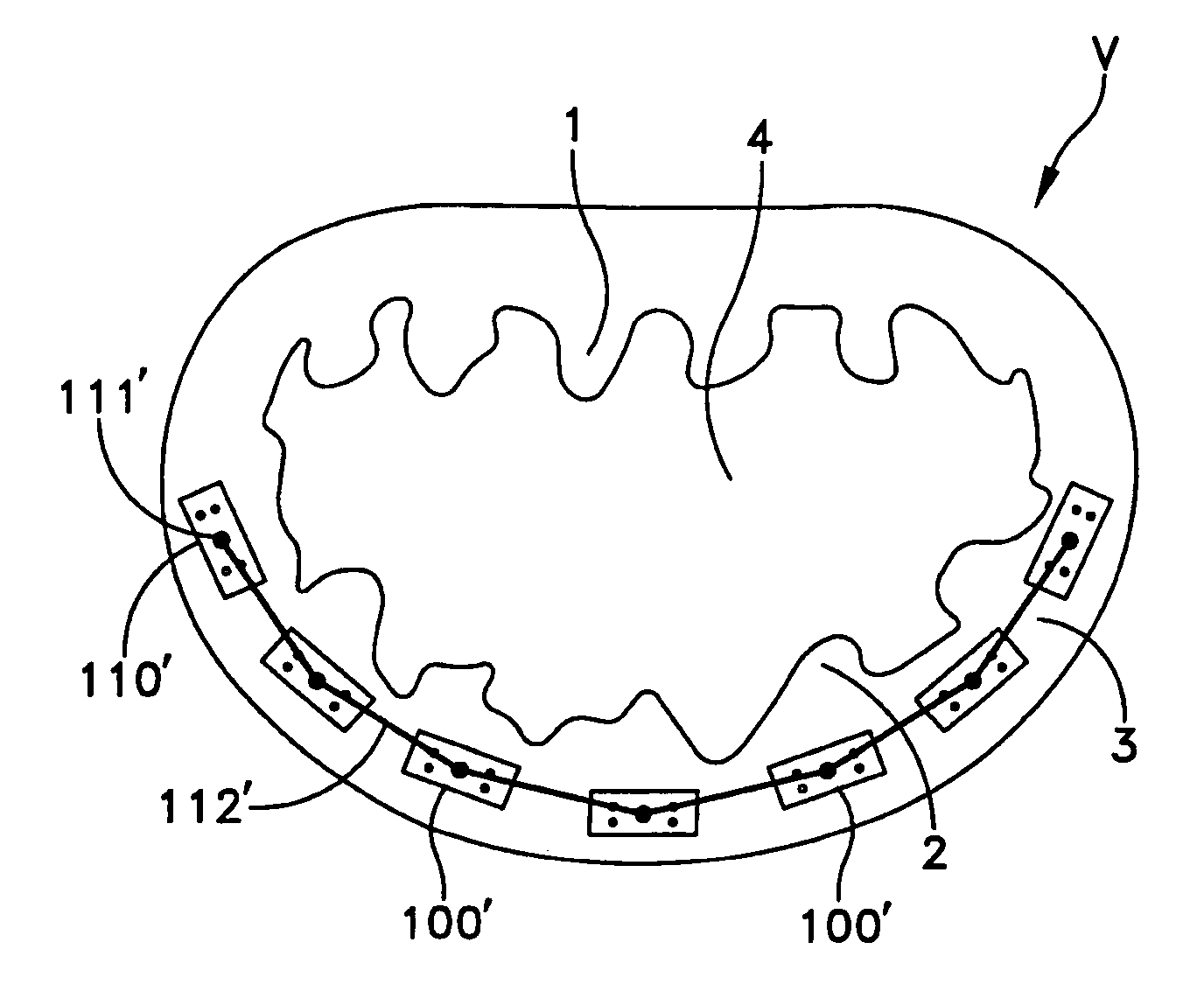
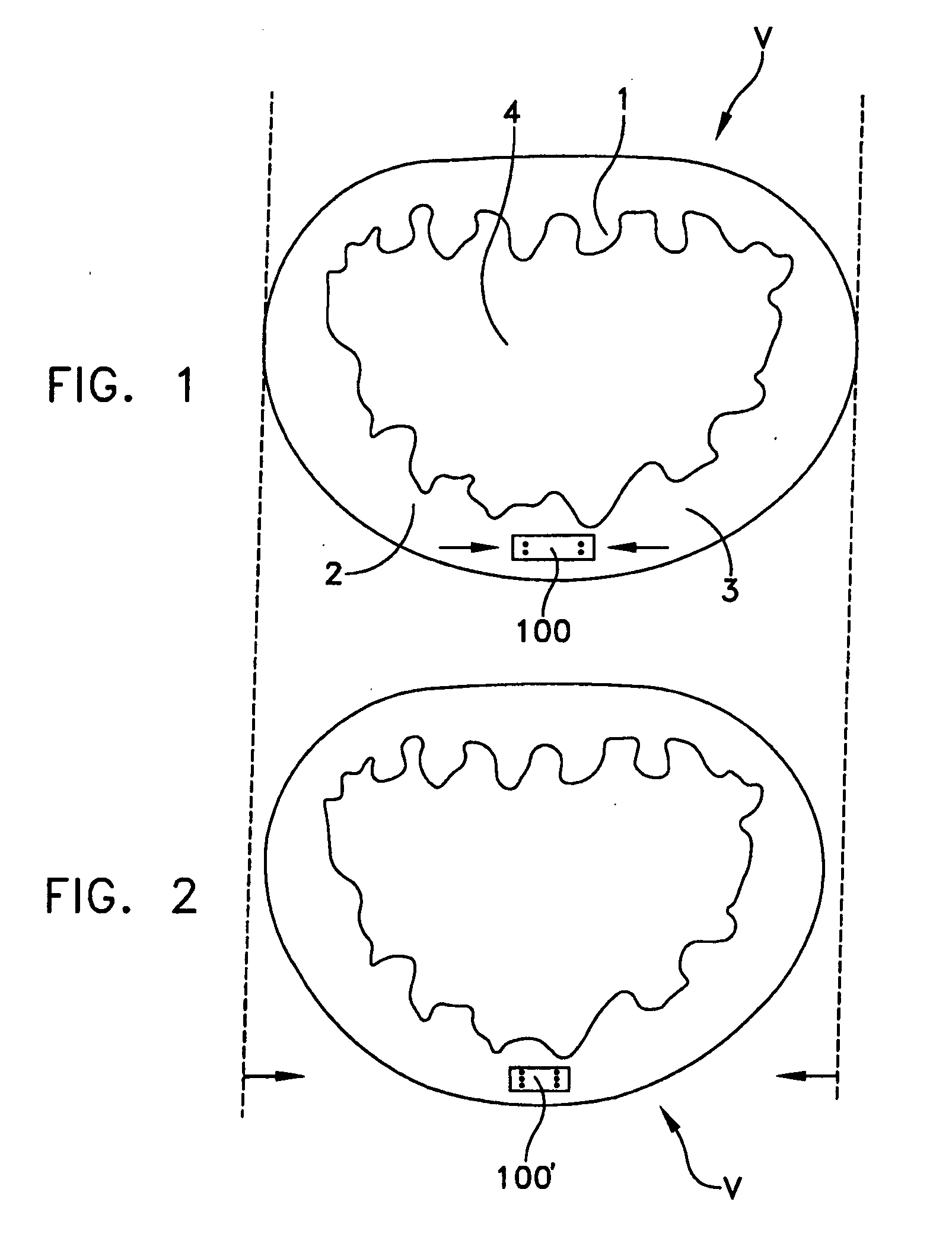
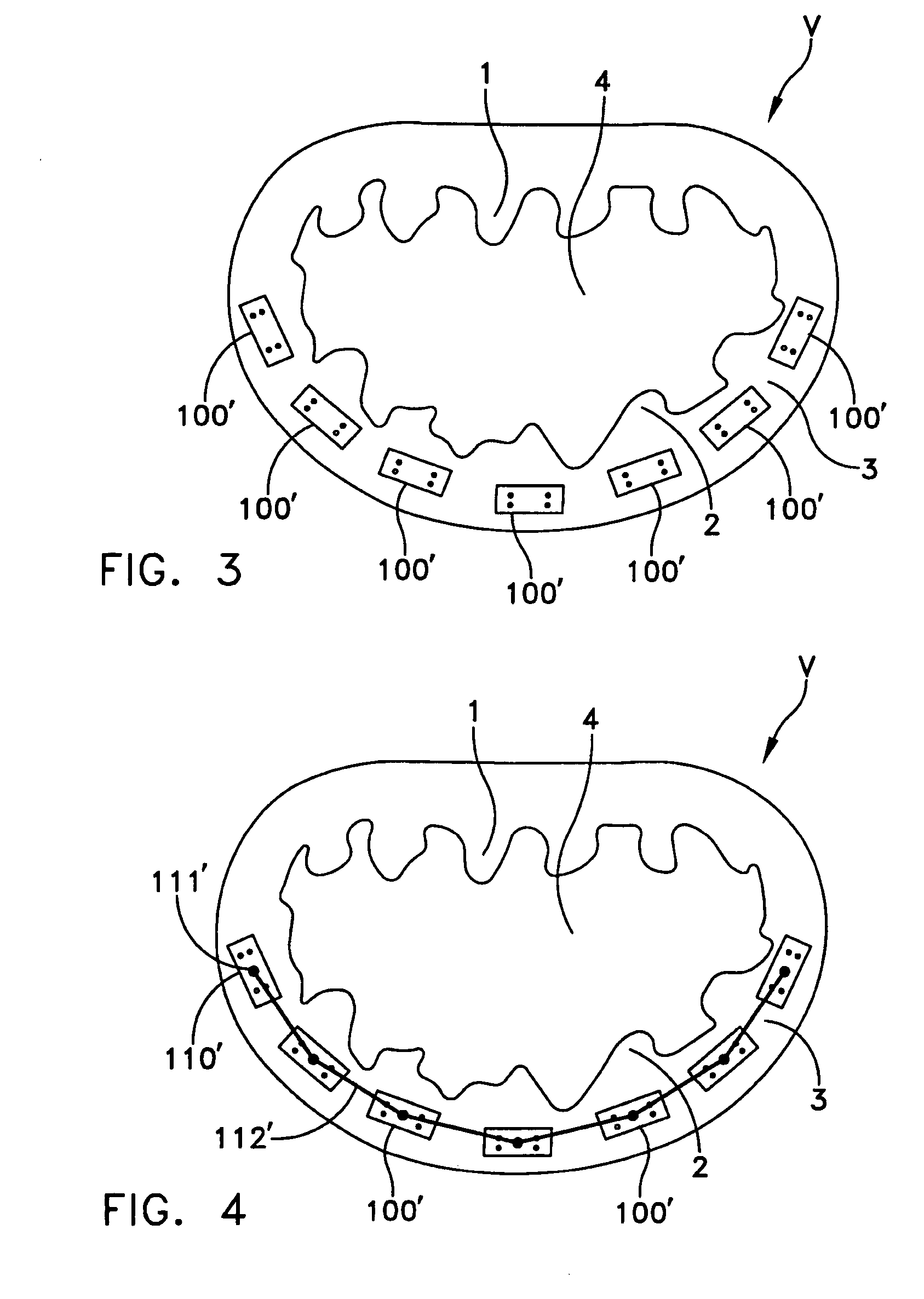
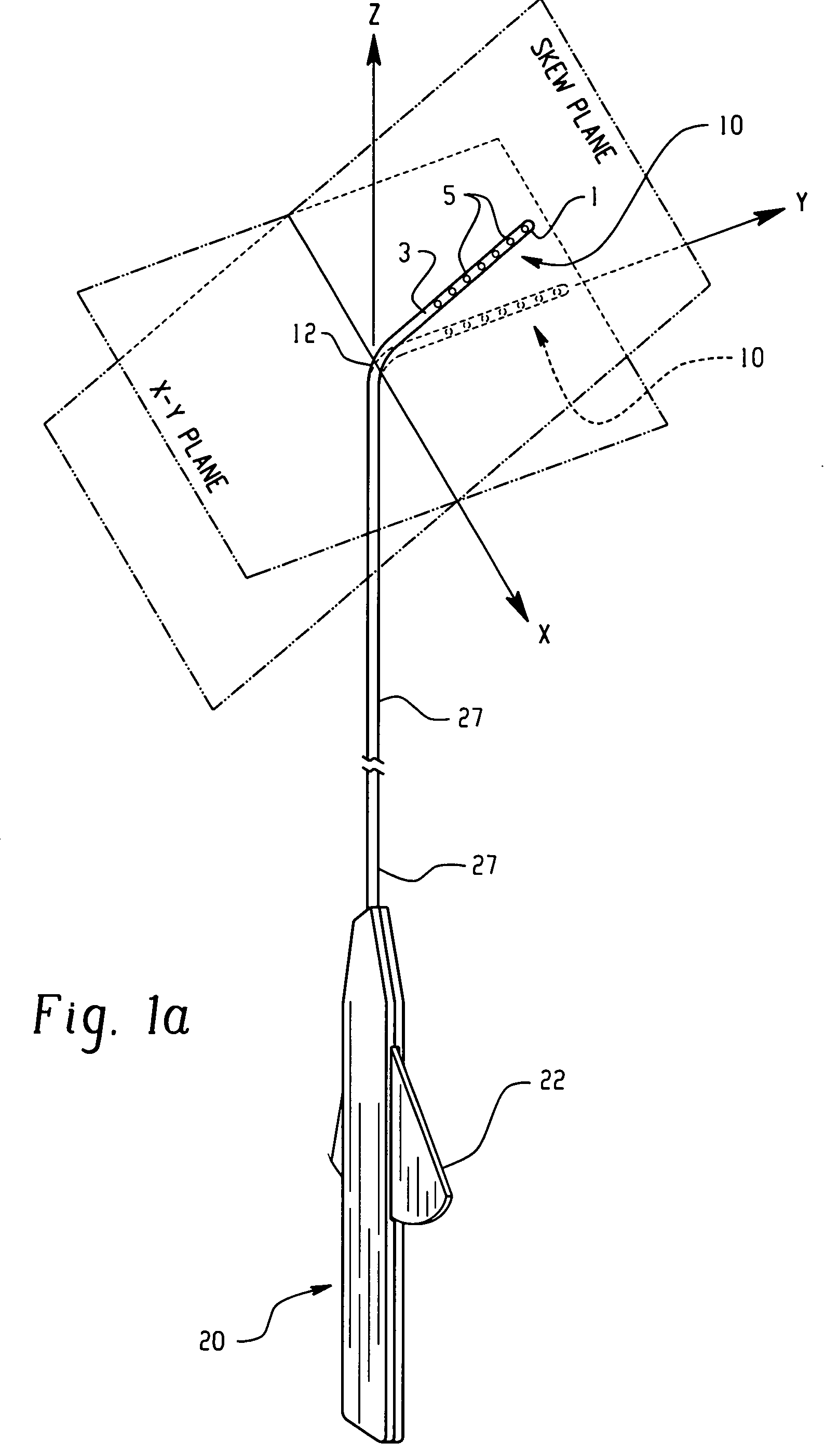
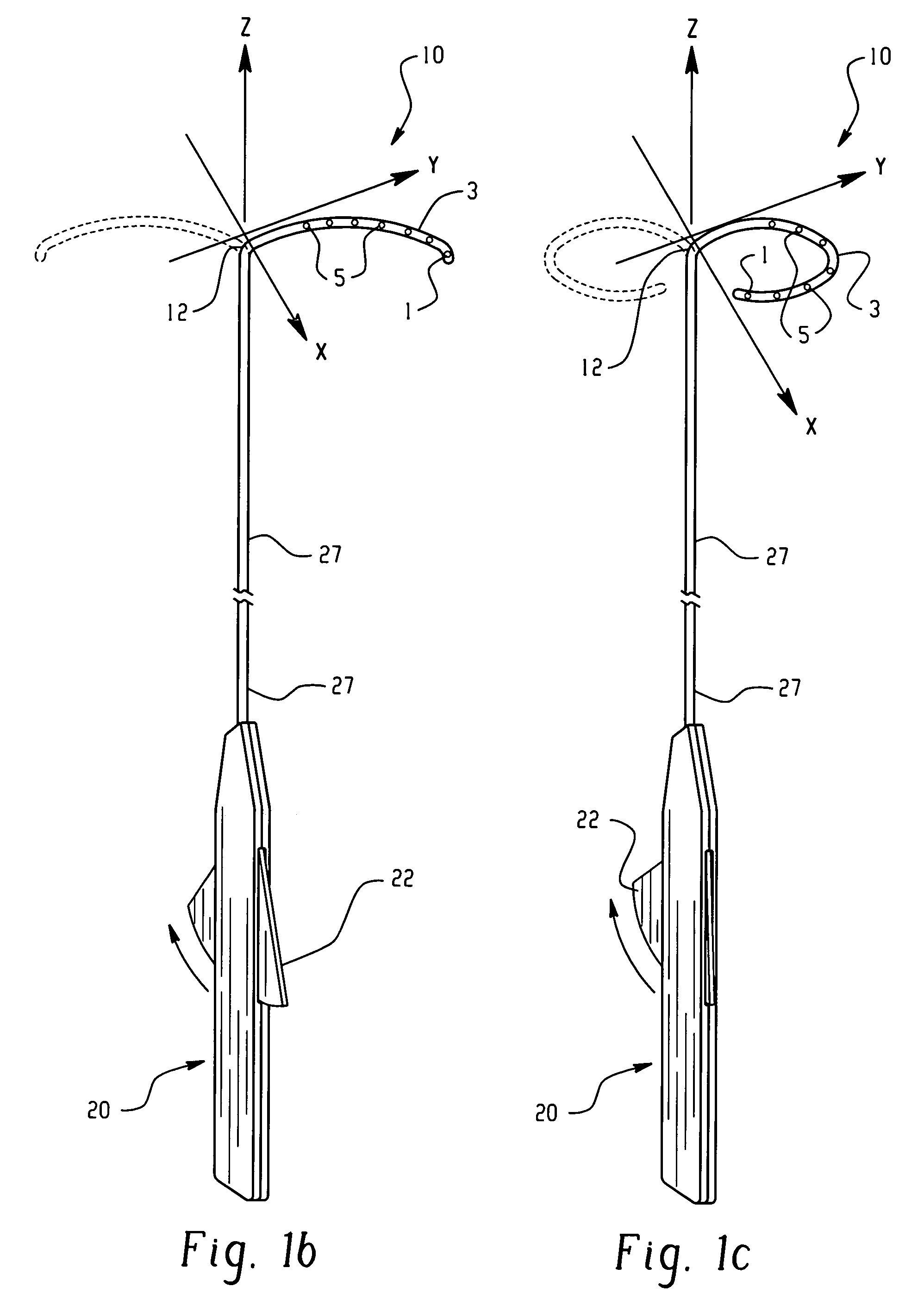
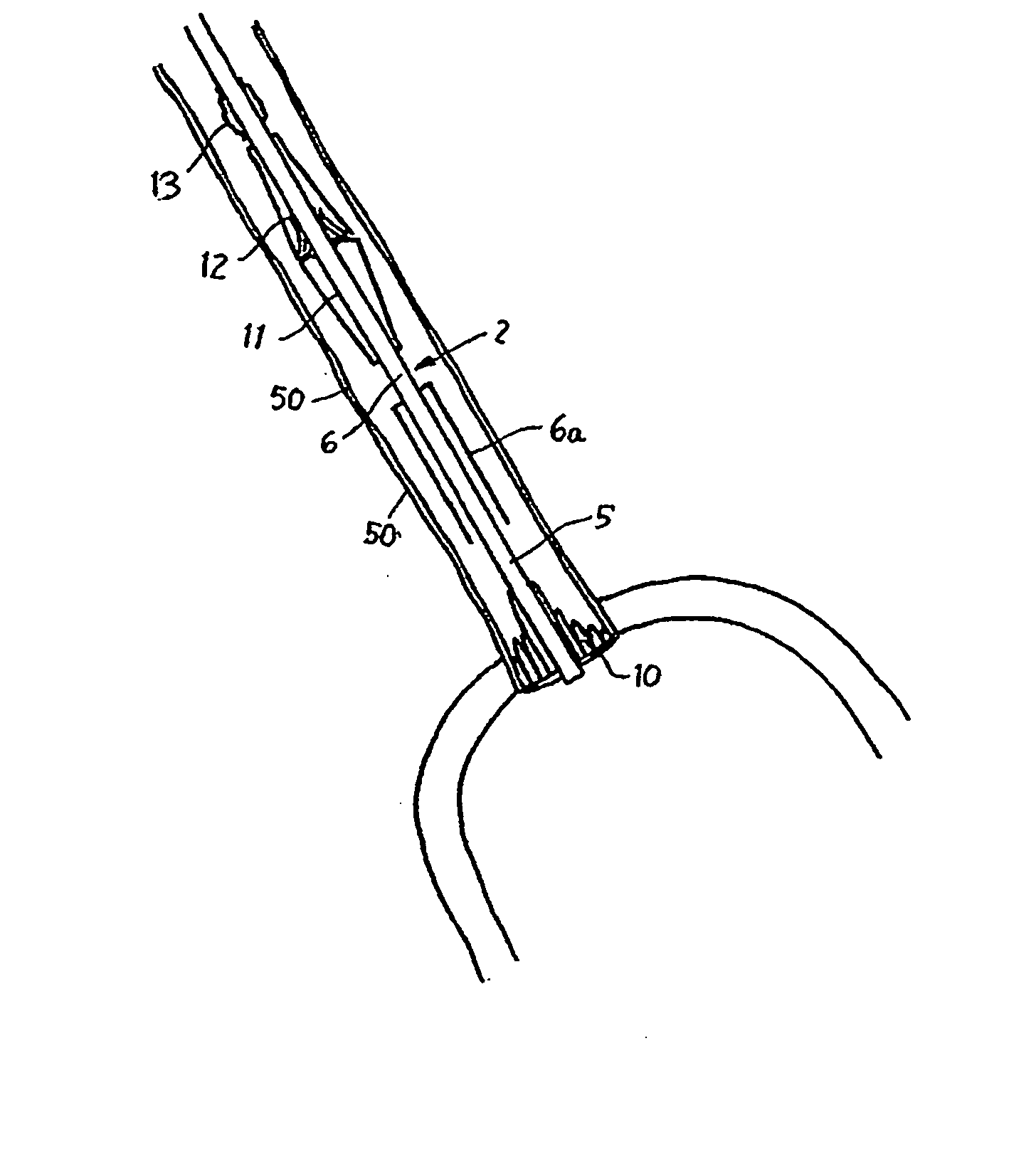
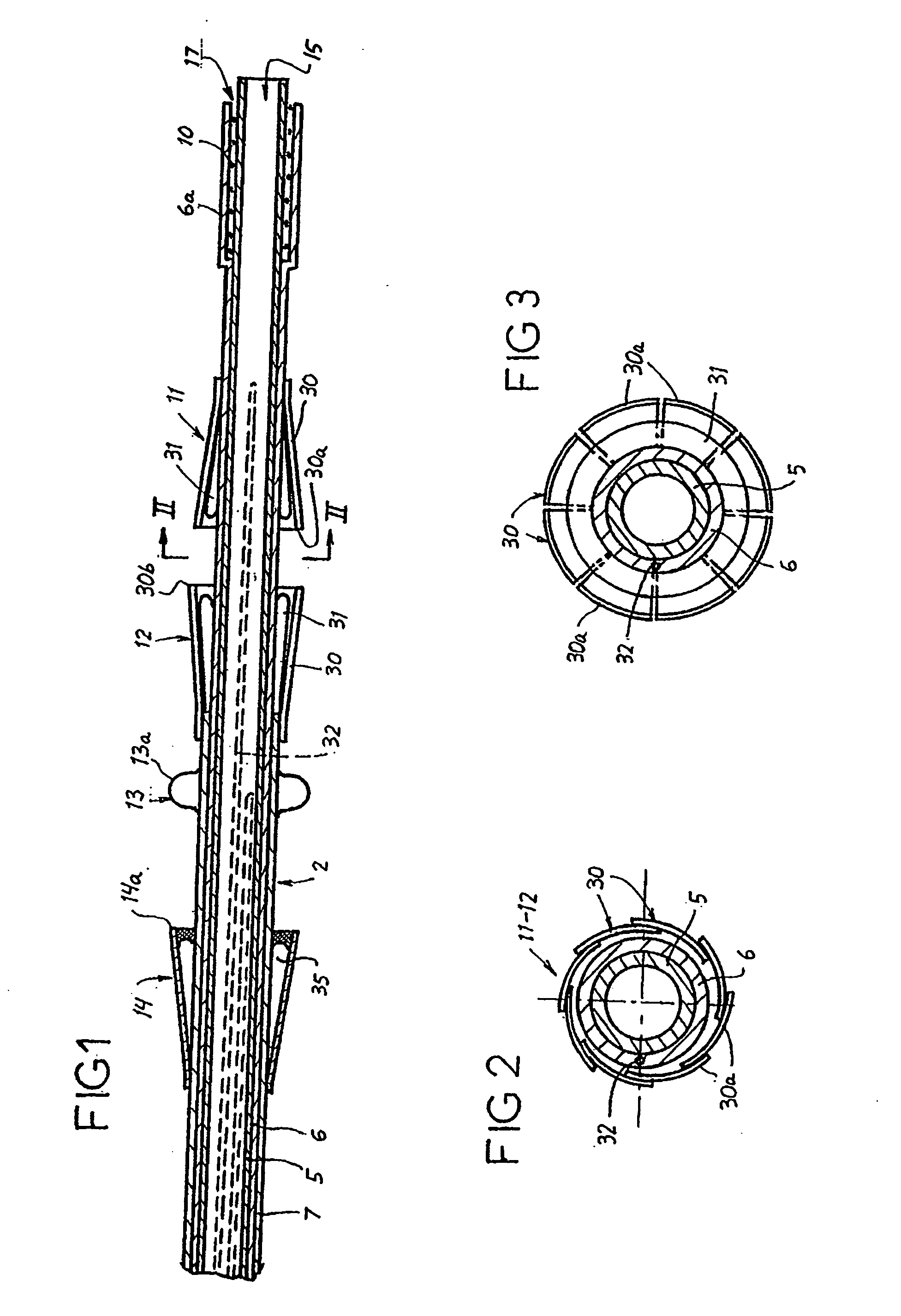
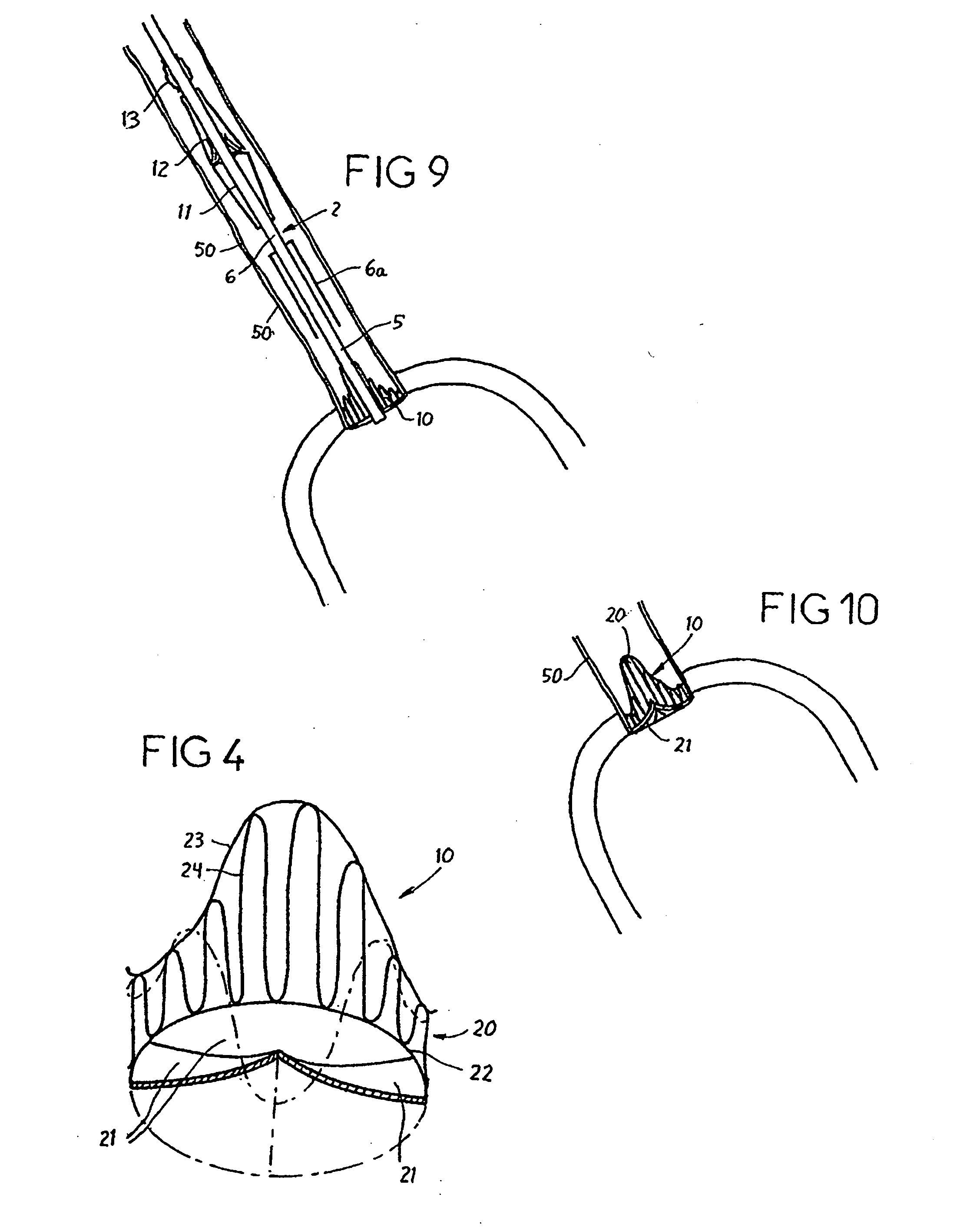
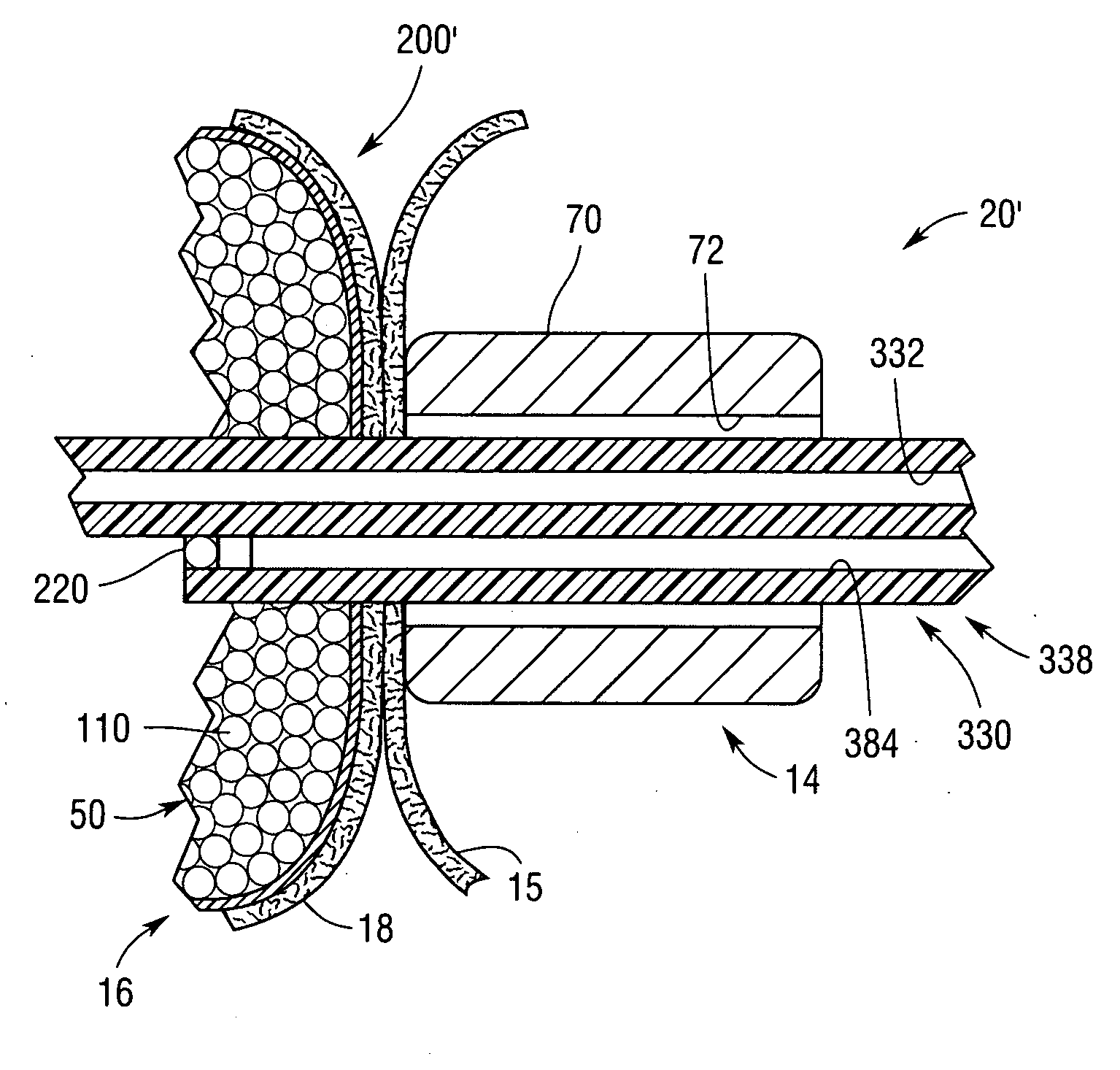
Catheter sheath for implant delivery
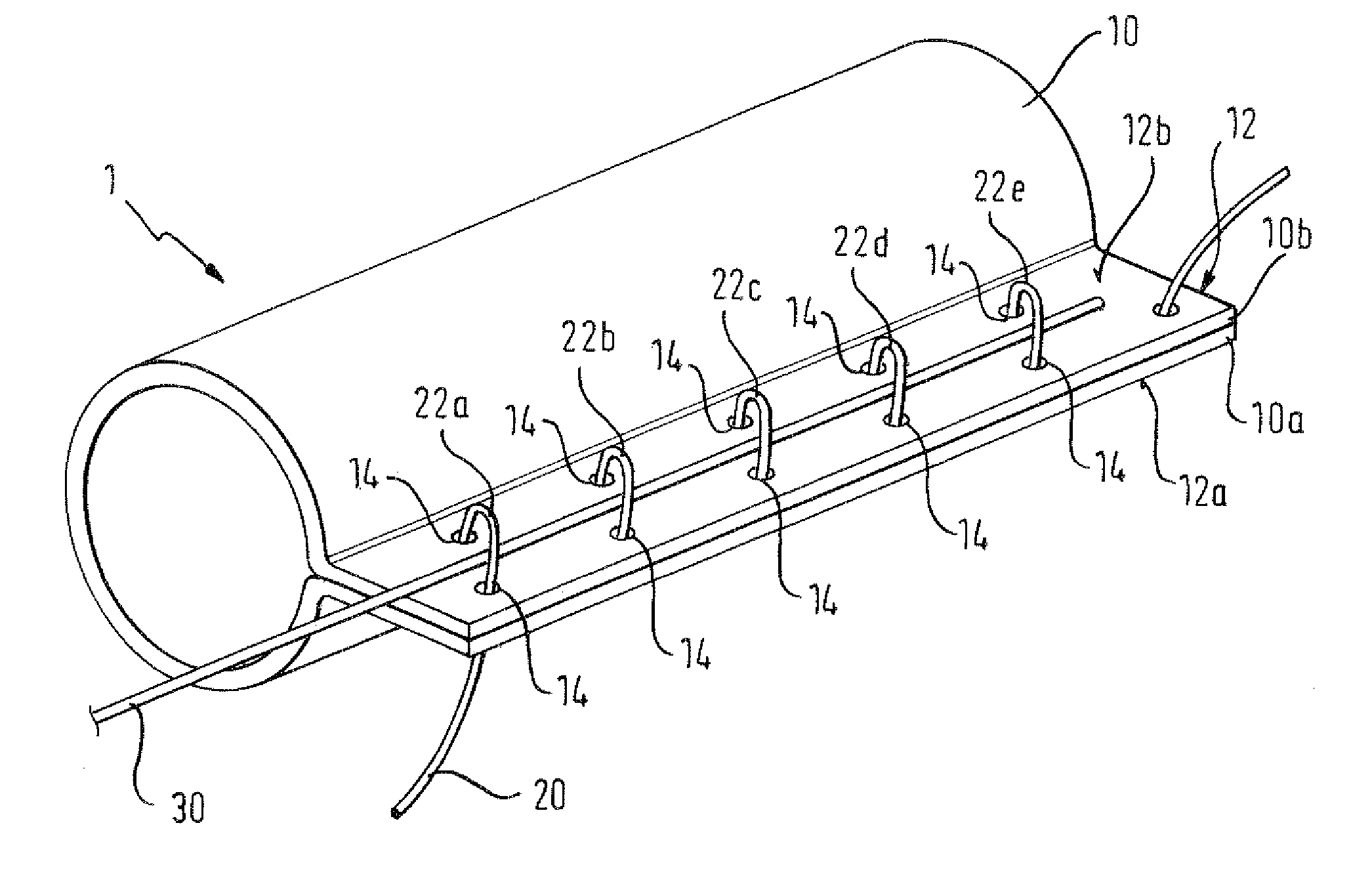
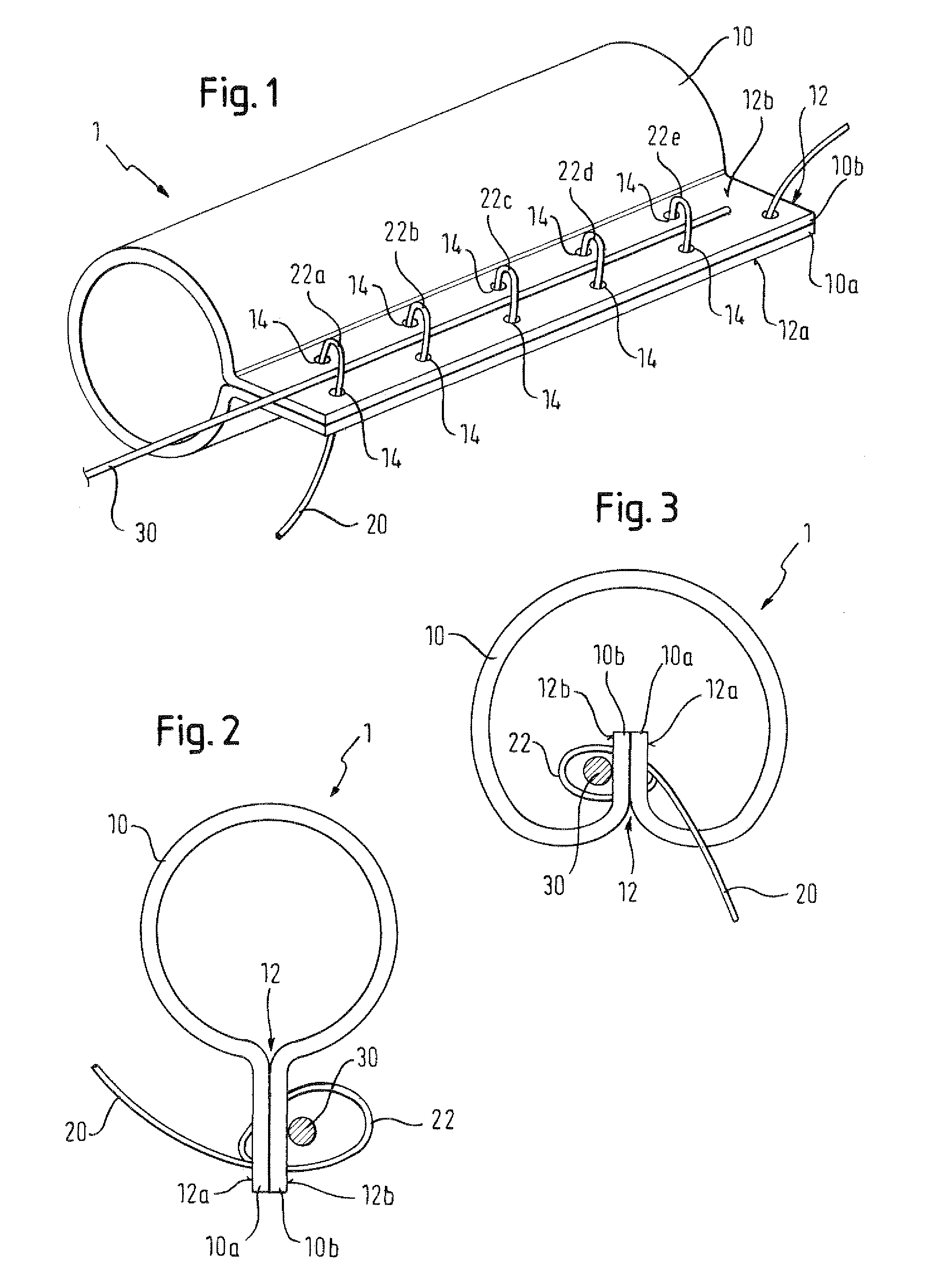
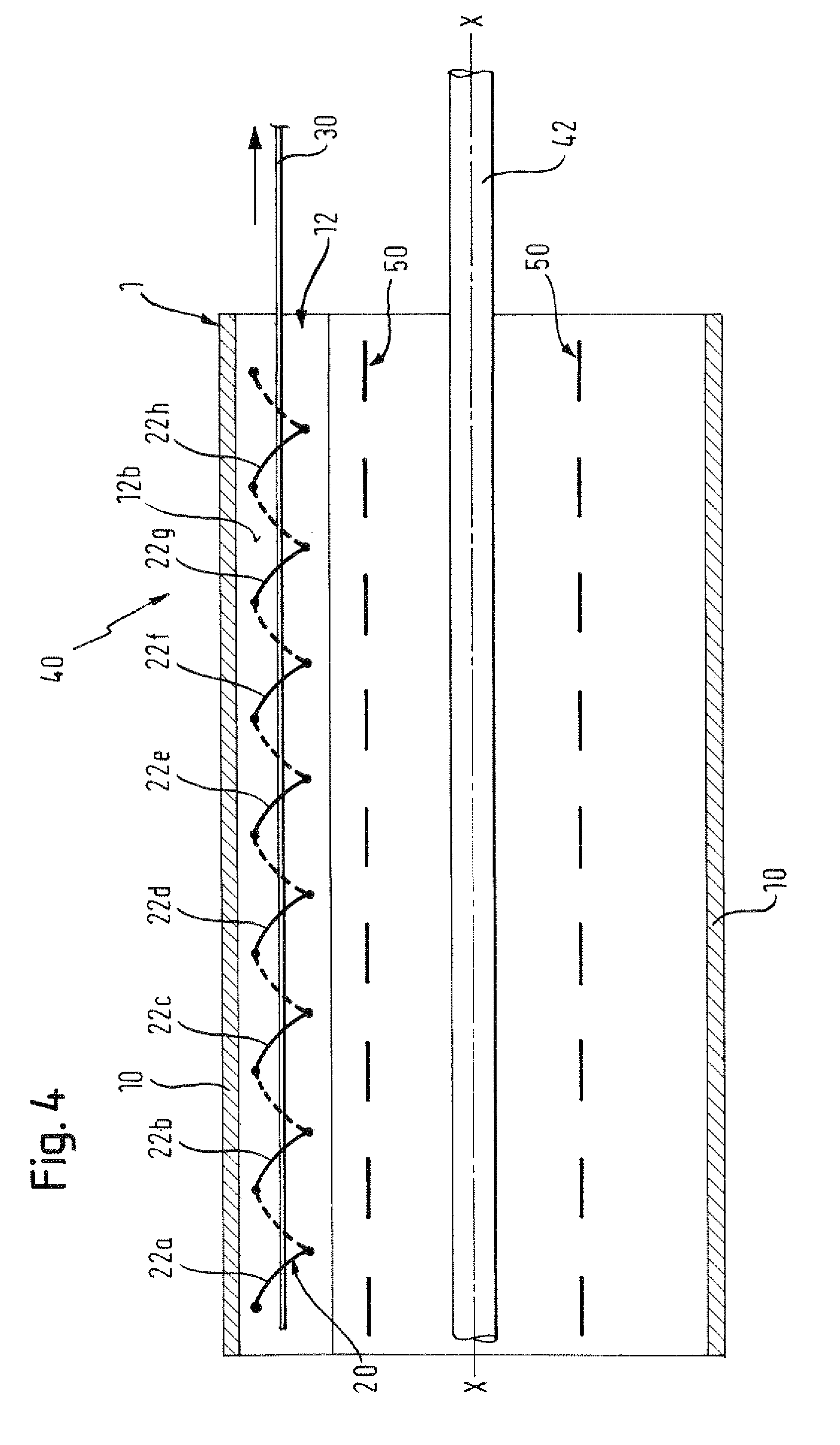
ActiveUS9060894B2Increase deflectionLarge radiusStentsBlood vesselsBiomedical engineeringCatheter sheath
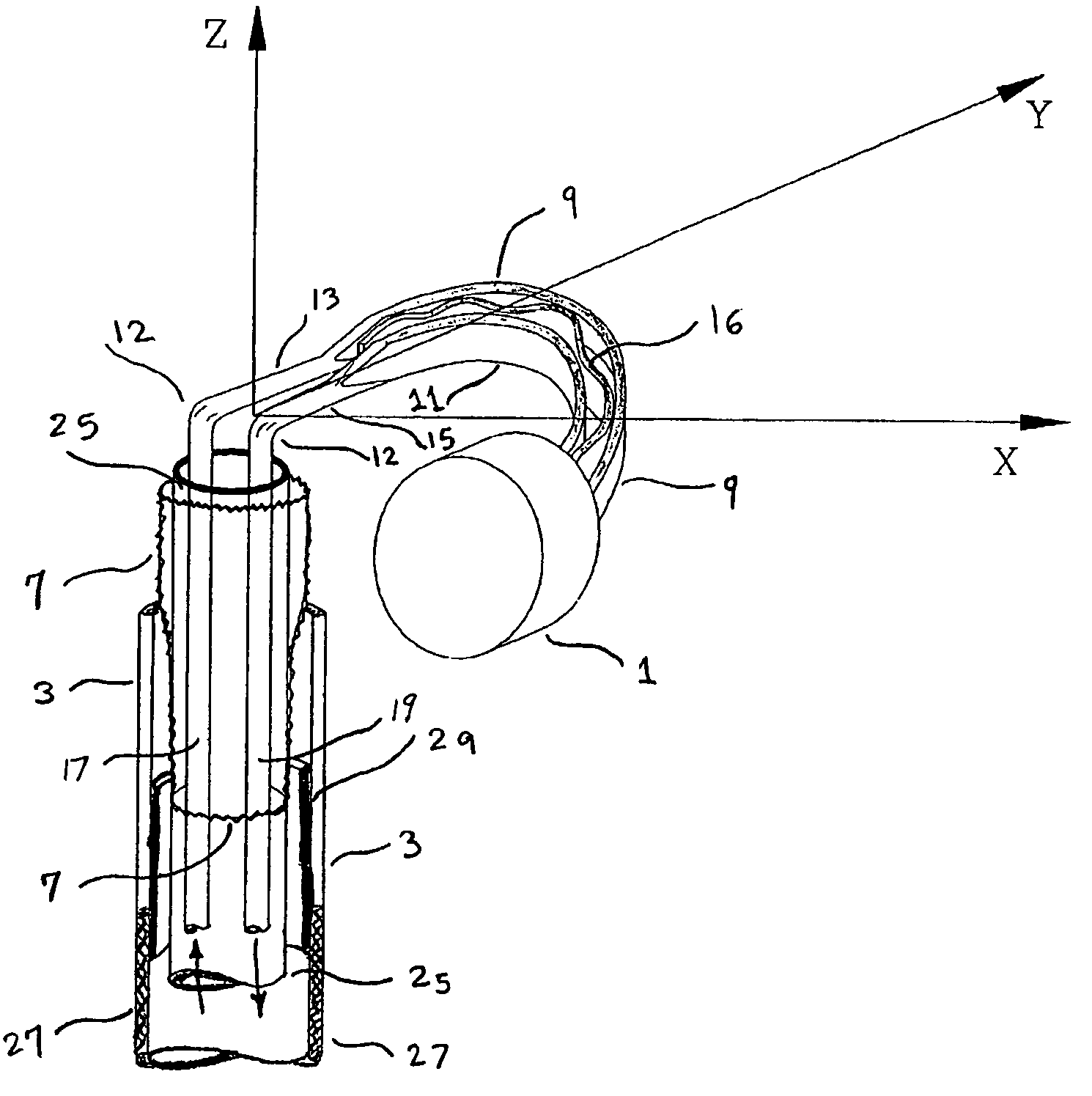
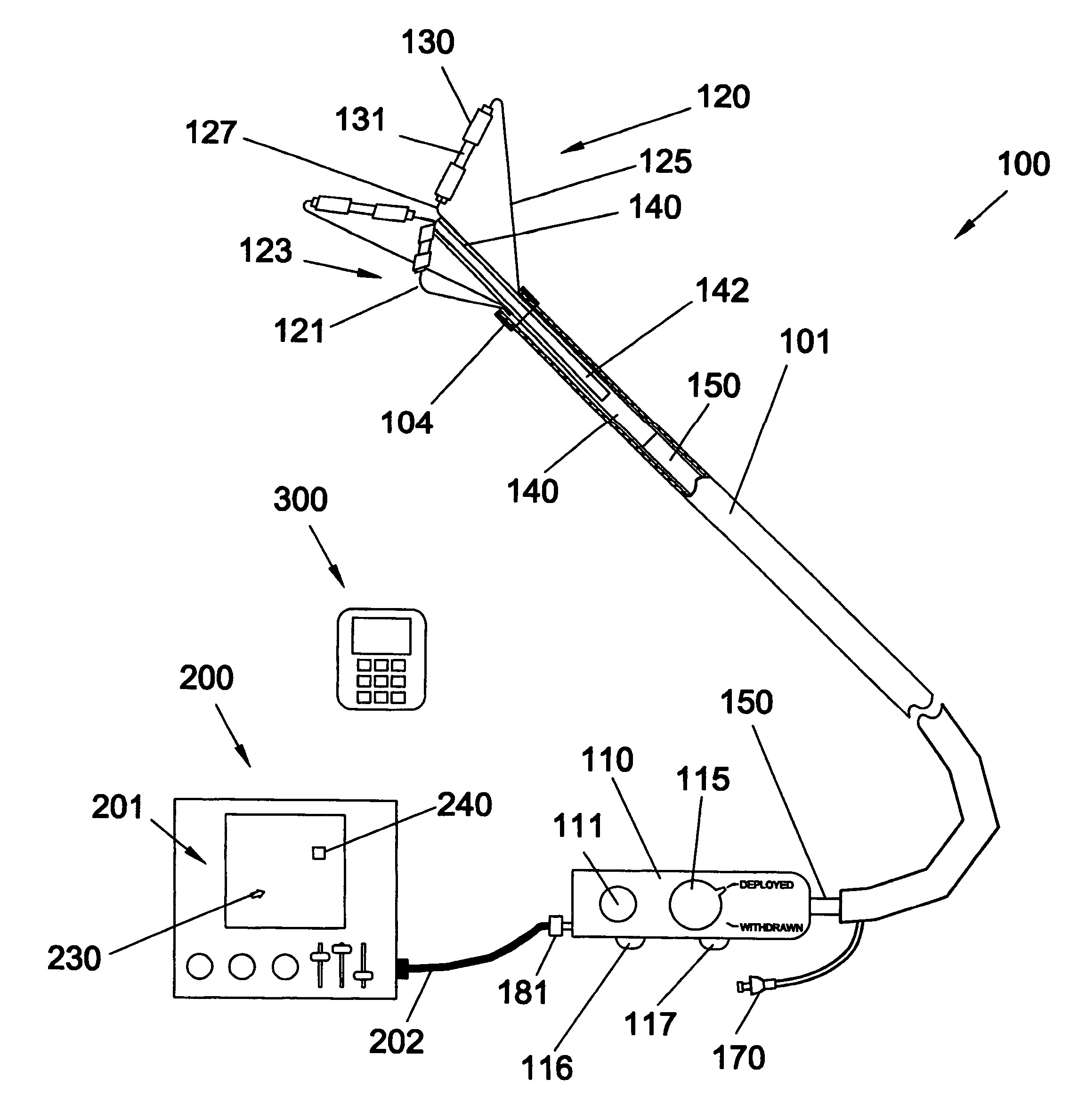
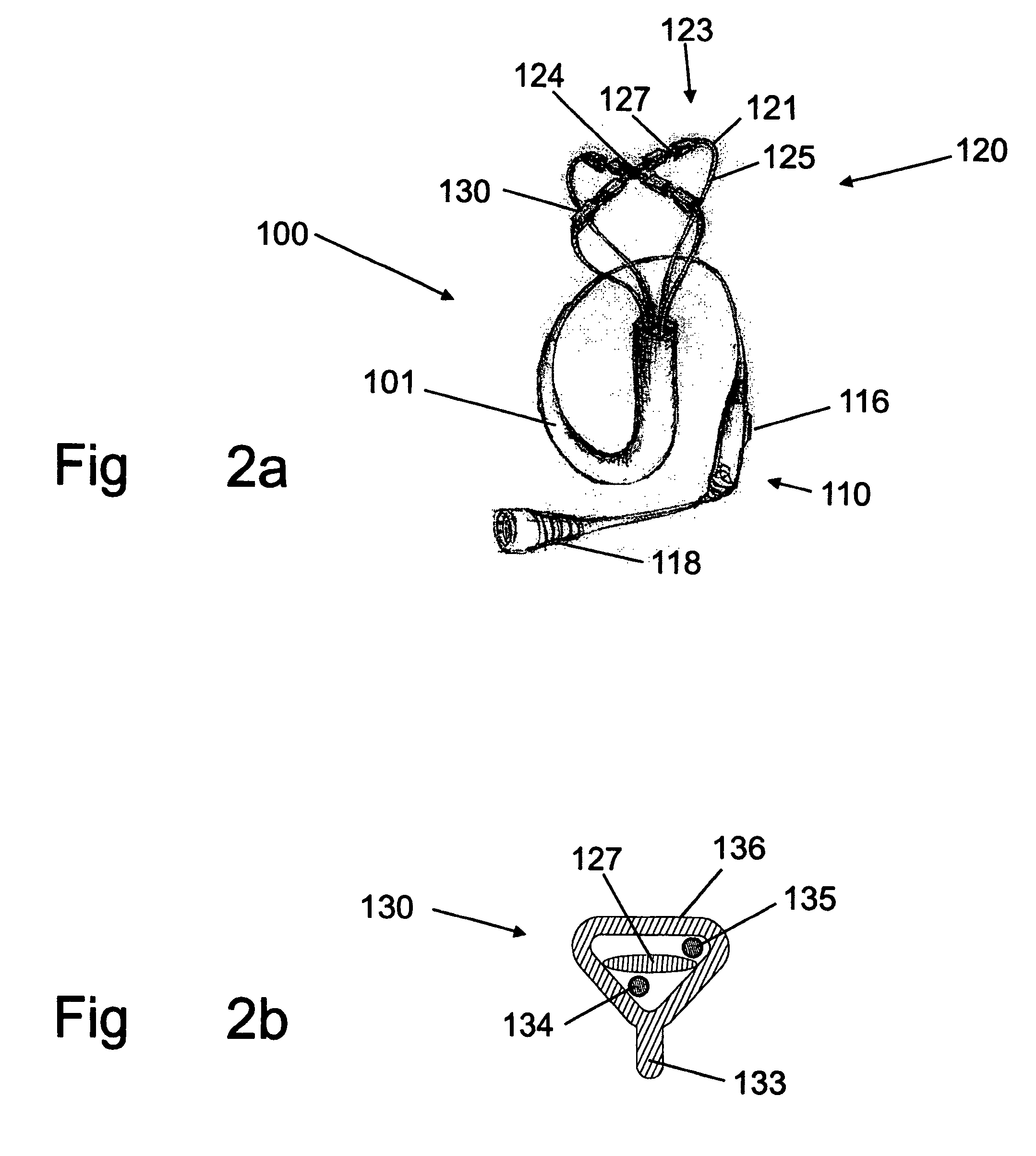
There is disclosed herein a tube of material (1) having a seam (12) extending between a proximal end and a distal end, two edges (10a, 10b) of the material meeting and at least partially overlapping along the seam (12) and being sewn together at the seam by stitches (21, 22, 24) of relatively flexible thread (20), the thread of one or more of the stitches (22, 24) passing from a first side (12a) of the overlapped edges (10a, 10b) of material, through both layers of overlapped material, crossing a relatively rigid member (30) which is disposed on a second, opposite side (12b) of the overlapped edges of material, and passing back to the first side (12a). There is further disclosed an implant delivery catheter (40) incorporating the tube of material (1), and associated methods for its manufacture.
Owner:CR BARD INC
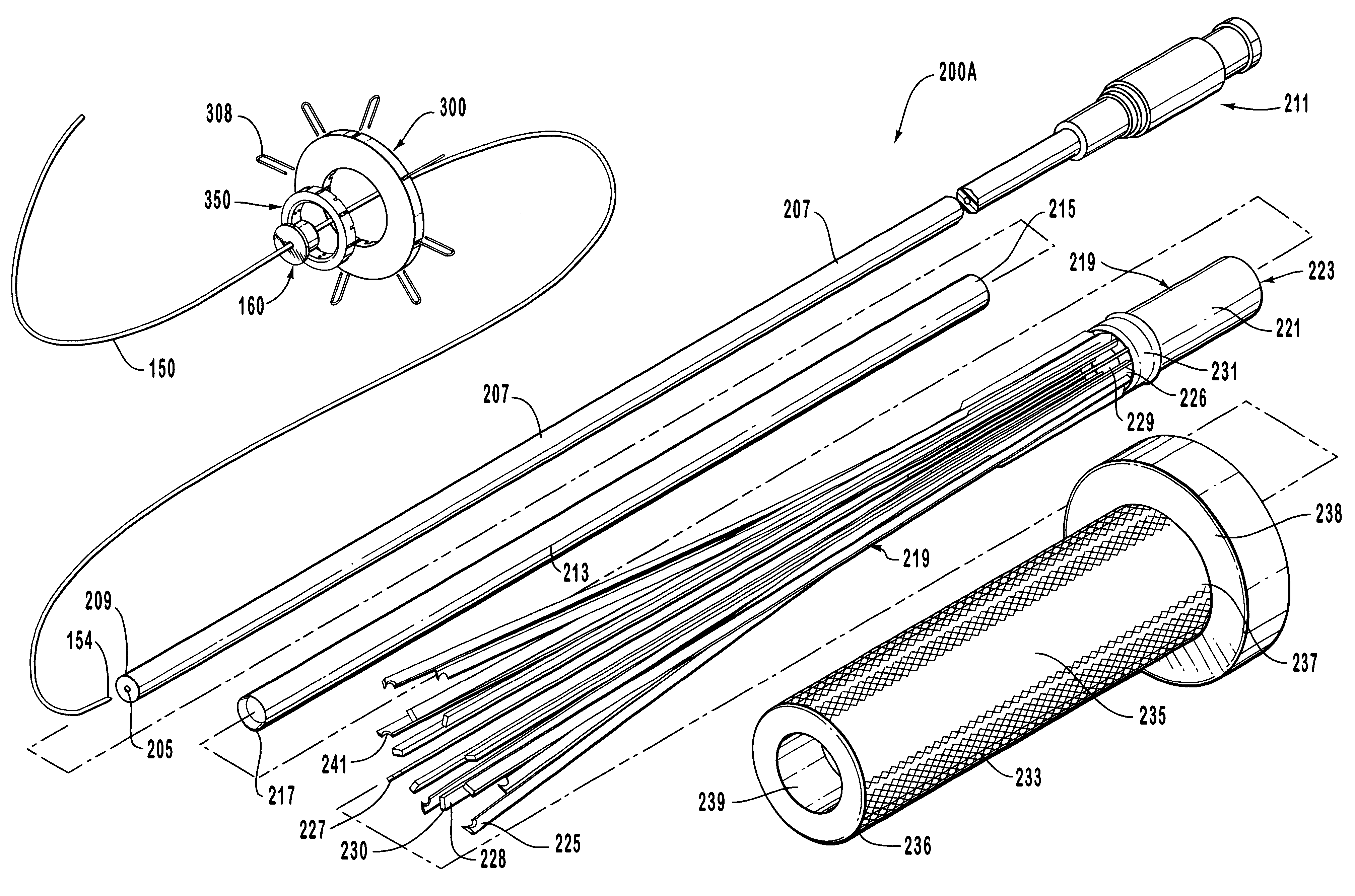
Flexible instrument
A method of performing a medical procedure on a patient comprises conveying control signals from a remote controller to a drive unit, intravenously introducing the catheter into a heart of the patient (e.g., via the vena cava into the right atrium), and creating a puncture within a wall between two chambers (e.g., the left and right atria) of the heart. The method further comprises creating a puncture within a wall between two chambers of the heart, and operating the drive unit in accordance with the control signals to advance a working catheter within the guide catheter through the puncture.
Owner:HANSEN MEDICAL INC
Surgical devices and methods using magnetic force to form an anastomosis
InactiveUS20080200934A1Maintain alignmentExcision instrumentsWound clampsMagnetic tension forceNatural orifice
A method for forming an anastomosis between first and second organs in a patient using a hollow receptacle that is inflatable with magnetic material. The method may include forming openings through the first and second organs utilizing a hole-forming instrument inserted into the organs through a natural orifice in the patient. The hollow receptacle may be supported on a catheter assembly that is also inserted through the patient's natural orifice and through the openings in the first and second organs and is positioned within the second organ. The hollow receptacle is then inflated with magnetic material and magnetic force is applied within the force organ to draw the inflated receptacle toward the first organ such that the inflated receptacle retains the second organ in sealing contact with the first organ while maintaining the alignment between the first and second openings to create an anastomosis between the first and second organs.
Owner:ETHICON ENDO SURGERY INC
Intravascular delivery system for therapeutic agents
Described herein is a system for intravascular drug delivery system, which includes a reservoir implantable a blood vessel, an intravascular pump fluidly coupled to the reservoir and an anchor expandable into contact with a wall of the blood vessel to retain the system within the vasculature. Delivery conduits may be extend from the reservoir and are positionable at select locations within the vasculature for target drug delivery to select organs or tissues.
Owner:WILLIAMS MICHAEL S +2
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com