Patents
Literature
4123 results about "Plasma viscosity" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
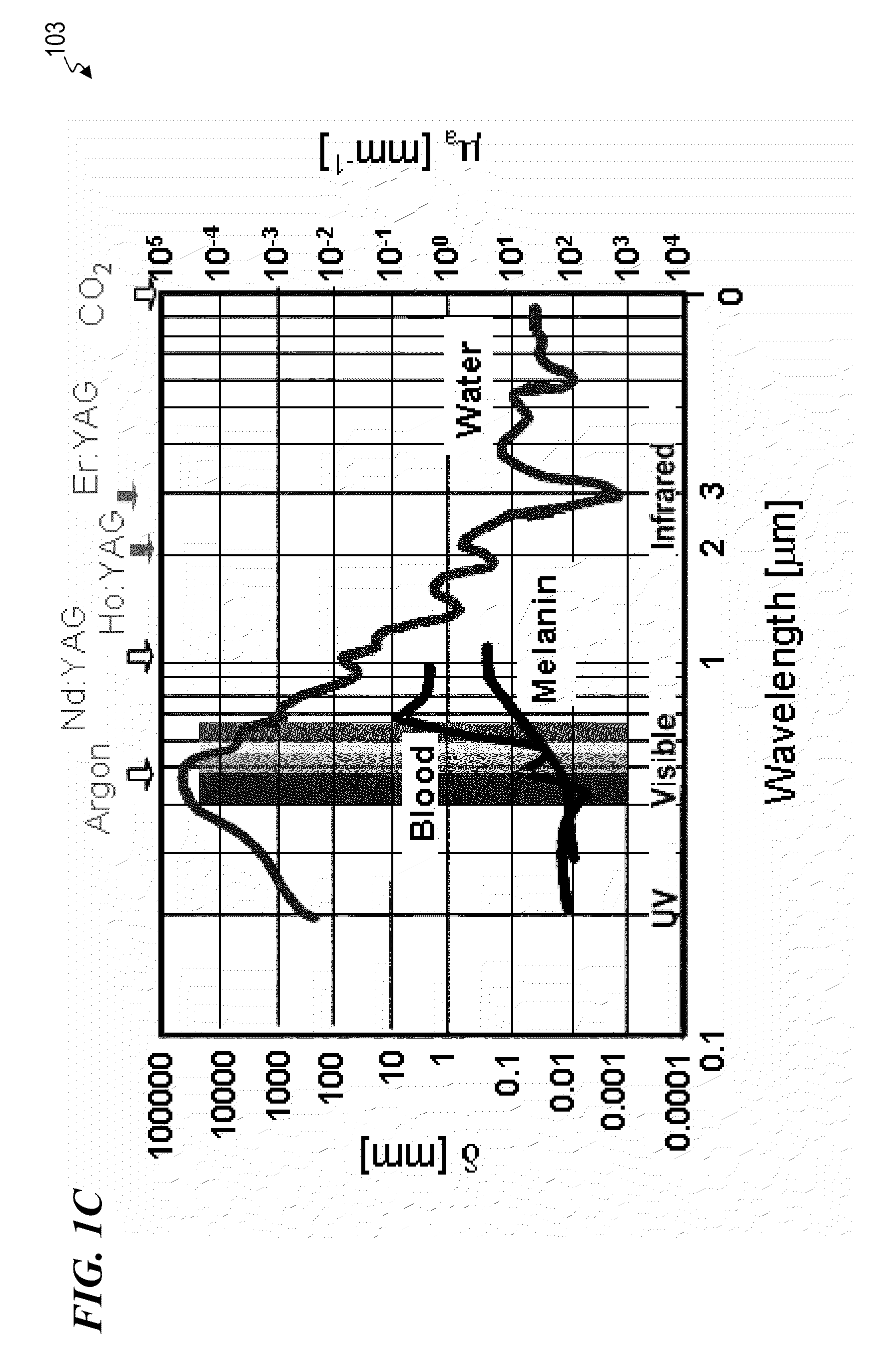
Typical values for the viscosity of normal human plasma at 37 °C is 1.4 mN·s/m2. The viscosity of normal plasma varies with temperature in the same way as does that of its solvent water; a 5 °C increase of temperature in the physiological range reduces plasma viscosity by about 10%.
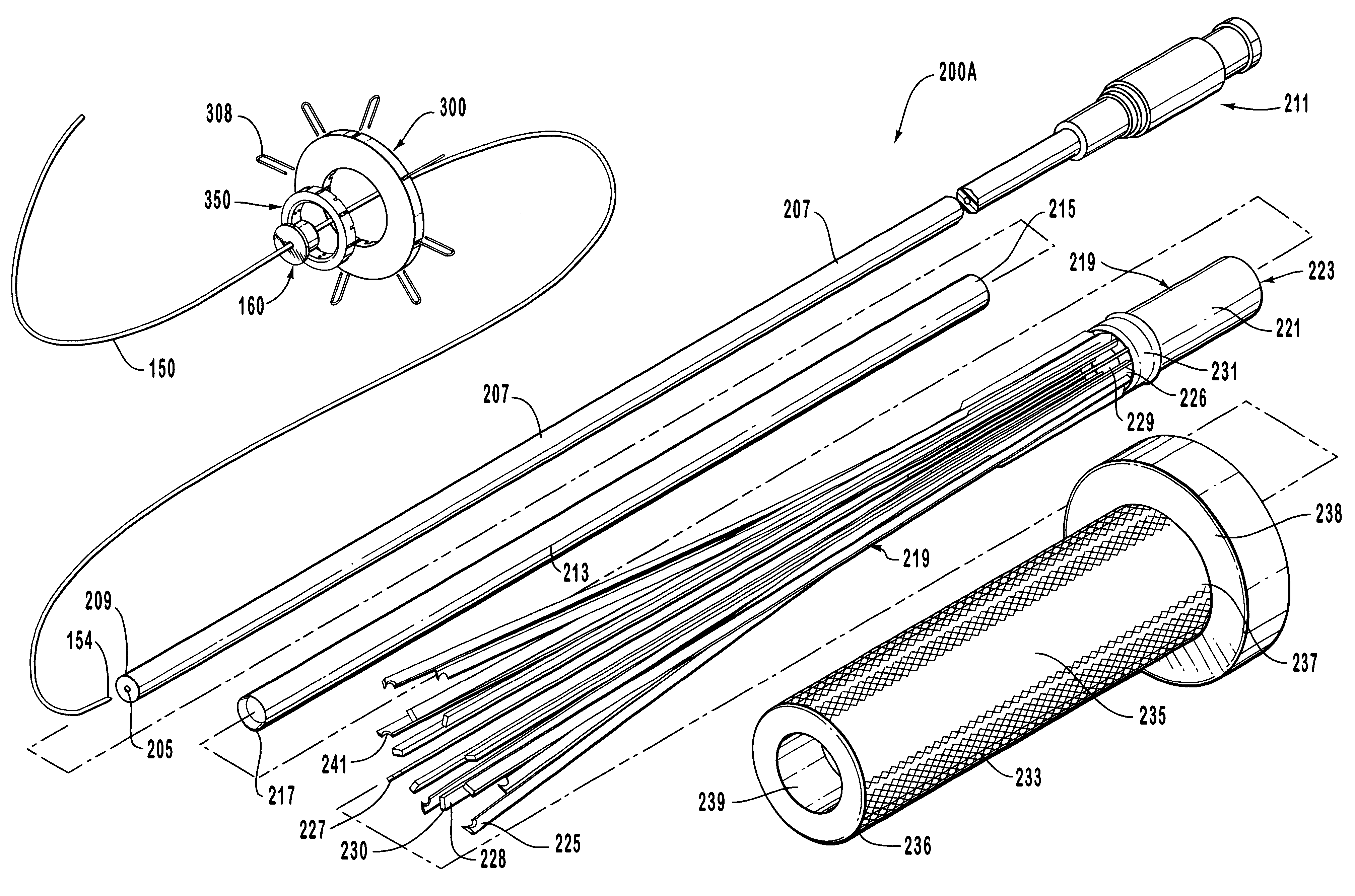
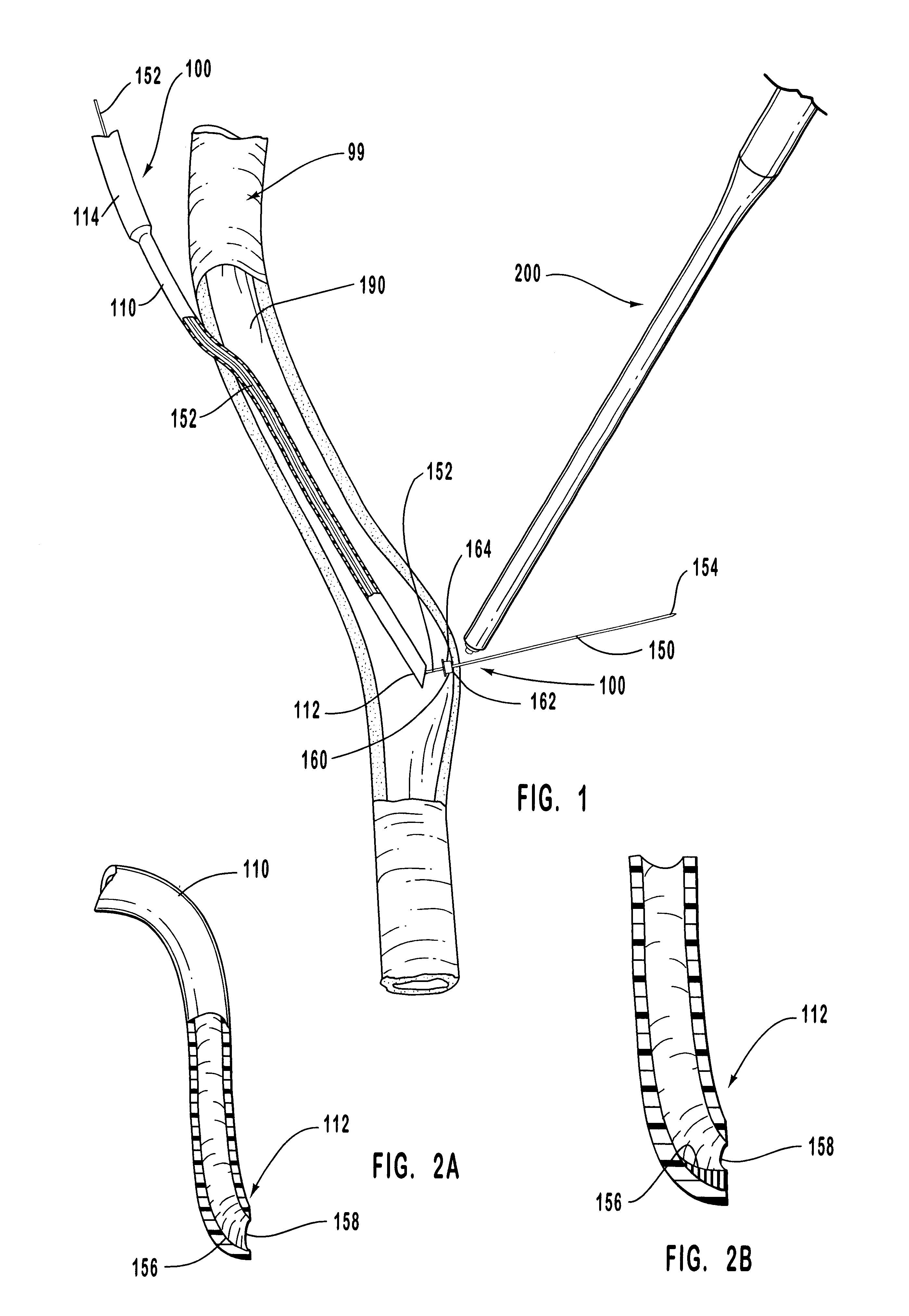
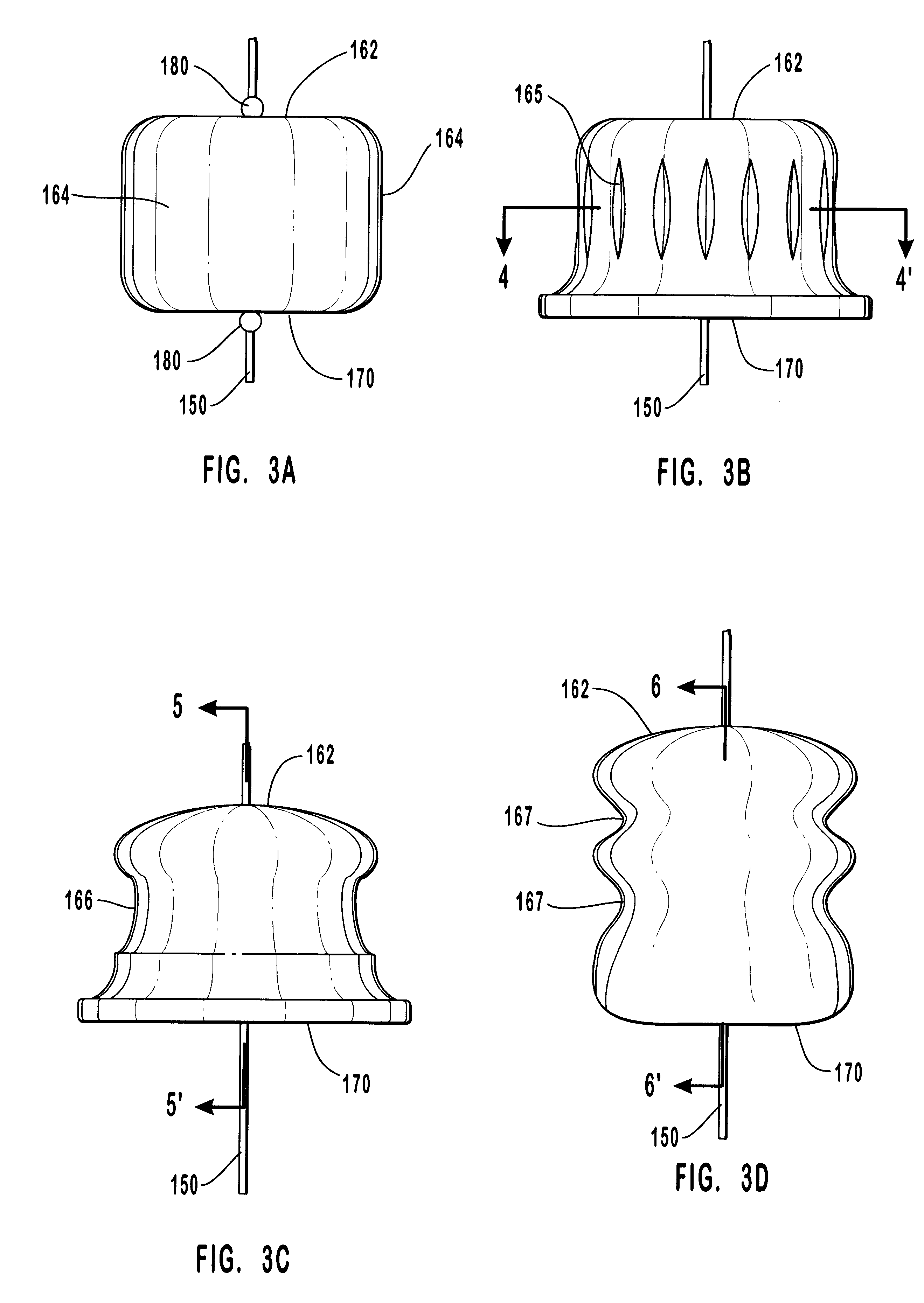
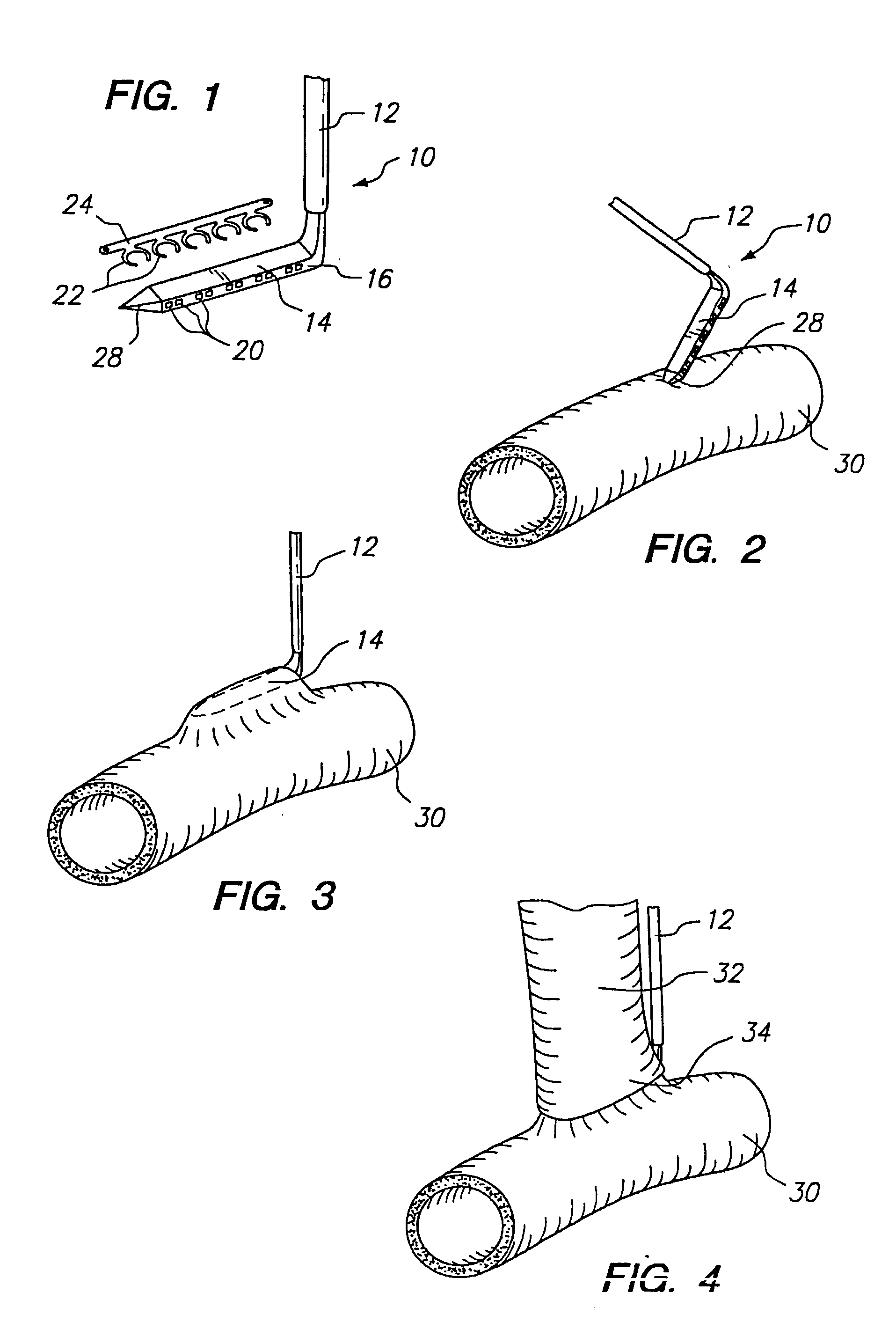
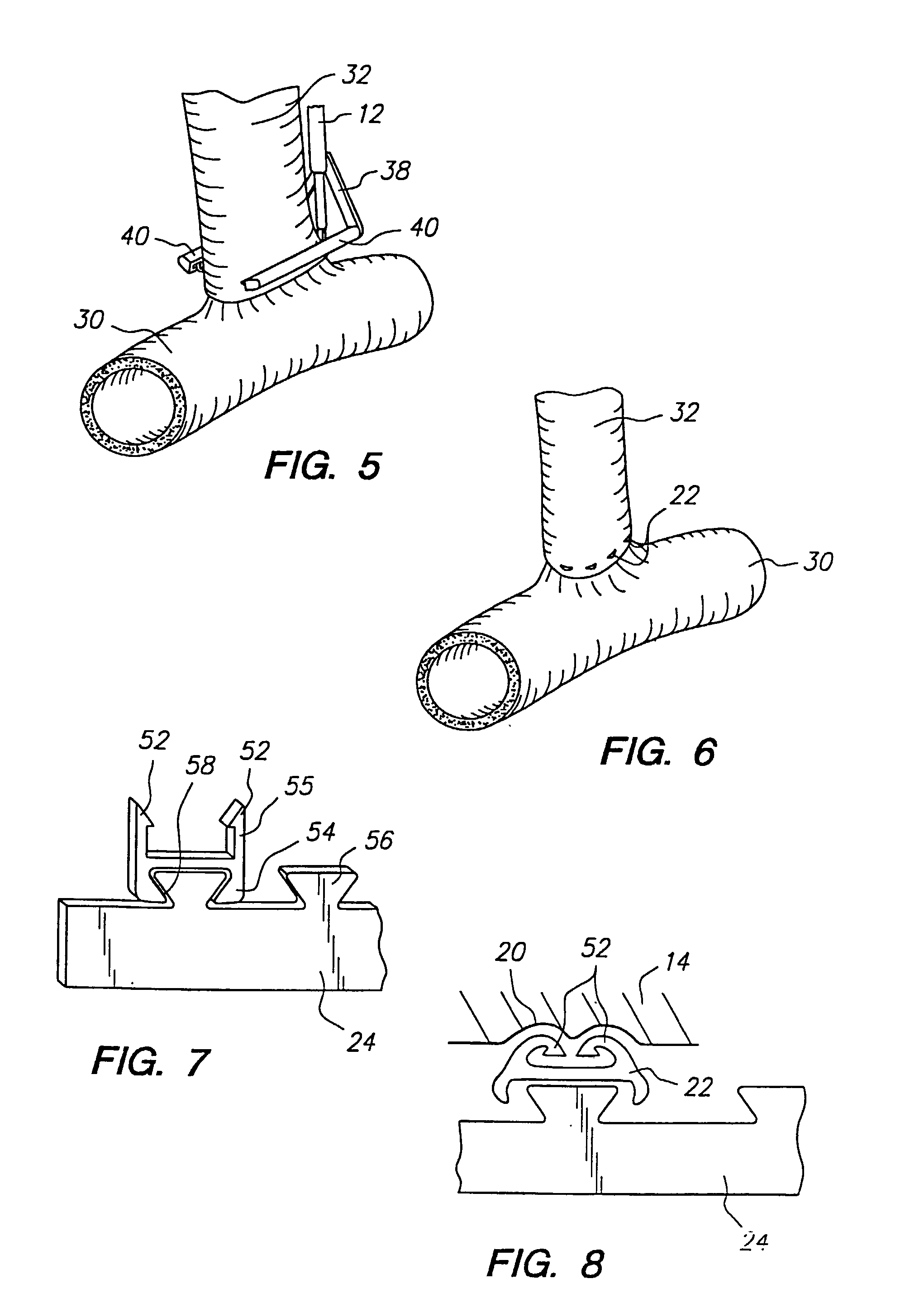
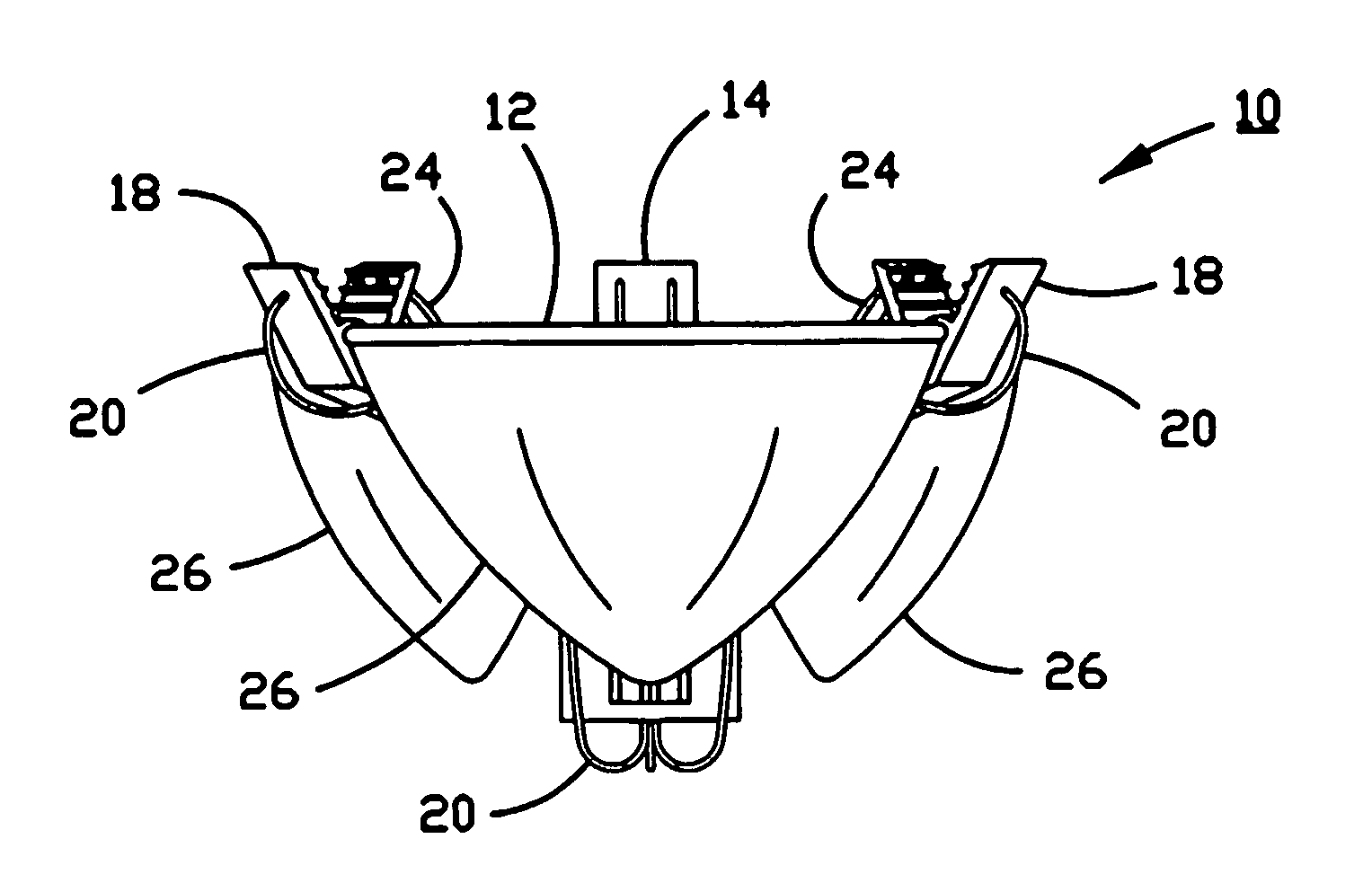
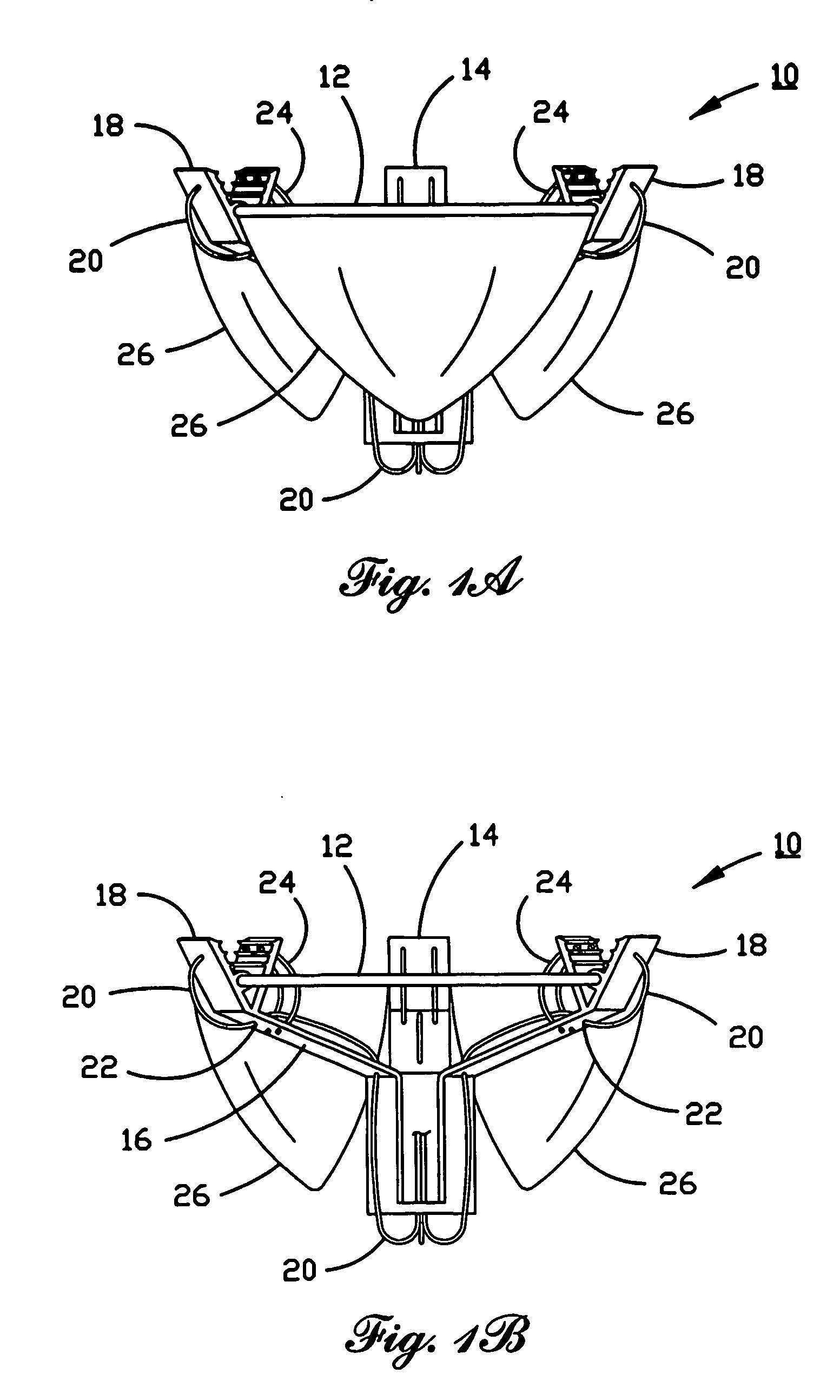
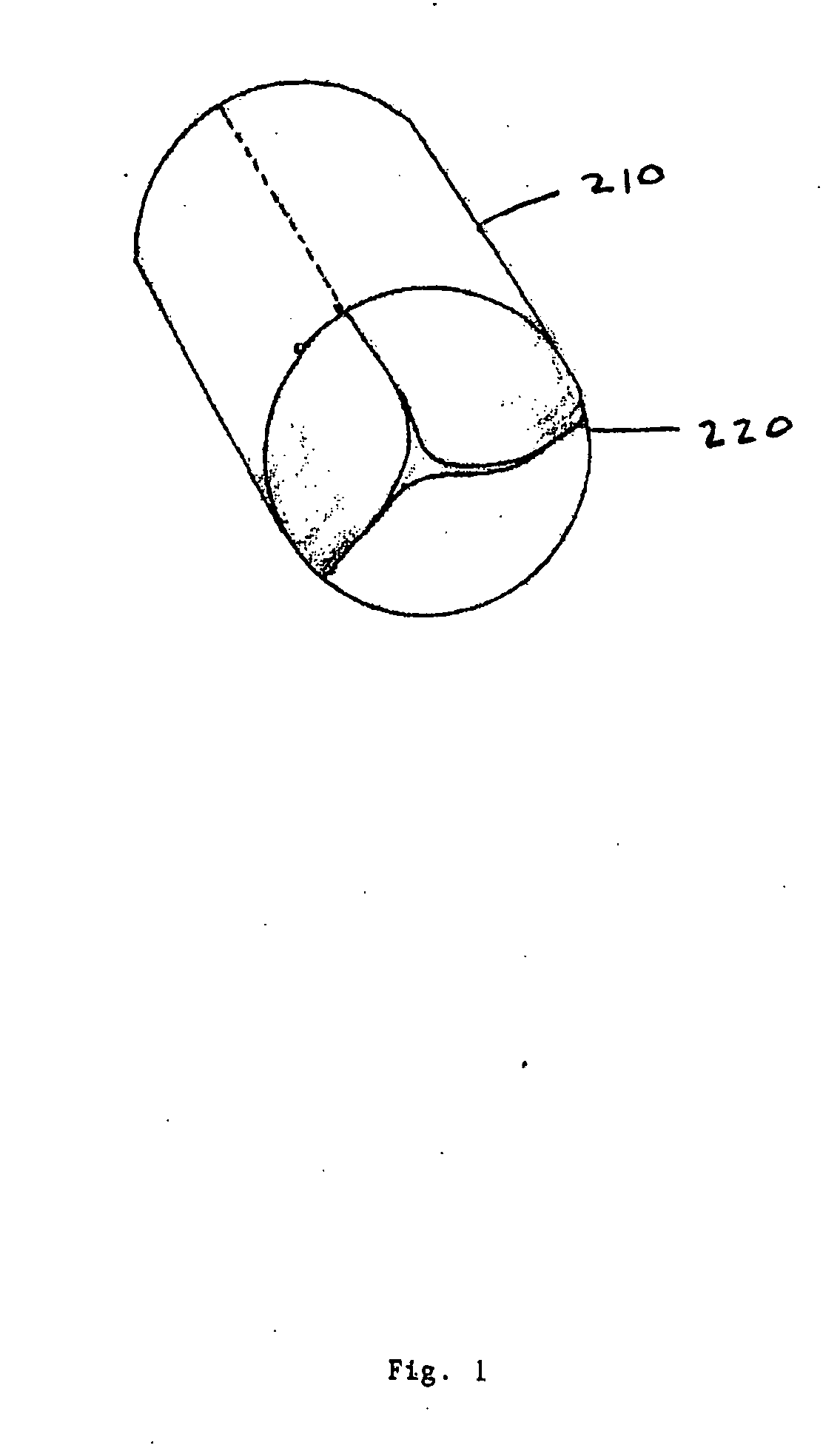
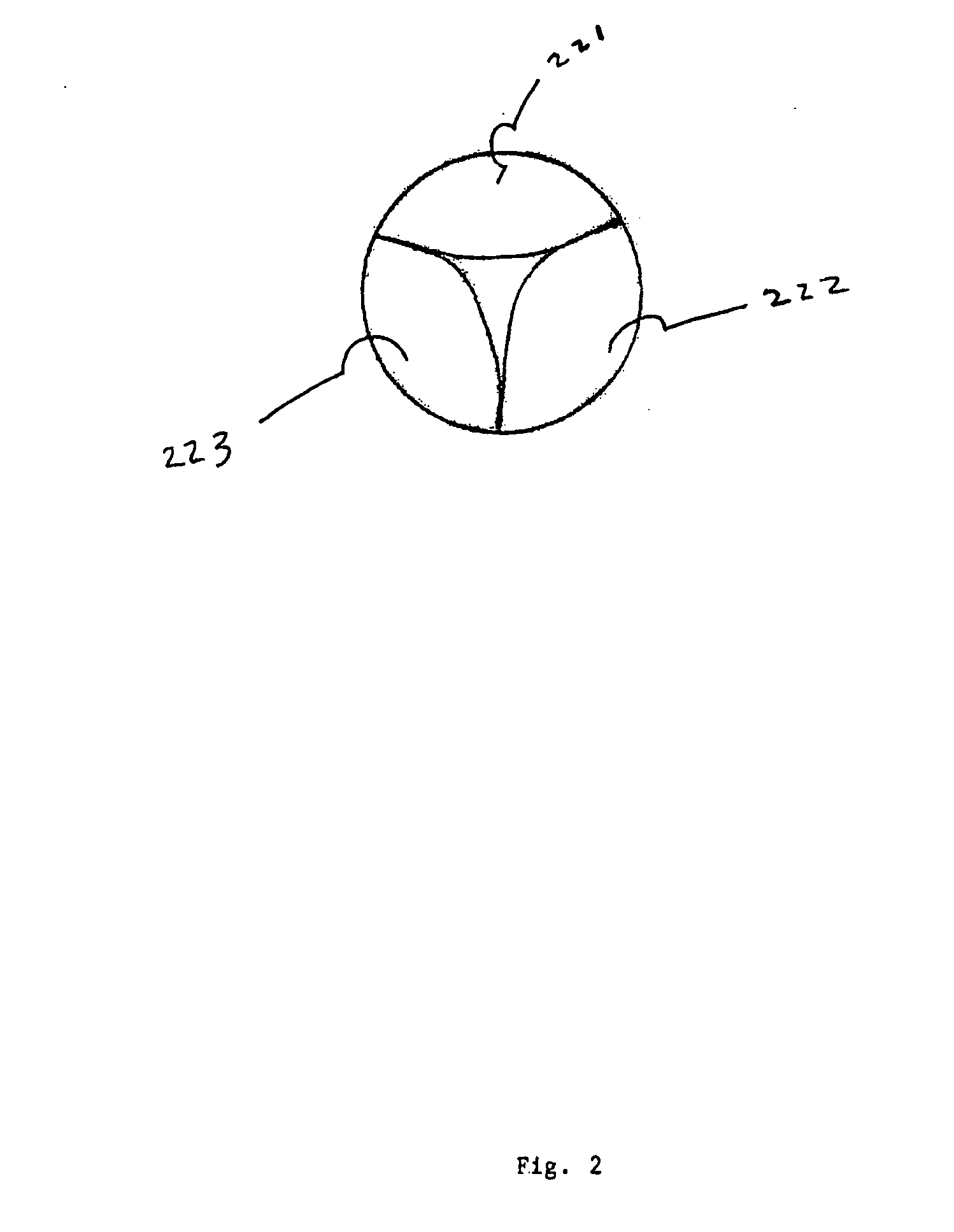
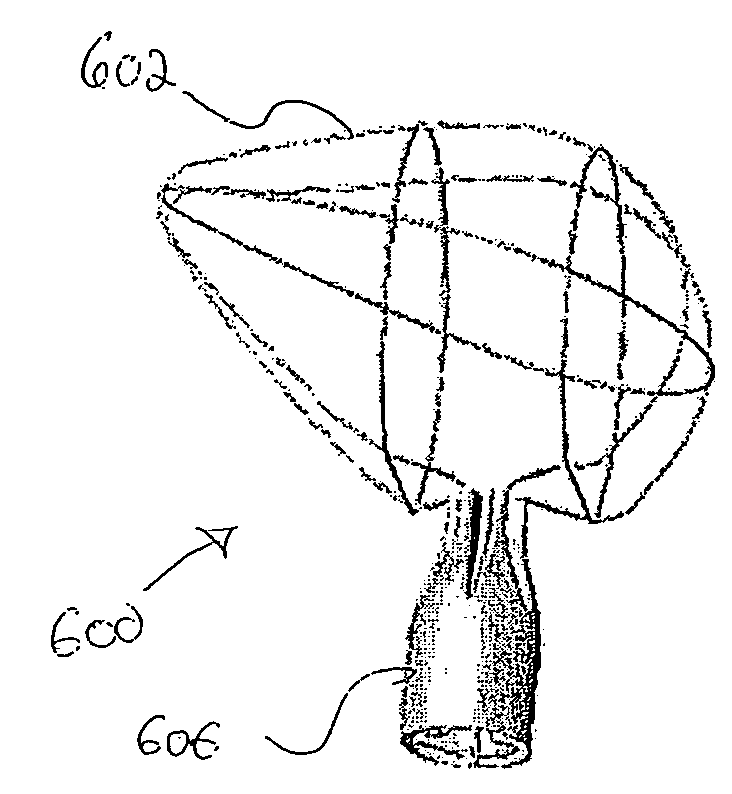
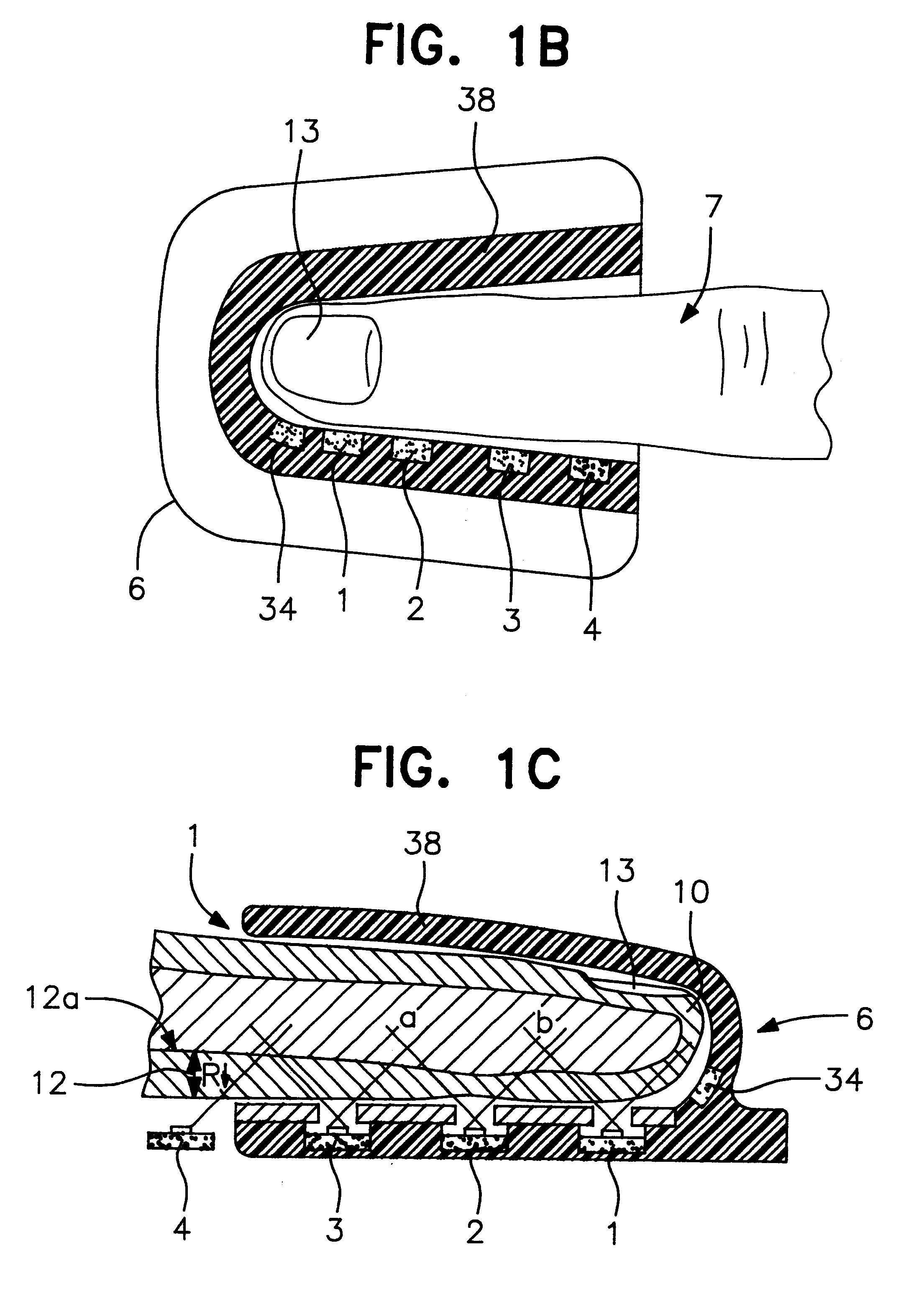
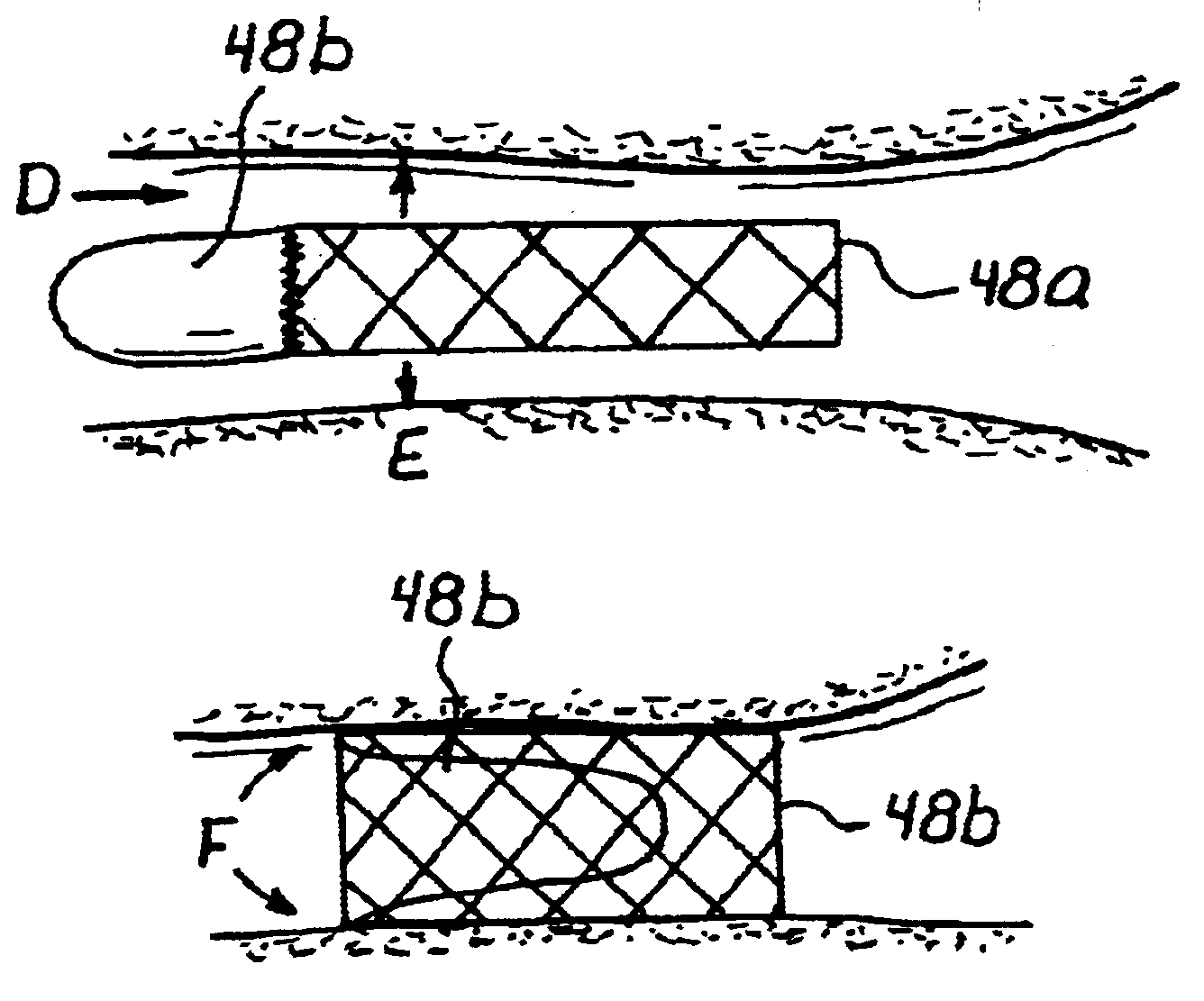
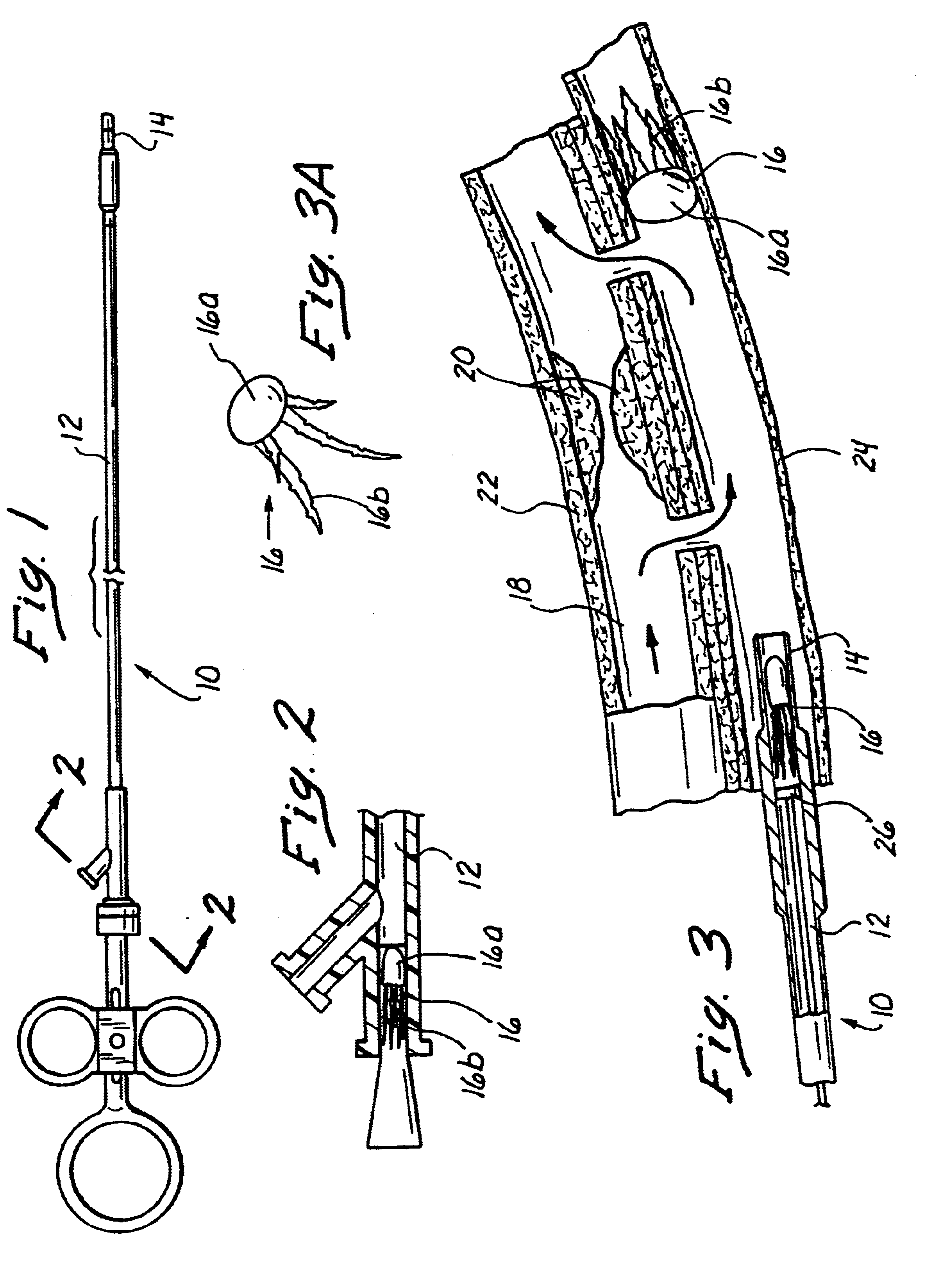
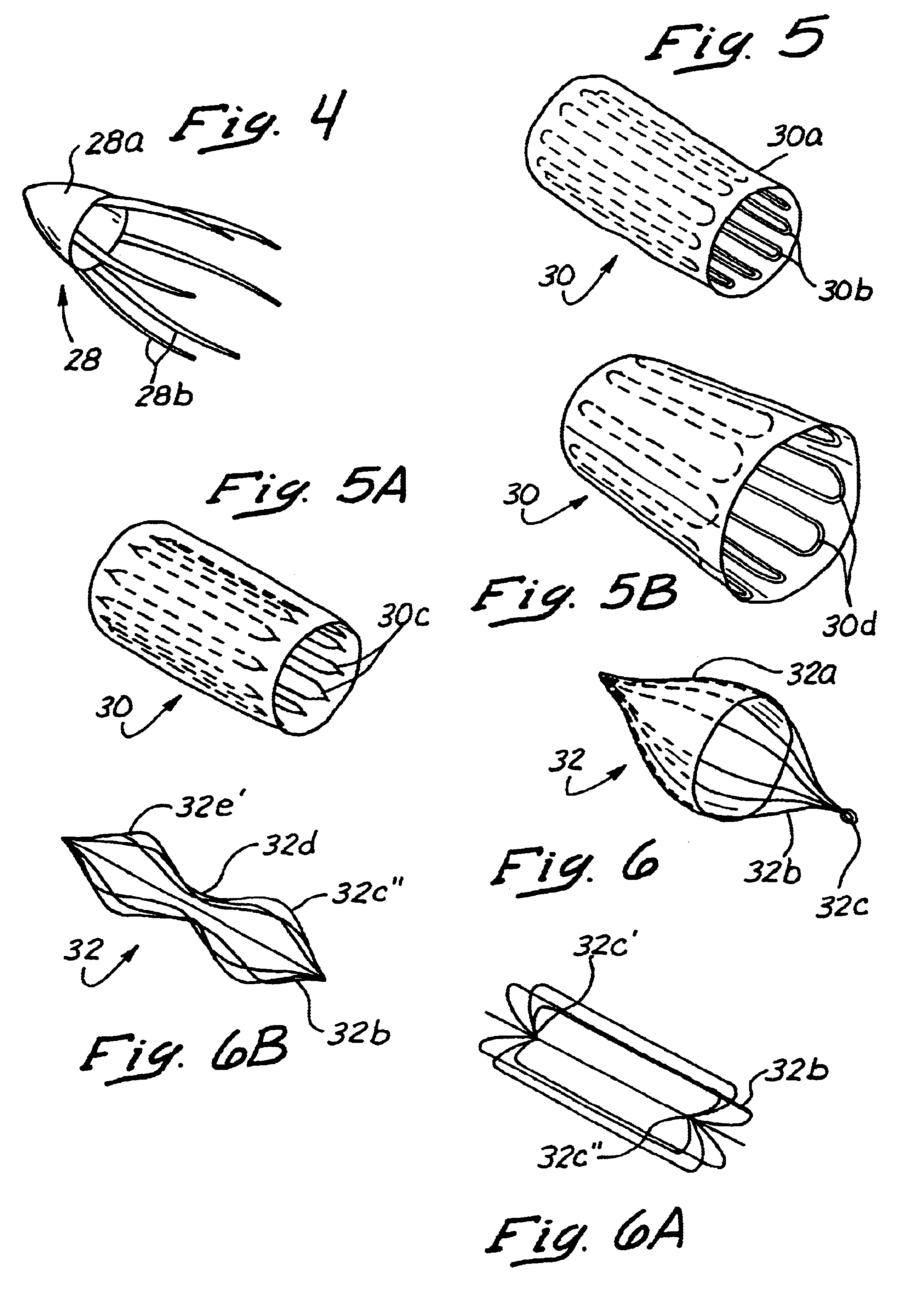
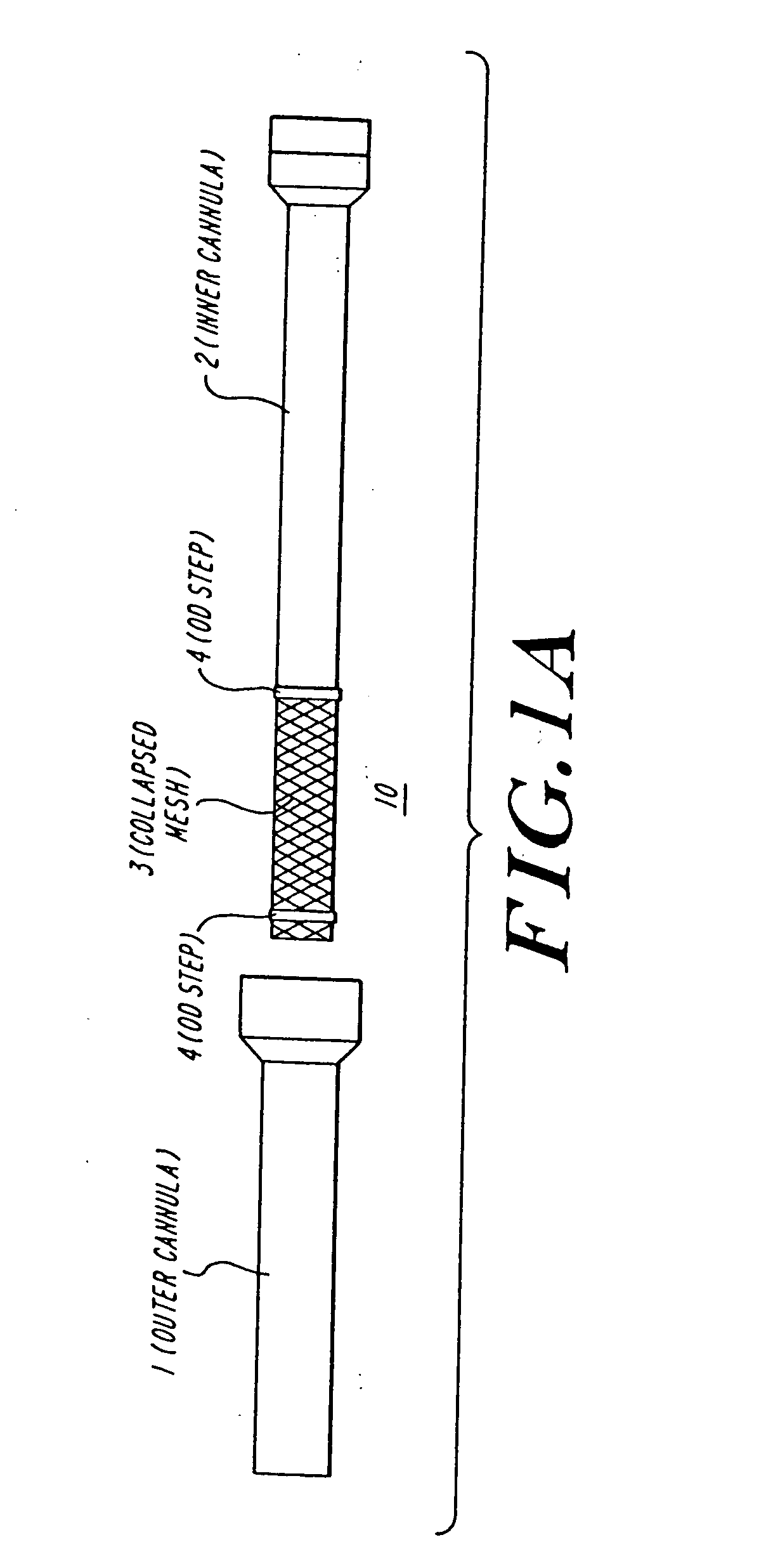
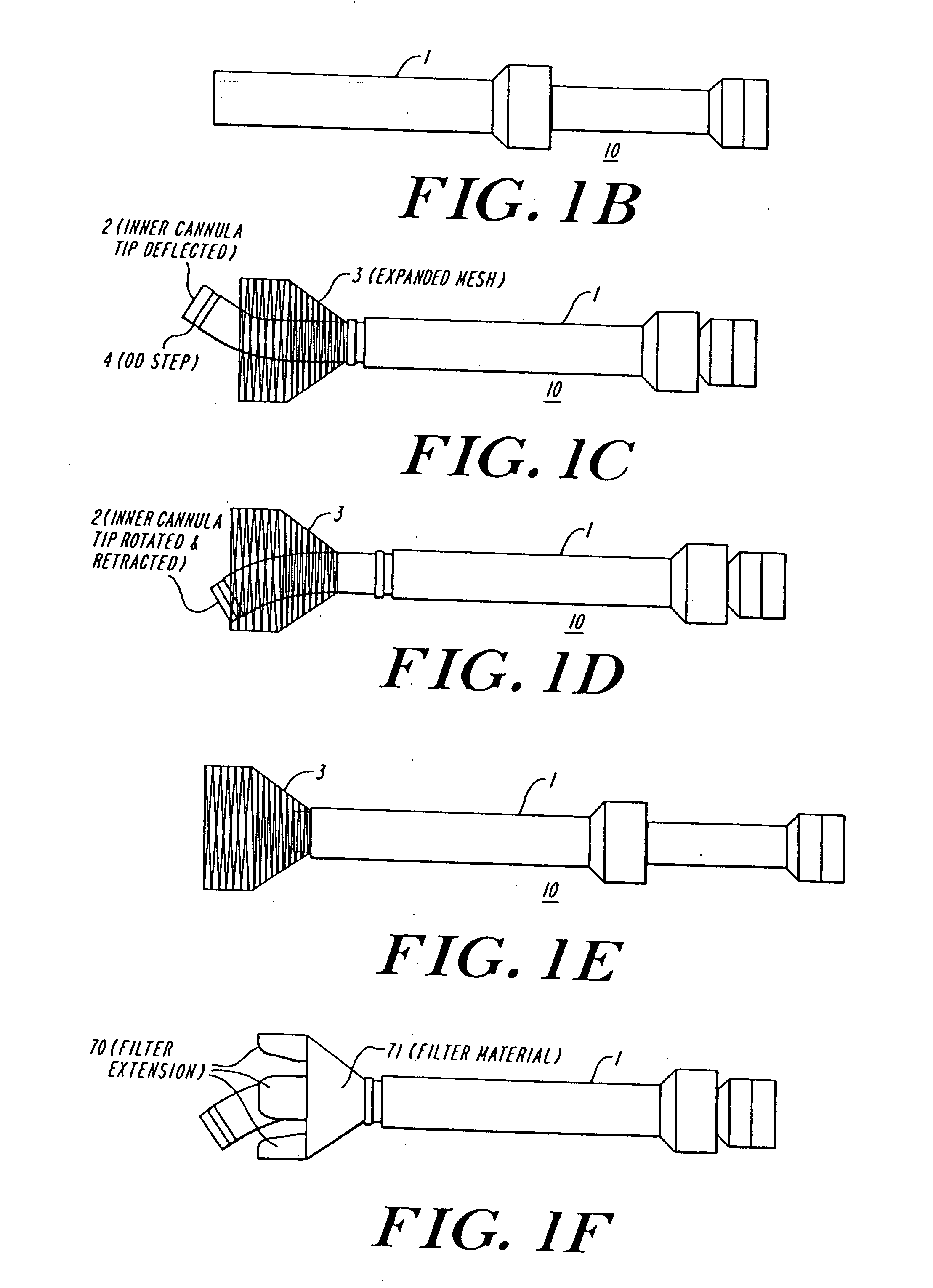
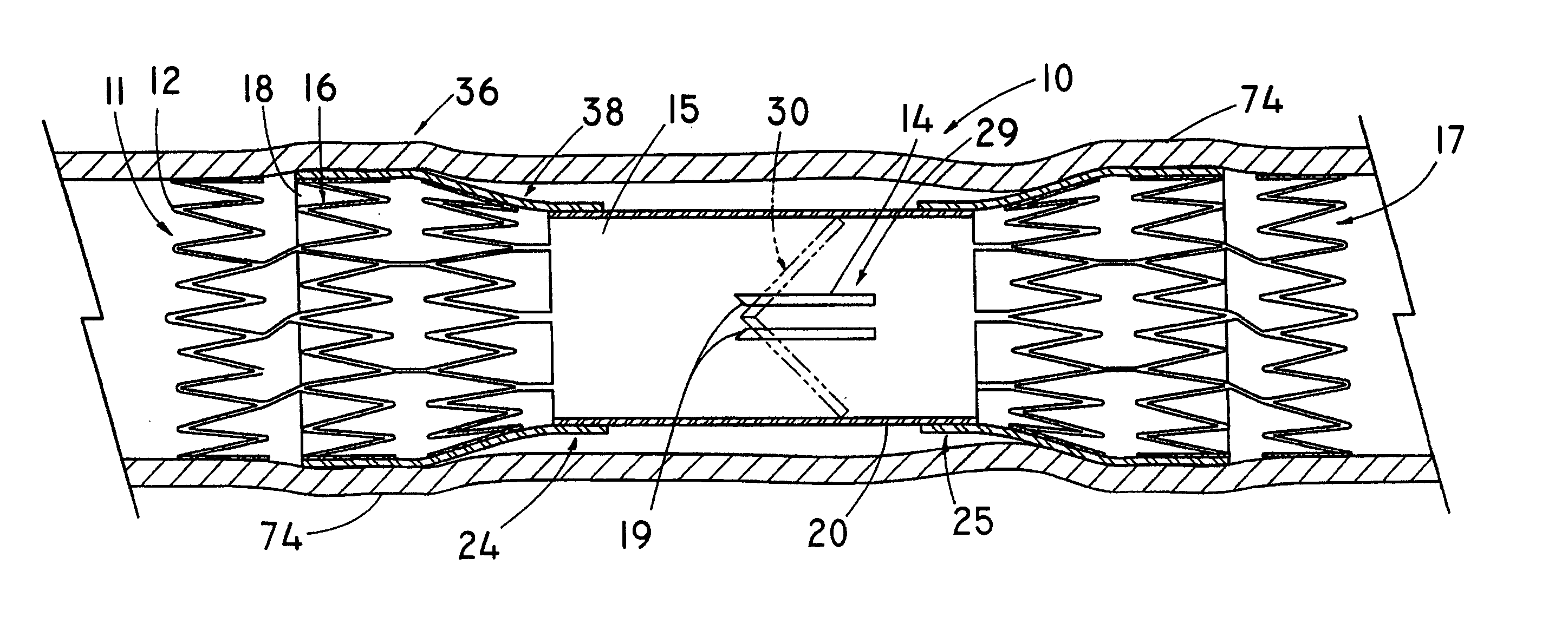
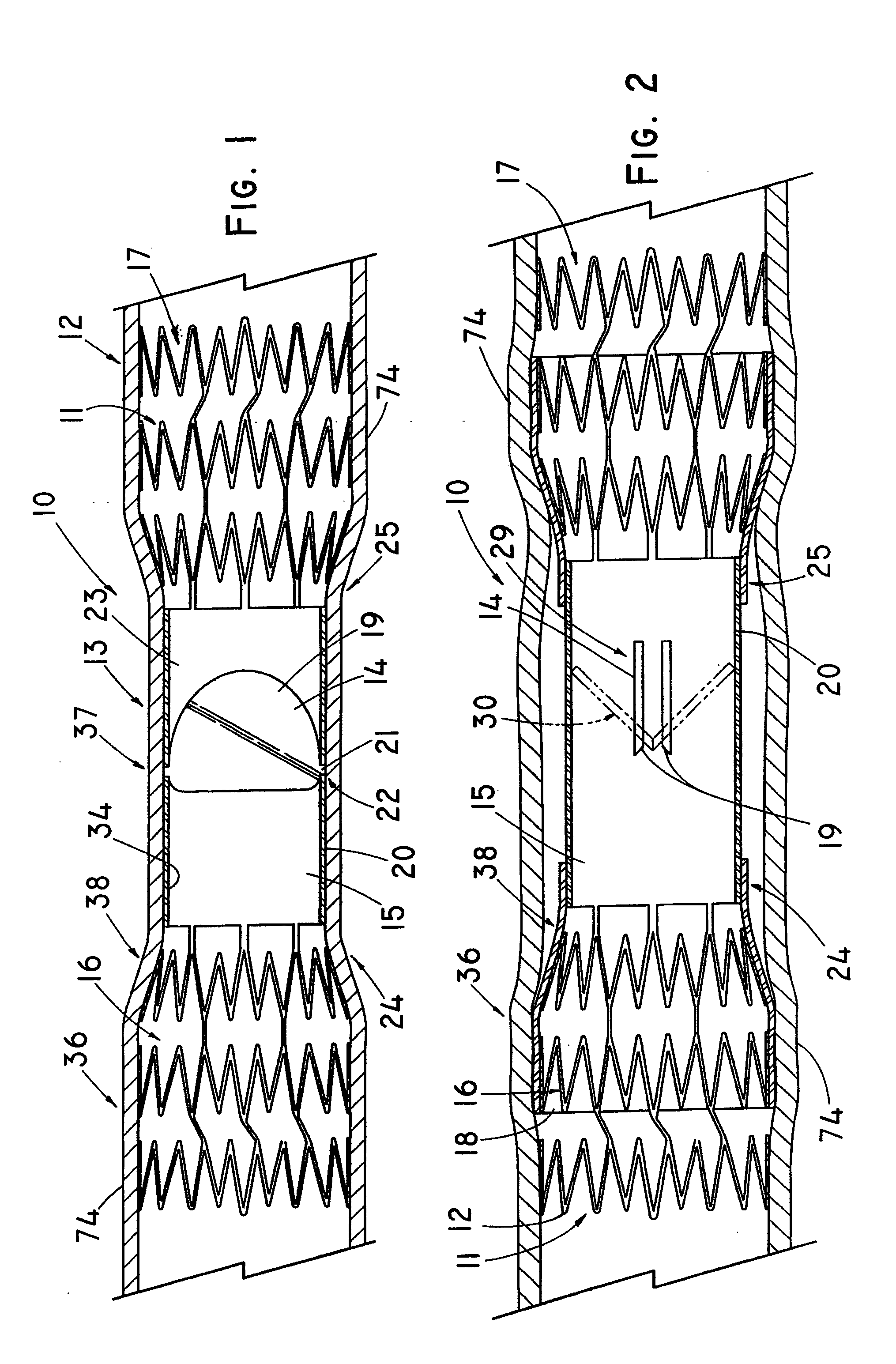
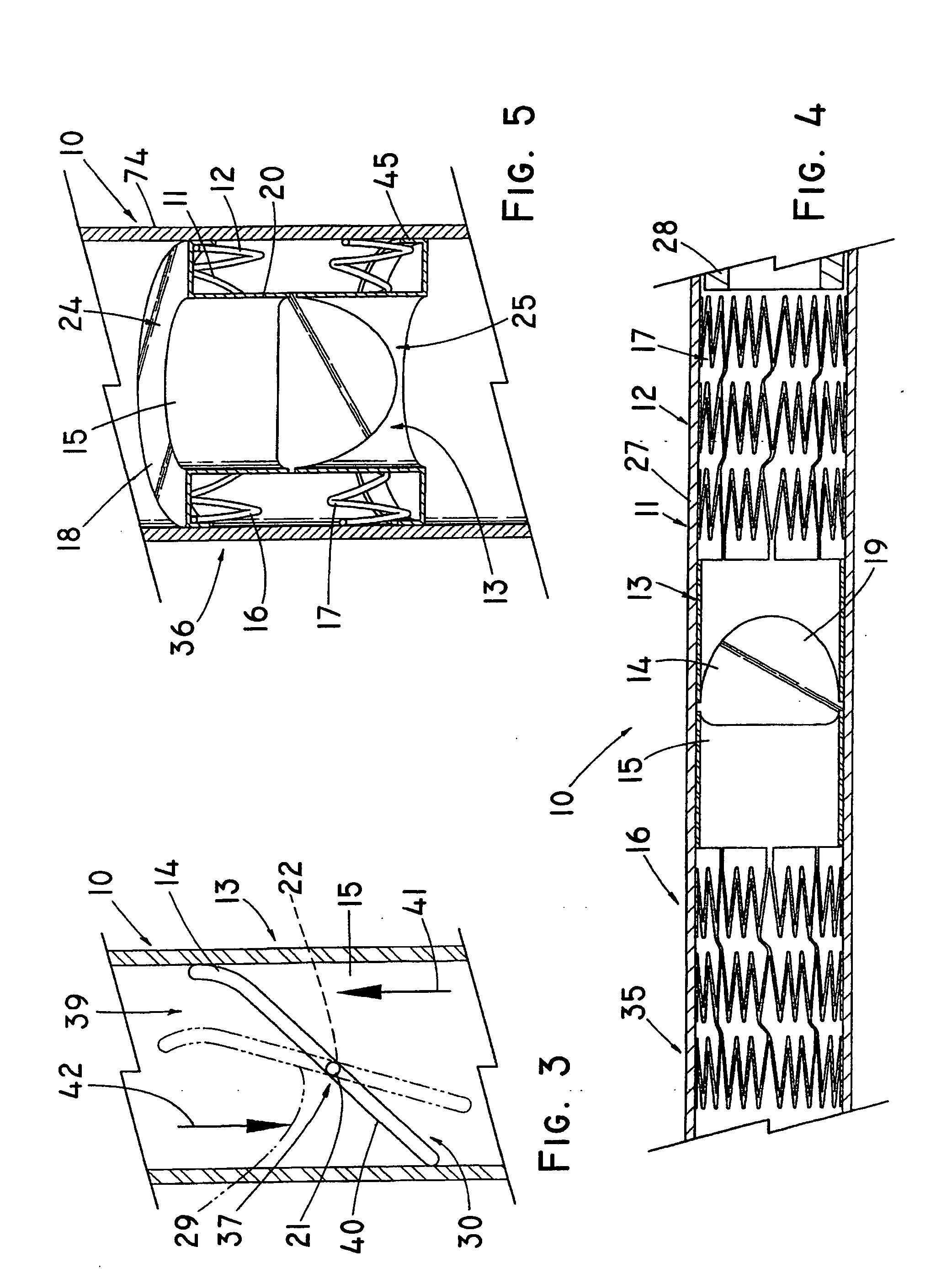
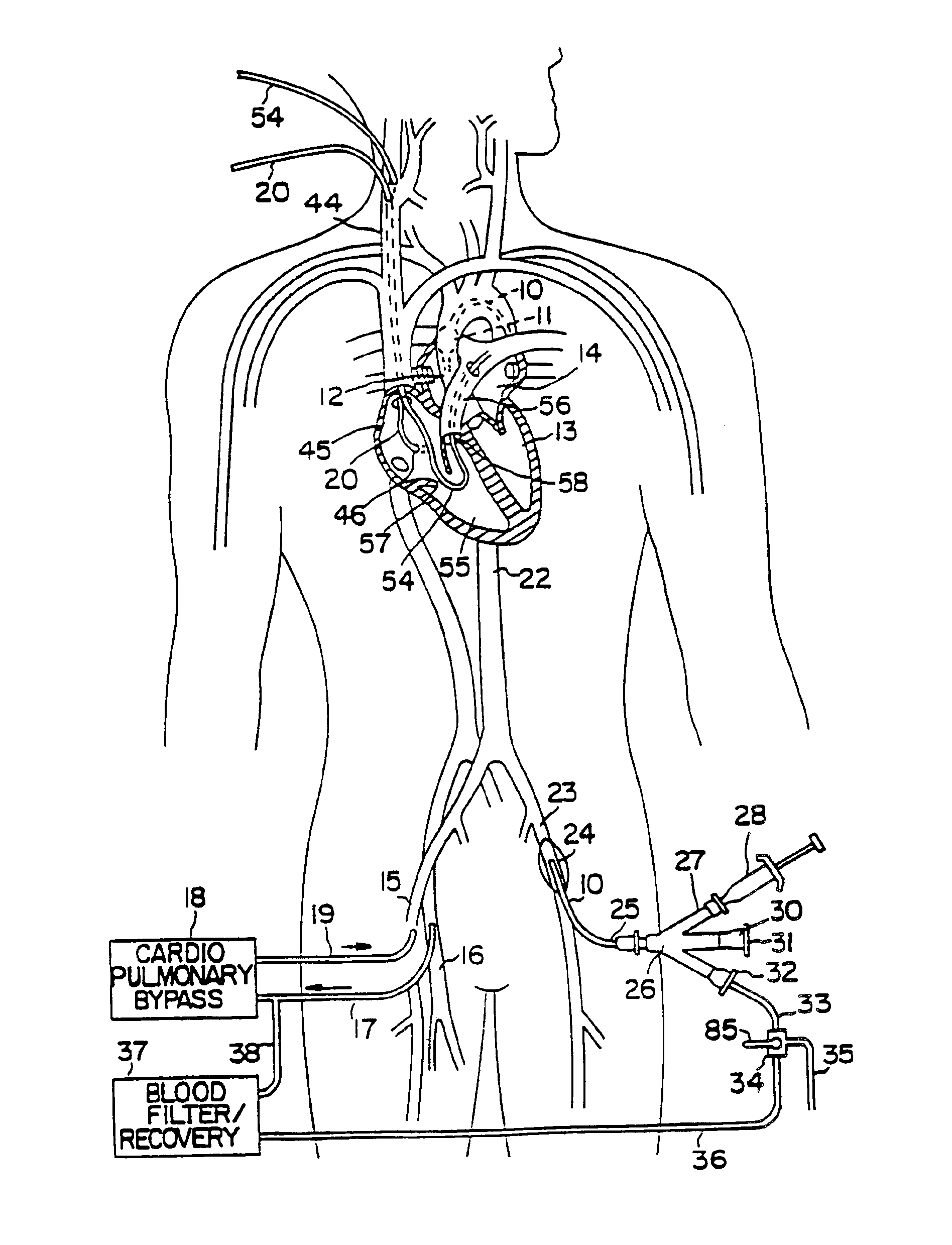
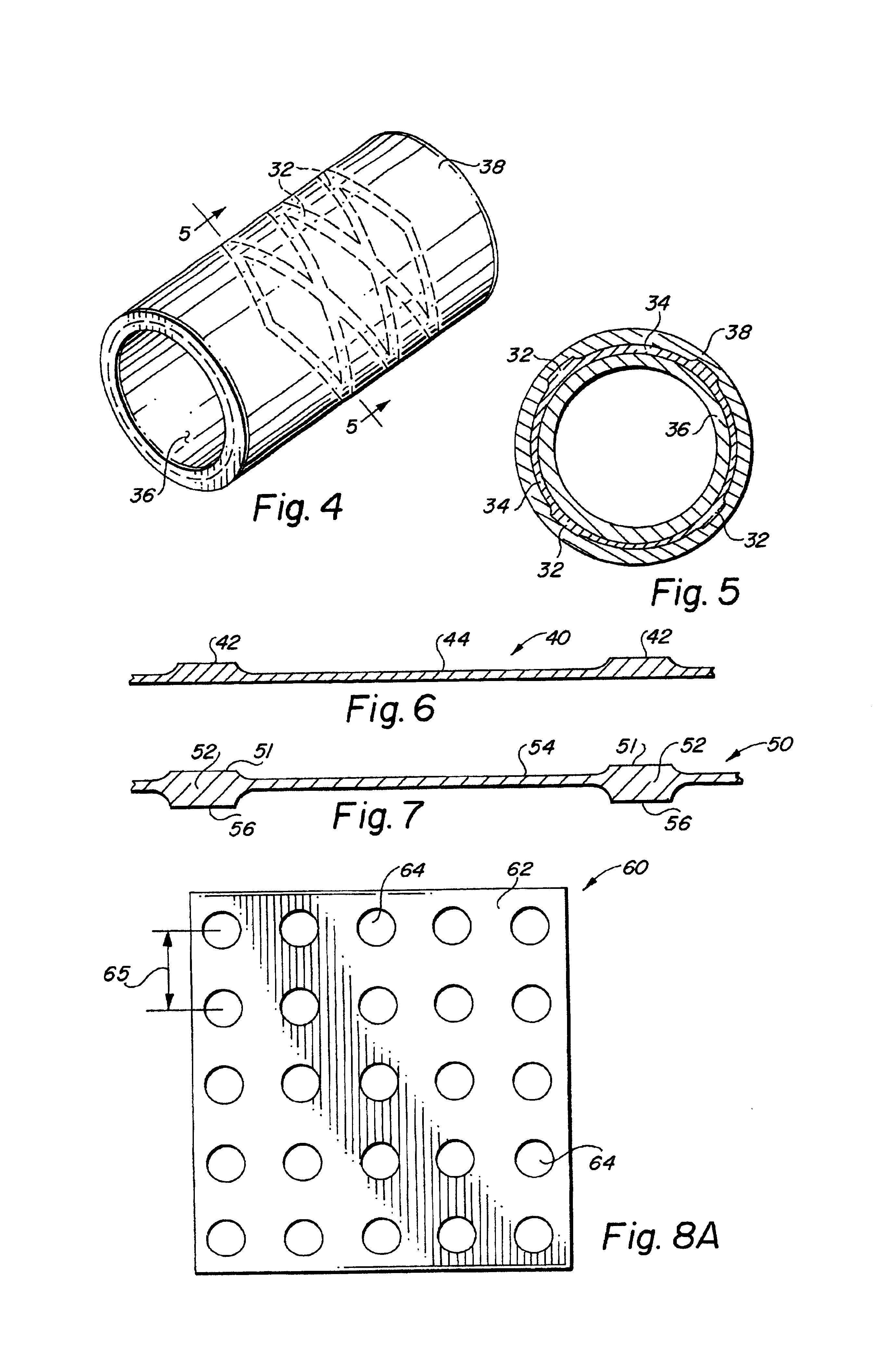
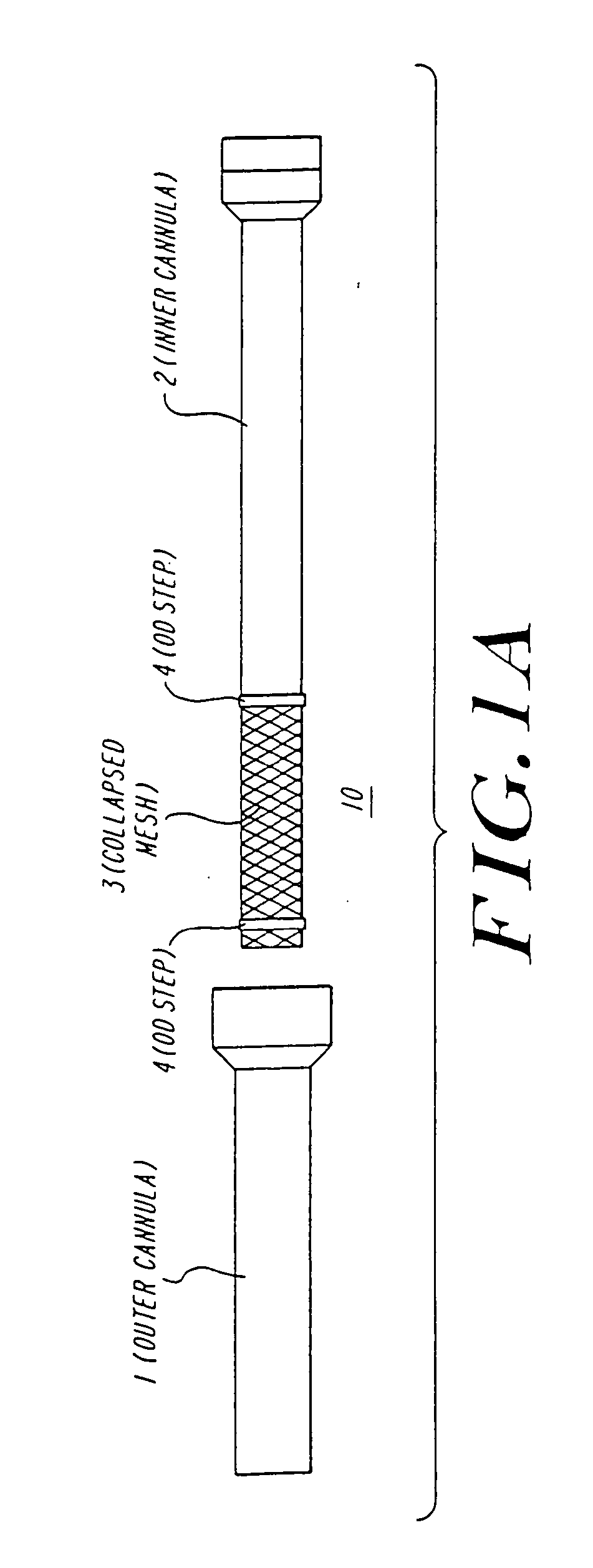
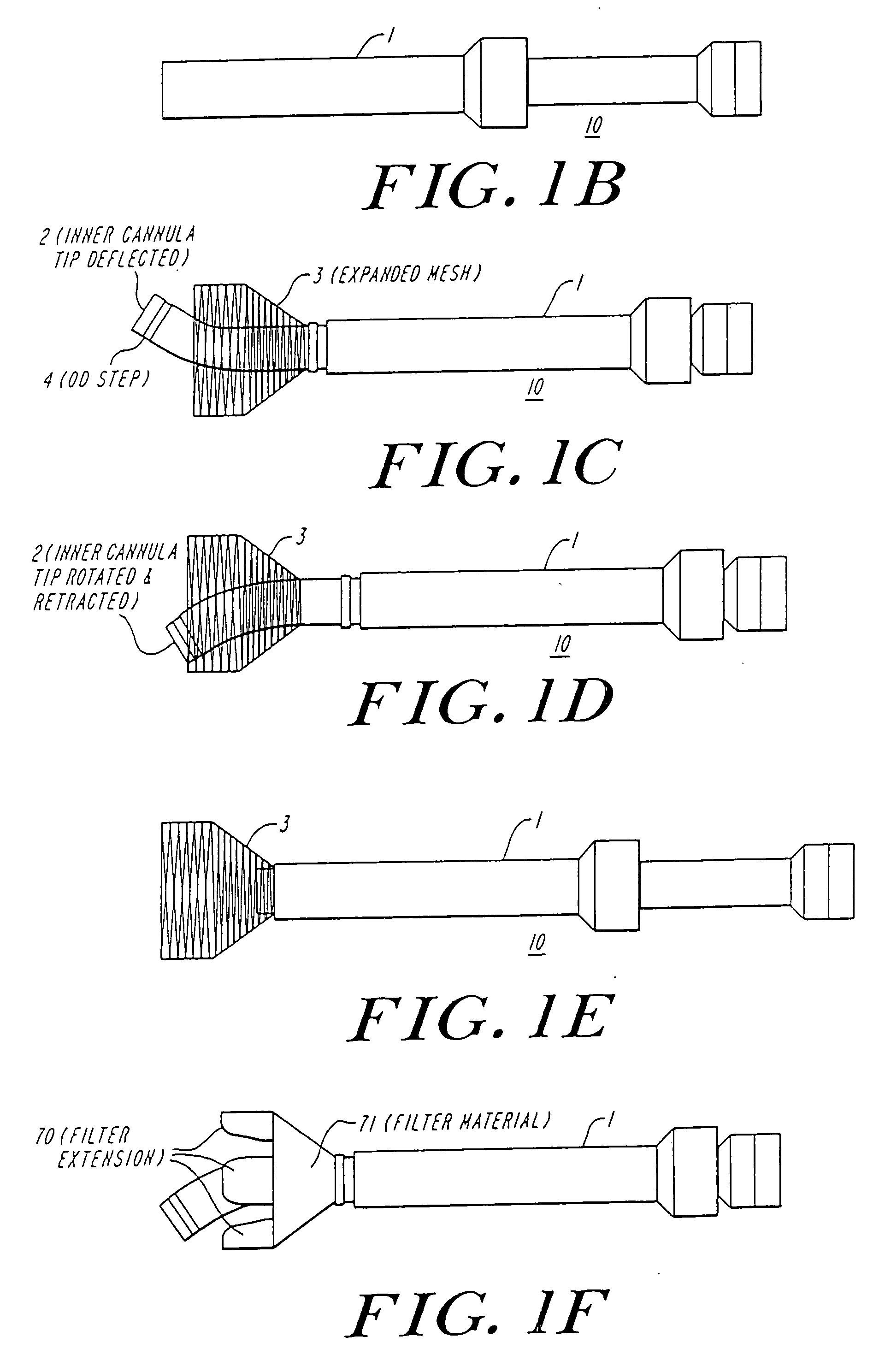
Anastomosis apparatus for use in intraluminally directed vascular anastomosis
InactiveUS6248117B1Surgical needlesSurgical staplesVascular anastomosisMinimally invasive procedures
The present invention relates to new and useful apparatus for use with an intraluminally directed anvil apparatus for intraluminally directed vascular anastomosis of an end of a graft vessel to the wall of a receiving blood vessel that is performed according to a minimally invasive procedure. The intraluminally directed vascular anastomosis does not require the interruption of blood flow in the receiving blood vessel and it is versatile enough to suitably combine a variety of cutting, welding, soldering, sealing, and joining techniques such as stapling. The intraluminally directed anvil apparatus comprises an anvil and a wire used for signaling the optimal anastomosis site; this signaling can be performed when the initial exploration is performed. The intraluminally directed anvil apparatus is typically used with a catheter.
Owner:VITAL ACCESS CORP
Anastomosis method
An anastomosis system and method uses an anvil to control and support a tissue site during an anastomosis procedure. The anvil is particularly useful for supporting a wall of a coronary artery during attachment of a graft vessel to the coronary artery because the wall of the coronary artery is very thin, difficult to grasp, and susceptible to tearing. In one method, the anvil is inserted into a pressurized or unpressurized target vessel and is pulled against an inner wall of the target vessel causing tenting of the thin tissue of the vessel wall. A graft vessel is then advanced to the anastomosis site and an end of the graft vessel is positioned adjacent and exterior of the target vessel. Staples are inserted through the tissue of the graft vessel and the target vessel by pivoting the arms of a staple holder towards the anvil. When the ends of the staples engage staple bending features on the anvil, the ends of the staples bend over securing the graft vessel and target vessel together. After stapling is complete, an incision is formed in the wall of the target vessel to allow blood flow between the target vessel and the graft vessel.
Owner:AESCULAP AG
Hemostatic compositions for arresting blood flow from an open wound or surgical site
A hemostatic composition for stopping or decreasing blood flow from an open wound or medical or surgical procedure. Compositions of the invention comprise a mixture of a cationic polymer and a cation exchange material. In one embodiment, the composition comprises a mixture: (1) a high molecular weight copolymer of diallyl dimethyl ammonium chloride (DADMAC) and acrylamide [DADMAC copolymer], and (2) the hydrogen form of a crosslinked, sulfonated polystyrene (hydrogen resin). In an exemplified embodiment, a composition of the invention comprises the mixture of DADMAC copolymer and hydrogen resin provided in a dry powdered form. The compositions of the invention may be applied directly to a wound or treatment site, or they may be incorporated into a wound dressing, such as a bandage. The seal formed at a wound or treatment site treated with the present invention is adhesive and exhibits considerable toughness.
Owner:BIOLIFE
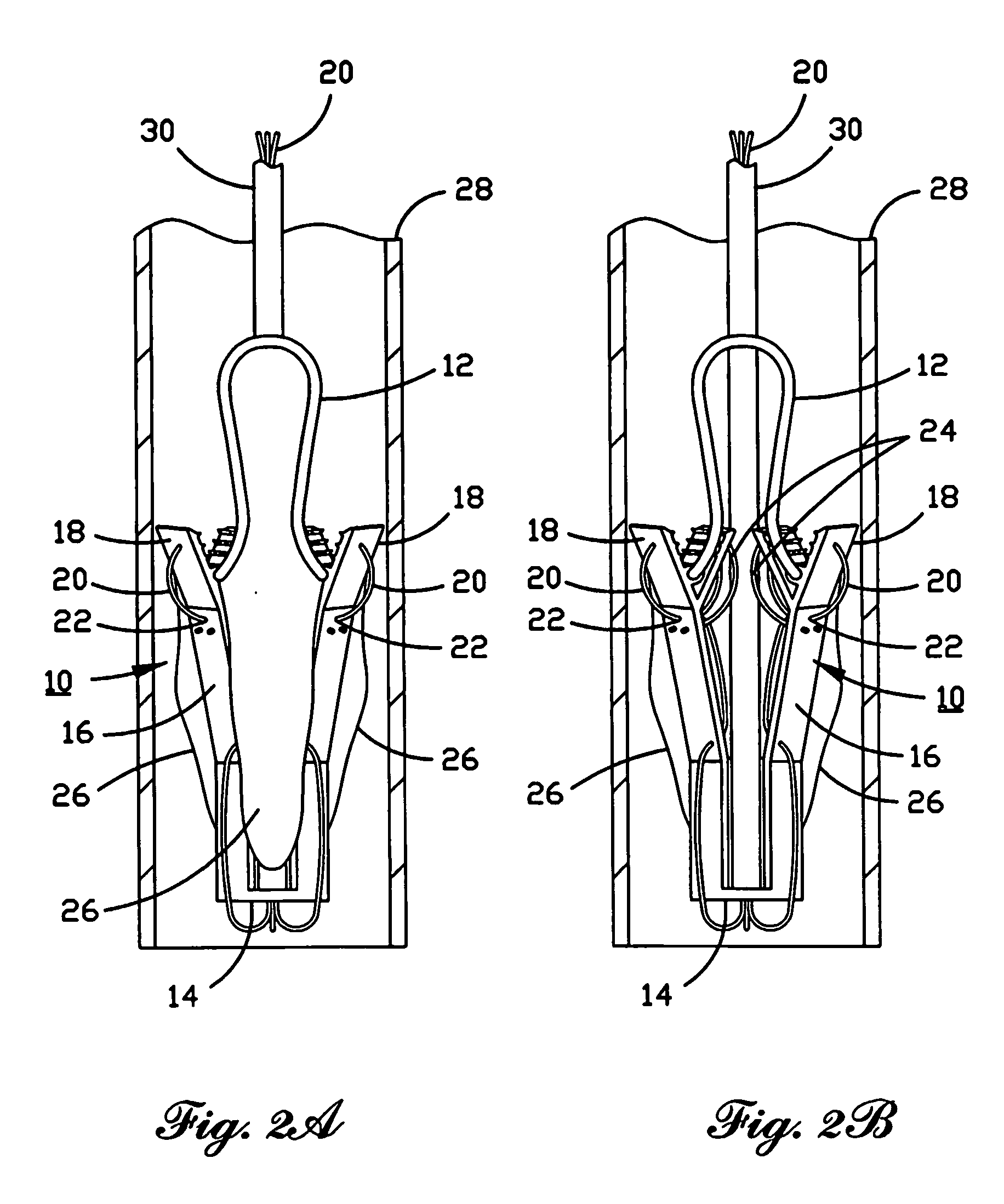
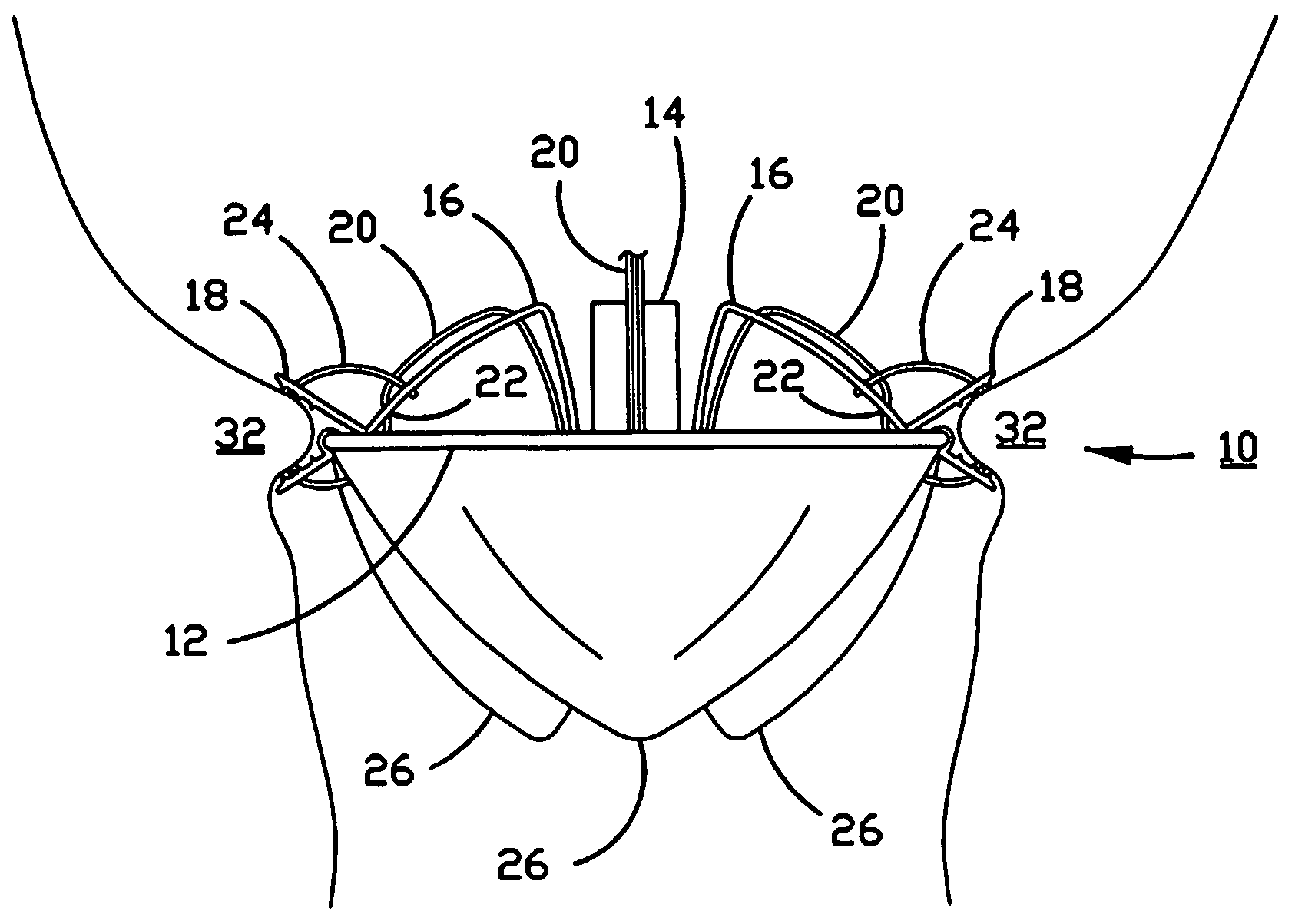
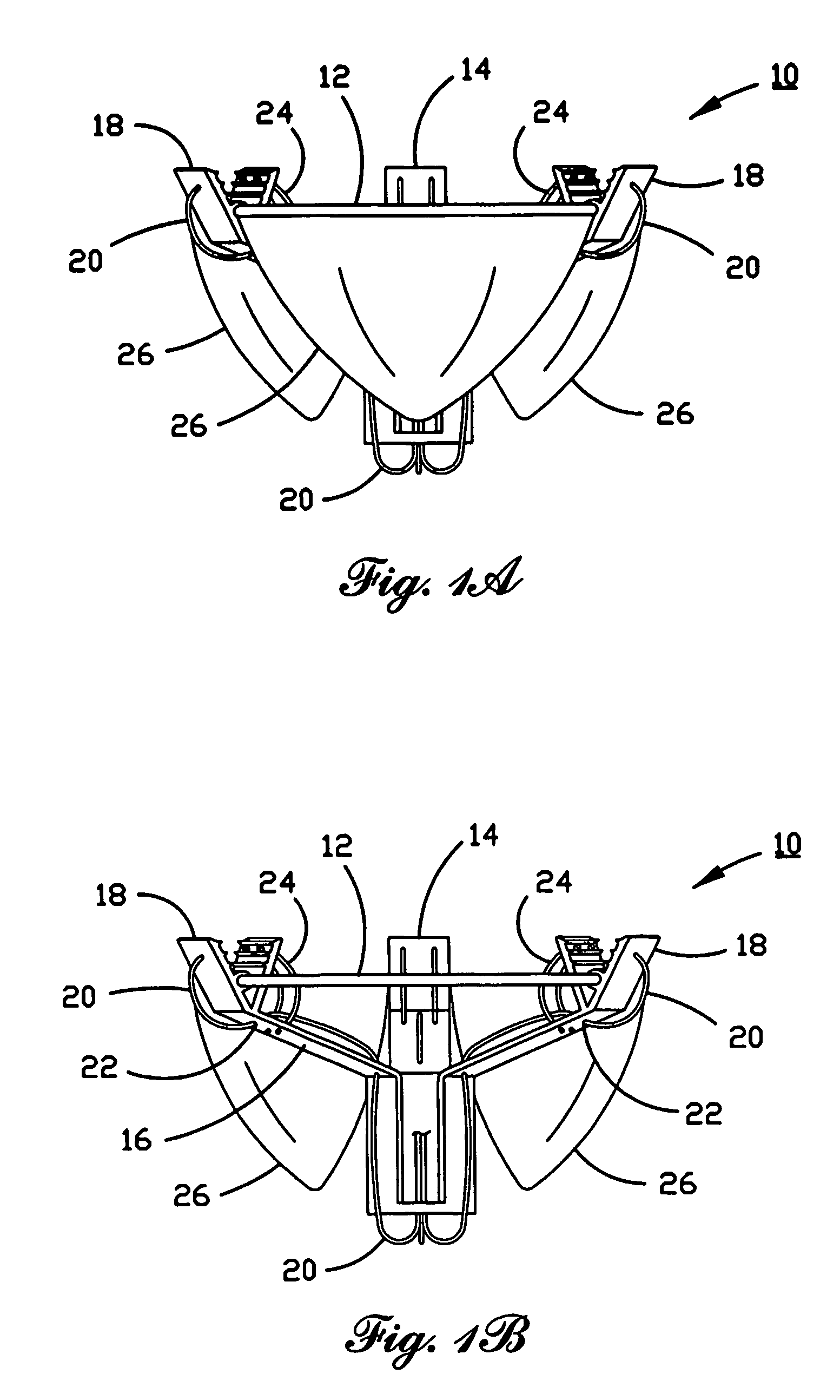
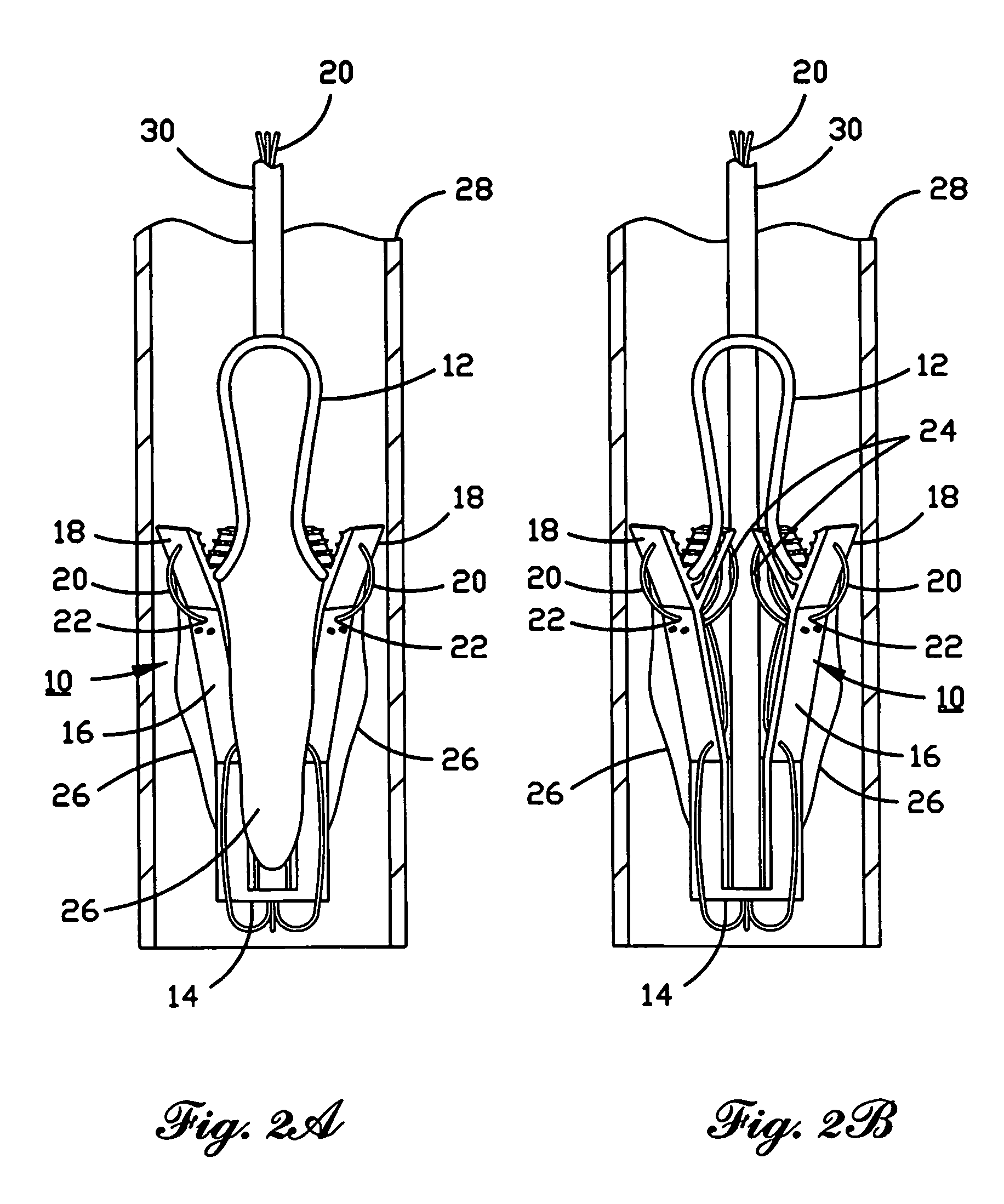
Percutaneous heart valve
ActiveUS20070016286A1Avoid flowStability and functioning of the heart valve are satisfactoryHeart valvesBlood flowValvular prosthesis
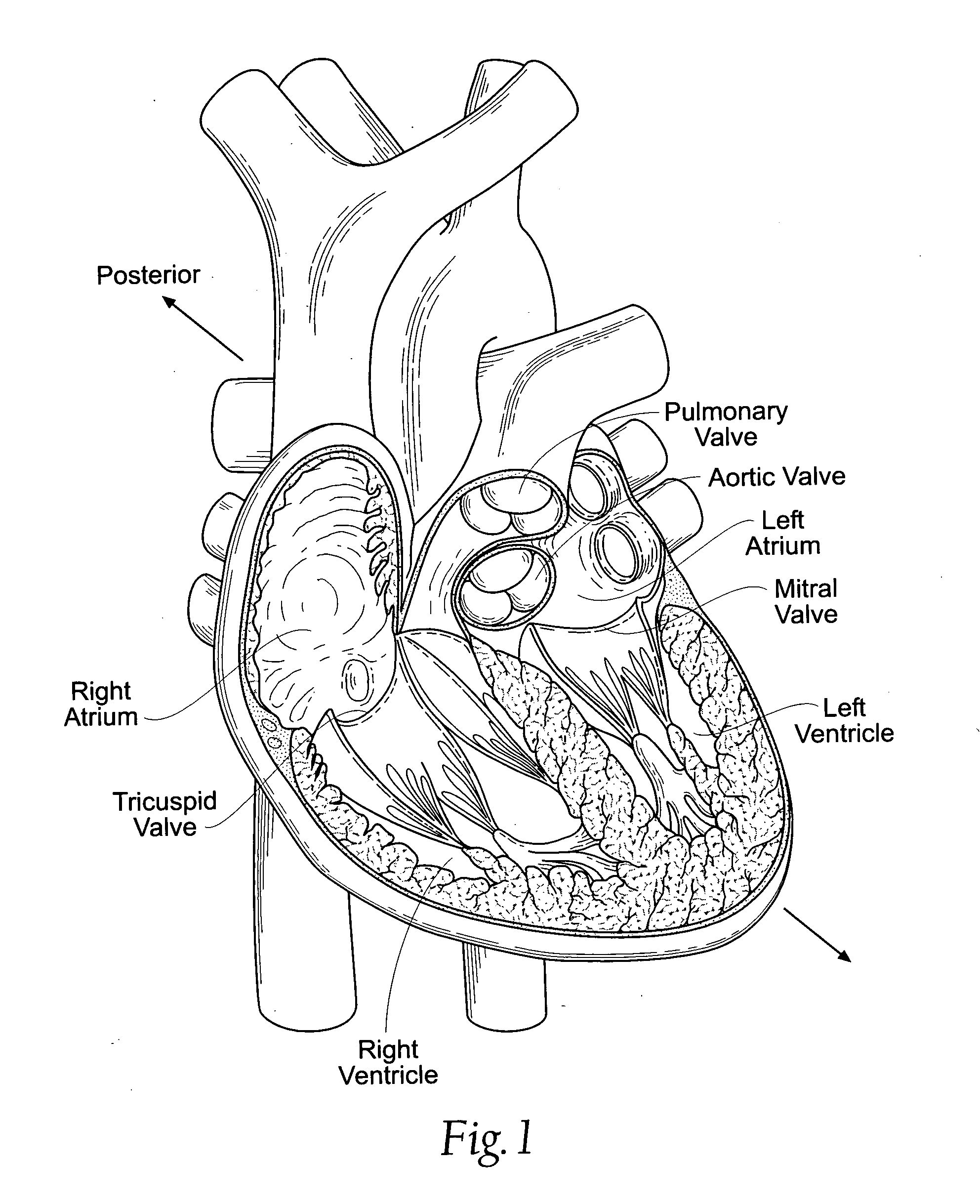
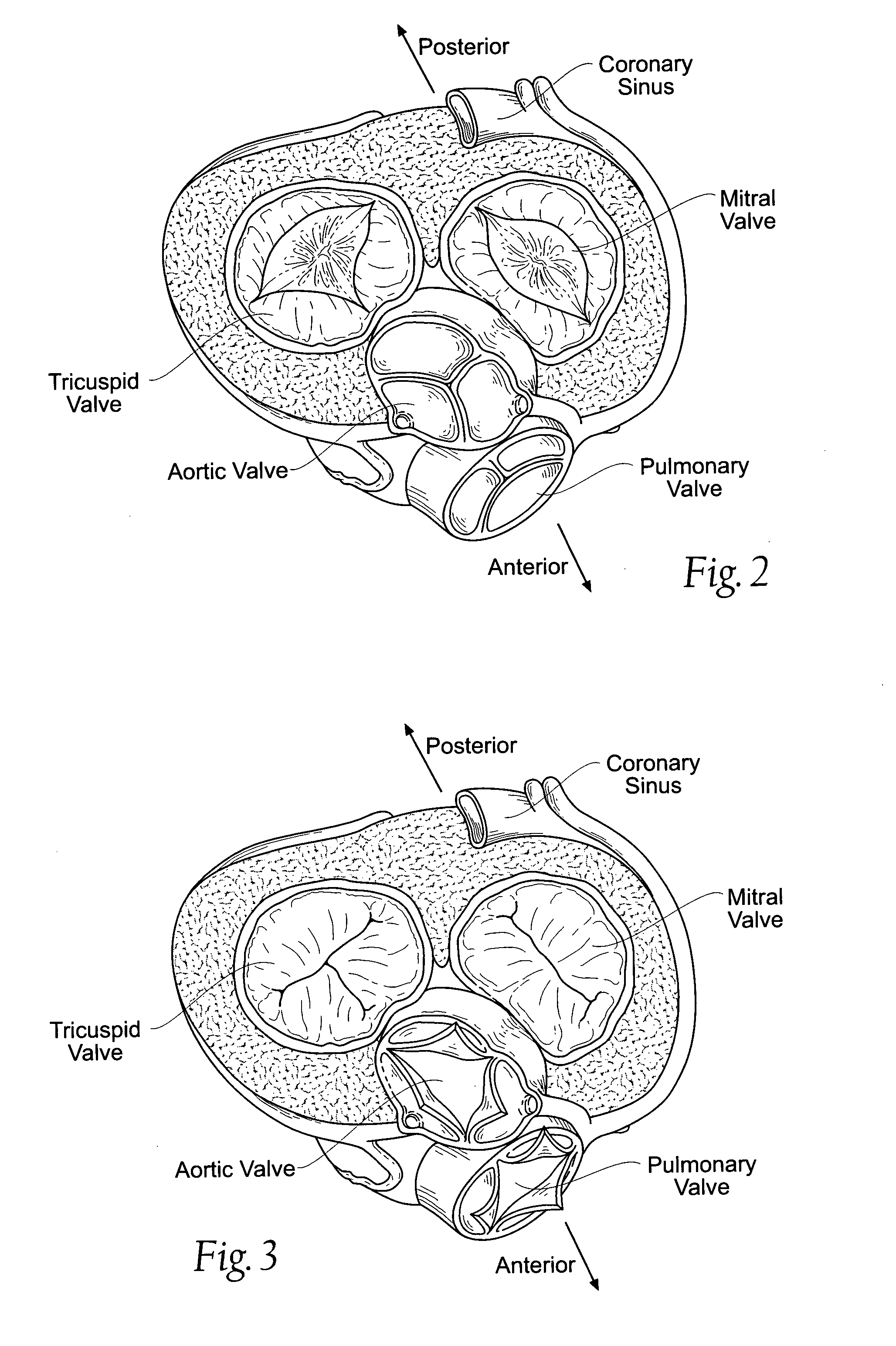
A percutaneously inserted bistable heart valve prosthesis is folded inside a catheter for transseptal delivery to the patient's heart for implantation. The heart valve has an annular ring, a body member having a plurality of legs, each leg connecting at one end to the annular ring, claws that are adjustable from a first position to a second position by application of external force so as to allow ingress of surrounding heart tissue into the claws in the second position, and leaflet membranes connected to the annular ring, the body member and / or the legs, the leaflet membranes having a first position for blocking blood flow therethrough and a second position for allowing blood flow therethrough. The heart valve is designed such that upon removal of the external force the claws elastically revert to the first position so as to grip the heart tissue positioned within the claws, thereby holding the heart valve in place. The body member and claws may be integrated into a one-piece design. The heart valve may be used as a prosthesis for the mitral valve, aortic valve, pulmonary valve, or tricuspid valve by adapting the annular ring to fit in a respective mitral, aortic, pulmonary, or tricuspid valve opening of the heart.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
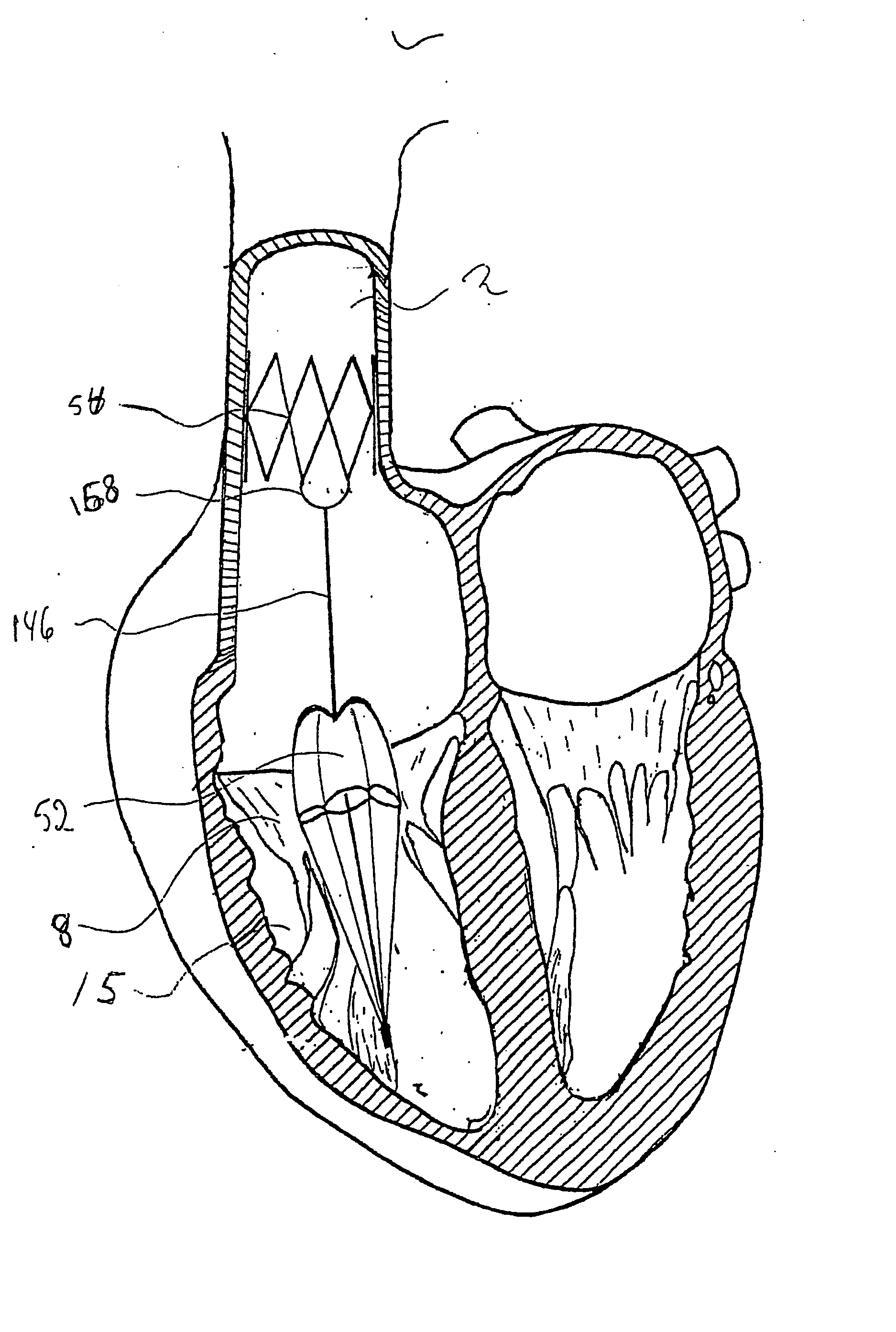
Blood flow controlling apparatus
InactiveUS20060241745A1Avoid improper sealingPrevent leakageSuture equipmentsAnnuloplasty ringsNative tissuePlasma viscosity
A blood flow controlling apparatus, which is configured to be implanted into a blood circulatory system of a patient, comprises an anchoring means, which is arranged to fix the position of the apparatus in the blood circulatory system, and a valve means being connected to the anchoring means. The valve means is configured to be arranged within the blood circulatory system and is configured to be extendable in a direction transverse to blood flow in order to make contact with native tissue when inserted in the blood circulatory system. The valve means is further configured to release said contact as a result of being exposed to blood flow in a permitted direction.
Owner:EDWARDS LIFESCIENCES AG
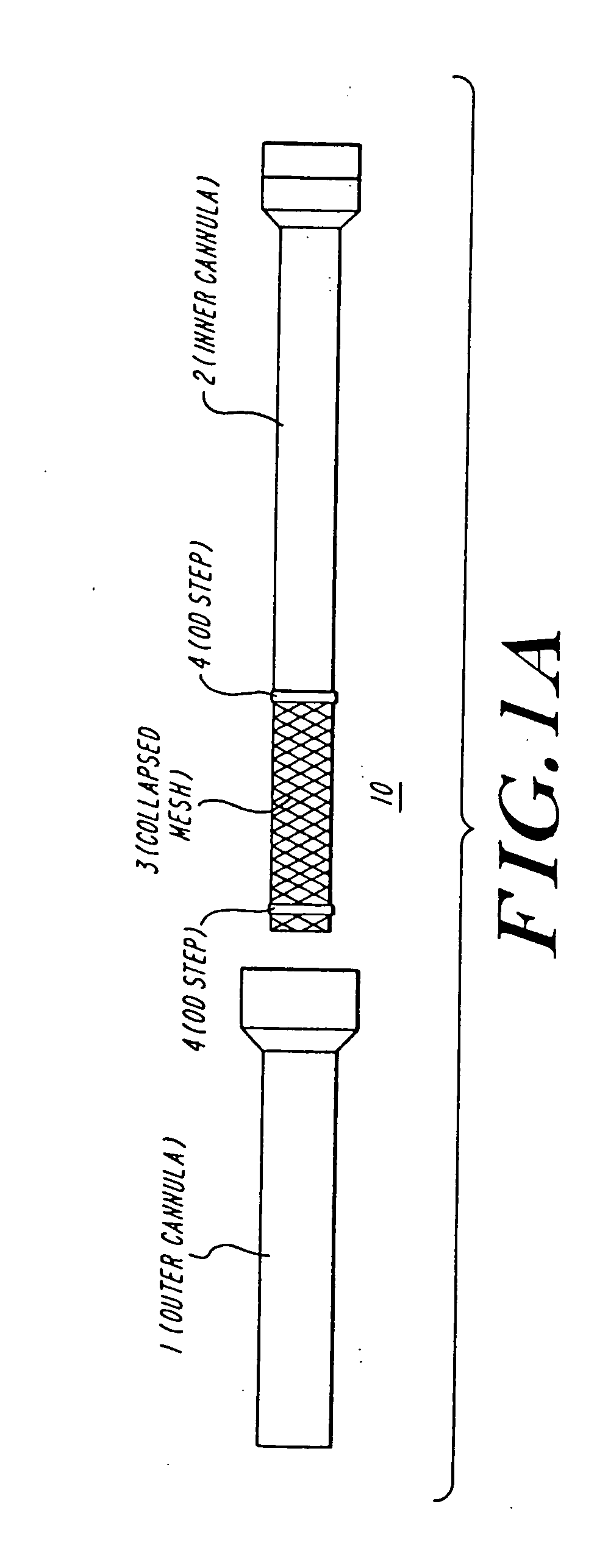
Cardiac valve procedure methods and devices
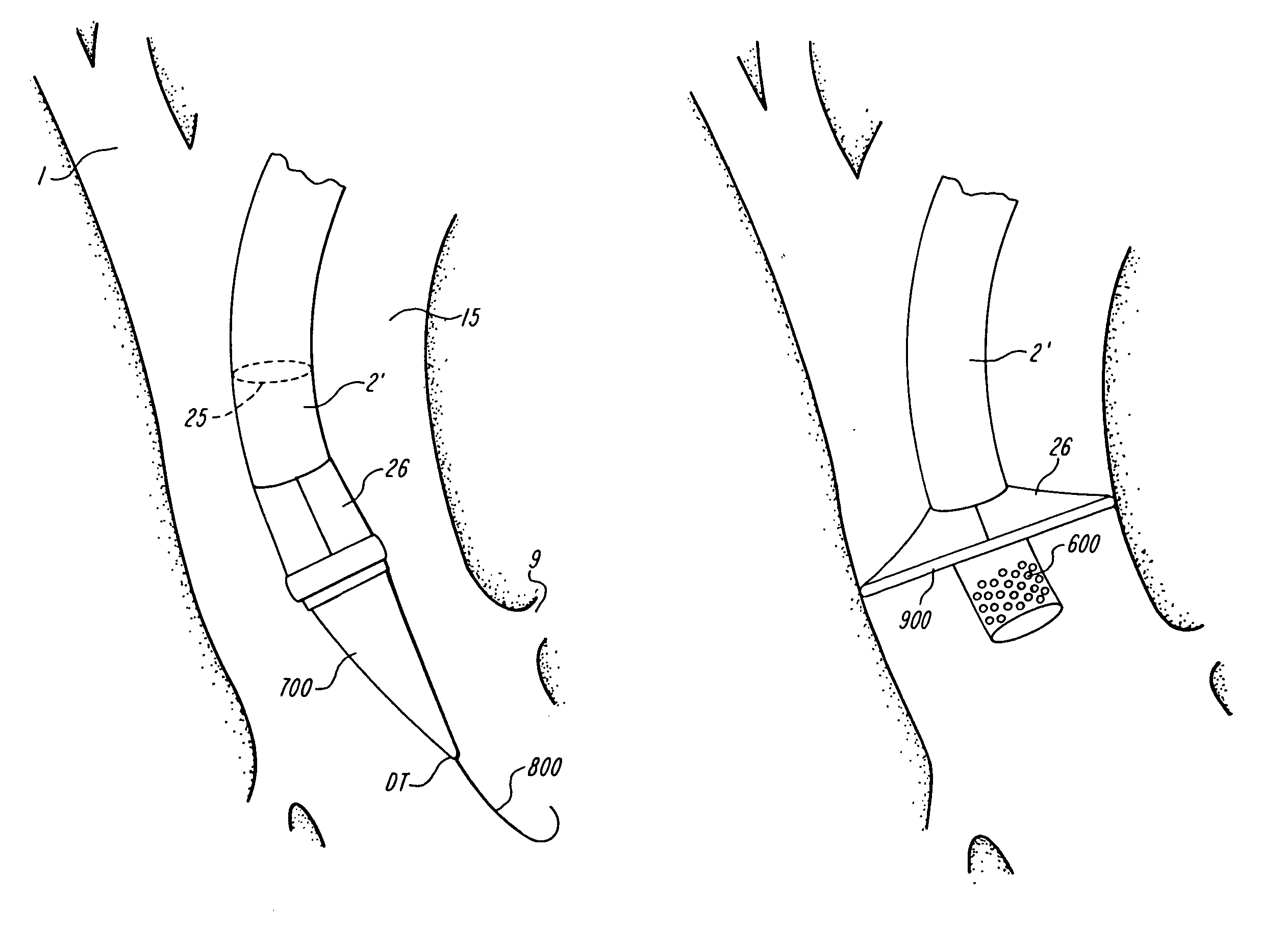
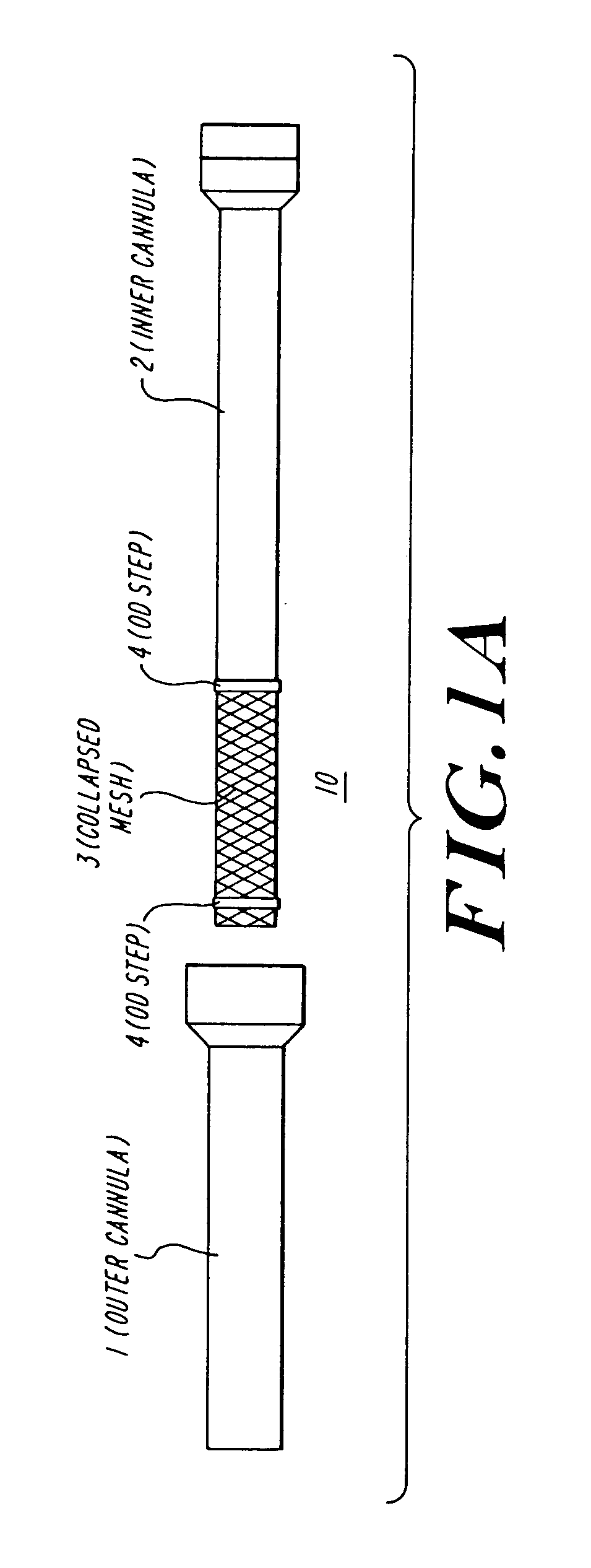
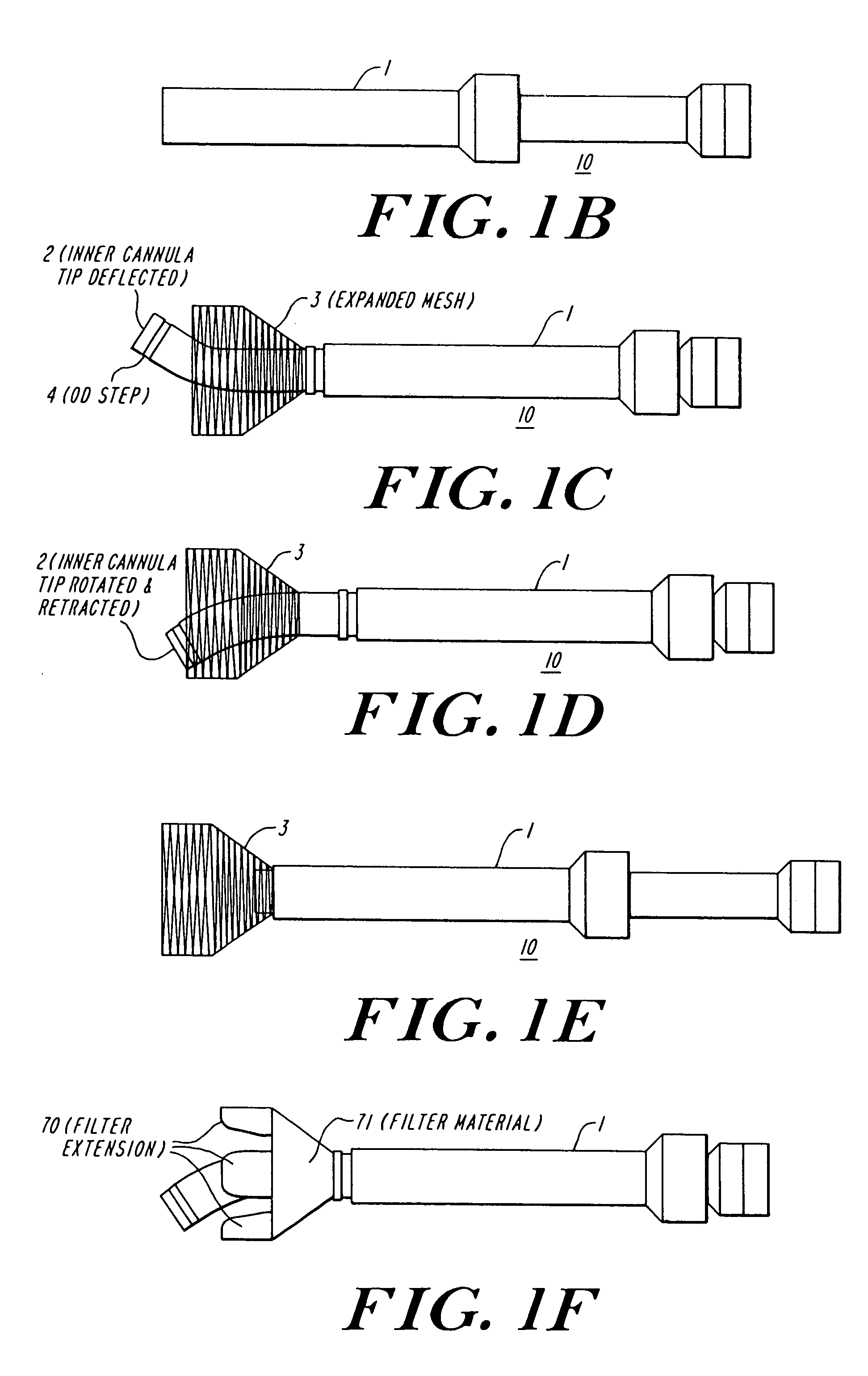
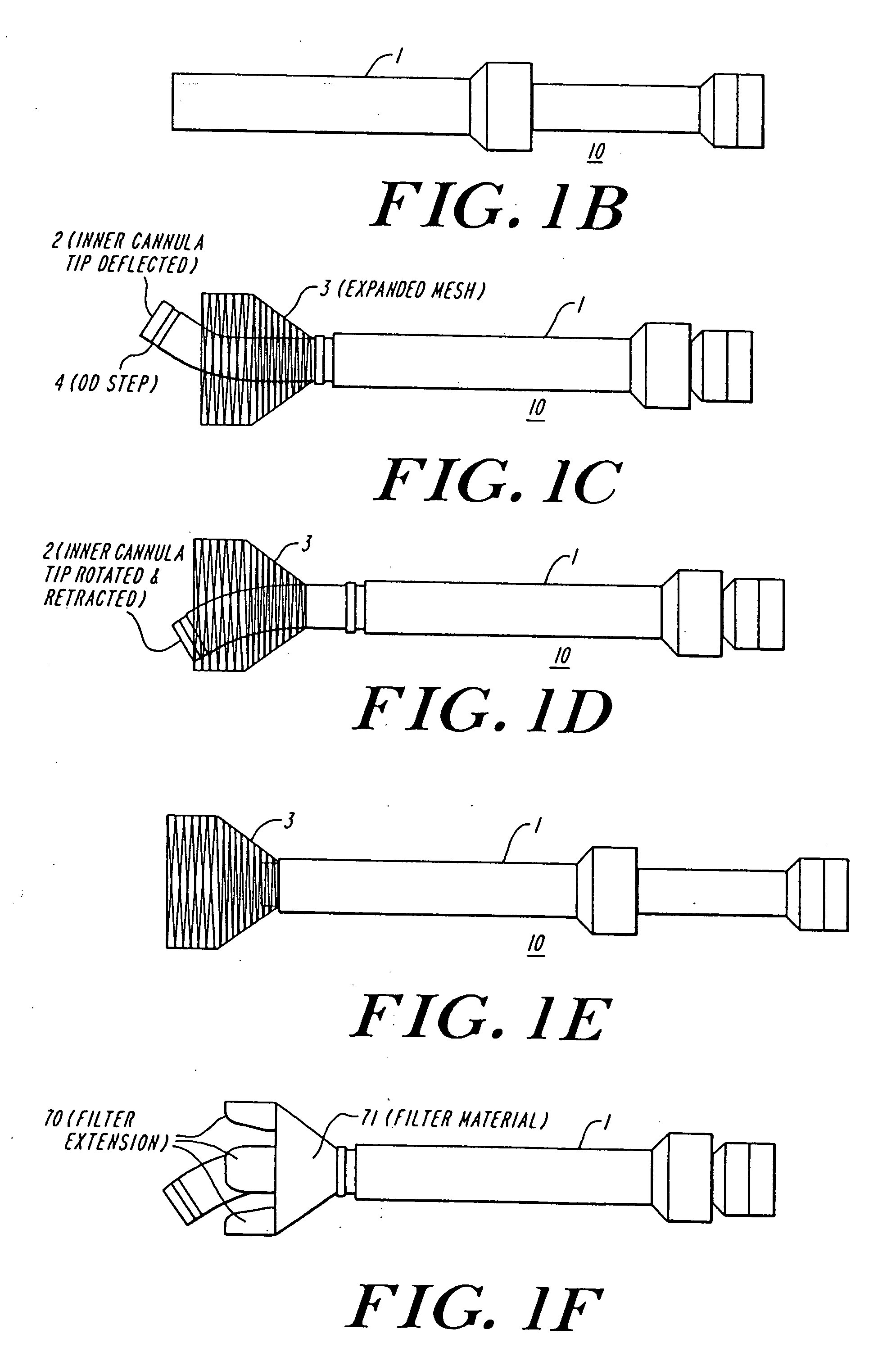
The present invention discloses devices and methods for performing intravascular procedures with out cardiac bypass. The devices include various embodiments of temporary filter devices, temporary valves, and prosthetic valves.The temporary filter devices have one or more cannulae which provide access for surgical tools for effecting repair of the cardiac valves. A cannula may have filters of various configurations encircling the distal region of the cannula, which prevent embolitic material from entering the coronary arteries and aorta.The temporary valve devices may also have one or more cannulae which guide the insertion of the valve into the aorta. The valve devices expand in the aorta to occupy the entire flow path of the vessel. In one embodiment, the temporary valve is a disc of flexible, porous, material that acts to filter blood passing therethrough. A set of valve leaflets extend peripherally from the disc. These leaflets can alternately collapse to prevent blood flow through the valve and extend to permit flow.The prosthetic valves include valve fixation devices which secure the prosthetic valve to the wall of the vessel. In one embodiment, the prosthetic valves have at least one substantially rigid strut, at least two expandable fixation rings located about the circumference of the base of the apex of the valve, and one or more commissures and leaflets. The prosthetic valves are introduced into the vascular system a compressed state, advanced to the site of implantation, expanded and secured to the vessel wall.
Owner:MEDTRONIC INC
Percutaneously implantable replacement heart valve device and method of making same
The present invention comprises a percutaneously implantable replacement heart valve device and a method of making same. The replacement heart valve device comprises a stent member made of stainless steel or self-expanding nitinol, a biological tissue artificial valve means disposed within the inner space of the stent member. An implantation and delivery system having a central part which consists of a flexible hollow tube catheter that allows a metallic wire guide to be advanced inside it. The endovascular stented-valve is created from a glutaraldehyde fixed biocompatible tissue material which has two or three cusps that open distally to permit unidirectional blood flow. The present invention also comprises a novel method of making a replacement heart valve by taking a fragment of biocompatible tissue material and treating, drying, folding and rehydrating it in such a way that forms a two- or three-leaflet / cusp valve with the leaflets / cusps formed by folding, thereby eliminating the extent of suturing required, providing improved durability and function.
Owner:COLIBRI HEART VALVE
Cardiac valve procedure methods and devices
The present invention discloses devices and methods for performing intravascular procedures with out: cardiac bypass. The devices include various embodiments of temporary filter devices, temporary valves, and prosthetic valves. The temporary filter devices have one or more cannulae which provide access for surgical tools for effecting repair of the cardiac valves. A cannula may have filters of various configurations encircling the distal region of the cannula, which prevent embolitic material from entering the coronary arteries and aorta. The temporary valve devices may also have one or more cannulae which guide the insertion of the valve into the aorta. The valve devices expand in the aorta to occupy the entire flow path of the vessel. In one embodiment, the temporary valve is a disc of flexible, porous, material that acts to filter blood passing therethrough. A set of valve leaflets extend peripherally from the disc. These leaflets can alternately collapse to prevent blood flow through the valve and extend to permit flow. The prosthetic valves include valve fixation devices which secure the prosthetic valve to the wall of the vessel. In one embodiment, the prosthetic valves have at least one substantially rigid strut, at least two expandable fixation rings located about the circumference of the base of the apex of the valve, and one or more commissures and leaflets. The prosthetic valves are introduced into the vascular system a compressed state, advanced to the site of implantation, expanded and secured to the vessel wall.
Owner:MEDTRONIC INC
System and method for monitoring a parameter associated with the performance of a heart
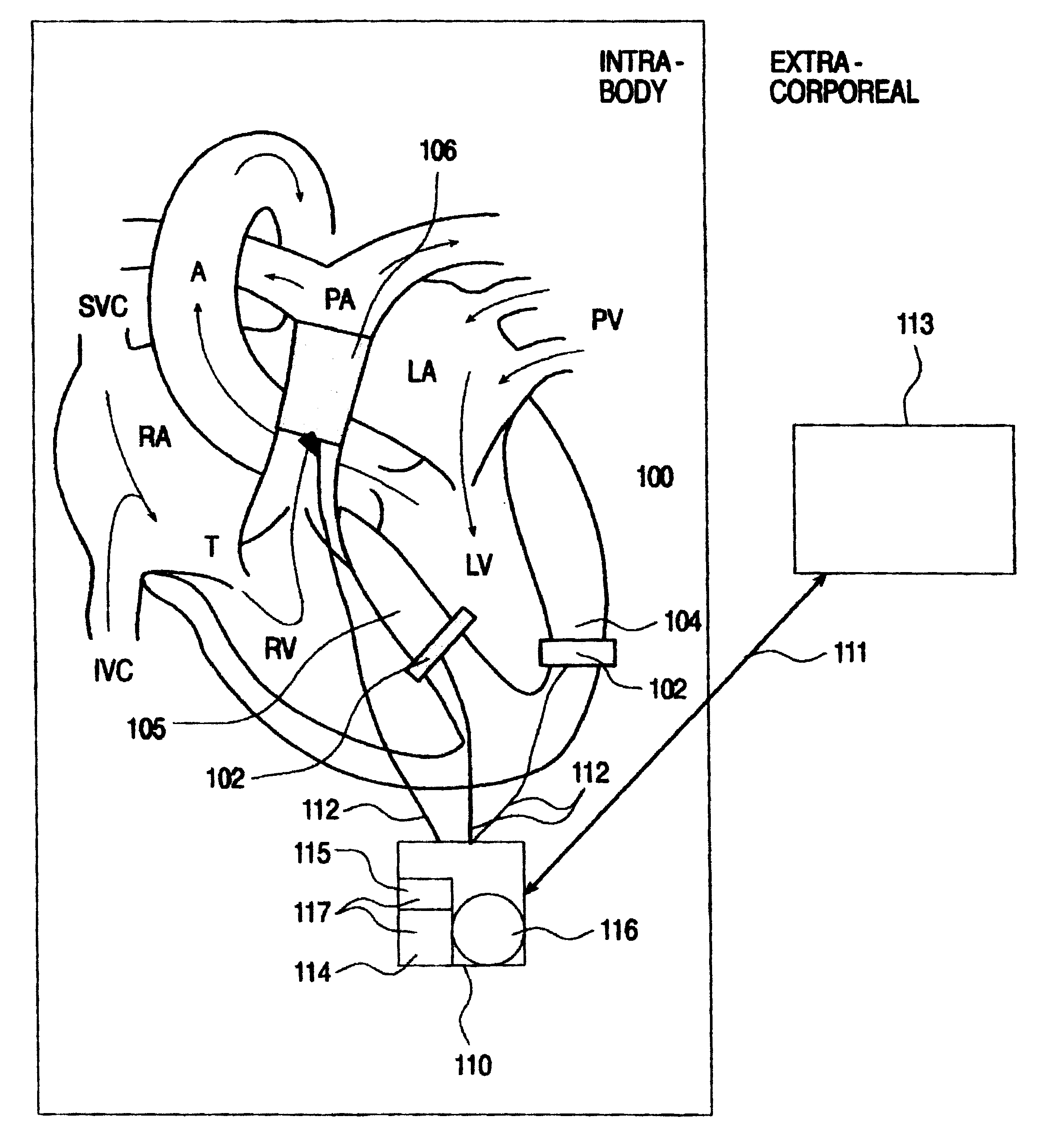
An intrabody implantable system for long-term, real time monitoring of at least one parameter associated with heart performance. The system includes (a) a first sensor being implantable within a heart and being for collecting information pertaining to a pressure in a first cavity of the heart; (b) at least one additional sensor being implantable in an blood vessel supporting blood flow into or out of a second cavity of the heart, the at least one additional sensor being for collecting information pertaining to a pressure and a flow within the blood vessel; and (c) at least one device implantable in the body and being in data communication with the first sensor and the at least one additional sensor, the at least one device being for receiving the information pertaining to the pressure in the first cavity of the heart and the information pertaining to the pressure and the flow within the blood vessel and for relaying the information pertaining to the pressure in the first cavity of the heart and the information pertaining to the pressure and the flow within the blood vessel outside the body.
Owner:REMON MEDICAL TECH
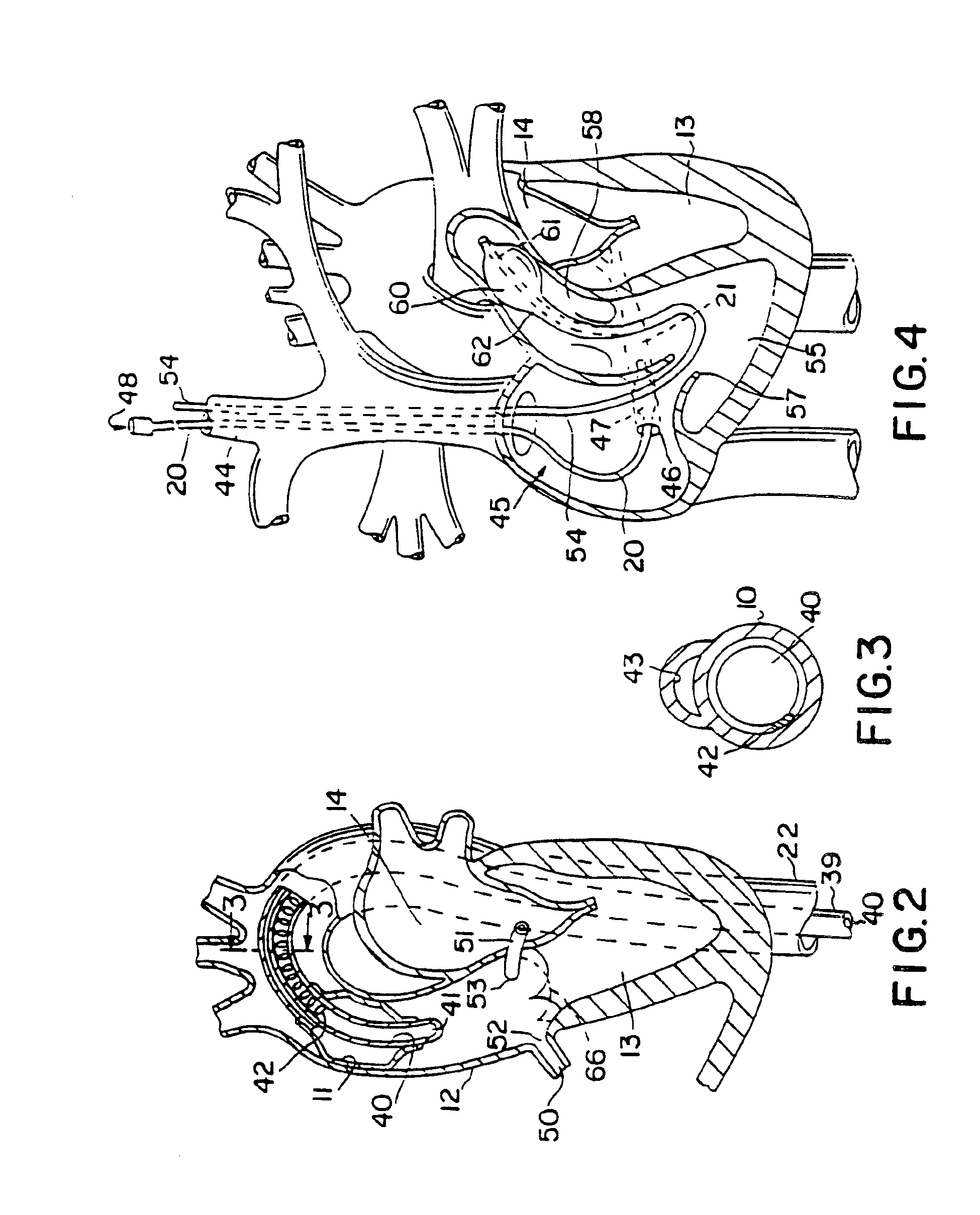
Device and method for treatment of heart valve regurgitation
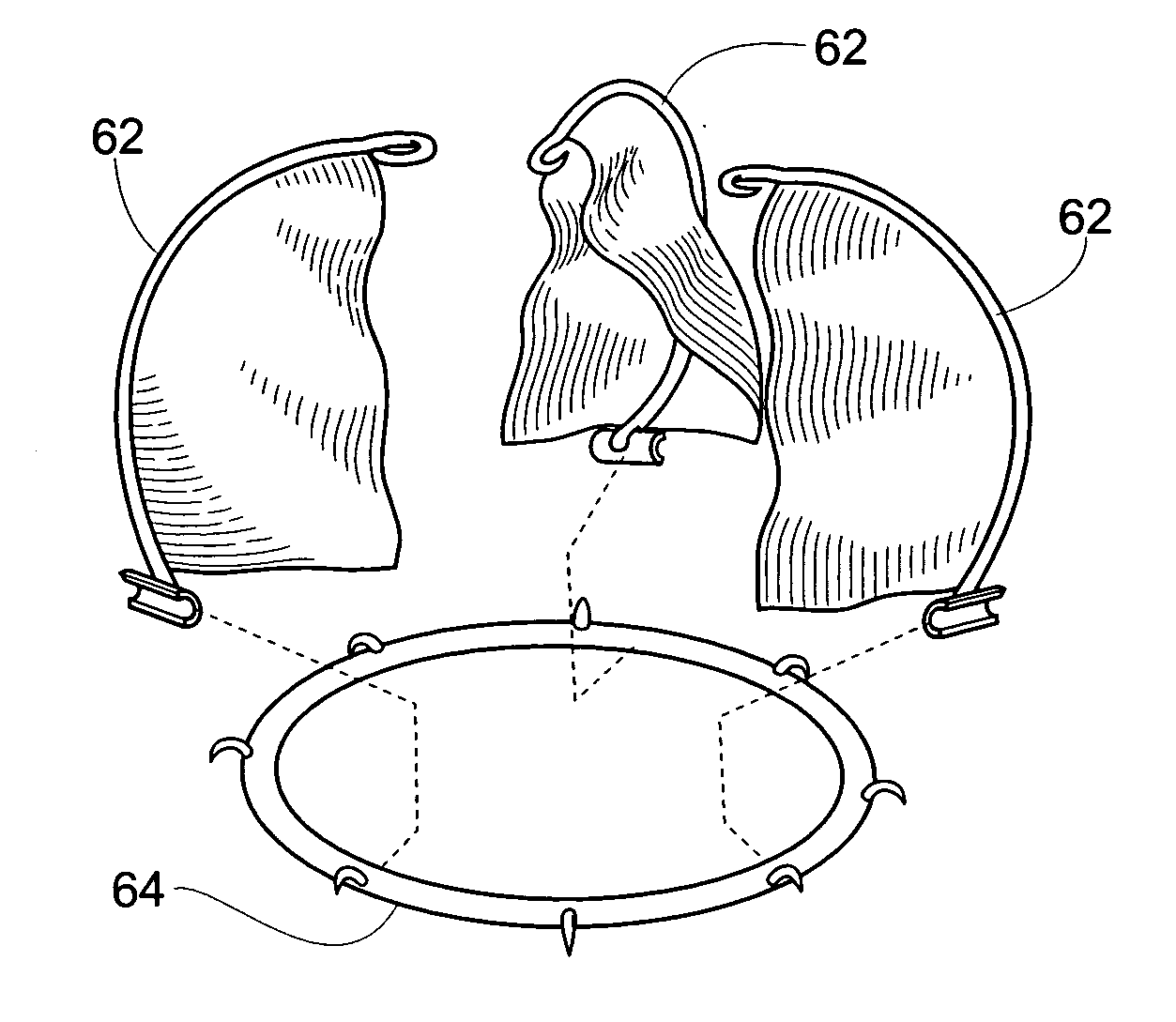
In one embodiment, the present invention provides a prosthesis that can be implanted within a heart to at least partially block gaps that may be present between the two mitral valve leaflets. In one preferred embodiment, the prosthesis includes an anchoring ring that expands within the left atrium to anchor the prosthesis and a pocket member fixed to the anchoring ring. The pocket member is positioned within the mitral valve, between the leaflets so that an open end of the pocket member is positioned within the left ventricle. When the mitral valve is open, blood flows past the pocket member, maintaining the pocket member in a collapsed state. When the mitral valve closes, the backpressure of the blood pushes into the pocket member, expanding the pocket member to an inflated shape. The mitral valve leaflets contact the expanded pocket member, allowing the prosthesis to block at least a portion of the openings between the leaflets, thereby minimizing regurgitated blood flow into the left atrium.
Owner:EDWARDS LIFESCIENCES AG
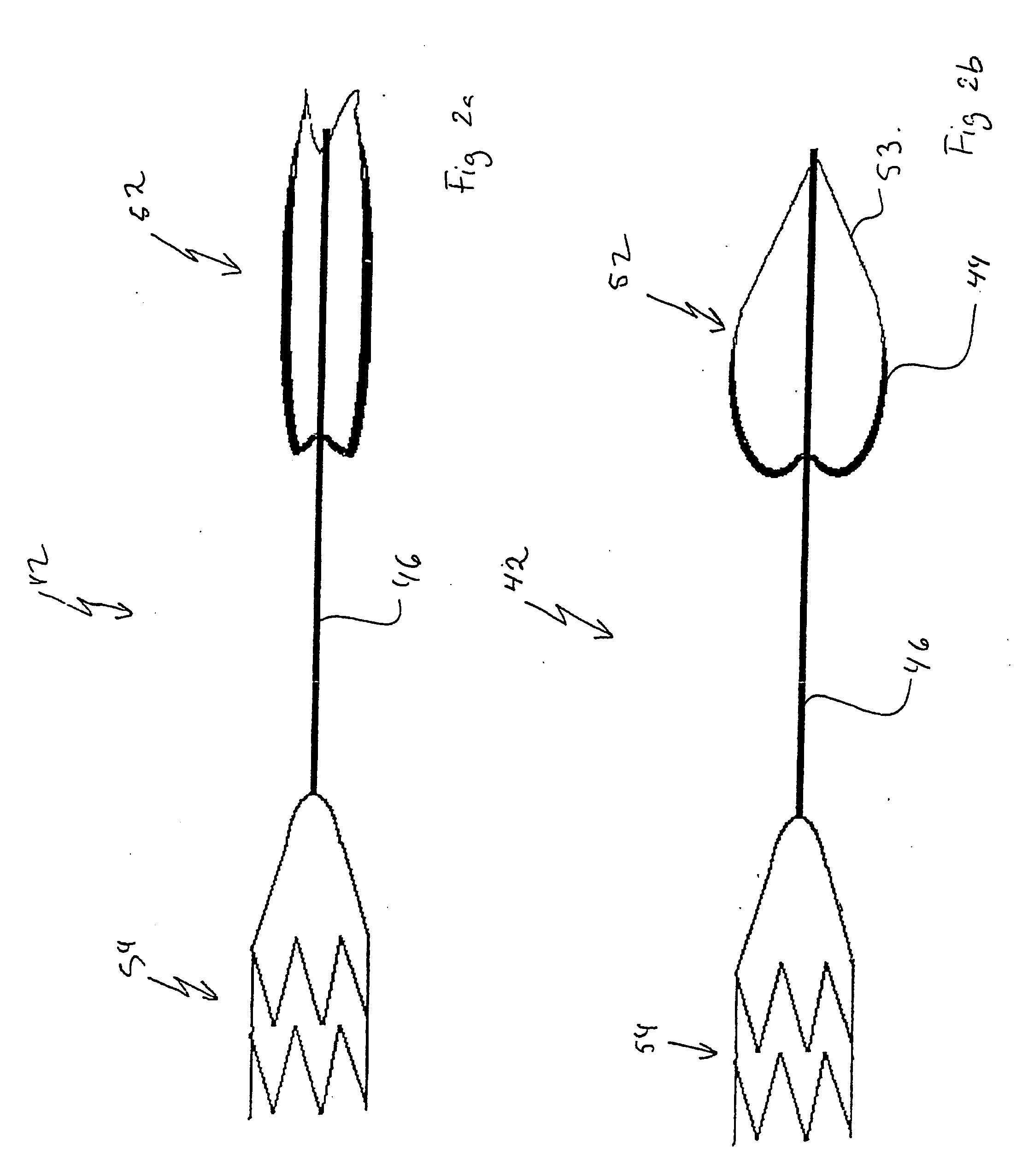
Suspended heart valve devices, systems, and methods for supplementing, repairing, or replacing a native heart valve
InactiveUS20050228495A1Good flexibility and compressibilityImprove foldabilityHeart valvesBlood vesselsHeart chamberBlood vessel
A valve prosthesis is sized and configured to rest within a blood path subject to antegrade and retrograde blood flow. A trestle element on the prosthesis extends across the blood path. A leaflet assembly is suspended from the trestle element and extends into the blood path in alignment with blood flow. At least one mobile leaflet member on the leaflet assembly is sized and configured to assume orientations that change according to blood flow direction. The mobile leaflet member has a first orientation that permits antegrade blood flow and a second orientation that resists retrograde blood flow. The valve prosthesis, when implanted in a heart chamber or great vessel, serves to supplement and / or repair and / or replace native one-way heart valve function.
Owner:AM DISCOVERY
System and method for conditioning animal tissue using laser light
InactiveUS20100049180A1Promote wound repairEnhances surgical wound healingSurgical instrument detailsLight therapyLaser lightHsp70 expression
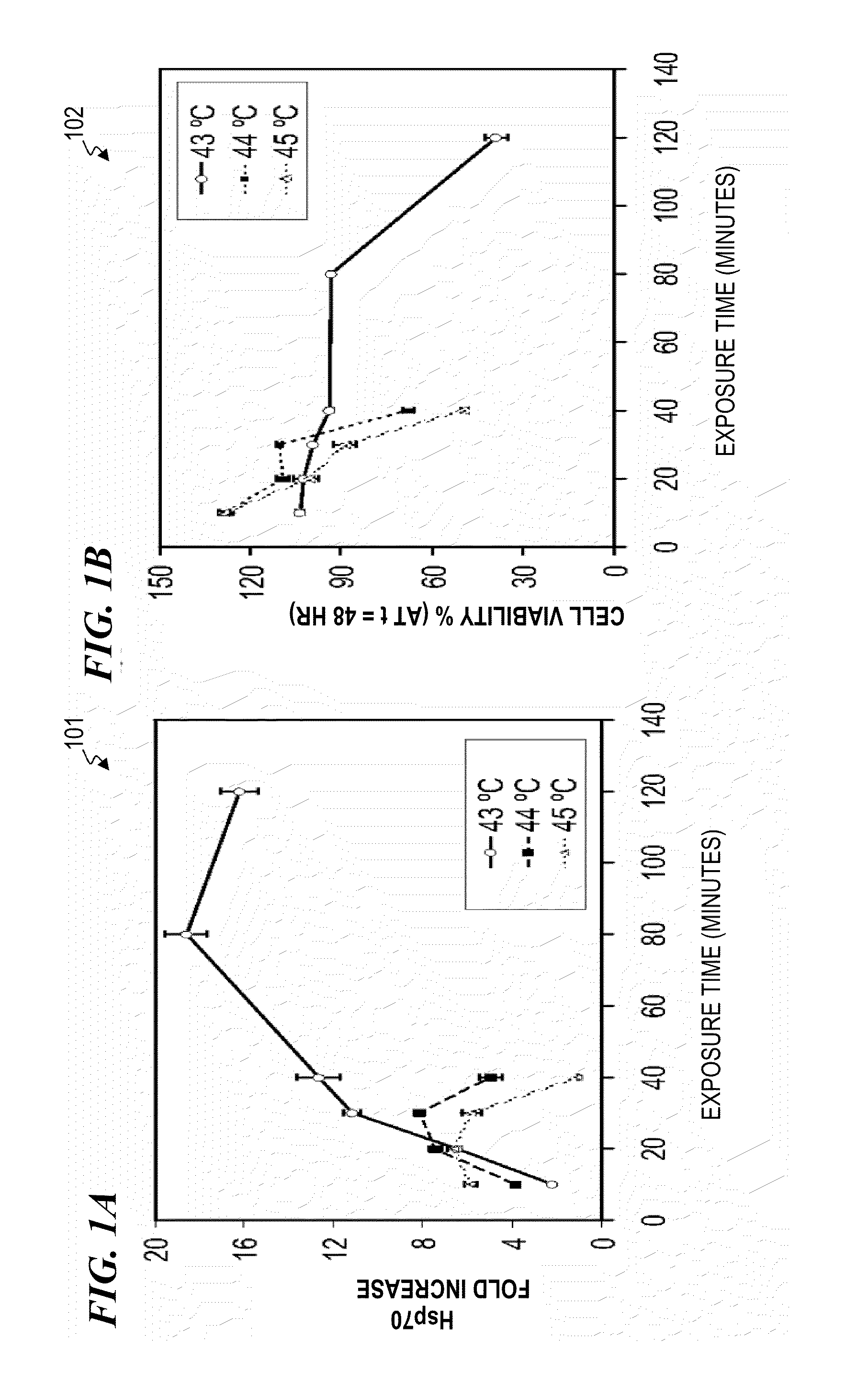
Systems and methods for prophylactic measures aimed at improving wound repair. In some embodiments, laser-mediated preconditioning would enhance surgical wound healing that was correlated with hsp70 expression. Using a pulsed laser (λ=1850 nm, Tp=2 ms, 50 Hz, H=7.64 mJ / cm2) the skin of transgenic mice that contain an hsp70 promoter-driven luciferase were preconditioned 12 hours before surgical incisions were made. Laser protocols were optimized using temperature, blood flow, and hsp70-mediated bioluminescence measurements as benchmarks. Bioluminescent imaging studies in vivo indicated that an optimized laser protocol increased hsp70 expression by 15-fold. Under these conditions, healed areas from incisions that were laser-preconditioned were two times stronger than those from control wounds. Our data suggest that these methods can provide effective and improved tissue-preconditioning protocols and that mild laser-induced heat shock that correlated with an expression of Hsp70 may be a useful therapeutic intervention prior to or after surgery.
Owner:LOCKHEED MARTIN CORP +2
Prosthetic heart valves
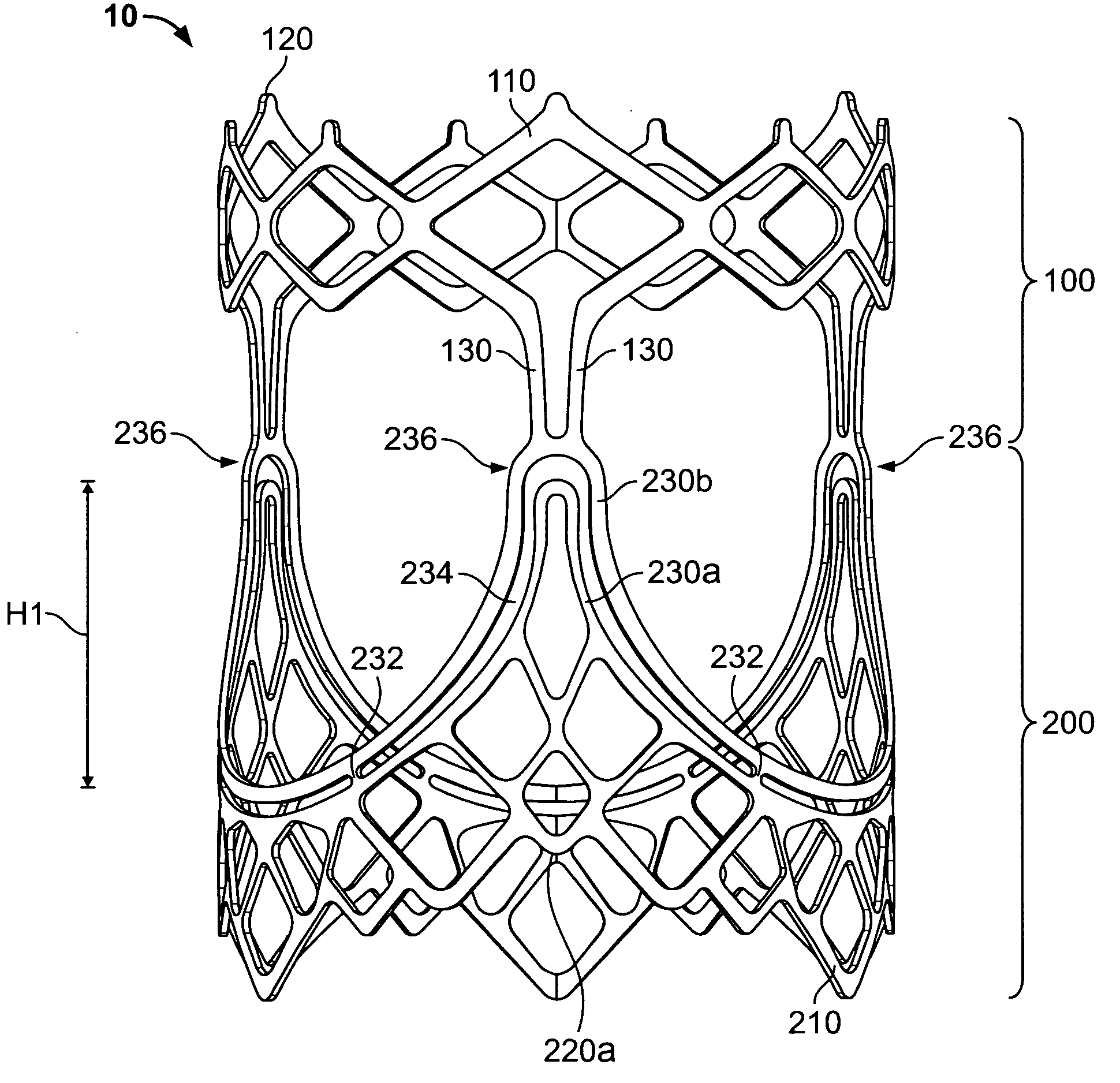
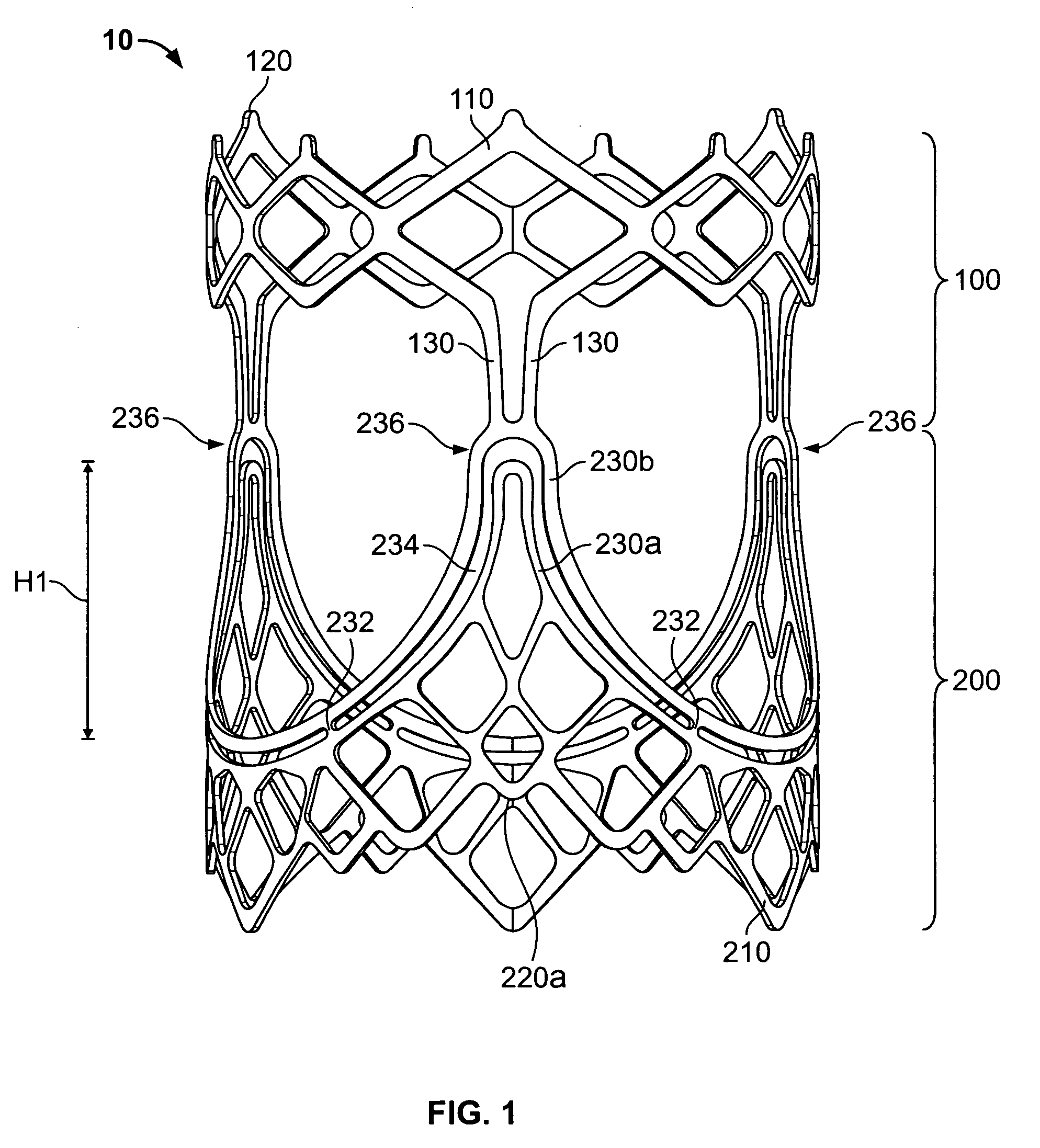
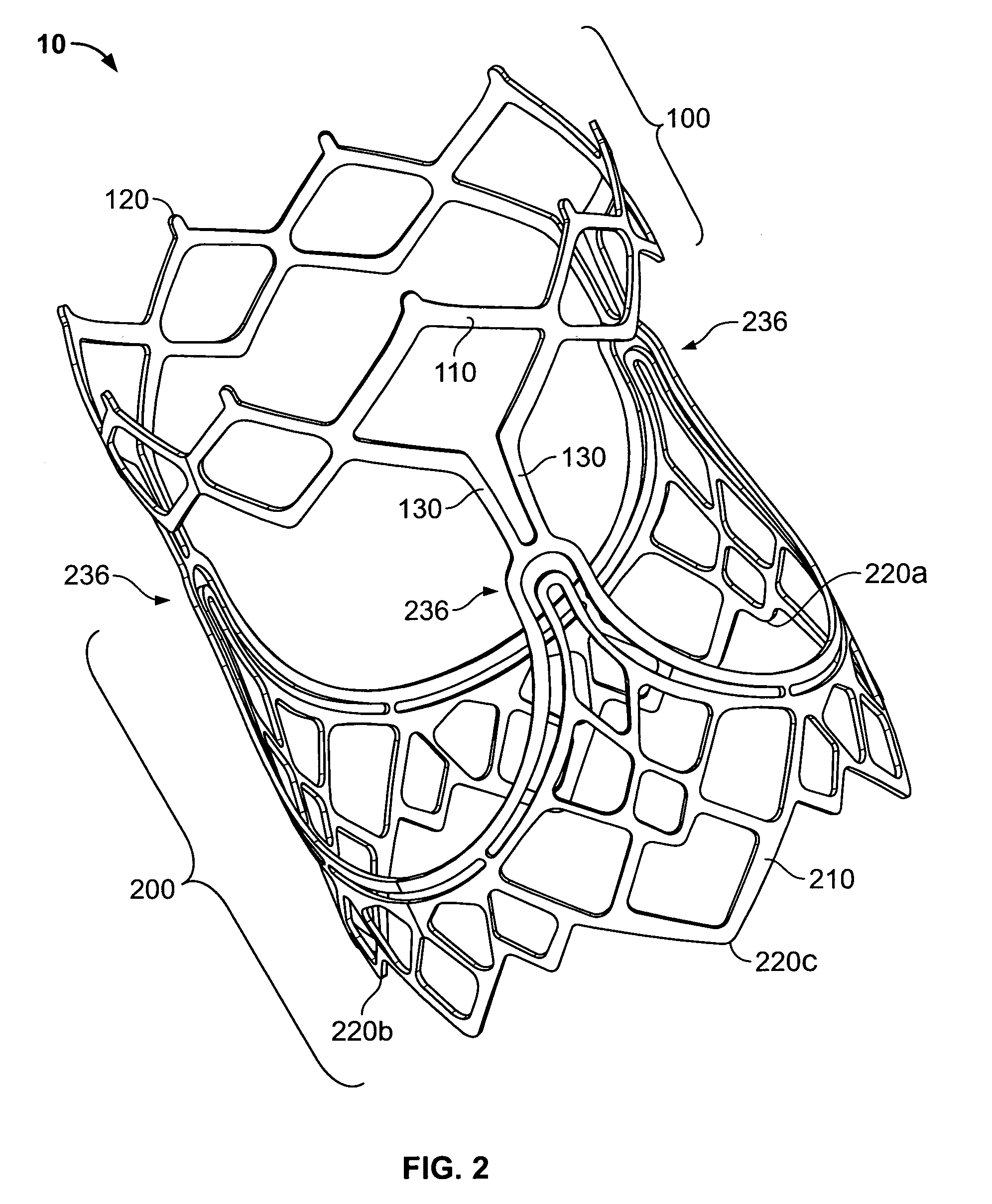
A prosthetic heart valve (10) (e.g., a prosthetic aortic valve) is designed to be somewhat circumferentially collapsible and then re-expandable. The collapsed condition may be used for less invasive delivery of the valve into a patient. When the valve reaches the implant site in the patient, it re-expands to normal operating size, and also to engage surrounding tissue of the patient. The valve includes a stent portion (200) and a ring portion (100) that is substantially concentric with the stent portion but downstream from the stent portion in the direction of blood flow through the implanted valve. When the valve is implanted, the stent portion engages the patient's tissue at or near the native valve annulus, while the ring portion engages tissue downstream from the native valve site (e.g., the aorta).
Owner:ST JUDE MEDICAL
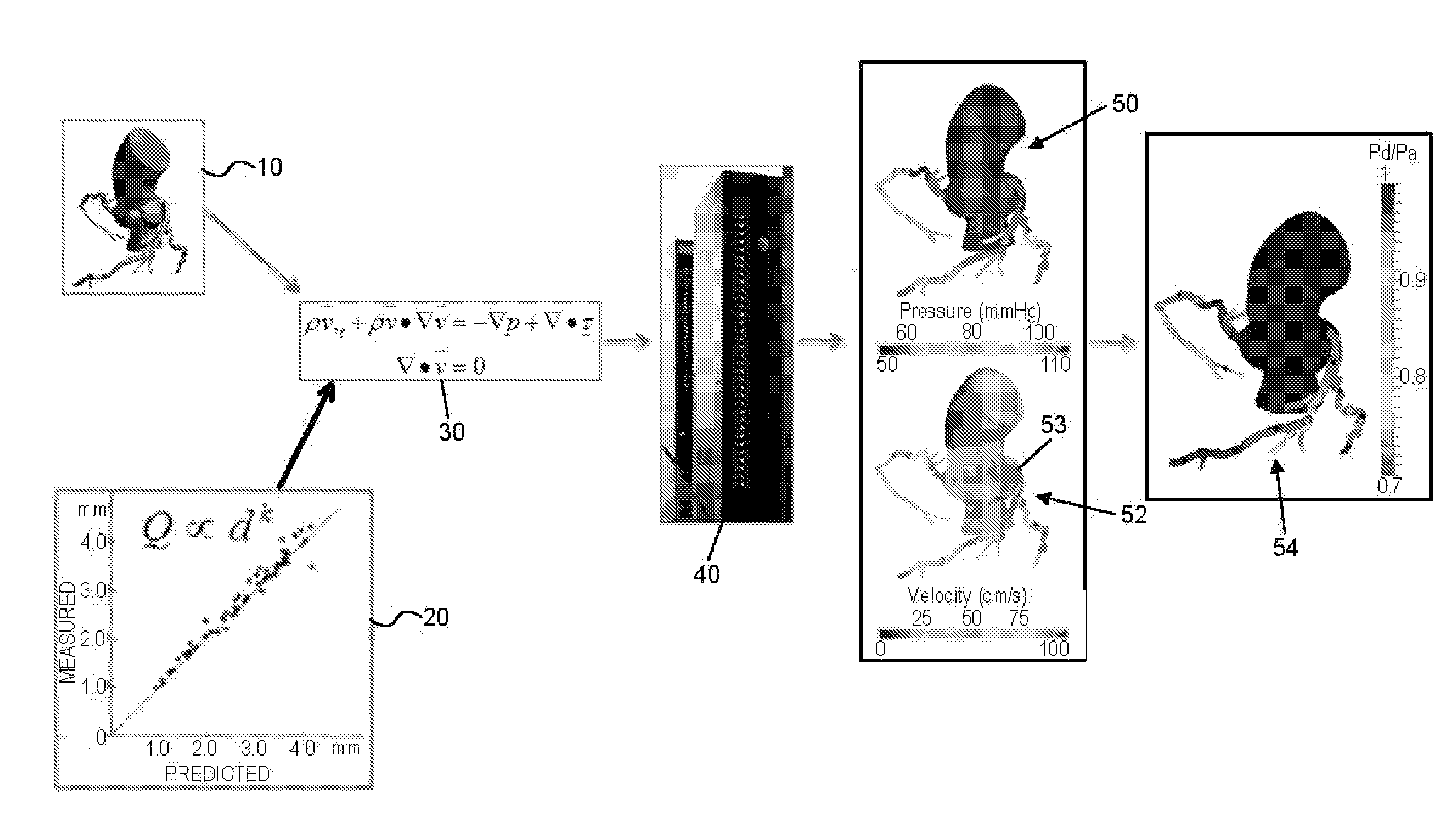
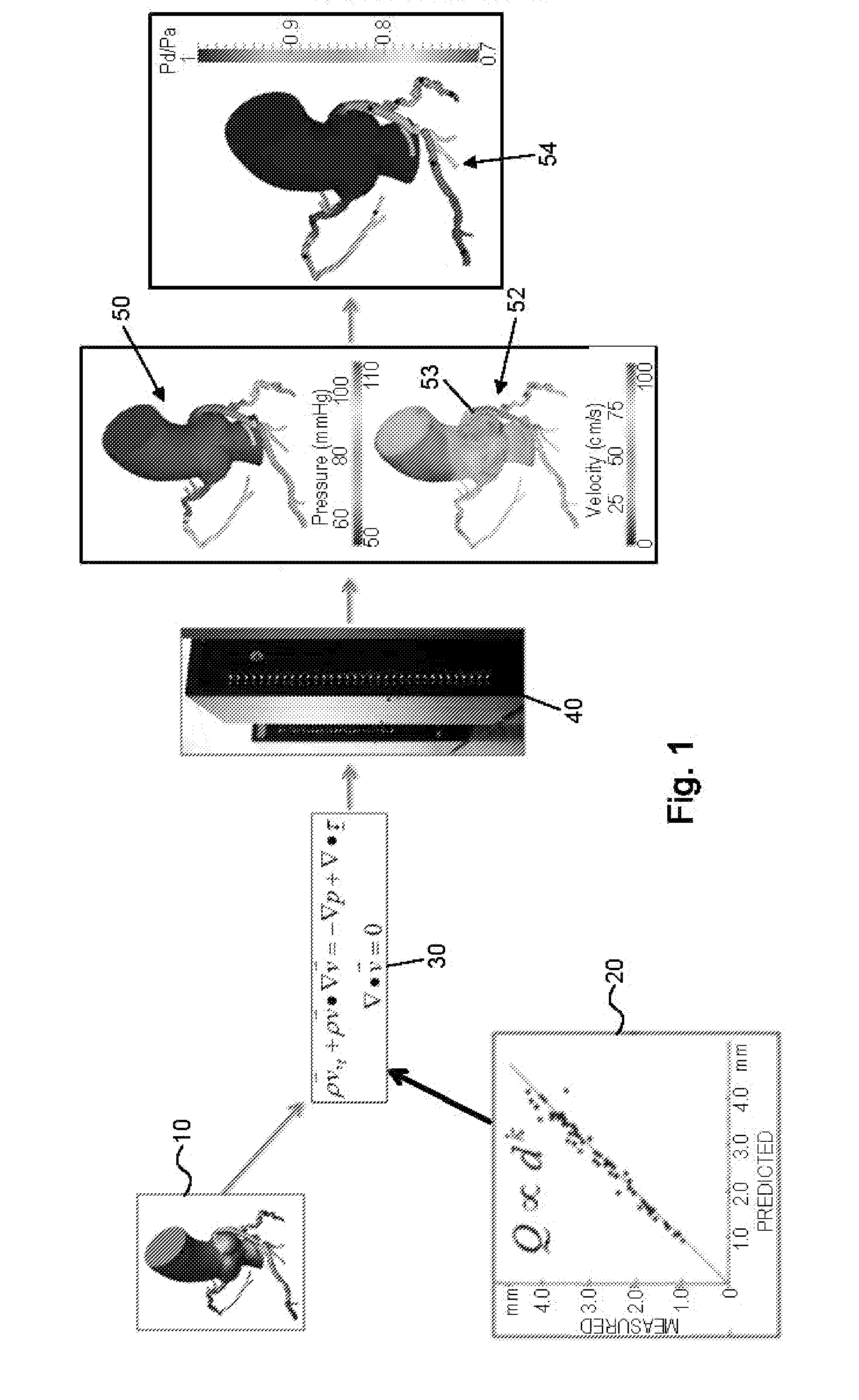
Method and system for patient-specific modeling of blood flow
Embodiments include a system for determining cardiovascular information for a patient. The system may include at least one computer system configured to receive patient-specific data regarding a geometry of the patient's heart, and create a three-dimensional model representing at least a portion of the patient's heart based on the patient-specific data. The at least one computer system may be further configured to create a physics-based model relating to a blood flow characteristic of the patient's heart and determine a fractional flow reserve within the patient's heart based on the three-dimensional model and the physics-based model.
Owner:HEARTFLOW
Prosthetic Valve for Transluminal Delivery
InactiveUS20100004740A1Preventing substantial migrationEliminate the problemBalloon catheterHeart valvesVenous accessImplantation Site
A prosthetic valve assembly for use in replacing a deficient native valve comprises a replacement valve supported on an expandable valve support. If desired, one or more anchors may be used. The valve support, which entirely supports the valve annulus, valve leaflets, and valve commissure points, is configured to be collapsible for transluminal delivery and expandable to contact the anatomical annulus of the native valve when the assembly is properly positioned. Portions of the valve support may expand to a preset diameter to maintain coaptivity of the replacement valve and to prevent occlusion of the coronary ostia. A radial restraint, comprising a wire, thread or cuff, may be used to ensure expansion does not exceed the preset diameter. The valve support may optionally comprise a drug elution component. The anchor engages the lumen wall when expanded and prevents substantial migration of the valve assembly when positioned in place. The prosthetic valve assembly is compressible about a catheter, and restrained from expanding by an outer sheath. The catheter may be inserted inside a lumen within the body, such as the femoral artery, and delivered to a desired location, such as the heart. A blood pump may be inserted into the catheter to ensure continued blood flow across the implantation site during implantation procedure. When the outer sheath is retracted, the prosthetic valve assembly expands to an expanded position such that the valve and valve support expand at the implantation site and the anchor engages the lumen wall. Insertion of the catheter may optionally be performed over a transseptally delivered guidewire that has been externalized through the arterial vasculature. Such a guidewire provide dual venous and arterial access to the implantation site and allows additional manipulation of the implantation site after arterial implantation of the prosthetic valve. Additional expansion stents may be delivered by venous access to the valve.
Owner:MEDTRONIC COREVALVE
Method and apparatus for non-invasive blood constituent monitoring
InactiveUS6181958B1Repeatable and reliableEasy to implementSensorsBlood characterising devicesNon invasiveHemoglobin G Szuhu
A system for determining a biologic constituent including hematocrit transcutaneously, noninvasively and continuously. A finger clip assembly includes including at least a pair of emitters and a photodiode in appropriate alignment to enable operation in either a transmissive mode or a reflectance mode. At least one predetermined wavelength of light is passed onto or through body tissues such as a finger, earlobe, or scalp, etc. and attenuation of light at that wavelength is detected. Likewise, the change in blood flow is determined by various techniques including optical, pressure, piezo and strain gage methods. Mathematical manipulation of the detected values compensates for the effects of body tissue and fluid and determines the hematocrit value. If an additional wavelength of light is used which attenuates light substantially differently by oxyhemoglobin and reduced hemoglobin, then the blood oxygen saturation value, independent of hematocrit may be determined. Further, if an additional wavelength of light is used which greatly attenuates light due to bilirubin (440 nm) or glucose (1060 nm), then the bilirubin or glucose value may also be determined. Also how to determine the hematocrit with a two step DC analysis technique is provided. Then a pulse wave is not required, so this method may be utilized in states of low blood pressure or low blood flow.
Owner:HEMA METRICS
Percutaneous heart valve
ActiveUS7621948B2Avoid flowStability and functioning of the heart valve are satisfactoryHeart valvesJoint implantsGuide tubeElastance
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
Methods and apparatus for blocking flow through blood vessels
This invention is methods and apparatus for occluding blood flow within a blood vessel (22). In a first series of embodiments, the present invention comprises a plurality of embolic devices (16) deployable through the lumen (12) of a conventional catheter (10) such that when deployed, said embolic devices (16) remain resident and occlude blood flow at a specific site within the lumen of the blood vessel (22). Such embolic devices (16) comprise either mechanical embolic devices that become embedded within or compress against the lumen of the vessel or chemical vaso occlusive agents that seal off blood flow at a given site. A second embodiment of the present invention comprises utilization of a vacuum / cauterizing device capable of sucking in the lumen of the vessel about the device to maintain the vessel in a closed condition where there is then applied a sufficient amount of energy to cause the tissue collapsed about the device to denature into a closure. In a third series of embodiments, the present invention comprises the combination of an embolization facilitator coupled with the application of an energy force to form an intraluminal closure at a specified site within a vessel.
Owner:MEDTRONIC VASCULAR INC
Cardiac valve procedure methods and devices
The present invention discloses devices and methods for performing intravascular procedures with out cardiac bypass. The devices include various embodiments of temporary filter devices, temporary valves, and prosthetic valves. The temporary filter devices have one or more cannulae which provide access for surgical tools for effecting repair of the cardiac valves. A cannula may have filters of various configurations encircling the distal region of the cannula, which prevent embolitic material from entering the coronary arteries and aorta. The temporary valve devices may also have one or more cannulae which guide the insertion of the valve into the aorta. The valve devices expand in the aorta to occupy the entire flow path of the vessel. In one embodiment, the temporary valve is a disc of flexible, porous, material that acts to filter blood passing therethrough. A set of valve leaflets extend peripherally from the disc. These leaflets can alternately collapse to prevent blood flow through the valve and extend to permit flow. The prosthetic valves include valve fixation devices which secure the prosthetic valve to the wall of the vessel. In one embodiment, the prosthetic valves have at least one substantially rigid strut, at least two expandable fixation rings located about the circumference of the base of the apex of the valve, and one or more commissures and leaflets. The prosthetic valves are introduced into the vascular system a compressed state, advanced to the site of implantation, expanded and secured to the vessel wall.
Owner:MEDTRONIC INC
Apparatus and methods of bioelectrical impedance analysis of blood flow
InactiveUS6095987AAccurately and non-invasively and continuously measuring cardiac outputCatheterSensorsBioelectrical impedance analysisCardiac pacemaker electrode
Apparatus and methods are provided for monitoring cardiac output using bioelectrical impedance techniques in which first and second electrodes are placed in the trachea and / or bronchus in the vicinity of the ascending aorta, while an excitation current is injected into the thorax via first and second current electrodes, so that bioelectrical impedance measurements based on the voltage drop sensed by the first and second electrodes reflect voltage changes induced primarily by blood flow dynamics, rather than respiratory or non-cardiac related physiological effects. Additional sense electrodes may be provided, either internally, or externally, for which bioelectrical impedance values may be obtained. Methods are provided for computing cardiac output from bioelectrical impedance values. Apparatus and methods are also provided so that the measured cardiac output may be used to control administration of intravenous fluids to an organism or to optimize a heart rate controlled by a pacemaker.
Owner:ECOM MED
Lumen Morphology and Vascular Resistance Measurements Data Collection Systems, Apparatus and Methods
A method and apparatus of automatically locating in an image of a blood vessel the lumen boundary at a position in the vessel and from that measuring the diameter of the vessel. From the diameter of the vessel and estimated blood flow rate, a number of clinically significant physiological parameters are then determined and various user displays of interest generated. One use of these images and parameters is to aid the clinician in the placement of a stent. The system, in one embodiment, uses these measurements to allow the clinician to simulate the placement of a stent and to determine the effect of the placement. In addition, from these patient parameters various patient treatments are then performed.
Owner:LIGHTLAB IMAGING
Percutaneously placed prosthesis with thromboresistant valve portion
InactiveUS20050182483A1Prevent and limit refluxAvoid poolingBall valvesVenous valvesVenous ValvesBlood flow
A venous valve prosthesis having a substantially non-expandable, valve portion comprising a valve-closing mechanism, such as a pair of opposing leaflets; and an anchoring portion, such as one or more self-expanding frames or stents that are expandable to anchor the prosthesis at the implantation site. In one embodiment, the rigid valve portion includes a deposition of material such as pyrolitic carbon to reduce the thrombogenecity of the blood-contacting surfaces. The anchoring portions preferably include a covering, such as a tubular construct of synthetic or collagen-derived material (such as a bioremodelable ECM material), which attaches about the support structure such that blood flow is directed through the valve mechanism as it transitions from the larger diameter anchoring portion to the intermediate, smaller-diameter portion of the prosthesis. In another embodiment, the valve support housing and valve-closing elements are delivered in a collapsed, folded, and / or dissembled state sized for delivery, then manipulated in situ to the second expanded configured following deployment.
Owner:COOK INC
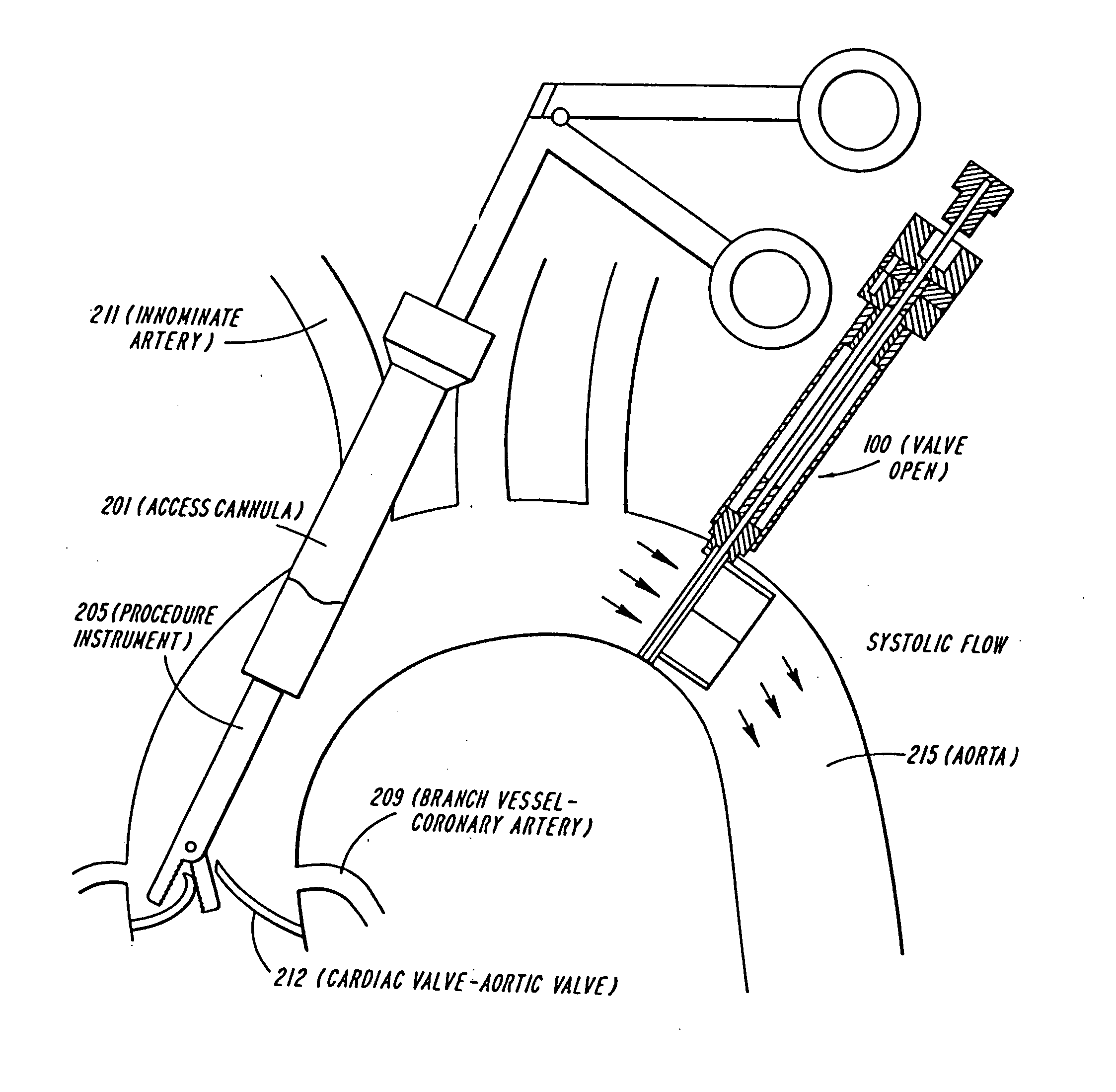
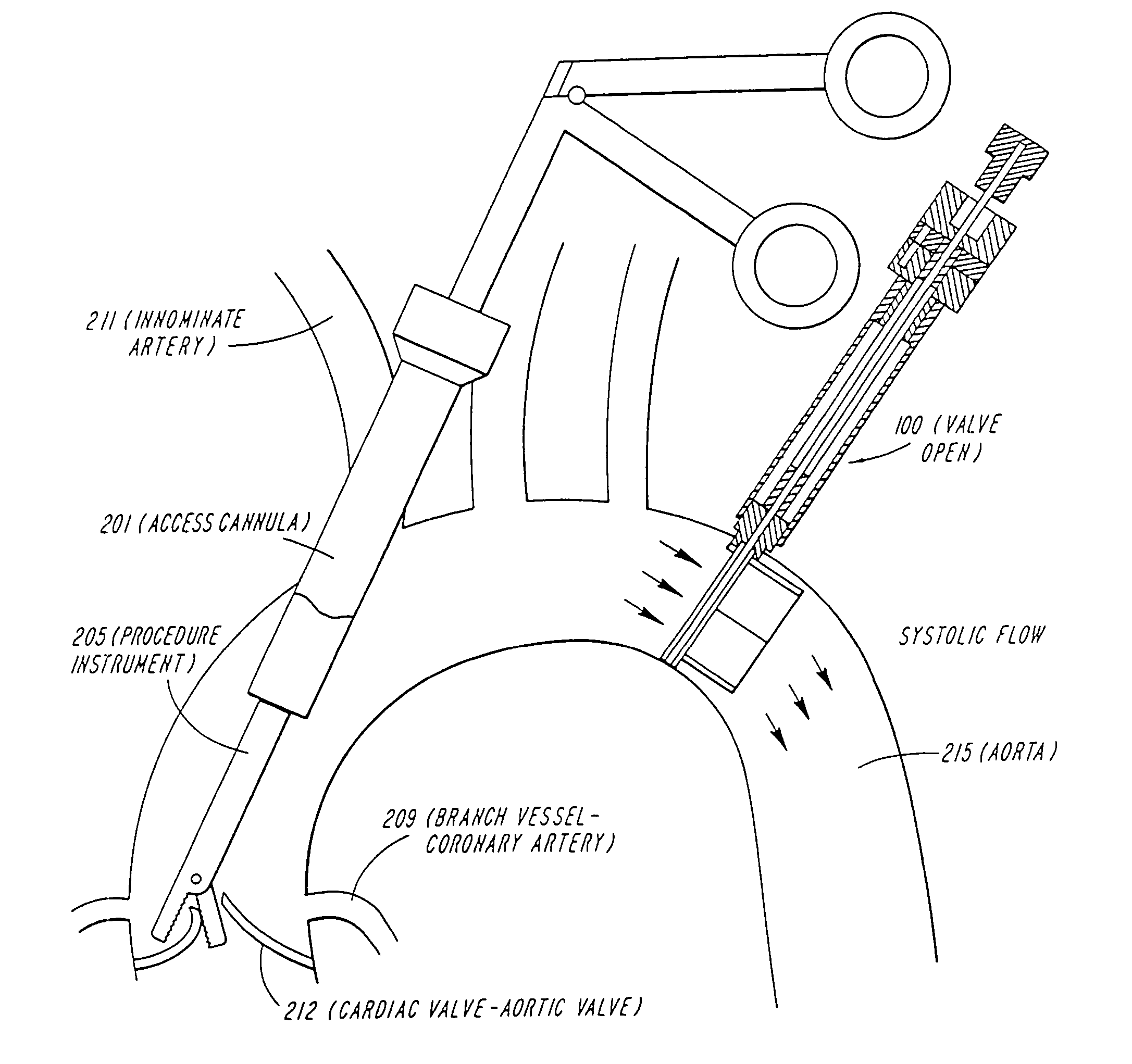
System for cardiac procedures
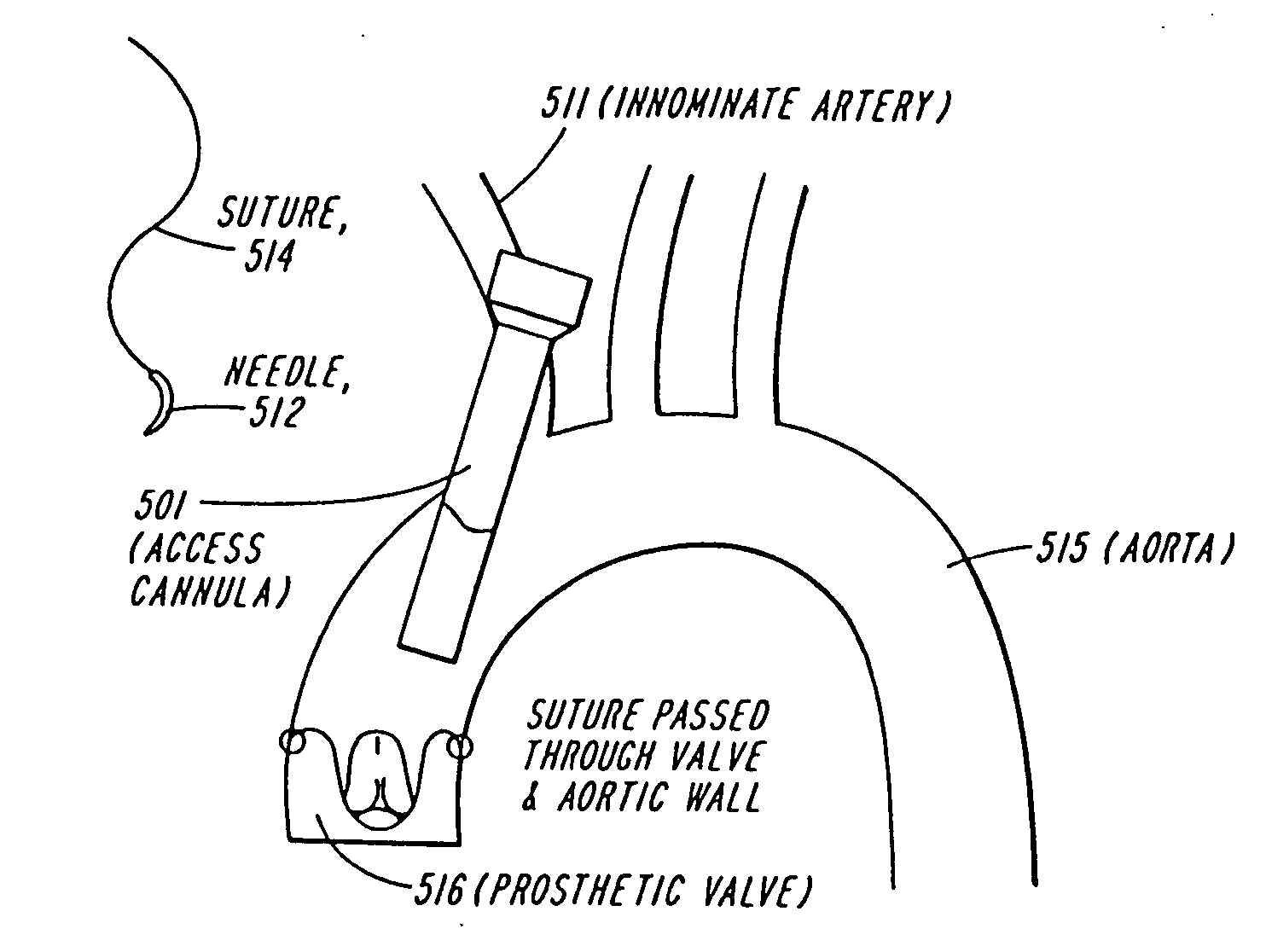
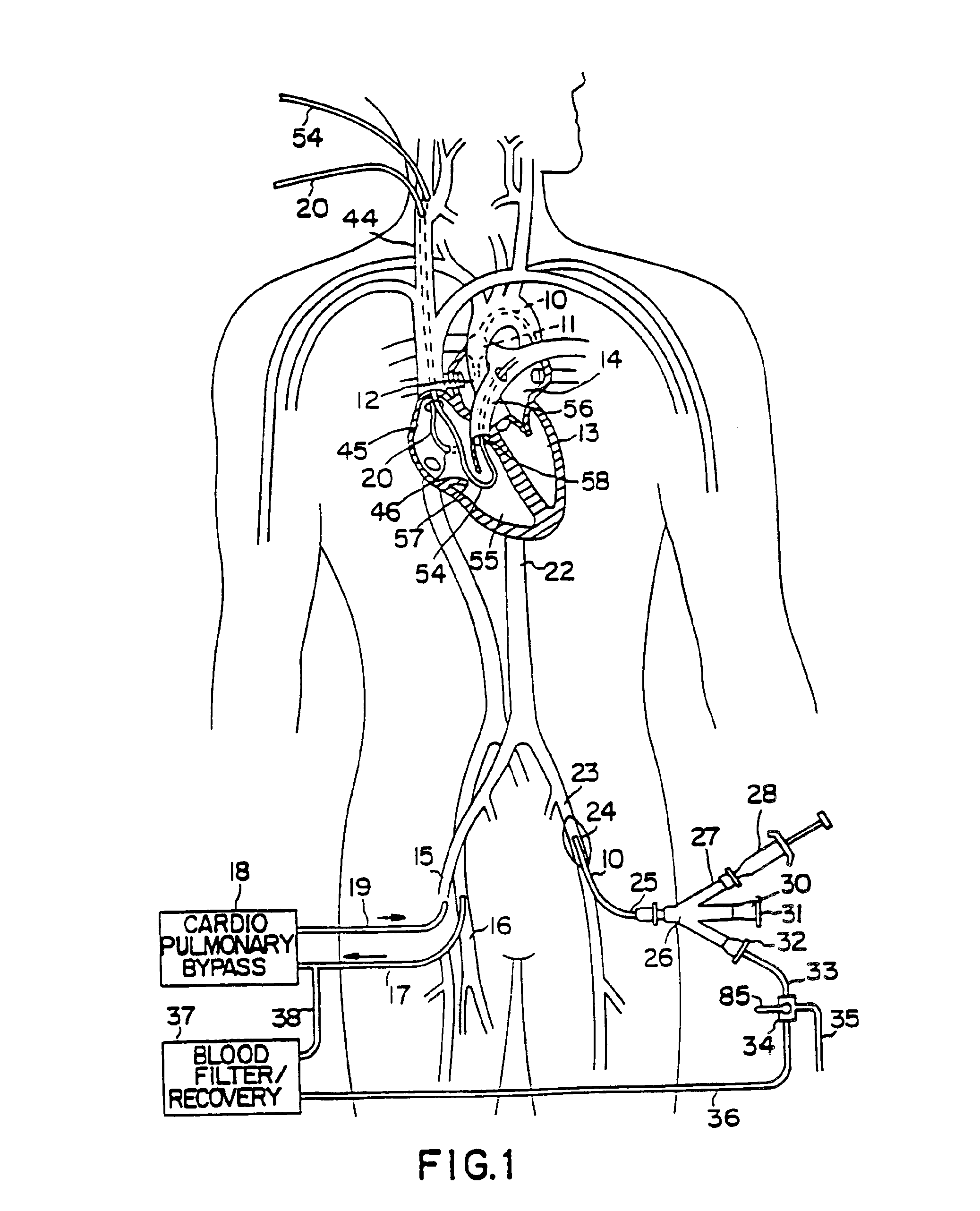
A system for accessing a patient's cardiac anatomy which includes an endovascular aortic partitioning device that separates the coronary arteries and the heart from the rest of the patient's arterial system. The endovascular device for partitioning a patient's ascending aorta comprises a flexible shaft having a distal end, a proximal end, and a first inner lumen therebetween with an opening at the distal end. The shaft may have a preshaped distal portion with a curvature generally corresponding to the curvature of the patient's aortic arch. An expandable means, e.g. a balloon, is disposed near the distal end of the shaft proximal to the opening in the first inner lumen for occluding the ascending aorta so as to block substantially all blood flow therethrough for a plurality of cardiac cycles, while the patient is supported by cardiopulmonary bypass. The endovascular aortic partitioning device may be coupled to an arterial bypass cannula for delivering oxygenated blood to the patient's arterial system. The heart muscle or myocardium is paralyzed by the retrograde delivery of a cardioplegic fluid to the myocardium through patient's coronary sinus and coronary veins, or by antegrade delivery of cardioplegic fluid through a lumen in the endovascular aortic partitioning device to infuse cardioplegic fluid into the coronary arteries. The pulmonary trunk may be vented by withdrawing liquid from the trunk through an inner lumen of an elongated catheter. The cardiac accessing system is particularly suitable for removing the aortic valve and replacing the removed valve with a prosthetic valve.
Owner:EDWARDS LIFESCIENCES LLC
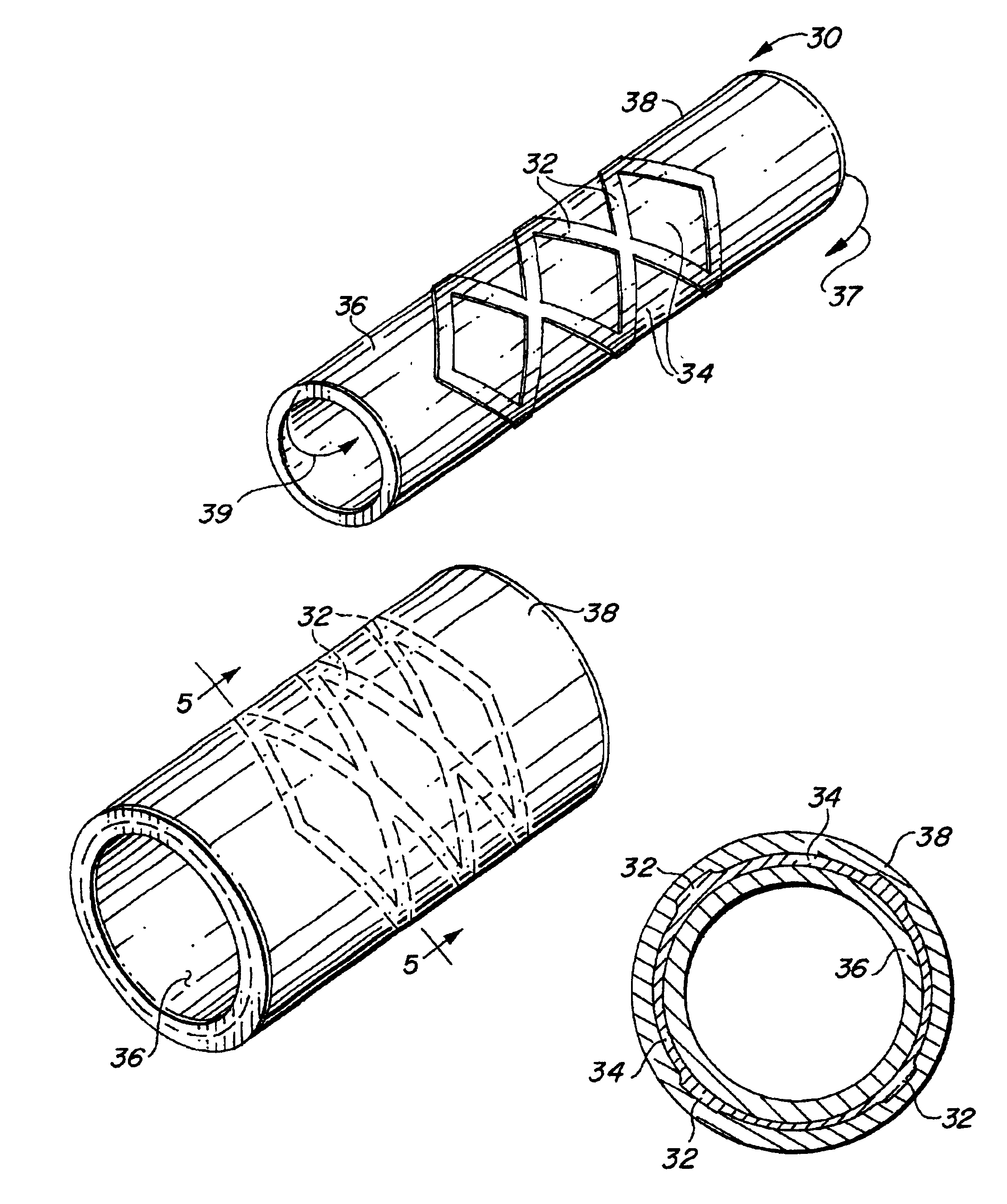
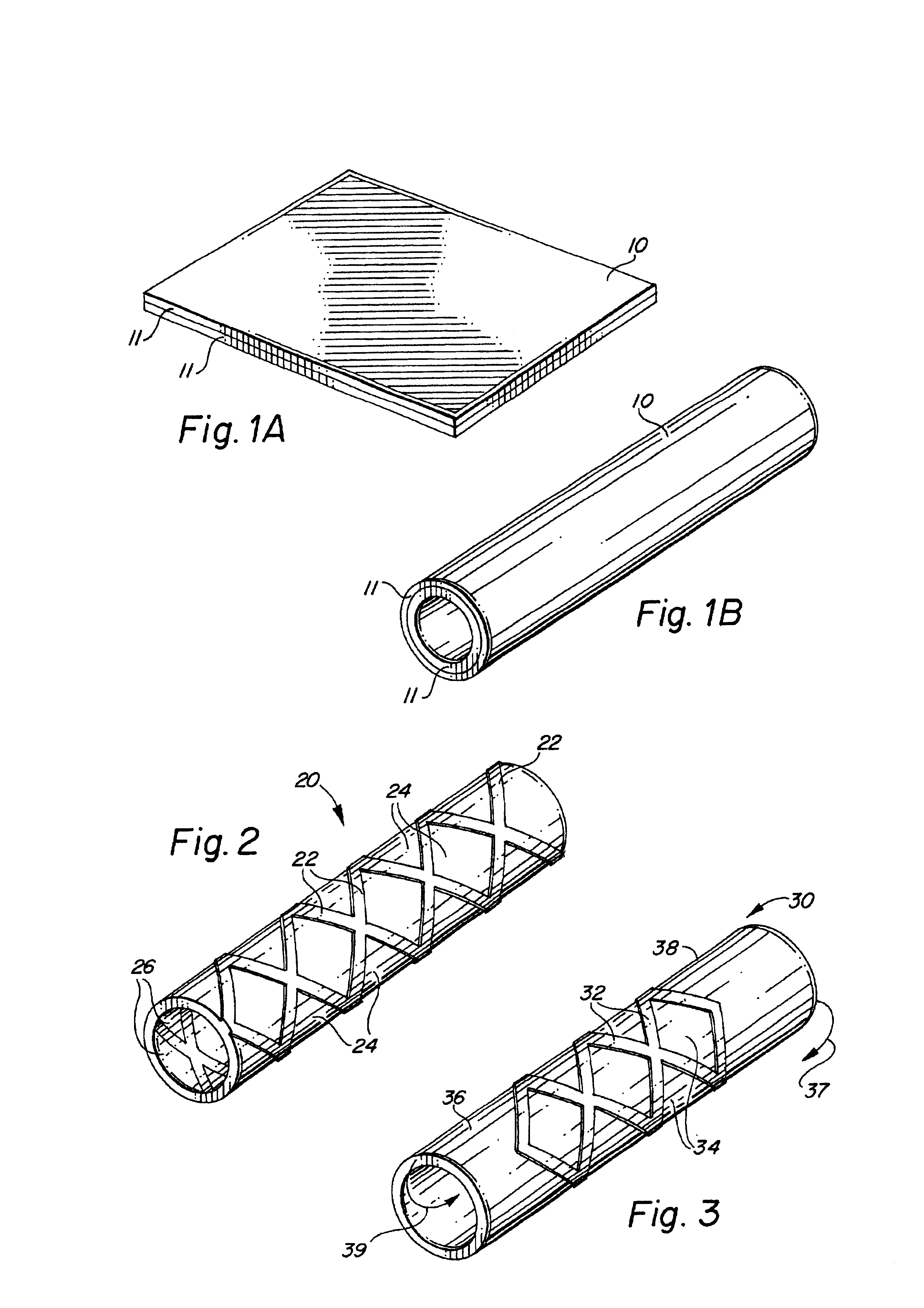
Self-supporting laminated films, structural materials and medical devices manufactured therefrom and methods of making same
Metal foils, wires, and seamless tubes with increased mechanical strength are provided. As opposed to wrought materials that are made of a single metal or alloy, these materials are made of two or more layers forming a laminate structure. Laminate structures are known to increase mechanical strength of sheet materials such as wood and paper products and are used in the area of thin films to increase film hardness, as well as toughness. Laminate metal foils have not been used or developed because the standard metal forming technologies, such as rolling and extrusion, for example, do not lend themselves to the production of laminate structures. Vacuum deposition technologies can be developed to yield laminate metal structures with improved mechanical properties. In addition, laminate structures can be designed to provide special qualities by including layers that have special properties such as superelasticity, shape memory, radio-opacity, corrosion resistance etc. Examples of articles which may be made by the inventive laminate structures include implantable medical devices that are fabricated from the laminated deposited films and which present a blood or body fluid and tissue contact surface that has controlled heterogeneities in material constitution. An endoluminal stent-graft and web-stent that is made of a laminated film material deposited and etched into regions of structural members and web regions subtending interstitial regions between the structural members. An endoluminal graft is also provided which is made of a biocompatible metal or metal-like material. The endoluminal stent-graft is characterized by having controlled heterogeneities in the stent material along the blood flow surface of the stent and the method of fabricating the stent using vacuum deposition methods.
Owner:VACTRONIX SCI LLC
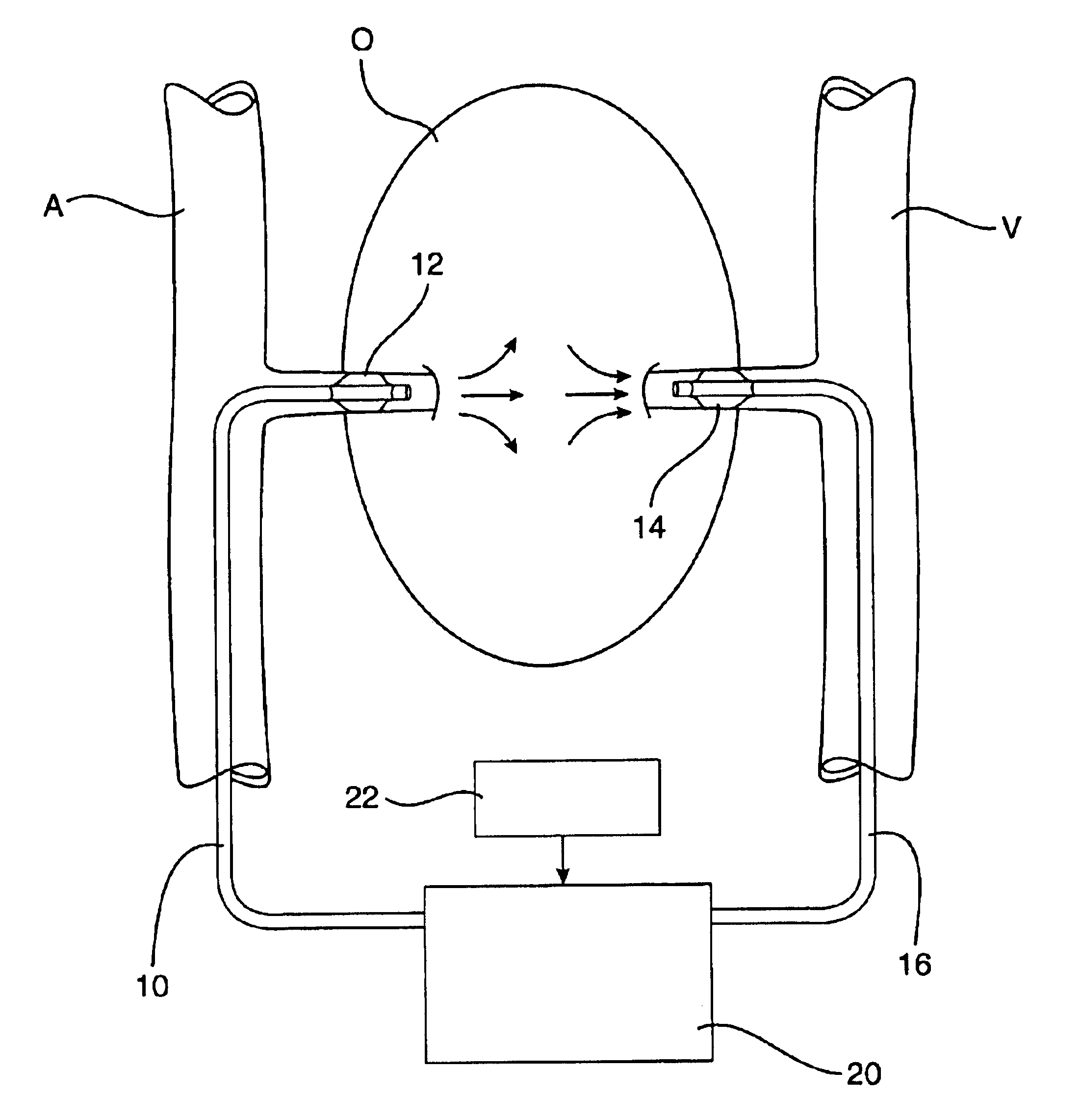
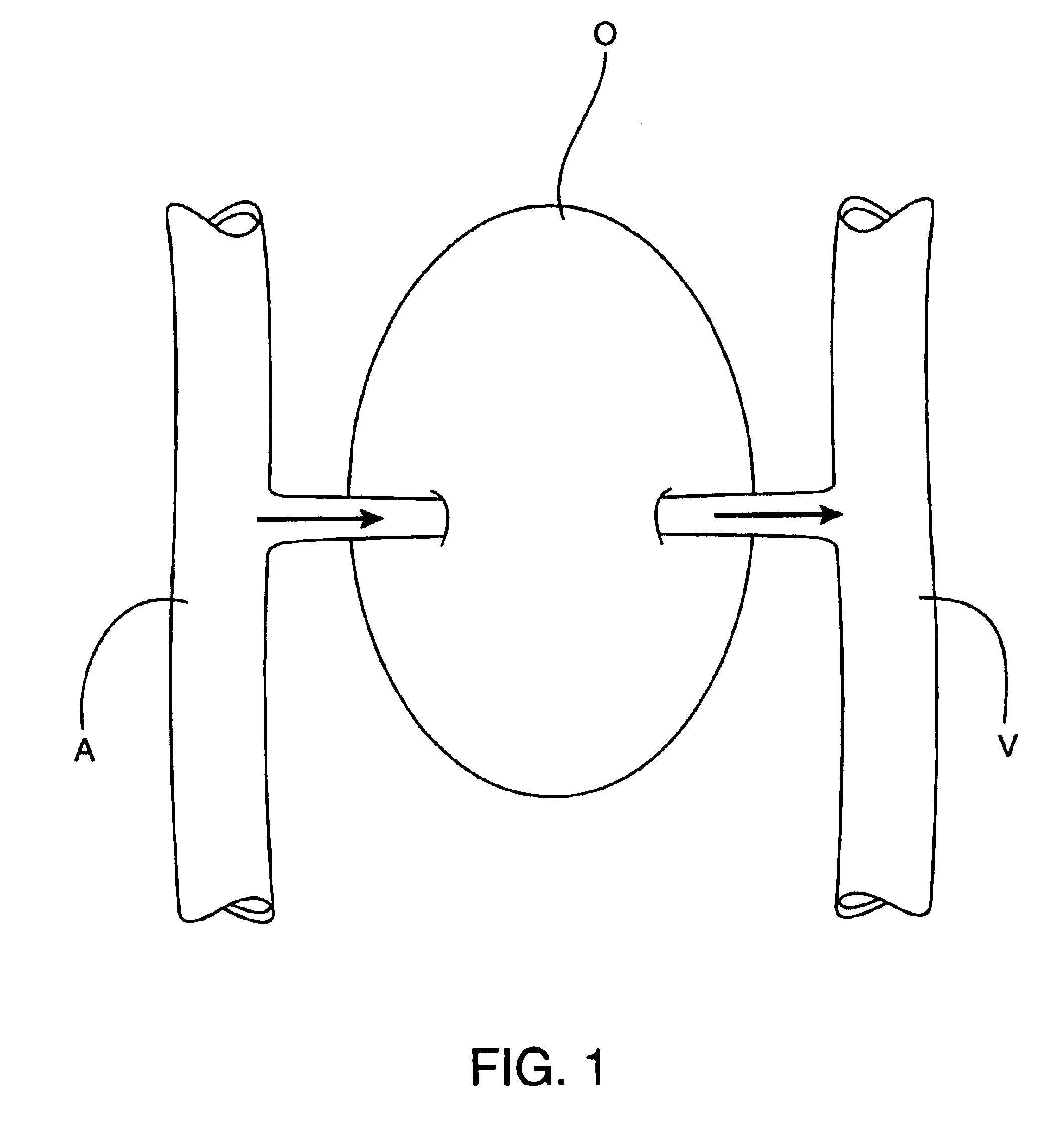
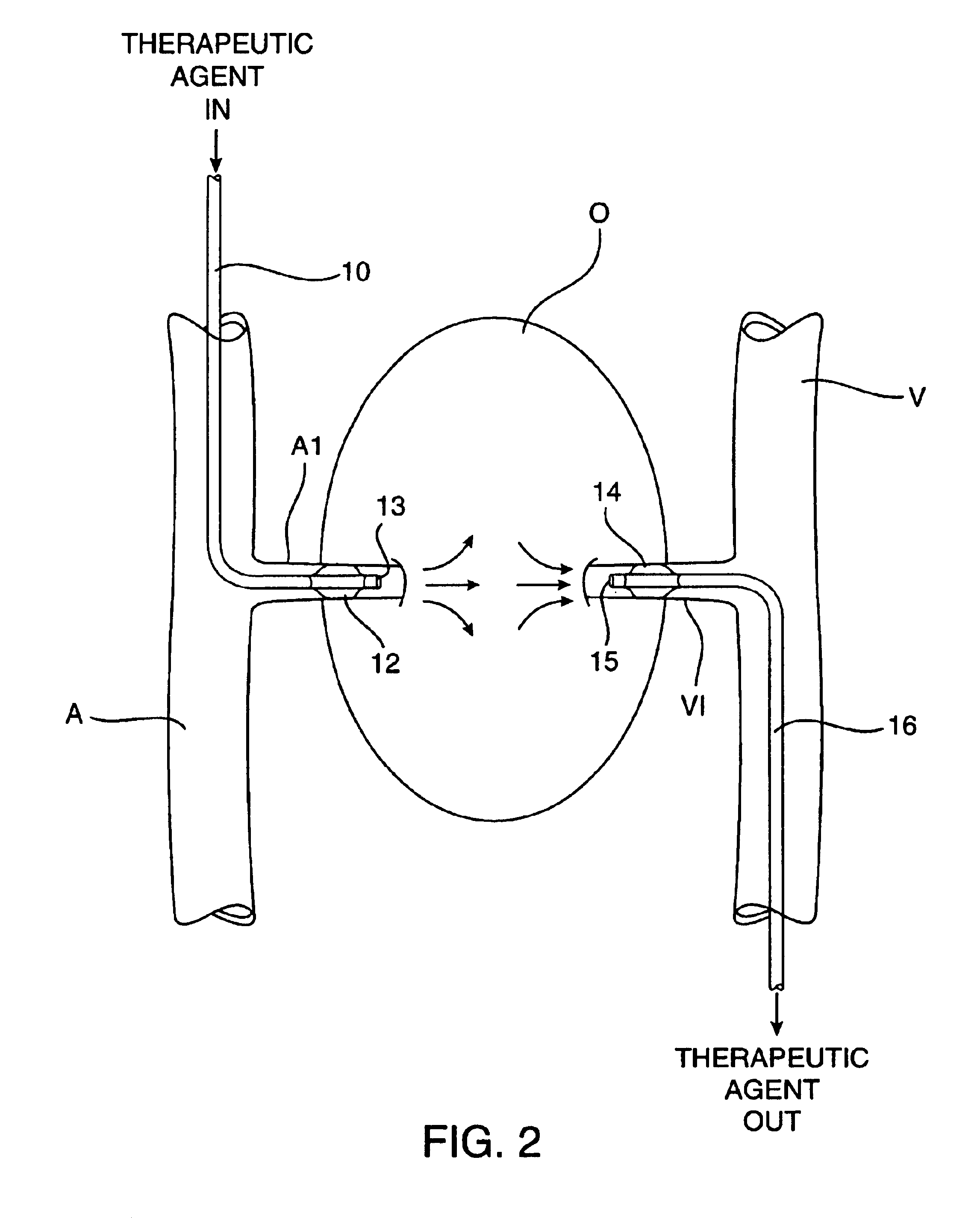
Methods and apparatus for perfusion of isolated tissue structure
Organs and other tissue structures are isolated and perfused with a therapeutic agent. Isolation is effected by endovascularly positioning catheters having occlusion balloons within the arteries or other blood vessels which supply blood to the organ. Similarly, blood flow from the organ back to the patient's circulatory system is blocked by endovascularly positioning one or more catheters carrying occlusion members within the veins or other blood vessels leading from the organ. The therapeutic agent may then be perfused through the organ in either an antegrade or retrograde fashion using the endovascularly positioned catheters while maintaining isolation.
Owner:PINPOINT THERAPEUTICS
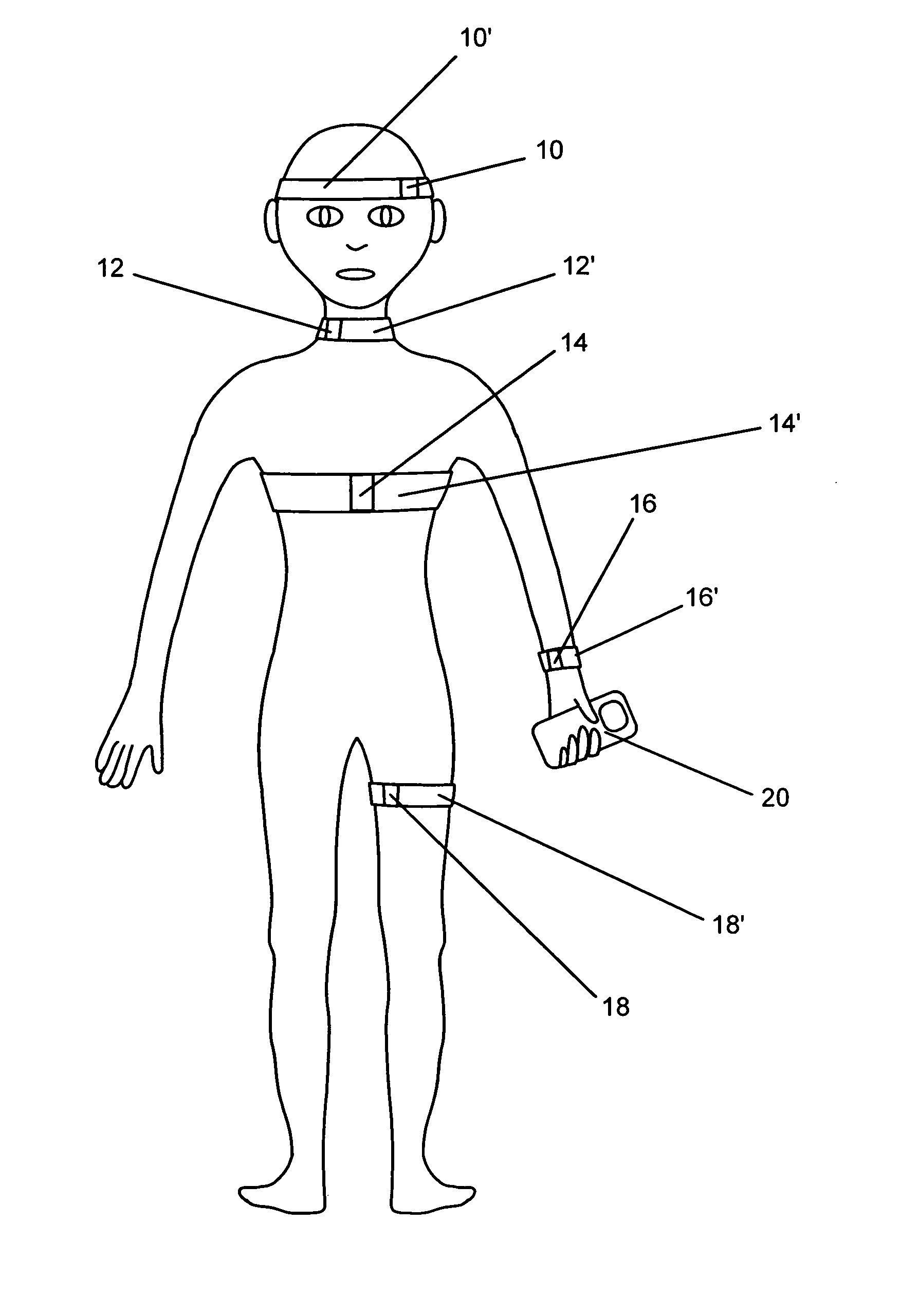
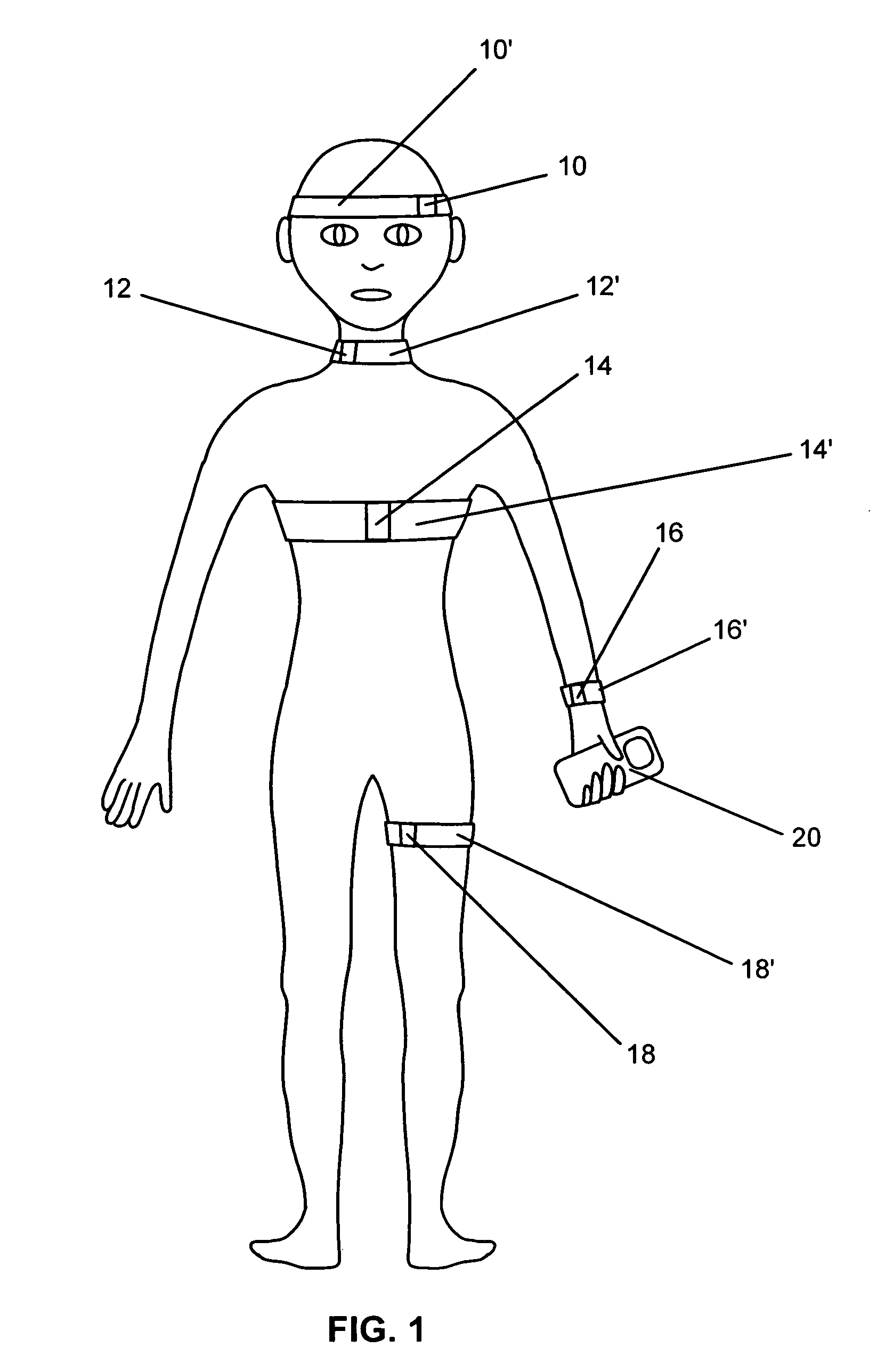
Systems and methods for non-invasive detection and monitoring of cardiac and blood parameters
InactiveUS20060100530A1Easy to detectEffective assessmentBlood flow measurement devicesCatheterData acquisitionNon invasive
Methods and systems for long term monitoring of one or more physiological parameters such as respiration, heart rate, body temperature, electrical heart activity, blood oxygenation, blood flow velocity, blood pressure, intracranial pressure, the presence of emboli in the blood stream and electrical brain activity are provided. Data is acquired non-invasively using ambulatory data acquisition techniques.
Owner:UNIV OF WASHINGTON +1
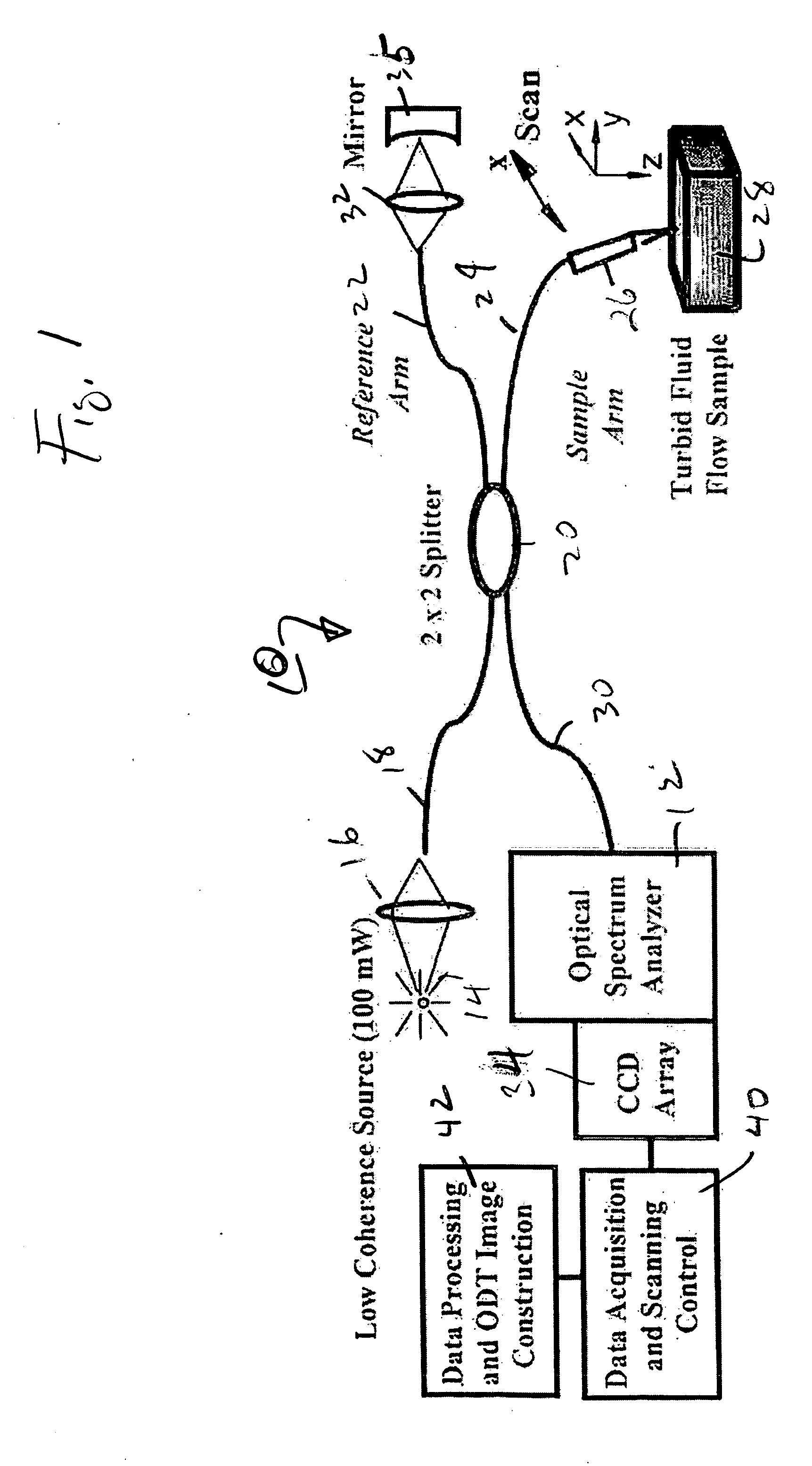
High speed spectral domain functional optical coherence tomography and optical doppler tomography for in vivo blood flow dynamics and tissue structure
ActiveUS20050171438A1Accurate settingImprove system sensitivityUltrasonic/sonic/infrasonic diagnosticsInfrasonic diagnosticsBlood flowIn vivo
A method for tomographic imaging comprises the steps of providing a source of at least partially coherent radiation and a frequency-swept laser source through an interferometer; phase modulating the radiation in the interferometer at a modulation frequency for elimination of DC and autocorrelation noises as well as the mirror image; detecting interference fringes of the radiation backscattered from the sample into the interferometer to obtain a spectral signal; transforming the spectral signal of the detected backscattered interference fringes to obtain a time and location dependent signal, including the Doppler shift and variance, at each pixel location in a data window; and generating a tomographic image of the fluid flow in the data window and of the structure of the scanned fluid flow sample in the data window from the time and location dependent signal. The apparatus comprises a system for tomographic imaging operating according to the above method.
Owner:RGT UNIV OF CALIFORNIA
Methods and devices for forming vascular anastomoses
Methods and devices for forming an anastomosis utilize a graft vessel secured to a vessel coupling that is fixed to a target vessel without using suture. The vessel coupling may be collapsed for introduction into the target vessel and then expanded to engage the vessel wall. The vessel coupling may be a stent attached to a graft vessel to form a stent-graft assembly. The anastomosis may be carried out to place the graft and target vessels in fluid communication while preserving native proximal flow through the target vessel, which may be a coronary artery. As a result, blood flowing through the coronary artery from the aorta is not blocked by the vessel coupling and thus is free to move past the site of the anastomosis.
Owner:MEDTRONIC INC
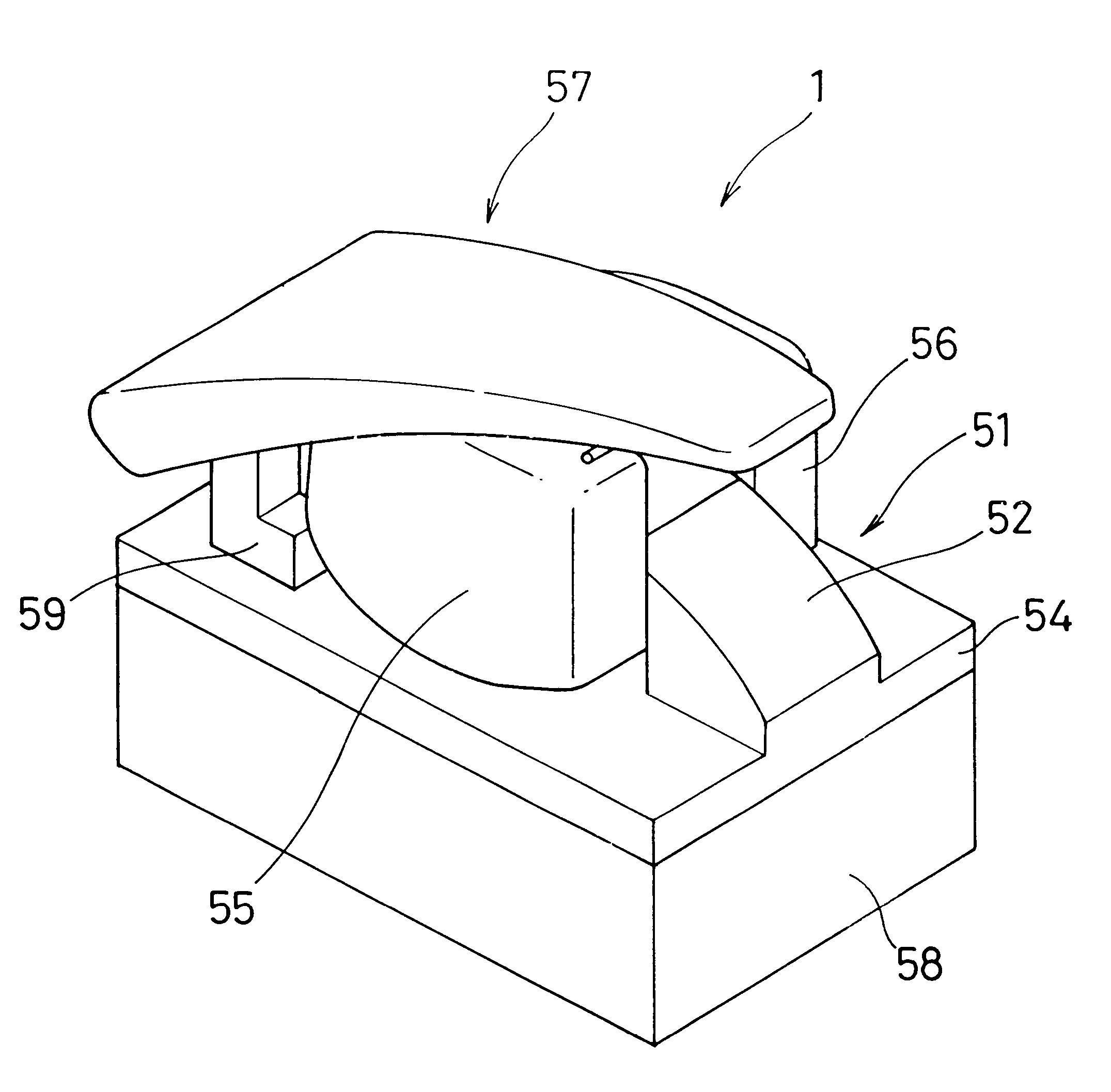
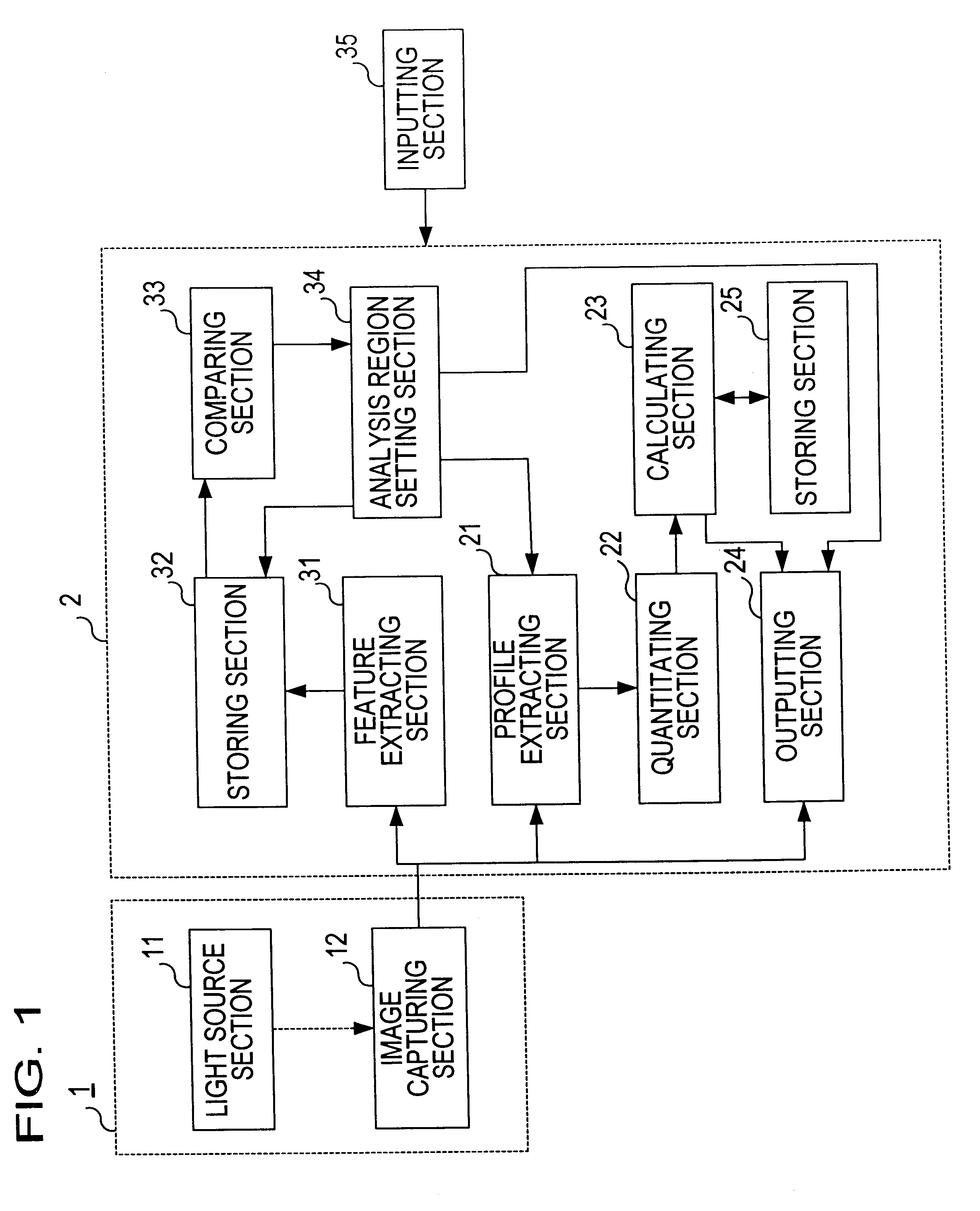
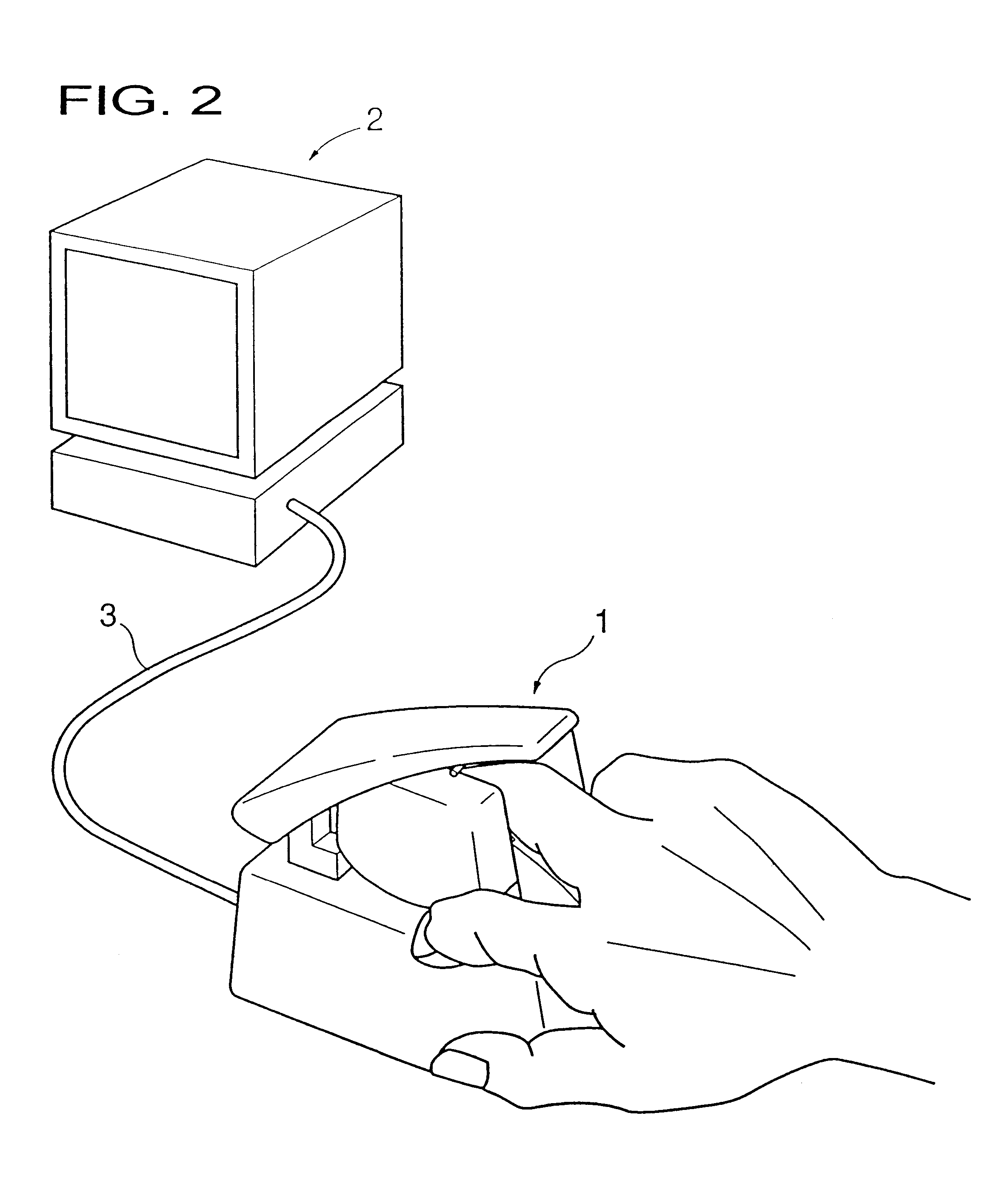
Living body inspecting apparatus and noninvasive blood analyzer using the same
The purpose is to fix a portion of a living body, which is an object of measurement, stably without strain and to acquire accurate inspection results with good reproducibility. An apparatus for capturing an image of a living body includes: a base for mounting a portion of a living body to be inspected; two sidewall members capable of holding the mounted portion of the living body therebetween from both sides; a light source section for supplying a light to the portion of the living body held on the base and between the sidewall members; and a light receiving section for detecting optical information from the portion of the living body supplied with the light, and a non-invasive apparatus for living body inspection including the above apparatus for capturing an image of a living body in which the light receiving section includes an image capturing element, and an analyzing section for calculating information on blood flowing through a blood vessel by analyzing an image of a tissue including the blood vessel obtained by the image capturing element.
Owner:SYSMEX CORP
Cardiac valve procedure methods and devices
ActiveUS20050015112A1Improve performanceReduce riskHeart valvesSurgeryCoronary arteriesProsthetic valve
Devices and methods for performing intravascular procedures without cardiac bypass include embodiments of temporary filter devices, temporary valves, and prosthetic valves. The temporary filter devices have a cannula which provides access for surgical tools for effecting repair of cardiac valves. The cannula may have filters which prevent embolitic material from entering the coronary arteries and aorta. The valve devices may also have a cannula for insertion of the valve into the aorta. The valve devices expand in the aorta to occupy the entire flow path of the vessel and operate to prevent blood flow and to permit flow through the valve. The prosthetic valves include valve fixation devices which secure the prosthetic valve to the wall of the vessel. The prosthetic valves are introduced into the vascular system in a compressed state, advanced to the site of implantation, and expanded and secured to the vessel wall.
Owner:MEDTRONIC INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com