Patents
Literature
133 results about "Endoluminal stent" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
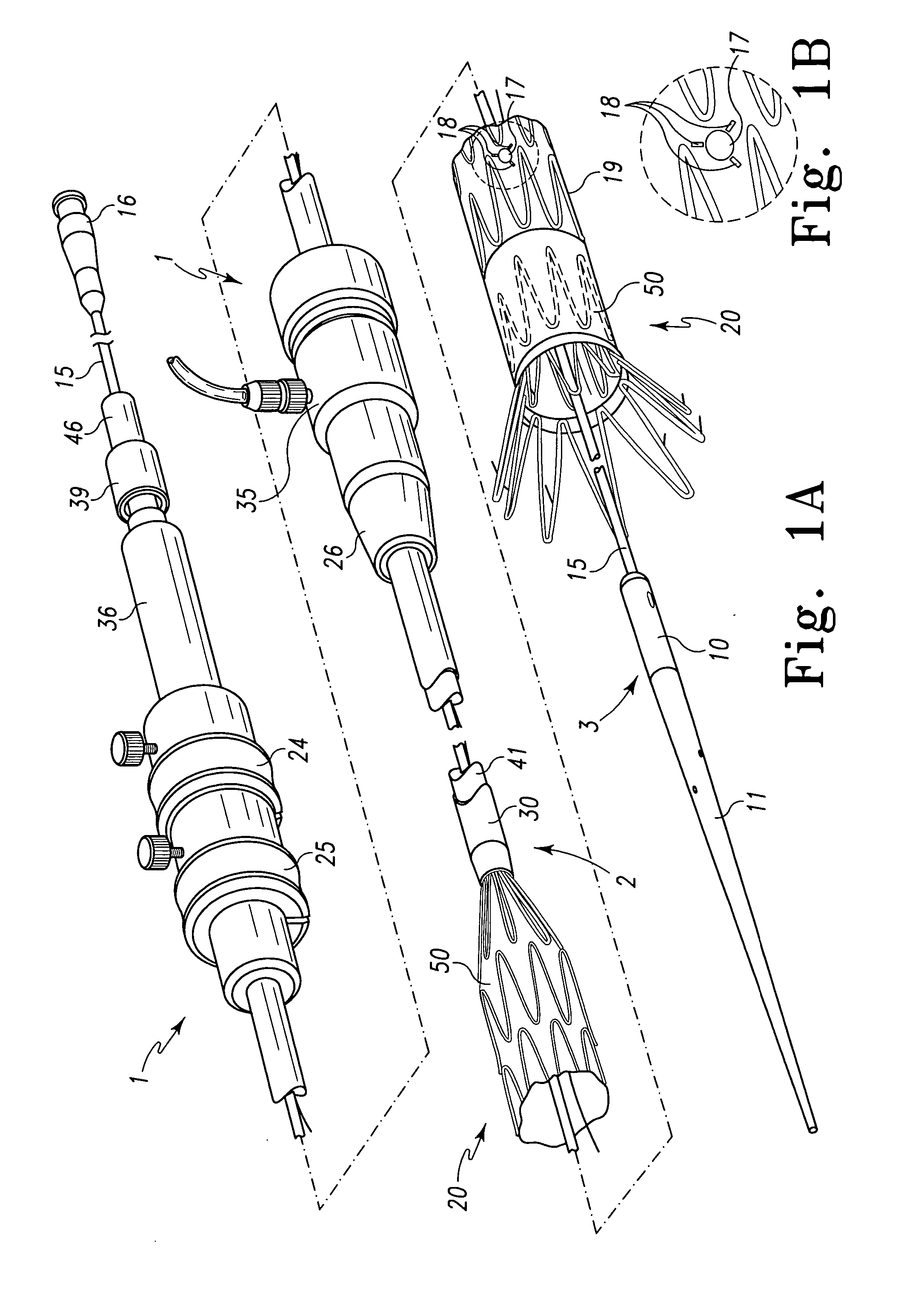
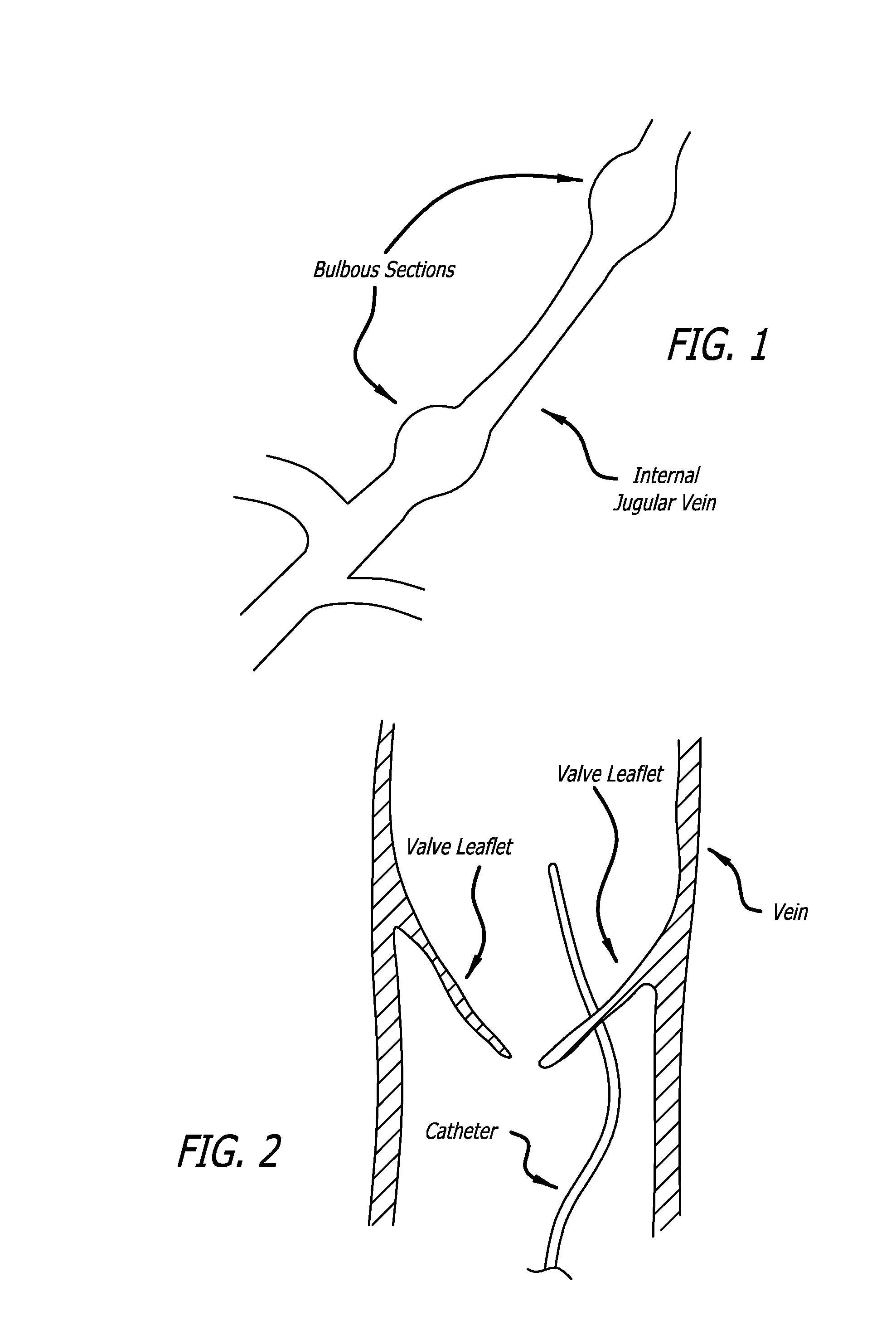
Endoluminal stenting is an endoscopic procedure that involves the placement of a thin tube (plastic or metal stent) to manage a blockage in the GI tract. Most commonly, endoluminal stents are placed in the bile duct, esophagus, colon and small bowel. Endoluminal stent placement may be used for non-cancerous or cancerous blockages.
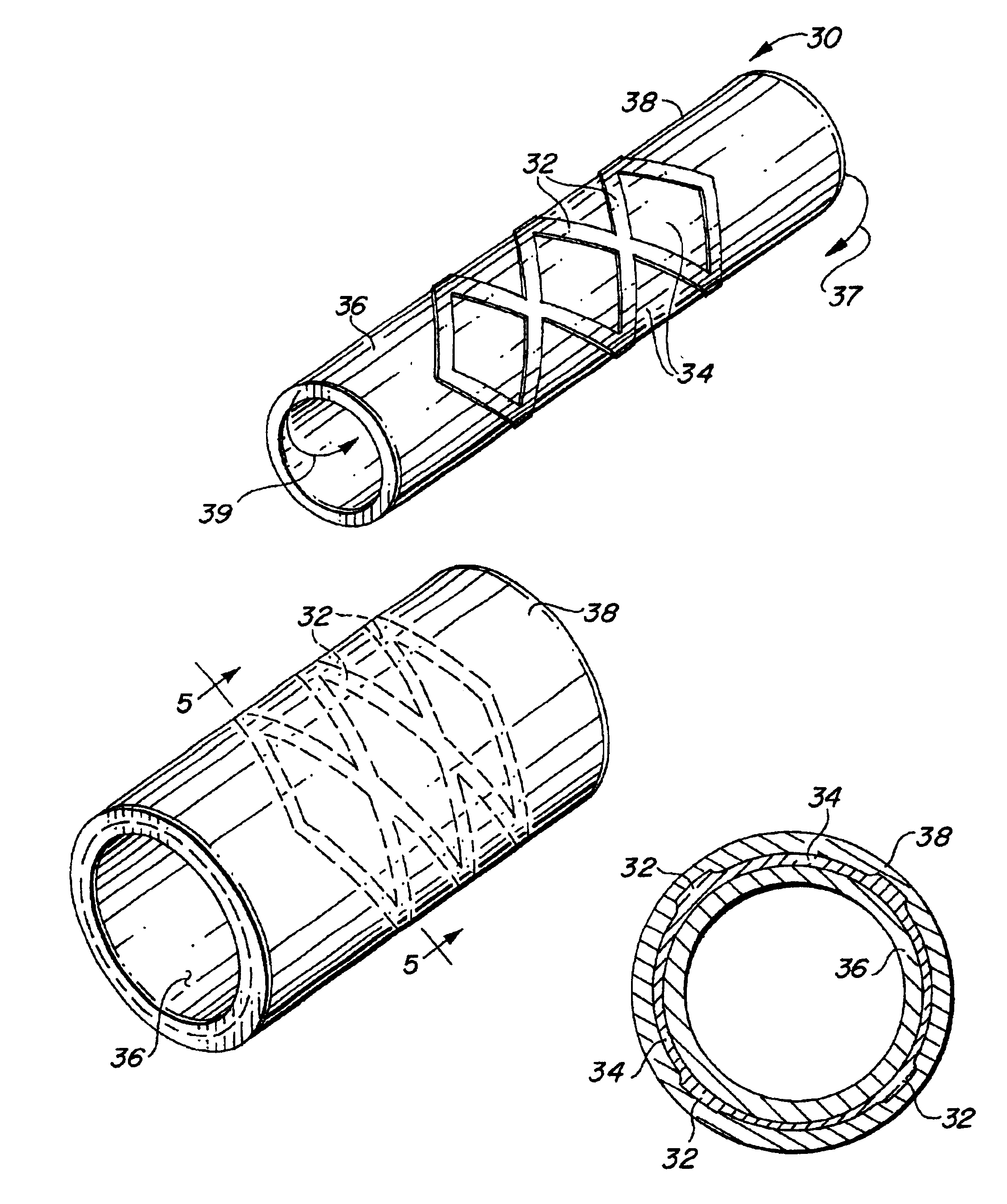
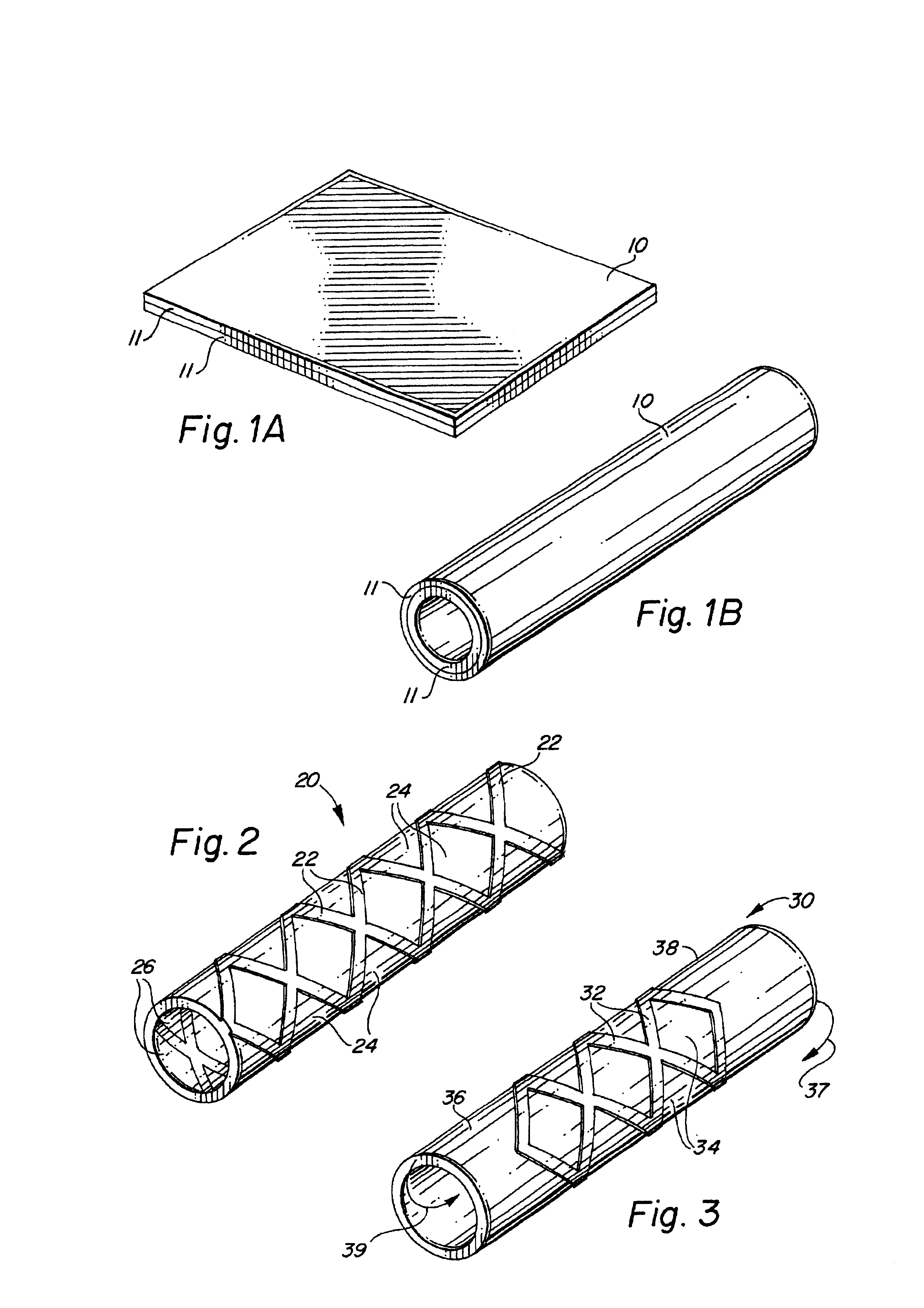
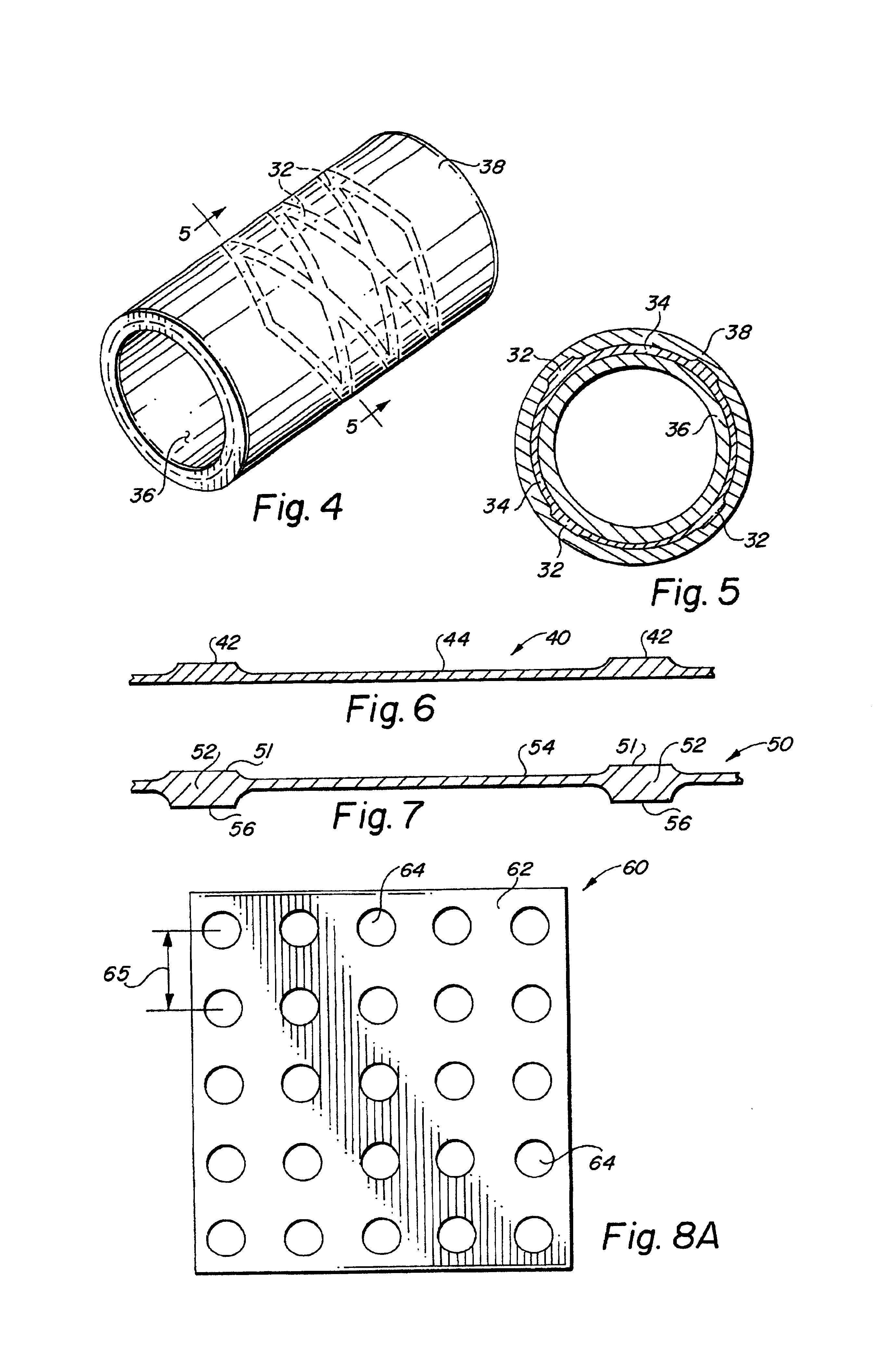
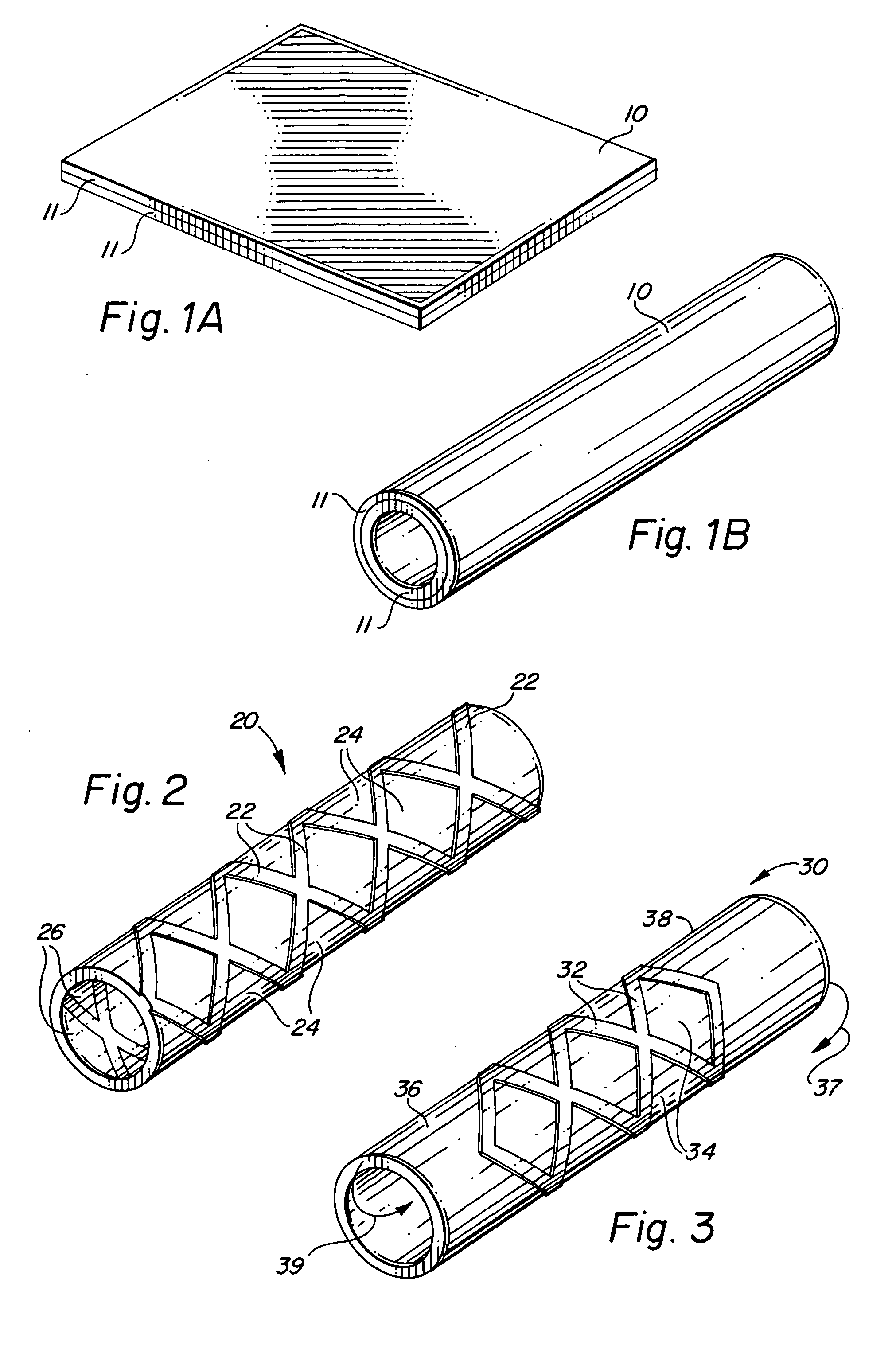
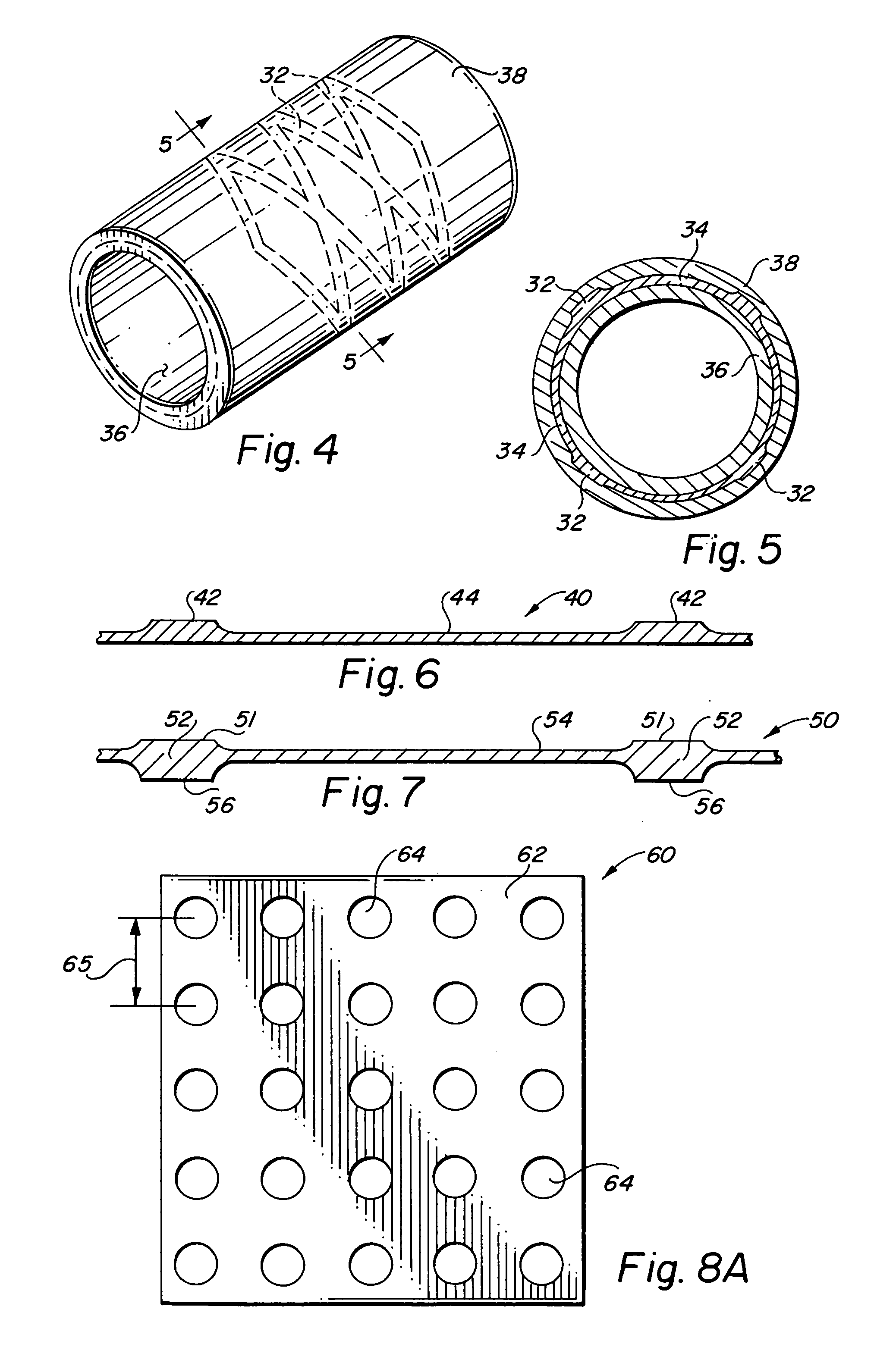
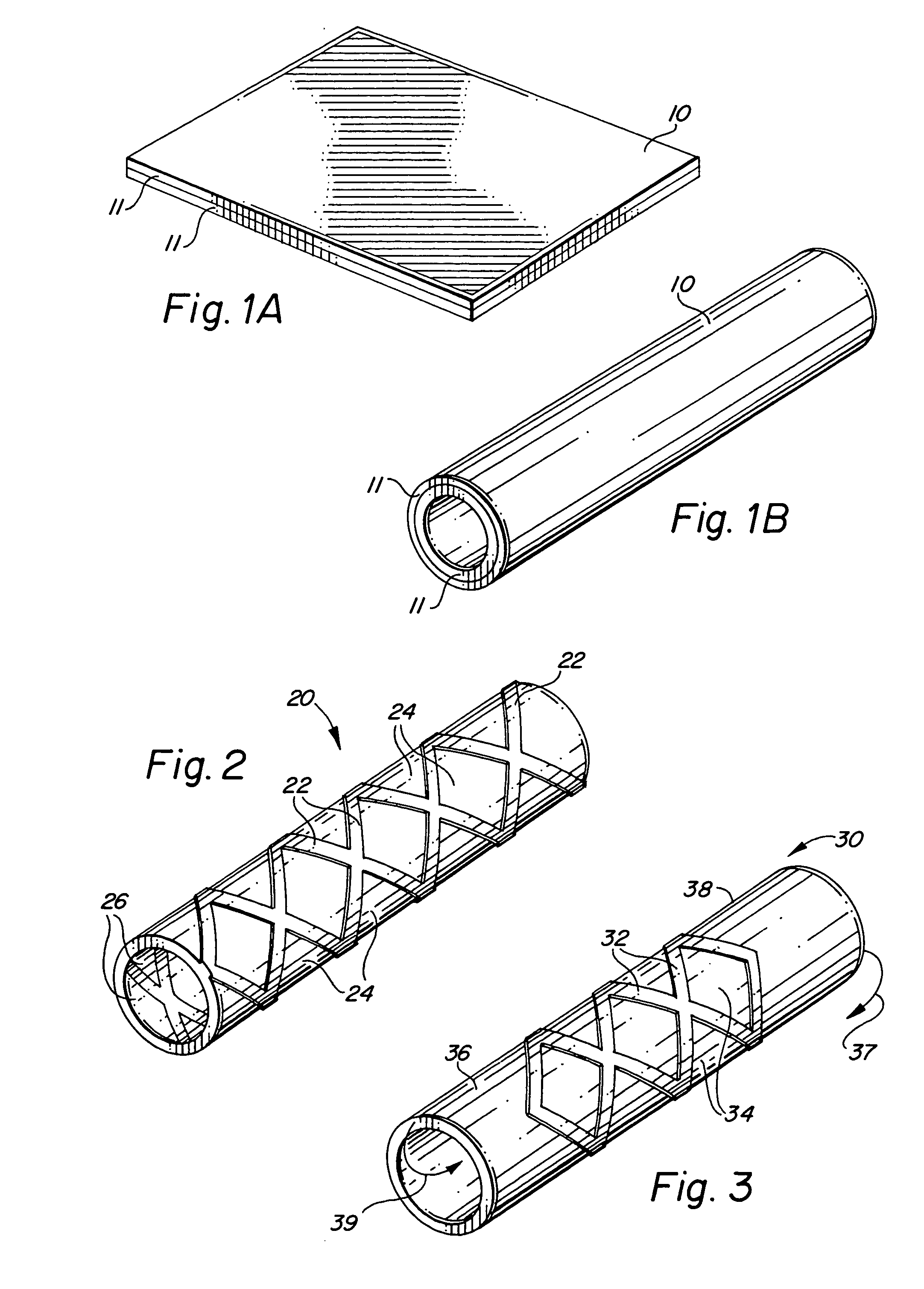
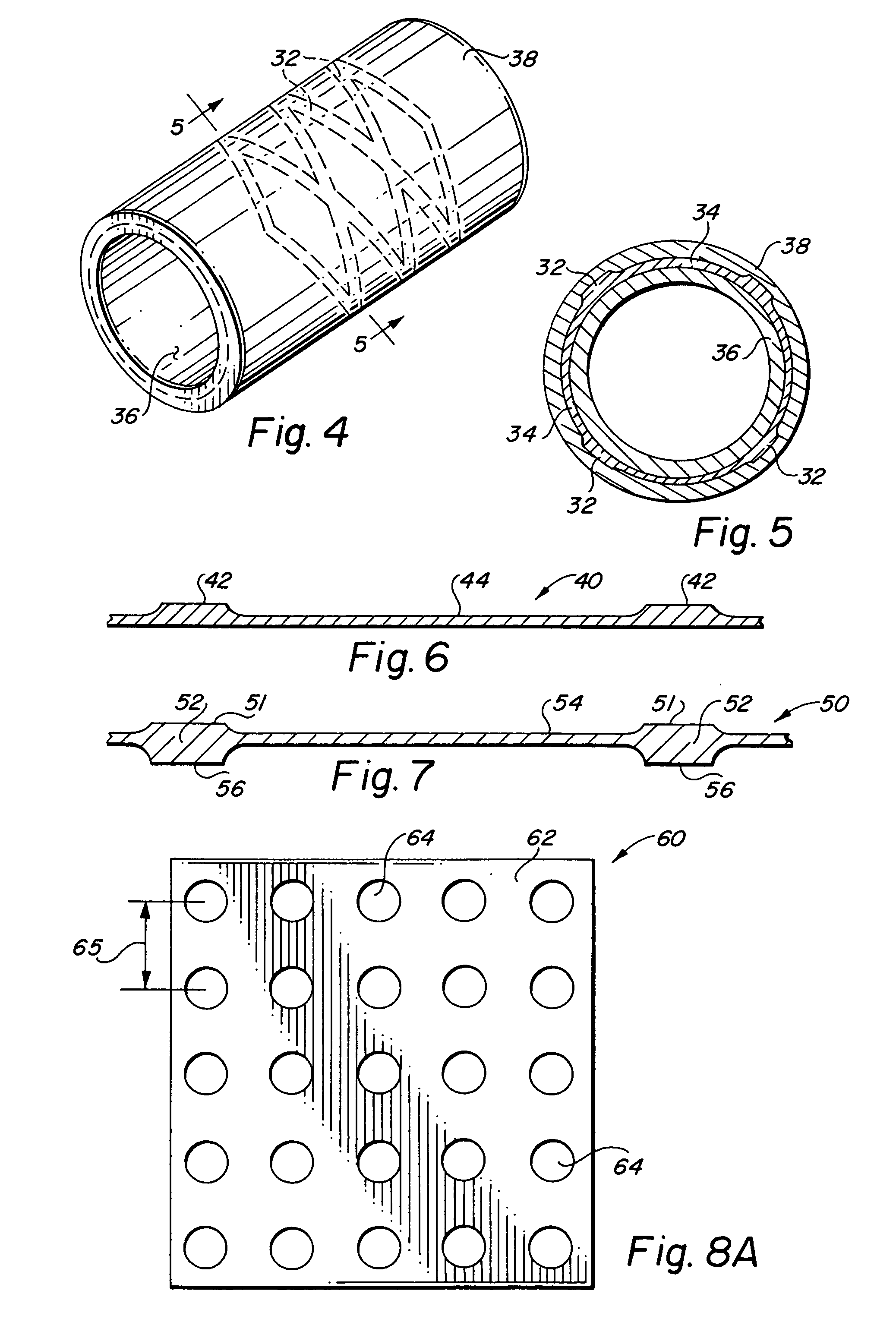
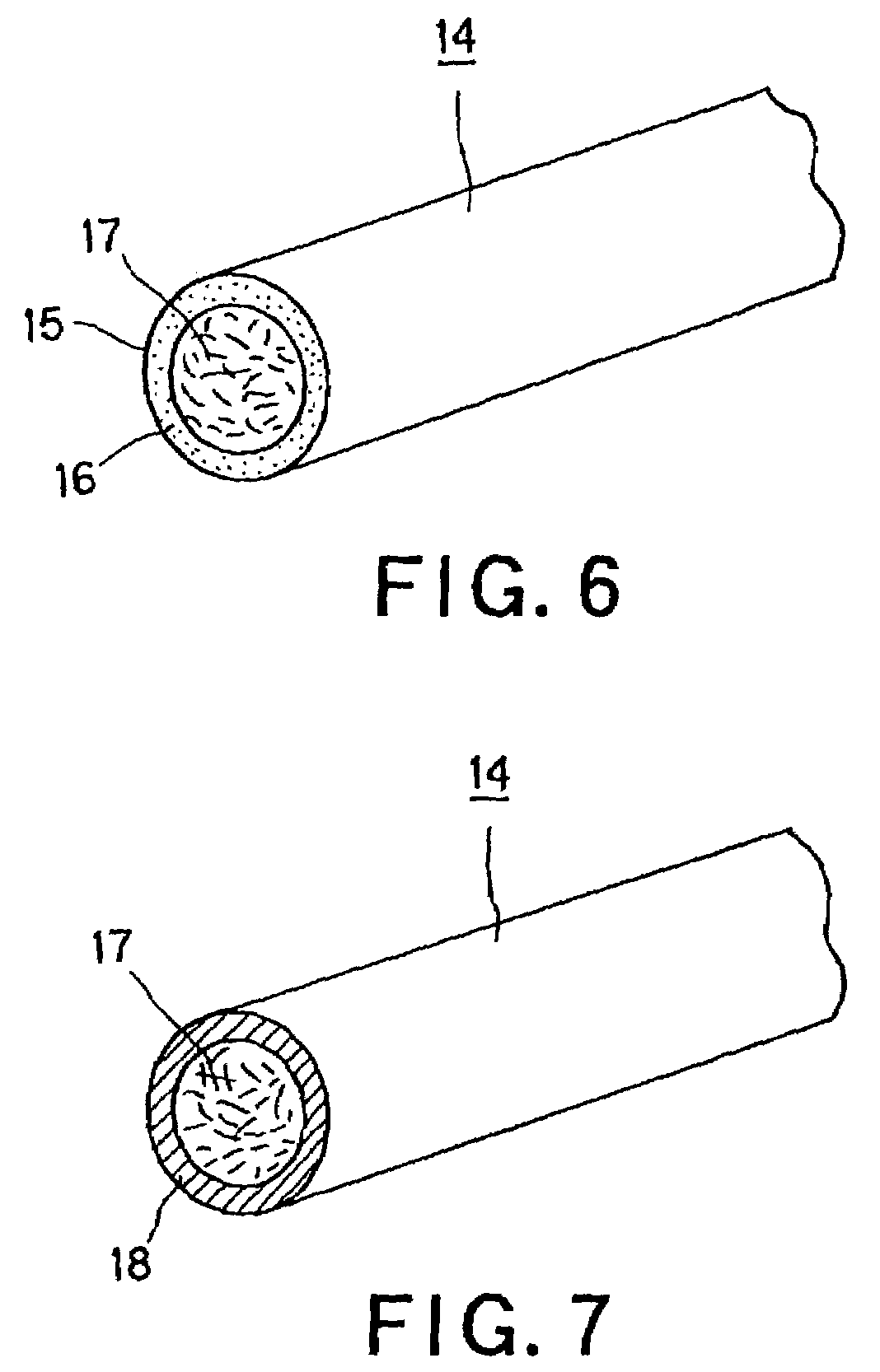
Self-supporting laminated films, structural materials and medical devices manufactured therefrom and methods of making same
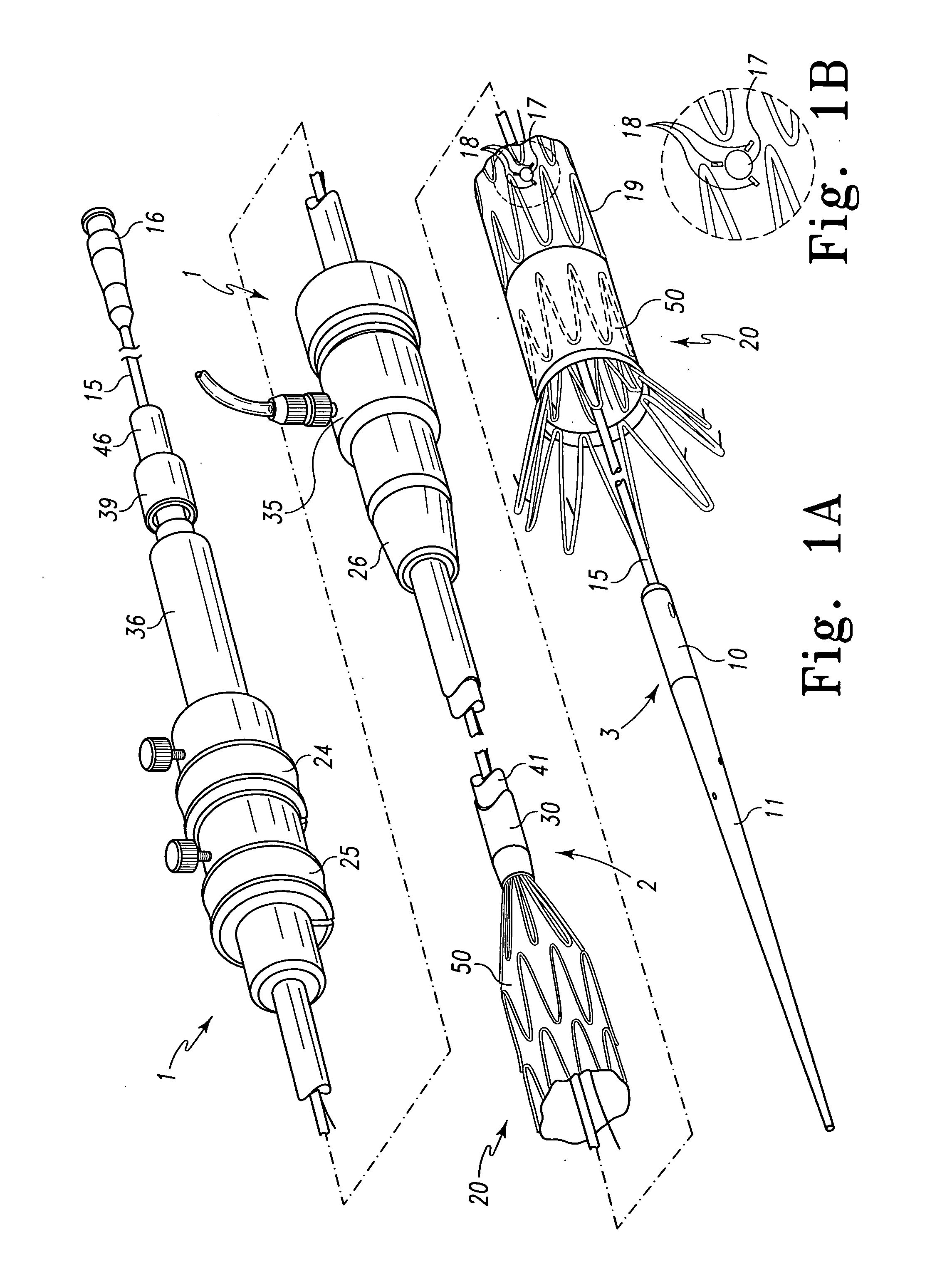
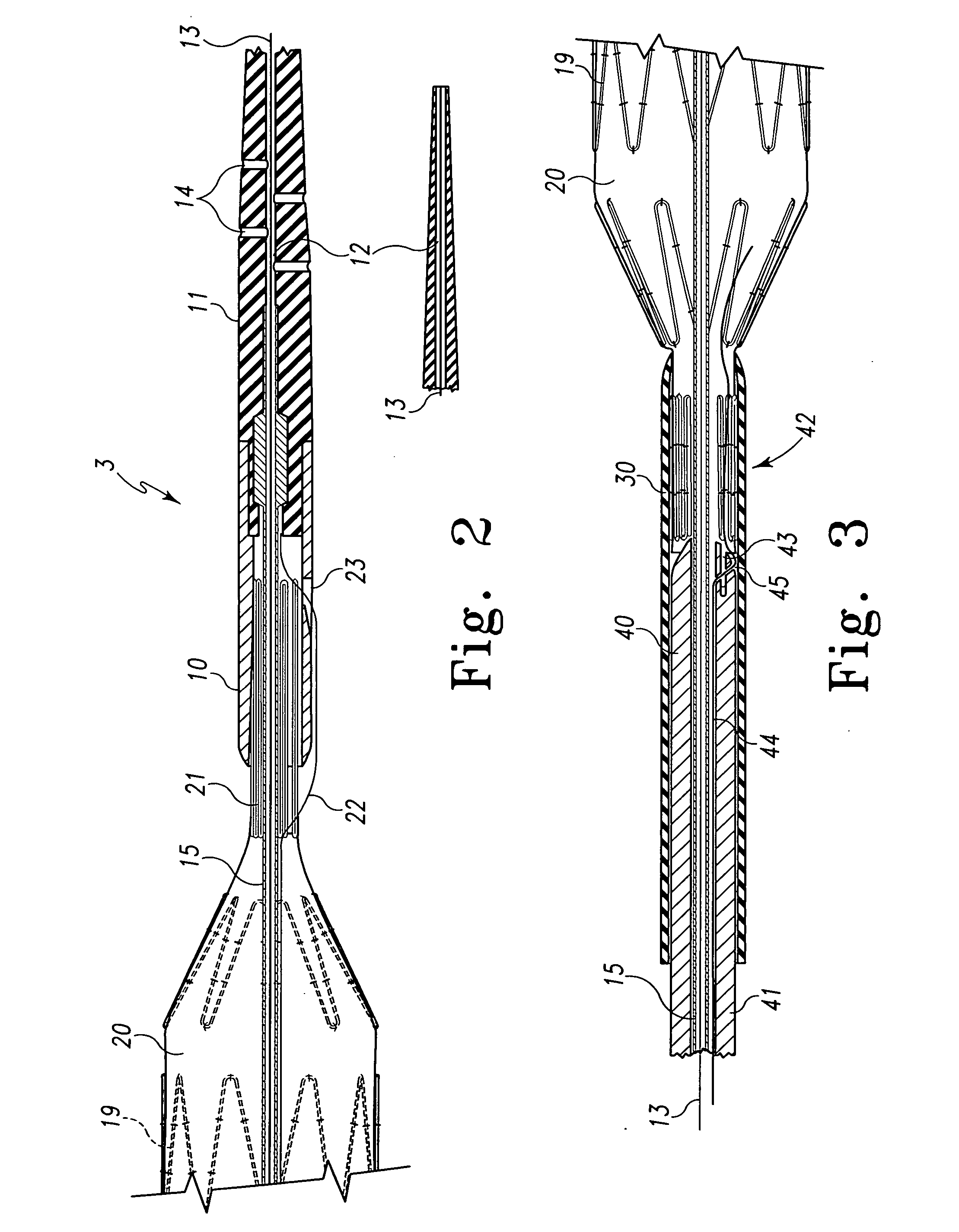
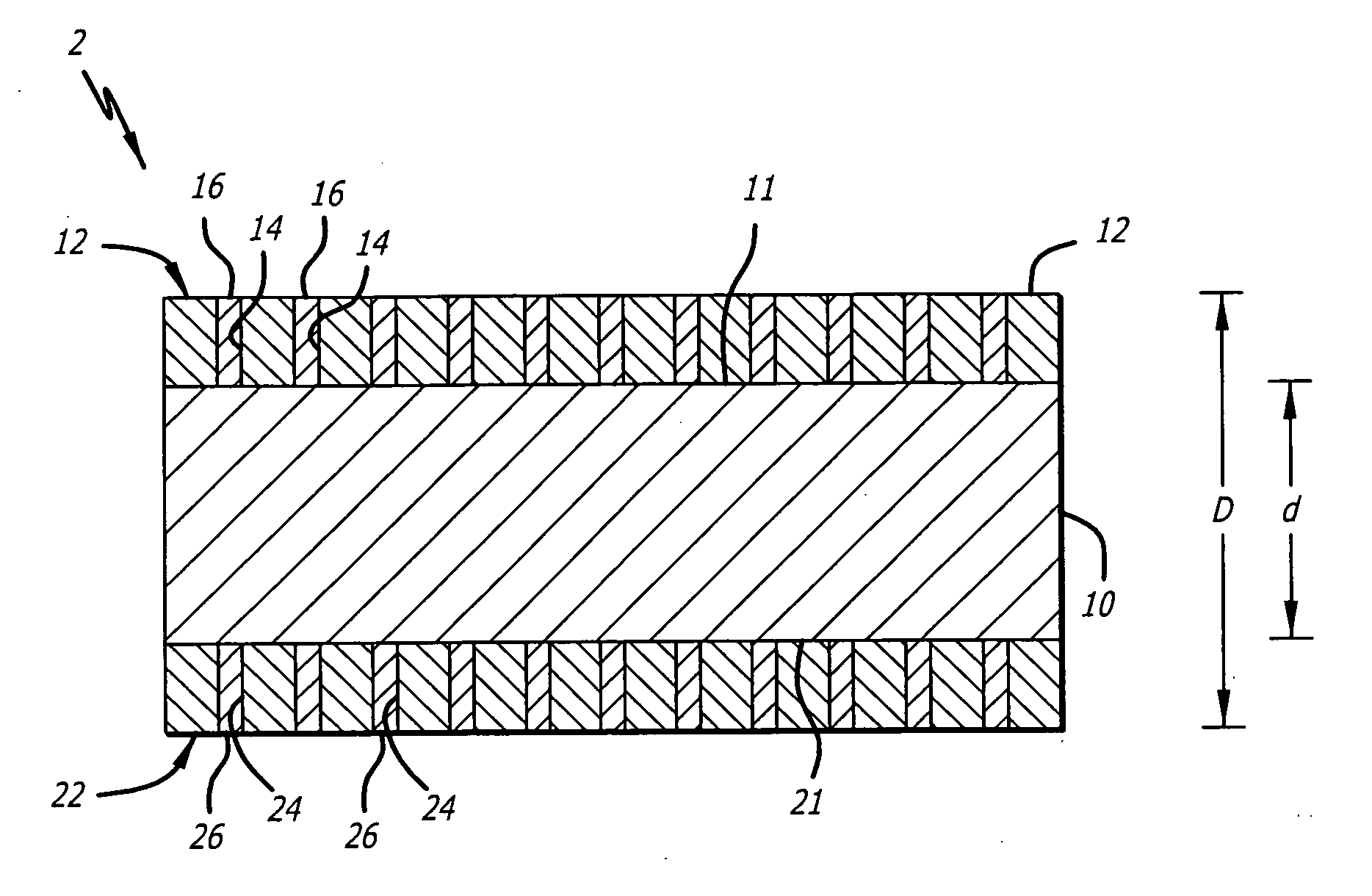
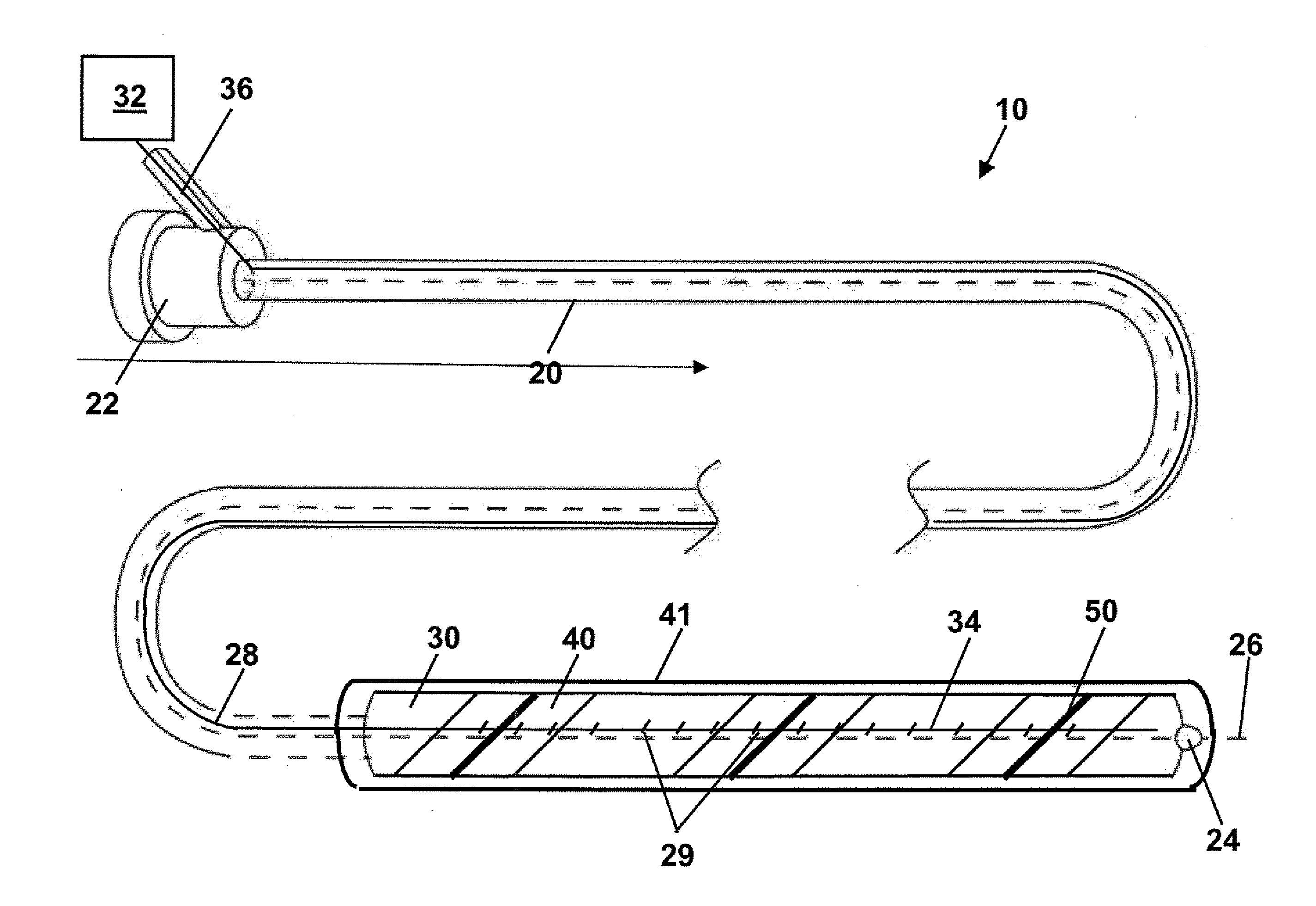
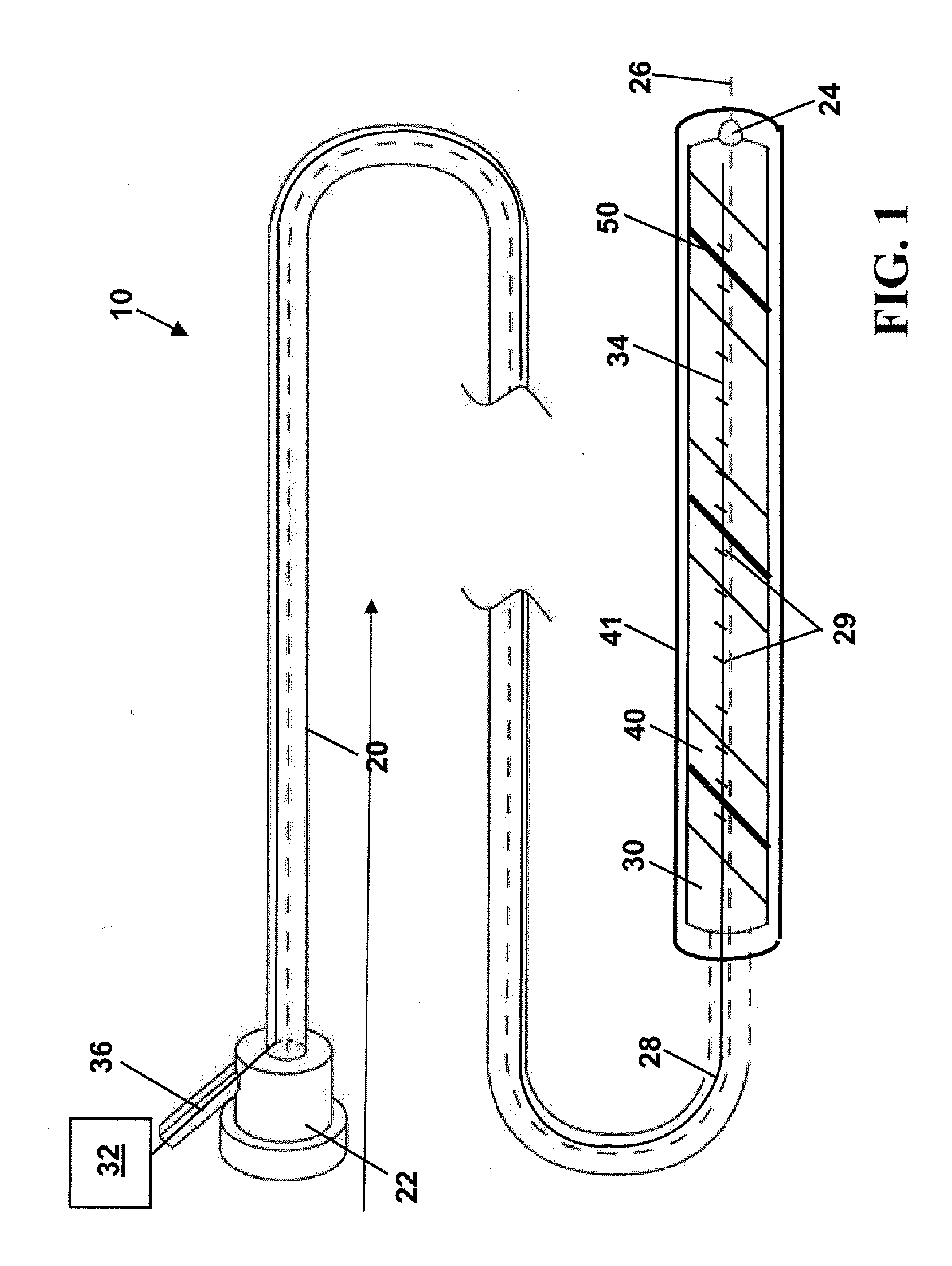
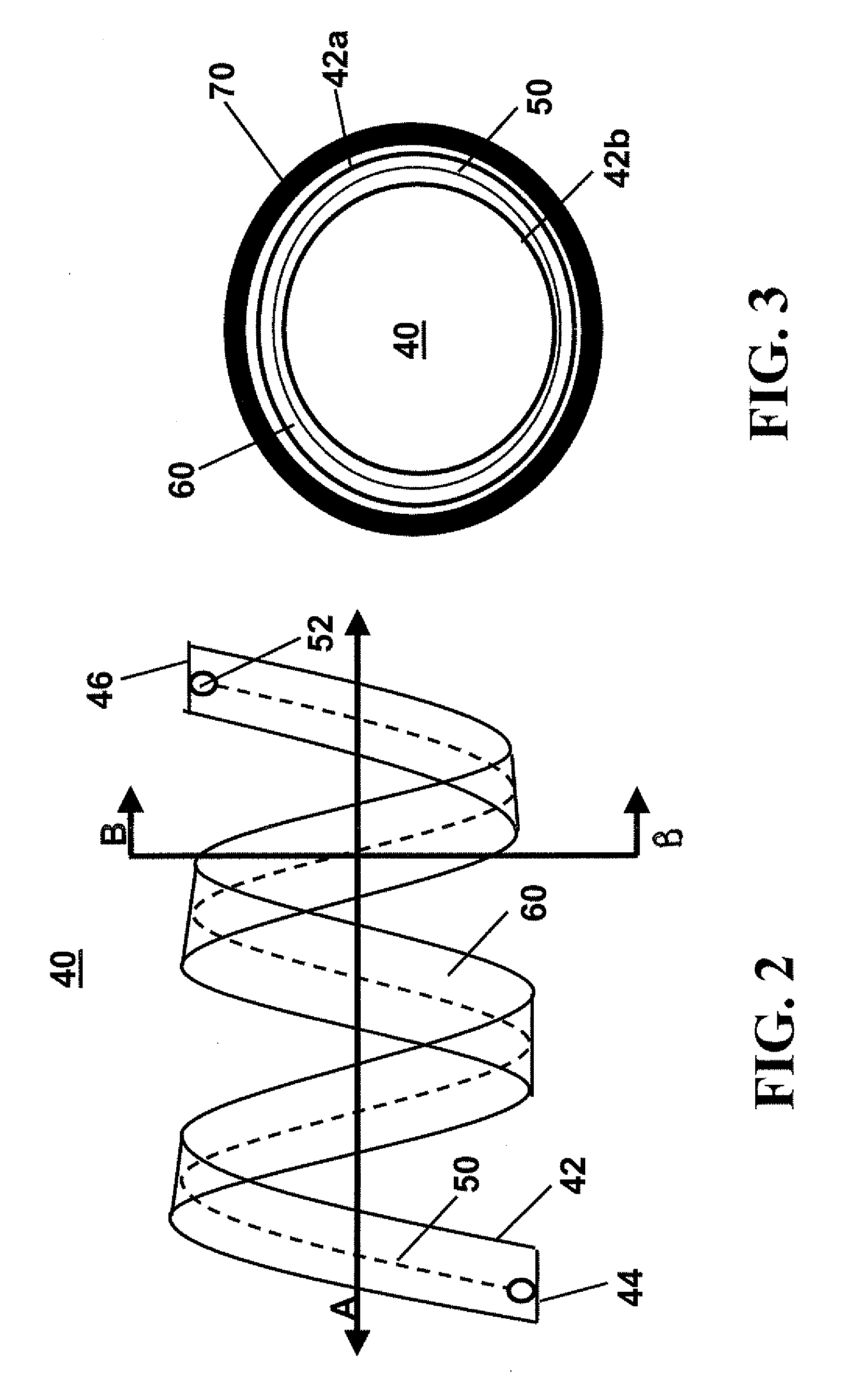
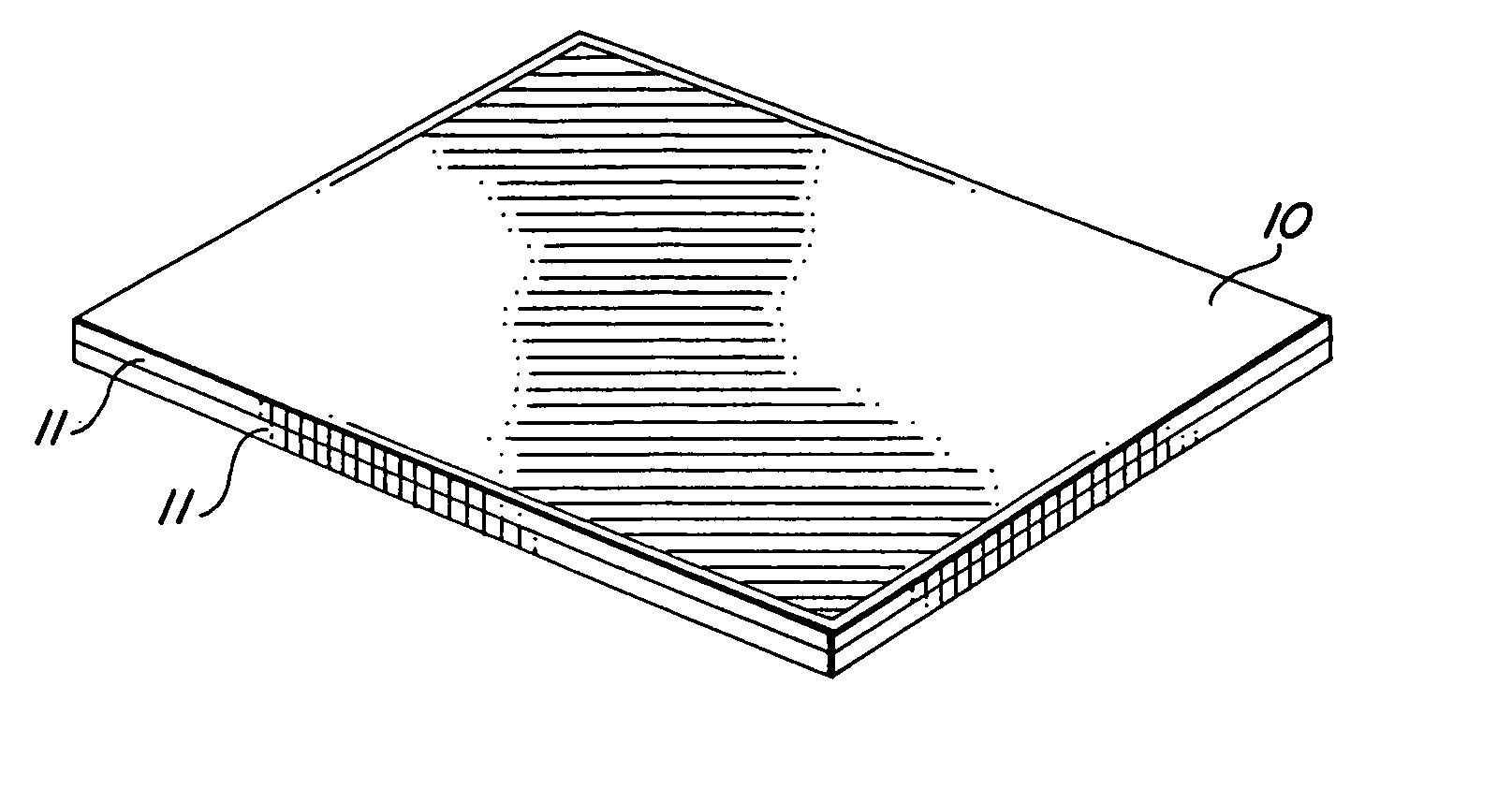
Metal foils, wires, and seamless tubes with increased mechanical strength are provided. As opposed to wrought materials that are made of a single metal or alloy, these materials are made of two or more layers forming a laminate structure. Laminate structures are known to increase mechanical strength of sheet materials such as wood and paper products and are used in the area of thin films to increase film hardness, as well as toughness. Laminate metal foils have not been used or developed because the standard metal forming technologies, such as rolling and extrusion, for example, do not lend themselves to the production of laminate structures. Vacuum deposition technologies can be developed to yield laminate metal structures with improved mechanical properties. In addition, laminate structures can be designed to provide special qualities by including layers that have special properties such as superelasticity, shape memory, radio-opacity, corrosion resistance etc. Examples of articles which may be made by the inventive laminate structures include implantable medical devices that are fabricated from the laminated deposited films and which present a blood or body fluid and tissue contact surface that has controlled heterogeneities in material constitution. An endoluminal stent-graft and web-stent that is made of a laminated film material deposited and etched into regions of structural members and web regions subtending interstitial regions between the structural members. An endoluminal graft is also provided which is made of a biocompatible metal or metal-like material. The endoluminal stent-graft is characterized by having controlled heterogeneities in the stent material along the blood flow surface of the stent and the method of fabricating the stent using vacuum deposition methods.
Owner:VACTRONIX SCI LLC
Method of recapturing a stent
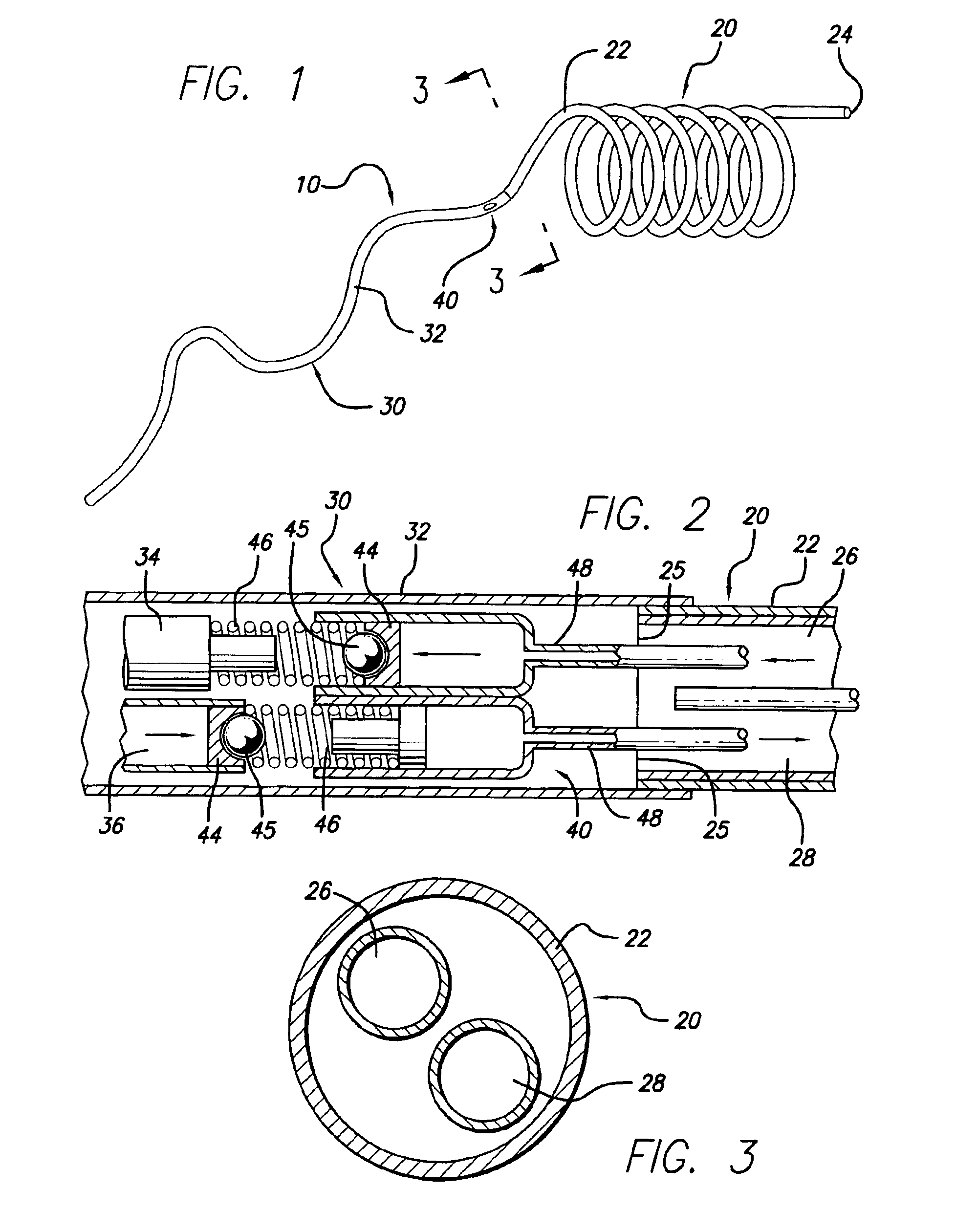
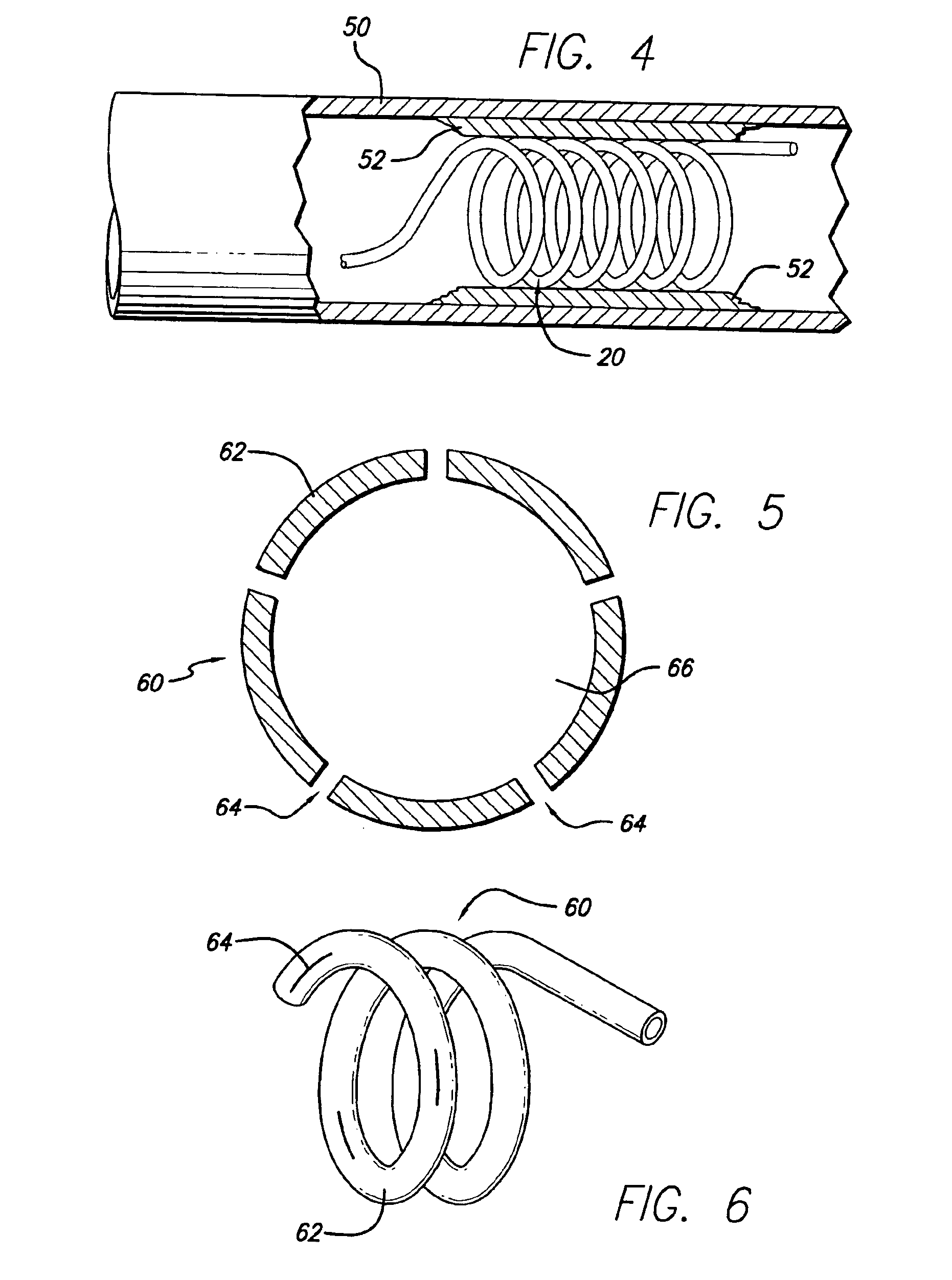
An endoluminal stent contains a hollow passageway for the circulation of heated and / or cryogenic fluids to recapture a previously implanted shape memory stent. The hollow passageway stent can have one or a plurality of passageways and is configured in a tubular shape with numerous coils, providing an empty tubular lumen through the center of the stent to allow blood flow. The stent is connected to a removable catheter that conducts fluid to the stent. Fluid flow may be regulated by valves incorporated in either the stent and / or the catheter.
Owner:BARD PERIPHERAL VASCULAR
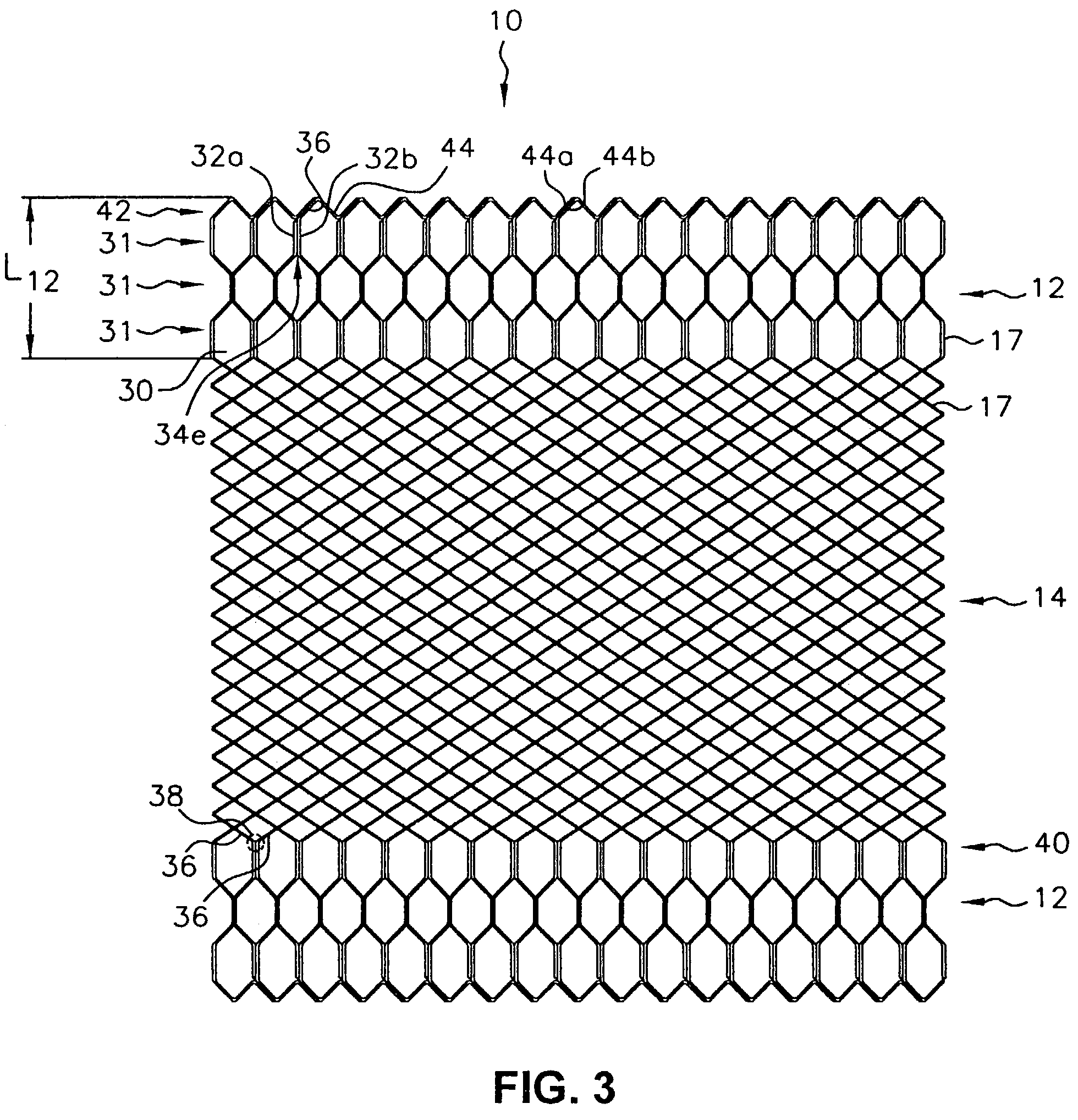
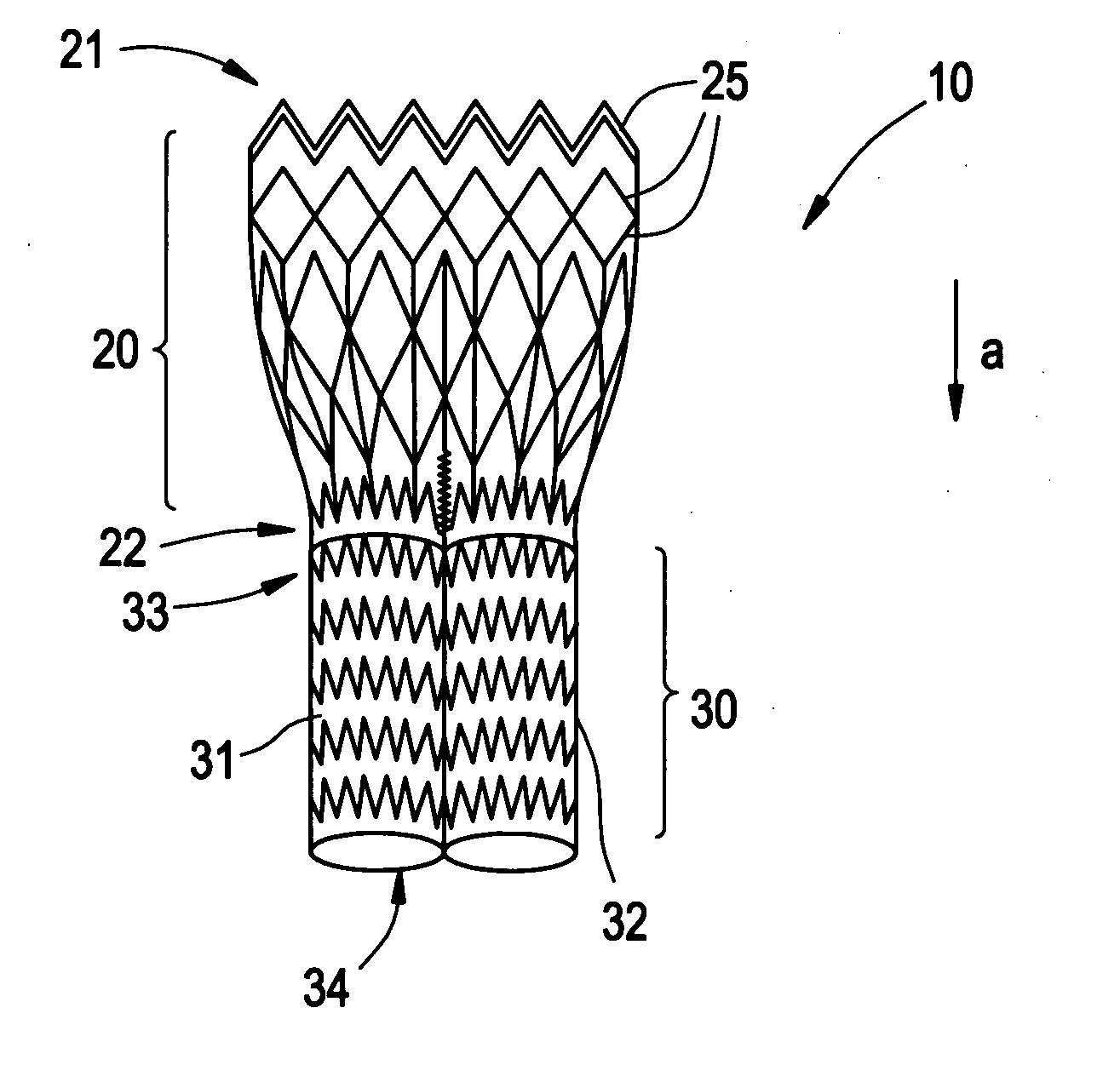
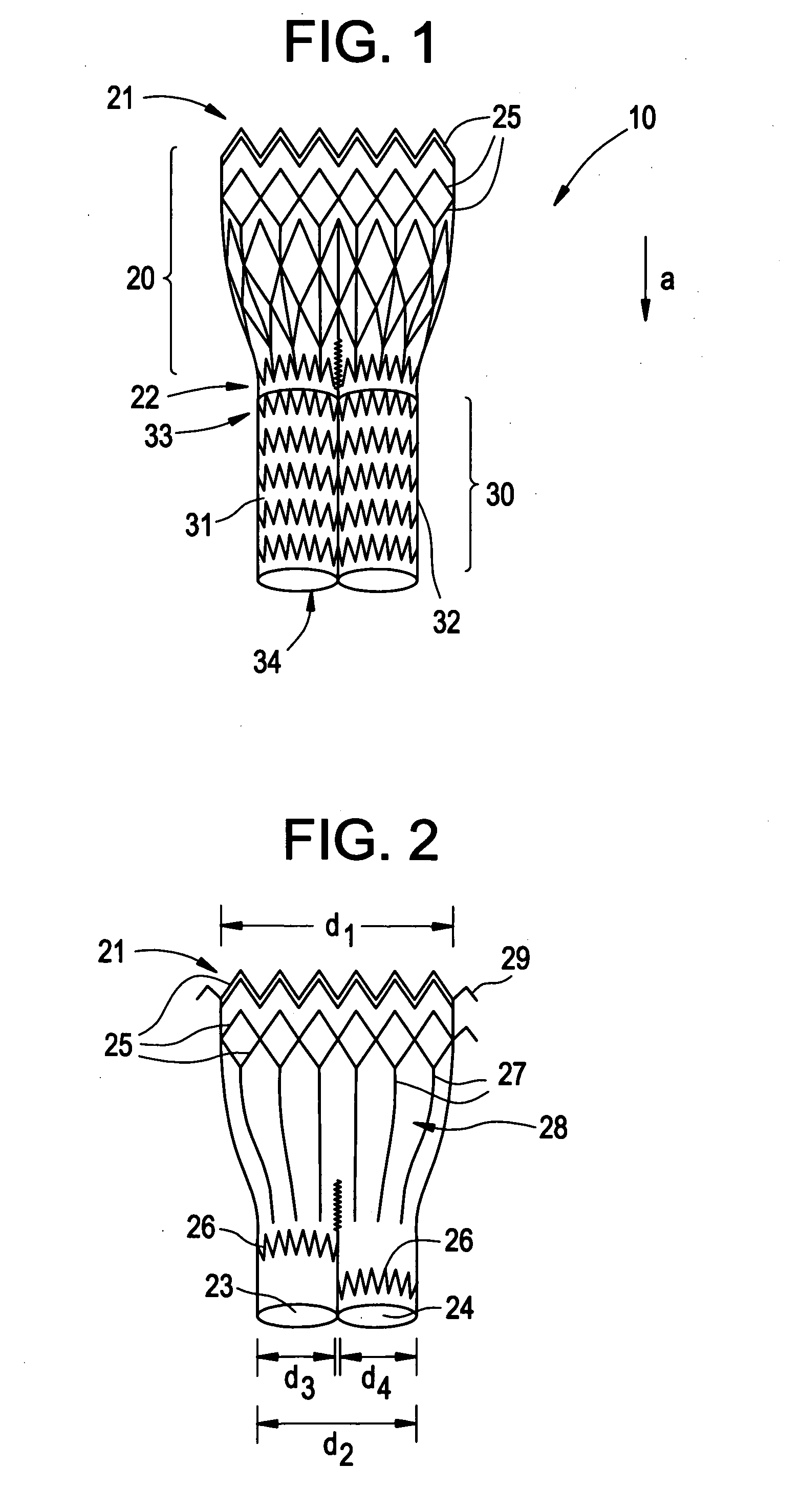
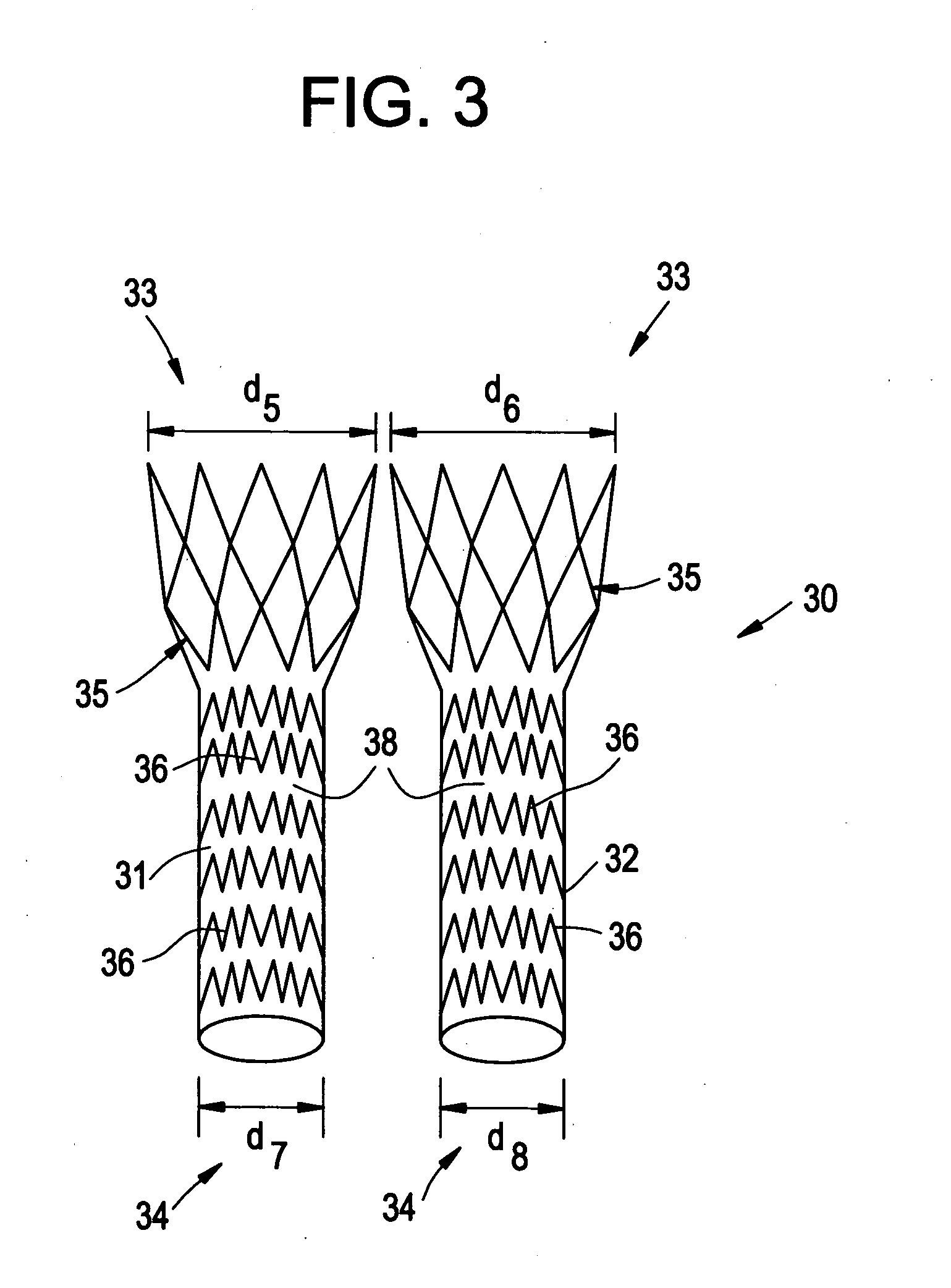
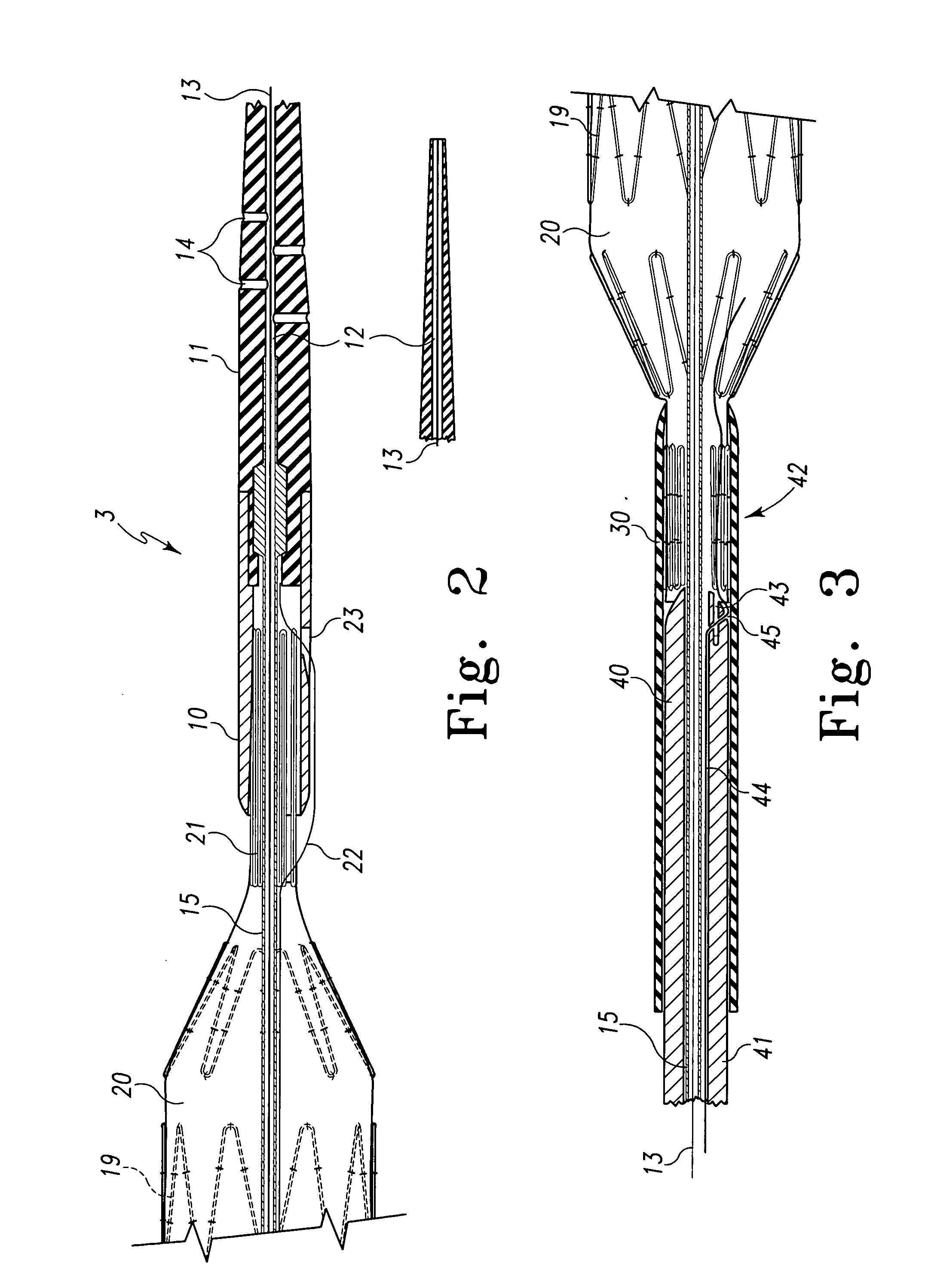
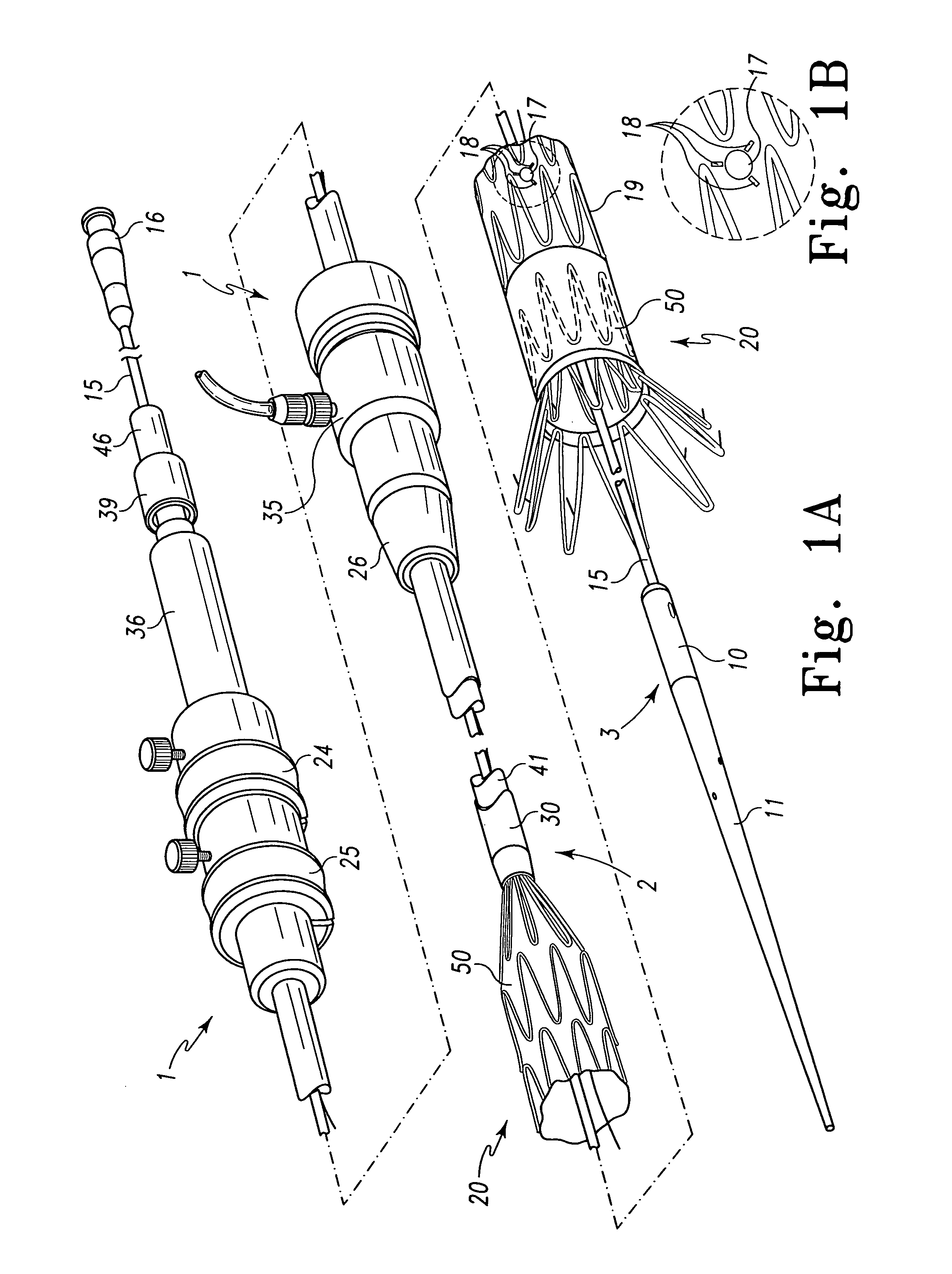
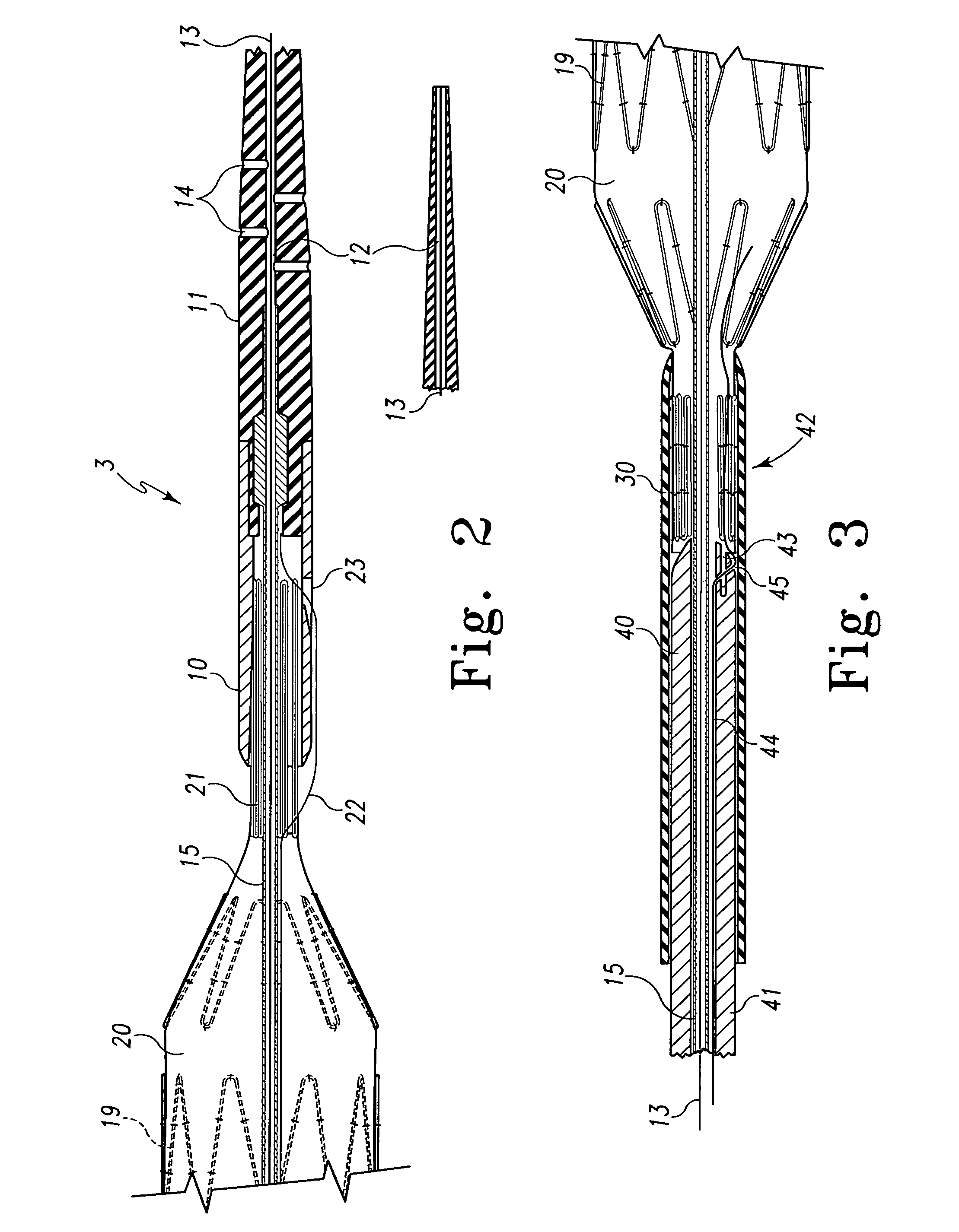
Multi-section filamentary endoluminal stent
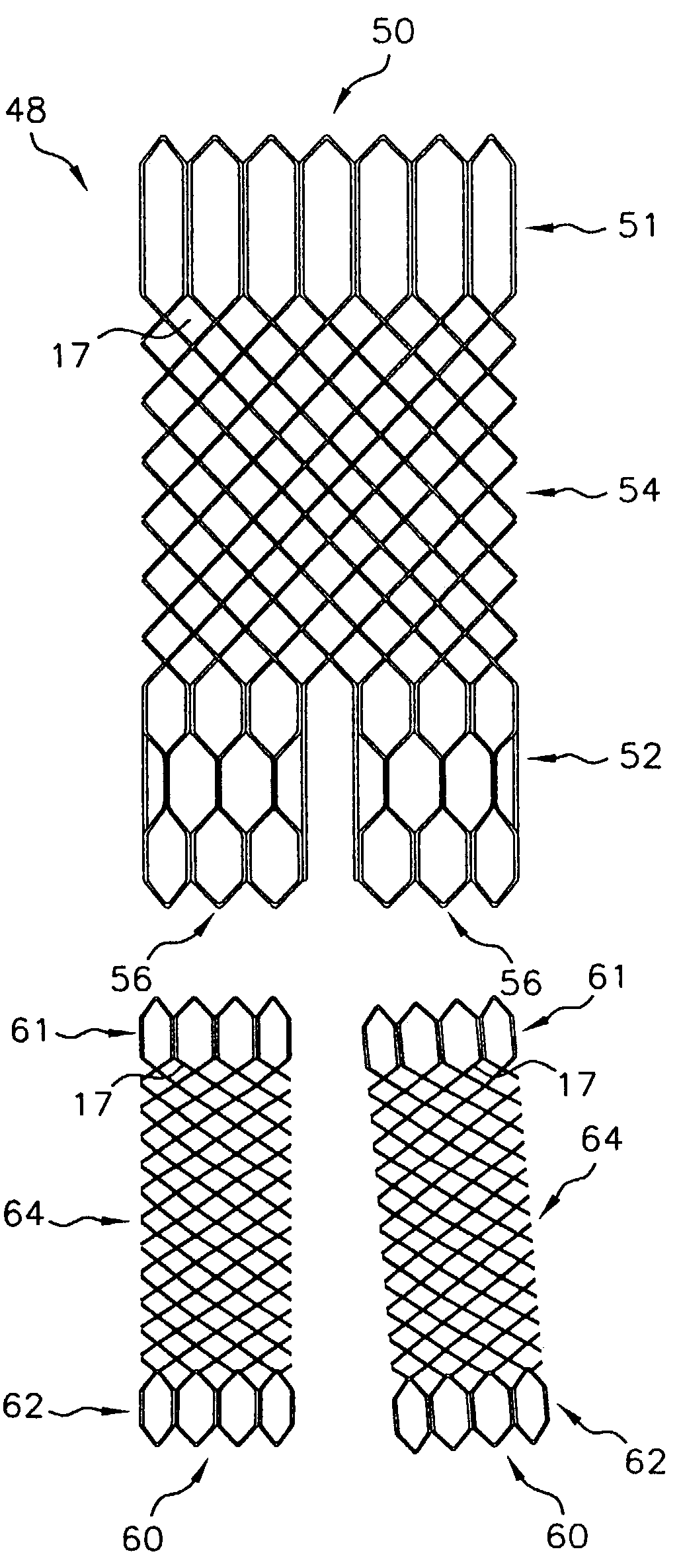
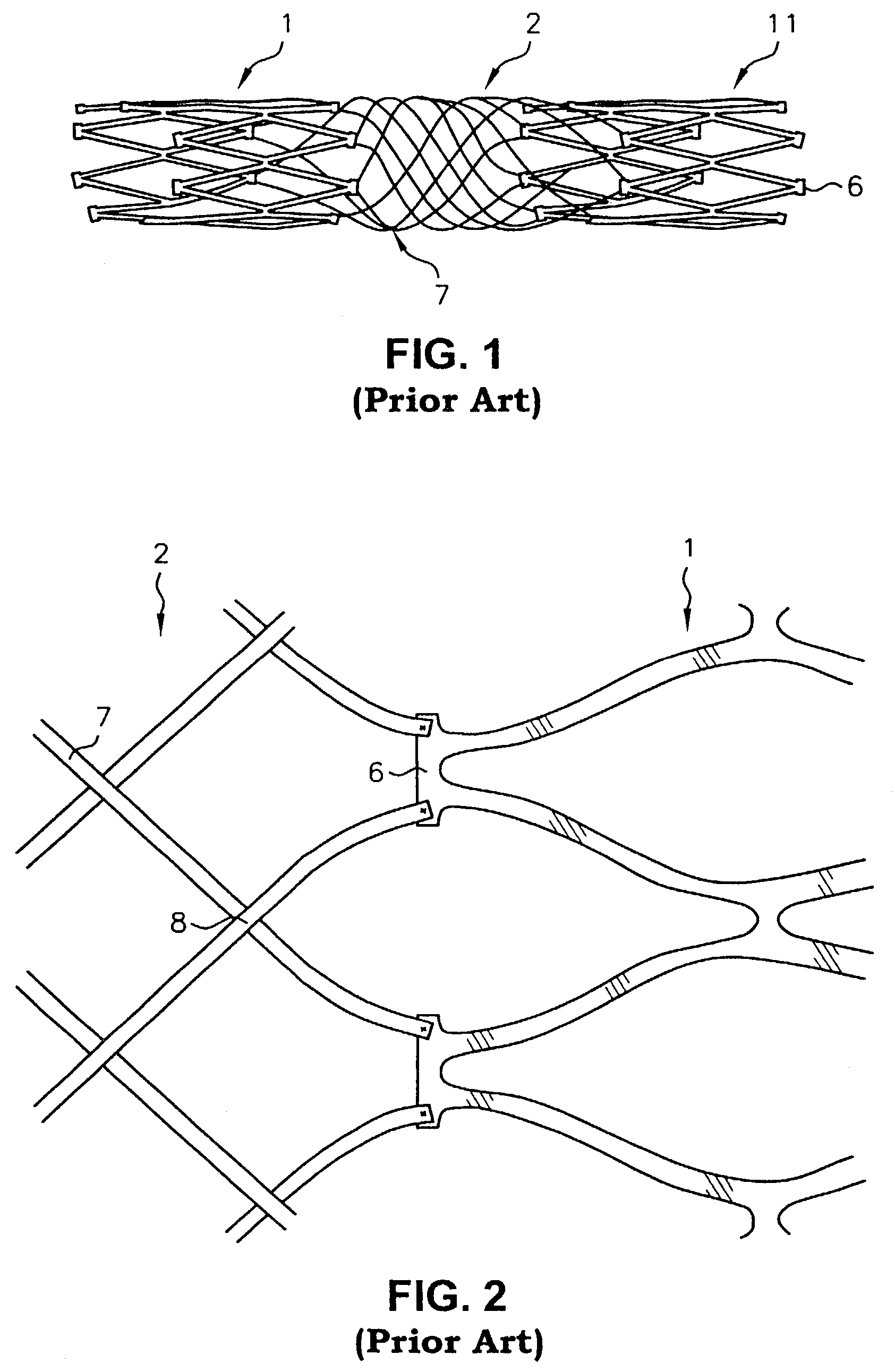
A multi-section filamentary stent comprises a braided section, which is a cylindrical mesh of a first set of filaments, connected to at least one wound section comprising a second set of one or more filaments having a repeating configuration with a bent portion. The two sections are preferably connected by at least one continuous filament extending into both sections. The two sections may be connected by a weld, a suture, a common graft, an overlapping portion of the two sections, or one or more filaments of one section looping through portions of the other section. The stent may comprise a first section, having a braided first stent architecture with a first flexibility and a first radial force, and a second section, having a non-braided second stent architecture with a second flexibility less than the first flexibility and a second radial force greater than the first radial force, in which at least one continuous filament is integral to both the first and second sections. The stent may have a radially compressed configuration and a radially expanded configuration, in which the first section has a first shortening ratio, and the second section has a second shortening ratio less than the first shortening ratio. Such multi-section stents may comprise modular components of a modular stent, such as a bifurcated modular stent, adapted for joining together in situ. The multi-section stent may comprise a first section having a first percentage of open area and a second section having a second percentage of open area. The stent may also comprise a first section having a first stent architecture with an end effect wherein the radial strength at the end is less than elsewhere in the stent, and a second section having a second stent architecture to counteract the end effect. Methods for treating body lumen by implanting the stents as described herein are also disclosed, as is a method for counteracting a stent architecture end effect.
Owner:LIFESHIELD SCI
Intraluminal stent graft
InactiveUS20070162109A1Minimizes delivery profileEasy to appreciateStentsBlood vesselsStent graftingEndoluminal stent
A modular intraluminal stent graft. The intraluminal stent graft is bifurcated having a primary section and a secondary section extending therefrom. The primary section tapers from a larger diameter at an upstream end to a smaller diameter at a downstream end. The downstream end of the primary section has a pair of independent openings each having an expanded diameter. The secondary section provides a first endoleg having an upstream end that is received through the expanded diameter of one opening of the primary section, and a second endoleg having an upstream end that is received through the second opening of the primary section. The upstream ends of each endoleg, in its expanded state, is larger than the downstream portion of the respective endolegs and expands within the primary section to help assemble the graft in situ. The first and second endolegs also expand within the respective openings each is received within to assemble the stent graft in situ as well. The primary section is positioned within a blood vessel trunk, whereas the endolegs of the secondary section are positioned within a blood vessel branched from the blood vessel trunk. A typical application would be to place the primary section within the abdominal aorta infrarenally, with the endolegs positioned in the ipsilateral and contralaterial iliacs, respectively. By minimizing the number of stent segments in the primary section of the stent graft a lower delivery profile is achieved.
Owner:CORDIS CORP
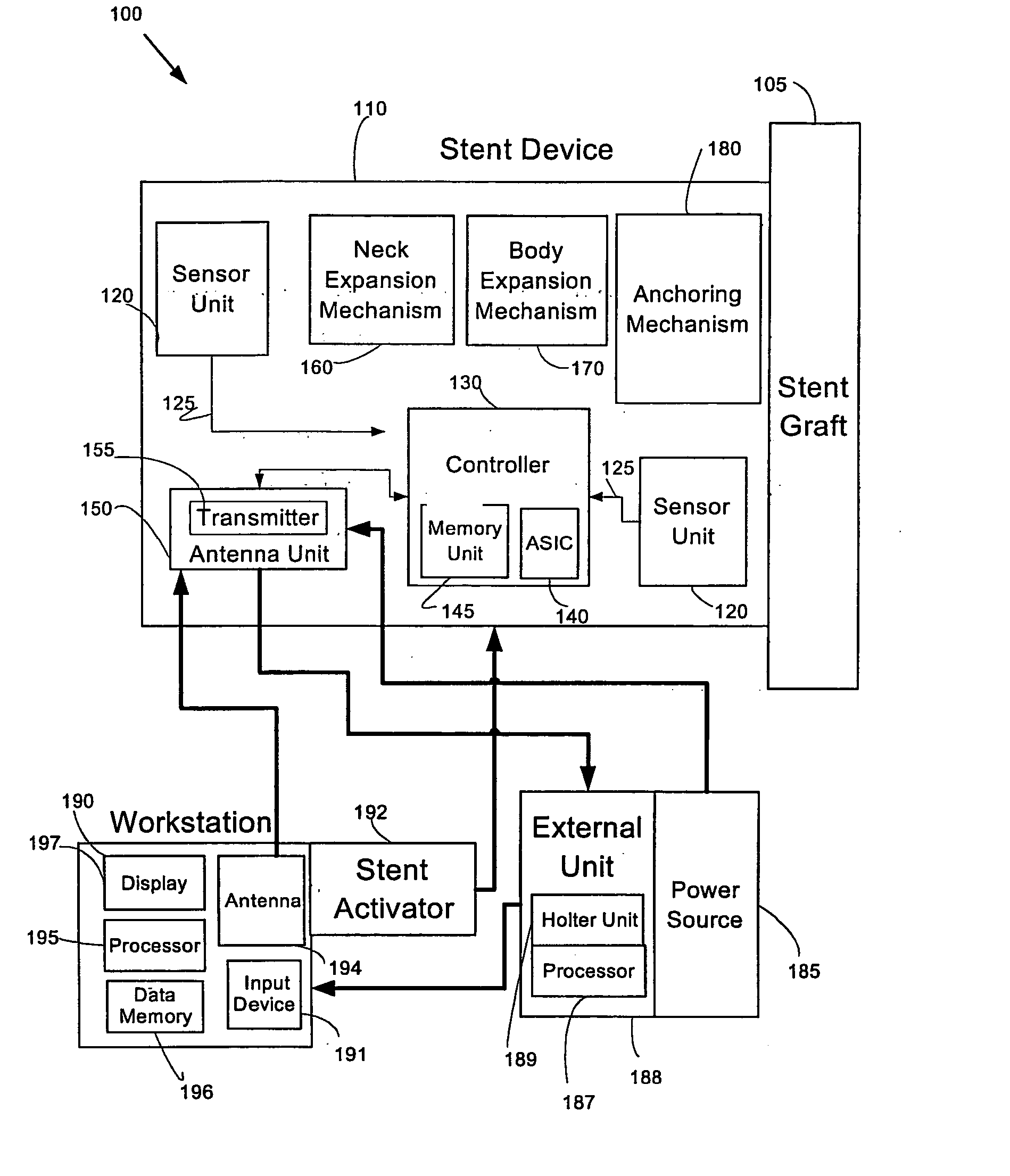
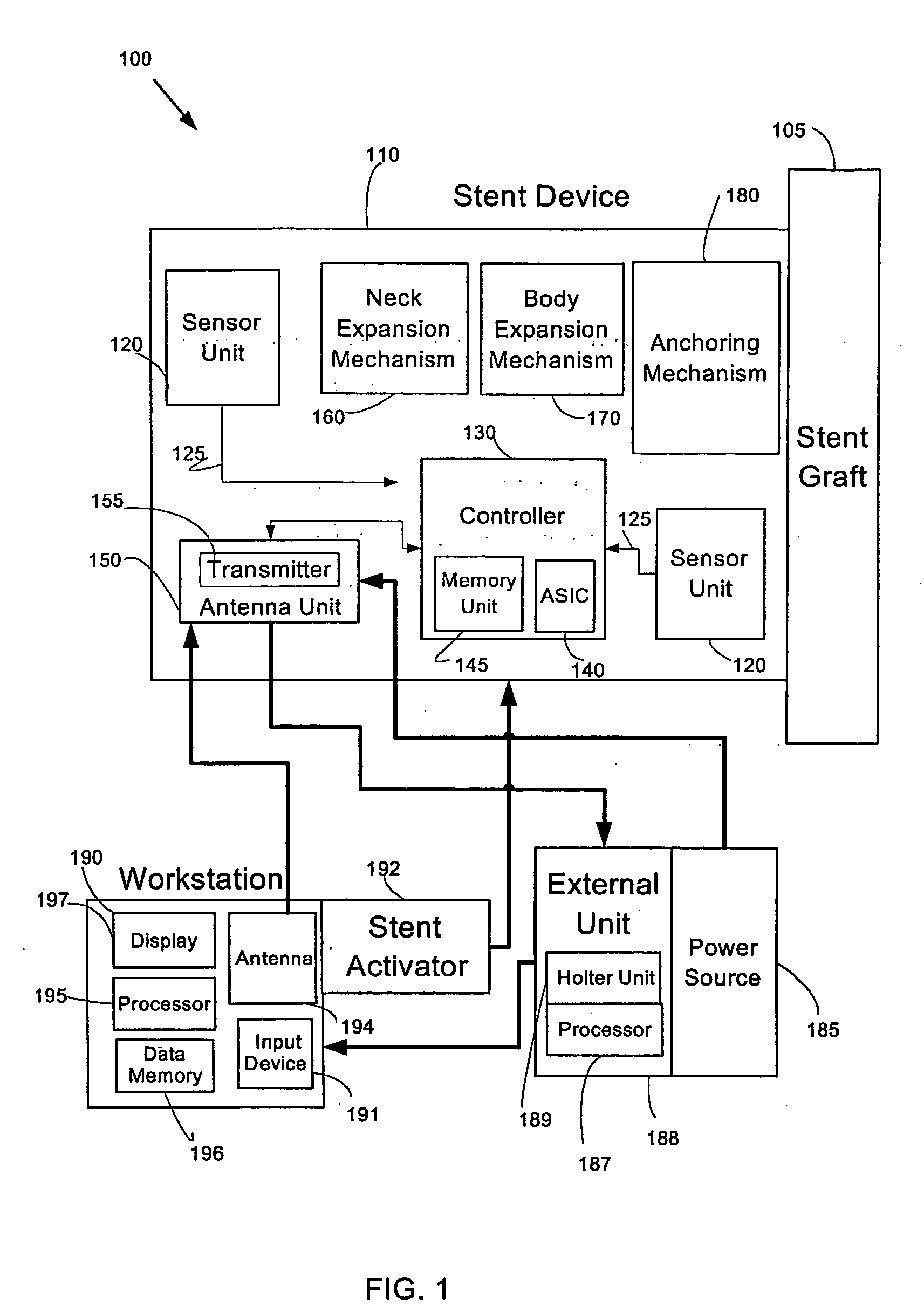
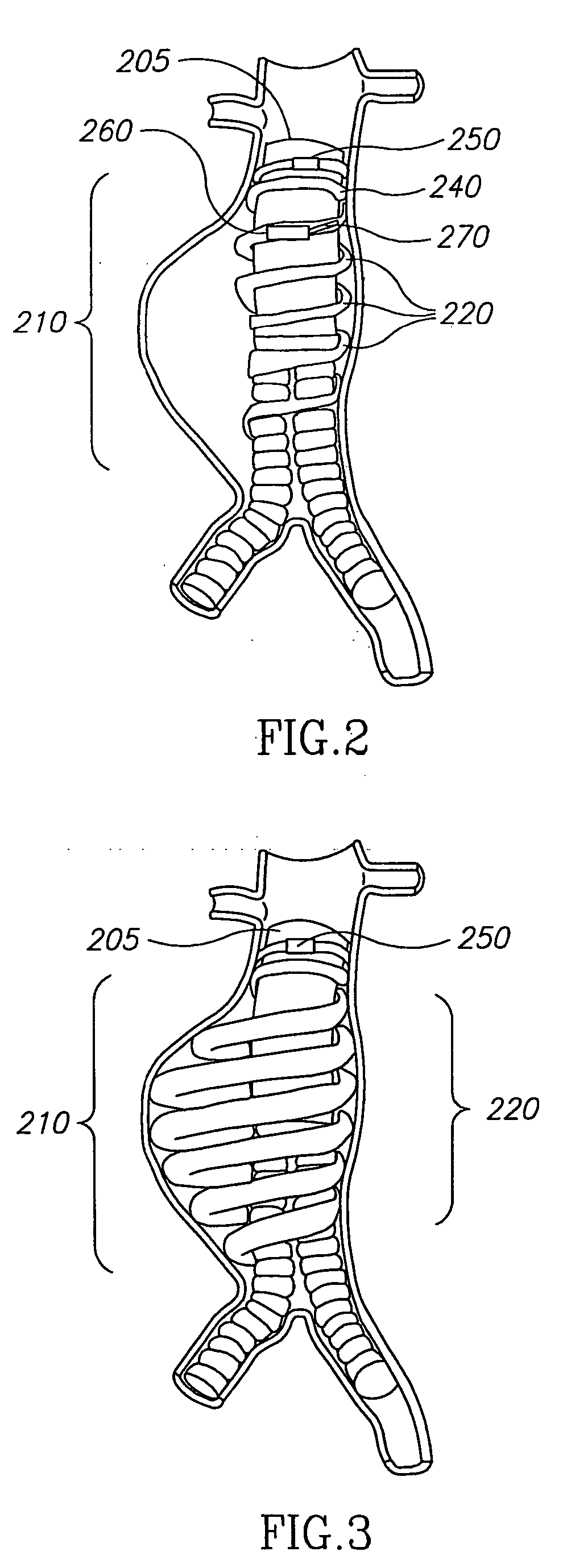
System and method of aneurism monitoring and treatment
InactiveUS20050065592A1Prevention of aneurism expansionImprove gripStentsCatheterEndoluminal stentInsertion stent
An endoluminal stent device, system, and method enabling localized detection and treatment of adverse stent-related conditions. Embodiments of the present invention may enable monitoring of endoluminal pressure in the region of an aneurism, and / or compliance in the neck region of a stent device. Other embodiments of the present invention may provide systems and methods for treating a variety of aneurism conditions, by externally activating at least one expansion mechanism associated with the stent device at a desired future time after an initial stent placement procedure.
Owner:HOLZER
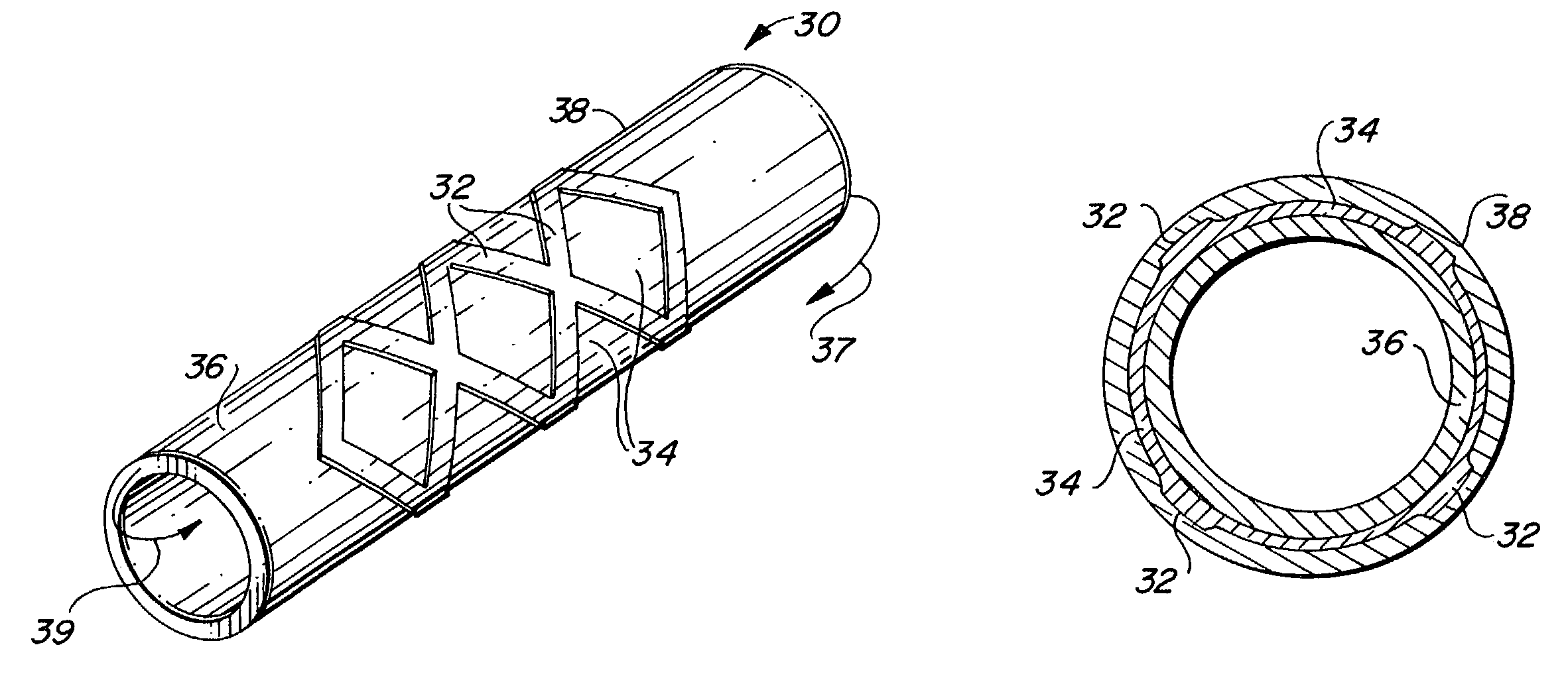
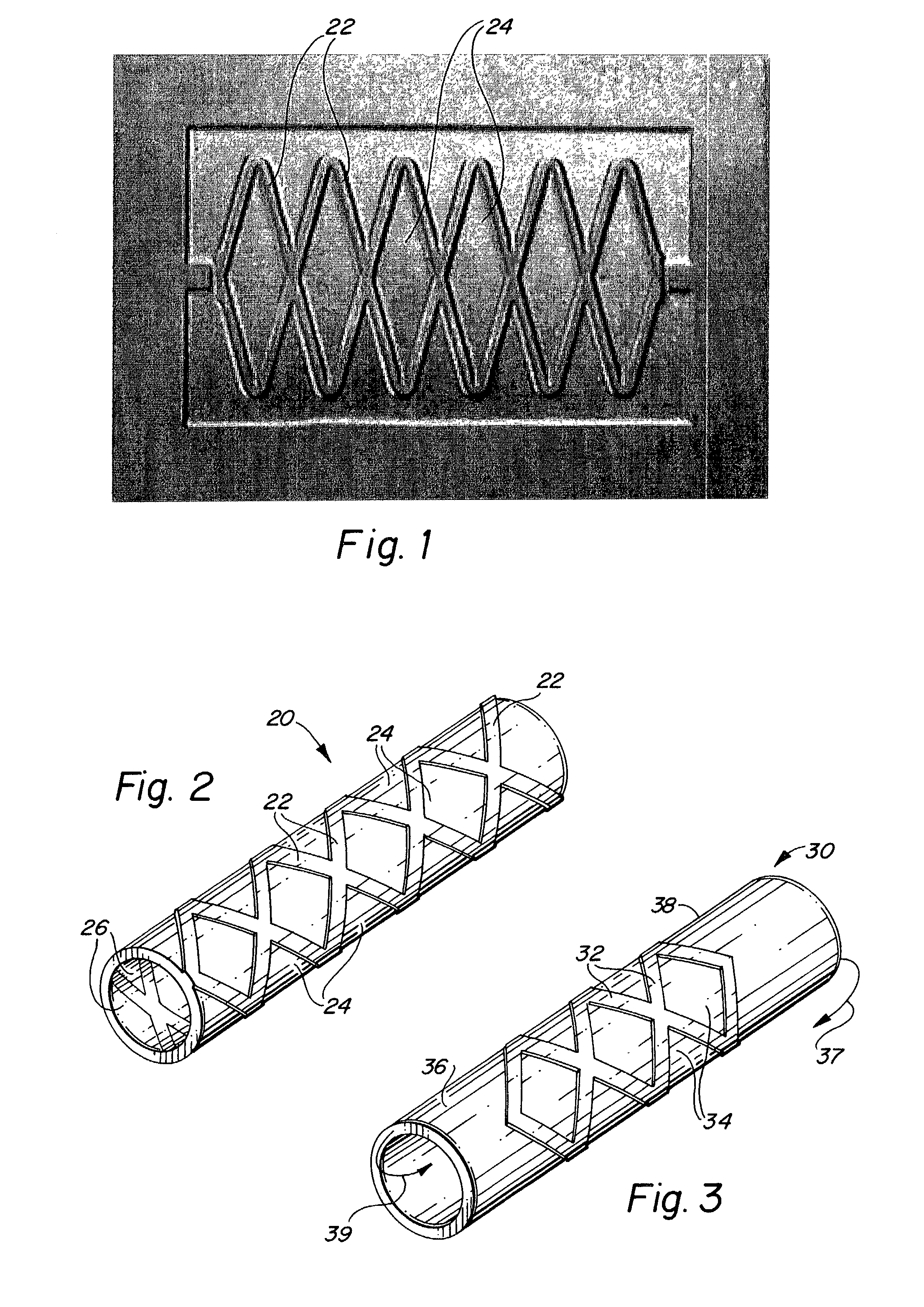
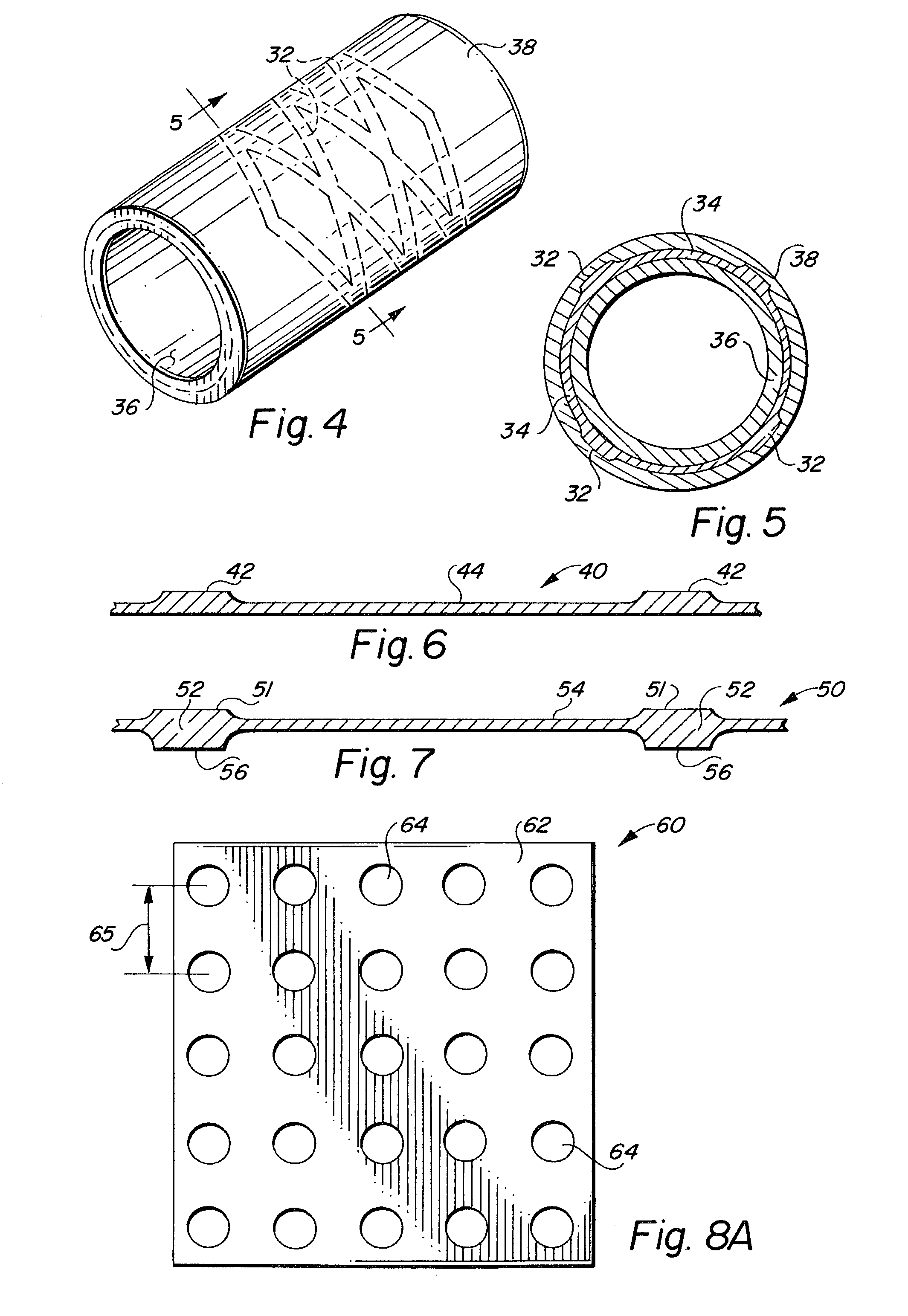
Balloon-assisted intraluminal stent graft
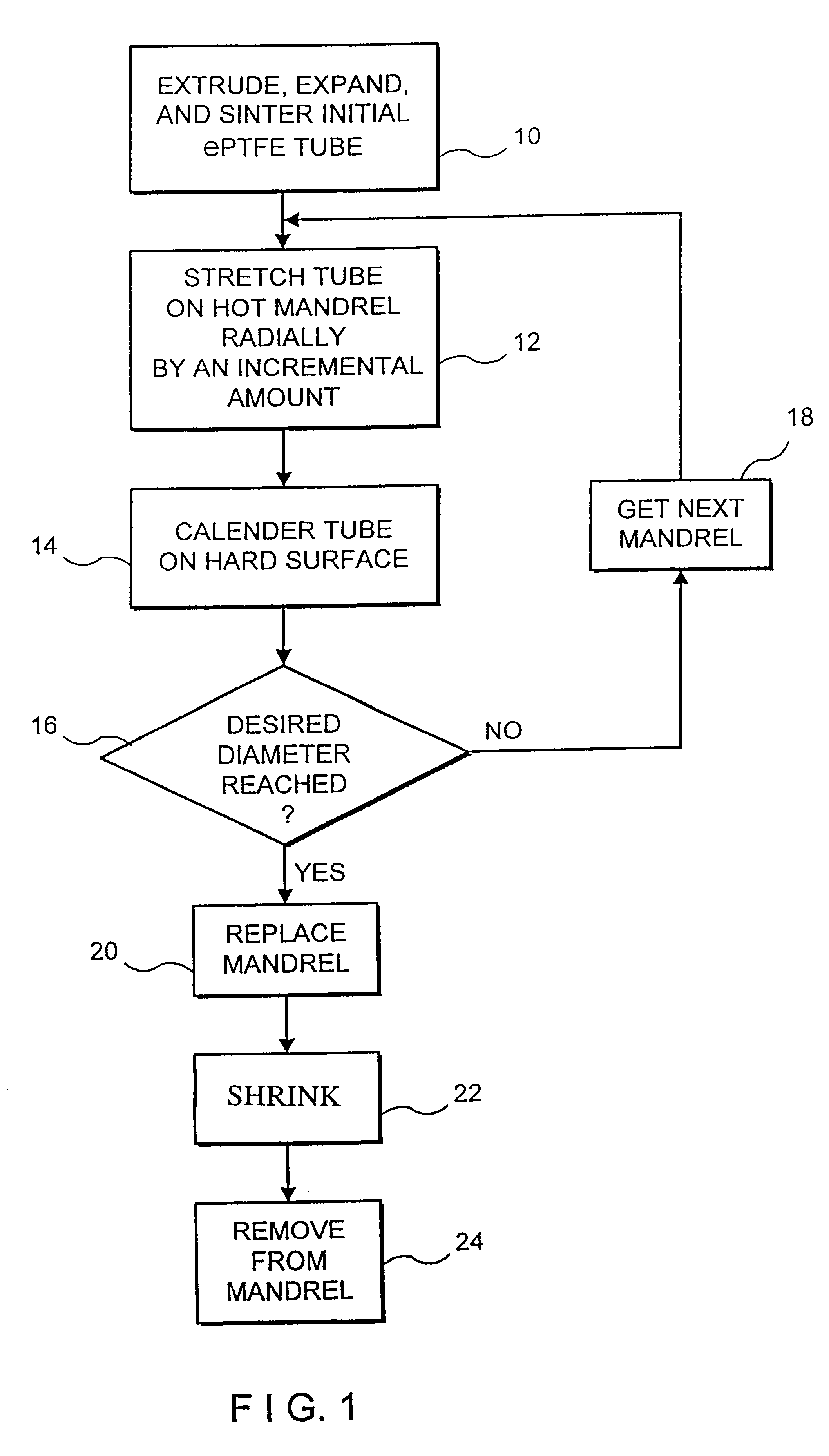
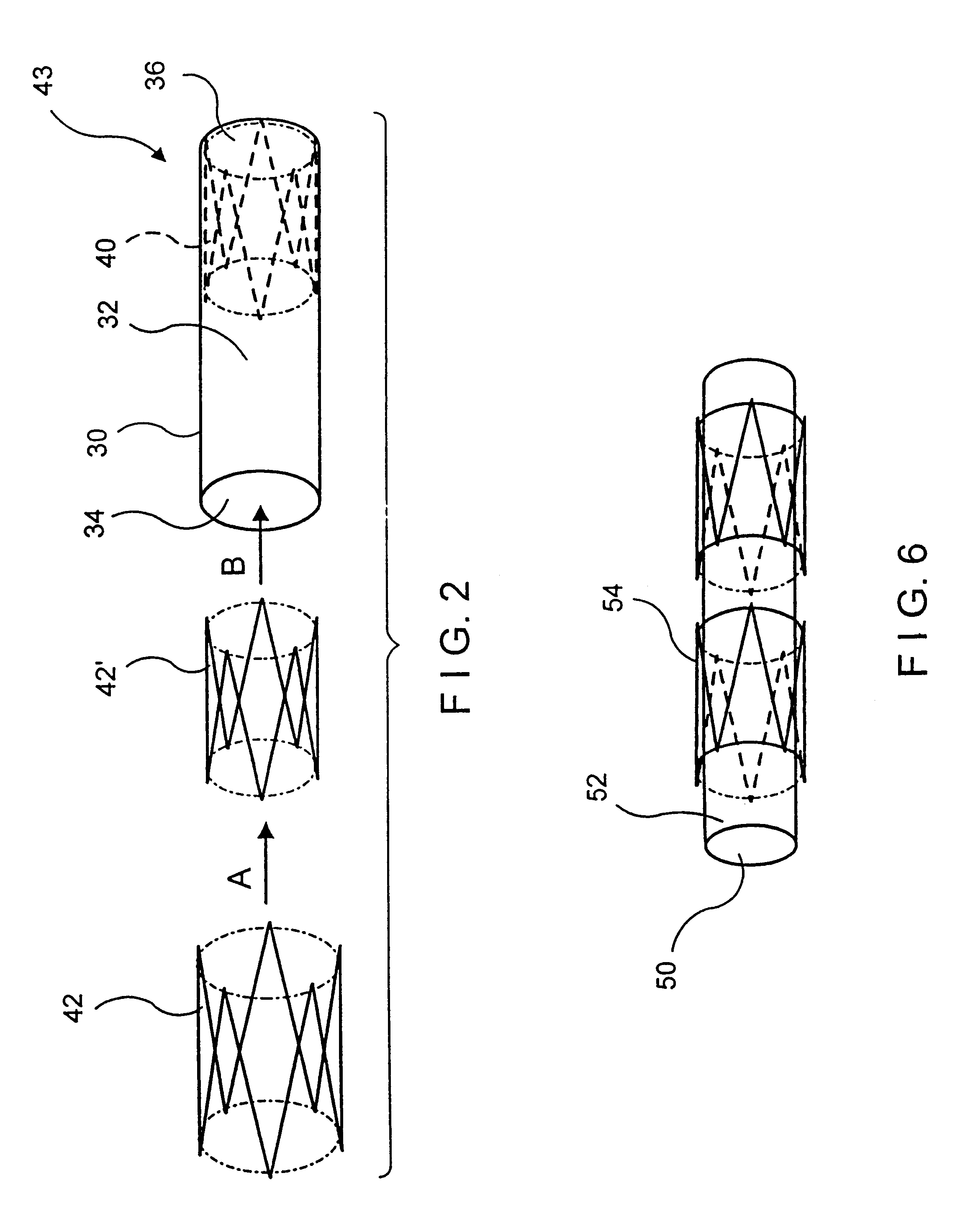
An intraluminal device such as a stent graft is formed of a conformable ePTFE tube and preferably a compressed self-expanding stent affixed to the tube. The conformable tube is made by a special process which insures that the tube is radially deformable up to a predetermined diameter without exceeding the plastic deformation limit of the tube. The stent has a relaxed diameter larger than the diameter of the expanded tube after insertion so that the tube is biased to a cylindrical shape. The process for making the tube involves progressively dilating a small diameter extruded tube until a desired diameter is achieved. The tube is then contracted on a small diameter mandrel by heating.
Owner:LEMAITRE ACQUISITION +1
Methods and apparatus for luminal stenting
Described herein are flexible implantable devices or stents that can, for example, navigate tortuous vessels of the neurovasculature. The devices can also conform to the shape of tortuous vessels of the vasculature. In some embodiments, the devices can direct blood flow within a vessel away from an aneurysm or limit blood flow to the aneurysm. Methods and structures are provided for adjusting, along a length of the device, the porosity of the device while maintaining a cross-sectional dimension. In some embodiments, a distal stent cover covers the distal end of the device and reduces friction between the stent and an inner surface of a delivery device, such as a catheter.
Owner:TYCO HEALTHCARE GRP LP
Stent grafts with bioactive coatings
Stent grafts are provided comprising an endoluminal stent and a graft, wherein the stent graft releases an agent which induces the in vivo adhesion of the stent graft to vessel walls, or, otherwise induces or accelerates an in vivo fibrotic reaction causing said stent graft to adhere to vessel wall. Also provided are methods for making and using such stent grafts.
Owner:ANGIOTECH INT AG (CH) +1
Fenestrated intraluminal stent system
An intraluminal prosthesis is provided for strengthening a main lumen and a branch lumen that branches from the main lumen. The intraluminal prosthesis can comprise two tubular grafts. The first tubular graft can have a flexible body with a fenestration. The second tubular graft can have a flexible body that is configured for intraluminal coupling to the fenestration of the first tubular graft. The flexible body of the second tubular graft can have an outer dimension that is about equal to an inner dimension of the fenestration of the first tubular graft. The second tubular graft can also have a terminal stent that curves outwardly from a proximal end of the flexible body of the second tubular graft, whereby the terminal stent acts to couple the second tubular graft to the first tubular graft.
Owner:MED INST INC +1
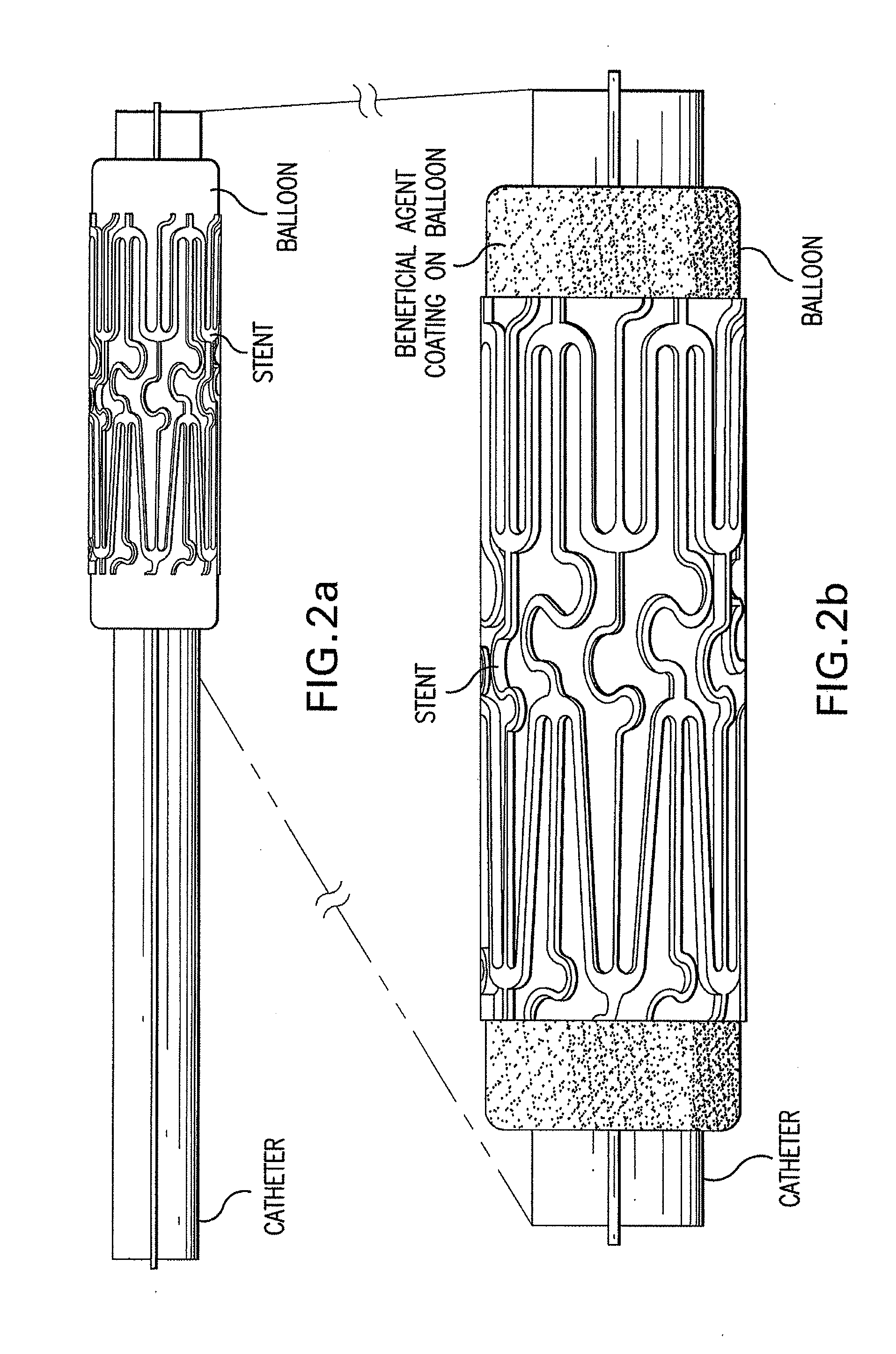
Multiple Drug Delivery From A Balloon And A Prosthesis
ActiveUS20100023108A1Low water solubilityGood film-forming propertiesStentsBalloon catheterAngioplasty balloonEndoluminal stent
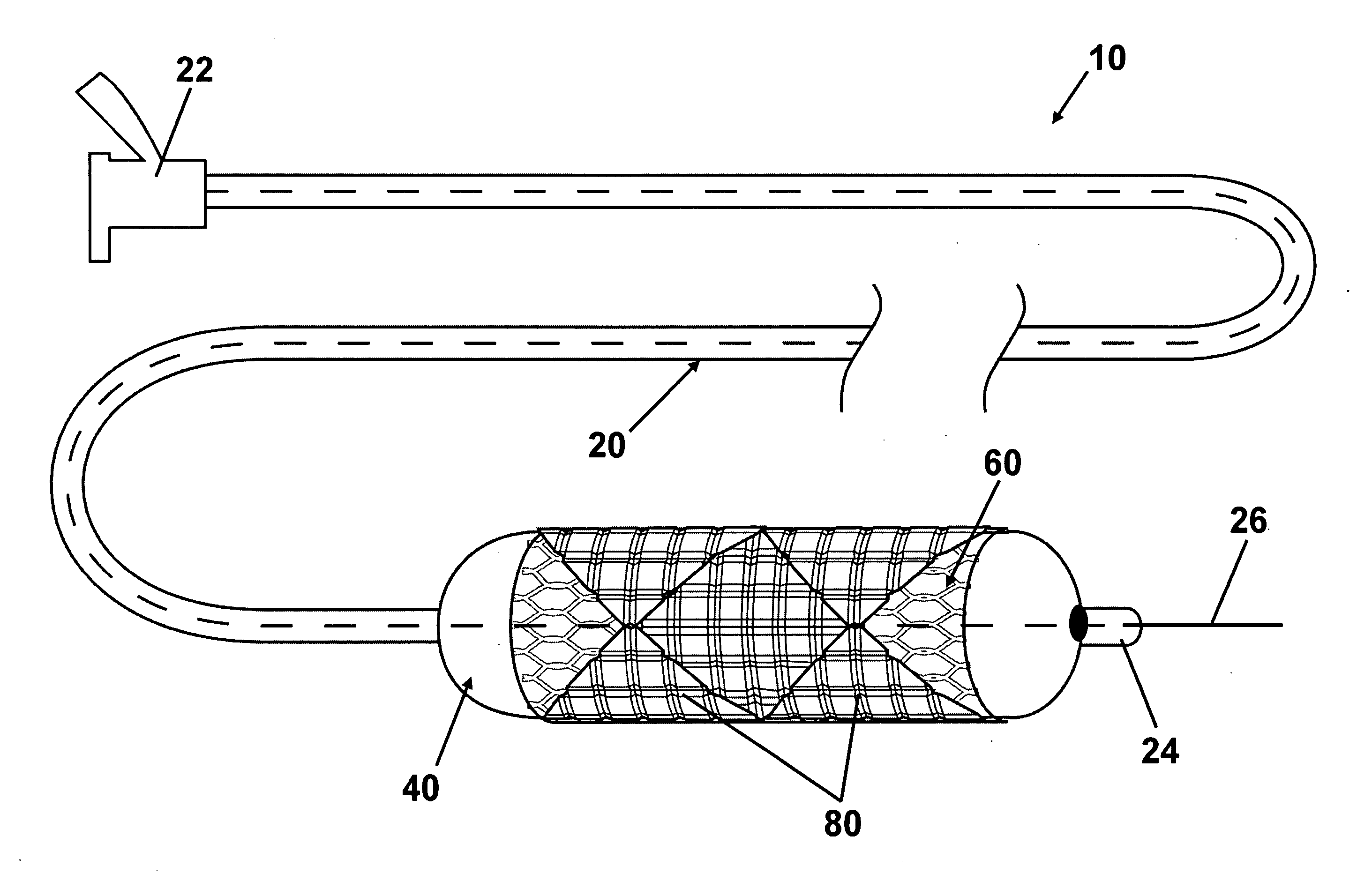
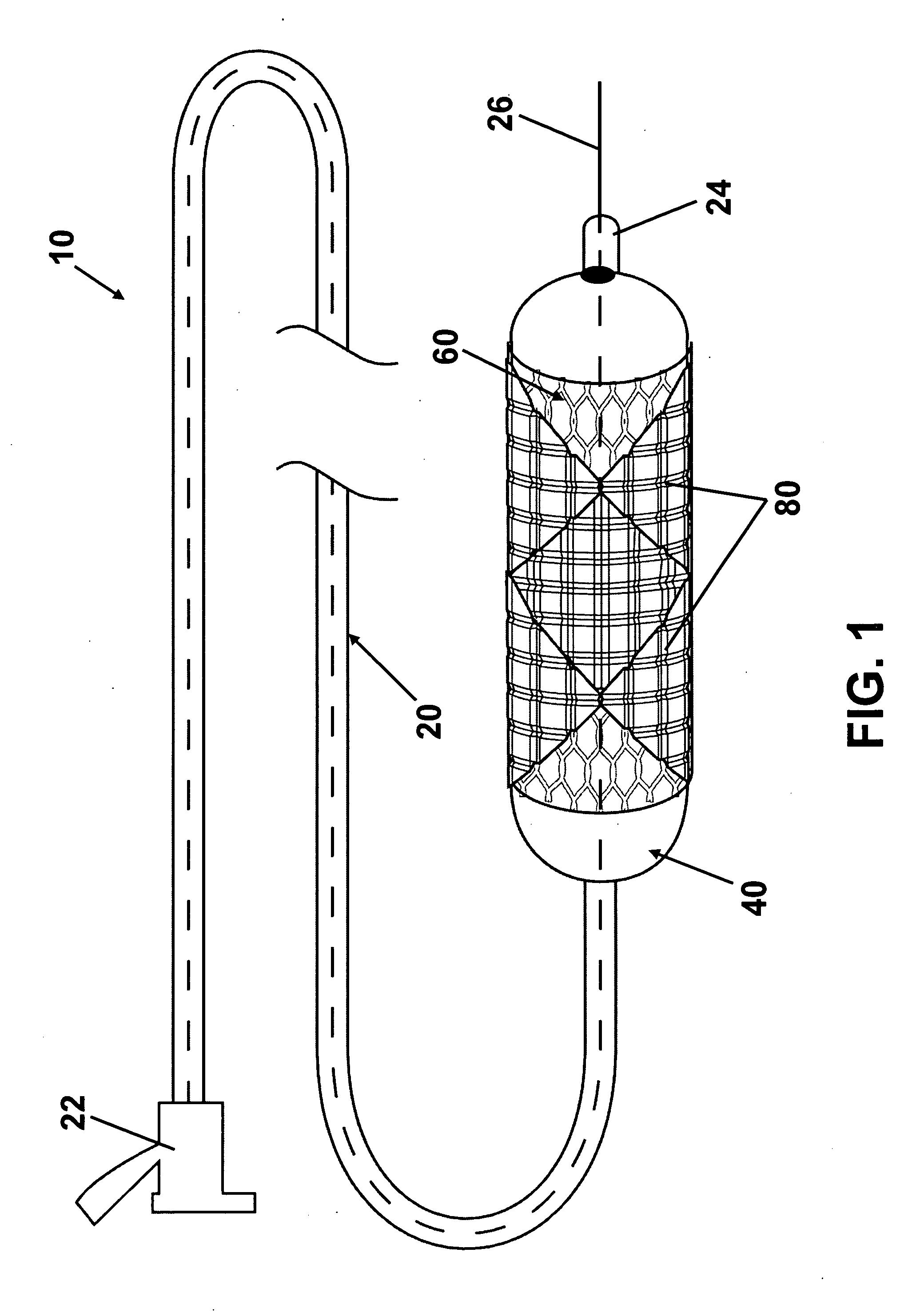
Disclosed is an interventional device for delivery of therapeutic agents from an angioplasty balloon and from a prosthesis such as an intraluminal stent. The invention also relates to the method of loading the beneficial agents onto the balloon and the device, as well as the method of delivery of the agents from separate surfaces. The invention also relates to an interventional device having a prosthesis surface that is loaded with a first beneficial agent, and a balloon surface loaded with a second beneficial agent. The invention also relates to a method of loading multiple beneficial agents onto the prosthesis surfaces and the balloon surfaces, and to a method of manufacturing an interventional device for the delivery of a first beneficial agent and a second beneficial agent from separate surfaces.
Owner:ABBOTT LAB INC
Endoluminal implantable stent-grafts
An implantable endoluminal device that is fabricated from materials that present a blood or body fluid and tissue contact surface that has controlled heterogeneities in material constitution. An endoluminal stent-graft and web-stent that is made of an monolithic material deposited into a monolayer and etched into regions of structural members and web regions subtending interstitial regions between the structural members. An endoluminal graft is also provided which is made of a biocompatible metal or metal-like material. The endoluminal stent-graft is characterized by having controlled heterogeneities in the stent material along the blood flow surface of the stent and the method of fabricating the stent using vacuum deposition methods.
Owner:VACTRONIX SCI LLC
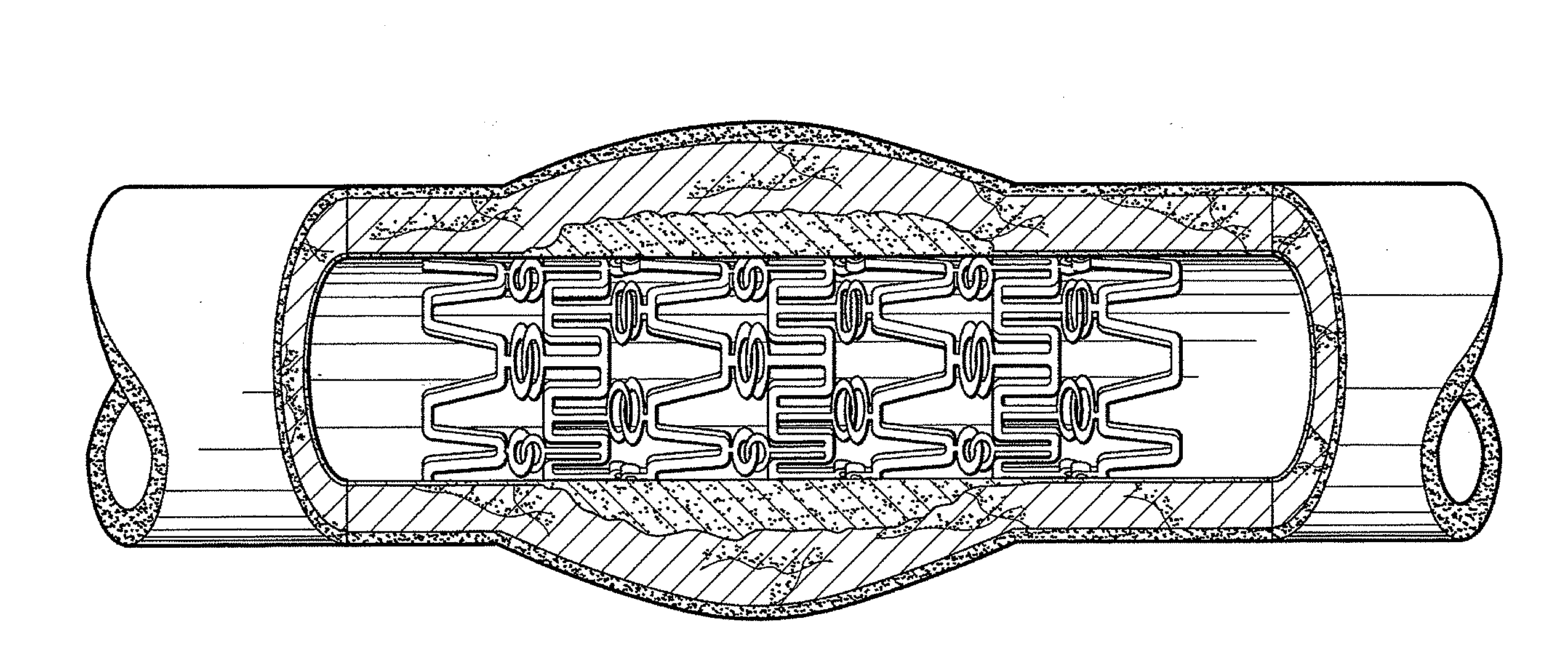
Tubular stent with oval struts
InactiveUS7153322B2Enhance crimping and symmetric expansion of stentLength of tubeStentsBlood vesselsLongest DiameterEndoluminal stent
A vascular or endoluminal stent is adapted for deployment in a vessel or tract of a patient to maintain an open lumen. The stent constitutes a scaffold formed from a single open-ended tube having a multiplicity of through-holes in its wall. The through-holes are defined by a plurality of struts that bound the holes. Each of the struts has an optimized cross-section of oval shape with a long diameter generally aligned with the length or circumference of the tube wall and a short diameter generally aligned with the thickness of the tube wall. The oval shape of the struts provide several advantages including enhancing flexibility of the stent, easing advancement of the stent through a lumen of the vessel or tract for deployment at a target site therein, protecting the balloon of a balloon catheter on which the stent is tightly crimped, and enhancing expansion of the stent during deployment while maintaining its capability to withstand compression in response to recoil of the vessel or tract following deployment.
Owner:BOSTON SCI SCIMED INC
Fenestrated endoluminal stent system
ActiveUS20070021822A1Encourage tissue prolapseMinimally invasive procedureStentsBlood vesselsInsertion stentMinimally invasive procedures
An intraluminal prosthesis is provided for strengthening a main lumen and a branch lumen in direct fluid communication with the main lumen. The prosthesis may comprise a tubular graft and a flexible stent. The tubular graft may include a flexible body having a wall with a fenestration. The flexible stent may include a body with at least one open space to encourage tissue prolapse. The flexible stent may be configured for intraluminal coupling to the fenestration of the tubular graft, so that the entire prosthesis may be assembled and anchored into a main lumen and a branch lumen with a minimally invasive procedure. The open space may encourage tissue prolapse, which may act to anchor the flexible stent to the branch lumen.
Owner:COOK MEDICAL TECH LLC
Implantable stent with endothelialization factor
InactiveUS20060085062A1Promote endothelializationStrong adhesionStentsSurgeryEndoluminal stentInsertion stent
A stent is provided in combination with a growth factor, specifically pleiotrophin or an analog or derivative thereof, which promotes endothelialization of the stent and re-endothelialization of the stented region of an injured site in a body lumen. In particular applications, the stent is an endolumenal stent and the growth factor promotes healing via endothelialization and substantially prevents restenosis. The growth factor is delivered from the stent formulated as a protein or peptide, or as a gene transfer vector. Methods for the treatment of vascular injury using pleiotrophin are also disclosed.
Owner:MEDLOGICS DEVICE CORP
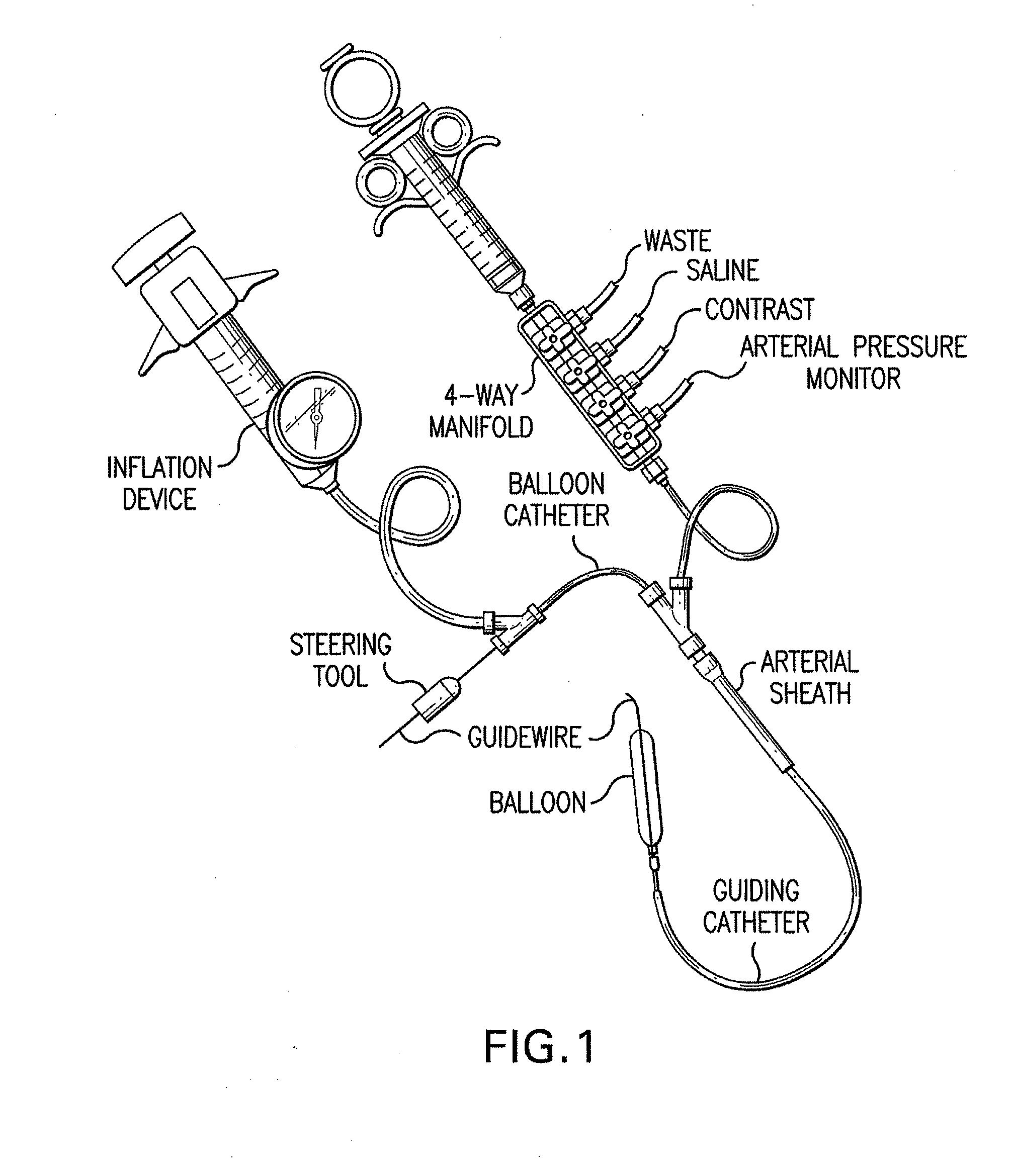
Method of delivering a medical device across a plurality of valves
Devices and methods for treating veins and venous conditions, such as chronic cerebrospinal venous insufficiency, are provided. In one aspect, the disclosed subject matter provides an intraluminal scaffold having a generally tubular body with a lumen defined therethrough, the tubular body having a compressed condition for delivery and an expanded condition for implant within a vessel having a distended portion, at least a length of the tubular body configured to form an enlarged portion in the expanded condition to engage a wall of the distended portion of the vessel. Methods for fabricating and using the scaffold, methods for remodeling a vein, and methods of deploying a medical device in a vessel without negatively impacting the function of a valve of the vessel, are also provided.
Owner:ABBOTT CARDIOVASCULAR
Stent, intraluminal stent delivery system, and method of treating a vascular condition
A stent, a stent delivery system, and a method of treating a vascular condition. The system includes a catheter, an inflatable member operably attached to the catheter, and a biodegradable stent disposed on the inflatable member. The stent includes a biodegradable flexible elongate member including an elongate member wall surrounding a cavity. A biodegradable reinforcing member is positioned within or adjacent the elongate member wall to support the biodegradable sleeve. A biodegradable photo-curable polymer positioned within the cavity. The method includes delivering a biodegradable stent having a cavity filled with a pre-polymer to a treatment site. A balloon is expanded to position the stent at the treatment site. The pre-polymer positioned within the stent cavity is photopolymerized. The deployed stent is supported in a radial direction with a biodegradable reinforcing member.
Owner:MEDTRONIC VASCULAR INC
Self-supporting laminated films, structural materials and medical devices manufactured therefrom and methods of making same
Metal foils, wires, and seamless tubes with increased mechanical strength are provided. As opposed to wrought materials that are made of a single metal or alloy, these materials are made of two or more layers forming a laminate structure. Laminate structures are known to increase mechanical strength of sheet materials such as wood and paper products and are used in the area of thin films to increase film hardness, as well as toughness. Laminate metal foils have not been used or developed because the standard metal forming technologies, such as rolling and extrusion, for example, do not lend themselves to the production of laminate structures. Vacuum deposition technologies can be developed to yield laminate metal structures with improved mechanical properties. In addition, laminate structures can be designed to provide special qualities by including layers that have special properties such as superelasticity, shape memory, radio-opacity, corrosion resistance etc. Examples of articles which may be made by the inventive laminate structures include implantable medical devices that are fabricated from the laminated deposited films and which present a blood or body fluid and tissue contact surface that has controlled heterogeneities in material constitution. An endoluminal stent-graft and web-stent that is made of a laminated film material deposited and etched into regions of structural members and web regions subtending interstitial regions between the structural members. An endoluminal graft is also provided which is made of a biocompatible metal or metal-like material. The endoluminal stent-graft is characterized by having controlled heterogeneities in the stent material along the blood flow surface of the stent and the method of fabricating the stent using vacuum deposition methods.
Owner:VACTRONIX SCI LLC
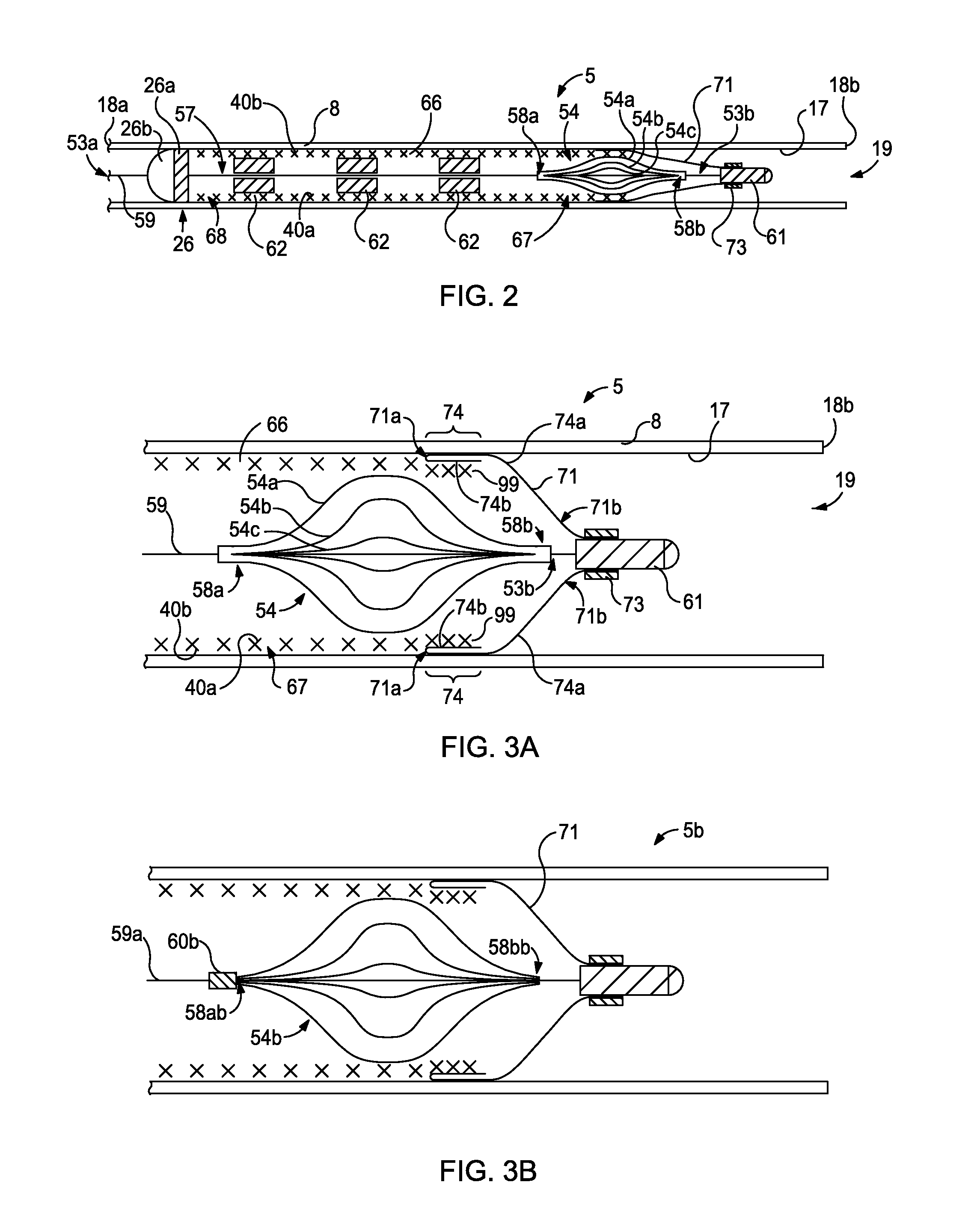
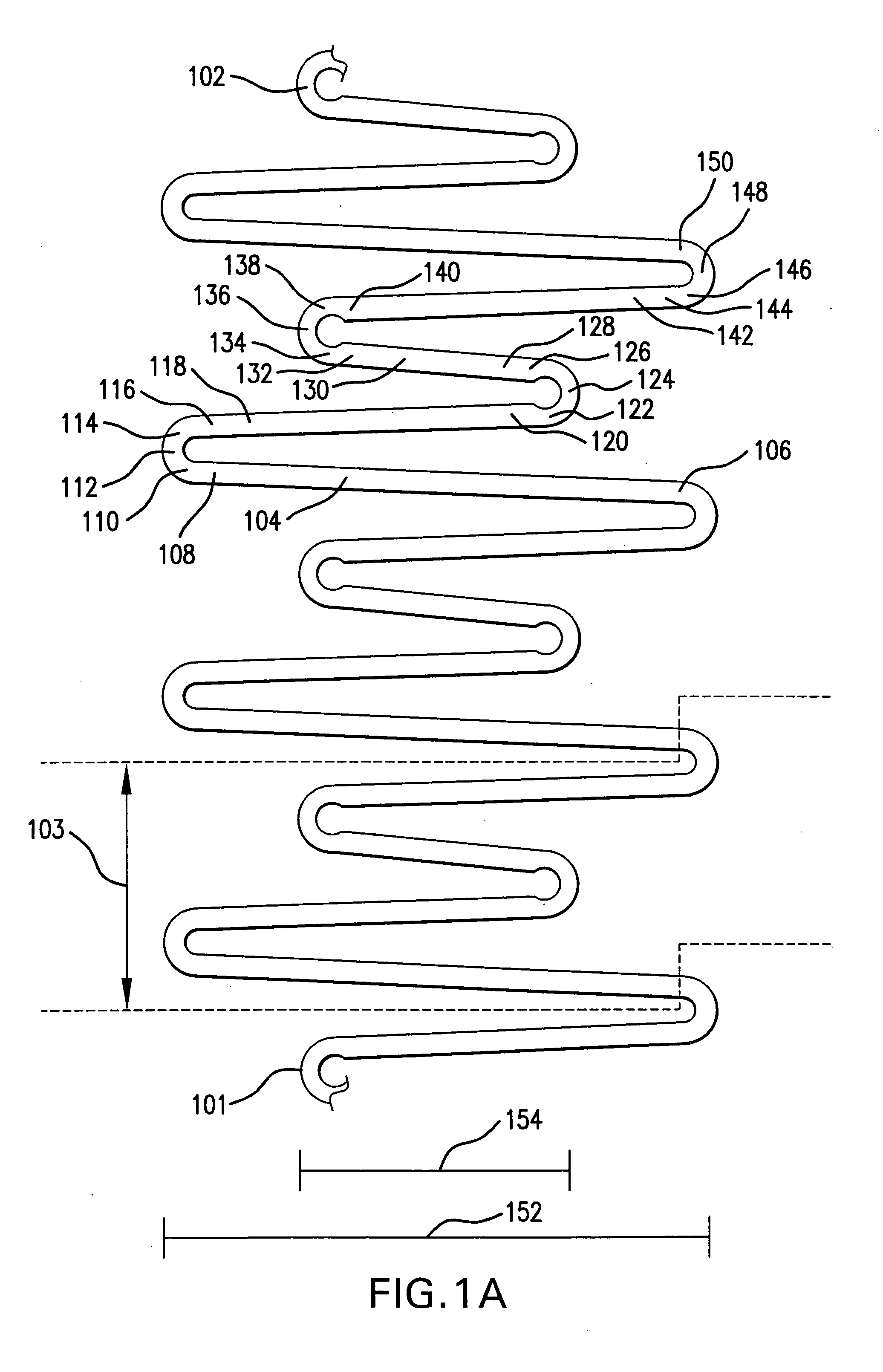
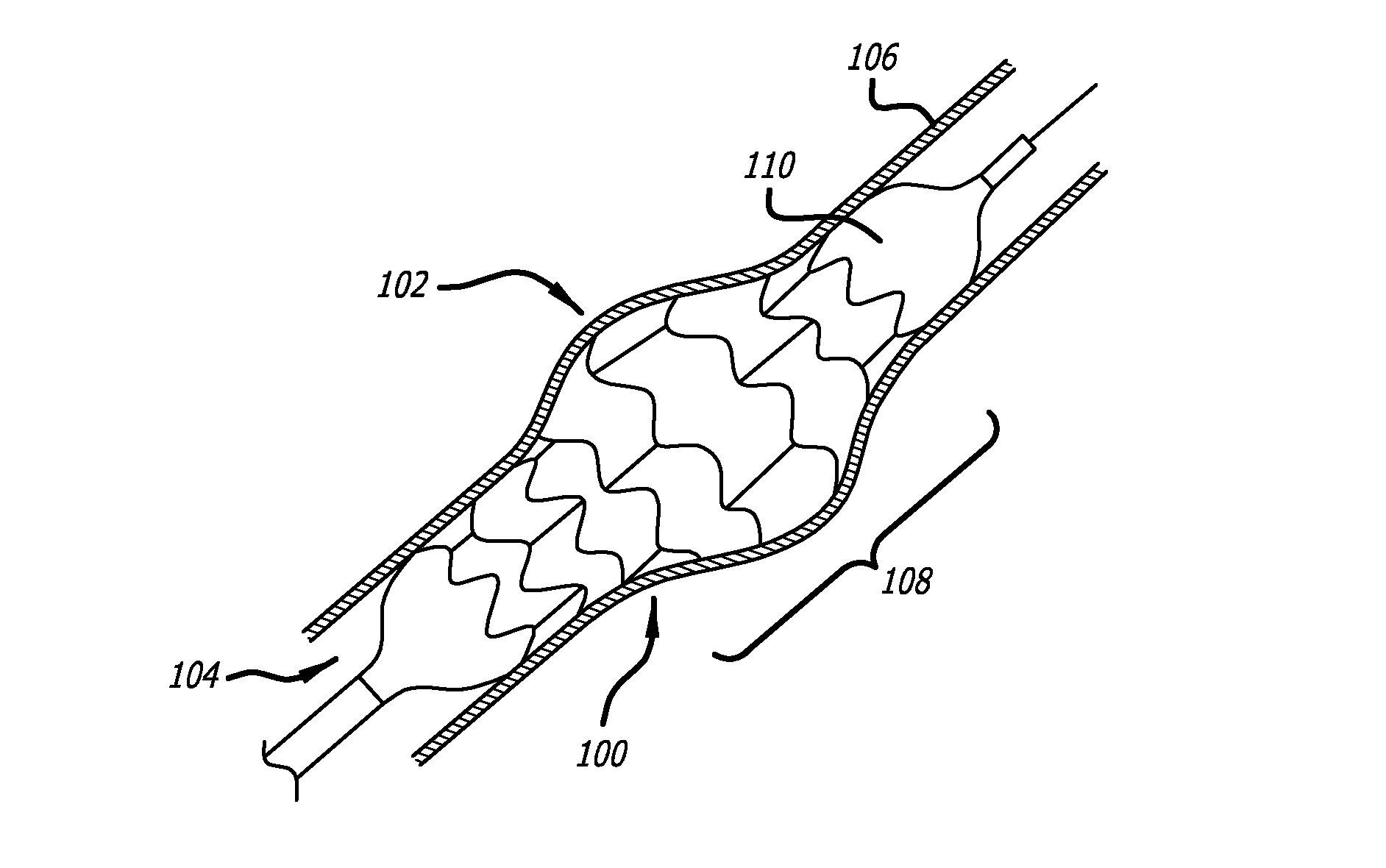
Intralumenal stent device for use in body lumens of various diameters
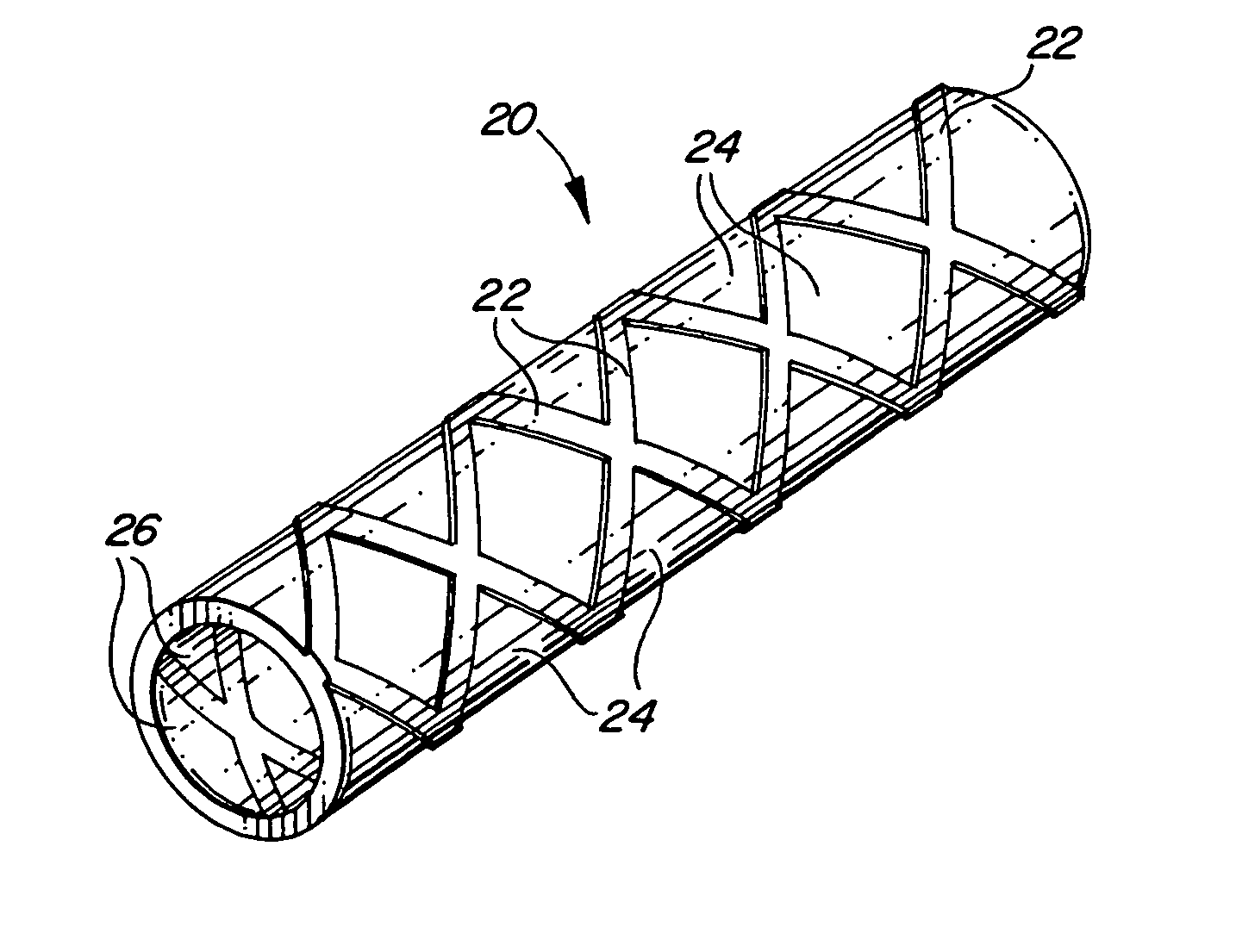
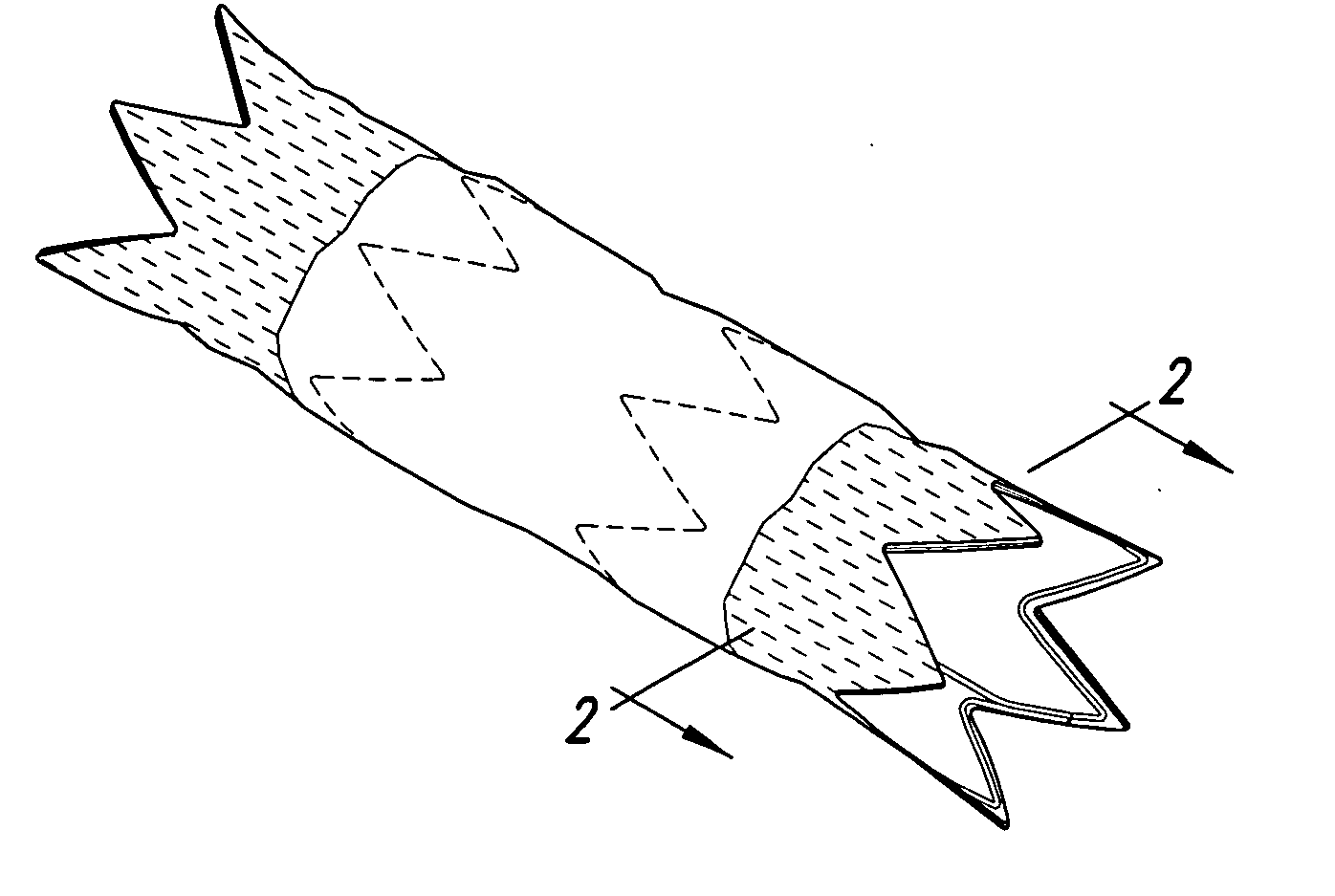
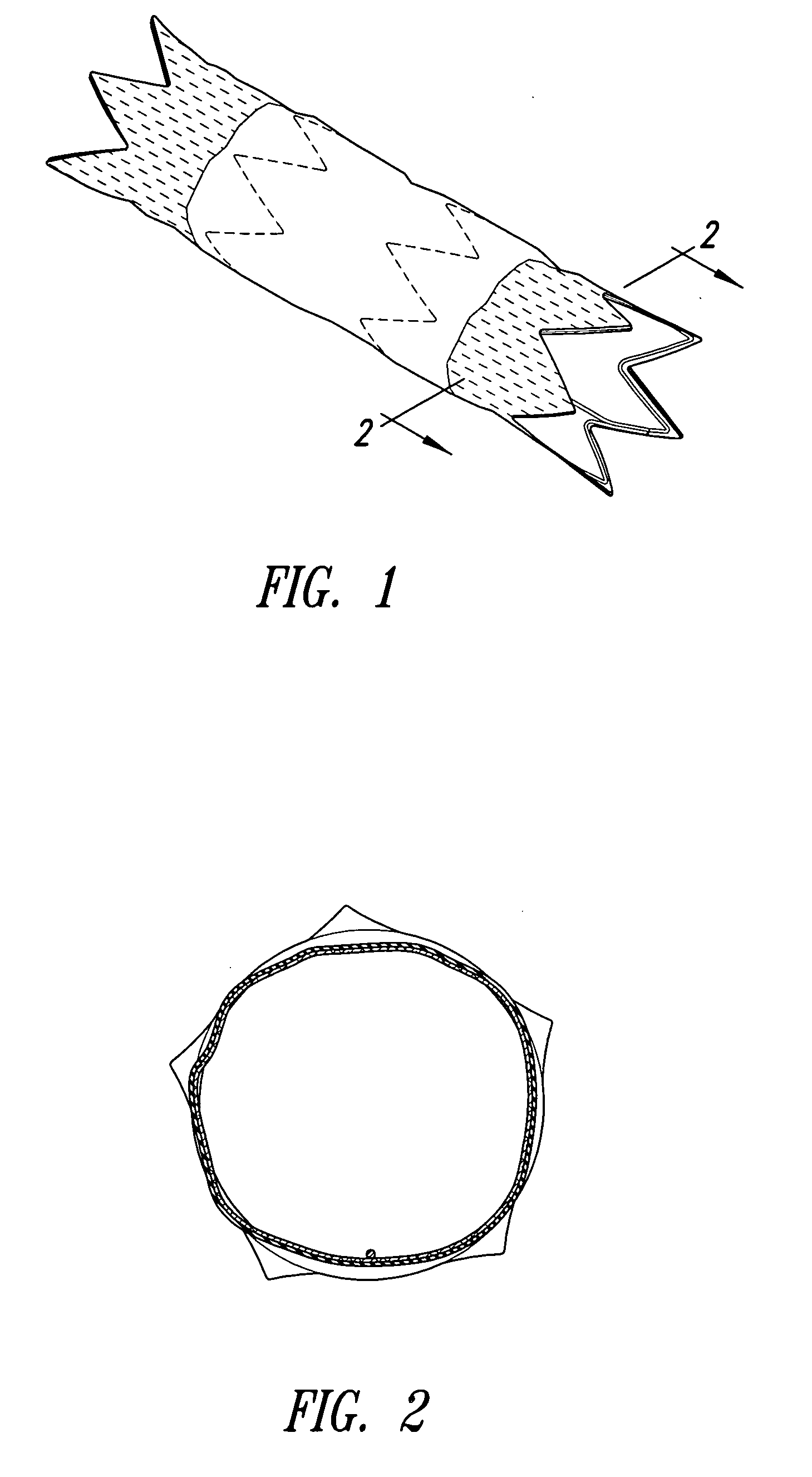
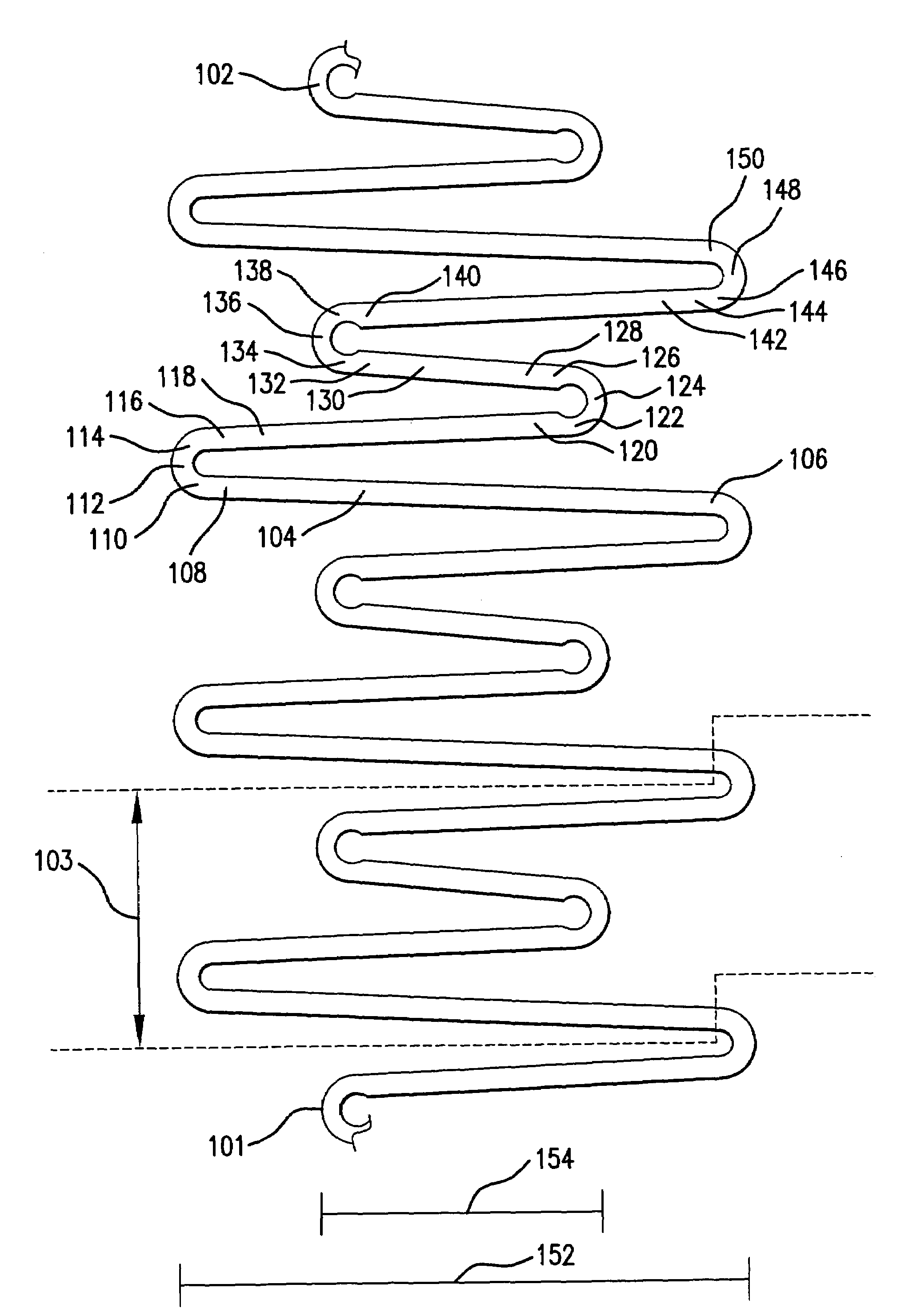
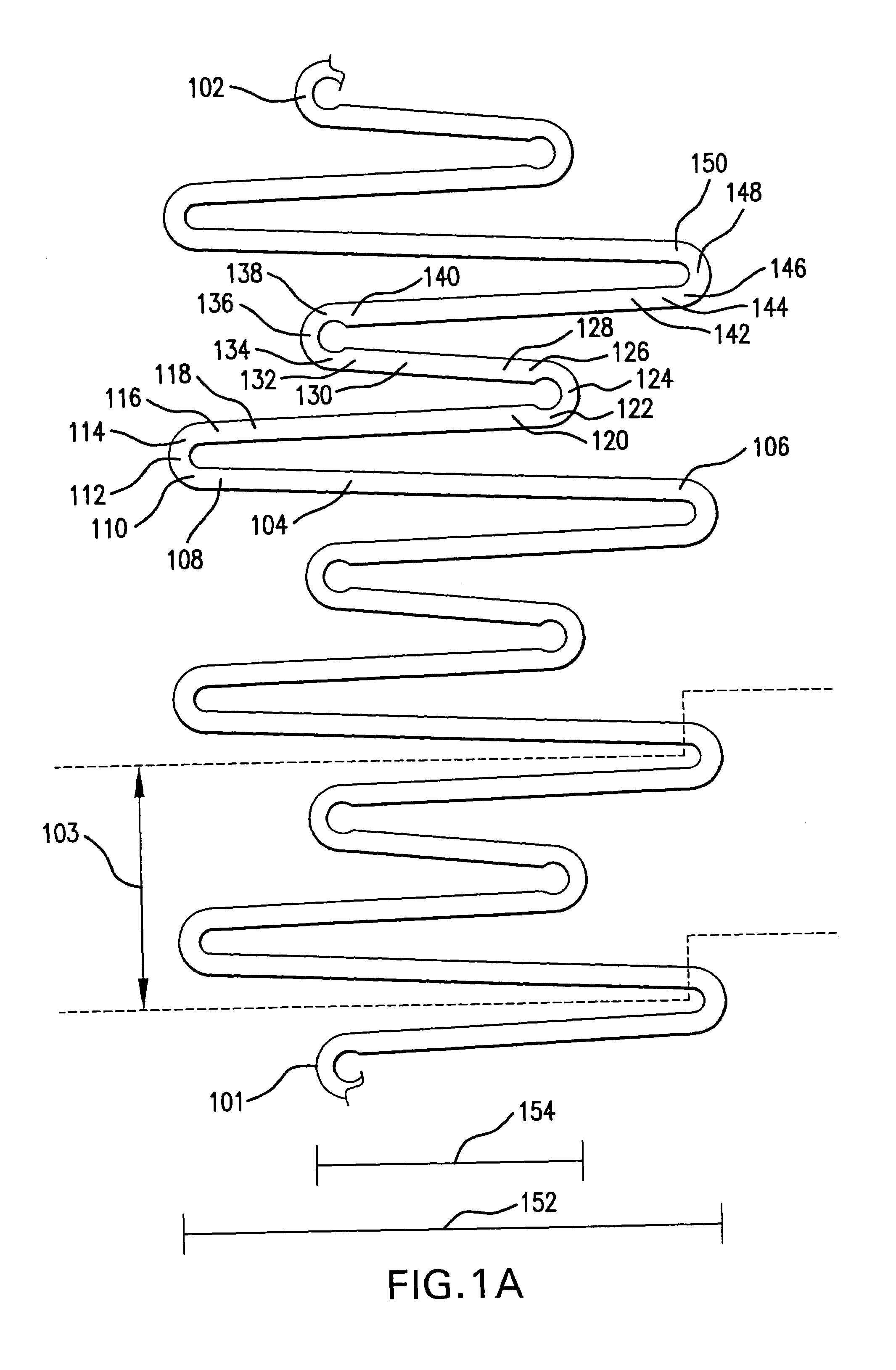
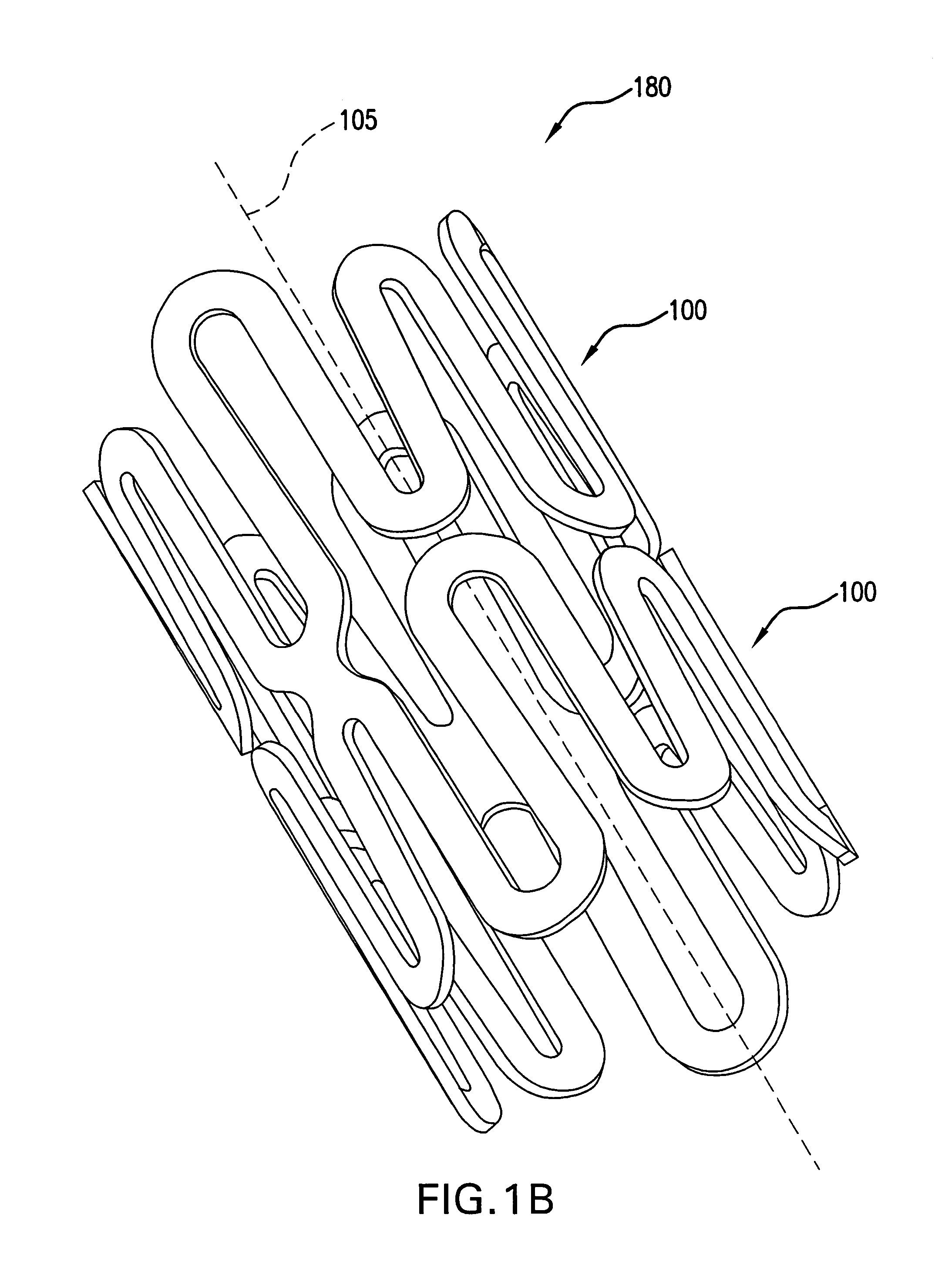
The present invention is an intralumenal stent device made up of two or more elements. Each element has undulations forming peaks and valleys and is formed from a repeating series including a long segment, a first midsized segment, a short segment, and a second midsized segment. These elements are aligned on a common axis and are connected directly to an adjacent element by a connection. The stent incorporates the advantages of having both long and short segments into a single element.
Owner:MEDTRONIC VASCULAR INC
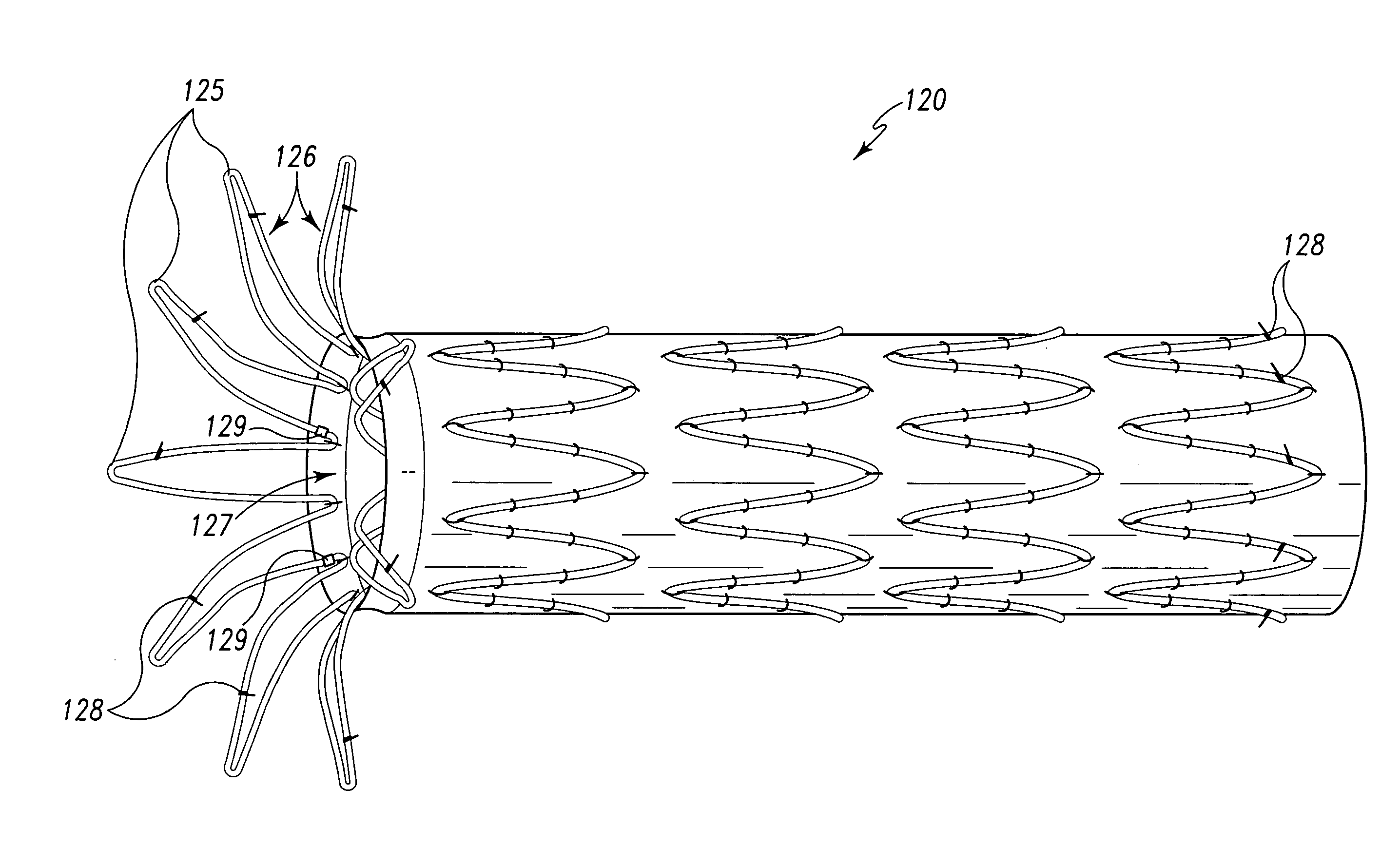
Flexible endoluminal stent and process of repairing a body lumen
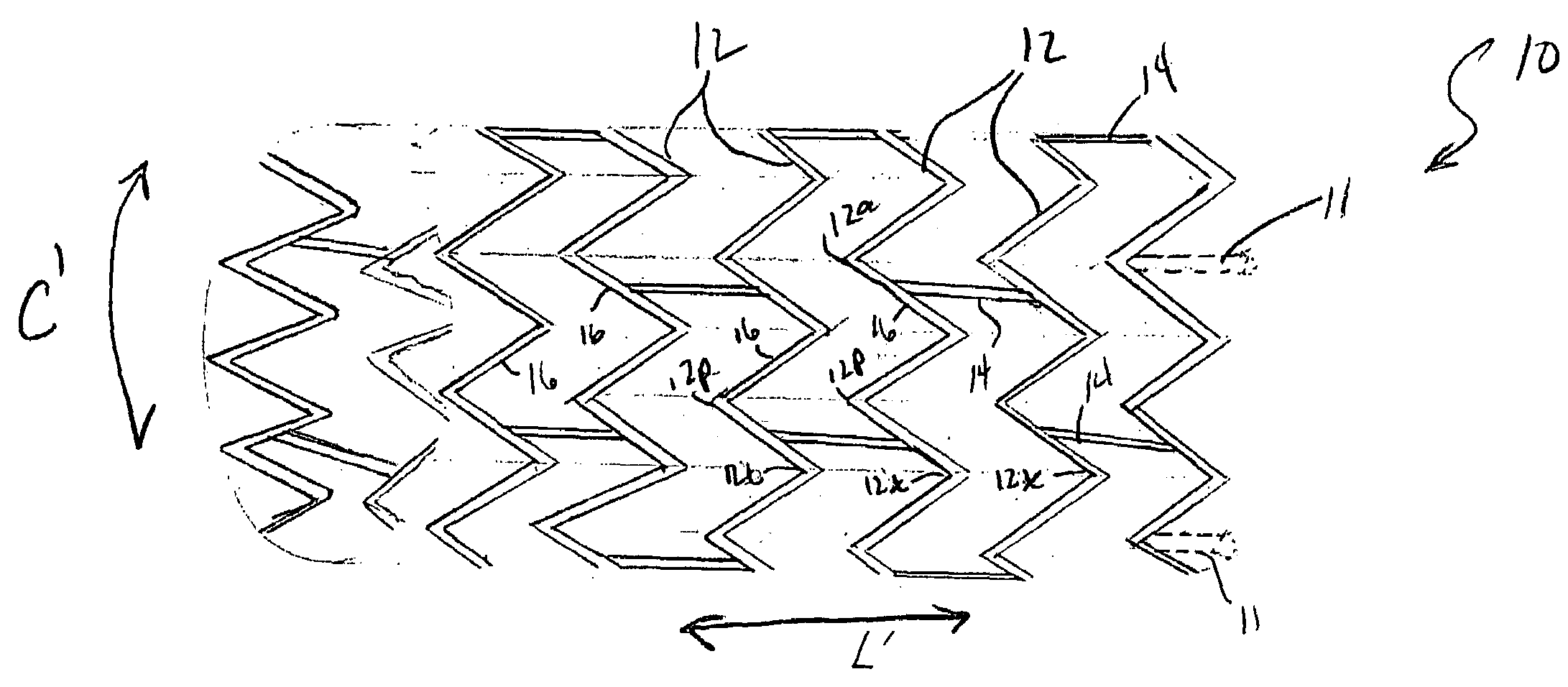
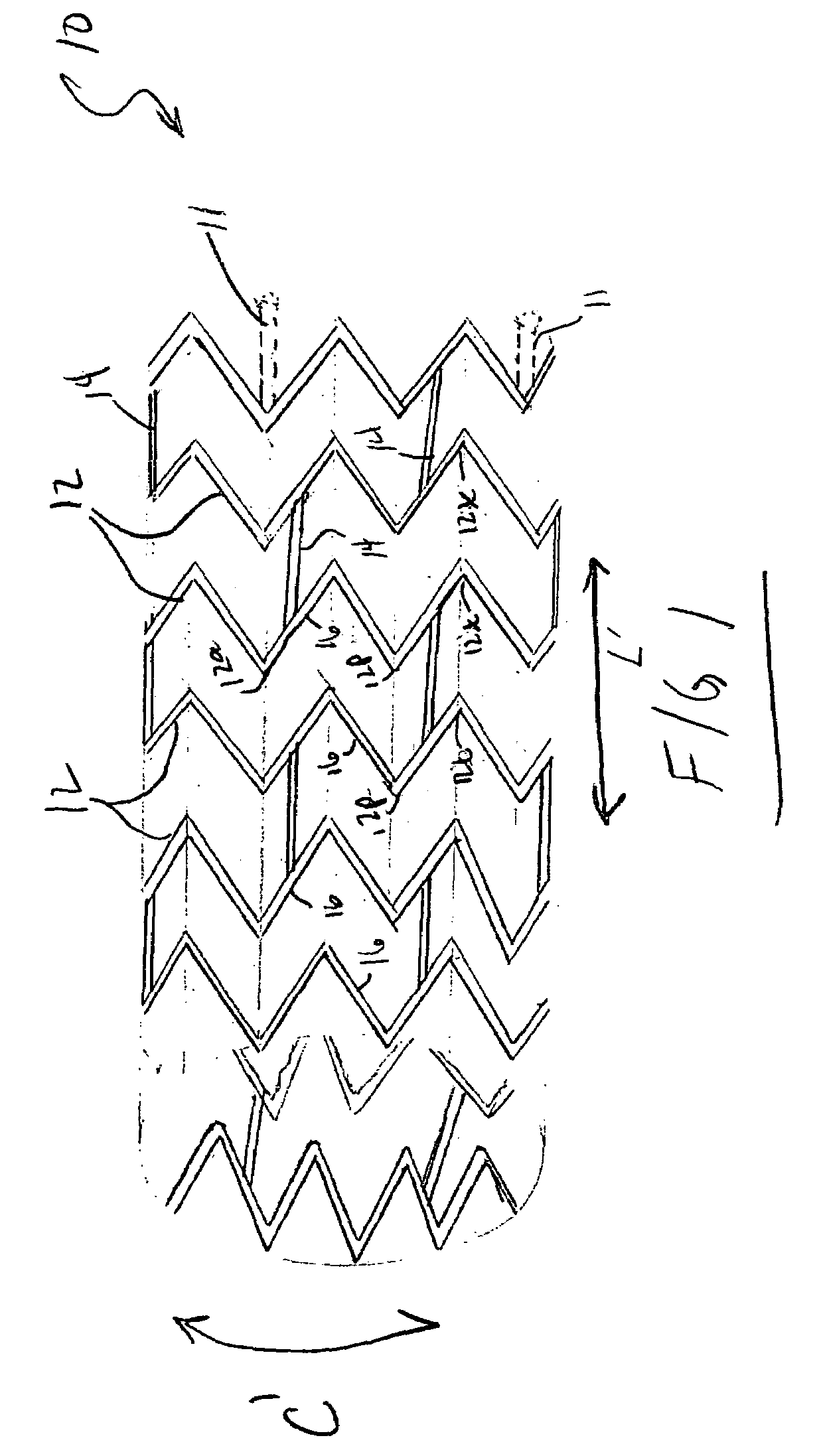
A generally tubular intraluminal compound stent comprising a plurality of component stents, each component stent having a length and a plurality of individual hoops axially disposed along the length and connected by a connecting spine, the component stents meshed with one another such that at least one hoop of one component stent is positioned between axially adjacent hoops of another component stent. The connecting spines may be helical, with at least one component stent spine oriented in a different helical direction than the spine of another component stent. Each hoop may further have a periphery comprising a pattern of zig-zags having apices, wherein adjacent hoops of the meshed component stents are aligned such that the apices of adjoining hoops abut or are interdigitated with one another. The compound stent may further comprise connectors, such as sutures, connecting at least some of the abutting or interdigitated apices. A process for manufacture of a flexible endoluminal compound stent is also disclosed.
Owner:SCI MED LIFE SYST
Drug-releasing stent with ceramic-containing layer
InactiveUS7713297B2Prevent proliferationPrevent restenosisStentsSurgeryEndoluminal stentAnti platelet
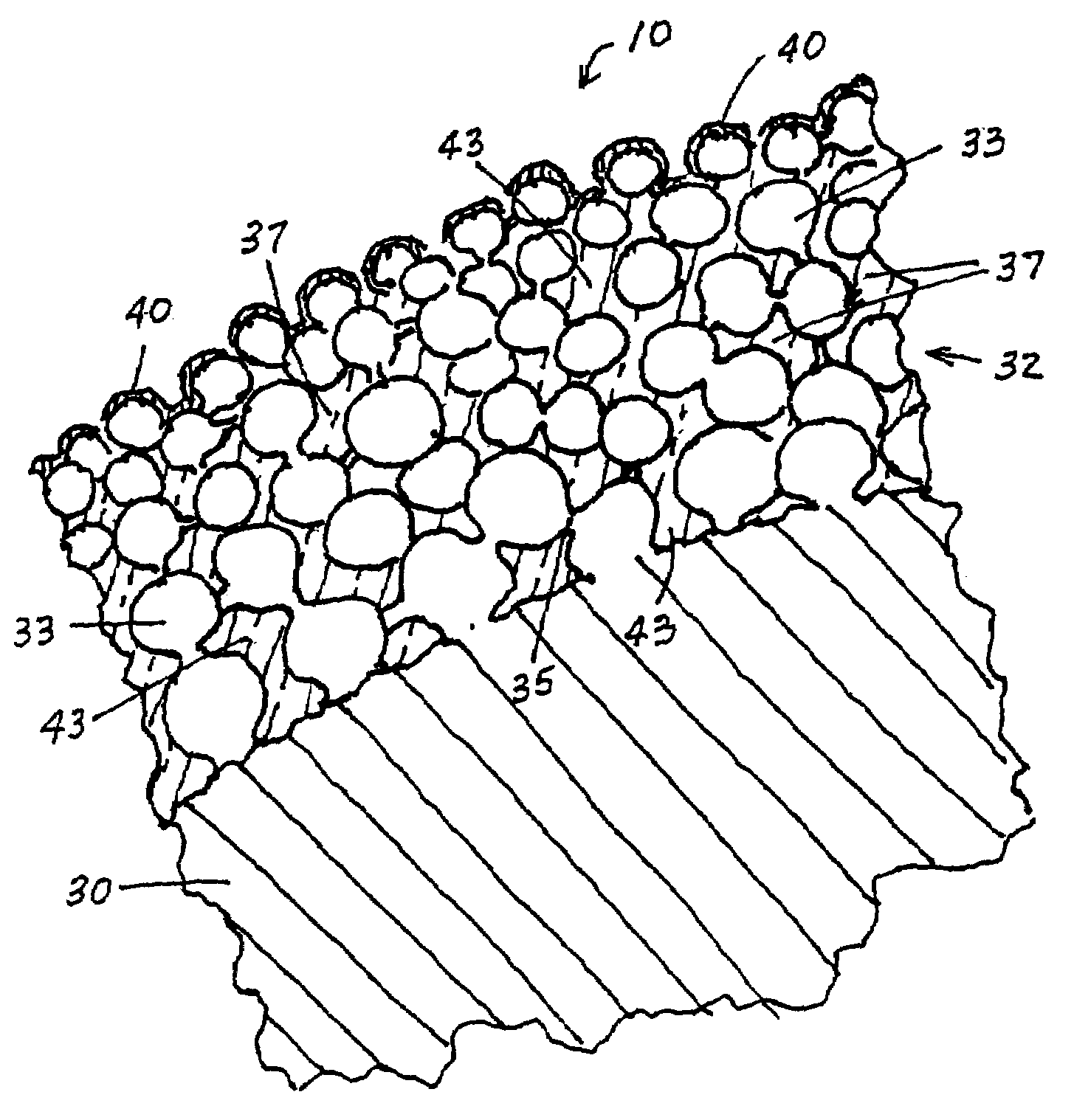
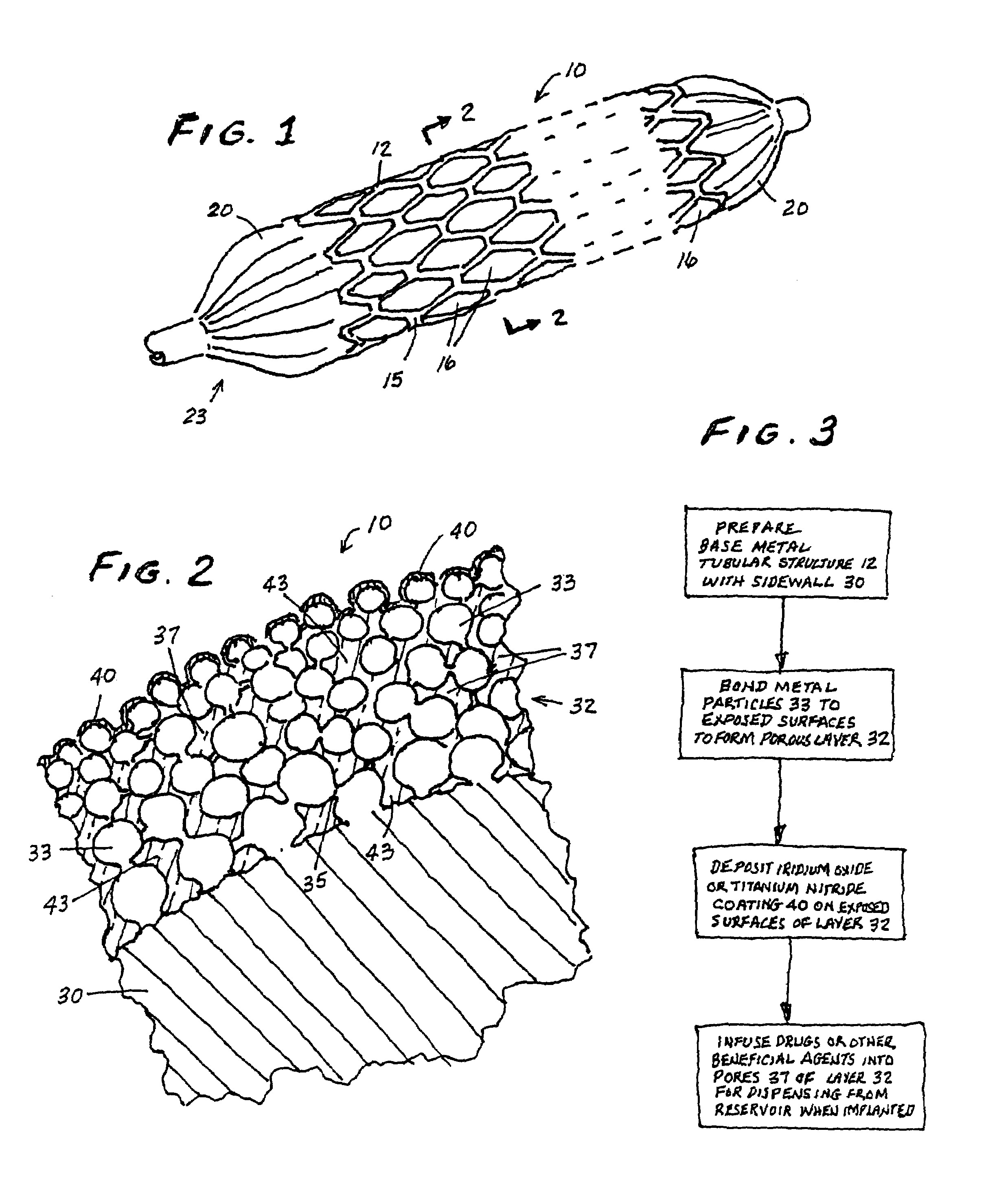
A vascular or endoluminal stent is adapted to be implanted in a vessel, duct or tract of a human body to maintain an open lumen at the site of the implant. The sidewall of the open-ended tubular structure of the stent is a base layer of a metal biologically compatible with blood and tissue of the human body. An intermediate metal particle layer of substantial greater radiopacity overlies the base layer, with particles bonded to the base layer and to each other to leave interstices therebetween as a repository for retaining and dispensing drugs or other agents for time release therefrom after the stent is implanted, to assist the stent in maintaining the lumen open. The particles are composed primarily of a noble metal—an alloy of platinum-iridium. The sidewall has holes extending therethrough, and the particle layer resides along the outward facing and inward facing surfaces, and the edges of the through holes and open ends of the sidewall. The larger particles are bonded to surfaces of the sidewall and progressively smaller particles are bonded to those and to each other up to the outer portion of the particle layer. Exposed surfaces of the particle layer are coated with ceramic-like iridium oxide or titanium nitrate, as a biocompatible material to inhibit irritation of tissue at the inner lining of the vessel when the stent is implanted. One or more anti-thrombotic, anti-platelet, anti-inflammatory and / or anti-proliferative drugs are retained in the interstices, together with a biodegradable carrier for time release therefrom. In an alternative embodiment, the intermediate layer is solid and the biodegradable carrier and drugs or agents therein are applied to the surface of the ceramic-like coating. Gene transfer is alternatively used to control tissue proliferation.
Owner:BOSTON SCI SCIMED INC
Intraluminal stent including therapeutic agent delivery pads, and method of manufacturing the same
An intraluminal stent, an intraluminal stent delivery system, and a method of manufacturing an intraluminal stent. The stent comprises a body and at least one pad operably attached to the stent body. The pad includes at least one therapeutic agent disposed thereon. The delivery system comprises a catheter and the stent disposed on the catheter. The manufacturing method includes providing a stent including a body, and at least one pad. The pad is operably attached to the stent body. The pad is treated with at least one therapeutic agent.
Owner:MEDTRONIC VASCULAR INC
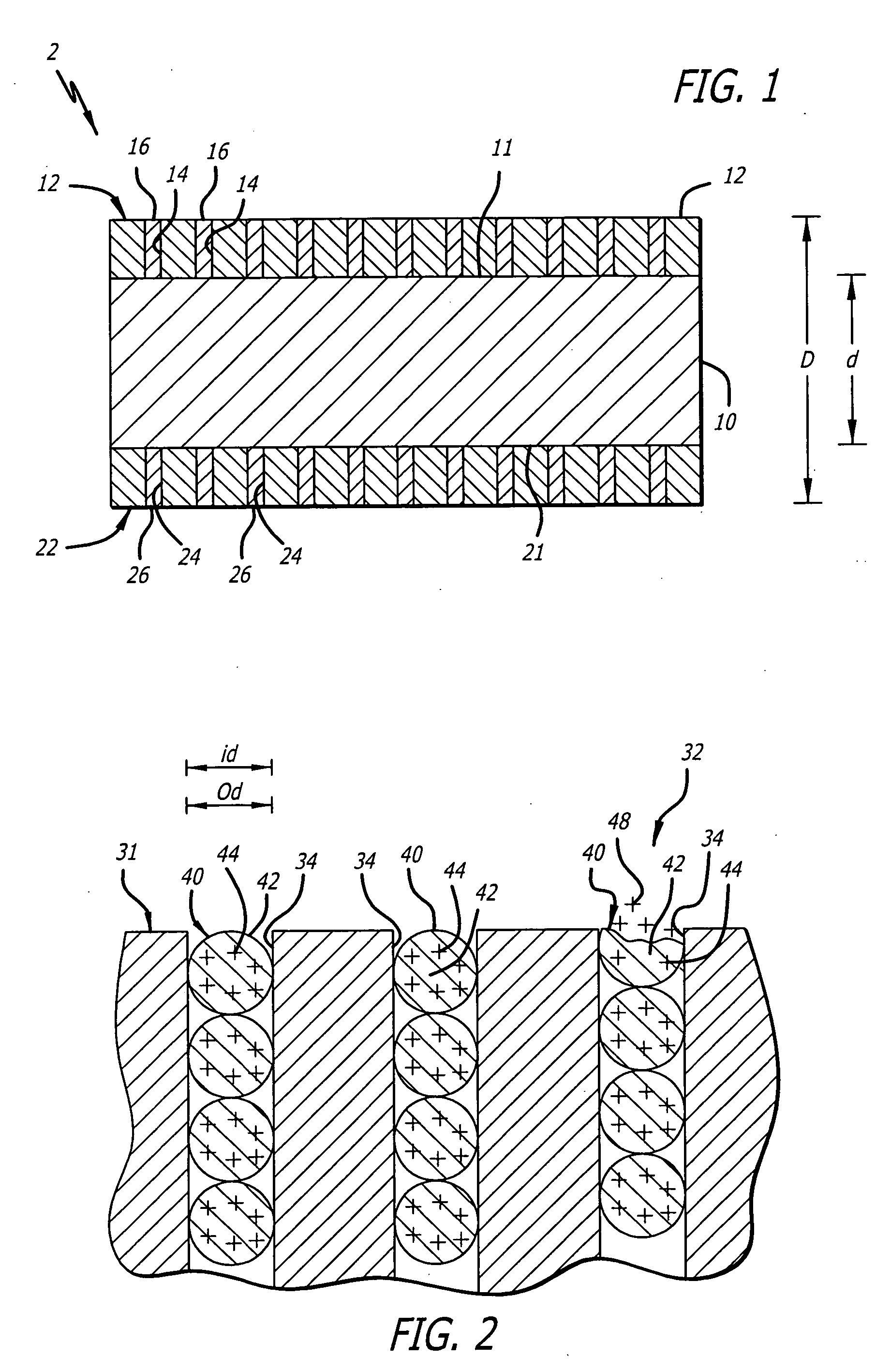
Self-supporting laminated films, structural materials and medical devices manufactured therefrom and methods of making same
Metal foils, wires, and seamless tubes with increased mechanical strength are provided. As opposed to wrought materials that are made of a single metal or alloy, these materials are made of two or more layers forming a laminate structure. Laminate structures are known to increase mechanical strength of sheet materials such as wood and paper products and are used in the area of thin films to increase film hardness, as well as toughness. Laminate metal foils have not been used or developed because the standard metal forming technologies, such as rolling and extrusion, for example, do not lend themselves to the production of laminate structures. Vacuum deposition technologies can be developed to yield laminate metal structures with improved mechanical properties. In addition, laminate structures can be designed to provide special qualities by including layers that have special properties such as superelasticity, shape memory, radio-opacity, corrosion resistance etc. Examples of articles which may be made by the inventive laminate structures include implantable medical devices that are fabricated from the laminated deposited films and which present a blood or body fluid and tissue contact surface that has controlled heterogeneities in material constitution. An endoluminal stent-graft and web-stent that is made of a laminated film material deposited and etched into regions of structural members and web regions subtending interstitial regions between the structural members. An endoluminal graft is also provided which is made of a biocompatible metal or metal-like material. The endoluminal stent-graft is characterized by having controlled heterogeneities in the stent material along the blood flow surface of the stent and the method of fabricating the stent using vacuum deposition methods.
Owner:VACTRONIX SCI LLC
Fenestrated endoluminal stent system
ActiveUS7833259B2Encourage tissue prolapseMinimally invasive procedureStentsBlood vesselsInsertion stentMinimally invasive procedures
An intraluminal prosthesis is provided for strengthening a main lumen and a branch lumen in direct fluid communication with the main lumen. The prosthesis may comprise a tubular graft and a flexible stent. The tubular graft may include a flexible body having a wall with a fenestration. The flexible stent may include a body with at least one open space to encourage tissue prolapse. The flexible stent may be configured for intraluminal coupling to the fenestration of the tubular graft, so that the entire prosthesis may be assembled and anchored into a main lumen and a branch lumen with a minimally invasive procedure. The open space may encourage tissue prolapse, which may act to anchor the flexible stent to the branch lumen.
Owner:COOK MEDICAL TECH LLC
Methods of treating a condition of a vessel in a patient
Devices and methods for treating veins and venous conditions, such as chronic cerebrospinal venous insufficiency, are provided. In one aspect, the disclosed subject matter provides an intraluminal scaffold having a generally tubular body with a lumen defined therethrough, the tubular body having a compressed condition for delivery and an expanded condition for implant within a vessel having a distended portion, at least a length of the tubular body configured to form an enlarged portion in the expanded condition to engage a wall of the distended portion of the vessel. Methods for fabricating and using the scaffold, methods for remodeling a vein, and methods of deploying a medical device in a vessel without negatively impacting the function of a valve of the vessel, are also provided.
Owner:ABBOTT CARDIOVASCULAR
Stent for blood vessel and material for stent for blood vessel
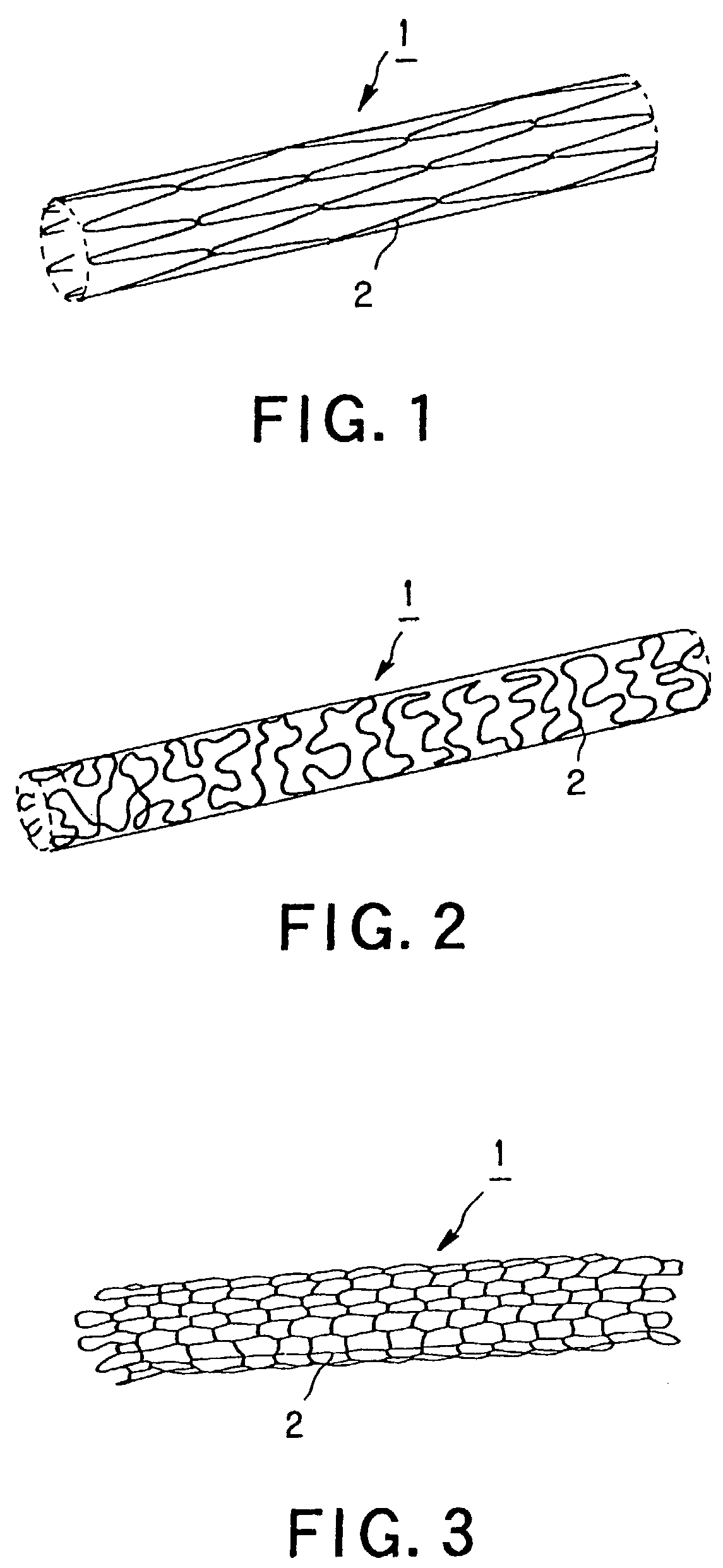
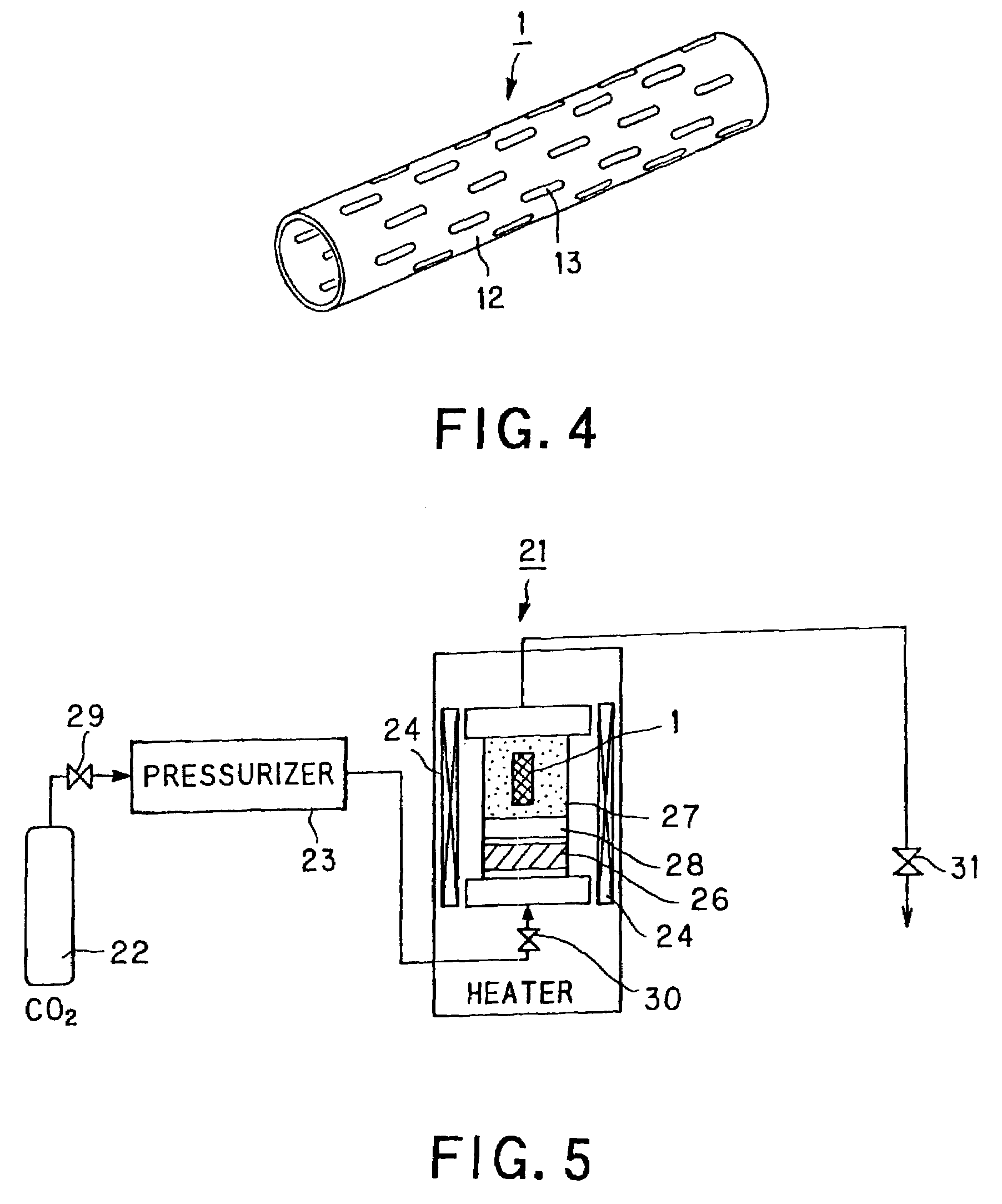
A luminal stent, implanted and implanted and left in the blood vessel, is disclosed. By permitting a stent (1), formed of a biodegradable polymer material (2), to be swollen, and by impregnating the swollen stent (1) with a drug, a sufficient quantity of the drug is impregnated in the stent. This drug is continuously released into the blood vessel over a prolonged time. A biodegradable polymer layer is formed on the surface of the stent (1) impregnated with the drug, and the release rate of the drug impregnated in the stent is controlled.
Owner:KYOTO MEDICAL PLANNING
Endoluminal device exhibiting improved endothelialization and method of manufacture thereof
An implantable endoluminal device which is fabricated from materials which present a blood or body fluid and tissue contact surface which has controlled heterogeneities in material constitution. An endoluminal stent which is made of a material having controlled heterogeneities in the stent material along the blood flow surface of the stent and the method of fabricating the stent using vacuum deposition methods.
Owner:VACTRONIX SCI LLC
Stent grafts with bioactive coatings
Stent grafts are provided comprising an endoluminal stent and a graft, wherein the stent graft releases an agent which induces the in vivo adhesion of the stent graft to vessel walls, or, otherwise induces or accelerates an in vivo fibrotic reaction causing said stent graft to adhere to vessel wall. Also provided are methods for making and using such stent grafts.
Owner:ANGIOTECH INERNAT
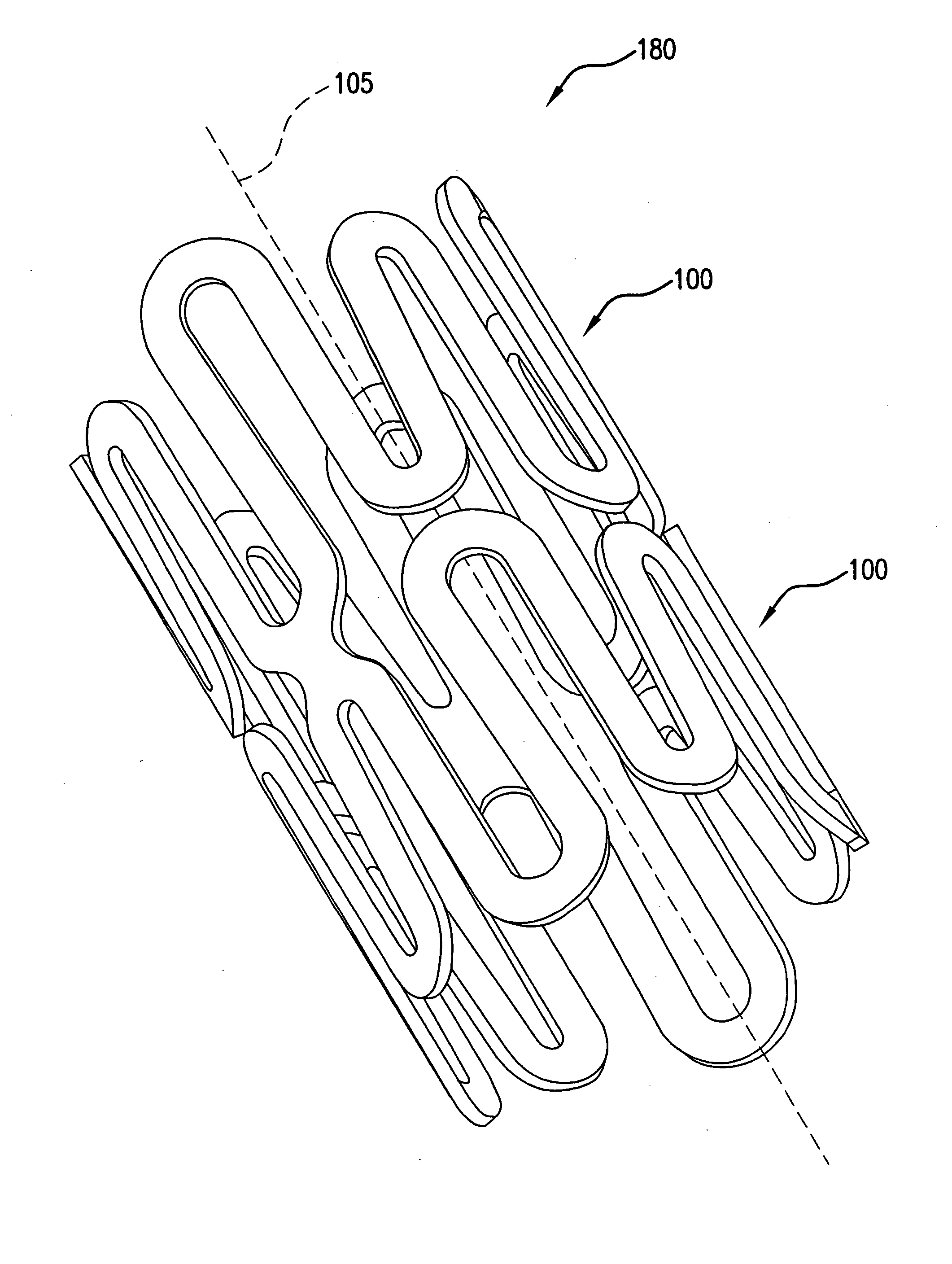
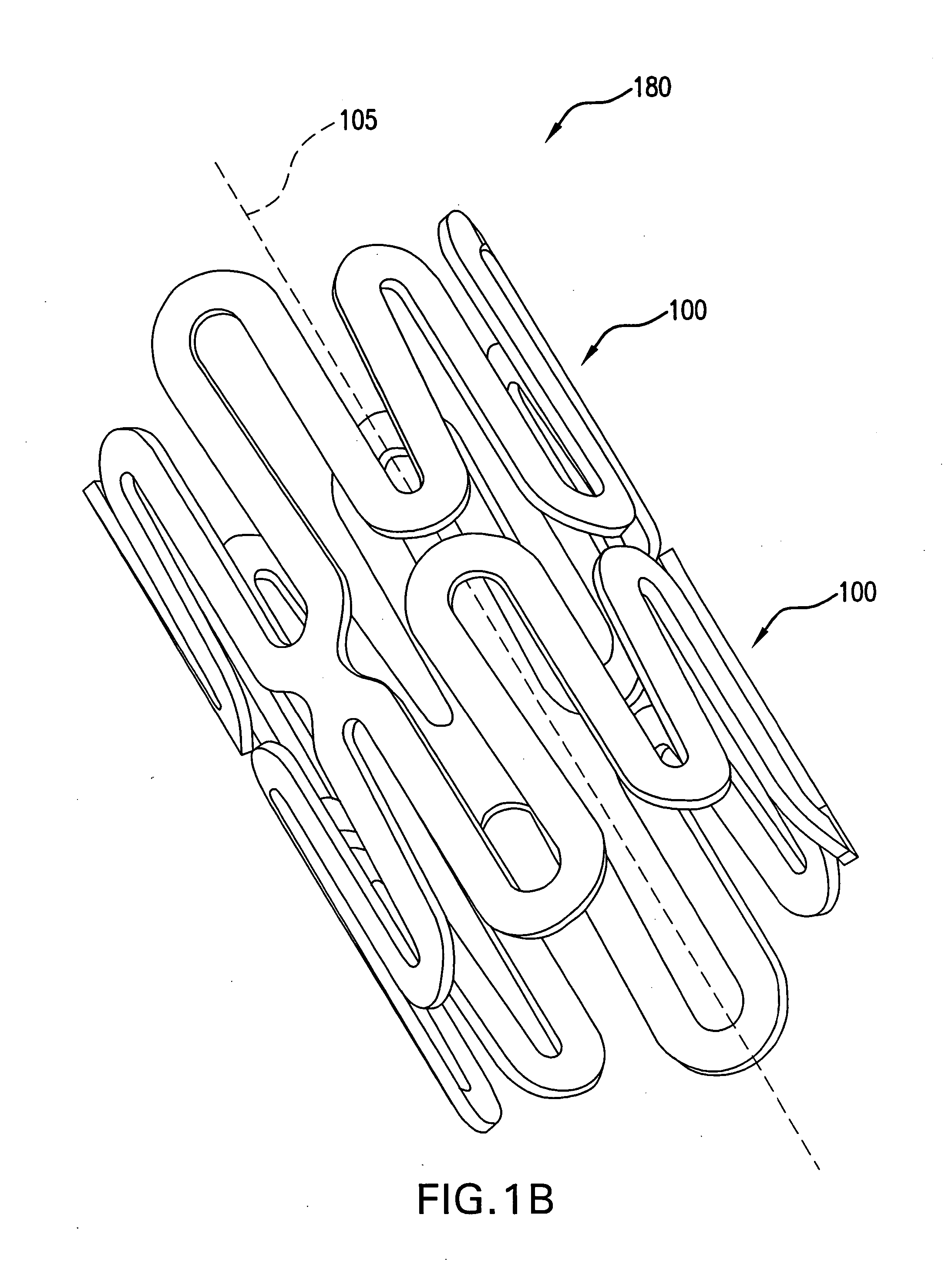
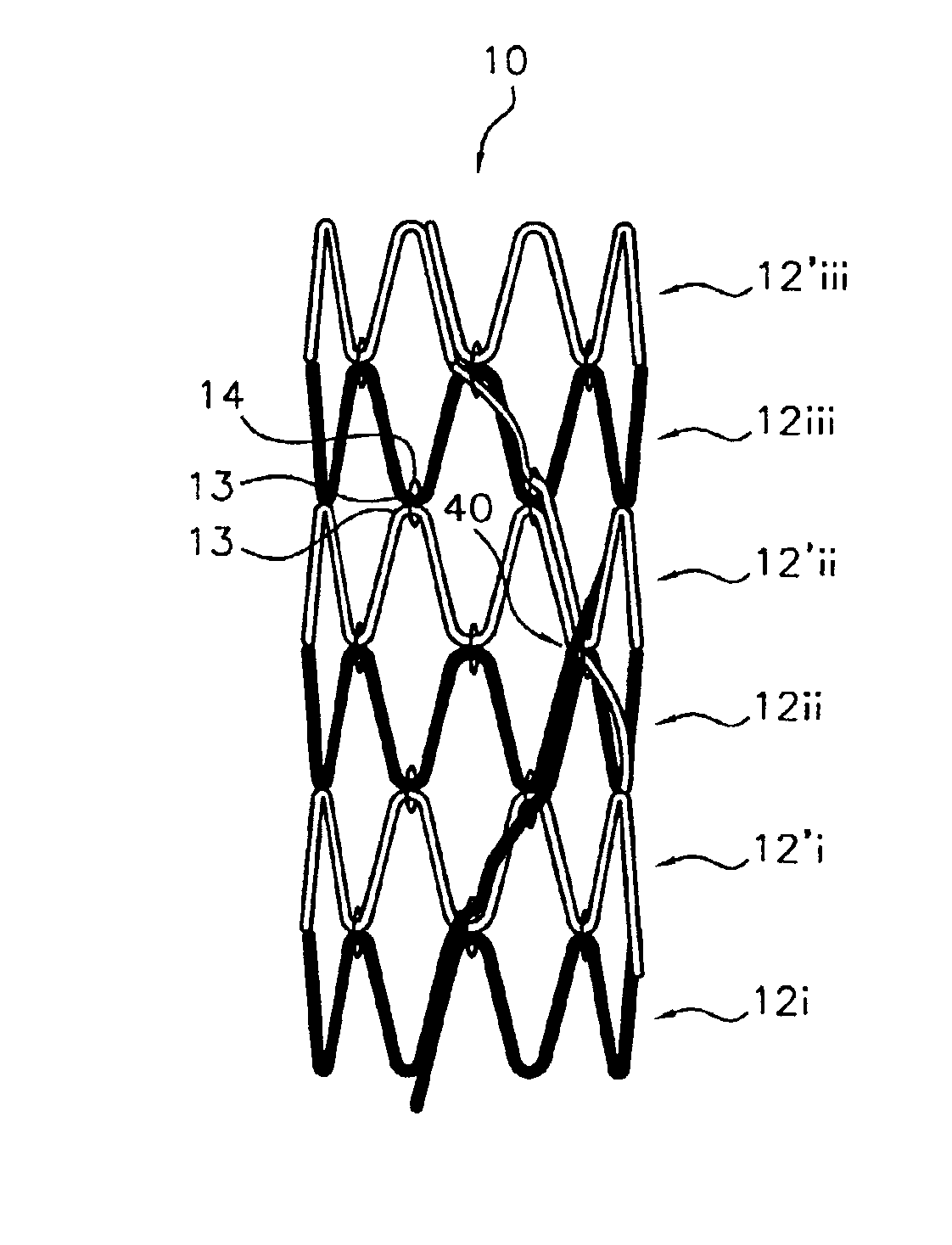
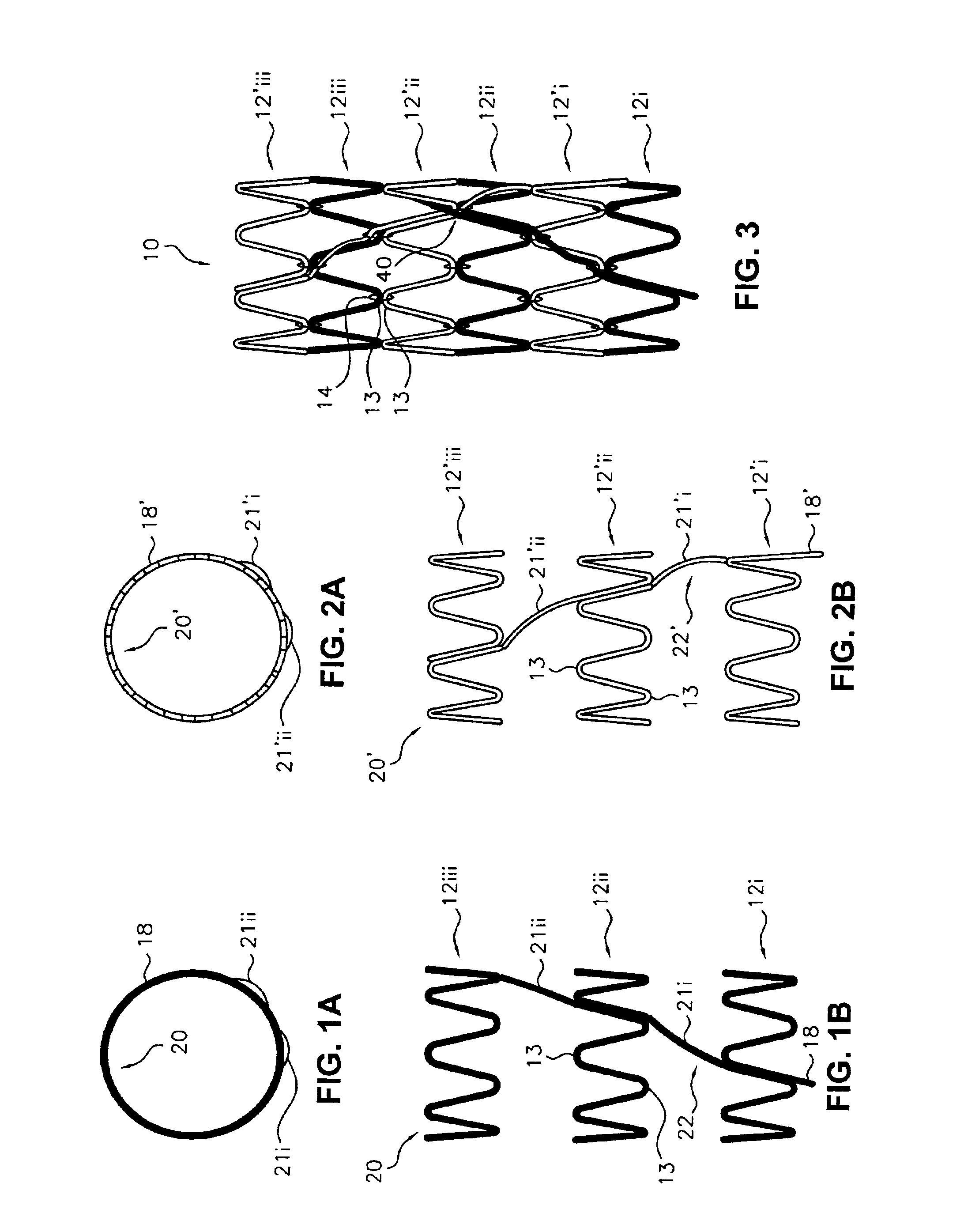
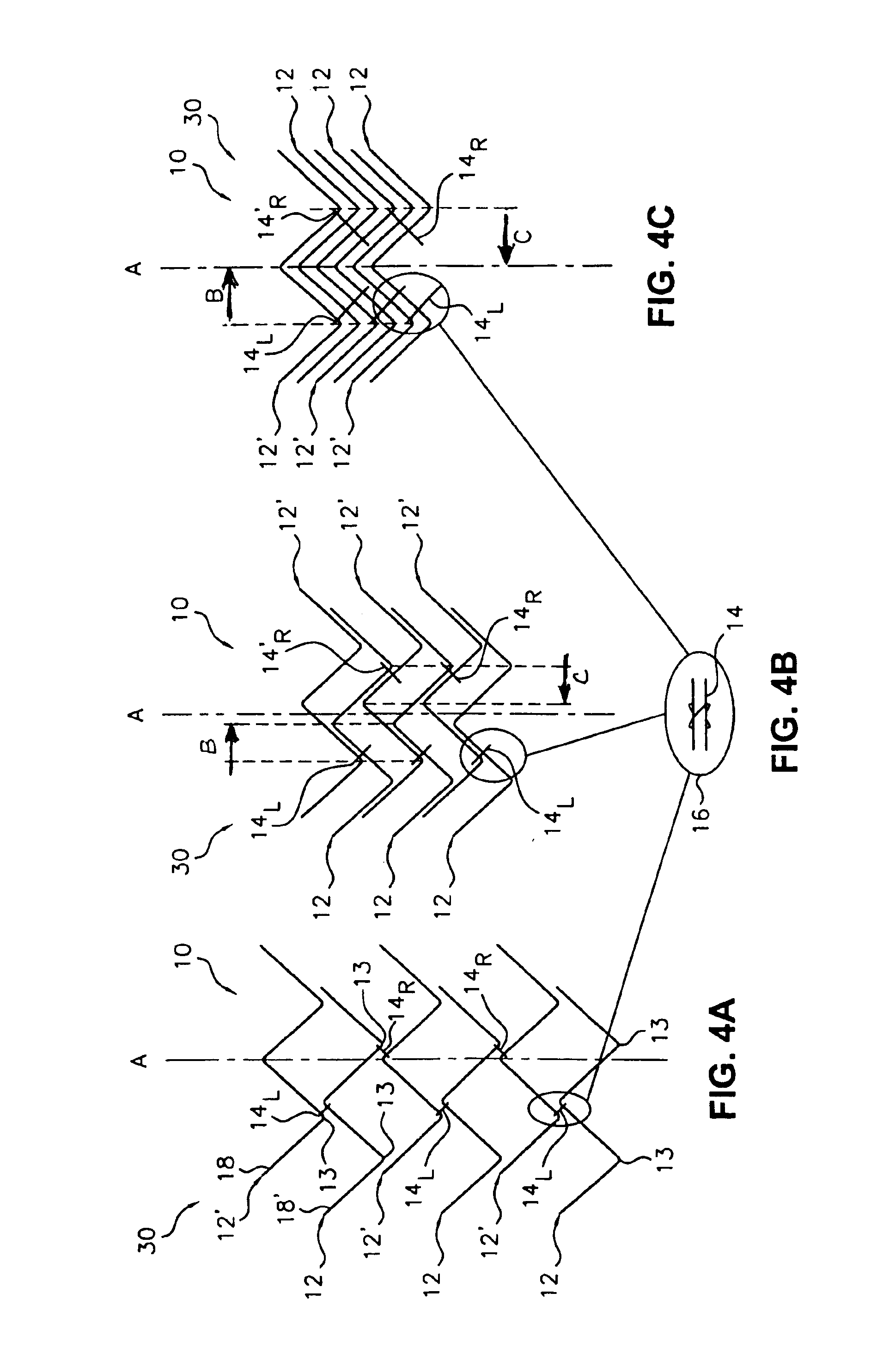
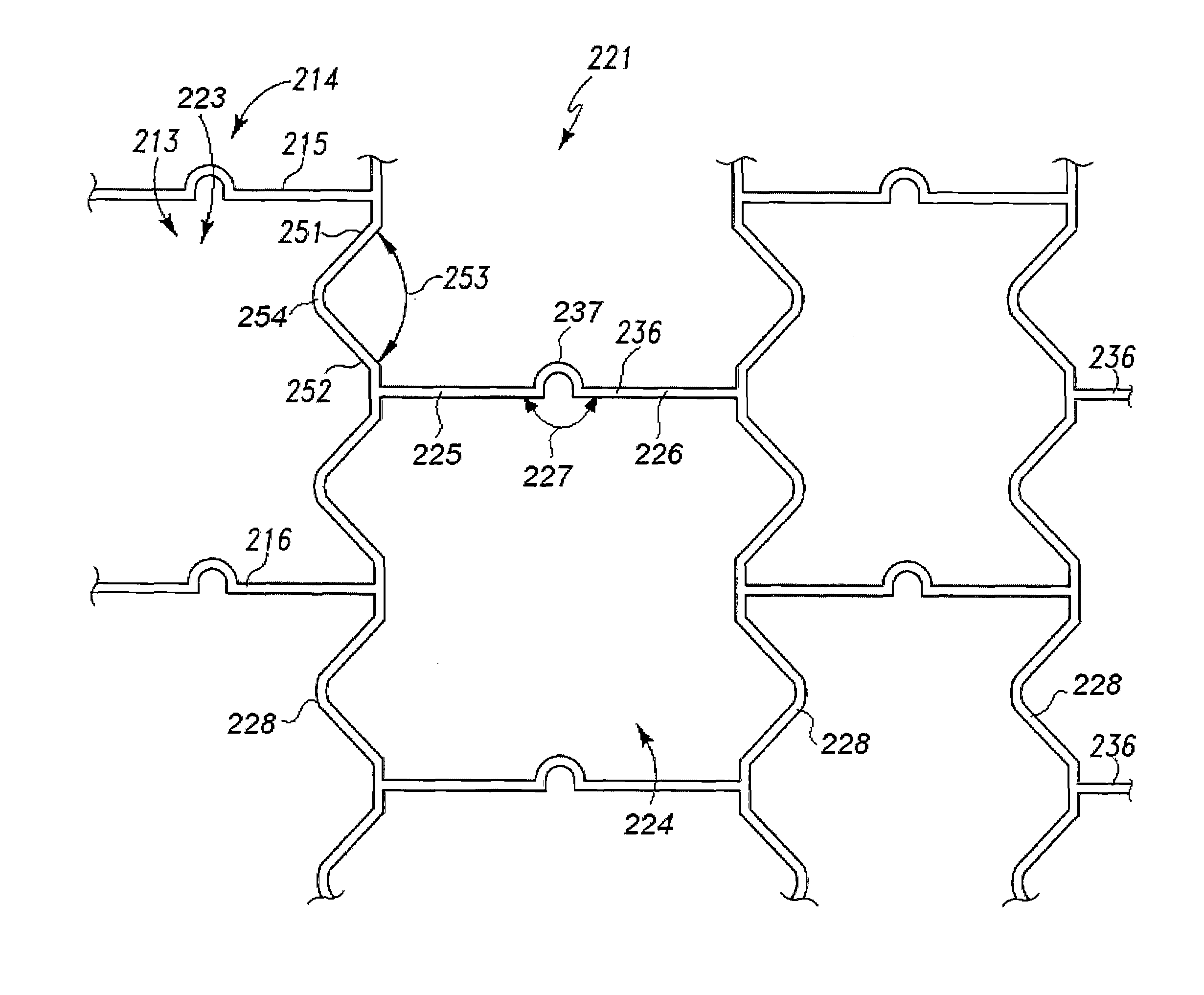
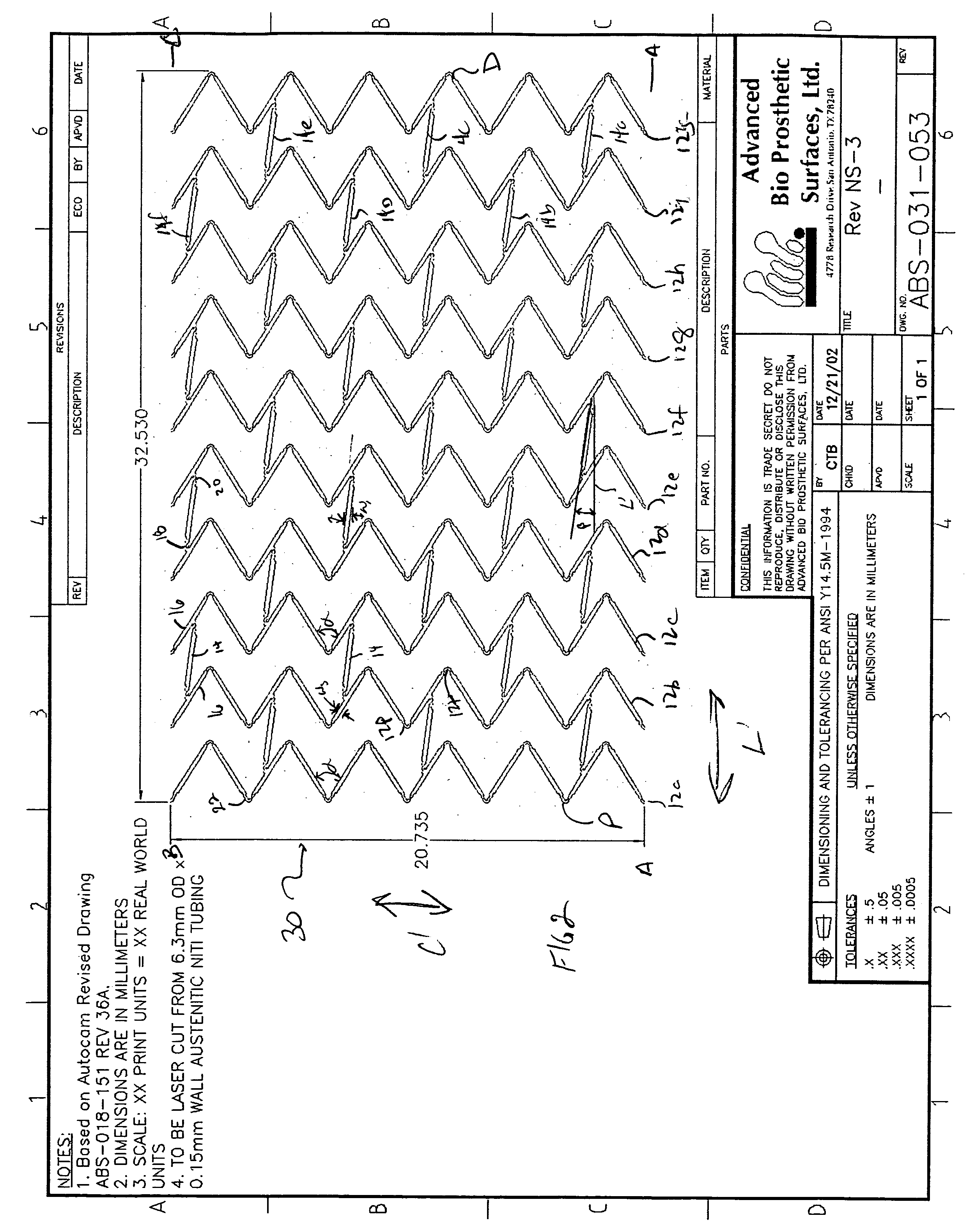
Endoluminal stent having mid-strut interconnecting members
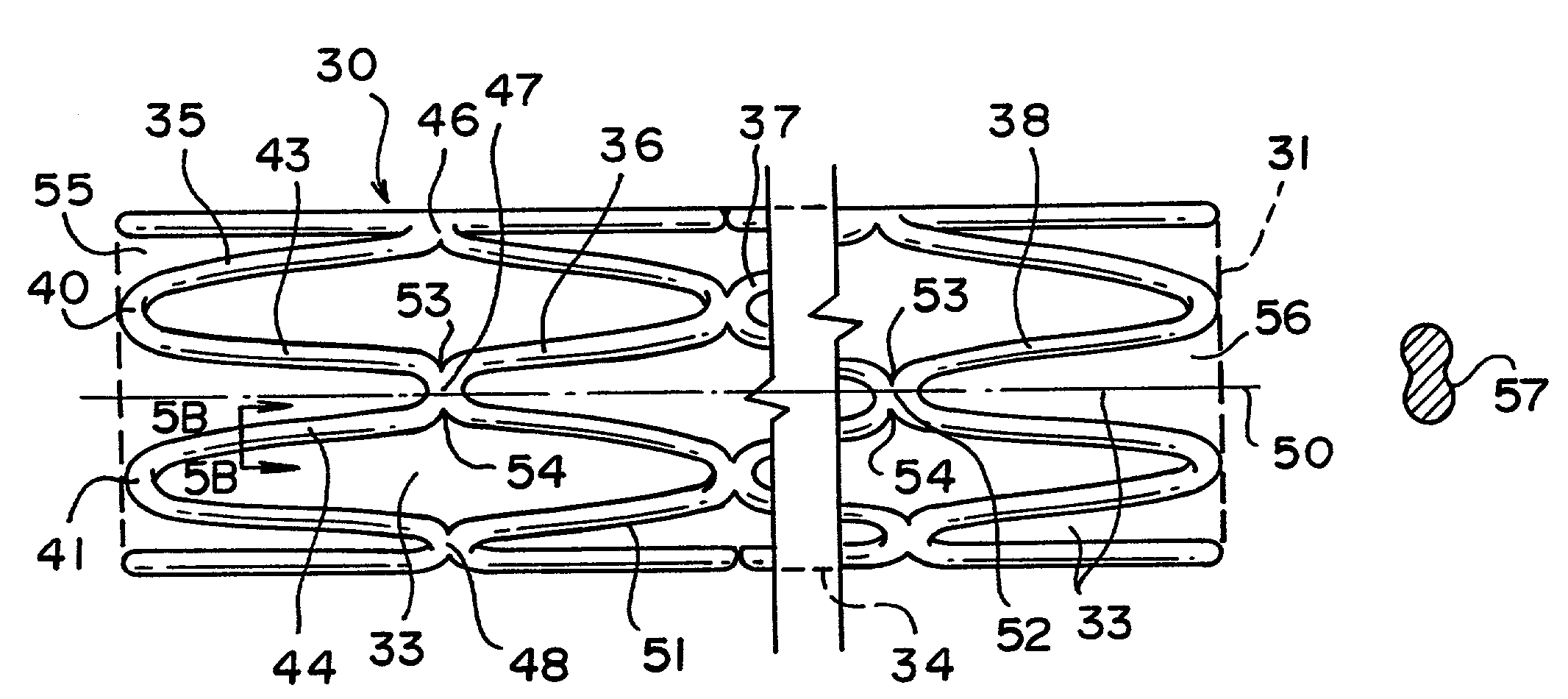
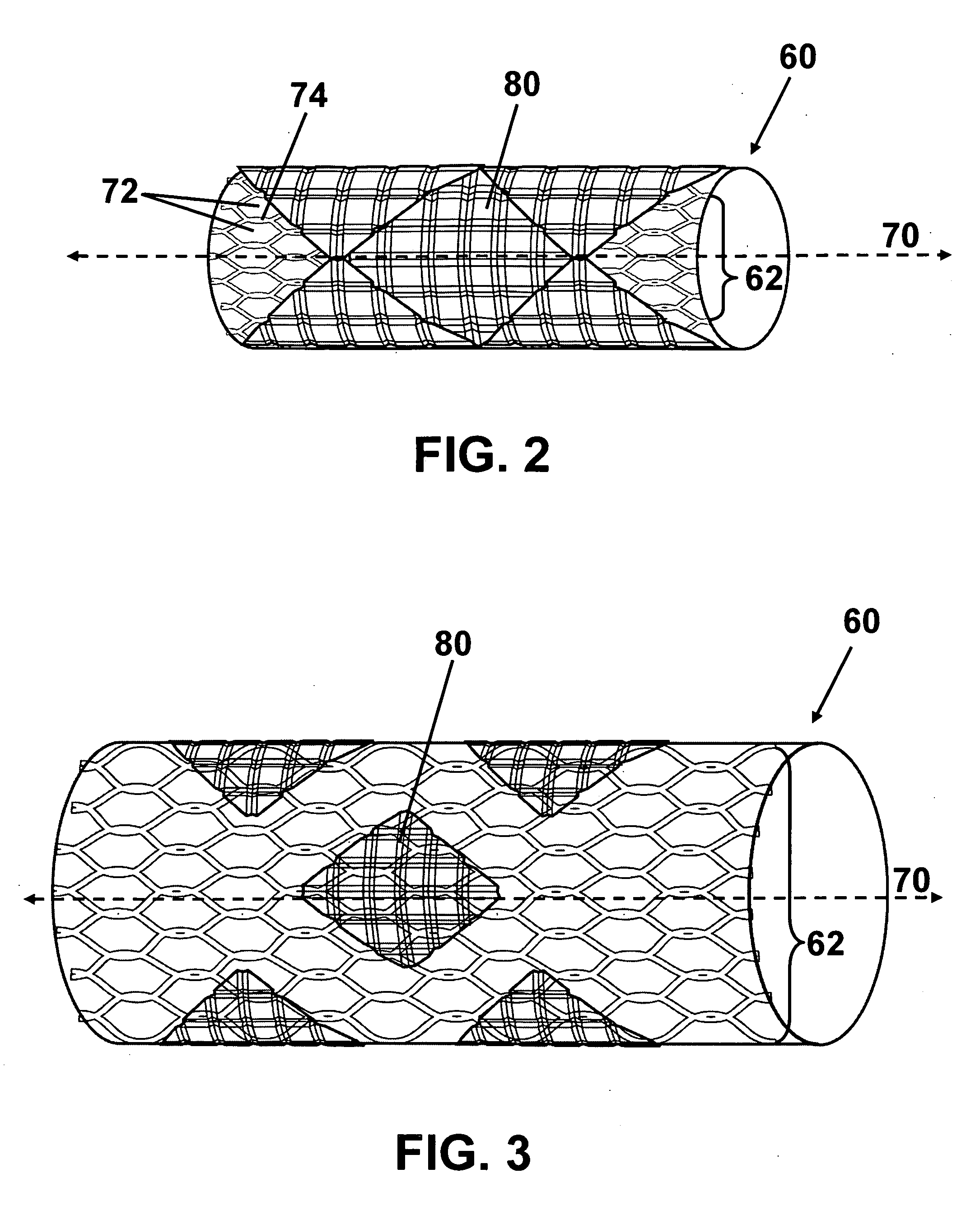
ActiveUS7122049B2Function optimizationMaximize functionalityStentsBlood vesselsEndoluminal stentEngineering
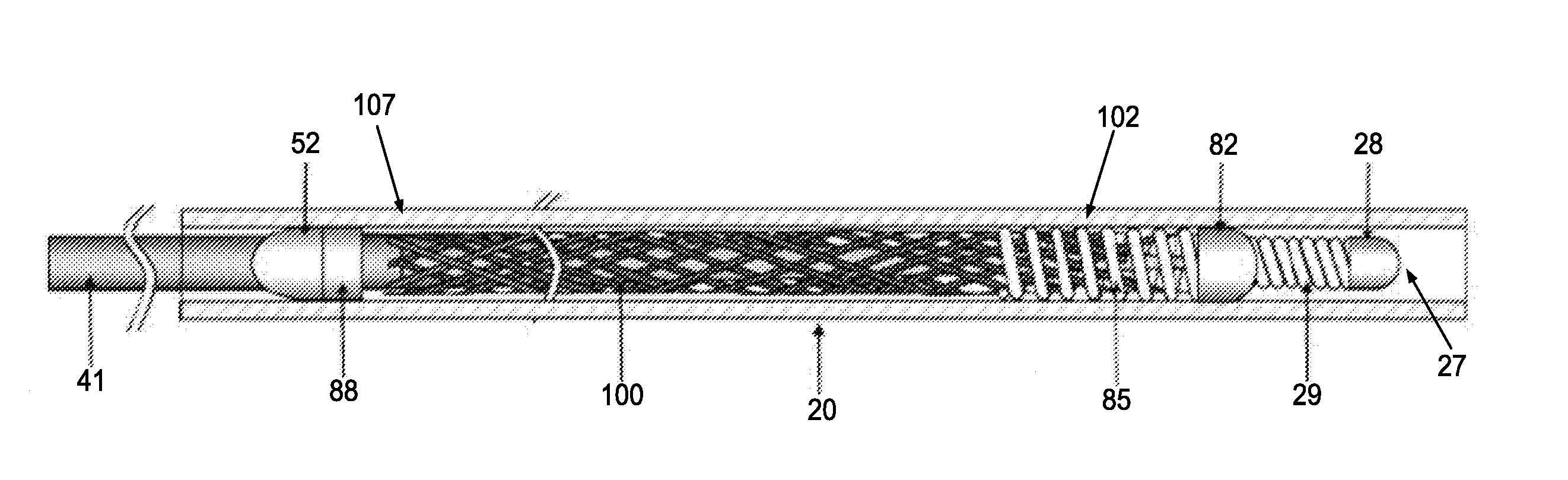
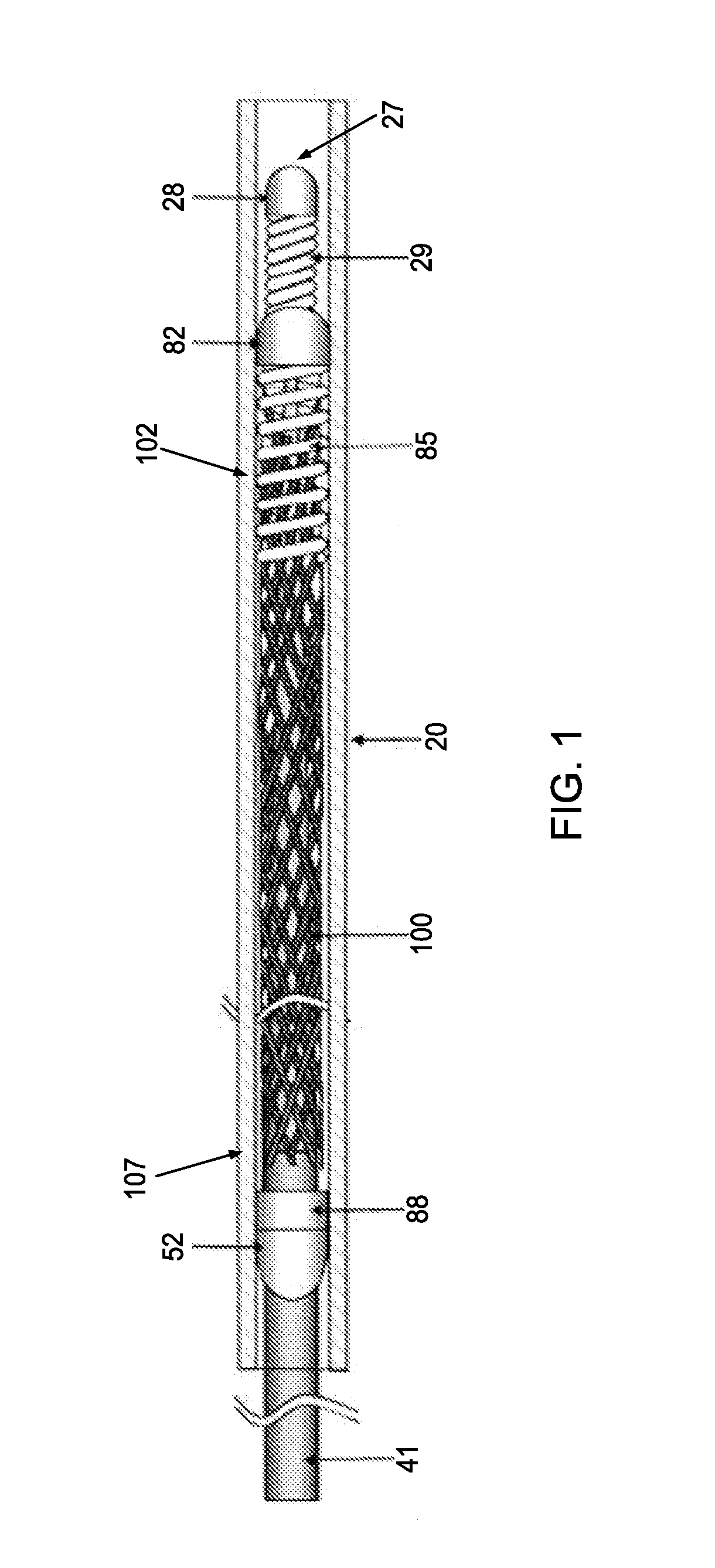
An endoluminal stent composed of a plurality of circumferential expansion elements arrayed to form the circumference of the stent and extending along the longitudinal axis of the stent, and a plurality of interconnecting members that interconnect adjacent pairs of circumferential expansion elements, the interconnecting members joining struts of adjacent pairs of interconnecting members at approximate mid-points of the struts.
Owner:VACTRONIX SCI LLC
Intralumenal stent device for use in body lumens of various diameters
The present invention is an intralumenal stent device made up of two or more elements. Each element has undulations forming peaks and valleys and is formed from a repeating series including a long segment, a first midsized segment, a short segment, and a second midsized segment. These elements are aligned on a common axis and are connected directly to an adjacent element by a connection. The stent incorporates the advantages of having both long and short segments into a single element.
Owner:MEDTRONIC VASCULAR INC
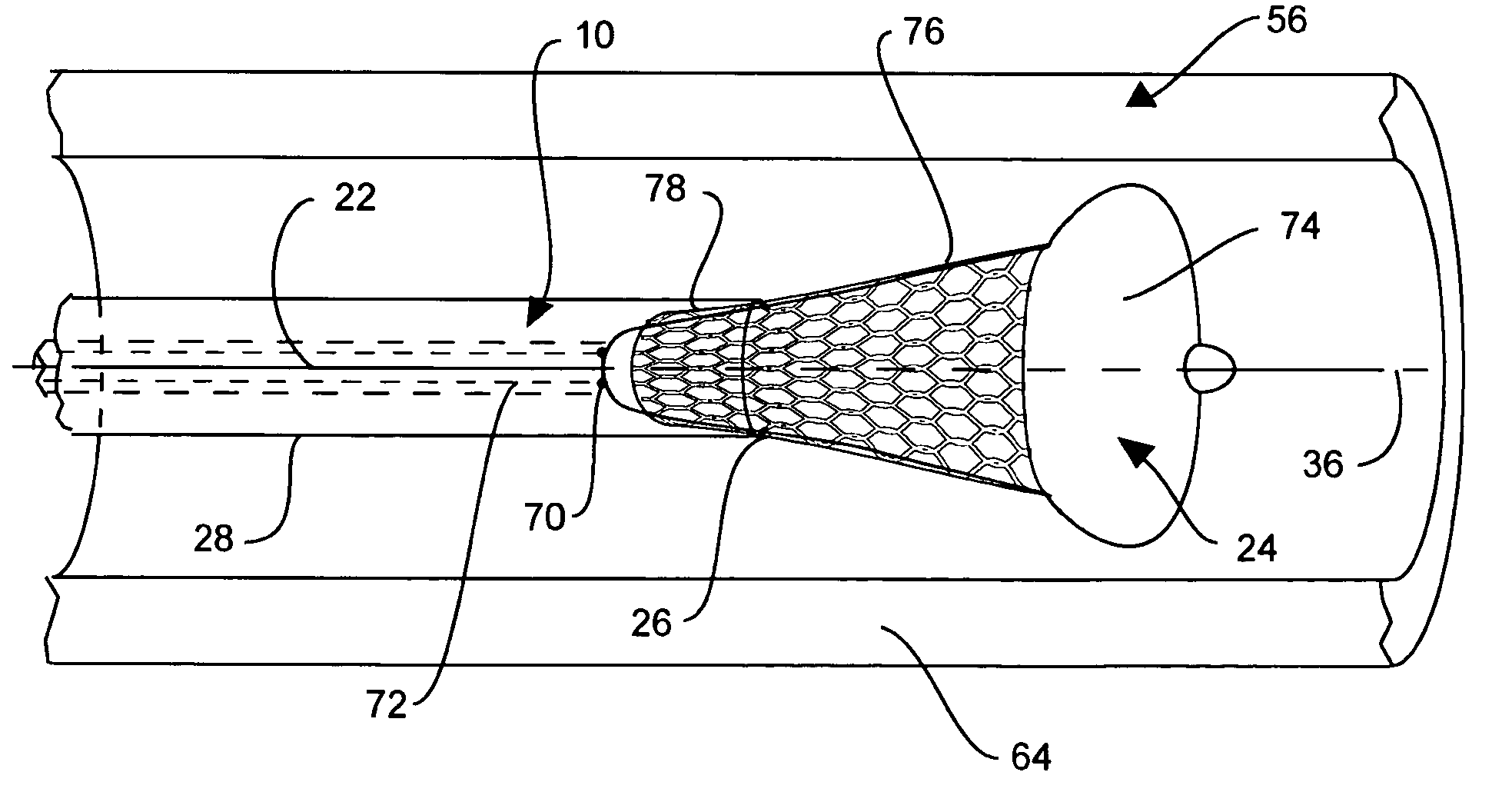
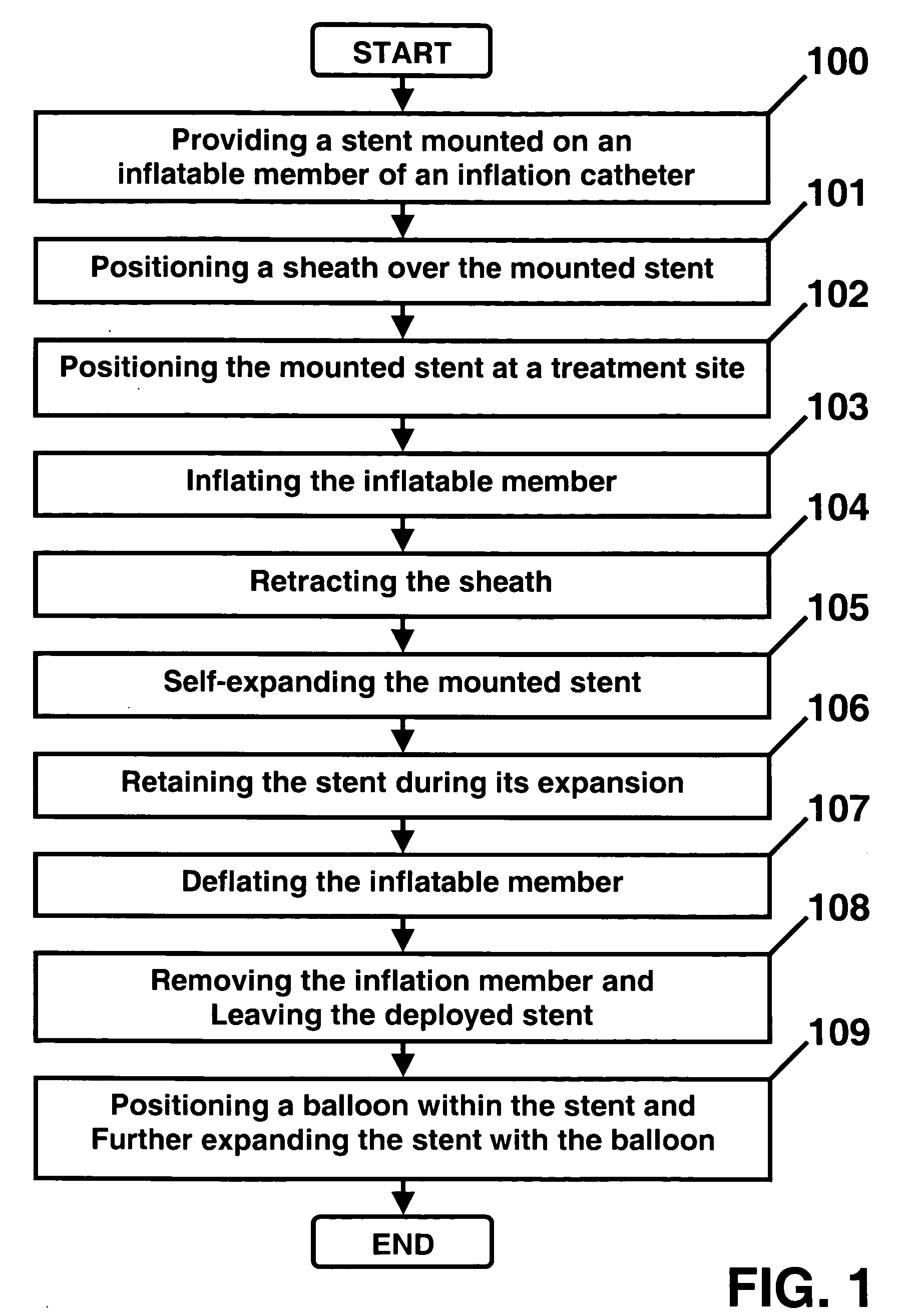
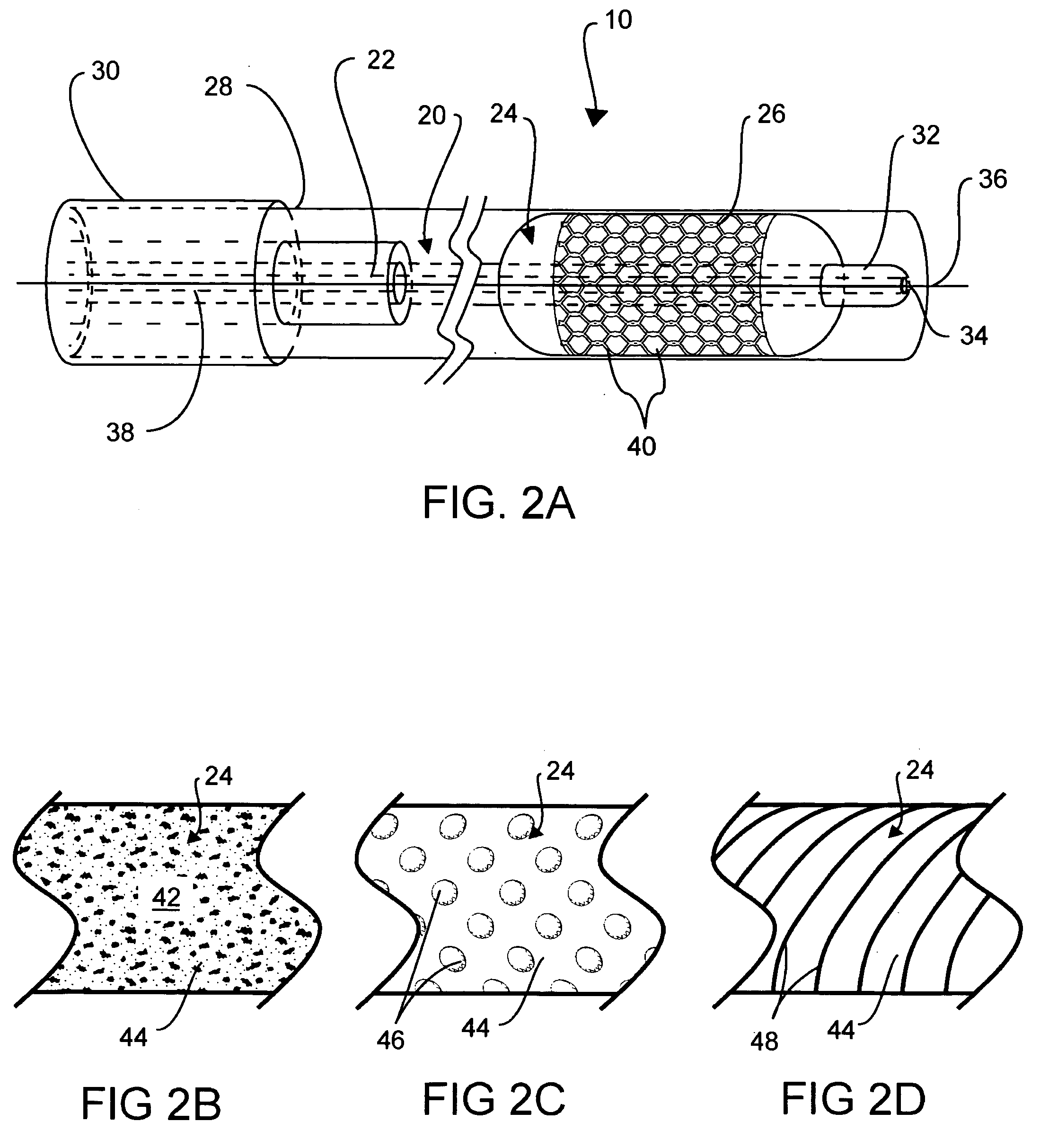
Intraluminal stent assembly and method of deploying the same
Intraluminal stent assemblies and methods of deploying the same. The assemblies include a catheter including at least one lumen formed therein. At least one inflatable member is disposed on the catheter and in communication with the lumen. The inflatable member includes at least one distal projection and / or at least one retention material. A stent is expandable from a compressed configuration to an expanded configuration. The stent is disposed on the inflatable member in the compressed configuration. A sheath is slidably positioned over the stent wherein the stent expands to the expanded configuration upon retraction of the sheath. The inflatable member is inflated with a fluid flowing through the lumen. The at least one distal projection and / or at least one retention material retain the stent in position on the inflatable member while the stent is being deployed.
Owner:MEDTRONIC VASCULAR INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com