Patents
Literature
117results about How to "Promote endothelialization" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
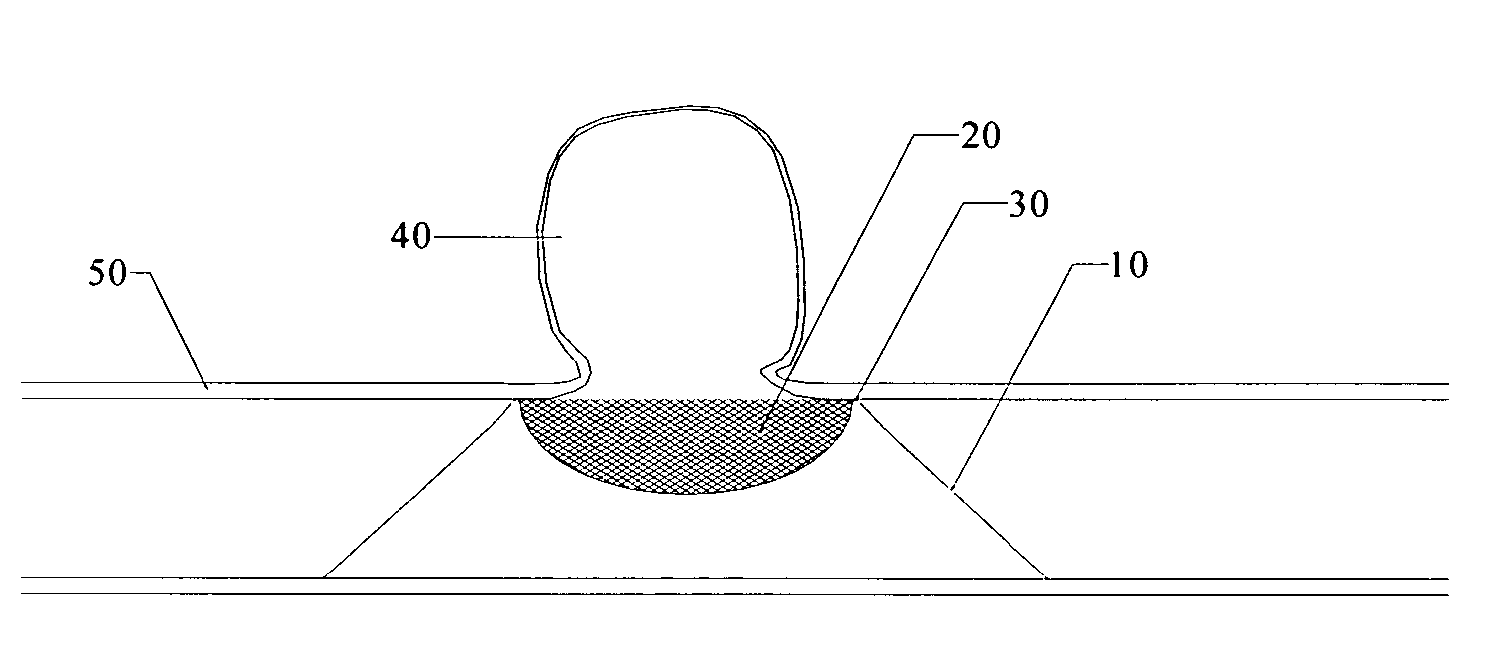
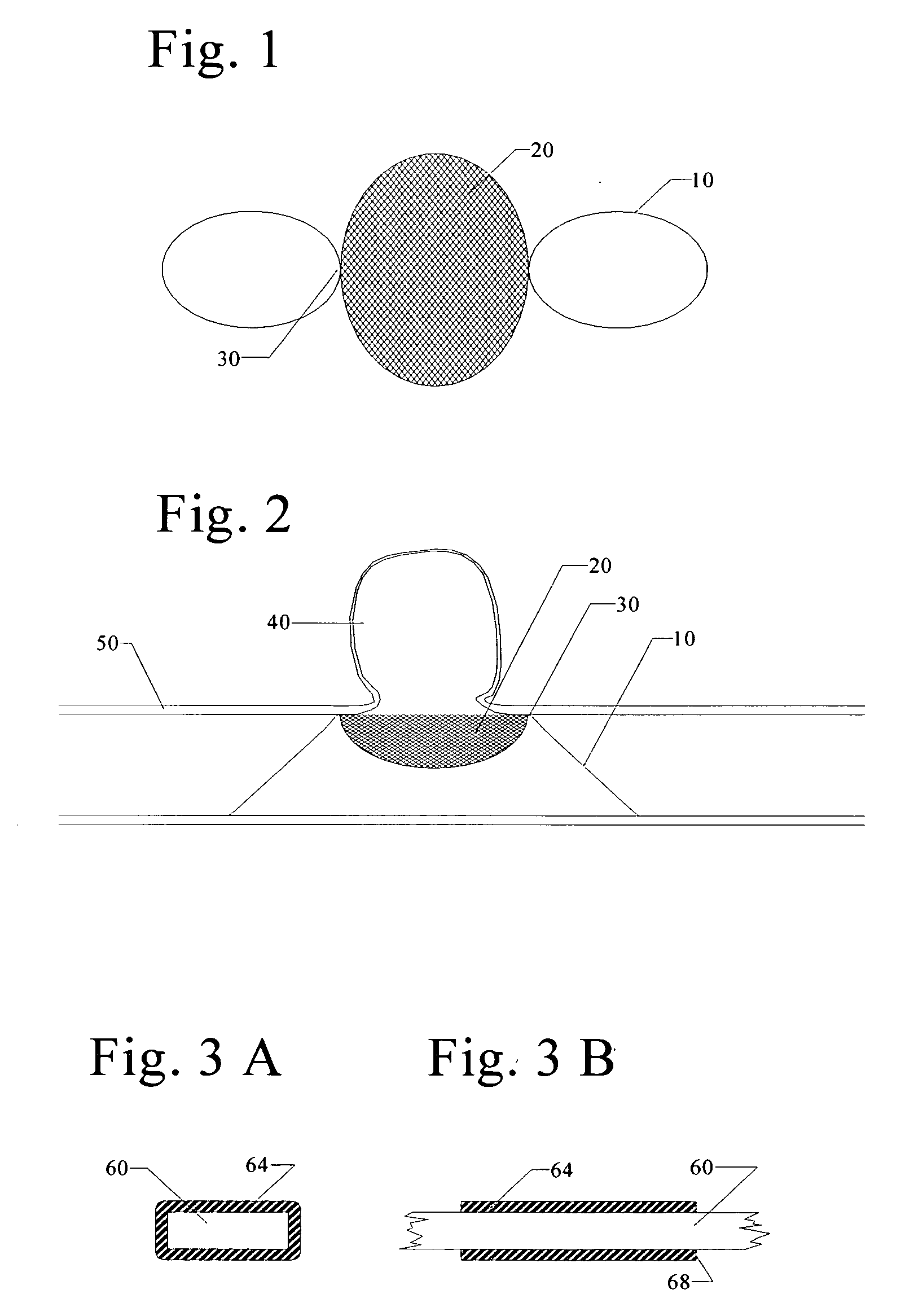
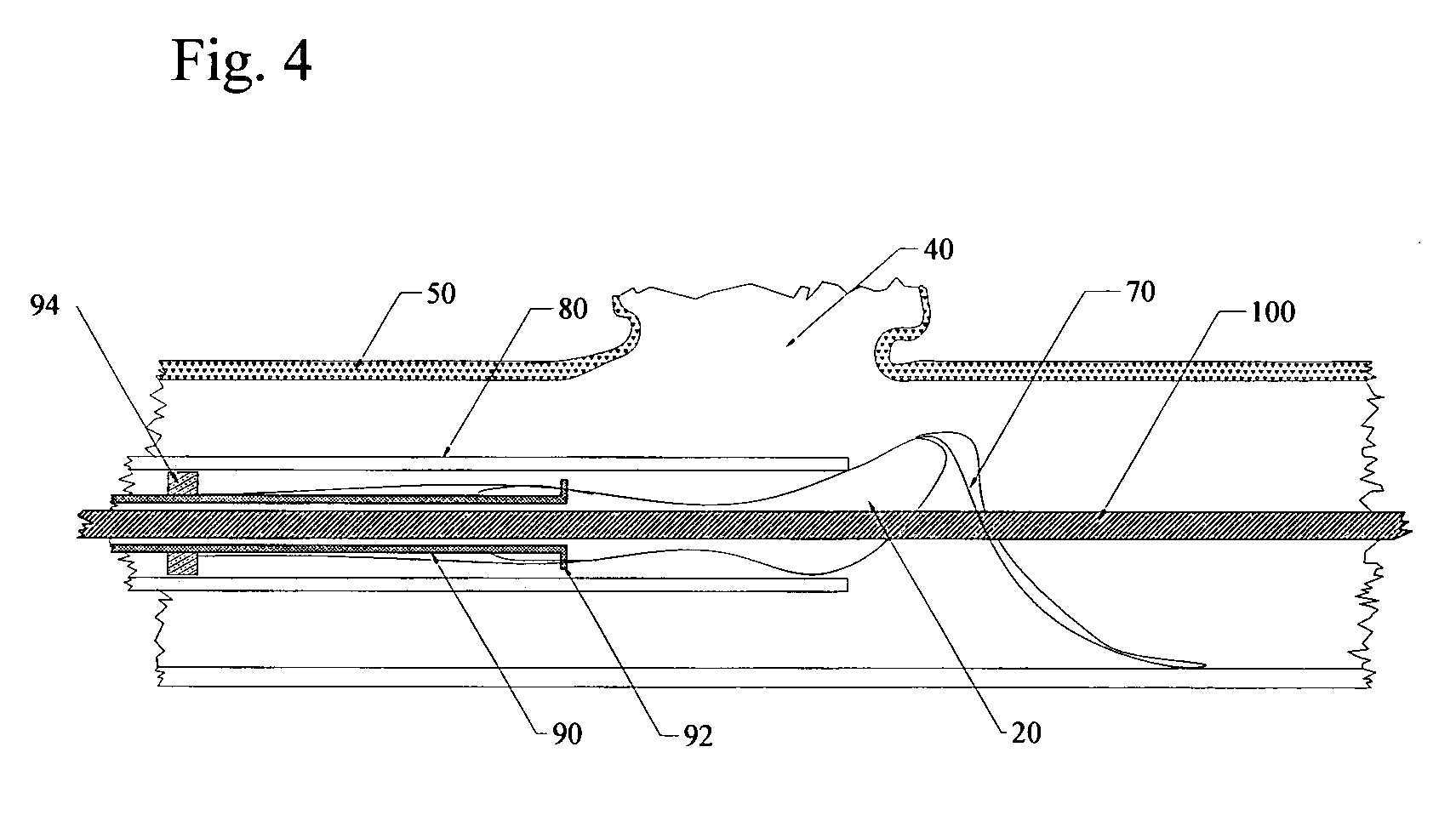
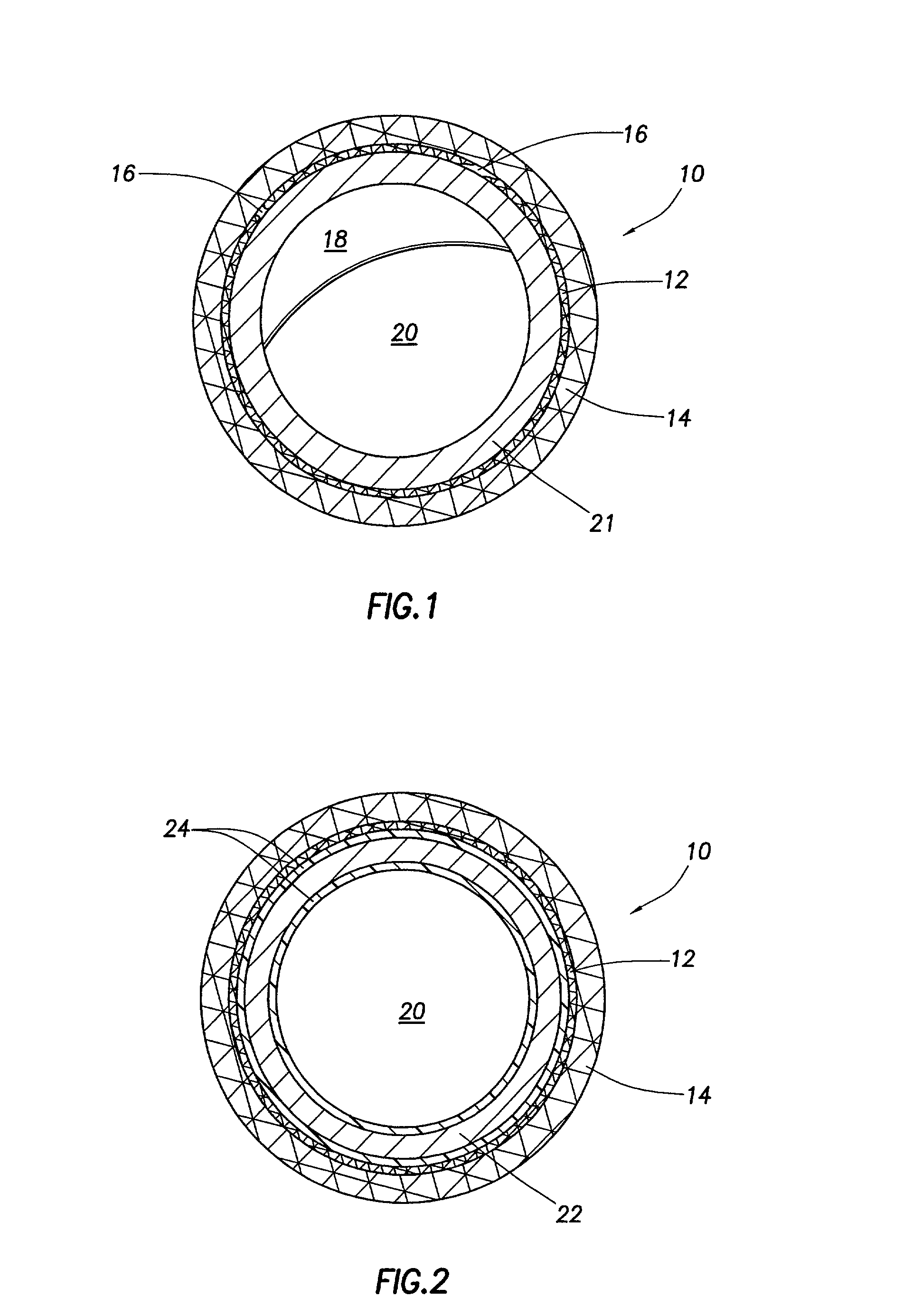
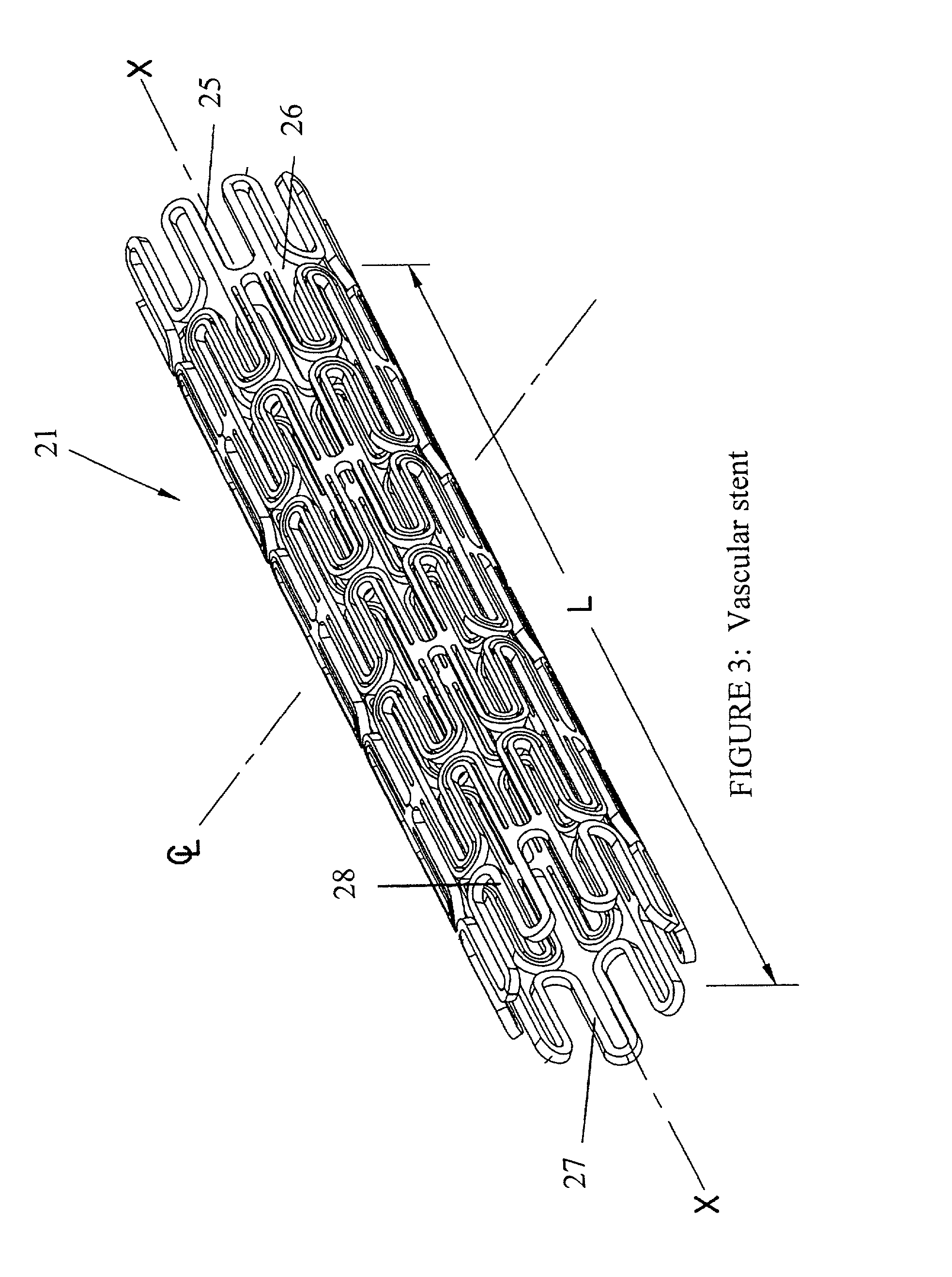
Endovascular aneurysm treatment device and delivery system
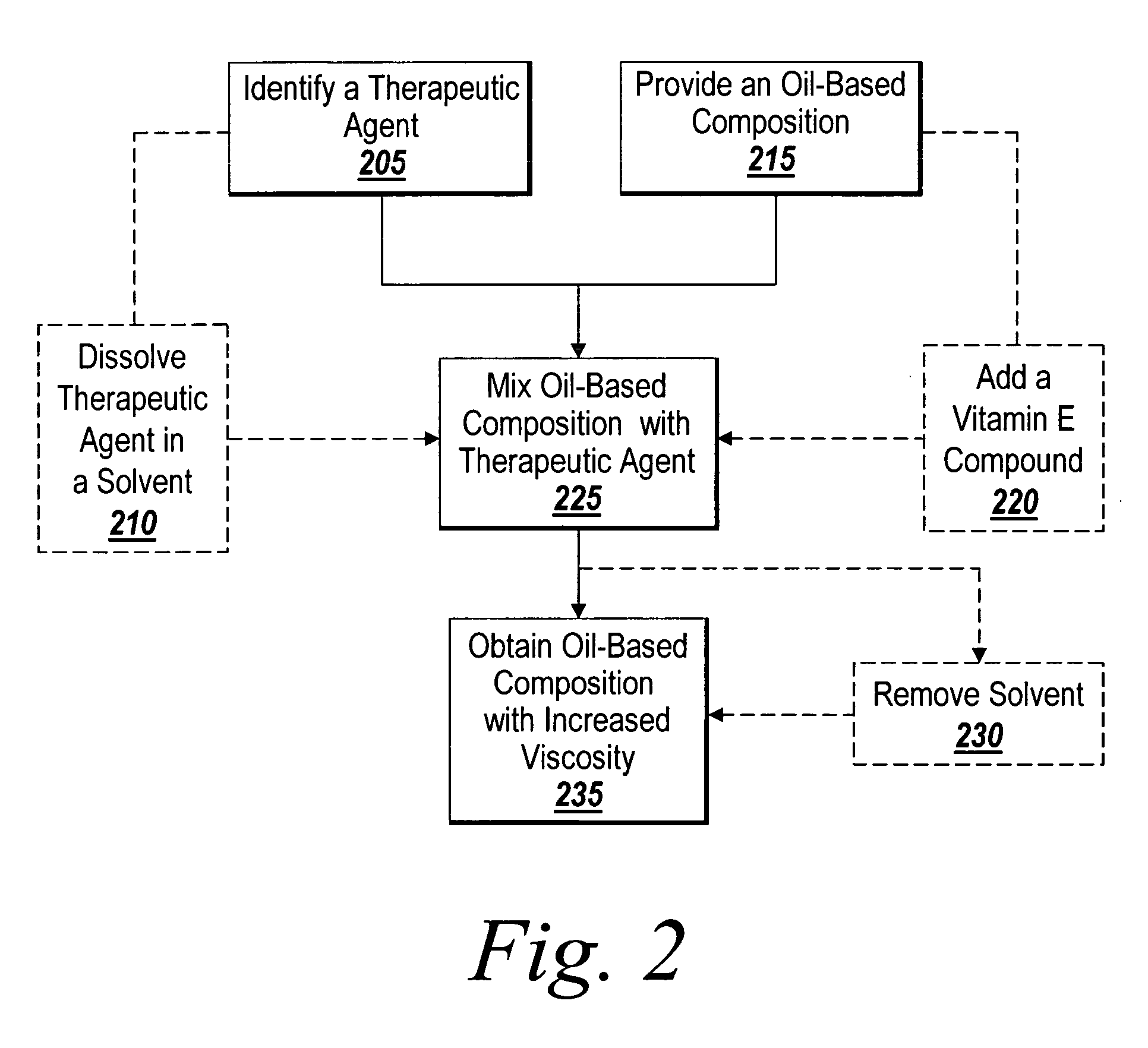
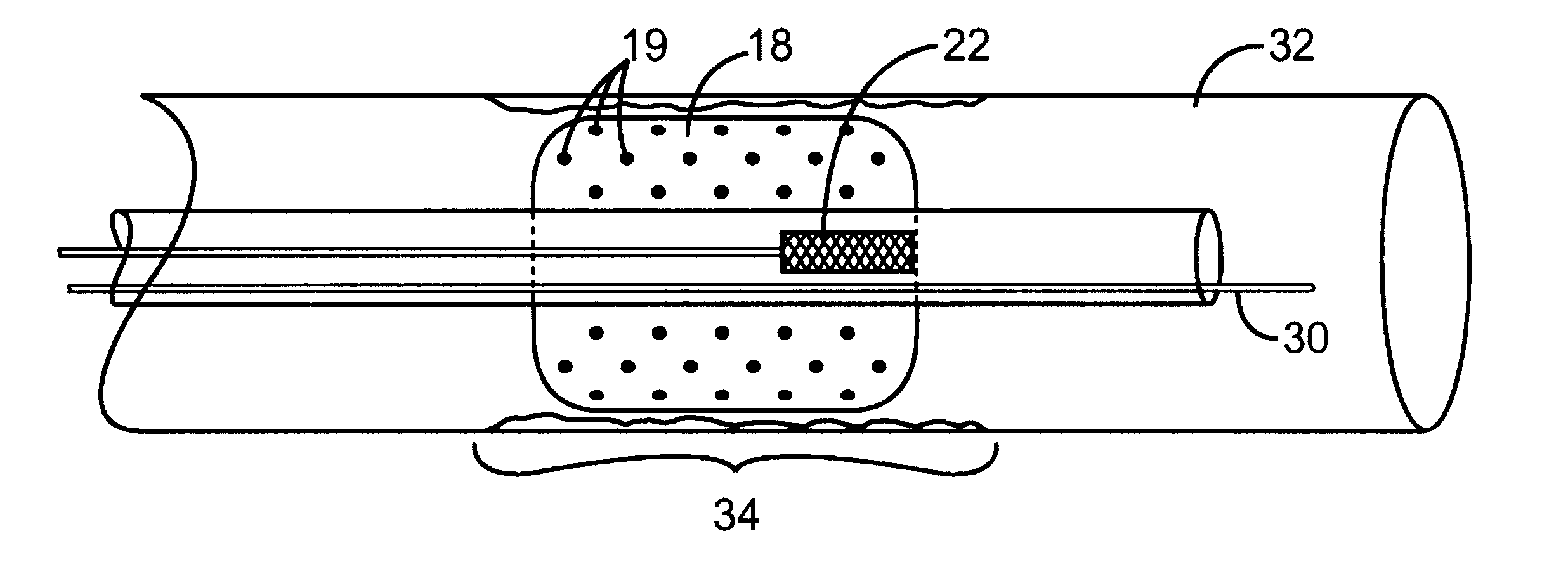
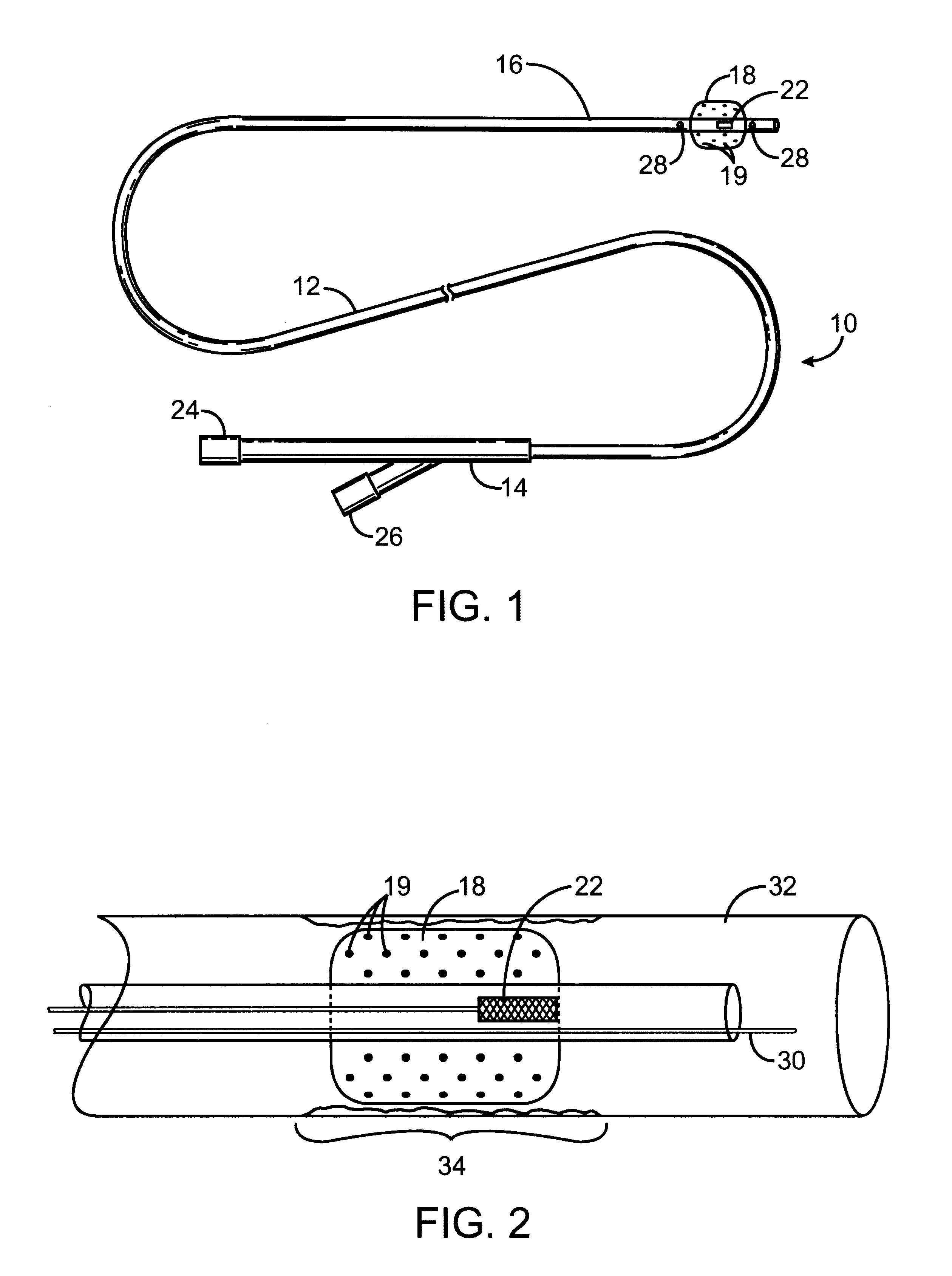
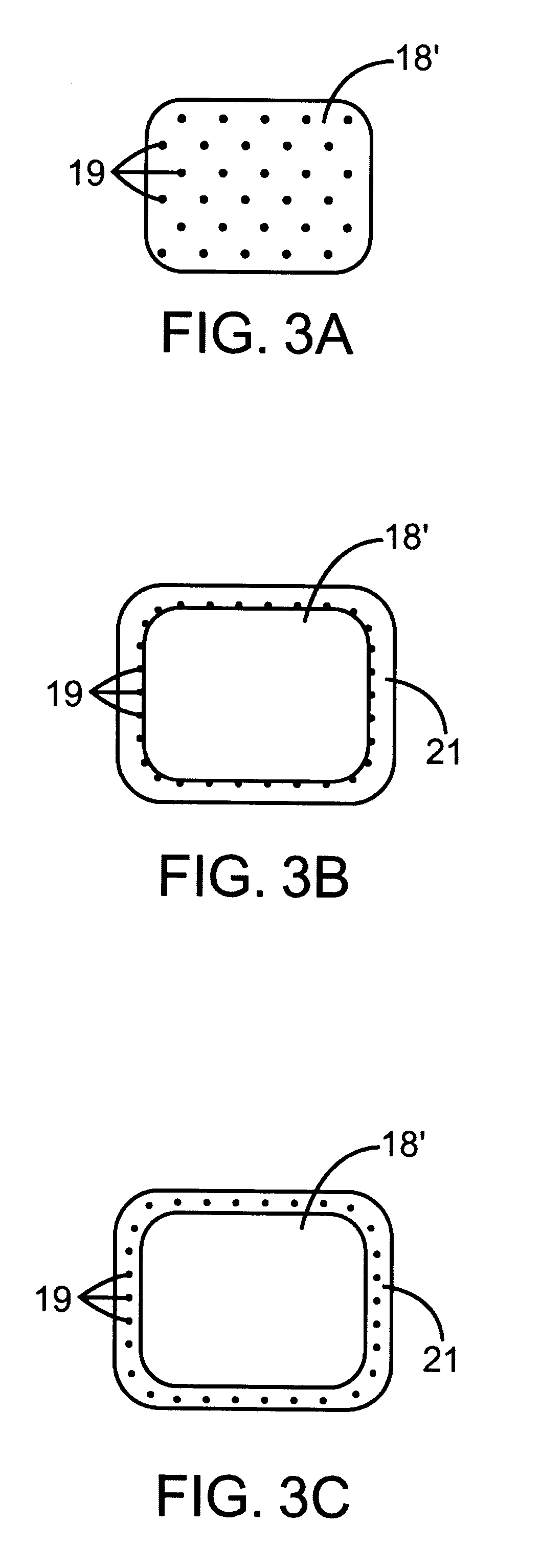
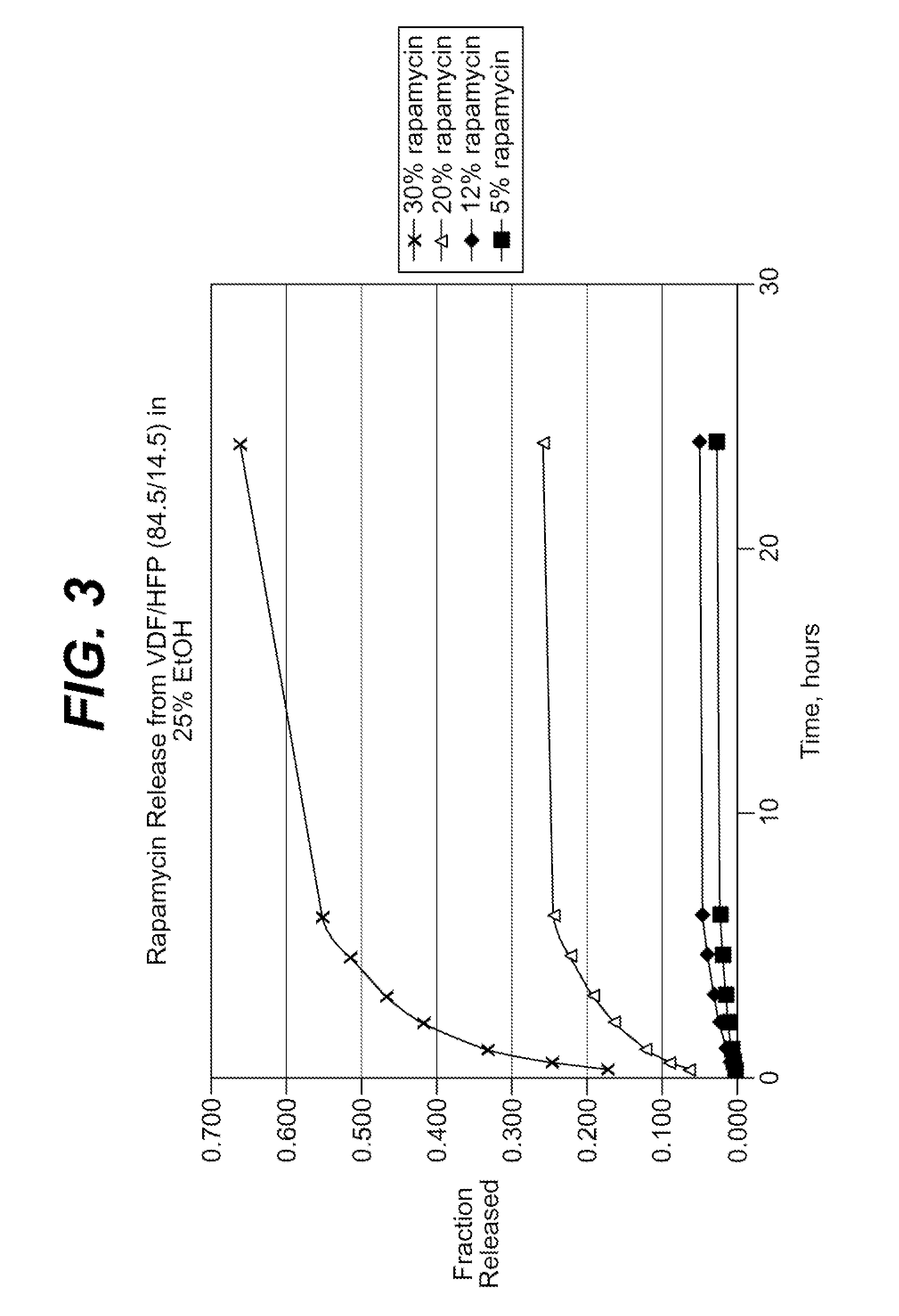
The present invention is directed to an intravascular treatment for intracranial aneurysms. The invention consists of a patch stent and, also, a patch stent delivery system allowing one to rotate the stent to align the patch with the neck of the aneurysm. A self-expanding nitinol framework holds the patch in place, a radiopaque agent allows the patch location to be visualized and a pusher tube that is mechanically locked into the unexpanded stent is used to push the stent out of the catheter with rotational and longitudinal control necessary to align the patch with the aneurysm. A self-expanding framework is used to support a patch at the neck of the aneurysm. The patch is designed to reduce the blood circulation in the aneurysm and the stagnant blood will clot or thrombus. The thrombus will stop any current blood leakage into the brain and will dramatically reduce the possibility of future leaks or potentially deadly ruptures. Over time the thrombus will be absorbed and the volume of the aneurysm will shrink reducing pressure on surrounding tissue.
Owner:HINES RICHARD ALLEN
Stent coatings containing HMG-CoA reductase inhibitors
InactiveUS20030077310A1Hydrolysis can be preventedAvoid accessStentsSurgeryHMG-CoA reductaseDepressant
Stents with coatings comprising a combination of a restenosis inhibitor comprising an HMG-CoA reductase inhibitor and a carrier. Also provided are methods of coating stents with a combination of an HMG-CoA reductase inhibitor and a carrier. A preferred example of a restenosis inhibitor is cerivastatin. The stent coatings have been shown to release restenosis inhibitors in their active forms.
Owner:ZIMMER ORTHOBIOLIGICS
Method of thickening a coating using a drug
InactiveUS20060083768A1Prevent restenosisInhibit neointimal growthBiocideStentsNuclear medicineMedical device
A method for the provision of a coating on an implantable medical device results in a medical device having a bio-absorbable coating. The coating includes a bio-absorbable carrier component. In addition to the bio-absorbable carrier component, a dissolved therapeutic agent component can also be provided. The coated medical device is implantable in a patient to effect controlled delivery of the coating, including the dissolved therapeutic agent, to the patient.
Owner:ATRIUM MEDICAL
Combination x-ray radiation and drug delivery devices and methods for inhibiting hyperplasia
InactiveUS6537195B2Promote endothelializationReduced dosages/concentrationsStentsElectrotherapyInsertion stentPercent Diameter Stenosis
The present invention provides improved devices, methods, and kits for inhibiting restenosis and hyperplasia after intravascular intervention. In particular, the present invention provides controlled drug delivery in combination with x-ray radiation delivery to selected locations within a patient's vasculature to reduce and / or inhibit restenosis and hyperplasia rates with increased efficacy. In one embodiment, the combination radiation and agent delivery catheter for inhibiting hyperplasia comprises a catheter body having a proximal end and distal end, an x-ray tube coupleable to the catheter body for applying a radiation dose to a body lumen, and a porous material, matrix, membrane, barrier, coating, infusion lumen, stent, graft, or reservoir for releasing an agent to the body lumen.
Owner:XOFT INC +1
Intraluminal device and therapeutic agent combination for treating aneurysmal disease
InactiveUS20070083258A1Avoid dilationReduce drug toxicityStentsSurgeryCompound (substance)Biocompatible material
Medical devices, and in particular implantable medical devices, may be coated to minimize or substantially eliminate a biological organism's reaction to the introduction of the medical device to the organism. The medical devices may be coated with any number of biocompatible materials. Therapeutic drugs, agents or compounds may be mixed with the biocompatible materials and affixed to at least a portion of the medical device. These therapeutic drugs, agents or compounds may also further reduce a biological organism's reaction to the introduction of the medical device to the organism. In addition, these therapeutic drugs, agents and / or compounds may be utilized to promote healing, including the formation of blood clots. The drugs, agents, and / or compounds may also be utilized to treat specific diseases, including vulnerable plaque. Therapeutic agents may also be delivered to the region of a disease site. In regional delivery, liquid formulations may be desirable to increase the efficacy and deliverability of the particular drug. Also, the devices may be modified to promote endothelialization. Various materials and coating methodologies may be utilized to maintain the drugs, agents or compounds on the medical device until delivered and positioned. In addition, the devices utilized to deliver the implantable medical devices may be modified to reduce the potential for damaging the implantable medical device during deployment. Medical devices include stents, grafts, anastomotic devices, perivascular wraps, sutures and staples. In addition, various polymer combinations may be utilized to control the elution rates of the therapeutic drugs, agents and / or compounds from the implantable medical devices.
Owner:CORDIS CORP
Coated aneurysmal repair device
InactiveUS20050249776A1Reduce drug toxicityGood curative effectBiocideStentsBlood vesselDisease cause
Medical devices, and in particular implantable medical devices, may be coated to minimize or substantially eliminate a biological organism's reaction to the introduction of the medical device to the organism. The medical devices may be coated with any number of biocompatible materials. Therapeutic drugs, agents or compounds may be mixed with the biocompatible materials and affixed to at least a portion of the medical device. These therapeutic drugs, agents or compounds may also further reduce a biological organism's reaction to the introduction of the medical device to the organism. In addition, these therapeutic drugs, agents and / or compounds may be utilized to promote healing, including the formation of blood clots. The drugs, agents, and / or compounds may also be utilized to treat specific diseases, including vulnerable plaque. Therapeutic agents may also be delivered to the region of a disease site. In regional delivery, liquid formulations may be desirable to increase the efficacy and deliverability of the particular drug. Also, the devices may be modified to promote endothelialization. Various materials and coating methodologies may be utilized to maintain the drugs, agents or compounds on the medical device until delivered and positioned. In addition, the devices utilized to deliver the implantable medical devices may be modified to reduce the potential for damaging the implantable medical device during deployment. Medical devices include stents, grafts, anastomotic devices, perivascular wraps, sutures and staples. In addition, various polymer combinations may be utilized to control the elution rates of the therapeutic drugs, agents and / or compounds from the implantable medical devices.
Owner:WYETH LLC
Implantable stent with endothelialization factor
InactiveUS20060085062A1Promote endothelializationStrong adhesionStentsSurgeryEndoluminal stentInsertion stent
A stent is provided in combination with a growth factor, specifically pleiotrophin or an analog or derivative thereof, which promotes endothelialization of the stent and re-endothelialization of the stented region of an injured site in a body lumen. In particular applications, the stent is an endolumenal stent and the growth factor promotes healing via endothelialization and substantially prevents restenosis. The growth factor is delivered from the stent formulated as a protein or peptide, or as a gene transfer vector. Methods for the treatment of vascular injury using pleiotrophin are also disclosed.
Owner:MEDLOGICS DEVICE CORP
Device for local and/or regional delivery employing liquid formulations of therapeutic agents
InactiveUS20080181927A1Elimination reactionReduce riskBiocideSurgeryDiseasePercent Diameter Stenosis
Medical devices may be utilized for local and regional therapeutic agent delivery. These therapeutic agents or compounds may reduce a biological organism's reaction to the introduction of the medical device to the organism. In addition, these therapeutic drugs, agents and / or compounds may be utilized to promote healing, including the prevention of thrombosis. The drugs, agents, and / or compounds may also be utilized to treat specific disorders, including restenosis, vulnerable plaque, and atherosclerosis in type 2 diabetic patients. In regional delivery, liquid formulations may be desirable to increase the efficacy and deliverability of the particular drug. Various materials and coating methodologies may be utilized to maintain the agents or compounds on the medical device until delivered and positioned.
Owner:CARDINAL HEALTH SWITZERLAND 515 GMBH
System for treating aneurysmal disease
InactiveUS20070026042A1Reduce drug toxicityGood curative effectSuture equipmentsBiocideCompound (substance)Biocompatible material
Medical devices, and in particular implantable medical devices, may be coated to minimize or substantially eliminate a biological organism's reaction to the introduction of the medical device to the organism. The medical devices may be coated with any number of biocompatible materials. Therapeutic drugs, agents or compounds may be mixed with the biocompatible materials and affixed to at least a portion of the medical device. These therapeutic drugs, agents or compounds may also further reduce a biological organism's reaction to the introduction of the medical device to the organism. In addition, these therapeutic drugs, agents and / or compounds may be utilized to promote healing, including the formation of blood clots. The drugs, agents, and / or compounds may also be utilized to treat specific diseases, including vulnerable plaque. Therapeutic agents may also be delivered to the region of a disease site. In regional delivery, liquid formulations may be desirable to increase the efficacy and deliverability of the particular drug. Also, the devices may be modified to promote endothelialization. Various materials and coating methodologies may be utilized to maintain the drugs, agents or compounds on the medical device until delivered and positioned. In addition, the devices utilized to deliver the implantable medical devices may be modified to reduce the potential for damaging the implantable medical device during deployment. Medical devices include stents, grafts, anastomotic devices, perivascular wraps, sutures and staples. In addition, various polymer combinations may be utilized to control the elution rates of the therapeutic drugs, agents and / or compounds from the implantable medical devices.
Owner:WYETH
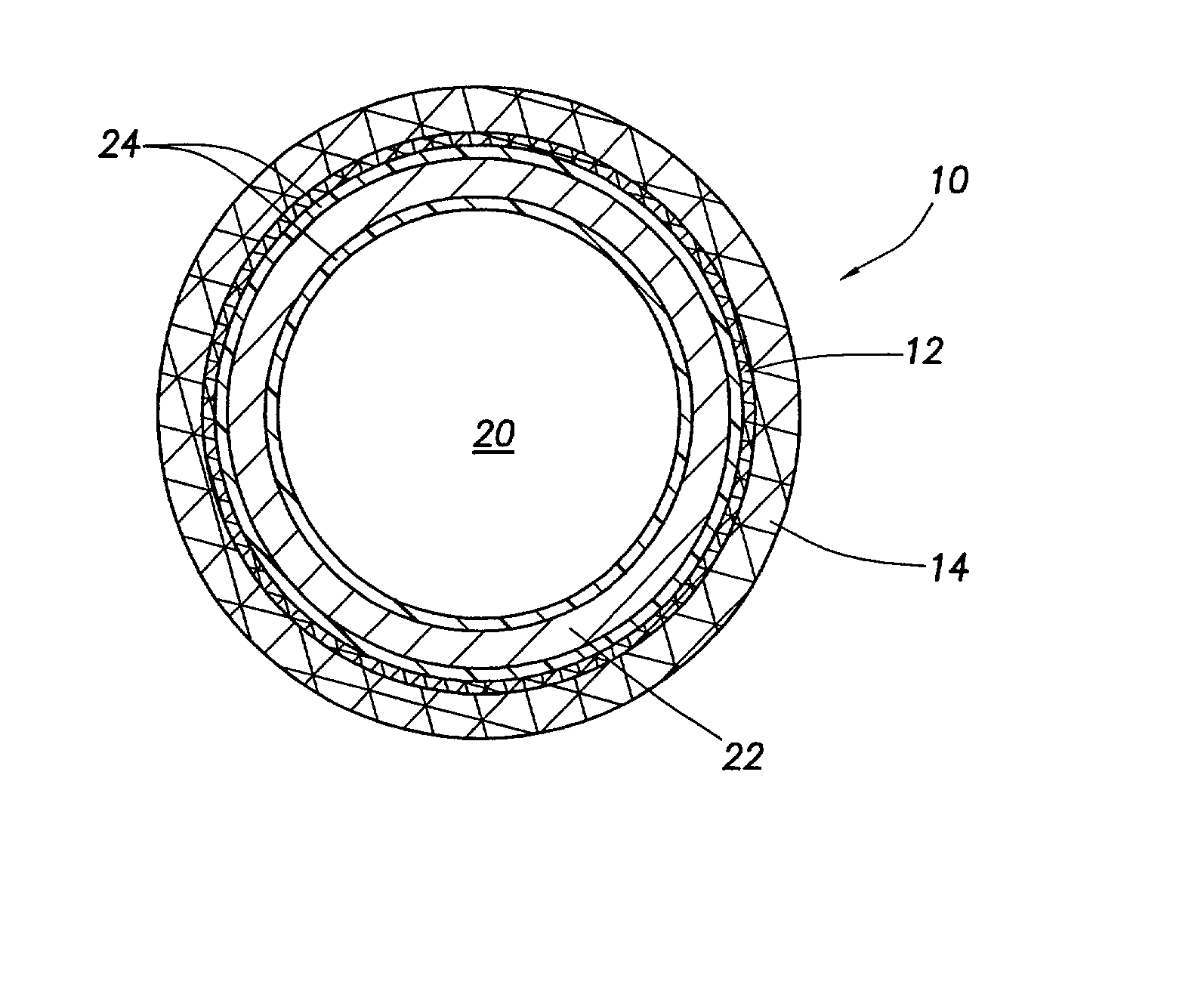
Metallic implantable grafts and method of making same
ActiveUS20050033418A1Promote endothelializationGive flexibilityStentsSurgerySurgical GraftLigament structure
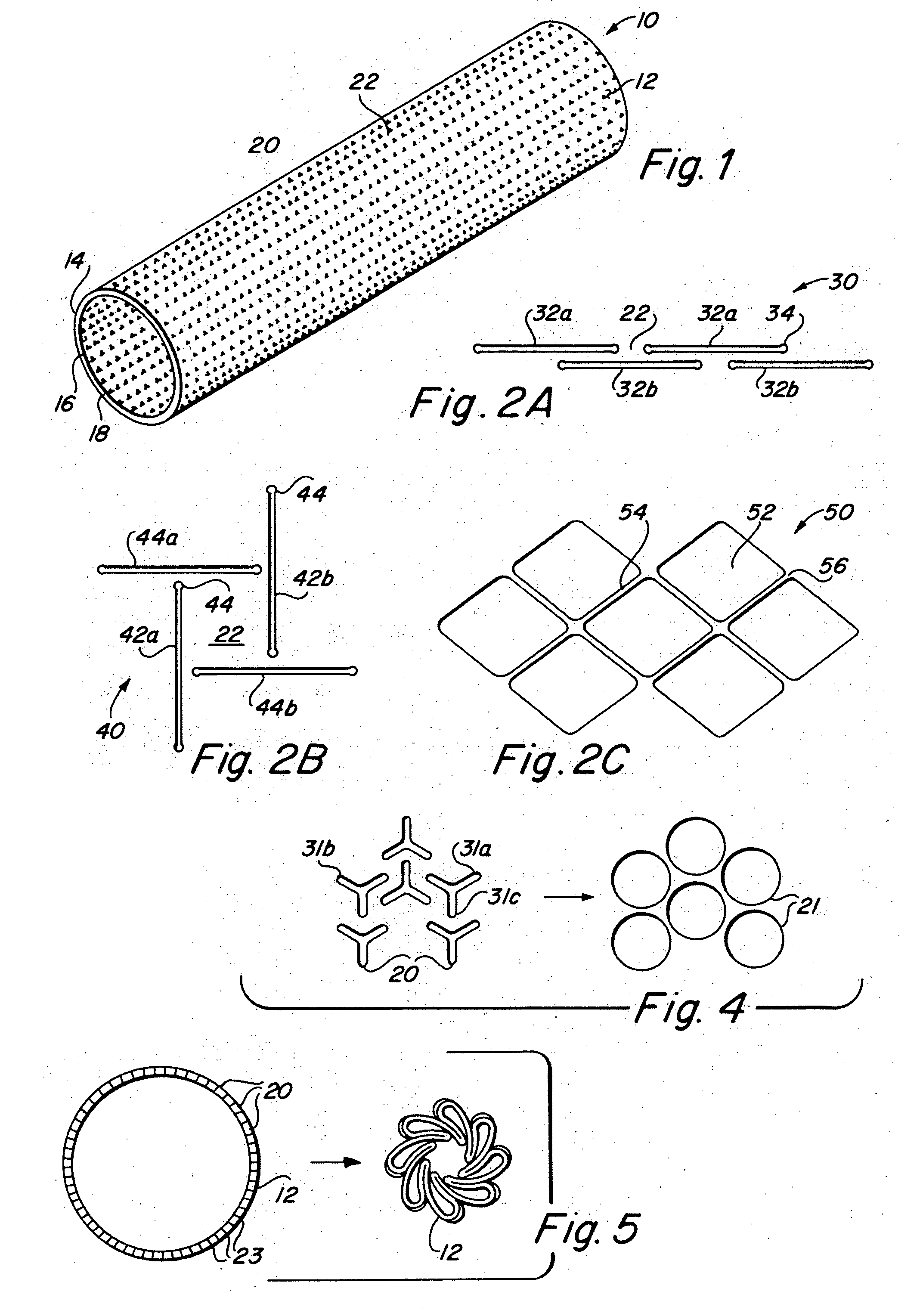
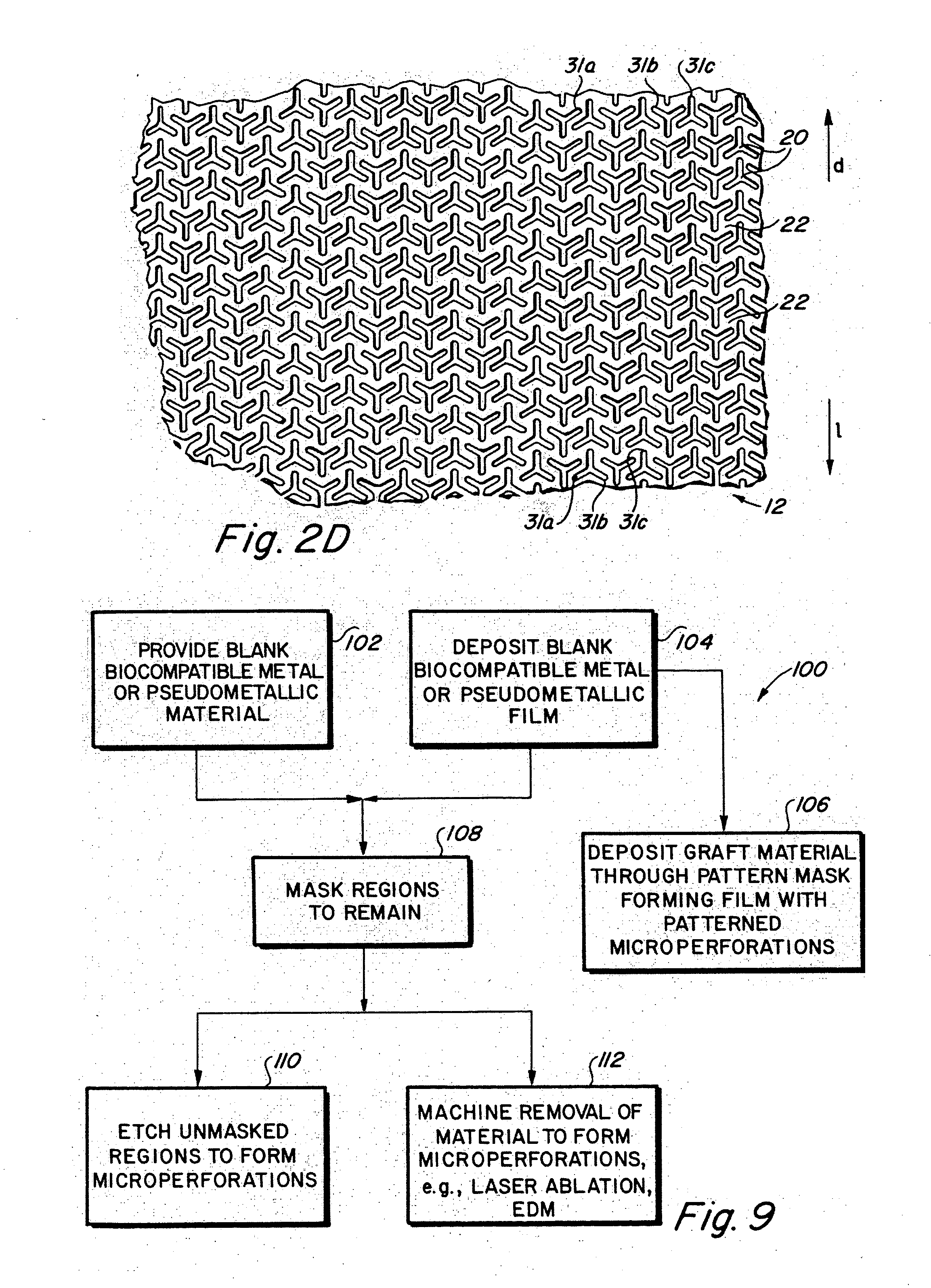
Implantable medical grafts fabricated of metallic or pseudometallic films of biocompatible materials having a plurality of microperforations passing through the film in a pattern that imparts fabric-like qualities to the graft or permits the geometric deformation of the graft. The implantable graft is preferably fabricated by vacuum deposition of metallic and / or pseudometallic materials into either single or multi-layered structures with the plurality of microperforations either being formed during deposition or after deposition by selective removal of sections of the deposited film. The implantable medical grafts are suitable for use as endoluminal or surgical grafts and may be used as vascular grafts, stent-grafts, skin grafts, shunts, bone grafts, surgical patches, non-vascular conduits, valvular leaflets, filters, occlusion membranes, artificial sphincters, tendons and ligaments.
Owner:VACTRONIX SCI LLC
Occlusive devices
ActiveUS20170079662A1Facilitate and healing effectPromote endothelializationOcculdersPorosityComputer graphics (images)
Owner:TYCO HEALTHCARE GRP LP
Stent and Method and Device for Fabricating the Stent
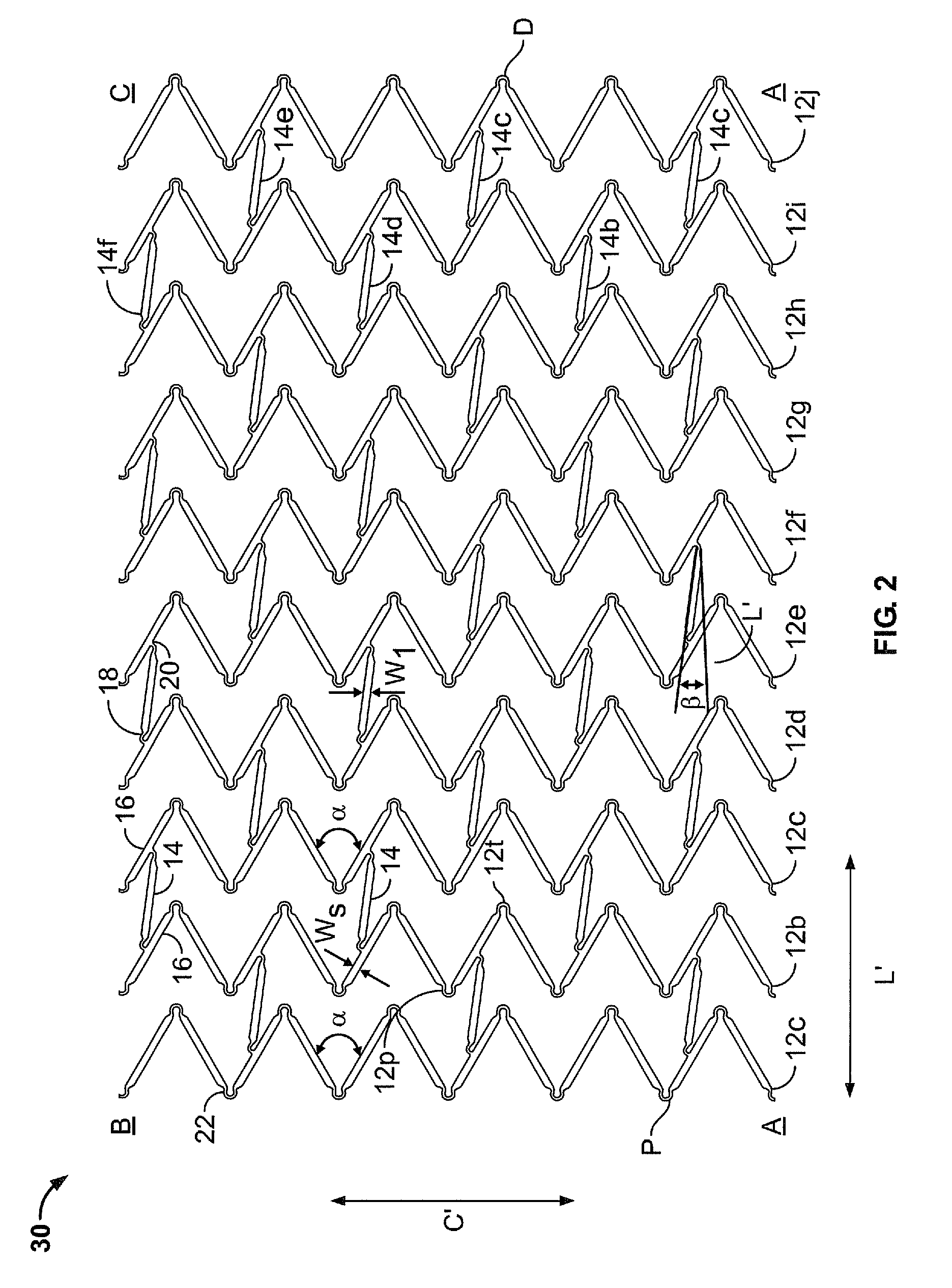
ActiveUS20100049300A1Stress minimizationAvoid injuryStentsElectrolysis componentsInsertion stentBiomedical engineering
Stent, as well as a method and device for fabricating the stent, wherein the stent has a tubular lattice structure comprising individual struts and at least one strut of which at least one longitudinal section runs with at least one directional component in the radial circumferential direction of the stent, wherein the surface of the longitudinal section facing the outside of the stent is curved only about the longitudinal axis of the stent. According to the invention, the surface of longitudinal section of the strut, which surface faces the inside of the stent, has such a curvature that the strut cross section is fluidically optimized.
Owner:BIOTRONIK AG
Endoluminal stent having mid-strut interconnecting members
ActiveUS7122049B2Function optimizationMaximize functionalityStentsBlood vesselsEndoluminal stentEngineering
An endoluminal stent composed of a plurality of circumferential expansion elements arrayed to form the circumference of the stent and extending along the longitudinal axis of the stent, and a plurality of interconnecting members that interconnect adjacent pairs of circumferential expansion elements, the interconnecting members joining struts of adjacent pairs of interconnecting members at approximate mid-points of the struts.
Owner:VACTRONIX SCI LLC
Endoluminal stent, self-supporting endoluminal graft and methods of making same
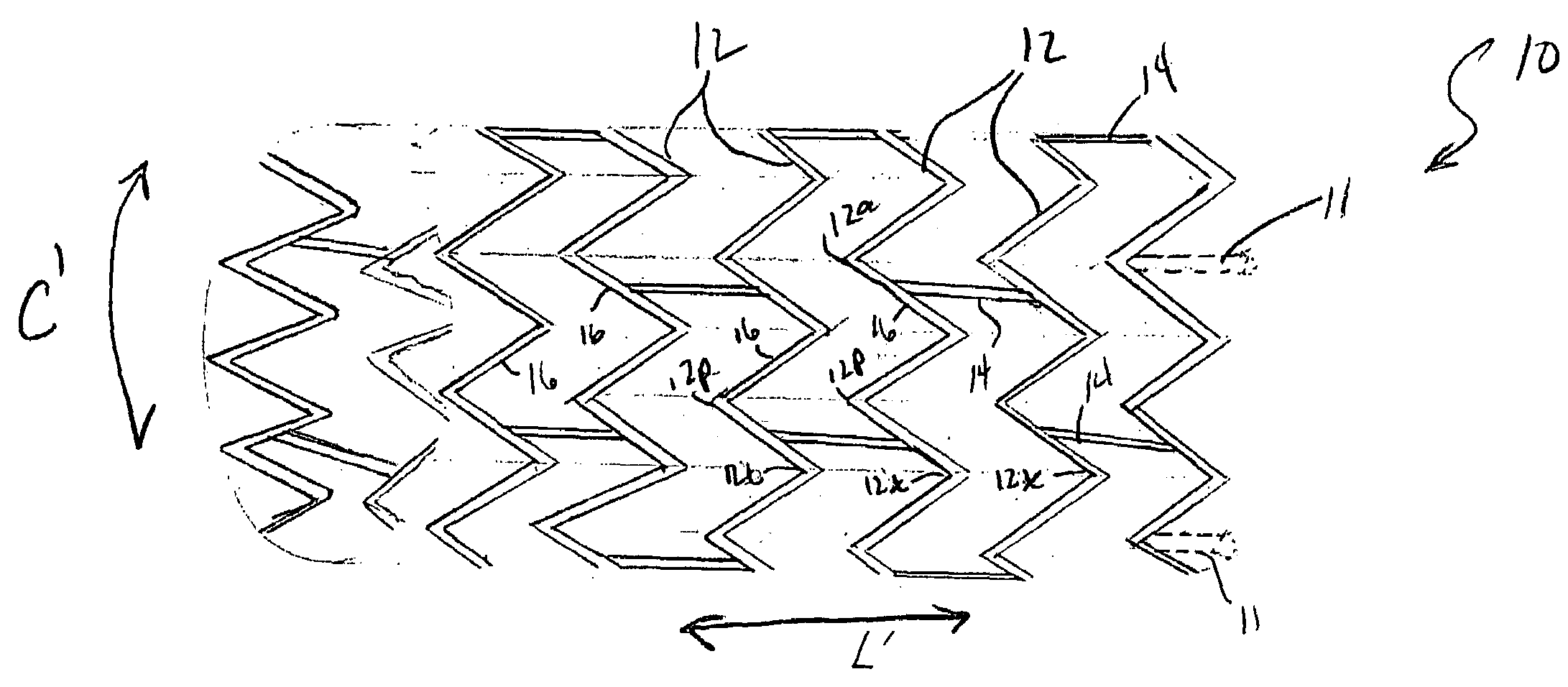
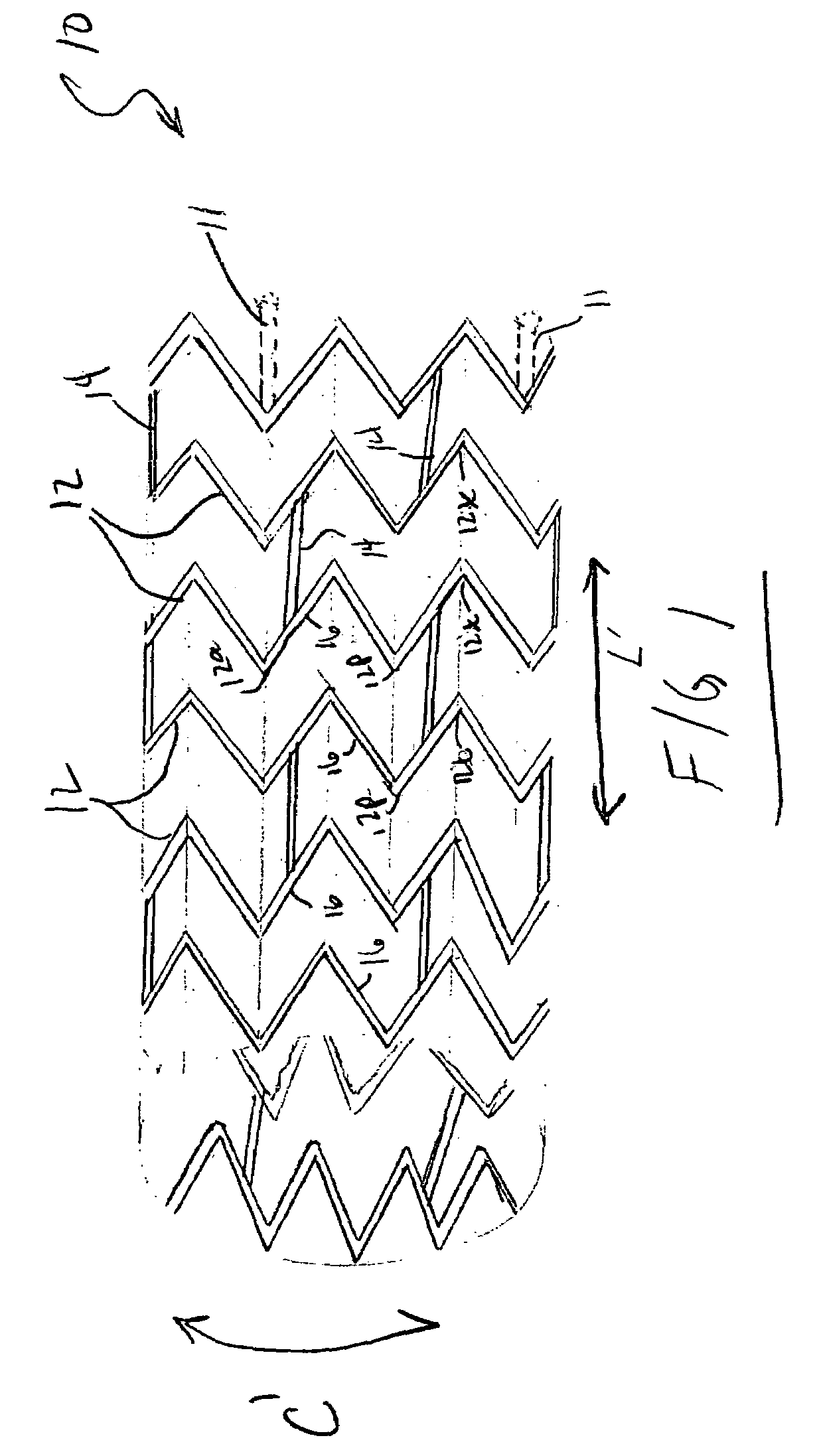
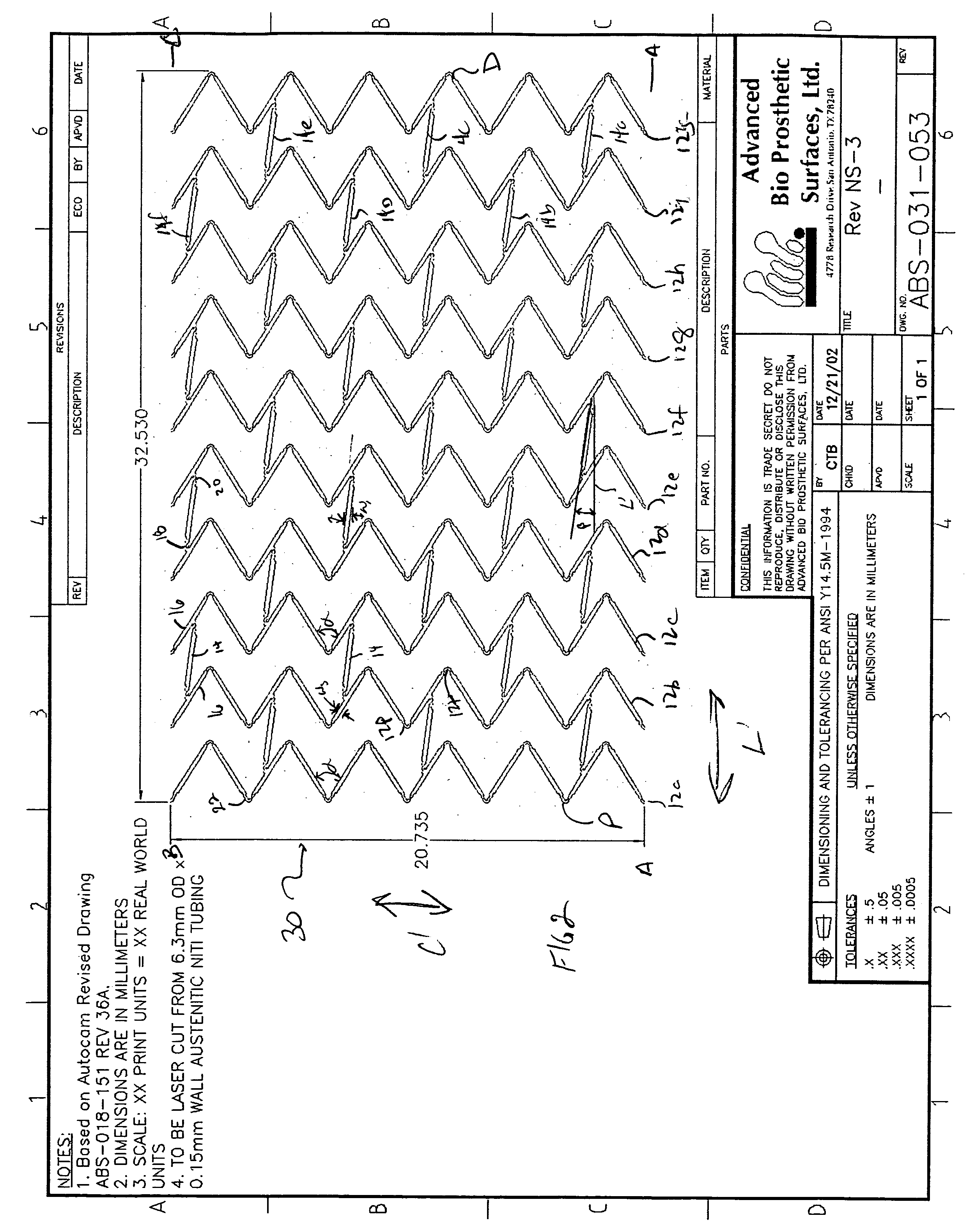
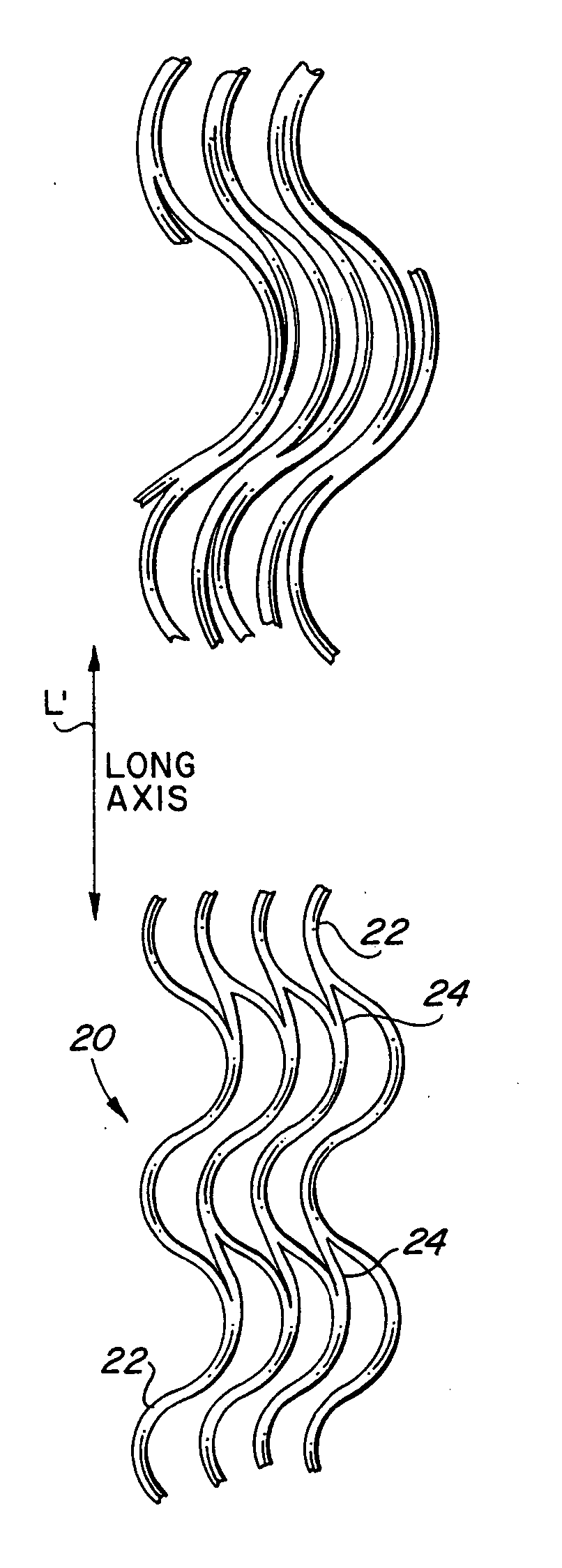
ActiveUS20060116751A1Promote endothelializationFunction optimizationStentsVacuum evaporation coatingStructural elementEngineering
An endoluminal stent composed of a plurality of first structural elements arrayed to form the circumference of the stent and extending along the longitudinal axis of the stent, and a plurality of second structural elements that interconnect adjacent pairs of first structural elements. The plurality of first structural elements have either a linear shape or a generally sinusoidal configuration with either a regular or irregular periodicity or regions of regular and regions of irregular periodicity between the peaks and troughs of the pattern, with the peaks and troughs projecting from the first structural elements in the circumferential axis. The plurality of second structural elements are generally linear or sinusoidal-shaped members which interconnect an apex of a peak of one of the plurality of first structural elements with an apex of a valley of a second and adjacent one of the plurality of first structural elements. Each of the plurality of second structural elements are generally oriented parallel to the circumferential axis of the stent.
Owner:VACTRONIX SCI LLC
Transcatheter left atrial appendage plugging system
ActiveCN104856741APhysiologically consistentReasonable specification designOcculdersIsosceles trapezoidMedicine
The invention relates to a transcatheter left atrial appendage plugging system. The system is composed of an inner plugging body, a connecting rod, a direction regulator and an outer plugging body, all of which are orderly arranged from the inner end to the outer end; the inner plugging body is provided with a mesh basket support and a first plugging film; the mesh basket support is formed by use of mesh basket wires and riveting hooks and protective balls are arranged on the mesh basket wires; the connecting rod is of a structure adjustable in length in the axial direction; the direction regulator is used for regulating the direction of the connecting rod; the outer plugging body is of a meshed hollow structure in a stand shape; the cross section of the outer plugging body is circular, while the longitudinal section of the outer plugging body is isosceles trapezoid-shaped; a second plugging film is arranged inside the outer plugging body; the first plugging film and the second plugging film both are microporous water-permeable films. According to the design ideal of inside plugging, the transcatheter left atrial appendage plugging system can be completely plugged without any residual cavity; the plugging system is suitable for the left atrial appendages different in shape and depth; besides, the plugging system is small in endothelialization area, steady in position after implantation and good in safety.
Owner:GUANGDONG PULSE MEDICAL SCI & TECH CO LTD
Implantable materials having engineered surfaces and method of making same
ActiveUS20090304772A1Promote endothelializationImproves drug loading/delivery capacityStentsBlood vesselsCompound (substance)Implant material
Owner:VACTRONIX SCI LLC
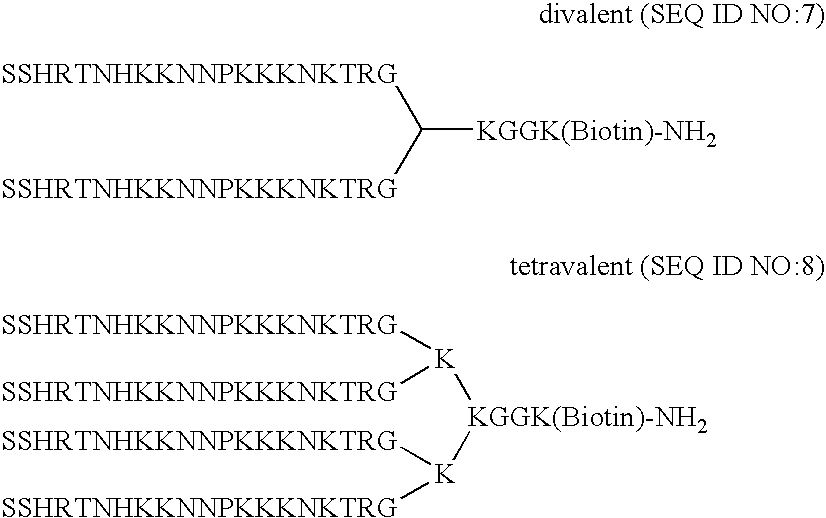
Methods and compositions for promoting attachment of cells of endothelial cell lineage to medical devices
InactiveUS20070160644A1Promote disseminationPromote one or more of healingPeptide/protein ingredientsPretreated surfacesCoated surfaceCell lineage
The present invention provides compositions and methods for an improved coating for medical devices. Provided is a biofunctional coating composition comprising at least one binding domain that has binding specificity for a surface material of a medical device, and at least one binding domain that has binding specificity for cells of endothelial cell lineage. Methods for coating a surface of a medical device, and for manufacturing of a medical device, comprise contacting the surface to be coated with the biofunctional coating material in an amount effective to form a coating, and may further comprise contacting the coated surface with cells of endothelial cell lineage to bind the cells of endothelial cell lineage to the coated surface.
Owner:AFFINERGY INC
Layer-by-layer stereocomplexed polymers as drug depot carriers or coatings in medical devices
Medical devices, and in particular implantable medical devices, may be coated to minimize or substantially eliminate a biological organism's reaction to the introduction of the medical device to the organism. The medical devices may be coated with any number of biocompatible materials. Therapeutic drugs, agents or compounds may be mixed with the biocompatible materials and affixed to at least a portion of the medical device. These therapeutic agents or compounds may also further reduce a biological organism's reaction to the introduction of the medical device to the organism. In addition, these therapeutic drugs, agents and / or compounds may be utilized to promote healing, including the prevention of thrombosis. The drugs, agents, and / or compounds may also be utilized to treat specific disorders, including vulnerable plaque, and atherosclerosis in type 2 diabetic patients. Therapeutic agents may also be delivered to the region of a disease site. In regional delivery, liquid formulations may be desirable to increase the efficacy and deliverability of the particular drug. Various materials and coating methodologies may be utilized to maintain the agents or compounds on the medical device until delivered and positioned.
Owner:CARDINAL HEALTH SWITZERLAND 515 GMBH
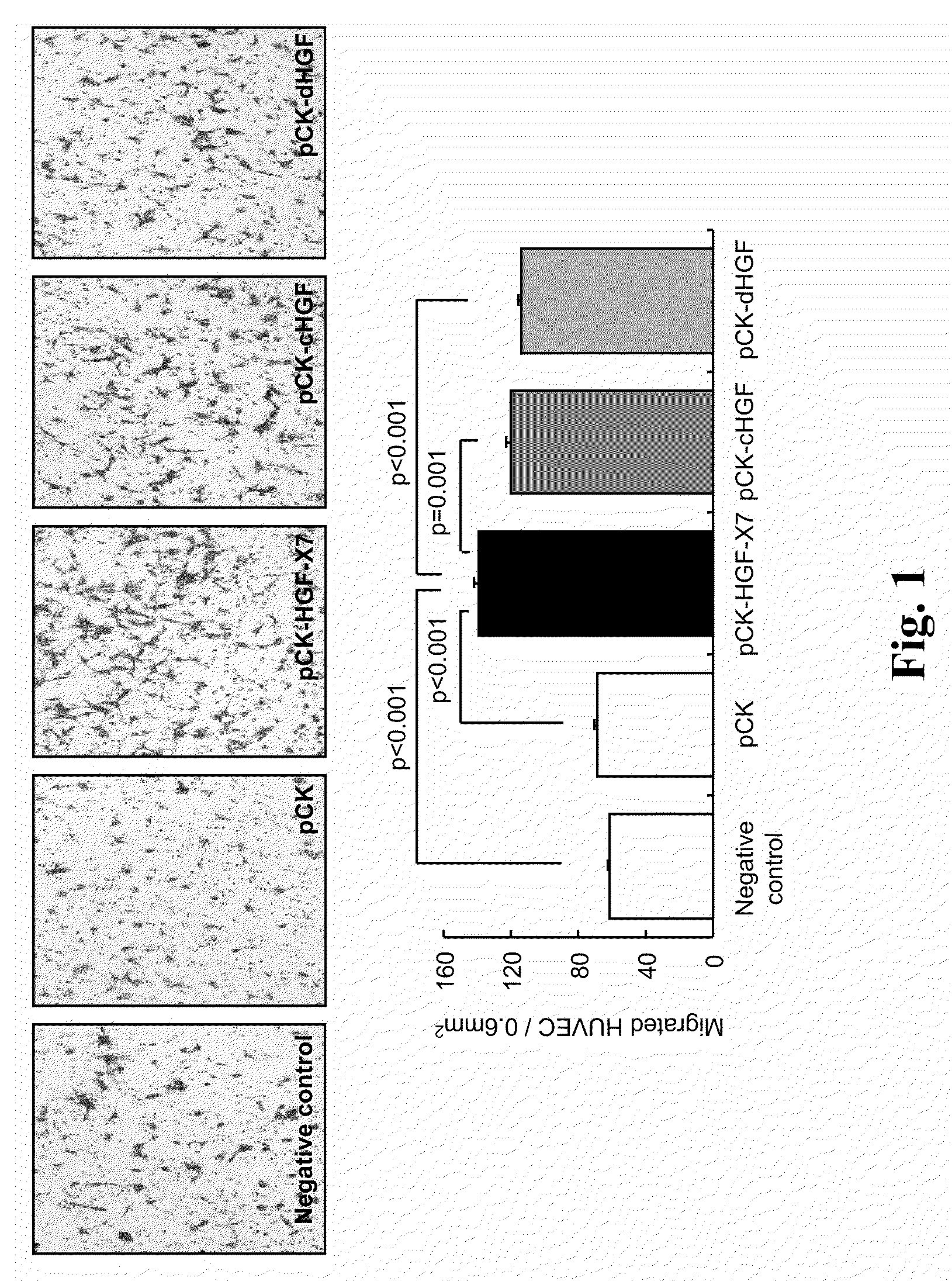
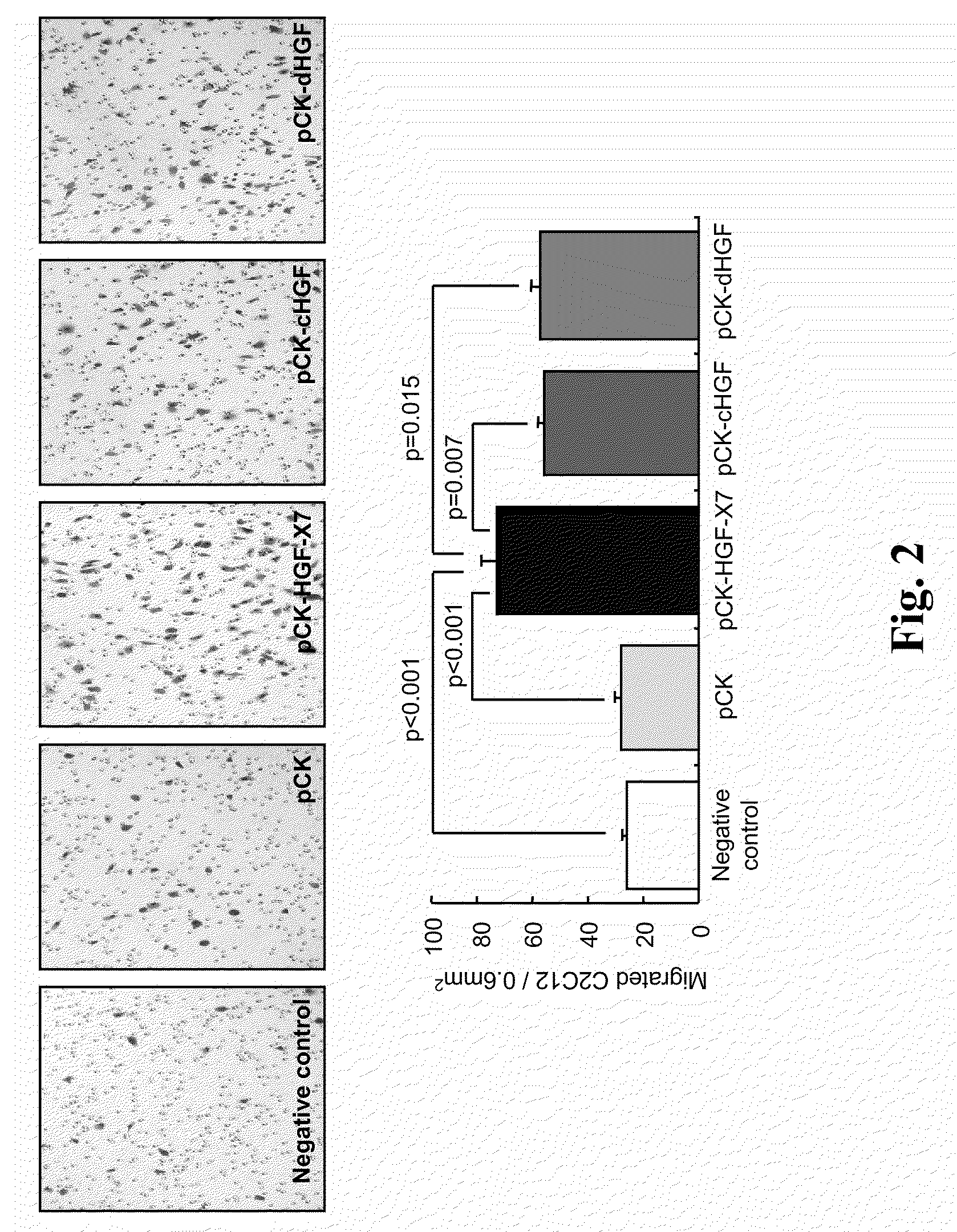
Treatment and Prevention of Cardiac Conditions Using Two or More Isoforms of Hepatocyte Growth Factor
InactiveUS20090202606A1Promote endothelializationPromotes and accelerates re-endothelializationPeptide/protein ingredientsCarbohydrate active ingredientsEndothelial NOSHepatocyte growth factor
The present invention relates to methods for treating or preventing cardiac conditions in a subject comprising administering to the subject two or more isoforms of hepatocyte growth factor (HGF). The present invention further relates to methods for promoting endothelial cell growth in a blood vessel comprising administering to the blood vessel two or more isoforms of hepatocyte growth factor (HGF). In one embodiment the two or more isoforms of HGF are administered as one or more polynucleotides encoding the isoforms.
Owner:HELIXMITH CO LTD
Compositions and methods for promoting attachment of cells of endothelial cell lineage to medical devices
InactiveUS20070166350A1Promote disseminationPromote one or more of healingPeptide/protein ingredientsAntibody mimetics/scaffoldsCoated surfaceCell lineage
The present invention provides compositions and methods for an improved coating for medical devices. Provided is a biofunctional coating composition comprising at least one binding domain that has binding specificity for a metallic surface material of a medical device, and at least one binding domain that has binding specificity for cells of endothelial cell lineage. Methods for coating a metallic surface of a medical device, and for manufacturing of a medical device, comprise contacting the metallic surface to be coated with the biofunctional coating material in an amount effective to form a coating, and may further comprise contacting the coated surface with cells of endothelial cell lineage to bind the cells of endothelial cell lineage to the coated surface.
Owner:AFFINERGY INC
Thromboresistant coatings for aneurysm treatment devices
ActiveUS10653426B2Good biological propertiesReduce thrombosisStentsSurgical needlesRadiologyVascular device
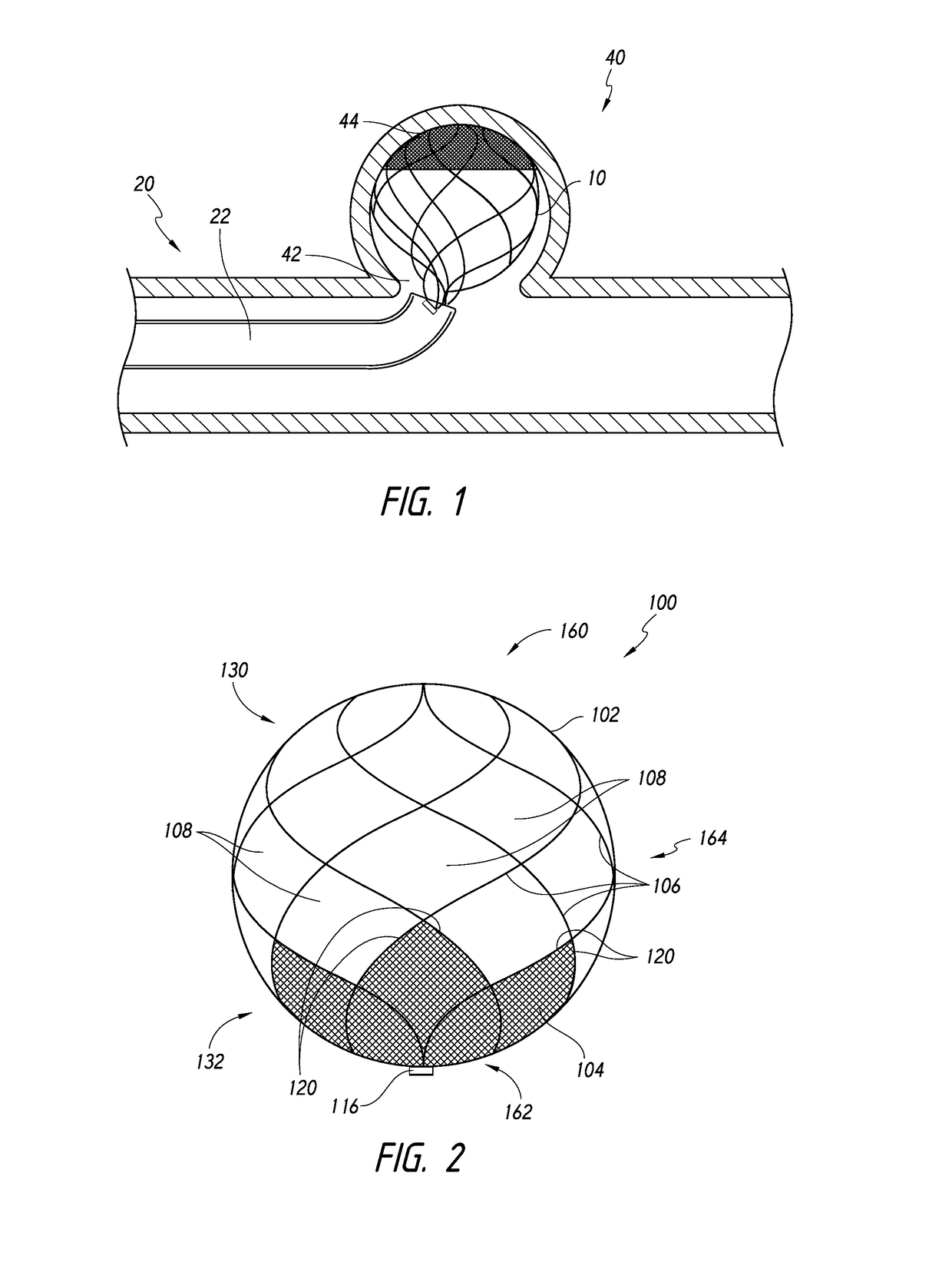
Disclosed are coating compositions, processes, and designs for endowing vascular devices with thromboresistant and endothelializing properties. Also disclosed are designs of vascular devices used as aneurysm treating devices for assisting in the delivery, packing, and maintenance of embolization coils within an aneurysm, particularly a neurovascular aneurysm.
Owner:INCEPT LLC
Endoluminal stent having mid-strut interconnecting members
ActiveUS20070088430A1Function optimizationMaximize functionalityStentsBlood vesselsEndoluminal stentEngineering
An endoluminal stent composed of a plurality of circumferential expansion elements arrayed to form the circumference of the stent and extending along the longitudinal axis of the stent, and a plurality of interconnecting members that interconnect adjacent pairs of circumferential expansion elements, the interconnecting members joining struts of adjacent pairs of interconnecting members at approximate mid-points of the struts.
Owner:VACTRONIX SCI LLC
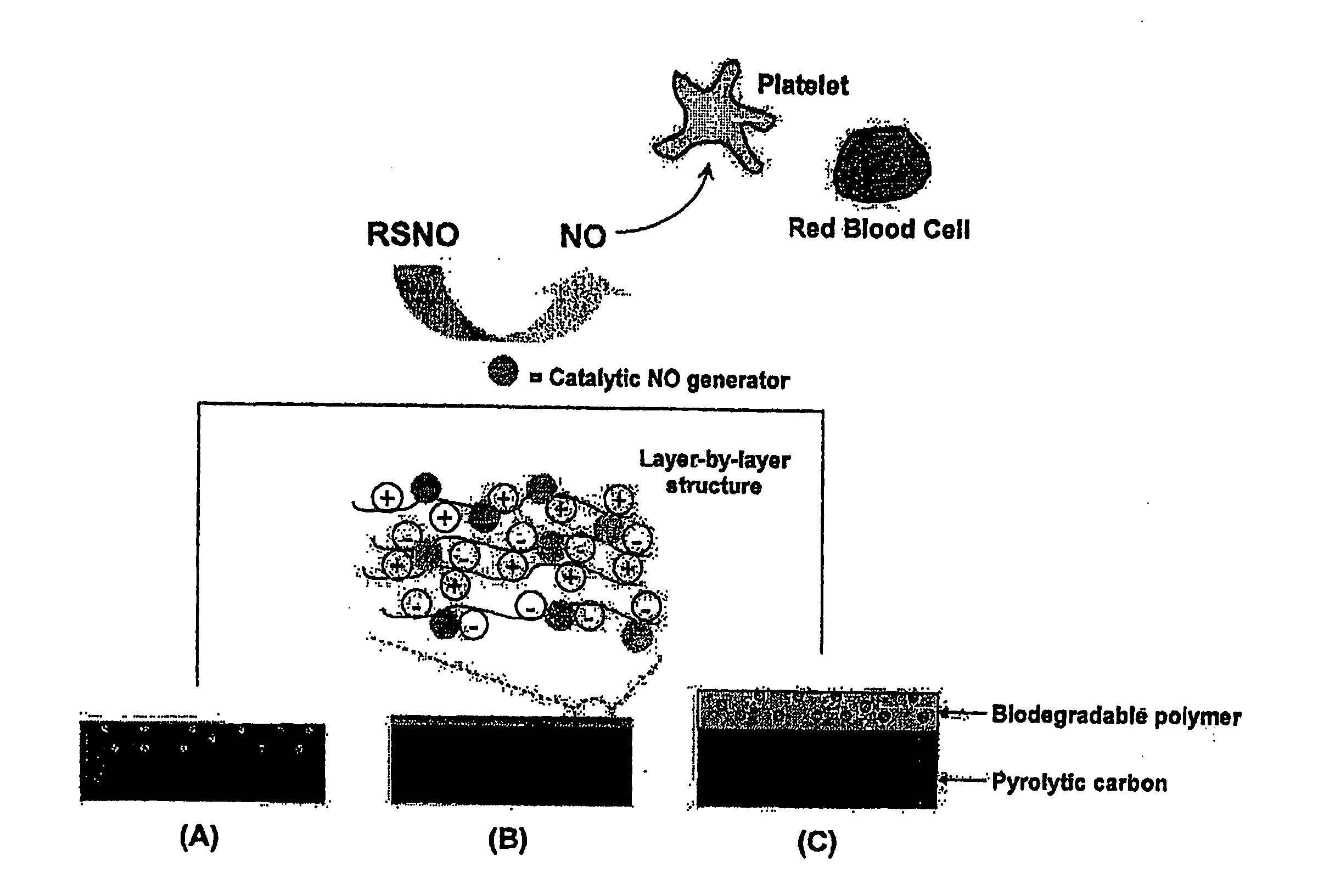
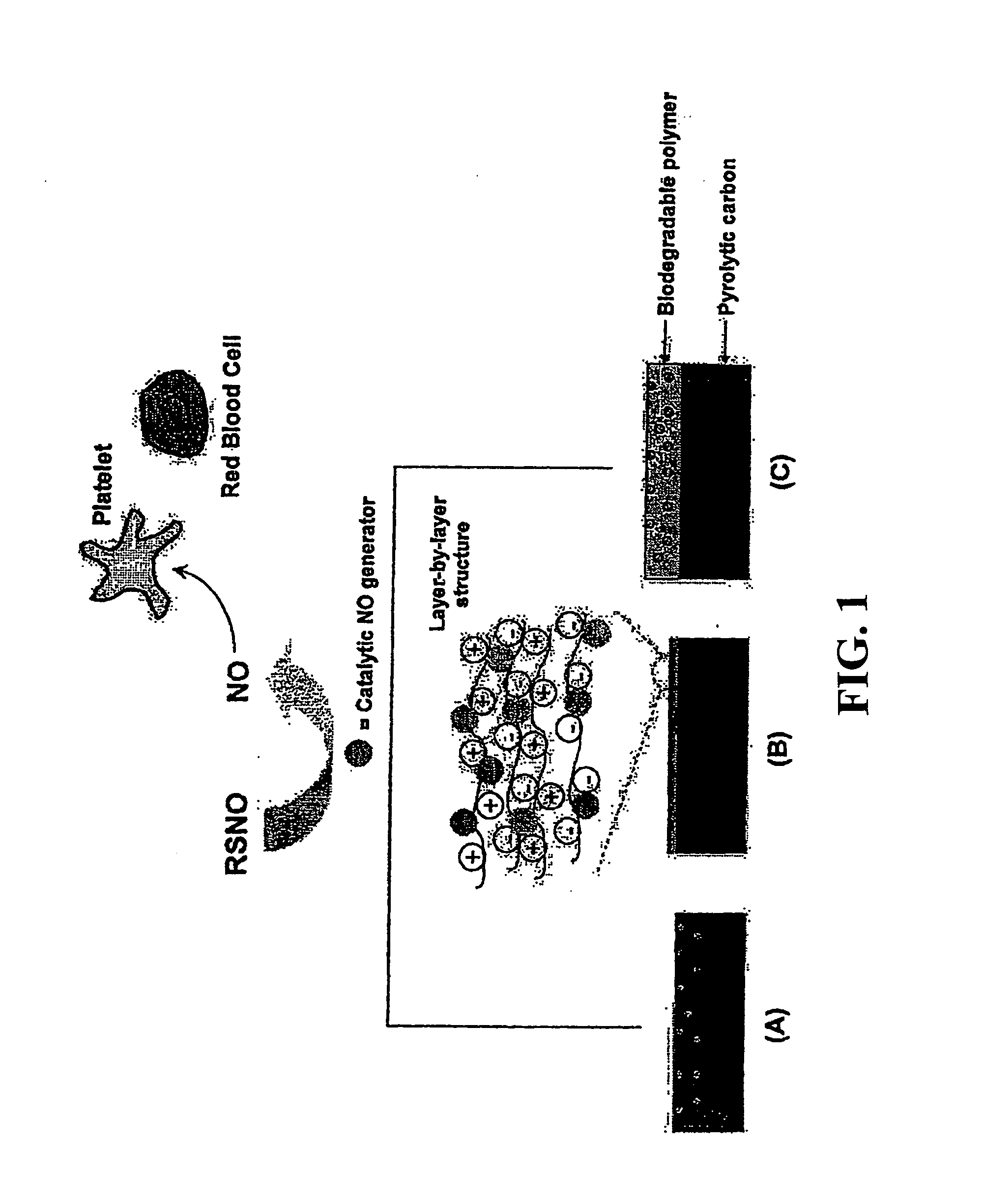
Enhancing biocompatibility of a medical device
ActiveUS20120121657A1Reduce morbidityGood biocompatibilityBiocideSurgeryPyrolytic carbonBiocompatibility
The present invention relates to a medical device comprising both pyrolytic carbon and an NO generator, methods of making same, and methods of using same.
Owner:ST JUDE MEDICAL LLC
Combination x-ray radiation and drug delivery devices and methods for inhibiting hyperplasia
InactiveUS20020165423A1Promote endothelializationReduced dosages/concentrationsStentsElectrotherapyPercent Diameter StenosisBlood vessel
The present invention provides improved devices, methods, and kits for inhibiting restenosis and hyperplasia after intravascular intervention. In particular, the present invention provides controlled drug delivery in combination with x-ray radiation delivery to selected locations within a patient's vasculature to reduce and / or inhibit restenosis and hyperplasia rates with increased efficacy. In one embodiment, the combination radiation and agent delivery catheter for inhibiting hyperplasia comprises a catheter body having a proximal end and distal end, an x-ray tube coupleable to the catheter body for applying a radiation dose to a body lumen, and means coupleable to the catheter body for releasing an agent to the body lumen.
Owner:XOFT INC
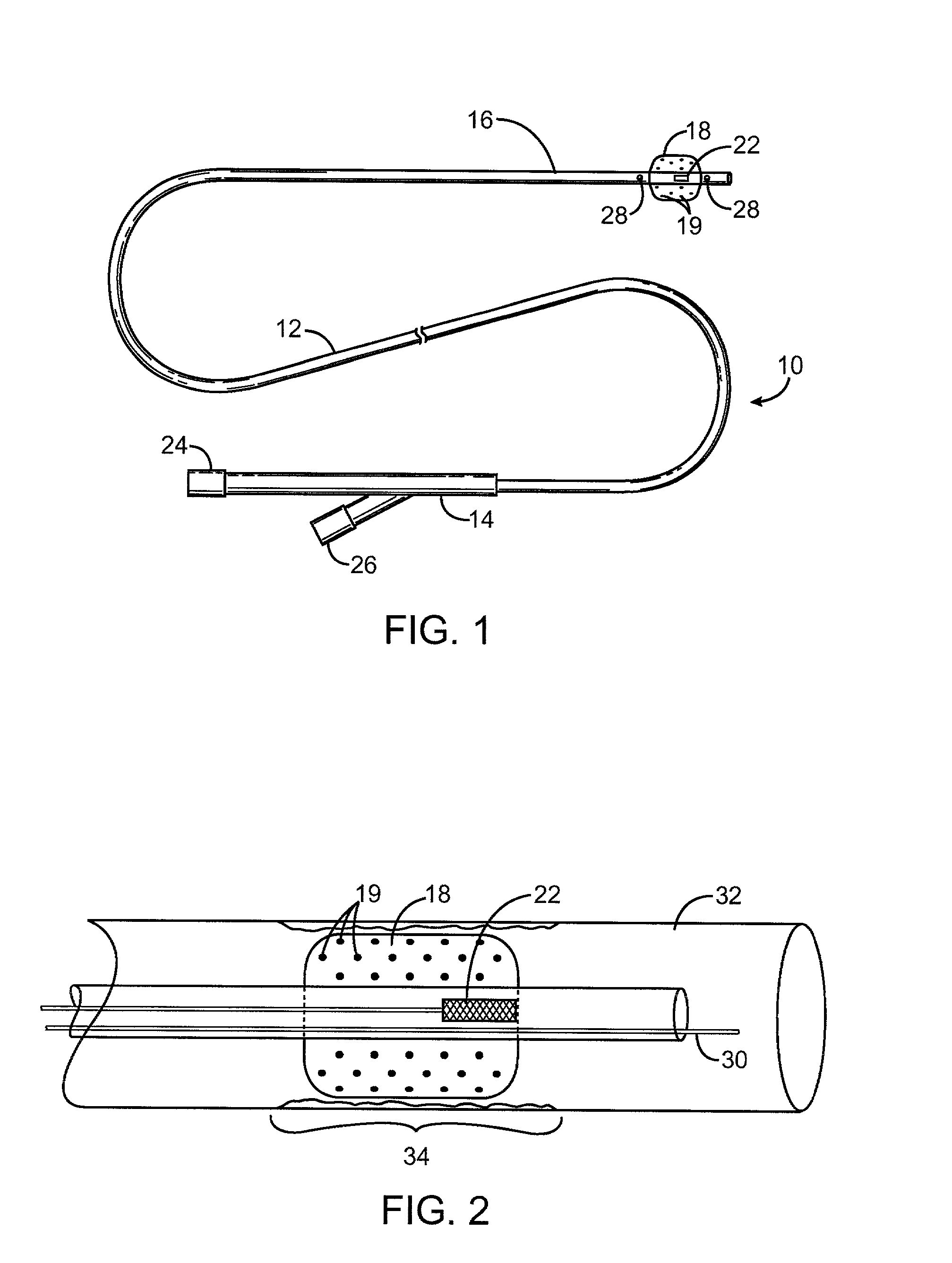
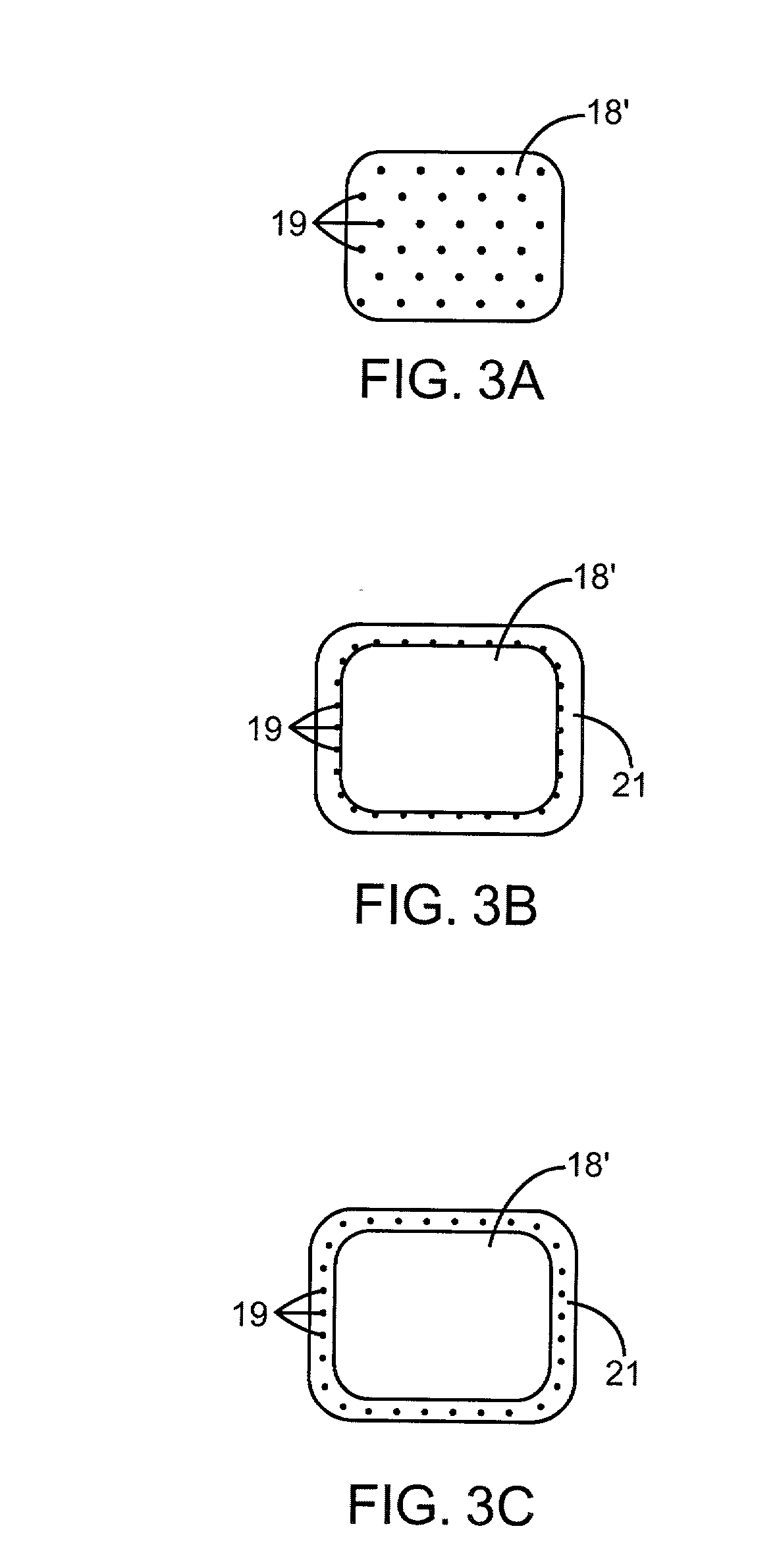
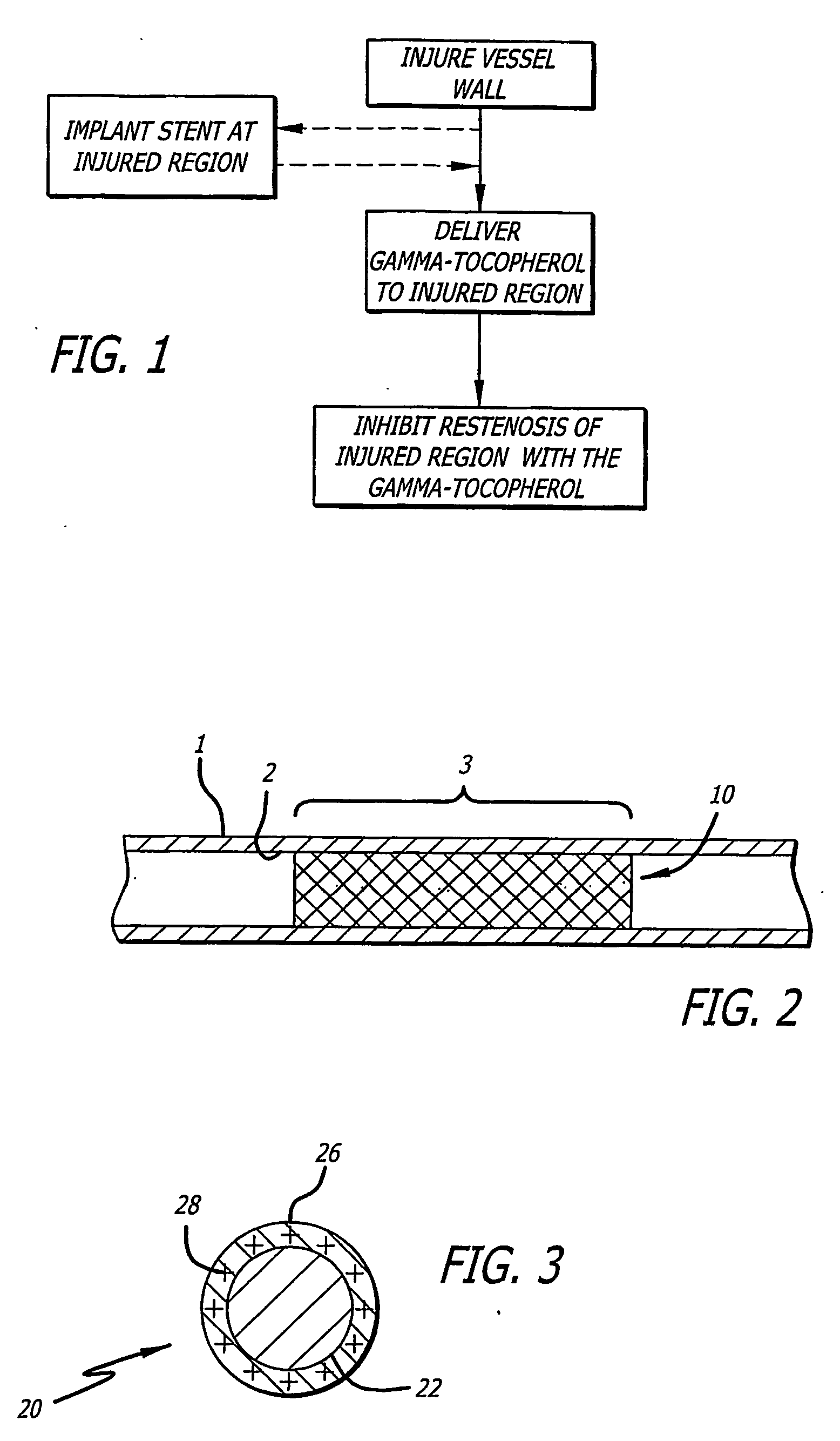
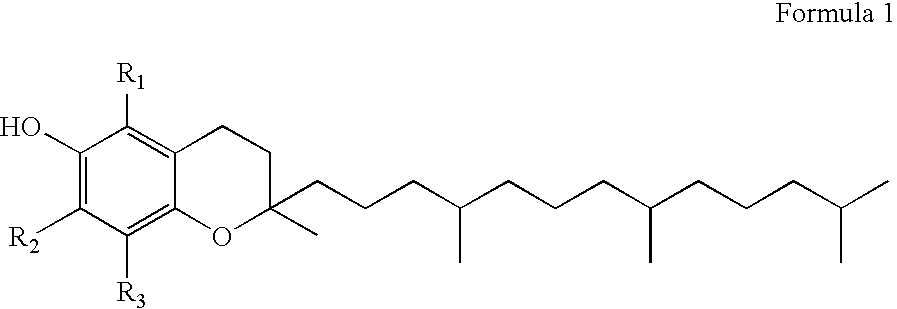
Gamma-tocopherol therapy for restenosis prevention
InactiveUS20070014827A1Reduce restenosisImprove biological activityOrganic active ingredientsSurgeryEverolimusPercent Diameter Stenosis
A stent is provided in combination with delivery of a tocopherol agent, and in particular a des-methyl tocopherol agent, and further beneficially a gamma-tocopherol agent, so as to reduce restenosis along the vessel or other lumenal wall where the stent is implanted. In particular applications, the stent is an endolumenal stent, and more specific beneficial applications is an endovascular stent, and the gamma-tocopherol elutes from a coating or carrier coupled with the stent. Certain combinations are provided with the des-methyl-tocopherol or phytyl substituted chromanol, e.g. gamma-tocopherol, combined with an additional agent such as an anti-restenosis agent, e.g. sirolimus, tacrolimus, everolimus, or paclitaxel, in order to provide synergistic benefit to tissues along the stented or recanalized regions. Other forms of tocopherol or tocotrienol, or other phytyl substituted chromanols, and other compounds such as palm oil, are also contemplated, for use to treat restenosis.
Owner:MEDLOGICS DEVICE CORP
Medical apparatus carrying gene and/or medicament and preparation method thereof
ActiveCN101502696AGood treatment effectReduce the incidence of restenosisMedical devicesProsthesisRestenosisPharmaceutical drug
The invention relates to a medical apparatus carrying gene and / or medicament and a preparation method thereof. The medical apparatus employs the electrostatic adhesion and / or micropore adhesion principle, the surface of the medical apparatus body is directly coated and / or fixed with active medicament and / or therapeutic genes, the invention has the advantages that various therapeutic genes and medicament are compounded on the same platform and the advantages of plural therapeutic genes and medicament are combined, thus radically solving the problems of restenosis and endothelialization delay. The preparation method is simple, the fixing effects of gene and medicament are good, the introduction of matrix carrier loaded with medicament and genes can be saved, the reaction hazard of restenosis and phlegmonosis caused by implantation can be reduced, and the cure effect is better than that of the current implanted medical apparatus.
Owner:LEPU MEDICAL TECH (BEIJING) CO LTD
Device for local and/or regional delivery employing liquid formulations of therapeutic agents
ActiveUS20110190876A1Elimination reactionReduce riskOrganic active ingredientsBiocideDiseaseThrombus
Medical devices may be utilized for local and regional therapeutic agent delivery. These therapeutic agents or compounds may reduce a biological organism's reaction to the introduction of the medical device to the organism. In addition, these therapeutic drugs, agents and / or compounds may be utilized to promote healing, including the prevention of thrombosis. The drugs, agents, and / or compounds may also be utilized to treat specific disorders, including restenosis, vulnerable plaque, and atherosclerosis in type 2 diabetic patients. In regional delivery, liquid formulations may be desirable to increase the efficacy and deliverability of the particular drug. Various materials and coating methodologies may be utilized to maintain the agents or compounds on the medical device until delivered and positioned.
Owner:CORDIS CORP
Vessel stent and production method thereof
InactiveCN104720941AIncrease mesh densityReduced risk of ruptureStentsSurgeryLesion siteInsertion stent
A vascular stent and a method for manufacturing same. The vascular stent has a mesh-shaped tubular structure. The mesh-shaped tubular structure is formed by braiding a metal wire and a biodegradable wire. The vascular stent has the following advantages: First, the stent has a thinner wall, facilitating implantation of the vascular stent into a narrower blood vessel and reducing the risk of restenosis after the stent is implanted in a small blood vessel. Secondly, the rejection caused by the presence of foreign bodies in the human body is reduced, and endothelialization of tissues is promoted. Thirdly, the vascular stent is allowed to have a greater mesh density, so that hemodynamics at the lesion site in the blood vessel can be improved after the vascular stent is implanted in the human body.
Owner:MICROPORT NEUROTECH SHANGHAI
Antithrombotic and restenosis blood vessel bracket and method for preparing the same
InactiveCN101195047AExcellent anticoagulant propertiesInhibits smooth muscle cell proliferationSurgeryCoatingsPercent Diameter StenosisAntibody
The invention relates to an effectively anti-thrombotic restenosis blood vessel inner support, and comprises a blood vessel inner support body. The invention is characterized in that the surface of the support body has a surface modifying layer which has A20 or A20 and correlation factors which can capture auto-endothelial progenitor cells in vivo, the A20 is A20 eukaryotic expression carriers, viral vectors, or A20 protein, and the correlation factors which can capture auto-endothelial progenitor cells in vivo are selected from esoderma growth factor II type receptor KDR antibodies, CD34 antibodies, or CD133 antibodies. The invention further relates to the process for preparing the support, the support can in vivo induce the endothelial progenitor cells and endothelial cells in the circulating blood to plant, and inhibit smooth muscle cells from breeding, and the performances of antithrombotic formation and anti-vessel restenosis are provided. The invention is used in the support plasty after the blood vessel is clinically provided with restenosis.
Owner:ARMY MEDICAL UNIV +1
Small and medium diameter artificial blood vessel with adjustable pressure and flow
The invention discloses a small and medium diameter artificial blood vessel with adjustable pressure and flow. The artificial blood vessel comprises a blood vessel wall, a blood vessel cavity is in the blood vessel wall, and a pressure and flow controller capable of adjusting the diameter of the artificial blood vessel is arranged outside the artificial blood vessel. Concave and convex grains are arranged on the blood vessel wall. The pressure and flow controller comprises a fixed shell and a control spring, wherein the fixed shell takes the shape of a cylindrical structure, and the control spring is configured to be a cylindrical structure, the control spring is arranged in the fixed shell and sleeved outside of the blood vessel wall of the artificial blood vessel, the control spring is provided with a limit spring handle for adjusting the diameter of the blood vessel, and the spring handle passes through a control frame. The fixed shell is provided with position fixing holes, and the control spring is provided with position fixing holes. The fixed shell is provided with a fastener. The pressure and flow controller comprises a mechanical pressure and flow controller, an in-vitro remote control pressure and flow controller and an in-vivo induction pressure and flow controller. The blood vessel is applicable to controlling the size of fractional flow according to an induced pressure of a hepatic portal vein after a portosystemic blood vessel is anastomosed and shunted so as to ensure the hepatic portal vein perfusion pressure required by individuals.
Owner:SECOND MILITARY MEDICAL UNIV OF THE PEOPLES LIBERATION ARMY
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com