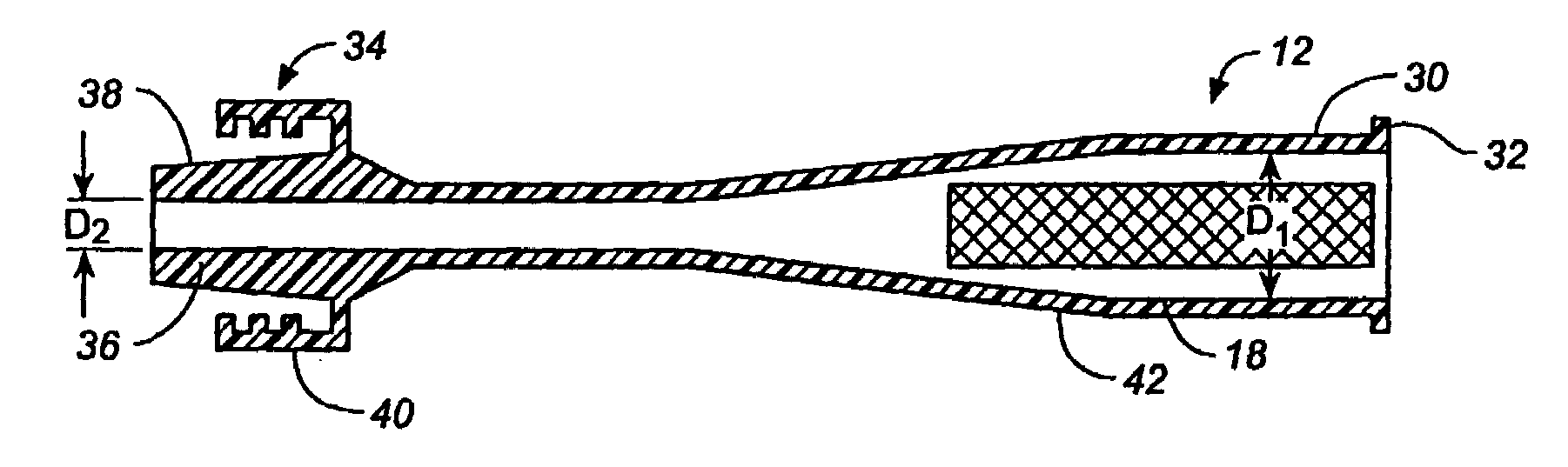Patents
Literature
43 results about "Radiopaque agent" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Radiopaque agents are drugs used to help diagnose certain medical problems. They contain iodine, which blocks x-rays. Depending on how the radiopaque agent is given, it localizes or builds up in certain areas of the body.
Drug delivery devices and methods
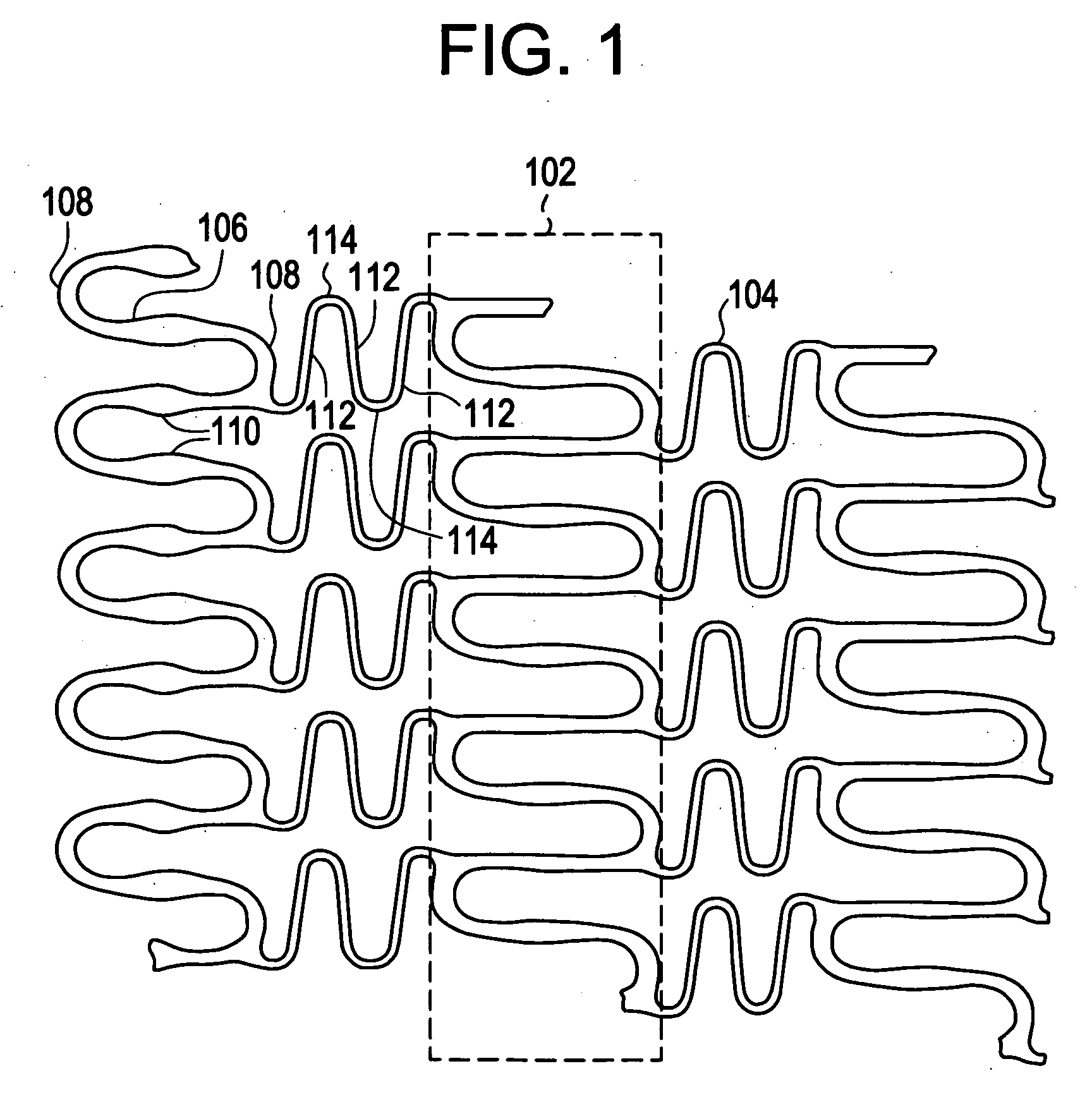
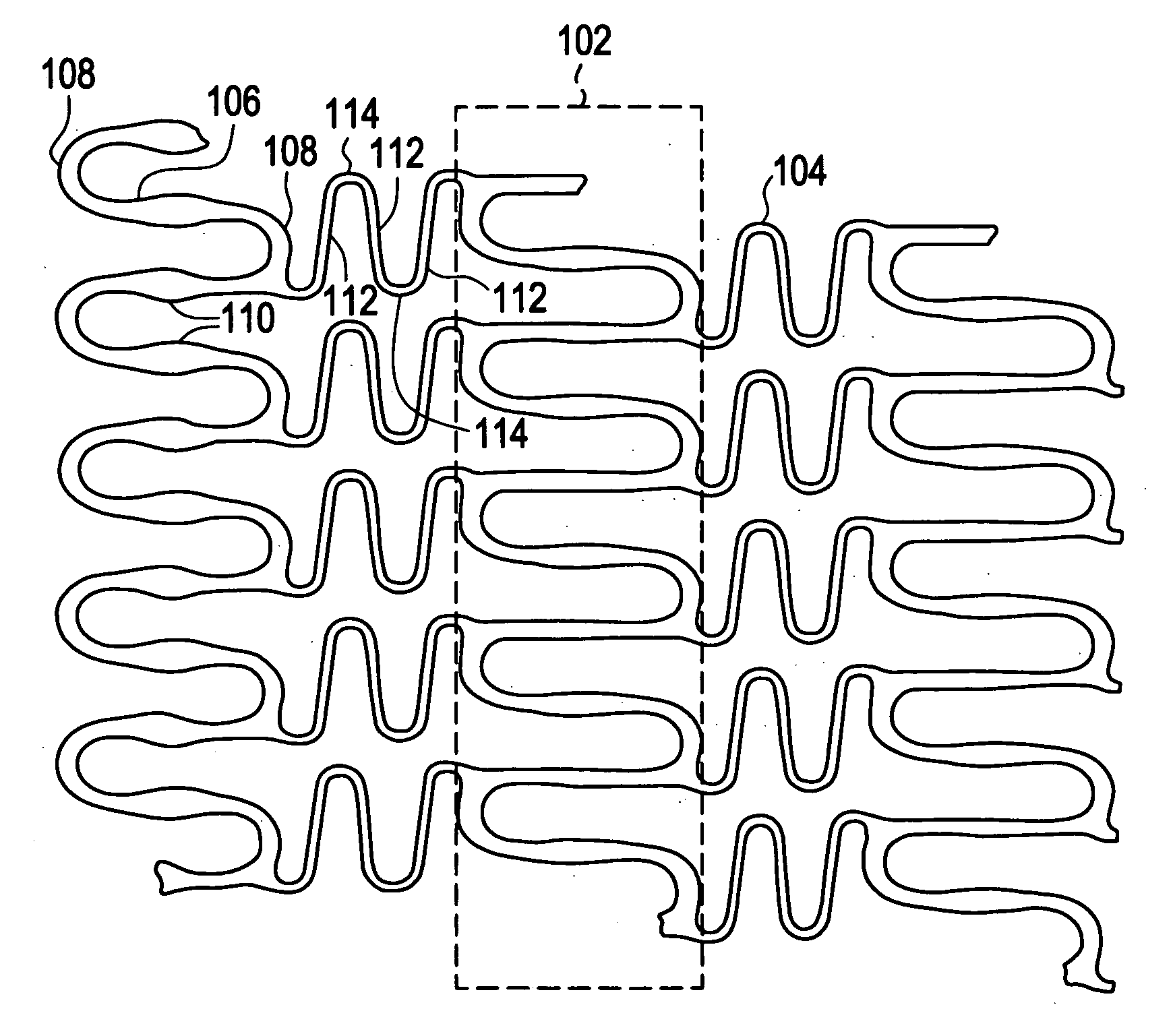
InactiveUS20080051866A1Designing can be facilitatedWide load rangeStentsHeart valvesRadiopaque agentActive agent
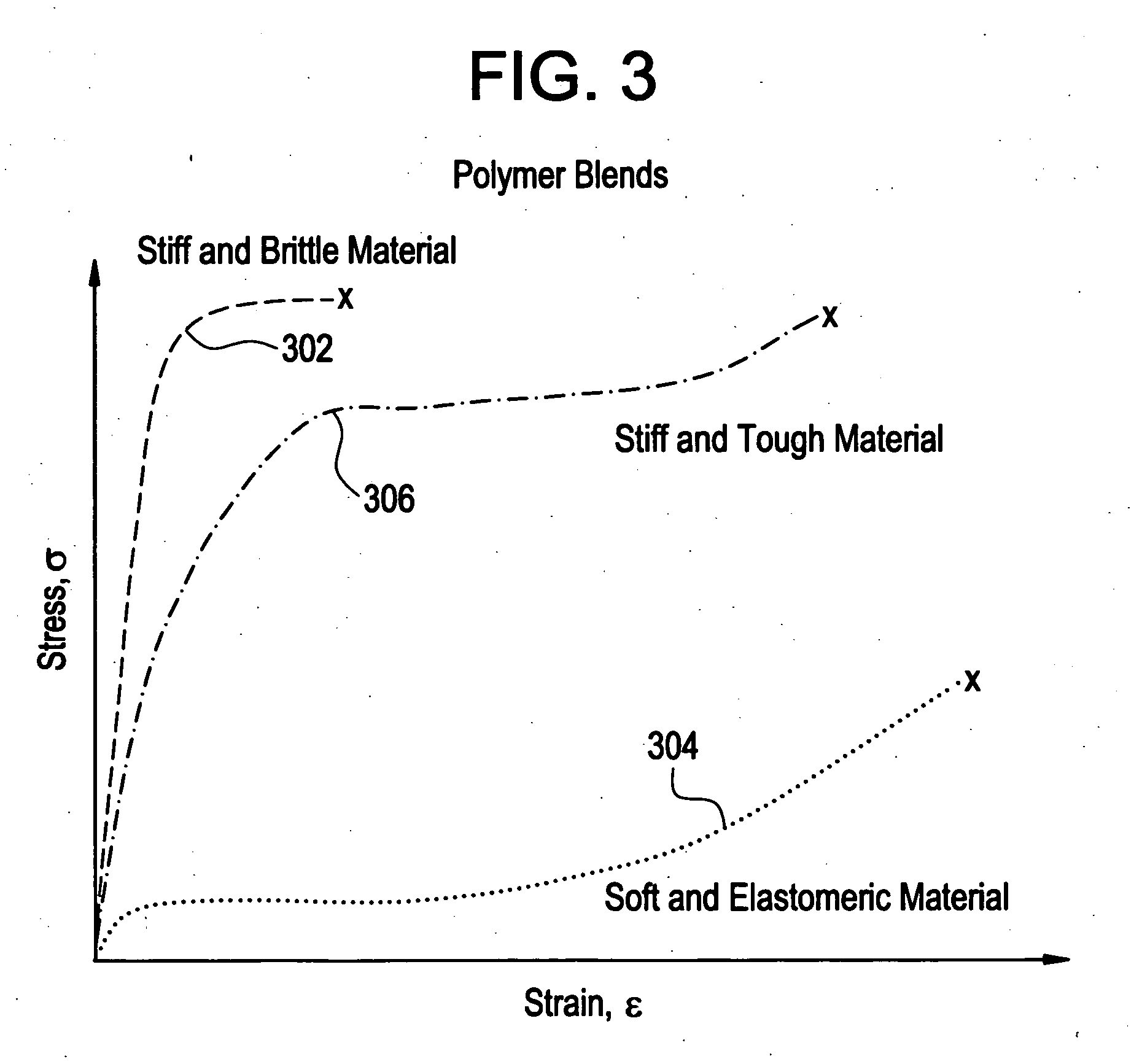
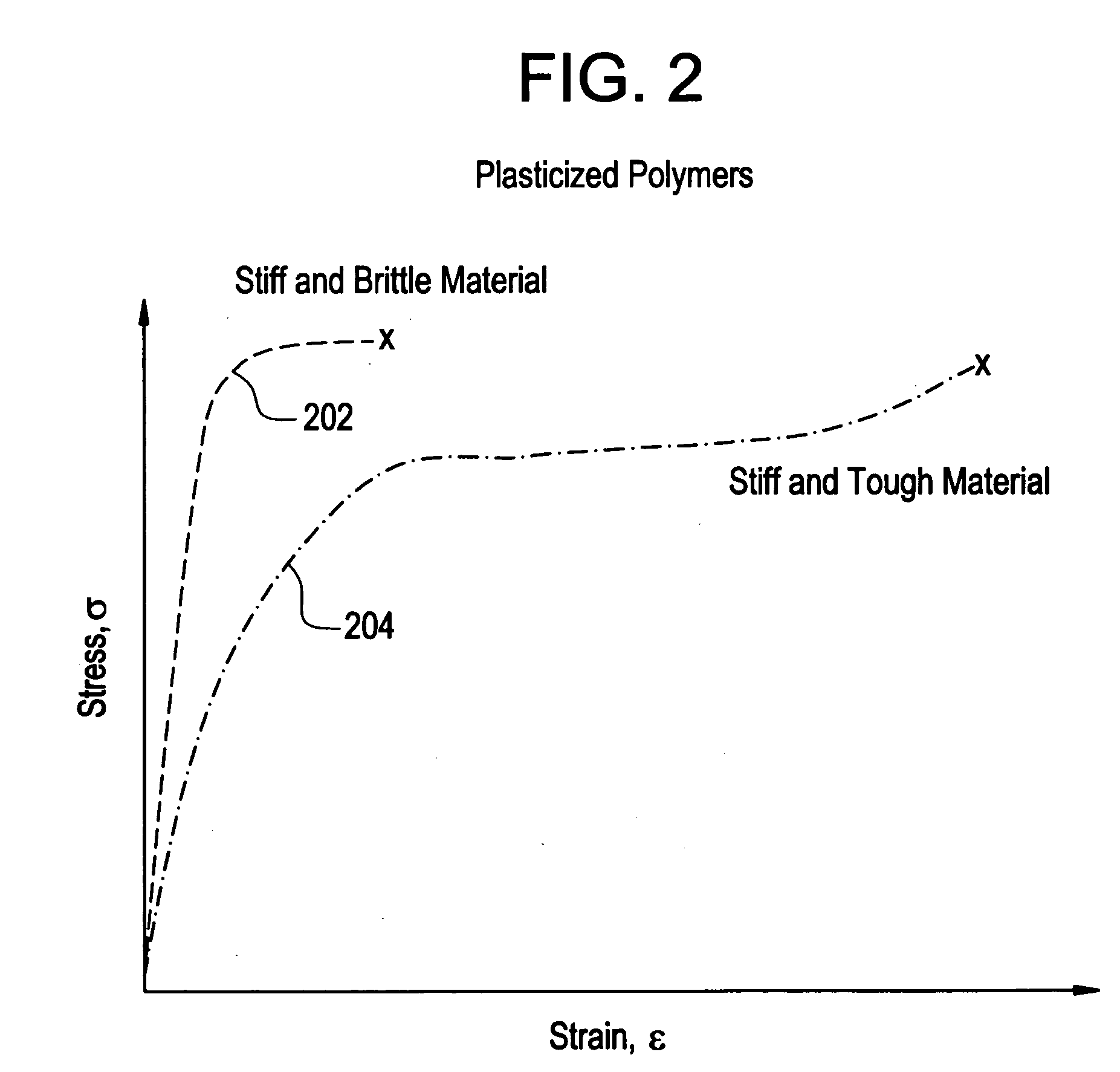
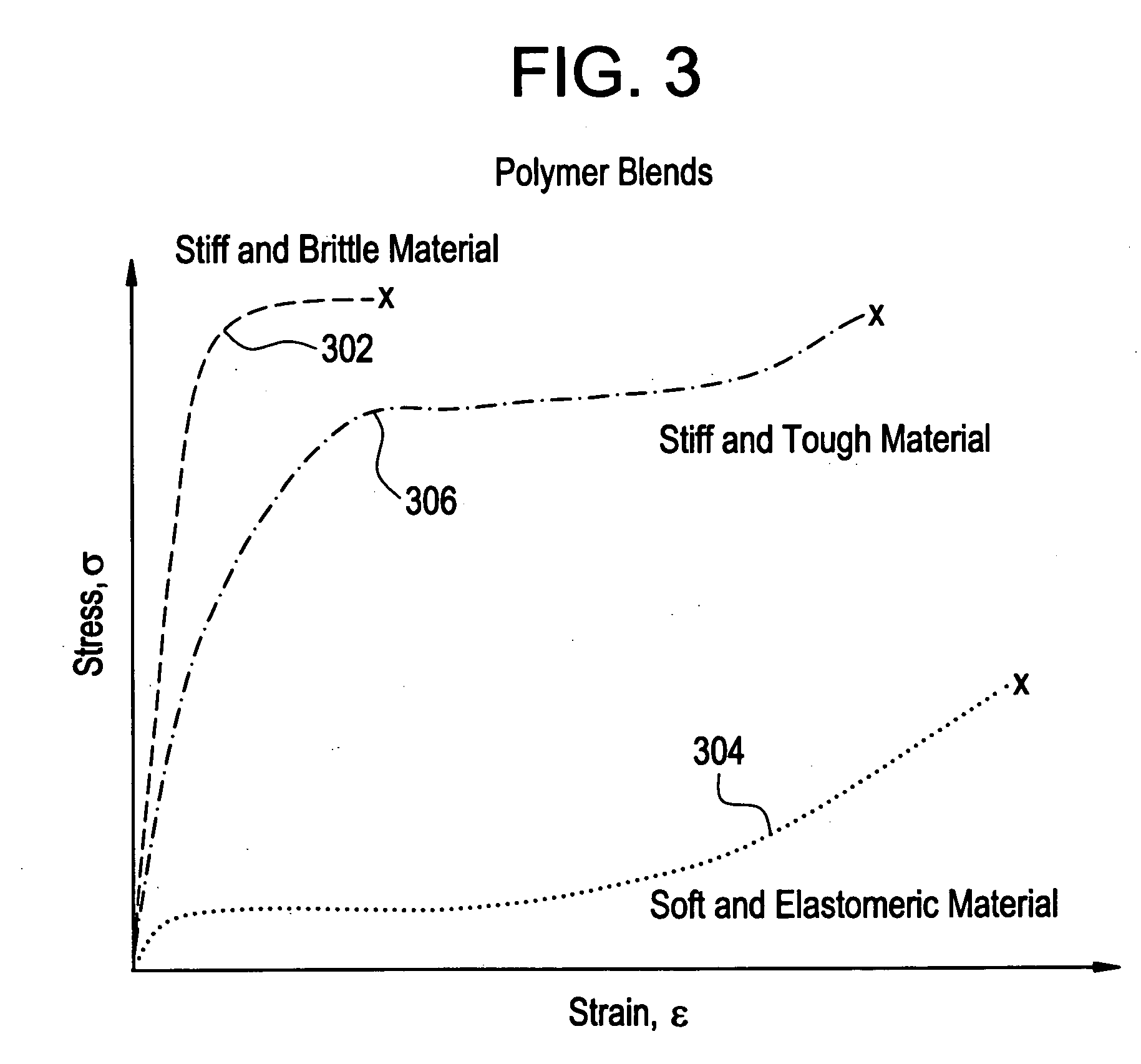
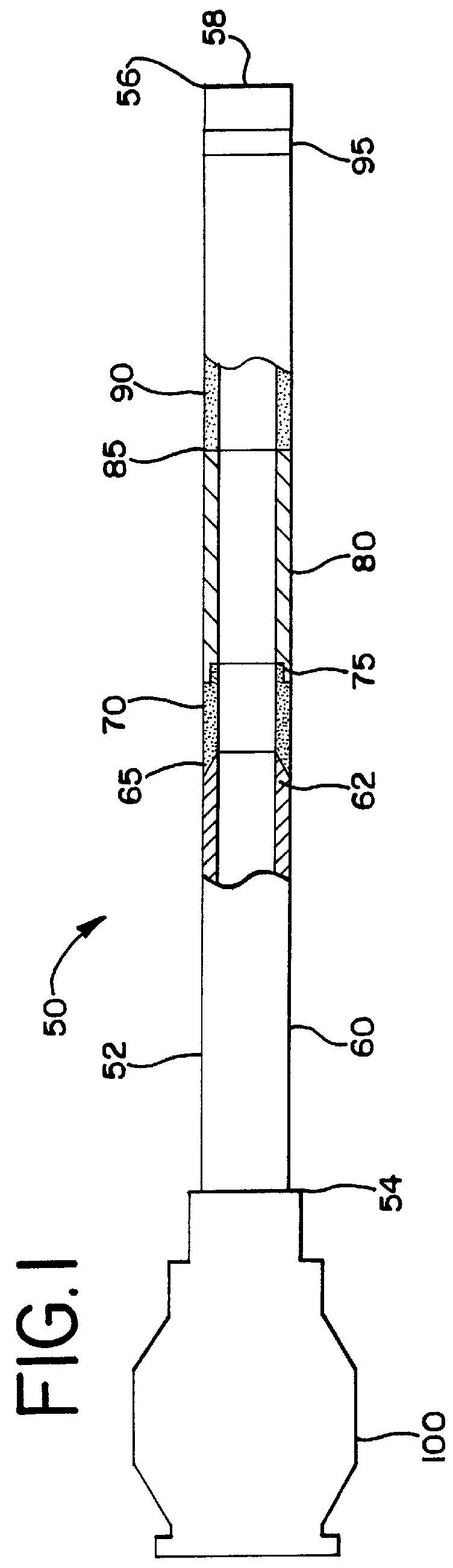
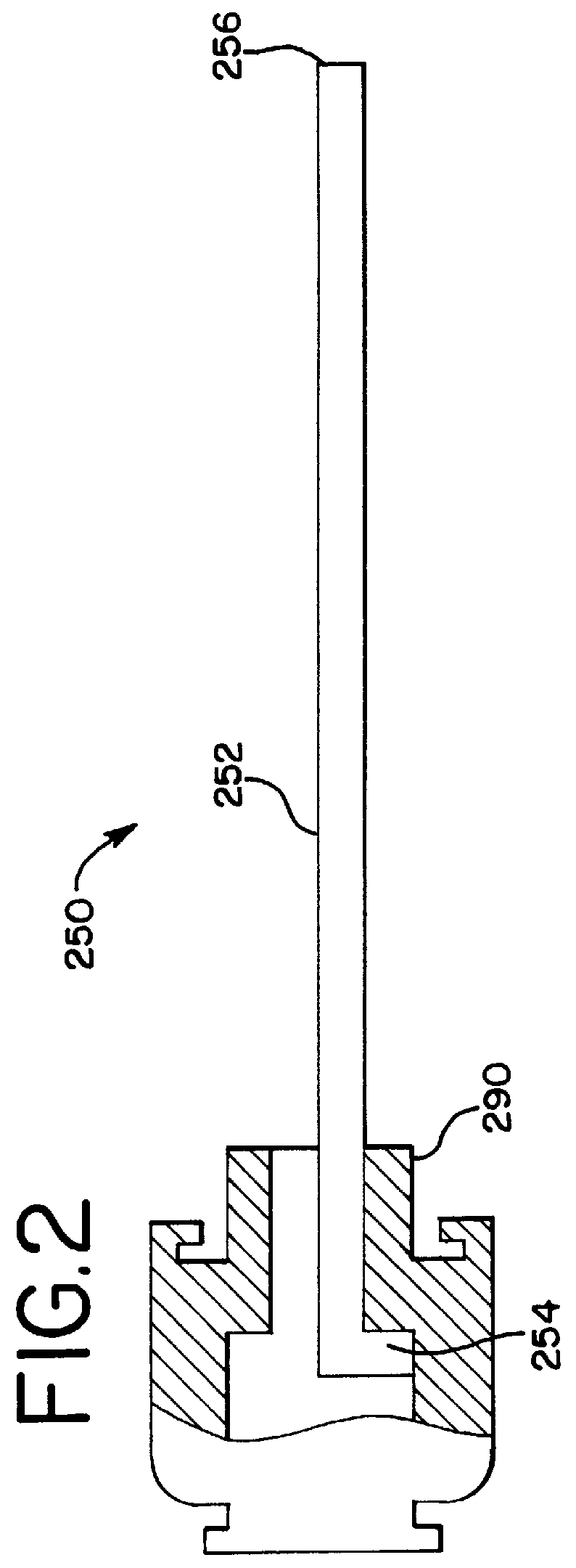
A biocompatible material may be configured into any number of implantable medical devices including intraluminal stents. Polymeric materials may be utilized to fabricate any of these devices, including stents. The stents may be balloon expandable or self-expanding. The polymeric materials may include additives such as drugs or other bioactive agents as well as radiopaque agents. By preferential mechanical deformation of the polymer, the polymer chains may be oriented to achieve certain desirable performance characteristics.
Owner:CORDIS CORP
Means and method for the treatment of cerebral aneurysms
Disclosed is a system for the treatment of cerebral aneurysms using a stent and a aneurysm pocket fill structure delivery system. One embodiment of the present invention uses a highly radiopaque, drug eluting stent that is deployed with its sidewall over the ostium of the aneurysm pocket. A fill structure delivery catheter is then advanced through the patient's vascular system until the catheter's distal end is situated within the aneurysm pocket. Compressed aneurysm pocket filling structures are then pushed through the fill structure delivery catheter. As the aneurysm pocket filling structures emerge from an opening in the catheter's distal end, they promptly expand so that their minimum dimension is sufficiently large so that they cannot pass through the spaces between the struts of the stent that cover the ostium of the aneurysm pocket.
Owner:FISCHELL ROBERT E +1
Endovascular aneurysm treatment device and delivery system
The present invention is directed to an intravascular treatment for intracranial aneurysms. The invention consists of a patch stent and, also, a patch stent delivery system allowing one to rotate the stent to align the patch with the neck of the aneurysm. A self-expanding nitinol framework holds the patch in place, a radiopaque agent allows the patch location to be visualized and a pusher tube that is mechanically locked into the unexpanded stent is used to push the stent out of the catheter with rotational and longitudinal control necessary to align the patch with the aneurysm. A self-expanding framework is used to support a patch at the neck of the aneurysm. The patch is designed to reduce the blood circulation in the aneurysm and the stagnant blood will clot or thrombus. The thrombus will stop any current blood leakage into the brain and will dramatically reduce the possibility of future leaks or potentially deadly ruptures. Over time the thrombus will be absorbed and the volume of the aneurysm will shrink reducing pressure on surrounding tissue.
Owner:HINES RICHARD ALLEN
Polymeric marker with high radiopacity for use in medical devices
InactiveUS20050255317A1Overcomes shortcomingImprove fill ratePowder deliverySurgeryPolymer resinRadiopaque agent
High radiopacity is achieved in a polymeric marker by combining a polymeric resin, a powdered radiopaque agent having uniformly shaped particles of a specific particle size distribution, and a wetting agent. The method to produce the marker calls for the blending and pelletization of these materials followed by extrusion onto support beading. The resulting supported tubing is subsequently cut to length with the beading still in place. After ejection of the beading remnant the marker is slipped into place on the device to be marked and attached by melt bonding. Marking of a guide wire allows lesions to be measured while the marking of balloon catheters allow the balloon to be properly positioned relative to a lesion.
Owner:ABBOTT CARDIOVASCULAR
Polymeric marker with high radiopacity for use in medical devices
ActiveUS7303798B2Overcomes shortcomingEnhance wetting and adhesive and flow propertyMedical devicesDischarge tube main electrodesPolymer resinRadiopaque agent
High radiopacity is achieved in a polymeric marker by combining a polymeric resin, a powdered radiopaque agent having uniformly shaped particles of a specific particle size distribution and a wetting agent. The method to produce the marker calls for the blending and pelletization of these materials followed by extrusion onto support beading. The resulting supported tubing is subsequently cut to length with the beading still in place. After ejection of the beading remnant the marker is slipped into place on the device to be marked and attached by melt bonding. Marking of a guidewire allows lesions to be measured while the marking of balloon catheters allow the balloon to be properly positioned relative to a lesion.
Owner:ABBOTT CARDIOVASCULAR
Polymeric marker with high radiopacity
InactiveUS20050064223A1Overcomes shortcomingImprove fill rateSurgeryConductive materialPolymer resinRadiopaque agent
High radiopacity is achieved in a polymeric marker by combining a polymeric resin, a powdered radiopaque agent having uniformly shaped particles of a specific particle size distribution and a vetting agent. The method to produce the marker calls for the blending and pelletization of these materials followed by extrusion onto support beading. The resulting supported tubing is subsequently cut to length with the beading still in place. After ejection of the beading remnant the marker is slipped into place on the device to be marked and attached by melt bonding. Marking of a guidewire allows lesions to be measured while the marking of balloon catheters allow the balloon to be properly positioned relative to a lesion.
Owner:ABBOTT CARDIOVASCULAR
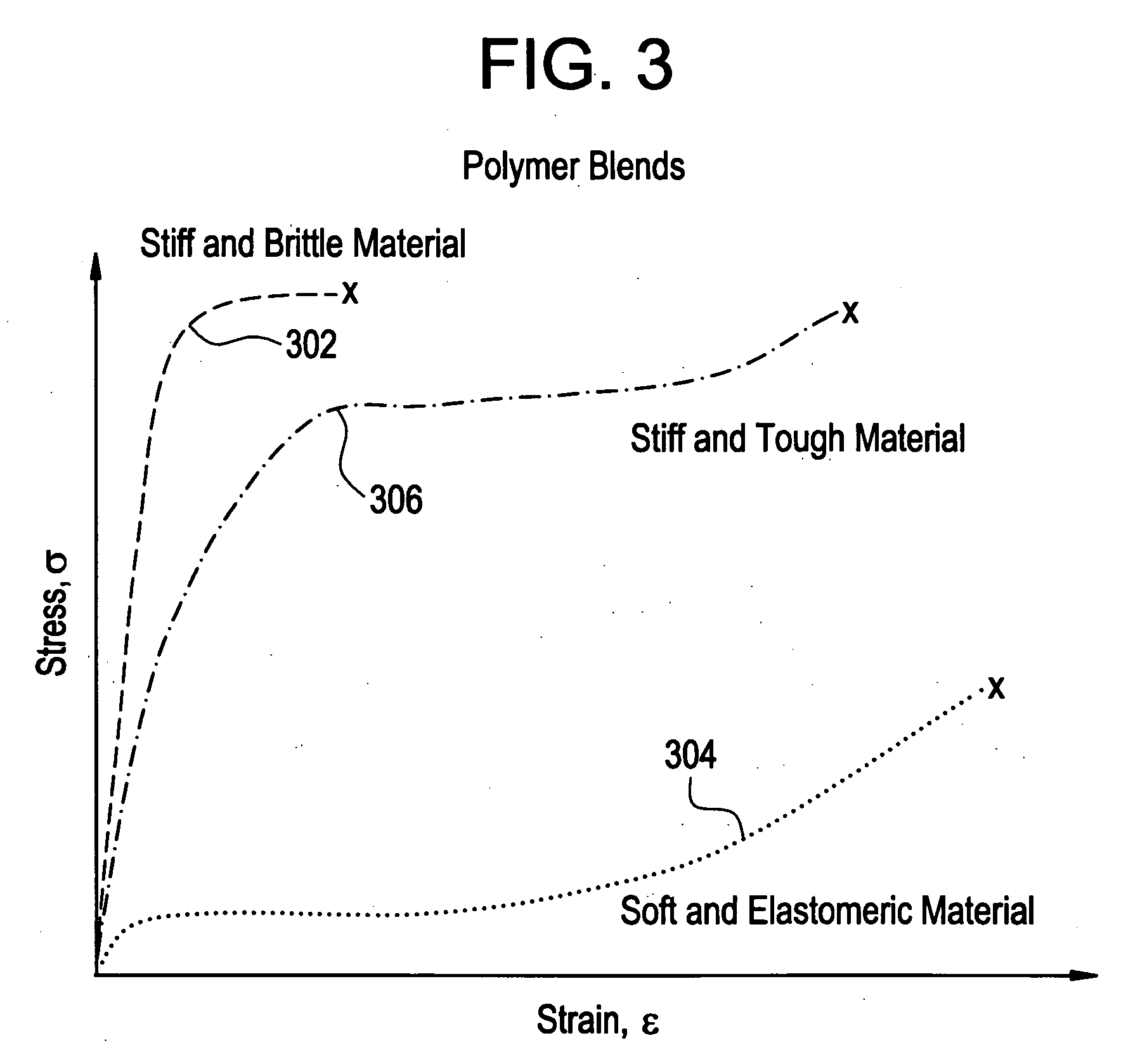
Implantable device formed from polymer blends
A biocompatible material may be configured into any number of implantable medical devices including intraluminal stents. Polymeric materials may be utilized to fabricate any of these devices, including stents. The stents may be balloon expandable or self-expanding. The polymeric materials may include additives such as drugs or other bioactive agents as well as radiopaque agents. By preferential mechanical deformation of the polymer, the polymer chains may be oriented to achieve certain desirable performance characteristics.
Owner:CORDIS CORP
Implantable device formed from polymer blends having modified molecular structures
InactiveUS20070203569A1Designing can be facilitatedWide load rangeStentsSurgeryDevice formActive agent
A biocompatible material may be configured into any number of implantable medical devices including intraluminal stents. Polymeric materials may be utilized to fabricate any of these devices, including stents. The stents may be balloon expandable or self-expanding. The polymeric materials may include additives such as drugs or other bioactive agents as well as radiopaque agents. By preferential mechanical deformation of the polymer, the polymer chains may be oriented to achieve certain desirable performance characteristics.
Owner:CORDIS CORP
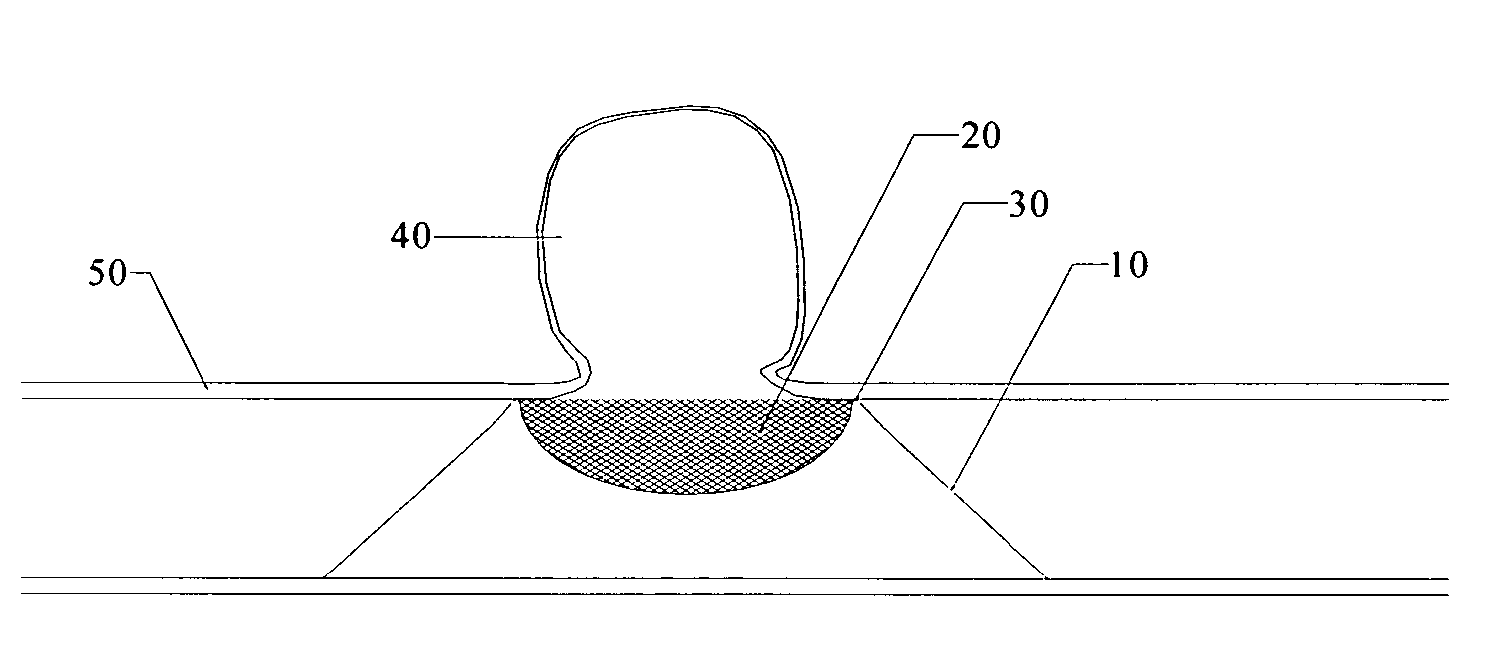
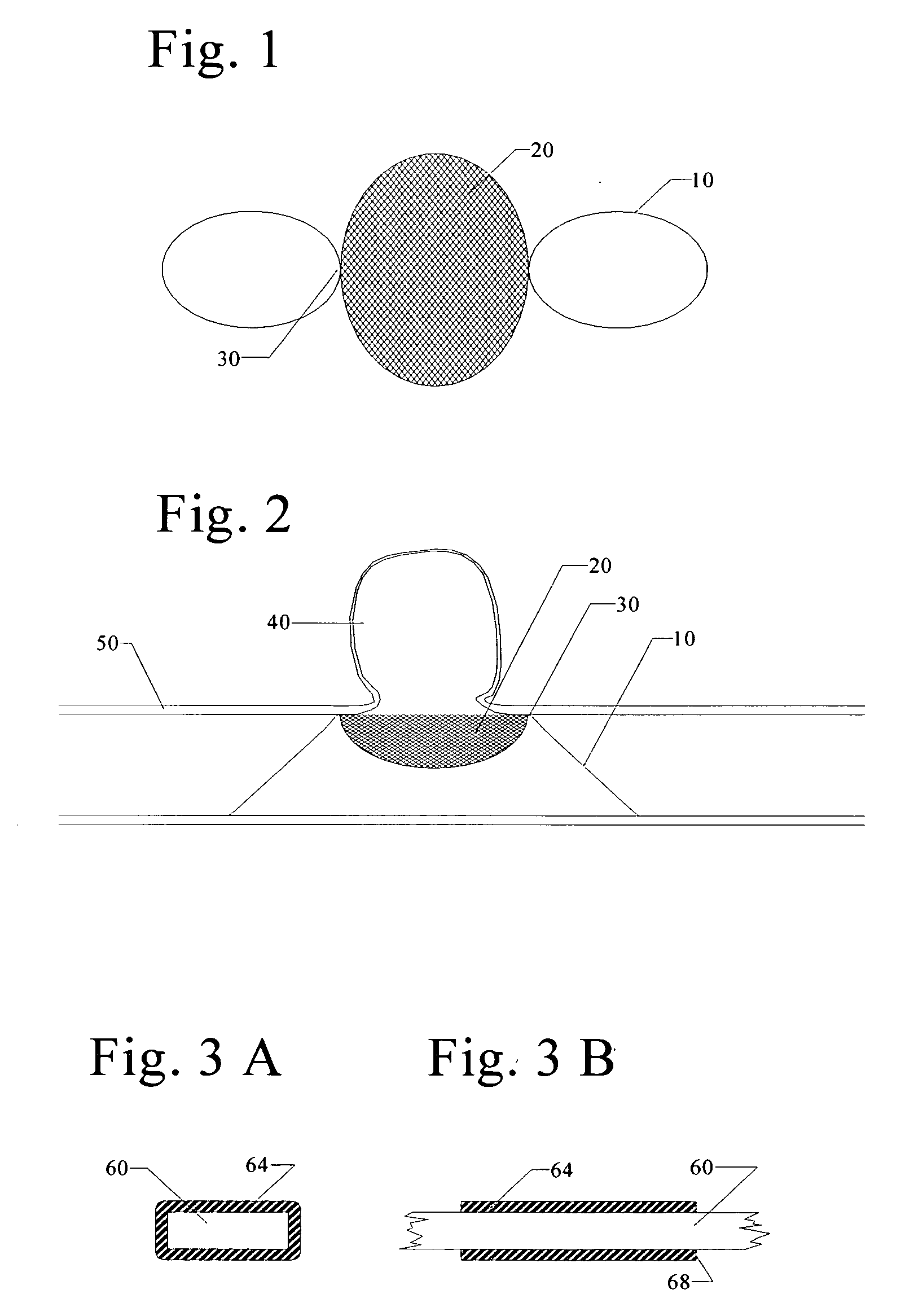
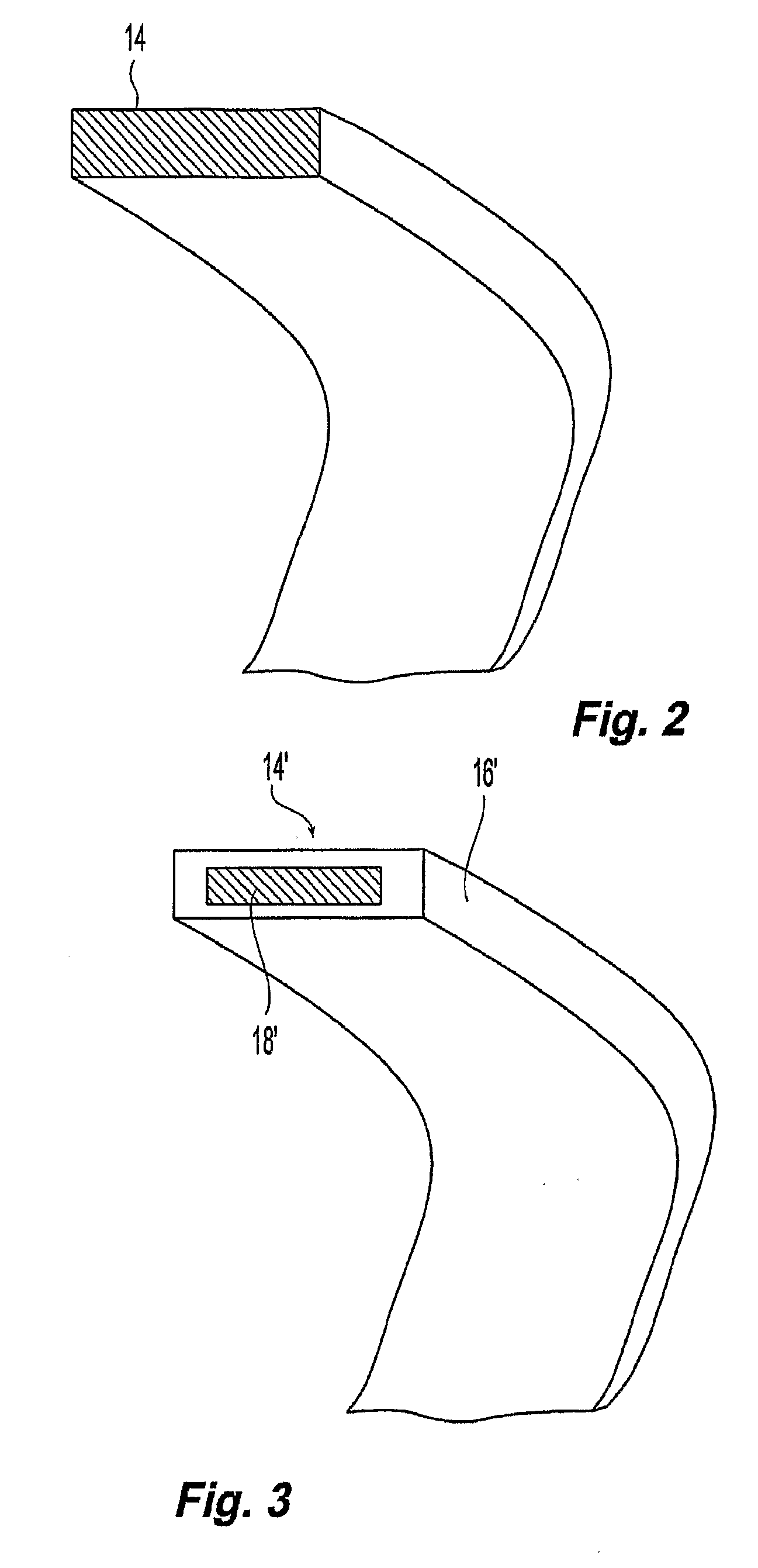
Grafts and stent grafts having a radiopaque beading
InactiveUS20090171436A1Meet needsProvide structural rigiditySurgeryDiagnostic recording/measuringRadiopaque agentStent grafting
A device and method provides a graft having a layer of synthetic non-metallic material including a first surface and a second surface spaced apart from the first surface. The device further includes a beading coupled to the layer and a radiopaque agent coupled to the beading. Another device and method provides a implantable prosthesis having a stent frame, a first inner layer and a second outer layer defining a central axis. The implantable prosthesis further includes a beading coupled to at least one the layers.
Owner:CR BARD INC
Polymeric marker with high radiopacity for use in medical devices
InactiveUS20050065434A1Overcomes shortcomingImprove fill ratePowder deliveryStentsRadiopaque agentBalloon catheter
High radiopacity is achieved in a polymeric marker by combining a polymeric resin, a powdered radiopaque agent having uniformly shaped particles of a specific particle size distribution, and a wetting agent. The method to produce the marker calls for the blending and pelletization of these materials followed by extrusion onto support beading. The resulting supported tubing is subsequently cut to length with the beading still in place. After ejection of the beading remnant the marker is slipped into place on the device to be marked and attached by melt bonding. Marking of a guide wire allows lesions to be measured while the marking of balloon catheters allow the balloon to be properly positioned relative to a lesion.
Owner:ABBOTT CARDIOVASCULAR
Polymeric marker with high radiopacity
ActiveUS20050064224A1Minimize and prevent flowingReduce wall thicknessMedical devicesDischarge tube main electrodesPolymer resinRadiopaque agent
High radiopacity is achieved in a polymeric marker by combining a polymeric resin, a powdered radiopaque agent having uniformly shaped particles of a specific particle size distribution and a wetting agent. The method to produce the marker calls for the blending and pelletization of these materials followed by extrusion onto support beading. The resulting supported tubing is subsequently cut to length with the beading still in place. After ejection of the beading remnant the marker is slipped into place on the device to be marked and attached by melt bonding. Marking of a guidewire allows lesions to be measured while the marking of balloon catheters allow the balloon to be properly positioned relative to a lesion.
Owner:ABBOTT CARDIOVASCULAR
Implantable device prepared from melt processing
InactiveUS20070200271A1Designing can be facilitatedWide load rangePeptide/protein ingredientsSurgeryActive agentInsertion stent
A biocompatible material may be configured into any number of implantable medical devices including intraluminal stents. Polymeric materials may be utilized to fabricate any of these devices, including stents. The stents may be balloon expandable or self-expanding. The polymeric materials may include additives such as drugs or other bioactive agents as well as radiopaque agents. By preferential mechanical deformation of the polymer, the polymer chains may be oriented to achieve certain desirable performance characteristics.
Owner:CORDIS CORP
Polymeric tissue sealant
ActiveUS20080253987A1High mechanical strengthReduces and prevents leakageOrganic active ingredientsImpression capsTissue sealantActive agent
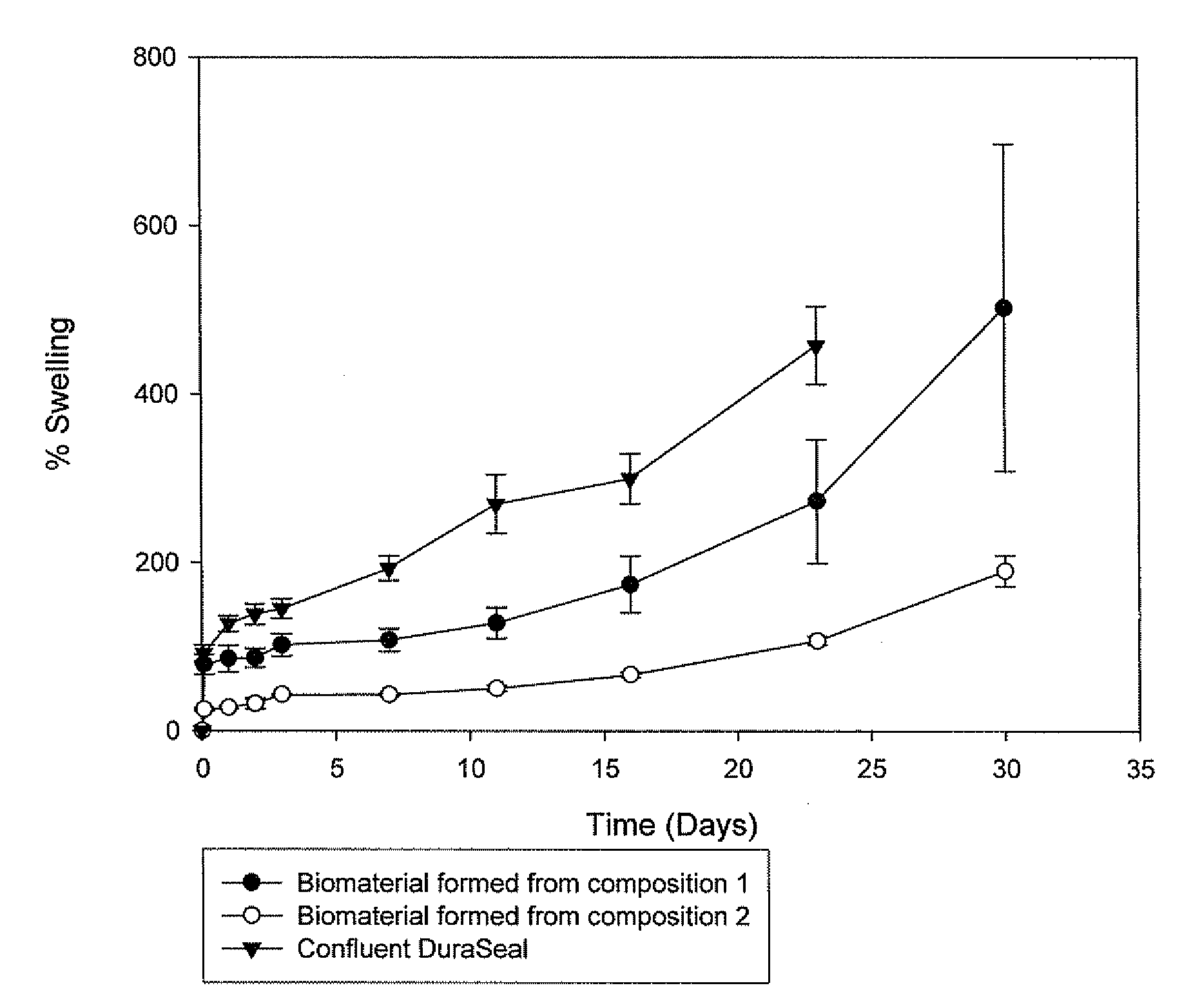
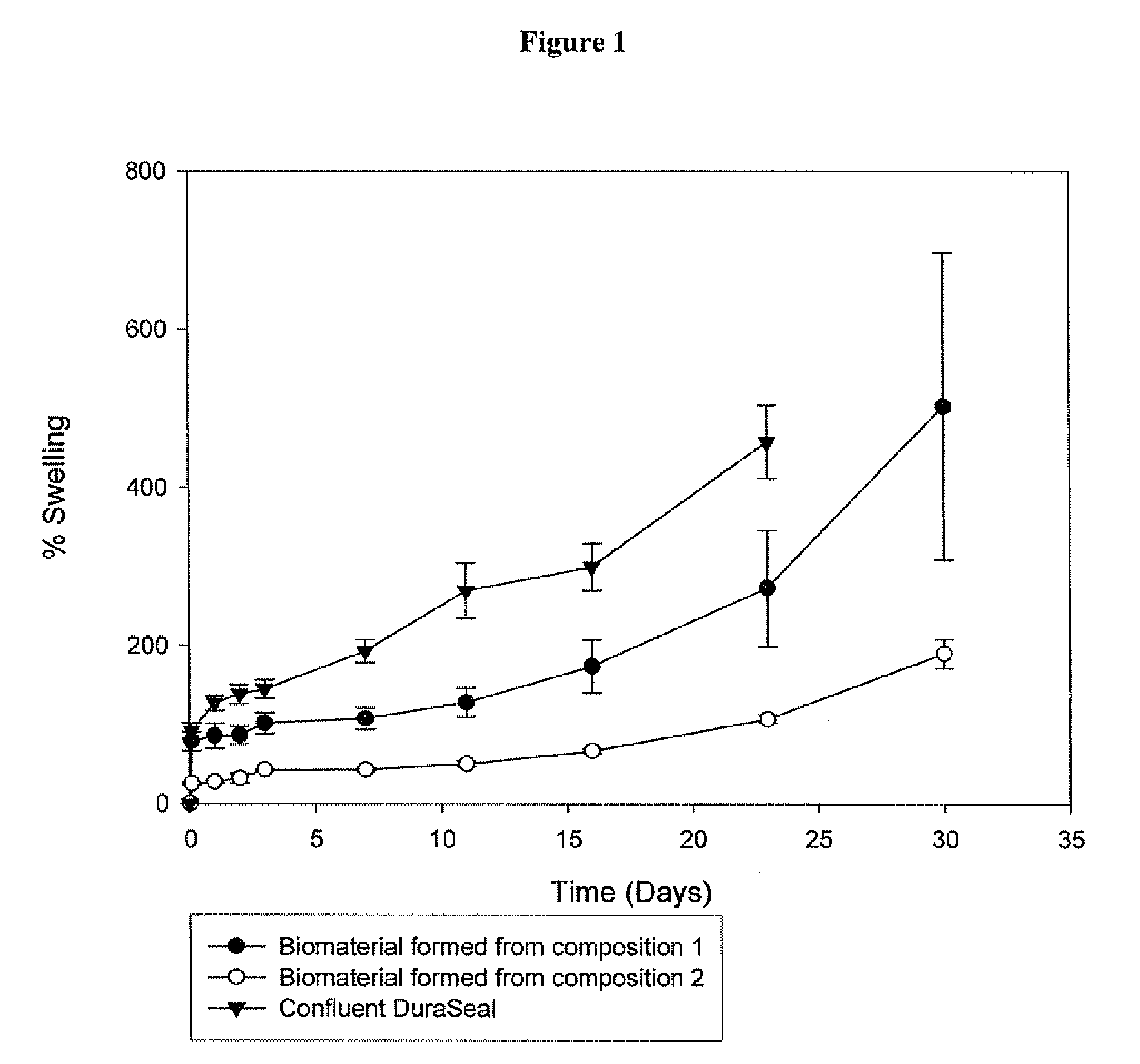
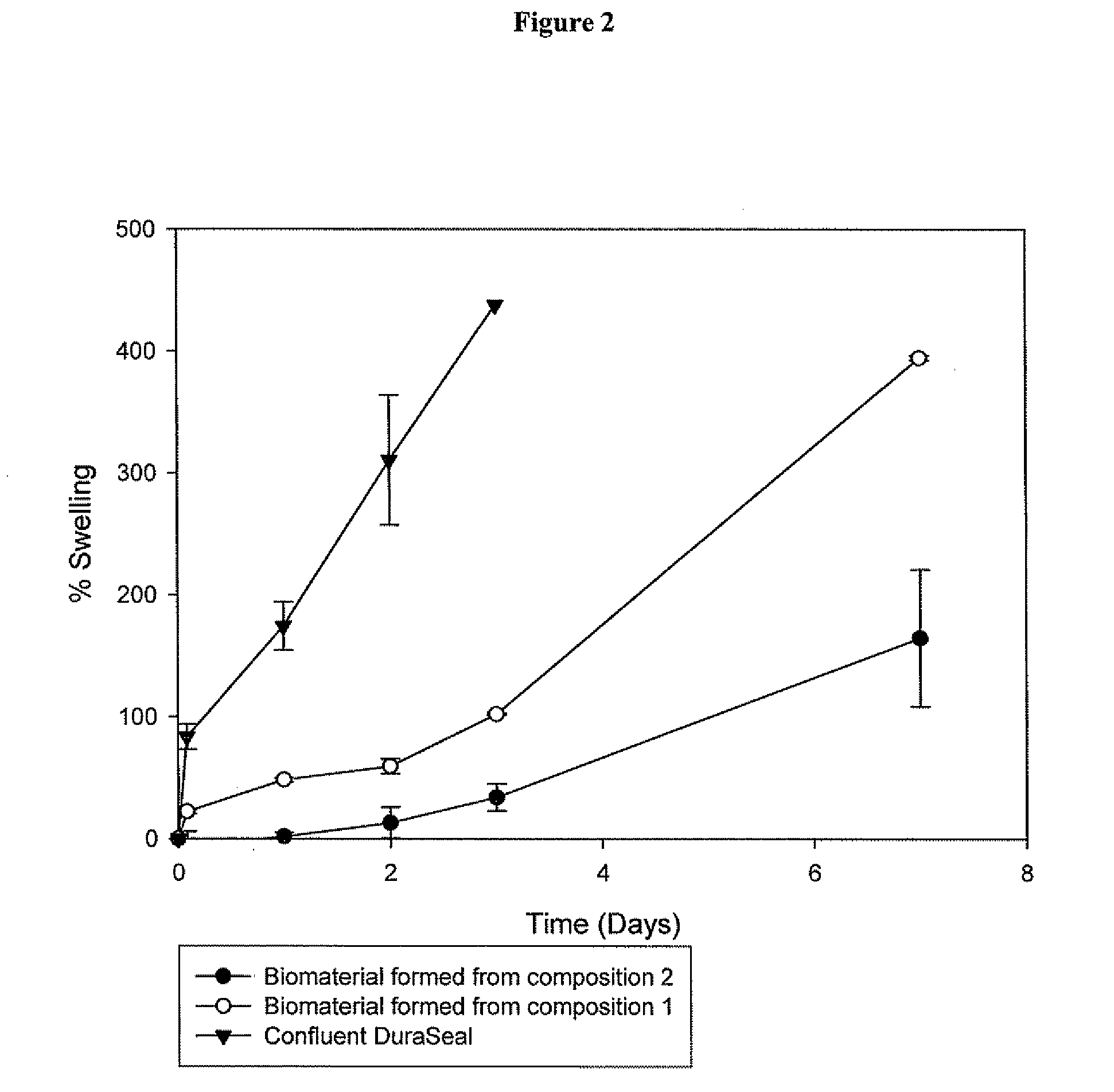
Methods for making biomaterials for use as a tissue sealant, kits containing precursors for forming the biomaterials, and the resulting biomaterials are described herein. The biomaterials are formed from a composition comprising at least a first and a second precursor molecule, wherein:i) the first precursor molecule is a poly(ethylene glycol) based polymer having x nucleophilic groups selected from the group consisting of thiol or amino groups, wherein x is greater than or equal to 2ii) the second precursor molecule is of the general formula:A-[(C3H6O)n—(C2H4O)m—B]i wherein m and n are integers from 1 to 200i is greater than 2A is a branch pointB is a conjugated unsaturated groupThe precursors are selected based on the desired properties of the biomaterial. Optionally, the biomaterials contain additives, such as thixotropic agents, radiopaque agents, or bioactive agents. In the preferred embodiment, the biomaterials are used to reduce, inhibit, or contain loss of a biological fluid or gas in a patient.
Owner:KUROS BIOSURGERY AG
Balloon expandable bioabsorbable drug eluting stent
ActiveUS20080132995A1Designing can be facilitatedWide load rangeStentsSurgeryEndoluminal stentActive agent
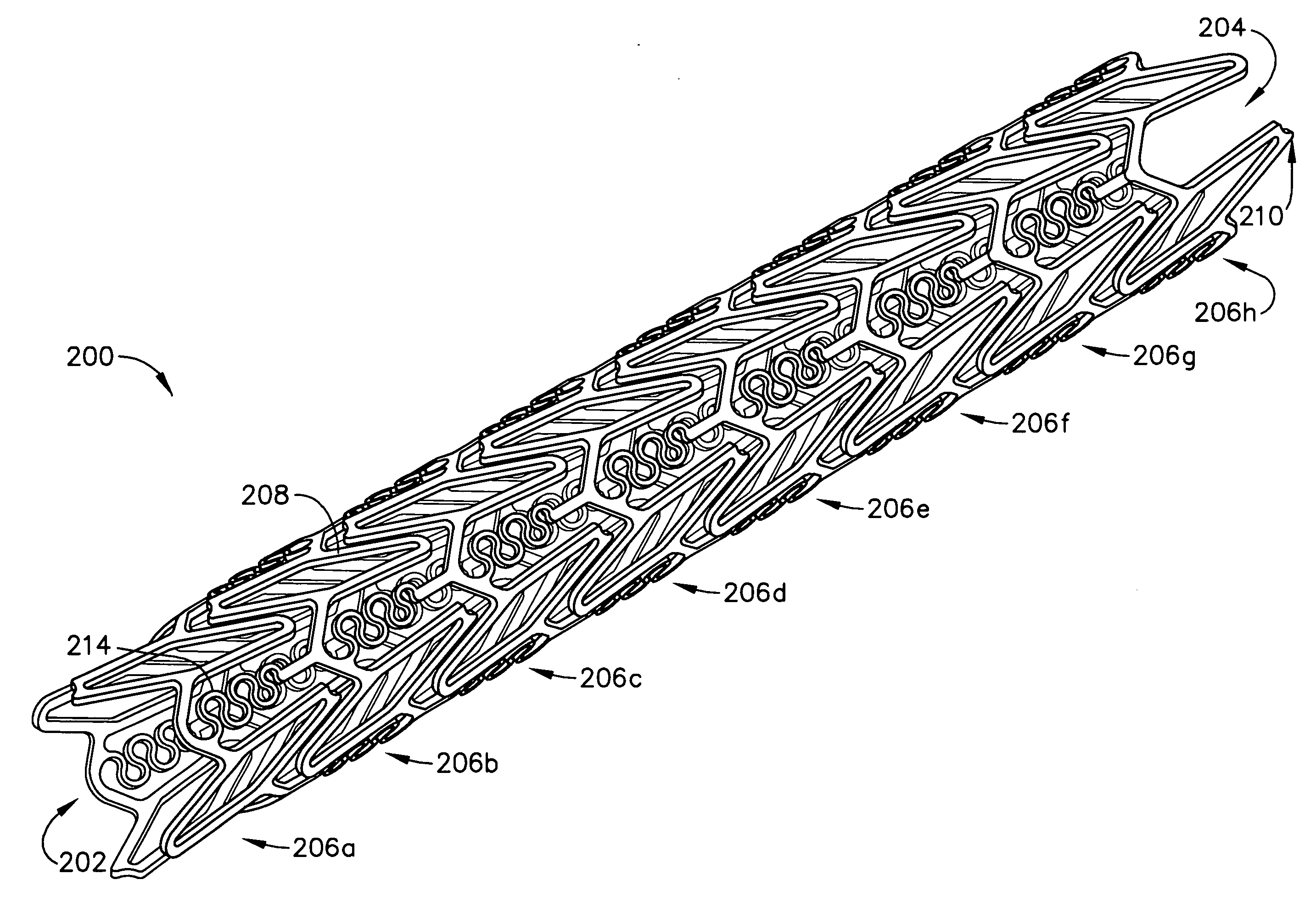
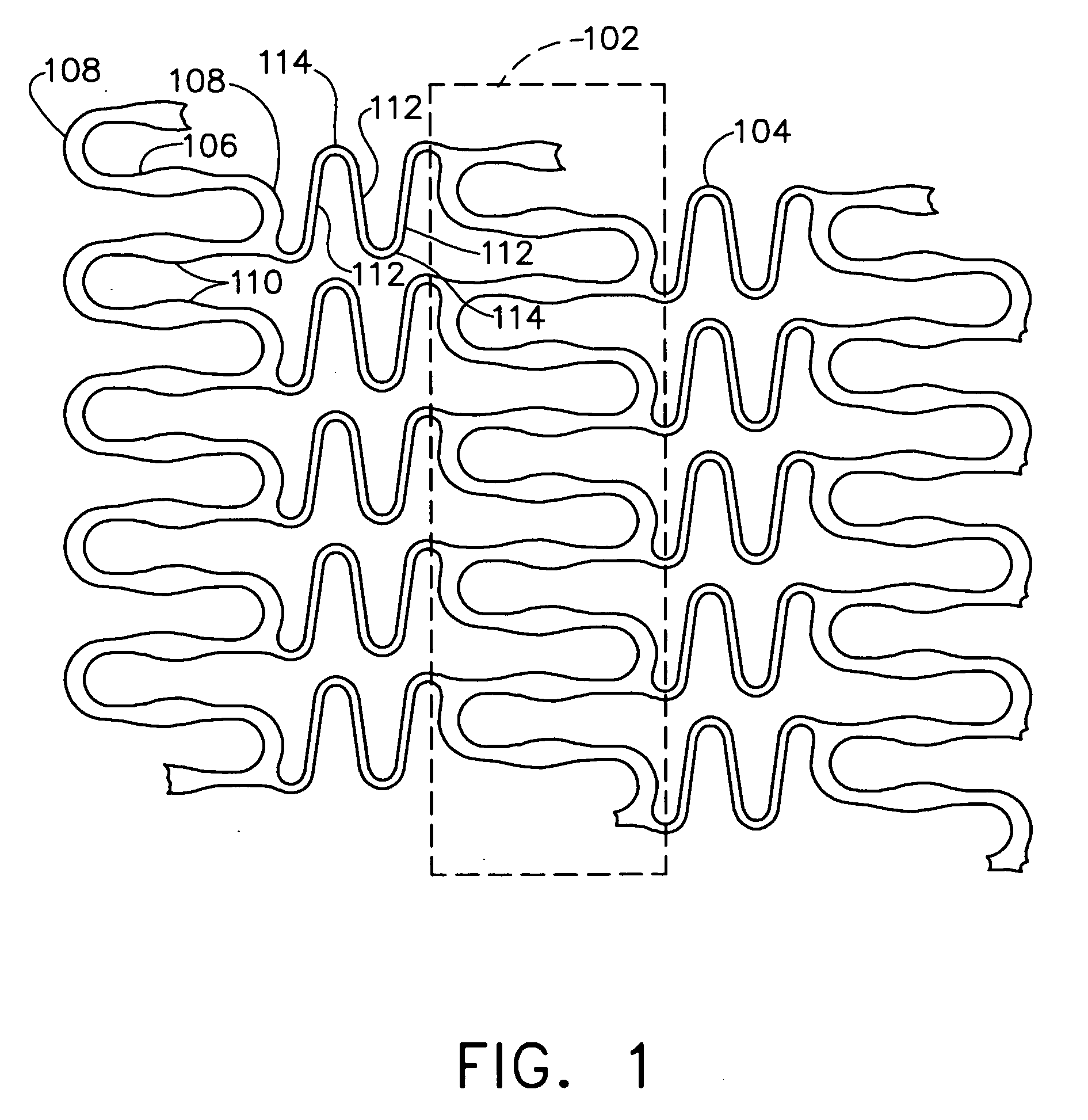
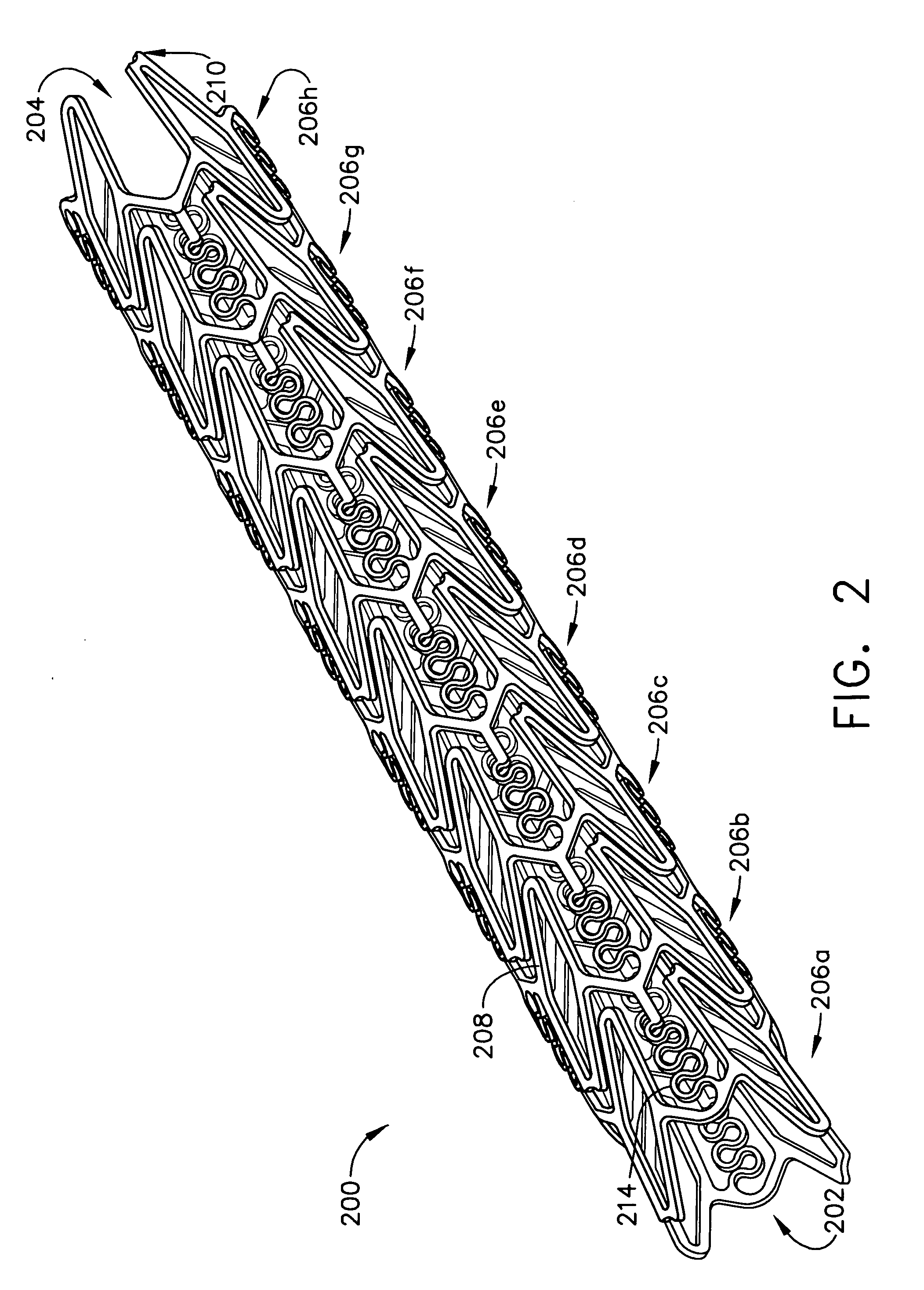
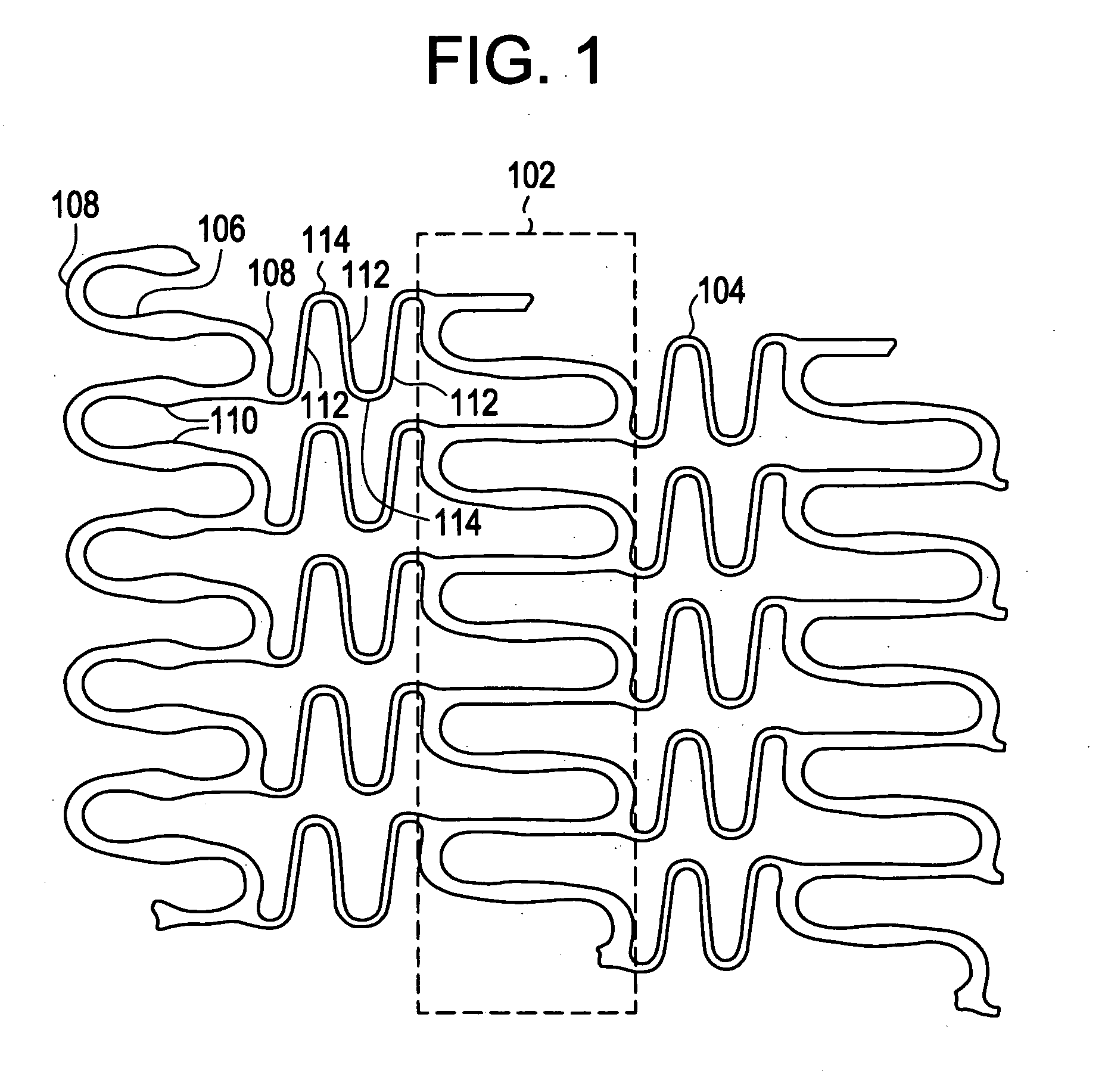
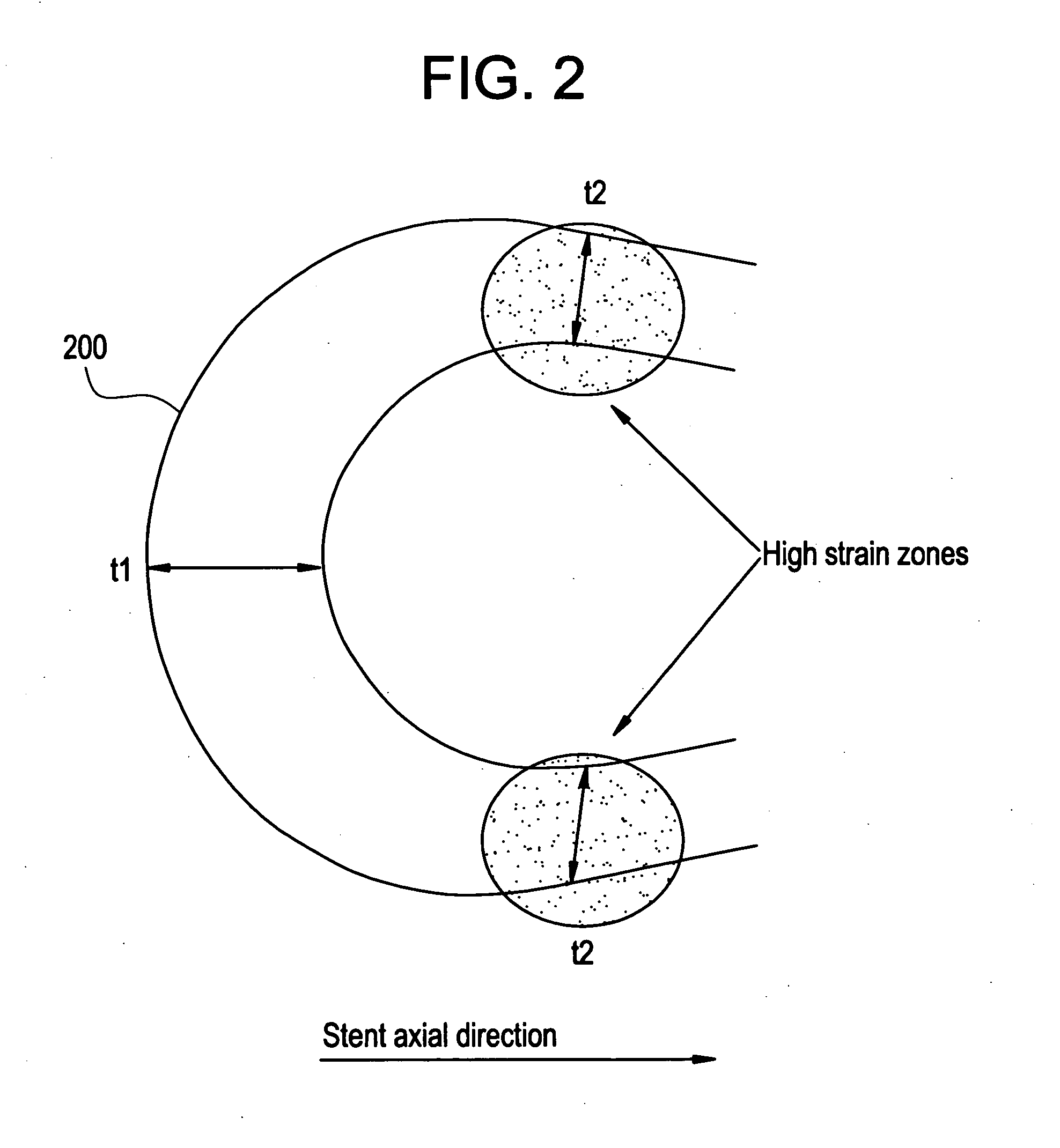
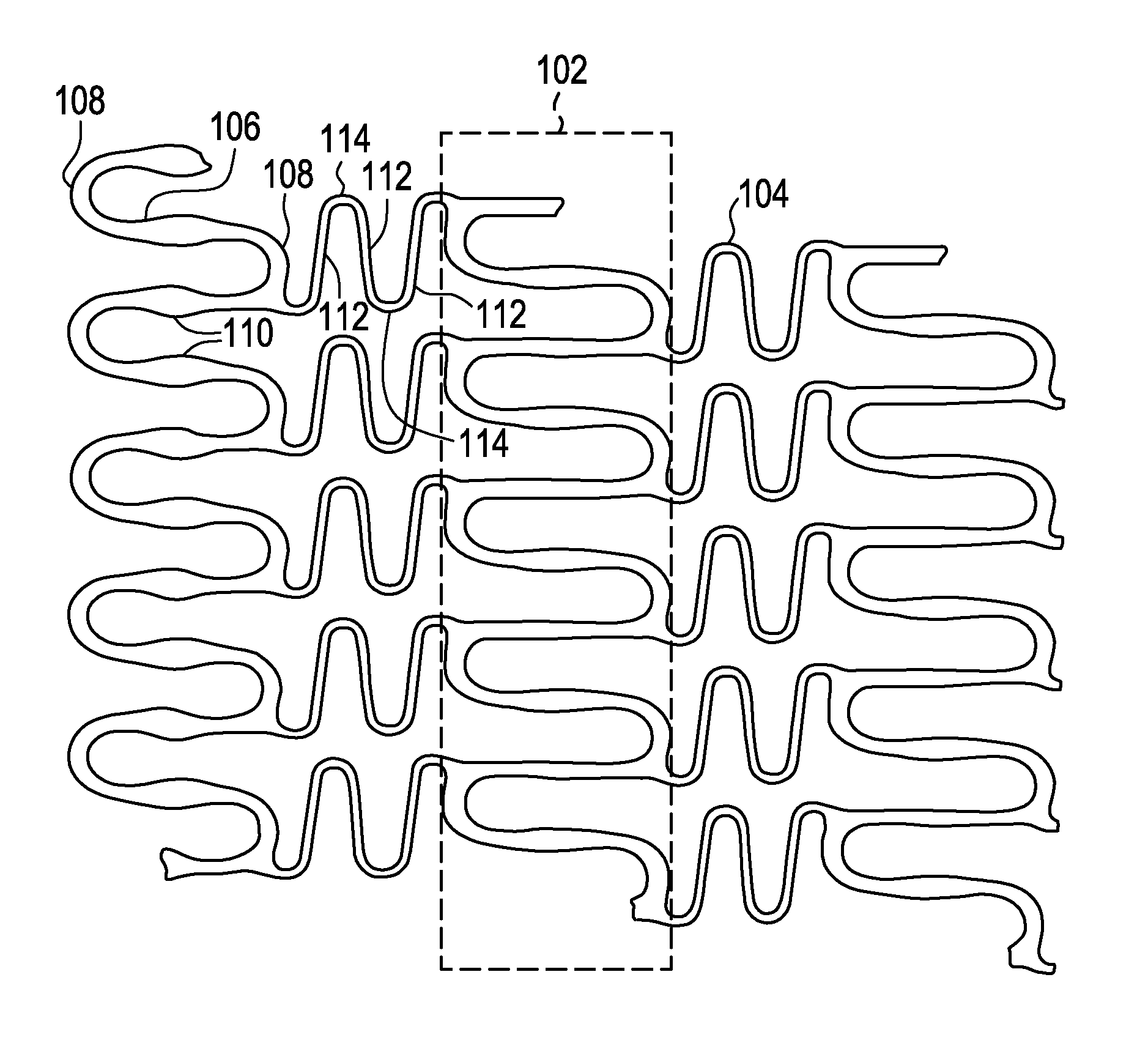
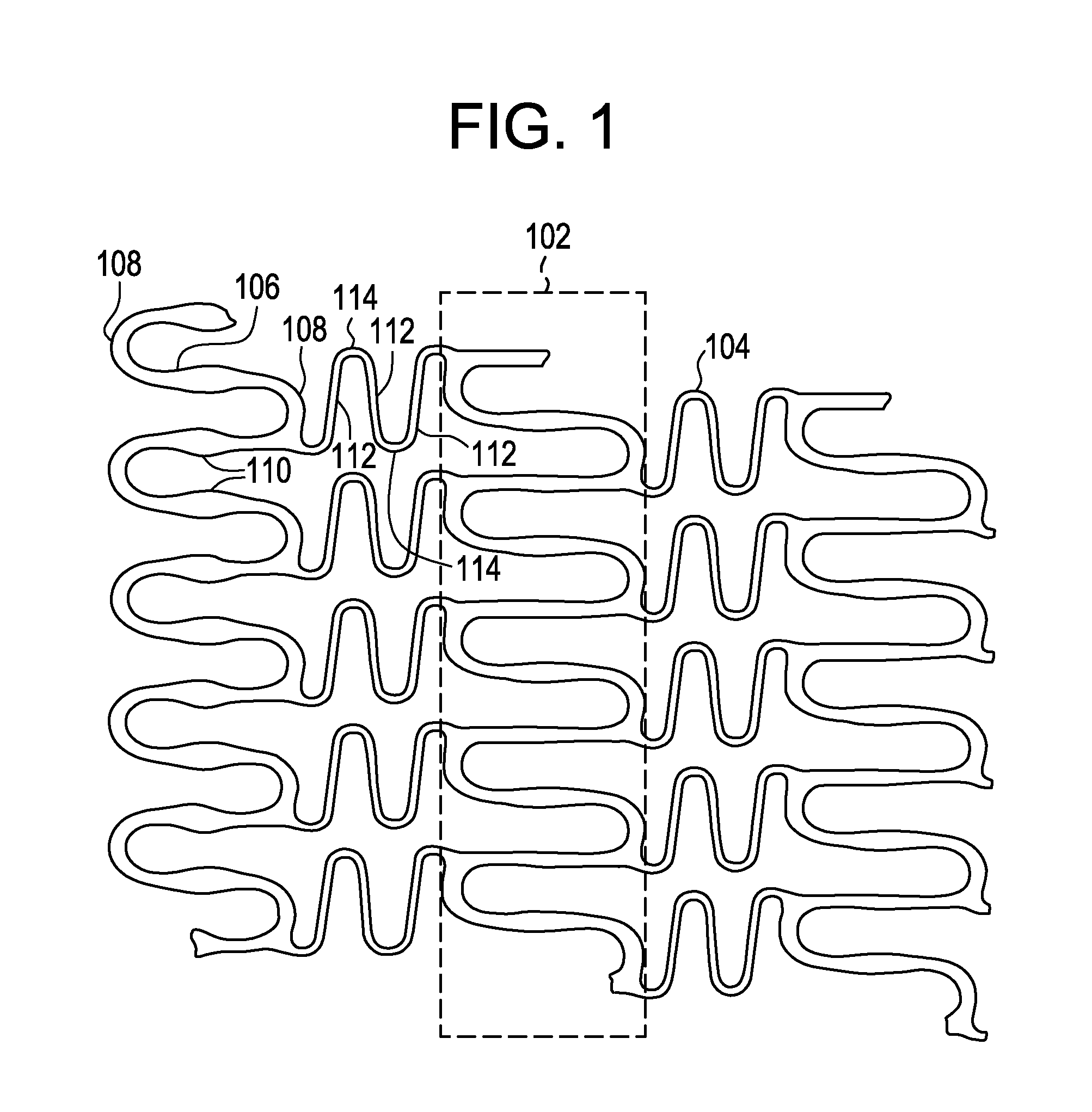
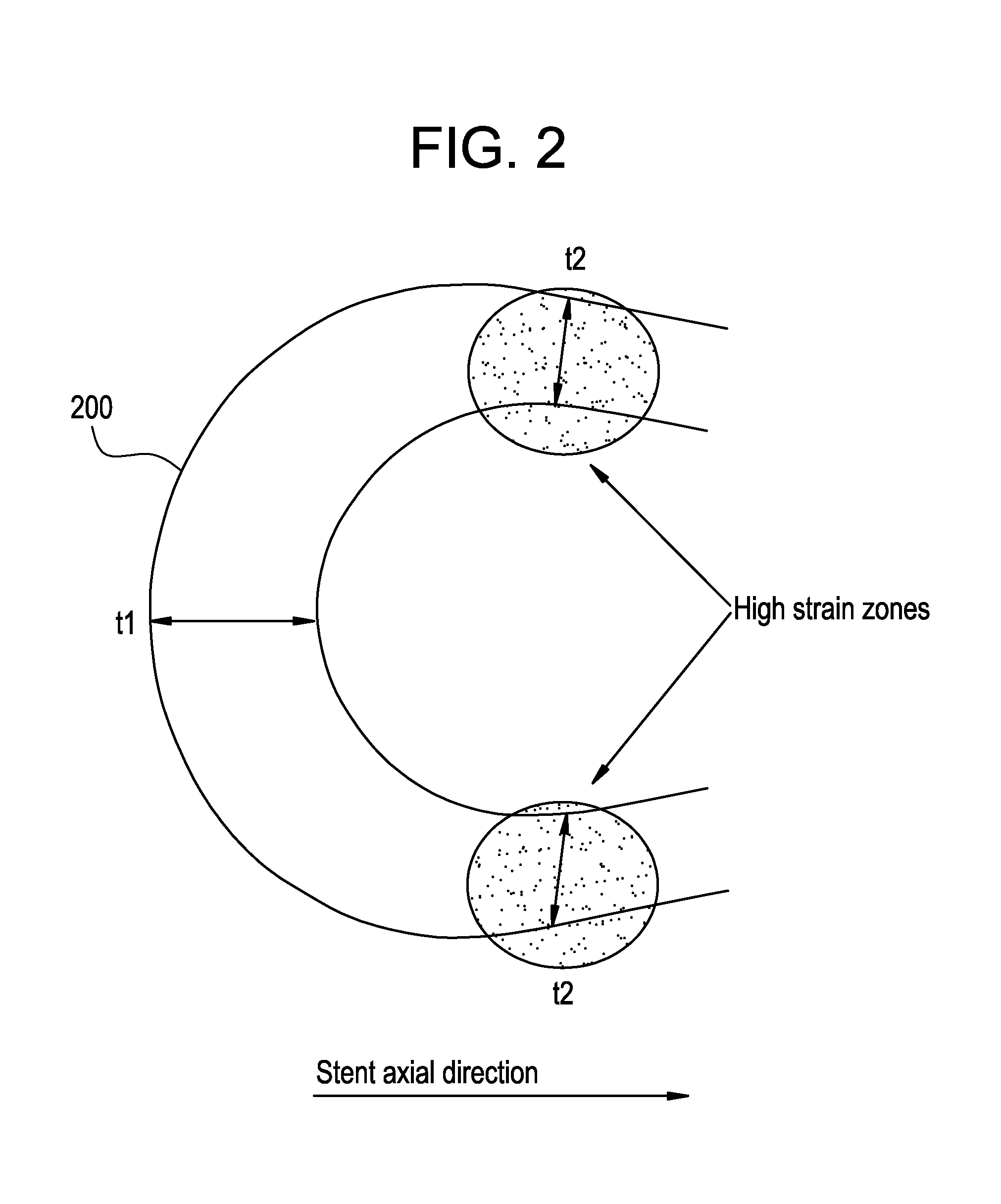
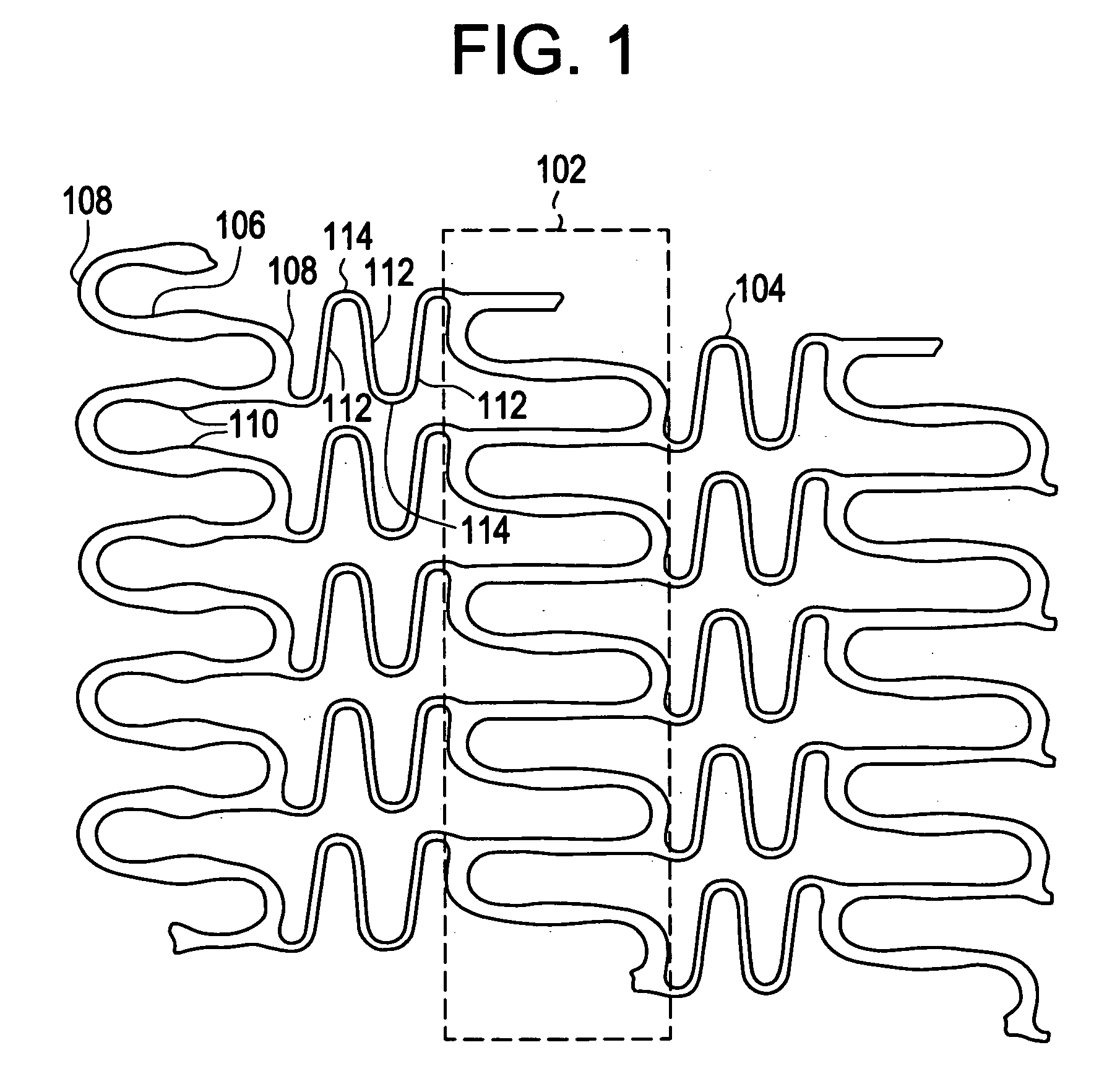
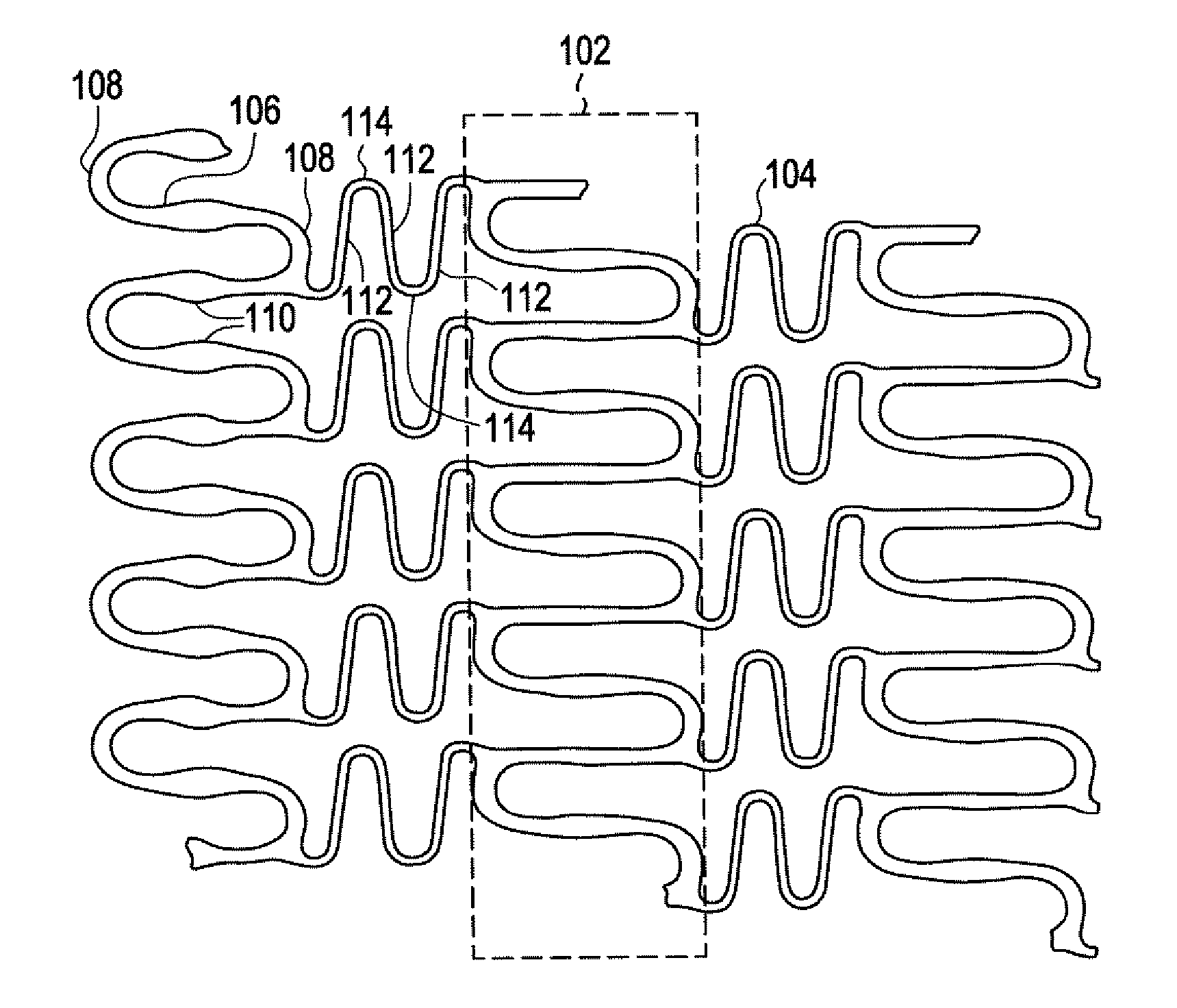
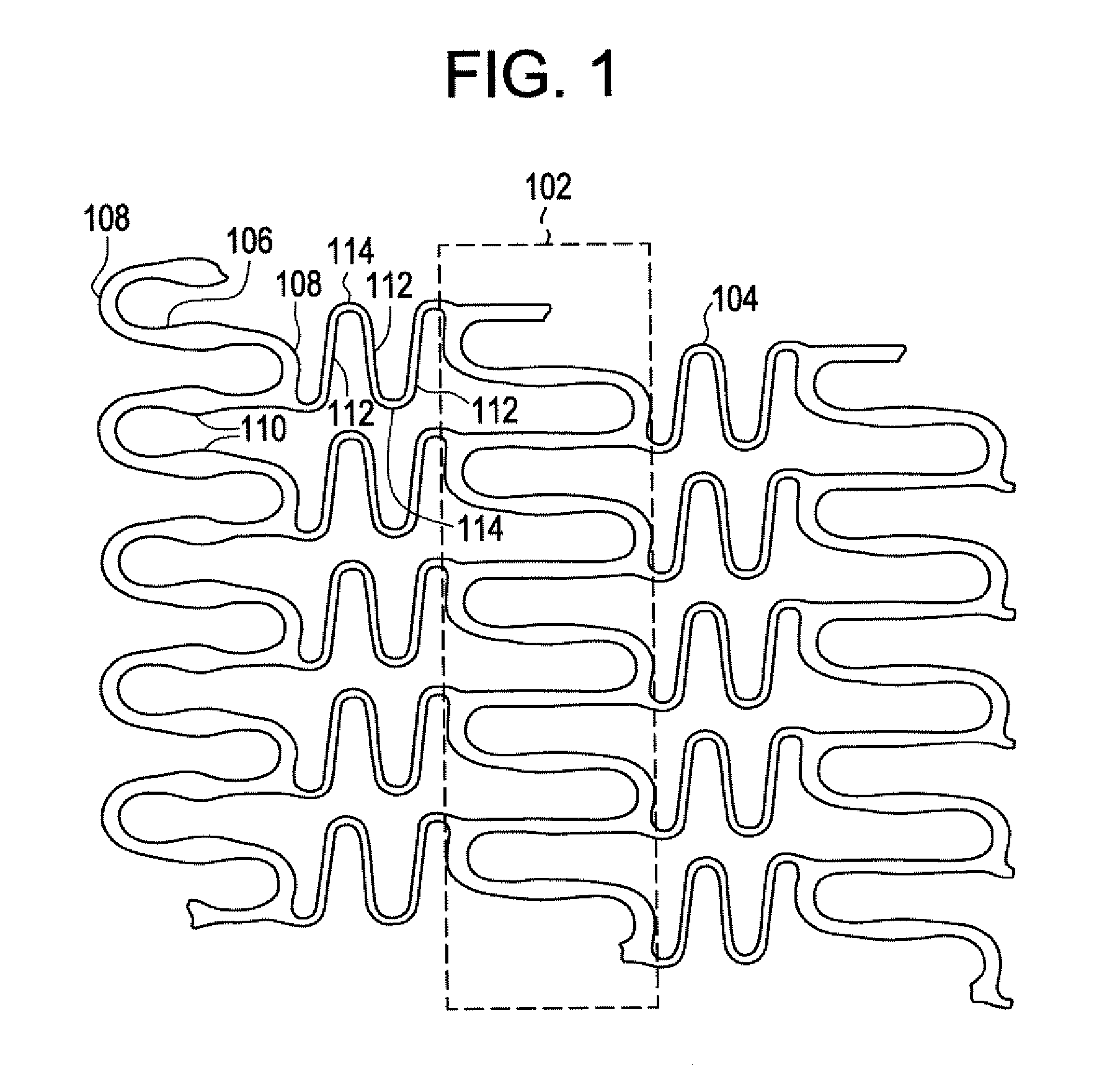
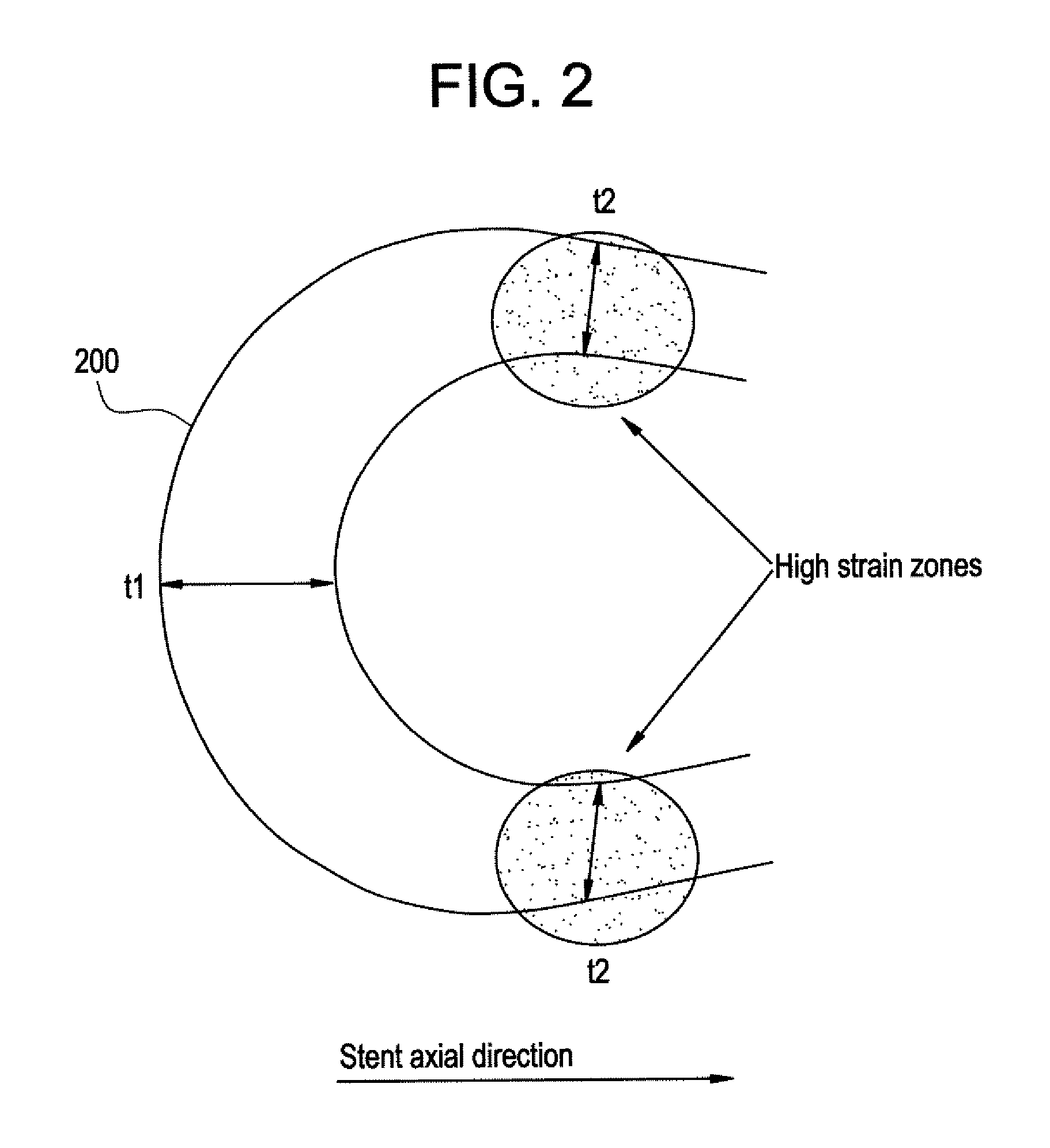
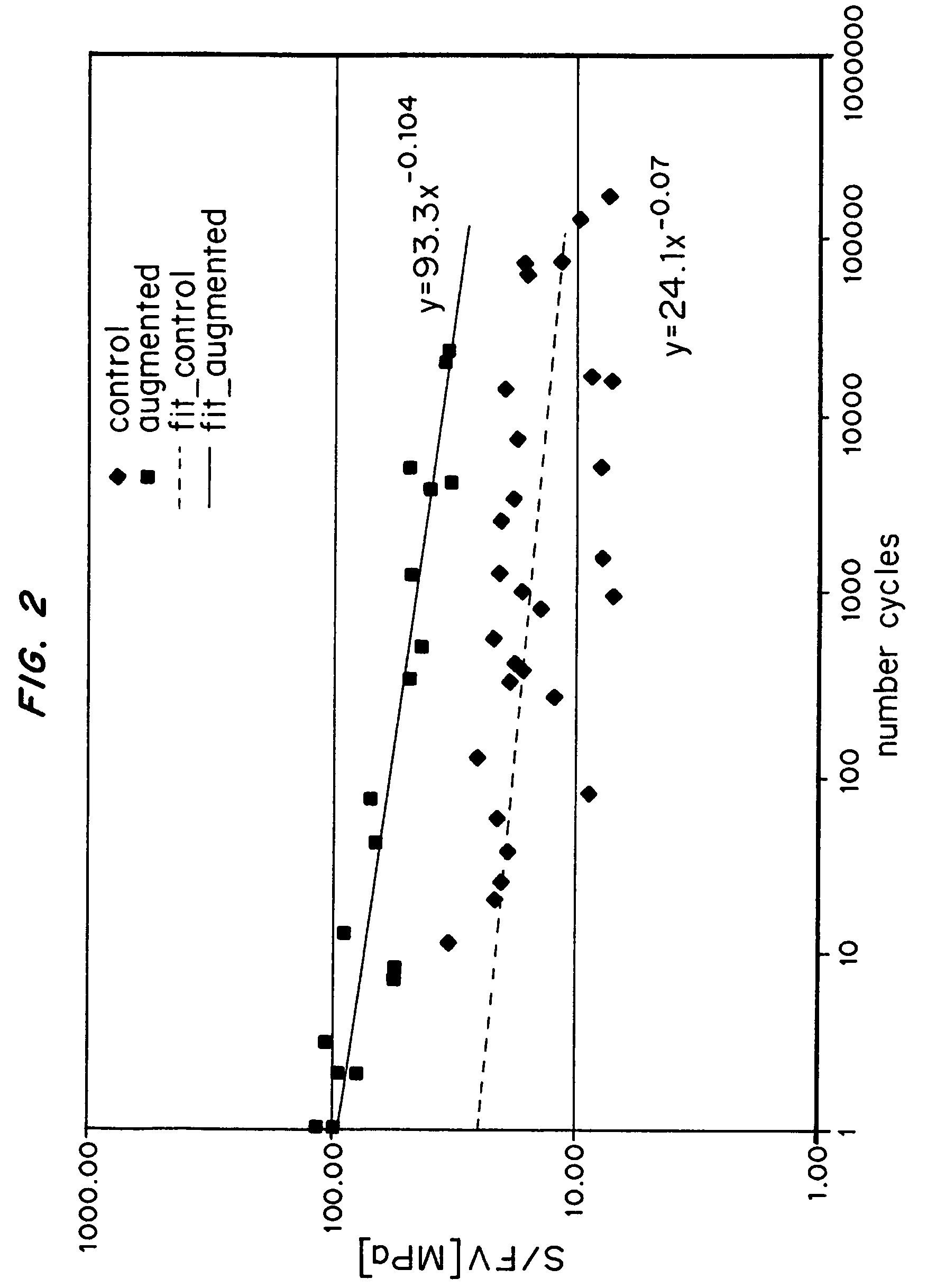
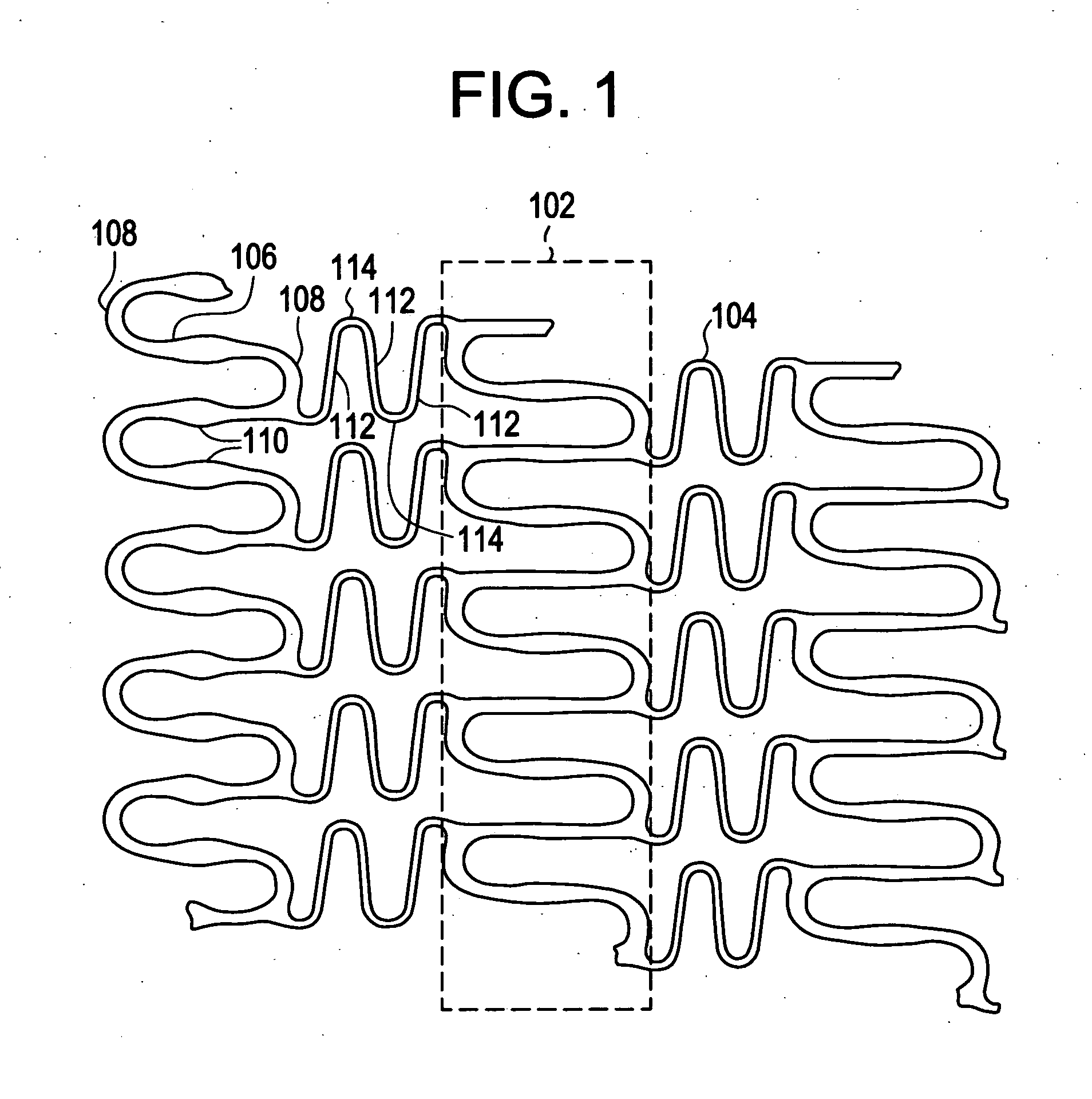
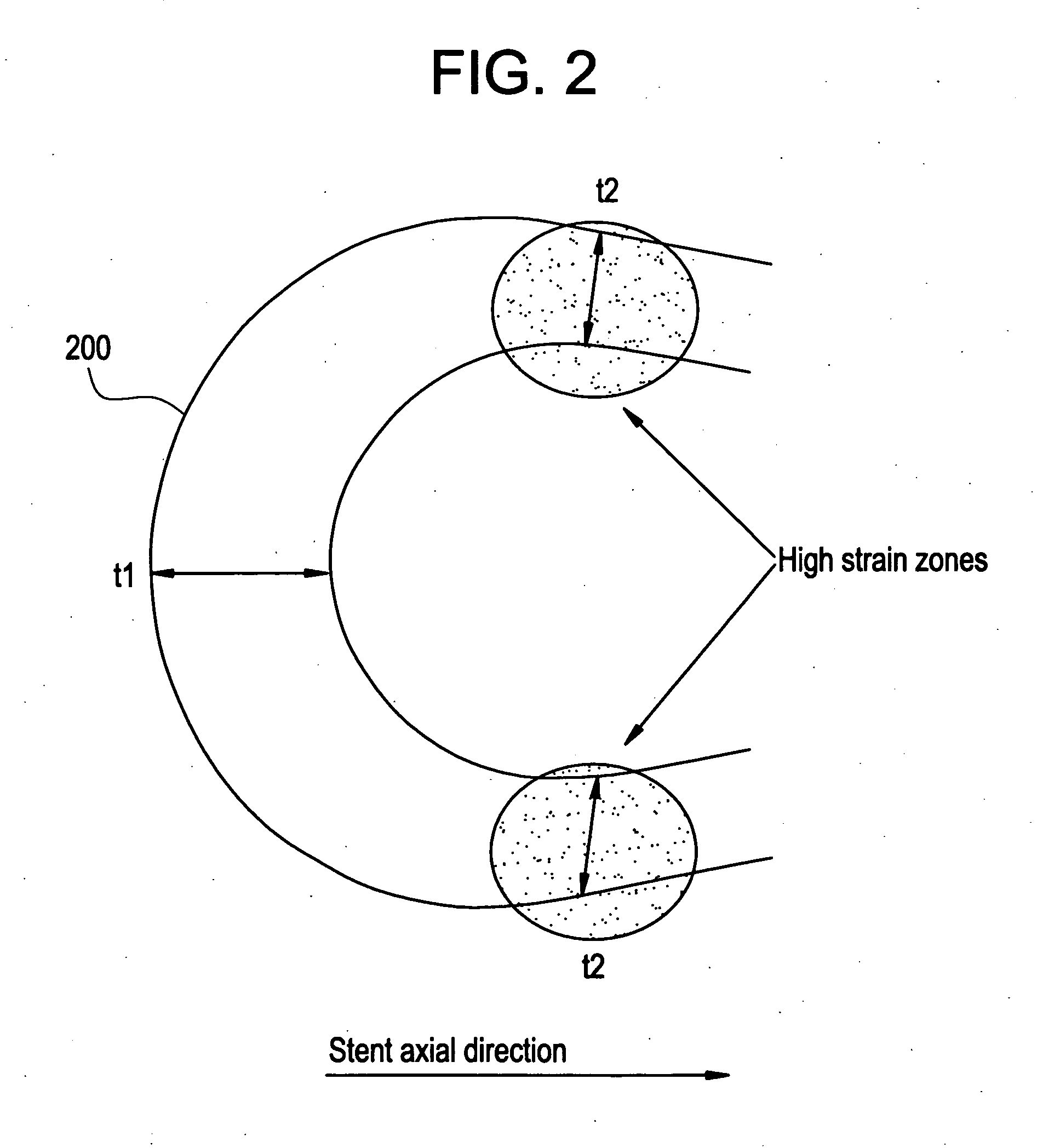
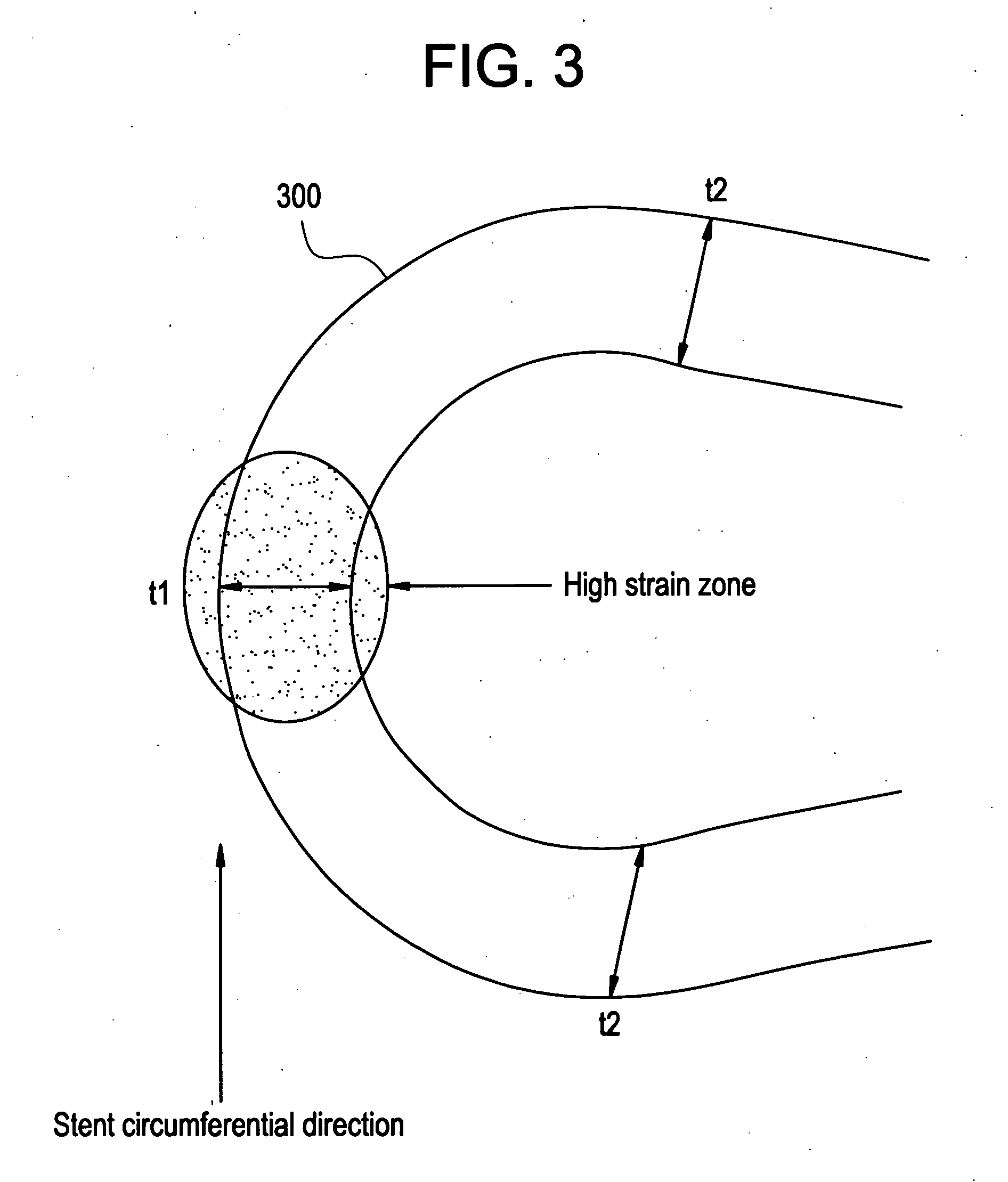
A biocompatible material may be configured into any number of implantable medical devices including intraluminal stents. Polymeric materials may be utilized to fabricate any of these devices, including stents. The stents may be balloon expandable or self-expanding. The polymeric materials may include additives such as drugs or other bioactive agents as well as radiopaque agents. By preferential mechanical deformation of the polymer, the polymer chains may be oriented to achieve certain desirable performance characteristics. The stent has a plurality of hoop components interconnected by a plurality of flexible connectors. The hoop components are formed as a continuous series of substantially longitudinally or axially oriented radial strut members and alternating substantially circumferentially oriented radial arc members. The geometry of the struts and arcs is such that when the stent is expanded, it has very high strains within a relatively small region. This strain localization results in what is often referred to as “hinging”, where the hinge is the small region within which the strains are very high.
Owner:CARDINAL HEALTH SWITZERLAND 515 GMBH
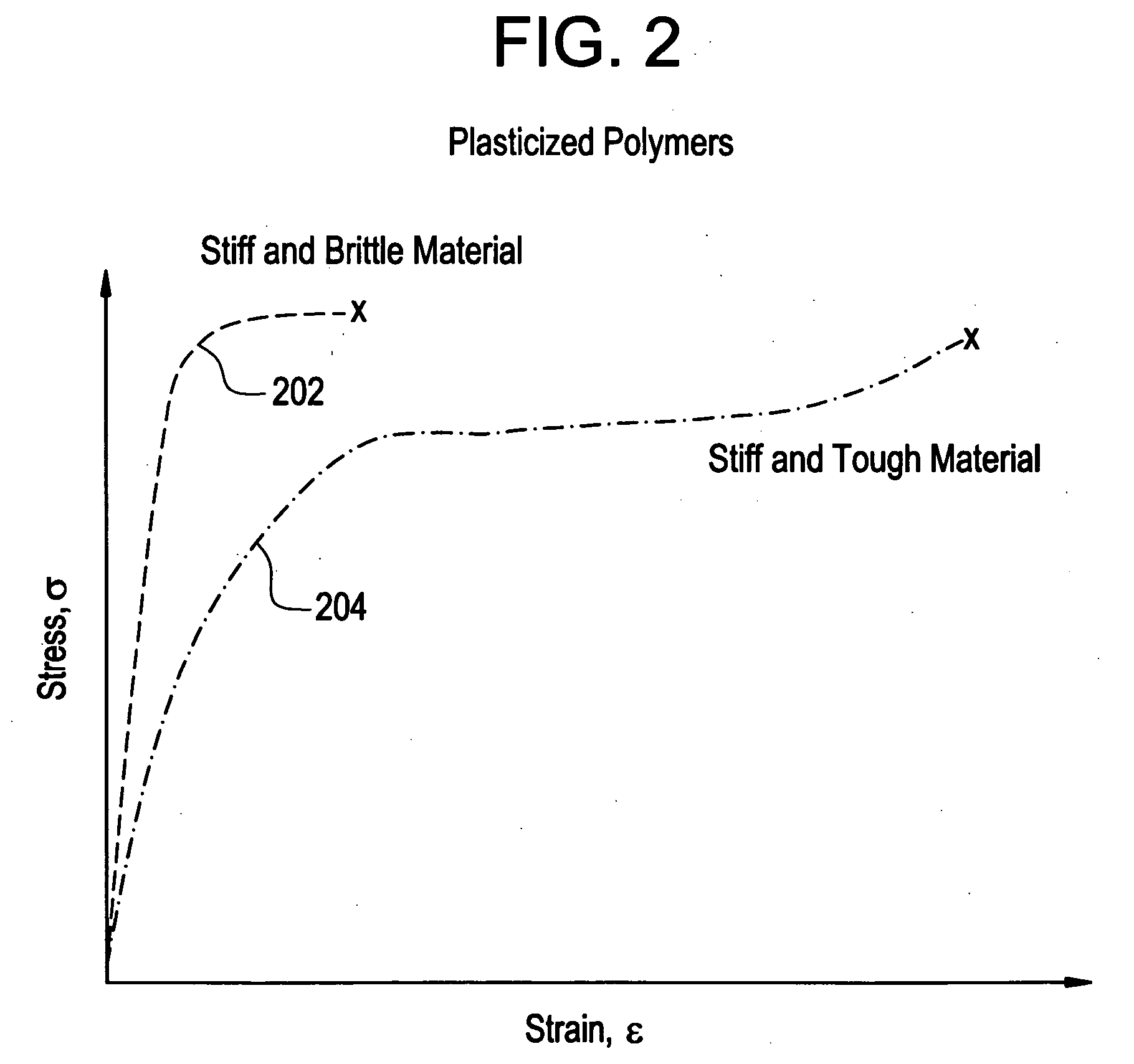
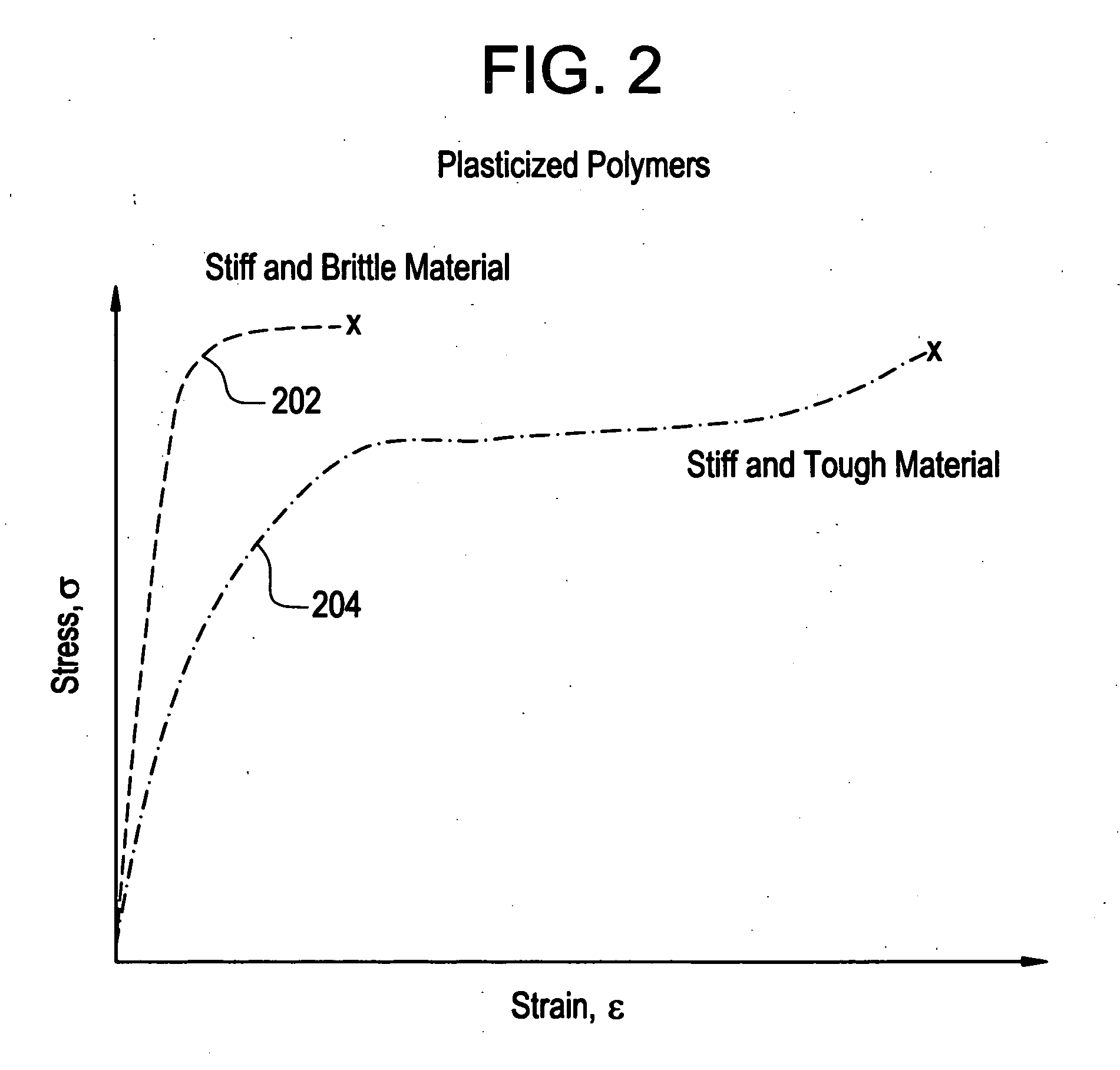
Implantable device formed from polymer and plasticizer blends
A biocompatible material may be configured into any number of implantable medical devices including intraluminal stents. Polymeric materials may be utilized to fabricate any of these devices, including stents. The stents may be balloon expandable or self-expanding. The polymeric materials may include additives such as drugs or other bioactive agents as well as radiopaque agents. By preferential mechanical deformation of the polymer, the polymer chains may be oriented to achieve certain desirable performance characteristics.
Owner:CORDIS CORP
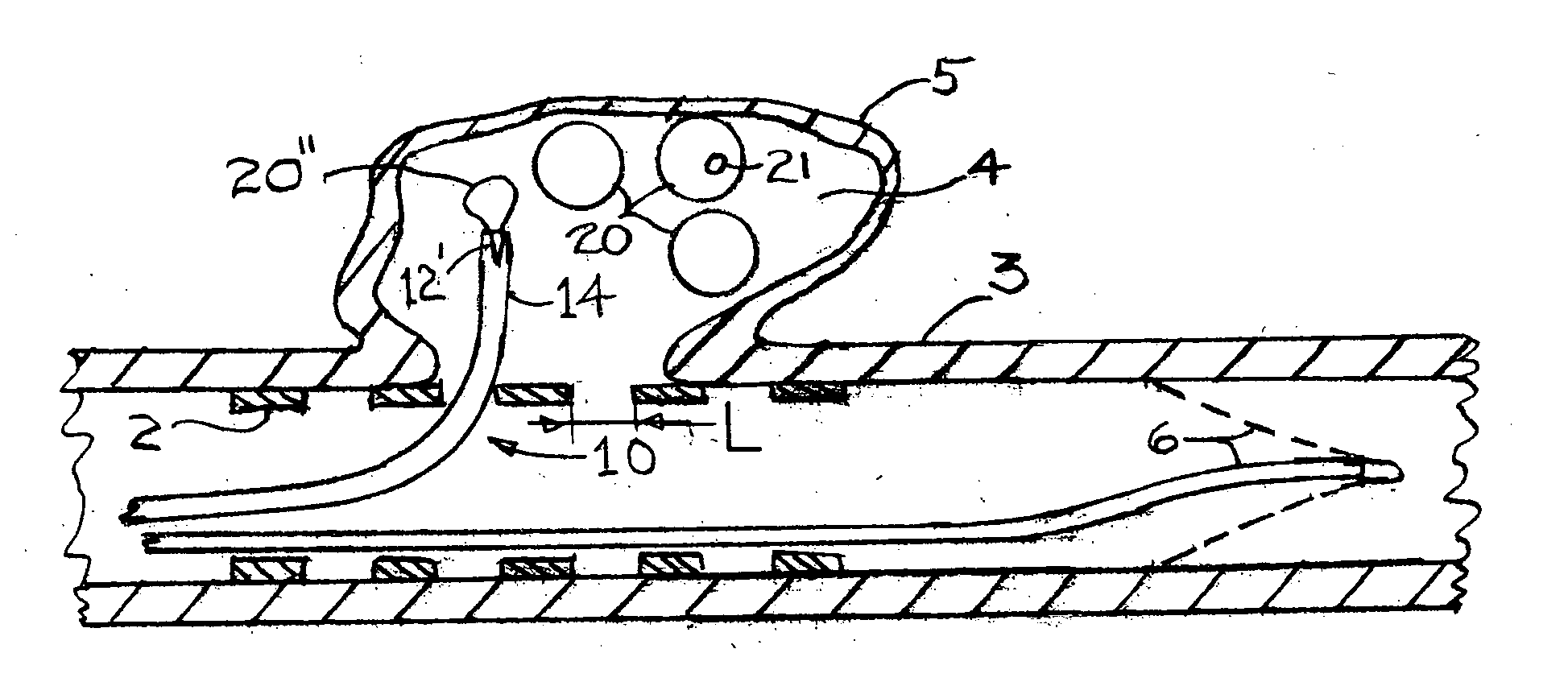
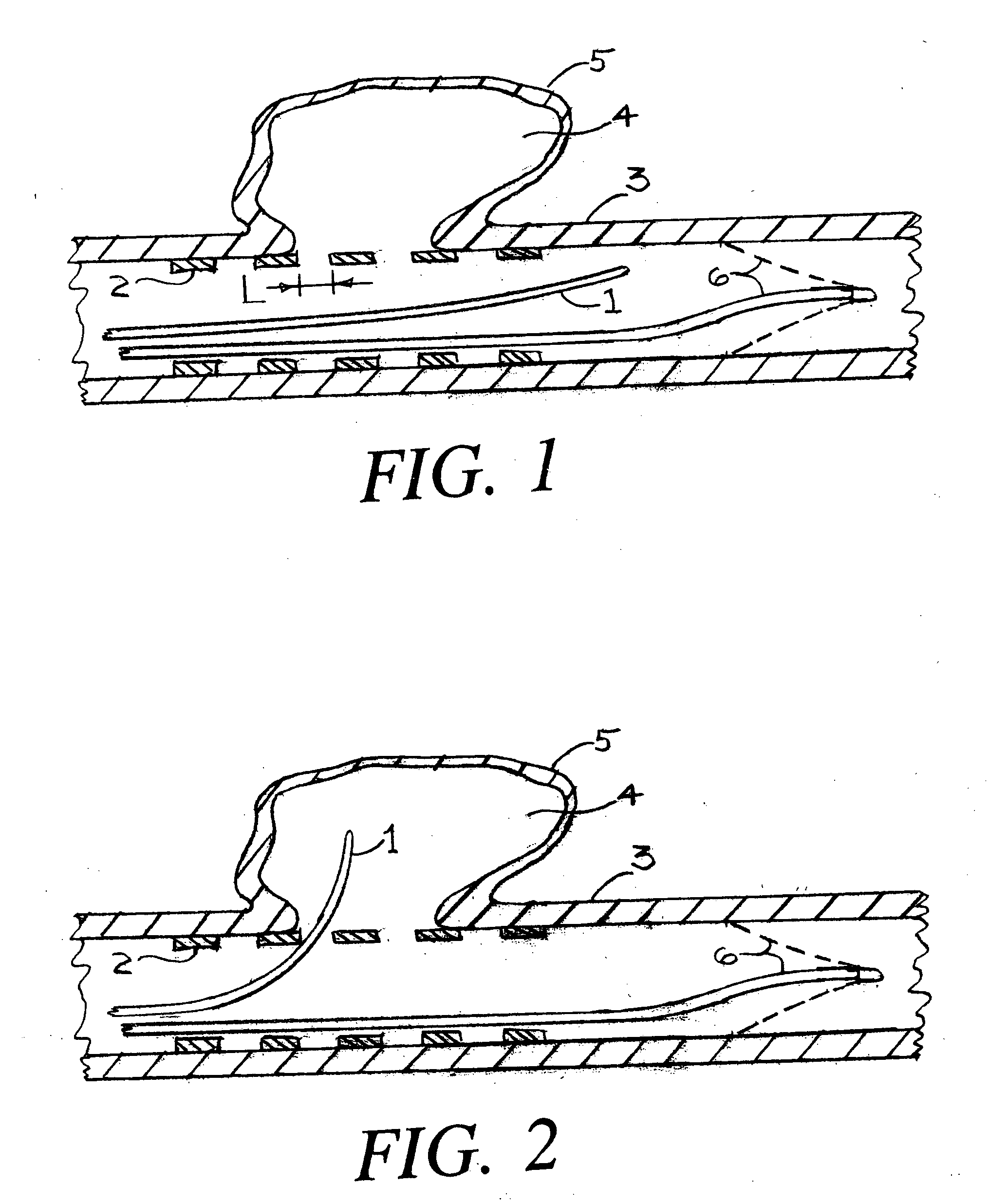
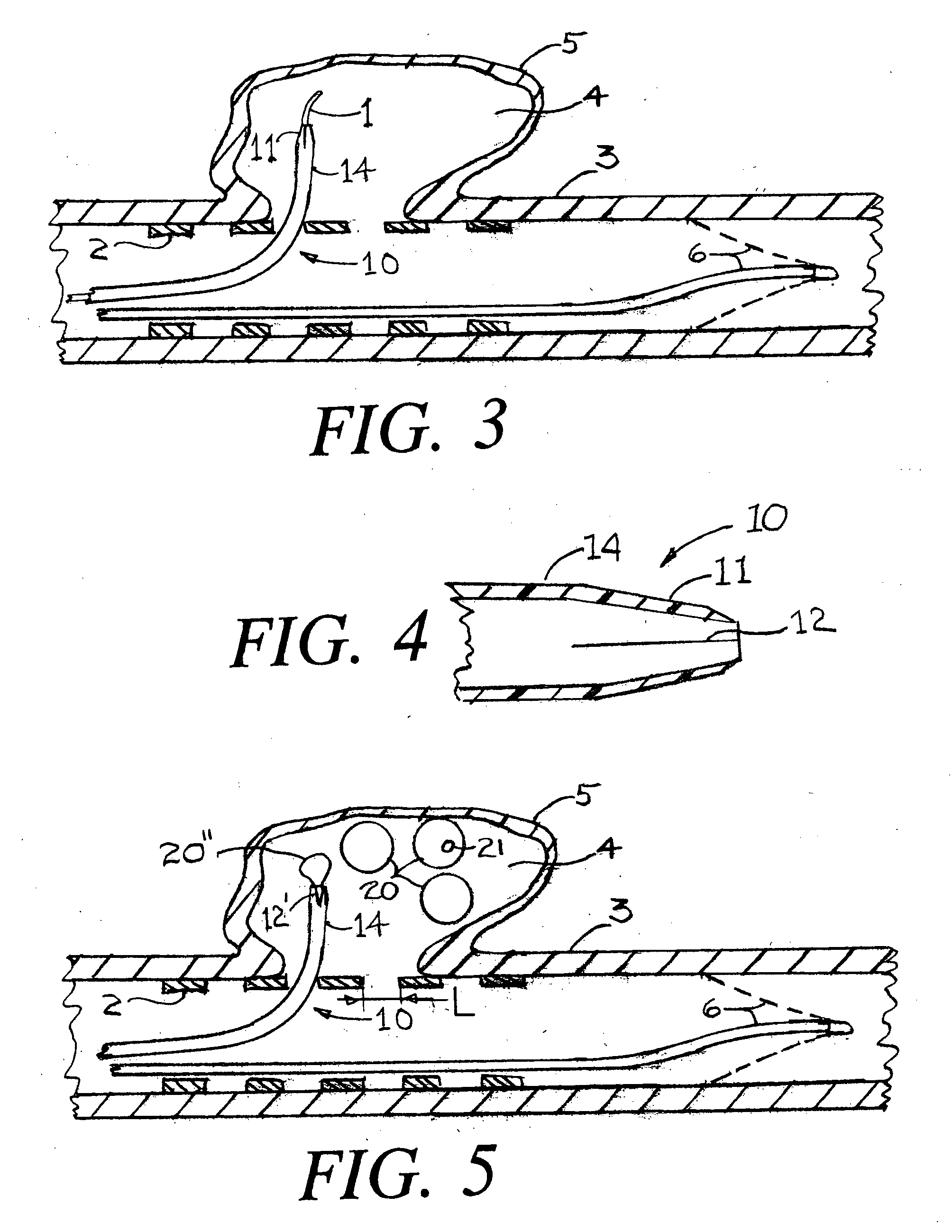
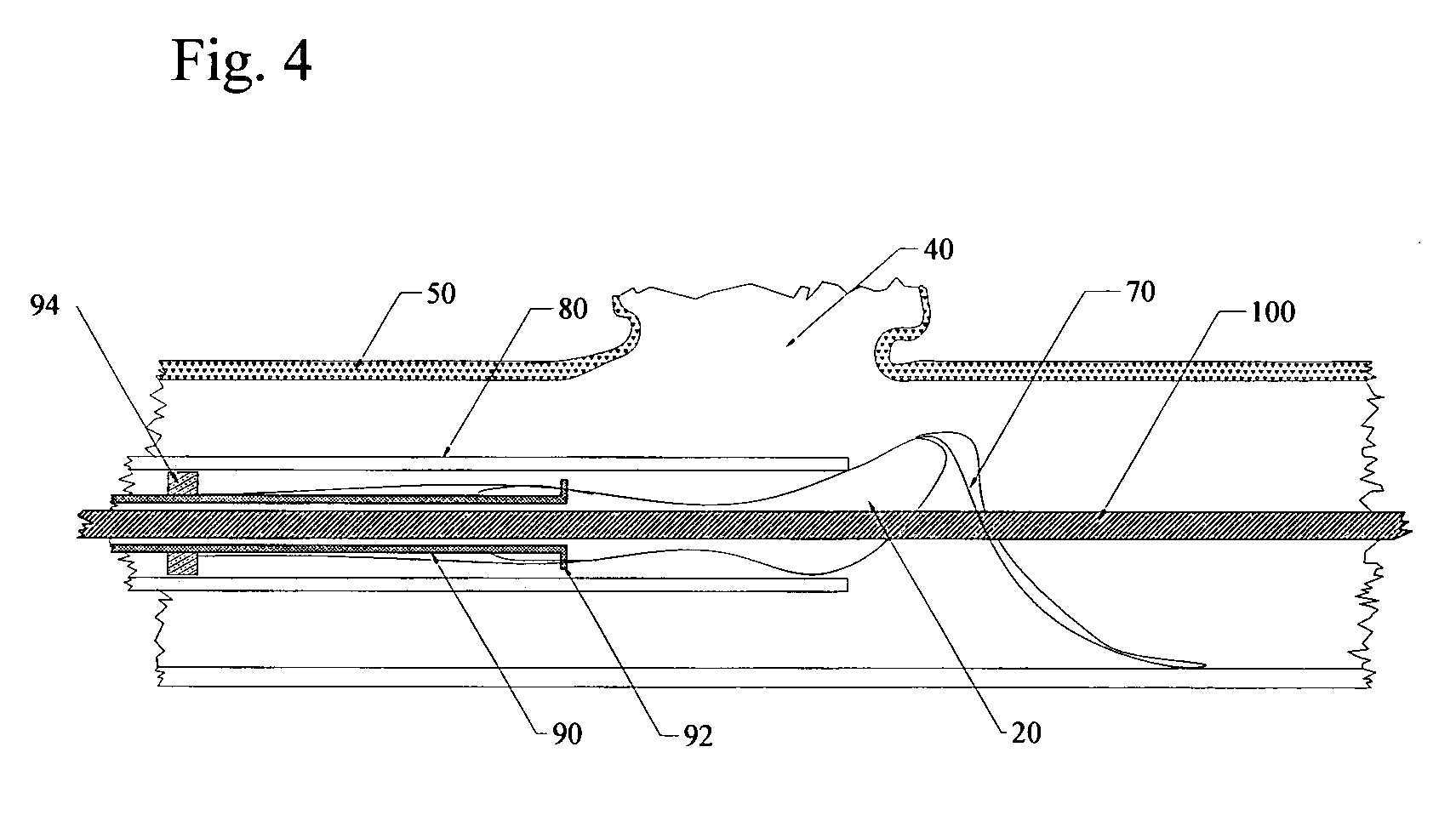
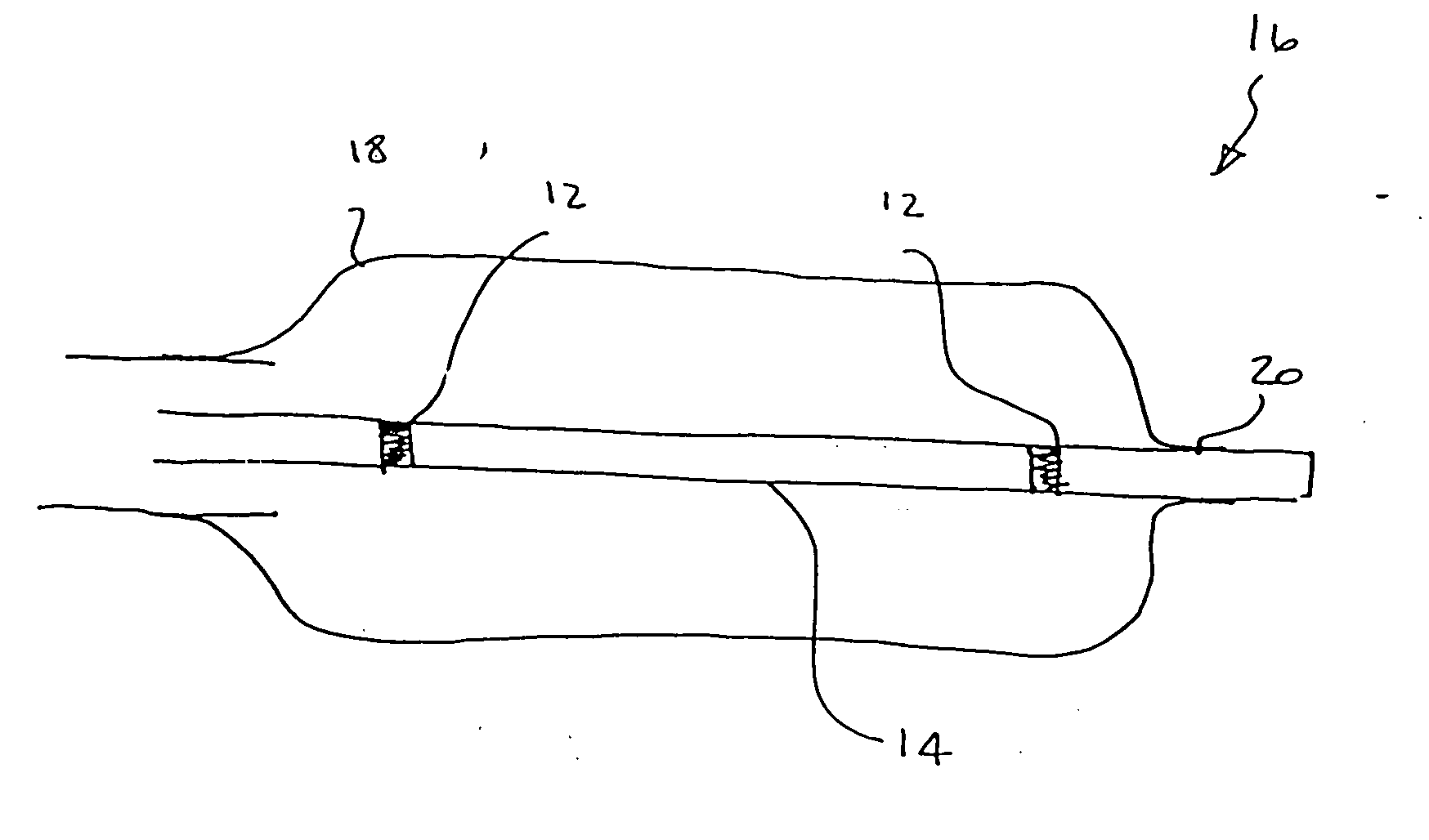
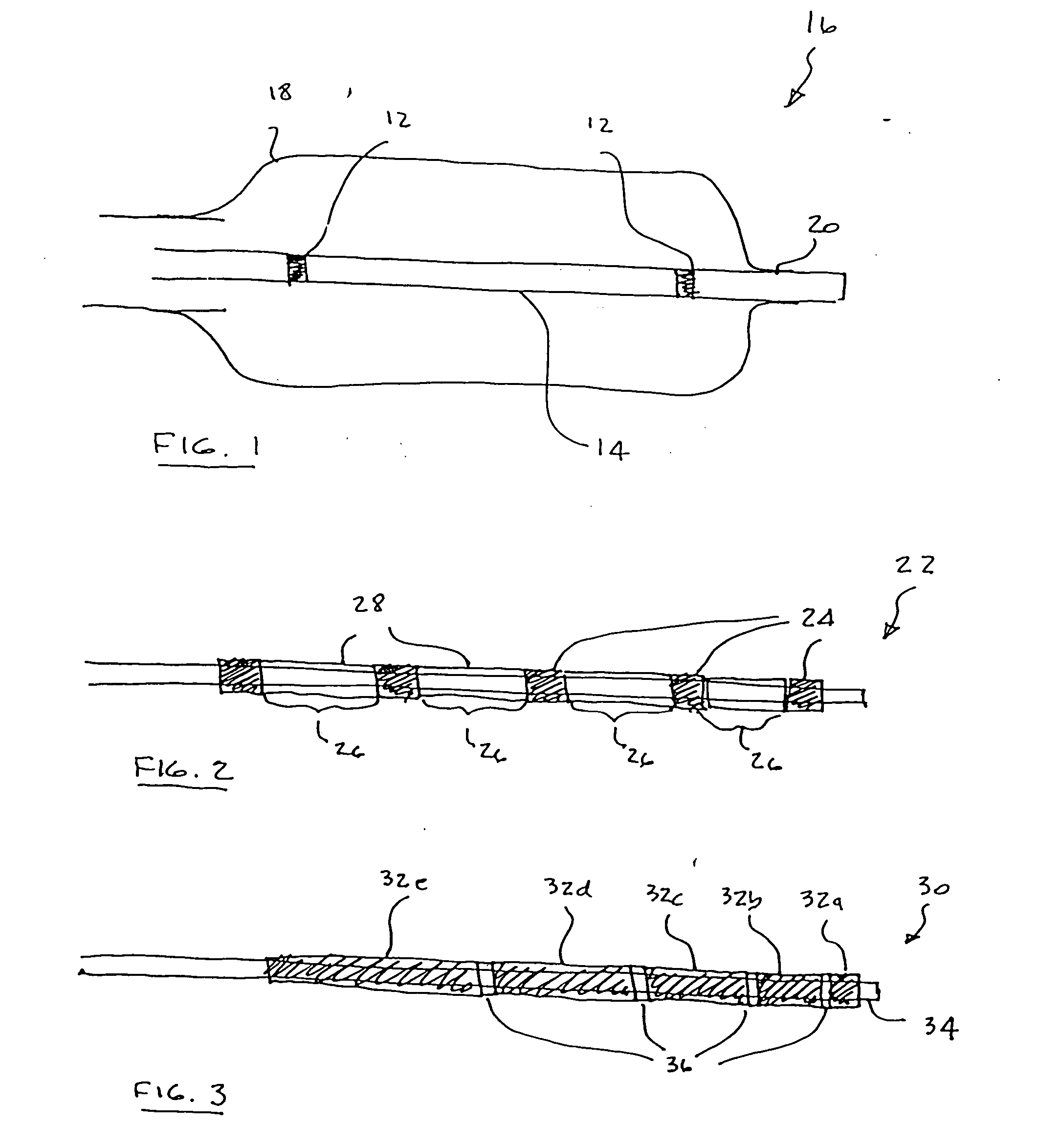
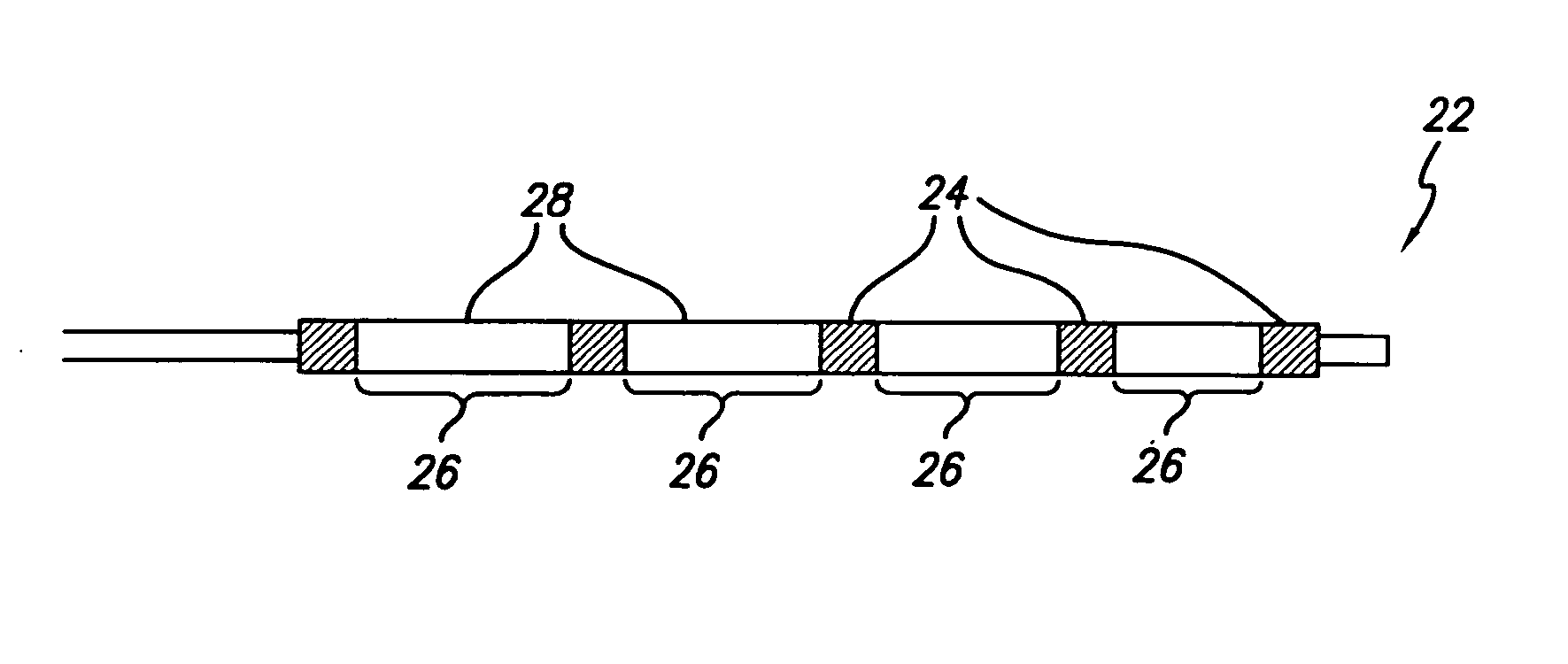
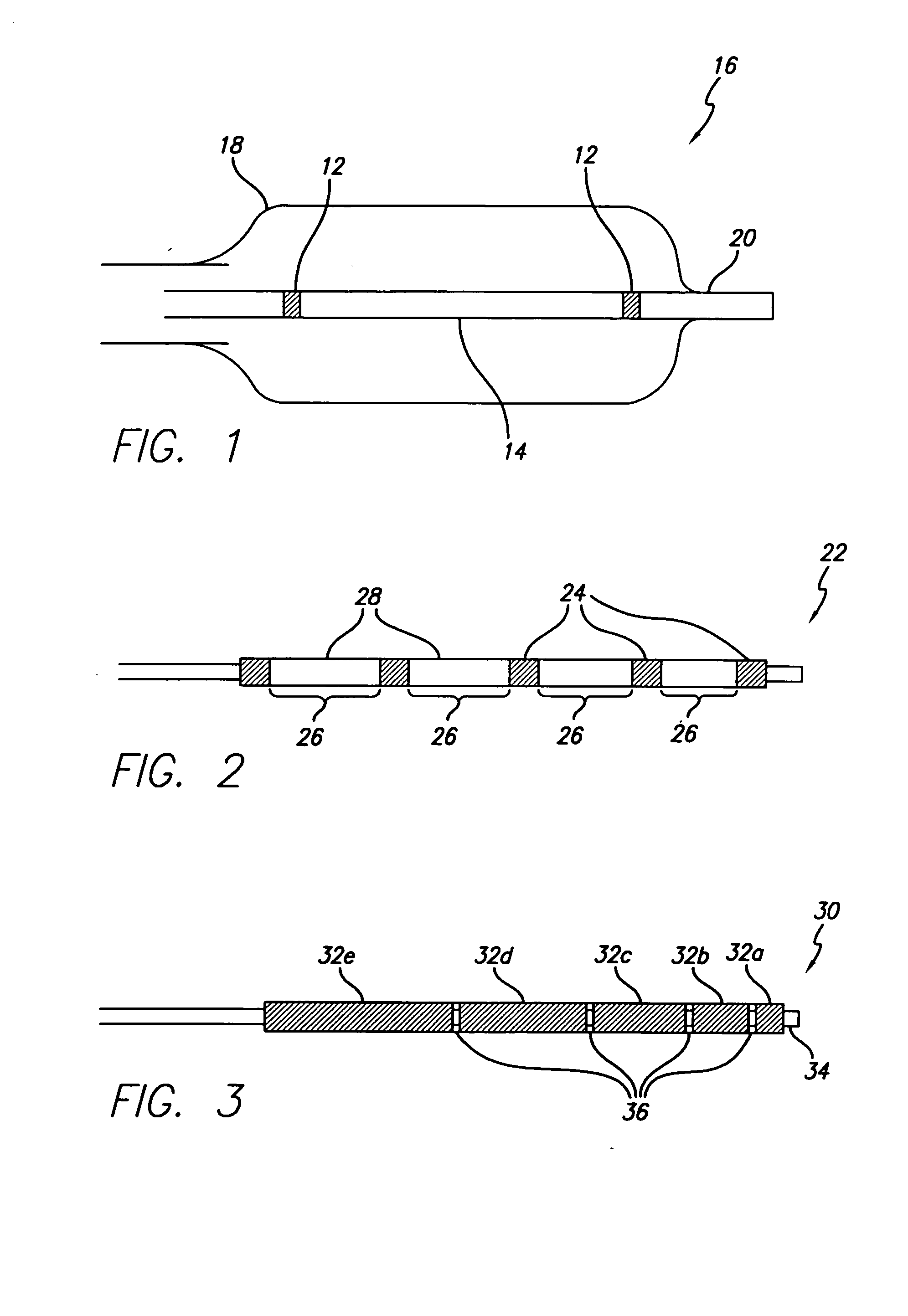
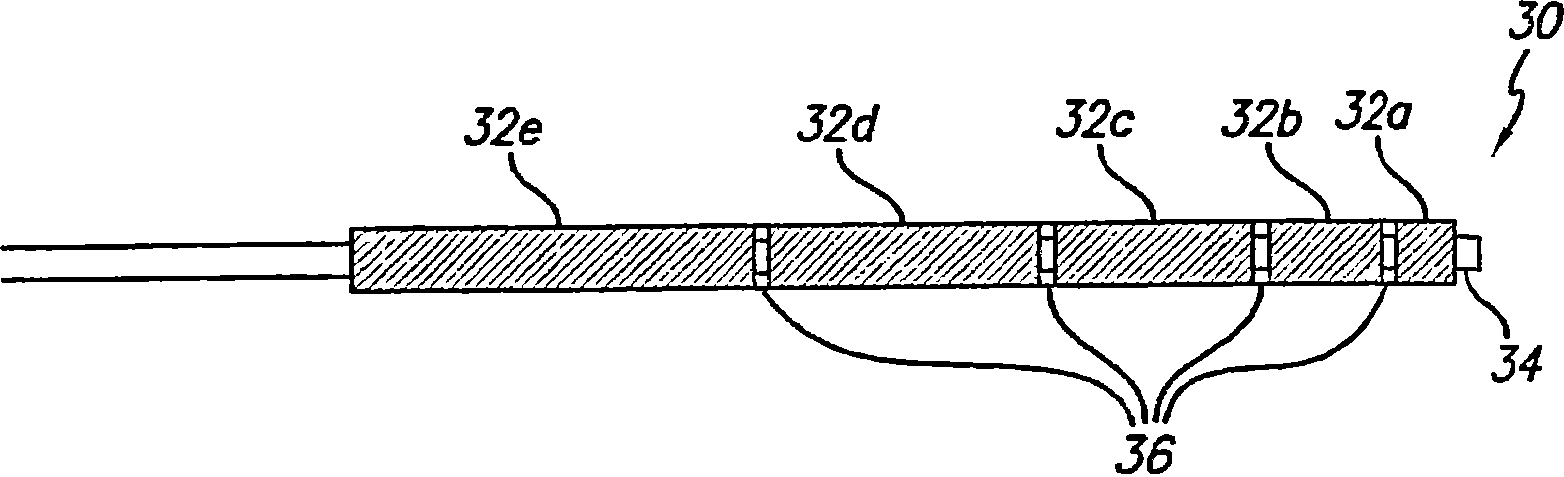
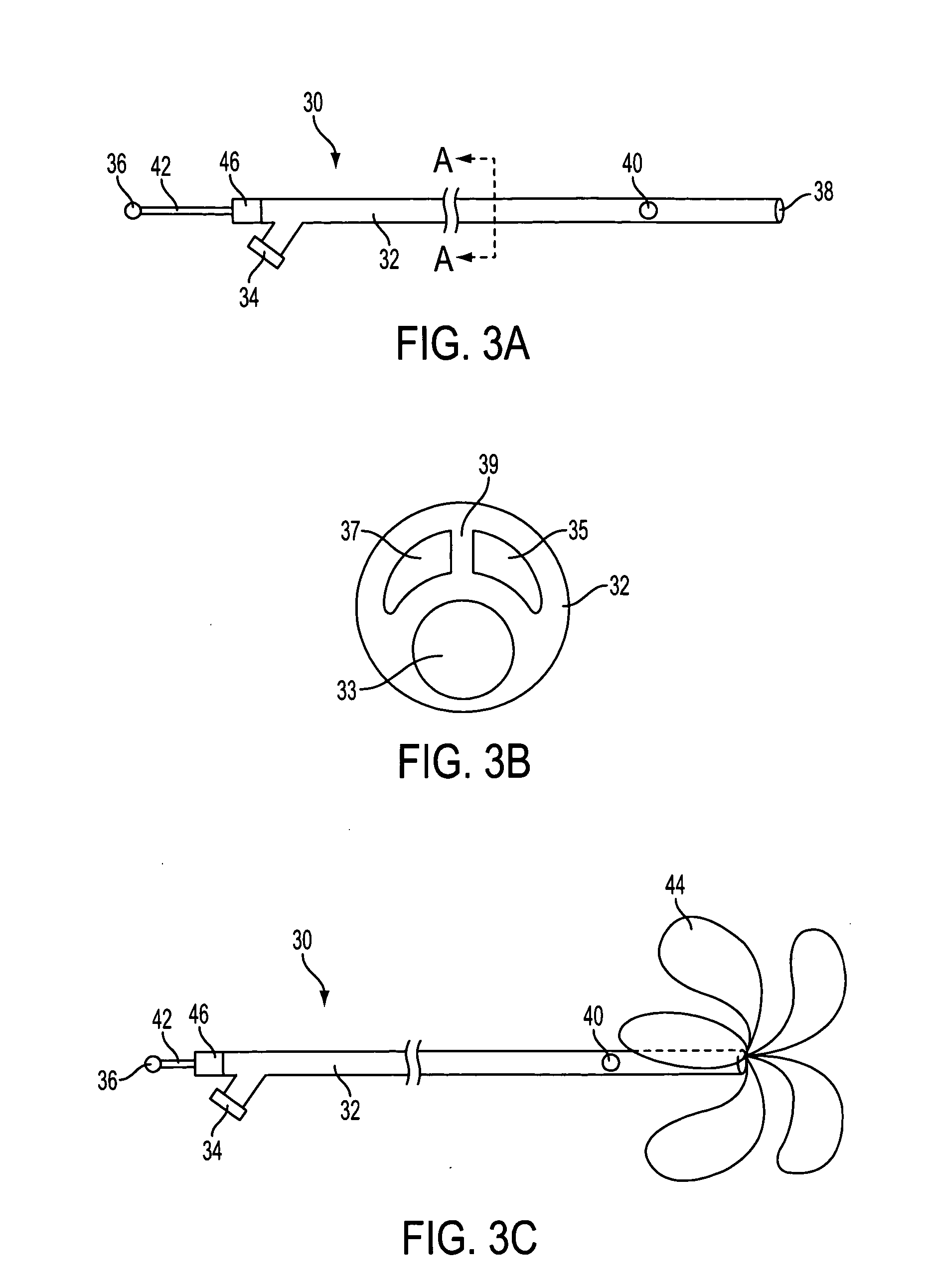
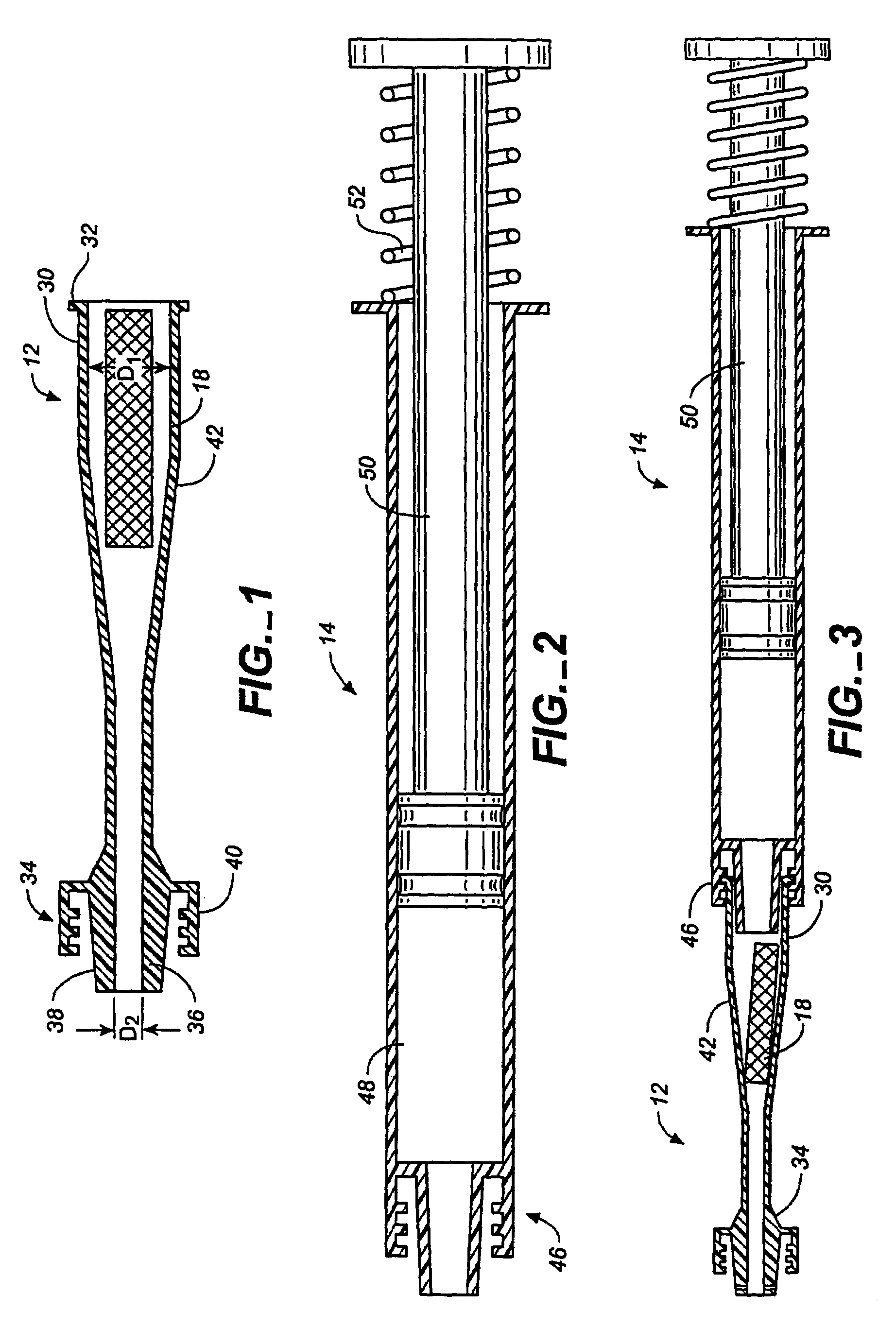
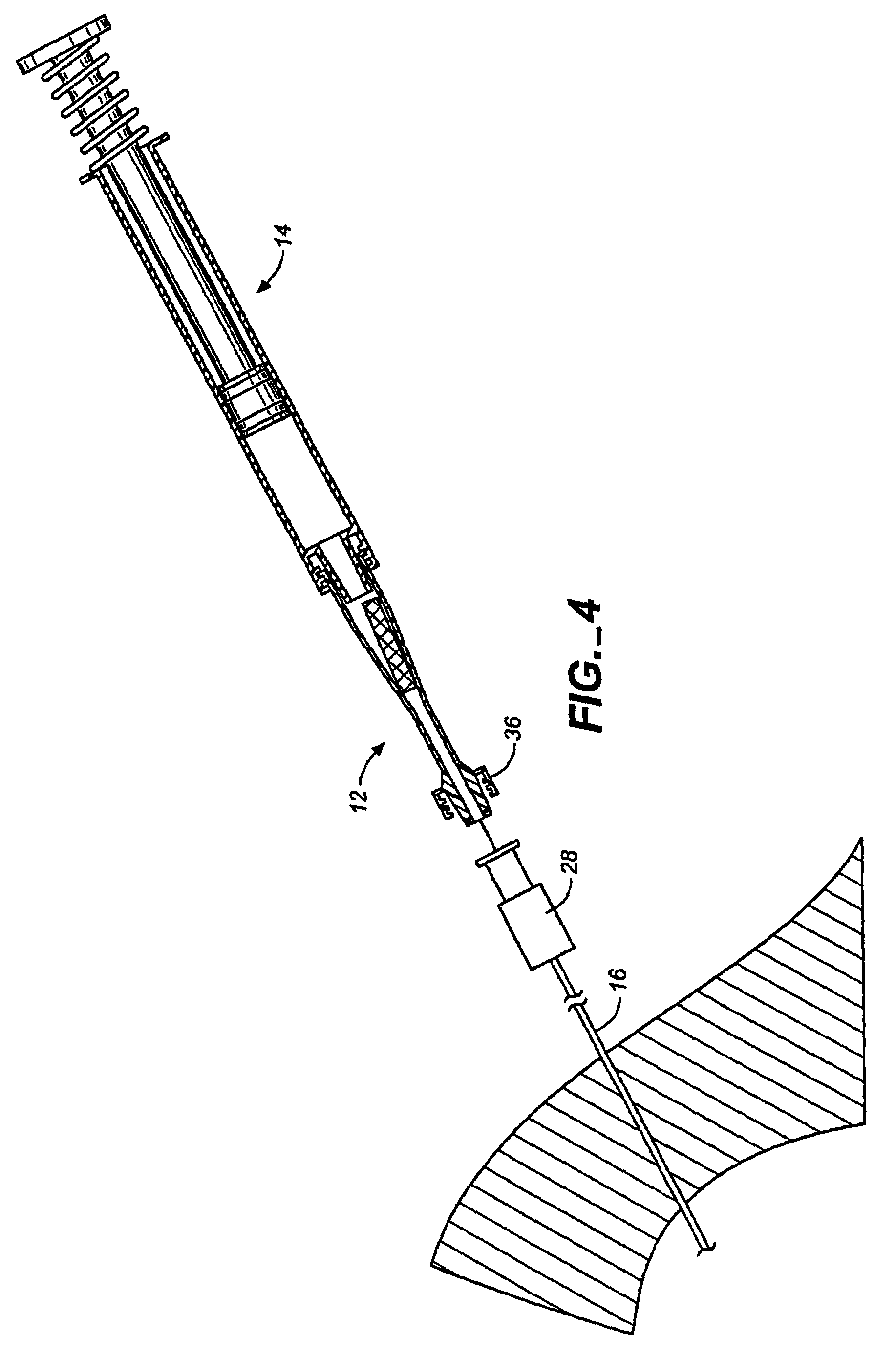
Flow directed catheter having radiopaque strain relief segment
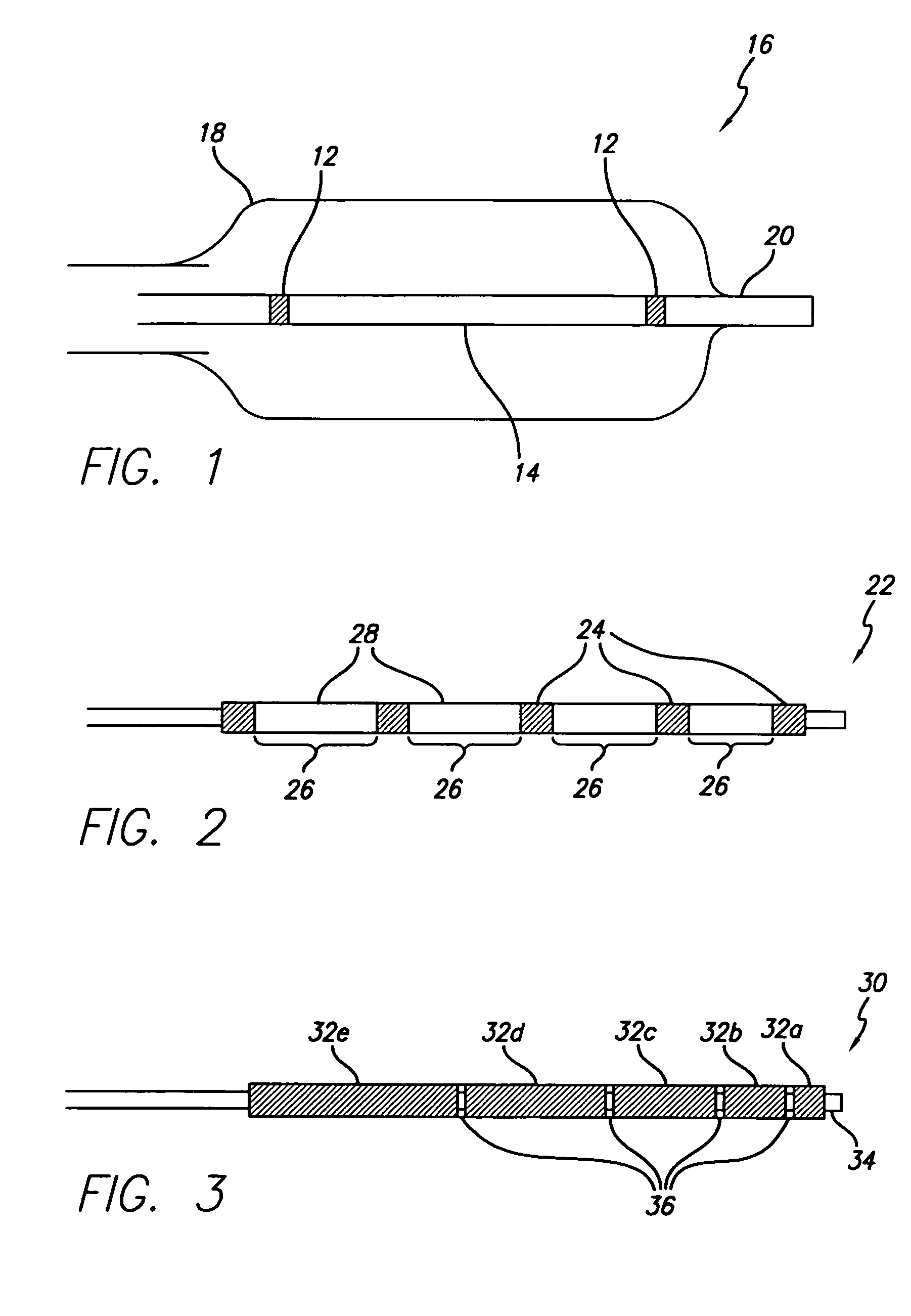
A flow directed catheter for use in medical diagnostic or therapeutic procedures having a strain relief segment between the relatively stiff proximal section of the catheter and the floppy distal tip portion in which the strain relief segment is formed of a polymeric material containing a radiopaque agent.
Owner:CODMAN & SHURTLEFF INC
Balloon expandable bioabsorbable drug eluting flexible stent
ActiveUS20080046068A1Improve toughnessEnhance of componentsStentsSurgeryEndoluminal stentActive agent
A biocompatible material may be configured into any number of implantable medical devices including intraluminal stents. Polymeric materials may be utilized to fabricate any of these devices, including stents. The stents may be balloon expandable or self-expanding. The polymeric materials may include additives such as drugs or other bioactive agents as well as radiopaque agents. By preferential mechanical deformation of the polymer, the polymer chains may be oriented to achieve certain desirable performance characteristics. The stent has a plurality of hoop components interconnected by at least one flexible connector. The hoop components are formed as a continuous series of alternating substantially longitudinally oriented strut members and connector junction struts, whereas the longitudinal strut is connected to the connector junction strut by alternating substantially circumferentially oriented arc members.
Owner:CARDINAL HEALTH SWITZERLAND 515 GMBH
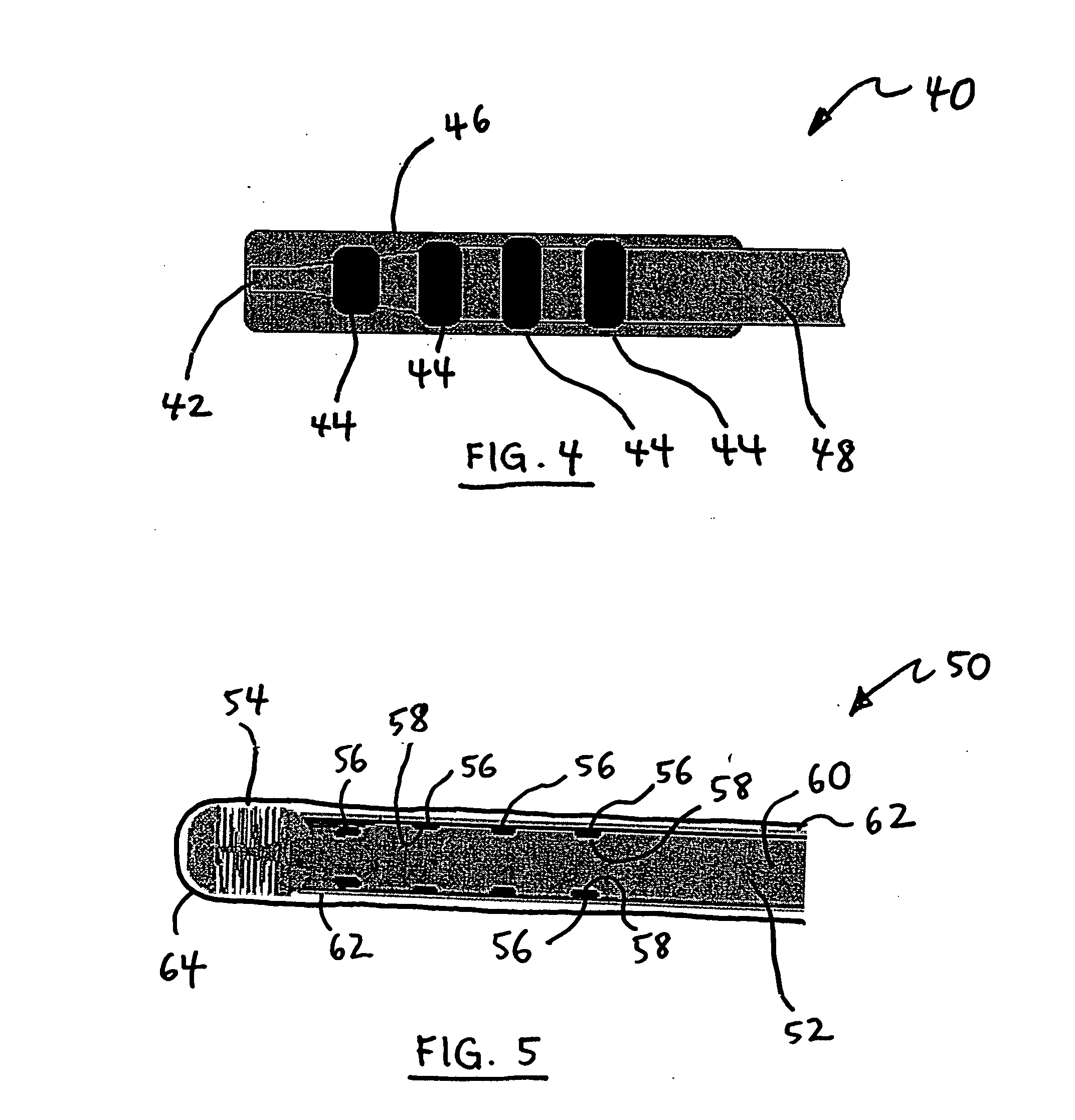
Medical Device Having Regions With Various Agents Dispersed Therein and a Method for Making the Same
InactiveUS20080167710A1Increase awarenessImprove radial stiffnessStentsSurgeryVisibilityRadiopaque agent
Owner:CARDINAL HEALTH SWITZERLAND 515 GMBH
Aluminous cement-based composition for application in endodontics and cementitious product obtained thereof
InactiveUS20110281241A1Improve rheologyImprove workabilityCosmetic preparationsImpression capsRadiopaque agentPlasticizer
The present invention provides a composition based on calcium aluminate cement (CAC) for application in endodontics, comprising: (a) a cement—Al2O3 (>68.5 wt %), CaO (<31 wt %), SiO2 (0.3-0.8 wt %), MgO (0.4-0.5 wt %), and Fe2O3 (<0.3 wt %); (b) additives: dispersant at a content of 0.4 to 0.8% by weight of the cement, a plasticizer at a content of 2.0 to 4.0% by weight of the cement, and a radiopaque agent at a content of 20 to 35% by weight of the cement; and (c) water, wherein water / cement ratio lies in the range of 0.19-0.24 in the presence of additives. Cementitious product obtained thereof, after setting time, is also disclosed and characterized by enhanced properties when compared to the most used commercial repair cement, MTA.
Owner:UNIVE FEDERAL DE SAO CARLOS
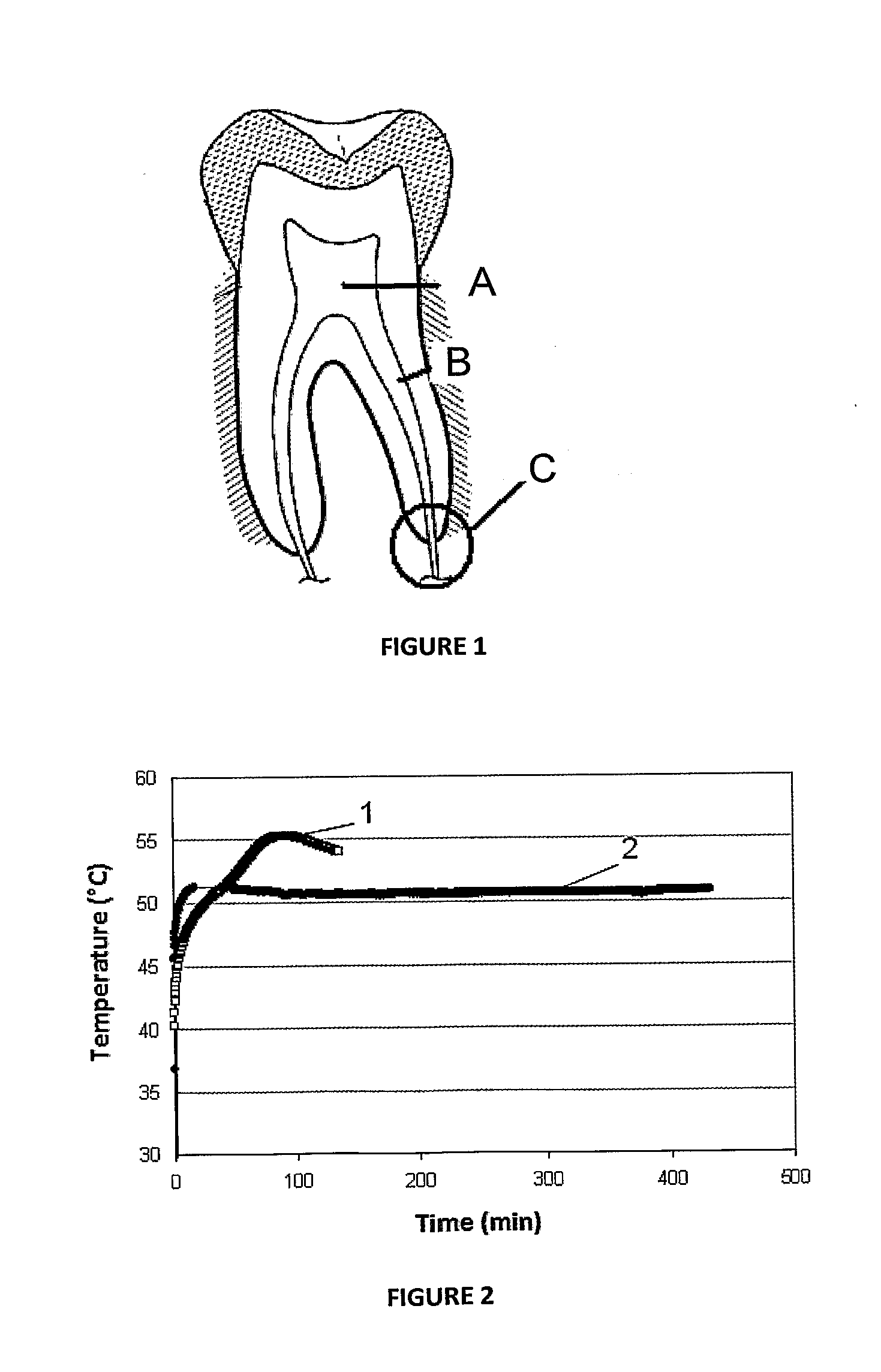
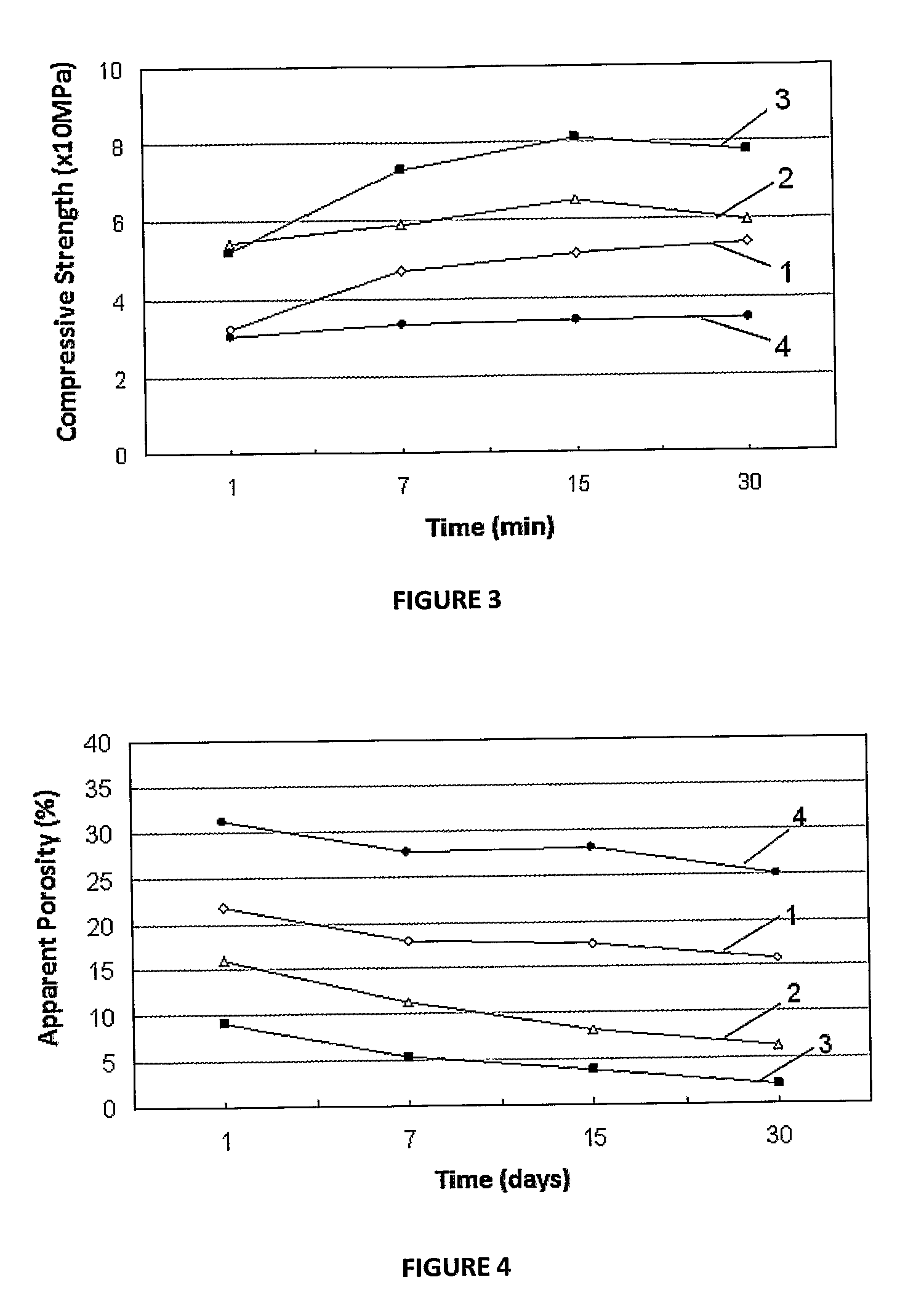
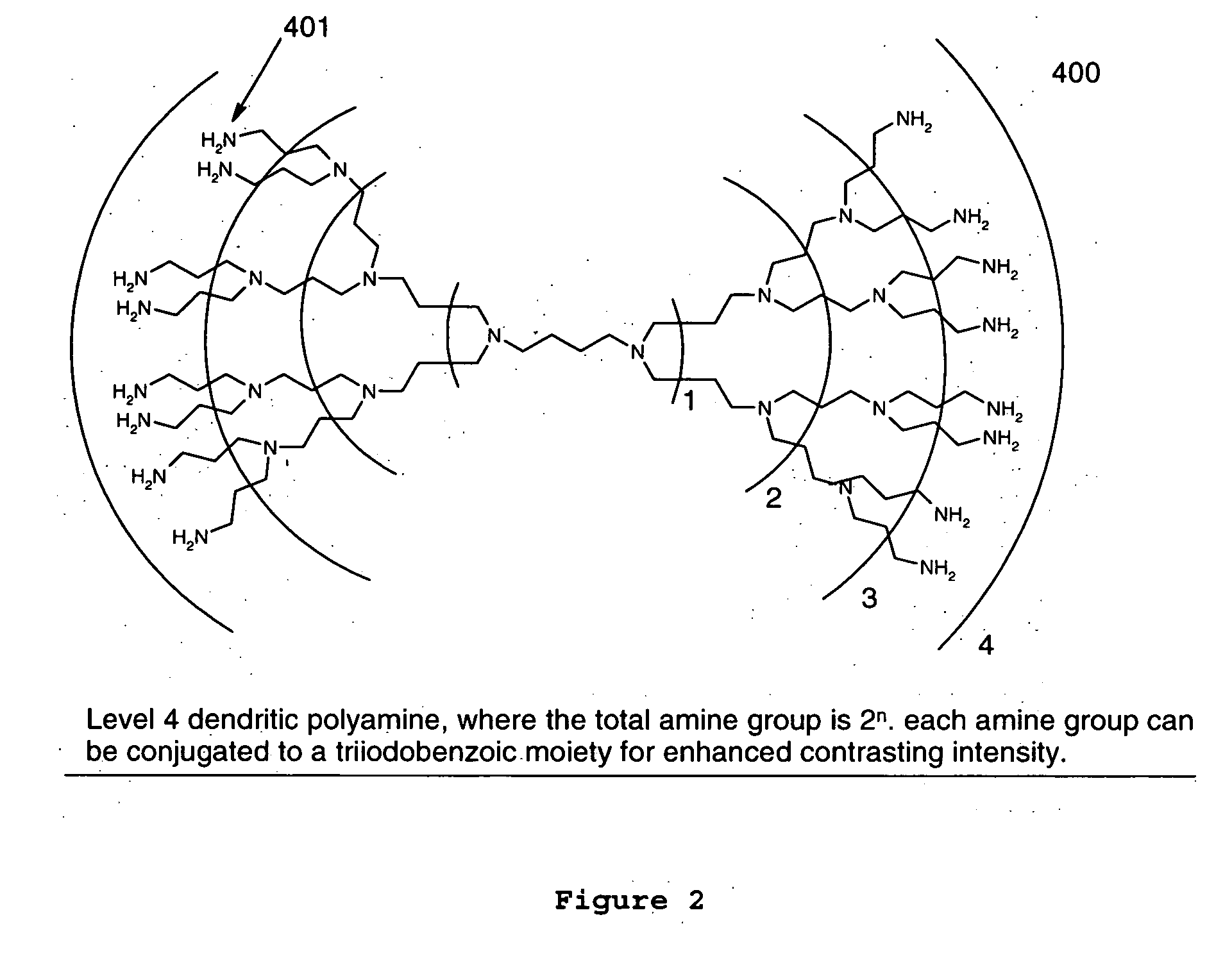
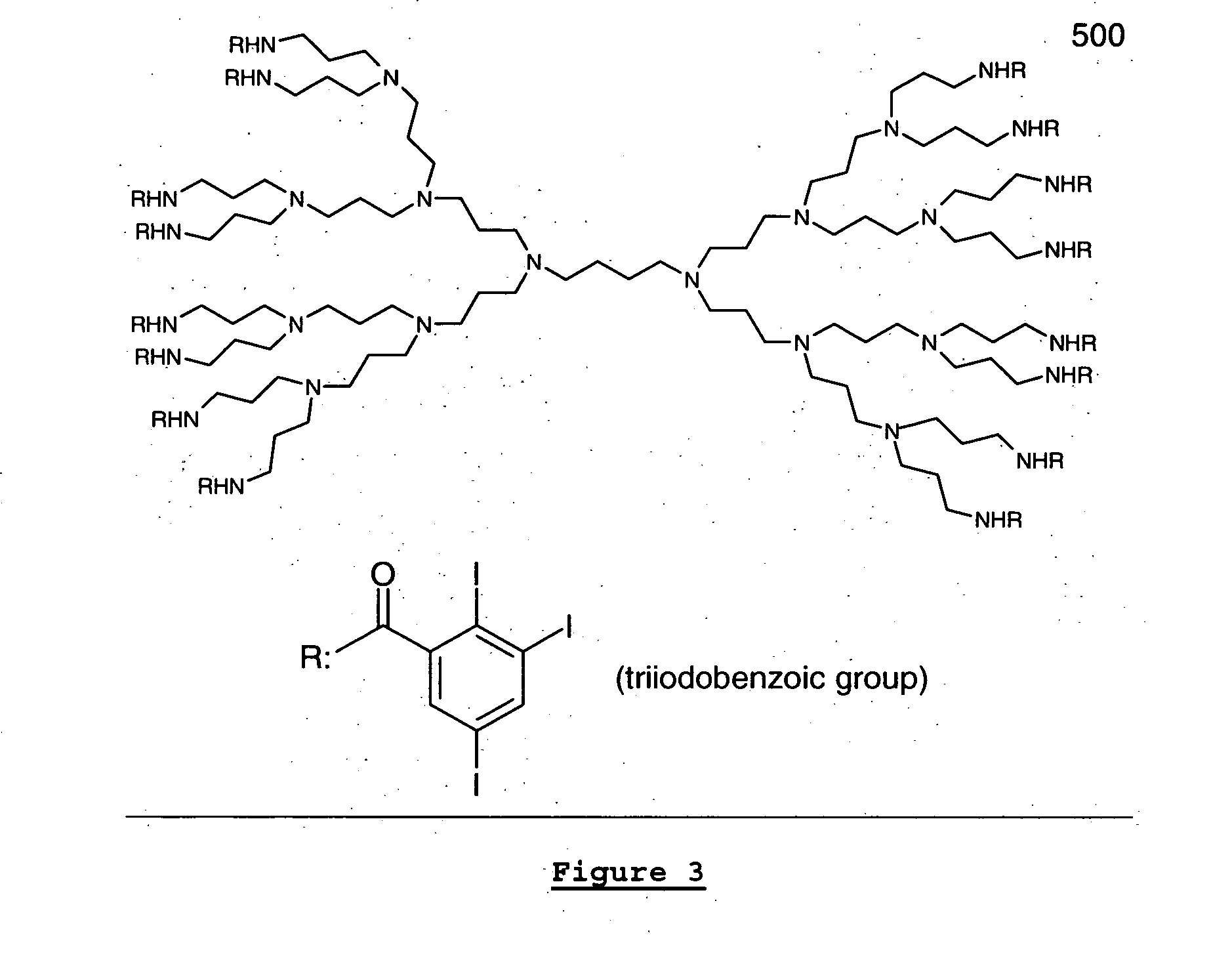
Dendritic and star-shaped contrast agents for medical devices and bioabsorbable radiopaque bulk material and method for producing same
InactiveUS20060292077A1Easy loadingPrevent thrombosisOrganic active ingredientsSurgeryDendrimerRadiopaque agent
In accordance with the present invention, a high intensity radiopaque contrast agent is disclosed. The agent may be coated on or incorporated within bulk material which may then be subsequently utilized to fabricate a radiopaque medical device. Primary effects through chemistry include higher radiopaque concentrations per unit weight of the radiopaque element or agent. Secondary effects include selective placement of the radiopaque elements which may further enhance the radiopacity of the device with reduced requirements of the radiopaque agent. Such a radiopaque contrast agent may be produced in various forms such as a dendrimer and / or incorporated as the end groups of polymeric chain. In addition one can incorporate biological and / or pharmaceutical agents in combination with the present invention.
Owner:CORDIS CORP
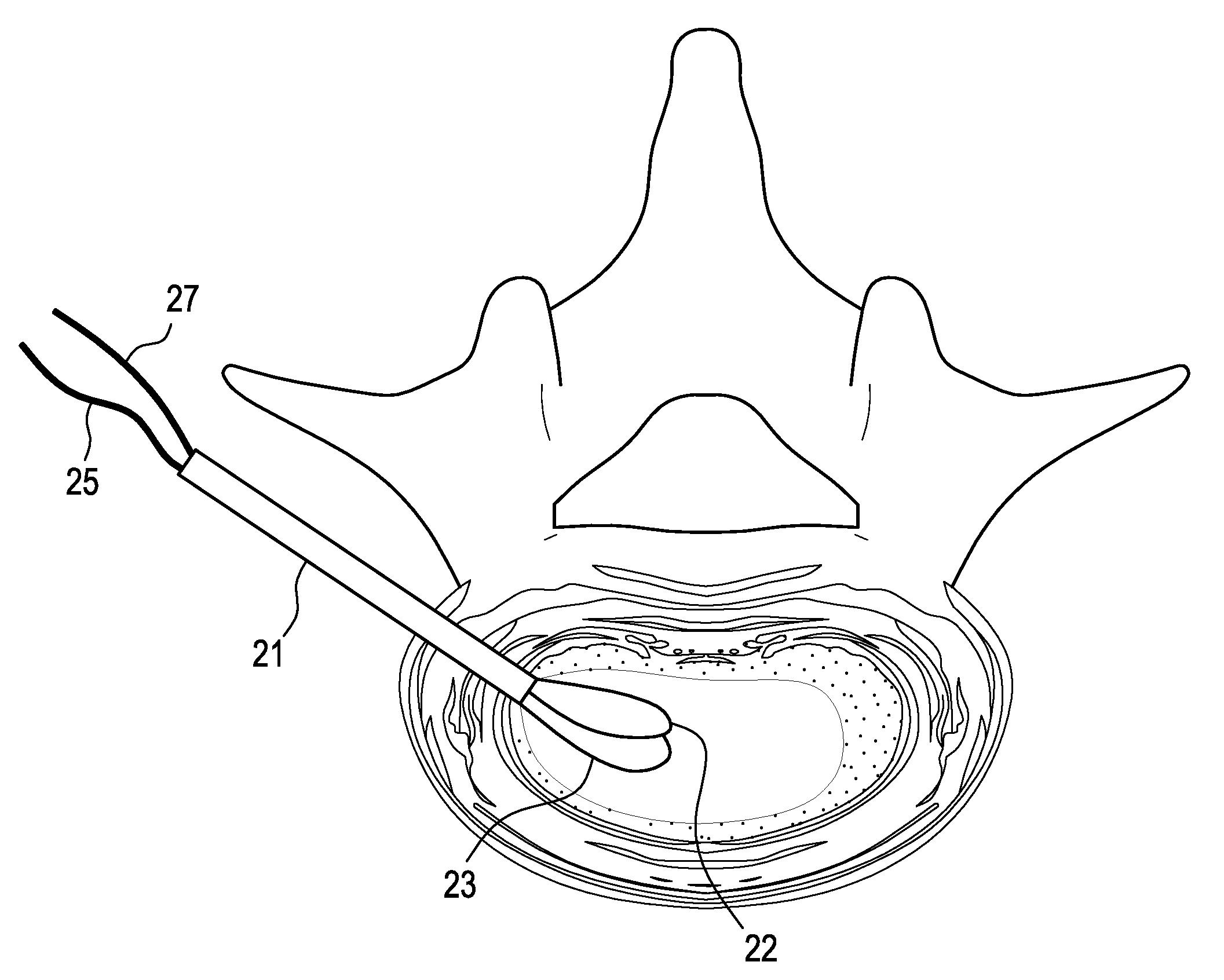
Nucleus replacement device and method
ActiveUS8979931B2Lower potentialMinimized in sizeJoint implantsSpinal implantsIntervertebral spacesRadiopaque agent
A method of replacing a nucleus pulposus material wherein curable nucleus pulposus material is injected into a balloon in an intervertebral space. The balloon has an inlet port and an outlet port that are connected to a pressure-measuring device. After the catheter is placed in the intervertebral space, the balloon is filled with a radio-opaque solution until the desired balloon volume is attained. The nucleus replacement material is then fed into the inlet port of the balloon, while the radiopaque agent is allowed to leave via the outlet port. Once the balloon is completely filled and curing of the curable nucleus replacement material is accomplished, the catheter and ports are removed, and the annular defect can be sealed.
Owner:DEPUY SYNTHES PROD INC
Polymeric marker with high radiopacity for use in medical devices
InactiveCN1901940ACapable of measuringEasy and fast measurementStentsPowder deliveryRadiopaque agentBalloon catheter
High radiopacity is achieved in a polymeric marker by combining a polymeric resin, a powdered radiopaque agent having uniformly shaped particles of a specific particle size distribution, and a wetting agent. The method to produce the marker calls for the blending and pelletization of these materials followed by extrusion onto support beading. The resulting supported tubing is subsequently cut to length with the beading still in place. After ejection of the beading remnant the marker is slipped into place on the device to be marked and attached by melt bonding. Marking of a guide wire allows lesions to be measured while the marking of balloon catheters allow the balloon to be properly positioned relative to a lesion.
Owner:ABBOTT CARDIOVASCULAR
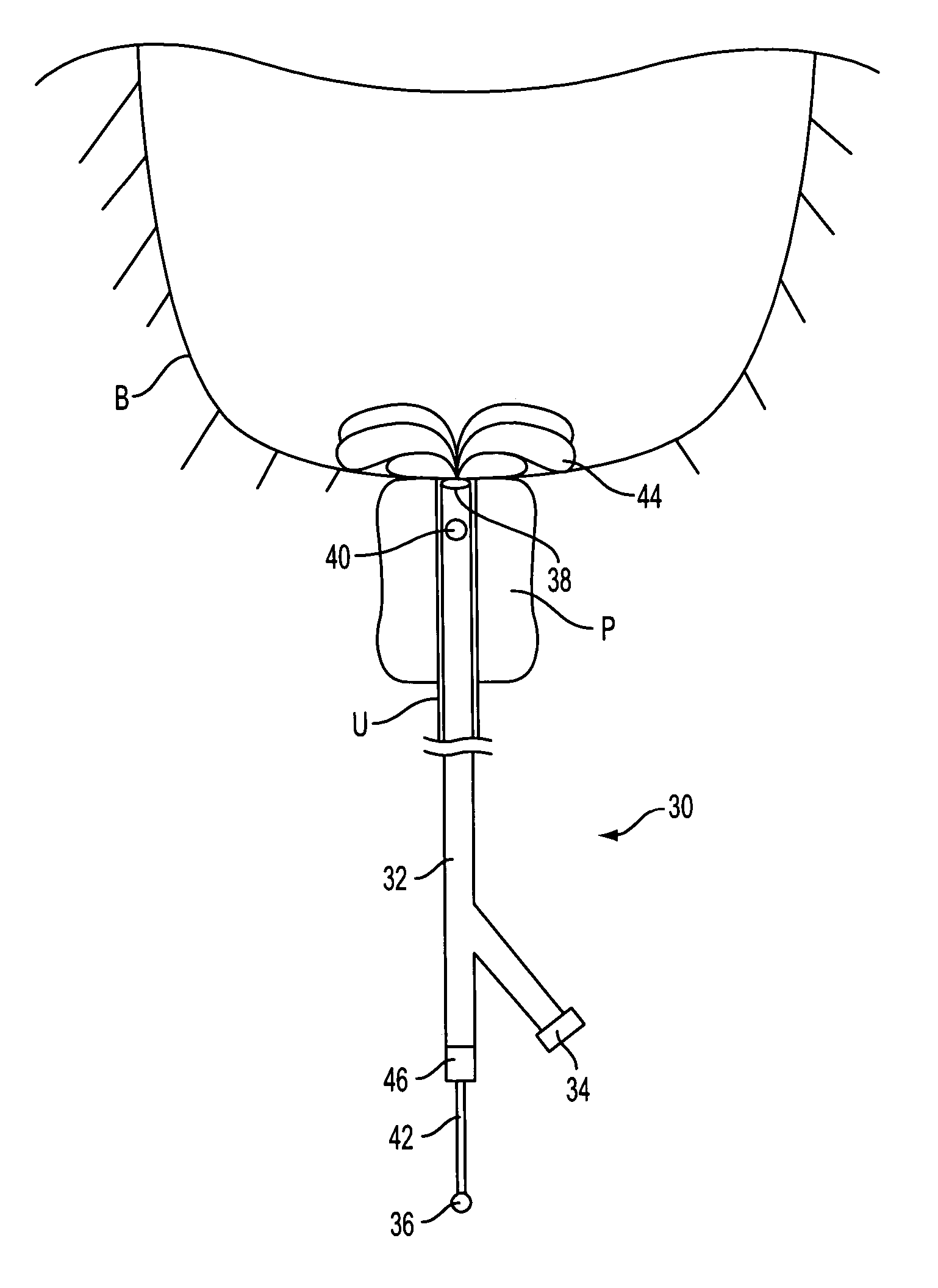
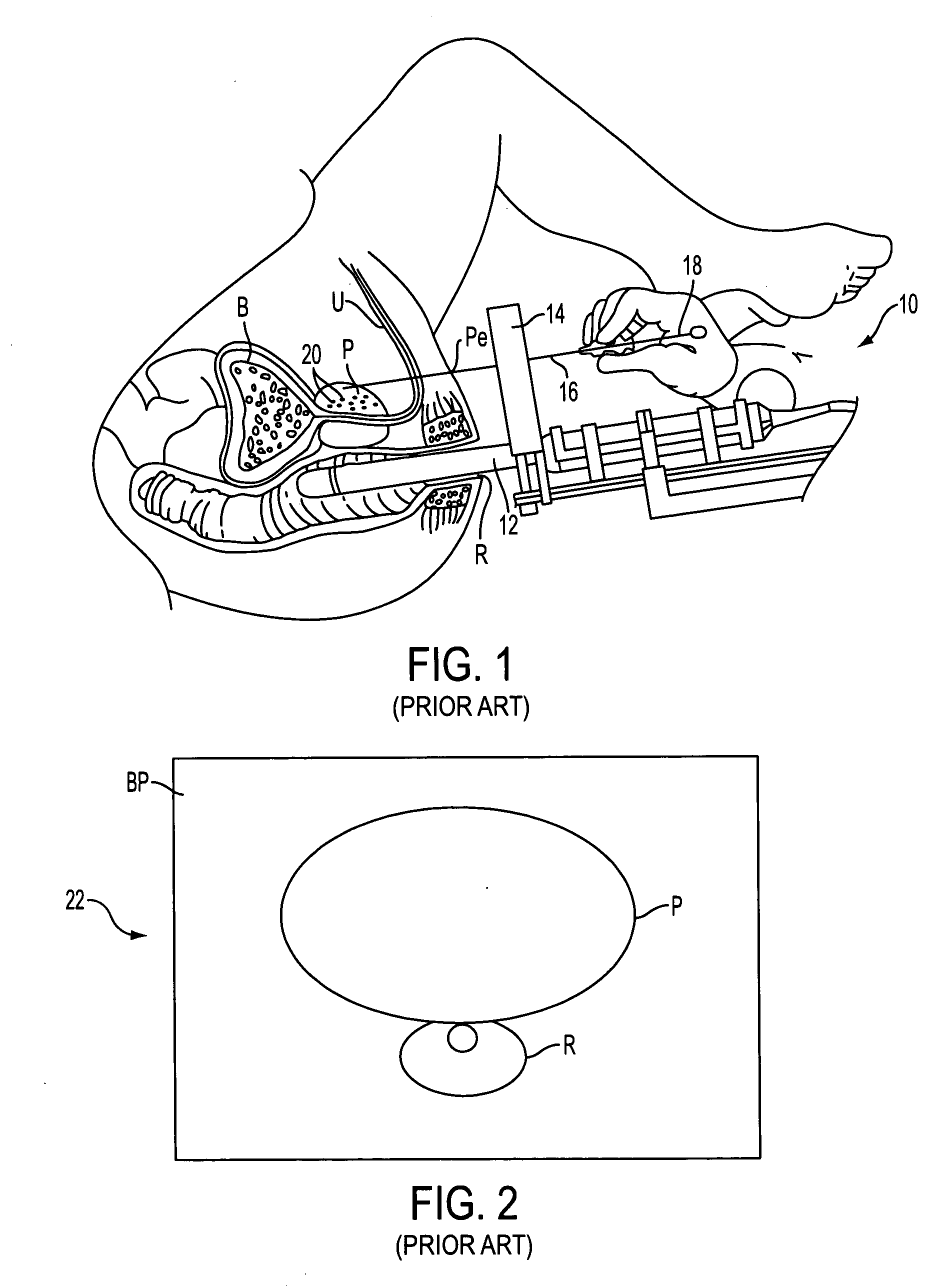
Prostate visualization device and methods of use
InactiveUS20050004471A1Reliable imagingIncrease awarenessStentsOrgan movement/changes detectionDiseaseUltrasonic imaging
Methods and apparatus are provided for improved administration of brachytherapy in the treatment of prostate disease. More particularly, a prostate visualization device is provided comprising a catheter coupled to at least one deployable member at the distal end of the catheter. The deployable member is preferably manufactured from a shape memory alloy having a petal-shaped configuration suitable for engaging and defining the proximal region of a patient's bladder. The deployable member may comprise tubing filled with air or other radiopaque agents to facilitate ultrasonic imaging of the deployable members near the bladder / prostate junction.
Owner:NEOSEED TECH
Compositions for tissue augmentation
ActiveUS7575740B2Skeletal disorderSilicon compound active ingredientsActive agentBiological materials
Methods for making biomaterials for augmentation of soft and hard tissues, kits containing precursors for forming the biomaterials, and the resulting biomaterials are described herein. The biomaterials are formed from at least a first and a second precursor component. The first precursor component contains at least two nucleophilic groups, and the second precursor component contains at least two electrophilic groups. The nucleophilic and electrophilic groups of the first and second precursor components form covalent linkages with each other at physiological temperatures. The precursors are selected based on the desired properties of the biomaterial. In the preferred embodiment, the first precursor is a siloxane. Optionally, the biomaterials contain additives, such as thixotropic agents, radiopaque agents, or bioactive agents. In the preferred embodiment, the biomaterials are used to augment at least one vertebra of the spine (vertebroplasty).
Owner:KUROS BIOSURGERY AG
Implantable device formed from polymer and plasticizer blends having modified molecular structures
InactiveUS20070202146A1Designing can be facilitatedWide load rangeStentsBiocideActive agentDevice form
A biocompatible material may be configured into any number of implantable medical devices including intraluminal stents. Polymeric materials may be utilized to fabricate any of these devices, including stents. The stents may be balloon expandable or self-expanding. The polymeric materials may include additives such as drugs or other bioactive agents as well as radiopaque agents. By preferential mechanical deformation of the polymer, the polymer chains may be oriented to achieve certain desirable performance characteristics.
Owner:CORDIS CORP
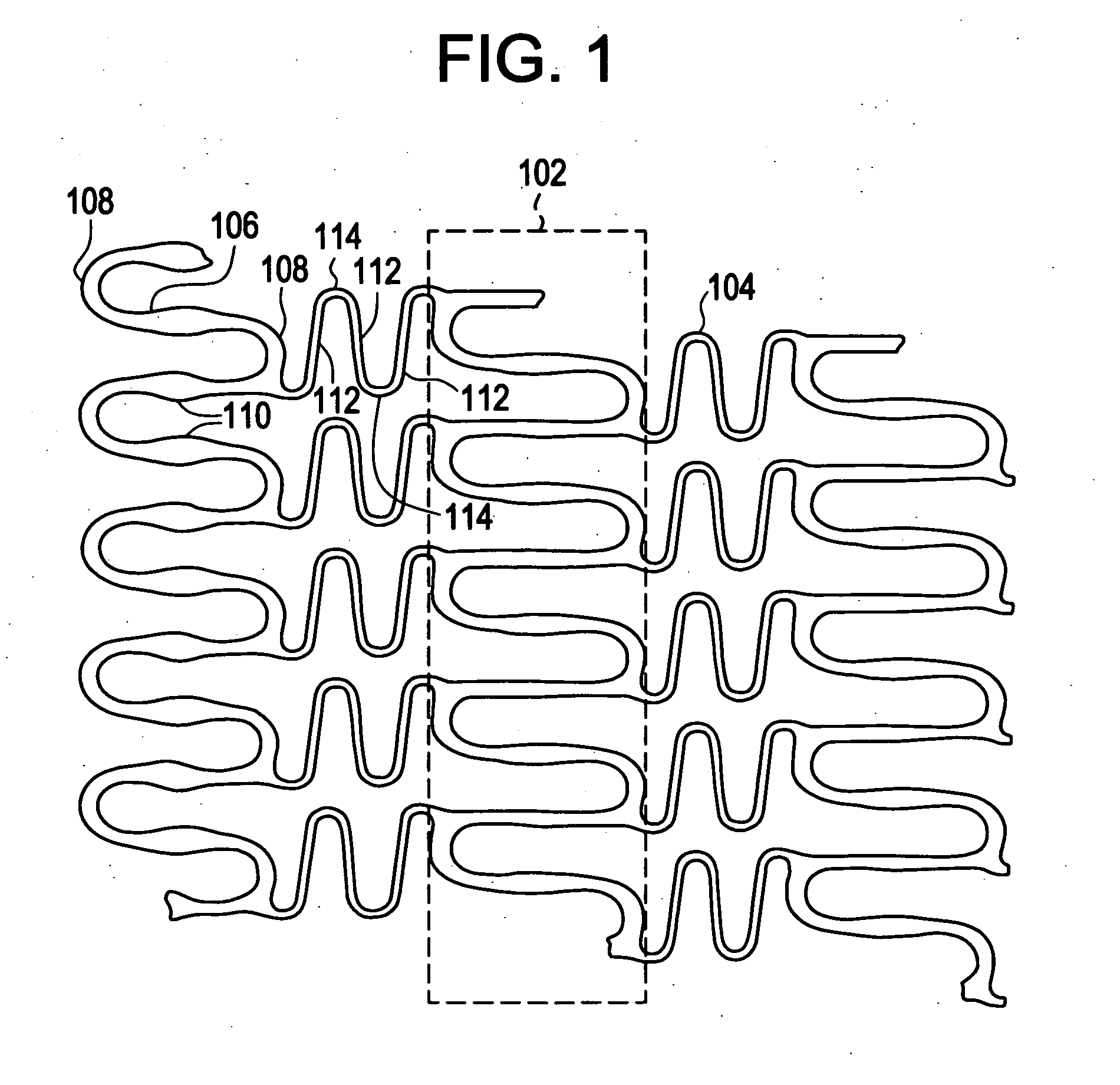
Method for making a device having discrete regions
InactiveUS20080102098A1Increase awarenessAbility to deliverPowder deliveryStentsVisibilityRadiopaque agent
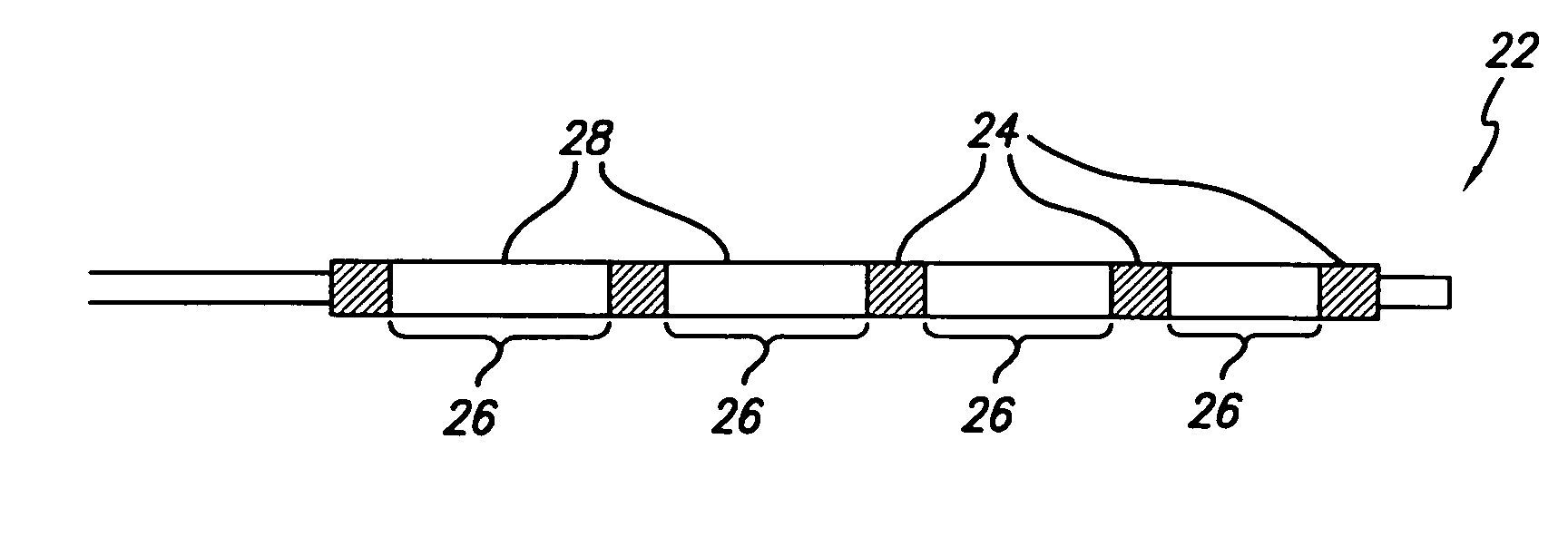
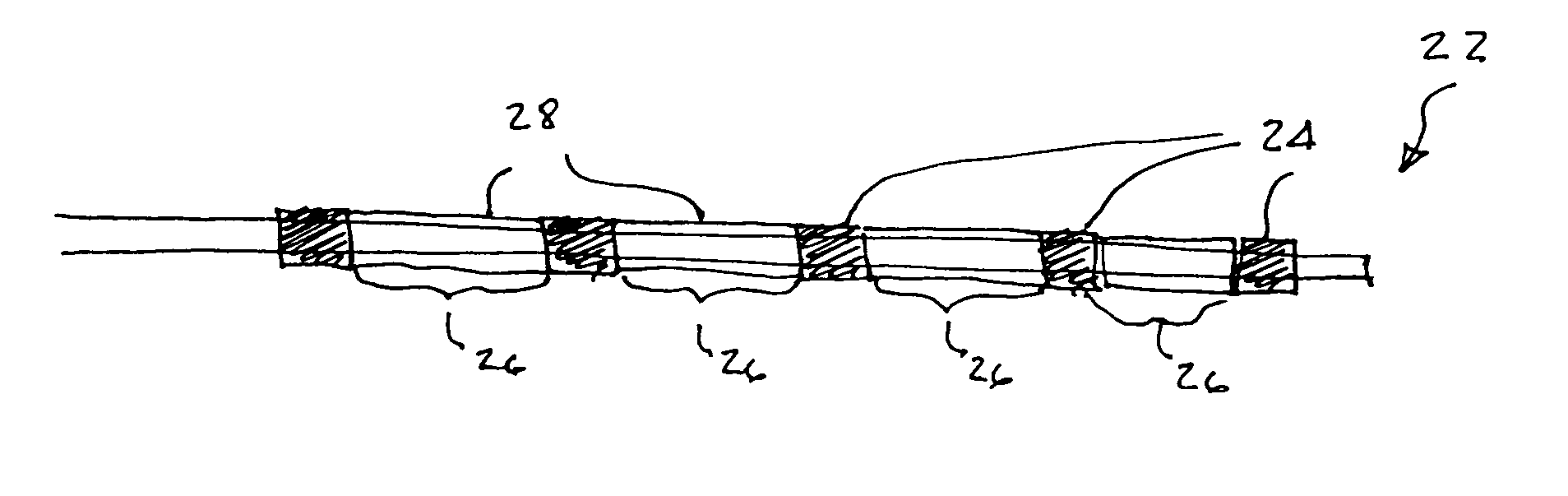
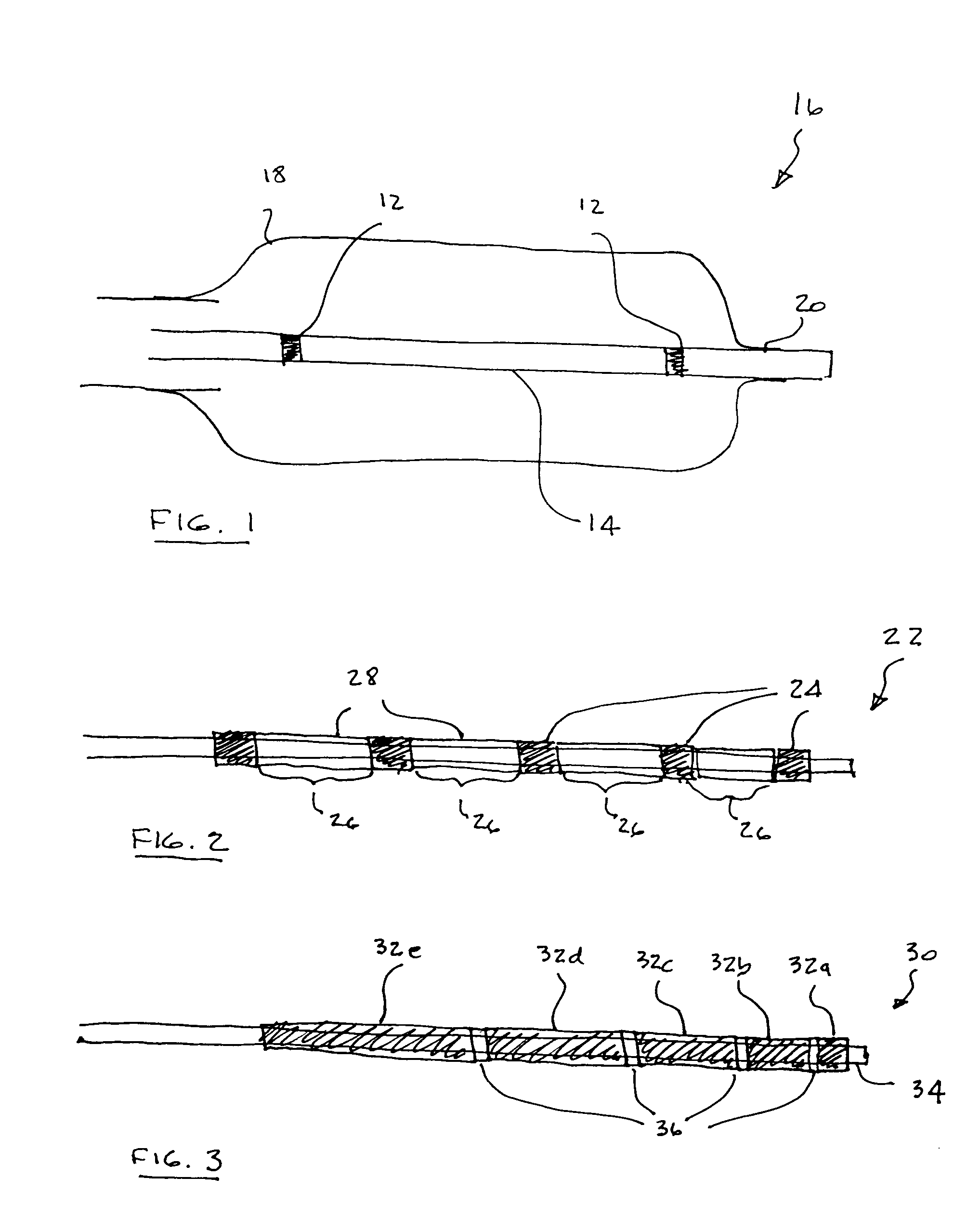
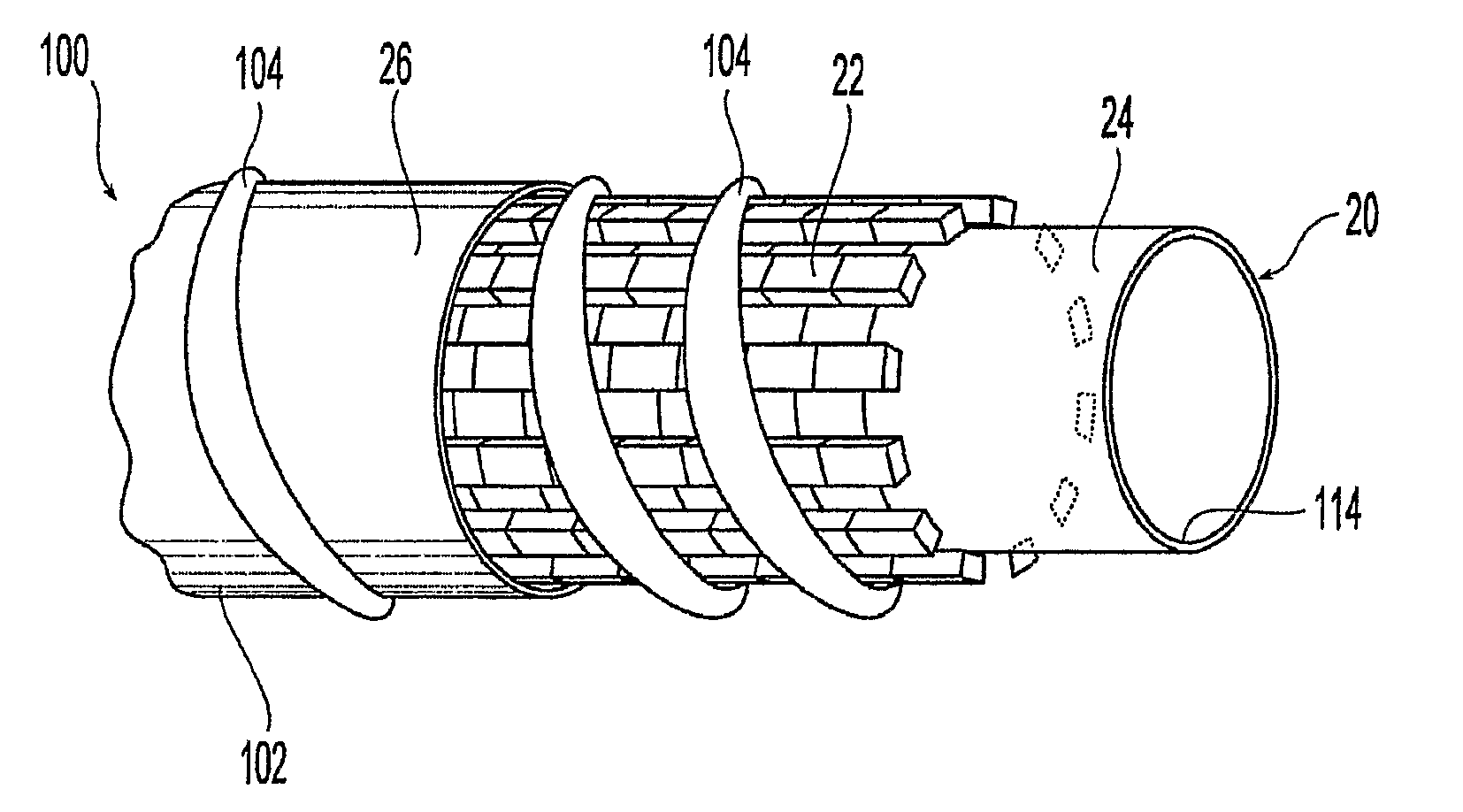
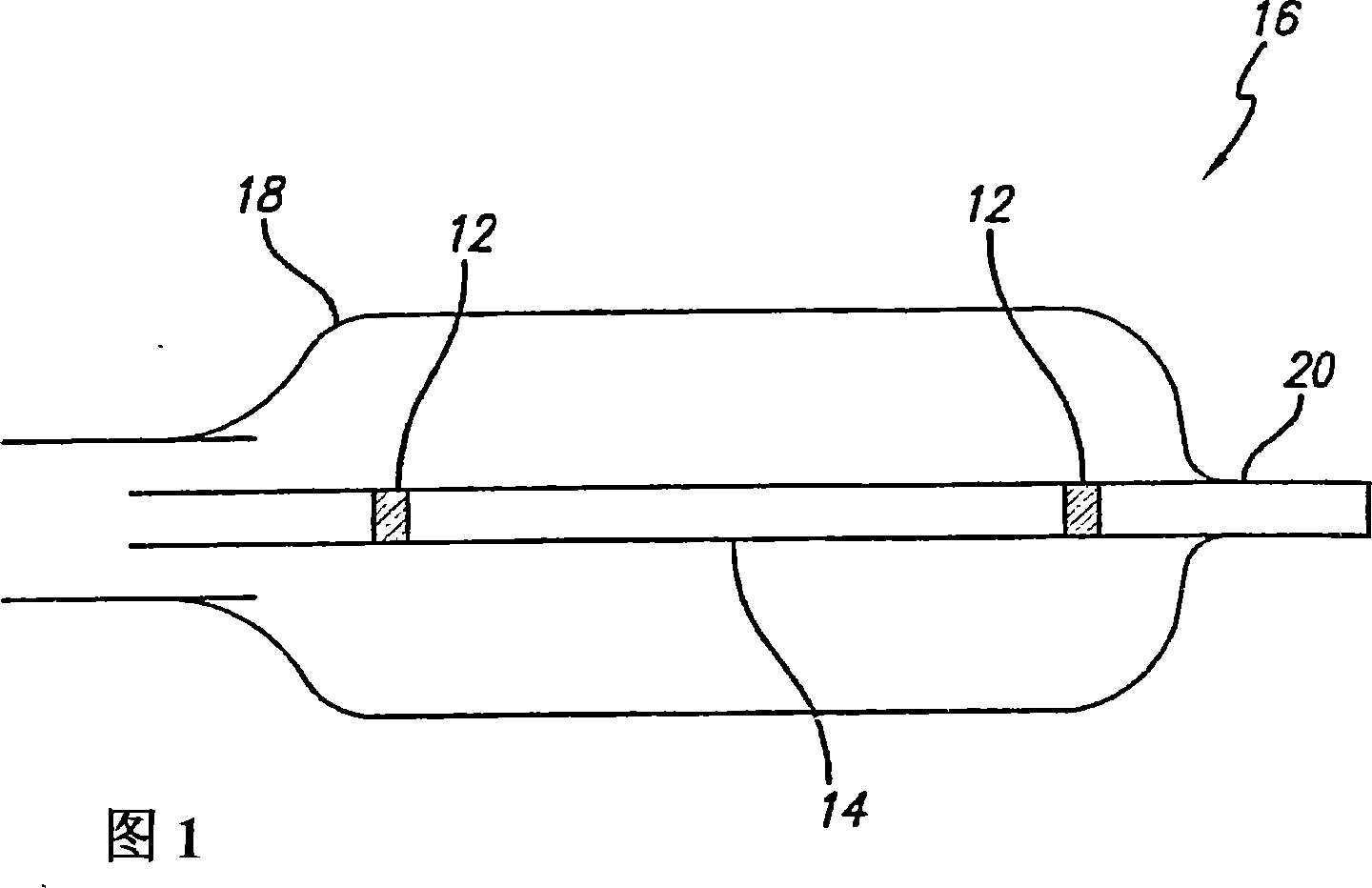
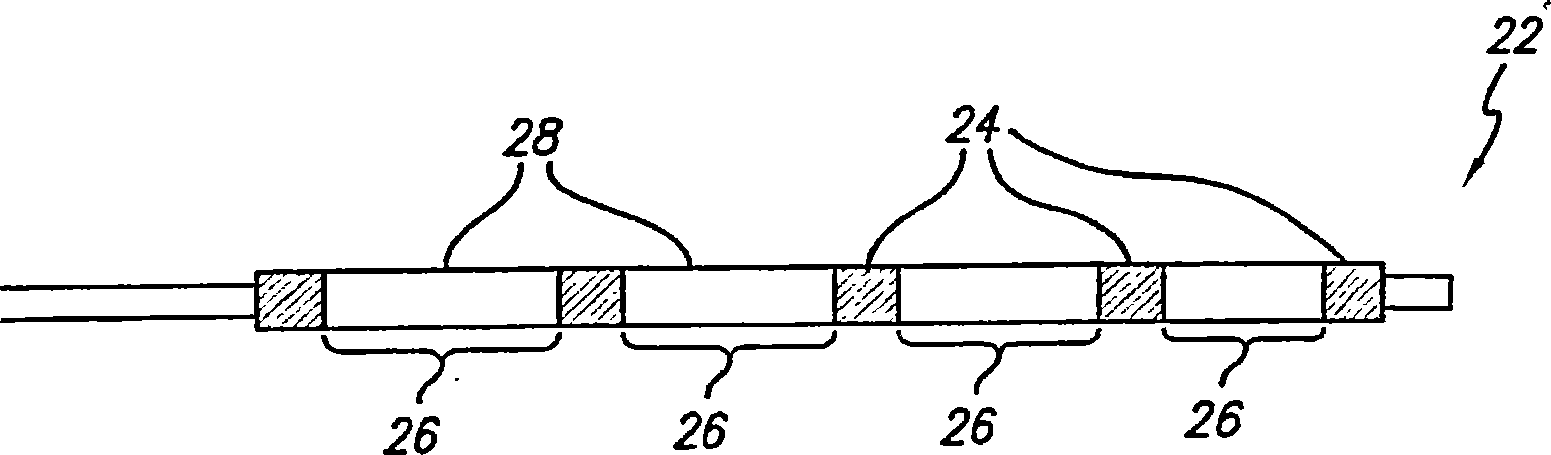
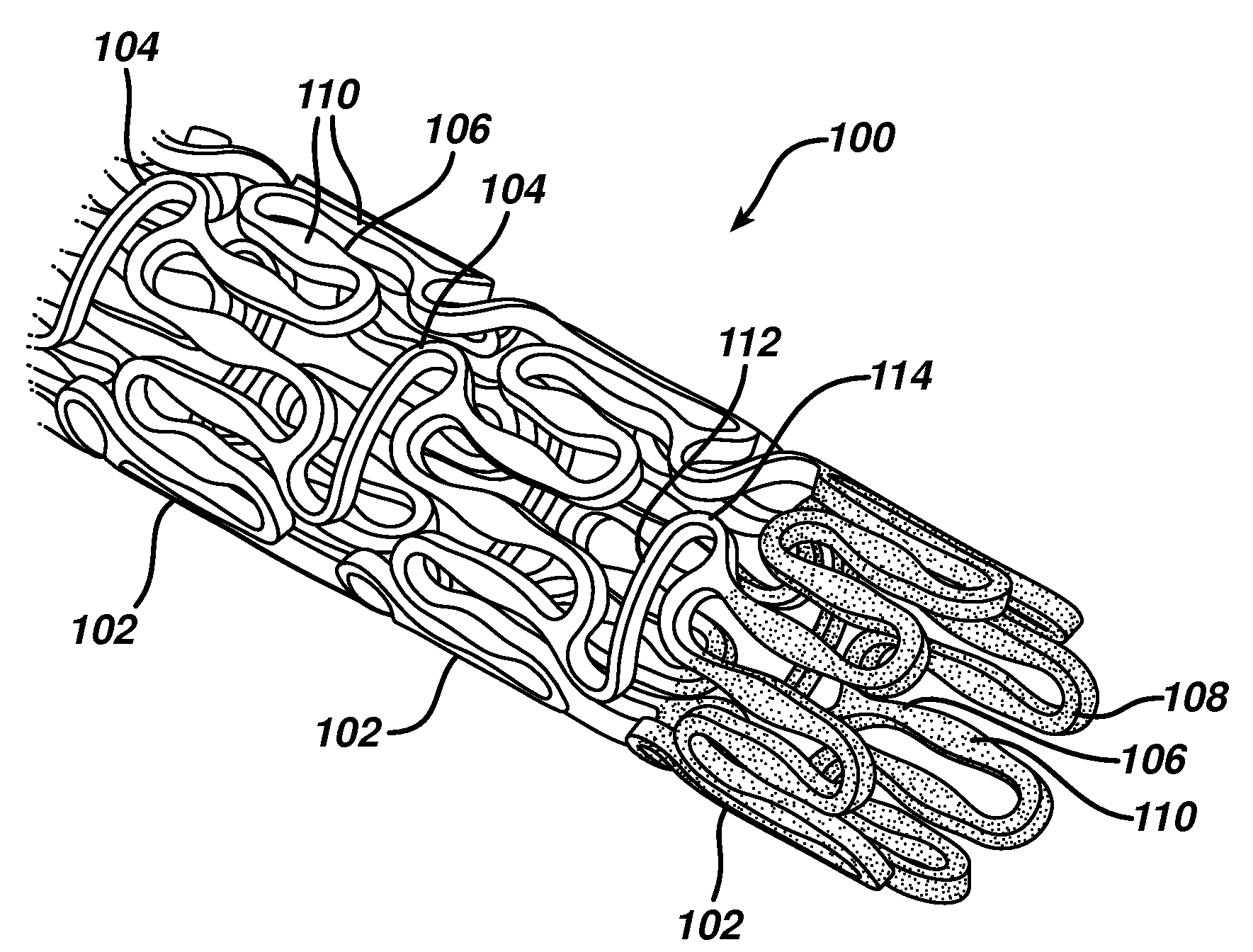
A medical device is constructed from a polymeric composition having properties such as increased visibility of the device and the ability to deliver therapeutic and other agents. The device is constructed with additives incorporated into the polymeric structure, such as a material that increases visibility of the device, while still maintaining desired mechanical characteristics such as high radial stiffness, minimized recoil values, and improved flexibility. The device can assume a wide range of geometries that are adaptable to various loading conditions In order to include performance-enhancing additives to the medical device without affecting mechanical performance, the additives are localized in discrete regions of a polymer structure from which a medical device will be formed. For example, a medical device can be prepared from a polymer form such as a tube containing radiopaque agent localized at its ends or in a desired pattern along the device.
Owner:CARDINAL HEALTH SWITZERLAND 515 GMBH
Monofilament allowing contrast x-ray radiography
InactiveUS20100329417A1Excellent in X-ray contrast capabilitySoft textureOther chemical processesDuplicating/marking methodsMedicineRadiopaque agent
Disclosed is a monofilament allowing contrast X-ray radiography. At least part of the monofilament is formed of a thermoplastic resin containing a radiopaque agent. The monofilament contains the radiopaque agent in the thermoplastic resin in a content of 30 to 80% by mass, and has a Young's modulus of 0.1 to 5.0 cN / dtex and a fineness of 500 to 20000 dtex.
Owner:UNITIKA LTD
Absorbable sponge with contrasting agent
InactiveUS7618567B2Good hemostasisAccurate identificationUltrasonic/sonic/infrasonic diagnosticsMicrobiological testing/measurementPuncture WoundRadiopaque agent
An absorbable sponge containing a contrasting agent (e.g, radiopaque agent) that can be introduced to a biopsy tract or other puncture wound site is provided. The contrasting agent permits identification of the site by fluoroscopy or other imaging techniques.
Owner:SUB Q
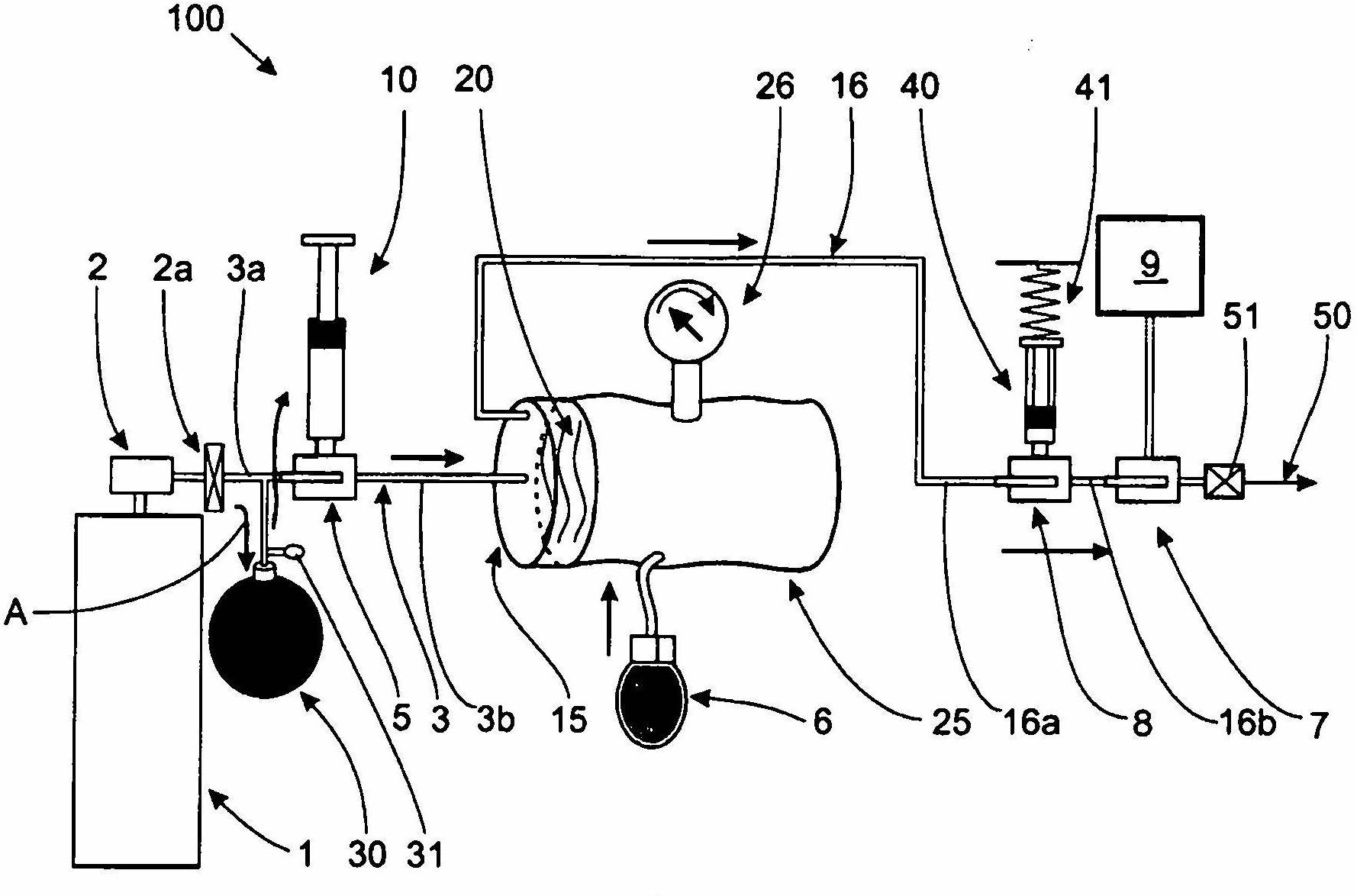
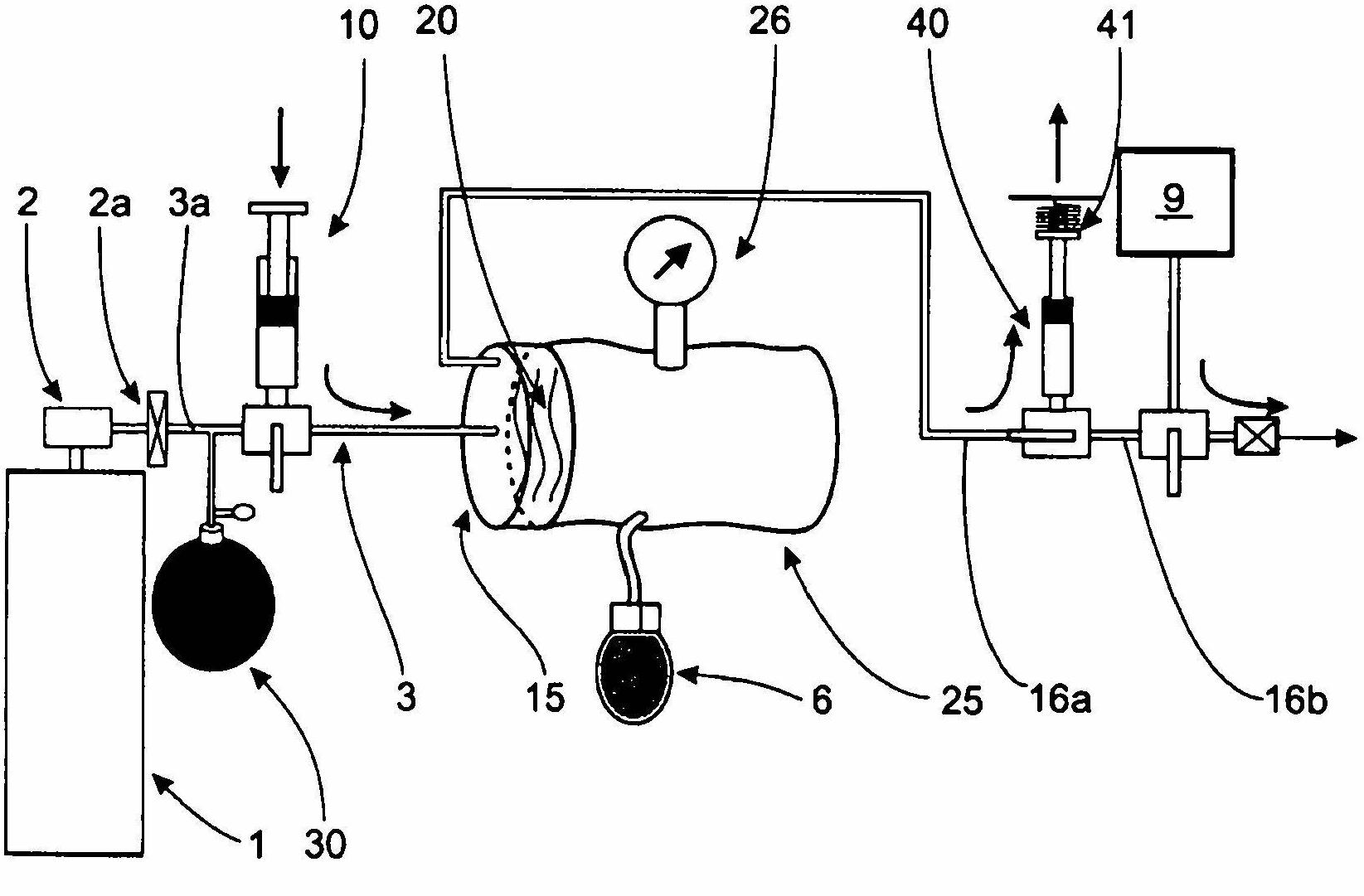
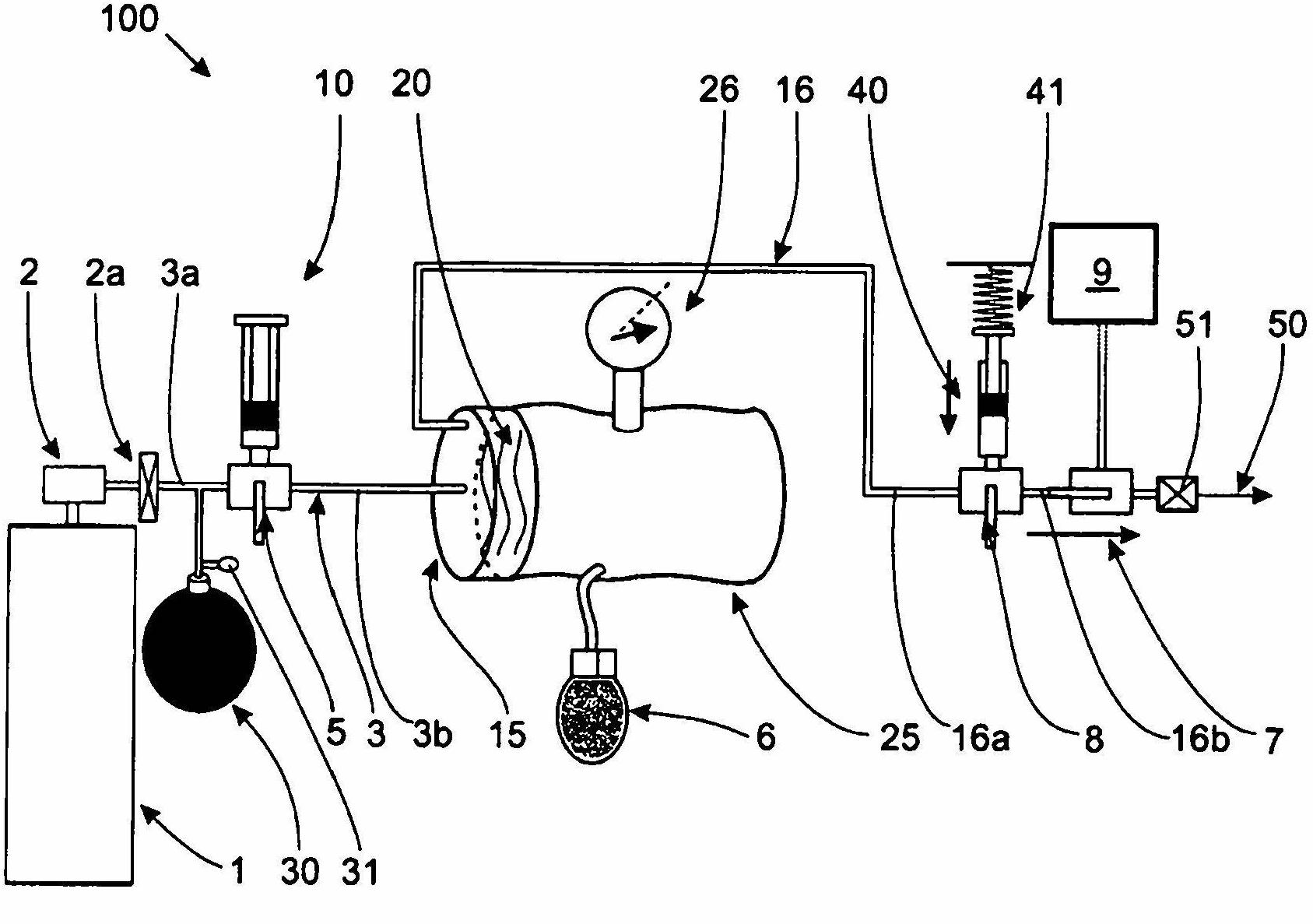
Device for dosing and adjusting the flow of a radiopaque agent to be used in performing an angiography
InactiveCN102655896ADetermine the exact doseNo contamination issuesPressure infusionFlow controlRadiopaque agentMedicine
Owner:SPARK
Compositions for tissue augmentation
InactiveUS8282912B2Pharmaceutical delivery mechanismSynthetic polymeric active ingredientsRadiopaque agentActive agent
Methods for making biomaterials for augmentation of soft and hard tissues, kits containing precursors for forming the biomaterials, and the resulting biomaterials are described herein. The biomaterials are formed from at least a first and a second precursor component. The first precursor component contains at least two nucleophilic groups, and the second precursor component contains at least two electrophilic groups. The nucleophilic and electrophilic groups of the first and second precursor components form covalent linkages with each other at physiological temperatures. The precursors are selected based on the desired properties of the biomaterial. In the preferred embodiment, the first precursor is a siloxane. Optionally, the biomaterials contain additives, such as thixotropic agents, radiopaque agents, or bioactive agents. In the preferred embodiment, the biomaterials are used to augment at least one vertebra of the spine (vertebroplasty).
Owner:KUROS BIOSURGERY AG
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com