[0017] The
high intensity X-
ray contrast agent in accordance with the present invention overcomes the disadvantages and shortcomings of what is currently available and satisfies the
unmet needs of imaging medical devices, particularly non-metallic medical devices by maximizing the intensity of the x-
ray contrast agent both through primary and secondary effects. Primary effects include incorporating the radiopaque element and maximizing the content of this element in the contrast agent through
chemistry, while secondary effects include optimizing the location of the radiopaque element within the
polymer. Essentially by selectively maximizing and incorporating the
iodine content within and dispersed throughout the
polymer one can tune the radiopacity of polymeric materials to levels previously not available. Moreover, the creation and optimization of this contrast agent allows for improved
processing characteristics when combined with polymeric materials and as such may further reduce manufacturing costs while providing a polymeric material with improved high intensity radiopacity with a satisfactory degradation profile.
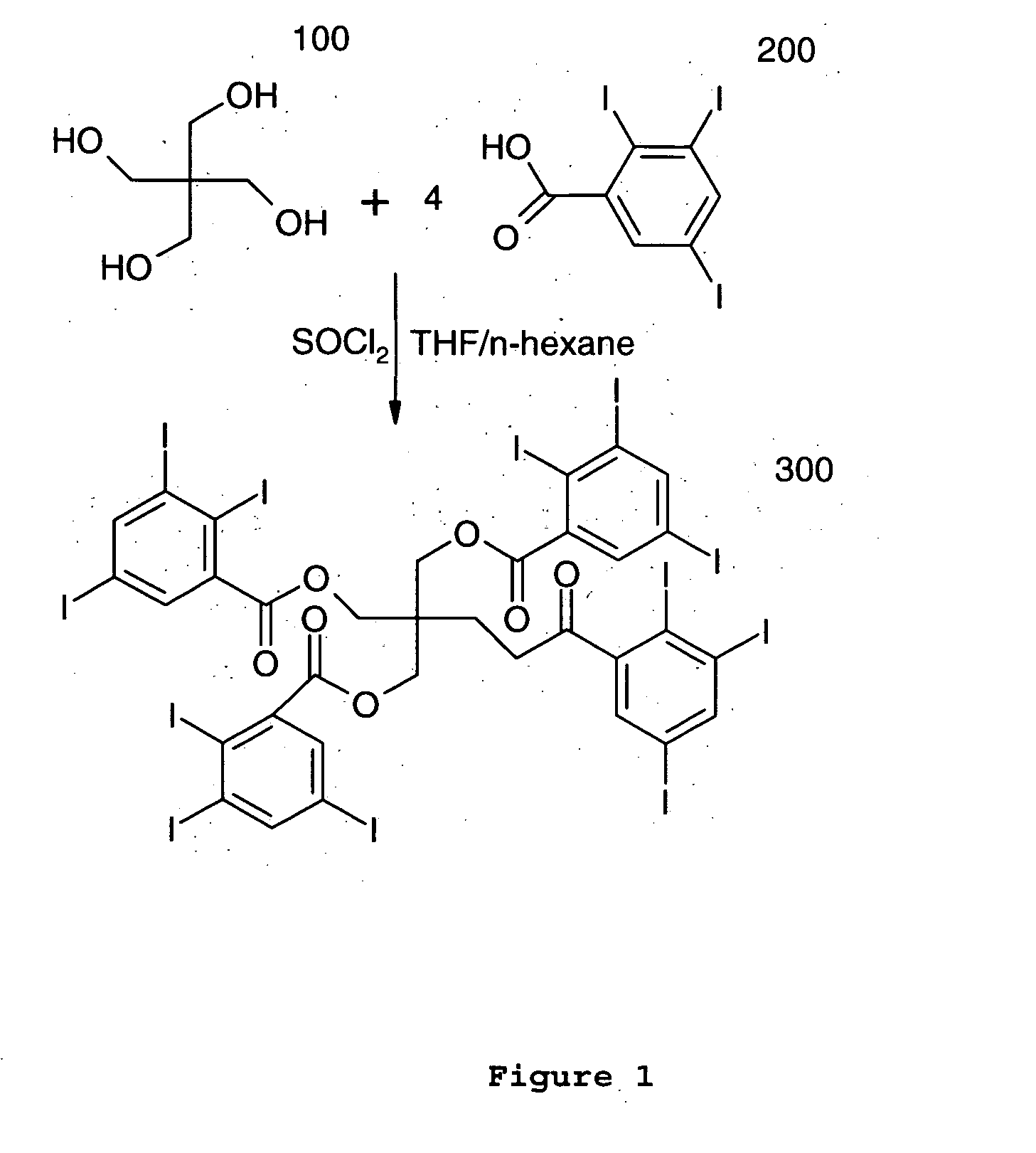
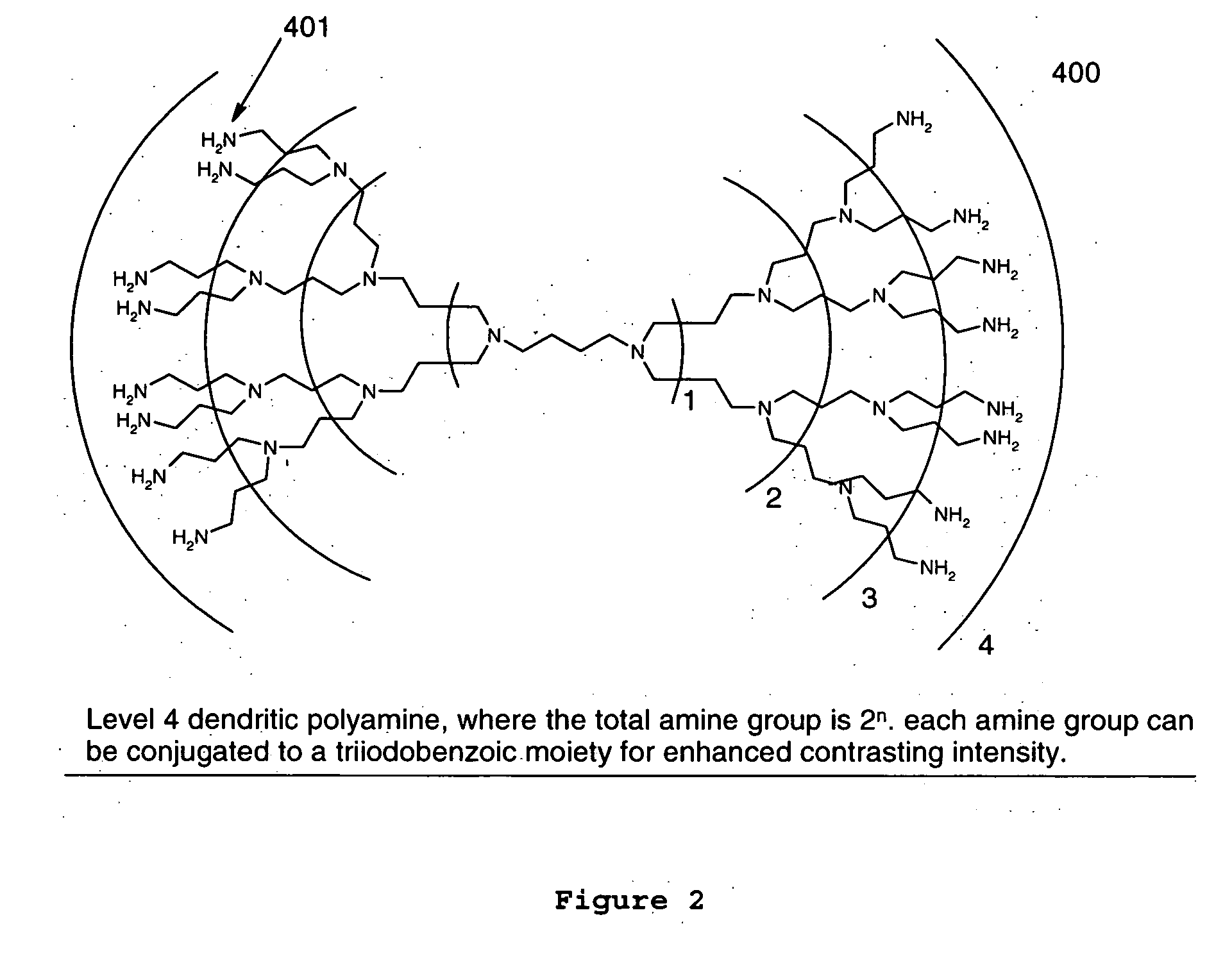
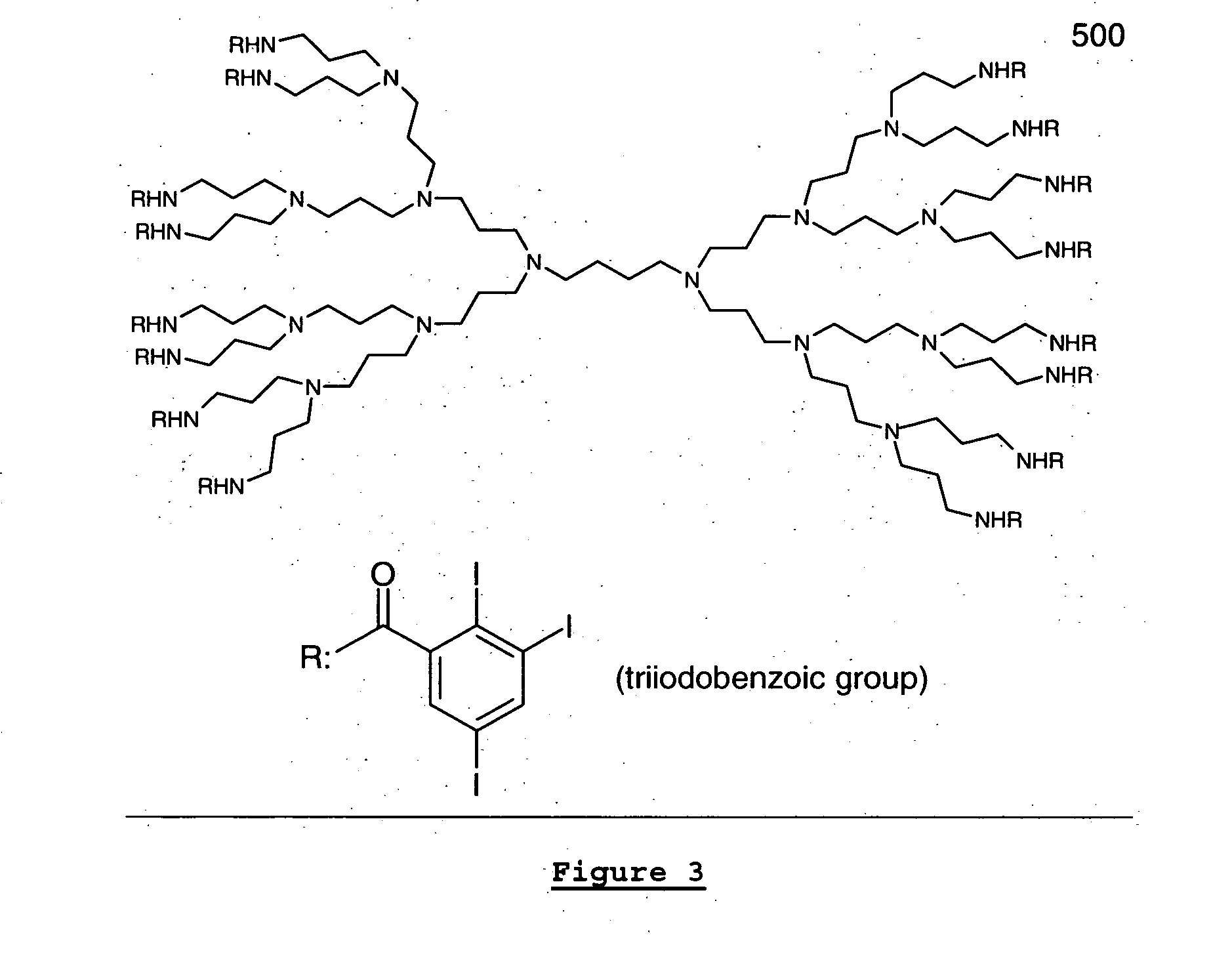
[0019] In an exemplary embodiment of the present invention, the contrast agent may contain a multiplicity of
iodine atoms or
bromine atoms or a combination of both in a single molecule in order to enhance the x-ray image produced by dispersing the agent throughout the material that either the device will be fabricated from or applied as a
coating to the device. In accordance with the present invention, the contrast agent can be constructed from any core of
dendrimer containing free functional groups such as amine, hydroxyl, sulfhydryl, isocyante, and result in a molecule containing a multiple of three (3)
iodine or
bromine or a combination of both atoms with each additional conjugation of small iodine or
bromine containing building block, such as triiodobenzoic acid or as triiodobenzoic acid
chloride. When constructed in this fashion, the contrast agent may be substantially soluble in common
organic solvent such as
acetone,
dimethylacetamide (DMA), dimethylsulfoxide (DMSO),
acetone, THF, 1,4-dioxane, DCM etc. and also has substantially good
miscibility with common organic polymers such as
PLGA, PLA etc. The contrast agent in accordance with the present invention can form a
solid solution with a polymer matrix that can then form the basis of a
medical device. The contrast agent in accordance with the present invention is substantially biocompatible and can be added to polymer or polymer mixtures, and / or inorganic / organic composite materials to enhance its X-ray
image quality.
[0021] In yet another exemplary embodiment of the present invention, selective incorporation of the contrast agent to a polymeric structure can be accomplished in a number of ways. By ensuring placement of the contrast agent in certain areas of the polymer structure and not in other areas, additional secondary improvements in radiopacity can be realized without affecting material and / or mechanical properties. One such example is incorporation of the contrast agent at the proximal and distal ends of the polymer chain. By utilizing methods such as orientrusion, which may provide for a high degree of molecular orientation of the polymer chains within the polymer, one can create a polymeric material with high intensity radiopacity at the select portions of the bulk material which would be significantly more radiopaque than the surrounding areas where the contrast agent was not present. Like wise the selective placement of the contrast agents in the
coating material can provide one with secondary benefits similar to those obtained with selective placement of the contrast agents in the bulk material.
[0024] The use of compounds in conjunction with the present invention can provide distinct clinical advantages over existing therapies and / or devices. More specifically, compounds that are capable of causing
lysis or degradation of the embolic debris can be incorporated into the filtering portion of the present invention. A factor to consider in the selection of such a compound is the origin of the debris be it
thrombus, plaque,
atheroma, or any other form representing an
embolus. As the mesh and or pore size of the filtering aspect of the present invention decreases, more embolic material may become trapped in the filtering mechanism of the present invention, thereby increasing the load on the filtering portion. While small emboli (typically smaller than 100 microns) are not a major concern because of the body's natural ability to enzymatically degrade, digest or lyse the emboli, the embolic load on the filter itself can be overloaded and result in formation of a
thrombus if the
blood flow is significantly slowed to the point which allows for a
thrombus formation. In this situation the incorporation or application of compounds, which can degrade trapped emboli, can be beneficial. Some exemplary suitable compounds may include:
Tissue Plasminogen activator (TPA);
Streptokinase(SK); Reteplase; Tenecteplase;
Urokinase; Lanoteplase;
Staphylokinase; and / or Nadroparin(anti-factor Xa). In addition, the filtering portion of the present invention may incorporate an
antithrombotic and / or antithrombogenic agent to prevent the formation of a thrombus. Some exemplary compounds may include:
Heparin; Fragmin (dalteparin, low MW
Heparin); a
monoclonal antibody such as ReoPro™ (abciximab, antiplatelet antibodies) Acenocoumarol; Anisindione; Dicumarol;
Warfarin; Enoxaparin (Lovenox);
Anagrelide (Agrylin); Indomethacin (Indocin);
Dipyridamole;
Clopidogrel; Aggrenox; and / or Coumadin. Furthermore, an affinity-binding compound may also be incorporated with the filtering aspect of the present invention by itself or in combination with other compounds. Affinity-binding compounds can promote the binding and / or adhesion of embolic material thus facilitating
entrapment of embolic material and subsequent removal from the
blood stream. Whether incorporated into the strut or membrane by methods such as chemical surface treatments, bombardment, placement into reservoirs, or in the case of polymeric struts and membranes, blended with the material itself, or by application of a
coating to the struts and / or membranes with a compound, any identified compound or combination of identified compounds may be used. Furthermore any number of compounds may suggest themselves to one who is skilled in the art and may be utilized in connection with the present invention alone or in combination with other compounds.
[0025] The foregoing exemplary embodiments of the present invention provide a high intensity radiopaque contrast agent which may be used independently, for example as a coating or may be incorporated within a polymeric material to be subsequently fabricated into medical devices in accordance with the present invention. Moreover, the incorporation of drugs and / or agents may be combined with the high intensity contrast agent to realize additional synergistic benefits. As noted above, the incorporation of biological and / or pharmaceutically active agents with the present invention can be utilized for the additional purposes of preventing thrombus formation, promotion of binding, and degradation of thrombus, all of which provide a patient benefit.
 Login to View More
Login to View More