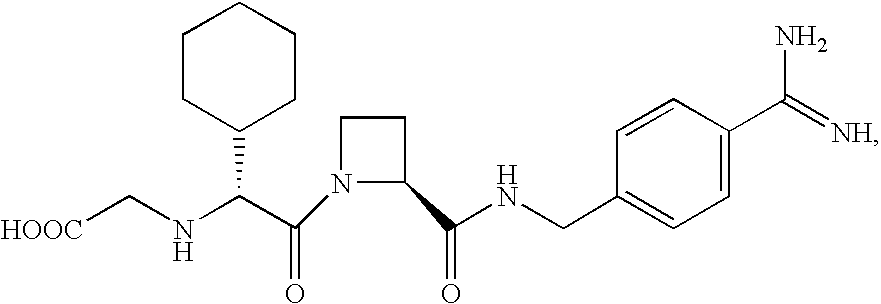
Patents
Literature
155 results about "Dipyridamole" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
This medication is used in combination with "blood thinners" such as warfarin to keep clots from forming after heart valve replacements.
Medical implants with a combination of compounds
InactiveUS20100074934A1Minimize formationImprove biological effectOrganic active ingredientsBiocideDipyridamoleFibrosis
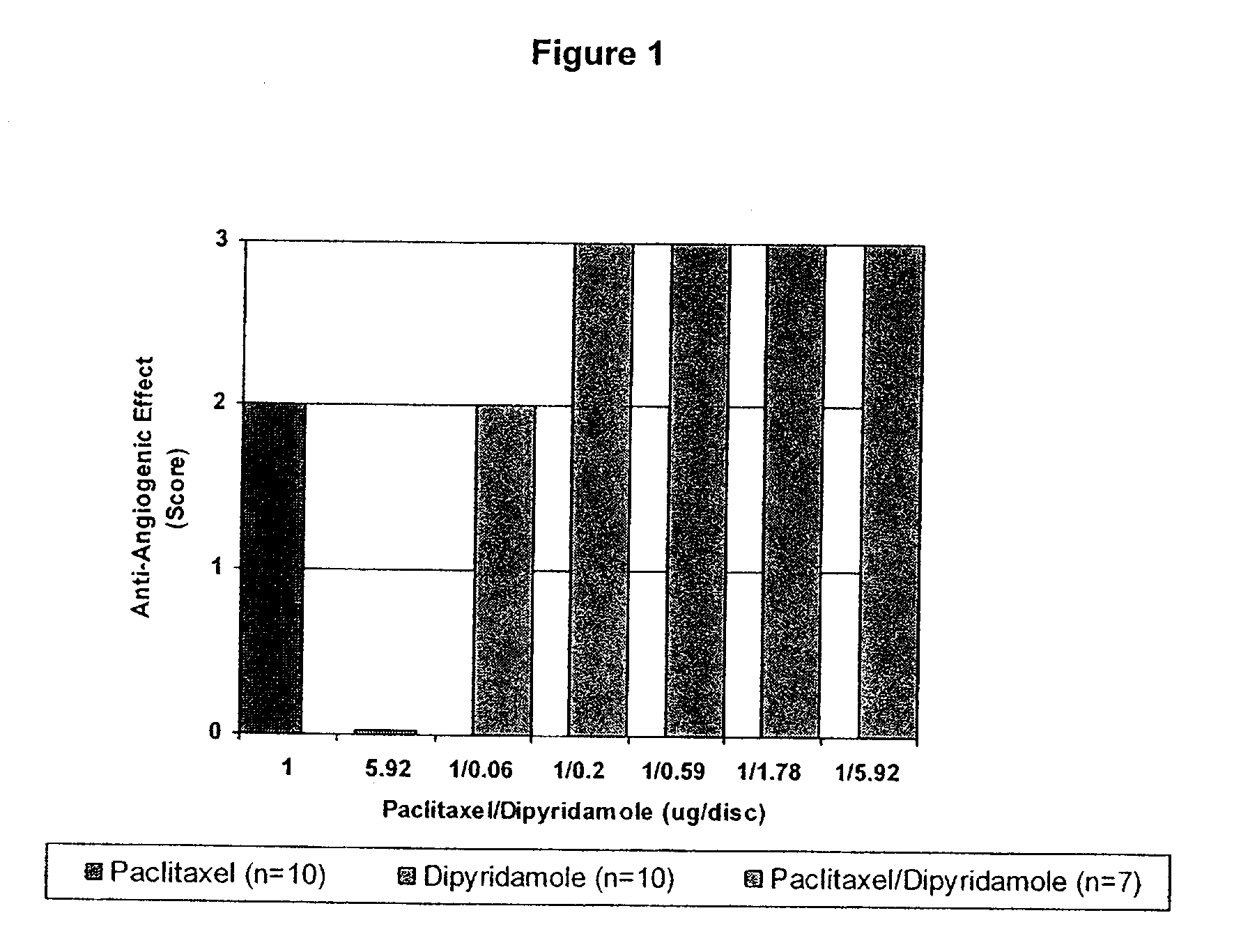
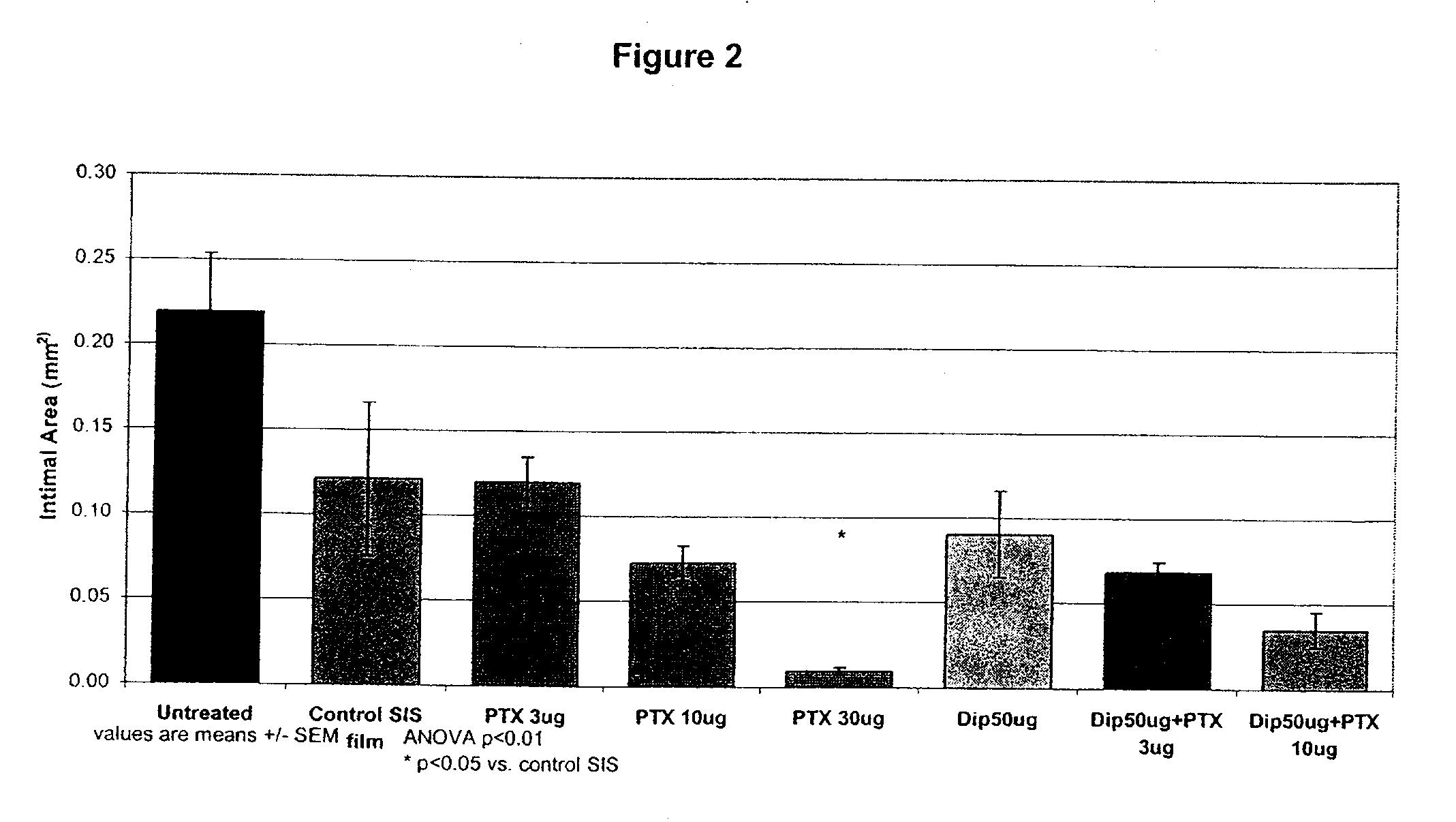
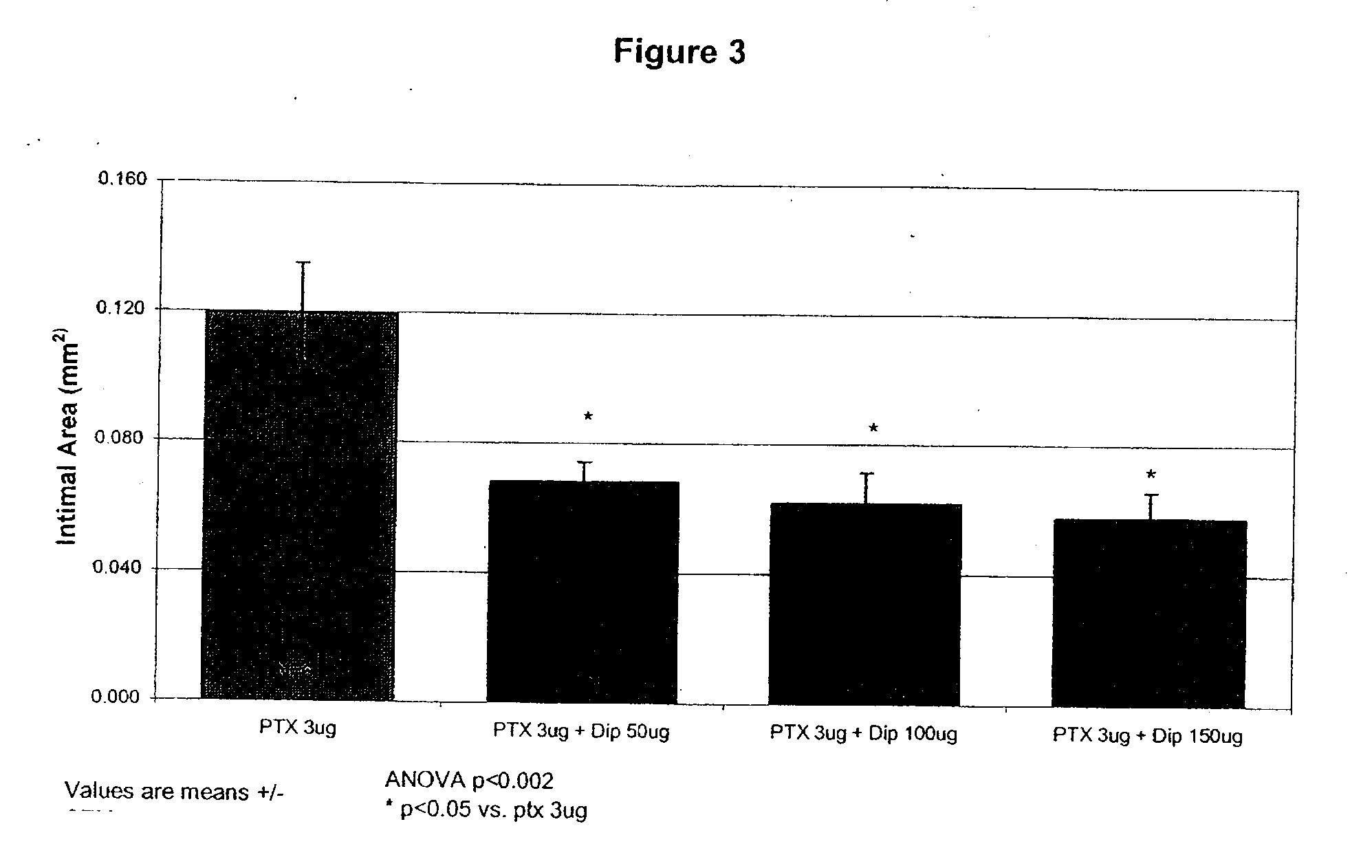
Implants are associated with a combination of paclitaxel or derivatives and dipyridamole or derivatives in order to inhibit fibrosis that may otherwise occur when the implant is placed within an animal. Exemplary implants include intravascular implants (e.g., coronary and peripheral vascular stents, catheters, balloons), non-vascular stents, pumps and sensors, vascular grafts, perivascular devices, implants for hemodialysis access, vena cava filters, implants for providing an anastomotic connection, electrical devices, intraocular implants, and soft tissue implants and fillers.
Owner:ANGIOTECH PHARMA INC
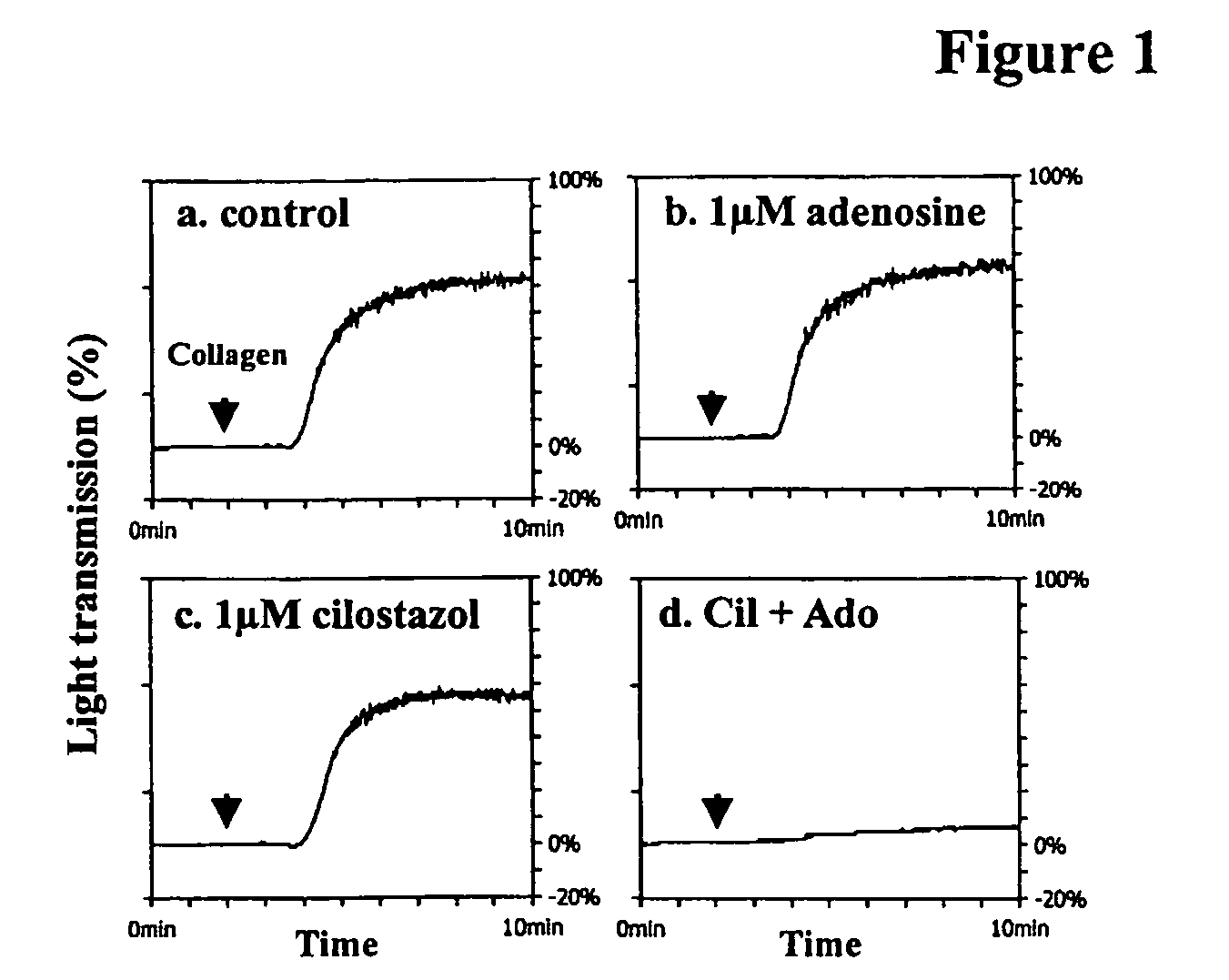
Pharmaceutical compositions comprising a multifunctional phosphodiesterase inhibitor and an adenosine uptake inhibitor
InactiveUS20050165030A1Increase antiplatelet effectIncrease vasodilationBiocideMuscular disorderDipyridamoleAdenosine
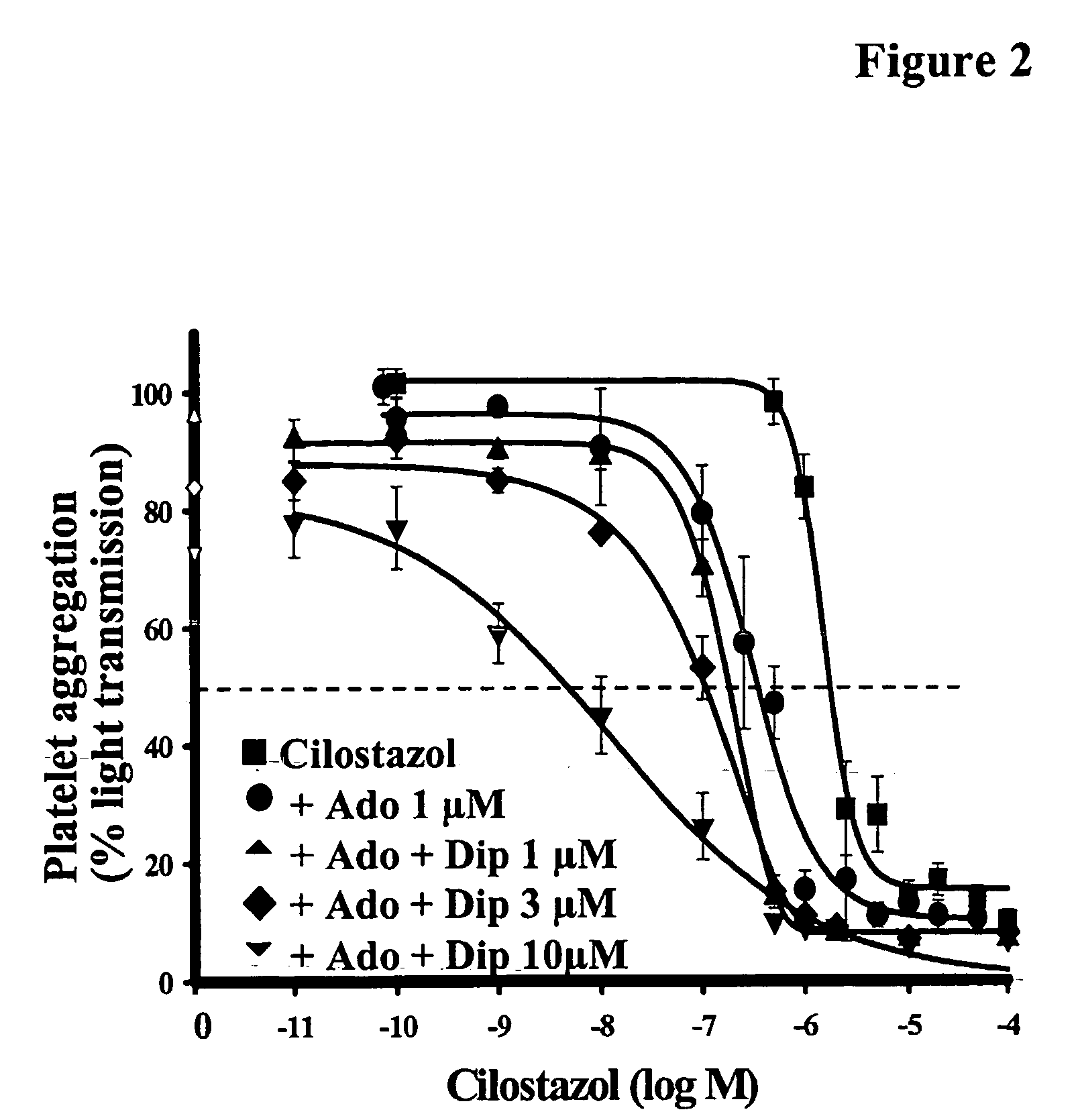
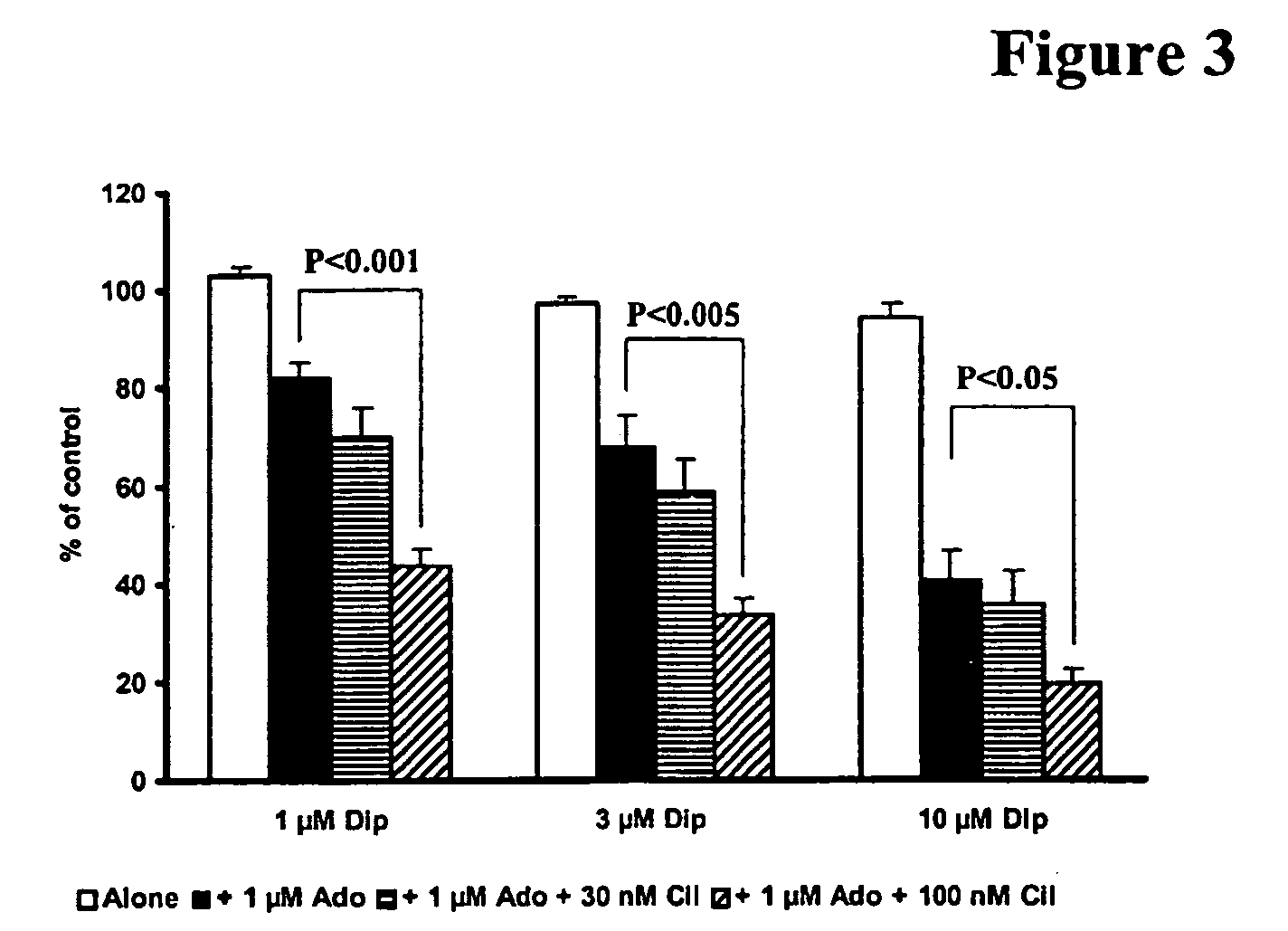
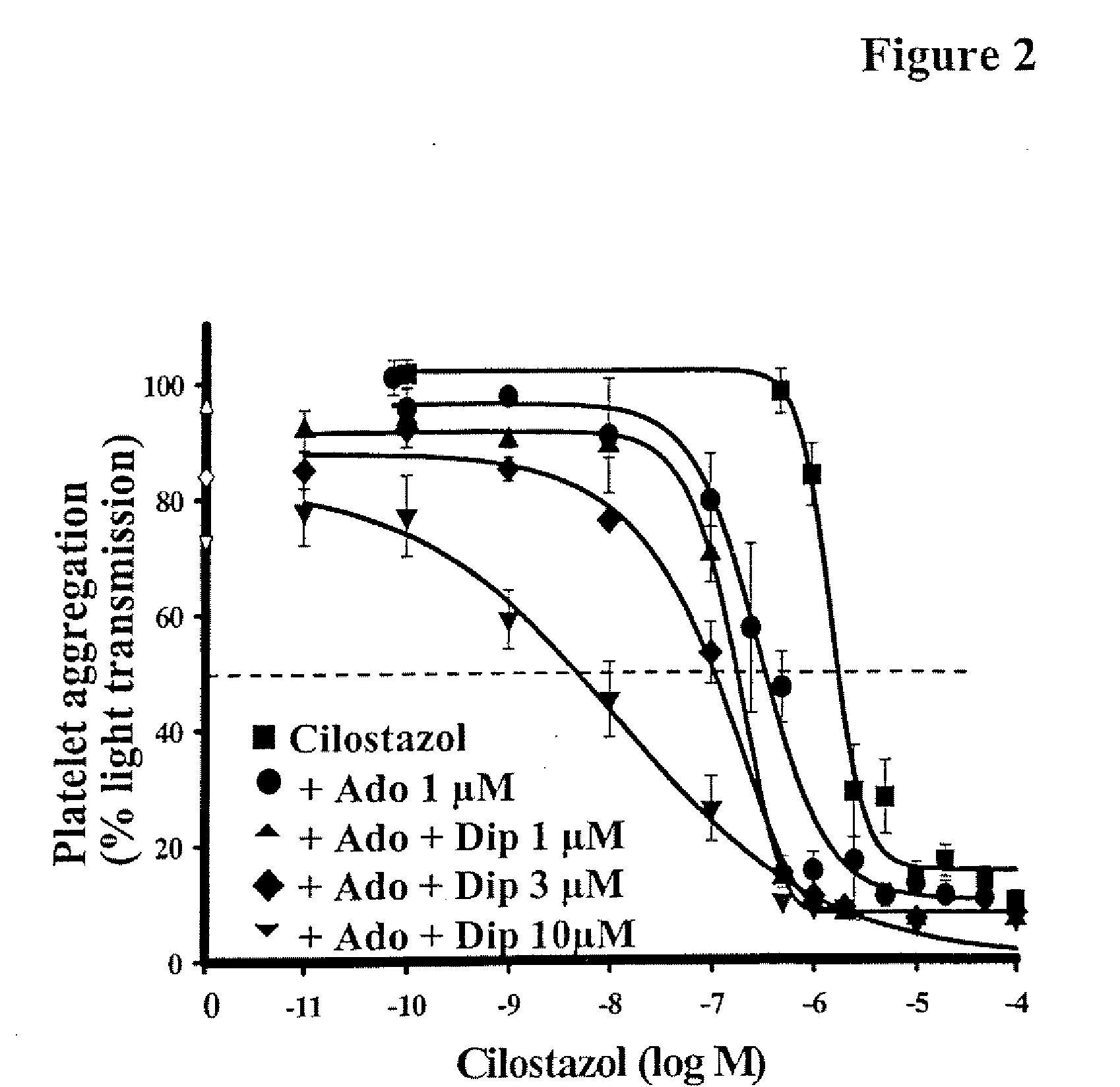
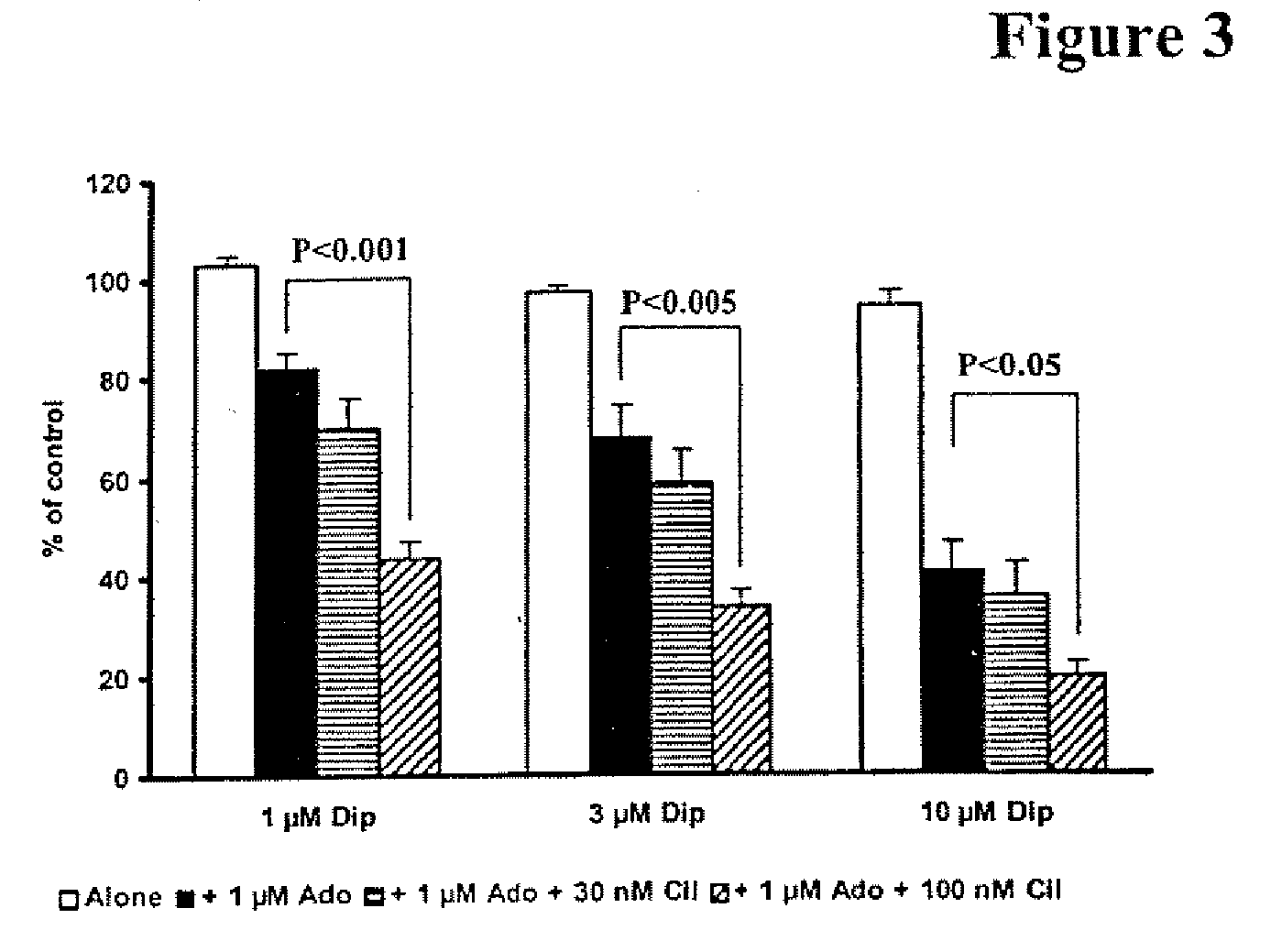
The present invention relates to pharmaceutical compositions comprising at least one multifunctional phosphodiesterase inhibitor (MPDEI) and at least one adenosine uptake inhibitor. The present invention also relates to compositions comprising cilostazol and dipyridamole and their use.
Owner:OTSUKA PHARM CO LTD
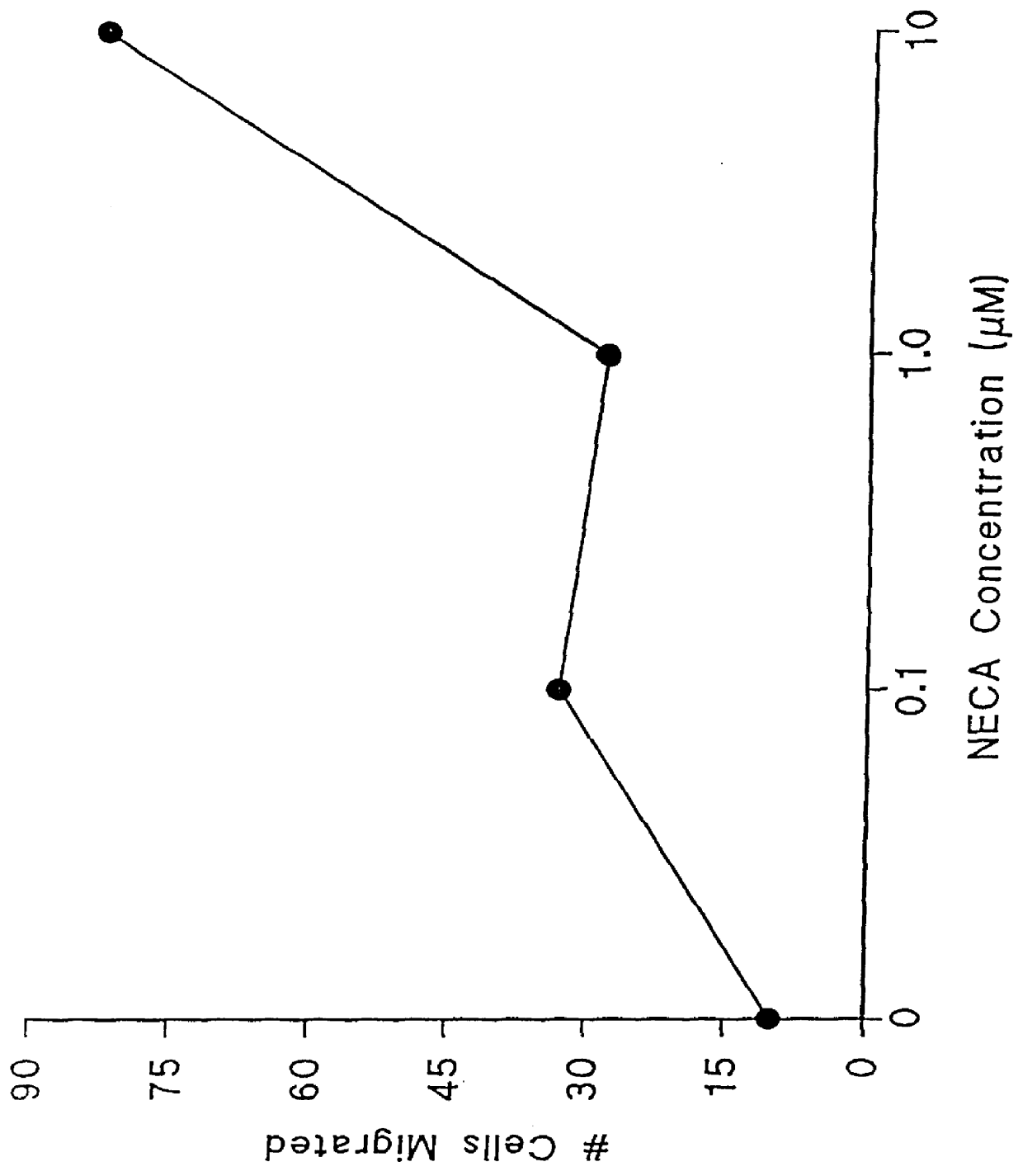
Adenosine receptor agonists for the promotion of wound healing
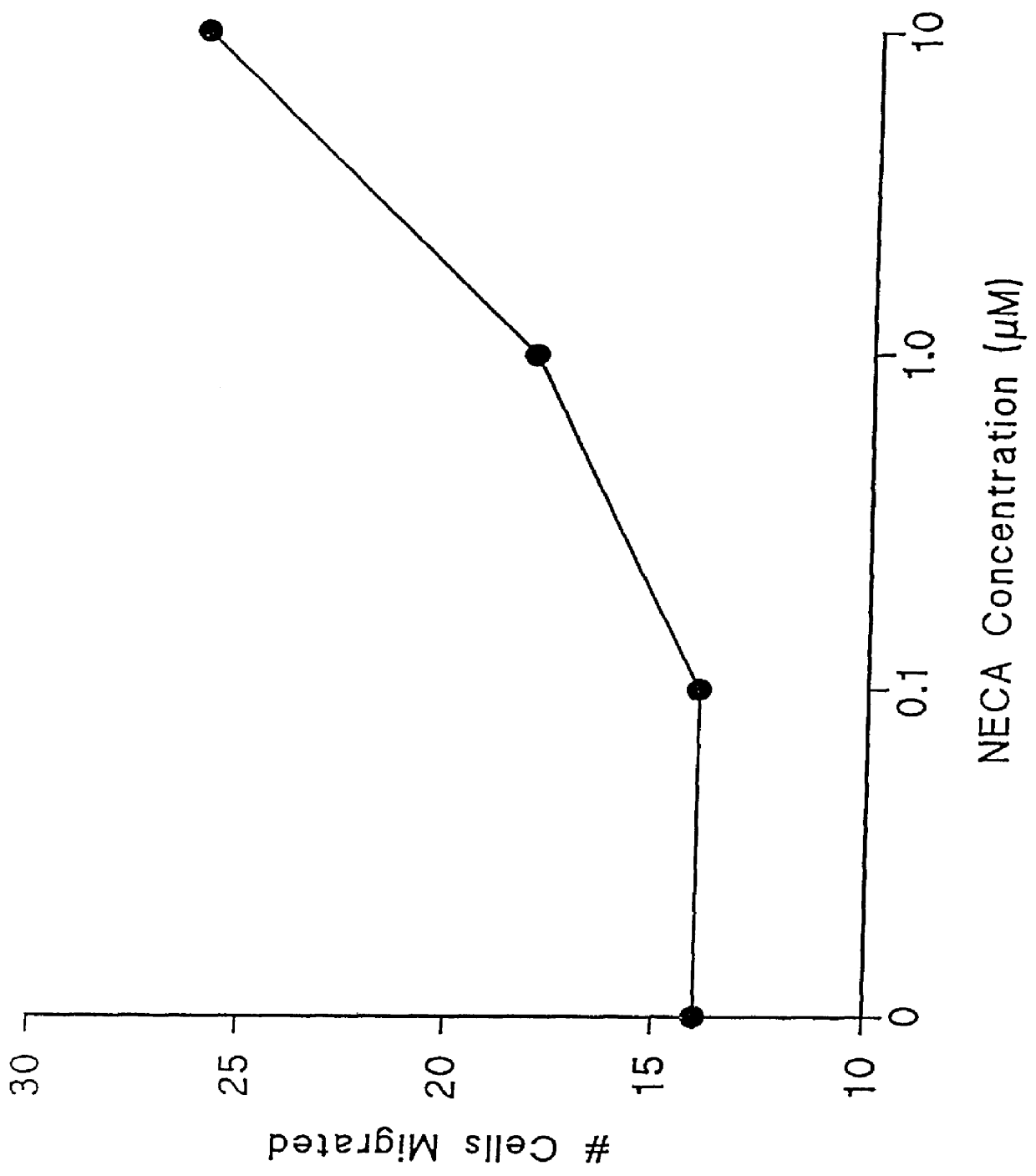
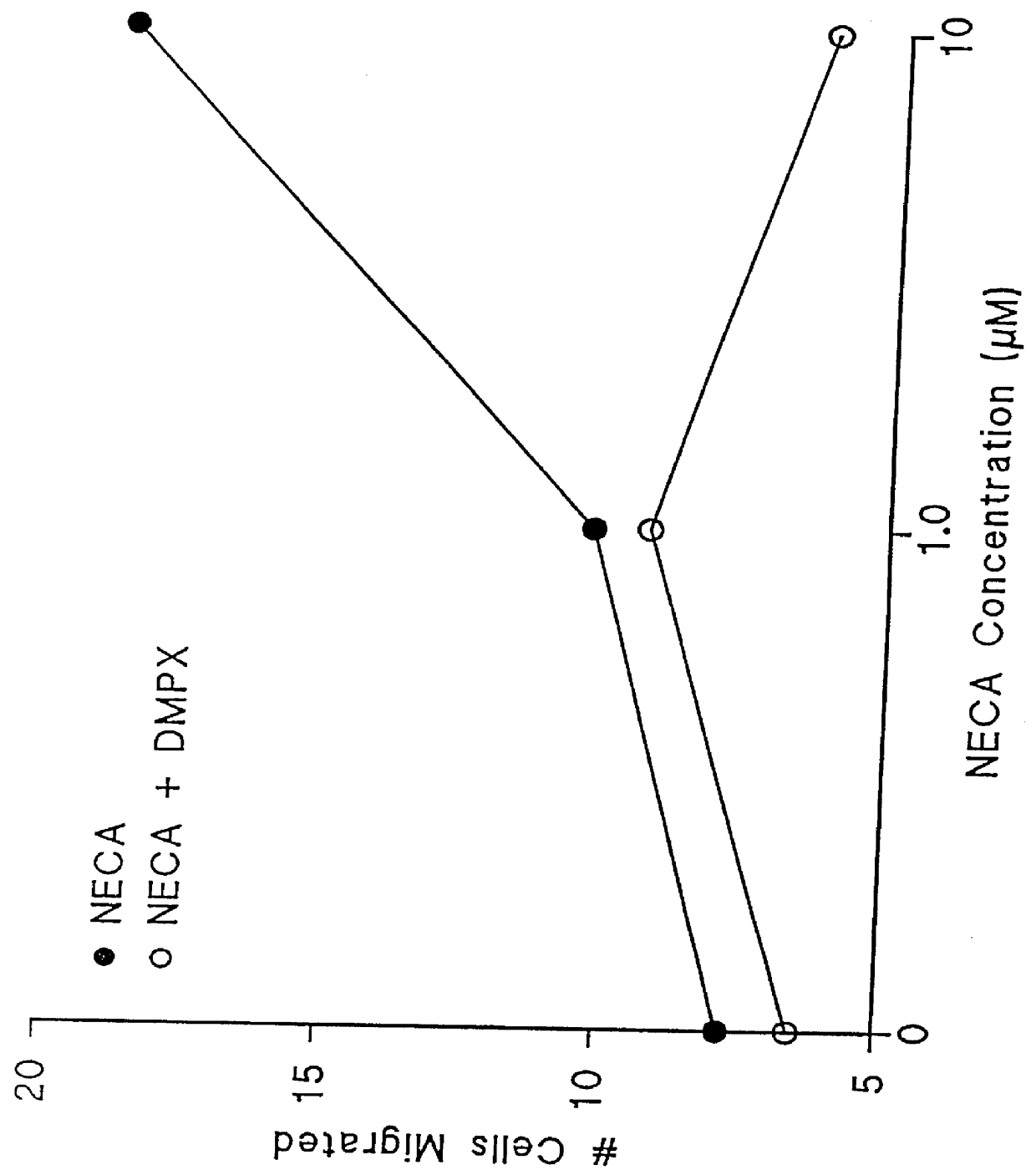
Agonists of the adenosine A2 receptor promote the migration of endothelial cells, fibroblasts and epithelial cells. Thus, methods and pharmaceutical compositions useful for treating wounds and promoting wound healing comprise agents which cause stimulation of the adenosine A2 receptor, preferably receptor agonists and adenosine uptake blockers. Preferred agonists include 2-phenylaminoadenosine, 2-para-2-carboxyethylphenyl-amino-5'N-ethylcarboxamidoadenosine, 5'N-ethylcarbox-amidoadenosine, 5'N-cyclo-propyladenosine, 5'N-methylcarboxamidoadenosine and PD-125944. Preferred uptake blockers include dipyridamole, nitrobenzylthio-inosine, dilazep and R75231.
Owner:NEW YORK UNIV
Use Of Dipyridamole For Treatment Of Resistance To Platelet Inhibitors
InactiveUS20090048173A1Reduce decreaseBiocidePeptide/protein ingredientsDipyridamolePlatelet inhibitor
Owner:EISERT WOLFGANG +1
Dipyridamole self-emulsifying medicament administration system and preparation method thereof
InactiveCN101780037AHigh dissolution ratePromote absorptionOrganic active ingredientsSenses disorderDipyridamolePeristalsis
The invention discloses a dipyridamole self-emulsifying medicament administration system, which is characterized by comprising the following components in percentage by mass: dipyridamole 0.5-10, an oil phase 20-80, a surfactant 10-70 and an auxiliary surfactant 0-50. The system has the advantages that: self-emulsifying capsules prepared by using the dipyridamole, the oil phase, the surfactant and the auxiliary surfactant change into emulsion by slightly stirring after disintegrating in water or spontaneously change into emulsion in vivo under the action of the peristalsis of the gastrointestinal tracts after being delivered by oral taking, wherein the particle size of the emulsion is 10 to 500 nanometers; the dissolution of the dipyridamole is improved greatly; after the system is taken orally, the absorption of the medicament is improved greatly, the bioavailability is improved and the individual difference is reduced; and the preparation process is simple and is suitable for large-scale production of medical enterprises.
Owner:SHENZHEN HEPALINK PHARMA GRP CO LTD
Medicament microsphere and preparation method thereof
InactiveCN103610649AReduce solubilityHigh encapsulation efficiencyOrganic active ingredientsAntipyreticDipyridamoleMicrosphere
The invention belongs to the technical field of pharmaceutical preparations, and particularly relates to a medicament microsphere and a preparation method thereof. The medicament microsphere comprises medicinal materials, a high polymer material and a surfactant, wherein the ratio of the medicament to the high polymer material is 1:(1-3); the medicament preferably is celecoxib, ketoprofen, dipyridamole and nimodipine. The medicament microsphere is prepared by adopting an O / W emulsified solvent diffusion-volatilization method. The O / W emulsified solvent diffusion-volatilization method comprises the following steps: respectively weighing the medicinal materials, the high polymer material and a release regulator according to the prescription amount, and then adding the weighted materials to an organic solvent; ultrasonically or mechanically stirring and dissolving as a disperse phase; taking a surfactant solution as a continuous phase; warming and stirring after low-temperature emulsification under an agitation state, so as to remove an organic solvent; and then carrying out solid separation, washing by distilled water, and drying, so as to obtain the medicament microsphere. Thus, the prepared microsphere is high in encapsulation efficiency and high in yield.
Owner:SHENYANG PHARMA UNIVERSITY
Use of dipyridamole in combination with antithrombotics for treatment and prevention of thromboembolic diseases
InactiveUS20090075949A1Good coagulationEnhancing thrombin formationBiocideSalicyclic acid active ingredientsAntithrombotic AgentDipyridamole
The invention relates to a method of treating and preventing thromboembolic disorders, comprising administering dipyridamole in combination with an antithrombotic selected from direct thrombin inhibitors, factor Xa inhibitors and combined thrombin / factor Xa inhibitors to a patient, pharmaceutical compositions suitable for this method of treatment as well as the use of dipyridamole for the manufacture of these pharmaceutical compositions.
Owner:EISERT WOLFGANG
Reagent for stabilization of blood sample cells
ActiveCN104381245AAvoid gatheringDoes not affect molecular analysisPreparing sample for investigationDead animal preservationDipyridamoleAdenosine
The invention discloses a reagent for stabilization of blood sample cells. The reagent comprises 100 micromoles per liter of L beta-cyclodextrin, 100 micromoles per liter of disodium cromoglycate, 0.05% of potassium dichromate, 50 micromoles per liter of triflusal, 100 micromoles per liter of Terutroban, 11 millimoles per liter of sodium citrate, 1.5 millimoles per liter of theophylline, 20 micromoles per liter of dipyridamole, 0.37 millimoles per liter of adenosine, 20 millimoles per liter of glucose and 1.25 millimoles per liter of LPEG. The reagent can stabilize blood cells for at least 36h and prevent blood cell aggregation. Through the reagent, a blood sample subjected to treatment does not influence follow-up sample molecule analysis. The reagent can be widely used for blood sample molecule analysis and detection and is especially suitable for cell or molecular detection such as a microfluidic technology with strict sample cell requirements and an analysis technology platforms such as a flow cytometer with strict sample cell requirements.
Owner:广东国盛医学科技有限公司 +1
Method of measuring platelet activation
InactiveUS7011938B2Reduce platelet activationInhibition of activationBiocideSamplingDipyridamoleAdenosine
Platelet activation is measured by determining Mean Platelet Component (MPC) of suspended blood platelets, using a specific anticoagulant composition. The composition comprises at least one component for effecting platelet sphering (for example EDTA), and at least one platelet antagonist (for example at least one of, and preferably all three of theophylline, adenosine and dipyridamole).
Owner:BARTS & THE LONDON NHS TRUST
Compound medicine of ginkgo leaf extract and dipyridamole and preparing method thereof
InactiveCN1454596AReduce lossesAdvanced preparation technologyOrganic active ingredientsBlood disorderFiberDipyridamole
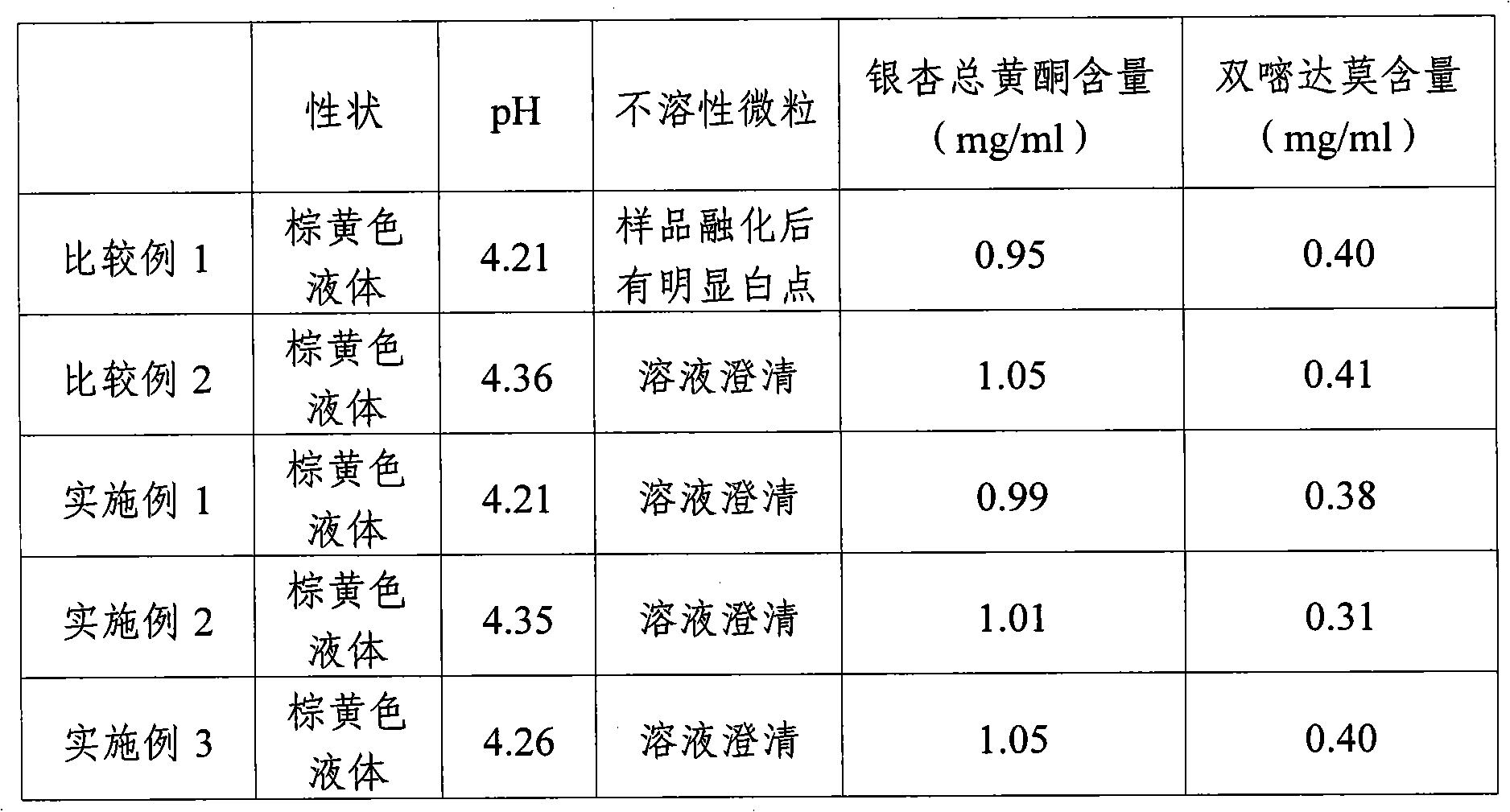
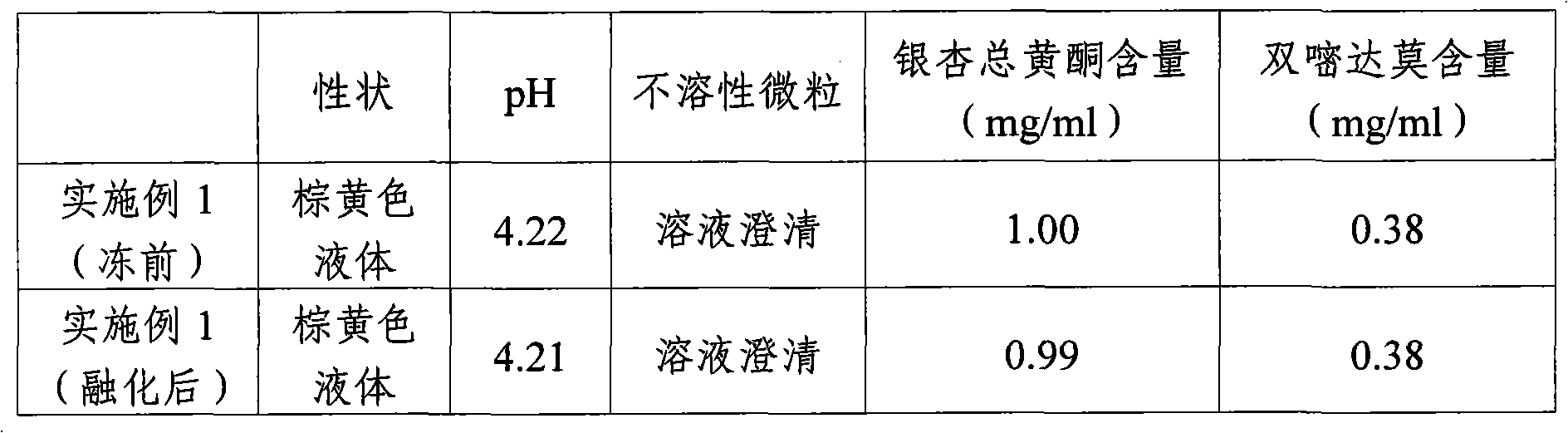
The invention is a manufacturing method for vein medicament of gingkgo leaves extraction materials and di-pyridine compound drug. It is based on that the stability of gingkgo flavone in low thickness aqueous solution is higher than in high thickness aqueous solution, the gingkgo leaves extraction materials and di-pyridine is dissolved at the same time, after the two are frozen and dried into even solid, the stability of gingko flavone is higher than the solid which is only frozen and dried from itself, and produces the infusion agent and powder pin agent. The manufacturing process doesn't use active carbon, but uses middle hole fiber film filtering new technology to wipe off impurities, enhances thep quality of the product. The method is simple, convenient, and good curative effect.
Owner:张哲峰
Compound preparation for treating and preventing thrombosis and apoplexia as well as its preparing method
InactiveCN1480144AReduce manufacturing costSmall fluctuations in performanceSalicyclic acid active ingredientsBlood disorderBenzoic acidDipyridamole
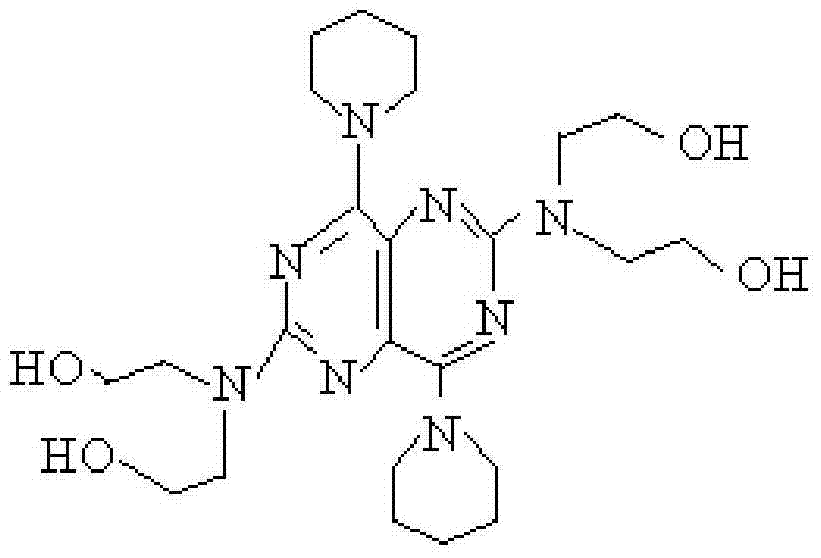
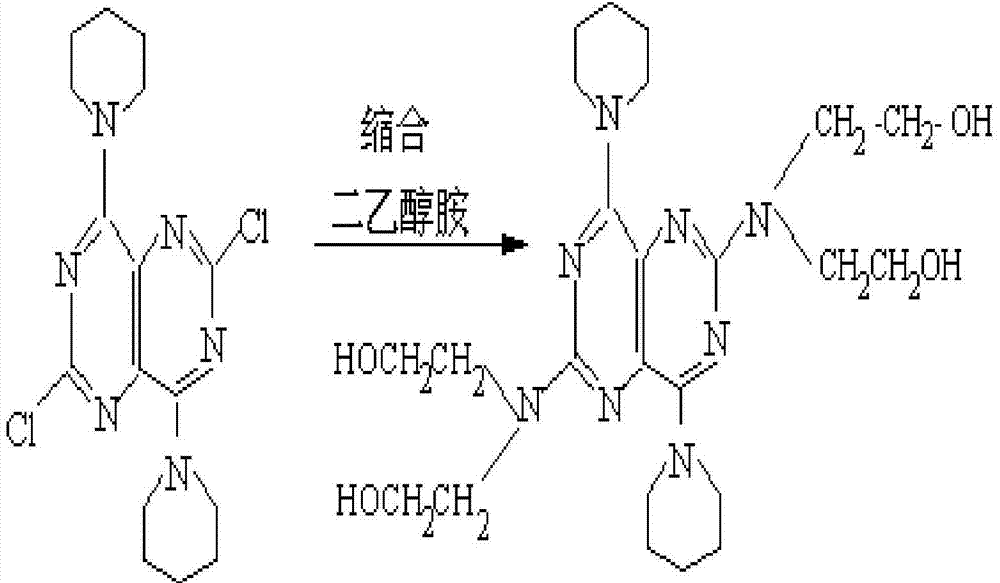
A slowly-releasing compound medicine in the form of tablet for treating and preventing thrombosis and apoplexy is prepared from aspirin, 2-(acetoxy) benzoic acid, slow-releasing dipyridamole, 2,2',2'',2''', [(4,8-dipiperidylpyrimido[5,4-d] pyrimidine-2,6-diyl) dihyponitido]-tetraethanol through granulating, tabletting and coating. Its advantages are high curative effect and equickly taking its effect.
Owner:CHENGDU LIST PHARMA
Pharmaceutical composition for treating cardiovascular and cerebrovascular diseases, preparation process and quality control method thereof
The invention provides a pharmaceutical composition for treating cardiovascular and cerebrovascular diseases, its preparing process and quality control method, wherein Dipyridamole and total glycosides of codonopsis pilosula are employed in combination to obtain various dose forms of injections and oral administration preparations. The composite preparation is mainly used for treating coronary disease, pulmonary heart disease, cerebral ischemia, nervous prostration, climacteric metancholia, The preparation of the invention has the advantages of high purity, ensured constituents, controllable quality, improved curative effect, wider range of safely, and less fluctuation of treatment effects.
Owner:BEIJING QI YUAN YI DE PHARMA RESEARCH CENTER
Preparation method of ginkgo dipyridolum injection with high content of ginkgo terpene lactones
ActiveCN102670670AHigh extraction rateAvoid damageOrganic active ingredientsMetabolism disorderDipyridamoleFlavones
The invention provides a preparation method of ginkgo dipyridolum injection with high content of ginkgo terpene lactones. The preparation method comprises the following steps: 1), collecting, processing, detecting and classifying ginkgo leaves; 2), extracting and preparing ginkgo terpene lactones extract from ginkgo leaves; 3), extracting ginkgo leaf flavonoid extract; 4), reflowing ginkgo leaf flavonoid extract and partial dipyridamole by adding water, and then filtering so as to be prepared into mixed liquor; 5), mixing the mixed liquor, ginkgo terpene lactones extract, the rest dipyridamole and auxiliary material to be prepared into ginkgo dipyridolum injection as per conventional preparation method; and in the ginkgo dipyridolum injection, the content of the total ginkgo leaf flavonoid and that of the dipyridamole conform to the conventional requirements, and the content of the ginkgo terpene lactones ranges from 0.5 to 1.2 mg / mL.
Owner:SHANXI PUDE PHARMA CO LTD
Dipyridamole soft capsule preparation and its preparing method
InactiveCN1586484AOral convenienceInhibition effectOrganic active ingredientsAntiviralsDipyridamoleMedicine
The present invention discloses one kind of soft diphyidamole capsule and its preparation process. The medicine consists of medicine liquid and capsule shell, the medicine liquid contains diphyidamole, matrix and stabilizer, and the capsule shell contains gelatin, glycerin, water, preservative and light screening agent. Compared with available diphyidamole preparations, the present invention is even suitable for children and other people incapable of taking care of himself to take and has the advantages of high bioavailability, high stability, accurate content, etc.
Owner:北京瑞伊人科技发展有限公司 +1
Pharmaceutical Compositions Comprising A Multifunctional Phosphodiesterase Inhibitor and An Adenosine Uptake Inhibitor
InactiveUS20080200484A1Limiting positive inotropic effectGood effectBiocideMuscular disorderAdenosine Deaminase InhibitorDipyridamole
Owner:OTSUKA PHARM CO LTD
Method for preparing ginkgodipyidamolum injection
ActiveCN101947247AImprove quality stabilityEfficient removalOrganic active ingredientsPharmaceutical delivery mechanismDipyridamoleGinkgo biloba
The invention provides a method for preparing ginkgodipyidamolum injection. The method comprises the following steps of: 1) extracting a ginkgo leaf extract from ginkgo leaves; 2) extracting the ginkgo leaf extract once again with over 90 percent ethanol so as to refine the ginkgo leaf extract; 3) adding water into the refined ginkgo leaf extract and dipyridamole for inverse flow and filtering so as to obtain ginkgo leaf extracting solution; and 4) mixing the ginkgo leaf extracting solution, the dipyridamole and auxiliary materials so as to obtain the ginkgodipyidamolum injection, wherein in the ginkgodipyidamolum injection, the weight ratio of the ginkgo leaf extracting solution to the dipyridamole based on total ginkgo flavone-glycoides is 0.9-1.1:0.3-0.5. The method of the invention has the advantages of effectively removing various impurities, enhancing medicament effectiveness, improving the quality stability of the ginkgodipyidamolum injection and reducing safety risk.
Owner:SHANXI PUDE PHARMA CO LTD
Pharmaceutical Capsules Comprising Extended Release Dipyridamole Pellets
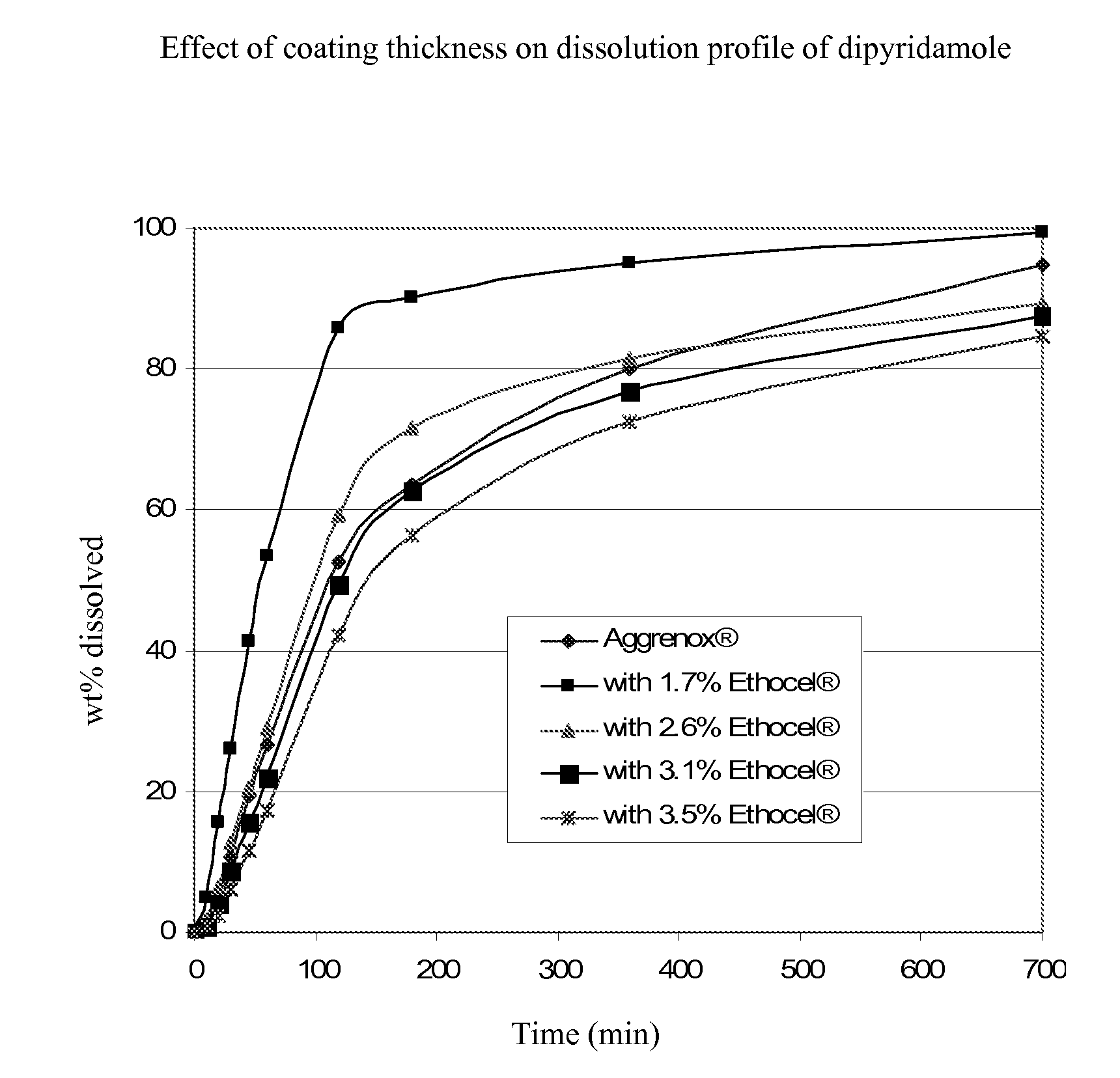
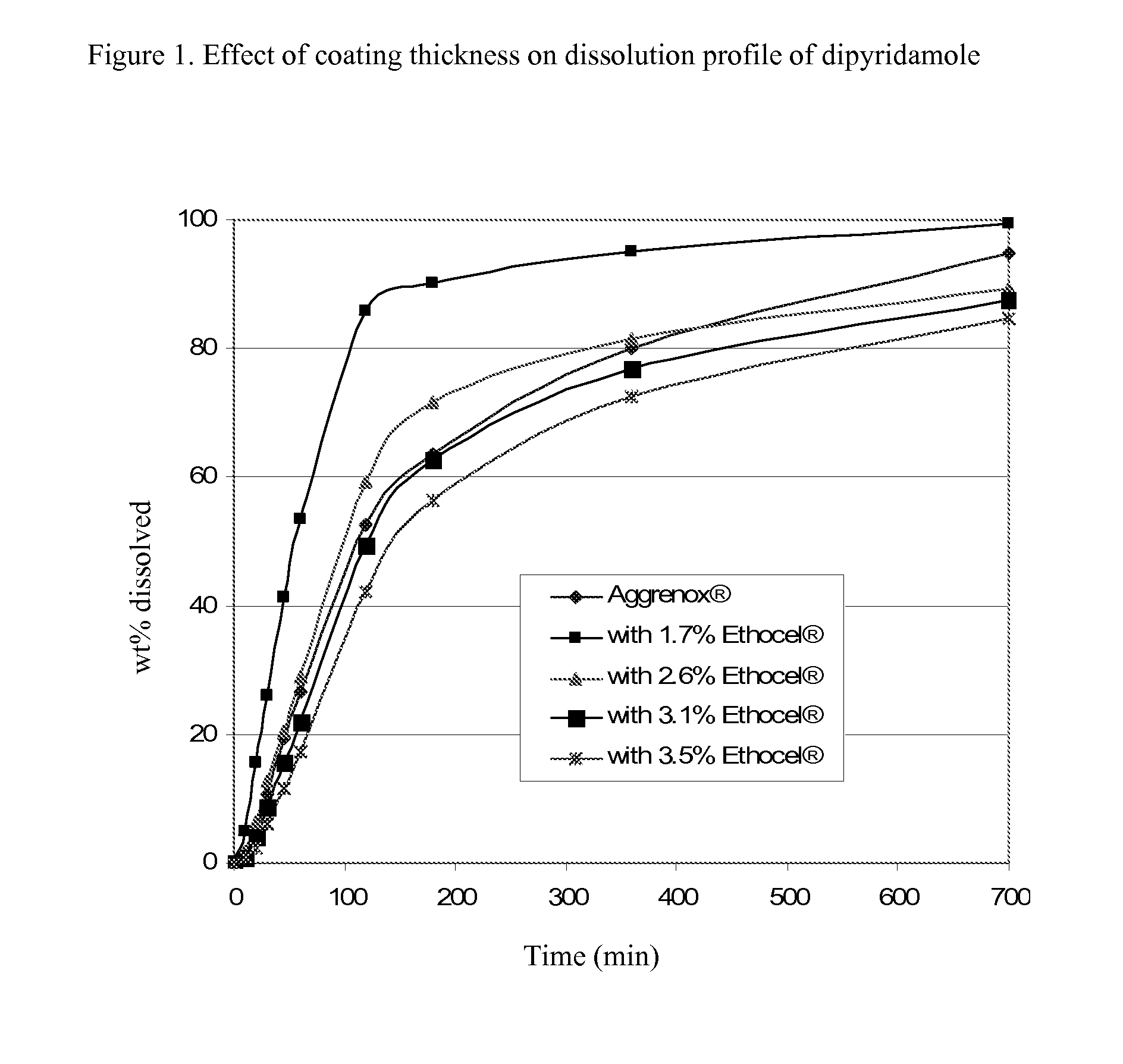
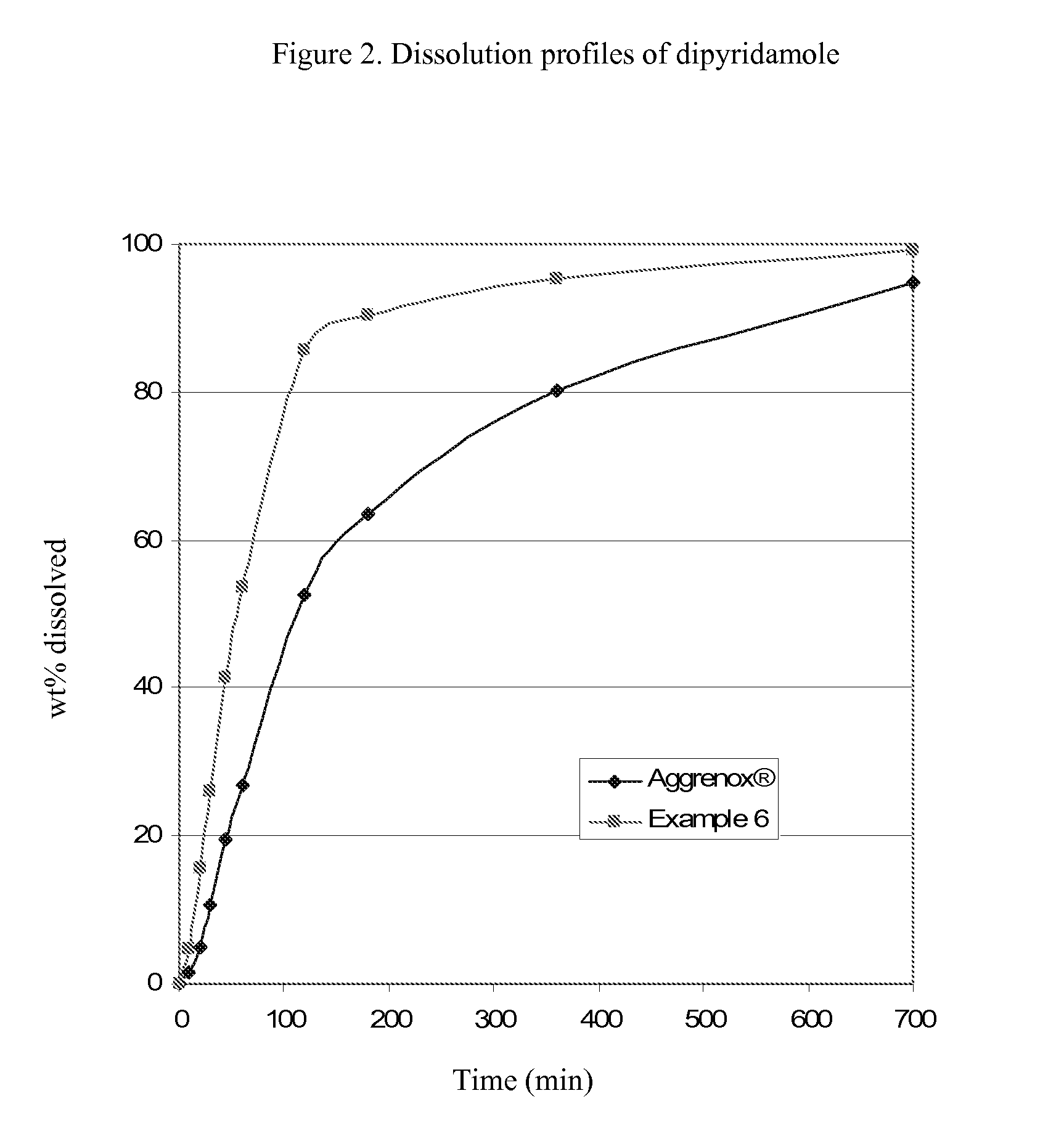
InactiveUS20090196935A1Improve bioavailabilityPowder deliveryOrganic active ingredientsDipyridamoleRisk stroke
The present invention is directed to pharmaceutical capsules comprising extended release formulations of dipyridamole, processes for preparing such dipyridamole extended release formulations and their use in the treatment of stroke.
Owner:BARR LAB
Composition preparation of ginkgo biloba extract and dipyridamole and preparation method thereof
ActiveCN101683363AReduce adverse reactionsSimple production processOrganic active ingredientsPowder deliveryDipyridamoleOrganic film
The invention discloses a composition preparation of ginkgo biloba extract and dipyridamole and preparation method thereof, which is a traditional Chinese medicine injection preparation prepared by combining 20 to 100 parts of ginkgo biloba extract and 2 to 10 parts of dipyridamole. Wherein the ginkgo biloba extract is obtained by proceeding respective alcohol extraction and water extraction to ginkgo biloba medicinal material, the extracting solution is then concentrated, micro-filtration by inorganic ceramic film, refining by polyamide resin and then being made by organic film ultrafiltration. Comparing with the prior art, the invention adopts a method of membrane separation technology combined with resin refining treatment to proceed purifying, separating and condensation to the extract, active ingredient is enriched, physical method is used to replace conventional chemical method can maintain characteristics of traditional Chinese medicine compound preparation better, which reducesadverse effects of drugs and improves curative effect, and thereby quality of the product is improved and assured.
Owner:GUIZHOU YIBAI PHARMA CO LTD
Purification technology of dipyridamole
The invention belongs to the field of drug preparation and particularly relates to a purification technology of a synthetic drug. The method comprises the steps of adding a methanol solvent and a dipyridamole crude product into methyl benzenesulfonic acid and 1-(3-aminobenzene)-3-methyl-2-imidazolone, stirring for reaction to form a sulfonate intermediate product, mixing the sulfonate intermediate product and a 70% methanol solution for dissolving, adding alkaline liquor to regulate pH (potential of hydrogen) of a system to 8-8.5, then adding active carbon, carrying out heating, decoloring, stirring and press filtration, cooling filtrate, and carrying out centrifuge, washing and drying to form purified high-purity dipyridamole.
Owner:常州康普药业有限公司
Method for preparing aspirin and dipyridamole multilayer tablets
The invention provides a method for preparing aspirin and dipyridamole multilayer tablets, which is characterized by comprising: preparing dipyridamole sustained-release tablet cores by tabletting; and coating a stomach soluble insulation layer, an aspirin quick-release layer and a stomach soluble protective layer in turn. The method has the advantage that aspirin common release and dipyridamole sustained-release compound preparations are prepared by using conventional pharmaceutical equipment.
Owner:SHANDONG XINHUA PHARMA CO LTD
Preparation of Ginkgo Damo injection
ActiveCN1939357AReduce permeabilityImprove deformation abilityOrganic active ingredientsSenses disorderDipyridamoleIschemic retinopathy
A gingko-dipyridamole injection for treating cerebral thrombus, cerebrovascular spasm, cerebral insufficiency, ischemic heart disease, ischemic retinosis, etc is prepared from gingko leaves through breaking, reflux extracting in alcohol, concentrating, filtering, resin adsorption, alcohol eluting to obtain liquid extract, mixing with dipyridamole in dark condition, filling N2, pouring in containers, sterilizing, lamp examining, and packing.
Owner:通化谷红制药有限公司
Dipyridamole and acetylsalicylic acid formulations and process for preparing same
The present invention provides pharmaceutical formulations of dipyridamole and acetylsalicylic acid, methods of making thereof, and methods of using thereof.
Owner:TEVA PHARM USA INC
Dipyridamole orally disintegrating tablet and preparation
InactiveCN101234094AQuick effectPromote dissolution and absorptionOrganic active ingredientsAntiviralsDipyridamoleMedicine
The invention relates to dipyradimole orally disintegrating tablet and a preparation method thereof, which pertains to a pharmaceutic preparation field. Components and mass percentages thereof of the orally disintegrating tablet are: 5-50 percent of dipyradimole, 20-70 percent of stuffing agent, 10-40 percent of disintegrating agent, 1-5 percent of effervescent, 0-3 percent of correctant, 0.5-1.8 percent of lubricant and 8-20 percent of adhesive. The method of the invention comprises the following steps: 1) the stuffing agent, the disintegrating agent, the correctant and the effervescent are respectively ground for 30-60min and then fully blended into a mixture; 2) the dipyradimole is ground and added in the mixture cumulatively; after fully blended, the mixture passes through a 100 mesh sieve for 2-3 times; 3) the mixture is made into soft material by the adhesive; then the soft material is pelletized through a 16 mesh sieve and forced-air dried for 4-6 hours at the temperature ranging from 40-50 DEG C; the pelletized granules are sifted by a 20 mesh sieve; the lubricant is added in the pelletized granules and fully blended to prepare the dipyradimole orally disintegrating tablet after stamping and slice production. The orally disintegrating tablet of the invention has the advantages of short disintegrating time, rapid effect and convenient using, etc.
Owner:BIOCHEM ENG COLLEGE OF BEIJING UNION UNIV
Methods for treating eye disorders using dipyridamole
ActiveUS9254289B2Organic active ingredientsPharmaceutical delivery mechanismDipyridamoleOcular disease
The present invention discloses methods for treating eye disorders. The methods include the step of administering an effective amount of a topically-administered dipyridamole. Preferably, the topically-administered dipyridamole is formulated as a solution. Preferably, the topically-administered dipyridamole is at least one agent selected from the group consisting of: dipyridamole, and a pharmaceutically-acceptable salt thereof. Preferably, the effective amount corresponds to a concentration of at least about 10−5 molarity. Preferably, the effective amount is based on a treatment administration of at least once every other day.
Owner:O D OCULAR DISCOVERY LTD
Antitumor drug with double active components and application thereof
ActiveCN102198150AGood antitumor activityLow toxicityOrganic active ingredientsHeavy metal active ingredientsDexamethasoneDipyridamole
The invention discloses an antitumor drug with double active components, and an application thereof. The antitumor drug comprises an independent first active component and an independent second active component. The independent first active component comprises at least two of A, B and C. The A comprises Dipyridamole and pharmaceutically acceptable derivatives of Dipyridamole or pharmaceutically acceptable analogues of Dipyridamole or pharmaceutically acceptable salts of Dipyridamole. The B comprises Ubenimex bestatin and pharmaceutically acceptable derivatives of Ubenimex bestatin or pharmaceutically acceptable analogues of Ubenimex bestatin or pharmaceutically acceptable salts of Ubenimex bestatin. The C comprises Dexamethasone and pharmaceutically acceptable derivatives of Dexamethasone or pharmaceutically acceptable analogues of Dexamethasone. The independent second active component comprises Taxol, ADM, DDP, MMC, 5-FM, Gemcitabine or a tyrosine kinase inhibitor. The antitumor drug provided by the invention can effect on multiple targets and multiple paths, and thus antineoplastic effects of the antitumor drug are clear and obvious.
Owner:MEDICINE & BIOENG INST OF CHINESE ACAD OF MEDICAL SCI +1
Dipyridamole oral emulsion administration system and preparation method thereof
ActiveCN106389329AImprove stabilityImprove bioavailabilityOrganic active ingredientsAntiviralsDipyridamolePediatric drug
The invention provides a dipyridamole emulsion which comprises the following components in parts by weight: 1-2.5 parts of dipyridamole, 5-75 parts of emulsifier, 0.1-15 parts of a co-emulsifier, 25-150 parts of oil, 250-500 parts of water, 0.5-1 part of a sweetening agent, 1-2 parts of an aromatic and 0.25-0.5 part of a preservative. The dipyridamole emulsion provided by the invention has the advantages of uniform texture, good stability, improved drug dissolution, easiness in absorption by the human body and high bioavailability. After high-pressure homogenization, the particle size of the emulsion is small in uniform distribution, the emulsion has good stability, the dissolution of dipyridamole is remarkably improved, and the oral bioavailability is enhanced; and flavoring agents such as the sweetening agent and the aromatic also can be added into the emulsion, so that disagreeable tastes of the drug can be effectively covered, and multiple tastes provide multiple options for children. Through a sterilization process, a little or no preservative can be added, so that the quality of the finished product of the emulsion is improved, and the safety of pediatric drugs is further enhanced.
Owner:黑龙江童医生儿童生物制药有限公司
Freeze-drying composition for injection
InactiveCN1466946AImprove stabilityEnsure safetyOrganic active ingredientsPowder deliverySolubilityDipyridamole
A freeze-dried composition for injection contains the extract of ginkgo leaf and dipyridamole. The process for prepairing it is also disclosed. Its advantages are better solubility and high stability.
Owner:北京东方天翔医药技术开发有限公司
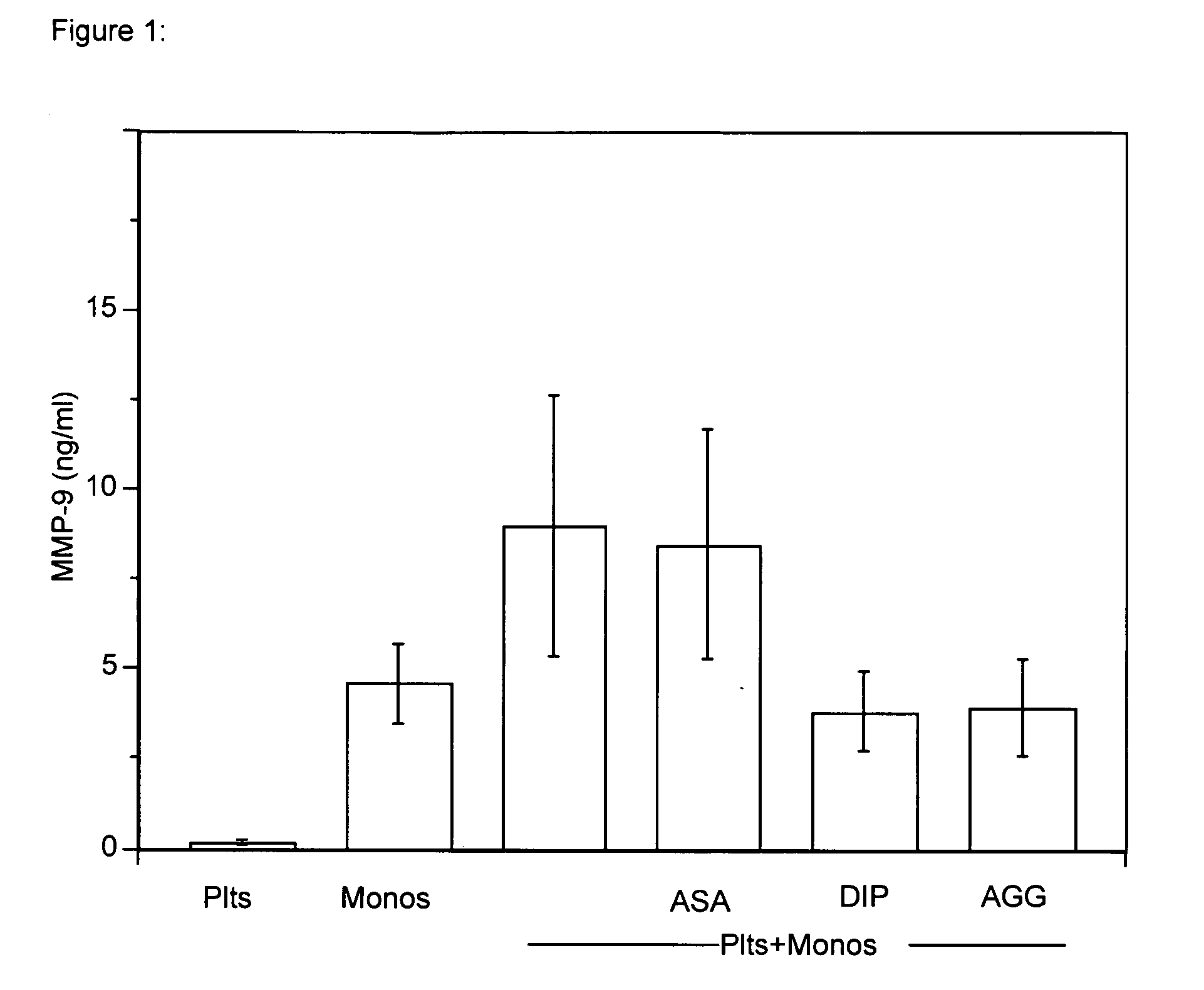
Use of dipyridamole or mopidamole for treatment and prevention of MMP-9-dependent disorders
InactiveUS20050282830A1Reduce MMP- gene expressionStabilize cell membraneAntibacterial agentsBiocideAutoimmune ReactionsHuman animal
A method of treatment of the human or non-human animal body for treating or preventing MMP-9-dependent disorders is disclosed, for example vascular syndromes, damages or diseases, atherosclerotic damages, arthritic conditions, inflammatory reactions, autoimmune reactions or proliferative diseases, which method comprises administering to a human or non-human animal body in need of such treatment an effective amount of a pharmaceutical composition containing dipyridamole, mopidamole or a pharmaceutically acceptable salt thereof, and the use of dipyridamole or mopidamole for the manufacture of a corresponding pharmaceutical composition.
Owner:BOEHRINGER INGELHEIM INT GMBH
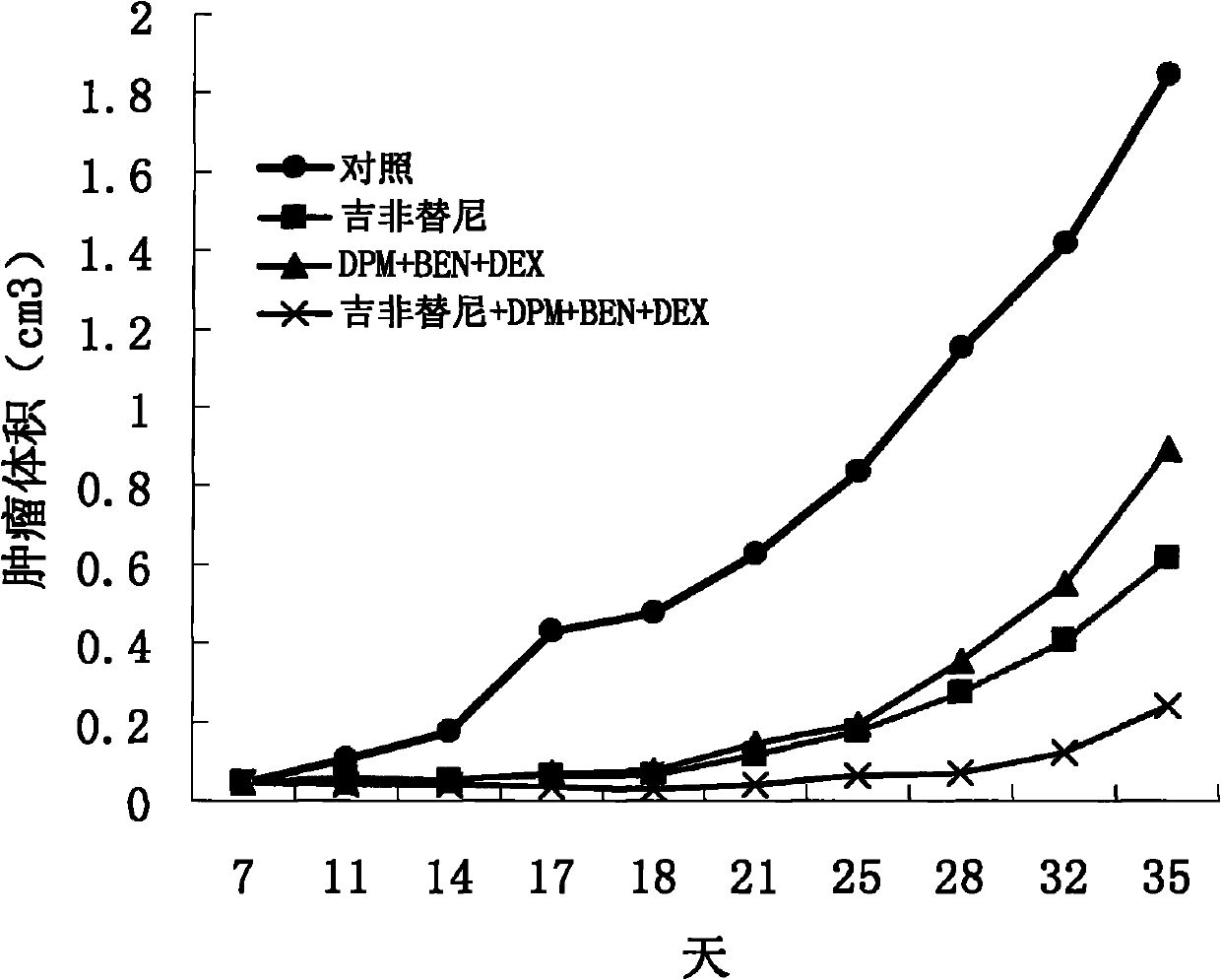
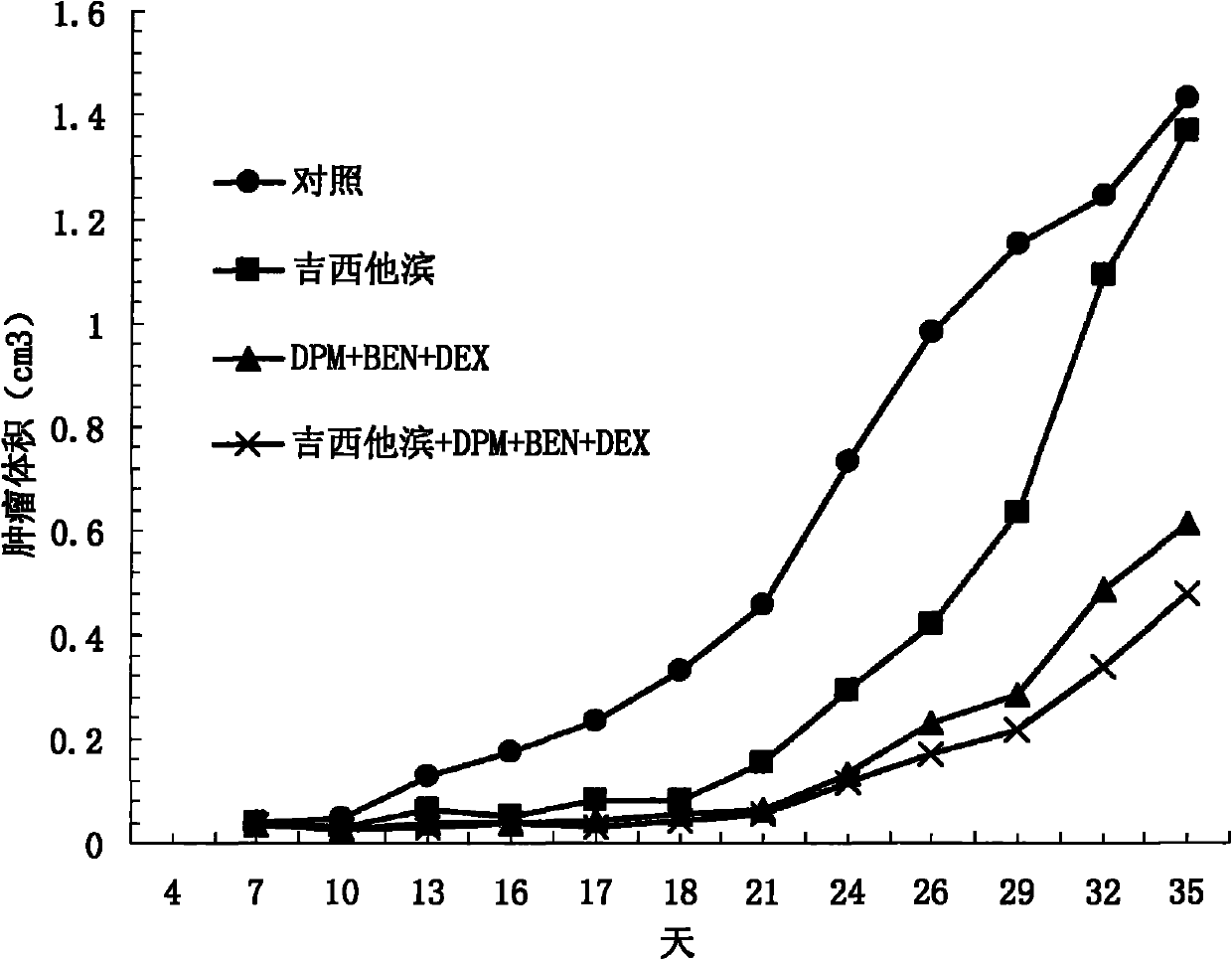
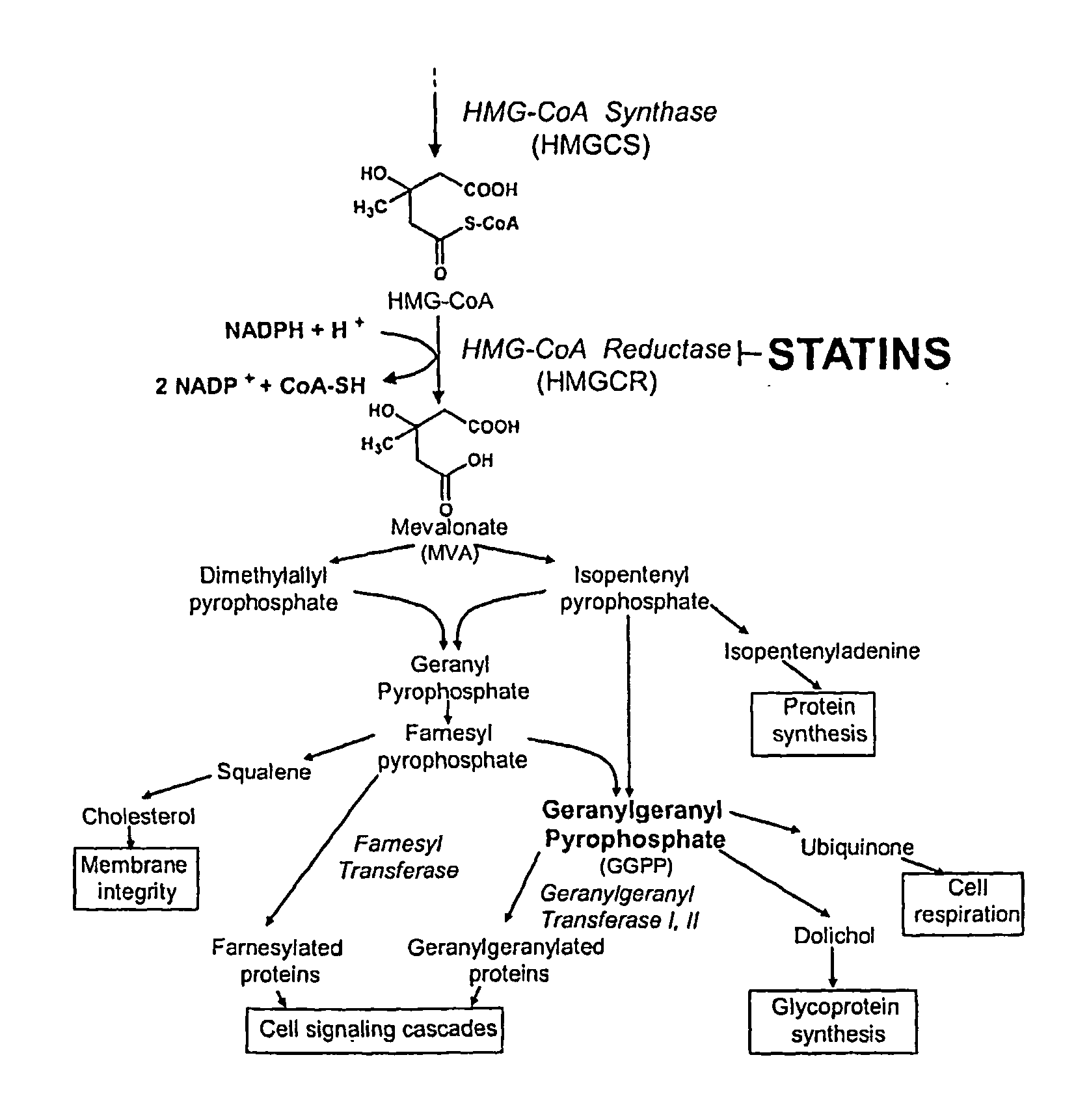
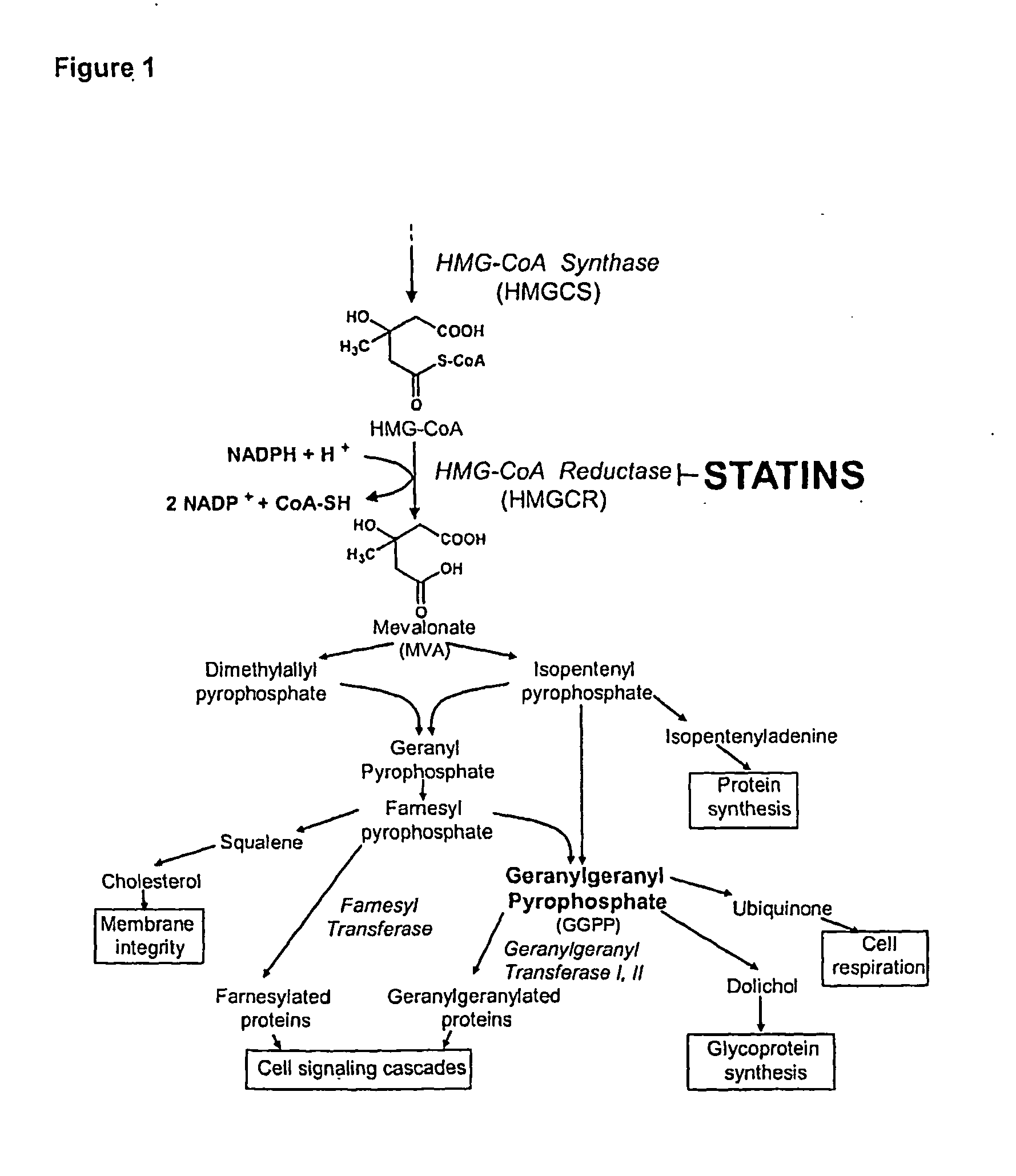
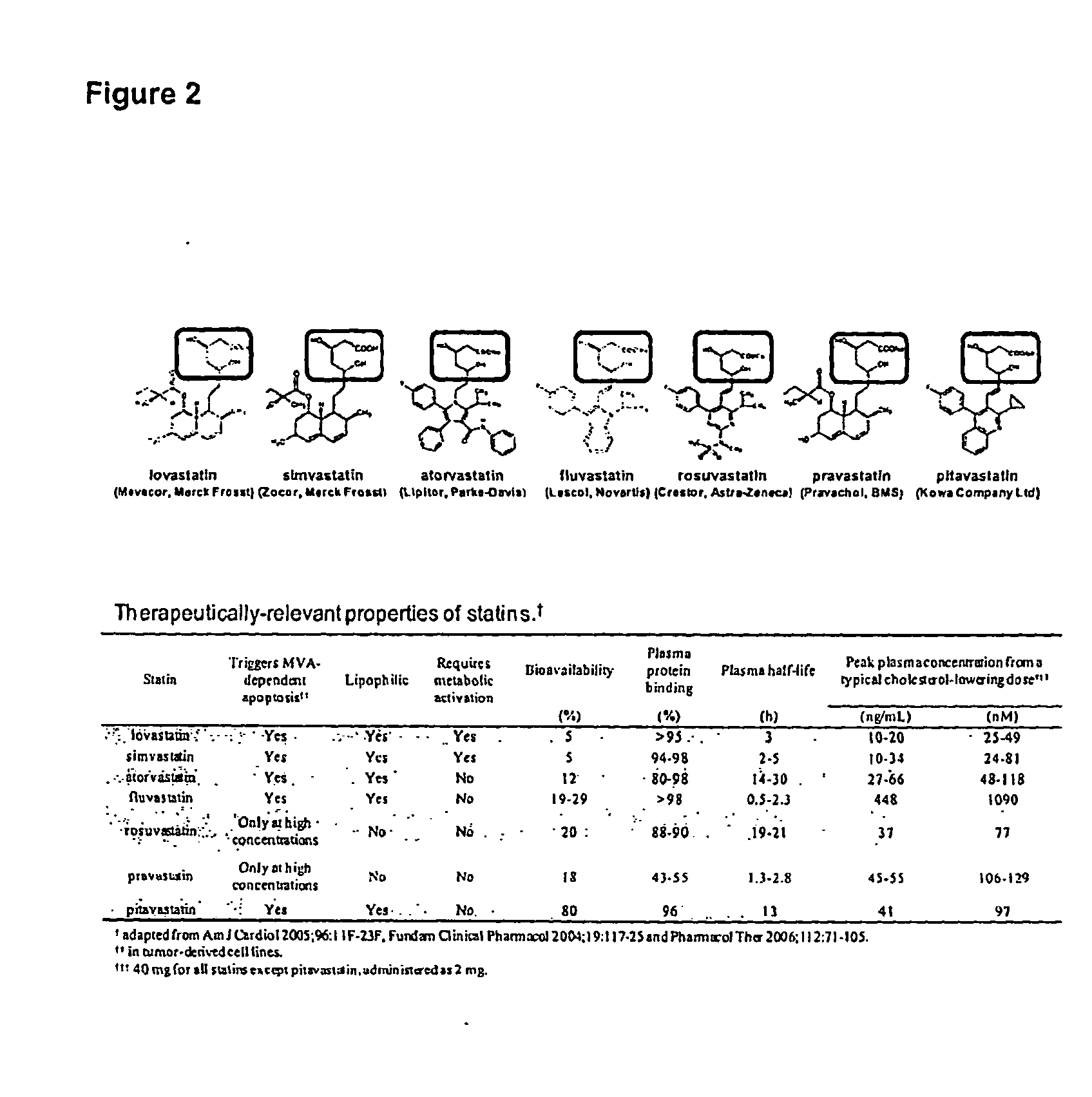
Treating cancer with statins and compounds having dipyridamole activity
The disclosure pertains to methods of treating a cancer comprising administering to a subject in need thereof an effective amount of a statin in combination with an effective amount of a dipyridamole and / or a compound that has dipyridamole activity.
Owner:UNIV HEALTH NETWORK
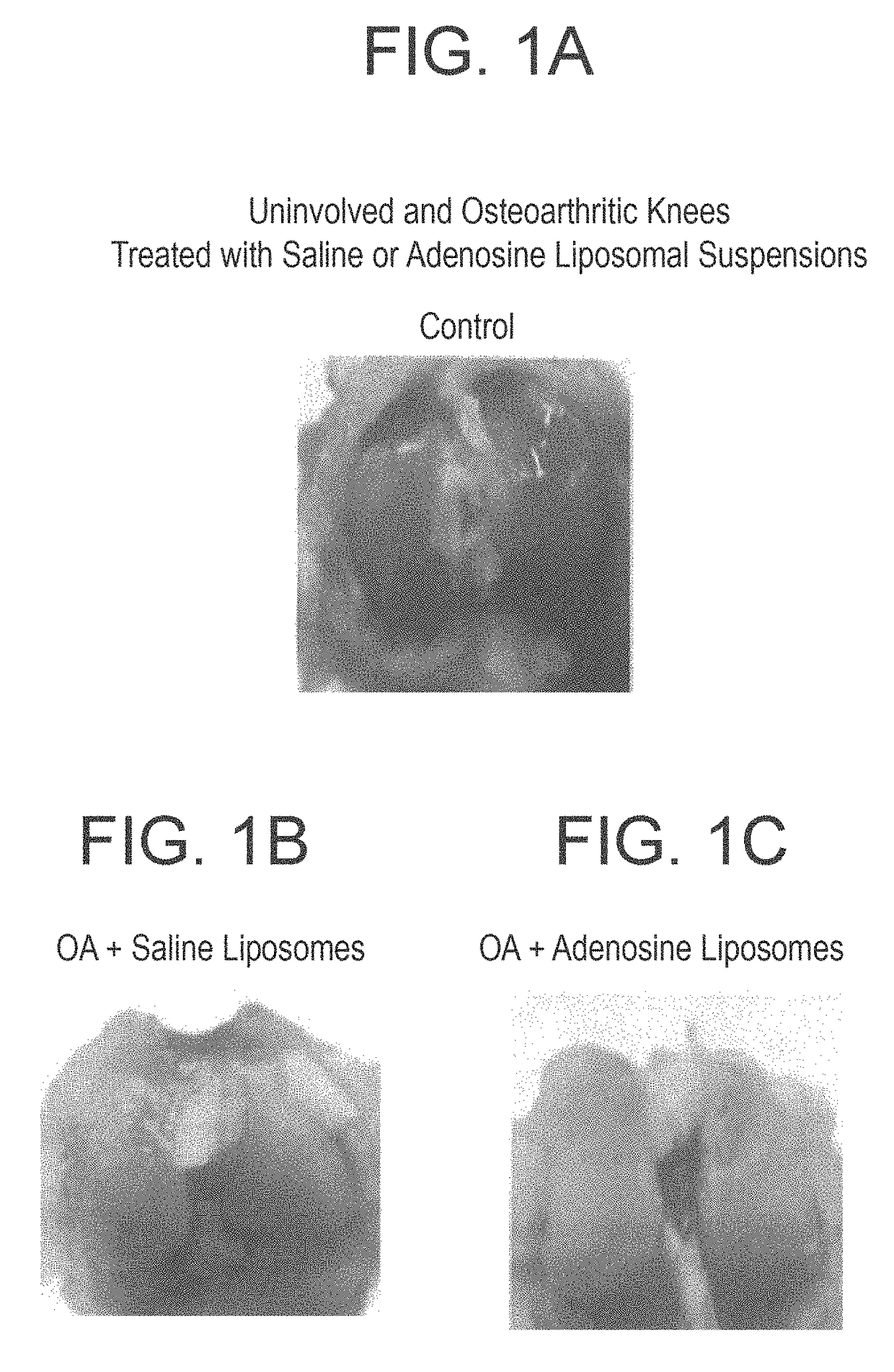
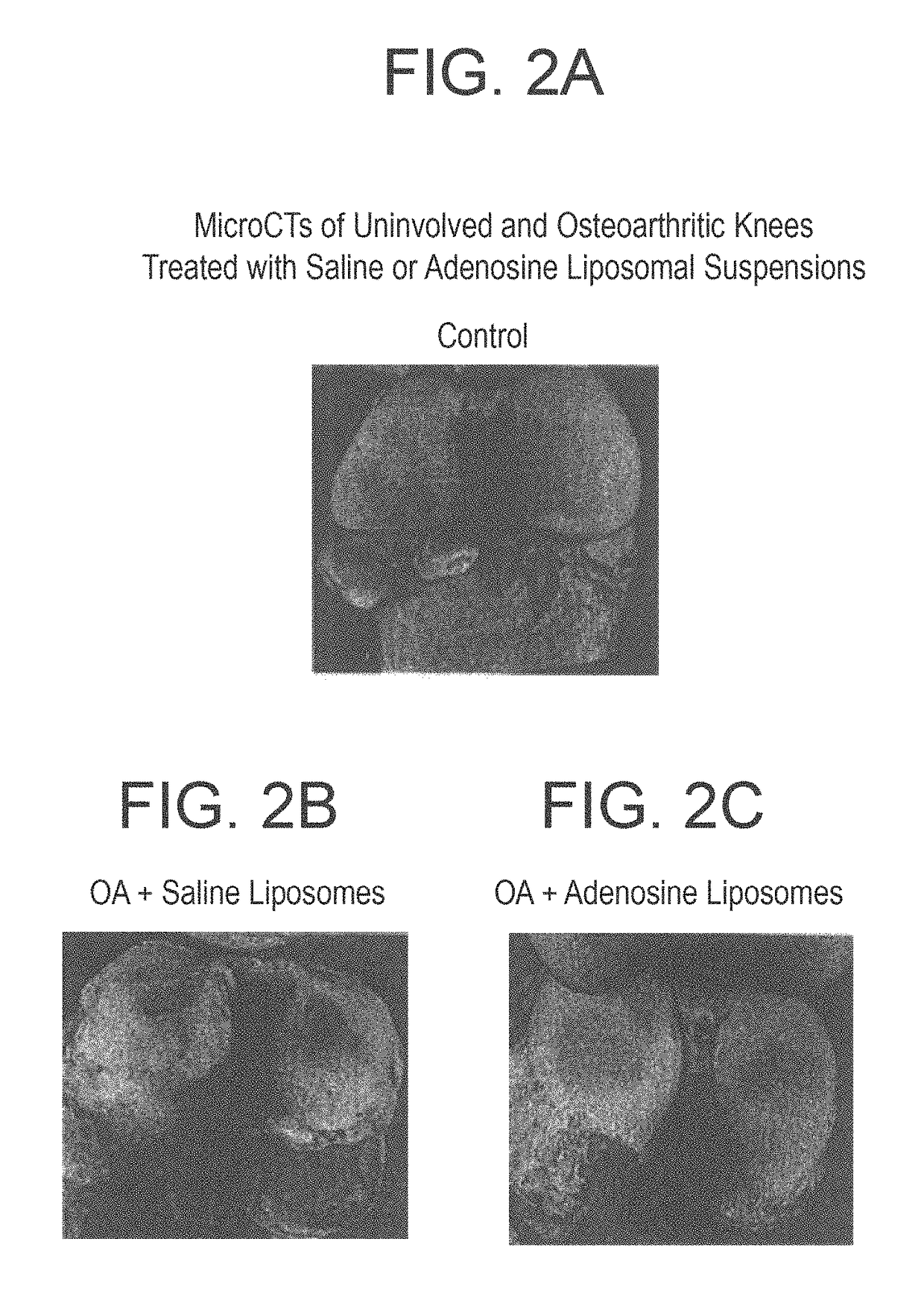
Methods and compositions for treating osteoarthritis and promoting cartilage formation
ActiveUS20180036238A1Minimize potential damageEliminate side effectsInorganic non-active ingredientsSkeletal disorderDipyridamoleTicagrelor
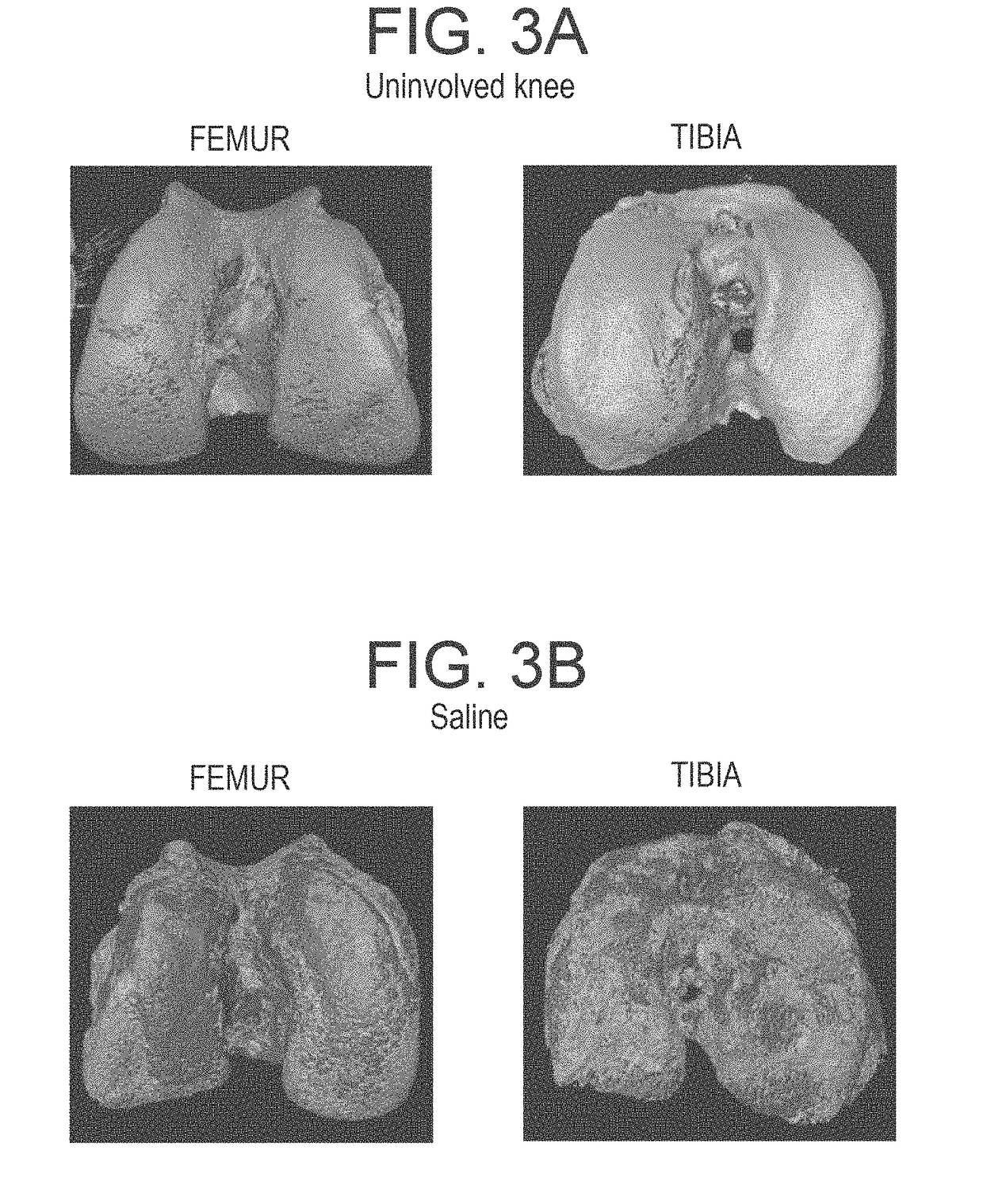
The invention provides methods and compositions for treating or inhibiting the development of osteoarthritis in a subject having osteoarthritis or at risk for developing osteoarthritis and for stimulating or increasing cartilage production or formation in a subject. The methods feature administering to the subject a therapeutically effective amount of a composition comprising one or more agent from among adenosine, an adenosine receptor agonist, and an agent that upregulates or increases the amount of or the biological activity of adenosine, or an analog or derivative thereof. The adenosine receptor may be an A1, A2A, A2B and A3 adenosine receptor. The agent that upregulates or increases the amount of or the biological activity of adenosine may be dipyridamole or ticagrelor. The composition may be administered via intraarticular injection such as injection into the synovial fluid of a joint. The composition may be effective to reduce or inhibit degeneration or damage to cartilage or to stimulate or increase cartilage production or formation. The composition may be a liposomal composition or contain a liposome.
Owner:NEW YORK UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com