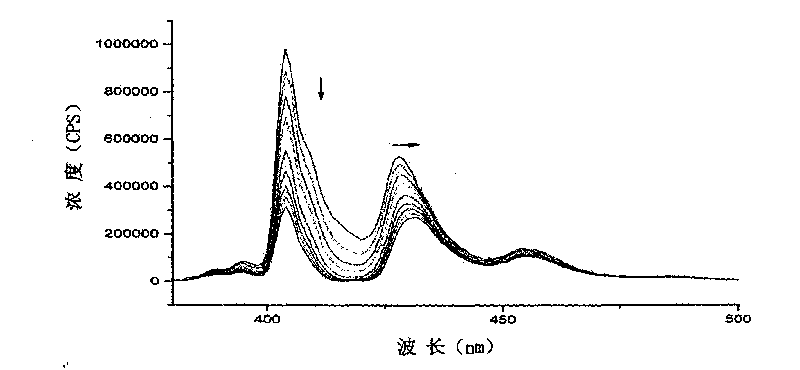
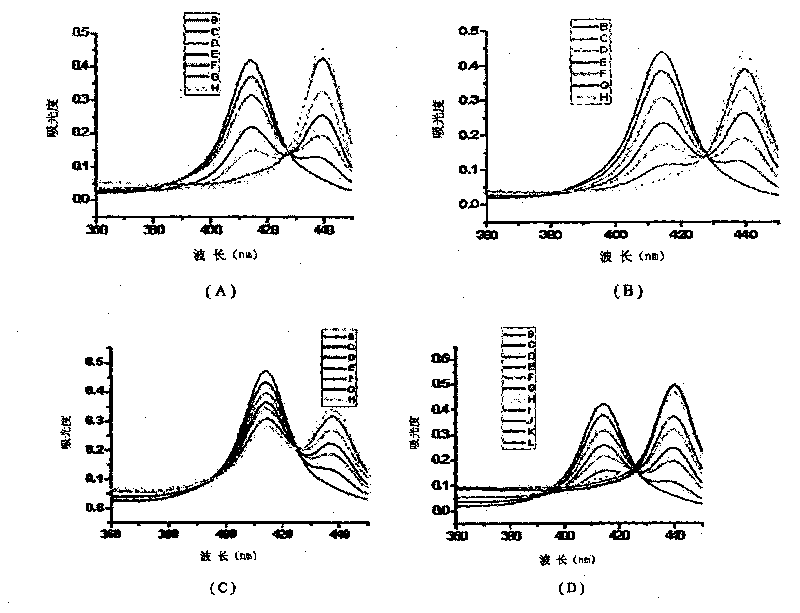
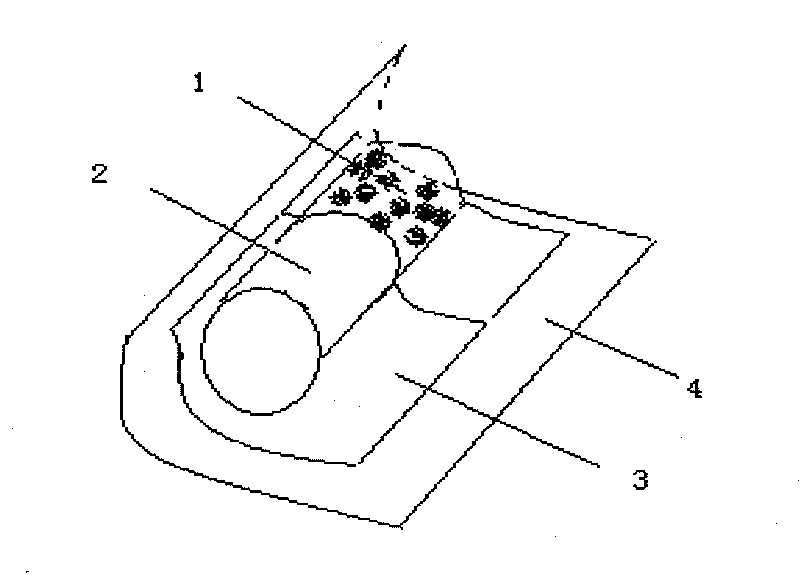
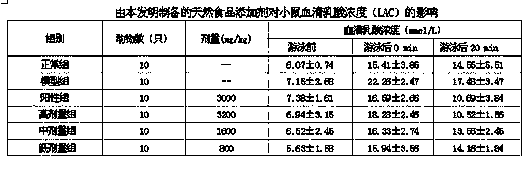
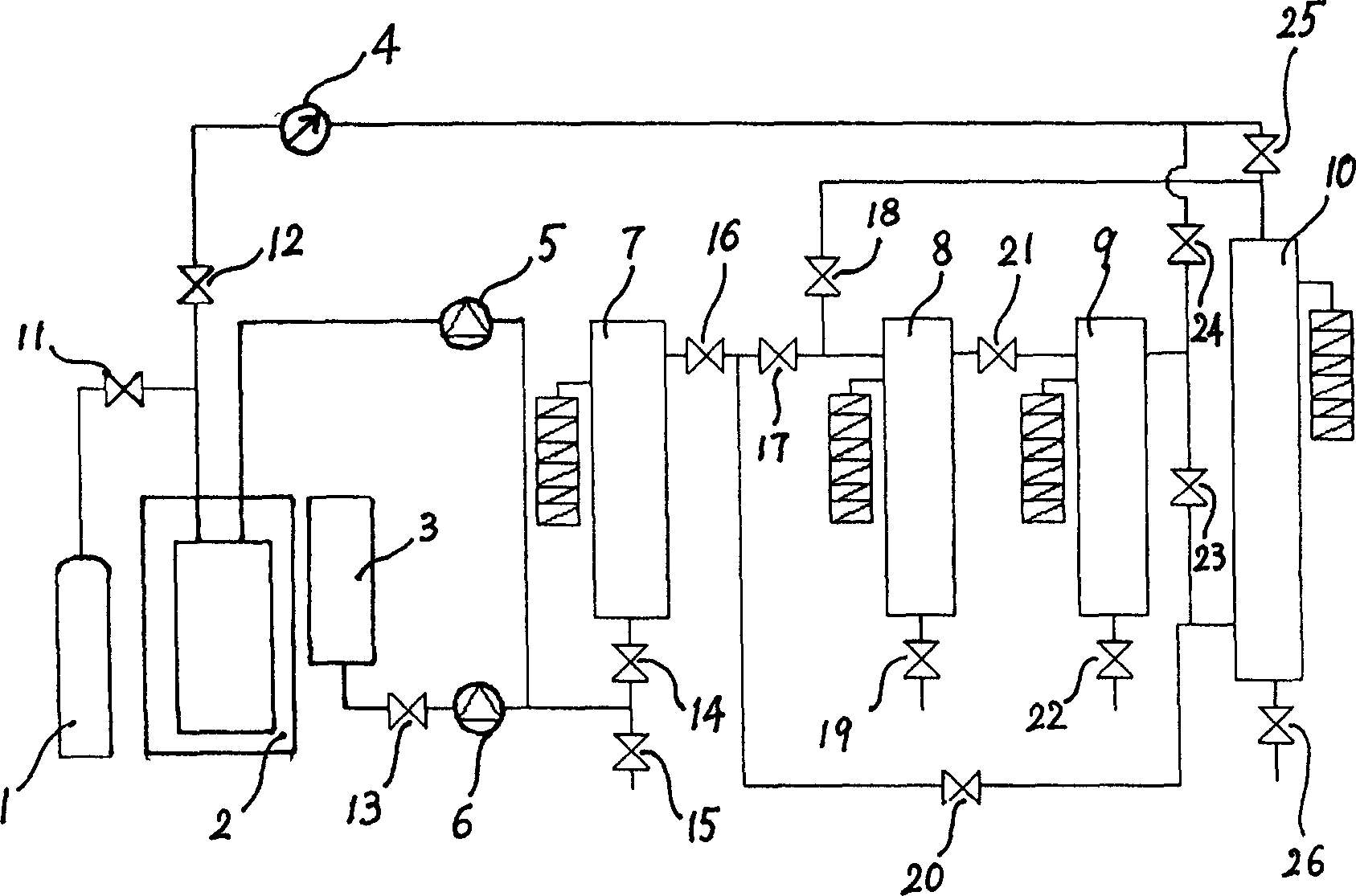
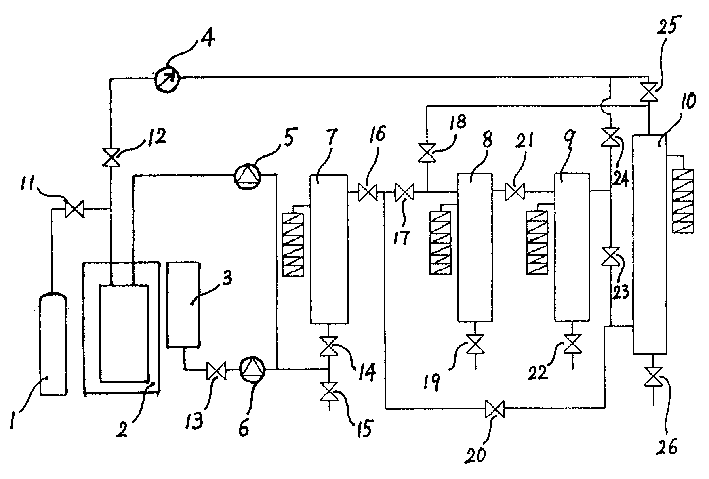
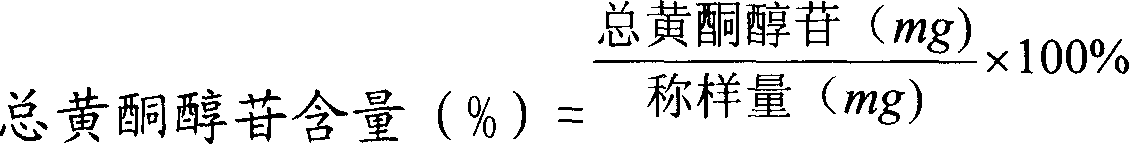Patents
Literature
804 results about "Ginkgo leaf extract" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Functional mixture for refreshing and remitting physical fatigue and visual fatigue and application thereof
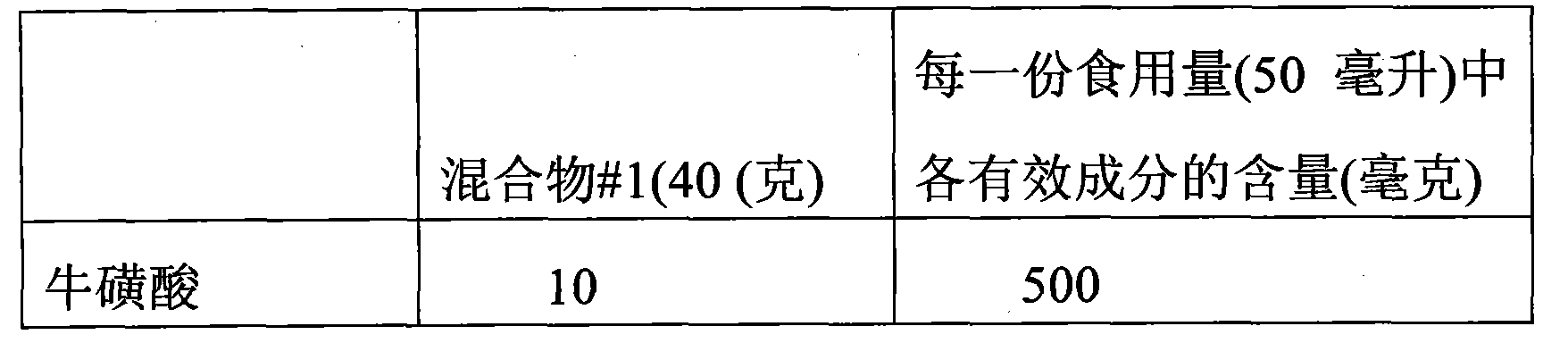
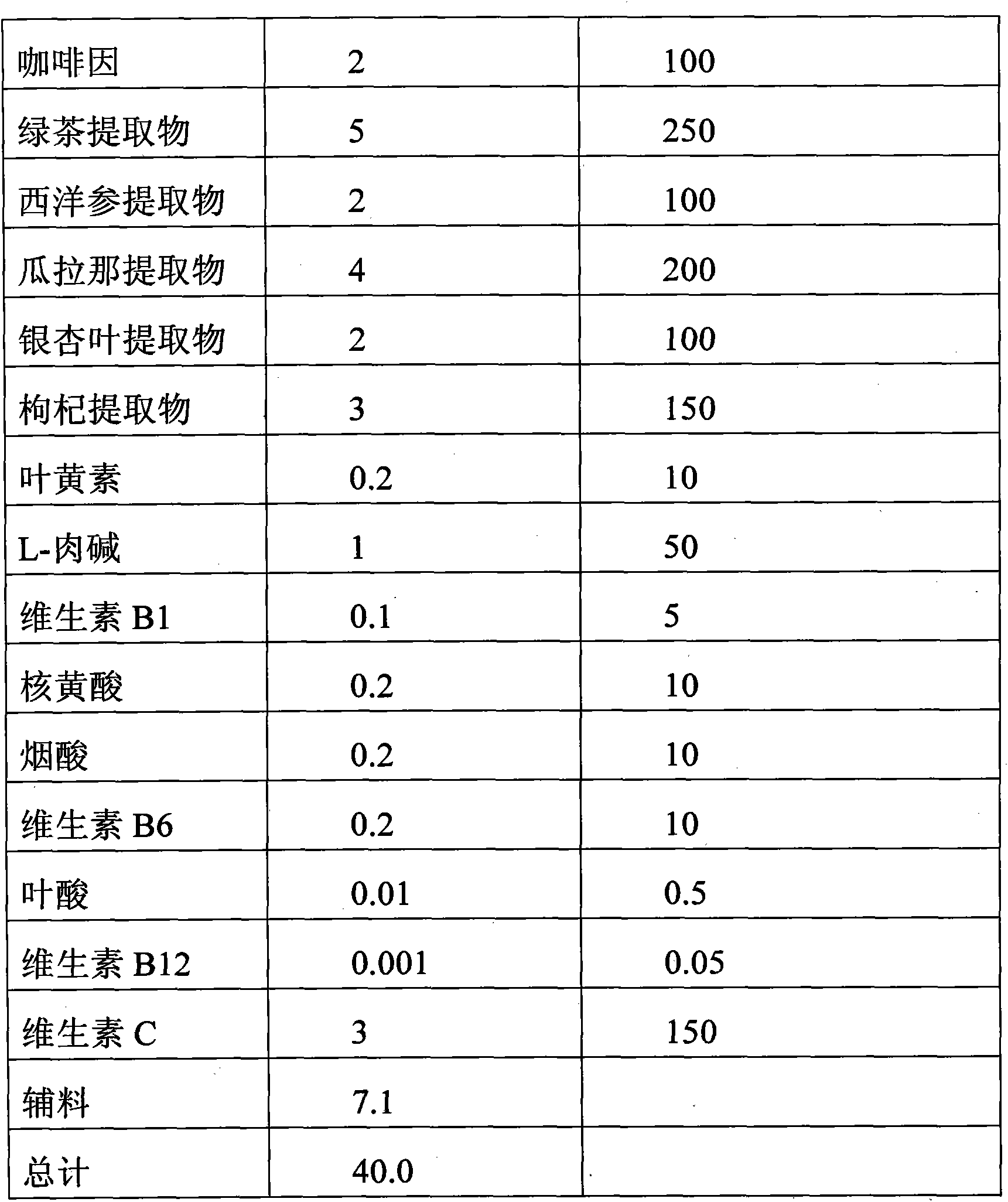
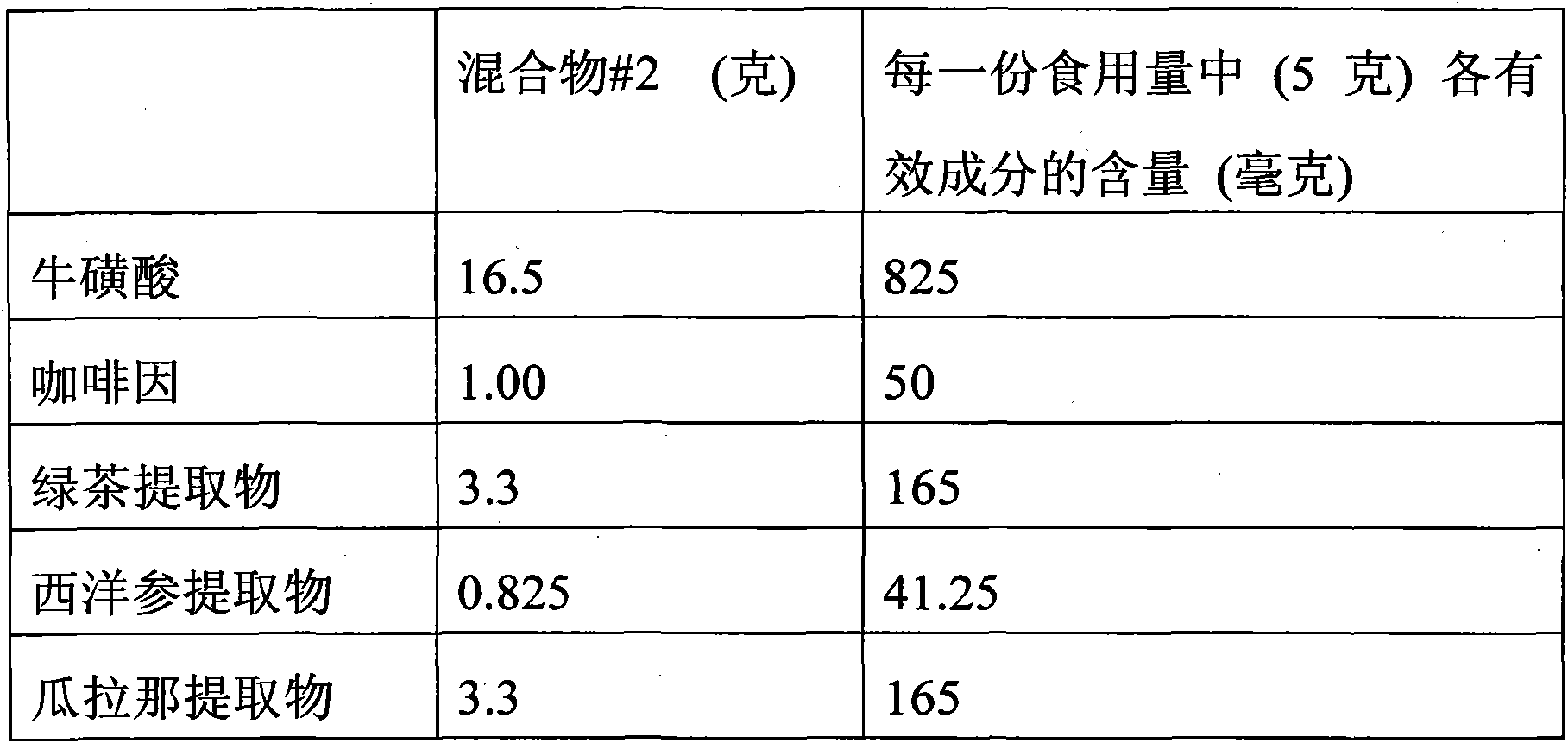
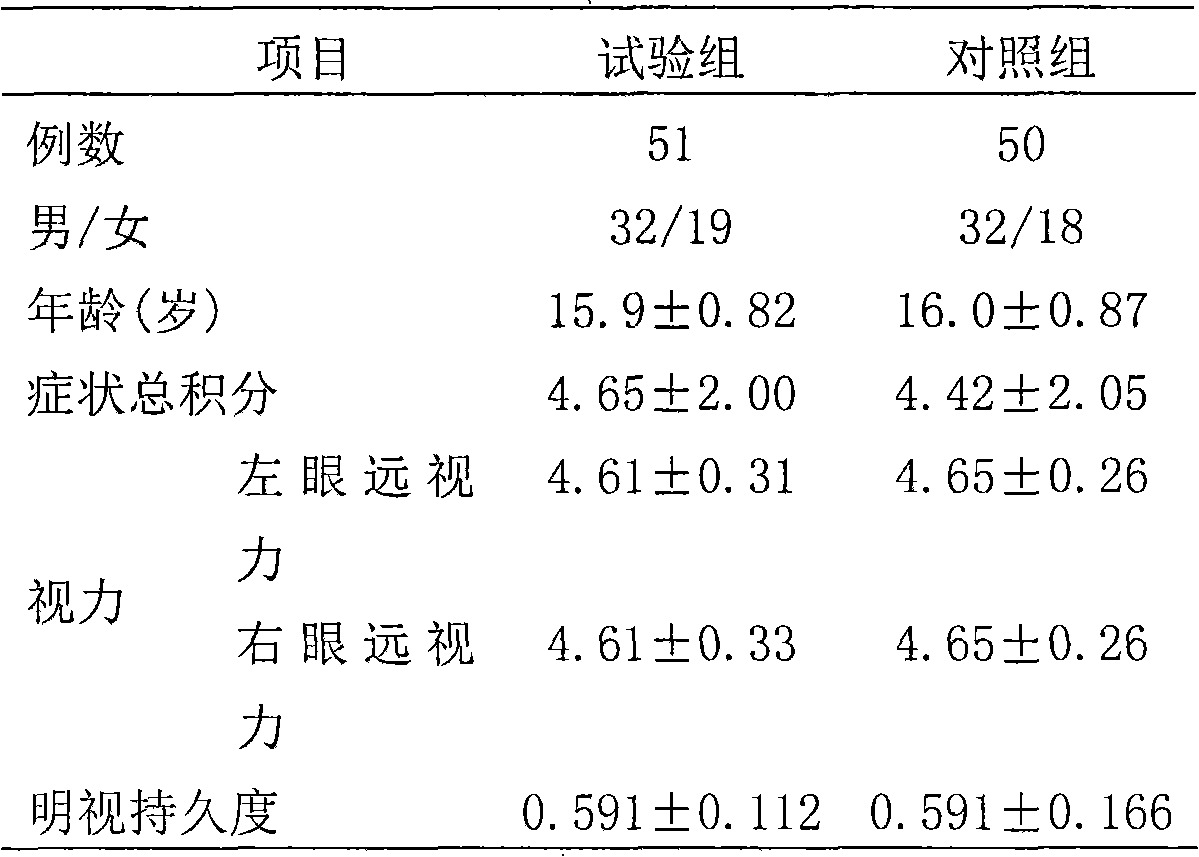
The invention relates to a functional mixture for refreshing and remitting the physical fatigue and the visual fatigue and application thereof. The mixture comprises the components such as taurine, caffeine, green tea extract, American ginseng extract, guarana extract, ginkgo leaf extract, wolfberry extract, lutein, L-carnitine, vitamin B1, nuclear yellow acid, nicotinic acid, vitamin, folic acid, vitamin B, vitamin C, auxiliary materials and the like. The mixture can be used for preparing the functional beverage, the effervescent preparation and the solid beverage electuary. The functional mixture has an application mode which is convenient to carry so as to meet the requirement, has the functions of remitting the physical fatigue and the brain fatigue, adds the nutrients for protecting the vision such as the wolfberry extract and the lutein, is particular suitable for personal who operates a computer for a long time, drivers and students, and plays roles in improving the brain power, protecting the vision, and remitting the physical fatigue and the visual fatigue.
Owner:姜天安
Health food with function of relieving visual fatigue and preparation method thereof
The invention relates to a health food with a function of relieving visual fatigue and a preparation method thereof, belonging to the technical field of health foods. The health food with the function of relieving visual fatigue is characterized in that active ingredients and additives are used for preparing the health food into soft capsules, hard capsules, oral liquid or tablets, wherein the active ingredients comprise the following raw materials: flaxseed oil or perilla oil, beta-carotene or vitamin A, lutein, zeaxanthin, vitamin E, ginkgo leaf extract and grape pip extract or cowberry extract; when the health food is prepared into the soft capsules, an emulsifying agent is used as the additive; when the health food is prepared into the hard capsules, a diluting agent is used as the additive; when the health food is prepared into oral liquid, a preservative, a flavoring agent, an emulsifying agent and water are used as the additives; and when the health food is prepared into the tablets, a diluting agent and a disintegrating agent are used as the additives. The health food prepared by the invention has the function of relieving visual fatigue.
Owner:INST OF OIL CROPS RES CHINESE ACAD OF AGRI SCI
Slow/controlled release pellet composition containing ginkgo leaf extracts and preparation method thereof
InactiveCN101375869ASmall toxicityStable blood concentrationGranular deliveryGinkgophyta medical ingredientsSustained release pelletsHard Capsule
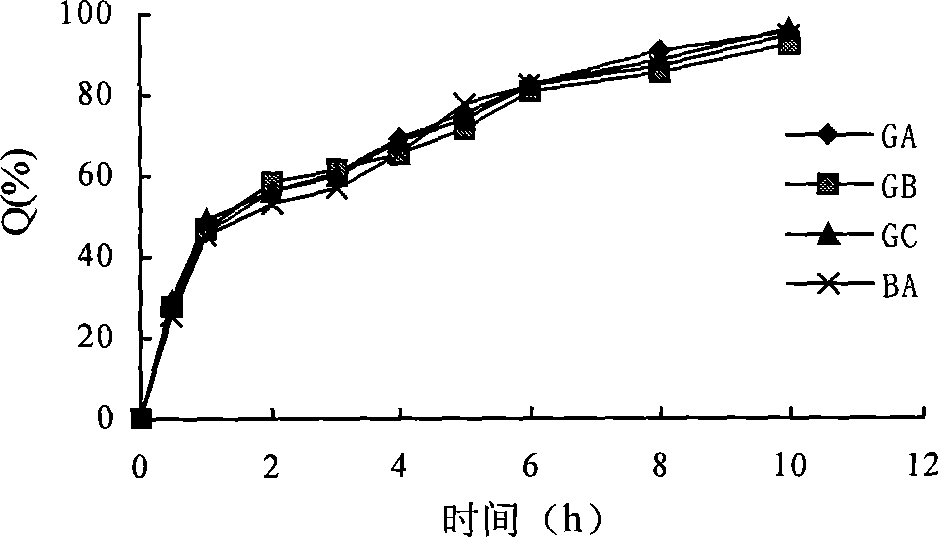
The invention belongs to the field sustained / controlled-release preparations, in particular to an oral sustained / controlled-release pellet combination containing ginkgo biloba extract and a preparation method. The oral sustained / controlled-release pellet combination is composed of (A) a core containing a pill; (B) an insulating coating layer; (C) a sustained-release coating layer; (D) and an enteric-coated coating layer. The invention is the traditional Chinese medicine multi-component sustained-release pellet combination which is taken once by 24 hours and the multi-unit sustained-release pellet combined preparation with the different drug release systems, the core containing the pill is prepared by adopting the extrusion pill rolling method, a novel sustained-release multi-layer coating technology and a fluidized bed are utilized for coating the sustained-release pellet, the rapid-release part and the sustained-release part of the coated pellet are mixedly filled into a hard capsule or pressed into a pellet tablet. The sustained-release pellet has stable coating process and good reproducibility, thereby being applicable to the industrial mass production; and the drug quality of the preparation is stable through the long-term storage. The in vitro release test shows that the multiple components of the traditional Chinese medicine can achieve the sustained-release role, the sustained-release preparation can significantly increase the transmembrane absorption and the stability of various effective active ingredients by oral drug administration, the curve of plasma drug concentration in vivo is smooth, and the design purpose of 24-hour sustained-release is achieved.
Owner:CHINA PHARM UNIV
Product having sobering up and liver protecting functions and its preparation method and usage
InactiveCN1698879APromote alcohol metabolismAvoid gatheringNervous disorderPeptide/protein ingredientsGreen Tea PolyphenolsVitamin C
The invention provides a product having sobering up and liver protecting functions and its preparation method and use, which comprises the following raw material (by weight ratio), soybean peptides and soybean extract 2.5-12:0.5, panaxoside or ginkgo leaf extract, green tea polyphenols, glutacid, arginine or lycine, vitamin C, vitamin B6, vitamin B1, vitamin B2 or vitamin B12. The medicament can be made into various dose forms including capsule, oral liquid, tablet, effervescent tablet or injection.
Owner:蔺益民
Use of gingko biloba extracts to promote neuroprotection and reduce weight loss
InactiveUS20050015263A1Extend your lifeDelay and decreases development of clinicalPharmaceutical non-active ingredientsGinkgophyta medical ingredientsAmyotrophic lateral sclerosisGingko biloba
The invention is directed to methods of preventing, delaying, or reducing motor neuron damage in an individual by administering to the individual a composition containing an extract of gingko biloba. The methods can be used to treat an individual having or at risk of having a condition characterized by motor neuron damage, e.g., amyotrophic lateral sclerosis. The invention also includes methods of preventing or reducing weight loss in an individual by administering to the individual a composition containing an extract of gingko biloba.
Owner:U S GOVERNMENT REPRESENTED BY THE DEPT OF VETERANS AFFAIRS +1
Composite filter tip containing biological composition
The invention relates to a composite filter tip containing a biological composition. A filament bundle filter stick of the filter tip comprises the biological composition, and after the biological composition is stuck to a carrier, when the filament bundle filter stick is formed, the carrier to which the biological composition is stuck is added into the filament bundle filter stick, wherein the biological composition comprises cobalt porphyrin and ginkgo leaf extract with the mass ratio being 1:1-80. The filter tip can not only obviously reduce the release amounts of harmful ingredients such as free radical, benzo[a]pyrene, peculiar nitrosamines of tobacco, and the like in smoke of cigarettes but also can enable the acute toxicity, subchronic toxicity, cytotoxicity and mutagenicity of the cigarettes to be lower as compared with comparison cigarettes, and at the same time has no adverse effects on the smoking quality of the cigarettes.
Owner:CHONGQING CHINA TOBACCO IND CO LTD +1
Natural food additive
ActiveCN103462037AImprove antioxidant capacityStrong anti agingFood preparationBiotechnologyFood additive
The invention discloses a natural food additive which belongs to the technical field of food additives. The natural food additive is characterized by comprising the following substances in percentage by weight: 20%-30% of a red bayberry anthocyanin extractive, 10%-20% of a purple sweet potato anthocyanin extractive, 10%-40% of a mulberry anthocyanin extractive, 10%-50% of an okra pectin and polysaccharide extractive, 10%-20% of a folium ginkgo extractive, 0.0005%-0.0008% of selenium yeast, 2%-10% of seaweed meal, and 5%-10% of ganoderma lucidum spore powder. The natural food additive has strong functions of oxidation resistance, aging resistance, fatigue resistance, invigorating the kidney, strengthening Yang, improving immunity and reducing blood sugar, cholesterol and triglyceride. After the natural food additive is detected, the total anthocyanin content is 15%-36%, the total flavone content is 16%-28% and the total phenolic acid content is 18%-29%.
Owner:ZHEJIANG FORESTRY UNIVERSITY
Method for extracting ginkgolide B from gingkgo leaf or gingkgo leaf extract
ActiveCN101054384AAvoid the problems of large loss and low extraction rateReduce dosageOrganic chemistryGinkgophyta medical ingredientsBilobalidesGinkgo leaf extract
The present invention discloses a preparation method for extracting bilobalide B from gingko leaf or its extract, which comprise: high speed countercurrent extraction, column chromatography for separation, recrystallization. The method can not only remove the harmful substance as alkylphenolic acids from gingko crude extract, but promote the yield of bilobalide B, promote the product content, and be suitable for industrial production.
Owner:GUILIN NATURAL INGREDIENTS CORP
Whitening cosmetic and preparation method thereof
ActiveCN104027293AHigh value-added utilizationSimple processCosmetic preparationsToilet preparationsBiotechnologyPhenolic content in tea
The invention discloses whitening cosmetic and a preparation method thereof. A composition of a plurality of plants is taken as a main raw material, and the whitening cosmetic mainly comprises the following components: a rice bran extract, a rhodiola rosea extract, a ginkgo leaf extract, tea polyphenol, a lemon juice concentrated liquor, chitosan, a puerarin extract, konjac powder, a sunflower faceplate extract, a sunflower seed extract, a saponin kernel, hemicelluloses and honey. In addition, the whitening cosmetic also contains the following raw materials: a little of glycerinum, 1,3-propylene glycol, squalane, sodium hyaluronate, carbomer, triethanolamine, triethanolamine, allantoin, nanometer titania, collagen powder, an antibacterial agent, a ferulic acid isooctyl ester and glutathione. The composition can be applied to a whitening formula, has the effects of whitening the skin, moisturizing, resisting bacteria and diminishing inflammation, removing free radicals, inhibiting melanin formation, and inhibiting hallachrome mutase and tyrosinase activity.
Owner:GLOBAL COSMETICS HONG KONG CO LTD
Efficient plant deodorant
ActiveCN105582805ANo side effectsNo secondary pollutionGas treatmentDispersed particle separationYucca Schidigera extractGinkgo biloba
Owner:HUNAN PUTAIER ENVIRONMENTAL CO LTD
Ginkgo leaf extract and production process of separating high purity effective component of the extract
InactiveCN1847237AAvoid pollutionAvoid destructionOrganic chemistryChromatographic separationAlcohol
The present invention discloses new process of extracting and separating high purity bilobalide and ginkgetin. By means of macroporous adsorption resin chromatographic separation technology, the water extract of ginkgo material is eluted in macroporous adsorption resin column successively with distilled water, 5-25 % concentration alcohol solution, 0.01-10 % concentration alkali solution, distilled water and 30-95 % concentration alcohol solution, and the elute of alkali solution and the elute of 30-95 % concentration alcohol solution are collected separately, concentrated and purified to obtain bilobalide or ginkgetin. The process has high efficiency, simple operate, low production cost and environment friendship, and the product has high quality and high yield.
Owner:厦门国宇知识产权研究有限公司
Anti-aging anti-wrinkle composition and applications thereof
PendingCN109602668AImprove stabilityImprove surface conditionCosmetic preparationsToilet preparationsAsiatic pennywortBaical Skullcap Root
The invention relates to the field of daily chemical products, in particular to an anti-aging anti-wrinkle composition and applications thereof. In view of the above technical problem, on the one hand, the present invention firstly provides the anti-aging anti-wrinkle composition, and the preparation raw materials include a moisturizing agent, a skin conditioning agent, and a thickening agent; theskin conditioning agent comprises a plant extract; and the plant extract is selected from one or more of glycine soja seed extract, swertia bimaculata extract, apple seed extract, ginkgo leaf extract, asiatic pennywort herb extract, giant knotweed rhizome extract, baical skullcap root extract, tea leaf extract, glycyrrhiza glabra root extract, mayweed flower extract, rosemary leaf extract and ceratonia siliqua seed extract.
Owner:ZHEJIANG KANGMANJIA DAILY NECESSITIES CO LTD
Method for removing ginkgolic acid from ginkgo leaf extract by extraction method
InactiveCN1470487ASave raw materialsSimple and fast operationOrganic compounds purification/separation/stabilisationSolid solvent extractionAlcoholOrganic solvent
The present invention discloses a method for removing ginkgolic acid from ginkgo leaf extract by orgnaic solvent extraction process, and said process mainly includes: extracting and drying. In which the refining procedure includes: adding methyl alcohol or ethyl alcohol to pulverized ginkgo leaf extract, stirring, dissolving, standing still, filtering, reduced pressure concentrating and drying; and its extracting procedure includes: stirring the pulverzed ginkgo leaf extract with ethyl alcohol or acetone, dissolving and placing said material into extract, adding paraffins material, uniformly mixing them, standing still and demixing, removing upper layer, repeatedly operating lower layer for several times, drying.
Owner:YANGTZE RIVER PHARM GRP CO LTD
Bilobalide B raw material and preparation thereof
ActiveCN101302222AHigh puritySimple processOrganic chemistryGinkgophyta medical ingredientsOrganic solventFiltration
The invention discloses a ginkgolide B raw material, which is characterized in that: the ginkgolide B raw material is made through the following method that: ginkgo leaves or ginkgo leaf extracts are taken and boiled in a hydrochloric acid solution; the boiled liquid undergoes organic extraction after filtration; when the obtained extract is condensed, 100 to 200-mesh silica gel is added in the extract to be mixed and dried; silica gel column chromatography is used to collect eluent rich in ginkgolide B, and the ginkgolide B raw material is obtained through crystallization; and the content of ginkgolide B in the ginkgolide B raw material is more than or equal to 90 percent. The ginkgolide B raw material made through adopting the method has high purity of ginkgolide B, simple preparation technological process, high transfer rate and low cost, and is suitable for industrial production.
Owner:YANGTZE RIVER PHARMA GRP JIA NGSU LONGFENGTANG TRADITIONAL CHINESE MEDICINE CO LTD +3
Production process of water soluble ginkgo leaf extractive
InactiveCN101254220ARetain and optimize the content of active ingredientsSimple production processGinkgophyta medical ingredientsCardiovascular disorderAlcohol freeGinkgo biloba
A production process of a water soluble ginkgo leaf extract comprises the following steps of pulverizing ginkgo leaf, and extracting with ethanol for 2-4 times to obtain concentrated solution I; diluting with purified water, filtering to obtain medicinal liquid II, washing the residue with purified water for several times, filtering the supernatant to obtain medicinal liquid III, mixing with the medicinal liquid II, diluting with water, and filtering to obtain clear medicinal liquid IV; adsorbing with macroporous adsorptive resin, pre-washing with purified water or ethanol, and eluting with ethanol to obtain medicinal liquid V; concentrating the medicinal liquid V to obtain alcohol-free concentrated extract VI; extracting the concentrated extract VI with ethanol for 2-4 times, filtering, and concentrating the filtrate to obtain concentrated extract VII; or alternatively completely dissolving conventional ginkgo leaf extract in ethanol, recovering ethanol to obtain concentrated extract, extracting with ethanol for 2-4 times, filtering, and concentrating the filtrate to obtain concentrated extract VII; and extracting the concentrated extract VII with purified water for 2-4 times, cooling the extractive solution, filtering, concentrating, and drying to obtain the water soluble ginkgo leaf extract. The inventive production process has the advantages of simple process, stable product quality, and good economic benefit.
Owner:ZHEJIANG CONBA PHARMA
Method for extracting bilobalide B from ginkgo leaf or ginkgo leaf extract
The invention discloses a novel process for extracting bilobalide B from ginkgo leaf or ginkgo leaf extract through liquid-liquid extraction and refining, wherein the extraction solvent being n-butanol or other solvents having similar dissolving property and polarity. The obtained bilobalide B has a purity higher than 95% and a yield higher than 1%.
Owner:孙毅
Whitening anti-aging essence liquid and preparation method thereof
ActiveCN105250184AAnti agingAging long-termCosmetic preparationsToilet preparationsPhosphoric Acid EstersMonosodium glutamate
The invention belongs to the technical field of cosmetics, and particularly relates to whitening anti-aging essence liquid and a preparation method thereof. The whitening anti-aging essence liquid is prepared from polyethylene glycol, polydimethylsiloxane, poly(sodium glutamate), glycerinum, hyaluronic acid, xylitol, C20-22 alcohol phosphate / C20-22 alcohol, ginkgo leaf extracts, ophiopogon root extracts, herba portulacae extracts, caulis spatholobi extracts, milk protein and the like. The whitening anti-aging essence liquid is good in permeability, easy to absorb and capable of effectively supplementing water, moisturizing and whitening, repairing the skin wrinkles, reducing the fine wrinkles and delaying skin aging.
Owner:江苏更美科技有限公司
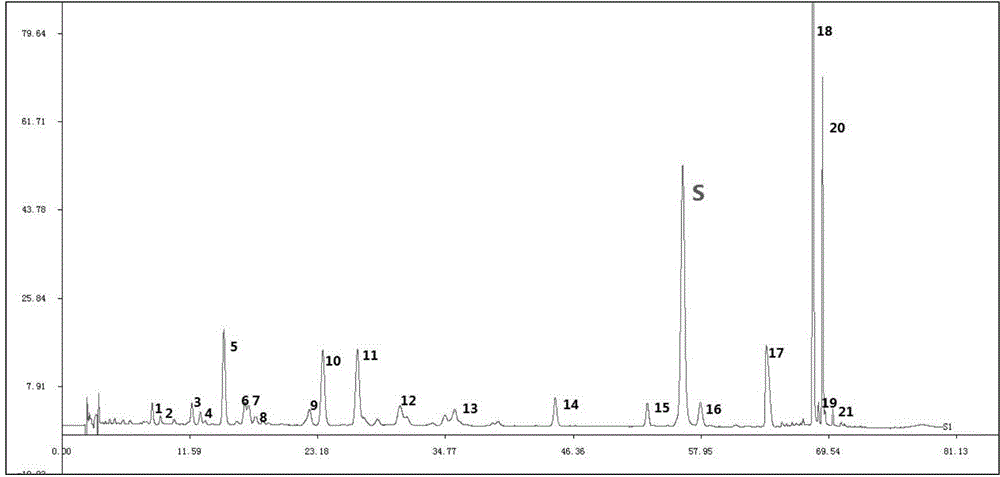
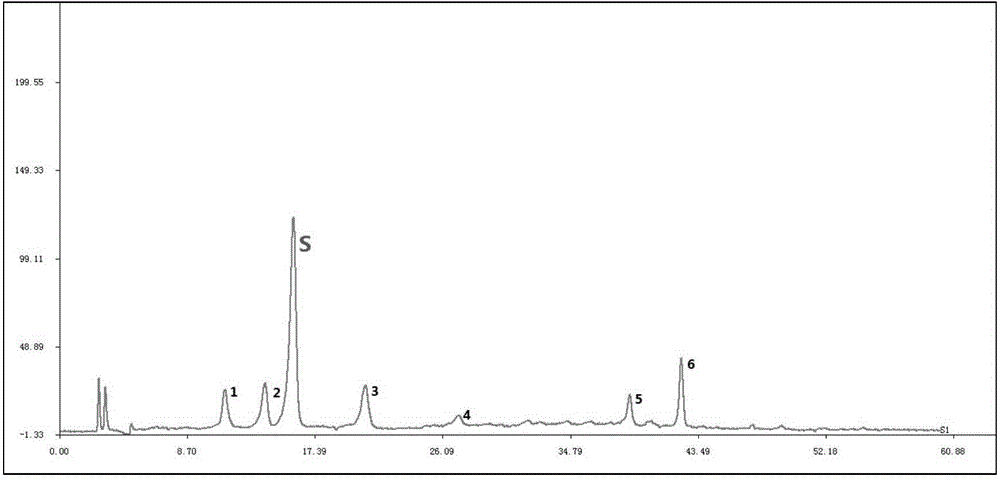
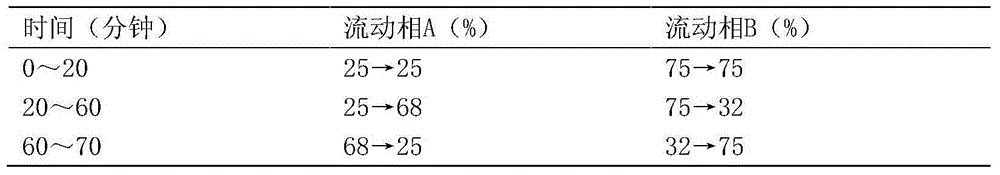
Detecting method for measuring content of flavonoid compounds and terpene lactone compounds in ginkgo leaf extract or preparations of ginkgo leaf extract at same time
ActiveCN105891355AAvoid frequent replacementImprove work efficiencyComponent separationGinkgo leaf extractAnalysis method
The invention relates to the field of medicine detection, in particular to a high-performance liquid chromatography detecting method for measuring the content of flavonoid compounds and terpene lactone compounds in ginkgo leaves (or ginkgo leaf extract) or preparations of the ginkgo leaves at the same time. The HPLC-DAD-ELSD method is set up, the method can measure the eight flavonoid compounds and three terpene lactone compounds in the ginkgo leaves (or the leaf extract) or preparations of the ginkgo leaves at the same time, the measurement result is analyzed, and a new analysis method and a basis are provided for quality control over the ginkgo leaf extract and the preparations of the ginkgo leaf extract.
Owner:HEBEI SHINEWAY PHARMA +2
Ginkgo leaf extract and its extracting method
ActiveCN1586548AScientifically feasibleHigh extraction rateUnknown materialsCardiovascular disorderVascular diseaseGinkgo leaf extract
The present invention belongs to the field of Chinese medicine preparing technology. The ginkgo leaf extract has total flavone and total terpene lactone content over 80 %, total flavone content over 70 %, flavonoid glycoside content over 40 % and total terpene lactone content over 10 %. The extraction process includes the following steps: extracting, purifying and separating, concentration, drying, etc. Compared with available technology, the present invention has the advantages of reasonable technological path, being suitable for industrial production, low production cost, high extracting rate of effective components, etc. The present invention also discloses the infusion liquid and powder for injection with the ginkgo leaf extract and their preparation process. The ginkgo leaf extract has wide application in medicine for treating cardiac vascular diseases.
Owner:AOLING BODA MEDICINE SCI & TECH DEV BEIJING
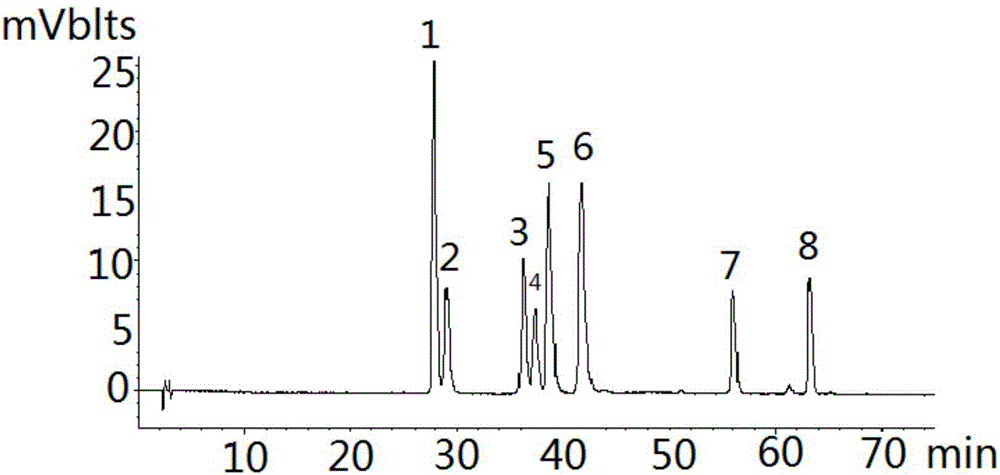
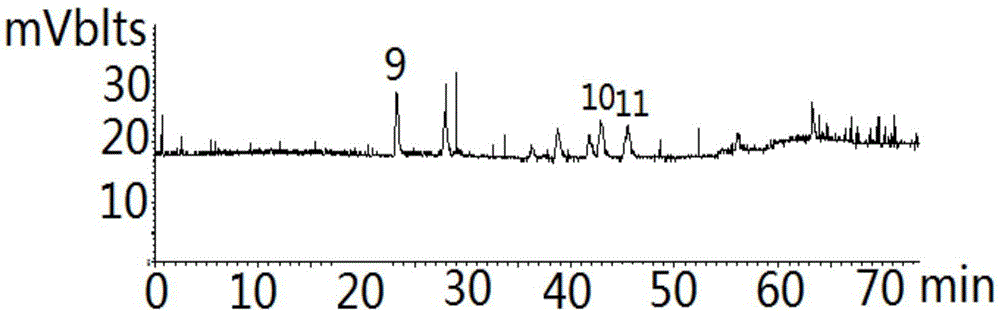
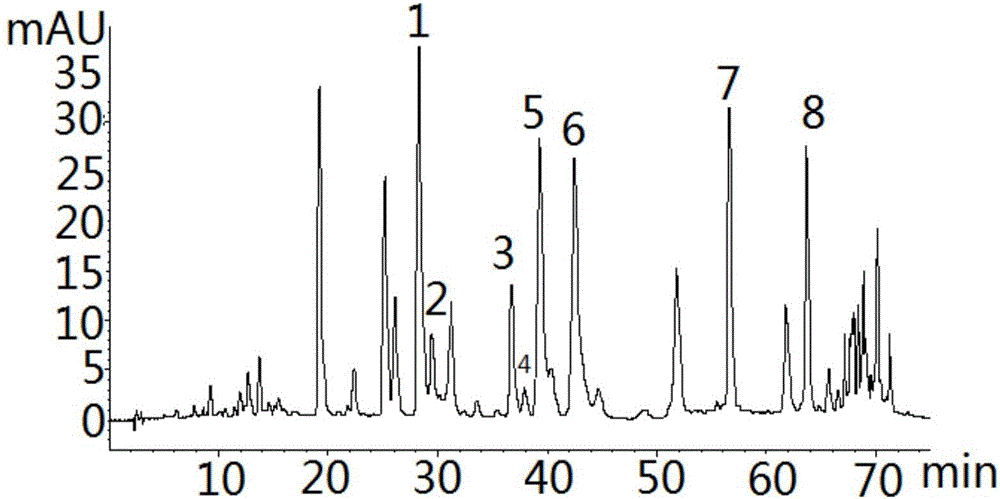
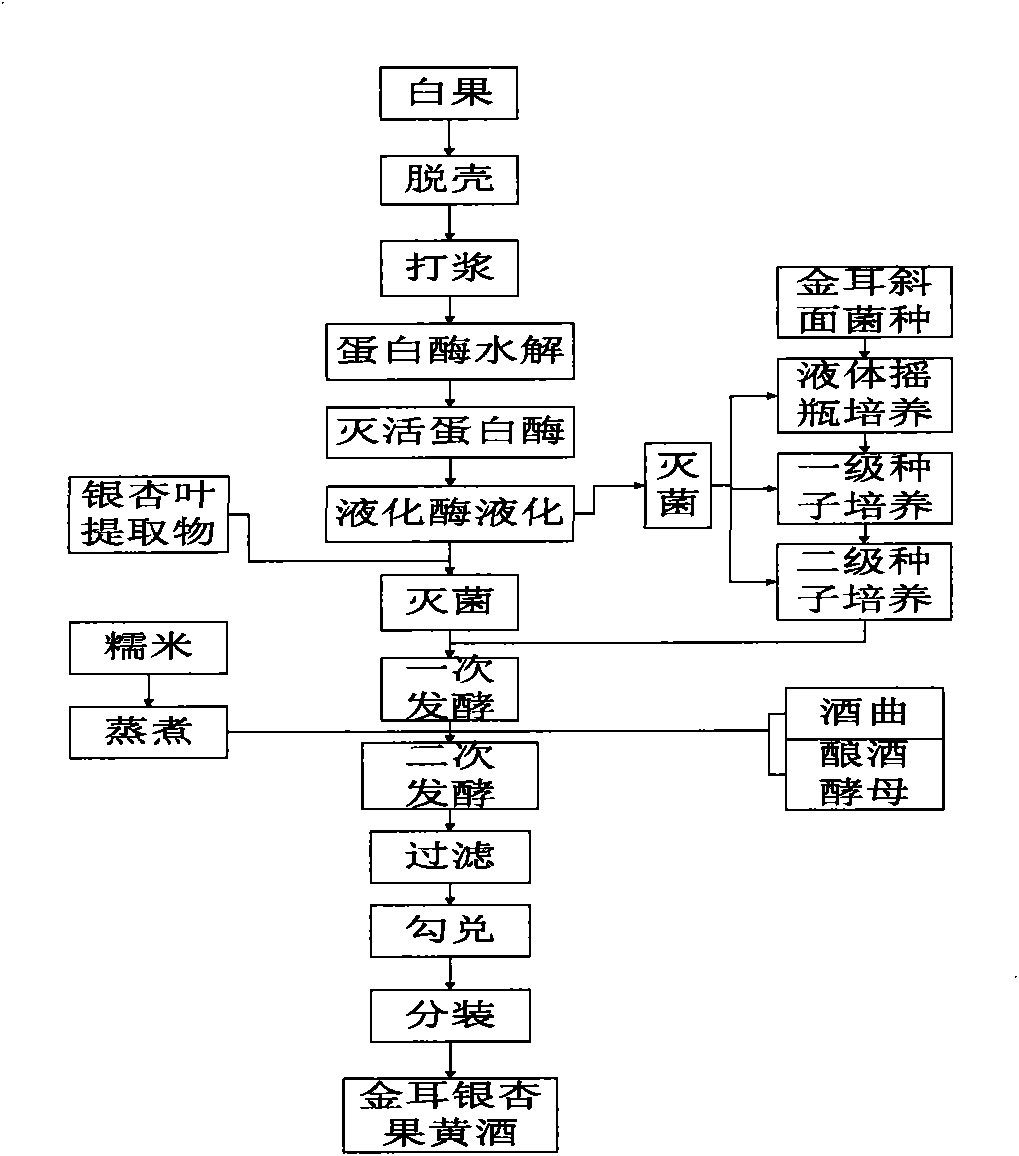
Golden fungus gingko yellow wine, preparation method and efficacy thereof
InactiveCN101831369AImprove immunityRegulate immunityMetabolism disorderMicroorganism based processesHydrolysateGinkgolide
The invention discloses a golden fungus gingko yellow wine. Alcoholic strength is 8-45%, general flavone content is more than or equal to 1-5 mg / L, ginkgolide is more than or equal to 1-6 mg / kg, total triterpenoid compound content is 30-800 mg / L, protein is more than or equal to 60 mg / kg, soluble sugar is less than or equal to 2.0%, gingko phenolic acid is less than or equal to 5 ppm, and hydrocyanic acid is less than or equal to 0.1 mu g / L. A double-enzyme method is adopted to hydrolyze gingko to obtain gingko hydrolysate which contains active compounds, such as ginkgo flavone, triterpenoid compound, amino acid, peptide, starch and the like, wherein the content of amino acid and peptide is more than or equal to 1%, and starch content is more than or equal to 2%. After being added with gingko leaf extract, the hydrolysate is converted by golden fungus strains, and rice koji is added to obtain fermentation liquor; the fermentation liquor is filtered and blended to obtain golden fungus gingko yellow wine. The wine contains gingko and golden fungus active ingredients and has the efficacy of preventing diabetes and hyperlipidemia, preventing oxidation and improving immunity of body.
Owner:江苏同源堂生物工程有限公司 +1
A high-content natural shikimic acid extract product and a preparing method thereof
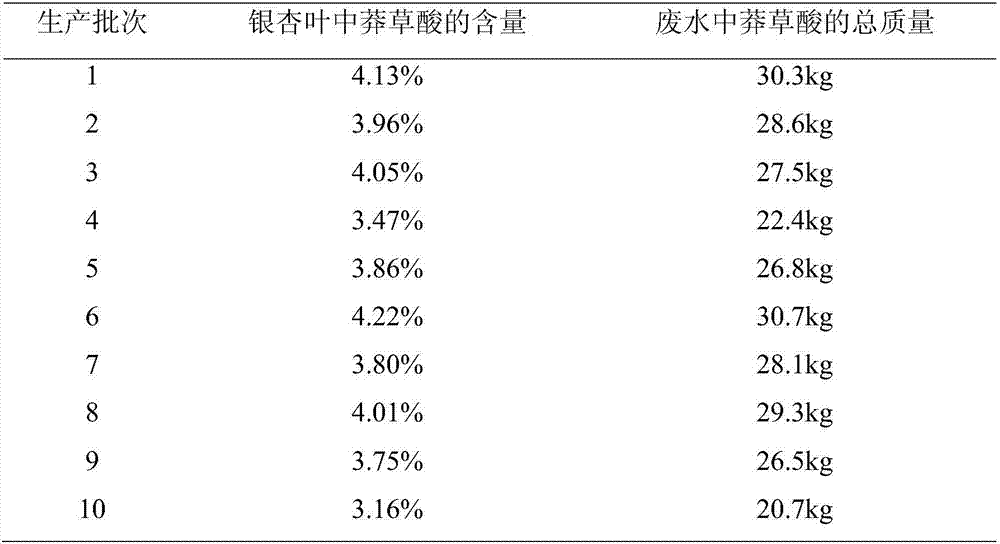
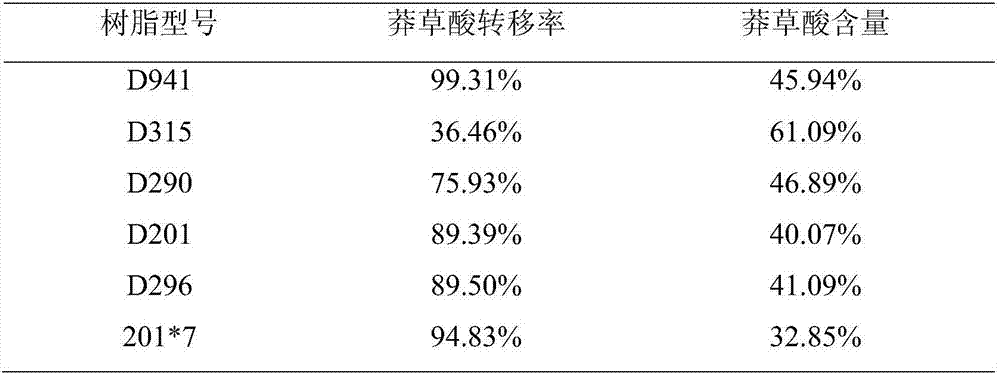
ActiveCN107353201AAlleviate resource shortagesLower requirementCarboxylic compound separation/purificationWastewaterShikimic acid
The invention discloses a high-content natural shikimic acid extract product separated and prepared from waste water generated in a ginkgo leaf extract product production process and a preparing method thereof. The content of shikimic acid is higher than 98%. The waste water generated in the ginkgo leaf extract product production process is adopted as a raw material of the method, and the method includes directly subjecting the waste water to adsorption separation with anion exchange resin, allowing the eluate obtained to pass through cation exchange resin to remove sodium, subjecting a solution after sodium removal to three combined steps of adding a shikimic acid high-purity product, performing high-temperature treatment, and allowing the mixture to stand at a low temperature in order to precipitate a shikimic acid precipitate, and recrystallizing the shikimic acid precipitate to obtain the shikimic acid extract product the shikimic acid content of which is 98% or above.
Owner:YUNNAN HIKON BIOTECH CO LTD +2
Technique for removing ginkgolic acid in folium ginkgo extract
ActiveCN101194918AEfficient removalSimple processIon-exchange process apparatusIon-exchanger regenerationPolyamideGinkgolide
The invention discloses a removal technique of ginkgolic acid in a ginkgo leaf extract, which utilizes ginkgo leaf extract which passes through a macroreticular resinous to be raw material, and the process comprises firstly utilizing polyamide which is pre-processed to be a removing agent of the ginkgolic acid, and then changing superpositions of resin pillar with pre-processing strong alkalinity macroporous ion to remove ginkgolic acid. The process has simple technique, stability and low cost, which can effectively remove ginkgolic acid in ginkgo leaf extract, and can not affect yield rates of ginkgo biloba flavonoids and ginkgolide.
Owner:石药银湖制药有限公司
Special diet food for patient with hyperlipidemia and hypertension
InactiveCN101507494ATo promote metabolismPeptide/protein ingredientsMetabolism disorderCorn silkLactalbumin
The invention discloses a special diet food eaten by the patients with hyperlipidemia / hypertension, or a nutritional food with health functions, which is characterized in that the nutritional food comprises the following raw materials in proportion by weight: 2 to 20 grams of soybean active oligopeptide (ACEI antihypertensive active peptide), 4 to 30 grams of oat flour, 5 to 15 grams of soybean isolated protein, 2 to 8 grams of evening primrose oil, 0.2 to 1.5 grams of ginkgo leaf extract, 2 to 10 grams of corn silk, 0.1 to 1 gram of agaric polysaccharide, 200 to 500 milligrams of tea polyphenol, 2 to 10 grams of pumpkin powder, 1 to 8 grams of lactalbumin, and 2 to 20 grams of fructo-oligosaccharide. The nutritional food is designed by taking the physiological nutrition characteristics of the patients with hyperlipidemia / hypertension and referring to the actual situations of diets of the people with hyperlipidemia, and can evenly replenish nutrition and assist in reducing blood lipid and blood pressure. The nutritional food not only is suitable for the demands of the patients with hyperlipidemia for evenly replenishing nutrition, but also can assist in reducing blood lipid and blood pressure, and improve metabolism.
Owner:刘志伟
Method for detecting ginkgo leaf extract and diphyridamole injection preparation
The invention provides a method for detecting a ginkgo leaf extract and diphyridamole injection preparation. The method comprises the step of fingerprint test and content test of the ginkgo leaf extract and diphyridamole injection preparation, wherein the fingerprint test refers to fingerprint test of flavonoid and terpene lactones in a gingko leaf extract; and the content test refers to test of flavonoid and terpene lactones in the gingko leaf extract. Compared with the prior art, the method can realize more effective product quality control, as well as authentication and content test of effective component monomers, of the ginkgo leaf extract and diphyridamole injection preparation, is very good in precision, stability and repeatability and is another excellent guarantee for the variety curative effect.
Owner:GUIZHOU YIBAI PHARMA CO LTD
Composition of plant extracts and application in skin whitening and moisture preservation
InactiveCN101836944AStrong whitening and moisturizing effectNatural colorCosmetic preparationsToilet preparationsCentella asiatica extractSide effect
The invention relates to a composition of plant extracts and application thereof in skin whitening and moisture preservation. The composition comprises the following active ingredients in percentage by weight: 5 to 90 percent of green tea extract, 5 to 50 percent of ginkgo leaf extract and 5 to 45 percent of centella extract, wherein the composition accounts for 0.5 to 20 percent when applied to preparing external products of skin, and the composition is uniformly mixed with medically-acceptable excipients or carriers in proportion to prepare products in forms of gel, cream, serosity, aqueous solution agents, emulsions or solids. The composition has the effects on skin whitening and moisture preservation without adding moisture preserving components; compared with products with similar application, the composition has stronger effects on skin whitening and moisture preservation and smaller dosage; the composition is safe, has no toxic or side effect, has low allergy rates and inflammation probability, penetrates into skin dermis, does not change the normal apoptosis process of cells, restrains tyrosinase, dopachrome tautomerase, DHICA oxidase and endothelin appropriately and balances the metabolism of melanin, so that the color and luster of the skin is more natural; and in addition, the generated skin whitening effect is definite, and the composition is convenient to use in cosmetics.
Owner:杭州千岛湖康诺邦健康产品有限公司
Radiation-resisting nourishing and health caring noodles
InactiveCN102334632AImprove immunityPromote healthy growthDough treatmentFood preparationPollenGinkgo leaf extract
The invention provides radiation-resisting nourishing and health caring noodles. The noodles are prepared from raw materials of flour, purple amaranth juice, dendranthema morifolium extract liquid, ginkgo leaf extract liquid, medlar virgin pulp, spiral algae powder, pollen, dietary alkali, and common salt. The product provided by the invention has abundant and enriched nutrients, and a good radiation-resisting effect. The noodles are tasty, smooth, soft, delicious, and boiling-tolerant. When eaten, the noodles do not break off, stick on teeth, or make soup turbid. The product is an ideal healthcare food for stuffs with computer careers.
Owner:朱广凡
Ginkgo leaf extract, injection containing said extract and its preparing method
InactiveCN1899323AEasy to useDefinite curative effectPharmaceutical delivery mechanismGinkgophyta medical ingredientsVascular diseaseSide effect
The present invention discloses a kind of ginkgo leaf extract, injection containing the said extract and its preparation process. The ginkgo leaf extract contains flavonol glycoside 18-40 wt%, bilobalide 2.5-25 wt%, and heavy metals less than 9.4 ppm, with the bilobalide comprising bilobalide A, bilobalide B and bilobalide C in the weight proportion of 7-27 to 3-20 to 4-40. The ginkgo leaf extract with high effective component content and high purity, and the prepared medicine preparation, especially injection, has obvious curative effect on cardiac and cerebral vascular diseases, clinical use safety and no side effect.
Owner:厦门国宇知识产权研究有限公司
Extractive of ginkgo tree leaves, prepn. method and application thereof
ActiveCN1911257AHigh extraction rateNon-irritatingPowder deliveryNervous disorderFreeze-dryingUltrafiltration
A gingko leaf extract used for preparing liquid injection or freeze-dried powder injection is prepared from gingko leaves through breaking, thermal reflux extracting with the aqueous solution of alcohol, vacuum volatilizing of solvent, depositing in ammonia water to remove polyphenol, extracting with n-hexane to remove ginkgolic acid, adsorbing by macroreticular resin, separating, refining, ultrafiltration and spray drying.
Owner:BEIJING SUNHO PHARMA
Compositions and methods for treating traumatic brain injury
ActiveUS20140170211A1Symptoms improvedPromote healingBiocideKetone active ingredientsL-GlutaminTaurine
The disclosure provides compositions treating traumatic brain injuries such as concussions. In one embodiment, the composition comprises phosphatidylserine, phosphatidylcholine, quercetin, astaxanthin, R-alpha lipoic acid, N-acetyl cysteine, taurine, L-glutamine, carnitine, D-ribose, creatine, epigallocatechin gallate, melatonin, ginkgo leaf extract, curcumin and L-glycine. The disclosure also provides methods for treating traumatic brain injuries such as concussions by administering an effective amount of the compositions described within.
Owner:HAVN LIFE SCI INC
Method for preparing ginkgo leaf extractive
InactiveCN1472206AQuality improvementSolve the real problemOrganic chemistryUnknown materialsAlcoholGinkgolide
Owner:山西振东泰盛制药有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com