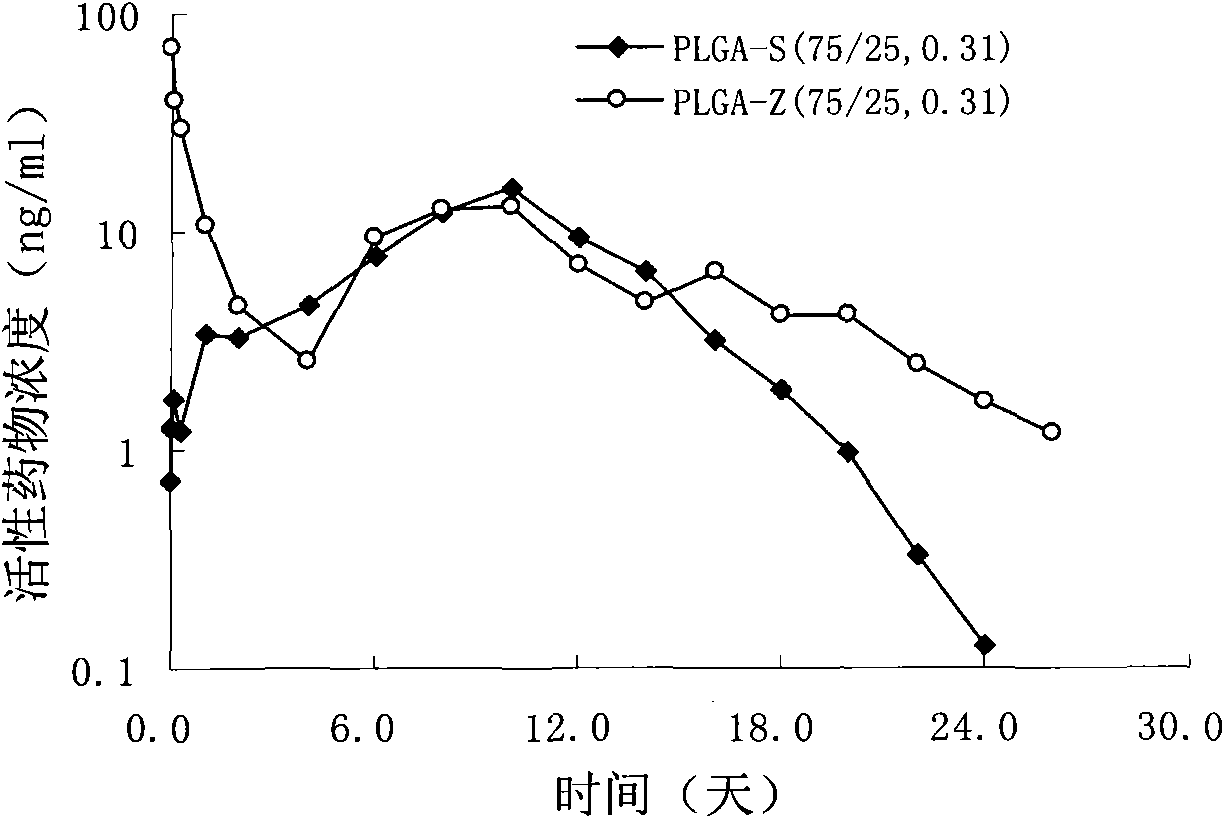
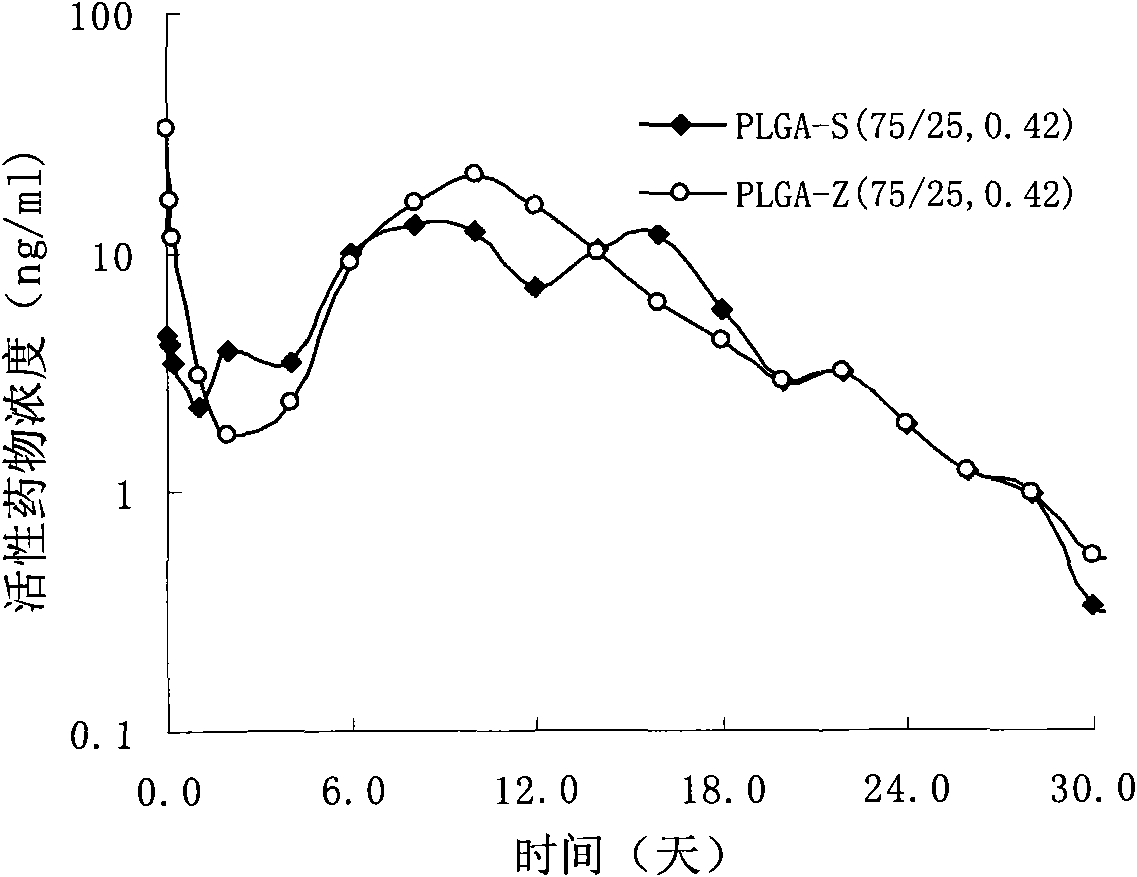
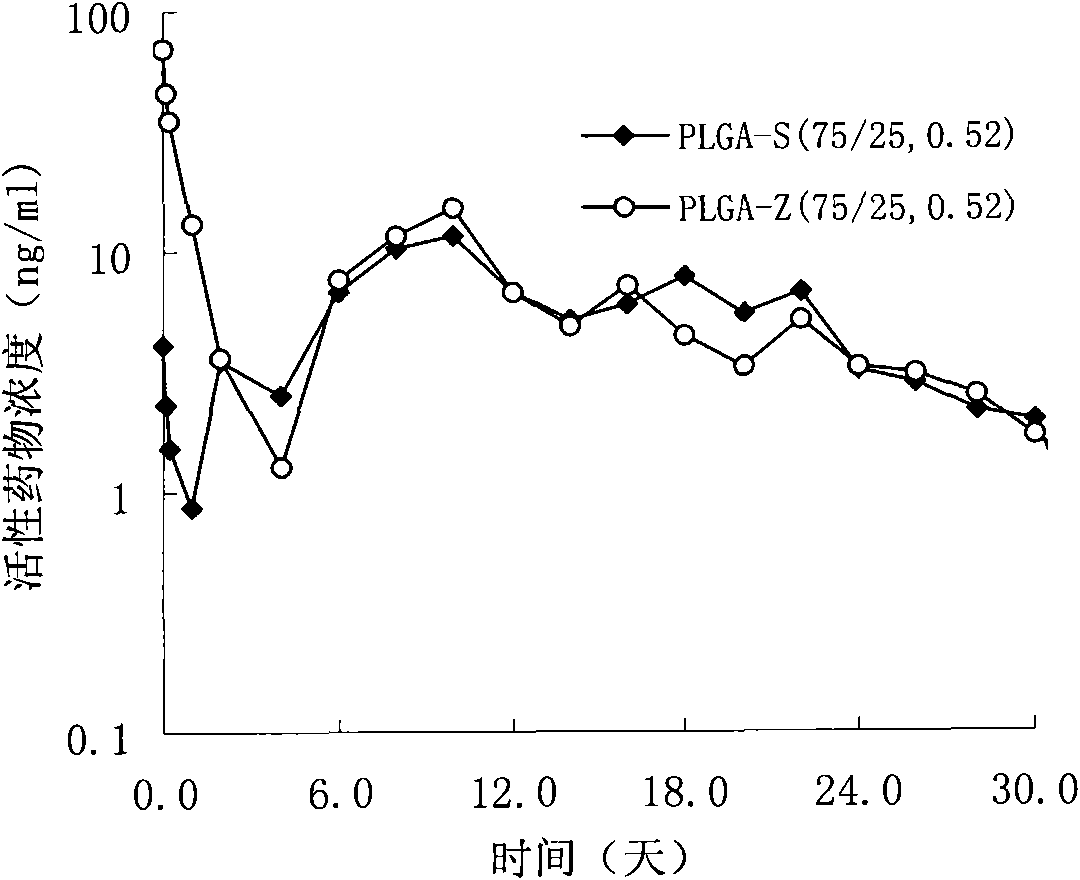
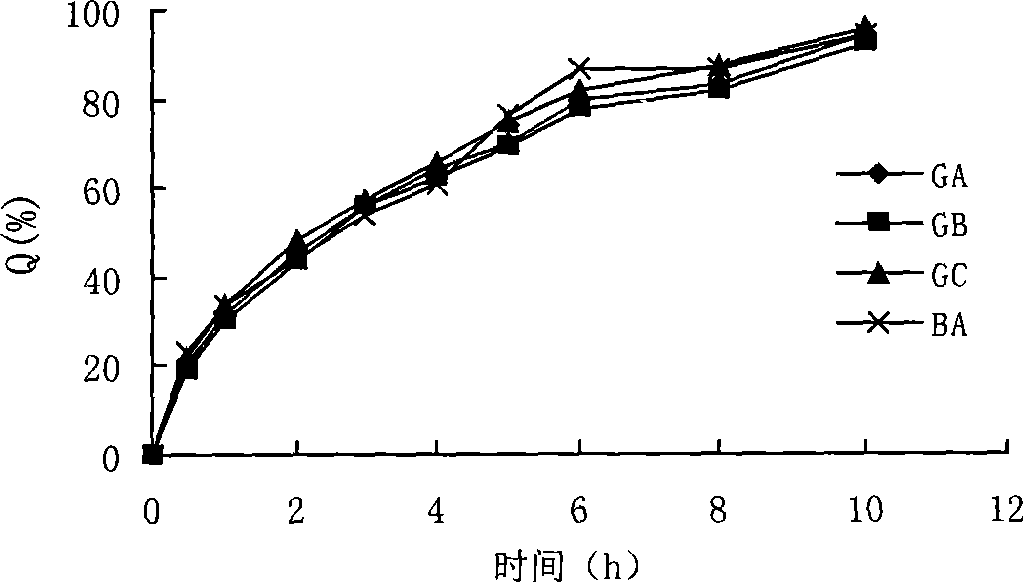
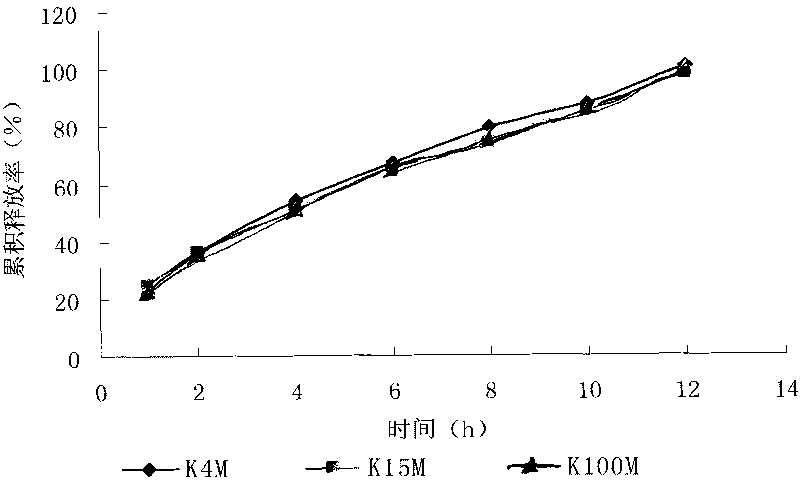
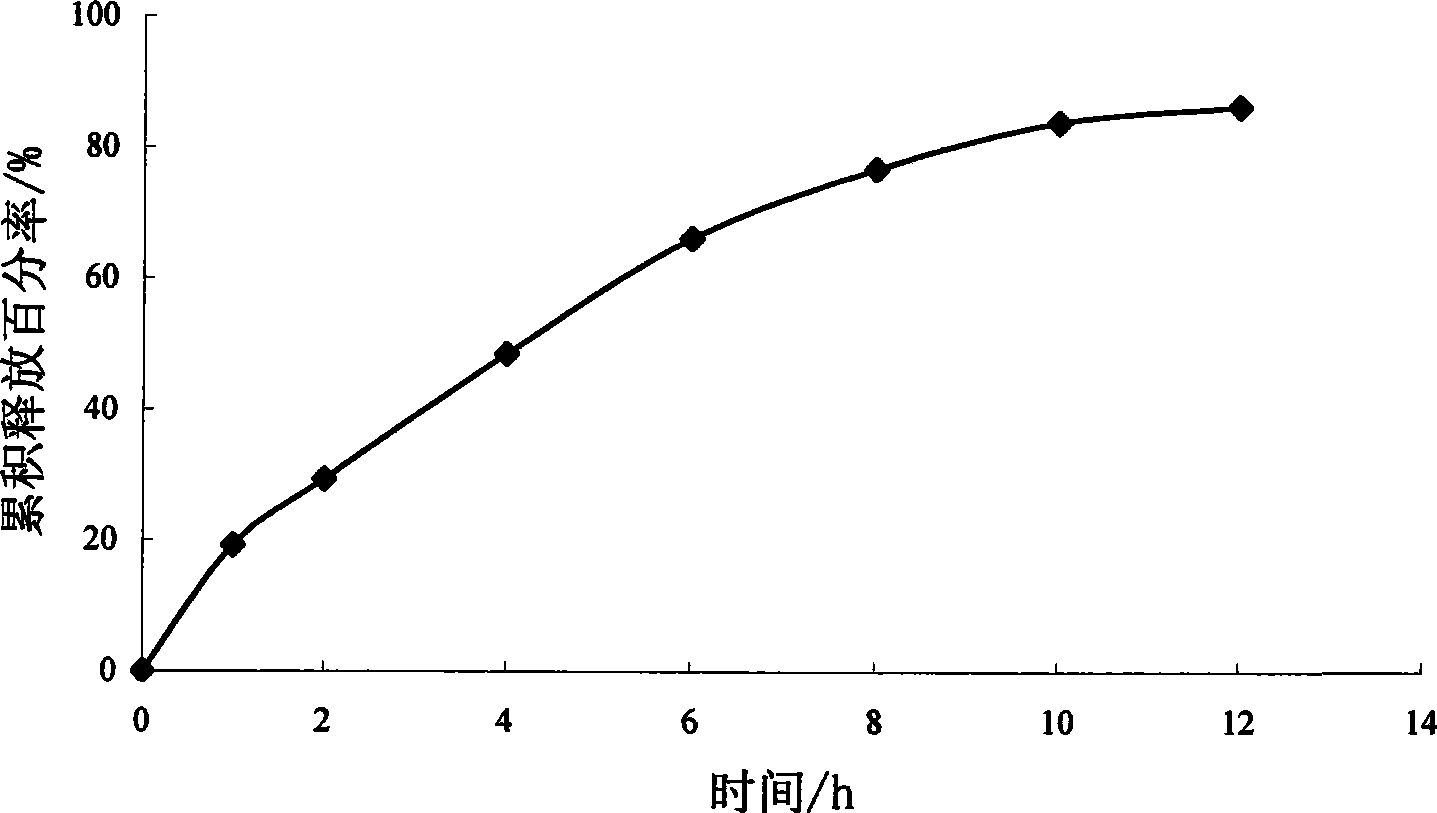
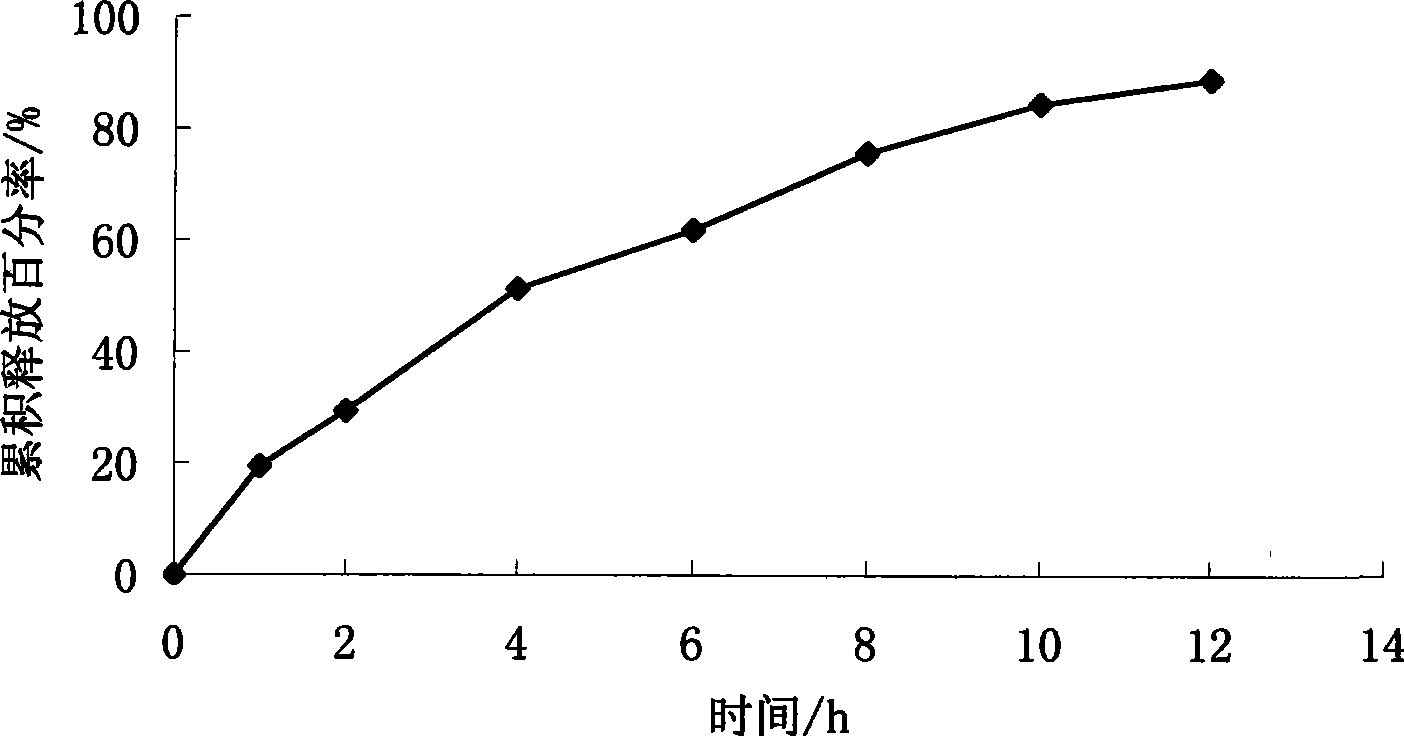
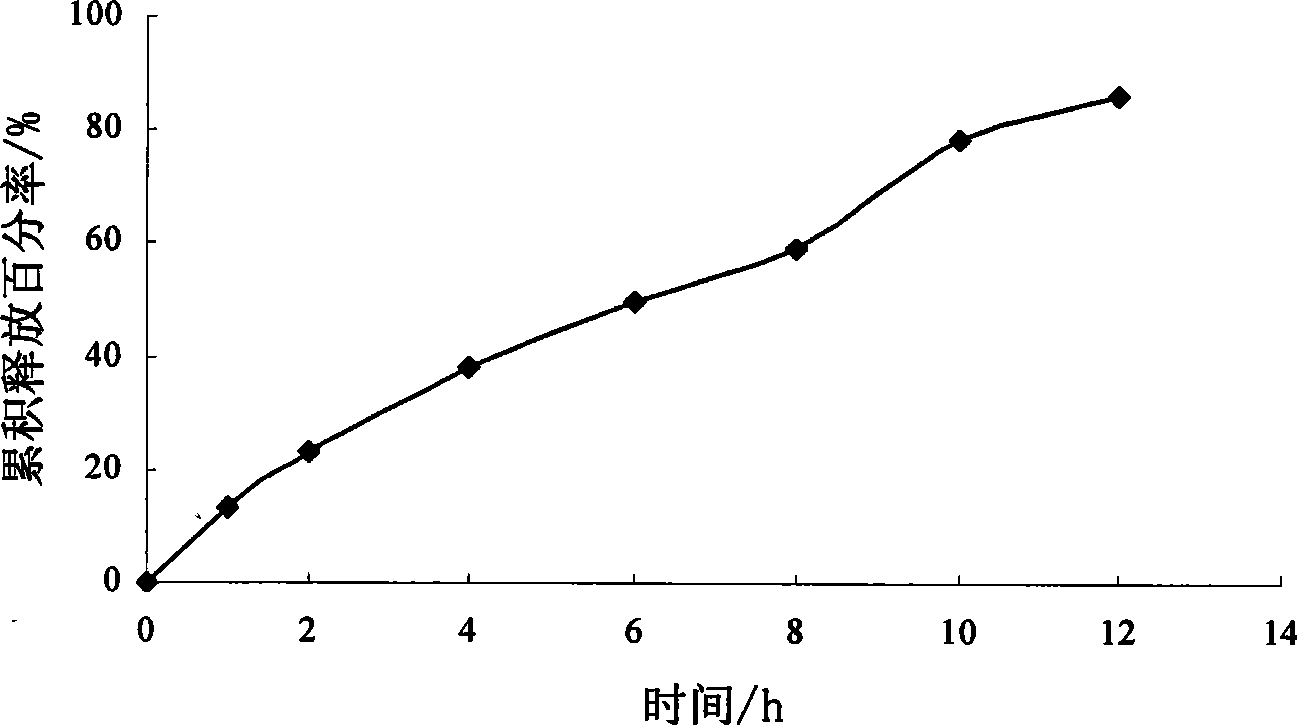
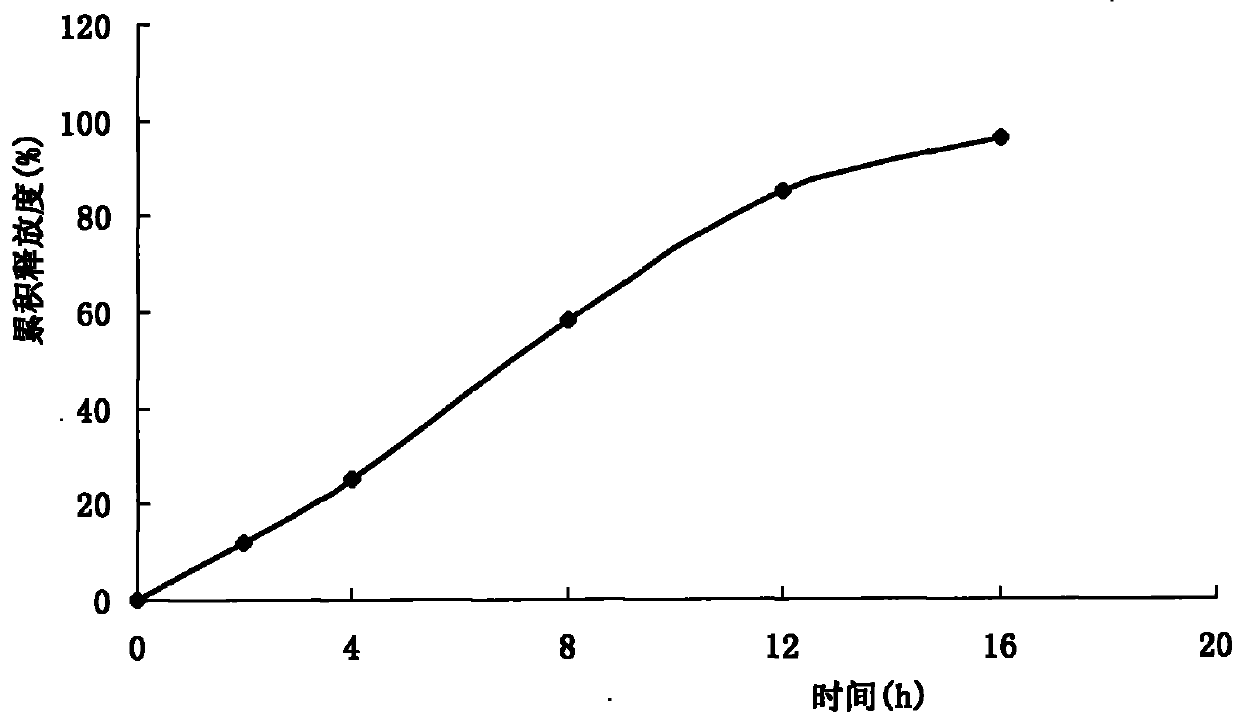
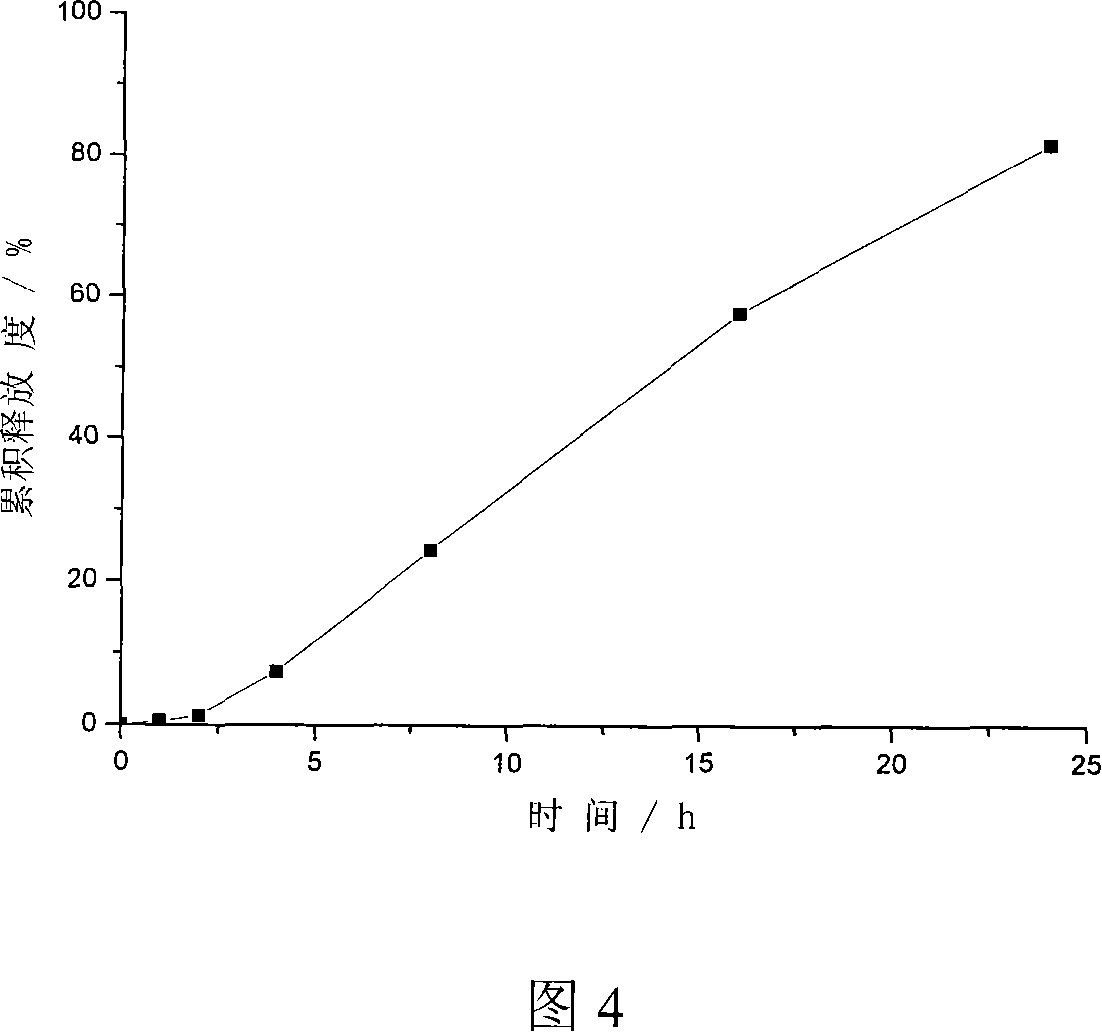
Patents
Literature
644results about How to "Stable blood concentration" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Risperidone slow-release microsphere, preparation method and application thereof
ActiveCN101653422AHigh drug loadingImprove complianceOrganic active ingredientsNervous disorderBlood concentrationMicrosphere
The invention provides a risperidone slow-release microsphere, a preparation method and an application thereof. The microsphere comprises risperidone or 9-hydroxy risperidone or the salt thereof and anon-end-capped lactide-glycollide copolymer. The risperidone slow-release microsphere provided by the invention has higher medicine-carrying quantity, no in-vivo sudden-release phenomenon, stable blood concentration and no medicine release lag period, reduces the administration frequency of a patient greatly, reduces the administration volume of each time, enhances the conformance of the patientand reduces the generation of adverse reactions.
Owner:SHANDONG LUYE PHARMA CO LTD +1
Gingko leaf slow-releasing table and preparation process thereof
InactiveCN1371744AReduce the number of dosesStable blood concentrationUnknown materialsPill deliveryDiseaseAnhydrous ethanol
The preparation method of ginkgo leaf slow-released tablet mainly for curing cerebrovascular disease includes the following steps: 1. preparing gingko leaf extract; and 2. preparing slow-released tablet: using gingko leaf extract 120 portions, hydroxypropyl methyl cellulose 80-100 portions, lactose 50-80 portions and starch 30-80 portions, mixing them uniformly, adding anhydrous ethanol 0.8-1.2 portions to make granulation, then adding magnesium stearate 2-5 portions, uniformly mixing them and tabletting to obtain said invented product.
Owner:YABAO PHARMA GRP CO LTD
Slow/controlled release pellet composition containing ginkgo leaf extracts and preparation method thereof
InactiveCN101375869ASmall toxicityStable blood concentrationGranular deliveryGinkgophyta medical ingredientsSustained release pelletsHard Capsule
The invention belongs to the field sustained / controlled-release preparations, in particular to an oral sustained / controlled-release pellet combination containing ginkgo biloba extract and a preparation method. The oral sustained / controlled-release pellet combination is composed of (A) a core containing a pill; (B) an insulating coating layer; (C) a sustained-release coating layer; (D) and an enteric-coated coating layer. The invention is the traditional Chinese medicine multi-component sustained-release pellet combination which is taken once by 24 hours and the multi-unit sustained-release pellet combined preparation with the different drug release systems, the core containing the pill is prepared by adopting the extrusion pill rolling method, a novel sustained-release multi-layer coating technology and a fluidized bed are utilized for coating the sustained-release pellet, the rapid-release part and the sustained-release part of the coated pellet are mixedly filled into a hard capsule or pressed into a pellet tablet. The sustained-release pellet has stable coating process and good reproducibility, thereby being applicable to the industrial mass production; and the drug quality of the preparation is stable through the long-term storage. The in vitro release test shows that the multiple components of the traditional Chinese medicine can achieve the sustained-release role, the sustained-release preparation can significantly increase the transmembrane absorption and the stability of various effective active ingredients by oral drug administration, the curve of plasma drug concentration in vivo is smooth, and the design purpose of 24-hour sustained-release is achieved.
Owner:CHINA PHARM UNIV
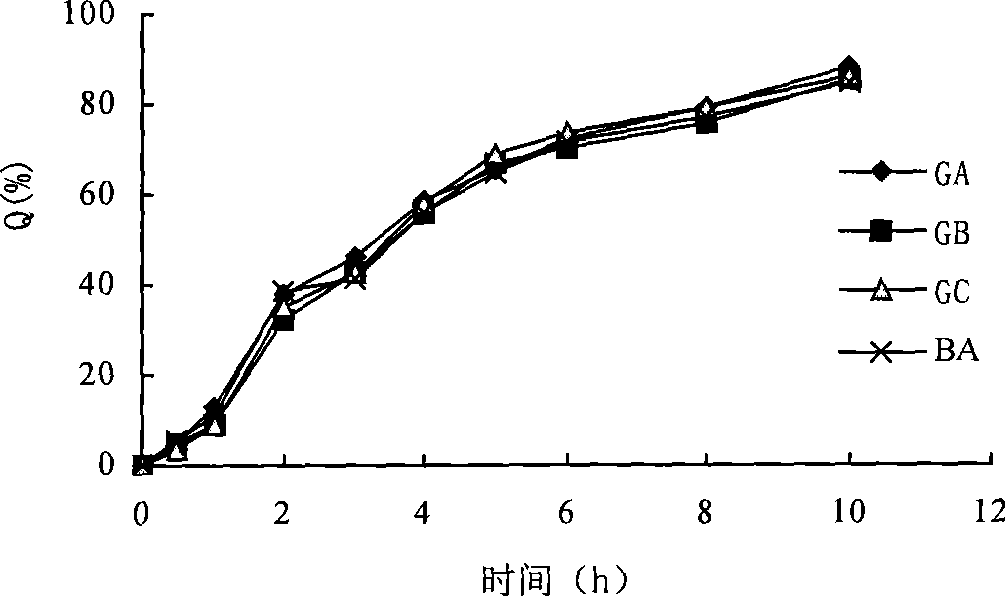
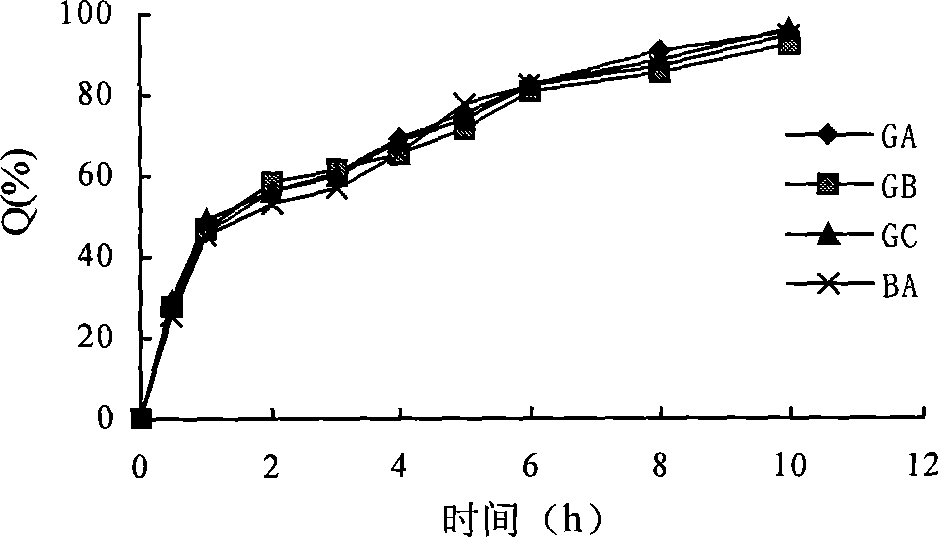
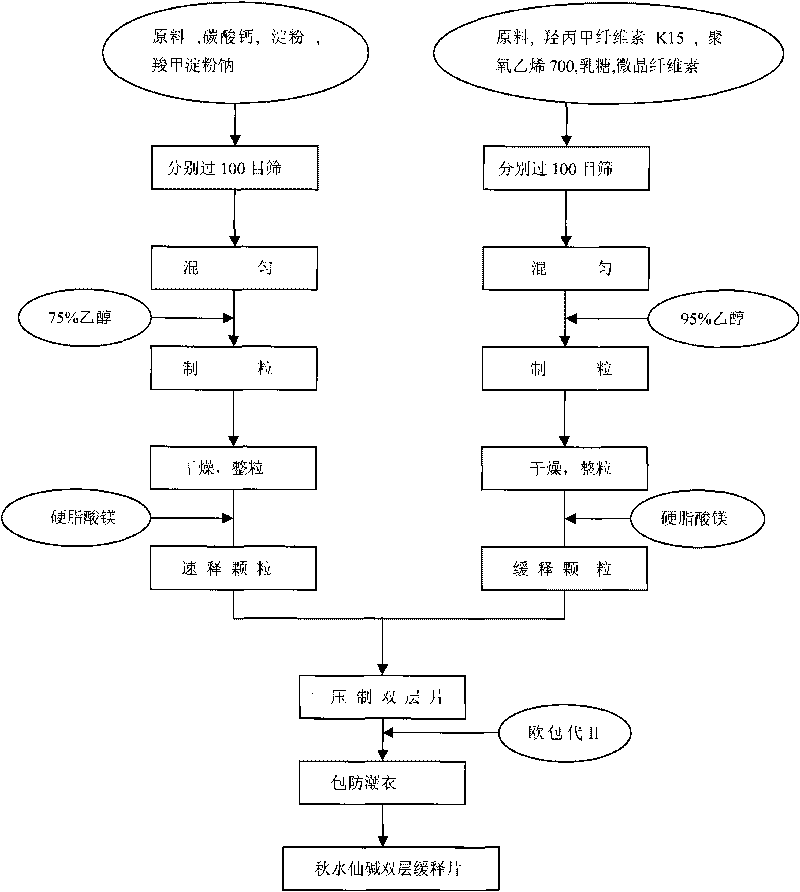
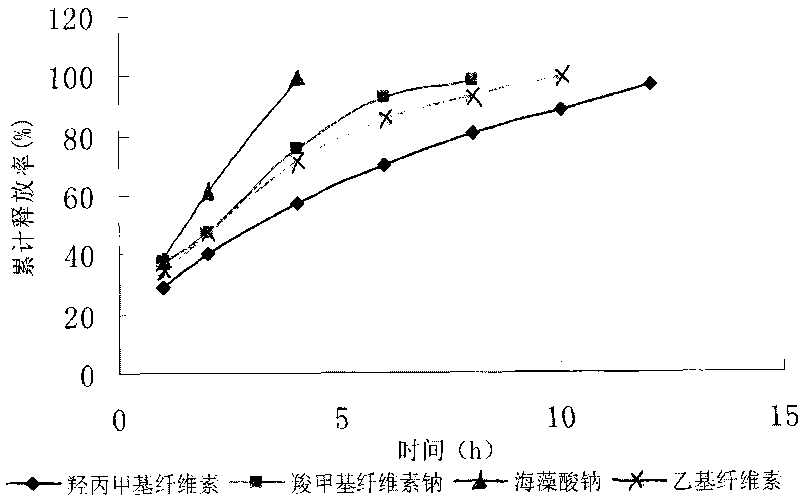
Colchicine bilayer sustained-release tablet and preparing method thereof
InactiveCN101732274AEffective plasma concentration stableStable blood concentrationOrganic active ingredientsPharmaceutical delivery mechanismSustained Release TabletMedicine
The invention discloses a colchicine bilayer sustained-release tablet which comprises a quick release layer and a sustained-release layer, wherein the quick release layer mainly consists of colchicine, a disintegrant, a filler, a lubricant and an adhesive; the sustained-release tablet mainly consists of colchicine, a sustained-release matrix, a retarding agent, a filler and an adhesive; the weight ratio of the colchicine in the quick release layer to the colchicine in the sustained-release layer is (0.25-0.42) : 1; and the weight ratio of the quick release layer to the sustained-release layer is 1: 2 to 1: 4. The invention also discloses a method for preparing the colchicine bilayer sustained-release tablet. The colchicine bilayer sustained-release tablet releases the drugs through the quick release layer to quickly achieve the effective blood drug concentration, slowly releases the drugs through the sustained-release layer to exert and sustain the stable and uniform effective blood drug concentration, reduces the drug taking times and lightens the toxic or side function.
Owner:普尔药物科技开发(深圳)有限公司
Sirolimos sustained and controlled release preparation and preparation method thereof
InactiveCN101361703AOvercome the problems of poor water solubility and narrow therapeutic windowImprove securityOrganic active ingredientsPharmaceutical delivery mechanismSolubilityCyclodextrin
The invention provides a Sirolimus slow controlled release preparation and a preparation method thereof. By adopting the solubilizing methods including a solid dispersion technology or cyclodextrin inclusion technology or adding one or a plurality of surface active agents after micronizing drugs and the like, the solubility is dramatically improved and further the bioavailability is improved, therefore, a matrix type slow controlled release preparation is made by adding one or a plurality of framework materials and other accessories, or a diaphragm-controlled or osmotic pump type slow controlled release preparation is made by adopting slow controlled release materials for coating. The Sirolimus slow controlled release preparation has better solubility and dissolution rate, high bioavailability as well as slow controlled release effect, thus being capable of maintaining steady blood concentration, bringing down the concentration of peak, reducing the occurrence of adverse reaction and improving the safety of clinical medicines, in addition, the raw materials can be acquired easily, the preparation process is simple and feasible with high yield rate and low cost, and the large-scale industrial production can be realized and the economic benefit is marked.
Owner:宋洪涛
Method for preparing an amlodipine microsphere
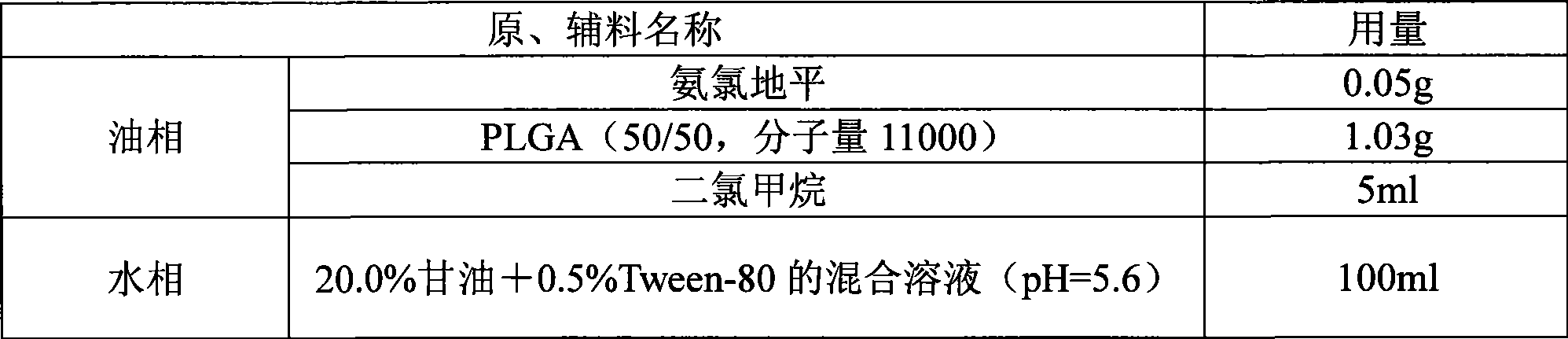
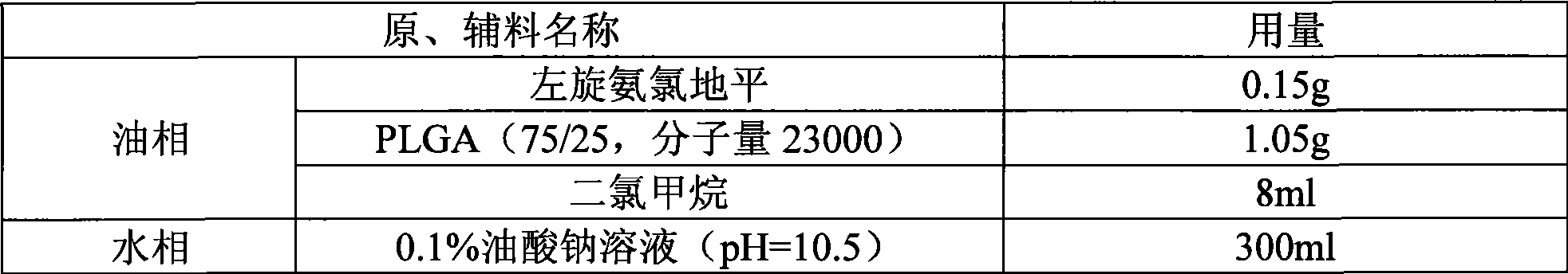
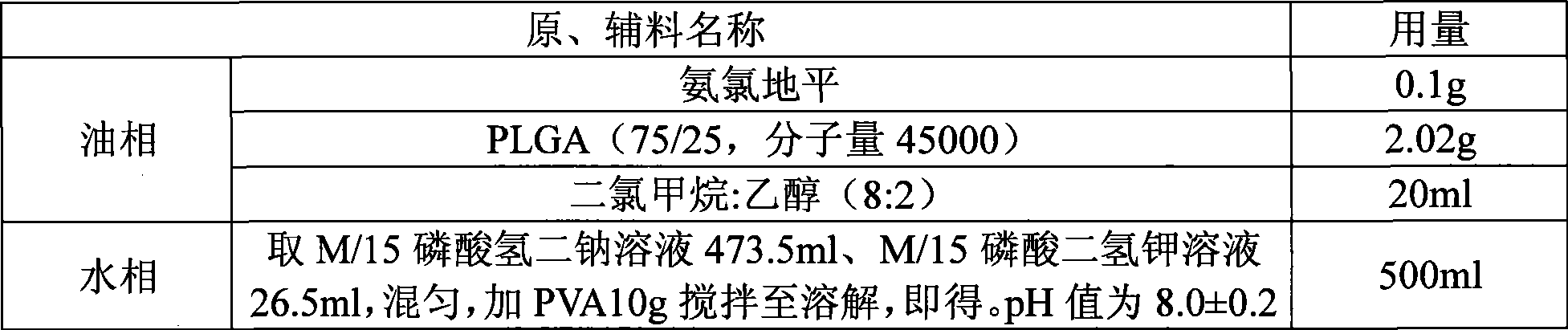
InactiveCN101530396ARound shapeUniform particle size distributionOrganic active ingredientsPharmaceutical non-active ingredientsCelluloseMicrosphere
The method discloses a method for preparing an amlodipine microsphere. Medicament wrapped by the prepared amlodipine microsphere is amlodipine and organic acid salts of the amlodipine; a carrier material of the microsphere is polylactic acid (PLA), a polylactic acid-glycolic acid copolymer (PLGA), a polylactic acid-glycol block copolymer (PLA-mPEG) or other biodegradable materials; a surfactant solution, a monosaccharide or amylose solution, a polyalcohol solution, a cellulose solution and a colloid solution are used as a dispersion medium; and through an emulsion solvent evaporation method, the amlodipine microsphere is prepared under the mechanical stirring or high-speed shearing action. The microsphere has a round shape and even distribution of grain diameter; the grain diameter is within the range between 1 and 125 mu m; the medicine loading capacity can reach more than 1.5 percent; and the encapsulating rate is more than 70 percent.
Owner:XIAN LIBANG PHARMA TECH
Chinese herbal medicine preparation
ActiveCN104117083AHas a long-lasting sustained-release effectStable blood concentrationPillowsPowder deliveryMedicineCurative effect
The invention relates to a pharmaceutical composition which is stored in bedding articles and capable of enabling people to obtain curative effects of tranquilizing and allaying excitement. The Chinese herbal medicine preparation is powder or granule which is prepared by taking one or more of lavender, twinings, jasmine flower and liquorice as materials. According to the invention, a scientific process is adopted for preparing one or more of lavender powder, twinings powder, jasmine flower powder and liquorice powder into powder with a proper grain size or into a similar pharmaceutical granule. The substances with effects of tranquilizing and allaying excitement have effects of slowly releasing for a long time.
Owner:汪建
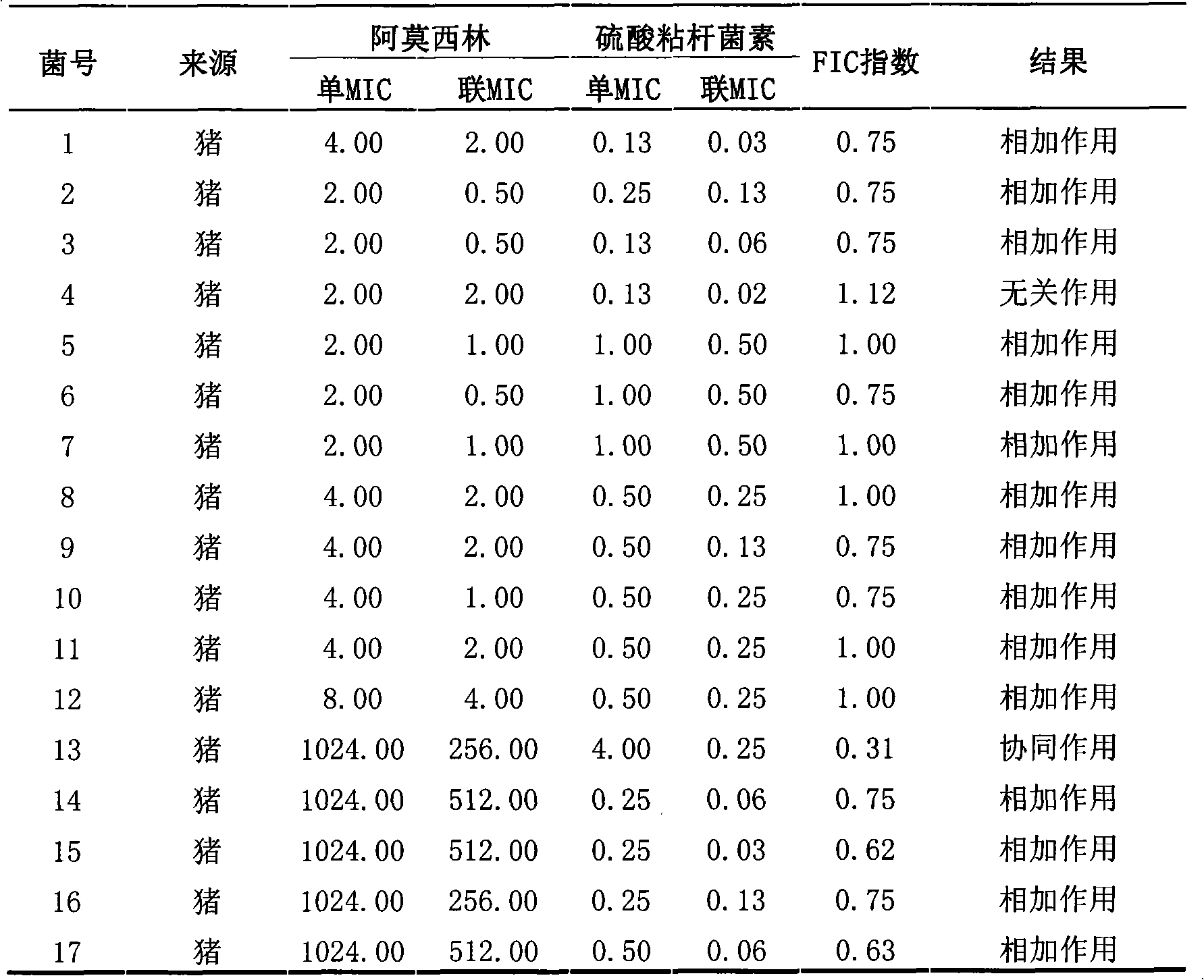
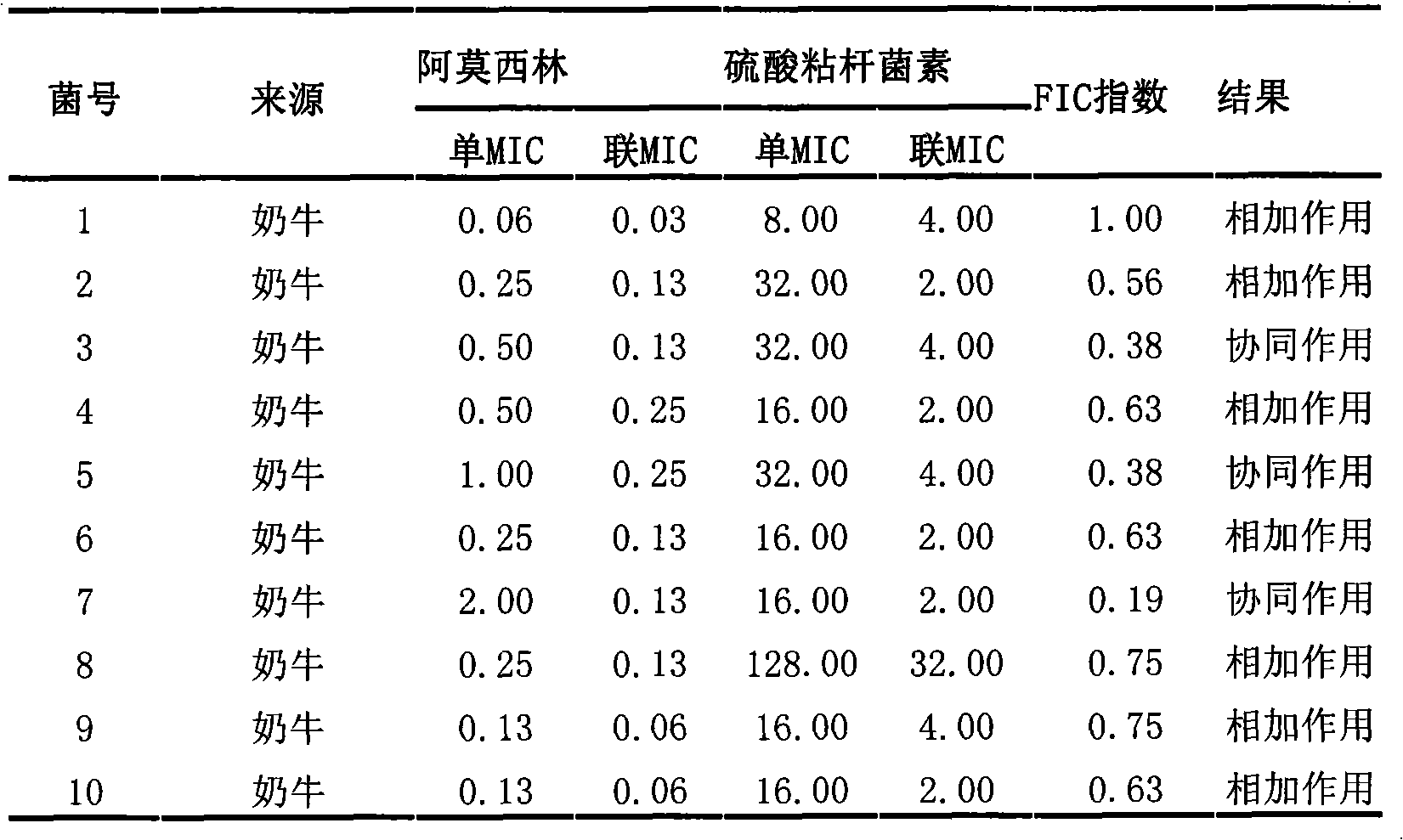
Veterinary suspension containing amoxicillin, colistin sulfate and prednisolone and preparation method thereof
InactiveCN101953784AExpanded antimicrobial spectrumHigh antibacterial efficacyAntibacterial agentsSolution deliveryPrednisoloneColistin Sulfate
The invention discloses a veterinary suspension containing amoxicillin, colistin sulfate and prednisolone and a preparation method thereof. The veterinary suspension adopts the amoxicillin, the colistin sulfate and the prednisolone as active ingredients. The preparation method of the veterinary suspension containing the amoxicillin, the colistin sulfate and the prednisolone comprises the following steps of: (1) dissolving or dispersing a suspending agent, an antioxidant and a preservative in a hot dispersion medium to obtain a solution (A); (2) adding 40-80 percent of dispersion medium in a formula ratio in a colloid mill, starting the colloid mill, then slowly adding the solution (A) and adding a wetting agent while stirring after the solution (A) is fully added; (3) sequentially adding the amoxicillin, the colistin sulfate and the prednisolone after fully adding all the accessories, and grinding by adopting two alternate modes, i.e. an endless grinding mode and a non-endless grinding mode; and (4) detecting the grain fineness, stopping grinding when the grain fineness accords with the requirement, adding the dispersion medium to the formula ratio, mixing, canning, sealing and sterilizing to obtain the veterinary suspension containing the amoxicillin, the colistin sulfate and the prednisolone.
Owner:CHINA AGRI UNIV +1
Tulobuterol containing pressure-sensitive adhesive, transdermal paster, and its preparing method and use
ActiveCN1640388ALow costImprove productivityOrganic active ingredientsRespiratory disorderTransdermal patchPolymer science
The present invention discloses a transcutaneous plaster containing tulobuterol, its preparation method and application. The pressure-sensitive adhesive used by said invention is a hot-melt pressure-sensitive adhesive whose melting point is 50-250 deg.C. the described hot-melt pressure-sensitive adhesive is an adhesive made up by using thermoplastic polymer as main raw material, and hs the hot-melting and pressure-sensitive double characteristics. Said product contains no organic solvent, and its coating speed is quick, and its cost is low.
Owner:YABAO PHARMA GRP CO LTD
Allopurinol dual-release preparation and preparation method thereof
ActiveCN102091051AInsufficient improvementEffective plasma concentrationOrganic active ingredientsSkeletal disorderSpecific gravityAdministration time
The invention relates to an allopurinol dual-release preparation and a preparation method thereof, which is characterized in that the dual-release preparation consists of a quick-release part and a slow-release part, wherein the ratio of the base allopurinol contained in the quick-release part to the base allopurinol contained in the slow-release part is 1:1 to 1:24. The invention belongs to the technical field of medicine preparation and aims at providing the allopurinol dual-release preparation with good patient compliance and small side effect and the preparation method thereof, wherein the allopurinol dual-release preparation has the advantages of taking effect quickly and maintaining effective blood concentration stably. The dual-release preparation can not only be used for reducing the side effect on the gastrointestinal tract, but also decreasing the administration times of a patient and adverse effect and promoting the patient compliance.
Owner:COSCI MED TECH CO LTD
Transdermal plaster of rivastigmine and preparation process thereof
InactiveCN1994290AEasy to useClear curative effectOrganic non-active ingredientsEster active ingredientsTransdermal patchRivastigmine
The invention relates to a method for preparing kabalatin adhere agent for treating senile dementia, wherein said adhere agent can stably hold blood drug density and reduce feeding time, with high safety.
Owner:SHANGHAI INST OF PHARMA IND CO LTD
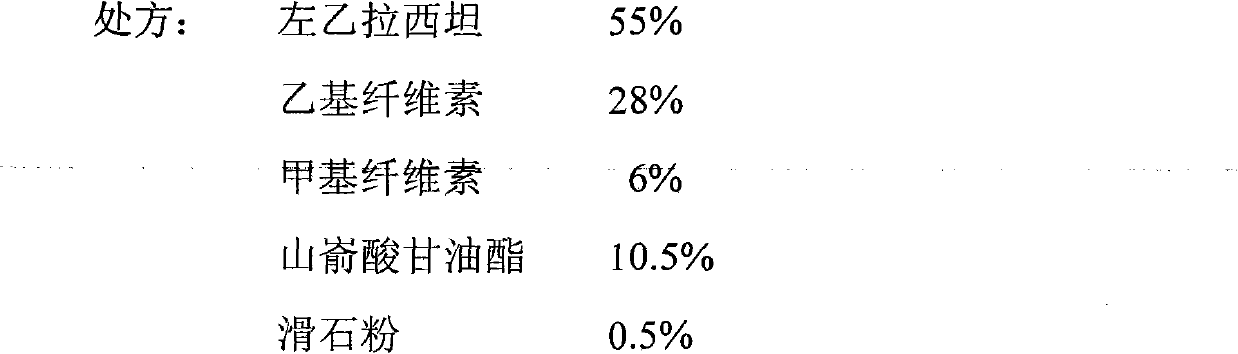
Levetiracetam sustained-release tablets and preparation method thereof
InactiveCN102068414AStable blood concentrationProlong the action timeOrganic active ingredientsNervous disorderSide effectOral medication
The invention discloses levetiracetam sustained-release tablets and a preparation method thereof. The levetiracetam sustained-release tablets consist of levetiracetam, a filler, a sustained-release agent, an adhesion agent and a lubricant according to the weight ratio of 100: 30: (3-60): (30-70): 1. The preparation method comprises the following steps of: pelletizing by the wet method, drying the granules, arranging the granules and adding a proper quantity of lubricant, uniformly mixing and pressing to obtain the levetiracetam sustained-release tablets. Compared with the common preparation, the levetiracetam sustained-release tablets prepared by the preparation method have the advantages of small blood concentration fluctuation range, reduction in toxin and side effect and increase in biddability of patients by oral administration once every day. The sustained-release preparation will be clinically applied to additional treatment on partial seizure of epilepsia patients.
Owner:HUZHOU LLISSY BIOLOGY TECH +1
Diclofenac sodium hydrogel microballoon with pH sensitivity, preparation method and application thereof
InactiveCN102018674ANo adverse reactionAvoid morning stiffnessOrganic active ingredientsAntipyreticStomach mucous membraneRheumatism
The invention relates to a diclofenac sodium hydrogel microballoon with pH sensitivity, a preparation method and application thereof. In the invention, chitosan and sodium alginate are used as drug carriers to prepare a diclofenac sodium hydrogel microballoon slow release preparation; the mass ratio of a used diclofenac sodium drug to the chitosan to the sodium alginate is (1-3):(5-8):(5-8); and the chitosan is N-succinyl-chitosan. The microballoon preparation can durably develop the drug effect to ensure that diclofenac sodium can be regularly and quantitatively released for a long time; after the microballoon preparation is taken, the blood concentration is stable, the fluctuation is small, and the duration time is long; the microballoon preparation has pH sensitivity, thereby irritations and toxic hazards to the stomach mucous membrane, which are caused by directly and orally taking diclofenac sodium tablets, are reduced; and the microballoon preparation has good effects of cooling down and easing pains and is suitable for various rheumatisms, rheumatoid arthritis, lupus erythematosus, ankylosing spondylitis, various pains caused after operations, fevers caused by various reasons, and the like.
Owner:TONGJI UNIV
Aspirin enteric-coated pellet
ActiveCN101596166AEasy accessAvoid stimulationOrganic active ingredientsAntipyreticBlood concentrationBioavailability
The invention discloses an aspirin enteric-coated pellet which comprises the following components in percentage by weight from inside to outside: 5-22 blank core pellet, 55-75 medicinal layer consisting of aspirin and medicinal excipients and 20-35 enteric-coated layer. The invention also discloses a method for preparing the aspirin enteric-coated pellet and an aspirin enteric-coated capsule containing the aspirin enteric-coated pellets. The aspirin enteric-coated capsule has the advantages of good stability, small stimulation, steady blood concentration, high bioavailability, and the like.
Owner:Yung Shin Pharm Ind (Kunshan) Co Ltd
Levetiracetam slow release pellet capsule preparation and preparation method thereof
InactiveCN101647789AImprove liquidityReduce or eliminate irritationNervous disorderPharmaceutical delivery mechanismMass compositionSide effect
The invention relates to a levetiracetam slow release pellet capsule preparation and a preparation method thereof. The levetiracetam slow release pellet capsule preparation comprises the following components in percentage by mass: 50-70 percent of levetiracetam, 15-30 percent of blank pellet, 5-10 percent of hydroxypropylmethyl cellulose or polyvidone, 7-15 percent of ethylcellulose or acrylic resin, 1-8 percent of talc powder and 0.5-2 percent of cataloid. The preparation method comprises the following steps: coating levetiracetam fine powder on the blank pellet by a binding agent to be madeinto a medicine-contained pellet; coating an isolating layer on the medicine-contained pellet; coating a slow release coating film on the medicine-contained pellet coated with the isolating layer to prepare a slow release pellet; and mixing the slow release pellet, the talc powder and the cataloid and filling the mixture into a capsule. The levetiracetam is made into the slow release capsule preparation by a pellet and slow release technology, and the slow release preparation can stabilize blood medicine concentration, reduce the generating frequency and degree of the side effect and fundamentally solve the problems of great influence on a tablet by gastric pyloric sphincter and big differences of gastric emptying individuals.
Owner:天津药物研究院药业有限责任公司
Stomach detention sustained and controlled release medicament releasing system and preparation method
ActiveCN101371822AEasy to swallowImprove complianceSurface/boundary effectCarbohydrate active ingredientsControlled releaseOrganic acid
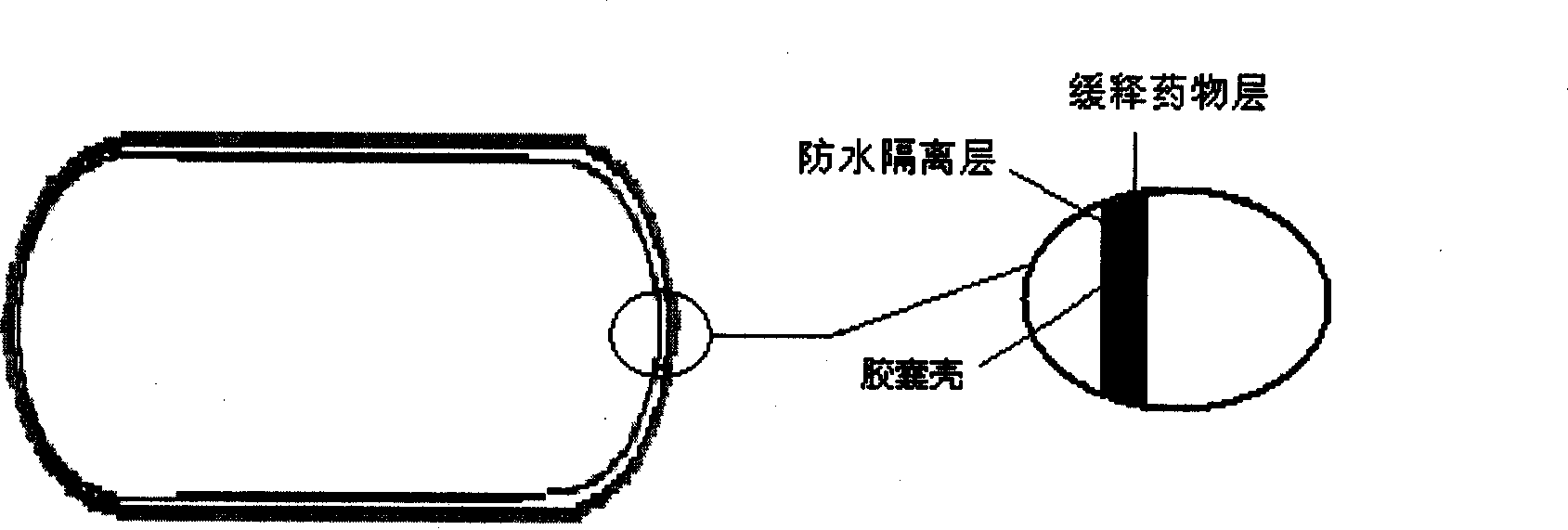
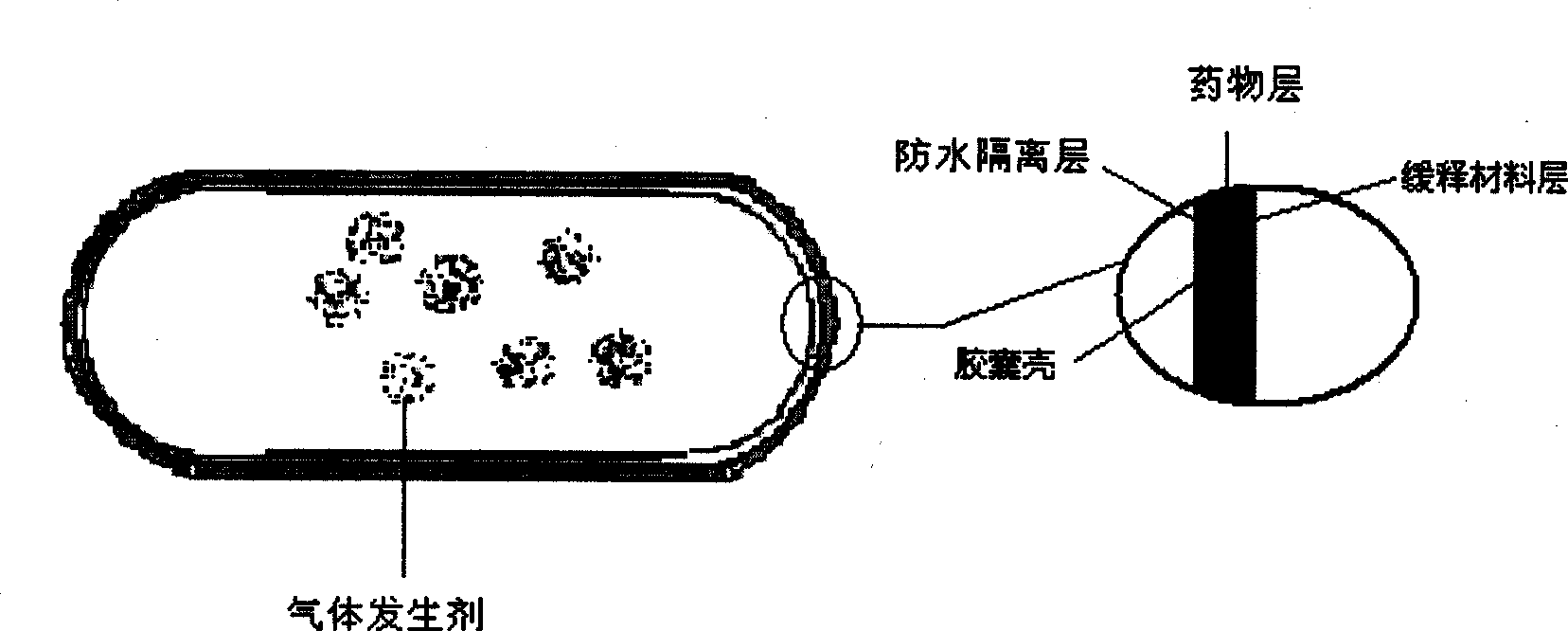
The invention provides a new air sac type gastric retention controlled-release medicine release system, including (1) an air sac which is formed by wrapping high molecular film-forming materials and hydrophobic materials outside a hollow sac; (2) a medicine containing layer which is composed of medicines and pharmaceutically acceptable excipient; the medicine containing layer covers the outside of the air sac and includes a medicine controlled release layer or a medicine slow release layer. If necessary, a quick release layer, which is composed of medicines and excipient and covers the outside of the controlled release layer or the slow release layer, can also be included. The air sac can also be internally filled with a certain amount of gas generant which includes carbonate and pharmaceutically acceptable organic acid. The average density of the whole formulation can be generally controlled below 0.6 / cm<3> and is obviously superior to the present gastro-floating formulation on sale, and the floating time in the stomach is rather longer than a common gastro-floating formulation.
Owner:北京天衡药物研究院有限公司
Gliclazide tablet and preparation method thereof
ActiveCN103191077AReduce the impactImprove solubilityMetabolism disorderSulfonylurea active ingredientsPolyvinyl acetateSide effect
The invention discloses a gliclazide tablet. The gliclazide tablet is directly tabletted by gliclazide solid dispersoid particles and auxiliary materials accepted pharmaceutically; and the gliclazide solid dispersoid particles are prepared according to the following method: citrate and polyvinyl acetate are heated and molten in a hot melt extruder, then gliclazide is added inside to melt, and the molten liquid is extruded and granulated. The preparation method of the invention is simple in preparation production technology; a patient just takes the medicine once every day, the active components slowly release in the body of the patient, and the plasma concentration is steady; and the gliclazide has long effectiveness, so that the times of administration are reduced, the toxic and side effects are lowered, and the treatment cost is greatly reduced.
Owner:广东彼迪药业有限公司
Metformin hydrochloride sustained-release tablets and preparation method thereof
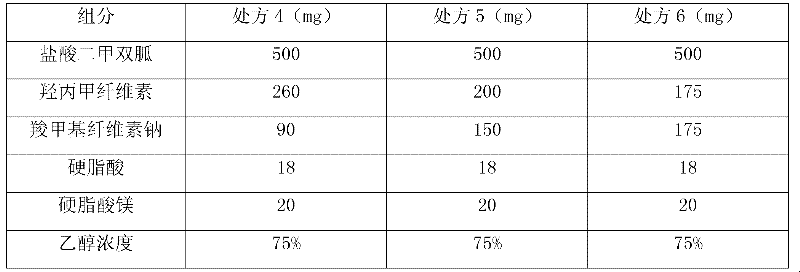
ActiveCN102440975AStable blood concentrationExtended half-lifeOrganic active ingredientsMetabolism disorderCarboxymethyl celluloseHalf-life
The invention discloses metformin hydrochloride sustained-release tablets which are characterized in that: every 10000 tablets are prepared from medicine materials and auxiliary materials of, by weight: 5000g of metformin hydrochloride, 1750g of hypromellose, 1750g of sodium carboxymethyl cellulose, 180g of stearic acid, 200g of magnesium stearate, and an appropriate amount of 75% ethanol. The metformin hydrochloride sustained-release tablets can be slowly released in vivo. With the tablets, stability of blood drug level can be maintained, and a half-life period is prolonged. The tablets are safe, can be used for treating type II diabetes, and are advantaged in high efficiency, low toxicity, and convenient administration. The invention also provides a preparation method of the metformin hydrochloride sustained-release tablets.
Owner:ZHEJIANG WAN SHENG PHARMA CO LTD
Venlafaxine hydrochloride controlled release tablets and preparation method thereof
ActiveCN101953822AReduce manufacturing costStable blood concentrationOrganic active ingredientsNervous disorderSustained-Release PreparationsSide effect
The invention discloses venlafaxine hydrochloride controlled release tablets and a preparation method thereof. The venlafaxine hydrochloride controlled release tablets are elementary osmotic pump (EOP) controlled release tablets which comprise single-layer tablet cores, insoluble semi-permeable membranes and medicament release holes. In the invention, the high-molecular-weight ethylene oxide, microcrystalline cellulose and sodium chloride three auxiliary materials mixed in a proper ratio form a balance system for medicament dissolution and osmotic pressure generation in a pump chamber, and tablet weight is adjusted by using detrix as a filler, so that a high-medicament-capacity osmotic pump preparation can be obtained and the stable and lasting release can be kept. The preparation effectively overcome the shortcomings that the conventional sustained-release preparation is limited in the stability and controllability of the medicament release and can generate stable and constant-speed release, and the medicament release is protected from the influences of stomach and intestine environments, so that individual difference is reduced and the medicament controlled release design aim of giving play to curing effects and reducing side effects is further fulfilled.
Owner:HEFEI LIFEON PHARMA
Controlled release dosing medicine core composition and osmotic pump preparation comprising medicine core composition
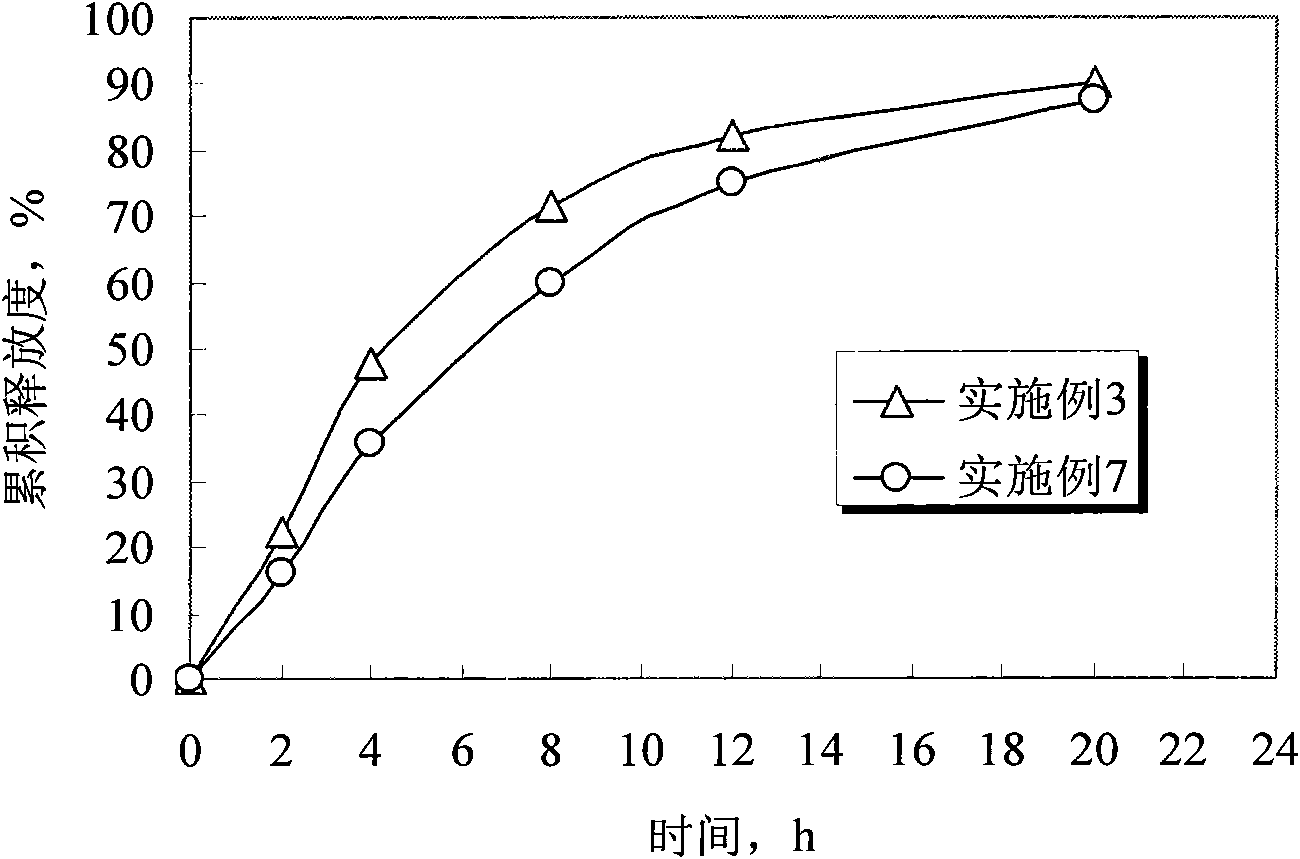
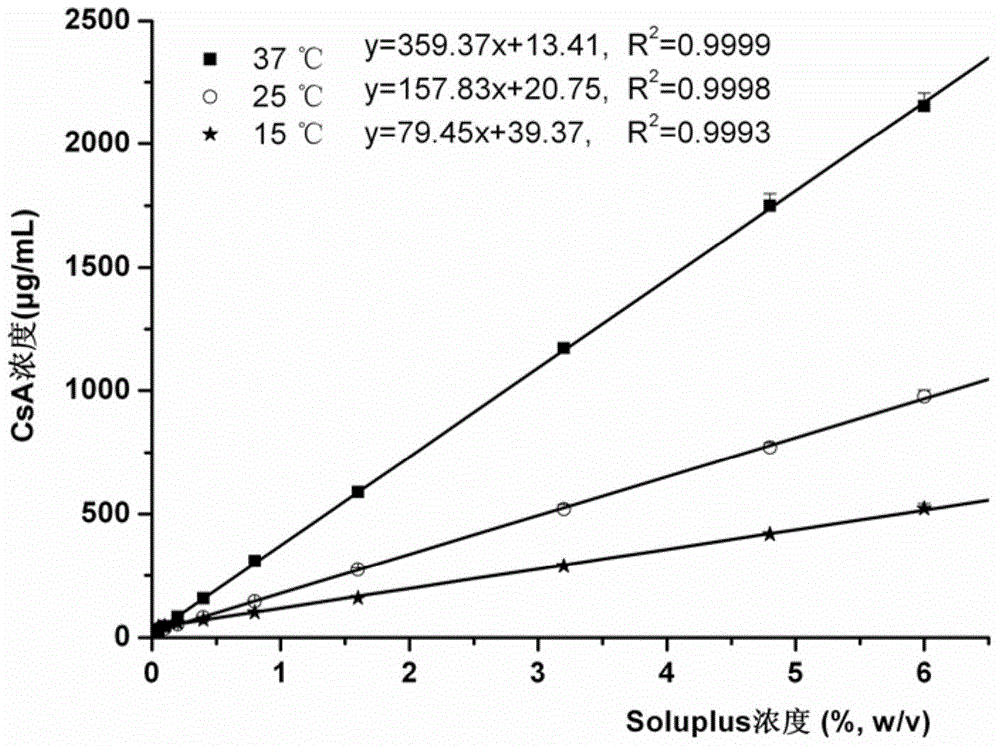
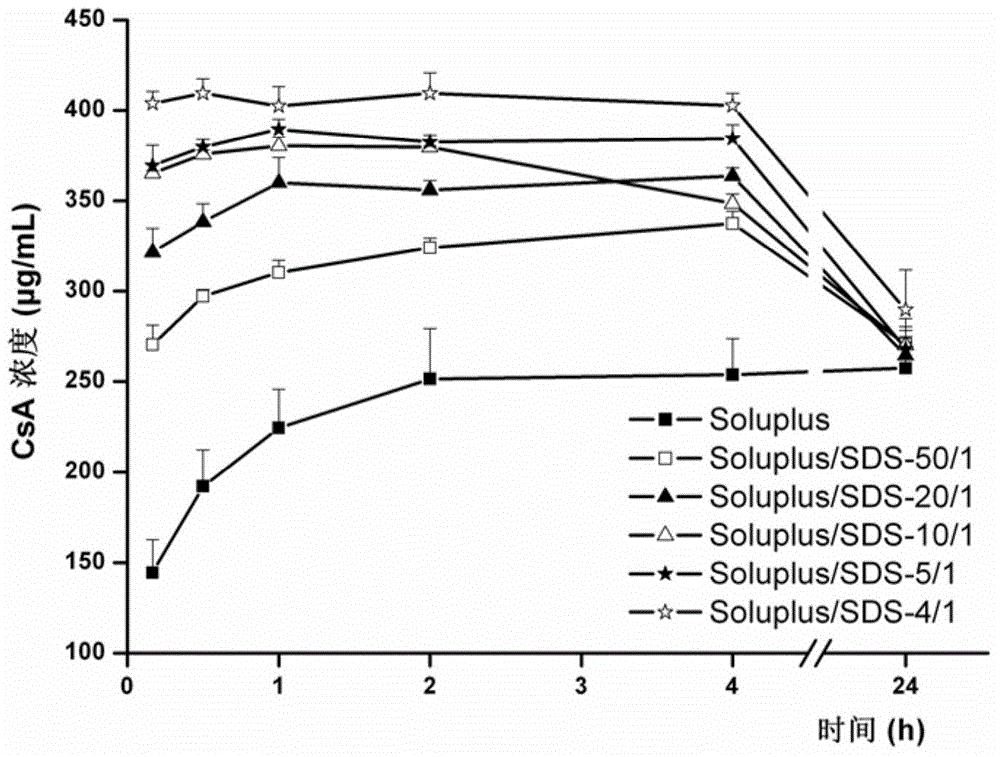
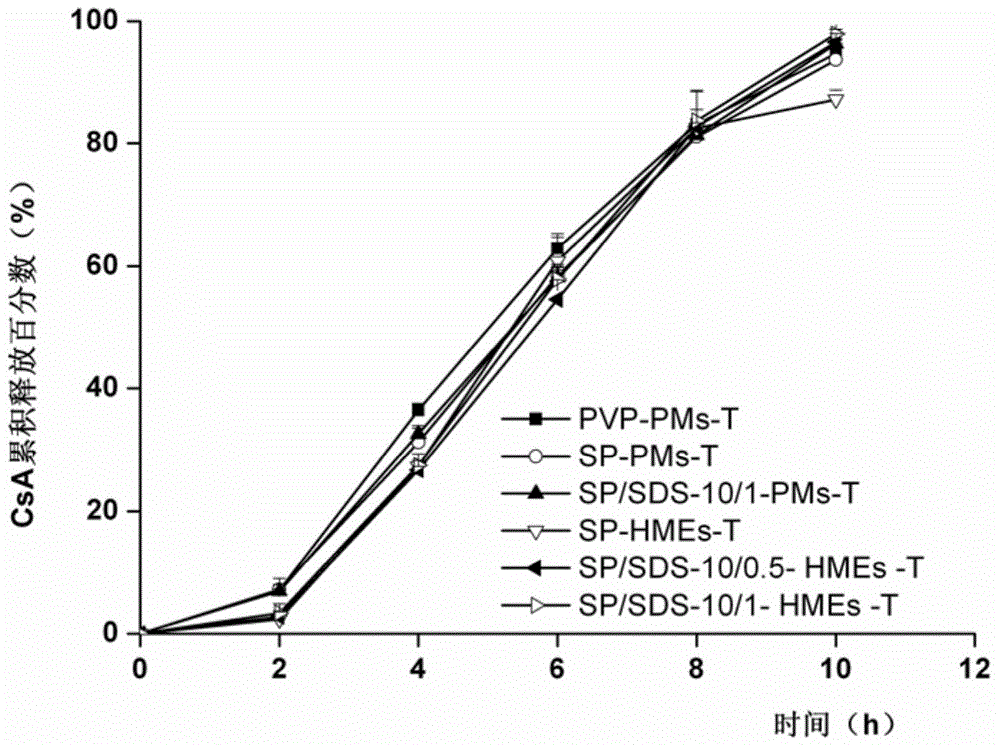
ActiveCN104857515AAchieve zero-order drug releaseStable blood concentrationPharmaceutical non-active ingredientsDrageesSolubilityMedicine
The invention provides a controlled release dosing medicine core composition and an osmotic pump preparation comprising the medicine core composition. The medicine core composition comprises, by weight, 100 parts of an amphiphilic polymer carrier Soluplus, 5-200 parts of medicines, 0-50 parts of a surface active agent, 0-200 parts of a framework material having the controlled release effect, 100-200 parts of an osmotic pressure accelerant having the controlled release effect and 0-20 parts of other auxiliary materials acceptable in pharmacy; the low-solubility medicines are medicines with the in-water solubility smaller than or equal to 1 mg / mL and comprise indissolvable or insoluble medicines with the solubility smaller than or equal to 0.1 mg / mL in water and medicines with the solubility larger than 0.1 mg / mL and very slightly soluble medicines smaller than or equal to 1 mg / mL; the surface active agent is an anion surface active agent; and the other auxiliary materials acceptable in pharmacy comprises one or more kinds of a bonding agent, a coloring agent and a lubricating agent.
Owner:SHANGHAI INST OF MATERIA MEDICA CHINESE ACAD OF SCI
Human serum albumin complex hydrophobically modified pullulan nanoparticles and preparation method thereof
InactiveCN102145173AReduce releaseStable blood concentrationSteroidsGranular deliveryPullulanCholesterol
The invention relates to human serum albumin complex hydrophobically modified pullulan nanoparticles and a preparation method thereof. Hydrophobic group cholesterol, pullulan polysaccharides, succinic anhydride and human serum albumin are taken as raw materials for synthesis; the specific synthesis process is as follows: enabling the cholesterol and the succinic anhydride in the mass ratio of (1-2.5): 2 to react to firstly synthesize succinic anhydride cholesterol, and enabling the succinic anhydride cholesterol to react with the pullulan polysaccharides to synthesize cholesterol hydrophobically modified pullulan polysaccharides in the presence of a catalyst, wherein the mass ratio of cholesterol succinate (CHS) to sugar moiety of the pullulan polysaccharides is (1-4): 20; and performing further self-assembly on the hydrophobically modified pullulan polysaccharides to form a solution of the nanoparticles, then incubating the nanoparticles and the human serum albumin in a shaker for 9 hours, and finally getting the human serum albumin complex hydrophobically modified pullulan nanoparticles. The nanoparticles after complexation of the human serum albumin and the hydrophobically modified pullulan polysaccharide nanoparticles are shaped like spheres, and can maintain slow release effect of the medicament-loaded nanoparticles in a long lasting manner and stabilize plasma concentration.
Owner:INST OF BIOMEDICAL ENG CHINESE ACAD OF MEDICAL SCI
Dimethyl biguanide sustained release capsule and preparation method thereof
InactiveCN1568950AReduce the frequency of takingStable blood concentrationOrganic active ingredientsMetabolism disorderDrugBiguanide
The invention discloses a dimethylbiguanide slow release capsule which includes capsule outer cover and slow release particles, wherein the slow release particles comprises dimethylbiguanide and hollow ball cores, retarding agent, slow release film forming material, bulking agent, plasticizing agent, adhesives and hole-forming agent.
Owner:范敏华
Tablet of isosorbide mononitrate
ActiveCN101732276AEasy to pumpStable release ratePill deliveryHeterocyclic compound active ingredientsMedicineSemipermeable membrane
The invention relates to a tablet of isosorbide mononitrate, in particular to a double-layer osmotic pump controlled release tablet of isosorbide mononitrate, which belongs to the field of medicine preparation. A single-chamber double-layer osmotic pump tablet of the isosorbide mononitrate is characterized in that a semi-transparent coating film cover a double-layer core body consisting of a medicine-containing layer and a boosting layer; and the coating film is provided with a medicine releasing pore on the surface of the medicine-containing layer. The gastrointestinal tract water enters a double-layer tablet chip through the semi-transparent film; the medicines forms a mixed suspension liquid or solution when contacting water in the medicine-containing layer; a penetration enhancer enables the solution of the medicine-containing layer to be hypertonic so that an osmotic pressure difference exists between the inner side and the outer side of the film, which is beneficial to pumping the medicines out; and the pressure is generated in the boosting layer through water absorption, dissolution and expansion of a penetrating agent so as to further boost a medicine liquid to eject the pore.
Owner:LUNAN BETTER PHARMA
Method of producing double-layer core permeation pump patch of medicament
InactiveCN101152158ASimple processLow costPharmaceutical non-active ingredientsPill deliveryDrugOsmotic pump
The invention discloses a drug double layer core osmotic pump tablet and the preparation method. The drug or the solid dispersion of the drug is mixed uniformly with penetrating agent, thickener and filler, added with adhesive to produce soft material and granulation, baked, and added with lubricant, and then drug layer core tablet material is produced. The penetrating agent, expansive agent and the filler are mixed uniformly, are added with adhesive to produce soft material and particles, are baked and are added with lubricant, and then promoting layer core tablet material is produced. The drug layer core tablet material and the promoting layer core tablet material are punched by a preforming machine with a needle punch to produce double layer core tablet of drug layer with concave holes. Coating liquid containing semi-permeable polymer changes the double layer core tablet of drug layer with concave holes into coated tablet in the coating pot, which is baked to produce double layer core osmotic pump tablet. The double layer core osmotic pump tablet prepared by the invention is similar to zero level release drugs. The invention disuses the slotting process after coating in preparing double layer core osmotic pump tablets without resorting to expensive laser drilling machine, simplifies the preparation process, reduces the cost and fits for industrial production.
Owner:ZHEJIANG UNIV
Levetiracetam slow release medicinal composite and preparation method thereof
ActiveCN102379857ASimple preparation processPromote reproductionOrganic active ingredientsNervous disorderControl releaseBiocompatibility Testing
The invention relates to a levetiracetam slow release medicinal composite and a preparation method thereof. The tablet core of the medicinal composite is mainly composed by 55-65% of levetiracetam in weight percentage and 35-45% of non hydrophilia auxiliary material with the slow release function in weight percentage, the non hydrophilia auxiliary material with the slow release function is mixed by one, two or three of ethyl cellulose, methyl cellulose or behenic acid glyceride, and the slow release medicinal composite does not need a functional controlled release coating to adjust the drug release. The levetiracetam medicinal composite has stable drug release performance, good biocompatibility and dissolution performance; and the preparation technology is simple, is easy to reproduce, and is applicable to industrialization production.
Owner:SHENZHEN SALUBRIS PHARMA CO LTD
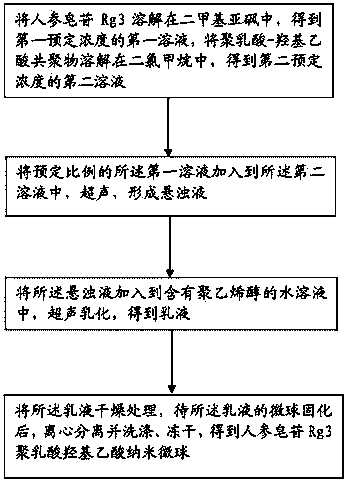
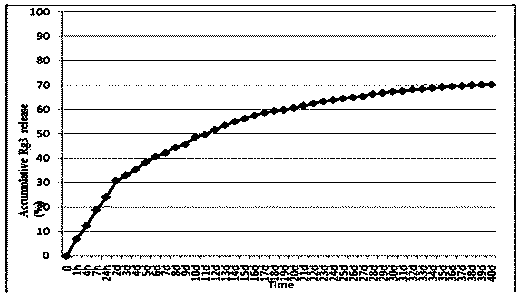
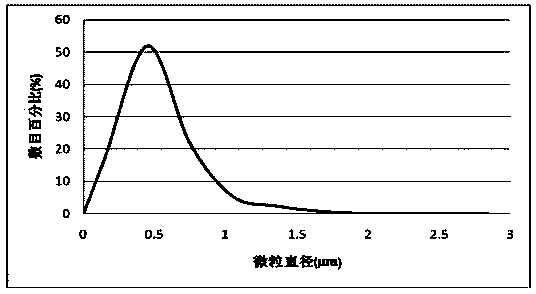
Ginsenoside Rg3 poly(lactic-co-glycolic acid) nano microsphere and preparation method thereof
InactiveCN104288111ASolve the shortcomings of poor water solubility and difficult absorption after oral administrationLittle side effectsPowder deliveryOrganic active ingredientsFreeze-dryingMicrosphere
The invention discloses a ginsenoside Rg3 poly(lactic-co-glycolic acid) (PLGA) nano microsphere and a preparation method thereof. The preparation method of the ginsenoside Rg3 poly(lactic-co-glycolic acid) nano microsphere comprises the following steps: S1, dissolving ginsenoside Rg3 in dimethyl sulfoxide to obtain a first liquid with a first predetermined concentration, and dissolving poly(lactic-co-glycolic acid) copolymer into dichloromethane to obtain a second liquid with a second predetermined concentration; S2, adding the first liquid in a predetermined proportion into the second liquid to perform ultrasonic treatment to form a turbid liquid; S3, adding the turbid liquid into an aqueous liquid containing polyvinyl alcohol to perform ultrasonic emulsion to obtain emulsion; and S4, drying the emulsion, centrifugally separating and washing, and freeze-drying the emulsion after curing the microspheres of the microsphere of the emulsion to obtain the ginsenoside Rg3 poly(lactic-co-glycolic acid) nano microsphere. According to the preparation method of the ginsenoside Rg3 poly(lactic-co-glycolic acid) nano microsphere of the embodiment of the invention, the prepared ginsenoside PLGA microsphere is good in pesticide effect.
Owner:BEIJING JIAOTONG UNIV
Rhizoma Gastrodiae nasal gel preparation
InactiveCN101053550AAvoid first pass effectImprove bioavailabilityNervous disorderPharmaceutical delivery mechanismDiseaseNeurasthenia
The invention relates to a gastrodin gel administrating by nasal cavity, which is composed of main medicine gastrodin and proper watercraft gel matrix and other additive medicine. The inventive gel can be adhered on the mucous membrane surface and extend the resorting period in the nasal cavity after administration. The released medicine can not only be absorbed into the blood circulation from mucous membrane, but also into the brain from the olfactory system, bypassing the blood-brain barrier to better do a treatment as gastrodin. The invention provides a new gastrodin without mucous membrane cilia toxicity has a high bioavailability, a strong patent compliance, a good stability and a simple production process and can be used for the treatments such as headache, blackout, neurasthenia and etc.
Owner:KPC PHARM INC
Medicine compounds for treating hypertension
ActiveCN101229372AGood treatment effectReduce incidencePharmaceutical non-active ingredientsHeterocyclic compound active ingredientsTreatment hypertensionLong term survival
The invention provides a medical composite for treating hypertension and a preparation thereof. The medical composite comprises antihypertensive drug, HMGCoA reductase inhibitor and insulin sensitizer. The medical composite of the invention not only can effectively reduce blood pressure, but also has good effect on the treatment and prevention of hypertension complications. The long term survival of patients can be improved obviously.
Owner:LUNAN PHARMA GROUP CORPORATION
Doxazosin-mesylate controlled-releasing tablet and preparation method thereof
ActiveCN101167728AImprove complianceStable blood concentrationOrganic active ingredientsPharmaceutical delivery mechanismSide effectGeneration rate
The invention discloses methanesulfonic acid doxazosin controlling releasing tablets and a process for preparation, which contains a single-layer core, insoluble semi-permeable membrane, single drug-releasing eyelet on each side of the tablet, and depends on the osmotic pressure difference between inside and outside of the semi-permeable membrane medium. The invention realizes the control of linear releasing of methanesulfonic acid doxazosin by single-layer core structure, and can remain effective and steady blood and drug concentration. The curative effect is improved, the generation rate of side effect is reduced, and the productive technology is greatly simplified.
Owner:HEFEI LIFEON PHARMA
Control releasing venlafaxine hydrochloride tablet and its prepn
ActiveCN1771921ALittle side effectsGuaranteed curative effectOrganic active ingredientsNervous disorderDrugSide effect
The present invention discloses one kind of control releasing venlafaxine hydrochloride tablet and its preparation process. The control releasing venlafaxine hydrochloride tablet consists of medicine core of venlafaxine hydrochloride and semi-permeable coating with pores. The medicine core consists of venlafaxine hydrochloride 75 weight portions, stuffing 30-250 weight portions, osmotic pressure promoter 1-50 weight portions, adhesive 1-20 weight portions and lubricant 0.1-20 weight portions. The preparation has reasonable composition, excellent control releasing effect, long effective time, less side effect on gastrointestinal tract, less differential personal effect, high safety and good compliance.
Owner:CHENGDU KANGHONG PHARMA GRP
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com