Method for preparing an amlodipine microsphere
A technology of amlodipine and microspheres, which is applied to medical preparations containing non-active ingredients, medical preparations containing active ingredients, and pharmaceutical formulas, etc. It can solve the problem of patients with large blood pressure fluctuations and increased incidence of cardiovascular events, etc. problems, to achieve the effect of solving missed doses, reducing the incidence rate, and rounding the shape
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
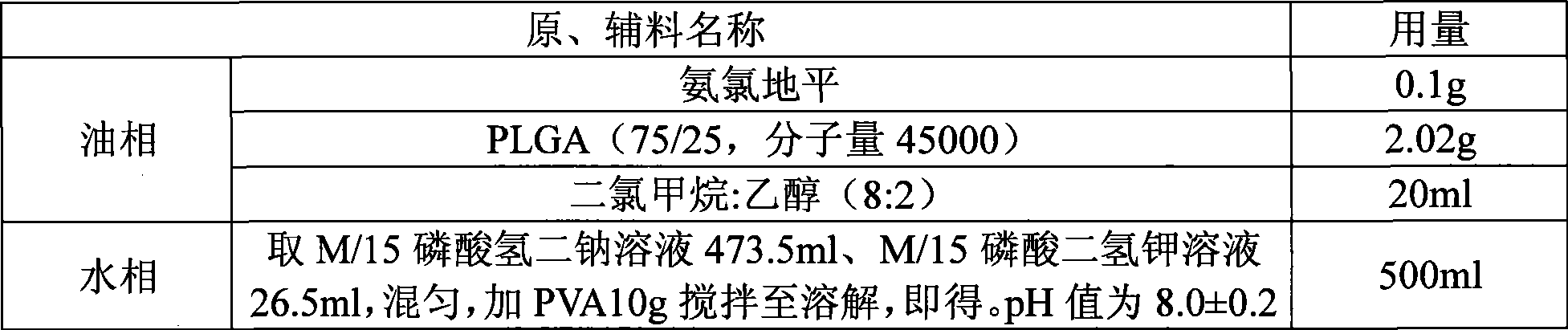
[0018] The preparation method of amlodipine microspheres of the present invention selects polylactic acid (PLA), polylactic acid-polyglycolic acid copolymer (PLGA), or polylactic acid-polyethylene glycol block copolymer (PLA-mPEG) as carrier The material encapsulates the drug amlodipine, and the relative molecular weight of the carrier material is 5×10 3 ~1.5×10 5 , the ratio to the drug is 50:1-3:1, the concentration of the carrier material in the oil phase is 1%-50% (g / ml); the carrier material and the drug are dissolved in an organic solvent to make an oil phase, and the oil phase The organic solvent in is dichloromethane, or the mixed solution of dichloromethane and ethanol, or the mixed solution of dichloromethane and ethyl acetate;
[0019] Add the oil phase to the water phase, and make the volume ratio of the oil phase to the water phase 1:300~1:5; fully emulsify through mechanical stirring or high-speed shearing to obtain an O / W emulsion, and then use the emulsion sol...
Embodiment 1
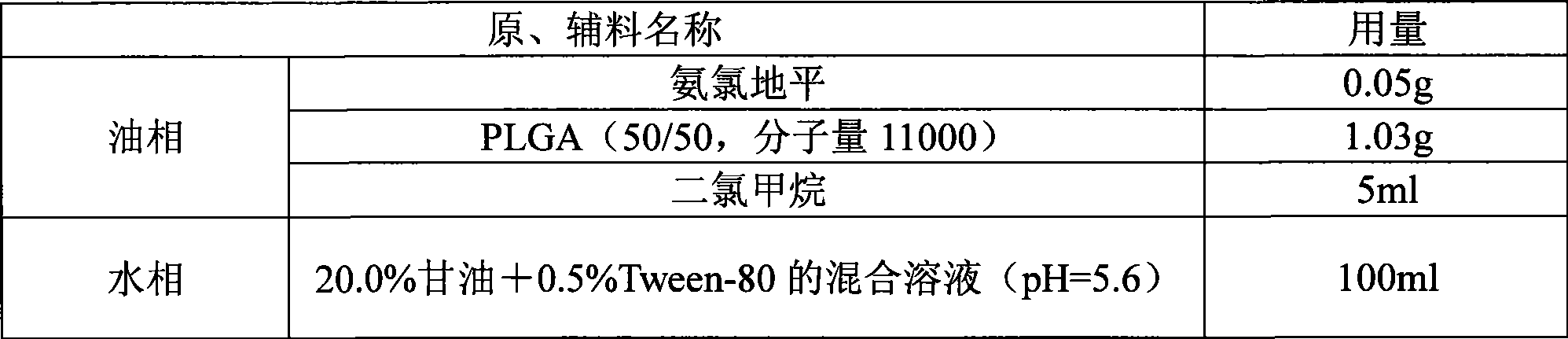
[0028] prescription:
[0029]
[0030] Preparation method:
[0031] Weigh PLGA and amlodipine according to the prescription amount, add 5ml of dichloromethane to dissolve, add to the water phase under the condition of stirring at 500rpm, continue to stir for 1 hour, then slowly raise the temperature to 40°C, and stir for 2 hours; Filter through a 125 μm sieve to collect the filtrate, then filter through a 1 μm sieve to collect the microspheres, wash the microspheres with an appropriate amount of water several times, filter out the water, and dry at 40°C to obtain the product.
Embodiment 2
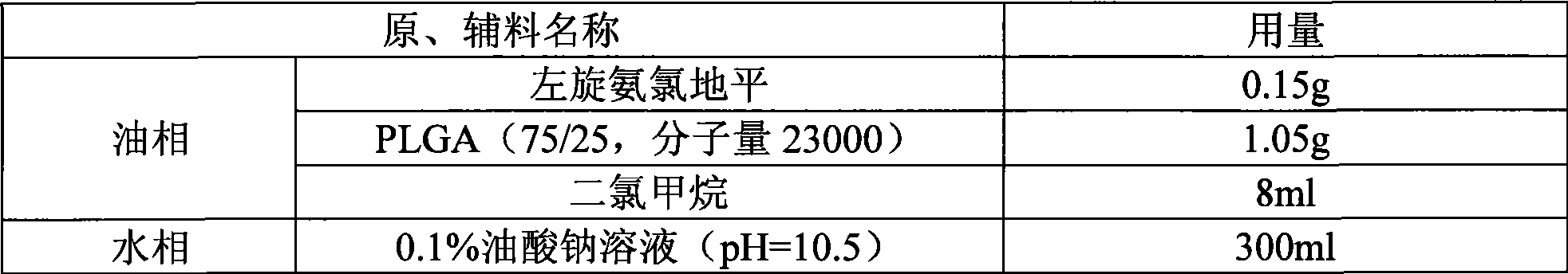
[0033] prescription:
[0034]
[0035] Preparation method:
[0036] Weigh PLGA and levamlodipine according to the prescription amount, add 8ml of dichloromethane to dissolve, slowly add to the water phase under 300rpm stirring condition, continue to stir for 0.5 hours, then slowly heat up to 40°C, stir for 3 hours, Then filter through a 125 μm sieve to collect the filtrate, then filter through a 1 μm sieve to collect the microspheres, wash the microspheres with an appropriate amount of water several times, filter out the water, and dry under reduced pressure at 40°C to obtain the product.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com