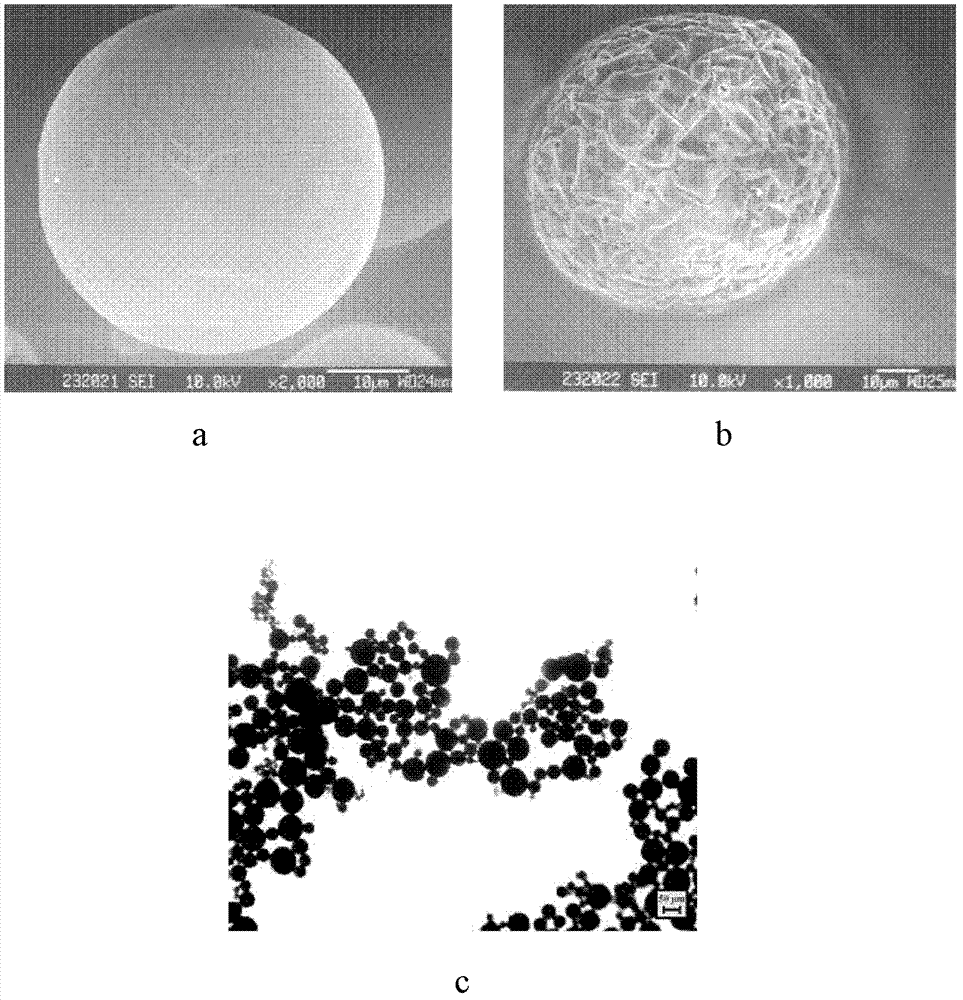
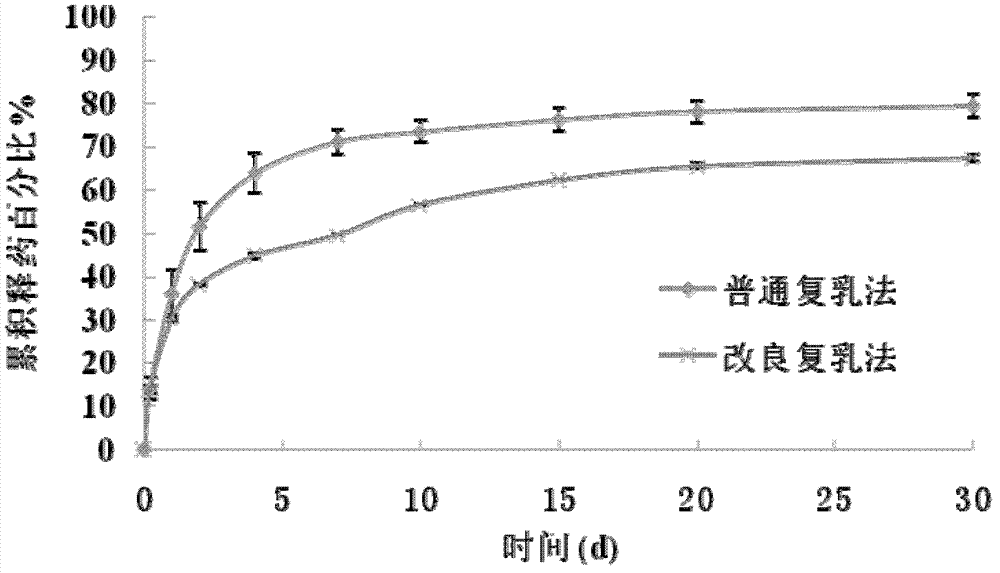
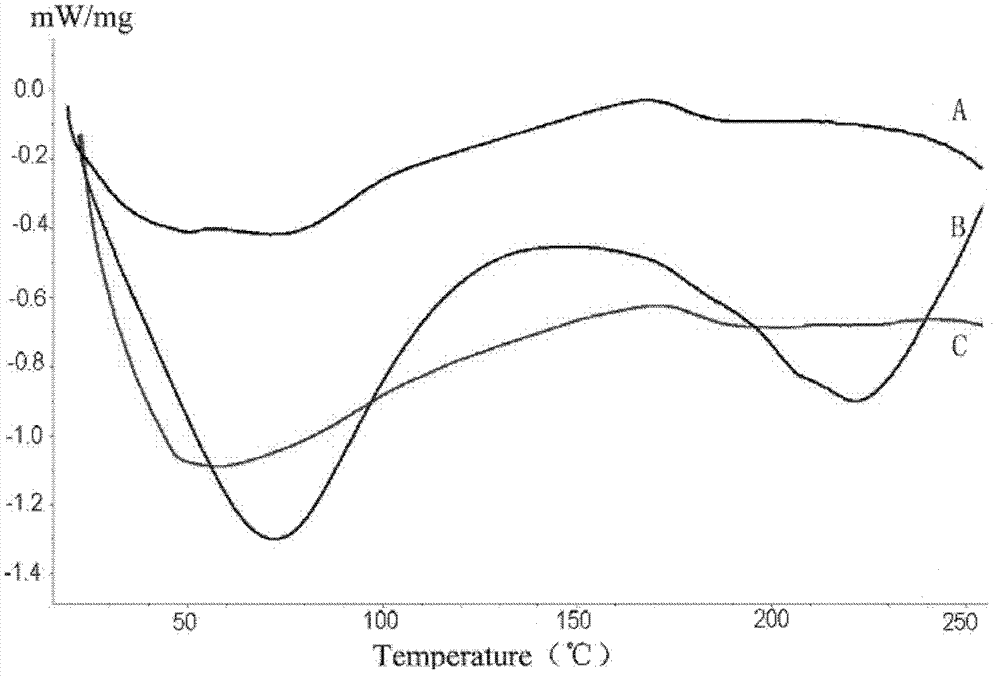
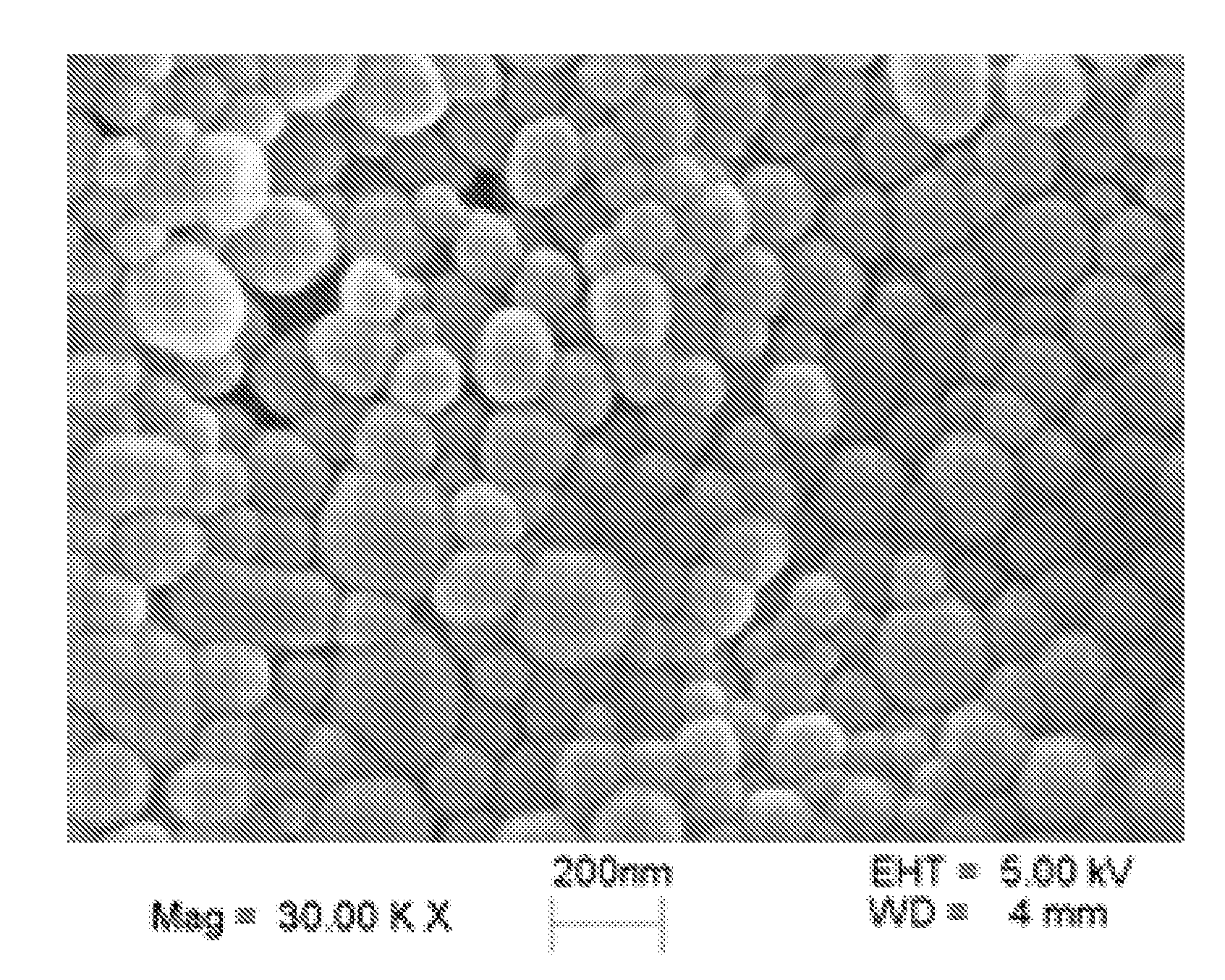
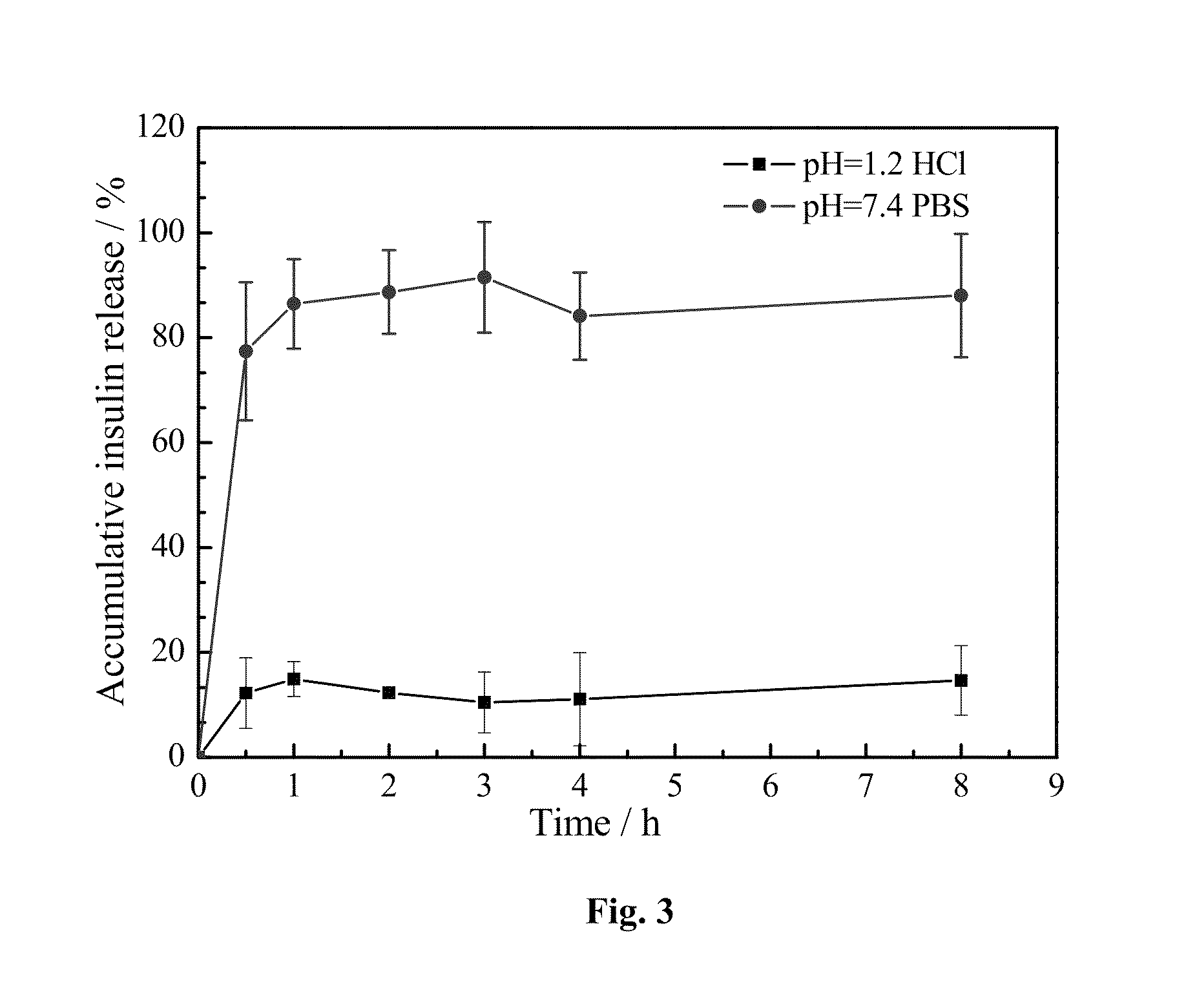
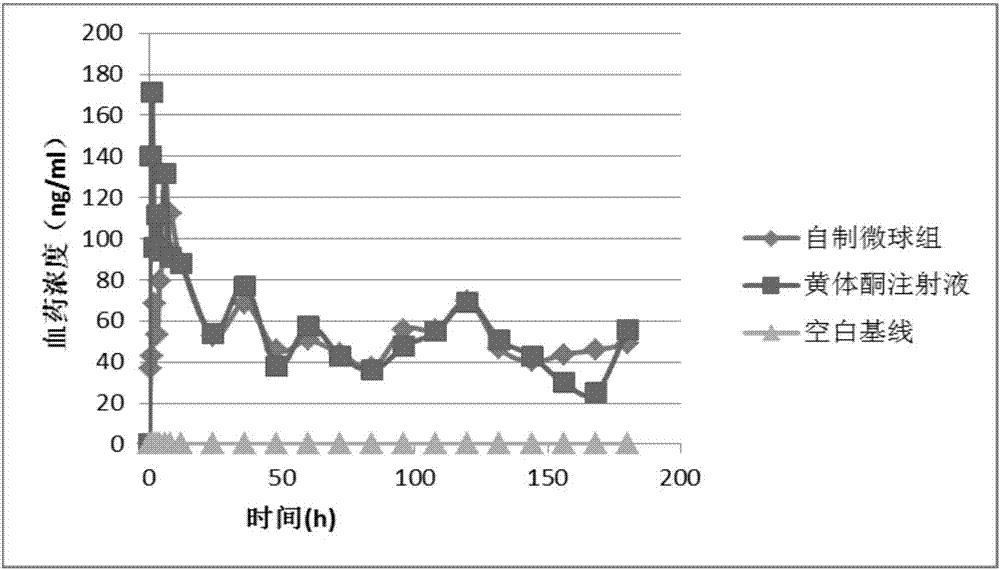
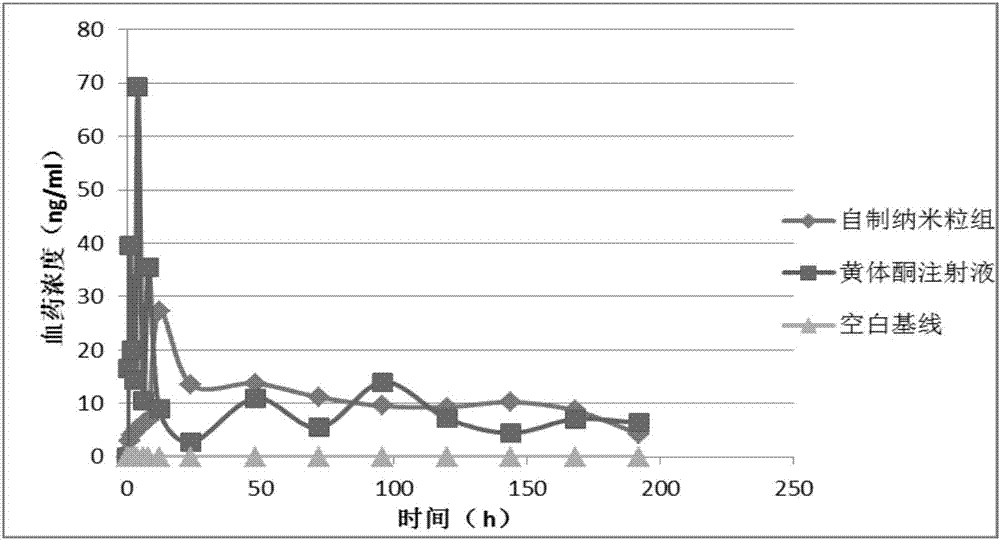
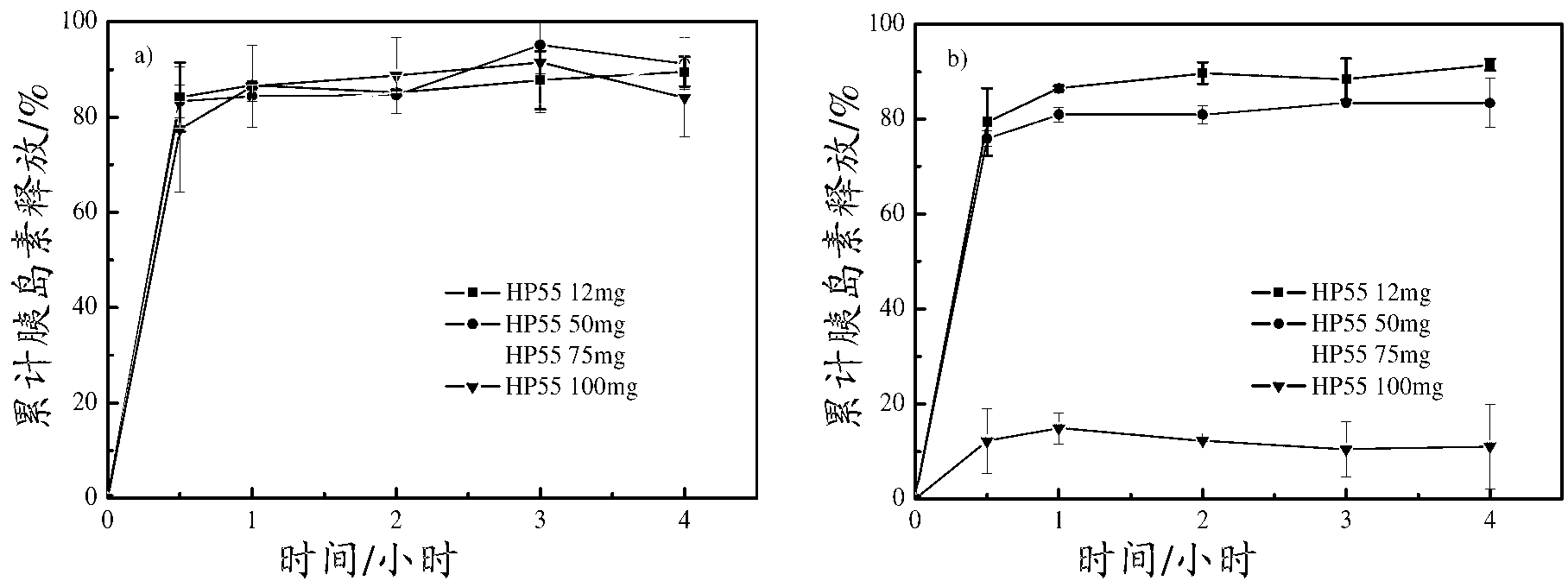
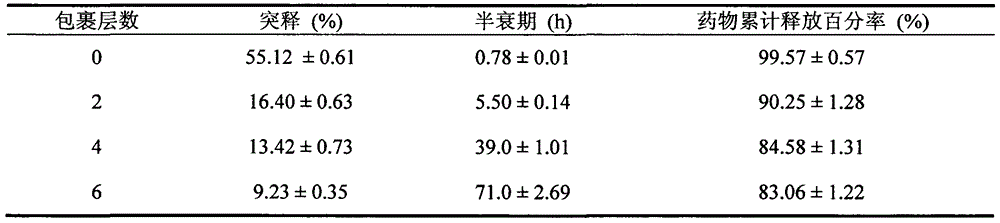
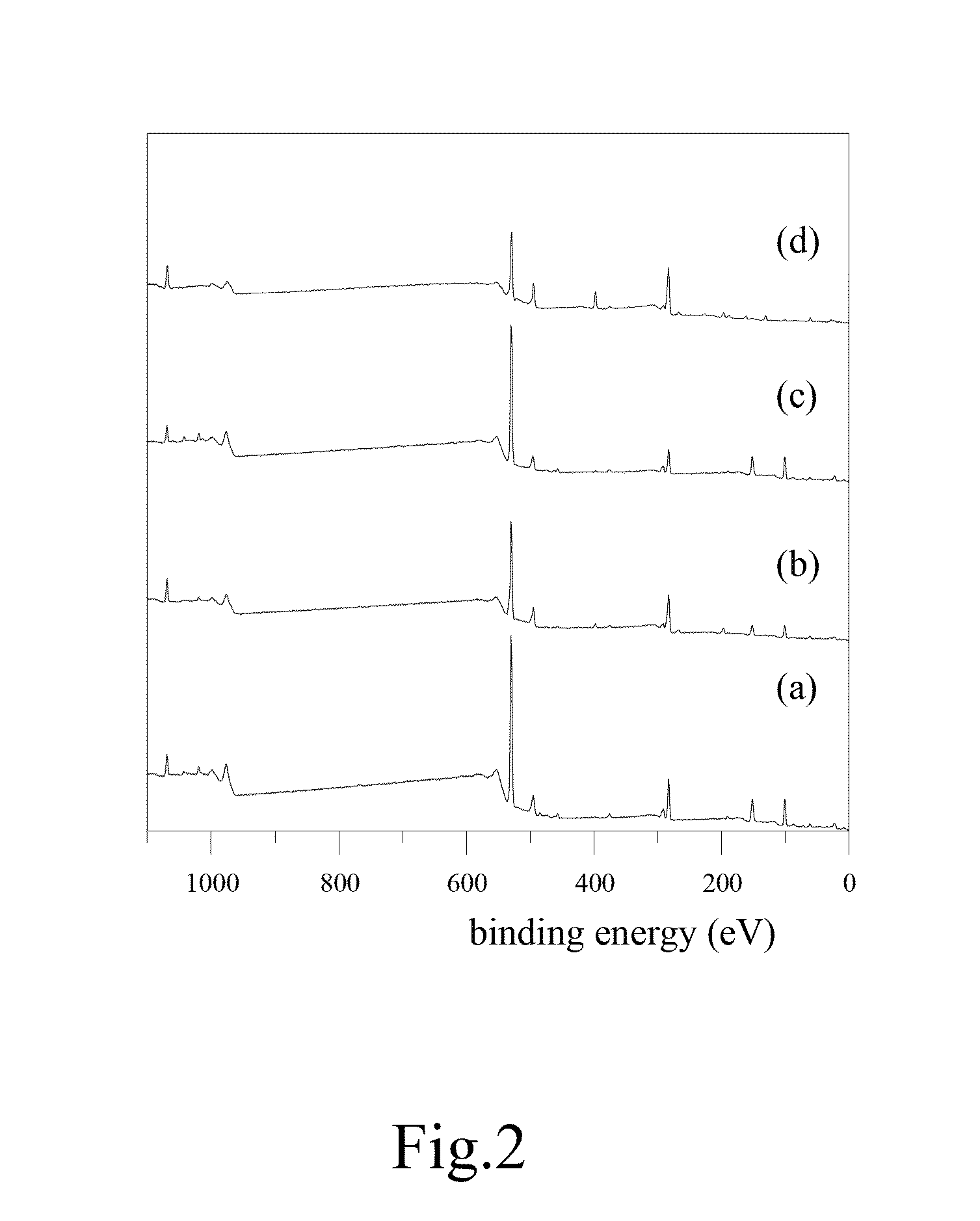
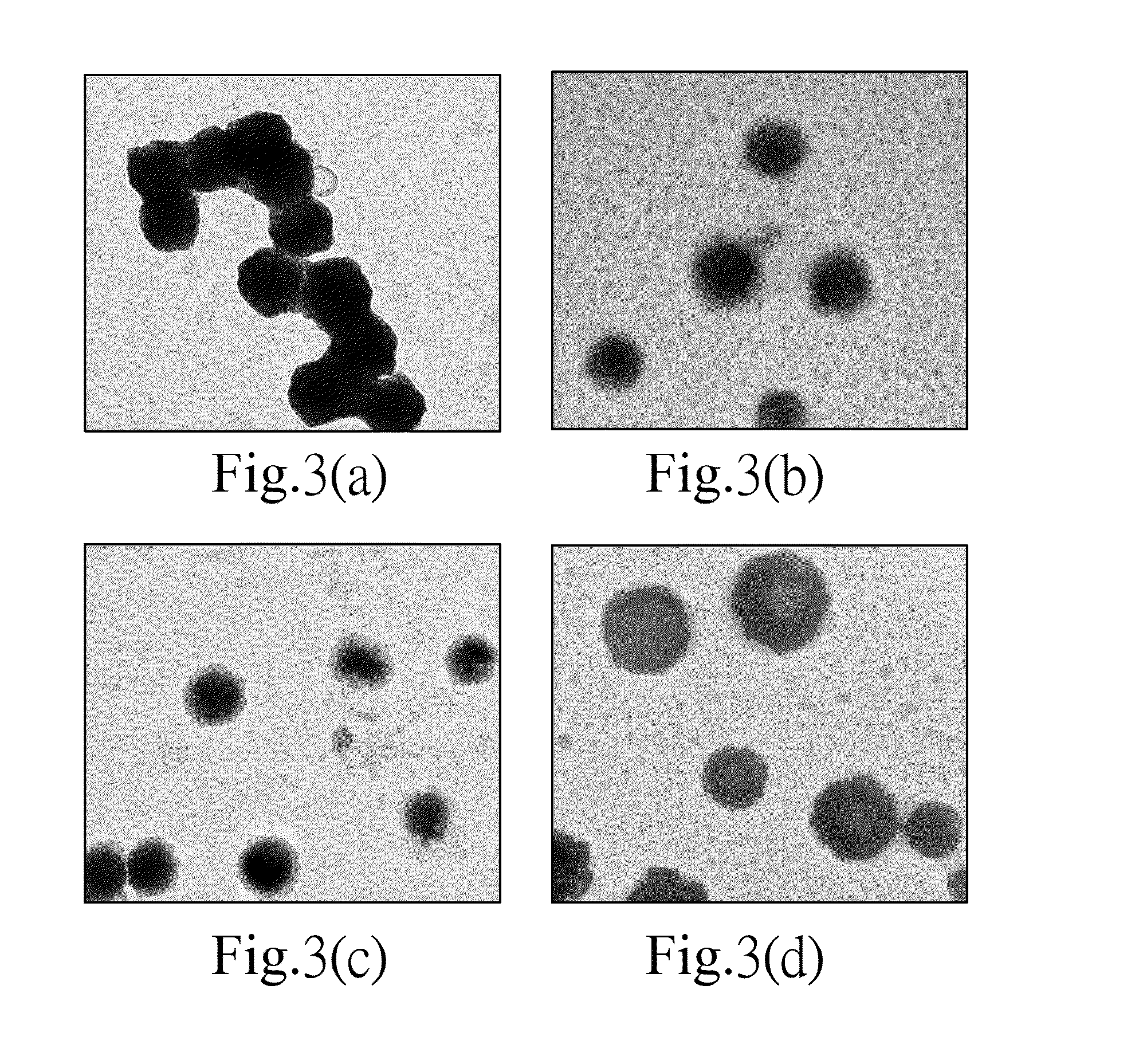
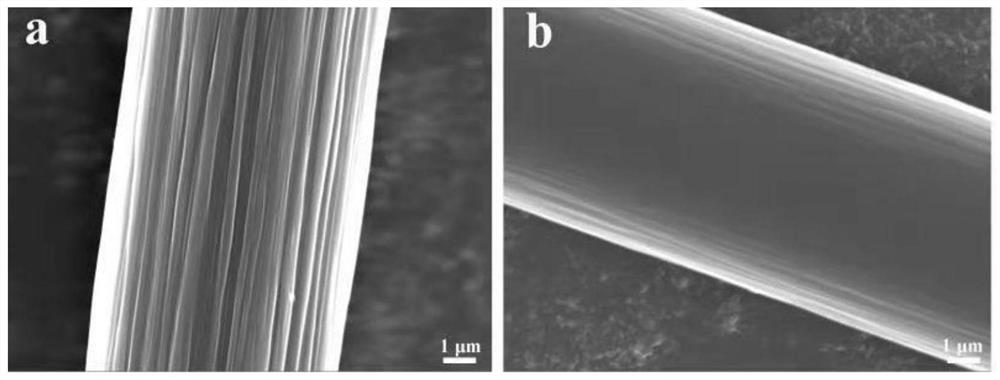
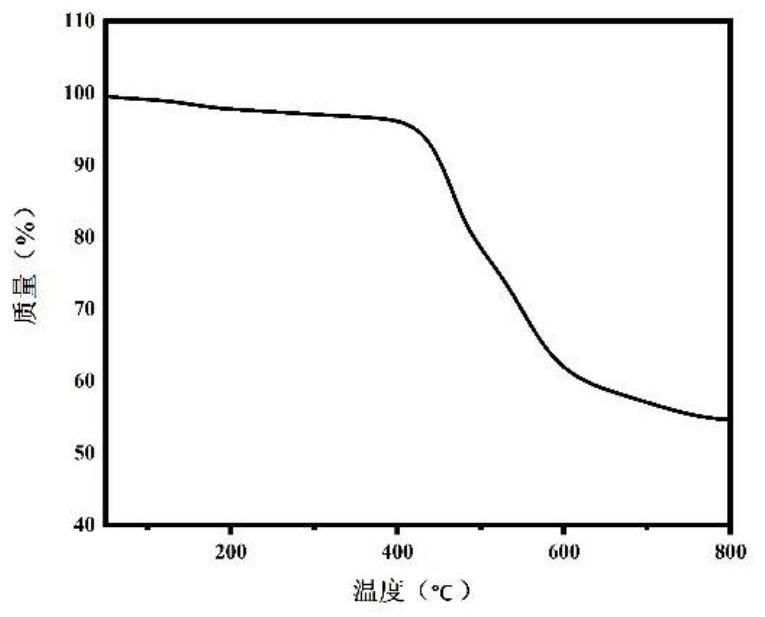
Patents
Literature
67 results about "Emulsion solvent evaporation" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
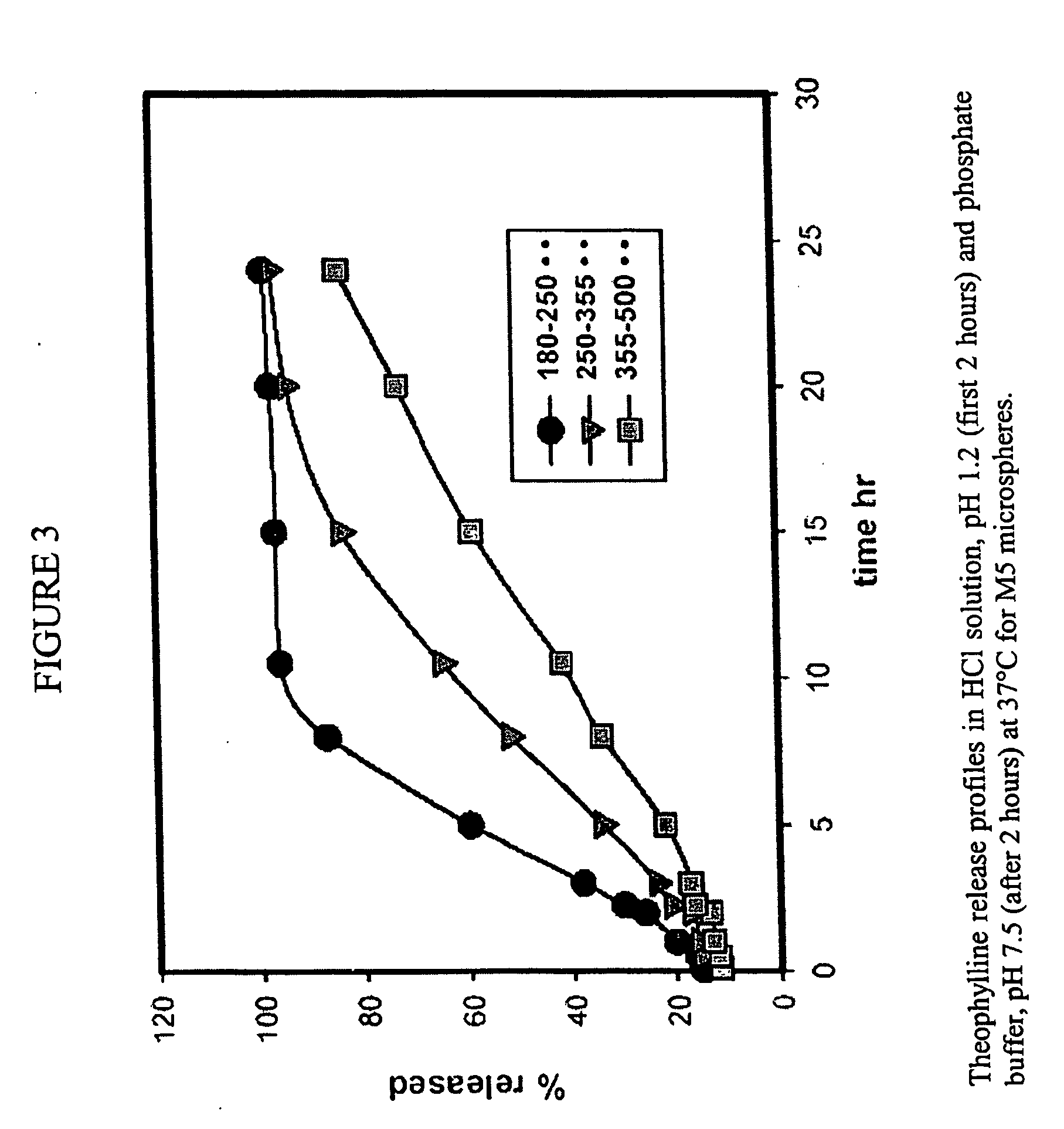
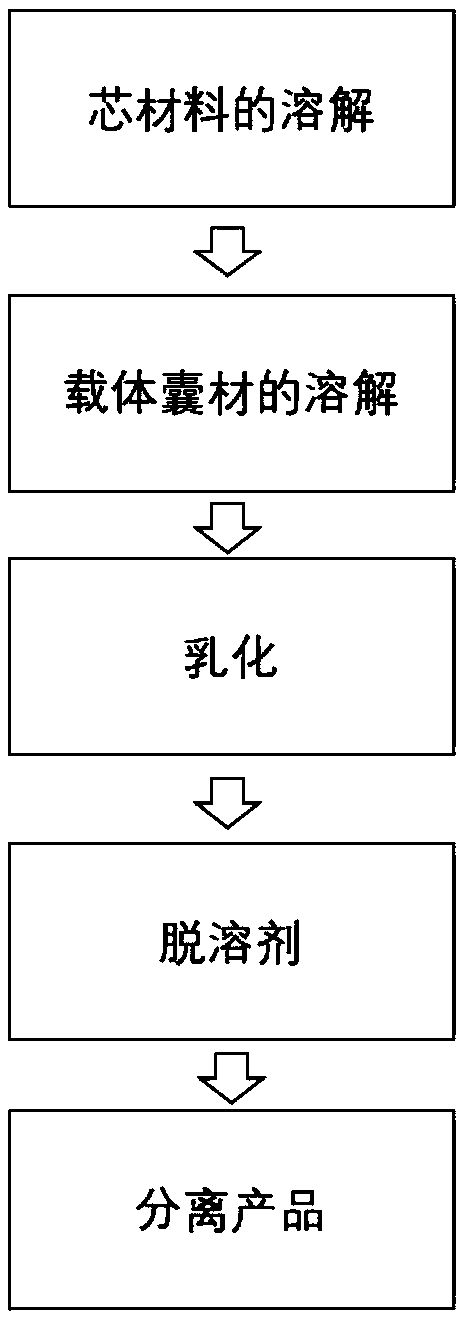
Solvent evaporation is defined as solvent removal from an emulsion consisting of a polymer volatile organic solvent in water (Poncelet, 2006). This technique is based on four major steps: (i) dissolution of polymer as coating and active compound in an organic solvent to form a suspension, an emulsion or a solution;
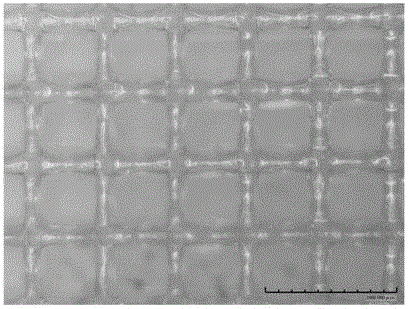
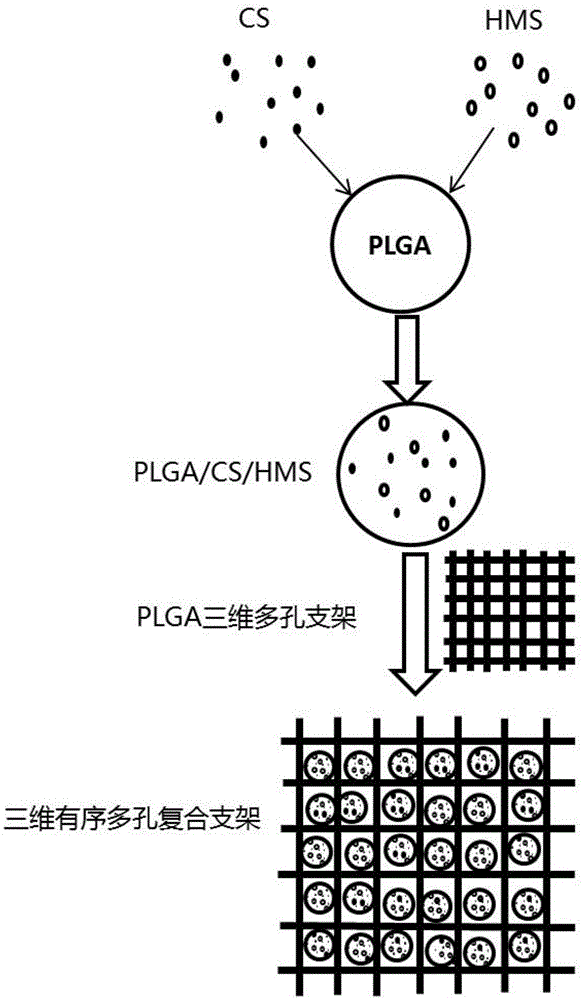
Bone repair porous compound scaffold based on 3D (three-dimensional)-Bioplotter printing technology and preparation method thereof
ActiveCN105031718APrecise Control of GeometryPrecise control of apertureProsthesisCompound aCalcium silicate
The invention discloses a bone repair porous compound scaffold based on 3D (three-dimensional)-Bioplotter printing technology and a preparation method thereof. The scaffold is formed by compounding a matrix with a 3D macroporous structure and a drug carrying microsphere. The preparation method comprises the following steps: printing a scaffold matrix with a regular 3D macroporous structure through 3D-Bioplotter; preparing a drug carrying microsphere compounding hexagonal mesoporous silica (HMS), calcium silicate (CS) powder and PLGA through an emulsion solvent evaporation method; finally, fixing the compound microsphere into the matrix through low-temperature sintering so as to obtain the bone repair porous compound scaffold based on 3D-Bioplotter printing technology. According to the invention, the 3D printed porous scaffold and the PLGA / HMS / CS compound microsphere with drug sustained release and bone repair effects are combined, so that the scaffold has a macroporous structure, has good drug carrying and drug release properties and osteogenic differentiation capability and can be used for effectively promoting the repair and reconstruction of bone tissues.
Owner:SOUTH CHINA UNIV OF TECH
Microspheres and related processes and pharmaceutical compositions
The instant invention provides microspheres and related processes and pharmaceutical compositions useful in the controlled delivery of a wide variety of active ingredients. In one embodiment, the microspheres comprise an active ingredient dispersed within a polymeric composition comprising a first pH insensitive hydrophobic polymer and second pH sensitive hydrophobic polymer, wherein the microspheres, in an aqueous environment having a pH of around 5 or greater, release the active ingredient in a substantially zero-order profile. In another embodiment, the microspheres comprise an active ingredient dispersed within a polymeric composition comprising a first pH insensitive hydrophobic polymer and second water-swellable polymer, wherein the microspheres, in an aqueous environment, release the active ingredient in a substantially zero-order profile. In both of these embodiments, the microspheres are prepared by a non-aqueous emulsion solvent evaporation method.
Owner:PRICE JAMES C +1
Nano drug carrier particles for improving bioavailability of rapamycin and preparation method thereof
InactiveCN102871966AGood water solubilityGood biocompatibilityPowder deliveryOrganic active ingredientsUltrafiltrationBiocompatibility Testing
The invention discloses nano drug carrier particles for improving the bioavailability of rapamycin and a preparation method thereof, so as to carry out a drug effect optimization. The nano drug carrier particles are prepared in the following steps that according to the principle of an emulsion solvent evaporation method, a determined amount of PEG-PLGA and rapamycin are respectively dissolved in acetone; after being mixed uniformly, the PEG-PLGA, the rapamycin and the acetone are slowly added into water to be stirred by magnetic force; after a certain period of time, the obtained liquid is ultrasonically homogenated, and organic phase is removed in a vacuum dryer; the obtained water phase removes free medicine in a centrifugal tube; and then an ultrafiltration tube is used for concentration, and nano particles are obtained after freezing and drying. The method has the advantages of convenience in operation, simplicity and feasibility, good repeatability and the like. The prepared nano drug carrier particles can improve the utilization rate of the medicine by improving the absorptivity of the medicine and prolonging the cycling time in a human body. Meanwhile, the nano particles which are prepared through the method have good biocompatibility, and surface active groups can further modify ligands or targeted groups.
Owner:SOUTHEAST UNIV
Method for preparing an amlodipine microsphere
InactiveCN101530396ARound shapeUniform particle size distributionOrganic active ingredientsPharmaceutical non-active ingredientsCelluloseMicrosphere
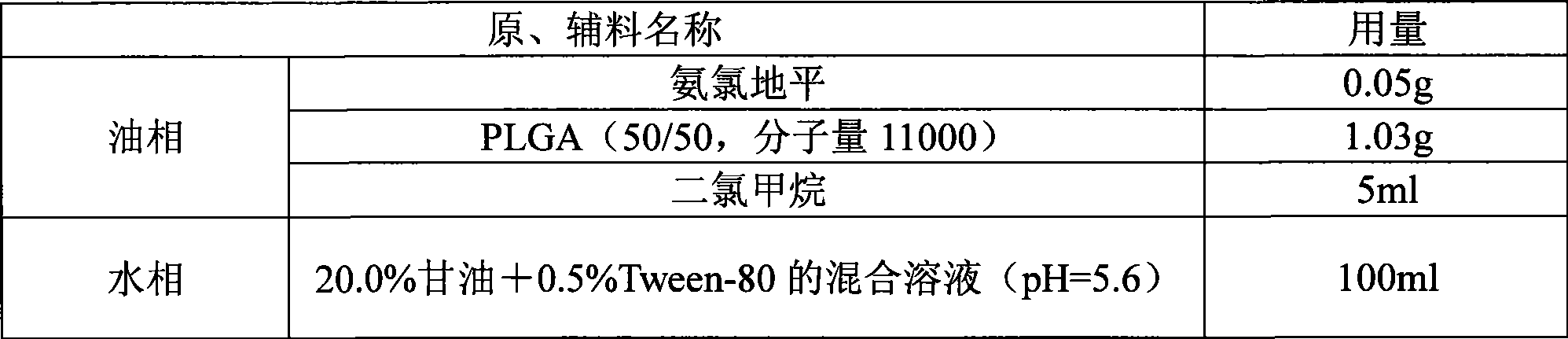
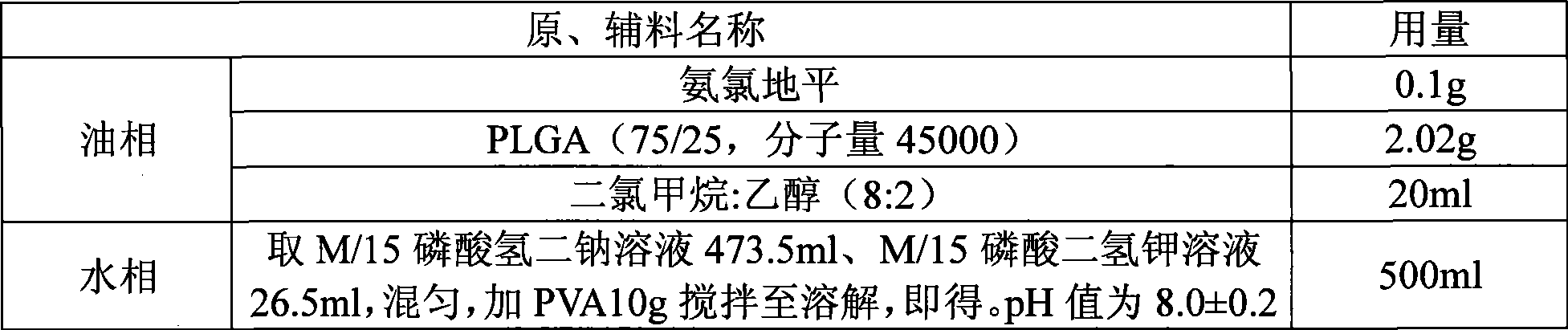
The method discloses a method for preparing an amlodipine microsphere. Medicament wrapped by the prepared amlodipine microsphere is amlodipine and organic acid salts of the amlodipine; a carrier material of the microsphere is polylactic acid (PLA), a polylactic acid-glycolic acid copolymer (PLGA), a polylactic acid-glycol block copolymer (PLA-mPEG) or other biodegradable materials; a surfactant solution, a monosaccharide or amylose solution, a polyalcohol solution, a cellulose solution and a colloid solution are used as a dispersion medium; and through an emulsion solvent evaporation method, the amlodipine microsphere is prepared under the mechanical stirring or high-speed shearing action. The microsphere has a round shape and even distribution of grain diameter; the grain diameter is within the range between 1 and 125 mu m; the medicine loading capacity can reach more than 1.5 percent; and the encapsulating rate is more than 70 percent.
Owner:XIAN LIBANG PHARMA TECH
Microbeads and preparing method and application thereof
ActiveCN105418944ALarge specific surface areaSoft textureCosmetic preparationsToilet preparationsMembrane emulsificationBiodegradable polymer
The invention provides microbeads. The microbeads are prepared from raw materials comprising biodegradable polymers and are spherical microbeads. The invention provides a preparing method of the microbeads in the technical scheme. The preparing method includes the step that the raw materials comprising the biodegradable polymers are prepared into the spherical microbeads with an emulsion-solvent evaporation method or a membrane emulsification method or a spray drying method or a polymerization method. Compared with the prior art, the microbeads are the degradable spherical microbeads, and are large in specific surface area, soft in texture, small in skin irritation and high in skin comfort. By means of the preparing method of the microbeads, the size, the size distribution, the microstructure and the feature of the microbeads can be controlled, functional substances can be led into the interiors or the surfaces of the microbeads in the microbead preparing process, and the prepared microbeads meet different application requirements accordingly. The invention further provides an application of the microbeads to preparing of cleaning care products and cosmetics.
Owner:CHANGCHUN SINOBIOMATERIALS
Method of manufacturing fulvestrant sustained-release microspheres
InactiveCN101108168ARound shapeUniform particle size distributionPowder deliveryOrganic active ingredientsMicrospherePolyvinyl alcohol
The invention discloses a faslodex controlled-release microballoon preparation method. The faslodex controlled-releasemicroballoon in the invention envelopes fulvestrant takes PLA or PLGA or PCL or other biodegradable materials as the carrier materials. Besides, the invention takes PVA or the miscible liquids of PCL and Tween 80 as the disperse medium. Then, with the emulsion solvent evaporation method, finish the preparation of fulvestrant controlled-release microballoon under the action of mechanical mixing or high-speed shearing. The microballoon has spherical shape and even distribution of grain size that is controllable within the scope of 15 to 125Mu m as well as reaches a drug-loading rate of over 7 per cent and encapsulation rate of over 80 per cent.
Owner:XIAN LIBANG PHARMA TECH
Protein-medicament-carrying PLGA composite microspheres and preparation method thereof
InactiveCN102727899AThe preparation process is stableThe preparation process is feasiblePowder deliveryPeptide/protein ingredientsProlonged-Action PreparationsMicrosphere
The invention discloses protein-medicament-carrying PLGA composite microspheres and a preparation method thereof. According to the invention, an improved multiple emulsion-solvent evaporation method is adopted. On the basis of a multiple emulsion method, a principle that sodium alginate and calcium ions are subjected to chelating and form sustained-release gel is adopted; PLGA is adopted as a microsphere carrier; lyophilized injection-use recombinant human interferon-alpha, bovine serum albumin and the like are adopted as encapsulation objects; and the medicament-carrying microspheres are prepared. The microspheres are advantaged in round appearance, and uniform particle size distribution. An average particle size distribution is 70 micrometers, a medicament-carrying rate is above 0.6%, an encapsulation rate reaches approximately 50%, and an in-vitro medicament releasing performance satisfies the characteristic of prolonged-action preparations.
Owner:THE THIRD AFFILIATED HOSPITAL OF SUN YAT SEN UNIV
Imidazole epoxy curing agent micro capsule and preparation method thereof
InactiveCN104193965ASimple processProcess conditions are easy to controlMicroballoon preparationMicrocapsule preparationEpoxySolvent evaporation
The invention relates to an imidazole epoxy curing agent micro capsule and a preparation method thereof, belonging to the technical field of latent epoxy curing agents. According to the curing agent micro capsule, polysulfone plastic is adopted as a wall raw material, an imidazole epoxy curing agent is adopted as a capsule core, and an emulsion-solvent evaporation method is adopted to prepare the polysulfone coated imidazole epoxy curing agent micro capsule. The preparation method of the micro capsule provided by the invention is simple in process and low in cost; the imidazole epoxy curing agent micro capsule is solid powder and has the characteristics of high coating rate and stability at normal temperature; as the imidazole curing agent is embedded into a container of a polysulfone polymer, the imidazole curing agent is not easy to release at normal temperature, is convenient to store and transport, can achieve the purpose of rapid curing by only being heated to be an appointed temperature when being used, and can be widely applied to the fields such as latent prepreg, self-repairing composite materials and semiconductor device packaging materials.
Owner:JIANGHAN UNIVERSITY
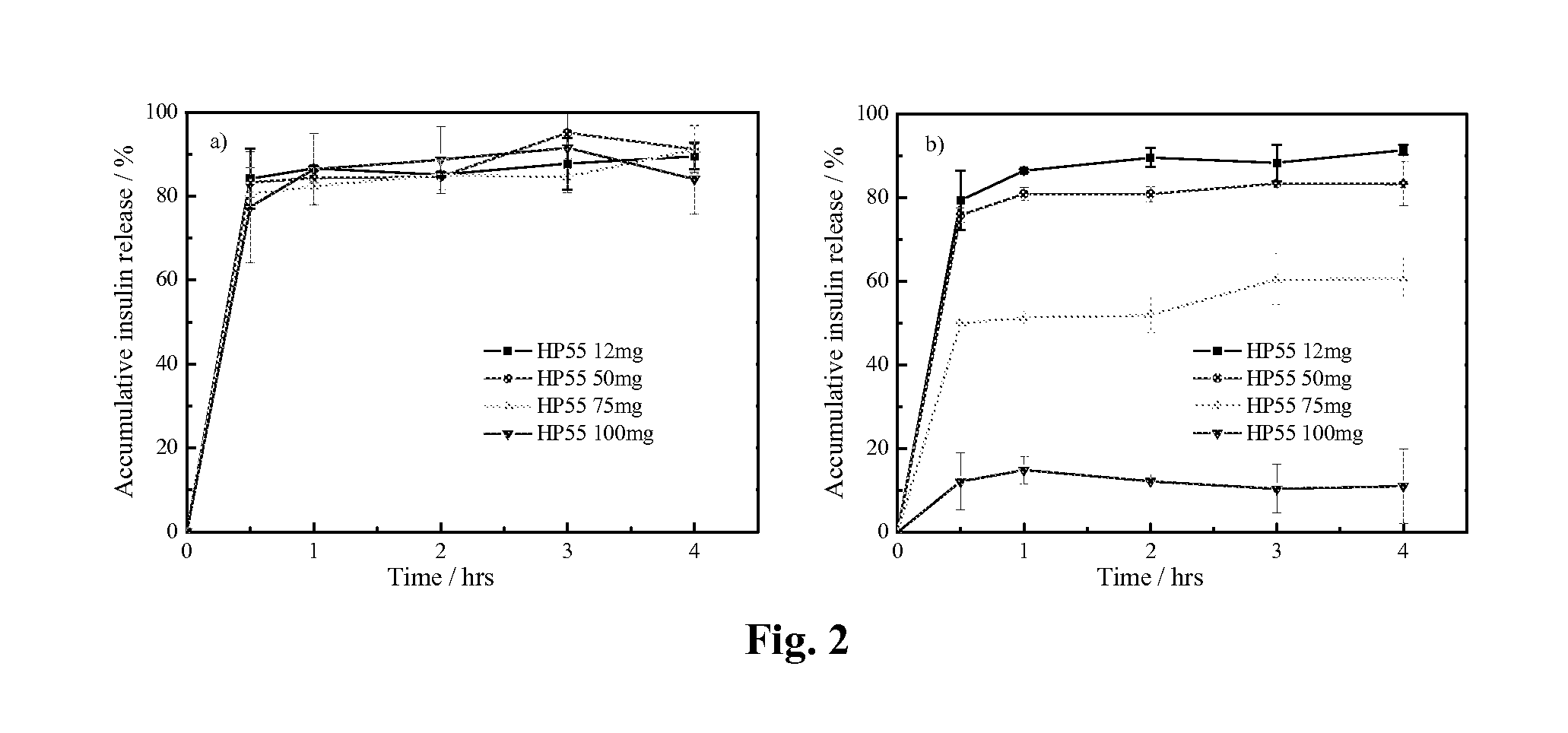
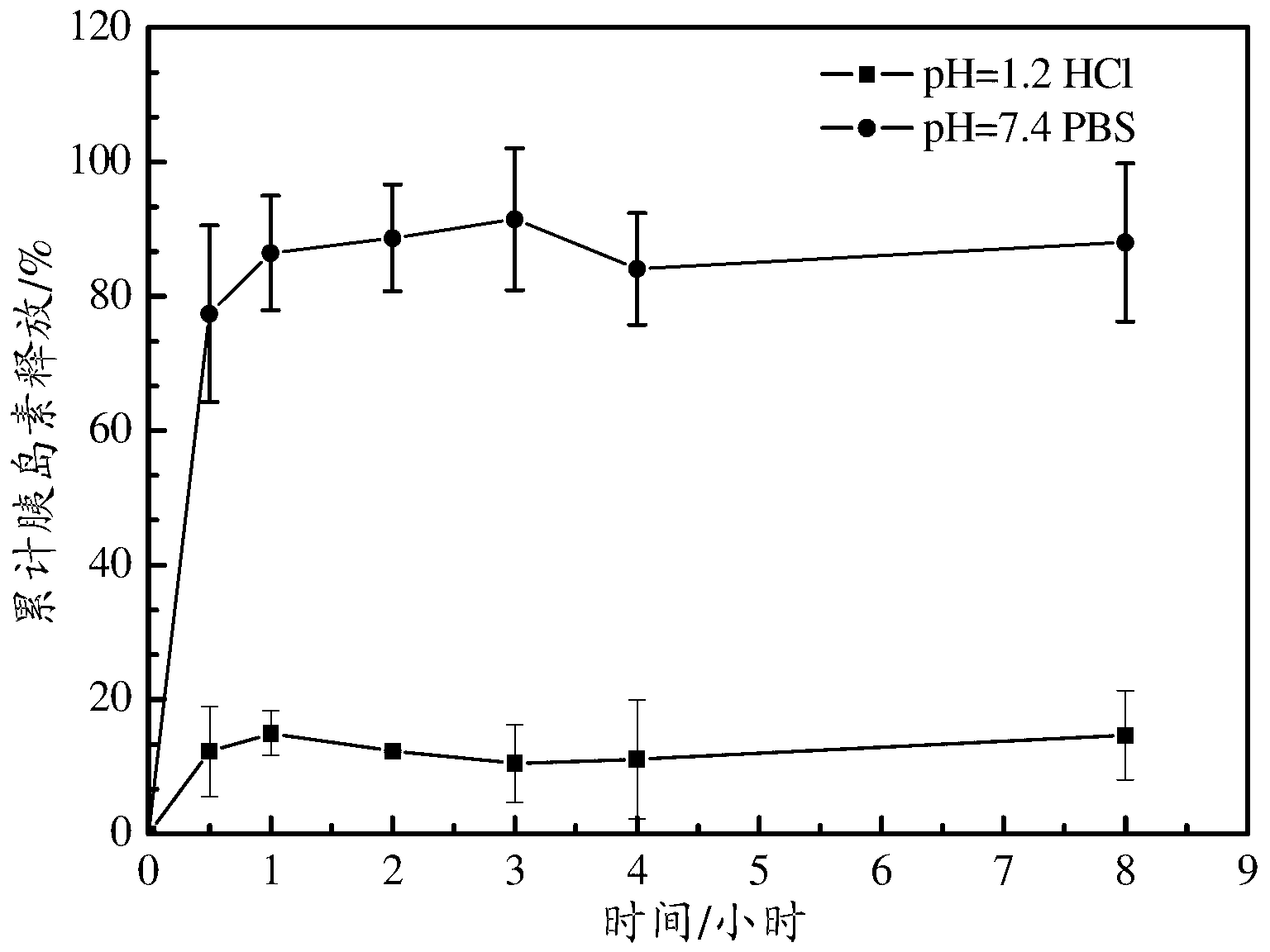
pH-SENSITIVE NANOPARTICLES FOR ORAL INSULIN DELIVERY
ActiveUS20130034589A1Novel and effective pH-sensitiveImprove oral bioavailabilityPowder deliveryPeptide/protein ingredientsPh sensitive nanoparticlesOral medication
The present invention discloses the pH-sensitive nanoparticles composed of pH-sensitive polymer, hydrophobic material, internal stabilizer, external stabilizer content and insulin drug. The present invention also includes a method for preparation of pH-sensitive nanoparticles, in particular, a multiple emulsions solvent evaporation method. The pH-sensitive nanoparticles of the present invention show good pH-sensitive property with 100-300 nanometer particle size. Significant decrease in blood glucose level is observed in streptozotocin (STZ)-induced diabetic rats and the bioavailability of insulin is more than 10% after oral administration of the insulin-loaded pH-sensitive nanoparticles.
Owner:NANO & ADVANCED MATERIALS INST
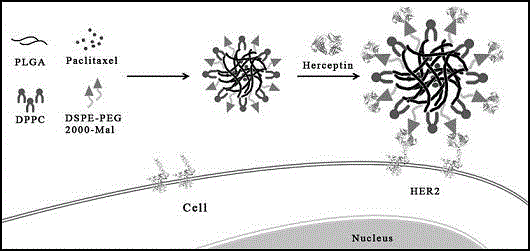
Herceptin modified paclitaxel-carried targeting nanoparticle transfer system
InactiveCN104814934AHigh encapsulation efficiencyTherapeutic level can be maintainedPowder deliveryOrganic active ingredientsCancer cellTumor therapy
The invention discloses a herceptin modified paclitaxel-carried targeting nanoparticle transfer system and a preparation method thereof. Nanoparticles (PLNs) with PLGA / Lipid core-shell structures are prepared by an improved classical emulsion solvent evaporation method, paclitaxel-carried polymer lipid nanoparticles are prepared by a binary organic solvent method, and herceptin modified paclitaxel-carried targeting nanoparticles (T=PCNs) are built by a chemical modification method. The prepared T=PCNs release drugs in vitro, sudden release rate within 24h is 41.2%, cumulative release rate within 120h is 73.4%, and cancer therapy is facilitated by drug release level. Cell experiment results indicate that the T=PCNs are fine in targeting property for highly expressed HER2 cancer cells and have an obvious killing function for the cancer cells. Safety and the targeting property of paclitaxel are greatly improved.
Owner:JILIN UNIV
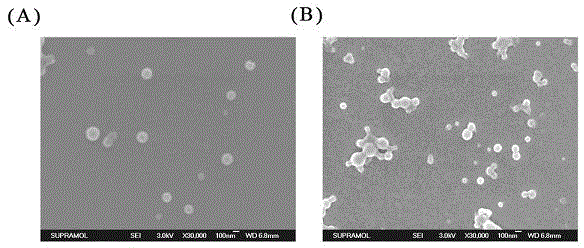
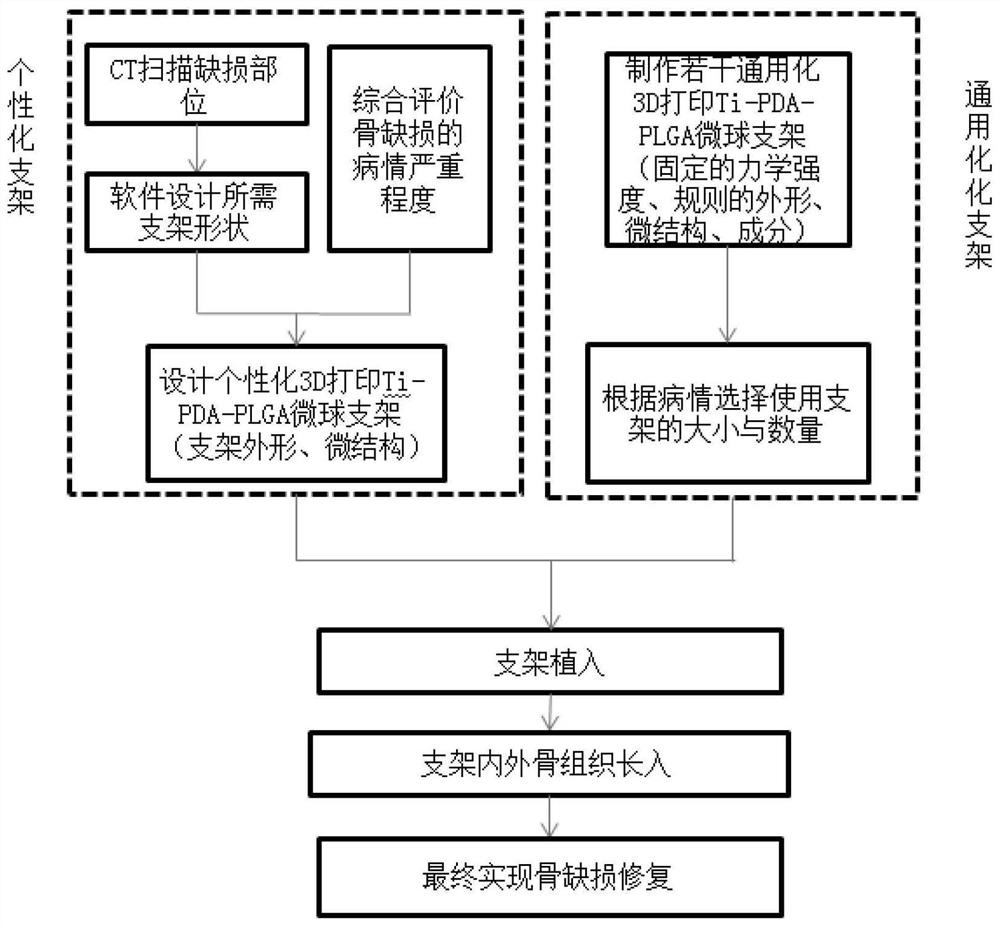
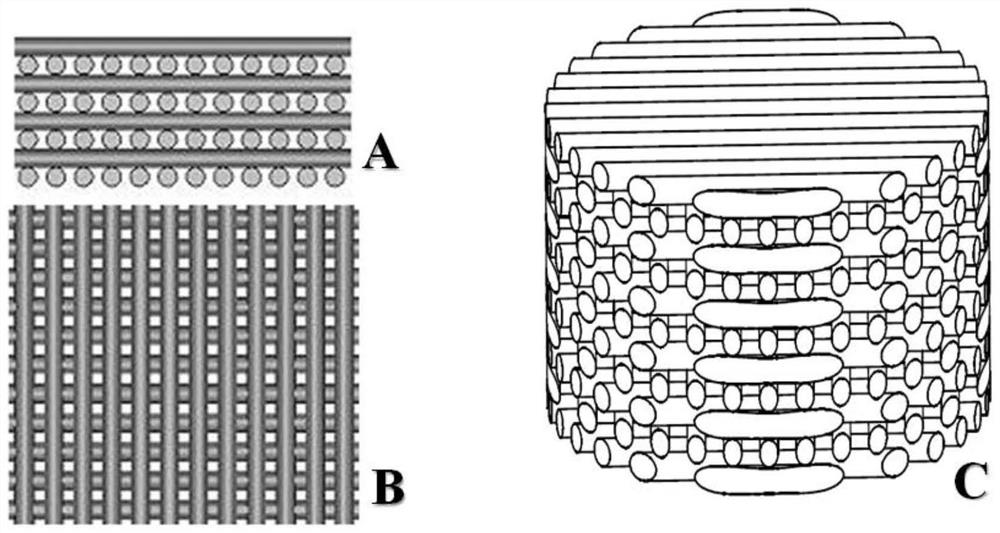
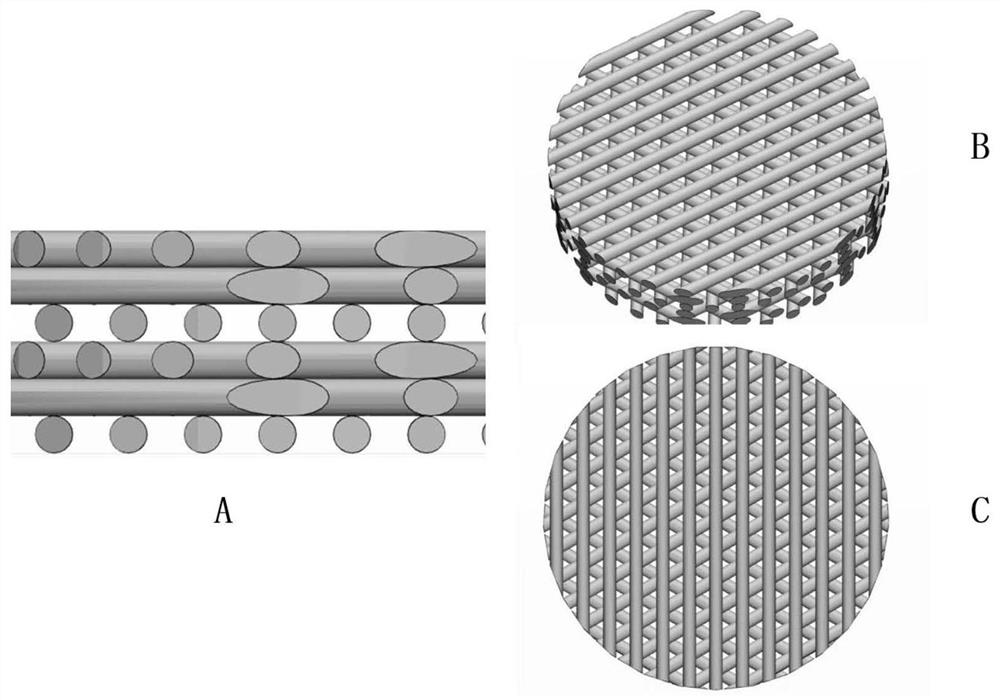
3D printed Ti-PDA-PLGA microsphere bone defect repair stent and preparation method thereof
InactiveCN112295014AAchieve fine controlAchieve sustained releaseAdditive manufacturing apparatusTissue regenerationRepair tissueMicrosphere
The invention discloses a 3D printed Ti-PDA-PLGA microsphere bone defect repair stent and a preparation method thereof. A 3D printed Ti stent is prepared through a laser sintering technology; then, under a certain condition, dopamine is self-polymerized on the fiber surface of the 3D printed Ti stent to form a PDA coating, so that the 3D printed Ti-PDA stent is prepared; and then VEGF-carrying PLGA microspheres is prepared by a multiple emulsion solvent evaporation method, and finally the BMP2 and the VEGF-carrying PLGA microspheres are absorbed and fixed on the surface of the stent by an adsorption method, finally the 3D printed Ti-PDA-PLGA microsphere bone defect repair stent is prepared. The bone defect repair tissue engineering stent of the invention has the advantages of reliable mechanical property, high biological activity and safety, convenience in implantation, small trauma and low cost, which can be used for repairing treatment of bone trauma, bone tumor and bone defect afterbone infection.
Owner:NANJING DONGSHANG BIOTECHNOLOGY CO LTD
Lixisenatide controlled-release microspheres and preparation method thereof
InactiveCN104248628AImproved particle size distributionNo obvious side effectsPeptide/protein ingredientsMetabolism disorderMicrosphereAmino acid
The invention related to the technical field of medicine and discloses lixisenatide controlled-release microspheres and a preparation method thereof. The lixisenatide controlled-release microspheres are prepared from lixisenatide, a biodegradable material and one or more stabilizing agents as controlled-release microsphere components and a surfactant aqueous solution as an external water phase by a water / oil / water multiple emulsion-solvent evaporation method, wherein the biodegradable material is polylactic acid, a lactic acid-glycolic acid polymer or polyglycolic acid and the one or more stabilizing agents are selected from polyol, sugar, inorganic salt, amino acids, polymers, human serum albumin and fatty glyceride. The lixisenatide controlled-release microspheres reduce administration frequency, can stably produce curative effects for a long time, have no toxic or side effect and good histocompatibility, can be applied by injection and non-injection methods and can be popularized and used in diabetes treatment.
Owner:HYBIO PHARMA
Preparation method of microcapsule-supported metal complex catalyst
ActiveCN103566971AHigh decolorization rateGood catalyticCatalyst carriersOrganic-compounds/hydrides/coordination-complexes catalystsEmulsionActive component
The invention relates to a preparation method of a microcapsule-supported metal complex catalyst. The preparation method comprises a multiple emulsion solvent evaporation method, an emulsion crosslinking method and an in-liquid drying method. When the supported metal complex catalyst provided by the invention and hydrogen peroxide are used for catalytic oxidation treatment of wastewater containing dyes, the catalyst is high in decolorization rate, remarkable in catalytic effect and recyclable. According to the technical scheme, catalytic active components can be favorably fixed, the stability of the catalyst is improved, the preparation method is simple in operation, and the catalyst is suitable for the catalytic oxidation treatment of the wastewater containing the dyes and can be recycled many times, so that the treatment cost is reduced.
Owner:DONGHUA UNIV
Nano drug carrier particles for improving bioavailability of rapamycin and preparation method thereof
InactiveCN102871966BGood water solubilityGood biocompatibilityPowder deliveryOrganic active ingredientsUltrafiltrationBiocompatibility Testing
The invention discloses nano drug carrier particles for improving the bioavailability of rapamycin and a preparation method thereof, so as to carry out a drug effect optimization. The nano drug carrier particles are prepared in the following steps that according to the principle of an emulsion solvent evaporation method, a determined amount of PEG-PLGA and rapamycin are respectively dissolved in acetone; after being mixed uniformly, the PEG-PLGA, the rapamycin and the acetone are slowly added into water to be stirred by magnetic force; after a certain period of time, the obtained liquid is ultrasonically homogenated, and organic phase is removed in a vacuum dryer; the obtained water phase removes free medicine in a centrifugal tube; and then an ultrafiltration tube is used for concentration, and nano particles are obtained after freezing and drying. The method has the advantages of convenience in operation, simplicity and feasibility, good repeatability and the like. The prepared nano drug carrier particles can improve the utilization rate of the medicine by improving the absorptivity of the medicine and prolonging the cycling time in a human body. Meanwhile, the nano particles which are prepared through the method have good biocompatibility, and surface active groups can further modify ligands or targeted groups.
Owner:SOUTHEAST UNIV
Reactive-oxygen-species sensitive nanoparticle capable of promoting vascularization of surface of wound and preparation method thereof
InactiveCN104587449ARelease stabilityTo achieve the effect of slow-release encapsulation proteinPowder deliveryPeptide/protein ingredientsMedicineActive component
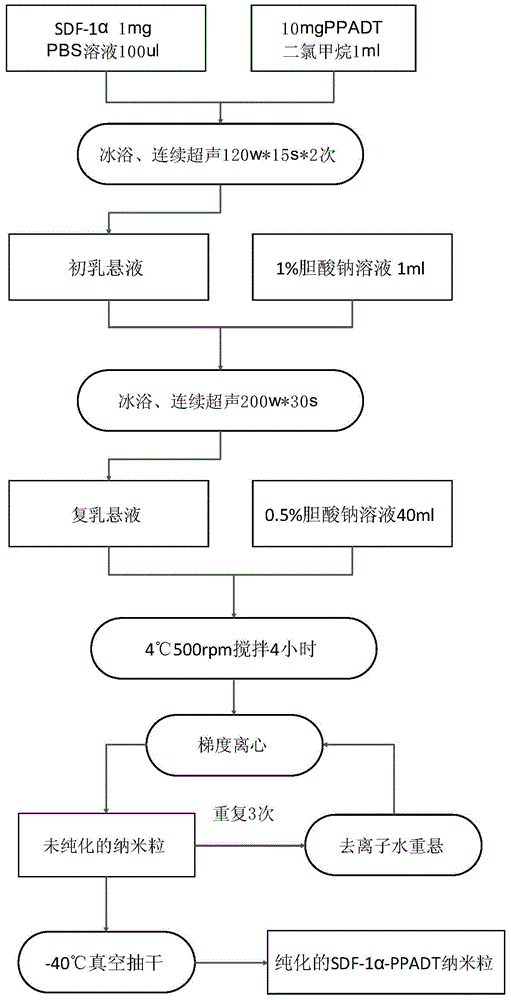
The invention belongs to the technical field of medicines and discloses a reactive-oxygen-species sensitive nanoparticle capable of promoting the vascularization of the surface of a wound and a preparation method thereof. The active component of the nanoparticle is a stromal cell derived factor 1 alpha (SDF-1alpha), and the medicine carrying nanomaterial is poly(1,4-phenyleneacetone dimethylenethioketal) (PPADT). The nanoparticle has a size of 70-130nm. The invention further discloses the preparation method of the nanoparticle. The medicine carrying nanoparticle is prepared by adopting a multiple emulsion-solvent evaporation method. The product has a good slow-releasing effect, is reduced in protein denaturation and improved in encapsulation rate and has good stability and low biotoxicity.
Owner:SECOND MILITARY MEDICAL UNIV OF THE PEOPLES LIBERATION ARMY
Latent light-sensitive microcapsule epoxy curing agent and preparation method thereof
The invention relates to the technical field of adhesives, in particular to a latent light-sensitive microcapsule epoxy curing agent and a preparation method thereof. The microcapsule epoxy curing agent takes a degradable polymer of a novel structure as a microcapsule wall material and an epoxy curing agent as a core material. The microcapsule wall material of the microcapsule epoxy curing agent can be degraded at a wavelength of 200-400 nm, and further the epoxy curing agent is released to cure epoxy resin; the problems that a system reaction takes a relatively long time, the rapid curing isnot sufficient and the like caused by reasons such as incomplete rupture of a microcapsule during curing, inadequate release of the curing agent after capsule rupture and the like in the curing reaction of epoxy resin performed by utilizing the microcapsule latent curing agent are solved; by means of an emulsion-solvent evaporation technology, the polymer or a mixture of other polymer materials doped with the polymer is used as the microcapsule wall material to encapsulate the epoxy resin curing agent, and the round microcapsule curing agent with a particle size of 1-100 mu m is prepared.
Owner:CHANGCHUN YONGGU TECH
A nanoparticle preparation for the treatment of brain diseases
ActiveCN102283812AGood treatment effectReduce moisture contentPowder deliveryOrganic active ingredientsDiseaseNasal cavity
The invention discloses a nanoparticle preparation for treating brain diseases. The nanoparticle preparation comprises the following raw materials in part by weight: 10 to 250mg of geniposide or total iridoid glycoside of cape jasmine fruit serving as a medicament carried by the nanoparticle preparation; 50 to 300mg of polylactic acid-glycolic acid copolymer serving as a carrier of the nanoparticle preparation; and 200 to 500mg of polyvinyl alcohol and 1 to 30mg of tween-80 or hydrogenated castor oil serving as emulsifiers of the nanoparticle preparation. The invention also discloses the nanoparticle preparation, which is modified by chitosan, wherein the geniposide or total iridoid glycoside of cape jasmine fruit serves as a carried medicament of the nanoparticle preparation. The nanoparticle preparation is prepared by a multiple emulsion-solvent evaporation method. The preparation is administrated through nasal cavities, so the concentration of the geniposide or total iridoid glycoside of cape jasmine fruit in brain tissues is effectively increased. Compared with preparations prepared from the geniposide or total iridoid glycoside of cape jasmine fruit, which are administrated through gastrointestinal tracts, intramuscular injection and the like, the nanoparticle preparation has the advantages of small dosage and high patient compliance.
Owner:JIANGXI UNIVERSITY OF TRADITIONAL CHINESE MEDICINE
pH-sensitive nanoparticles for oral insulin delivery
ActiveUS8859004B2Improve bioavailabilityImprove oral bioavailabilityBiocideNanotechPh sensitive nanoparticlesPH-sensitive polymers
Owner:NANO & ADVANCED MATERIALS INST
Novel injection self-condensing composite artificial bone carrying rhBMP_2 micro spheres
The invention provides a novel injection self-condensing composite artificial bone carrying rhBMP_2 micro spheres. The artificial bone comprises raw materials of composite micro spheres carrying rhBMP_2, modified calcium phosphate bone cement CPC, collagen and nano bacterial cellulose, wherein the rhBMP_2 composite micro spheres are prepared by PLGA, rhBMP_2 and PAV by adopting a multiple emulsion-solvent evaporation technology; the modified calcium phosphate bone cement is prepared by the mixing of hydroxyl poly calcium sodium phosphate powder and physiological saline with dissolved carbodimide. According to the composite micro spheres carrying the rhBMP_2, the volume ratio of the modified calcium phosphate bone cement is 50-70:30-50, the collagen adding amount is 5-10% of the total mass,the adding amount of the nano bacterial cellulose is 2-6% of the total mass. The composite artificial bone has good bone forming osteoinductive performance, and the mechanical strength is high.
Owner:PLA NO 5 HOSPITAL
Sustained -release antibacterial compound bone grafting material and preparation method thereof
ActiveCN107469155ALong local release timeImprove the bactericidal effectAntibacterial agentsInorganic active ingredientsMicrosphereMedicine
The invention discloses a sustained-release antibacterial compound bone grafting material and a preparation method thereof. The sustained-release antibacterial compound bone grafting material is mainly prepared by mixing nanosilver / TMC-207 sustained release microspheres, nano-hydroxyapatite and calcium phosphate cement powder. The preparation method comprises the following steps: firstly, preparing the nanosilver / TMC-207 sustained release microspheres by an O / O emulsion-solvent evaporation method, wherein PLGA is used as a carrier material; secondly, mixing the nanosilver / TMC-207 sustained release microspheres, the nano-hydroxyapatite and the calcium phosphate cement powder, and blending an obtained mixture with deionized water to obtain paste; setting and drying the paste to obtain the slow-release antibacterial compound bone grafting material. The sustained-release antibacterial compound bone grafting material disclosed by the invention has the advantages of long local drug release time, obvious sterilizing effect and high bone repairing capability. The preparation method has the advantages of being simple and convenient to operate and lower in cost.
Owner:XIANGYA HOSPITAL CENT SOUTH UNIV
Progestin sustained-release microsphere and nanoparticle and preparation method thereof and progestin sustained-release injection
InactiveCN107157957ARound shapeUniform particle size distributionOrganic active ingredientsPharmaceutical non-active ingredientsLife qualityMedicine
The invention provides a progestin sustained-release microsphere and nanoparticle and a preparation method thereof and a progestin sustained-release injection. The preparation provided by the invention comprises progestin and biodegradable polymers PLGA, PLA, PCL and PGA; and the progestin microsphere is prepared by adopting an emulsion-solvent evaporation method and the progestin nanoparticle is prepared by adopting a nano-emulsification method in the process aspect. According to the prepared microsphere, the particle size is within a range of 10-20 microns, the drug loading capacity is 30-40% and the encapsulation efficiency is greater than 90%; and according to the nanoparticle, the particle size is within a range of 100-400nm, the drug loading capacity can reach 10-20% and the encapsulation efficiency is 80-90%. The progestin preparation requires frequent administration for multiple times; and the sustained-release injection has good correlation in vivo and vitro, the sustained-release effect of more than a week is reached, the medication compliance of a patient can be improved, and the living quality is improved.
Owner:CHANGCHUN GENESCIENCE PHARM CO LTD
pH-sensitive nanoparticles for oral insulin delivery
ActiveCN102908627AFacilitated releaseLow encapsulation efficiencyNanotechPeptide/protein ingredientsPh sensitive nanoparticlesOral medication
The present invention discloses the pH-sensitive nanoparticles composed of pH-sensitive polymer, hydrophobic material, internal stabilizer, external stabilizer content and insulin drug. The present invention also includes a method for preparation of pH-sensitive nanoparticles, in particular, a multiple emulsions solvent evaporation method. The pH-sensitive nanoparticles of the present invention show good pH-sensitive property with 100-300 nanometer particle size. Significant decrease in blood glucose level is observed in streptozotocin (STZ)-induced diabetic rats and the bioavailability of insulin is more than 10% after oral administration of the insulin-loaded pH-sensitive nanoparticles.
Owner:NANO & ADVANCED MATERIALS INST
Radiosensitizer and preparation method thereof
InactiveCN104415348AReduce radiosensitivityImprove hypoxiaOrganic active ingredientsGenetic material ingredientsRadiation sensitizersHypericin
The invention relates to a radiosensitizer and a preparation method thereof, and the radiosensitizer can improve a radiation resistance problem of solid tumors; the radiosensitizer comprises 50%-80% of PLGA-PEI / hypericin nano colloidal particles, 5%-8% of HIF-1alpha-siRNA, and 12%-45% of hyaluronic acid, wherein the hyaluronic acid molecular weight can be any one of 11000, 6600 and 800. The preparation method comprises the steps of firstly preparing the PLGA-PEI / hypericin nano colloidal particles by an emulsion solvent evaporation method, then dropwise adding the HIF-1alpha-siRNA, and finally wrapping with HA. The preparation process is simple and easy to implement.
Owner:汪步海
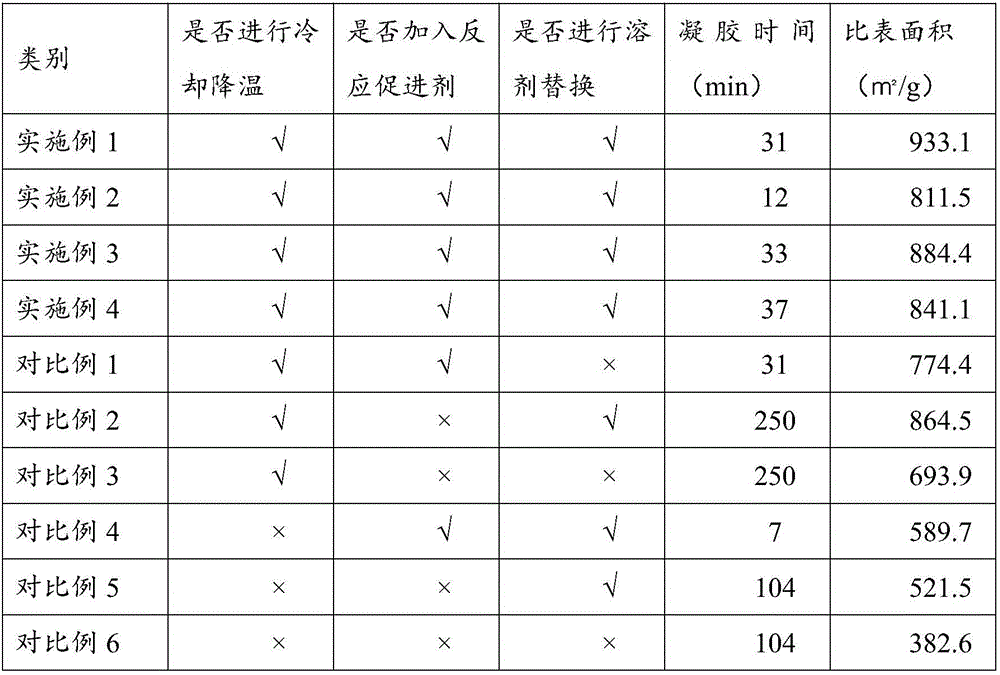
Silicon dioxide aerogel with high specific surface area and fast preparation method thereof
The invention discloses a silicon dioxide aerogel with a high specific surface area and a fast preparation method thereof. The preparation method comprises the following steps: cooling all raw materials, in a cooling condition, mixing a silicone precursor, a solvent, an acidic catalyst and water, then adding an alkaline catalyst and a reaction accelerant, leaving the mixture to stand to gelatinize the mixture, and aging the mixture in the condition; transferring the aged gel into an exchange liquid to be soaked for solvent exchange; and performing supercritical drying on the gel after solvent exchange to obtain the silicon dioxide aerogel. By adopting a mode of cooling preparation, collapse of holes of the aerogel in a nano-structure due to solvent evaporation in the preparation and gel aging processes is avoided, and meanwhile, by using the reaction accelerant, the reaction rate at a low temperature is accelerated and the preparation efficiency is improved. In addition, an exchange liquid is used to replace the solvent in the surface layer of the gel before supercritical drying, so that the damage of the aerogel structure due to solvent evaporation in the temperature-raising and pressure-raising process is reduced, and the prepared silicon dioxide aerogel has a good specific surface area.
Owner:GUANGDONG ALISON HI TECH
Polylactic acid/nano-hydroxyapatite composite material as well as preparation method and application thereof
ActiveCN111286074AAdvantages of preparation methodEvenly dispersedPharmaceutical delivery mechanismProsthesisMicrosphereNano hydroxyapatite
The invention provides a polylactic acid / nano-hydroxyapatite composite material as well as a preparation method and application thereof. The preparation method comprises the following steps: firstly,preparing polylactic acid / nano-hydroxyapatite composite microspheres as a powder raw material by adopting an emulsion solvent evaporation method, then putting the powder raw material into a mold, heating, pressurizing and molding the powder raw material to obtain the polylactic acid / nano-hydroxyapatite composite material. By adopting the preparation method disclosed by the invention, uniform dispersion of the nano-hydroxyapatite in a polylactic acid matrix is facilitated, so that the mechanical property and the impact strength of the product are improved, the microstructure of the molded product is easy to control, and a microsphere bonded porous structure and a microsphere fused compact structure can be obtained by adjusting the temperature and the pressure or adding a pore-forming agent.
Owner:WUHAN UNIV OF TECH
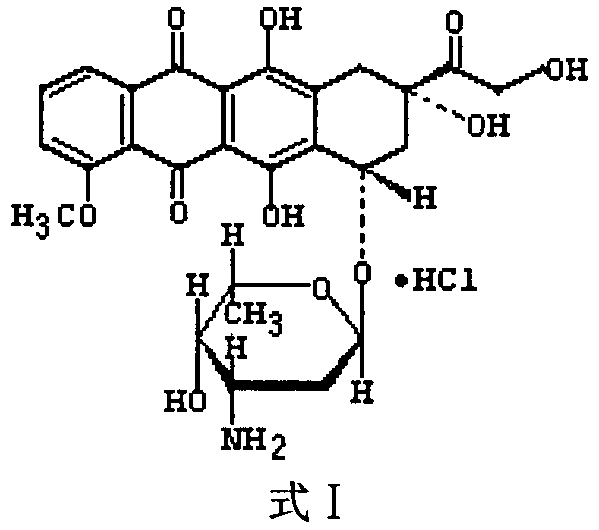
Preparation method for surface-laminated and self-assembled doxorubicin hydrochloride copolymer nano-particles
ActiveCN106491559AOrganic active ingredientsPharmaceutical non-active ingredientsPolyelectrolyteRelease time
The invention relates to the technical field of a pharmaceutical preparation and specifically relates to surface-laminated and self-assembled doxorubicin hydrochloride copolymer nano-particles and a preparation method thereof. According to the invention, a multiple emulsion-solvent evaporation process is adopted for preparing doxorubicin hydrochloride-loaded lactic acid-glycolic acid copolymer nano-particles, and then natural hydrophilic polyelectrolyte chitosan and alginic acid are selected for laminating and self-assembling on the surfaces of the nano-particles. The self-assembled nano-particles prepared according to the invention have particle sizes within the scope of 100-300nm and are uniformly distributed; the burst release of the medicine can be reduced, and the release time of the medicine can be prolonged; the nano-particles are slowly released under a physiological pH value, so that the nano-particles are beneficial to anti-tumor application; and the nano-particles have certain long circulation effects and excellent market prospects.
Owner:JIANGSU HEALTH VOCATIONAL COLLEGE
Solid-state avermectin particle preparation and preparation method and application thereof
ActiveCN105851022ASolve the problem of unstable storage processEasy to prepareBiocideAnimal repellantsMicrosphereMembrane emulsification
The invention discloses a solid-state avermectin particle preparation and a preparation method and application thereof. Particles comprise microspheres and microcapsules. A preparation method of the microspheres includes following steps: 1), dissolving avermectin in an organic solvent to obtain an organic solution of avermectin as an oil phase; 2), dissolving a stabilizer in water to obtain a stabilizer water solution as an outer water phase; 3), mixing the oil phase with the outer water phase for mechanical stirring to obtain an oil-in-water primary emulsion; 4), utilizing a membrane emulsification device to perform membrane filtering on the oil-in-water primary emulsion under action of nitrogen to obtain an oil-in-water emulsion; 5), sequentially stirring and centrifuging the oil-in-water emulsion to collect precipitate, washing with water, and drying to obtain the solid-state avermectin microspheres. A membrane emulsification method is combined with an emulsion solvent evaporation method to obtain the solid-state avermectin microspheres and microcapsule preparation which has the advantages of stability in storage, safety and convenience in transport and use and low cost.
Owner:INST OF CHEM CHINESE ACAD OF SCI
BMP-2/PPLA (bone morphogenetic protein-2/polylactic acid and polyethylene glycol block copolymer) microspheres and preparation method thereof
ActiveCN106620654AImprove hydrophilicityReduce adsorptionPeptide/protein ingredientsSkeletal disorderMicrospherePolyethylene glycol
The invention discloses BMP-2 / PPLA (bone morphogenetic protein-2 / polylactic acid and polyethylene glycol block copolymer) microspheres and a preparation method thereof. The method comprises stages as follows: a stage of preparing an initial emulsion by dissolving or dispersing BMP-2 in an emulsion phase with PPLA dissolved; a stage of preparing a compound emulsion by dispersing the initial emulsion in a PVA solution; a stage of preparing the microspheres encapsulated with BMP-2 after an organic solvent in the compound emulsion is removed. The PPLA is taken as a supporter material, BMP-2 is subjected to entrapment with a compound emulsion solvent evaporation method, and the microspheres are prepared; the obtained microspheres have smooth surfaces, uniform sizes and high stability and can be used for local injection administration, so that BMP-2 acts on application parts in a long-acting and stable manner, and bone growth is promoted.
Owner:SHANDONG UNIV +1
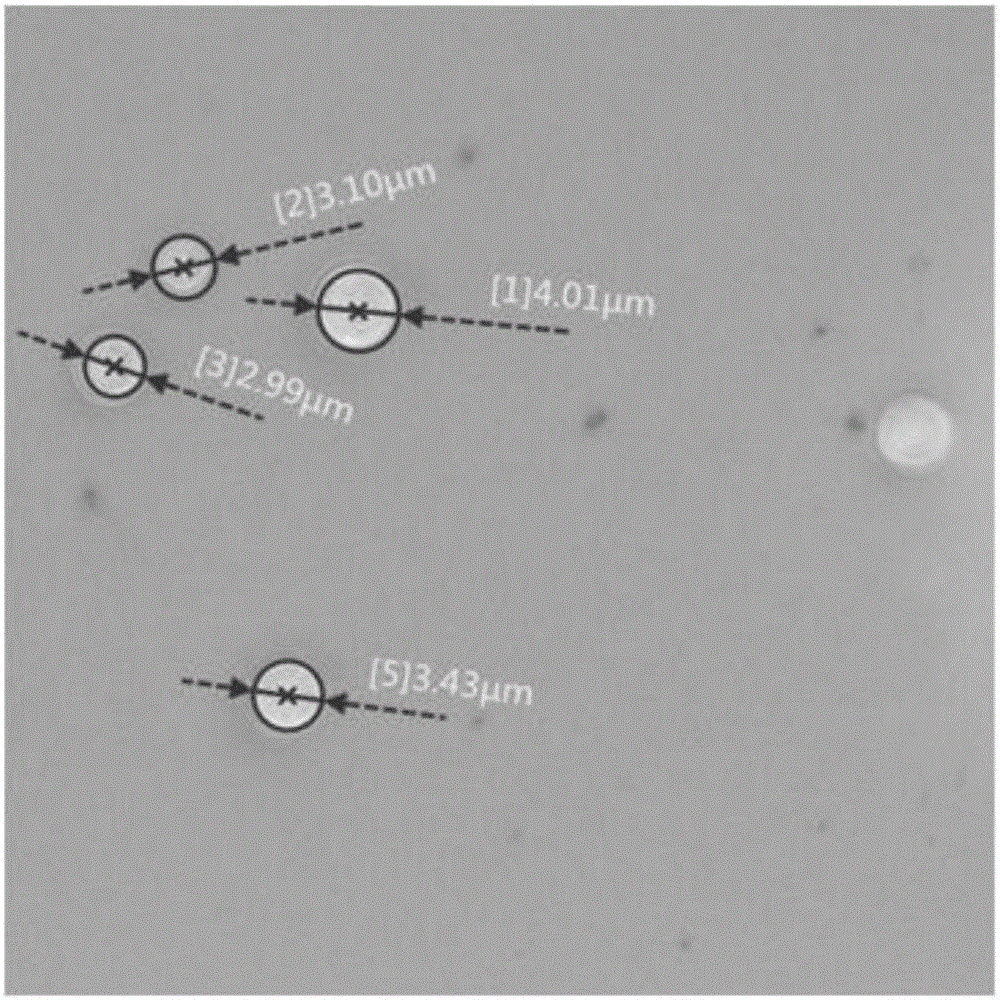
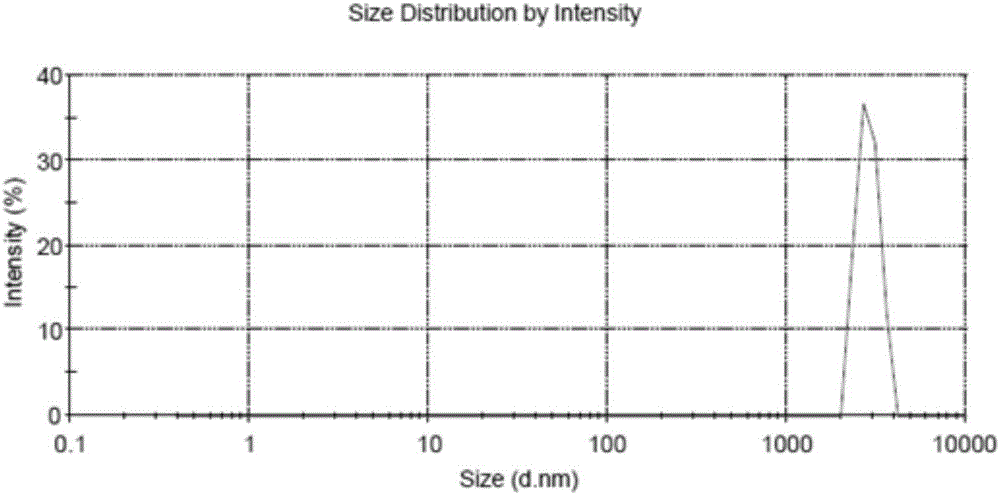
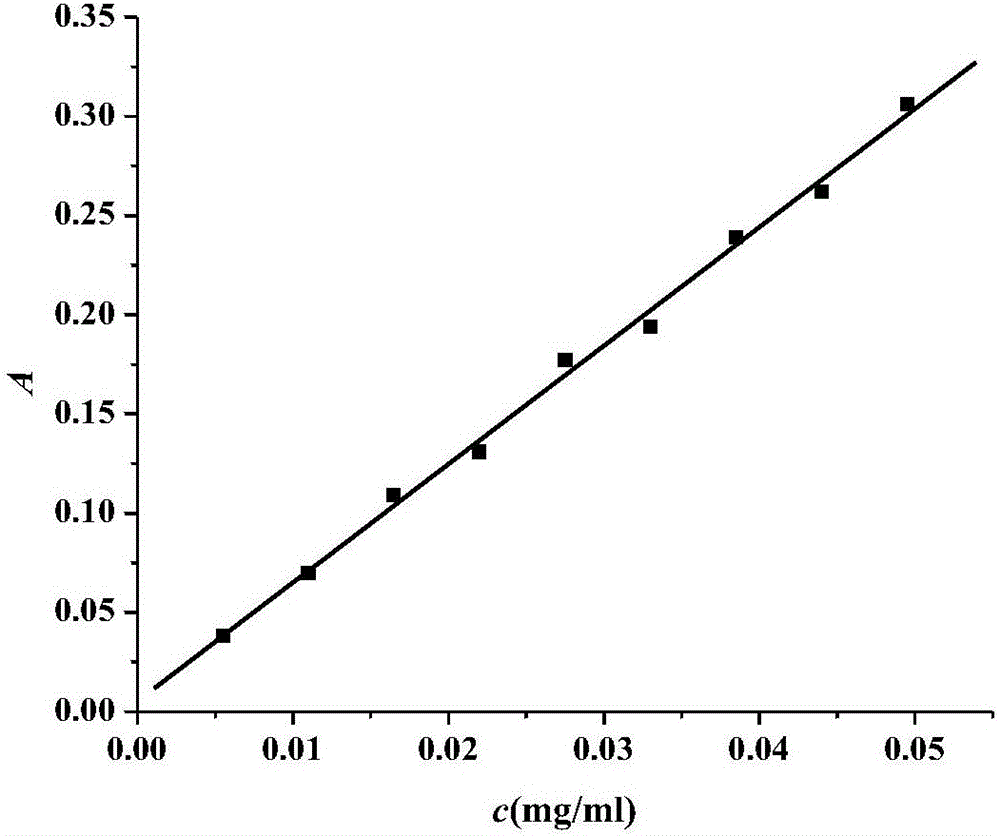
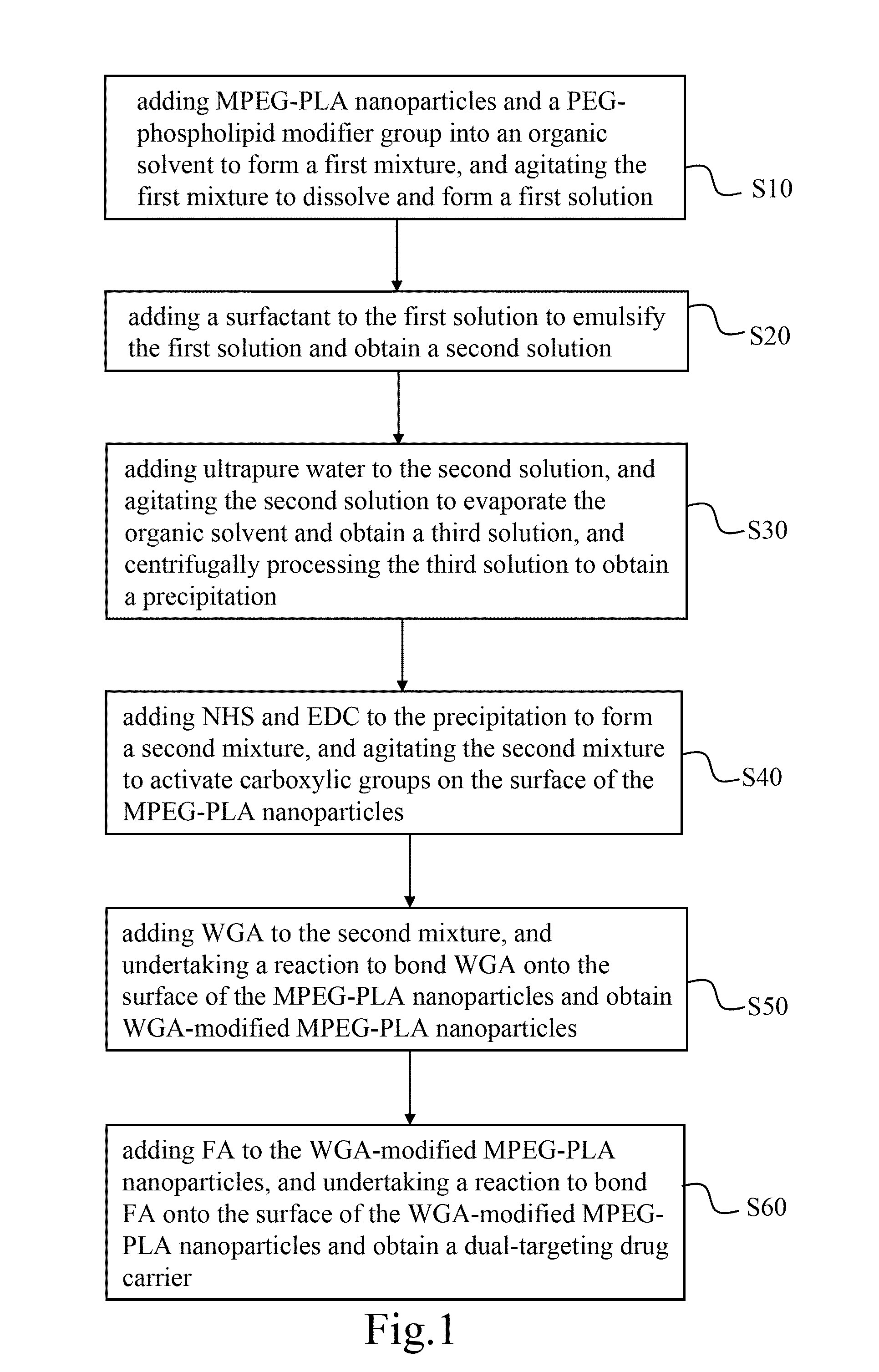
Dual-targeting drug carrier and method for fabricating the same
The present invention discloses a dual-targeting drug carrier and a method for fabricating the same, wherein WGA- and FA-modified MPEG-PLA nanoparticles of the carrier enable the anticancer drugs encapsulated thereinside to pass through BBB and target human glioblastoma cells. The dual-target drug carrier is fabricated in an emulsion-solvent evaporation technology and verified with an in-vitro BBB model formed of HBMECs, HAs and HBVPs. The present invention can increase the permeability of the in-vitro BBB model to the dual-target drug carrier and promote the glioblastoma-inhibition effect. Therefore, the present invention would contribute to the clinical therapy of brain cancers substantially in the future.
Owner:NATIONAL CHUNG CHENG UNIV
Phenolphthalein modified polyaryletherketone water-based sizing agent as well as preparation method and application thereof
PendingCN113563577AImprove solubilityImprove dynamic stabilityCarbon fibresPolymer scienceActive agent
The invention provides a phenolphthalein modified polyaryletherketone water-based sizing agent as well as a preparation method and application thereof. The sizing agent is prepared from the following components in percentage by mass: 0.5 to 3 parts of phenolphthalein modified polyaryletherketone resin, 0.2 to 5 parts of a surfactant, 0.1 to 1 part of a flatting agent, 0.1 to 1 part of a lubricating agent, 0.1 to 1 part of a de-foaming agent, 0.1 to 2 parts of an adhesive, 0.2 to 1 part of an antistatic agent and 86 to 98.7 parts of de-ionized water. According to the principle of 'like dissolves like',the main slurry of the sizing agent provided by the invention is polyaryletherketone resin containing a distorted non-coplanar phenolphthalein structural unit with a carboxyl functional side group, so that excellent compatibility of the sizing agent and polyaryletherketone matrix resin can be ensured, and the sizing agent also has relatively good thermal stability. The water-based sizing agent is prepared through an emulsion solvent evaporation method, interface adhesion can be enhanced through diffusion and physical entanglement effects between the sizing agent and the matrix resin; and carboxyl contained in phenolphthalein can further form hydrogen bonds with ketone groups in the matrix resin, and interface adhesion between the sizing agent and the matrix resin is further improved.
Owner:CHANGCHUN UNIV OF TECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com