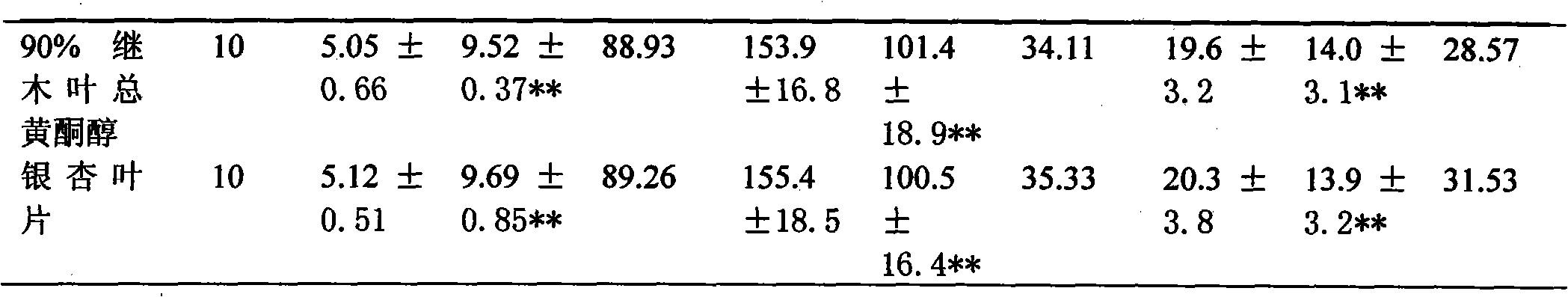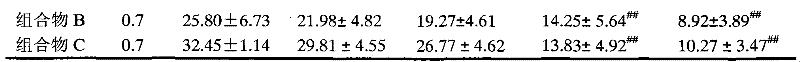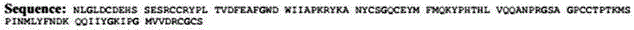Patents
Literature
110 results about "Streptozotocin" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Streptozotocin or streptozocin (INN, USP) (STZ) is a naturally occurring alkylating antineoplastic agent that is particularly toxic to the insulin-producing beta cells of the pancreas in mammals. It is used in medicine for treating certain cancers of the islets of Langerhans and used in medical research to produce an animal model for hyperglycemia in a large dose, as well as type 2 diabetes or type 1 diabetes with multiple low doses.
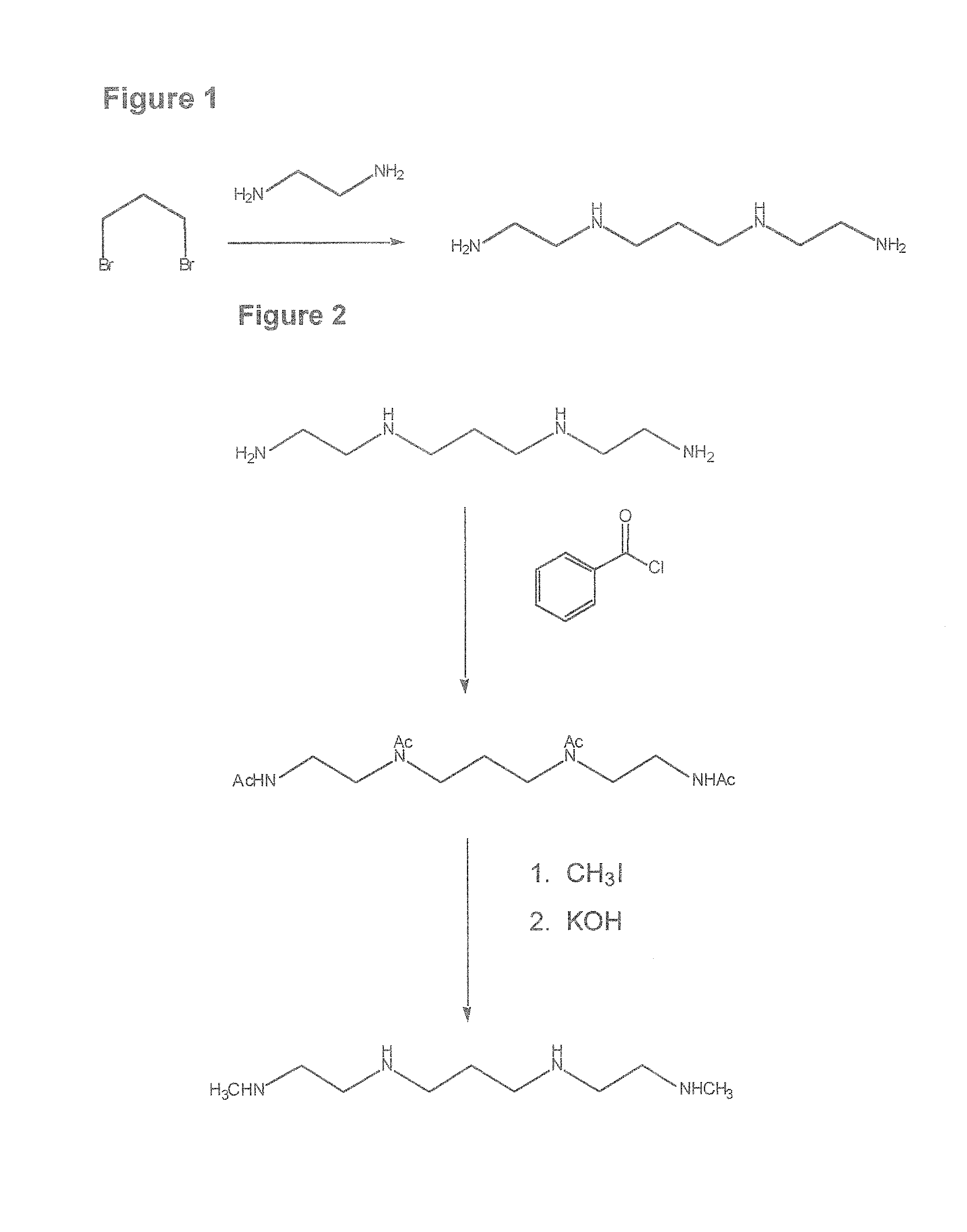
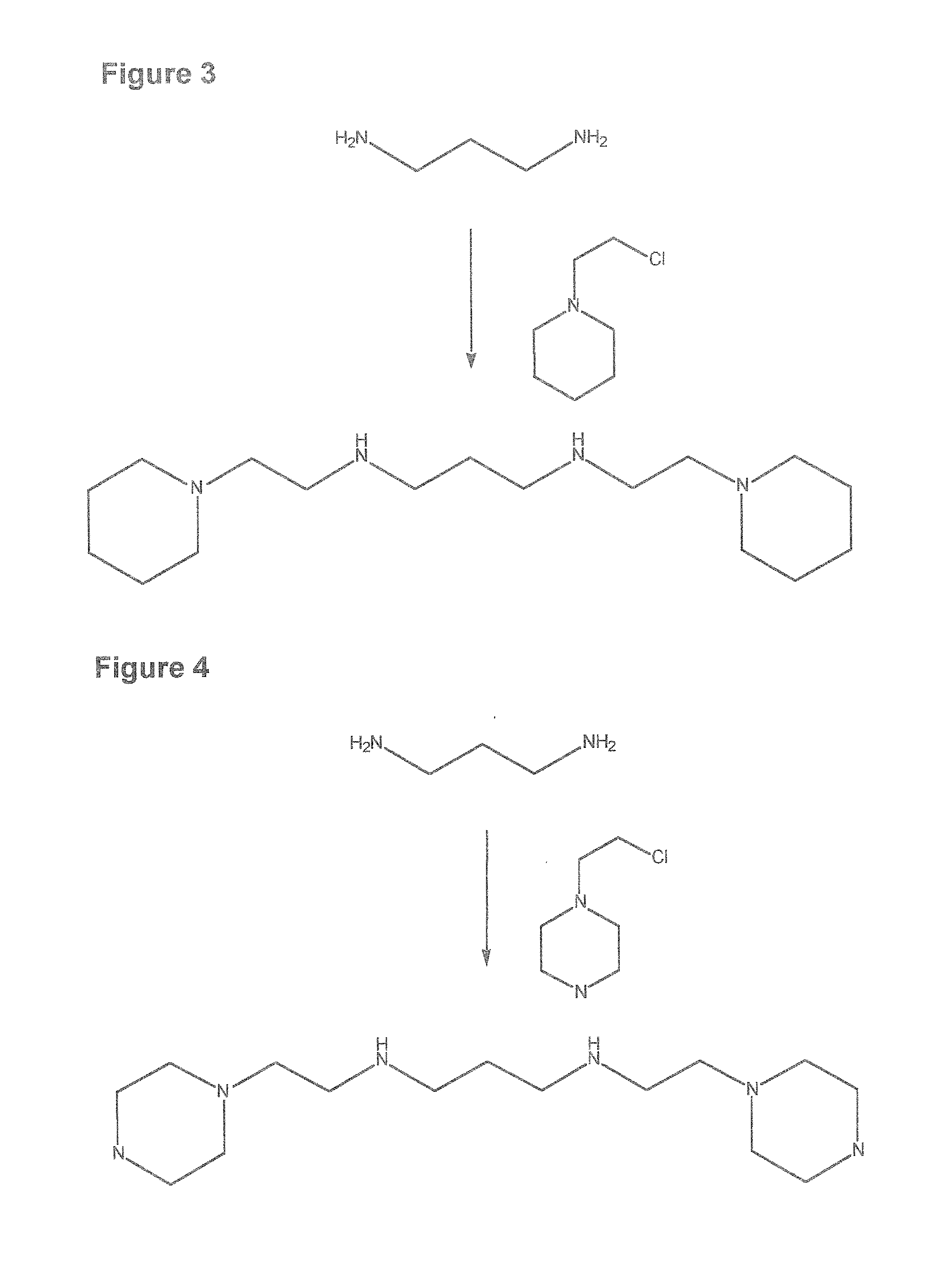
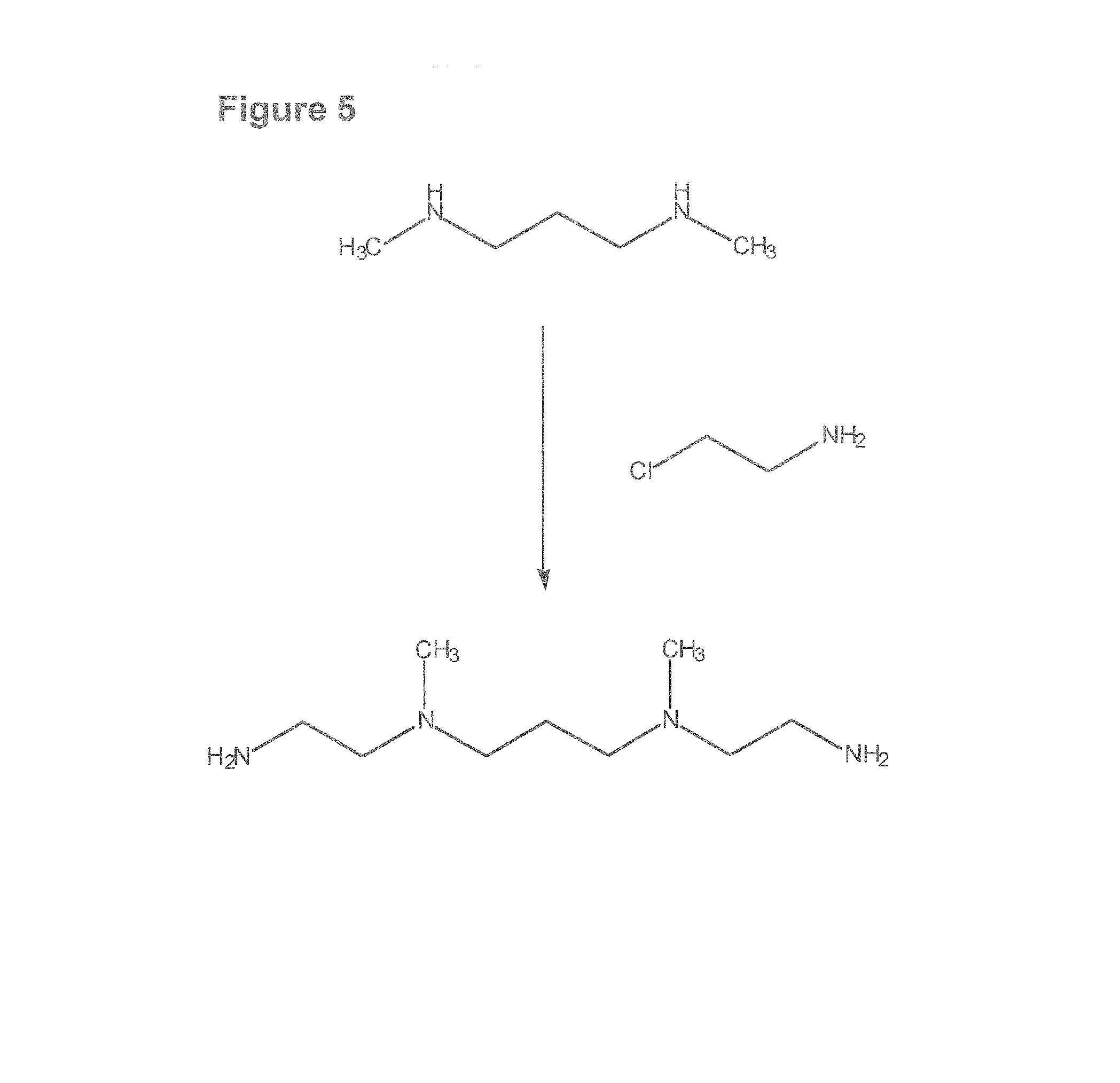
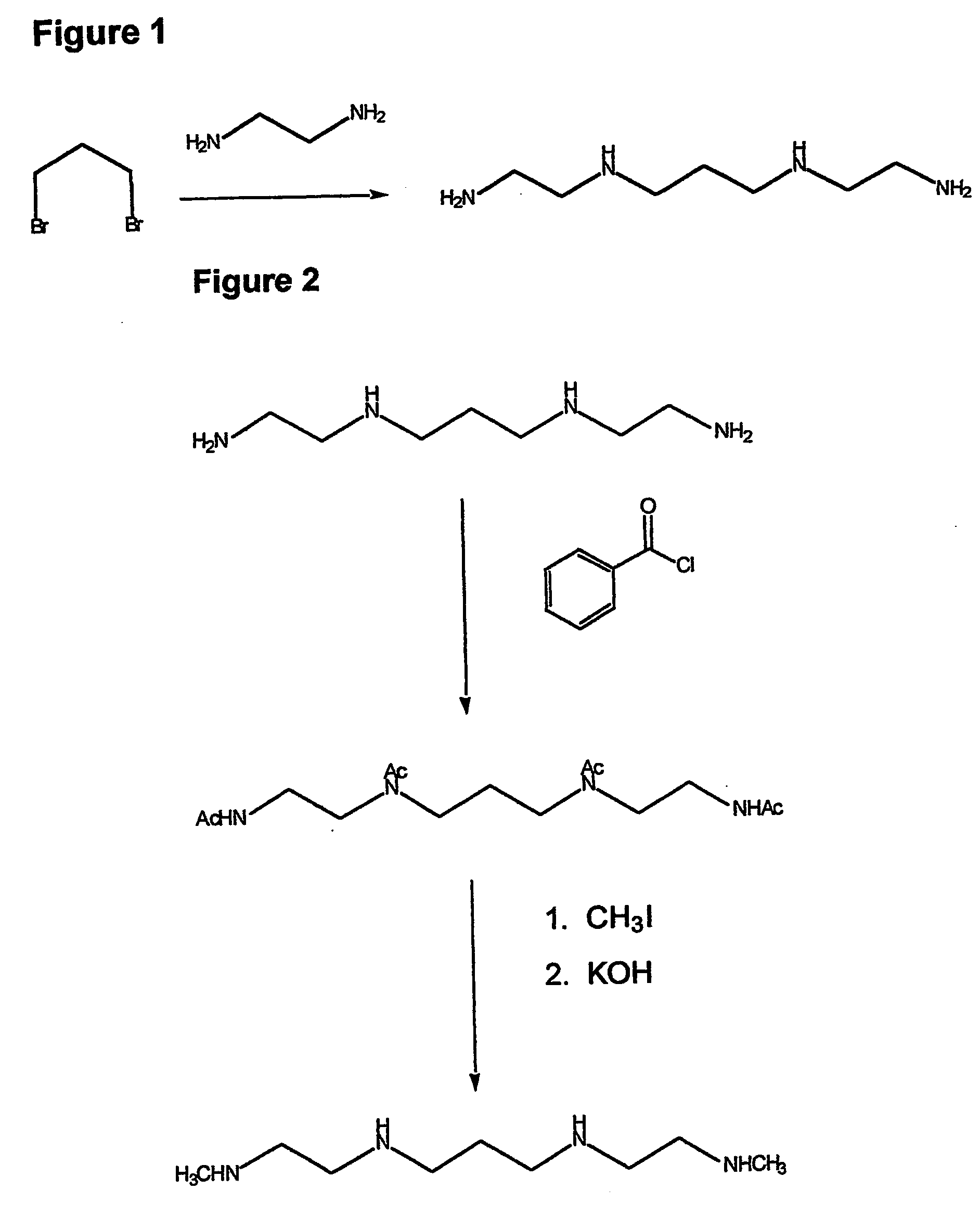
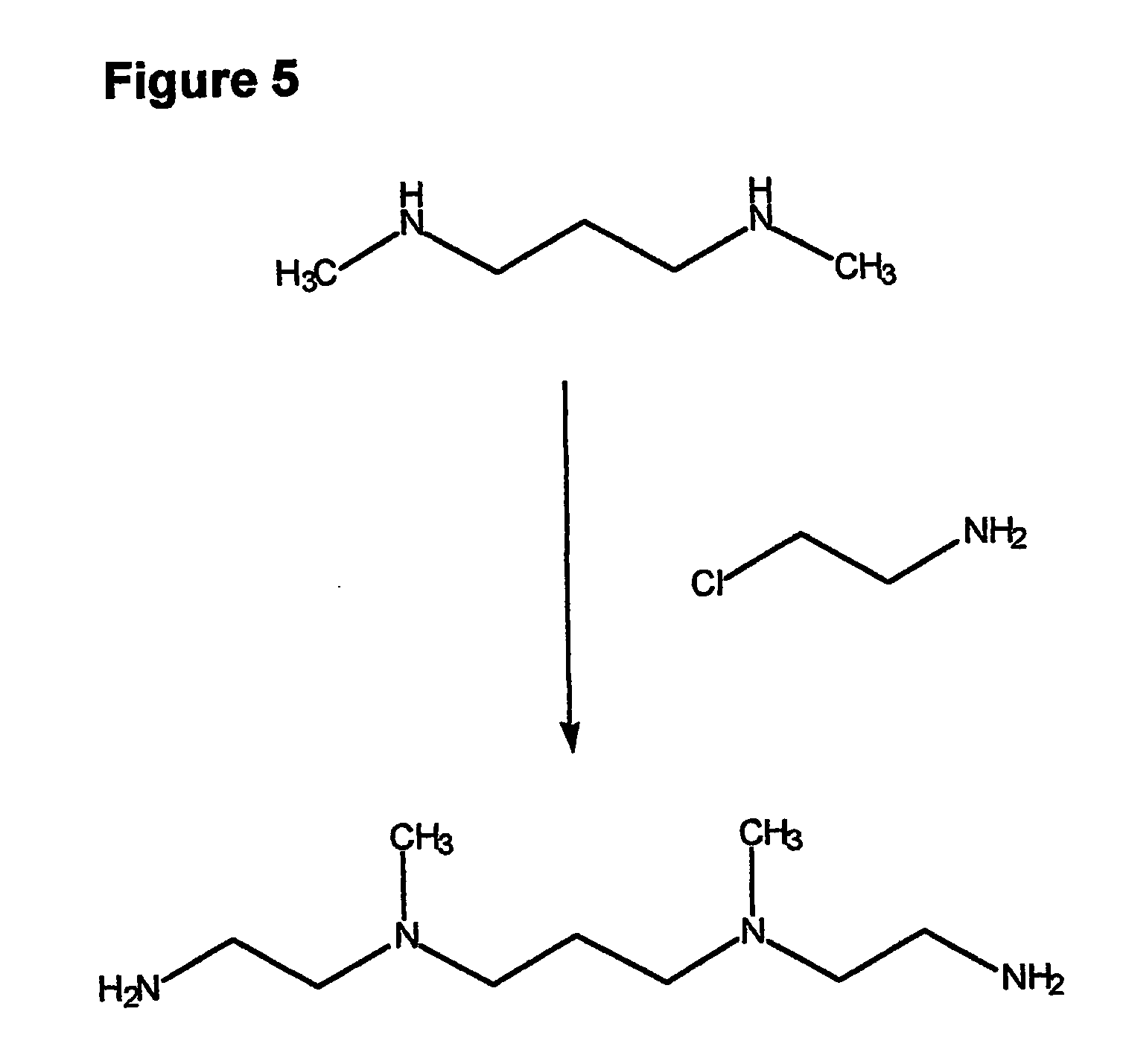
Composition, synthesis and therapeutic applications of polyamines
InactiveUS20050085555A1Chromium concentration were decreasedIncrease excretionBiocideGroup 5/15 element organic compoundsAntidoteRisk stroke
This invention relates to a process of synthesis and composition of open chain (ring), closed ring, linear branched and or substituted polyamines, polyamine derived tyrosine phosphatase inhibitors and PPAR partial agonists / partial antagonists via a series of substitution reactions and optimizing the bioavailability and biological activities of the compounds. Polyamines prevent the toxicty of neutoxins and diabetogenic toxins including paraquat, methyphenyl pyridine radical, rotenone, diazoxide, streptozotocin and alloxan. These polyamines can be to treat neurological, cardiovascular, endocrine acquired and inherited mitochondrial DNA damage diseases and other disorders in mammalian subjects, and more specifically to the therapy of Parkinson's disease, Alzheimer's disease, Lou Gehrig's disease, Binswanger's disease, Olivopontine Cerebellar Degeneration, Lewy Body disease, Diabetes, Stroke, Atherosclerosis, Myocardial Ischemia, Cardiomyopathy, Nephropathy, Ischemia, Glaucoma, Presbycussis, Cancer, Osteoporosis, Rheumatoid Arthritis, Inflammatory Bowel Disease, Multiple Sclerosis and as Antidotes to Toxin Exposure.
Owner:MURPHY MICHAEL A
Fatty-liver-related liver cancer model building method based on knockout mice
ActiveCN105052830AIncrease incidenceMicrobiological testing/measurementAnimal husbandryKnockout animalGenotype
The invention relates to a fatty-liver-related liver cancer model building method based on knockout mice. The method is characterized in that APOE- / - and LDLR- / - knockout mice including female mice and male mice are selected, the female mice and the male mice are matched in number, and double knockout mice are obtained; a primer is designed, and the genotypes in mice are identified through a PCR (polymerase chain reaction) system; the PCR (polymerase chain reaction) system comprises an ApoE gene identification PCR conditions and LDLR gene identification PCR conditions; the double knockout mice are pregnant, and suckling mice are obtained; streptozotocin is injected to the suckling mice, the suckling mice begin a high-fat diet after ablactation, and finally the suckling mice surfer from liver cancer; it is identified that the suckling mice begin the high-fat diet after ablactation, fatty liver occurs six weeks later, NASH occurs 8-10 weeks later, dysplastic nodule occurs 13-16 weeks later, and the liver cancer occurs 18-24 weeks later. The occurrence rate of the liver cancer is about 100% 24 weeks later. The time when pure-gene APOE- / - and LDLR- / - knockout mice suffer from NASH and HCC due to the high-fat diet is advanced by 24-30 weeks, and the occurrence rate is high.
Owner:施军平
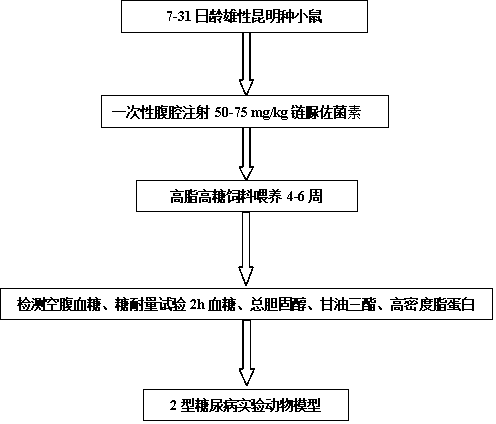
Construction method for mouse type 2 diabetes mellitus (T2DM) animal experiment model
ActiveCN104257671ASmall doseHigh molding rateOrganic active ingredientsAnimal husbandryIntraperitoneal routeHigh fat
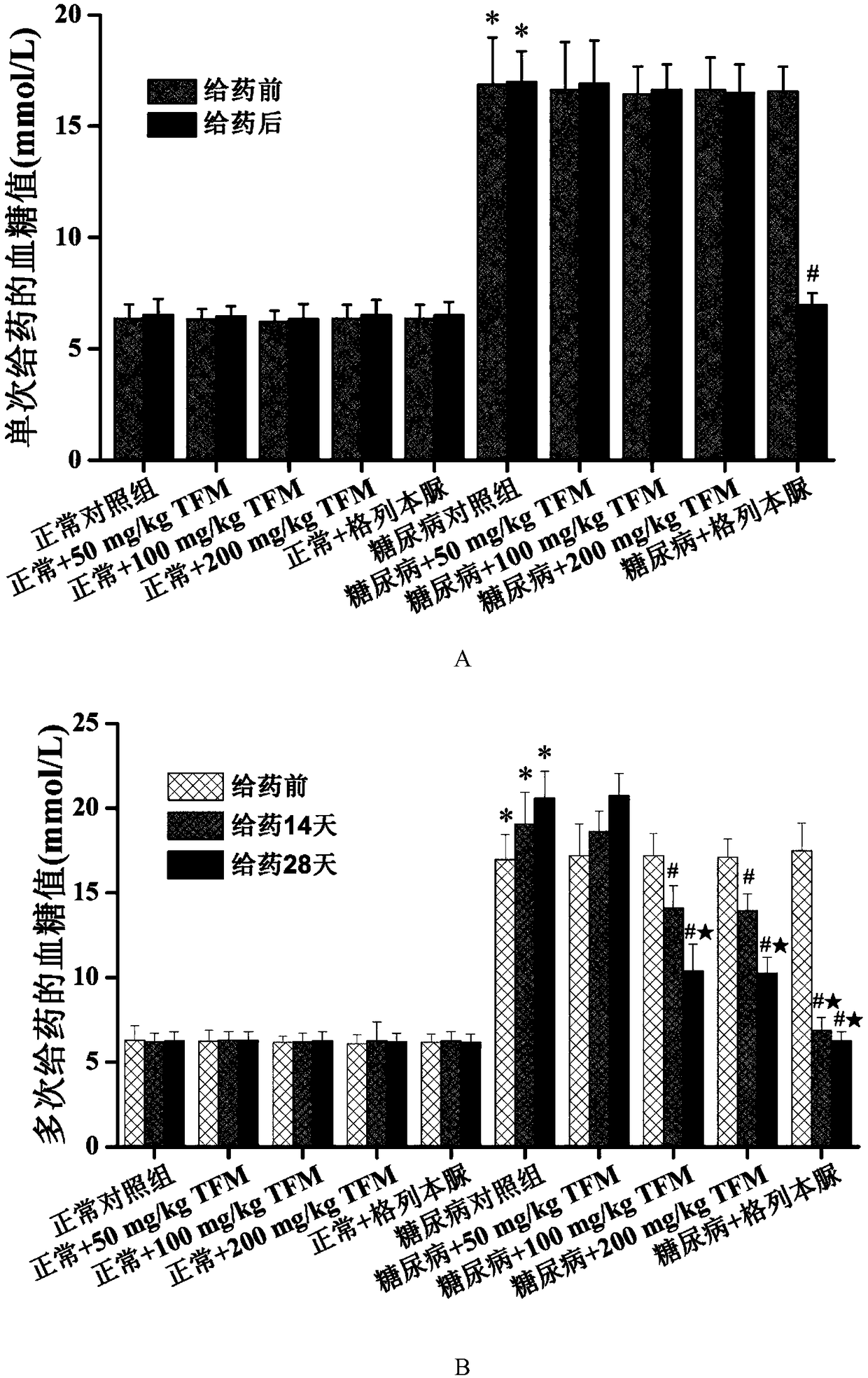
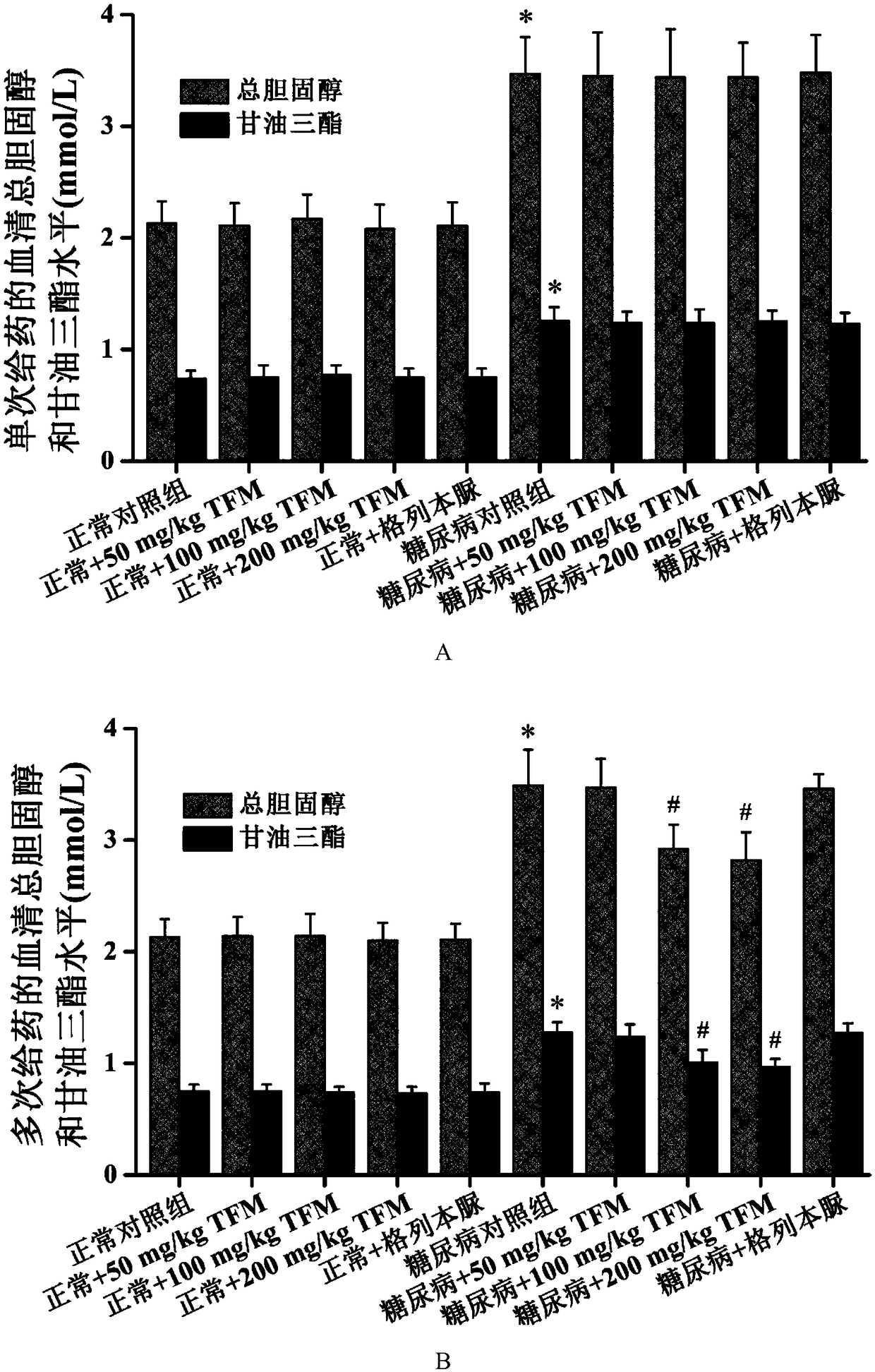
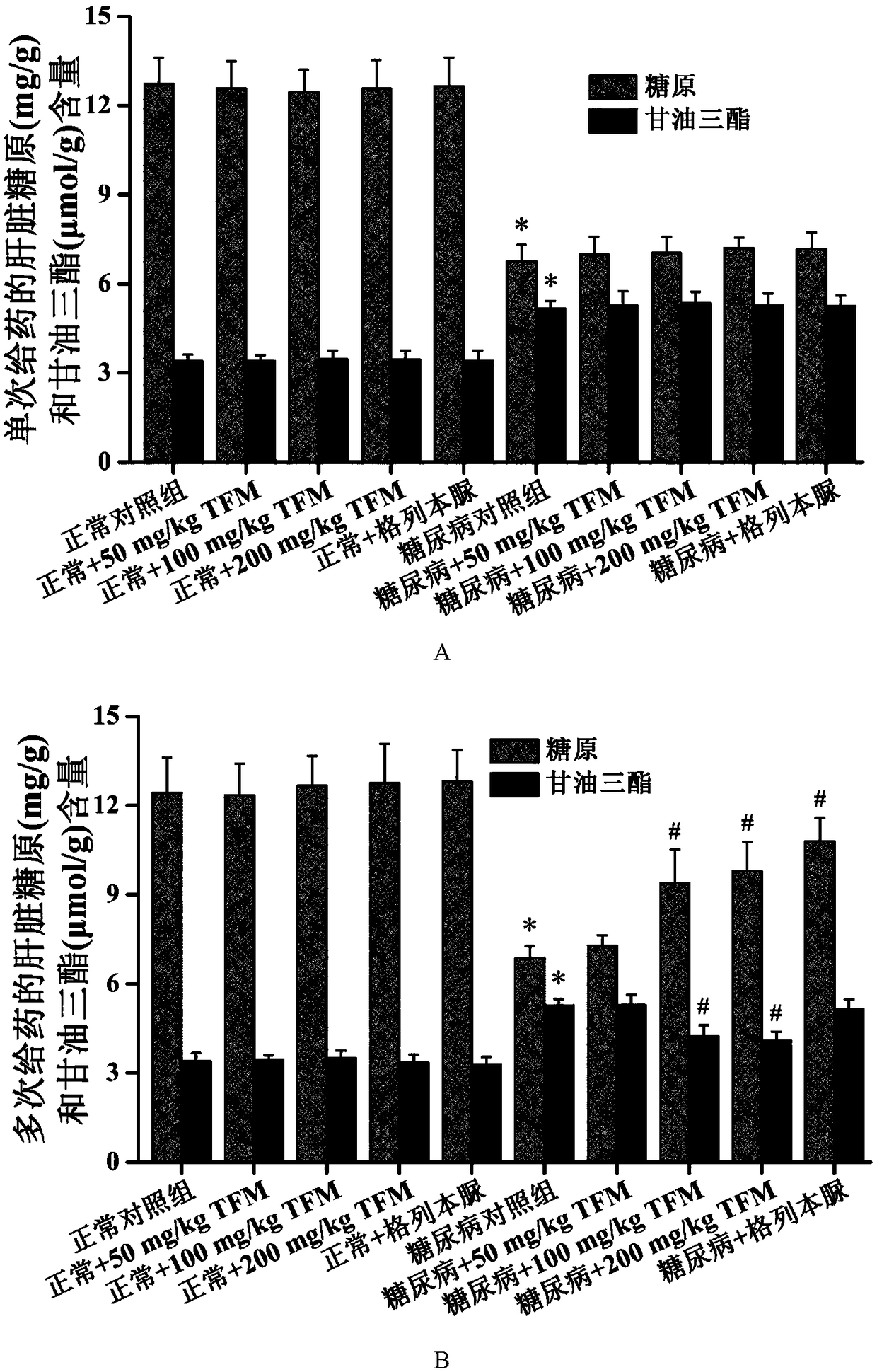
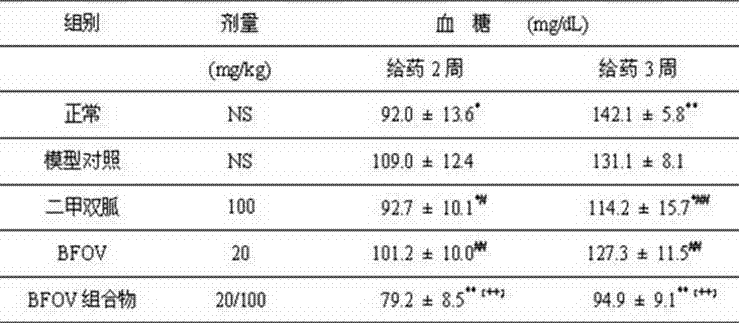
The invention patent relates to the technical field of animal experiment model construction, in particular to a construction method for a mouse type 2 diabetes mellitus (T2DM) animal experiment model. The method includes the following steps: male Kunmin mice at the age of 7 to 31 days are selected; 50 to 75 mg / Kg (milligrams per kilogram) of streptozotocin is taken by the mice through intraperitoneal injection at a time; after the mice are continuously fed with high-fat high-glucose feed for 4 to 6 weeks upon free eating, the mice of which fasting blood-glucose is above 7.0 millimoles per liter, blood glucose in a 2-hour oral glucose tolerance test is above 11.1 millimoles per liter, cholesterol is above 5.7 millimoles per liter, triglyceride is above 1.7 millimoles per liter, and high-density lipoprotein is below 1.0 millimole per liter at the same time are defined as T2DM animal experiment models. The model can simulate the T2DM characteristics of humans, and adopts multiple testing indicators to accurately define T2DM; due to the light weights of the underage animals, the streptozotocin dosage is small, so that the experiment cost is reduced.
Owner:QINGHAI NORMAL UNIV
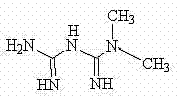
4-amino-6-(3-(3-bromophenyl)phenyl)-5-cyano-7-(β-l-xylofuranose)pyrrolo[2, 3-d]pyrimidine, similar derivatives And for the preparation of antitumor drugs
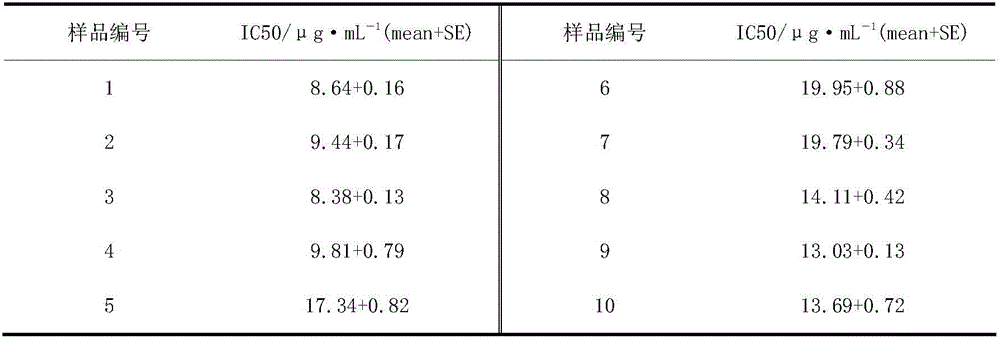
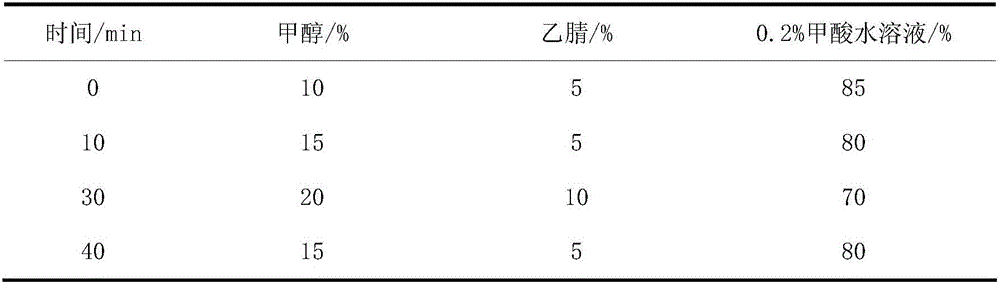
The invention belongs to the technical field of new drug synthesis, in particular to a class of 4-amino-6-(3-(3-bromophenyl)phenyl)-5-cyano-7-(β-L-xylofuranose)pyrrolo [2,3-d]pyrimidine, its similar derivatives, and drugs for preparing anti-human liver cancer cells, human lung cancer cells, human breast cancer cells, human cervical cancer cells, and human gastric cancer cells. The compounds of the present invention are directed against 4-amino-6-(3-(3-bromophenyl)phenyl)-5-cyano-7-(β-L-xylofuranose)pyrrolo[2,3 -d] The C6 position of pyrimidine is modified and synthesized by chemical methods. Experiments have shown that this type of compound can significantly inhibit the proliferation of various cancer cells, effectively induce apoptosis of cancer cells, including highly metastatic cancer cells, and can be used as drugs and drug components for treating cancer.
Owner:JILIN UNIV
Production and use for kaki-leaf extract
An extract of persimmon leaf is prepared from persimmon leaf through pulverizing, extracting in water or alcohol, and separating. Said extract along with proper medicinal additives can be used to prepare tablet, mixture, capsule, particle, or injection for decreasing blood pressure and blood fat, and treating arrhythmia, and diabetes and its complications.
Owner:黄仁彬
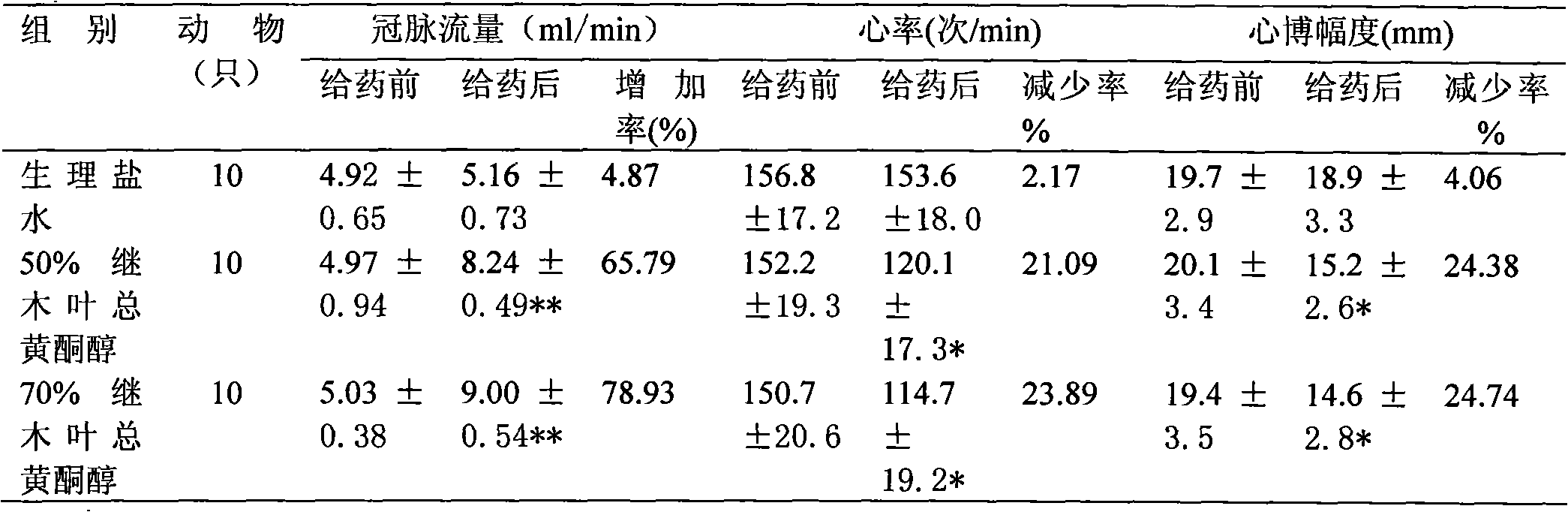
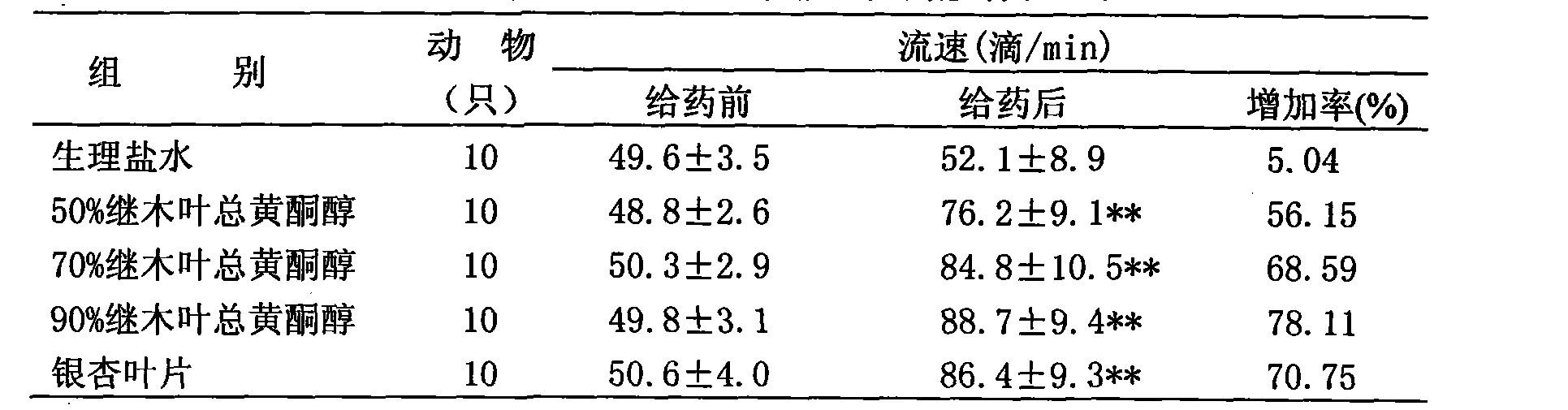
Chinese loropetalum leaf total flavonol extract and medical application thereof
ActiveCN101940605AImprove lesionHigh trafficCardiovascular disorderPlant ingredientsDiabetes retinopathySide effect
The invention discloses a Chinese loropetalum leaf total flavonol extract, which is characterized in that: the total flavonol content is over 50 percent, and the Chinese loropetalum leaf total flavonol extract is extracted and refined from a traditional Chinese medicine, namely Chinese loropetalum leaves. Pharmacodynamic and toxicological tests prove that the Chinese loropetalum leaf total flavonol has the advantages of obviously improving the diabetic retinopathy of rats caused by streptozotocin, expanding coronary arteries and peripheral vessels, increasing the blood flow of the coronary arteries, inhibiting cardiac muscles, reducing the heart rate and the aggregation of blood platelets, inhibiting the formation of thrombi, and obviously protecting cerebral ischemia, along with small toxic and side effects. The invention also discloses medical application of the Chinese loropetalum leaf total flavonol extract.
Owner:湖南药圣堂中药科技有限公司
Medicine for treating diabetes mellitus and its production
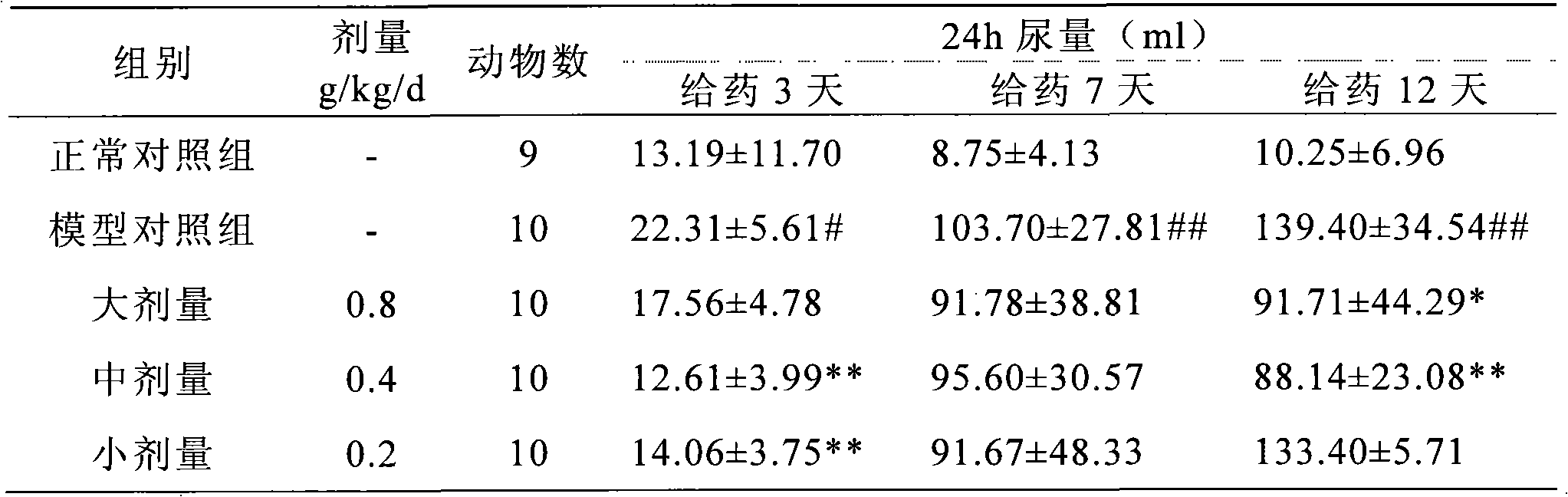
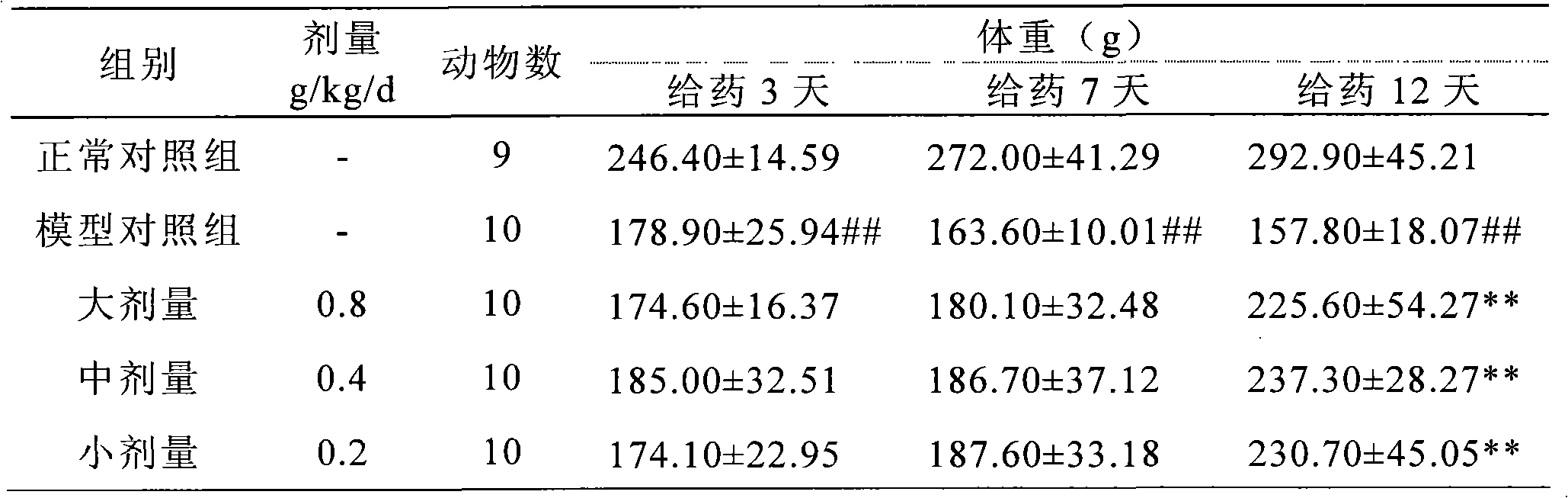
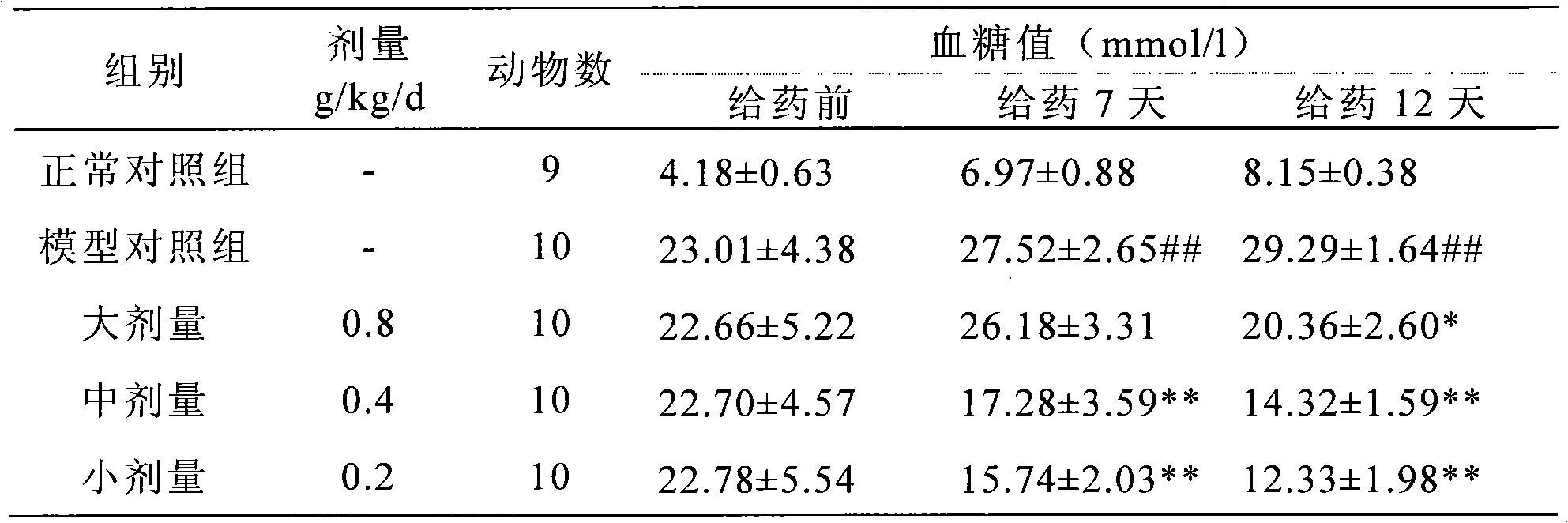
InactiveCN101028316AClear blood sugar lowering effectBlood sugar effect is clearMetabolism disorderPill deliveryAlcoholStreptozotocin
Owner:黄仁彬 +1
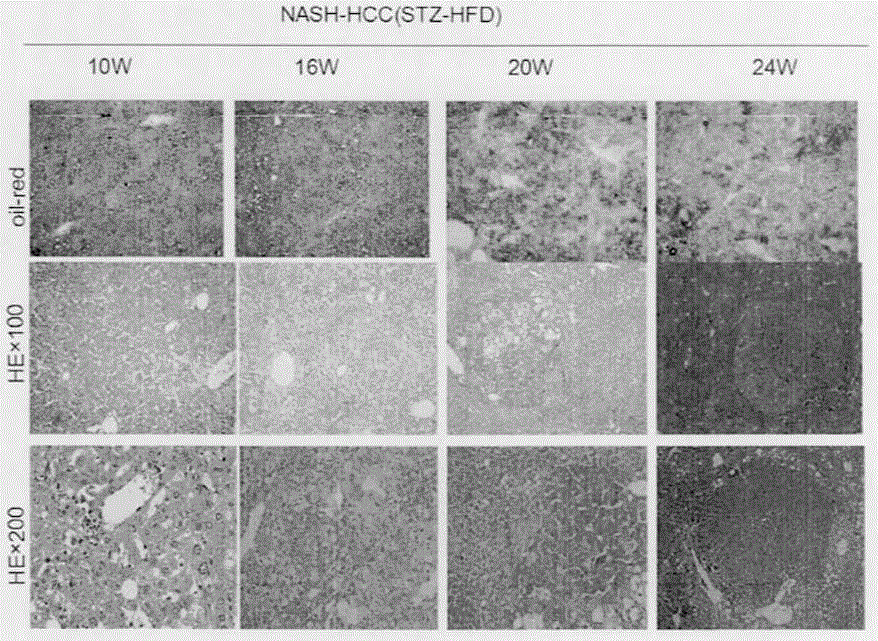
Experimental animal model with non-alcoholic steatohepatitis transforming into hepatic carcinoma
The present invention relates to the field of biotechnology and in particular to a construction method of an experimental animal model, specifically a mouse model, with non-alcoholic steatohepatitis (NASH) transforming into hepatic carcinoma (HCC), and use thereof. The present invention provides a construction method of a mouse model with NASH and chronic steatohepatitis developing into chronic hepatic fibrosis, cirrhosis and HCC. The construction method comprises the following steps: newborn C57BL / 6 mice (including male and female newborn mice) are subjected to subcutaneous single injection of streptozotocin (STZ) (100-400 [mu]g / newborn mouse) and the mice begin to be fed with experiment use high-fat feed after the completion of female mouse breastfeeding to construct the NASH mouse model with hepatic fibrosis, cirrhosis and HCC. The provided mouse model is relatively simple in construction process, relatively simple in technical means, and low in construction cost, has an extremely high value as the needed animal model in scientific research and drug research and development, and belongs to a domestic initiative.
Owner:凯斯艾生物科技(苏州)有限公司
RH (Rhein) liposome nanoparticles with kidney targeted distribution characteristics and application
ActiveCN109432049AHas sustained release propertiesGood target distribution propertiesOrganic active ingredientsMetabolism disorderLysosomeTarget peptide
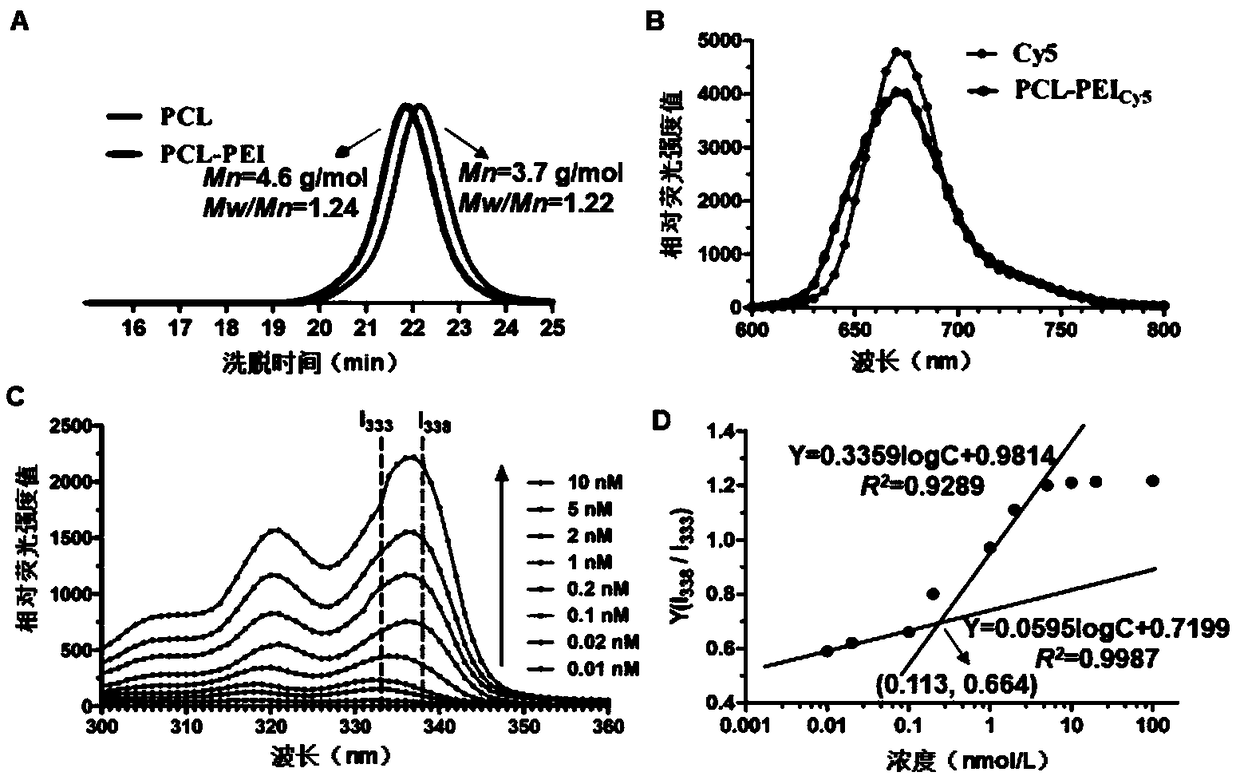
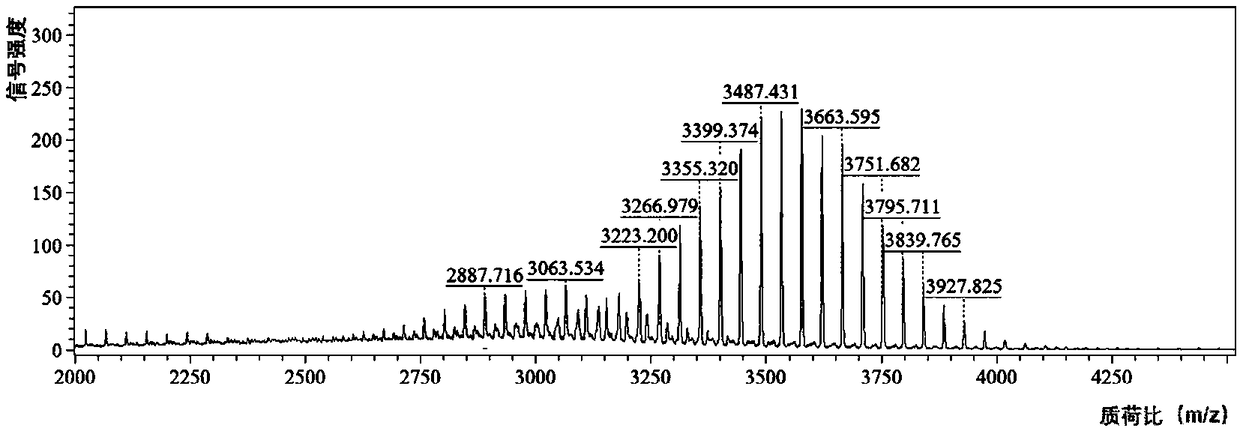
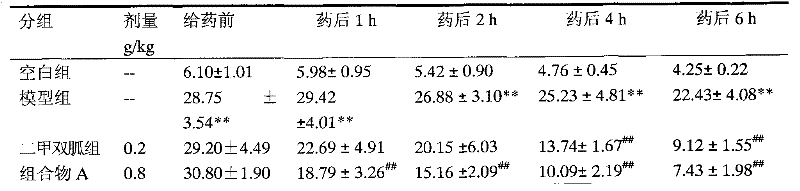
The invention relates to RH (rhein) liposome nanoparticles with kidney targeted distribution characteristics and an application of the RH liposome nanoparticles. The lipid capsule nanoparticles have the characteristics of higher encapsulation efficiency and drug loading capacity for RH, uniform particle size distribution, slow release with adoption of in-vitro release, rapid cell uptake, avoidanceof entering lysosome and good biological safety. In a streptozotocin-induced diabetic nephropathy mouse model, KLPPR (Kidney targeting peptide modified Liposomal PCL-PEI nanoparticle loading Rhein) has the good kidney targeted distribution characteristics, can significantly improve a plurality of pathological indexes of the diabetic nephropathy mouse and alleviate and reverse deterioration of thecourse of diabetic nephropathy, and effectively improves the therapeutic effect of RH on diabetic nephropathy. According to the nanoparticles, a brand-new strategy is provided for targeted treatmentof diabetic nephropathy, clinical transformation of RH drugs is further promoted, and an important reference is provided for research of novel nano-preparations of RH and similar drugs.
Owner:浙江省中医院、浙江中医药大学附属第一医院
Chinese medicinal composition for treating diabetes mellitus
The invention discloses a Chinese medicinal composition for treating diabetes mellitus and a preparation thereof. The Chinese medicinal composition is prepared to form a certain preparation in the following steps: extracting and separating mulberry twig, mulberry leaf, dogwood, rhizoma anemarrhenae, prepared rehmannia and golden thread with 0 to 75 percent ethanol. The six medicaments are combined, so that the Chinese medicinal composition has the functions of tonifying kidney and consolidating vital energy, nourishing yin and gathering essence, clearing heat and purging intense heat, and reducing blood sugar and quenching thirst, and is used for treating light and moderate diabetes mellitus. Pharmacodynamic researches prove that: the medicinal composition can remarkably reduce the high blood sugar value of a streptozotocin and alloxan induced diabetes mellitus experimental diabetic model, and can remarkably reduce the sugar tolerance of a mice.
Owner:王宇红 +1
Method for studying mechanism of gynura divaricata influencing inflammatory factors in type-II diabetic mice
InactiveCN110279873ACompounds screening/testingMetabolism disorderIntraperitoneal routeInflammatory factors
The invention discloses a method for studying the mechanism of gynura divaricata influencing inflammatory factors in type-II diabetic mice, and relates to diabetes research. The method comprises the following steps: adaptively feeding 50 mice for one week, then randomly dividing into a blank control group and a model group, and feeding with normal feed and high-fat and high-sugar feed; after feeding for 4 weeks, fasting and but feeding with water for 12h overnight, weighing and marking; and injecting STZ for three days, fasting and but feeding with water for 12h overnight, firstly experimenting in advance by using a glucometer and test paper, then monitoring the tail blood glucose of the mice by using a microglucometer, and determinating, wherein the fasting blood glucose being greater than or equal to 11.1mmol / L is used as a model standard for the type-II diabetes. Type-II diabetes mouse models are replicated through coordination of a high-fat diet and intraperitoneal injection of streptozotocin, and influence on the inflammatory factors in the mice after intervention of the fresh gynura divaricate is evaluated, so that a clearer theory is provided for the treatment of the type-II diabetes by the gynura divaricate and further development of a Guangxi-characteristic Chinese herbal medicine.
Owner:GUANGXI UNIV OF CHINESE MEDICINE
Establishment of rhesus autoimmune 1 type diabetes model
The invention provides application of streptozotocin with low dosage in preparing an animal model for sieving drugs for treating autoimmune 1 type diabetes. The application method and dosage of the streptozotocin is 15-30mg / kg once by veins administration. The invention also provides method for preparing a rhesus autoimmune 1 type diabetes model, and autoimmune 1 type diabetes animal model prepared by the method. The animal model can be used for the evaluation of the biotechnology new drug which can not be evaluated by the rodent animal model, and the evaluation of the stem cell transplantation treating technology.
Owner:WEST CHINA HOSPITAL SICHUAN UNIV +1
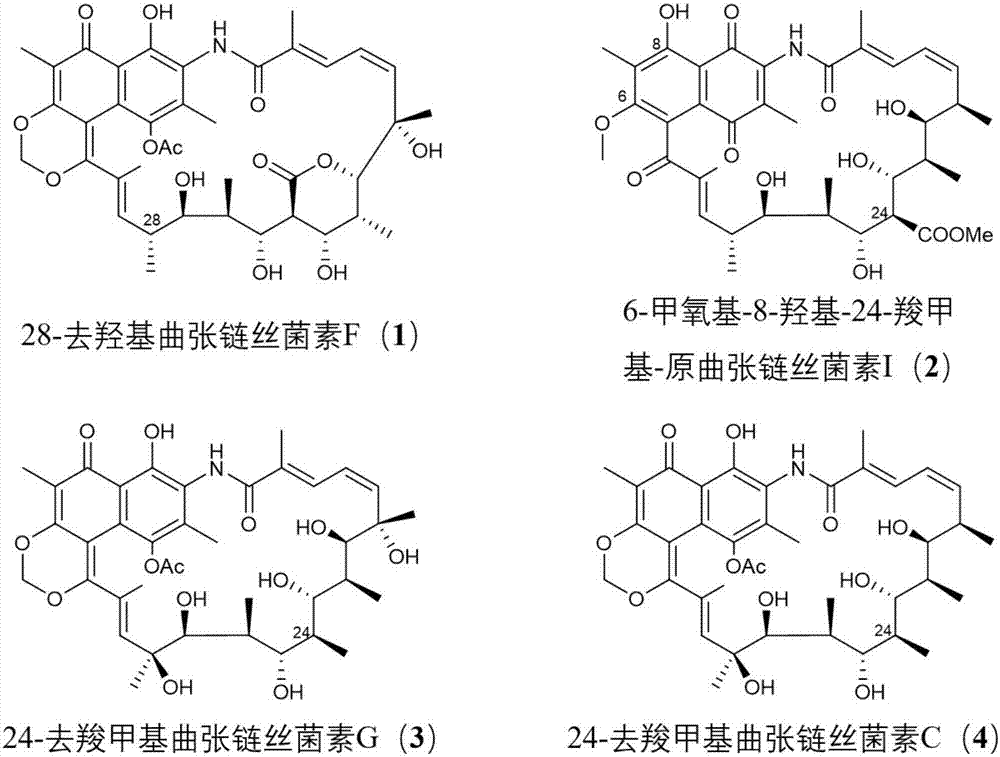
Streptovaricin derivative and preparation method and application thereof
ActiveCN107540682ARich diversityImprove antibacterial propertiesAntibacterial agentsOrganic active ingredientsBiosynthetic genesAntibiotic resistance
Owner:WUHAN UNIV
Novel antidiabetic
InactiveCN101204466AReduce food intakeLose weightPowder deliveryMetabolism disorderBlood sugarExcipient
The invention belongs to the field of medicine, in particular to a new drug for anti-diabetes, which takes a wild plant discolorous clinquefoil root and tea as the raw materials, the wild plant discolor cinquefoil herb and the tea are in the proportion of 1 to 1 through 1 to 10 or 10 to 1, producing the material powder through 8 steps, resloving in the normal saline to get the oral liquid of the new drug for anti-diabetes. The material powder can be produced with the medical carrier or excipient to troche, water agent, capsule, drop pills for anti-diabetes by tradtional method. The oral liquid is applied to animal test to prove that the oral liquid can reduce the food quantity, weight, the content of blood sugar and glycosylation haematoglobin in the blood of tested II-type diabetes mouse induced by the streptozotocin, increase the tolerance of the grape sugar and reduce the blood sugar level of the I-type diabetes mouse induced by the tetraoxypyrimidine.
Owner:邱日辉 +1
Chicken feed additive for improving meat quality
InactiveCN107397071ACompatibility is reasonableAdequately meet growth needsFodderBiotechnologyMicrobial agent
The invention discloses a chicken feed additive for improving meat quality. The chicken feed additive contains the following components in parts by weight: 5-8 parts of kelp powder, 10-18 parts of gracilaria powder, 40-60 parts of peptides streptozotocin, 20-30 parts of microbial agents and 30-36 parts of spiral seaweed powder. The chicken feed additive disclosed by the invention is reasonable in matching, growth demands of chickens can be sufficiently met, no antibiotic hormone is used, the immunity of the chickens can be improved, and the slaughtering rate of the chickens is increased; and meanwhile, the components are absorbed by chicken bodies while the additive is taken by the chickens, so that the physique of the chickens is improved, and chicken meat can be fresh and tender when being taken, and thus is good in taste.
Owner:合肥先智商贸有限责任公司
Mirabilis jalapa root general flavone extractive for preventing and curing diabetes mellitus type 2 and complication and preparation method and application thereof
ActiveCN108143755ALower blood sugarLower blood fatMetabolism disorderPlant ingredientsMirabilis jalapaLipid lowering
The invention relates to the field of medicine, in particular to a mirabilis jalapa root general flavone extractive for preventing and curing diabetes mellitus type 2 and a preparation method and application thereof. In the extractive, the content of general flavone is greater than 40%, when the dosage of the extractive is 100 mg / kg, the extractive has obvious effects on decreasing the content ofblood glucose, total cholesterol, triglycerides, tissue triglycerides and increasing glycogen content, prophylaxis and remedial medication have similar effects on blood glucose and lipid lowering of diabetes mellitus type 2 induced by streptozotocin, also reduce blood glucose and lipid type 2 diabetes (db / db) mice of gene knockout, and do not affect glucolipid metabolism of normal mice. Accordingto the preparation method of mirabilis jalapa root general flavone extractive, mirabilis jalapa root general flavone is separated and purified by macroporous adsorption resin, in the prepared mirabilis jalapa root general flavone extractive, the content of the general flavone is greater than 40 %, the transfer rate reaches up to 70 %, and the yield rate can reach up to 2 %.
Owner:THE SECOND AFFILIATED HOSPITAL ARMY MEDICAL UNIV
Preparation method for fermentative production of marine peptides streptozotocin by using sea cucumber leftovers
InactiveCN105385748AEliminate heavy pollutionIncrease productivityMicroorganism based processesFermentationPeptideStreptozotocin
The invention discloses a preparation method for fermentative production of marine peptides streptozotocin by using sea cucumber leftovers, belonging to the technical field of food processing. The preparation method comprises the steps of preprocessing raw materials, crushing corn grits, saccharifying, fermenting, emulsifying, homogenizing and freeze-drying. The sea cucumber leftovers are fermented by adopting cheap grains and adopting the bioengineering technology, the preparation method which is low in cost, simple in process and high in production efficiency is provided for producing the marine peptides streptozotocin rich in micro-molecular functional polypeptides and probiotics, and a novel way is provided for further processing and comprehensively utilizing the sea cucumber resource.
Owner:QINGDAO BEIBAO OCEAN TECH CO LTD
Animal model of hypertension and construction method and application of animal model
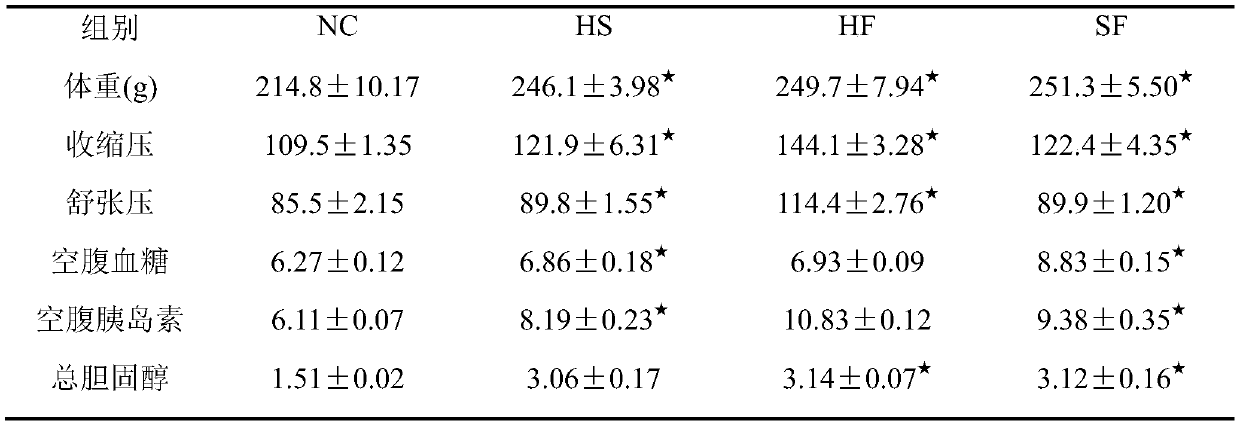
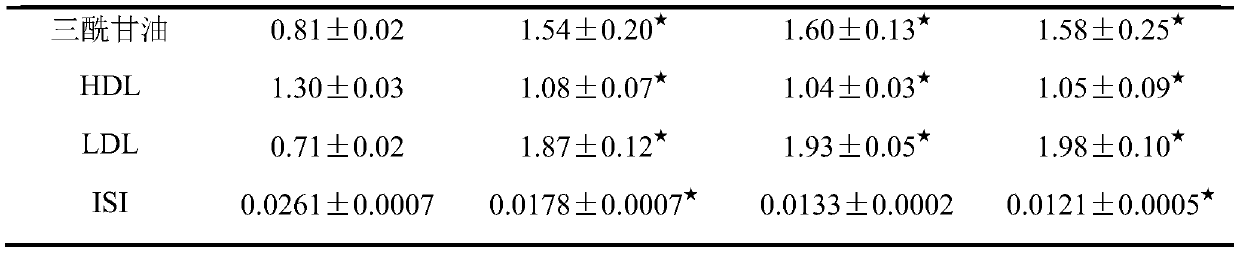
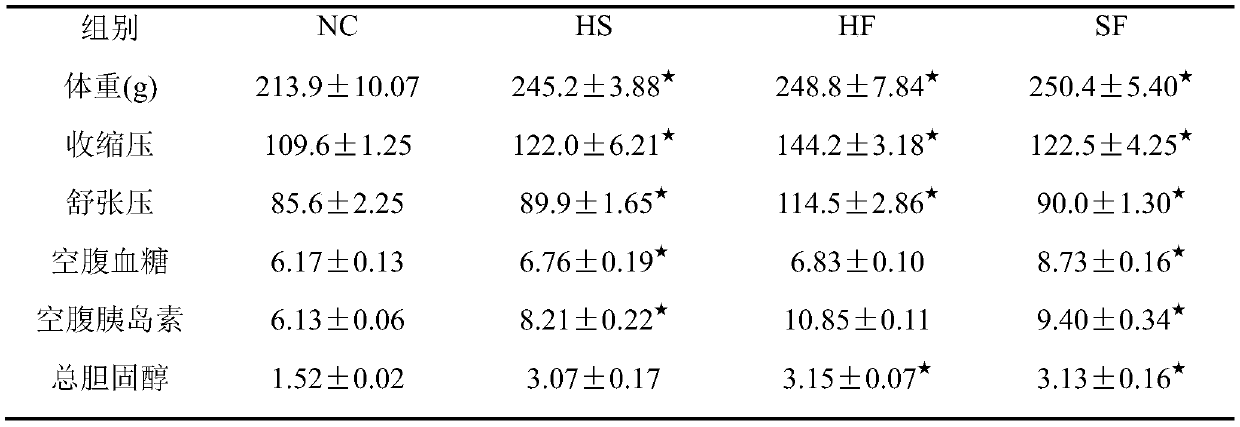
The invention relates to the technical field of medicament, and provides an animal model of hypertension and a construction method and application of the animal model. The animal model of hypertensioncan be used for studying pathogenesis of the hypertension and rules of occurrence and development of complications. The construction method includes: on the basis of a spontaneously hypertensive rat,feeding high-sugar and high-fat diets to the model rat, wherein every high-sugar and high-fat diet comprises, by weight, 47.9-49 parts of normal feed, 2-5 parts of cholesterol, 20-25 parts of streptozotocin, 20-25 parts of lard oil, 0.1-5 parts of sodium tauroglycocholate and 10-15 parts of yolk powder; after feeding the rat for 12 weeks, constructing a hypertensive rat model. Biochemical criterions of the constructed hypertensive rat model include systolic pressure, diastolic pressure, blood glucose and blood fat, and blood fat inspection indexes include serum total cholesterol, triacylglycerol, high-density lipoprotein and low-density lipoprotein. The construction method of the animal model is simple to operate, convenient to control and high in model forming rate.
Owner:山东科诺德生物医药科技有限公司
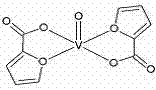
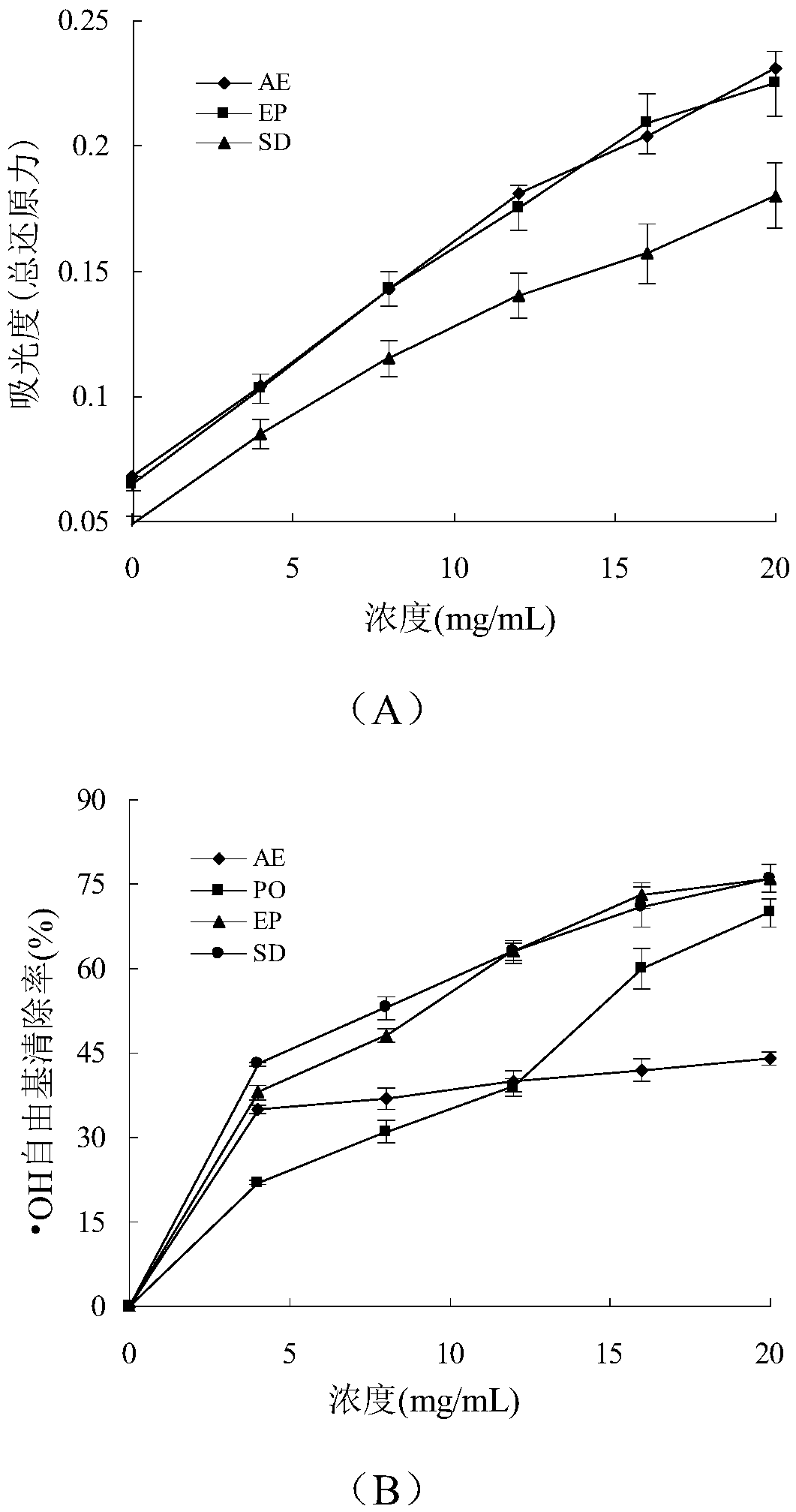
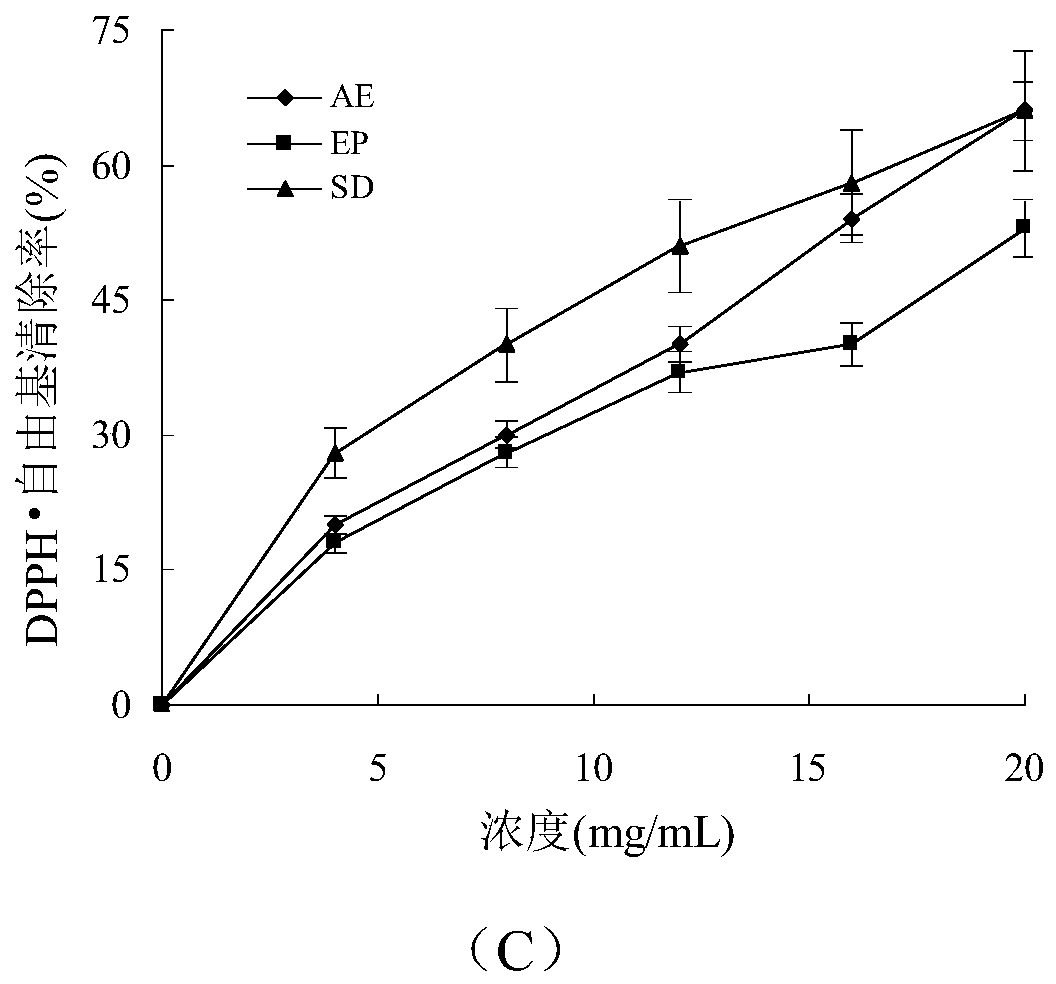
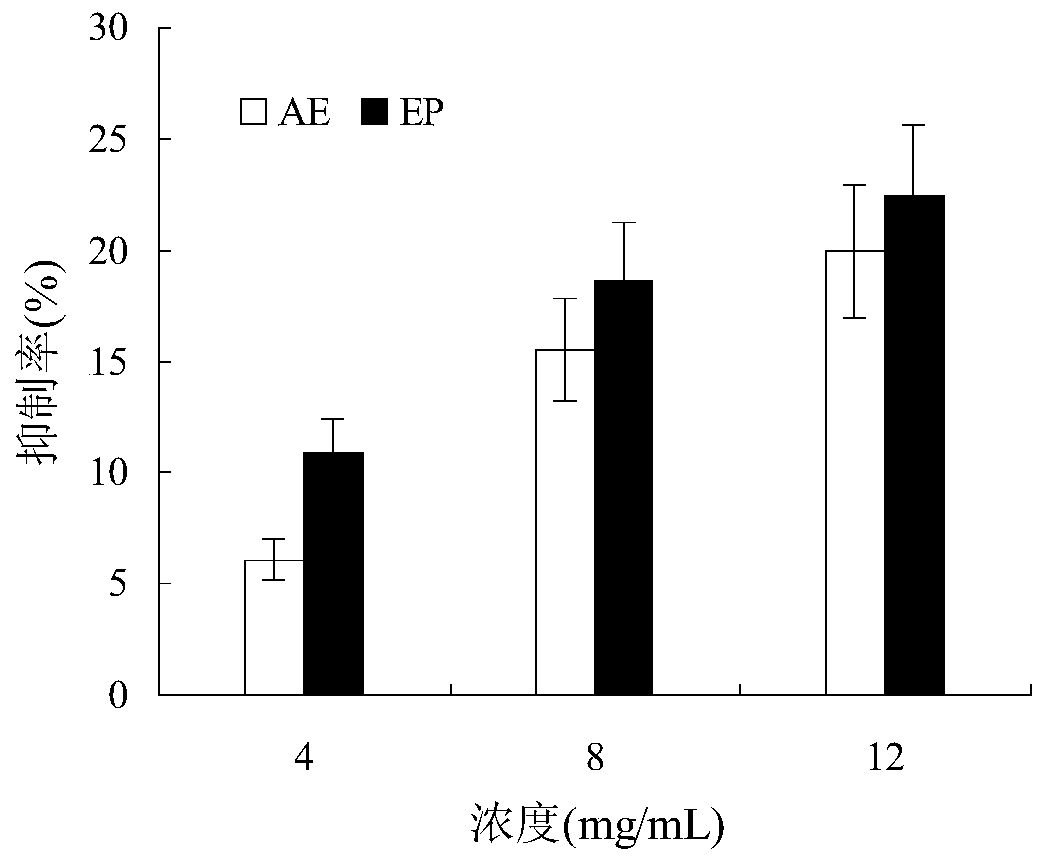
Bis(alpha-furancarboxylato)oxovanadium composition for treatment of diabetes
InactiveCN103316017AImprove stabilityReduced stabilityOrganic active ingredientsMetabolism disorderPhysiologyPharmaceutical Aids
The invention discloses a bis(alpha-furancarboxylato)oxovanadium composition for treatment of diabetes, and belongs to the field of pharmaceutics. The composition of the invention is prepared from bis(alpha-furancarboxylato)oxovanadium and melbine at a ratio of 1:5 to 50, and appropriate accessories are added to produce pills, powders and capsules. Recommended dosage for human is 10mg: (50 to 500mg) per day, and preferable dosage is 10mg:200mg or 10mg:500mg. The results of animal experiment show that: the composition is capable of decreasing blood glucose levels of alloxan-induced diabetic mice, streptozotocin-induced diabetic mice and high fat feed-induced insulin-resistant mice greatly, improving lipid metabolism disorders of insulin-resistant mice, and increasing insulin sensitivity.
Owner:KUNMING MEDICAL UNIVERSITY +2
Polygonatum kingianum solid beverage assisting in decreasing blood sugar and preparation method thereof
ActiveCN110326731AAbundant resourcesIncrease productionNatural extract food ingredientsFood ingredient functionsBULK ACTIVE INGREDIENTBlood sugar
The invention discloses a polygonatum kingianum solid beverage assisting in decreasing blood sugar. The polygonatum kingianum solid beverage assisting in decreasing the blood sugar is prepared from the following components in parts by mass: 55.00-75.50 parts of polygonatum kingianum aqueous extract, 27.00-38.00 parts of polygonatum kingianum polysaccharide, 0.60-4.00 parts of stevioside, 1.30-12.00 parts of citric acid and 0.40-0.80 part of magnesium carbonate. The invention further discloses a preparation method of the solid beverage. The preparation method of the solid beverage comprises thesteps of preparing, weighing, mixing and granulating of the polygonatum kingianum aqueous extract and the polygonatum kingianum polysaccharide and the like. The main active ingredient, the polygonatum kingianum aqueous extract, has antioxidant activity and alpha-glucosidase inhibition, the polygonatum kingianum polysaccharide can reduce fasting a blood glucose level of streptozotocin-induced diabetic mice and relieve the symptoms of "drink more, eat more, urinate more and weight loss" in the diabetic mice. The solid beverage is reasonable in formula, has a better effect in assisting in decreasing the blood sugar, is easy to carry and store, and all quality indexes meet the requirements of standards and specifications such as "solid beverage".
Owner:SHAANXI NORMAL UNIV
Neo-islets comprising stem and islet cells and treatment of diabetes mellitus therewith
ActiveUS20170073641A1Organic active ingredientsMetabolism disorderInsulin dependent diabetesIslet cells
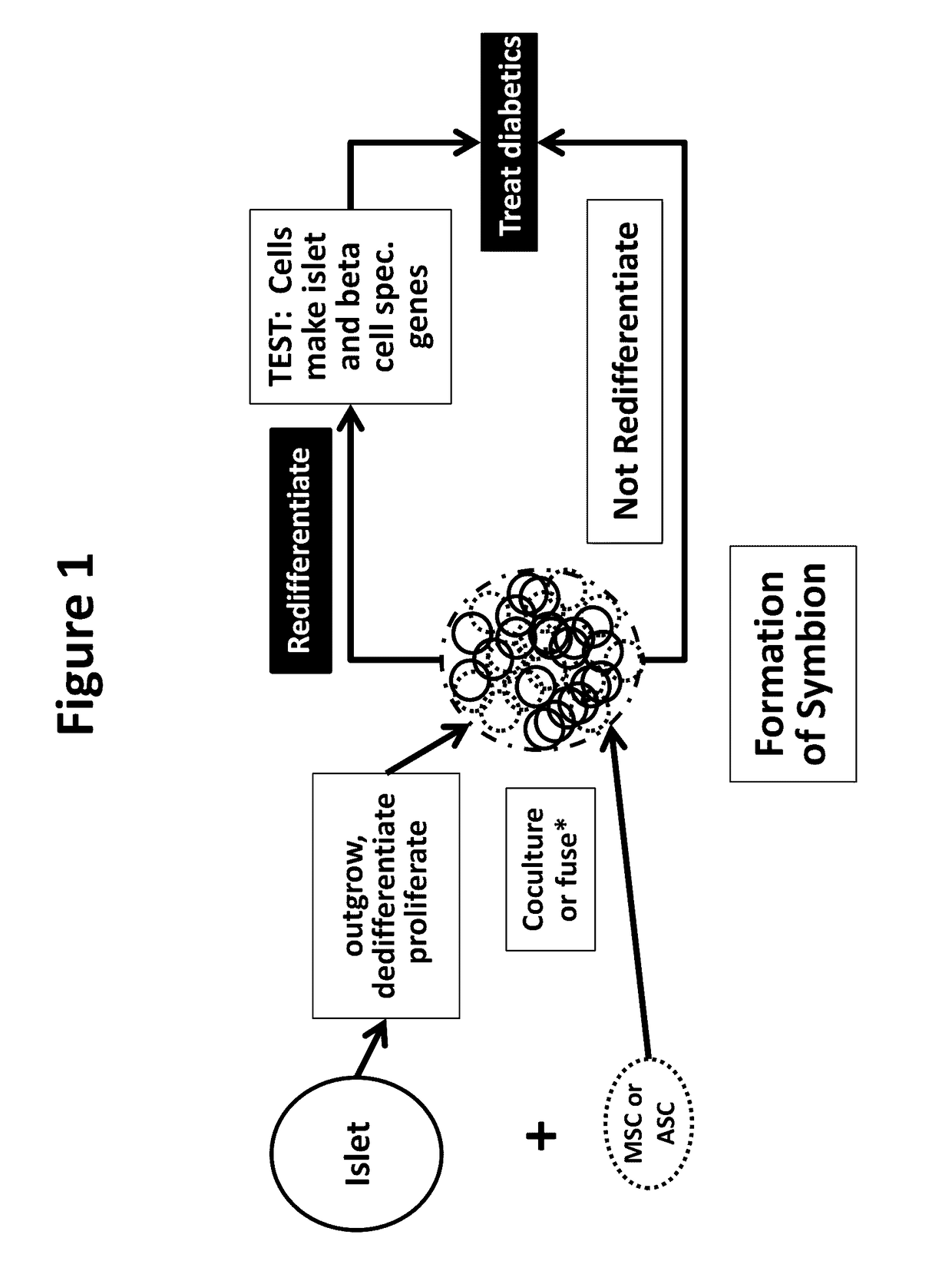
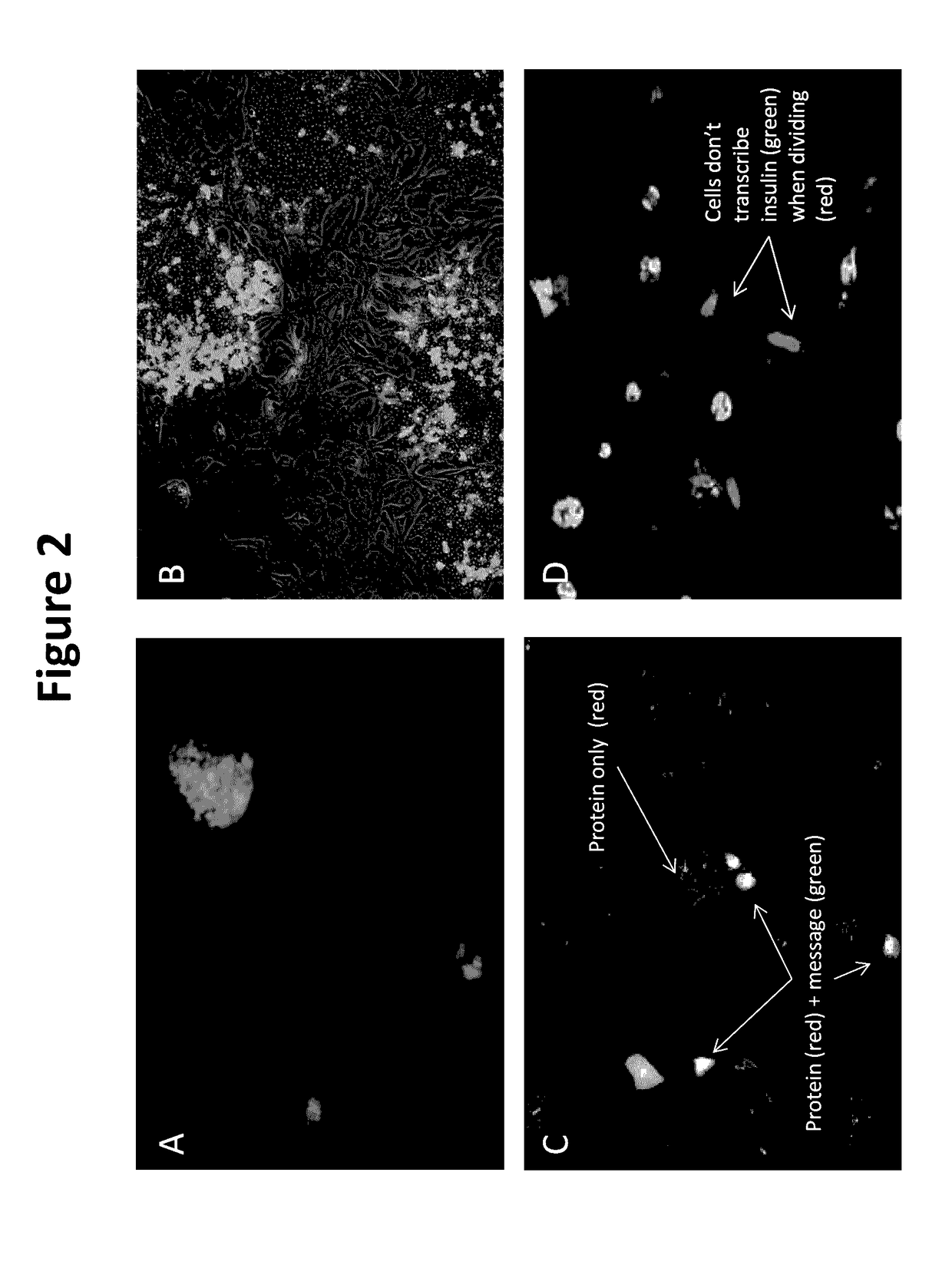
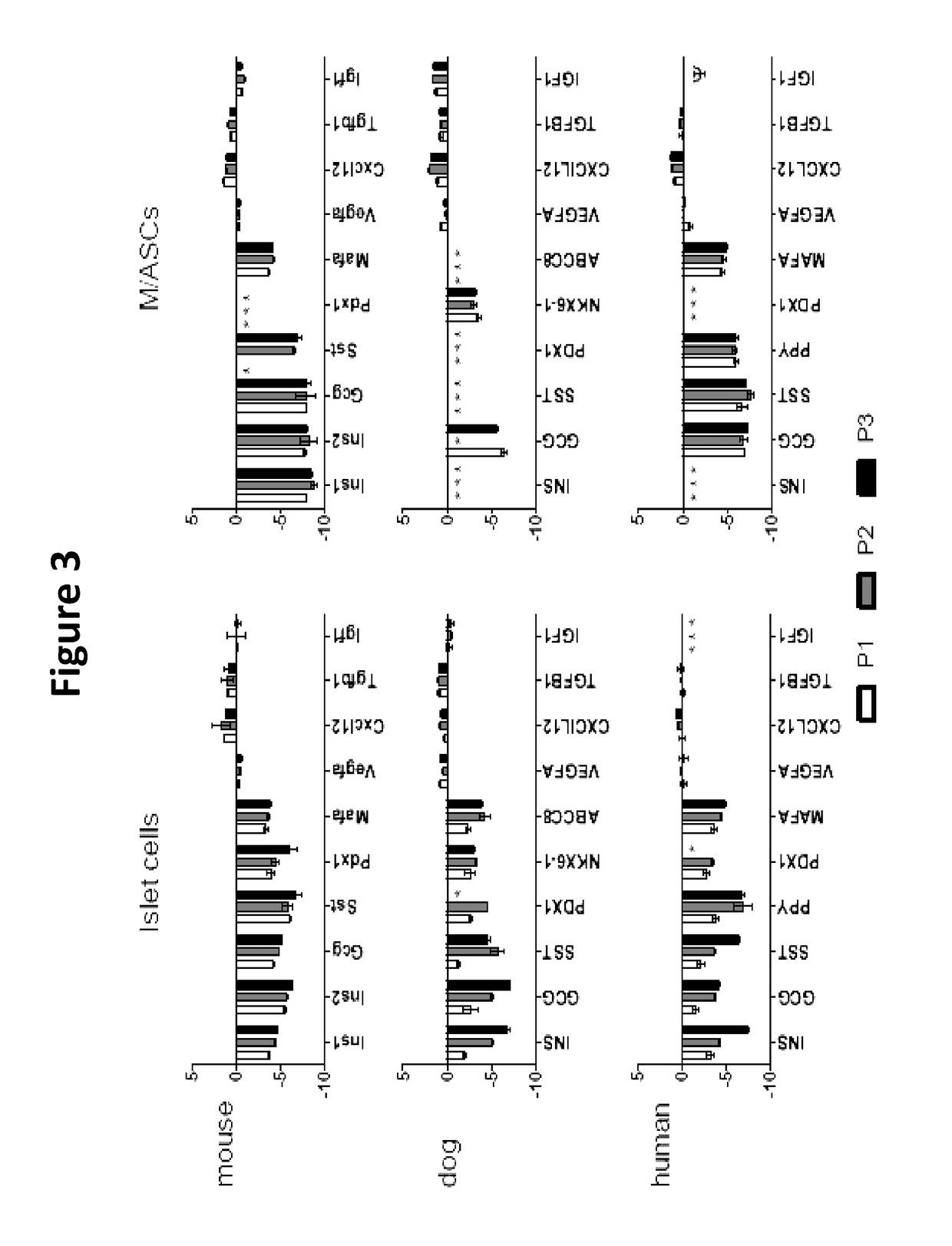
Described are Neo-Islets comprising: a) dedifferentiated islet cells and mesenchymal and / or adipose stem cells; or b) redifferentiated islet cells and mesenchymal and / or adipose stem cells where the cells have been treated so as to facilitate redifferentiation. Further described herein are methods of generating Neo-Islets, the methods comprising: culturing a) dedifferentiated islet cells and mesenchymal and / or adipose stem cells; or b) redifferentiated islet cells and mesenchymal and / or adipose stem cells; on a surface that promotes the formation of cell clusters. Also described are methods of treating a subject, the methods comprising: providing to the subject Neo-Islets described herein. Additionally described are methods of treating a subject suffering from Type 1 Diabetes Mellitus, Type 2 Diabetes Mellitus, and other types of insulin-dependent diabetes mellitus, or impaired glucose tolerance by providing to the subject Neo-Islet as described herein. Additionally described are methods of treatment in which intraperitoneal administration of islet-sized Neo-Islets composed of high numbers of mesenchymal stem cells and cultured islet cells, durably and reversibly treats, without hypoglycemia, both streptozotocin-induced and spontaneous Type 1 Diabetes Mellitus, Type 2 Diabetes Mellitus, and other types of insulin-dependent diabetes mellitus, or impaired glucose tolerance.
Owner:SYMBIOCELLTECH
Applications of safflower extract with definite spectrum-effect relationship
ActiveCN105920071AComprehensive and accurate spectrum effect basisMetabolism disorderFood ingredient functionsAdjuvantProtein Tyrosine Phosphatase 1B
The invention relates to applications of safflower extract with the definite spectrum-effect relationship. An hypoglycemic extract is obtained from safflower carthamus through distilled water reflux extraction and macroporous resin separation and purification, and the medical applications of the hypoglycemic extract are illustrated preliminarily; the sum of the peak areas of hydroxysafflor yellow A and 6-hydroxyl kaempferol-3,6-bis-O-glucose-7-O-glucoside in an HPLC (High Performance Liquid Chromatography) fingerprint of the extract is not less than 50%; through calculation, the peak area ratio of hydroxysafflor yellow A to 6-hydroxyl kaempferol-3,6-bis-O-glucose-7-O-glucoside is 5.0-8.0. The activity screening test proves that the extract obtained by adopting the method provided by the invention has the obvious effect of inhibiting protein-tyrosine-phosphatase 1B (PTP-1B), has the excellent effect of reducing blood glucose on SD (Sprague Dawley) rats suffering from diabetes induced by high-fat diet and streptozotocin (STZ), and can be taken as the raw material or adjuvant to be used for preparing medicines or health products for preventing and treating diabetes.
Owner:XINJIANG TECHN INST OF PHYSICS & CHEM CHINESE ACAD OF SCI
Application of sarpogrelate containing pharmaceutical composition in treatment of diabetic nephropathy
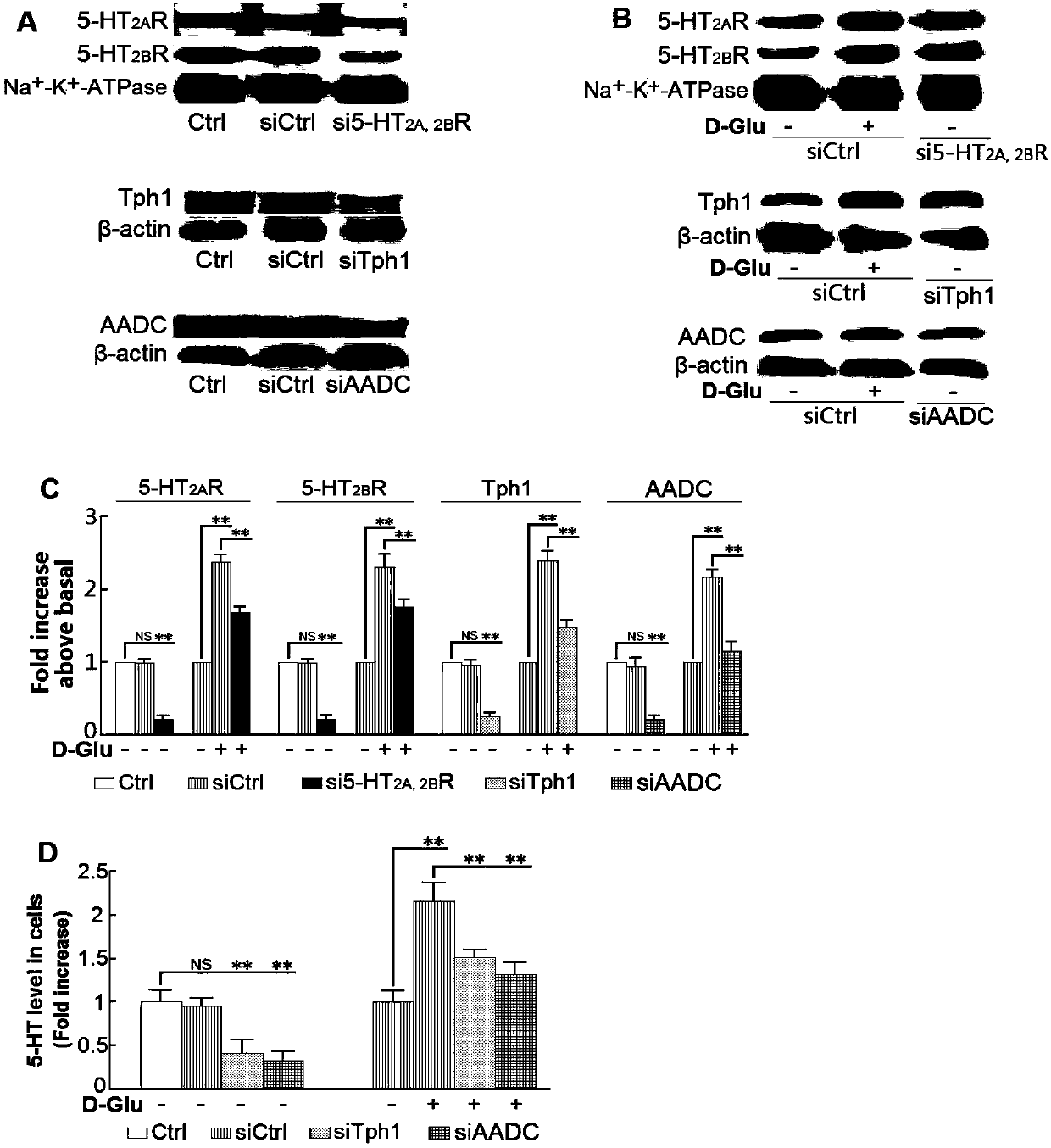
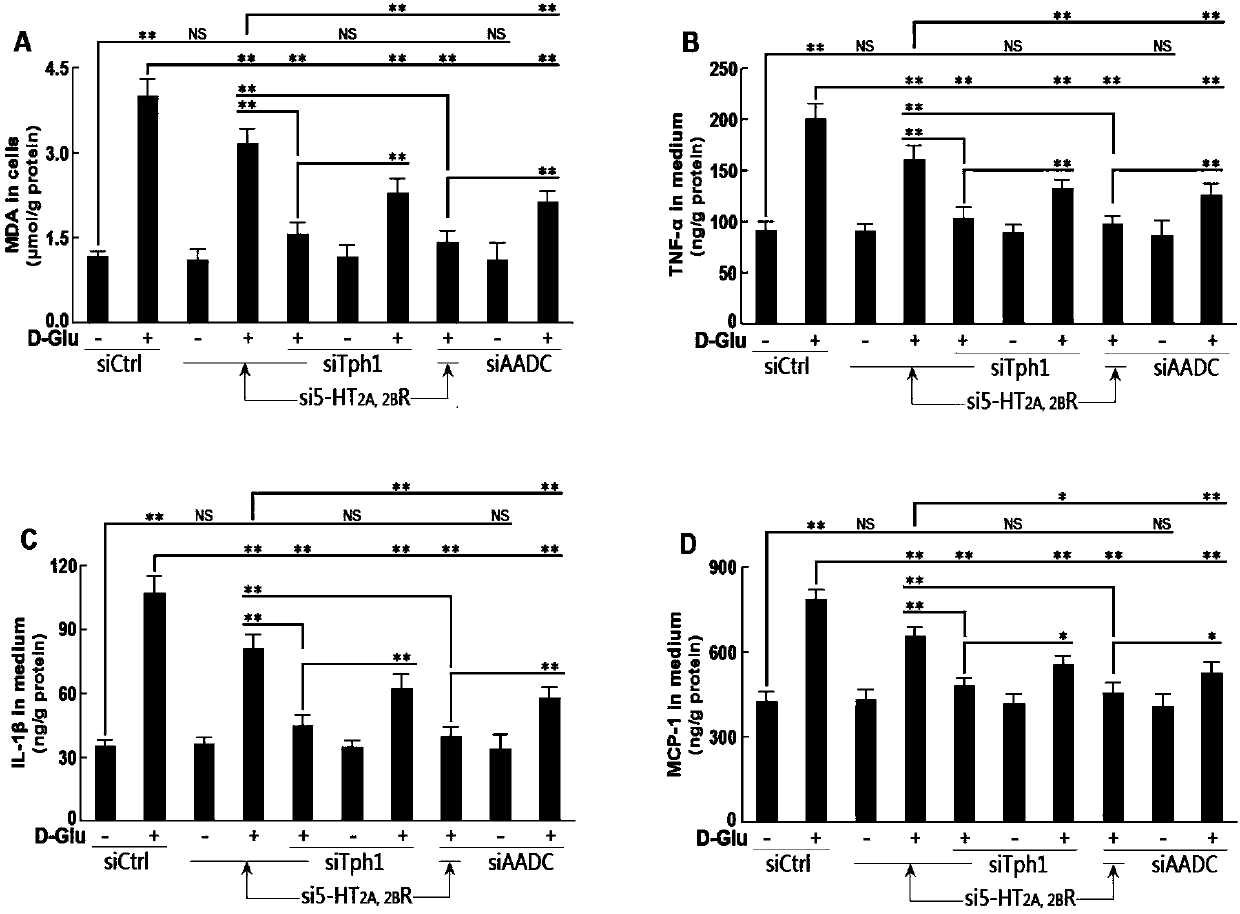
The invention belongs to the technical field of pharmaceuticals, discloses application of a sarpogrelate containing pharmaceutical composition in treatment of diabetic nephropathy, and specifically discloses the application of a compound pharmaceutical composition taking sarpogrelate and 5-hydroxytryptamine synthesizing inhibitor as pharmaceutical active components in preparation of drugs for treating diabetic nephropathy. The test shows that gene silencing 5-HT synthetase and a 5-HT 2 receptor show good effect on inhibiting in-vitro culture and high glucose-induced oxidative stress of human renal mesangial cell strain, proinflammatory factor and chemotactic factor, and meanwhile, the gene silencing 5-HT synthetase system (Tph1 or AADC) and a 5HT 2 receptor are capable of greatly inhibiting high glucose-inducing effect and performing synergistic effect. With the adoption of the pharmaceutical composition, the diabetic nephropathy caused by high-fat feed feeding and streptozotocin induced diabetic nephropathy can be obviously treated; compared with simple prescription, the compound prescription is high in attenuation, and the lethal amount of mice orally taking the drug is obviouslyhalved.
Owner:CHINA PHARM UNIV
Organic vanadium complexes with insulin mimetic activity, and preparation method thereof
The invention relates to three organic vanadium complexes with insulin mimetic activity, and preparation method thereof, and belongs to the technical field of complex synthesis and medicine chemistry.Vanadium ions are taken as the center ions of the three complexes. (E)-N'-(2-hydroxybenzylidene)-3-methoxybenzhydrazide and ethyl maltol are taken as the ligands of a complex 1; (E)-N'-(2-hydroxybenzylidene)-3, 5-dimethoxybenzoyl hydrazine and methanol are taken as the ligands of a complex 2; and (E)-N'-(3-ethoxy-2-hydroxybenzylidene)-3,5-dimethoxybenzhydrazide and ethyl maltol are taken as the ligands of a complex 3. The three complexes are of sole-core structures, and hexa-coordinate octahedral coordination configurations are adopted. It is found in tests that the complexes 1, 2, and 3 possess relatively high insulin mimetic activity, are capable of reducing blood sugar of type I diabetes Kunming mouse obviously, and can be used for preparing hypoglycemic drugs.
Owner:SHANDONG UNIV OF TECH
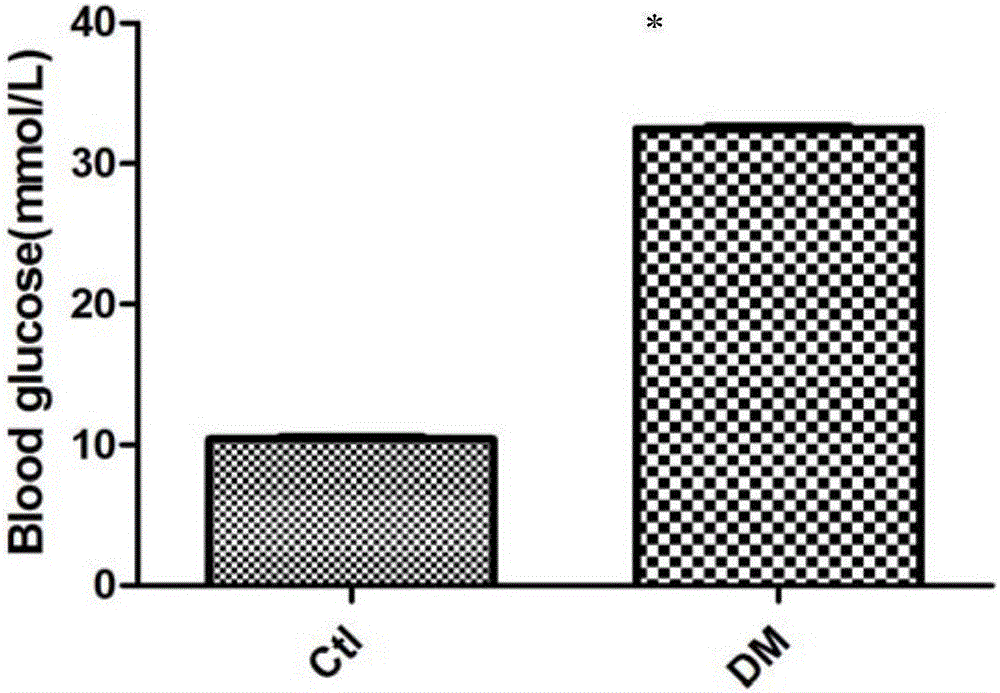
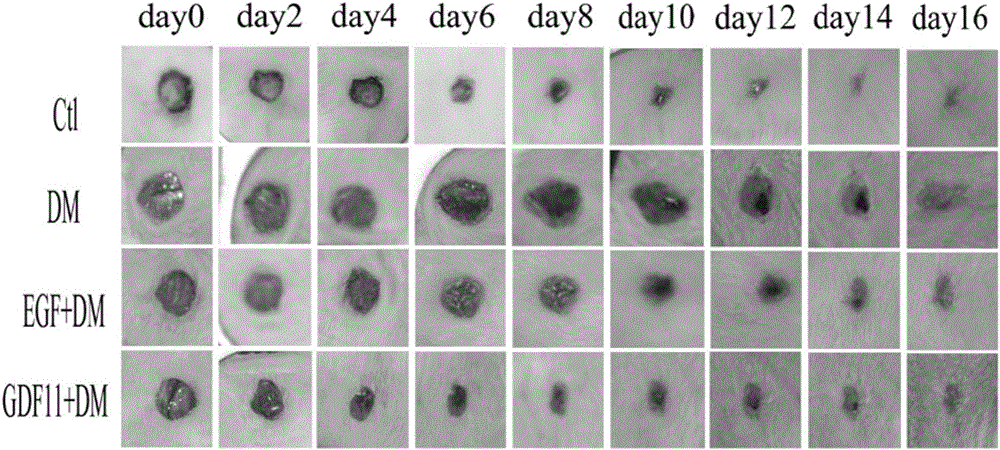
Use of growth differentiation factor 11 in promoting healing diabetic wound
ActiveCN105770860APromote healingPeptide/protein ingredientsMetabolism disorderPositive controlFactor ii
The invention discloses use of growth differentiation factor 11 in promoting healing of a diabetic wound and belongs to the technical field of medicine. By establishing a Type-I diabetic mouse wound model induced with streptozotocin (STZ) of effective concentration, the influence of the growth differentiation factor 11 upon the healing of the diabetic mouse wound model is estimated, the study discovers that the growth differentiation factor 11 can accelerate the healing of the diabetic mouse wound, and the healing speed of the wound is higher than that of both a positive control group (EGF) and a blank control group. Therefore, an effective technical means to heal and treat diabetic wounds is provided herein.
Owner:HARBIN MEDICAL UNIVERSITY
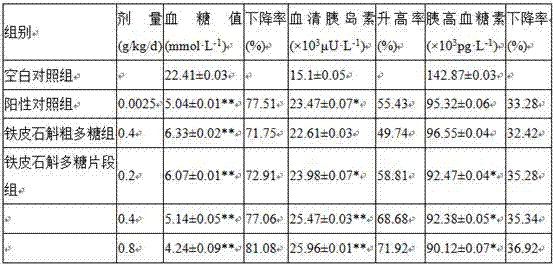
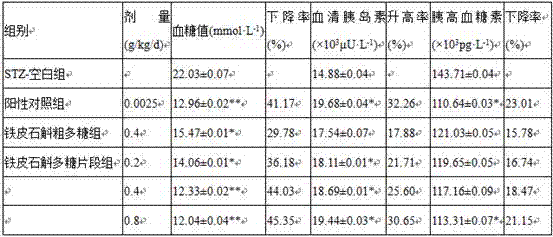
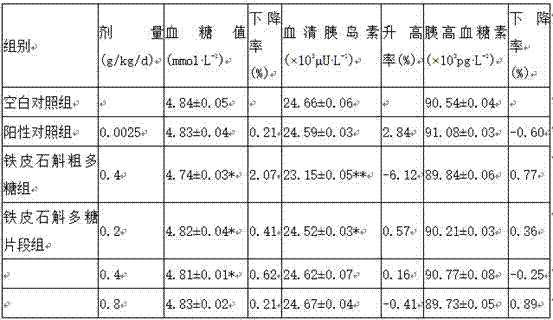
Polysaccharide fragment of Dendrobium officinale and extraction method thereof
ActiveCN107267574ANo stimulation of secretionGood hypoglycemic effectFermentationBulk chemical productionBlood glucose decreasedInsulin secretion
The present invention relates to the technical field of Dendrobium officinale, and in particular relates to a polysaccharide fragment of Dendrobium officinale and an extraction method thereof, the extraction methods includes water extraction, deproteinization, enzymatic modification, purification, drying and other steps, the extracted polysaccharide fragment comprises six monosaccharides such as D-glucose, D-mannose, D-galactose, L-rhamnose, D-xylose and D-fucose, experiments prove that the polysaccharide fragment of Dendrobium officinale has non-stimulating effect on insulin secretion of normal mice has higher safety, compared with use of glipizide hypoglycemic drugs, blood glucose decreased rate and glucagon rising rate by use of the polysaccharide fragment of Dendrobium officinale all good, and the polysaccharide fragment of Dendrobium officinale has a significant hypoglycemic effect on a SD rat with streptozotocin induced diabetes.
Owner:连南瑶族自治县欣胜堂生物科技有限公司
Use of a pharmaceutical composition containing sargrelate for treating or preventing fatty liver, liver fibrosis and/or liver damage
The invention belongs to the technical field of medicines, and discloses application of medicine compositions with sarpogrelate to treating or preventing fatty livers, liver fibrosis and / or liver injury. As proved from experiments, the application has the advantages that the fatty livers due to high fat and high cholesterol can be obviously reversed by the aid of the medicine compositions, and the liver fibrosis and the liver injury due to combination effects of the high fat, the high cholesterol and streptozotocin irritation can be obviously reversed.
Owner:CHINA PHARM UNIV
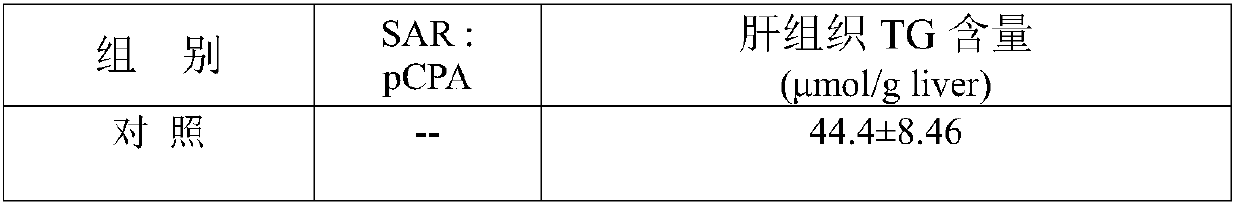
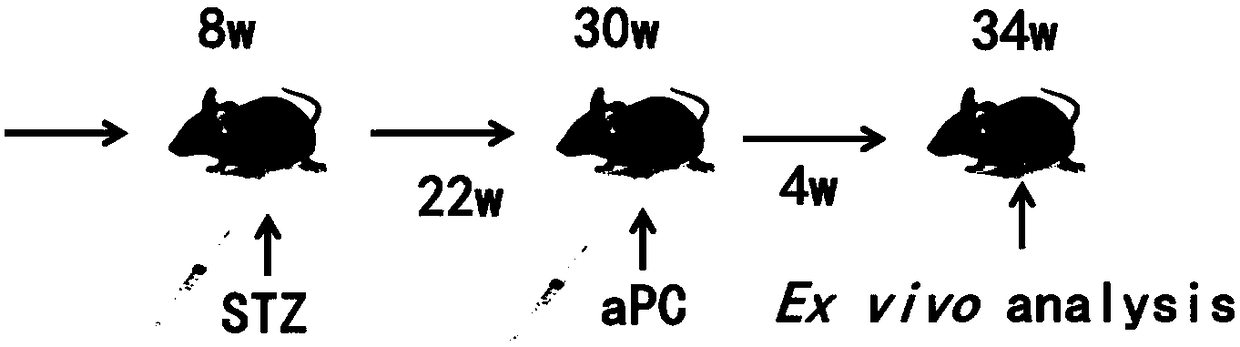
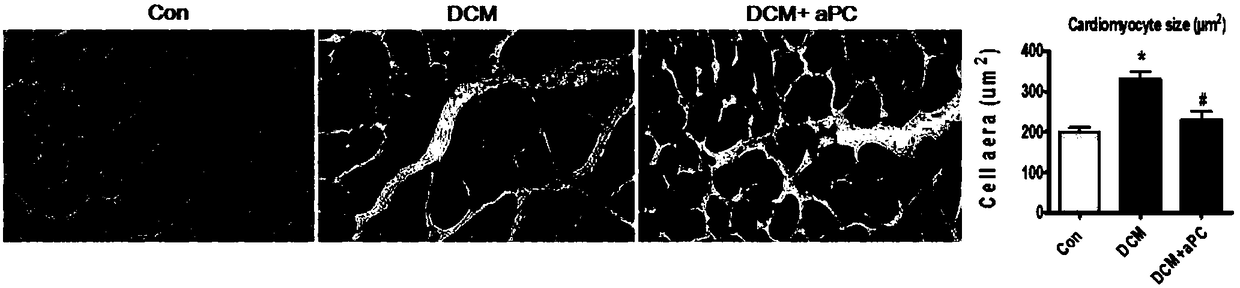
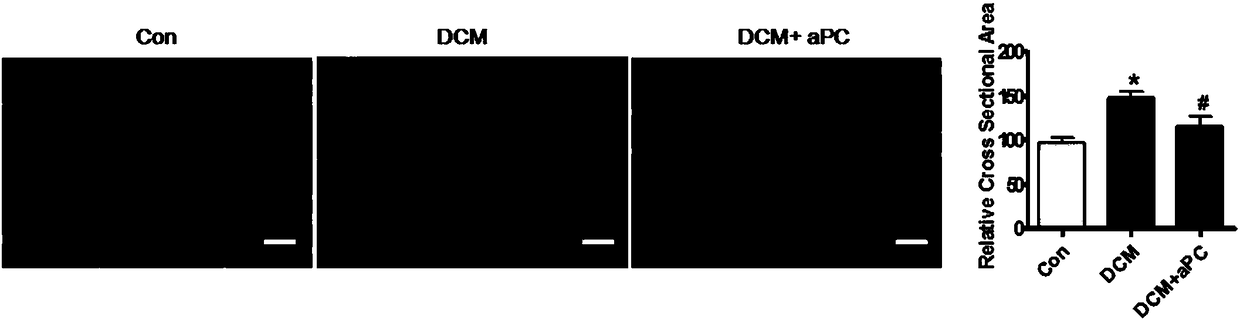
Application of thrombin aPC in drugs for prevention and treatment of diabetic cardiomyopathy
ActiveCN108159399AReduce hypertrophyReduce fibrosisPeptide/protein ingredientsMetabolism disorderFibrosisCurative effect
The invention discloses an application of thrombin aPC in drugs for prevention and treatment of diabetic cardiomyopathy induced by streptozotocin. The thrombin aPC can stabilize the expression of YB1by inhibiting ubiquitination of a cardiac muscle cell YB1 in a hyperglycemic state, so as to alleviate cardiomyocyte hypertrophy and fibrosis. The therapeutic study results indicate that the thrombinaPC has significant curative effects of alleviating cardiomyocyte hypertrophy and fibrosis, improving myocardial contractile functions and increasing ejection fraction.
Owner:TONGJI HOSPITAL ATTACHED TO TONGJI MEDICAL COLLEGE HUAZHONG SCI TECH
Application of traditional Chinese indian lettuce in preparation of diabetes drugs
The invention relates to a new application of traditional Chinese indian lettuce in the pharmaceutical field, in particular to the application of drugs for curing or preventing diabetes. Experiments prove that rats suffering from diabetes due to streptozotocin significantly decrease urinary output, put on weight gradually and significantly decrease blood sugar level after being fed with the aqueous extract of traditional Chinese indian lettuce. Experiments also prove that no evident toxic side effect produces after rats are fed with the traditional Chinese indian lettuce by 200 times more thanan adult equivalent dose by the maximum gavage amount to the rat (0.4 ml / 10g).
Owner:SHANXI UNIV OF CHINESE MEDICINE
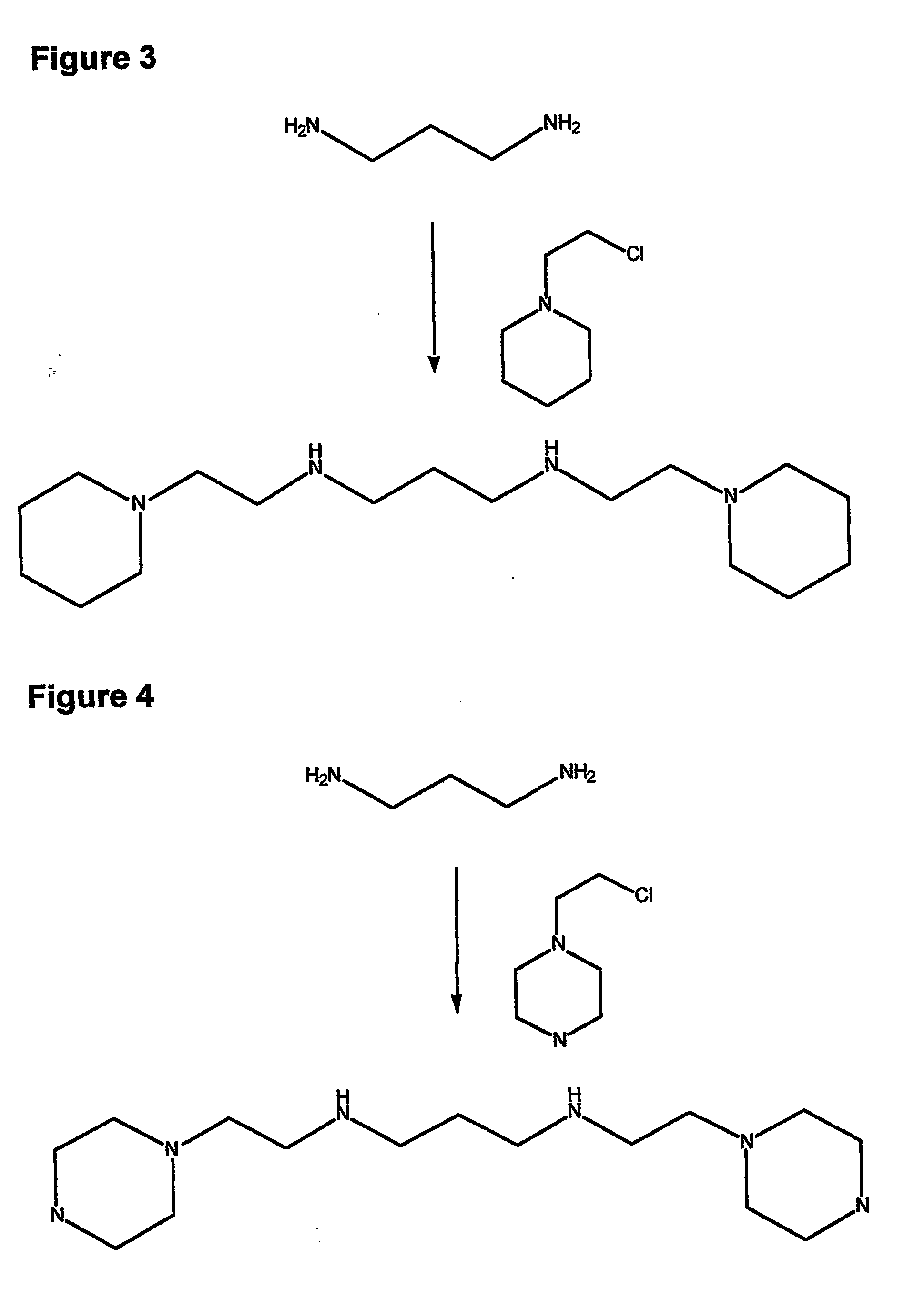
Therapeutic polyamine compositions and their synthesis
InactiveUS20140057877A1Chromium concentration were decreasedIncrease excretionBiocideCopper organic compoundsAntidoteRisk stroke
This invention relates to a process of synthesis and composition of open chain (ring), closed ring, linear branched and or substituted polyamines, polyamine derived tyrosine phosphatase inhibitors and PPAR partial agonists / partial antagonists via a series of substitution reactions and optimizing the bioavailability and biological activities of the compounds. Polyamines prevent the toxicity of neurotoxins and diabetogenic toxins including paraquat, methyphenyl pyridine radical, rotenone, diazoxide, streptozotocin and alloxan. These polyamines can be utilized to treat neurological, cardiovascular, endocrine acquired and inherited mitochondrial DNA damage diseases and other disorders in mammalian subjects, and more specifically to the therapy of Parkinson's disease, Alzheimer's disease, Lou Gehrig's disease, Binswanger's disease, Olivopontine Cerebellar Degeneration, Lewy Body disease, Diabetes, Stroke, Atherosclerosis, Myocardial Ischemia, Cardiomyopathy, Nephropathy, Ischemia, Glaucoma, Presbycussis, Cancer, Osteoporosis, Rheumatoid Arthritis, Inflammatory Bowel Disease, Multiple Sclerosis and as Antidotes to Toxin Exposure.
Owner:MURPHY MICHAEL A +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com

![4-amino-6-(3-(3-bromophenyl)phenyl)-5-cyano-7-(β-l-xylofuranose)pyrrolo[2, 3-d]pyrimidine, similar derivatives And for the preparation of antitumor drugs 4-amino-6-(3-(3-bromophenyl)phenyl)-5-cyano-7-(β-l-xylofuranose)pyrrolo[2, 3-d]pyrimidine, similar derivatives And for the preparation of antitumor drugs](https://images-eureka.patsnap.com/patent_img/03cb45ce-a15e-469a-98f4-94d24b90d681/HDA0000071034700000011.png)
![4-amino-6-(3-(3-bromophenyl)phenyl)-5-cyano-7-(β-l-xylofuranose)pyrrolo[2, 3-d]pyrimidine, similar derivatives And for the preparation of antitumor drugs 4-amino-6-(3-(3-bromophenyl)phenyl)-5-cyano-7-(β-l-xylofuranose)pyrrolo[2, 3-d]pyrimidine, similar derivatives And for the preparation of antitumor drugs](https://images-eureka.patsnap.com/patent_img/03cb45ce-a15e-469a-98f4-94d24b90d681/HDA0000071034700000012.png)
![4-amino-6-(3-(3-bromophenyl)phenyl)-5-cyano-7-(β-l-xylofuranose)pyrrolo[2, 3-d]pyrimidine, similar derivatives And for the preparation of antitumor drugs 4-amino-6-(3-(3-bromophenyl)phenyl)-5-cyano-7-(β-l-xylofuranose)pyrrolo[2, 3-d]pyrimidine, similar derivatives And for the preparation of antitumor drugs](https://images-eureka.patsnap.com/patent_img/03cb45ce-a15e-469a-98f4-94d24b90d681/HDA0000071034700000013.png)