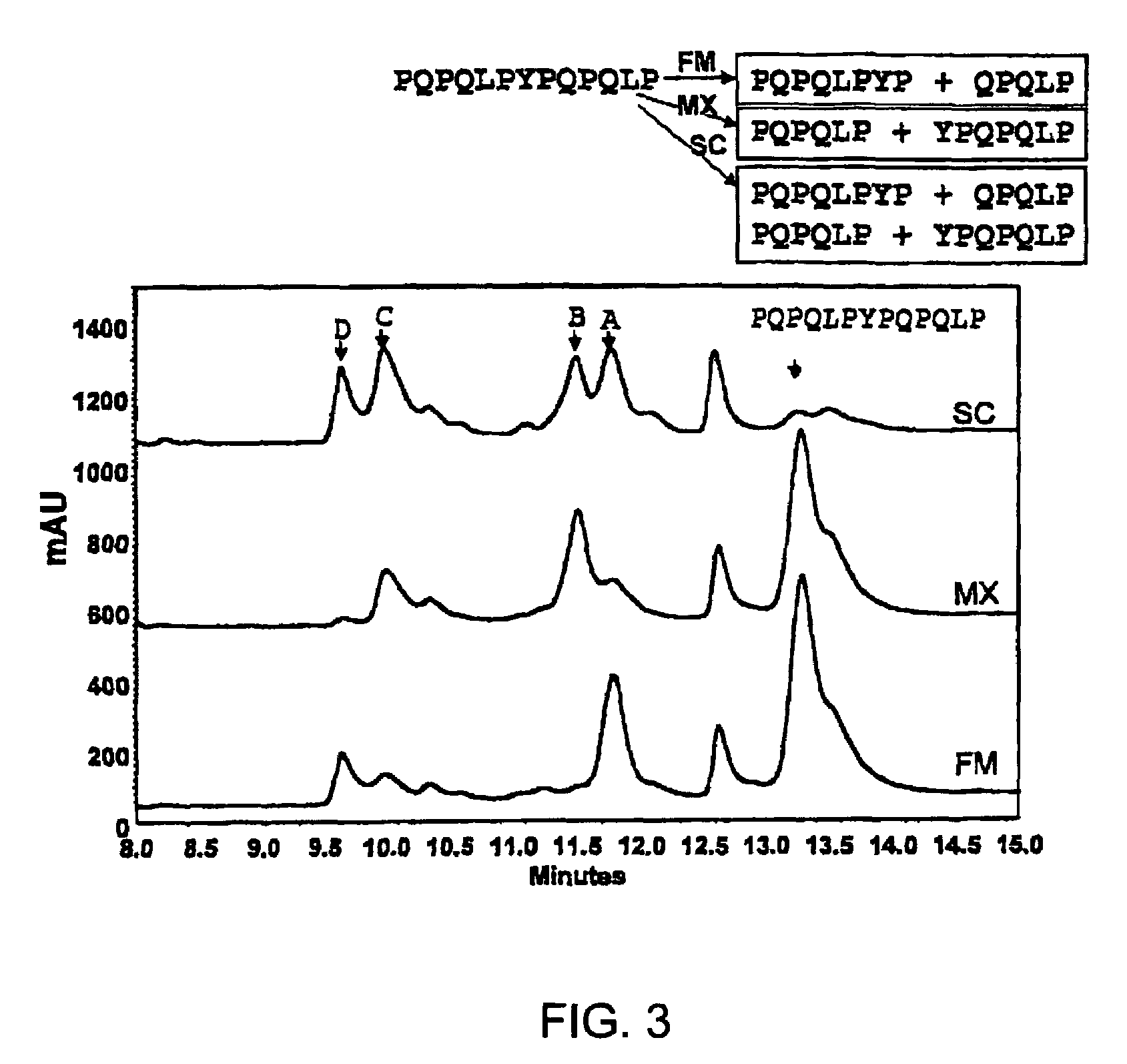
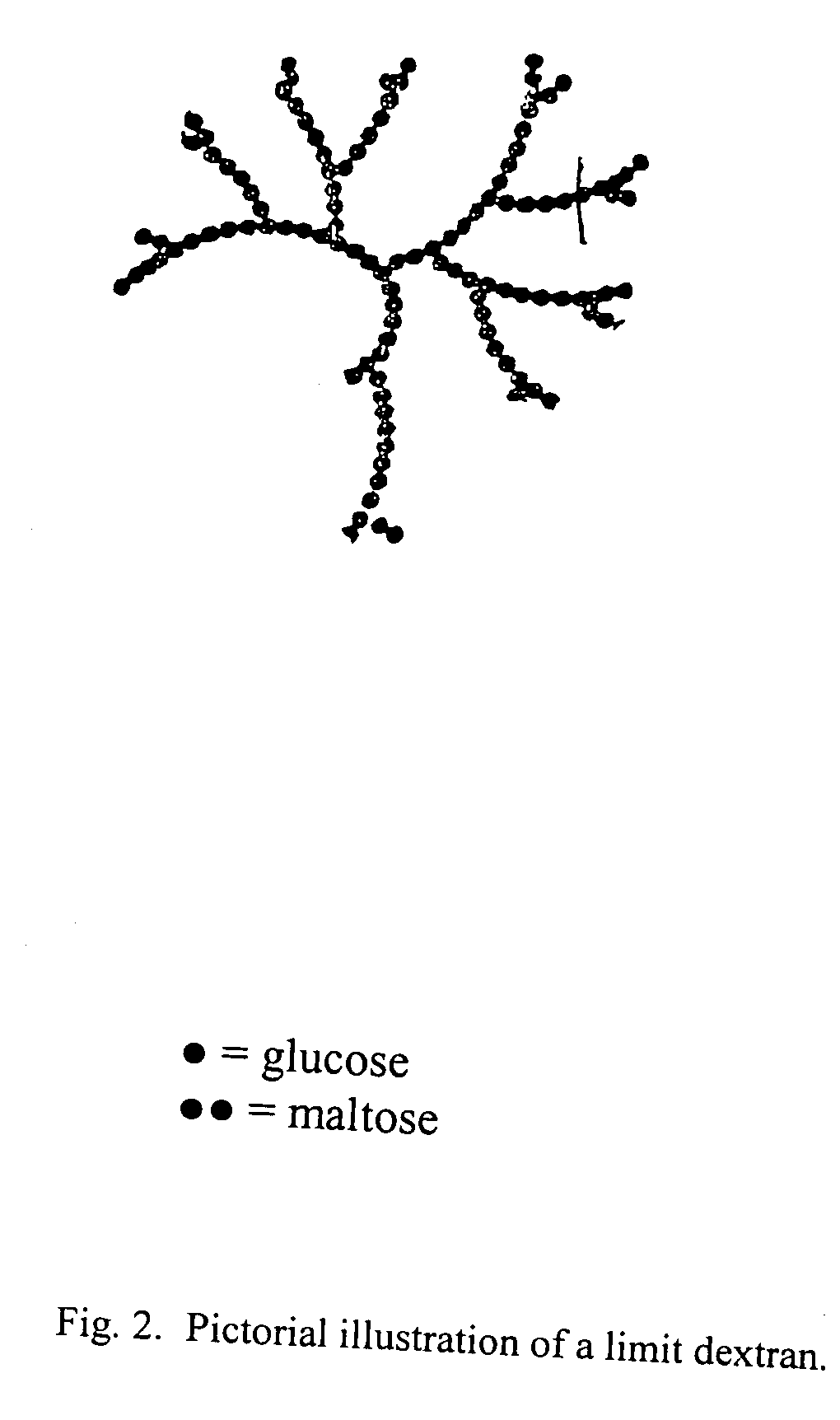
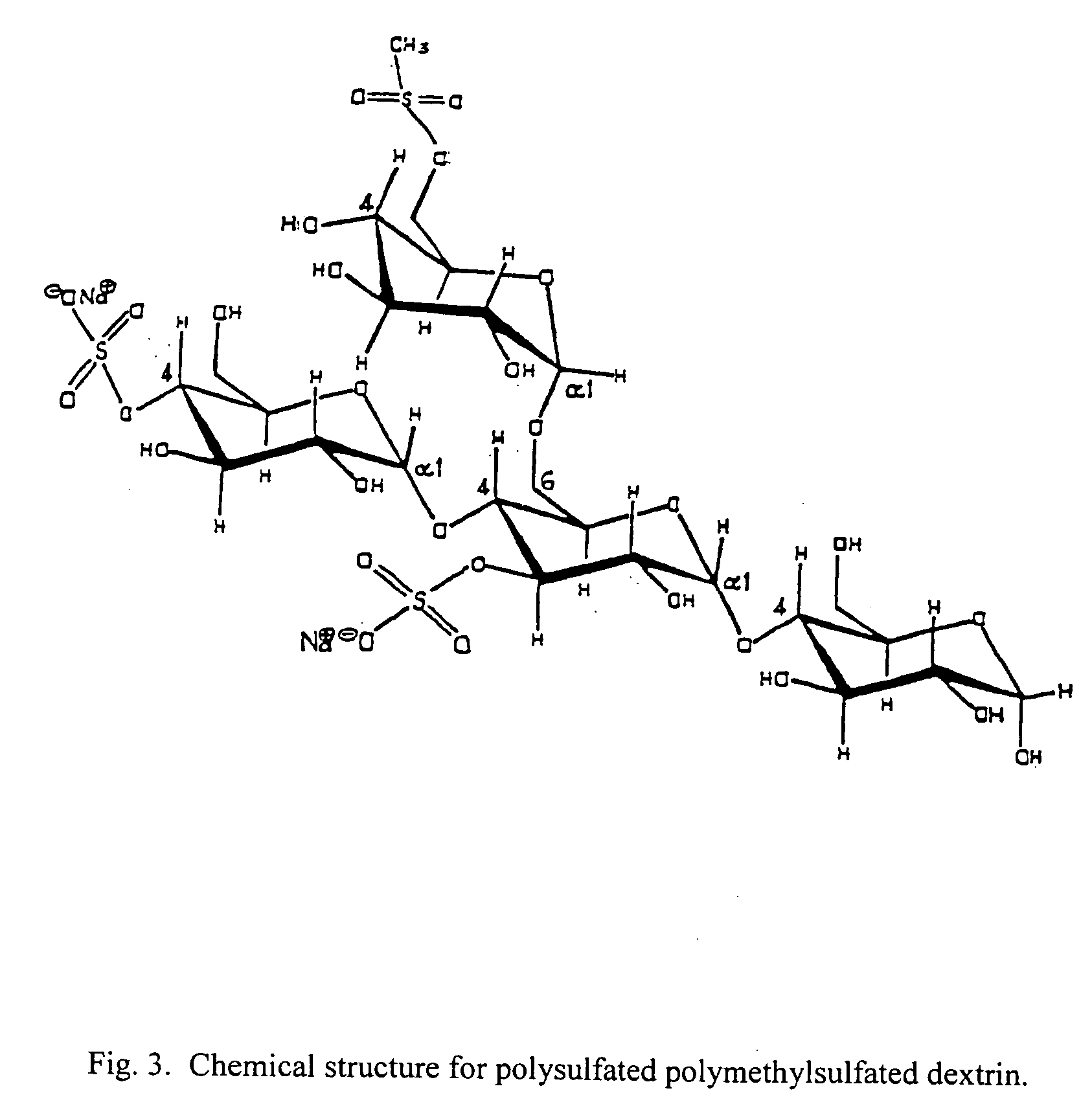
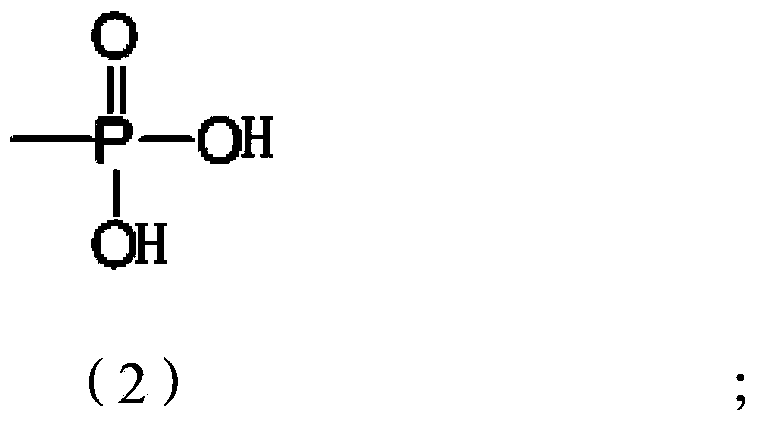Patents
Literature
538 results about "Intraperitoneal injection" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Intraperitoneal injection or IP injection is the injection of a substance into the peritoneum (body cavity). It is more often applied to animals than to humans. In general, it is preferred when large amounts of blood replacement fluids are needed or when low blood pressure or other problems prevent the use of a suitable blood vessel for intravenous injection.
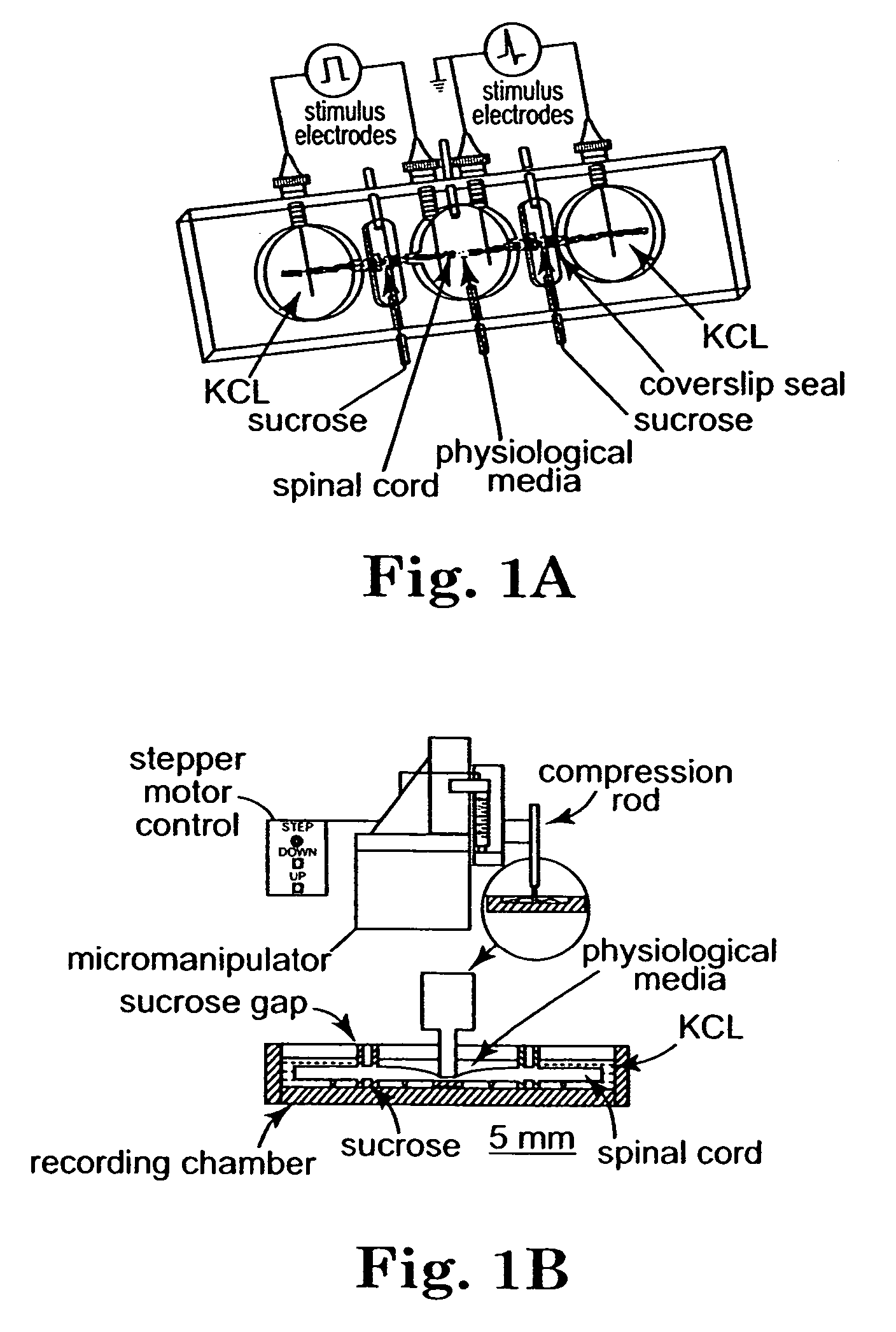
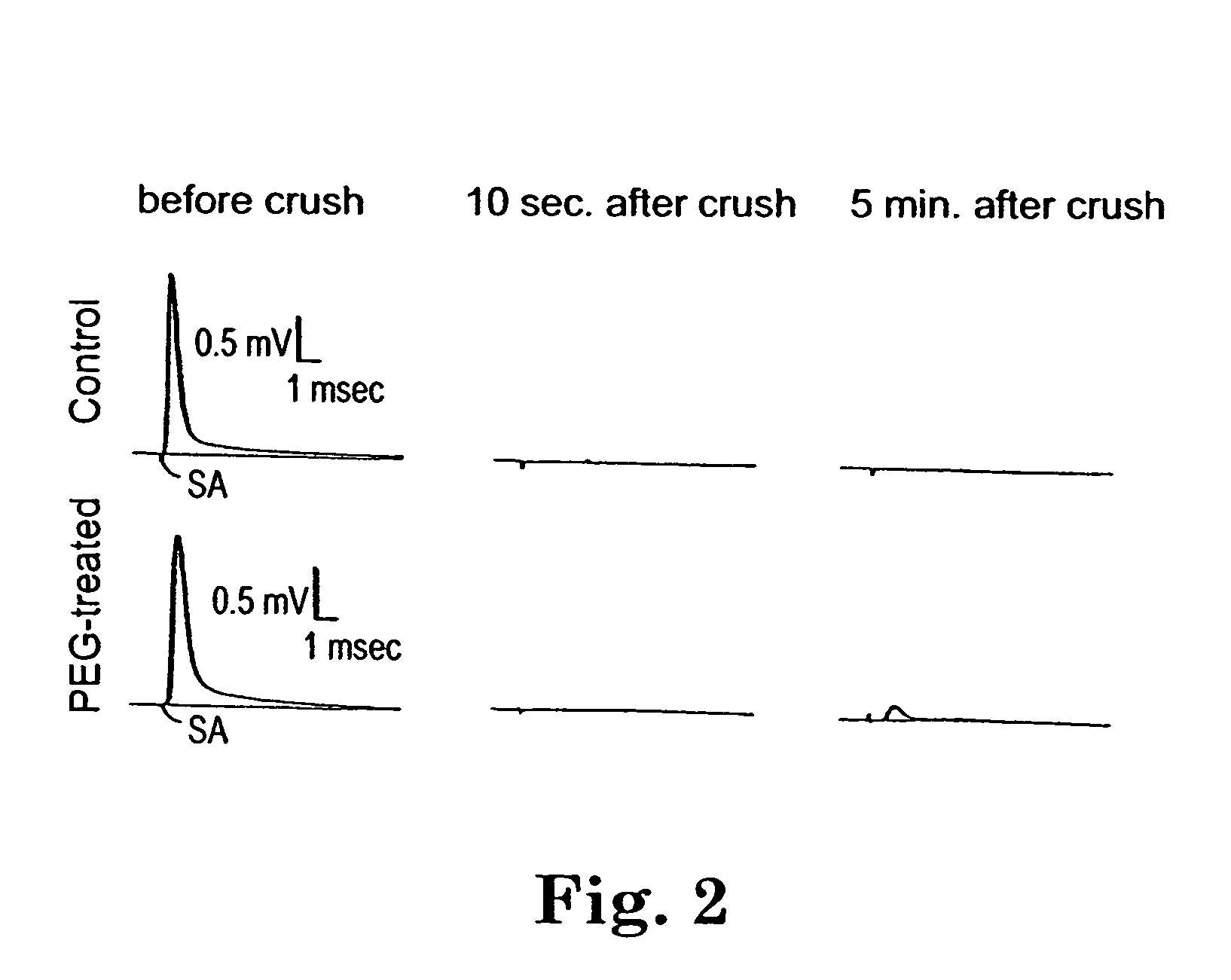
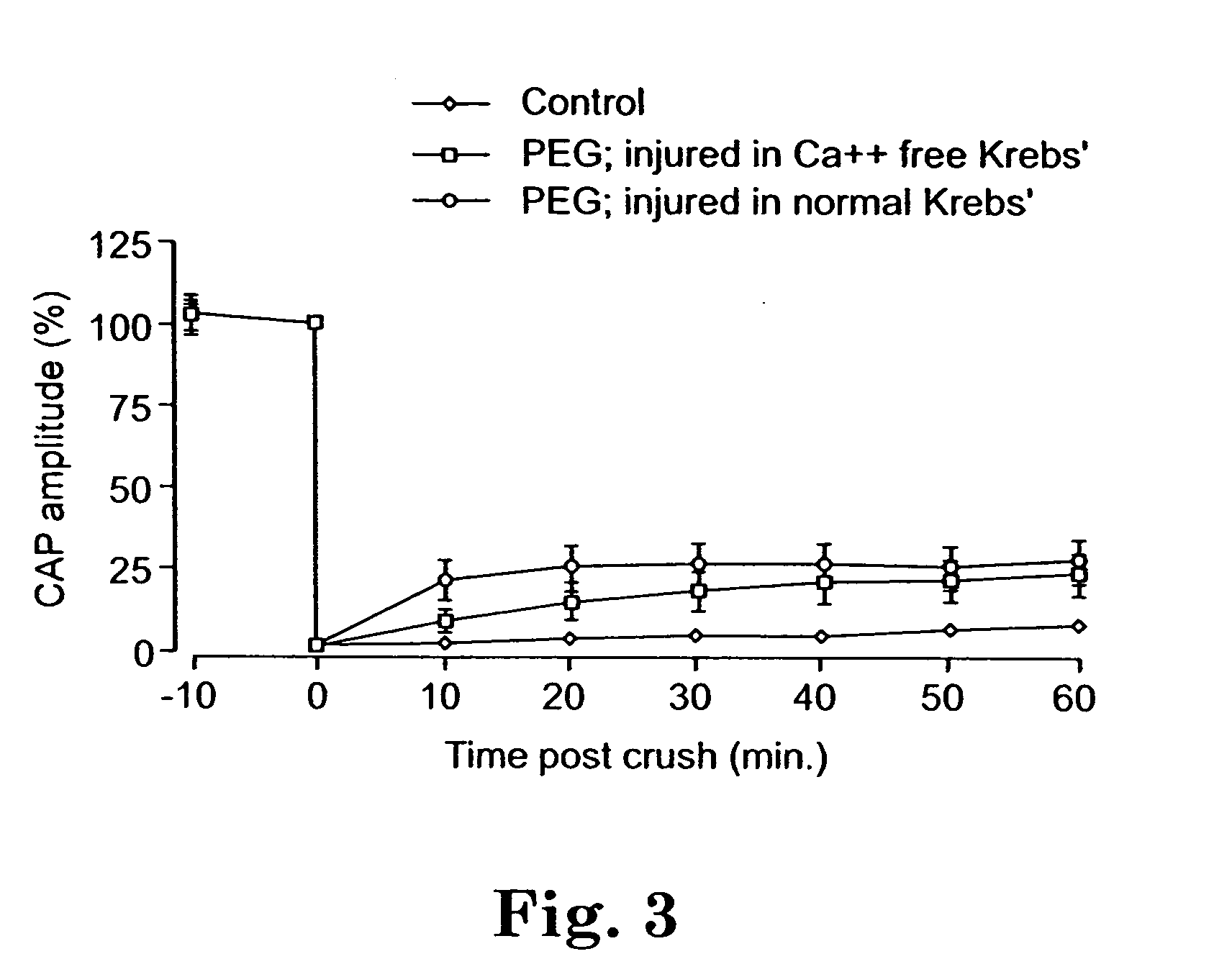
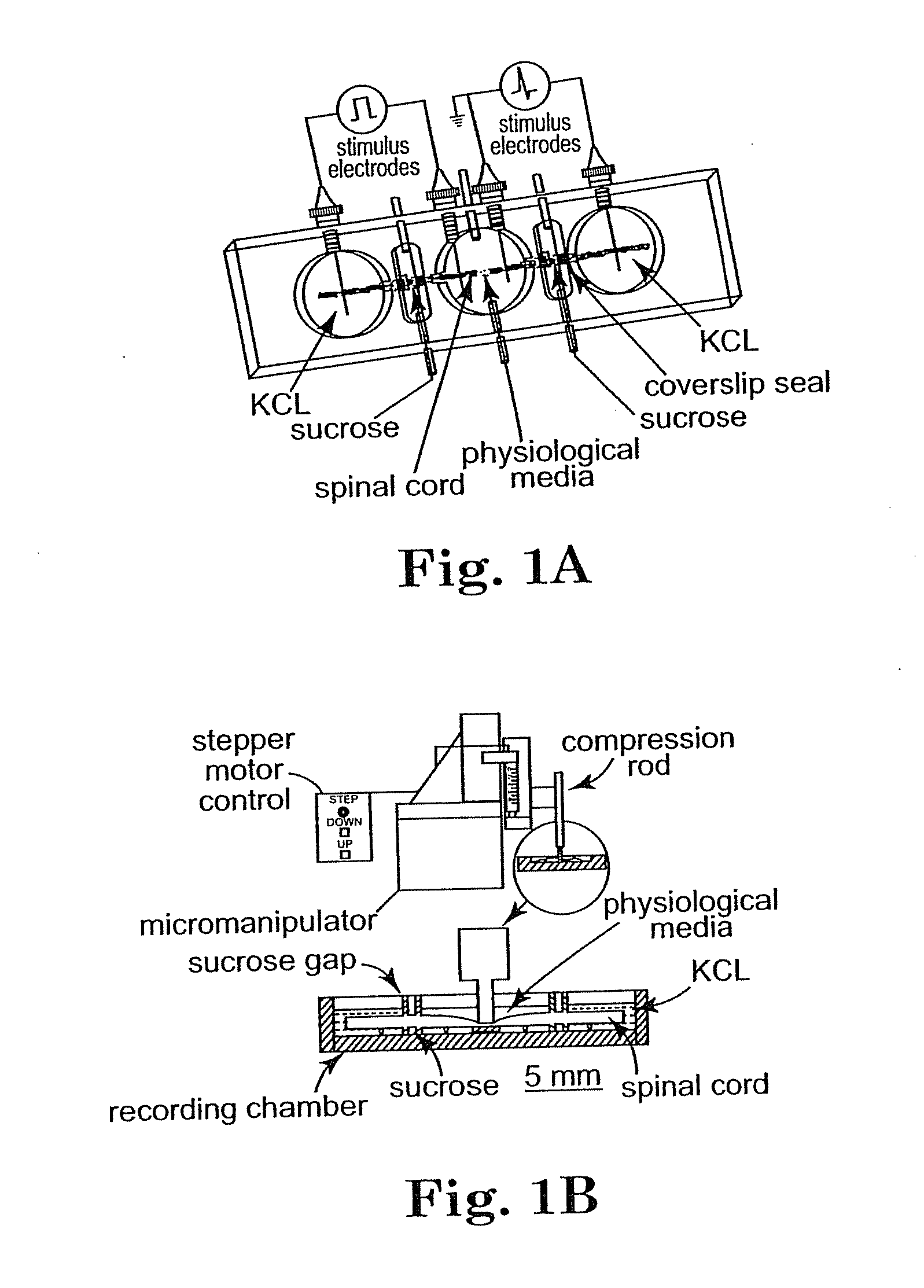
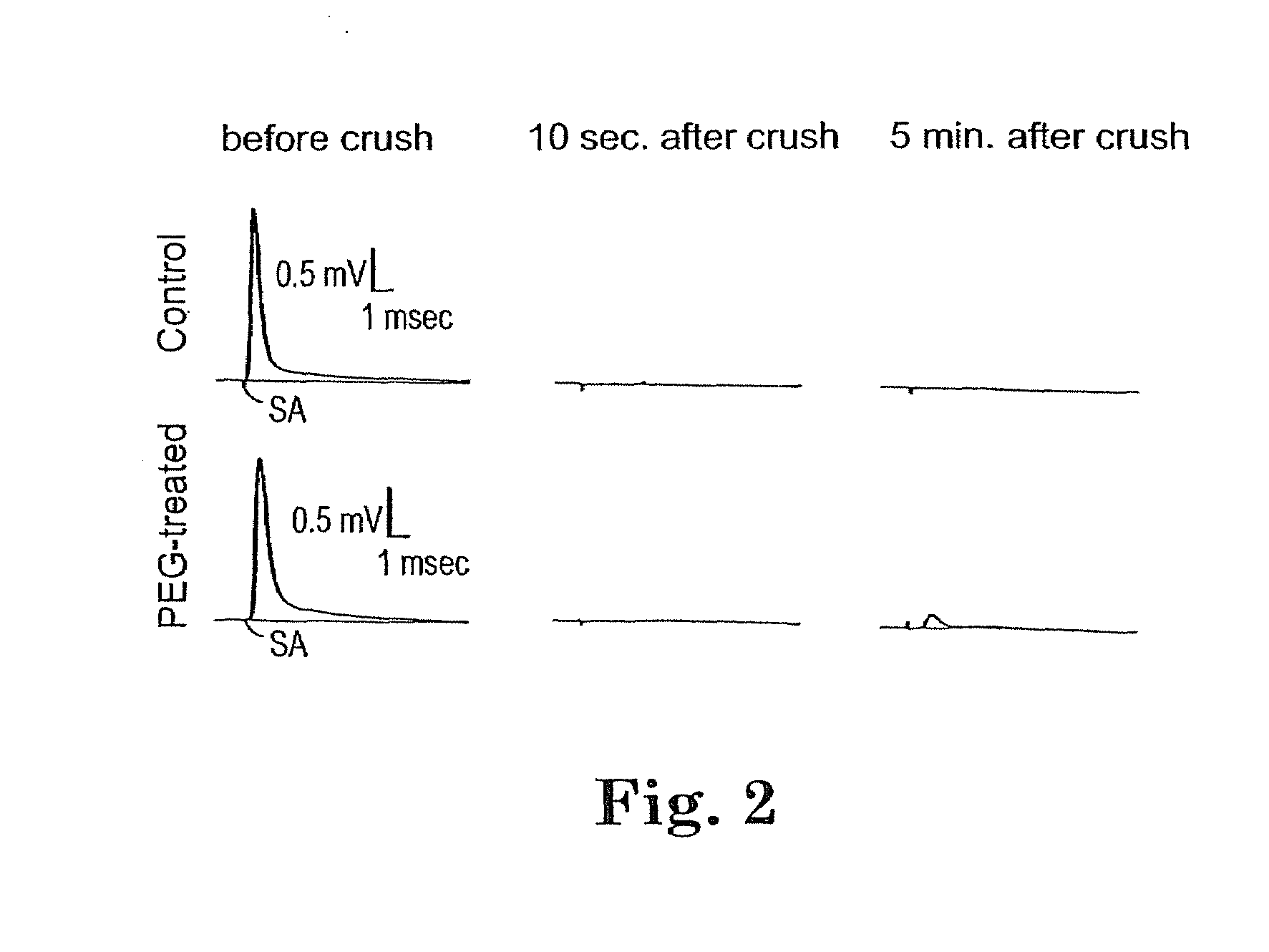
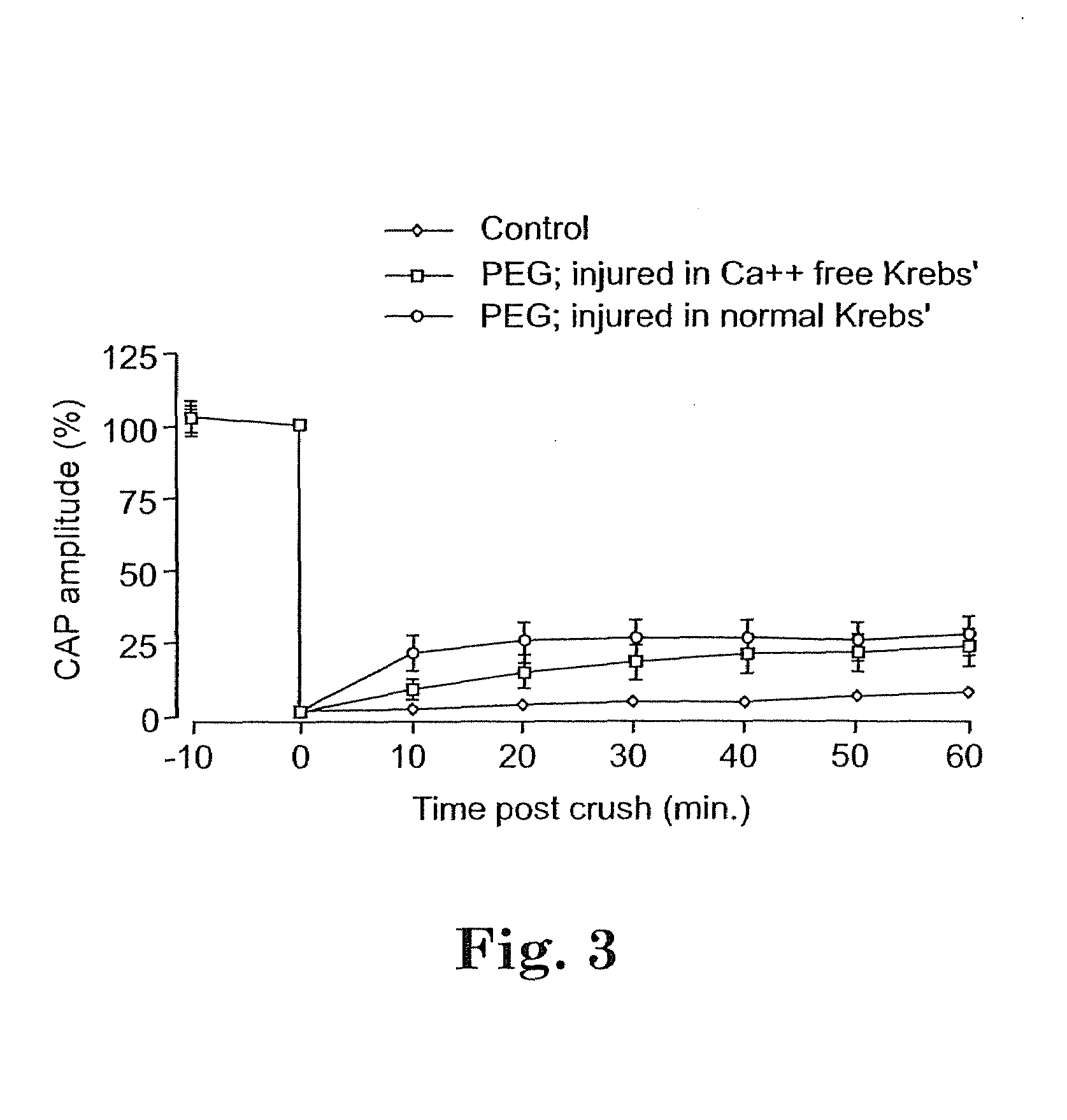
Methods and compositions for treating mammalian nerve tissue injuries
ActiveUS20050069520A1Promote recoveryLoss in its effectivenessNervous disorderSynthetic polymeric active ingredientsIntraperitoneal routeIn vivo
To achieve, an in vivo repair of injured mammalian nerve tissue, an effective amount of a biomembrane fusion agent is administered to the injured nerve tissue. The application of the biomembrane fusion agent may be performed by directly contacting the agent with the nerve tissue at the site of the injury. Alternatively, the biomembrane fusion agent is delivered to the site of the injury through the blood supply after administration of the biomembrane fusion agent to the patient. The administration is preferably by parenteral administration including including intravascular, intramuscular, subcutaneous, or intraperitoneal injection of an effective quantity of the biomembrane fusion agent so that an effective amount is delivered to the site of the nerve tissue injury.
Owner:PURDUE RES FOUND INC +1
Therapy with clostridium perfringens enterotoxin to treat ovarian and uterine cancer
ActiveUS20060084594A1Low toxicityGood curative effectBacterial antigen ingredientsIn-vivo radioactive preparationsIntraperitoneal routeCancer cell
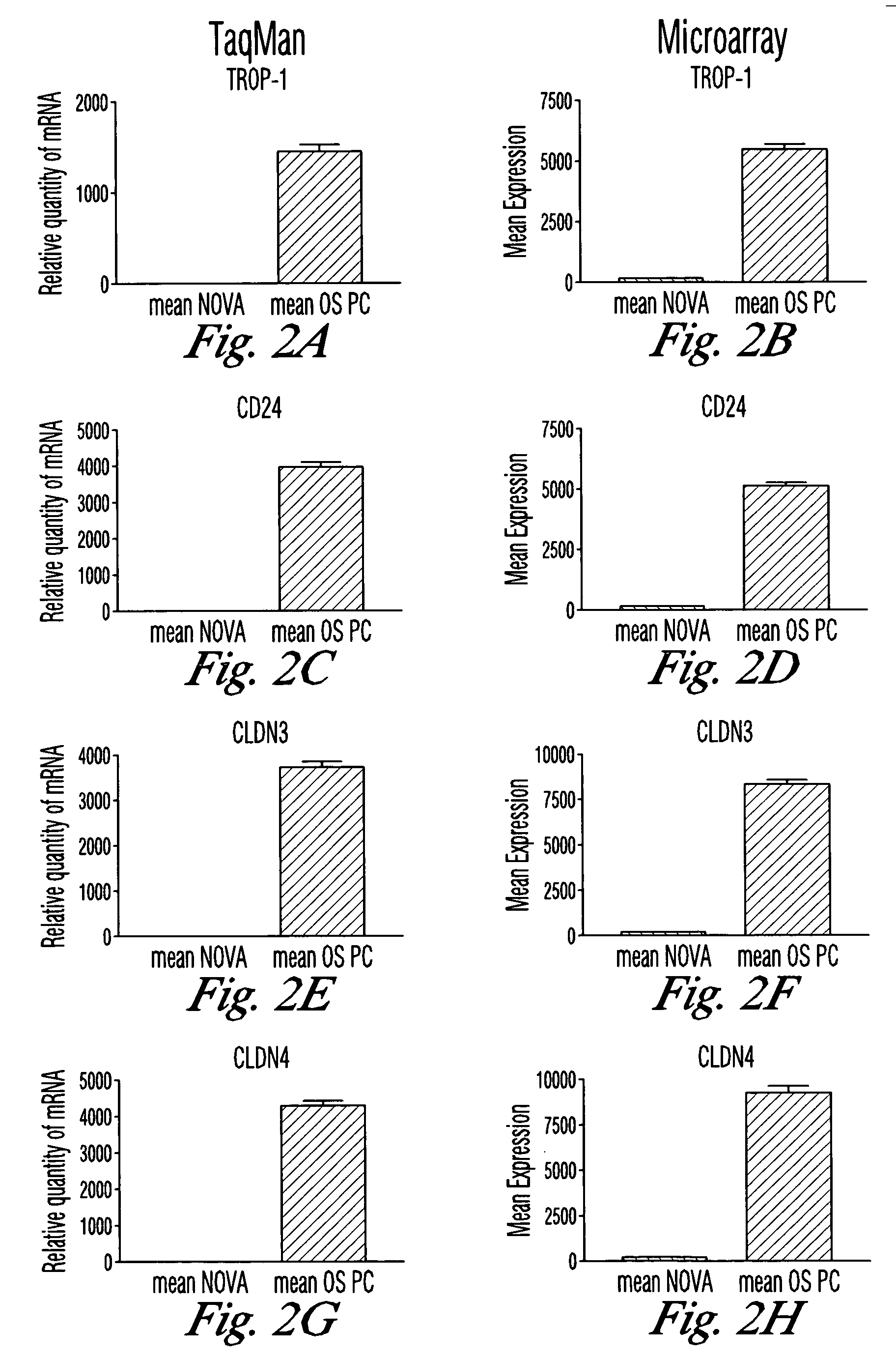
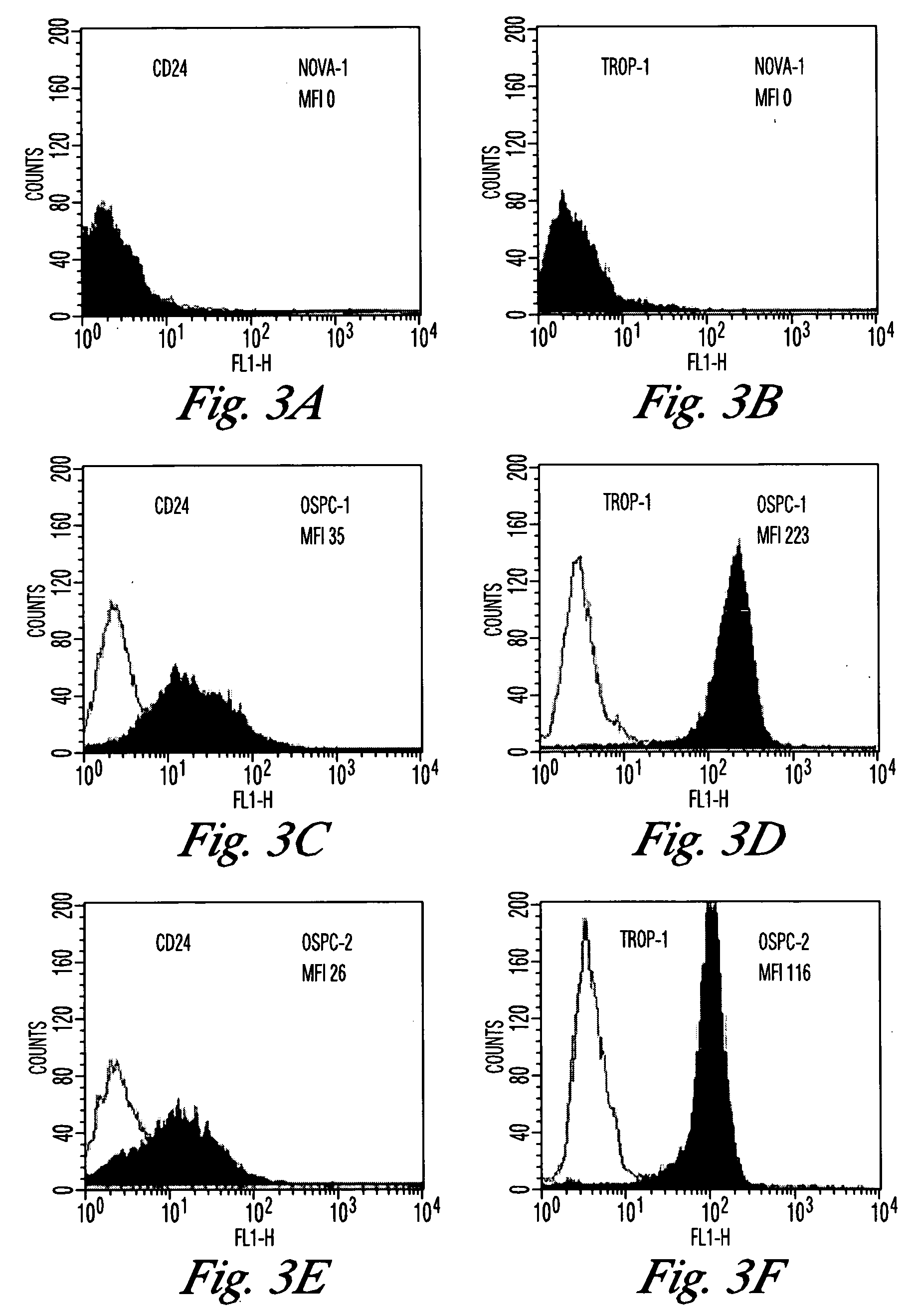
The invention discloses high levels of receptors for Clostridium perfringens enterotoxin (CPE) have been found in ovarian cancer and uterine cancer tissue samples. In addition, successful in vivo treatment of a mouse model of ovarian cancer with intraperitoneal injection of CPE is disclosed. High levels of Ep-CAM protein is also disclosed in ovarian cancer tissue samples. Thus, the invention provides a method of treating ovarian cancer and uterine cancer by administering CPE. The invention also provides a method of treating cancer in a mammal involving intraperitoneal administration of CPE, where at least some cancerous cells are located in or adjacent to the peritoneal cavity of the mammal. The invention also provides a method of treating ovarian cancer involving administering an anti-Ep-CAM antibody. The invention also provides a method of treating cancers expressing claudin-3 or claudin-4 by administering an antibody against claudin-3 and / or an antibody against claudin-4. The invention also provides a method of protecting a mammal from CPE toxicity involving administering a protective agent that binds to claudin-3 and / or claudin-4 and inhibits CPE binding to claudin-3 and / or claudin-4.
Owner:YALE UNIV
Therapeutic enzyme formulations and uses thereof in celiac sprue and/or dermatitis herpetoformis
InactiveUS7628985B2Decrease in levelAvoid toxicityOrganic active ingredientsPeptide/protein ingredientsDermatitis herpetiformisPharmaceutical formulation
Pharmaceutical formulations of glutenase enzymes are provided. The enzymes find particular use in the treatment of a Celiac or dermatitis herpetiformis patient.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
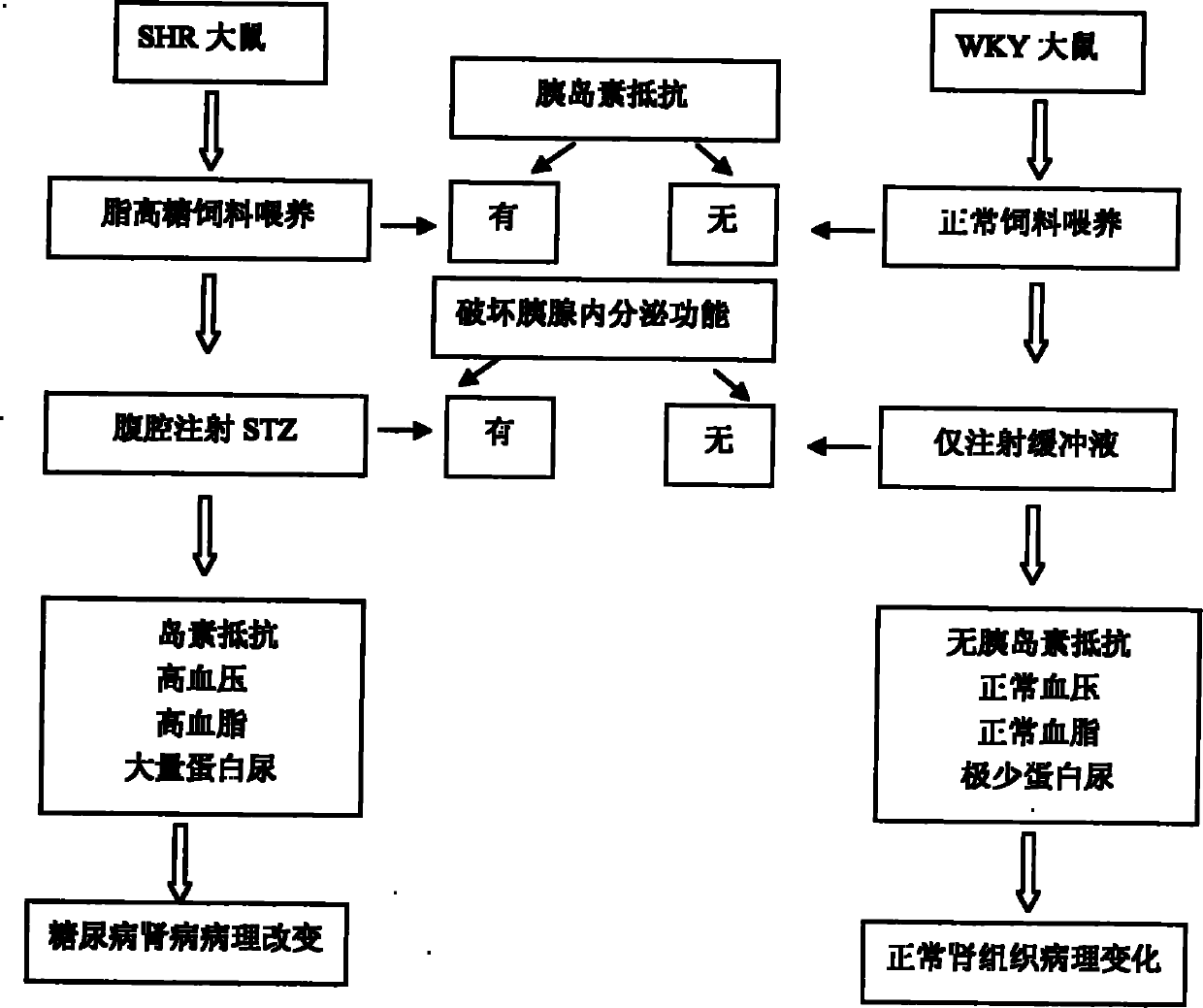
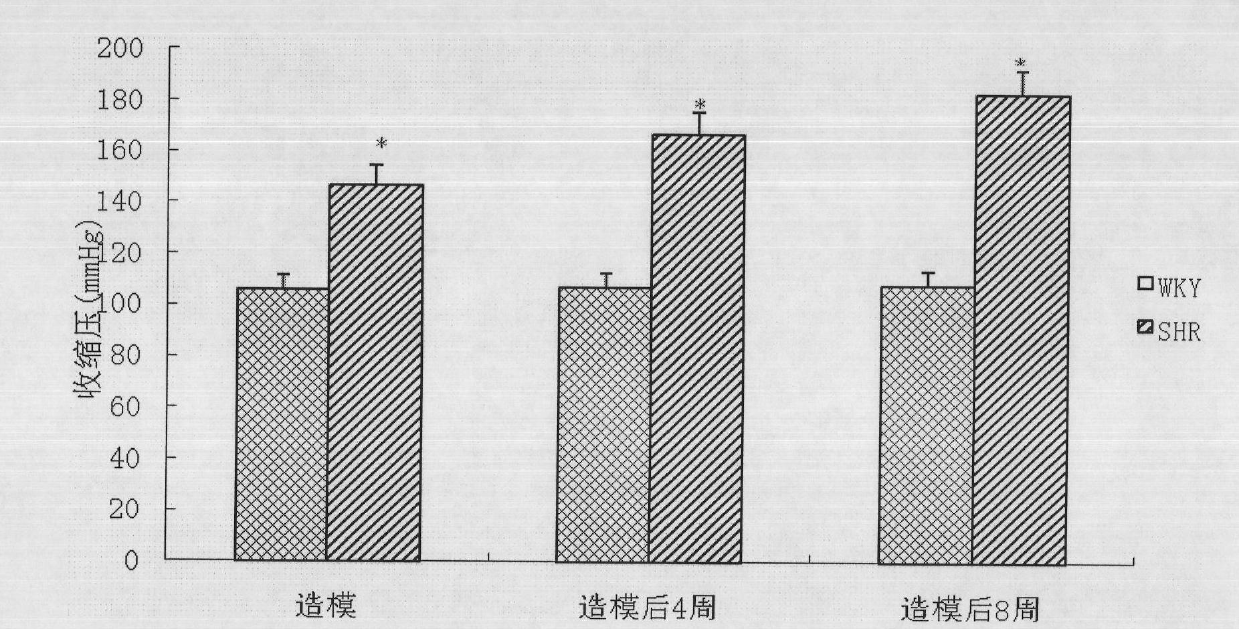
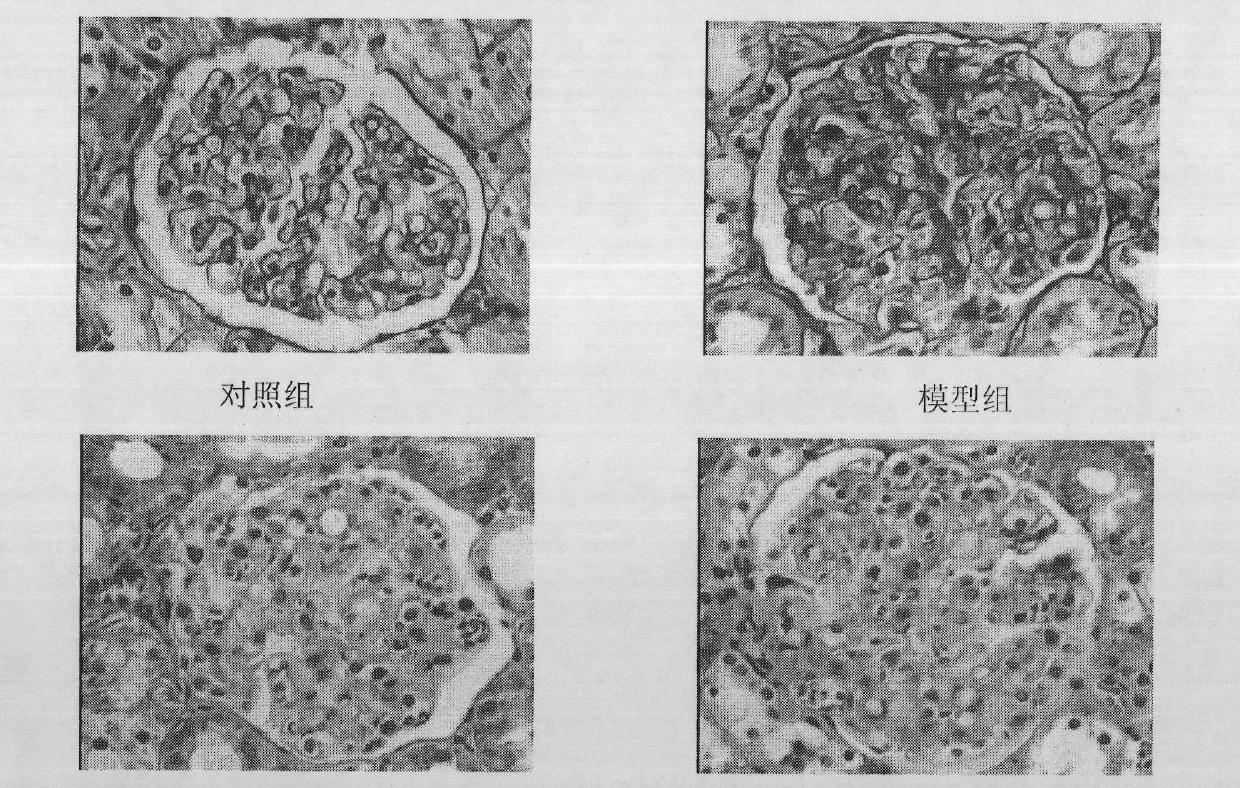
Preparation method of 2-type diabetic nephropathy model
InactiveCN101766149AIncrease concentrationEasy to operateAnimal feeding stuffIntraperitoneal routeSucrose
The invention relates to a preparation method of a 2-type diabetic nephropathy model. The method comprises the following steps of: selecting a male SHR (spontaneously hypertensive rat) rat with an age of 6 weeks, wherein the blood pressure of the male SHR rat at birth is normal, and at the time of 4 weeks, the blood pressure begins to rise; after adaptively feeding the SHR rat selected in the step (1) for one week, feeding a high sugar and high fat feed for 2 weeks; detecting the weight, the blood fat, the blood sugar and the insulin concentration and calculating an insulin resistance index evaluated by a steady-state model, wherein the insulin resistance index is not smaller than 3.8, and the high fat and high sugar feed comprises the following components in percentage by weight: 10.0 percent of lard, 20.0 percent of cane sugar, 2.5 percent of cholesterol, 2.0 percent of cholate and 66.5 percent of conventional feed; after fasting the insulin resistant SHR rat for 12 hours, carrying out a small-dose one-time STZ (streptozocin) (35mg / kg) intraperitoneal injection on the SHR rat, wherein the small dose is 35mg / kg; after 72 hours, measuring the random blood sugar; and judging the 2-type diabetic nephropathy model to be successfully prepared if the plasma glucose is not smaller than 16.7mmol / L.
Owner:SOUTHEAST UNIV
Drug therapy for celiac sprue
InactiveUS20050256054A1Data processing applicationsPeptide/protein ingredientsDermatitis herpetiformisBinding peptide
Celiac Sprue and / or dermatitis herpetiformis arc treated by interfering with HLA binding of immunogenic gluten peptides. The antigenicity of gluten oligopeptides and the ill effects caused by an immune response thereto are decreased by administration of an HLA-binding peptide inhibitor. Such inhibitors are analogs of immunogenic gluten peptides and (i) retain the ability to bind tightly to HLA molecules; (ii) retain the protcolytic stability of these peptides; but (iii) are unable to activate disease-specific T cells.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
Methods and uses of leptin in immune modulation and hepatocellular carcinoma
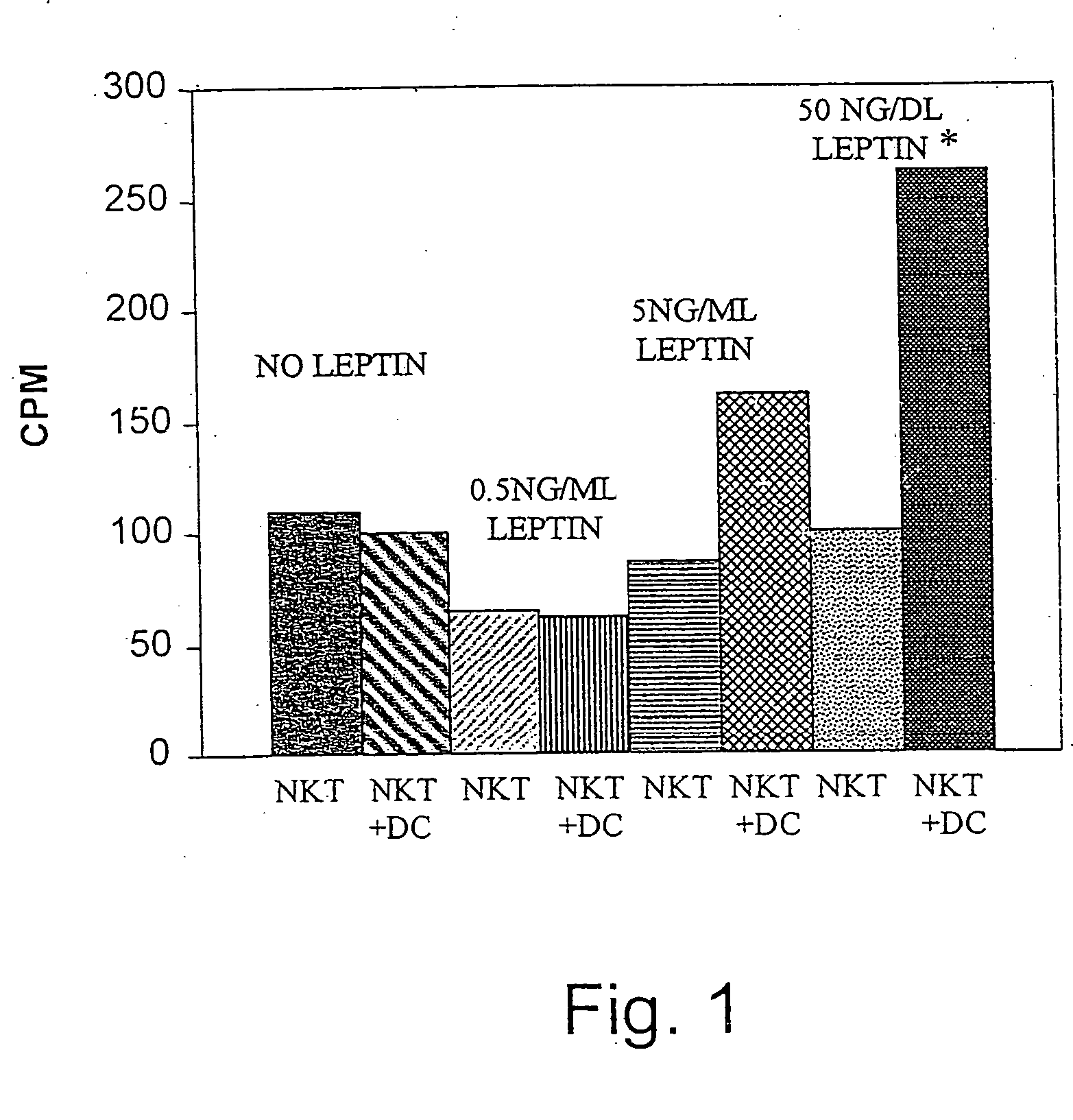
Leptin was previously demonstrated to exert potent immune modulatory properties in several immune mediated disorders. The aim of the study was to determine leptin's anti-tumor effect in a murine model of human hepatocellular carcinoma (HCC). In vivo, Athymic T cell deficient (nude) mice transplanted with 1×106 human Hep3B cells, followed by administration of two daily intraperitoneal doses of 0.5 mg / gram leptin for 6 weeks. Leptin administration induced a significant reduction in tumor size and improved survival in nude mice. Histologically, tumors of leptin-administered mice featured increased inflammatory exudate in interphase areas. Leptin-induced tumor suppression was associated with a significant increase in peripheral natural killer (NK) cell number. Splenocytes from leptin-treated mice featured decreased expression of CIS mRNA. To determine which lymphocyte subset is a prerequisite for the anti tumor effect of leptin, T&B cell deficient (Scid) mice and T,B& NK deficient (Scid-Beige) mice were subcutaneously implanted with Hep3B tumor cells, with and without the daily intraperitoneal administration of 0.5 mg / gram leptin for 6 weeks. SCID mice featured leptin-associated tumor suppression similar to those of nude mice. In contrast, NK-deficient SCID-Beige mice developed larger tumors. To further establish natural killer cell's central role in mediation of leptin's anti-tumor effect, NK cells were incubated in vitro with increasing doses of leptin, demonstrating a dose-dependent increase in cytotoxic activity. Incubation of leptin with hepatoma cell line was found to induce a dose-dependent reduction in hepatoma cell proliferation, suggesting an additive direct anti-tumor effect. Further synergism in inhibition of hepatoma cell proliferation in vitro was achieved following addition of natural killer cells. HCC cells expressed leptin receptor mRNA, while addition of leptin induced increased mRMA expression of STAT2 and SOCS1 on tumor cell lines. Leptin administration induces a significant suppression of human HCC. This effect is mediated by induction of natural killer cell proliferation and activation, and by direct inhibition of tumor growth. Decreased natural killer cell expression of inhibitory CIS protein and over expression of the anti-proliferative STAT2 and SOCS1 proteins in HCC lines may underline both anti cancerous effects of leptin.
Owner:ENZO THERAPEUTICS
Antiviral composition
InactiveUS20050209189A1Low toxicityEliminate the effects ofBiocideOrganic active ingredientsIntraperitoneal routeCyclodextrin
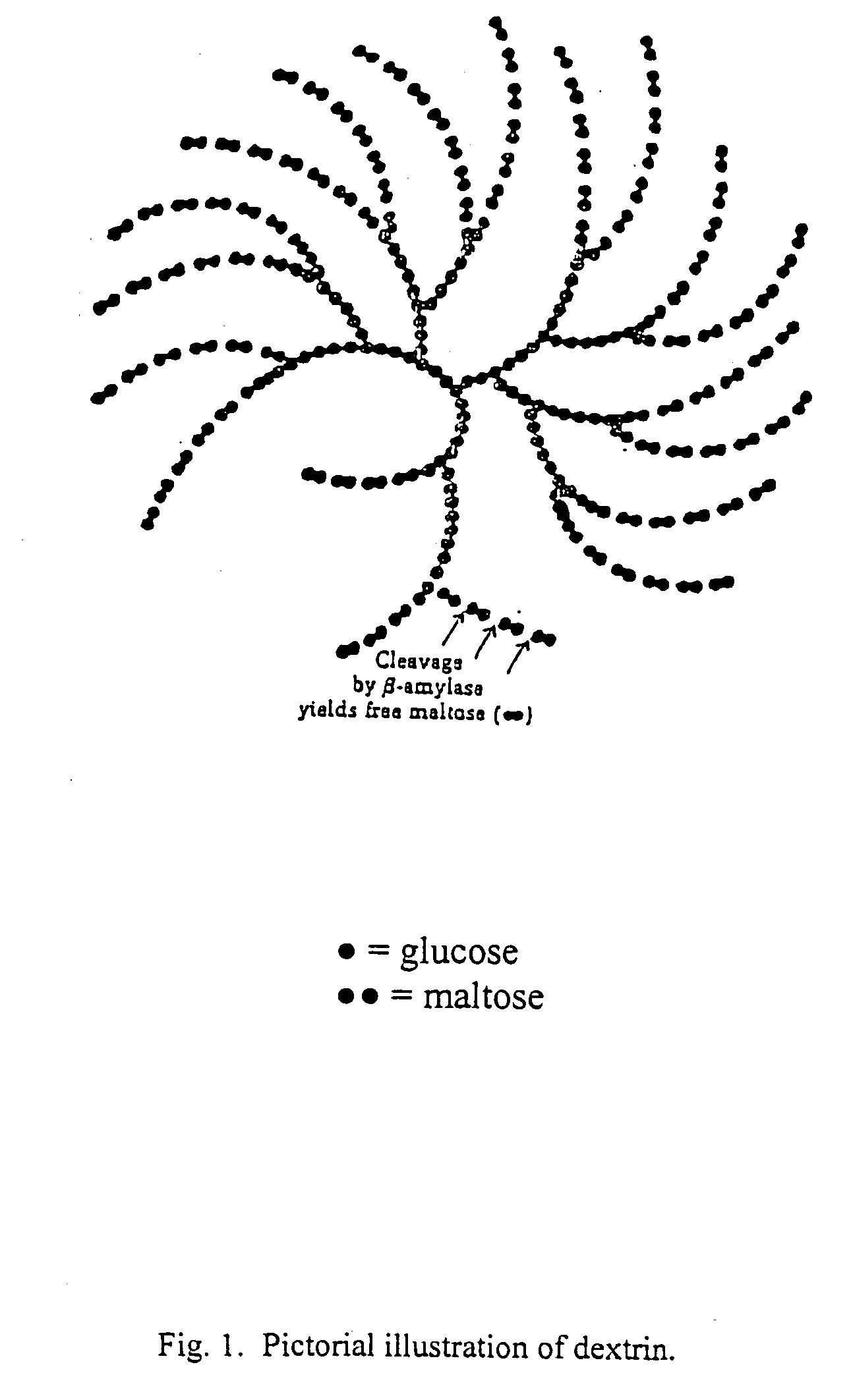
Chemical compounds, being the alkyl sulfate of sulfated saccharides, particularly, dextrin, dextran, and cyclodextrin, and pharmaceutical compositions containing these compounds. The compounds of the invention provide antiviral activity, particularly in the treatment and prevention of sexually-transmitted diseases. Methods of treating viral infection and preventing viral transmission include administration include administration of the compounds of the invention orally, topically, subcutaneously, by muscular injection, by intraperitoneal injection and by intravenous injection.
Owner:HERSHLINE ROGER
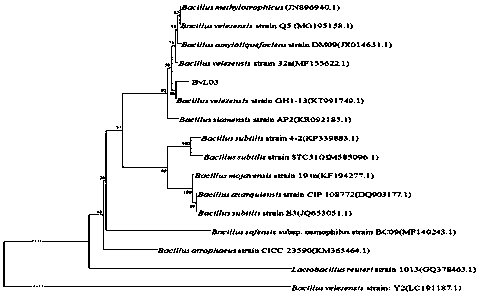
Bacillus velezensis, and micro-ecological preparation and preparation method thereof
ActiveCN110016451AGrowth inhibitionReduced activityAntibacterial agentsBacteriaIntraperitoneal routeFresh water organism
The invention relates to a Bacillus velezensis, and a micro-ecological preparation and preparation method thereof. The Bacillus velezensis is preserved in China Center for Type Culture Collection, hasa strain preservation number of CCTCC NO: M 2019218, and is classified and named as Bacillus velezensis BvL03, and the Latin academic name is Bacillus velezensis BvL03. The micro-ecological preparation suitable for aquiculture prepared by the strain has broad-spectrum effect of inhibiting fish pathogenic bacteria through drug-delivery routes such as feed addition, water body sprinkling or intraperitoneal injection, and the protection rate reaches 85 percent. After acting on fish bodies, the preparation can effectively prevent fish pathogenic bacteria such as aeromonas hydrophila from infecting freshwater fishes such as grass carps and crucian carps, and particularly has the best protection effect on grass carps.
Owner:HUNAN NORMAL UNIVERSITY
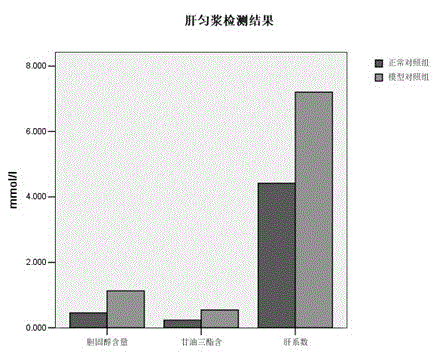
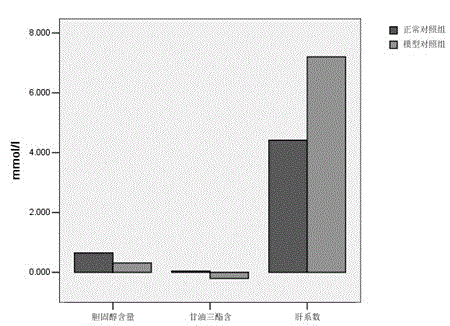
Modeling processing method of non-alcoholic fatty liver mouse model
InactiveCN102972345AIncrease aromaIncrease appetiteIn-vivo testing preparationsAnimal husbandryMortality rateHigh fat
The invention discloses a modeling method of a non-alcoholic fatty liver mouse model, and the method is characterized by consisting of the following steps that (1) a healthy mouse is caught and first adaptively raised for 3 to 7 days in an animal house, a backing material and clean water in a mouse cage are changed every day, and basic fodder is fed during that period of time; (2) after adaptive raising, composite high-fat fodder is fed every day; (3) 5 percent CC14 liquid is injected into the abdominal cavity of the mouse two weeks later, the liquid carbon tetrachloride is diluted to 5 percent emulsion with deionized water, and the liquid which is injected into the abdominal cavity shall be newly prepared; the abdominal cavity injection state continues for the 28th day, and meanwhile the composite high-fat fodder is fed; and the model is obtained. The modeling method has the advantages of short time, high modeling success rate, extremely low mortality rate and low cost.
Owner:GUIZHOU UNIV
Diagnostic method for Celiac Sprue
ActiveUS7776545B2Less invasiveLess expensivePeptide/protein ingredientsDisease diagnosisDigestionT cell
Detection of toxic gluten oligopeptides refractory to digestion and antibodies and T cells responsive thereto can be used to diagnose Celiac Sprue.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
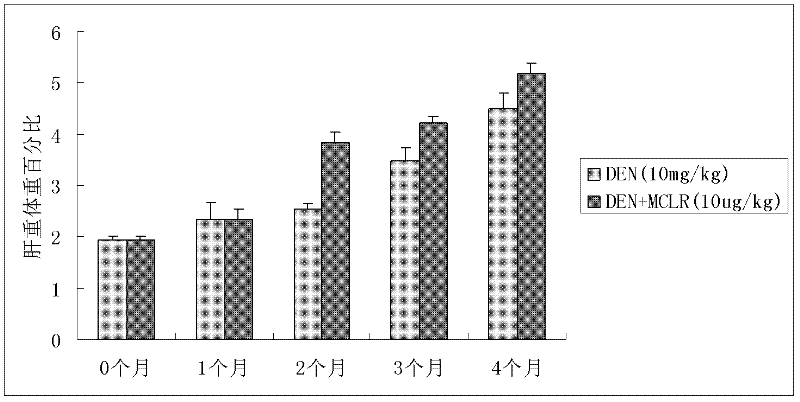
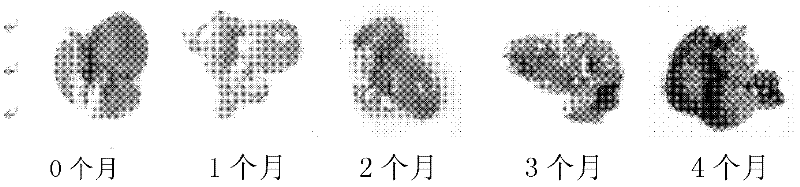
Method for establishing microcystin MC-LR promoted diethyl nitrosamine DEN induced rat liver cancer model
InactiveCN102524180ACyclic peptide ingredientsVeterinary instrumentsIntraperitoneal routeProcess mechanism
The invention discloses a method for inducing a rat liver cancer model by injecting diethyl nitrosamine DEN in coordination with microcystin MC-LR into a rat body through intraperitoneal injection. Indexes such as liver weight to body weight ratio, liver appearance, liver histopathological slicing and GST-Pi (Glutathione S-transferase) protein expression level clearly show liver cell injury phase, liver cell hyperplasia-hepatocirrhosis phase and liver cell carcinomatous change process in a rat liver cancer occurring process, and find that the liver cancer model induced by the DEN in coordination with the microcystin MC-LR has a more obvious effect at the same time than that induced by DEN independently; the tumor formation time is shorter; and the model is an ideal model for researching occurrence of the liver cancer of a human body. The model has the characteristic of clearly showing each stage for occurrence of the liver cancer and contributing to research of a liver cancer occurrence process mechanism, detection of precancerous lesion and development of treatment medicaments.
Owner:ANHUI UNIVERSITY
Pelteobagrus fulvdraco sperm ultralow temperature freezing and recovery technology
InactiveCN101904329AShort operating timeImprove the induction effectClimate change adaptationDead animal preservationIntraperitoneal routeWater baths
The invention relates to pelteobagrus fulvdraco sperm ultralow temperature freezing storage and recovery method. Parent pelteobagrus fulvdraco which is in good shape and good growth, has no disease and is sexually mature is selected, 30Mug / kg LHRH-A2 intraperitoneal injection is carried out to induce sperm increasing, after 36 hours, dissection is carried out to obtain spermary, and the spermary is cut into pieces in a clean mortar cooled in a refrigerator at 4 DEG C in advance, the pieces are diluted by Ringers solution in the proportion of 1:6, balancing is carried out for 30min at 4 DEG C, 10% methyl alcohol is added, and mixing to be uniform is carried out, packaging is carried out, balancing is carried out for 10min 6cm above liquid nitrogen surface, and balancing is carried out for 5min on the liquid nitrogen surface, and then the product is thrown into liquid nitrogen to be stored. Water bath ate 37 DEG C is carried out, activation solution SM is used for activation, pelteobagrus fulvdraco roe is extruded into a container, frozen sperm is added in drops in the sperm roe quantity ratio of 105:1, stirring to be uniform is carried out, then appropriate activation solution SM is added in drops, and stirring to be uniform is carried out, so that fertilization of roe is carried out. The sperm stored by the invention has high quality, has insemination capability and has important theoretical significance and application value on seed quality saving, inheritance breeding, sustainable culture and disease control of pelteobagrus fulvdraco.
Owner:FRESHWATER FISHERIES RES INSITUTE OF JIANGSUPROVINCE +1
Duplication method of rat acute or persistent hyperuricemia model
InactiveCN1698906AReduce the wayShort copy cycleIn-vivo testing preparationsIntraperitoneal routeHypodermoclysis
The invention discloses a duplication method of rat acute or persistent hyperuricemia model which comprises, (1) selecting male, female healthy rats with the specie of SD and Wistar and 150-450g of body weight, simultaneously administering purines by 100-2000 mg / kg and uric acid depressant by 5-1000mg / kg through stomach filling, hypodermic injection or abdominal cavity injection, (2) after 5-24 hours, administering the rats by the same mode and medicament dose, (3) administering the rats daily continually according to the step (1) and (2), continuing administration for one to two weeks.
Owner:NANJING UNIVERSITY OF TRADITIONAL CHINESE MEDICINE
Imaging model in small animal living bodies with echinococcus granulosus and construction method thereof
InactiveCN105641716AHigh incidenceIncreased mortalityCompounds screening/testingLuminescence/biological staining preparationIntraperitoneal routePositive control
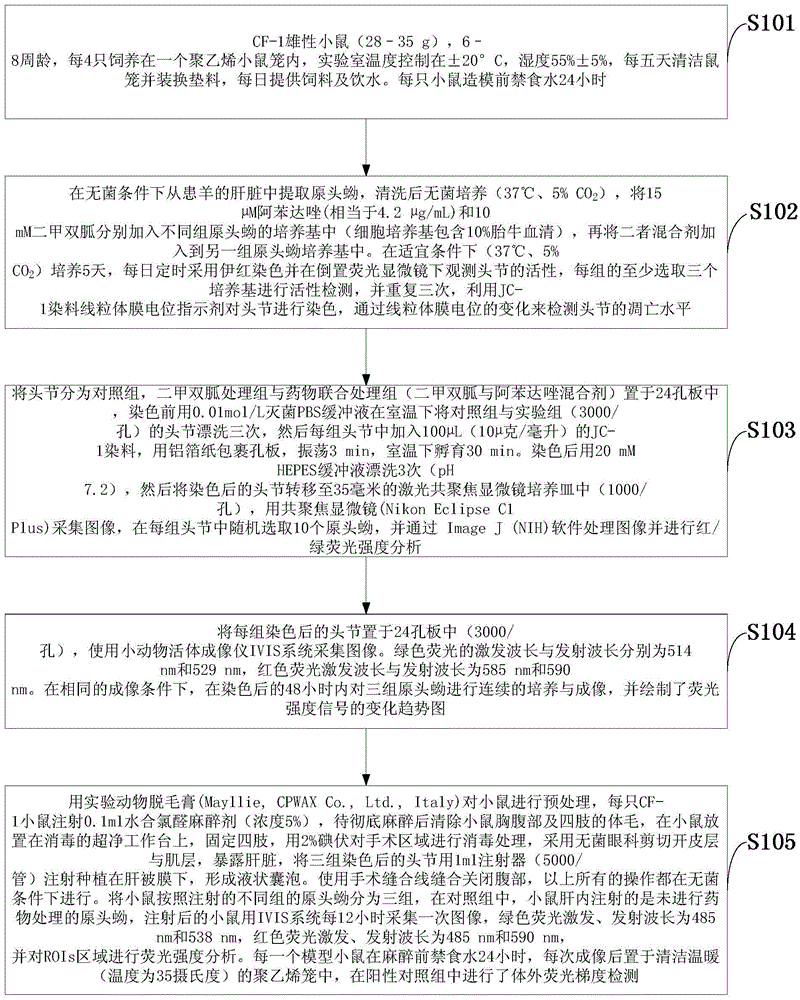
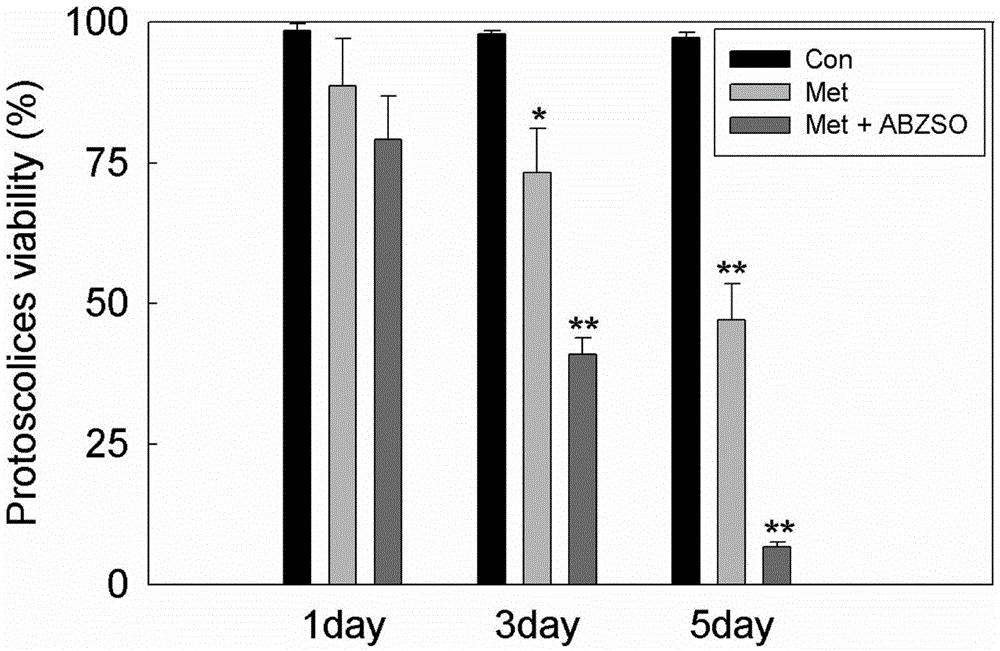
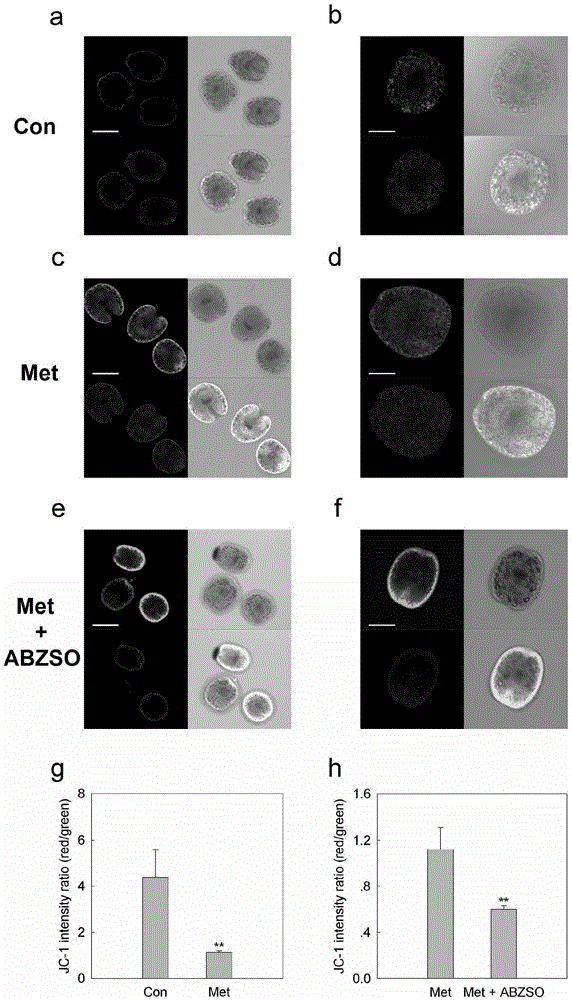
The invention discloses an imaging model in small animal living bodies with echinococcus granulosus and a construction method thereof. The construction method comprises the steps of: feeding CF-1 male mice; carrying out in vitro culture and drug treatment on protoscolex; carrying out in vitro fluorescence imaging and fluorescence gradient analysis; pretreating mice with depilatory paste for experimental animals, injecting the abdominal cavity of each mouse with chloral hydrate anesthetic, implanting three groups of stained scoleces under Glisson capsules by injection, dividing the mice into three groups according to different groups of injected protoscolex; in a control group, injecting the livers of mice with protoscolex on which drug treatment is not carried out, collecting images every 12 hours after injection, carrying out fluorescence intensity analysis on the ROIs region, putting into a polyethylene cage after imaging, and carrying out in vitro fluorescence gradient detection in the positive control group. The construction method constructs a mouse model with echinococcus granulosus which can express luciferase, provides a platform for drug sensitive tests in living bodies with echinococcosis, and can monitor the growth and transfer of hydatid in the living model dynamically in a long term.
Owner:王思博
Construction method for mouse type 2 diabetes mellitus (T2DM) animal experiment model
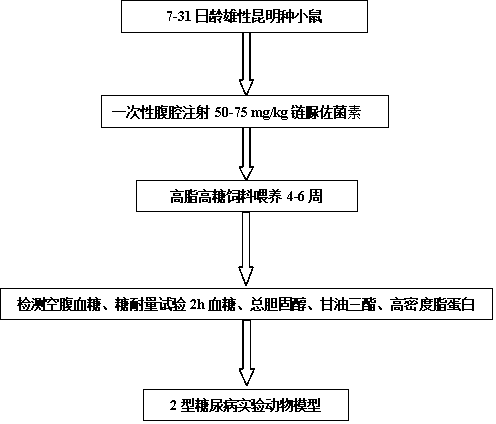
ActiveCN104257671ASmall doseHigh molding rateOrganic active ingredientsAnimal husbandryIntraperitoneal routeHigh fat
The invention patent relates to the technical field of animal experiment model construction, in particular to a construction method for a mouse type 2 diabetes mellitus (T2DM) animal experiment model. The method includes the following steps: male Kunmin mice at the age of 7 to 31 days are selected; 50 to 75 mg / Kg (milligrams per kilogram) of streptozotocin is taken by the mice through intraperitoneal injection at a time; after the mice are continuously fed with high-fat high-glucose feed for 4 to 6 weeks upon free eating, the mice of which fasting blood-glucose is above 7.0 millimoles per liter, blood glucose in a 2-hour oral glucose tolerance test is above 11.1 millimoles per liter, cholesterol is above 5.7 millimoles per liter, triglyceride is above 1.7 millimoles per liter, and high-density lipoprotein is below 1.0 millimole per liter at the same time are defined as T2DM animal experiment models. The model can simulate the T2DM characteristics of humans, and adopts multiple testing indicators to accurately define T2DM; due to the light weights of the underage animals, the streptozotocin dosage is small, so that the experiment cost is reduced.
Owner:QINGHAI NORMAL UNIV
Use hydrolyzed medium containing microorganisms medicinally
InactiveUS20080102061A1OptimizationPromote high vitalityBiocideMilk preparationIntraperitoneal routeDisease
Owner:TECH COMMLIZATION
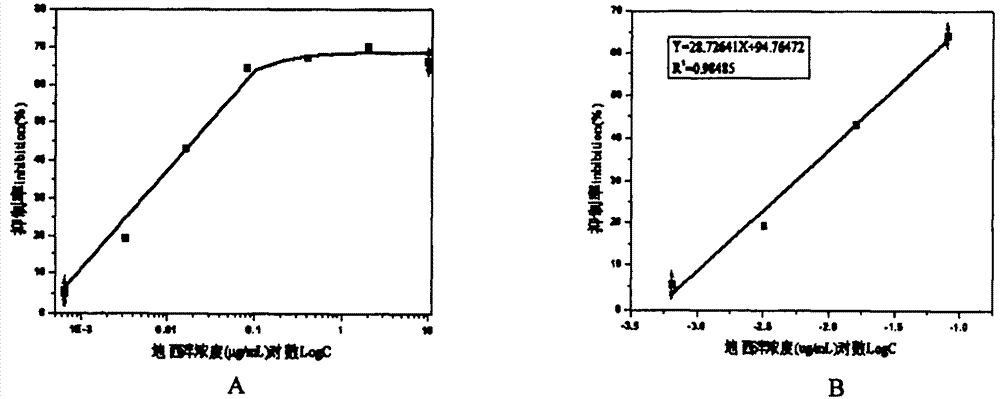
Diazepam monoclonal antibody screening and application
Belonging to the field of biotechnologies, the invention discloses a preparation method of a diazepam monoclonal antibody, and especially discloses application of the diazepam monoclonal antibody in the rapid detection method of ELISA. The method includes: coupling carboxylation reformed diazepam with carrier protein by an EDC technique, immunizing 5-6-week-old Balb / c mice by intraperitoneal injection, taking spleen from the mouse with positive binding activity and competitive activity to conduct cell fusion and carrying out screening to prepare the monoclonal antibody, and optimizing reaction conditions, determining the concentration of optimal coating antigen DZP-OVA at 1.25 microgram / mL and the optimal antibody dilution factor at 1:16000, thus establishing the ELISA method for the rapid detection of diazepam. The lowest detection limit IC10 of the method is 0.0011 microgram / mL. The invention provides the basic reagent antibody for immunoassay of diazepam and the detection method.
Owner:INST OF HYGIENE & ENVIRONMENTAL MEDICINE PLA ACAD OF MILITARY MEDICAL
Method for constructing model of animal with chronic cardiac failure heart qi deficiency and blood stasis edema
InactiveCN104000834AImprove stabilityGood repeatabilityOrganic active ingredientsWestern medicineAbdominal cavity
The invention discloses a method for constructing a model of an animal with chronic cardiac failure heart qi deficiency and blood stasis edema. The method includes the following steps that a clean adult male SD rat is selected, the rat is placed in a cleansing cage, the temperature ranges from 19 DEG C to 23 DEG C, the relative humidity ranges from 40% to 50%, a natural lighting mode is adopted, and the rat is fed through the rat standard feeds and can drink water freely; after adaptive feeding is conducted for one week, lavage is conducted through adriamycin of 0.2 % of the fodder weight, and propylthiouracil injection is conducted on the abdominal cavity one time every week according to the proportion of 3.5 mg / kg and continues for six weeks. The method for constructing the model of the animal with the chronic cardiac failure heart qi deficiency and the blood stasis edema has the advantages that the model can better simulate the chronic cardiac failure heart qi deficiency and the blood stasis edema, meet the pathological characters, for example, LVEF and LVFS reduce remarkably, and BNP increases, of the chronic cardiac failure in western medicine, and show the chronic cardiac failure heart qi deficiency and the blood stasis edema in traditional Chinese medicine, and the copy method is good in stability and repeatability, simple and objective.
Owner:周杰
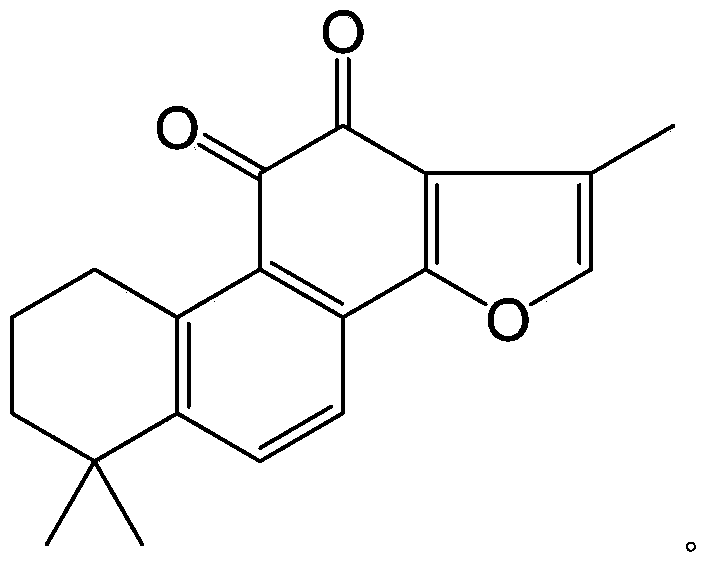
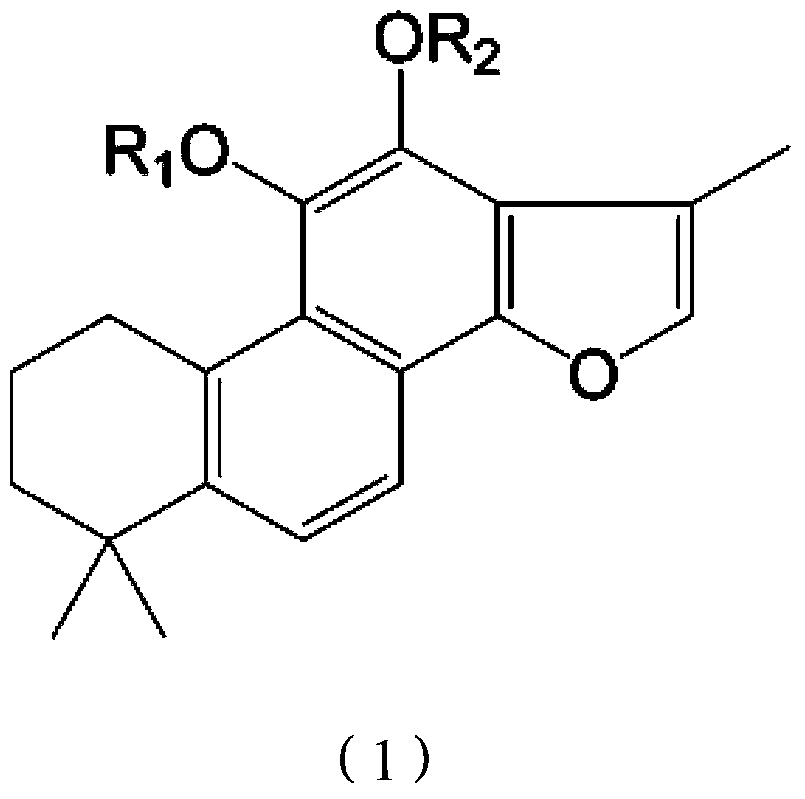
Tanshinone IIA phosphate phenolic ester derivative and preparation process thereof
ActiveCN103382214AGood water solubilityEasy to makeOrganic active ingredientsSteroidsDiseaseIntraperitoneal route
The invention relates to a tanshinone IIA phosphate phenolic ester derivative and a preparation process thereof. The derivative and the preparation process thereof have the advantages that firstly, the new derivative of tanshinone IIA is provided, and the new substance can serve as a drug for treating cerebrovascular diseases; secondly, the tanshinone IIA phosphate phenolic ester derivative is good in water solubility and can be produced into injections conveniently. Animal experiments indicate that the tanshinone IIA phosphate phenolic ester derivative has a good pesticide effect on mouse middle cerebral artery occlusion (MCAO) through intraperitoneal injection and can be developed into the new drug for treating the cerebrovascular diseases, particularly the injection-type drug for treating the cerebrovascular diseases. The derivative serves as an important prodrug and has the high application value clinically.
Owner:江苏新本草医药研究院有限公司
Edwardsiella tarda recombinant subunit vaccine and application thereof
InactiveCN101991844AHigh protection rateAntibacterial agentsBacterial antigen ingredientsEscherichia coliEdwardsiella tarda
The invention relates to the field of molecular biology and immunology and particularly discloses an Edwardsiella tarda recombinant subunit vaccine as well as a preparation method and application thereof. The vaccine has a base sequence in a sequence table SEQ ID No.1. The preparation method of the Edwardsiella tarda recombinant subunit vaccine comprises the following steps of constructing a vaccine antigen expression plasmid pETXV21, converting the vaccine antigen expression plasmid pETXV21 into escherichia coli BL21 (DE3), recovering a recombinant protein after induced expression, mixing the recombinant protein with a strain B187, and dissolving the mixture in PBS (Phosphate Buffer Saline) to obtain a vaccine mixed liquor which has the function of immune protection to Edwardsiella tarda. The vaccine mixed liquor is intraperitoneally injected to fishes to achieve the purpose of immune protection.
Owner:INST OF OCEANOLOGY - CHINESE ACAD OF SCI
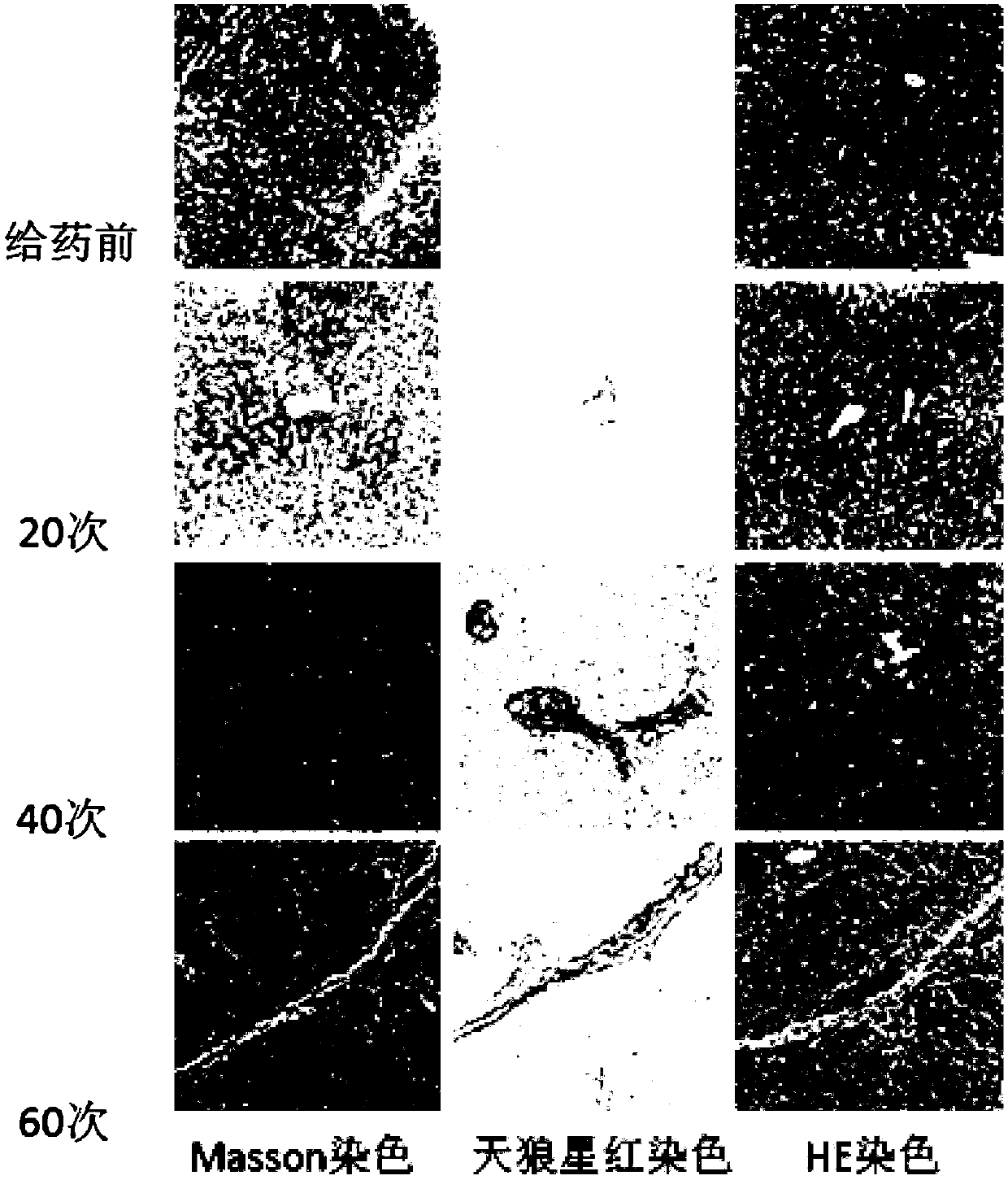
Method for establishment of macaca mulatta liver fibrosis model by intraperitoneal injection
ActiveCN107693507ASimple methodShort modeling timeHalogenated hydrocarbon active ingredientsPharmaceutical delivery mechanismIntraperitoneal routeTolerability
The invention provides a method for establishment of a macaca mulatta liver fibrosis model by intraperitoneal injection. The method includes the steps of: selecting 5-9kg male macaca mulattas of 2-3 years old as the model animals, performing feeding with high-fat feed during modeling, supplying an alcohol water solution with a volume concentration of 9%-11% for drinking, and injecting CCl4 injection to each macaca mulatta during modeling twice a week by intraperitoneal injection continuously for 20-30 weeks, and controlling the injecting amount at 0.8-1.5mL / kg each time, thus achieving successful modeling. The animal model establishment method provided by the invention is simple, safe and reliable, has the characteristics of short modeling time, high success rate, good repeatability, goodanimal tolerance and low death rate, and can be used for studying the pathogenesis, pathogenic factors and treatment means of chronic liver diseases.
Owner:SICHUAN PROVINCIAL PEOPLES HOSPITAL
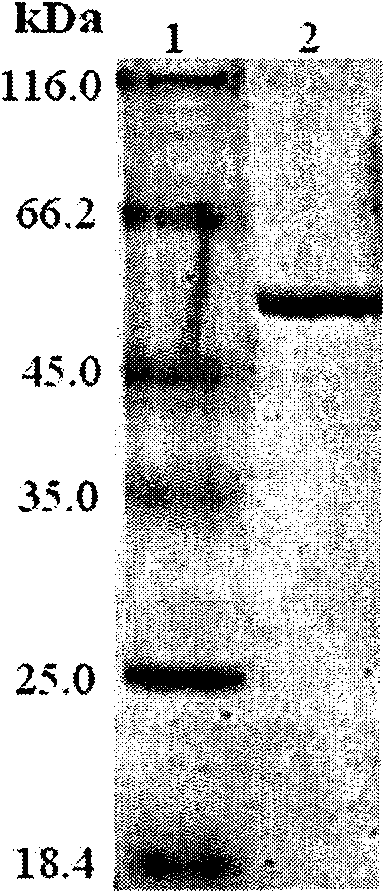
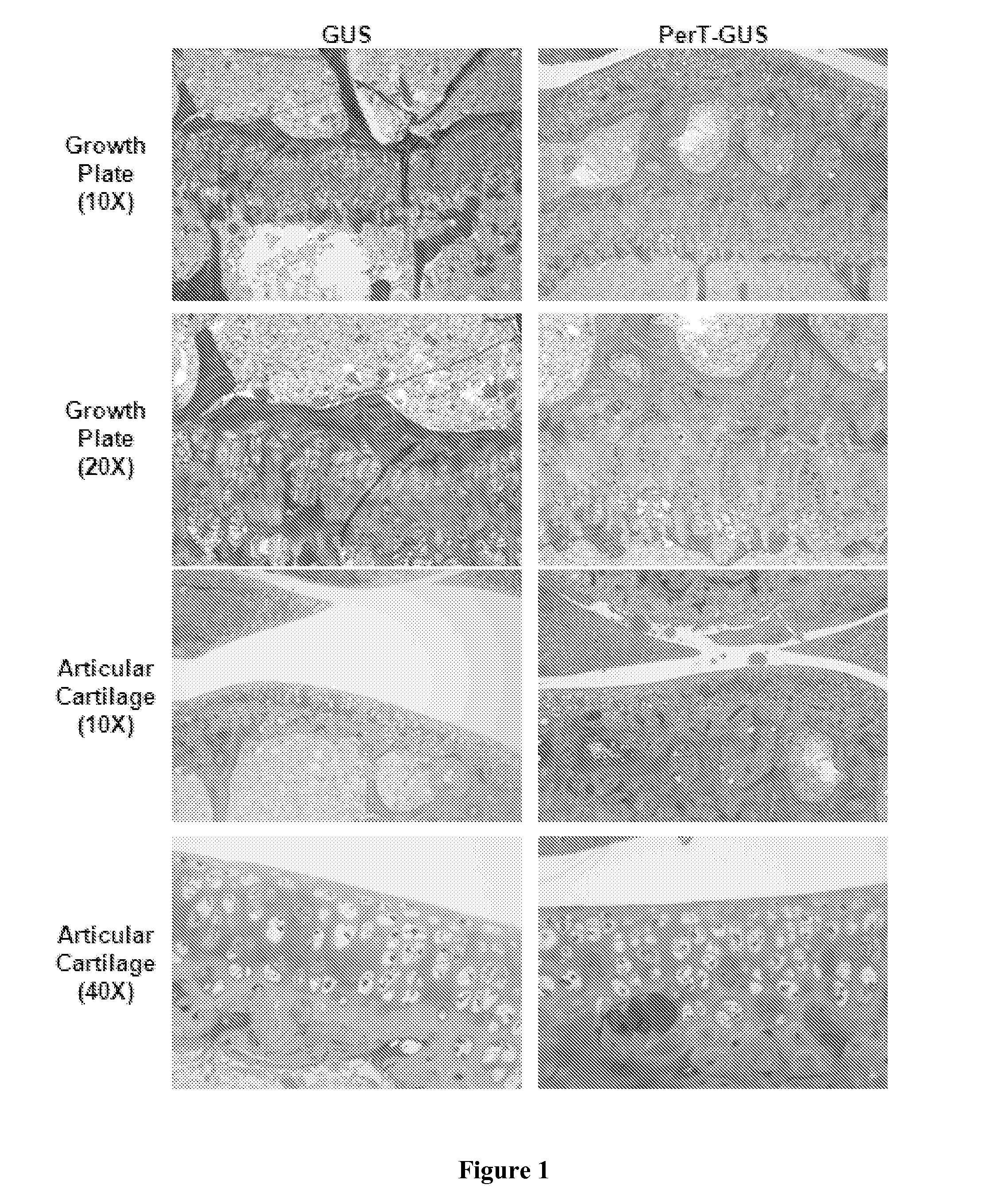
Enzyme replacement therapy for treating MPS vii related bone lesions using a chemically modified enzyme
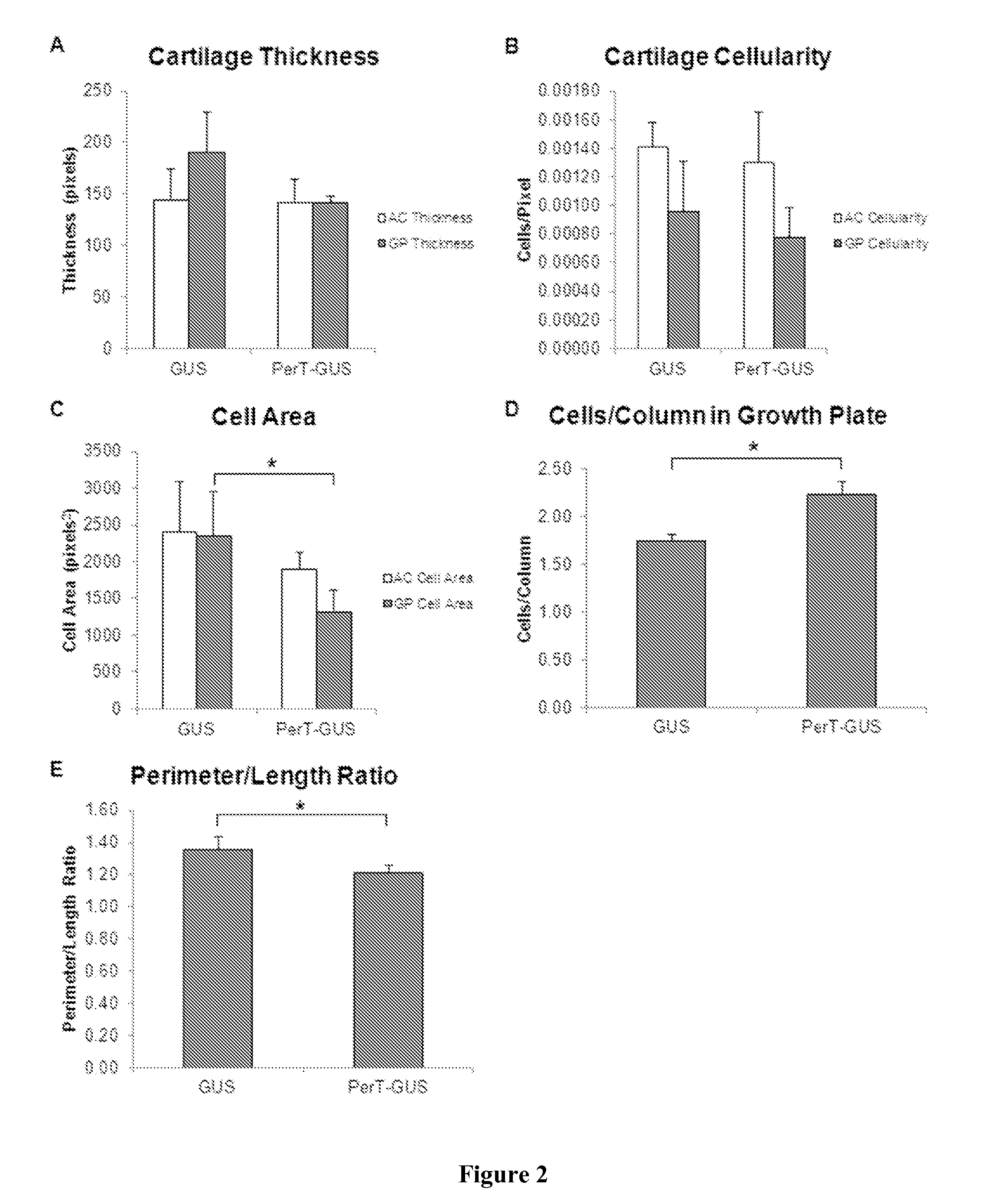
The invention relates to a method of treating mucopolysaccharidoses using enzyme replacement therapy with chemically modified lysosomal enzymes. More specifically the method relates to administering chemically modified lysosomal enzymes intraperitoneal injection. In addition, the invention relates to treating type VII mucopolysaccharidoses or mucopolysaccharidoses type VII related bone lesions with a chemical modified β-glucuronidase, wherein the modified β-glucuronidase may be administered 5 weeks after birth, and or may be administered intraperitoneally.
Owner:CAROL ANN FOUND & INT MORQUIO ORG
Method for establishing damp-heat diarrhea animal model
The invention relates to a method for establishing a damp-heat diarrhea animal model. The method includes the steps of putting a Wistar rat in a metabolism cage and letting the rat freely drink hydromel; alternatively carrying out fasting, sufficient forage feeding, and lard gavage every other day for 10 days; filling a proper amount of liquor on the 10-15th days, and placing the rat in high temperature and humidity environment for 5 days; and injecting Escherichia coli into the abdominal cavity on the 16th day, and injecting again in 24 hours. By observing the general condition of the rat and detecting the blood, blood viscosity, blood biochemistry and serum cytokines, it is shown that the damp-heat diarrhea animal model is established successfully by the method. The animal model constructed by the invention has good reproducibility and stability, and can withstand the test of drug test, so as to provide an ideal animal model for developing new therapeutic drugs.
Owner:GANSU AGRI UNIV
B-group epidemic neisseria meningitidis recombinant protein vaccine and preparing method thereof
ActiveCN104888209AGood antigenicityImproving immunogenicityAntibacterial agentsAntibody medical ingredientsIntraperitoneal routeProtein detection
The invention provides a B-group epidemic neisseria meningitidis recombinant protein vaccine and a preparing method thereof, and belongs to the field of biological products. The vaccine contains three mutant recombinant proteins of a human H factor binding protein. The invention further provides a preparing method of the B-group epidemic neisseria meningitidis recombinant protein vaccine. The method includes the steps of human H factor binding protein V1, V2 and V3 recombinant expression carrier construction, expression strain screening and identification, fermental cultivation, recombinant protein antigen inducible expression and recombinant protein antigen purification and protein detection; and according to the concentrations of the three antigen components, quantified mixing is carried out, and the B-group epidemic neisseria meningitidis recombinant protein vaccine is prepared in cooperation with an aluminum adjuvant. By means of the B-group epidemic neisseria meningitidis recombinant protein vaccine, intraperitoneal injection is carried out on an immune mice, the mice body can be stimulated to generate a B-group epidemic neisseria resisting peculiar antibody, and the alexin mediated serum sterilization antibody can rise by more than 8 times.
Owner:BEIJING MINHAI BIOTECH
Hybridoma cell line and chloramphenicol-resistant monoclonal antibody produced by same
ActiveCN101942414AStrong specificityHigh sensitivityTissue cultureImmunoglobulinsIntraperitoneal routeCarrier protein
The invention relates to a chloramphenicol-resistant monoclonal antibody, which is mainly used for detecting chloramphenicol residue in livestock products and milk products. The chloramphenicol-resistant monoclonal antibody is secreted from a hybridoma cell line 7C10; and the preparation method comprises the following steps of: coupling EDC-activated chloramphenicol and carrier protein to obtain immunogen and coating antigen; performing intraperitoneal injection of mouse immunogen and enhancing the immunity; screening out a mouse who is subjected to immunity enhancement and has high serum titer; fusing spleen cells and SP2 / 0 myeloma cells; performing sub-clone to obtain the monoclonal antibody hybridoma cell line 7C10 capable of stably secreting the chloramphenicol; performing intraperitoneal injection on the mouse with the hybridoma cell line 7C10; collecting ascitic fluid; and performing liquid-phase chromatographic purification to obtain the chloramphenicol-resistant monoclonal antibody 7C10'. The chloramphenicol-resistant monoclonal antibody has the advantages of high specificity, high flexibility and affinity, large-scale production and effect of quickly and sensitively detecting the chloramphenicol residue in foods such as milk, meats, aquatic products and the like.
Owner:JIANGSU HUACHUANG MEDICINE RES & DEV PLATFORM MANAGEMENT CO LTD
Method for establishing fish inflammatory bowel disease model and established model thereof
ActiveCN103301162AAvoid mechanical damageAvoid enteringBacteria material medical ingredientsIntraperitoneal routePerfusion
The invention relates to the technical field of establishment of animal experiment models and in particular relates to a method for establishing a fish inflammatory bowel disease model and the established model thereof. The method comprises the following steps: injecting aeromonas hydrophila with the collection number of CCTCC NO.M2013089 into a soaked anaesthetic healthy fish through anus perfusion, breeding and selecting fish with the symptom of the inflammatory bowel disease as the fish inflammatory bowel disease model. According to the method, the aeromonas hydrophila is directly filled in the intestinal tract of the fish, and the process that the pathogenic bacteria enter blood through intramuscular injection or intraperitoneal injection and act on the intestinal tract through blood circulation is avoided, so that the pathogenic bacteria directly act on the intestinal tract, and the pertinence is improved; furthermore, the established model has high stability and repeatability, and because the aeromonas hydrophila is wide in infection spectrum, the established method can be widely applied to multiple fishes.
Owner:苏州培恩特生物科技有限公司
Use of methylene blue in prevention of acute cerebral ischemia damage
InactiveCN104027338AImprove performanceReduced infarct volumeOrganic active ingredientsFood preparationIntraperitoneal routeModerate-Dose
The invention relates to novel use of methylene blue (MB) in prevention of acute cerebral ischemia. MB can remarkably reduce cerebral ischemia infarction caused by permanent middle cerebral artery occlusion (middle cerebral artery occlusion MCAO) and alleviate damage of neurological function. The inventors of the invention permanently occlude middle cerebral artery of a mouse by adopting a suture method and after intraperitoneal injection of MB at the moment and at different time points after cerebral ischemia, changes on degree and ethology of permanent cerebral ischemia infarction of the mouse are observed. Researches discover that MB can remarkably reduce infarct volume caused by cerebral ischemia and improve the active ability of the mouse after cerebral ischemia. Through the method provided by the invention, moderate doses of MB are delivered for many times after cerebral ischemia can remarkably alleviate damage degree after permanent cerebral ischemia of the mouse so as to improve the active ability of the mouse after cerebral ischemia. The use of MB is expected to provide a rescue therapeutic measure to cerebral apoplexy and alleviate permanent neurological function deficit after golden treatment period.
Owner:INST OF BASIC MEDICAL SCI ACAD OF MILITARY MEDICAL SCI OF PLA
Building method of ulcerative colitis transformation animal model
PendingCN110680830ARaise the ratioIncrease contentOrganic active ingredientsUnknown materialsIntraperitoneal routePerfusion
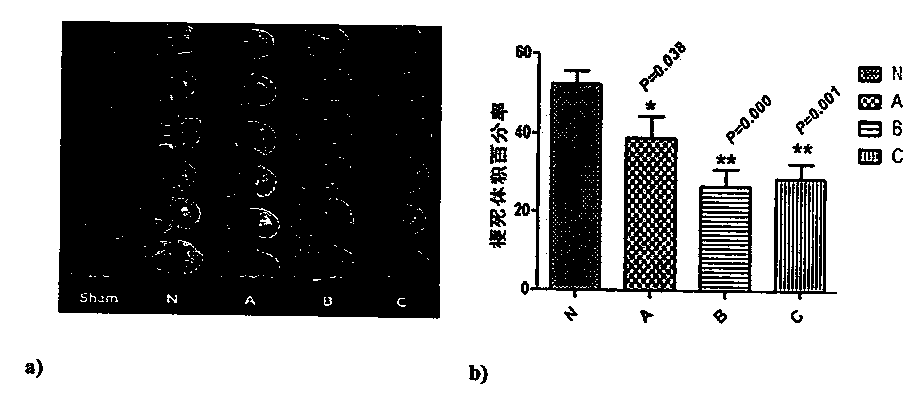
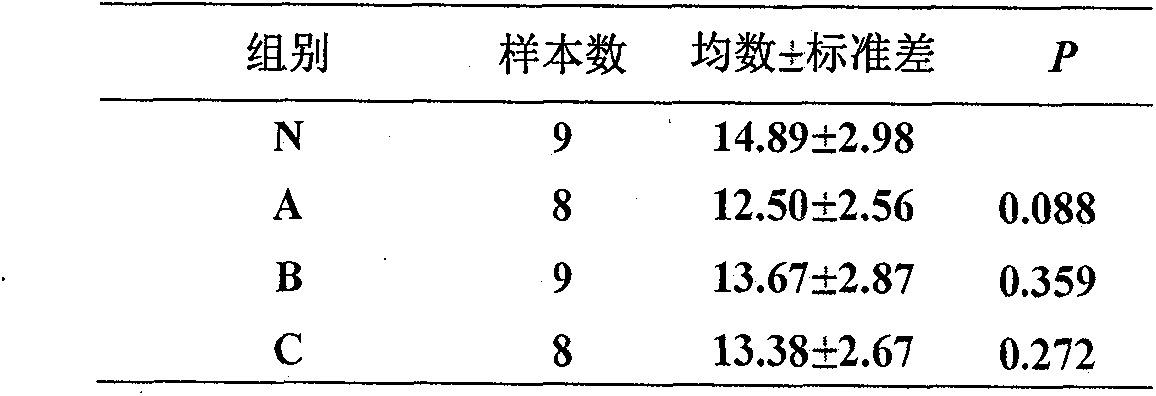
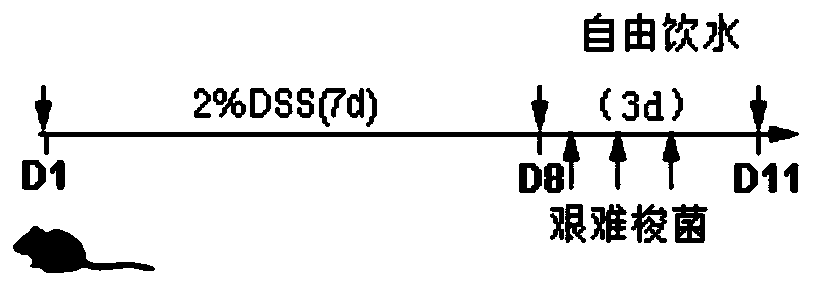
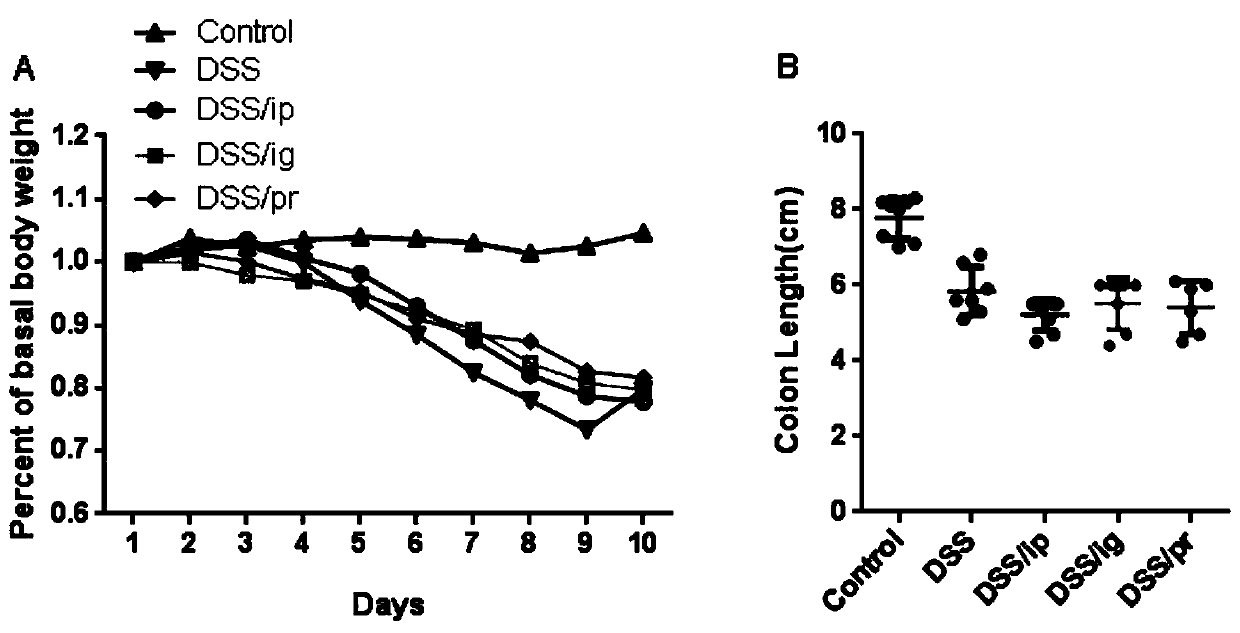
The invention discloses a building method of an ulcerative colitis transformation animal model. By using the method, an inflammatory transformation model combining with clostridium difficile in a DSSacute colitis model is built; and different bacterium feeding modes, including three measures of intraperitoneal injection, gastric perfusion and enema are used. The model has the advantages that theproportion of mouse mesenteric lymphocytes Th17 can be obviously increased by the model; the secretion of IL-17 is increased; the result is more similar to the pathological features of clinic patientswith ulcerative colitis; and the clostridium difficile feeding effect on the mice through gastric perfusion is best.
Owner:CHINA PHARM UNIV
Methods and Compositions for Treating Mammalian Nerve Tissue Injuries
InactiveUS20100016444A1Promote recoveryLoss in its effectivenessBiocideNervous disorderIntraperitoneal routeAnesthesia
To achieve, an in vivo repair of injured mammalian nerve tissue, an effective amount of a biomembrane fusion agent is administered to the injured nerve tissue. The application of the biomembrane fusion agent may be performed by directly contacting the agent with the nerve tissue at the site of the injury. Alternatively, the biomembrane fusion agent is delivered to the site of the injury through the blood supply after administration of the biomembrane fusion agent to the patient. The administration is preferably by parenteral administration including intravascular, intramuscular, subcutaneous, or intraperitoneal injection of an effective quantity of the biomembrane fusion agent so that an effective amount is delivered to the site of the nerve tissue injury.
Owner:PURDUE RES FOUND INC
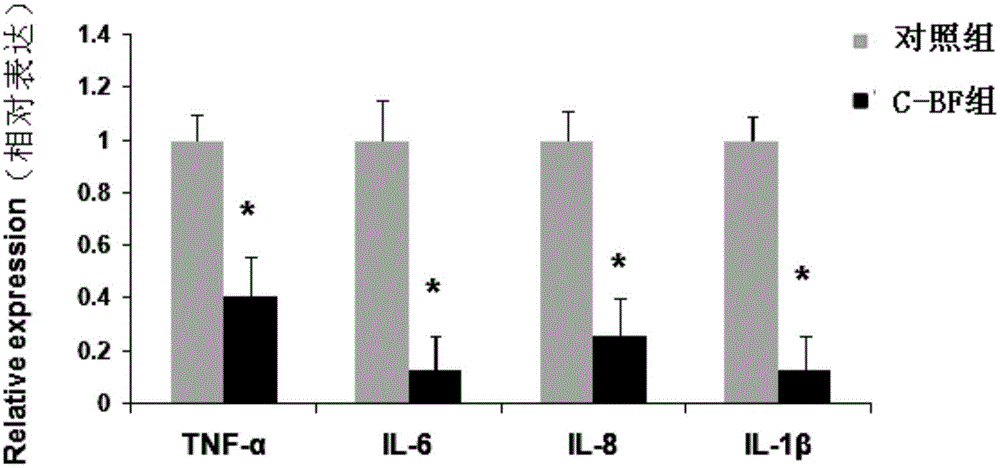
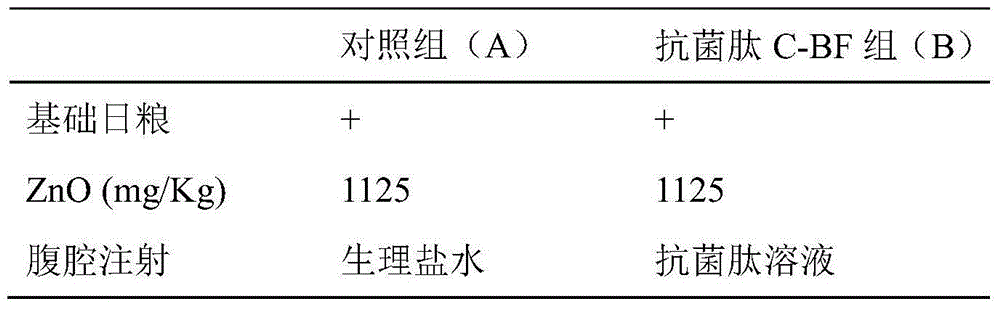
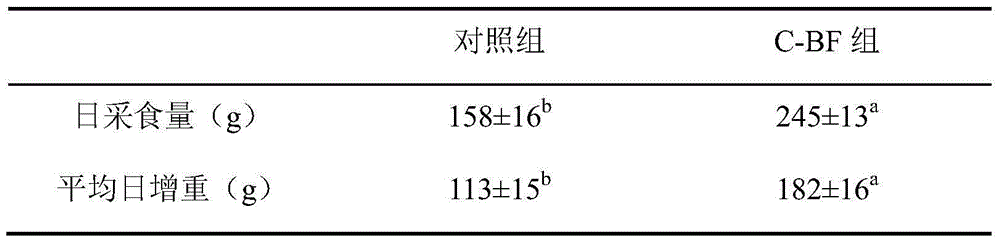
Application of antibacterial peptide C-BF in preparation of medicines for preventing and treating weanling stress of piglets
InactiveCN104645314AImprove growth performanceIncrease the height of jejunal villiPeptide/protein ingredientsDigestive systemIntraperitoneal routeIntestinal morphology
The invention discloses an application of an antibacterial peptide C-BF in preparation of medicines for preventing and treating weanling stress of piglets. The intestinal morphology can be improved by the antibacterial peptide C-BF; the villus height can be increased; the intestinal inflammation is relieved; the growth performance of weaned pigs is improved; the diarrhea rate is lowered; and the antibacterial peptide C-BF is dosed through intraperitoneal injection.
Owner:ZHEJIANG UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com