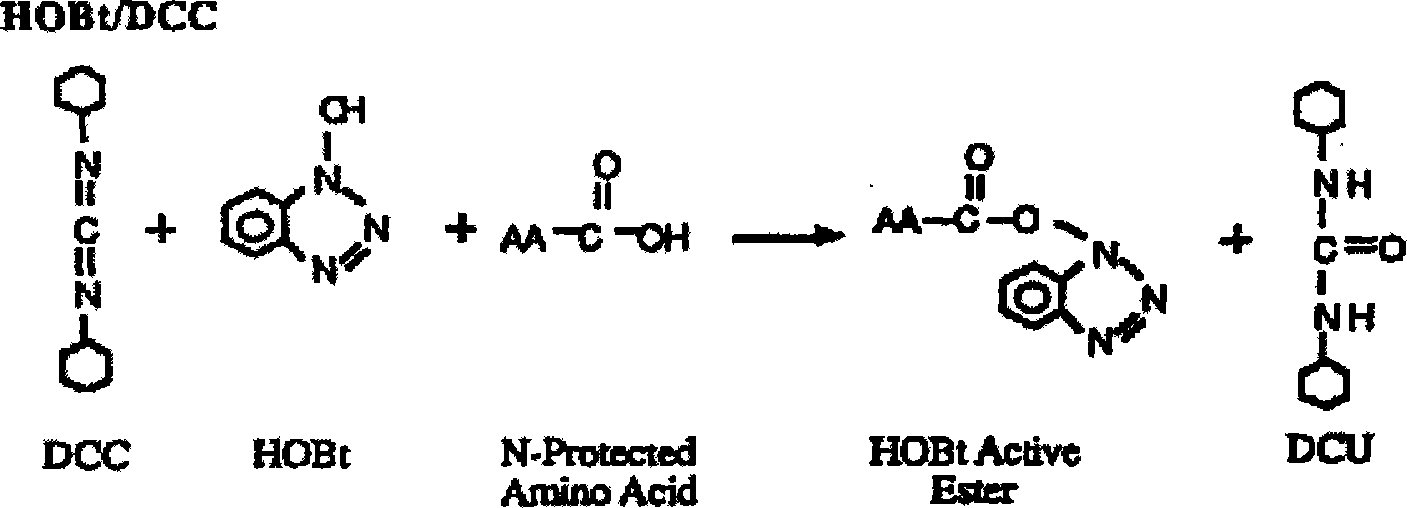
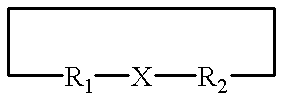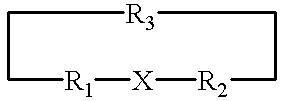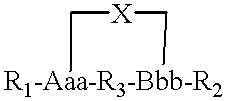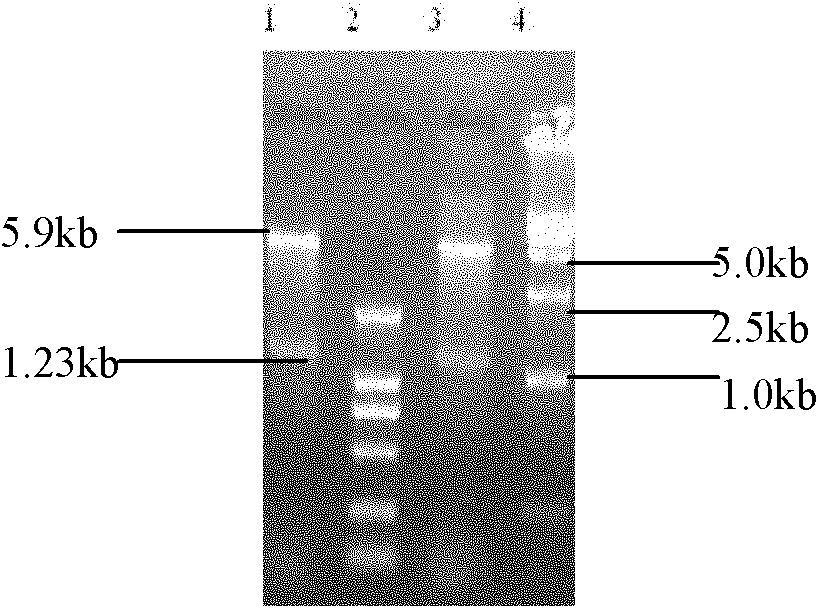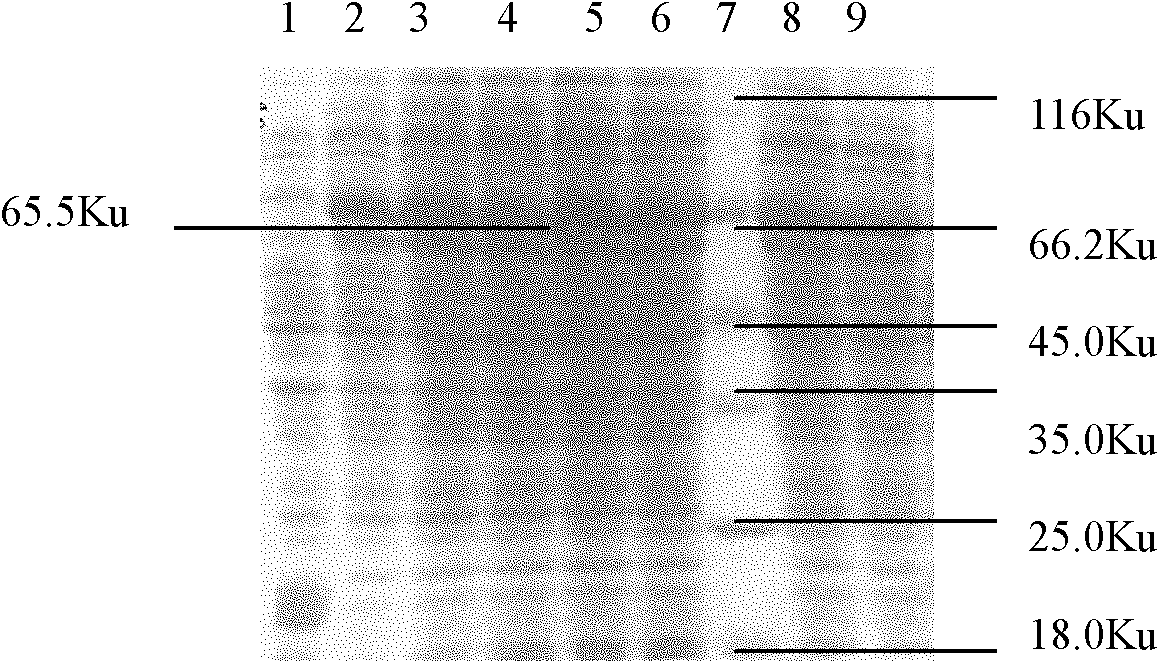Patents
Literature
466results about How to "Good antigenicity" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
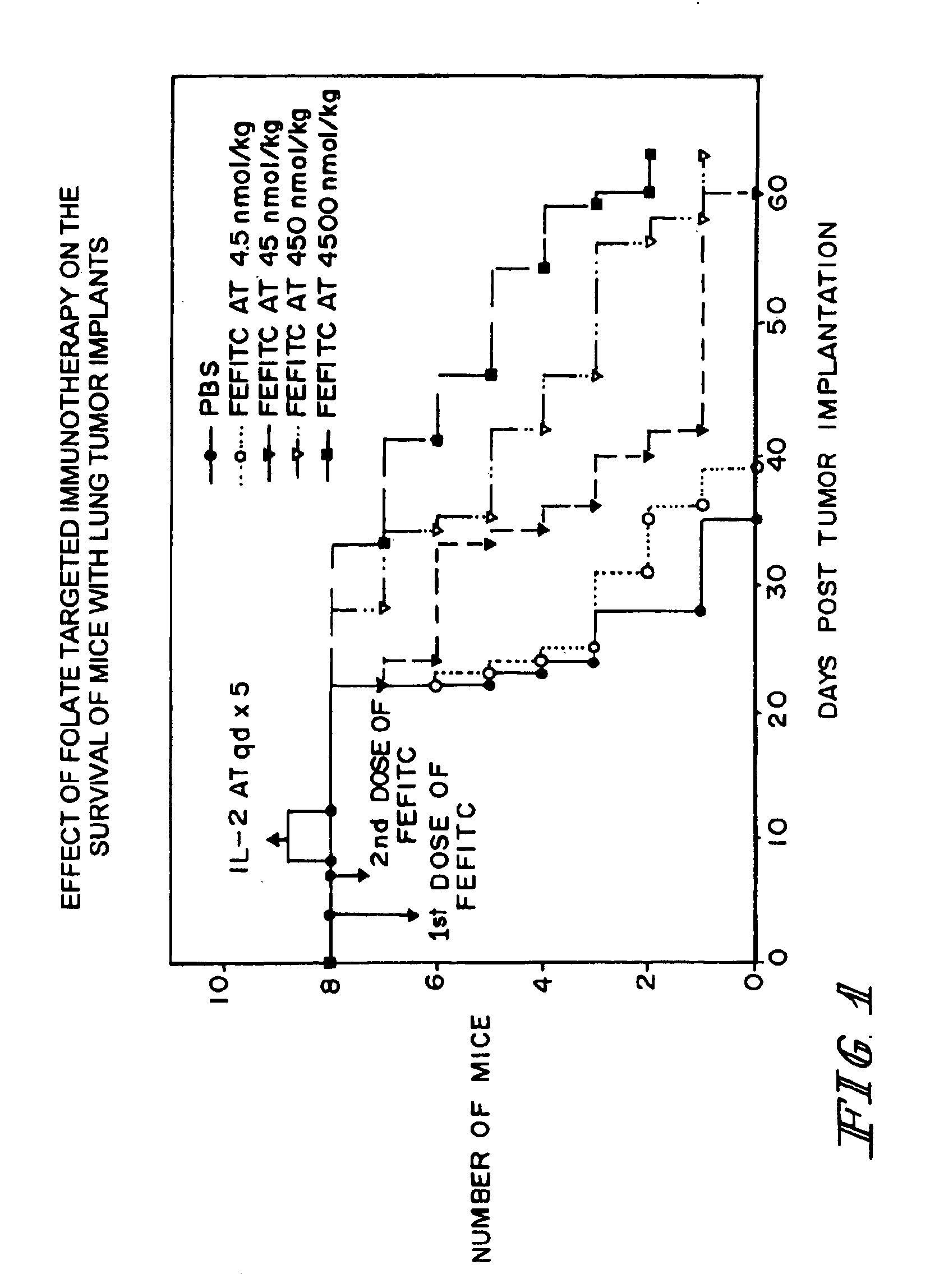
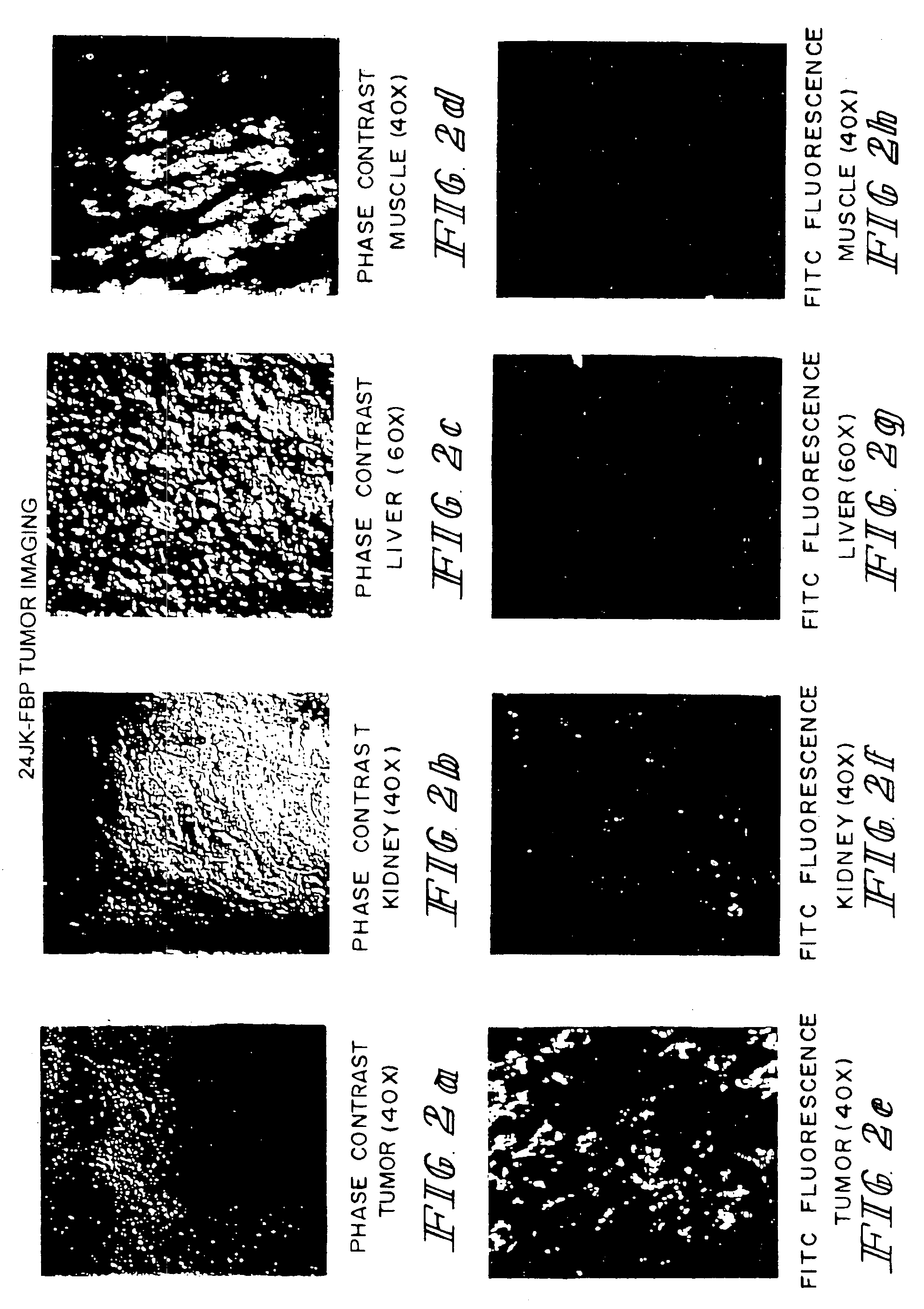
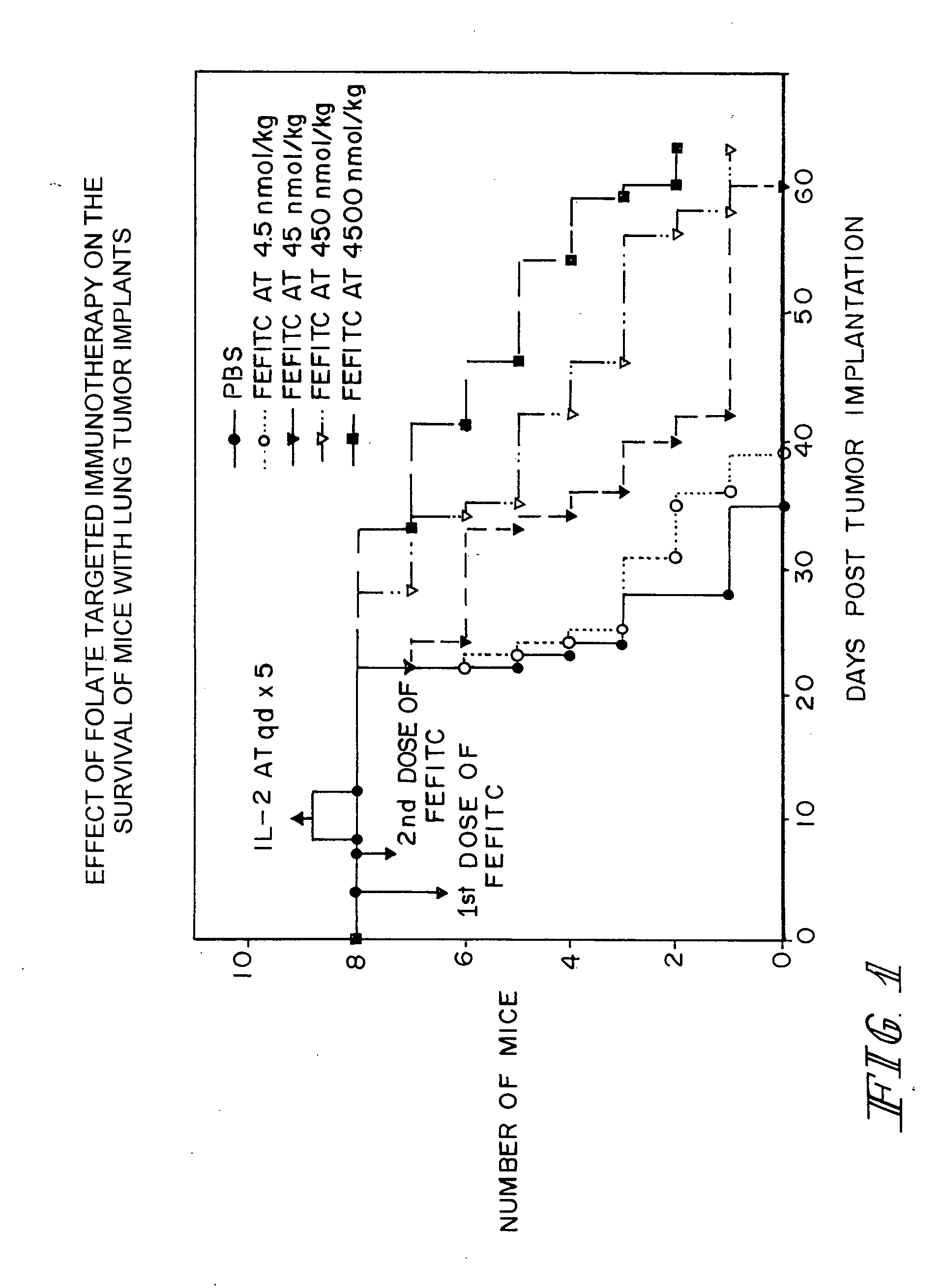
Method of treatment using ligand-immunogen conjugates
InactiveUS7033594B2Improve recognitionEnhance endogenous immune response-mediated eliminationAntibacterial agentsBiocideBinding siteCytotoxicity
A method and pharmaceutical composition are provided for enhancing the endogenous immune response-mediated elimination of a population of pathogenic cells in a host animal wherein the pathogenic cells preferentially express, uniquely express, or overexpress a binding site for a particular ligand. The invention comprises administering the ligand conjugated to an immunogen to a host animal harboring the population of pathogenic cells. Antibodies, preexisting or administered to the host animal to establish a passive immunity, directed against the immunogen bind to the ligand-immunogen conjugate resulting in elimination of the pathogenic cells by the host's immune response. At least one additional therapeutic factor is administered selected from the group consisting of a cell killing agent, a tumor penetration enhancer, a chemotherapeutic agent, antimicrobial agent, a cytotoxic immune cell, and a compound capable of stimulating an endogenous immune response wherein the compound does not bind to the ligand-immunogen conjugate.
Owner:PURDUE RES FOUND INC
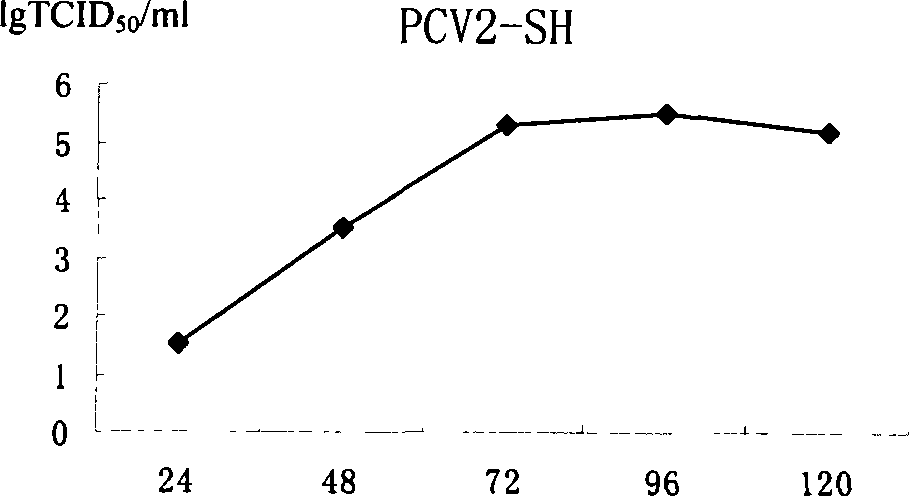
Porcine circovirus 2 type inactivated vaccine
InactiveCN101240264ASimple processEasy to operateViral antigen ingredientsMicroorganism based processesAdjuvantVaccine Production
The pig circular ring virus 2 type (PVC2) inactivated vaccine (SH individual plant) of the invention belongs to biotechnology field. The pig circular ring virus 2 type poisonous individual plant SH belongs to circular ring virus section circular ring virus genus which has been preserved in Wuhan institute of virology, Chinese academy of sciences. The shanghai separated individual plant SH of purified PCV2 virus is obtained by gathering raw material from hogpen which happened bad weaning piglet multisystem exhaustion failure syndrome in Shanghai in 2002 year, separating, appraising and purifying virus. The PCV2-SH plant is proliferated in mass in PK-15 cell, inactivated through methyl aldehyde and emulsified with liquid paraffine adjuvant to prepare conventional liquid paraffin(e) adjuvant immunomodulators for vaccines. The laboratory has trial-manufactured five lots vaccines successfully which are good safety and also can induce pig bring immune protection effect, made out a draft rules for vaccines production and testing. The inactivated vaccine proved by every aspects experiment has met state biological products standard completely.
Owner:NANJING AGRICULTURAL UNIVERSITY
Structurally determined cyclic metallo-constructs and applications
InactiveUS6331285B1Improve the level ofLess susceptible to proteolysisPeptide sourcesRadioactive preparation carriersProtein secondary structureMedicinal chemistry
A metallo-construct, which may be a peptide, is provided for use as a biological, therapeutic, diagnostic imaging, or radiotherapeutic agent, and for use in library or combinatorial chemistry methods. The construct has a conformationally constrained global secondary structure obtained upon complexing with a metal ion. The peptide constructs are of the general formula:where X is a plurality of amino acids and includes a complexing backbone for complexing metal ions, so that substantially all of the valences of the metal ion are satisfied upon complexation of the metal ion with X, resulting in a specific regional secondary structure forming a part of the global secondary structure; and where R1 and R2 each include from 0 to about 20 amino acids, the amino acids being selected so that upon complexing the metal ion with X at least a portion of either R1 or R2 or both have a structure forming the balance of the conformationally constrained global secondary structure. All or a portion of the global secondary structure, which may be sychnologic or rhegnylogic, may form a ligand or mimic a known biological-function domain. The construct has substantially higher affinity for its target upon labeling with a metal ion.
Owner:PALATIN TECH INC
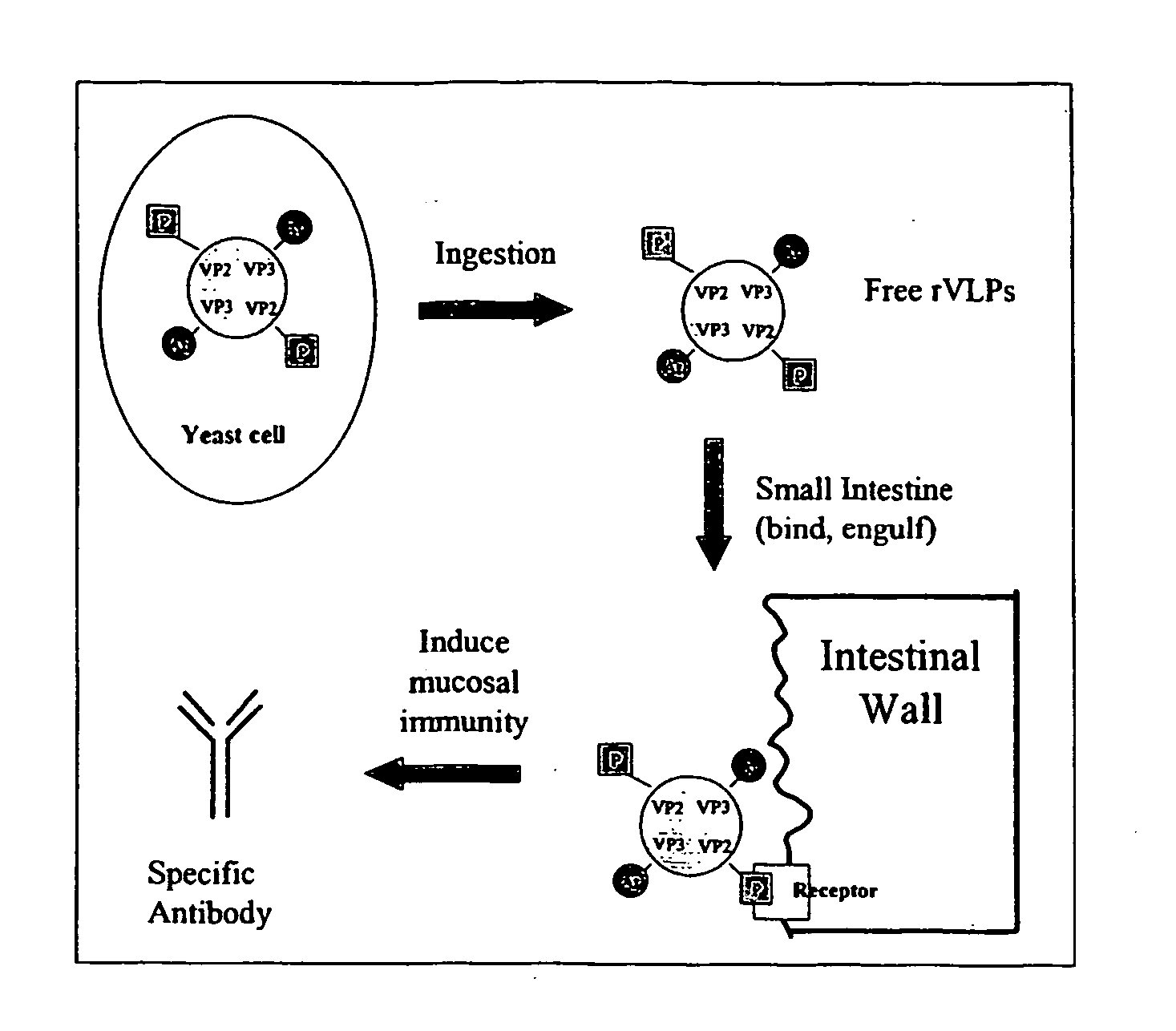
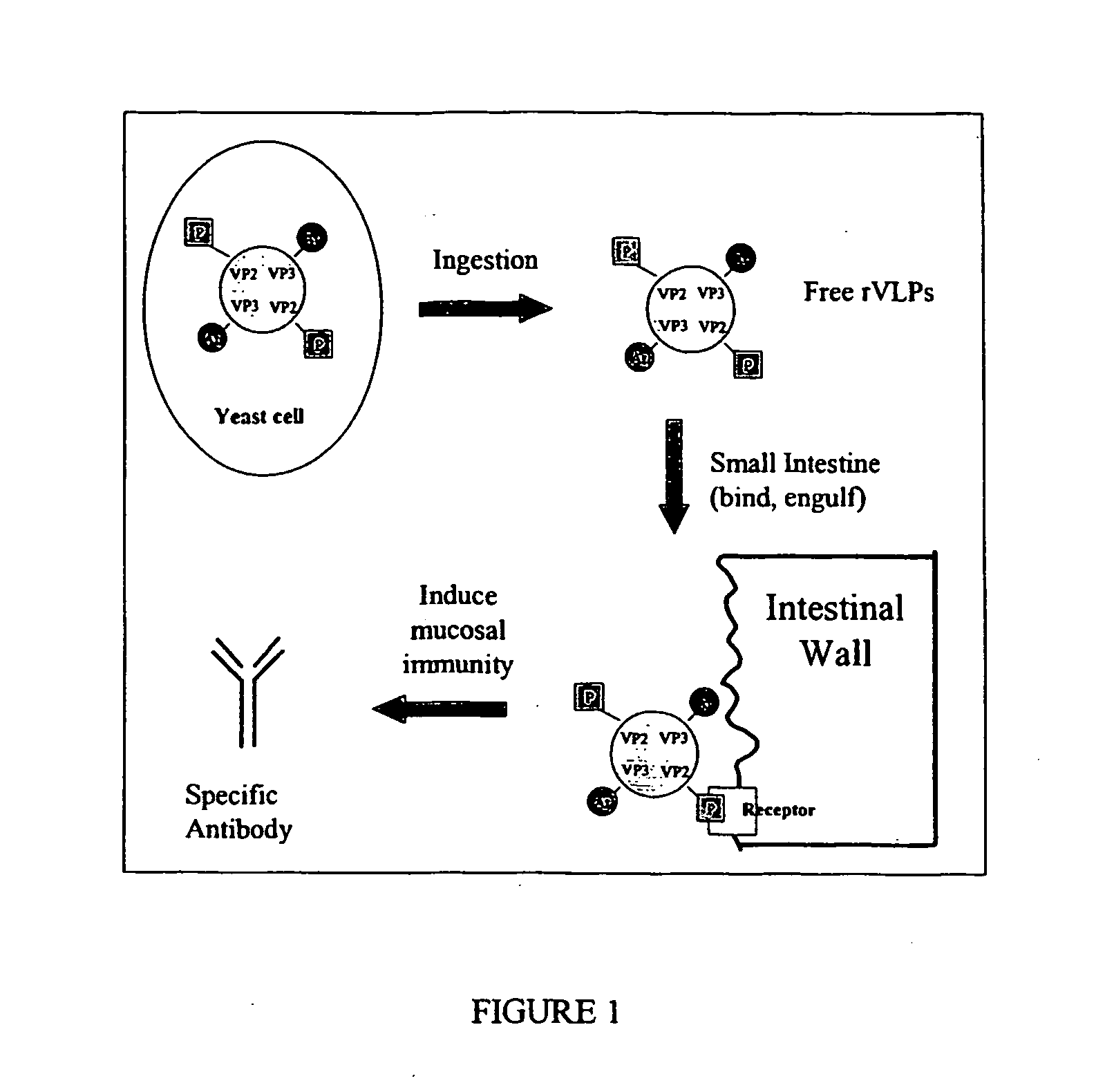
Viruses and virus-like particles for multiple antigen and target display
InactiveUS20060121468A1Improve effectivenessImprove responseSsRNA viruses positive-senseVectorsTissue targetingVaccine Immunogenicity
The present invention relates to the display of antigenic or allergenic components along with a tissue-targeting component on viruses or virus-like particles. Capsid protein genes are recombinantly modified to contain the specified components then expressed within a host organism, such as yeasts, bacteria, or algae, and allowed to spontaneously form active virus particles or virus-like particles. The recombinant complexes (virus or virus-like particle) can then be purified or used in situ as a therapeutic tool for disease or allergy prevention. The expression of multivalent and multifunctional components to increase the immunogenicity of the recombinant complexes, especially on oral administration, is provided.
Owner:ADVANCED BIONUTRITION CORP
Compositions comprising heat shock proteins or alpha(2) macroglobulin, antigenic molecules and saponins, and methods of use thereof
InactiveUS20020037290A1Growth inhibitionEffective amountBiocideSenses disorderAutoimmune ReactionsAutoimmune responses
The present invention relates to pharmaceutical compositions and methods for the prevention and treatment of autoimmune diseases, infectious diseases, neurodegenerative diseases, and primary and metastatic neoplastic diseases. In the practice of the invention, the compositions are employed comprising: (a) a heat shock protein (hsp) or an alpha(2)macroglobulin (alpha2M); (b) a saponin; and, optionally, (c) an antigenic molecule. The antigenic molecule displays the antigenicity of an antigen of: (a) a cell that elicits an autoimmune response; (b) an agent of an infectious disease; (c) a cancerous cell; or (d) a cell or structure associated with a neurodegenerative or amyloid disease. The hsps that can be used in the practice of the invention include but are not limited to hsp70, hsp90, gp96, calreticulin, hsp 110, grp 170, and PDI, alone or in combination with each other. The antigenic molecule can be covalently or noncovalently bound to the hsp or alpha2M, free in solution, and / or covalently bound to the saponin. The compositions of the invention can be administered alone or in combination with the administration of antigen presenting cells sensitized with an hsp- or alpha2M-antigenic molecule complex.
Owner:ANTIGENICS
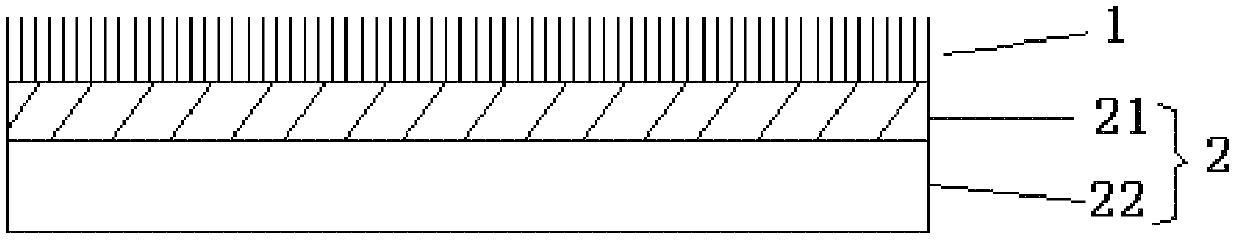
Medical flocking hemostasis material, preparation thereof and application
ActiveCN102600013AAvoid burnsNo toxicityAbsorbent padsMacromolecular non-active ingredientsFiberVein
The invention relates to a medical flocking hemostasis material for stopping bleeding and protecting arteries, veins and wounds of tissues, application of the medical flocking hemostasis material and a method for preparing the medical flocking hemostasis material. The medical flocking hemostasis material comprises a flocking layer and a base layer, the flocking layer is used as a wound contacting layer and made of functional fibers, and the functional fibers are fixed on the upper surface of the base layer in a flocking method. The flocking method comprises a mechanical flocking method and an electrostatic flocking method. The medical flocking hemostasis material can be used for stopping bleeding and protecting the arteries, the veins and the wounds of the tissues, and further can be used as a medicine slow-release carrier. The invention further discloses method for controlling bleeding of the arteries, bleeding of the veins and bleeding of the wounds of the tissues by the aid of the medical flocking hemostasis material. Compared with a hemostasis material in the prior art, the medical flocking hemostasis material has the advantages that a hemostasis performance, comfortableness and the like are greatly improved.
Owner:SUZHOU BOCHUANG TONGKANG PHARM TECH CO LTD
Diagnostic drugs for autoimmune diseases
InactiveUS20020009749A1More sensitiveMore objectiveFungiPeptide/protein ingredientsAutoimmune diseaseDisease cause
A diagnostic drug and a diagnostic kit for autoimmune diseases including at least one of a polypeptide selected from an HMG-1 family, a polypeptide selected from an HMG-2 family, a fragment thereof which is reactable with an antibody of an autoimmune disease patient, and a method for detecting an antibody of an autoimmune disease patient using the same are provided.
Owner:KANEKA CORP
Soluble fragments of the SARS-CoV spike glycoprotein
InactiveUS20060240515A1Easy to produceImprove resistance to degradationSsRNA viruses positive-senseAntibody mimetics/scaffoldsAptamerVaccination
The invention relates to the spike protein from the virus (SARS-CoV) that is etiologically linked to severe acute respiratory syndrome (SARS); polypeptides and peptide fragments of the spike protein; nucleic acid segments and constructs that encode the spike protein, polypeptides and peptide fragments of the spike protein, and coupled proteins that include the spike protein or a portion thereof; peptidomimetics; vaccines; methods for vaccination and treatment of severe acute respiratory syndrome; antibodies; aptamers; and kits containing immunological compositions, or antibodies (or aptamers) that bind to the spike protein.
Owner:HEALTH & HUMAN SERVICES GOVERNMENT OF US SEC THE DEPT OF
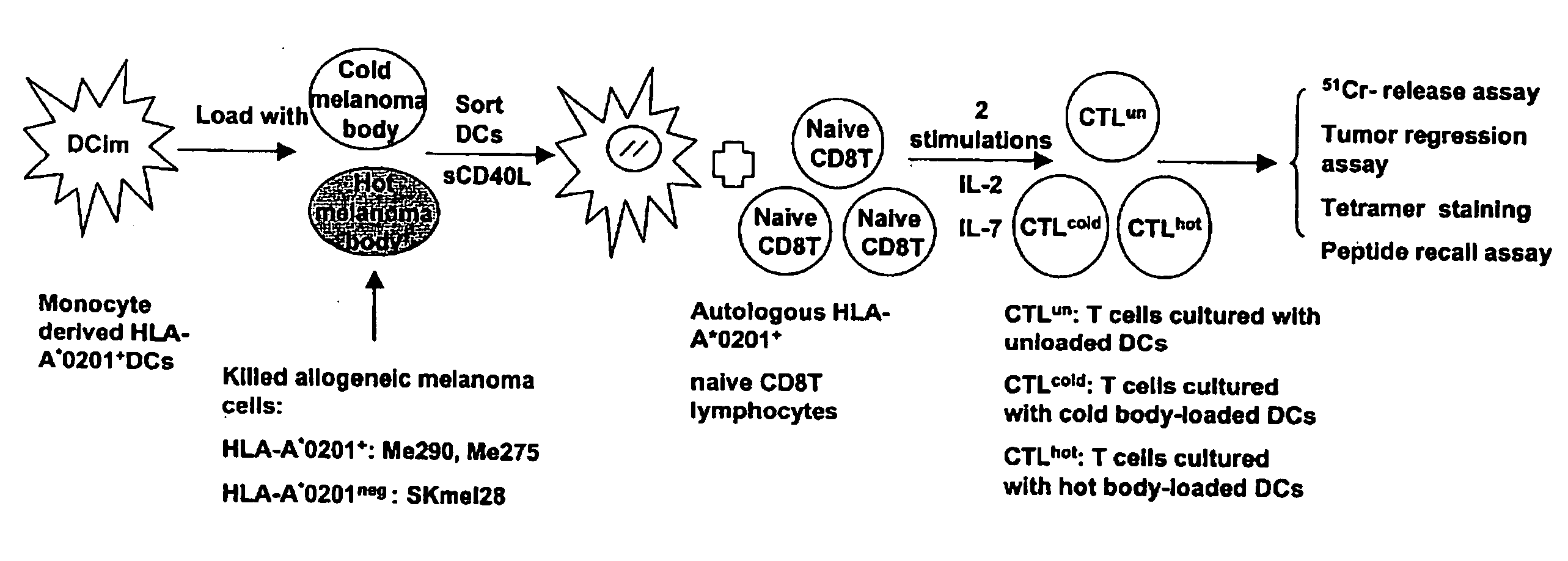
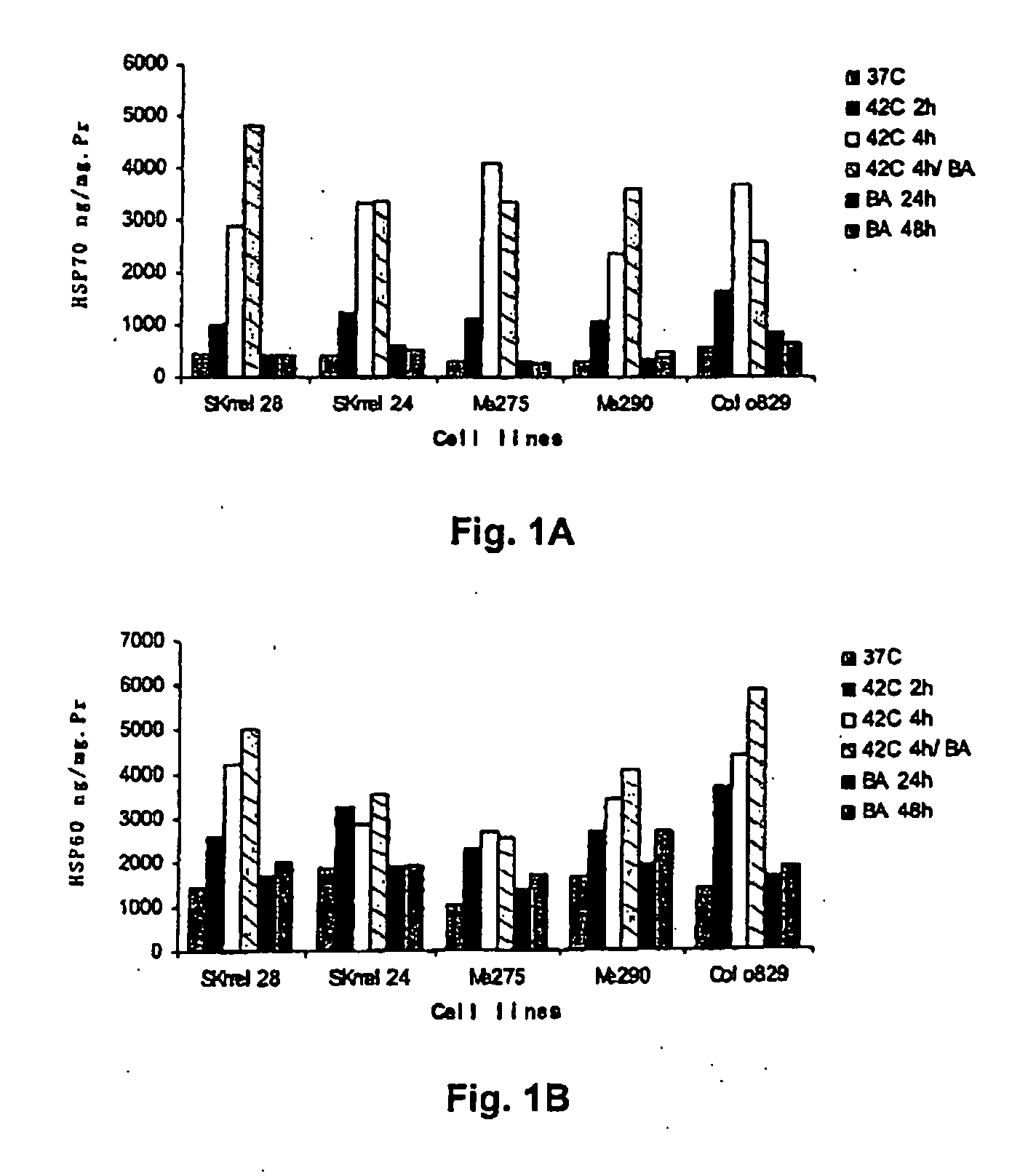
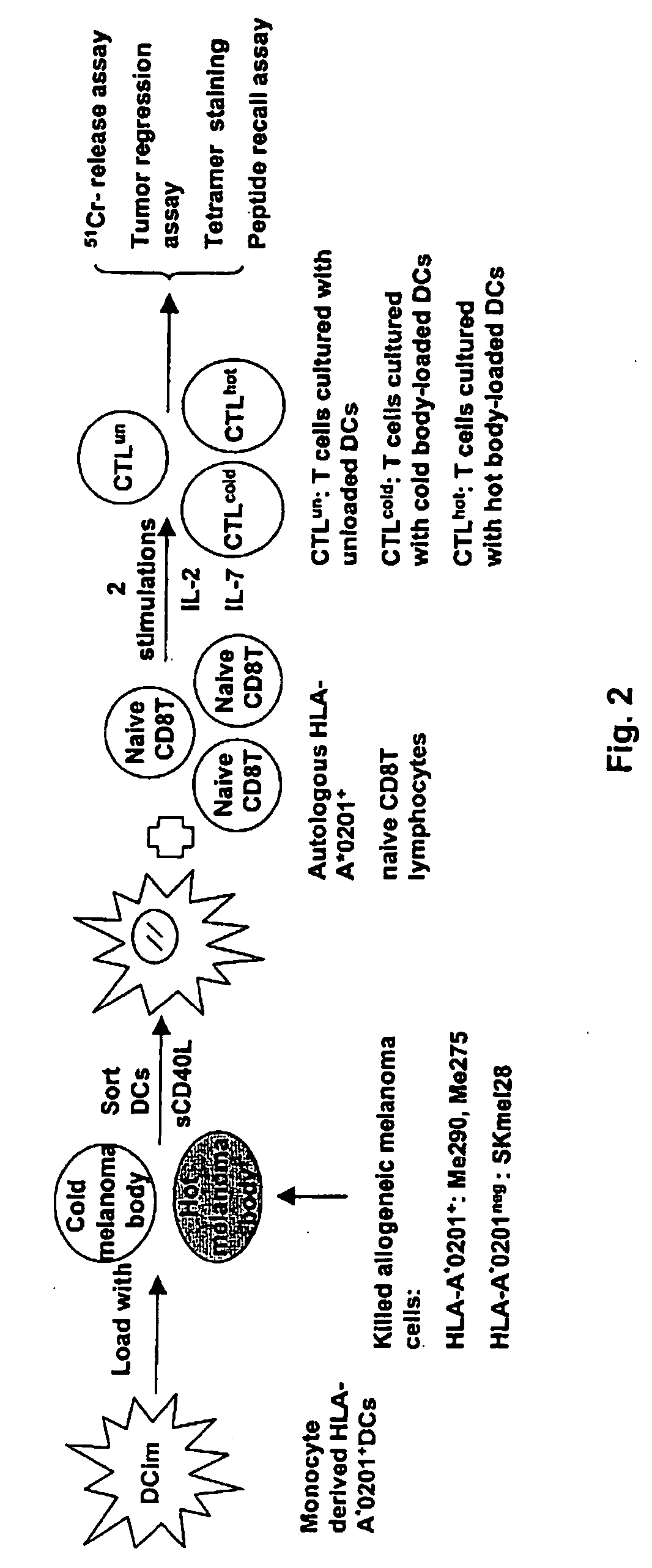
Dendritic cells loaded with heat shocked melanoma cell bodies
InactiveUS20060140983A1Reduce or eliminate needIncrease antigenicityVertebrate cellsArtificial cell constructsMolecular biologyCancer vaccine
Owner:BAYLOR RES INST
Method of treatment using ligand-immunogen conjugates
InactiveUS20060067946A1Improve recognitionEnhance endogenous immune response-mediated eliminationAntibacterial agentsBiocideBinding siteCytotoxicity
A method and pharmaceutical composition are provided for enhancing the endogenous immune response-mediated elimination of a population of pathogenic cells in a host animal wherein the pathogenic cells preferentially express, uniquely express, or overexpress a binding site for a particular ligand. The invention comprises administering the ligand conjugated to an immunogen to a host animal harboring the population of pathogenic cells. Antibodies, preexisting or administered to the host animal to establish a passive immunity, directed against the immunogen bind to the ligand-immunogen conjugate resulting in elimination of the pathogenic cells by the host's immune response. At least one additional therapeutic factor is administered selected from the group consisting of a cell killing agent, a tumor penetration enhancer, a chemotherapeutic agent, antimicrobial agent, a cytotoxic immune cell, and a compound capable of stimulating an endogenous immune response wherein the compound does not bind to the ligand-immunogen conjugate.
Owner:PURDUE RES FOUND INC
Foot and mouth disease virus-like particle, preparation method and application thereof
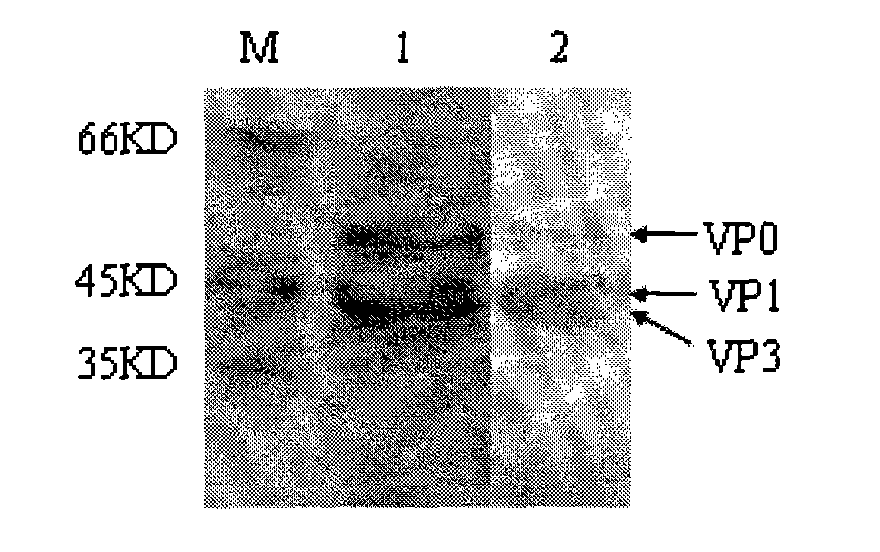
ActiveCN101914501AImproving immunogenicityImprove biological activityInactivation/attenuationAntiviralsEnzyme digestionStructural protein
The invention discloses an Asia I type foot and mouth disease virus-like particle, a preparation method and an application thereof. The Asian I type foot and mouth disease virus-like particle comprises structural proteins of VP0, VP3 and VP1 of Asia I type foot and mouth disease virus, wherein the gene sequence of VP0 is shown in SEQ 1, the gene sequence of VP3 is shown in SEQ 3, and the gene sequence of VP1 is shown in SEQ 2. The preparation method of the Asian I type foot and mouth disease virus-like particle has the following steps: performing amplification to obtain the VP0, VP3 and VP1, performing enzyme digestion to obtain a recombinant expression vector, performing enzyme digestion on fusion protein by small ubiquitin-like modifier (SUMO), and carrying out in-vitro assembling to obtain the foot and mouth disease virus-like particle.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
Methods and products based on oligomerization of stress proteins
InactiveUS20050221395A1Guaranteed economic efficiencyImproving immunogenicityAntibacterial agentsBiocideHeat shockStress Proteins
In one aspect, the invention provides methods for determining the biological activity of heat shock proteins or heat shock protein-peptide complexes based on the ATPase activity or the multimeric structure of the heat shock proteins or heal shock protein-peptide complexes, and methods for screening agents that modulate the biological activity of heat shock proteins or heat shock protein-peptide complexes. In another aspect, the invention provides complexes, compositions and methods for enhancing the immunogenicity of a heat shock protein or a complex comprising a heal shock protein and an antigenic molecule.
Owner:CONNECTICUT HEALTH CENT UNIV OF +1
Methods and compositions for immunization against virus
ActiveUS20100247571A1Increase immunogenicityMaximize responseSsRNA viruses negative-senseSsRNA viruses positive-senseImmunogenicityViral glycoprotein
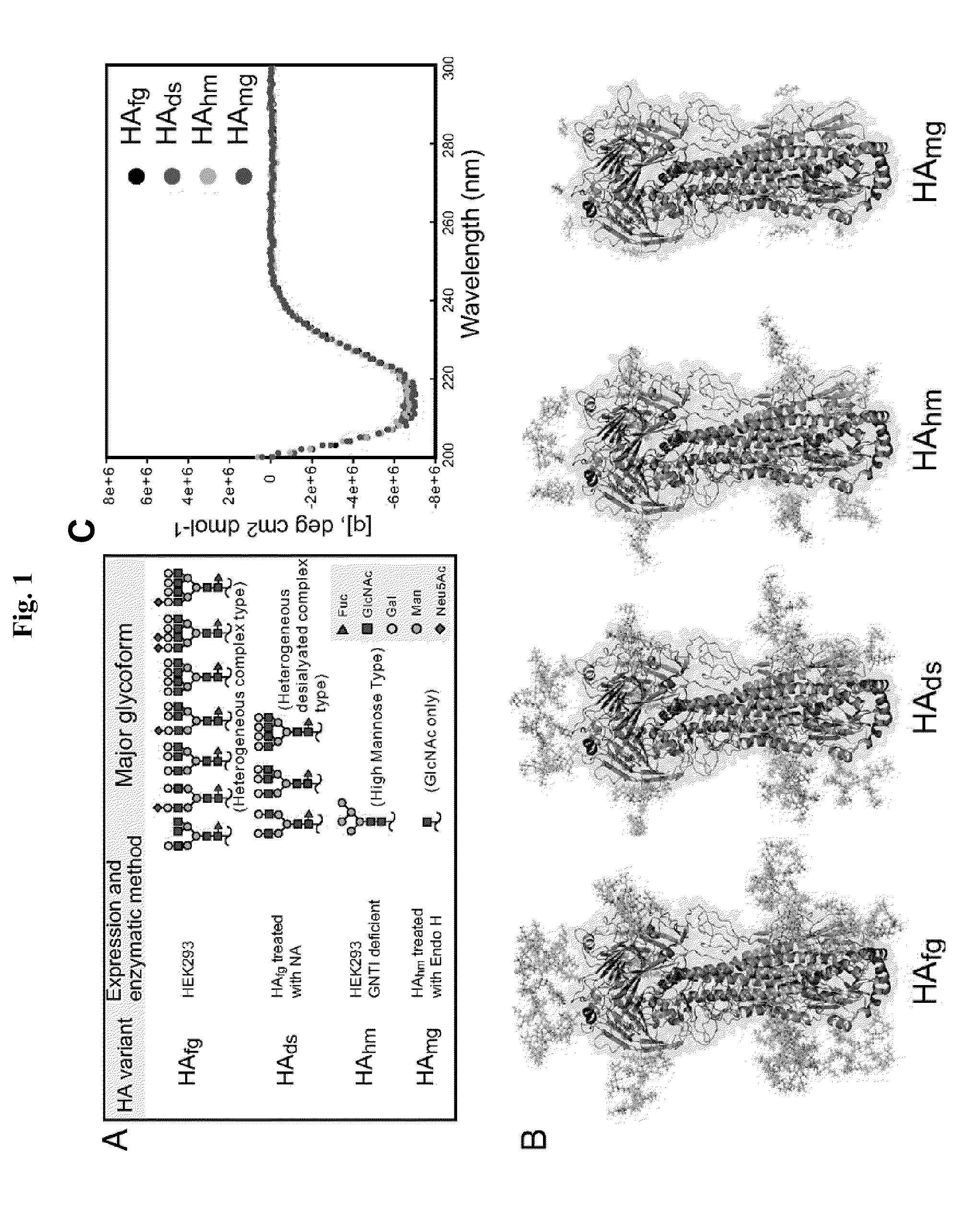
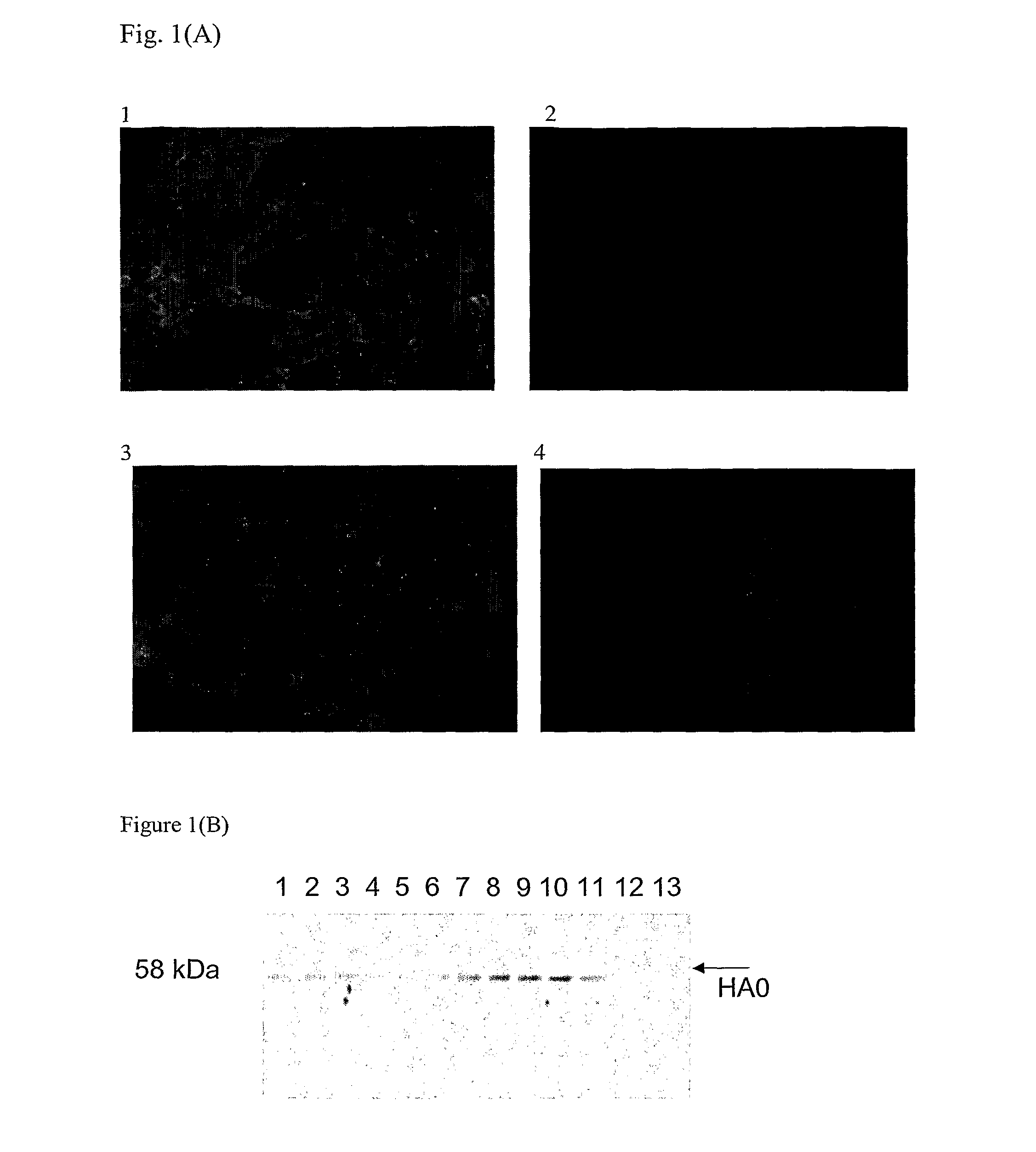
Immunogenic compositions comprising partially glycosylated viral glycoproteins for use as vaccines against viruses are provided. Vaccines formulated using mono-, di-, or tri-glycosylated viral surface glycoproteins and polypeptides provide potent and broad protection against viruses, even across strains. Pharmaceutical compositions comprising monoglycosylated hemagglutinin polypeptides and vaccines generated therefrom and methods of their use for prophylaxis or treatment of viral infections are disclosed. Methods and compositions are disclosed for influenza virus HA, NA and M2, RSV proteins F, G and SH, Dengue virus glycoproteins M or E, hepatitis C virus glycoprotein E1 or E2 and HIV glycoproteins gp120 and gp41.
Owner:ACAD SINIC
Injected bone repairing material and its prepn and application
InactiveCN1846789ABiologically activeClosed woundPeptide/protein ingredientsSkeletal disorderFiberBlood plasma
The present invention discloses one kind of injected bone repairing material and its preparation and application. The injected bone repairing material consists of solution I and solution II, the solution I is the mixture of platelet rich plasma (PRP) and 80-100 mg / ml concentration fibrinogen solution in the ratio of 1 to 3-6; and the solution II is solution mixture of calcium chloride in 40 mmol / ml concentration and thrombin in 400-1000 U / ml concentration. When the injected bone repairing material is used, solution I and solution II in the volume ratio of 1-9 are sucked with two syringes and injected into body to form gel with high adhesive force, with PRP accounting for 10-30 vol%. The injected bone repairing material is used in preparing implant for treating bone defect and bone nonunion and promoting fracture healing.
Owner:NAN FANG HOSPITAL
Indirect ELISA diagnostic reagent kit for pig B type circular virus antibody
The invention discloses an indirect ELISA diagnosing reagent box for pig II type circular virus (PCV2) antibody. There arranged with antibody detecting board in the reagent box, enzyme combination liquid, positive comparison, negative comparison, sample diluter, 10X condensed detergent, developing liquid A and B, and stopping liquid, the detecting board of the reagent box is removable 96-apertures enzyme labeling board enclosed with PCV2 reconstructed enucleation localizing signal capsid protein (dCap), the enzyme combination liquid is goat-anti-pig IgG multi-clone antibody labeled with HRP, the positive comparison is pig PCV 2 standard positive serum, the negative comparison is pig standard negative serum. The sensitivity of the invention is high, simple, and easy to be wide applied.
Owner:ZHEJIANG UNIV
Riemerella anatipestifer antibody indirect ELISA method detection kit and application thereof
InactiveCN102323428AEasy to operateLow instrument requirementsBiological testingAntiendomysial antibodiesEnzyme binding
The invention relates to a riemerella anatipestifer antibody indirect ELISA method detection kit and an application thereof; the kit comprises: a) an antibody detection plate, b) enzyme conjugate working fluid, c) a positive control, d) a negative control, e) a sample diluent, f) 10X concentrated washing liquid, g) a substrate coloured solution A, h) a substrate coloured solution B, and i) a stopping solution; the beneficial and positive effects of the invention are that: the kit is simple in operation; the requirements for apparatuses needed are not high; the kit can be operated by everyone, can meet the requirements with different levels such as epidemic disease monitoring, hygienic epidemic prevention, intensive culture, and individual culture, is suitable for large-scope popularization and application, and has wide market prospects.
Owner:INST OF ANIMAL SCI & VETERINARY HUBEI ACADEMY OF AGRI SCI
Korean novel duck hepatitis viral antibody ELISA (Enzyme-Linked Immunosorbent Assay) detection kit
InactiveCN102127531ASolve the difficulty of purificationImprove securityRecombinant DNA-technologyFermentationDuck hepatitis A virusViral antibody
The invention discloses a Korean novel duck hepatitis viral antibody ELISA (Enzyme-Linked Immunosorbent Assay) detection kit. The detection kit contains an ELISA board coated by Korean novel duck hepatitis VP1 (Phenotypic Variance1) recombination protein, a sample diluent, concentrated washing liquid, an enzyme conjugate working solution, a chromogenic reagent (A), a chromogenic reagent (B), a stopping solution, a positive contrast solution and a negative contrast solution. The VP1 recombination protein is obtained by using the following method: using Korean novel duck hepatitis viruses as a material, augmenting and cloning the VP1 gene through an RT-PCR (Reverse Transcriptase-Polymerase Chain Reaction) method to obtain recombinant expression plasmid pMD (physical medium dependent)-VP1; then, directionally inserting to an expression vector pET-32a (+) and screening to obtain recombinant expression plasmid pET-32a(+)-VP1; and inducing, expressing and purifying by ITPG (Isopropyl beta-D-Thiogalactopyranoside) to obtain VP1 recombination protein. The detection kit is used for detecting the Korean novel duck hepatitis and has strong specificity, high sensitivity, simplicity of operation, easiness of popularization and application in a large-area range and wide market prospects.
Owner:POULTRY INST SHANDONG ACADEMY OF AGRI SCI
Antigen modified cancer cell vaccines for cancer therapy
InactiveUS20050106130A1Rapid and increased anamnestic responseGood antigenicityBiocideBacteriaAbnormal tissue growthCancer cell
Disclosed are methods for treating cancers, particularly tumorigenic types. Cancer cells are modified to express highly immunogenic antigens so that the cells will generate a defensive response in a mammal that exhibits the cancer or is predisposed to cancer and prevent or ameliorate proliferation of cancer cells. The novel cancer cell vaccines are expected to be effective against a wide range of tumors and leukemias.
Owner:MORPHOGENESIS
Peony polypeptide as well as preparation method and application thereof
ActiveCN103937865AEnhance nutritional propertiesPromote digestion and absorptionPeptide preparation methodsPlant peptidesFood industryCholesterol
The invention relates to a method for preparing peony polypeptide from cake meal which is obtained through preparing peony seed oil by squeezing peony seeds, the polypeptide prepared by the method and application of the polypeptide. During the preparation of the polypeptide, various microbes are added to ferment or various proteases are added to be subjected to enzymolysis, so as to obtain the peony polypeptide product. The product can be in the form of powder, granules, tablets, capsules, oral liquid and the like. The peony polypeptide has the efficacy advantages of lowering cholesterol, lowering blood pressure, promoting fat metabolism, resisting fatigue, enhancing human immunity, regulating human physiological functions and the like and has broad development and application prospect in the fields of food industry, health care, medicine and the like.
Owner:李杰
Genotype VII Newcastle disease virus marker vaccine strain and application thereof
ActiveCN104988124AHigh growth titerHigh biological propertiesViral antigen ingredientsMicroorganism based processesViral MarkersChick embryos
The invention discloses a genotype VII Newcastle disease virus marker vaccine strain and an application thereof, and belongs to the field of genotype VII Newcastle disease virus marker vaccine strain rescue and application. A built Newcastle disease virus reverse genetic operating platform is utilized for enabling NP protein of a G7 strain to miss 18 amino acids and conducting mutation on F-protein cleavage loci, and the highly-weak virulence and high-virus titer genotype VII Newcastle disease virus marker vaccine strain MG7-NPdelta18+Fmut is rescued through screening. The microbial preservation serial number is CCTCC NO: V201505. The marker vaccine strain has the biological characteristics of high growth titer and low virulence in chick embryos and is genetically stable. The immune protection test result shows that the marker vaccine strain is good in immunogenicity, capable of inducing high-level protective antibodies, and capable of completely protecting immunized chicken, can be used for preventing and controlling a currently-popular genotype VII Newcastle disease virus and lays the foundation of identifying vaccine immunity and wild virus infection.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
Recombinant cell line for stable expression of porcine epidemic diarrhea virus S1 protein, vaccine and application
InactiveCN107619819AEase of mass productionGood antigenicityAntiviralsAntibody medical ingredientsEpidemic diarrheaAdjuvant
The invention discloses a recombinant cell line for stable expression of porcine epidemic diarrhea virus S1 protein, a vaccine and an application. The recombinant cell line is constructed by transfecting HEK-293T cells by virtue of recombinant plasmid which is constructed by carrying a target gene on a lentiviral vector, and then transfecting the HEK-293T cells by virtue of generated high-titer virus particles; and the recombinant cell line, which can achieve stable expression, can still keep an excellent protein expression level after several passages. The recombinant cell line for stable expression of the porcine epidemic diarrhea virus S1 protein provided by the invention has the characteristics of being easy for culture, rapid in proliferation, unlimited in expansion, stable in property and high in protein expression amount; and when the vaccine, which is prepared from the expression protein and adjuvants, is used for immunizing pigs, the generation of a high-titer porcine epidemicdiarrhea virus neutralizing antibody can be induced from animal bodies, and the piglets (the pigs) can resist strong attack of porcine epidemic diarrhea viruses.
Owner:GUANGZHOU BONIZZI BIOTECH CO LTD
Human AD7C-NTP antigen determinant polypeptide, antibody and application thereof in diagnosis kit
ActiveCN101215323AGood antigenicityEasy to synthesizeImmunoglobulins against animals/humansBiological testingAntigenAntibody
The invention relates to a human AD7C-NTP antigen determinant polypeptide antibody and the application on a diagnosis reagent case. The AD7C-NTP antigen determinant polypeptide of the invention is one of polypeptide sections which are stated by the sequence table SEQ ID NO.1 to SEQ ID NO.6. The antibody of the invention is prepared by any AD7C-NTP antigen determinant polypeptide of the invention. The AD7C-NTP antibody of the invention can be applied to prepare an AD7C-NTP external diagnosis reagent case.
Owner:XUANWU HOSPITAL OF CAPITAL UNIV OF MEDICAL SCI +1
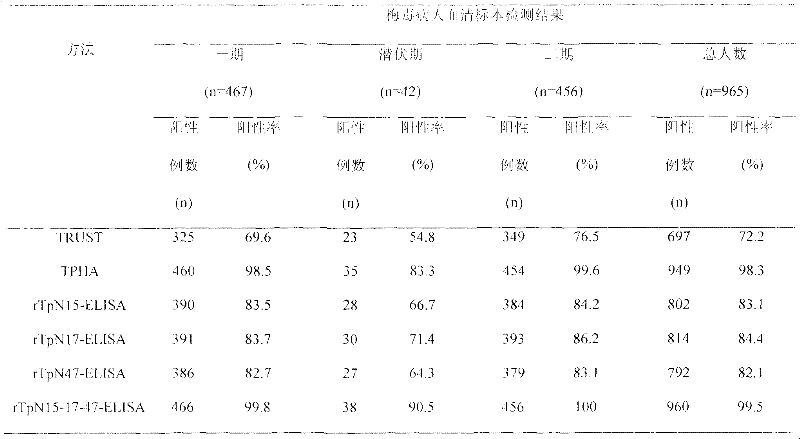
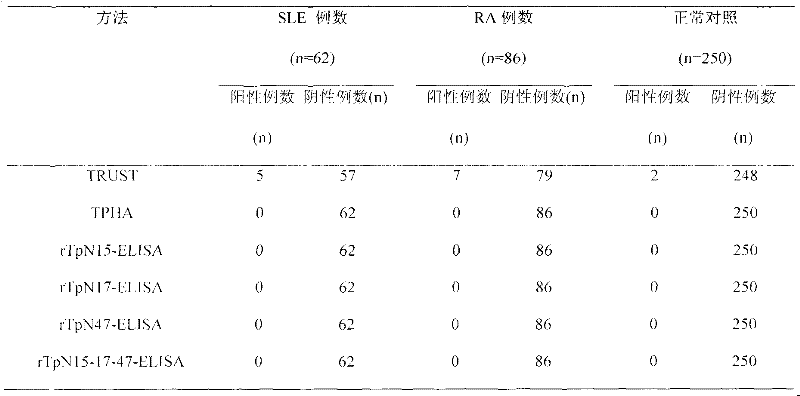
Preparation method of rTpN15-17-47-ELISA for detecting syphilis serum antibody
InactiveCN102183646AOvercome the disadvantage of high costGood antigenicityBacteriaFermentationSyphilisNucleotide
The invention relates to a syphilis serological screening or diagnosis method. The purpose is that the method has the characteristics of low price, high speed, sensitivity and specificity. The technical scheme provided by the invention is that: a preparation method of rTpN15-17-47-ELISA for detecting syphilis serum antibodies comprises the following steps in turn: constructing an artificial fusion gene tpN15-17-47 of tpN15, tpN17 and tpN47 and a prokaryotic expression system E.coliBL21DE3Pet42a-tpN15-17-47 by gene technology, purifying the recombinant expression product rTpN15-17-47 as a coating antigen, establishing rTpN15-17-47-ELISA for detecting syphilis serum antibodies. The fusion gene tpN15-17-47 has a nucleotide sequence and an amino acid sequence as shown in sequence table 5.
Owner:孙爱华 +1
Indirect ELISA kit for detecting avian infectious bronchitis virus antibody
InactiveCN102093999AGood securityGood antigenicityRecombinant DNA-technologyMaterial analysisProtein servingsSorbent
The invention discloses an indirect enzyme-linked immuno sorbent assay (ELISA) kit for detecting avian infectious bronchitis virus (IBV) antibody. The kit contains an ELISA plate enveloped by IBV-N recombinant protein serving as antigen. The IBV-N recombinant protein is obtained by the following method: designing a pair of specific primers according to an IBV-N gene sequence; amplifying the N gene of IBV by using a reverse transcription polymerase chain reaction (RT-PCR) method, directionally inserting the N gene into a pET-32a(+) expression vector, and screening to obtain a positive recombinant expression plasmid of pET-32a(+)-IBV-N; and transferring the plasmid to a BL21 competent cell, and performing isopropyl thiogalactoside (ITPG) induction expression to obtain the IBV-N recombinant protein. The kit is low in cost, easy, convenient and quick to operate, and particularly suitable for detecting batch samples, and greatly improves the efficiency of serodiagnosis of the avian infectious bronchitis.
Owner:POULTRY INST SHANDONG ACADEMY OF AGRI SCI
Immunological combination compositions and methods
InactiveUS6251405B1Extended shelf lifeImproving immunogenicityAntibacterial agentsOrganic active ingredientsAdjuvantImmunogenicity
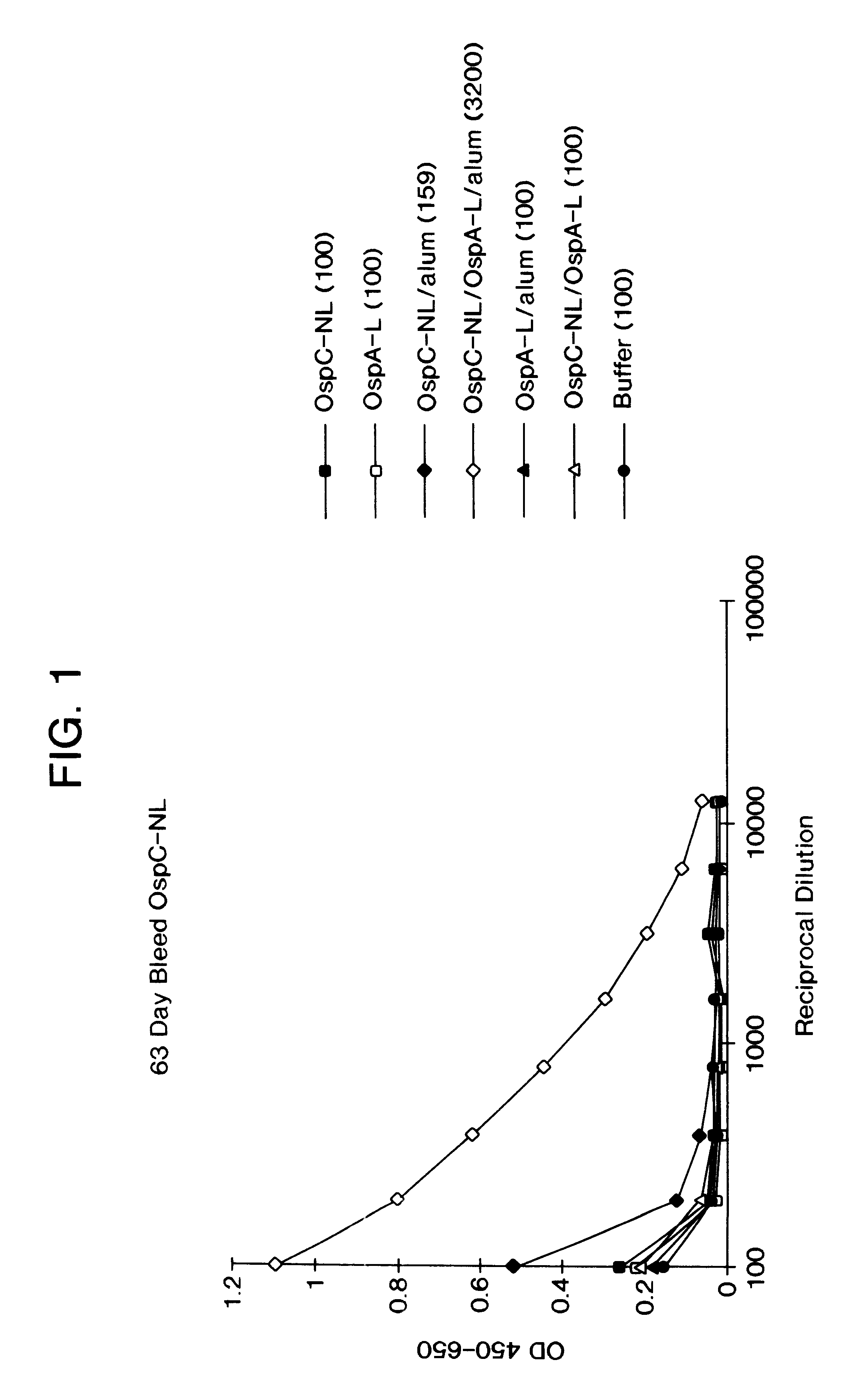
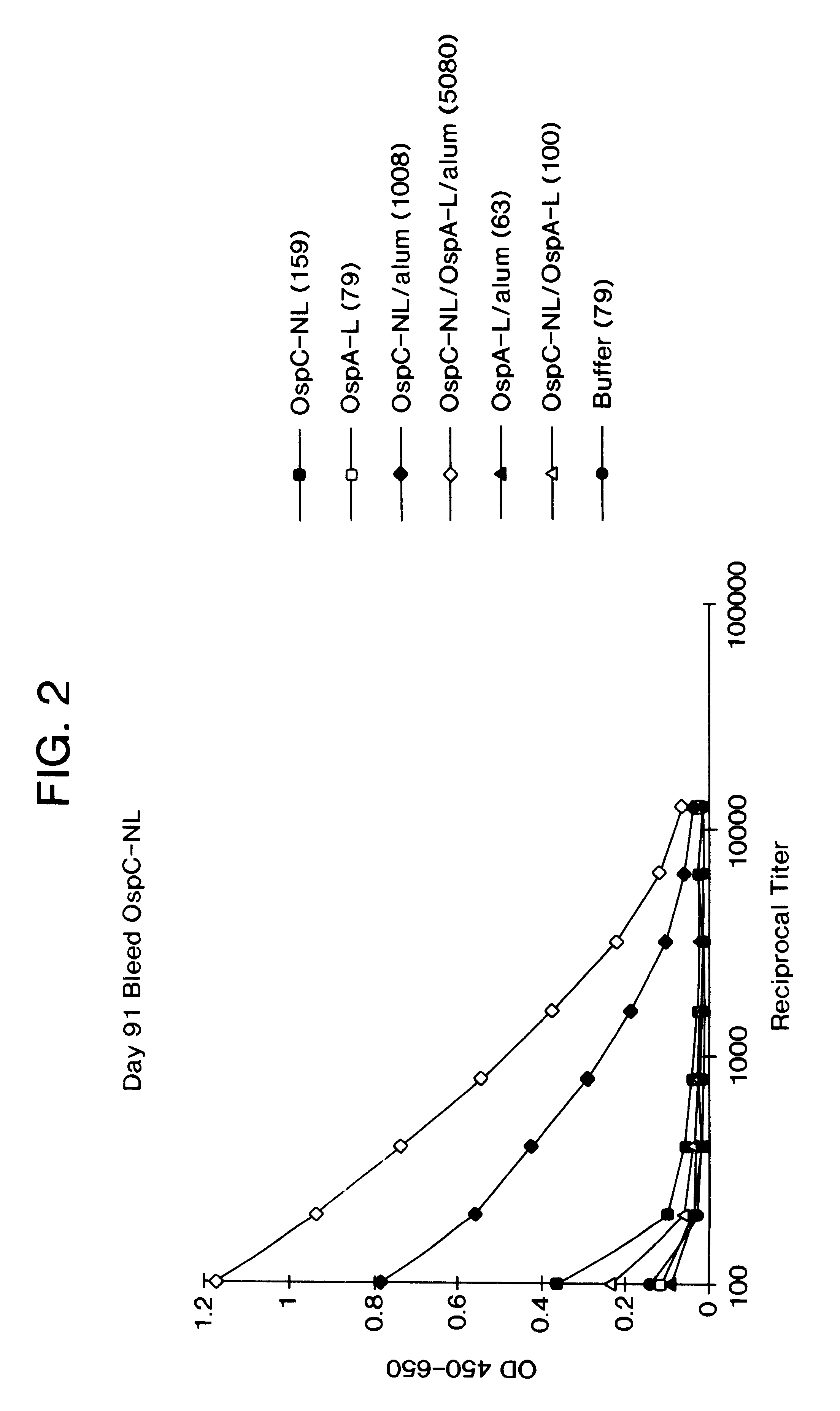
Immunological compositions and methods for making and using them. The compositions contain an antigen and a lipoprotein and optionally an adjuvant. The lipoprotein can itself be antigenic or immurogenic. The antigen can be influenza HA and the lipoprotein a recombinantly expressed product having an OspA leader for lipidation and PspA for the protein portion. The antigen can be OspC and the lipoprotein OspA. The components of the composition are co-administered. A potentiated immunological response is obtained by the compositions and methods.
Owner:CONNAUGHT LAB
Preparation method of heavy metallic lead resistant monoclonal antibody
InactiveCN101041696AEfficient synthesisPromote rapid developmentImmunoglobulins against animals/humansTissue cultureAntigenDiethylenetriamine
The invention discloses a making method of heavy-metal lead monoclonal antibody in the biological technical domain, which is characterized by the following: coupling lead ion and carrier protein through p-aminobenzene-diethylenetriamine pentoacetic acid as bifunctional metal chelant; obtaining full antigen; crossing splenocyte and Sp2 / 0 marrow tumour cell to make crossed tumour cell; sieving; obtaining stable strong secretory specificity anti-lead monoclonal monomer to inhibit Pb-DTPA.
Owner:NANJING AGRICULTURAL UNIVERSITY
Binding protein and epitope-blocking ELISA for the universal detection of H5-subtype influenza viruses
ActiveUS8574830B2Process stabilityGood antigenicityMicrobiological testing/measurementBiological material analysisSerodiagnosesSerum ige
Monoclonal antibodies and related binding proteins specific to influenza H5 subtype HA protein can be used in serological diagnosis of influenza H5 infection in mammalian and avian serum samples, including human serum samples. Each antibody reacts strongly with a wide variety of strains of H5 subtype and does not show cross-reactivity with non-H5 influenza subtypes.
Owner:TEMASEK LIFE SCIENCES LABORATORY
Preparation method of heavy metal mercury monoclonal antibody
InactiveCN101139398AFast pushStrong antigenicityImmunoglobulins against animals/humansBiological testingBALB/cSpleen cell
The present invention relates to a preparation method of heavy metal mercury monoclonal antibody, which belongs to the field of biotechnology. The present invention is particularly used in the preparation of specificity-recognition heavy metal mercury monoclonal antibody and in the fast mercury high-sensitivity measurement remained in the agricultural production and the agricultural production environment. The heavy metal mercury ion and the carrier albumen are coupled into full antigens through bifunctional metal chelate 1-(4-separate-cyano phenyl)-EDTA; the full antigens are used on Balb / C mouse and the spleen cells and the Sp2 / 0 myeloma cells are used to prepare hybridoma cells through the hybridoma technology. And monoclonal antibody which can stably excrete anti-Hg-EDTA is generated. The preparation technology in the present invention is easy and practical: the whole preparation process of the antigen requires no special instrument. The present invention is suitable for factory-scale production.
Owner:NANJING AGRICULTURAL UNIVERSITY
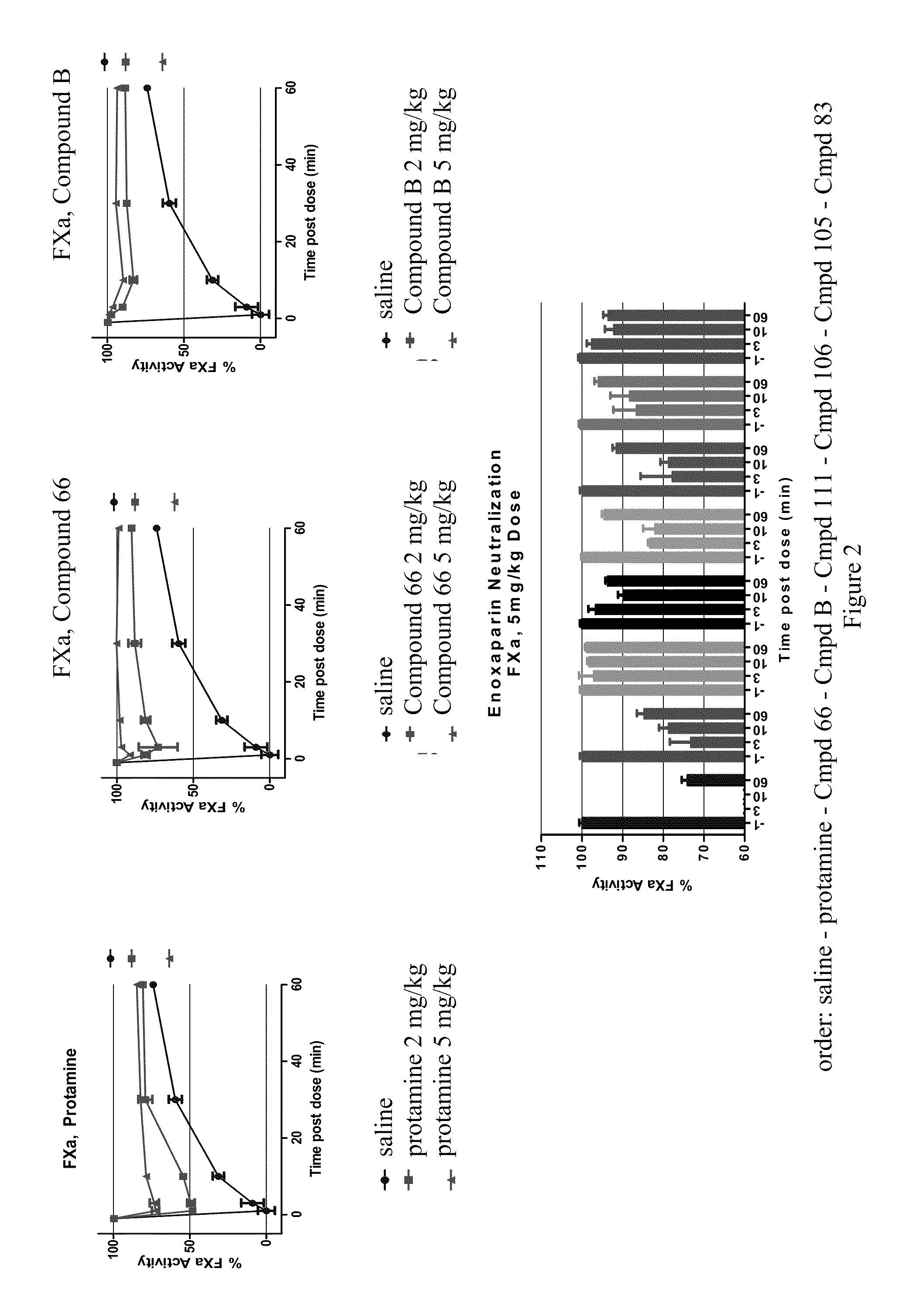
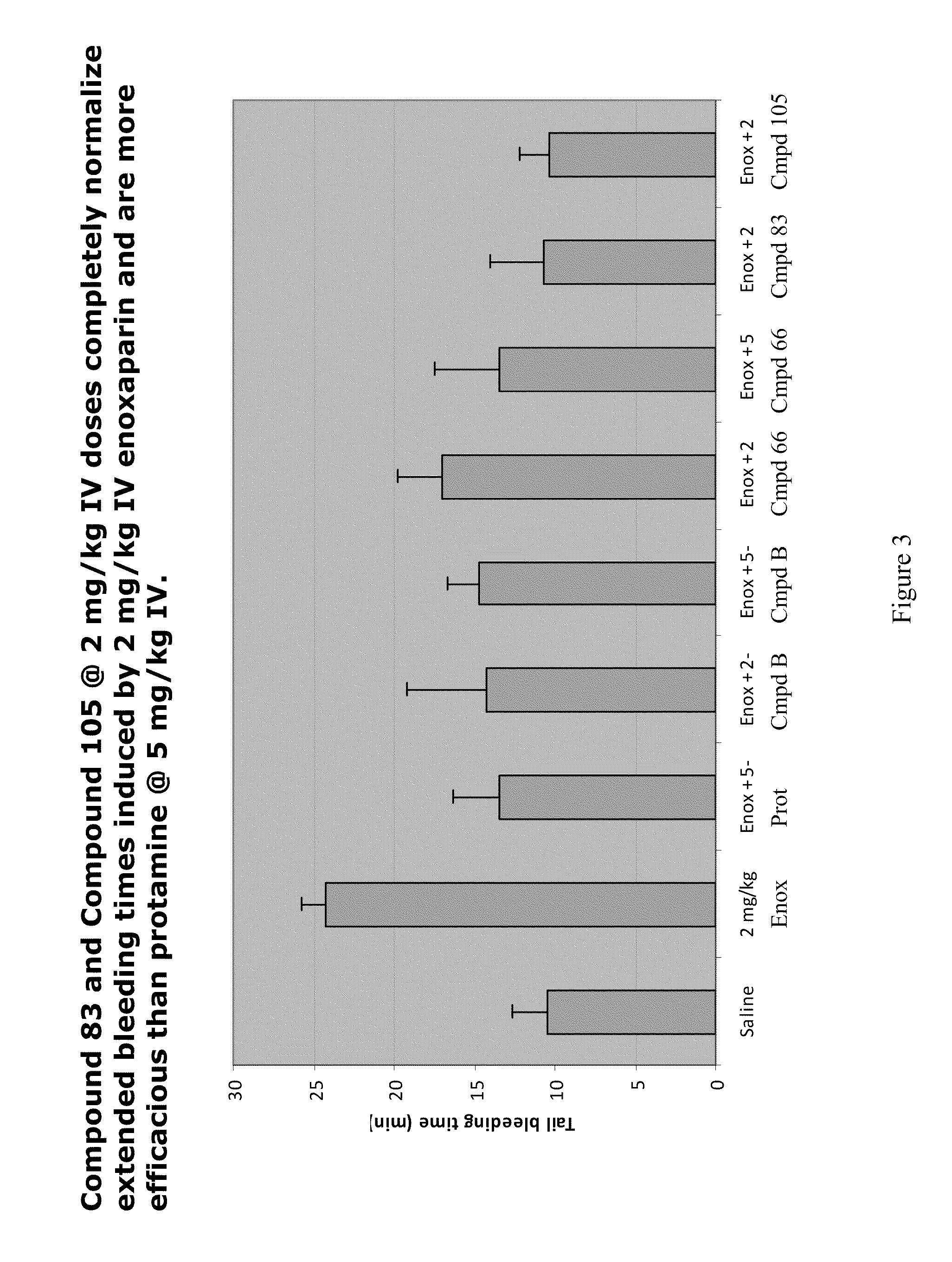
Anti-Heparin Compounds
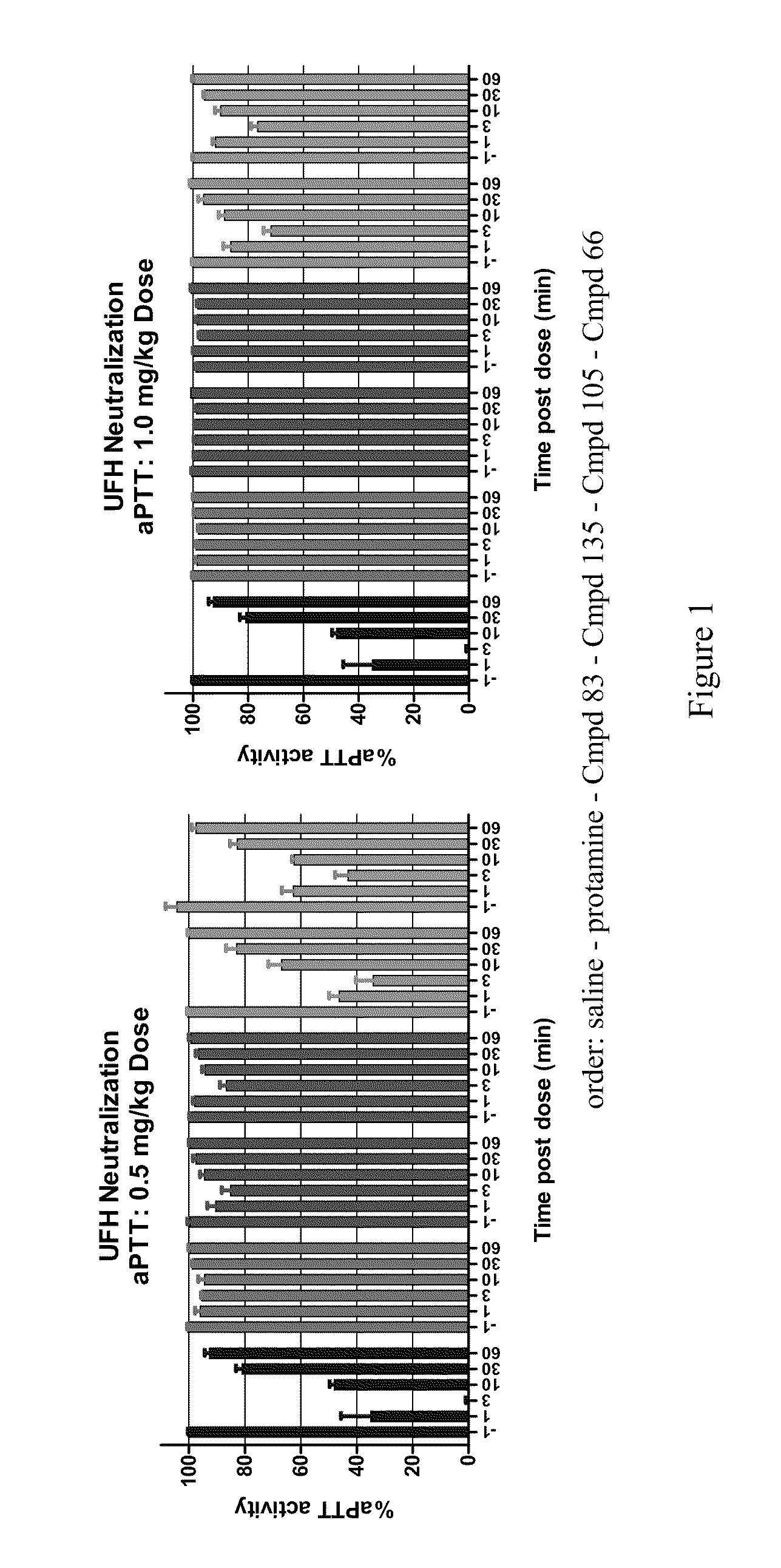
InactiveUS20110178104A1Effectively antagonizeRapidly antagonizeBiocideOrganic chemistryAnticoagulant effectMedicinal chemistry
The present invention provides compounds and methods for antagonizing the anticoagulant effect of an anticoagulant agent that is selected from UFH, LMWH, and a heparin / LMWH derivative in a patient comprising administering to the patient a compound of the invention or a salt thereof, or a composition comprising the same.
Owner:POLYMEDIX
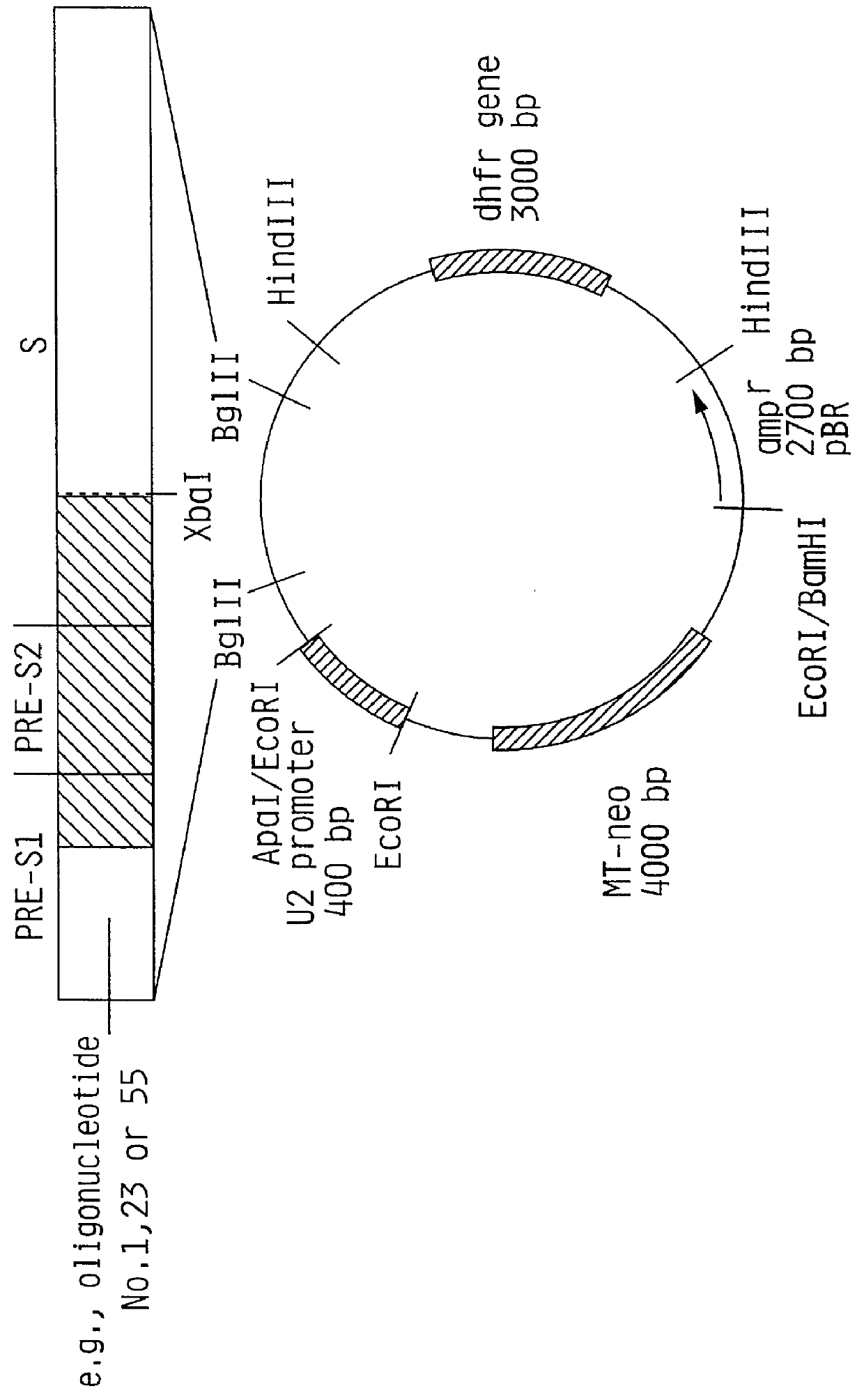
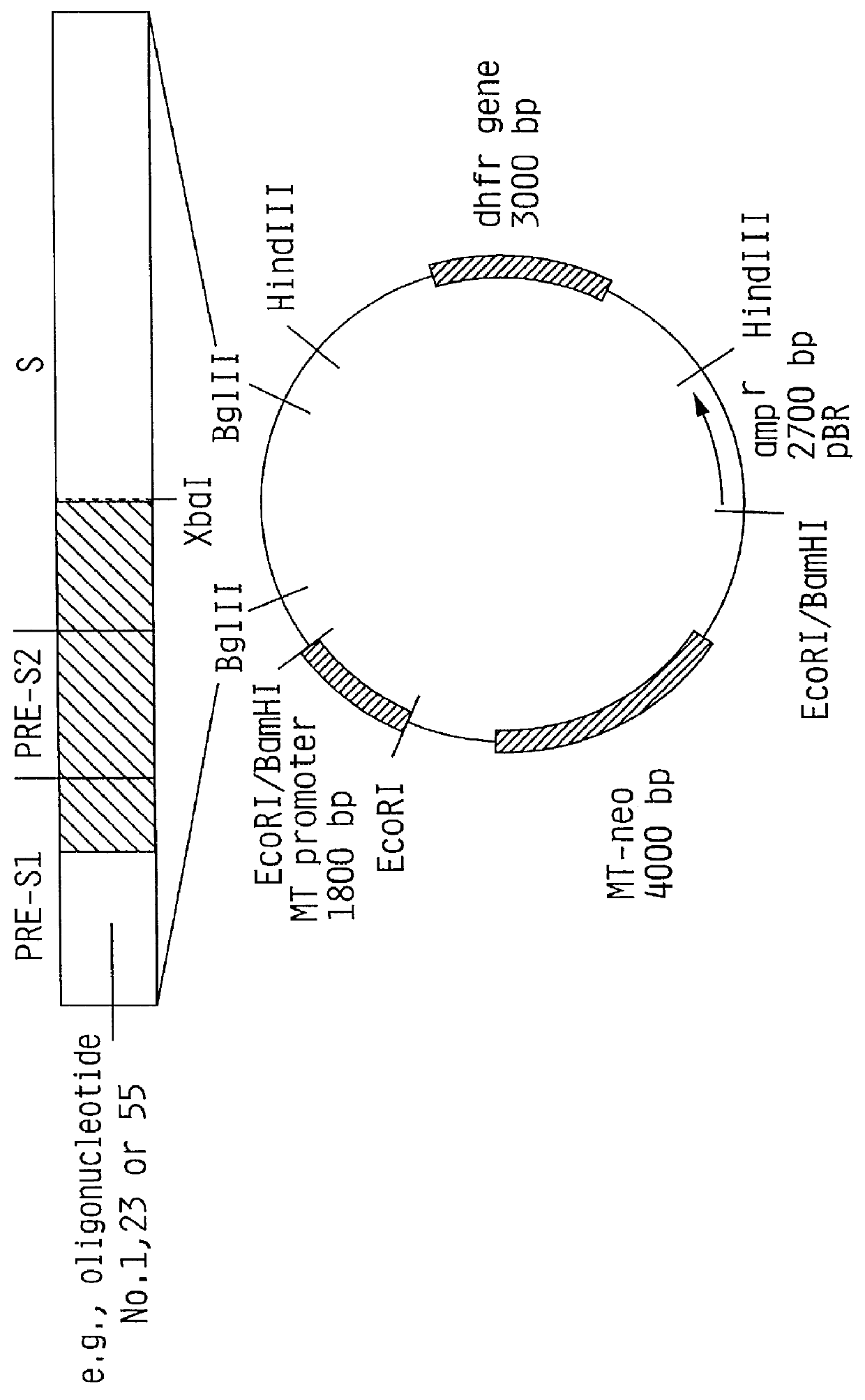
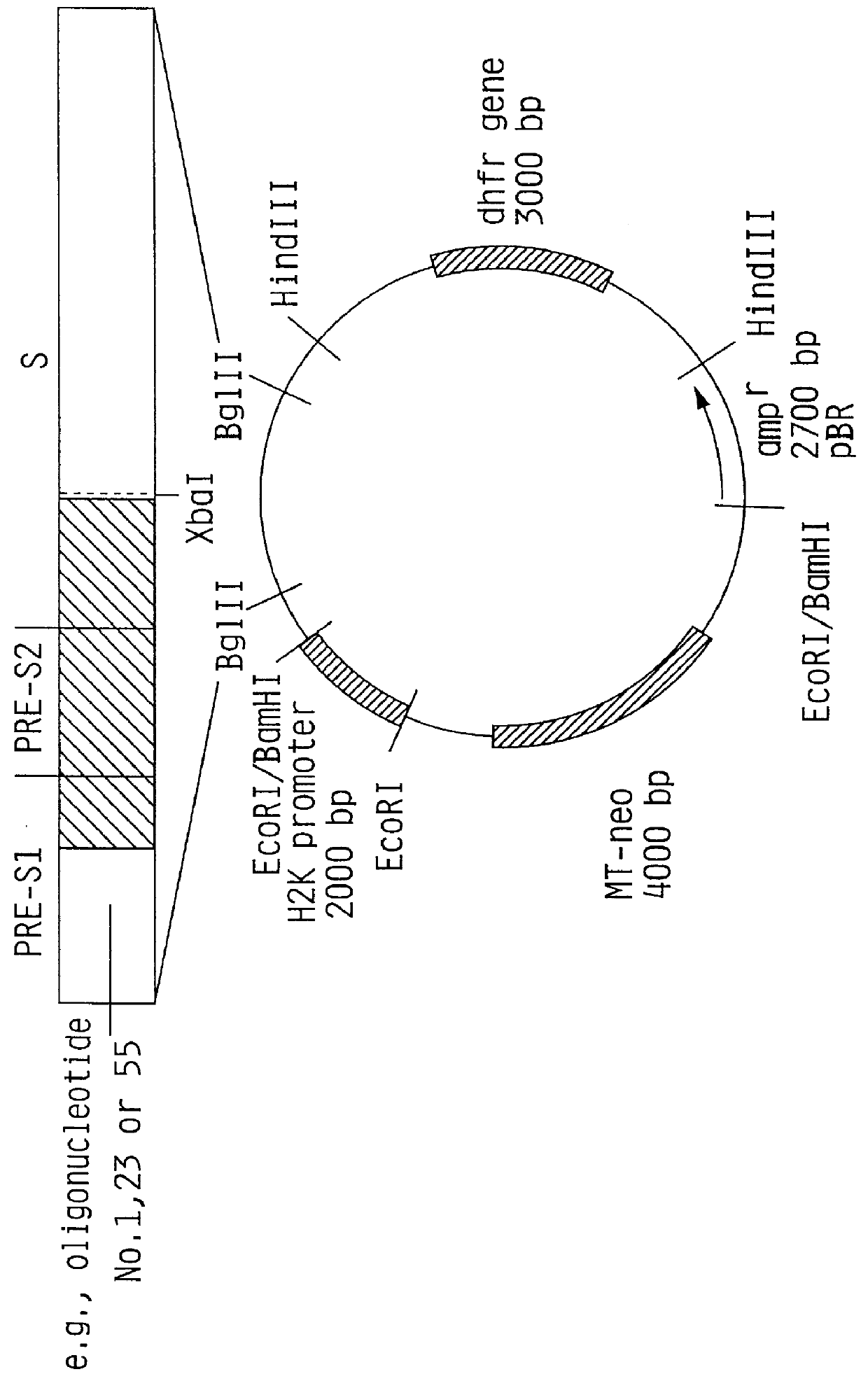
Hepatitis B surface antigen vaccine
InactiveUS6072049AGood antigenicityStimulate immune responseVirusesBacteriaEukaryotic plasmidsHepatitis B virus
HBV surface antigen particles, prepared by recombinant DNA technology are described, said particles being composed of epitopes from the group of surface peptides and / or core peptide of non-A, non-B hepatitis virus, hepatitis virus A and / or hepatitis virus B. Respective particles are especially characterized by a composition of different epitopes selected from pre-S and S peptides. There are also described DNA-sequences, plasmids and cell lines coding for respective HBV surface antigen particles as well as a new vaccine containing the same.
Owner:MEDEVA HLDG
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com