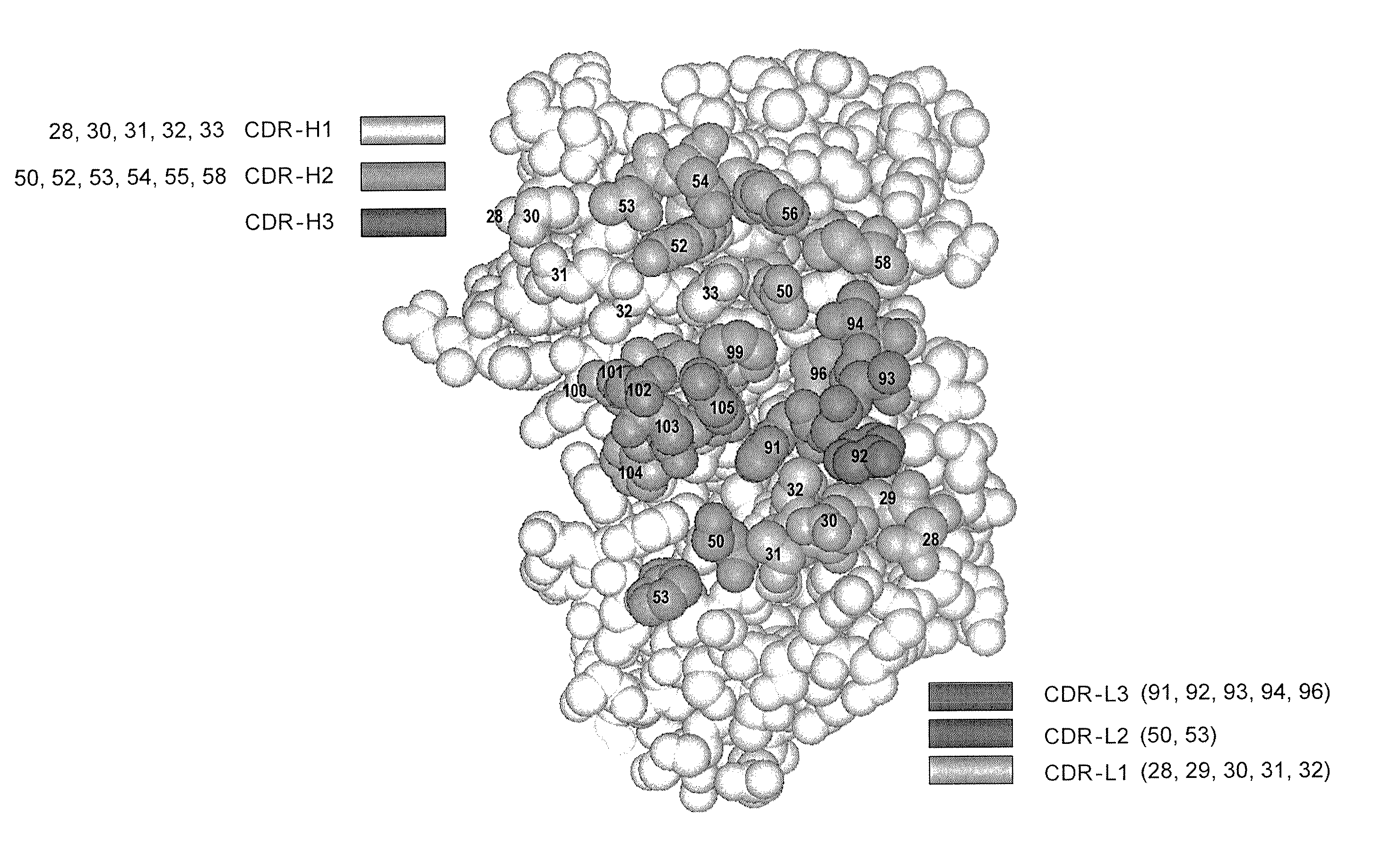
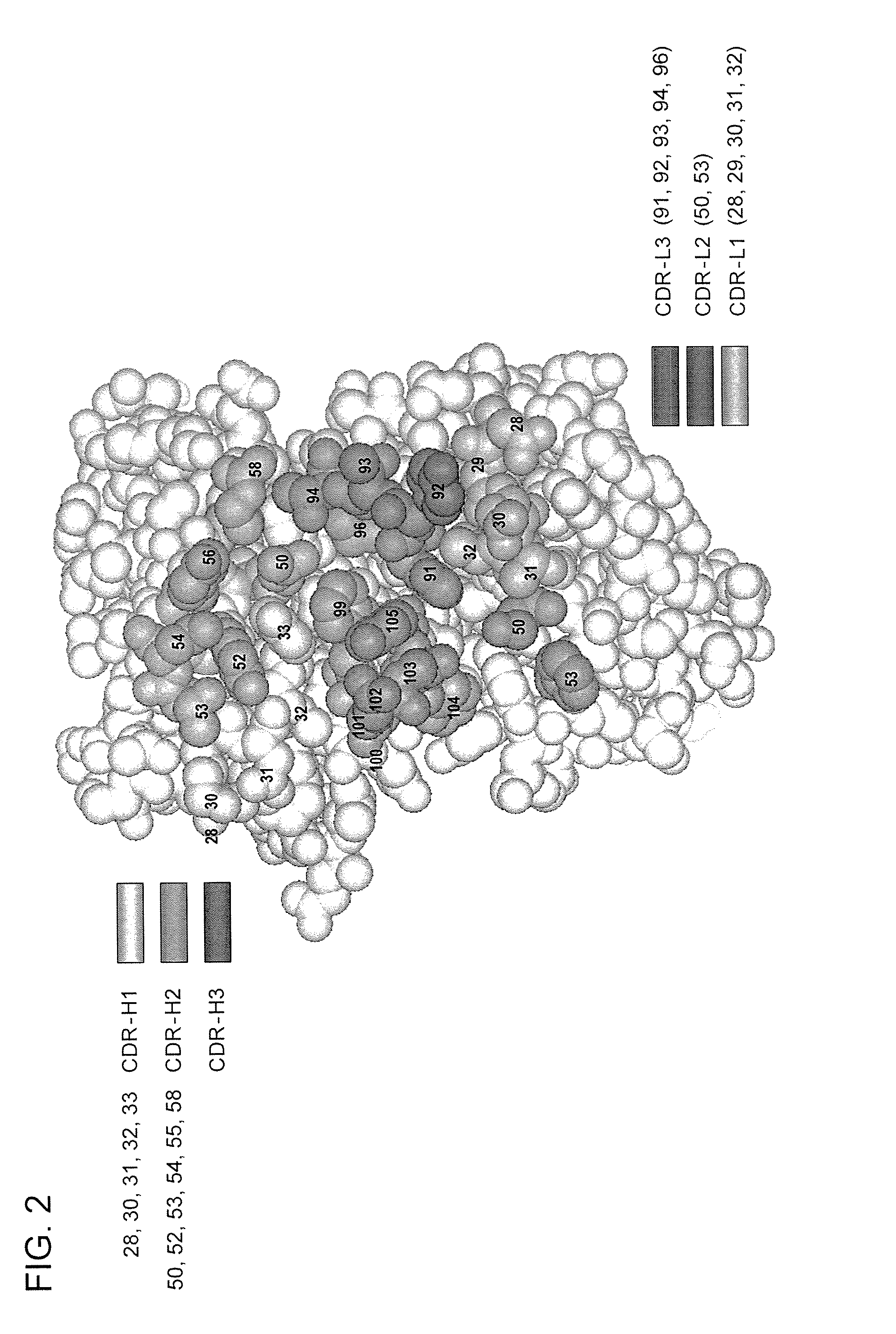
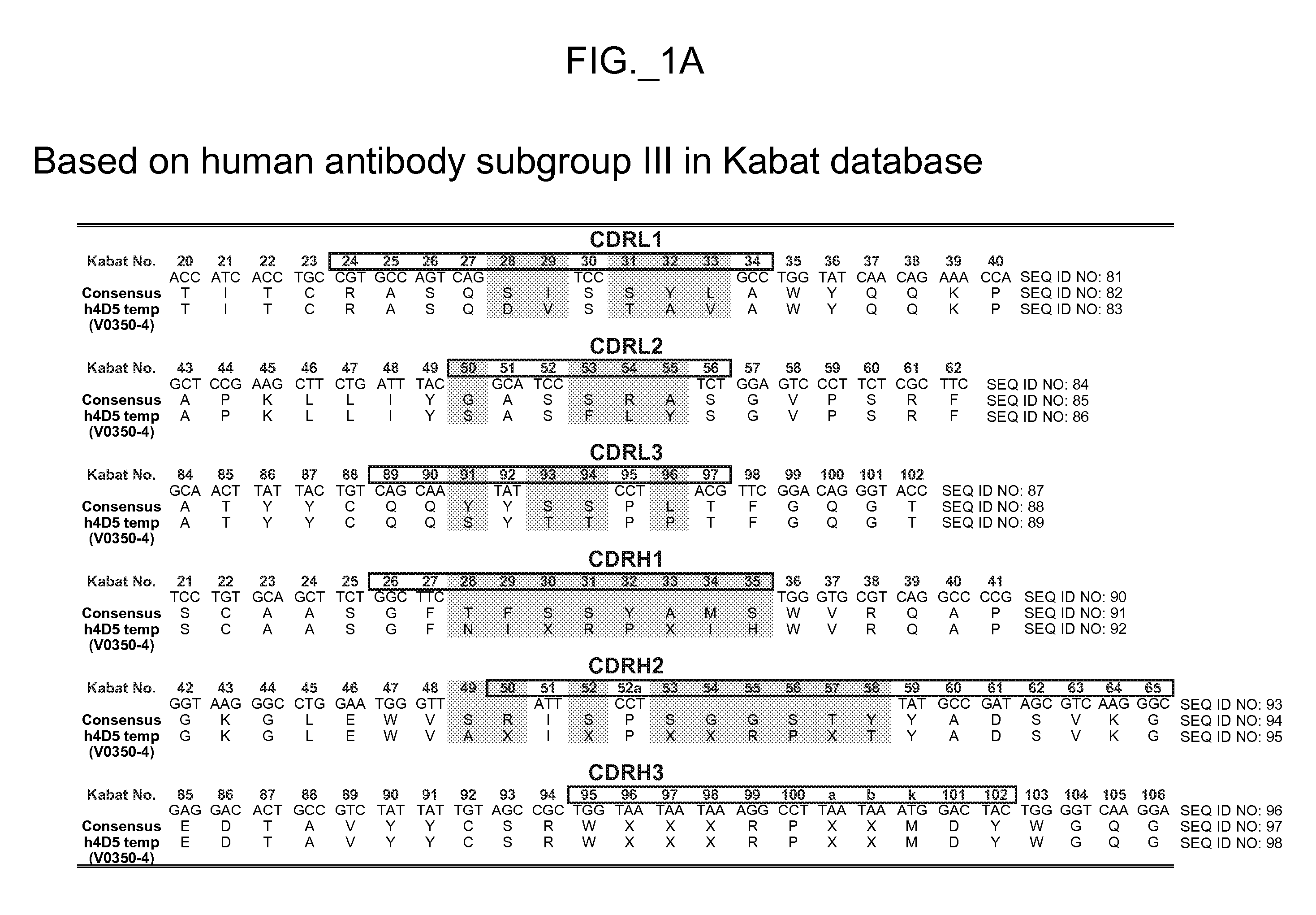
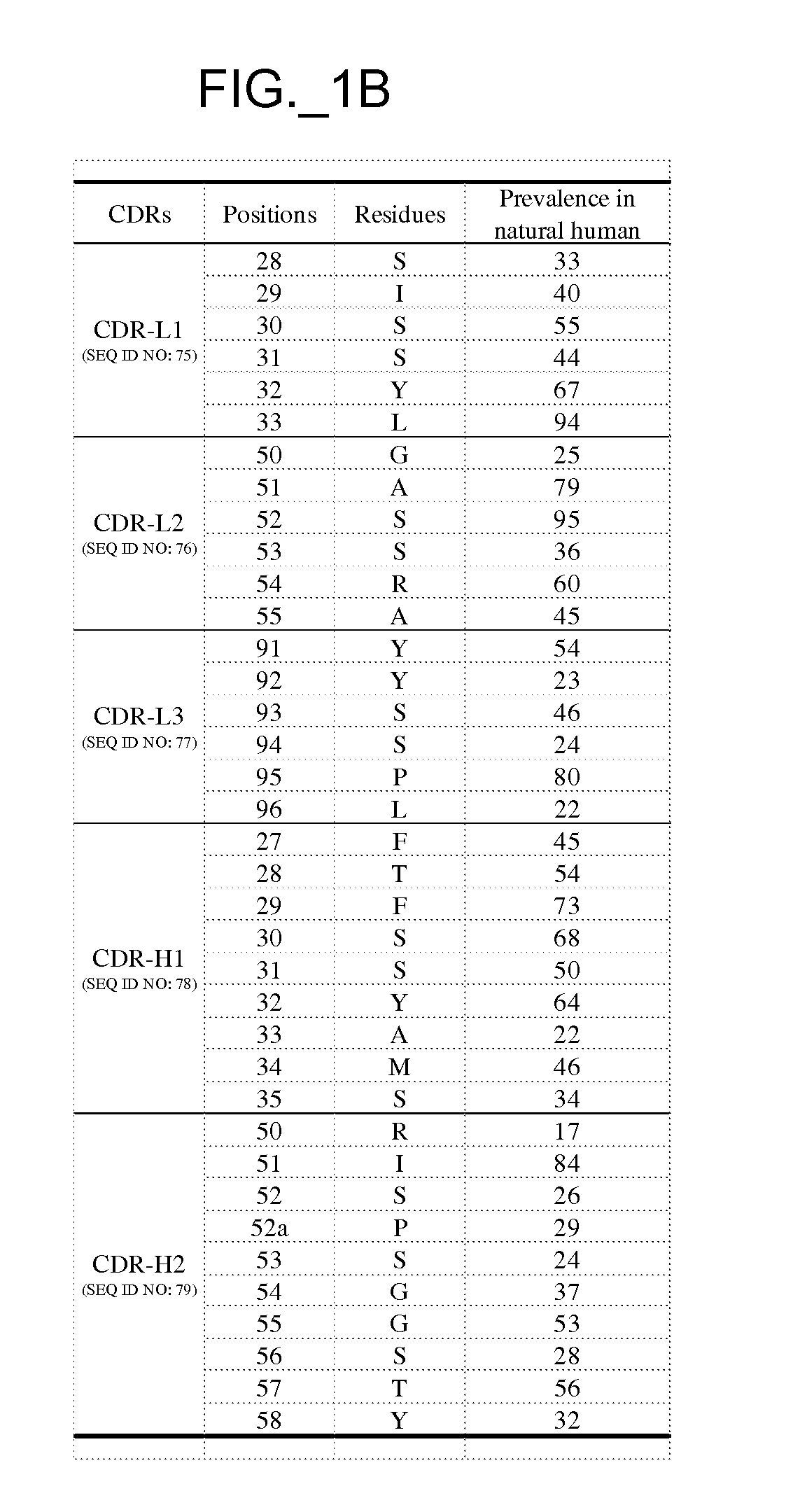
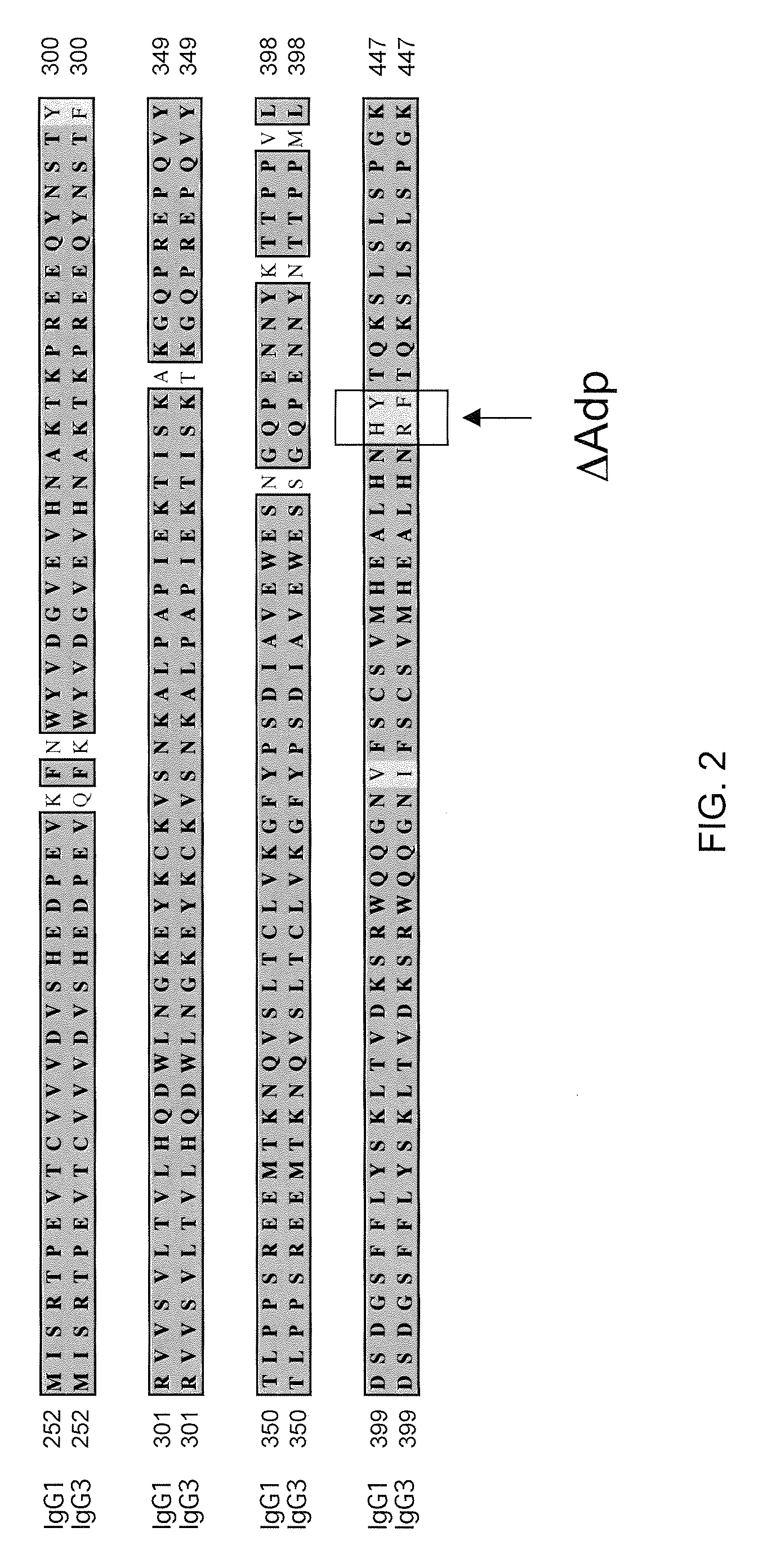
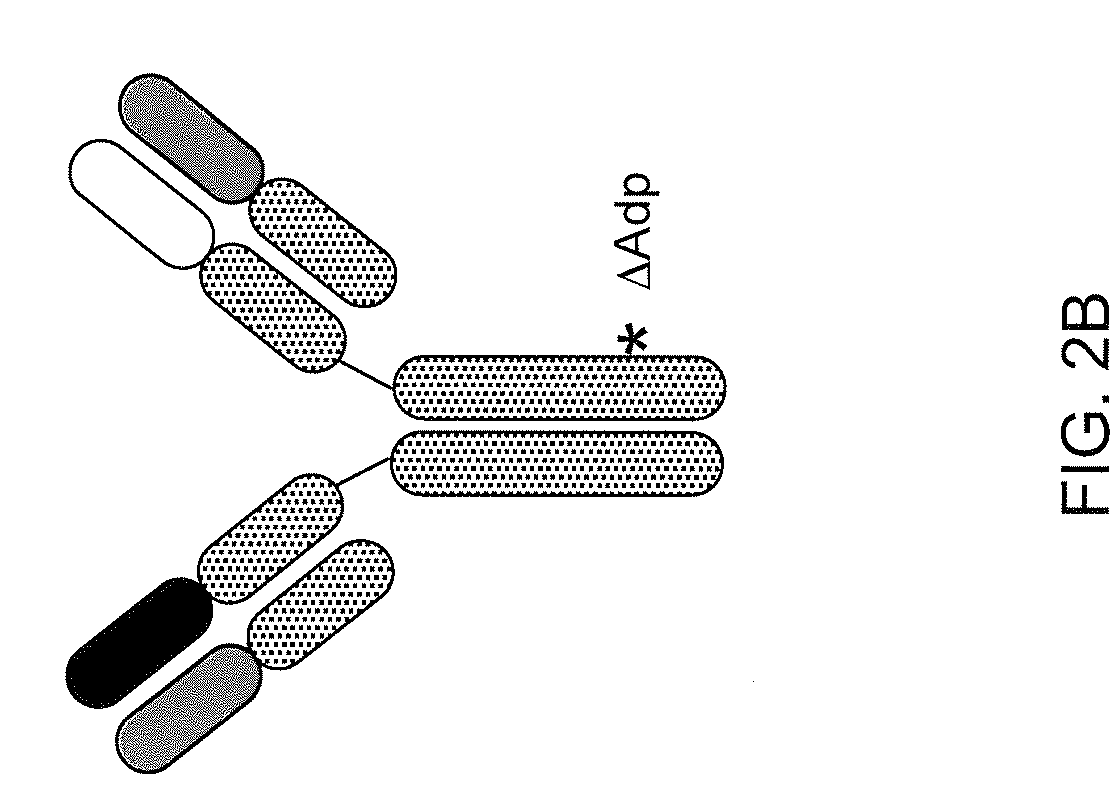
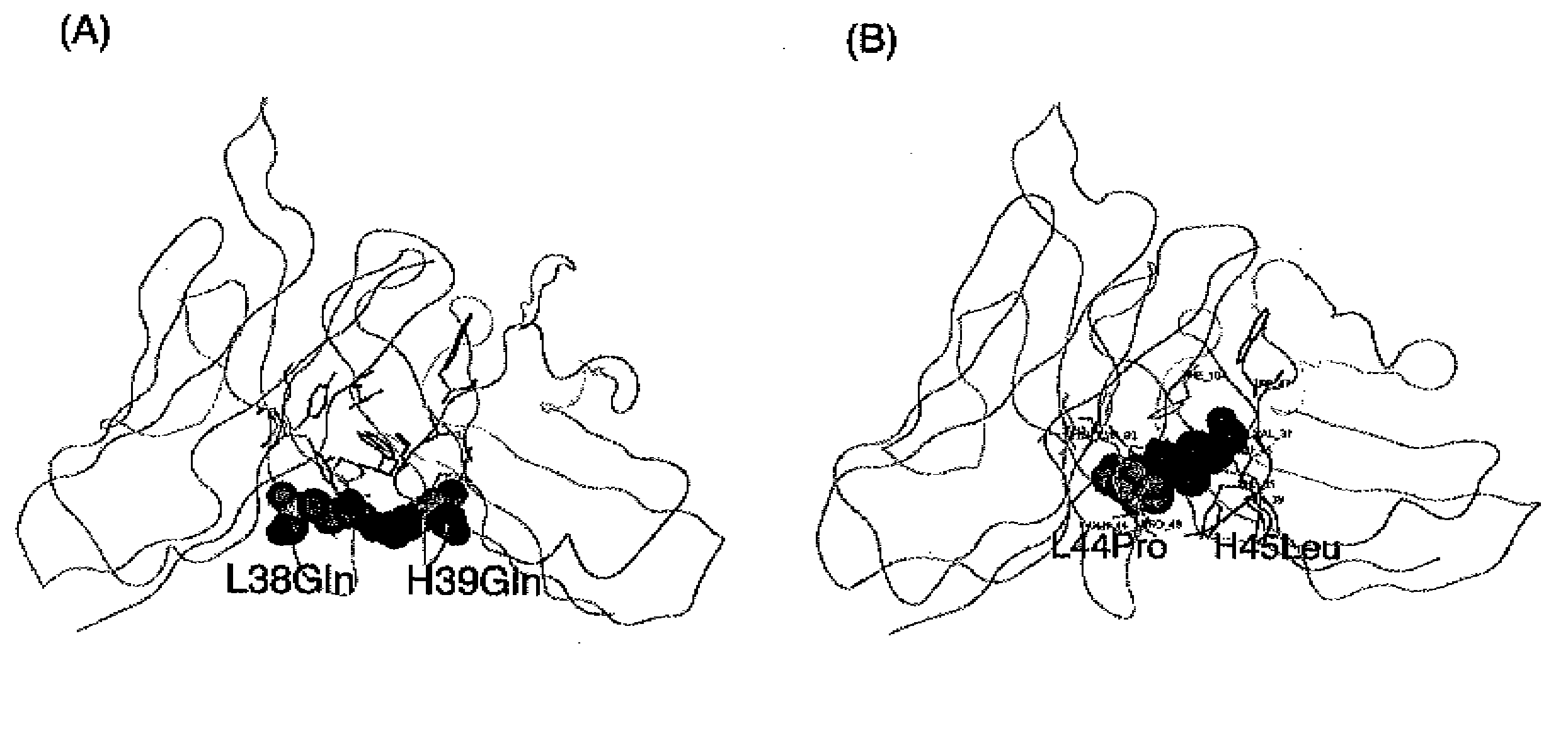
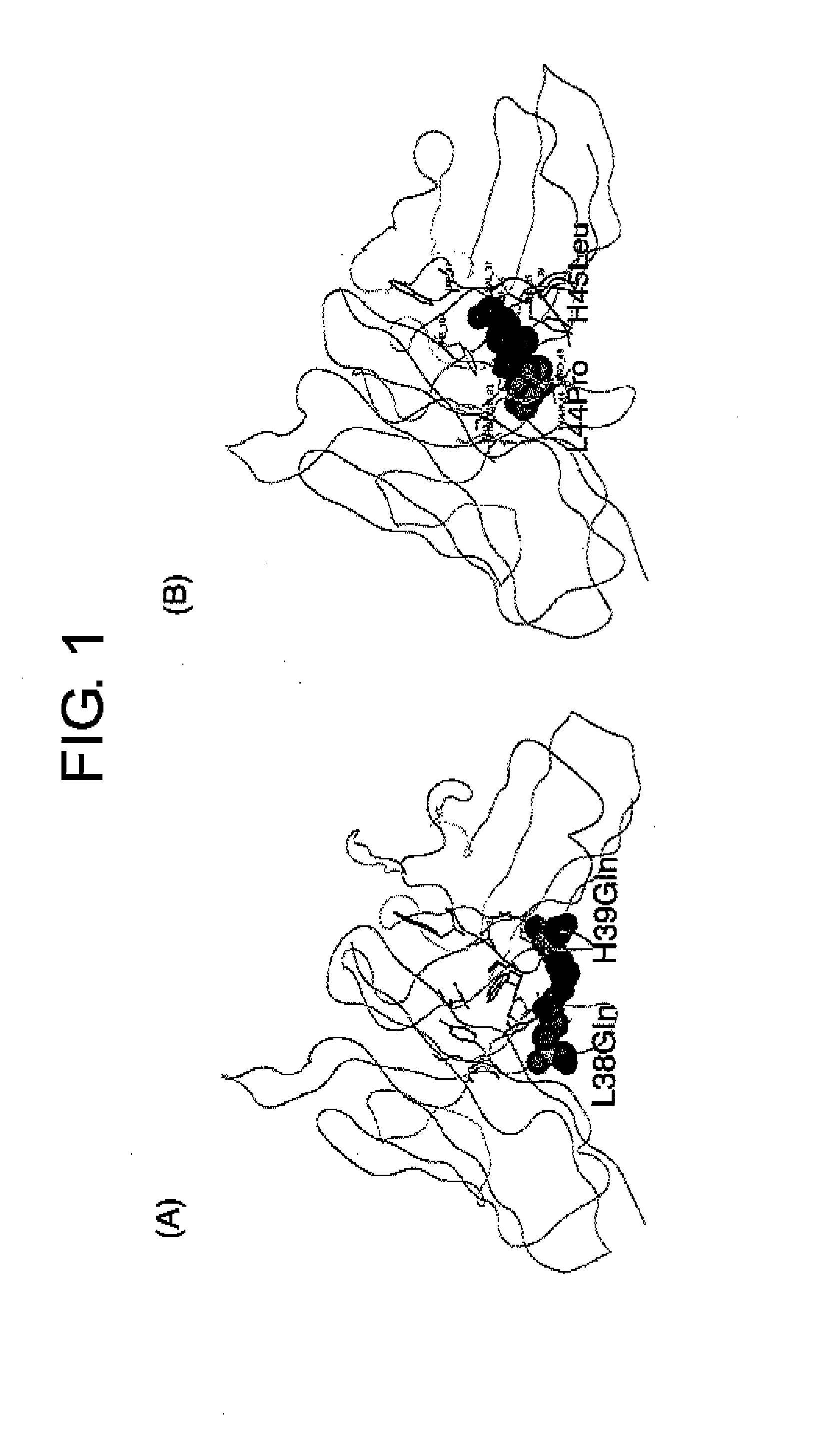
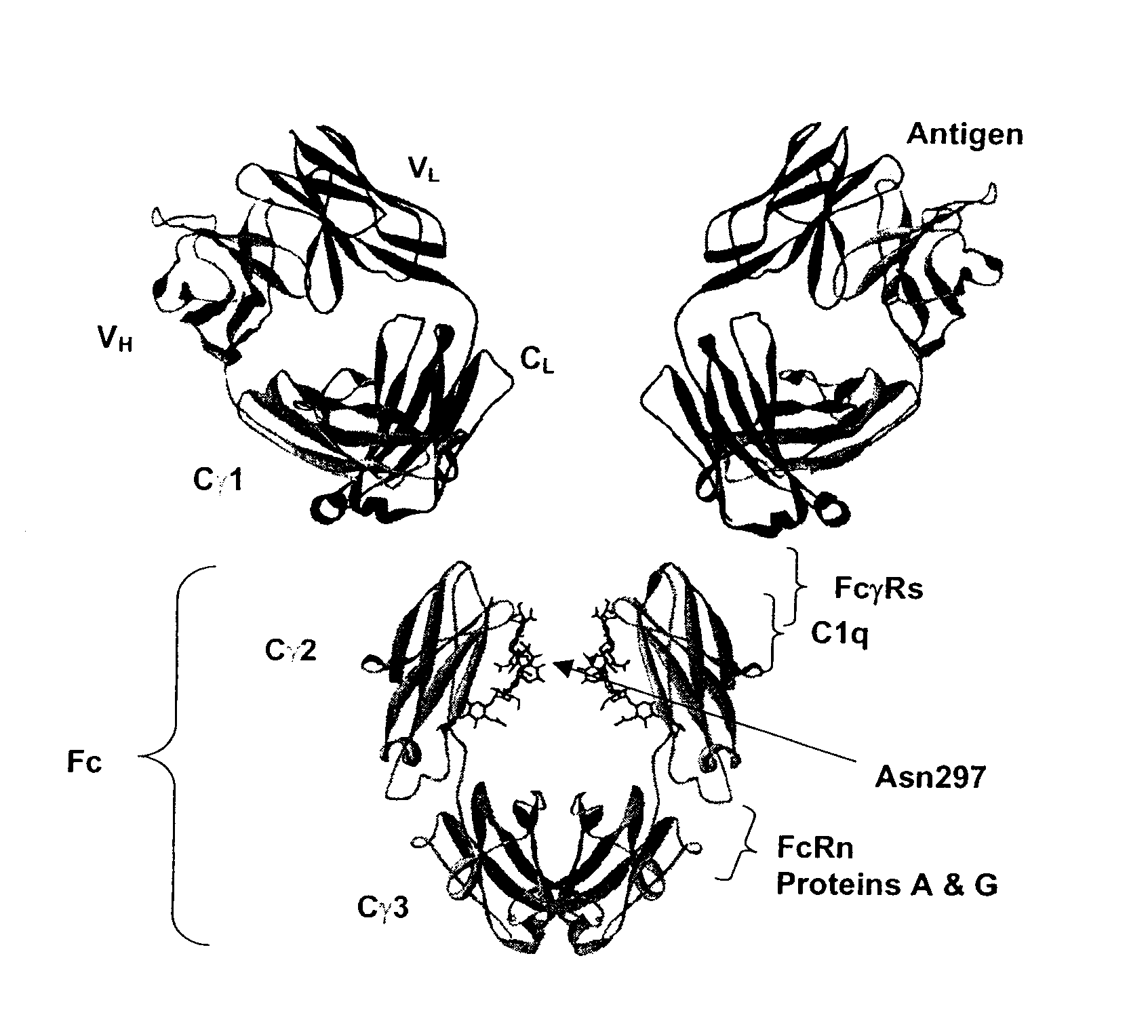
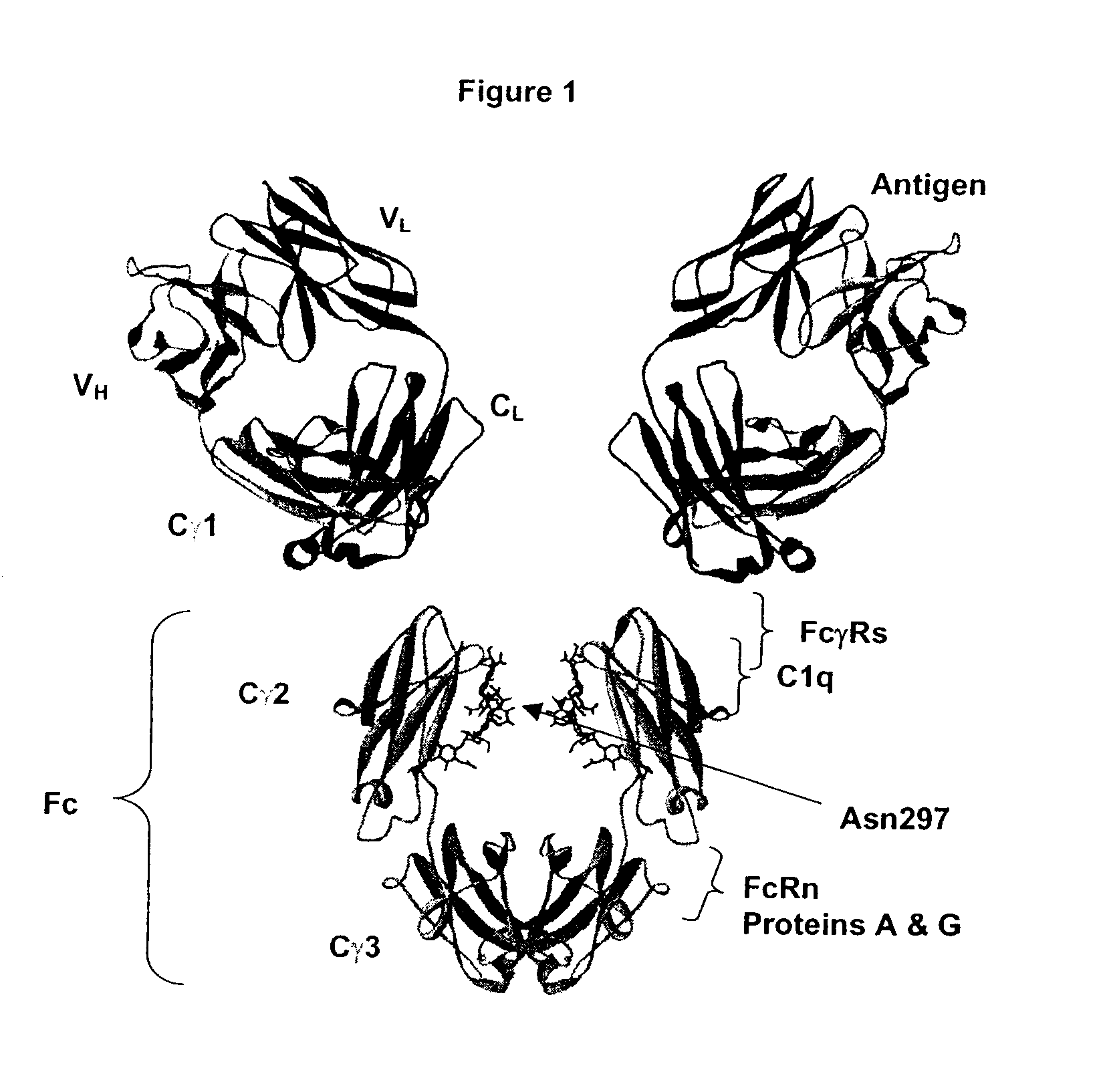
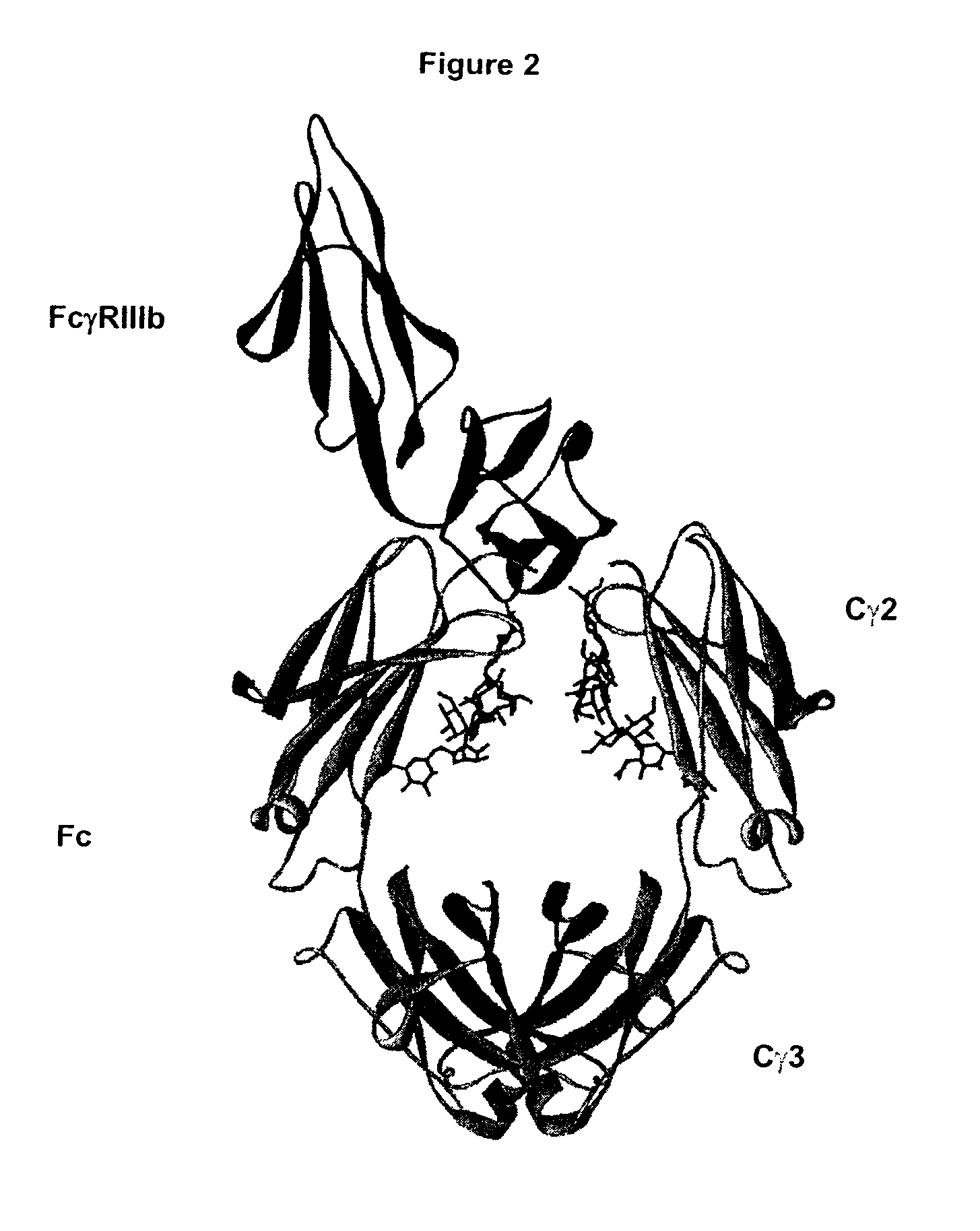
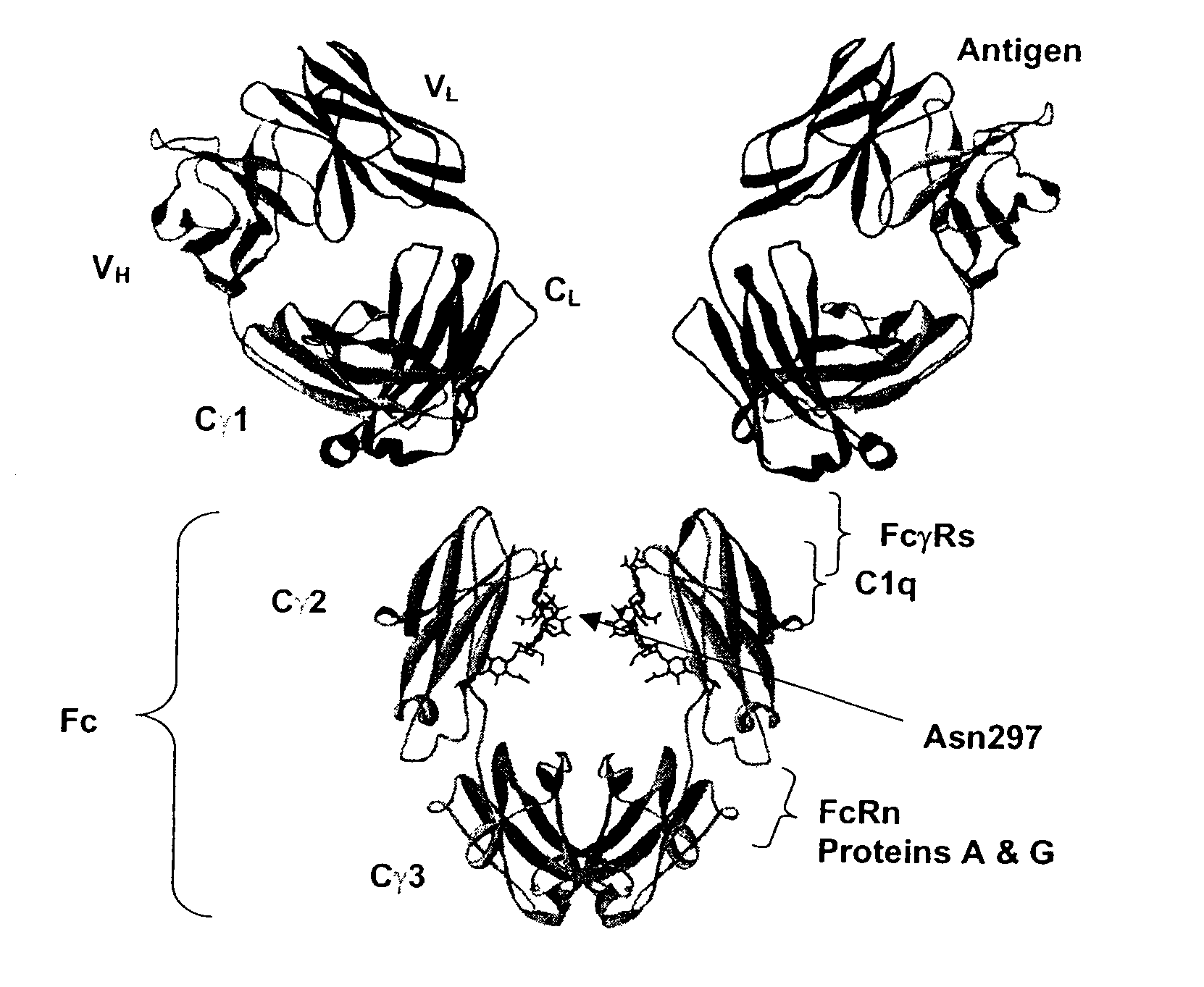
Patents
Literature
7016results about "Immunoglobulins against animals/humans" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
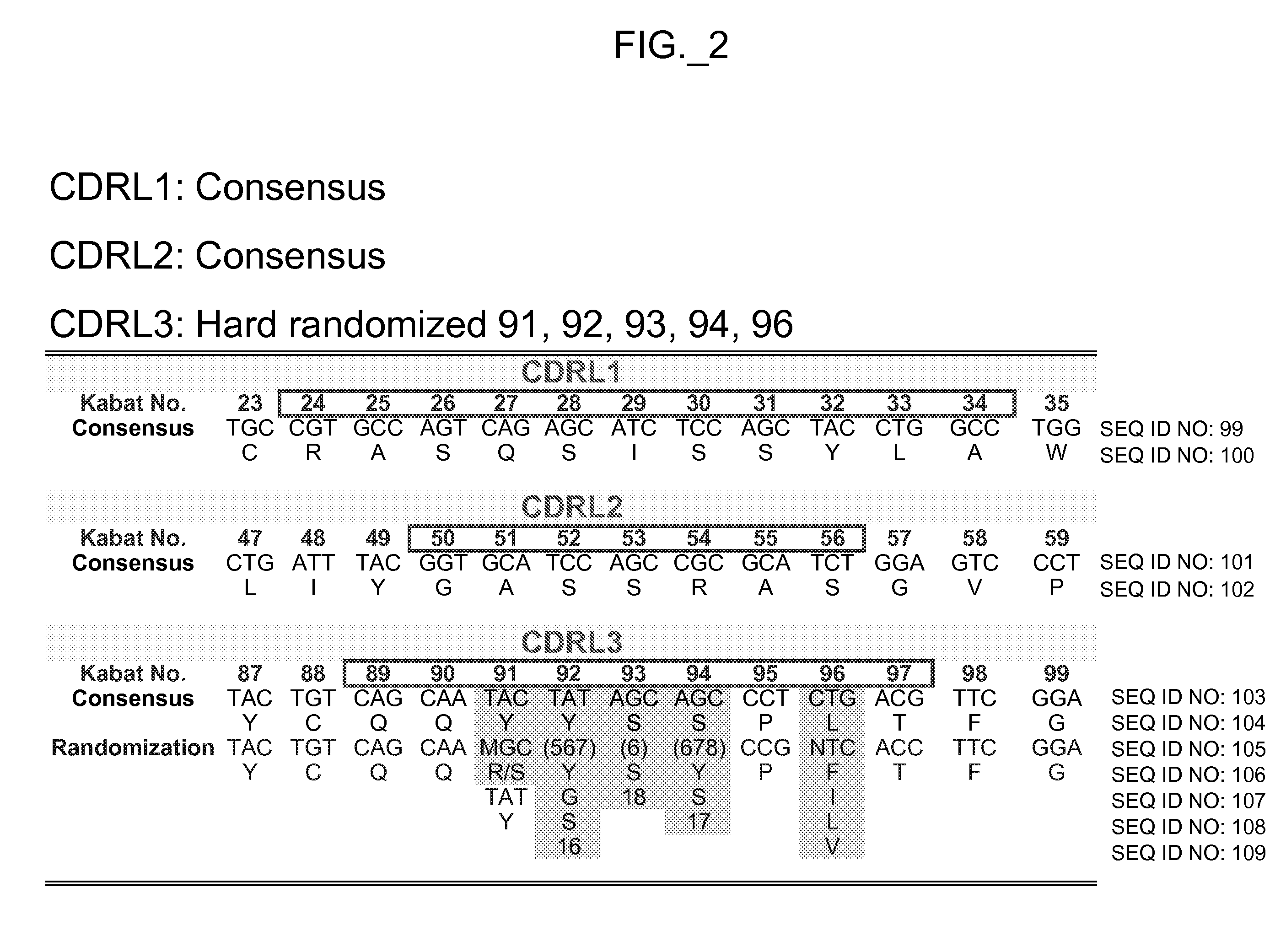
Binding polypeptides with restricted diversity sequences
InactiveUS20070237764A1Small sizeHigh-quality target binding characteristicFermentationVector-based foreign material introductionHeterologousAntigen binding
The invention provides variant CDRs comprising highly restricted amino acid sequence diversity. These polypeptides provide a flexible and simple source of sequence diversity that can be used as a source for identifying novel antigen binding polypeptides. The invention also provides these polypeptides as fusion polypeptides to heterologous polypeptides such as at least a portion of phage or viral coat proteins, tags and linkers. Libraries comprising a plurality of these polypeptides are also provided. In addition, methods of and compositions for generating and using these polypeptides and libraries are provided.
Owner:GENENTECH INC
Binding polypeptides with diversified and consensus vh/vl hypervariable sequences
ActiveUS20070160598A1Raise the possibilitySmall sizeAnimal cellsSugar derivativesAntibody hypervariable regionBioinformatics
The invention provides variant hypervariable regions comprising selected amino acid sequence diversity. Libraries comprising a plurality of these polypeptides are also provided. In addition, methods of and compositions for generating and using these polypeptides and libraries are provided.
Owner:GENENTECH INC
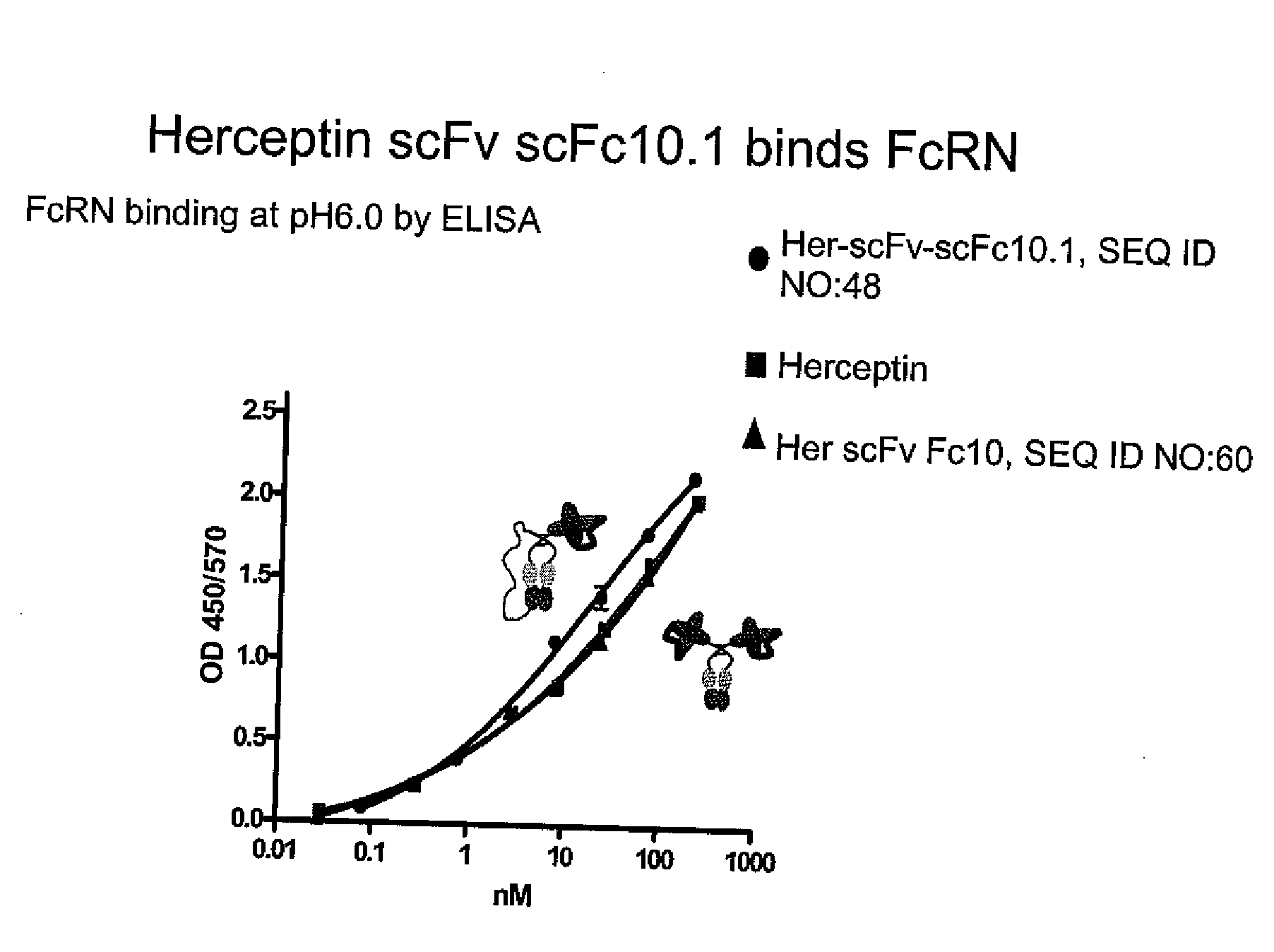
Molecules with extended half-lives, compositions and uses thereof
The present invention provides molecules, including IgGs, non-IgG immunoglobulin, proteins and non-protein agents, that have increased in vivo half-lives due to the presence of an IgG constant domain, or a portion thereof that binds the FcRn, having one or more amino acid modifications that increase the affinity of the constant domain or fragment for FcRn. Such proteins and molecules with increased half-lives have the advantage that smaller amounts and or less frequent dosing is required in the therapeutic, prophylactic or diagnostic use of such molecules.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST +1
Method of targeting specific cell populations using cell-binding agent maytansinoid conjugates linked via a non-cleavable linker, said conjugates, and methods of making said conjugates
The present invention discloses a method for targeting maytansinoids to a selected cell population, the method comprising contacting a cell population or tissue suspected of containing the selected cell population with a cell-binding agent maytansinoid conjugate, wherein one or more maytansinoids is covalently linked to the cell-binding agent via a non-cleavable linker and the cell-binding agent binds to cells of the selected cell population.
Owner:IMMUNOGEN INC
Readily Isolated Bispecific Antibodies with Native Immunoglobulin Format
ActiveUS20100331527A1Improve abilitiesReduces and eliminates bindingHybrid immunoglobulinsSerum immunoglobulinsImmunoglobulin heavy chainHeavy chain
A bispecific antibody format providing ease of isolation is provided, comprising immunoglobulin heavy chain variable domains that are differentially modified in the CH3 domain, wherein the differential modifications are non-immunogenic or substantially non-immunogenic with respect to the CH3 modifications, and at least one of the modifications results in a differential affinity for the bispecific antibody for an affinity reagent such as Protein A, and the bispecific antibody is isolable from a disrupted cell, from medium, or from a mixture of antibodies based on its affinity for Protein A.
Owner:REGENERON PHARM INC
Methods for Producing Polypeptides by Regulating Polypeptide Association
ActiveUS20100015133A1Efficient productionEfficient formationAnimal cellsSugar derivativesHeterologousAntiendomysial antibodies
In the course of the present invention, it was discovered that one could regulate association between polypeptides by modifying amino acid residues that form the interface during the association to amino acids carrying the same type of charge. In this context, the present invention enables efficient formation of heterologous molecules. For example, the present invention can be suitably applied to the preparation of bispecific antibodies.
Owner:CHUGAI PHARMA CO LTD
PD-1 Antibodies and PD-L1 Antibodies and Uses Thereof
ActiveUS20120039906A1Reduced activityStrong cytotoxicityAntibacterial agentsAnimal cellsPD-L1Antibody
Owner:INST JEAN PAOLI & IRENE CALMETTES +2
Molecules with extended half-lives, compositions and uses thereof
InactiveUS20030190311A1High affinityExtended half-lifeCompounds screening/testingFungiIntravenous gammaglobulinIn vivo
The present invention provides molecules, including IgGs, non-IgG immunoglobulin, proteins and non-protein agents, that have increased in vivo half-lives due to the presence of an IgG constant domain, or a portion thereof that binds the FcRn, having one or more amino acid modifications that increase the affinity of the constant domain or fragment for FcRn. Such proteins and molecules with increased half-lives have the advantage that smaller amounts and or less frequent dosing is required in the therapeutic, prophylactic or diagnostic use of such molecules.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST +1
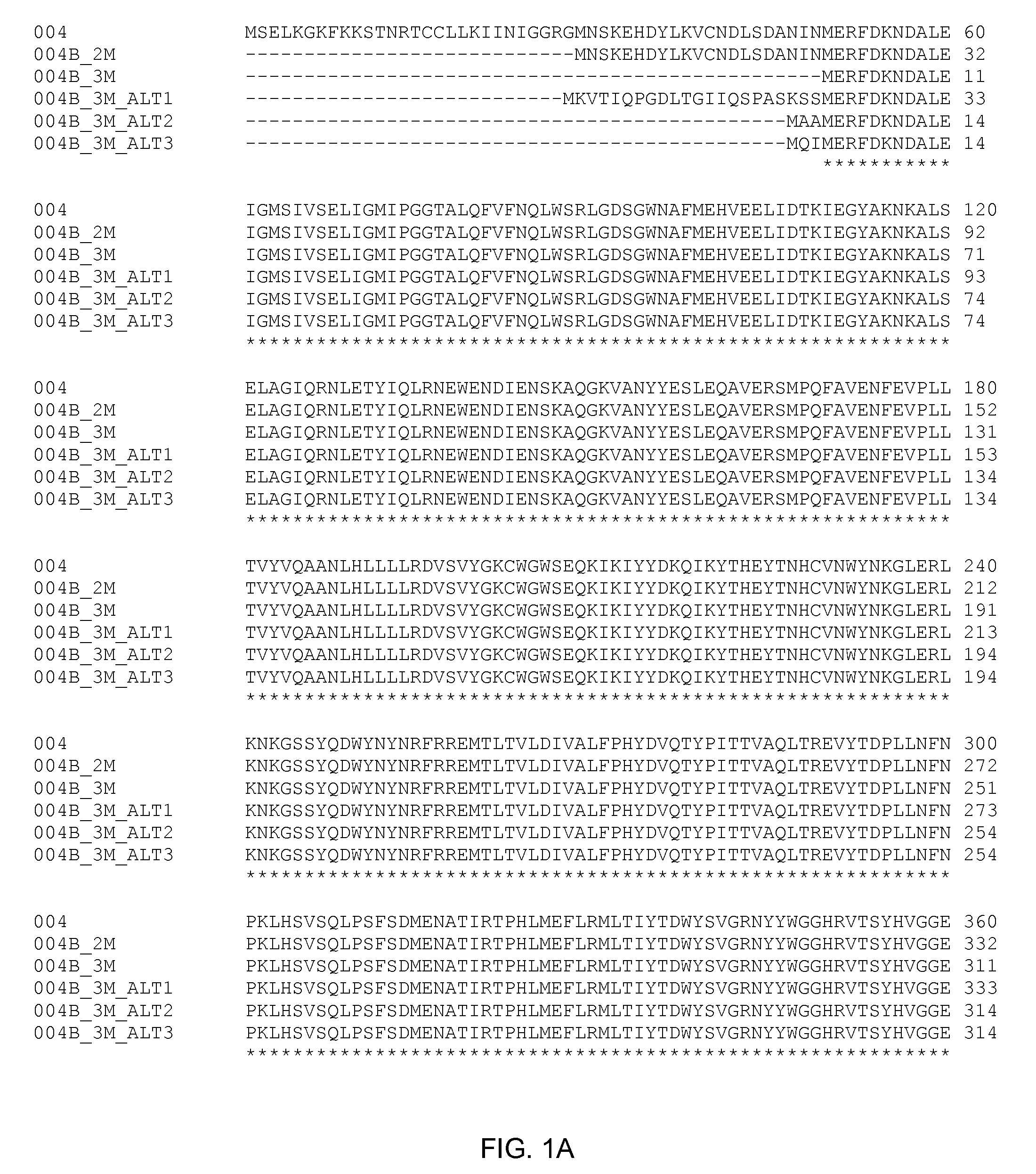
Synthetic axmi-004 delta-endotoxin genes and methods for their use
Compositions and methods for conferring pesticidal activity to bacteria, plants, plant cells, tissues and seeds are provided. Compositions comprising a coding sequence for a delta-endotoxin polypeptide are provided, particularly synthetically-derived coding sequences. The coding sequences can be used in DNA constructs or expression cassettes for transformation and expression in plants and bacteria. Compositions also comprise transformed bacteria, plants, plant cells, tissues, and seeds. In particular, isolated delta-endotoxin nucleic acid molecules are provided. Additionally, amino acid sequences corresponding to the polynucleotides are encompassed, and antibodies specifically binding to those amino acid sequences. In particular, the present invention provides for isolated nucleic acid molecules comprising nucleotide sequences encoding the amino acid sequence shown in SEQ ID NO:9, 11, 13, 15, or 18, or the nucleotide sequence set forth in SEQ ID NO:1, 2, 4, 6, 7, 8, 10, 12, 14, 16, or 17, as well as variants and fragments thereof.
Owner:BASF AGRICULTURAL SOLUTIONS SEED LLC
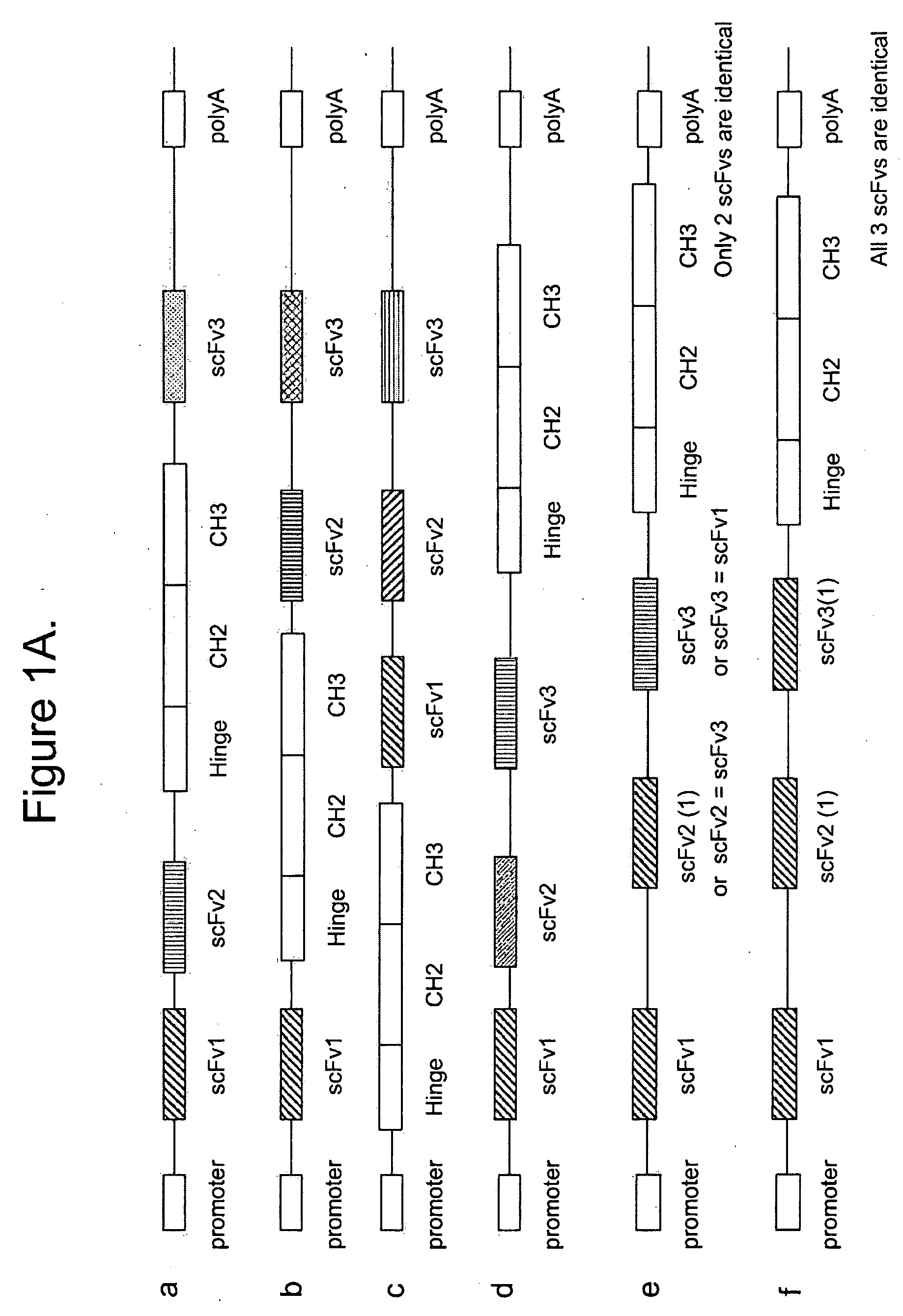
Multispecific epitope binding proteins and uses thereof
InactiveUS20090155275A1Stimulate immune responseEnhanced interactionAntipyreticAnalgesicsEpitopeDisease
The present invention relates to multispecific epitope binding proteins, methods of making, and uses thereof in the prevention, management, treatment or diagnosis of acute or chronic diseases.
Owner:MEDIMMUNE LLC
Antibody
InactiveUS20070274985A1Generate efficientlyEffective isolationAntibody mimetics/scaffoldsImmunoglobulins against animals/humansNatural antibodySingle-Chain Antibodies
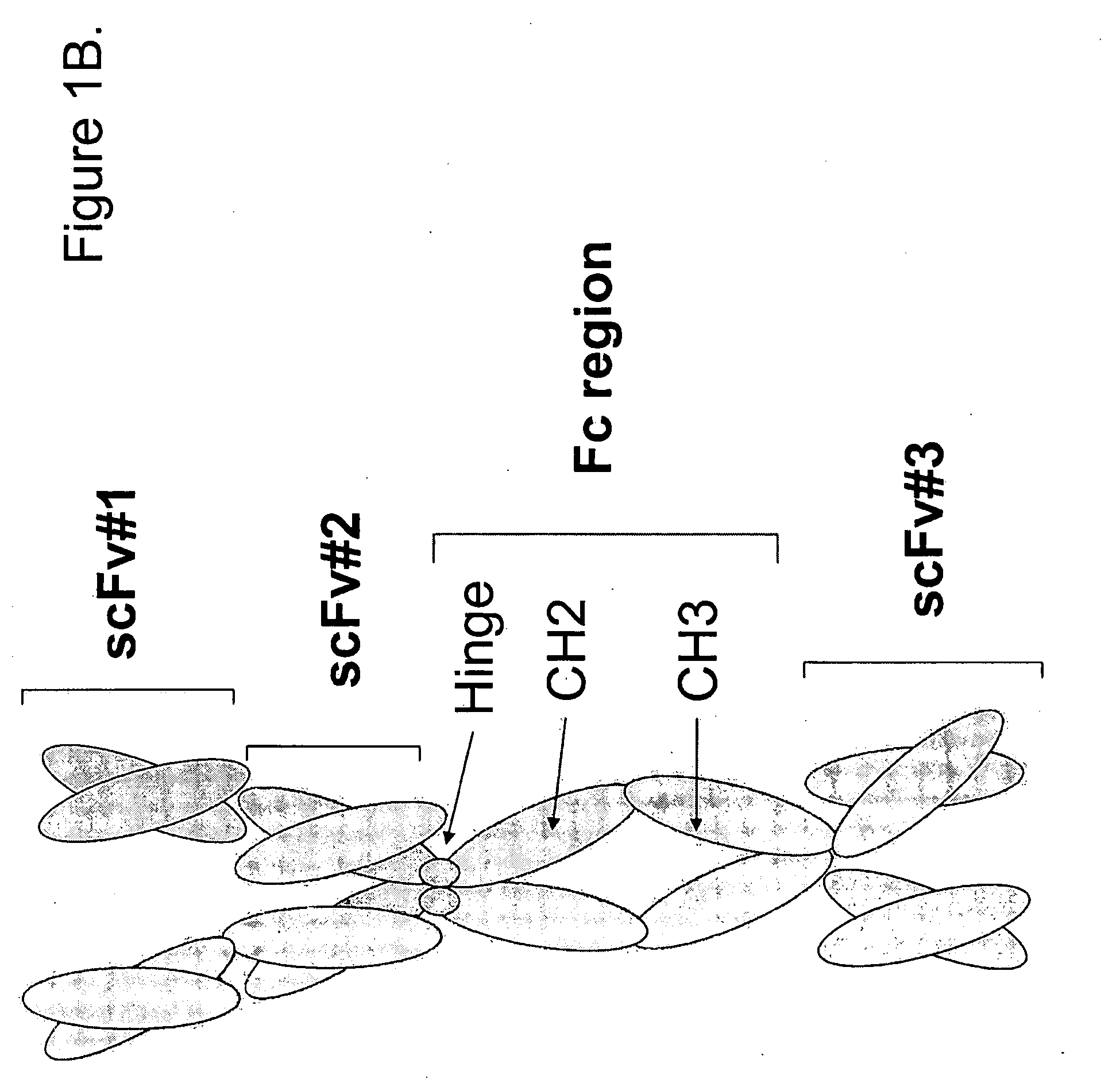
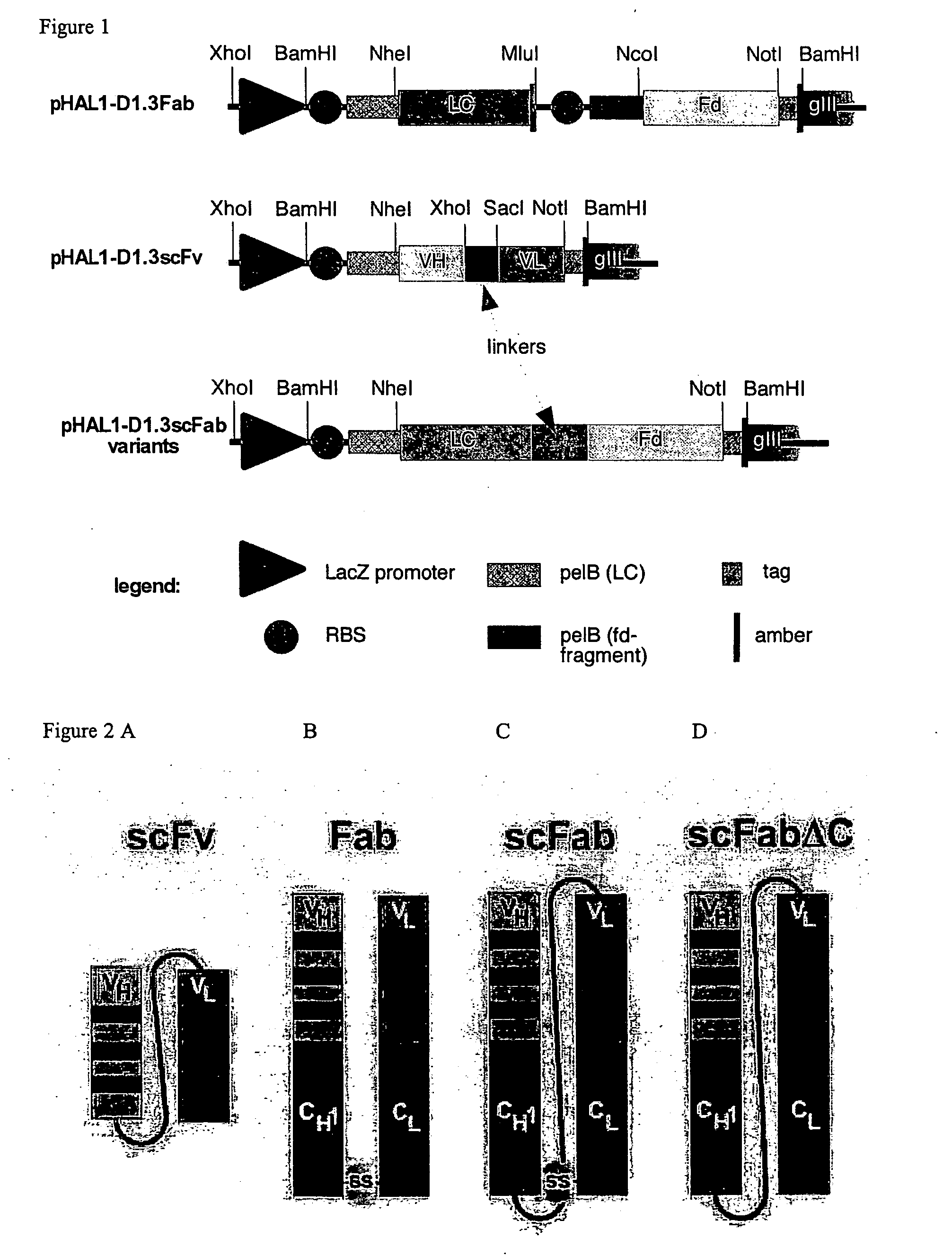
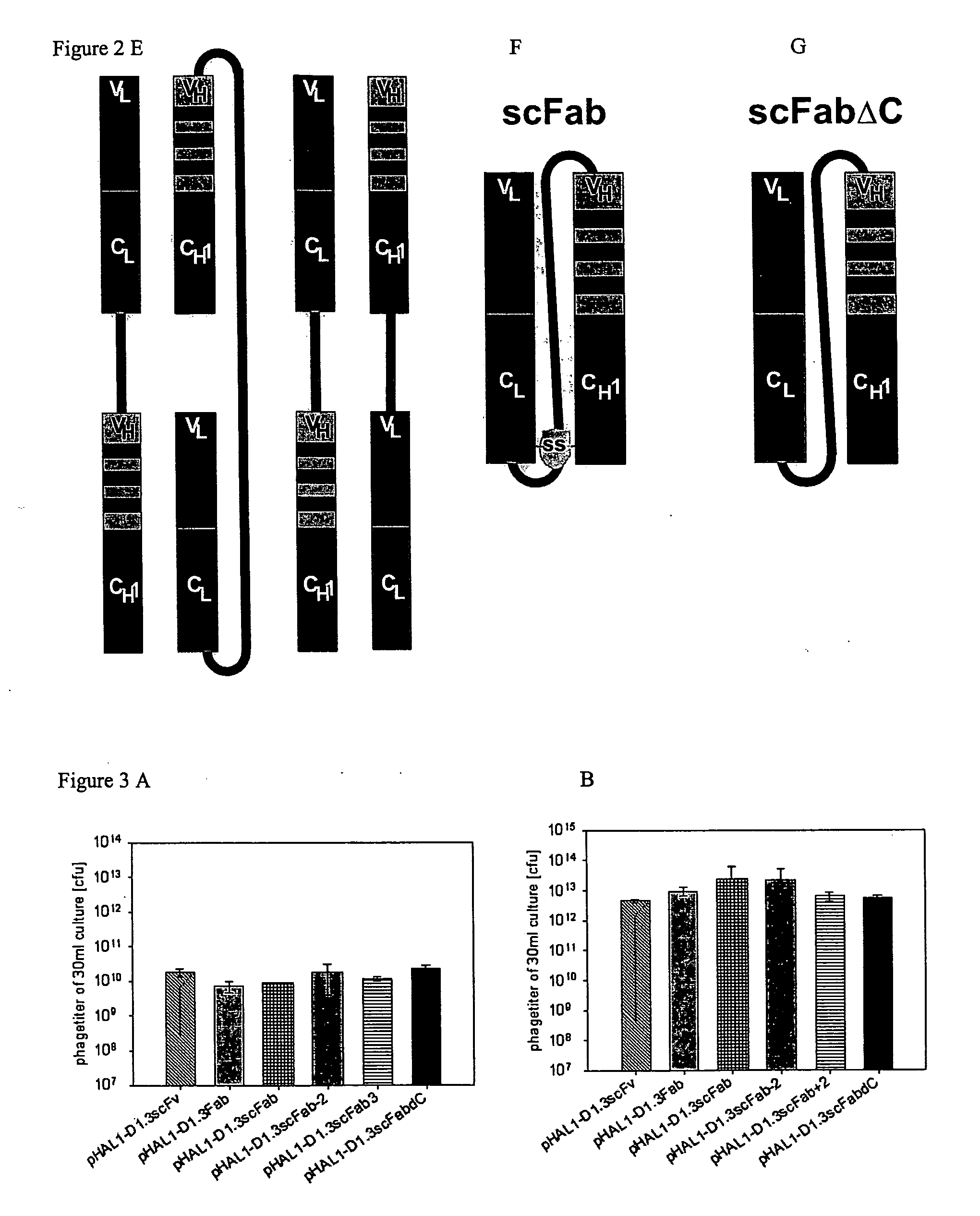
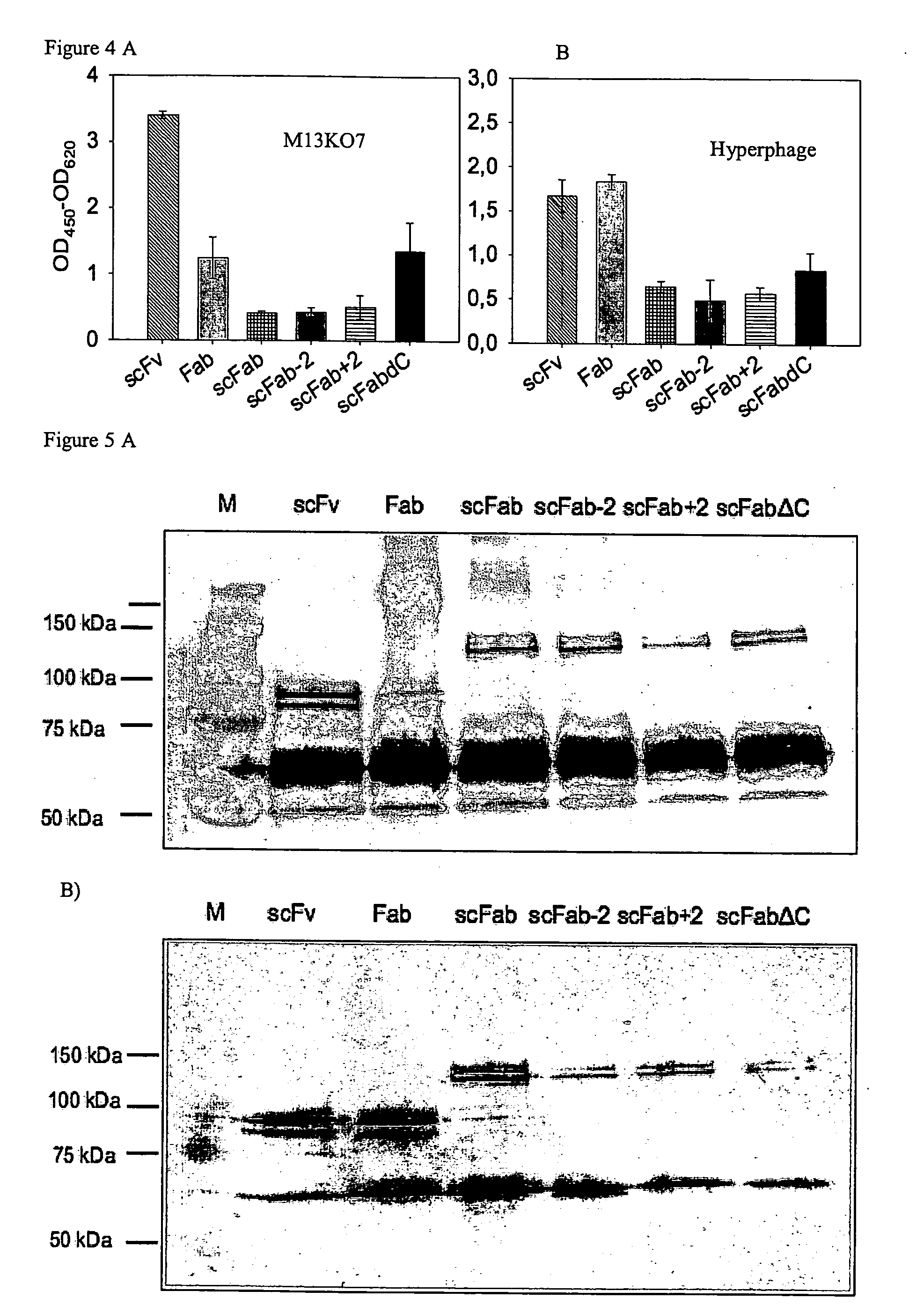
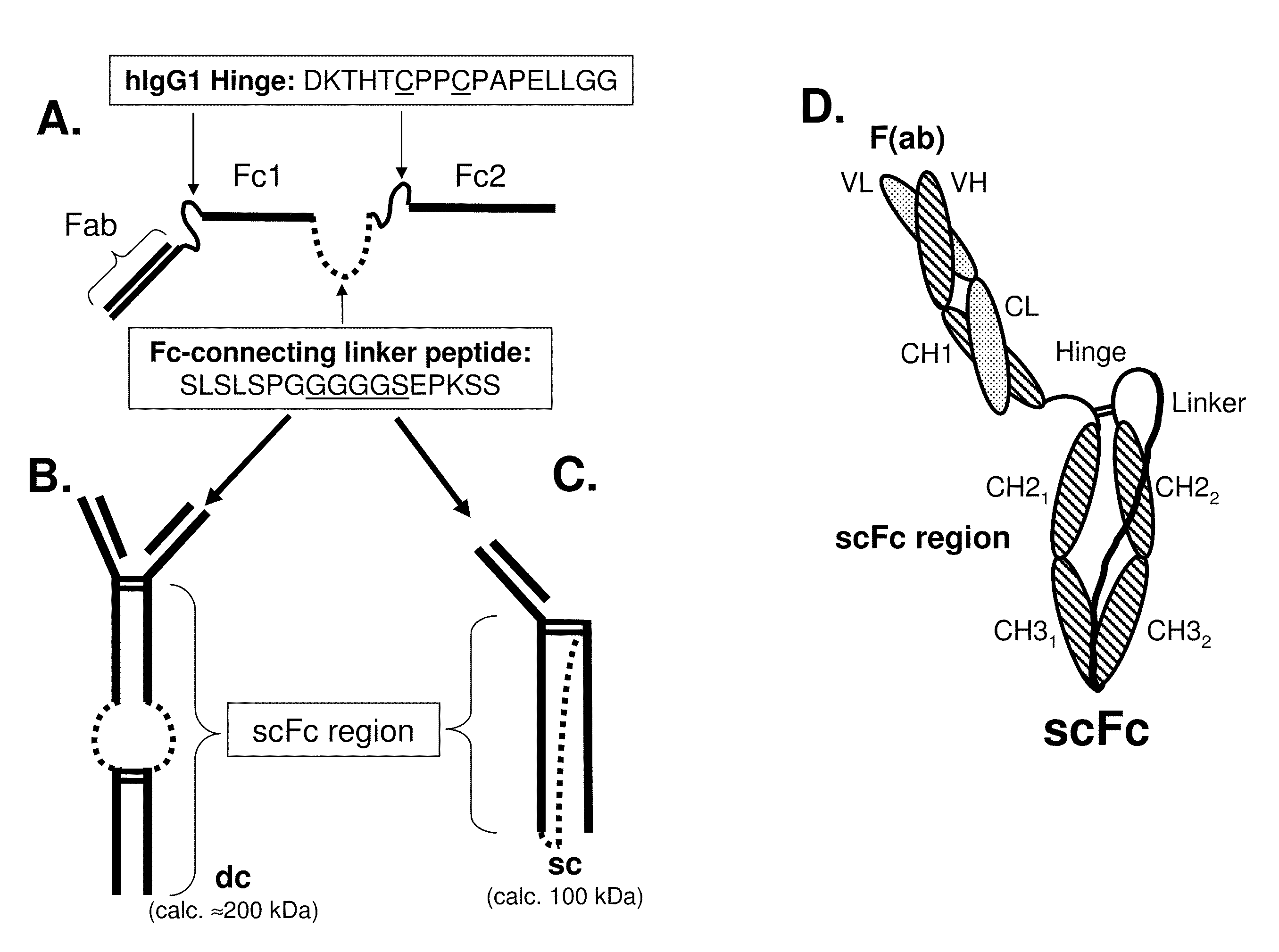
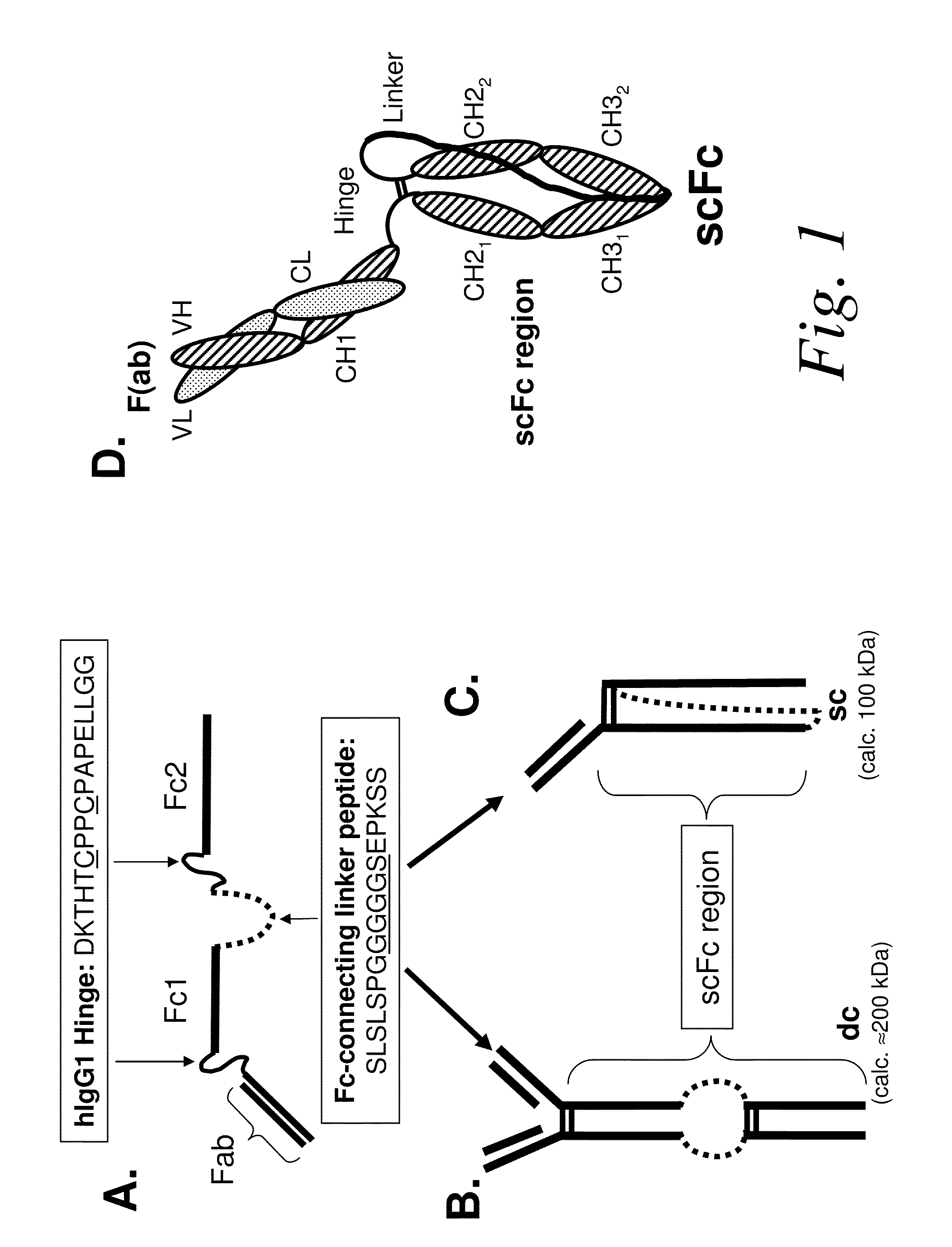
The present invention refers to synthetic antibody molecules which comprise domains from naturally occuring antibodies, e.g. domains derivable from IgG, preferably of human origin, in a novel arrangement. Single chain molecules are provided which are suitable for expression in micro-organisms in their active conformation, which single chain molecules generally comprise a VL domain, a CL domain, and a VH domain, a CH1 domain, linked by a linker arranged between VUCL and VH / CH1. Accordingly, these antibody molecules can be termed single chain Fabs (scFabs). These antibody molecules are single chain proteins, which can also be associated to dimers, including heteromeric antibodies, wherein at least two single chain antibody molecules are associated.
Owner:TECH UNIV BRAUNSCHWEIG
Single chain fc, methods of making and methods of treatment
InactiveUS20080260738A1Improve solubilityImprove stabilityAnimal cellsFungiAntigen bindingStereochemistry
The present invention relates generally to scFc molecules. The scFc molecules comprise at least two Fc regions and at least one linker, and can be produced in a variety of single chain configurations. The scFc molecules can further comprise at least one binding entity and / or at least one functional molecule. Binding entities can be fused to the scFc molecule in a variety of configurations. The present invention also relates generally to methods for making such molecules and methods for their use. The scFc molecules provided herein can be recombinantly produced. Also provided are monovalent forms of the scFc molecules that have an equivalent or superior ADCC and / or CDC response than do bivalent molecules targeting the same antigens. Provided herein are improved antigen binding compositions. Methods for using the scFc molecules of the present inventions are provided
Owner:ZYMOGENETICS INC
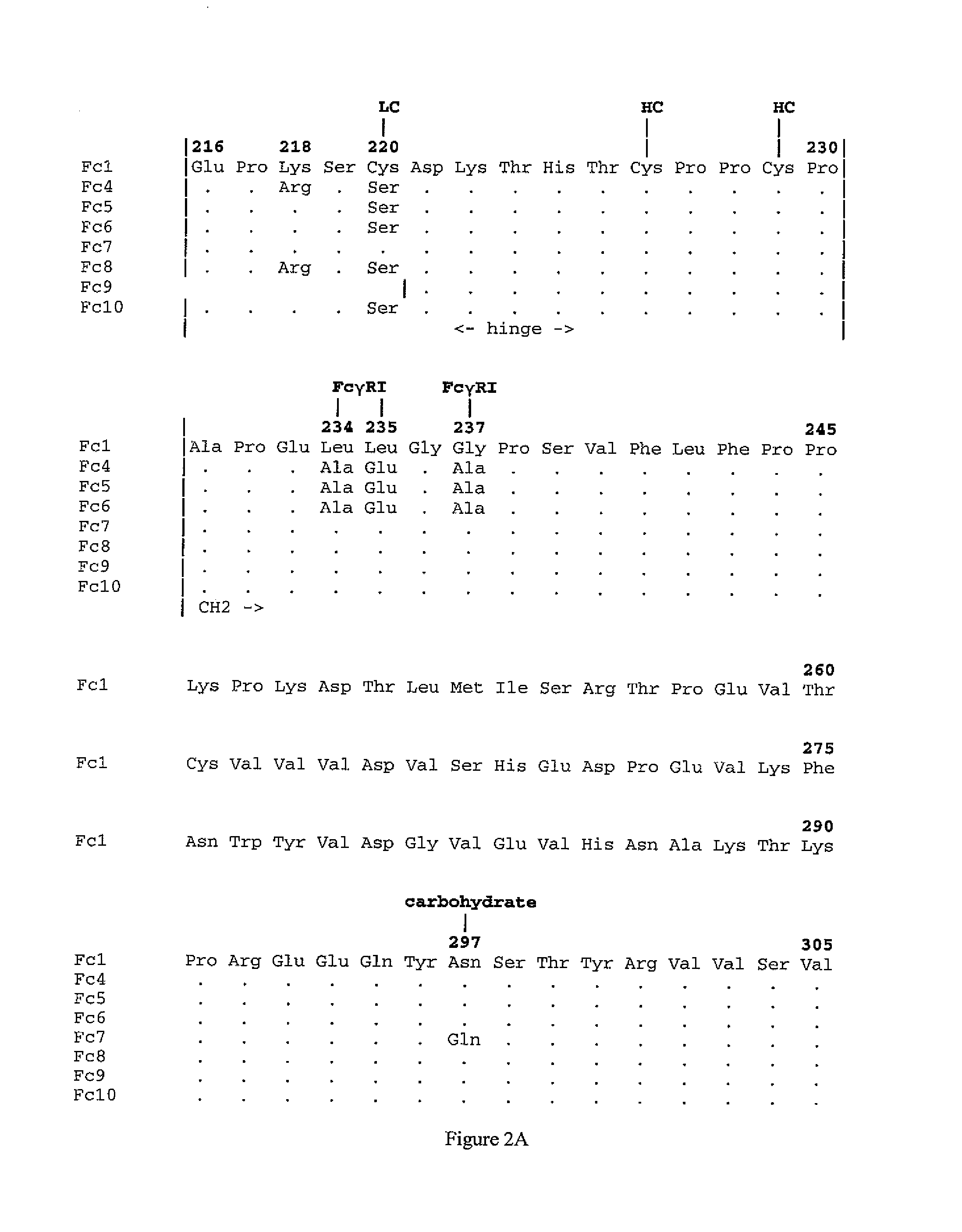
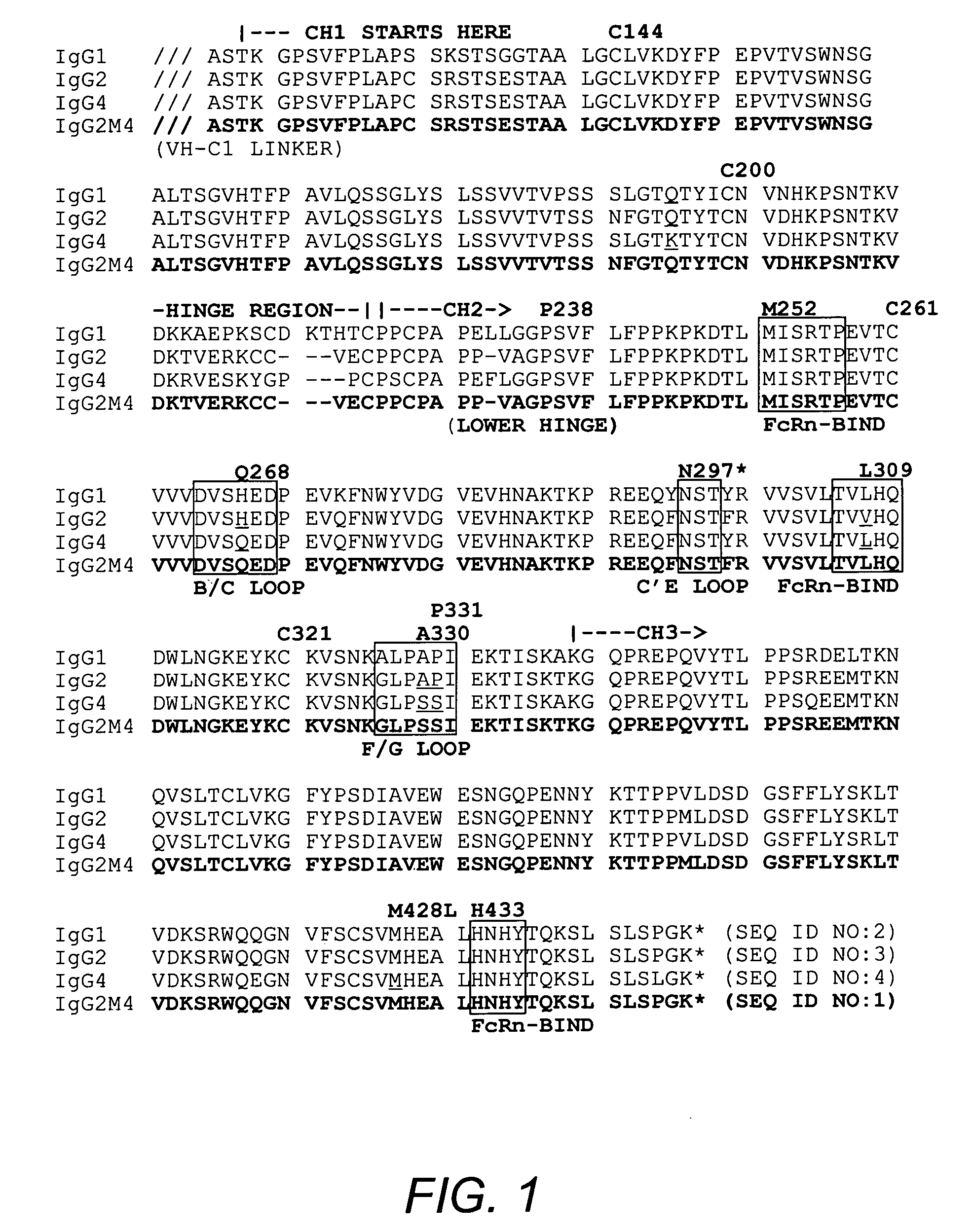
Non-immunostimulatory antibody and compositions containing the same
ActiveUS20070148167A1Immunoglobulins against animals/humansEnzymologyTherapeutic antibodyFc-Gamma Receptor
The present invention relates to a non-immunostimulatory antibody which lacks antibody-dependent cell-mediated cytotoxicity, Fc gamma receptor binding and complement-mediated cytotoxicity. In some embodiments, the antibody contains a modified immunoglobulin G2 (IgG2) Fc region with at least one substitution in the B / C loop, FcRn binding domain, and the F / G loop. The antibody of the invention is useful in the preparation of therapeutic antibodies and pharmaceutical compositions and kits containing the same.
Owner:MERCK SHARP & DOHME CORP
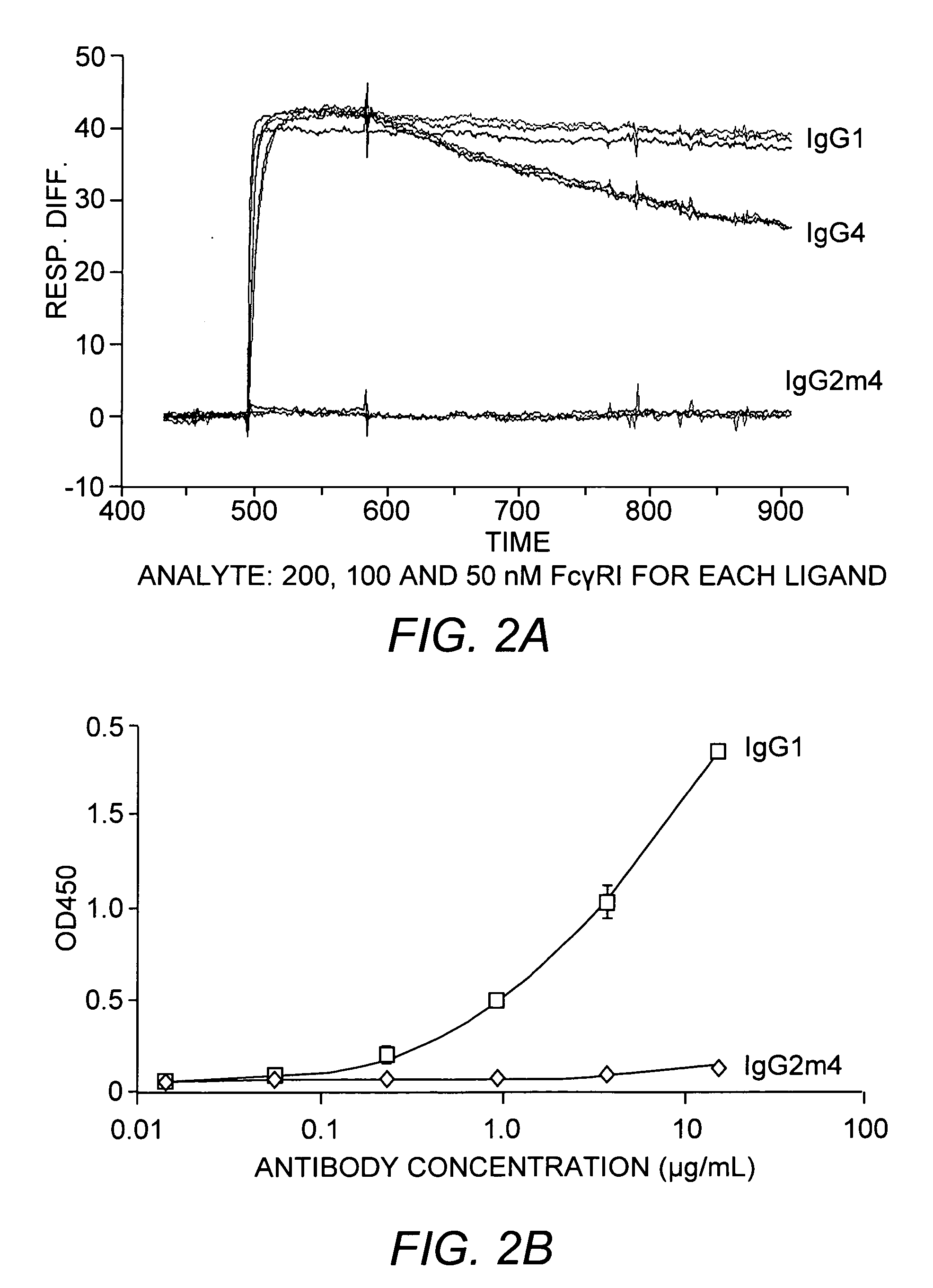
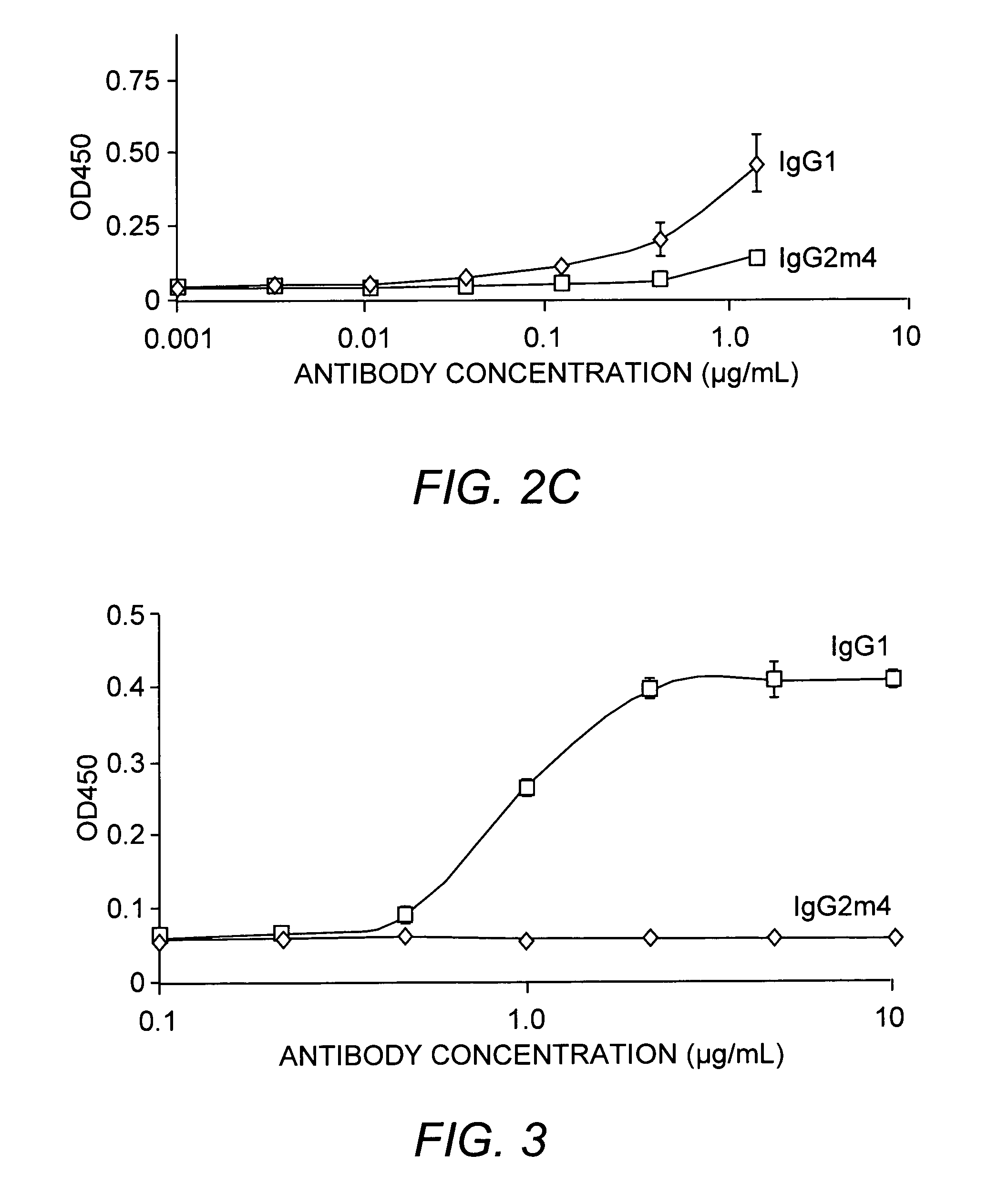
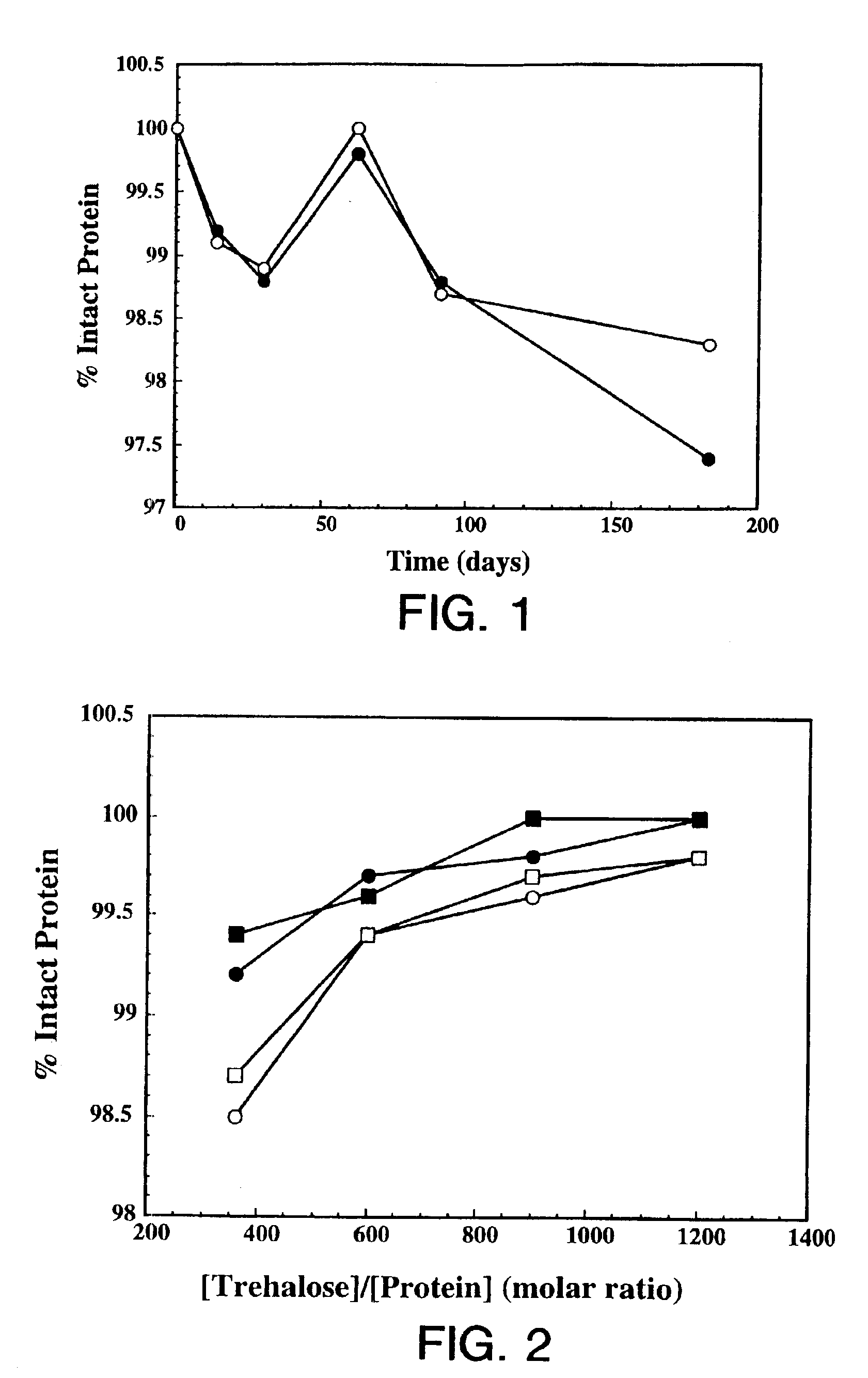
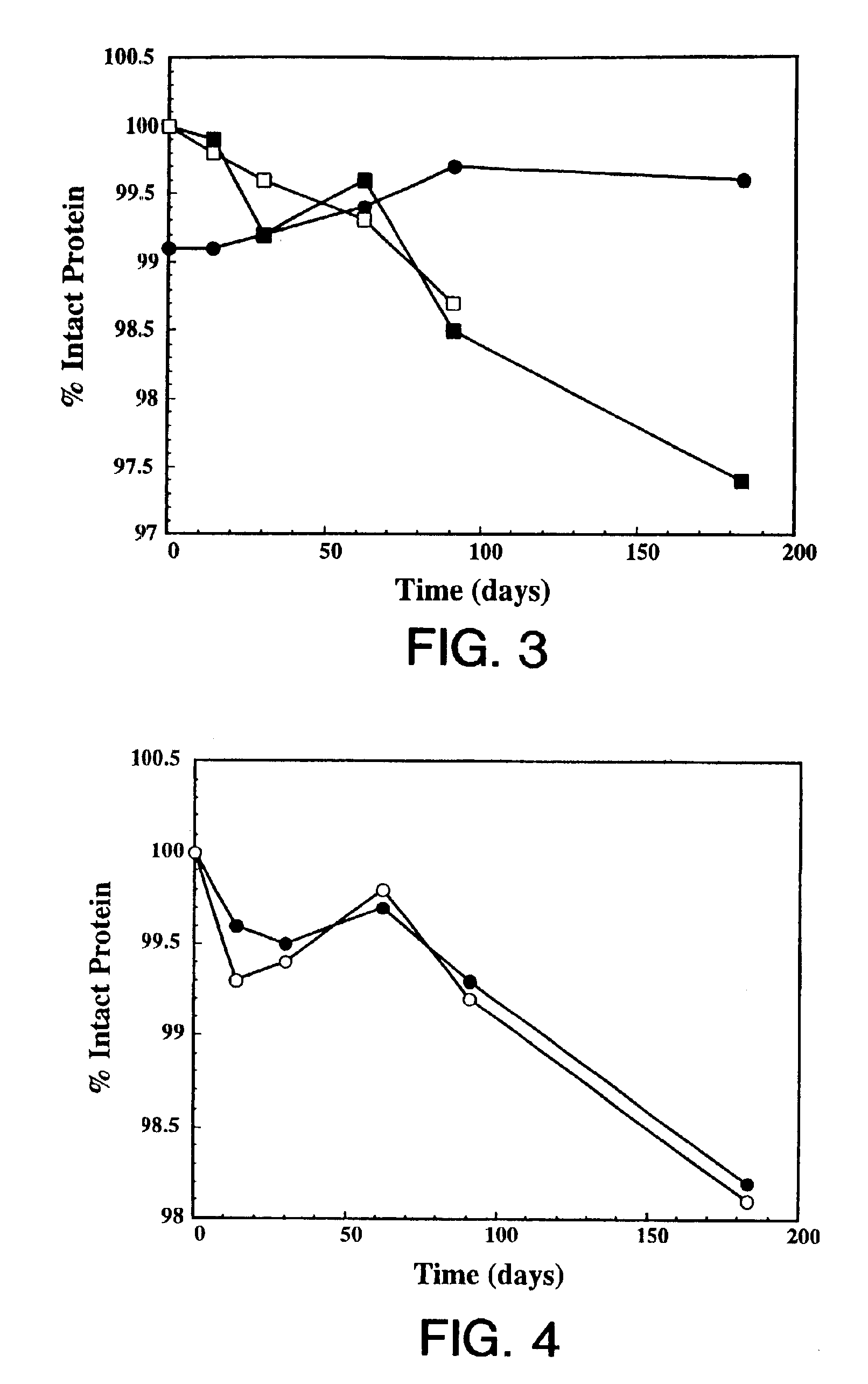
Protein formulation
InactiveUS7060268B2Reduce aggregationReduce formation of particulateBiocideOrganic active ingredientsDiluentHigh protein
A stable lyophilized protein formulation is described which can be reconstituted with a suitable diluent to generate a high protein concentration reconstituted formulation which is suitable for subcutaneous administration. For example, anti-IgE and anti-HER2 antibody formulations have been prepared by lyophilizing these antibodies in the presence of a lyoprotectant. The lyophilized mixture thus formed is reconstituted to a high protein concentration without apparent loss of stability of the protein.
Owner:GENENTECH INC
Hybrid immunoglobulins with moving parts
Hybrid immunoglobulins containing moving parts are provided as well as related compositions and methods of use and methods of production. In addition, analogous genetic devices are provided as well as related compositions and methods of use and methods of production.
Owner:BIOMOLECULAR HLDG LLC
Single-chain Fc (scFc) regions, binding polypeptides comprising same, and methods related thereto
InactiveUS20090252729A1Fine-tune such effector functionHigh expressionNervous disorderAntibody mimetics/scaffoldsDiseaseChemistry
The present invention features inter alia polypeptides comprising an Fc region comprising genetically-fused Fc moieties. In addition, the instant invention provides, e.g., methods for treating or preventing a disease or disorder in subject by administering the binding polypeptides of the invention to said subject.
Owner:BIOGEN MA INC
Serum albumin binding proteins with long half-lives
InactiveUS20070269422A1Increased serum half-lifeReduce dosing frequencyOrganic active ingredientsPeptide/protein ingredientsSerum igePrimate
The present invention relates to amino acid sequences that are capable of binding to serum albumin; to compounds, proteins and polypeptides comprising or essentially consisting of such amino acid sequences; to nucleic acids that encode such amino acid sequences, proteins or polypeptides; to compositions, and in particular pharmaceutical compositions, that comprise such amino acid sequences, proteins and polypeptides; and to uses of such amino acid sequences, proteins and polypeptides. Particularly, the amino acid sequences and compounds of the present invention bind to or otherwise associate with serum albumin in such a way that, when the amino acid sequence or compound is bound to or otherwise associated with a serum albumin molecule in a primate, it exhibits a serum half-life of at least 50% of the natural half-life of serum albumin in said primate.
Owner:ABLYNX NV
Fc Variants Having Decreased Affinity for FcyRllla
InactiveUS20070237767A1Weak affinityFunction increaseImmunoglobulins against animals/humansAntibody ingredientsChemistry
Owner:XENCOR INC
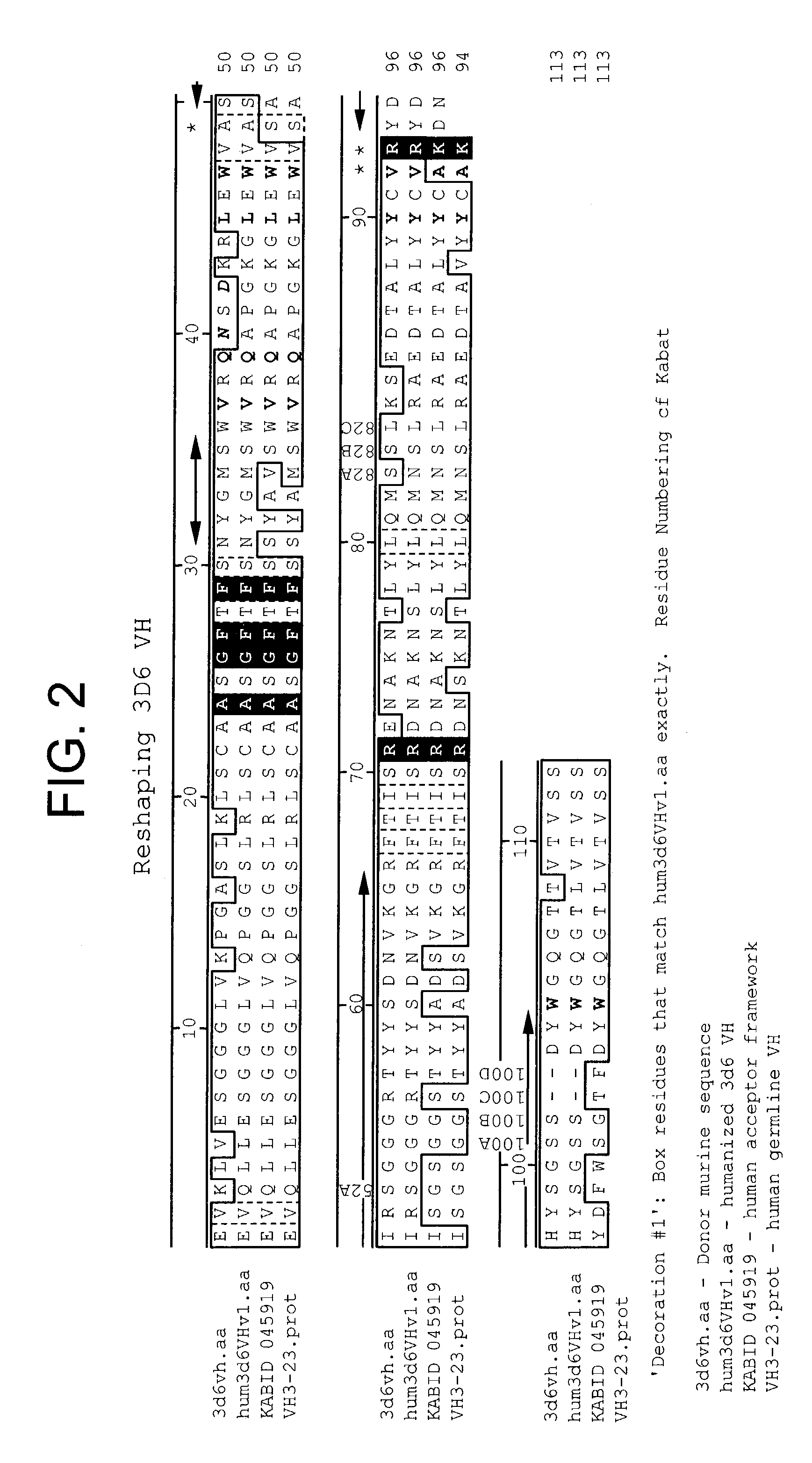
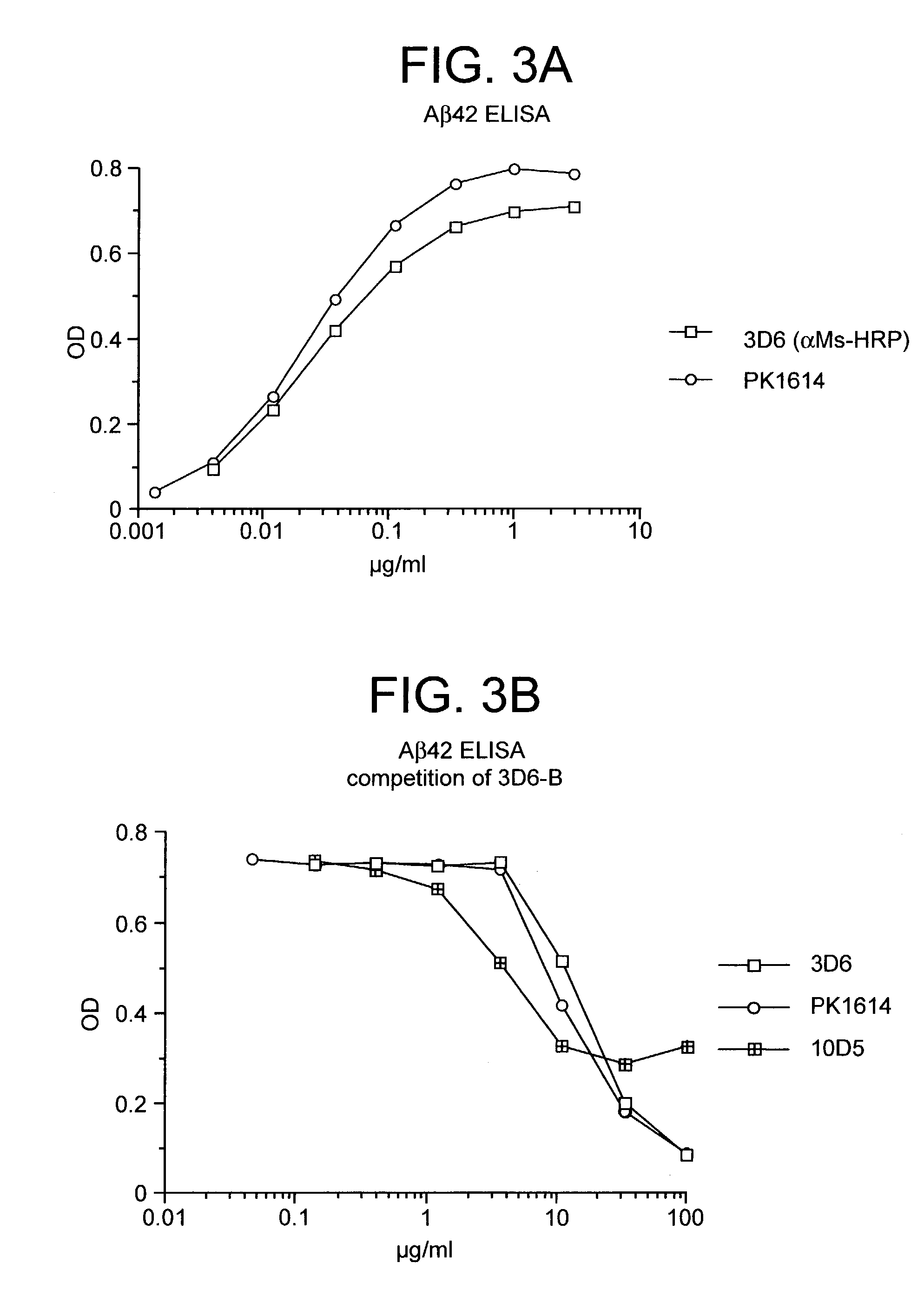
Humanized antibodies that sequester abeta peptide
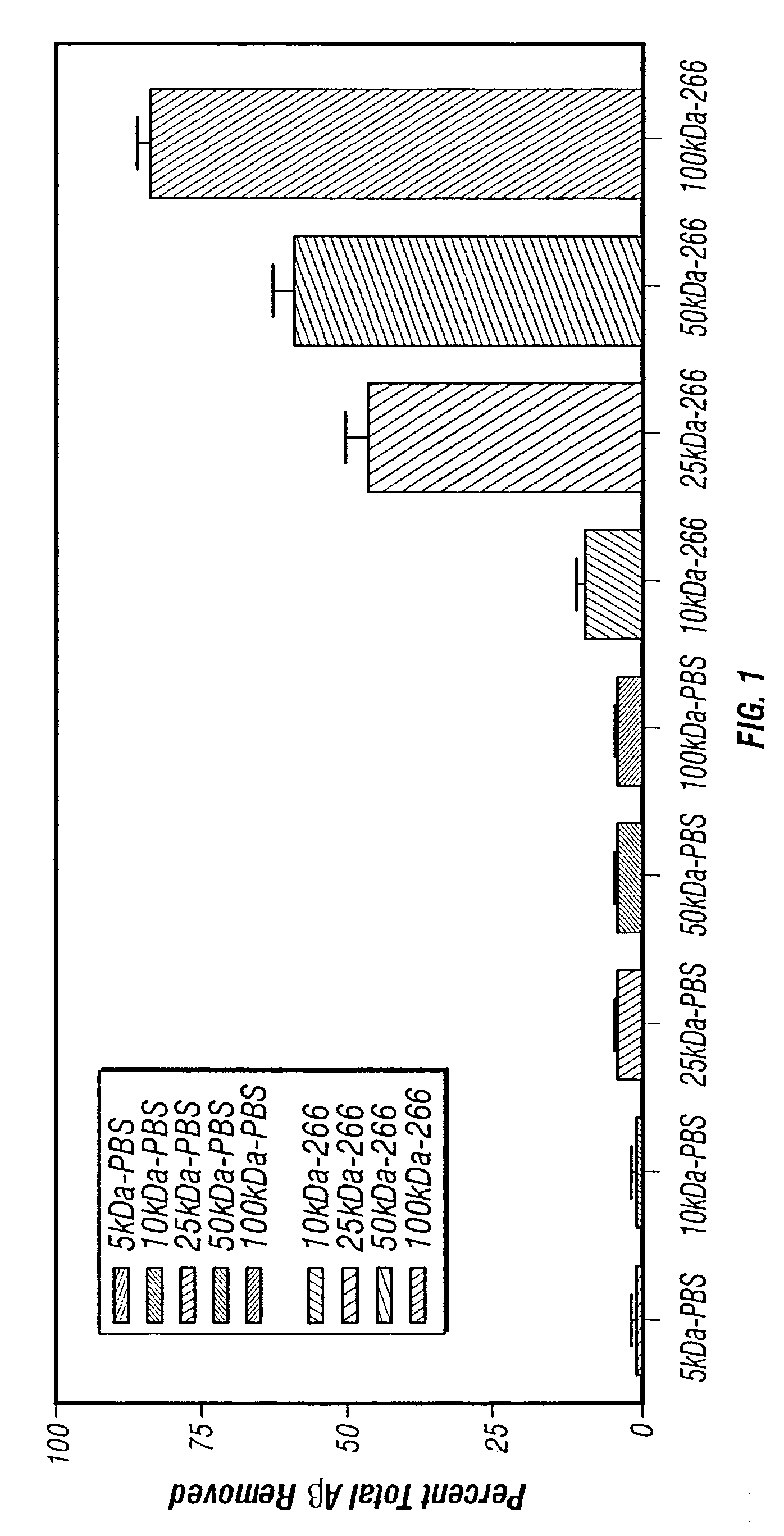
A method to treat conditions characterized by formation of amyloid plaques both prophylactically and therapeutically is described. The method employs humanized antibodies which sequester soluble Aβ peptide from human biological fluids or which preferably specifically bind an epitope contained within position 13–28 of the amyloid beta peptide Aβ.
Owner:ELI LILLY & CO +1
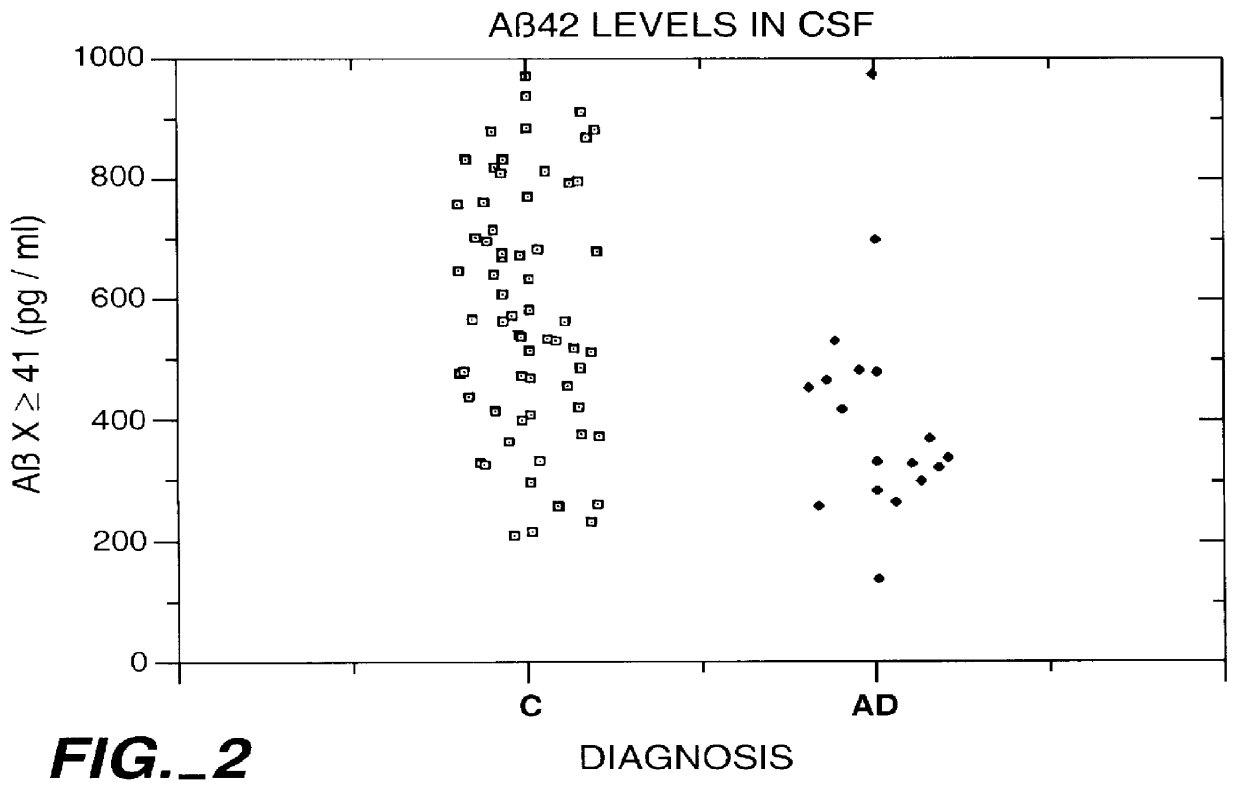
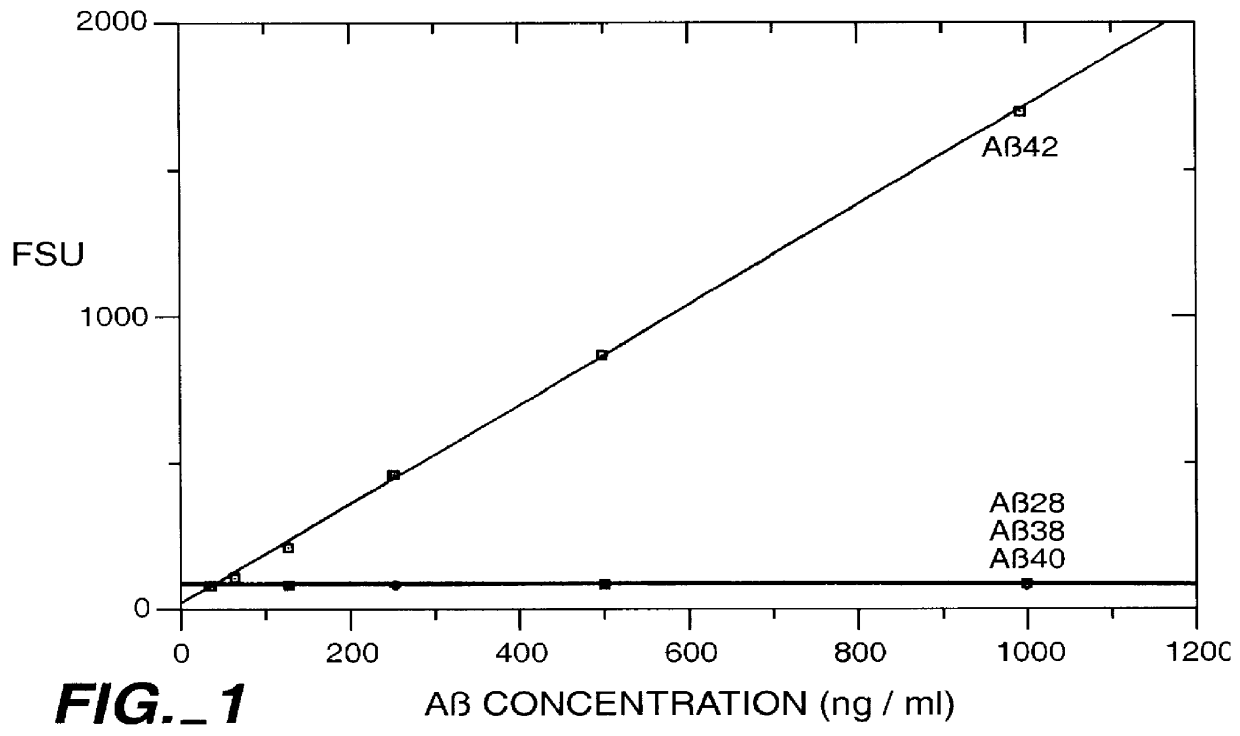
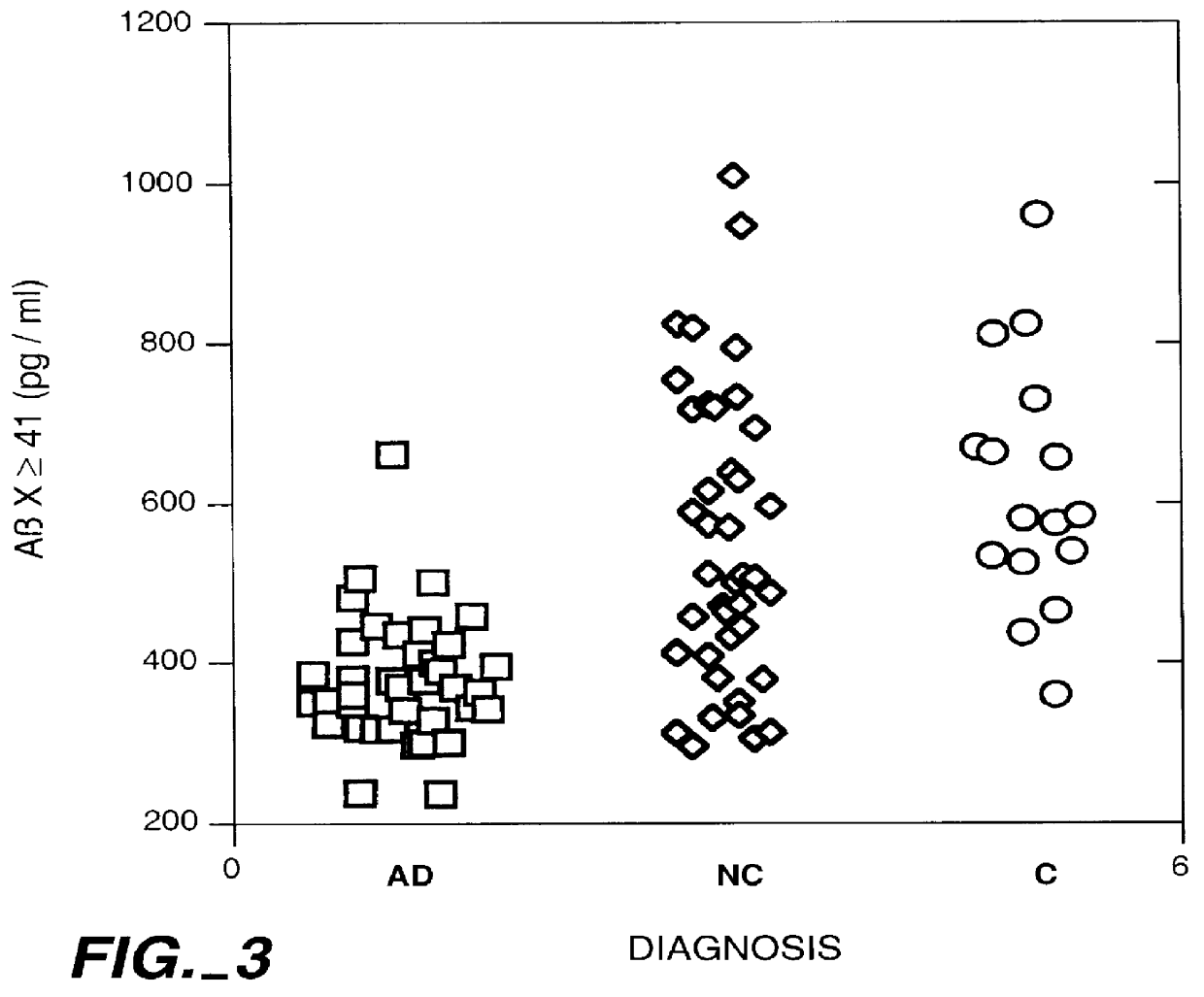
Methods for aiding in the diagnosis of Alzheimer's disease by measuring amyloid- beta peptide (x->/=41)
InactiveUS6114133AImmunoglobulins against animals/humansDisease diagnosisAlzheimer SyndromeDisease cause
This invention provides methods useful in aiding in the diagnosis of Alzheimer's disease. The methods involve measuring the amount of amyloid- beta peptide (x-> / =41) in the cerebrospinal fluid of a patient. High levels of the peptide generally are inconsistent with a diagnosis of Alzheimer's. Low levels of the peptide are consistent with the disease and, with other tests, can provide a positive diagnosis.
Owner:ELAN PHARM INC
Antibody purification
Owner:ABBVIE BIOTECHNOLOGY LTD
Fc Variants Having Decreased Affinity for FcyRlla
InactiveUS20070243188A1Weak affinityFunction increaseImmunoglobulins against animals/humansAntibody ingredientsChemistry
Owner:XENCOR INC
Fc Variants Having Increased Affinity for FcyRl
InactiveUS20070237765A1Weak affinityFunction increaseImmunoglobulins against animals/humansAntibody ingredientsChemistry
Owner:XENCOR INC
Fc Variants Having Increased Affinity for FcyRllla
InactiveUS20070237766A1Weak affinityFunction increaseImmunoglobulins against animals/humansAntibody ingredientsChemistry
Owner:XENCOR INC
Methods for refolding of recombinant antibodies
ActiveUS20060194280A1Efficient and economic productionImproved pharmaceuticalImmunological disordersFermentationRecombinant antibodiesCoupling reagent
The present invention is generally directed to methods of producing an increase in the enrichment or recovery of preferred forms of IgG proteins. More particularly, the invention relates to subjecting preparations of such recombinant IgG proteins with a reduction / oxidation coupling reagent and optionally a chaotropic agent.
Owner:AMGEN INC
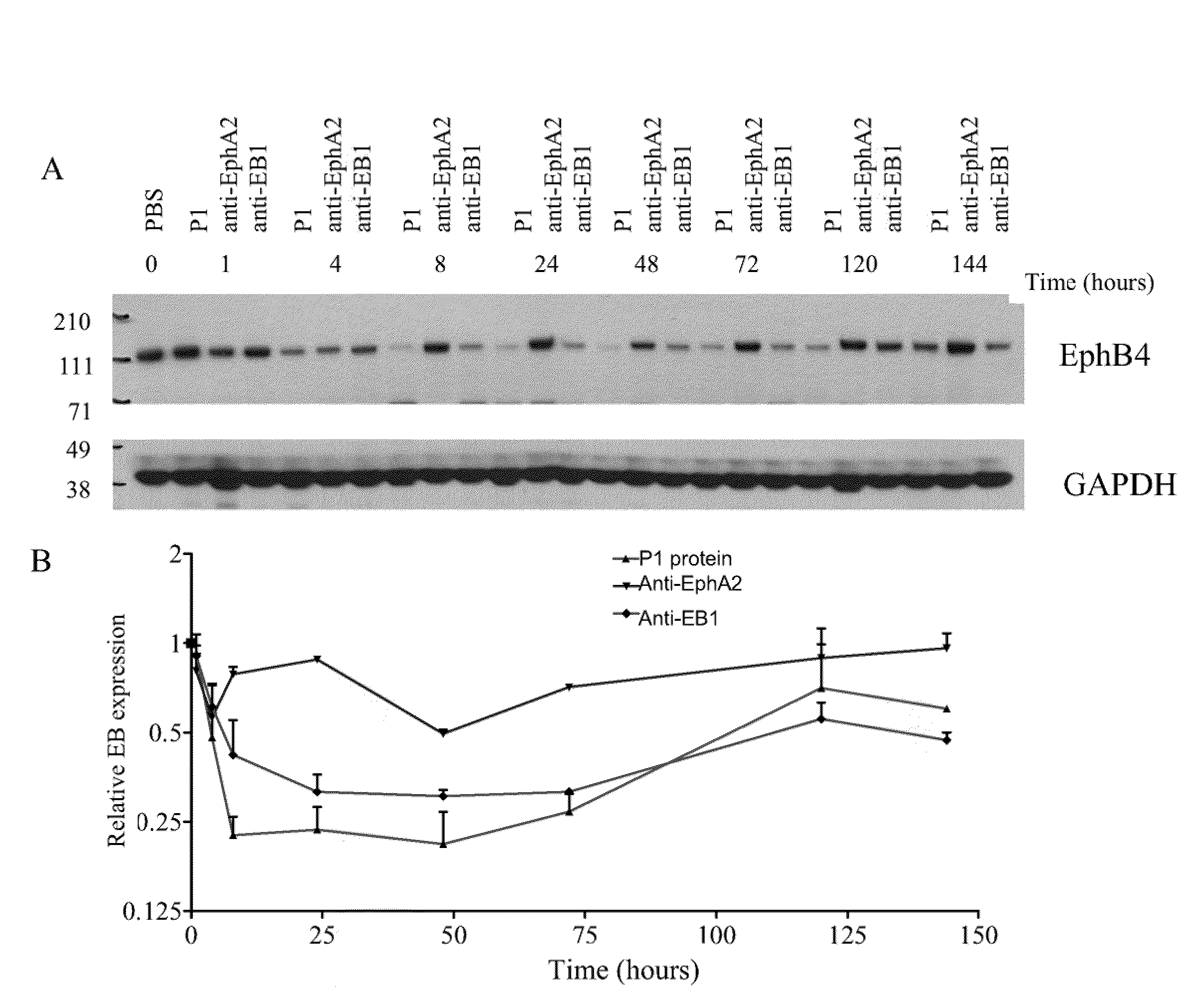
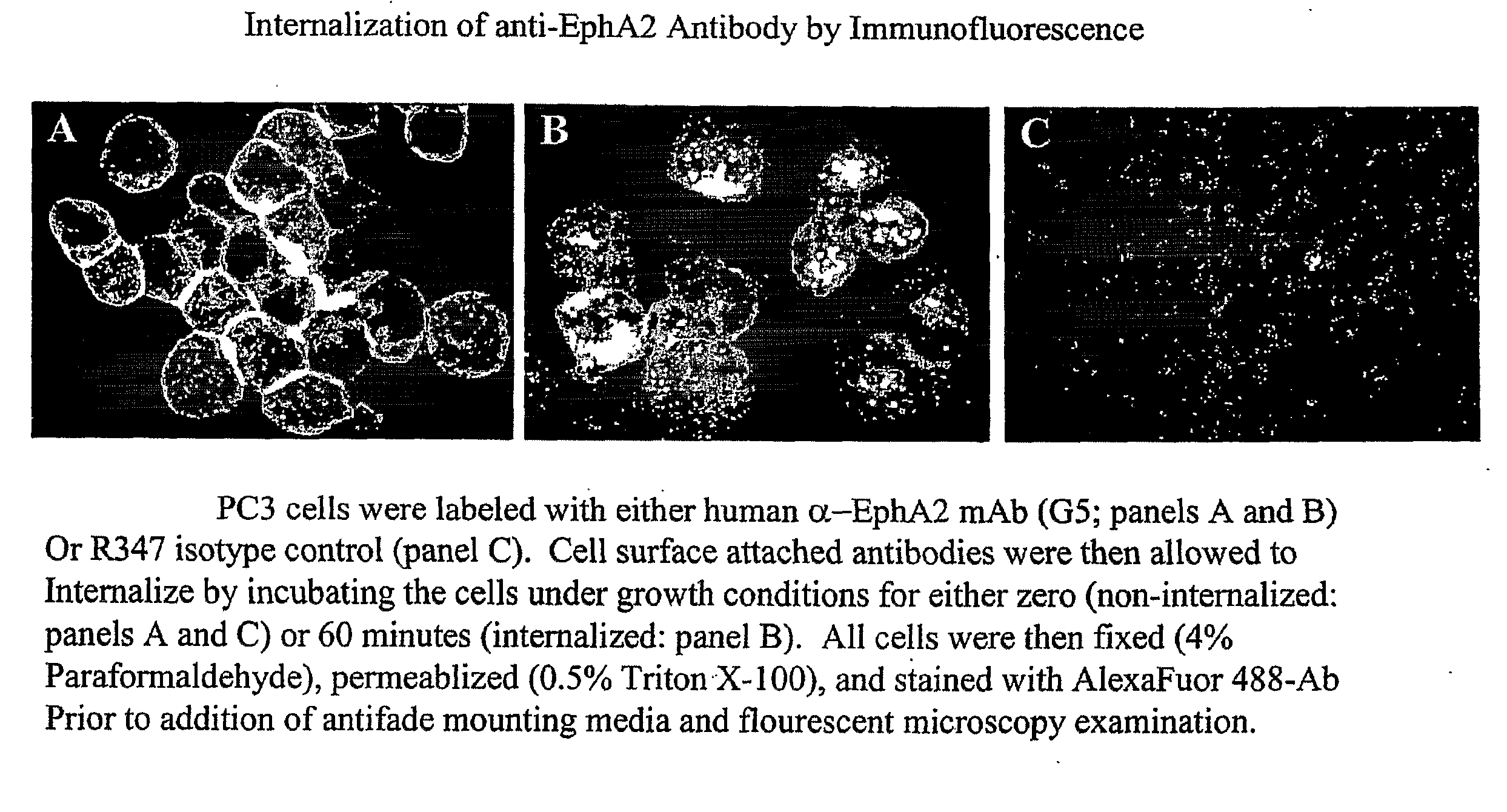
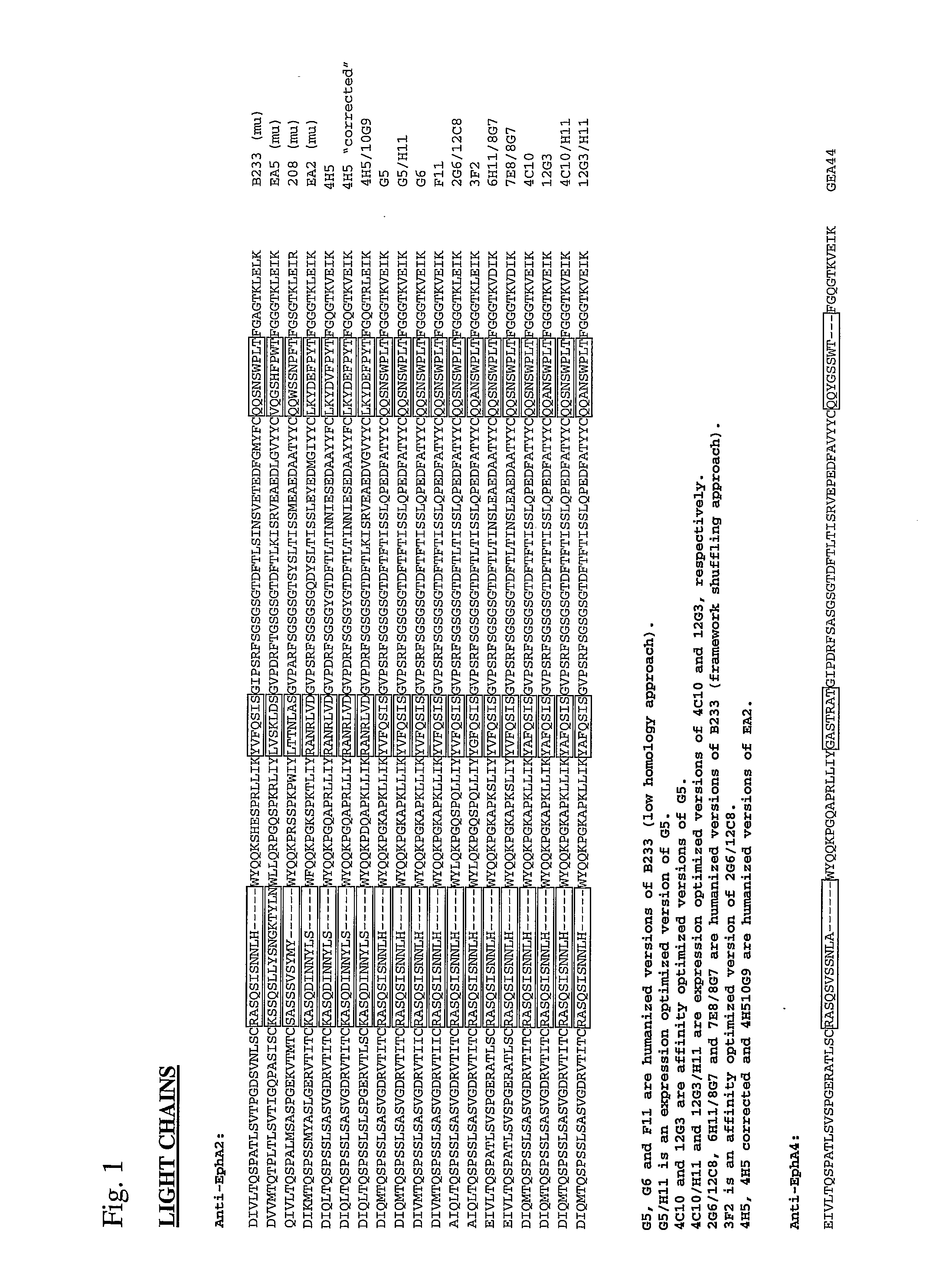
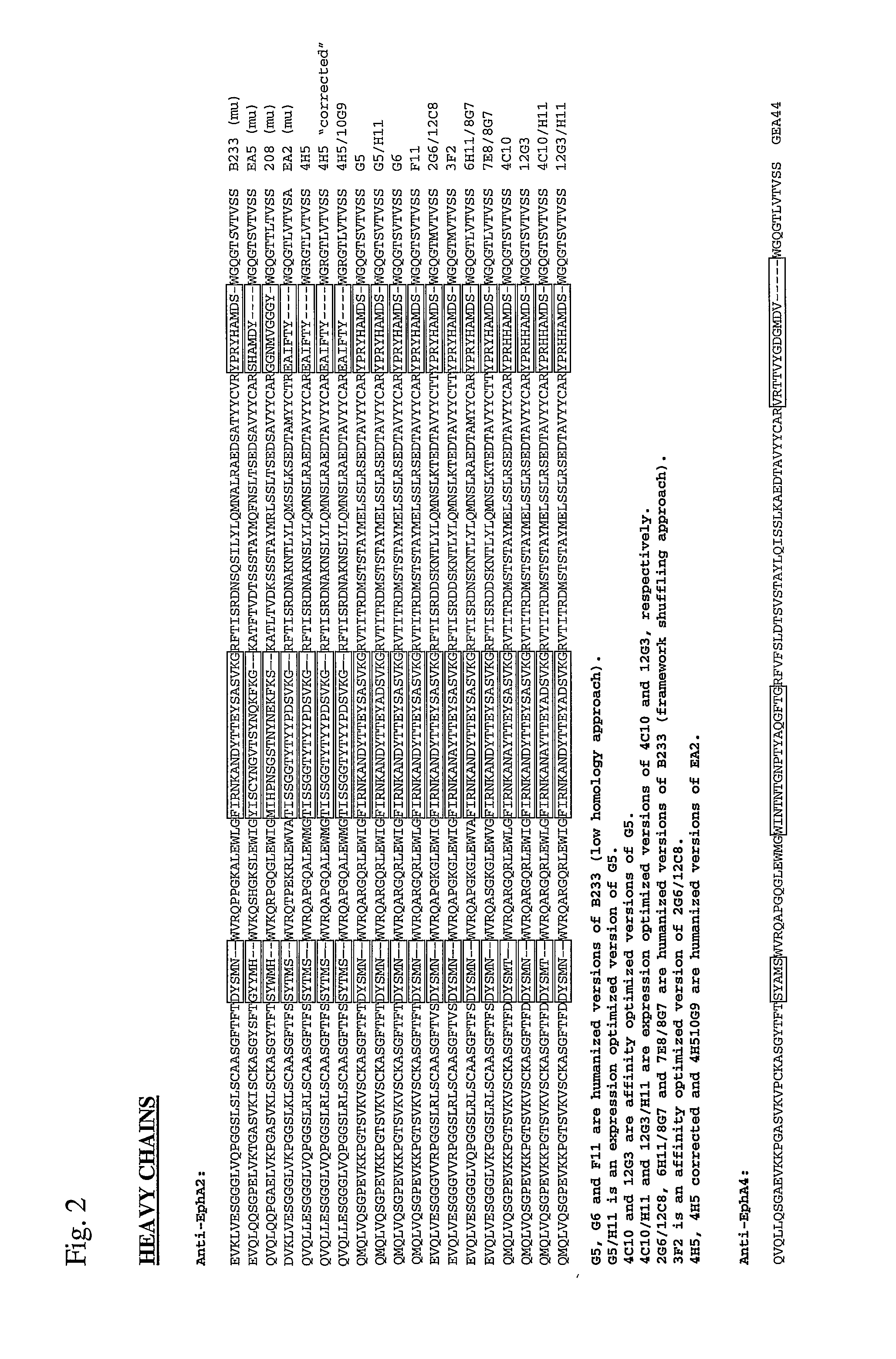
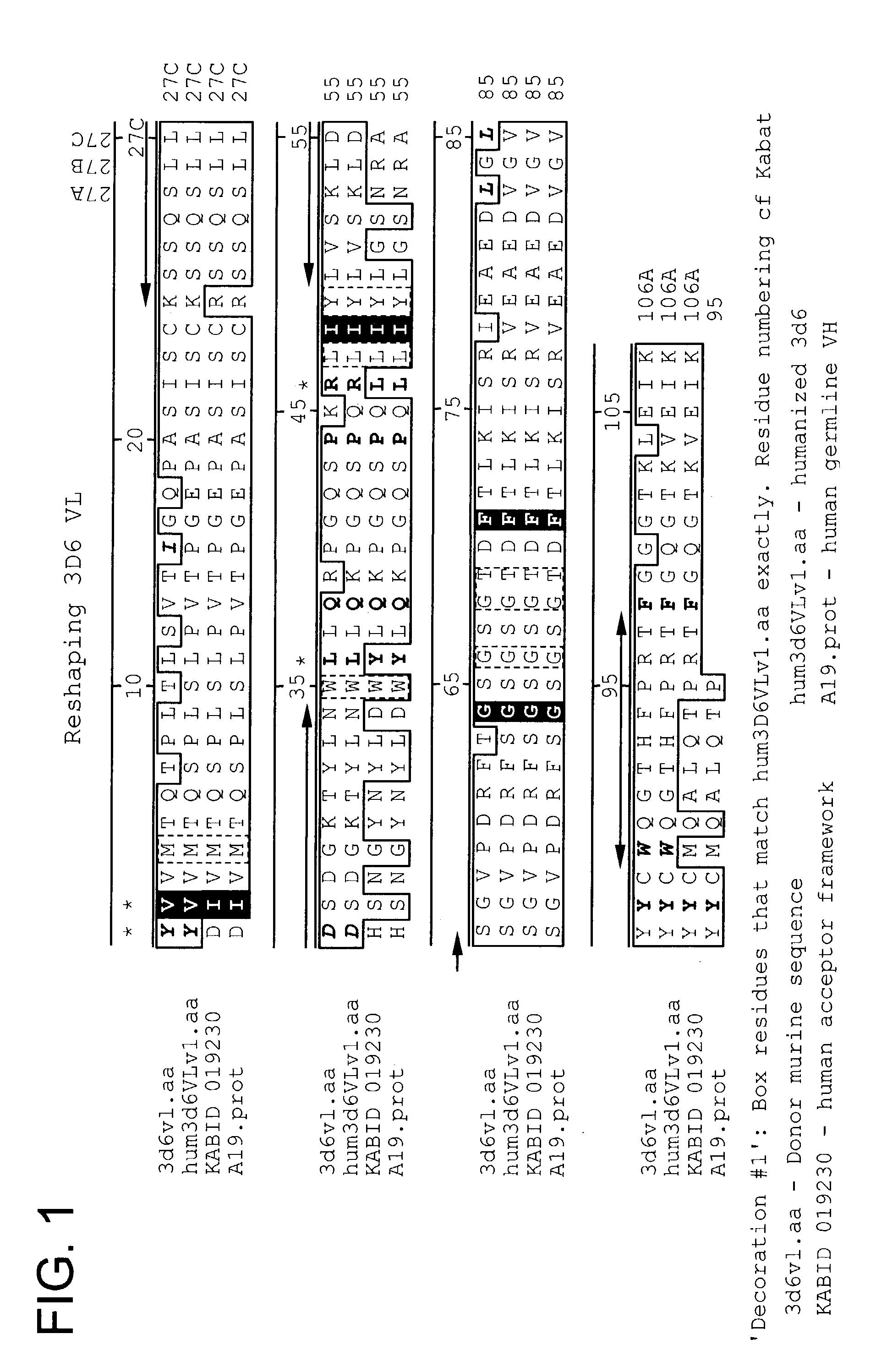
Toxin conjugated eph receptor antibodies
InactiveUS20090304721A1Prolong half-life in vivoIncrease local concentrationAnimal cellsImmunoglobulins against animals/humansCancer cellToxin Conjugates
Owner:SEATTLE GENETICS INC +1
Enrichment of circulating fetal DNA
InactiveUS20070243549A1High accuracy of resultsMicrobiological testing/measurementImmunoglobulins against animals/humansNon invasiveMolecular diagnostics
A non-invasive screening or diagnostic method for determining the likelihood of a fetus with a genetic abnormality or a potential pregnancy complication, which utilizes a liquid blood sample from a pregnant woman. Antibodies specific to a section of histone 3.1 which is exposed to a far greater extent in chromatin of fetal origin than in chromatin of maternal origin are used to sequester and isolate such fetal nucleosomes including the associated fetal DNA. Following isolation / enrichment of such fetal DNA, genetic analysis is carried out using known molecular diagnostics.
Owner:BAYLOR COLLEGE OF MEDICINE
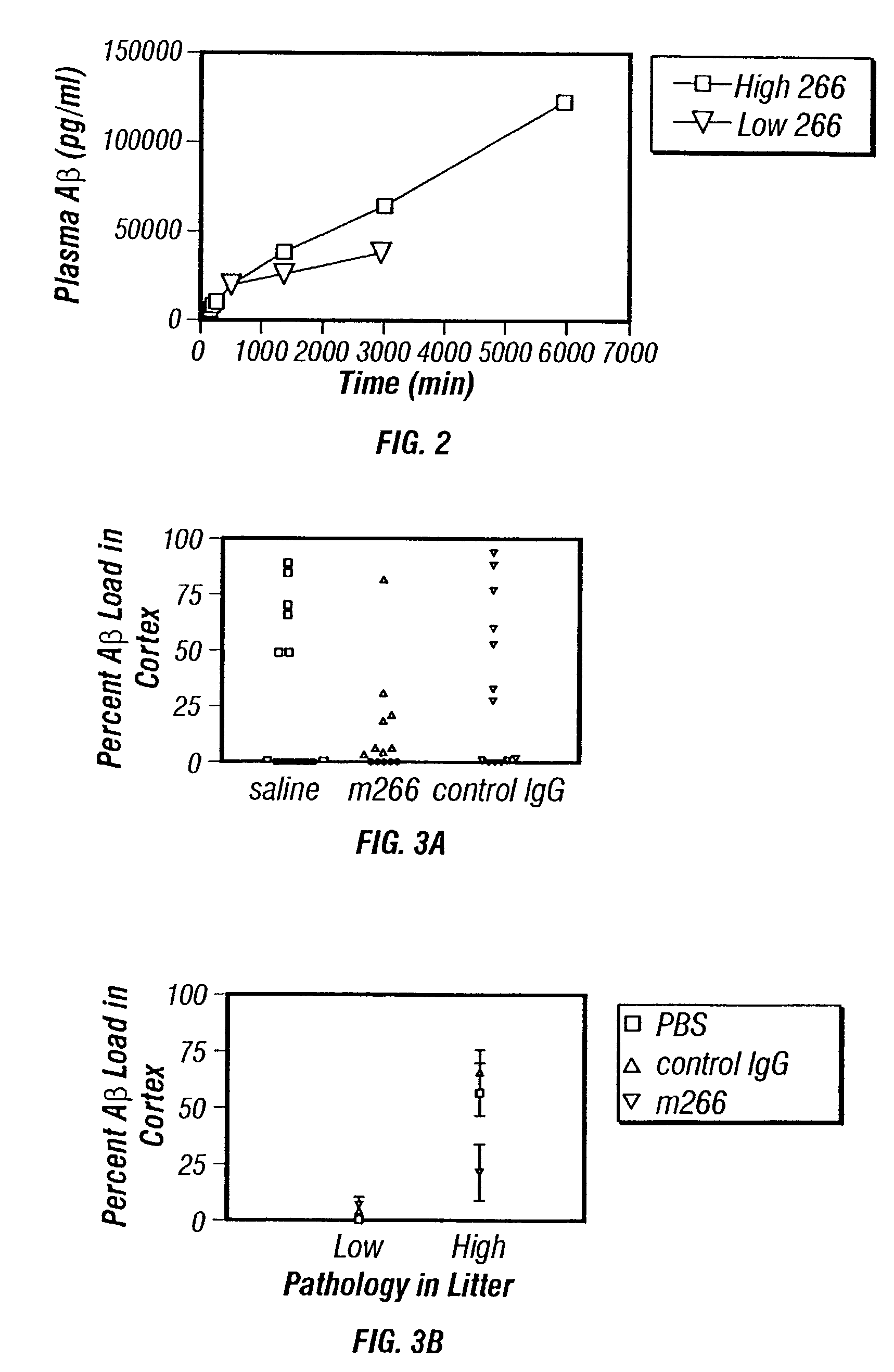
Humanized antibodies that recognize beta amyloid peptide
InactiveUS7179892B2Reduce the burden onReducing the neuritic dystrophyNervous disorderGenetically modified cellsHumanized antibodyΒ amyloid peptide
The invention provides improved agents and methods for treatment of diseases associated with amyloid deposits of Aβ in the brain of a patient. Preferred agents include humanized antibodies.
Owner:WYETH LLC +1
Polyclonal antibody composition for treating allergy
InactiveUS6849259B2Efficient removalPotential clinical advantageImmunoglobulins against animals/humansImmunoglobulins against plantsMicrosphereBULK ACTIVE INGREDIENT
A pharmaceutical composition for treating allergy is described. The composition comprises as an active ingredient a recombinant polyclonal antibody or a mixture of different monoclonal antibodies capable of reacting with or binding to an allergen together with one or more pharmaceutically acceptable excipients. The composition may be used topically as a solution, dispersion, powder, or in the form of microspheres. The polyclonal antibody is preferably a recombinant polyclonal antibody produced by phage display technology. The pairing of specific immunoglobulin variable region light chain and heavy chain maintained from the original polyclonal immune response or selected by panning using the allergen in question is preferably maintained by bulk transfer of the pairs into an expression vector.
Owner:SYMPHOGEN AS
Rationally Designed, Synthetic Antibody Libraries and Uses Therefor
ActiveUS20090181855A1Peptide librariesImmunoglobulins against animals/humansPolynucleotideSynthetic antibody
The present invention overcomes the inadequacies inherent in the known methods for generating libraries of antibody-encoding polynucleotides by specifically designing the libraries with directed sequence and length diversity. The libraries are designed to reflect the preimmune repertoire naturally created by the human immune system and are based on rational design informed by examination of publicly available databases of human antibody sequences.
Owner:ADIMAB LLC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com