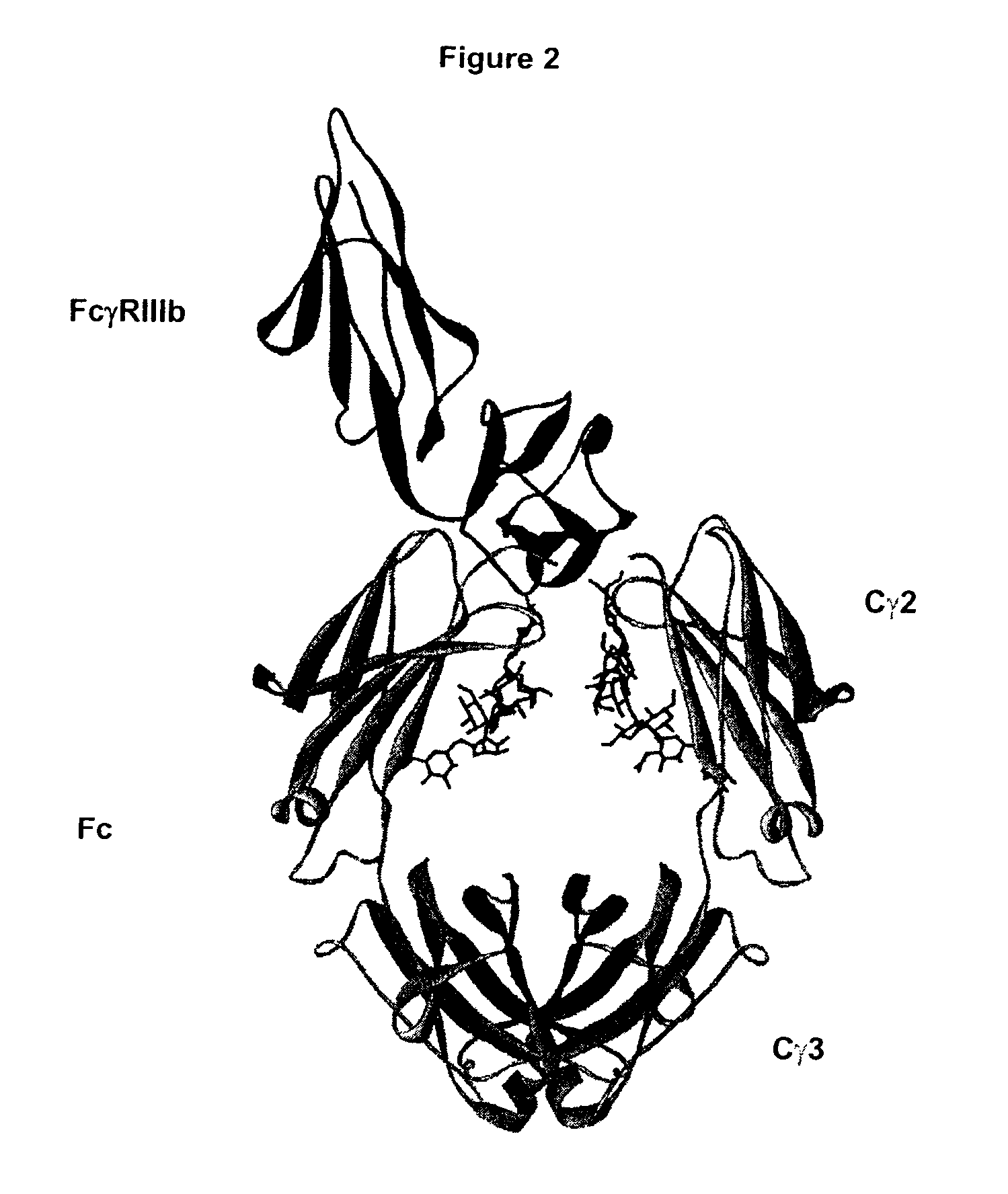
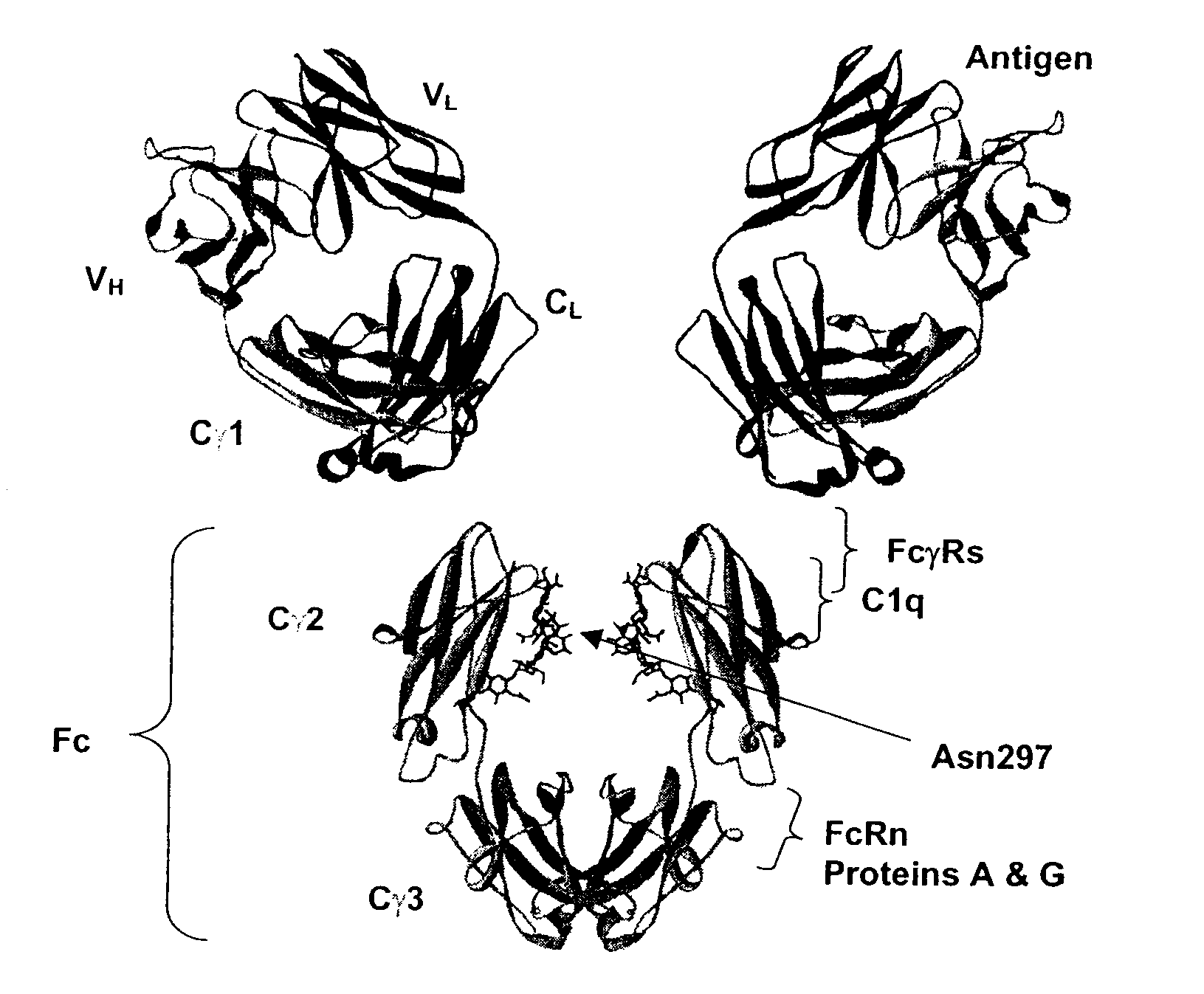
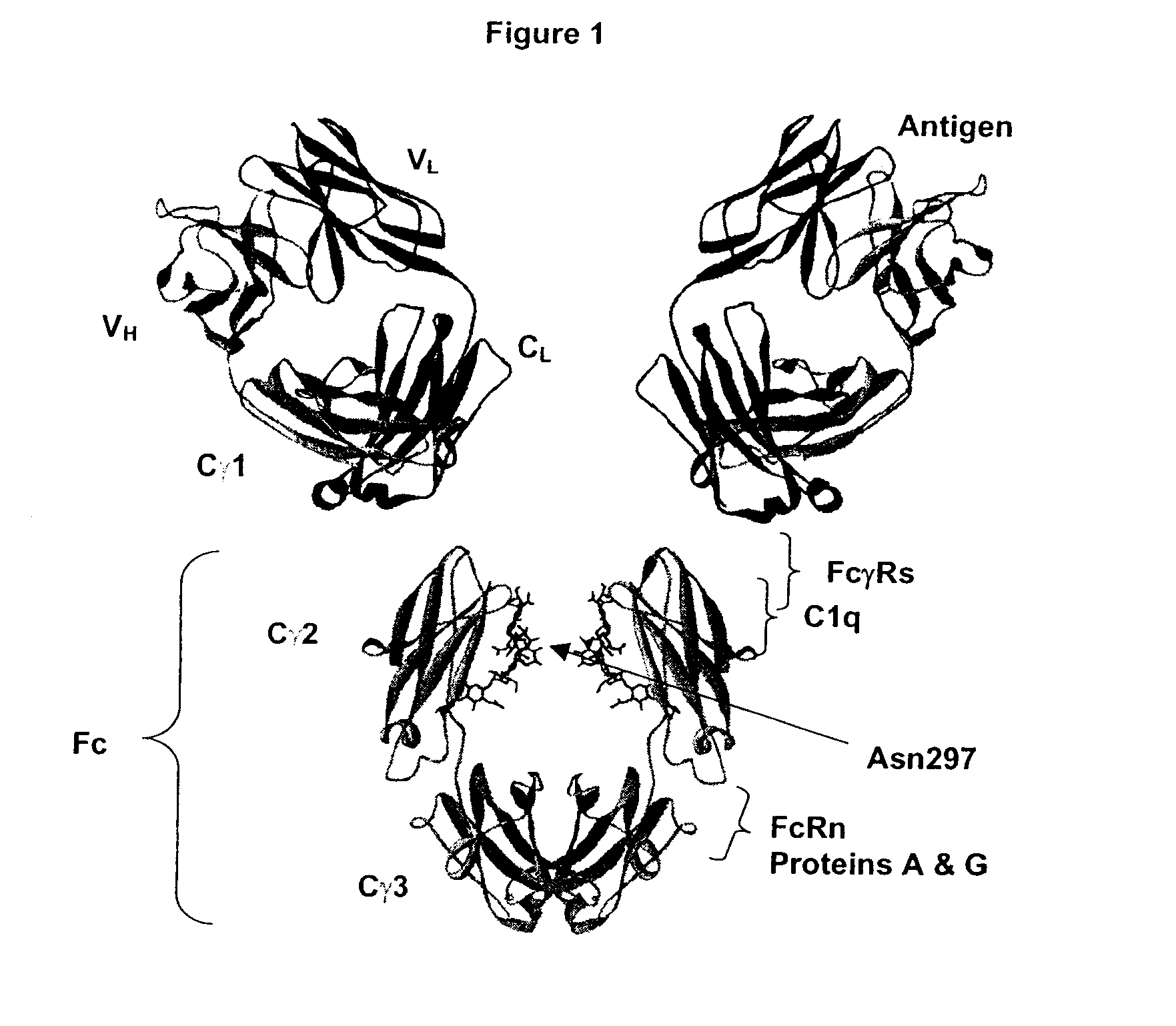
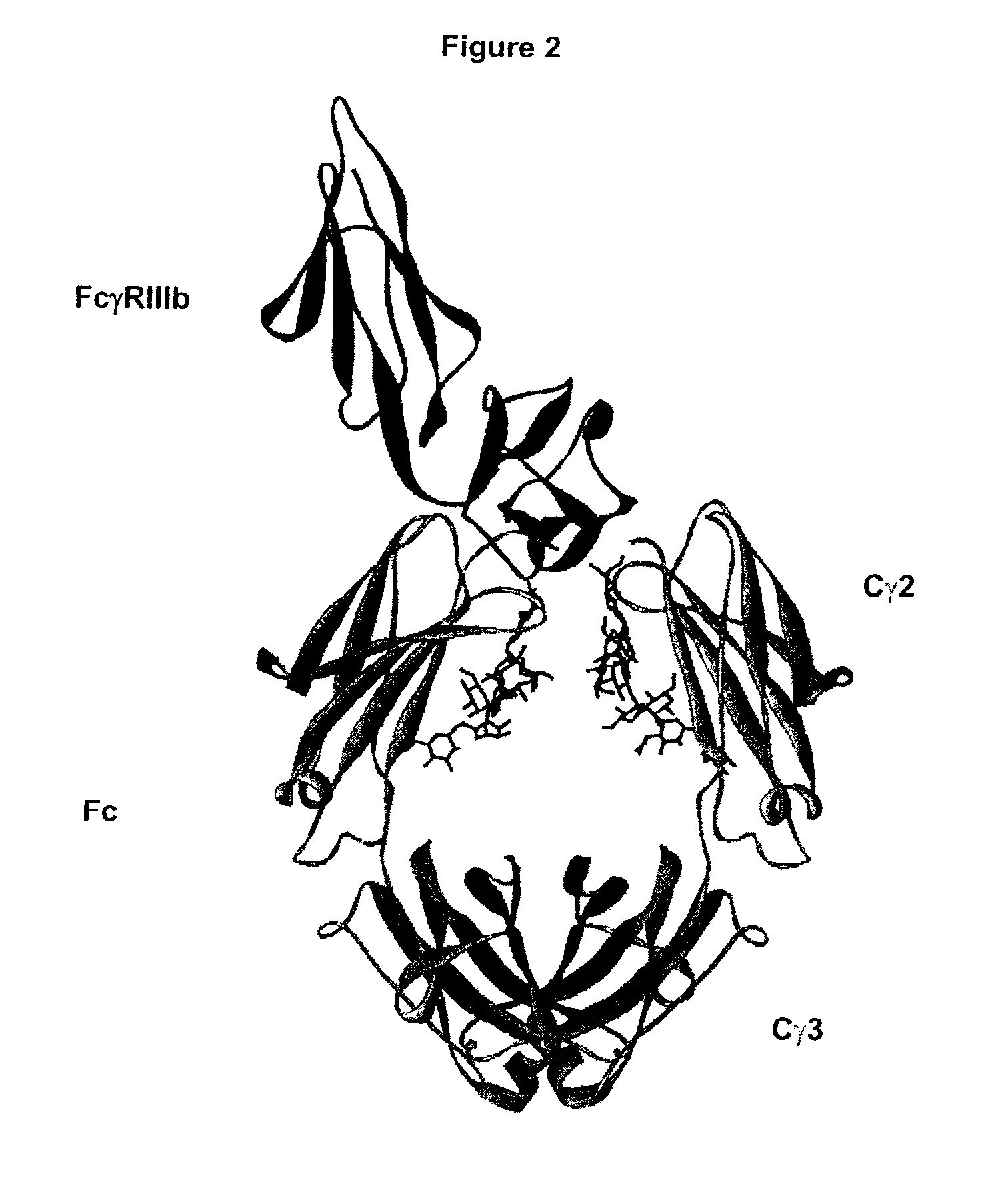
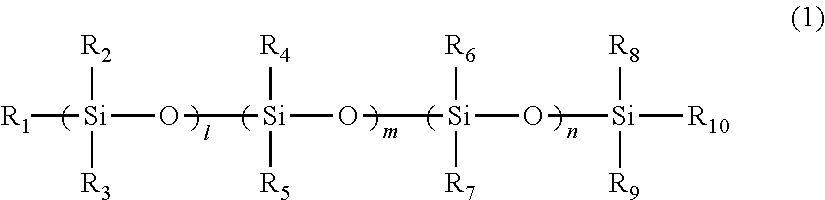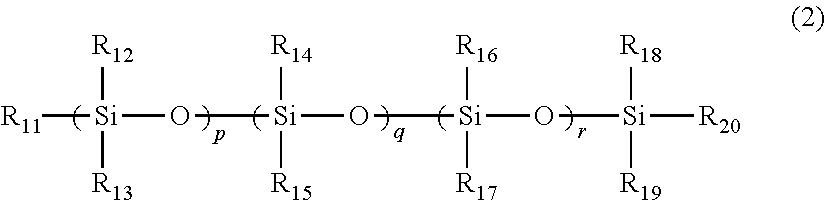Patents
Literature
172results about How to "Weak affinity" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
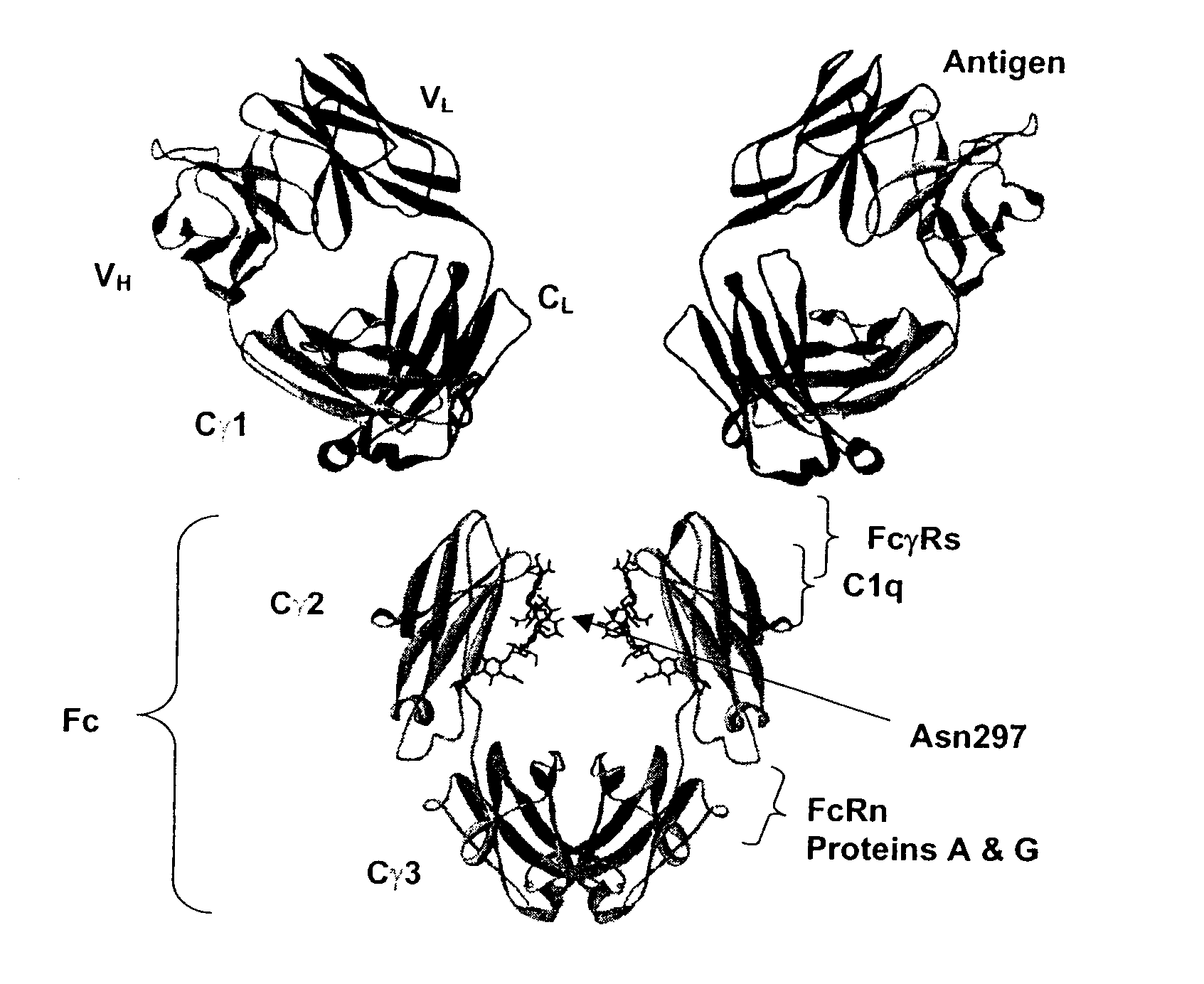
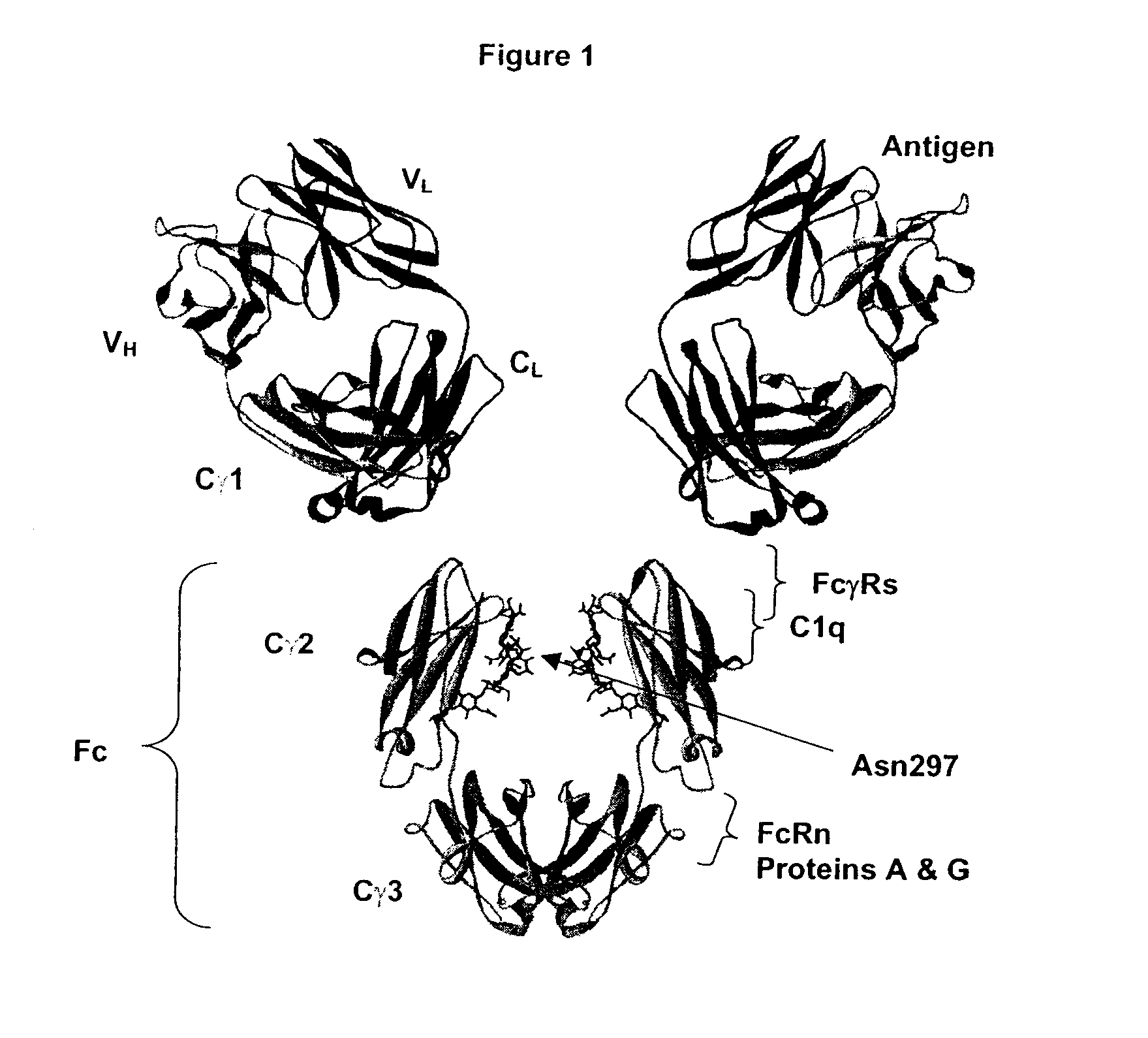
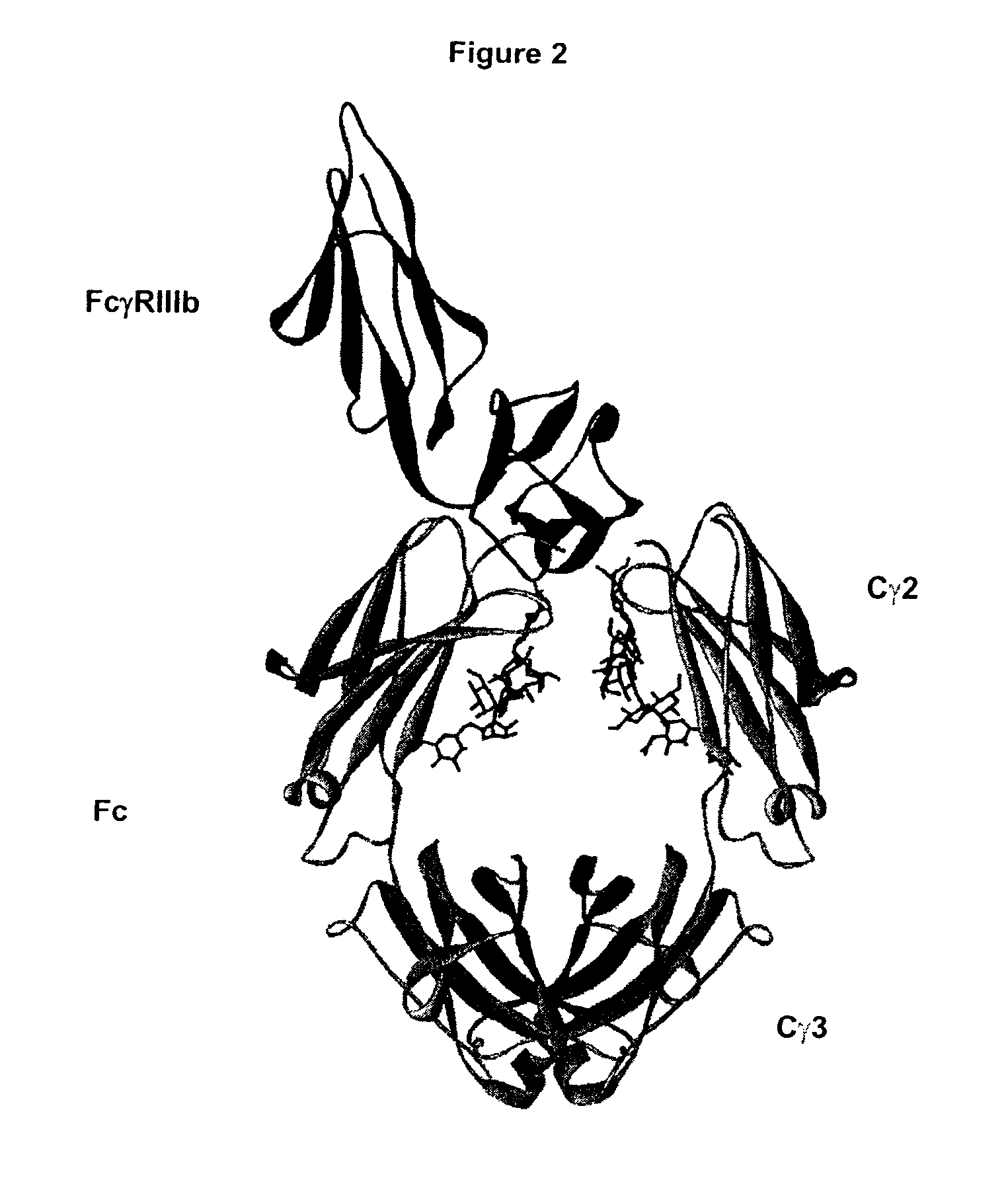
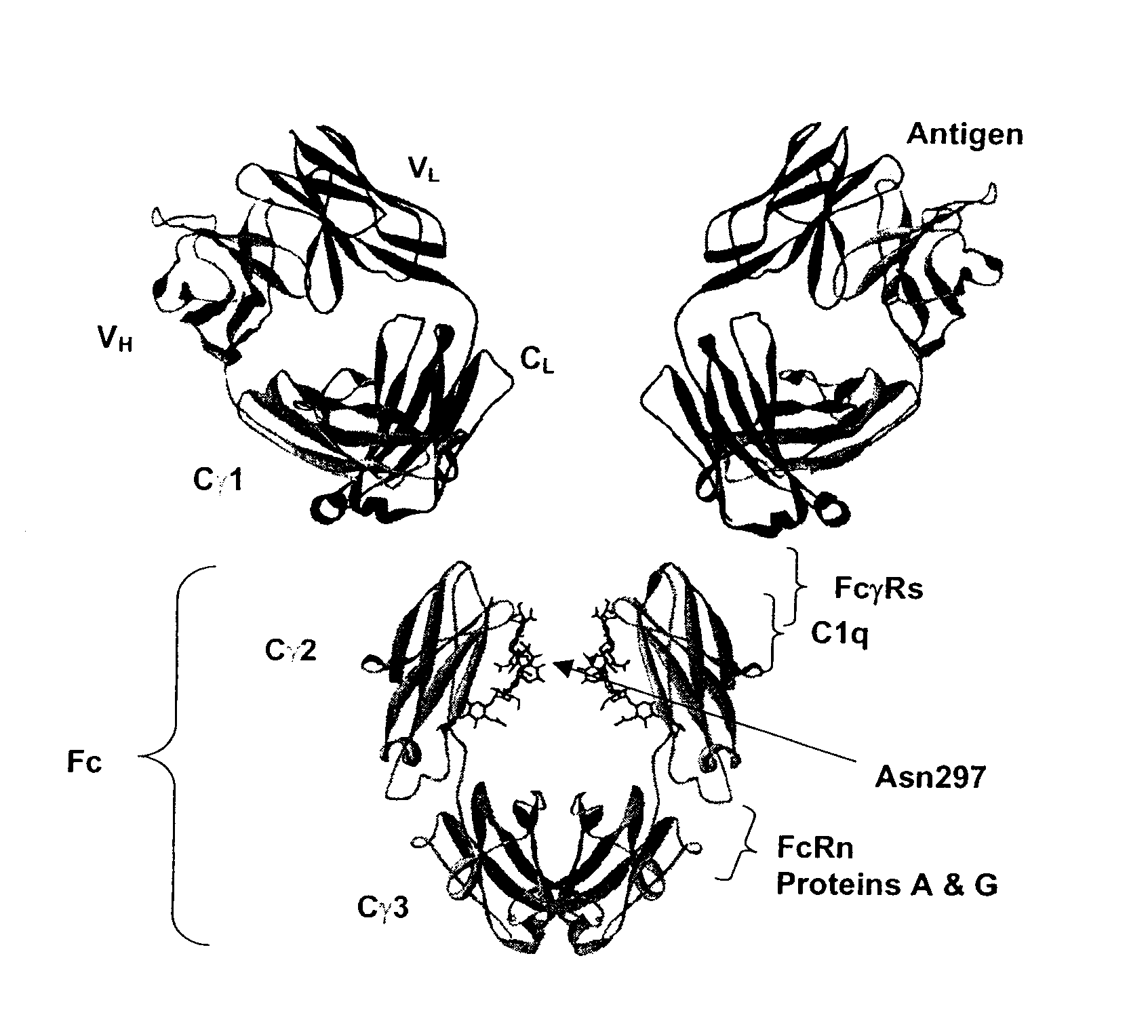
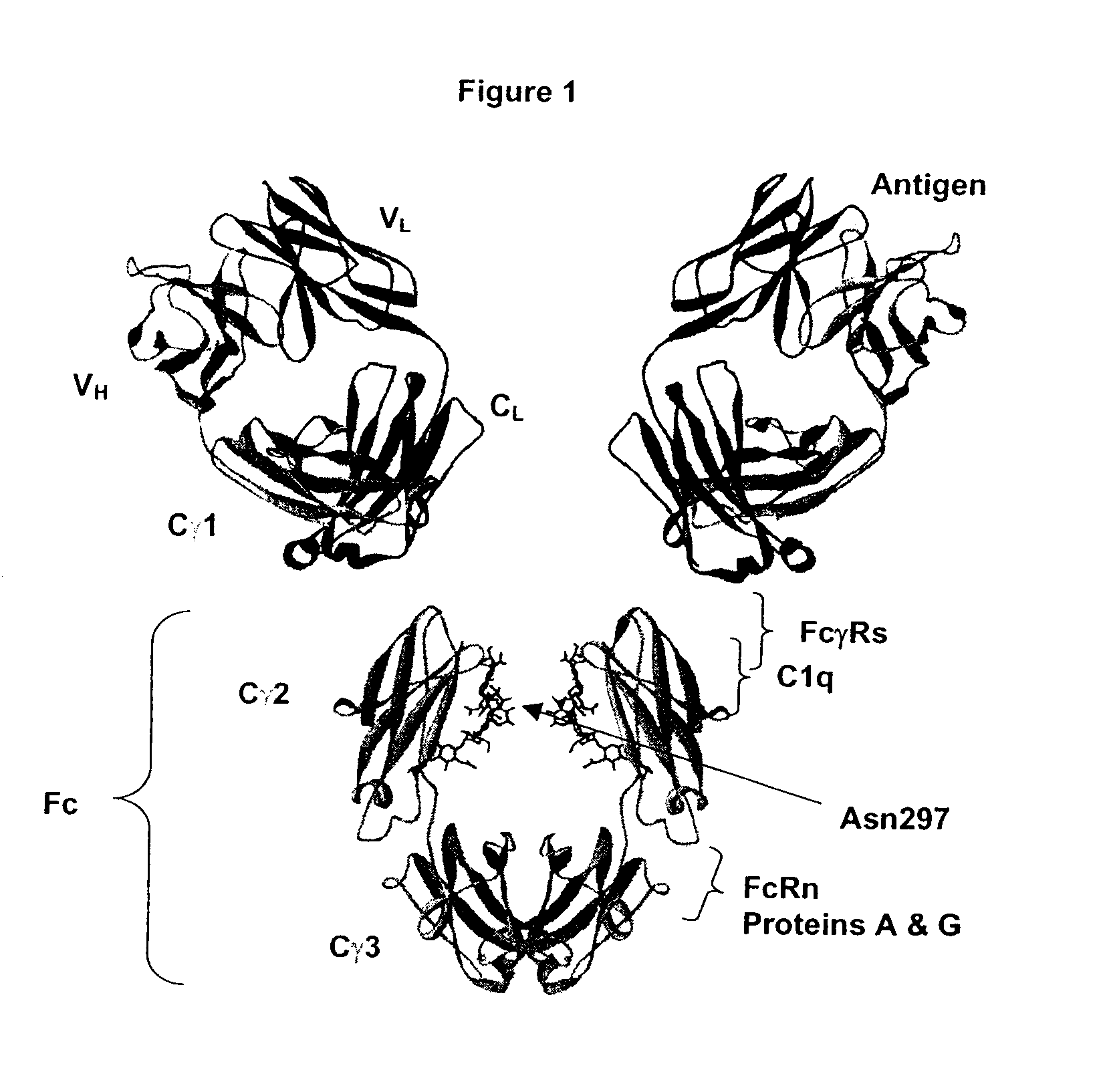
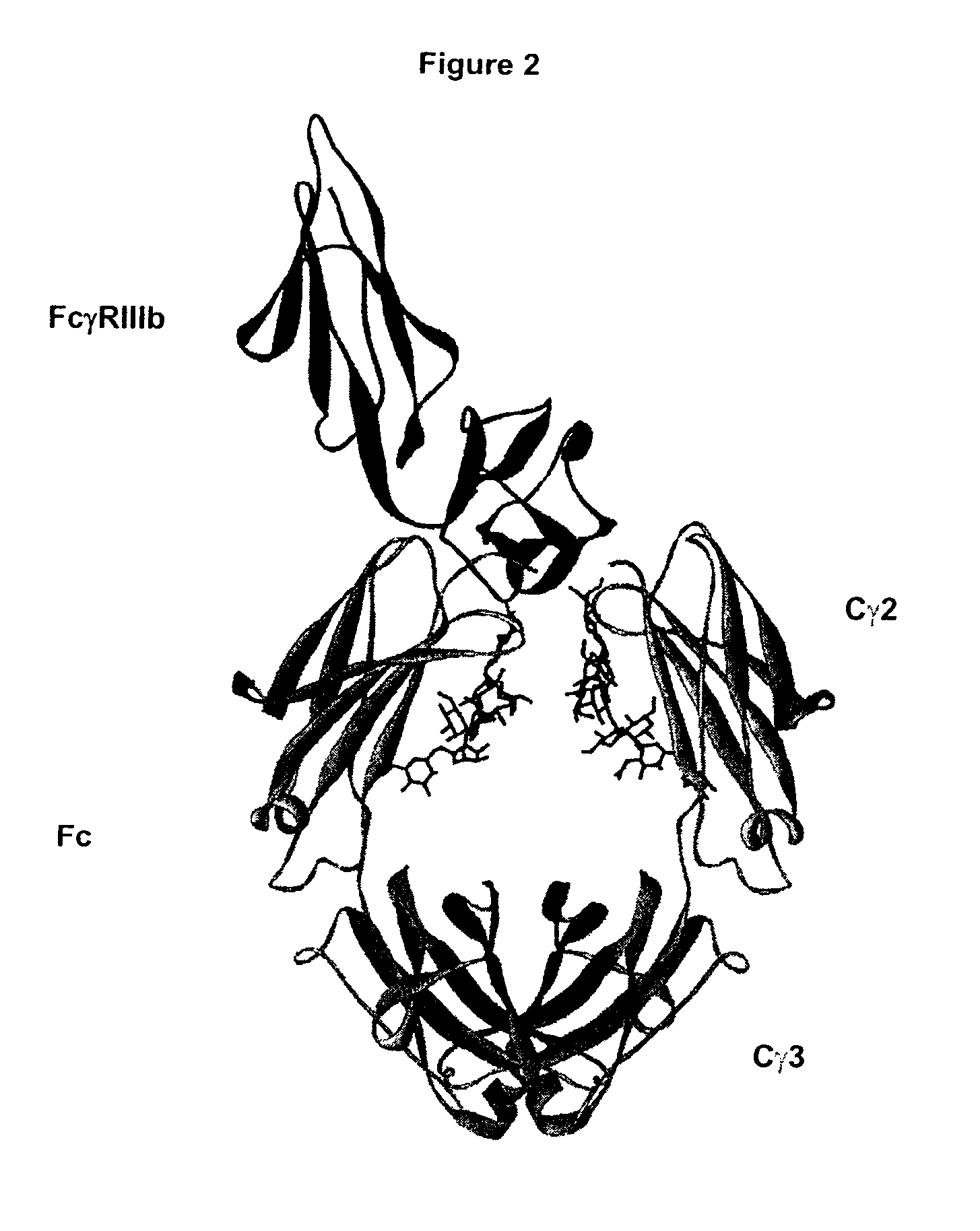
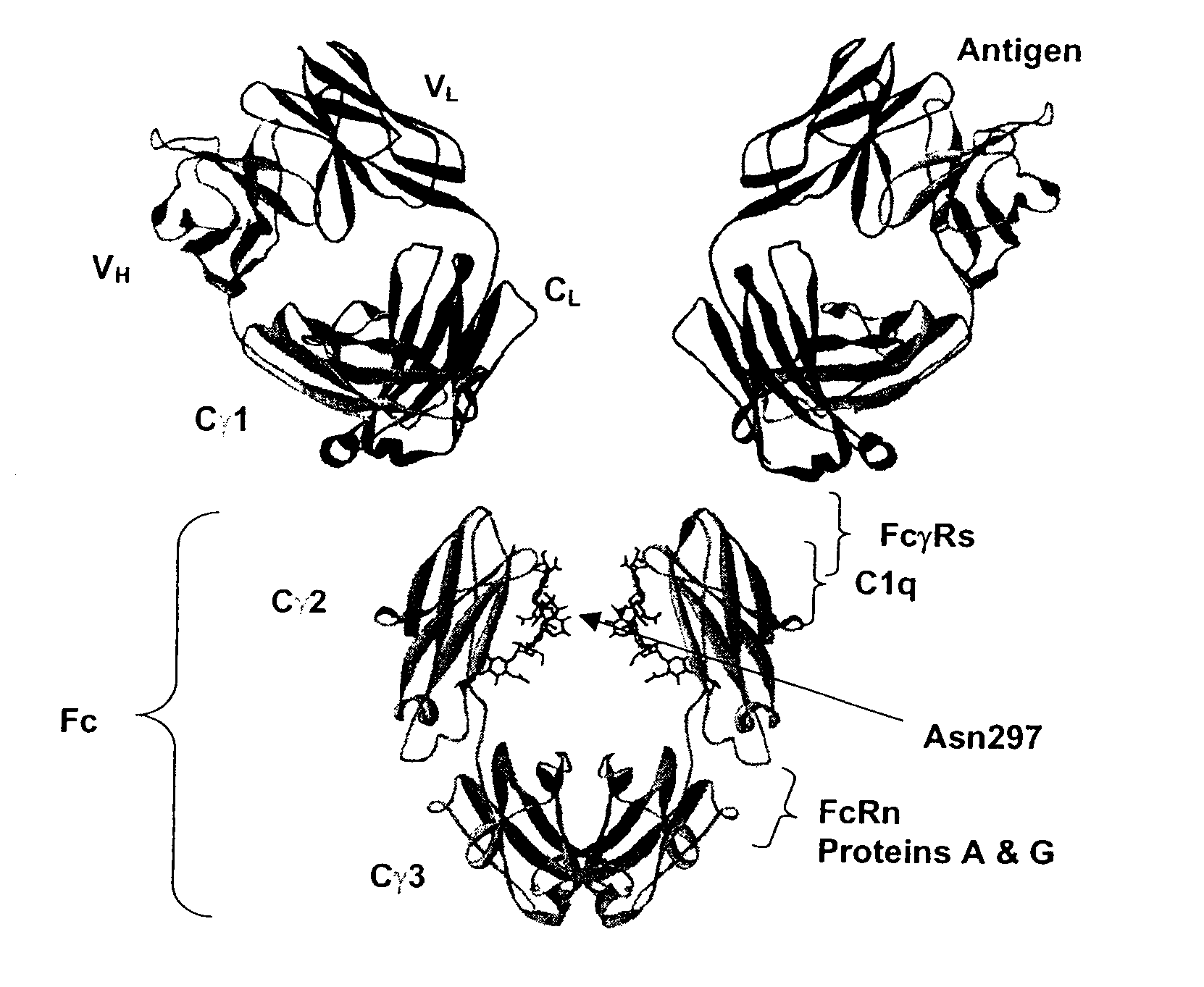
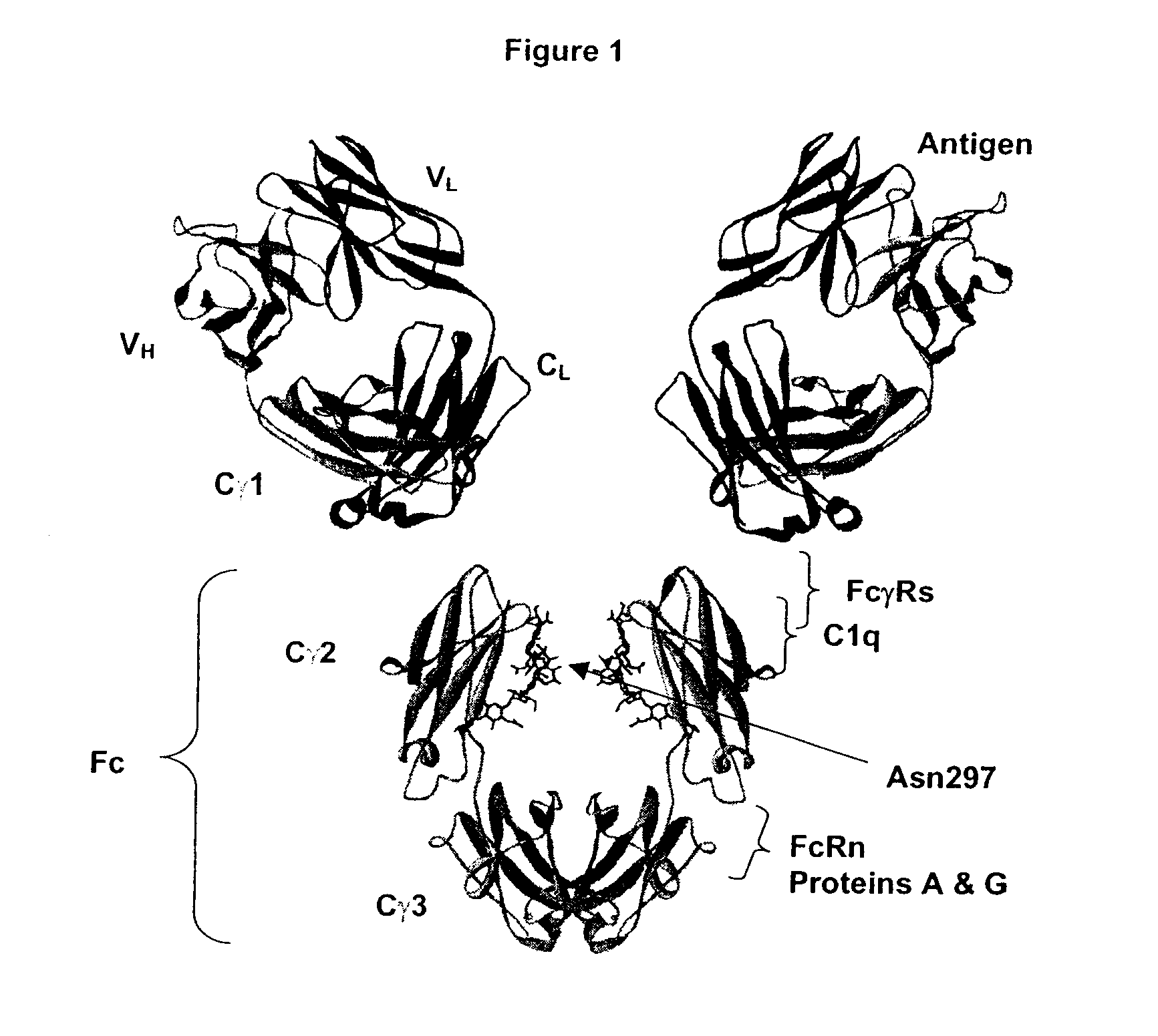
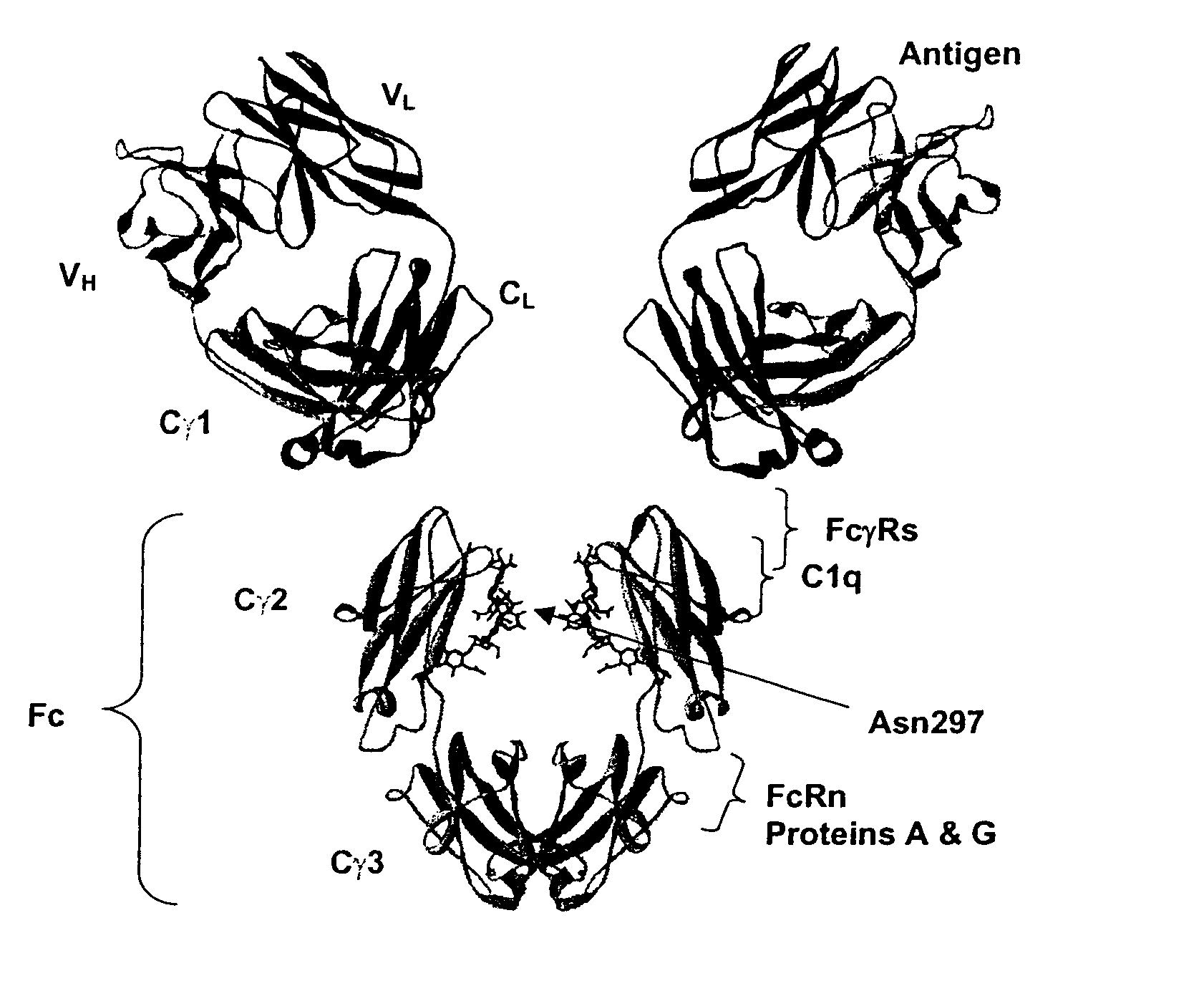
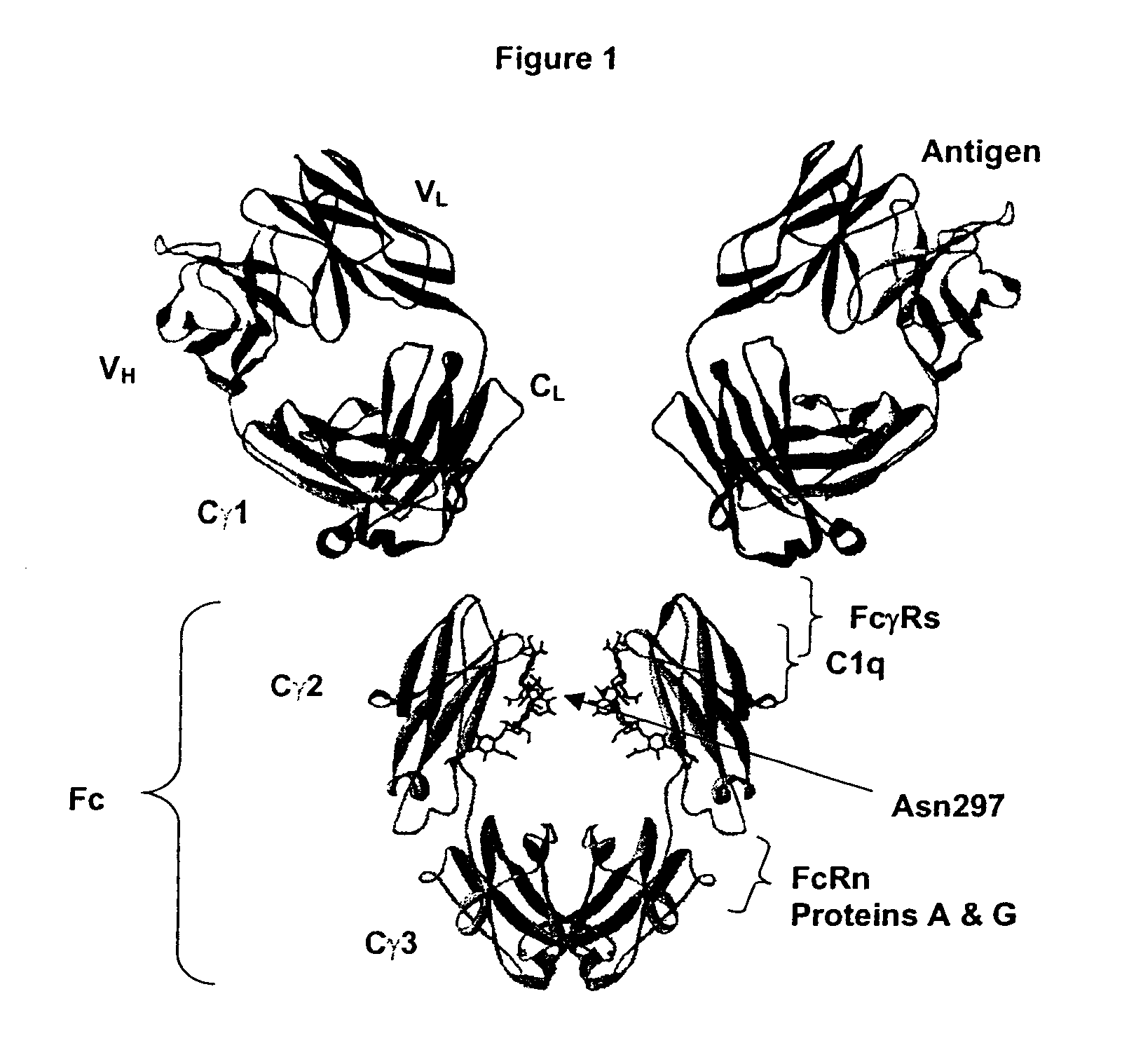
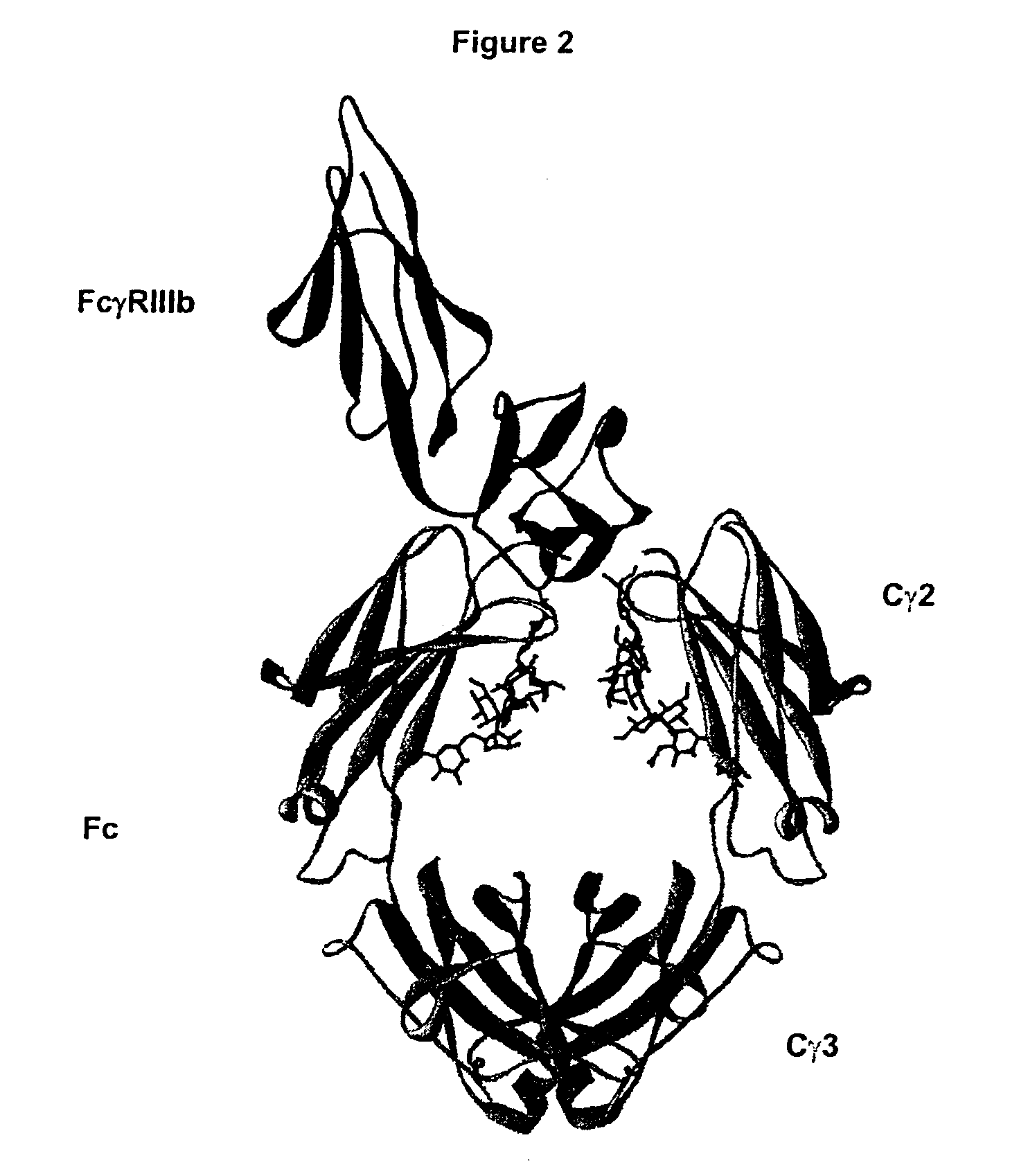
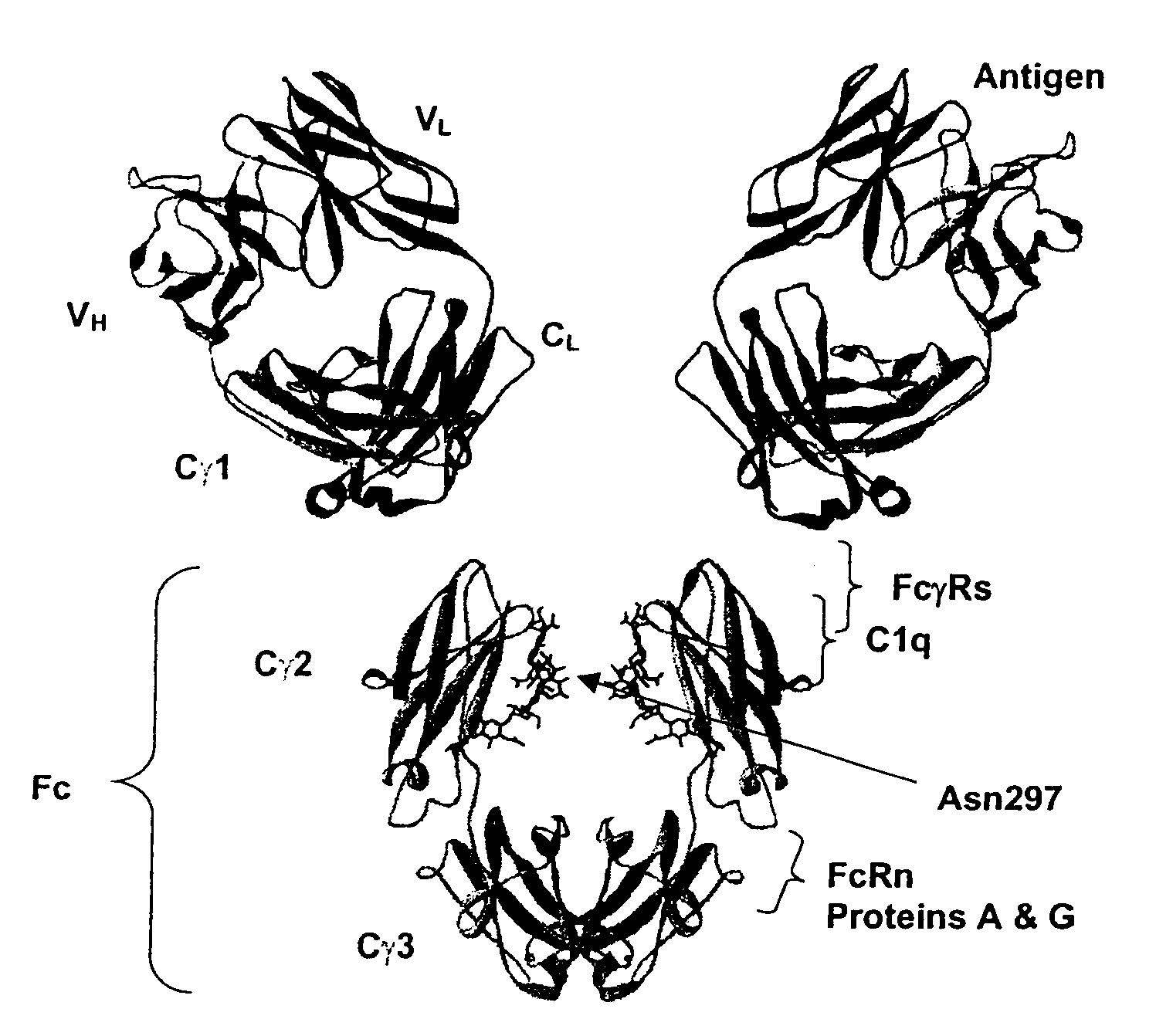
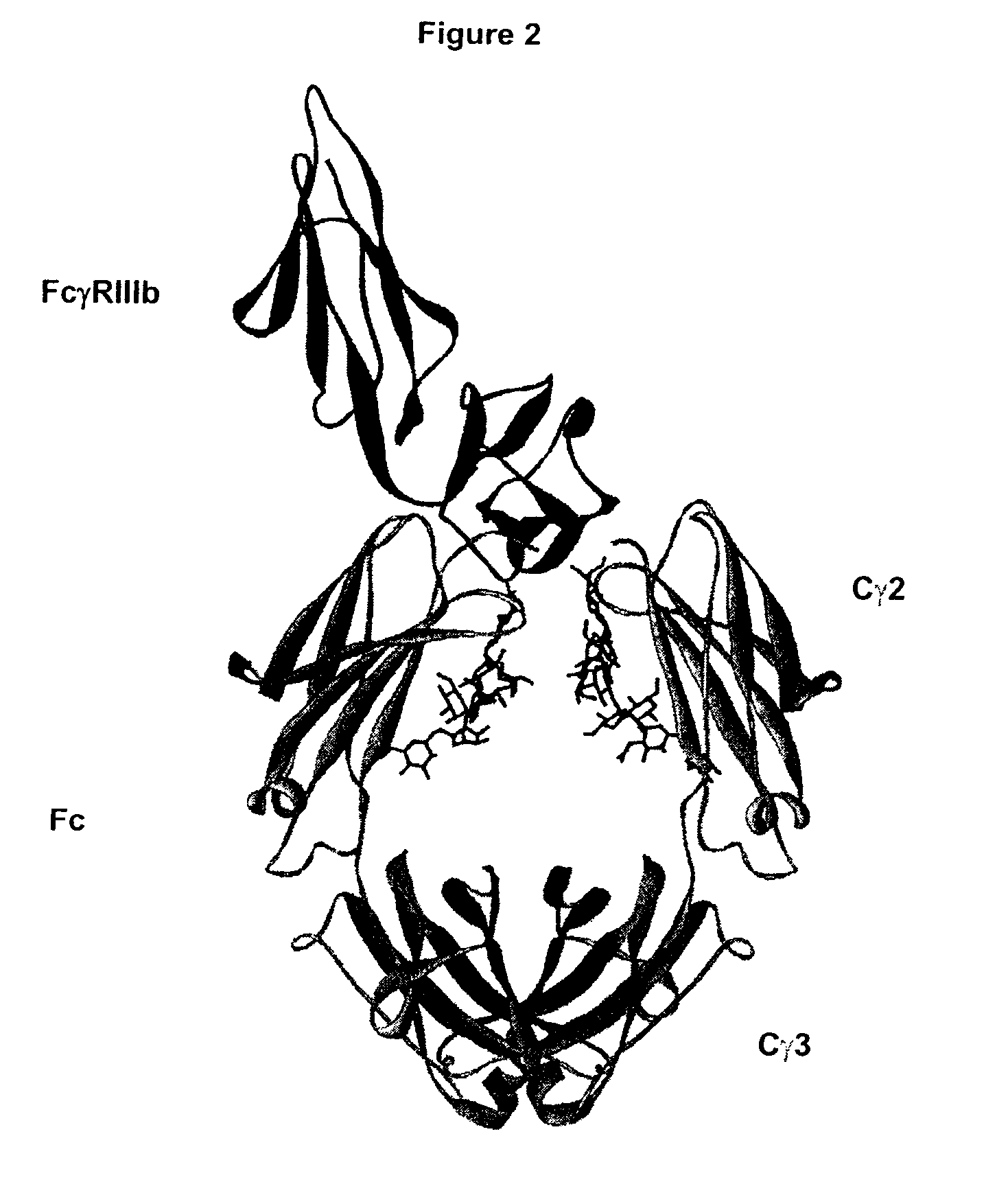
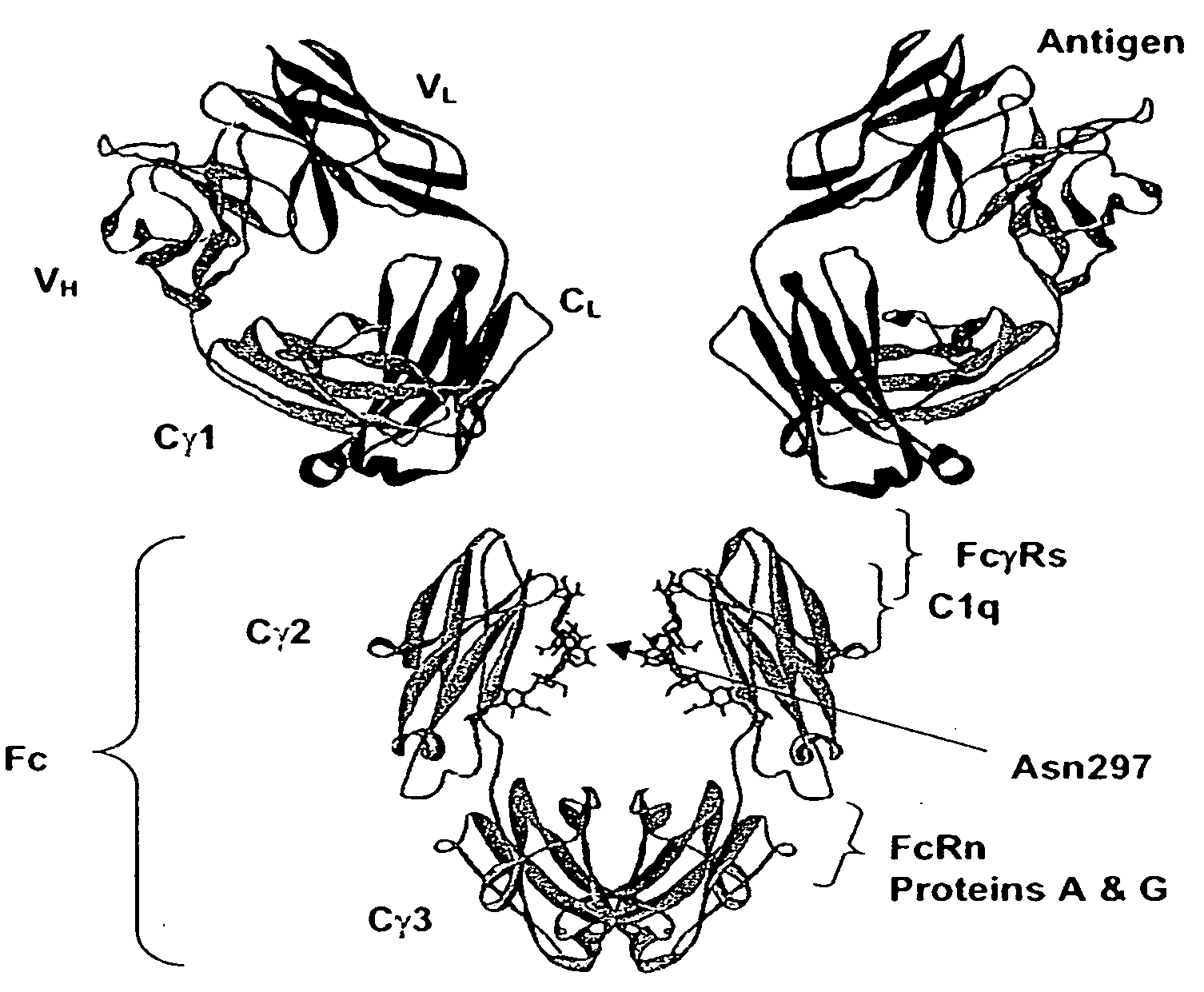
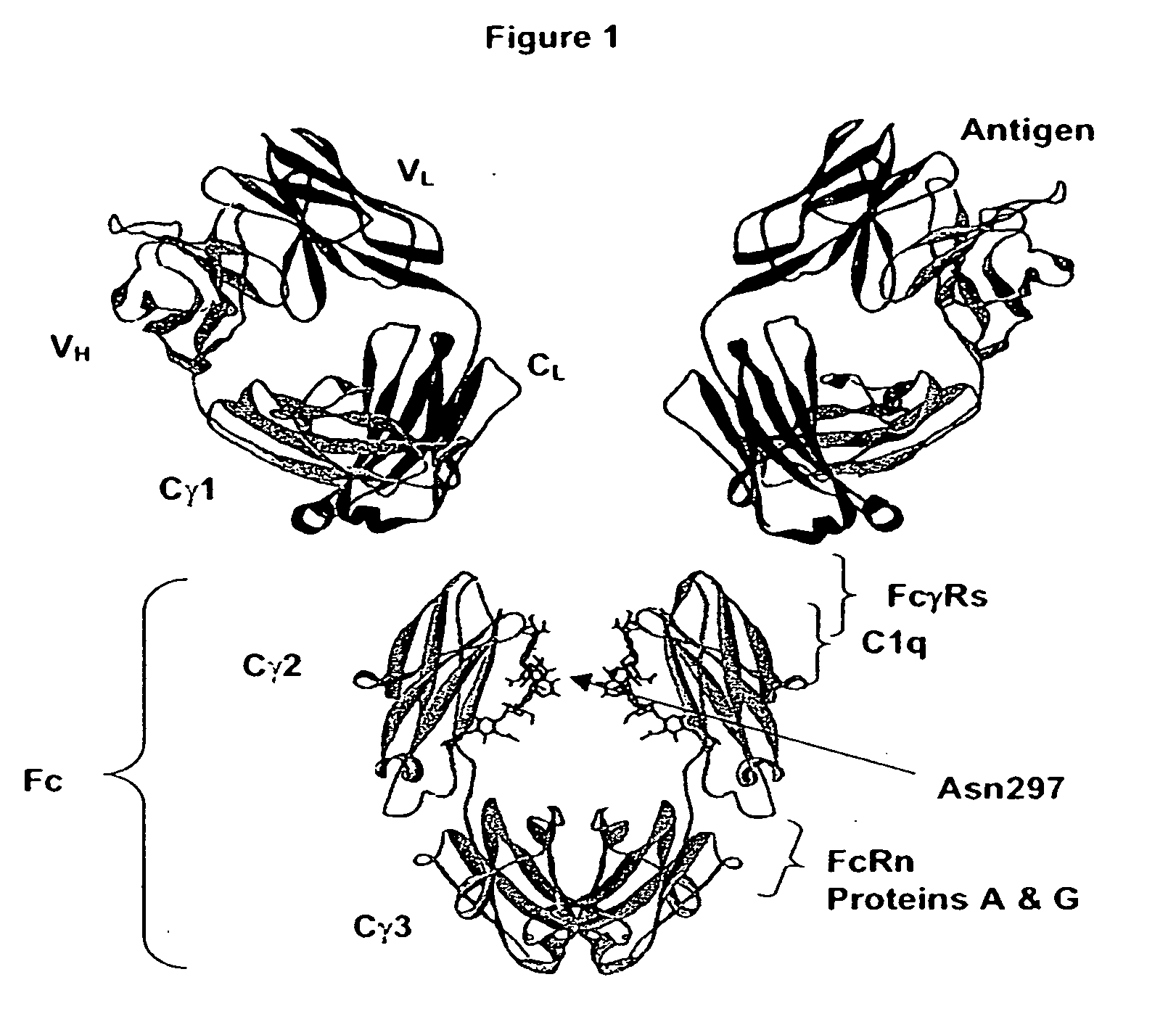
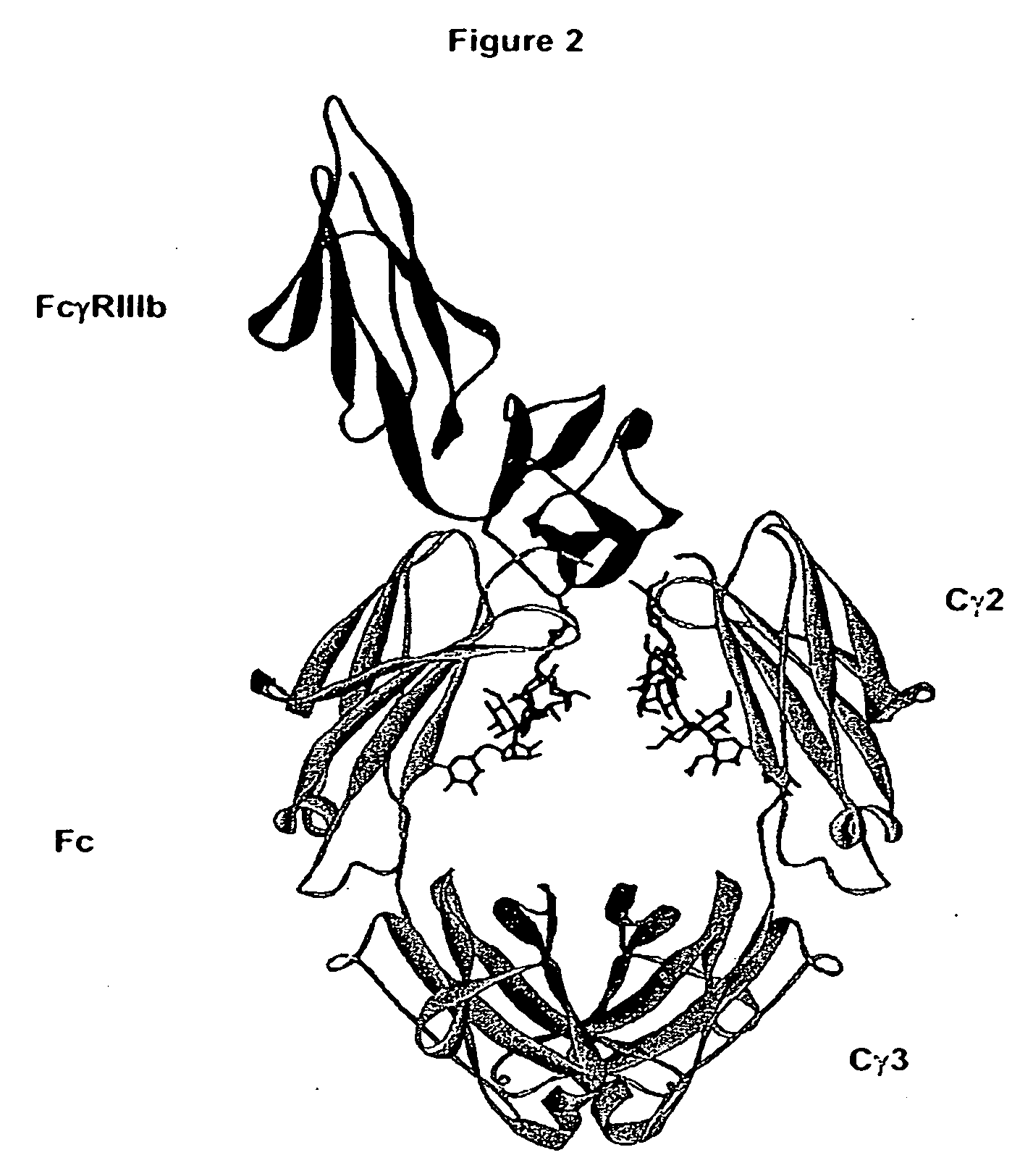
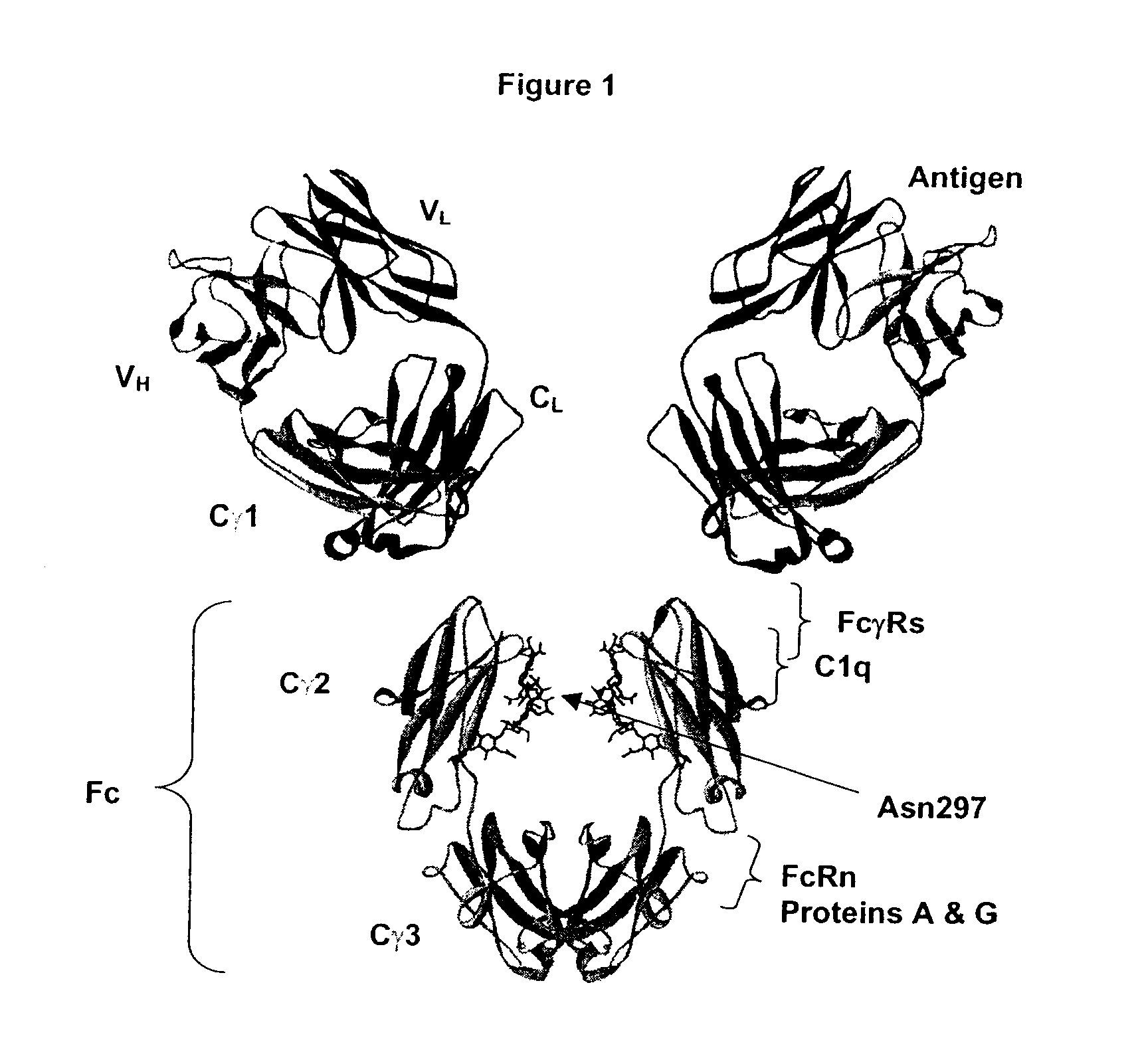
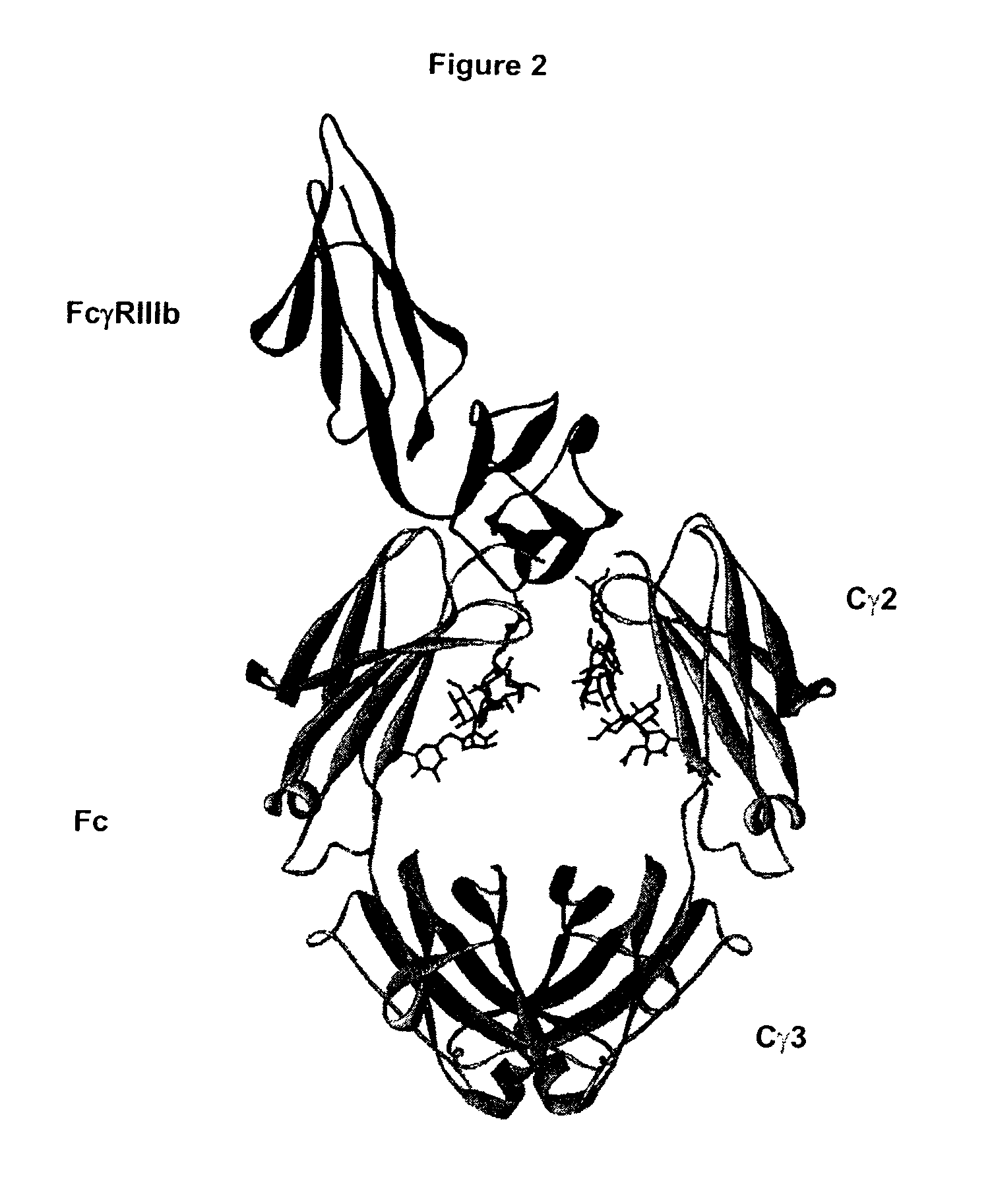
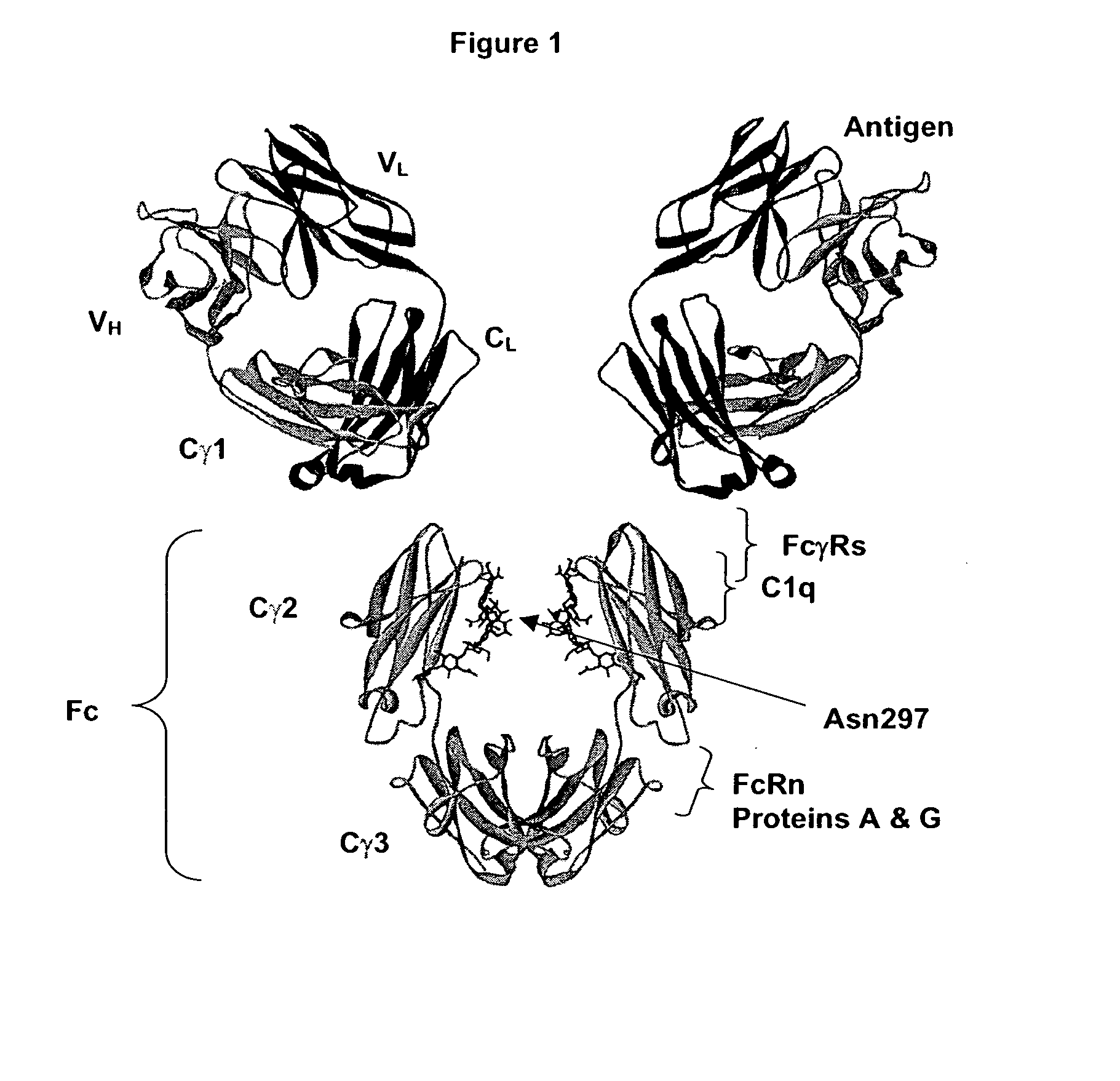
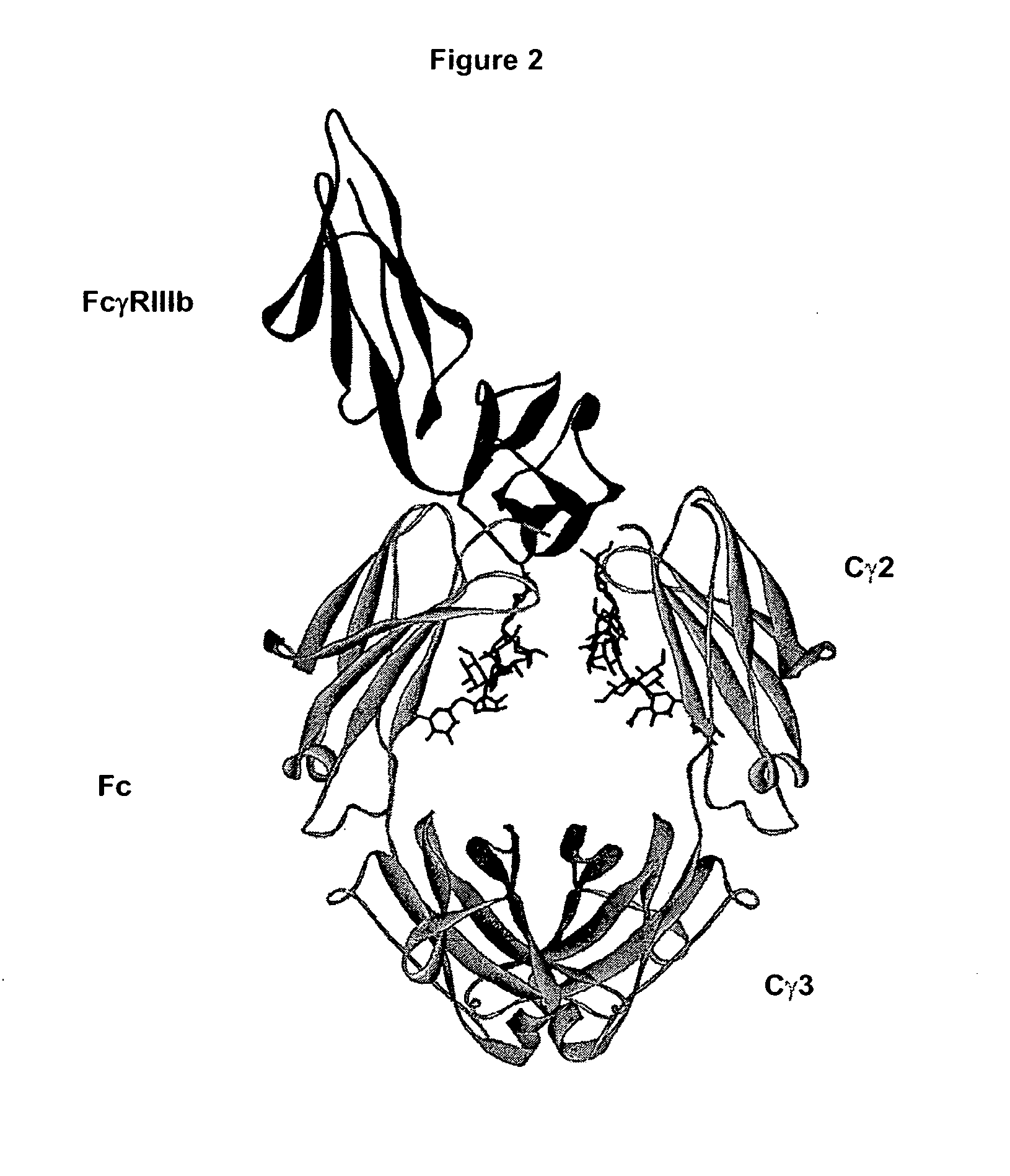
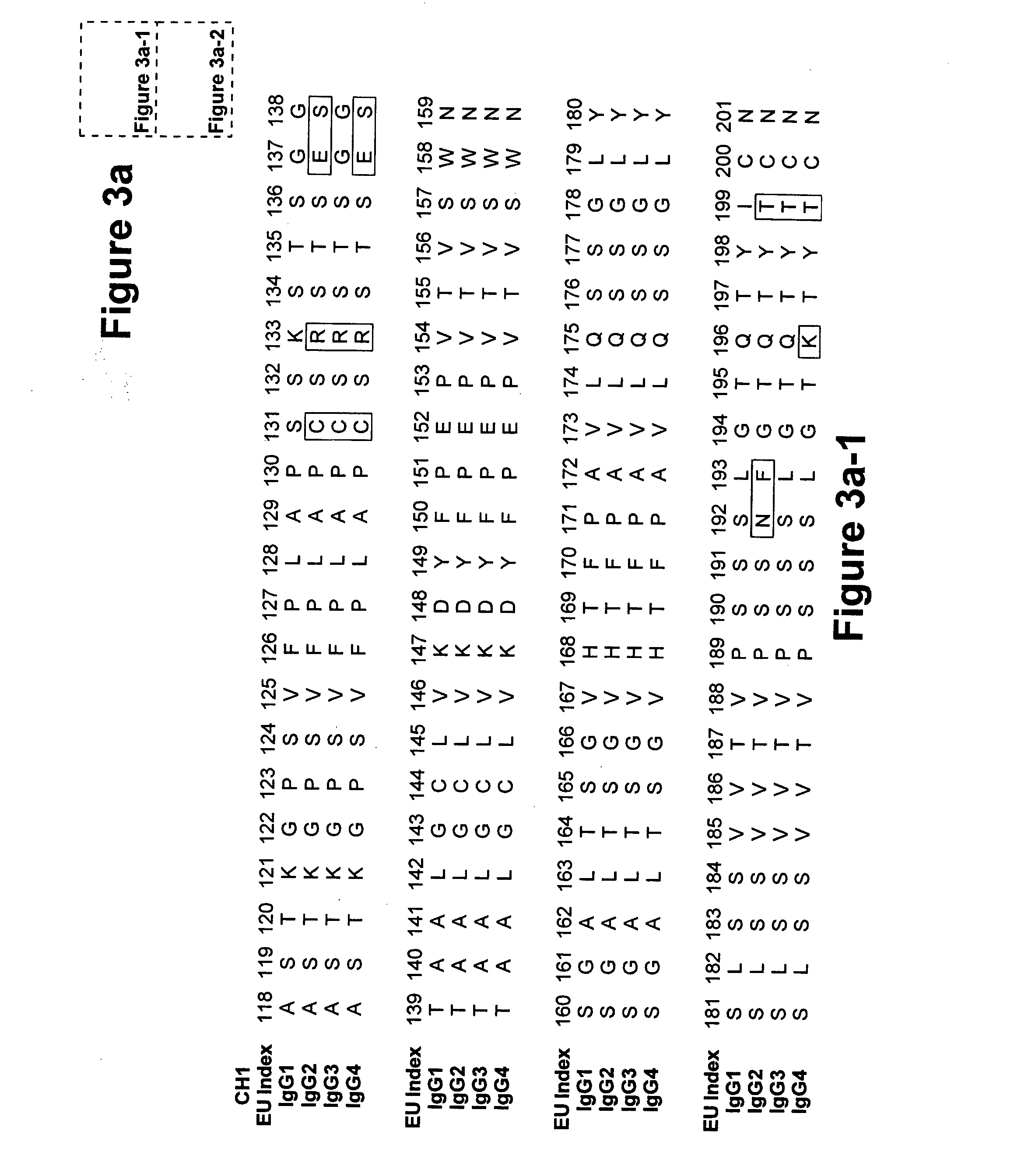
Fc Variants Having Decreased Affinity for FcyRllla
InactiveUS20070237767A1Weak affinityFunction increaseImmunoglobulins against animals/humansAntibody ingredientsChemistry
Owner:XENCOR INC
Fc Variants Having Decreased Affinity for FcyRlla
InactiveUS20070243188A1Weak affinityFunction increaseImmunoglobulins against animals/humansAntibody ingredientsChemistry
Owner:XENCOR INC
Fc Variants Having Increased Affinity for FcyRl
InactiveUS20070237765A1Weak affinityFunction increaseImmunoglobulins against animals/humansAntibody ingredientsChemistry
Owner:XENCOR INC
Fc Variants Having Increased Affinity for FcyRllla
InactiveUS20070237766A1Weak affinityFunction increaseImmunoglobulins against animals/humansAntibody ingredientsChemistry
Owner:XENCOR INC
Fc Variants with Increased Affinity for FcyRIIC
InactiveUS20080057056A1Weak affinityFunction increaseSugar derivativesImmunoglobulins against cell receptors/antigens/surface-determinantsChemistryBiochemistry
Owner:XENCOR INC
Fc Variants with Increased Affinity for FcyRIIc
InactiveUS20080154025A1Weak affinityFunction increaseImmunoglobulins against cell receptors/antigens/surface-determinantsDepsipeptidesAvidityChemistry
Owner:XENCOR INC
Fc Variants Having Decreased Affinity for FcyRIIc
Owner:XENCOR INC
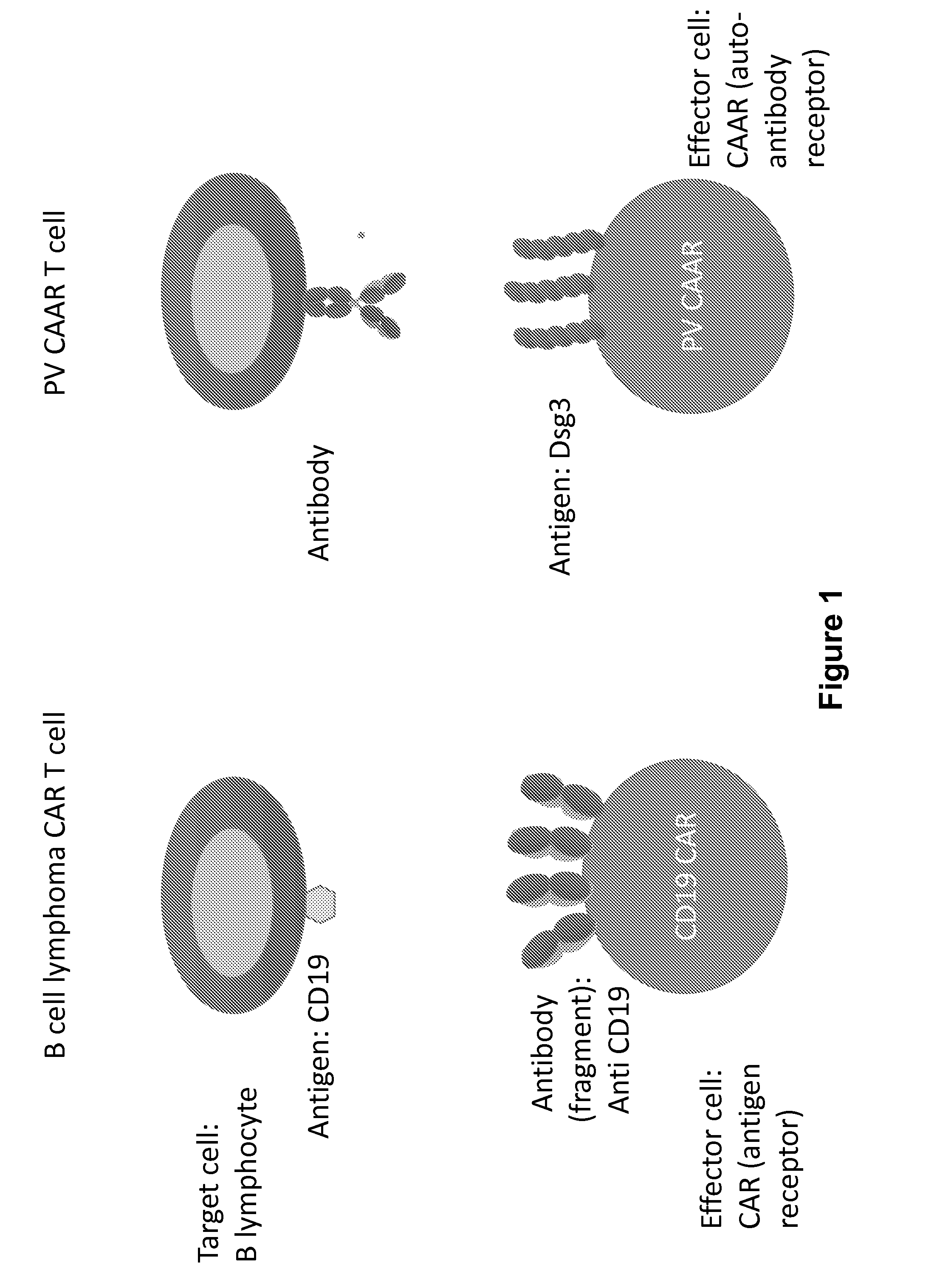
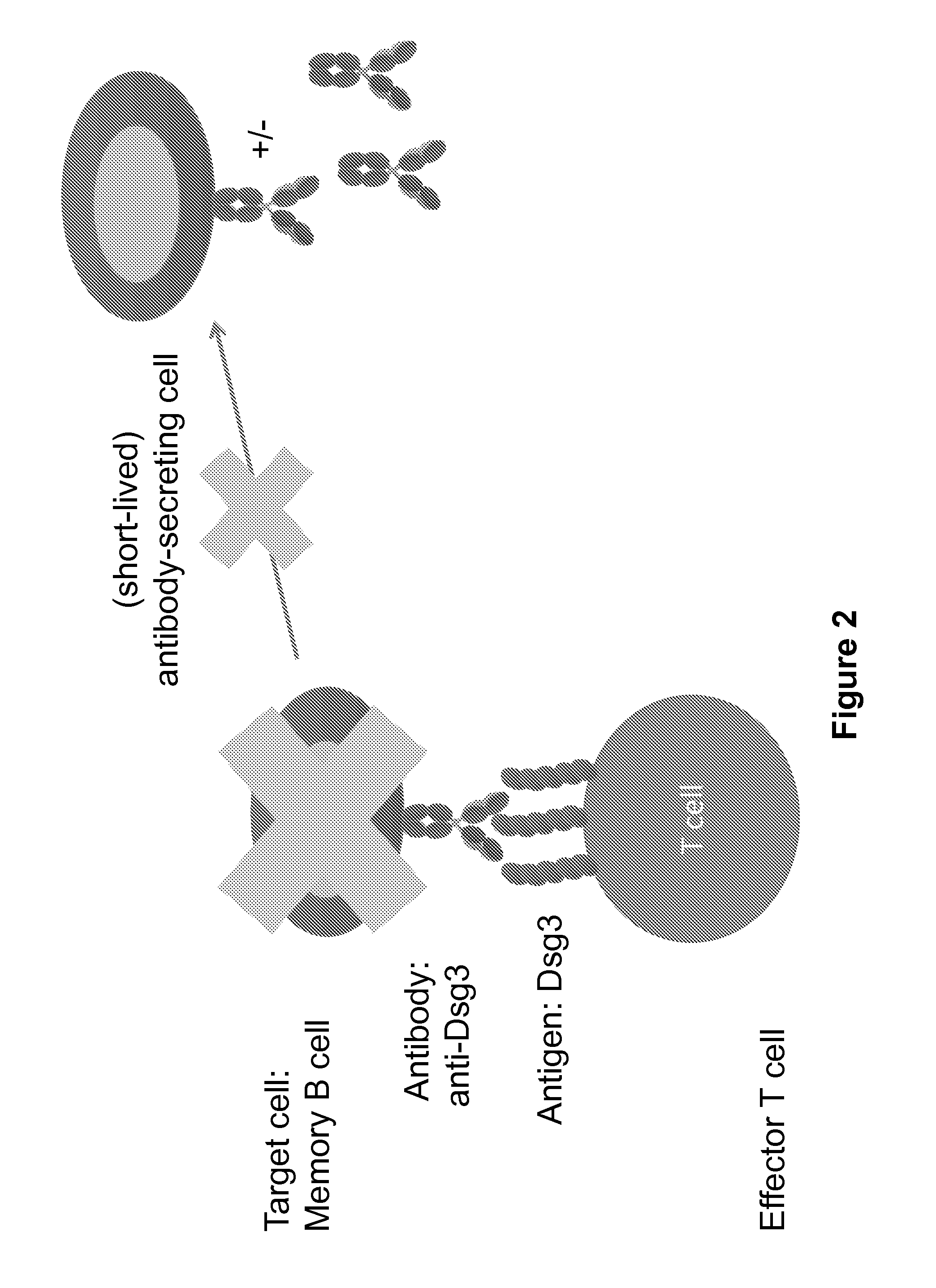
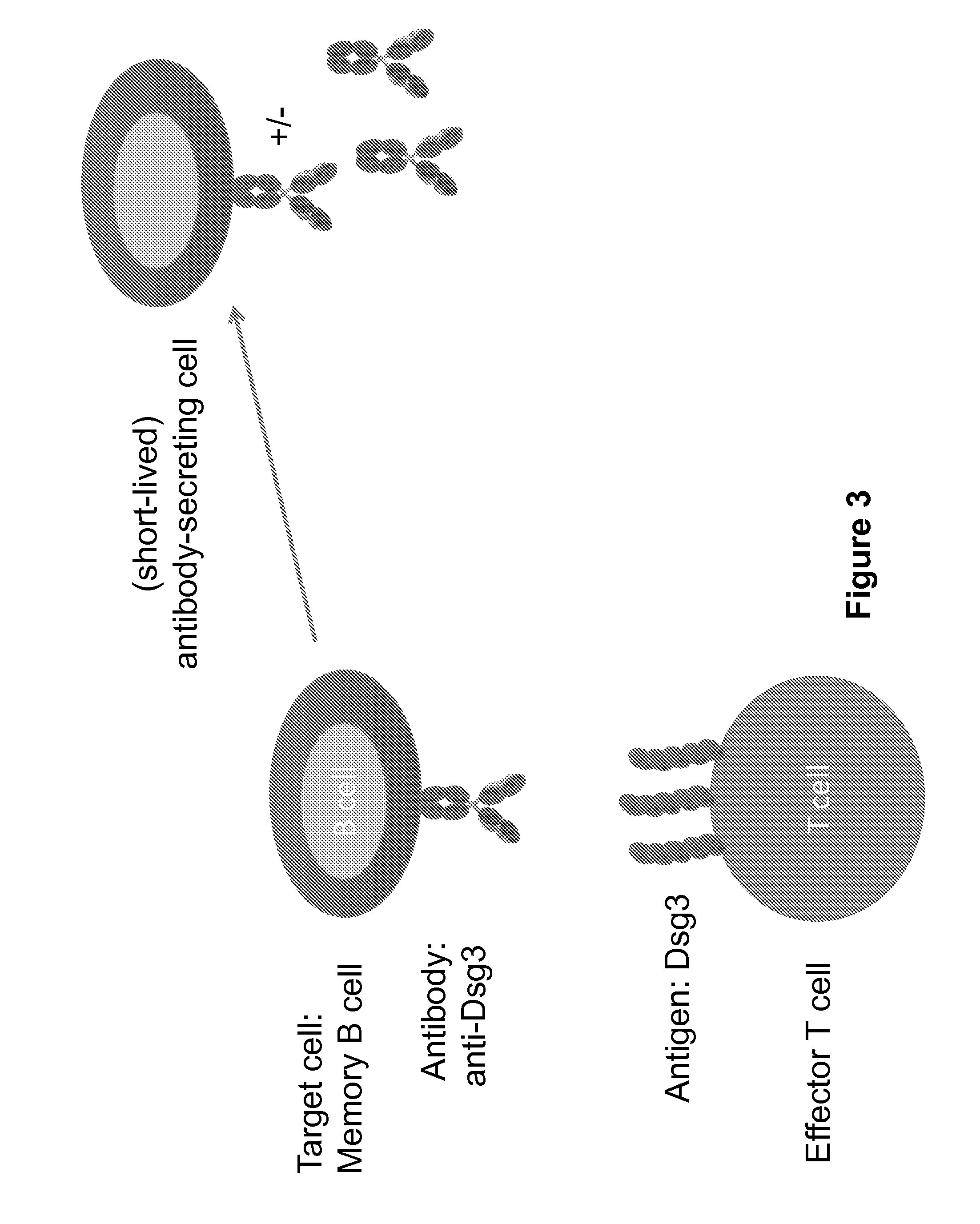
Compositions and methods of chimeric autoantibody receptor t cells
ActiveUS20170051035A1Low toxicityHigh affinityPolypeptide with localisation/targeting motifImmunoglobulin superfamilyDesmoyokinAntibody receptor
The invention includes compositions comprising at least one chimeric autoantibody receptor (CAAR) specific for an autoantibody, vectors comprising the same, compositions comprising CAAR vectors packaged in viral particles, and recombinant T cells comprising the CAAR. The invention also includes methods of making a genetically modified T cell expressing a CAAR (CAART) wherein the expressed CAAR comprises a desmoglein extracellular domain.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
Optimized Fc variants
Owner:XENCOR INC
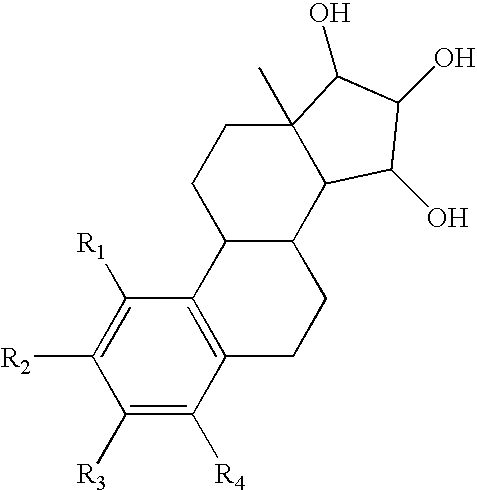
Use of compositions comprising an estrogenic component for the treatment and prevention of musculoskeletal pain
InactiveUS20060276414A1Increasing cell proliferationHigh affinityOrganic active ingredientsBiocideGynecologyPresent method
The present invention relates to a method of treating or preventing musculoskeletal pain in a mammal receiving administration of an estrogen. suppressant selected from the group consisting of aromatase inhibitors, GnRH analogues, cyclo-oxy-genase 2 (COX-2) inhibitors, 17β-hydroxysteroid dehydrogenase type 1 inhibitors, progestogens, anti-estrogens and combinations thereof, said method comprising the administration of an effective amount of an estrogenic component, wherein the estrogenic component is selected from the group consisting of: substances represented by the following formula (1) in which formula R1, R2, R3, R4 independently are a hydrogen atom, a hydroxyl group or an alkoxy group with 1-5 carbon atoms; precursors capable of liberating a substance according to the aforementioned formula when used in the present method; and mixtures of one or more of the aforementioned substances and / or precursors.
Owner:COELINGH BENNINK HERMAN JAN TIJMEN +1
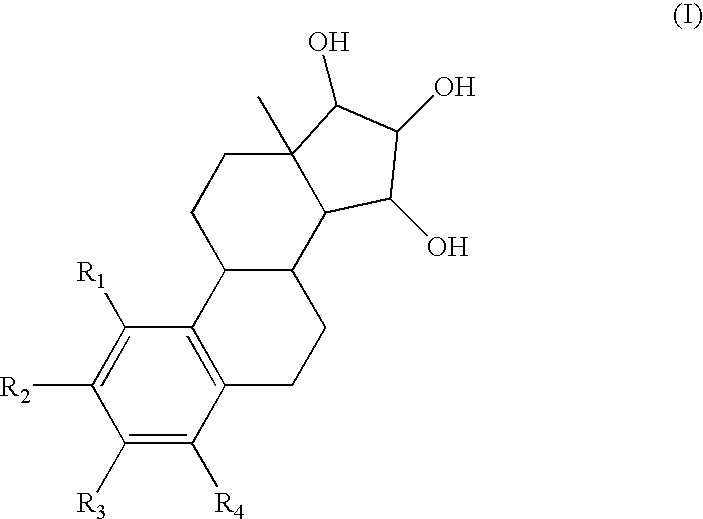
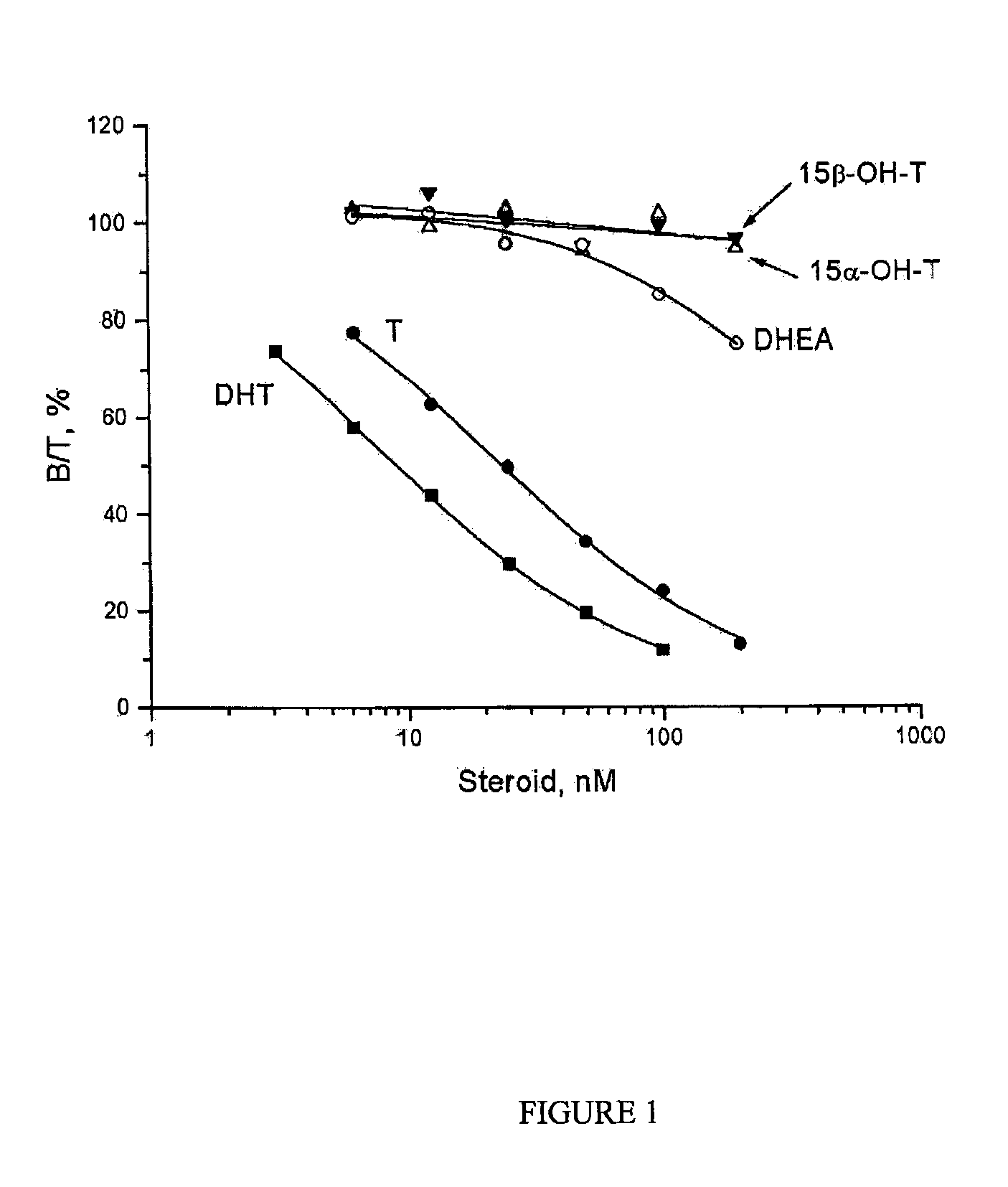
Pharmaceutical application of 15- or 16-substituted testosterone analogues
InactiveUS7943602B2Minimal impactAdequate androgenic potencyBiocideOrganic active ingredientsPhysiology4-androsten-3-one
The invention relates to pharmaceutical dosage units for oral, transmucosal or transdermal administration containing 15- or 16-substituted testosterone analogues, as well as to therapeutic methods that employ these testosterone analogues. More particularly, the invention is concerned with such pharmaceutical dosage units containing at least 10 μg of an androgenic steroid selected from the group consisting of 15-hydroxytestosterones, 16-hydroxytestosterones, precursors thereof and mixtures of these hydroxytestosterones and / or their precursors; and a pharmaceutically acceptable excipient. The term “15-hydroxytestosterones” encompasses both 15α-hydroxytestosterone (15α, 17β-dihydroxy-4-androsten-3-one) and 15β-hydroxytestosterone (15β, 17β-dihydroxy-4-androsten-3-one). Similarly, the term “16-hydroxytestosterones” encompasses both 16α-hydroxytestosterone hydroxytestosterone (16α, 17β-dihydroxy-4-androsten-3-one) and 16β-hydroxytestosterone (16β, 17β-dihydroxy-4-androsten-3-one). The androgenic steroids according to the invention are advantageously employed in e.g. a method of treating or preventing androgen deficiency or a method of hormonal contraception.
Owner:PANTARHEI BIOSCI
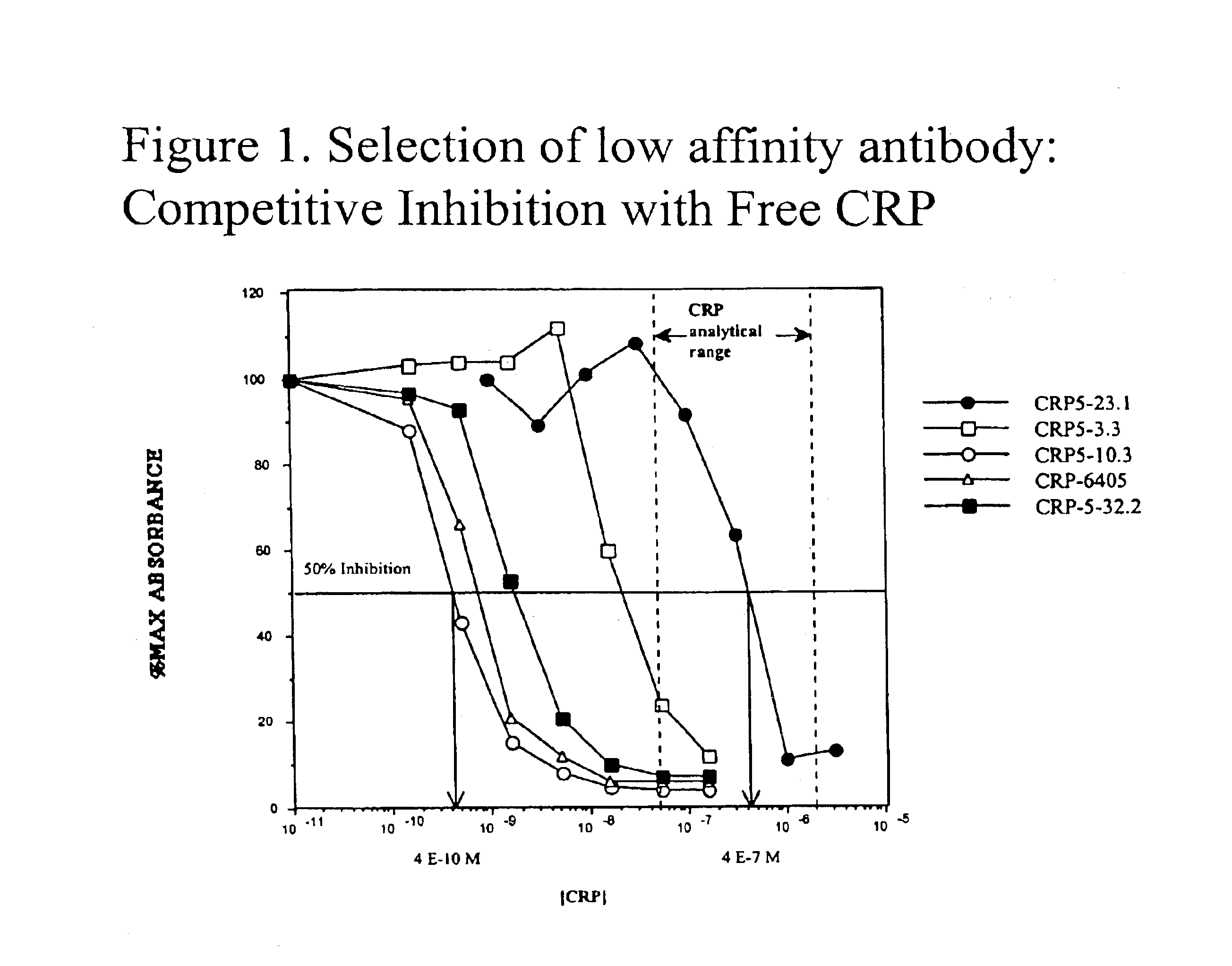
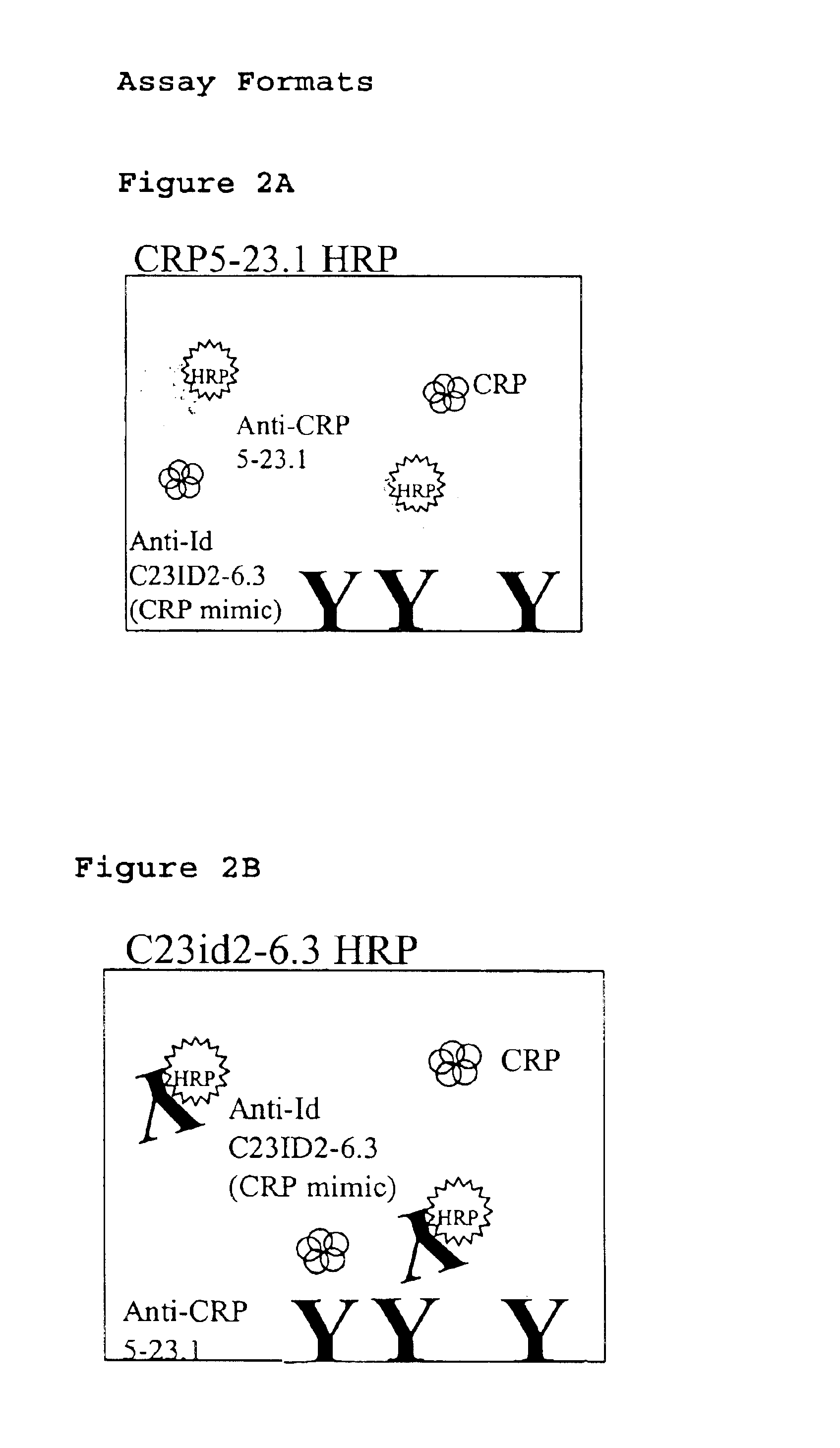
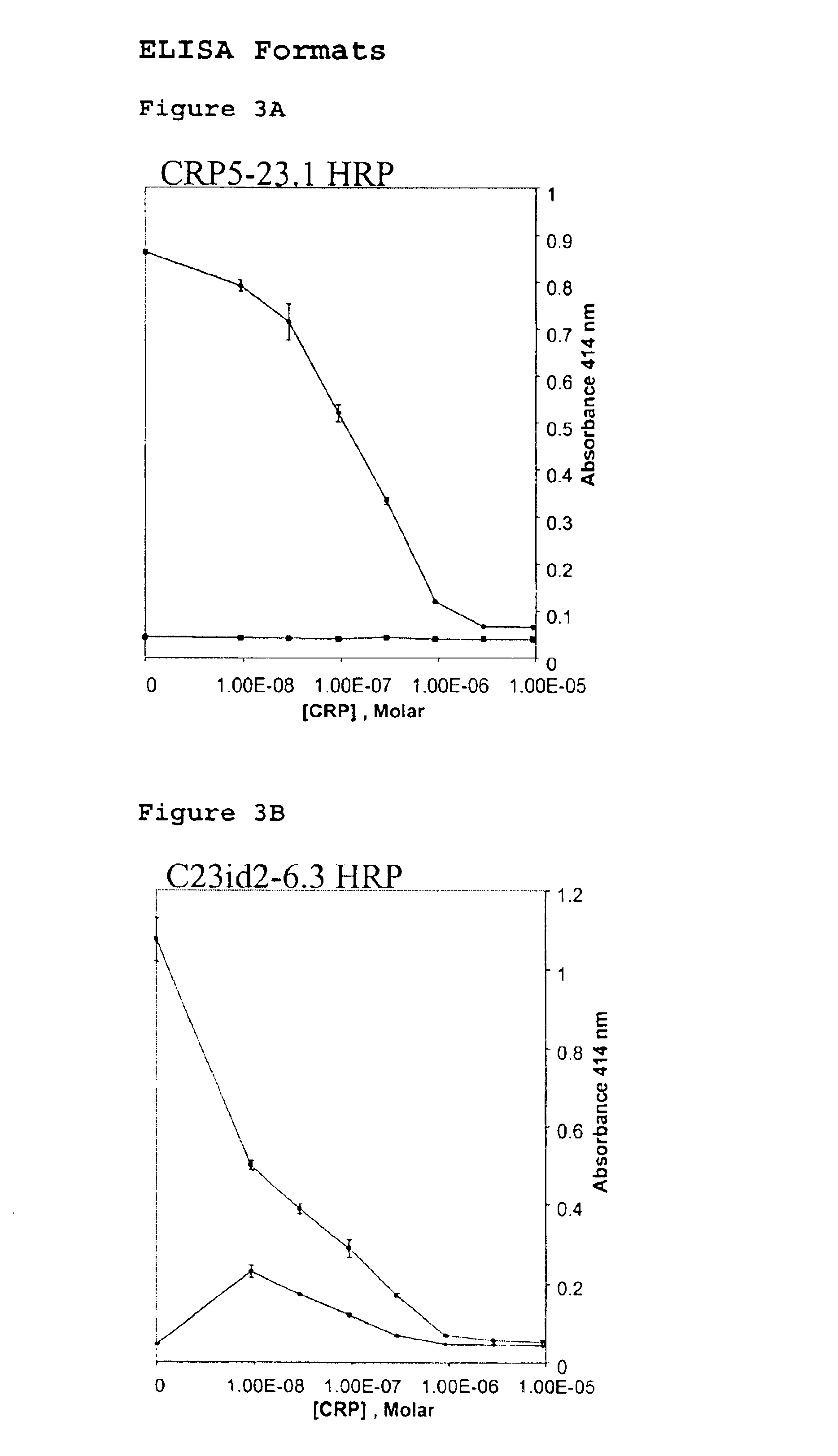
Immunoassay for C-reactive protein
InactiveUS6838250B2High affinityWeak affinityAnimal cellsImmunoglobulins against animals/humansLow affinityAntigen
The present invention relates to new CRP immunoassay compositions. The compositions include a low affinity anti-human CRP monoclonal antibody, and an antiidiotypic antibody raised against it. The invention further provides a method for obtaining antiidiotypic monoclonal antibody populations directed to an antibody that is specific for a high concentration, high molecular weight target antigen.
Owner:ORTHO-CLINICAL DIAGNOSTICS
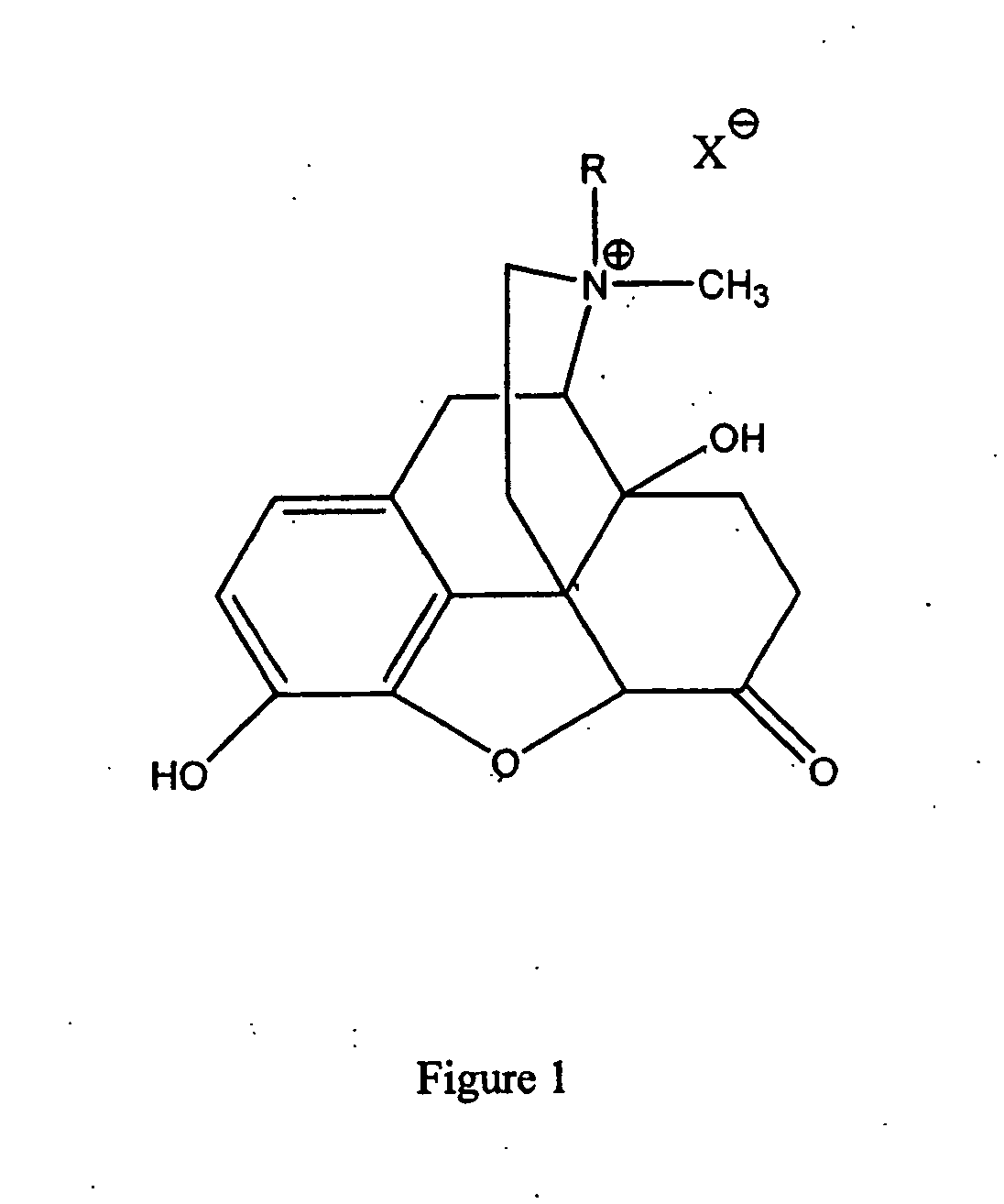
Use of methylnaltrexone and related compounds to treat post-operative gastrointestinal dysfunction
InactiveUS20060205753A1Restoring gastrointestinal activityPromote recoveryBiocideDigestive systemSegmental colectomyBowel dysfunction
Methods and compositions for treating post-surgical gastrointestinal dysfunction are provided. Methods include administering a quaternary derivative of noroxymorphone (e.g., methylnaltrexone) to a patient after a segmental colectomy is performed on the patient.
Owner:PROGENICS PHARMA INC
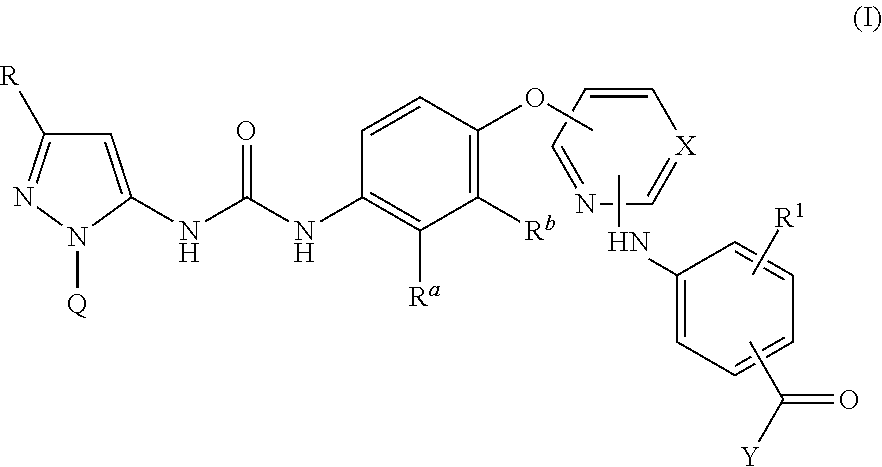
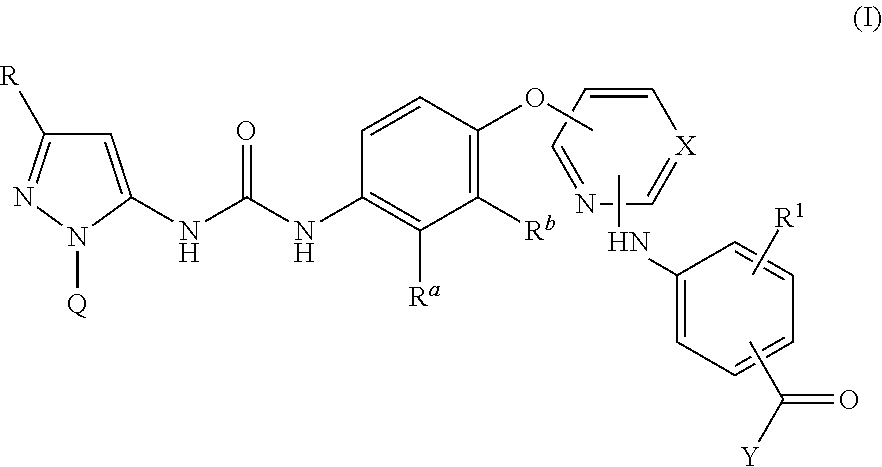
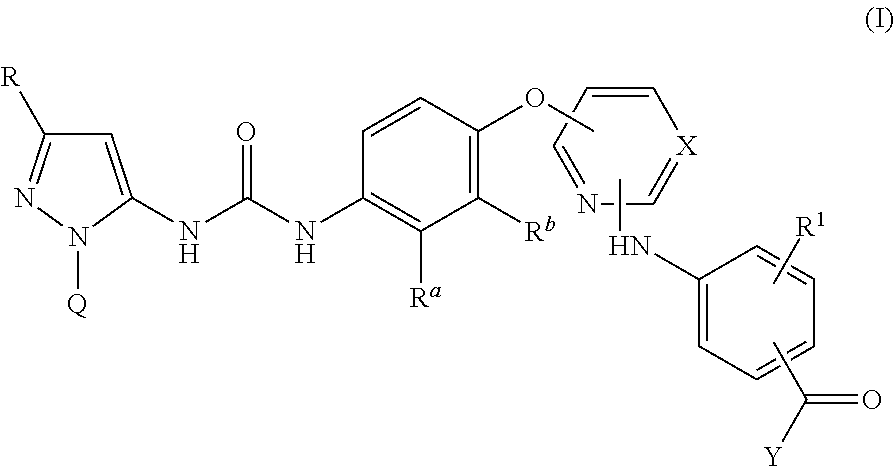
Kinase inhibitors
InactiveUS20140057915A1Weak affinityImproved profileBiocideOrganic active ingredientsTyrosineP38 Mitogen Activated Protein Kinase
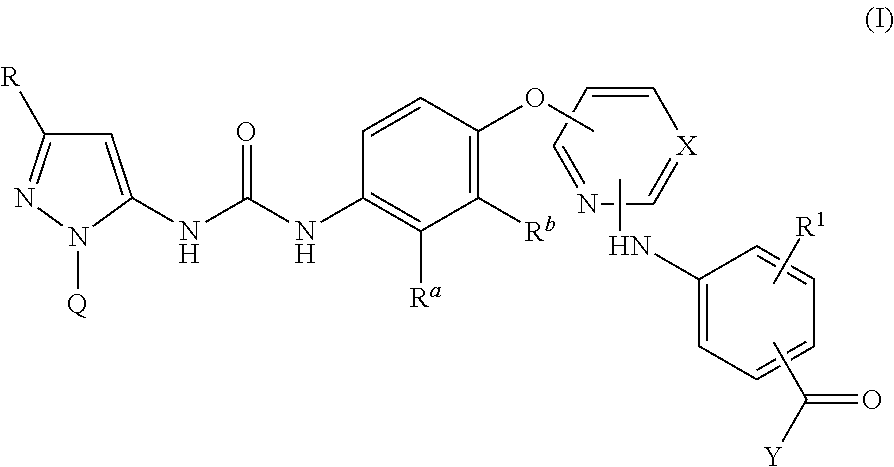
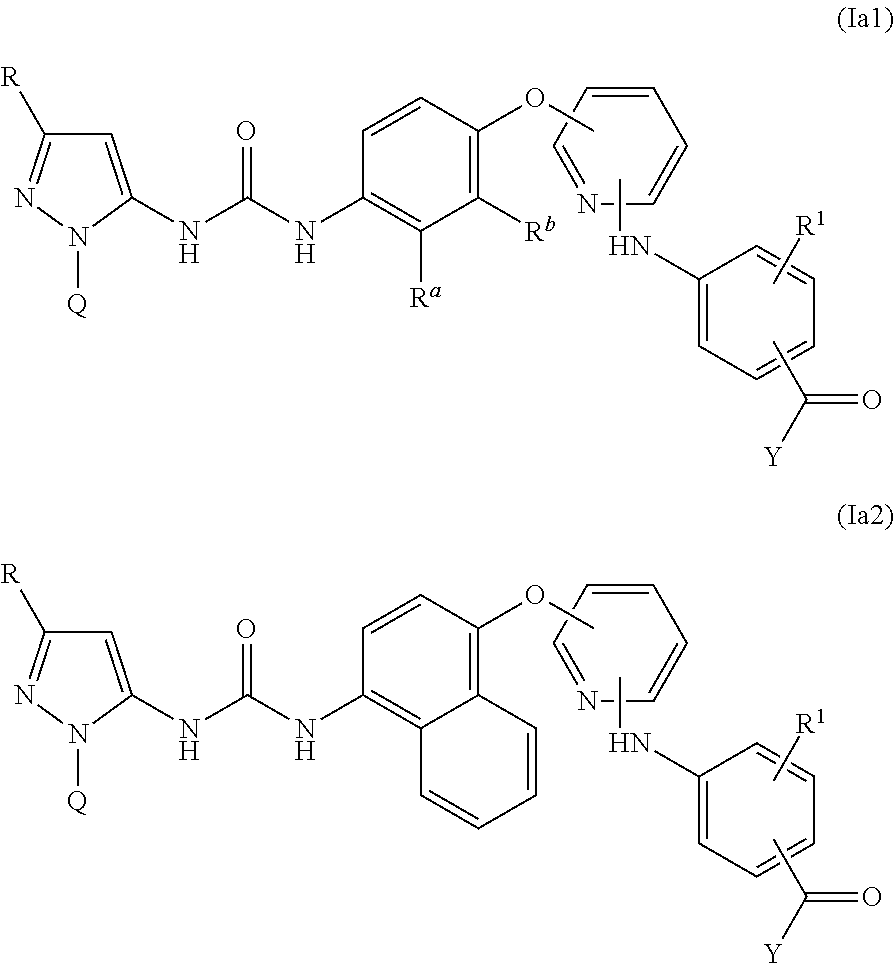
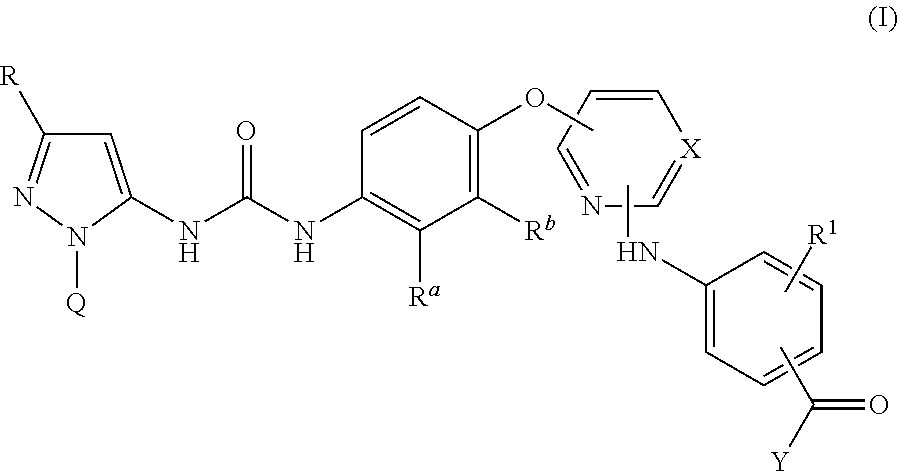
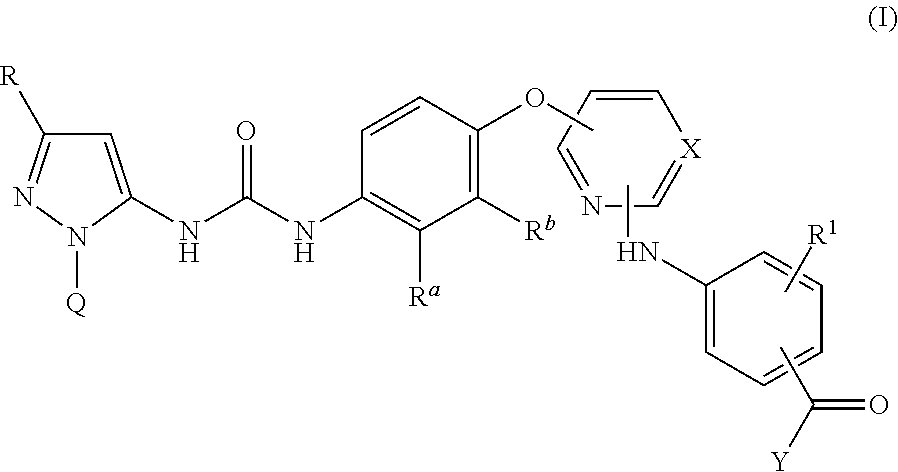
There are provided compounds of formula I,wherein R, R1, Ra, Rb, Q, X and Y have meanings given in the description, which compounds have antiinflammatory activity (e.g. through inhibition of one or more of members of: the family of p38 mitogen-activated protein kinase enzymes; Syk kinase; and members of the Src family of tyrosine kinases) and have use in therapy, including in pharmaceutical combinations, especially in the treatment of inflammatory diseases, including inflammatory diseases of the lung, eye and intestines.
Owner:RESPIVERT +1
Kinase inhibitors
InactiveUS20160016934A1Low affinityWeak affinityBiocideAntipyreticEnzymeP38 Mitogen Activated Protein Kinase
There are provided compounds of formula I,wherein:Y represents NR2R3;one of R2 and R3 represents —[C2-4 alkylene-O]1-12—[C2-4 alkylene]-R2a and the other of R2 and R3 has a meaning given in the description; andR, R1, R2a, Ra, Rb, Q, X and Y have meanings given in the description,which compounds have antiinflammatory activity (e.g., through inhibition of one or more of members of: the family of p38 mitogen-activated protein kinase enzymes; Syk kinase; and members of the Src family of tyrosine kinases) and have use in therapy, including in pharmaceutical combinations, especially in the treatment of inflammatory diseases, including inflammatory diseases of the lung, eye and intestines.
Owner:RESPIVERT +1
Optimized Fc variants
InactiveUS9051373B2Weak affinityFunction increaseAnimal cellsBacteriaAmino acid substitutionAmino acid
The present invention relates to a variant Fc region comprising an amino acid substitution at position 238 of the Fc region as compared to a human parent Fc region, wherein the variant Fc region comprises a 238D substitution, wherein the variant Fc region binds FcγRIIb with increased binding affinity compared to a human parent Fc region.
Owner:XENCOR INC
Polypeptide variants
InactiveUS20060194954A1Weak affinityPeptide/protein ingredientsAntibody mimetics/scaffoldsAmino acid substitutionComplement-dependent cytotoxicity
A variant of a polypeptide comprising a human IgG Fc region is described, which variant comprises an amino acid substitution at one or more of amino acid positions 270, 322, 326, 327, 329, 331, 333 or 334 of the human IgG Fc region. Such variants display altered effector function. For example, C1q binding and / or complement dependent cytotoxicity (CDC) activity may be altered in the variant polypeptide. The application also discloses a variant of a parent polypeptide comprising a human IgG Fc region, which variant has a better binding affinity for human C1q than the parent polypeptide
Owner:GENENTECH INC
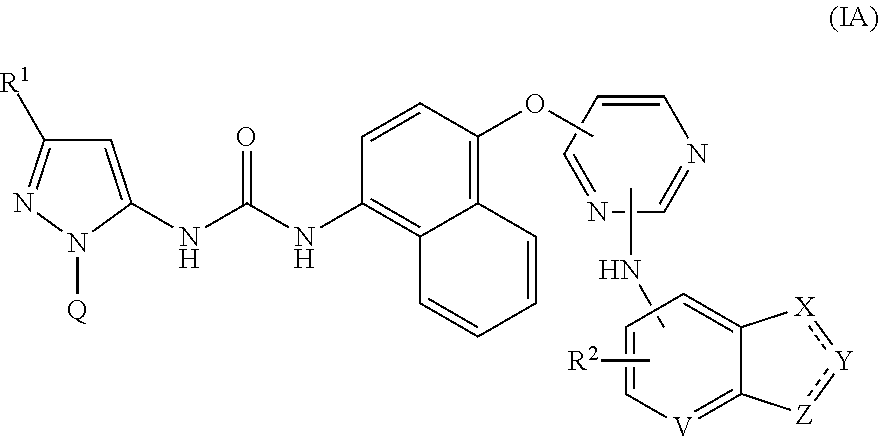
Pyrazolyl-ureas as kinase inhibitors
There are provided compounds of formula I,wherein R, R1, Ra, Rb, Q, X and Y have meanings given in the description, which compounds have antiinflammatory activity (e.g., through inhibition of one or more of members of: the family of p38 mitogen-activated protein kinase enzymes; Syk kinase; and members of the Src family of tyrosine kinases) and have use in therapy, including in pharmaceutical combinations, especially in the treatment of inflammatory diseases, including inflammatory diseases of the lung, eye and intestines.
Owner:OXULAR ACQUISITIONS LTD
Optimized Fc variants
Owner:XENCOR INC
Pyrazole derivatives as p38 MAP inhibitors
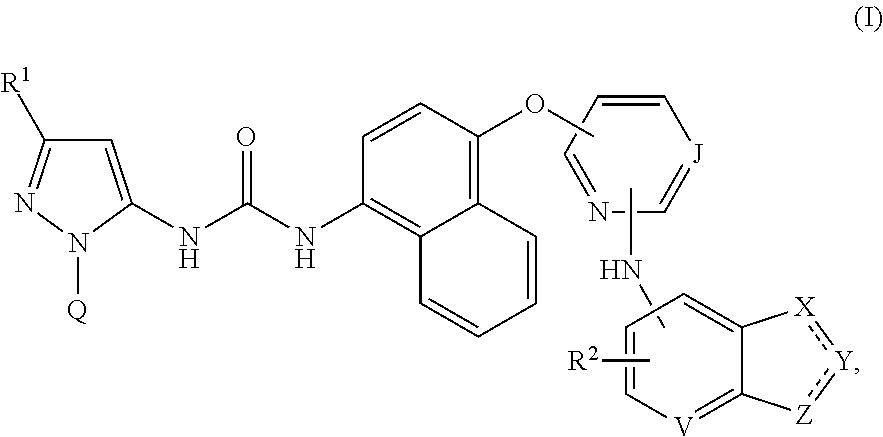
InactiveUS9249125B2Weak affinityImproved profileSenses disorderOrganic chemistryUveitisUlcerative colitis
Compounds of formula (I):wherein R1, R2, J, Q, V, X, Y and Z are defined herein are disclosed. The compounds are inhibitors of the family of p38 mitogen-activated protein kinase enzymes (referred to herein as p38 MAP kinase inhibitors), particularly the alpha sub-type thereof, and of Syk kinase and the Src family of tyrosine kinases. The compounds may be used in therapy, including in pharmaceutical combinations, especially in the treatment of inflammatory diseases, in particular inflammatory diseases of the lung, such as asthma and COPD, as well as those of the gastrointestinal tract, such as ulcerative colitis and Crohn's disease and of the eye, such as uveitis.
Owner:RESPIVERT +1
Novel inhibitor compounds of phosphodiesterase type 10a
InactiveUS20130116241A1High selectivityGood metabolic stabilityBiocideNervous disorderDiseaseMedical disorder
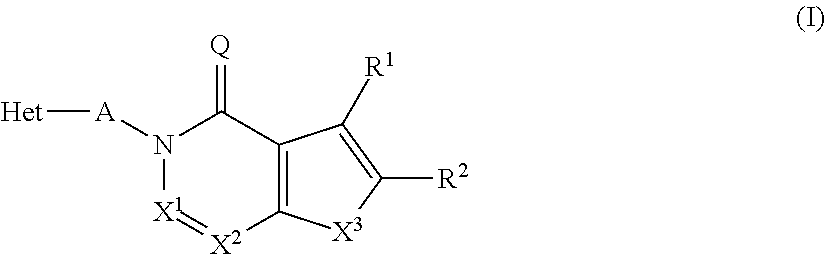
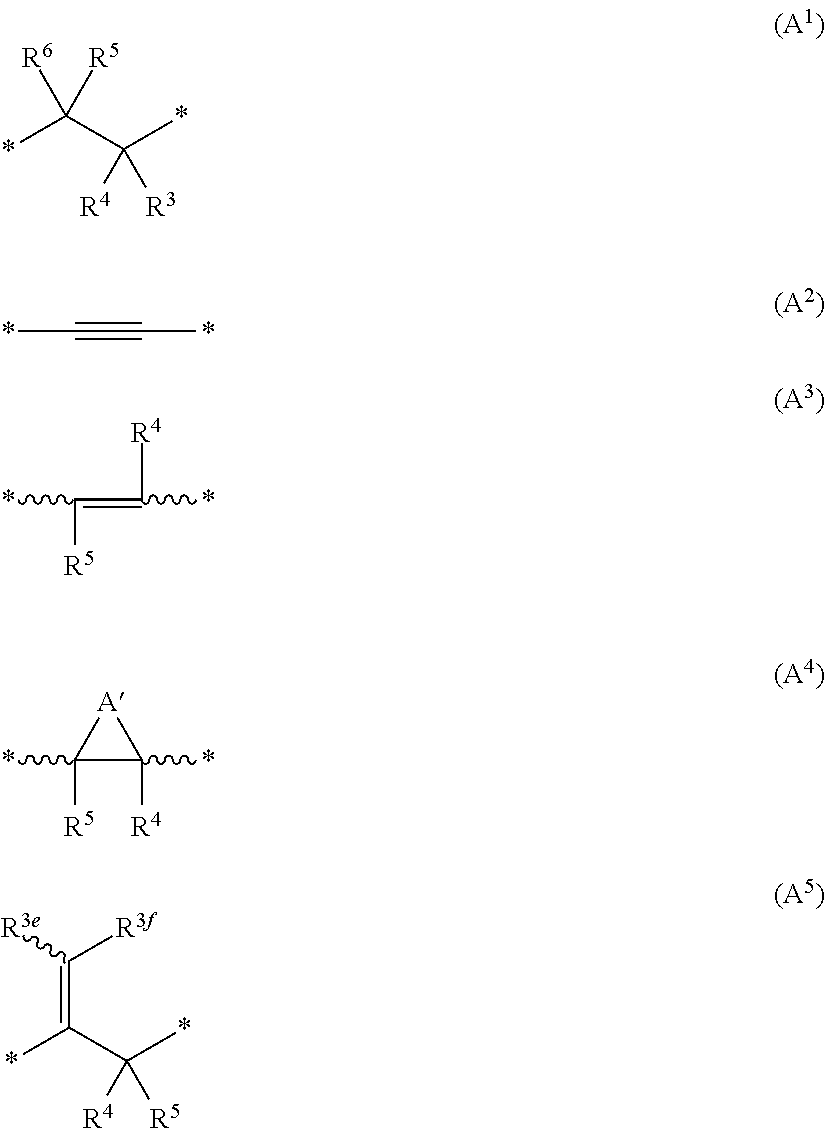
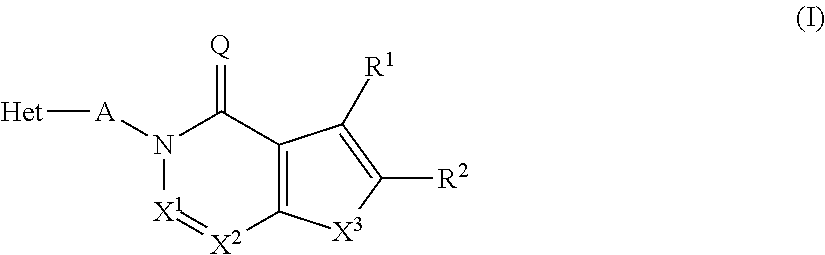
The present invention relates to novel compounds of the formula I which are inhibitors of phosphodiesterase type 10A and to their use for the manufacture of a medicament and which thus are suitable for treating or controlling of medical disorders selected from neurological disorders and psychiatric disorders, for ameliorating the symptoms associated with such disorders and for reducing the risk of such disorders.whereinQ is O or S, X1 is N or CH, X2 is N or C—R7; X3 is O, S—X4═C(R8)—, where C(R8) is bound to the carbon atom which carries R2, or —X5═C(R9)—, where X5 is bound to the carbon atom which carries R2; X4 is N or C—R9; X5 is N;Het is selected from optionally substituted phenyl, monocyclic hetaryl and fused bicyclic hetaryl;R1 is selected inter alia from hydrogen, halogen, OH, C1-C4-alkyl, trimethylsilyl, C1-C4-alkylsulfanyl, C1-C4-alkoxy-C1-C4-alkyl, C1-C4-alkoxy, C1-C4-alkoxy-C1-C4-alkoxy, the moiety Y1-Cyc1;R2 is selected inter alia from hydrogen, halogen, OH, C1-C4-alkyl, trimethylsilyl, C1-C4-alkoxy-C1-C4-alkyl, C1-C4-alkoxy, C1-C4-alkoxy-C1-C4-alkoxy, C2-C4-alkenyloxy, etc;A represents one of the following groups A1, A2, A3, A4 or A5:where * indicates the points of attachment to Het and to the nitrogen atom, respectively;and where R3 to R9, R3e, R3f, A′, Y1 and Cyc1 are defined in the claims.
Owner:ABBVIE INC +1
Survivin-derived peptides and use thereof
InactiveUS20040210035A1Easy to filterWeak affinityPeptide/protein ingredientsDepsipeptidesMHC class IWilms' tumor
MHC Class I-restricted peptides derived from the tumor associated antigen, survivin, which peptides are capable of binding to Class I HLA molecules at a high affinity, capable of eliciting INF-gamma-producing cells in a PBL population of a cancer patient and capable of in situ detection of cytotoxic T cells in a tumor tissue, therapeutic and diagnostic composition comprising the peptide and uses hereof.
Owner:SURVAC
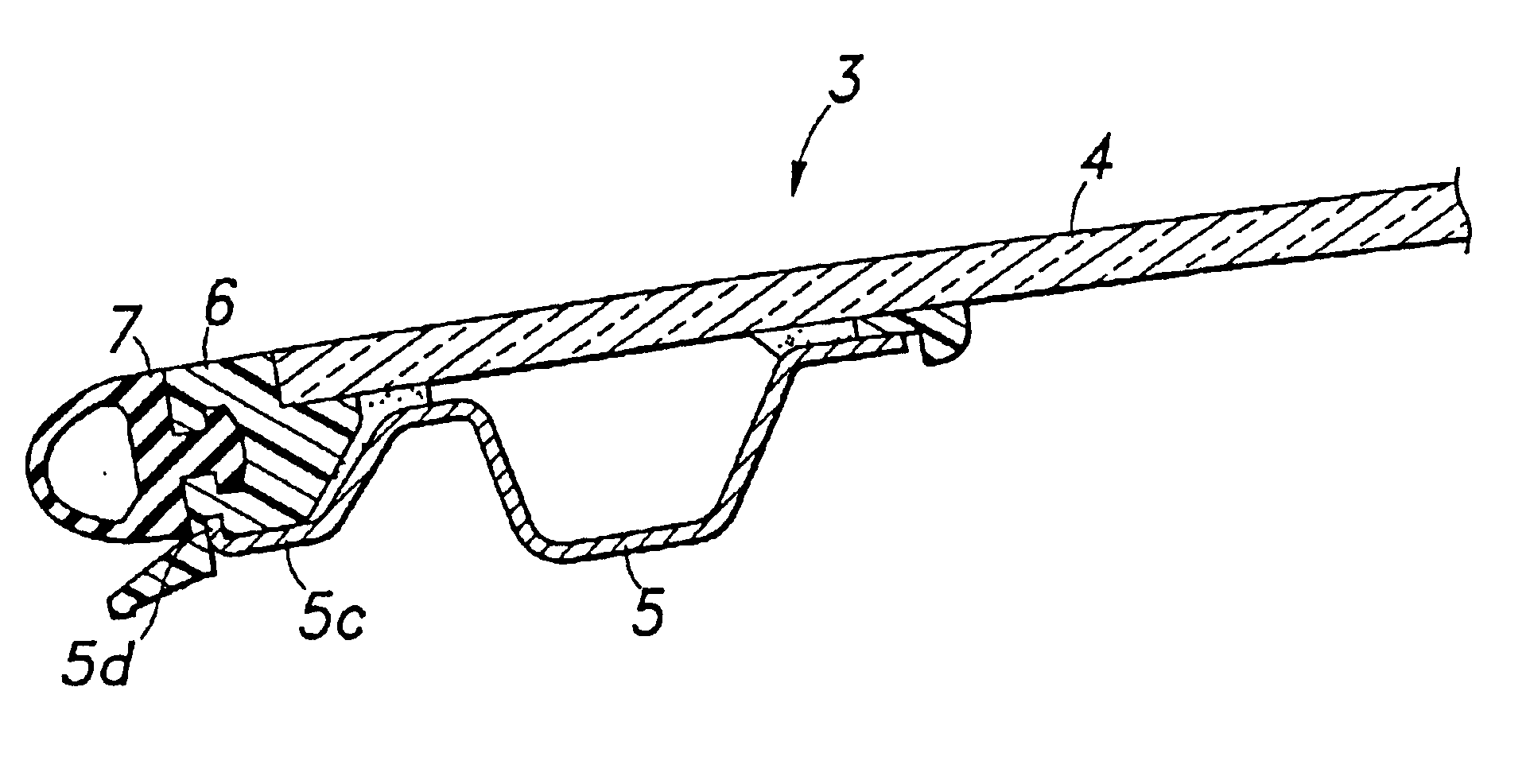
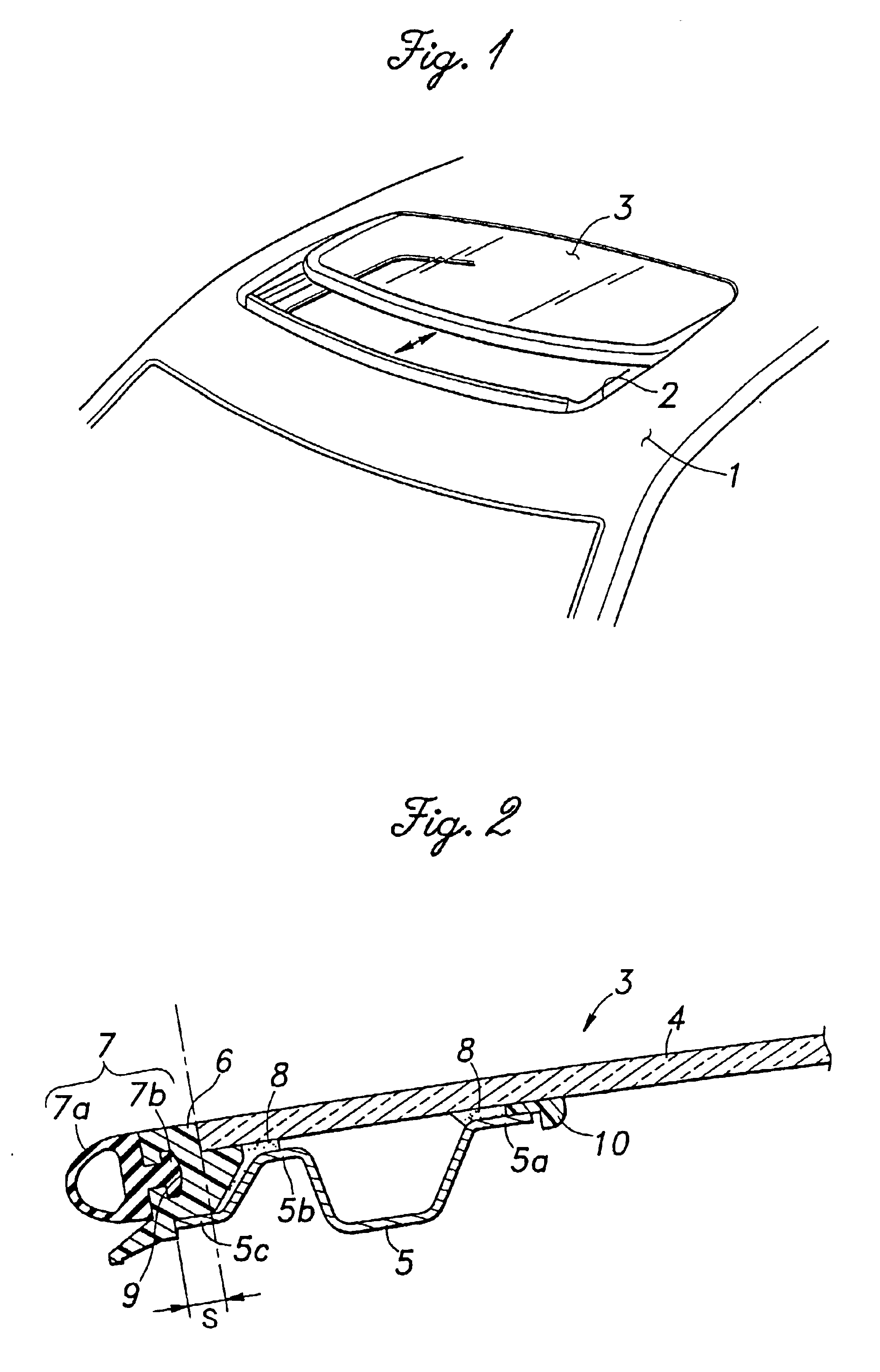
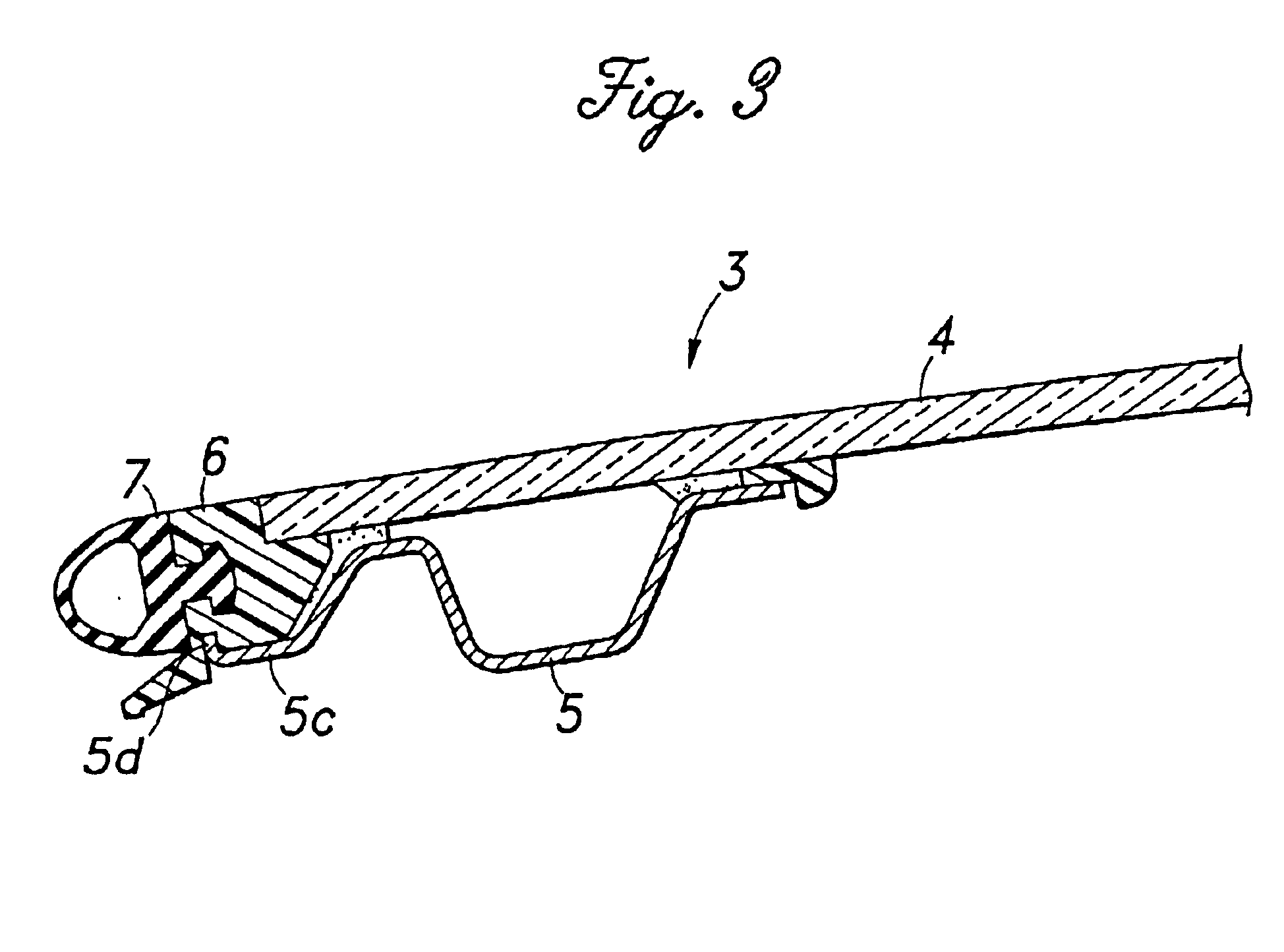
Glass panel arrangement for automotive sun roof systems
InactiveUS6893084B2Secure attachmentWeak affinityEngine sealsSuperstructure subunitsBraced framePlastic materials
In a glass panel arrangement for automotive sun roof systems having a weather strip frame which is disposed substantially flush with the glass panel, a support frame made of metallic material and attached to a lower surface of the glass panel along a peripheral part of the glass panel is provided with an outer extension for supporting a part of the weather strip frame extending away from the edge of the glass panel from below so as to resist the tendency for the weather strip frame to get detached from the outer periphery of the glass panel. Therefore, the weather strip frame can be made of plastic material which has a relatively low affinity with the glass panel. In particular, if the outer extension is provided with an upwardly directed flange, not only the retaining force of the outer extension is improved but also the bending rigidity of the outer extension is improved.
Owner:HONDA MOTOR CO LTD +1
Targeted intracellular delivery of antiviral agents
InactiveUS20100129437A1High affinitySpecificity of bindingPowder deliveryBiocideInternalizationChemical agent
The invention relates to methods of targeted drug delivery of antiviral compounds, including, chemical agents (like nucleoside analogs or protease inhibitors) and nucleic acid based drugs (like DNA vaccines, antisense oligonucleotides, ribozymes, catalytic DNA (DNAzymes) or RNA molecules, siRNAs or plasmids encoding thereof). Furthermore, the invention relates to targeted drug delivery of antiviral compounds to intracellular target sites within cells, tissues and organs, in particular to target sites within the central nervous system (CNS), into and across the blood-brain barrier, by targeting to internalizing uptake receptors present on these cells, tissues and organs. Thereto, the antiviral compounds, or the pharmaceutical acceptable carrier thereof, are conjugated to ligands that facilitate the specific binding to and internalization by these receptors.
Owner:TO BBB HLDG
Pyrazole derivatives as p38 map inhibitors
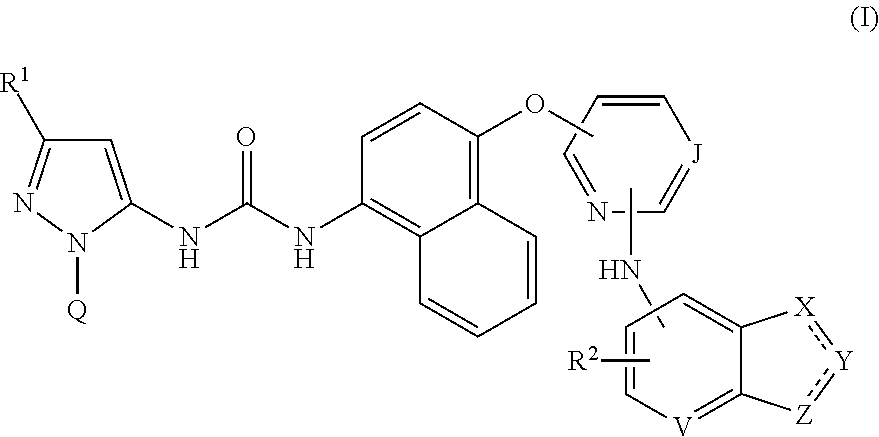
InactiveUS20150203475A1Weak affinityImproved profileBiocideOrganic active ingredientsUveitisUlcerative colitis
Compounds of formula (I):wherein R1, R2, J, Q, V, X, Y and Z are defined herein are disclosed. The compounds are inhibitors of the family of p38 mitogen-activated protein kinase enzymes (referred to herein as p38 MAP kinase inhibitors), particularly the alpha sub-type thereof, and of Syk kinase and the Src family of tyrosine kinases. The compounds may be used in therapy, including in pharmaceutical combinations, especially in the treatment of inflammatory diseases, in particular inflammatory diseases of the lung, such as asthma and COPD, as well as those of the gastrointestinal tract, such as ulcerative colitis and Crohn's disease and of the eye, such as uveitis.
Owner:RESPIVERT +1
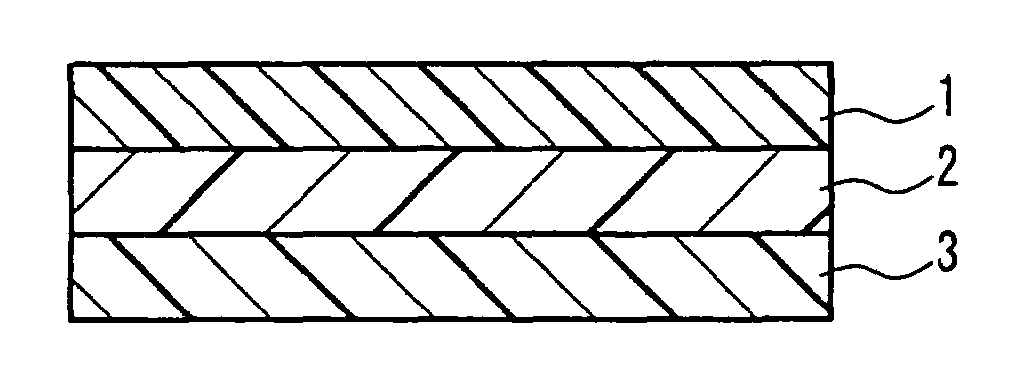
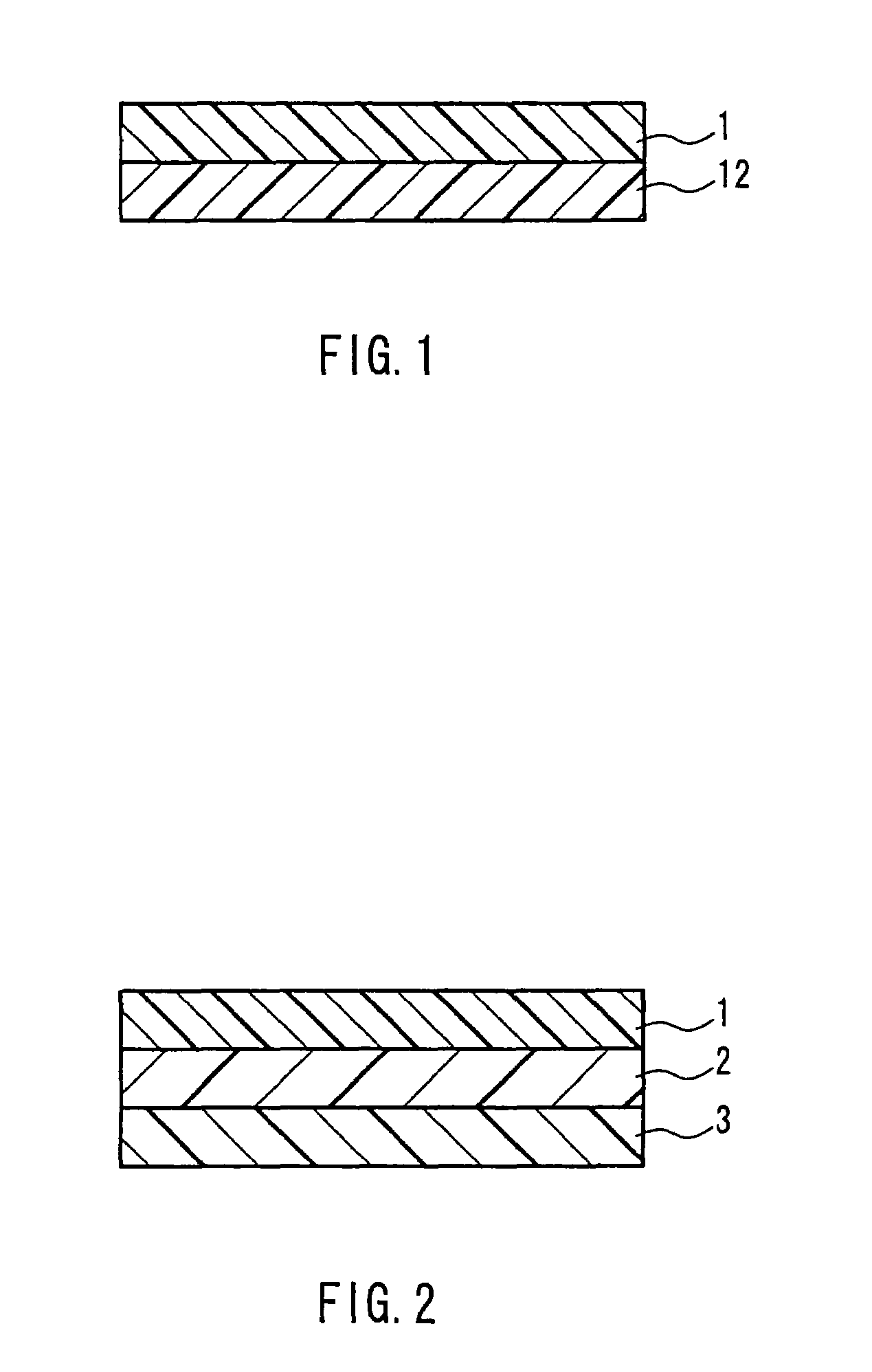
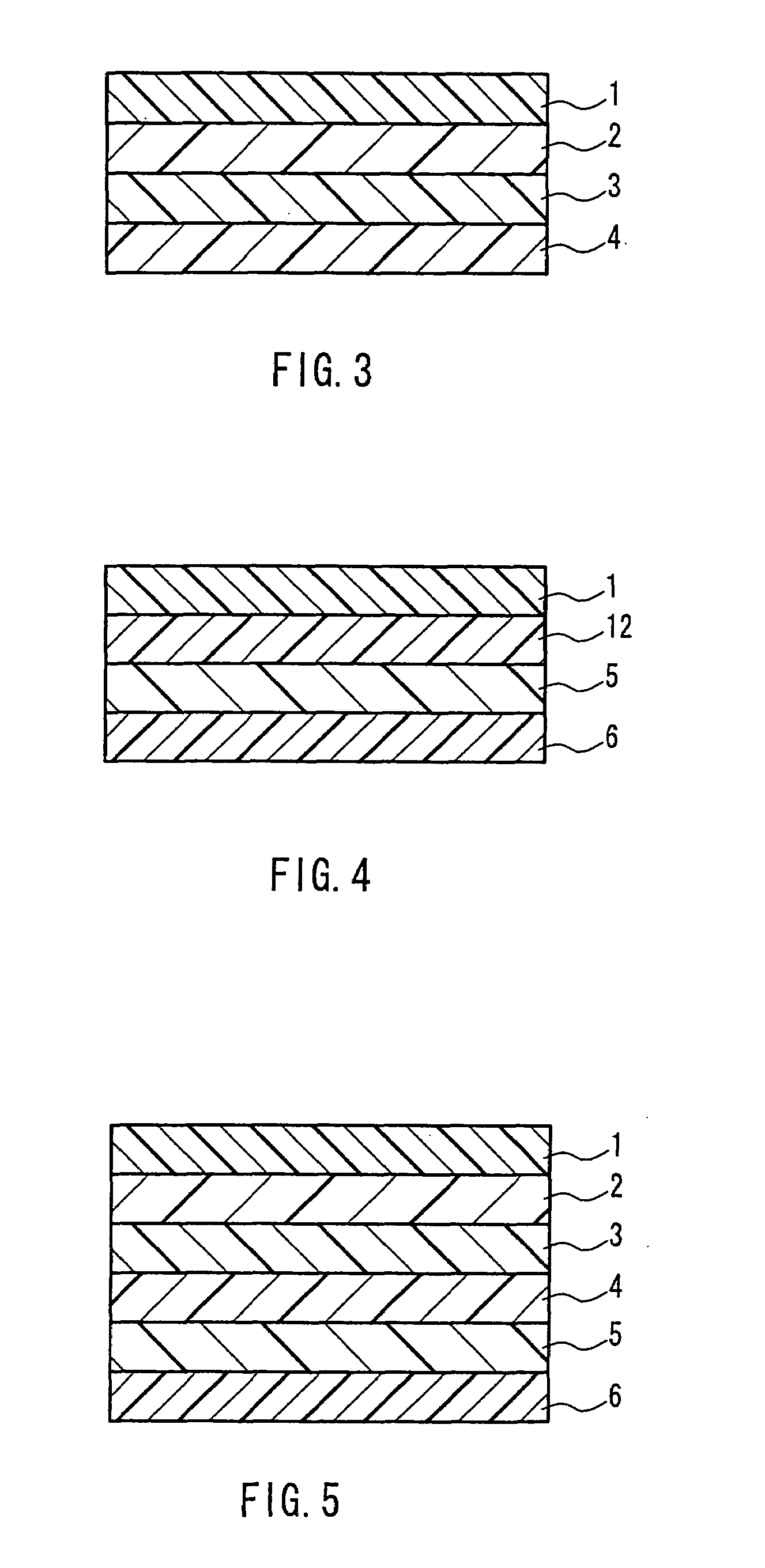
Laminate for printing and, printing method and printed matter using the same
InactiveUS7238644B2Inhibit migrationWeak affinityLayered productsAblative recordingEngineeringDyeing
A laminate for printing for coloring a resin layer by allowing a sublimable dyeing agent to permeate into the inside of the resin layer through heating, which comprises, in the order from the surface, a surface resin layer A(1) having a weak affinity with the sublimable dyeing agent and allowing the dyeing agent to pass through it and a coloring resin layer B(12) having strong affinity with the dyeing agent and preventing the transfer of the dyeing agent; and a printing method and a printed mater using the lamainate. The laminate for printing has, formed as an inner layer, a coloring resin layer having strong affinity with a sublimable dyeing agent and capable of preventing the transfer of the dyeing agent, which allows the prevention of the transfer of a sublimable dyeing agent having been printed.
Owner:KIWA CHEM IND CO LTD
Device and method for preparing washed red blood cells for newborn transfusions
InactiveUS20050274679A1Small volumeMinimizes recipient 's donor exposureWater/sewage treatment by centrifugal separationOther blood circulation devicesMedicinePotassium
A newborn transfusion cell washing device generally comprising a disposable, graduated test tube shaped container having a cap with an inlet port, an injection / sampling port, a suction port, and a vent. The container is capable of being inserted into a conventional clinical centrifuge. The device requires a relatively small volume to operate, 25 ml or less per procedure, and can be performed easily by any hospital blood bank technologist without any special skills. Washed RBCs can be provided to the patient in a timely manner, without the need for “fresh blood.” Any in-dated RBCs can be washed to remove excessive potassium and other toxins. The main RBC aliquot can be saved and repeatedly sampled until the unit is expired or exhausted. This provides a cost savings to the hospital and more importantly, minimizes the recipient's donor exposure.
Owner:THE BOARD OF SUPERVISORS OF LSU A&M
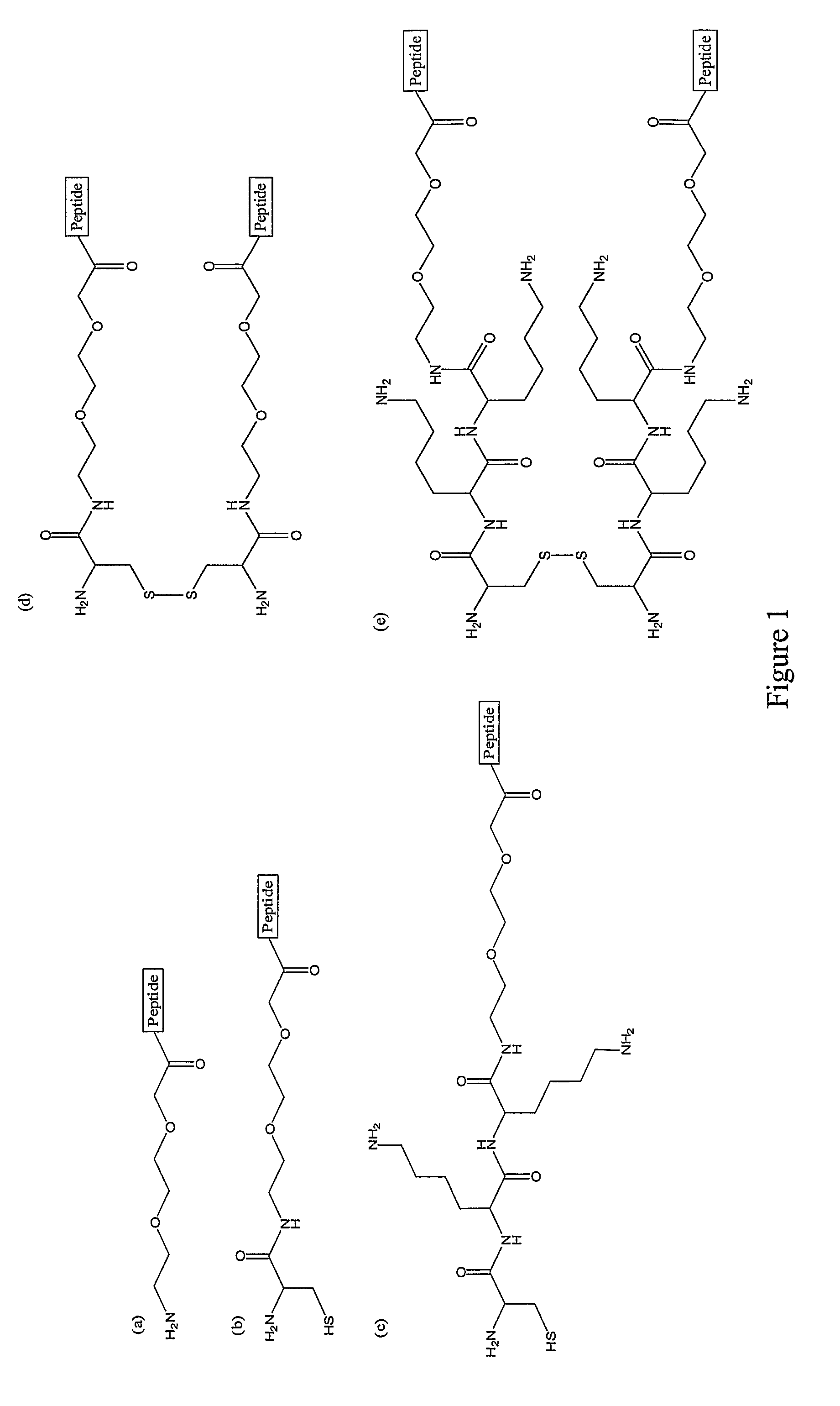
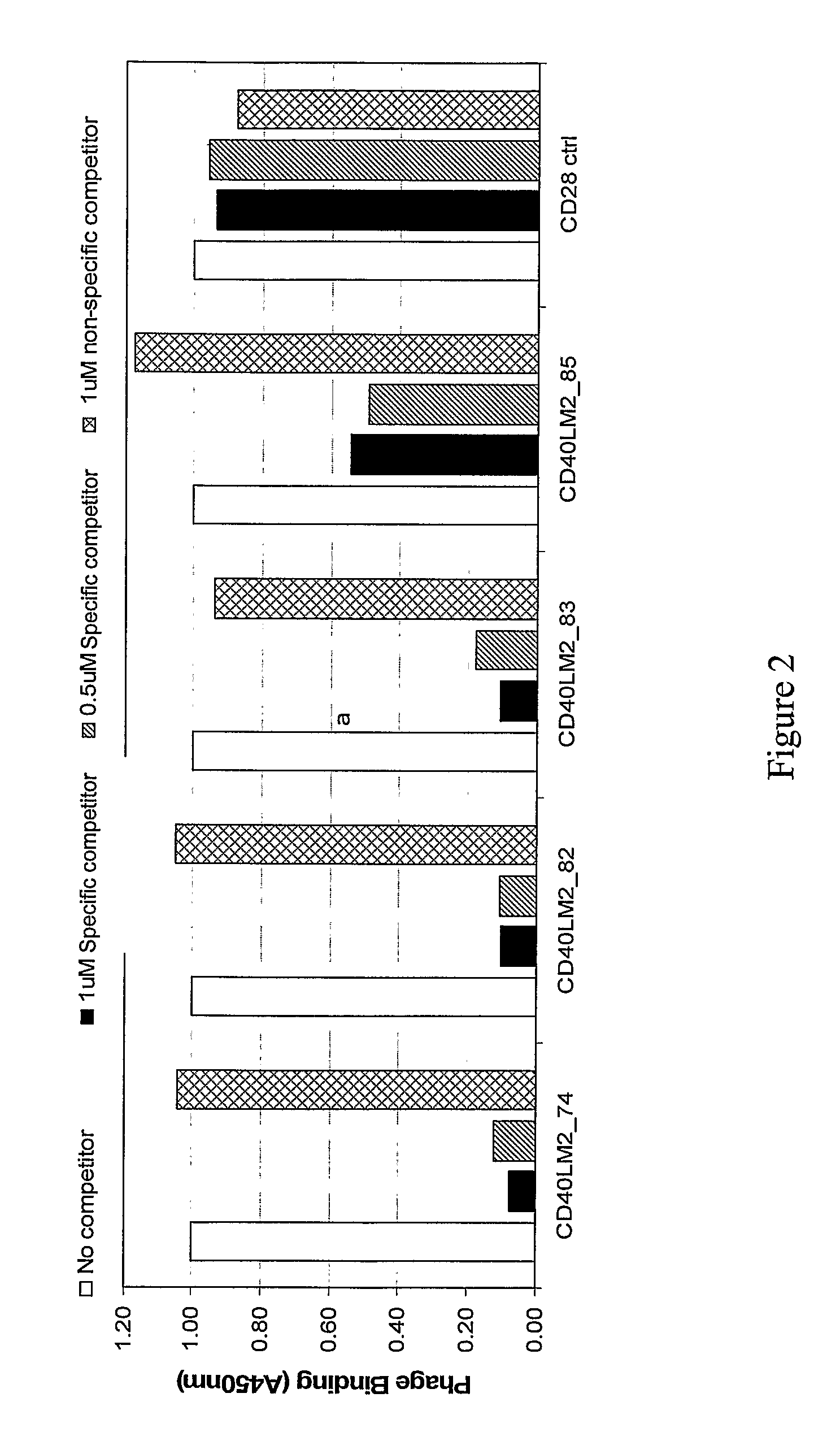
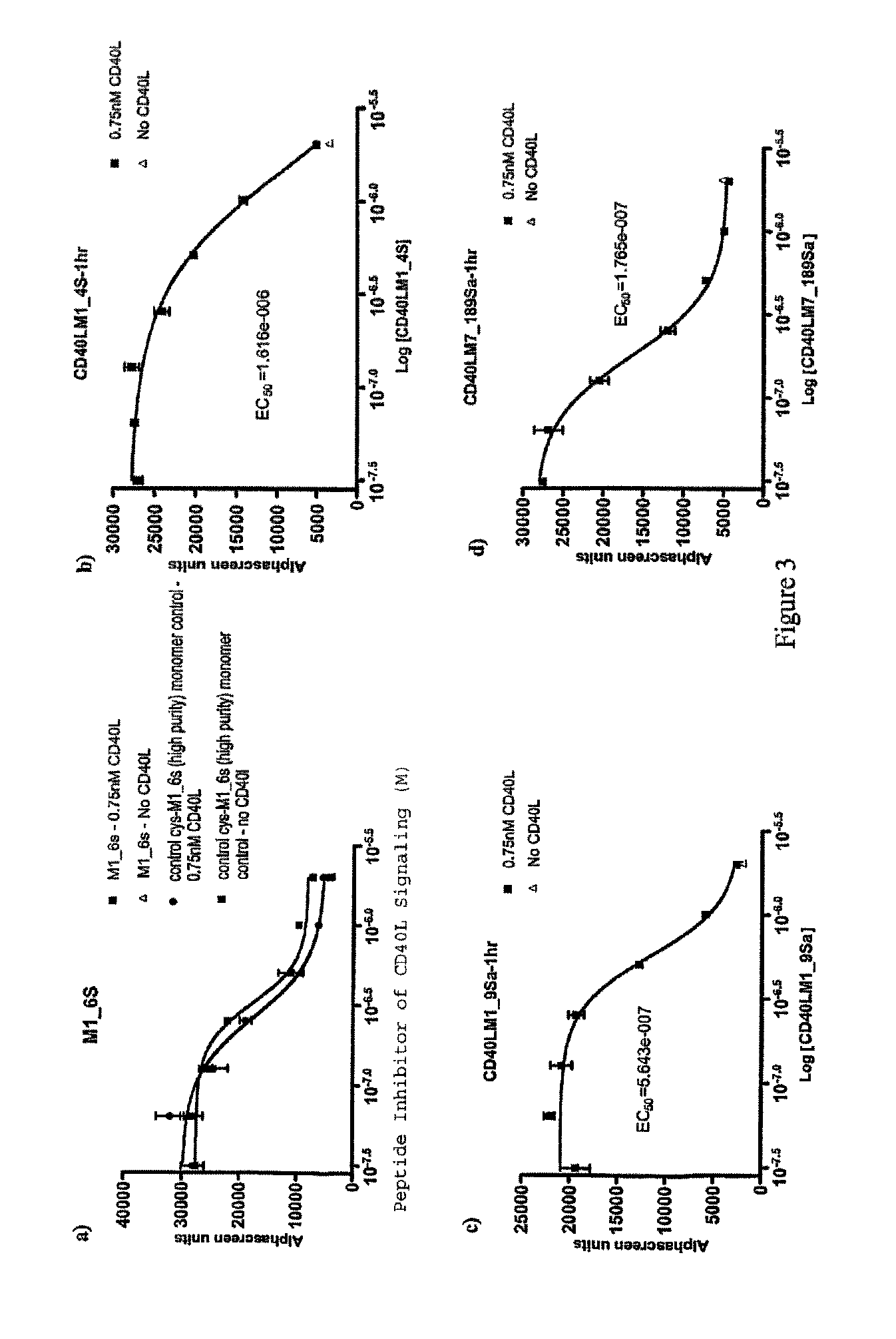
CD40-L Inhibitory Peptides
InactiveUS8802634B2Weak affinityInhibitory activityAntipyreticAnalgesicsNatural sourceSynthetic derivatives
The present invention provides compositions comprising peptidyl inhibitors of CD40L-dependent signalling that are not derived from a natural binding partner of CD40L such as CD40, or from a native CD40-CD40L interface. More particularly, the peptidyl inhibitors of the present invention are derived from natural sources that do not express CD40-CD40L costimulatory pathways. The invention also provides synthetic derivatives and analogs of the peptidyl inhibitors having enhanced binding affinity for CD40L or enhanced inhibitory activity relative to their parent molecules.
Owner:PHYLOGICA
Toner and method for producing toner
A toner including: a binder resin containing an ethylene-vinyl acetate copolymer; a polysiloxane derivative A represented by structural formula 1; and a polysiloxane derivative B represented by structural formula 2:(in structural formula 1, R1 to R10 each independently represent a methyl group or a phenyl group, and l, m and n each independently represent an integer of at least 1)(in structural formula 2, at least one of R11 to R20 is an organic group having a C4-30 alkyl group, a C4-30 alkoxy group, an acrylic group, an amino group, a methacrylic group or a carboxyl group, the remaining groups among R11 to R20 each independently represent a methyl group or a phenyl group, and p, q and r each independently represent an integer of at least 1).
Owner:CANON KK
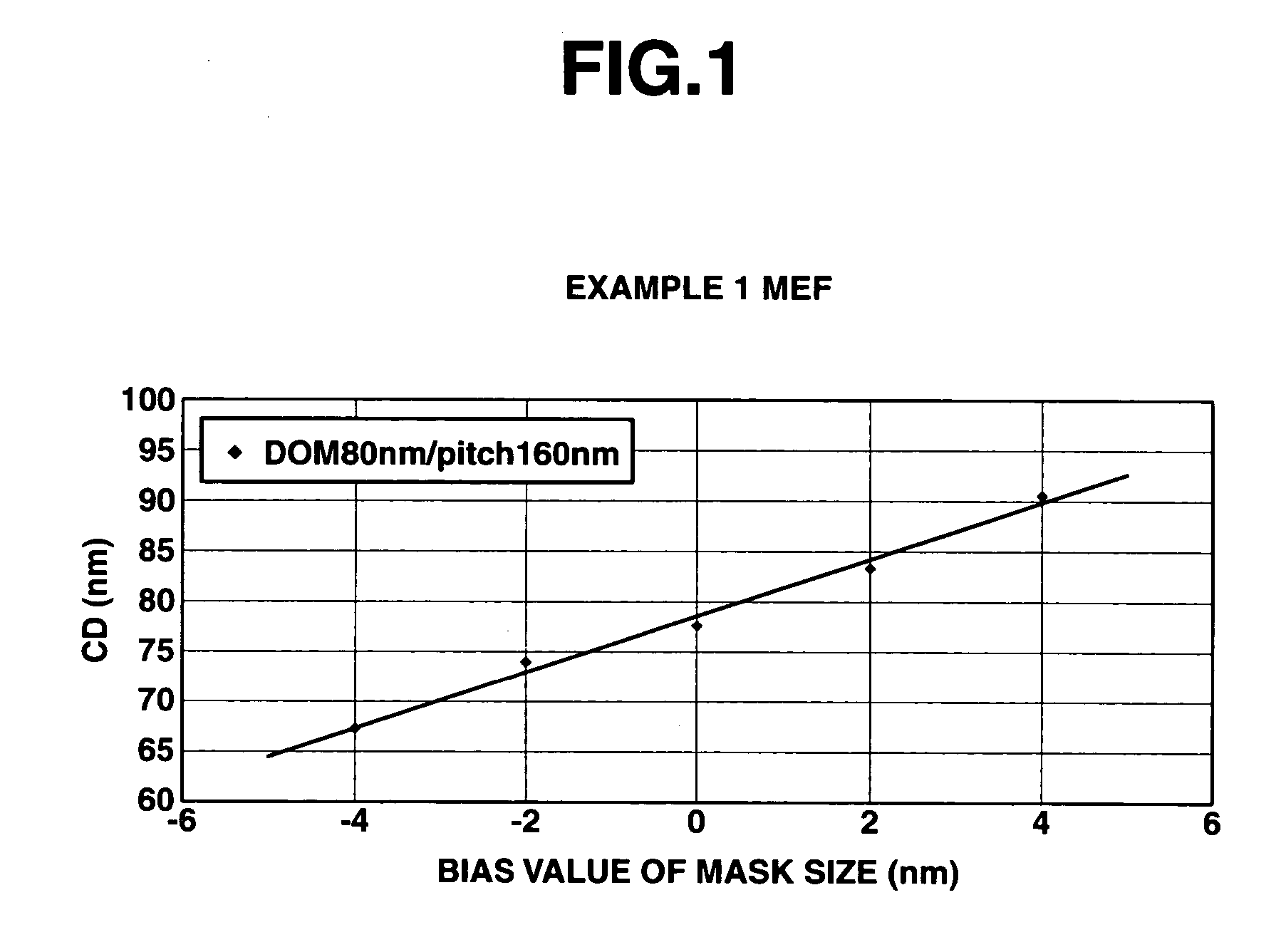
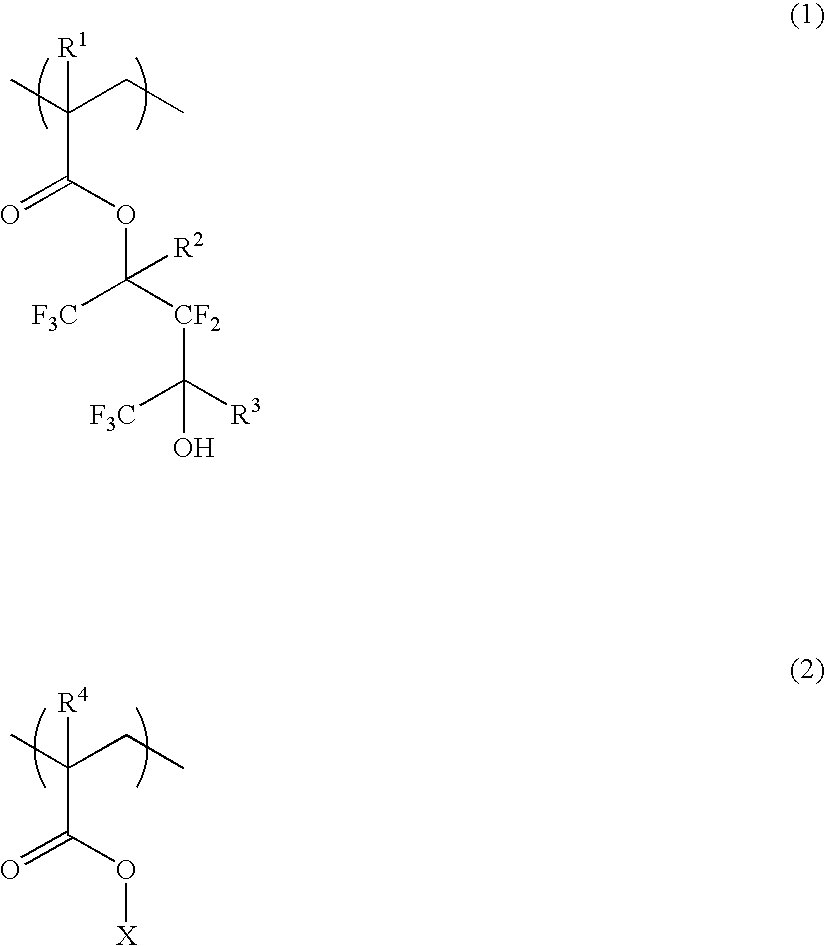
Polymer, resist composition and patterning process
ActiveUS20070099114A1High affinityWeak affinityPhotosensitive materialsPhotomechanical apparatusResistImage resolution
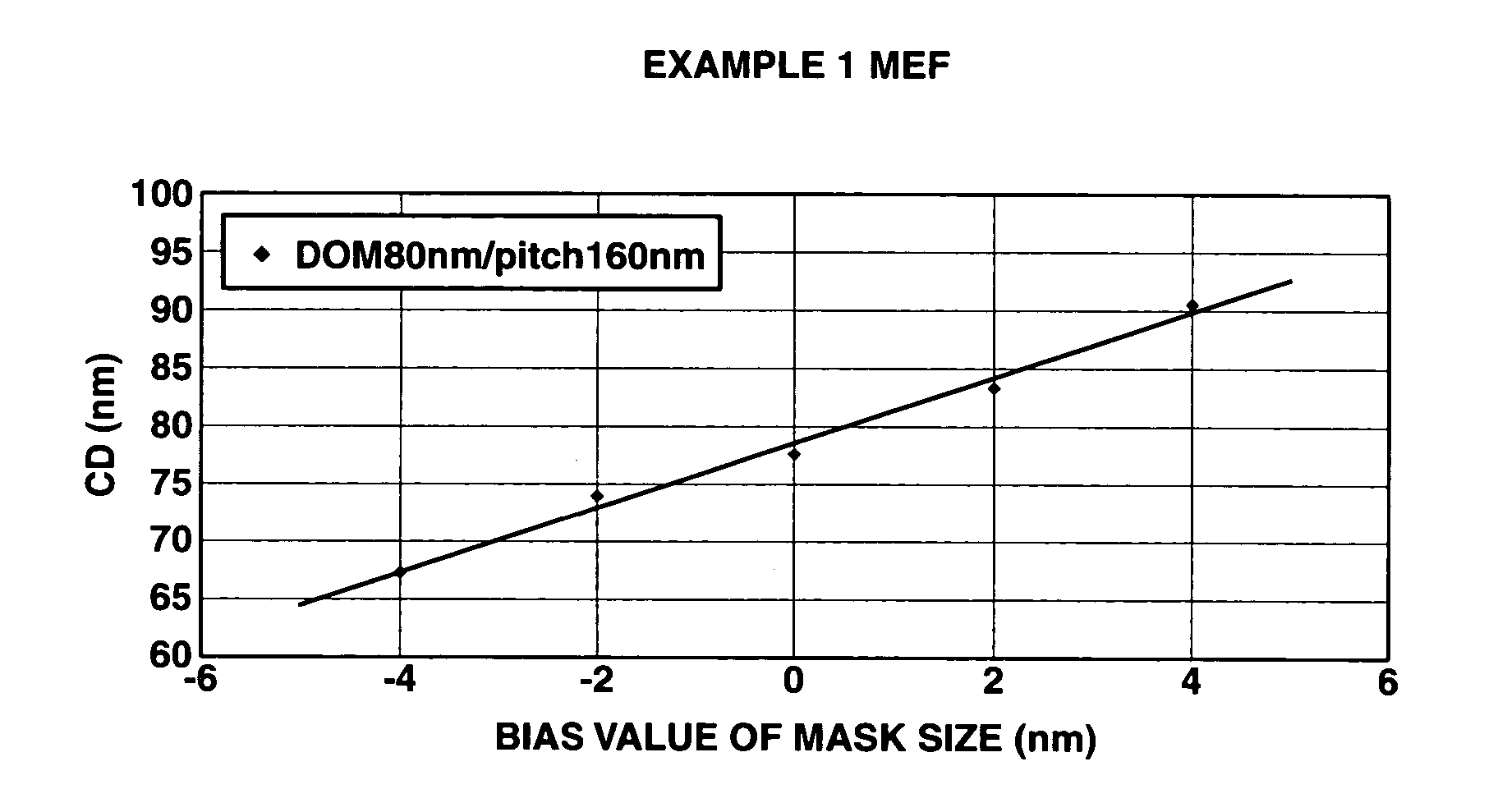
A polymer of which dissolution rate in an alkaline developer increases under the action of acid comprises recurring units having formulae (1) and (2) wherein R1, R2, and R4 are H or methyl, R3 is difluoromethyl or trifluoromethyl, and X is tertiary alkyl. A resist composition comprising the polymer has a high sensitivity and resolution, decreased pattern collapse during development, and minimized MEF and is best suited as micropatterning material for the VLSI manufacture.
Owner:SHIN ETSU CHEM IND CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com