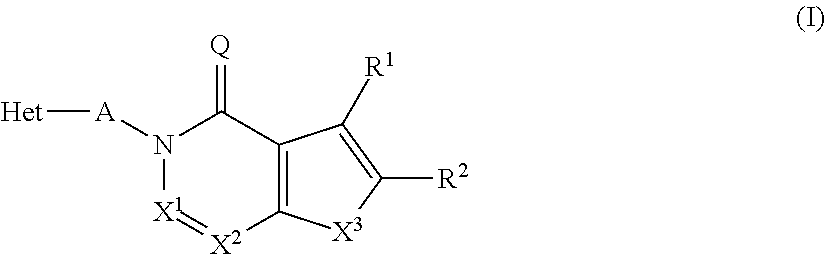
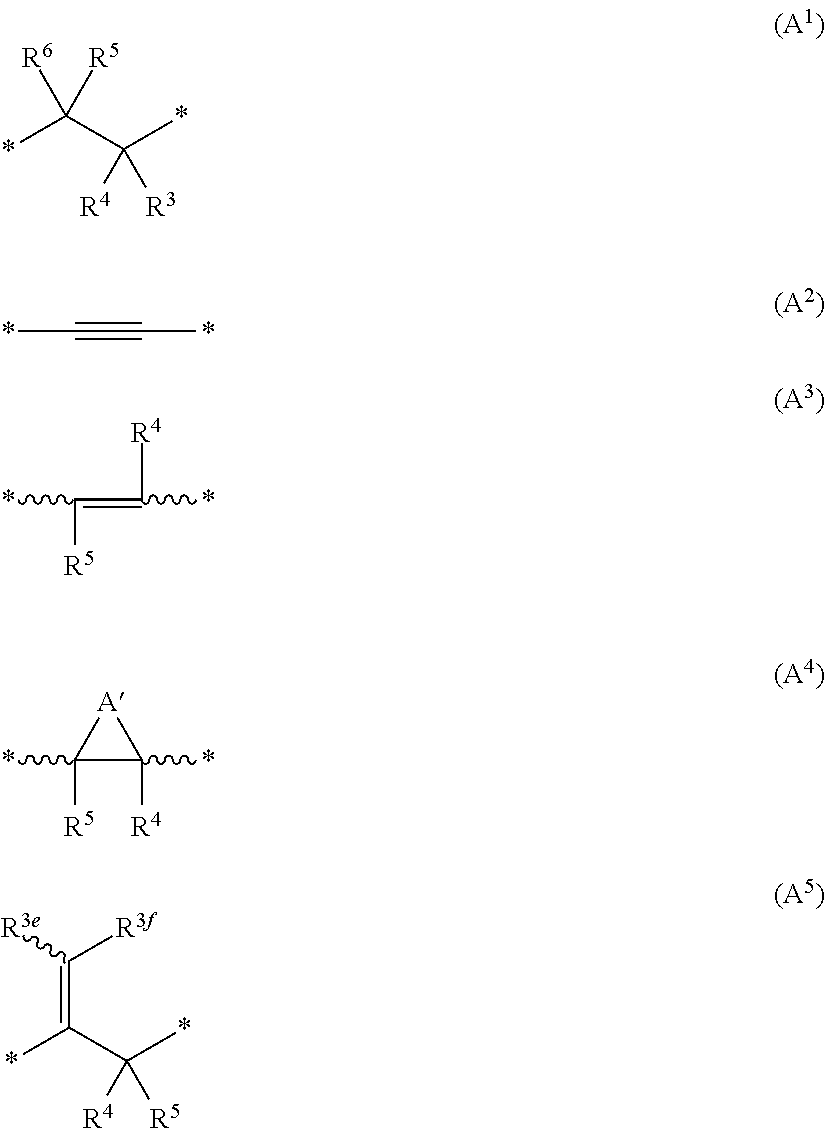
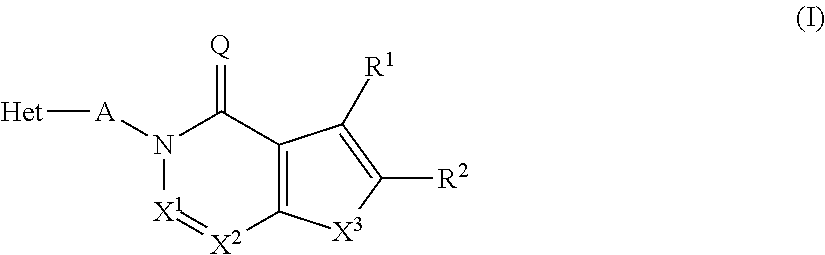
Novel inhibitor compounds of phosphodiesterase type 10a
a technology of phosphodiesterase and inhibitor compounds, which is applied in the direction of biocide, drug compositions, metabolic disorders, etc., can solve the problems of short exposure half-life after systemic administration, poor potency and selectivity of papaverine, and significant limitations in this regard, and achieve enhanced metabolic stability, high selectivity, and high selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
preparation examples
I. Preparation of Intermediates
[0957]The starting materials used in the examples are either commercially available or can be synthesized by the average skilled person trained in organic chemistry following routine laboratory practice as outlined, for example in the examples below.
a) Preparation of Compounds of the General Formula Het-A′-OH
a1) 2-Quinolin-2-yl-ethanol
[0958]
a1.1) Quinolin-2-yl-acetic acid ethyl ester
[0959]To a suspension of vacuum dried Zn dust (6.0 g, 93.8 mmol) in dry THF (100 mL) was added TMSCl (0.5 mL) dropwise over 5 min under N2 atmosphere and under stiffing. The mixture was stirred for 30 min and warmed to 45° C. Ethyl bromoacetate (5.2 mL, 46.9 mmol) was added dropwise via a syringe. After addition, the mixture was stirred at the same temperature for 1 h. After sedation at room temperature for 2 h, a clear orange solution was formed. The orange solution (50 mL) was carefully sucked into a syringe through a long needle and added to a mixture of 2-bromoquinoline...
example 1
3,7-Di(pyridin-4-yl)-5-[2-(quinolin-2-yl)ethyl]thieno[2,3-d]pyridazin-4(5H)-one
[1029]
1.1 4-Bromothiophene-3-carboxylic acid
[1030]To a mixture of Mg (1.4 g, 60 mmol) and I2 (0.1 g) in anhydrous THF (2 mL) was added dropwise a solution of 2-bromo-propane (7.4 g, 60 mmol) in anhydrous THF (60 mL) at room temperature under nitrogen during a period of 30 min After the addition, the mixture was refluxed until the most of magnesium was consumed. The resulting Grignard reagent was added dropwise to a solution of 3,4-dibromo-thiophene (12.1 g, 50 mmol) in anhydrous THF (60 mL) at 0° C. under nitrogen within about 30 min. The mixture was allowed to stir at 0° C. for 1.5 h. Excessive CO2 was purged into the mixture at −30° C. and the reaction mixture was stirred until the temperature rose to room temperature. Then the reaction was quenched with water (30 mL) and basified with 8% aq. NaOH solution to pH 11 and was washed with ethyl acetate (3×60 mL). The aqueous layer was acidified with 5% aq. ...
example 2
7-(Pyridin-4-yl)-5-[2-(quinolin-2-yl)ethyl]thieno[2,3-d]pyridazin-4(5H)-one
[1041]
2.1 2-Isonicotinoylthiophene-3-carboxylic acid
[1042]To a solution of diisopropylamine (5.2 g, 51.5 mmol) in anhydrous THF (40 mL) at −30° C. was added n-BuLi (23.2 mL, 56.2 mmol, 2.5M in THF) dropwise. The mixture was stirred at the same temperature for 0.5 h, then cooled to −78° C. and HMPA (0.8 g, 4.7 mmol) was added slowly. Then a solution of thiophene-3-carboxylic acid (3.0 g, 23.4 mmol) in anhydrous THF (50 mL) was added slowly. The mixture was stirred at the same temperature for 1 h, N-methoxy-N-methyl-4-pyridinecarboxamide (5.0 g, 46.9 mmol) was added drop wise into the stirring mixture at −78° C. The reaction mixture was stirred for another 1 h at room temperature and was then quenched with H2O (10 mL). The aqueous layer was acidified with 5% aq. HCl to pH 1˜2, the precipitate was collected by filtration. The filter cake wash with DCM and the filtrate was extracted with DCM (3×200 mL). The organ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| enantiomeric excess | aaaaa | aaaaa |
| chemical | aaaaa | aaaaa |
| chemical bond | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com