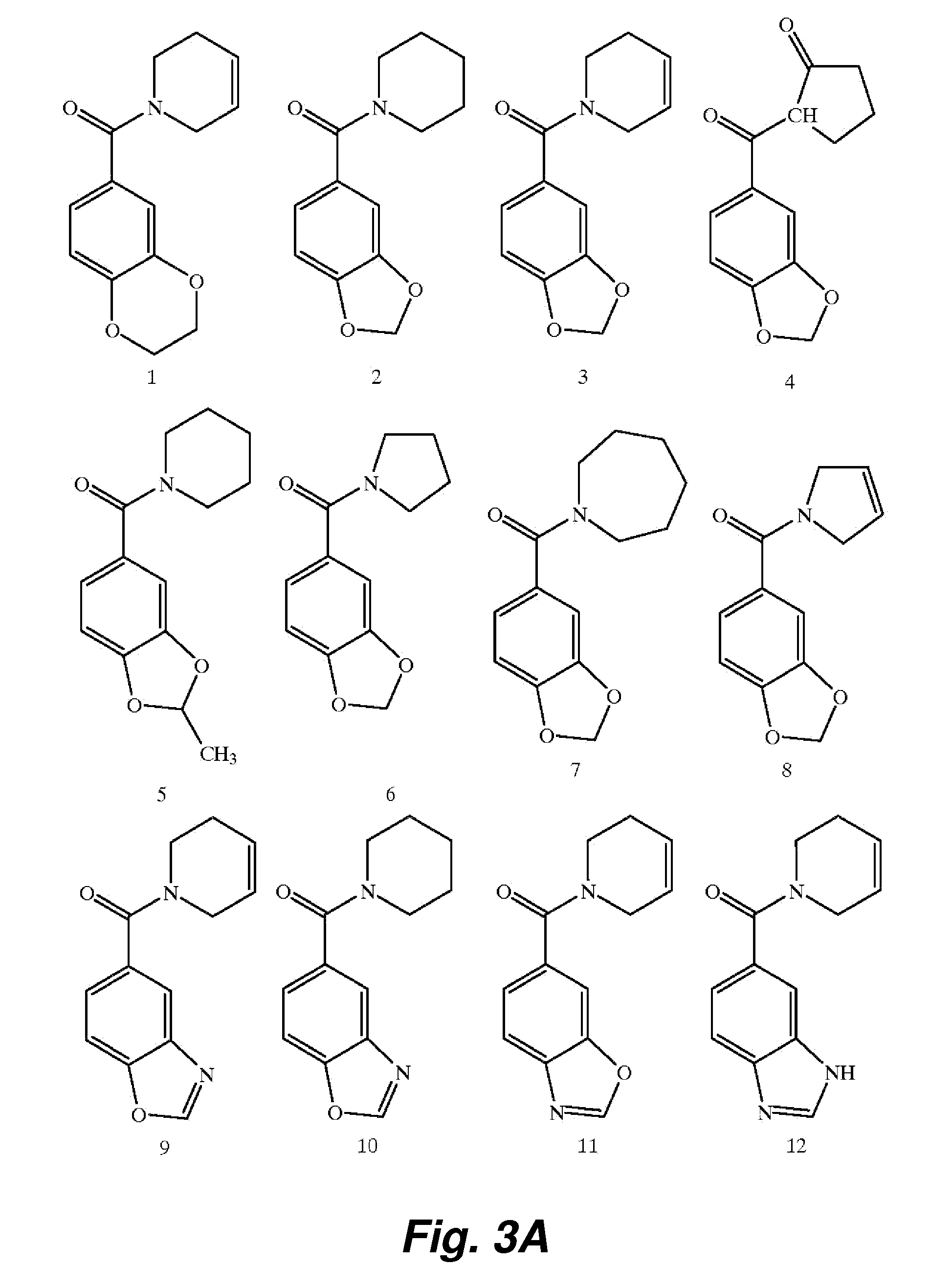
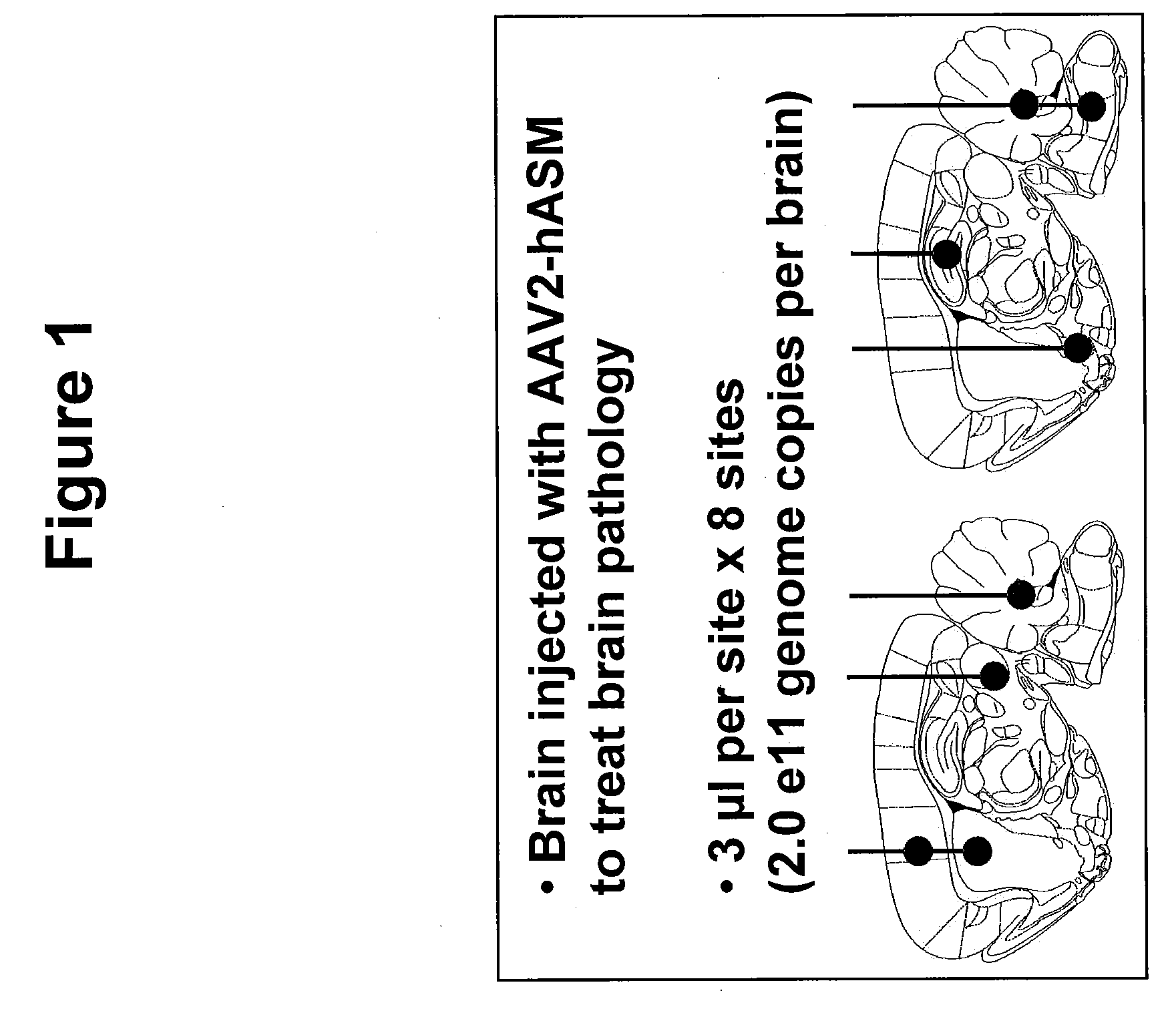
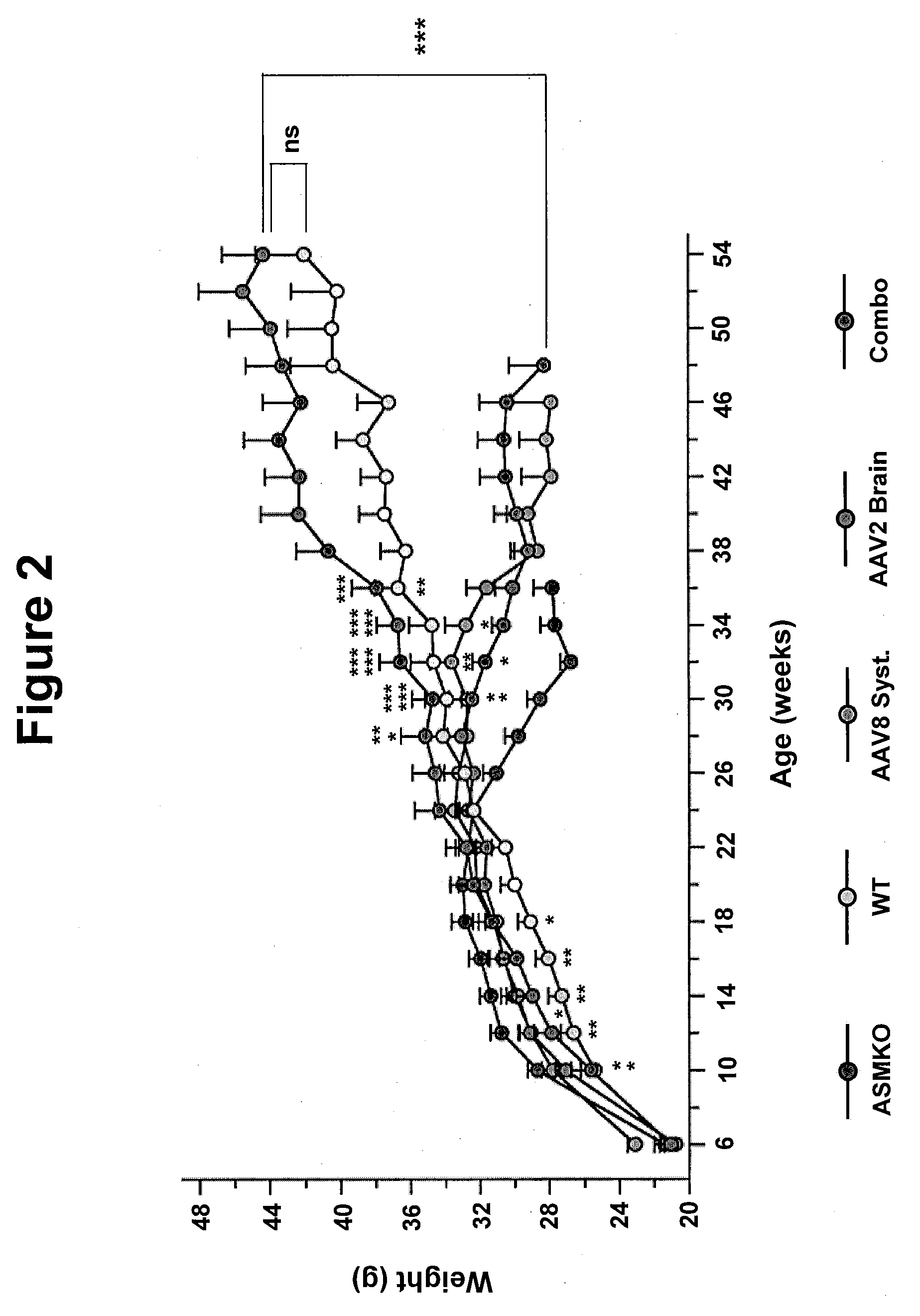
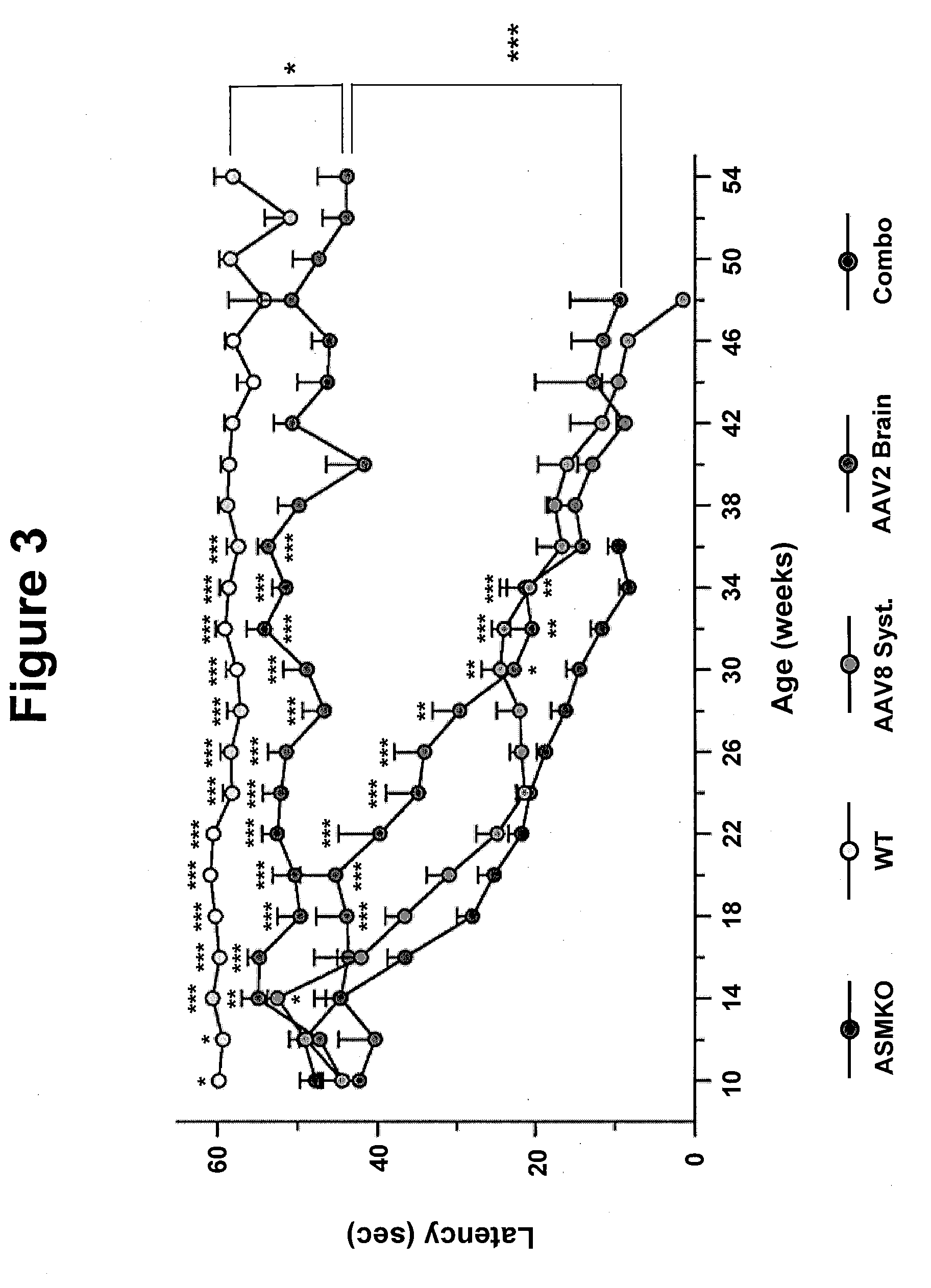
Patents
Literature
58 results about "Mammalian brain" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
The Limbic System or Mammalian Brain. The mammalian brain was the second brain to evolve and is comprised of, among other parts, the brain stem and cerebrum. It is involved with emotions, memory formation and long-term memory; it connects events with feelings and controls hormones and temperature.
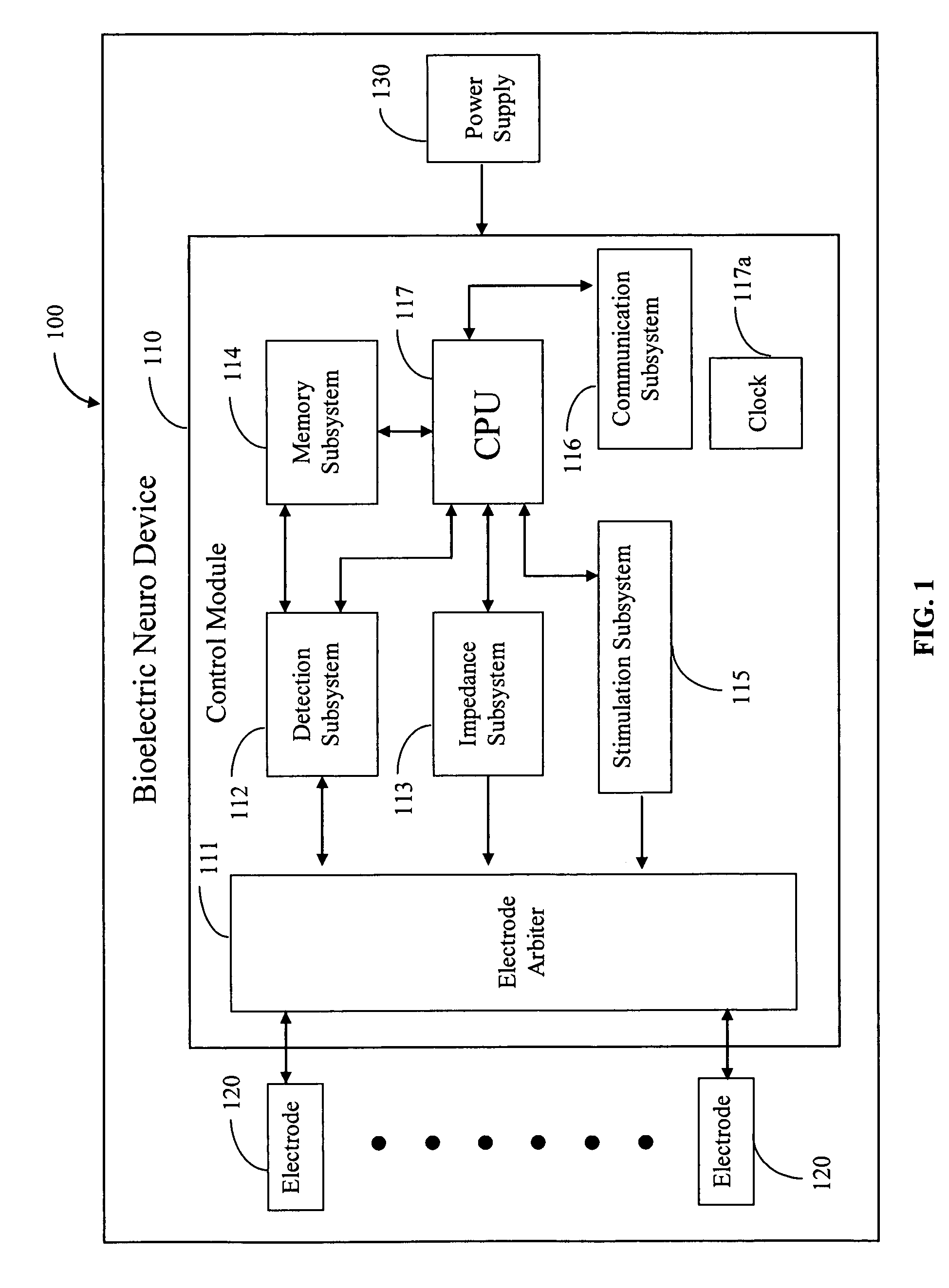
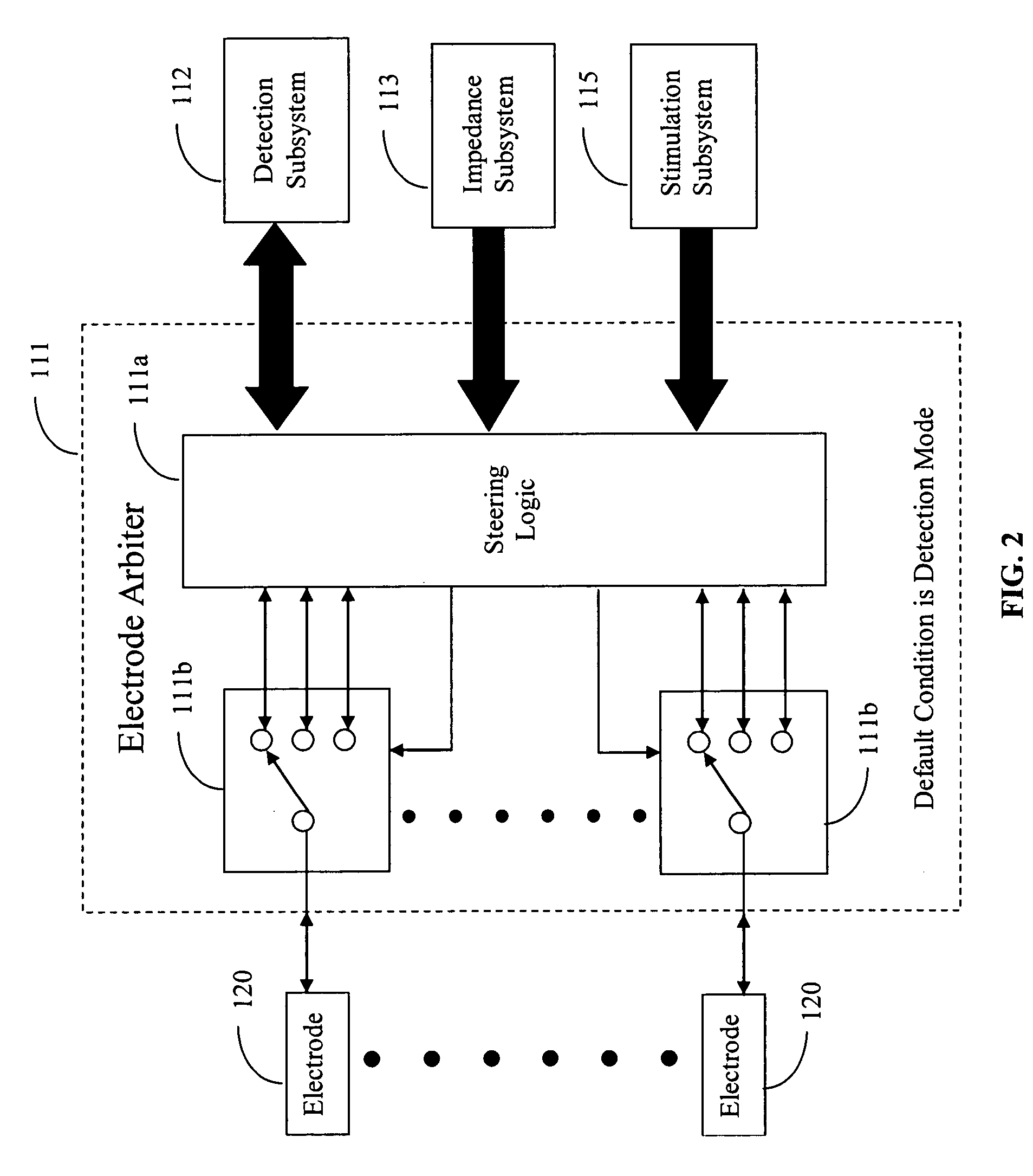
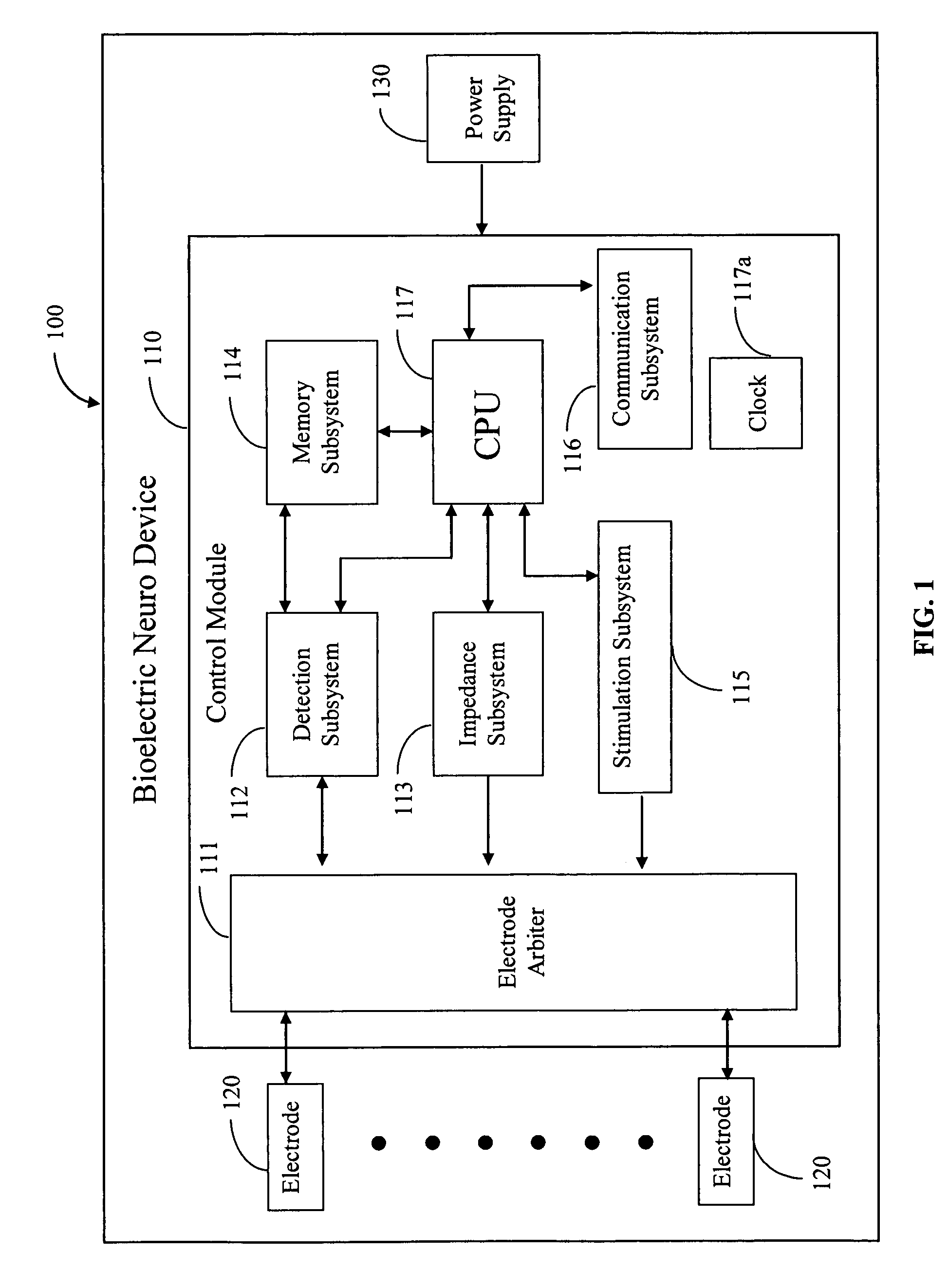
Medical devices for the detection, prevention and/or treatment of neurological disorders, and methods related thereto
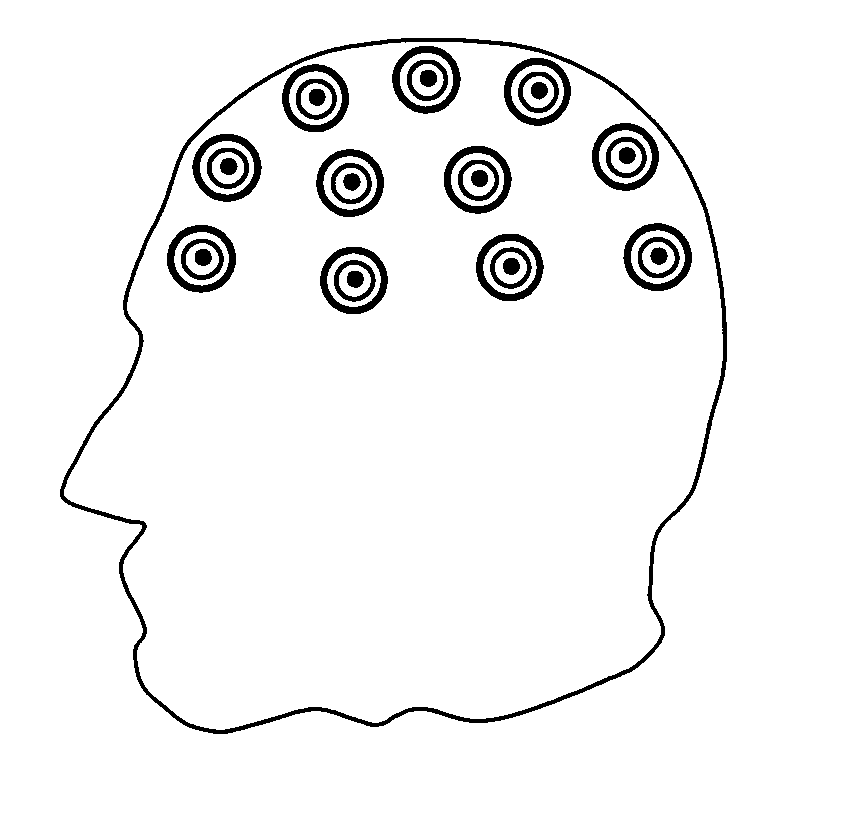
ActiveUS20060173510A1Avoid detectionMinimal invasionElectroencephalographyHead electrodesSubstance abuserTranscranial Electrical Stimulations
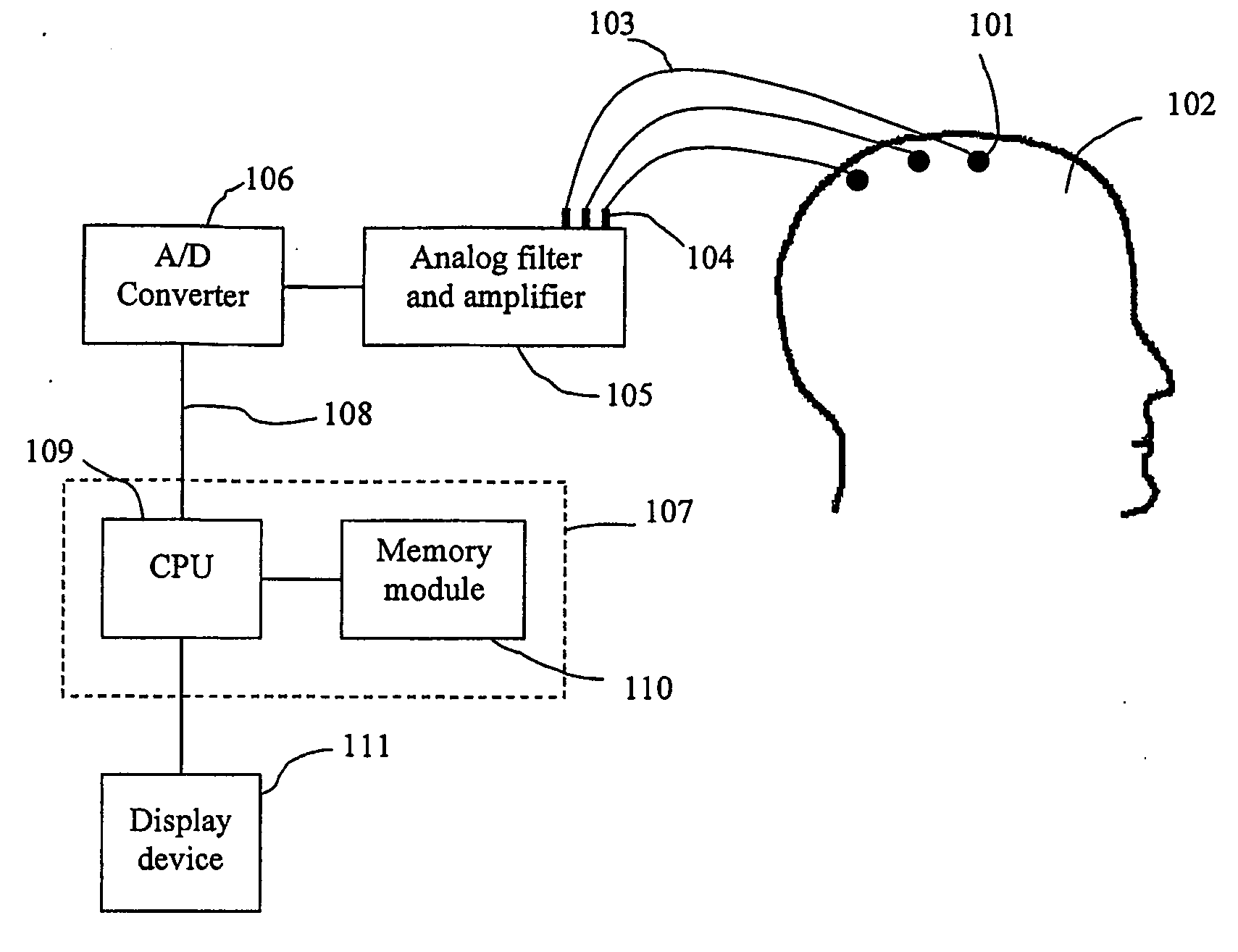
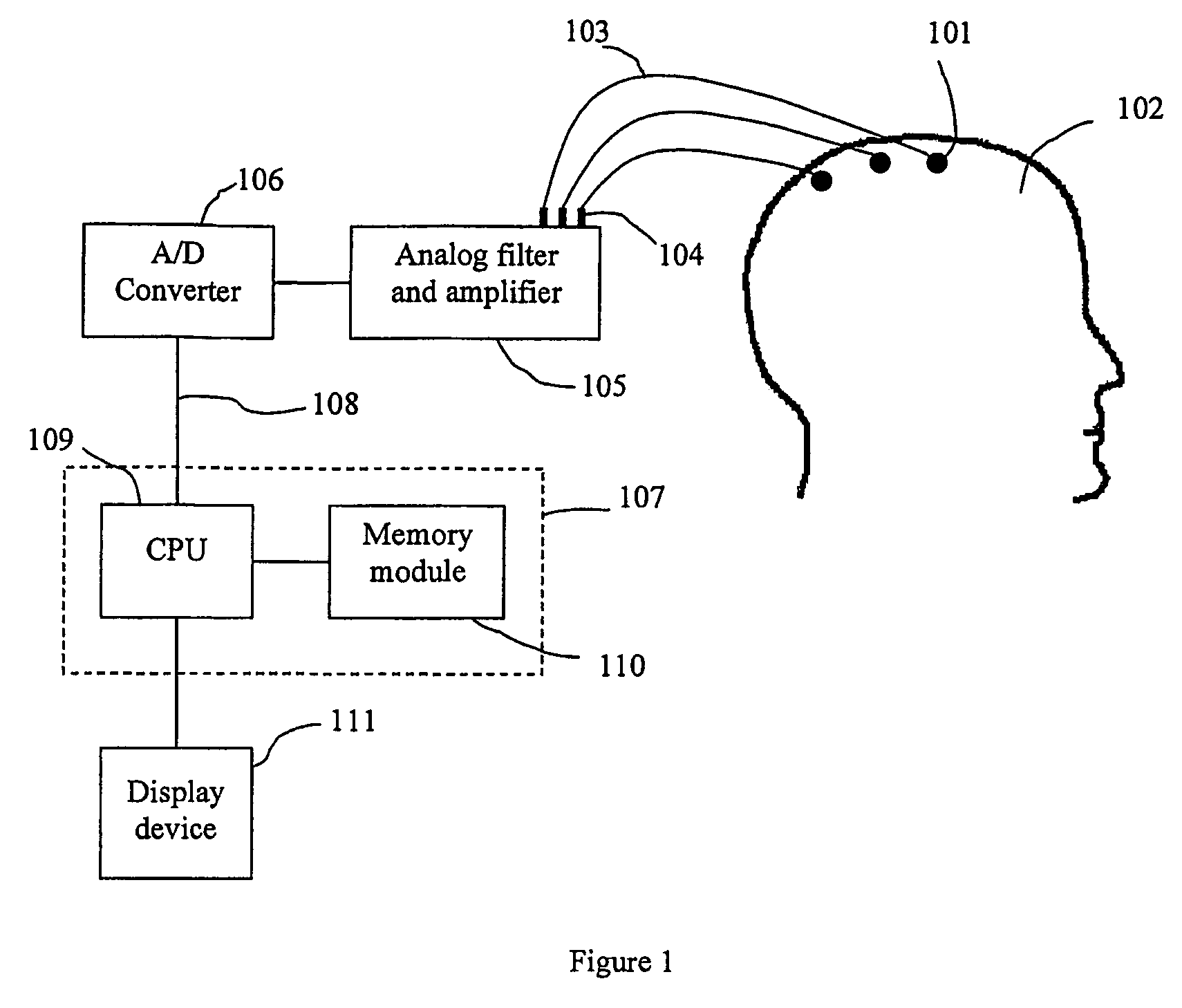
Disclosed are devices and methods for detecting, preventing, and / or treating neurological disorders. These devices and methods utilize electrical stimulation, and comprise a unique concentric ring electrode component. The disclosed methods involve the positioning of multiple electrodes on the scalp of a mammal; monitoring the mammal's brain electrical patterns to identify the onset of a neurological event; identifying the location of the brain electrical patterns indicative of neurological event; and applying transcutaneous or transcranial electrical stimulation to the location of the neurological event to beneficially modify brain electrical patterns. The disclosed methods may be useful in the detection, prevention, and / or treatment of a variety of indications, such as epilepsy, Parkinson's Disease, Huntington's disease, Alzheimer's disease, depression, bipolar disorder, phobia, schizophrenia, multiple personality disorder, migraine or headache, concussion, attention deficit hyperactivity disorder, eating disorder, substance abuse, and anxiety. The disclosed methods may also be used in combination with other peripheral stimulation techniques.
Owner:LOUISIANA TECH UNIV RES FOUND A DIV OF LOUISIANA TECH UNIV FOUND +1
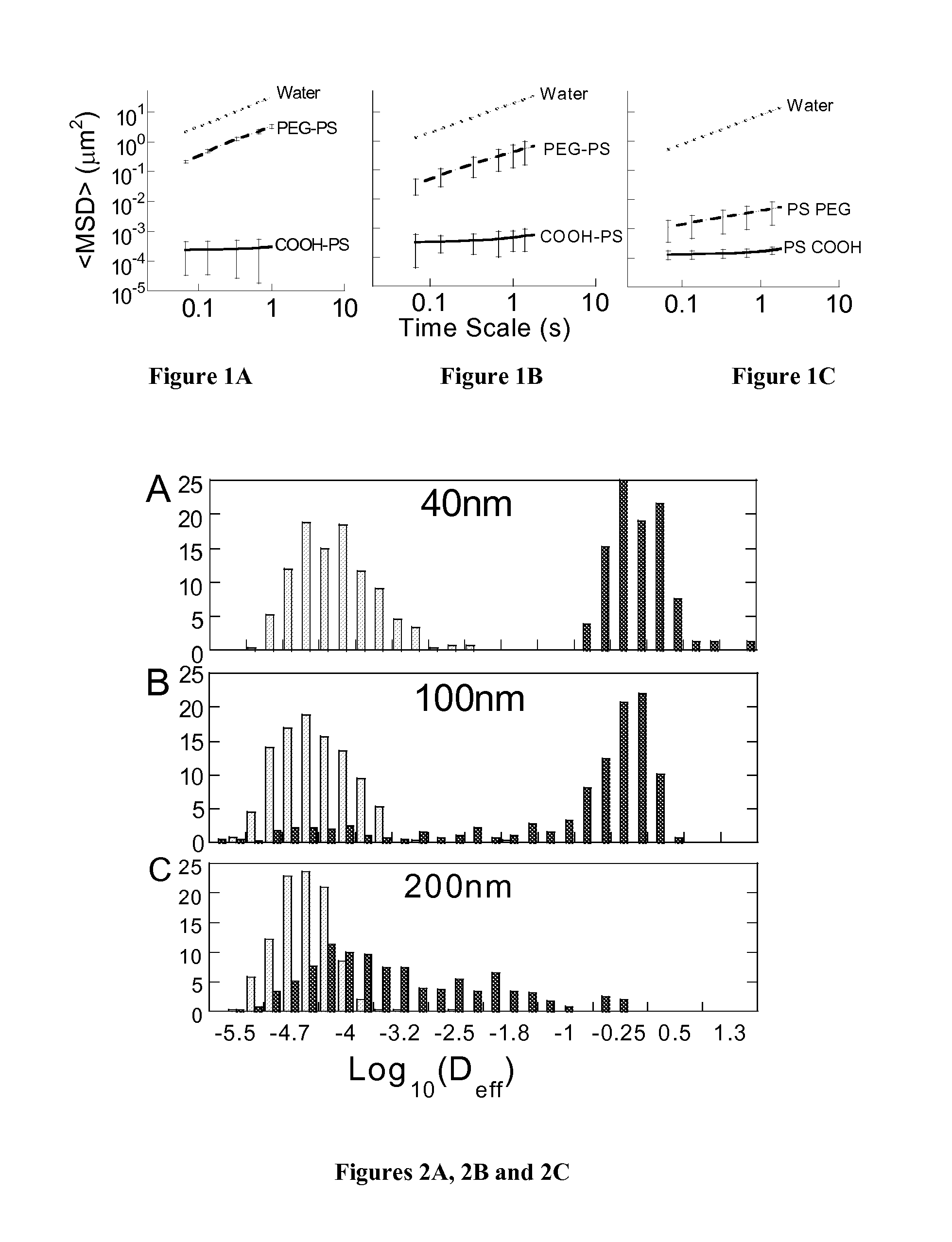
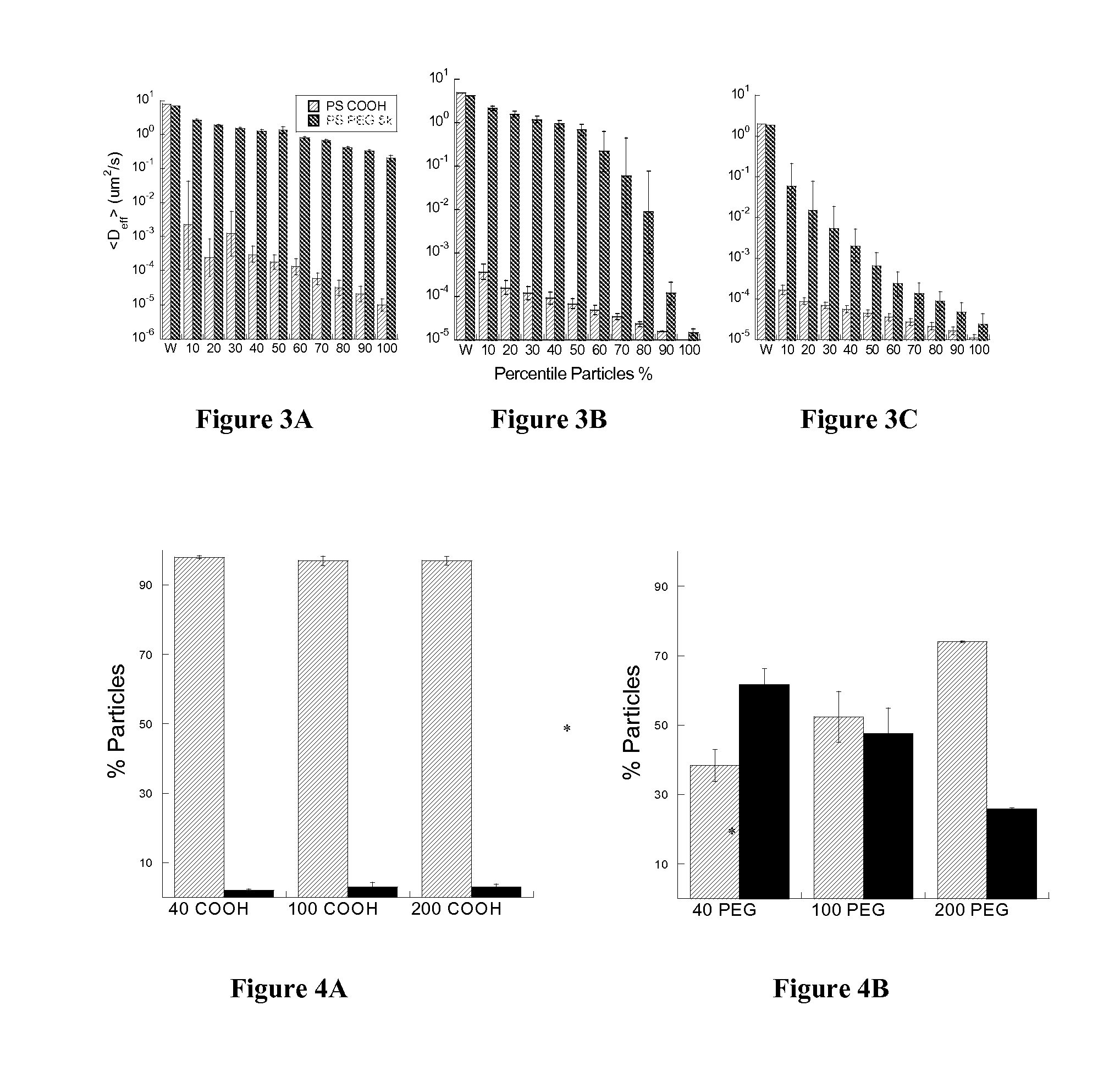
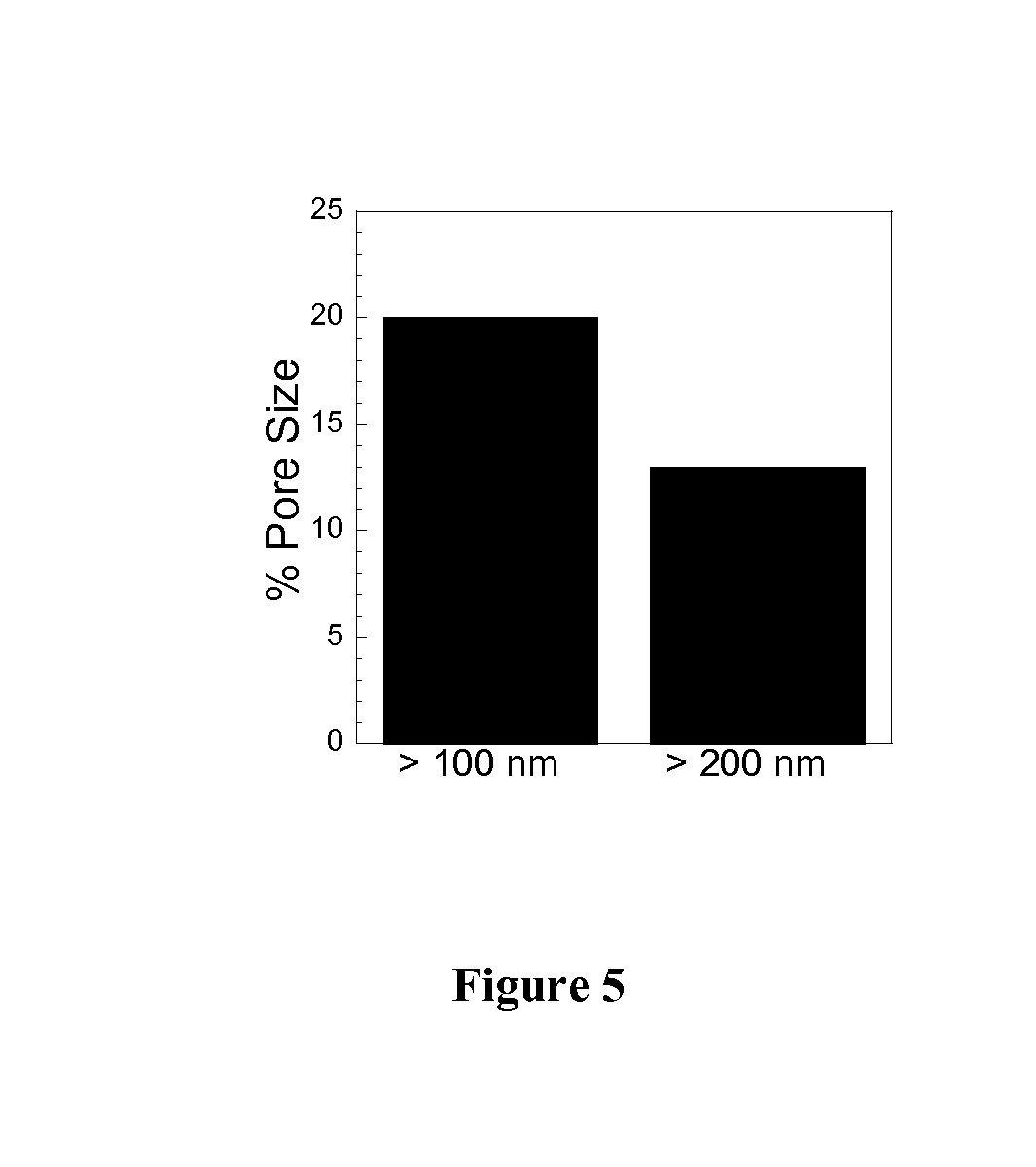
Rapid Diffusion of Large Polymeric Nanoparticles in the Mammalian Brain
ActiveUS20130183244A1Reduce deliveryHigher drug payloadPowder deliveryNervous disorderGene deliveryHydrophilic coating
Non-adhesive particles as large as 110 nm can diffuse rapidly in the brain ECS, if coated with hydrophilic coatings such as PEG coatings and preferably having neutral surface charge. The ability to achieve brain penetration with larger particles will significantly improve drug and gene delivery within the CNS since larger particles offer higher drug payload, improved drug loading efficiency, and significantly longer drug release durations.
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE
Medical devices for the detection, prevention and/or treatment of neurological disorders, and methods related thereto
ActiveUS8190248B2Improve localizationMinimal invasionElectroencephalographyHead electrodesSubstance abuserDisease
Disclosed are devices and methods for detecting, preventing, and / or treating neurological disorders. These devices and methods utilize electrical stimulation, and comprise a unique concentric ring electrode component. The disclosed methods involve the positioning of multiple electrodes on the scalp of a mammal; monitoring the mammal's brain electrical patterns to identify the onset of a neurological event; identifying the location of the brain electrical patterns indicative of neurological event; and applying transcutaneous or transcranial electrical stimulation to the location of the neurological event to beneficially modify brain electrical patterns. The disclosed methods may be useful in the detection, prevention, and / or treatment of a variety of indications, such as epilepsy, Parkinson's Disease, Huntington's disease, Alzheimer's disease, depression, bipolar disorder, phobia, schizophrenia, multiple personality disorder, migraine or headache, concussion, attention deficit hyperactivity disorder, eating disorder, substance abuse, and anxiety. The disclosed methods may also be used in combination with other peripheral stimulation techniques.
Owner:LOUISIANA TECH UNIV RES FOUND A DIV OF LOUISIANA TECH UNIV FOUND +1
Method and system for identification of source of chronic pain and treatment
A method for identifying and treating a neural pathway associated with chronic pain via nerve stimulation and brain wave monitoring of a mammalian brain includes positioning a probe to stimulate a target nerve, wherein the target nerve is suspected of being a source of chronic pain; delivering a first nerve stimulation from the probe to the target nerve, wherein the first nerve stimulation is sufficient to elicit a chronic pain response in the brain; and monitoring for evoked potential activity in the brain as a result of the first nerve stimulation. The method can also include delivering second and third nerve stimulations to confirm the correct identification of the neural pathway and to treat the chronic pain, respectively. A system and apparatus for performing a procedure to identify and treat a nerve that is the source of chronic pain are also described.
Owner:AVENT INC
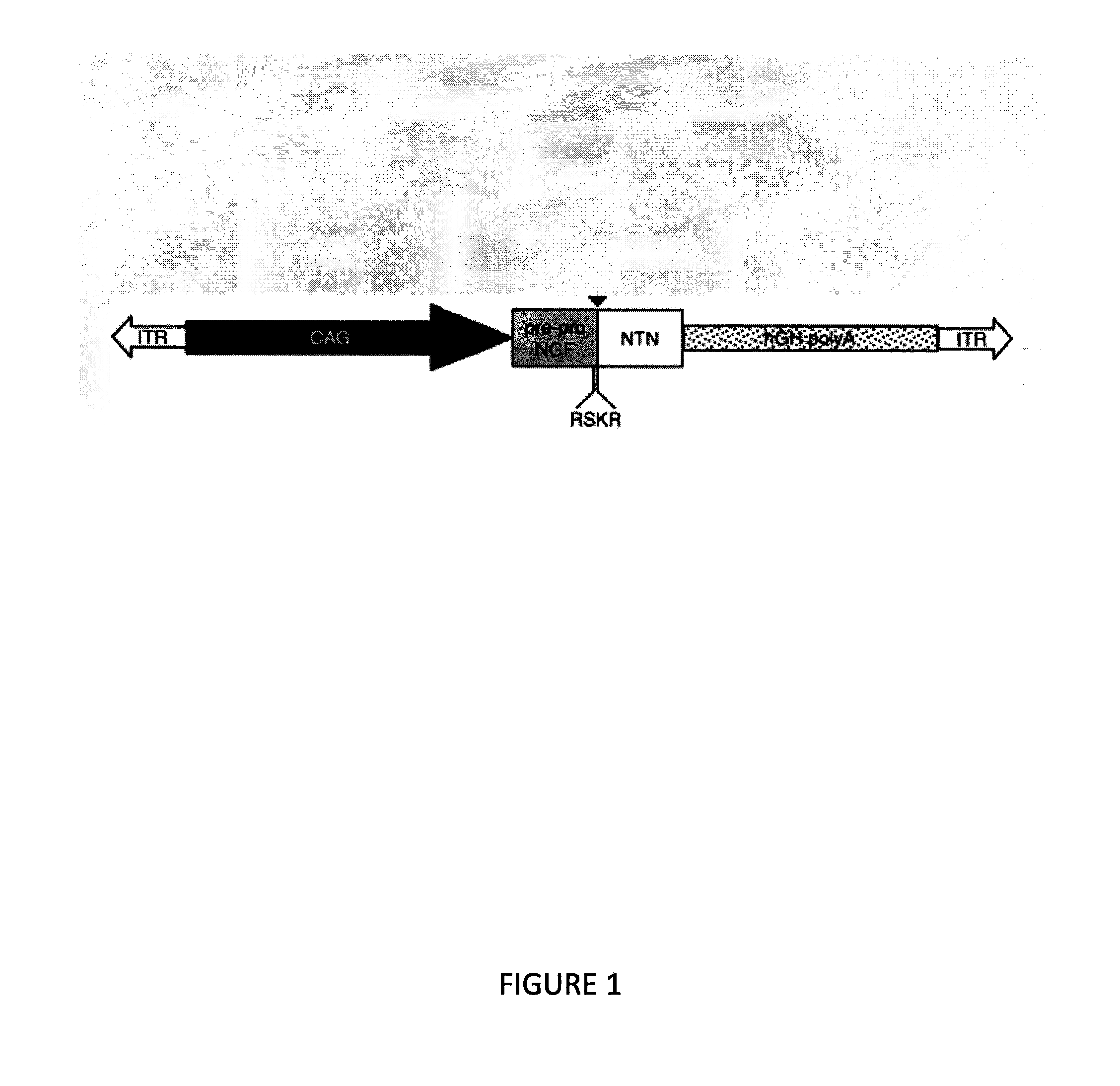
Methods for therapy of neurodegenerative disease of the brain
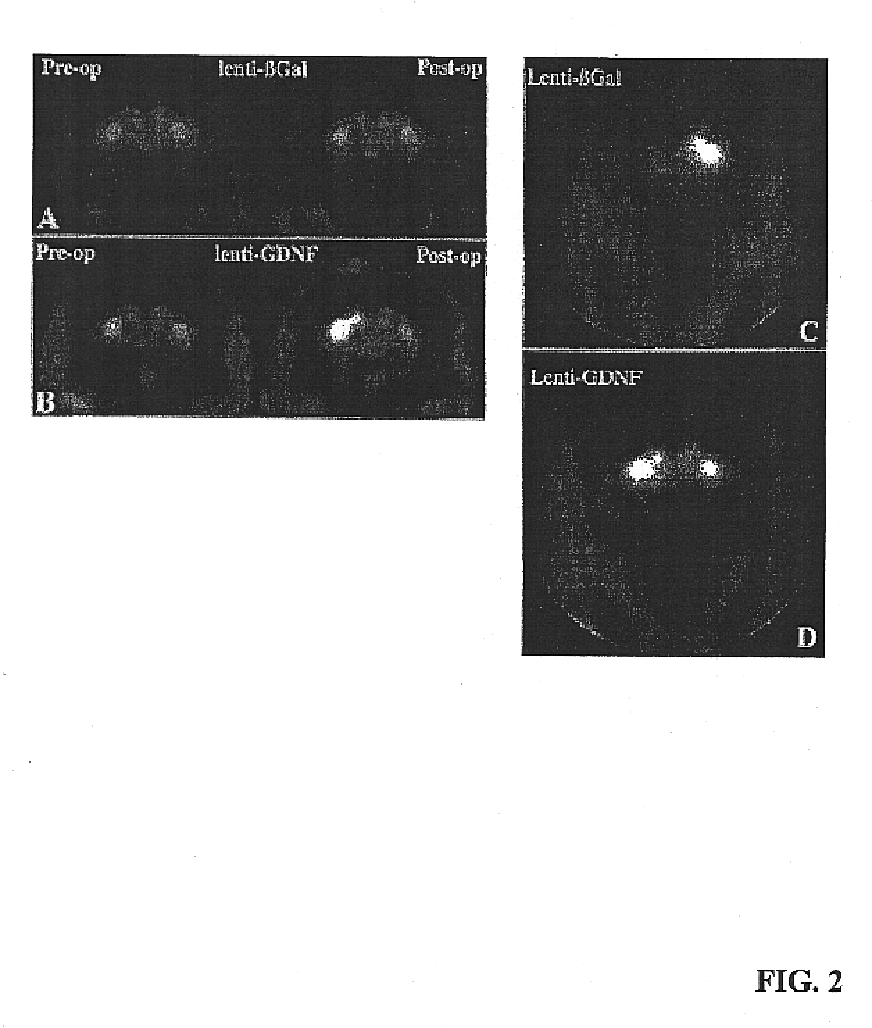
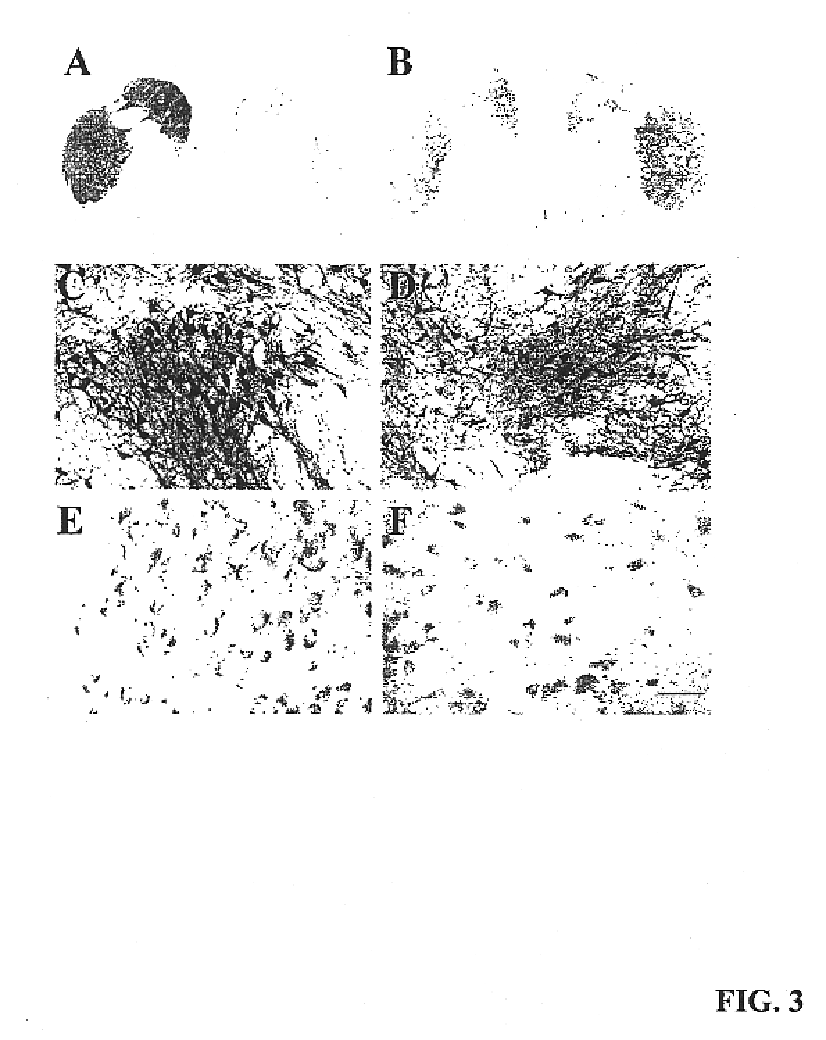
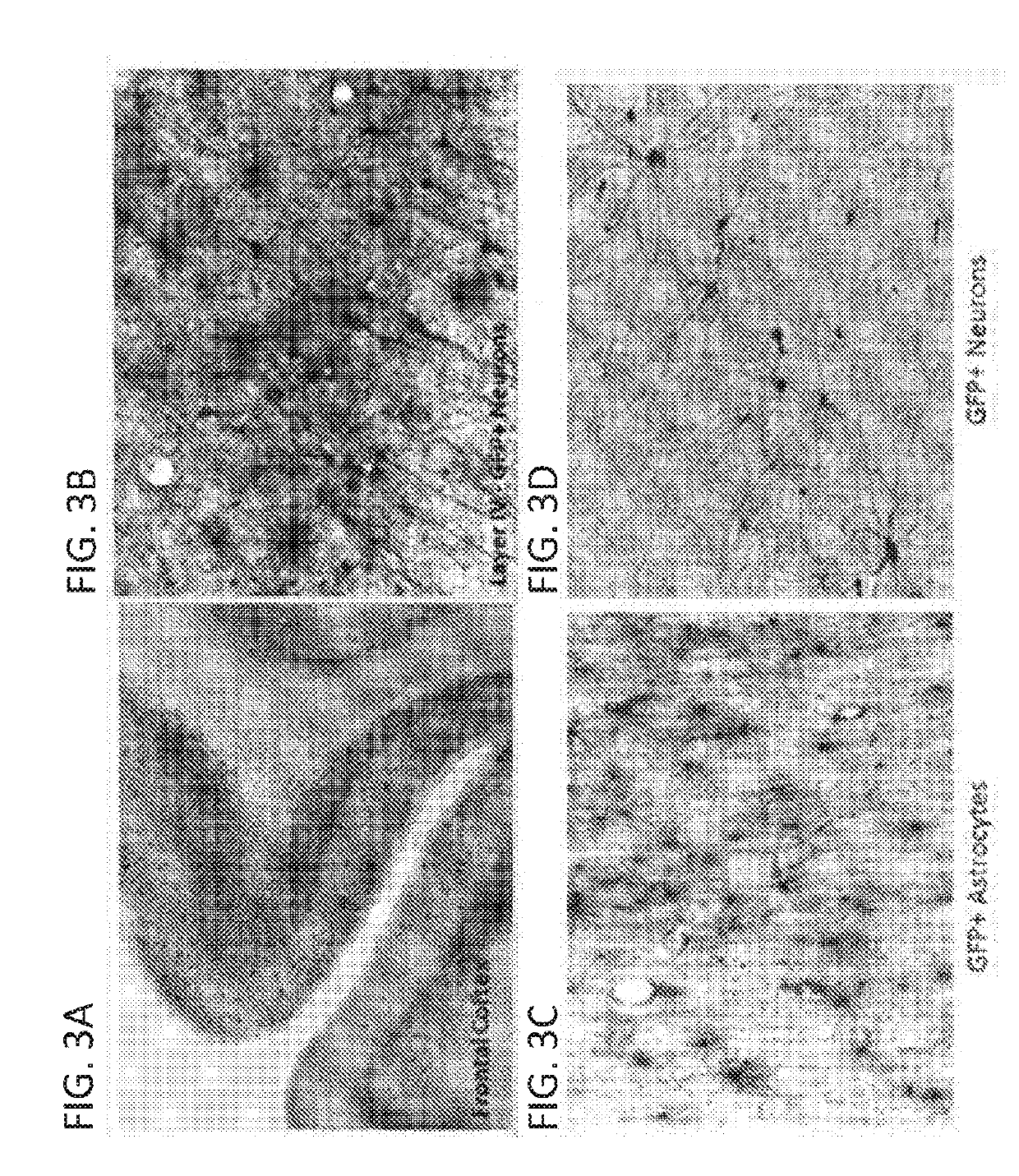
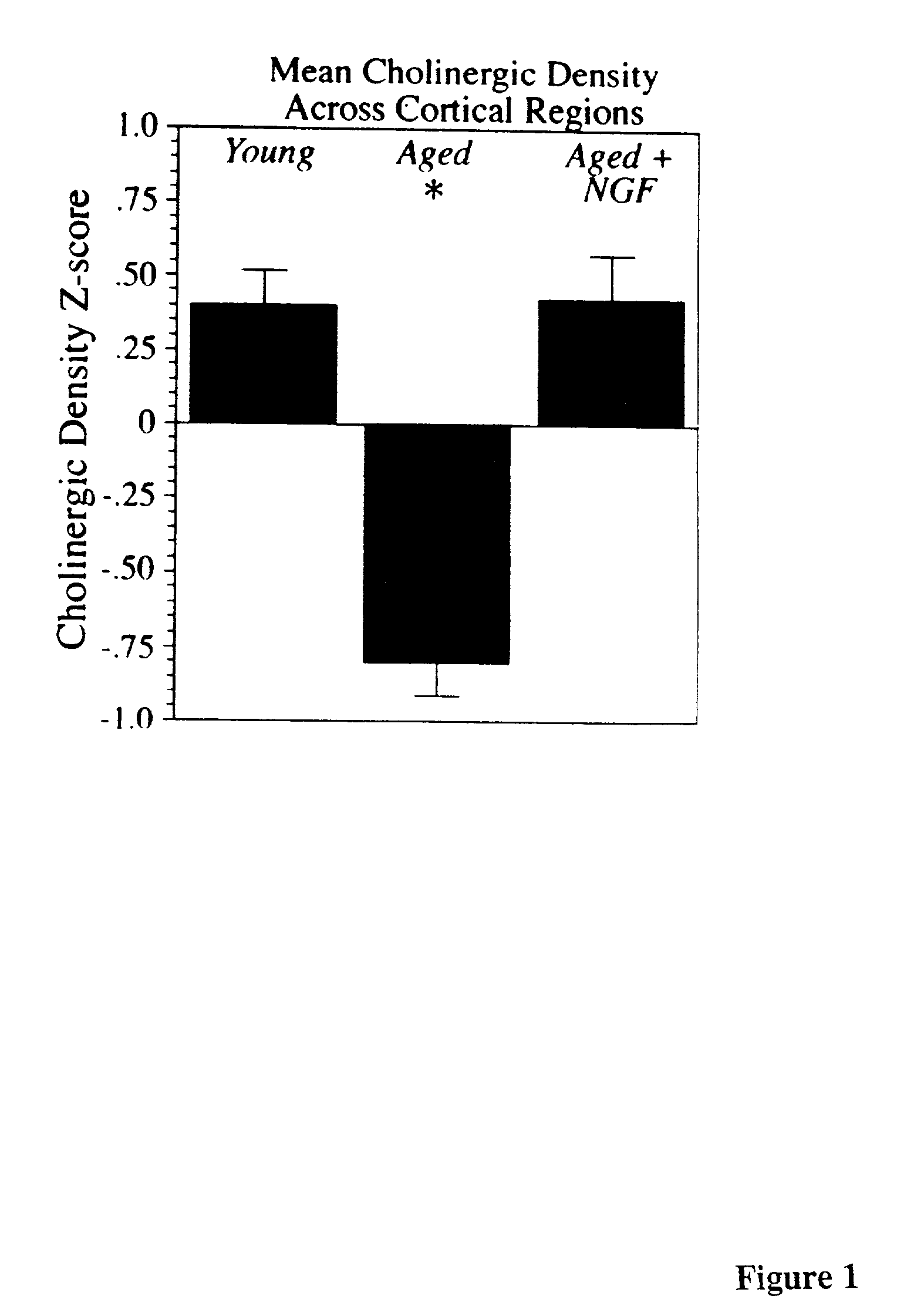
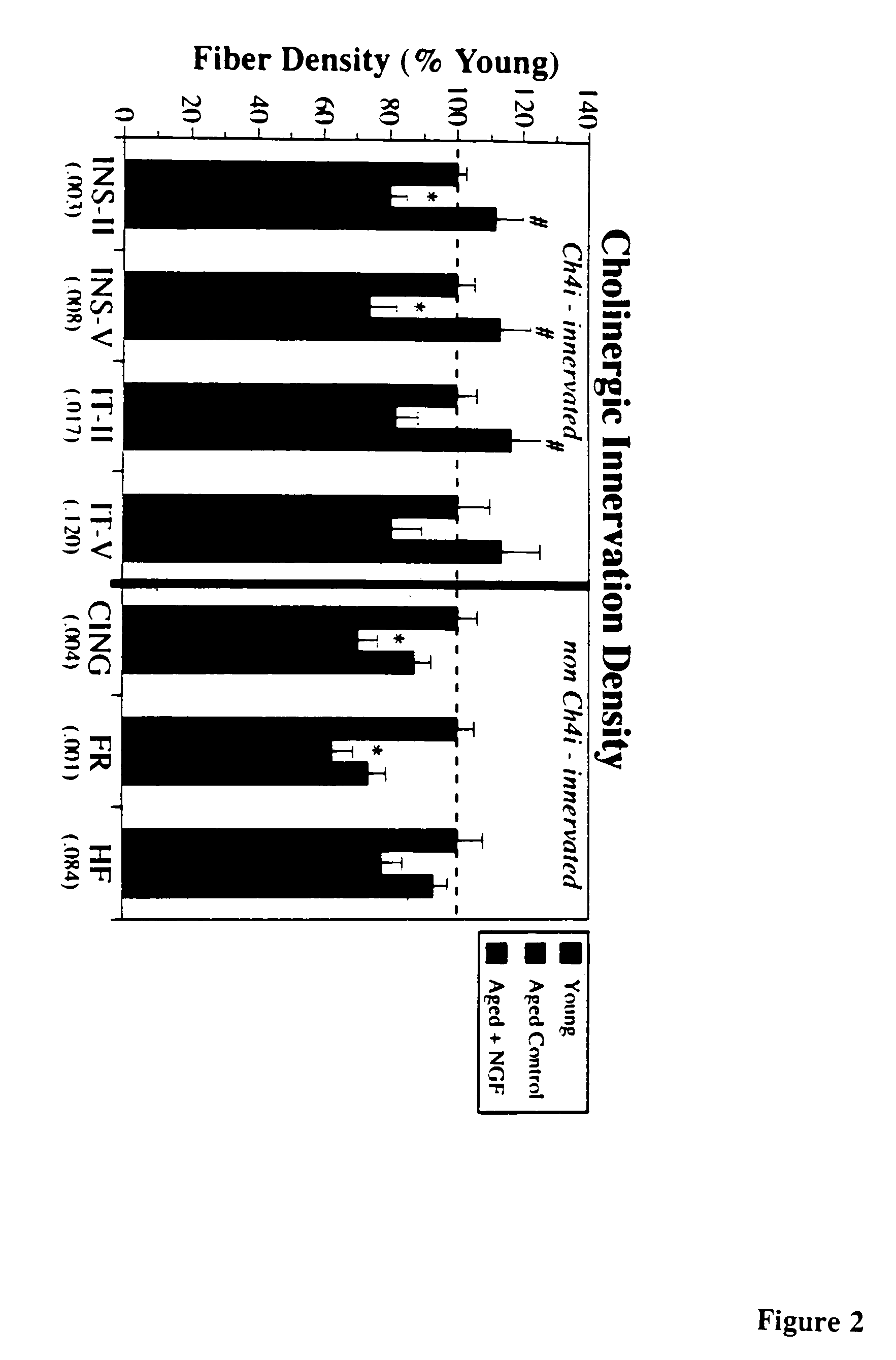
A specific clinical protocol for use toward therapy of defective, diseased and damaged cholinergic neurons in the mammalian brain, of particular usefulness for treatment of neurodegenerative conditions such as Alzheimer's disease. The protocol is practiced by delivering a definite concentration of recombinant neurotrophin into, or within close proximity of, identified defective, diseased or damaged brain cells. Using a viral vector, the concentration of neurotrophin delivered as part of a neurotrophic composition varies from 10<10 >to 10<15 >neurotrophin encoding viral particles / ml of composition fluid. Each delivery site receives from 2.5 mul to 25 mul of neurotrophic composition, delivered slowly, as in over a period of time ranging upwards of 10 minutes / delivery site. Each delivery site is at, or within 500 mum of, a targeted cell, and no more than about 10 mm from another delivery site. Stable in situ neurotrophin expression can be achieved for 12 months, or longer.
Owner:RGT UNIV OF CALIFORNIA
Brain Activity as a Marker of Disease
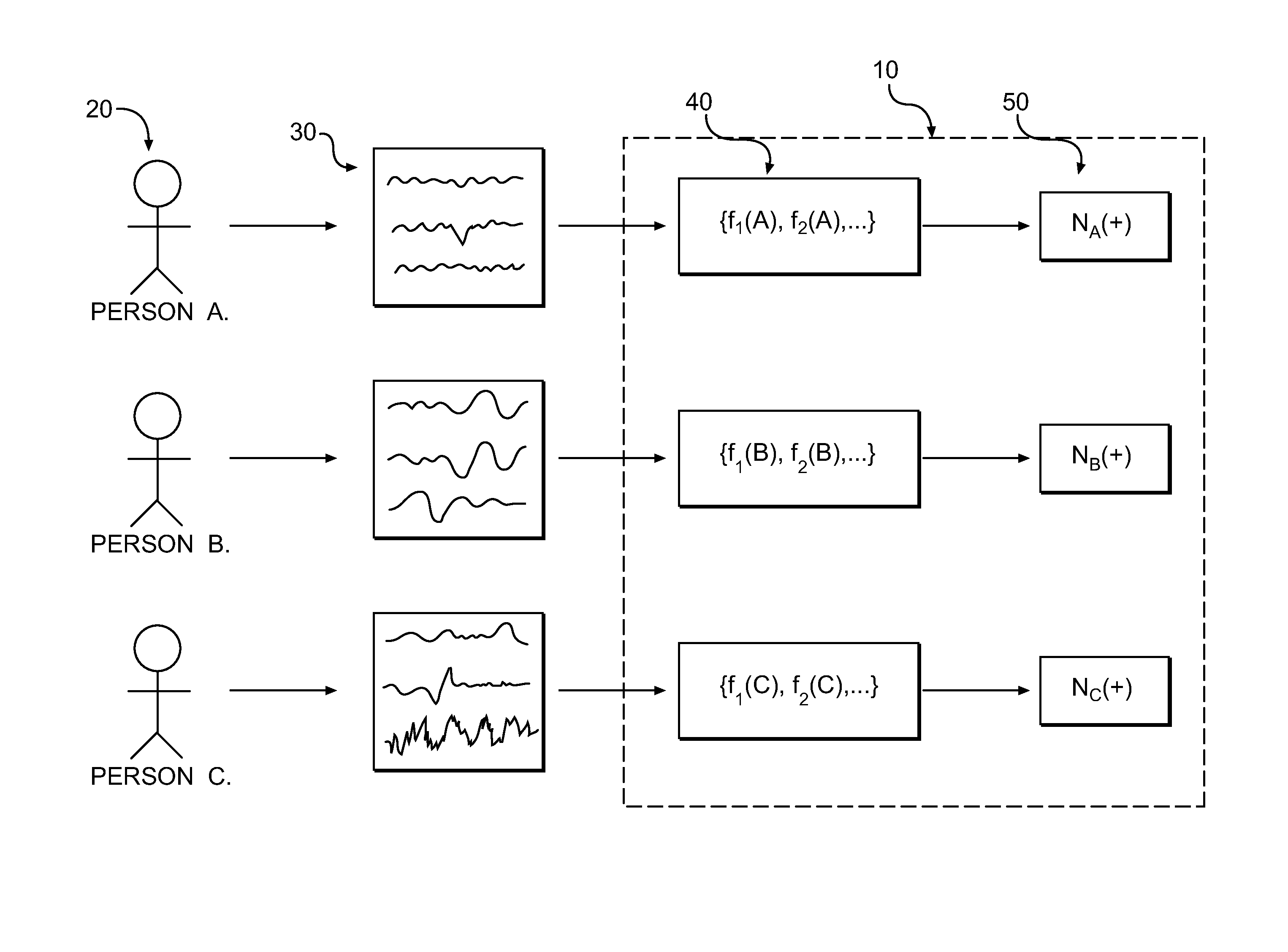
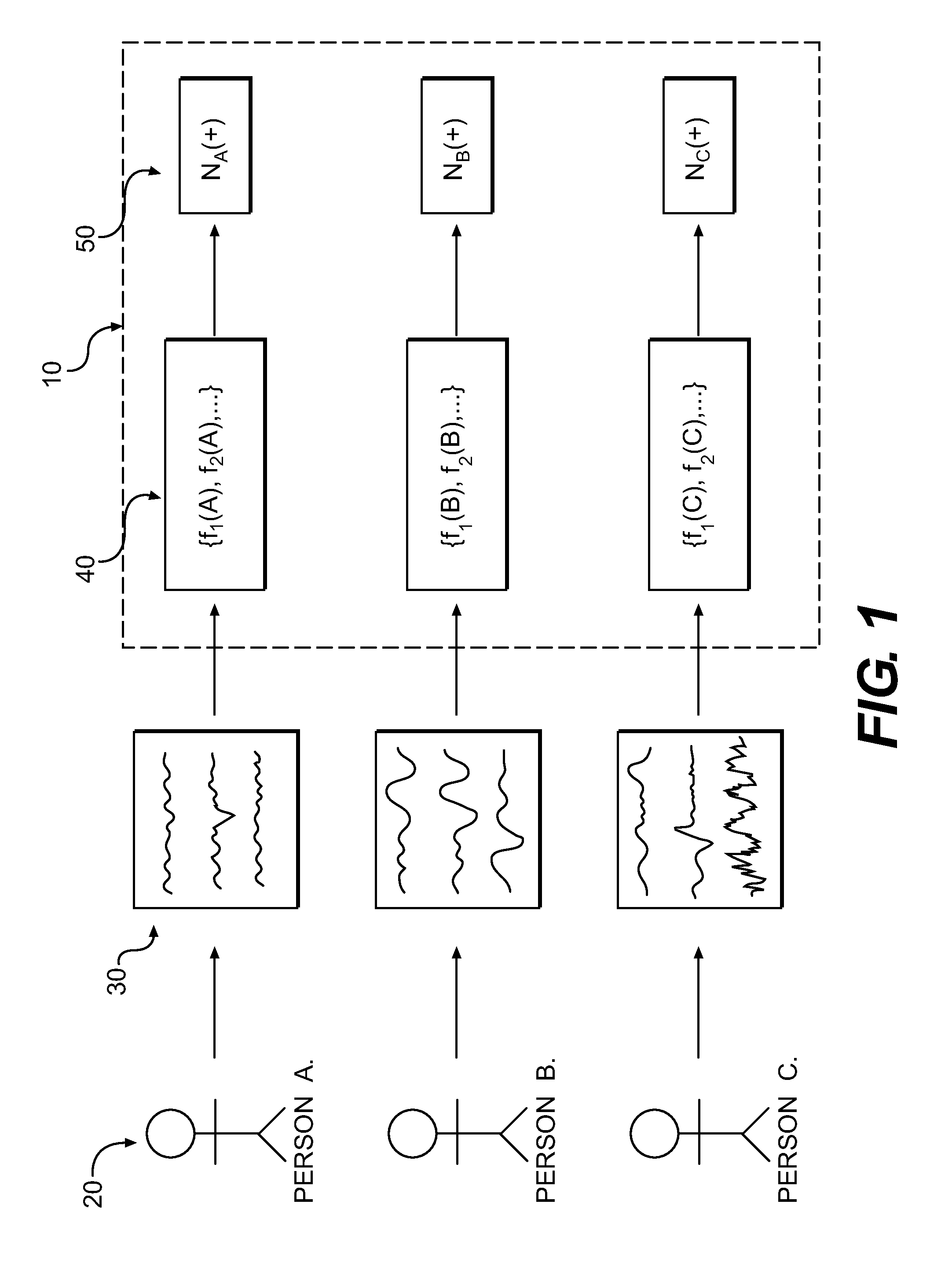
A method of monitoring brain activity is provided, wherein the method includes receiving a signal associated with neuronal activity of a mammalian brain. The method also includes processing the signal using a linear, non-linear, or combination algorithm to extract a signal feature. A neuromarker may be determined based on an association between the signal feature and a library of features, wherein the library includes a plurality of signal features correlated with a plurality of disease states.
Owner:BRAINSCOPE SPV LLC
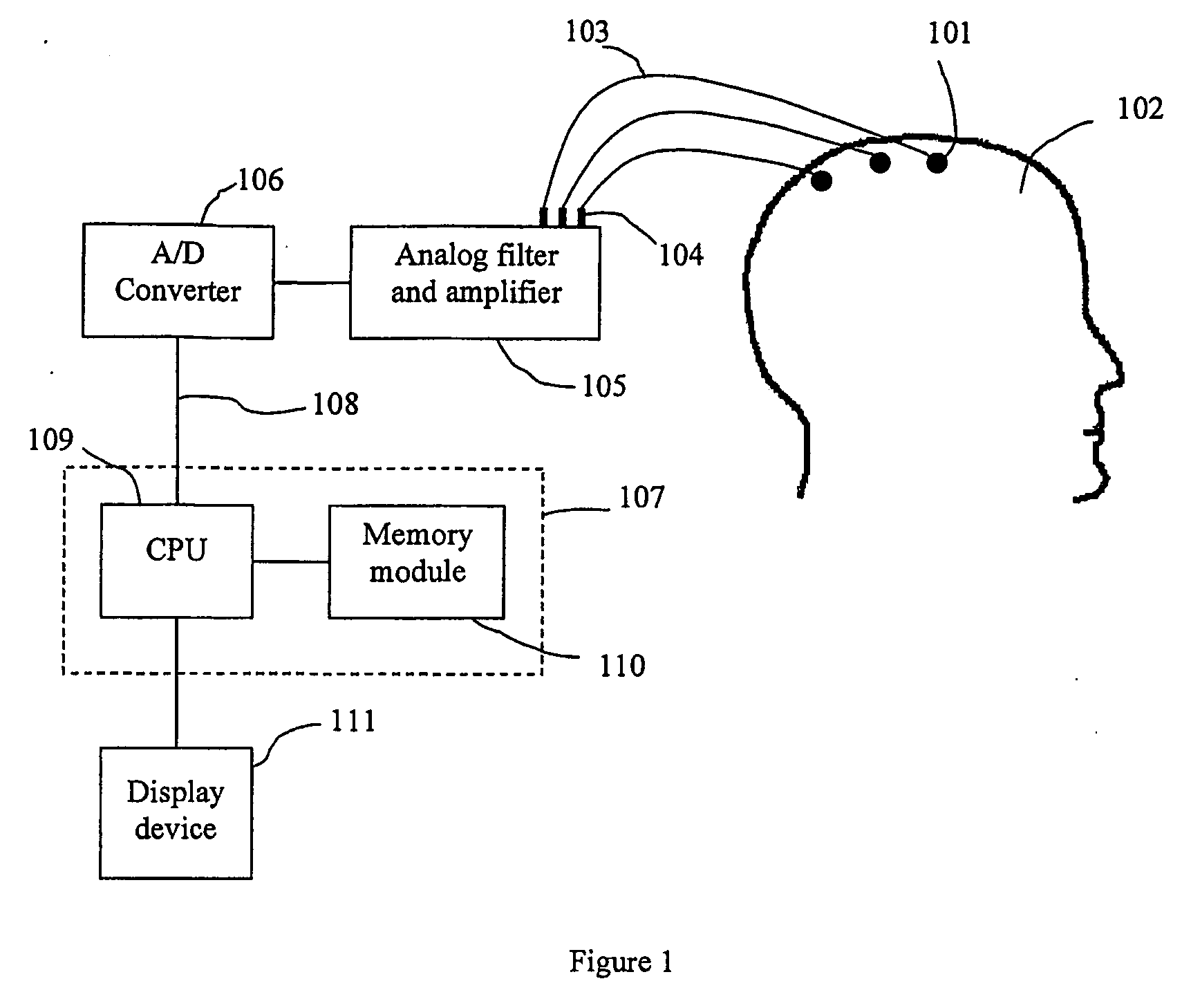
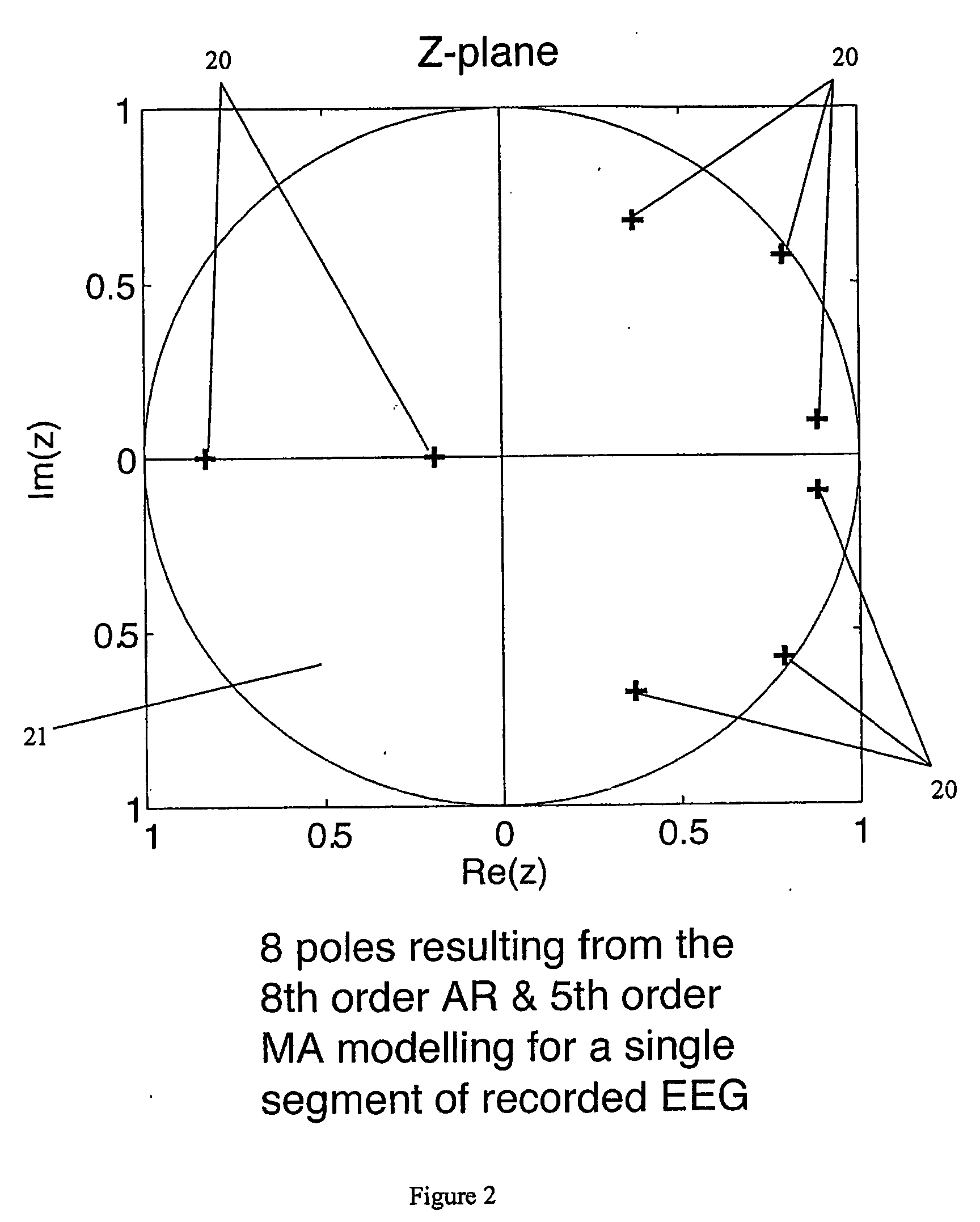
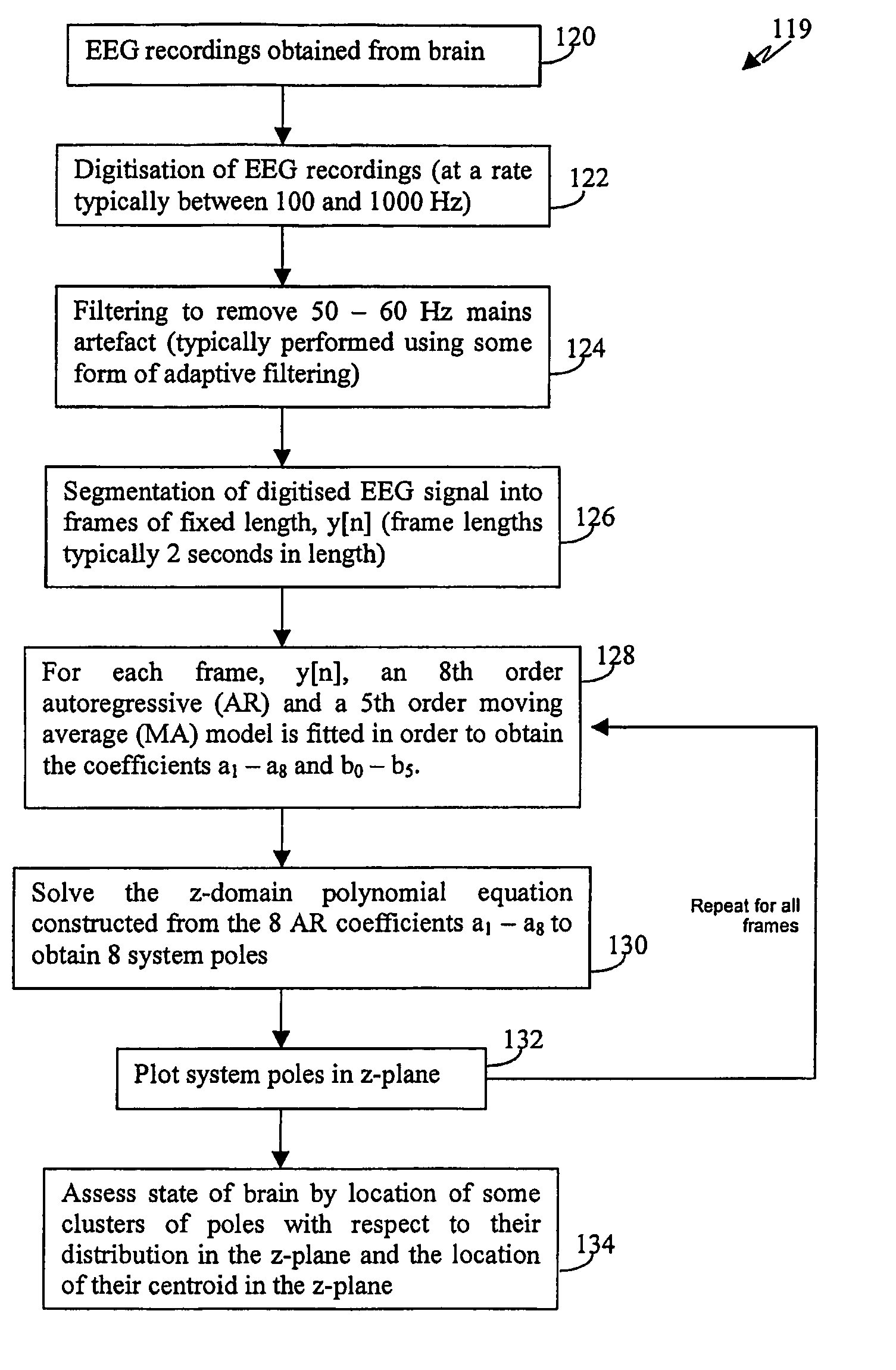
Method of monitoring brain function
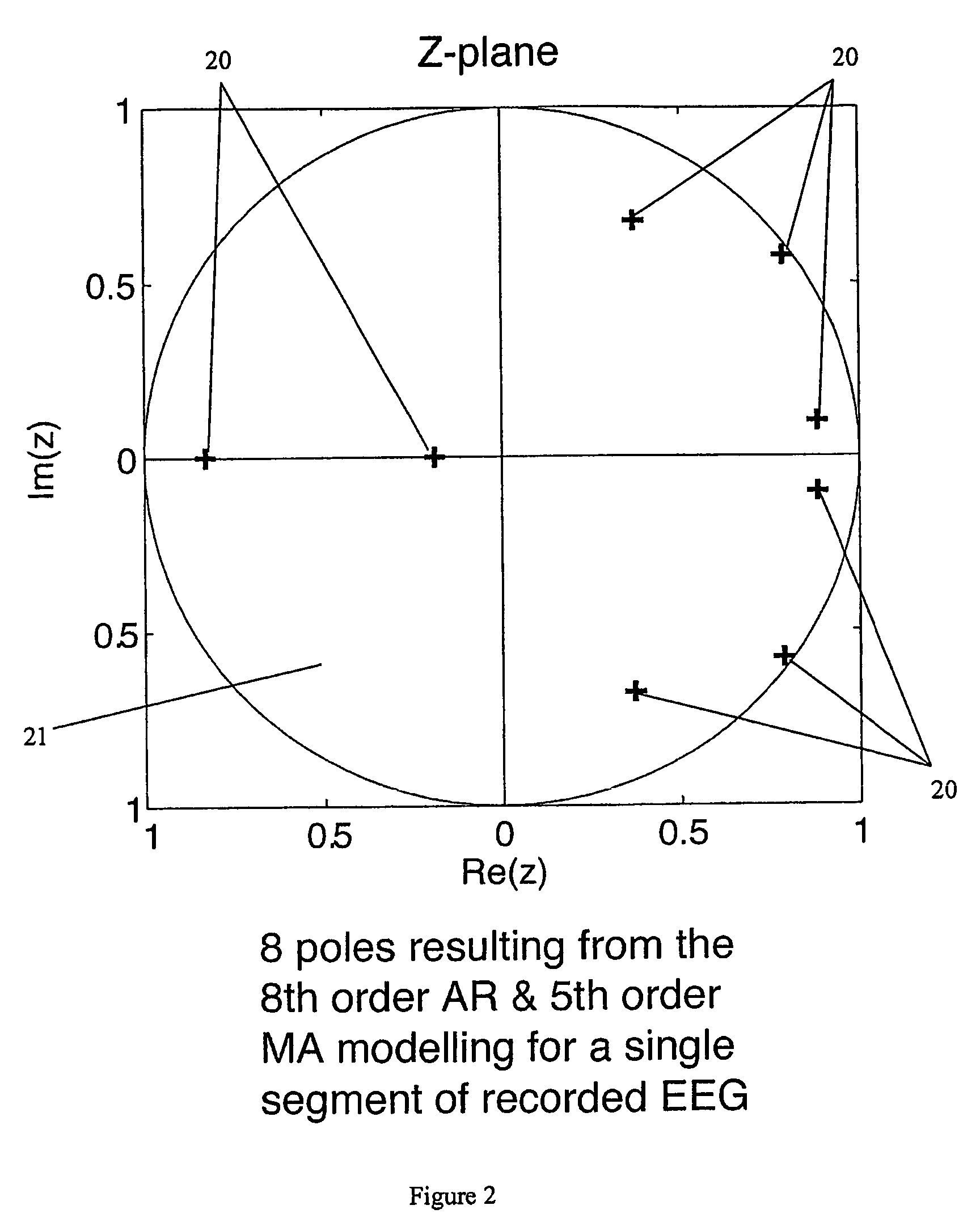
A method for assessing brain state by analysing mammalian brain electroencephalogram (“EEG”) recordings using an eighth order autoregressive and fifth order moving average discrete time equation.
Owner:CORTICAL DYNAMICS
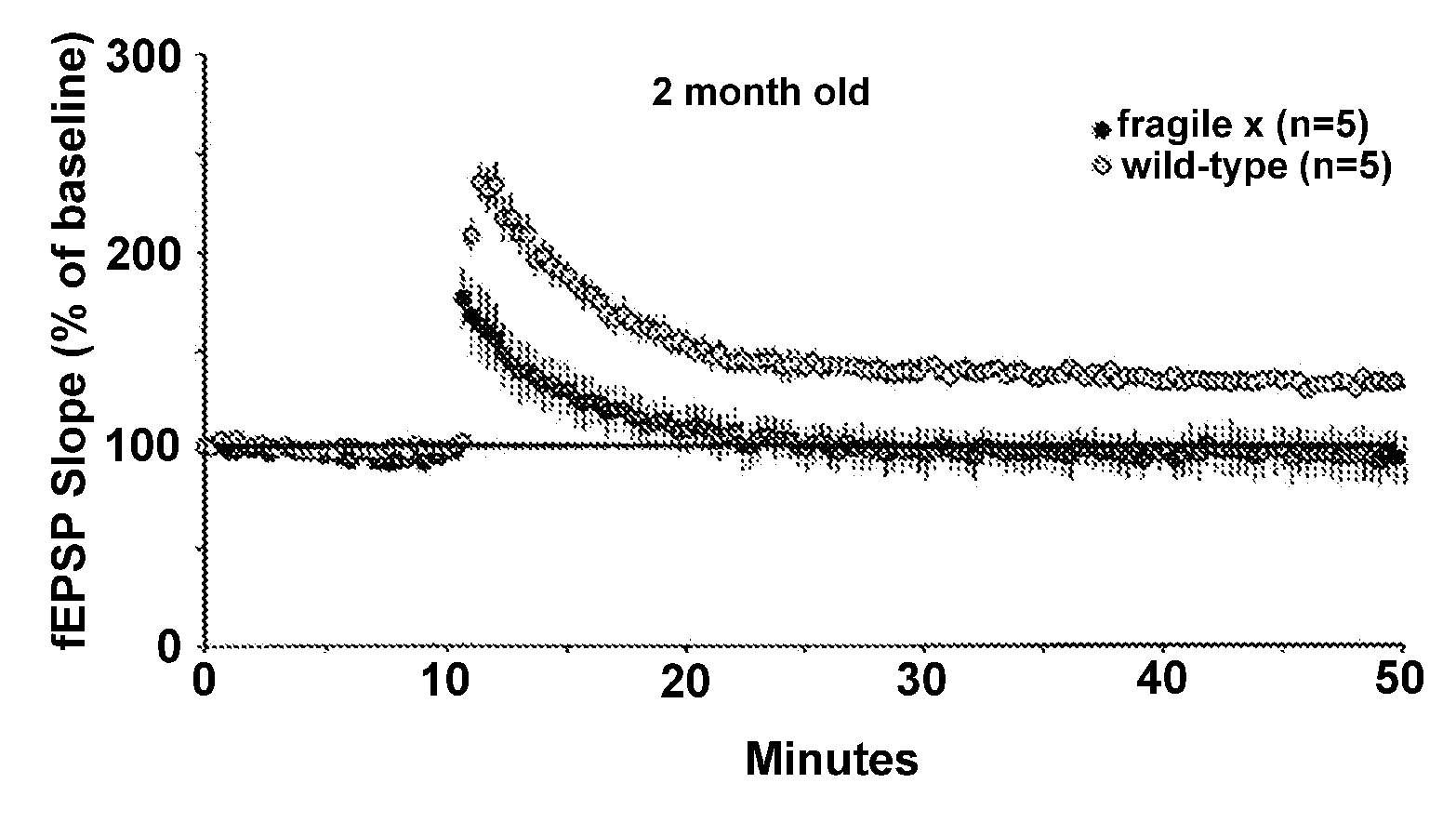
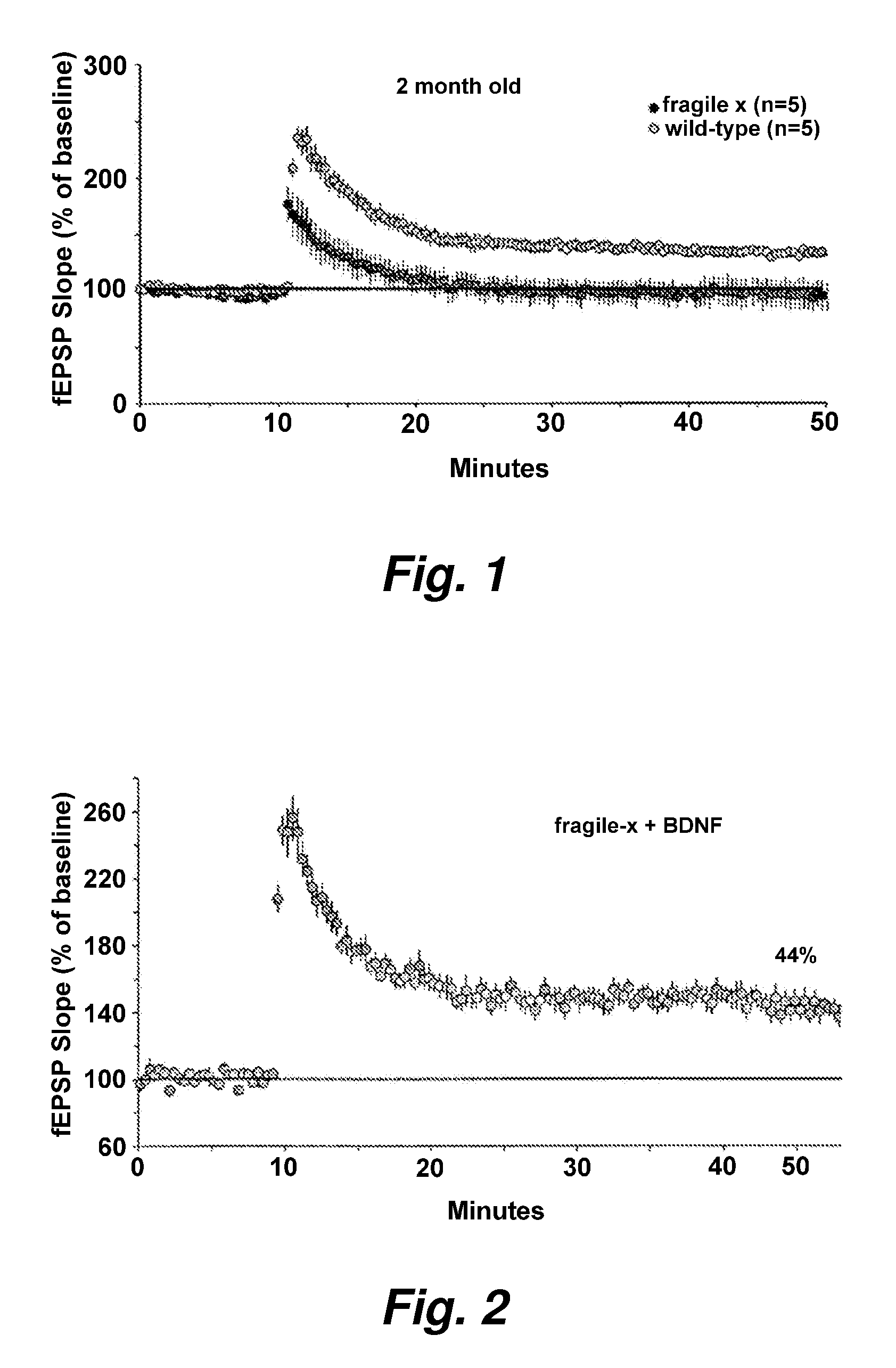
Upregulating bdnf levels to mitigate mental retardation
InactiveUS20080139472A1High activityImprove abilitiesBiocideHormone peptidesFragile X chromosomeMammal
This invention provides methods of preserving, improving, or restoring cognitive function in mammal having one or more mutations in the FMR1 gene (e.g. at risk for or having fragile x syndrome), where the methods involve the brain derived neurotrophic factor (BDNF) level or activity in the brain of said mammal. In certain embodiments the methods involve administering one or more AMPA potentiators (e.g., ampakines) to the mammal in an amount sufficient to increase BDNF levels in the brain of the mammal.
Owner:RGT UNIV OF CALIFORNIA
Gene therapy for niemann-pick disease type a
InactiveUS20090117156A1Symptoms improvedCompound screeningOrganic active ingredientsMedicineTransgene
This disclosure pertains to methods and compositions for tolerizing a mammal's brain to exogenously administered acid sphingomyelinase polypeptide by first delivering an effective amount of a transgene encoding the polypeptide to the mammal's hepatic tissue and then administering an effective amount of the transgene to the mammal's central nervous system (CNS).
Owner:GENZYME CORP
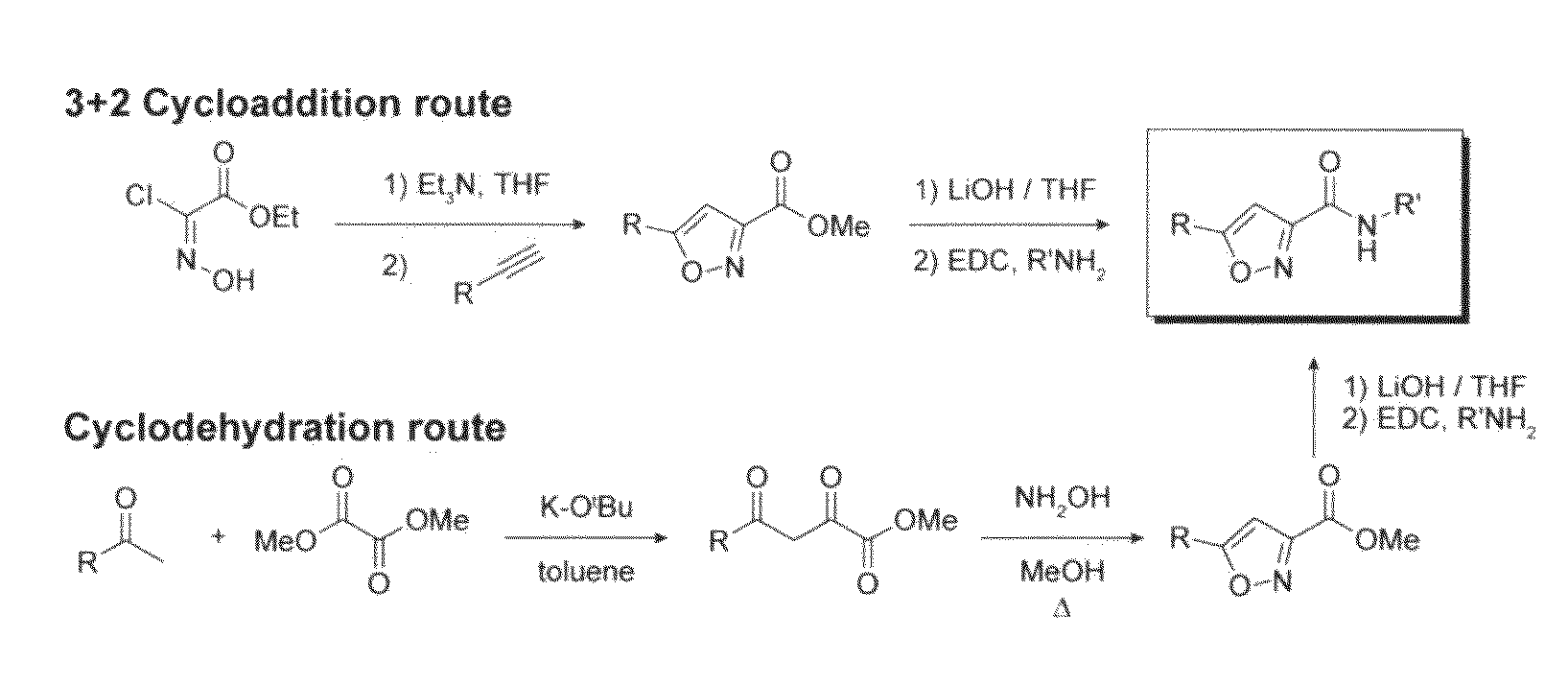
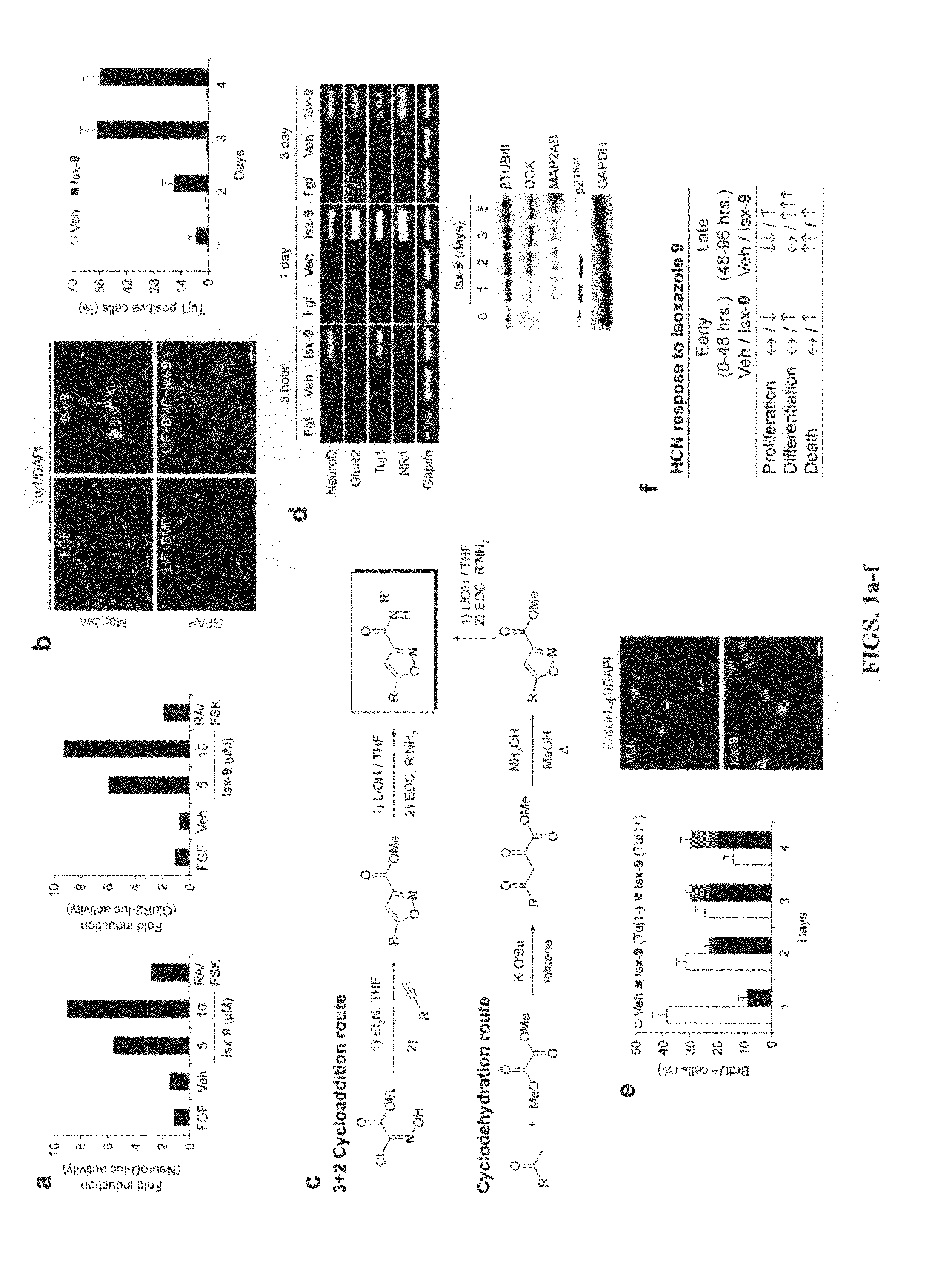
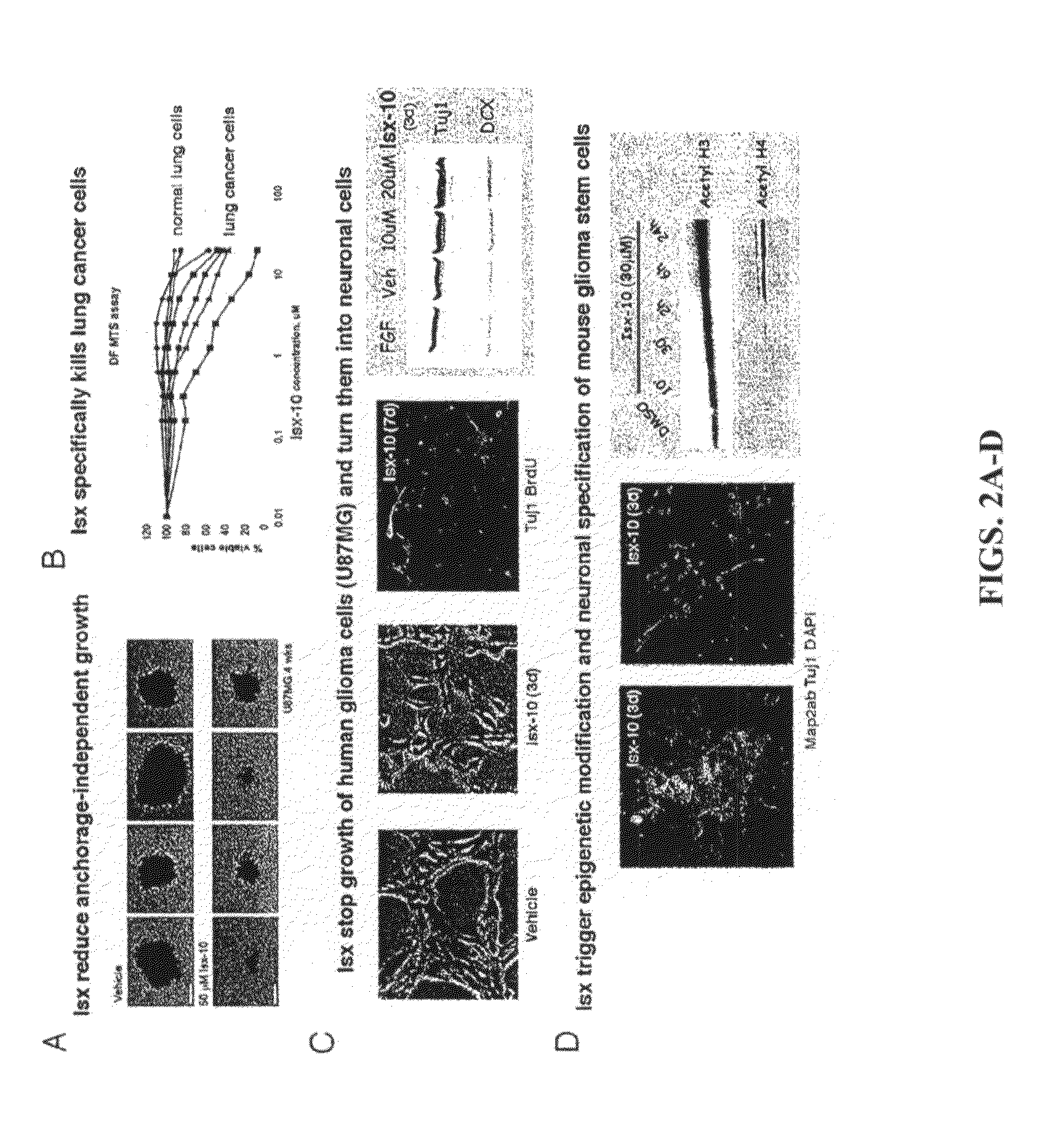
Chemical inducers of neurogenesis
InactiveUS20090036451A1Promotes and enhances well-beingReduce frequencyBiocideNervous disorderNervous systemNeurogenesis
The present invention relates to compounds and methods for inducing neuronal differentiation in normal neural stem cells and brain cancer stem cells. The methods may take place in vitro, such as in isolates from the adult mammalian brain, or in vivo. Compounds and methods described herein may find use in the treatment of neurodegenerative and psychiatric diseases, the repair and regeneration of the nervous system, and in treatment of neurologic malignancy.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
Methods for therapy of neurodegenerative disease of the brain
InactiveUS6815431B2Improve the level ofMinimal toxicityBiocidePeptide/protein ingredientsMammalMammalian brain
Owner:RGT UNIV OF CALIFORNIA
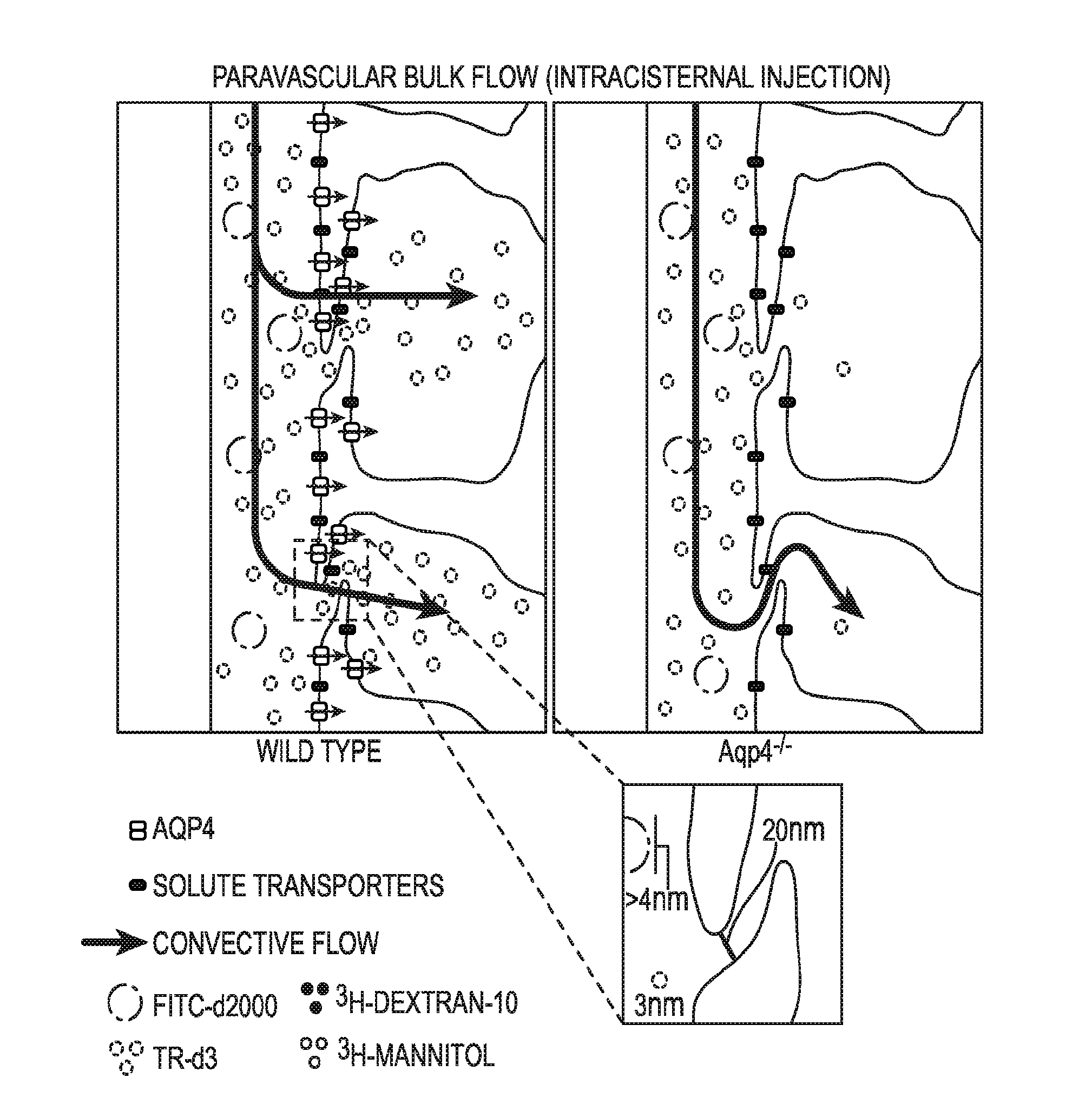
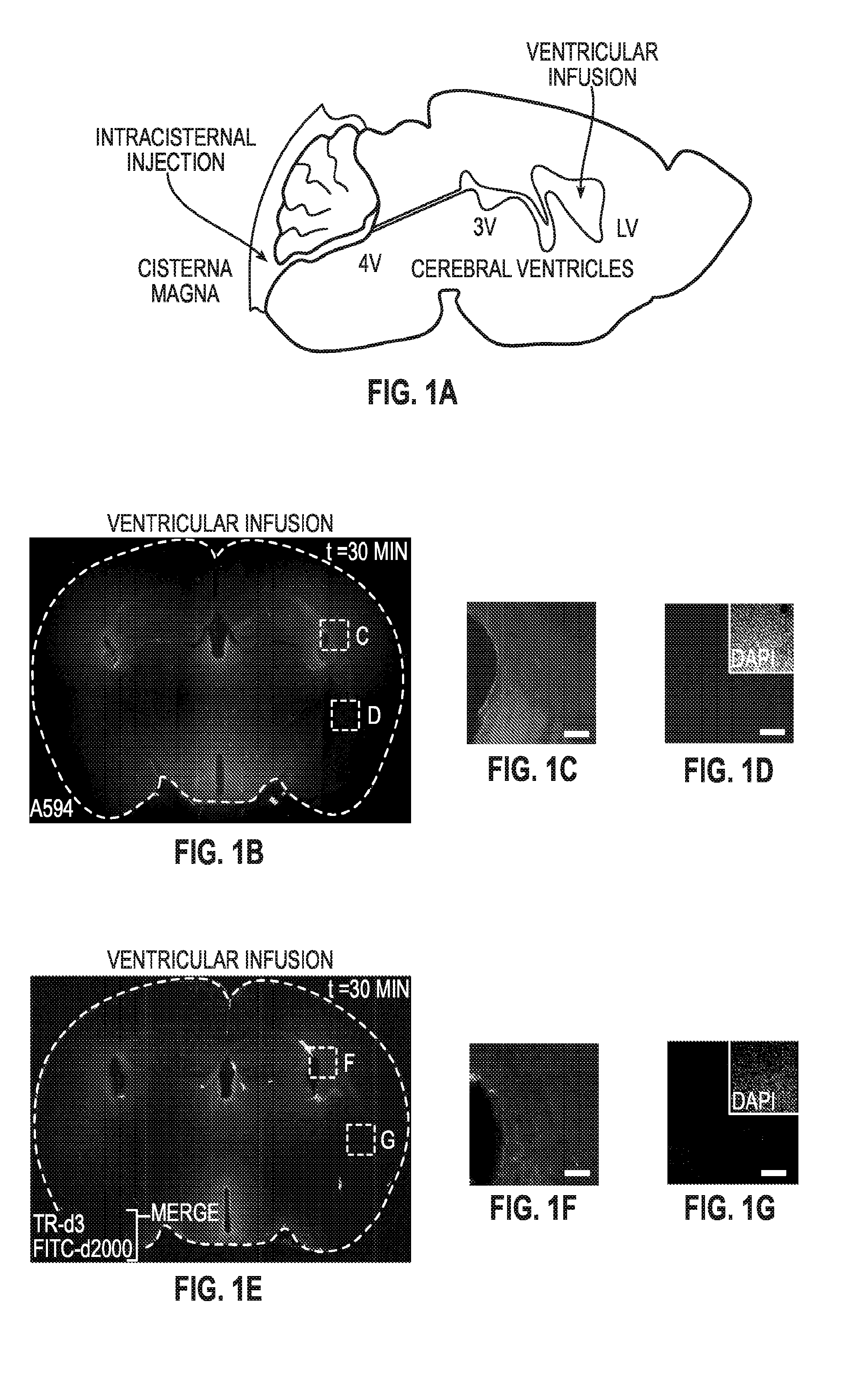
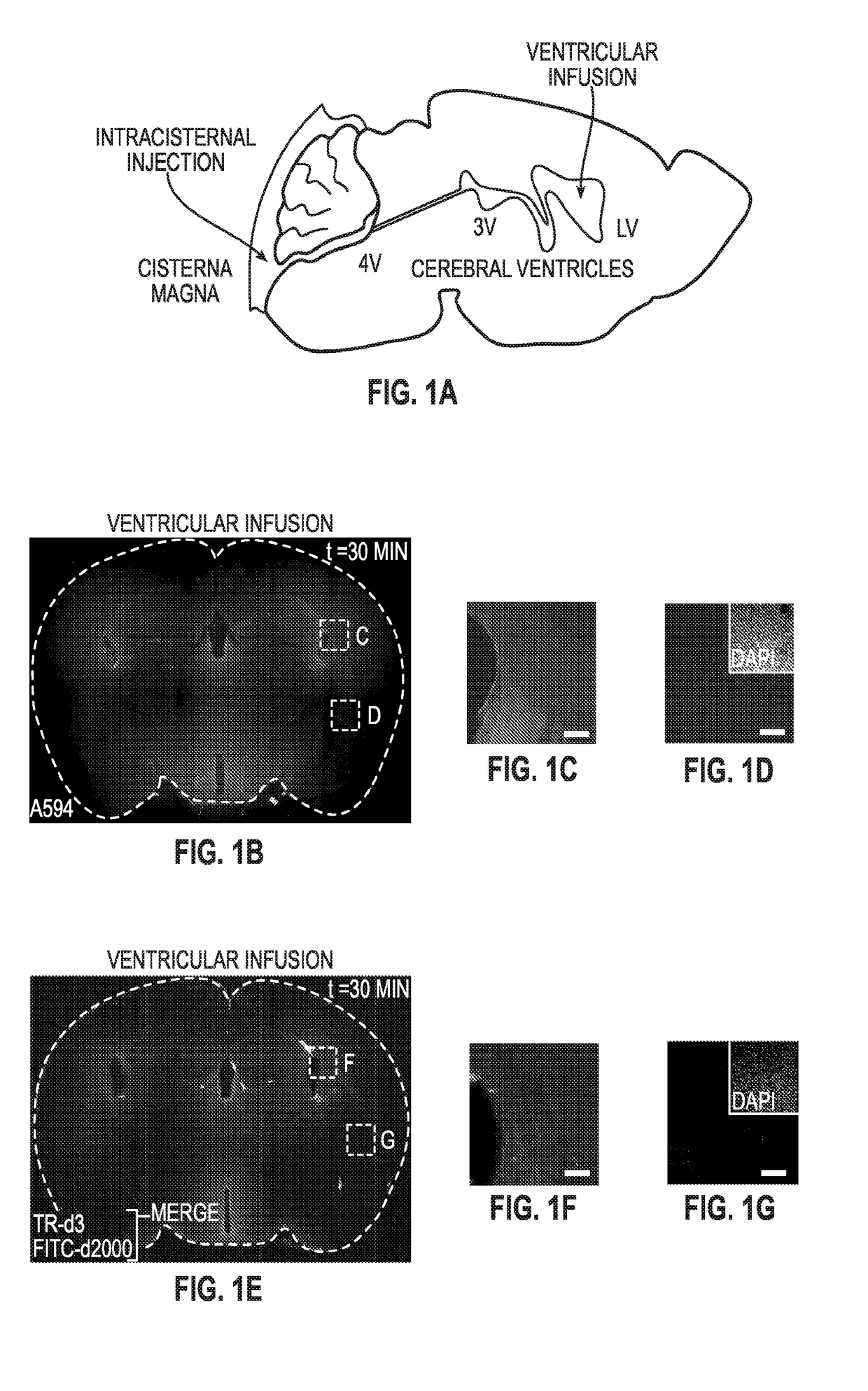
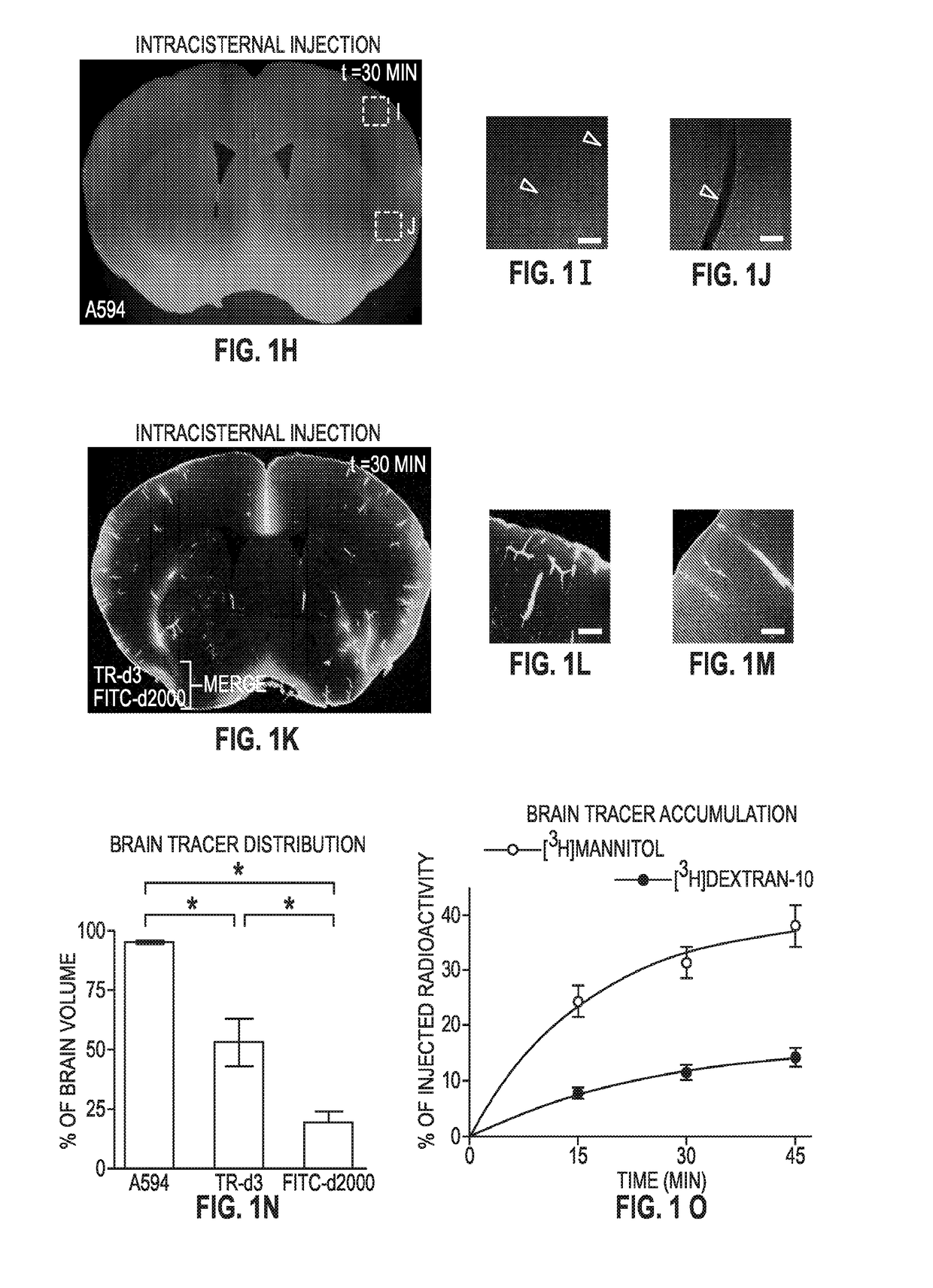
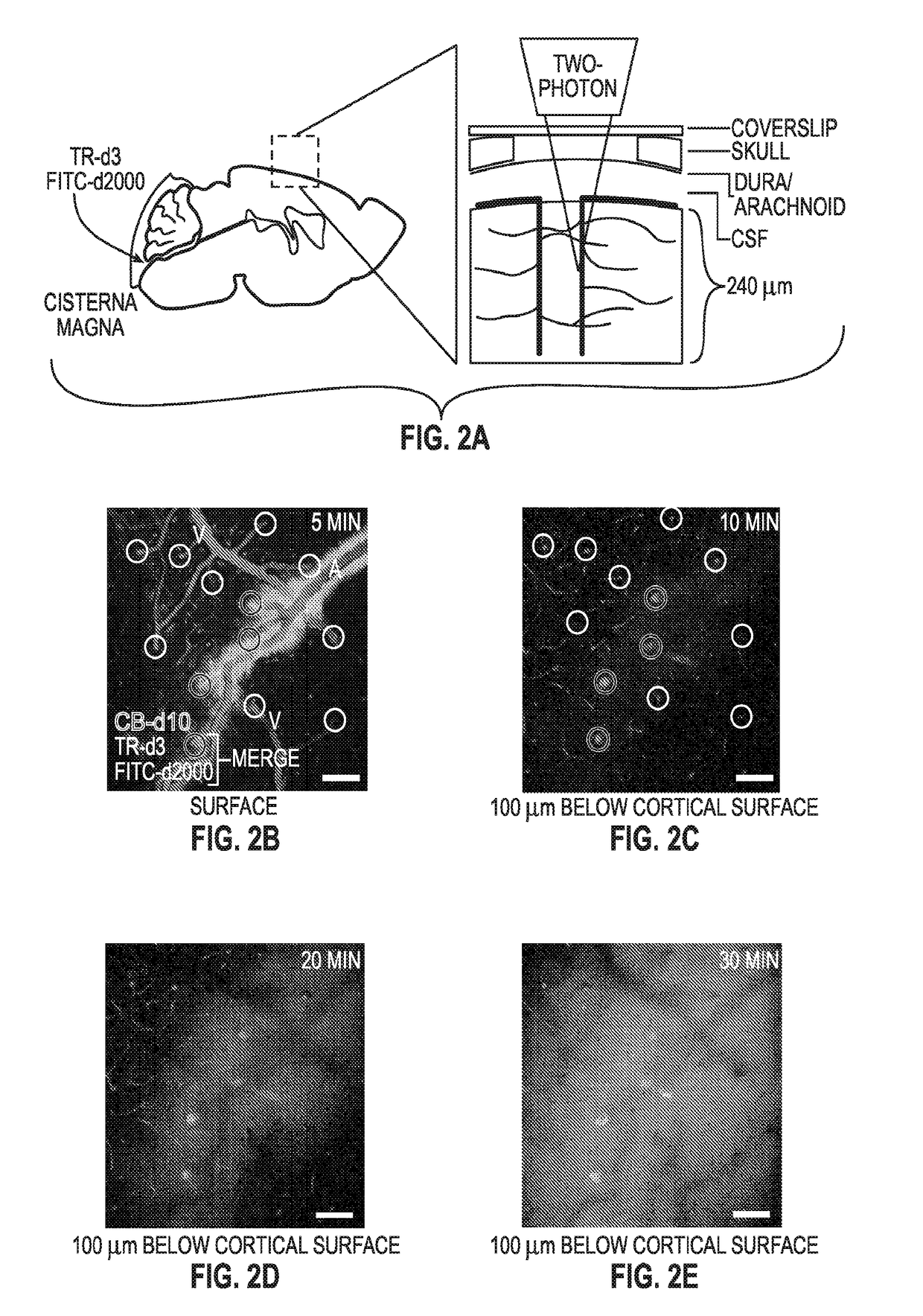
Methods for evaluating brain-wide paravascular pathway for waste clearance function and methods for treating neurodegenerative disorders based thereon
ActiveUS20160000945A1Increase and promotes glymphatic clearanceIncrease and promotes clearanceBiocideMedical imagingContrast-enhanced Magnetic Resonance ImagingImaging agent
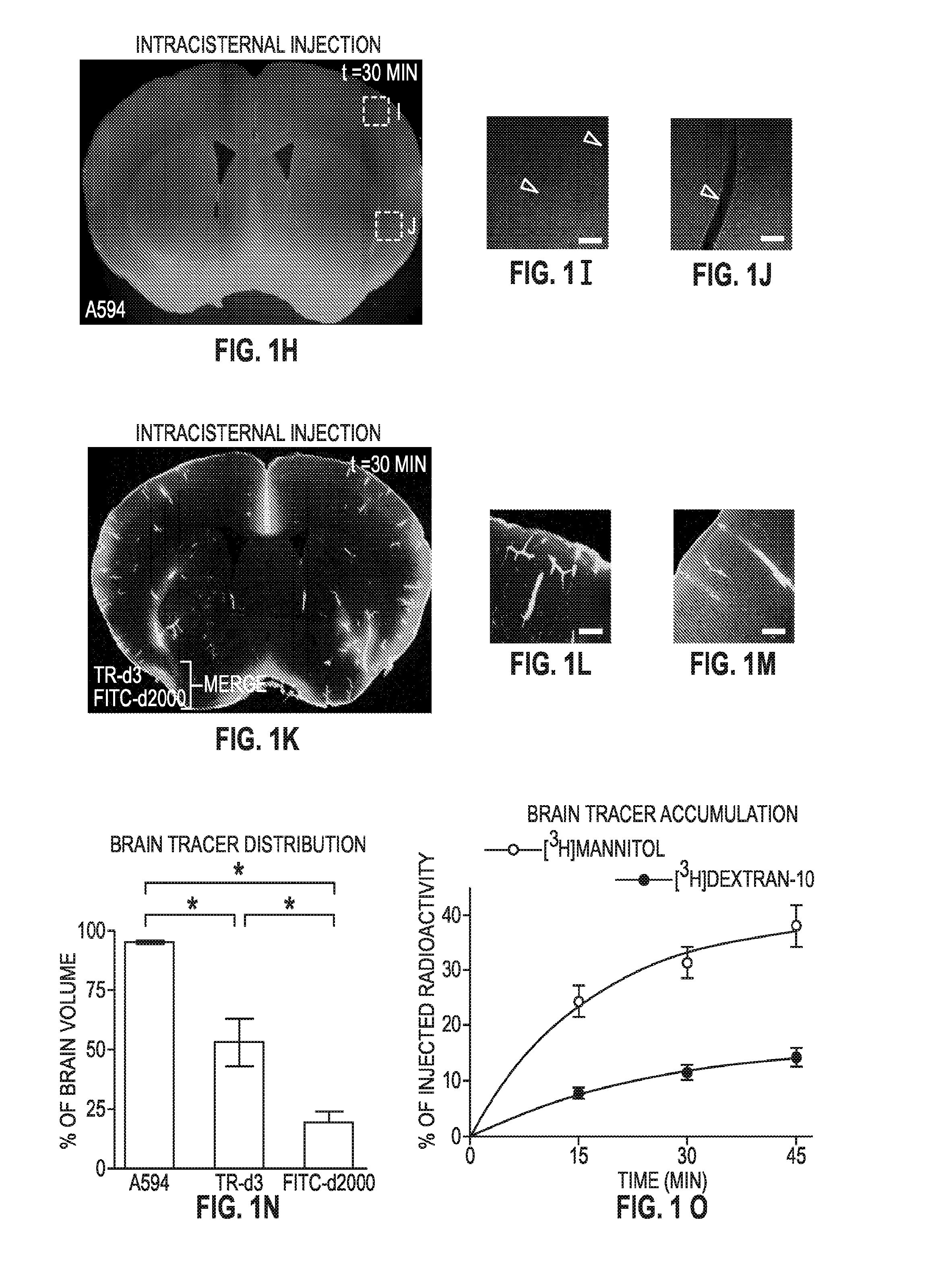
Methods are provided for measuring glio-vascular pathway (“glymphatic system”) function in the brain of a mammal which include performing imaging of the brain and measuring cerebrospinal fluid-interstitial fluid (CSF-ISF) exchange in the brain. The methods can be used to track the exchange between CSF and ISF compartments. An imaging agent is optionally administered intrathecally. The imaging agent can be a negative or positive (paramagnetic) contrast agent and dynamic or contrast-enhanced magnetic resonance imaging (MRI) of the brain can be performed. The imaging agent can be a positron-emitting radionuclide tracer and positron emission tomography (PET) can be performed. Methods for treating diseases or disorders of the mammalian brain are also provided, in which the methods increase or decrease glymphatic clearance.
Owner:THE RES FOUND OF STATE UNIV OF NEW YORK +1
Glycerophospholipids for the improvement of cognitive functions
ActiveUS7935365B2Improve cognitive functionReduce dosageBiocideNervous disorderAcyl groupMammalian brain
This invention provides a preparation comprising serine glycerophospholipids which comprise a mixture of serine glycerophospholipids comprising eicosapentaenoic acid (EPA) and serine glycerophospholipids comprising docosahexaenoic acid (DHA), wherein each such serine glycerophospholipid comprising EPA and each such serine glycerophospholipid comprising DHA has the formula (I):wherein R″ is serine; wherein one of R or R′ is acyl EPA or acyl DHA and the other of R or R′ is hydrogen or an acyl group; wherein the combined amount of EPA and DHA present in such mixture of serine glycerophospholipids constitutes 10-50% by weight of the total fatty acids content of the serine glycerophospholipids in said preparation; and wherein the mixture is not identical to naturally occurring human or mammalian brain PS.
Owner:ENZYMOTEC
Myelination of congenitally dysmyelinated forebrains using oligodendrocyte progenitor cells
Owner:CORNELL RES FOUNDATION INC
Method of counteracting seizures
InactiveUS20120095524A1High pulse rateReducing synchronyElectrotherapyArtificial respirationElectricityMedicine
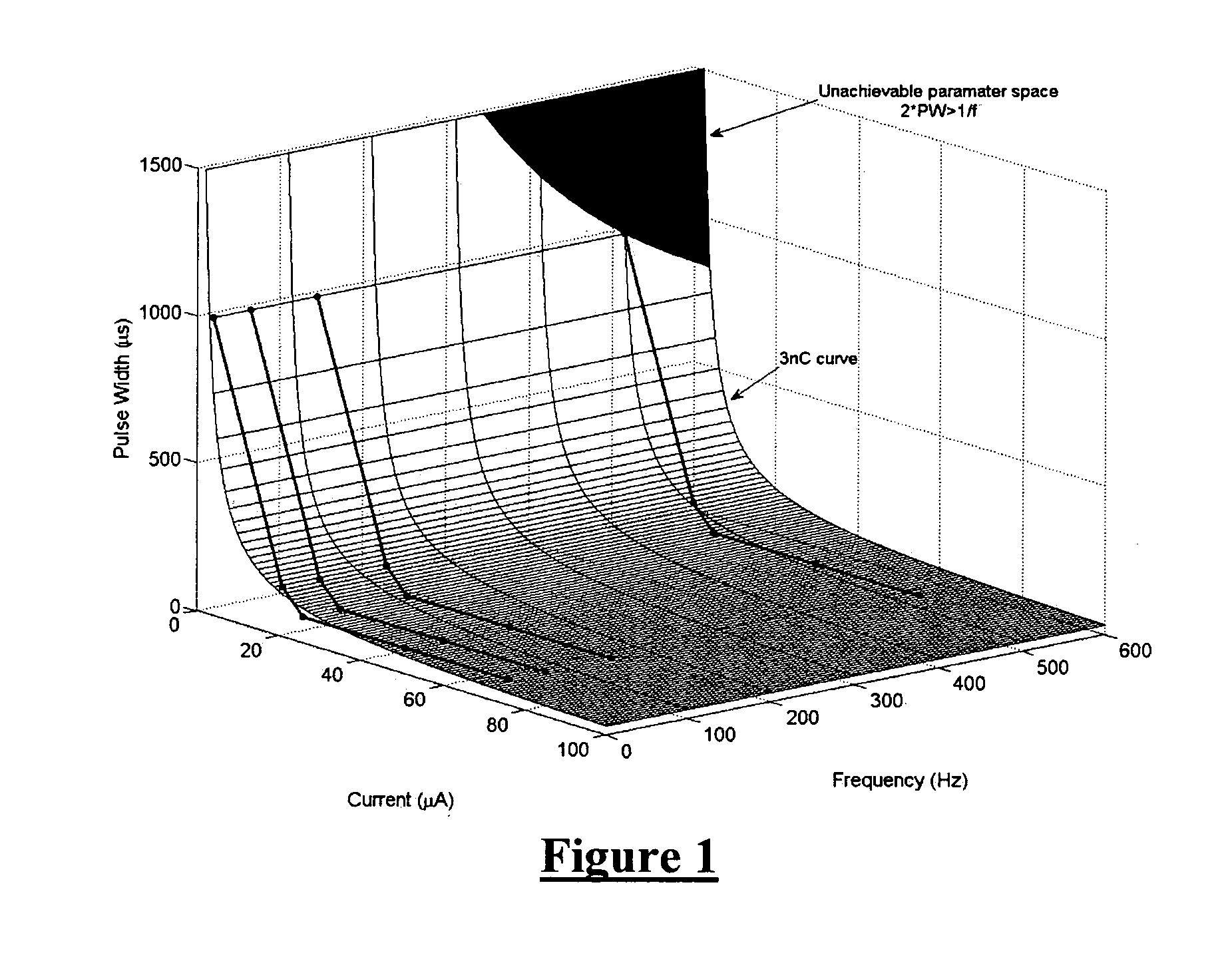
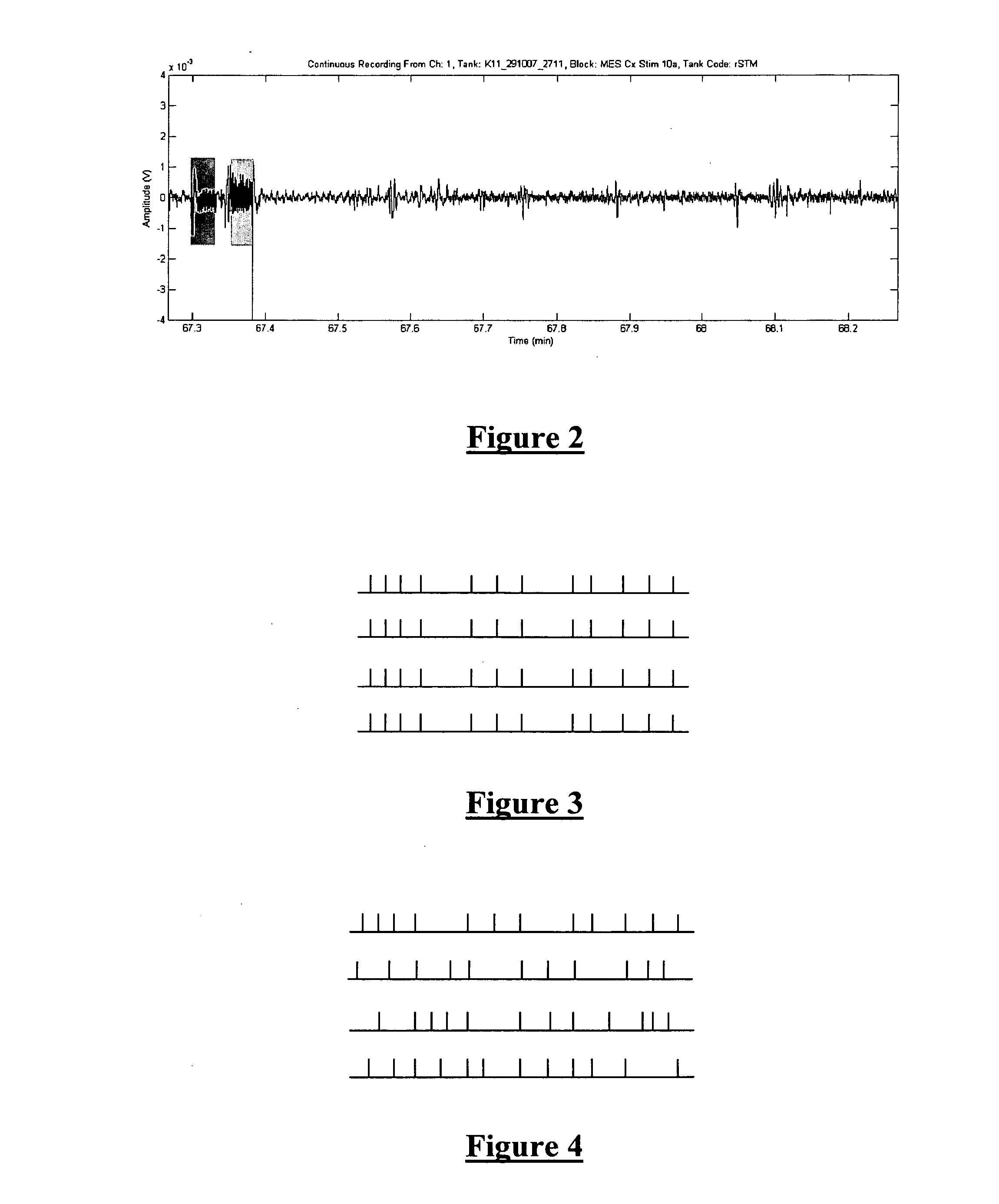
The present invention provides a method for counteracting seizure events in a mammalian brain, the method comprising applying an electrical stimulus to the brain, the electrical stimulus being pulsatile and comprising pulses forming a pulse train. In order to effectively “desynchronize” the neural activity patterns in the brain, the pulse train can be at a frequency greater than substantially 300 Hz and at a duty cycle greater than substantially 20%, the pulse train can have an inconstant inter pulse interval such that the pulse rate is not constant throughout the pulse train, and the pulses can have a pulse width greater than substantially 300 μsec. Apparatus for carrying out the method is also described.
Owner:THE BIONICS INST OF AUSTRALIA
Methods for therapy of neurodegenerative disease of the brain
A specific clinical protocol for use toward therapy of defective, diseased and damaged cholinergic neurons in the mammalian brain, of particular usefulness for treatment of neurodegenerative conditions such as Alzheimer's disease. The protocol is practiced by delivering a definite concentration of recombinant neurotrophin into, or within close proximity of, identified defective, diseased or damaged brain cells. Using a viral vector, the concentration of neurotrophin delivered as part of a neurotrophic composition varies from 1010 to 1015 neurotrophin encoding viral particles / ml of composition fluid. Each delivery site receives from 2.5 μl to 25 μl of neurotrophic composition, delivered slowly, as in over a period of time ranging upwards of 10 minutes / delivery site. Each delivery site is at, or within 500 μm of, a targeted cell, and no more than about 10 mm from another delivery site. Stable in situ neurotrophin expression can be achieved for 12 months, or longer.
Owner:RGT UNIV OF CALIFORNIA
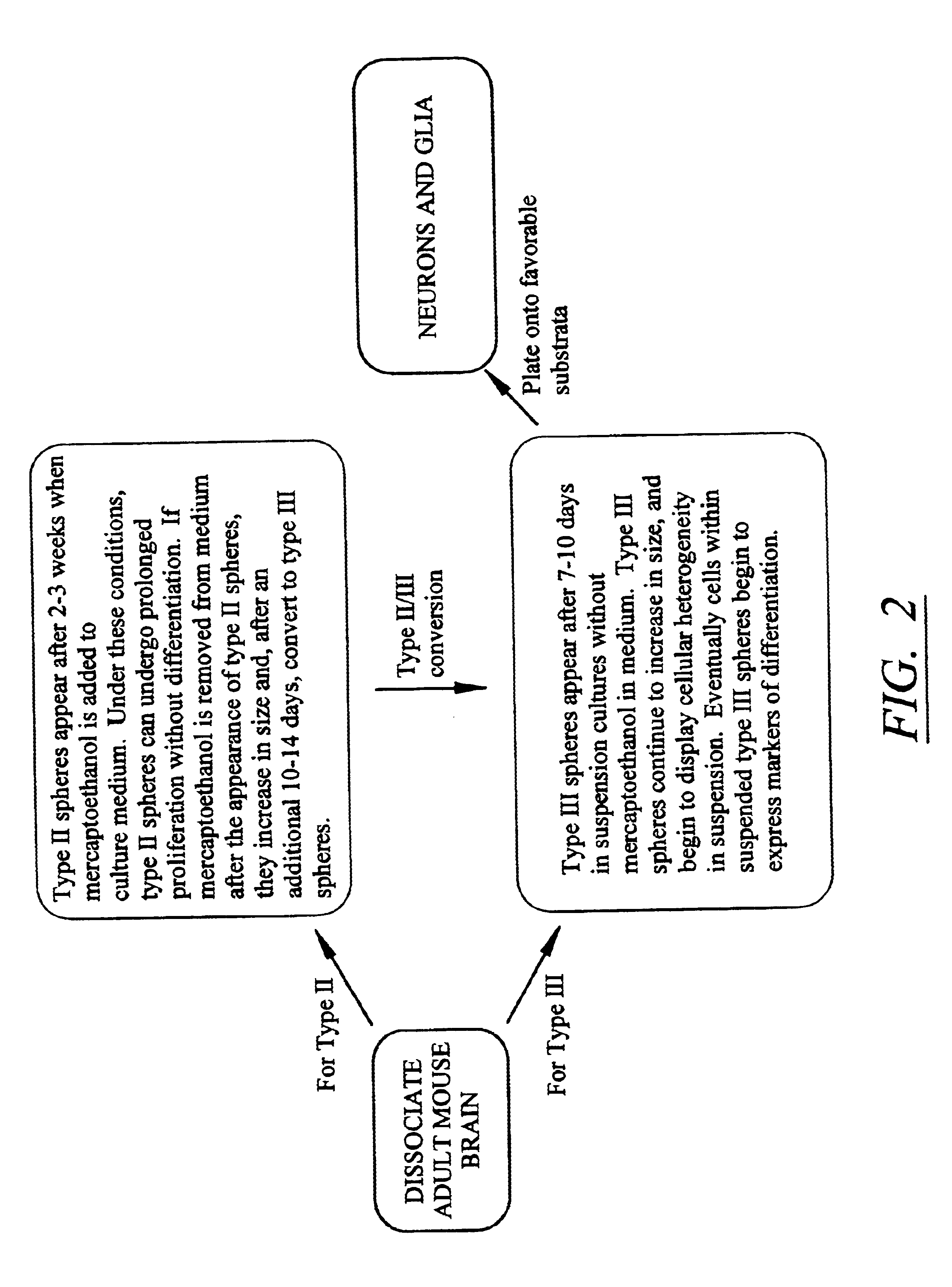
Isolated mammalian neural stem cells, methods of making such cells
Using a novel culture approach, previously unknown populations of neural progenitor cells have been found within an adult mammalian brain. By limiting cell-cell contact, dissociated adult brain yields at least two types of cell aggregates. These aggregates or clones of stem / precursor cells can be generated from adult brain tissue with significantly long postmortem intervals. Both neurons and glia arise from stem / precursor cells of these cultures, and the cells can survive transplantation to the adult mammalian brain.
Owner:UNIV OF TENNESSEE RES FOUND
Method of monitoring brain function
A method for assessing brain state by analysing mammalian brain electroencephalogram (“EEG”) recordings using an eighth order autoregressive and fifth order moving average discrete time equation.
Owner:CORTICAL DYNAMICS
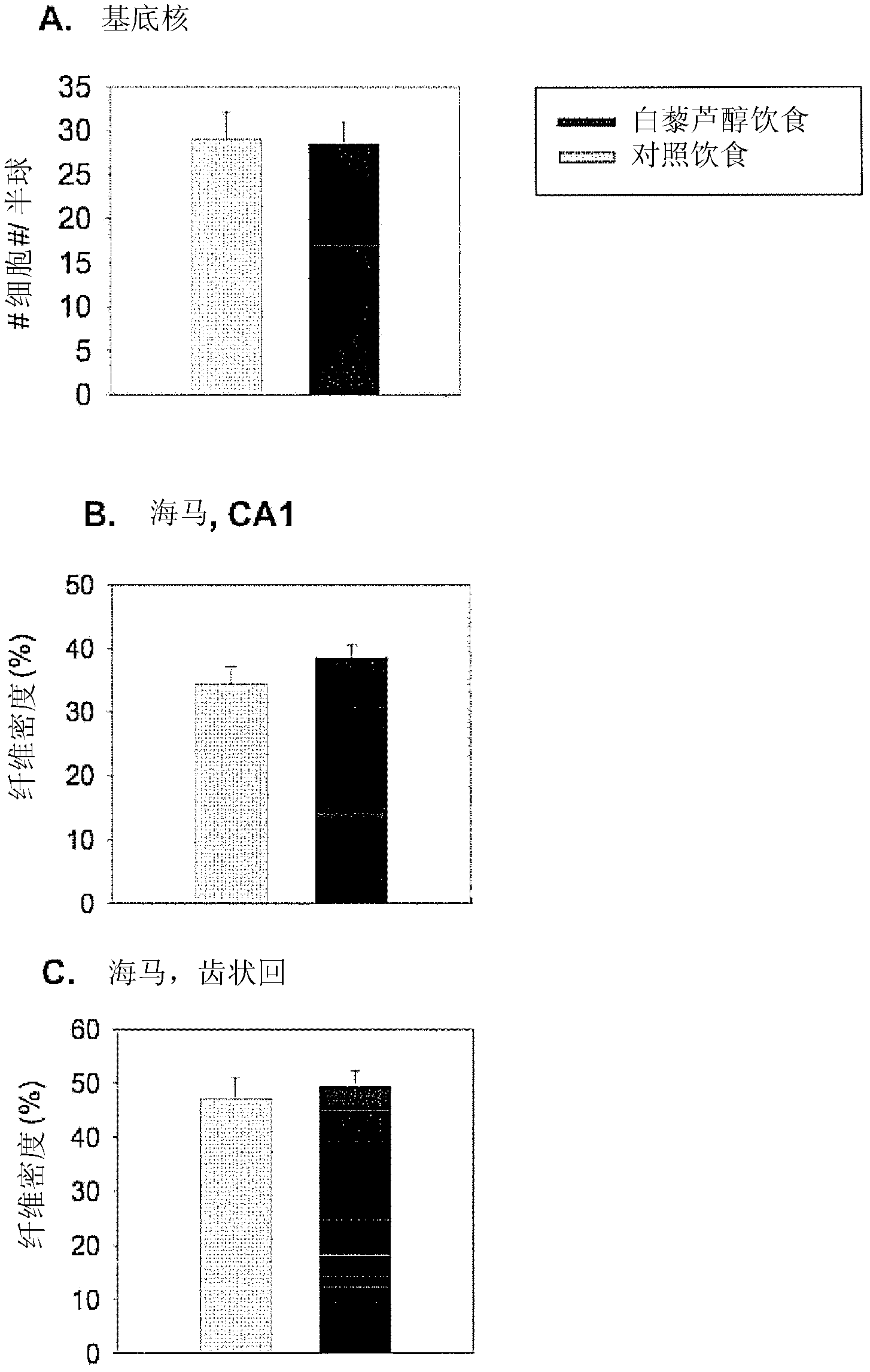
Use of resveratrol or another hydroxylated stilbene for preserving cognitive functioning
The present invention relates to the use of an hydroxylated stilbene, in particular resveratrol, in the manufacture of a neutraceutical composition for increasing the mi- crovascular plasticity and / or microvessel density, and / or decreasing the microvessel abnormalities in the brain, in particular in the hippocampus of a mammal, in particular for the treatment of age- and condition-related decline in brain neuronal function and / or cognitive functioning in a mammal. In particular, the condition is selected from the group of Alzheimer's Disease, dementia, depression, sleep disorders, impaired memory function, psychoses, Parkinson's disease, Huntington's chorea, epilepsy, schizophrenia, paranoia, ADHD and anxiety.
Owner:NUTRICIA
Methods for evaluating brain-wide paravascular pathway for waste clearance function and methods for treating neurodegenerative disorders based thereon
ActiveUS9901650B2Increase the gapPromoting faster CSF clearanceMedical imagingNervous disorderDiseaseContrast-enhanced Magnetic Resonance Imaging
Methods are provided for measuring glio-vascular pathway (“glymphatic system”) function in the brain of a mammal which include performing imaging of the brain and measuring cerebrospinal fluid-interstitial fluid (CSF-ISF) exchange in the brain. The methods can be used to track the exchange between CSF and ISF compartments. An imaging agent is optionally administered intrathecally. The imaging agent can be a negative or positive (paramagnetic) contrast agent and dynamic or contrast-enhanced magnetic resonance imaging (MRI) of the brain can be performed. The imaging agent can be a positron-emitting radionuclide tracer and positron emission tomography (PET) can be performed. Methods for treating diseases or disorders of the mammalian brain are also provided, in which the methods increase or decrease glymphatic clearance.
Owner:THE RES FOUND OF STATE UNIV OF NEW YORK +1
Enzymatically synthesized marine phospholipids
InactiveUS20060177486A1Increase ratingsReduce inhibitionFatty acid esterificationClimate change adaptationEnzymatic synthesisRed blood cell
This invention discloses an improved enzymatic process, under organic solvent free conditions, for the incorporation of fatty acids such as omega-3 fatty acids into phospholipids. The rate of transesterification is increased 4 times by adding a base to the reaction mixture, typically an amine. The invention also discloses novel phospholipid compositions as well as novel use of the phospholipid compositions as a food supplement, a fish feed, animal feed and human food. In addition to methods for enriching prey organisms used in aquaculture, methods of reducing arachidonic acid levels in mammalian plasma / red blood cells and methods for increasing DHA levels in the mammalian brain.
Owner:AKER BIOMARINE ASA
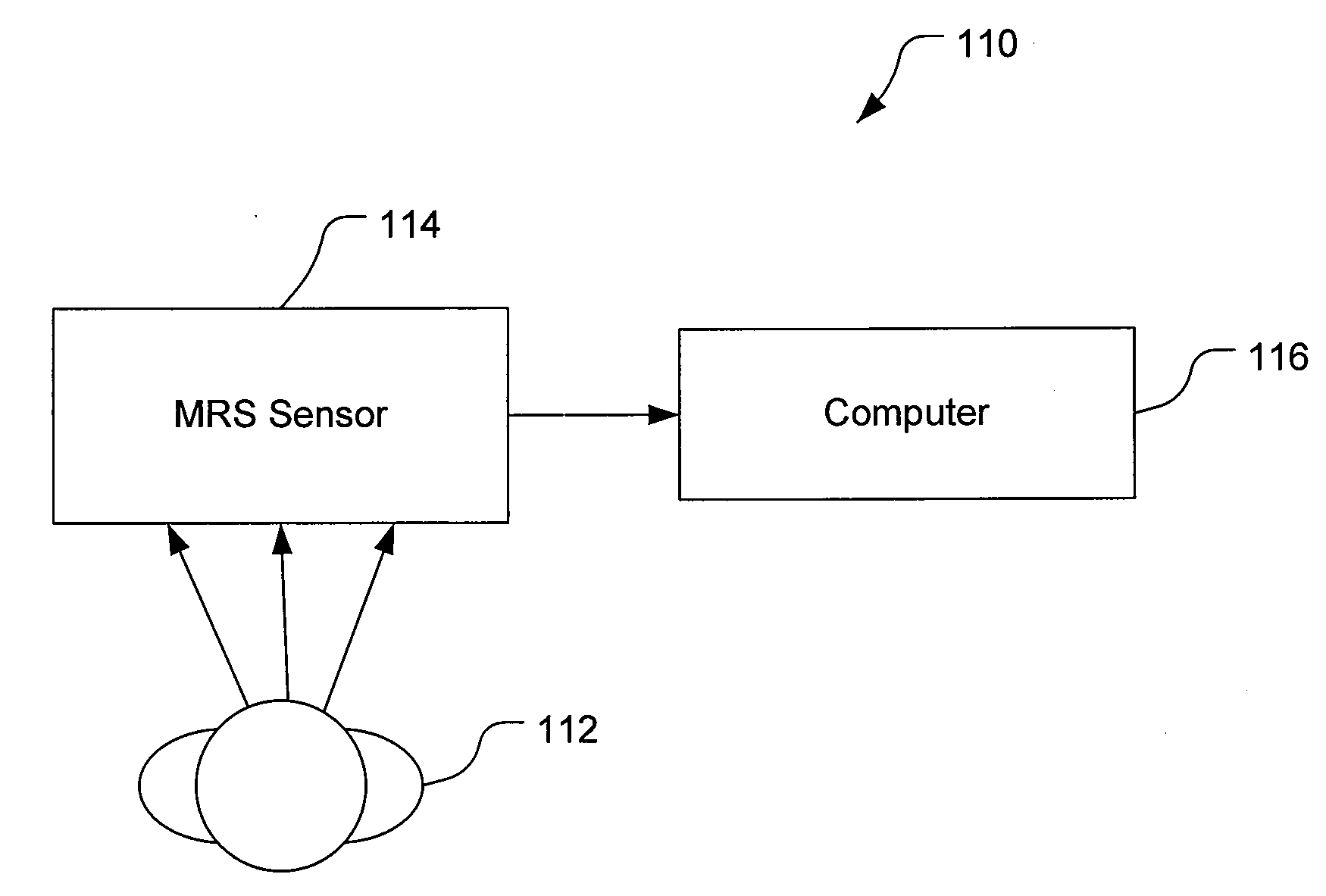
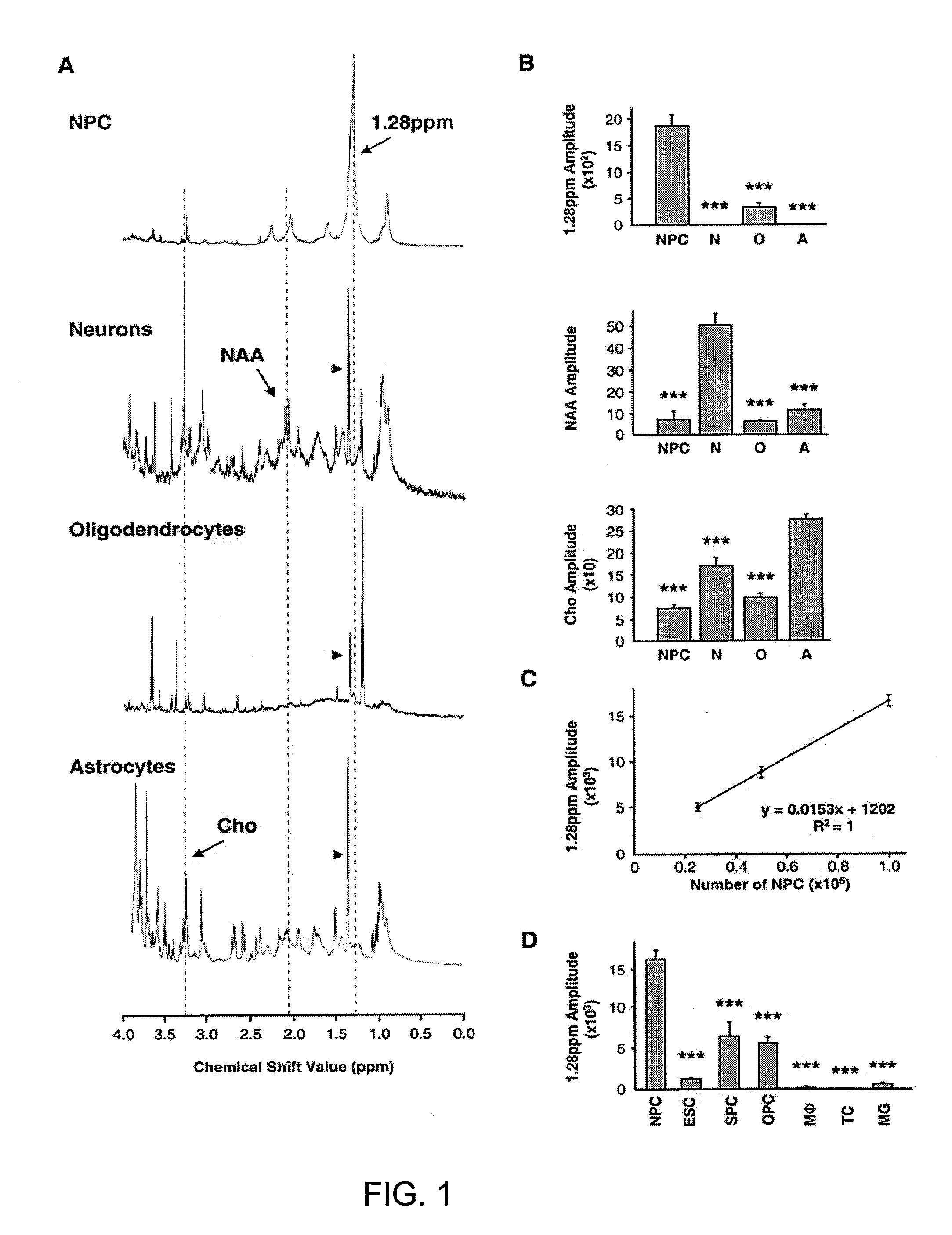
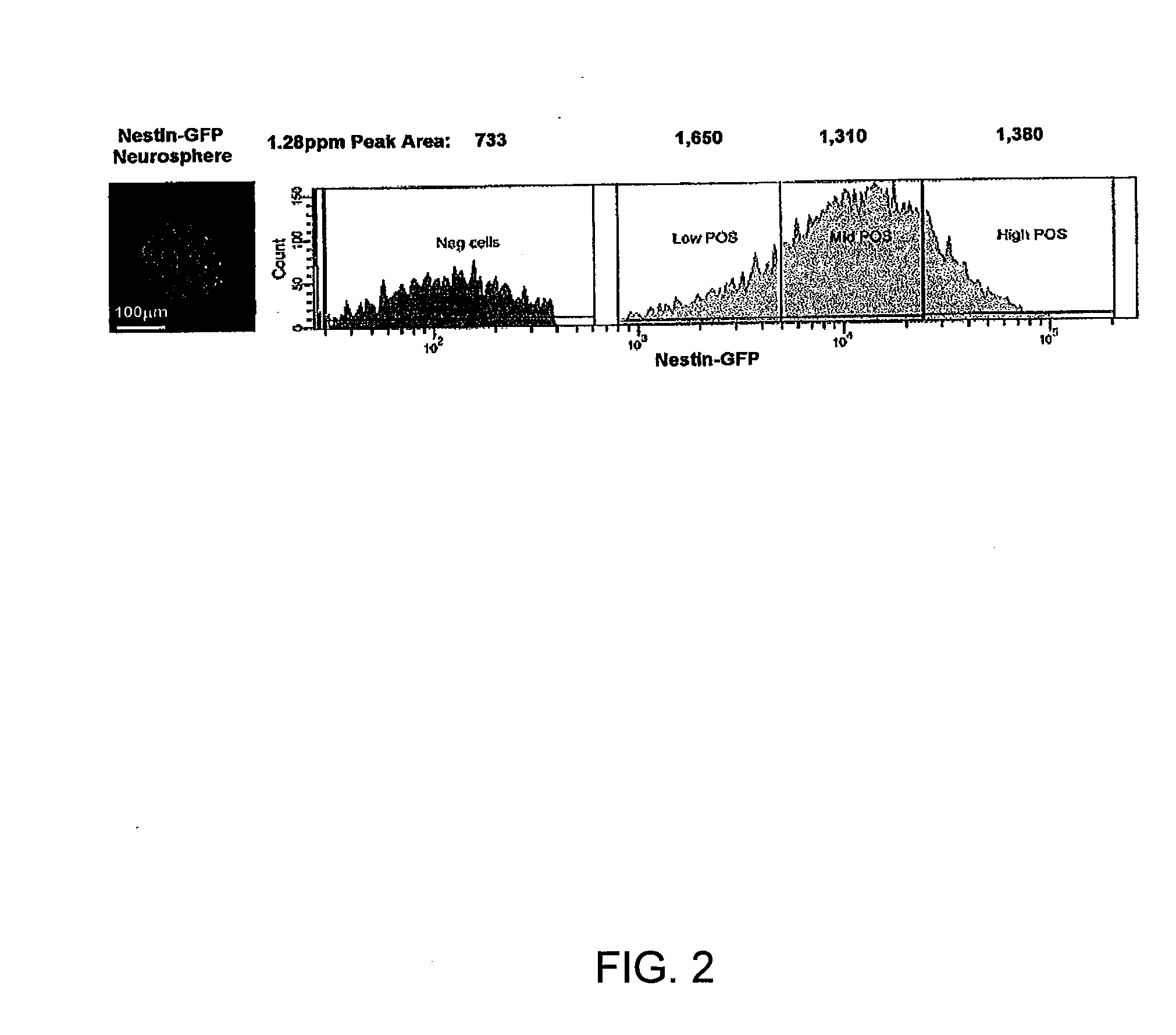
Spectral biomarker and algorithm for the identification and detection of neural stem and progenitor cells and their use in studying mammalian brains
InactiveUS20090247860A1Reduce the impactReduce impactMagnetic measurementsDisease diagnosisProgenitorNeurogenesis
The disclosure provides a biomarker and algorithm for identifying and detecting neural stem and progenitor cells and their use in studying mammalian brains. The disclosure further provides magnetic resonance spectroscopy methods and an image enhancing algorithm for the study of the proliferation of these cells and the associated neurogenesis in the live mammalian brain.
Owner:THE RES FOUND OF STATE UNIV OF NEW YORK +1
Methods for therapy of neurodegenerative disease of the brain
InactiveUS20070249554A1Avoid restrictionsReduce adverse effectsOrganic active ingredientsNervous disorderCholinergic cellsMammalian brain
A specific clinical protocol for use toward therapy of defective, diseased and damaged cholinergic neurons in the mammalian brain, of particular usefulness for treatment of neurodegenerative conditions such as Alzheimer's disease. The protocol is practiced by delivering a definite concentration of recombinant neurotrophin into, or within close proximity of, identified defective, diseased or damaged brain cells. Using a viral vector, the concentration of neurotrophin delivered as part of a neurotrophic composition varies from 1010 to 1015 neurotrophin encoding viral particles / ml of composition fluid. Each delivery site receives form 2.5 μl to 25 μl of neurotrophic composition delivered slowly, as in over a period of time ranging upward of 10 minutes / delivery site. Each delivery site is at, or within 500 μm of, a targeted cell, and no more than about 10 mm from another delivery site. Stable in situ neurotrophin expression can be achieved for 12 months, or longer.
Owner:RGT UNIV OF CALIFORNIA
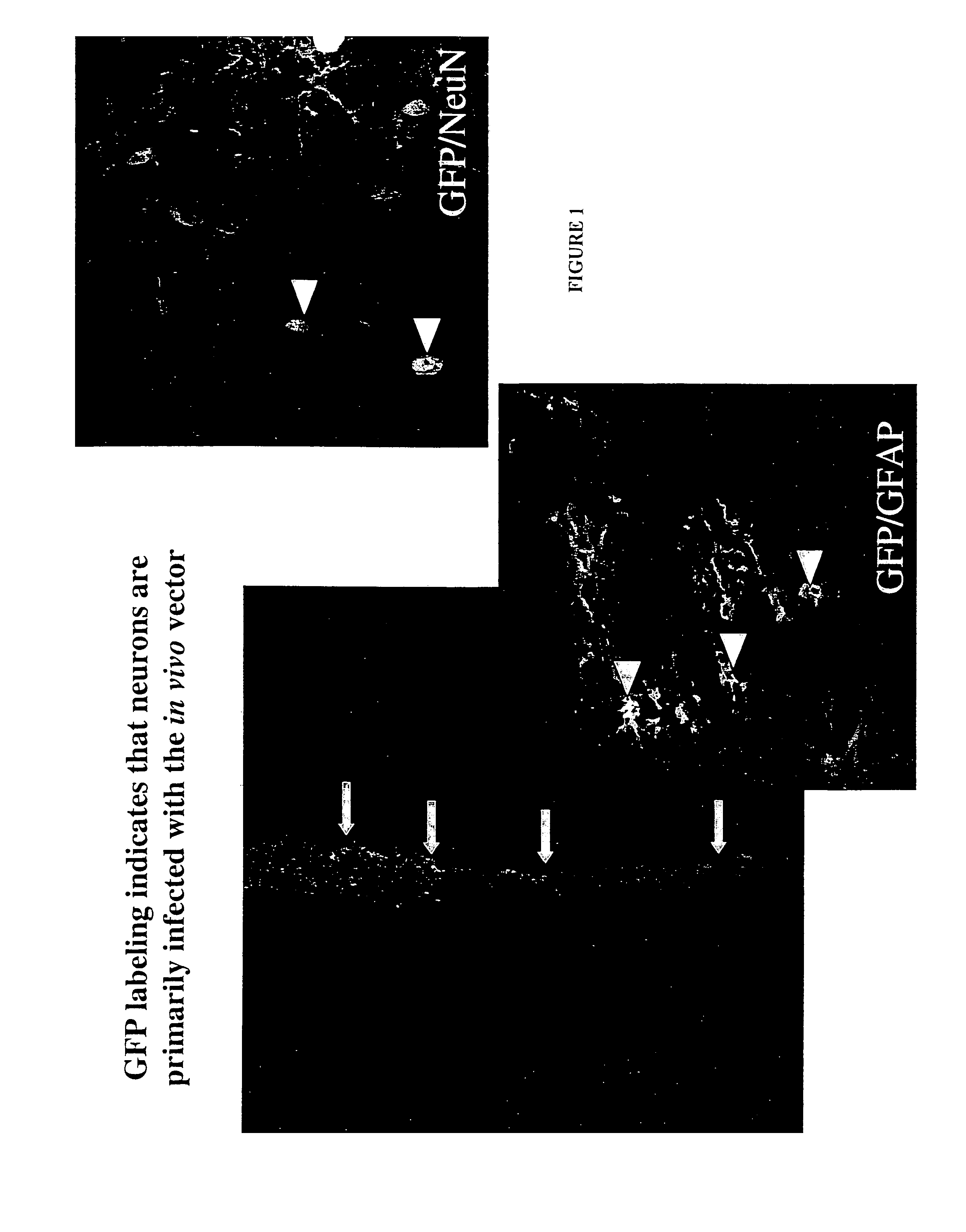
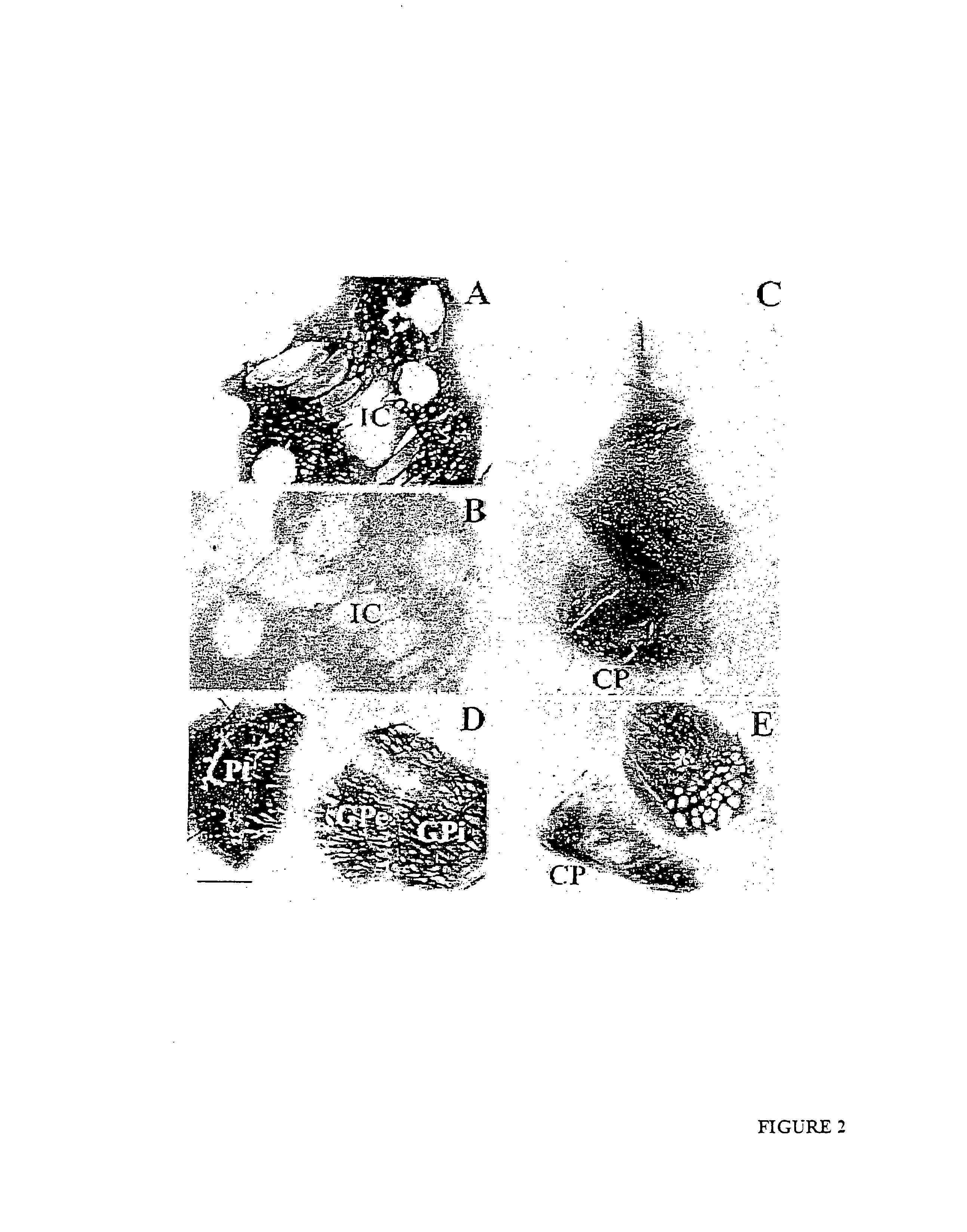
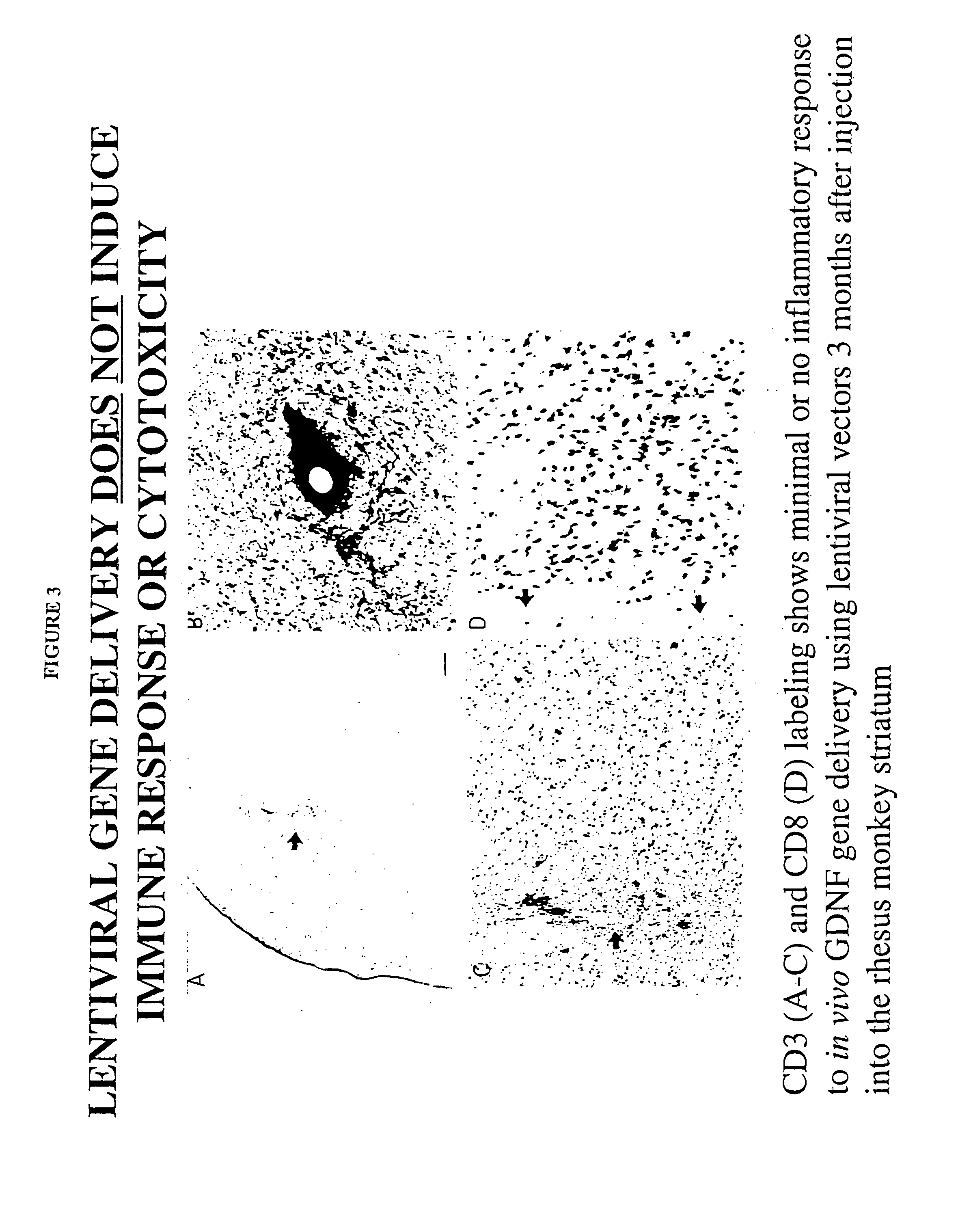
Enhanced delivery of viral particles to the striatum and cortex
Provided herein are novel methods for delivering recombinant adeno-associated viral (rAAV) particles to the central nervous system of a mammal (e.g., a human). In aspects, the methods involve administering rAAV particles containing a heterologous nucleic acid to the striatum and causing expression of the heterologous nucleic acid in at least the cerebral cortex and the striatum of the mammal.
Owner:GENZYME CORP
Methods for therapeutic use of brain derived neurotrophic factor in the entorhinal cortex
InactiveUS20030124095A1Measured cognitive functionImprove cognitive functionBiocideSenses disorderFactor iiCortical tissue
A protocol for use of growth factors to stimulate neuronal cell growth and activity in trkB receptor containing cortical tissues, including the entorhinal and hippocampal cortices. The method introduces exogenous growth factor, such as BDNF, NT-4 / 5 and NT-3, into the EC. The method is useful in therapy of defective, diseased and damaged neurons in the mammalian brain, of particular usefulness for treatment of neurodegenerative conditions such as Alzheimer's disease or for normal aging.
Owner:RGT UNIV OF CALIFORNIA
Methods for modulation of the effects of aging on the primate brain
InactiveUS7157435B2Minimizing surgical invasionImprove neurological functionBiocideVirusesNeurulationNervous system
Owner:RGT UNIV OF CALIFORNIA
Method for therapy of neurodegenerative disease of the brain
InactiveUS20060051322A1Reduce adverse effectsAvoid restrictionsBiocideNervous disorderCholinergic cellsNeuro-degenerative disease
A specific clinical protocol for use toward therapy of defective, diseased and damaged cholinergic neurons in the mammalian brain, of particular usefulness for treatment of neurodegenerative conditions such as Alzheimer's disease. The protocol is practiced by delivering a definite concentration of recombinant neurotrophin into, or within close proximity of, identified defective, diseased or damaged brain cells. Using a viral vector, the concentration of neurotrophin delivered as part of a neurotrophic composition varies from 1010 to 1015 neurotrophin encoding viral particles / ml of composition fluid. Each delivery site receives form 2.5 μl to 25 μl of neurotrophic composition, delivered slowly, as in over a period of time ranging upwards of 10 minutes / delivery site. Each delivery site is at, or within 500 μm of, a targeted cell, and no more than about 10 mm from another delivery site. Stable in situ neurotrophin expression can be achieved for 12 months, or longer.
Owner:TUSZYNSKI MARK H
Methods for treating parkinson's disease and other disorders of dopaminergic neurons of the brain
A specific clinical protocol for use toward therapy of defective, diseased and damaged neurons in the mammalian brain, of particular usefulness for treatment of neurodegenerative conditions such as Parkinson's disease. The protocol is practiced by directly delivering a definite concentration of a nerve growth factor via delivery of the protein, an expression vector operably encoding the nerve growth factor, or grafting a donor cell containing such an expression vector into the substantia nigra and preferably also the striatum. The method stimulates growth of targeted neurons, and reversal of functional deficits associated with the neurodegenerative disease being treated.
Owner:CEREGENE
Methods for therapy of neurodegenerative disease of the brain
InactiveUS20040141953A1Reduce adverse effectsAvoid restrictionsBiocideVirusesCholinergic cellsMammal
A specific clinical protocol for use toward therapy of defective, diseased and damaged cholinergic neurons in the mammalian brain, of particular usefulness for treatment of neurodegenerative conditions such as Alzheimer's disease. The protocol is practiced by delivering a definite concentration of recombinant neurotrophin into, or within close proximity of, identified defective, diseased or damaged brain cells. Using a viral vector, the concentration of neurotrophin delivered as part of a neurotrophic composition varies from 10<10 >to 10<15 >neurotrophin encoding viral particles / ml of composition fluid. Each delivery site receives from 2.5 mul to 25 mul of neurotrophic composition, delivered slowly, as in over a period of time ranging upwards of 10 minutes / delivery site. Each delivery site is at, or within 500 mum of, a targeted cell, and no more than about 10 mm from another delivery site. Stable in situ neurotrophin expression can be achieved for 12 months, or longer.
Owner:RGT UNIV OF CALIFORNIA
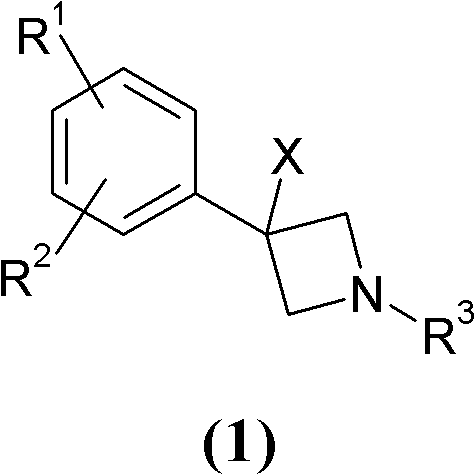
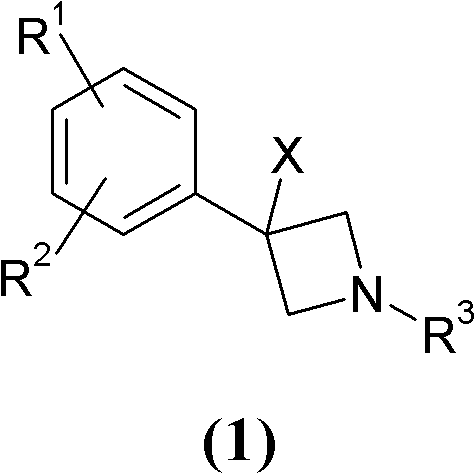
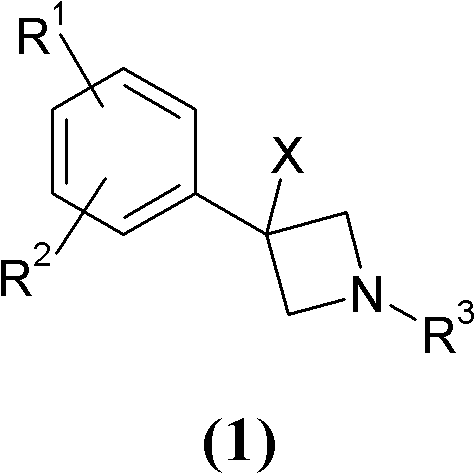
Novel 3-phenyl-azetidine derivatives useful as modulators of cortical catecholaminergic neurotransmission
InactiveCN102224135AOrganic active ingredientsNervous disorderNeurological impairmentMammalian brain
The present invention relates to novel 3-phenyl-azetidine derivatives, useful for modulating extracellular levels of catecholamines, dopamine and norepinephrine, in cerebral cortical areas of the mammalian brain, and more specifically for the treatment of central nervous system disorders. In other aspects the invention relates to pharmaceutical compositions comprising the 3-phenyl-azetidine derivatives of the invention and to the use of these compounds for therapeutic applications.
Owner:NSAB FILIAL AF NEUROSEARCH SVERIGE
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com