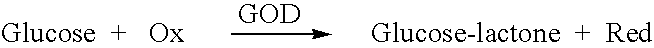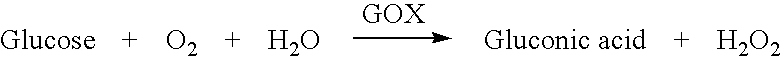Patents
Literature
315 results about "Interstitial fluid" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Interstitial fluid is a solution that bathes and surrounds the cells of multicellular animals. It is the main component of the extracellular fluid, which also includes plasma and transcellular fluid. The interstitial fluid is found in the interstitial spaces, also known as the tissue spaces. On average, a person has about 10 litres of interstitial fluid, providing the cells of the body with nutrients and a means of waste removal.
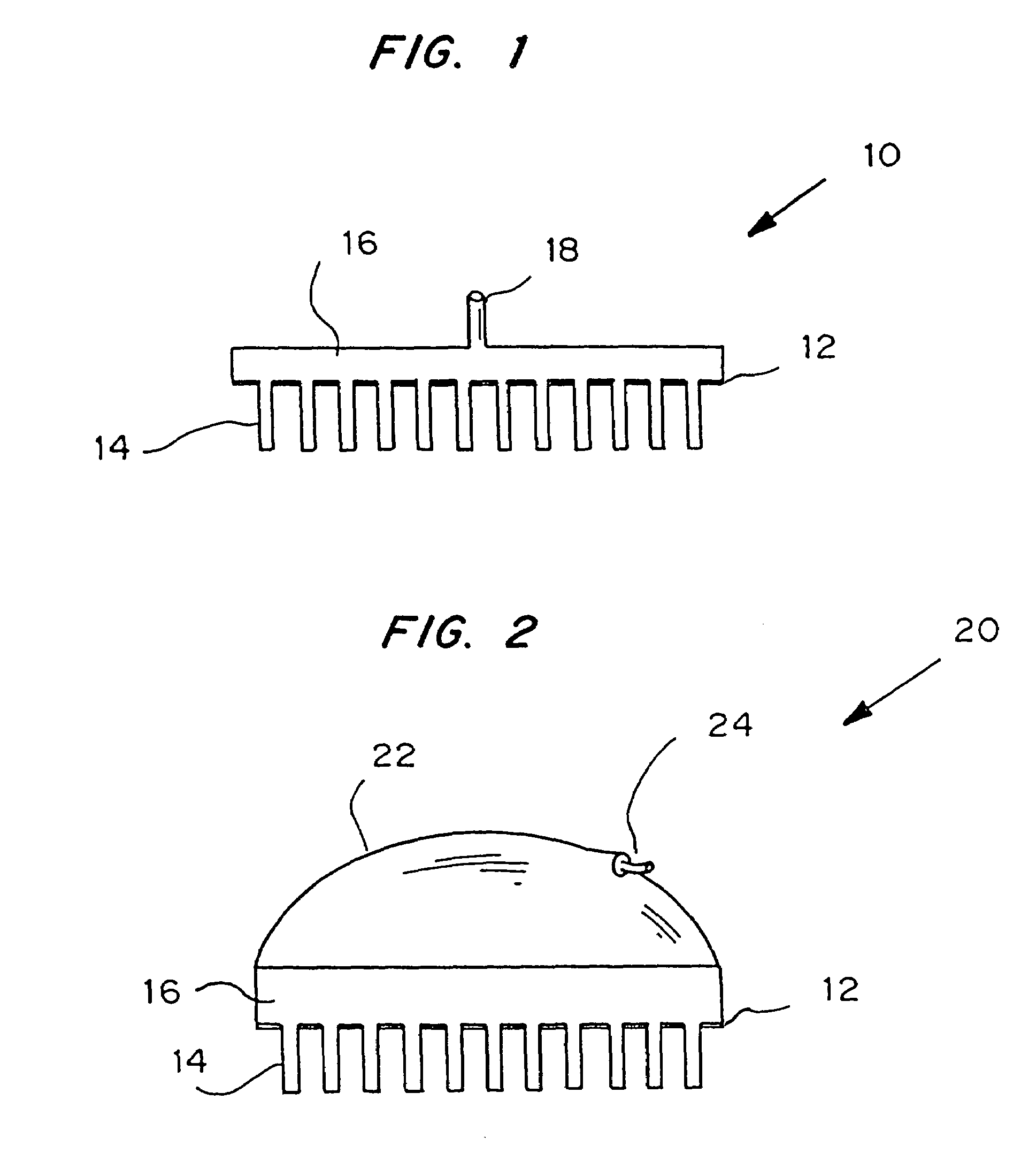
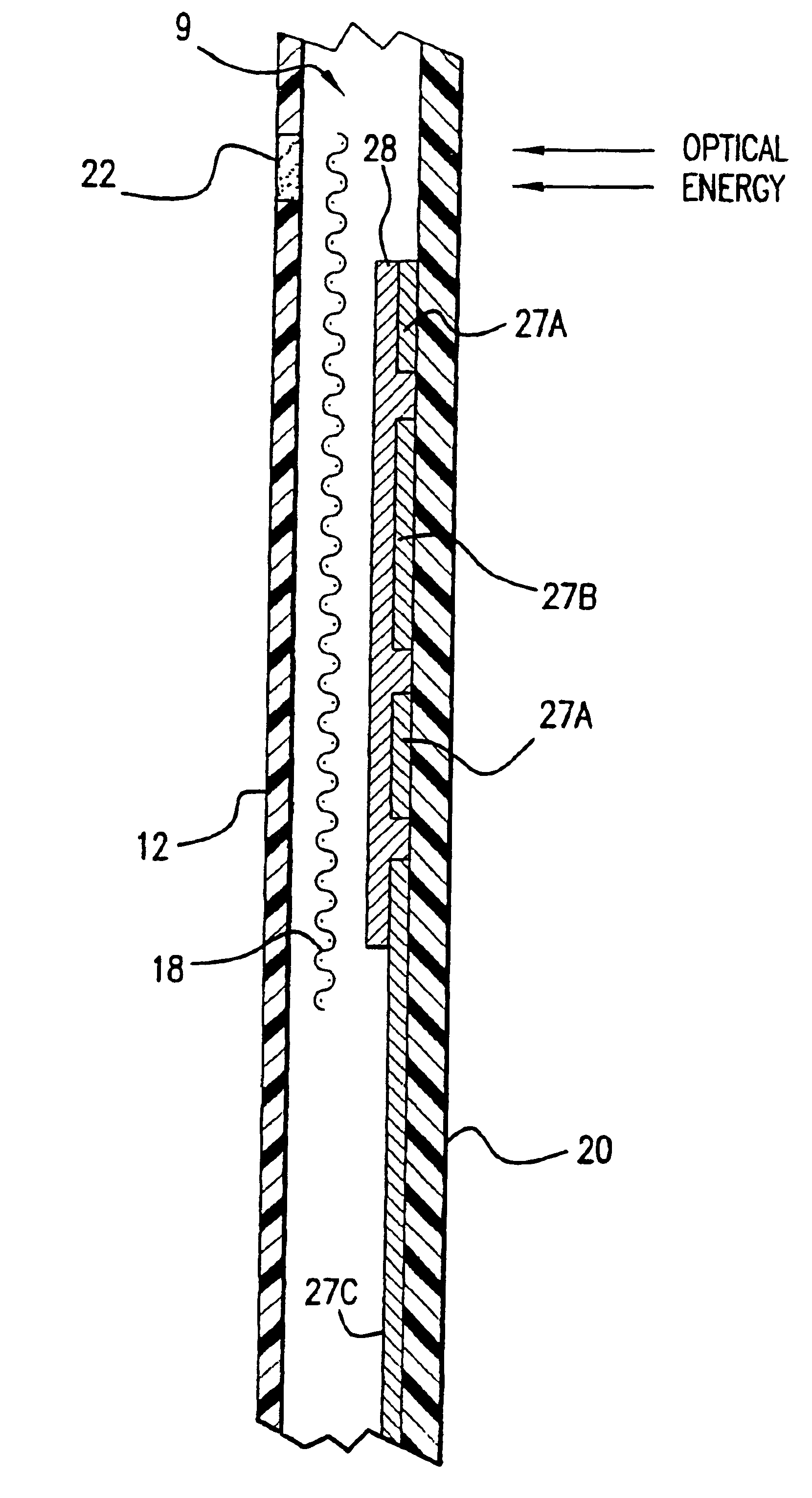
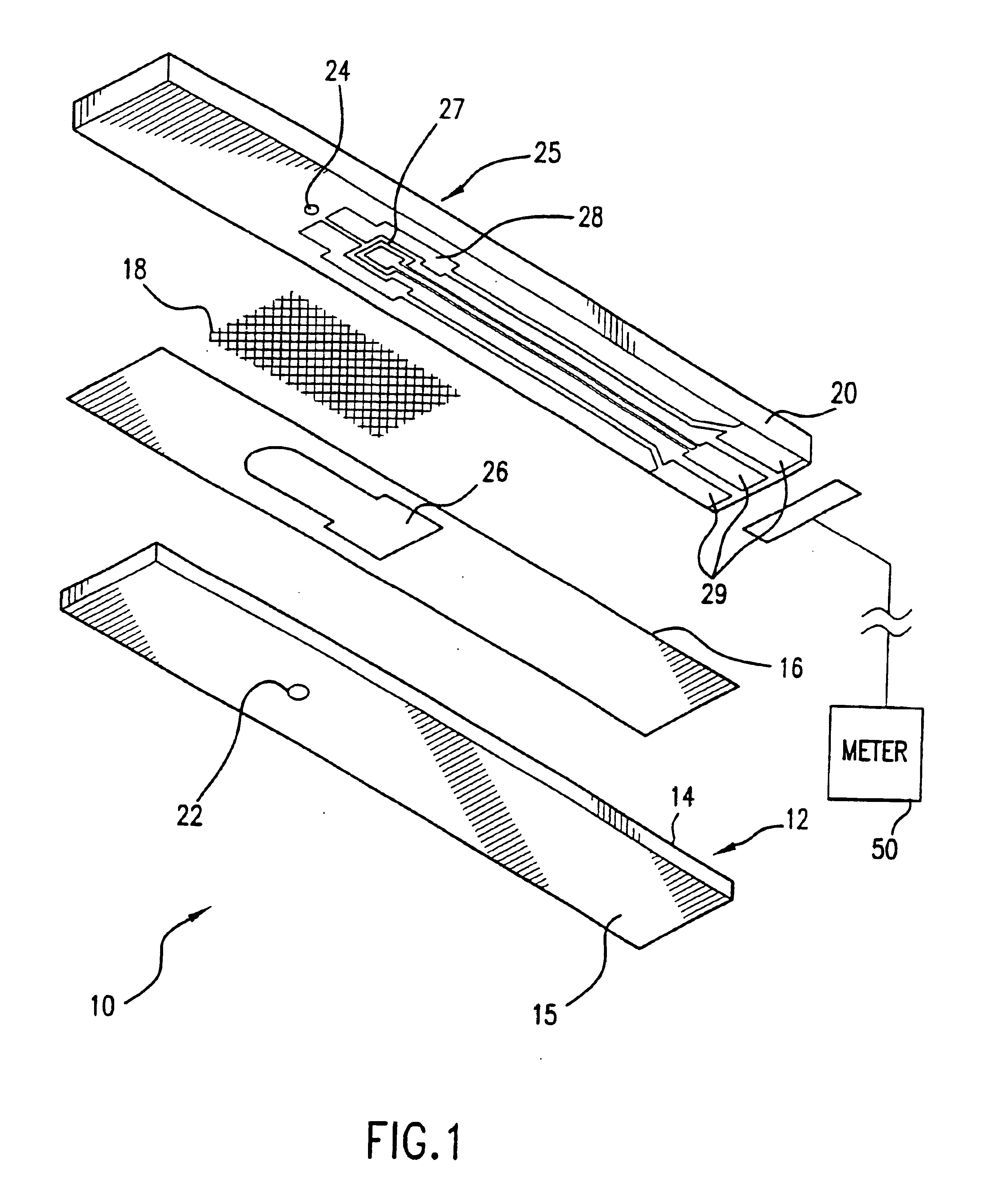
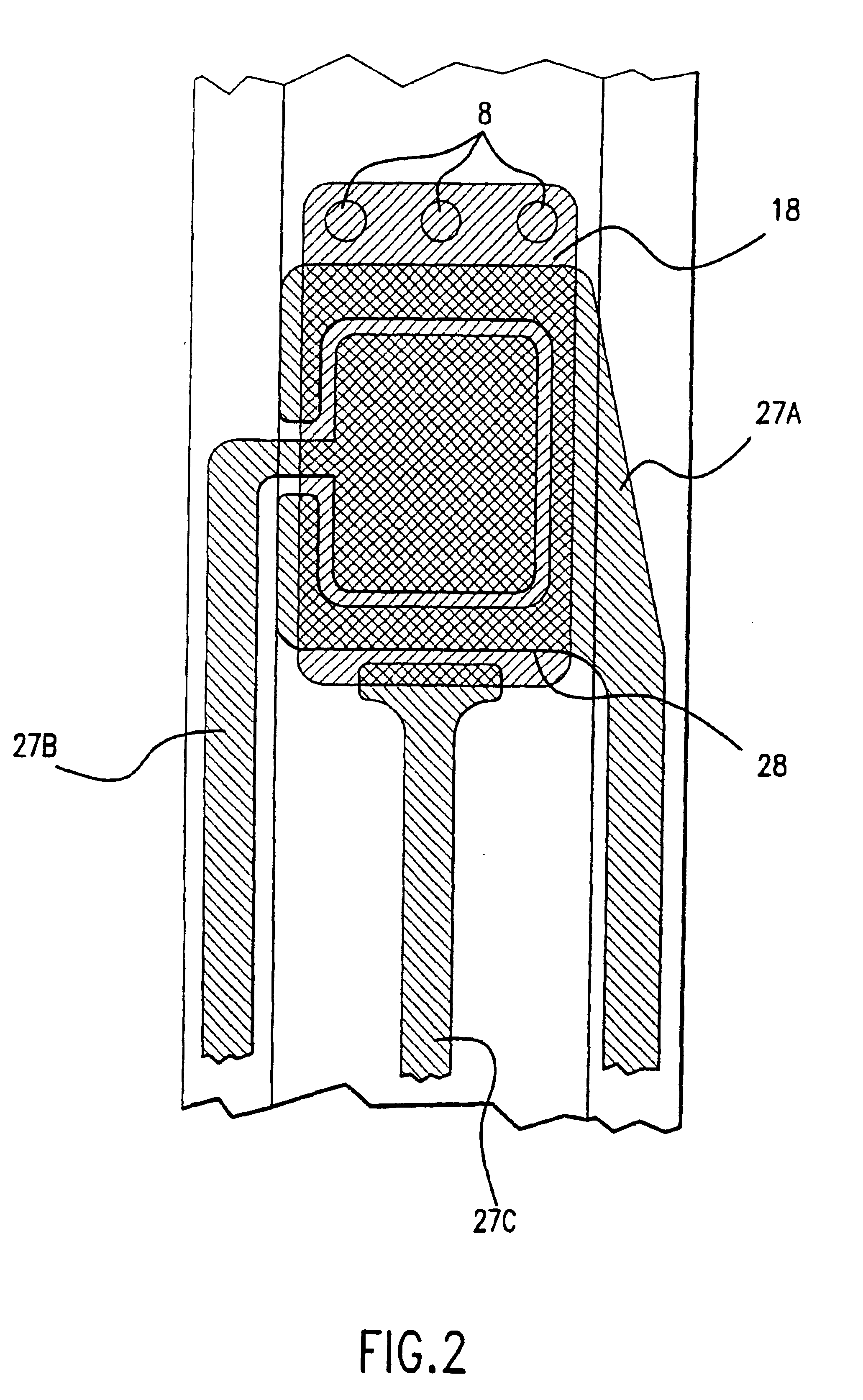
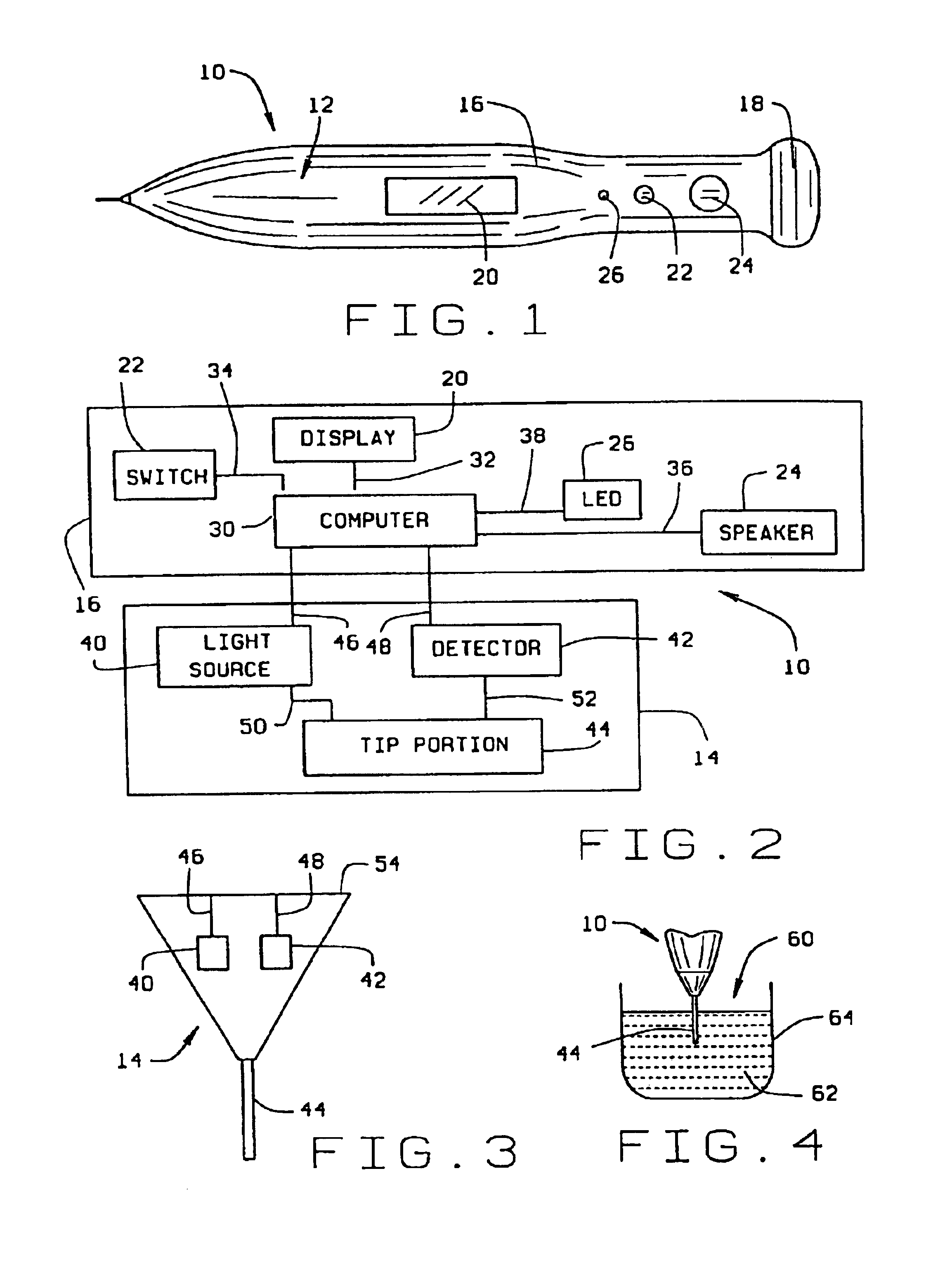
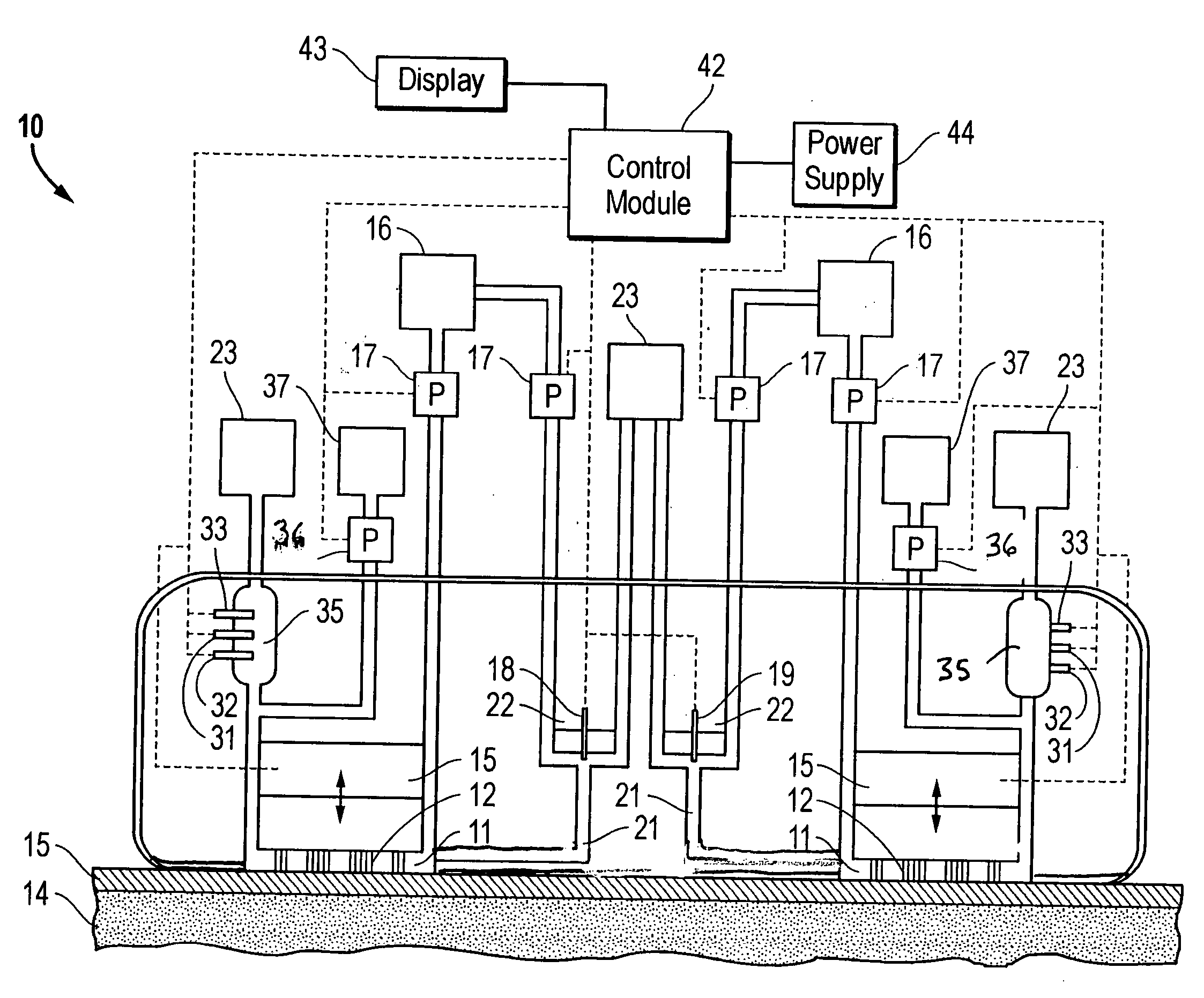
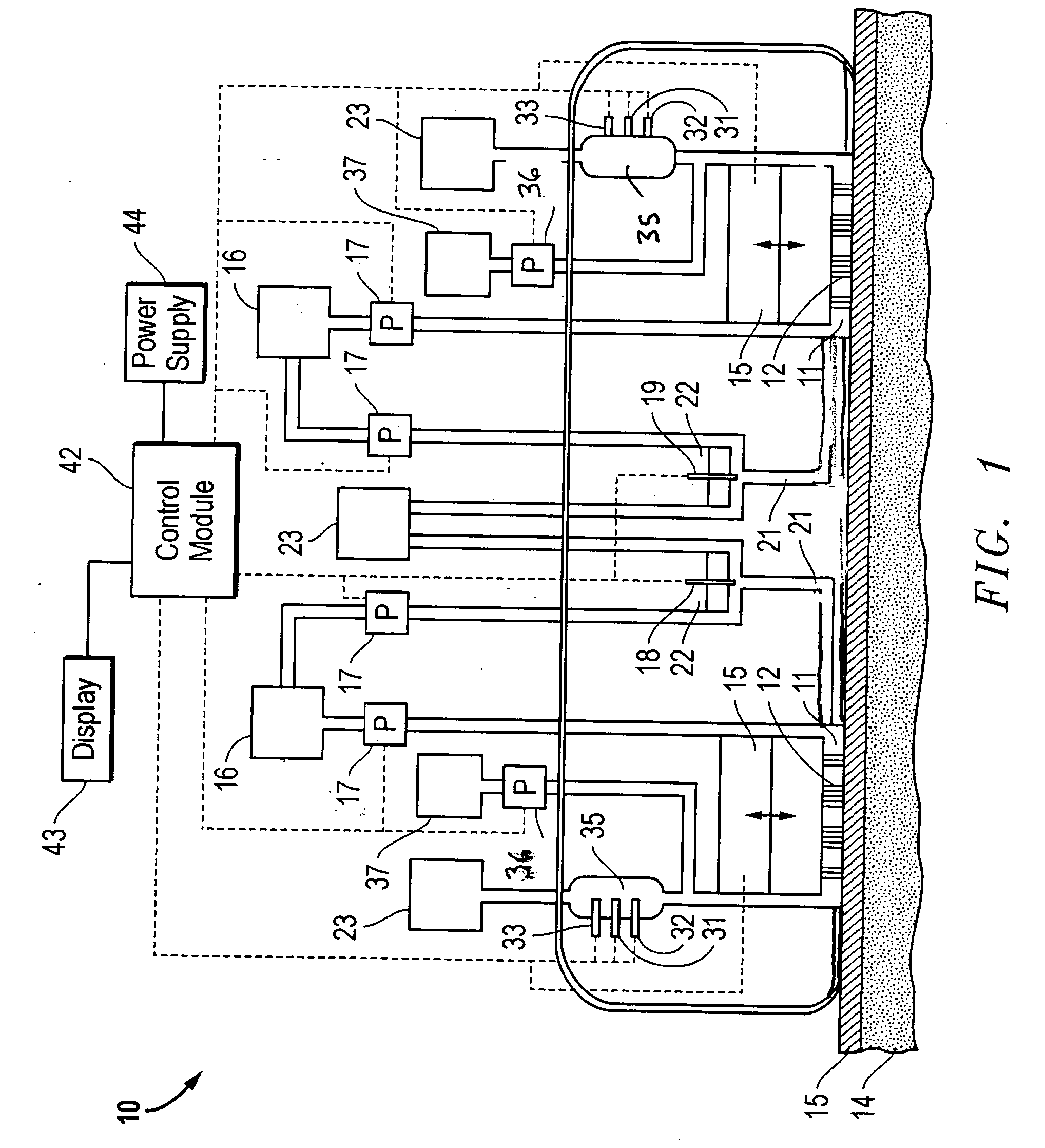
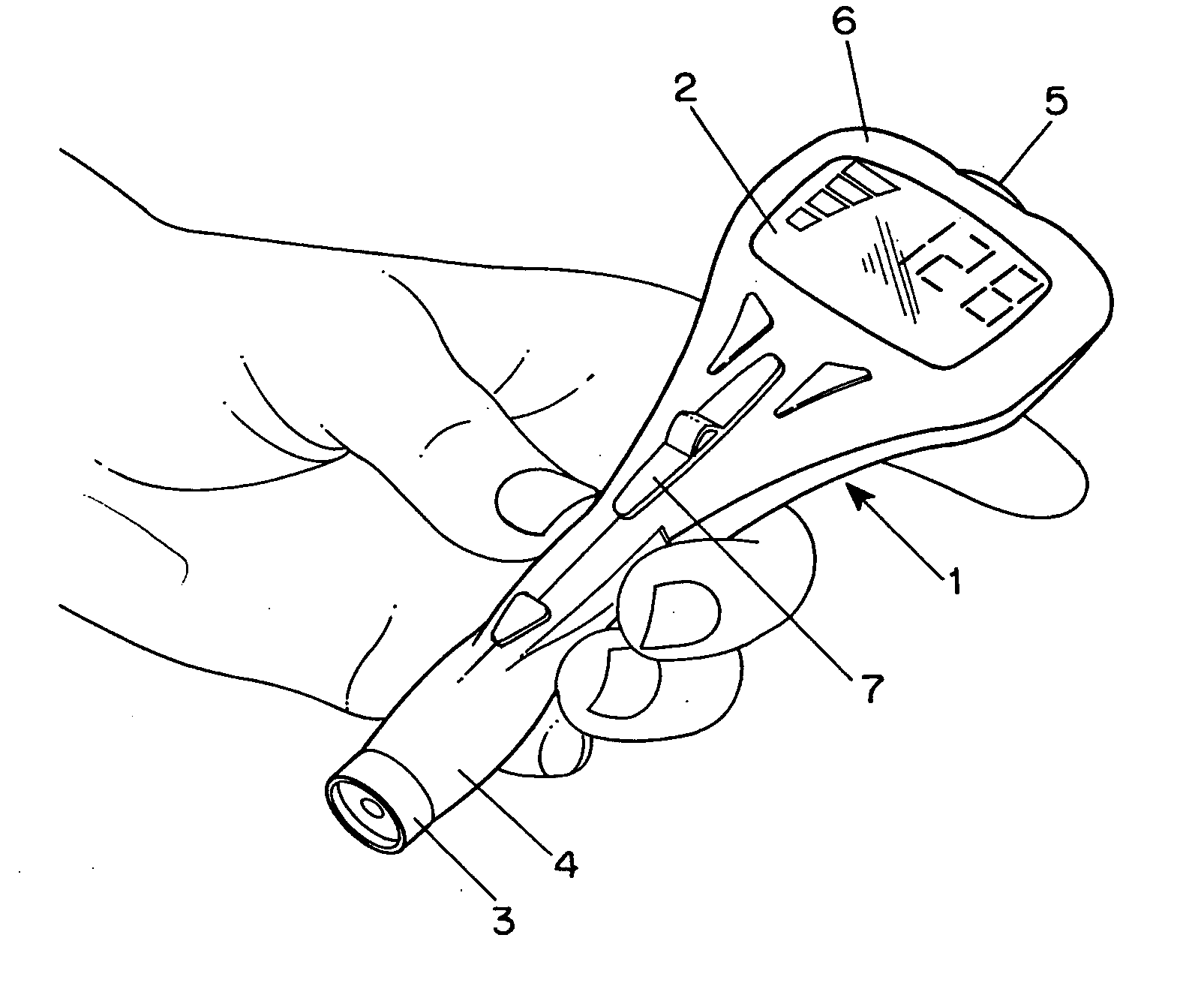
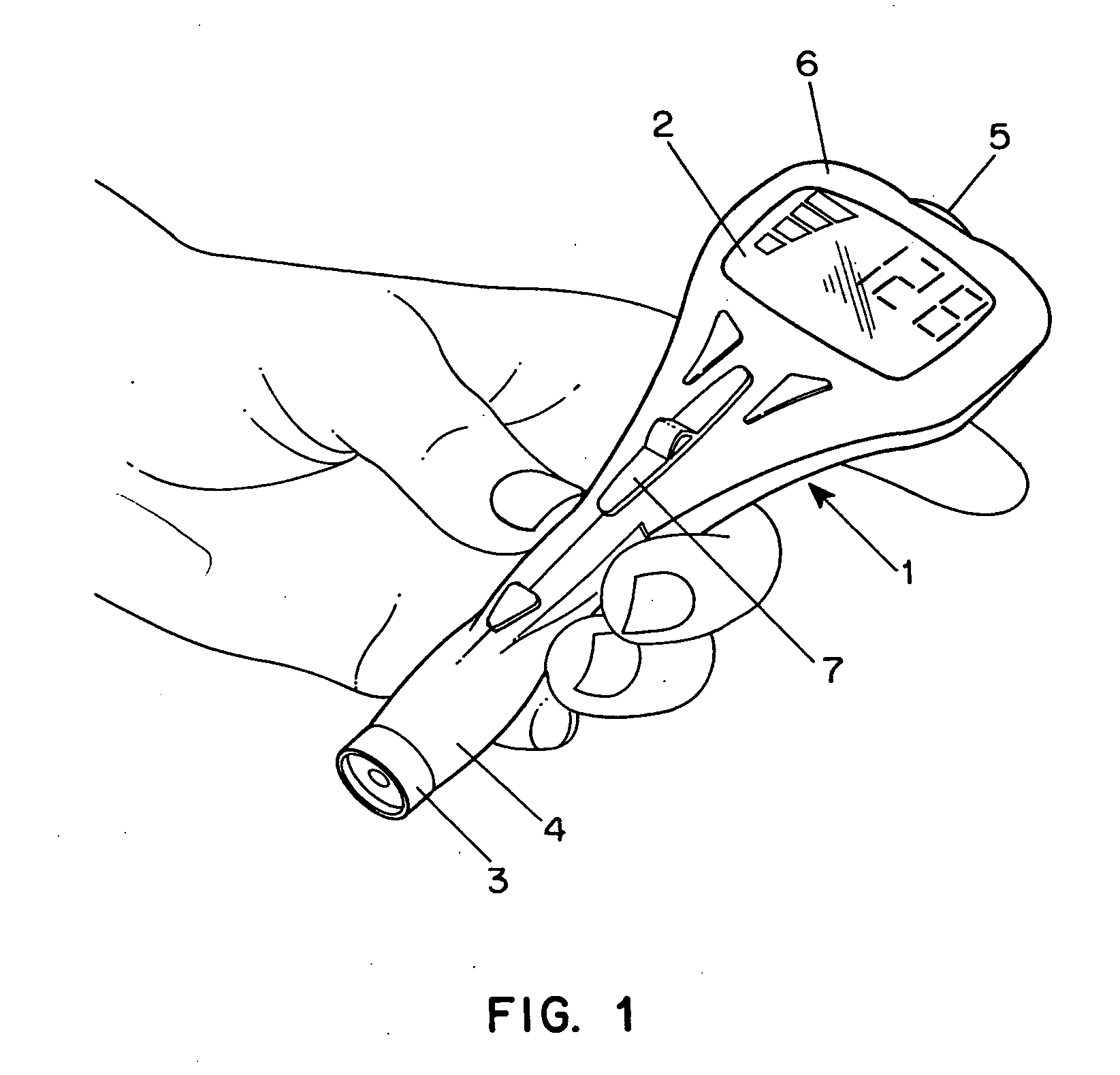
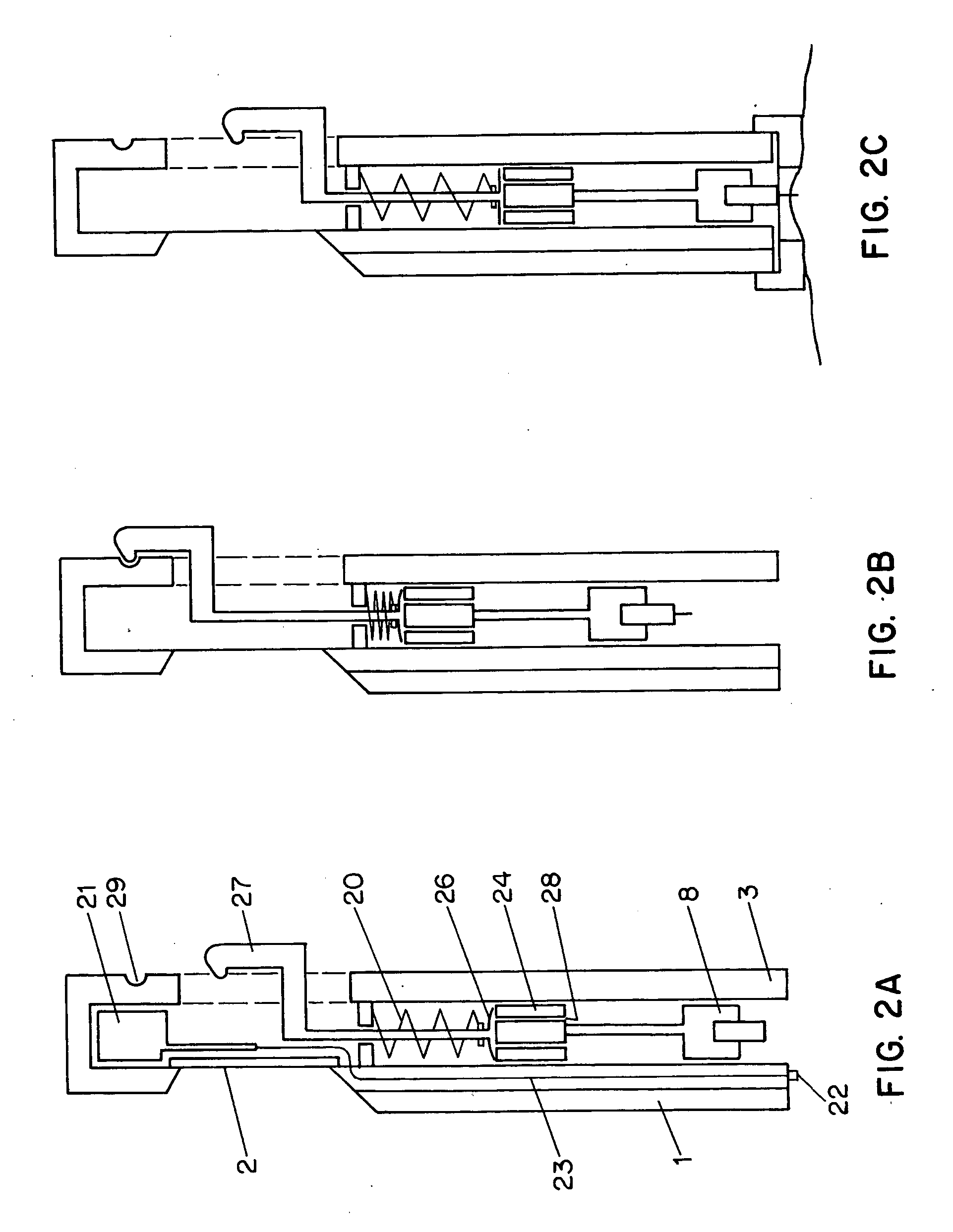
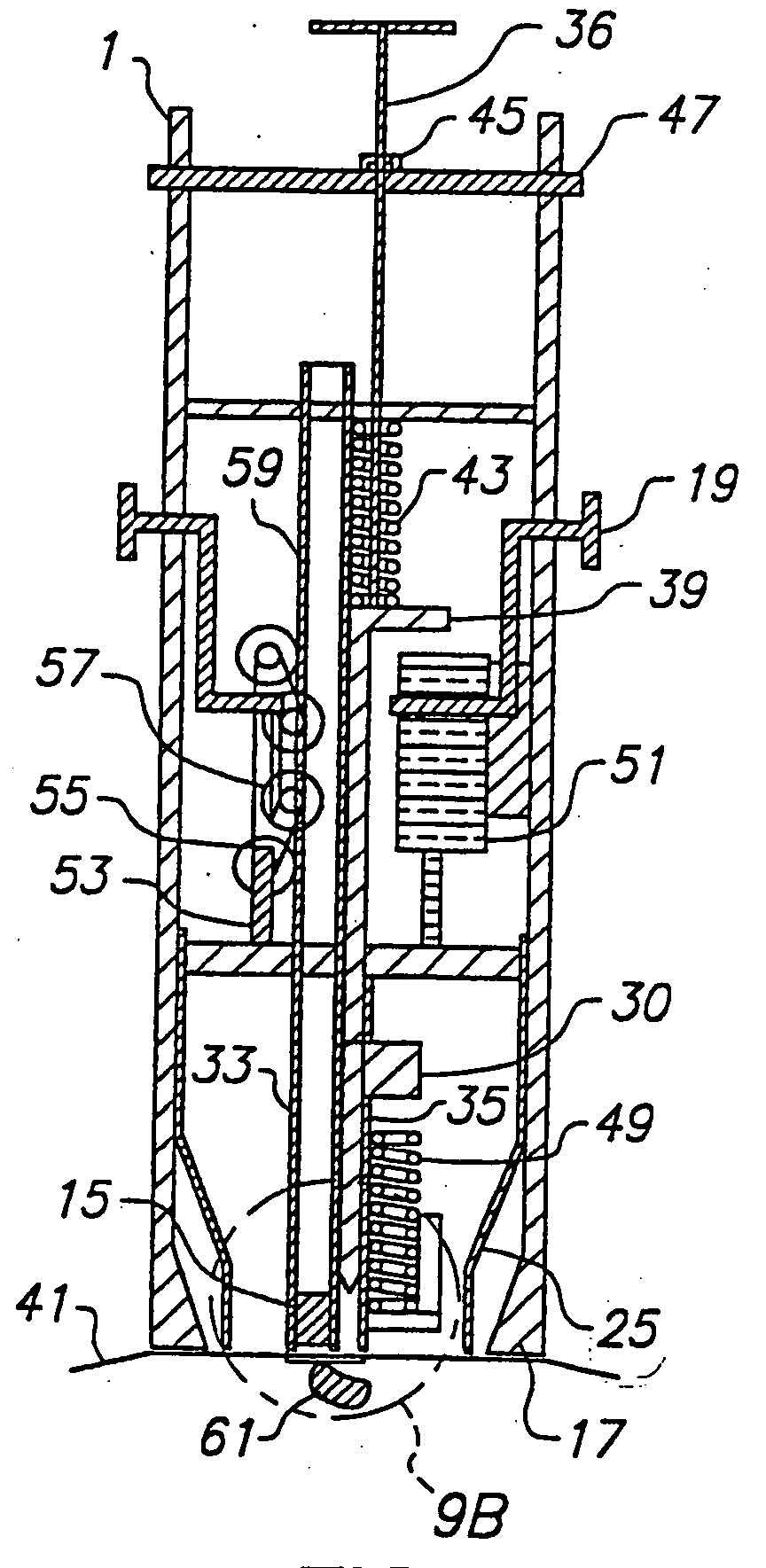
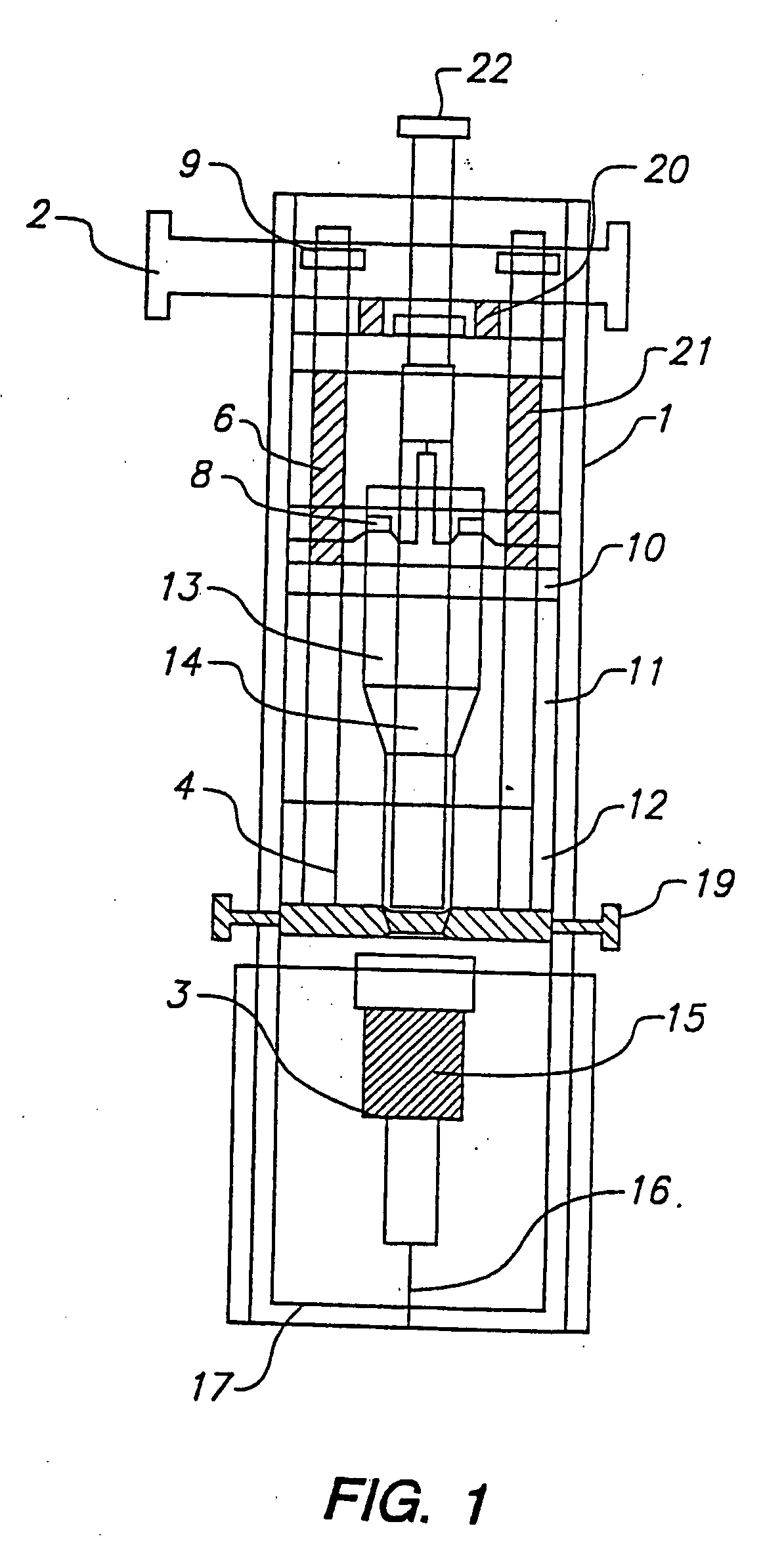
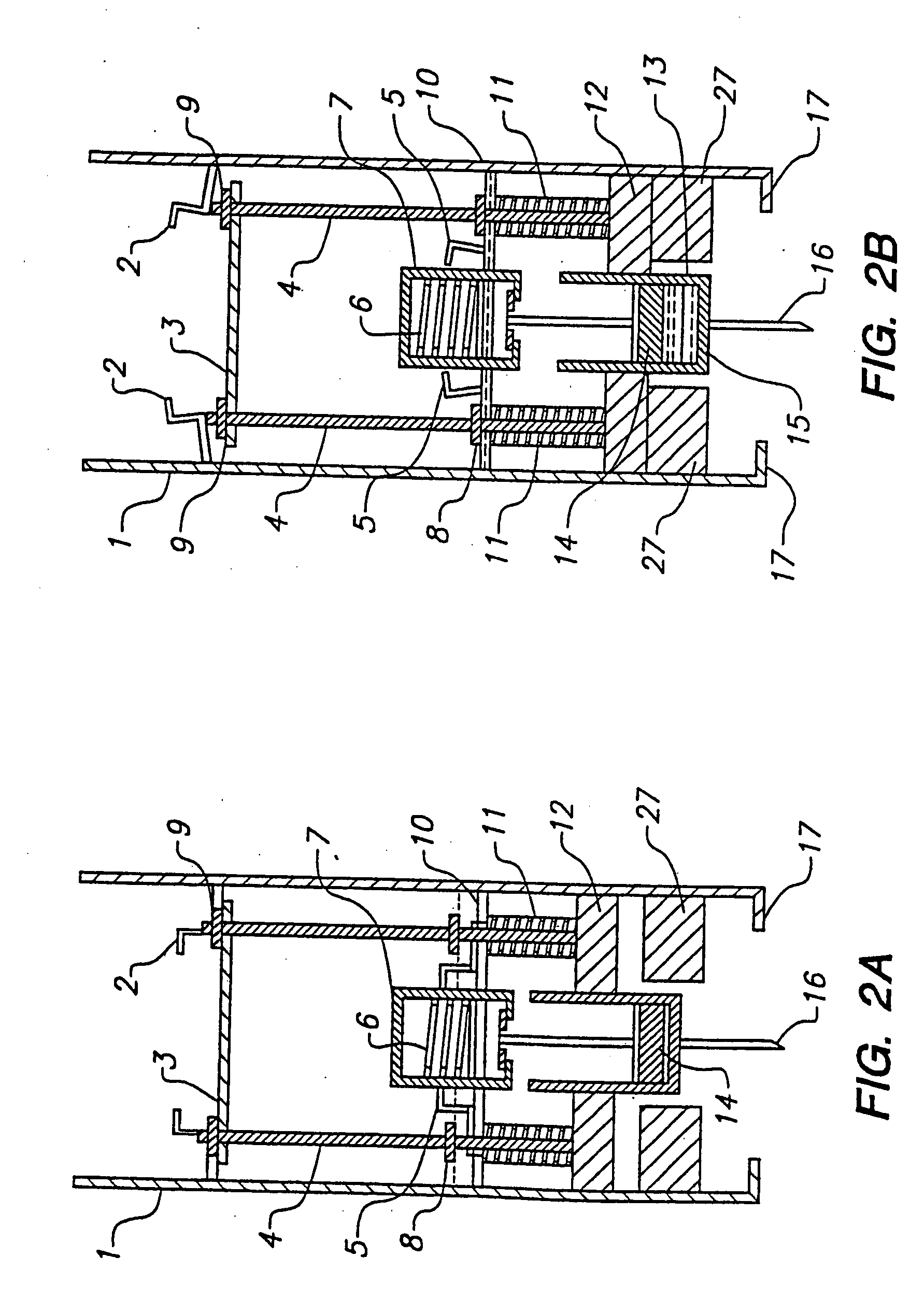
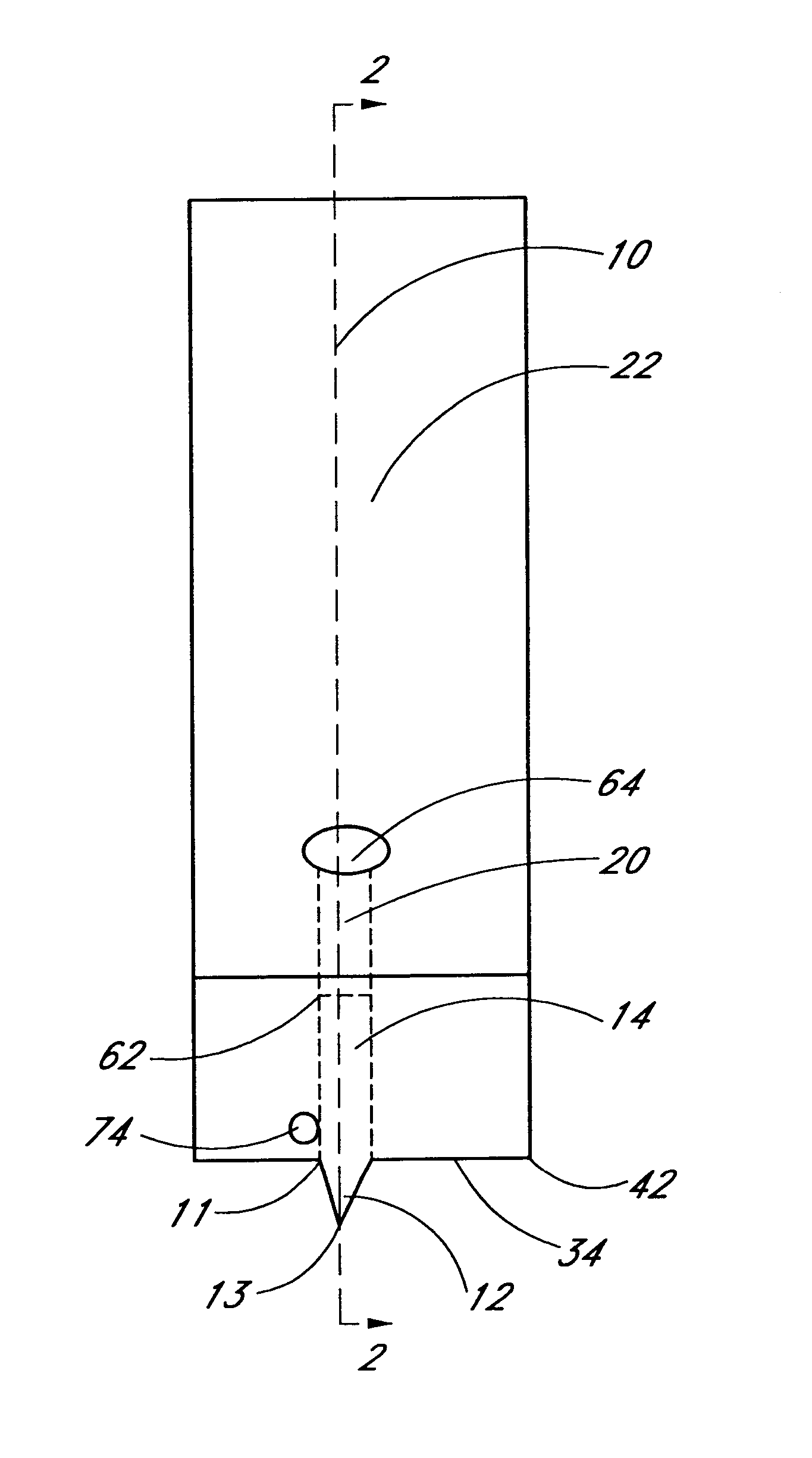
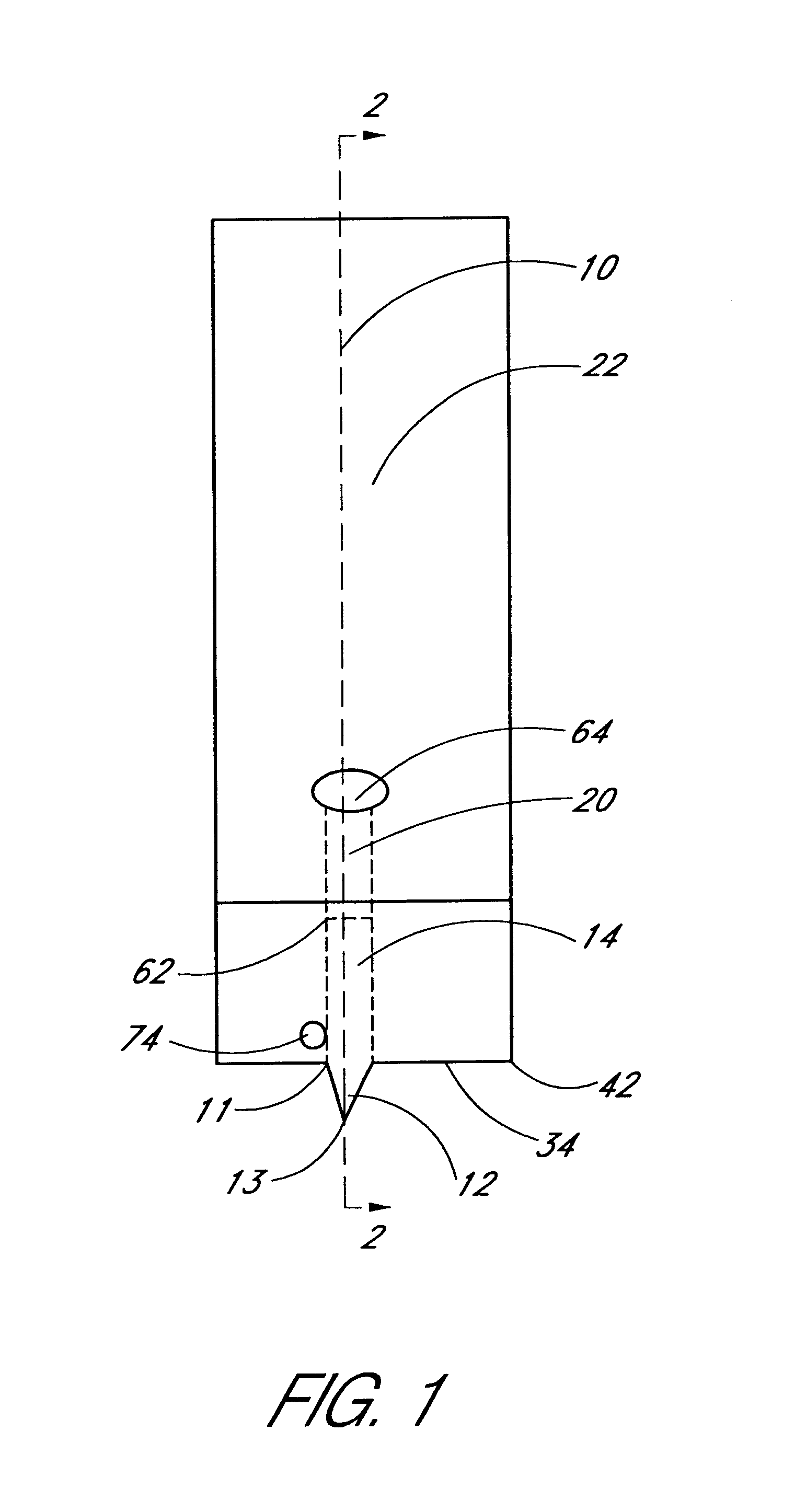
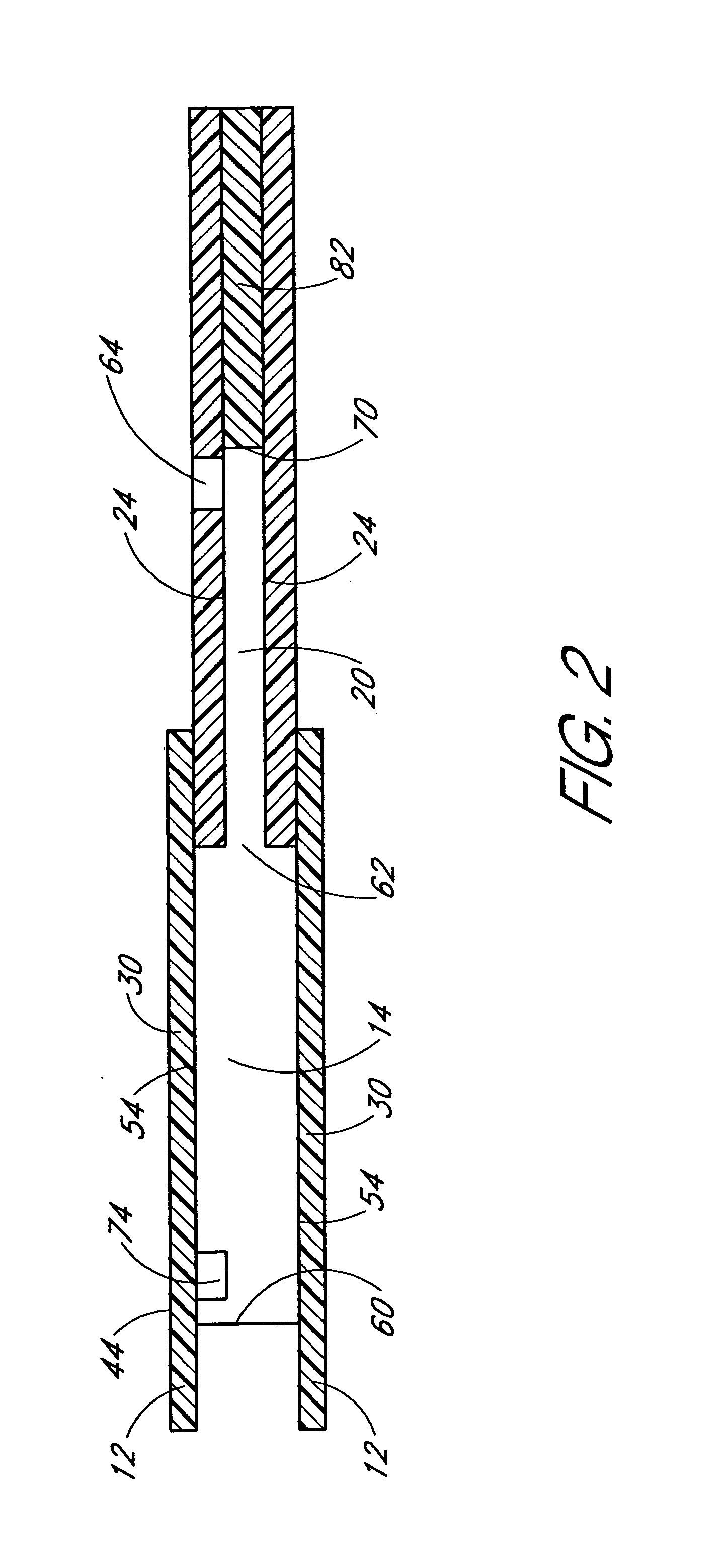
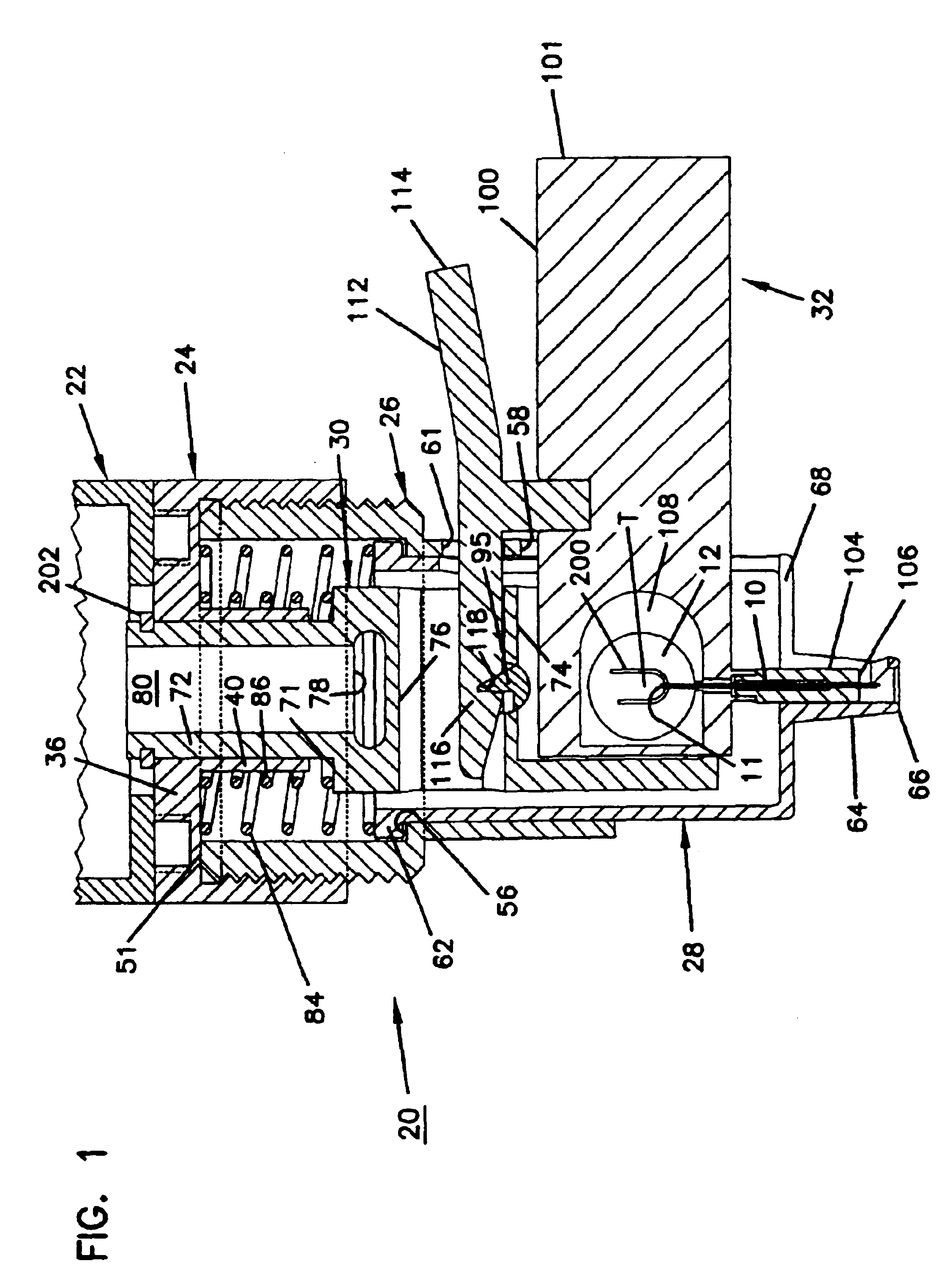
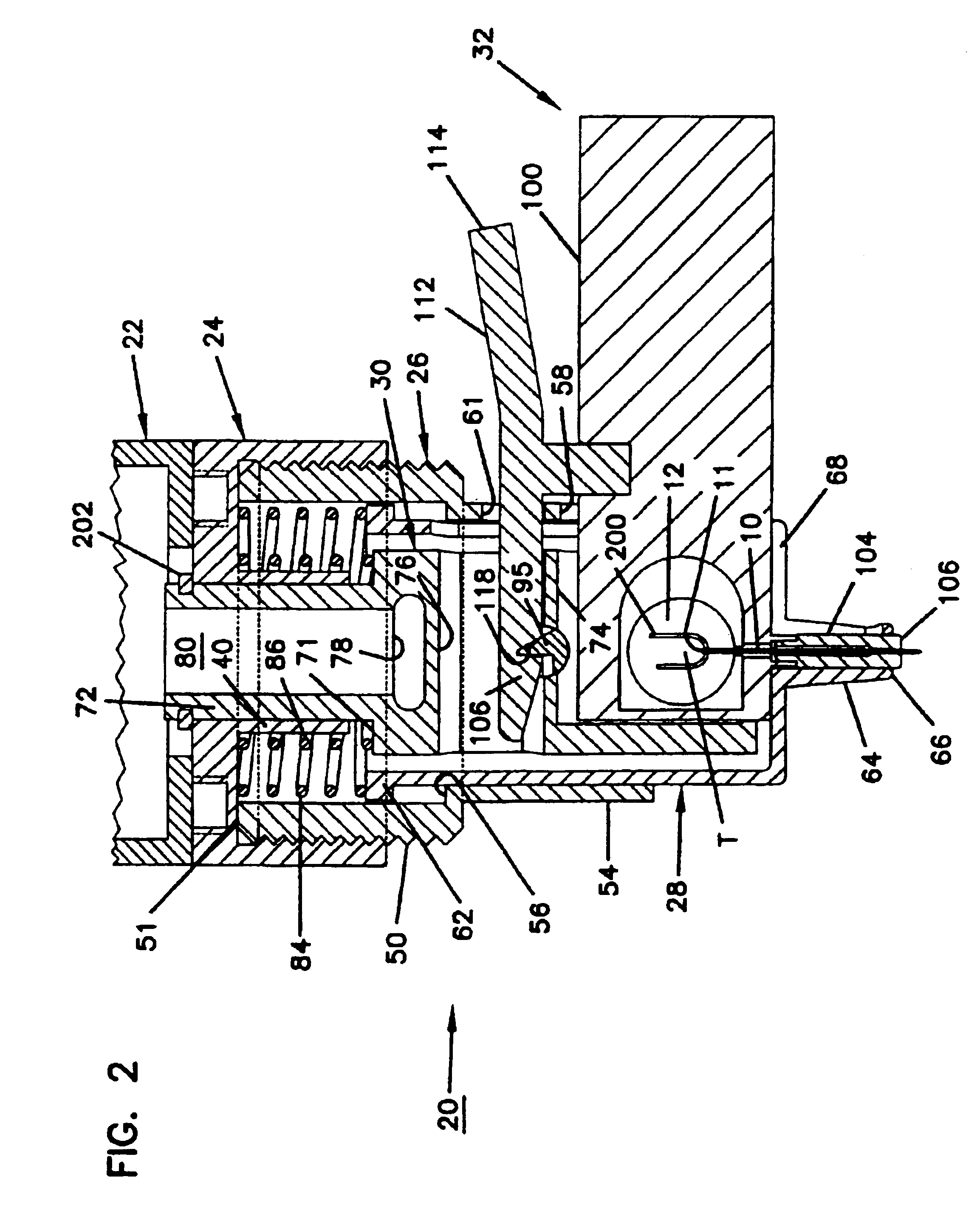
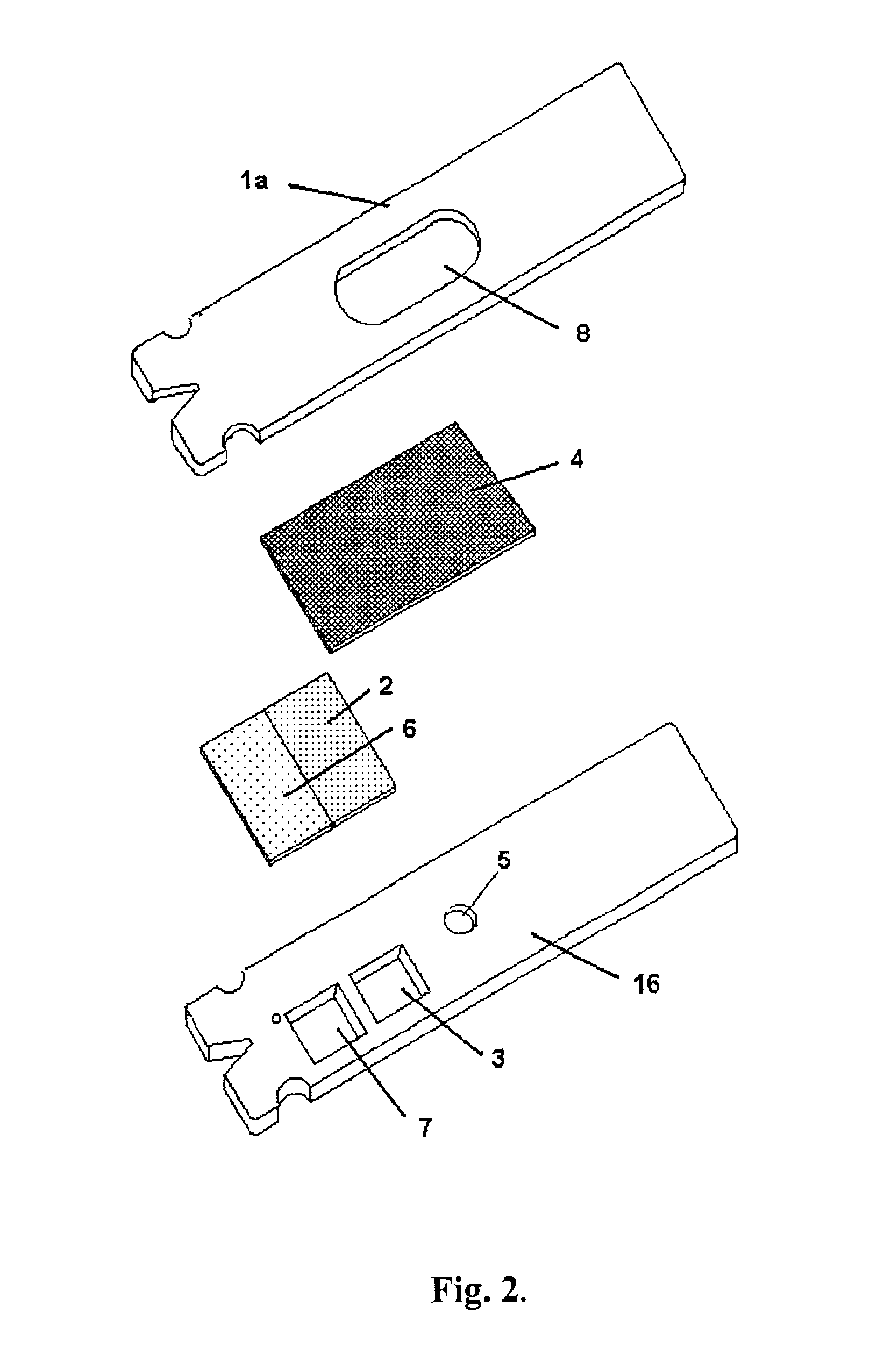
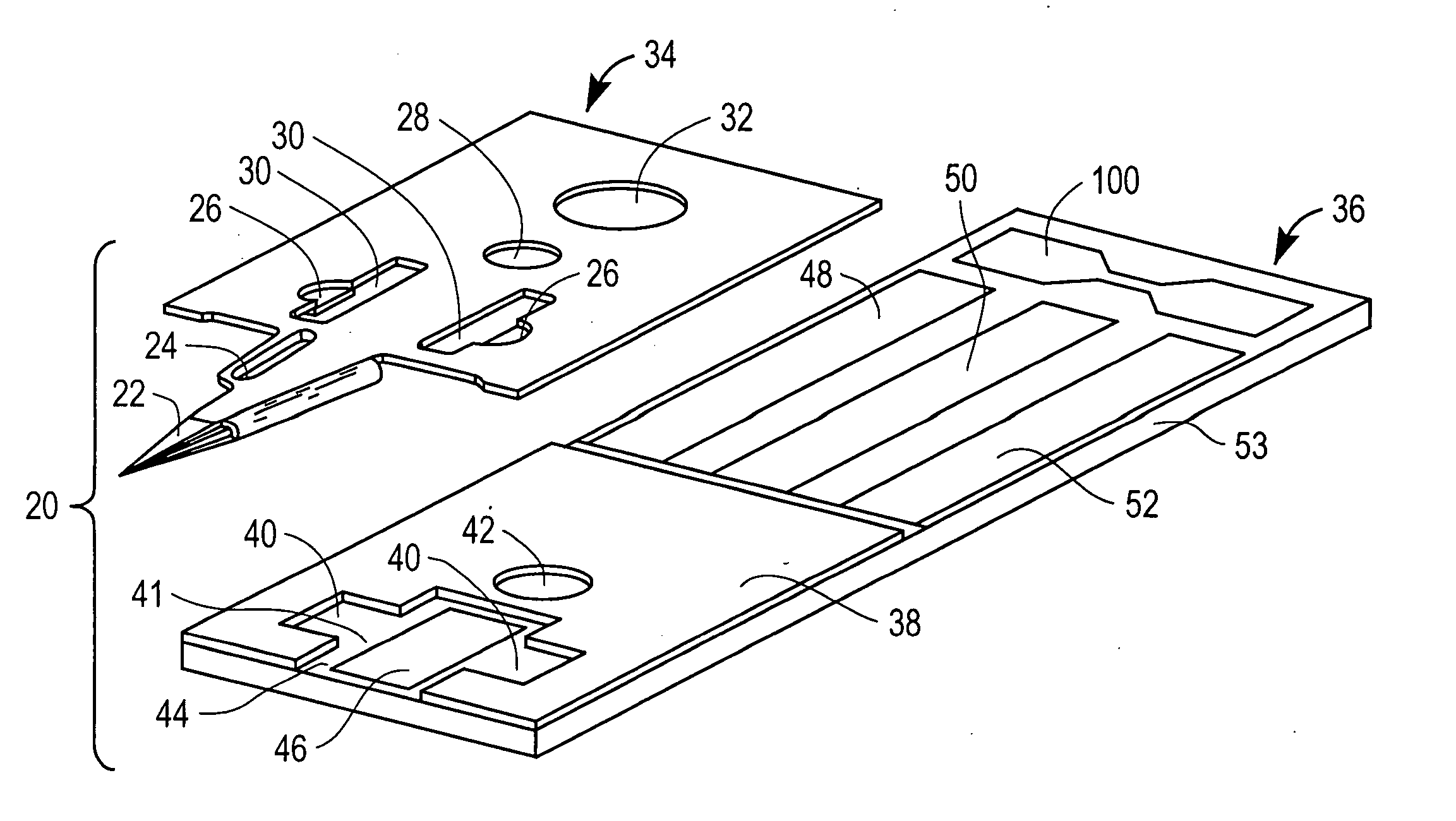
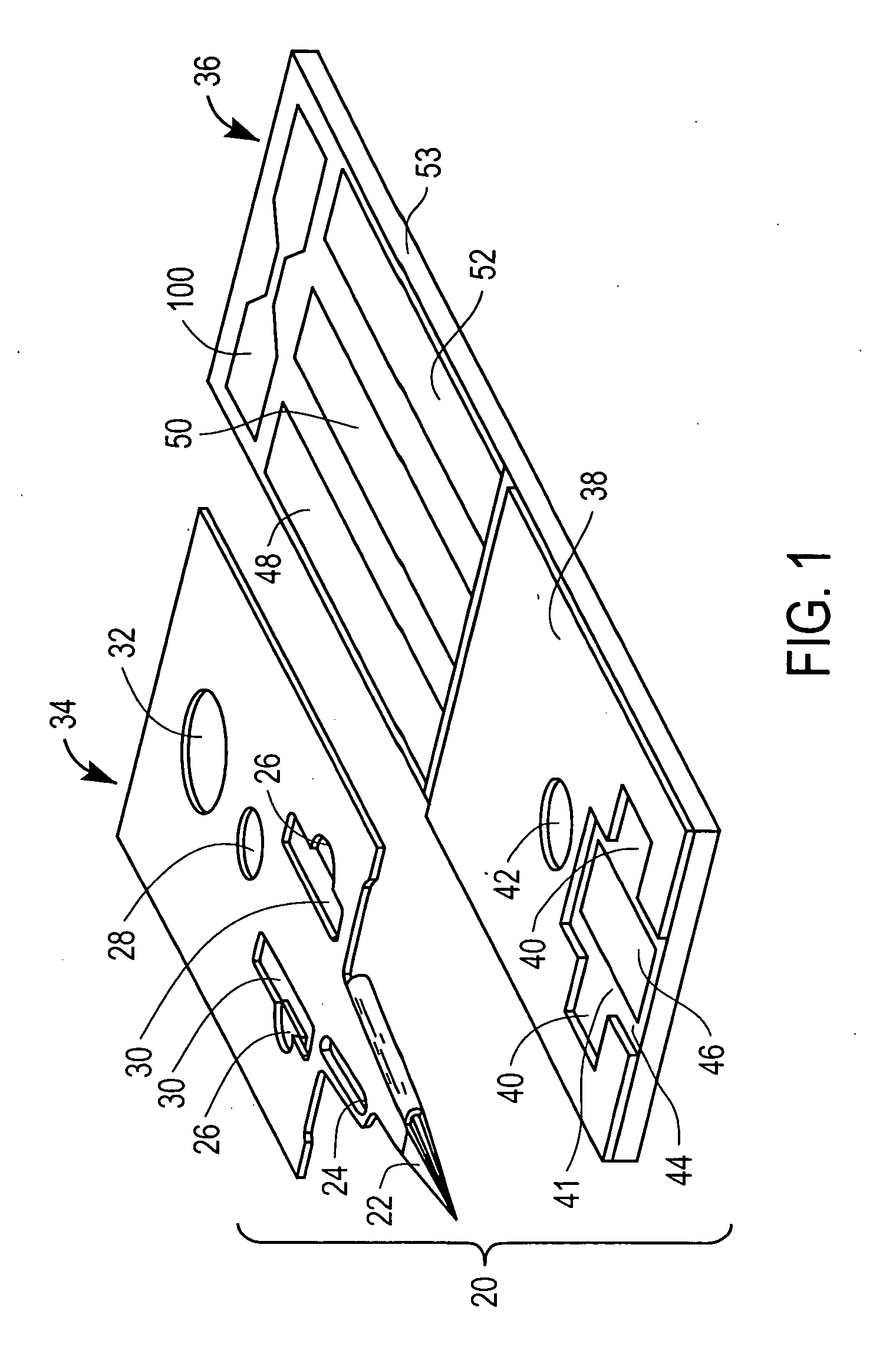
Combined lancet and electrochemical analyte-testing apparatus
InactiveUS20020130042A1Easy to takeReduces and eliminates disposal issueImmobilised enzymesBioreactor/fermenter combinationsAnalyteDisplay device
An apparatus for detection and quantitation of an electrochemically-detect- able analyte, such as glucose, in blood or interstitial fluid includes a meter unit, a lancet and an electrochemical sensor. Of these components, the meter is preferably reusable, while the lancet and the electrochemical sensor are preferably incorporated in assemblies intended for single-use. The meter unit has a housing, within which a lancet is engaged with a mechanism for moving then lancet; a connector disposed within the housing for engaging an electrochemical sensor specific for the analyte and transmitting a signal indicative of the amount of analyte, and a display operatively-associated with a connector for displaying the amount of the analyte to user. The electrochemical sensor is adapted for detection of a particular analyte. In addition, the electrochemical sensor has an absorptive member for uptake of a sample of blood or interstitial fluid. In one version, the lancet moves from a initial position to a piercing position in which skin of the user is pierced and optionally back to a retracted position. The electrochemical sensor is disposed such that the absorptive member takes up a sample from the pierced skin of the user when it is pierced by the lancet without movement of the apparatus. In an alternative version, the lancet is a hollow cannula through which blood or interstitial fluid is transported from the puncture site to an absorbent portion of the electrochemical sensor. In either version, the apparatus provides single-step operation in which sample acquisition and analysis occur as a result of the single action of pressing the apparatus against the users skin.
Owner:LIFESCAN IP HLDG LLC
Microneedle device for extraction and sensing of bodily fluids
InactiveUS7344499B1Simple wayMinimal and no damageAdditive manufacturing apparatusMicroneedlesMetaboliteIrritation
Microneedle devices are provided for controlled sampling of biological fluids in a minimally-invasive, painless, and convenient manner. The microneedle devices permit in vivo sensing or withdrawal of biological fluids from the body, particularly from or through the skin or other tissue barriers, with minimal or no damage, pain, or irritation to the tissue. The microneedle device includes one or more microneedles, preferably in a three-dimensional array, a substrate to which the microneedles are connected, and at least one collection chamber and / or sensor in communication with the microneedles. Preferred embodiments further include a means for inducing biological fluid to be drawn through the microneedles and into the collection chamber for analysis. In a preferred embodiment, this induction is accomplished by use of a pressure gradient, which can be created for example by selectively increasing the interior volume of the collection chamber, which includes an elastic or movable portion engaged to a rigid base. Preferred biological fluids for withdrawal and / or sensing include blood, lymph, interstitial fluid, and intracellular fluid. Examples of analytes in the biological fluid to be measured include glucose, cholesterol, bilirubin, creatine, metabolic enzymes, hemoglobin, heparin, clotting factors, uric acid, carcinoembryonic antigen or other tumor antigens, reproductive hormones, oxygen, pH, alcohol, tobacco metabolites, and illegal drugs.
Owner:GEORGIA TECH RES CORP +1
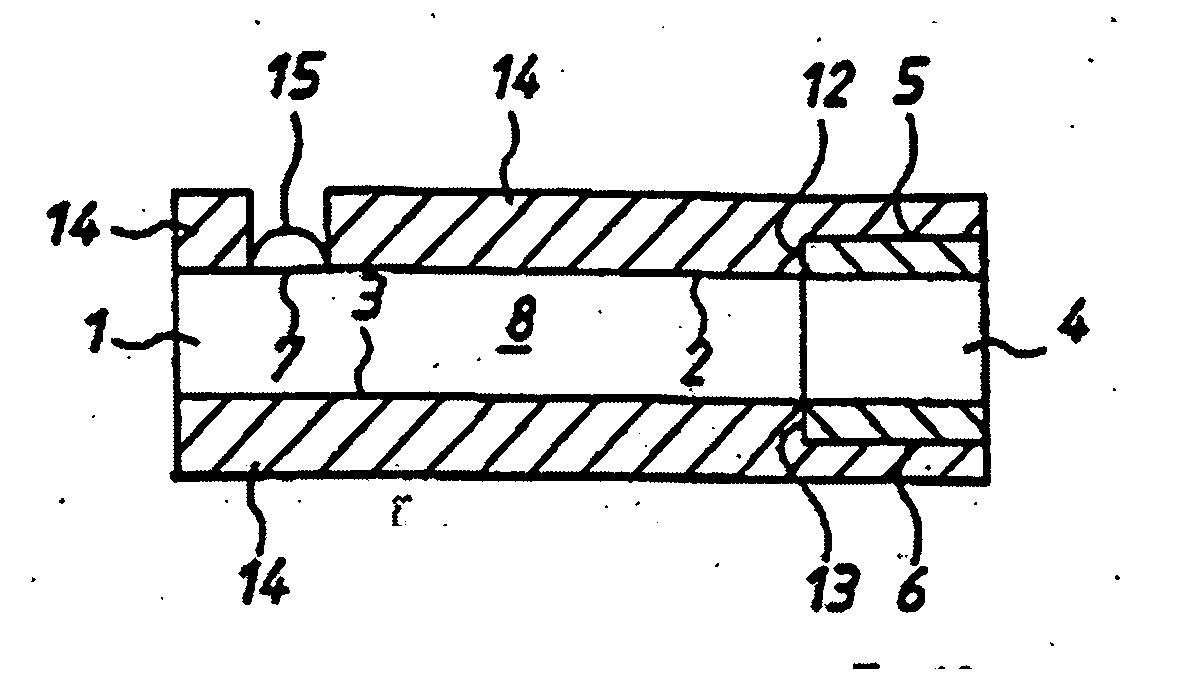
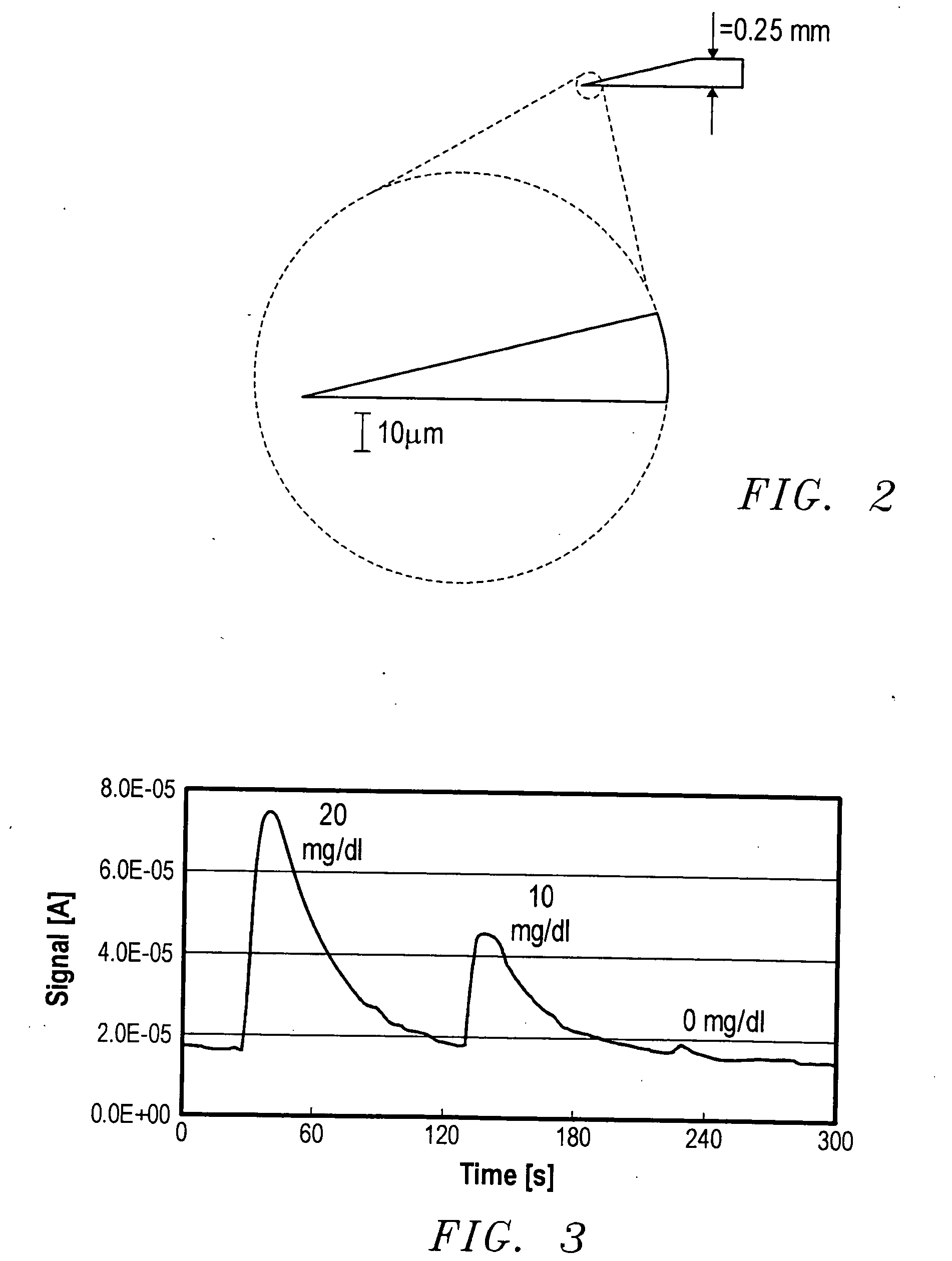
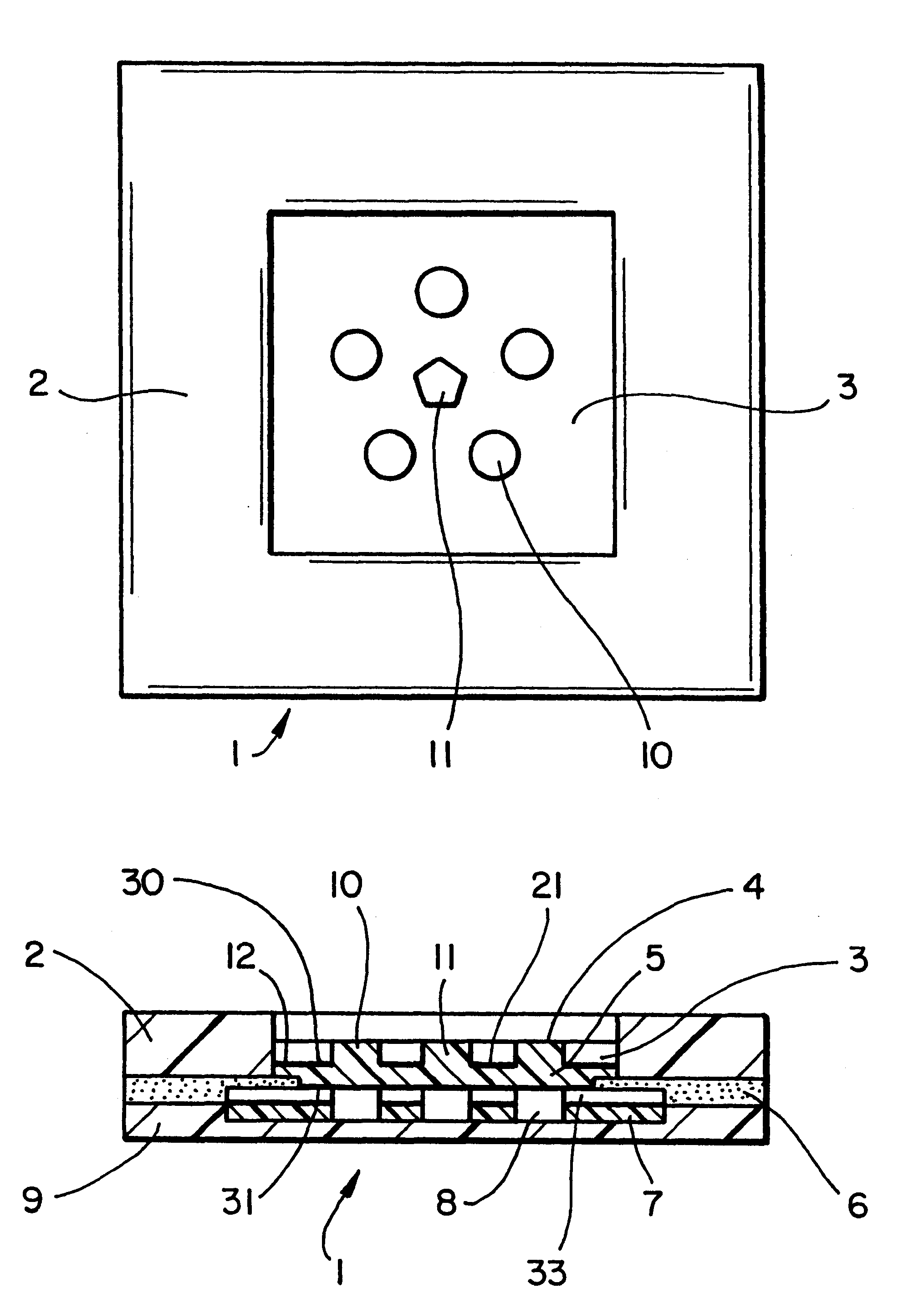
Analyte measurement
InactiveUS20040096959A1Low viscosityMore suitedBioreactor/fermenter combinationsBiological substance pretreatmentsElectrochemical detectorAnalyte
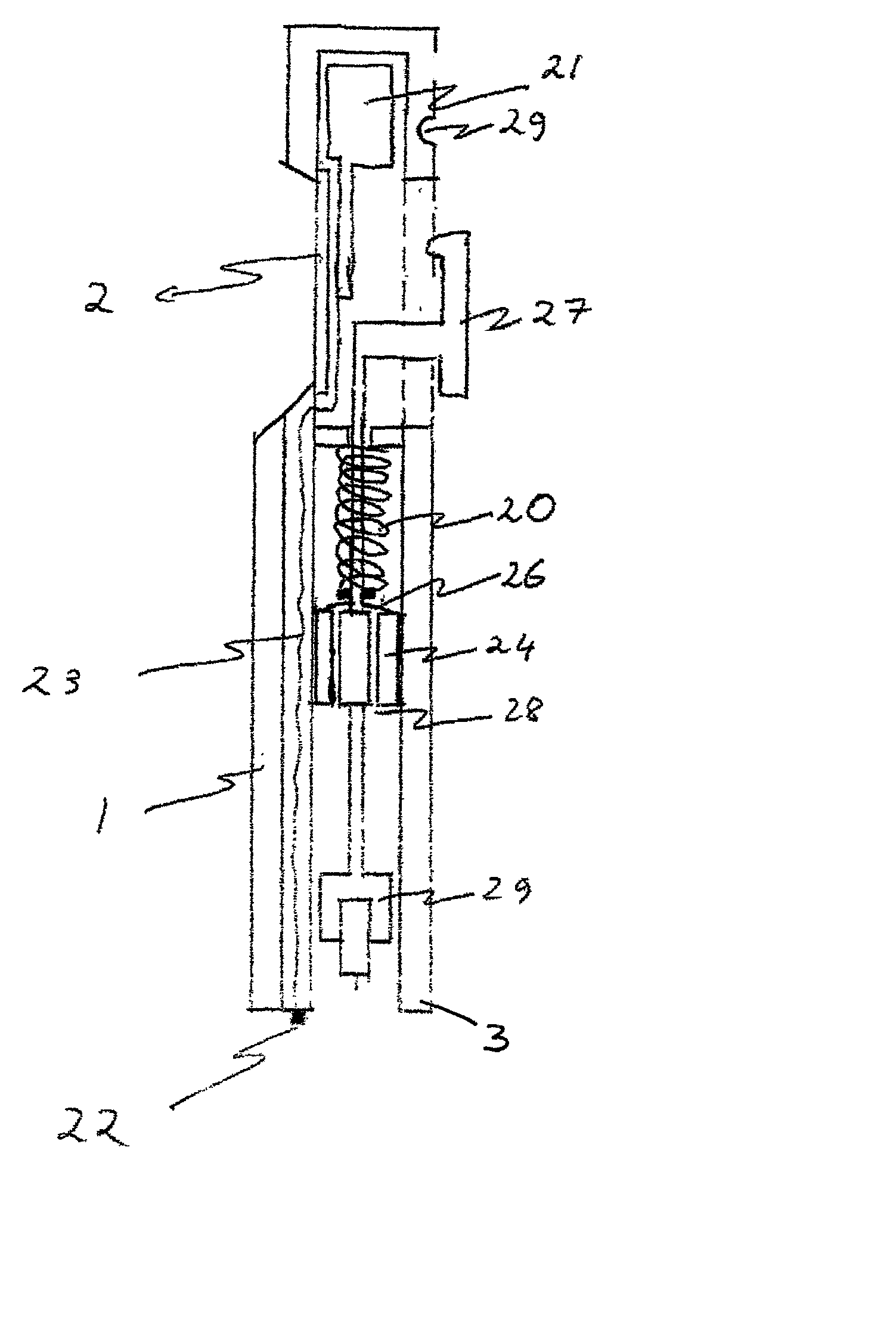
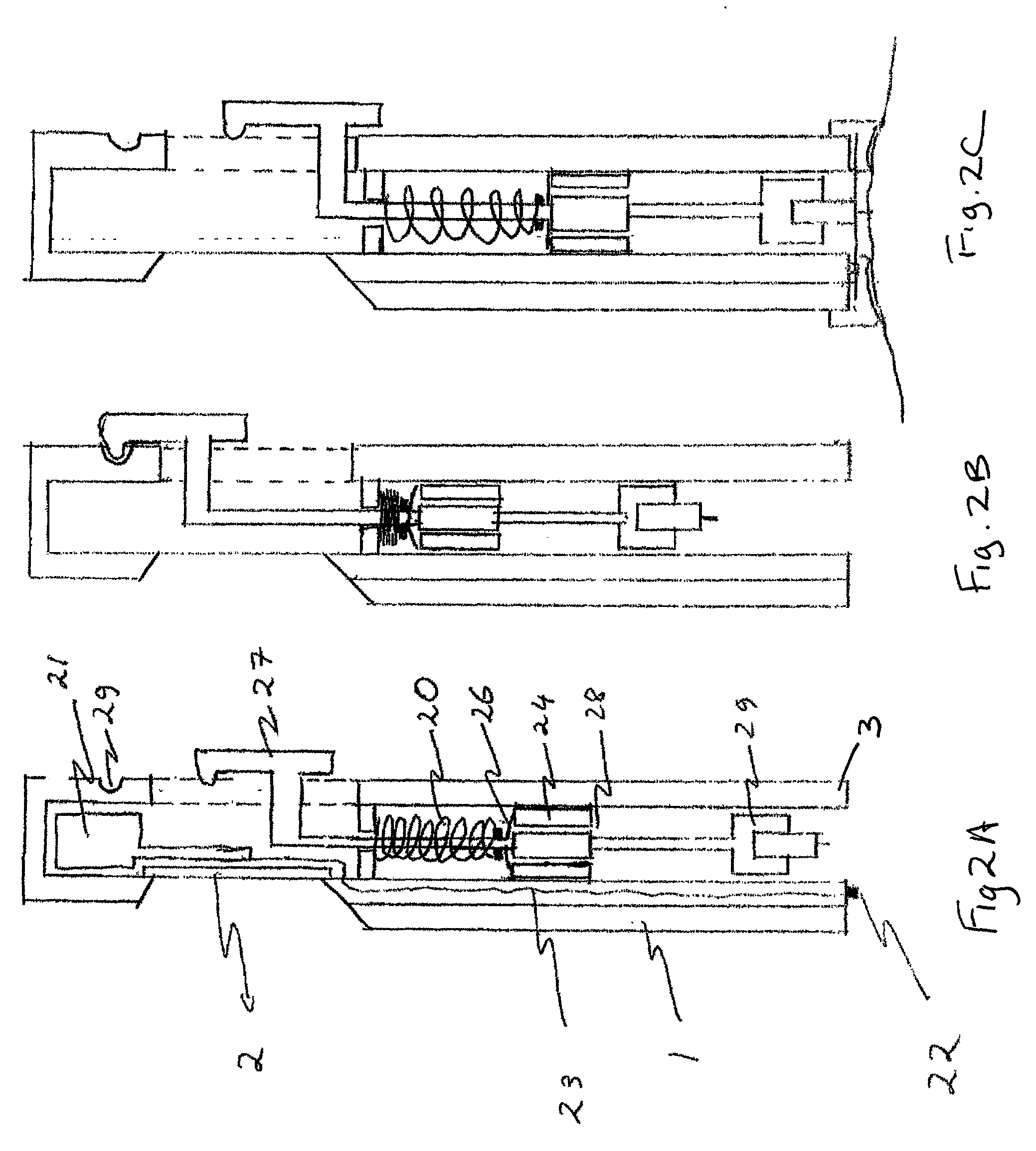
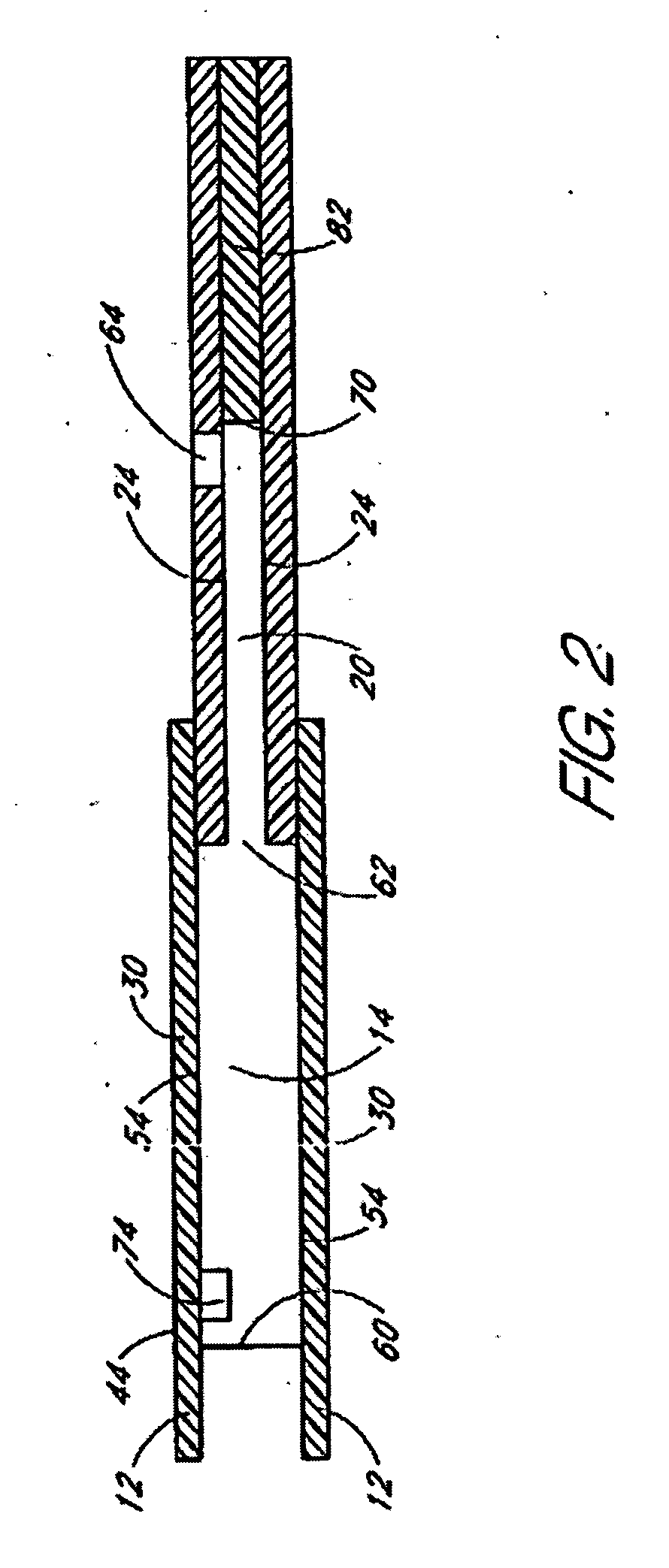
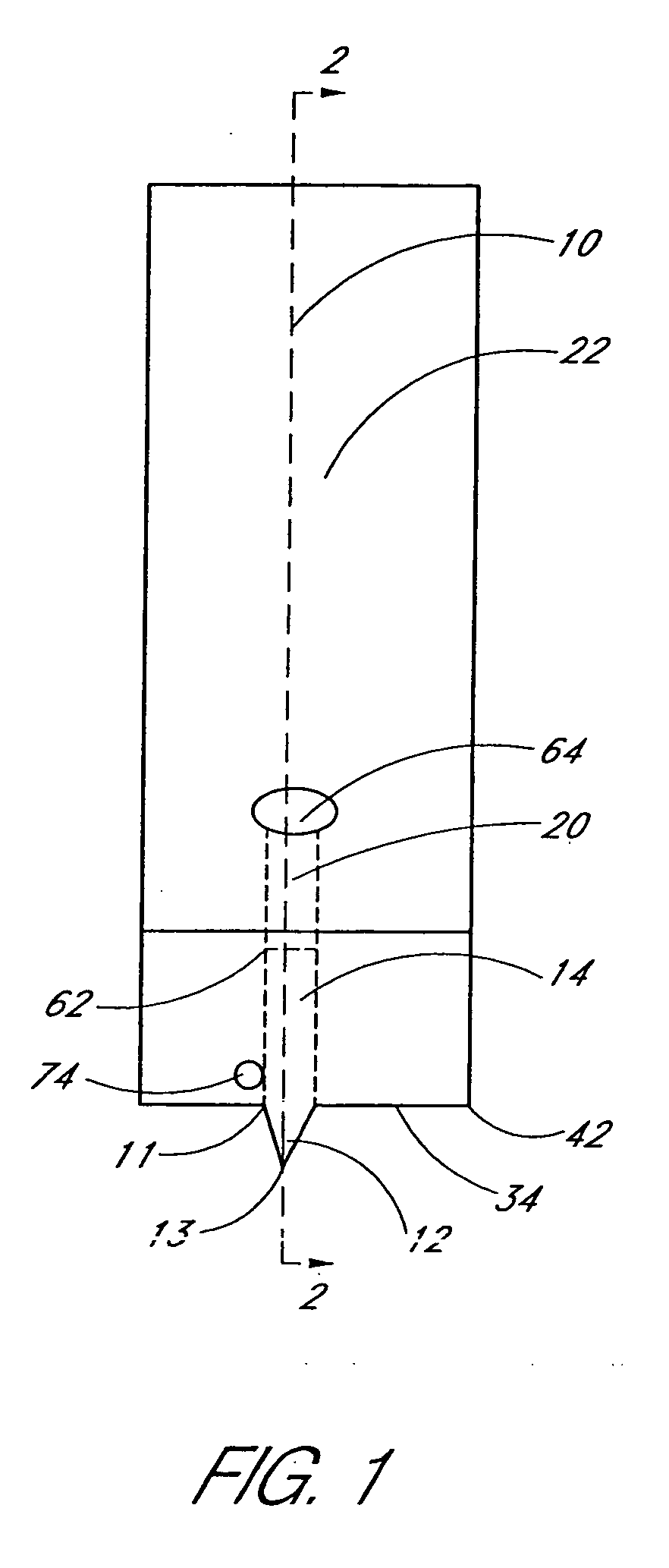
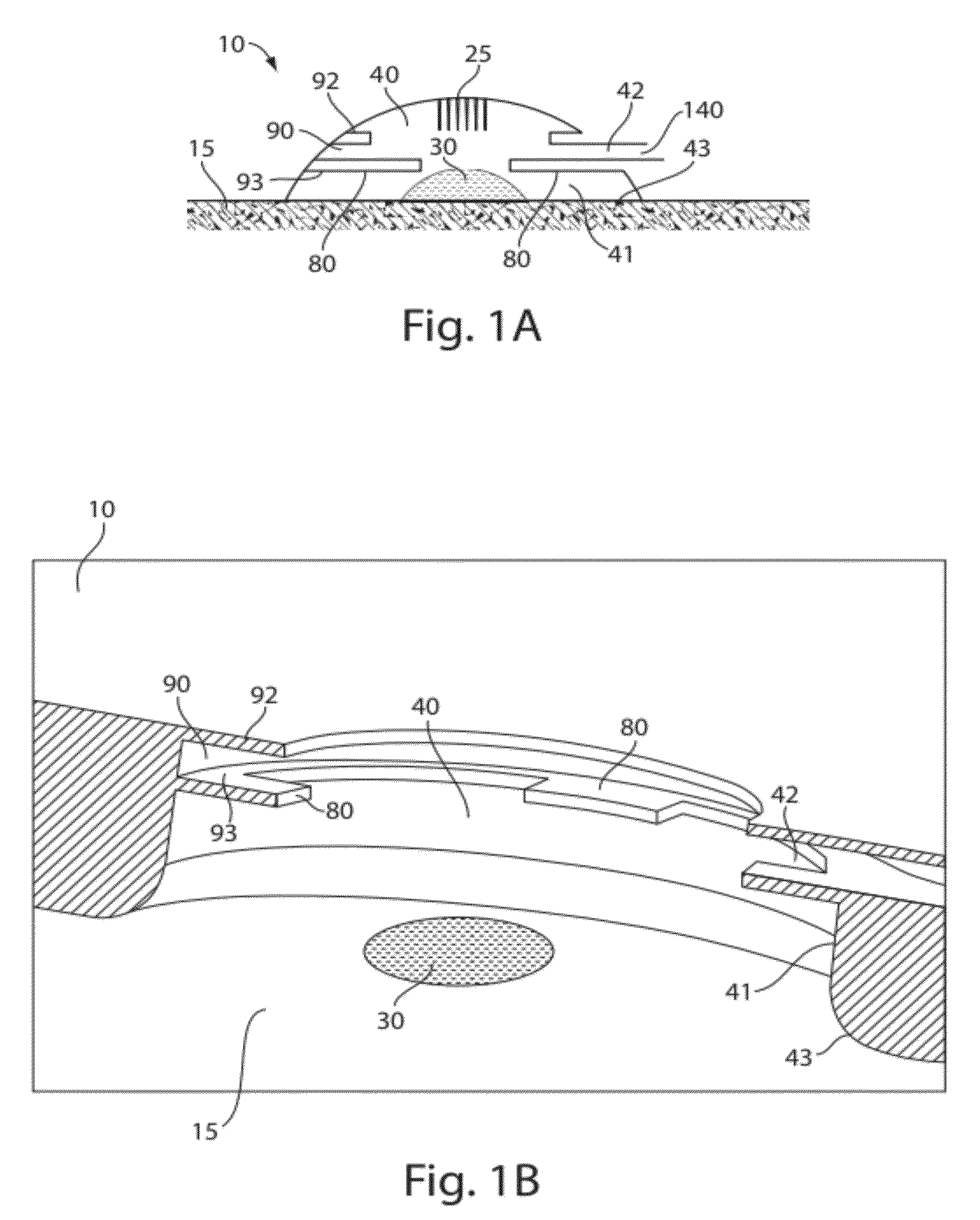
A glucose sensor in the form of a skin patch 2 has a microneedle 4 which painlessly penetrates the skin to draw out interstitial fluid. The interstitial fluid passes to a common entrance port 7. A series of microchannels 8 is provided on the skin patch. The fluid drawn onto the patch is selectively switched between a number of microchannels 8 by means of electro-osmotic pumps 10 and hydrophobic gates 12. Each microchannel 8 has an electrochemical detector 11 for sensing gluocse concentration. Also disclosed is a monlithic device with an integrated lance 83.
Owner:LIFESCAN IP HLDG LLC +1
Devices, systems and methods for extracting bodily fluid and monitoring an analyte therein
ActiveUS20040249253A1Easy to useLittle painWithdrawing sample devicesEvaluation of blood vesselsAnalyteElectronic communication
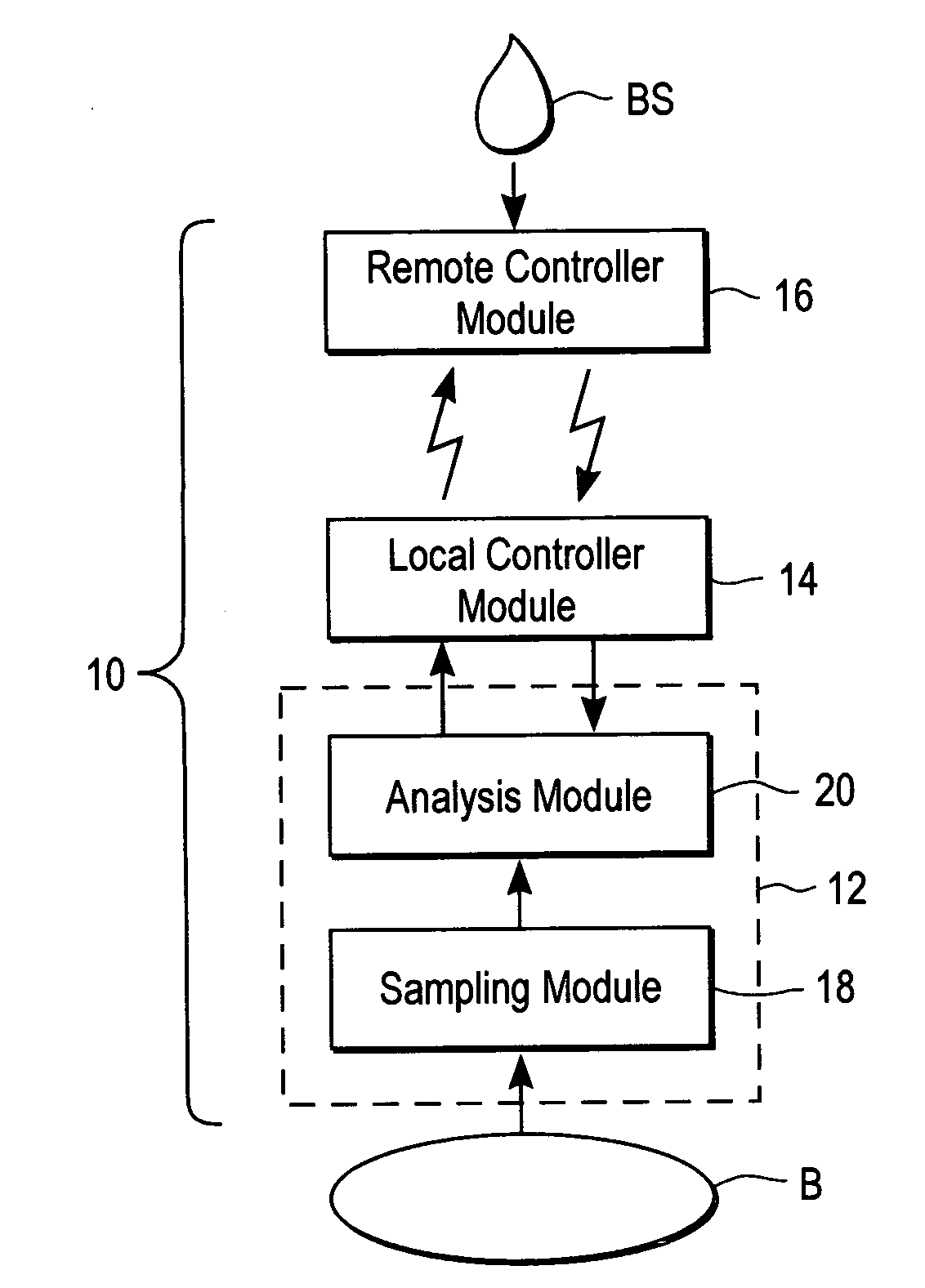
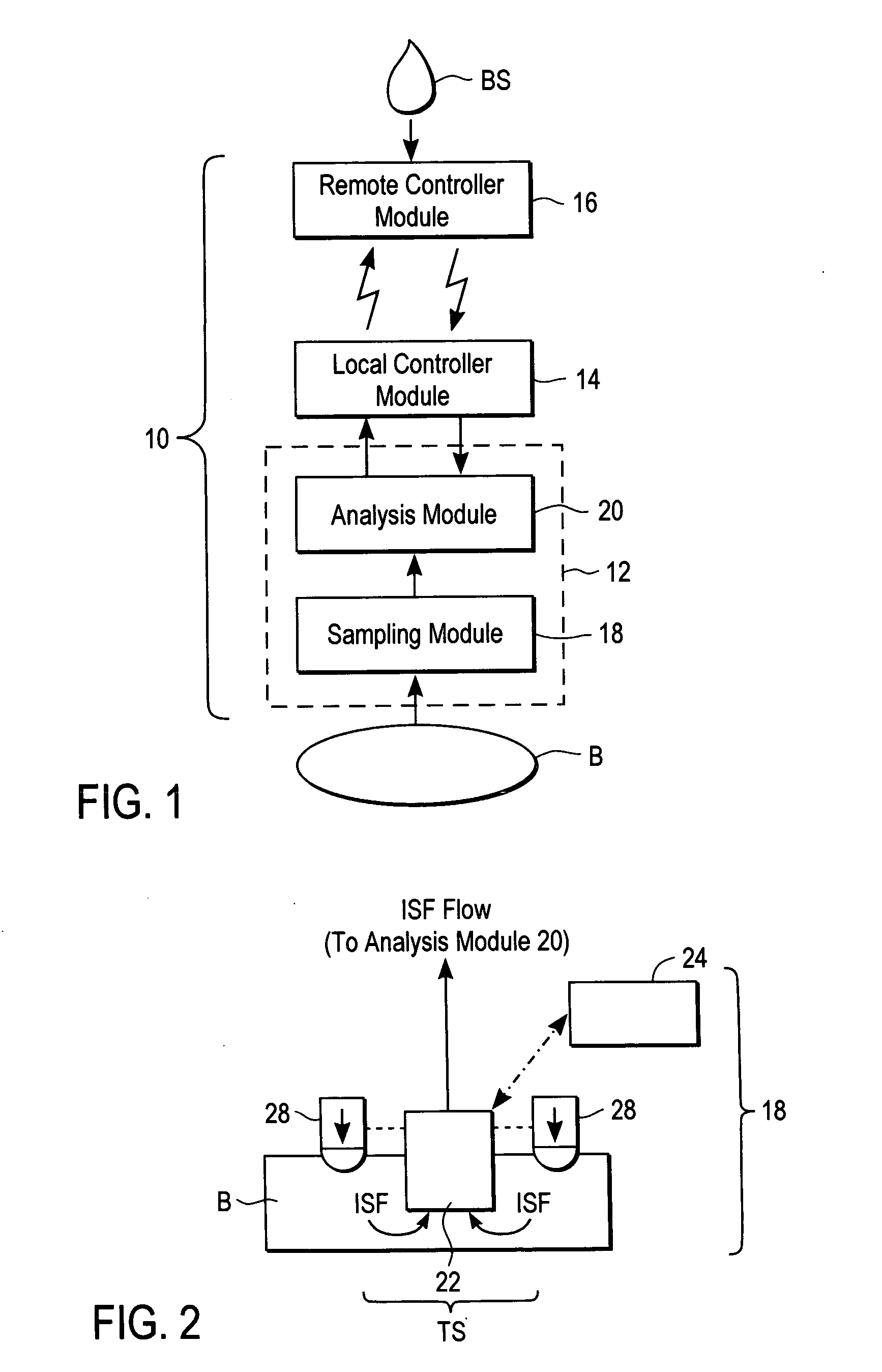
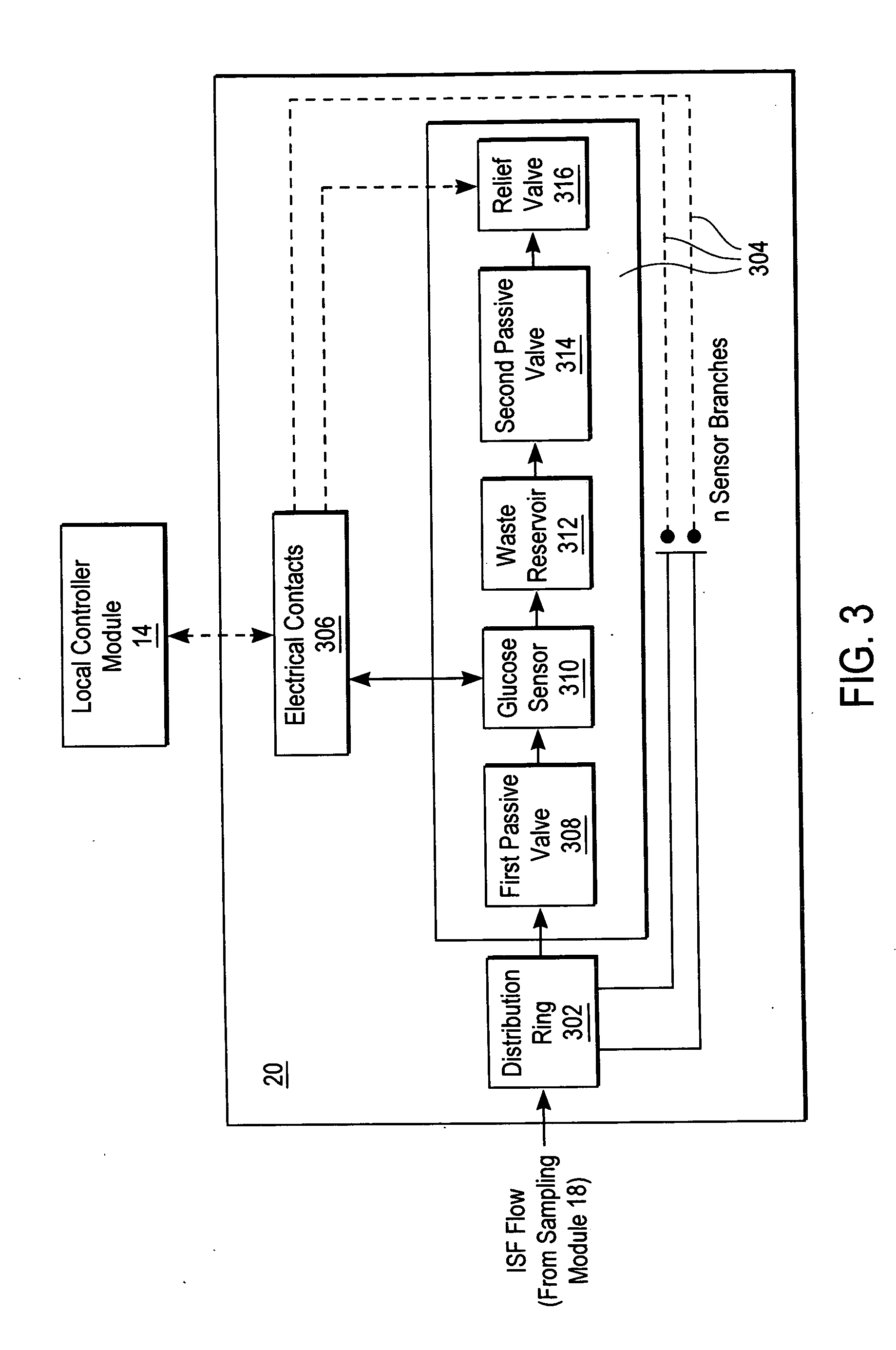
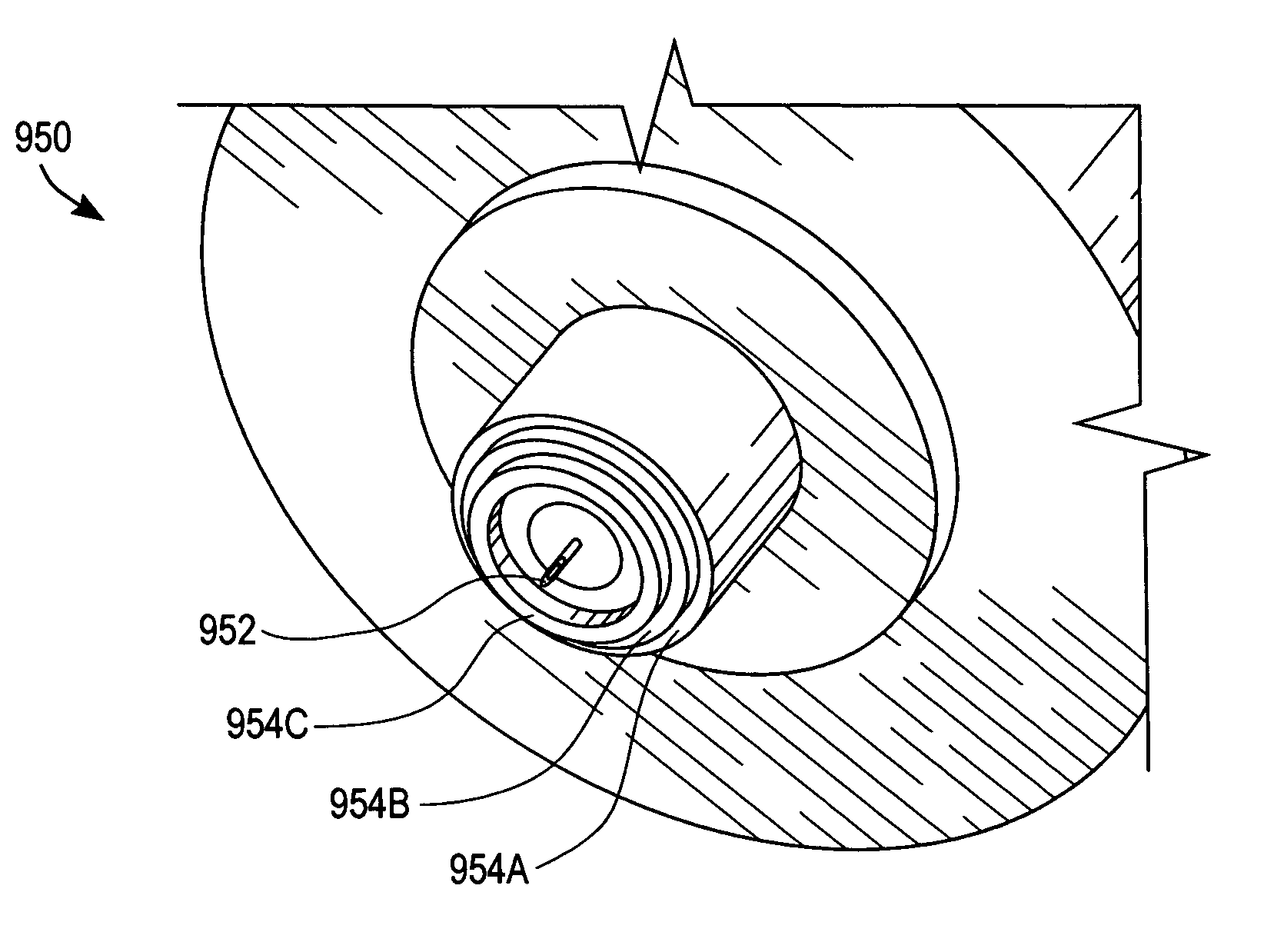
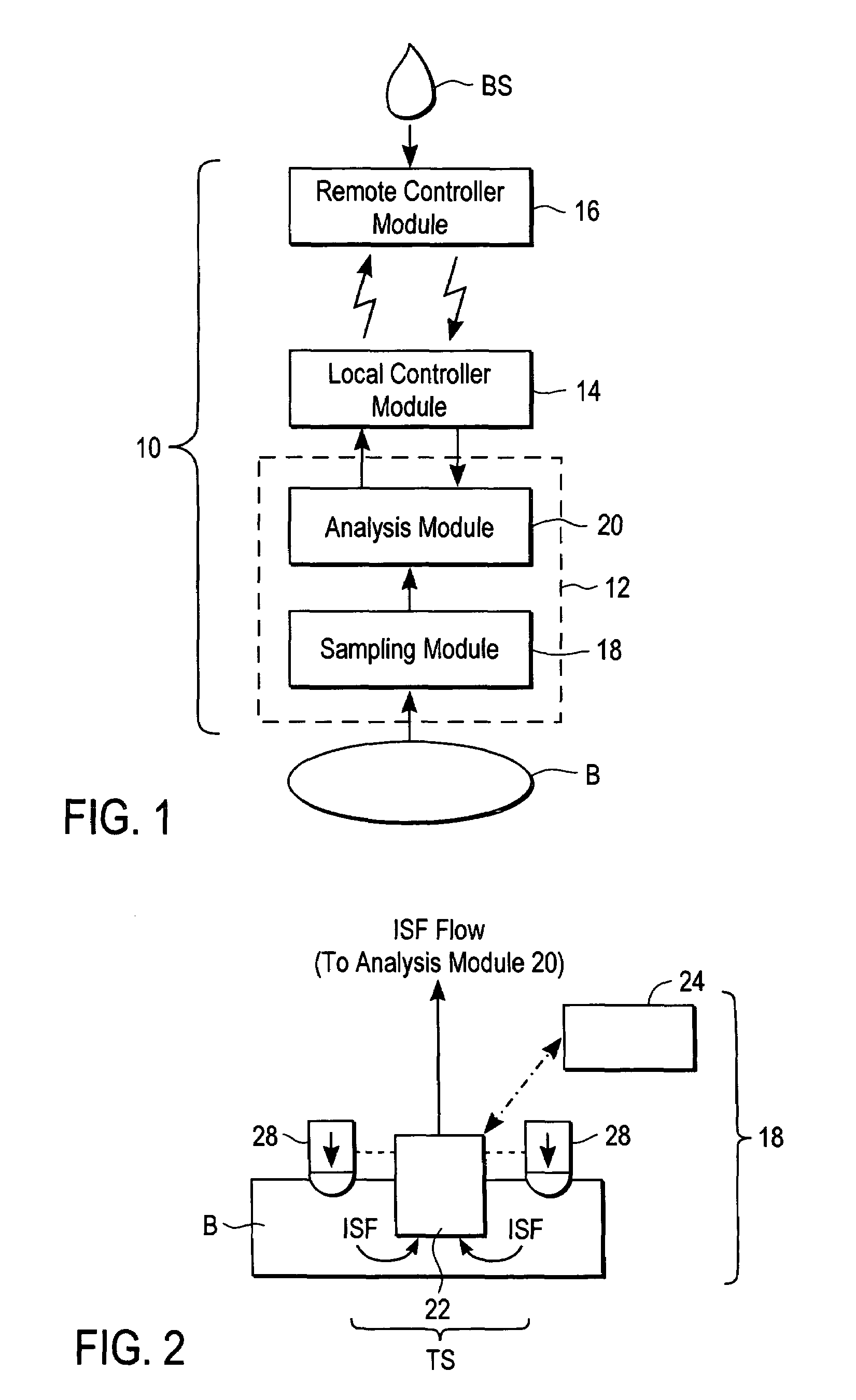
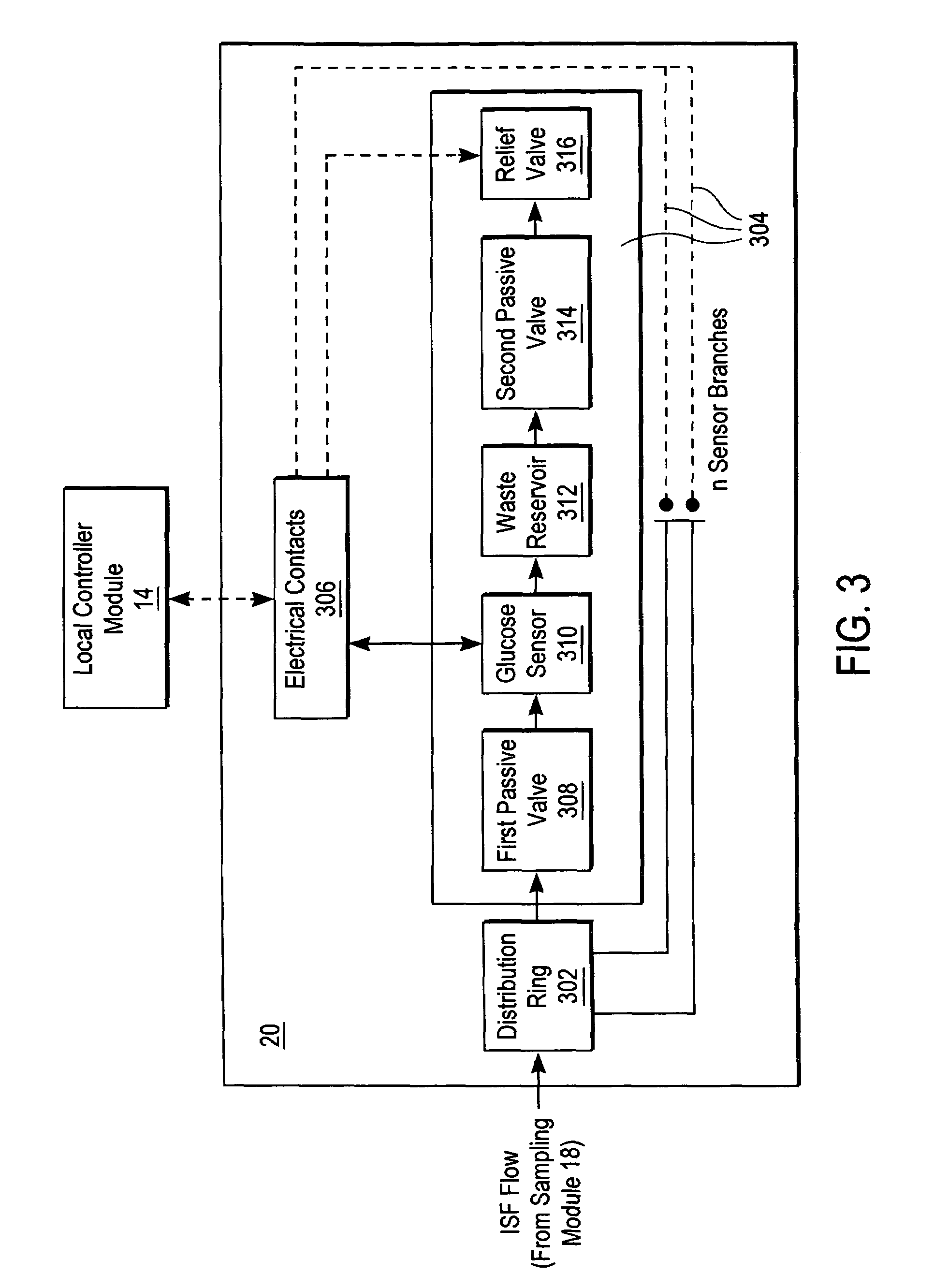
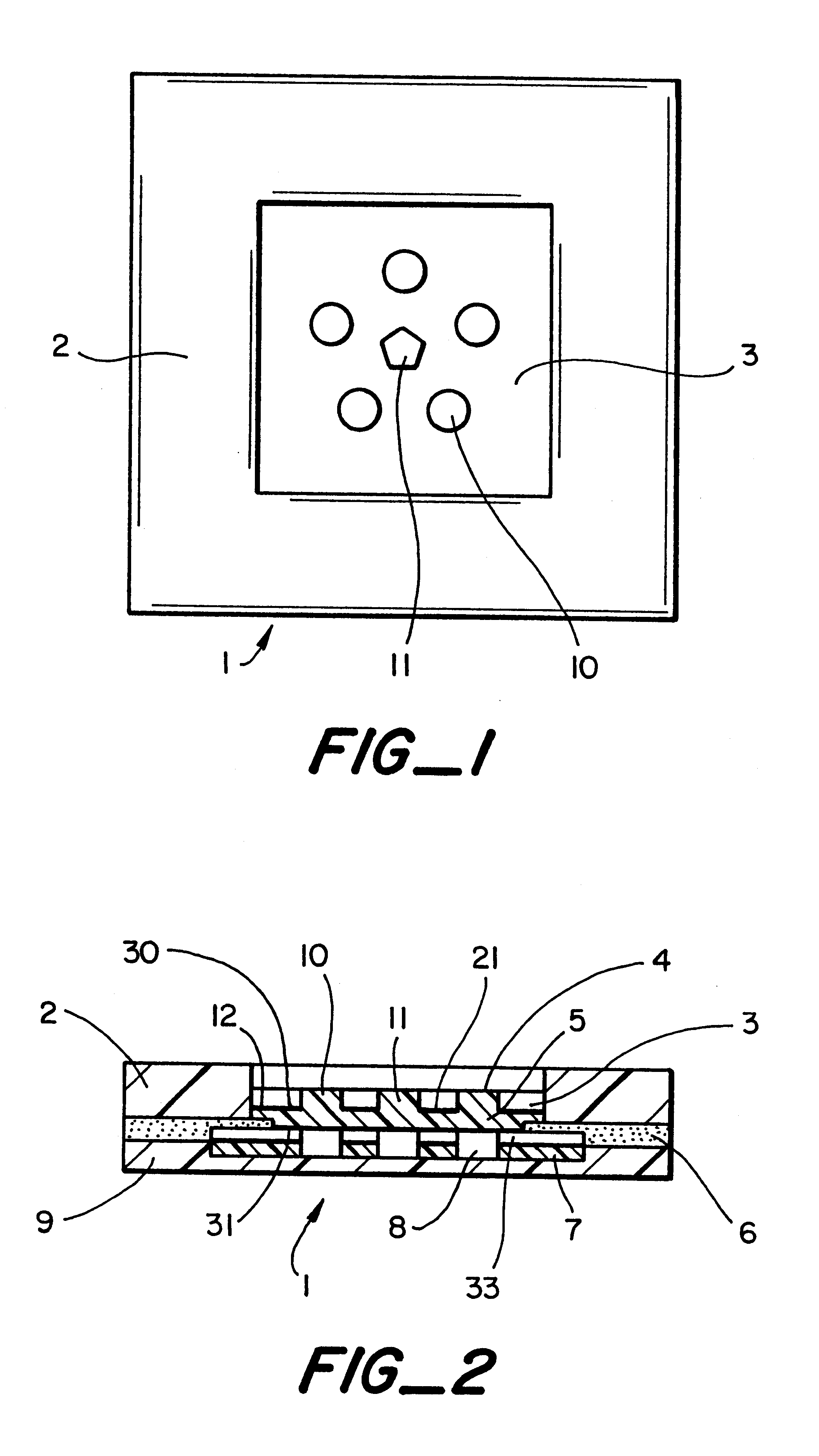
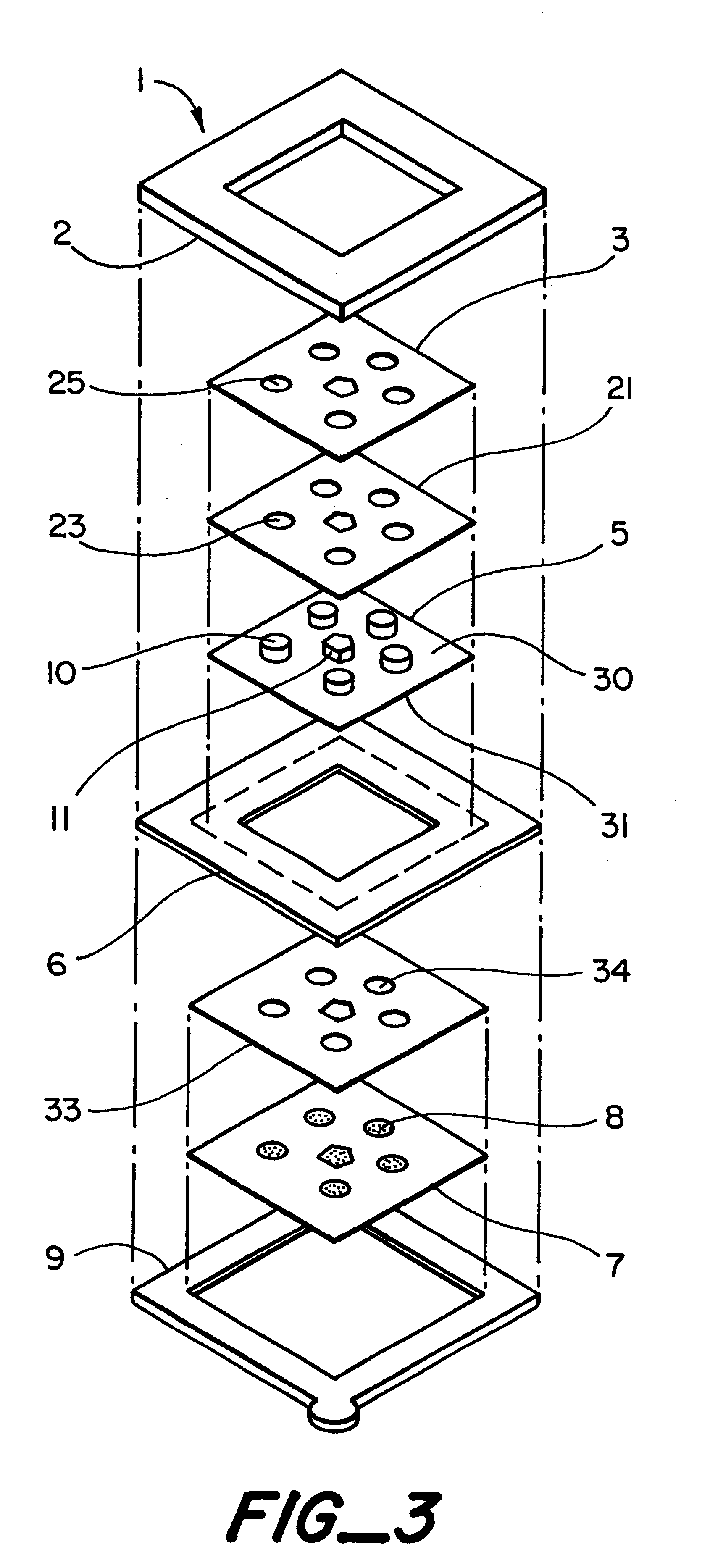
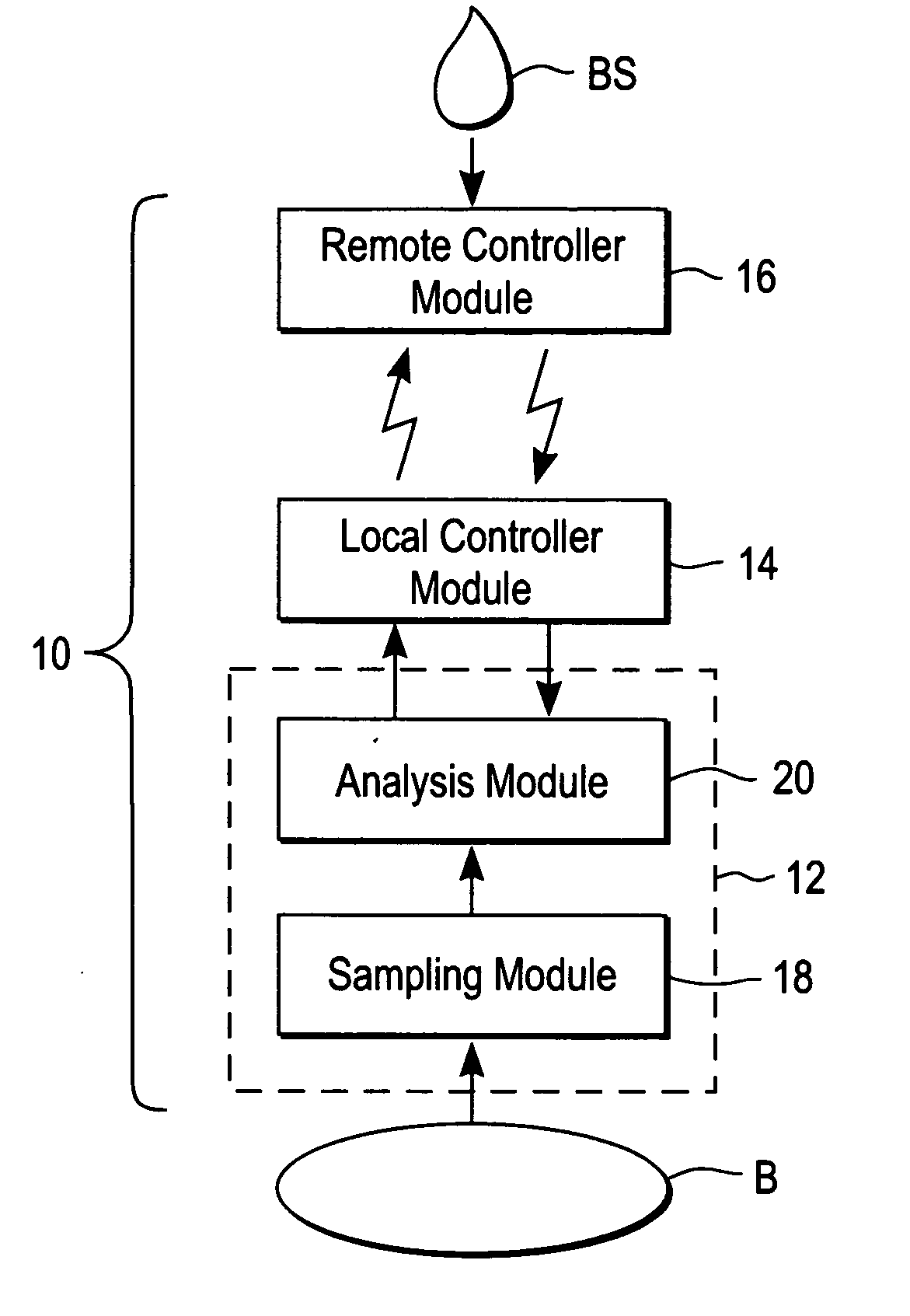
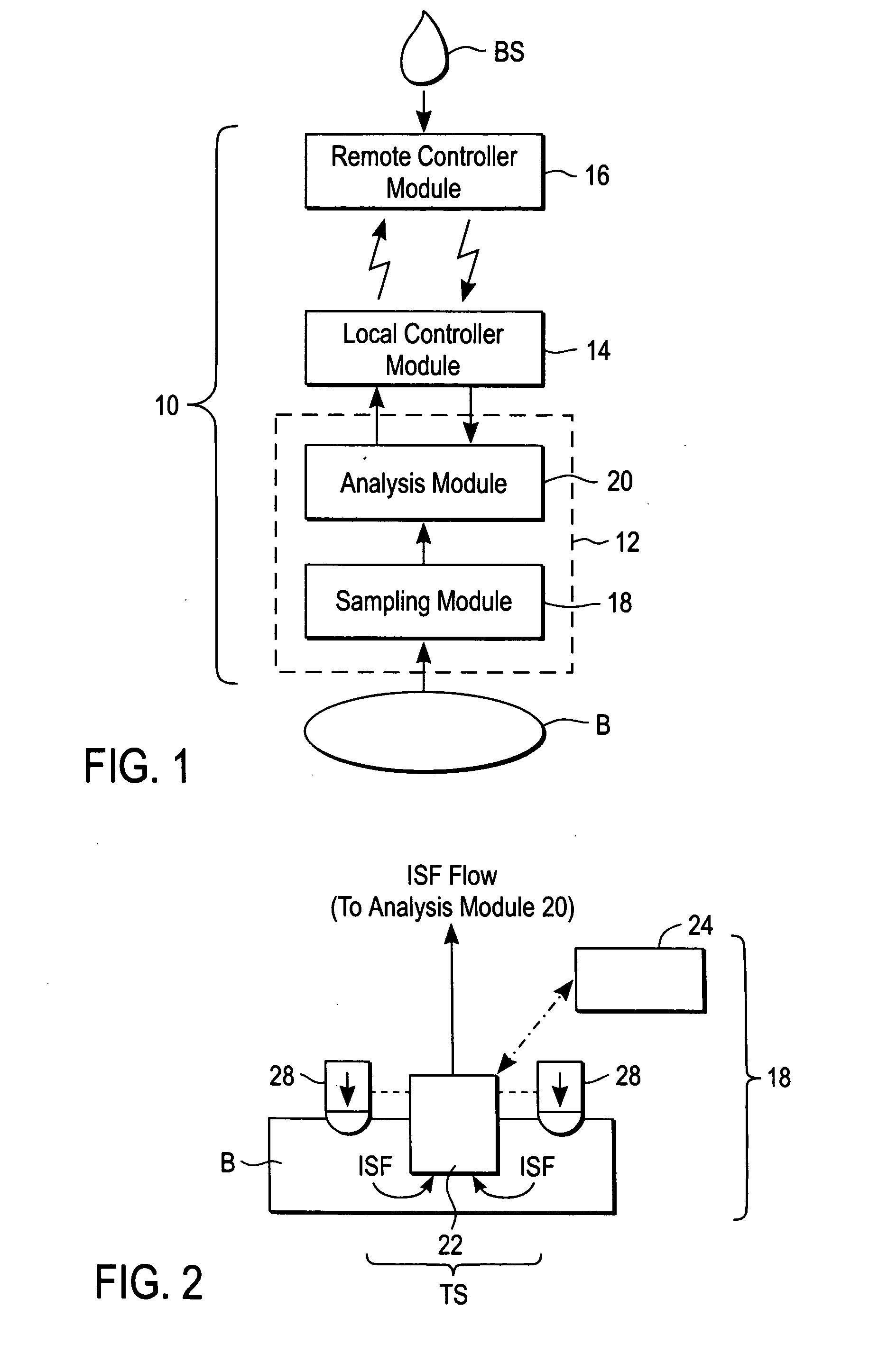
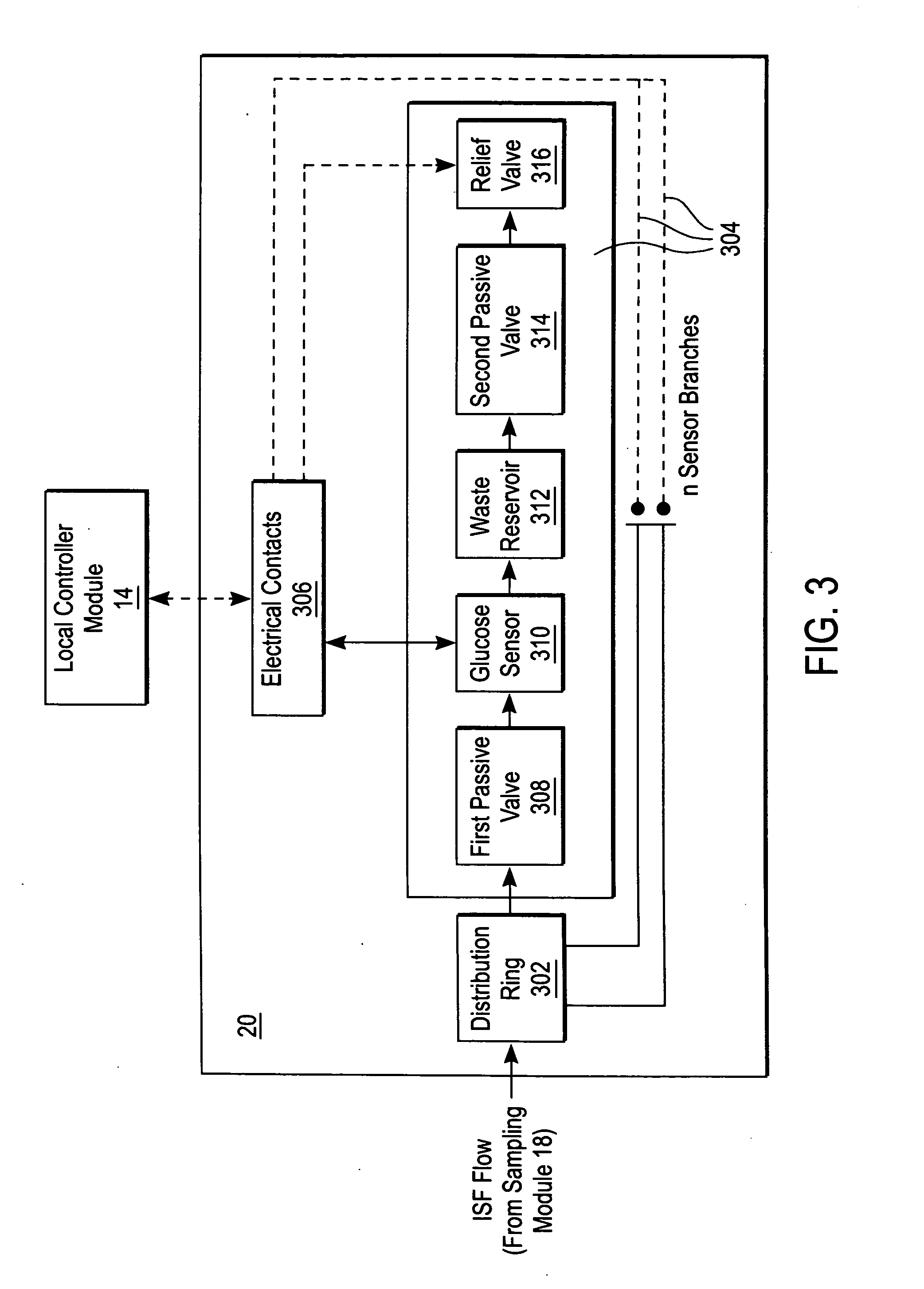
A system for extracting a bodily fluid sample (e.g., an interstitial fluid [ISF] sample) and monitoring an analyte therein includes a disposable cartridge and a local controller module. The disposable cartridge includes a sampling module adapted to extract a bodily fluid sample and an analysis module adapted to measure an analyte (e.g., glucose) in the bodily fluid sample. The local controller module is in electronic communication with the disposable cartridge and is adapted to receive and store measurement data from the analysis module. An ISF extraction device includes a penetration member configured for penetrating and residing in a target site of a user's skin layer and, subsequently, extracting an ISF sample therefrom. The device also includes a pressure ring(s) adapted for applying pressure to the user's skin layer in the vicinity of the target site. The device is configured such that the pressure ring(s) is capable of applying pressure in an oscillating manner whereby an ISF glucose lag of the ISF sample extracted by the penetration member is mitigated. A method for extracting ISF includes providing an ISF fluid extraction device with a penetration member and a pressure ring(s). Next, a user's skin layer is contacted by the pressure ring(s) and penetrated by the penetration member. An ISF sample is then extracted from the user's skin layer while pressure is being applied in an oscillating manner by the pressure ring(s). The oscillating pressure mitigates an ISF glucose lag of the extracted ISF sample extracted.
Owner:LIFESCAN IP HLDG LLC
Devices, systems and methods for extracting bodily fluid and monitoring an analyte therein
ActiveUS7258673B2Little painSlight discomfortWithdrawing sample devicesEvaluation of blood vesselsAnalyteD-Glucose
An interstitial fluid (ISF) extraction device includes a penetration member configured for penetrating a target site of a user's skin layer and, subsequently, residing in the user's skin layer and extracting an ISF sample therefrom and at least three concentrically-arranged pressure rings, each adapted for applying pressure to the user's skin layer in the vicinity of the target site while the penetration member is residing in the user's skin layer. In addition, the ISF extraction device is configured such that (i) the pressure rings apply pressure in an oscillating manner with asymmetric deployment and retraction cycles and (ii) only one of the at least three concentrically-arranged pressure rings is deployed at a time, thereby mitigating an ISF glucose lag of the ISF sample extracted by the penetration member.
Owner:LIFESCAN IP HLDG LLC
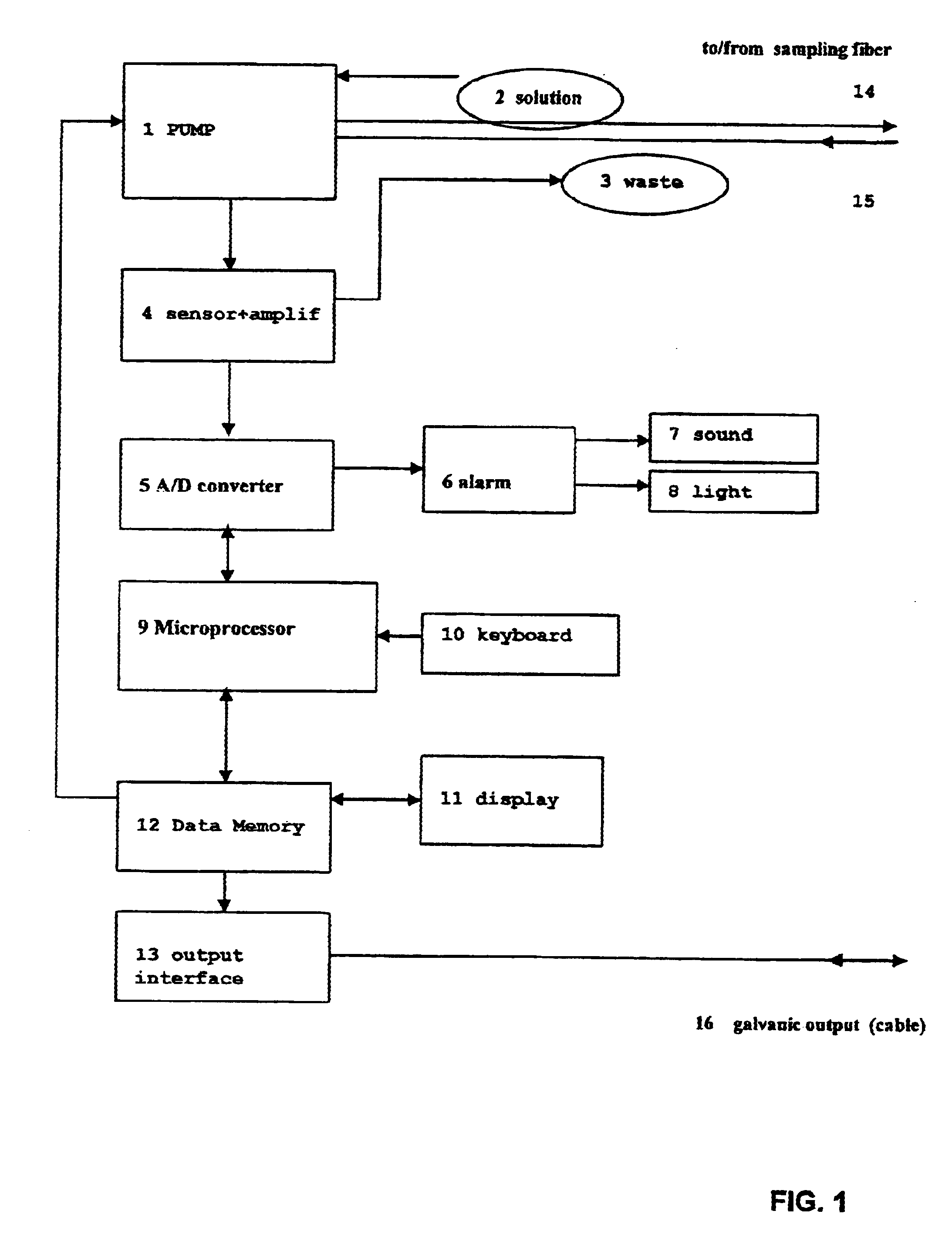
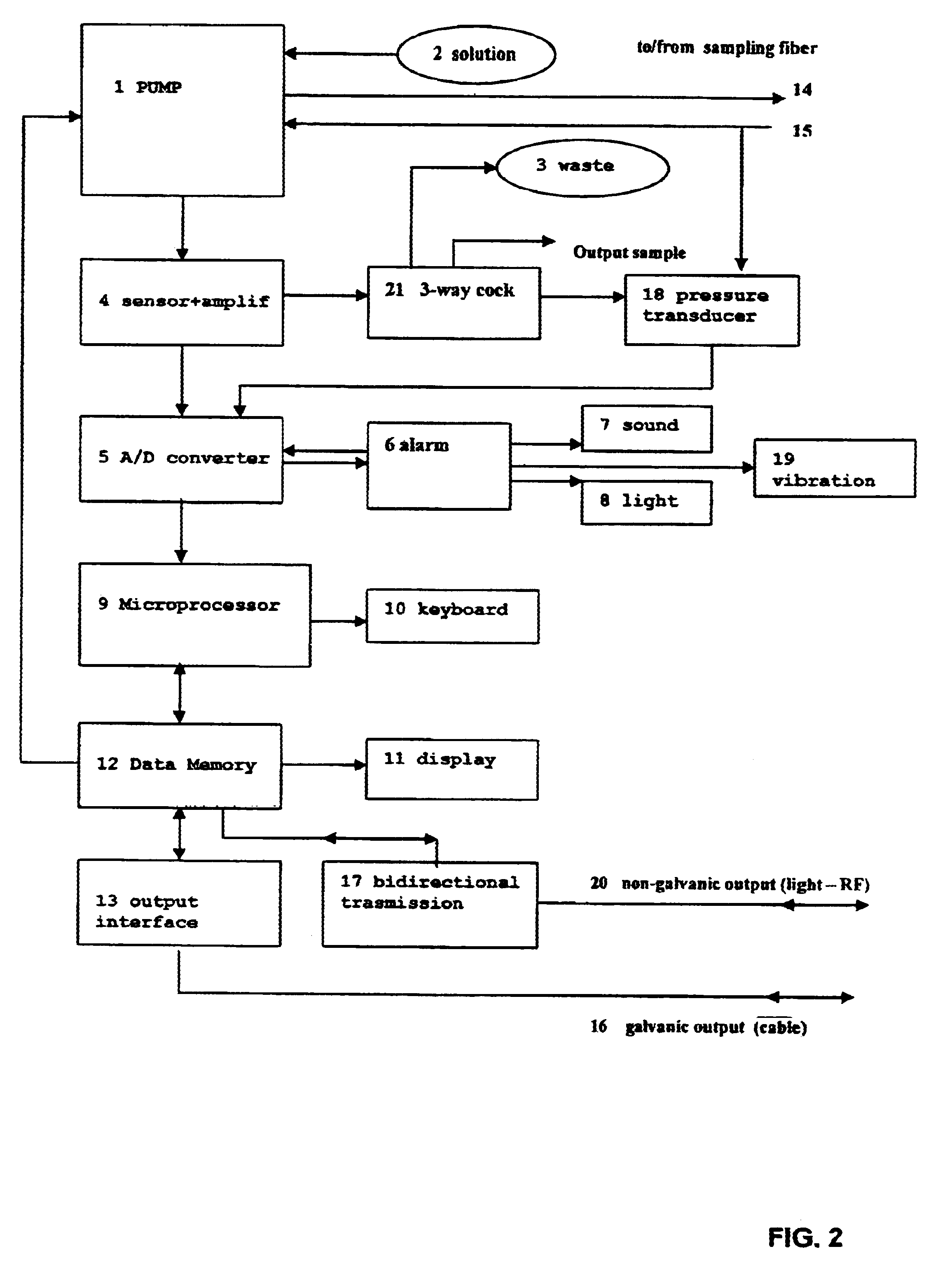
Apparatus for measurement and control of the content of glucose, lactate or other metabolites in biological fluids
InactiveUS6618603B2Immobilised enzymesBioreactor/fermenter combinationsMetaboliteGlucose Measurement
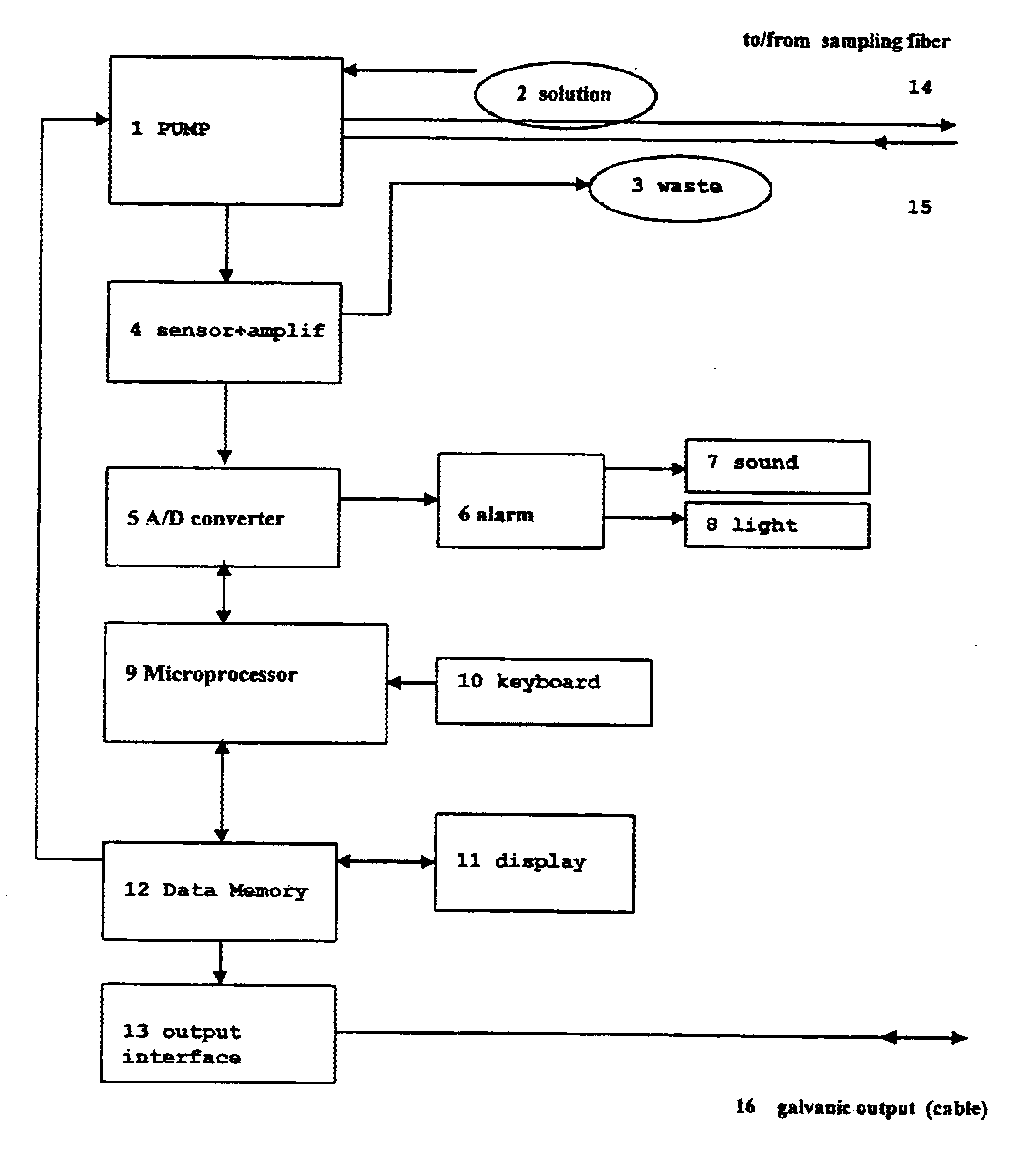
An apparatus for the continuous measurement of glucose and lactate in interstitial fluids including a glucose measurement cell, an A / D conversion block, a memory block and a bi-directional communication between the interface block and an external calculation unit.
Owner:A MENARINI IND FARM RIUNITE SRL
Method and device for sampling and analyzing interstitial fluid and whole blood samples
InactiveUS20070017805A1Less riskLess invasiveSurgeryVaccination/ovulation diagnosticsAnalyteWhole blood sample
Owner:LIFESCAN IP HLDG LLC
Intracutaneous microneedle array apparatus
InactiveUS20050209565A1Sufficient separation distanceGreater transdermal fluxElectrotherapySurgical needlesEngineeringBiological fluids
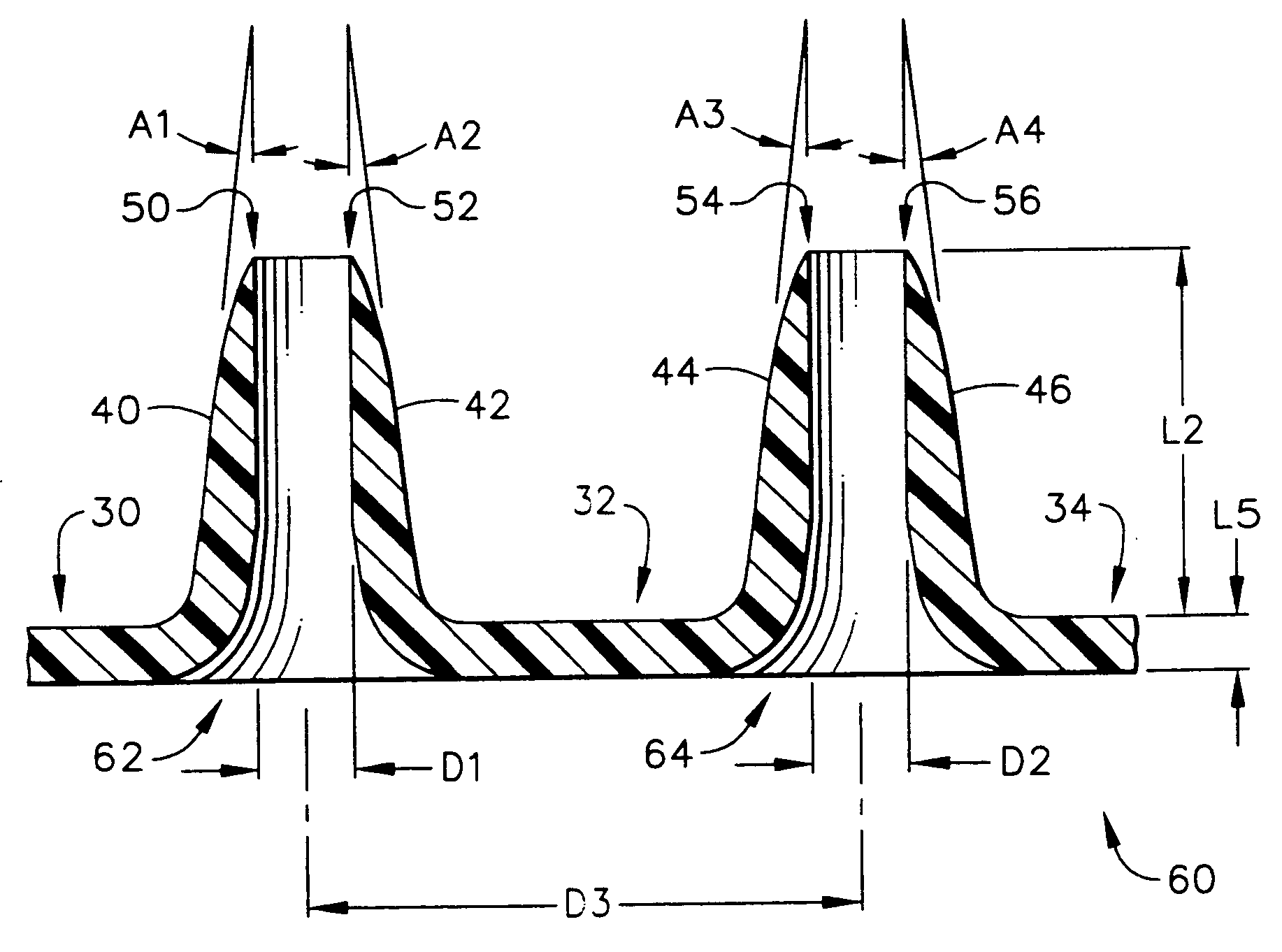
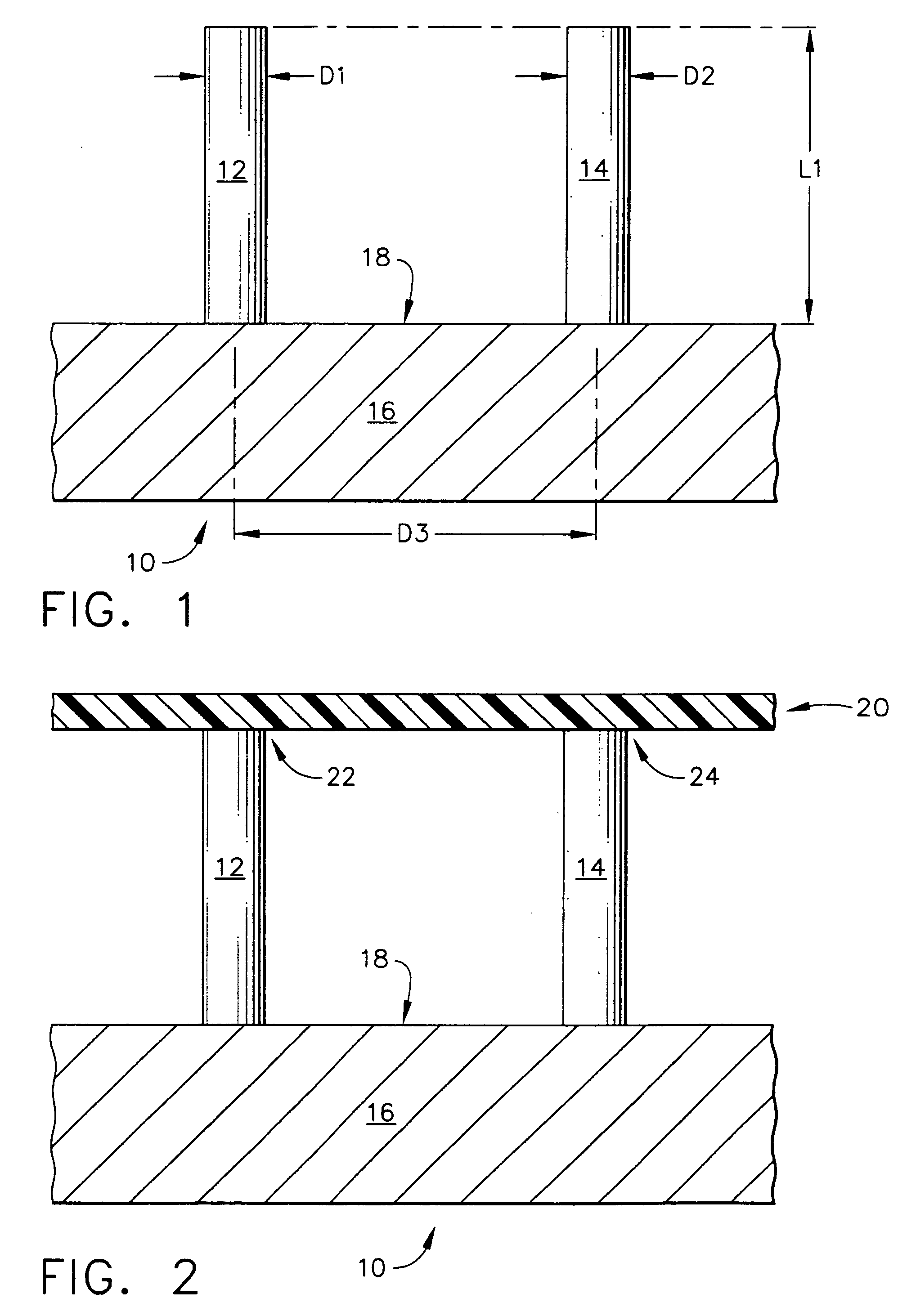
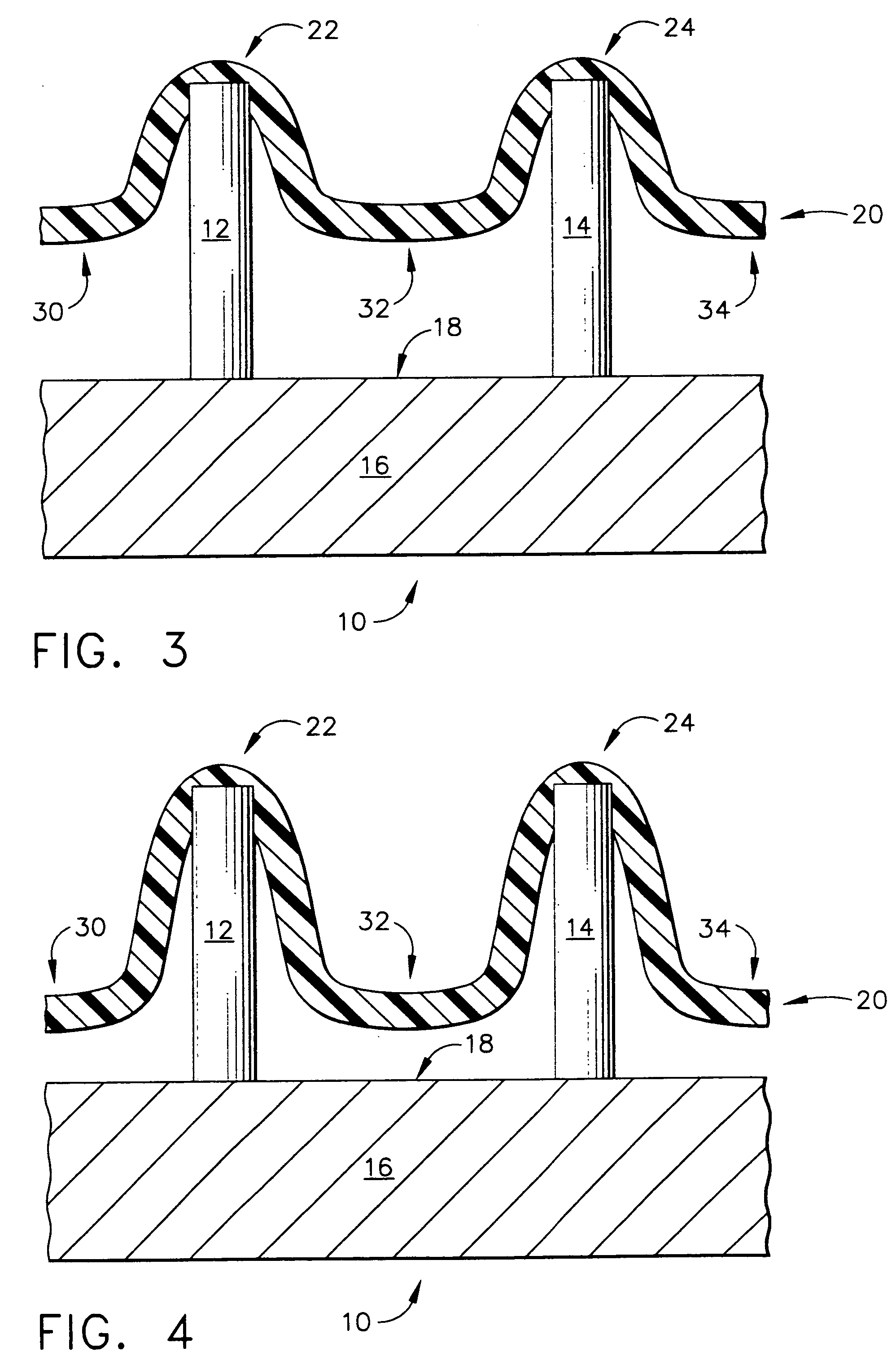
Improved microneedle arrays are provided having a sufficiently large separation distance between each of the individual microneedles to ensure penetration of the skin while having a sufficiently small separation distance to provide high transdermal transport rates. A very useful range of separation distances between microneedles is in the range of 100-300 microns, and more preferably in the range of 100-200 microns. The outer diameter and microneedle length is also very important, and in combination with the separation distance will be crucial as to whether or not the microneedles will actually penetrate the stratum corneum of skin. For circular microneedles, a useful outer diameter range is from 20-100 microns, and more preferably in the range of 20-50 microns. For circular microneedles that do not have sharp edges, a useful length for use with interstitial fluids is in the range of 50-200 microns, and more preferably in the range of 100-150 microns; for use with other biological fluids, a useful length is in the range of 200 microns—3 mm, and more preferably in the range of 200-400 microns. For circular microneedles having sharp side edges, a useful length for use with interstitial fluids is in the range of 50-200 microns, and more preferably in the range of 80-150 microns; for use with other biological fluids, a useful length is again in the range of 200 microns—3 mm, and more preferably in the range of 200-400 microns. For solid microneedles having a star-shaped profile with sharp edges for its star-shaped blades, a useful length for use with interstitial fluids is in the range of 50-200 microns, and more preferably in the range of 80-150 microns; for use with other biological fluids, a useful length is again in the range of 200 microns—3 mm, and more preferably in the range of 200-400 microns, while the radius of each of its blades is in the range of 10-50 microns, and more preferably in the range of 10-15 microns.
Owner:CORIUM INC
Integrated poration, harvesting and analysis device, and method therefor
An integrated device for poration of biological tissue, harvesting a biological fluid from the tissue, and analysis of the biological fluid. The device comprises a tissue-contacting layer having an electrically or optically heated probe to heat and conduct heat to the tissue to form at least one opening, such as a micropore to collect biological fluid from the opening, and a detecting layer responsive to the biological fluid to provide an indication of a characteristic of the biological fluid, such as the concentration of an analyte in interstitial fluid. In the embodiment in which, the probe comprises a photosensitizing assembly designed for the uniform application of a photosensitizing material, such as, for example, a dye or a pigment, to a tissue, e.g., the stratum comeum. In one embodiment, the photosensitizing assembly comprises photosensitizing material combined with a carrier, such as, for example, an adhesive or an ink, and the resulting combination is applied to a substrate, such as, for example, an inert polymeric substrate to form a photosensitizing assembly. In another embodiment, the photosensitizing assembly comprises photosensitizing material incorporated into a film-forming polymeric material.
Owner:NITTO DENKO CORP +1
Micro-invasive method for painless detection of analytes in extracellular space
InactiveUS6904301B2Reduces and eliminates delay timeAvoid destructionAdditive manufacturing apparatusSurgeryAnalyteStratum basale
A method of detecting at least one analyte in extra-cellular spaces includes the step of inserting a microprobe through the stratum corneum toward the stratum basale of the skin of a subject into extra-cellular spaces containing interstitial fluid having at least one analyte to be detected, said microprobe having a diameter at its tip no larger than approximately 10-50 microns. The method further includes optically testing for a predetermined analyte in the extra-cellular space adjacent the distal end of the microprobe without drawing a sample of the interstitial fluid. Preferably the microprobe body includes a sensor layer covering the distal optical tip of the microprobe body, the sensor layer being adapted to interact with a predetermined analyte to be detected in the interstitial fluid, and an optical detector responsive to interaction of the sensor layer with the predetermined analyte to signal detection of said predetermined analyte.
Owner:BECTON DICKINSON & CO
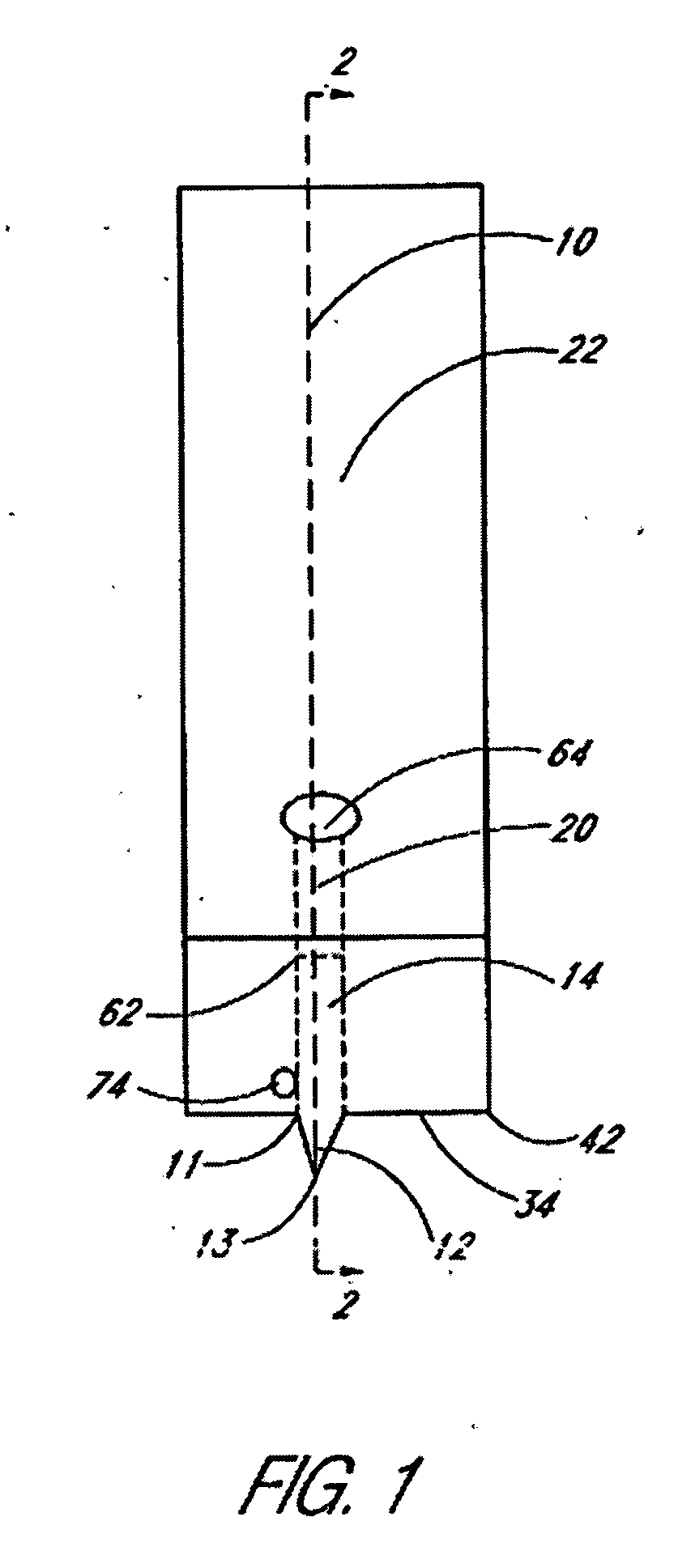
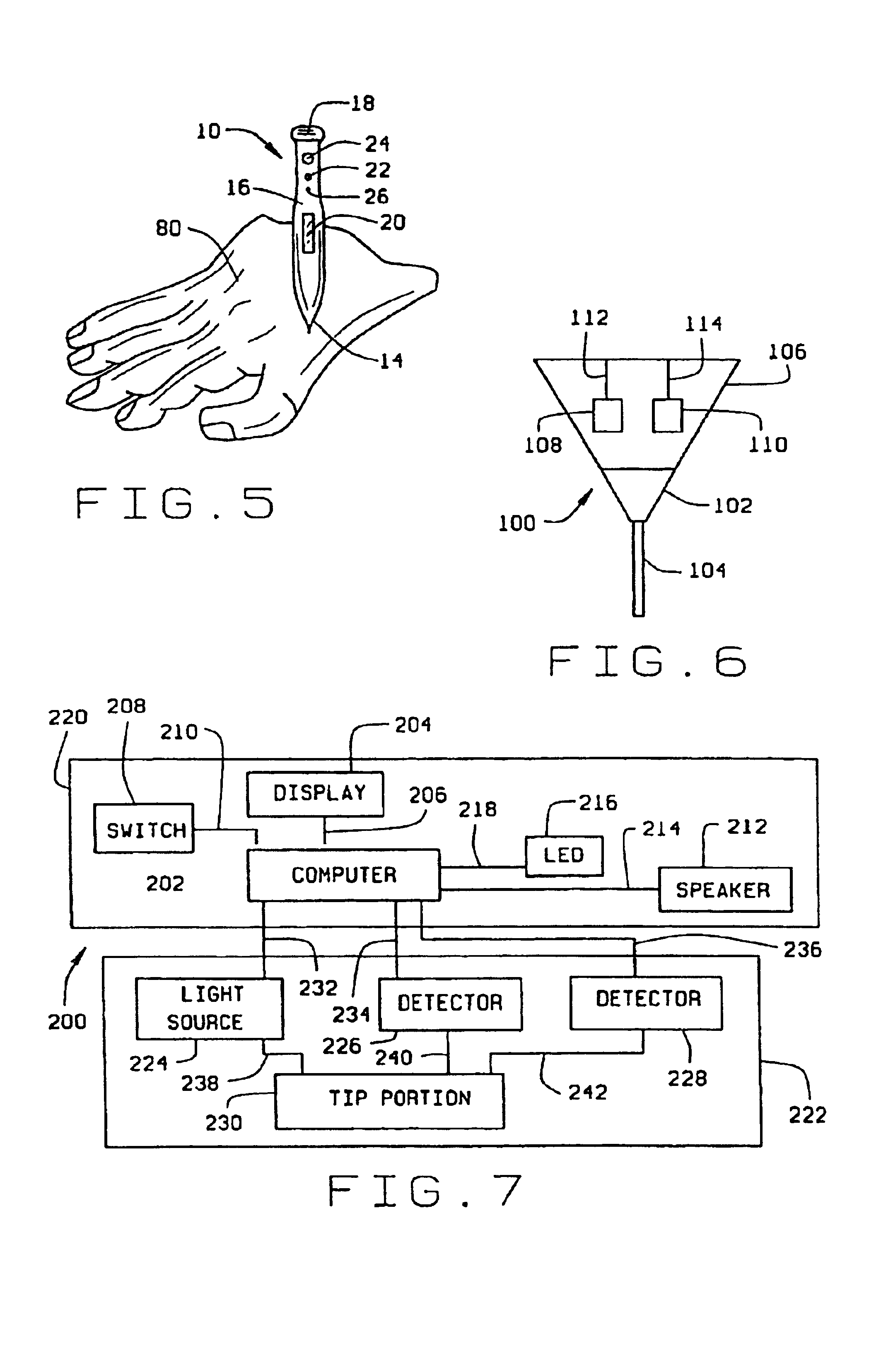
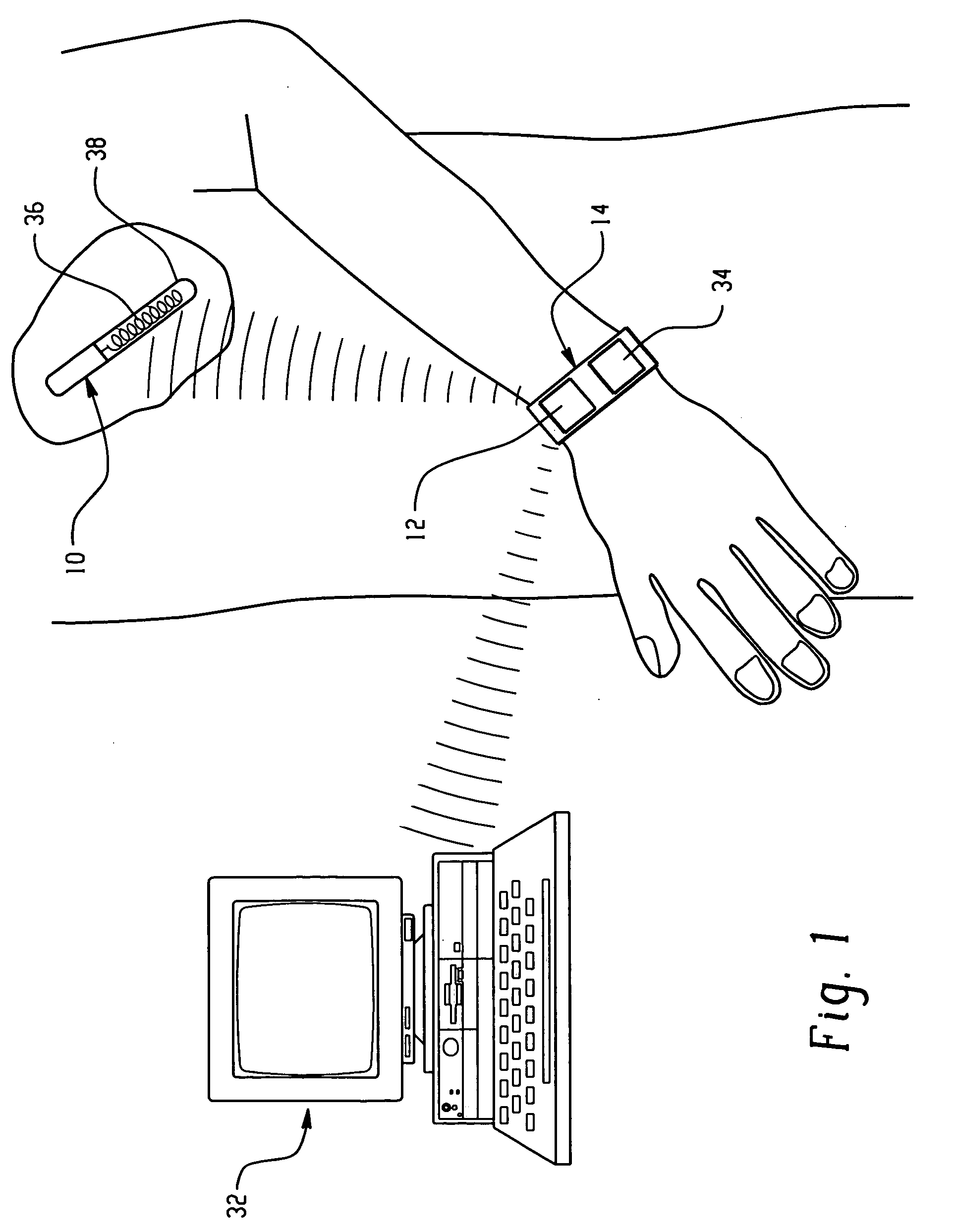
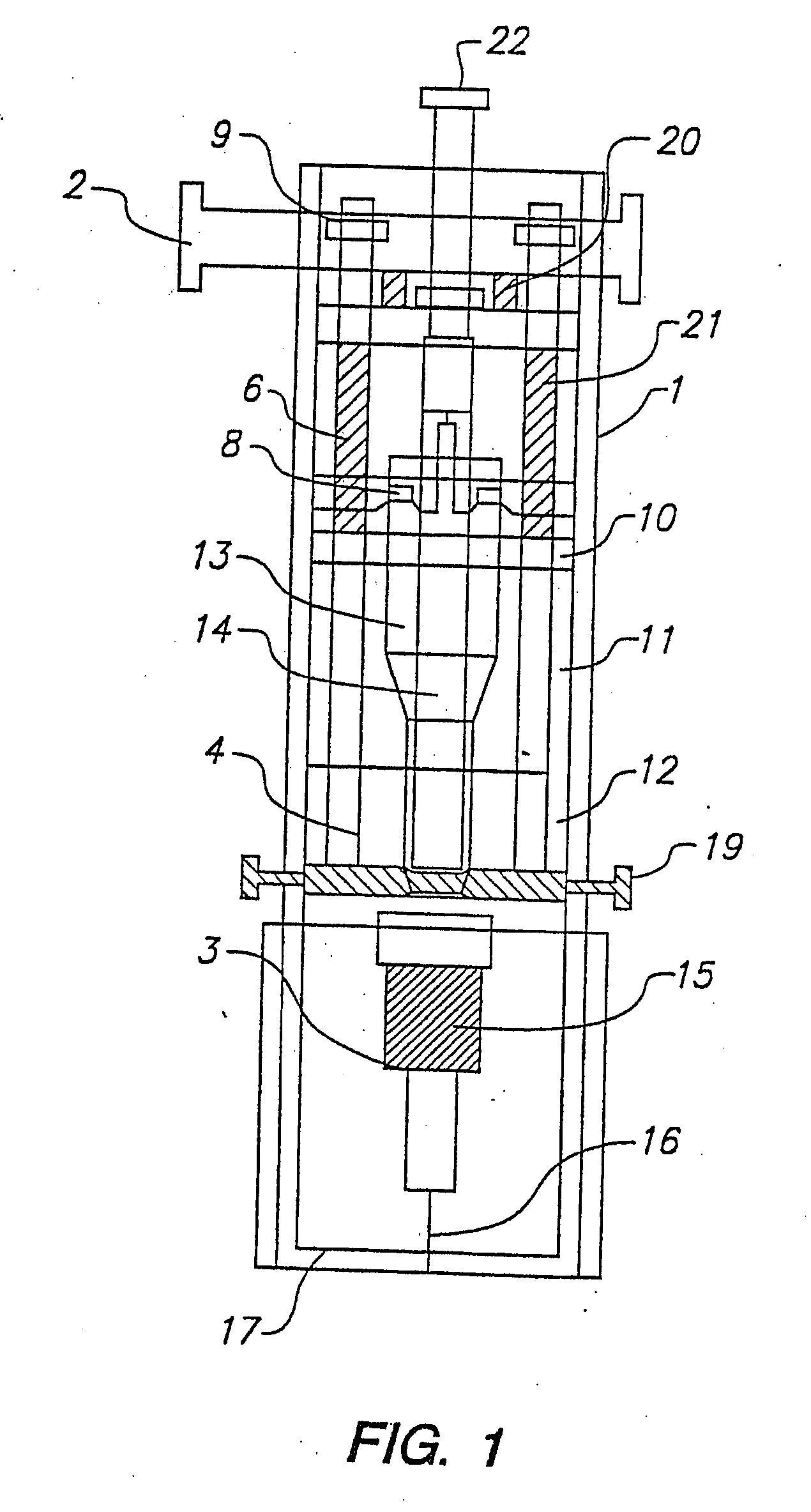
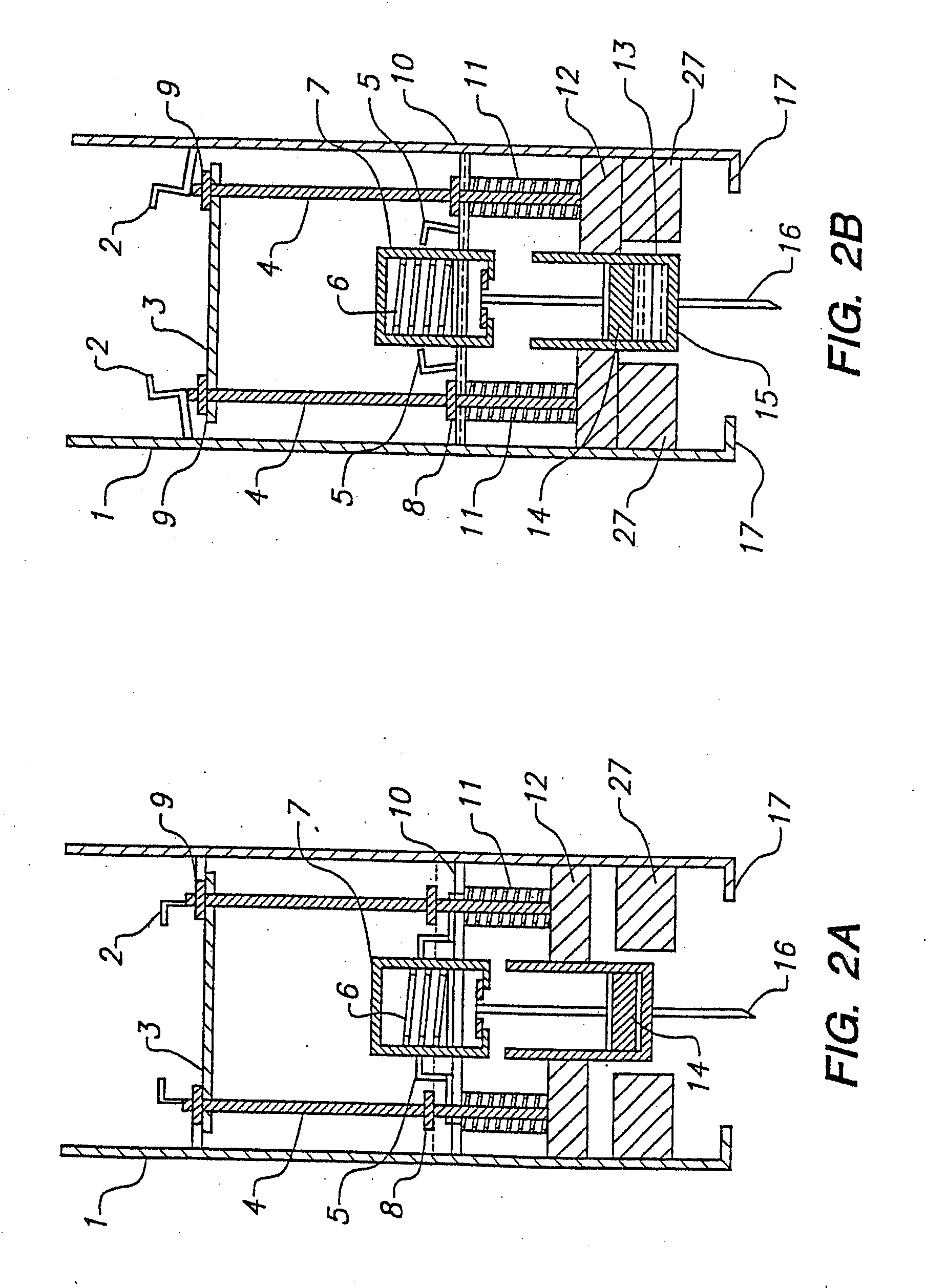
Sliver type autonomous biosensors
InactiveUS20040180391A1Bioreactor/fermenter combinationsBiological substance pretreatmentsIn vivoElectrochemistry
In vivo or in vitro monitoring of chemical and biochemical species (e.g., pH, or glucose levels) in the interstitial fluid of patients or in a sample of a fluid to be analyzed is provided by a probe (10, 70, 210, 270). For in vivo monitoring, the probe is readily inserted by a minimally invasive method. Optical or electrochemical sensing methods are employed to detect a physical or chemical change, such as pH, color, electrical potential, electric current, or the like, which is indicative of the concentration of the species or chemical property to be detected. Visual observation by the patient may be sufficient to monitor certain biochemicals (e.g., glucose) with this approach. A CAP membrane allows high enzyme loadings, and thus enables use of microminiature probes, and / or diagnosis of low levels of the analyte(s), with sufficient signal-to-noise ratio and low background current.
Owner:CASE WESTERN RESERVE UNIV
Interstitial fluid analyzer
A device useful for measuring an analyte in the interstitial fluid of an animal comprising an array chamber having an array of one or more microprojections and a detection compartment comprising a sensor in selective fluid communication with the array chamber. Also included are two extraction electrodes for inducing electrotransport of the interstitial fluid from the animal into the array chamber. A method includes the steps of forming a plurality of microchannels through a stratum corneum layer of an epidermis of the animal, inducing electrotransport of interstitial fluid containing the analyte through the microchannels and mixing one or more materials with the interstitial fluid to form a mixture, contacting the mixture with detection electrodes and analyzing the mixture with the detection electrodes.
Owner:LYNNTECH
Interstitial fluid methods and devices for determination of an analyte in the body
InactiveUS6251083B1Simpler to useFacilitate increased patient complianceDiagnostic recording/measuringSensorsAnalytePorous membrane
Devices and methods for utilizing dry chemistry dye indicator systems for body fluid analysis, such as glucose level provided by incorporating a porous membrane in a disposable patch. The devices also provide for microtitration of fluid samples in fixed volumetric openings containing indicator reagent. The devices provided are low cost due to efficient manufacturing methods provided.
Owner:ROCHE DIABETES CARE INC
Devices, systems and methods for extracting bodily fluid and monitoring an analyte therein
InactiveUS20040249254A1Easy to useLittle painWithdrawing sample devicesEvaluation of blood vesselsAnalyteElectronic communication
A system for extracting a bodily fluid sample (e.g., an interstitial fluid [ISF] sample) and monitoring an analyte therein includes a disposable cartridge and a local controller module. The disposable cartridge includes a sampling module adapted to extract a bodily fluid sample and an analysis module adapted to measure an analyte (e.g., glucose) in the bodily fluid sample. The local controller module is in electronic communication with the disposable cartridge and is adapted to receive and store measurement data from the analysis module. An ISF extraction device includes a penetration member configured for penetrating and residing in a target site of a user's skin layer and, subsequently, extracting an ISF sample therefrom. The device also includes a pressure ring(s) adapted for applying pressure to the user's skin layer in the vicinity of the target site. The device is configured such that the pressure ring(s) is capable of applying pressure in an oscillating manner whereby an ISF glucose lag of the ISF sample extracted by the penetration member is mitigated. A method for extracting ISF includes providing an ISF fluid extraction device with a penetration member and a pressure ring(s). Next, a user's skin layer is contacted by the pressure ring(s) and penetrated by the penetration member. An ISF sample is then extracted from the user's skin layer while pressure is being applied in an oscillating manner by the pressure ring(s). The oscillating pressure mitigates an ISF glucose lag of the extracted ISF sample.
Owner:LIFESCAN INC
Method and apparatus for enhancement of transdermal transport
InactiveUS20040236268A1Effective to induce immune responseCompounds screening/testingElectrotherapyPharmaceutical drugTGE VACCINE
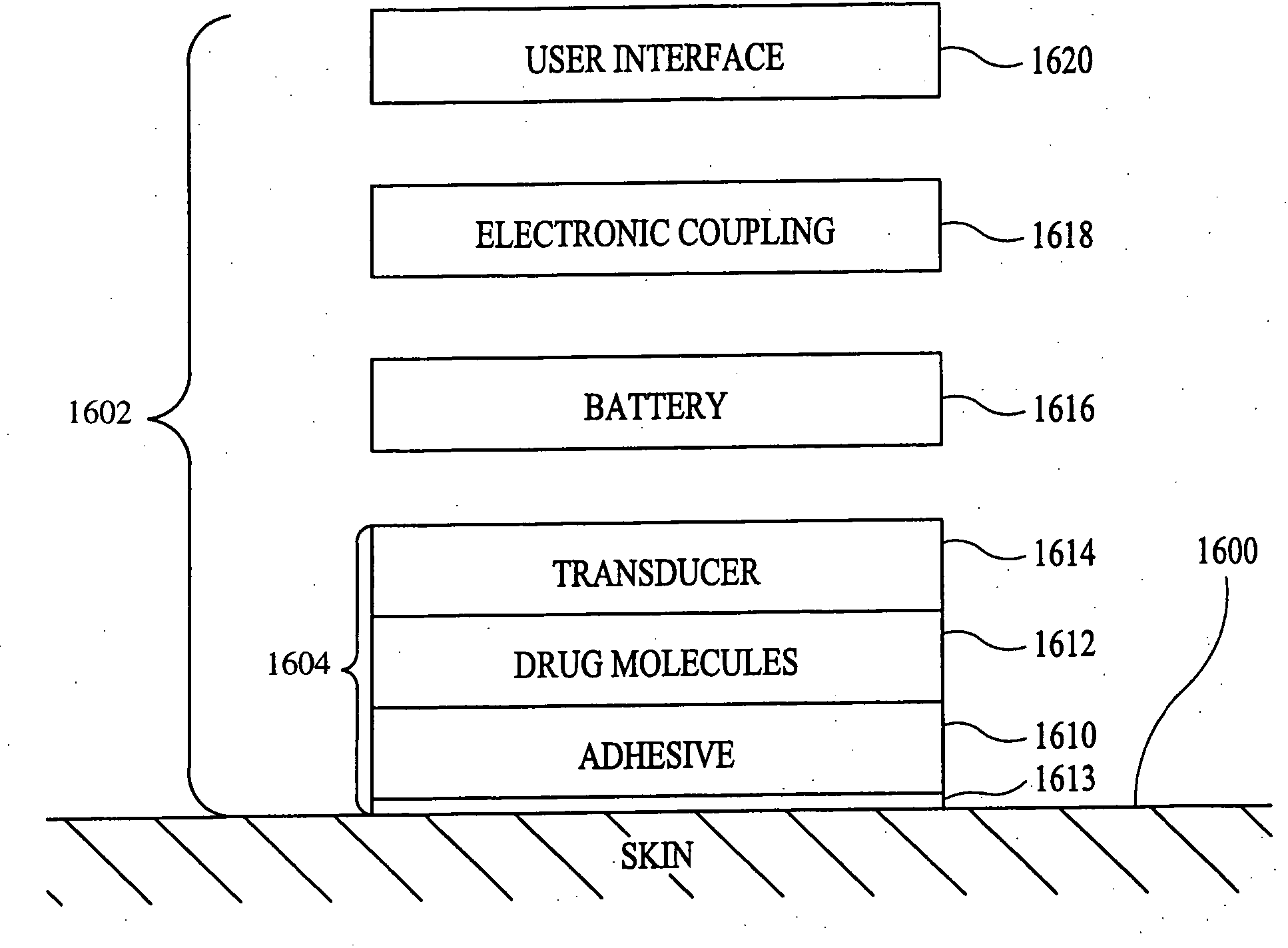
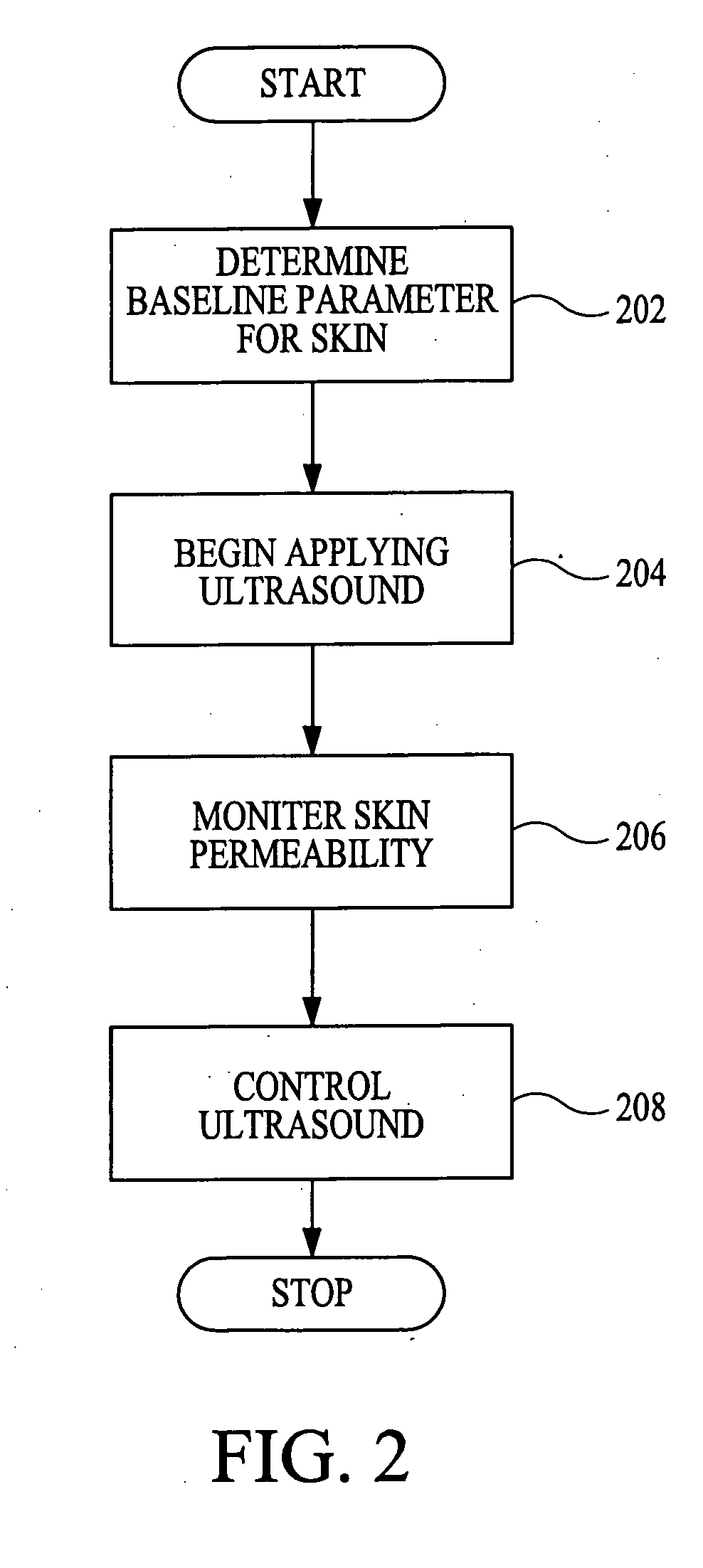
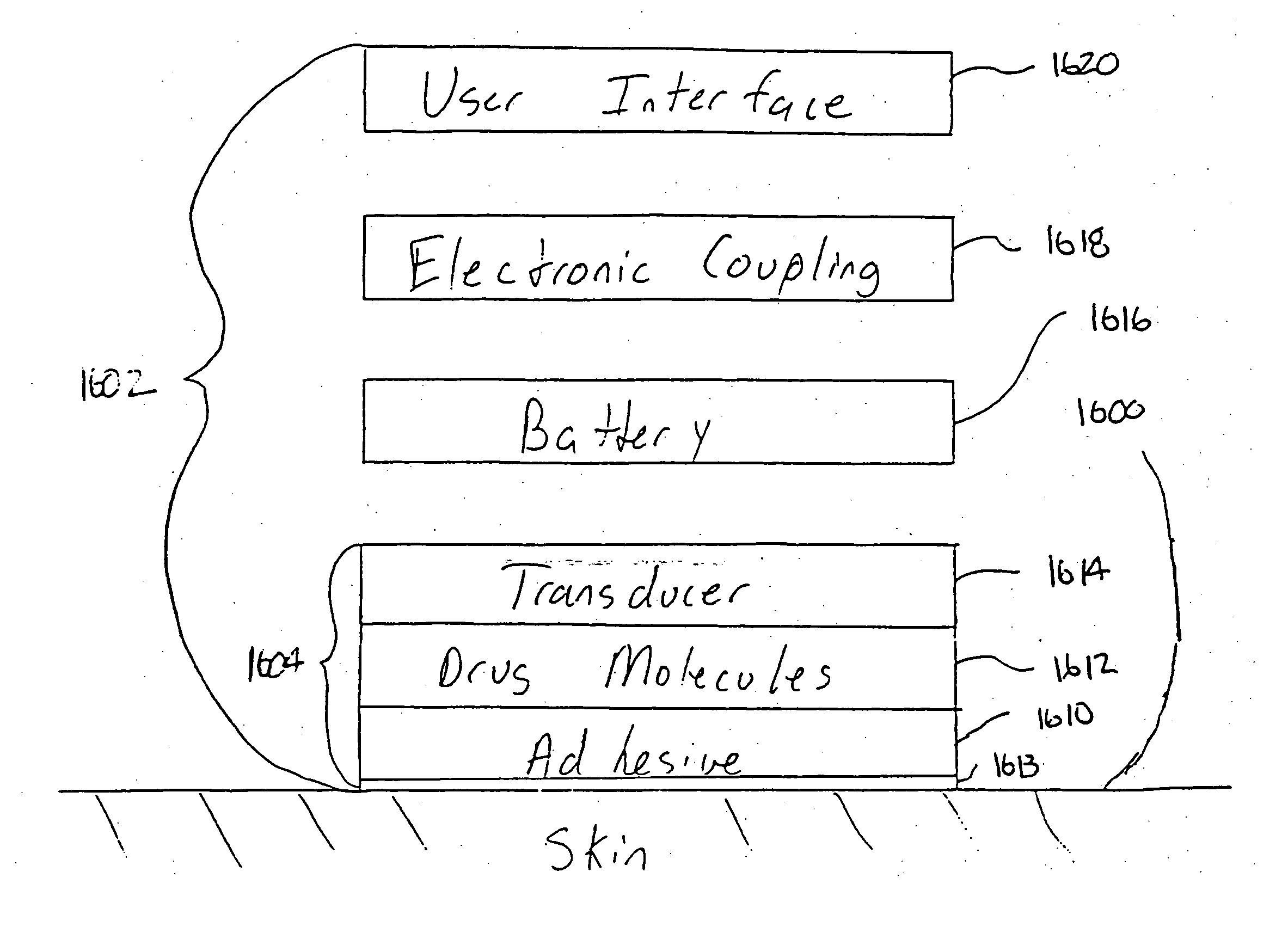
According to the present invention, a method for enhancing transdermal transport is disclosed. The method includes the steps of increasing a permeability of an area of a membrane with a permeabilizing device. The membrane may be, inter alia, biologic skin or synthetic skin. The permeabilizing device may be an ultrasound-producing device. A substance is transported into and through the area of the membrane. The substance may be a drug, a vaccine, or a component of interstitial fluid.
Owner:ECHO THERAPEUTICS INC
Combined lancet and electrochemical analyte-testing apparatus
InactiveUS20050011759A1Easy to takeReduces and eliminates disposal issueImmobilised enzymesBioreactor/fermenter combinationsTissue fluidDisplay device
An apparatus for detection and quantitation of an electrochemically-detectable analyte, such as glucose, in blood or interstitial fluid includes a meter unit, a lancet and an electrochemical sensor. Of these components, the meter is preferably reusable, while the lancet and the electrochemical sensor are preferably incorporated in assemblies intended for single-use. The meter unit has a housing, within which a lancet is engaged with a mechanism for moving then lancet; a connector disposed within the housing for engaging an electrochemical sensor specific for the analyte and transmitting a signal indicative of the amount of analyte, and a display operatively-associated with a connector for displaying the amount of the analyte to user. The electrochemical sensor is adapted for detection of a particular analyte. In addition, the electrochemical sensor has an absorptive member for uptake of a sample of blood or interstitial fluid. In one version, the lancet moves from a initial position to a piercing position in which skin of the user is pierced and optionally back to a retracted position. The electrochemical sensor is disposed such that the absorptive member takes up a sample from the pierced skin of the user when it is pierced by the lancet without movement of the apparatus. In an alternative version, the lancet is a hollow cannula through which blood or interstitial fluid is transported from the puncture site to an absorbent portion of the electrochemical sensor. In either version, the apparatus provides single-step operation in which sample acquisition and analysis occur as a result of the single action of pressing, the apparatus against the users skin.
Owner:LIFESCAN IP HLDG LLC
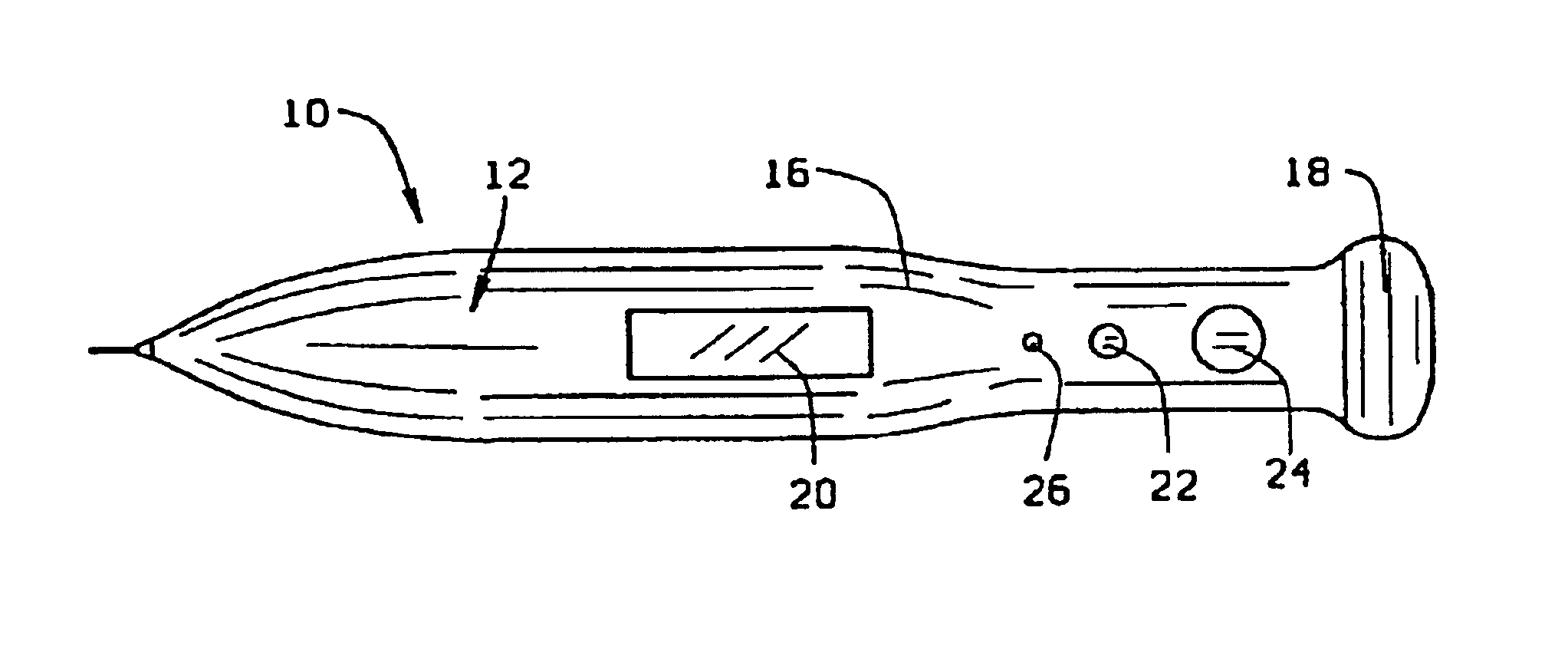
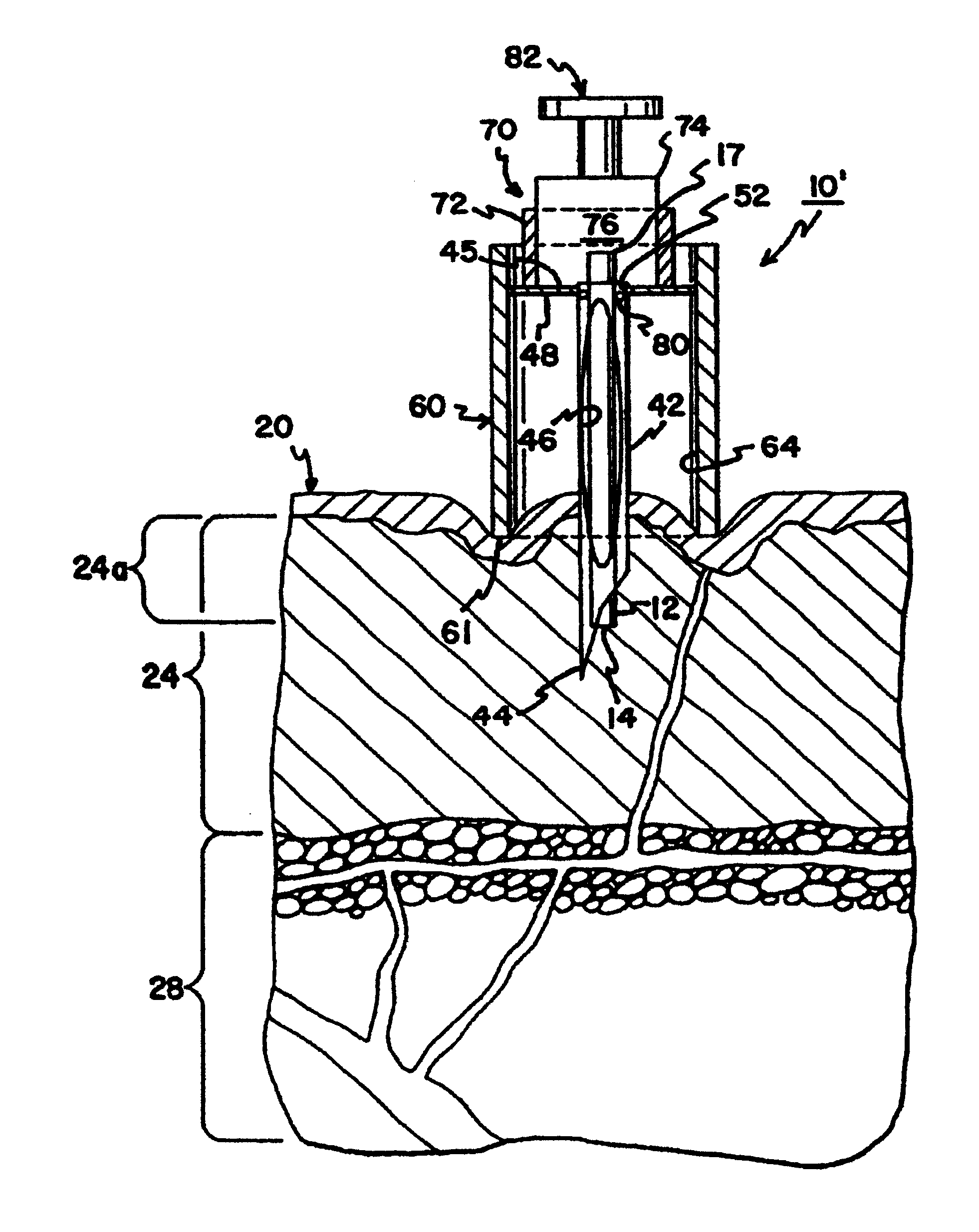
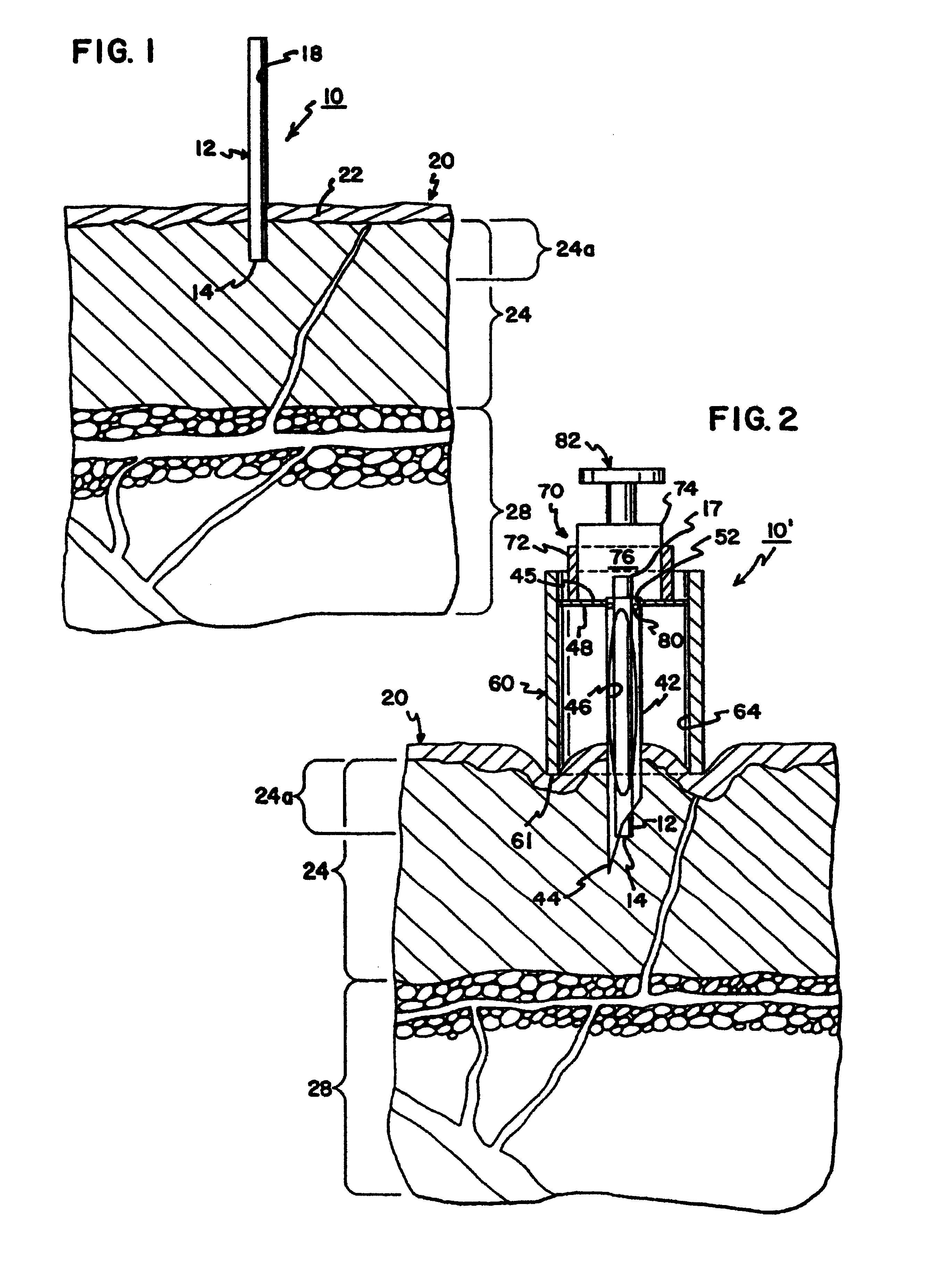
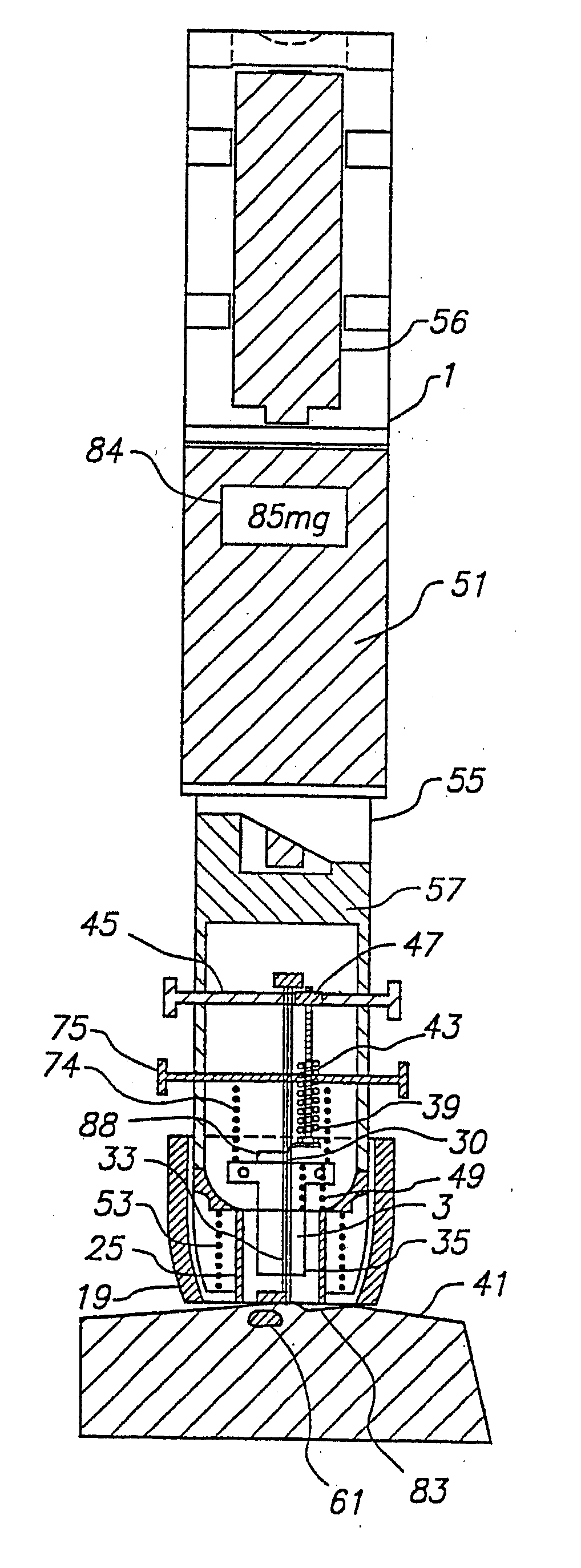
Blood and interstitial fluid sampling device
InactiveUS20050010134A1Improve overall senseIncrease sampling volumeSurgeryVaccination/ovulation diagnosticsBlood collectionElectricity
A device and method for lancing a patient, virtually simultaneously producing and collecting a small fluid sample from a body. The device comprises a blood collection system including a lancing needle (16), drive mechanism (11), kneading or vibration mechanism (25), optional suction system (7), and sample ejection mechanism. The device is preferably sized to be hand-held in one hand and operable with one hand. The device can optionally contain integral testing or analysis component (83) for receiving the sample and providing testing or analysis indication or readout for the user. A method involves piercing the skin at a rapid rate, kneading the surrounding area by ultrasonic action, piezoelectric or mechanical oscillation to stimulate the blood flow from the wound, drawing the fluid using a pumping system.
Owner:ROCHE DIAGNOSTICS OPERATIONS INC
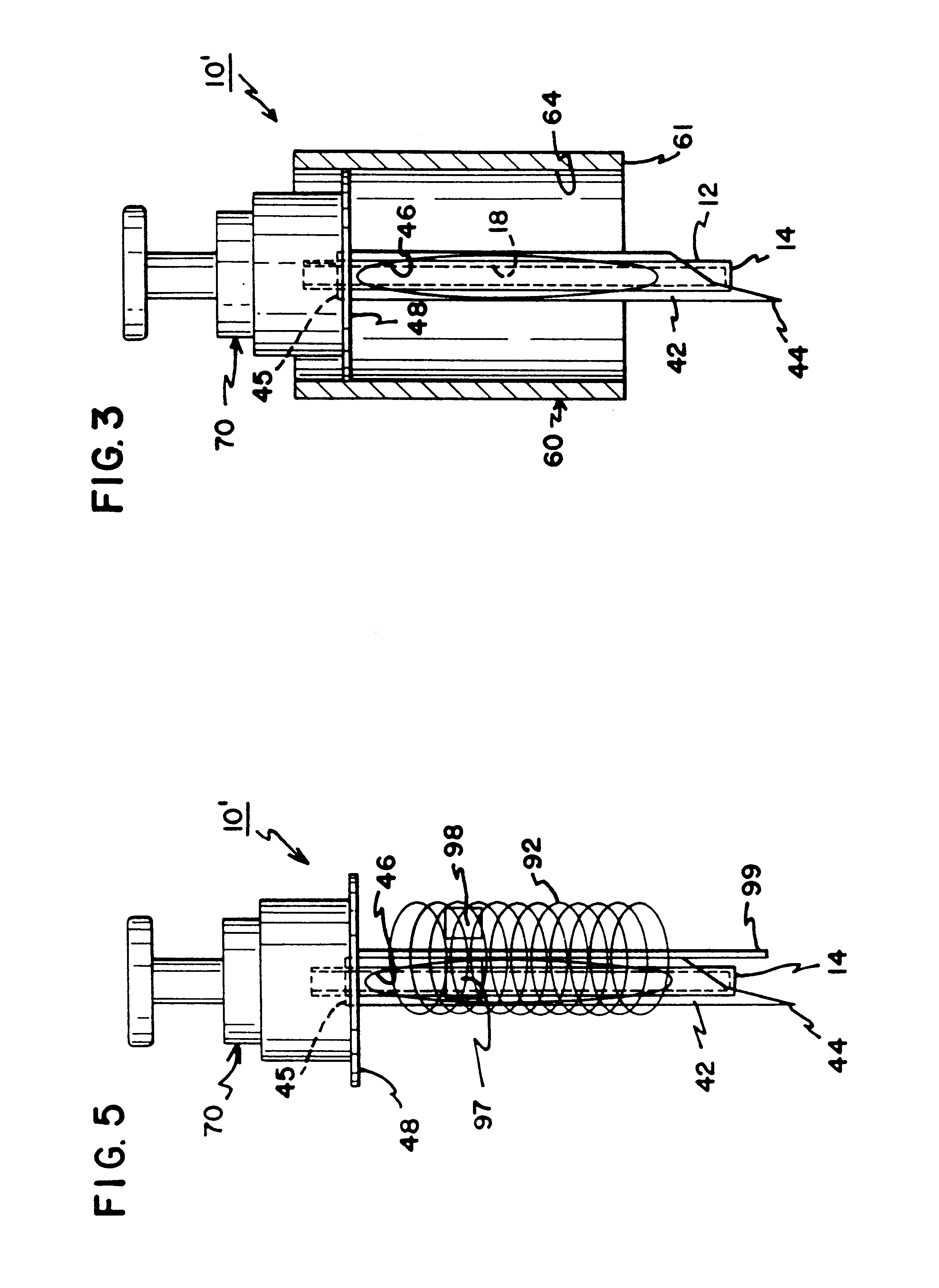
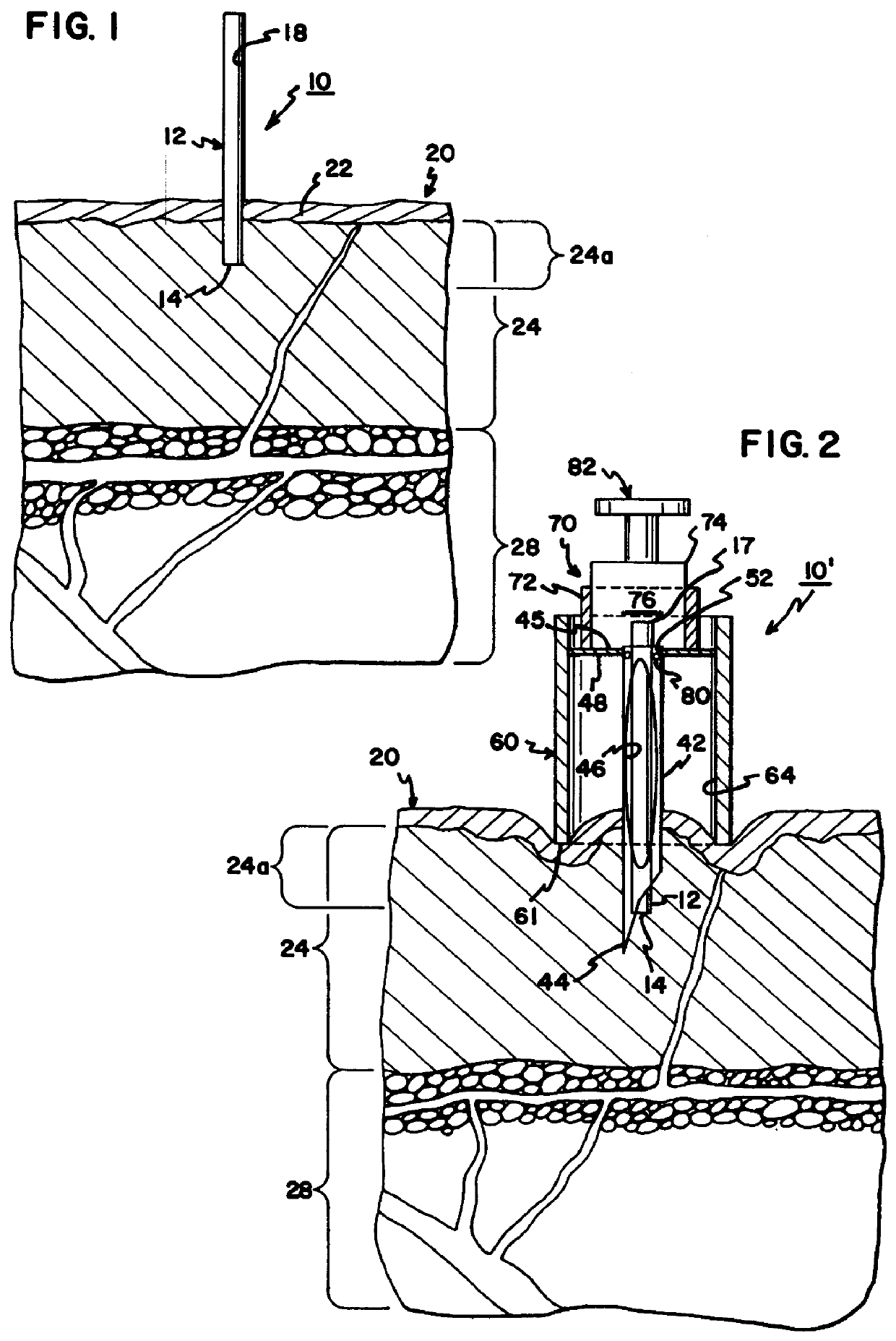
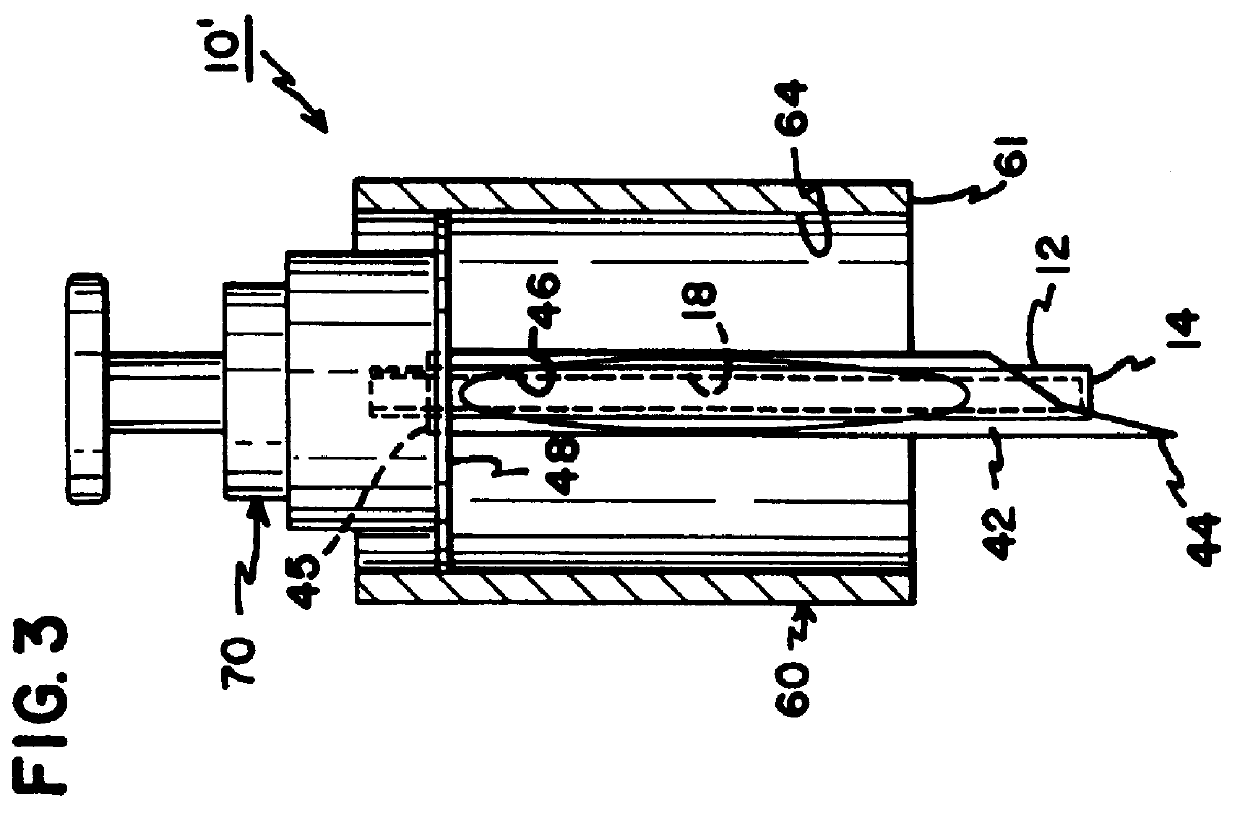
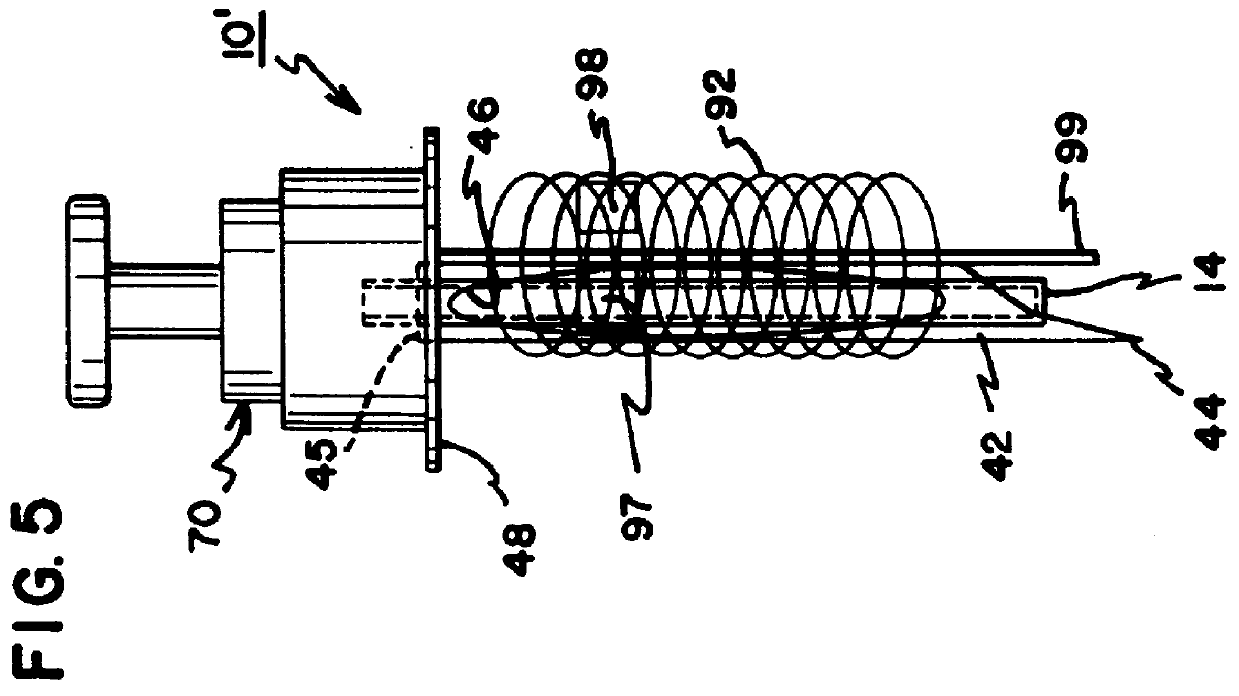
Interstitial fluid collection and constituent measurement
An apparatus and method is disclosed for obtaining and measuring constituents in a sample of body fluid. The apparatus includes a member which is sized to penetrate into at least the dermal layer of skin to collect a sample of body fluid located within the dermal layer.
Owner:INTEG
Method and apparatus for enhancement of transdermal transport
InactiveUS20040171980A1Effective to induce immune responseCompounds screening/testingElectrotherapyBiomedical engineeringMembrane configuration
According to the present invention, a method for enhancing transdermal transport is disclosed. The method includes the steps of increasing a permeability of an area of a membrane with a permeabilizing device. The membrane may be, inter alia, biologic skin or synthetic skin. The permeabilizing device may be an ultrasound-producing device. A substance is transported into and through the area of the membrane. The substance may be a drug, a vaccine, or a component of interstitial fluid.
Owner:SONTRA MEDICAL
Method and device for sampling and analyzing interstitial fluid and whole blood samples
InactiveUS20050010137A1Less riskLess invasiveSurgeryVaccination/ovulation diagnosticsAnalyteGlucose polymers
The invention disclosed in this application is a method and device for combining the sampling and analyzing of sub-dermal fluid samples, e.g., interstitial fluid or whole blood, in a device suitable for hospital bedside and home use. It is applicable to any analyte that exists in a usefully representative concentration in the fluid, and is especially suited to the monitoring of glucose.
Owner:HODGES ALASTAIR +2
Method and kit for the transdermal determination of analyte concentration in blood
InactiveUS7004901B2Efficient extractionTesting is superfluousDiagnostic recording/measuringSensorsAnalyteMedicine
A method is provided for determining the level of an analyte in the blood of an individual by measuring the level of the analyte in an interstitial fluid or in any other non blood fluid which does not contain red blood cells and adjusting the measurement value by the concentration of at least one reference analyte.
Owner:FISH FALK
Method of preventing short sampling of a capillary or wicking fill device
InactiveUS7043821B2Line/current collector detailsElectrical transducersMedicineBiomedical engineering
Owner:LIFESCAN INC
Method and device for sampling and analyzing interstitial fluid and whole blood samples
The invention disclosed in this application is a method and device for combining the sampling and analyzing of sub-dermal fluid samples, e.g., interstitial fluid or whole blood, in a device suitable for hospital bedside and home use. It is applicable to any analyte that exists in a usefully representative concentration in the fluid, and is especially suited to the monitoring of glucose.
Owner:LIFESCAN INC
Body fluid sampler
A sampling apparatus for sampling interstitial fluid includes a sampler having an external geometry selected to mate with an internal geometry of a testing apparatus such that the sampler may be inserted within the testing apparatus in a predetermined alignment and with a sampling location positioned accurately within a light path for detecting an amount of a desired constituent collected by the sampler.
Owner:INTEG
Surface-modified wick for diagnostic test strip
InactiveUS6967105B2Improve accuracyGood precisionAnalysis using chemical indicatorsMaterial analysis by observing effect on chemical indicatorAlkaneBlood sugar
A wicking material is disclosed that exhibits a horizontal wicking velocity of at least about 1.0 millimeter per second when contacted with a physiological fluid such as blood, lymph or cellular interstitial fluid. This high wicking rate is achieved by means of treatment of a fibrous wicking material candidate with a low temperature gas plasma, particularly a glow discharge gas plasma formed in a gaseous blend made up predominantly of a mixture of oxygen with a saturated alkane chosen from the group consisting of methane, ethane and propane. Diagnostic test strips made with the surface-modified wicking material, and containing an immobilized reagent means for analysis of an analyte in a physiological fluid, show improved performance in terms of increased accuracy, finer precision of analyses, reduced time of analysis, a smaller fluid sample size requirement, and improved compliance with manufacturing standards resulting in lower manufacturing costs blood sugar determinations.
Owner:QUESTSTAR MEDICAL
Blood and interstitial fluid sampling device
InactiveUS20070093728A1Improve overall senseIncrease sampling volumeCatheterDiagnostic recording/measuringBlood collectionElectricity
A device and method for lancing a patient, virtually simultaneously producing and collecting a small fluid sample from a body. The device comprises a blood collection system including a lancing needle (16), drive mechanism (11), kneading or vibration mechanism (25), optional suction system (7), and sample ejection mechanism. The device is preferably sized to be hand-held in one hand and operable with one hand. The device can optionally contain integral testing or analysis component (83) for receiving the sample and providing testing or analysis indication or readout for the user. A method involves piercing the skin at a rapid rate, kneading the surrounding area by ultrasonic action, piezoelectric or mechanical oscillation to stimulate the blood flow from the wound, drawing the fluid using a pumping system.
Owner:ROCHE DIABETES CARE INC
Devices for physiological fluid sampling and methods of using the same
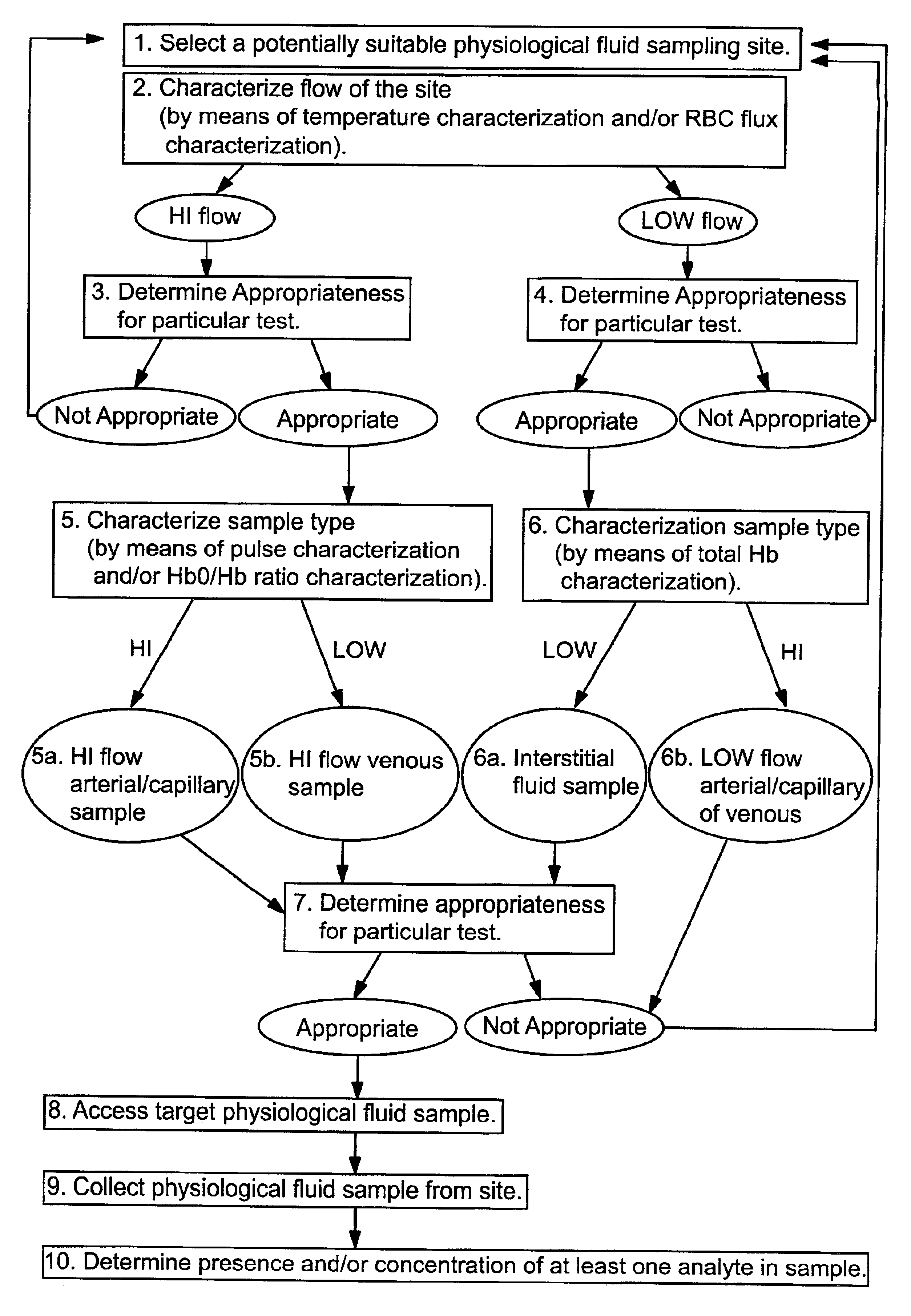
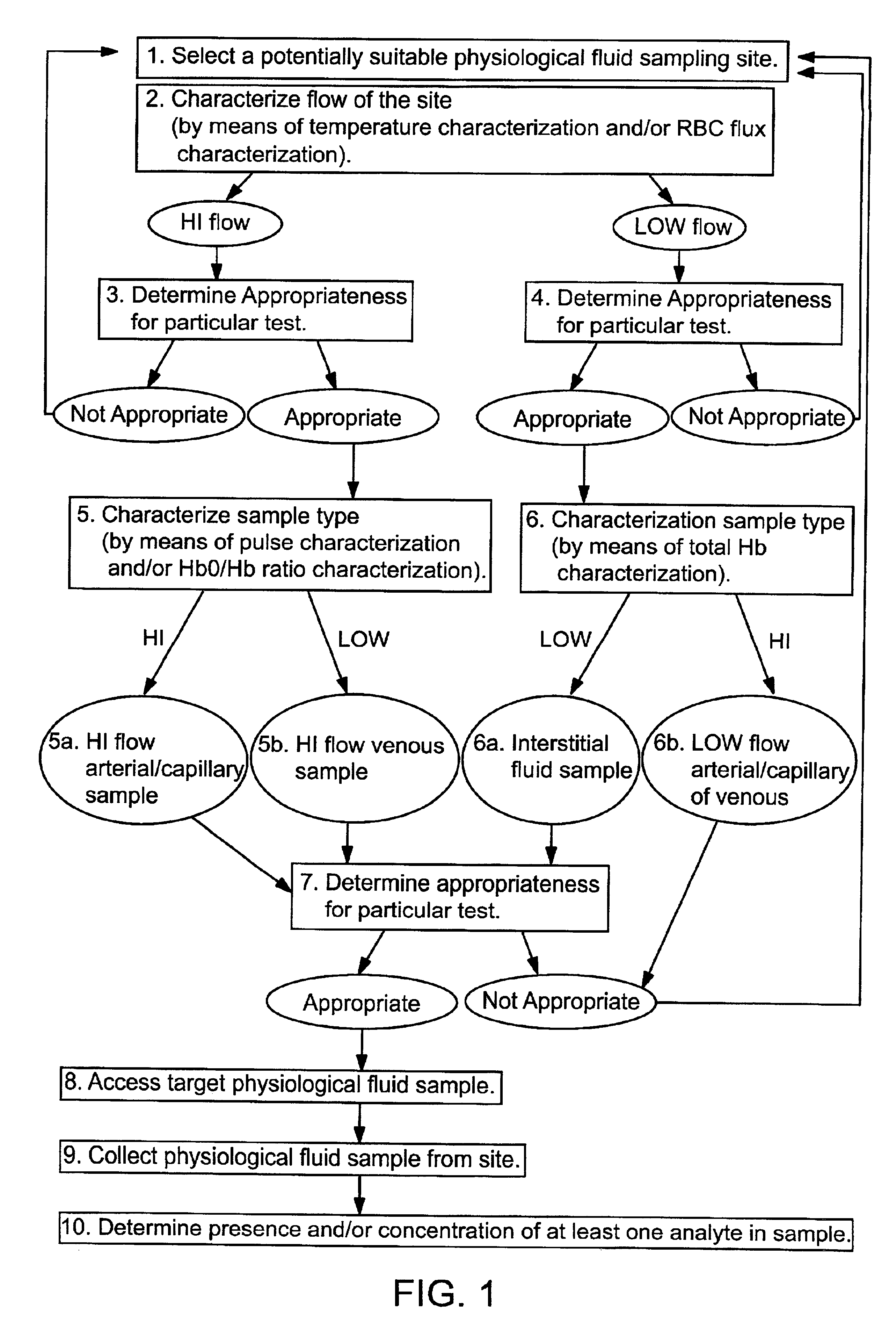
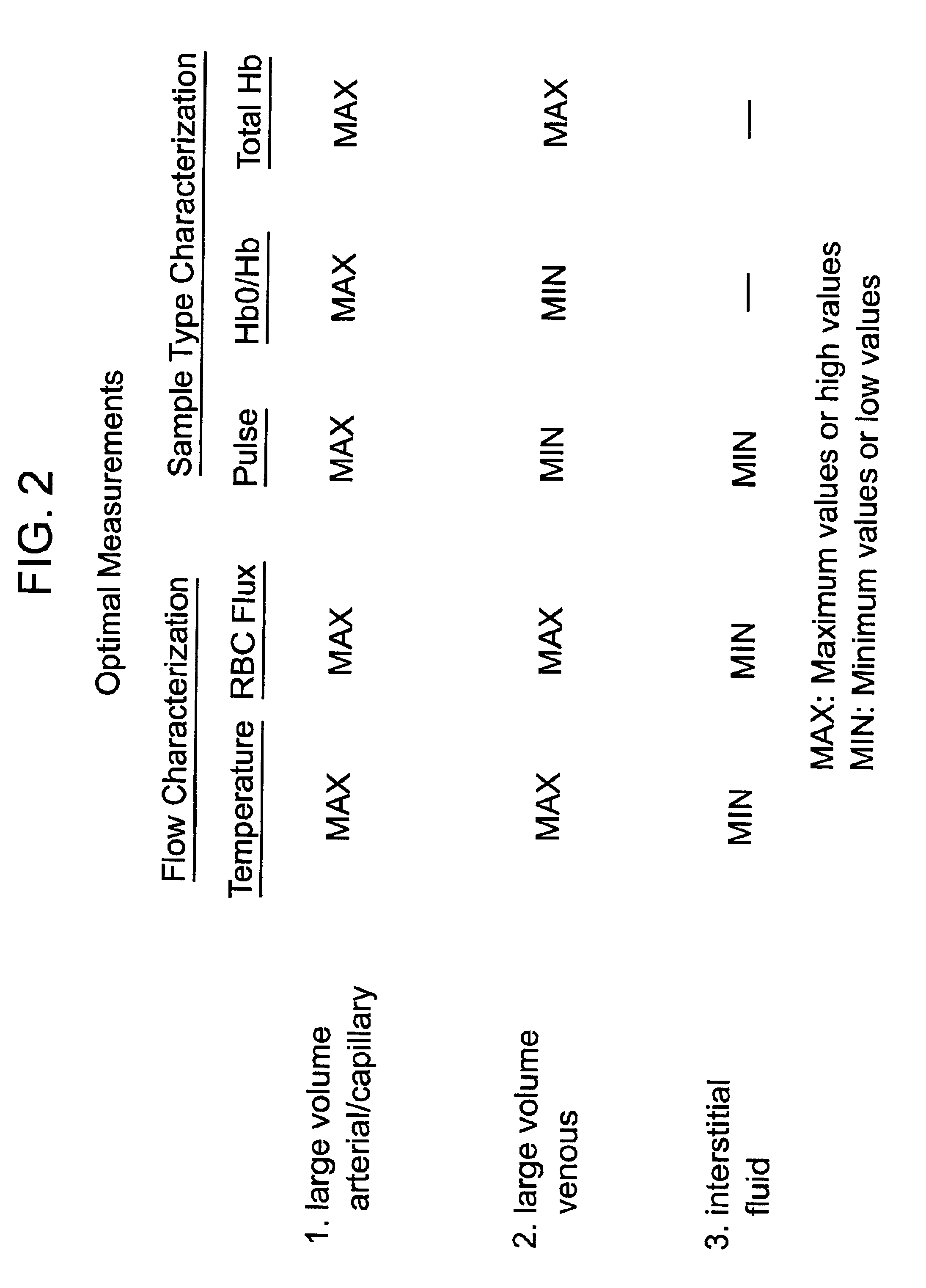
Methods and devices are provided for determining a suitable site for sampling physiological fluid. In the subject methods, a potentially suitable physiological sampling site is selected, the fluid flow of the site is characterized and the site is then determined to be suitable based on the whether the site has high or low flow. Suitability may also be determined based on the type of sample obtainable from the site, where the order of the above-described steps may be altered. The subject devices include at least one site flow characterization element for determining the flow characteristics of a potential physiological sampling site and / or at least one sample type characterization element for determining whether the vasculature is arterial, venous or neither, i.e., an interstitial fluid sampling site. The subject methods and devices are particularly suited for use in the detection of physiological sampling sites in the fingers, arms, legs, earlobes, heels, feet, nose and toes. Also provided are kits that include the subject devices for use in practicing the subject methods.
Owner:LIFESCAN IP HLDG LLC
Systems and methods for collecting fluid from a subject
ActiveUS20120277697A1Small sizeEasy to handleMicroneedlesMedical devicesInterstitial fluidIntensive care medicine
Systems and methods for delivering to and / or receiving fluids or other materials, such as blood or interstitial fluid, from subjects, e.g., from the skin. Beading disruptors and / or capillaries may be used for facilitating the transport of fluids from a subject into a device. Beading disruptors may disrupt the “pooling” of bodily fluids such as blood on the surface of the skin and help influence flow in a desired way. A capillary may conduct flow of fluid in the device, e.g., to an inlet of a channel or other flow path that leads to a storage chamber. A vacuum (reduced pressure relative to ambient) may be used to receive fluid into the device, e.g., by using relatively low pressure to draw fluid into the inlet of a channel leading to a storage chamber. The vacuum source may be part of the device.
Owner:YOURBIO HEALTH INC
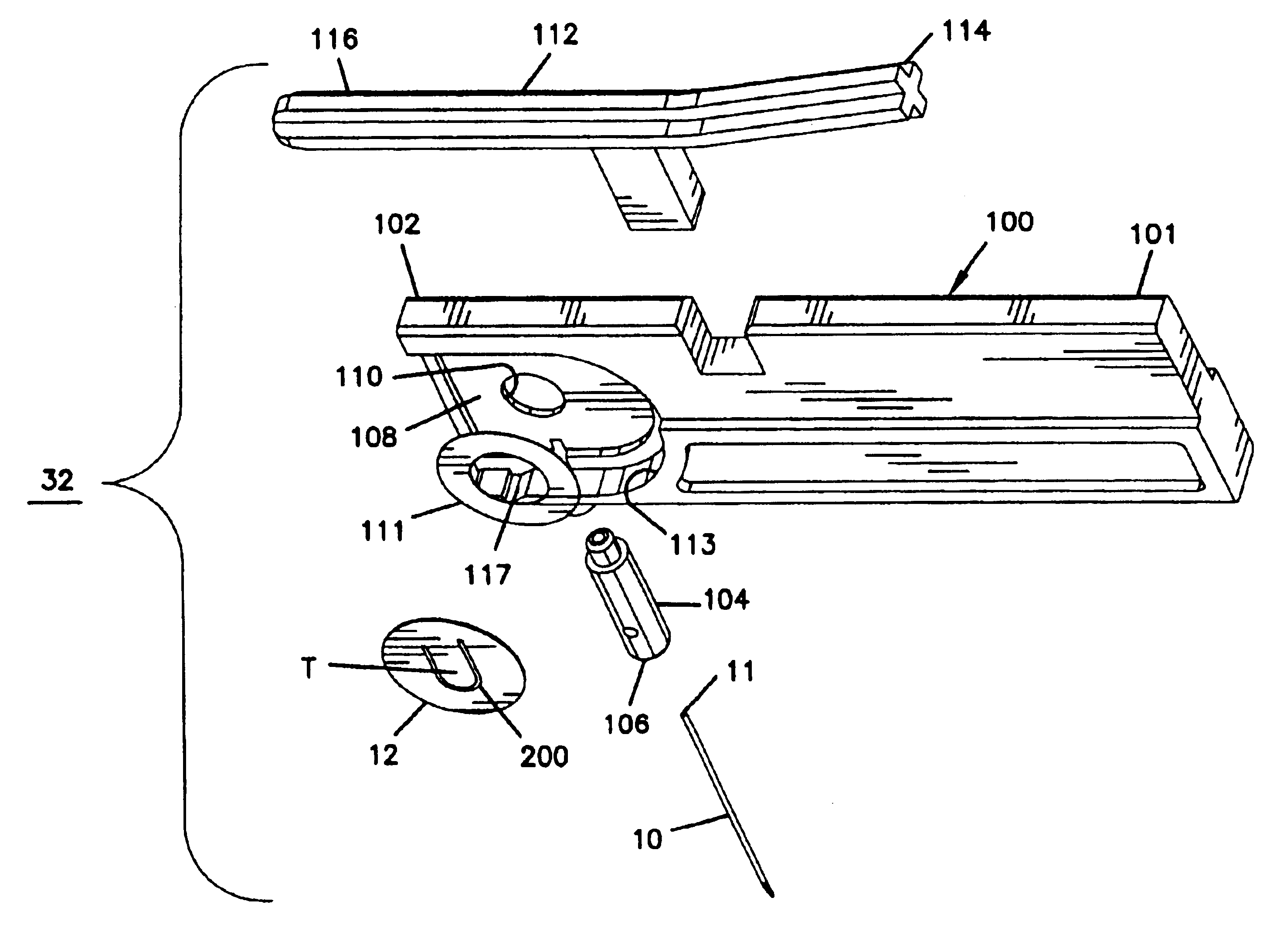
Analyte measuring system which prevents the reuse of a test strip
InactiveUS20050284757A1Microbiological testing/measurementLaboratory glasswaresAnalytePhysiological fluid
The present invention may be used in test strips for measuring an analyte or indicator such as glucose in a physiological fluid such as blood, interstitial fluid, or urine. The present invention also relates to test strips incorporating an integrated lance such as a needle, blade, or other sharp or skin puncturing device. In particular, in one embodiment of the present invention, a fused link is incorporated into the test strip. The fused link may be destroyed once the test is completed, preventing reuse of the strip.
Owner:LIFESCAN INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com