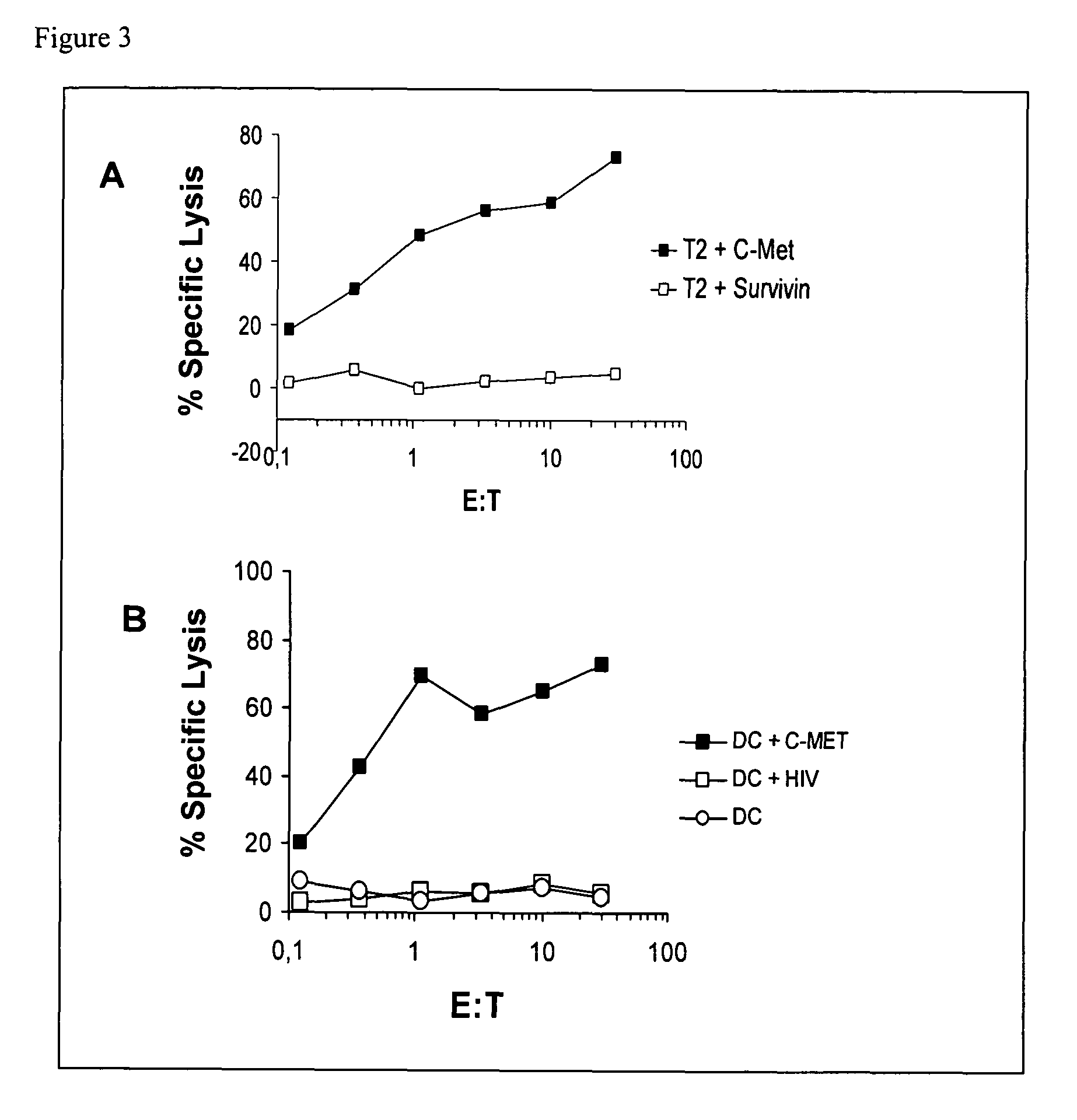
Patents
Literature
12298 results about "TGE VACCINE" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
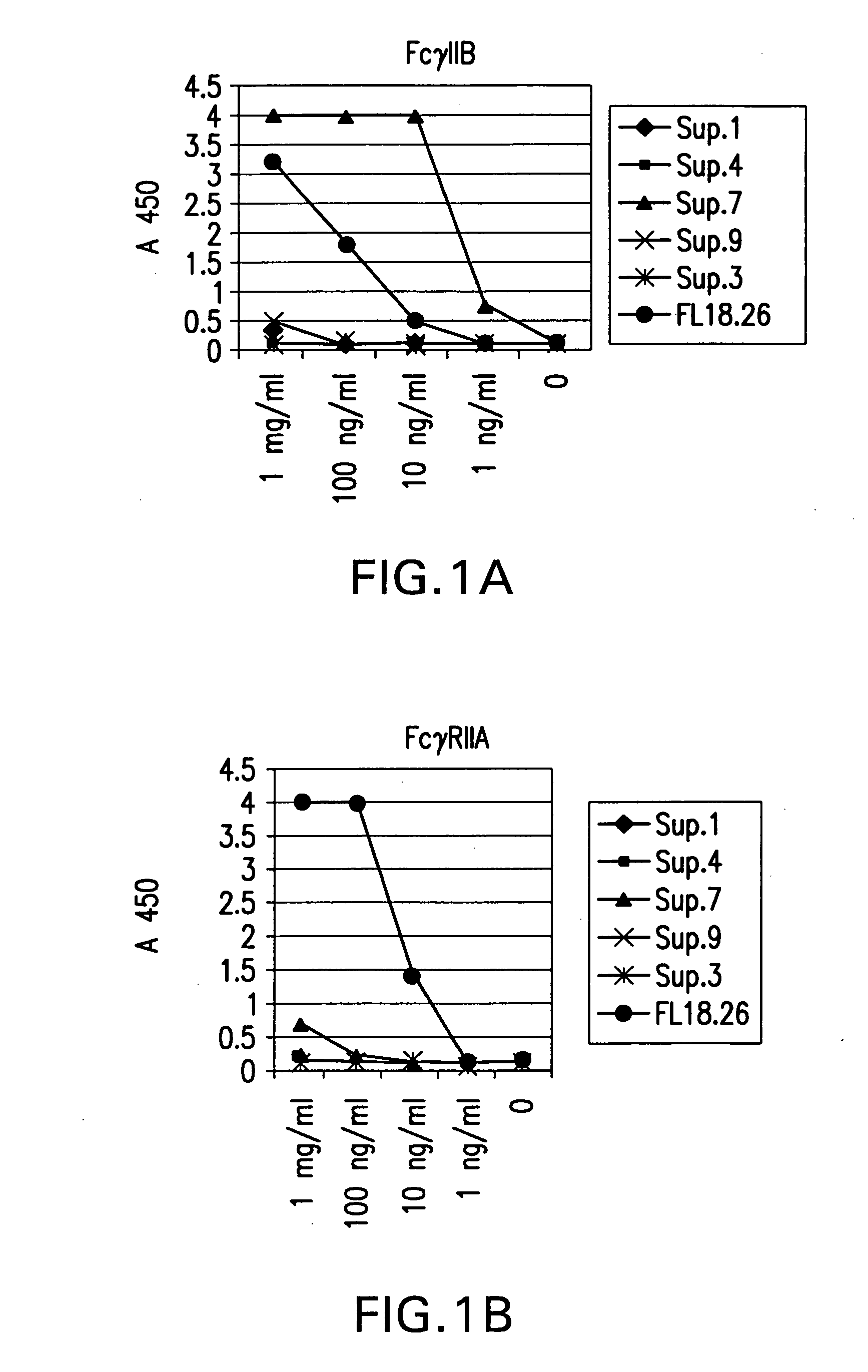
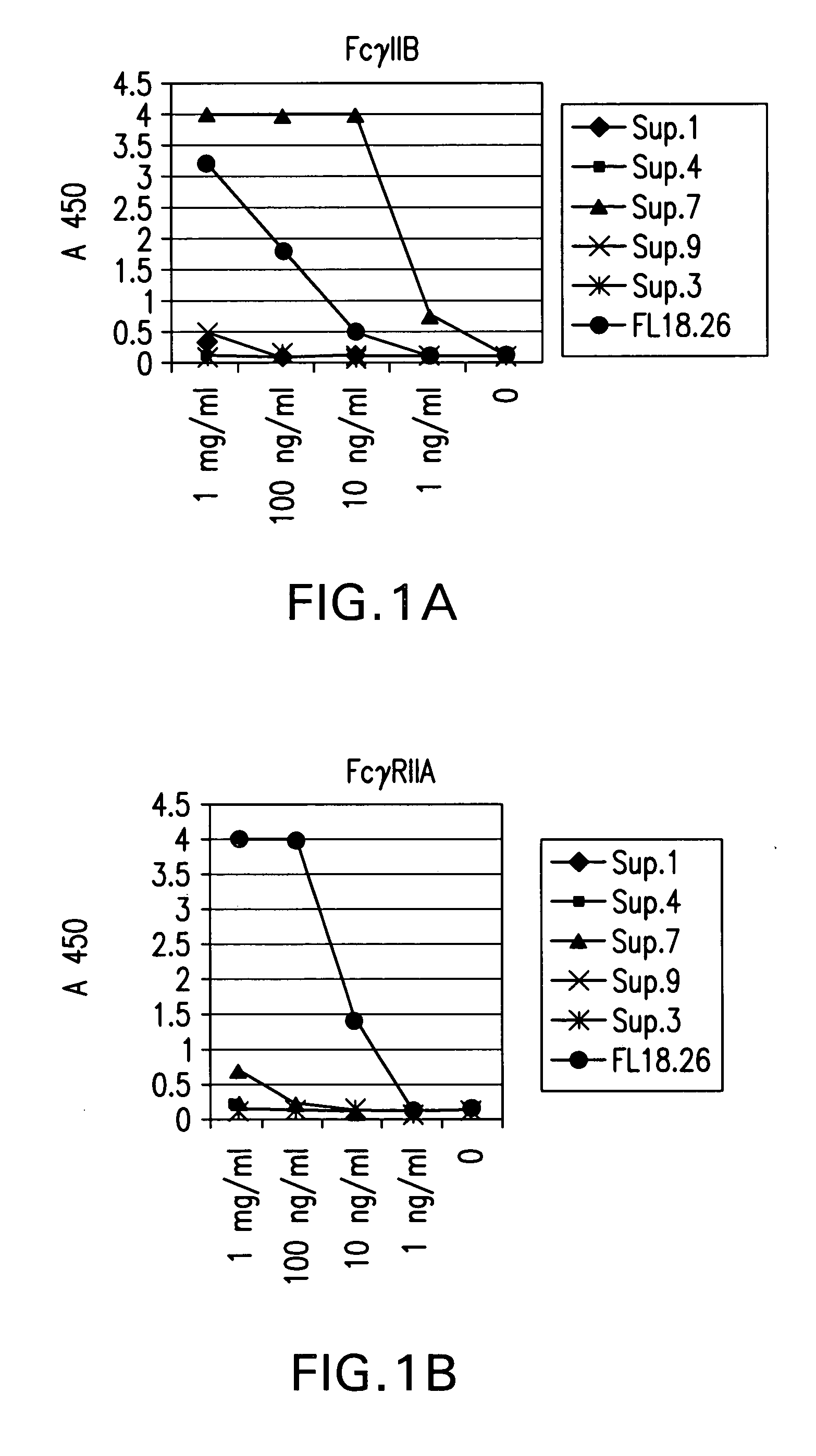
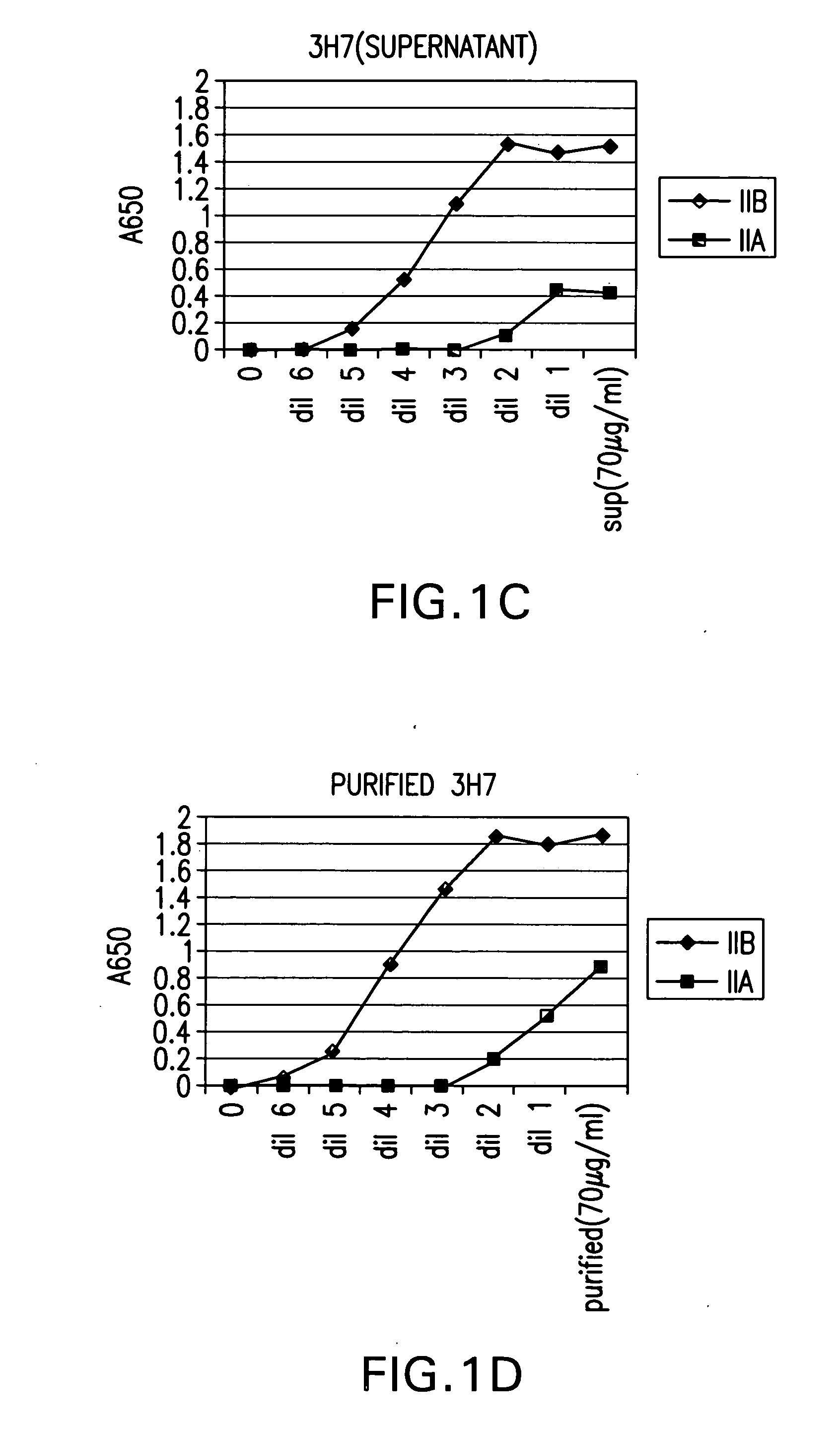
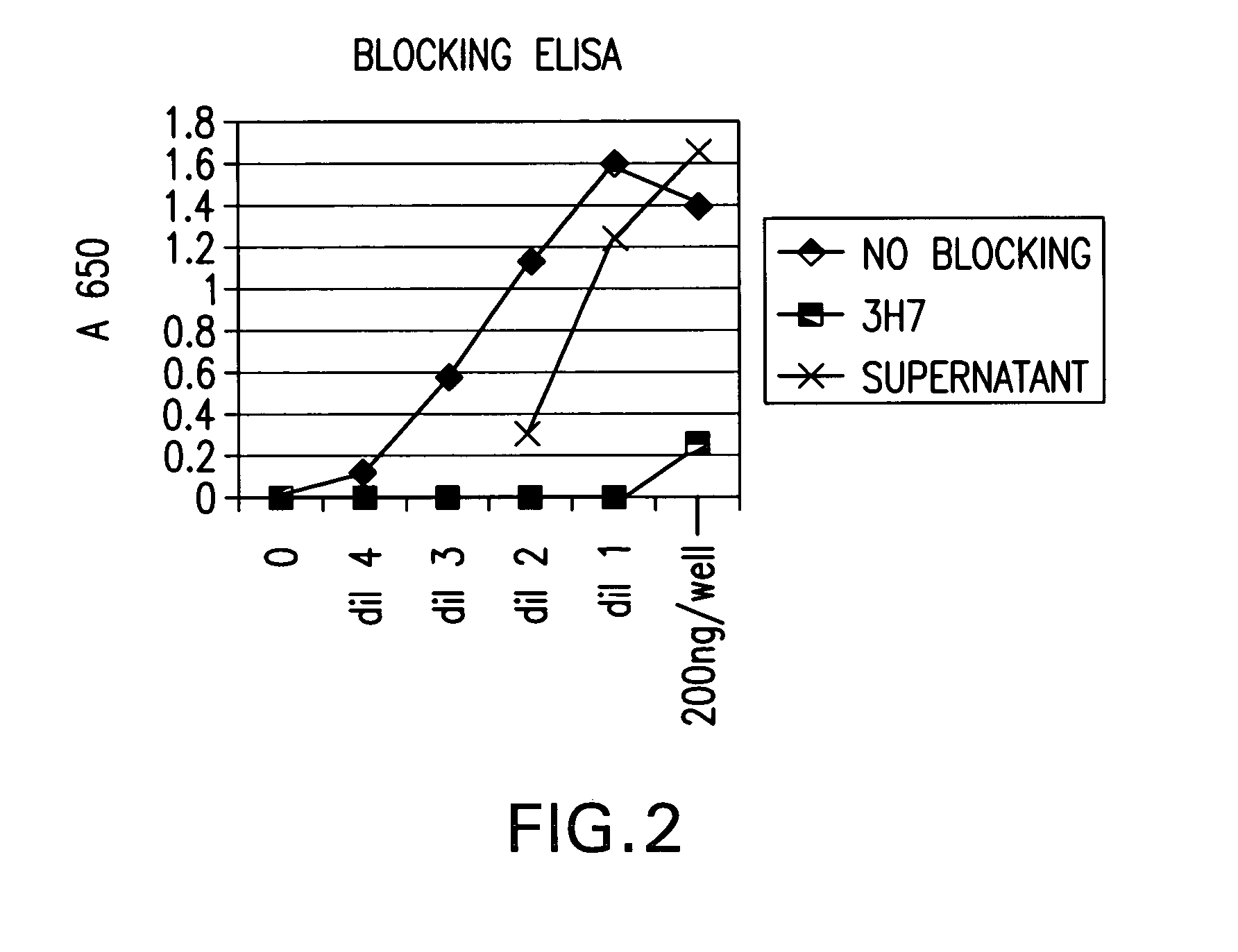
FcgammaRIIB-specific antibodies and methods of use thereof
ActiveUS20040185045A1Strong therapeutic activityEnhancing antibody-mediated effector functionSenses disorderAntipyreticTherapeutic antibodyTreatment effect
The present invention relates to antibodies or fragments thereof that specifically bind FcgammaRIIB, particularly human FcgammaRIIB, with greater affinity than said antibodies or fragments thereof bind FcgammaRIIA, particularly human FcgammaRIIA. The invention provides methods of enhancing the therapeutic effect of therapeutic antibodies by administering the antibodies of the invention to enhance the effector function of the therapeutic antibodies. The invention also provides methods of enhancing efficacy of a vaccine composition by administering the antibodies of the invention.
Owner:MACROGENICS INC
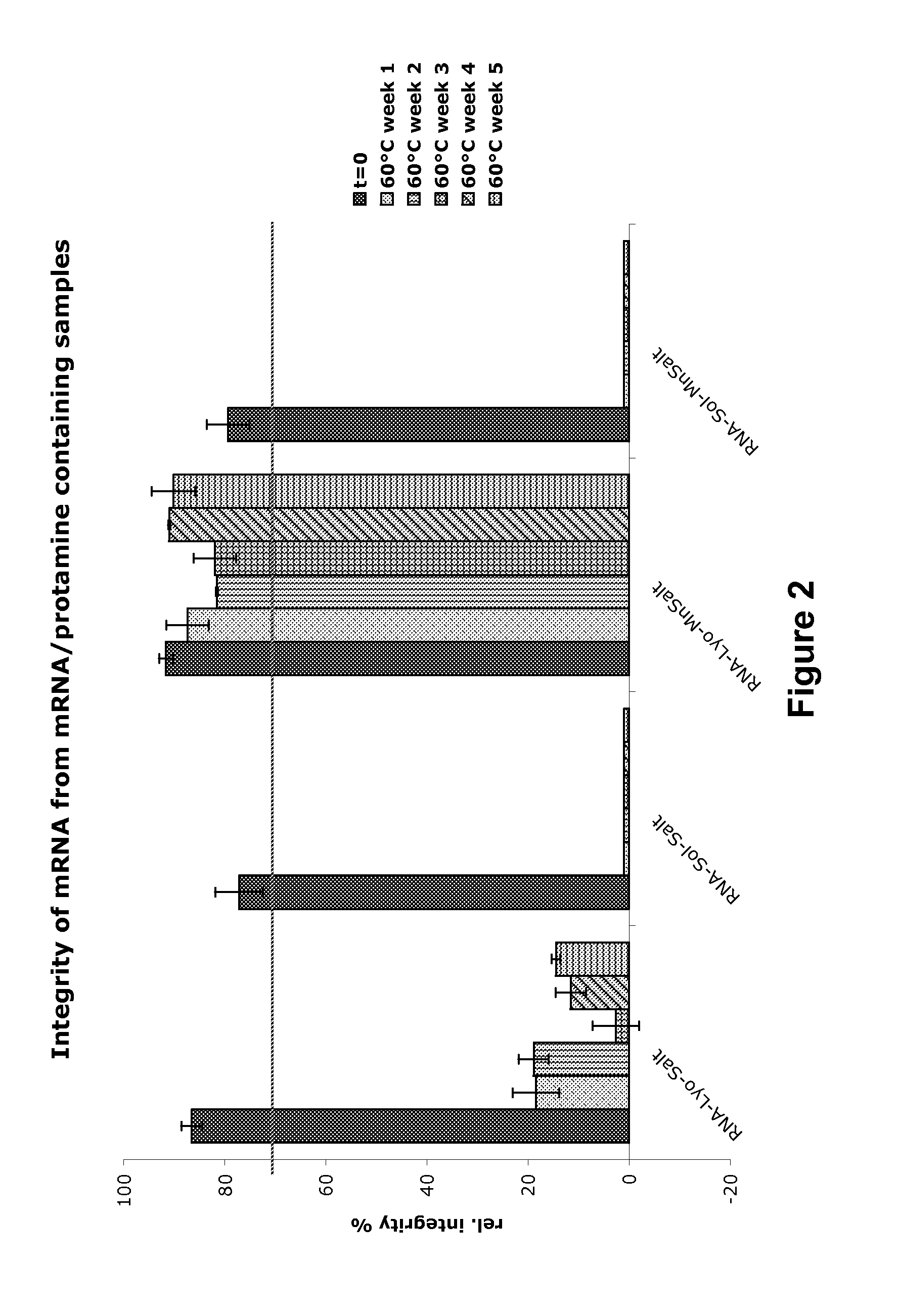
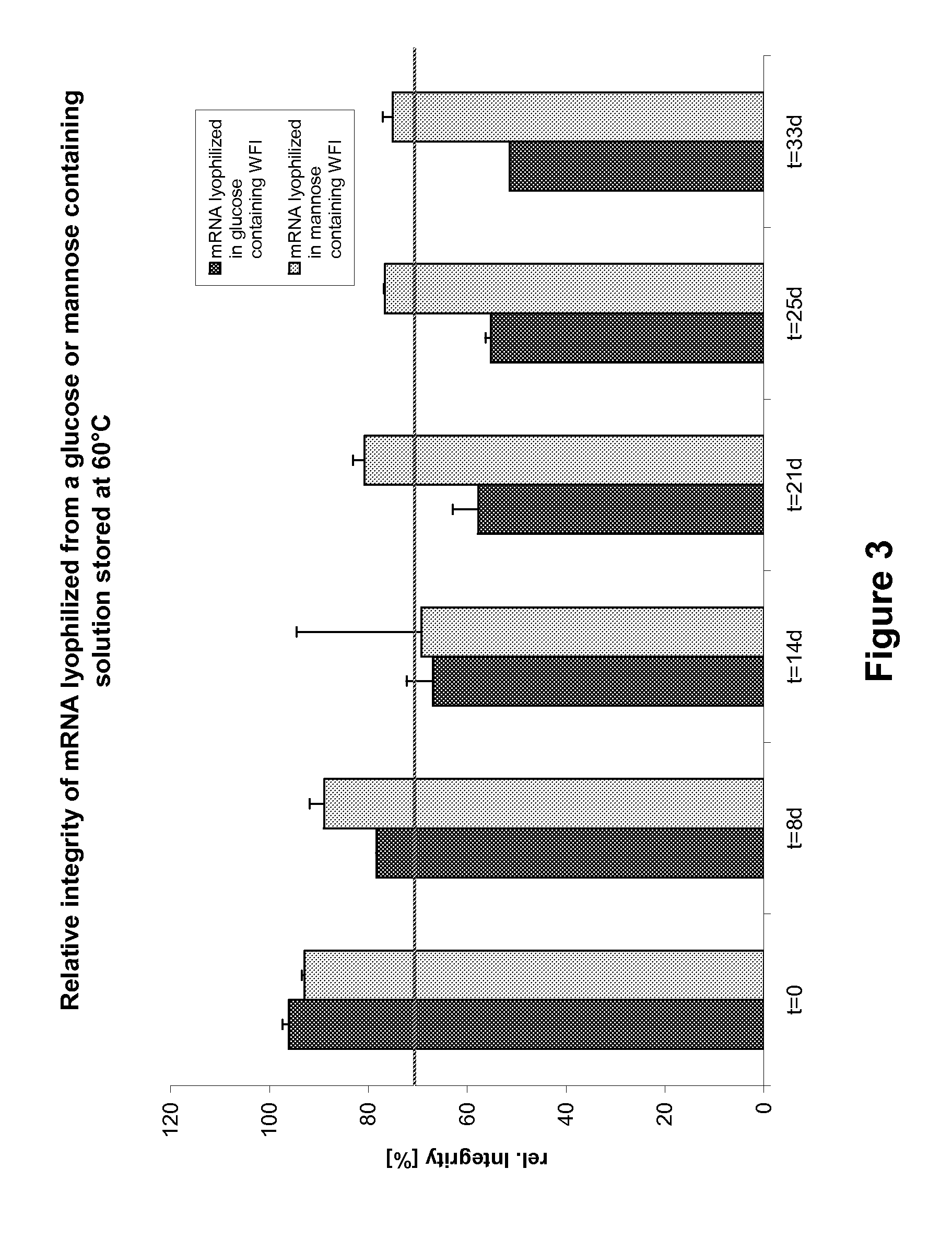
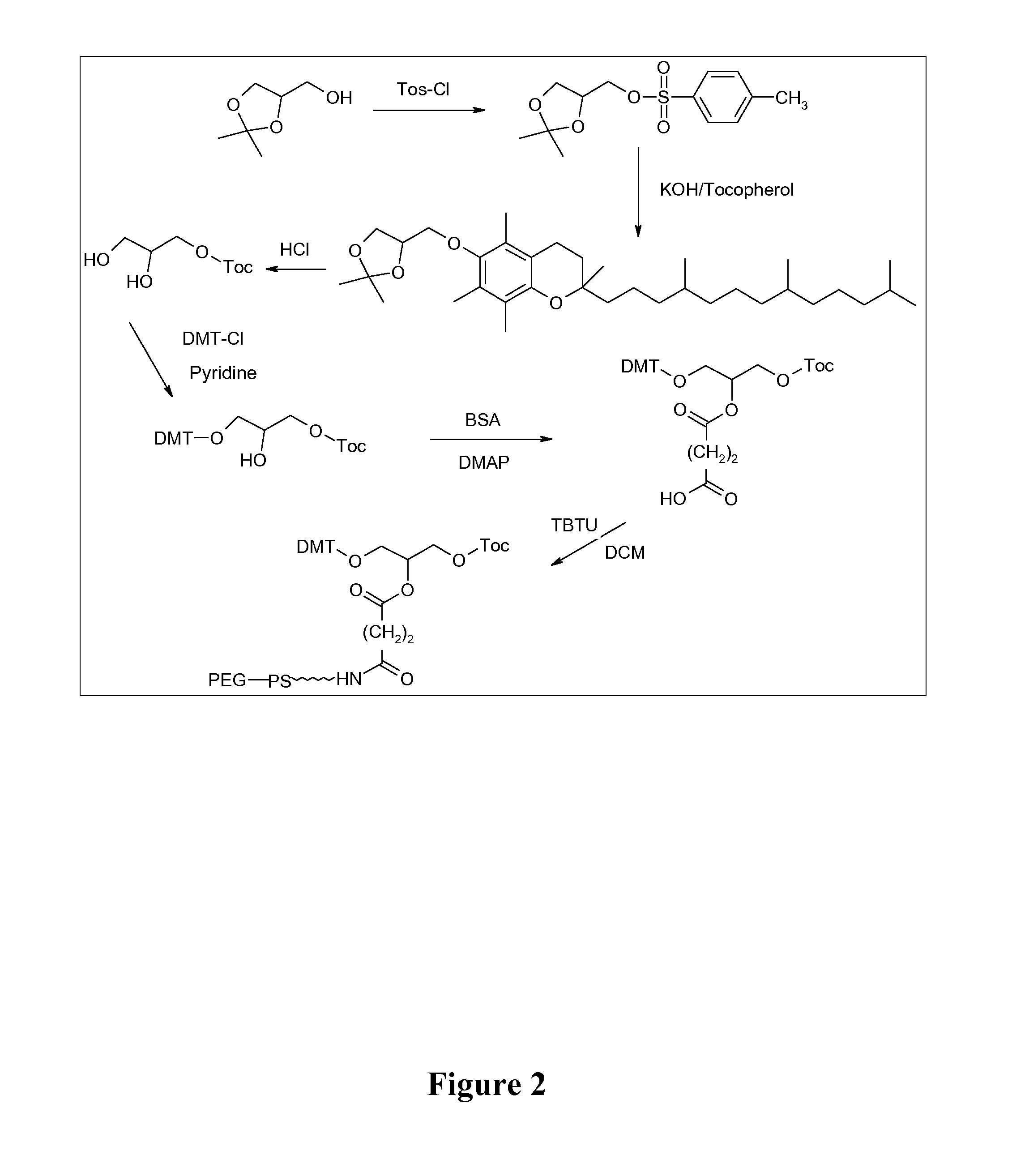
Mannose-containing solution for lyophilization, transfection and/or injection of nucleic acids
InactiveUS20120258046A1High transfection efficiencyEnhance protein expressionOrganic active ingredientsGenetic material ingredientsIn vivoTransfection
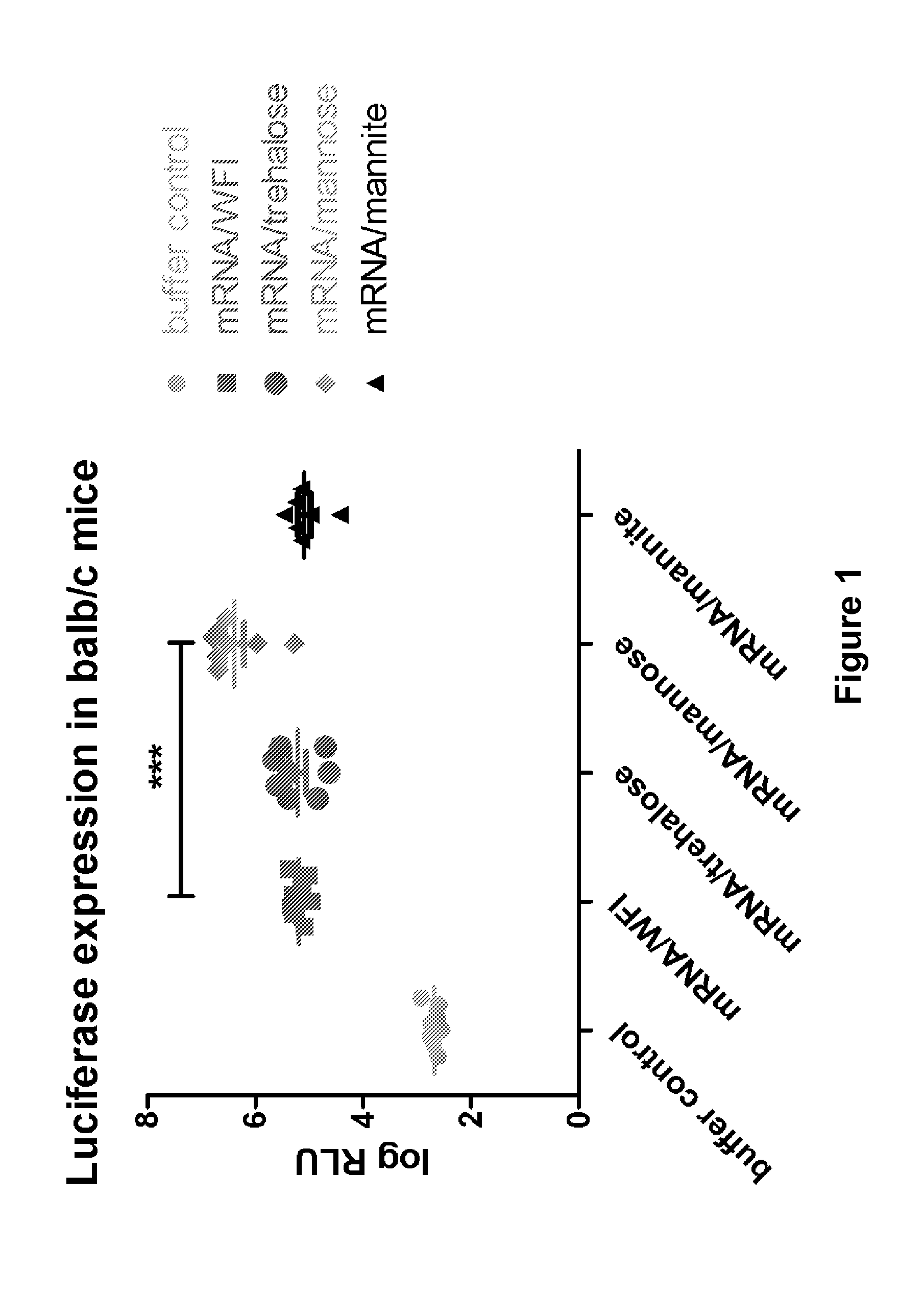
The present invention is directed to (the use of) a solution containing at least one nucleic acid (sequence) and free mannose for lyophilization, transfection and / or injection, particularly of RNA and mRNA. The inventive solution exhibits a positive effect on stabilization of the nucleic acid (sequence) during lyophilization and storage but also leads to a considerable increase of the transfection efficiency of a nucleic acid. It thus also increases in vivo expression of a protein encoded by such a nucleic acid upon increased transfection rate. The present invention is furthermore directed to a method of lyophilization using the mannose-containing solution, to pharmaceutical compositions, vaccines, kits, first and second medical uses applying such a mannose-containing solution and / or a nucleic acid (sequence) lyophilized or resuspended with such a solution.
Owner:CUREVAC SE
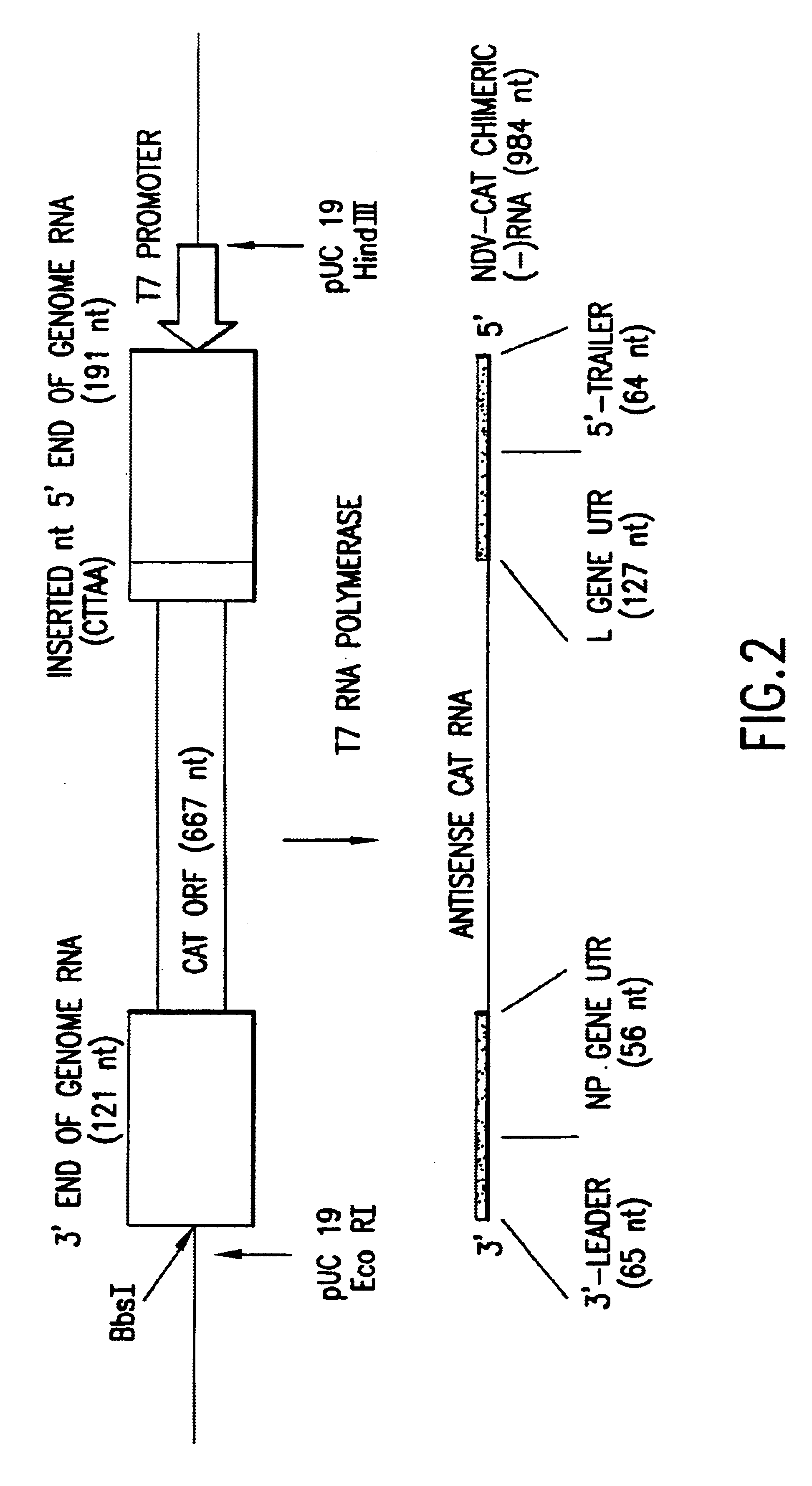
Helper-free rescue of recombinant negative strand RNA virus
Owner:MT SINAI SCHOOL OF MEDICINE
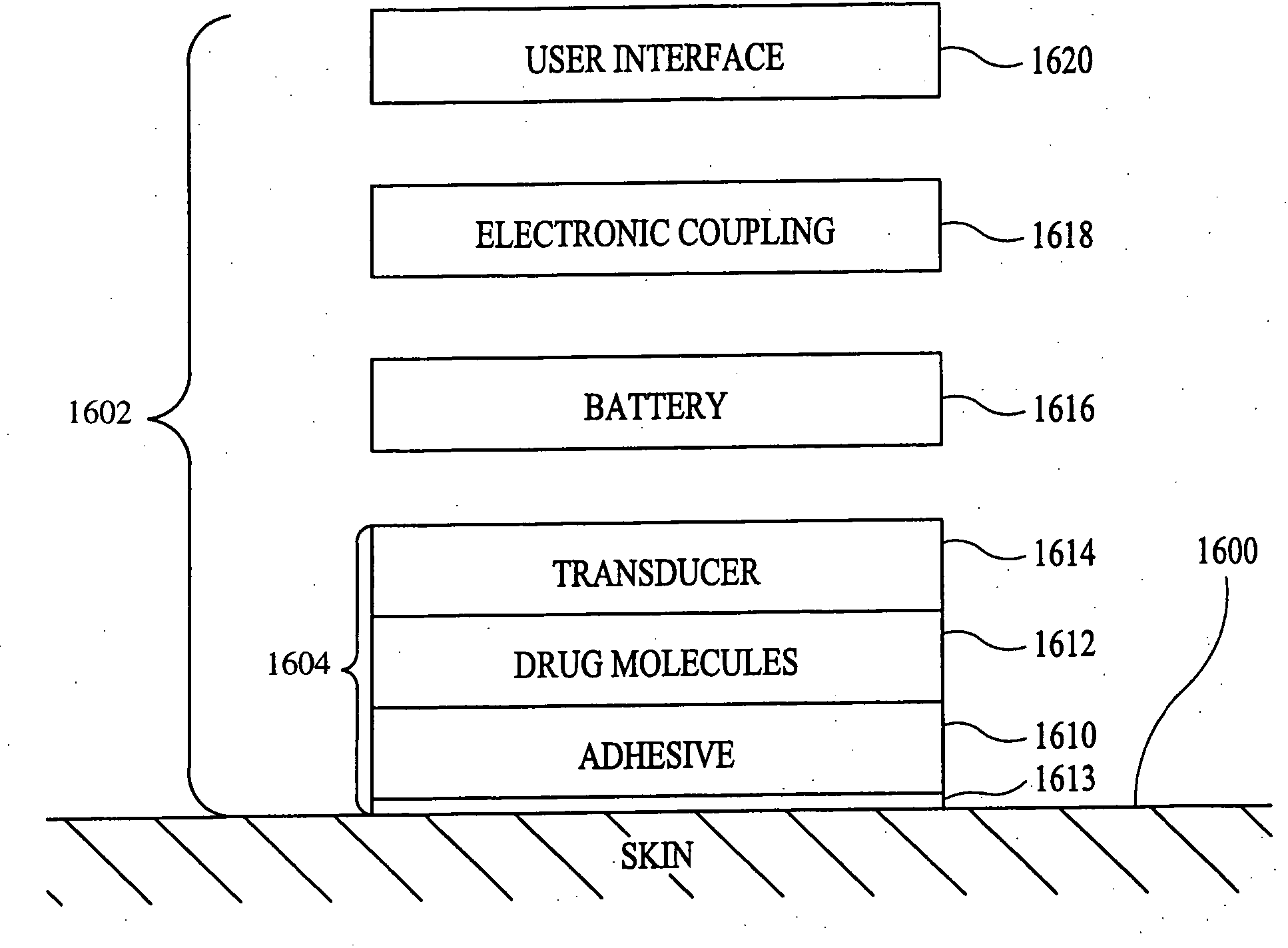
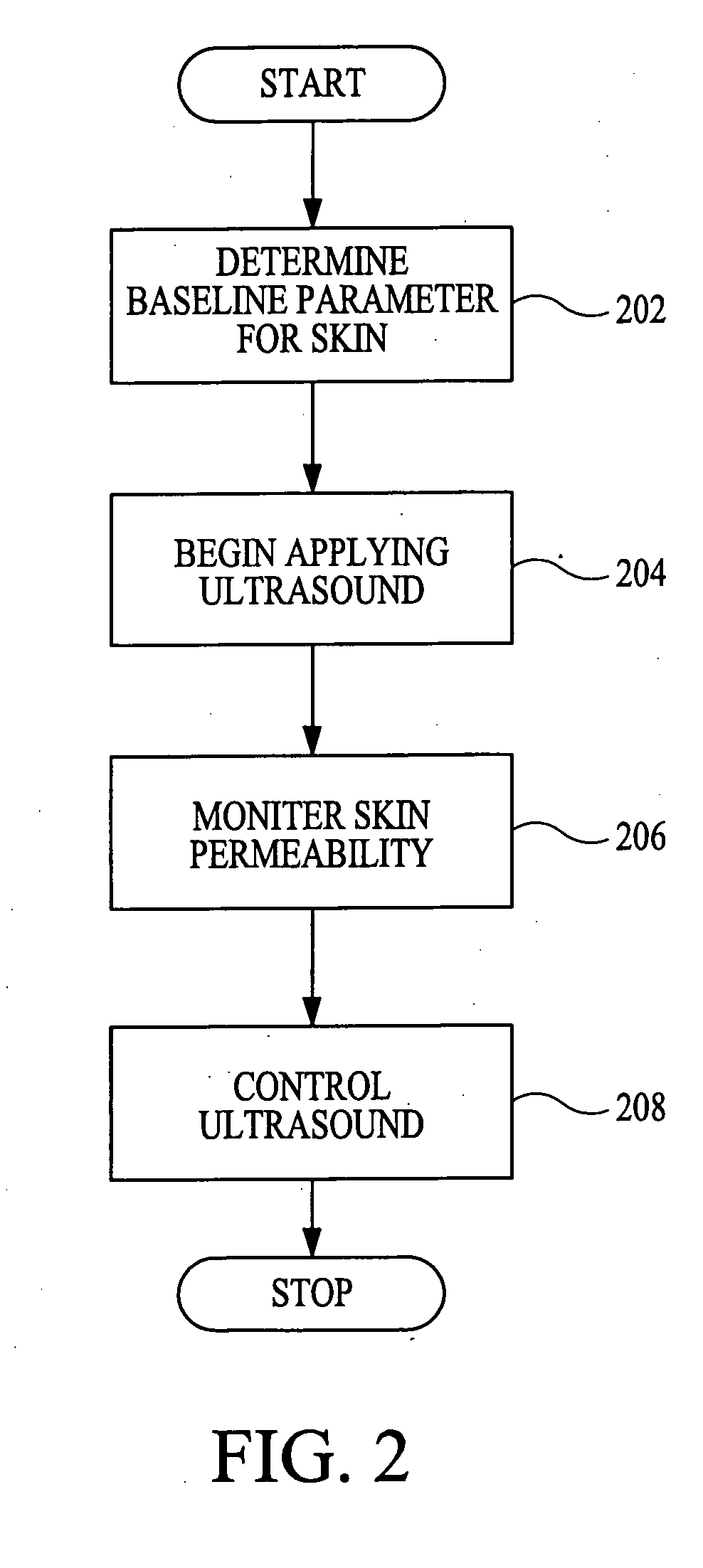
Method and apparatus for enhancement of transdermal transport
InactiveUS20040236268A1Effective to induce immune responseCompounds screening/testingElectrotherapyPharmaceutical drugTGE VACCINE
According to the present invention, a method for enhancing transdermal transport is disclosed. The method includes the steps of increasing a permeability of an area of a membrane with a permeabilizing device. The membrane may be, inter alia, biologic skin or synthetic skin. The permeabilizing device may be an ultrasound-producing device. A substance is transported into and through the area of the membrane. The substance may be a drug, a vaccine, or a component of interstitial fluid.
Owner:ECHO THERAPEUTICS INC
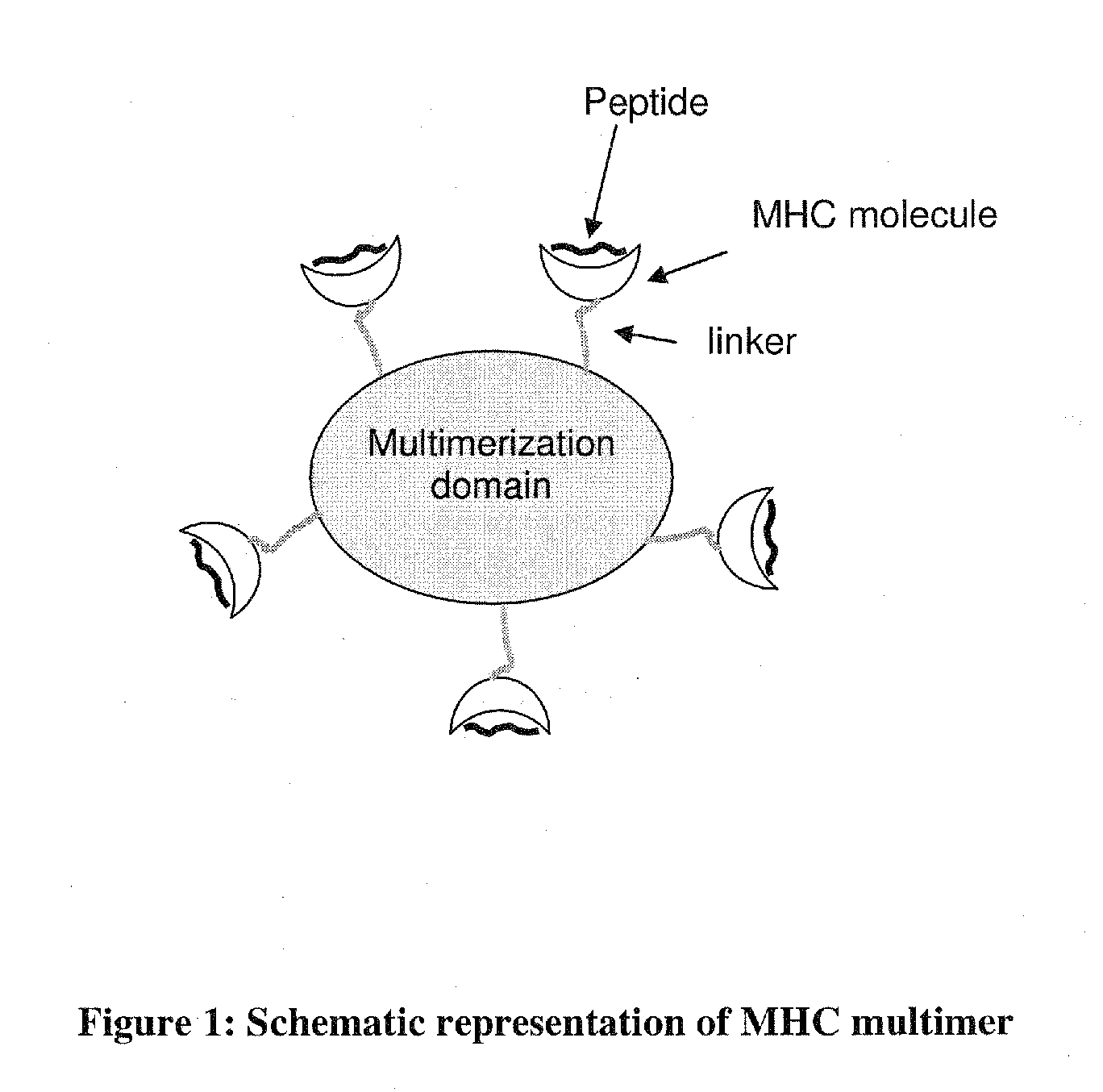
MHC Multimers in Cancer Vaccines and Immune Monitoring
InactiveUS20110318380A1Reduces infectious titerImprove efficacyPeptide/protein ingredientsImmunoglobulinsAntigenDisease
The present invention relates to MHC-peptide complexes and uses thereof in the diagnosis of, treatment of or vaccination against a disease in an individual. More specifically the invention discloses MHC complexes comprising cancer antigenic peptides and uses there of.
Owner:AGILENT TECH INC
Antigen delivery system and method of production
The present invention concerns polymer particle vaccine delivery systems in which a water insoluble protein antigen, e.g. a lipidated HpaA protein, is incorporated with particles comprising a polymer matrix. The present invention also concerns a method for incorporating such a water insoluble protein antigen with a polymer matrix in order to produce a polymer particle vaccine delivery system. In addition, the invention also provides a vaccine composition comprising the polymer particle delivery system. The vaccine can be used to treat and / or reduce the risk of for example Helicobacter infection.
Owner:ASTRAZENECA AB
Fcgamma riib specific antibodies and methods of use thereof
InactiveUS20050215767A1Enhance immune responseImprove responseSenses disorderAntipyreticTherapeutic antibodyAntiendomysial antibodies
The present invention relates to antibodies or fragments thereof that specifically bind FcγRIIB, particularly human FcγRIIB, with greater affinity than said antibodies or fragments thereof bind FcγRIIA, particularly human FcγRIIA. The invention provides methods of enhancing the therapeutic effect of therapeutic antibodies by administering the antibodies of the invention to enhance the effector function of the therapeutic antibodies. The invention also provides methods of enhancing efficacy of a vaccine composition by administering the antibodies of the invention.
Owner:MACROGENICS INC
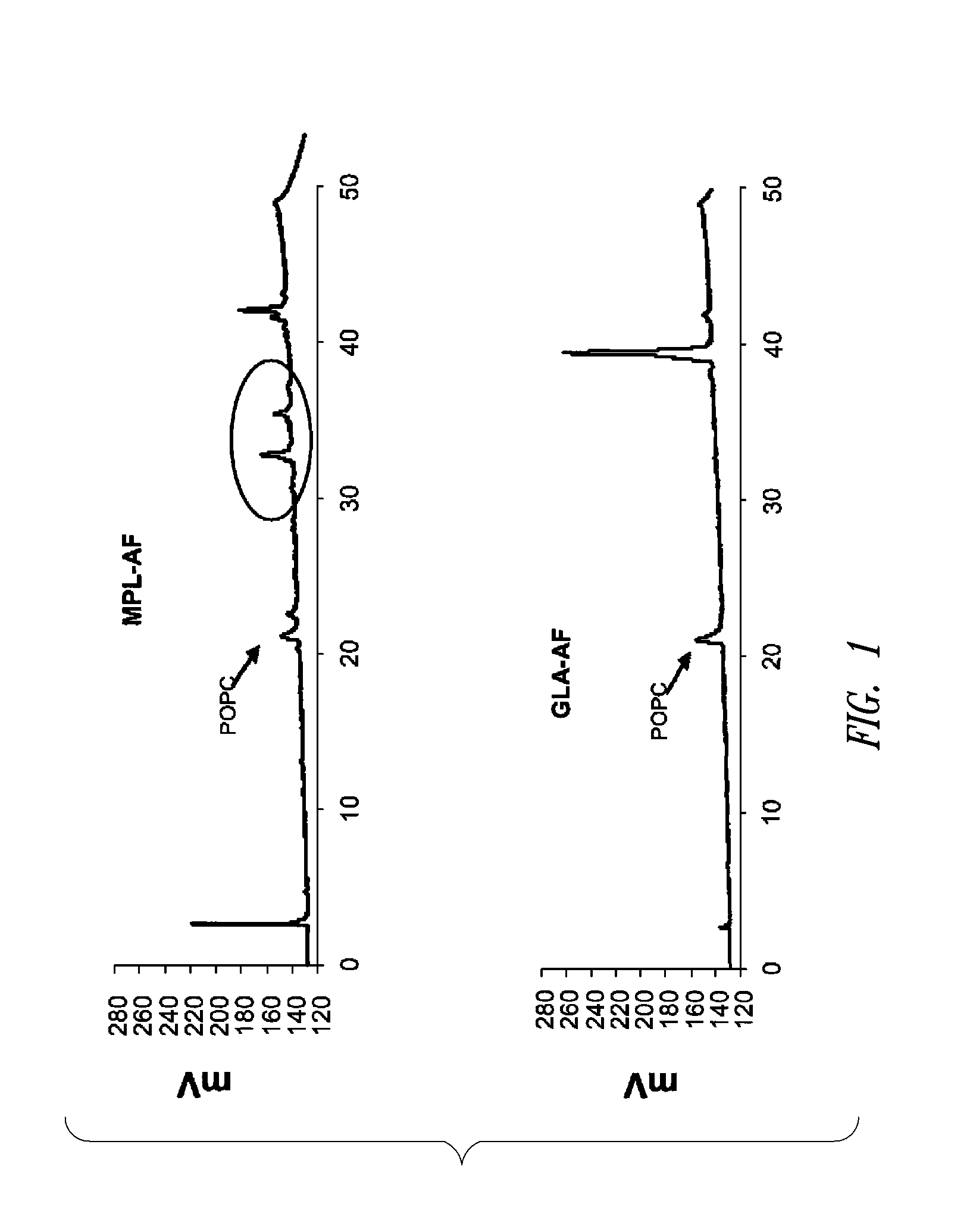
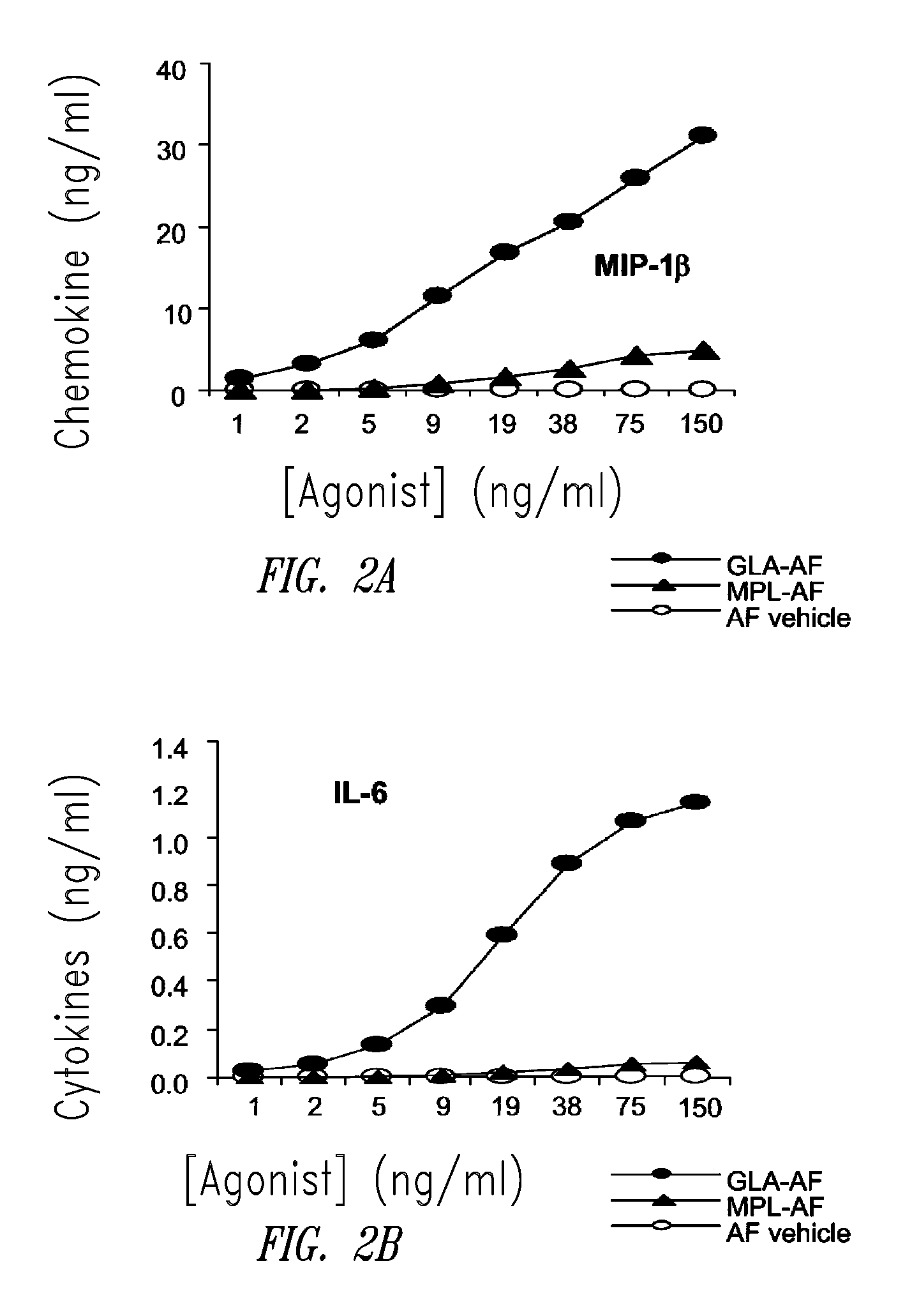
Vaccine composition containing synthetic adjuvant
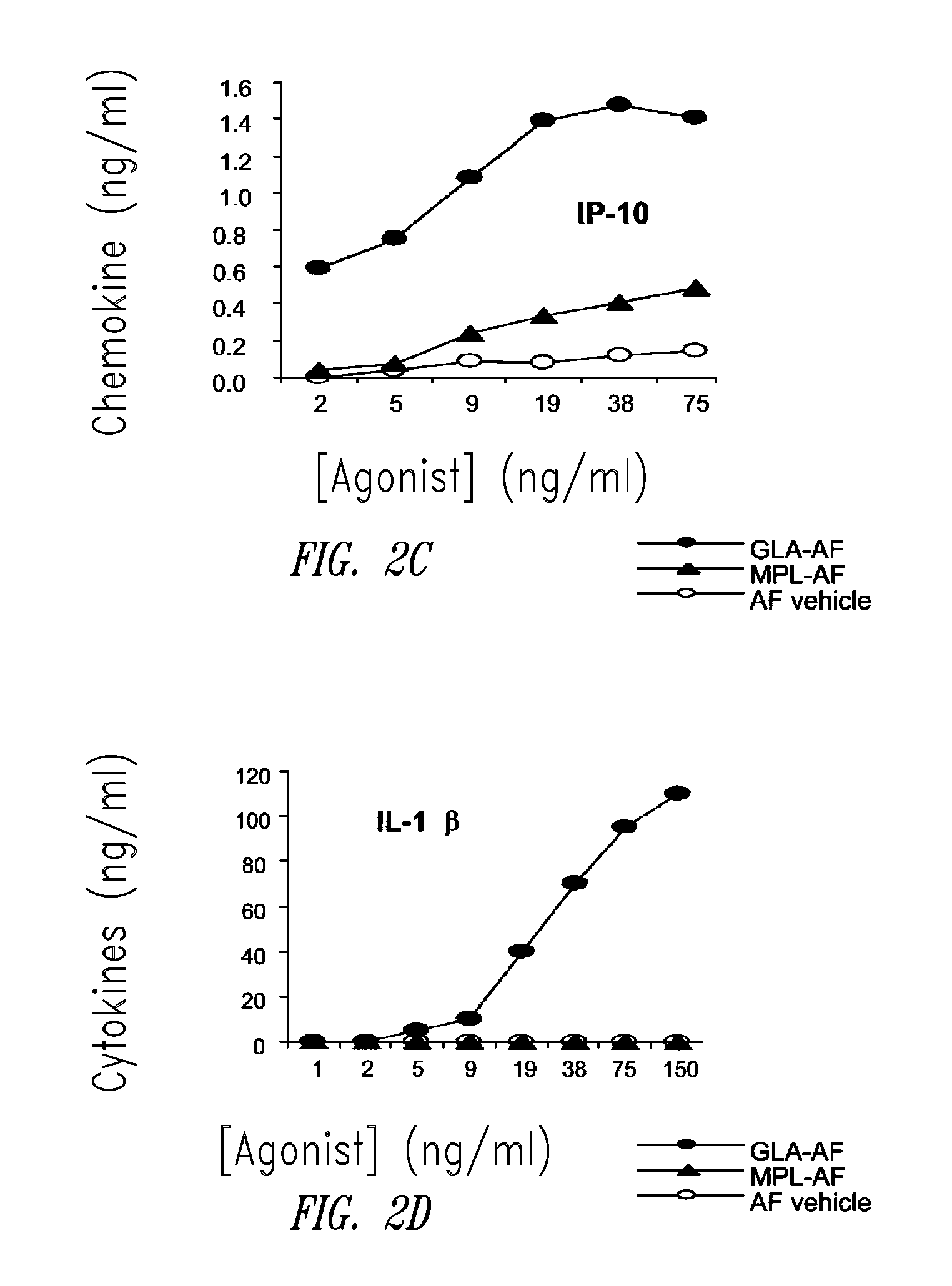
ActiveUS20080131466A1Elicit immune responseAntibacterial agentsBacterial antigen ingredientsNatural productAdditive ingredient
Compositions and methods, including vaccines and pharmaceutical compositions for inducing or enhancing an immune response are disclosed based on the discovery of useful immunological adjuvant properties in a synthetic, glucopyranosyl lipid adjuvant (GLA) that is provided in substantially homogeneous form. Chemically defined, synthetic GLA offers a consistent vaccine component from lot to lot without the fluctuations in contaminants or activity that compromise natural-product adjuvants. Also provided are vaccines and pharmaceutical compositions that include GLA and one or more of an antigen, a Toll-like receptor (TLR) agonist, a co-adjuvant and a carrier such as a pharmaceutical carrier.
Owner:ACCESS TO ADVANCED HEALTH INST
Functional influenza virus-like particles (VLPs)
ActiveUS20050009008A1SsRNA viruses negative-senseVirus peptidesMultiple copyVirus Structural Proteins
Recombinant influenza virus proteins, including influenza capsomers, subviral particles, virus-like particles (VLP), VLP complexes, and / or any portions of thereof, are provided as a vaccine for influenza viruses. The invention is based on the combination of two vaccine technologies: (1) intrinsically safe recombinant vaccine technology, and (2) highly immunogenic, self-assembled protein macromolecules embedded in plasma membranes and comprised of multiple copies of influenza virus structural proteins exhibiting neutralizing epitopes in native conformations. More specifically, this invention relates to the design and production of functional homotypic and heterotypic recombinant influenza virus-like particles (VLPs) comprised of recombinant structural proteins of human influenza virus type A / Sydney / 5 / 94 (H3N2) and / or avian influenza virus type A / Hong Kong / 1073 / 99 (H9N2) in baculovirus-infected insect cells and their application as a vaccine in the prevention of influenza infections and as a laboratory reagent for virus structural studies and clinical diagnostics.
Owner:NOVAVAX
Recombinant poxviruses having foreign DNA expressed under the control of poxvirus regulatory sequences
InactiveUS6998252B1SsRNA viruses negative-senseViral antigen ingredientsTranscriptional regulationVaccinia
Recombinant poxviruses, such as vaccinia, are provided that comprises a segment comprised of (A) a first DNA sequence encoding a polypeptide that is foreign to poxvirus and (B) a poxvirus transcriptional regulatory sequence, wherein (i) said transcriptional regulatory sequence is adjacent to and exerts transcriptional control over said first DNA sequence and (ii) said segment is positioned within a nonessential genomic region of said recombinant poxvirus. Vaccines, carriers, cells, and media comprising recombinant poxviruses, and methods of immunization with recombinant poxviruses also are provided.
Owner:DEPT OF HEALTH & HUMAN SERVICES UNITED STATES OF AMERICA AS REPRESENTED BY THE SEC
Bioinformatic processes for determination of peptide binding
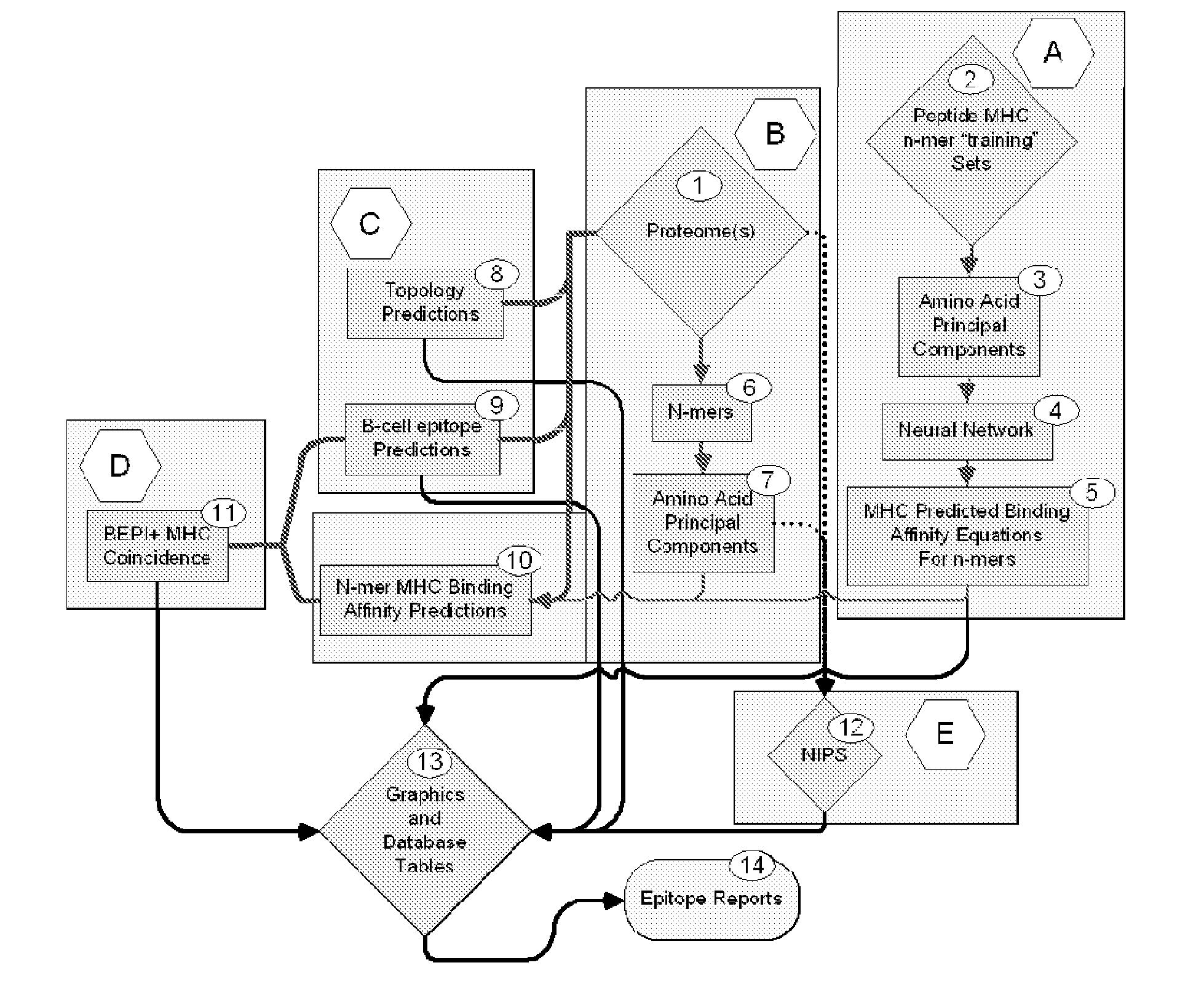
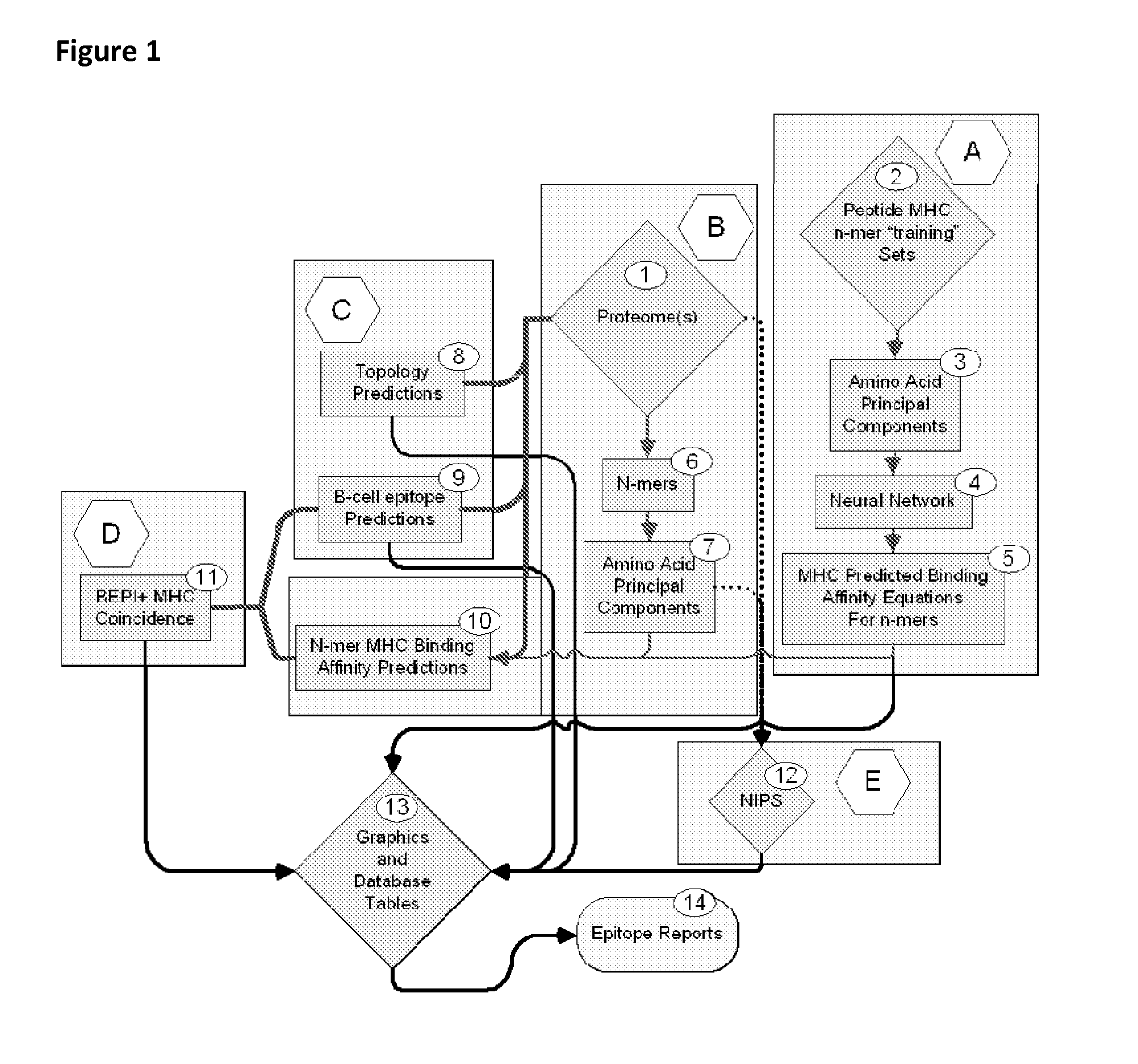
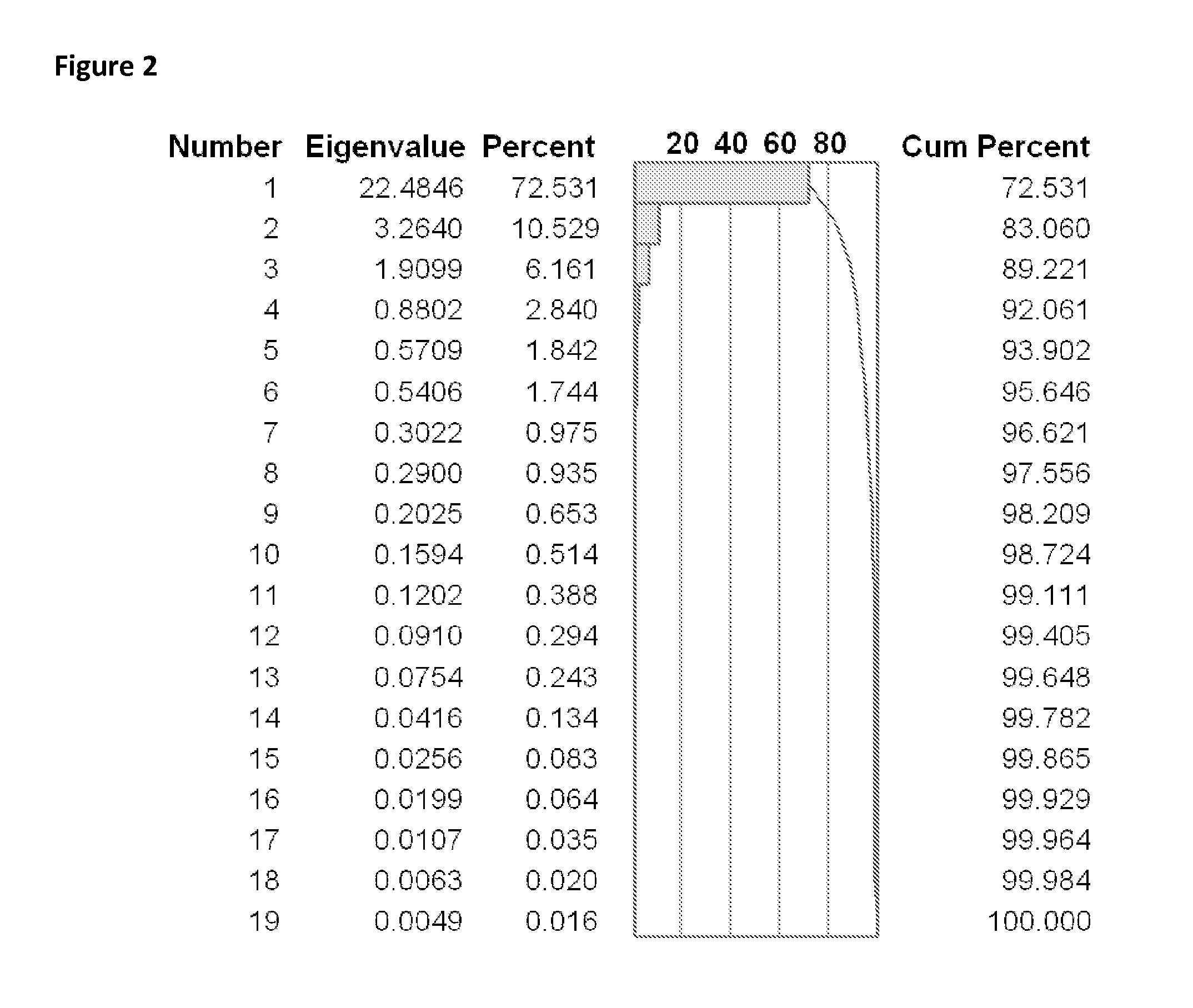
ActiveUS20130330335A1Increase probabilityHigh responsePeptide/protein ingredientsBiostatisticsEpitopeMicroorganism
This invention relates to the identification of peptide binding to ligands, and in particular to identification of epitopes expressed by microorganisms and by mammalian cells. The present invention provides polypeptides comprising the epitopes, and vaccines, antibodies and diagnostic products that utilize or are developed using the epitopes.
Owner:IOGENETICS
Individualized vaccines for cancer
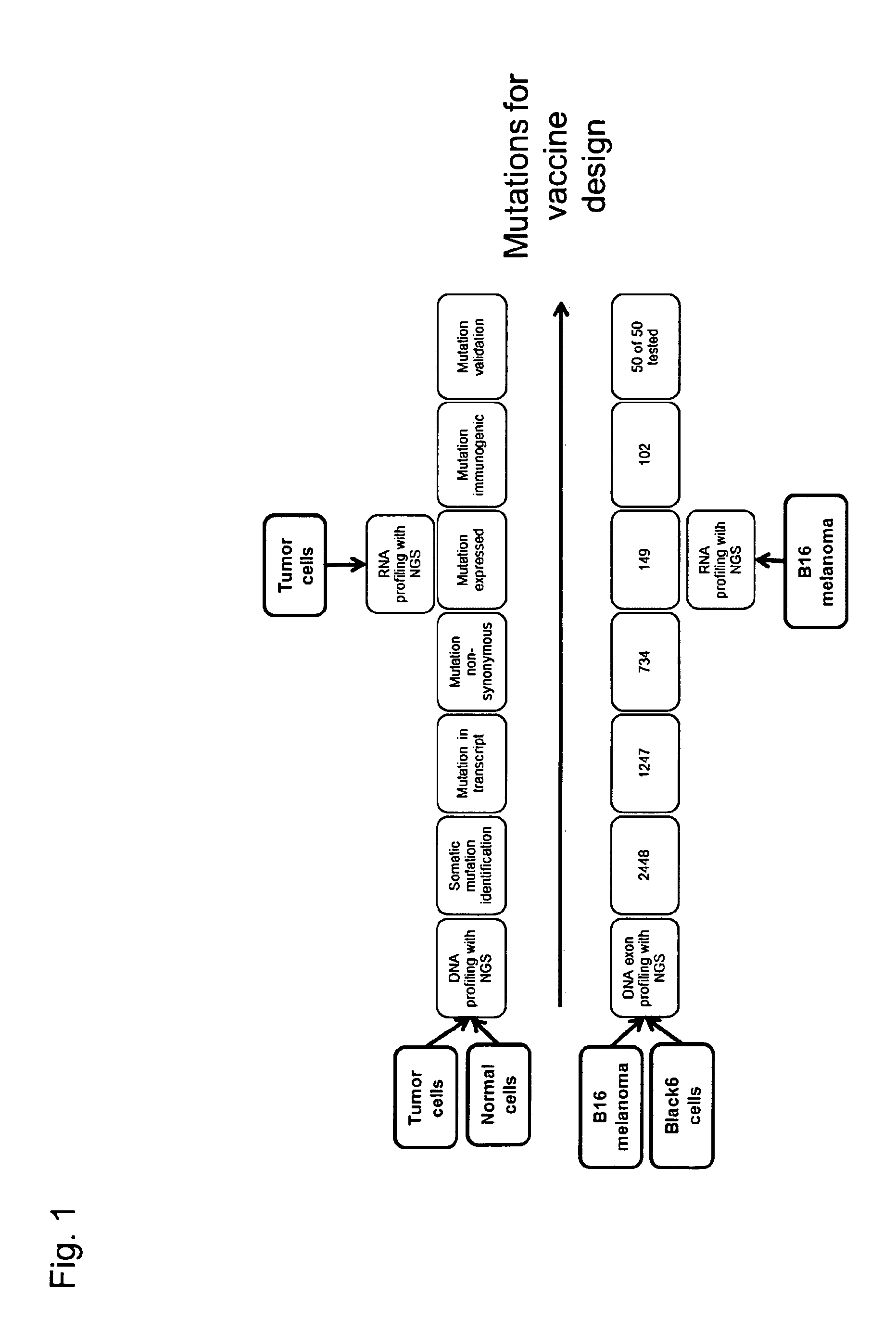
ActiveUS20140178438A1Reduces steric hindranceImprove translationVaccinesPharmaceutical delivery mechanismPrimary tumorTumour metastasis
The present invention relates to the provision of vaccines which are specific for a patient's tumor and are potentially useful for immunotherapy of the primary tumor as well as tumor metastases. In one aspect, the present invention relates to a method for providing an individualized cancer vaccine comprising the steps: (a) identifying cancer specific somatic mutations in a tumor specimen of a cancer patient to provide a cancer mutation signature of the patient; and (b) providing a vaccine featuring the cancer mutation signature obtained in step (a). In a further aspect, the present invention relates to vaccines which are obtainable by said method.
Owner:TRANSLATIONALE ONKOLOGIE AN DER UNIVSMEDIZIN DER JOHANNES GUTENBERG UNIV MAINZ GGMBH +1
Adjuvant in the form of a lipid-modified nucleic acid
The present invention relates to an immune-stimulating adjuvant in the form of a lipid-modified nucleic acid, optionally in combination with further adjuvants. The invention relates further to a pharmaceutical composition and to a vaccine, each containing an immune-stimulating adjuvant according to the invention, at least one active ingredient and optionally a pharmaceutically acceptable carrier and / or further auxiliary substances and additives and / or further adjuvants. The present invention relates likewise to the use of the pharmaceutical composition according to the invention and of the vaccine according to the invention for the treatment of infectious diseases or cancer diseases. Likewise, the present invention includes the use of the immune-stimulating adjuvant according to the invention in the preparation of a pharmaceutical composition for the treatment of cancer diseases or infectious diseases.
Owner:CUREVAC GMBH
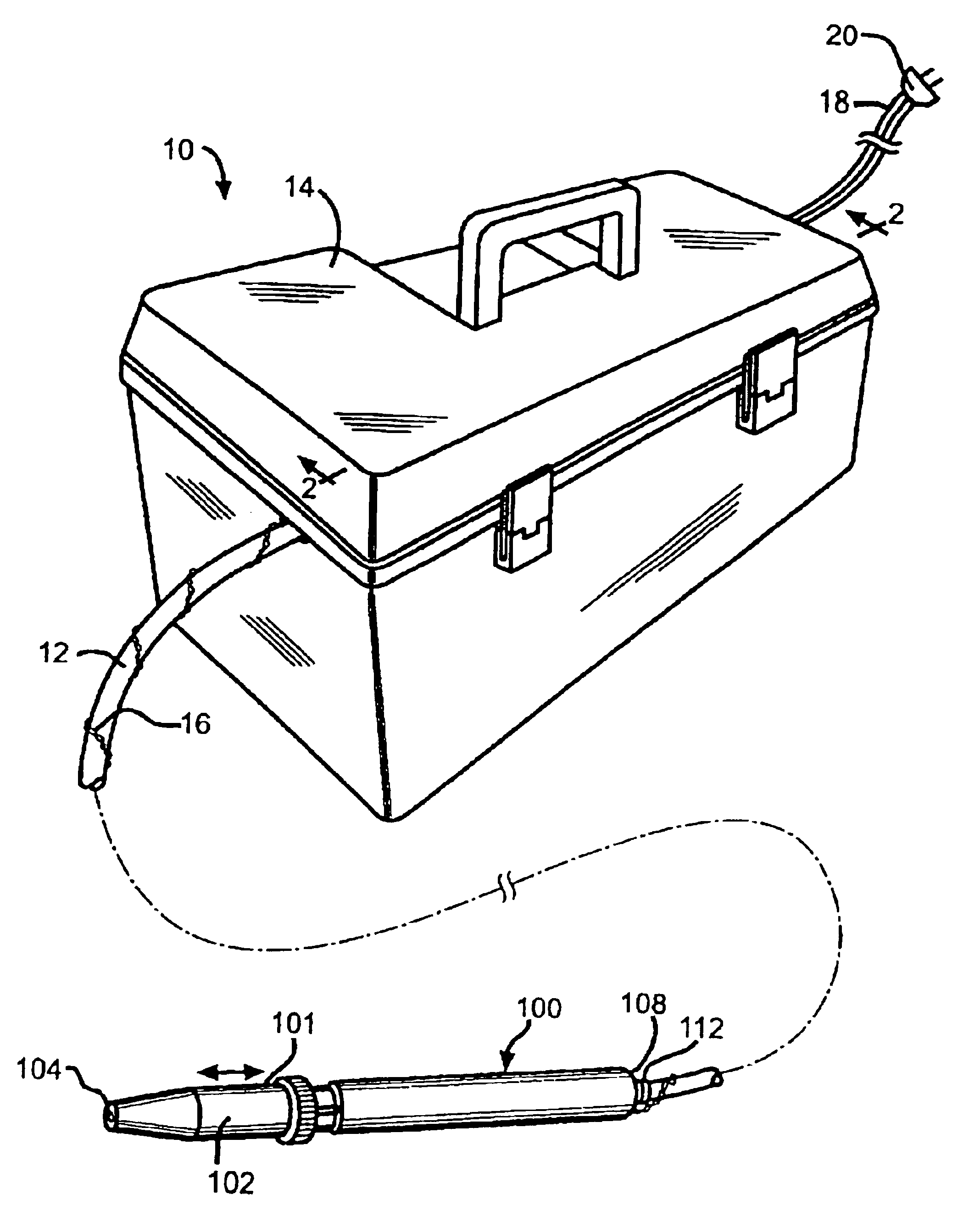
Vaccinator device
InactiveUS6858020B2Prevent leakageEliminates continual hand motionCannulasAutomatic syringesHand heldEngineering
An automatic repeater vaccinator apparatus (10) for dispensing a predetermined volume of a fluid into an animal, particularly a fluid which is a vaccine, and reloading after each volume of fluid is dispensed. The apparatus (10) comprises a handheld syringe (100) for dispensing the fluid, a flexible conduit (12) for transferring the fluid from a dispensing means to the syringe (100), and a reservoir (48) for providing the fluid to the dispensing means. The dispensing means is electrically activated by a magnetically closeable switch (preferably a reed switch (142)) in the syringe (100), which enables a predetermined volume of the fluid to be dispensed from the syringe (100) when the dispensing means is activated and reloading fluid from the reservoir (48) to replace the volume of fluid which has been dispensed from the syringe (100) when the dispensing means is deactivated. Preferably, the dispensing means comprises a pump (200 or 500) operated by a linear actuator such as a solenoid (300), which is electrically activated by the magnetically closeable switch in the syringe (100). The apparatus (10) is particularly useful for inoculating poultry, particularly inoculating the poultry by the wing web method using the needle and hub assembly (400) which comprises the filament (402) to prevent leakage between inoculations.
Owner:NEOGEN CORP
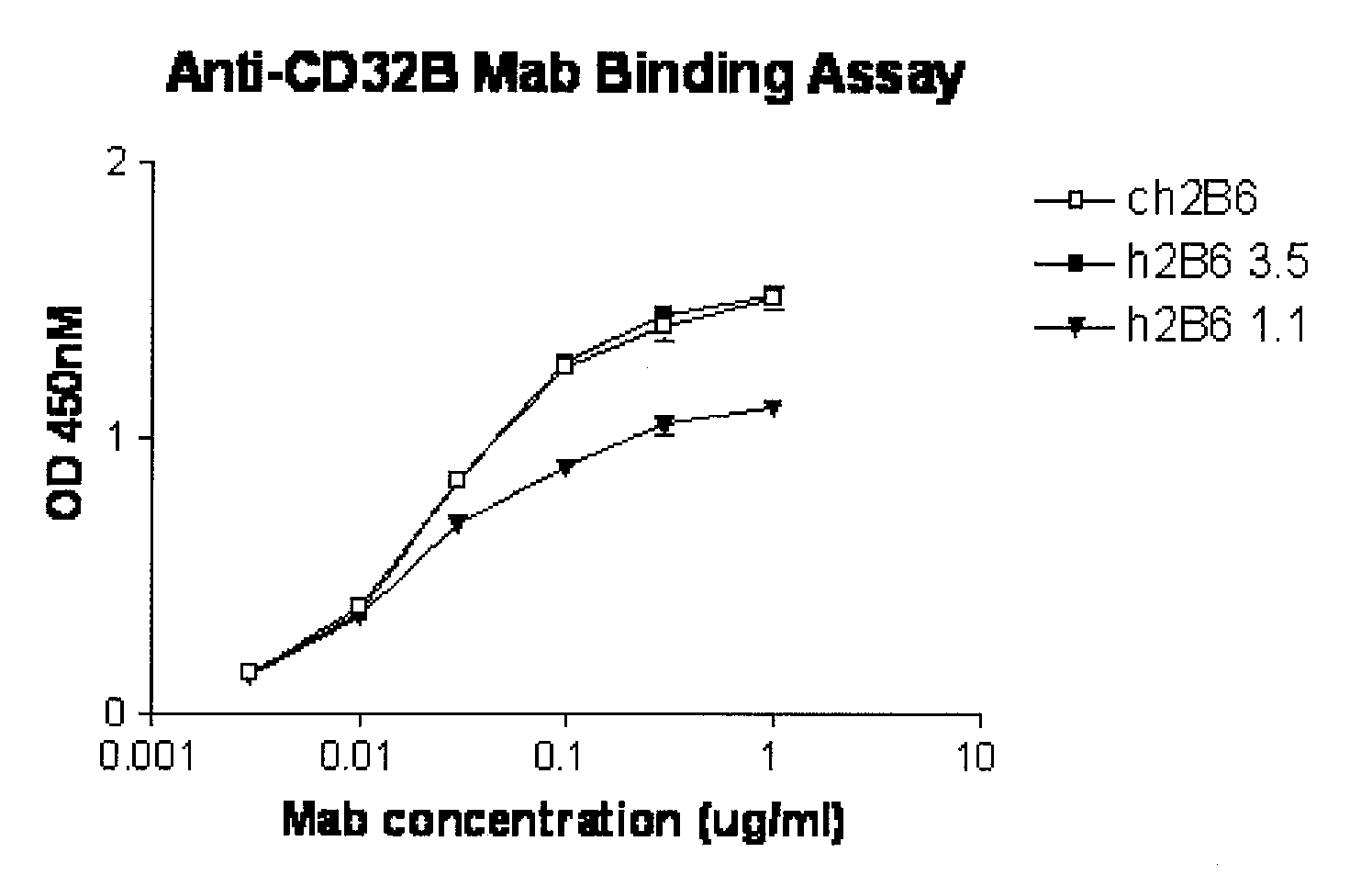
Humanized FcgammaRIIB-specific antibodies and methods of use thereof
ActiveUS20060013810A1Good curative effectConvenient treatmentSenses disorderNervous disorderFc(alpha) receptorDisease
The present invention relates to humanized FcγRIIB antibodies, fragments, and variants thereof that bind human FcγRIIB with a greater affinity than said antibody binds FcγRIIA. The invention encompasses the use of the humanized antibodies of the invention for the treatment of any disease related to loss of balance of Fc receptor mediated signaling, such as cancer, autoimmune and inflammatory disease. The invention provides methods of enhancing the therapeutic effect of therapeutic antibodies by administering the humanized antibodies of the invention to enhance the effector function of the therapeutic antibodies. The invention also provides methods of enhancing the efficacy of a vaccine composition by administering the humanized antibodies of the invention. The invention encompasses methods for treating an autoimmune disease and methods for elimination of cancer cells that express FcγRIIB.
Owner:MACROGENICS INC
Chimeric infectious DNA clones, chimeric porcine circoviruses and uses thereof
InactiveUS7279166B2Facilitate cell culture growthEnsure vaccine safetyFungiBacteriaSpecific immunityADAMTS Proteins
The present invention relates to infectious DNA clones, infectious chimeric DNA clones of porcine circovirus (PCV), vaccines and means of protecting pigs against viral infection or postweaning multisystemic wasting syndrome (PMWS) caused by PCV2. The new chimeric infectious DNA clone and its derived, avirulent chimeric virus are constructed from the nonpathogenic PCV1 in which the immunogenic ORF gene of the pathogenic PCV2 replaces a gene of the nonpathogenic PCV1, preferably in the same position. The chimeric virus advantageously retains the nonpathogenic phenotype of PCV1 but elicits specific immune responses against the pathogenic PCV2. The invention further embraces the immunogenic polypeptide expression products. In addition, the invention encompasses two mutations in the PCV2 immunogenic capsid gene and protein, and the introduction of the ORF2 mutations in the chimeric clones.
Owner:IOWA STATE UNIV RES FOUND +1
Saponin compositions and uses thereof
Owner:ANTIGENICS
Tumour-associated peptides binding to human leukocyte antigen (HLA) class i or ii molecules and related Anti-cancer vaccine
InactiveUS20090274714A1Organic active ingredientsPeptide/protein ingredientsHla class iiAdditive ingredient
The present invention relates to immunotherapeutic methods, and molecules and cells for use in immunotherapeutic methods. In particular, the present invention relates to the immunotherapy of cancer. The present invention furthermore relates to tumour-associated T-helper cell peptide epitopes, alone or in combination with other tumour-associated peptides, that serve as active pharmaceutical ingredients of vaccine compositions which stimulate anti-tumour immune responses. In particular, the present invention relates to two novel peptide sequences derived from HLA class II molecules of human tumour cell lines, which can be used in vaccine compositions for eliciting anti-tumour immune responses.
Owner:IMMATICS BIOTECHNOLOGIES GMBH
Compositions and Methods for Immunomodulation in an Organism
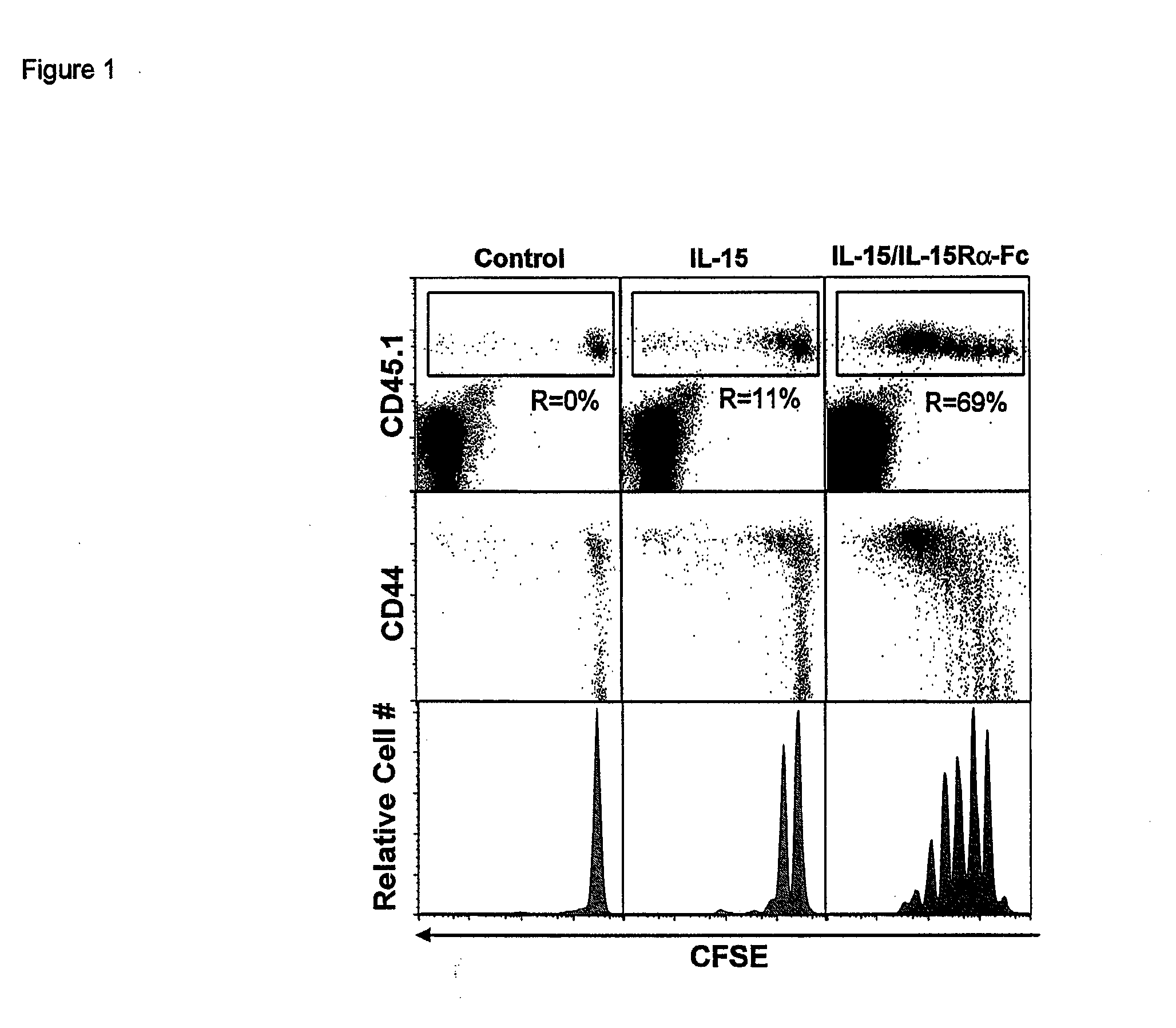
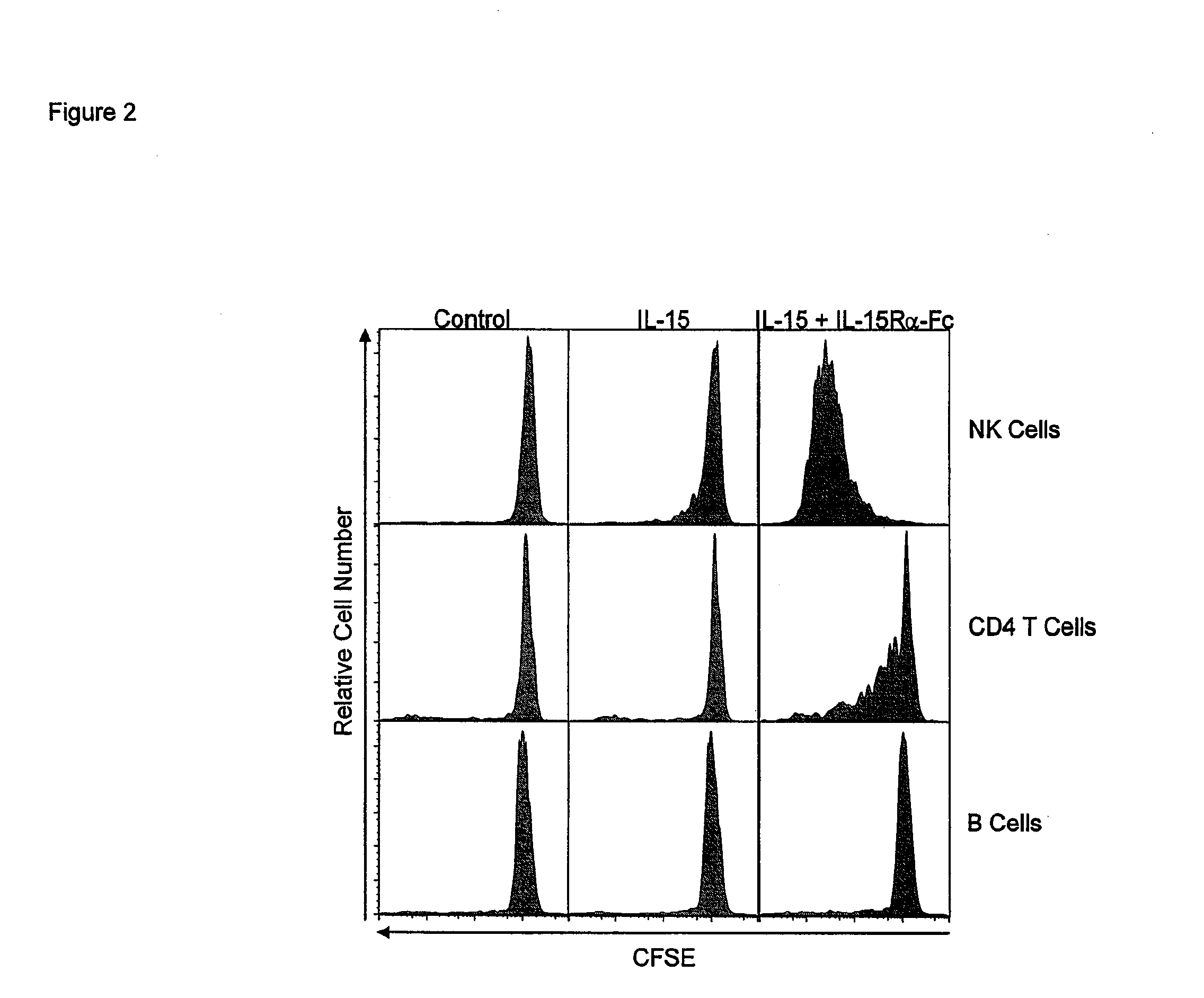
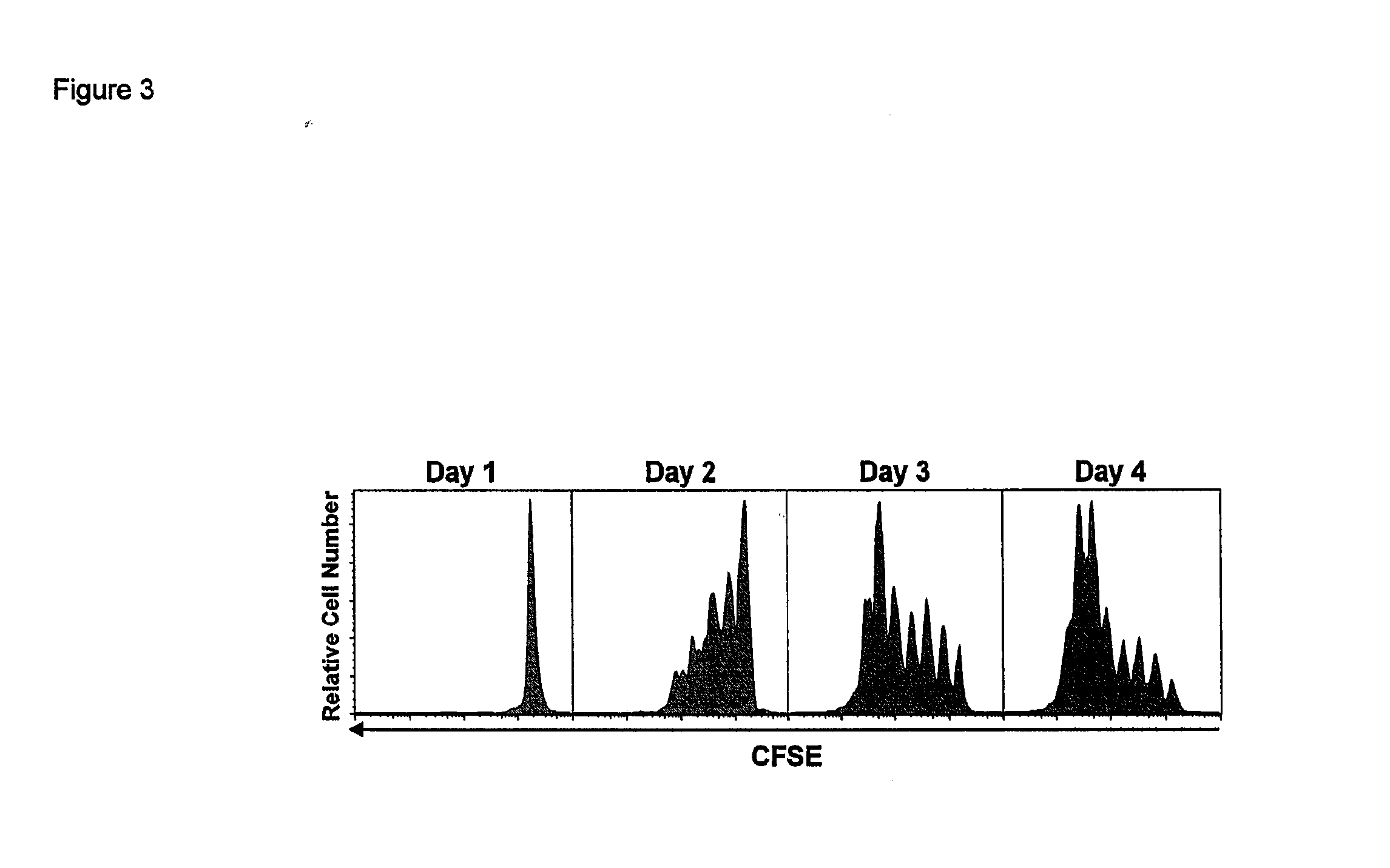
ActiveUS20120177598A1Long half-lifeGood treatment effectPolypeptide with localisation/targeting motifPeptide/protein ingredientsVaccinationHalf-life
The present invention relates to a therapeutic polypeptide and methods for its creation and use for modulating an immune response in a host organism in need thereof. In particular, the invention relates to the administration to an organism in need thereof, of an effective amount of a pre-coupled polypeptide complex comprising a lymphokine polypeptide portion, for example IL-15 (SEQ ID NO: 5, 6), IL-2 (SEQ ID NO: 10, 12) or combinations of both, and an interleukin receptor polypeptide portion, for example IL-15Ra (SEQ ID NO: 7, 8), IL-2Ra (SEQ ID NO: 9, 11) or combinations of both, for augmenting the immune system in, for example, cancer, SCID, AIDS, or vaccination; or inhibiting the immune system in, for example, rheumatoid arthritis, or Lupus. The therapeutic complex of the invention surprisingly demonstrates increased half-life, and efficacy in vivo.
Owner:UNIV OF CONNECTICUT
Humanized Fc.gamma.RIIB-Specific Antibodies and Methods of Use Thereof
InactiveUS20080044417A1Good curative effectEnhanced effector functionDisease diagnosisTissue cultureFc(alpha) receptorFc receptor
The present invention relates to humanized FcγRIIB antibodies, fragments, and variants thereof that bind human FcγRIIB with a greater affinity than said antibody binds FcγRIIA. The invention encompasses the use of the humanized antibodies of the invention for the treatment of any disease related to loss of balance of Fc receptor mediated signaling, such as cancer, autoimmune and inflammatory disease. The invention provides methods of enhancing the therapeutic effect of therapeutic antibodies by administering the humanized antibodies of the invention to enhance the effector function of the therapeutic antibodies. The invention also provides methods of enhancing the efficacy of a vaccine composition by administering the humanized antibodies of the invention. The invention encompasses methods for treating an autoimmune disease and methods for elimination of cancer cells that express FcγRIIB.
Owner:MACROGENICS INC
Immunostimulatory Combinations for Vaccine Adjuvants
This invention discloses immunostimulatory combinations of Tumor Necrosis Factor Receptor Superfamily (TN-FRSF) agonists, Toll-Like Receptor (TLR) agonists, “domain present in NAIP, CIITA, HET-E, TP-I (NACHT)-Leucine Rich Repeat (LRR)” or “NLR” agonists, RIG-I-Like Helicase or “RLH” agonists, purinergic receptor agonists and cytokine / chemokine receptor agonists, together with delivery methods. The combinations, when used alone at the site of pathology, provide immunostimulation that induces host humoral and cellular immunologic responses to eliminate pathogens or neoplasms. Alternatively, when the combinations are used with a defined antigens, these combinations can induce focused humoral and cellular immunologic responses useful as prophylactic and / or ameliorative therapeutic modalities for infections and the treatment of neoplastic disorders.
Owner:RGT UNIV OF CALIFORNIA
rPA optimization
An optimized synthetic polynucleotide encoding a Bacillus anthracis protective antigen and an anthrax vaccine based on the encoded protective antigen. Furthermore, heterologous expression in a host Pseudomonas fluorescens bacteria of an optimized polynucleotide sequence encoding a Bacillus anthracis protective antigen.
Owner:PELICAN TECH HLDG INC
Codon-optimized polynucleotide-based vaccines against human cytomegalovirus infection
InactiveUS20080085870A1Reduce in quantityDecreased immunological responseOrganic active ingredientsPeptide/protein ingredientsAntigenAdjuvant
The invention is related to polynucleotide-based cytomegalovirus vaccines. In particular, the invention is plasmids operably encoding HCMV antigens, in which the naturally-occurring coding regions for the HCMV antigens have been modified for improved translation in human or other mammalian cells through codon optimization. HCMV antigens which are useful in the invention include, but are not limited to pp65, glycoprotein B (gB), IE1, and fragments, variants or derivatives of either of these antigens. In certain embodiments, sequences have been deleted, e.g., the Arg435-Lys438 putative kinase in pp65 and the membrane anchor and endocellular domains in gB. The invention is further directed to methods to induce an immune response to HCMV in a mammal, for example, a human, comprising delivering a plasmid encoding a codon-optimized HCMV antigen as described above. The invention is also directed to pharmaceutical compositions comprising plasmids encoding a codon-optimized HCMV antigen as described above, and further comprising adjuvants, excipients, or immune modulators.
Owner:VICAL INC
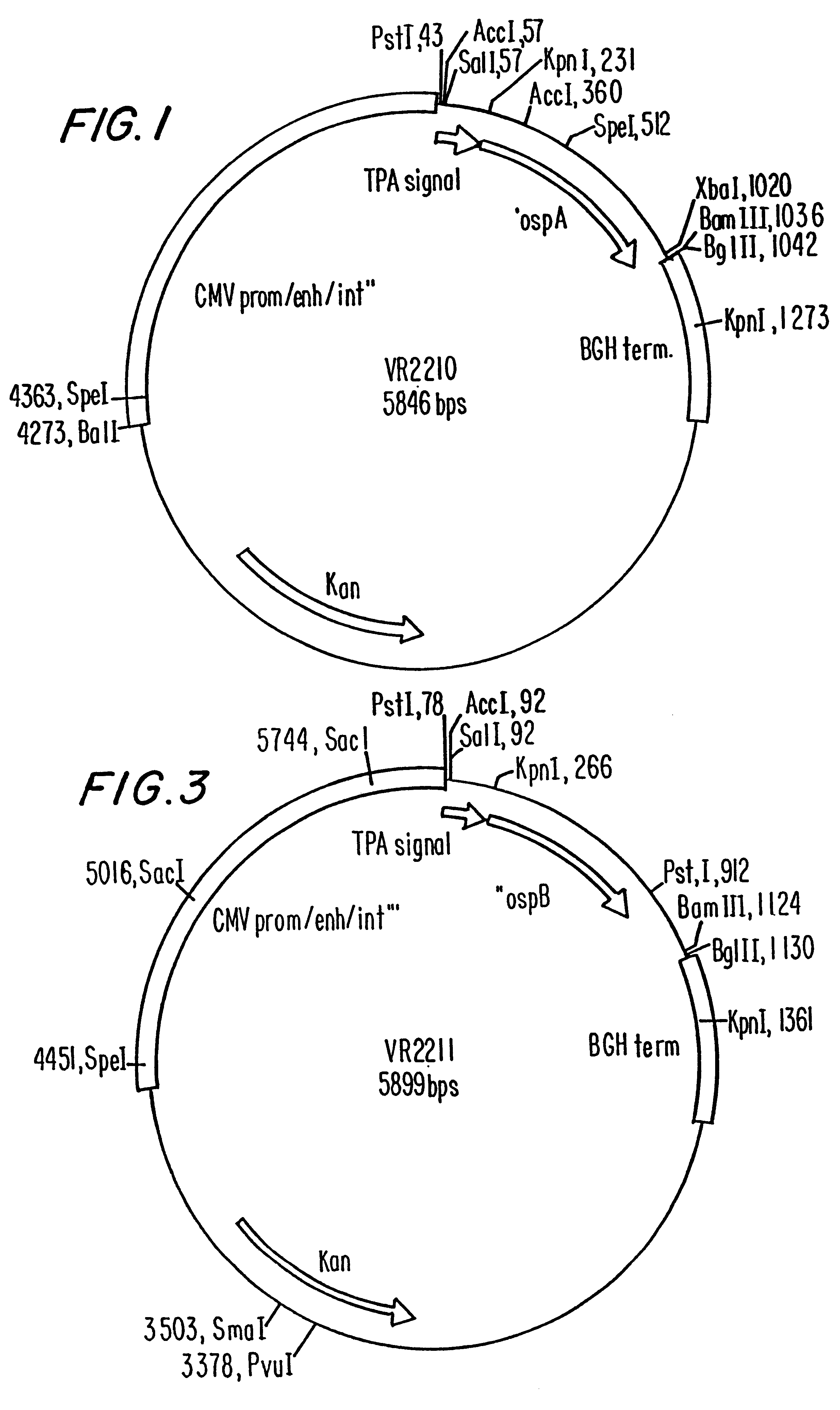
Compositions and methods for administering Borrelia DNA
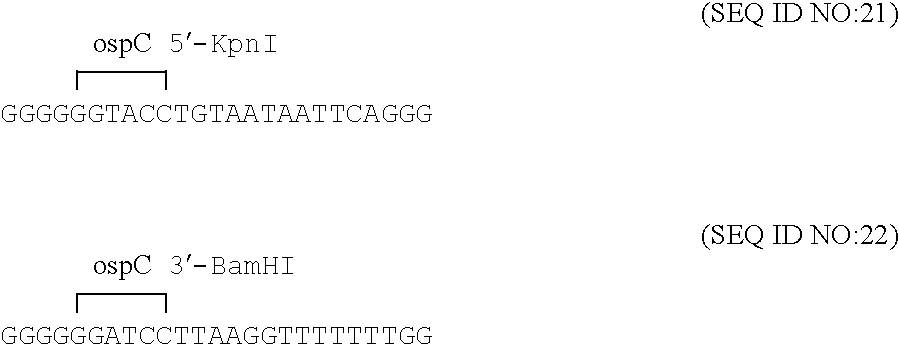
Disclosed is a vaccine against Lyme Disease or its causative agent Borrelia burgdorferi (sensu stricto or sensu lato) containing a plasmid a DNA encoding a promoter for driving expression in a mammalian cell, DNA encoding a leader peptide for facilitating secretion / release of a prokaryotic protein sequence from a mammalian cell, a DNA encoding Borrelia OspA or OspB, and a DNA encoding a terminator. Disclosed too is an immunogenic composition against Lyme Disease or its causative agent Borrelia burgdorferi (sensu stricto or sensu lato) containing a plasmid comprising a DNA encoding a promoter for driving expression in a mammalian cell, DNA encoding a leader peptide for facilitating secretion / release of a prokaryotic protein sequence from a mammalian cell, a DNA encoding a Borrelia OspC, and a DNA encoding a terminator. And, methods for making and using such vaccines and the immunogenic composition are also disclosed.
Owner:PASTEUR MERIEUX SERUMS & VACCINS SA
Screening for west nile virus antiviral therapy
InactiveUS20050058987A1Improve efficiencySsRNA viruses positive-senseVectorsHigh-Throughput Screening MethodsImmunogenicity
The instant invention provides stable and novel lineage I WNV reverse genetics systems, and methods for making the reverse genetics systems, specifically, a fully-infectious lineage I WNV cDNA or replicon system engineered with one or more nucleotide sequences each encoding a reporter gene to be used in high throughput cell-based screening assays for the identification of novel antiflaviviral chemotherapeutics and / or vaccines effective to treat and / or immunize against infections by WNV and other emerging flaviviruses, such as, for example, JEV, SLEV, AV, KV, JV, CV, YV, TBEV, DENV-1, DENV-2, DENV-3, DENV-4, YFV and MVEV. The present invention further provides methods of high throughput screening of antiflaviviral compounds or improved derivatives thereof using novel lineage I WNV reverse genetics systems and / or cell lines stably containing the reverse genetics systems. Also, the invention provides novel pharmaceutical compositions comprising an attenuated lineage I WNV that is less virulent but similarly immunogenic as the parent WNV and is capable of providing a protective immune response in a host.
Owner:HEALTH RES INC
Dock-and-lock (DNL) vaccines for cancer therapy
Owner:IBC PHARMACEUTICALS INC
Novel peptides and combination of peptides for use in immunotherapy against small cell lung cancer and other cancers
ActiveUS20170096461A1High error rateIncrease in motilityNervous disorderAntibody mimetics/scaffoldsPeptideDrug
The present invention relates to peptides, proteins, nucleic acids and cells for use in immunotherapeutic methods. In particular, the present invention relates to the immunotherapy of cancer. The present invention furthermore relates to tumor-associated T-cell peptide epitopes, alone or in combination with other tumor-associated peptides that can for example serve as active pharmaceutical ingredients of vaccine compositions that stimulate anti-tumor immune responses, or to stimulate T cells ex vivo and transfer into patients. Peptides bound to molecules of the major histocompatibility complex (MHC), or peptides as such, can also be targets of antibodies, soluble T-cell receptors, and other binding molecules.
Owner:IMMATICS BIOTECHNOLOGIES GMBH
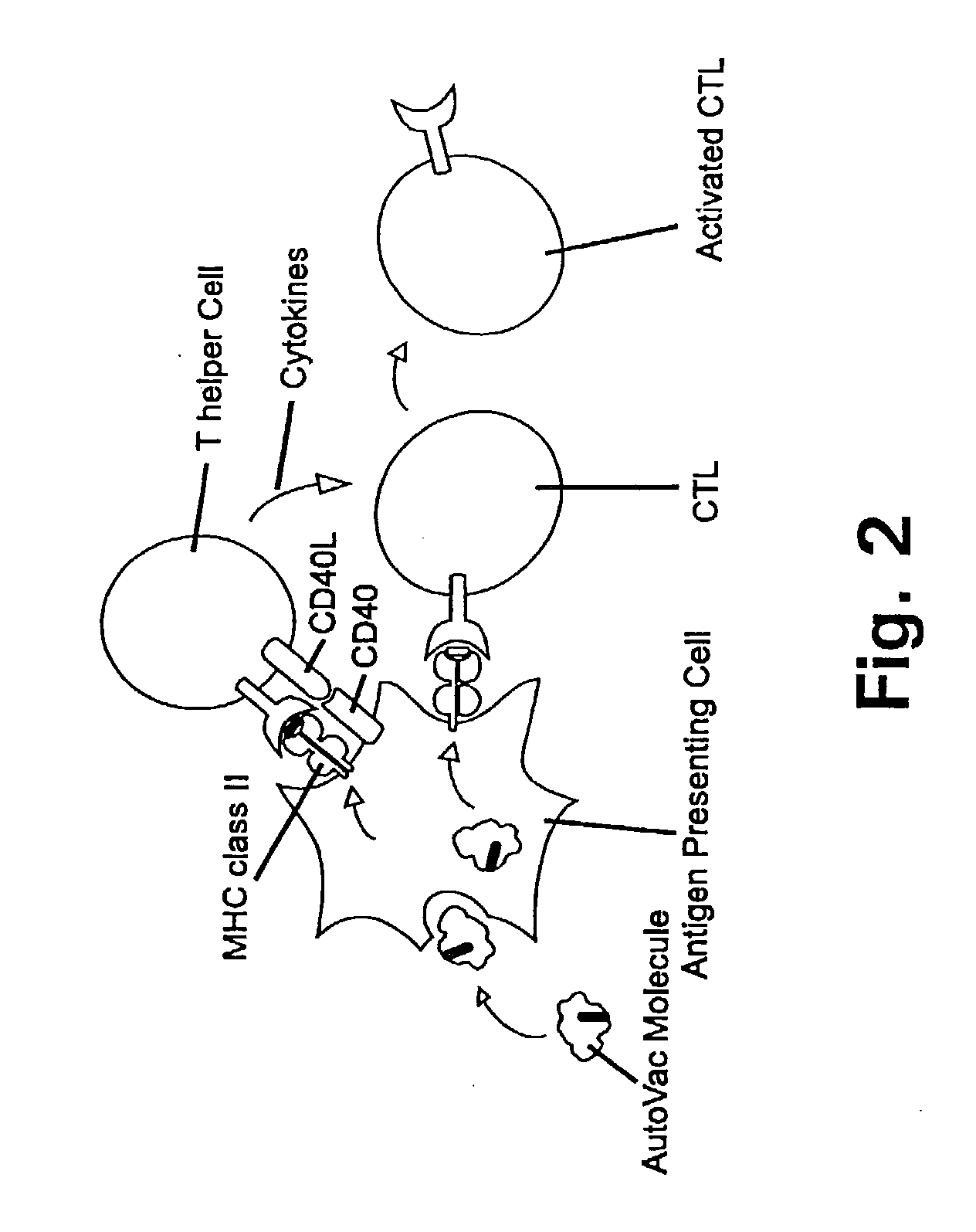
Novel methods for therapeutic vaccination
A method is disclosed for inducing cell-mediated immunity against cellular antigens. More specifically, the invention provides for a method for inducing cytotoxic T-lymphocyte immunity against weak antigens, notably self-proteins. The method entails that antigen presenting cells are induced to present at least one CTL epitope of the weak antigen and at the same time presenting at least one foreign T-helper lymphocyte epitope. In a preferred embodiment, the antigen is a cancer specific antigen, e.g. PSM, Her2, or FGF8b. The method can be exercised by using traditional polypeptide vaccination, but also by using live attenuated vaccines or nucleic acid vaccination. The invention furthermore provides immunogenic analogues of PSM, Her2 and FGF8b, as well as nucleic acid molecules encoding these analogues. Also vectors and transformed cells are disclosed. The invention also provides for a method for identification of immunogenic analogues of weak or non-immunogenic antigens.
Owner:BAVARIAN NORDIC AS
Vaccines comprising aluminium adjuvants and histidine
To improve the stability of vaccines comprising aluminum salt(s), the invention uses the amino acid histidine. This can improve pH stability and adjuvant adsorption and can be reduce antigen hydrolysis. Histidine is preferably presen during adsorption to the aluminium salt(s). The antigen in the vaccine may be a protein or a saccharide and is preferably from N. meningitidis.
Owner:GLAXOSMITHKLINE BIOLOGICALS SA
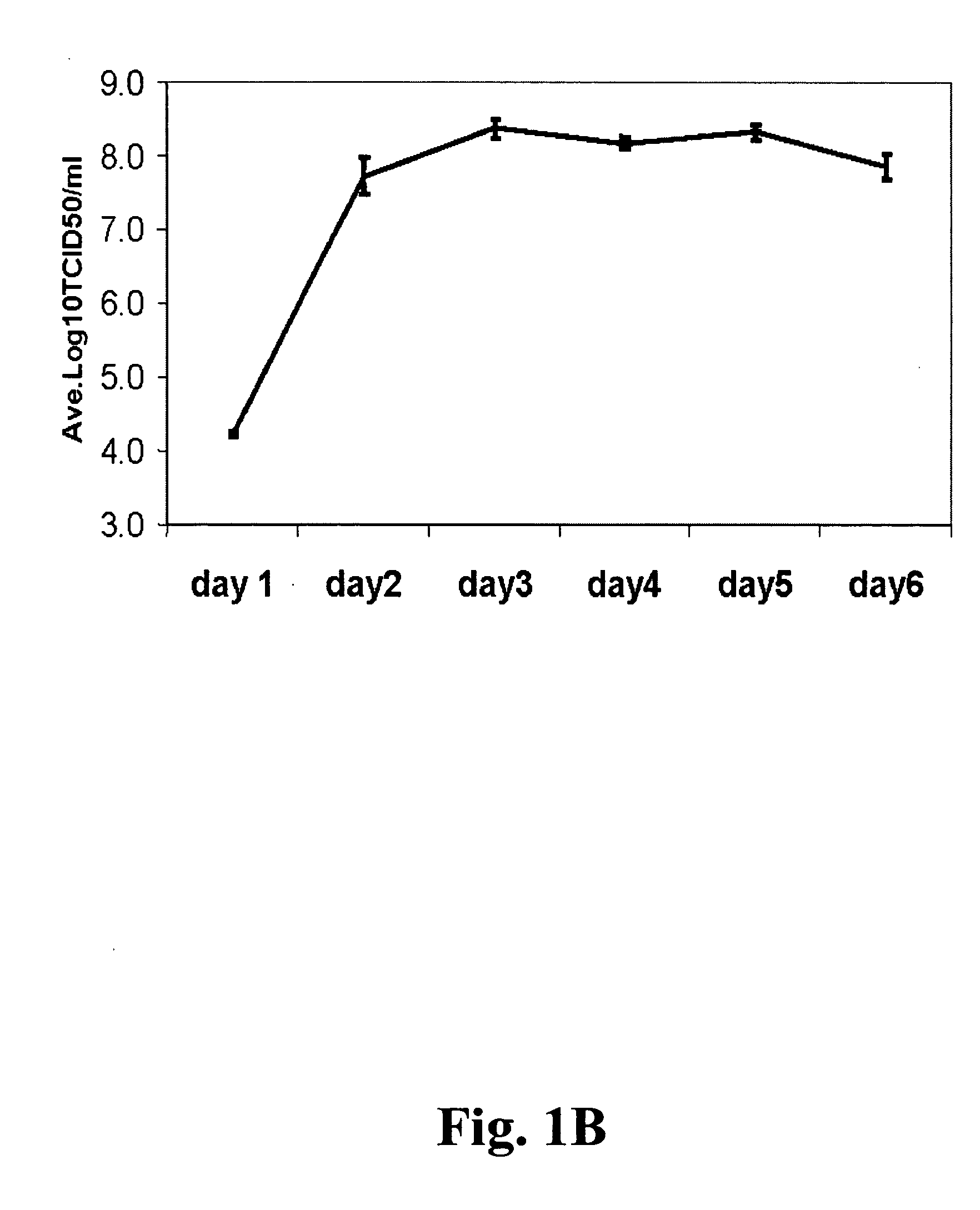
Non-tumorigenic MDCK cell line for propagating viruses
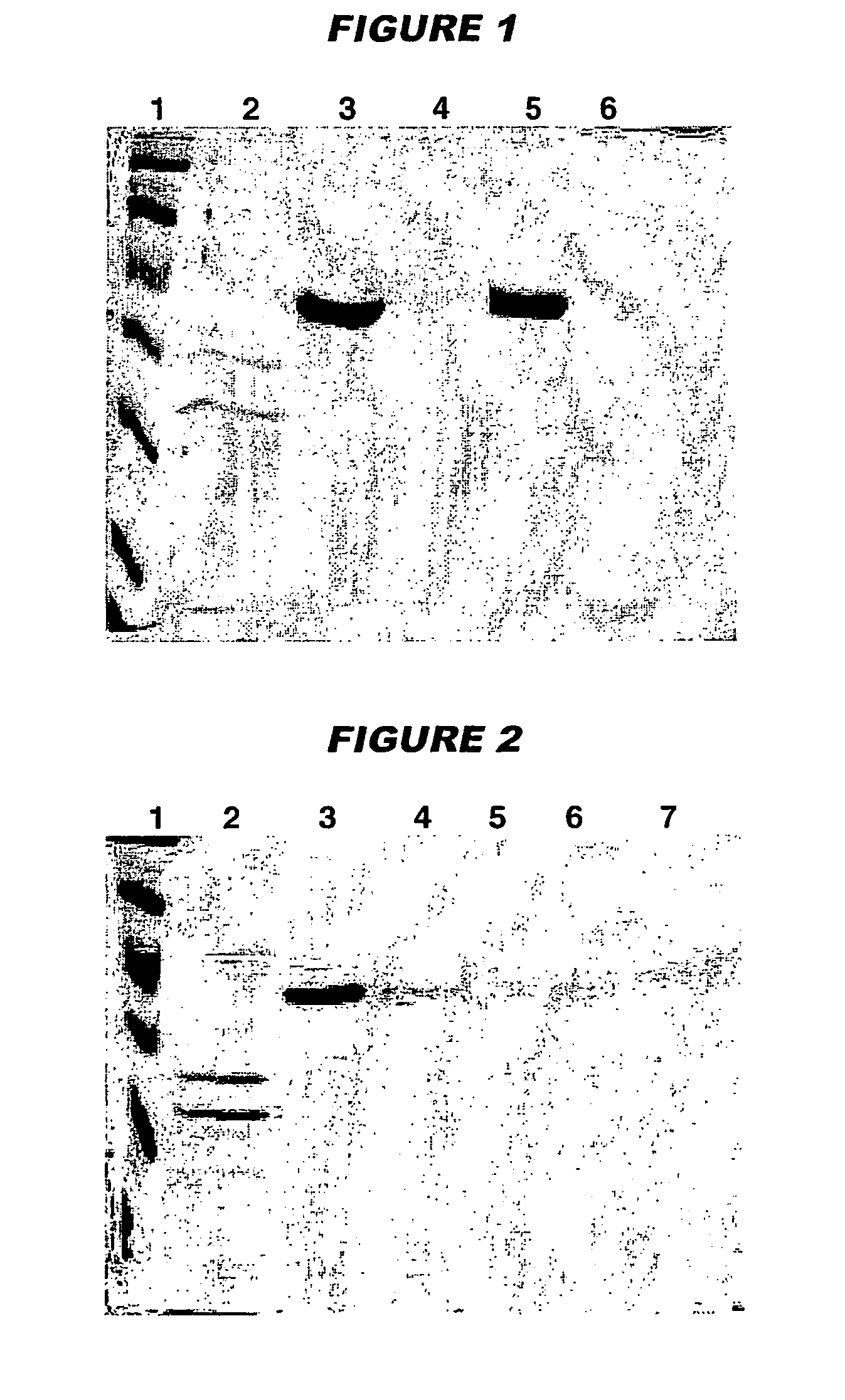
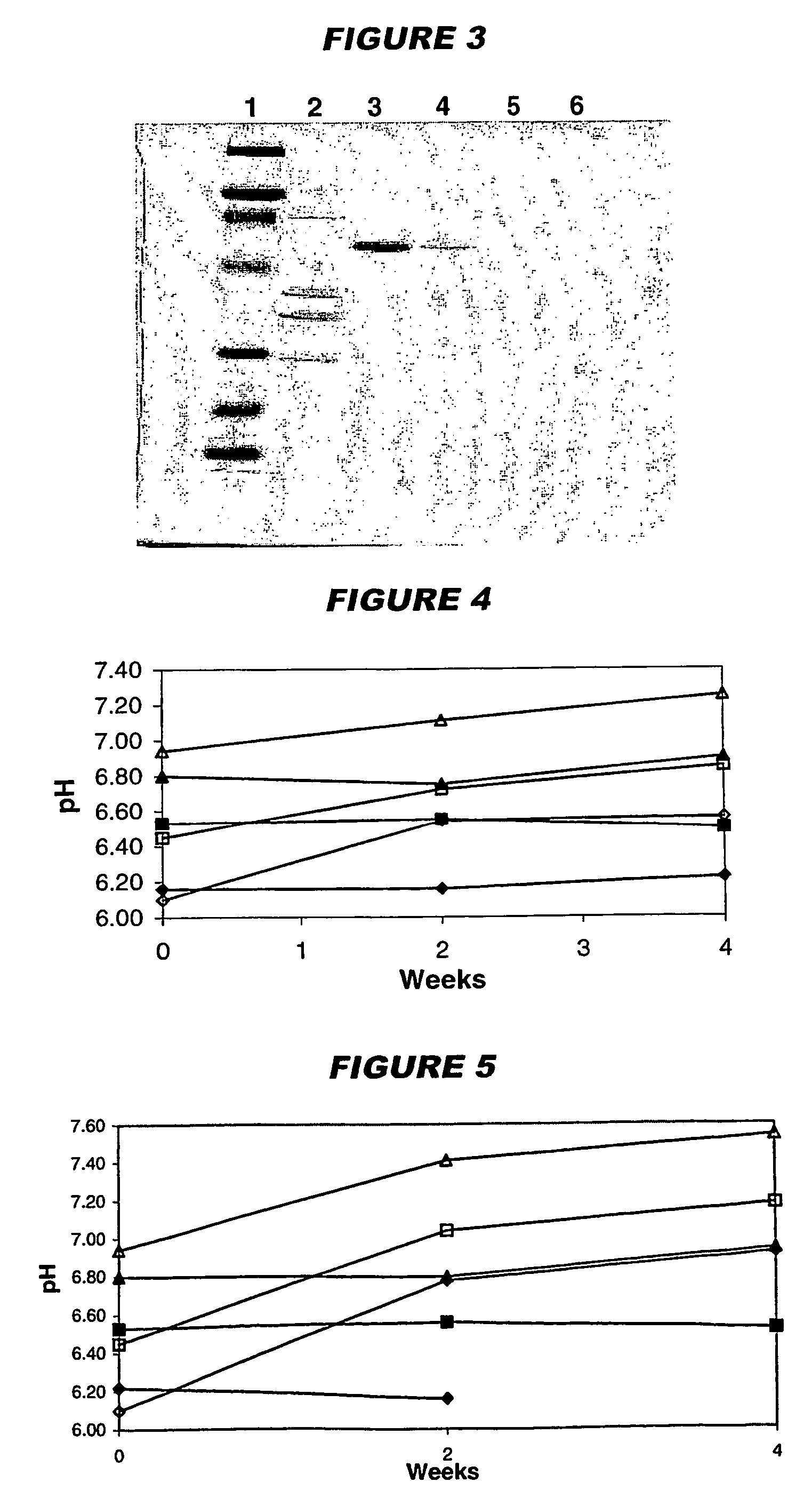
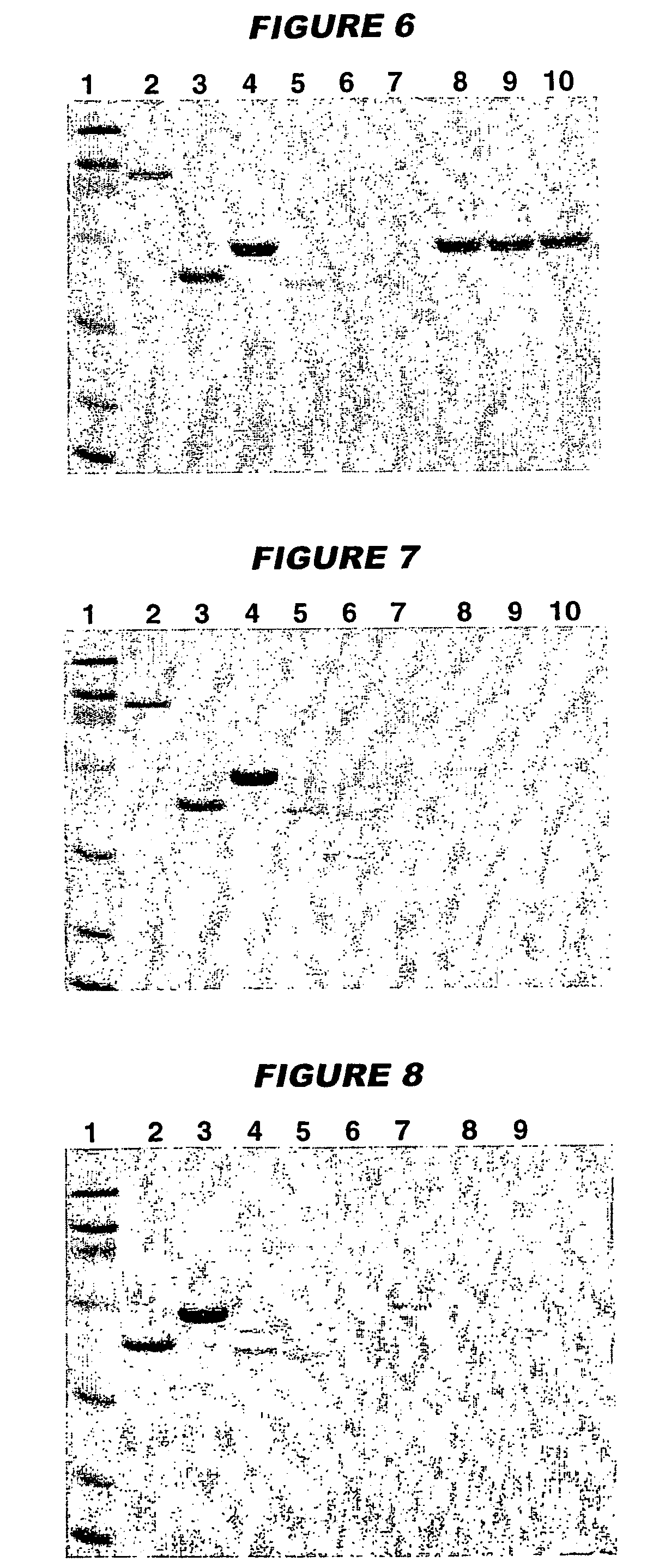
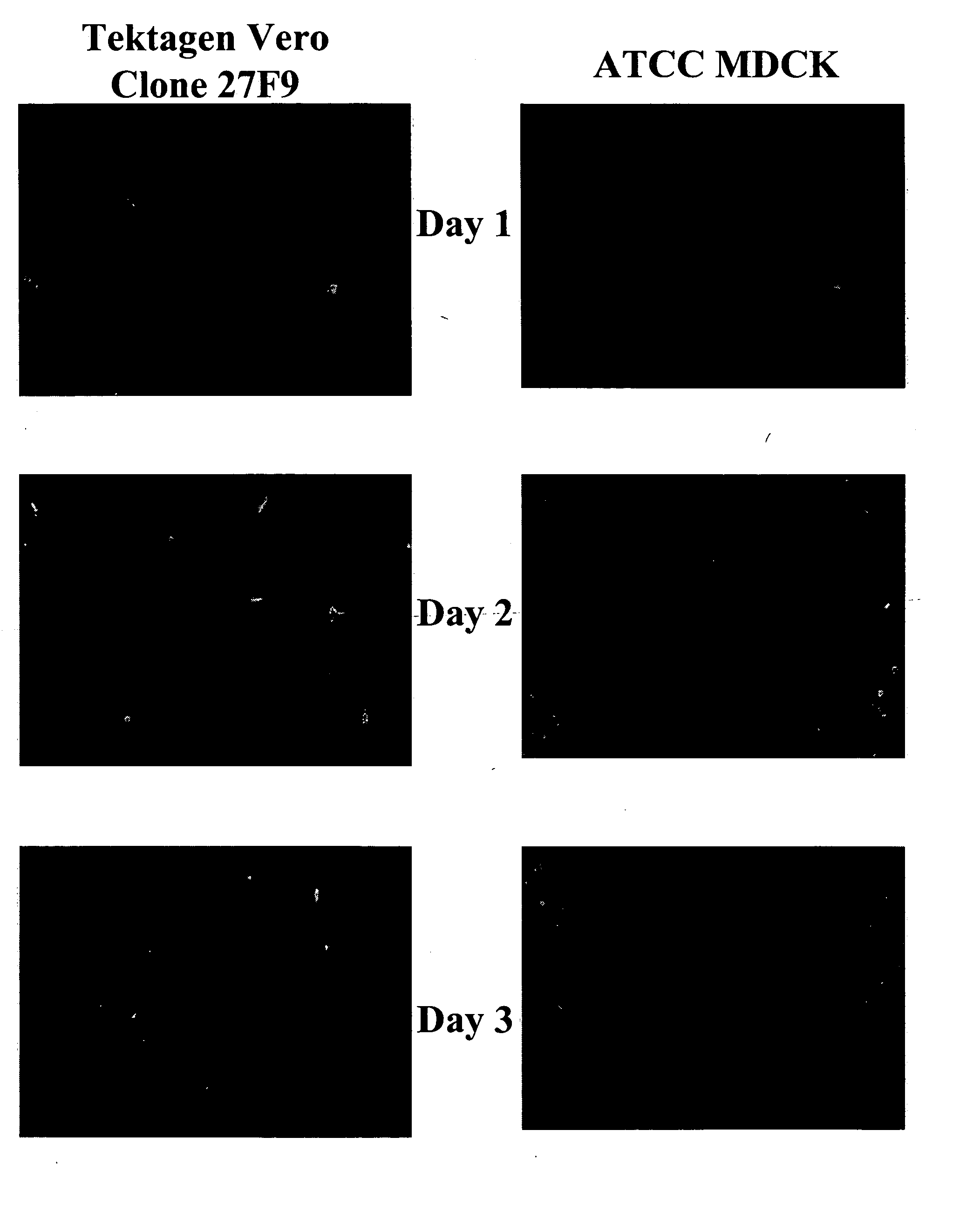
The present invention provides novel MDCK-derived adherent non-tumorigenic cell lines that can be grown in the presence or absence of serum. The cell lines of the present invention are useful for the production of vaccine material (e.g., viruses). More specifically, the cell lines of the present invention are useful for the production of influenza viruses in general and ca / ts influenza viruses in particular. The invention further provides methods and media formulations for the adaptation and cultivation of MDCK cells such that they remain non-tumorigenic. Additionally, the present invention provides methods for the production of vaccine material (e.g., influenza virus) in the novel cell lines of the invention.
Owner:MEDIMMUNE LLC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com