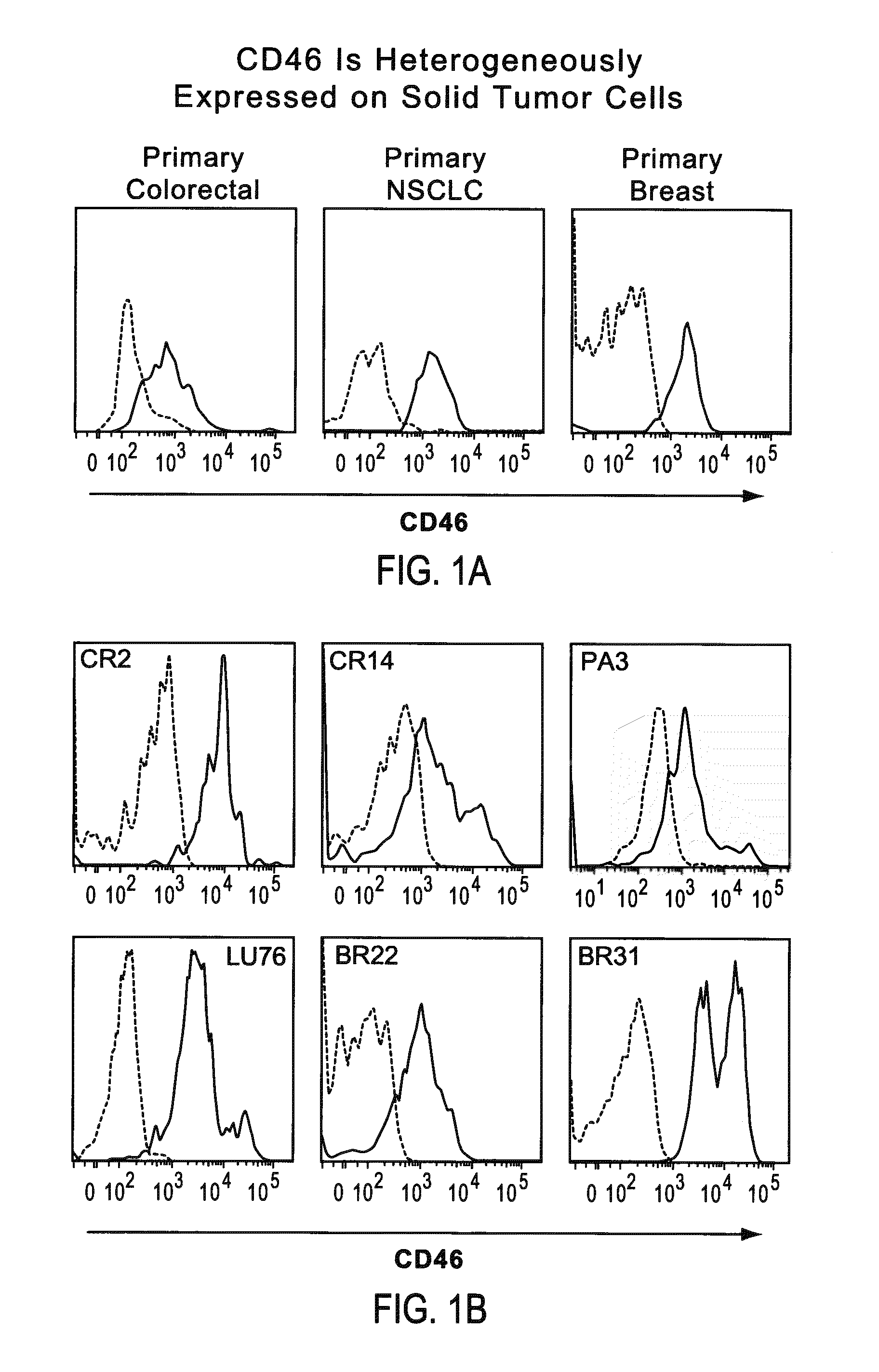
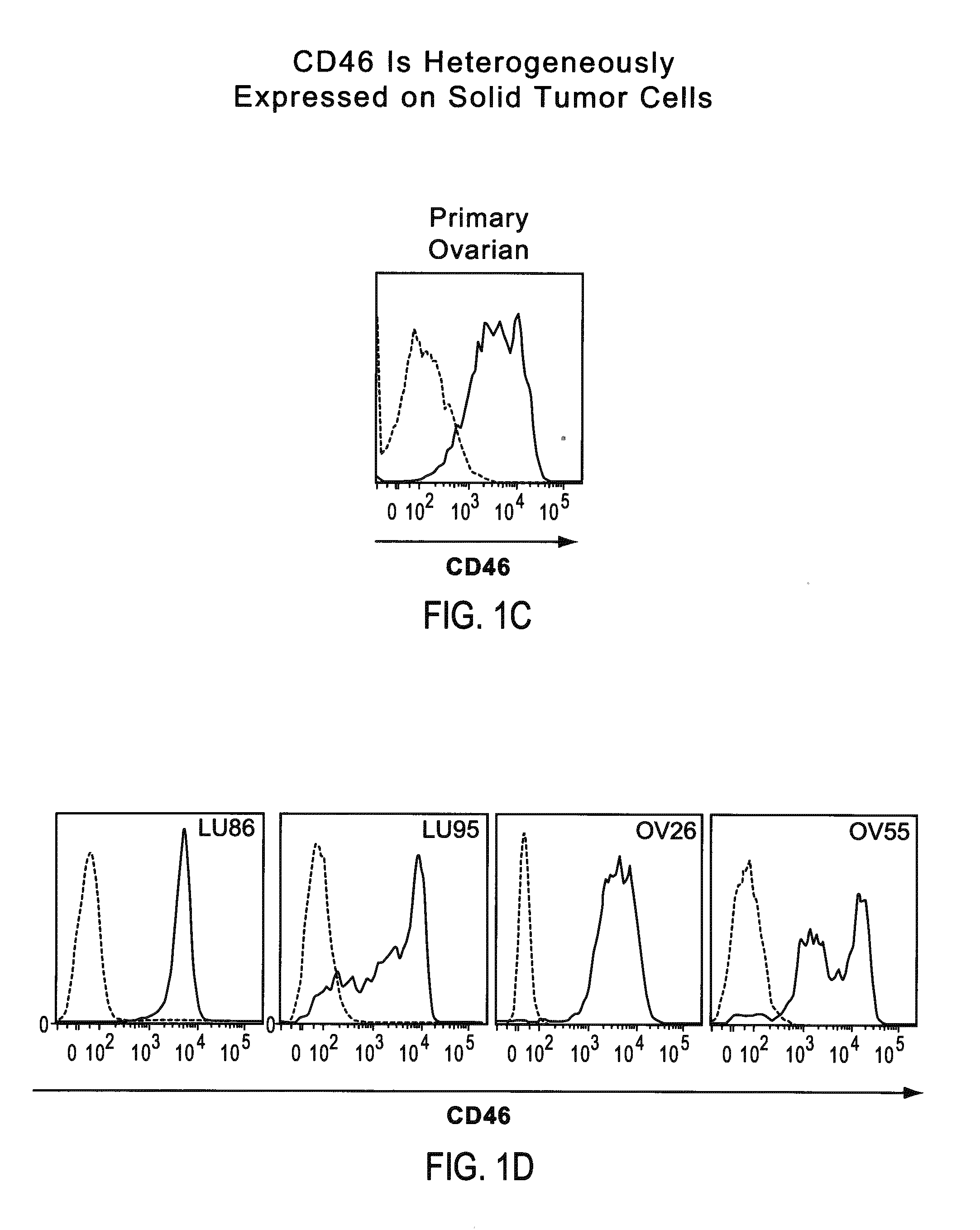
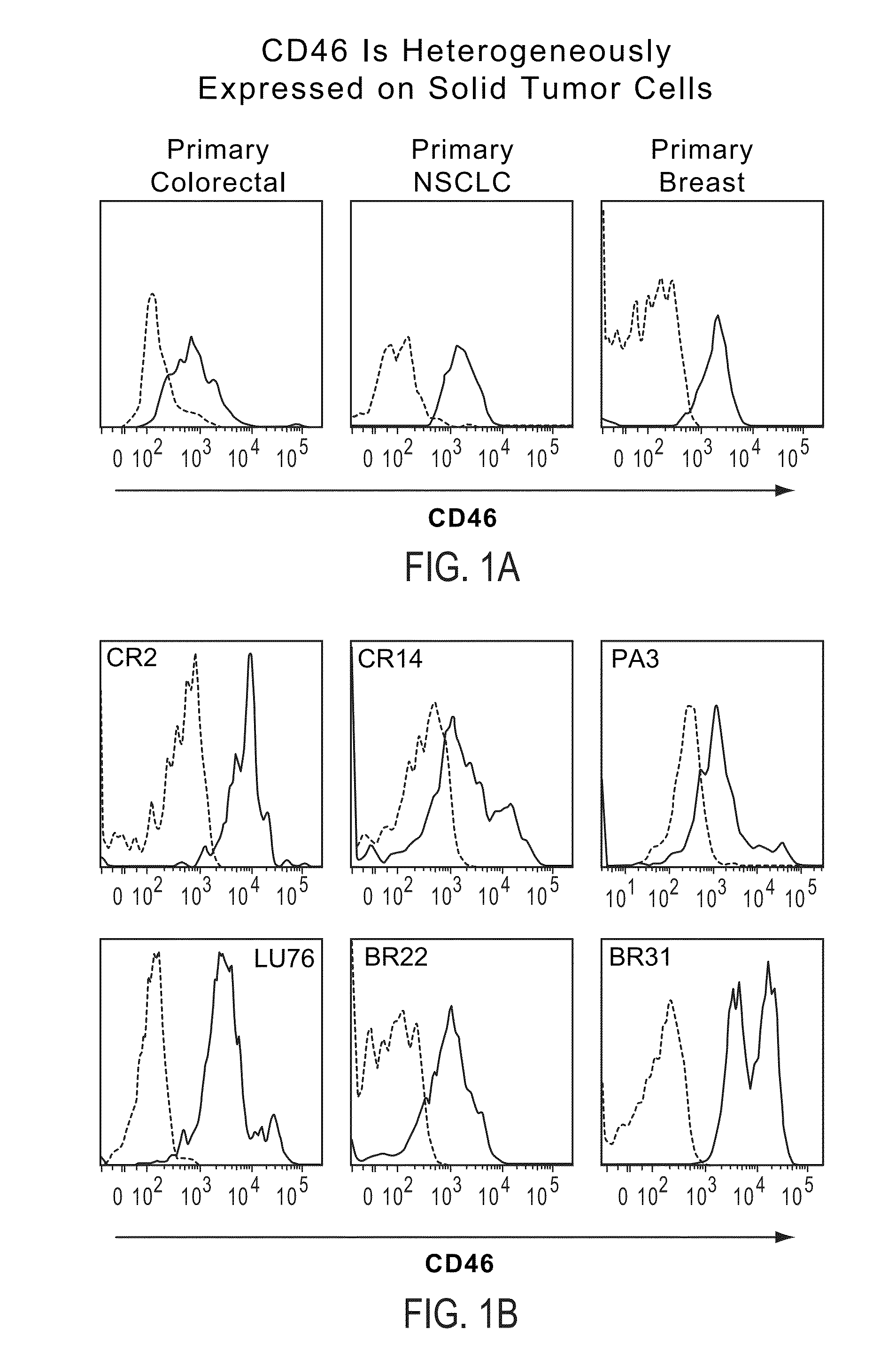
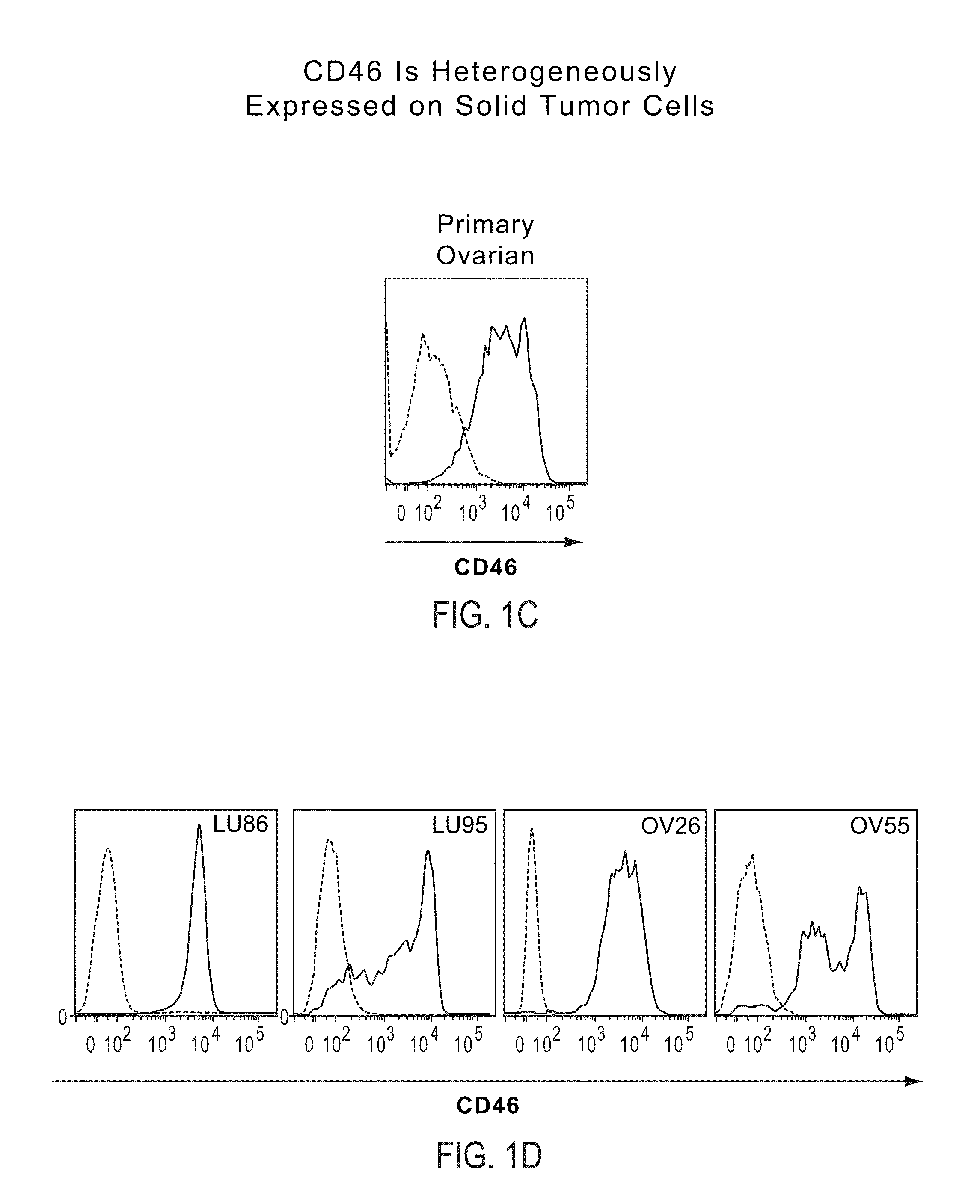
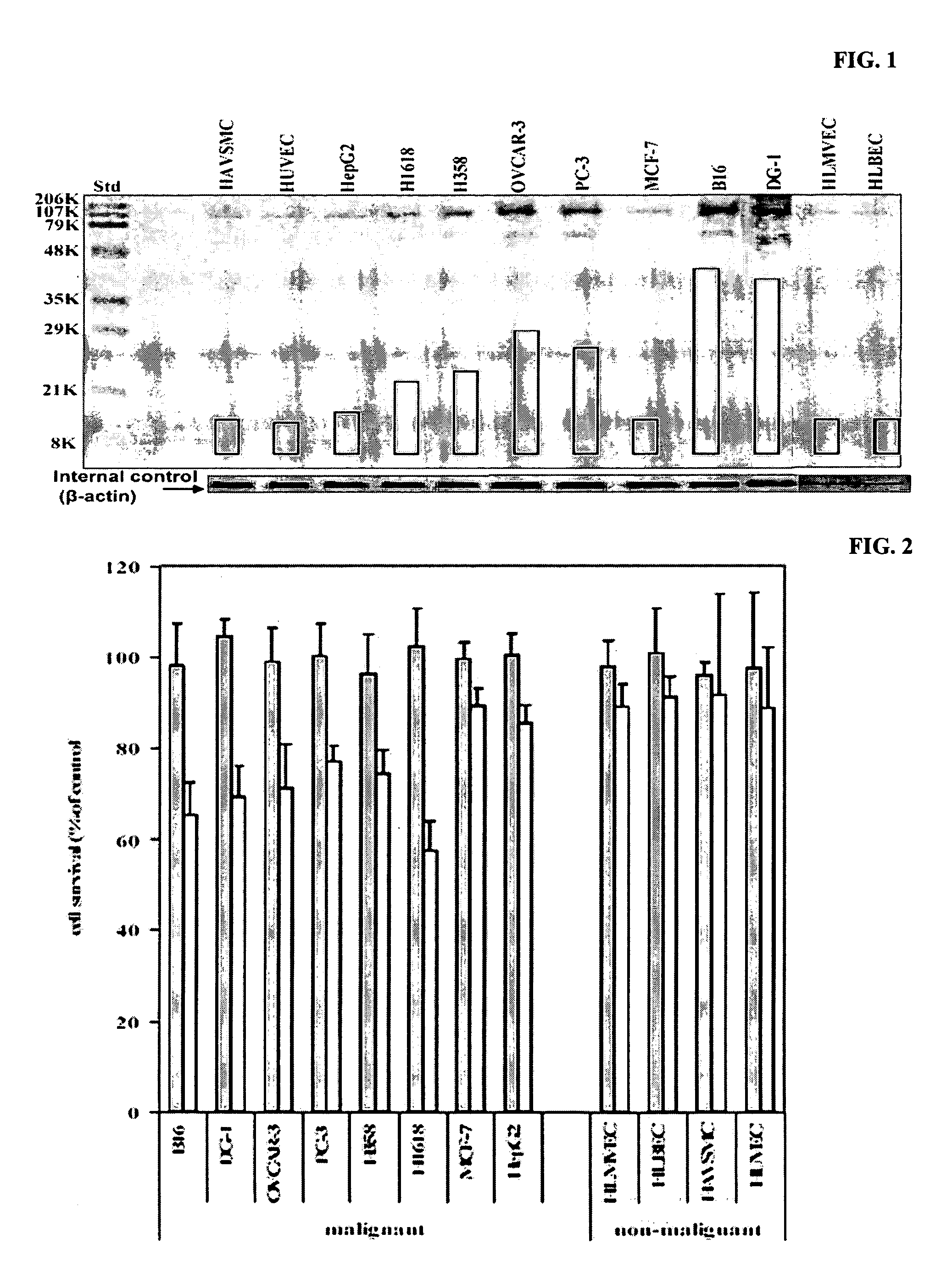
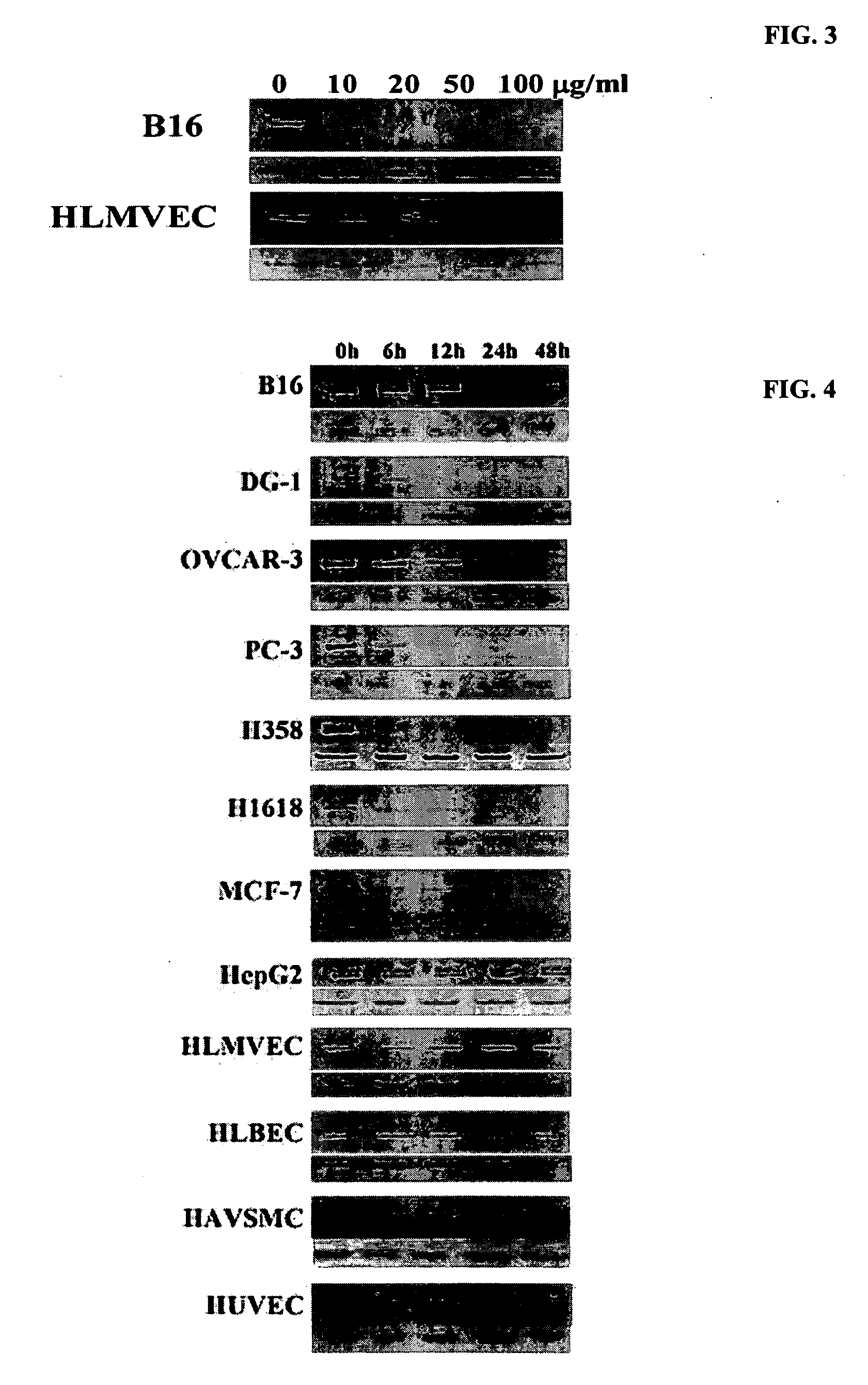
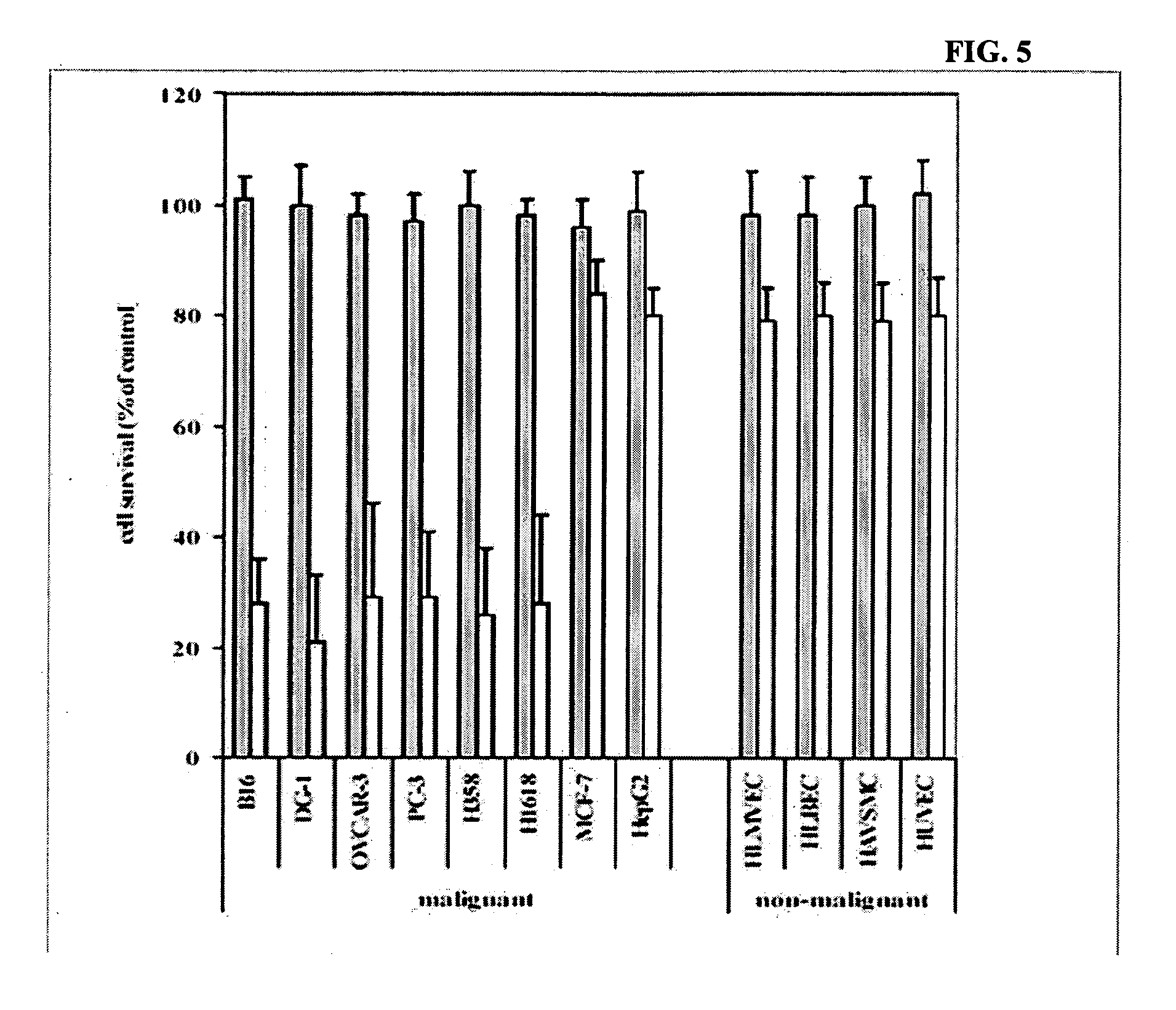
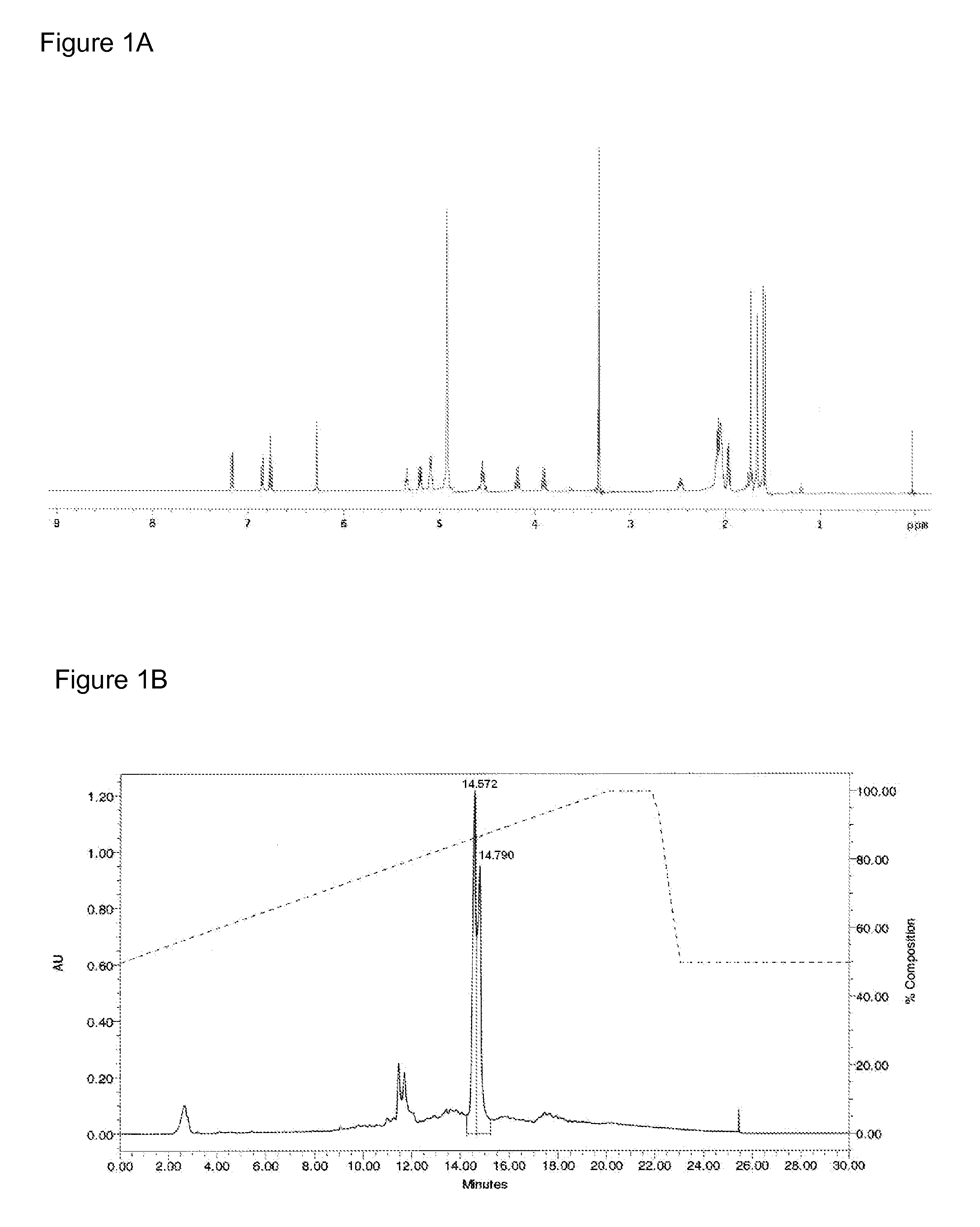
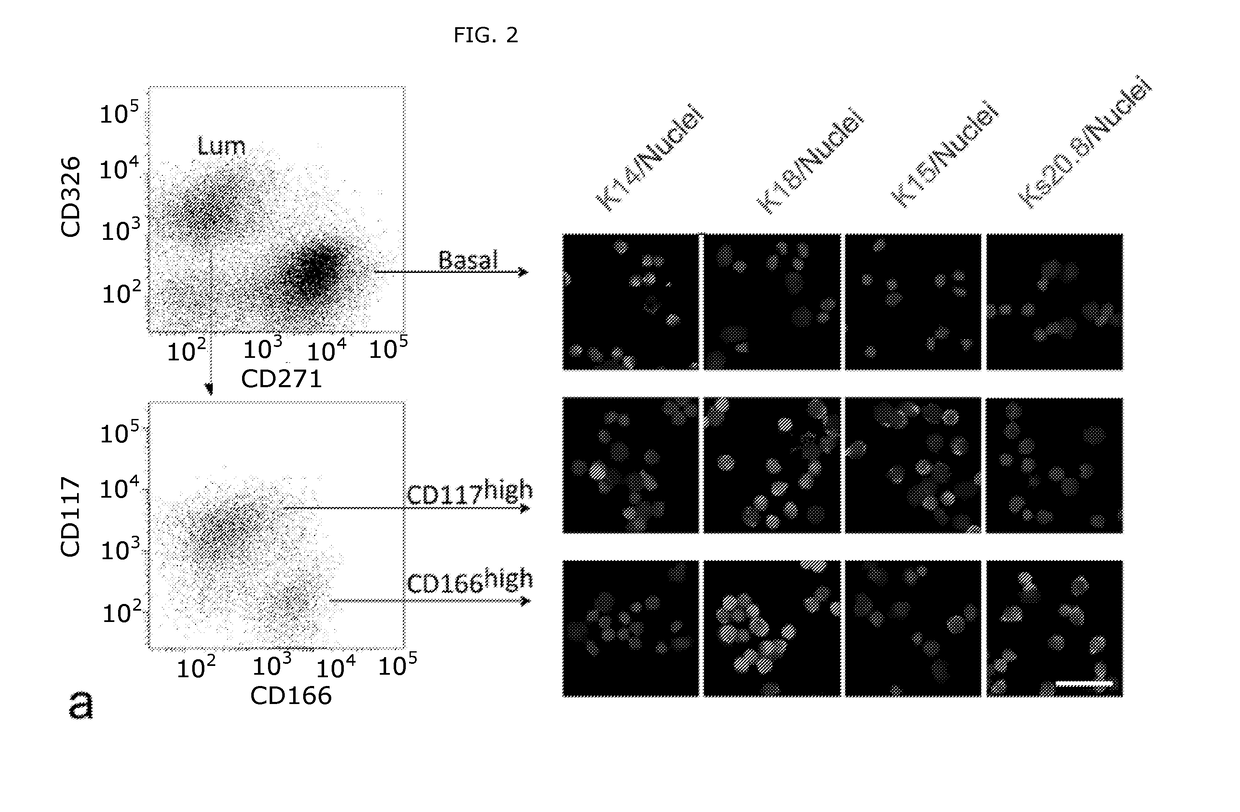
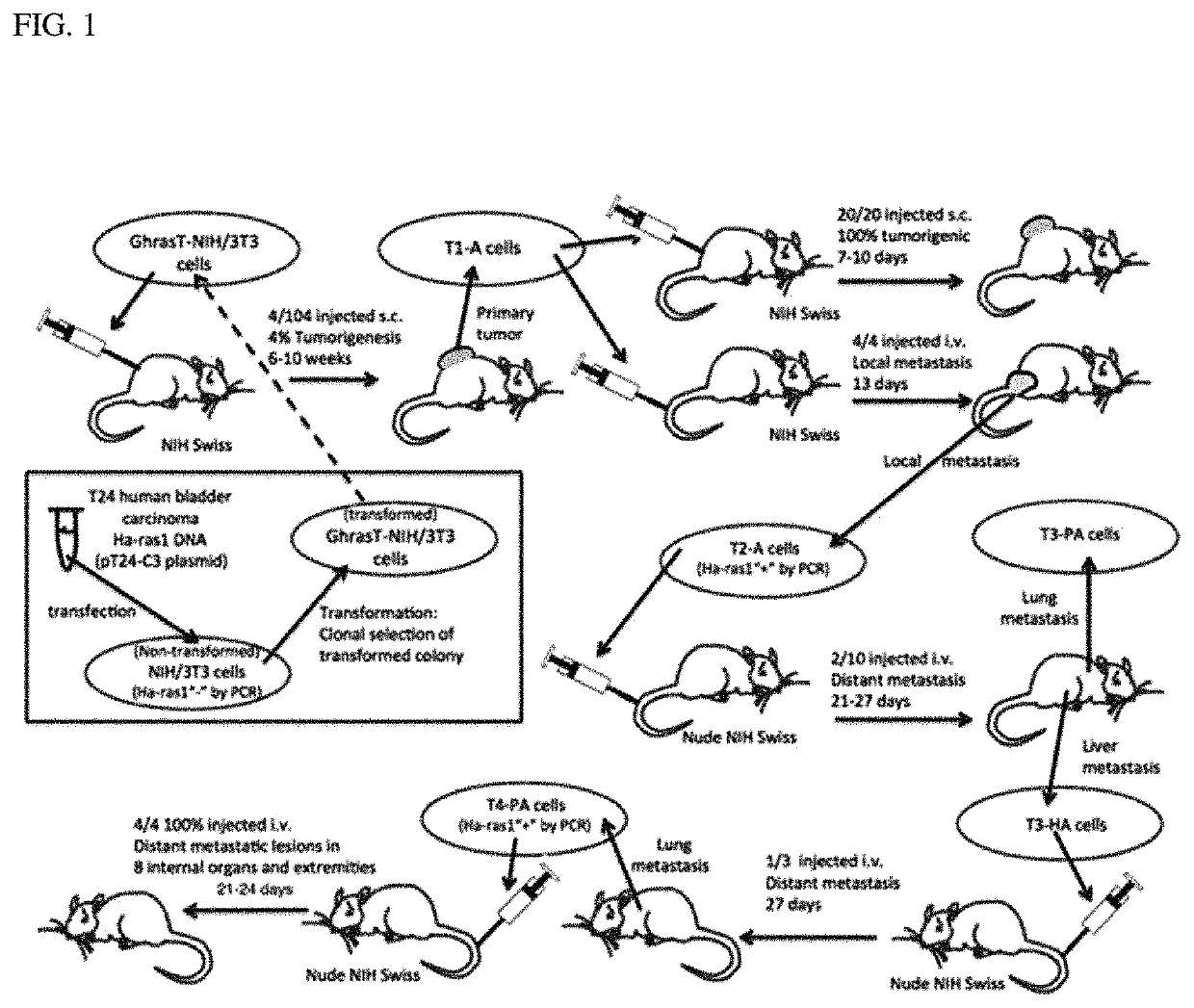
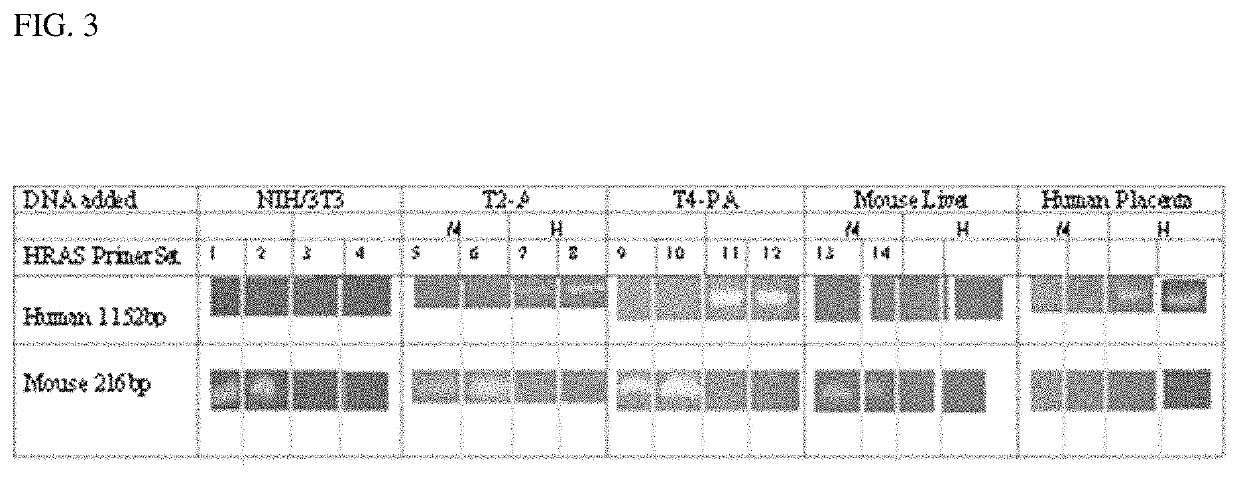
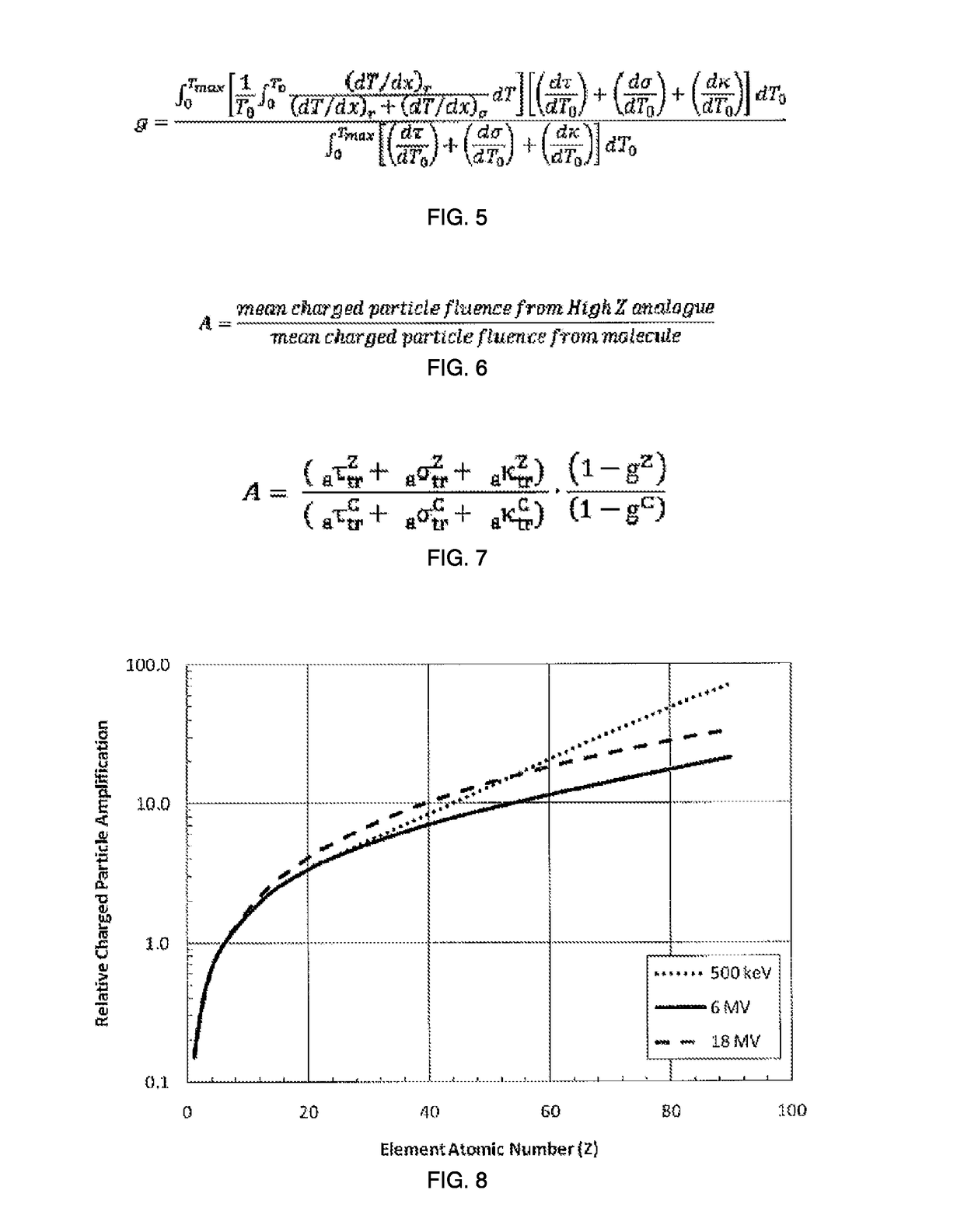
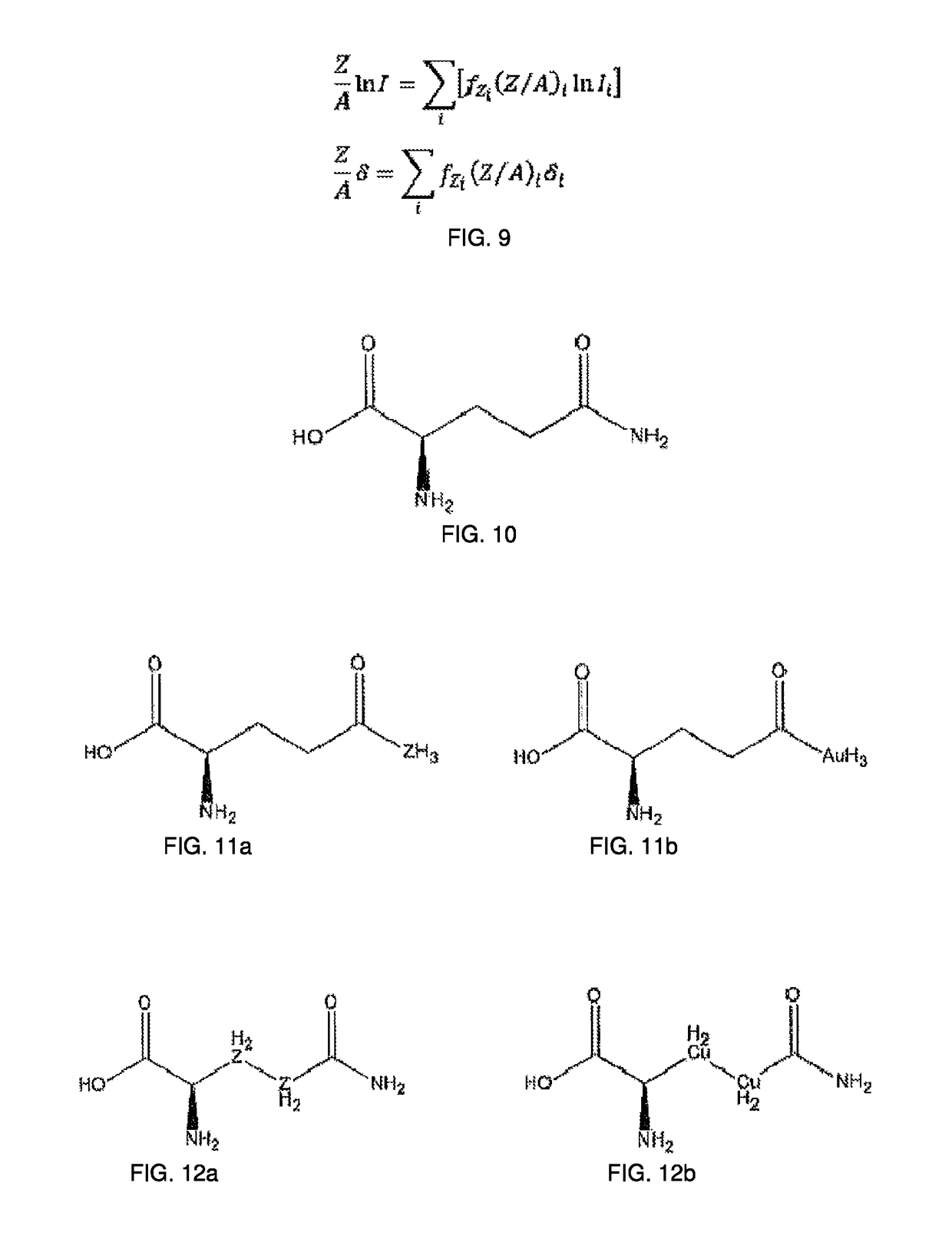
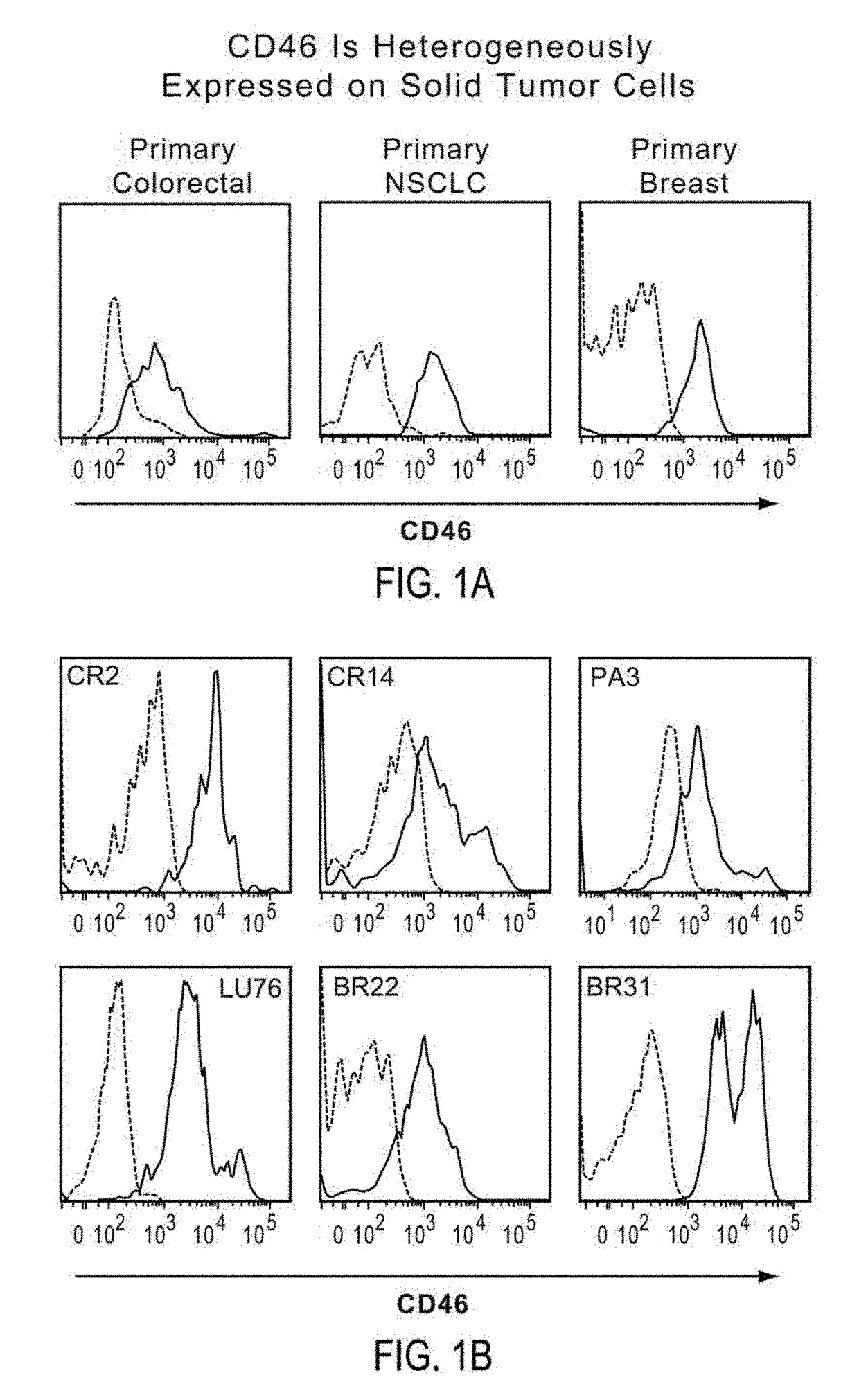
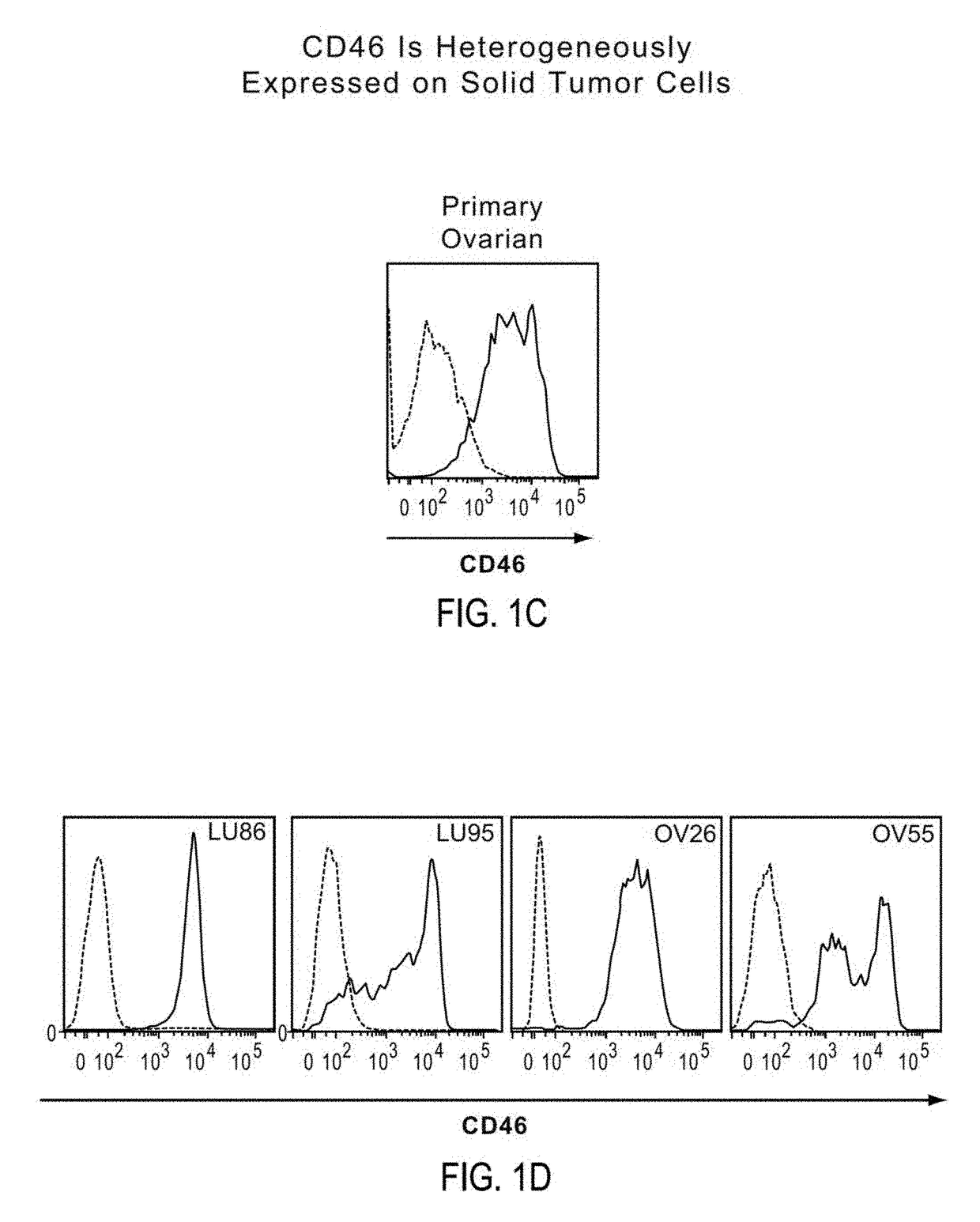
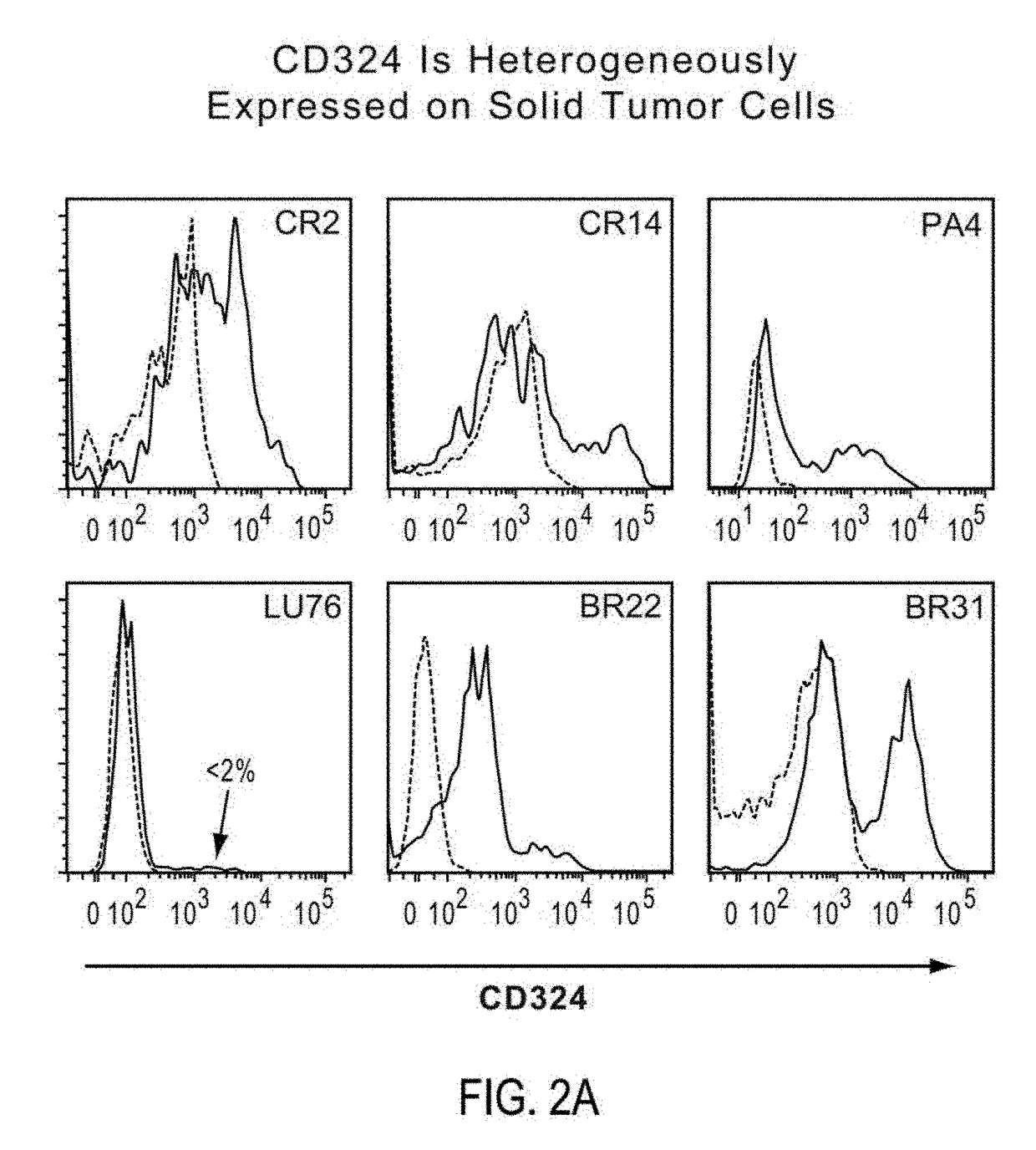
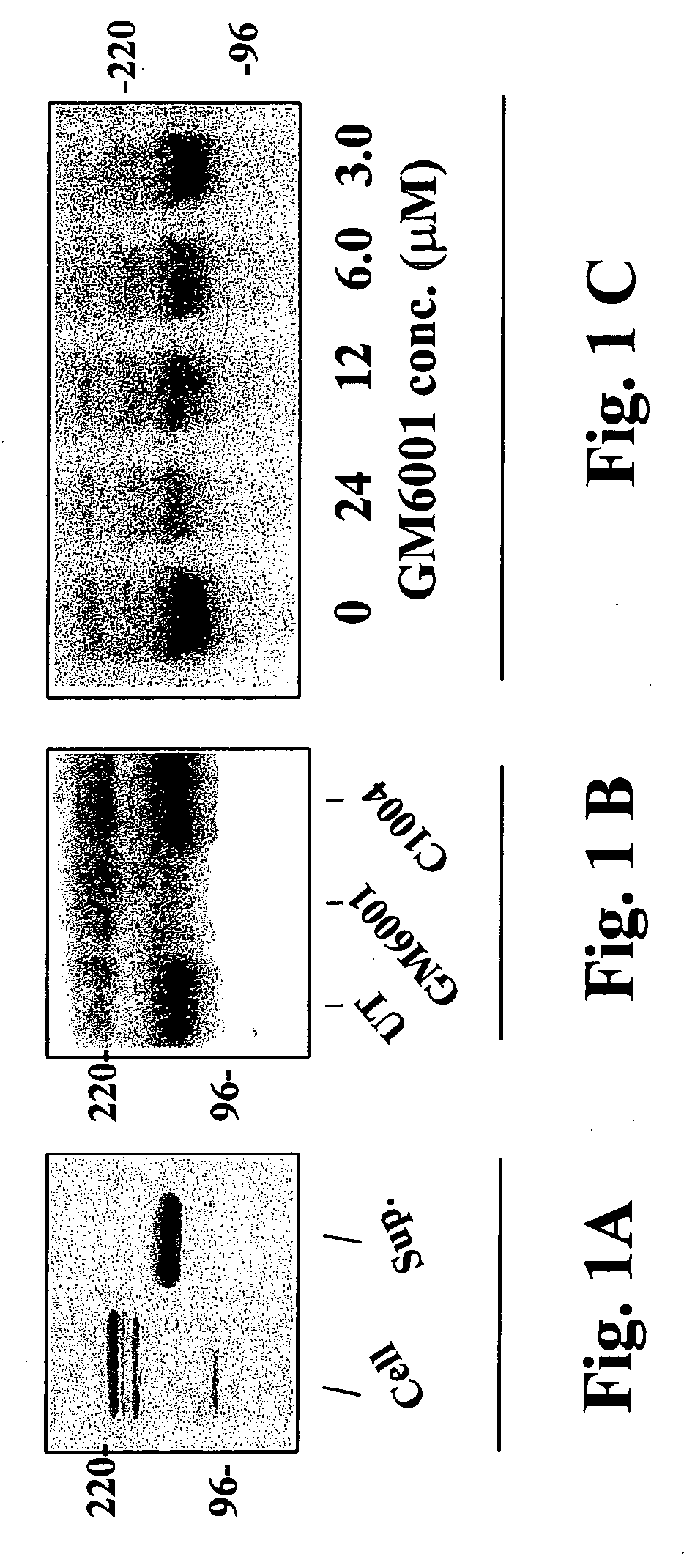
Patents
Literature
32 results about "Tumorigenic cell" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
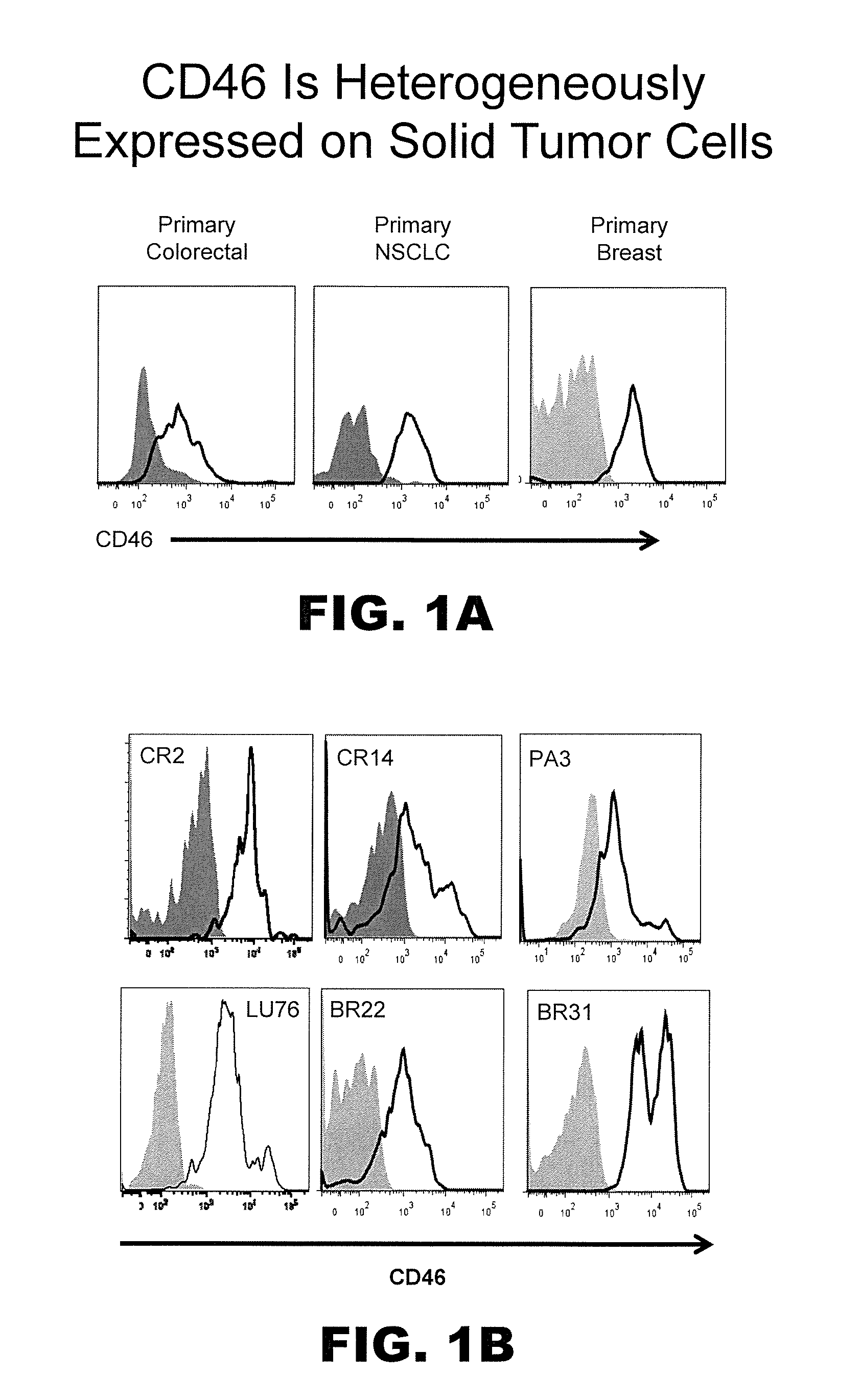
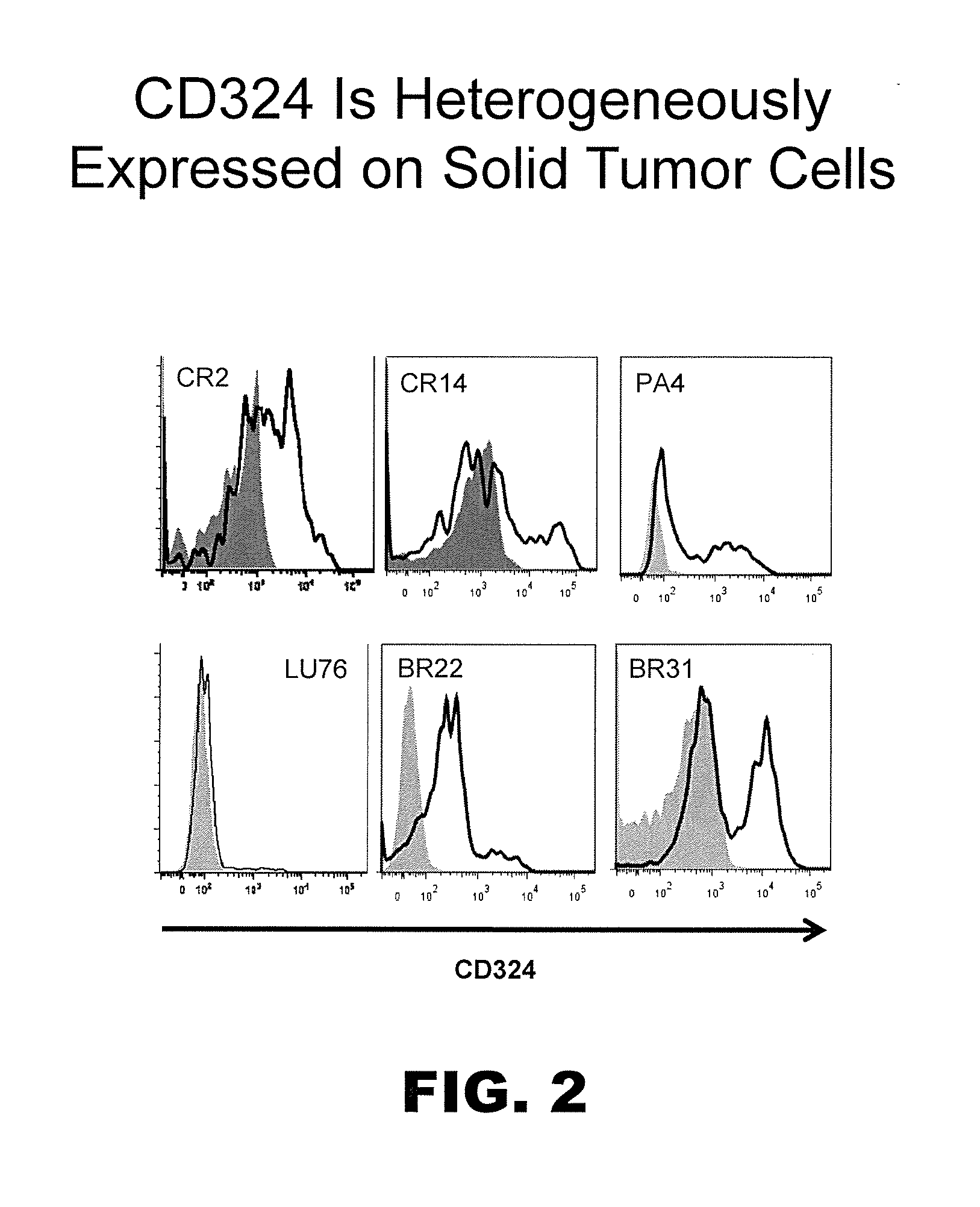
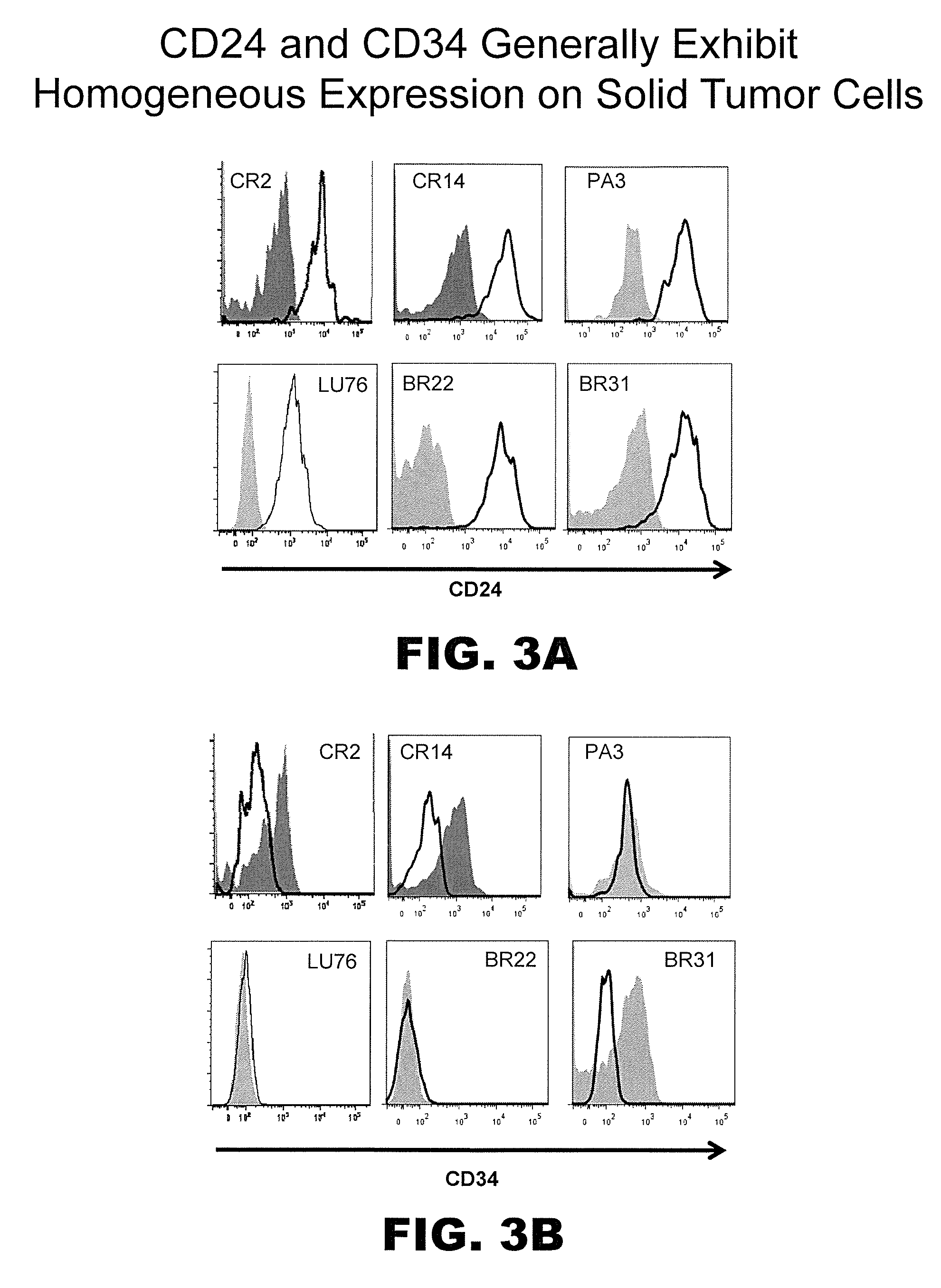
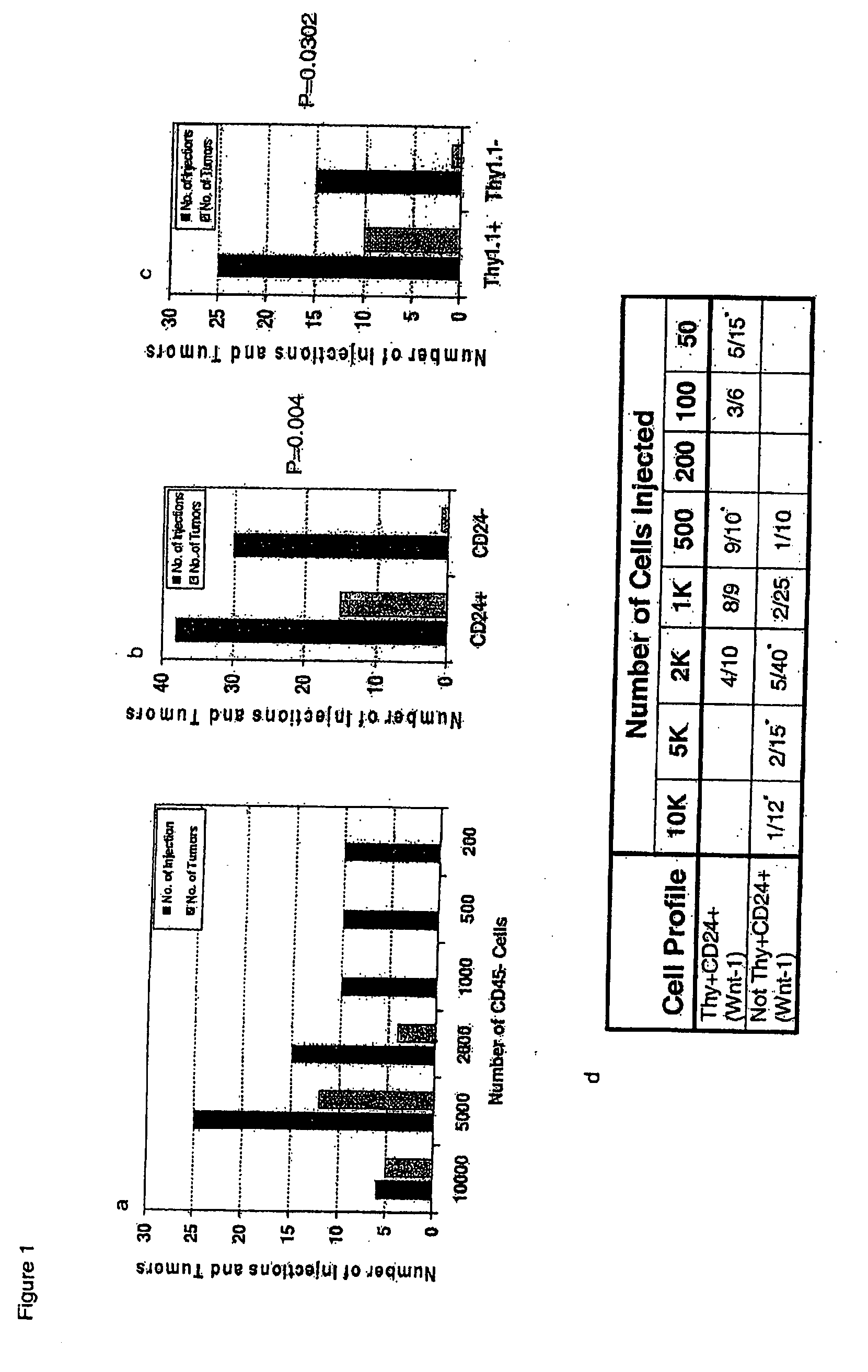
Prospective identification and characterization of breast cancer stem cells
InactiveUS20050089518A1Capacity loseOrganic active ingredientsPeptide/protein ingredientsAbnormal tissue growthSurface marker
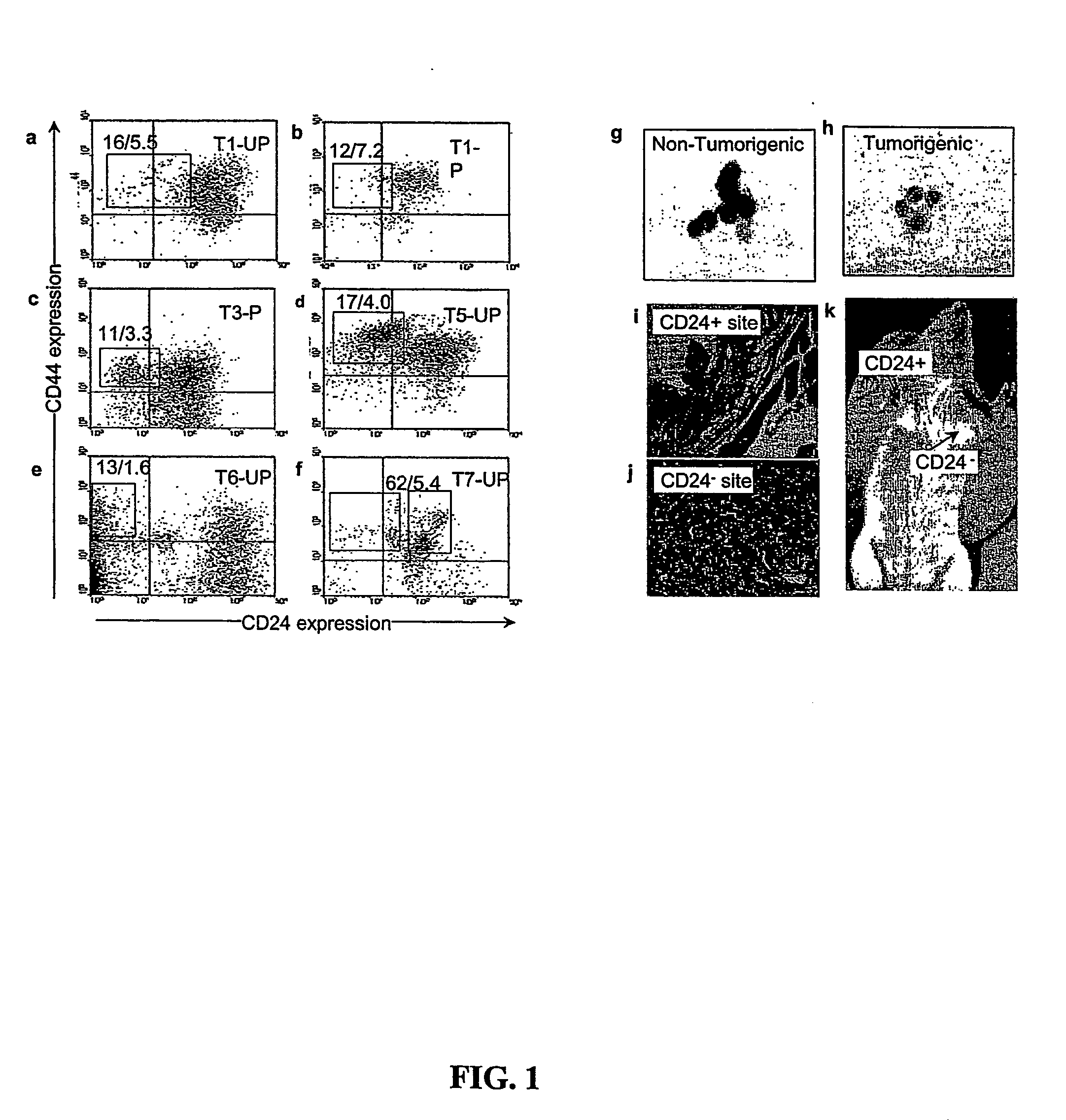
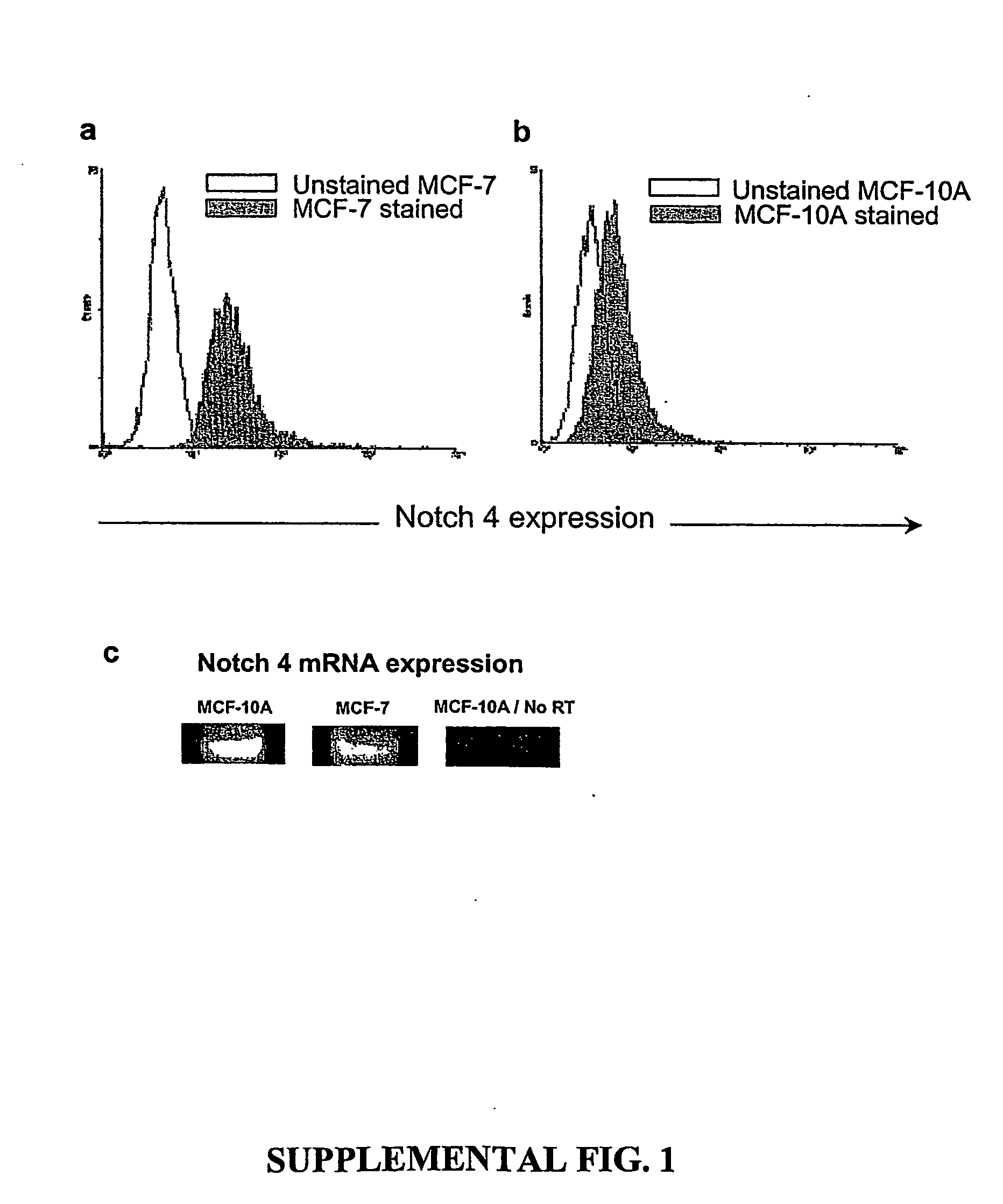
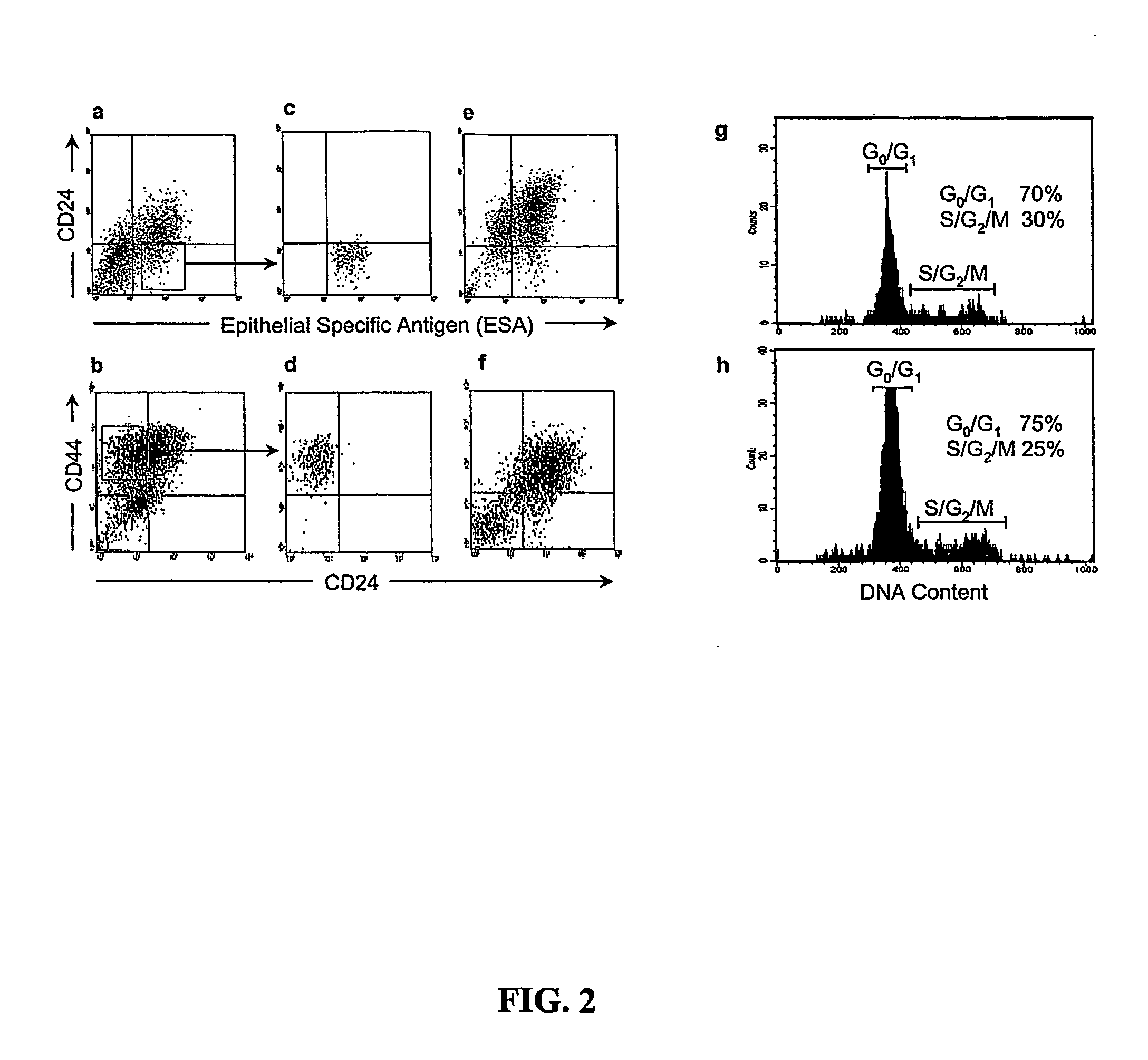
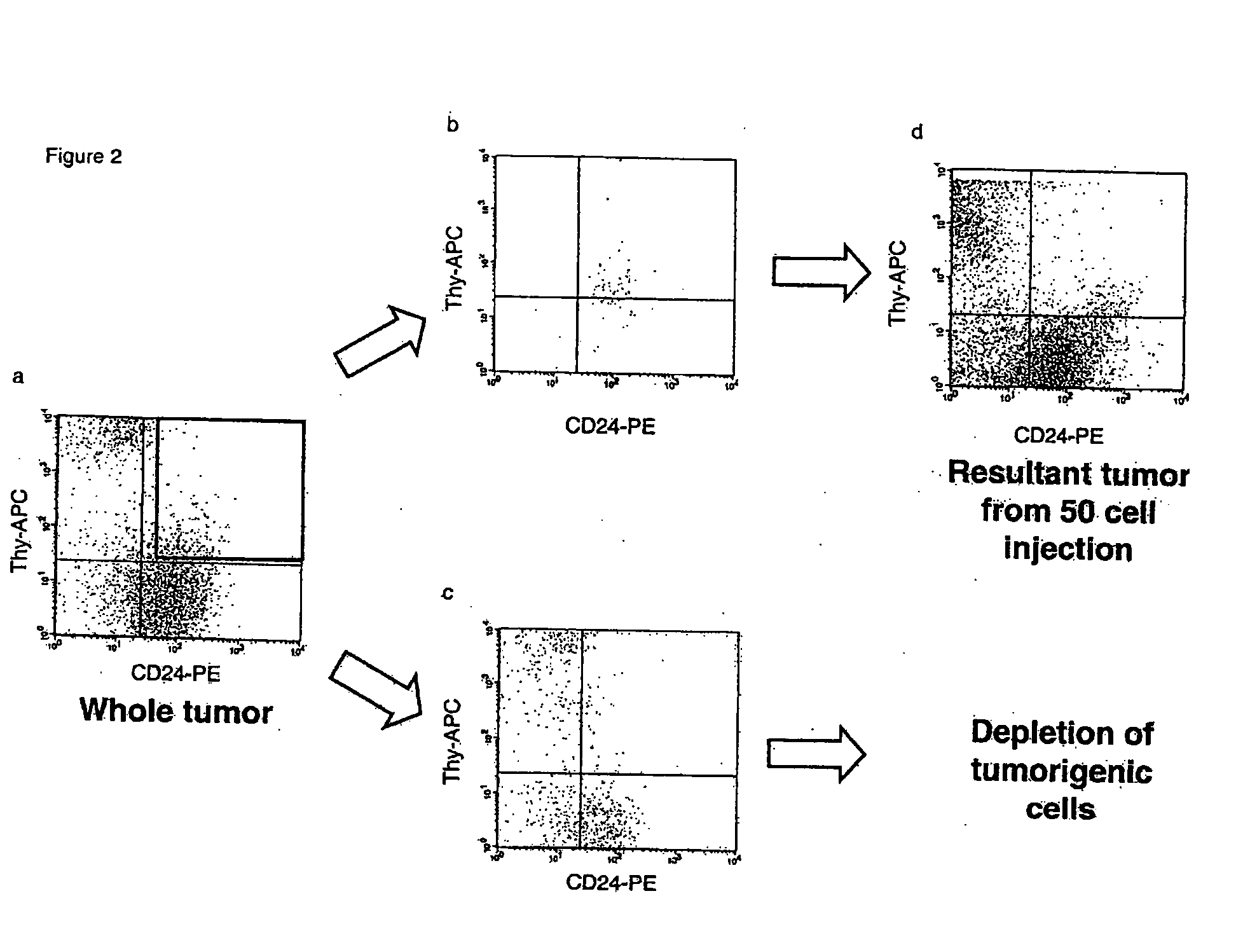
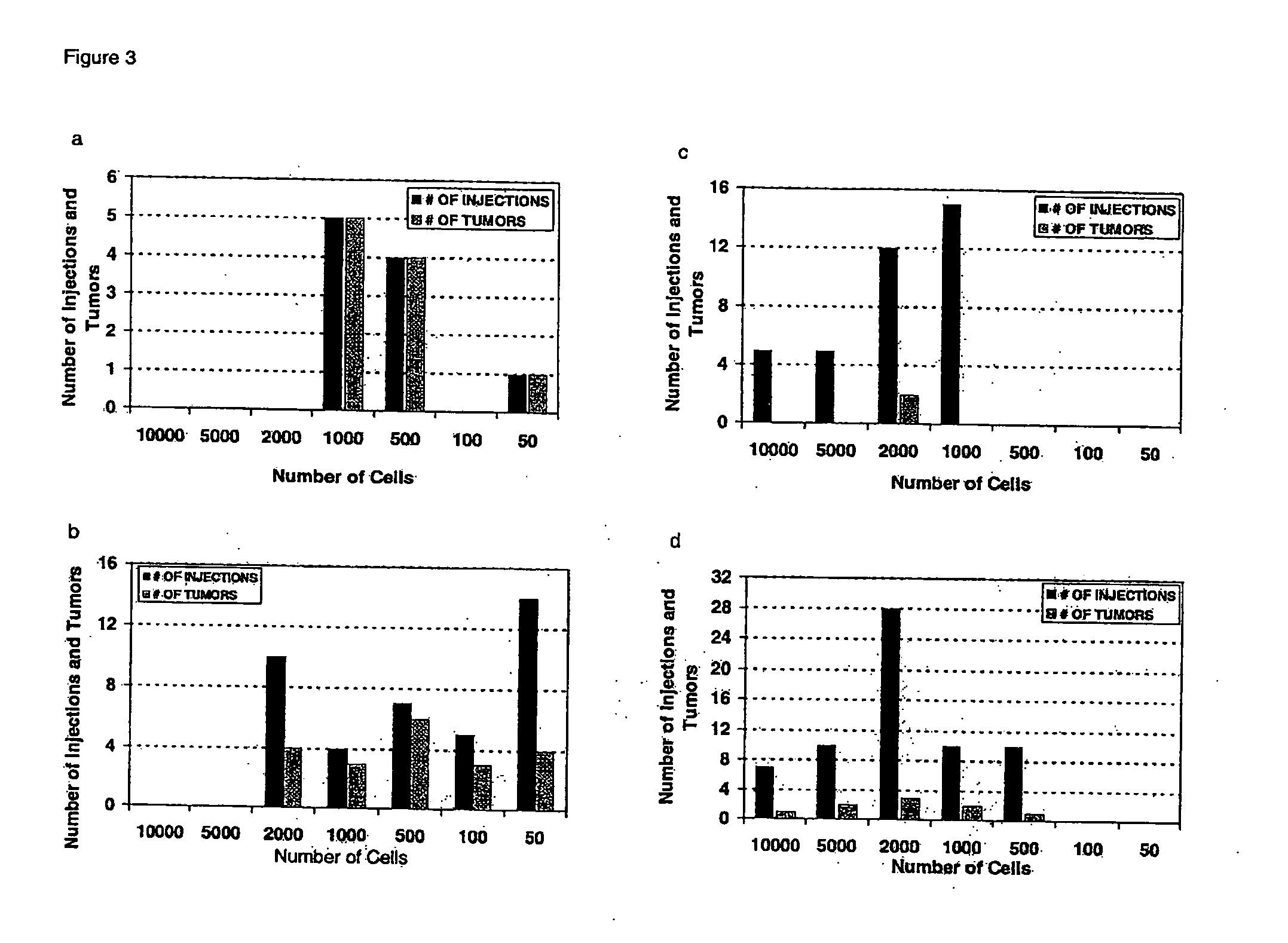
Human breast tumors contain hetrogeneous cancer cells. using an animal xenograft model in which human breast cancer cells were grown in immunocompromised mice we found that only a small minority of breast cancer cells had capacity to form new tumors. The ability to form new tumors was not a slochastic property, rather certain populations of cancer cells were depleted for the ability to form new tumors, while other populations were enriched for the ability to form new tumors. Tumorigenic cells could be distinguished from non-tumorigenic cancer cells based on surface marker expression. We prospectively identified and isolated the tumorigenic cells as CD4430CD24− / lowLINEAGE A few as 100 cells from this population were able to form tumors the animal xenograft model, while tens of thousands of cells from non-tumorigenic populations failed to form tumors. The tumorigenic cells could be serially passaged, each time generating new tumors containing and expanded numbers of CD44+CD24 Lineage tumorigenic cells as well as phenotypically mixed populations of non-tumorigenic cancer cells. This is reminiscent of the ability of normal stem cells to self-renew and differentiate. The expression of potential therapeutic targets also differed between the tumorigenic and non-tumorigenic populations. Notch activation promoted the survival of the tumorigenic cells, and a blocking antibody against Notch 4 induced tumorigenic breast cancer cells to undergo apoptosis.
Owner:RGT UNIV OF MICHIGAN
Identification and Enrichment of Cell Subpopulations
InactiveUS20130061340A1Efficiently employedCompound screeningApoptosis detectionBiochemistryCell subpopulations
Markers useful for the identification, characterization and, optionally, the enrichment or isolation of tumorigenic cells or cell subpopulations are disclosed.
Owner:ABBVIE STEMCENTRX LLC
Identification and Enrichment of Cell Subpopulations
InactiveUS20130061342A1Efficiently employedOther foreign material introduction processesFermentationBiochemistryCell subpopulations
Markers useful for the identification, characterization and, optionally, the enrichment or isolation of tumorigenic cells or cell subpopulations are disclosed.
Owner:ABBVIE STEMCENTRX LLC
Identification and enrichment of cell subpopulations
InactiveUS20150030636A1Efficiently employedLibrary screeningCancer antigen ingredientsBiochemistryCell subpopulations
Markers useful for the identification, characterization and, optionally, the enrichment or isolation of tumorigenic cells or cell subpopulations are disclosed.
Owner:ABBVIE STEMCENTRX LLC
Identification and enrichment of cell subpopulations
InactiveUS20130260385A1Convenient researchFacilitate drug development effortMicrobiological testing/measurementBiological material analysisBiochemistryCell subpopulations
Markers useful for the identification, characterization and, optionally, the enrichment or isolation of tumorigenic cells or cell subpopulations are disclosed.
Owner:ABBVIE STEMCENTRX LLC
Genetic characterization and prognostic significance of cancer stem cells in cancer
InactiveUS20070220621A1Predictive of survivalReduce spreadNew breed animal cellsMicrobiological testing/measurementSolid tumorGene expression
The present invention is related to the identification of cancer stem cells using the MMTV-Wnt-1 transgenic mouse model. These cancer stem cells have a gene expression signature that allows them to be distinguished from their non-tumorigenic counterparts. Moreover, the gene expression pattern can also predict survival in a diverse group of solid tumors.
Owner:CLARKE MICHAEL F +2
Non-tumorigenic MDCK cell line for propagating viruses
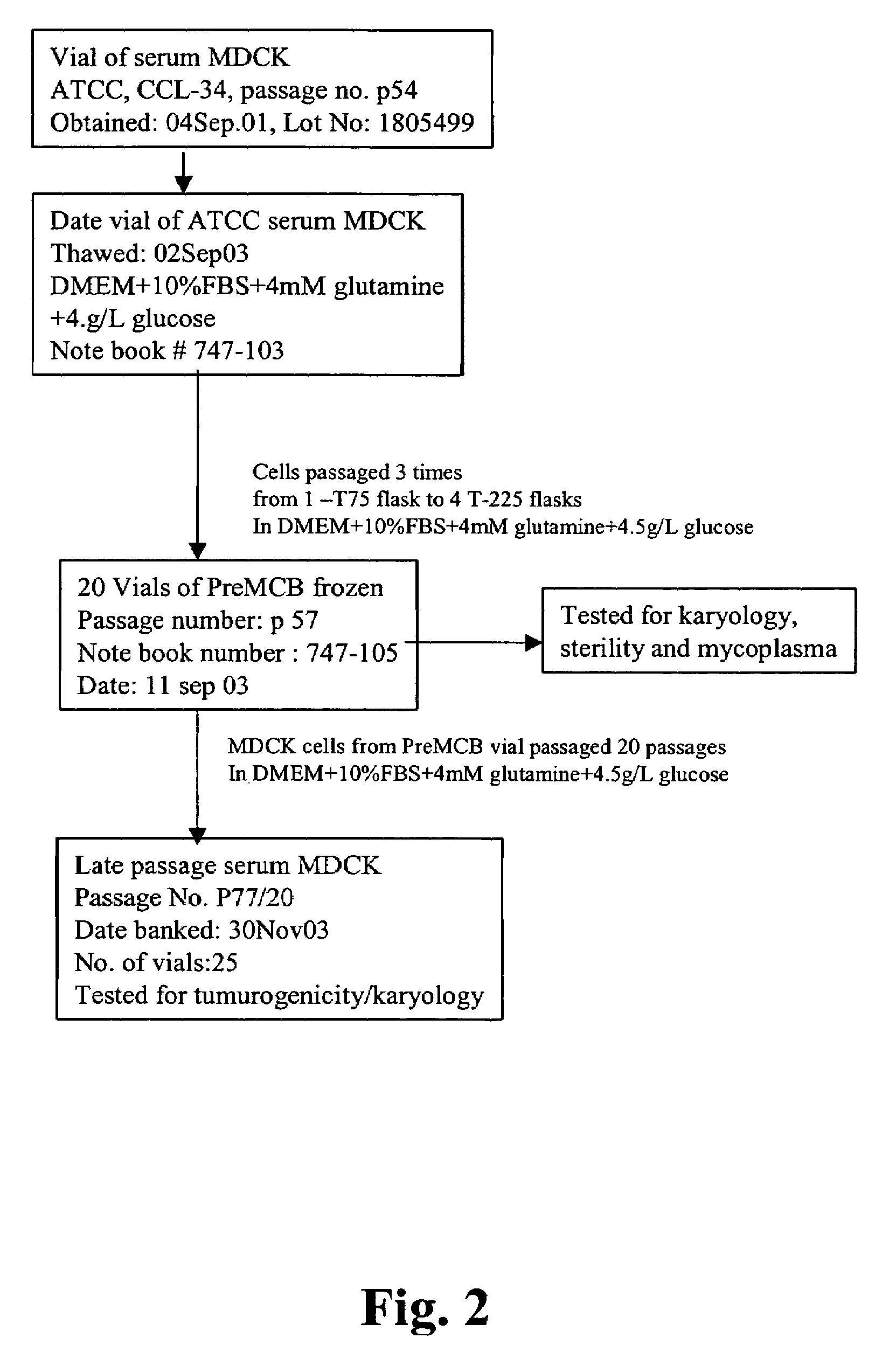
The present invention provides novel MDCK-derived adherent non-tumorigenic cell lines that can be grown in the presence or absence of serum. The cell lines of the present invention are useful for the production of vaccine material (e.g., viruses). More specifically, the cell lines of the present invention are useful for the production of influenza viruses in general and ca / ts influenza viruses in particular. The invention further provides methods and media formulations for the adaptation and cultivation of MDCK cells such that they remain non-tumorigenic. Additionally, the present invention provides methods for the production of vaccine material (e.g., influenza virus) in the novel cell lines of the invention.
Owner:MEDIMMUNE LLC
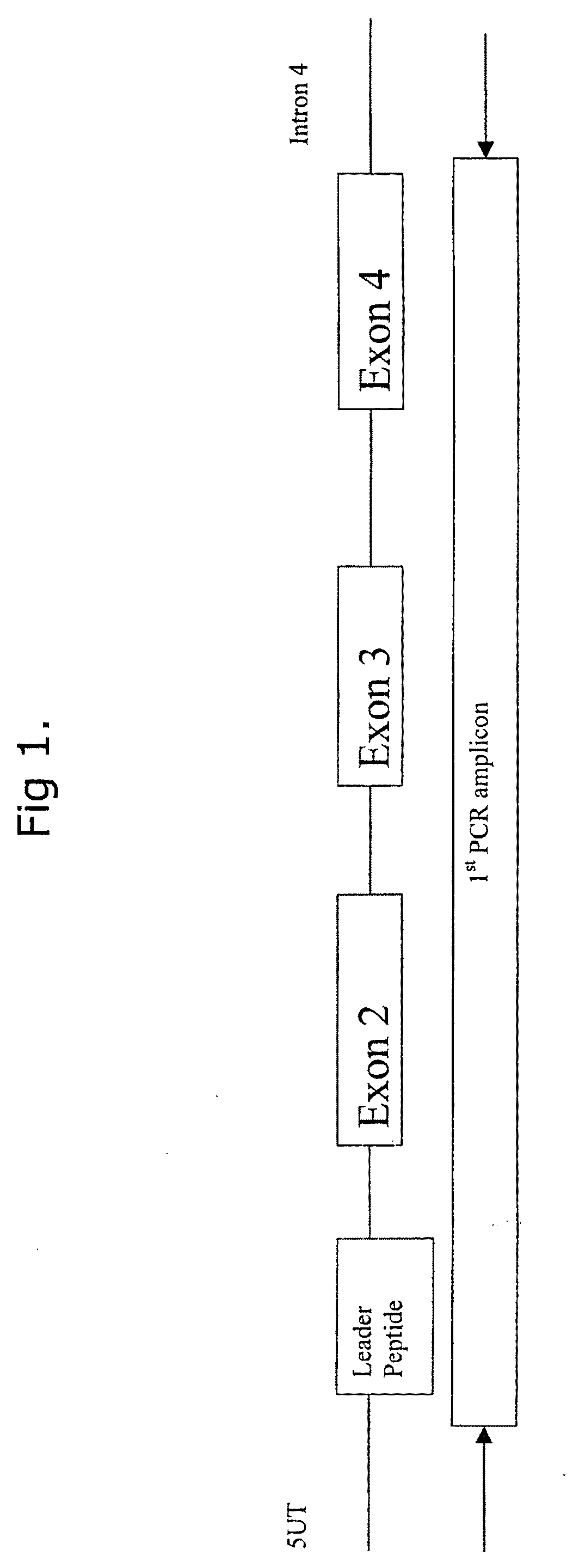
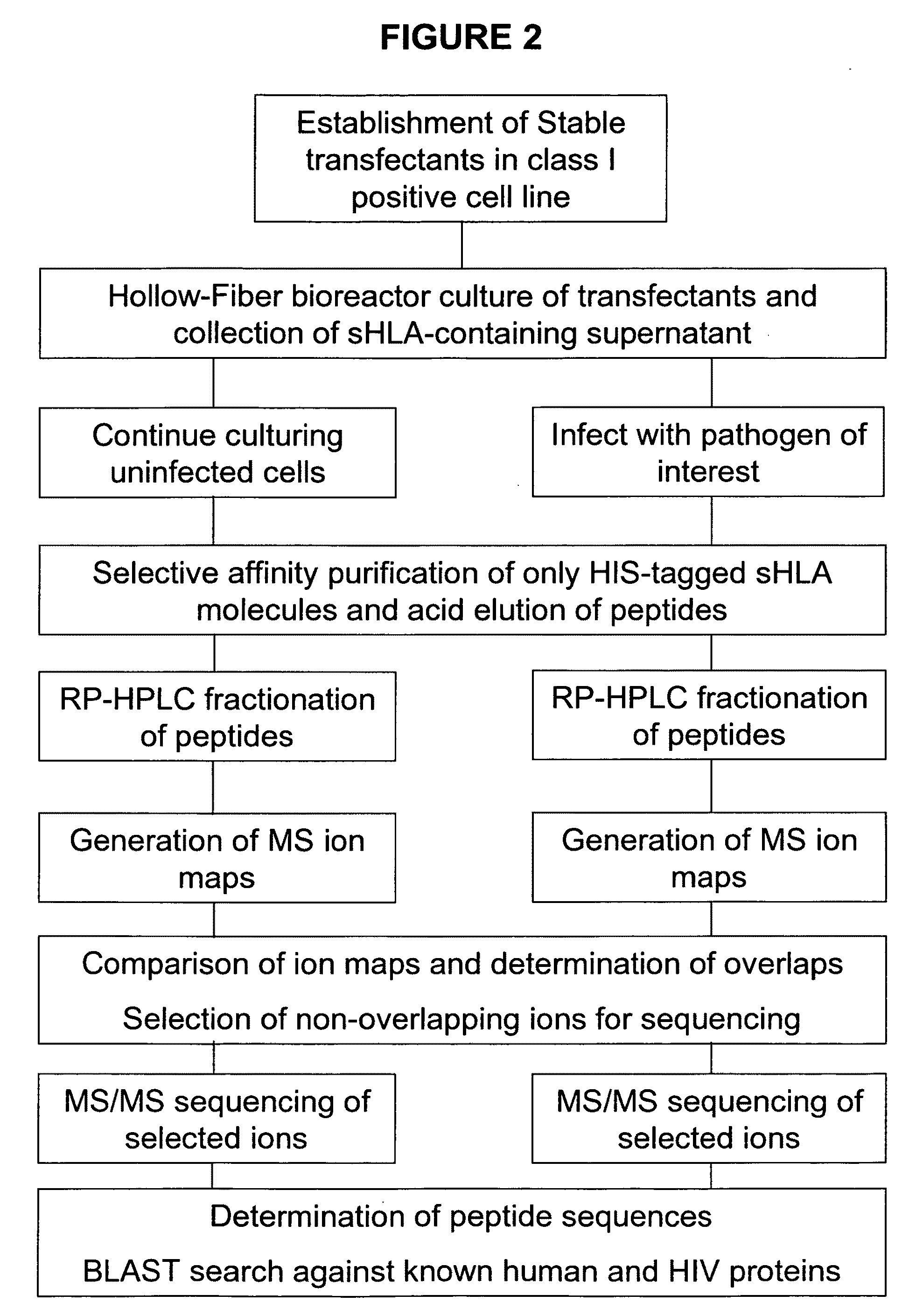
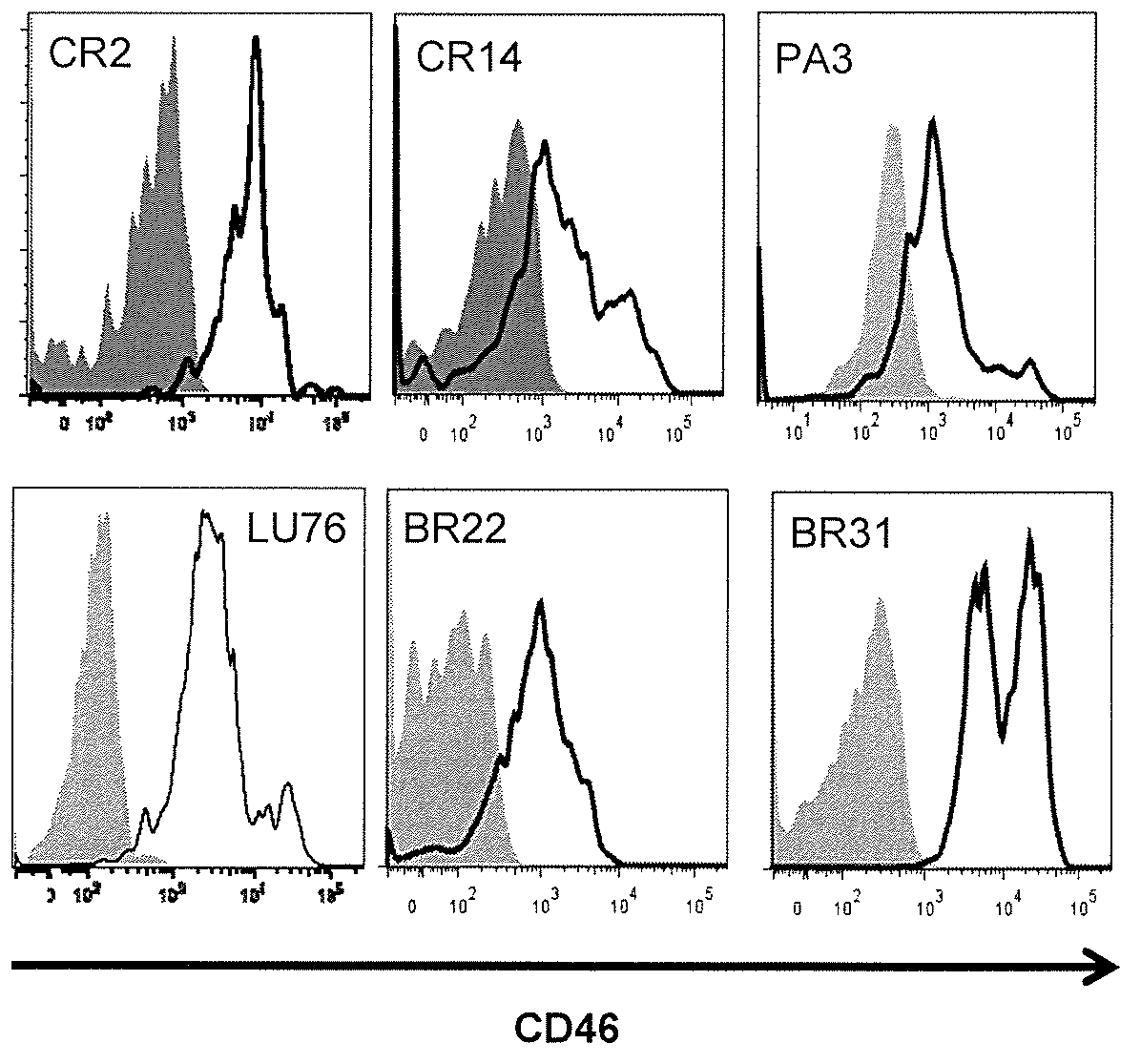
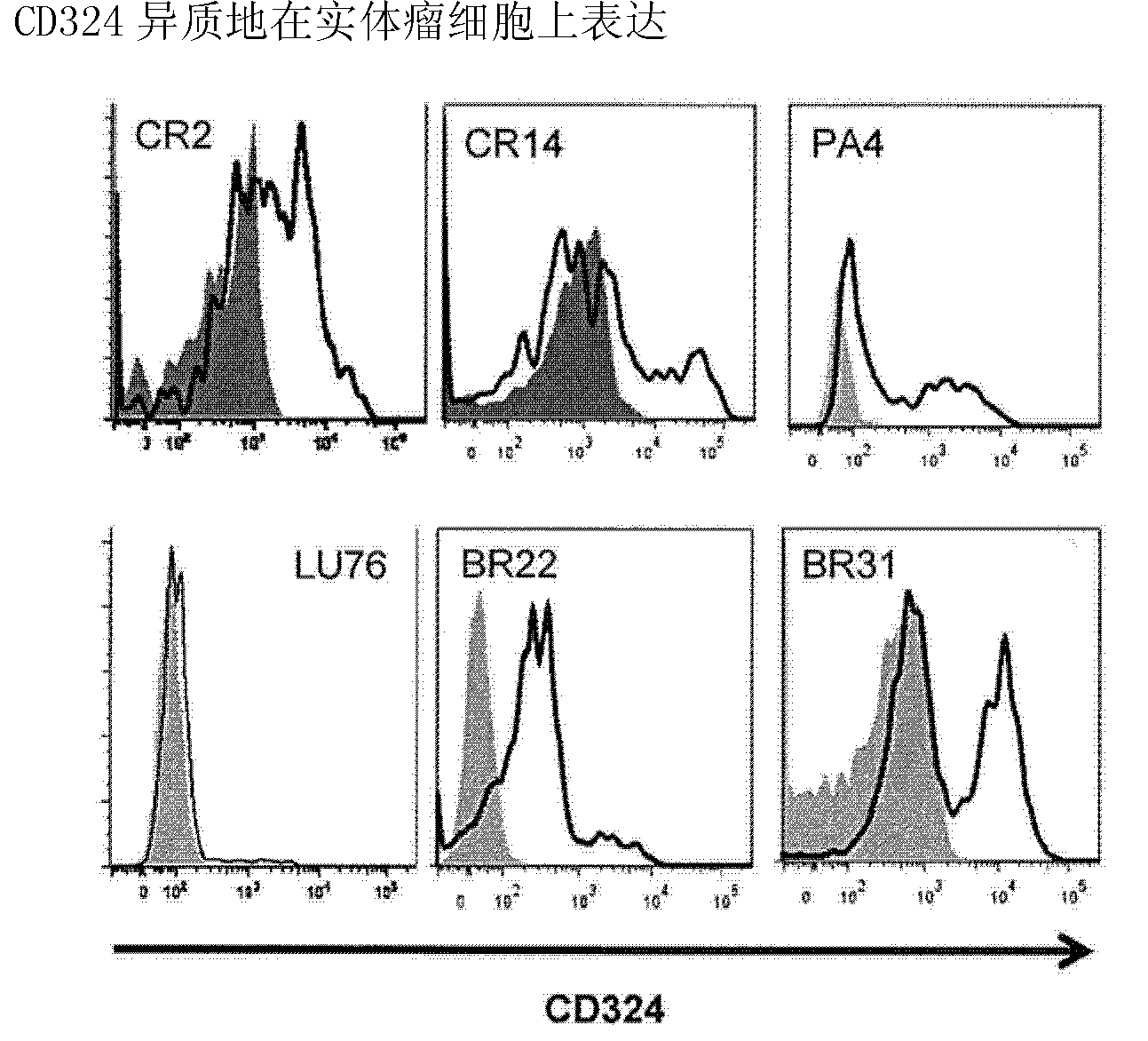
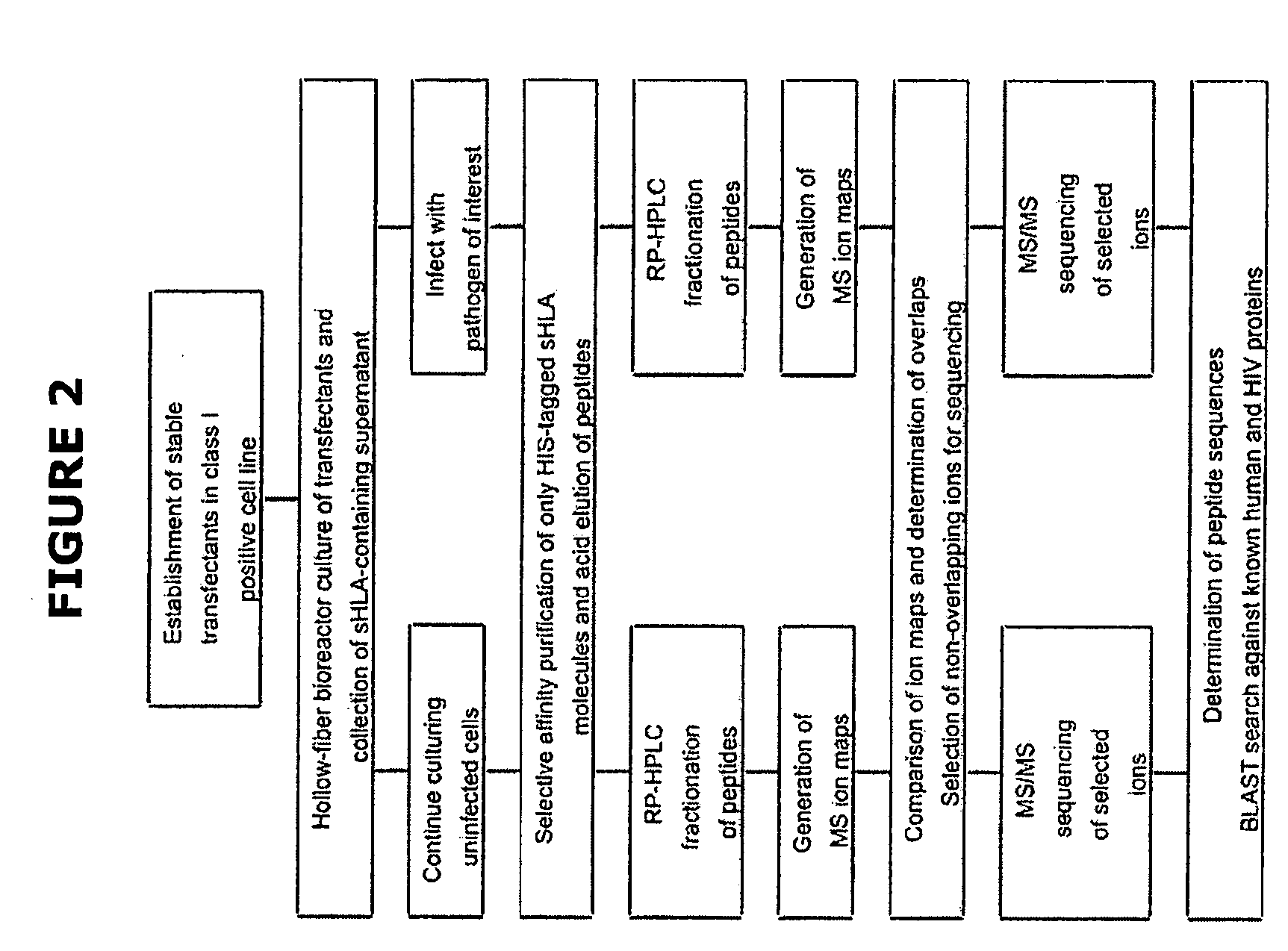
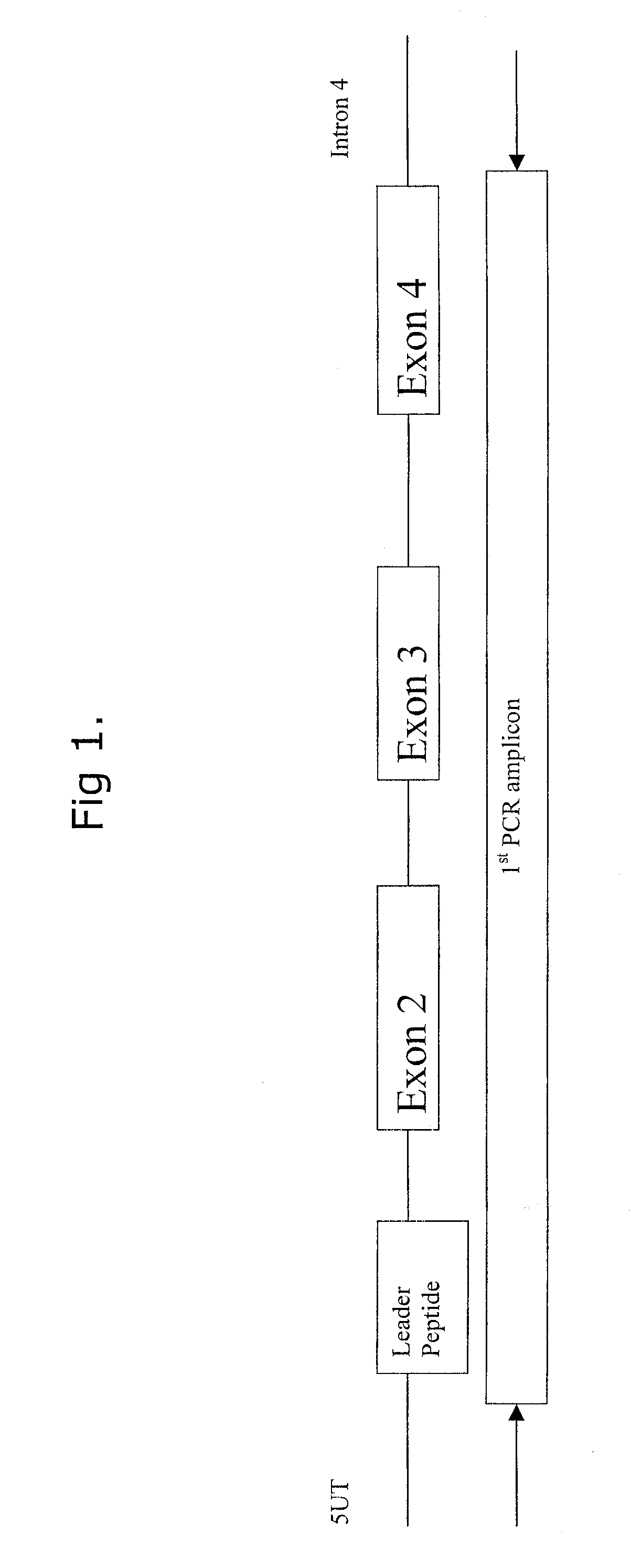
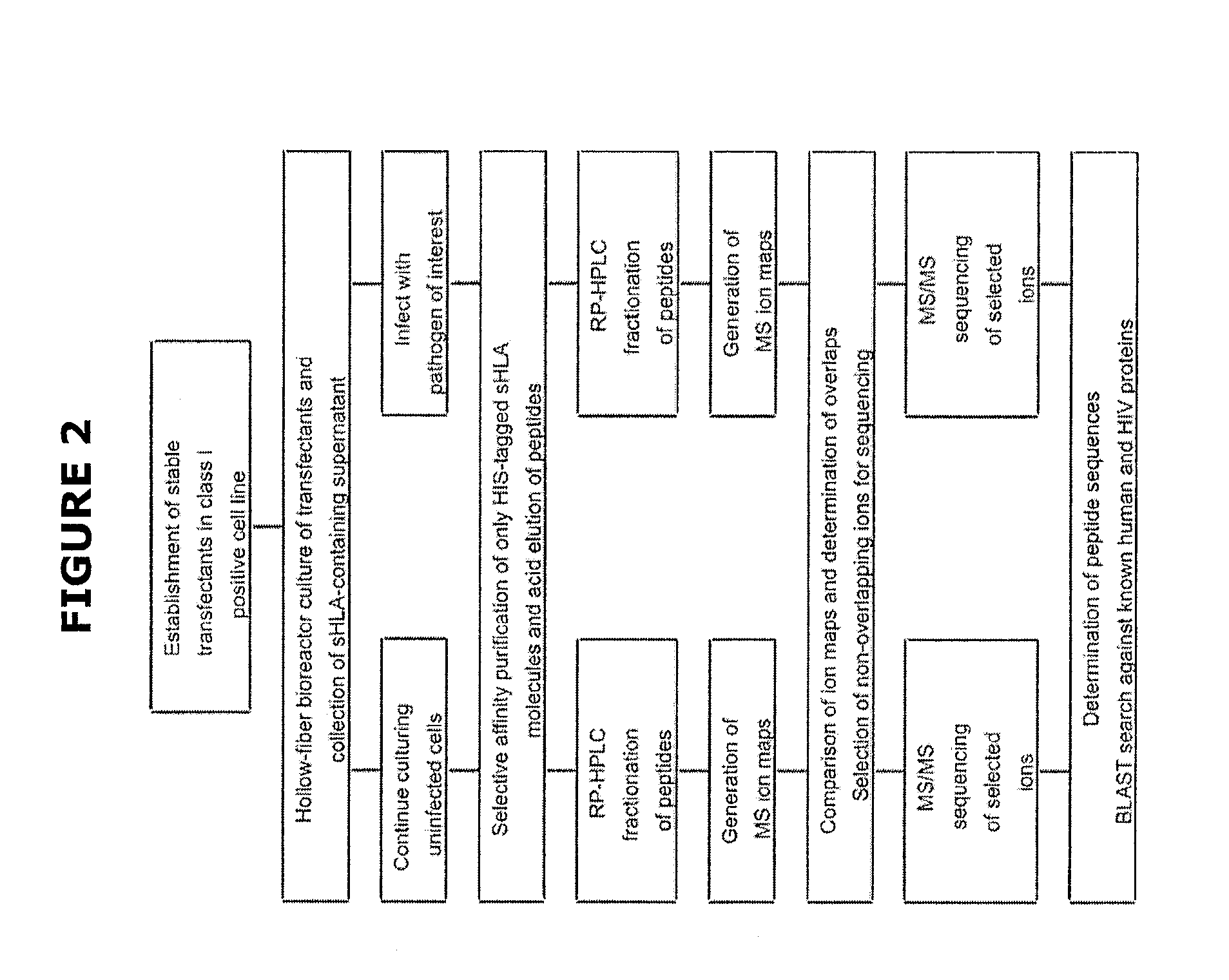
Comparative ligand mapping from MHC class I positive cells
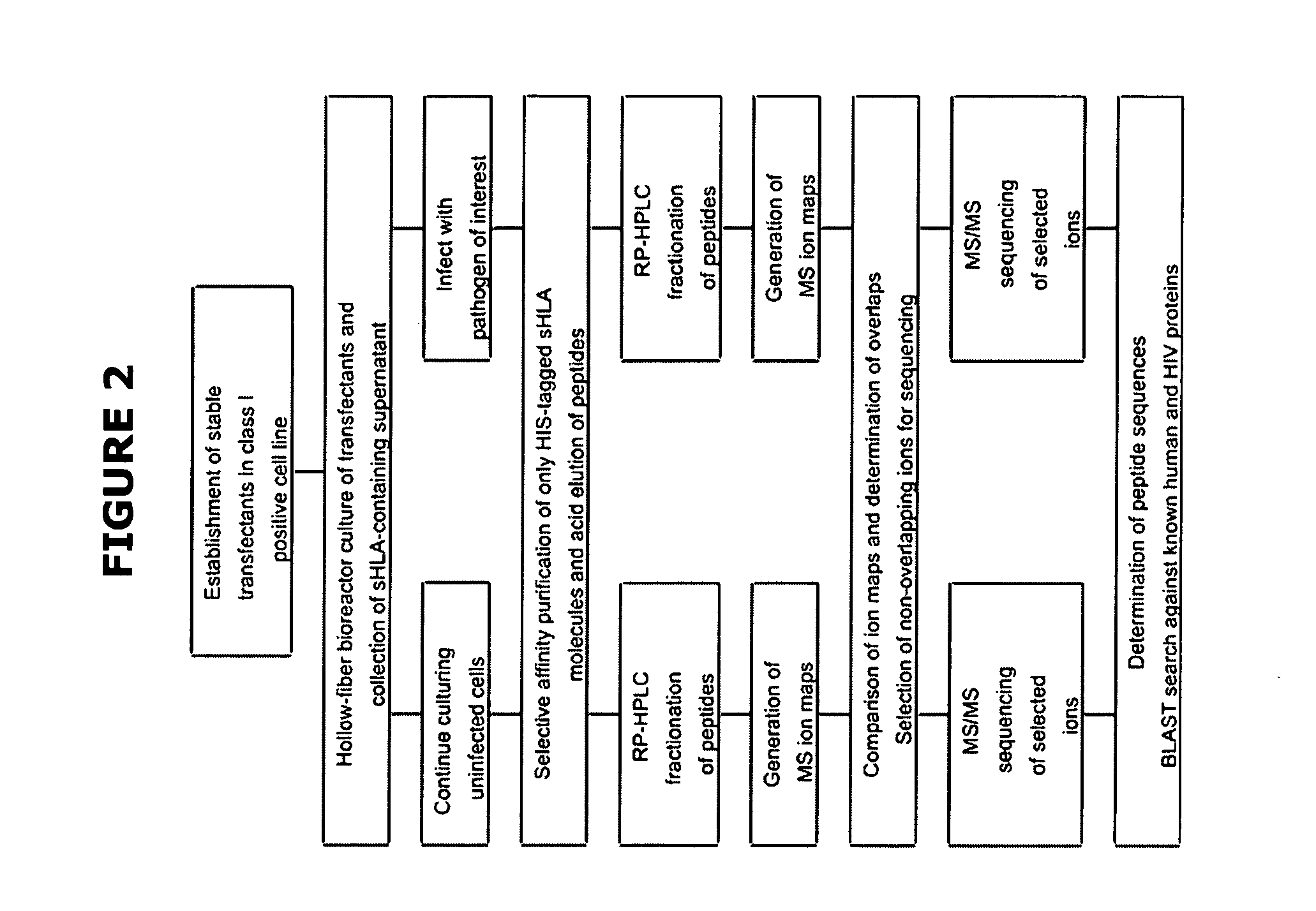
The present invention relates generally to a methodology for the isolation, purification and identification of peptide ligands presented by MHC positive cells. In particular, the methodology of the present invention relates to the isolation, purification and identification of these peptide ligands from soluble class I and class II MHC molecules which may be from uninfected, infected, or tumorigenic cells. The methodology of the present invention broadly allows for these peptide ligands and their cognate source proteins thereof to be identified and used as markers for infected versus uninfected cells and / or tumorigenic versus nontumorigenic cells, with said identification being useful for marking or targeting a cell for therapeutic treatment or priming the immune response against infected / tumorigenic cells.
Owner:THE BOARD OF RGT UNIV OF OKLAHOMA
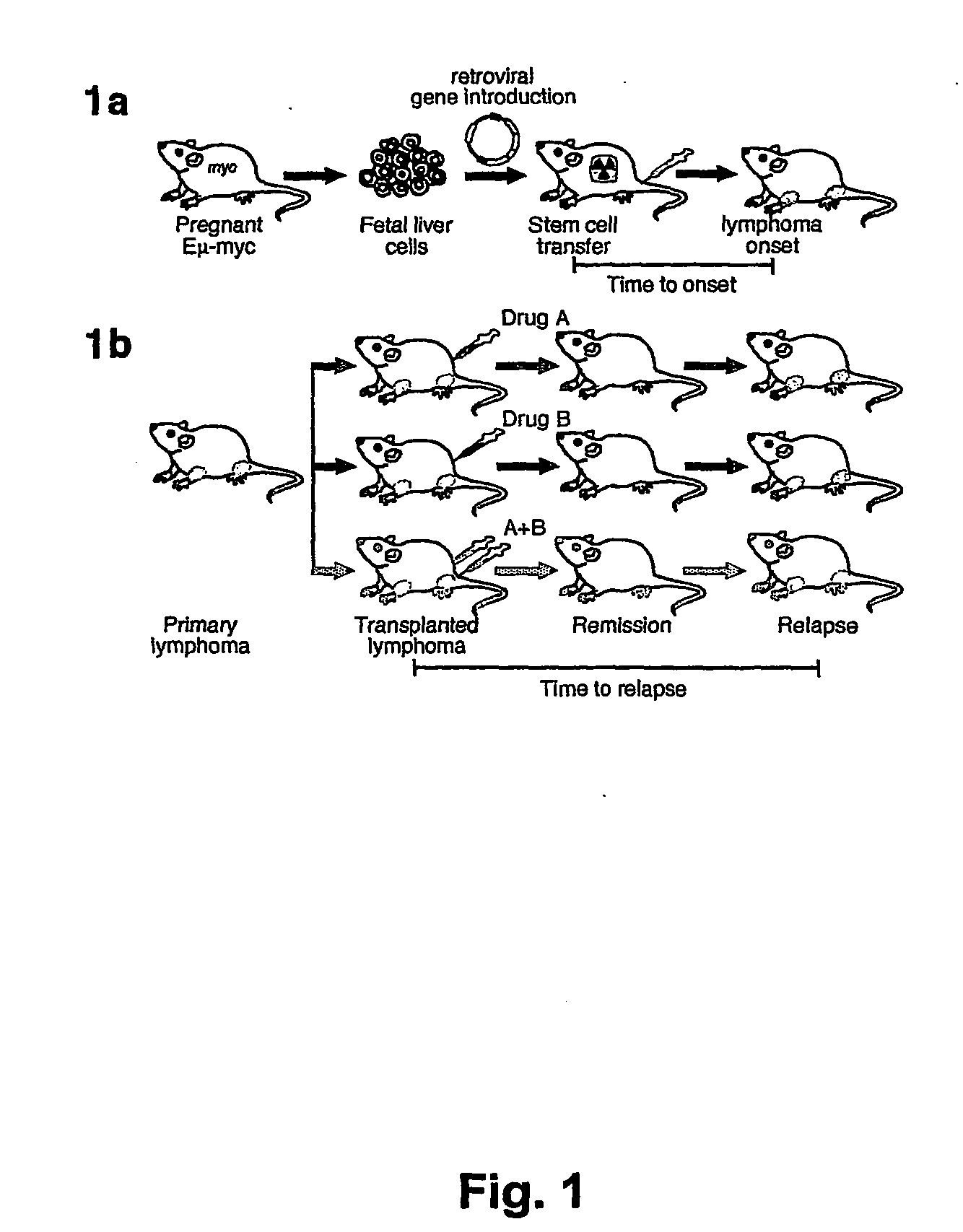
Model for studying the role of genes in tumor resistance to chemotherapy
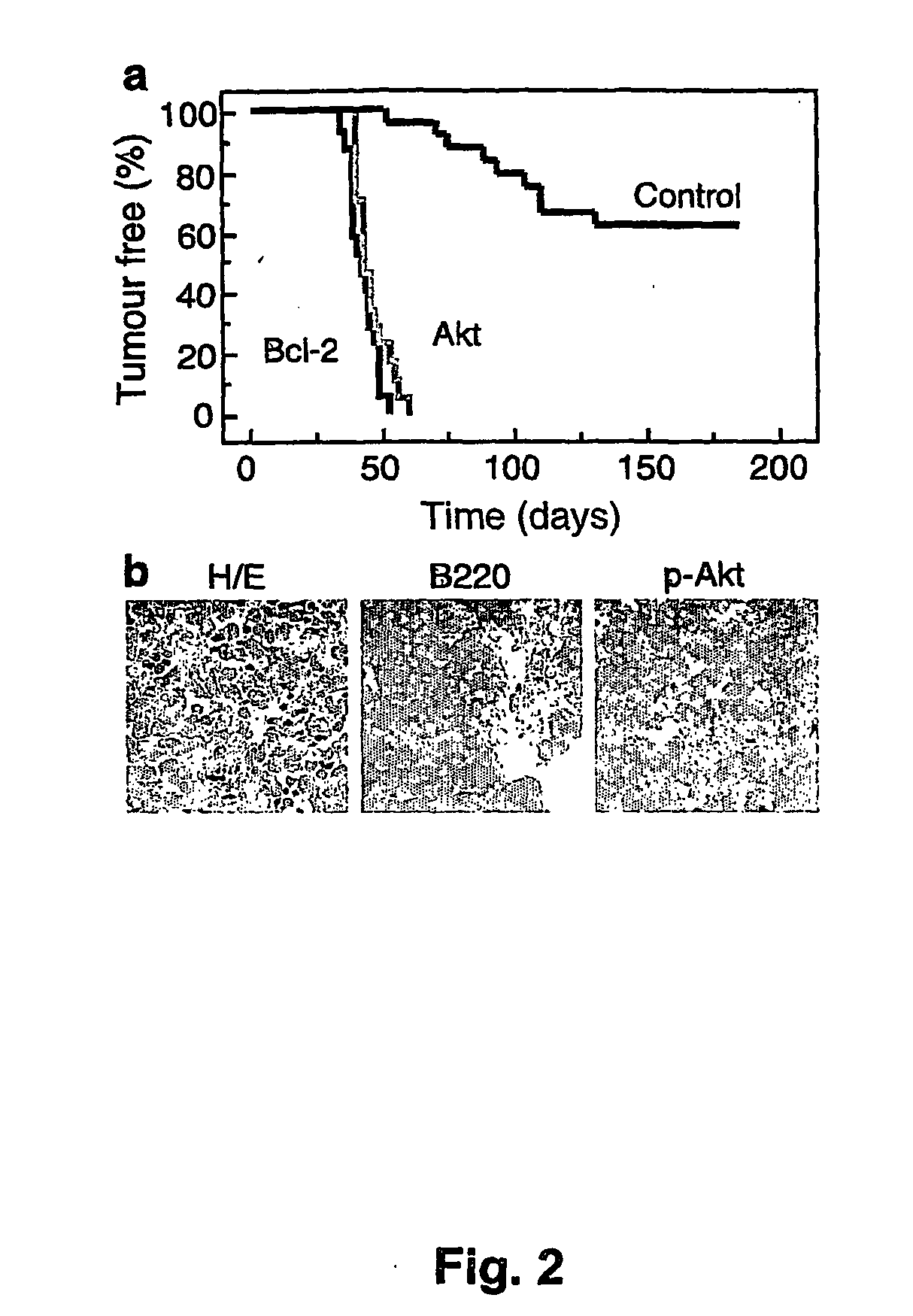
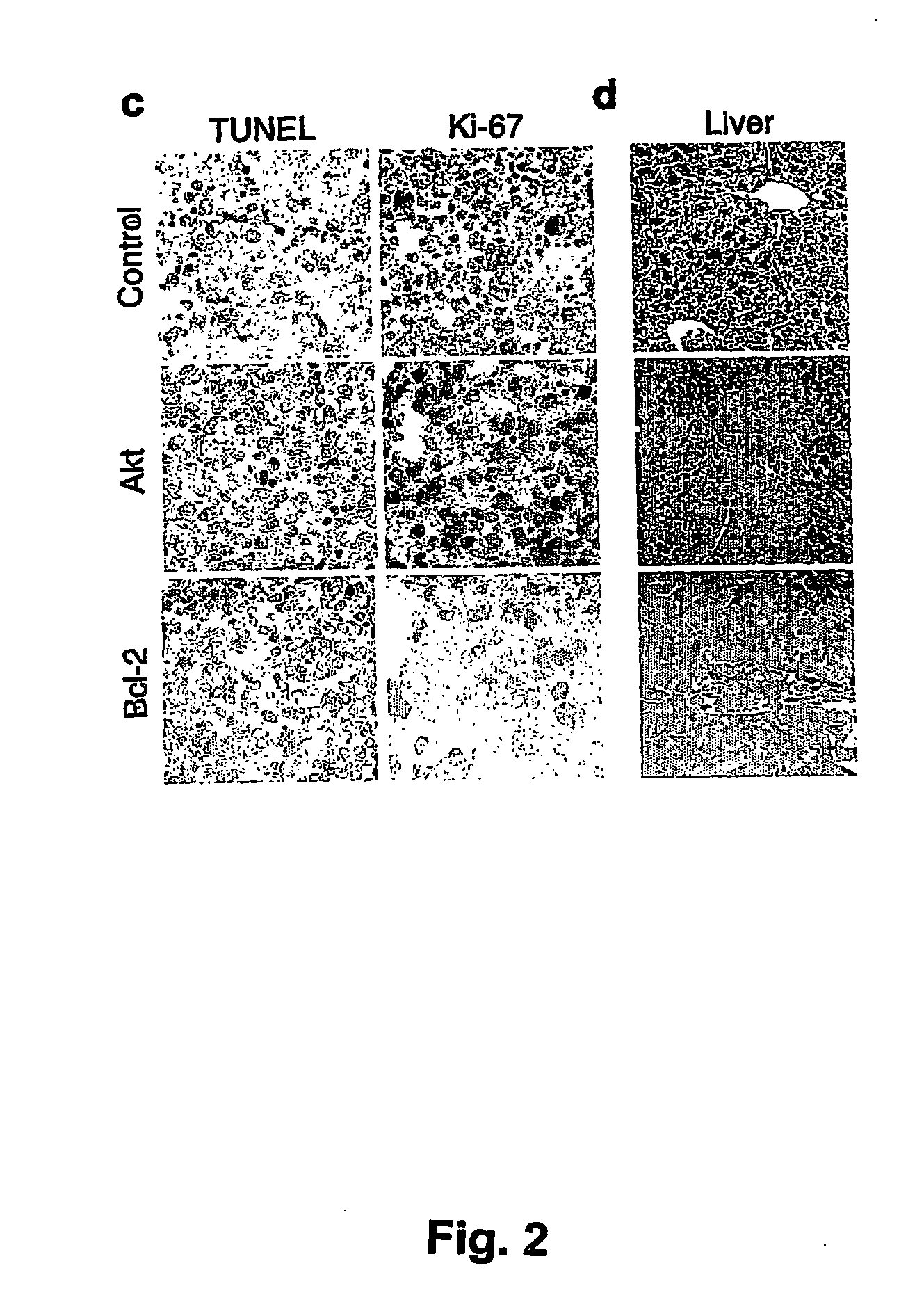
The invention provides the components of in vivo and in vitro systems and methods which use them to study the effects of altered expression of a gene activity, such as the human akt, bcl-2, eIF4E or PTEN activities, on the descendants of stem cells that have been engineered to give rise to hematopoietic tumorigenic or tumor cells, such as lymphomas, with a high frequency. The present invention provides vectors, cells and mammals, and methods which in part depend on such products, useful for understanding tumorigenesis and its treatments, and in particular, for identifying and studying inhibitors and activators associated with tumor cell growth and growth inhibition, cell death through apoptotic pathways, and changes in apoptotic pathway components that affect drug sensitivity and resistance in tumorigenic cells. Methods for identifying molecular targets for drug screening, identifying interacting gene activities, for identifying therapeutic treatments and for identifying candidates for new therapeutic treatments are provided.
Owner:COLD SPRING HARBOR LAB INC +1
Comparative ligand mapping from MHC class 1 positive cells
The present invention relates generally to a methodology for the isolation, purification and identification of peptide ligands presented by MHC positive cells. In particular, the methodology of the present invention relates to the isolation, purification and identification of these peptide ligands from soluble class I and class II MHC molecules which may be from uninfected, infected, or tumorigenic cells. The methodology of the present invention broadly allows for these peptide ligands and their cognate source proteins thereof to be identified and used as markers for infected versus uninfected cells and / or tumorigenic versus nontumorigenic cells with said identification being useful for marking or targeting a cell for therapeutic treatment or priming the immune response against infected cells.
Owner:THE BOARD OF RGT UNIV OF OKLAHOMA
Therapies for cancer using RLIP76
InactiveUS20060182749A1Reduce the numberReduced potencyOrganic active ingredientsCell receptors/surface-antigens/surface-determinantsRalA binding protein 1Cancer research
The present invention is a composition identified as a region of ralA binding protein 1, wherein the region neighbors a membrane-associated portion of the ralA binding protein 1, reduces transport activity and membrane association of the ralA binding protein 1 and kills cells undergoing uncontrolled cell growth in a subject that has cells undergoing uncontrolled cell growth. The region is used to generate medicines that kill malignant cells and tumorigenic cells. Medicines may be in the form of antibodies, si-RNA and small molecules that recognize the region.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
Methods for treating ras driven cancer in a subject
The invention relates to the discovery that the dibenzodiazepinone analogues have growth inhibiting activities on tumorigenic cells that are driven by expression of RAS or mutated RAS. Thus the invention includes methods for inhibiting the activity of RAS using dibenzodiazepinone analogues; methods for inhibiting the growth of a RAS driven cancer cell using dibenzodiazepinone analogues; methods for inhibiting the growth of a RAS driven cancer using dibenzodiazepinone analogues; and methods for treating a subject having a RAS driven cancer using dibenzodiazepinone analogues.
Owner:THALLION PHARMA
Compounds with modifying activity enhanced under hypoxic conditions
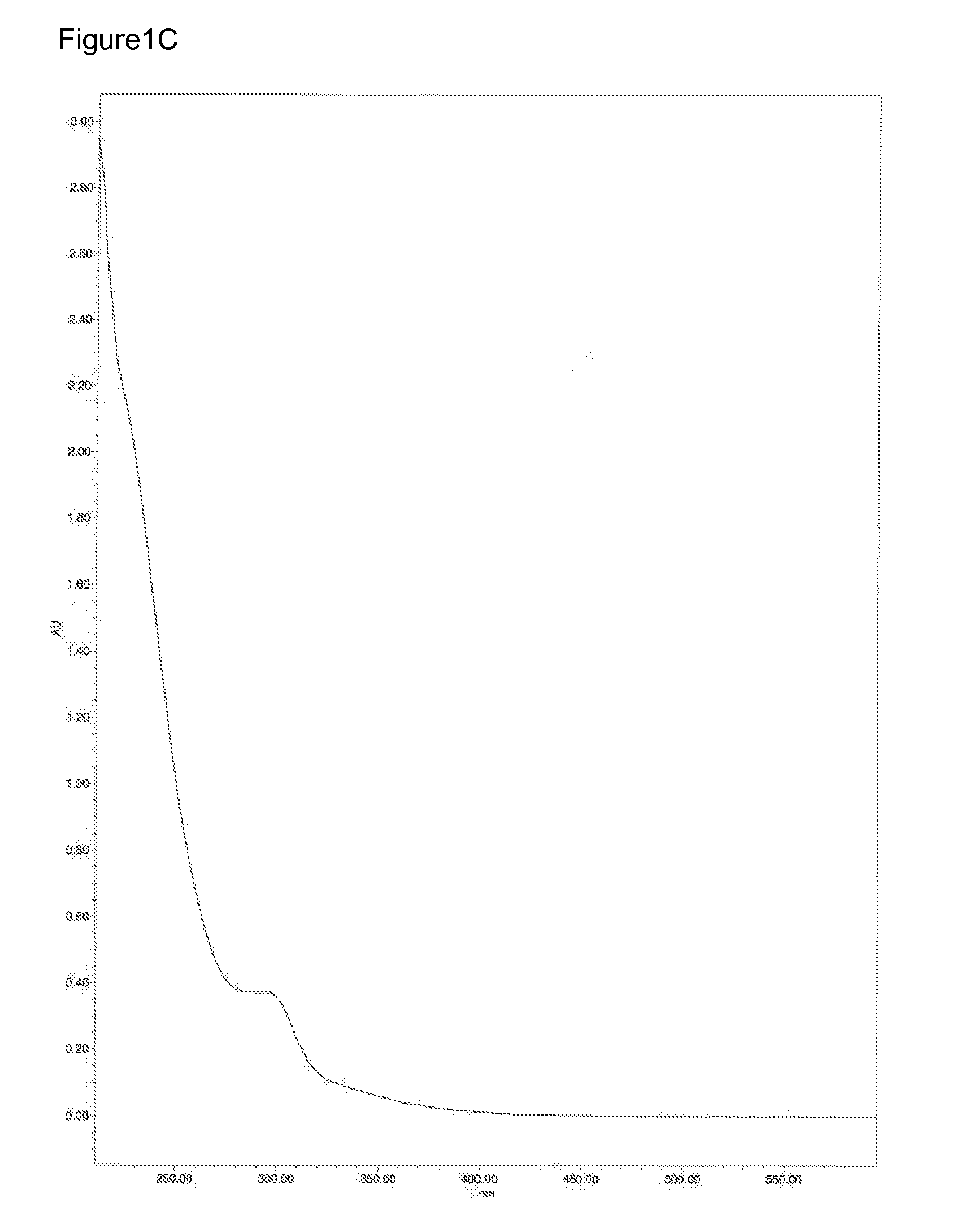
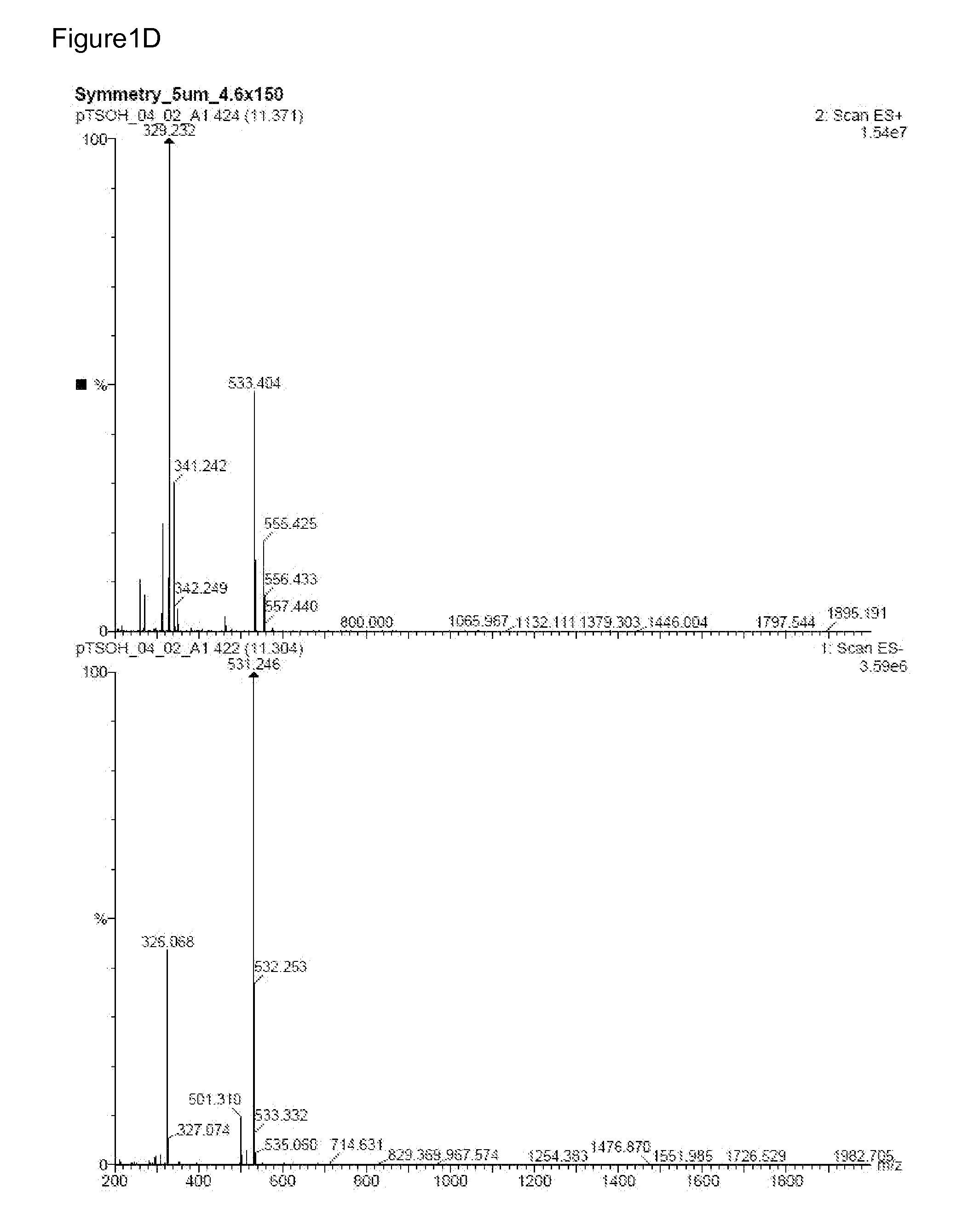
InactiveUS20070082881A1Improved biologic modifying activityHigh activityBiocideOrganic active ingredientsRutheniumCompound (substance)
Compositions and methods for modifying one or more biologic targets are provided. Suitable targets include cells, DNA, proteins, enzymes, and / or a subject in need thereof. The compositions may exist as a monomer or multimer and are active in a biologic environment with enhanced activity in hypoxic environments and, thus, exhibit improved specificity for hypoxic biologic targets (e.g., tumorigenic cells and those undergoing uncontrolled cell growth). A composition typically comprises a complex with an overall charge of 2+ or greater having at least one ruthenium atom attached to a redox active ligand. The redox active ligand helps maintain separation of more than one ruthenium atom. Suitable compositions may further include a terminal ligand comprising a heterocyclic aromatic compound. When provided to a biologic target, the composition modifies the biologic target and no additional compounds need be provided. Suitable compositions are typically catalytic and regenerative in the presence of a reducing agent.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
Cancer Starvation Therapy
The present invention is a glutamine analogue which enters the mitochondrion and is subsequently exposed to ionizing radiation. When exposed to ionizing radiation, the present invention damages mitochondrial (as well as other) substructures such as mtDNA, the outer membrane, the inner membrane, cristae, ribosomes, etc., and causes the effective destruction of such mitochondrion. Tumorigenic cells without mitochondria cannot produce the energy they need to subsist and replicate, effectively starving them of energy and causing their destruction.
Owner:SERRANO OJEDA PEDRO ANASTACIO
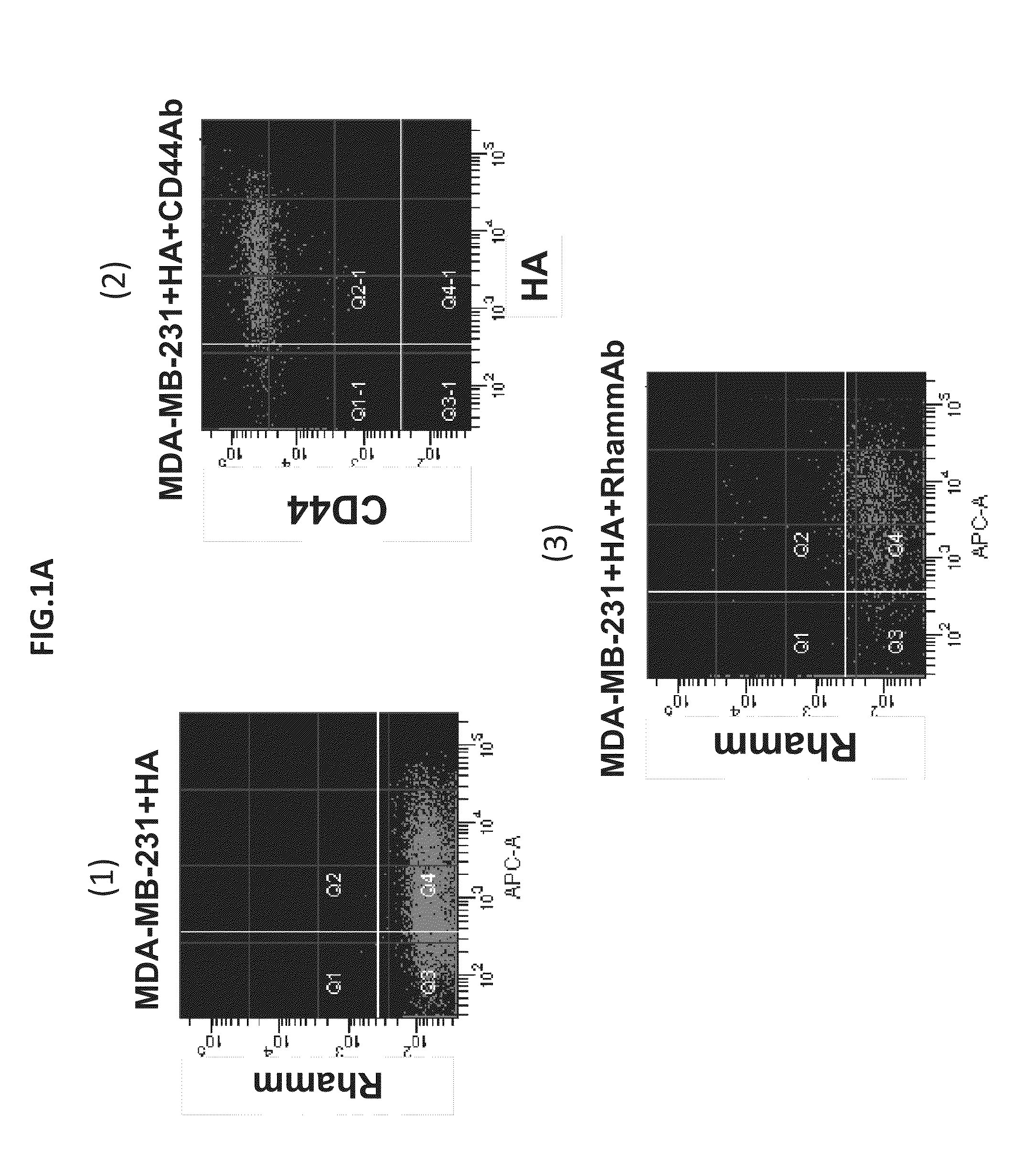
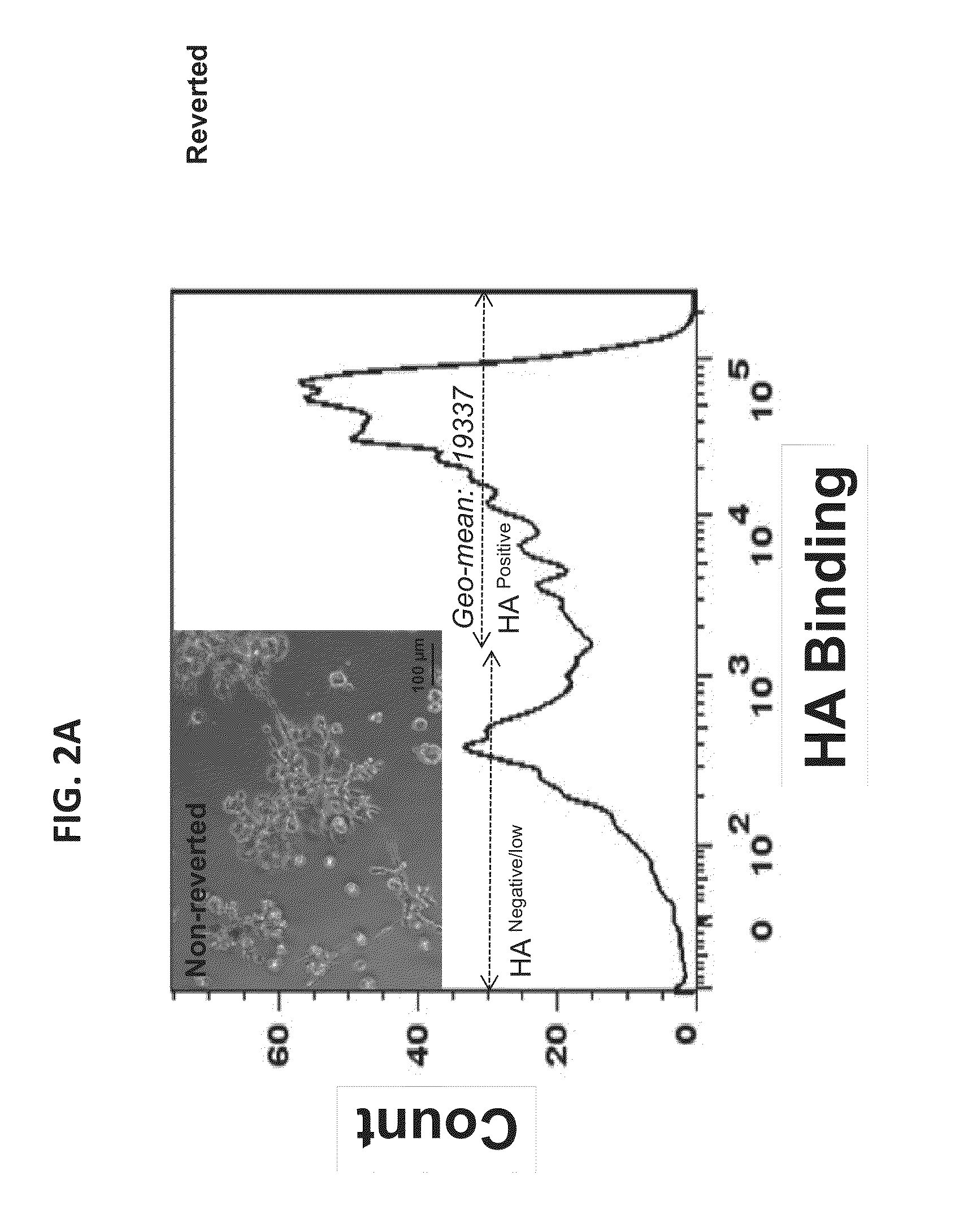
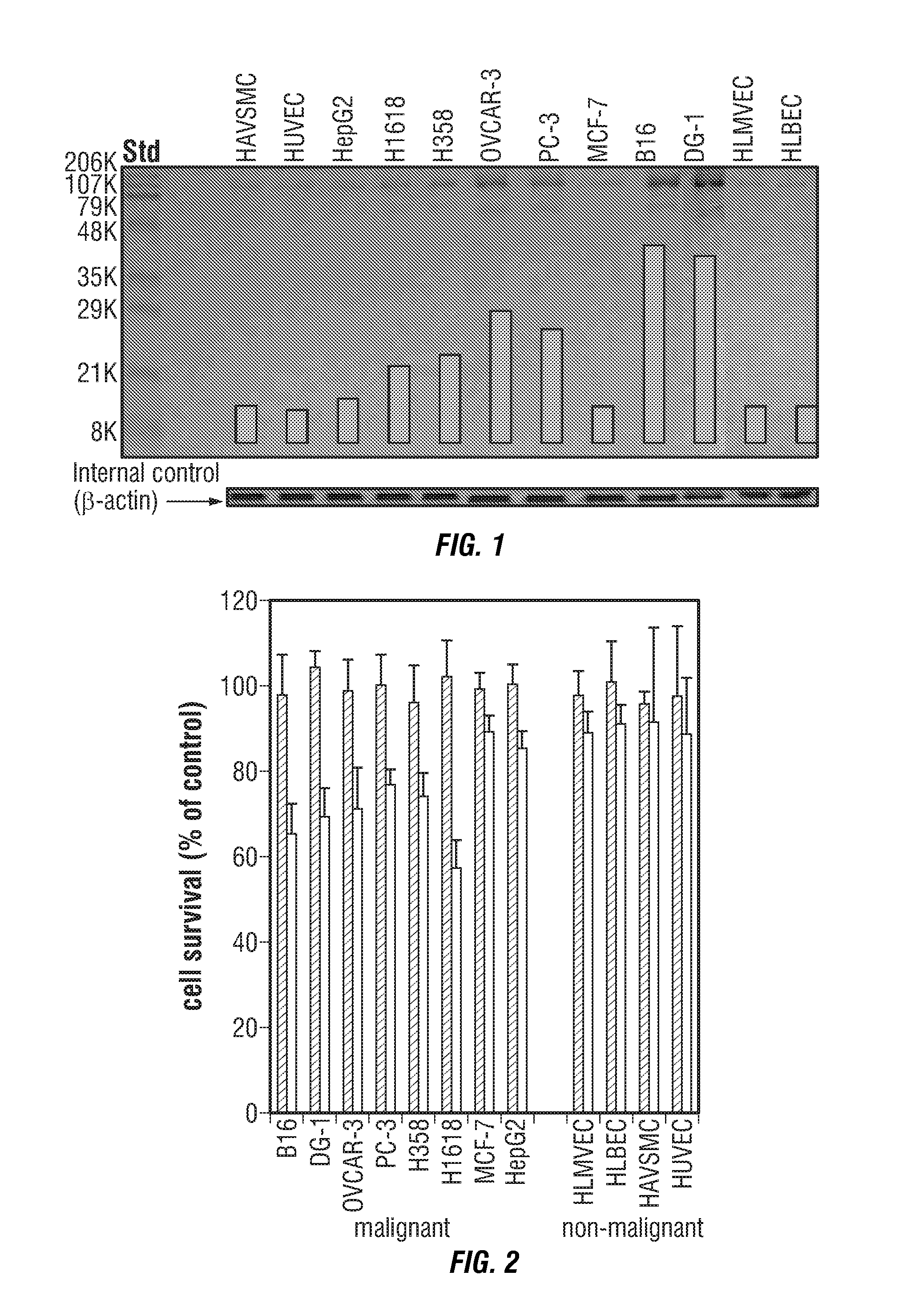
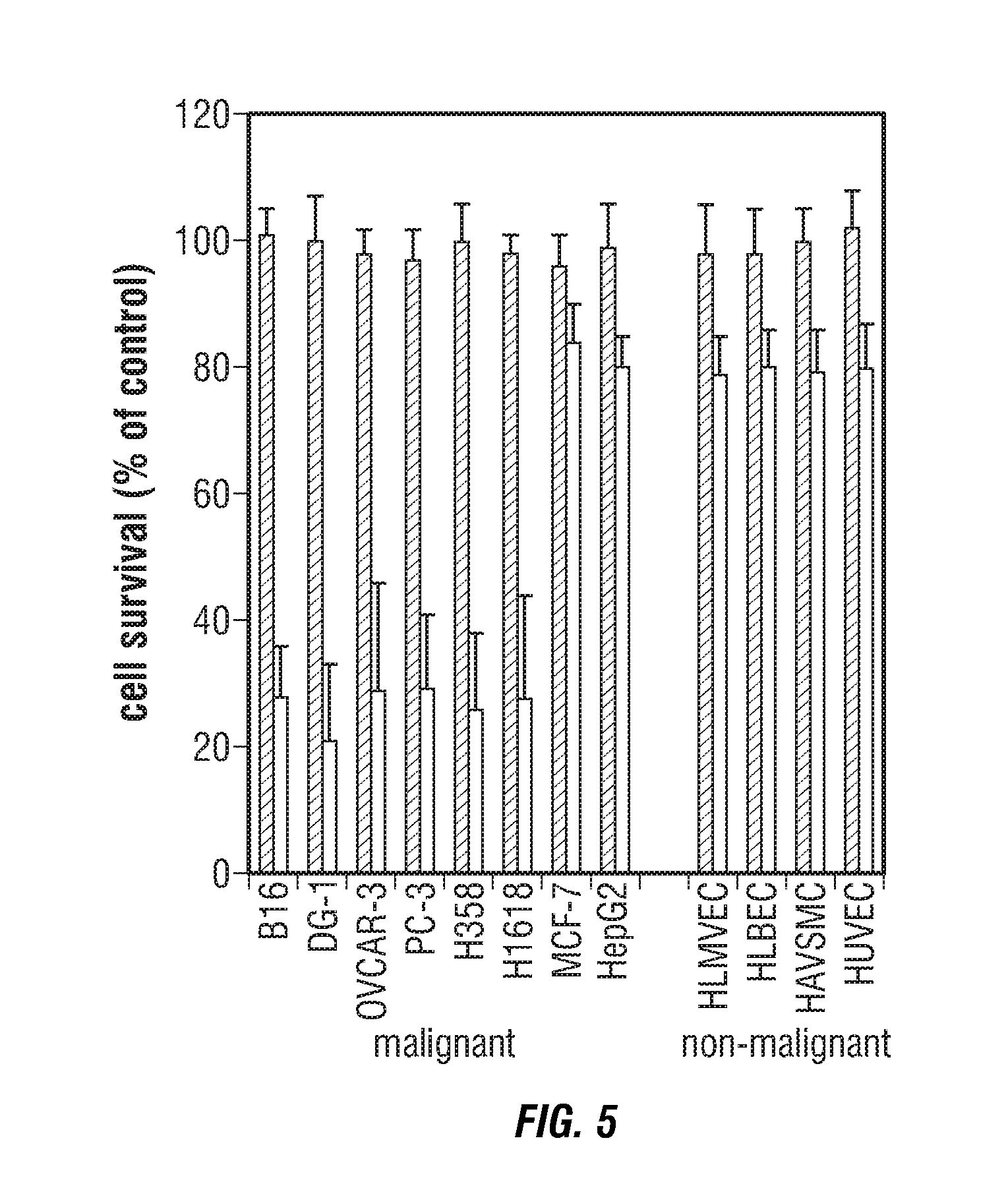
Identification of invasive and slow-growing tumorigenic cell subsets in tumors
InactiveUS20130157285A1Convenient treatmentLow costSugar derivativesMicrobiological testing/measurementDiseaseSafety profile
HA-based functional probes and a multiplexed targeting strategy for detection and isolation of invasive subpopulations in breast cancer cell lines. Methods for using HA metabolism for profiling and sorting breast cancer heterogeneity. As such, HA-based functional probes have appropriate targeting capacity and safety profiles for development as imaging and therapeutic agents for following repair and neoplastic disease processes such as breast cancer.
Owner:RGT UNIV OF CALIFORNIA
Identification and enrichment of cell subpopulations
InactiveCN103313726ABiological material analysisImmunoglobulins against growth factorsCell subpopulationsCell biology
Markers useful for the identification, characterization and, optionally, the enrichment or isolation of tumorigenic cells or cell subpopulations are disclosed.
Owner:ABBVIE STEMCENTRX LLC
Comparative ligand mapping from MHC class I positive cells
InactiveUS20070099182A1Peptide/protein ingredientsMicrobiological testing/measurementMHC class IPeptide ligand
The present invention relates generally to a methodology for the isolation, purification and identification of peptide ligands presented by MHC positive cells. In particular, the methodology of the present invention relates to the isolation, purification and identification of these peptide ligands from soluble class I and class 11 MHC molecules which may be from uninfected, infected, or tumorigenic cells. The methodology of the present invention broadly allows for these peptide ligands and their cognate source proteins thereof to be identified and used as markers for infected versus uninfected cells and / or tumorigenic versus nontumorigenic cells, with said identification being useful for marking or targeting a cell for therapeutic treatment or priming the immune response against infected cells.
Owner:THE BOARD OF RGT UNIV OF OKLAHOMA
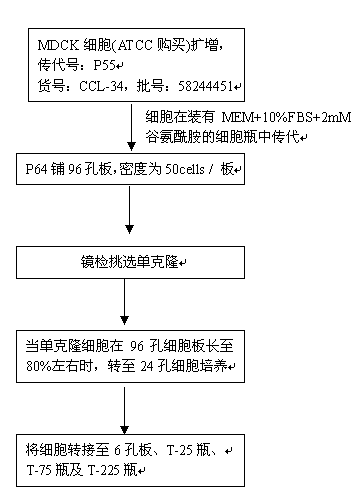
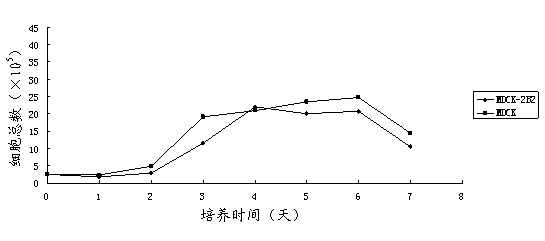
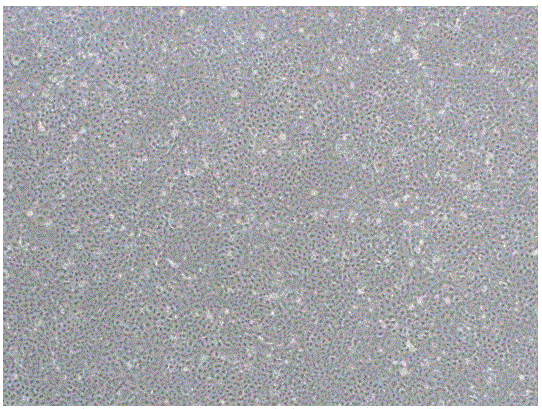
Non-tumorigenic MDCK cell line used for amplifying influenza viruses and screening method thereof
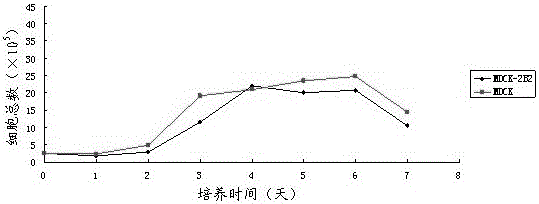
ActiveCN104059873AReduced tumorigenicityIncreased tumorigenicityMicroorganism based processesArtificial cell constructsScreening methodMorphologic change
The invention discloses a non-tumorigenic MDCK cell line used for amplifying influenza viruses and a screening method thereof. The cell line is MDCK-2B2 with an accession number of CCTCC 201323. The invention provides the non-tumorigenic MDCK-2B2 cell line and a subclone cell line thereof; and the cell line can grow into adherent cells, and / or has an epithelioid form and / or can support duplication of a variety of viruses, and is capable of amplifying viruses with high titers. The invention also provides the screening method for the non-tumorigenic cell line. The growth speed and morphologic changes of cells can be used to assist in determination of tumorigenicity; i.e., simple-pore cells with a slow growth speed have substantially reduced tumorigenicity, and occurrence of giant cells enables tumorigenicity to be substantially increased; during large-scale screening of non-tumorigenic cell lines, such an auxiliary determination method can shorten time and save cost.
Owner:SHANGHAI INST OF BIOLOGICAL PROD CO LTD
Isolation and long-term culturing of estrogen receptor-positive human breast epithelial cells
The present invention describes methods for long-term culturing of ERpos cells, cell lines, and / or cell strains with exetended lifespan and / or cell strain as well as culture medium compositions. The invention further describes methods for isolating cells which may be used as starting point for long-term culturing of ERpos cells, cell lines, and / or cell strains with extended lifespan and / or cell strain. The invention further discloses various methods for generating ERpos tumorigenic cells, cell lines, and / or cell strains with extended lifespan and / or cell strain as well as various assays for their use.
Owner:UNIVERSITY OF COPENHAGEN
Progressively tumorigenic cell lines
ActiveUS10525081B2Genetically modified cellsMammal material medical ingredientsBiochemistryAnti neoplastic
Provided herein, inter alia, are tumorigenic cell lines and methods for using the same for deriving a series of cell culture and non-human animal-based models which exhibit increasingly aggressive tumorigenic potential as well as use of the same for evaluating the anti-neoplastic properties of candidate therapeutic agents.
Owner:GROVE CITY COLLEGE
Non-tumorigenic mdck cell line and screening method for amplifying influenza virus
ActiveCN104059873BReduced tumorigenicityIncreased tumorigenicityMicroorganism based processesVertebrate cellsScreening methodCell growth rate
The invention discloses a non-tumor-causing MDCK cell line for amplifying influenza virus and a screening method thereof. The cell line is MDCK‑2B2, and the preservation number is CCTCC C201323. The present invention provides a non-tumorigenic cell line MDCK-2B2 and its subclone cell line, which grow into adherent cells and / or have epithelial-like morphology and / or can support the replication of various viruses, and can amplify Higher titer virus; In addition, the present invention has found a method for screening non-tumorigenic cell lines, which can assist the judgment of tumorigenicity through the growth rate of cells and the morphological changes of cells, that is, relatively slow-growing single-pore cells, which The tumorigenicity will be significantly reduced, and the appearance of giant cells will significantly increase the tumorigenicity. When screening non-tumorigenic cell lines on a large scale, such auxiliary judgment methods can shorten time and save costs.
Owner:SHANGHAI INST OF BIOLOGICAL PROD CO LTD
Glutamine-high z element compounds for treating cancer
ActiveUS20190076527A9Organic active ingredientsPharmaceutical non-active ingredientsRadio isotopesChemical compound
The present invention is a glutamine compound having a non-radioisotope high Z element attached via a linker, which enters the mitochondrion and is subsequently exposed to ionizing radiation. When exposed to ionizing radiation, the present invention damages mitochondrial (as well as other) substructures such as mtDNA, the outer membrane, the inner membrane, cristae, ribosomes, etc., and causes the effective destruction of such mitochondrion. Tumorigenic cells without mitochondria cannot produce the energy they need to subsist and replicate, effectively starving them of energy and causing their destruction.
Owner:SERBIG PHARMA CORP
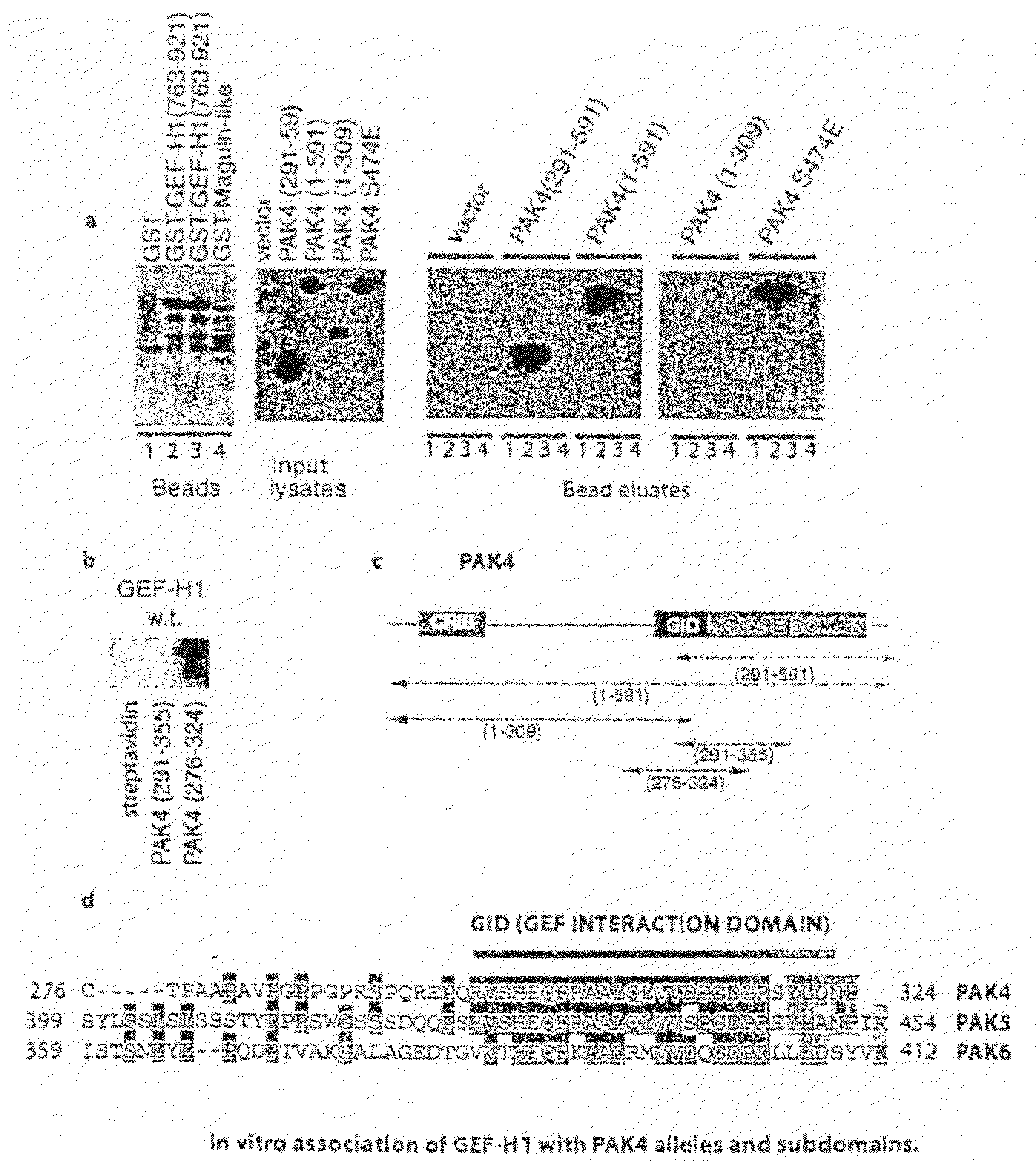
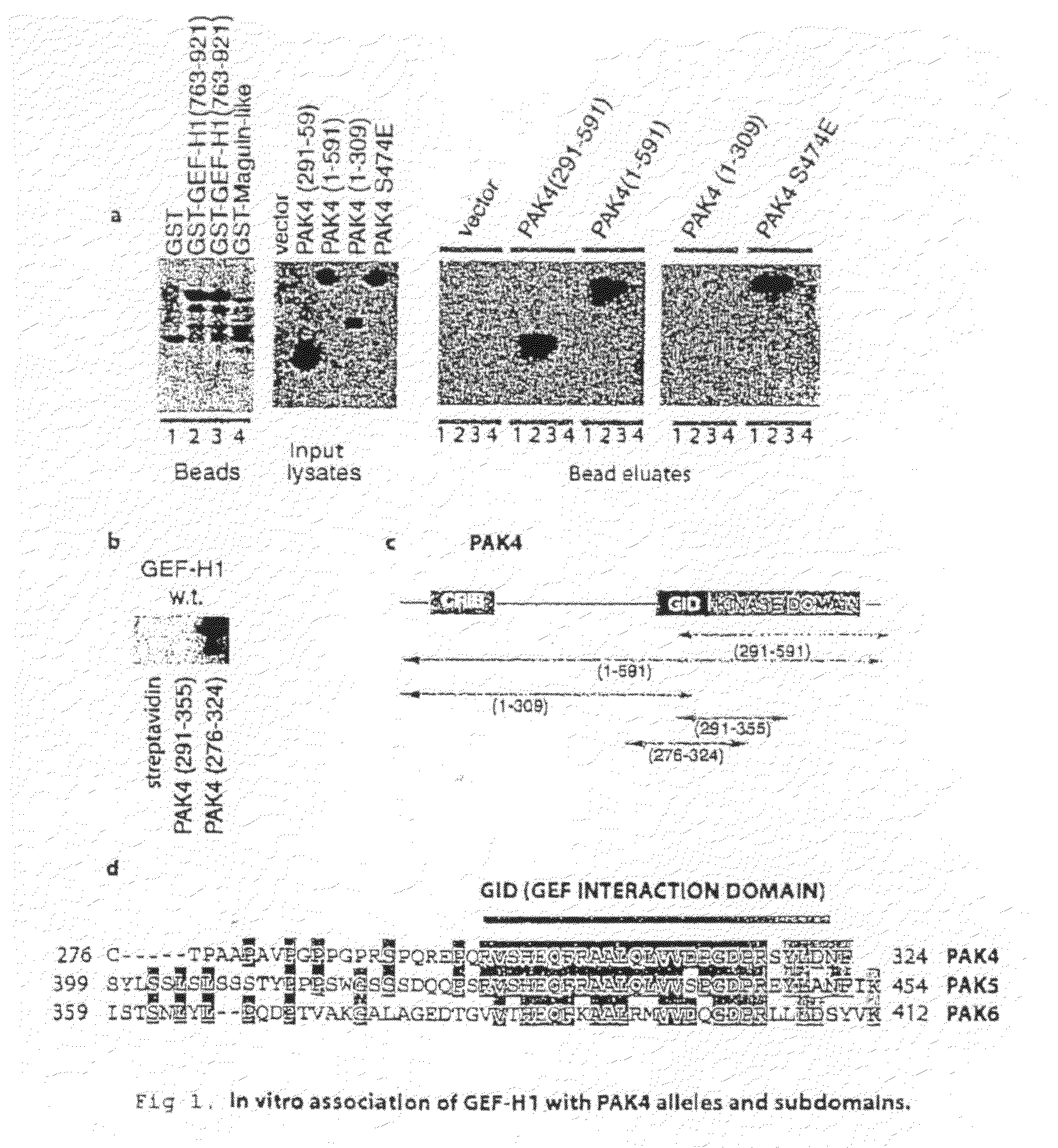
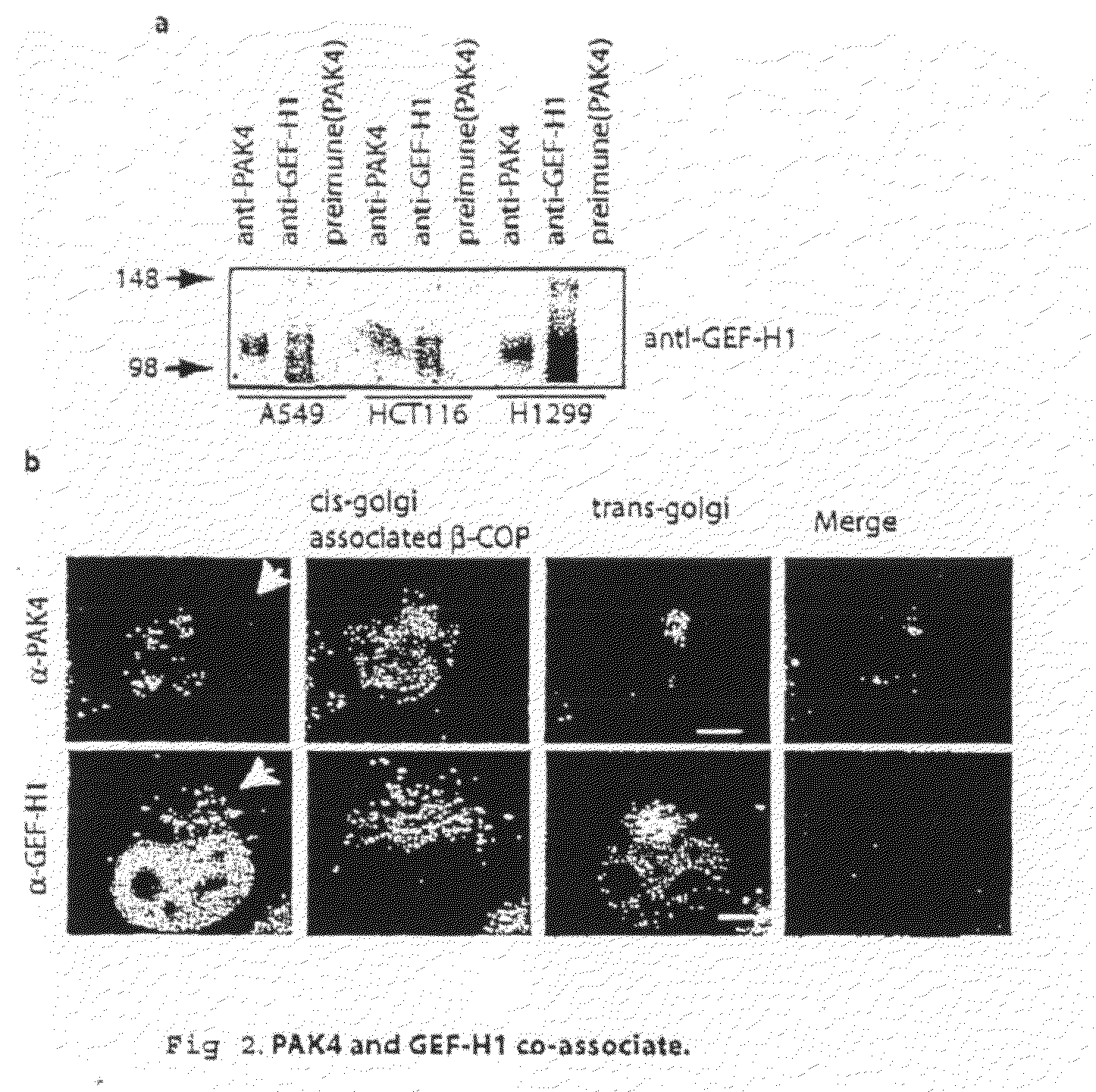
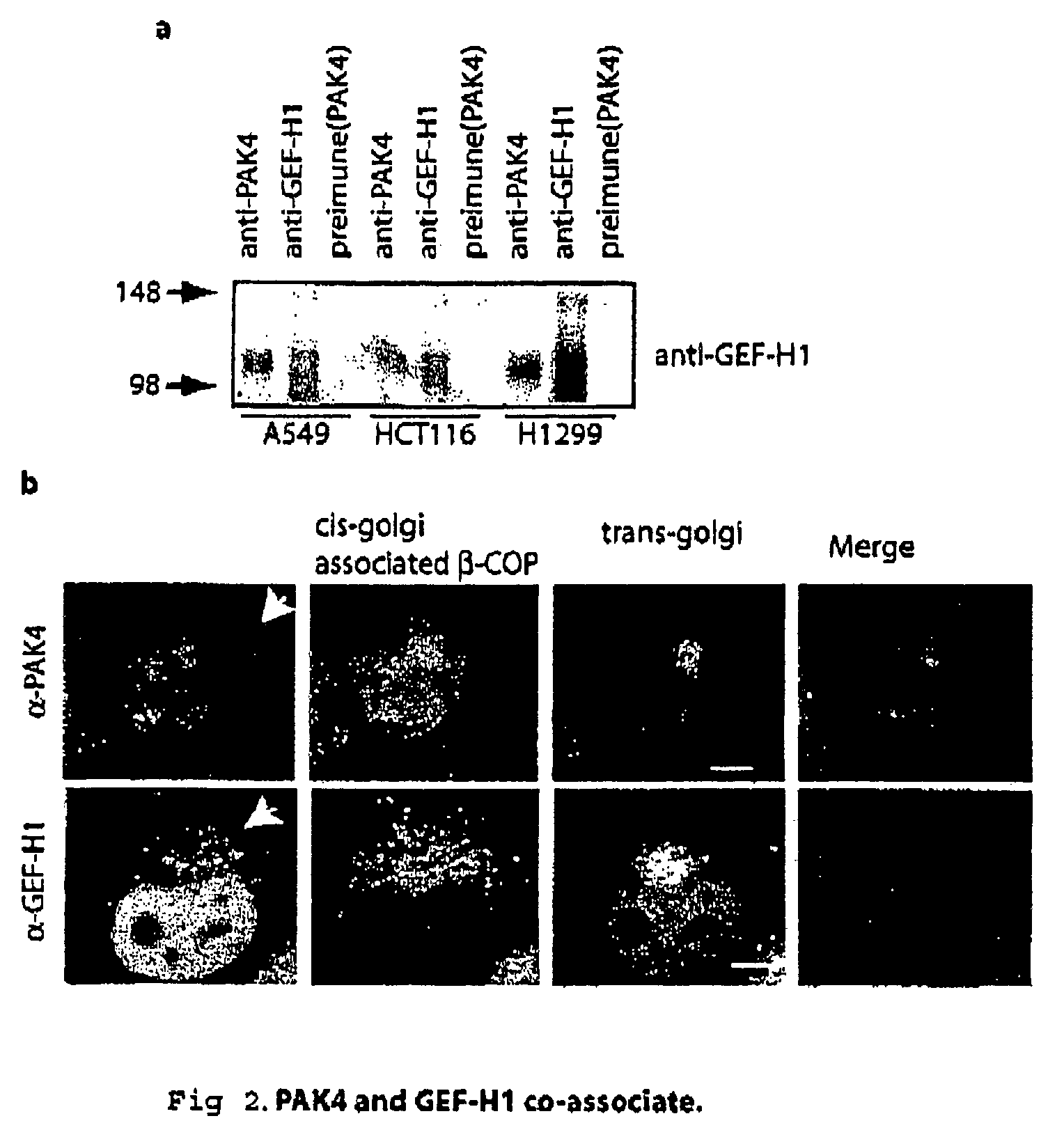
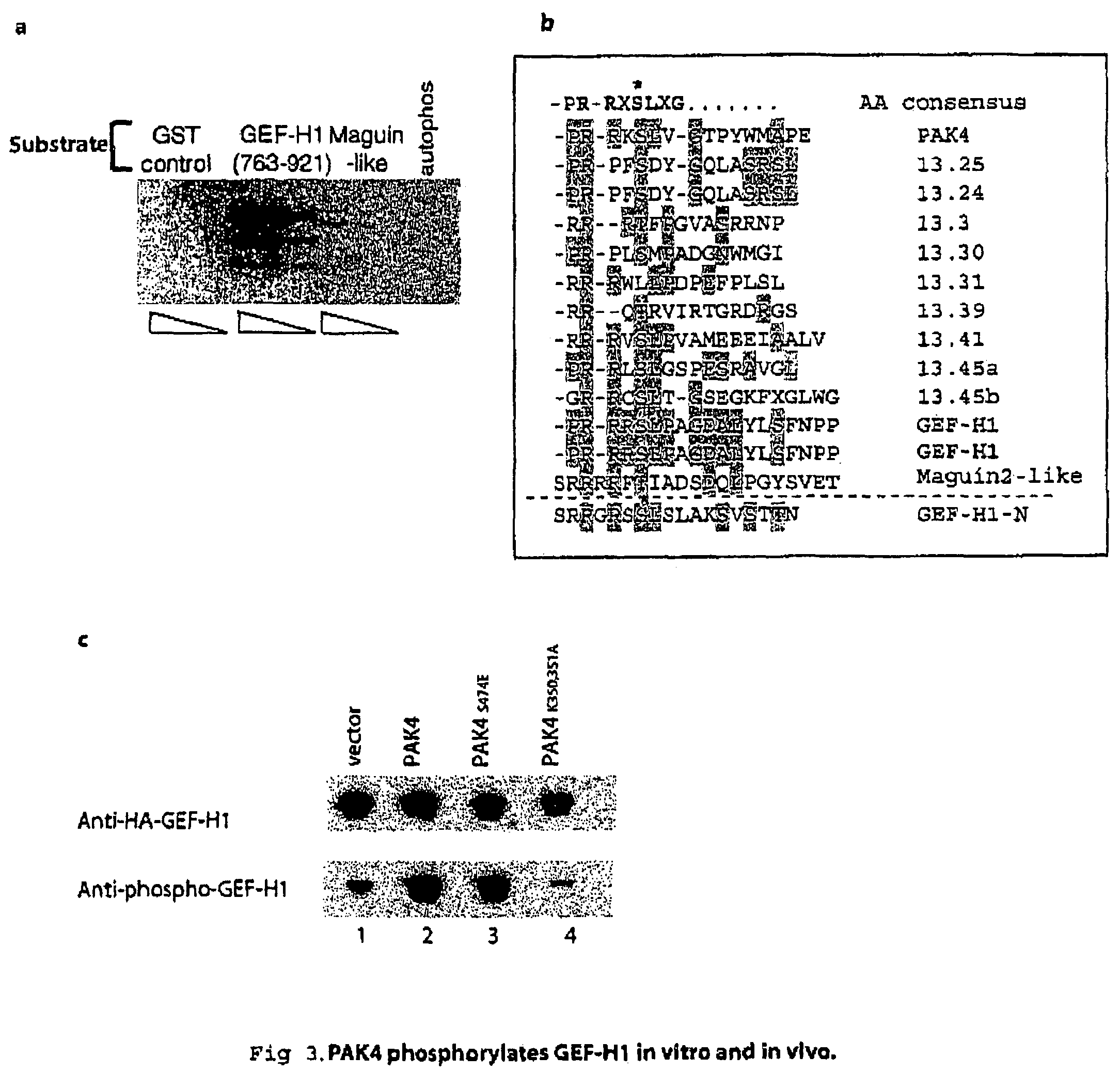
GEF-H1b: biomarkers, complexes, assays and therapeutic uses thereof
InactiveUS20090130695A1Lower Level RequirementsPrevents and reduces level of phosphorylationFungiBacteriaAssayPhosphorylation
The present invention relates to diagnosing abnormal cell proliferation in biological samples and screening for drugs which inhibit, reduce or abolish cell growth, especially tumorigenic cell growth, by detecting a phosphovariant isoform of a guanine nucleotide exchange factor biomarker, such as the novel GEF-H1S.
Owner:SUGEN INC
Therapies For Cancer Using RLIP76
ActiveUS20100183702A1Reduce in quantityReduce the numberOrganic active ingredientsPeptide/protein ingredientsRalA binding protein 1Cancer research
The present invention is a composition identified as a region of ralA binding protein 1, wherein the region neighbors a membrane-associated portion of the ralA binding protein 1, reduces transport activity and membrane association of the ralA binding protein 1 and kills cells undergoing uncontrolled cell growth in a subject that has cells undergoing uncontrolled cell growth. The region is used to generate medicines that kill malignant cells and tumorigenic cells. Medicines may be in the form of antibodies, si-RNA and small molecules that recognize the region.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
Comparative ligand mapping from mhc class i positive cells
The present invention relates generally to a methodology for the isolation, purification and identification of peptide ligands presented by MHC positive cells. In particular, the methodology of the present invention relates to the isolation, purification and identification of these peptide ligands from soluble class I and class II MHC molecules which may be from uninfected, infected, or tumorigenic cells. The methodology of the present invention broadly allows for these peptide ligands and their cognate source proteins thereof to be identified and used as markers for infected versus uninfected cells and / or tumorigenic versus nontumorigenic cells, with said identification being useful for marking or targeting a cell for therapeutic treatment or priming the immune response against infected / tumorigenic cells.
Owner:HILDEBRAND WILLIAM H +1
GEF-H1b: biomarkers, complexes assays and therapeutic uses thereof
The present invention relates to diagnosing abnormal cell proliferation in biological samples and screening for drugs which inhibit, reduce or abolish cell growth, especially tumorigenic cell growth, by detecting a phosphovariant isoform of a guanine nucleotide exchange factor biomarker, such as the novel GEF-H1S.
Owner:SUGEN INC
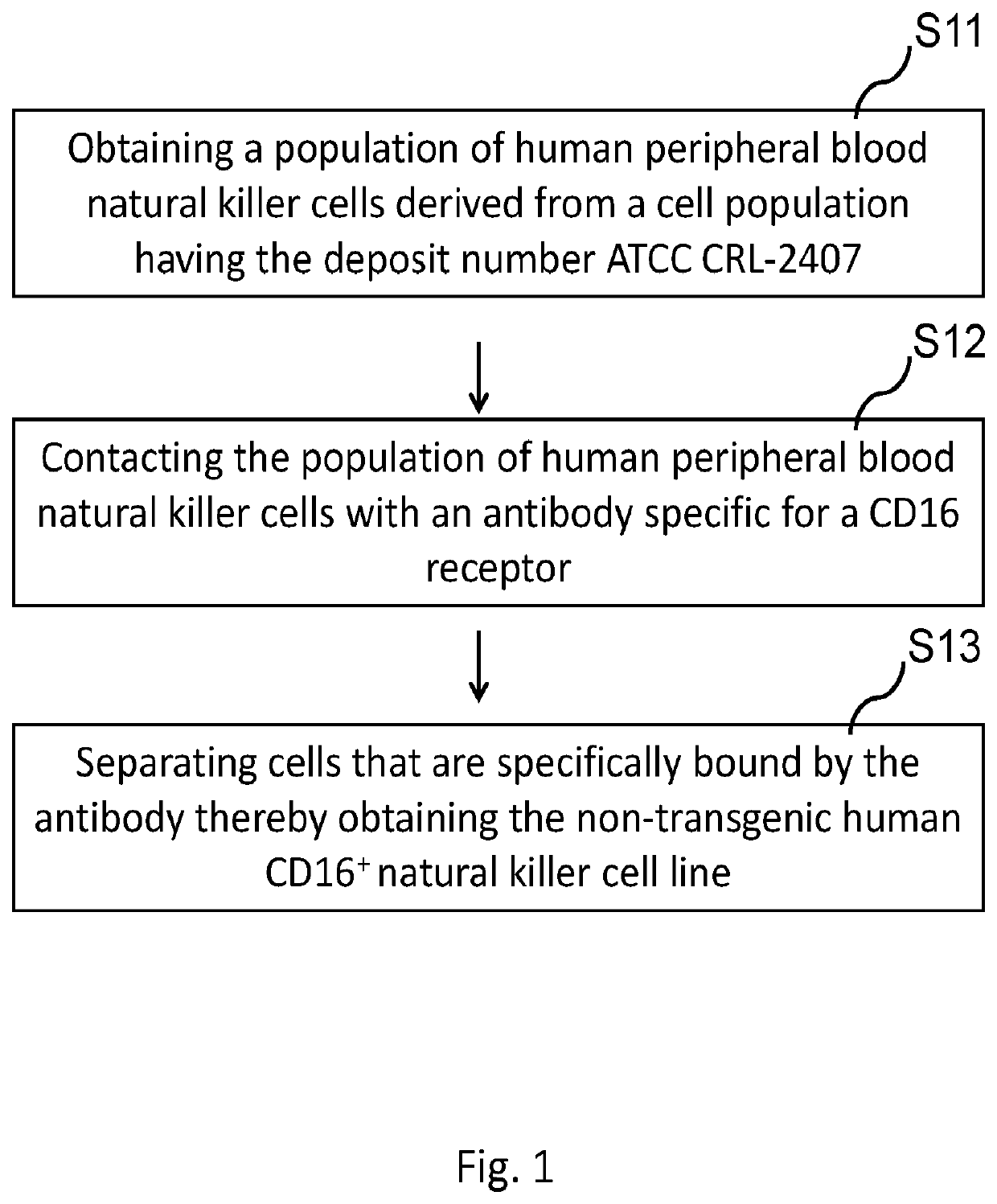
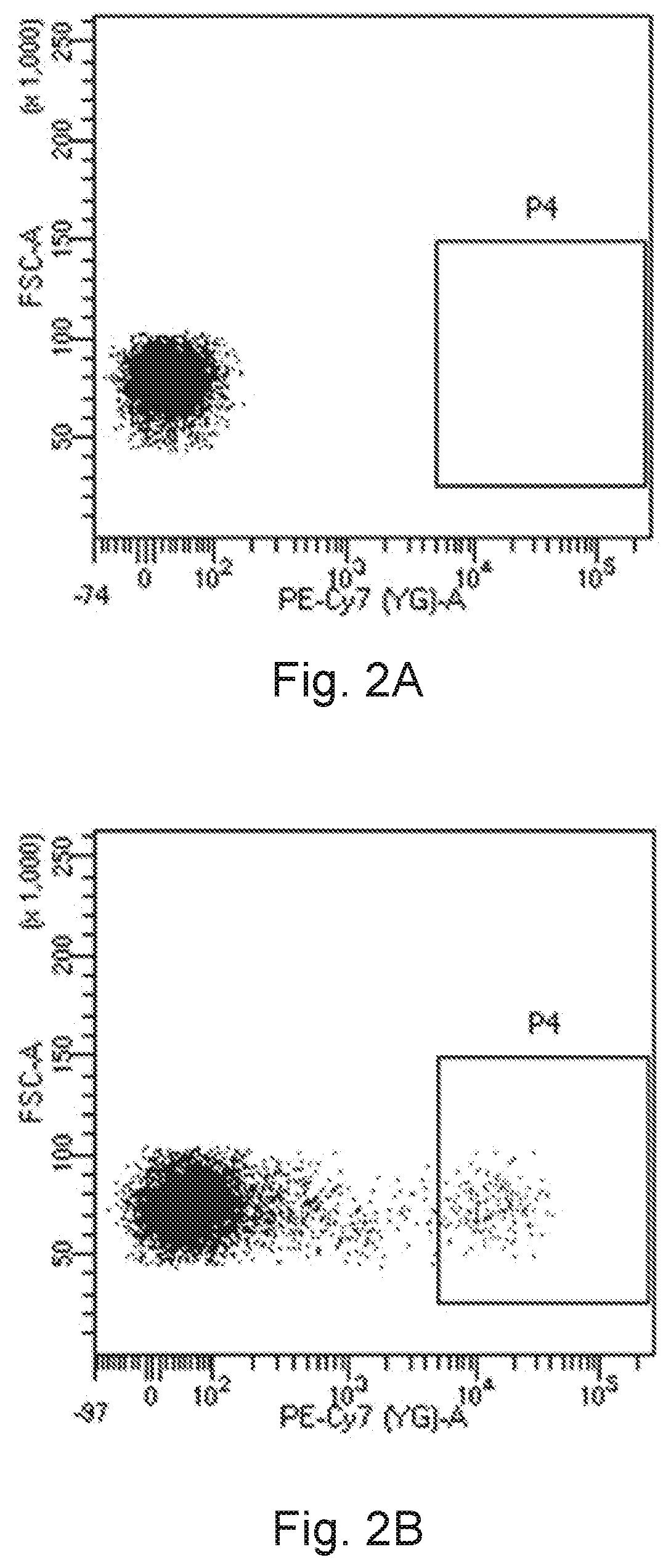
A novel cd16+ natural killer cell and a method of culturing cd16+ natural killer cell
PendingUS20220073878A1Increase concentrationReduce concentrationCulture processMammal material medical ingredientsCD16Natural killer cell
The present invention provides a human CD16+ natural killer cell line. This human CD16+ natural killer cell line does not include synthetic, genetically modified or deliberately delivered polynucleotide encoding the CD16 receptor and is a non-tumorigenic cell line. Therefore, this human CD16+ natural killer cell line might provide considerable long-term safety for disease treatment.
Owner:ACEPODIA BIOTECHNOLOGIES LTD
Identification and enrichment of cell subpopulations
InactiveUS9945842B2Convenient researchExtensive characterizationMicrobiological testing/measurementBiological material analysisBiochemistryCell subpopulations
Markers useful for the identification, characterization and, optionally, the enrichment or isolation of tumorigenic cells or cell subpopulations are disclosed.
Owner:ABBVIE STEMCENTRX LLC
Identification and enrichment of cell subpopulations
InactiveUS9778264B2Efficiently employedPeptide/protein ingredientsCancer antigen ingredientsBiochemistryCell subpopulations
Owner:ABBVIE STEMCENTRX LLC
Design of novel drug screens based on the newly found role of dystroglycan proteolysis in tumor cell growth
InactiveUS20050250164A1Suppressing abnormal growth of tumor cellLow or no tumorigenicityBiological material analysisBiological testingNormal growthΑ dystroglycan
The present invention provides methods and compositions for the diagnosis and treatment of cells lacking normal growth arresting characteristic. The present invention demonstrates that many tumor cells lack normal cell surface α-dystroglycan and thereby lack dystroglycan function. Dystroglycan can be lost from the cell surface by proteolytic shedding of a fragment of α-dystroglycan into the surrounding medium. Upon restoration of dystroglycan function and over-expression of the dystroglycan gene, the once tumorigenic cells revert to non-tumorigenic cells which polarize and arrest cell growth in the presence of basement membrane proteins, demonstrating that dystroglycan functions as a tumor marker and suppressor.
Owner:RGT UNIV OF CALIFORNIA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com