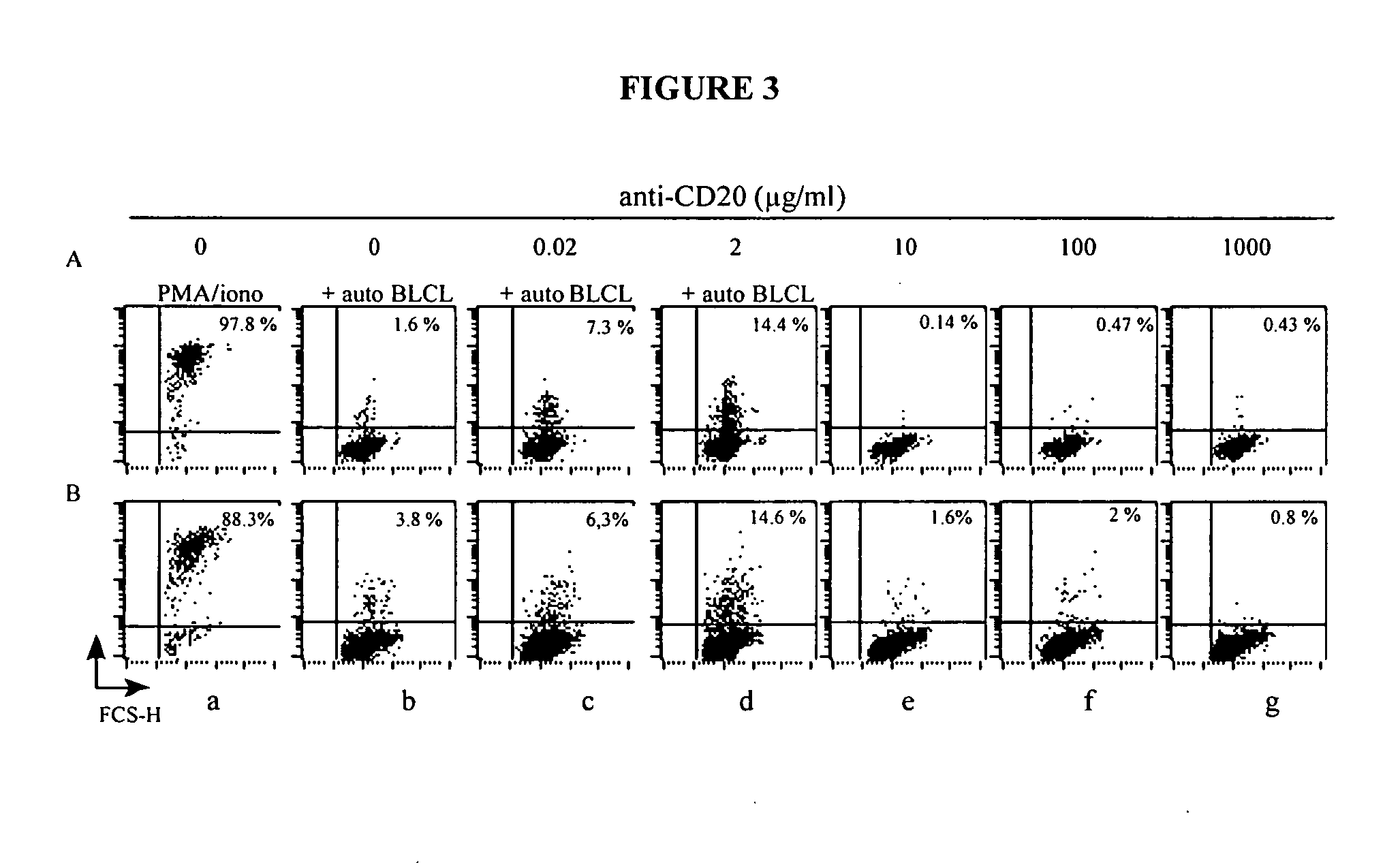
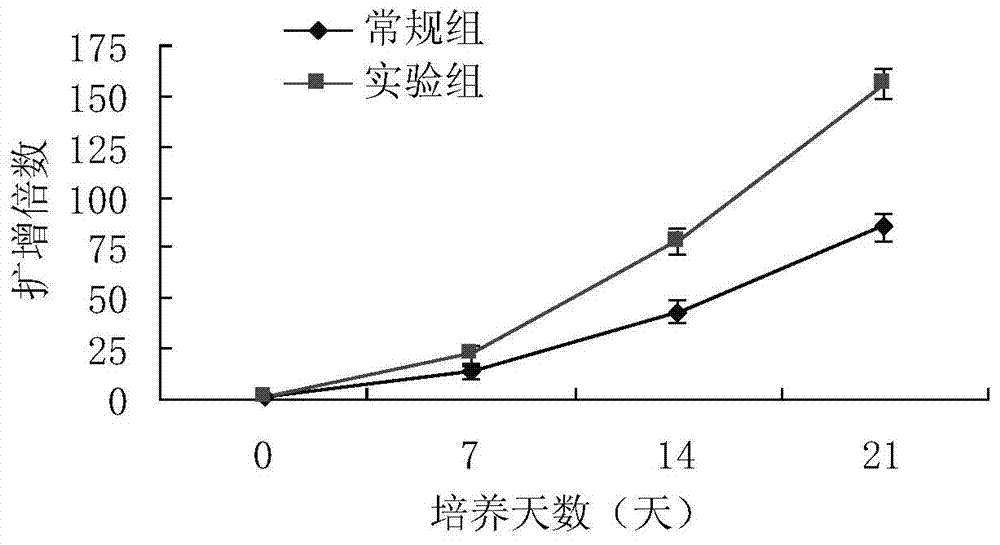
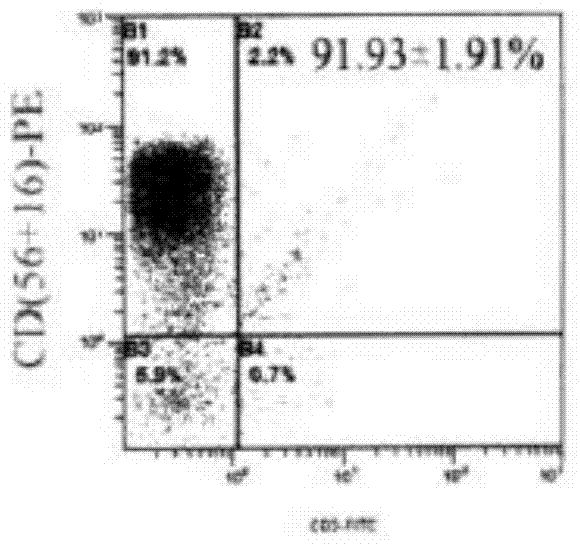
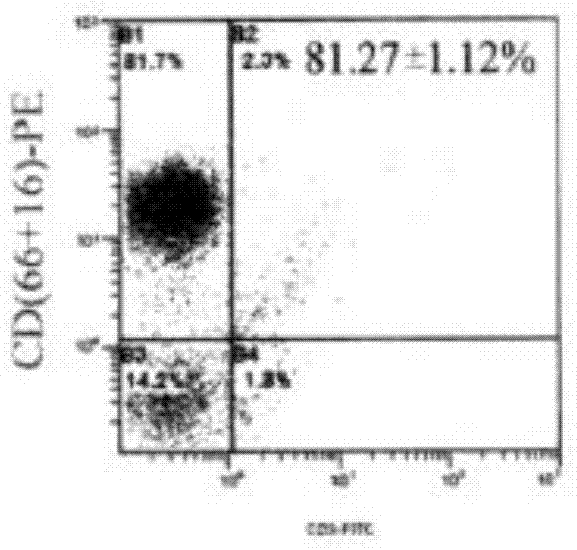
Patents
Literature
183 results about "CD16" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
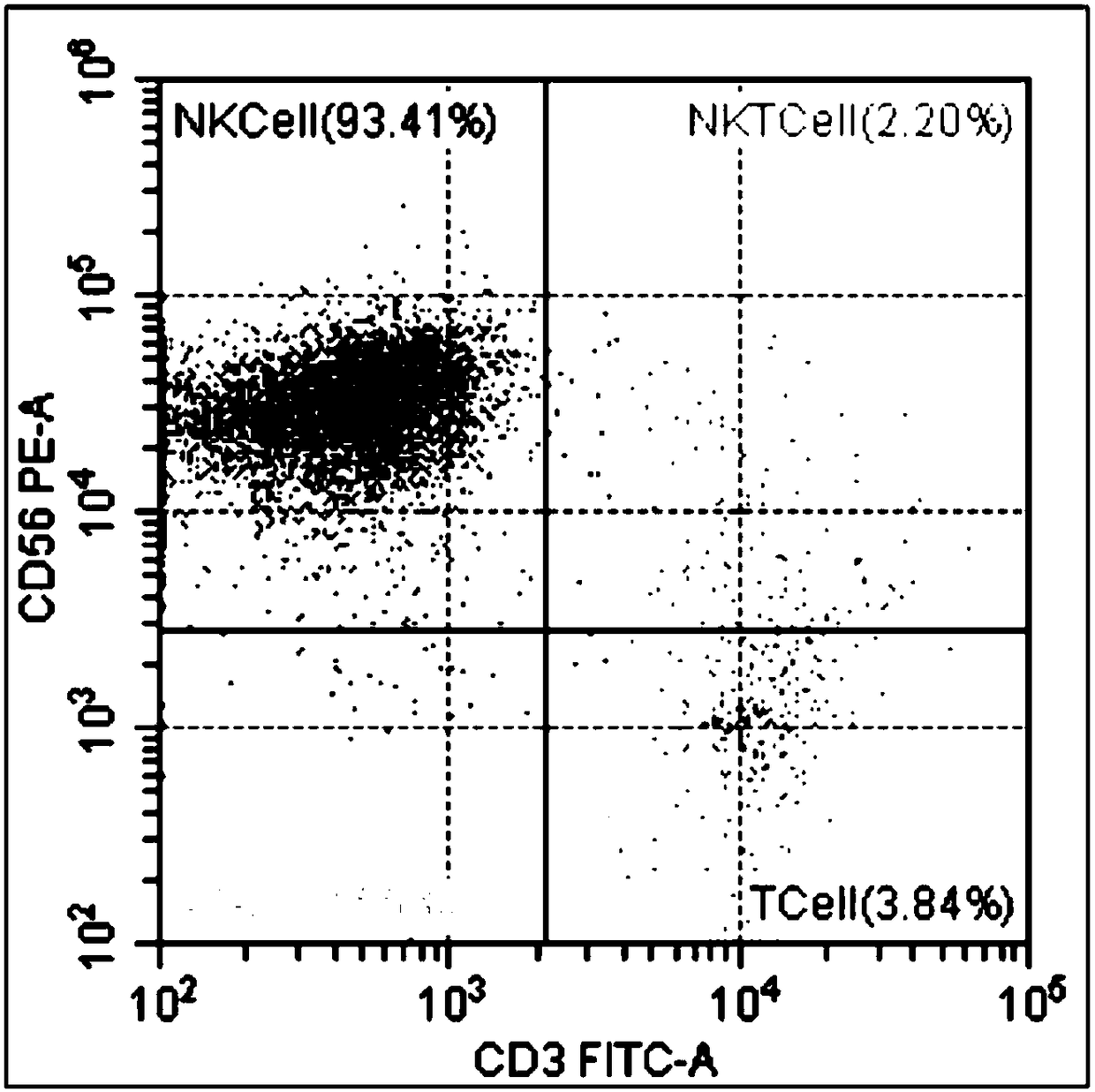
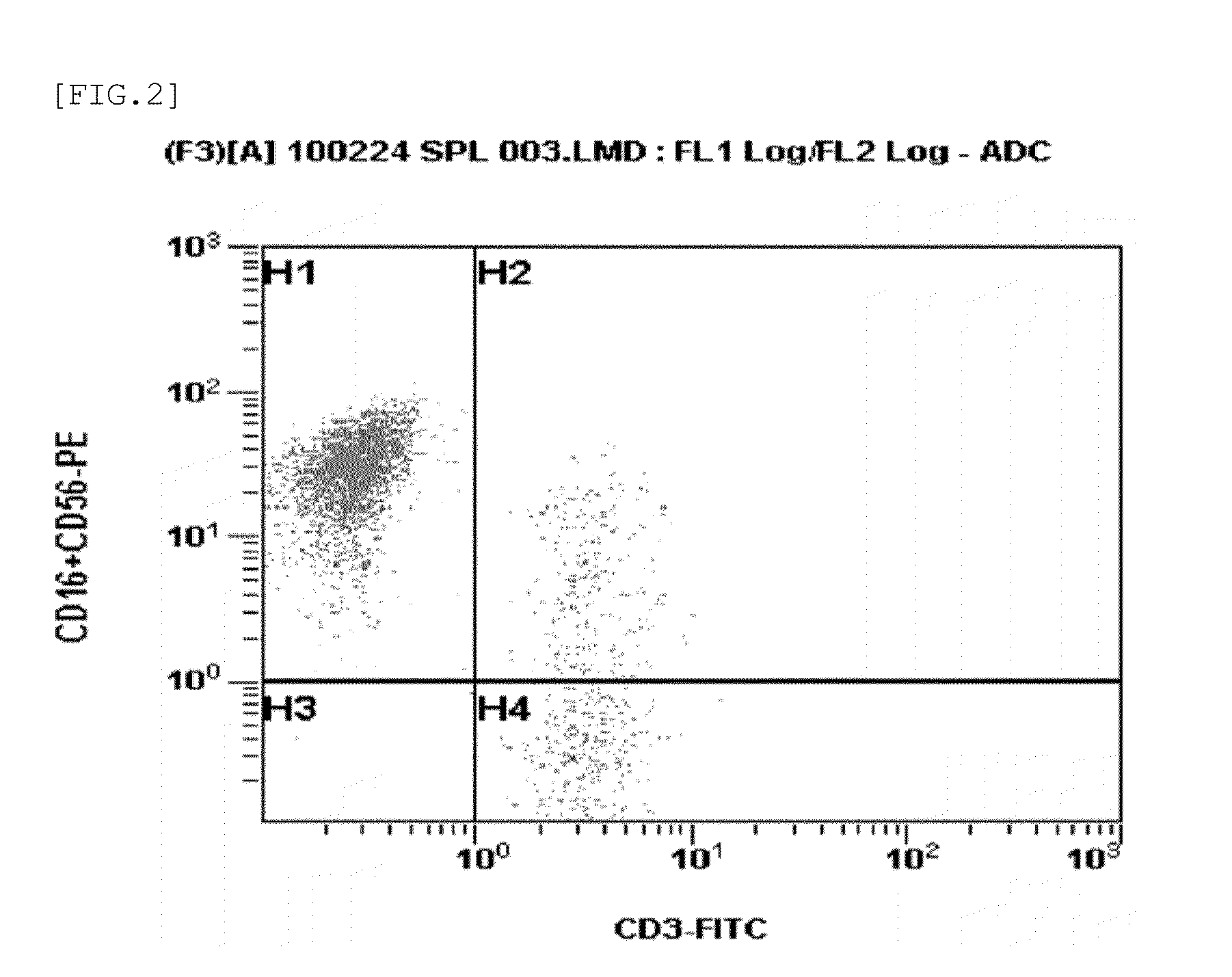
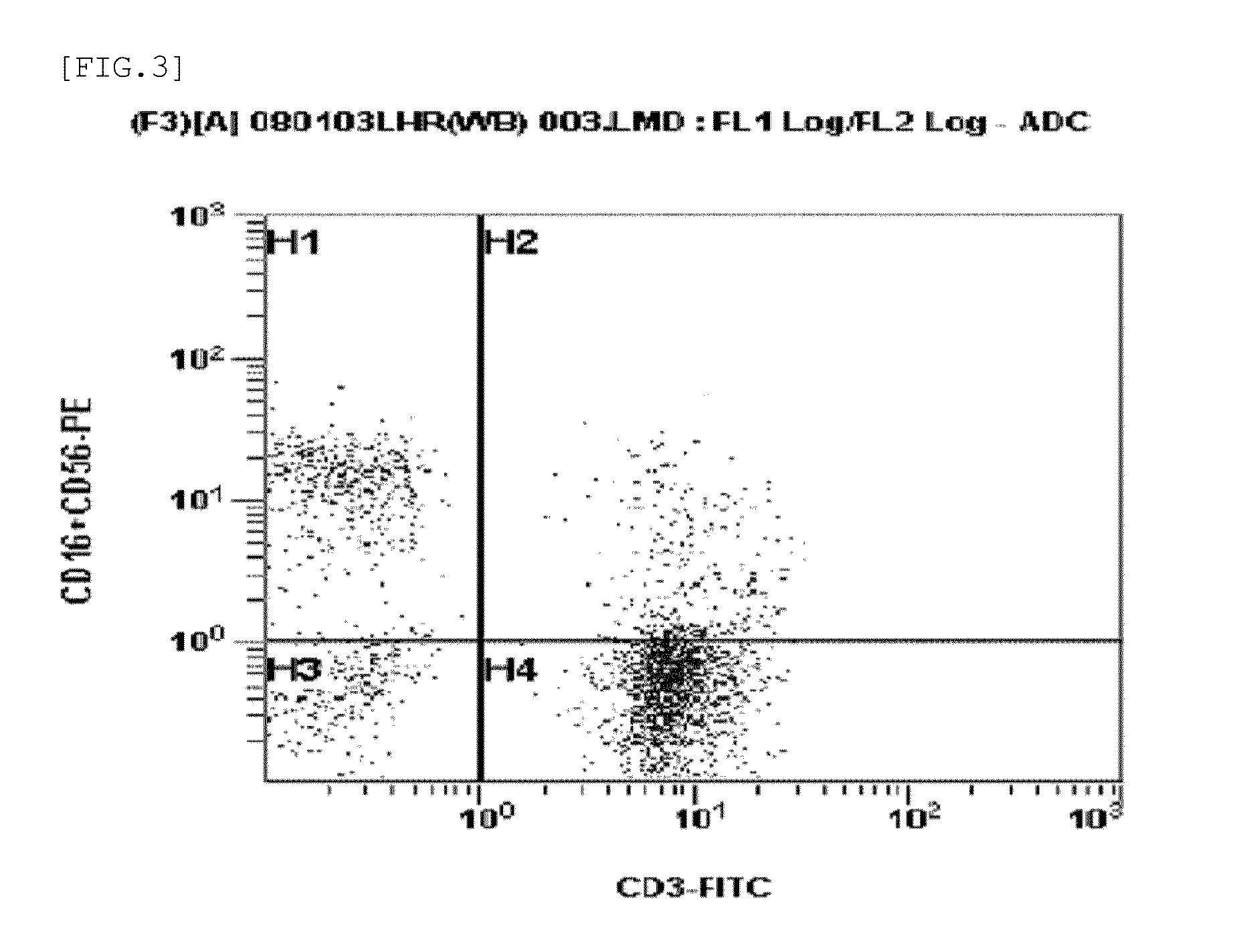
CD16, also known as FcγRIII, is a cluster of differentiation molecule found on the surface of natural killer cells, neutrophils, monocytes, and macrophages. CD16 has been identified as Fc receptors FcγRIIIa (CD16a) and FcγRIIIb (CD16b), which participate in signal transduction. The most well-researched membrane receptor implicated in triggering lysis by NK cells, CD16 is a molecule of the immunoglobulin superfamily (IgSF) involved in antibody-dependent cellular cytotoxicity (ADCC). It can be used to isolate populations of specific immune cells through fluorescent-activated cell sorting (FACS) or magnetic-activated cell sorting, using antibodies directed towards CD16.
Genetically modified human natural killer cell lines
ActiveUS8313943B2Increase rangeHelp studyDrug screeningImmunoglobulins against cell receptors/antigens/surface-determinantsFc(alpha) receptorFc receptor
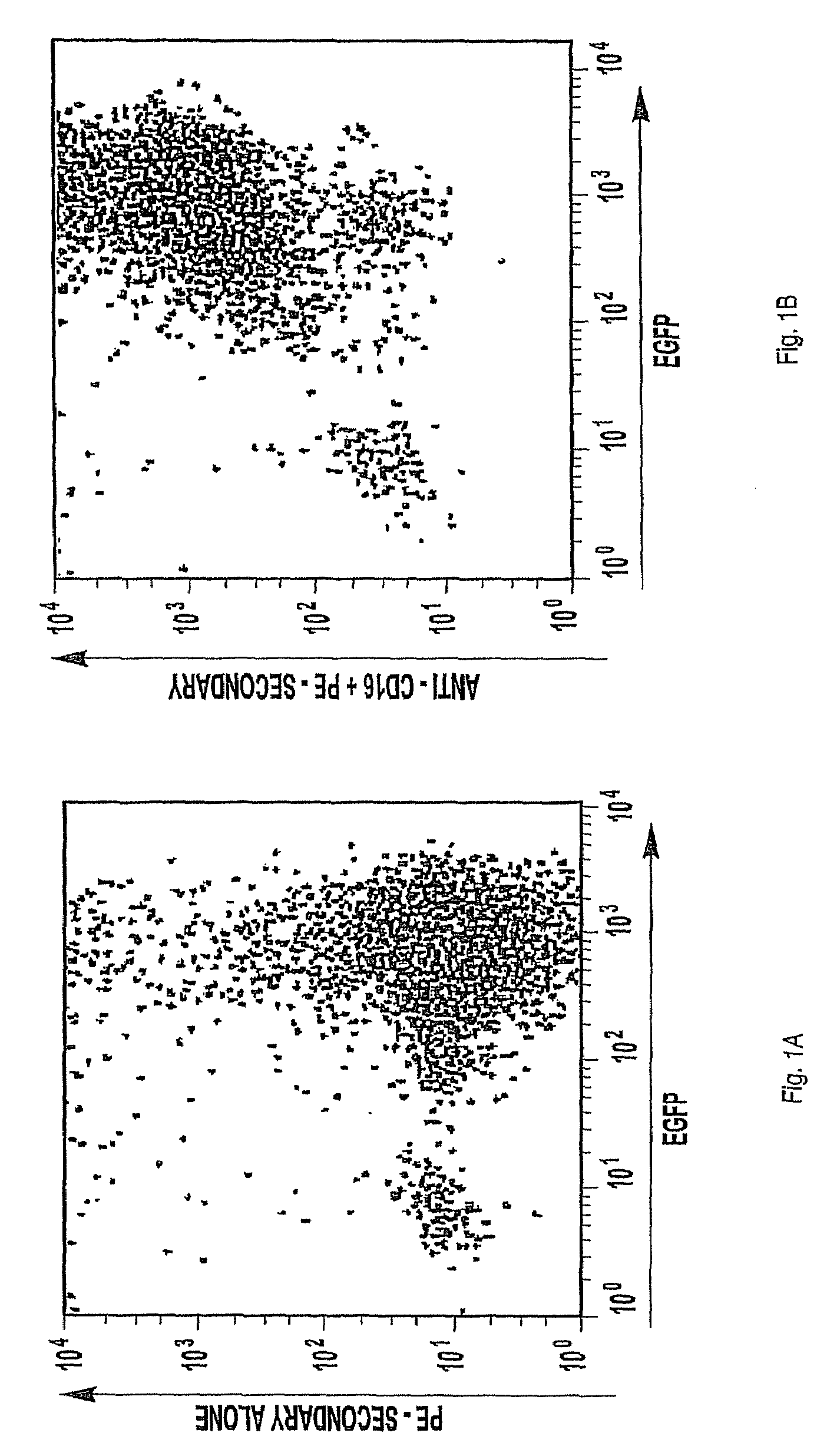
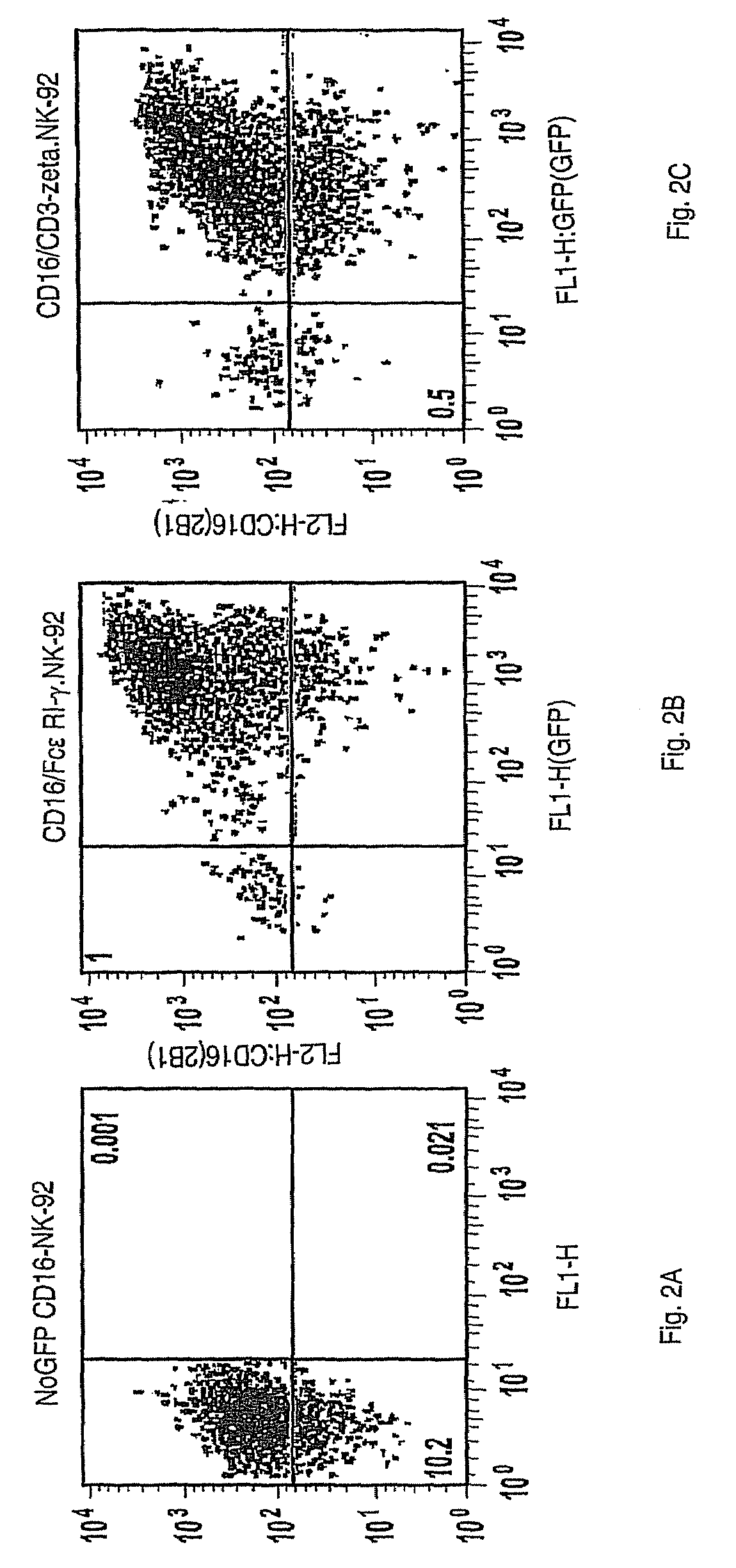
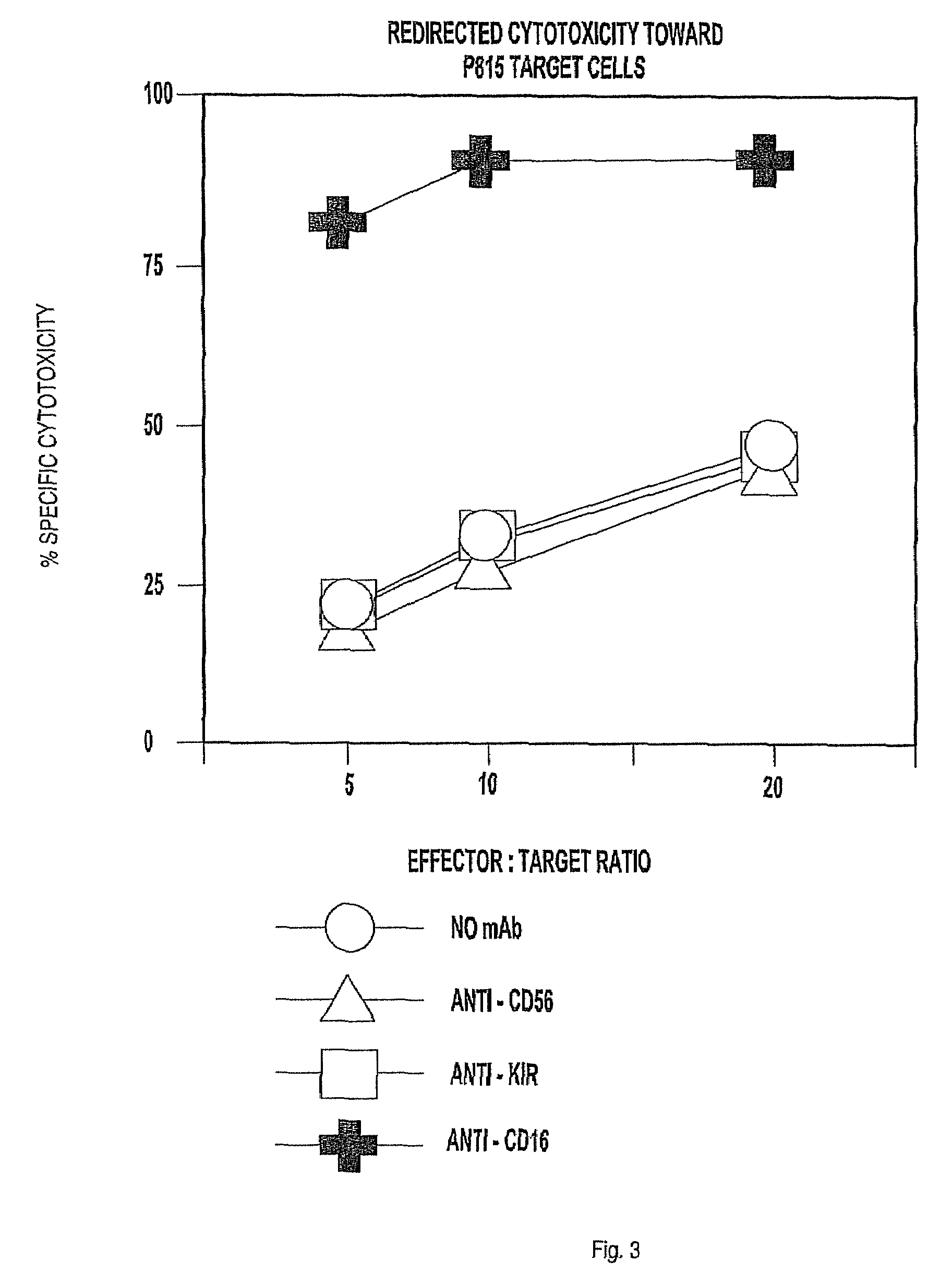
The invention provides a natural killer cell, NK-92, modified to express an Fc receptor on the surface of the cell, such as CD16 (FcγRIII-A), or other Fcγ or Fc receptors. The modified NK-92 cell can be further modified to concurrently express an associated accessory signaling protein, such as FcεRI-γ, TCR-ζ, or to concurrently express interleukin-2 (IL-2) or other cytokines. Additional methods are disclosed for various assays, assessments, and therapeutic treatments with the modified NK-92 cells.
Owner:INST FOR CANCER RES
Identification and isolation of multipotent cells from non-osteochondral mesenchymal tissue
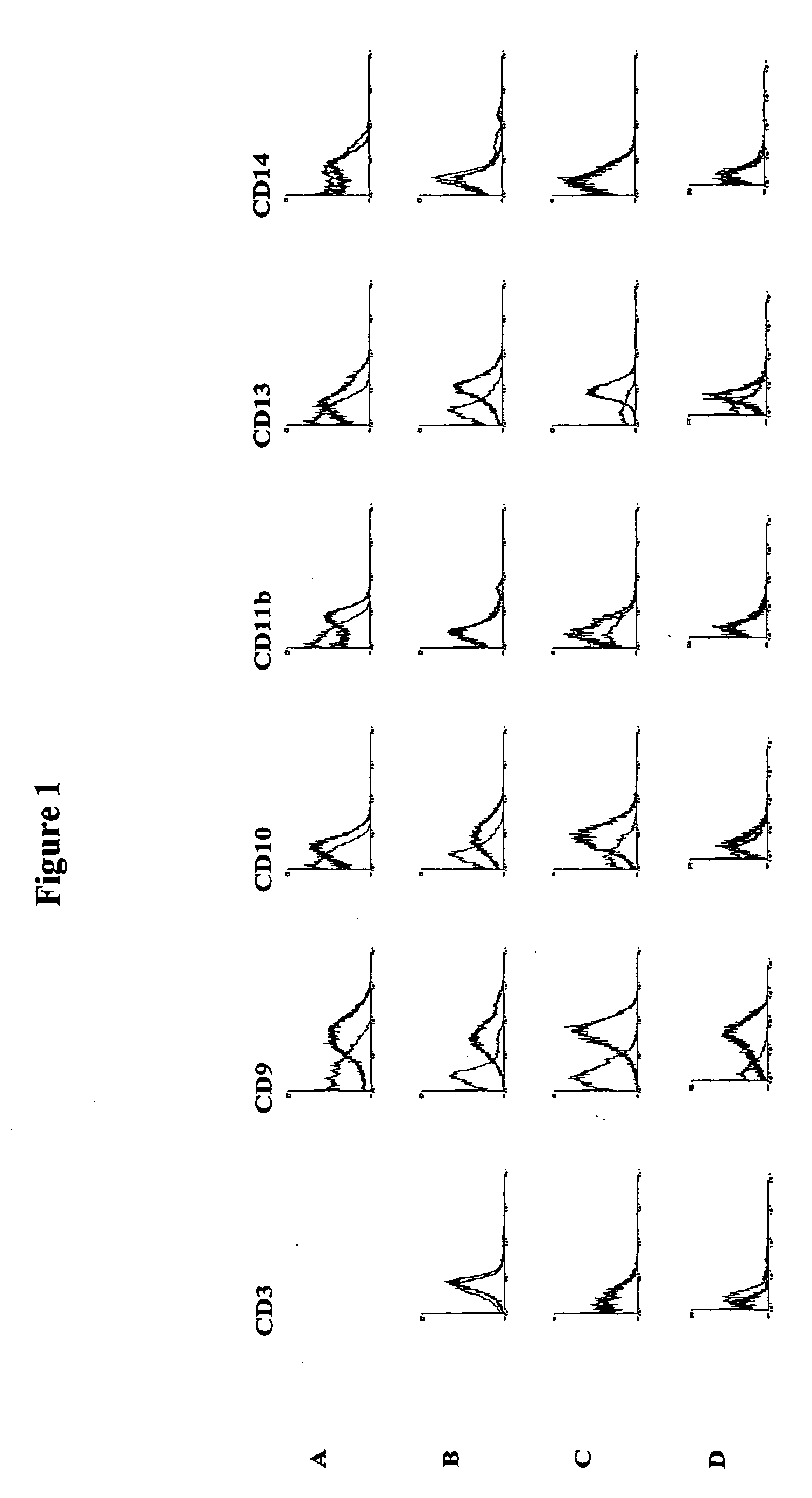
Methods for the identification and isolation of multipotent cells from non-osteochondral mesenchymal tissue. Specifically, this invention relates to an adult multipotent cell or a cell population or composition comprising said cell, isolated from non-osteochondral mesenchymal tissue, characterized in that the cell is positive for the following markers: CD9, CD10, CD13, CD29, CD44, CD49A, CD51, CD54, CD55, CD58, CD59, CD90 and CD105 and because it lacks expression of the following markers: CD11b, CD14, CD15, CD16, CD31, CD34, CD45, CD49f, CD102, CD104, CD106 and CD133.
Owner:AUTONOMOUS UNIVERSITY OF MADRID +1
Method for enhancing the antibody-dependent cellular cytotoxicity (ADCC) and uses of T cells expressing CD16 receptors
A method is provided for enhancing ADCC in an individual in need thereof, comprising the administration of T lymphocytes expressing a CD16-like receptor in said individual. Said method for enhancing ADCC may be used to treating cancers, autoimmune diseases or infections.
Owner:INST NAT DE LA SANTE & DE LA RECHERCHE MEDICALE (INSERM)
Method used for in vitro proliferation of NK cells
ActiveCN103756963AIncrease lethalityEasy to synthesizeBlood/immune system cellsSerum free mediaPeripheral blood mononuclear cell
Owner:SHANGHAI CLAISON BIOTECH
Identification and Isolation of Multipotent Cells From Non-Osteochondral Mesenchymal Tissue
Identification and isolation of multipotent cells from non-osteochondral mesenchymal tissue. This invention relates to the identification and isolation of multipotent cells from non-osteochondral mesenchymal tissue. Specifically, it relates to an adult multipotent cell or a cell population or composition comprising said cell, isolated from non-osteochondral mesenchymal tissue, characterized in that it is positive for the following markers: CD9, CD10, CD13, CD29, CD44, CD49A, CD51, CD54, CD55, CD58, CD59, CD90 and CD105 and because it lacks expression of the following markers: CD11b, CD14, CD15, CD16, CD31, CD34, CD45, CD49f, CD102, CD104, CD106 and CD133.
Owner:AUTONOMOUS UNIVERSITY OF MADRID +1
Use of ADCC-optimized antibodies for treating low-responder patients
ActiveUS7713524B2Good treatment effectGood effectAntibacterial agentsImmunoglobulins against blood group antigensCD16Monoclonal antibody
The invention concerns the use of human or humanized chimeric monoclonal antibodies which are produced in selected cell lines, said antibodies bringing about a high ADCC activity as well as a high secretion of cytokines and interleukins, for treating underpopulations of so-called weak-response patients exhibiting CD16 FCGR3A-158F homozygote or FCGR3A-158V / F heterozygote polymorphism.
Owner:LABE FR DU FRACTIONNEMENT & DES BIOTECH SA
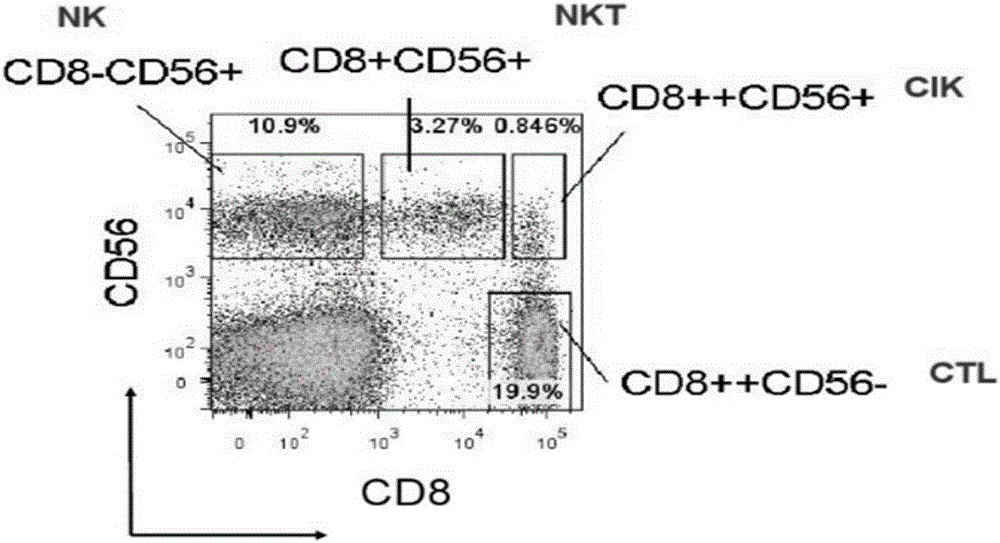
Method for simultaneous and efficient amplification of CD<3+>CD<56+>CIK cells and CD<3->CD<56+>NK cells
ActiveCN104357390AHigh purityHigh activityBlood/immune system cellsAdoptive cellular immunotherapySerum free media
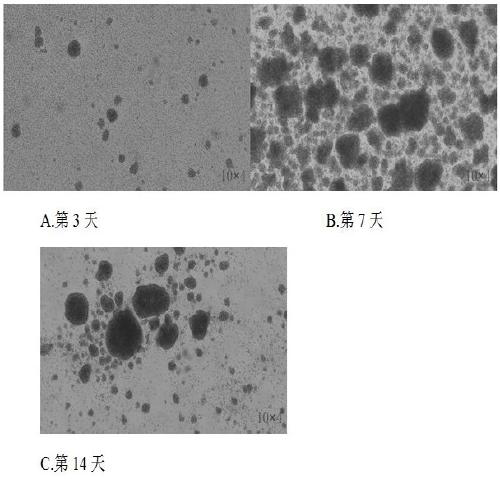
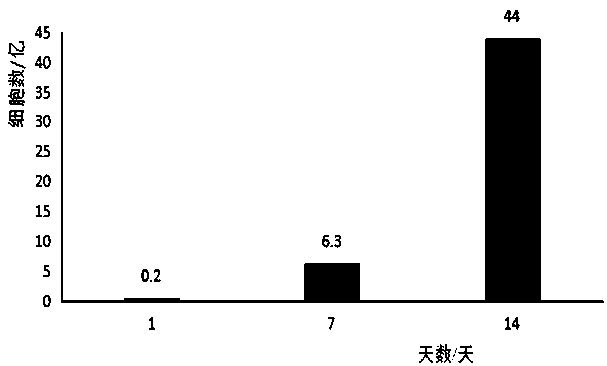
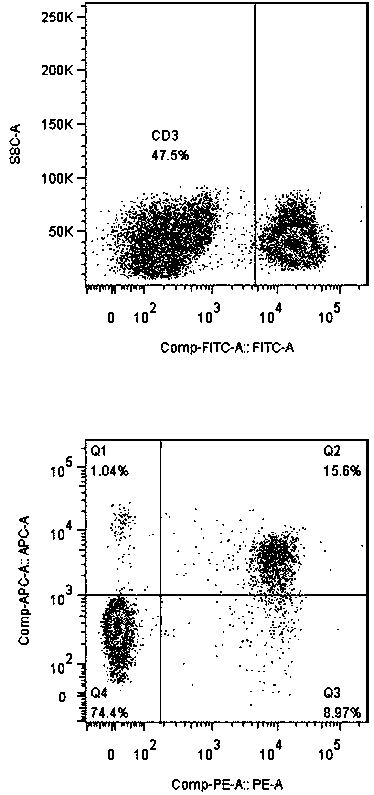
The invention discloses a method for simultaneous and efficient amplification of CD<3+>CD<56+>CIK cells and CD<3->CD<56+>NK cells. The method comprises the steps as follows: the concentration of separated PBMC (peripheral blood mononuclear cells) is adjusted by a serum-free medium containing autologous plasma, an Anti-CD16 antibody, IL-2 and IL-15 are added, and then the mixture is transferred into a T175 culture flask for culture; an Anti-CD3 antibody and an Anti-CD137 antibody are added; a serum-free medium containing the autologous plasma, IL-2 and IL-15 is supplemented every two days according to the cell growth condition; the cell concentration is controlled to be about 1.5*10<6> / ml; and after culture is performed for 14-21 days, large quantities of high-purity CD<3+>CD<56+>CIK cells and CD<3->CD<56+>NK cells can be obtained simultaneously, and the total cell quantity can reach an effective value of the cell quantity required for adoptive cellular immunotherapy clinically for tumor. The method for simultaneous and efficient amplification of the CD<3+>CD<56+>CIK cells and the CD<3->CD<56+>NK cells is simple, convenient, effective and high in cell killing activity.
Owner:HRYZ (SHENZHEN) BIOTECH CO +1
Method for differentiating human pluripotent stem cells into natural killer cells and application
ActiveCN111235105ALow costAvoid expensiveCulture processDead animal preservationHematopoietic progenitor cell differentiationNatural Killer Cell Inhibitory Receptors
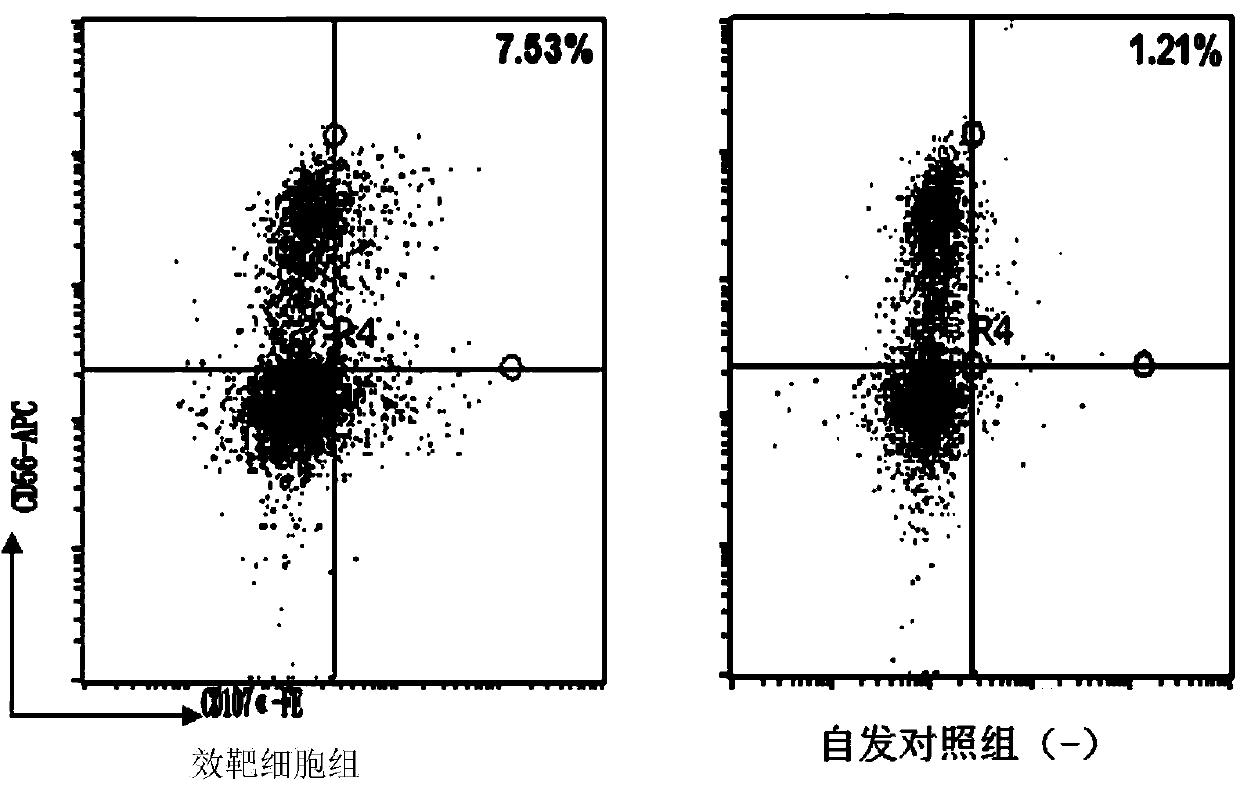
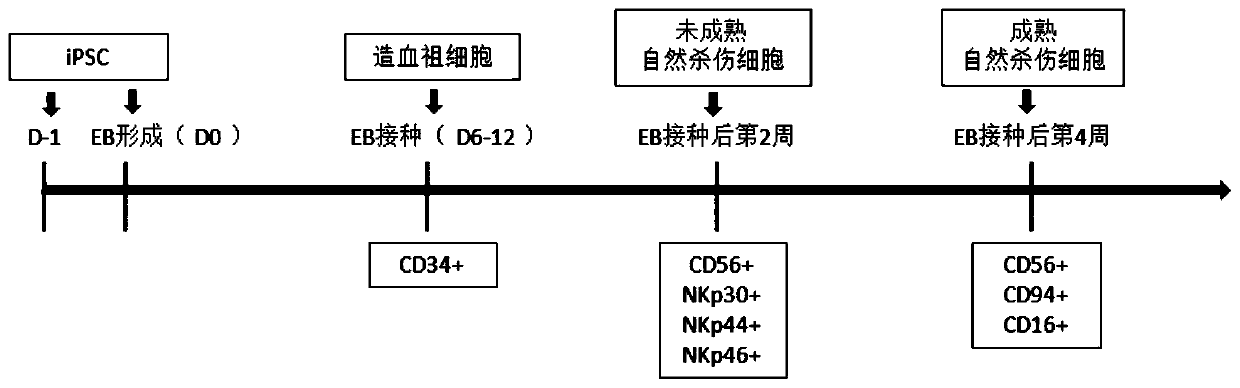
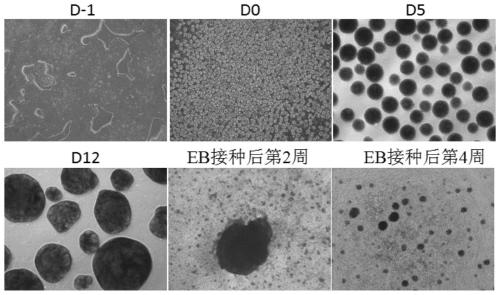
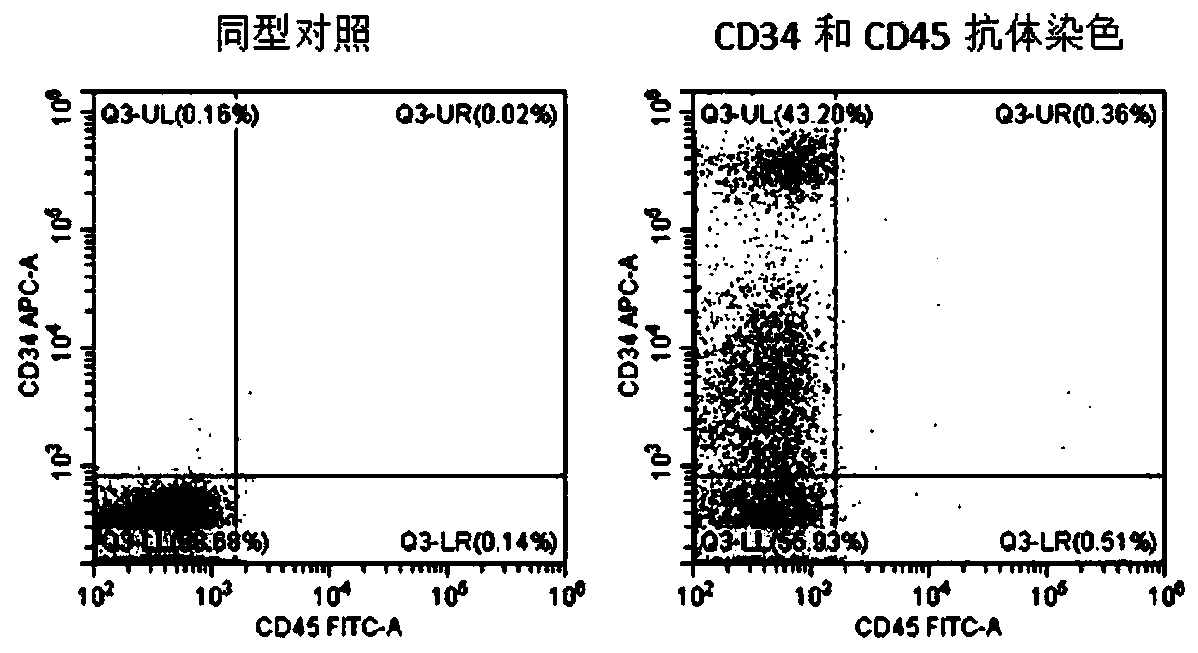
The invention relates to the field of stem cell biology, specifically to a method for differentiating human pluripotent stem cells into natural killer cells and an application. The invention disclosesa pluripotent stem cell-derived natural killer cell. The pluripotent stem cell-derived natural killer cell expresses CD56, Nkp30, Nkp44 and Nkp46, and also expresses markers CD16 and CD94 of a maturenatural killer cell. The invention also discloses a method for preparing the natural killer cell. The method comprises the following steps: S1, formation of an embryoid body; S2, differentiation of the embryoid body into hematopoietic progenitor cells; S3, differentiation of the hematopoietic progenitor cells into NK cells; and S4, maturation and expansion of the NK cells. With the differentiation method provided by the invention, the pluripotent stem cells can be rapidly, efficiently, simply and conveniently induced to be differentiated into the natural killer cells with low cost on the basis of a culture medium with definite components and an optimized cell factor combination.
Owner:安徽中盛溯源生物科技有限公司
Monocyte cell
ActiveUS20080057043A1Inhibit angiogenesisSimple procedureBiocideGenetic material ingredientsCD16CD14
Owner:OSPEDALE SAN RAFFAELE SRL +1
Method for abundantly amplifying NK (natural killer) cells from mononuclear cell of peripheral blood
ActiveCN107022524AAvoid insecurityHigh purityBlood/immune system cellsPeripheral blood mononuclear cellCD16
The invention relates to a method for abundantly amplifying NK (Natural Killer) cells from a mononuclear cell of peripheral blood. The method comprises the following steps of collecting the mononuclear cell of the peripheral blood, first putting the mononuclear cell into a culture bottle enveloped by a CD16 monoclonal antibody, culturing the mononuclear cell, and adding IL-2, IL-15, OK432 and inactivated autoserum into a culture medium; subsequently, transferring the cell into a culture bottle which is not enveloped by the CD16 monoclonal antibody, culturing the cell, adding the IL-2, the IL-15 and the inactivated autoserum into the culture medium; afterwards, transferring the cell into a culture bag, culturing the cell, and adding IL-4 into the culture medium on the last third day, and continuously culturing the cell, so as to obtain abundant NK cells. According to the method, components of animal serum and a tumor cell are not contained; therefore, the safety and the reliability of the NK cells are improved to a great extent; the method has a favorable application prospect.
Owner:SHANGHAI LIFE SCI & TECH CO LTD
Method for efficiently amplifying natural killer cells from peripheral blood in high purity manner
ActiveCN107326008AAvoid insecurityHigh purityBlood/immune system cellsCD16Natural Killer Cell Inhibitory Receptors
The invention relates to a method for efficiently amplifying natural killer cells from peripheral blood in a high purity manner. The method comprises the following steps: collecting 100ml-200ml of peripheral blood, separating mononuclear cell, putting the mononuclear cell into a culture bottle coated with CD16 monoclonal antibody and CD226 monoclonal antibody for culturing, and activating the cell by virtue of IFN-gamma; after the activation is carried out for 24 hours, adding IL-2, IL-15, IL-21, OK432, CD2 monoclonal antibody and inactivated autoserum into a culture medium; transferring the cell into a culture bottle which is not coated with CD16 monoclonal antibody and CD226 monoclonal antibody for culturing, and supplementing liquid to the cell once in each 2-3 days; and finally transferring the cell into a culture bag for culturing, and collecting NK cell in the 21th day. According to the method, no any heterologous serum component or trophoblast cell is used, so that the safety and reliability of the prepared NK cell are guaranteed; and the method has good clinical application values.
Owner:SHANGHAI LIFE SCI & TECH CO LTD
Enumeration of CD4+ lymphocytes
The invention provides a method of enumerating the number of cells of a cell type in a cell sample by (a) counting the white blood cells in the cell sample to obtain the white blood cell population of the sample; (b) determining the proportion or percentage of the cells of the cell type in the white blood cell population in the sample; and (c) calculating the number of cells of the cell type in the sample. The cell type may be a lymphocyte sub-set selected from the group comprising CD4+ lymphocytes, CD 45 cells, CD19 cells, CD16 and CD56 positive cells, CD8 cells, CD3 cells or any combination thereof. The method is particularly useful in monitoring the immune status of a patient infected with HIV or other immune deficiency state or disease or condition where CD4+ lymphocytes or CD4+ T cells are monitored or counted.
Owner:NAT HEALTH LAB SERVICE
In-vitro pure culture method for natural killer cells
ActiveCN109666640AReduce harmHigh purityMammal material medical ingredientsBlood/immune system cellsHeterologousCD16
The invention relates to an in-vitro pure culture method for natural killer cells. The method includes the following steps that peripheral blood is collected, single karyocyte is separated, magnetic beads combined with specific antibodies are adopted to remove the NK cells, and NK cell populations are obtained; the NK cell populations are put into a culture bottle coated with multiple antibodies,and are subjected to activation culture with a complete medium containing IL-2, IL-7, IL-15, anti-CD16, OK432 and inactivated autologous serum, and inactivated NK cell populations are obtained; the inactivated NK cell populations are put into a culture bottle free of antibody coating and continue to carry out multiplication culture, and the natural killer cells are obtained. Any heterologous serumingredients and trophoblast cells are not used in the in-vitro pure culture method, and high amplification efficiency can be wholly obtained; meanwhile, the purity of the amplified NK cells reaches 90% or above, in-vitro efficient pure culture is achieved, and the clinical application requirement is conveniently met.
Owner:IREGENE THERAPEUTICS LTD
Preparation method used for high efficiency amplification of natural killer cells
InactiveCN108103020AFix security issuesEasy to operateBlood/immune system cellsCell culture active agentsSerum igeCD16
The invention relates to a preparation method used for high efficiency amplification of natural killer (NK) cells. The preparation method comprises following steps: peripheral blood is collected, andsingle karyocytes are separated; the karyocytes are introduced into a culture bottle treated via coating with CD16 monoclonal antibody and HER2 monoclonal antibody for culturing; IL-2, IL-15, IL-21, and inactivated autologous serum are introduced into a culture medium at the same time; the cells are transformed into a culture bottle free of coating treatment, liquid supply is carried out every 2 to 3 days; and at last the obtained NK cells are collected. According to the preparation method, no heterogenous serum or trophoblastic cell is adopted, operation process is simple, high NK cell yieldis achieved, and the clinical application value is high.
Owner:上海莱馥生命科学技术有限公司
Method for in vitro multiplication culture of NK cells
ActiveCN109628397AStrong expansion abilityImprove securityCulture processBlood/immune system cellsSerum free mediaCD16
The invention relates to a method for in vitro multiplication culture of NK cells. The method comprises coating a culture bottle with heparin sodium, CD16 and human immunoglobulin, collecting peripheral blood, inactivating plasma, separating mononuclear cells, using a serum-free medium (containing autologous plasma, IL-2, IL-15 and IL-18) to culture multiplication cells, harvesting and detecting the cells and the like. Trophoblast cells or magnetic beads are not used for sorting and purifying, and the method has simple operation, high safety, good amplification effect and high clinical application value.
Owner:福建省海西细胞生物工程有限公司
A method for cultivating self activated lymphocyte
InactiveCN101603029ALittle side effectsImproved prognosisCancer antigen ingredientsBlood/immune system cellsCancer cellCD16
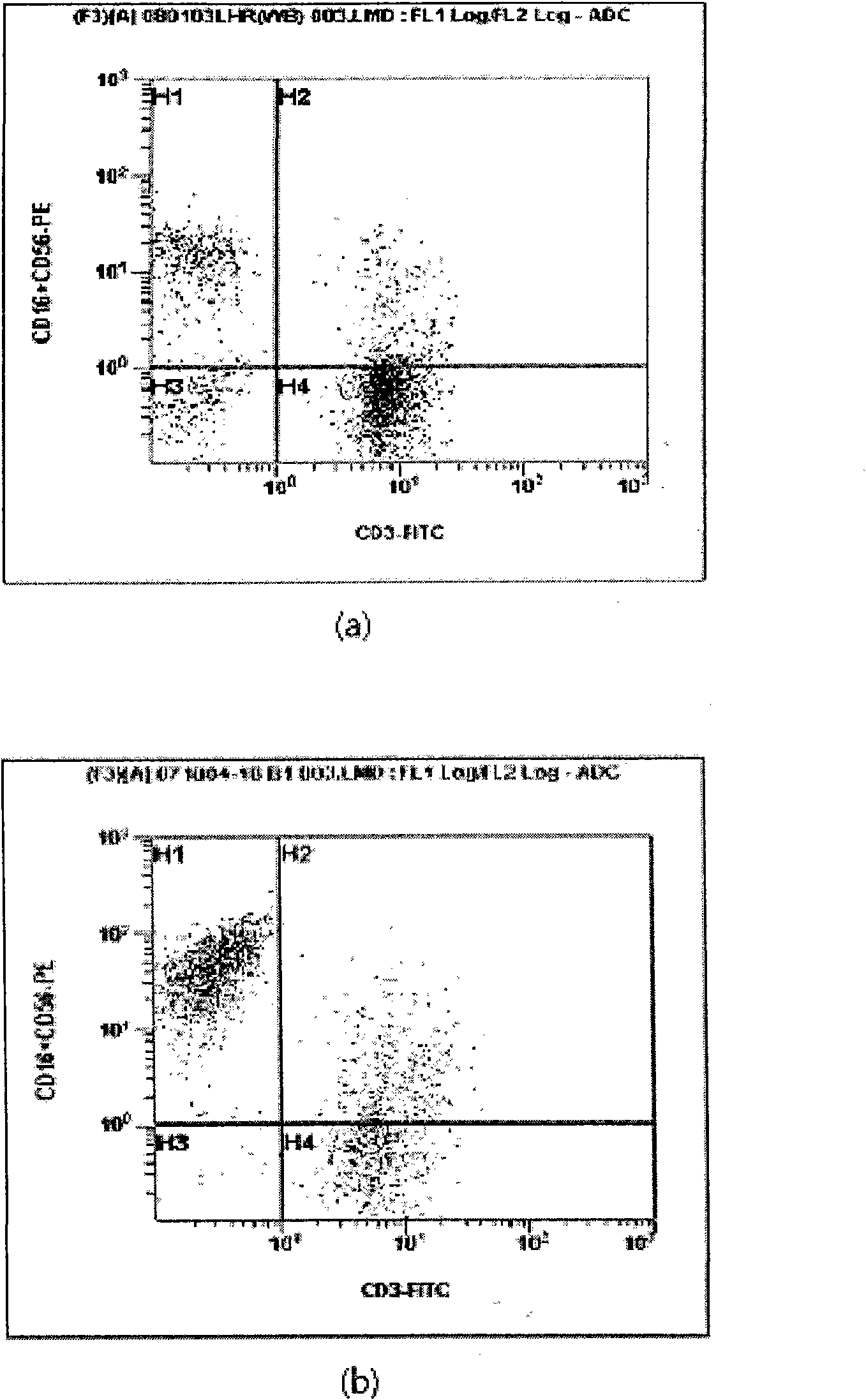
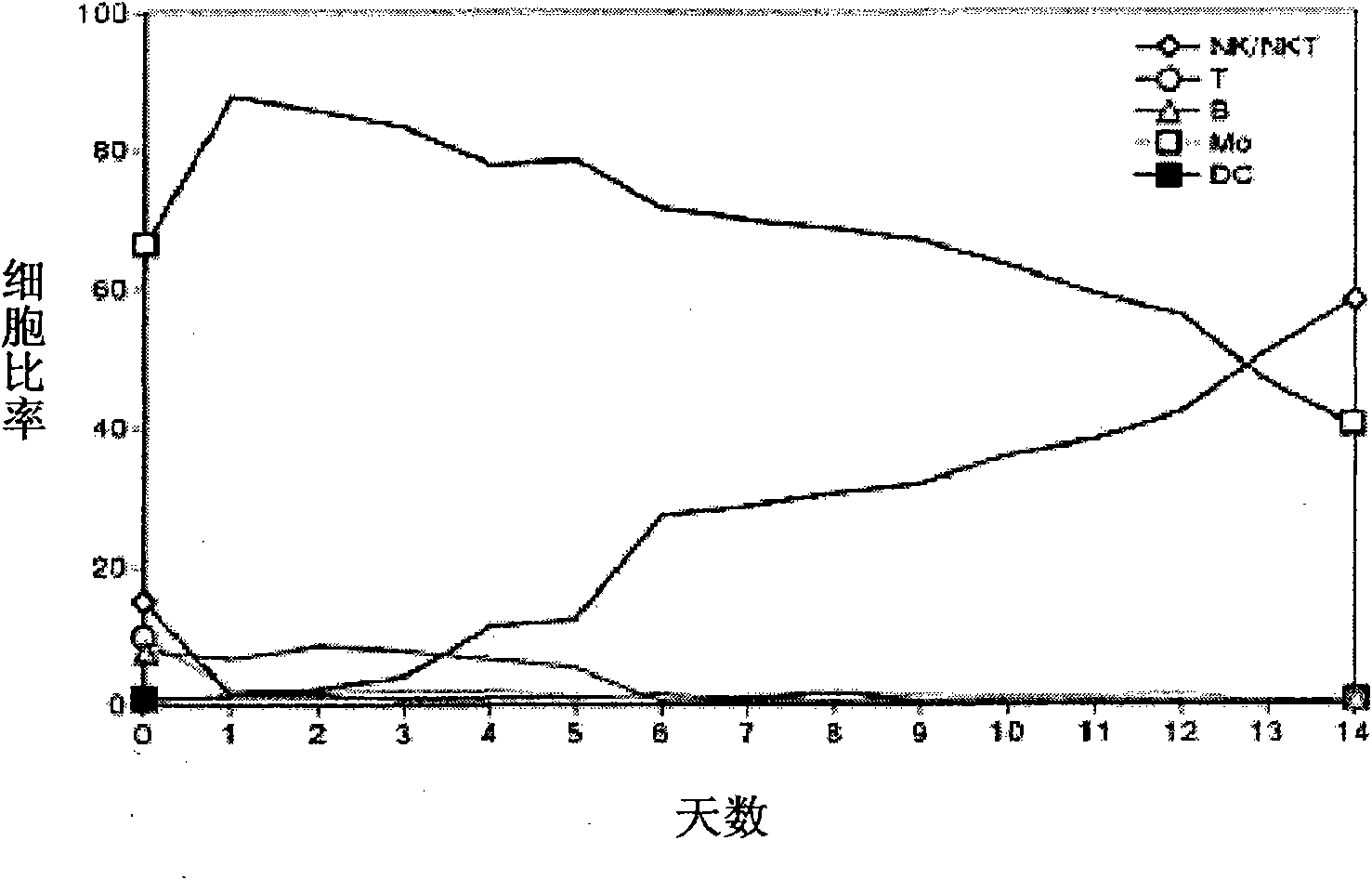
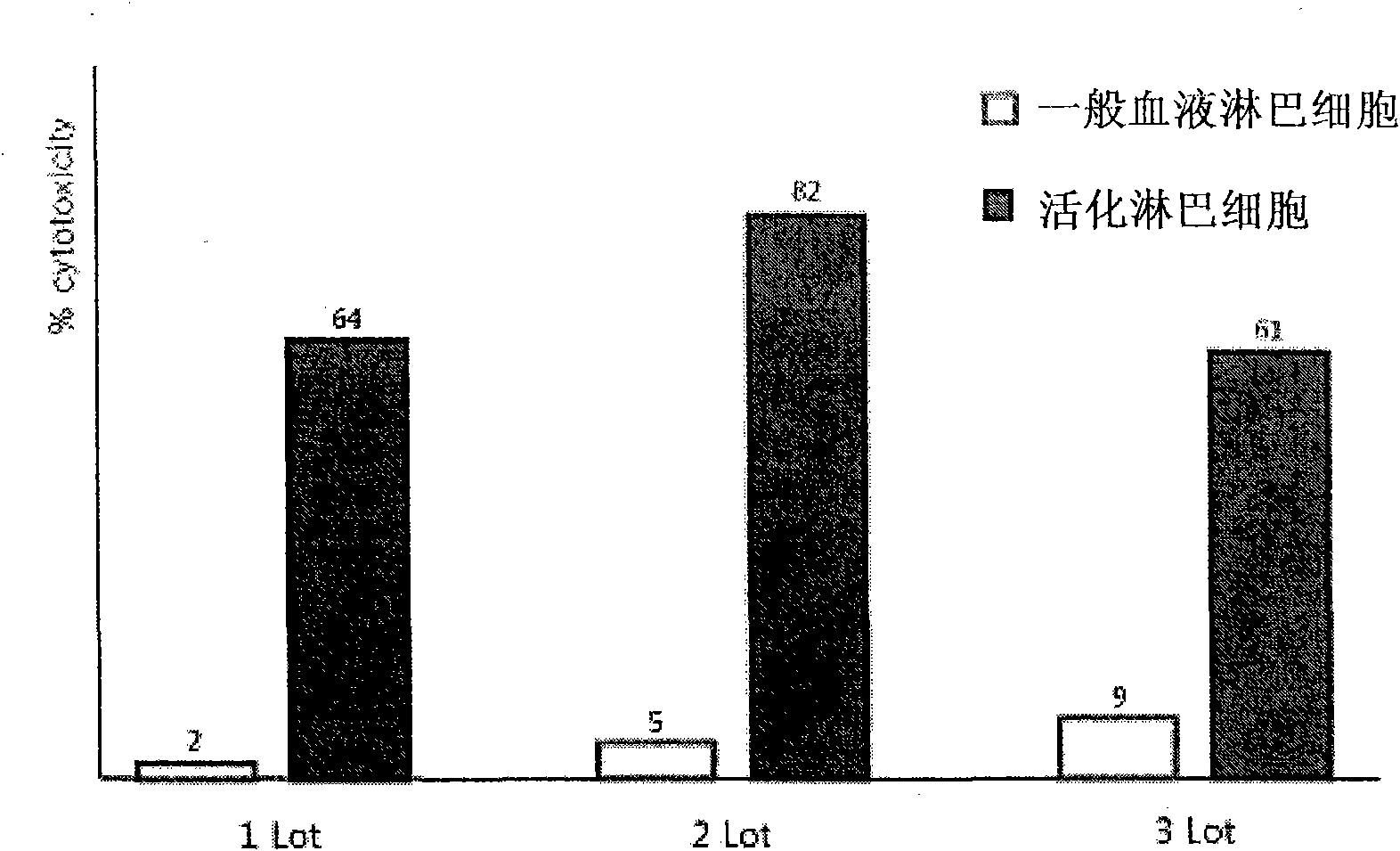
Provided is a method of culturing self-activated lymphocytes applicable to the treatment of malignant tumors. The method raises the percentage's of natural killer (NK) cells of the lymphocytes and evenly activate the NK cells, T cells and natural killer T (NKT) cells, and thus can be used to effectively eliminate various kinds of cancer cells. The method of culturing self- activated lymphocytes involves: extracting lymphocytes from human peripheral blood; performing a first culturing step of culturing the extracted lymphocytes in a culture fluid to which IL-2, L-glutamine and autochthonous plasma are added, in the presence of anti-CD3, anti- CD 16, and anti-CD56 antibodies; and performing a second culturing step of culturing the mixed culture fluid resulting from the first culturing step in the presence of anti-CD3, anti-CD16, and anti-CD56 antibodies after being admixed with a culture fluid to which IL-2, L-glutamine and autochthonous plasma are added.
Owner:NKBIO
Method for producing nk cell-enriched blood product
InactiveUS20120308986A1Increase probabilityPromote growthCulture processMammal material medical ingredientsBiological bodyWhole blood product
It is intended to provide a method for producing an NK cell-enriched blood product, the method being less invasive and capable of conveniently and rapidly growing NK cells, etc. in blood collected from an organism. The NK cells in blood are stimulated with NK cell growth-stimulating factors comprising an anti-CD16 antibody, OK432, a bisphosphonate derivative or a salt thereof, or a hydrate thereof, and a cytokine. Then, the blood is cultured at a physiological cell temperature to produce an NK cell-enriched blood product.
Owner:BIOTHERAPY INST OF JAPAN
Method for preparing natural killer cells using irradiated pbmcs, and Anti-cancer cell therapeutic agent comprising the nk cells
InactiveUS20180155690A1High purityCell dissociation methodsMammal material medical ingredientsCancer cellCD16
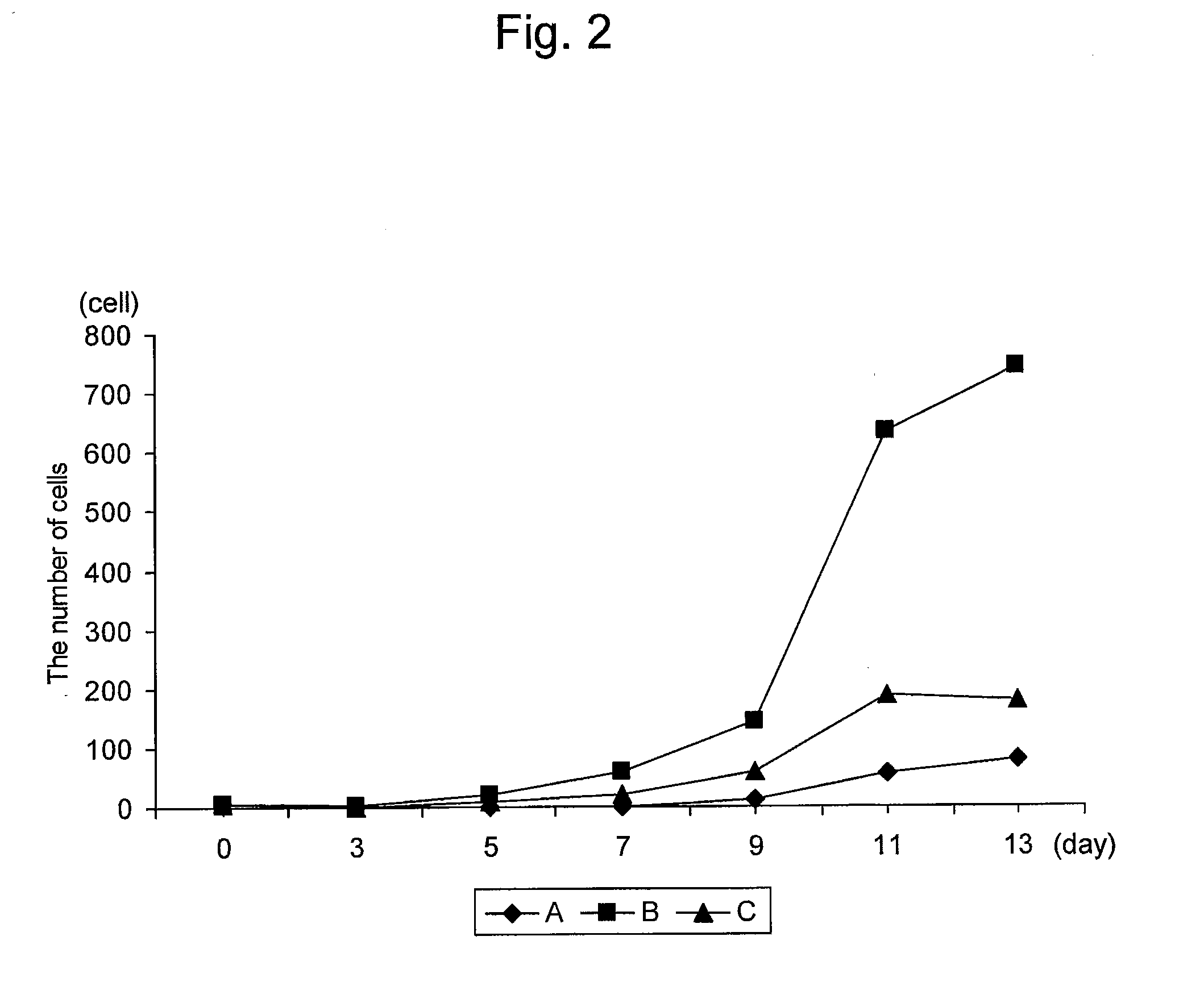
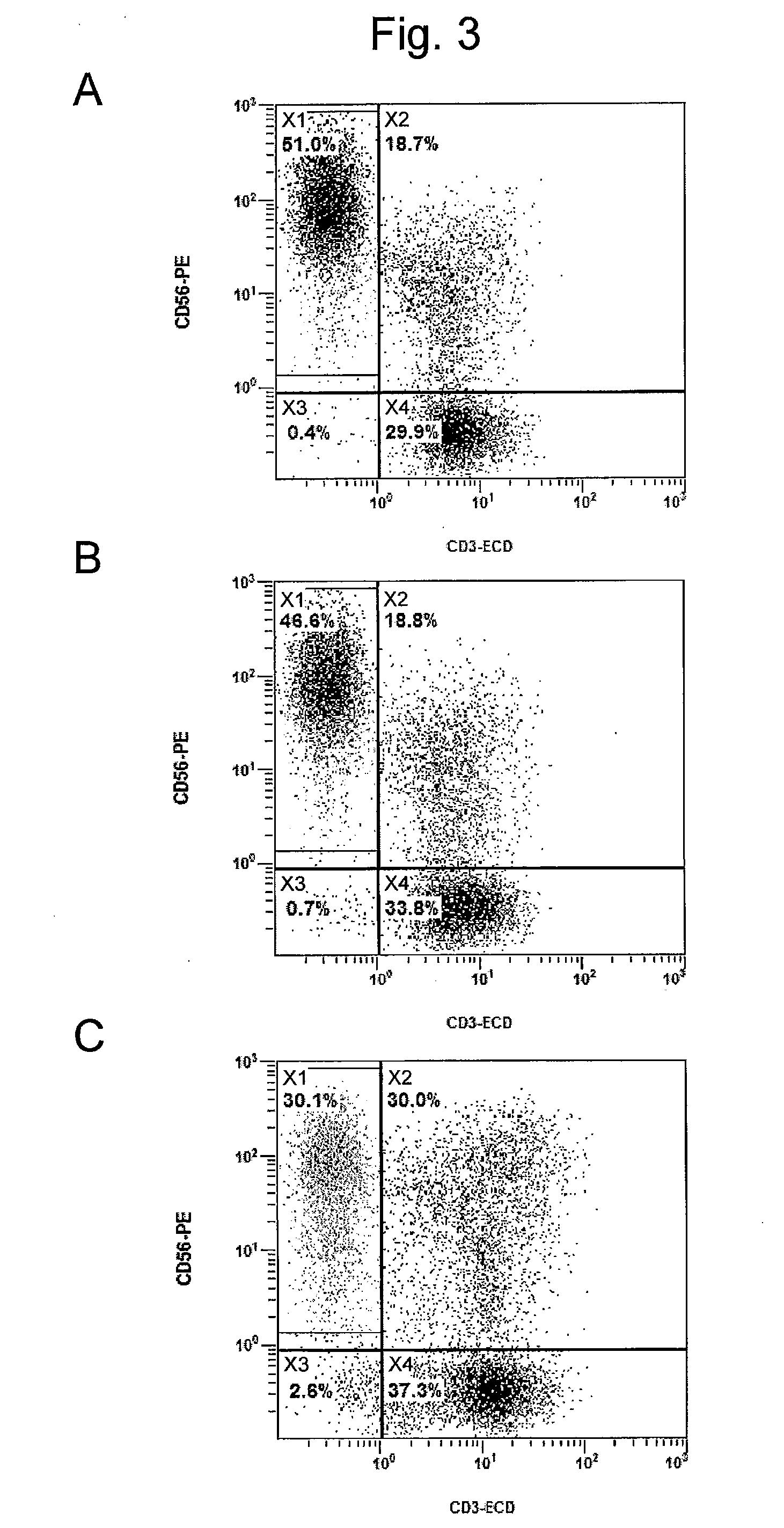
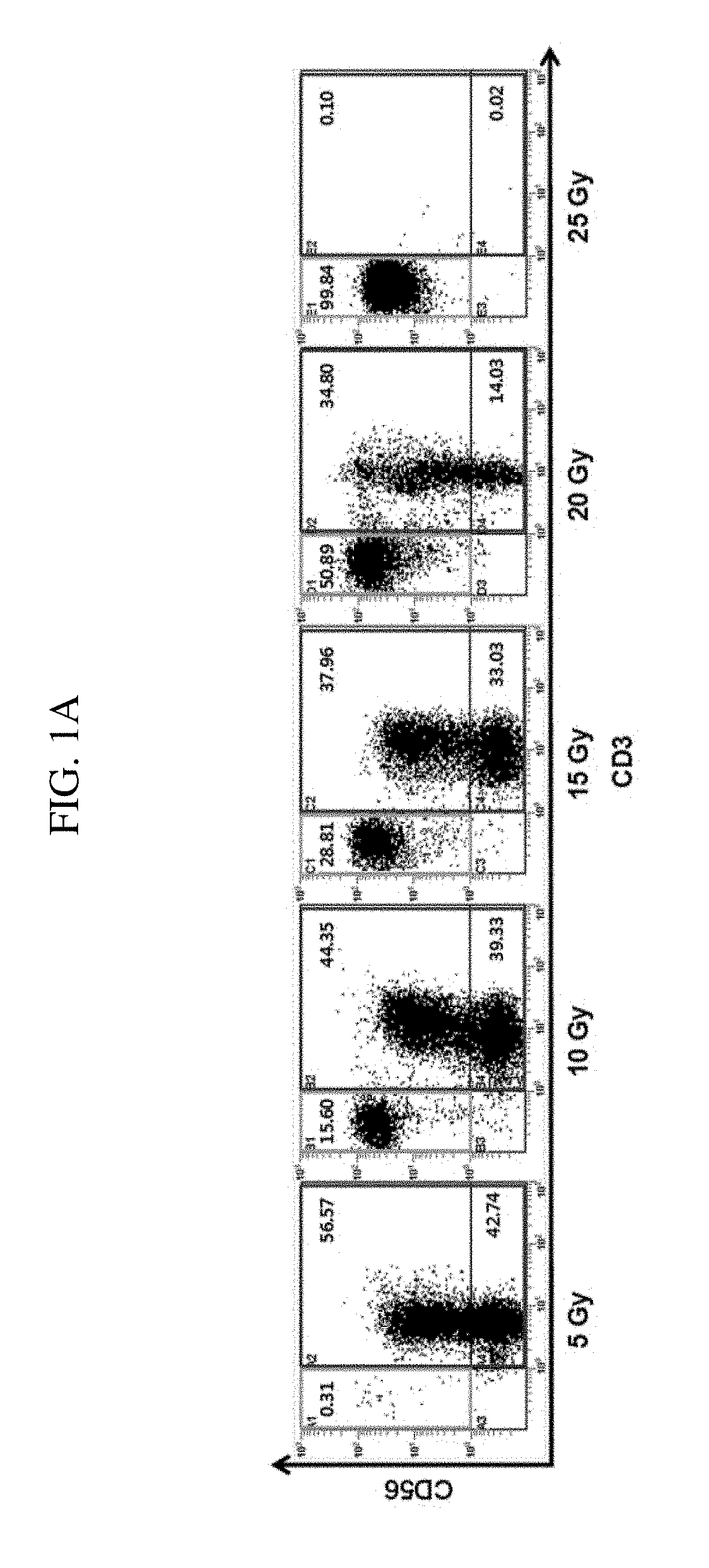
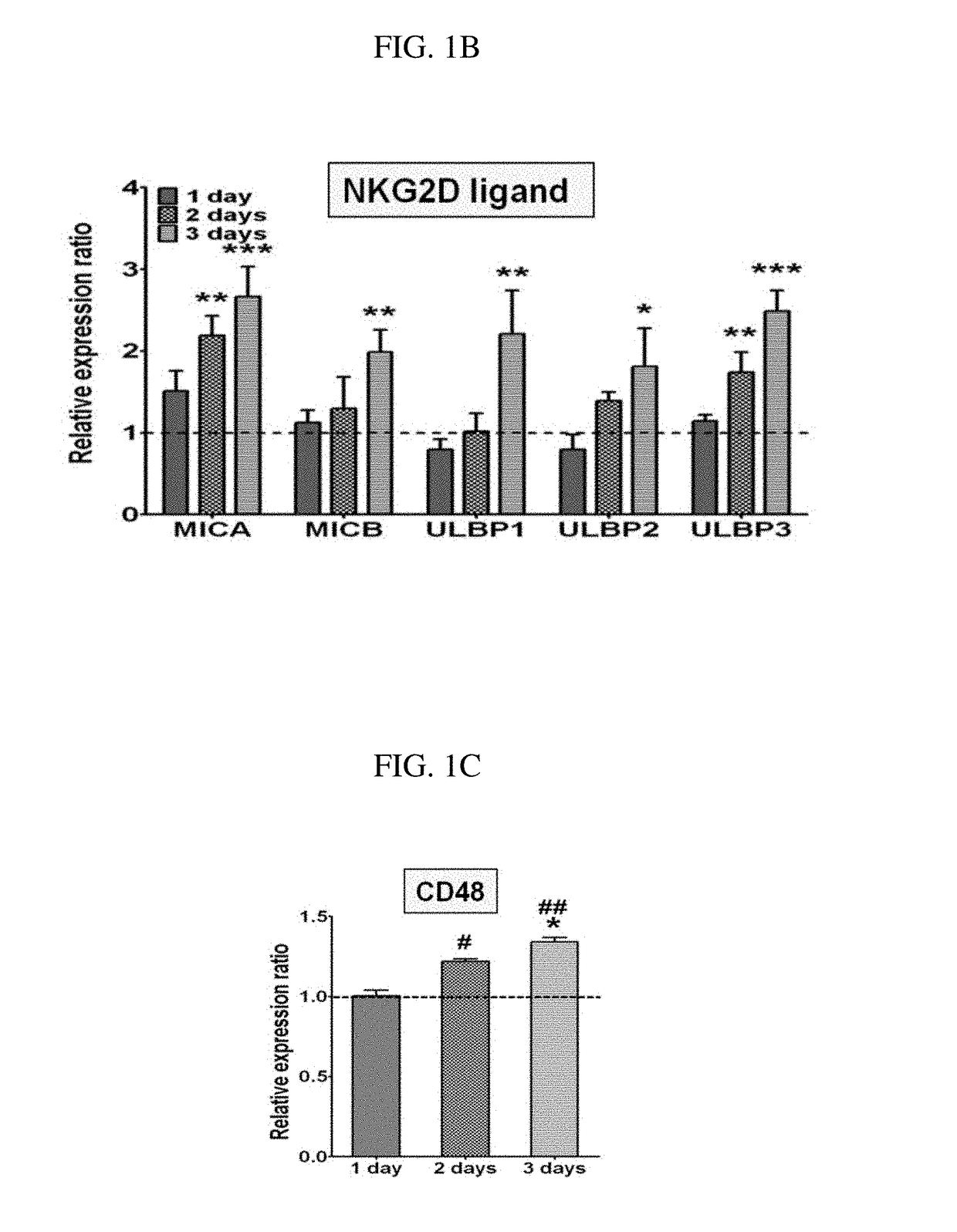
Provided is a method for preparing natural killer cell with high efficiency using irradiated peripheral blood mononuclear cells, more particularly to a method for proliferating highly activated NK cells using a combination of irradiated peripheral blood mononuclear cells (PBMCs) and a CD16 antibody and an anti-cancer cell therapeutic composition containing the natural killer cells (NK cells) prepared thereby. Further provided is a method for large-scale proliferation of activated NK cells with high efficiency using a combination of irradiated peripheral blood mononuclear cells (PBMCs) and a CD16 antibody without the use of cancer cells or genetically modified feeder cells having safety issues as feeder cells. The highly purified and highly cytotoxic NK cells proliferated in large quantities can be used as an active ingredient of a cancer immunotherapeutic composition.
Owner:KOREA INST OF RADIOLOGICAL & MEDICAL SCI
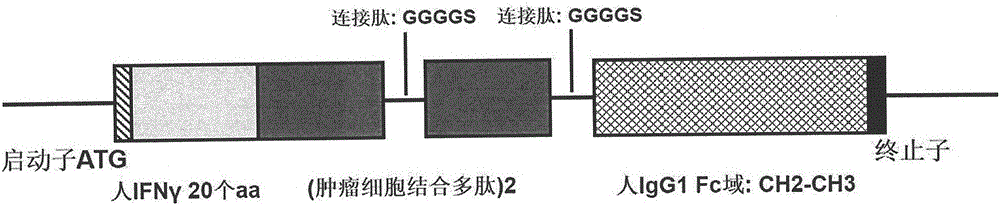
Preparation method and kit of cytokine-induced killing cell for inducing antibody-dependent cellular cytotoxicity
The invention provides a preparation method of a cytokine-induced killing cell for inducing antibody-dependent cellular cytotoxicity. The preparation method comprises constructing an interferon gamma signal peptide-tumor cell combined peptide, bonding 2th and 3th constant region gene segments of a crystallizable fragment domain heavy chain to the combined peptide, recombining the combined chain to a viral vector, transfecting a human cytokine-induced killing cell and carrying out high expression of a 2th and 3th constant region fusion protein of a novel polypeptide-crystallizable fragment domain heavy chain. The preparation method can cause antibody-dependent cellular cytotoxicity, improve a cytokine-induced killing cell proliferation multiple to 1000 times or more, a CD3+ / CD56+ expression rate to 40% or more and a CD16+ expression rate to 30% or more. In-vivo and in-vitro tests prove that the cytokine-induced killing cell has strong tumor killing toxicity. The invention also provides a kit for autologous cytokine-induced killing cell proliferation culture and has a high-efficiency anti-tumor function.
Owner:ZICHENG RUISHENGHUI BEIJING BIOTECH DEV CO LTD
Fusion protein of Her2 antibody and interleukin 2 and application thereof
InactiveCN102199218AImprove biological activityIncrease productionFungiBacteriaAntiendomysial antibodiesCD16
The invention discloses a fusion protein of Her2 antibody and interleukin 2 and application thereof, and belongs to the technical fields of medical science and oncology phymatology. The fusion protein comprises CD16 monoclonal antibody heavy chain signal peptide, ErbB2 antibody heavy chain variable region, a Linker 1, a light chain variable region, a human antibody Fc segment, a Linker 2 and IL-2 mature peptide, and has the amino acid sequence shown as SEQ ID No.1. The fusion protein can inhibit Herceptin resistant breast cancer cell proliferation, and can kill Her2 high-expression breast cancer cells. The invention lays a foundation for clinical application of the fusion protein of the Her2 antibody and the interleukin 2 in the anti-tumor aspect.
Owner:INST OF BASIC MEDICAL SCI ACAD OF MILITARY MEDICAL SCI OF PLA
A medium composition for cultivating self activated lymphocyte
InactiveCN101603028AEnhance killing activityLittle side effectsCancer antigen ingredientsBlood/immune system cellsCD16Cell culture media
Provided is a medium composition for culturing self- activated lymphocytes applicable to the treatment of malignant tumors, to which anti-CD3, anti-CD16 and anti-CD56 antibodies are added along with interleukin2 (IL-2) to evenly activate natural killer (NK) cells, T cells and natural killer T (NKT) cells, and thus the medium composition can be used to culture im- munocytes that can effectively treat various kinds of malignant tumors. The medium composition includes a cell culture medium and additives added to the cell culture medium, wherein the additives include interleukin2 (IL-2), anti-CD3 antibodies, anti-CD16 antibodies, and anti-CD56 antibodies.
Owner:NKBIO
NK cell culture solution and cell culture method
ActiveCN106222141APromote proliferationAvoid importingCulture processBlood/immune system cellsCD16Culture fluid
The invention belongs to the technical field of cells, and particularly relates to a cell culture solution and cell culture method and application thereof. The cell culture solution comprises a first culture solution and a second culture solution, the first culture solution contains cell factors such as OKT3, CD16, IL-2, IL-15 and 4-1BBL, and the second culture solution contains cell factors such as IL-2, IL-15 and 4-1BBL. The invention further provides the cell culture method. The method comprises the steps that cells are inoculated into the first culture solution for culture, and then the first culture solution is supplemented on the next day; the second culture solution is supplemented when culture is conducted on the ninth day for continuous culture, and the second culture solution is supplemented on the next day. According to the cell culture solution and cell culture method and application thereof, the cell factor combination and culture technological process is optimized, the culture period is shortened, only 14-21 days are needed for culture, and the purity of NK cells obtained through culture reaches 70% or above; the NK cells do not need to be purified, the steps are simple and convenient, and the operability is high.
Owner:湖南丰晖生物科技有限公司
Compositions and methods for enhancing the killing of target cells by nk cells
PendingCN112384534AImmunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsCancer cellCD16
Owner:COMPASS THERAPEUTICS LLC
Medium composition for culturing self-activated lymphocytes and method for culturing self-activated lymphocytes using same
InactiveUS20130157364A1Raise the ratioLess side effectsCancer antigen ingredientsImmunoglobulinsNatural Killer Cell Inhibitory ReceptorsCD16
Disclosed is a medium composition for culturing self-activated lymphocytes, which contains anti-CD3 antibody and anti-CD16 antibody in addition to interleukin 2 (IL-2), interleukin 12 (IL-12) and interleukin 18 (IL-18) in a medium, and thus can efficiently proliferate and activate NK cells, T cells and NKT cells and, at the same time, can significantly increase the ratio of NK cells in lymphocytes so as to provide immunocytes having excellent effects on the treatment of various kinds of malignant tumors, and a method for culturing self-activated lymphocytes using the medium composition.
Owner:CELLTECH LTD
Method for preparing efficient clinical-level CD 56<+> group immune cell
InactiveCN106554942AIncrease the number ofComply with GMP requirementsMammal material medical ingredientsBlood/immune system cellsRabbit anti-human thymocyte immunoglobulinCD16
The invention provides a method for preparing efficient clinical-level CD 56<+> group immune cell. The method comprises the following steps: a) separating single karyocyte from peripheral blood or umbilical cord blood; b) inoculating the single karyocyte in the step a) to an anti-CD16 antibody-coated culture dish; c) adding rabbit-anti-human thymic cell immune globulin, Lifein and animal origin-free cytokine in the culture dish for inducing and stimulating the single karyocyte inoculated in the step b); d) supplementing the culture dish and the animal origin-free cytokine according to the cells counting results every 2-3 days; and e) obtaining the CD 56<+> group immune cell. The method can reduce the cost, accords with the requirement of clinical-level preparation, and has good killing activity in vitro and vivo.
Owner:JILIN TUO HUA BIOTECH
Method for producing nk cell-enriched blood preparation
InactiveUS20130295671A1Increase probabilityAdvantageously low invasiveAntimycoticsMammal material medical ingredientsBiological bodyCD16
It is intended to provide a method for producing an NK cell-enriched blood preparation, which is low invasive and is capable of conveniently and rapidly growing NK cells, etc. in blood collected from an organism. The NK cells in blood are stimulated with NK cell growth-stimulating factors comprising an anti-CD16 antibody, OK432, an anti-CD3 antibody, and a cytokine. Then, the blood is cultured at a physiological cell temperature to produce an NK cell-enriched blood preparation.
Owner:BIOTHERAPY INST OF JAPAN
Medium composition for culturing self-activated lymphocytes and method for culturing self-activated lymphocytes using same
InactiveCN103080302ARaise the ratioLittle side effectsImmunoglobulins against cell receptors/antigens/surface-determinantsCancer antigen ingredientsCD16Interleukin II
Disclosed is a medium composition for culturing self-activated lymphocytes, which contains anti-CD3 antibody and anti-CD16 antibody in addition to interleukin 2 (IL-2), interleukin 12 (IL-12) and interleukin 18 (IL-18) in a medium, and thus can efficiently proliferate and activate NK cells, T cells and NKT cells and, at the same time, can significantly increase the ratio of NK cells in lymphocytes so as to provide immunocytes having excellent effects on the treatment of various kinds of malignant tumors, and a method for culturing self-activated lymphocytes using the medium composition.
Owner:CELLS SCI CORP
Detection method of human peripheral blood lymphocytes
InactiveCN110487706AOptimizing the right titerCombine accuratelyIndividual particle analysisFluorescenceCD16
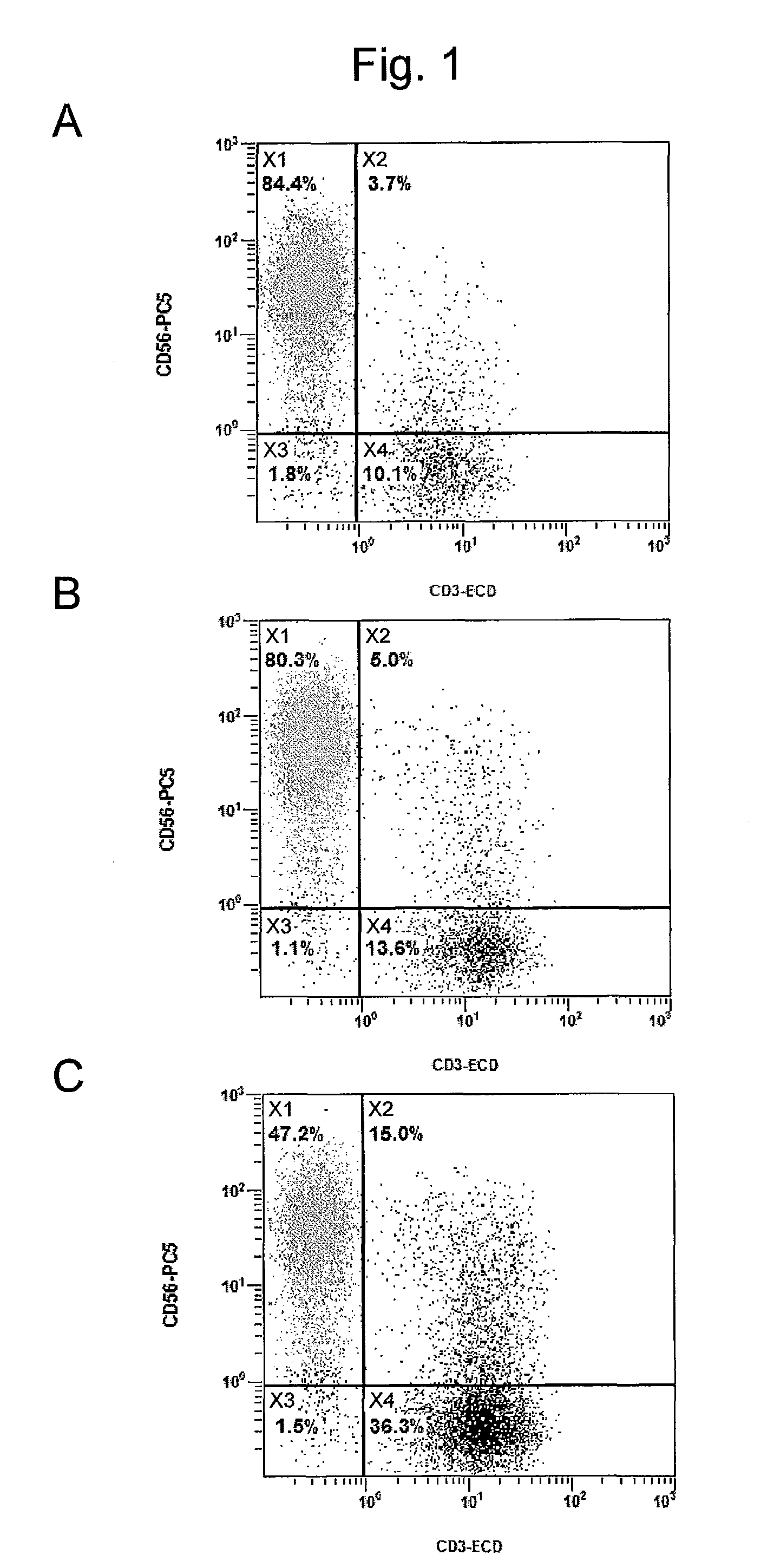
The invention discloses a detection method of human peripheral blood lymphocytes. The preparation method comprises the following steps of: taking human anticoagulation peripheral blood; adding anti-human CD3, CD4, CD8 and CD45 monoclonal antibodies and anti-human CD3, CD56, CD16 and CD45 monoclonal antibodies, then adding a hemolysin working solution containing a fluorescent probe to respectivelyobtain the percentage and absolute count value of a human peripheral blood T lymphocyte subset and an NK lymphocyte subset and achieve the detection of human peripheral blood lymphocytes. The method has the advantages of simplicity and convenience in operation, long sample stable preservation time, high accuracy, multi-index output and the like.
Owner:UB BIOTECHNOLOGY ZHEJIANG CO LTD
Efficient in-vitro amplification method of human natural killer cells
ActiveCN105462923AReduce manufacturing costSimple and fast operationBlood/immune system cellsIrritationCD16
The invention discloses an efficient in-vitro amplification method of human natural killer (NK) cells. The efficient in-vitro amplification method includes the steps that CD16 monoclonal antibodies, ATG and IL-2 are added into a processed culturing system, incubation is carried out in an incubator at the temperature of 37 DEG C, counting and solution changing are carried out, and fresh IL-2 is added; a culture solution is changed at regular intervals, culturing continues for a proper time, and the human NK cells are obtained. The efficient in-vitro amplification method has the advantages that the NK cells can be efficiently amplified through the ATG and the CD16 antibodies in a short time without being subjected to separation of magnetic beads and irritation of tumor modification cells, production cost is greatly saved, and operation is easy and convenient; the number of amplification times of the NK cells is one thousand or more, and the final proportion ranges from 80% to 93%; production efficiency is high, and the efficient in-vitro amplification method can be entirely applied to large-scale clinical researching and the like; the growth process of the NK cells is completed under the GMP condition, sterile operation is guaranteed, various kinds of security detection are carried out at the final production stage, and the efficient in-vitro amplification method can be applied to clinical researching.
Owner:THE SECOND HOSPITAL OF SHANDONG UNIV
Preparation method and application of autologous natural killer cell proliferation
The invention provides a preparation method of autologous natural killer cell proliferation and culture. The preparation method of the autologous natural killer cell proliferation and culture is characterized in that mononuclear cells of a cancer patient are amplified and activated to obtain natural killer cells after the mononuclear cells are transfected by 3- phosphoinositide-dependent protein kinase-1 and CD122 and under the effect of K562 cells which are expressed stably serve as trophoblast cells and interleukin 2, proliferation times of the natural killer cells are above 2500, phenotype of CD16+ / CD56+ is above 90%, and in-vivo and in-vitro tests show that the natural killer cells have an anti-tumor and have quite high killer toxicity. The invention also provides a kit which is used for autologous natural killer cell proliferation and culture and is obtained by using the preparation method. The kit has an efficient anti-tumor function.
Owner:ZICHENG RUISHENGHUI BEIJING BIOTECH DEV CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com