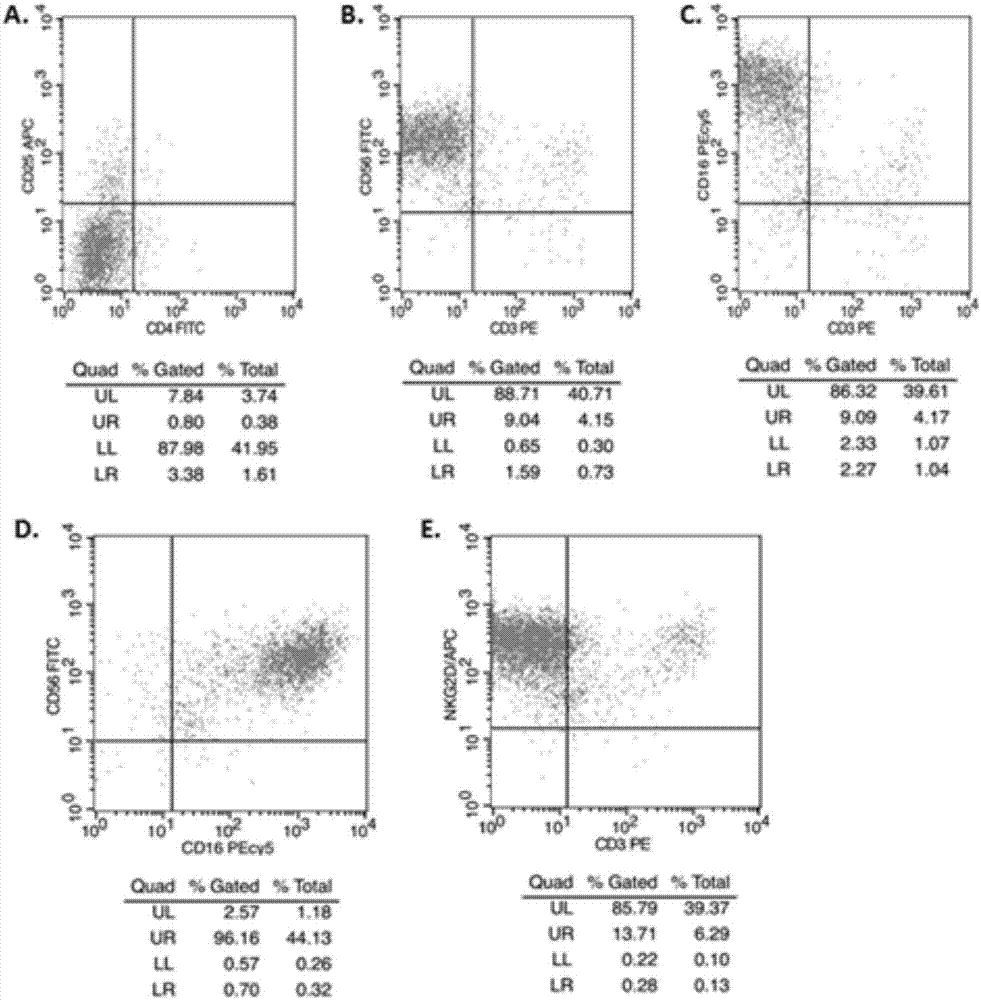
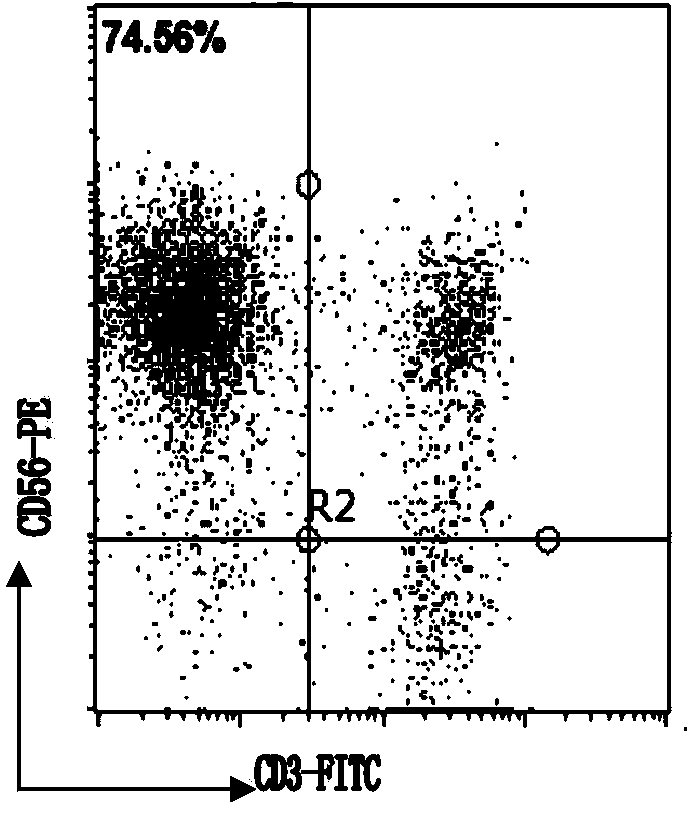
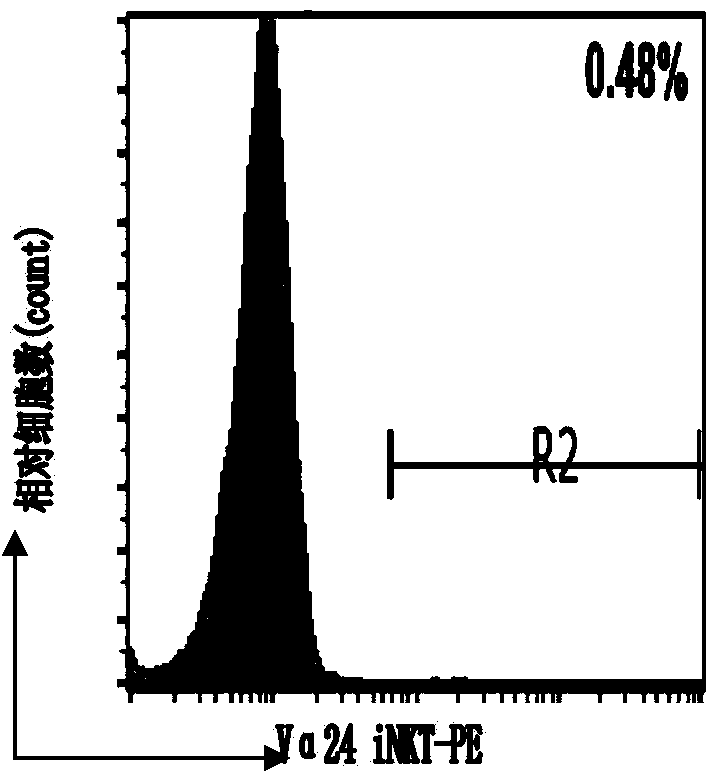
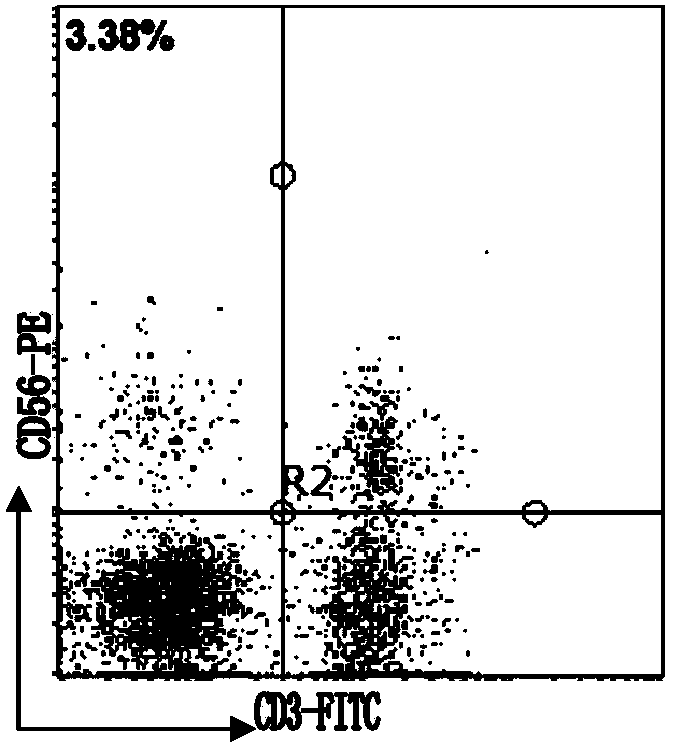
Patents
Literature
110 results about "Autologous plasma" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
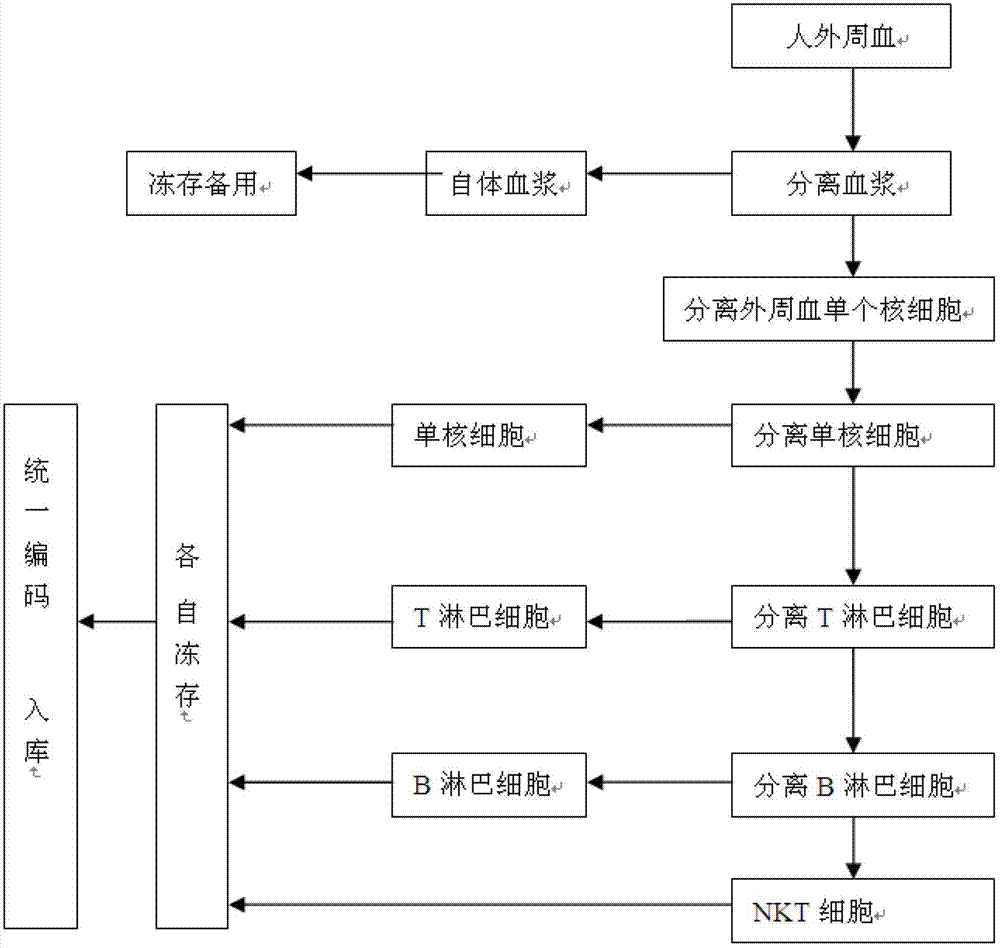
Method for constructing human peripheral blood immune cell bank
ActiveCN102758259AHigh activityHigh purityMicroorganism librariesBlood/immune system cellsPeripheral blood mononuclear cellNatural Killer Cell Inhibitory Receptors
The invention discloses a method for constructing a human peripheral blood immune cell bank. The method comprises the following steps of: collecting human peripheral blood, separating autologous plasma, separating a peripheral blood mononuclear cell, separating a mononuclear cell by the peripheral blood mononuclear cell and freezing, separating a T lymphocyte by the peripheral blood mononuclear cell and freezing, separating a B lymphocyte by the peripheral blood mononuclear cell and freezing, separating an NK cell by the peripheral blood mononuclear cell and freezing, and encoding and puttingin storage. According to the invention, immune cells of health or young people are separated and are respectively independently frozen, and relative numbers are stored and put into storage, so that the stored human immune cells have the characteristics of high activity, high purity and convenience for use, and fetal calf serum can be replaced by the human autologous plasma, so that introduction of a foreign protein can be avoided. Meanwhile, the method, provided by the invention, has the advantages of low cost, low requirements on laboratory conditions, and wide application.
Owner:济南赛尔生物科技股份有限公司
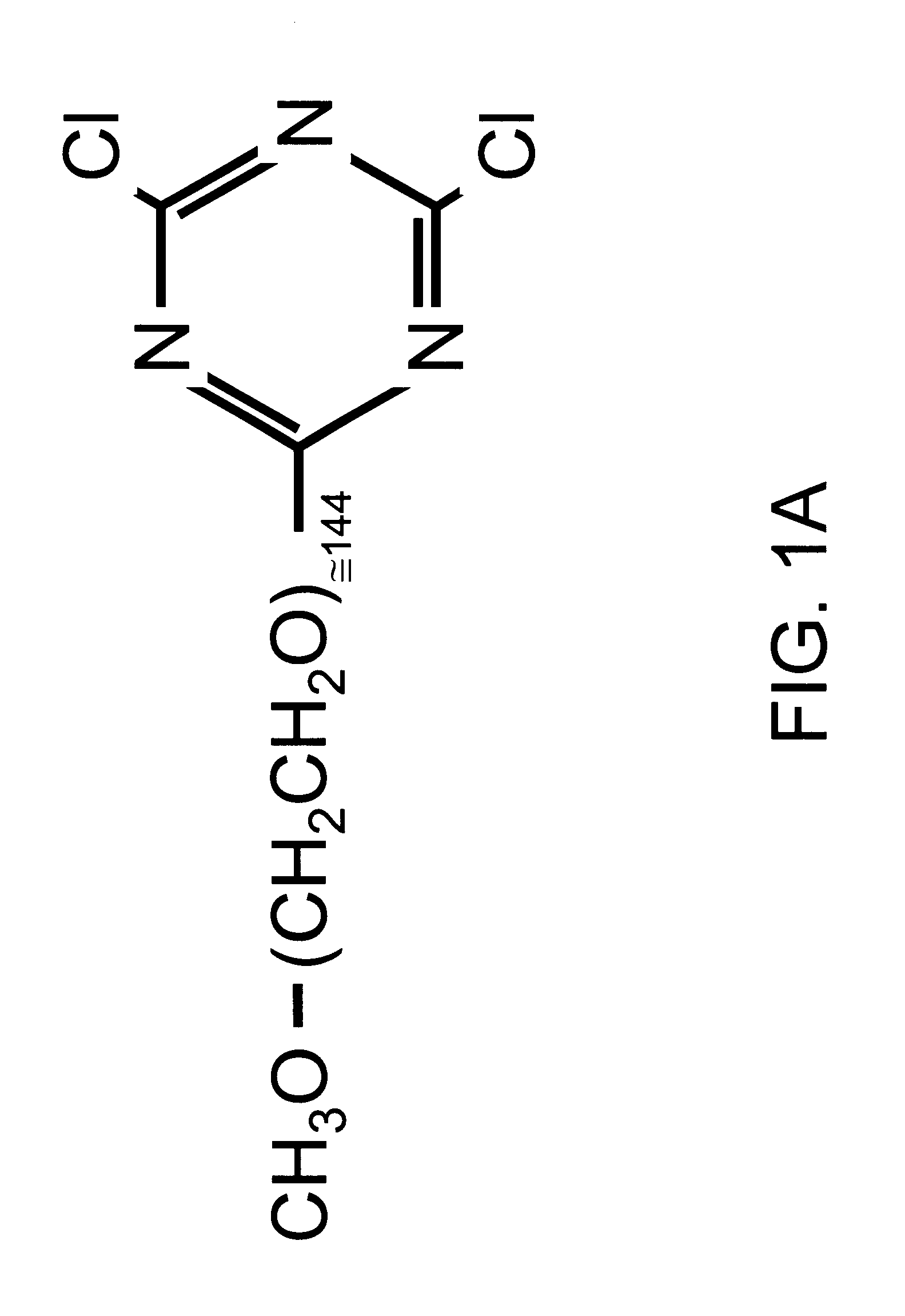
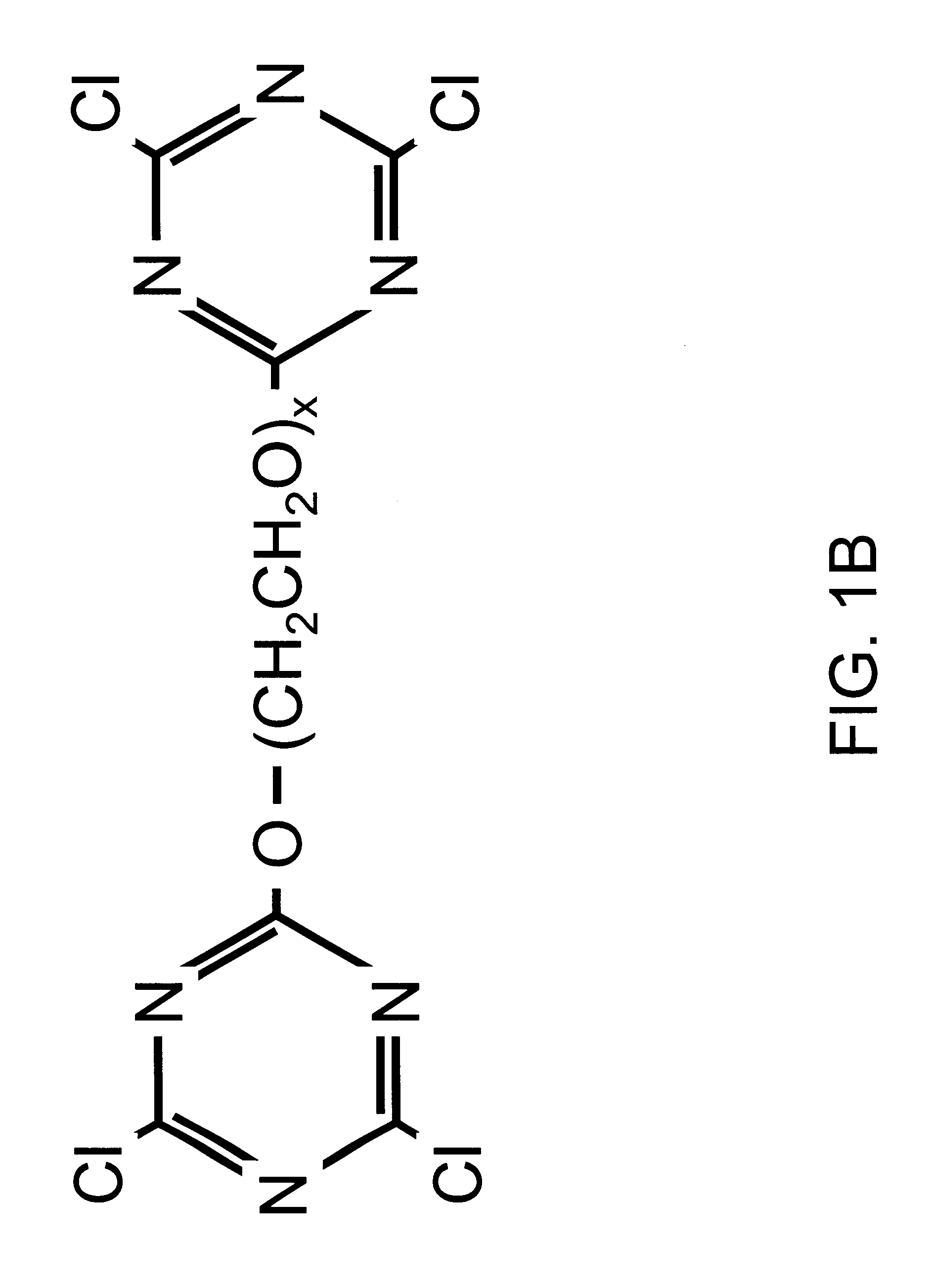
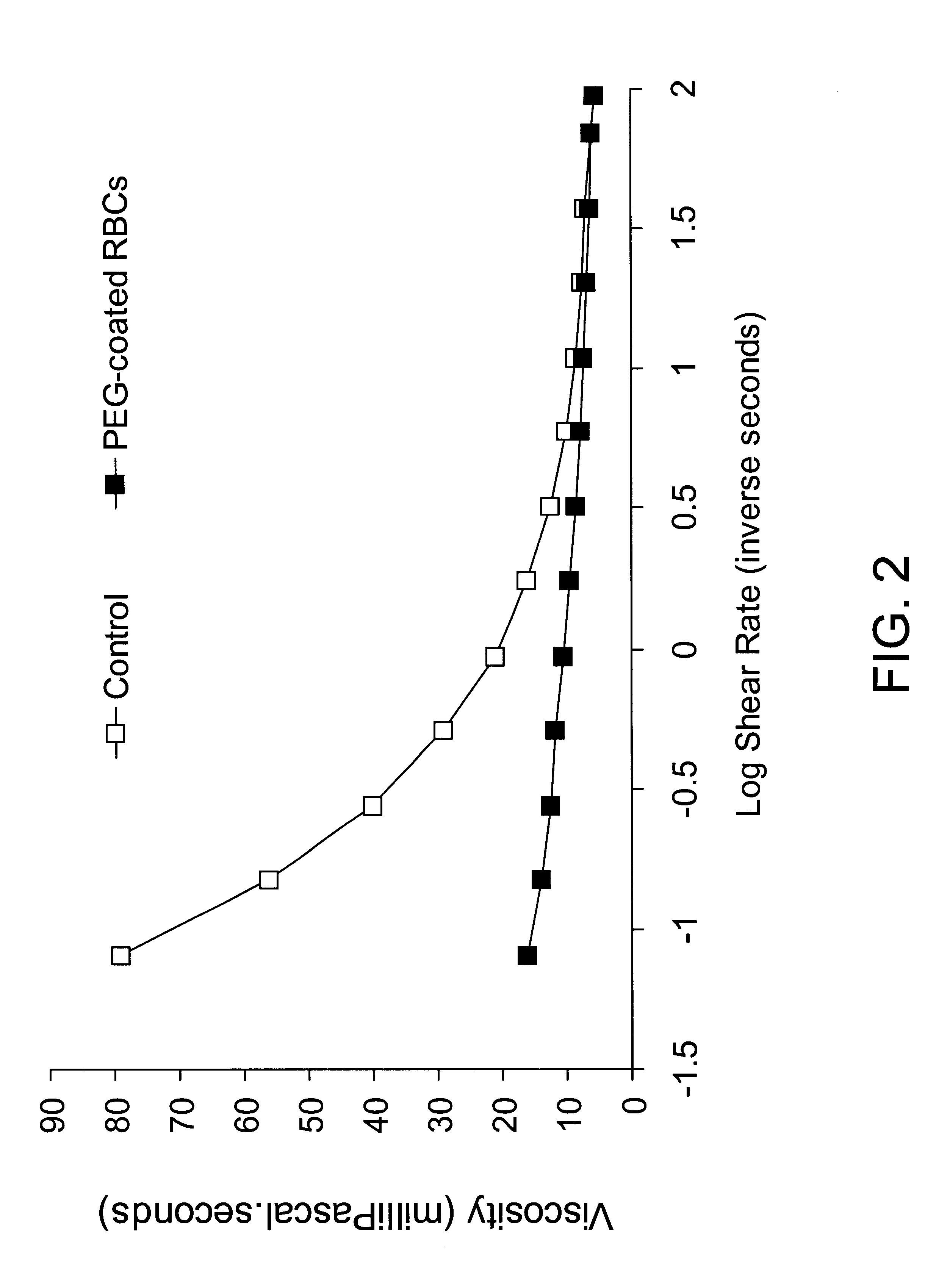
Red blood cells covalently bound with two different polyethylene glycol derivatives
InactiveUS6312685B1Reduce decreaseSignificant comprehensive benefitsBiocideDead animal preservationDiseaseBlood plasma
Living cells are modified at their surface with specially selected polymers. Covalently attaching specially selected polyethylene glycol (PEG) derivatives to the surface of red blood cells (RBC) in aqueous media under mild conditions is a preferred example. The selected PEG derivatives dramatically reduced aggregation and low shear viscosity of RBC resuspended in autologous plasma, and inhibited RBC agglutination by blood group-specific antibodies. The morphology and deformability of the PEG-treated cells were unaltered. PEG coating of the RBC surface is applicable to the treatment of a variety of diseases characterized by vaso-occlusion or impaired blood flow, e.g., myocardial infarction, shock, and sickle cell disease. An infusion solution is prepared containing red blood cells covalently bound to a PEG derivative having a molecular weight of between 2,000 and 5,000 Daltons and a PEG derivative having a molecular weight between 10,000 and 35,000 Daltons.
Owner:FISHER TIMOTHY C +1
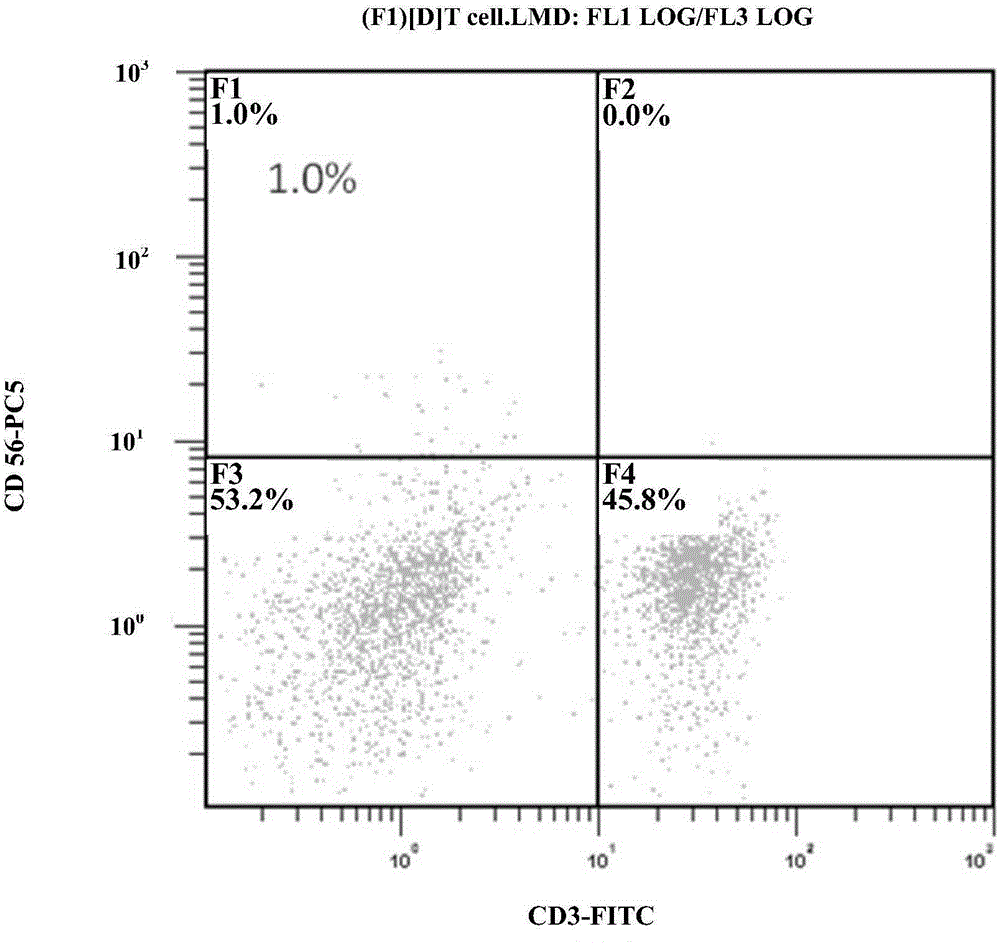
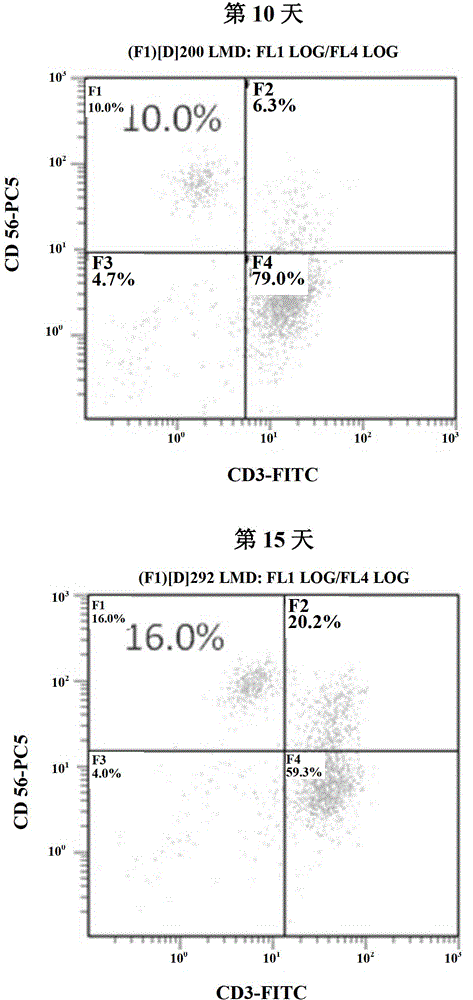
Method for simultaneous and efficient amplification of CD<3+>CD<56+>CIK cells and CD<3->CD<56+>NK cells
ActiveCN104357390AHigh purityHigh activityBlood/immune system cellsAdoptive cellular immunotherapySerum free media
The invention discloses a method for simultaneous and efficient amplification of CD<3+>CD<56+>CIK cells and CD<3->CD<56+>NK cells. The method comprises the steps as follows: the concentration of separated PBMC (peripheral blood mononuclear cells) is adjusted by a serum-free medium containing autologous plasma, an Anti-CD16 antibody, IL-2 and IL-15 are added, and then the mixture is transferred into a T175 culture flask for culture; an Anti-CD3 antibody and an Anti-CD137 antibody are added; a serum-free medium containing the autologous plasma, IL-2 and IL-15 is supplemented every two days according to the cell growth condition; the cell concentration is controlled to be about 1.5*10<6> / ml; and after culture is performed for 14-21 days, large quantities of high-purity CD<3+>CD<56+>CIK cells and CD<3->CD<56+>NK cells can be obtained simultaneously, and the total cell quantity can reach an effective value of the cell quantity required for adoptive cellular immunotherapy clinically for tumor. The method for simultaneous and efficient amplification of the CD<3+>CD<56+>CIK cells and the CD<3->CD<56+>NK cells is simple, convenient, effective and high in cell killing activity.
Owner:HRYZ (SHENZHEN) BIOTECH CO +1
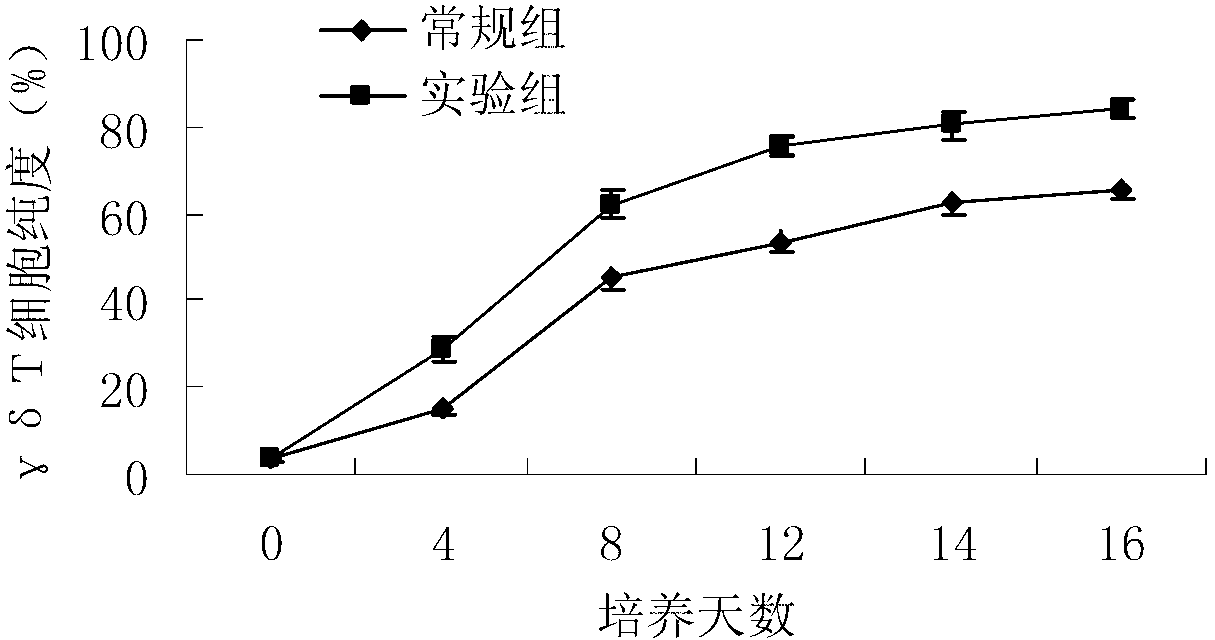
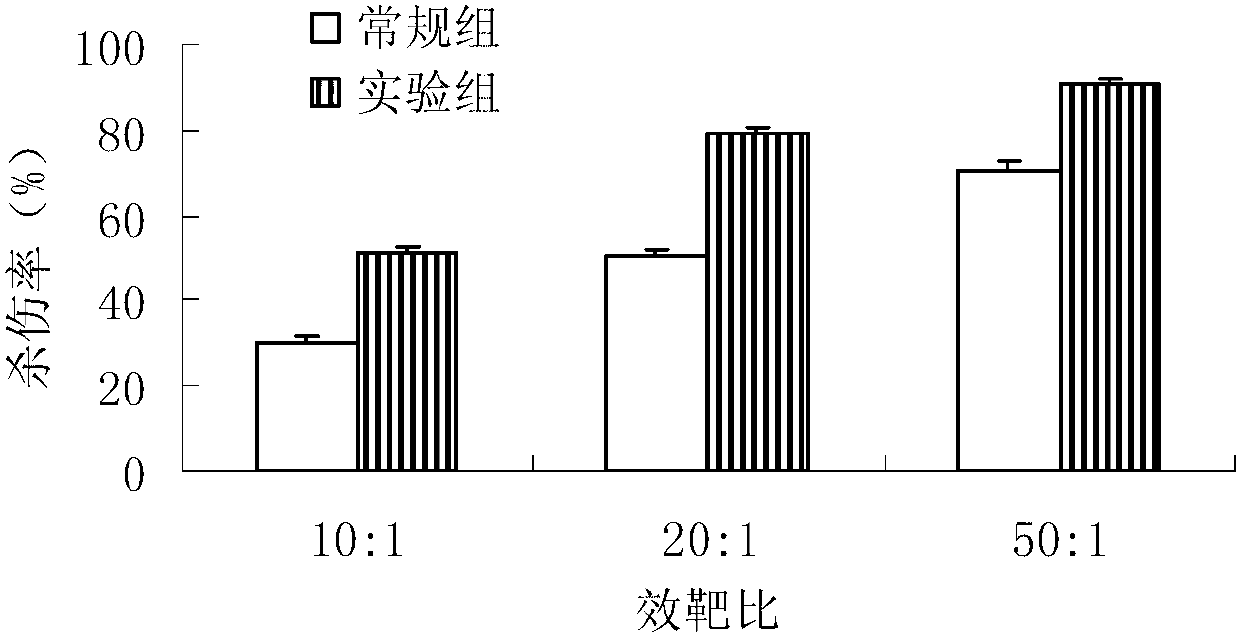
Method for in-vitro amplification of gamma-delta-T cells
InactiveCN102994448AFully stimulatedInhibit apoptosisBlood/immune system cellsPeripheral blood mononuclear cellMicrobiology
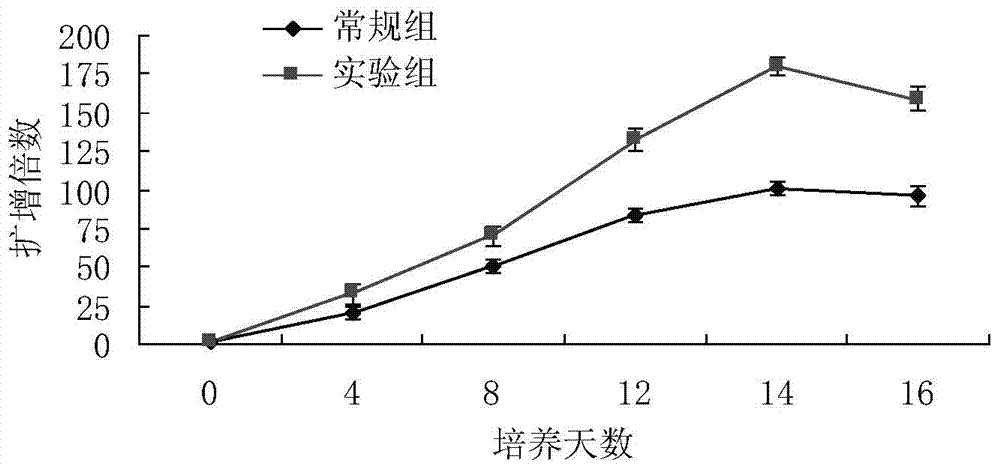
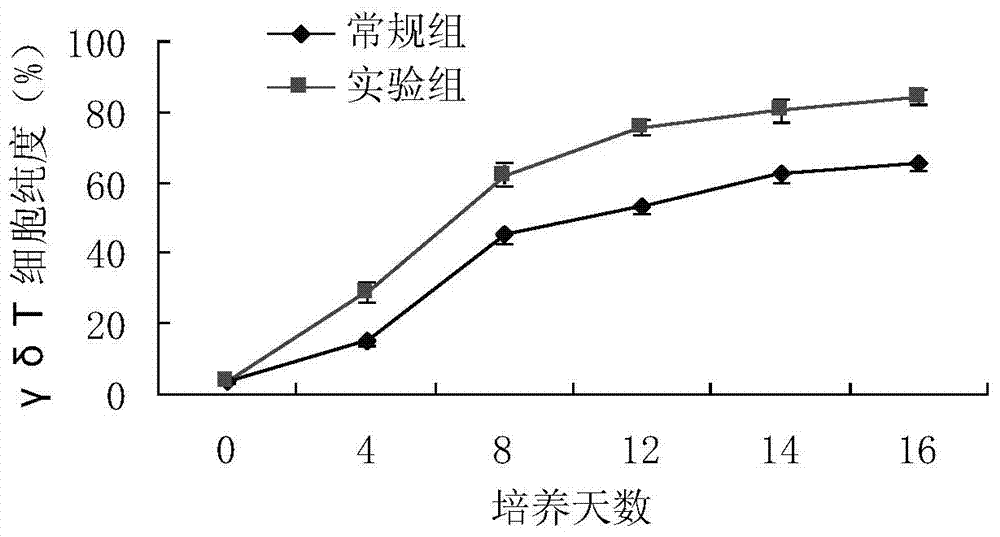
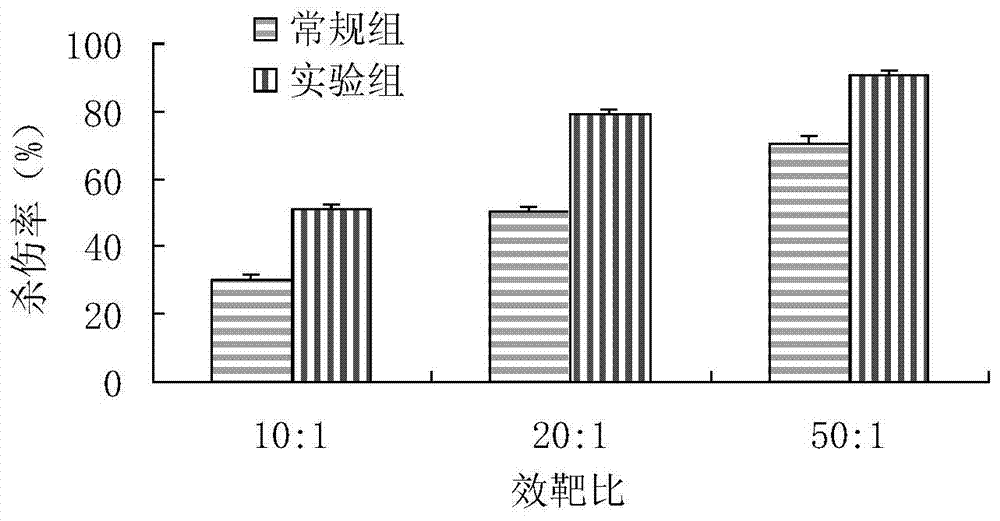
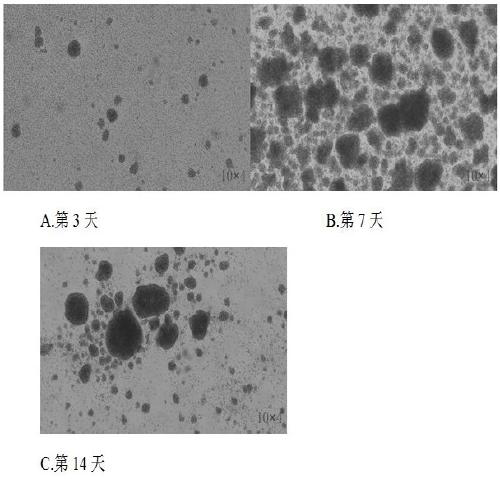
The invention relates to a method for culturing gamma-delta-T cells, and in particular relates to a method for in-vitro amplification of gamma-delta-T cells, wherein the method comprises the following operating steps of: pre-coating a T75 culture bottle by a TCR-gamma-delta resisting antibody and CD28McAb for later use use; isolating the peripheral blood mononuclear cell (PBMC) of a patient; regulating the PBMC concentration to 1*10<6> 6 / ml by a serum-free culture medium which contains 5% of autologous plasma, and transferring PBMC cell suspension into the T75 culture bottle; adding an initial culture medium which contains proper concentrations of Zoledronat, HSP70, 1L-2, 1L-7 and 1L-15; culturing in a saturated humid environment containing 5% of CO2 at 37 DEG C; depending on growth situation of the cell, changing the culture medium every 2-3days, to control the cell concentration at about 2.5*10<6> / ml; meanwhile, compensating full doses of Zoledronat, HSP70, 1L-2, 1L-7 and 1L-15; and continuously culturing for 12-16days, to obtain a great amount of gamma-delta-T cells which are comparatively high in purity.
Owner:SHANGHAI CLAISON BIOTECH
A hemopoietic stem cell cryopreserving solution, and a hemopoietic stem cell cryopreserving method
InactiveCN107094753AImprove protectionDrop in recovery rateDead animal preservationHydroxyethyl starchCell culture media
The invention relates to the technical field of cytobiology, and particularly relates to a hemopoietic stem cell cryopreserving solution, and a hemopoietic stem cell cryopreserving method. The cryopreserving solution includes following raw materials by weight: 10-20 mL of dimethyl sulfoxide, 6-10 g of hydroxyethyl starch, 4-8 mL of a cell culture medium, 6-10 mL of human serum albumin, and 60-100 mL of human autologous plasma. The formula of the cryopreserving solution is simple, and the raw materials are cheap. The cryopreserving solution can effectively protect hemopoietic stem cells from freezing injuries, is high in safety and low in cell injuries, can increase the resuscitation survival rate of hemopoietic stem cells. The survival rate after resuscitation can be 96% or above, and after resuscitation, the cell viability is high, the quantity of cell proliferation is high, the cells are not liable to age, physiological functions and biological characteristics of the hemopoietic stem cells after resuscitation can be ensured, and microbe detection indexes meet requirements. The survival time of the hemopoietic stem cells is prolonged.
Owner:DONGGUAN BOALAI BIOLOGICAL TECH CO LTD
Culturing method of NK (natural killer) cell
InactiveCN104928242AGuaranteed amplification factorGuaranteed cytotoxicityBlood/immune system cellsHuman bodyLymphocyte culture
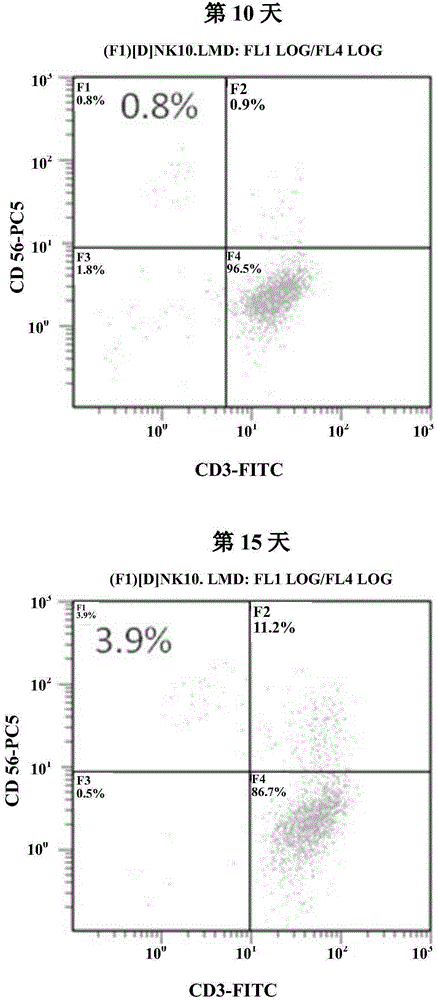
The invention discloses a culturing method of an NK (natural killer) cell. The culturing method comprises the following steps of (1) separating a mononuclear cell from peripheral blood or umbilical cord blood of a human body; (2) inoculating the mononuclear cell into a culture medium suitable for culturing lymphocyte, adding a CD3 monoclonal antibody, recombinant human interleukin 2 and autologous plasma, and culturing for 3 to 5 days; (3) adding the recombinant human interleukin 2 and the autologous plasma, and culturing; (4) harvesting the NK cell. According to the culturing method of the NK cell, the culturing cost is reduced, the amplification multiple and cell toxicity of the NK cell are guaranteed, the operation time is saved, meanwhile the probability of error operation is reduced, the obtaining efficiency of the NK cell is higher, and the safety of the NK cell is better.
Owner:WUHAN HAMILTON BIOTECH
CIK (cytokine-induced killer) frozen stock solution and frozen preservation method
ActiveCN104938477AImprove survival rateSimplified cryopreservation methodDead animal preservationHigh cellCvd risk
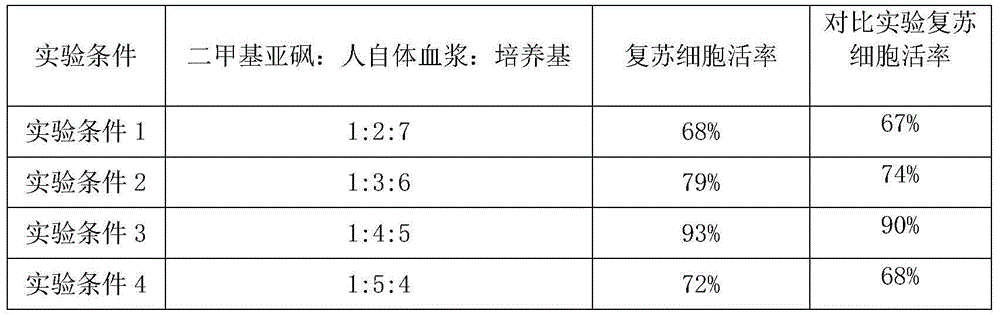
The invention provides a frozen stock solution for CIK (cytokine-induced killer) cells and a frozen preservation method. According to the frozen stock solution, serum is replaced with autologous plasma of a user, so that the risk of an infectious disease in exogenous serum is avoided. The adopted reagent is suitable for a simple programmed cooling method of a programmed cooling box. The reagent provided by the invention contains dimethyl sulfoxide, autologous plasma and a culture medium at the ratio of 1 to 4 to 5. The frozen stock solution has the characteristics of simple method and high cell resuscitation activity, and is suitable for storage of immune cells by patients at an earlier stage of chemoradiotherapy or healthy people in a manner of the CIK cells for later CIK cell therapy.
Owner:ADLAI NORTYE BIOPHARMA CO LTD
Storage method of immune cells and cell freezing medium
InactiveCN105685017AGuaranteed sterilityAvoid safety hazardsDead animal preservationPeripheral blood mononuclear cellBlood plasma
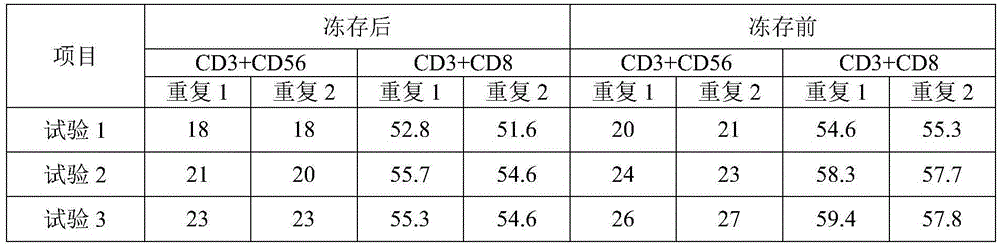
The invention relates to the technical field of cell preservation, in particular to a storage method of immune cells and a cell freezing medium. The method includes: separating and collecting peripheral blood mononuclear cells, and adding the cell freezing medium to store the cells in a frozen manner, wherein the cell freezing medium comprises, by weight percentage, 0-62% of serum-free liquid culture medium, 0.5-1.5% of penicillin-streptomycin solution, 5-10% of dimethyl sulfoxide and 30-92% of autologous plasma. The storage method of the immune cells has the advantages that the method is simple and effective, the infectious disease risks of exogenous serum are avoided, the survival rate of the revived freezing-stored cells after induction culture is above 90%, the various biological indexes of the cells satisfy the requirements, and a good storage effect is achieved; the method can monitor and trace cell bank samples in real time, accurately record information such as cell storage positions, temperature and cell sources, each cell has a unique identifier, and the method is convenient to use and safe and reliable.
Owner:DONGGUAN BOALAI BIOLOGICAL TECH CO LTD
Natural killer cell culture substrate and amplification culture method for natural killer cells
ActiveCN107475196APromote activation and proliferationStrong cytotoxicityCulture processBlood/immune system cellsPeripheral blood mononuclear cellCell culture media
The invention provides a natural killer cell culture substrate and an amplification culture method for natural killer cells, and relates to the technical field of cell culture. The natural killer cell culture substrate is mainly composed of a serum-free culture medium, autologous plasma and cytokines; according to the amplification culture method for the natural killer cells with the natural killer cell culture substrate, the IL-2, IL-12, IL-15, IL-21 cytokines in the cell culture substrate are combined through Lymactin-NK antibodies to induce mononuclear cells of human peripheral blood to release dangerous signals, the natural killer cells are activated, and the killing activity of the cells is enhanced. According to the method, a large quantity of high-quality natural killer cells can be obtained in a short period through efficient amplification, and the problems are effectively solved that in existing culture methods for the natural killer cells, the production process is complex, the production cost is relatively high, and natural killer cells are low in amplification multiple, not high in purity and difficult to produce in a large-scale mode.
Owner:TIANJIN CHANGHE BIOLOGICAL TECH
Method for simultaneously inducing and amplifying V alpha<24+>iNKT cells and CD<3->CD<56+>NK cells
ActiveCN104357391AHigh purityHigh activityBlood/immune system cellsAdoptive cellular immunotherapySerum free media
The invention discloses a method for simultaneously inducing and amplifying V alpha<24+>iNKT cells and CD<3->CD<56+>NK cells. The method comprises the steps as follows: PBMC (peripheral blood mononuclear cells) are separated from peripheral blood, the concentration of the PBMC is adjusted to 2*10<6> / ml by a serum-free medium containing autologous plasma; an Anti-CD<16> antibody, -GalCer, IL-2, IL-18 and IL-21 are added, and then the mixture is transferred into a culture flask for culture; an Anti-CD3 antibody is added in a cell suspension in 24 hours; a serum-free medium containing IL-2, IL-18 and IL-21 is supplemented every two days according to the cell growth condition; the cell concentration is controlled to be 1.5*10<6> / ml; and after continuous culture is performed for 14-21 days, large quantities of high-purity V alpha<24+>iNKT cells and CD<3->CD<56+>NK cells can be obtained simultaneously, and the total cell quantity can reach an effective value of the cell quantity required for adoptive cellular immunotherapy clinically for tumor. The method for simultaneous and efficient amplification of the V alpha<24+>iNKT cells and the CD<3->CD<56+>NK cells is simple, convenient and effective.
Owner:HRYZ (SHENZHEN) BIOTECH CO +1
Method used for in vitro amplification of gamma-delta-T cells
InactiveCN103756962AFully stimulatedInhibit apoptosisBlood/immune system cellsPeripheral blood mononuclear cellLevamisole
The invention relates to a method for culturing gamma-delta-T cells, and more specifically relates to a method for in-vitro amplification of gamma-delta-T cells. The method comprises the following operating steps: pre-coating a T75 culture bottle with a TCR-gamma-delta resisting antibody and CD28McAb for later use; isolating peripheral blood mononuclear cells (PBMC) of patients; regulating PBMC concentration to 1*10<6> / ml with a serum-free culture medium which contains 5% of autologous plasma, and transferring the PBMC cell suspension into the T75 culture bottle; adding an initial culture medium which contains proper concentrations of Zoledronat, HSP70, Toll-like Receptors7 (TLR7) ligand, Levamisole (LMS), IL-2, IL-7 and IL-15; culturing in a saturated humid environment containing 5% of CO2 at 37 DEG C; according to growth situation of the cell, changing the culture medium every 2 to 3 days so as to control the cell concentration at about 2.5*10<6> / ml; meanwhile, compensating full doses of Zoledronat, HSP70, Toll-like Receptors7 (TLR7) ligand, Levamisole (LMS), IL-2, IL-7 and IL-15;; and continuously culturing for 12to 16 days so as to obtain a great amount of gamma-delta-T cells which are comparatively high in purity.
Owner:SHANGHAI CLAISON BIOTECH
Method for in vitro multiplication culture of NK cells
ActiveCN109628397AStrong expansion abilityImprove securityCulture processBlood/immune system cellsSerum free mediaCD16
The invention relates to a method for in vitro multiplication culture of NK cells. The method comprises coating a culture bottle with heparin sodium, CD16 and human immunoglobulin, collecting peripheral blood, inactivating plasma, separating mononuclear cells, using a serum-free medium (containing autologous plasma, IL-2, IL-15 and IL-18) to culture multiplication cells, harvesting and detecting the cells and the like. Trophoblast cells or magnetic beads are not used for sorting and purifying, and the method has simple operation, high safety, good amplification effect and high clinical application value.
Owner:福建省海西细胞生物工程有限公司
Culture matrix and multiplication culture method for natural killer cells
ActiveCN107460168APromote activation and proliferationAvoid potential dangersCulture processCell culture supports/coatingPeripheral blood mononuclear cell3D cell culture
The invention provides a culture matrix and multiplication culture method for natural killer cells and relates to the technical field of cell culture. The culture matrix for the natural killer cells mainly contains a serum-free culture medium, autologous plasma and cell factors. According to the multiplication culture method utilizing the culture matrix for the natural killer cells, a Lymactin-NK antibody is combined with cell factors IL-2, IL-12 and IL-15 to induce a human-derived peripheral blood mononuclear cell to release a danger signal, so that the natural killer cells are activated, and the killing activity of the cells is enhanced; and a large number of high-quality natural killer cells can be efficiently multiplied in a short period, so that the problems that a production process is tedious, the production cost is relatively high, the multiplication fold of the natural killer cells is low, the purity of the natural killer cells is low, and the mass production is difficultly realized in an existing natural killer cell culture method are effectively relieved.
Owner:TIANJIN CHANGHE BIOLOGICAL TECH
Method for preparing autologous beautifying micro-needle preparation and application thereof
PendingCN106692196AEasy to prepareLow costPharmaceutical delivery mechanismMammal material medical ingredientsProgenitorWrinkle skin
The invention belongs to the technical field of cosmetic mini-plastic surgery and specifically discloses a method for preparing autologous beautifying micro-needle preparation and application thereof. The preparation of the micro-needle preparation comprises the following steps: separating autologous platelet-rich plasma and peripheral blood mononuclear cells; culturing by using an autologous activated cell factor solution and a cell culture medium; replacing a fresh autologous activated cell factor solution and a cell culture medium the next day; collecting circulating fibrocytes, endothelial progenitor cells and immune cells on the fifth day, resuspending by using the autologous platelet-rich plasma and autologous plasma, thereby obtaining the autologous beautifying micro-needle preparation. Compared with the prior art, the method disclosed by the invention has the advantages that a composition of the circulating fibrocytes, endothelial progenitor cells, immune cells and platelet-rich plasma is used for cosmetic preparations, the circulating fibrocytes are capable of reconstructing skin tissues, secreting collagens and eliminating wrinkles; the endothelial progenitor cells can achieve the effects of local skin microcirculation and improving the skin nutrition state; the immune cells can achieve the effects of swallowing and taking away metabolic products; and the combination of the endothelial progenitor cells and the immune cells can achieve the effects of repairing spotted dull skin.
Owner:SHENZHEN HORNETCORN BIOTECH
Clinical mesenchymal stem cell cryopreservation solution and preparation method thereof
InactiveCN109329271AImprove the living environmentImprove protectionDead animal preservationAmino Acid InjectionTrehalose
The invention discloses clinical mesenchymal stem cell cryopreservation solution and a preparation method thereof. The preservation solution comprises the following components in percentage by weight:1-5% of autologous plasma lysate mixed liquor, 1-2% of human serum albumin, 0-1% of VC injection, 5-10% of glucose injection, 20-30% of electrolyte-like solution, 20-30% of glucosamine injection, 20-30% of compound amino acid injection, 2-5% of dimethyl sulfoxide and 2.5-5% of trehalose. The prepared cryopreservation solution can be used for deep low-temperature and long-term storage of cells, and can realize long-distance transportation, so that the distribution range of cells is expanded, the long-distance treatment of stem cells is realized, and the cells can be directly returned after recovery so as to reduce the potential safety hazards caused by complicated operations.
Owner:CHENGDU QINGKE BIOTECH
Multi-cell immune preparation for treating tumors and preparation method of multi-cell immune preparation
InactiveCN105296425AMaintain nutrientsSignificant effectMammal material medical ingredientsBlood/immune system cellsAbnormal tissue growthPeripheral blood mononuclear cell
The invention provides a multi-cell immune preparation for treating tumors and a preparation method of multi-cell immune preparation and relates to an immune preparation and a preparation method thereof. According to the multi-cell immune preparation and the preparation method, the problems that the treatment of existing immune cell preparations to the tumors is relatively limited, and the infusion safety cannot be guaranteed are solved. The multi-cell immune preparation comprises immune cells, human serum albumin and compound electrolyte. The preparation method comprises the following steps: (1) preparation of autologous plasma; (2) separation of peripheral blood mononuclear cells PBMC; (3) preparation of DC; (4) preparation of CIK; (5) preparation of NK cells; (6) preparation of CD3AK; (7) preparation of gamma delta T; (8) preparation of the multi-cell immune preparation. The multi-cell immune preparation has broad spectrum activity to the treatment of the tumors, the immune cells are derived from the body of a patient, and exogenous substances such as fetal calf serum, trophoblast cells and the like are not introduced, so that the infusion safety is guaranteed. The multi-cell immune preparation is applied to the field of tumor treatment.
Owner:天晴干细胞股份有限公司
Multifunctional menstrual blood stem cell culture method
ActiveCN105112358AArtificially induced pluripotent cellsNon-embryonic pluripotent stem cellsQuality cultureHigh concentration
The invention relates to a multifunctional menstrual blood stem cell culture method which comprises the following steps: menstrual blood and plasma acquisition, menstrual blood bactericidal cleaning, menstrual blood filtration, autoplasma preparation, single karyocyte separation, menstrual blood stem cell culture, menstrual blood menstruation purification and amplification, and menstrual blood stem cell cryopreservation. The method overcomes the defects caused by the required use of high-concentration fetal calf serum for culture in the prior art; and by using the own plasma of the customer to culture the cells, the customer can feel safe and acceptable, thereby implementing the safe, stable and quick high-quality culture system for amplifying the mesenchymal stem cells in deed.
Owner:深圳新赛尔生物科技有限公司
Mesenchymal stem cell preserving fluid for clinic local injection and method for preserving mesenchymal stem cell
ActiveCN109511648AImprove the living environmentMild living environmentDead animal preservationCell activityAntioxidant
The invention discloses mesenchymal stem cell preserving fluid for clinic local injection and a method for preserving mesenchymal stem cells. The preserving fluid contains the following components inpercentage by volume: 1%-5% of autologous plasma lysate, 1%-5% of an autologous stem cell conditional medium extract, 0.1%-1% of an antioxidant, 0.1%-0.4% of hyaluronic acid and the balance of a normal saline injection. By preserving the mesenchymal stem cells through the preserving fluid, the cell viability is high, the maintenance time of cell activity is long, and the preserving fluid foes notexogenous component and is high in safety.
Owner:CHENGDU QINGKE BIOTECH
CAR-NK cell, and preparation method and application thereof
ActiveCN108300698AStrong ability to secrete factorsImprove anti-tumor effectMammal material medical ingredientsNucleic acid vectorPeripheral blood mononuclear cellBiological activation
The invention relates to a CAR-NK cell, and a preparation method and an application thereof. The preparation method comprises the following steps: S1, synthesizing a CAR sequence; S2, integrating theCAR sequence into a lentiviral target vector plasmid to obtain a target plasmid; S3, mixing the target plasmid with a 293T cell, performing culturing, collecting the obtained virus liquid, and concentrating the virus liquid to obtain the virus concentrate of the target plasmid; S4, collecting autologous plasma and peripheral blood mononuclear cells (PBMC), adjusting the cell density with an activation culture medium, performing cell culturing, transferring obtained cells into a new cell culturing bag, continuing culturing, adding a proliferation culturing medium, and obtaining the NK cell after the culturing is finished; and S5, adding the virus concentrate of the target plasmid to the NK cell, and performing incubation to obtain the CAR-NK cell. The CAR-NK cell has very strong factor secretion ability and tumor inhibition ability, and the transfection method for preparing the CAR-NK cell is efficient and stable.
Owner:深圳市沃英达生命科学有限公司
Biological beautifying preparation containing autologous stroma cells
ActiveCN104173252AImprove wrinklesAcne scar repairCosmetic preparationsToilet preparationsBlood plasmaAcne scarring
The invention provides a method for preparing a biological beautifying preparation containing autologous stroma cells. The method comprises the following steps: acquiring autologous plasma from autologous peripheral blood and culturing to acquire autologous stroma cells; and adding the autologous stroma cells into the autologous plasma to prepare the biological beautifying preparation. The invention simultaneously provides the biological beautifying preparation prepared from the autologous stroma cells and the autologous plasma, which is added with growth factors and anti-oxidation factors, and is capable of removing skin wrinkles, repairing acne scarring, repairing and improving burns, trauma and hypertrophic scars, resisting senescence and the like.
Owner:蔡贤芬
Umbilical cord blood stem cell injection solution with anti-aging effect and preparation method thereof
InactiveCN105816481ANo negative impactQuality controlledAntinoxious agentsPharmaceutical delivery mechanismHuman bodyTissue repair
The invention discloses an umbilical cord blood stem cell injection solution with an anti-aging effect and a preparation method thereof. The umbilical cord blood stem cell injection solution contains the following components by volume or concentration: 5X10<5> to 10X10<5> umbilical cord blood stem cells per ml, 2wt% of human albumin, 10-20v% of umbilical cord blood autologous plasma and 78-88v% of a compound electrolyte solution. According to the umbilical cord blood stem cell injection solution, the used stem cells are blood related stem cells and have the characteristics of amplification and directional differentiation; the cells return to an injured part when receiving an injured part signal; the cells are subjected to amplification and induced differentiation at the injured part and the stem cells of the injured part are promoted to recover functions, thereby quickly repairing and replacing injured cells and tissues; and cell factors, growth factors and tissue related factors are secreted quickly in time, thereby improving blood circulation of a human body, metabolic function, tissue repair capability and immunity, and further achieving the purpose of aging resistance.
Owner:SHANGHAI HUAYAN MEDICINE TECH CO LTD
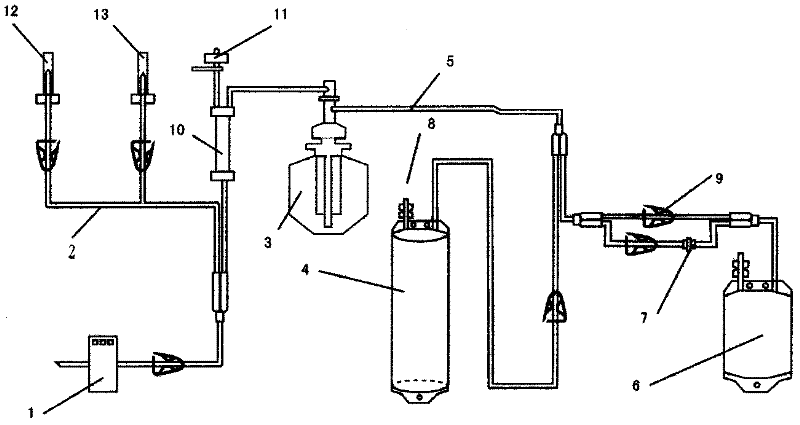
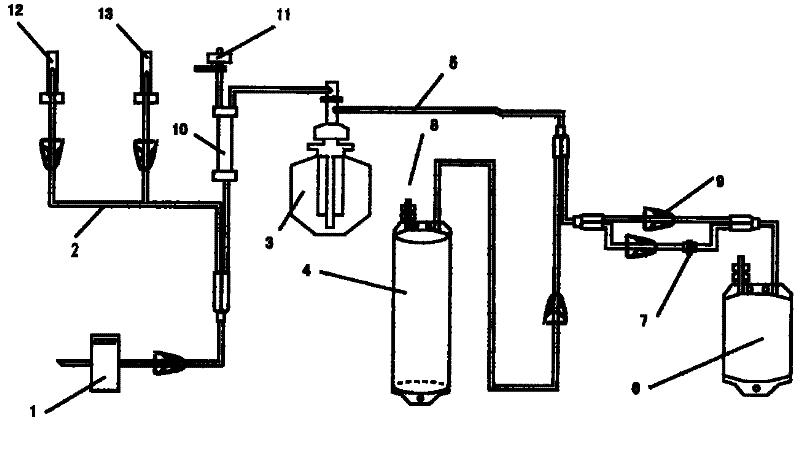
Non-drug treatment system for inactivation of HIV (human immunodeficiency virus) and other viruses
ActiveCN102389595AReduce loadAchieve antiviralOther blood circulation devicesLipid formationHuman immunodeficiency
The invention relates to a treatment system for carrying out autologous plasma virus inactivation for non-drug antivirus treatment on patients infected by lipid-enveloped viruses including HIV (human immunodeficiency virus) and the like caused by drug-resistant strains and variant strains. The treatment system comprises a plasma collector, a disposable separation and virus inactivation multi-purpose processor for collected plasma, an MB virus inactivator and an HHP virus inactivator, wherein the disposable separation and virus inactivation multi-purpose processor for collected plasma comprises a blood collector, a pipeline, a plasma separation cup, a lengthened plasma pipeline, a photochemical processing bag for pancake plasma collection and inactivation, a processing bag for tubular plasma collection and extra-high-pressure virus inactivation, an MB self-medicating pot, a puncture cap, a retaining clip, a blood filter, a pressure monitor, a saline water reinfusion needle and protection cap thereof, and an anticoagulant and protection cap thereof. The invention has the advantages of wide application range, good virus inactivation effect, wide spectrum of inactivated viruses, convenient operation process, high safety and efficiency and low cost, thereby being suitable to be popularized.
Owner:ZHENGZHOU FEILONG MEDICAL EQUIP CO LTD
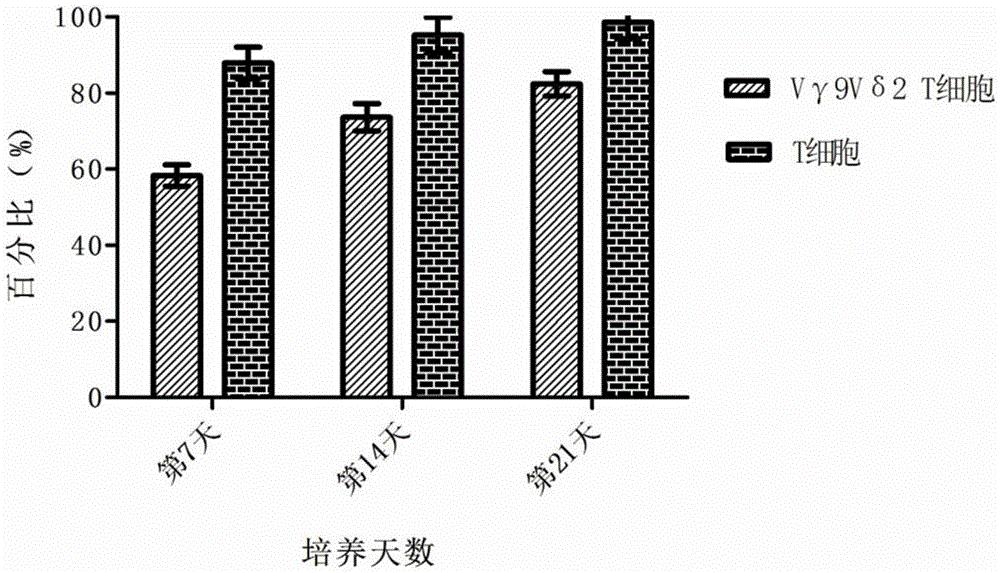
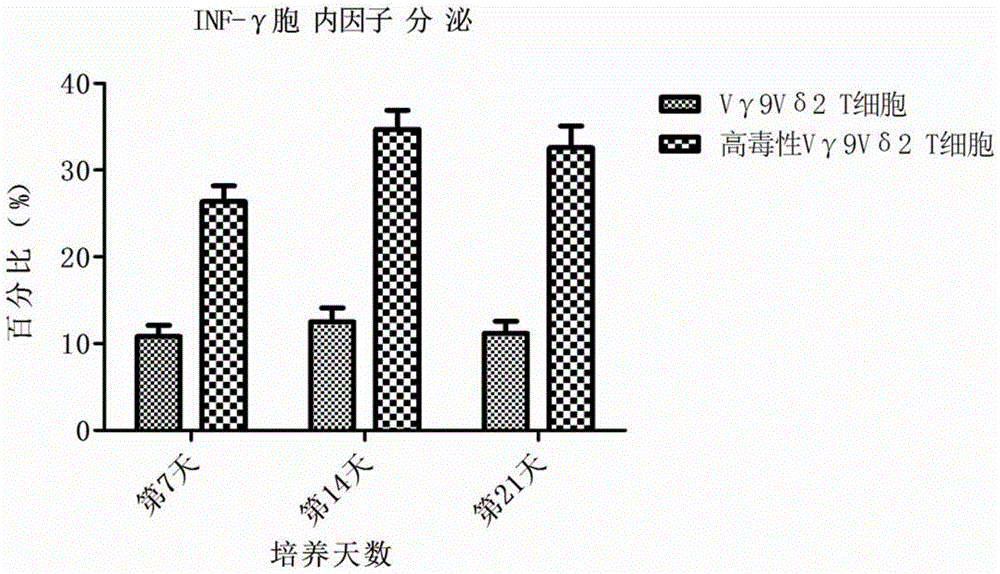
Preparing method for high-toxicity human Vgamma9Vdelta2 T cells induced by PD-1 antibody
InactiveCN105462925AImprove secretion capacityEasy to prepareBlood/immune system cellsCell culture active agentsAntigenPeripheral blood mononuclear cell
The invention discloses a preparing method for high-toxicity human Vgamma9Vdelta2 T cells induced by a PD-1 antibody. The preparing method comprises the following steps that firstly, human peripheral blood mononuclear cells are obtained through saccharosan-glucosamine diatrizoate density gradient centrifugation; secondly, separated PBMCs are put into an RPMI1640 culture medium containing an irritant mycobacterium tuberculosis heat-resistant antigen, cell factors rhIL-2 and autologous plasma accounting for 5-10%; rhIL-12 and the PD-1 antibody are added at the same time to culture PBMCs; thirdly, the fresh RPMI1649 culture medium is supplemented every other 2-3 days according to the growing condition of the cells; fourthly, the cells are collected on the 10th day to the 16th day according to the growing condition of the cells, and the high-toxicity human Vgamma9Vdelta2 T cells are obtained. The preparing method for the Vgamma9Vdelta2 T cells which are fast in cell proliferation, high in cell purity, high in cell toxicity, strong in tumor damage capability and low in cost is provided, and a basis for clinical application of the Vgamma9Vdelta2 T cells on tumor treatment medicine is laid.
Owner:SHENZHEN HORNETCORN BIOTECH
Method for preparing CIK cells
ActiveCN107988155AInduced proliferationSuitable for growthCulture processCell culture supports/coatingPeripheral blood mononuclear cellMonoclonal antibody
The invention relates to in-vitro culture of immune cells and in particular relates to in-vitro induction culture of CIK cells. The preparation method disclosed by the invention comprises the following steps: coating a cell culture bottle by fibronectin and a CD3 monoclonal antibody; preparing autologous plasma; acquiring peripheral blood mononuclear cells PBMC; inducing the PBMC by IL-2, IFN-gamma and the autologous plasma; and performing multiplication culture. The CIK cells prepared by the preparation method disclosed by the invention are great in quantity of CIK cells, high in cell viability, high in ratio of CD3+CD56+effector cells and high in cytolytic activity, can meet clinical requirements and have high anti-tumor abilities.
Owner:暨赛国际再生医学科技有限公司
Process to cary out a cellular cardiomyoplasty
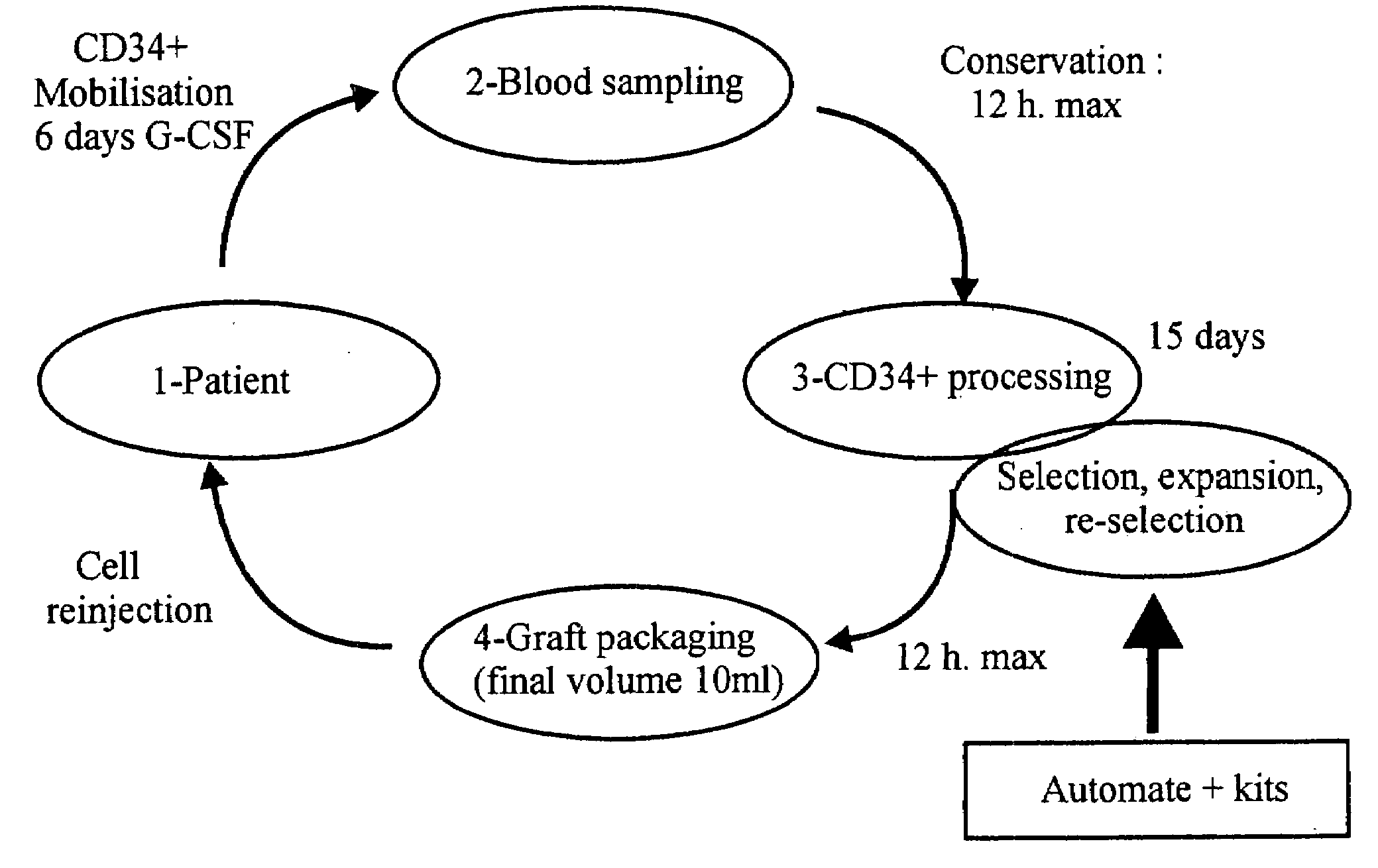
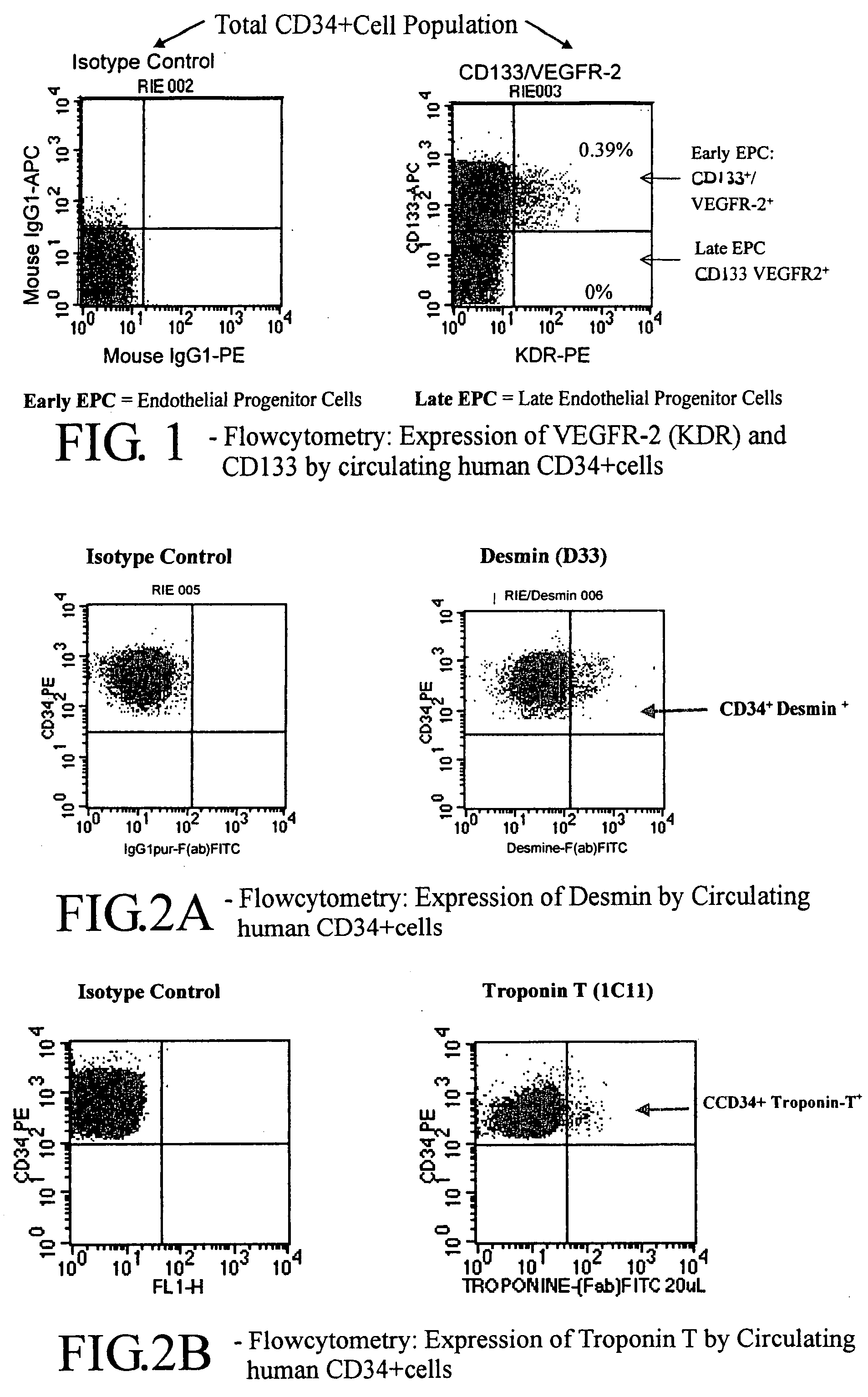
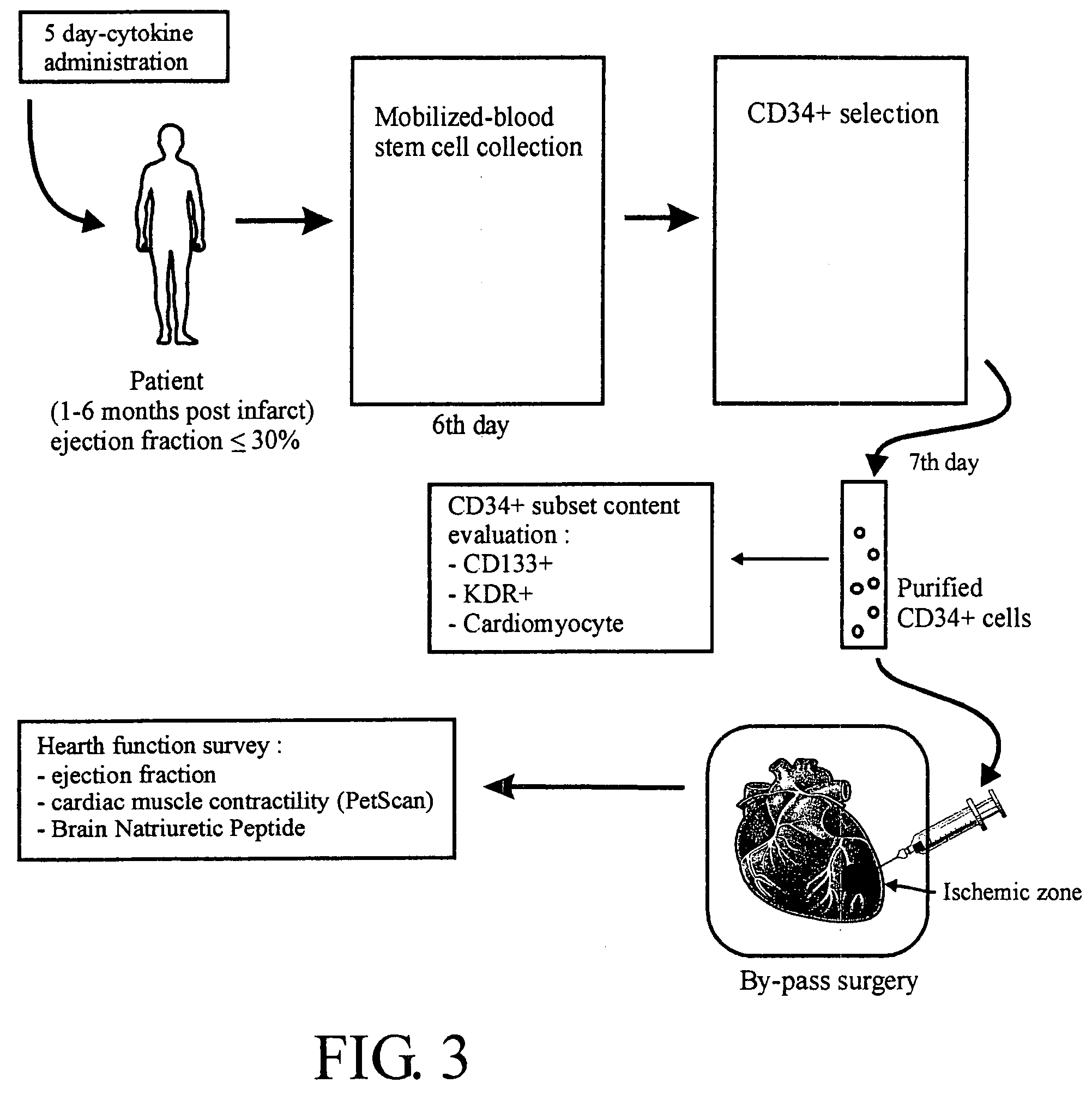
InactiveUS20080118977A1Efficient and reliableLow costPeptide/protein ingredientsArtificial cell constructsCell processingMedicine
A cellular cardiomyoplasty process based on the potential capacity of CD34+ cells to regenerate myocardium after acute myocardial infarct (AMI) and on their collection in blood in which the following phases are performed: Phase 1 a G-CSF-mobilization phase of CD-34+ cell is started as soon as the infarct is stabilized and its impact on heart function has been evaluated; Phase 2 a cell collecting phase is undertaken after G-CSF-mobilization; Phase 3 a cell processing phase is performed to select ex-vivo CD34+ cells and expand them in vitro to achieve around a 20-fold increase of the total number of CD34+ cells; Phase 4 a resuspension phase of the amplified-cell product in a final predetermined volume of autologous plasma, and Phase 5 a packaging phase of the cell suspension in a sterile syringue for reinjection to the patient.
Owner:INSTITUT DE RECH & HEMATOLOGIE & TRANSPLANTATION
Culture method of DC-CIK (Dendritic Cell-Cytokine-Induced Killer) cells loaded with tumor cell exosomes
InactiveCN109456941AHigh cytotoxic activityLethalNervous system cellsBlood/immune system cellsDendritic cellMicrobiology
The invention relates to the field of DC-CIK (Dendritic Cell-Cytokine-Induced Killer) cell culture and particularly relates to a culture method of DC-CIK cells loaded with tumor cell exosomes. The culture method comprises the following steps of: commonly culturing CIK cells and peripheral blood DC cells loaded with the tumor cell exosomes, after culturing for 65-75 hours, adding IL-2 with the amount of 1000-1500 IU / mL by volume of culture solution, and continuously culturing for 65-75 hours to obtain the DC-CIK cells; culturing by a CIK culture medium comprising the components of GT-T581 culture medium, autologous plasma and IL-2, wherein the autologous plasma is 5%+ / -0.5% of the volume of the GT-T581 culture medium and the concentration of the IL-2 is 1000+ / -100 IU / mL. The culture methodhas the beneficial effects that by culturing the CIK cells and the peripheral blood DC cells loaded with the tumor cell exosomes in a certain mode, the obtained DC-CIK cells loaded with the tumor cellexosomes have good killing capability.
Owner:见多视光(北京)科技有限公司
Natural killer T cell culture medium and multiplication culture method for natural killer T cells
ActiveCN107488630AHigh activityPromote proliferationCulture processCell culture supports/coatingHeterologousSerum free media
The invention provides a natural killer T cell culture medium and a multiplication culture method for natural killer T cells and relates to the technical field of cell culture. The natural killer T cell culture medium, disclosed by the invention, is mainly composed of a serum-free medium, autologous plasma and a cell factor IL-2. According to the multiplication culture method for natural killer T cells by using the cell culture medium, human-derived peripheral blood mononuclear cells are induced to release a danger signal by virtue of the antibody binding cell factor IL-2, and further the natural killer T cells are activated. According to the method, high-quality natural killer T cells can be obtained by massive high-efficiency multiplication in a short period on premise of ensuring the security. The potential security risk caused by culture of heterogenous animal serum or tumor cells in the conventional natural killer T cell culture method and the problems that the conventional natural killer T cell culture method is low in multiplication times and low in purity and is difficult to use on a large scale are effectively solved.
Owner:TIANJIN CHANGHE BIOLOGICAL TECH
Formulation, apparatus, and methods for treatment of brain trauma
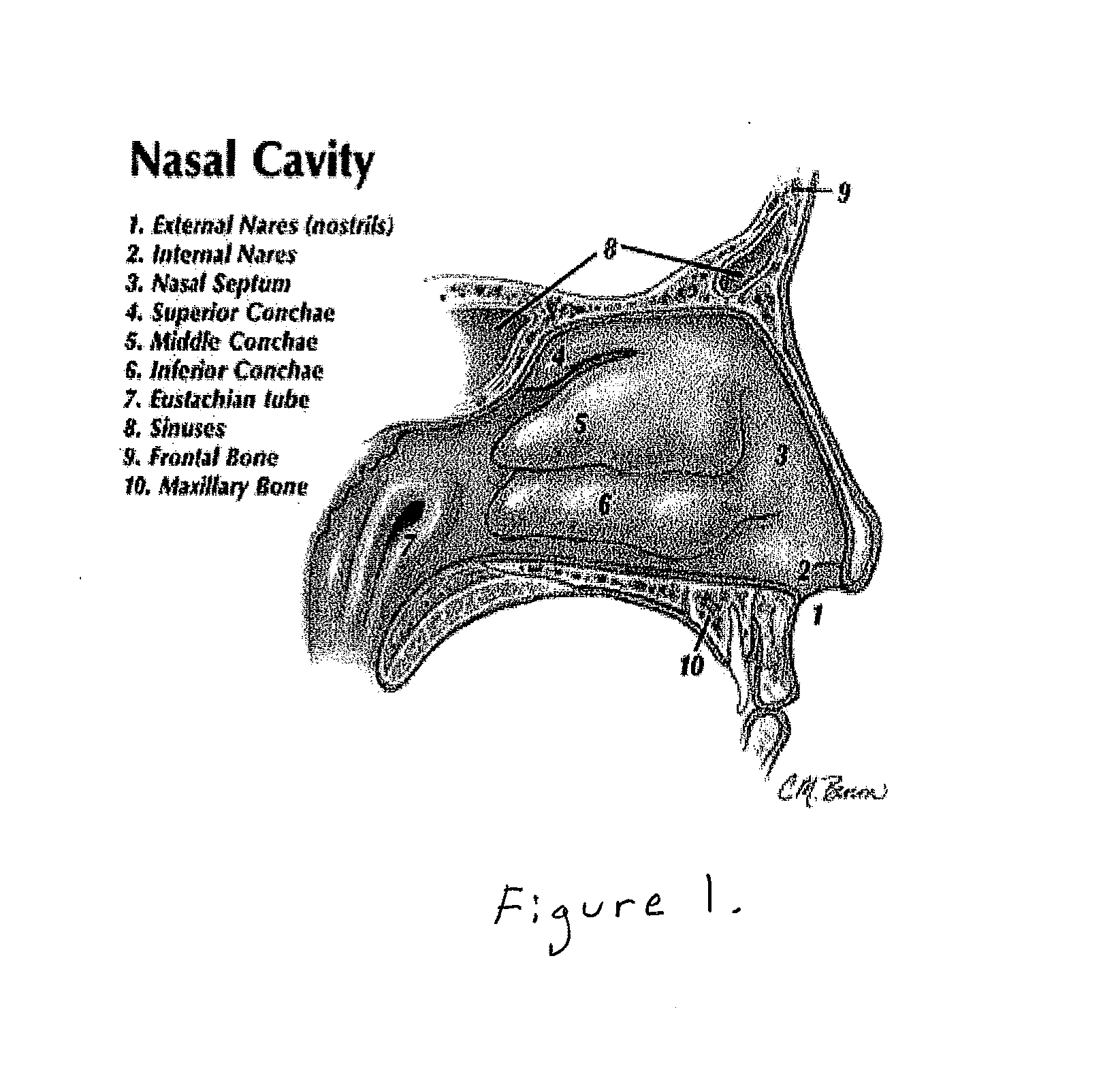
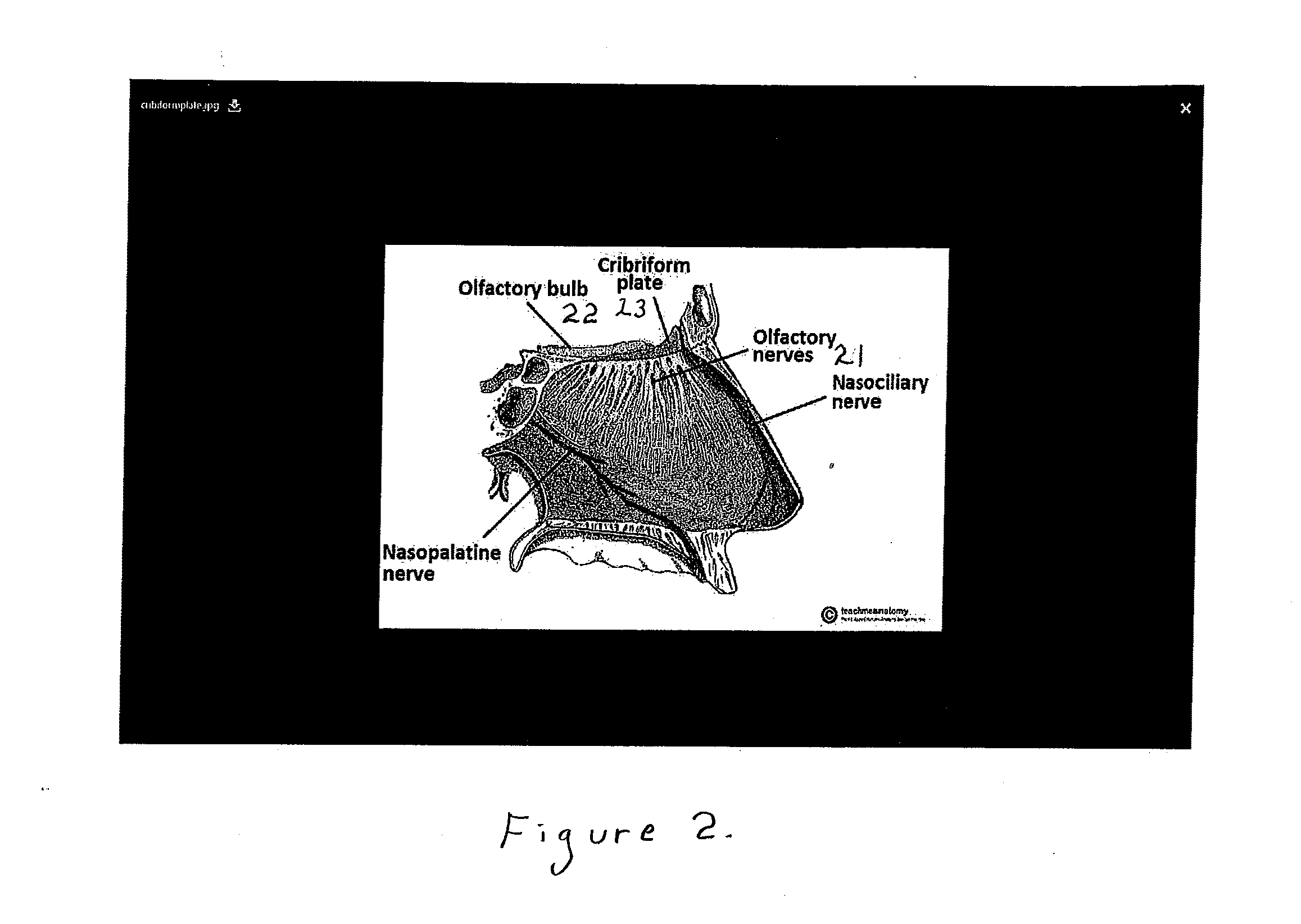
ActiveUS20160235786A1Promote recoveryEfficacy in of injuryBreathing protectionOrganic active ingredientsNasal cavityNostril
A formulation comprising platelet rich plasma (PRP) for treatment of patients who have experienced brain injury, consisting of a mixture of human autologous plasma, D5W, glutathione, methylcobalamin, and regular insulin. The formulation is infused directly adjacent to a patient's brain through the nostrils or nares of the nasal cavity. Treatment using the formulation may be supplemented with one or more therapies including hyperbaric oxidation therapy (HBOT), cranial osteopathic therapy, intravenous (IV) nutrition, electroencephalographic (EEG) biofeedback, low level laser therapy (LLLT), transcranial magnetic stimulation (TMS), adult stem cell treatments, and a ketogenic diet and medium-chain triglyceride (MCT) oil therapy.
Owner:HUGHES JOHN C
An efficient in-vitro amplification culture method for natural killer cells having high killing capability
InactiveCN107099503AEasy to obtainAvoid damageCell culture supports/coatingBlood/immune system cellsNatural Killer Cell Inhibitory ReceptorsBlood plasma
An efficient in-vitro amplification culture method for natural killer cells having high killing capability is disclosed and belongs to the technical field of cell culture. The method includes (1) preparing a medium; (2) sampling; (3) centrifuging mononuclear cells with 400 g of double-antibody PBS having a concentration of 500 U / mL for 10 min, fully rinsing the mononuclear cells for three times, and inoculating the mononuclear cells to a culture dish; (4) in the second day to the sixth day, observing the color of the medium and counting, and supplementing the fresh medium every 2-3 days; (5) after the seventh day, reducing the autologous plasma content by 1%; (6) purifying the NK cells; and (7) cryopreserving the purified CD56<+>NK cells in liquid nitrogen by utilizing a serum-free cryopreserving liquid. The method has advantages of easily available raw materials, capability of preparing a high number of NK cells having activity from a small amount of peripheral blood or umbilical blood, suitability for large-scale culture, low damage to bodies, and the like. The sources of the materials are wide, and in-vitro storage quantity is high. The prepared natural killer cells are suitable for autologous treatment and free of immunogenicity.
Owner:溯源生命科技股份有限公司
Medical cosmetic product containing autologous peripheral blood vascular endothelial progenitor cells
ActiveCN104622772APromote repairEasy to fillCosmetic preparationsToilet preparationsProgenitorPlatelets blood
The invention provides a preparation method of a medical cosmetic product containing autologous peripheral blood vascular endothelial progenitor cells. The method comprises the following steps: acquiring platelet-rich plasma from autologous peripheral blood and cultivating the platelet-rich plasma so as to obtain autologous endothelial progenitor cells; adding the obtained autologous endothelial progenitor cells to autologous plasma so as to prepare a biological cosmetic preparation; and promoting the differentiation of the endothelial progenitor cells into endothelial cells by virtue of the autologous plasma so as to form blood vessels. Meanwhile, the invention provides a medical cosmetic product prepared from the autologous endothelial progenitor cells and the autologous plasma, and the medical cosmetic product is applicable to repairing and improving dark spots in skin, acnes and cicatrices, scars caused by burn or trauma and the like.
Owner:广州赛琅生物技术有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com