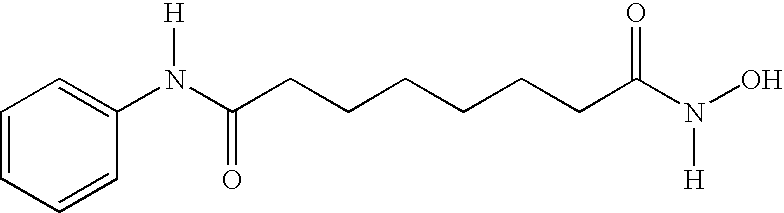
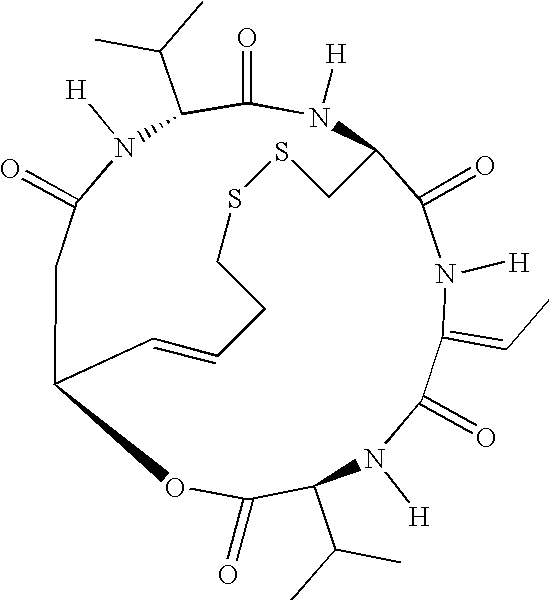
Patents
Literature
747 results about "Peripheral blood mononuclear cell" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
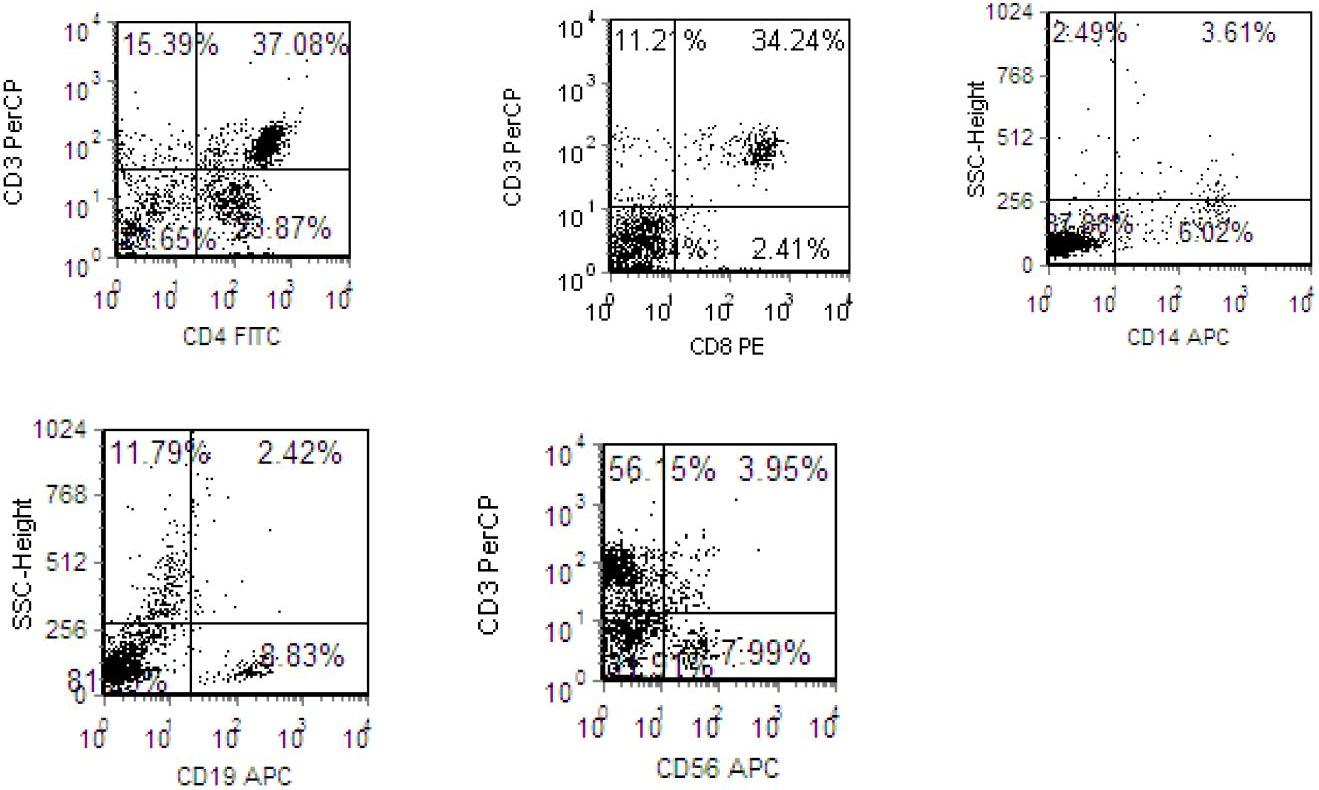
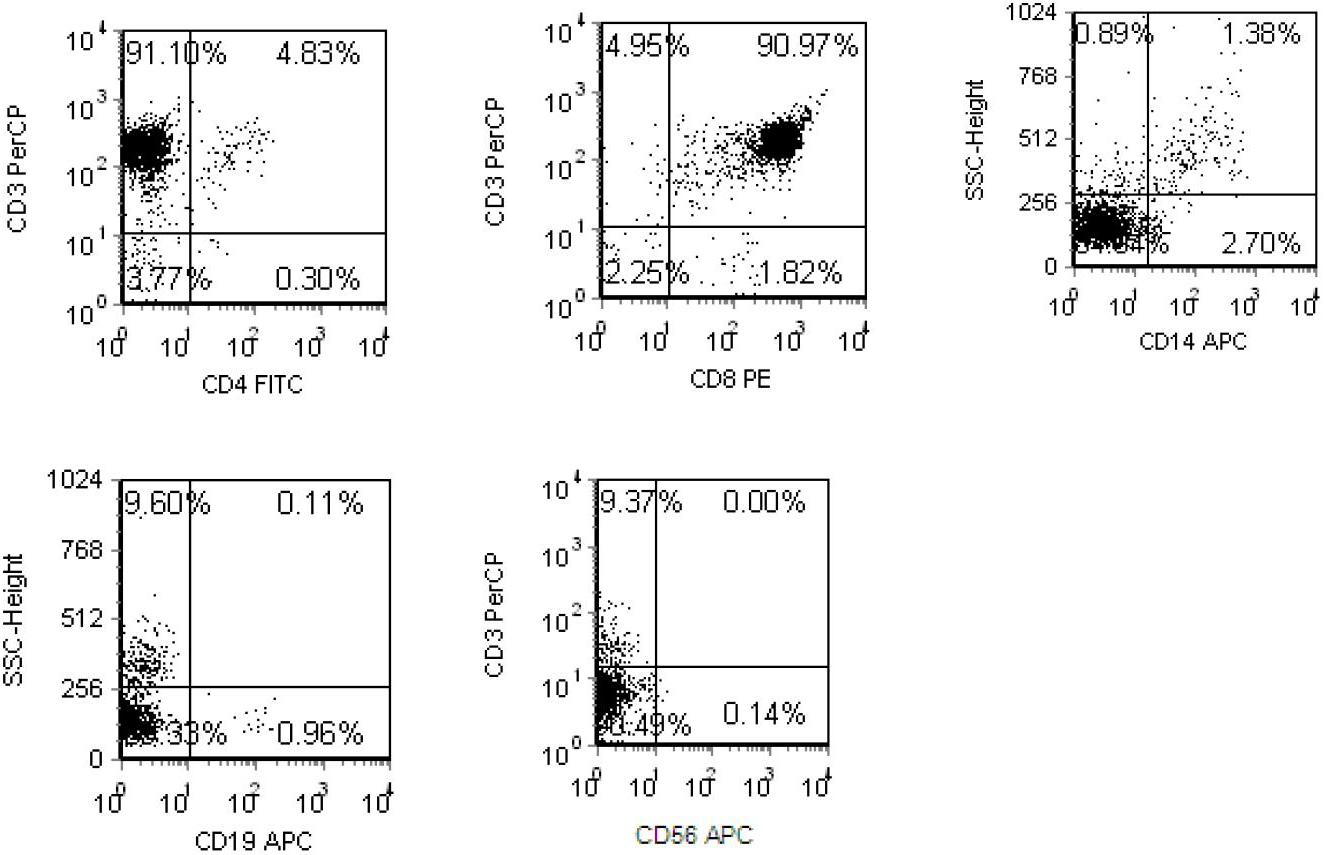
A peripheral blood mononuclear cell (PBMC) is any peripheral blood cell having a round nucleus. These cells consist of lymphocytes (T cells, B cells, NK cells) and monocytes, whereas erythrocytes and platelets have no nuclei, and granulocytes (neutrophils, basophils, and eosinophils) have multi-lobed nuclei. In humans, lymphocytes make up the majority of the PBMC population, followed by monocytes, and only a small percentage of dendritic cells.
Methods of producing enriched populations of tumor reactive T cells from peripheral blood
ActiveUS9844569B2Mammal material medical ingredientsBlood/immune system cellsAbnormal tissue growthPeripheral blood mononuclear cell
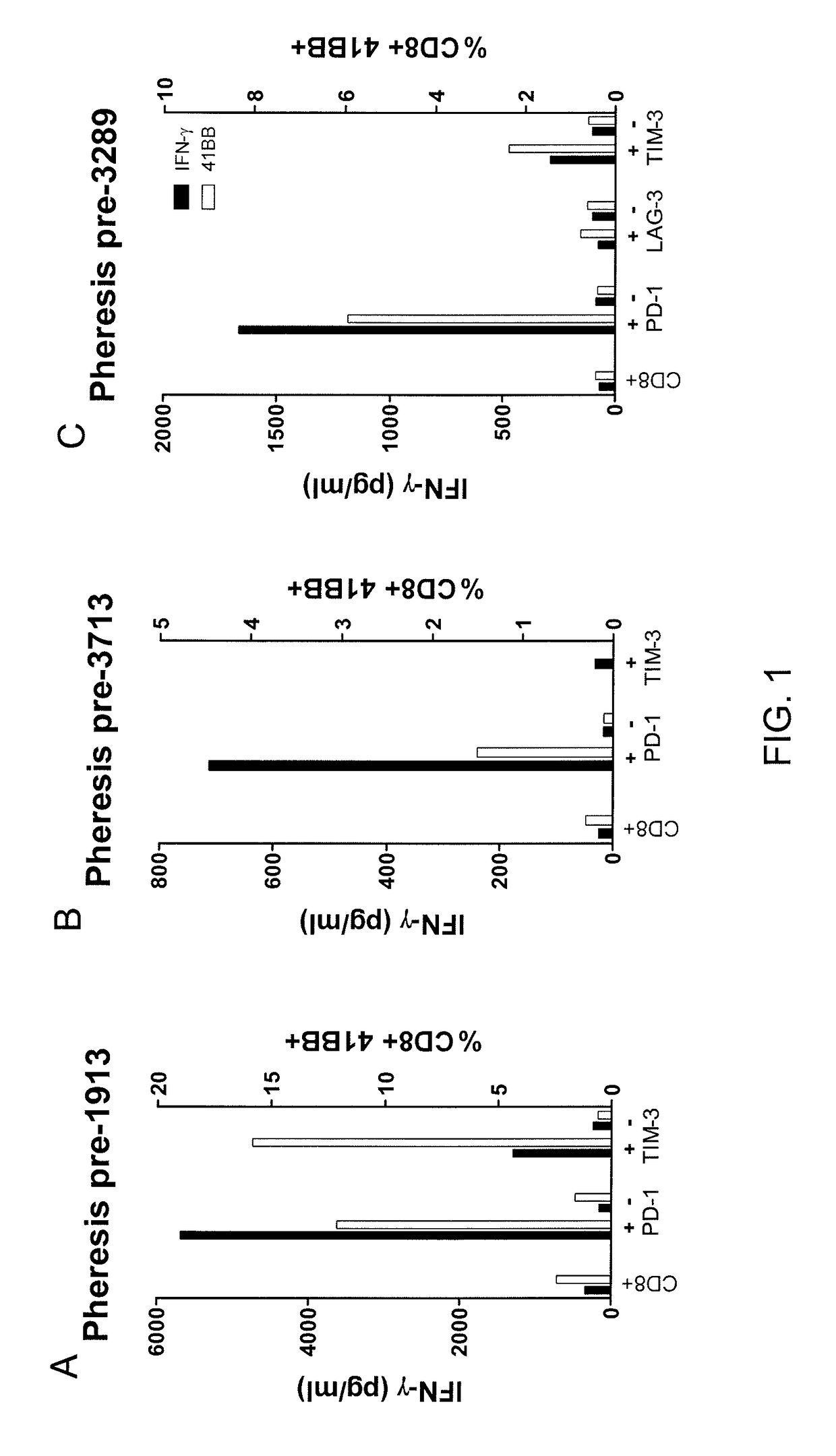
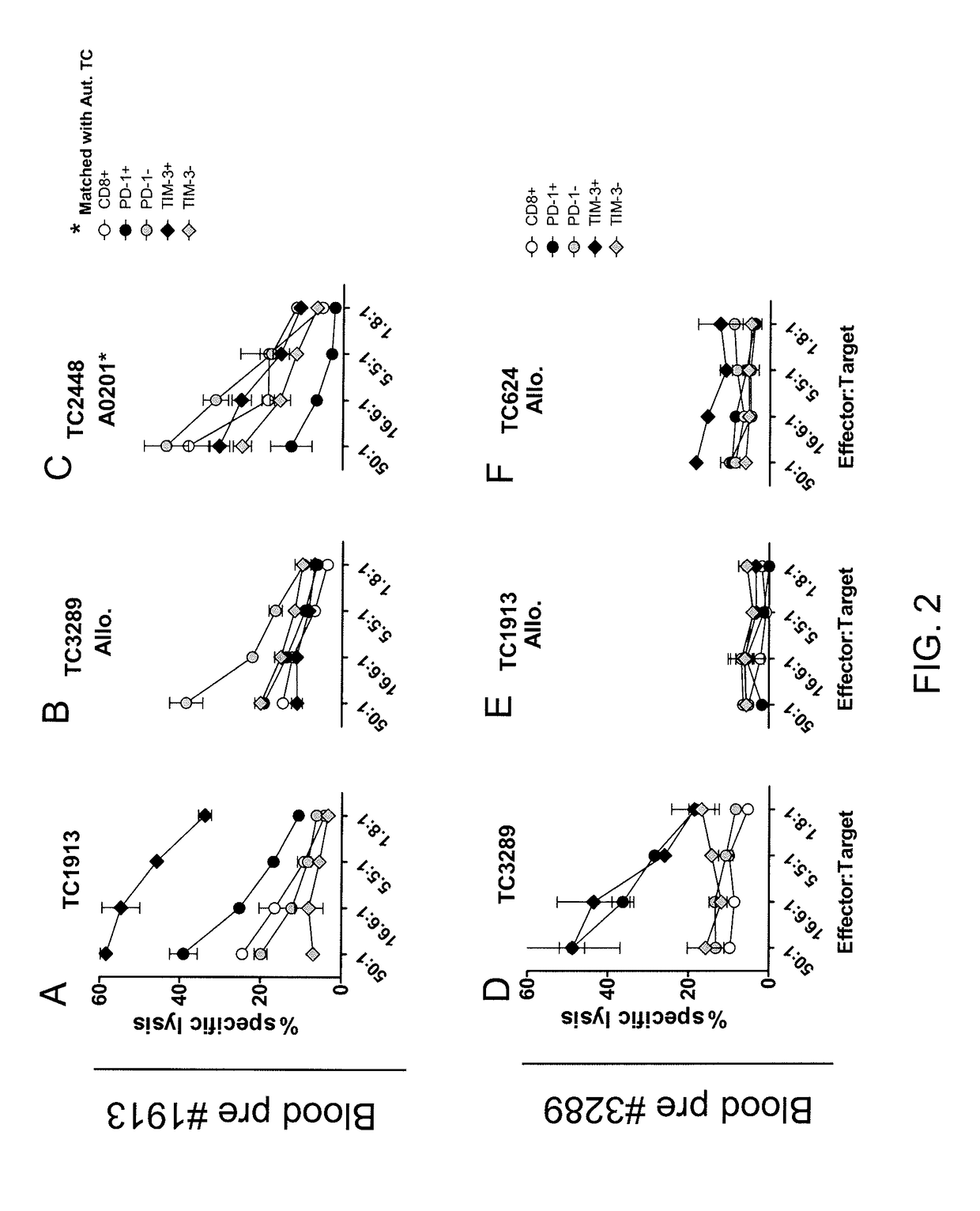
Methods of obtaining a cell population enriched for tumor-reactive T cells, the method comprising: (a) obtaining a bulk population of peripheral blood mononuclear cells (PBMCs) from a sample of peripheral blood; (b) specifically selecting CD8+T cells that also express PD-1 and / or TIM-3 from the bulk population; and (c) separating the cells selected in (b) from unselected cells to obtain a cell population enriched for tumor-reactive T cells are disclosed. Related methods of administering a cell population enriched for tumor-reactive T cells to a mammal, methods of obtaining a pharmaceutical composition comprising a cell population enriched for tumor-reactive T cells, and isolated or purified cell populations are also disclosed.
Owner:UNITED STATES OF AMERICA
Generation of fully mature and stable dendritic cells from leukaphereses products for clinical applications
InactiveUS20040072347A1Bioreactor/fermenter combinationsBiological substance pretreatmentsWhite blood cellPeripheral blood monocyte
The present invention provides a method for producing mature and stable dendritic cells or immature dendritic cells which comprises cultivating hematopoietic progenitor cells in a sterile cultivating apparatus, an apparatus suitable for said method and a method for preparing peripheral blood mononuclear cells, which are suitable for cultivation of dendritic cells.
Owner:MERIX BIOSCI
Method for amplifying NK cells of human beings under condition of in vitro culture
InactiveCN101684456AIncrease the amplification factorHigh purityBlood/immune system cellsForeign genetic material cellsPeripheral blood mononuclear cellNatural Killer Cell Inhibitory Receptors
The invention discloses a method for amplifying NK cells of human beings under the condition of in vitro culture, which is characterized in that a peripheral blood mononuclear cell (PBMC) is taken asthe original culturing material, and the PBMC is cultured together with an engineering cell which is built by adopting the genetic engineering method and is used for stimulating the growth of the NK cell. The built engineering cell which is used for stimulating the growth of the NK cell expresses several cytokines (IL-2, IL-12, IL-15, IL-18, 4-1BB) which can promote the growth of the NK cell on the surface of the K562 cell; after irradiation and inactivation with gamma-rays, the engineering cell is cultured with the PBMC in vitro, as a result, the amplification effect of the stimulation methodin the invention is hundreds of times stronger than that of the currently universal method in which soluble cytokines are added purely to the culture solution; and after cultured for 3 weeks, the non-NK cells in the PBMC are mainly dead and disappeared, the NK cells are proliferated in great quantity, the purity of the NK cells reaches over 96% and the total number of the NK cells is amplified byover 1500 times.
Owner:JIANGMEN LUOSEN BIO PHARMA
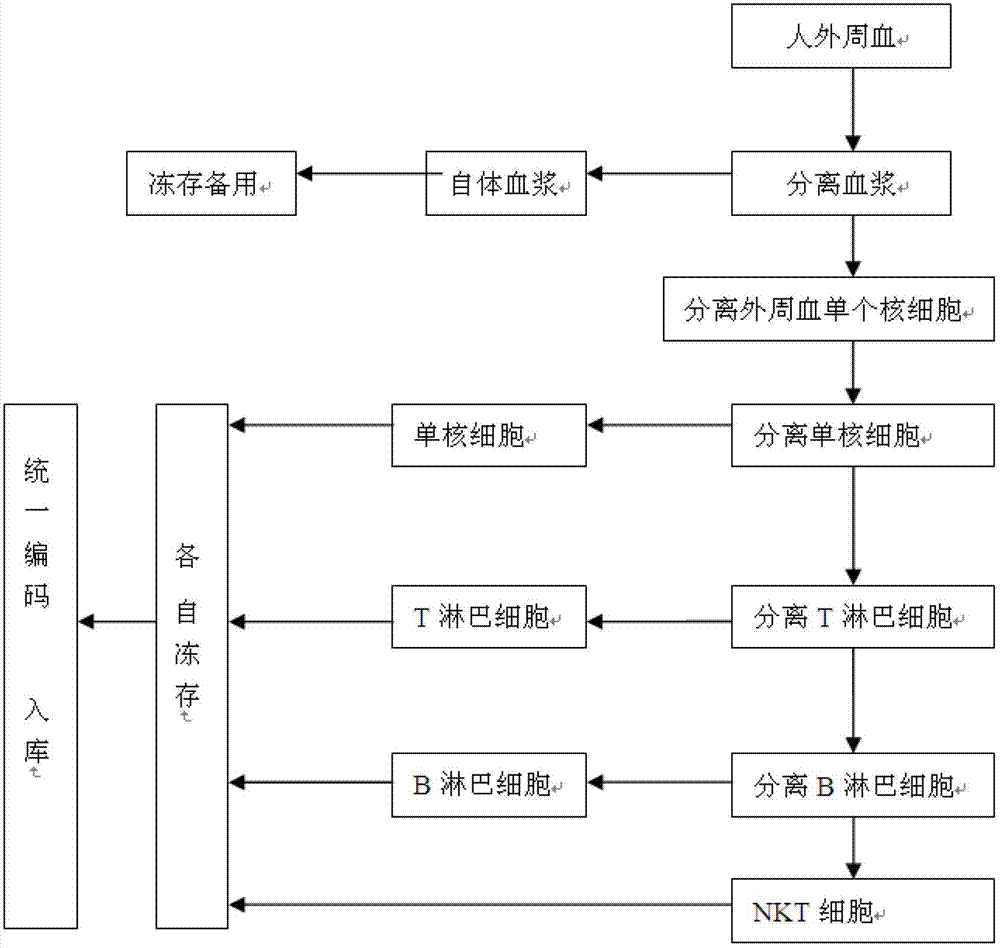
Method for constructing human peripheral blood immune cell bank
ActiveCN102758259AHigh activityHigh purityMicroorganism librariesBlood/immune system cellsPeripheral blood mononuclear cellNatural Killer Cell Inhibitory Receptors
The invention discloses a method for constructing a human peripheral blood immune cell bank. The method comprises the following steps of: collecting human peripheral blood, separating autologous plasma, separating a peripheral blood mononuclear cell, separating a mononuclear cell by the peripheral blood mononuclear cell and freezing, separating a T lymphocyte by the peripheral blood mononuclear cell and freezing, separating a B lymphocyte by the peripheral blood mononuclear cell and freezing, separating an NK cell by the peripheral blood mononuclear cell and freezing, and encoding and puttingin storage. According to the invention, immune cells of health or young people are separated and are respectively independently frozen, and relative numbers are stored and put into storage, so that the stored human immune cells have the characteristics of high activity, high purity and convenience for use, and fetal calf serum can be replaced by the human autologous plasma, so that introduction of a foreign protein can be avoided. Meanwhile, the method, provided by the invention, has the advantages of low cost, low requirements on laboratory conditions, and wide application.
Owner:济南赛尔生物科技股份有限公司
Method used for in vitro proliferation of NK cells
ActiveCN103756963AIncrease lethalityEasy to synthesizeBlood/immune system cellsSerum free mediaPeripheral blood mononuclear cell
Owner:SHANGHAI CLAISON BIOTECH
Method for preparing II-type pig's ring-virus nucleic vaccine and the use thereof
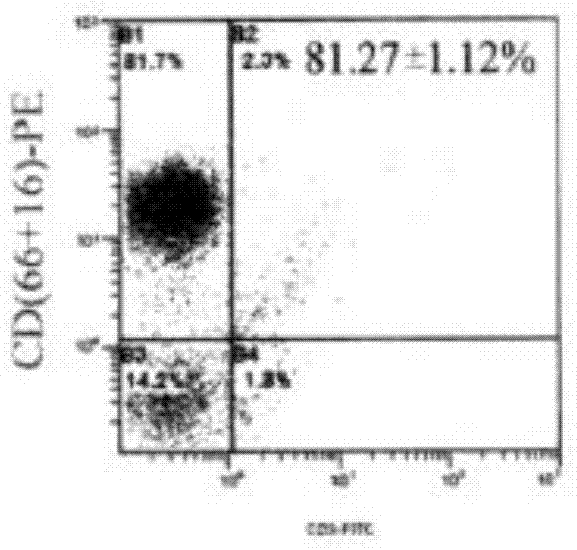
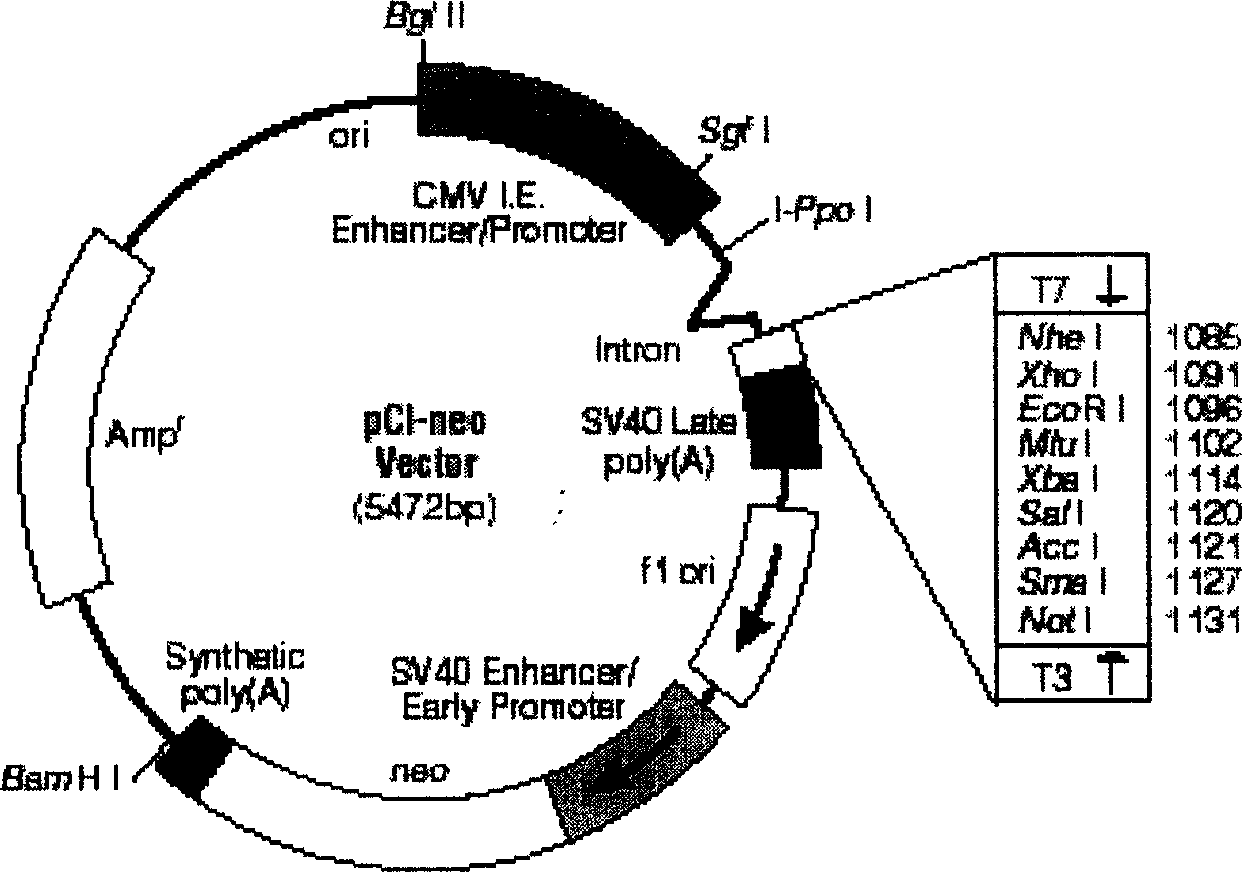
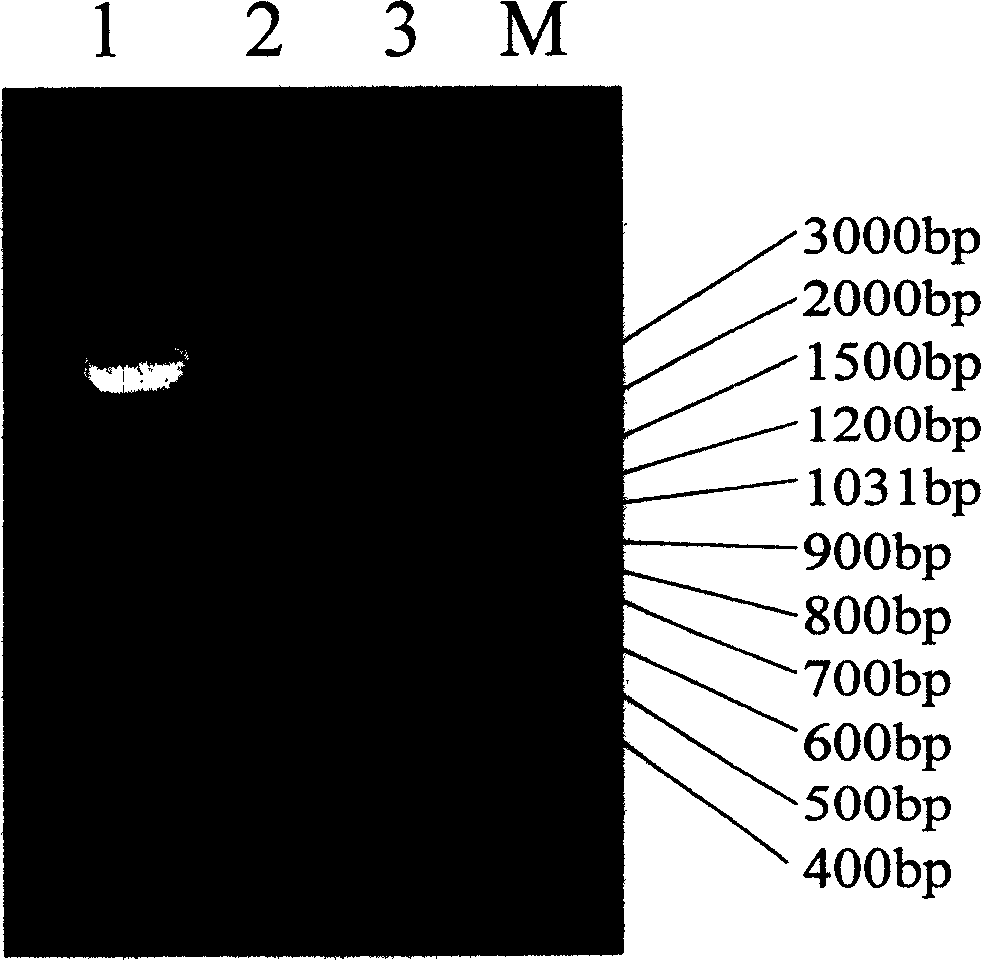
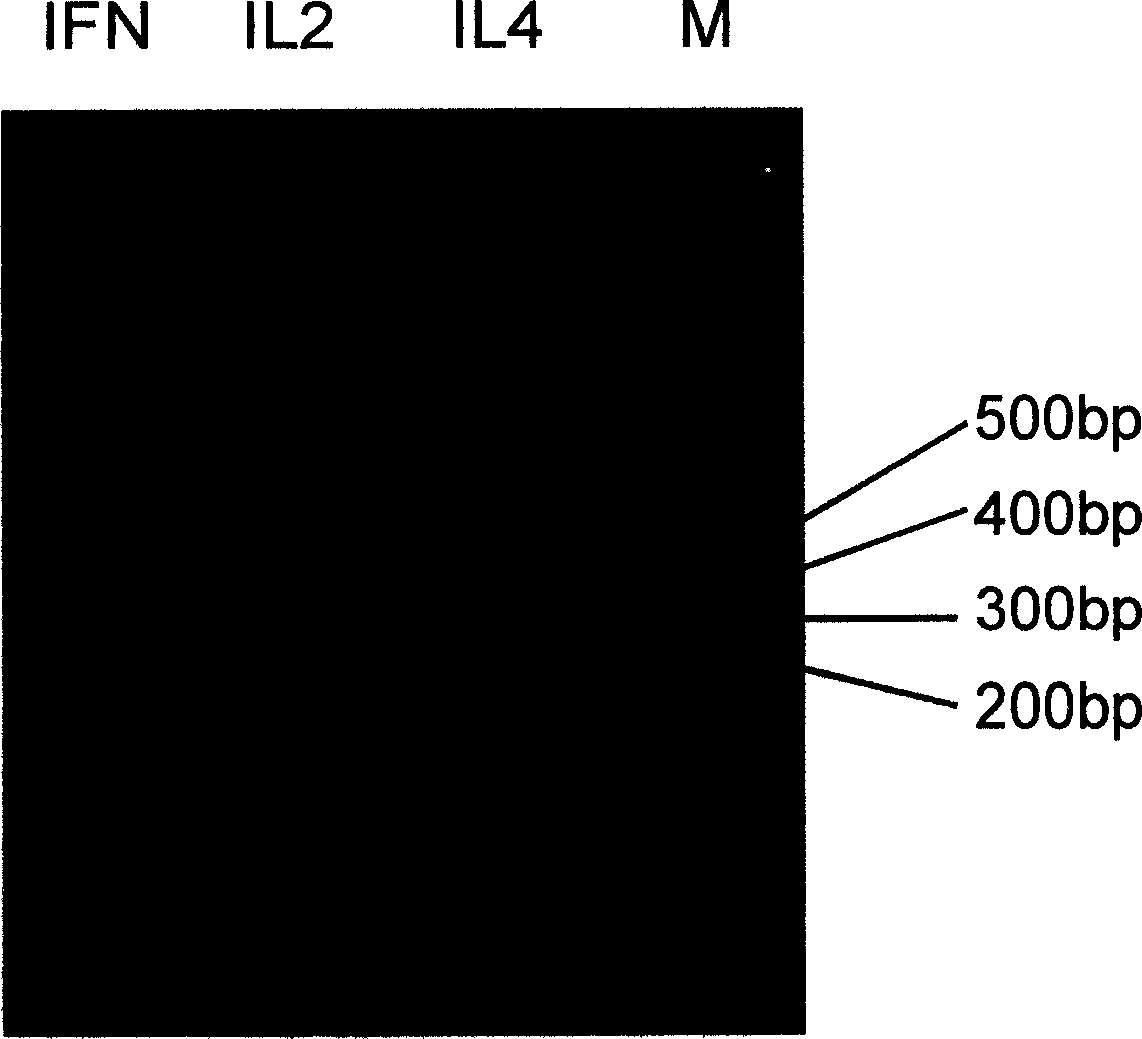
InactiveCN1579553AShorten the production cycleAvoid pollutionViral antigen ingredientsGenetic material ingredientsPeripheral blood mononuclear cellBiology
The invention discloses a manufacturing method and application of II type pig ring virus nucleic acid vaccine. The method is: 1) designs the specific primer, uses PCV2 Hangzhou (HZ201) gene group as template, closes ORF1, ORF2, ORF3 and ORF4 genes of PCV-2 with PCR method, and they are constructed into eucaryon expressing carrier with pCI-neo; 2) separates the pig external blood single nucleus cell, clones the genes IFN, IL-2 and IL-4 with RT-PCR method, and they are constructed into eucaryon expressing carrier with pCI-neo; 3) based on above mentioned reconstructed carrier, constructs the fused expressing carrier of the other genes of PCV2, ORF2 and PCV2 or pig cell factor gene; the merits of the invention lie in: (1) it needs not to culture virus, the producing period is short; (2) it needs not cell culture, thus can prevent the contamination from other pig source virus; (3) it does not express the pathogenesis protein which is harmful to body, the safety is high. (4) it can activates body secretion and cell immunity replay at the same time.
Owner:ZHEJIANG UNIV
Specific epitope based immunological diagnosis of tuberculosis
ActiveUS20060115847A1Easy to identifyTesting is superfluousBacterial antigen ingredientsMicrobiological testing/measurementSkin allergy testPeripheral blood mononuclear cell
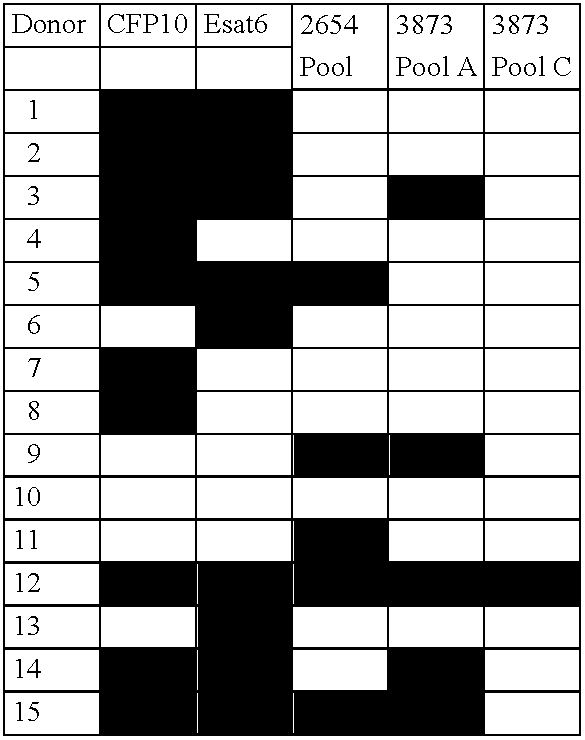
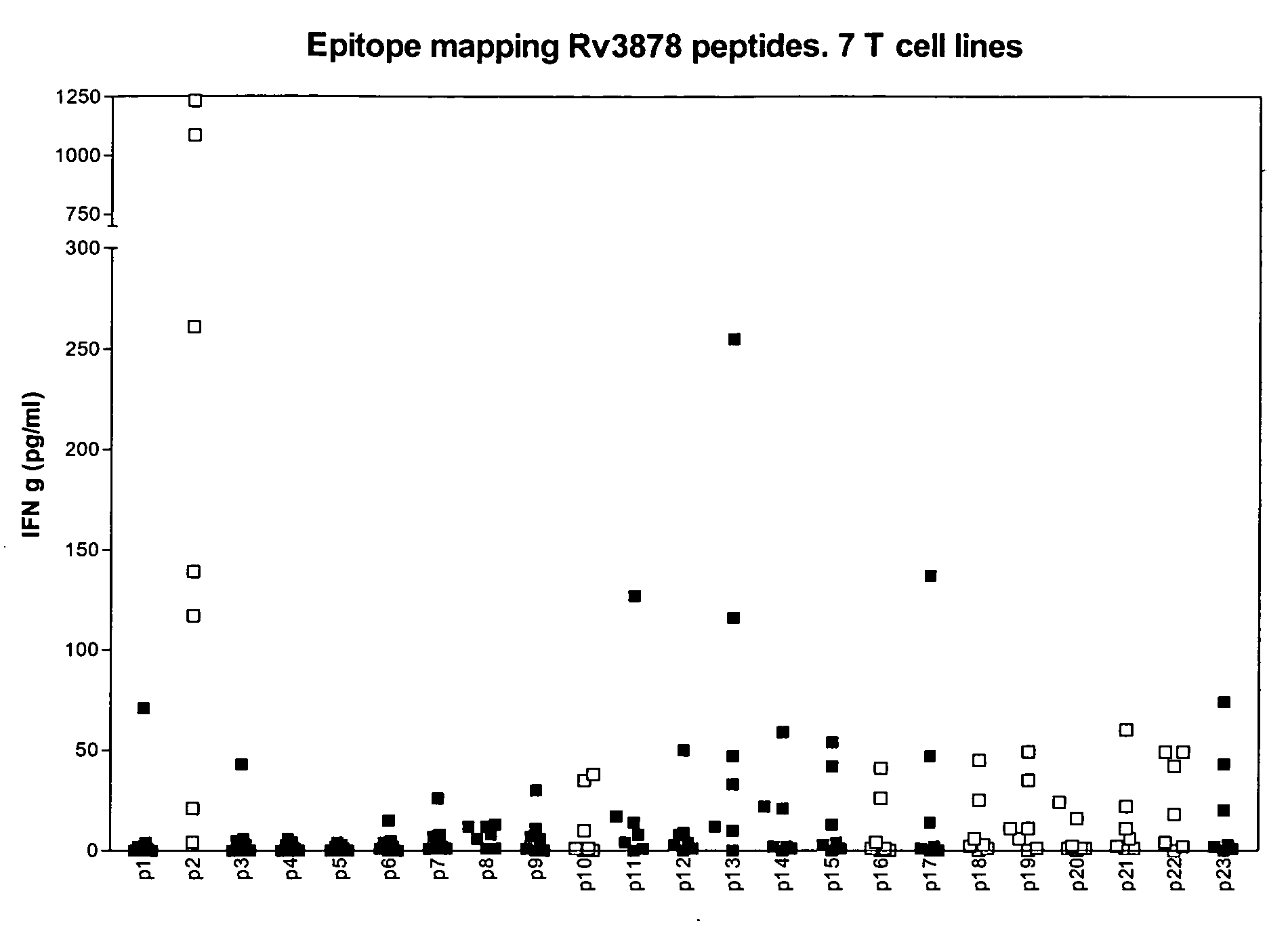
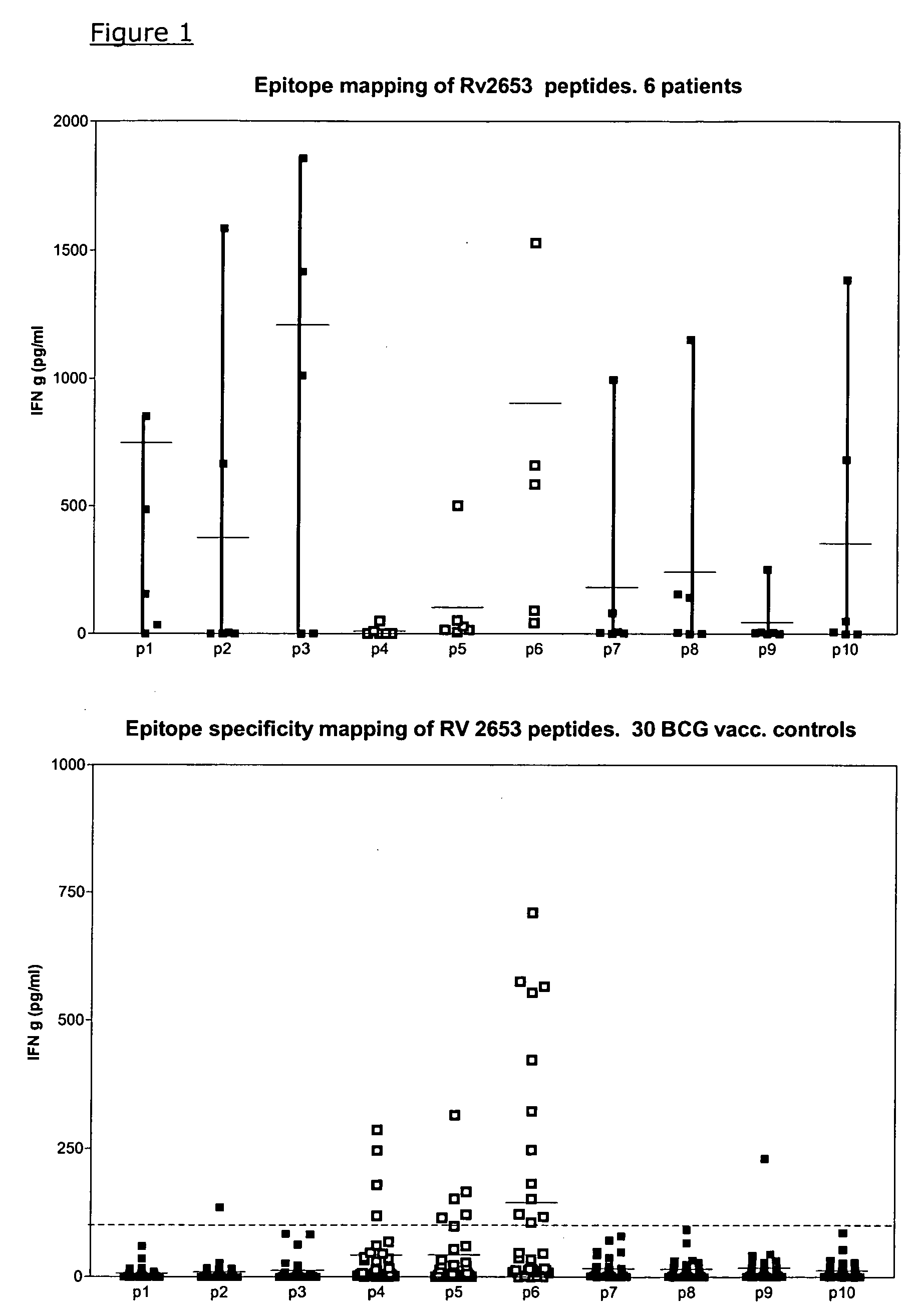
The currently used method for immunological diagnosis of tuberculosis infection, the tuberculin skin test, is problematic for a number of reasons; it has low specificity in BCG vaccinated individuals, a high interobserver variance and requires skill to be read and interpreted. Furthermore it requires an extra visit to the clinic to have the test read. Both people vaccinated with BCG and those exposed to non-tuberculosis mycobacteria give a positive skin test result similar to that seen in a TB infected individual. This also applies for purified protein derivative (PPD) when used in a blood cell based test. The present invention discloses the development of an immunological TB diagnostic tool based on a combination of epitopes from proteins encoded by regions of the M. Tuberculosis (M. tub.) genome, that are not present in the BCG vaccine strain or in the most common non-tuberculosis mycobacteria. Four recently characterized proteins with this diagnostic potential were selected. Peptides from these proteins were tested one by one with peripheral blood mononuclear cells from microscopy or culture confirmed TB patients as well as from healthy BCG vaccinated controls. Some combinations of peptides showed a sensitivity level comparable to the level seen with the two wellknown M. tuberculosisspecific proteins ESAT 6 and CFP 10. An epitope combination with these peptides combined with ESAT 6 and CFP 10 gave a sensitivity of 93%, representing a raise in sensitivity of about 26-33% compared to using ESAT6 or CFP 10 alone. The results from a panel of TB patients, using a collection of the new specific epitopes clearly demonstrates, that addition of other specific epitopes to the already known specific antigens, increases the sensitivity of a diagnostic assay based on cell mediated immune response.
Owner:STATENS SERUM INST
Fusion protein construct and method for inducing HIV-specific serum IgG and secretory IgA antibodies in-vivo
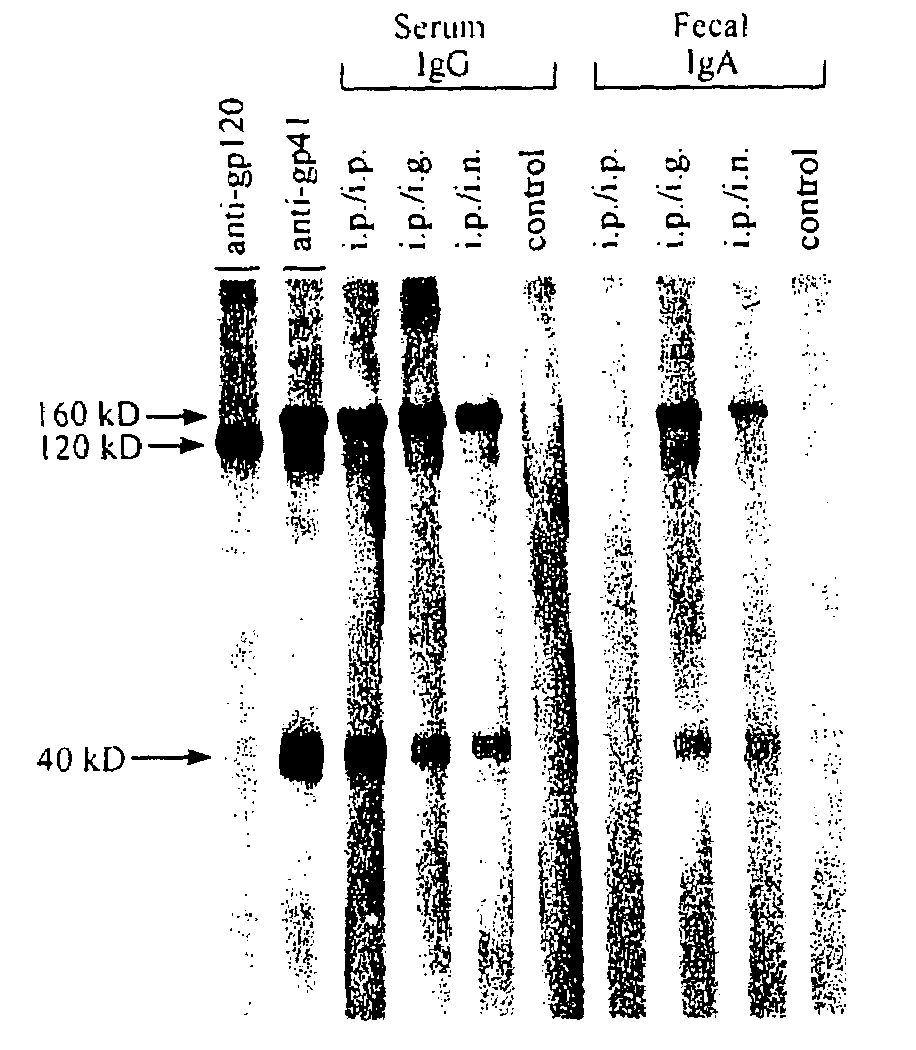
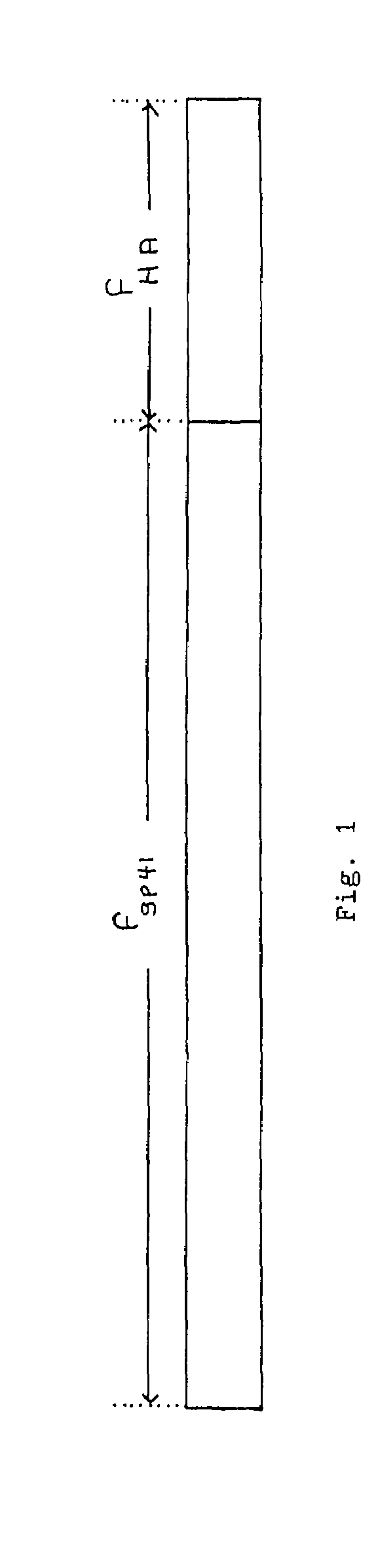
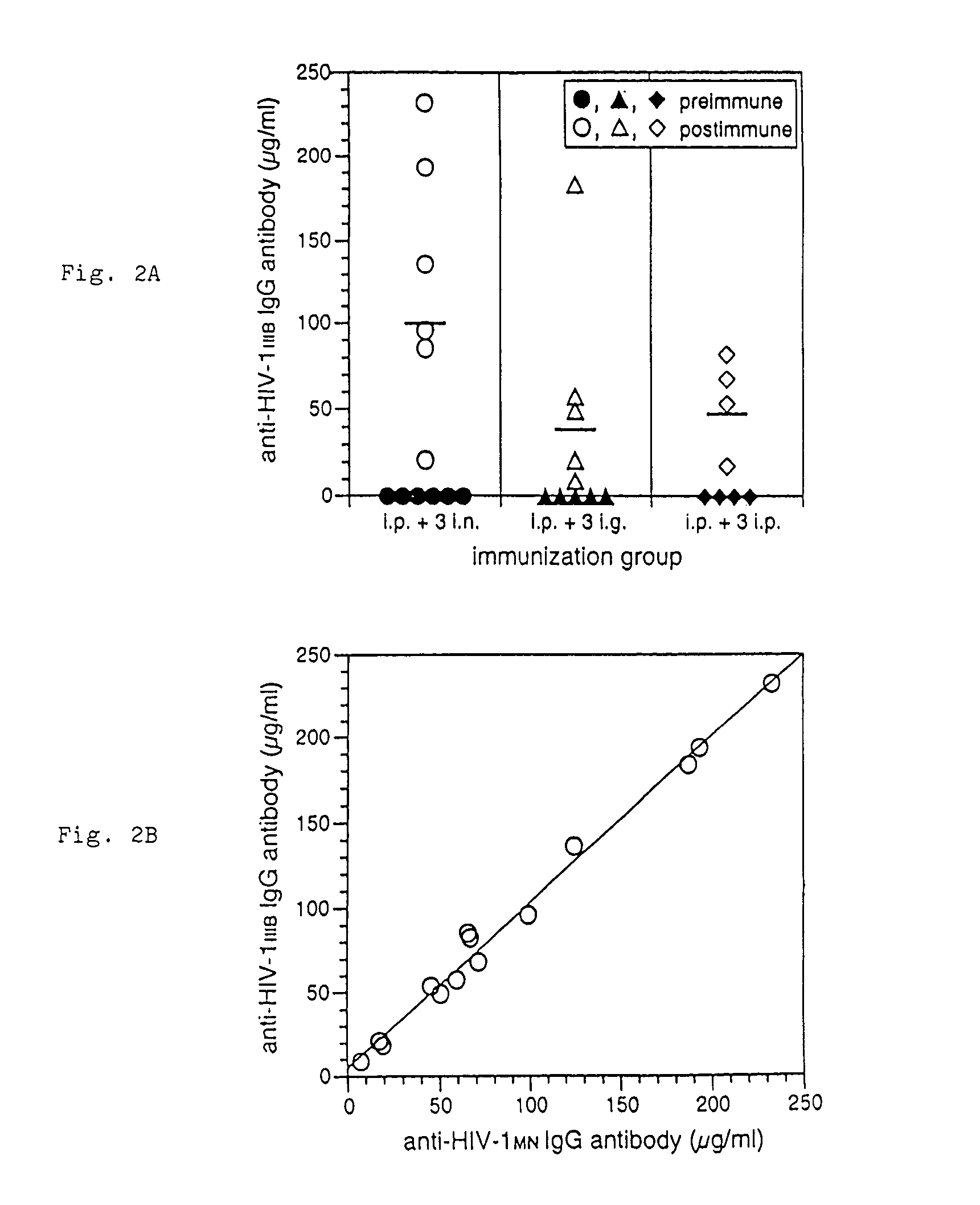
The present invention provides a fusion protein construct (gp41HA) consisting of the ectodomain of the HIV-1IIIB envelope glycoprotein gp41 fused to a fragment of the influenza virus HA2 hemagglutinin protein. Immunization in-vivo via an intraperitoneal prime followed by intranasal or intragastric boosts with gp41HA induces high concentrations of serum IgG antibodies and fecal IgA antibodies that reacted with gp41 in HIV-1IIIB viral lysate and are cross-reactive with gp41 in HIV-1MN lysate. Followup analyses by indirect immunofluorescence showed that both serum IgG and fecal IgA recognized human peripheral blood mononuclear cells infected with either syncytium-inducing (SI) or non-syncytium-inducing (NSI) North American HIV-1 field isolates, but not uninfected cells.
Owner:CHILDRENS MEDICAL CENT CORP
Method for in-vitro amplification of NK cells
InactiveCN102994449AEasy to synthesizeIncrease lethalityBlood/immune system cellsSerum free mediaPeripheral blood mononuclear cell
The invention relates to a method for in-vitro amplification of NK cells, and in particular relates to a method for massive in-vitro amplification of NK cells, wherein the method comprises the following steps of: a, inoculating a peripheral blood mononuclear cell in a CD3McAb and CD226McAb pre-coated culture bottle for coculture; b, adding 1L-2 and 1L-18, coculturing for 72hours to stimulate amplification of NK cells; c, transferring the NK cells, K562 cells after lethal treatment and a serum-free medium containing 1L-2 and 1L-18 in a cell culture bag for coculture; and d, collecting the NK cells. According to the method for in-vitro amplification of the NK cells, two antibodies CD3McAb and CD226McAb are simultaneously coated, so the cell factor synthesis and ADCC effect are promoted, and killing toxicity of the NK cells is remarkably improved; the activation and amplification on the NK cells are achieved just by the 1L-2 and 1L-18 cell factors, so the amplification multiple and cell toxicity of the NK cells are guaranteed, and the cost of cell culture is reduced.
Owner:SHANGHAI CLAISON BIOTECH
Method for simultaneous and efficient amplification of CD<3+>CD<56+>CIK cells and CD<3->CD<56+>NK cells
ActiveCN104357390AHigh purityHigh activityBlood/immune system cellsAdoptive cellular immunotherapySerum free media
The invention discloses a method for simultaneous and efficient amplification of CD<3+>CD<56+>CIK cells and CD<3->CD<56+>NK cells. The method comprises the steps as follows: the concentration of separated PBMC (peripheral blood mononuclear cells) is adjusted by a serum-free medium containing autologous plasma, an Anti-CD16 antibody, IL-2 and IL-15 are added, and then the mixture is transferred into a T175 culture flask for culture; an Anti-CD3 antibody and an Anti-CD137 antibody are added; a serum-free medium containing the autologous plasma, IL-2 and IL-15 is supplemented every two days according to the cell growth condition; the cell concentration is controlled to be about 1.5*10<6> / ml; and after culture is performed for 14-21 days, large quantities of high-purity CD<3+>CD<56+>CIK cells and CD<3->CD<56+>NK cells can be obtained simultaneously, and the total cell quantity can reach an effective value of the cell quantity required for adoptive cellular immunotherapy clinically for tumor. The method for simultaneous and efficient amplification of the CD<3+>CD<56+>CIK cells and the CD<3->CD<56+>NK cells is simple, convenient, effective and high in cell killing activity.
Owner:HRYZ (SHENZHEN) BIOTECH CO +1
Preparation method of human cytokine-induced killer cells
InactiveCN102732481APromote proliferationRaise the ratioBlood/immune system cellsHybrid peptidesPeripheral blood mononuclear cellCytotoxicity
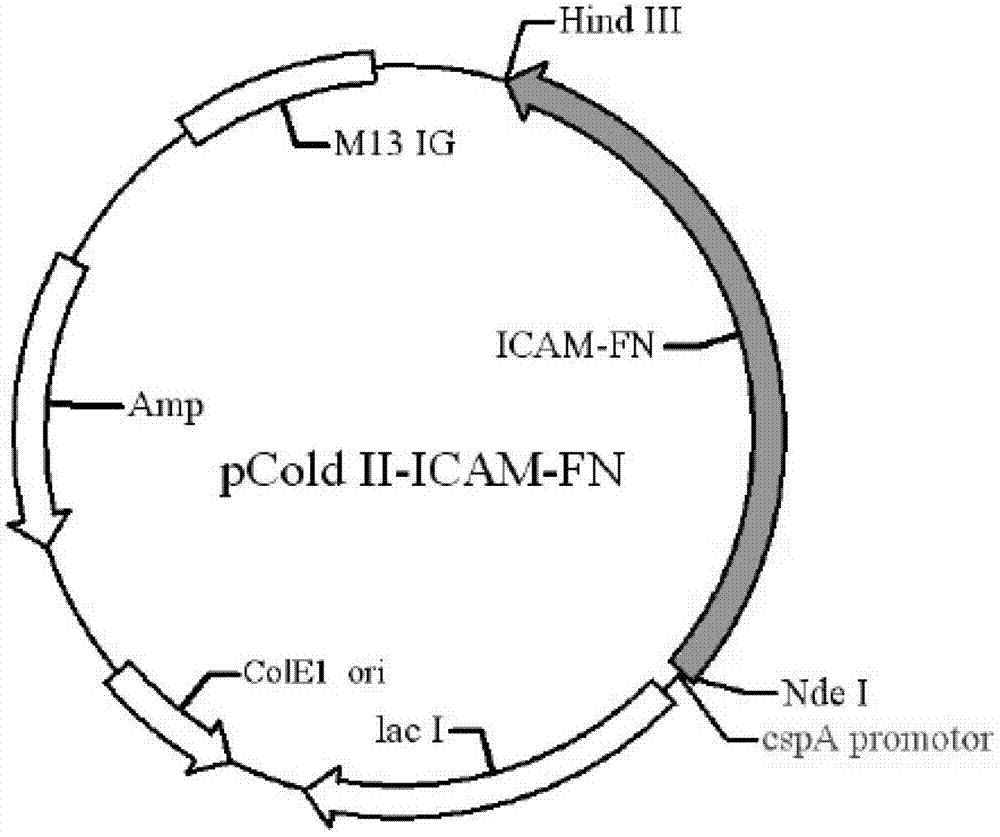
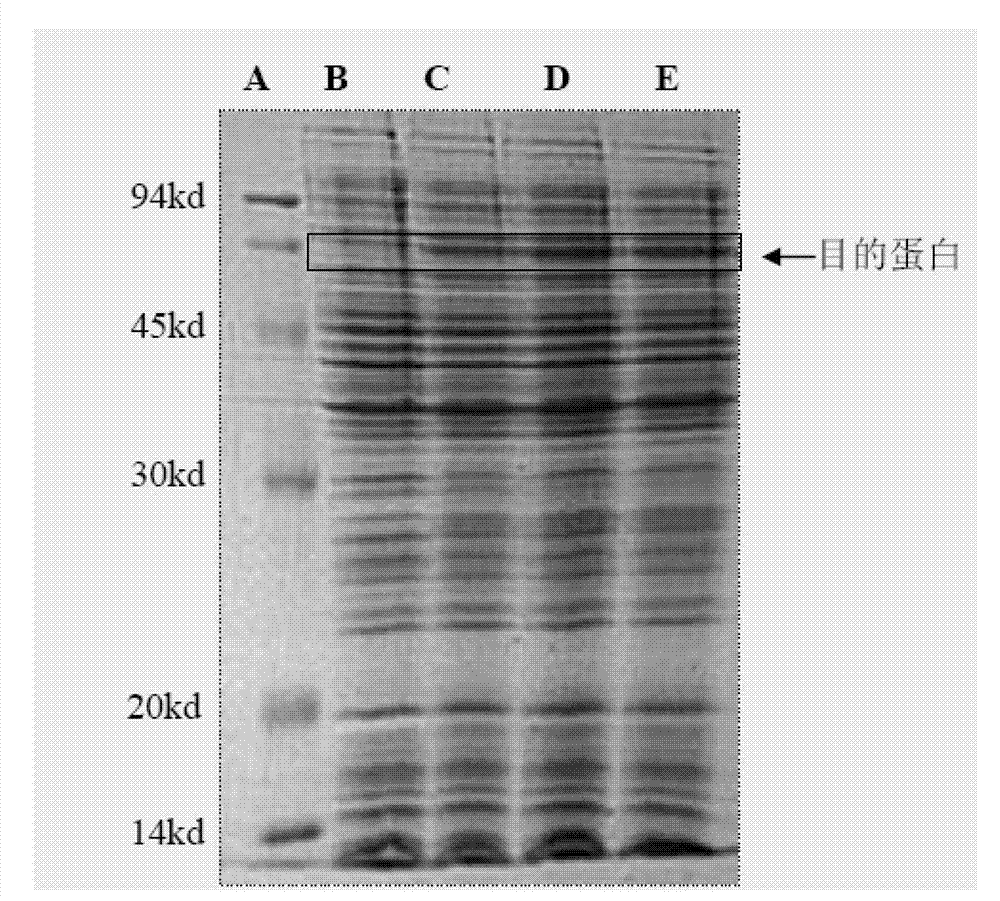
The invention discloses a preparation method of human cytokine-induced killer cells, comprising the following steps: coating a cell culture flask with a coating buffer containing effective amount of fusion protein and human CD3 monoclonal antibody before culturing precursor cells of human CIK cells, and adding the human CD3 monoclonal antibody in the whole process of inducing and culturing the human CIK cells, wherein the fusion protein is human intercellular adhesion molecule-1 functional domain and human fibronectin functional domain fusion protein, and the concentration of the human CD3 monoclonal antibody in the cell culture solution is lower than the concentration of the human CD3 monoclonal antibody in the coating buffer. According to the invention, ex-vivo expansion efficiency of peripheral blood mononuclear cells and the proportion of CD3 / CD56 double positive cells in the CIK cells are significantly raised, the cytotoxicity activity of the CIK cells is enhanced, thus the effect of cellular immunity treatment is raised.
Owner:SHENZHEN YOUNGCELL BIO TECH
Use of thioredoxin measurements for diagnostics and treatments
InactiveUS20050288227A1Increase doseLower Level RequirementsPeptide/protein ingredientsMicrobiological testing/measurementPeripheral blood mononuclear cellAutoimmune condition
The invention relates to methods for monitoring patient response to histone deacetylase inhibitors (e.g., suberoylanilide hydroxamic acid (SAHA)) or other therapeutic agents by measuring the level of thioredoxin in body fluids, tissues, and / or cells, such as peripheral blood mononuclear cells, plasma, or serum. The invention also relates to methods of monitoring and / or assisting with the diagnosis of a wide variety of thioredoxin-related diseases and conditions, such as inflammatory diseases, allergic diseases, autoimmune diseases, diseases associated with oxidative stress or diseases characterized by cellular hyperproliferation.
Owner:SLOAN KETTERING INST FOR CANCER RES
Treatment and prevention of immunodeficiency virus infection by administration of non-pyrogenic derivatives of lipid A
InactiveUS20040120924A1BiocideBacterial antigen ingredientsPeripheral blood mononuclear cellImmunodeficiency virus
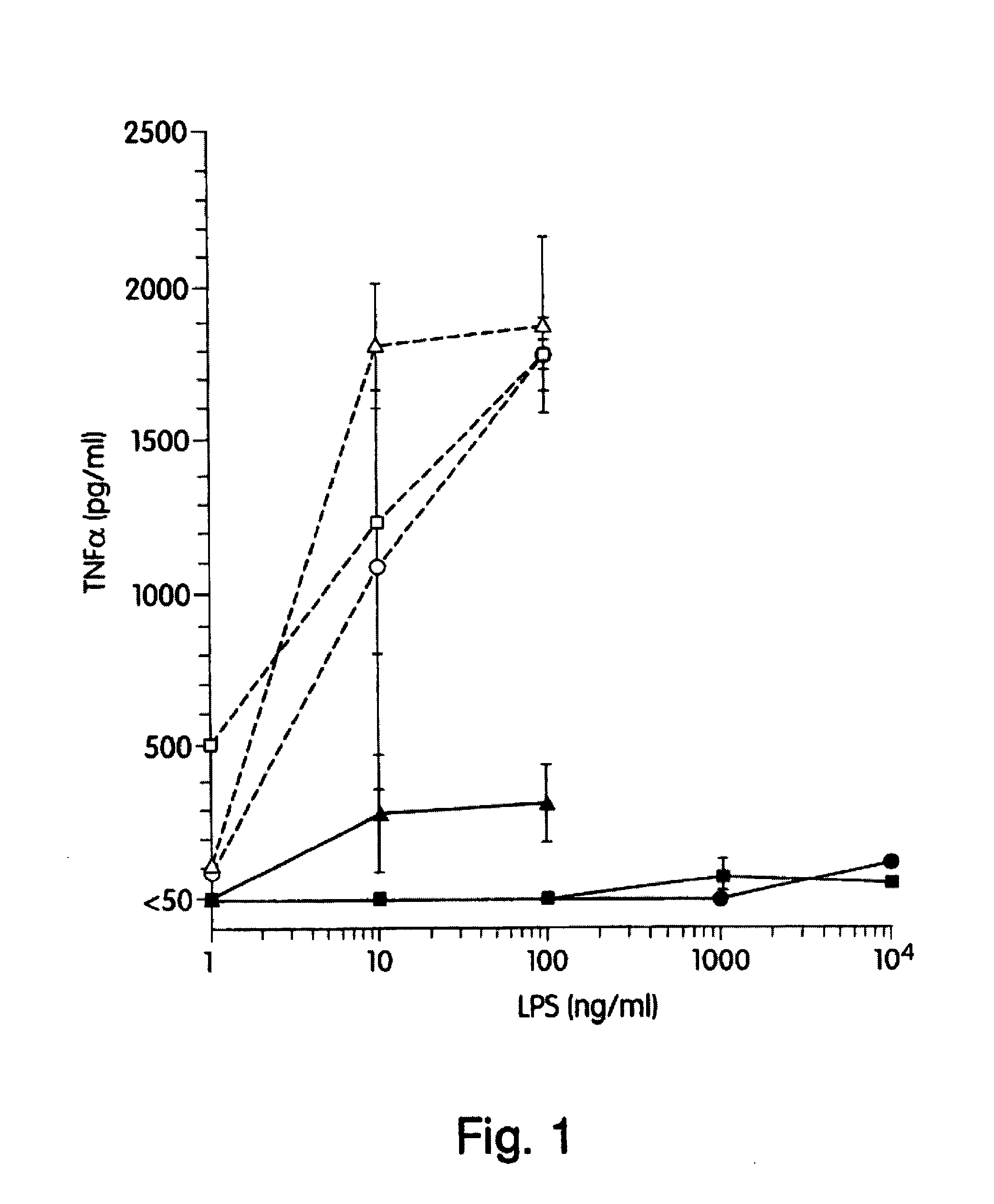
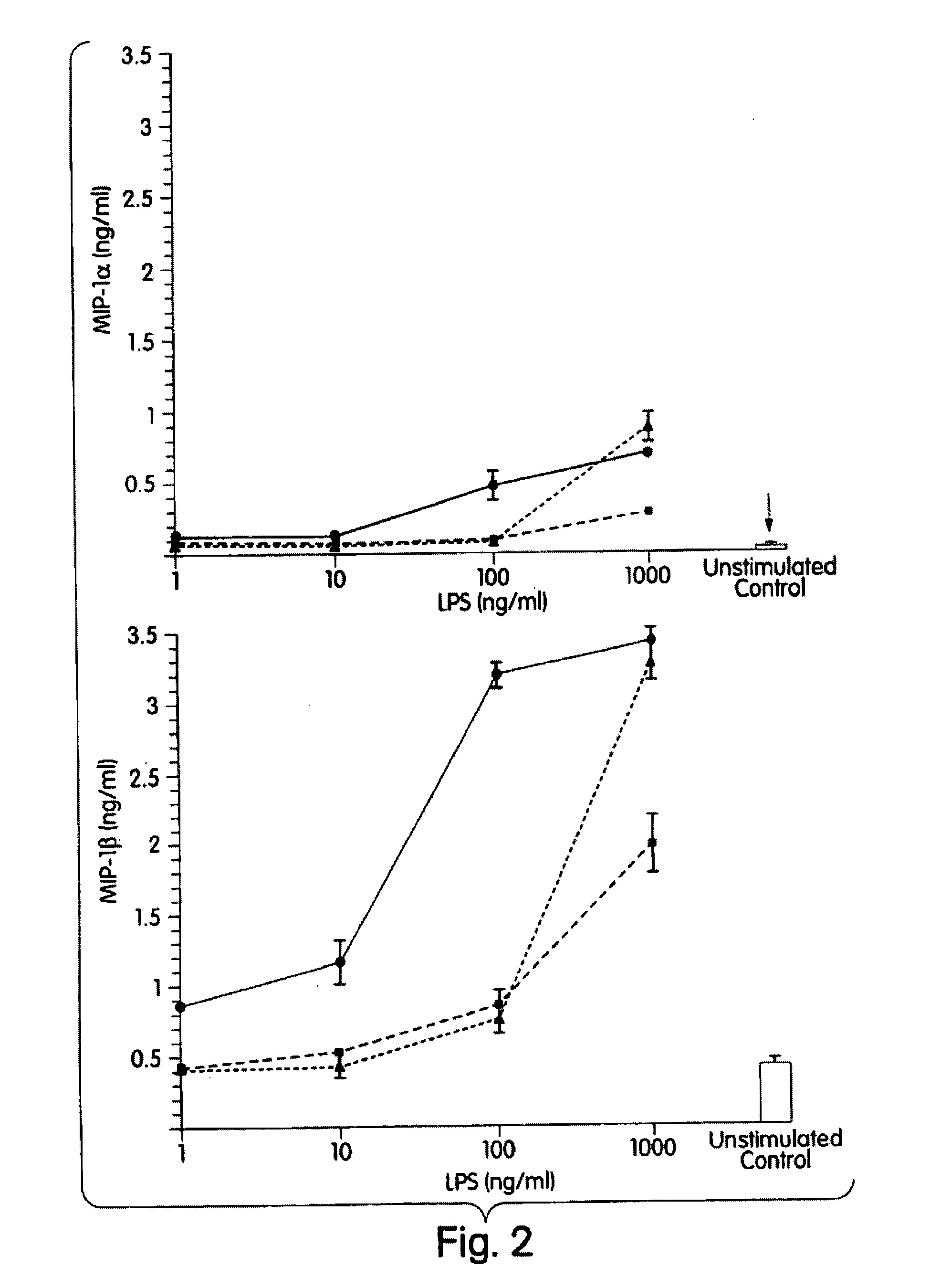
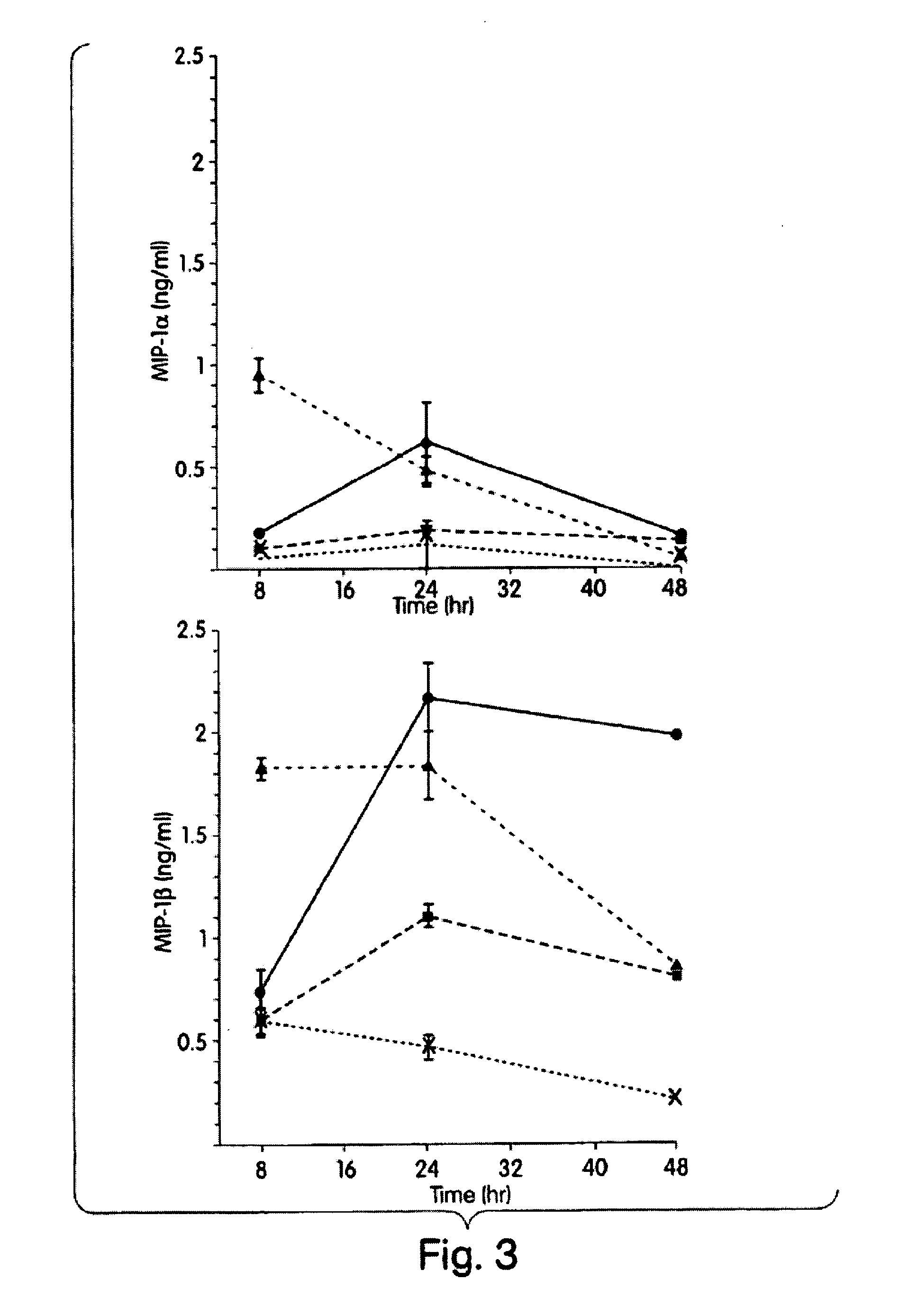
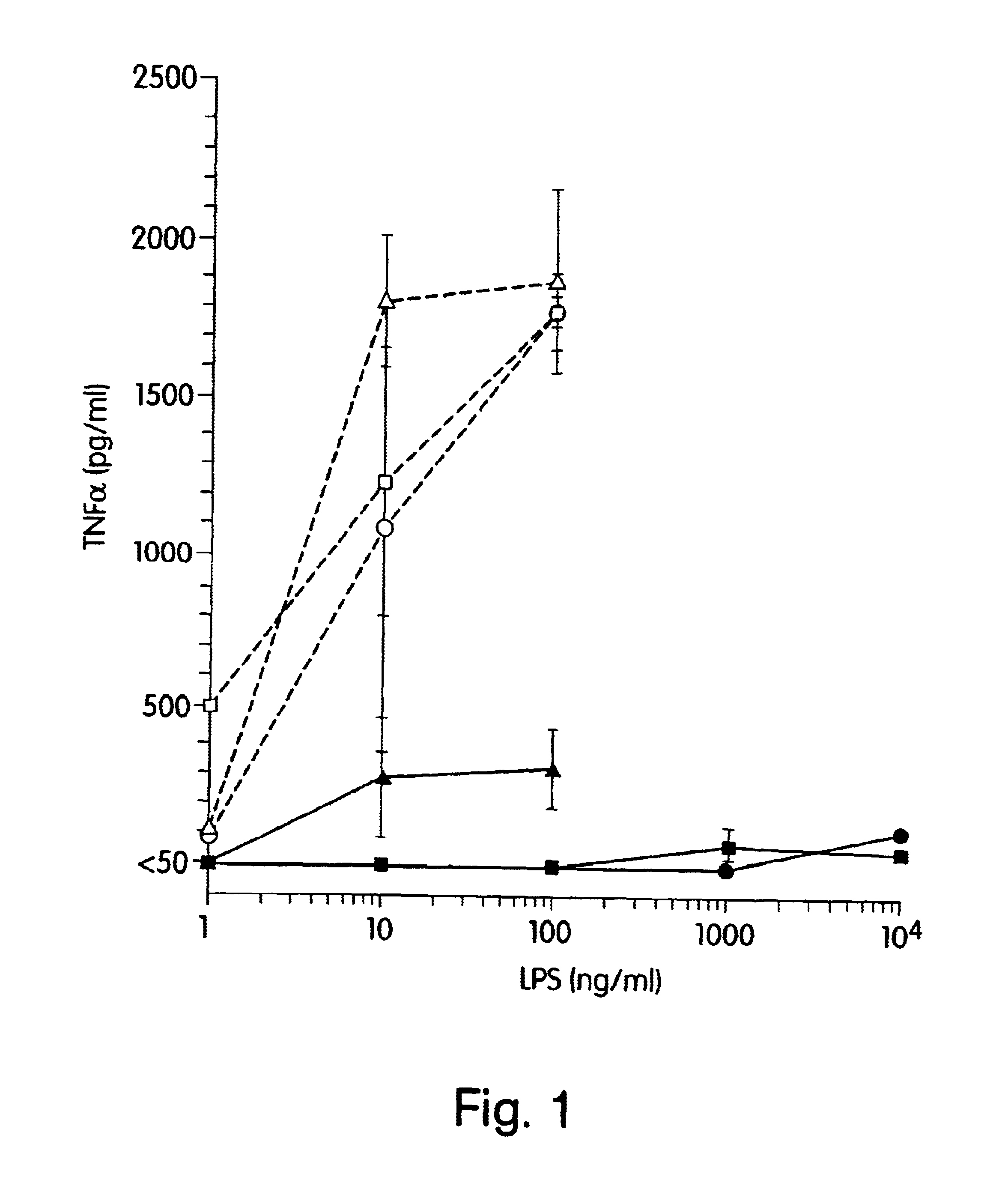
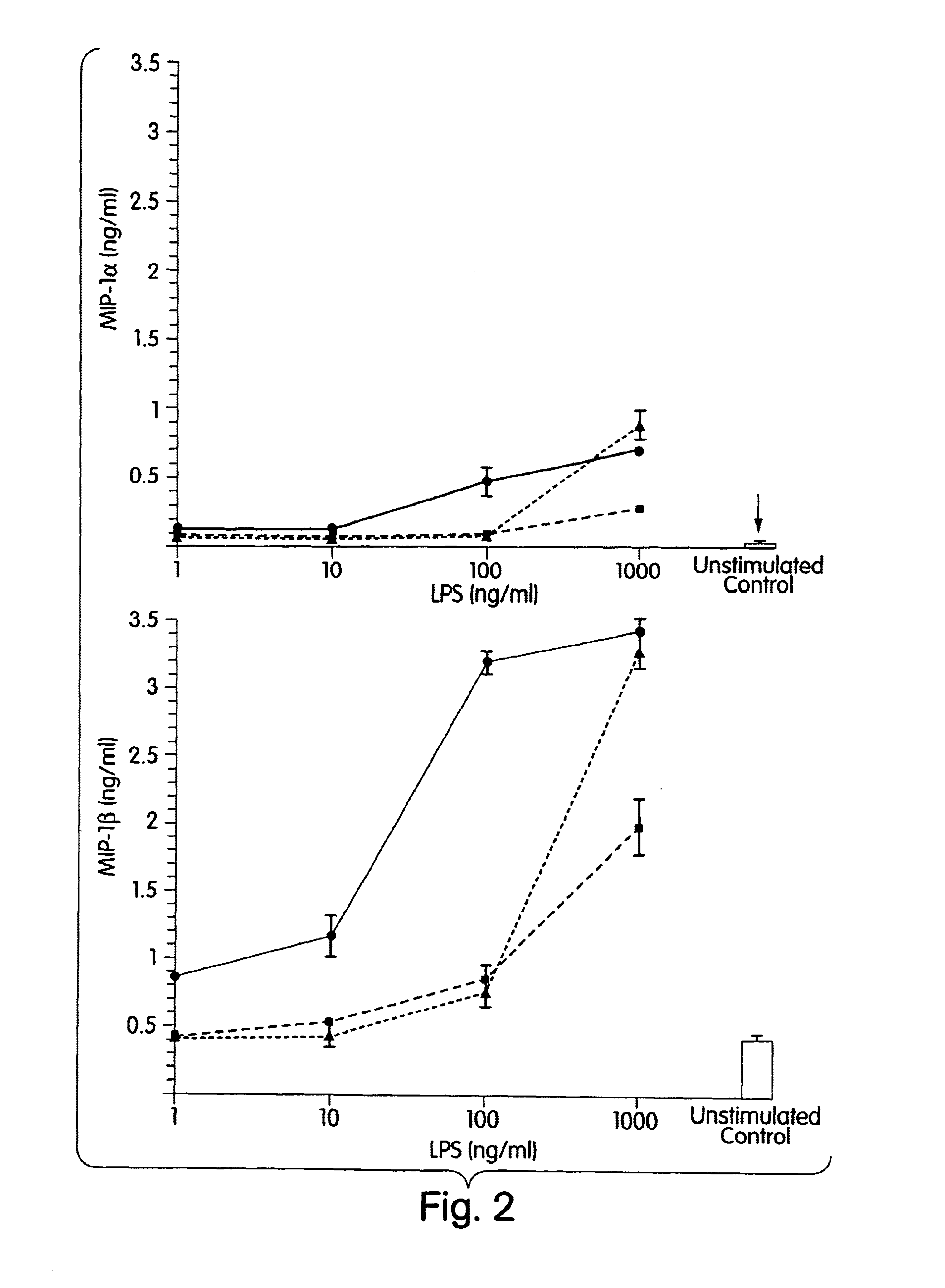
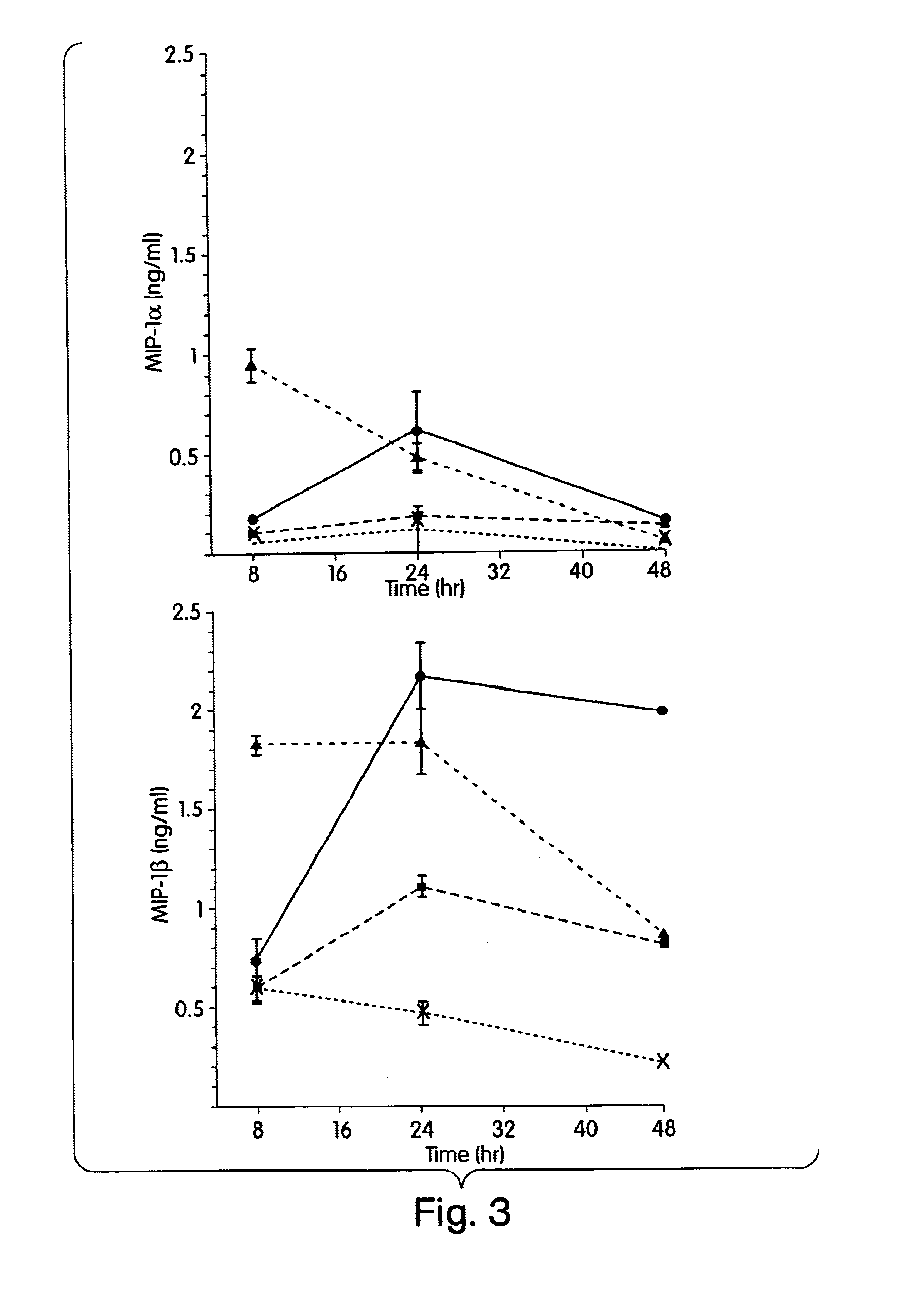
The present inventors have found that certain preparations containing LPS and / or lipid A variants, derivatives, and / or analogs demonstrate non-pyrogenic properties and exhibit anti-viral activities. In particular, non-pyrogenic preparations of LPS, lipid A, LPS antagonists and lipid A antagonists, and derivatives thereof induce beta chemokine secretion, such as MIP-1beta, but not proinflammatory cytokines, such as TNFalpha, IL-1beta and IL-6. Non-pyrogenic preparations of the invention have been demonstrated by the Applicant to suppress HIV replication in human peripheral blood monocytes, as described by way of example herein. The present invention provides preparations of LPS or lipid A variants, analogs and derivatives of decreased or absent pyrogenicity which can be used as therapeutics for the treatment or prevention of immunodeficiency virus infection and its consequences.
Owner:HONE DAVID M +2
Preparation method and application of tumour cell specific polyclonal T cells
InactiveCN104630146AHigh killing efficiencyApparent tumor cell specificityMammal material medical ingredientsBlood/immune system cellsCell specificPeripheral blood mononuclear cell
The invention relates to the technical fields of molecular biology and cellular immunology and in particular relates to a preparation method of tumour cell specific polyclonal T cells. The preparation method of the tumour cell specific polyclonal T cells comprises the following steps: (1) obtaining peripheral blood mononuclear cells; (2) mixing the obtained peripheral blood mononuclear cells with gene modifying tumour cells; and (3) resuspending the obtained mixed cells in a culture medium containing cell factors which can maintain cell growth, proliferation and differentiation. The prepared tumour cell specific polyclonal T cells are mainly applied to medicines which are sued for preventing or treating cancers. The preparation method of the tumour cell specific polyclonal T cells has the advantages that the prepared tumour cell specific polyclonal T cells have the characteristics of large quantity, high specificity and strong killing capability; besides, a technology is simple, and the preparation cost is obviously reduced compared with the prior art.
Owner:马飞
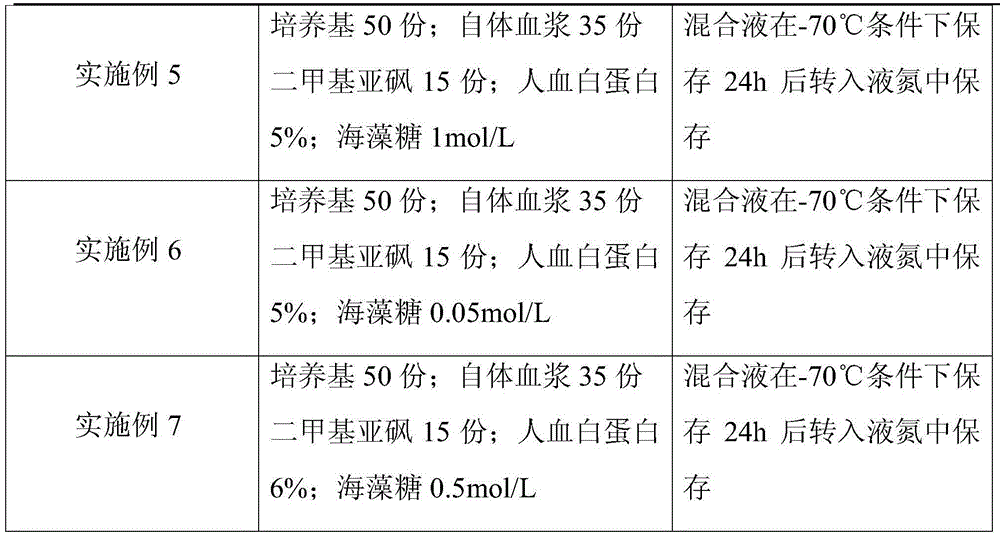
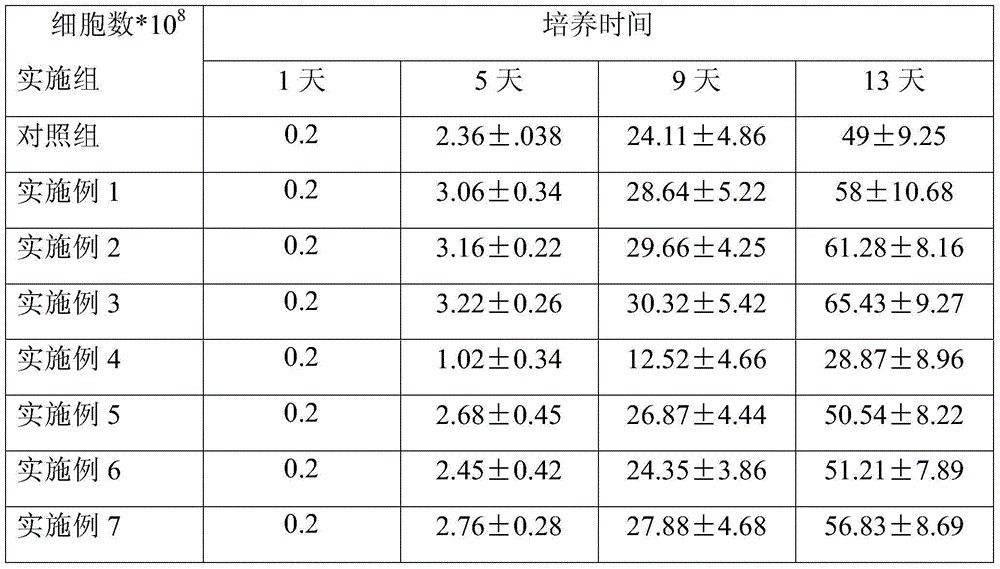
Peripheral blood mononuclear cell serum-free freezing medium and freezing method
ActiveCN104719282AImprove securityTo ensure the effect of freezingDead animal preservationSerum igePeripheral blood mononuclear cell
The invention discloses a peripheral blood mononuclear cell serum-free freezing medium. The peripheral blood mononuclear cell serum-free freezing medium is prepared from the following components in percentage by volume: 5 to 20% of dimethyl sulfoxide, 0.5 to 10% of dextran 40, and the balance of plasmalyte A. The invention further discloses a method for freezing peripheral blood mononuclear cells through the peripheral blood mononuclear cell serum-free freezing medium. Compared with the prior art, the freezing medium is free of animal serum, human serum and a cell culture medium, and has a good freezing effect; the cells can be directly applied to the clinic after resuscitation and can be also induced in vitro into immune cells NKT and CIK and have high application value in the clinic.
Owner:GUANGZHOU SALIAI STEMCELL SCI & TECH CO LTD
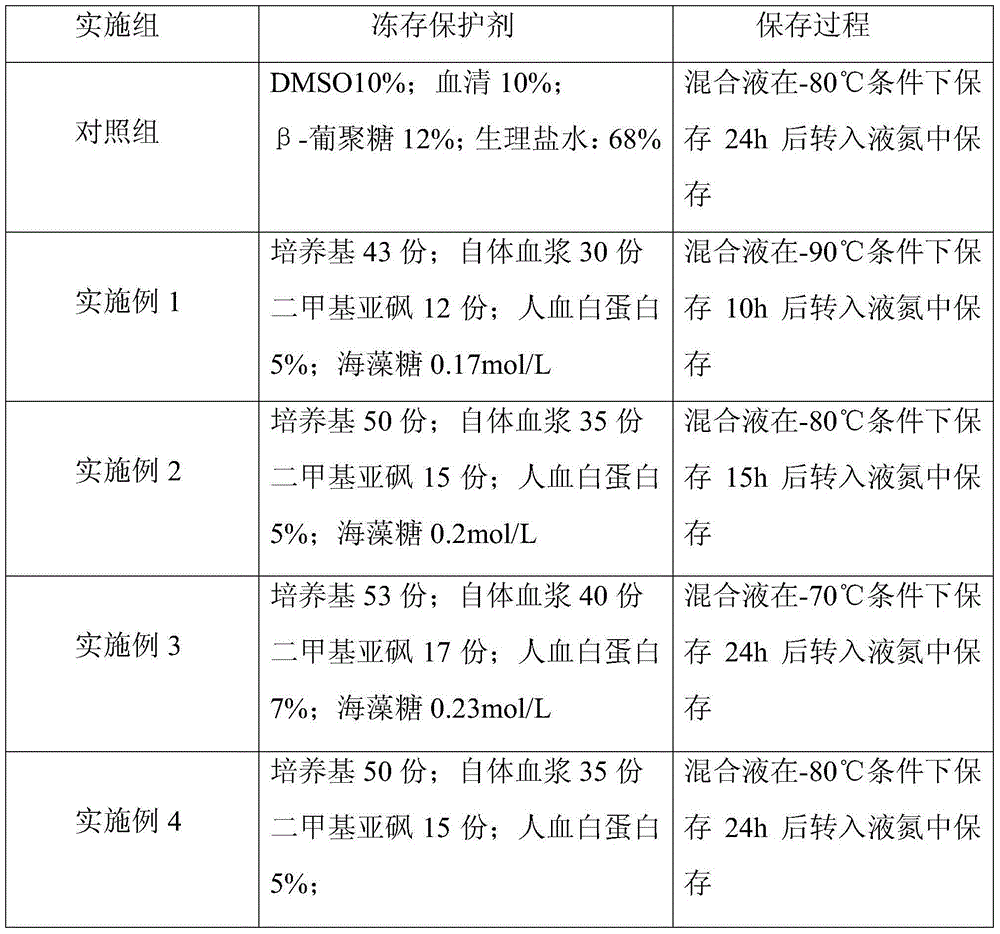
Cryoprotective agent of peripheral blood mononuclear cells and preservation method of cryoprotective agent
ActiveCN104082277AImprove survival rateImprove cell activityDead animal preservationPeripheral blood mononuclear cellMotility
The invention discloses a cryoprotective agent of peripheral blood mononuclear cells. The cryoprotective agent is prepared from the following ingredients in parts by weight: 43-53 parts of a culture medium, 30-40 parts of auto-plasma, 12-17 parts of dimethyl sulfoxide, 5-7% of human serum albumin, and the balance being 0.17-0.23mol / L trehalose. The invention discloses a preservation method of the cryoprotective agent. The preservation method comprises the steps of collecting fresh anticoagulation blood, preparing peripheral mononuclear cells into cell suspension by using human serum albumin, adding the cryoprotective agent with the same volume as that of the cell suspension, mixing uniformly, preserving for 10-20h at below 90-below-70-below DEG C, and preserving in liquid nitrogen. According to the cryoprotective agent and the preservation method thereof, a cryopreserved cell has the high motility rate and the strong cell activity and can shorten the Cytokine Induced Killer (CIT) cell induced amplification period.
Owner:CHENGDU QINGKE BIOTECH
Efficient multiplication CTL preparation method killing tumors in targeted mode
InactiveCN103923880AEfficient proliferative abilityInhibition of differentiationBlood/immune system cellsSerum free mediaPeripheral blood mononuclear cell
The invention discloses an efficient multiplication CTL preparation method killing tumors in a targeted mode. The CTL preparation method comprises the following steps: (a) removing CD4+CD25+Treg cells through immunomagnetic bead negative sorting; (b) arranging mixed cells in a serum-free medium for cultivation, and obtaining suspension cells and adherent cells; (c) adding GM-SCF and IL-4 in the adherent cells, culturing the cells for five days; in the sixth day, adding a tumour cell holoantigen, and in the seventh day, adding TNF-alpha and IL-27; (d) transferring the suspension cells to a culture flask wrapped by a CD3 monoclonal antibody and recombinant human fibronectin, adding IFN-gamma, in the second day, adding IL-2, IL-12 and the IL-27, and culturing the mixture till the eighth day to obtain CIK cells; (e) mixing the CIK cells and mature DC cells, and adding the IL-12, IL-7 and an anti-CD 28 monoclonal antibody for cultivation; in the third day, adding an anti-CTLA-4 monoclonal antibody, and then culturing the mixture for four days. According to the efficient multiplication CTL preparation method killing tumors in the targeted mode, efficiency of in-vitro CTL cell proliferation is improved, activity of killing the tumor cells in the targeted mode is improved, transformation of peripheral blood mononuclear cells to the CD4+CD25+Treg cells is inhibited.
Owner:四川全组生命科技有限公司
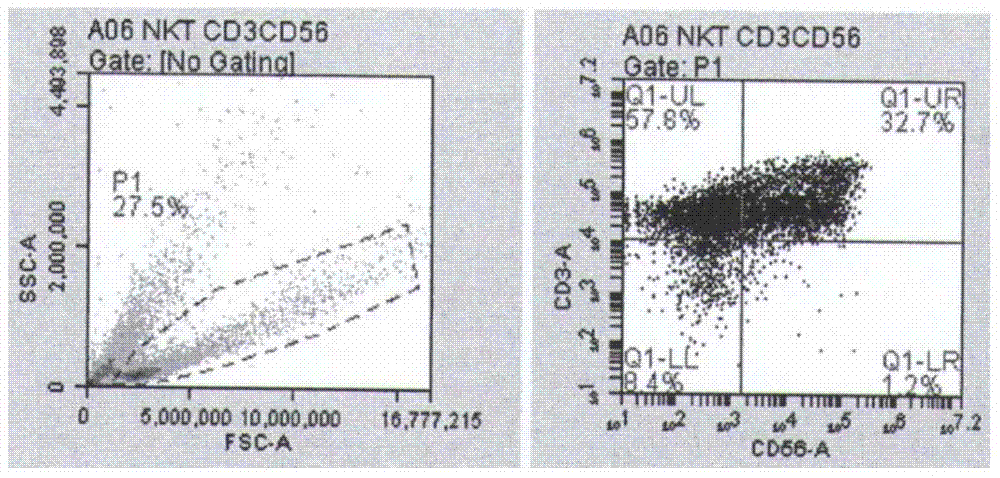
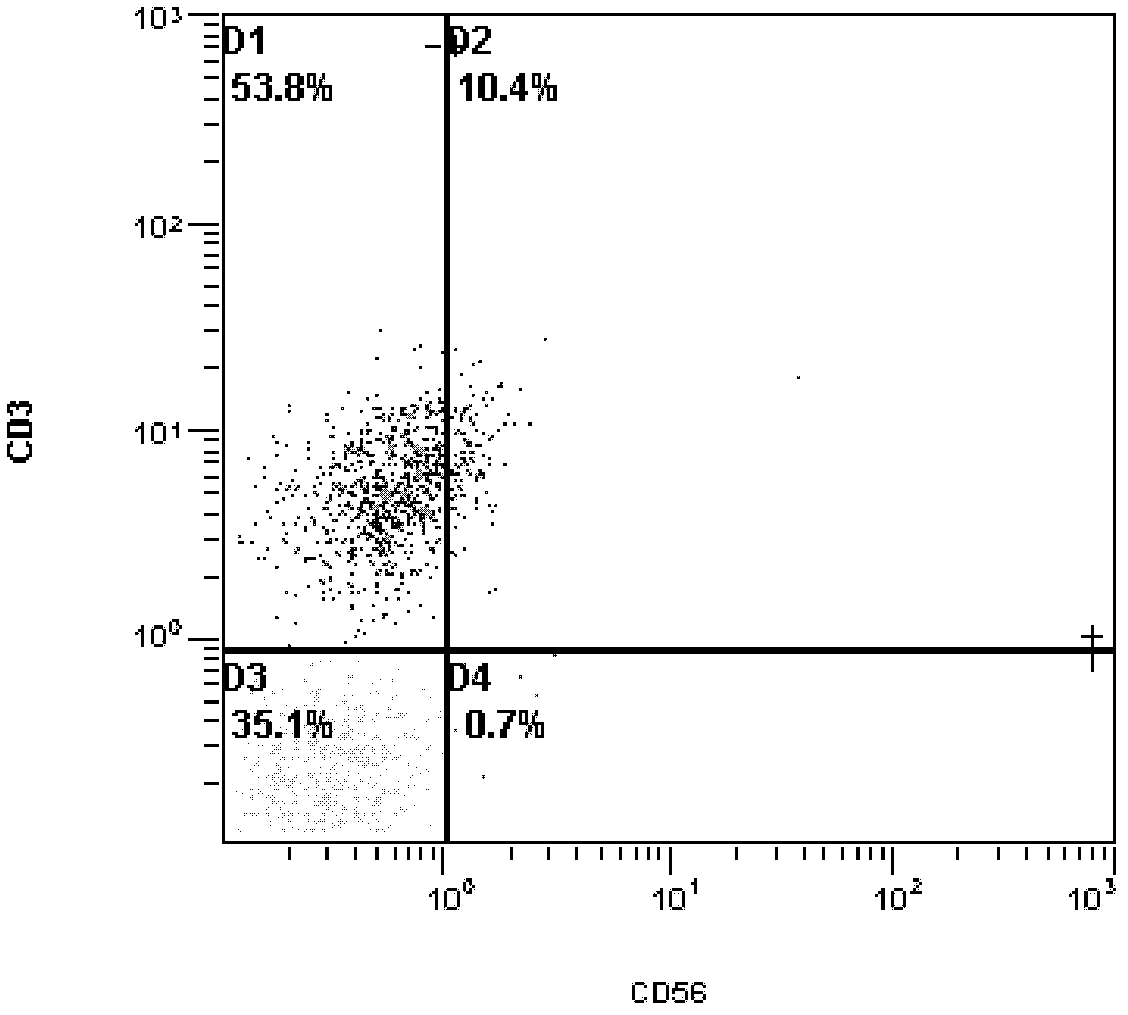
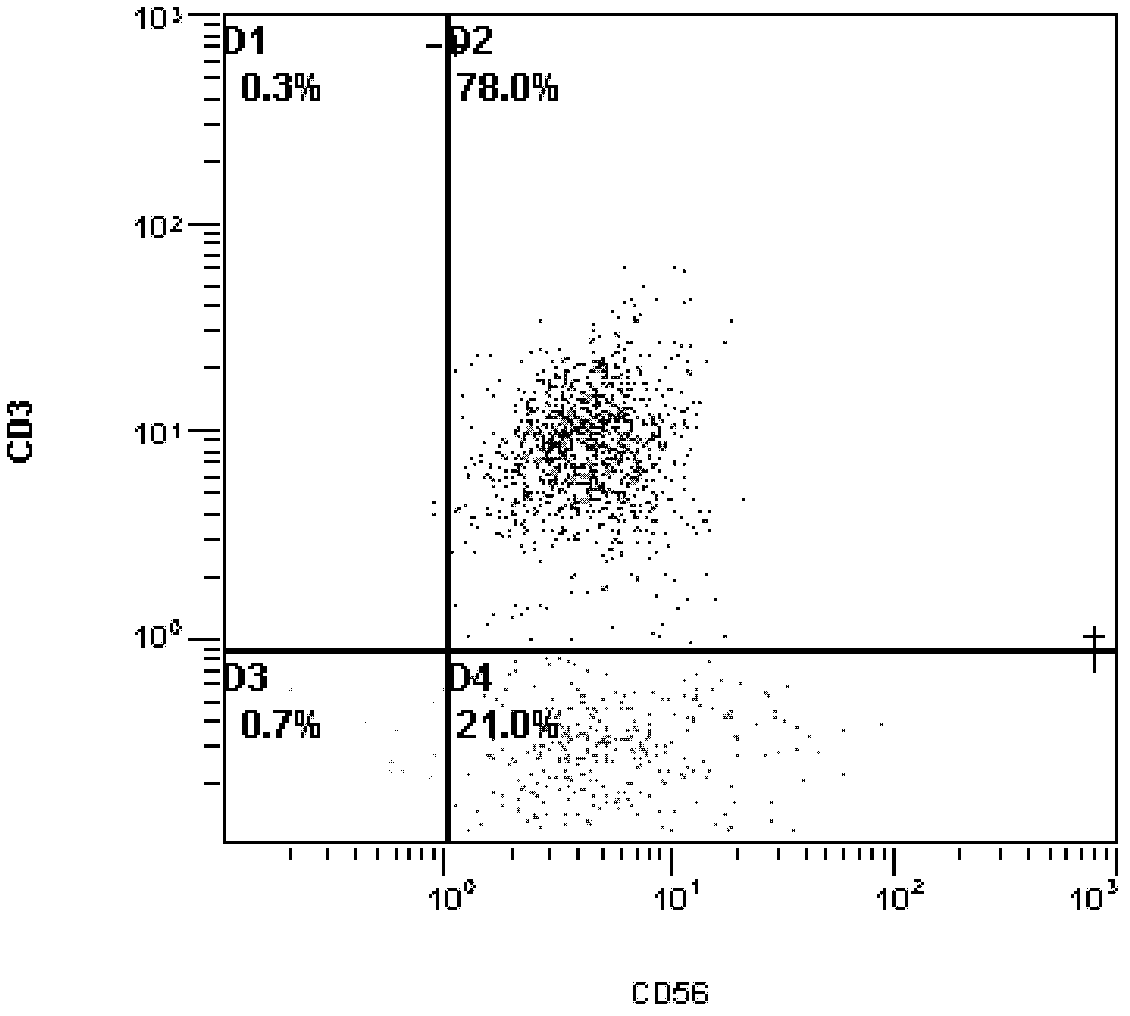
Method for culturing natural killer (NK) and/or natural killer T (NKT) cells
InactiveCN102676453ATo proliferateHigh purityBlood/immune system cellsPeripheral blood mononuclear cellMagnetic bead
The invention discloses a method for culturing natural killer (NK) and / or natural killer T (NKT) cells. The method comprises the following step: inoculating isolated NK and / or NKT cells into a culture system A for culture to obtain propagated NK and / or NKT cells, wherein the culture system A consists of a buffer solution containing CD3 antibody and / or Retronectin and inducing factors. Experiments prove that peripheral blood mononuclear cells (PBMC) extracted from peripheral blood are separated and enriched through magnetic beads, high-purity CD56+ cells are obtained, two proteins, namely Retronectin and CD3mAb are added into an in-vitro culture system for joint stimulation, and IL-2 and IL-5 factors are used for assisting in induction, so that a culture method capable of obtaining massive NK and NKT cells with high killing activity is established.
Owner:CANCER INST & HOSPITAL CHINESE ACADEMY OF MEDICAL SCI
Methods for prognosis and treatment of solid tumors
InactiveUS20060194211A1Microbiological testing/measurementImmunoglobulins against animals/humansFavorable prognosisPeripheral blood mononuclear cell
Solid tumor prognosis genes, and methods, systems and equipment of using these genes for the prognosis and treatment of solid tumors. Prognosis genes for a solid tumor can be identified by the present invention. The expression profiles of these genes in peripheral blood mononuclear cells (PBMCs) are correlated with clinical outcome of the solid tumor. The prognosis genes of the present invention can be used as surrogate markers for predicting clinical outcome of a solid tumor in a patient of interest. These genes can also be used to select a treatment which has a favorable prognosis for the solid tumor of the patient of interest.
Owner:WYETH LLC
Treatment and prevention of immunodeficiency virus infection by administration of non-pyrogenic derivatives of lipid A
The present inventors have found that certain preparations containing LPS and / or lipid A variants, derivatives, and / or analogs demonstrate non-pyrogenic properties and exhibit anti-viral activities. In particular, non-pyrogenic preparations of LPS, lipid A, LPS antagonists and lipid A antagonists, and derivatives thereof induce β chemokine secretion, such as MIP-1β, but not proinflammatory cytokines, such as TNFα, IL-1β and IL-6. Non-pyrogenic preparations of the invention have been demonstrated by the Applicant to suppress HIV replication in human peripheral blood monocytes, as described by way of example herein. The present invention provides preparations of LPS or lipid A variants, analogs and derivatives of decreased or absent pyrogenicity which can be used as therapeutics for the treatment or prevention of immunodeficiency virus infection and its consequences.
Owner:UNIV OF MARYLAND BIOTECH INST
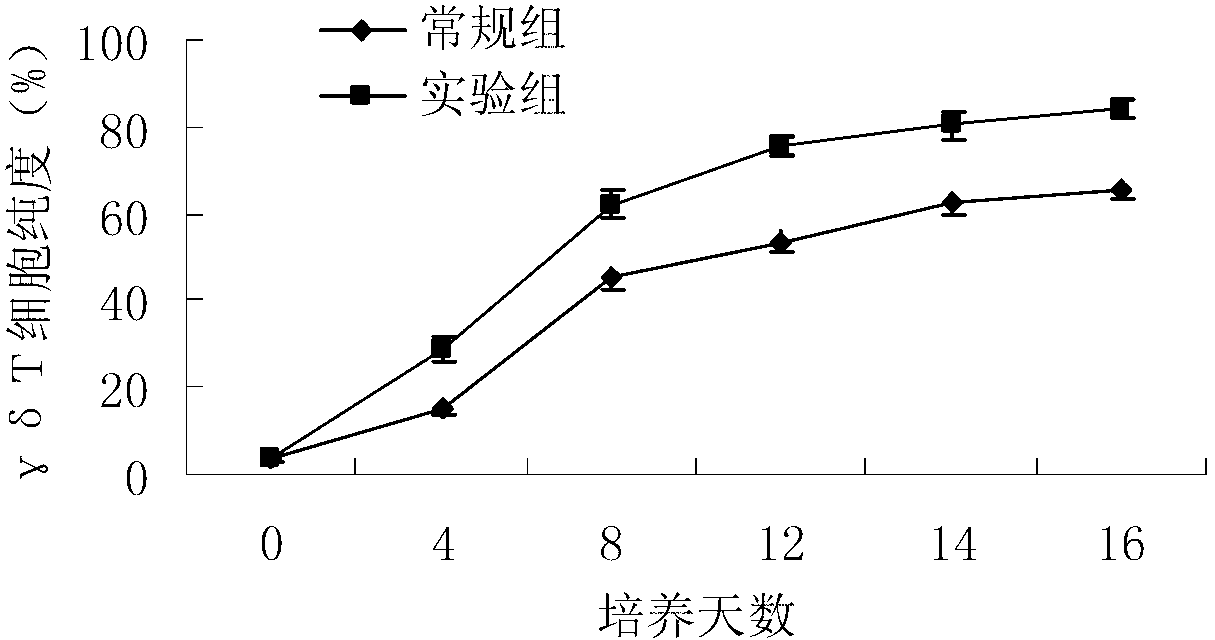
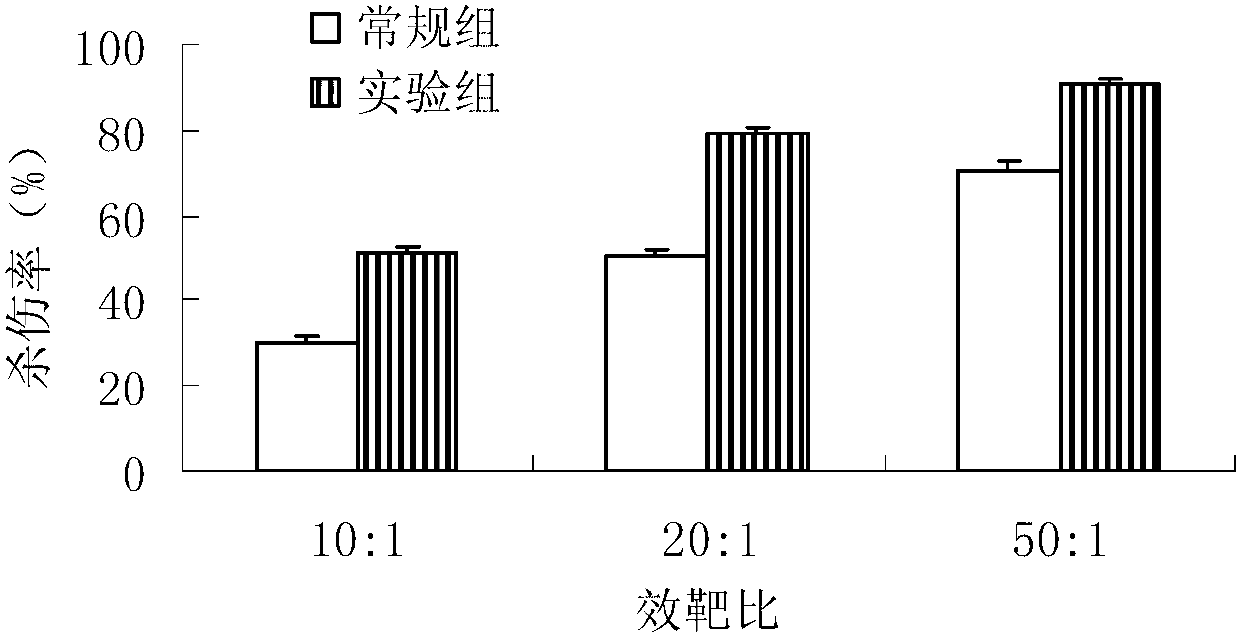
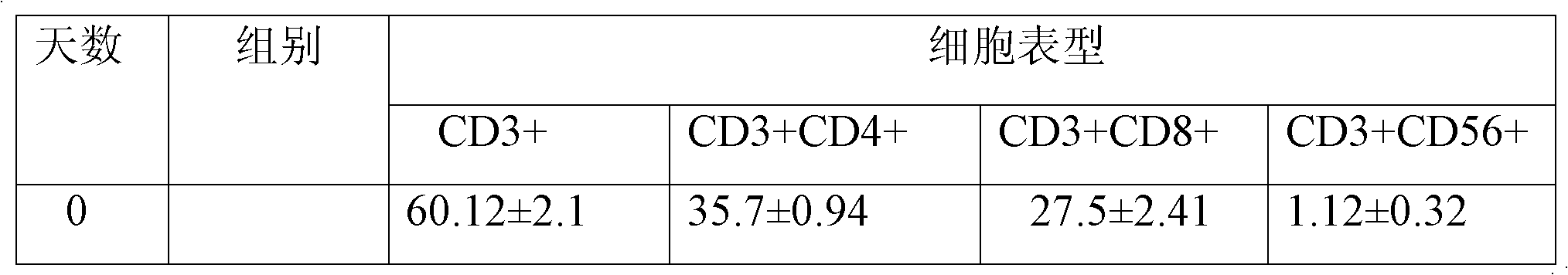
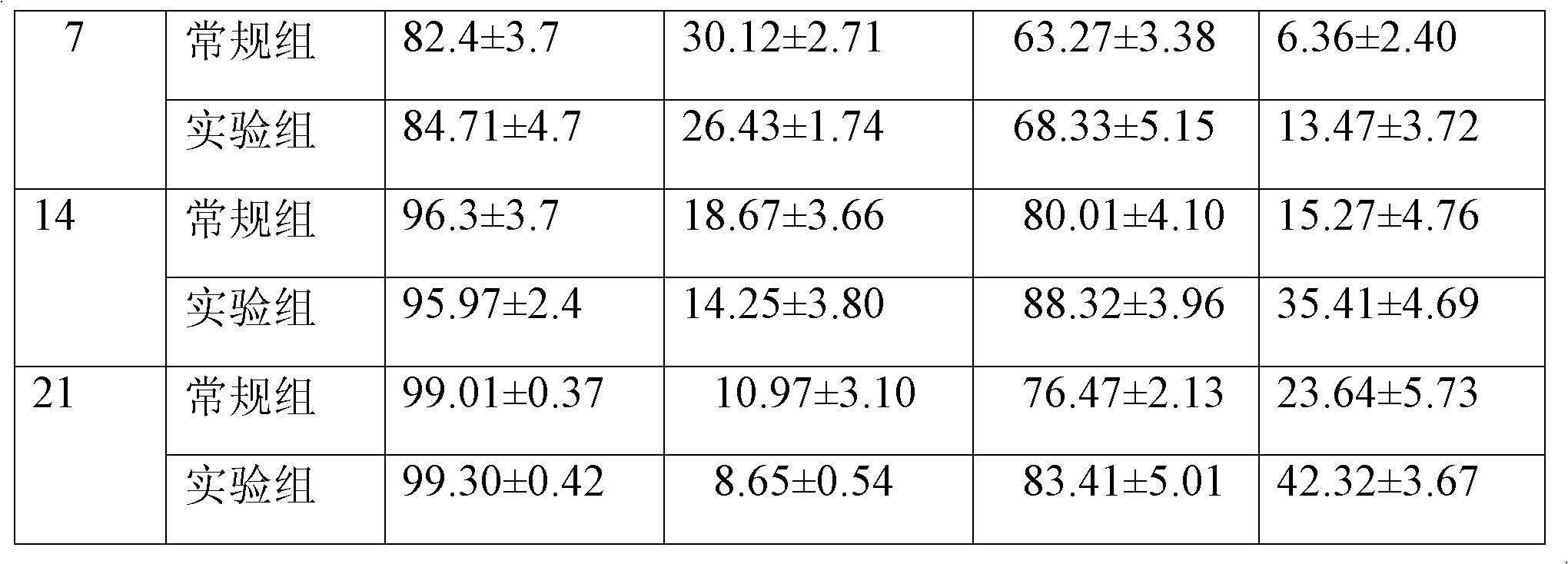
Method for in-vitro amplification of gamma-delta-T cells
InactiveCN102994448AFully stimulatedInhibit apoptosisBlood/immune system cellsPeripheral blood mononuclear cellMicrobiology
The invention relates to a method for culturing gamma-delta-T cells, and in particular relates to a method for in-vitro amplification of gamma-delta-T cells, wherein the method comprises the following operating steps of: pre-coating a T75 culture bottle by a TCR-gamma-delta resisting antibody and CD28McAb for later use use; isolating the peripheral blood mononuclear cell (PBMC) of a patient; regulating the PBMC concentration to 1*10<6> 6 / ml by a serum-free culture medium which contains 5% of autologous plasma, and transferring PBMC cell suspension into the T75 culture bottle; adding an initial culture medium which contains proper concentrations of Zoledronat, HSP70, 1L-2, 1L-7 and 1L-15; culturing in a saturated humid environment containing 5% of CO2 at 37 DEG C; depending on growth situation of the cell, changing the culture medium every 2-3days, to control the cell concentration at about 2.5*10<6> / ml; meanwhile, compensating full doses of Zoledronat, HSP70, 1L-2, 1L-7 and 1L-15; and continuously culturing for 12-16days, to obtain a great amount of gamma-delta-T cells which are comparatively high in purity.
Owner:SHANGHAI CLAISON BIOTECH
Method for amplifying cytokine induced kill cells (CIK) and CIK cell preparation
ActiveCN102352342AStrong mitogenic effectIncrease the level of amplificationMammal material medical ingredientsBlood/immune system cellsPeripheral blood mononuclear cellCell separation
The invention relates to a method for amplifying cytokine induced kill (CIK) cells and a CIK cell preparation, which belong to the field of in-vitro culture of immune cells. The method concretely adopts the following procedures that: a, lymphocyte cell separation liquid is used for separating out peripheral blood mononuclear cells (PBMC), a culture bag is covered by CD3mAb and CD137mAb in advance, the concentration of the PBMC obtained through separation is regulated to 1*10<6> / ml by a serum-free culture medium, in addition, IFN-gamma is added to obtain the final concentration being 1000 mu / ml, and the materials are transferred to the culture bag to be cultured; b, CD3mAb, CD28mAb and CD137mAb are added after the culture for 24h, in addition, the prepared serum-free culture medium is added, IL-1alpha, IL-2, IL-12 and IL-15 are added into the prepared serum-free culture medium, and obtained CIK cells are collected through centrifugation after the continuous culture for 7 to 21 days; and c, in the culture process of the step b, the cells in the culture bag are counted every three days, in addition, the culture medium is supplemented according to the concentration of the cells, and the CD3mAb, the CD28mAb and the CD137mAb are added to the corresponding concentration every six days, so the CIK cell generative cell times and the cytotoxin activeness are improved.
Owner:SHANGHAI CLAISON BIOTECH
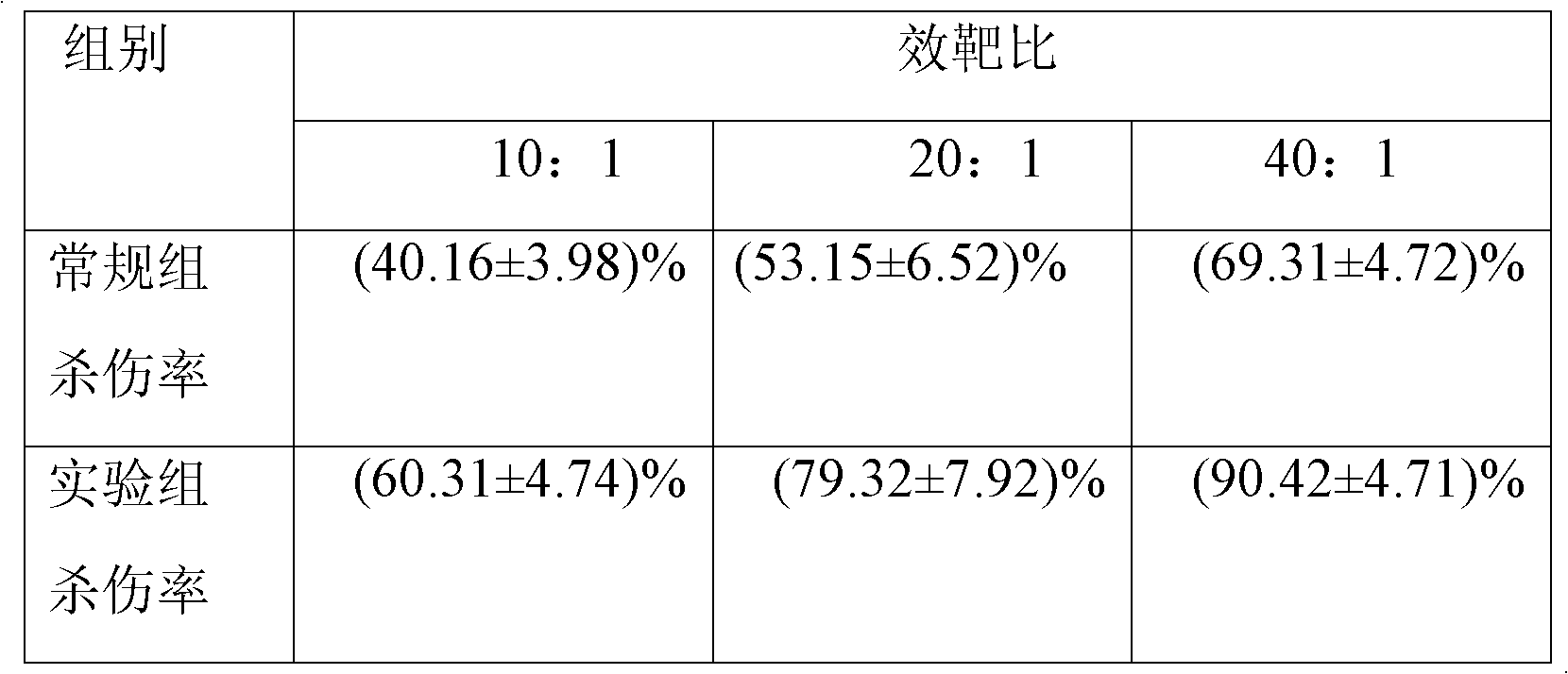
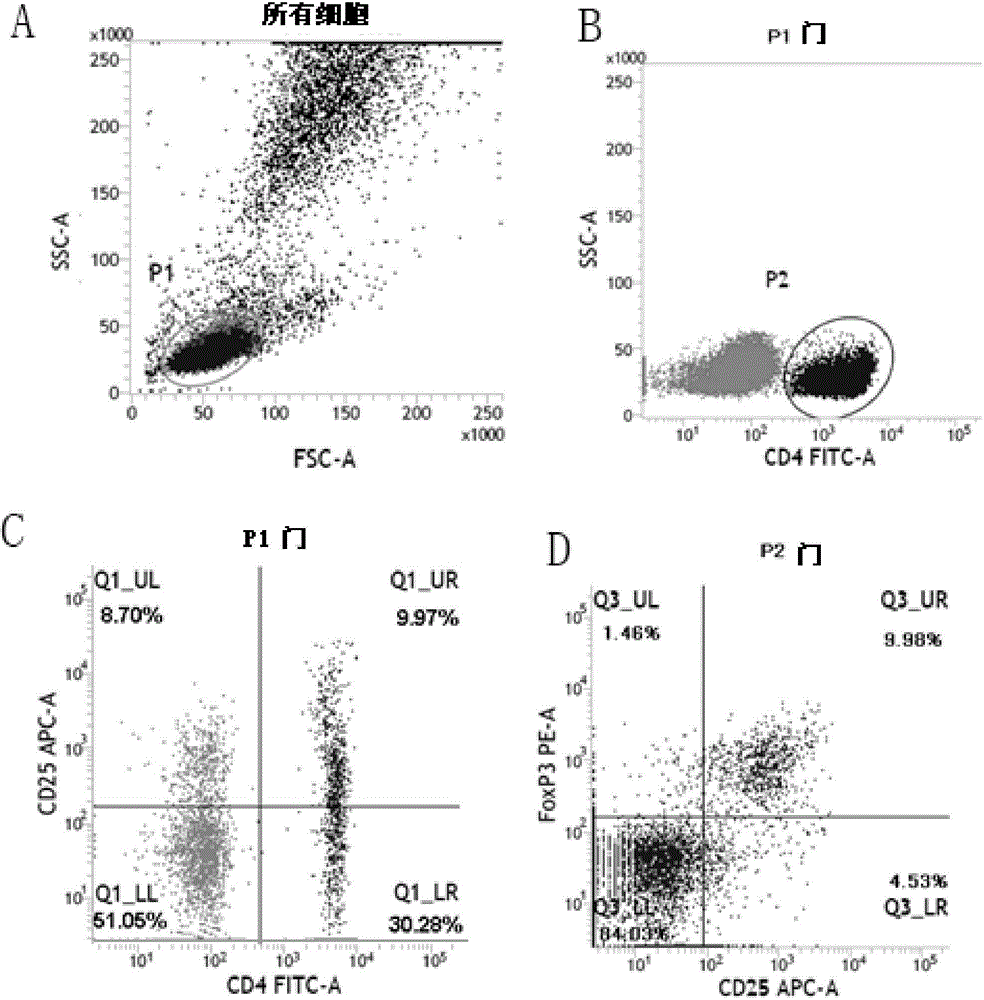
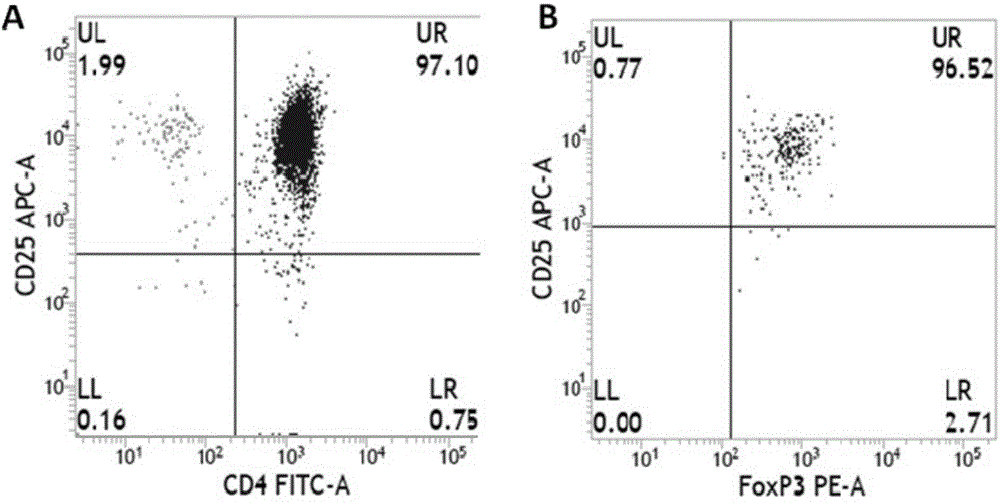
Sorting and amplification method of human peripheral blood CD4+CD25+Foxp3+ regulatory T cells
The invention discloses a sorting and amplification method of human peripheral blood CD4+CD25+Foxp3+ regulatory T cells, and belongs to the field of cell sorting amplification. The sorting and amplification method of the human peripheral blood CD4+CD25+Foxp3+ regulatory T cells specifically comprises the following steps: (1) separating peripheral blood mononuclear cells; (2) performing MACS (magnetic activated cell sorting) on CD4+CD25+Foxp3+Treg cells; and (3) performing in-vitro culture and amplification on the CD4+CD25+Foxp3+Treg cells, and finally performing amplification and culture to obtain the human peripheral blood CD4+CD25+Foxp3+ regulatory T cells. According to the sorting and amplification method disclosed by the invention, human peripheral blood CD4+CD25+Treg cells are successfully separated by using an immuno-magnetic bead two-step process, and the purity of the human peripheral blood CD4+CD25+Treg cells is subjected to flow identification to ensure that the purity of the finally-obtained CD4+CD25+Treg cells can reach 97%, and the activity of the cells can reach more than 95%.
Owner:SUN YAT SEN MEMORIAL HOSPITAL SUN YAT SEN UNIV
Preparation method for HLA-A0201 limited antigen specificity CTL (cytotoxic T lymphocyte)
ActiveCN102618498ASimple and fast operationHigh purityBlood/immune system cellsDendritic cellPeripheral blood mononuclear cell
The invention belongs to the field of biotechnology development and application research, and discloses a preparation method for an HLA-A0201 limited antigen specificity CTL (cytotoxic T lymphocyte). The method comprises the following steps: collecting eripheral mononuclear cells one by one, so as to enrich and purify a CD8+T lymphocyte; stimulating the CD8+T lymphocyte with a mature dendritic cell bearing an HLA-A0201 limited target antigen polypeptide, and promoting the growth of the T lymphocyte through the combination of rhIL-2 and rhIL-7; purifying the target CTL according to a Tetramer marking method and a flow cell sorting method; stimulating the growth of the target CTL according to a solid phase coated anti-human-CD3mAb and IL-2; adding an autologous PBMC (peripheral blood mononuclear cell) to enhance the actification of the target CTL; and adding rhIL-15 for enlarging cultivation, collecting and identifying. The CTL prepared according to the method has the advantage that the purity, the proliferation capability, the cytotoxicity and the CTL-CM proportion are high, so as to be used for immunological therapy of tumors and the like.
Owner:江苏得康生物科技有限公司
Method for preparing NK (natural killer) cell
InactiveCN103484429AHigh purityBlood/immune system cellsPeripheral blood mononuclear cellMultiplication rate
The invention provides a method for efficiently preparing an NK cell, which can improve the multiplication rate and the purity of the NK cell through combination of stimulation effects of cell factors and feeder cells. The method is mainly characterized by comprising the steps as follows: NCR3LG1 and m IL-15 are transfected to a K562 cell simultaneously, m IL-15 can be used for adjusting activation and multiplication of the NK cell, NCR3LG1 serving as a ligand of NKp30 which is one of main activated receptors on the surface of the NK cell can effectively stimulate activation of the NK cell, and NCR3LG1 and m IL-15 have a synergistic effect; and PBMC(peripheral blood mononuclear cells) can be multiplied over 500 times in 21 cultivation days through stimulation of factors of freely added IL-2, IL-21 and the like, and a proportion of CD3-CD56+NK cell exceeds 70%; and up to now, a research report that NCR3LG1 and m IL-15 are transfected to the feeder cells simultaneously and jointly stimulate and activate the NK cell in combination of free cell factors is absent. The invention firstly provides a method for jointly cultivating and preparing the NK cell in combination of the feeder cells and the free factors.
Owner:青岛麦迪赛斯生物科技有限公司
Preparation method of high-purity, high-multiplication capacity and high-cytotoxin activity CIK (cytokine induced kill) cell
ActiveCN102154206AIncrease the number ofHigh activityMammal material medical ingredientsBlood/immune system cellsPhytohemagglutininsPeripheral blood mononuclear cell
The invention belongs to the technical field of cell culture in vitro, and particularly relates to a preparation method of high-purity, high-multiplication capacity and high-cytotoxin activity CIK (cytokine induced kill) cell. The method comprises the following steps: collecting and separating peripheral blood mononuclear cell of a patient, eliminating CD4+CD25+Treg cell by means of Mini MACS (magnetic active cell sorting) method, and sorting to obtain CD3+, CD4+ and CD8+T cells; and putting the obtained cells into culture solution containing phytohemagglutinin (PHA), so that the PHA concentration in the suspension liquid is 100ng / ml, hatching for 24h under the culture condition of 5% CO2 at 37 DEG C, transferring the hatched suspension liquid into a cell culture bottle coated by CD3 monoclonal antibody (1mug / ml), adding IFN (interferon)-gamma (1000U / ml), adding IL (interleukin)-2(500U / ml) and IL (interleukin)-21(1000U / ml) after 48h, compensating sodium selenite-containing (0.005mg / L)cell culture after four days, and continuously culturing for 7-14 days to obtain the high-purity, high-multiplication capacity and high-cytotoxin activity CIK (cytokine induced kill) cell. The quantity, the activity and the purity of the CIK cell which is prepared by the method and amplified in vitro are improved, so that the antineoplastic function of the CIK is enhanced.
Owner:郑骏年
Method for in-vitro amplification of NK cells and NK cells obtained by same
InactiveCN105754942AHigh purityReduce usageBlood/immune system cellsCell culture active agentsPeripheral blood mononuclear cellNatural Killer Cell Inhibitory Receptors
The invention discloses a method for in-vitro amplification of NK cells and the NK cells obtained by the same. The method comprises the following steps: taking peripheral blood mononuclear cells (PBMC) separated out from peripheral blood as seed cells, adding the cells into a culture bottle pre-coated with CD16Mab when induced culture begins and enlarged culture is carried out every time, performing continuous stimulation on the cells, and finally obtaining a large amount of high-purity NK cells. Under the situation that the stimulation is performed without using trophoblast cells, the large amount of high-purity NK cells is finally obtained through the method of performing continuous stimulation by coating the culture bottle with CD16Mab, and the use amount of cell factors is reduced.
Owner:TIANJIN PURUI SAIER BIOLOGICAL TECH CO LTD
In-vitro large-scale amplification method of natural killer cells
PendingCN106011061ASolve the problem of low efficiency of in vitro amplificationHigh purityCulture processBlood/immune system cellsPeripheral blood mononuclear cellMicrobiology
The invention discloses an in-vitro large-scale amplification method of natural killer cells. The method comprises the following steps: collecting and separating peripheral blood mononuclear cells; sorting natural killer cells; culturing the natural killer cells; and collecting the natural killer cells. The method utilizing immunomagnetic beads to sort the natural killer cells has the advantages of simplicity in establishing and operation, and high comprehensive efficiency; and the effect of the method adopting in-vitro amplification of the immunomagnetic bead shorted NK cells is 2-3 times the effect of present amplification methods, so the method disclosed in the invention has great application values in the field of in-vitro large-scale amplification.
Owner:GUANGDONG NO 2 PROVINCIAL PEOPLES HOSPITAL
Method and device for separating single karyocyte
ActiveCN101560495ASolve pollutionReduce the amount of mixingBioreactor/fermenter combinationsBiological substance pretreatmentsHuman bodyCord blood stem cell
The invention provides a method and a device for separating a single karyocyte, in particular a method and a device for separating single karyocyte of cord blood, marrow and peripheral blood. The single karyocyte can be separated without a hundred grades purification environment, and the method and the device are not limited by sample numbers and volumes, and has high efficiency, simple structure and no pollution, thereby being applied to laboratory research and human body treatment, and being easy to produce in batch.
Owner:SHENZHEN BEIKE BIOTECH +1
Method for abundantly amplifying NK (natural killer) cells from mononuclear cell of peripheral blood
ActiveCN107022524AAvoid insecurityHigh purityBlood/immune system cellsPeripheral blood mononuclear cellCD16
The invention relates to a method for abundantly amplifying NK (Natural Killer) cells from a mononuclear cell of peripheral blood. The method comprises the following steps of collecting the mononuclear cell of the peripheral blood, first putting the mononuclear cell into a culture bottle enveloped by a CD16 monoclonal antibody, culturing the mononuclear cell, and adding IL-2, IL-15, OK432 and inactivated autoserum into a culture medium; subsequently, transferring the cell into a culture bottle which is not enveloped by the CD16 monoclonal antibody, culturing the cell, adding the IL-2, the IL-15 and the inactivated autoserum into the culture medium; afterwards, transferring the cell into a culture bag, culturing the cell, and adding IL-4 into the culture medium on the last third day, and continuously culturing the cell, so as to obtain abundant NK cells. According to the method, components of animal serum and a tumor cell are not contained; therefore, the safety and the reliability of the NK cells are improved to a great extent; the method has a favorable application prospect.
Owner:SHANGHAI LIFE SCI & TECH CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com