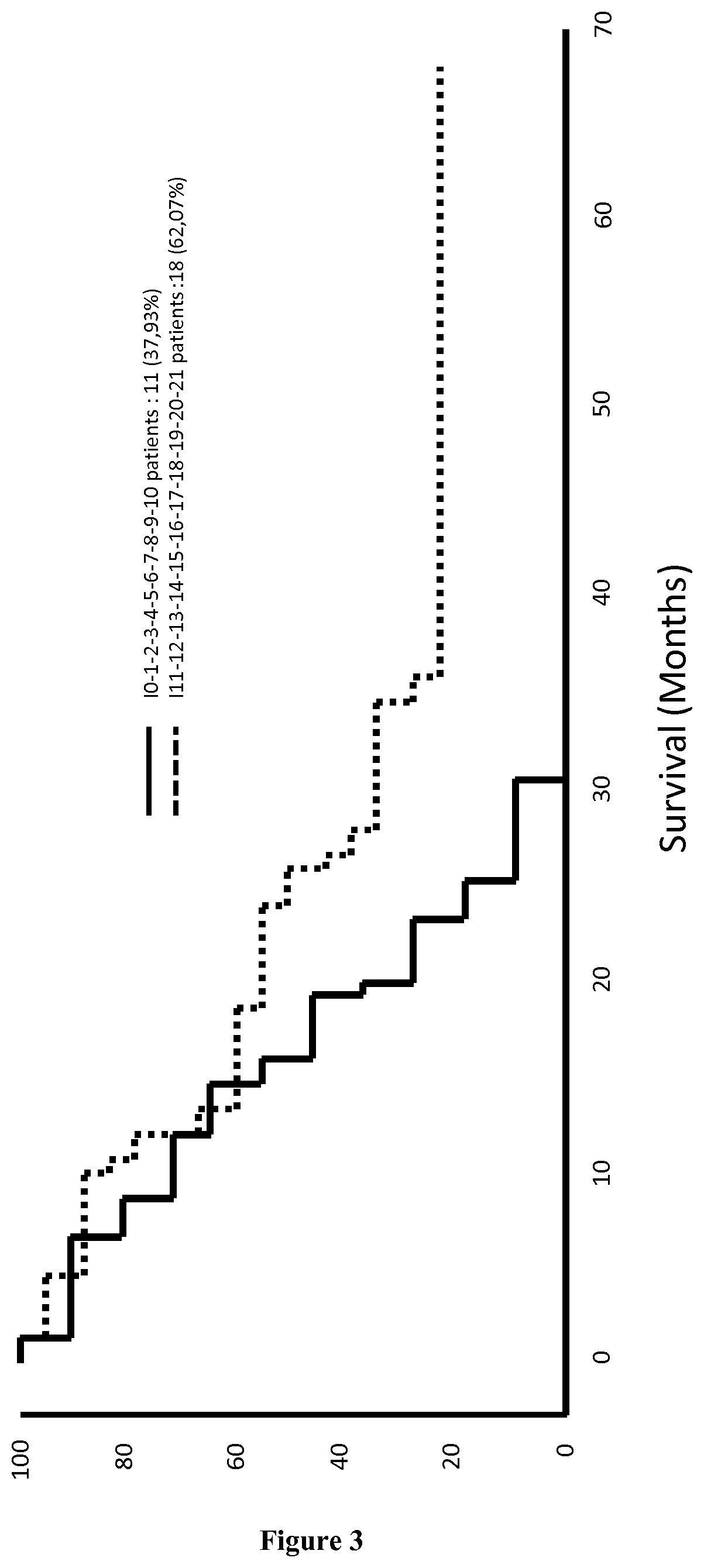
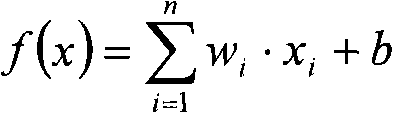Patents
Literature
37 results about "Favorable prognosis" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
A favorable prognosis means a good chance of treatment success. For example, the overall 5-year relative survival rate for testicular cancer is 95%. This means that most men diagnosed with the disease have a favorable prognosis.
Methods for prognosis and treatment of solid tumors
InactiveUS20060194211A1Microbiological testing/measurementImmunoglobulins against animals/humansFavorable prognosisPeripheral blood mononuclear cell
Solid tumor prognosis genes, and methods, systems and equipment of using these genes for the prognosis and treatment of solid tumors. Prognosis genes for a solid tumor can be identified by the present invention. The expression profiles of these genes in peripheral blood mononuclear cells (PBMCs) are correlated with clinical outcome of the solid tumor. The prognosis genes of the present invention can be used as surrogate markers for predicting clinical outcome of a solid tumor in a patient of interest. These genes can also be used to select a treatment which has a favorable prognosis for the solid tumor of the patient of interest.
Owner:WYETH LLC
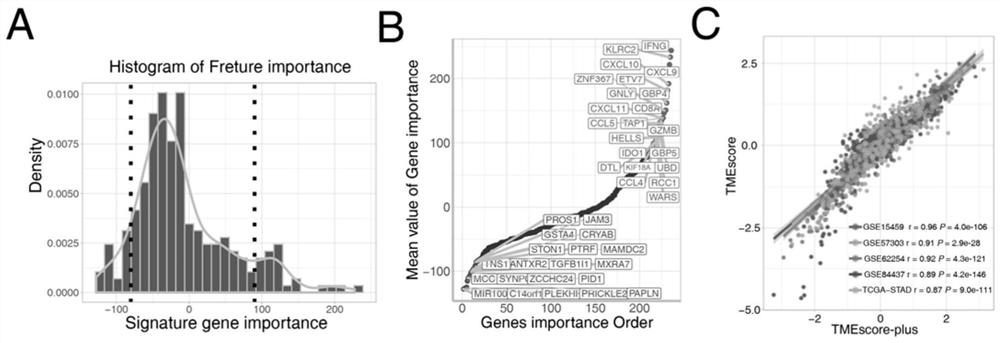
Gene sets and scoring model for evaluating tumor microenvironment, and application of gene sets
ActiveCN112133365ASignificant prognostic differenceGood forecastMicrobiological testing/measurementBiostatisticsFavorable prognosisPrognosis biomarker
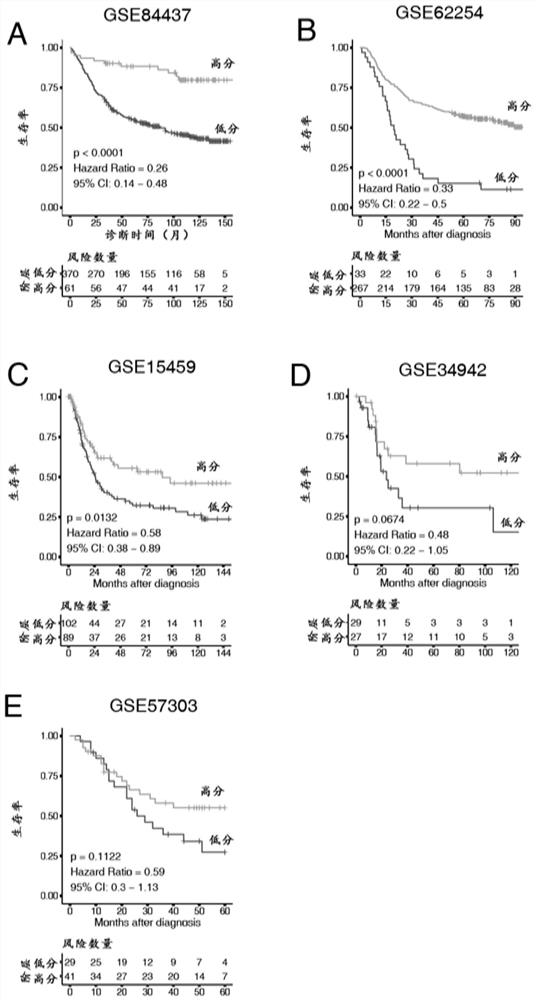
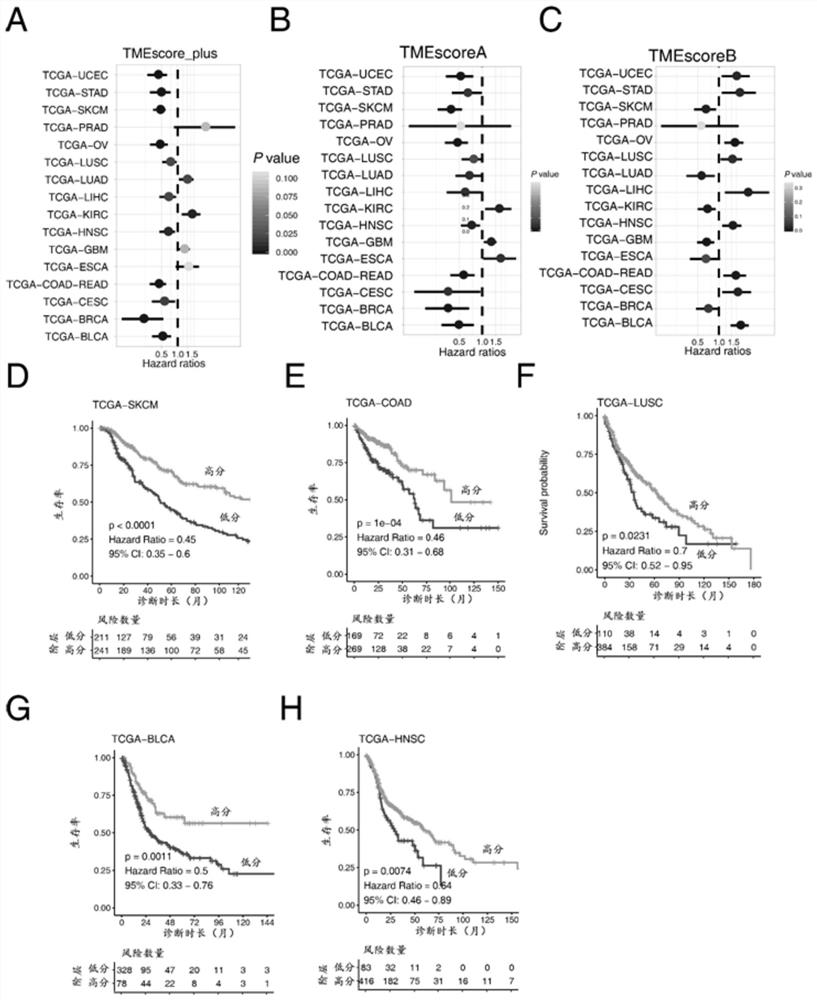
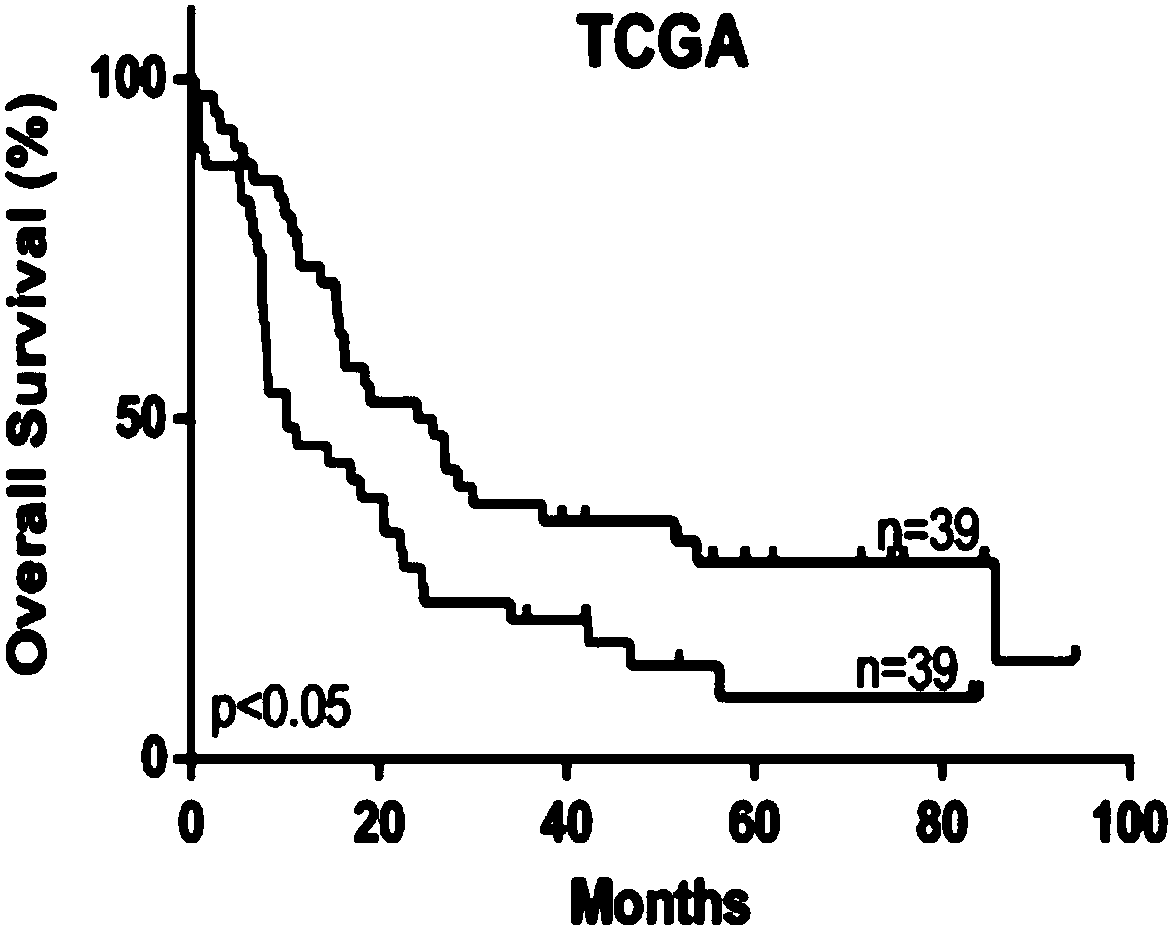
The invention discloses gene sets and a scoring model for evaluating a tumor microenvironment, and application of the gene sets, and belongs to the field of biological information. It is proved for the first time that the gastric cancer has remarkable tumor microenvironment typing, and prognosis difference of gastric cancer patients with different typing is remarkable. According to a constructionmethod of the multi-gene scoring model for evaluating the gastric cancer tumor microenvironment, 44 gene sets capable of representing different microenvironment types are screened out, and the prediction efficiency of the tumor microenvironment multi-gene scoring model corresponding to the gene sets is remarkably better than that of other existing models; and the model is not only a prognostic marker of a gastric cancer patient, but also has a quite remarkable prediction effect in pan-cancer. The model provided by the invention not only can be used as a good prognosis biomarker, but also can be used for predicting checkpoint inhibitor immunotherapy response of various tumors by effectively evaluating the tumor microenvironment, and has important significance and clinical application valuefor better screening immunotherapy benefiting people.
Owner:广东隆宇医疗科技有限公司
Diagnostic methods for determining prognosis of non-small cell lung cancer
InactiveUS20090258350A1Accurate identificationEnhanced informationMicrobiological testing/measurementFavorable prognosisNucleic Acid Probes
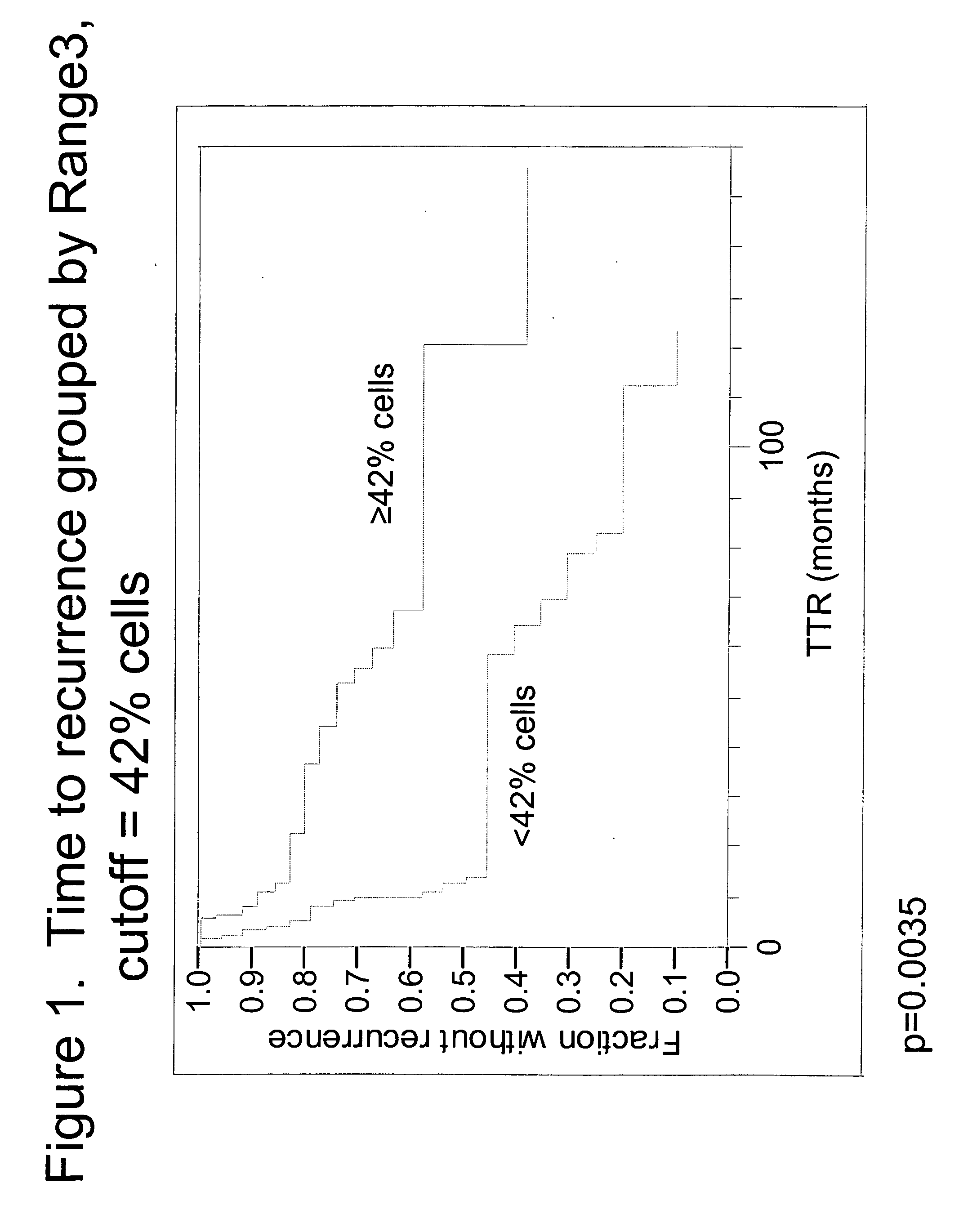
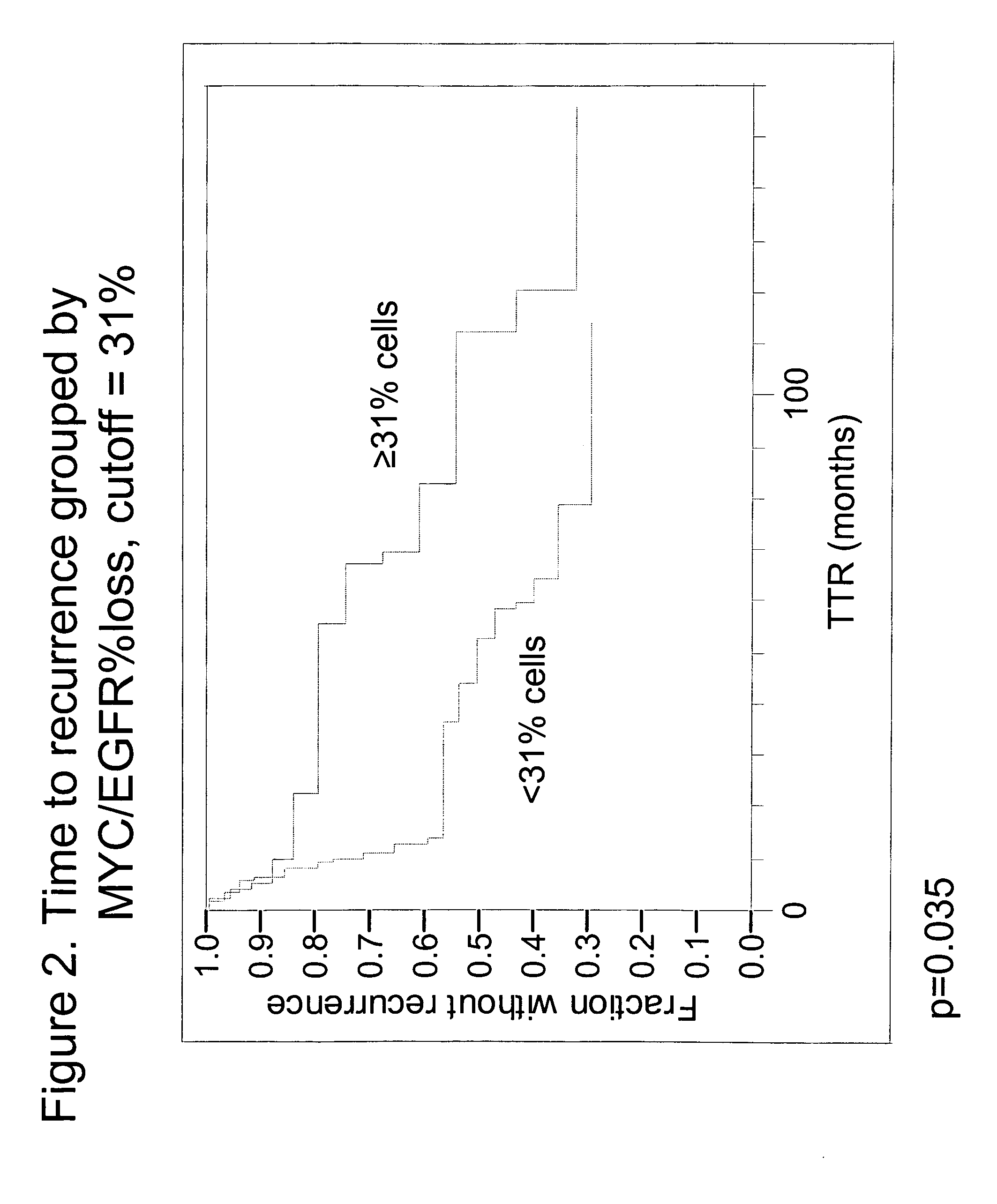
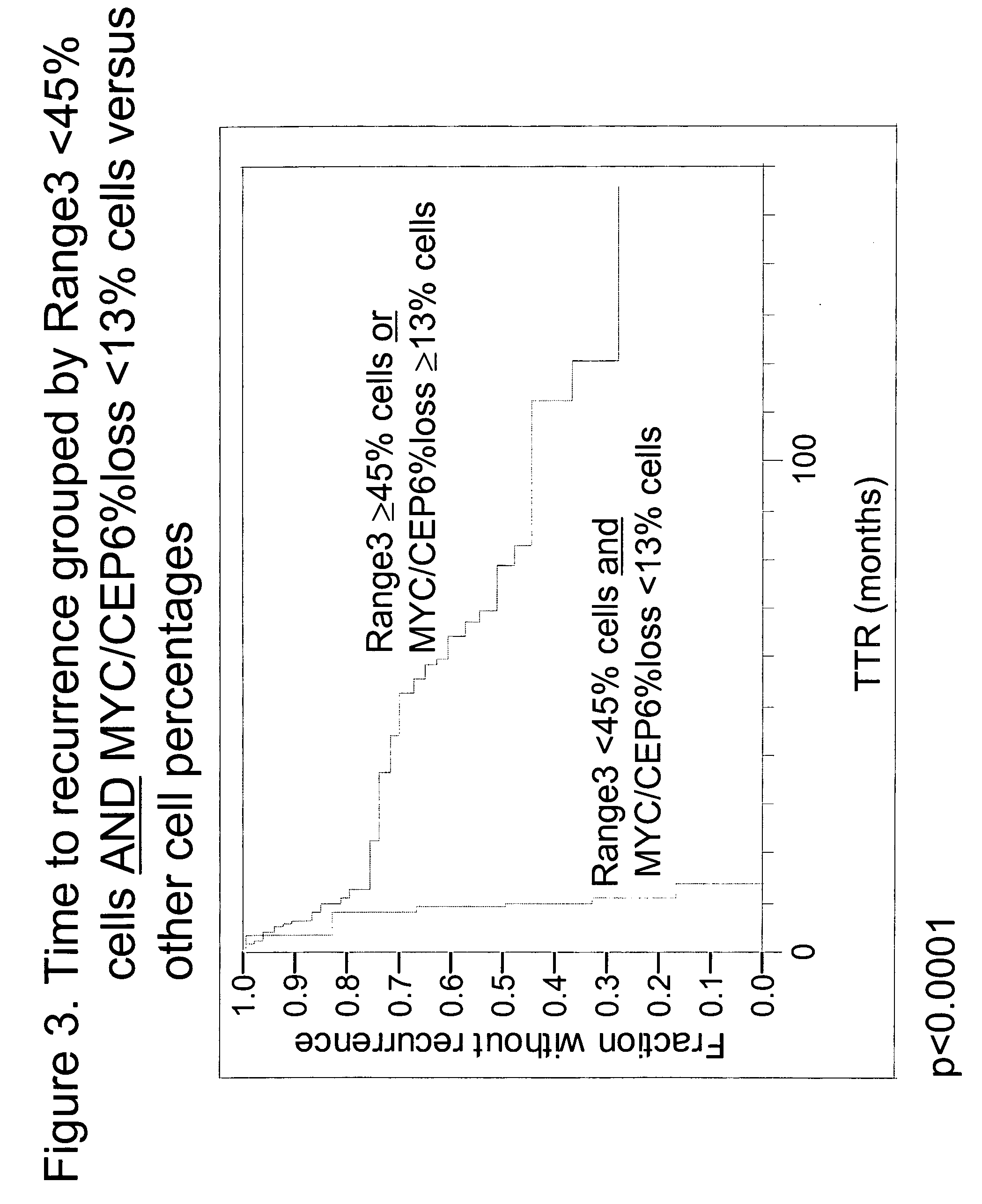
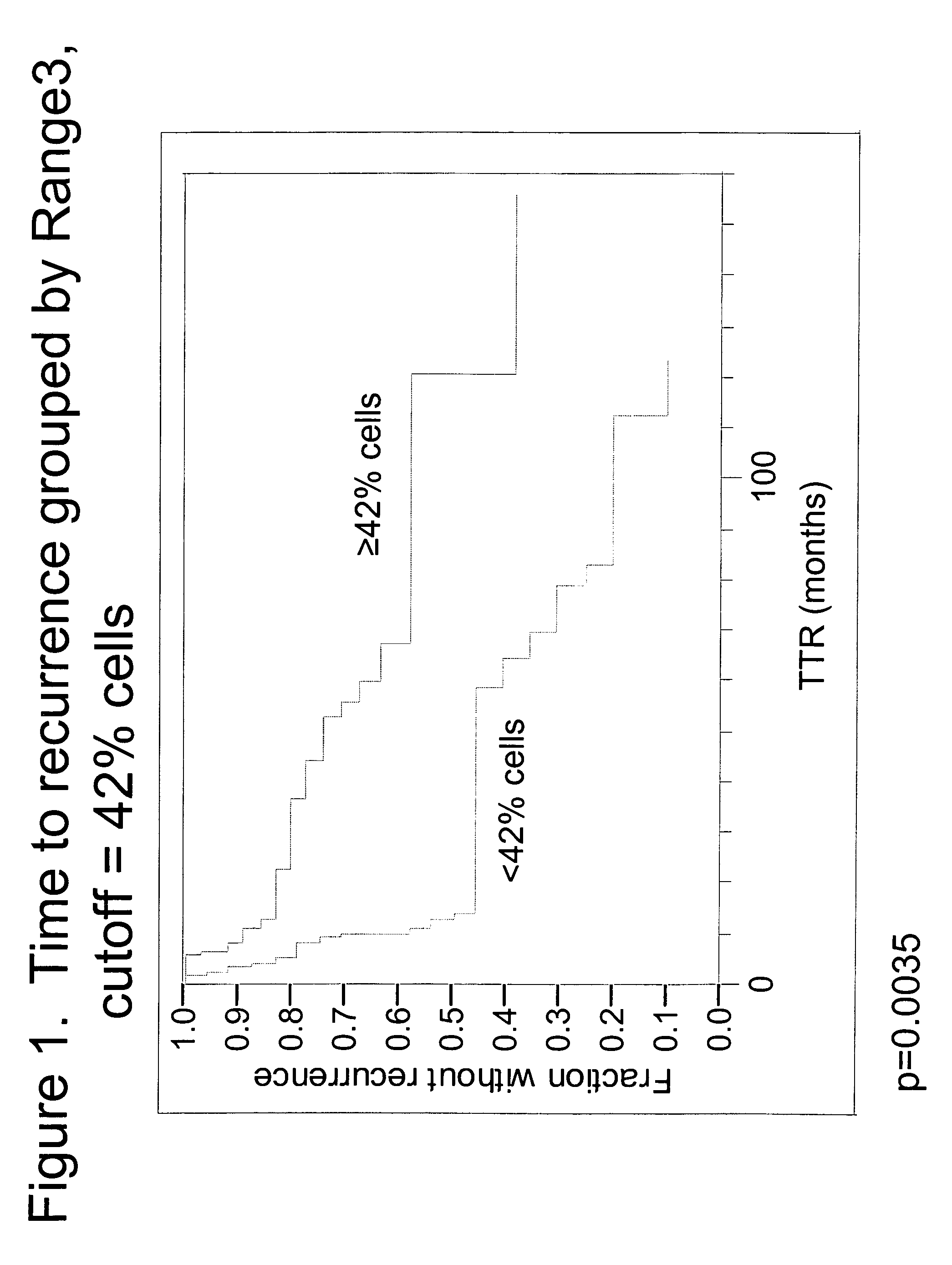
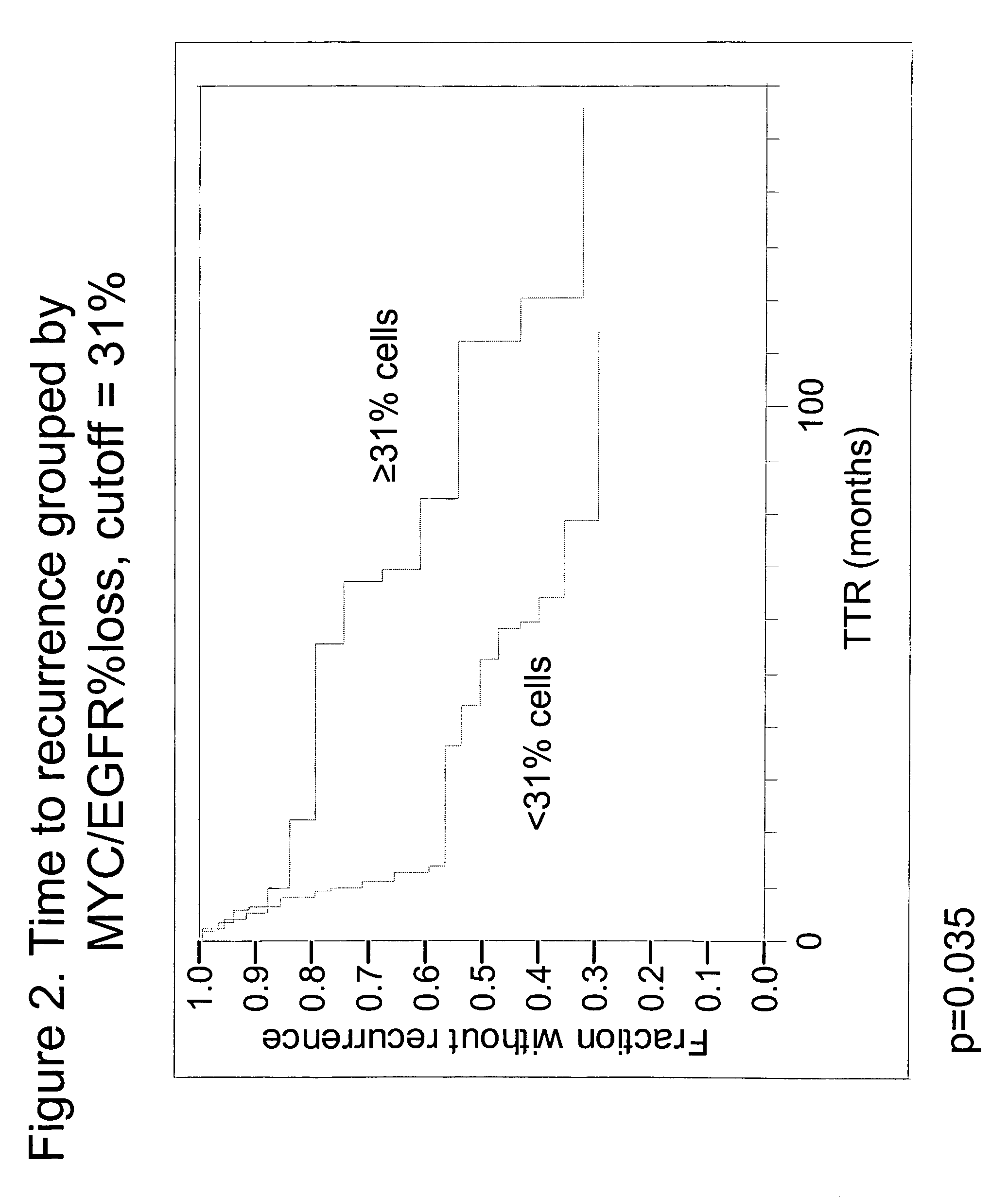
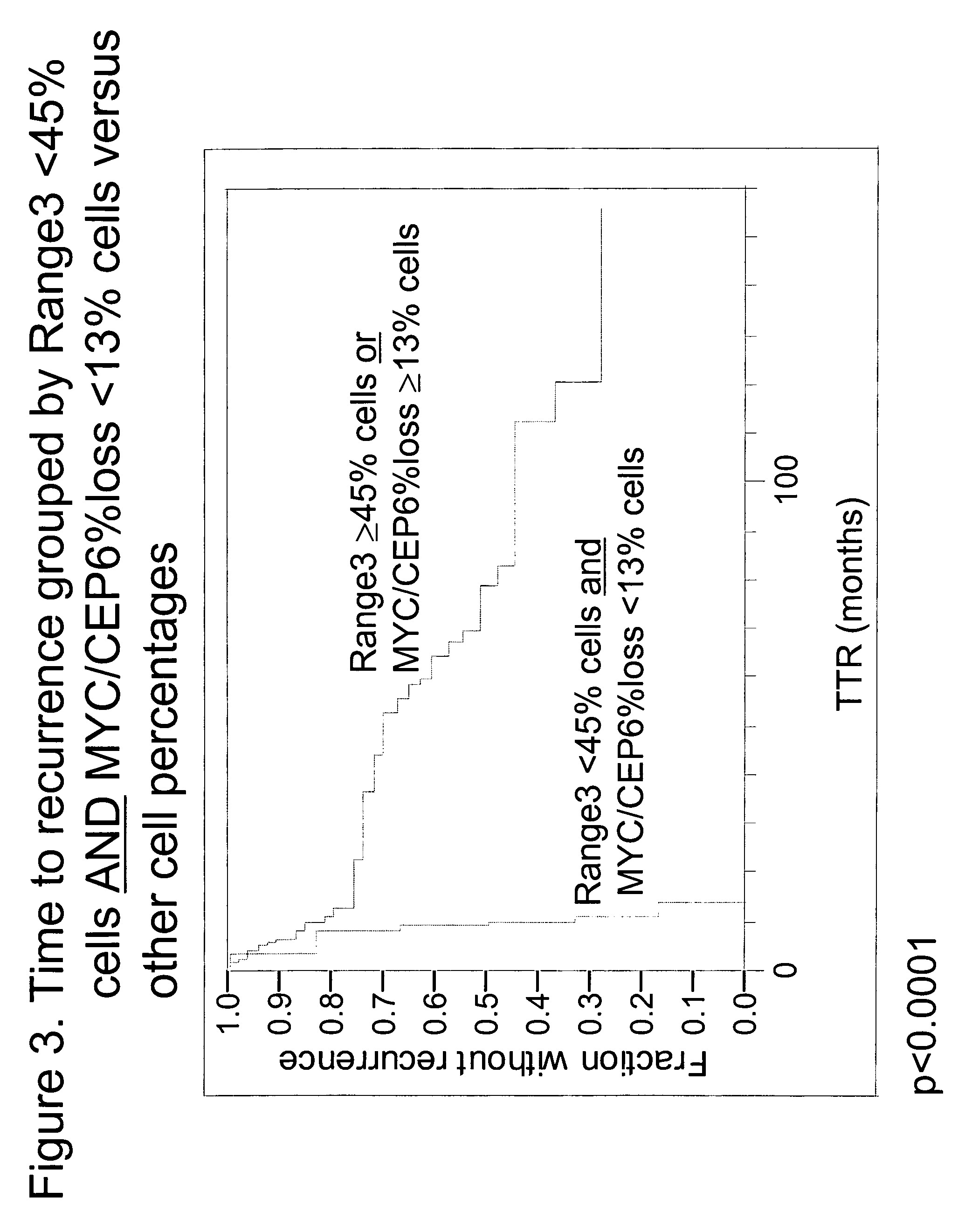
The invention provides methods for identifying early stage non-small cell lung cancer (NSCLC) patients who will have a favorable prognosis for the recurrence of lung cancer after surgical resection. The invention is based on the discovery that assessment of chromosomal copy number abnormalities at two or more of chromosome 5p15, 7p12, 8q24 and centromere 6 can be used for prognostic classification. The invention preferably uses fluorescence in situ hybridization with fluorescently labeled nucleic acid probes to hybridize to patient samples to quantify the chromosomal copy number of the these genetic loci. Assessment of the copy number abnormality patterns using four classifiers produced statistically significant prognostic classification for NSCLC: (i) the Range3 pattern of cells showing a difference on a cell by cell basis, of at least three FISH probe signals between the FISH signals at the chromosomal locus with the largest number of FISH signals minus the FISH signals at the chromosomal locus with the lowest number of FISH signals; (ii) the MYC / EGFR % loss pattern assessing the percentage of cells showing fewer MYC FISH probe signals than EGFR FISH probe signals; (iii) a combination of the Range3 pattern and the MYC / CEP6 ratio pattern of a percentage of cells showing a relative loss of MYC FISH probe signals to the FISH probe signal for CEP6; (iv) the combination of the MYC / 5p15 ratio pattern showing the relative ratio of MYC and 5p15 locus signals of ≧0.80 and the 5p15 / CEP6 ratio pattern assessing percentage of cells having a relative ratio of 5p15 FISH probe signals to CEP6 FISH probe signals ≧1.1 versus MYC / 5p15 ratio of <0.80 or 5p15 / CEP6<1.1; and (v) a combination of the average range of probe signal differences of equal to or greater than about 2.5 with the Range3 pattern in a percentage of the cells. The invention can be used to identify those early stage NSCLC patients at higher risk of recurrence who should be treated with neoadjuvant chemotherapy before surgery or with adjuvant chemotherapy after surgery.
Owner:ABBOTT MOLECULAR INC
Methods and compositions for diagnosing pulmonary fibrosis subtypes and assessing the risk of primary graft dysfunction after lung transplantation
InactiveUS20130029873A1Improved prognosisPoor prognosisNucleotide librariesMicrobiological testing/measurementGraft dysfunctionFavorable prognosis
A method for determining pulmonary fibrosis subtype and / or prognosis in a subject having pulmonary fibrosis comprising: a. determining an expression profile by measuring the gene expression levels of a plurality of genes selected from genes listed in Table 1, 2, 3, 4 7, 8, 9, and / or 10, in a sample from the subject; and b. classifying the subject as having a good prognosis or a poor prognosis based on the expression profile; wherein a good prognosis predicts decreased risk of post lung transplant primary graft dysfunction, and wherein a poor prognosis predicts an increased risk of post lung transplant primary graft dysfunction.
Owner:UNIV HEALTH NETWORK
Prognostic test for early stage non small cell lung cancer (NSCLC)
InactiveUS20100120027A1Accurate identificationEnhanced informationMicrobiological testing/measurementFavorable prognosisFluorescence
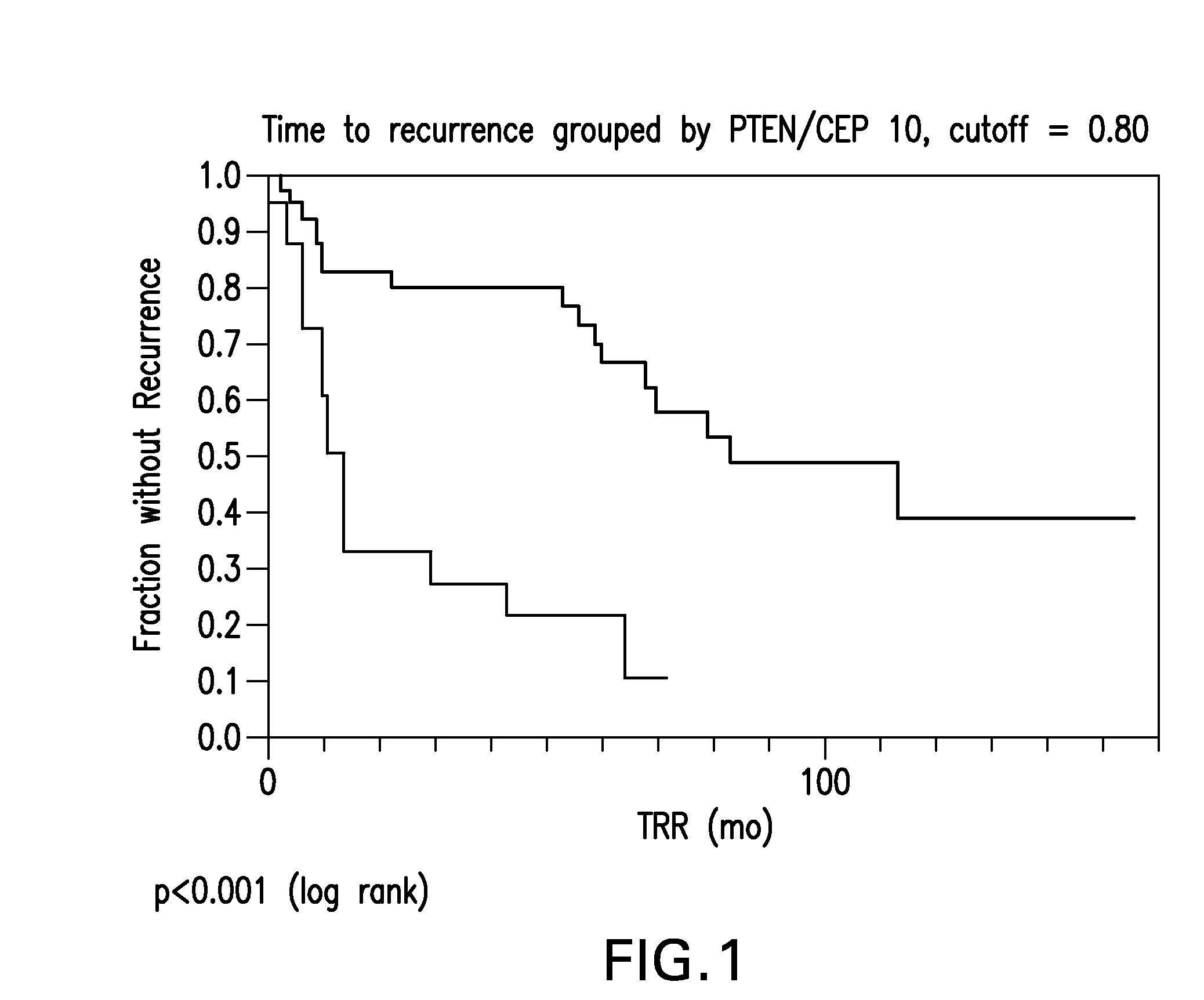
The invention provides methods for identifying early stage non-small cell lung cancer (NSCLC) patients who will have a favorable prognosis for the recurrence of lung cancer after surgical resection. The invention is based on the discovery that assessment of chromosomal copy number abnormalities at chromosome 10q23.3 and centromere 10 can be used for prognostic classification. The invention preferably uses fluorescence in situ hybridization with fluorescently labeled nucleic acid probes to hybridize to patient samples to quantify the chromosomal copy number of the these genetic loci. The chromosome copy number can also be determined using, for example, PCR or array CGH. Assessment of the copy number abnormality patterns with a classifier based on the relative loss of 10q23.3 signals compared to the centromere 10 signals produced statistically significant prognostic classification for NSCLC. The ratio of PTEN / CEP 10 signals, using a cutoff of 0.80, was capable of dividing patients into a group of 41 (≧0.80) in which 33 (80.5%) had the favorable prognosis, and a group of 18 (<0.80) in which 6 (33.3%) had the favorable prognosis (p=0.0008). Median times to recurrence in the former and latter groups were 83.0 and 13.0 months, respectively (p<0.0001).
Owner:ABBOTT MOLECULAR INC
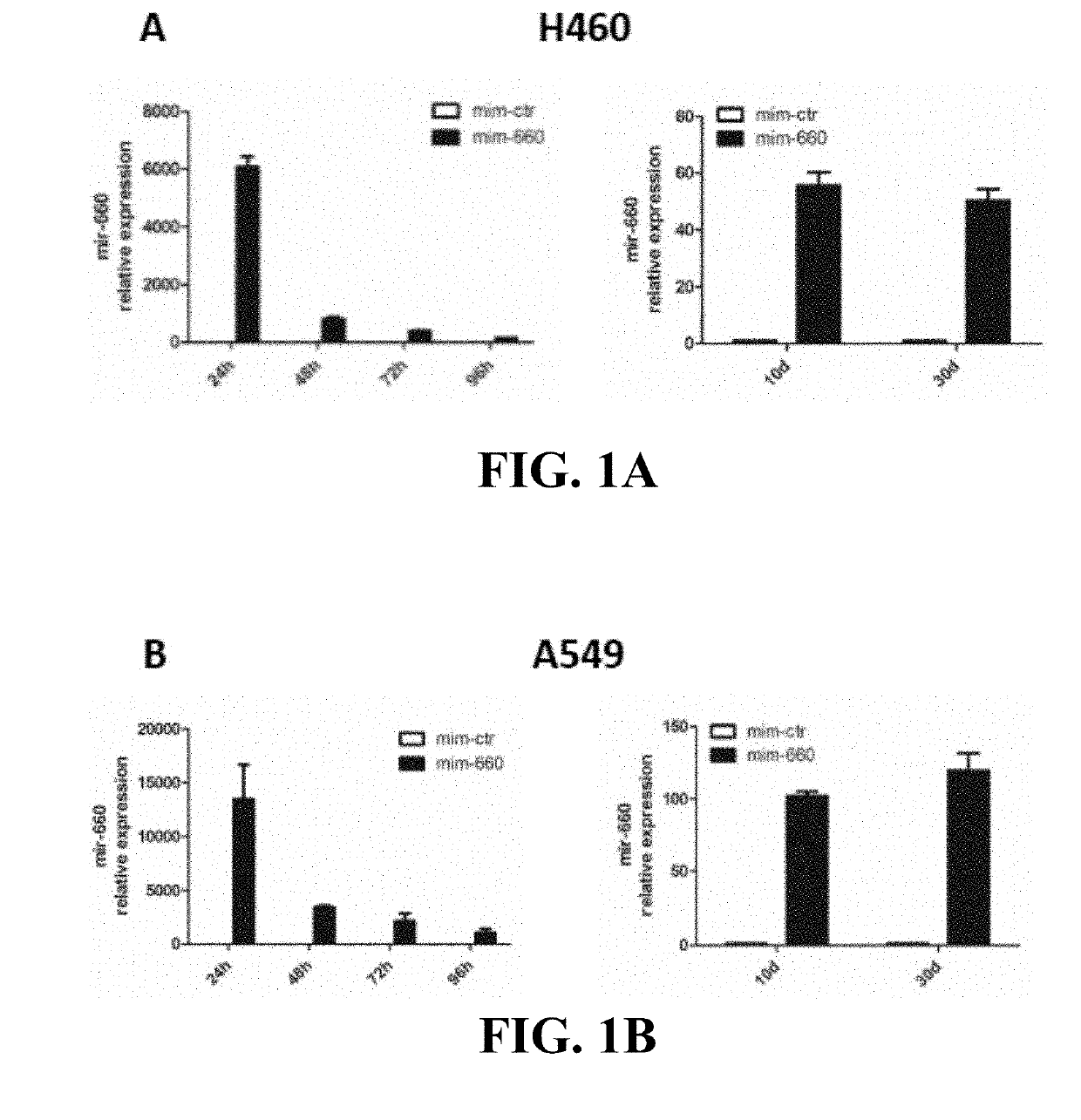
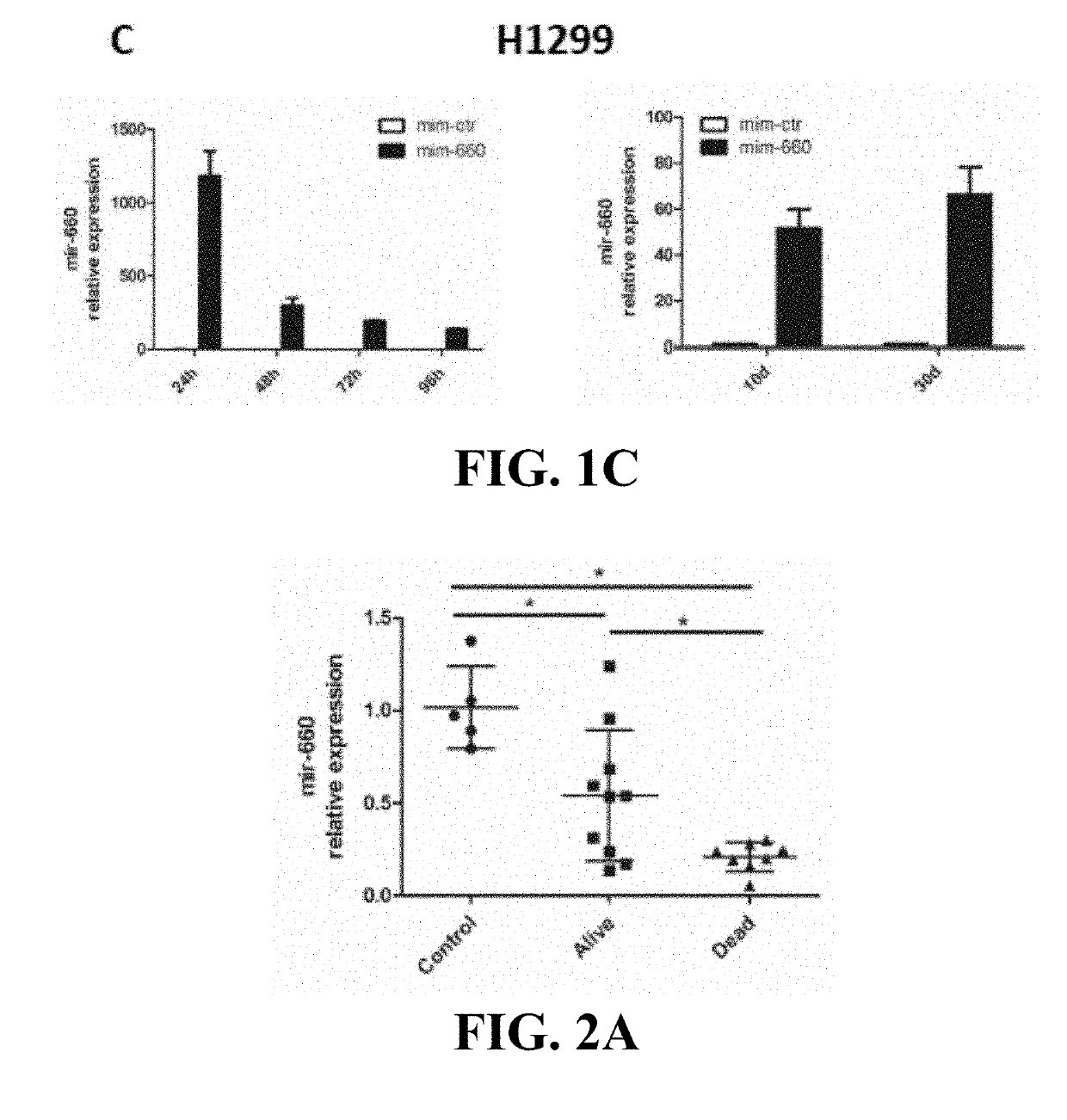
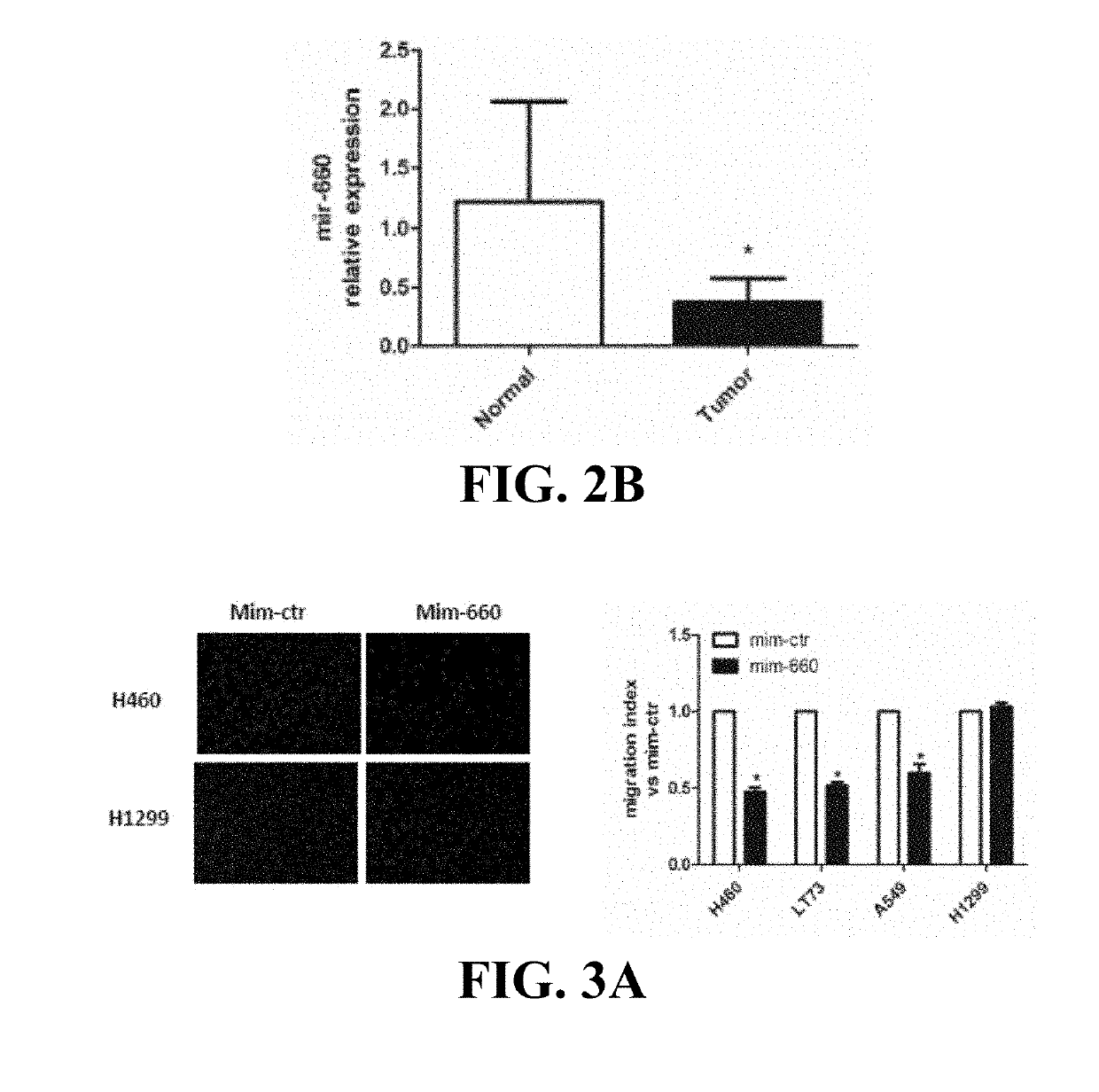
Lung cancer diagnostics and therapeutics with mir-660
InactiveUS20160108405A1Microbiological testing/measurementFermentationFavorable prognosisUbiquitin-Protein Ligases
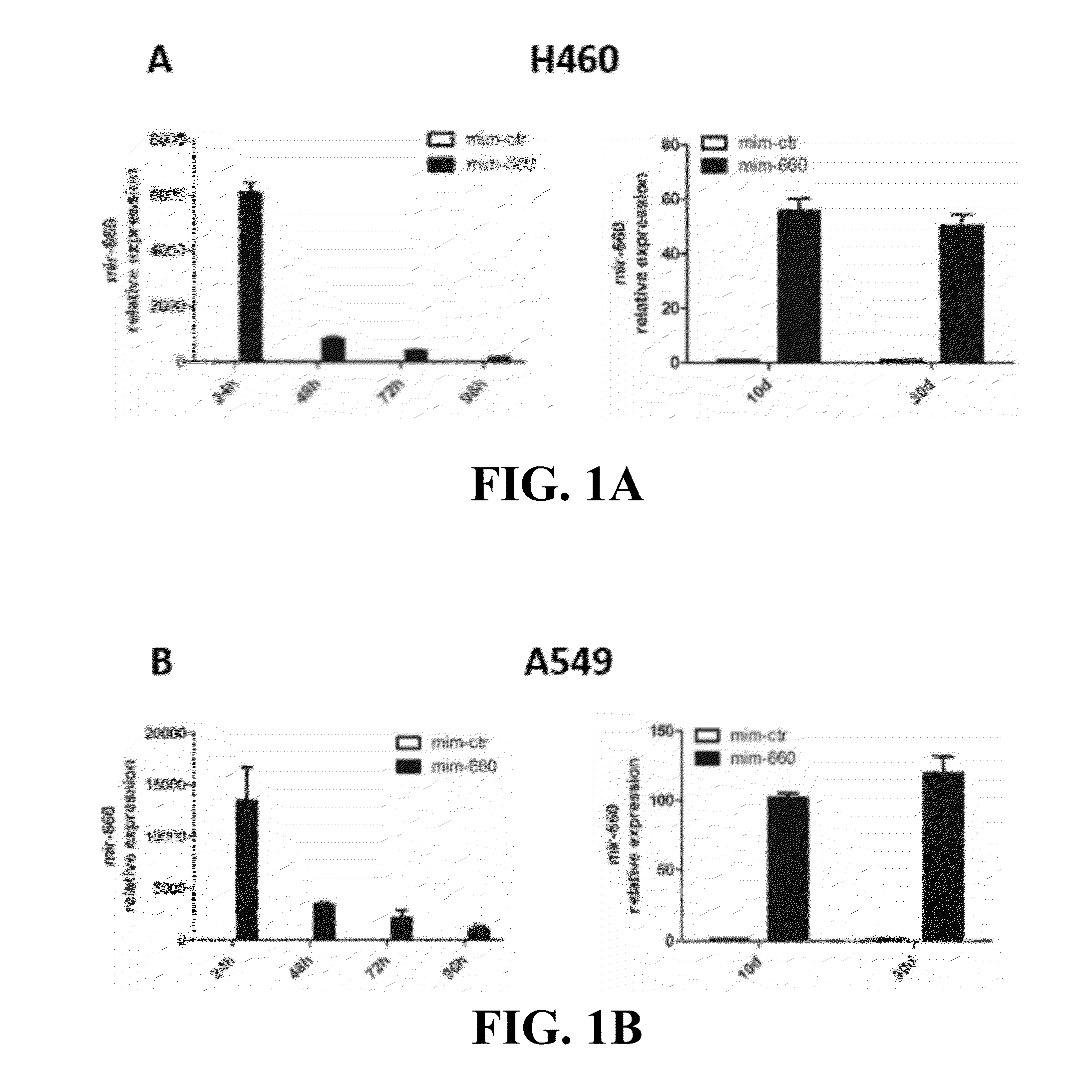
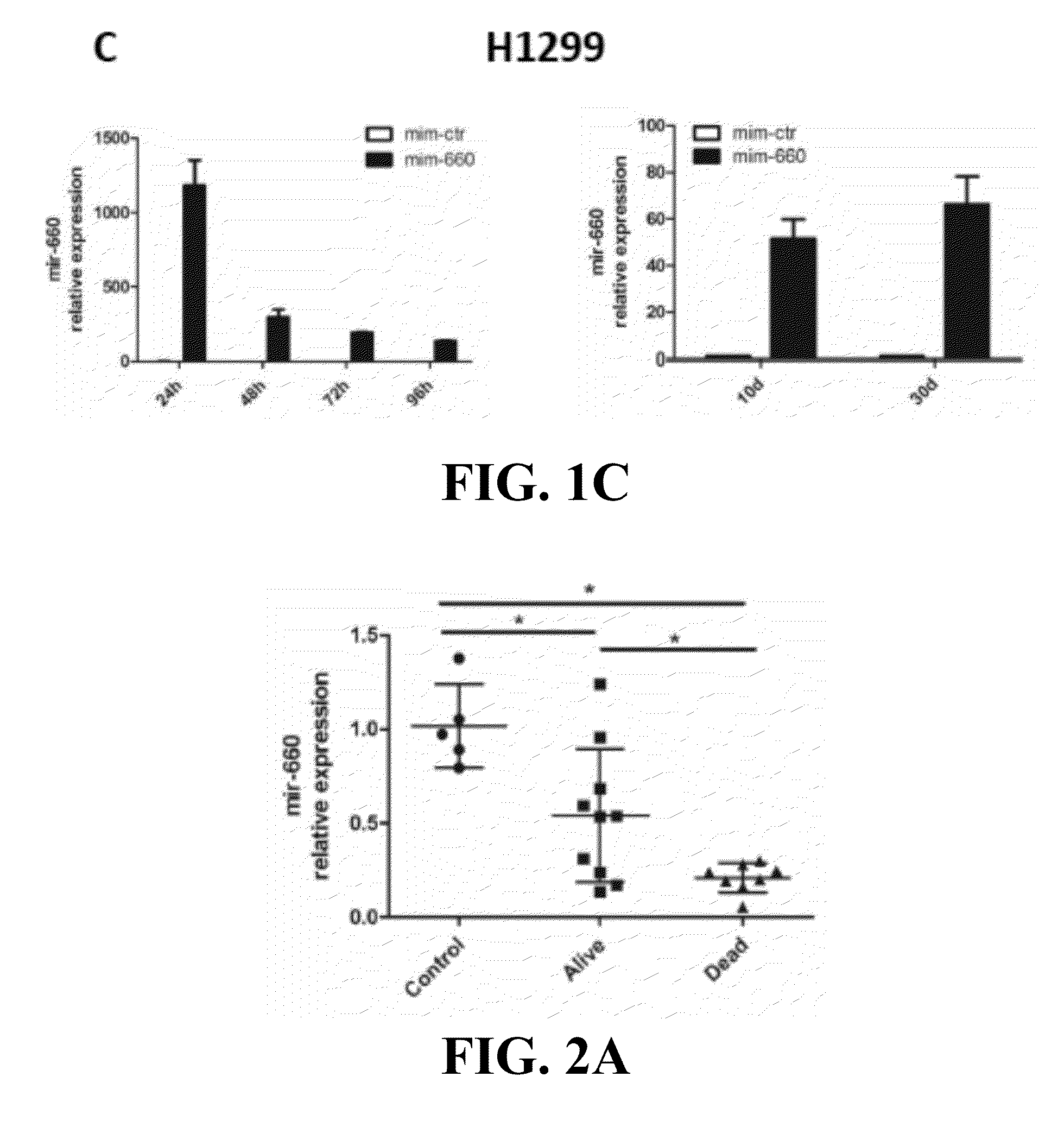
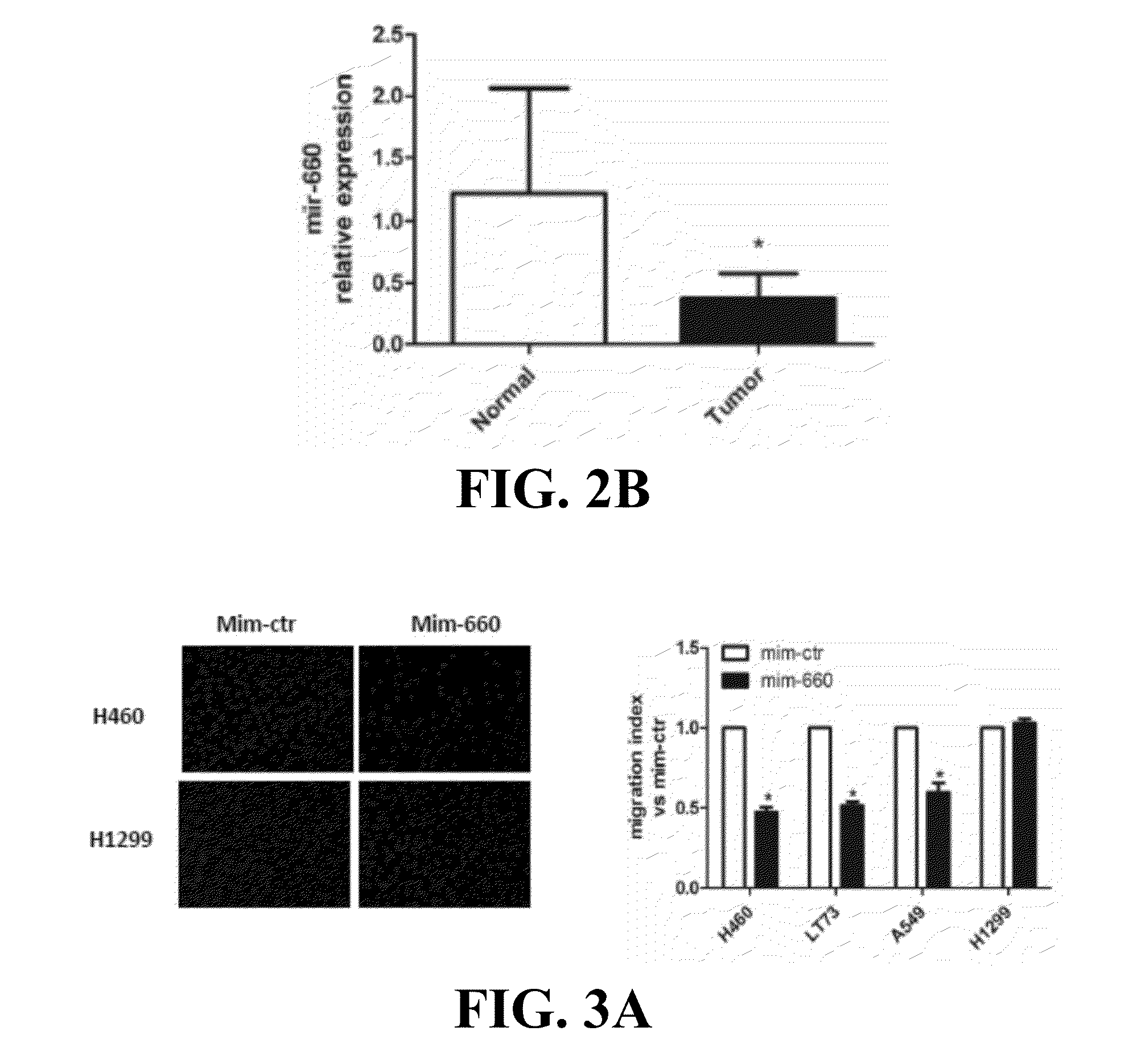
Provided are methods of treating lung cancer in a patient in need thereof. The method includes administration to the patient a composition comprising a therapeutically effective amount of a compound that reduces the expression level of E3 ubiquitin-protein ligase MDM2. The compound in certain instances is a miR-660 miRNA, or a functional variant thereof. The patient in need of treatment in certain instances expresses miR-660 in a lung tissue sample or biological fluid sample at a level lower as compared to a control level derived from a subject or plurality of subjects that do not have lung cancer, or as compared to a control level derived from a lung cancer patient or plurality thereof, that have been given a favorable prognosis; expresses MDM2 at a higher level in a lung tissue sample or biological fluid sample as compared to a control level derived from a subject or plurality of subjects that do not have lung cancer, or as compared to a control level derived from a lung cancer patient or plurality thereof that have been given a favorable prognosis; and / or expresses p53 in a lung tissue sample or a biological fluid sample below a control level derived from a lung tumor tissue sample, or plurality thereof, or a biological fluid sample, or plurality thereof, obtained from a patient that has a favorable lung cancer prognosis; or a control level derived from a healthy subject.
Owner:BIOMIRNA HLDG
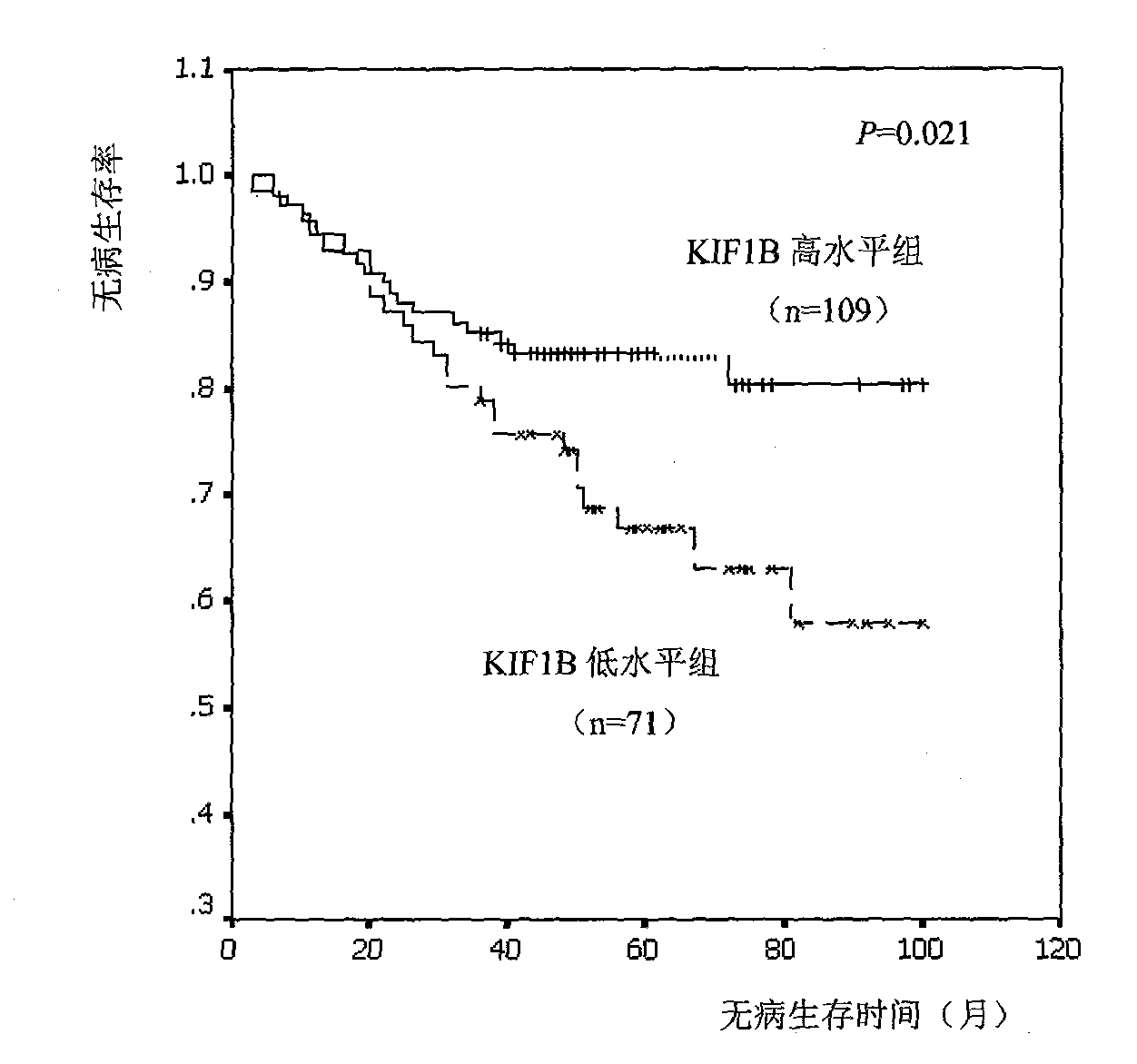
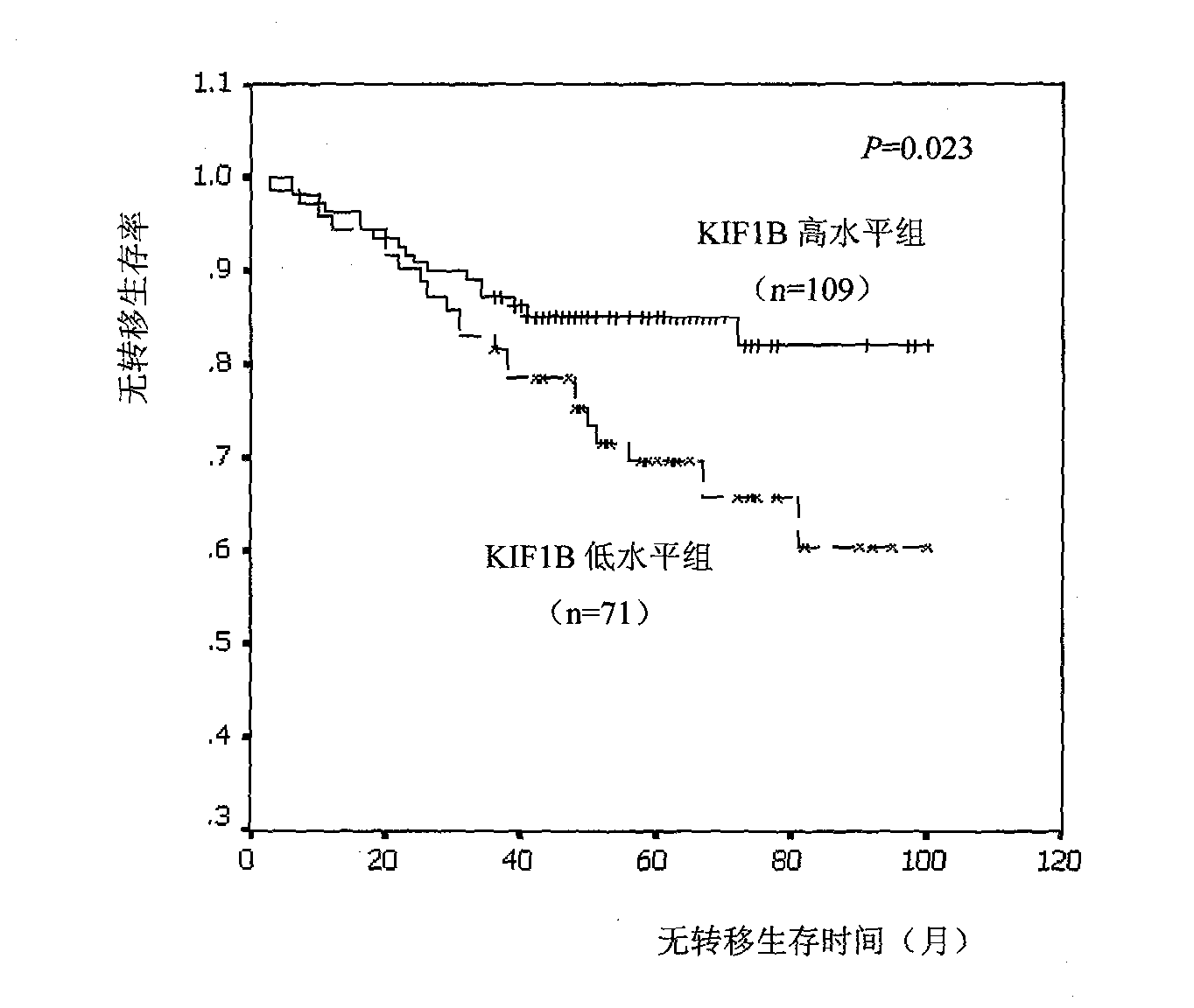
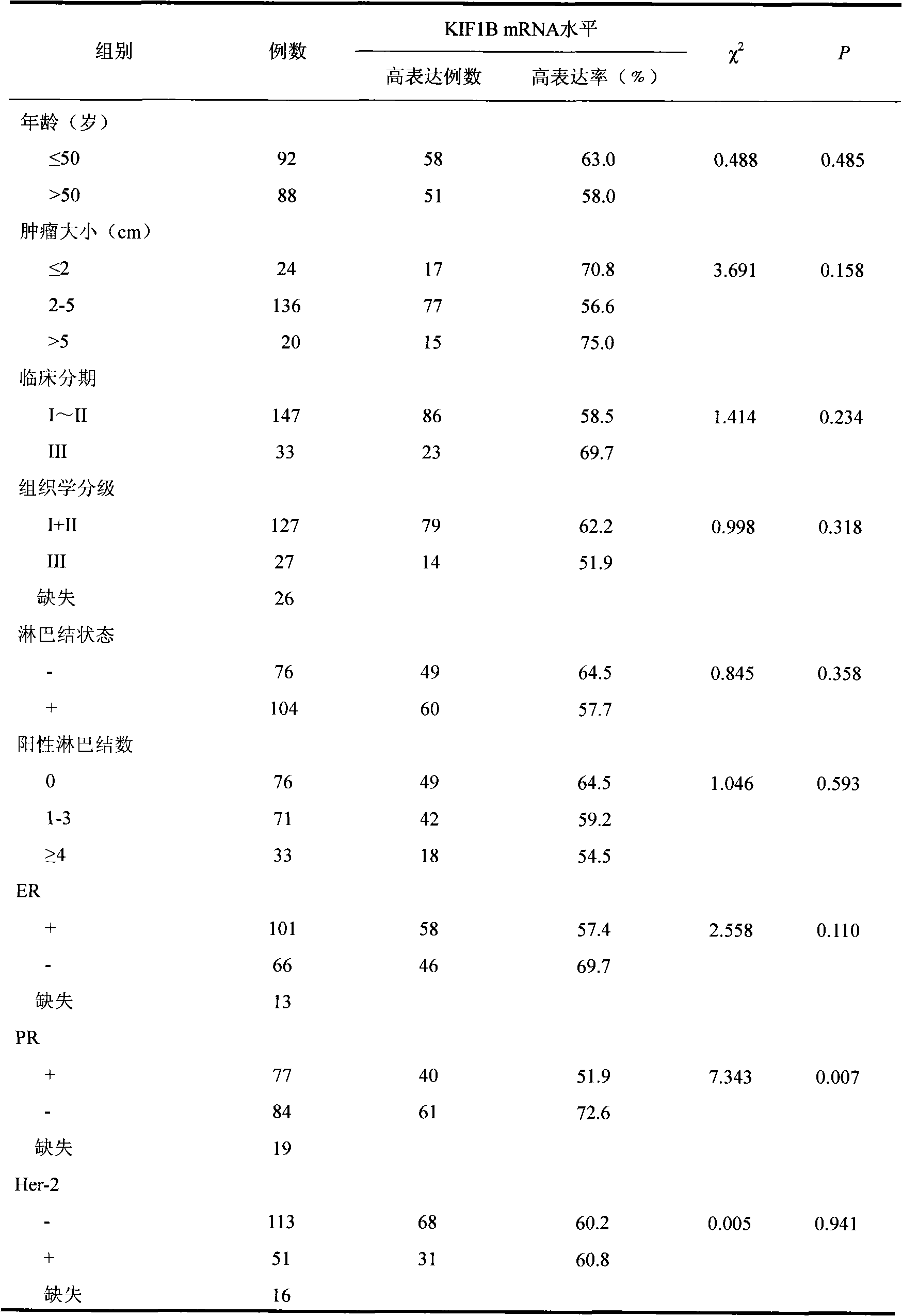
Relative transferring function of kinesin family member KIF1B, landmark application thereof in predicting tumor patient prognosis and application method thereof
The invention discloses a relative transferring function of kinesin family member KIF1B, a landmark application thereof in predicting tumor patient prognosis and an application method thereof, belonging to the technical field of tumor molecular biology. The invention discloses a relative transferring function of kinesin family member KIF1B, a landmark application of KIF1B expression level in predicting tumor patient prognosis and a detection and application method based on the KIF1B expression level in predicting tumor patient prognosis. In the method, real-time quantitative RT-PCR is carried out to detect the method for KIF1B mRNA expression level; quantitative PCR amplification comprises relative quantitative PCR amplification and absolute quantitative PCR amplification; the KIF1B expression level detection can also adopt a molecular hybridization method to detect the KIF1B mRNA expression level; an immunostaining method and / or immunoblotting method can be adopted to detect the KIF1B protein expression level; a critical value for prognosis judgment is determined according to the KIF1B gene or protein expression level in the primary cancer tissues of recurrent and metastatic positive cases and recurrent and metastatic negative cases of tumor patients; and the patient the critical value of whom is higher than the threshold value has favorable prognosis, and the patient the critical value of whom is lower than the threshold value has poor prognosis.
Owner:TIANJIN MEDICAL UNIV CANCER HOSPITAL
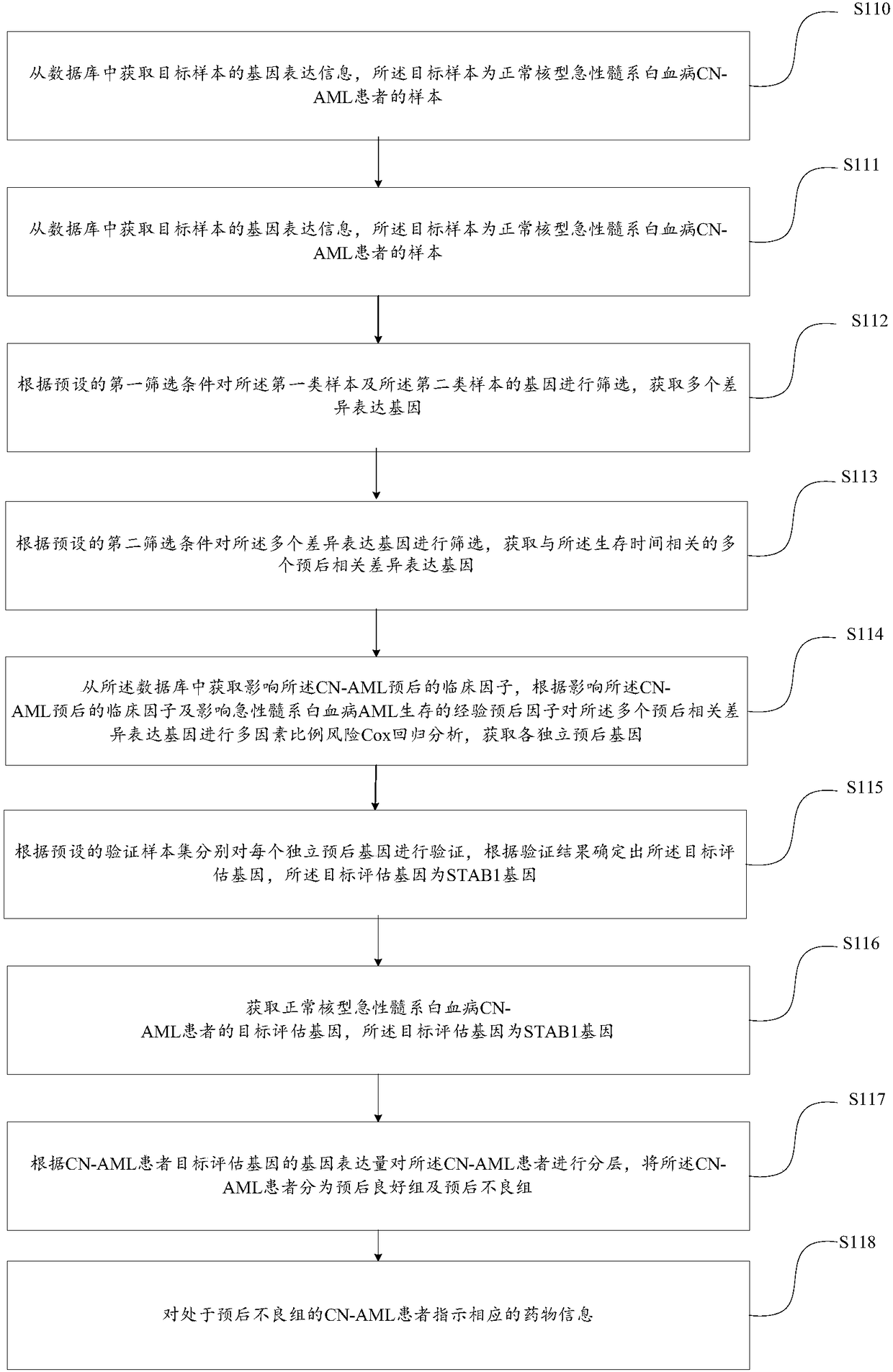
Method and device for indicating acute myelogenous leukemia medicine
InactiveCN108130372ASimplify the screening processAvoid delays in treatmentMicrobiological testing/measurementProteomicsDiseaseFavorable prognosis
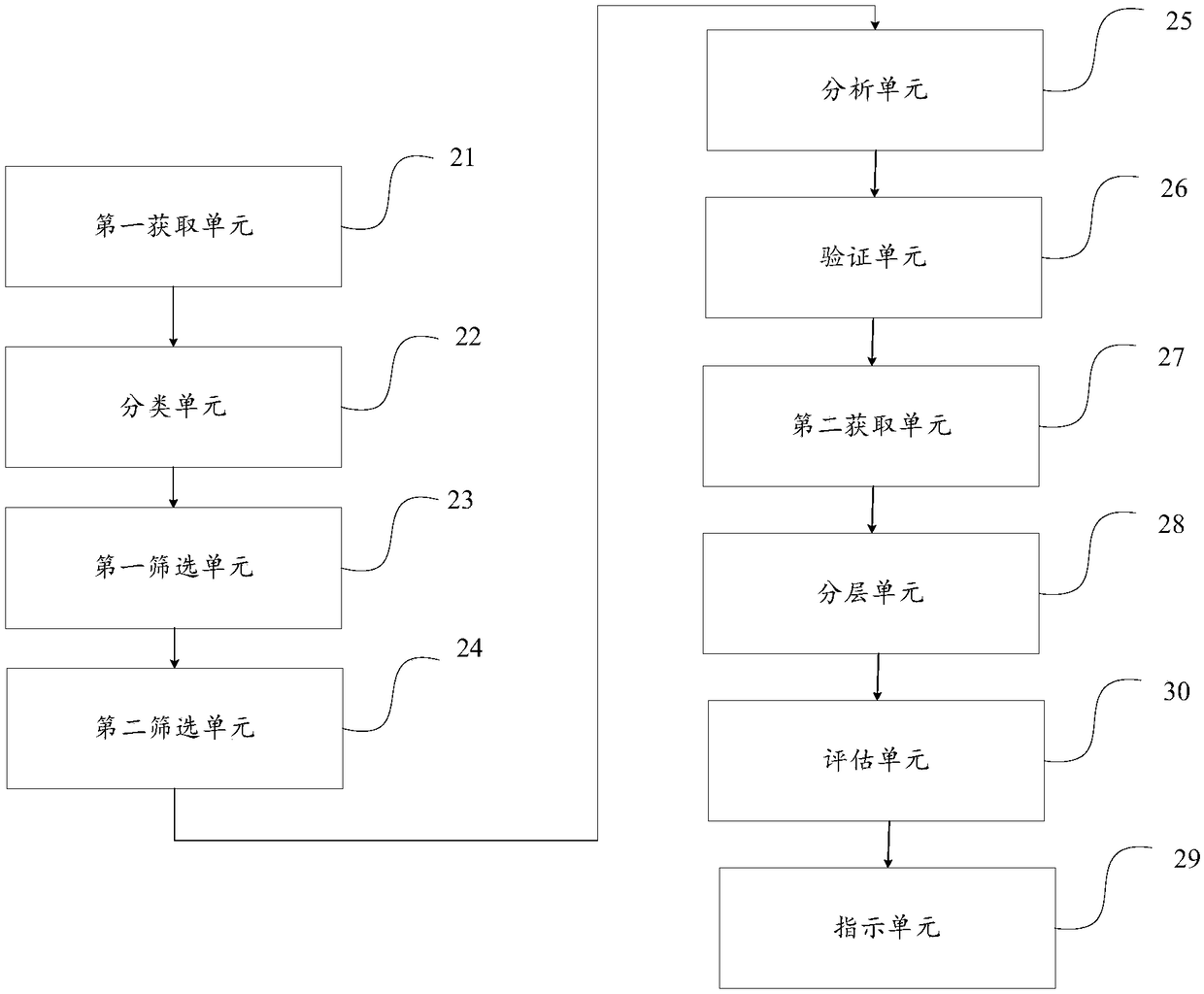
The embodiment of the invention provides a method and a device for indicating an acute myelogenous leukemia medicine. The method comprises the steps of screening a target evaluation gene from a presettarget sample, wherein the target evaluation gene is an STAB1 gene; acquiring target evaluation genes of normal karyotype acute myelogenous leukemia CN-AML patients; layering the CN-AML patients according to gene expression levels of the target evaluation genes of the CN-AML patients, and dividing the CN-AML patients into a favorable prognosis group and an unfavorable prognosis group; indicatingcorresponding medicine information for the CN-AML patients in the unfavorable prognosis group; only one target evaluation gene exists, so that the CN-AML patients can be simply subjected to prognosisevaluation and disease layering; in addition, the target evaluation gene is the STAB1 gene which is a membrane protein gene, so that the gene can be quickly detected by utilizing an existing detectiondevice, and the detection efficiency is improved.
Owner:EZHOU INST OF IND TECH HUAZHONG UNIV OF SCI & TECH
Composition and method for diagnosing kidney cancer and estimating kidney cancer patient's prognosis
InactiveCN101300348AEffective treatmentTreatment progressMicrobiological testing/measurementRecombinant DNA-technologyFavorable prognosisLymphatic Spread
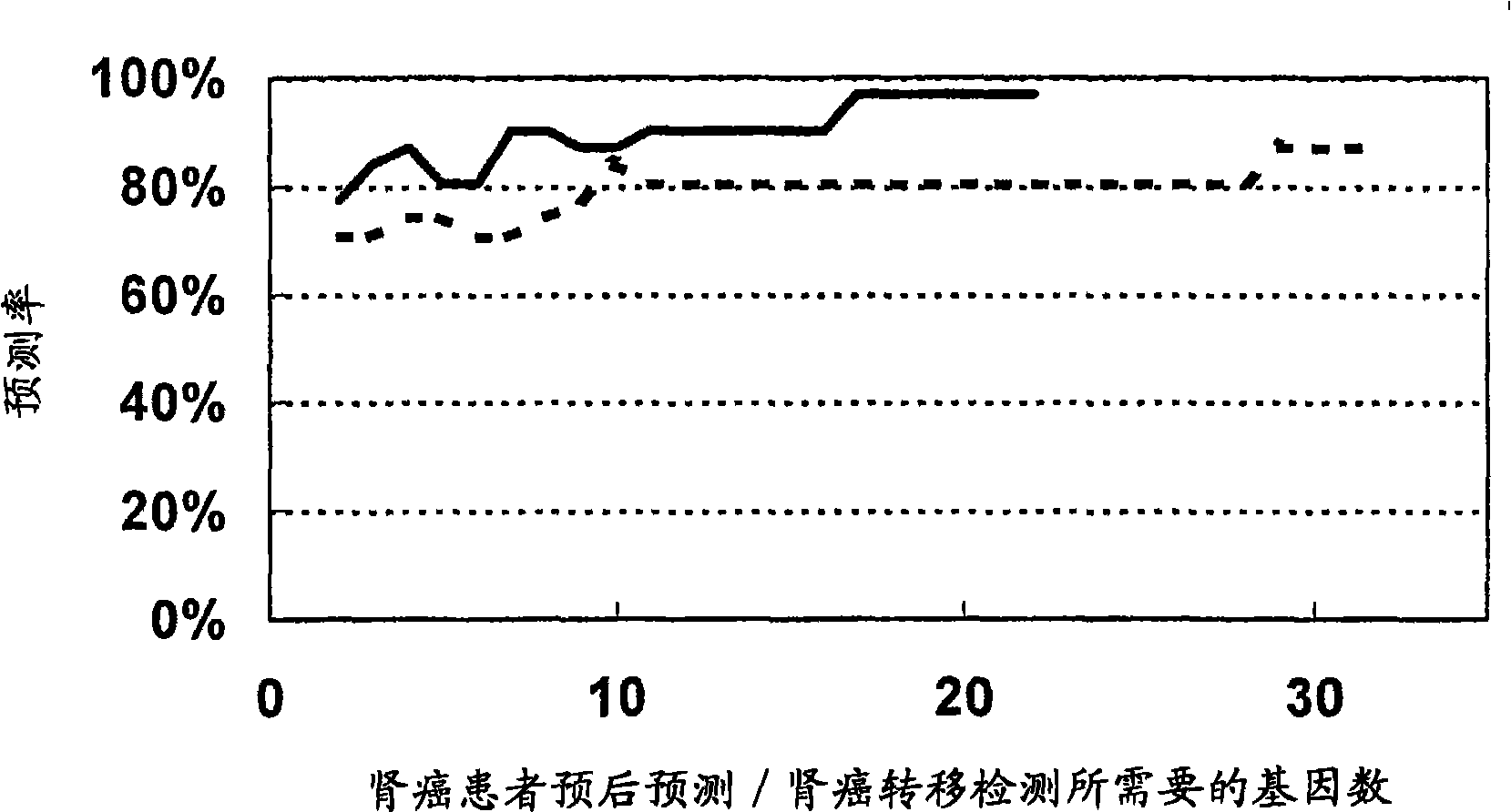
A composition for detecting and diagnosing kidney cancer, diagnosing the metastasis of kidney cancer and / or estimating the prognosis of kidney cancer containing one or more polynucleotides selected from the group consisting of a polynucleotide, which shows a variation in the expression level in a kidney cancer cell originating in a patient with poor prognosis compared with a kidney cancer cell of a patient having favorable prognosis, its variant or a fragment thereof, or an antibody, which binds specifically to such a polypeptide showing a variation in the expression level as described above or its fragment, or a fragment thereof; a kit; a DNA chip; and utilization of the same.
Owner:TORAY IND INC +1
MicroRNA-Based Methods for Prognosis of Hepatocellular Carcinoma
InactiveUS20140004521A1Increase probabilityMicrobiological testing/measurementBiological testingFavorable prognosisLiver tissue
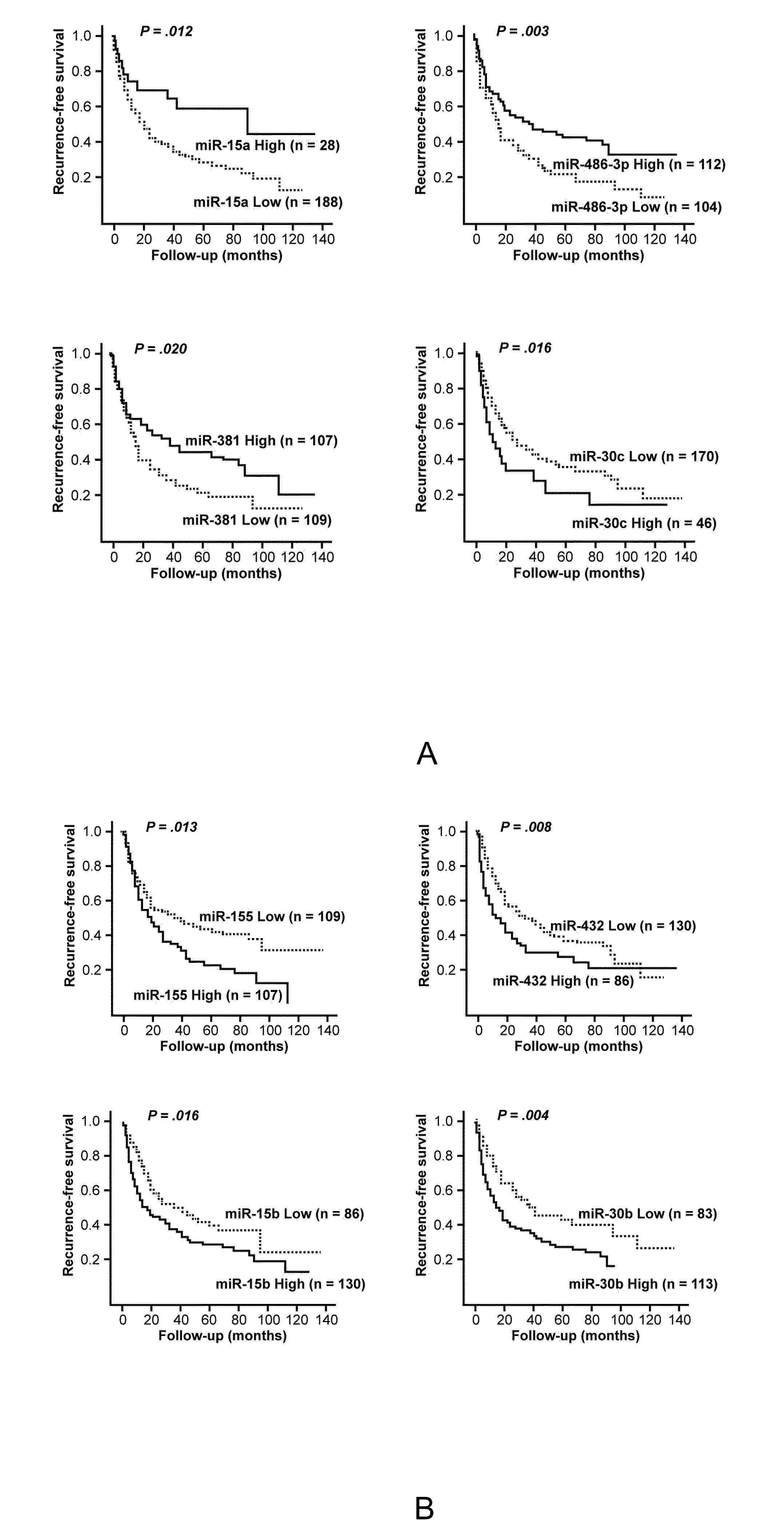
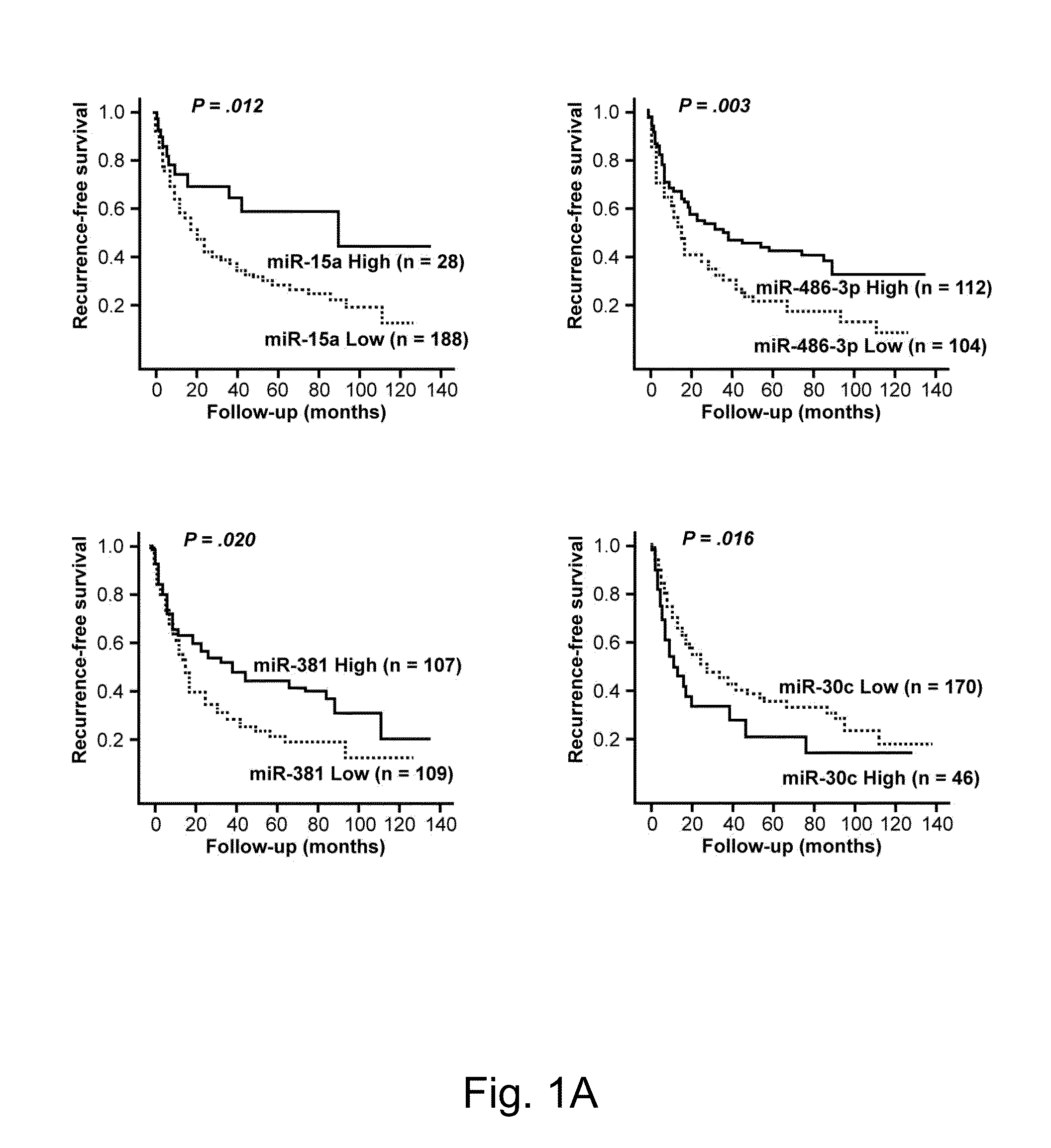
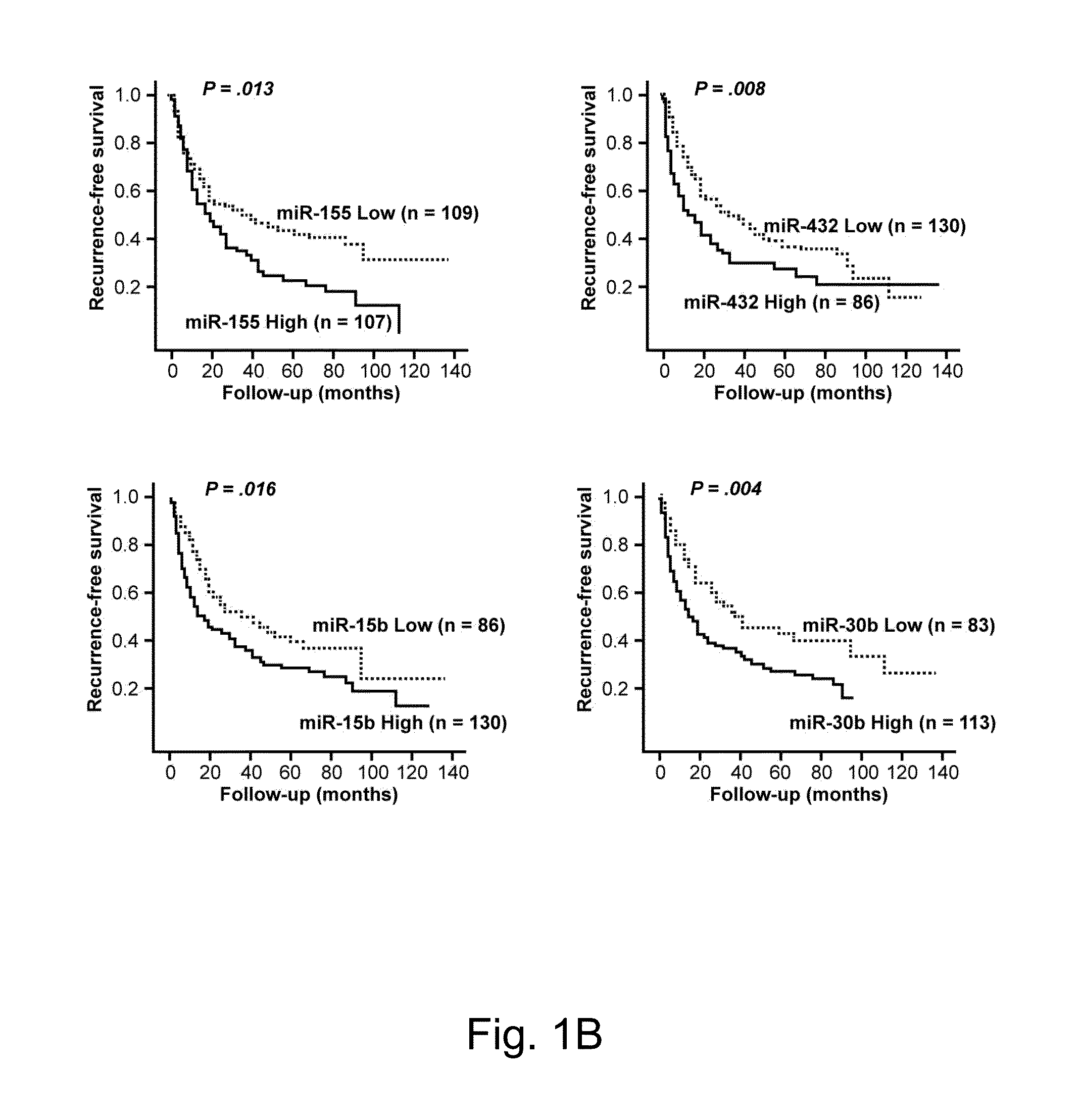
Disclosed herein is a method for determining the prognosis of a subject diagnosed with hepatocellular carcinoma based on the expression level of at least one microRNA in the non-cancerous liver tissue of the subject. According to various embodiments of the present disclosure, expression levels of miR-486-3p, miR-876-5p, and miR-381 are positively associated with a favorable prognosis, while expression levels of miR-30c, miR-432, and miR-15b are negatively associated with a favorable prognosis.
Owner:YEH CHAU TING +3
Construction method of tumor microenvironment scoring system for predicting gastric cancer immunotherapy and molecular probe
InactiveCN113528509AHigh detection throughputImprove accuracyMicrobiological testing/measurementCharacter and pattern recognitionFavorable prognosisNucleotide
The invention provides a group of molecular probes for detecting and evaluating a gene set of a tumor microenvironment. The molecular probes comprise 44 nucleotide sequences as shown in SEQ ID NO: 1-44. A screened probe is used for carrying out NanoString digital expression detection on the expression of a target gene in a same hole, a digital gene expression profile is drawn, and the probe can be used for evaluating the immunotherapy effect. The invention further provides a construction method of a multi-gene scoring model for evaluating the gastric cancer tumor microenvironment, and 44 gene sets capable of representing different microenvironment types are screened out and can be used for establishing the tumor microenvironment multi-gene scoring model for evaluating patients. By effectively evaluating the tumor microenvironment, the model not only can be used as a good prognosis biomarker, but also can predict checkpoint inhibitor immunotherapy response of various tumors, and has important significance and clinical application value for realizing individualized medical treatment and saving medical resources at the same time.
Owner:释科生物科技(广州)有限公司
Oral medicament for treatment of oral leukoplakia
InactiveCN105031284AImprove chappedImprove ulcerSalicyclic acid active ingredientsHydroxy compound active ingredientsAdditive ingredientAdemetionine
The invention discloses oral medicament for treatment of oral leukoplakia. The oral medicament comprises 5%-30% of Chinese medical extract, 1%-5% of Western medicine ingredients and the balance adjuvant materials. The Chinese medical extract is prepared from bulbophyllum inconspicuum maxim, stems and leaves of hookedhairypod tickclover, nephelium chryseum, edible manna lichen, fragrant sarcococca herbs, fermented Chinese gall herb and tea leaves, dye yam, shaddock ped, asiatic cudweeds, white perilla leaves, seguin chinkapin cup bark or roots, elsholtzia blanda, caragana microphylla, fleshy-root gentian roots, ulmus parvifolia leaves, cucumber gourd seeds, artemisia japonica, averrhoa carambola L., humulus japonicus, feather cockscomb seeds, herbs of herba cirsii and desmodium triquetrum. The Western medicine ingredients include disodium succinate, vitamin A acetic ester, astaxanthin, sodium chloride, vitamin A palmitate, salicylic acid and urea. The oral medicament can achieve antisepsis and anti-inflammation, promote blood circulation and urinate with diuretics, promote production of body fluid and nourish the lung, reduce abscess and nurse the health from inside to outside. Thorough treatment can be achieved fundamentally, and the symptoms such as chapping, anabrosis or base hardening and surface thickening of a leukoplakia area are reduced obviously. The oral medicament can be taken conveniently, stimulation from local smearing is avoided, no toxic and side effects exist, favorable prognosis is realized, and relapse tends not to happen.
Owner:王学宽
Method for the Prognosis of Survival Time of a Patient Suffering from a Solid Cancer
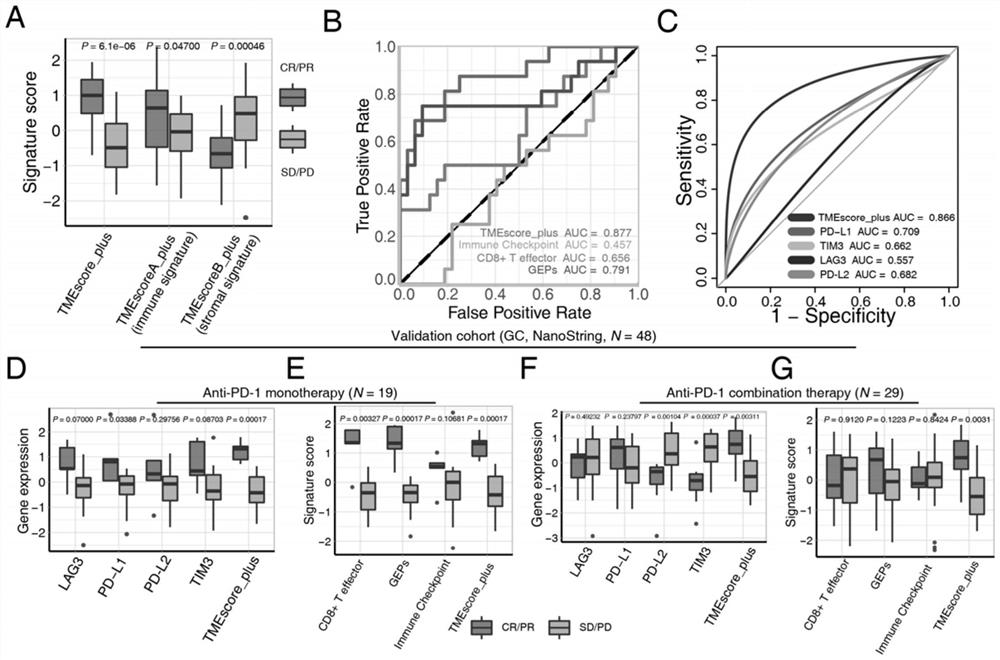
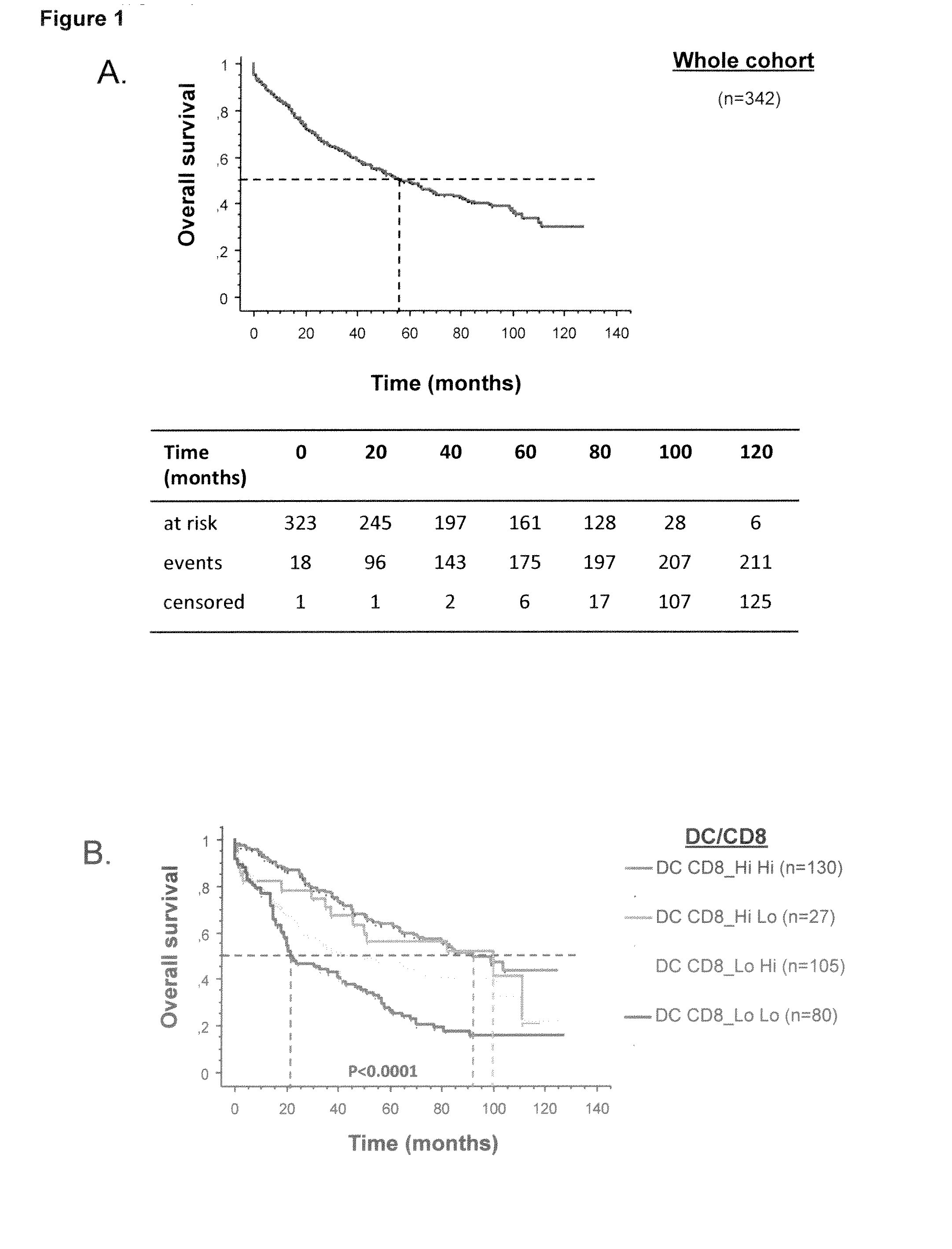
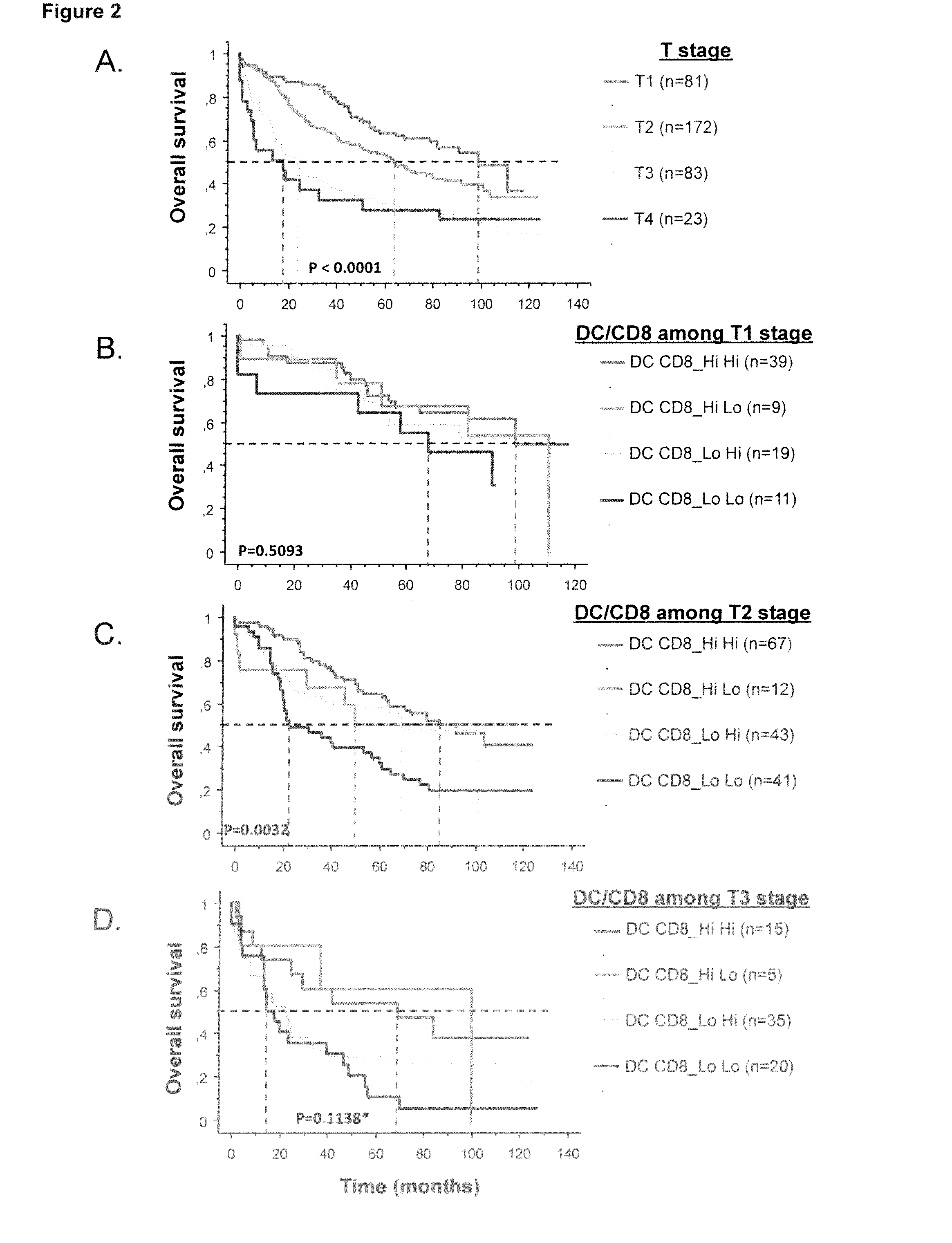
ActiveUS20160146820A1Avoid and minimize side effectIncrease probabilityBiocideIn-vivo radioactive preparationsDendritic cellFavorable prognosis
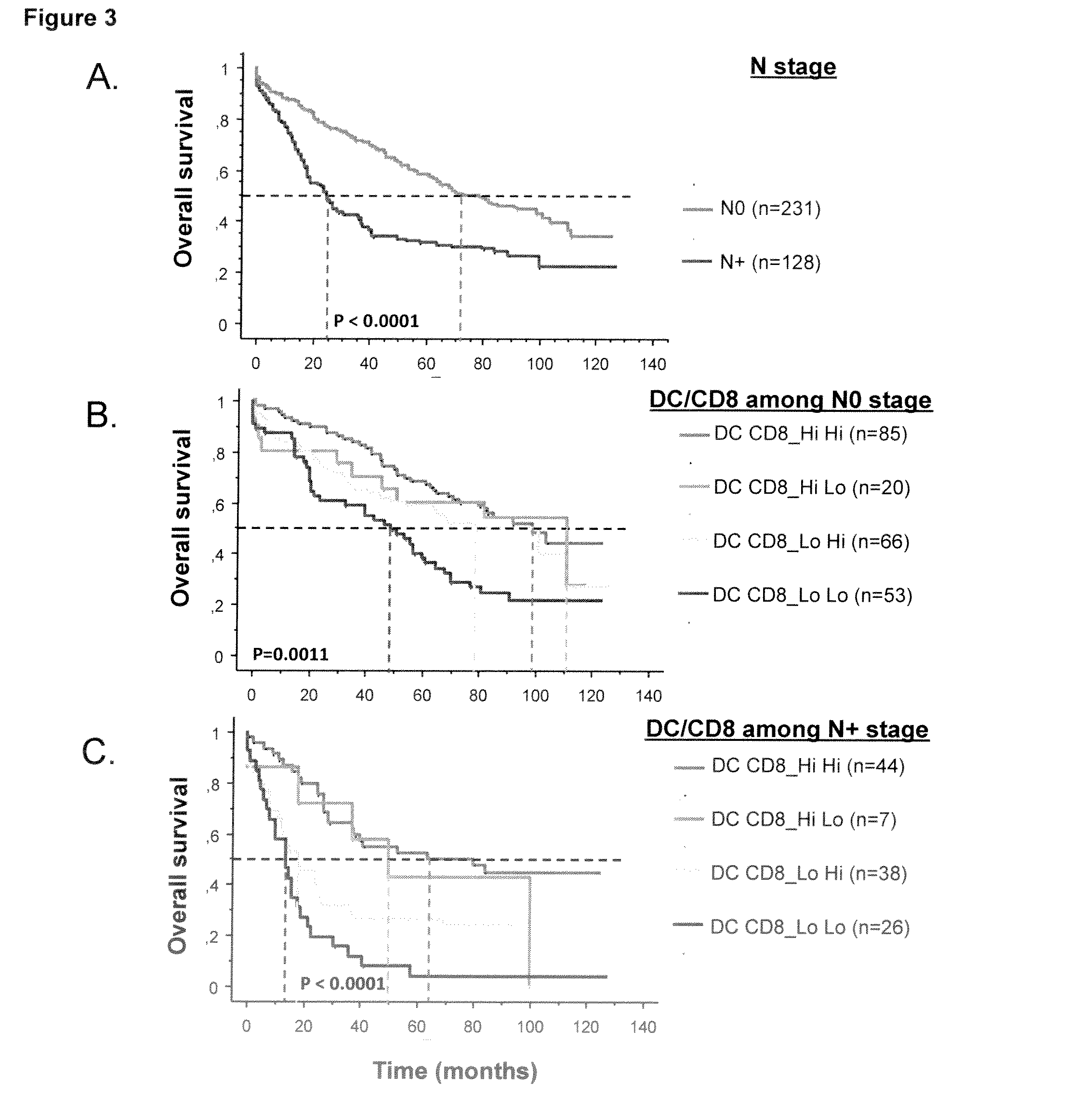
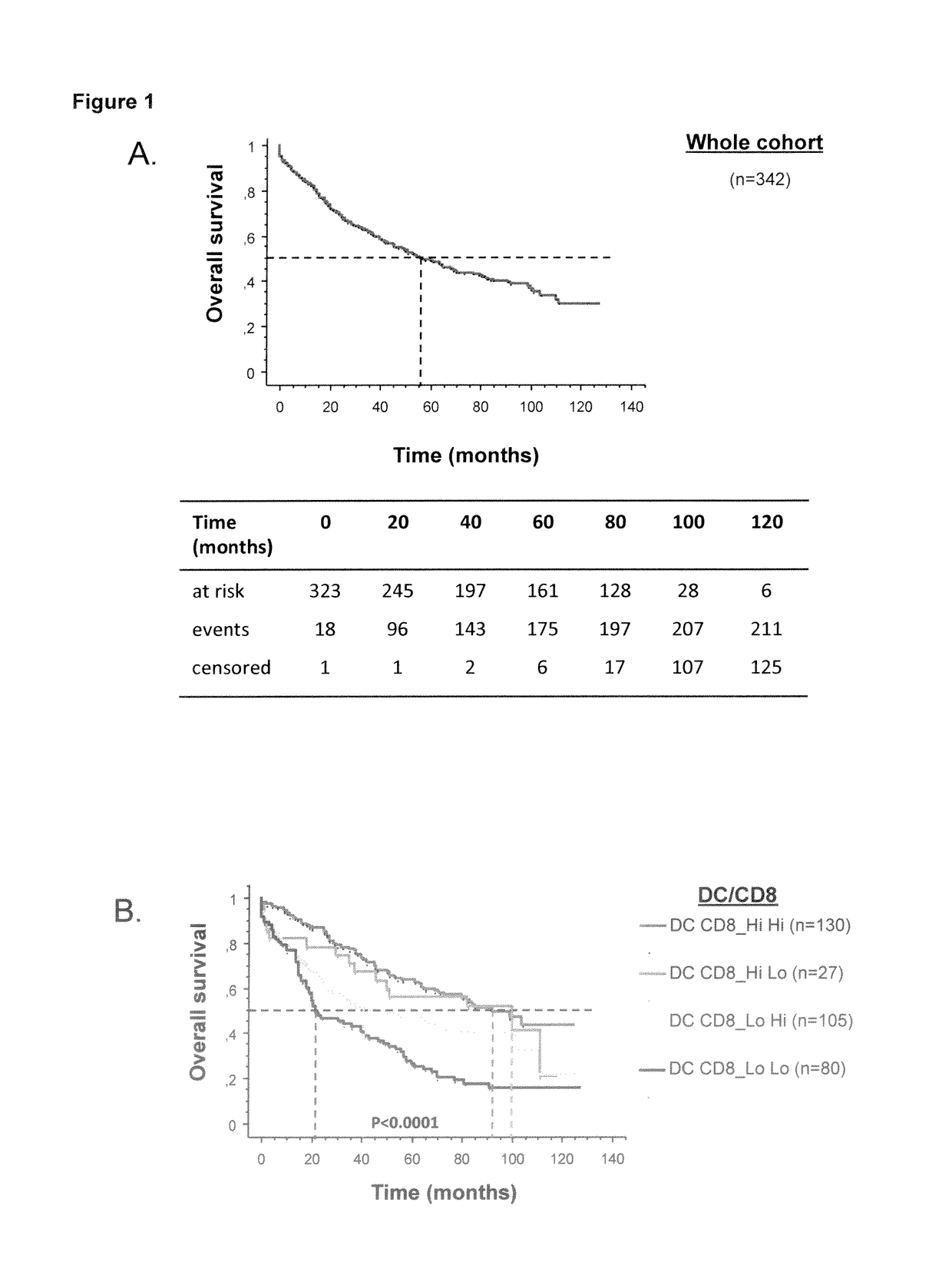
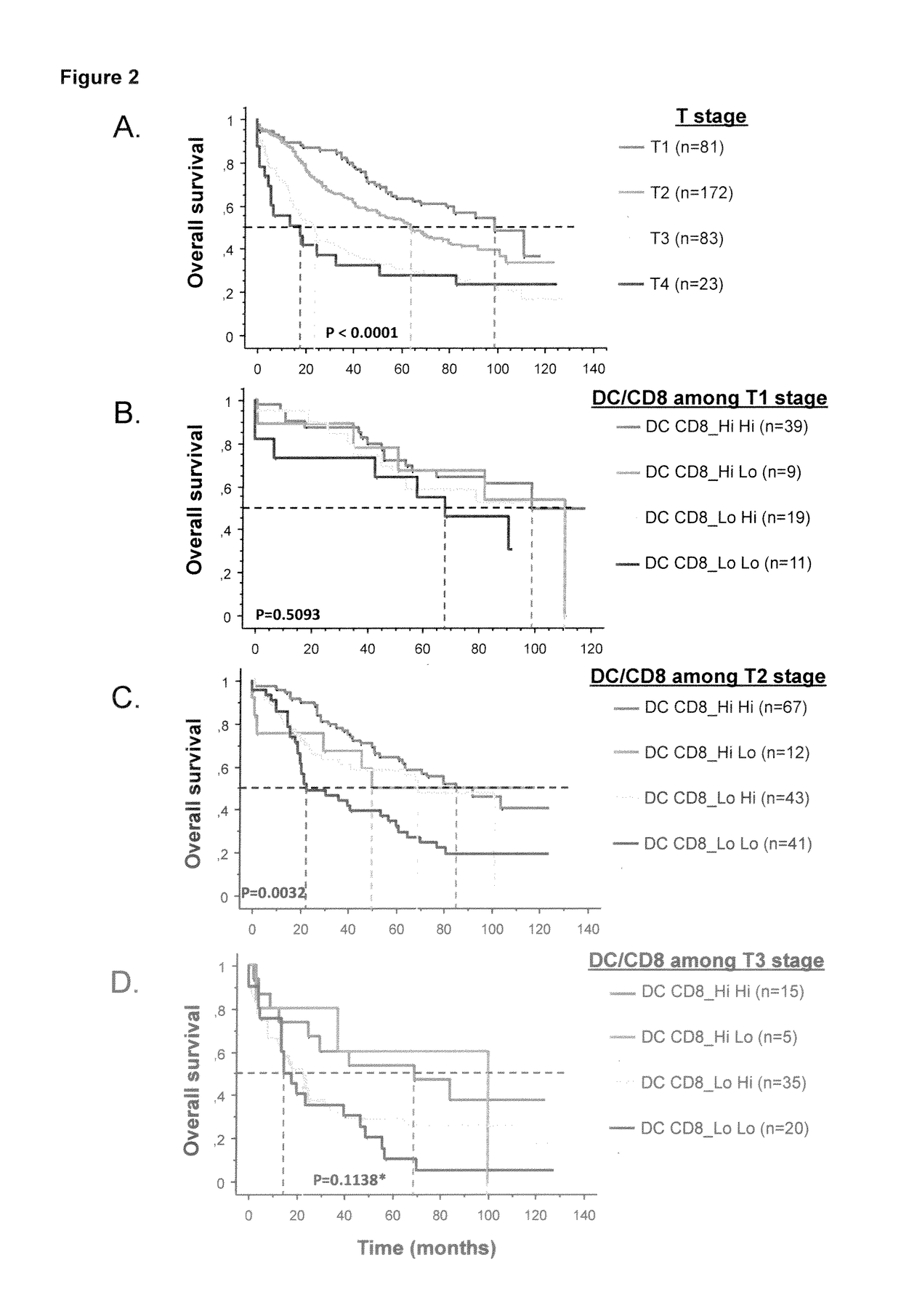
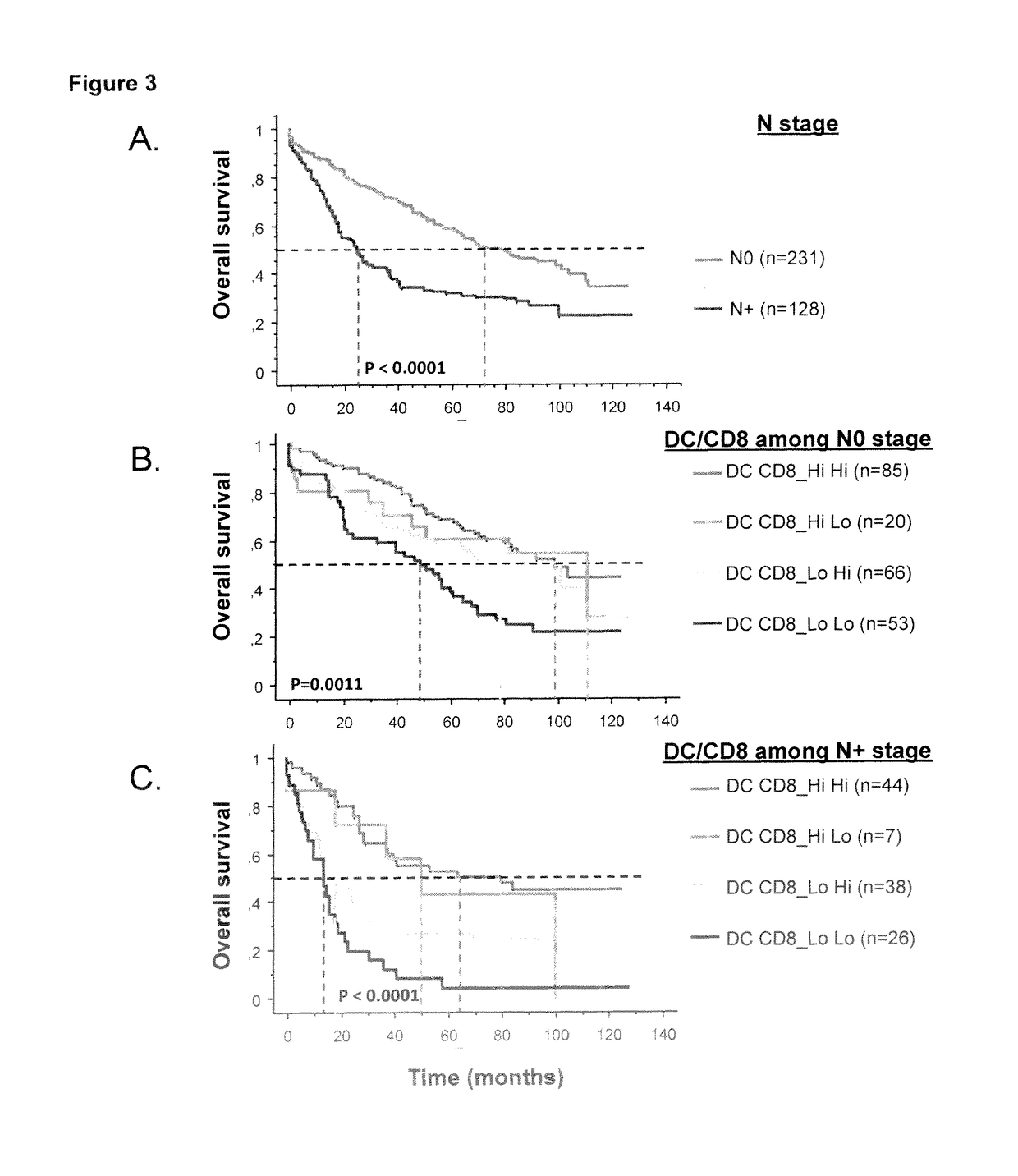
The present invention relates to an in vitro method for the prognosis of survival of a patient suffering from a solid cancer, comprising the quantification of the cell density of CD8+ cells and DC-LAMP+ dendritic cells present in a tumor tissue sample from said patient, wherein a high density of CD8+ cells and DC-LAMP+ dendritic cells indicates that the patient has a favorable prognosis, a high density of CD8+ cells and a low density of DC-LAMP+ dendritic cells indicates that the patient has a poor prognosis, and a low density of CD8+ cells and DC-LAMP+ dendritic cells indicates that the patient has the worst prognosis.
Owner:UNIVERSITÉ PARIS CITÉ +3
Nucleic acid sequences having characteristics of enhanced expression in human neuroblastoma with favorable prognosis based on comparison between human neuroblastoma with favorable prognosis and human neuroblastoma with unfavorable prognosis
InactiveUS20060188919A1Considerable numberImproved prognosisSugar derivativesMicrobiological testing/measurementFavorable prognosisNucleic acid sequencing
There are disclosed a nucleic acid which is derived from the gene expressed in human neuroblastoma, and which comprises any sequence selected from the group consisting of the nucleic acid sequences set forth SEQ ID NO:1 to NO:104 in the Sequence Listing, or its complementary nucleic acid; a fragment of the nucleic acid; their use as probes or primers; and the diagnosis of neuroblastoma prognosis using any of the foregoings.
Owner:HISAMITSU PHARM CO INC +1
Diagnostic methods for determining prognosis of non-small cell lung cancer
InactiveUS8951725B2Accurate identificationEnhanced informationBioreactor/fermenter combinationsBiological substance pretreatmentsFavorable prognosisFluorescence
The invention provides methods for identifying early stage non-small cell lung cancer (NSCLC) patients who will have a favorable prognosis for the recurrence of lung cancer after surgical resection. The invention is based on the discovery that assessment of chromosomal copy number abnormalities at two or more of chromosome 5p15, 7p12, 8q24 and centromere 6 can be used for prognostic classification. The invention preferably uses fluorescence in situ hybridization with fluorescently labeled nucleic acid probes to hybridize to patient samples to quantify the chromosomal copy number of the these genetic loci. Assessment of the copy number abnormality patterns using four classifiers produced statistically significant prognostic classification for NSCLC: (i) the Range3 pattern of cells showing a difference on a cell by cell basis, of at least three FISH probe signals between the FISH signals at the chromosomal locus with the largest number of FISH signals minus the FISH signals at the chromosomal locus with the lowest number of FISH signals; (ii) the MYC / EGFR % loss pattern assessing the percentage of cells showing fewer MYC FISH probe signals than EGFR FISH probe signals; (iii) a combination of the Range3 pattern and the MYC / CEP6 ratio pattern of a percentage of cells showing a relative loss of MYC FISH probe signals to the FISH probe signal for CEP6; (iv) the combination of the MYC / 5p15 ratio pattern showing the relative ratio of MYC and 5p15 locus signals of ≧0.80 and the 5p15 / CEP6 ratio pattern assessing percentage of cells having a relative ratio of 5p15 FISH probe signals to CEP6 FISH probe signals ≧1.1 versus MYC / 5p15 ratio of <0.80 or 5p15 / CEP6<1.1; and (v) a combination of the average range of probe signal differences of equal to or greater than about 2.5 with the Range3 pattern in a percentage of the cells. The invention can be used to identify those early stage NSCLC patients at higher risk of recurrence who should be treated with neoadjuvant chemotherapy before surgery or with adjuvant chemotherapy after surgery.
Owner:ABBOTT MOLECULAR INC
Method for the prognosis of survival time of a patient suffering from a solid cancer
InactiveUS20150004253A1Improve accuracyPrognosis of the survival timeHeavy metal active ingredientsDisease diagnosisHigh densityFavorable prognosis
The present invention relates to an in vitro method for the prognosis of the survival time of a patient suffering from a solid cancer, comprising the quantification of the cell density of follicular B cells present in tumor-induced lymphoid structures from said patient, wherein a high density of follicular B cells indicates that the patient has a favorable prognosis and a low density of follicular B cells indicates that the patient has a poor prognosis.
Owner:INST NAT DE LA SANTE & DE LA RECHERCHE MEDICALE (INSERM) +4
Methods for predicting the survival time and treatment responsiveness of a patient suffering from a solid cancer with a signature of at least 7 genes
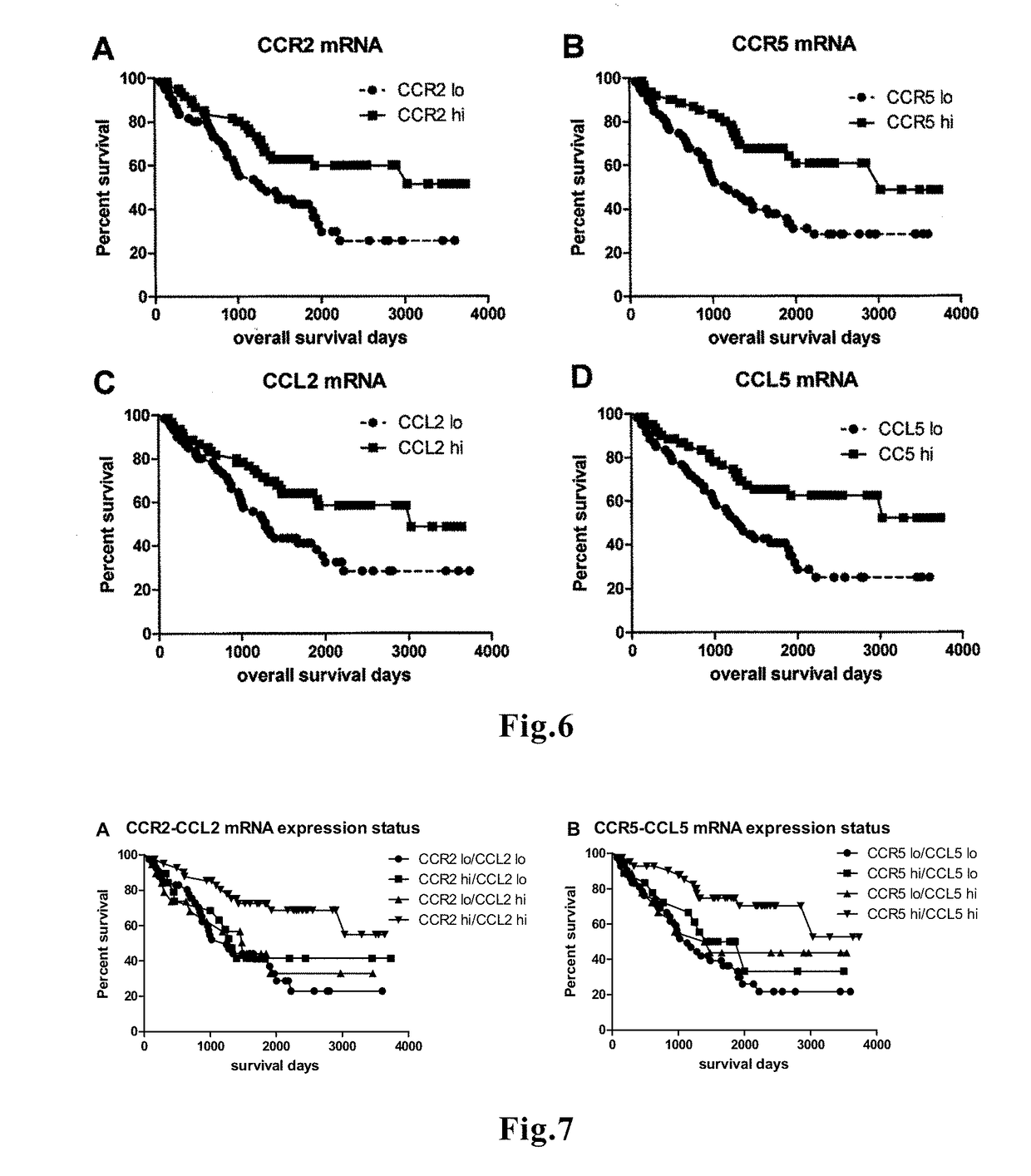
ActiveUS11242564B2Increase productionEasier for the immune system to recognise and destroyMicrobiological testing/measurementAntineoplastic agentsCCL2Favorable prognosis
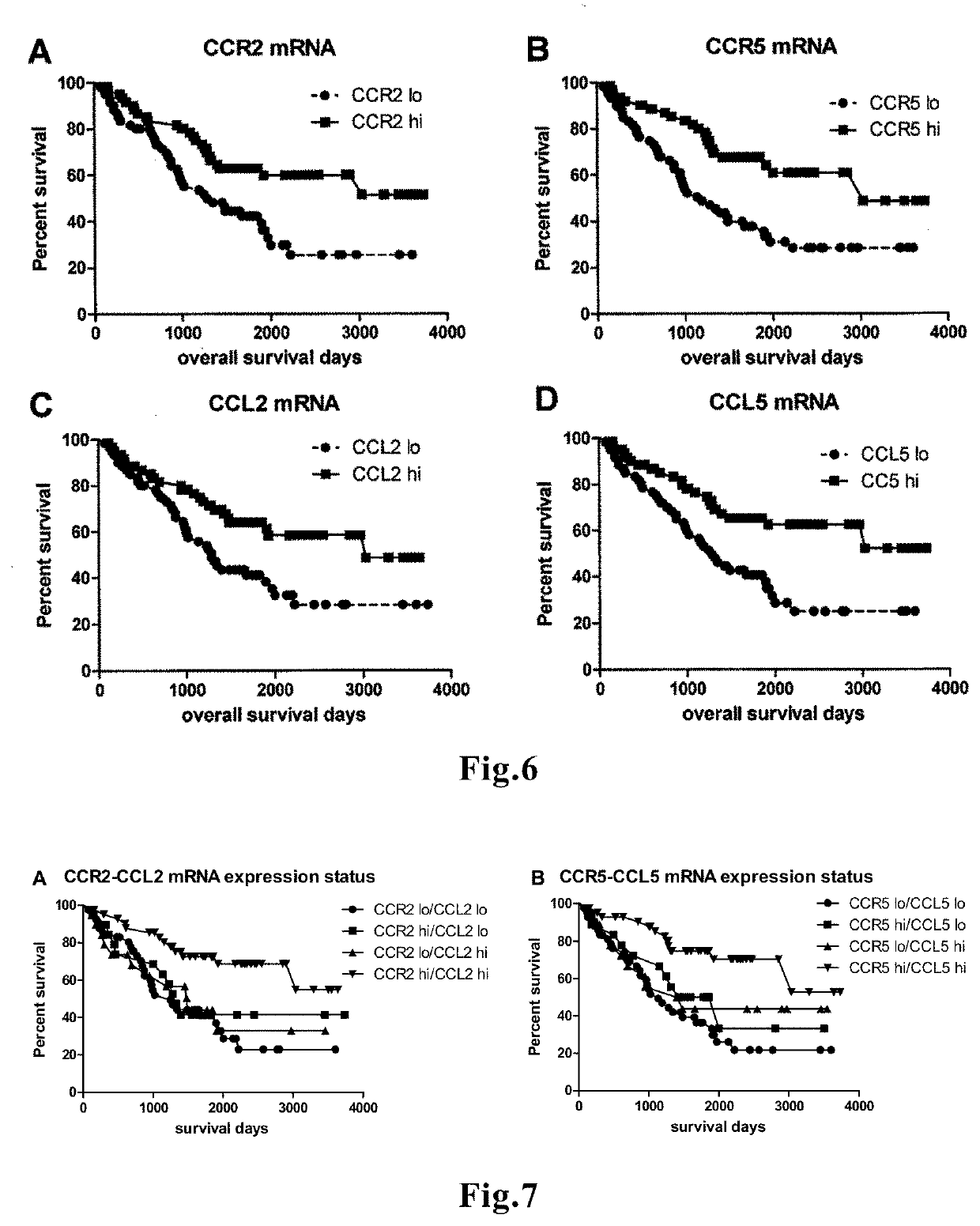
The present invention relates to a method for predicting the survival time of a patient suffering from a solid cancer comprising i) determining in a tumor sample obtained from the patient the gene expression level of at least 7 genes selected from the group consisting of CCR2, CD3D, CD3E, CD3G, CD8A, CXCL10, CXCL11, GZMA, GZMB, GZMK, GZMM, IL15, IRF1, PRF1, STAT1, CD69, ICOS, CXCR3, STAT4, CCL2, and TBX21, ii) comparing every expression level determined at step i) with their predetermined reference value and iii) providing a good prognosis when all expression levels determined at step i) are higher than their predetermined reference values, or providing a bad prognosis when all expression levels determined at step i) are lower than their predetermined reference values or providing an intermediate prognosis when at least one expression level determined value is higher than its predetermined value. The method is also particularly suitable for predicting the responsiveness of the patient to a treatment.
Owner:INST NAT DE LA SANTE & DE LA RECHERCHE MEDICALE (INSERM) +2
Nucleic acids isolated in neuroblastoma
There are disclosed a nucleic acid whose expression is enhanced in human neuroblastoma with unfavorable prognosis based on comparison between human neuroblastoma with favorable prognosis and human neuroblastoma with unfavorable prognosis, the nucleic acid comprising any one of base sequences set forth in SEQ ID NO:1 to NO:69 in the Sequence Listing, a nucleic acid comprising a portion of any of those base sequences, and an isolated nucleic acid capable of hybridizing to a complementary base sequence of the foregoing under stringent conditions. It discloses gene sequences relating to favorable or unfavorable prognosis of neuroblastoma and will enable the provision of their genetic information and the diagnosis of favorable or unfavorable prognosis.
Owner:HISAMITSU PHARM CO INC +1
Nucleic acids isolated in neuroblastoma
InactiveUS20050059001A1Considerable numberImproved prognosisSugar derivativesHydrolasesFavorable prognosisNeuroblastoma
There are disclosed a nucleic acid whose expression is enhanced in human neuroblastoma with favorable prognosis based on comparison between human neuroblastoma with favorable prognosis and human neuroblastoma with unfavorable prognosis, the nucleic acid comprising any one of base sequences set forth in SEQ ID NO:1 to NO:366 in the Sequence Listing, a nucleic acid comprising a portion of any of those base sequences, and an isolated nucleic acid capable of hybridizing to a complementary base sequence of the foregoing under stringent conditions. It discloses gene sequences relating to favorable or unfavorable prognosis of neuroblastoma and will enable the provision of their genetic information and the diagnosis of favorable or unfavorable prognosis.
Owner:HISAMITSU PHARM CO INC +1
Chromosome copy number gain as a biomarker of urothelial carcinoma lethality
ActiveUS20120295807A1Improved prognosisHigh risk of developingMicrobiological testing/measurementLibrary screeningFavorable prognosisTissue sample
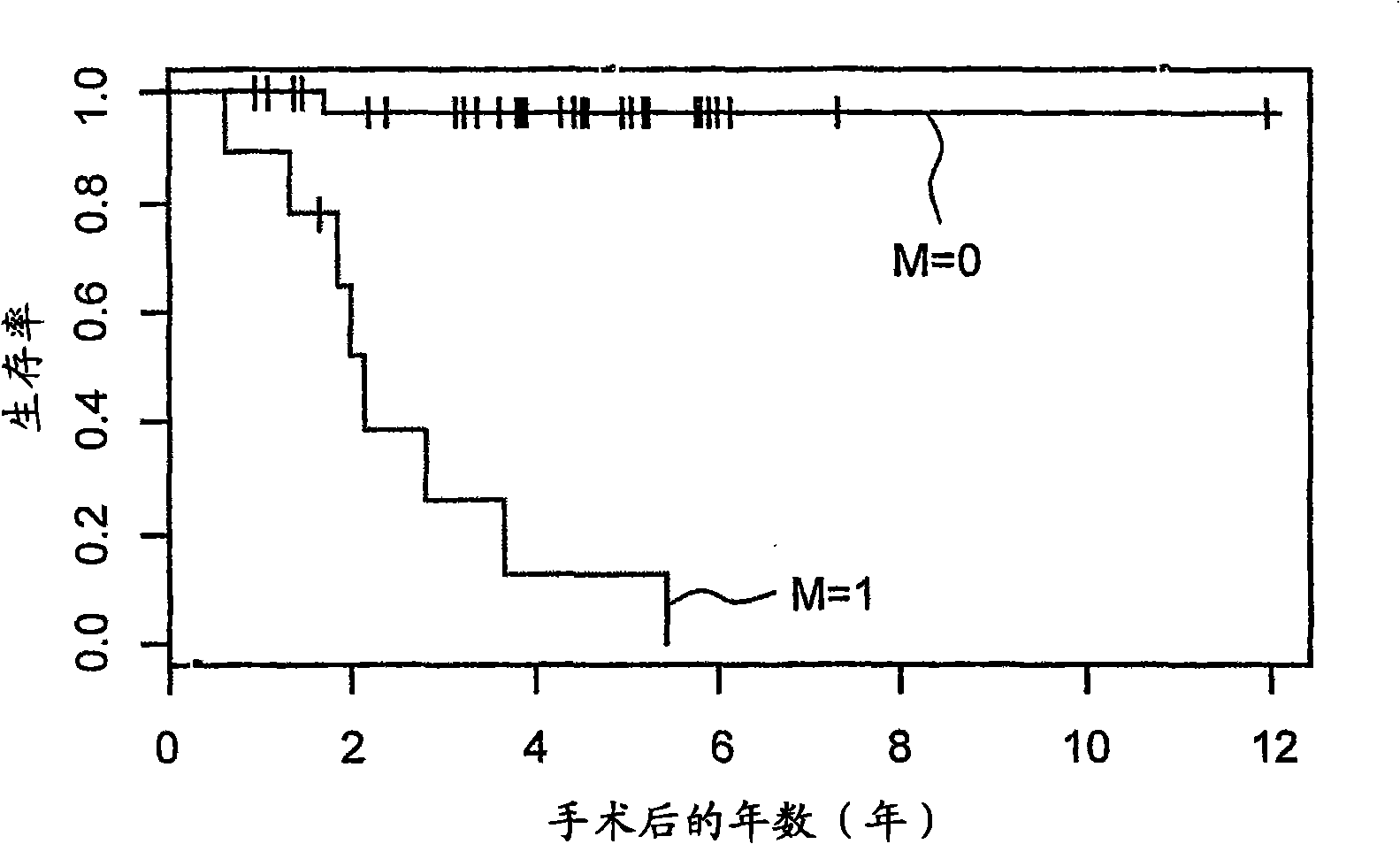
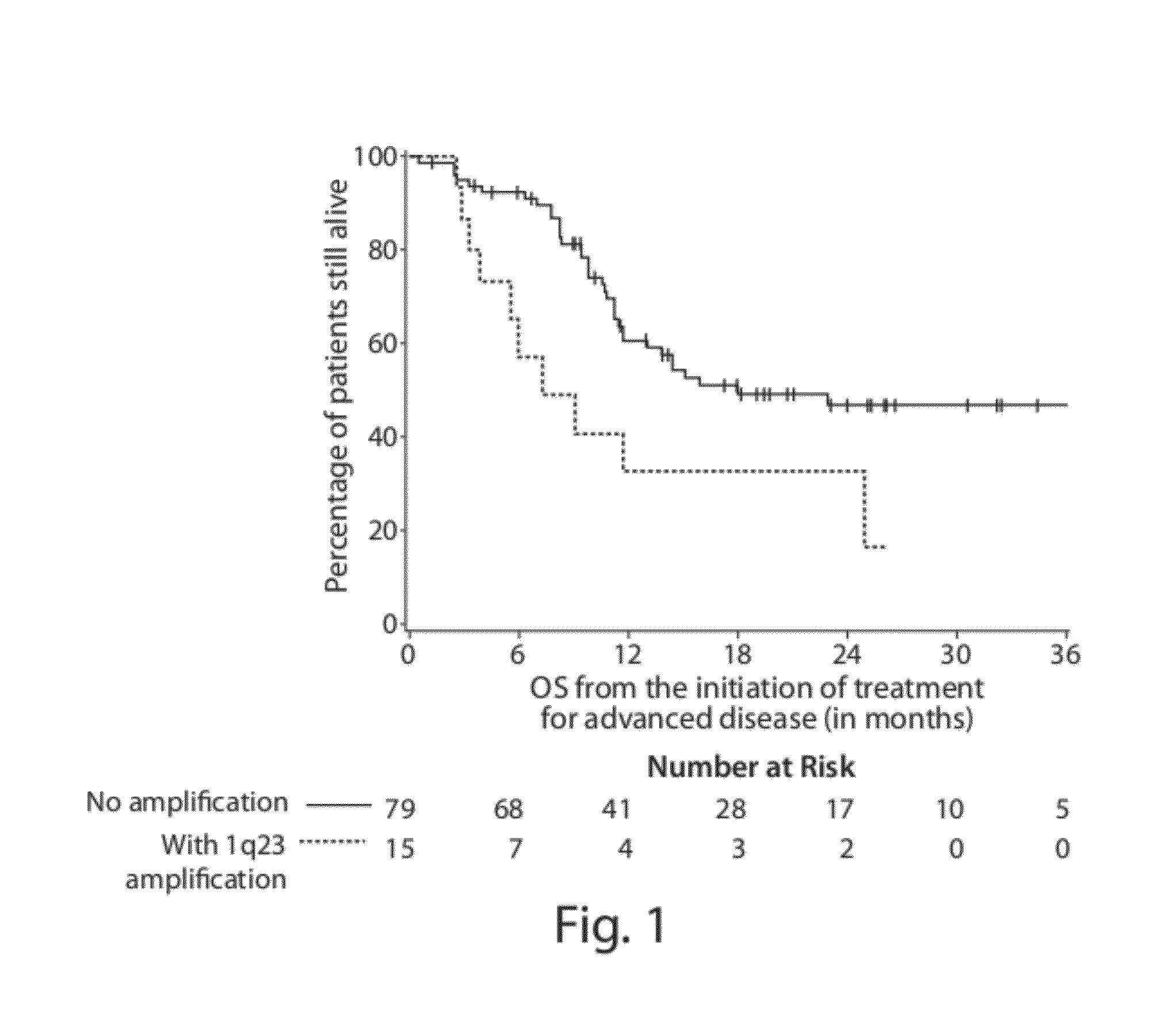
Diagnostic assays for medically classifying cancer patients are provided. The method comprises assessing a tissue sample of the patient for the presence of a copy number gain of chromosome regions 1q23.3 and / or 1q21.2. Copy number gain of chromosome regions 1q23.3 and / or 1q21.2 is indicative of a less favorable prognosis as compared to the prognosis if there was no copy number gain in the same regions.
Owner:DANA FARBER CANCER INST INC
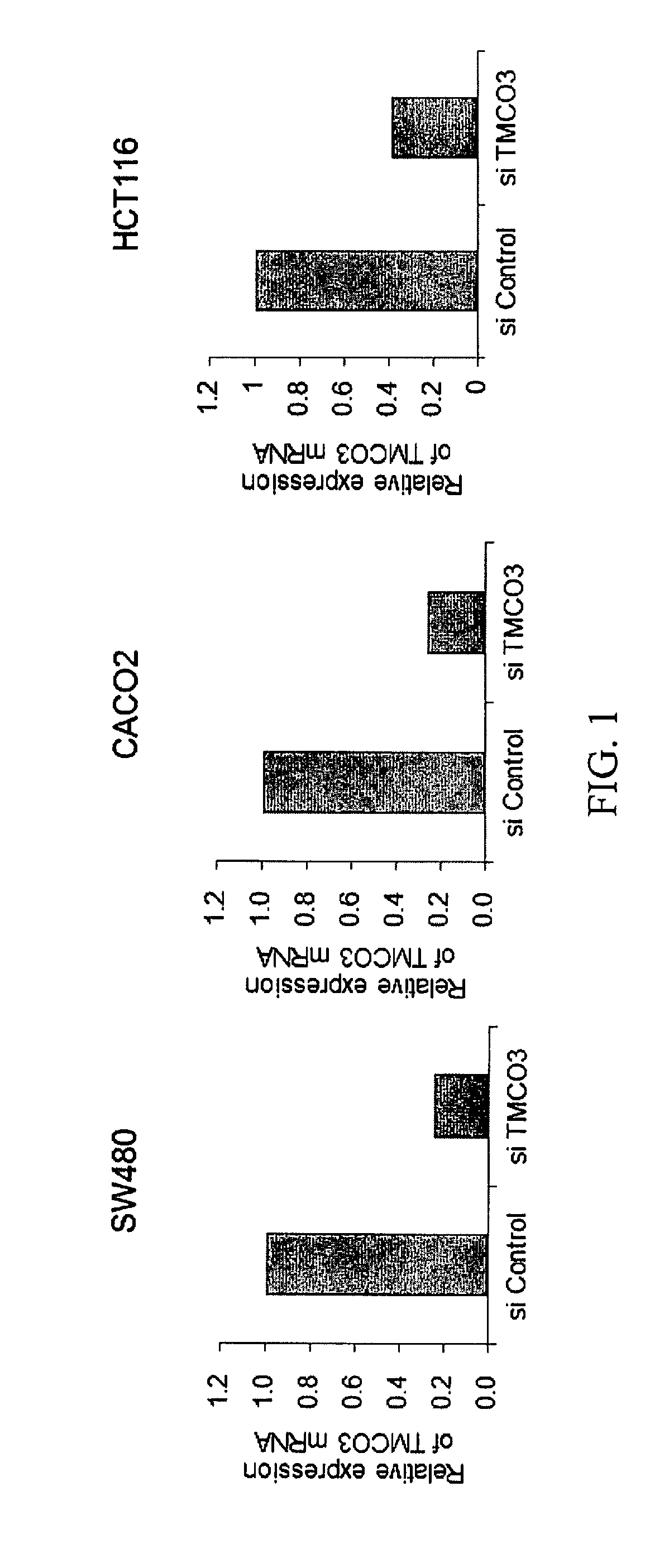
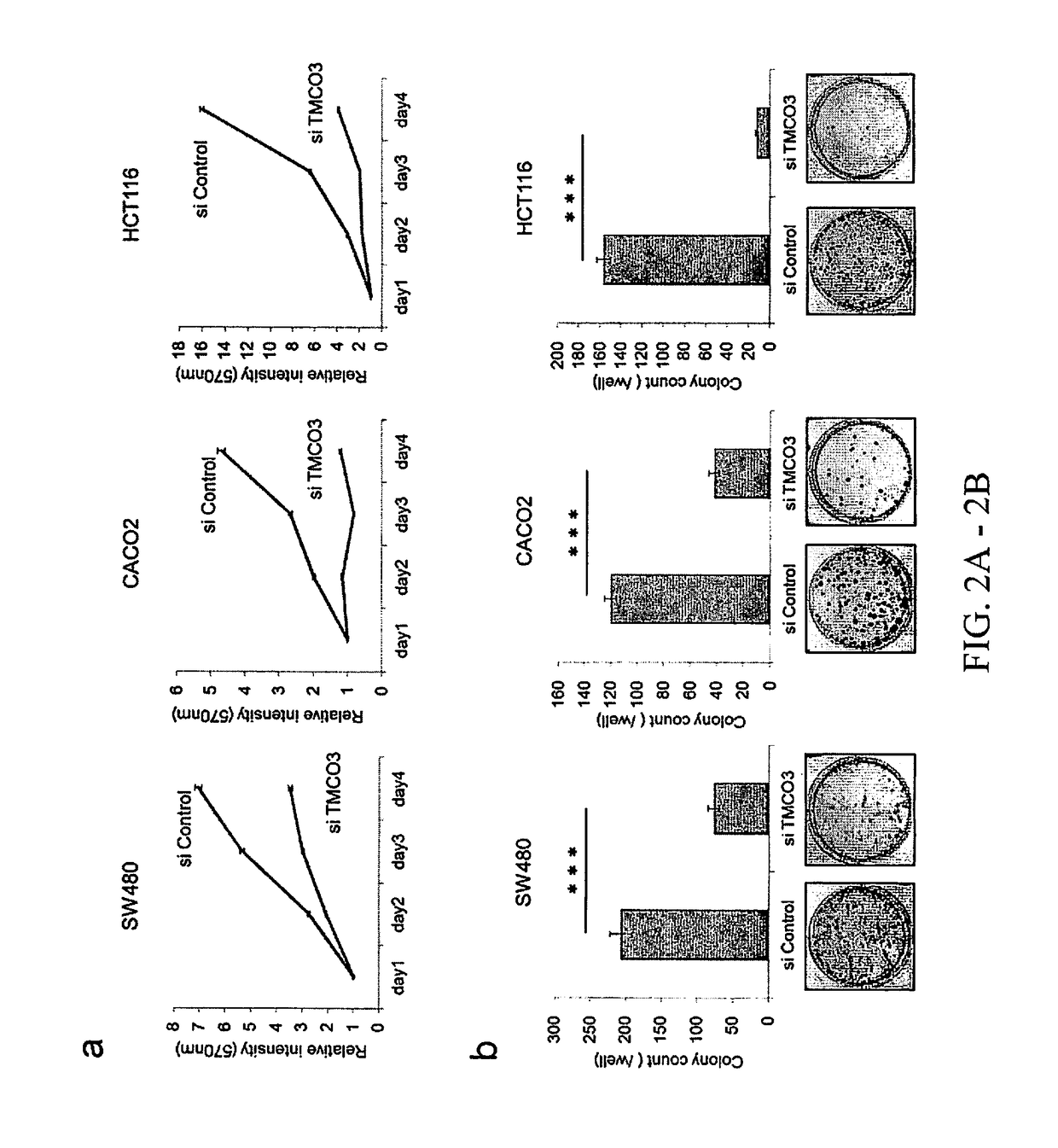
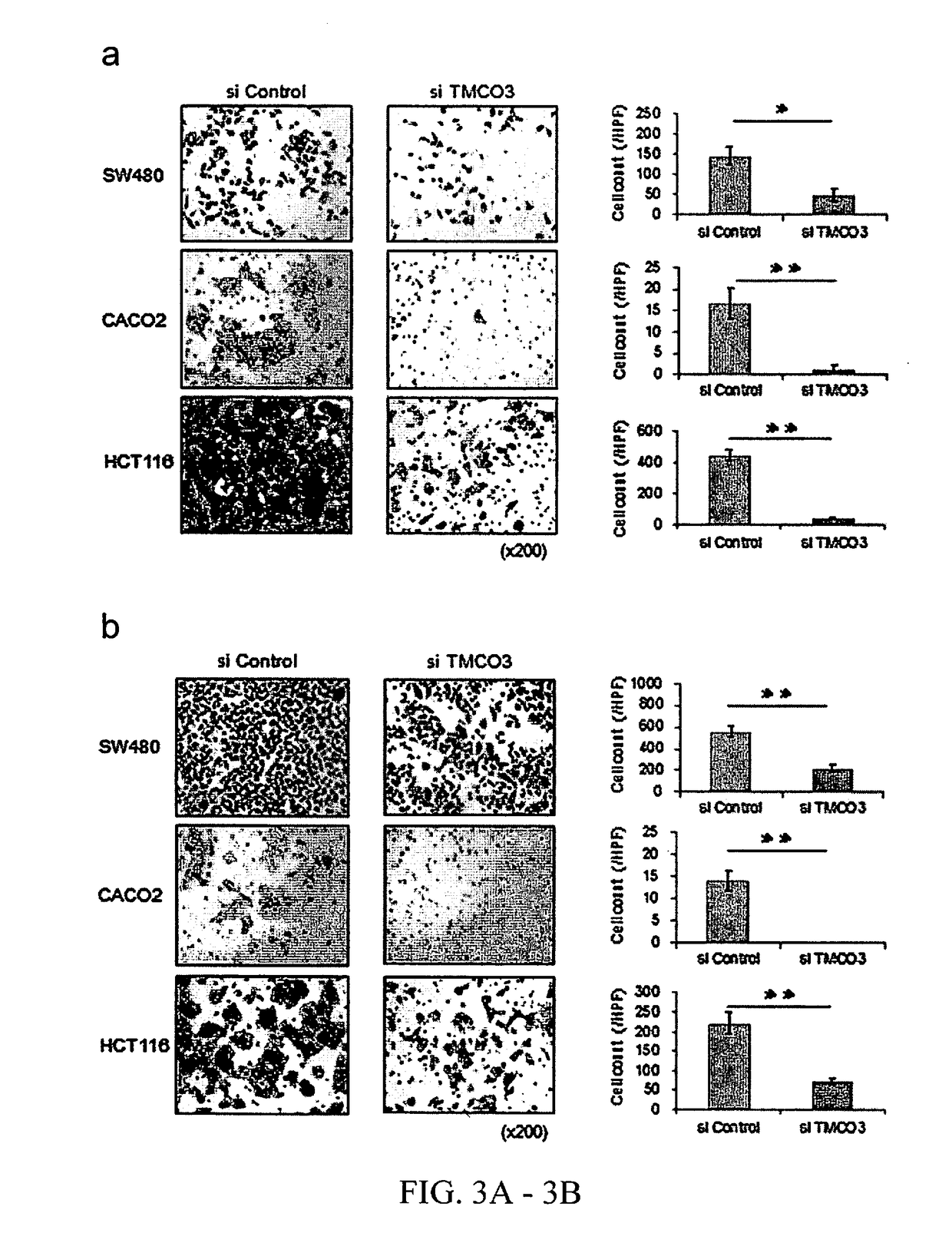
Methods and compositions involving transmembrane and coiled-coil domains 3 (tm-co3) in cancer
ActiveUS20180298386A1Organic active ingredientsMicrobiological testing/measurementFavorable prognosisOncology
Certain embodiments provide methods and compositions related to clinical management of cancer patients based on the expression level of TMCO3. Further embodiments involve methods and compositions related to treatment of cancer patients or patients determined to have an increased TMCO3 level relative to a control or a reference level that is normal or indicating favorable prognosis.
Owner:BAYLOR RES INST
Medicine for treating infantile enterorrhea
ActiveCN1251682CSlow down the peristalsisReduce stimulationOrganic active ingredientsDigestive systemFavorable prognosisGlucomannan
Owner:GUANGZHOU TENGJIAN MARINE LIFE SCI TECH
Lung cancer diagnostics and therapeutics with mir-660
InactiveUS20190276831A1Microbiological testing/measurementAntineoplastic agentsFavorable prognosisUbiquitin-Protein Ligases
Owner:BIOMIRNA HLDG
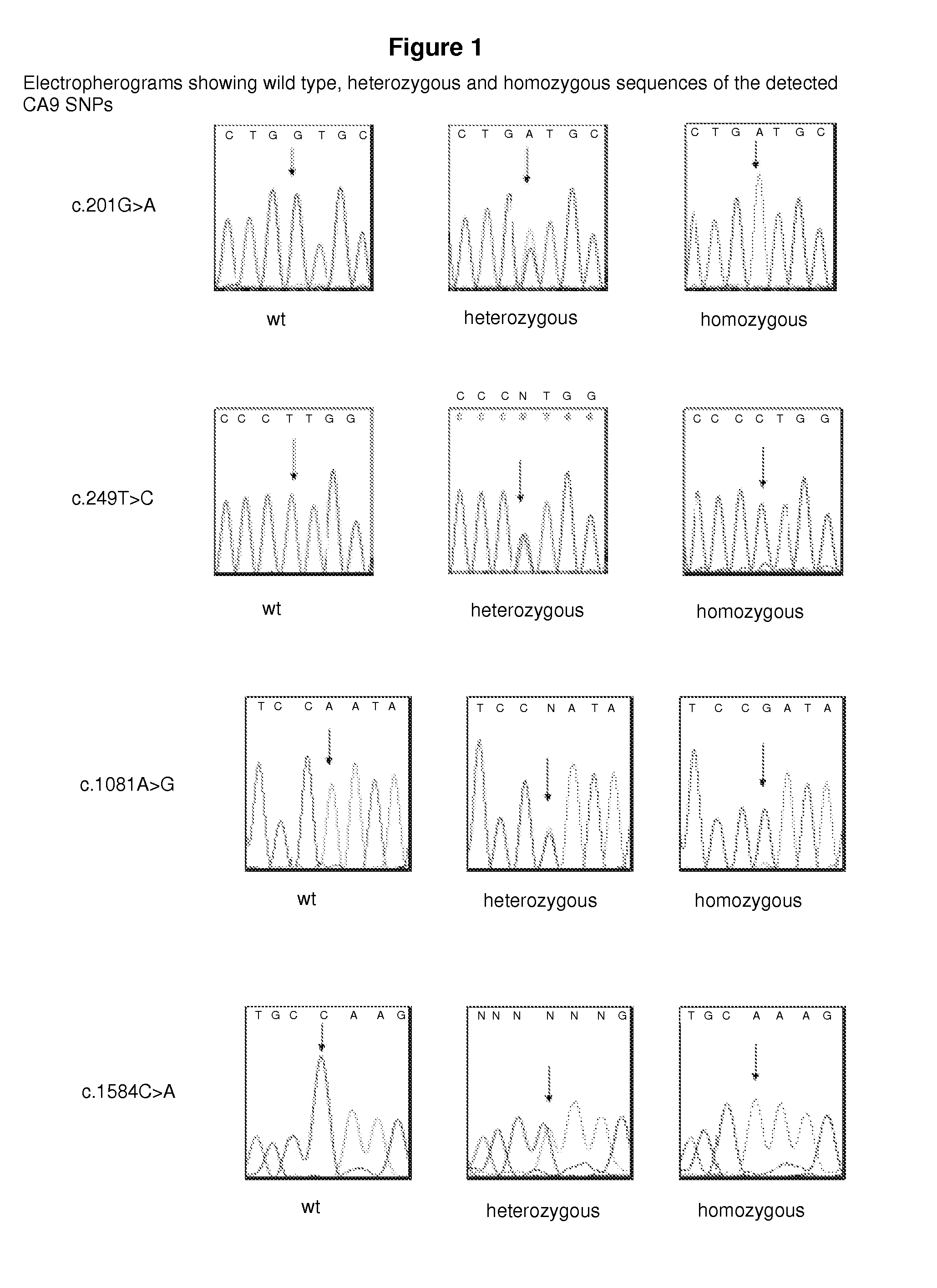
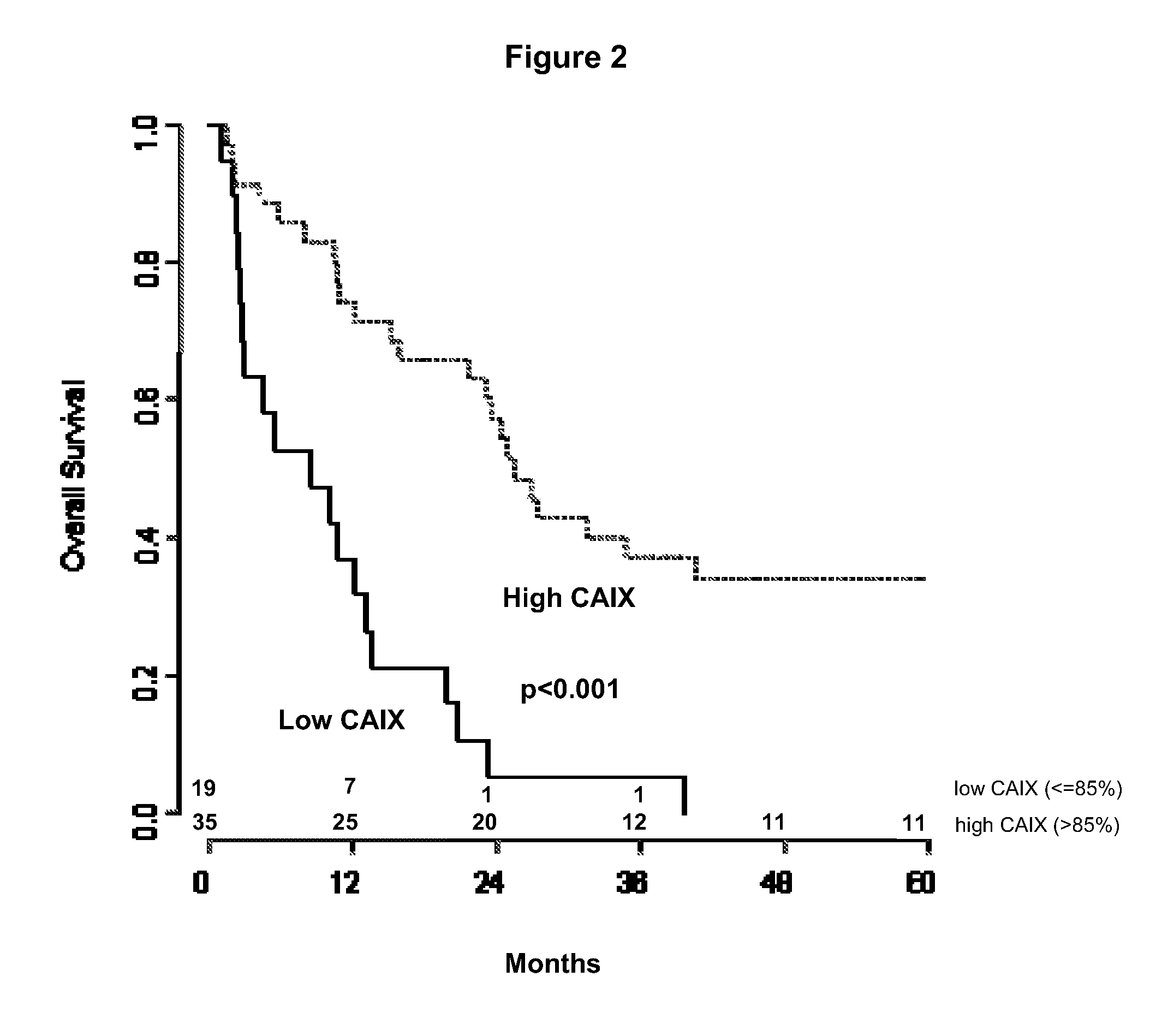
Ca9 gene single nucleotide polymorphisms predict prognosis and treatment response of metastatic renal cell carcinoma
InactiveUS20110262391A1Improve survivalGreat likelihoodSugar derivativesPeptide/protein ingredientsMetastatic renal cell cancerFavorable prognosis
Methods and compositions for providing a prognosis or diagnosis for a human patient having renal cell cancer are provided. The method relates to the discovery of SNPs which are associated with a favorable prognosis and response to therapy in RCC.
Owner:RGT UNIV OF CALIFORNIA
Method for the prognosis of survival time of a patient suffering from a solid cancer
InactiveUS20200041519A1Improve accuracyPrognosis of the survival timeDisease diagnosisCancer antigen ingredientsFavorable prognosisGood prognosis
The present invention relates to an in vitro method for the prognosis of the survival time of a patient suffering from a solid cancer, comprising the quantification of the cell density of follicular B cells present in tumor-induced lymphoid structures from said patient, wherein a high density of follicular B cells indicates that the patient has a favorable prognosis and a low density of follicular B cells indicates that the patient has a poor prognosis.
Owner:UNIVERSITÉ PARIS CITÉ +3
Method for predicting prognosis of patient with cancer or inflammatory disease
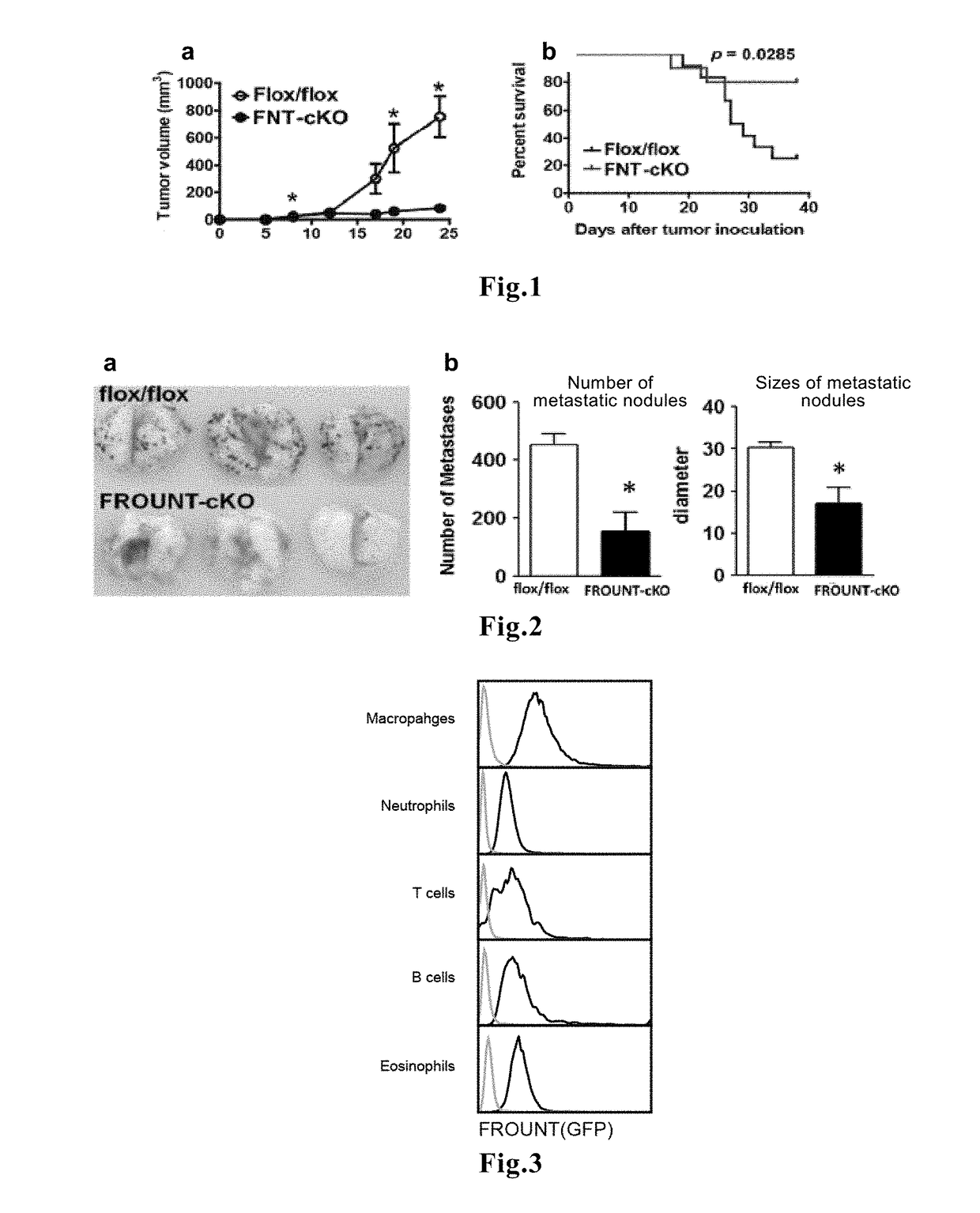
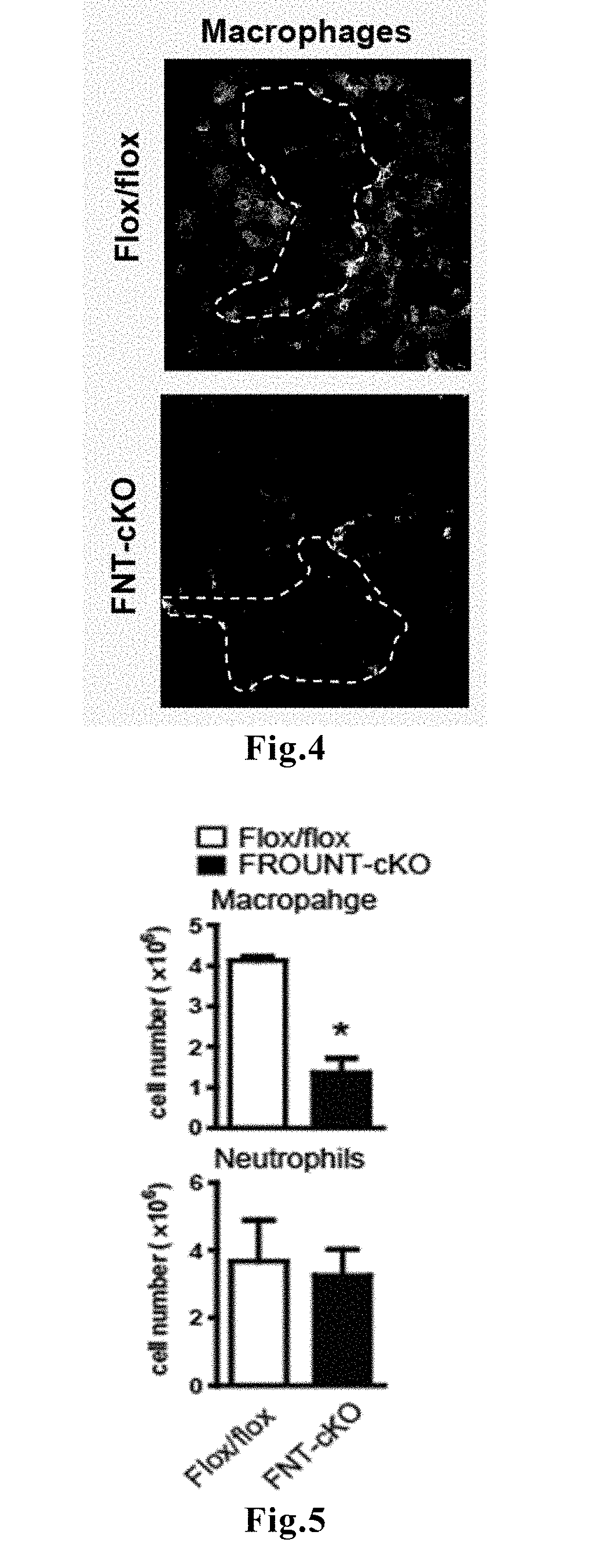
ActiveUS11193172B2Predict prognosisImproved prognosisMicrobiological testing/measurementDisease diagnosisDiseaseFavorable prognosis
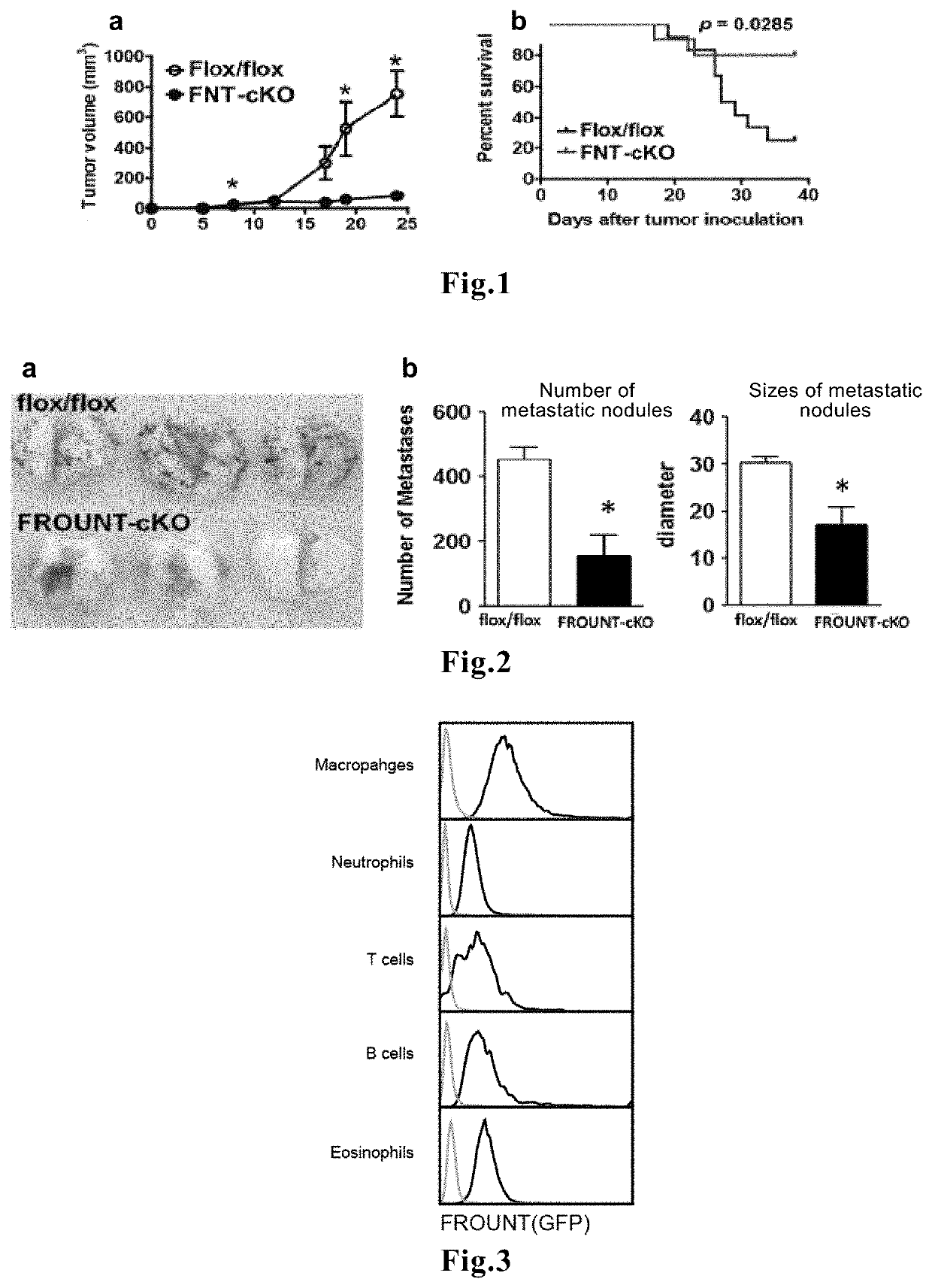
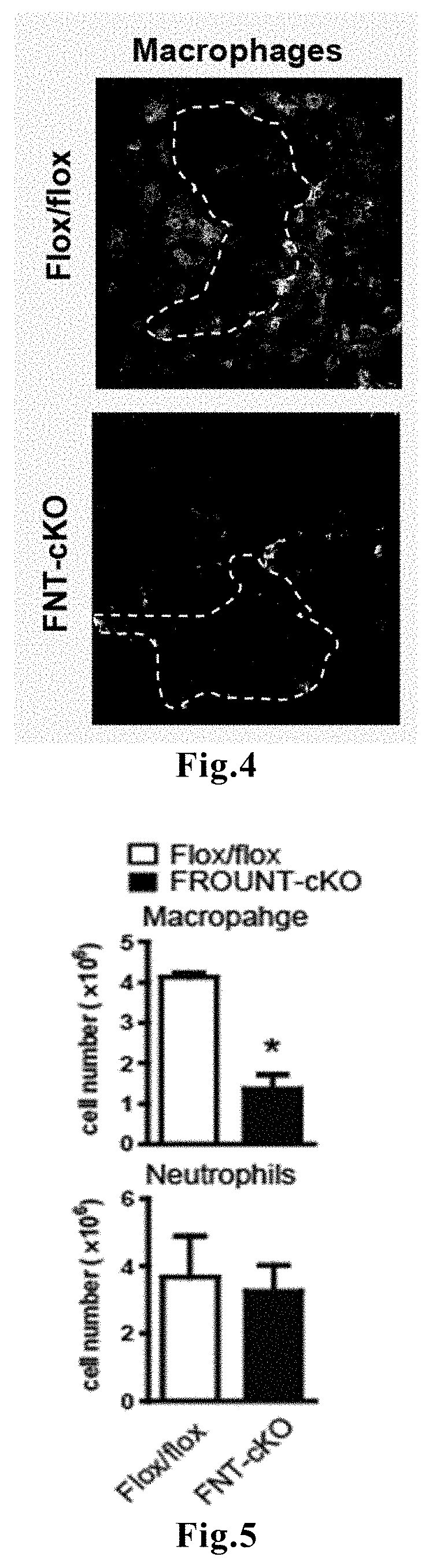
Novel means that enables prediction of prognosis of a patient with cancer or inflammatory disease is disclosed. In the method for prediction of prognosis of a patient with cancer or inflammatory disease according to the present invention, the expression level of the FROUNT gene in a sample collected from the patient is measured. Since FROUNT is a poor prognostic factor, the lower the expression level of the FROUNT gene is, the better the prognosis in the patient is predicted to be. Or, the expression level of the CC chemokine receptor / ligand gene in a sample collected from the patient is measured. Since the CC chemokine receptor / ligand gene such as CCR2 or CCR5 is a good prognostic factor, the higher the expression level of the CC chemokine receptor / ligand gene is, the better the prognosis in the patient is predicted to be.
Owner:TOKYO UNIVERSITY OF SCIENCE
Multi-sinus combined cerebral venous thrombosis animal model and construction method thereof
The invention relates to the technical field of medicines, and particularly discloses a multi-sinus combined cerebral venous thrombosis animal model and a construction method thereof. A severe CVT animal model with multiple venous sinus combined thrombus (such as superior sagittal sinus + transverse sinus, superior sagittal sinus + transverse sinus + internal jugular vein), large-area venous cerebral infarction, cerebral hemorrhage and other pathological changes, and with more serious neurological dysfunction and longer thrombus duration is induced through a method of combining semi-ligation with ferric chloride and thrombin; and the model can be used for research on pathological and physiological mechanisms of severe CVT as well as research and evaluation on treatment strategies, so that treatment of patients with severe CVT and promotion of good prognosis are possibly facilitated, new targets are explored, novel drugs are researched and developed, and the death rate of the patients with severe CVT is reduced.
Owner:XUANWU HOSPITAL OF CAPITAL UNIV OF MEDICAL SCI
Method for predicting prognosis of patient with cancer or inflammatory disease
ActiveUS20180237860A1Improved prognosisAccurately carry-outMicrobiological testing/measurementDisease diagnosisFavorable prognosisCC chemokine
Novel means that enables prediction of prognosis of a patient with cancer or inflammatory disease is disclosed. In the method for prediction of prognosis of a patient with cancer or inflammatory disease according to the present invention, the expression level of the FROUNT gene in a sample collected from the patient is measured. Since FROUNT is a poor prognostic factor, the lower the expression level of the FROUNT gene is, the better the prognosis in the patient is predicted to be. Or, the expression level of the CC chemokine receptor / ligand gene in a sample collected from the patient is measured. Since the CC chemokine receptor / ligand gene such as CCR2 or CCR5 is a good prognostic factor, the higher the expression level of the CC chemokine receptor / ligand gene is, the better the prognosis in the patient is predicted to be.
Owner:TOKYO UNIVERSITY OF SCIENCE
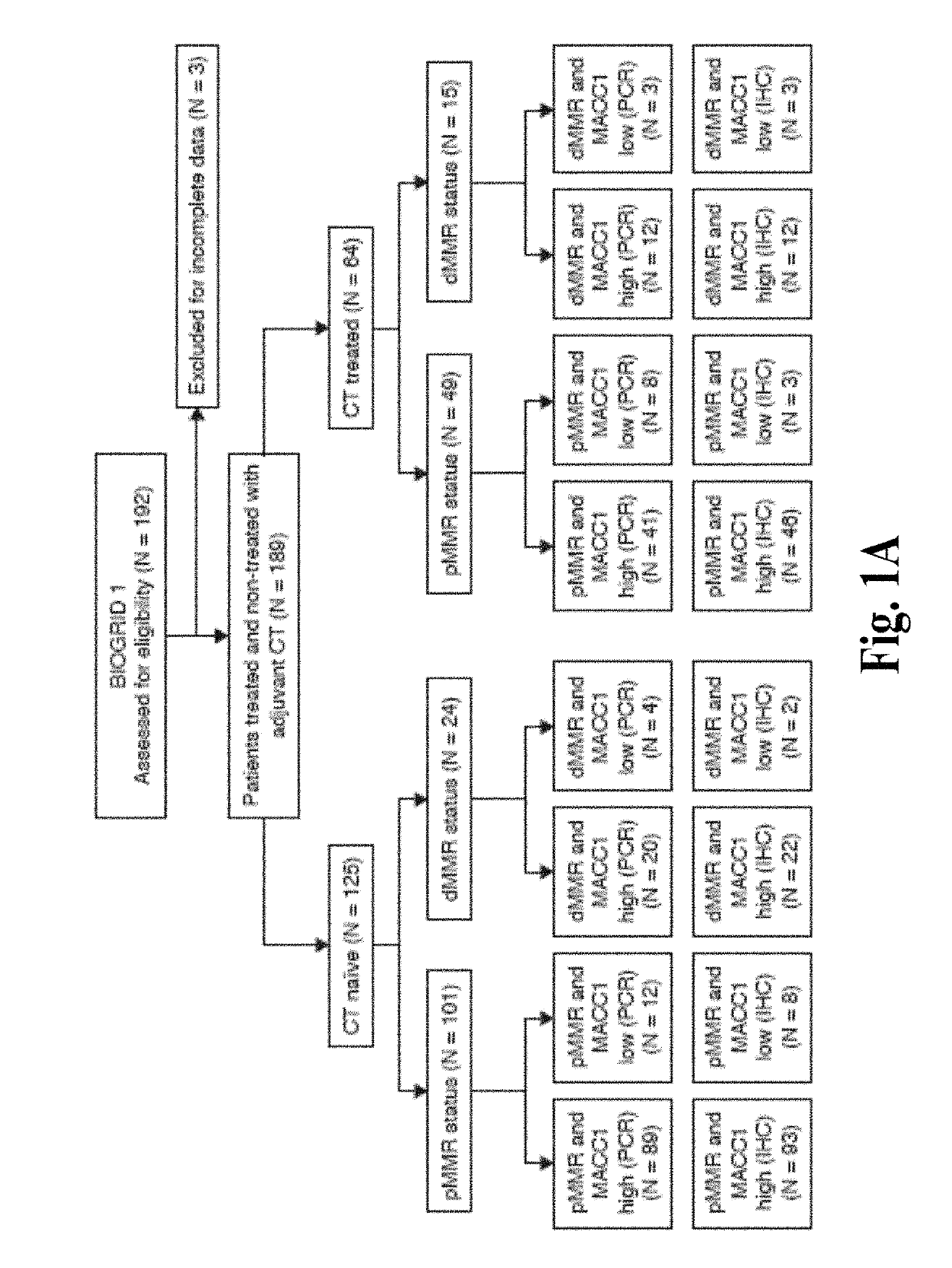
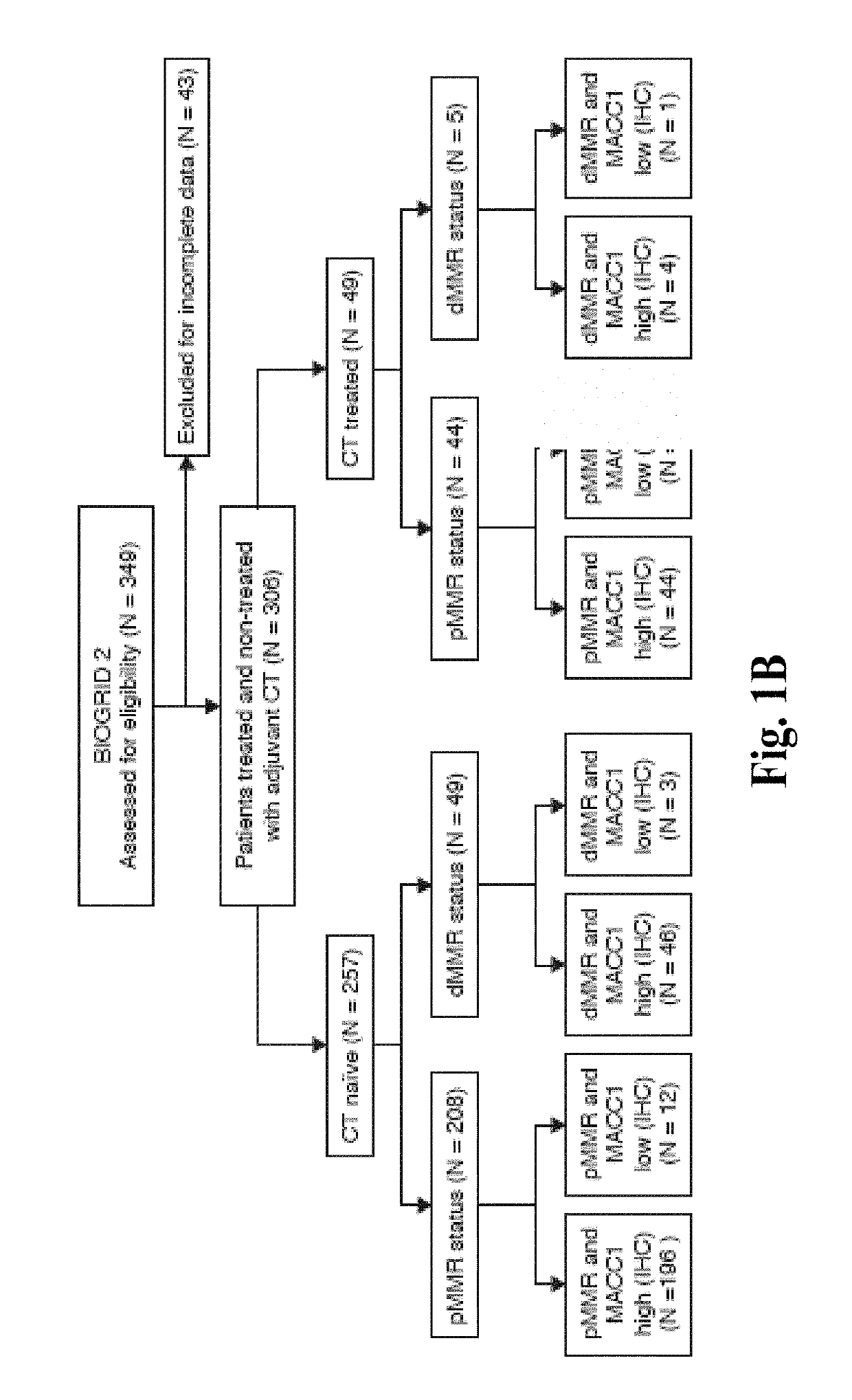
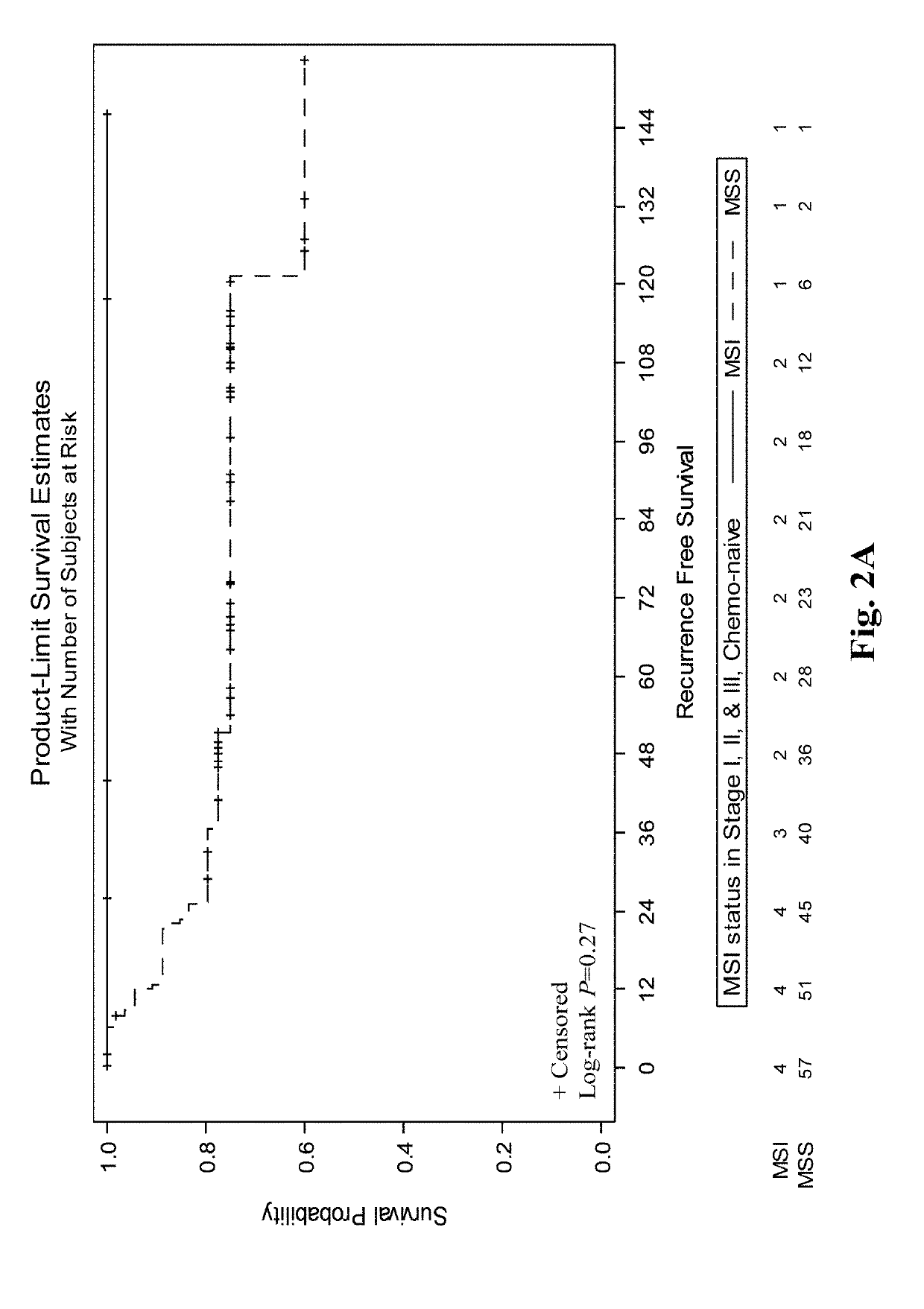
Compositions and methods for prognosing and treating colorectal cancer
InactiveUS20190269716A1Microbiological testing/measurementPreparing sample for investigationPatient survivalFavorable prognosis
A combination of mismatch repair (MMR) and Metastasis Associated in Colon Cancer 1 (MACC1) gene expression status of the patient serve as a basis for risk stratification of early stage colon cancer patients. Patients with defective MMR (dMMR) status have improved survival and do not benefit from 5-fluorouracil (5-FU) therapies. In contrast, patients with a proficient MMR (pMMR) status have a higher risk of recurrence and worse survival. The pMMR patients are then further stratified on the basis of MACC1 gene expression. Patients with a pMMR status and a low MACC1 expression have a favorable prognosis similar to patients having a dMMR status, whereas patients having a pMMR status and high MACC1 expression have a less favorable prognosis.
Owner:VENTANA MEDICAL SYST INC +2
Method for the prognosis of survival time of a patient suffering from a solid cancer
ActiveUS9851357B2Correctly accurately predictIncrease probabilityDisease diagnosisBiological testingDendritic cellFavorable prognosis
The present invention relates to an in vitro method for the prognosis of survival of a patient suffering from a solid cancer, comprising the quantification of the cell density of CD8+ cells and DC-LAMP+ dendritic cells present in a tumor tissue sample from said patient, wherein a high density of CD8+ cells and DC-LAMP+ dendritic cells indicates that the patient has a favorable prognosis, a high density of CD8+ cells and a low density of DC-LAMP+ dendritic cells indicates that the patient has a poor prognosis, and a low density of CD8+ cells and DC-LAMP+ dendritic cells indicates that the patient has the worst prognosis.
Owner:UNIVERSITÉ PARIS CITÉ +3
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com