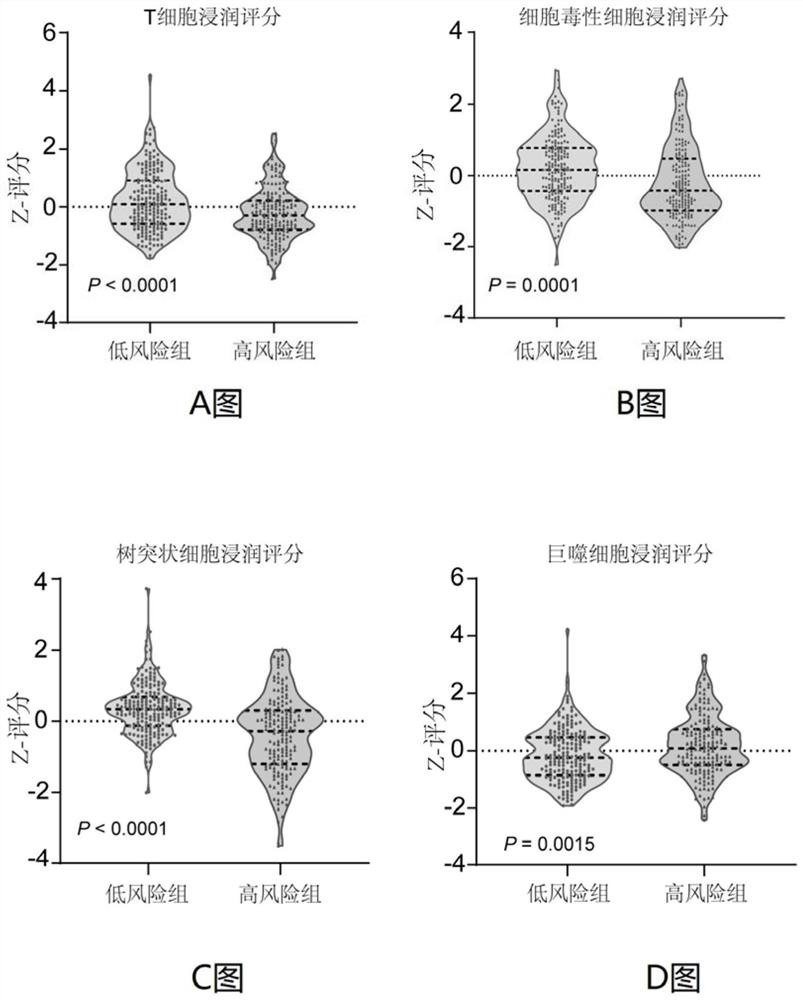
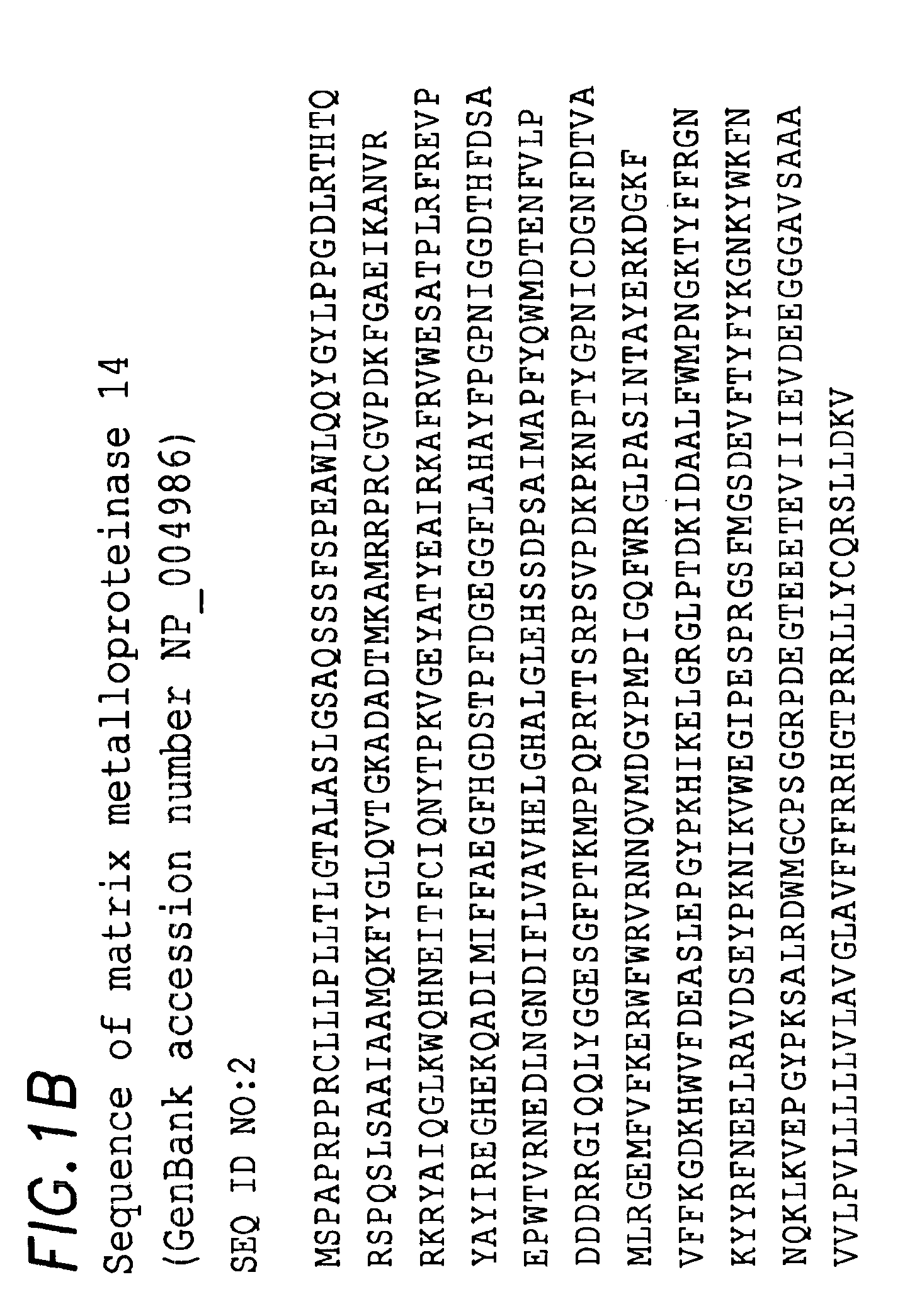
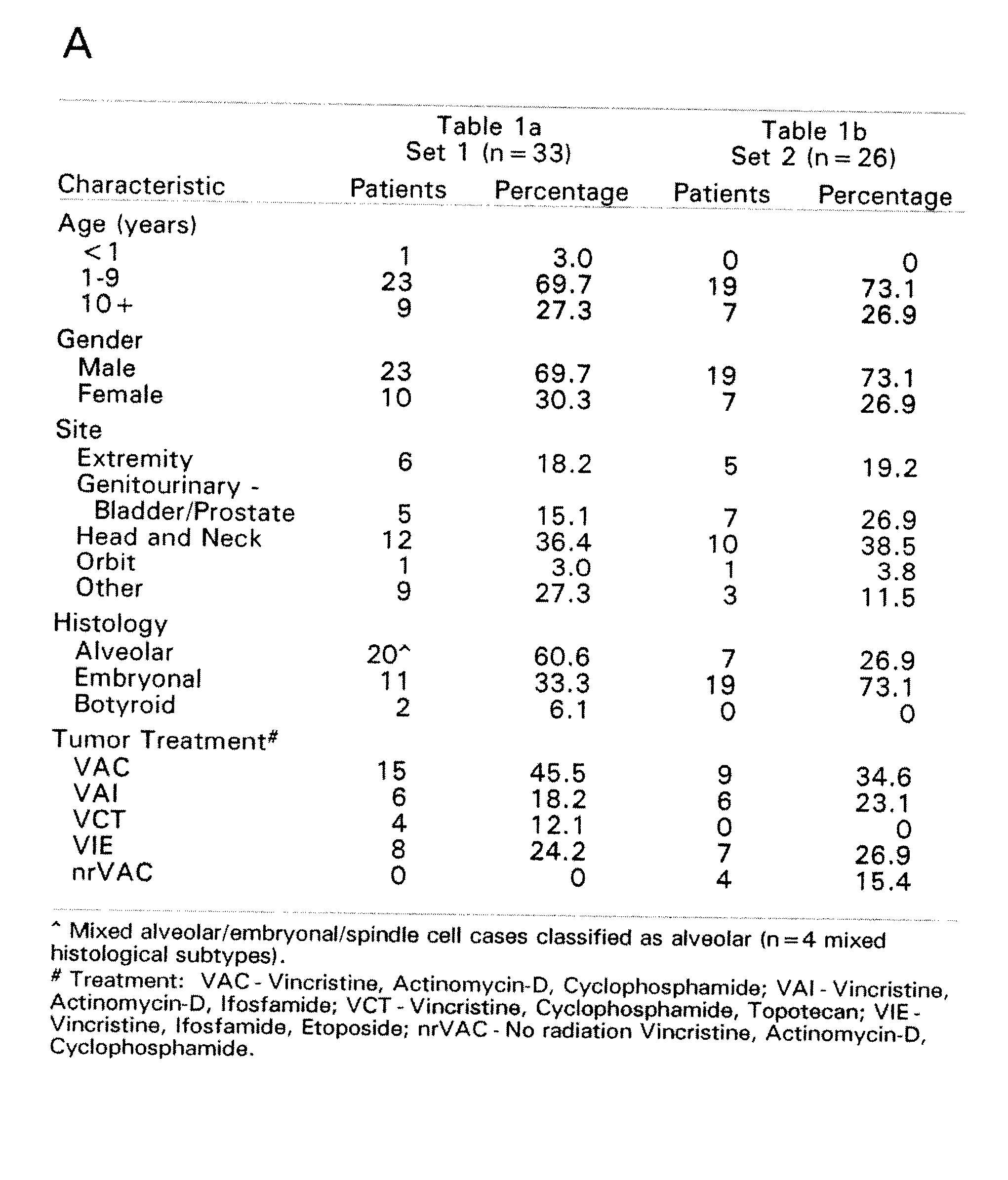
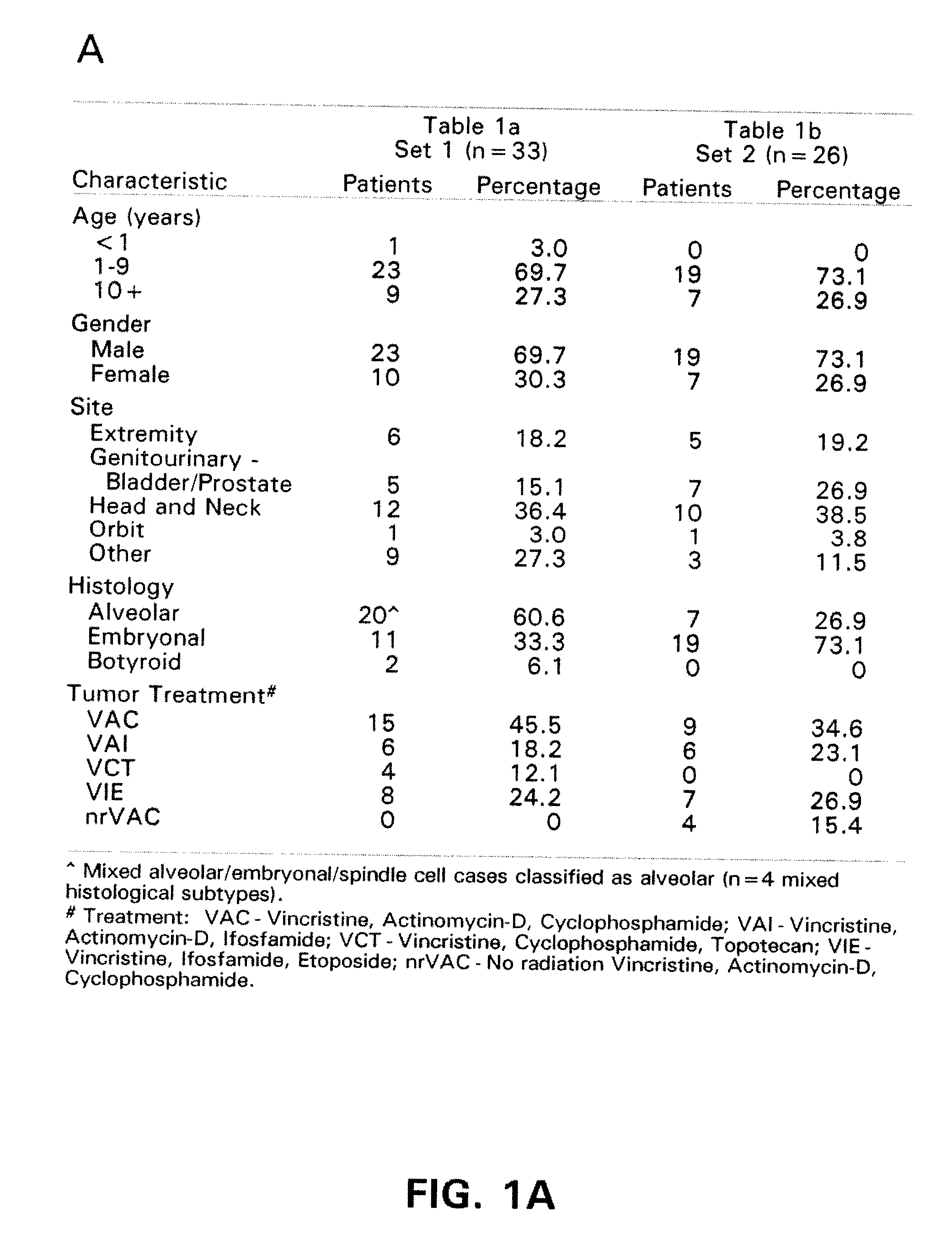
Patents
Literature
321 results about "Poor prognosis" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
A prognosis is an educated forecast of a disease’s possible progress. A good prognosis expects that the patient will do well based on current or previous test results. A poor prognosis expects that there will be complications or negative progress based on test results.
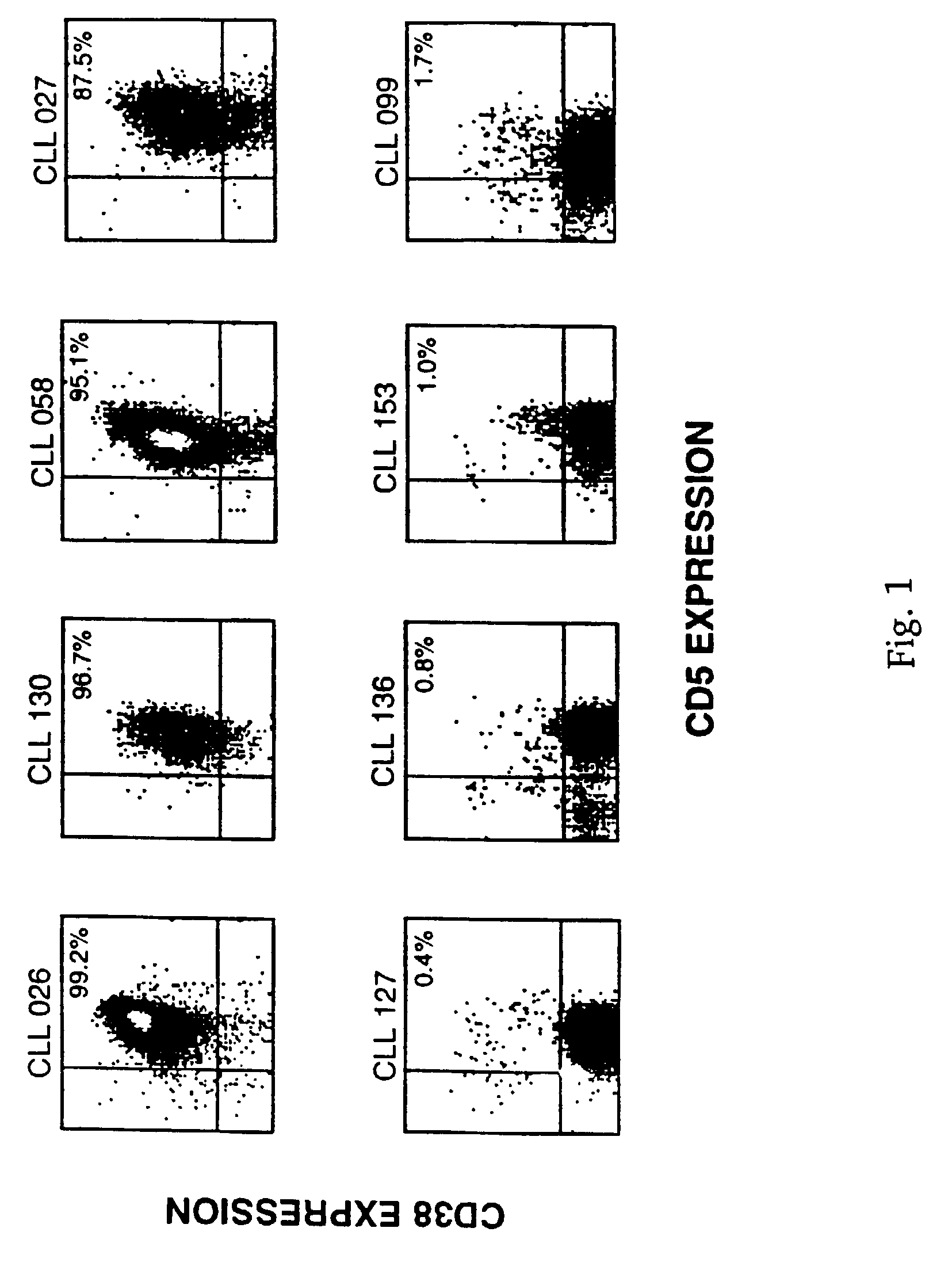
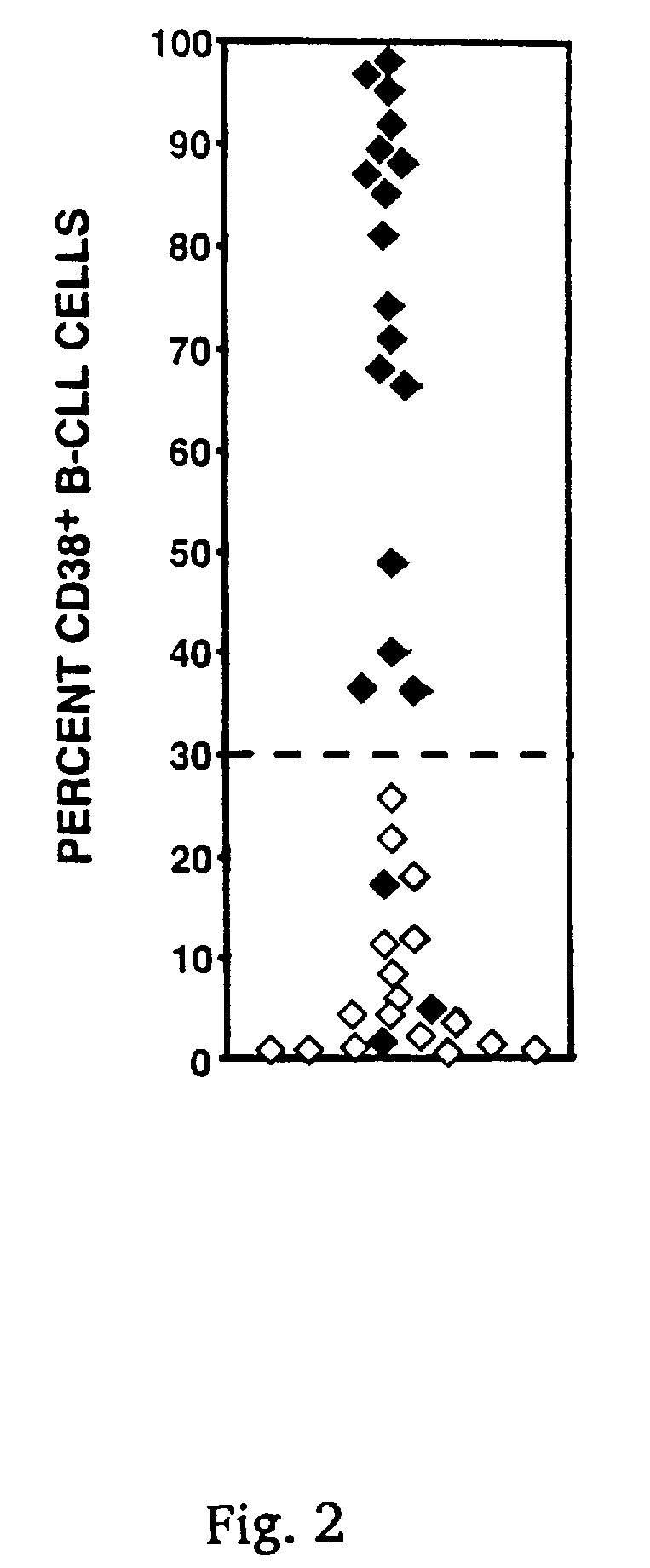
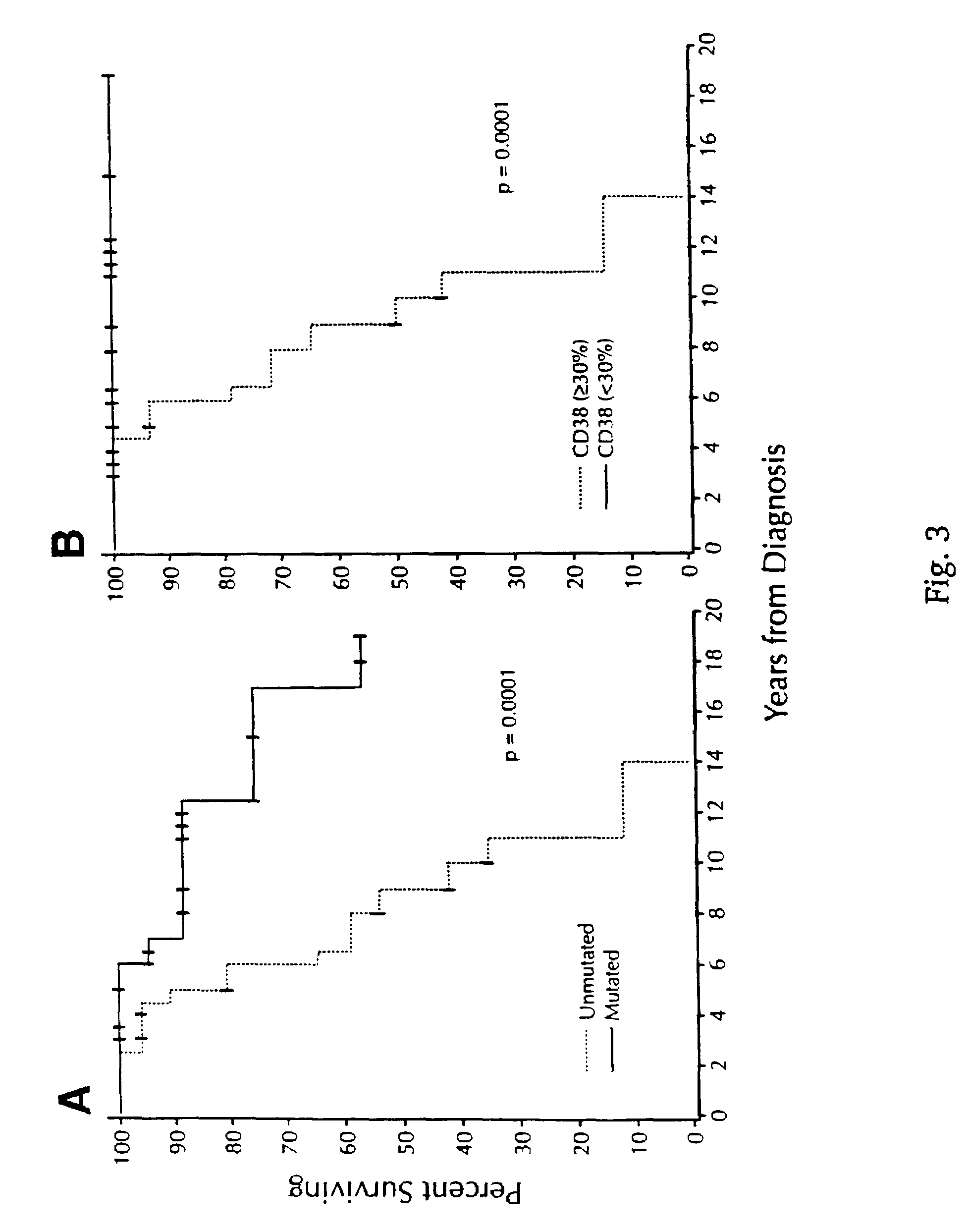
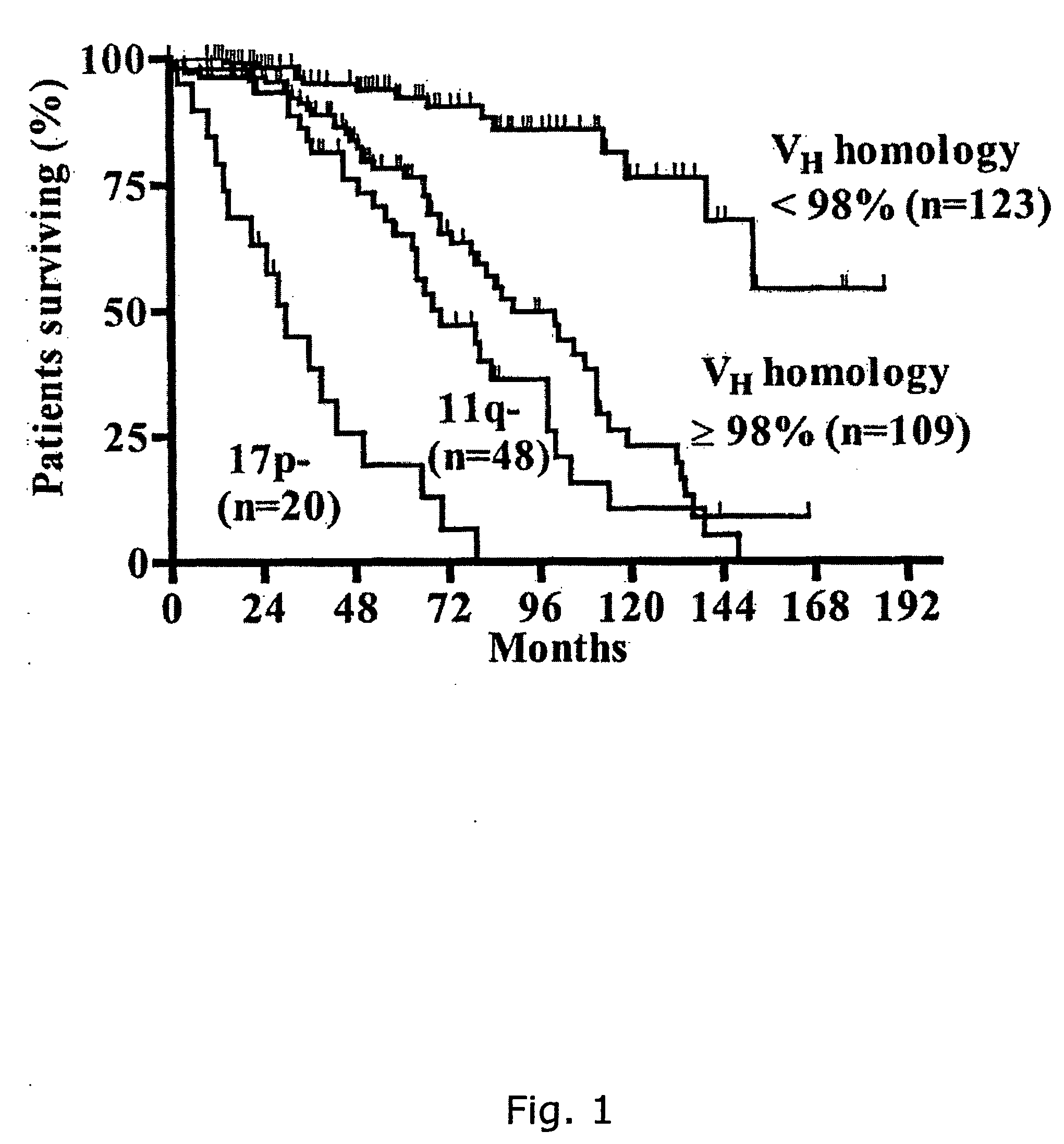
CD38 as a prognostic indicator in B cell chronic lymphocytic leukemia
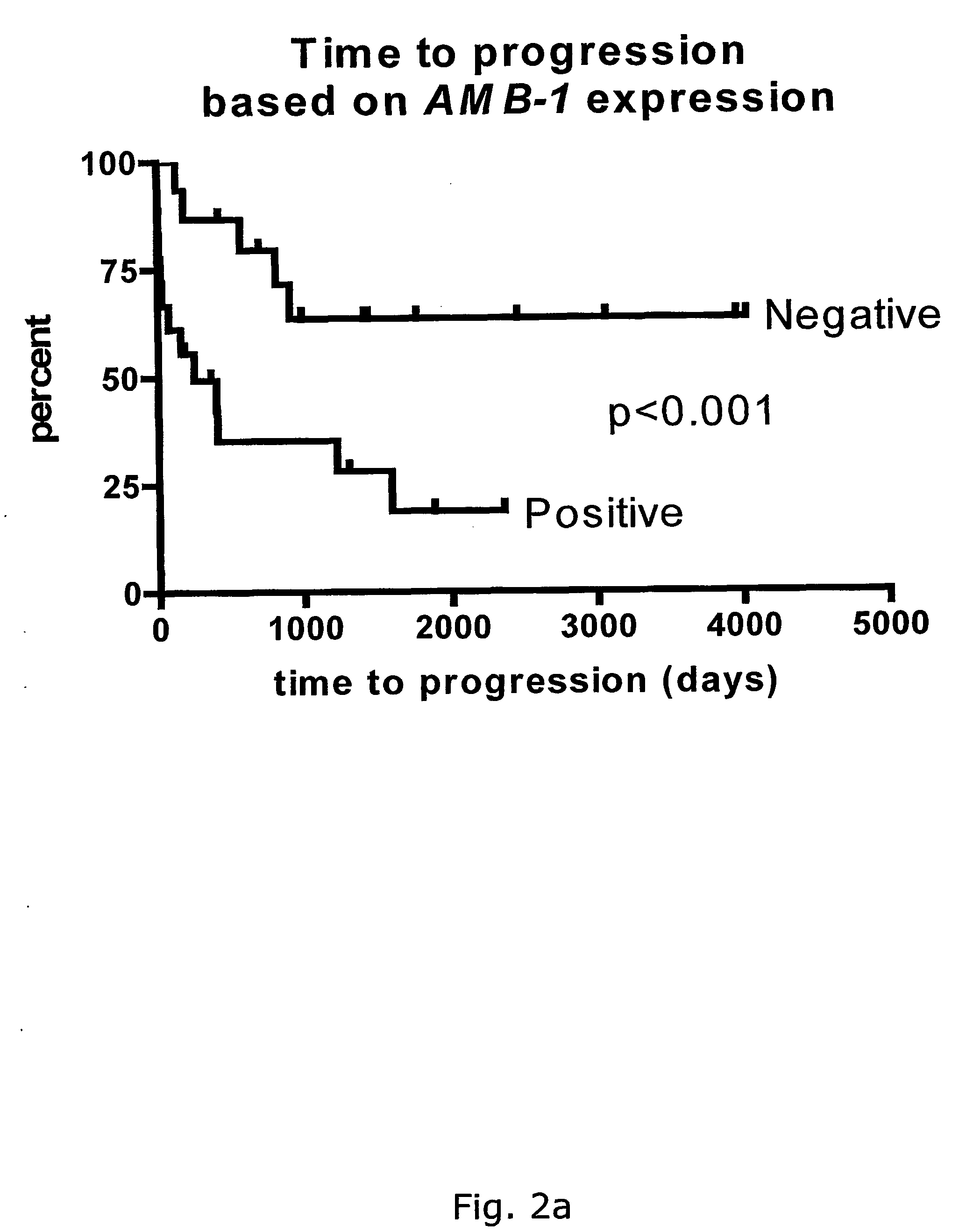
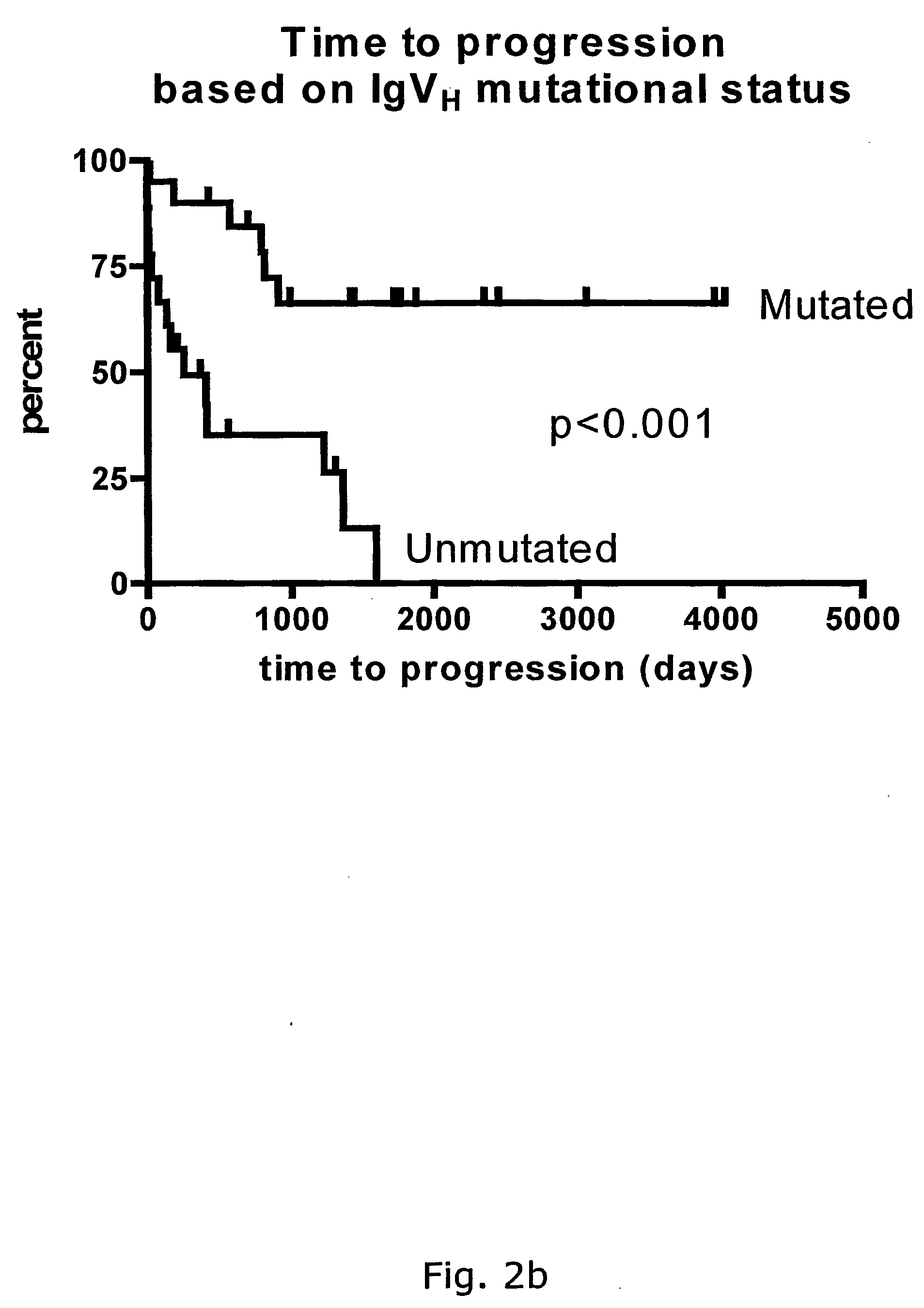
The subject invention discloses a method for determining the prognosis and probable clinical course of a subject diagnosed with B-CLL. Specifically, the invention involves comparing CD38 expression in a biological sample from the subject containing B-CLL cells to a baseline level of CD38 expression, wherein an elevated level of CD38 expression in relation to the baseline level of CD38 expression may indicate poor prognosis or aggressive course of disease in the subject. Also disclosed is a method for determining whether the Ig V genes of the B-CLL cells of a B-CLL patient are mutated, comprising comparing CD38 expression in a biological sample from the subject containing B-CLL cells to a baseline level of CD38 expression, wherein a lower level of CD38 expression in relation to the baseline level indicates IG V gene mutation.
Owner:THE FEINSTEIN INST FOR MEDICAL RES
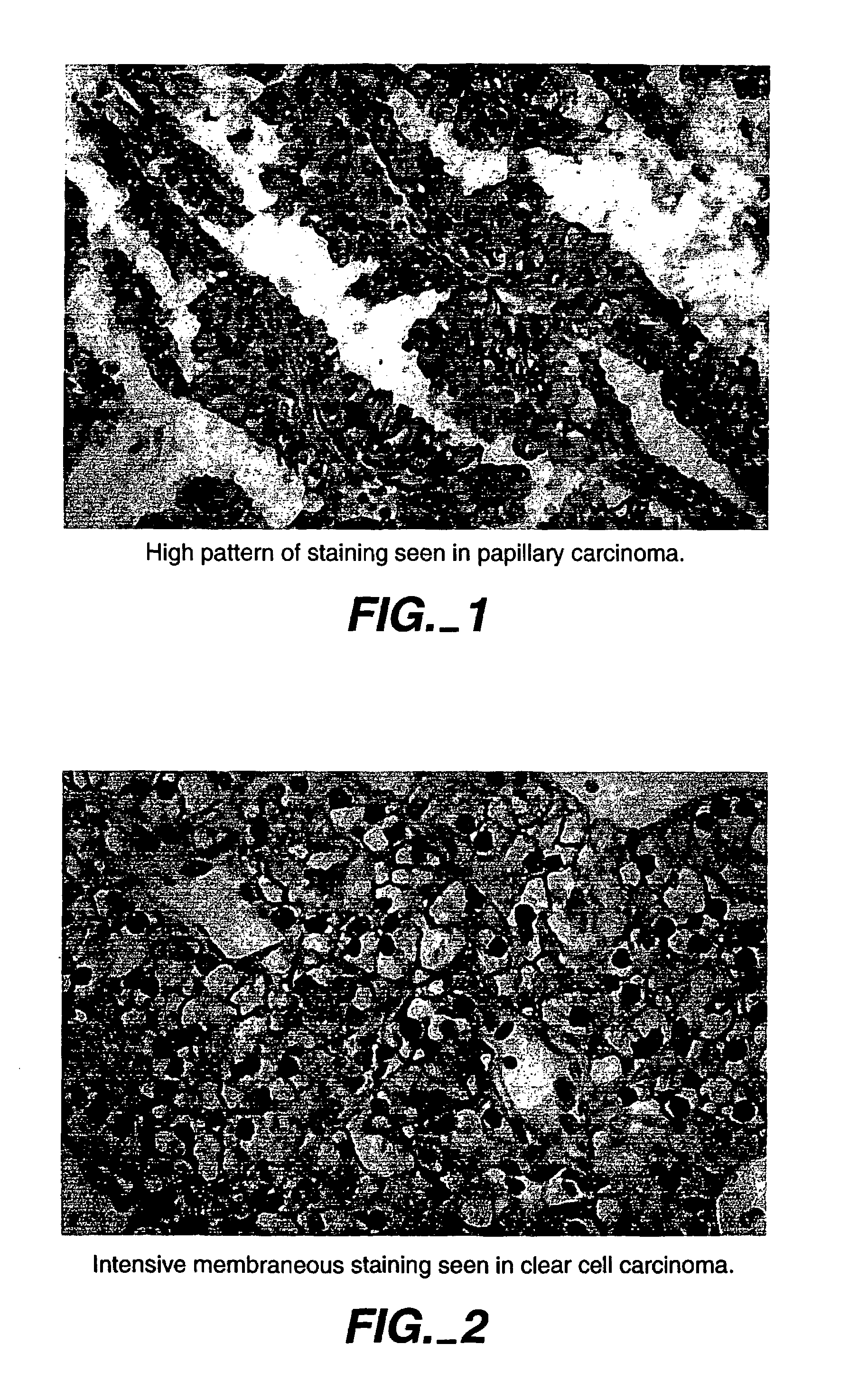
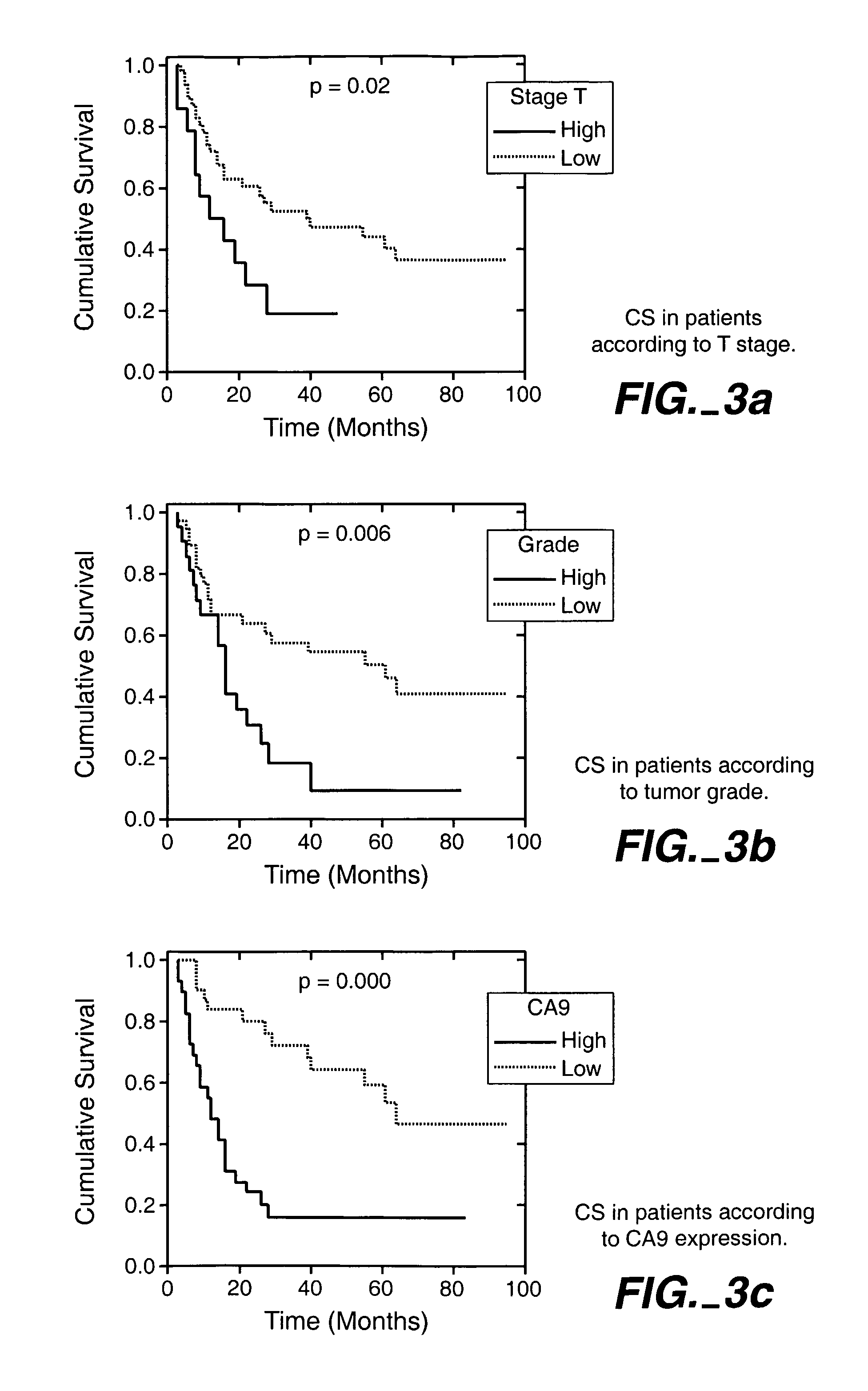
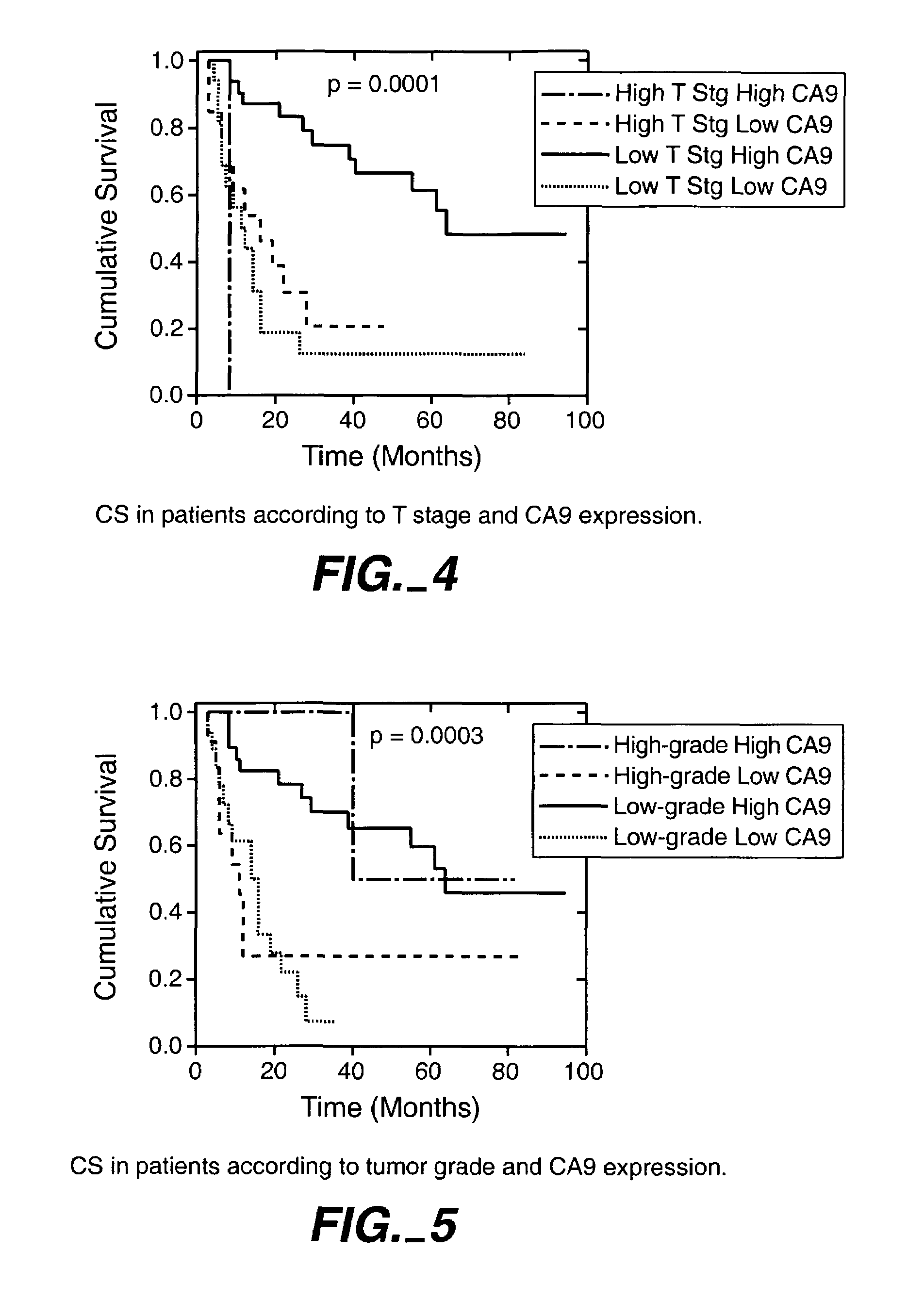
MN/CA IX/CA9 and Renal Cancer Prognosis
InactiveUS7482129B2Microbiological testing/measurementBiological testingRegimenRenal clear cell carcinoma
Herein disclosed are methods that are prognostic for renal cell carcinoma, particularly renal clear cell carcinoma, afflicting a vertebrate. An exemplary prognostic method comprises detecting the presence of, and quantitating the level and / or extent of a MN / CA9 gene expression product in a sample from the affected subject, wherein if 50% or fewer cells are found to express the MN / CA9 gene, then the subject is considered to have a poorer prognosis. MN / CA9 gene expression products useful in the prognostic methods include MN protein, MN polypeptide and / or MN nucleic acids. The methods are useful as an aid in the selection of treatment for a patient with renal cell carcinoma, alone or in combination with conventional tumor stage and / or grade information. The methods of the invention can be used, for example, to identify those patients requiring more aggressive therapy regimens, or those patients most likely to respond to adjuvant immunotherapies, particularly MN / CA IX / CA9-targeted therapies.
Owner:BIOMEDICAL RES CENT OF THE SLOVAK ACADEMY OF SCI +1
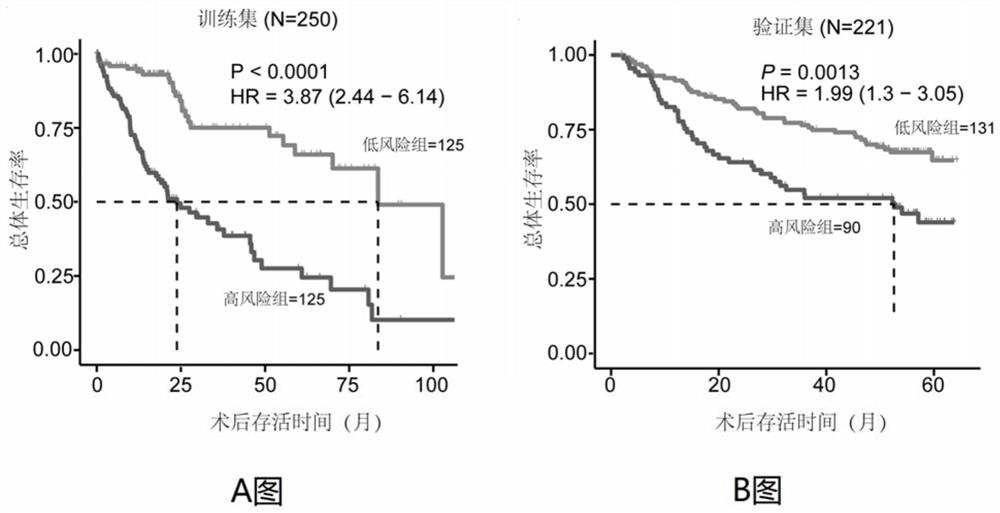
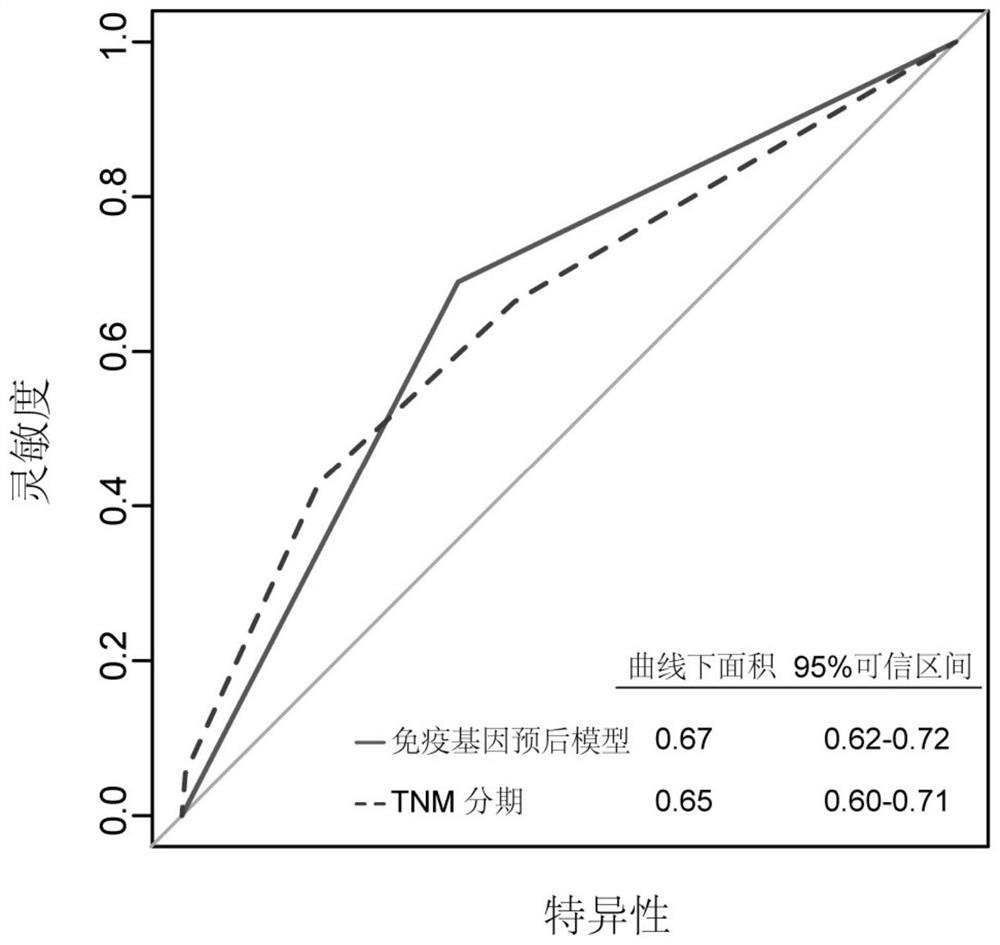
Immune gene prognosis model for predicting hepatocellular carcinoma tumor immune infiltration and postoperative survival time
ActiveCN112011616APromote the implementation of precision medicineObjective assessment of infiltrationMicrobiological testing/measurementBiostatisticsTNM staging systemMicroarray cgh
The invention relates to an immune gene prognosis model for predicting hepatocellular carcinoma tumor immune infiltration and postoperative survival time, and belongs to the technical field of biological medicines. The model can be used for evaluating the infiltration degree of immune cells in a tumor in clinical practice by detecting the expression levels of 22 specific immune related genes of ahepatocellular carcinoma patient, so that the model can be used for predicting hepatocellular carcinoma tumor immune infiltration in clinical practice and improve the prediction capability of the liver cancer immunotherapy response. The model can be used for judging the postoperative overall survival risk of a patient and guiding the formulation of a postoperative treatment strategy, and the corresponding microarray chip kit can realize the standardization and convenience of detection. Meanwhile, the immune gene prognosis model provided by the invention can increase the prediction accuracy andthe clinical net income of a hepatocellular carcinoma TNM staging system on the total survival time of three years and five years after operation. As a molecular marker for objectively and accuratelyevaluating the tumor immune state and poor prognosis risk of hepatocellular carcinoma, the model can realize accurate implementation of hepatocellular carcinoma immunotherapy and accurate prognosis prediction.
Owner:上海顿慧医疗科技发展有限公司
Methods of classifying, diagnosing, stratifying and treating cancer patients and their tumors
InactiveUS7118853B2Tumor rejection antigen precursorsPeptide/protein ingredientsAbnormal tissue growthKeratin
Owner:APPL GENOMICS INC +1
Stat3 as a theranostic indicator
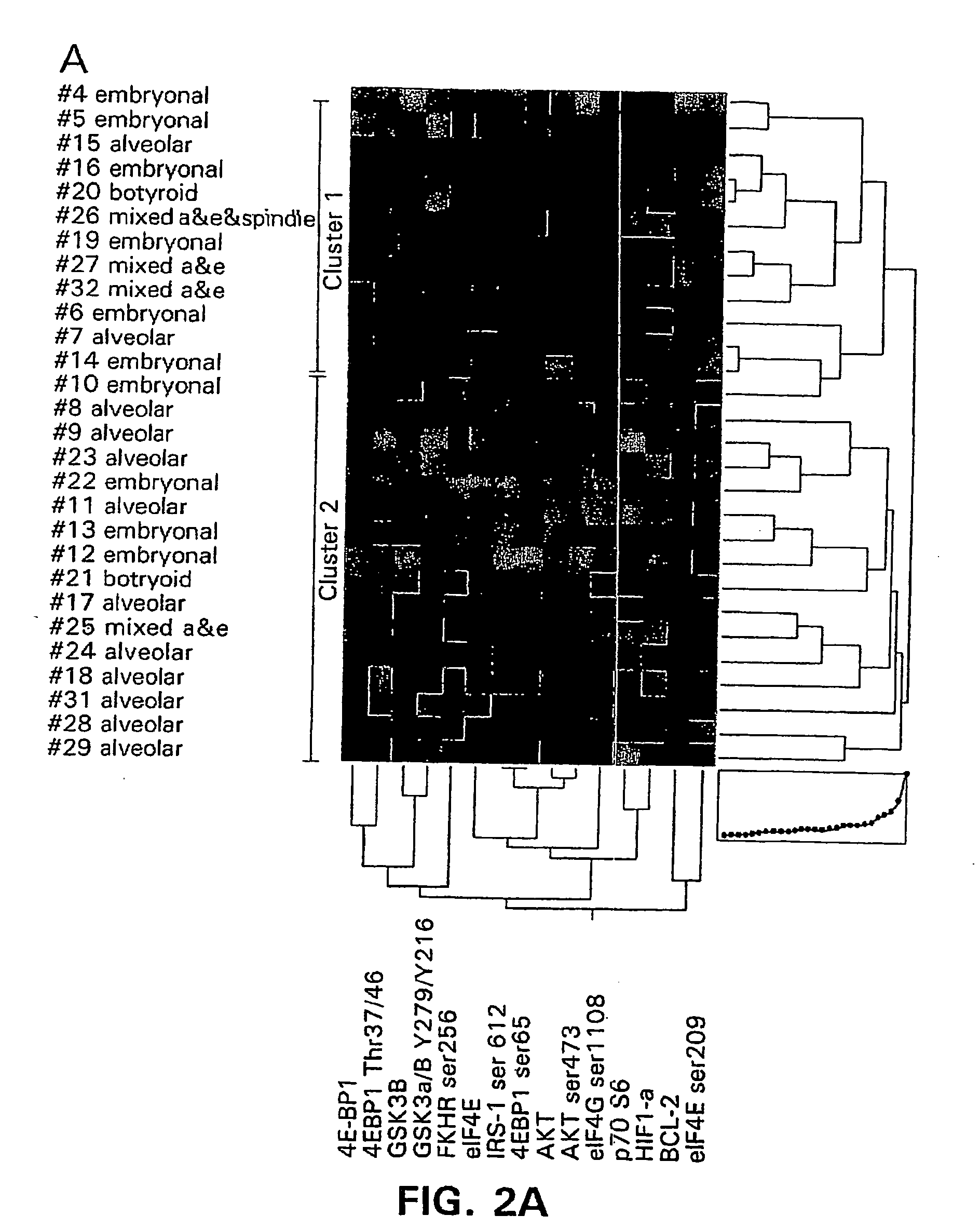
This invention relates, e.g., to a method for predicting the response of a subject having estrogen-receptor-positive breast cancer to an inhibitor of the estrogen signaling pathway (e.g. tamoxifen), comprising measuring in a cancer sample from the subject the level of phosphorylation, compared to a baseline value, of one or more of the following members of an interconnected intracellular signaling pathway: (a) 4EBP1, and / or (b) p70S6, and / or (c) STAT3, and / or (d) FAK, wherein a significantly elevated level of phosphorylation of 4EBP1, and / or p70S6 and / or STAT3, and / or a significantly decreased level of phosphorylation of FAK, compared to the baseline value, indicates that the subject is likely to be a non-responder to the inhibitor and / or has a poor prognosis. Additional members of the intracellular signaling pathway whose phosphorylation can be measured are also described. Also described is a method for treating breast cancer in a subject in need thereof, wherein the subject exhibits an elevated level of phosphorylation of these markers, comprising administering to the subject an effective amount of one or more inhibitors of members of the interconnected intracellular signaling pathway.
Owner:GEORGE MASON INTPROP OF FAIRFAX VIRGINIA
Methods of classifying, diagnosing, stratifying and treating cancer patients and their tumors
InactiveUS20060040302A1Tumor rejection antigen precursorsPeptide/protein ingredientsAbnormal tissue growthCytokeratin
The invention provides a variety of reagents for use in the diagnosis and management of cancer, particularly breast cancer. cDNA microarray technology was used to identify genes whose expression profile across a large group of tumor samples correlates with that of cytokeratin 5 and cytokeratin 17, markers for basal cells of the normal mammary lactation gland. The invention demonstrates that tumors that express cytokeratin 5 / 6 and / or 17 have a poor prognosis relative to tumors overall. The invention provides basal marker genes and their expression products and uses of these genes for diagnosis of cancer and for identification of therapies for cancer. In particular, the invention provides basal marker genes including cadherin3, matrix metalloproteinase 14, and cadherin EGF LAG seven-pass G-type receptor 2. The invention provides antibodies to the polypeptides expressed by these genes and methods of use thereof.
Owner:APPL GENOMICS INC +1
Method for selecting a high risk patient for participation in a care management program for patients having poor prognoses
InactiveUS20090240529A1Reduce the amount requiredImprove the quality of lifeComplete banking machinesFinanceNursing managementPoor prognosis
The method of the present invention is used to select high risk or co-morbid patients for participation in a care management program. An acuity score determines which patients are selected for care management.
Owner:ENHANCED CARE INITIATIVES
Assay for metastatic colorectal cancer
InactiveUS20100003247A1Easy to operateShort maintenance periodBiocideMicrobiological testing/measurementLymphatic SpreadAssay
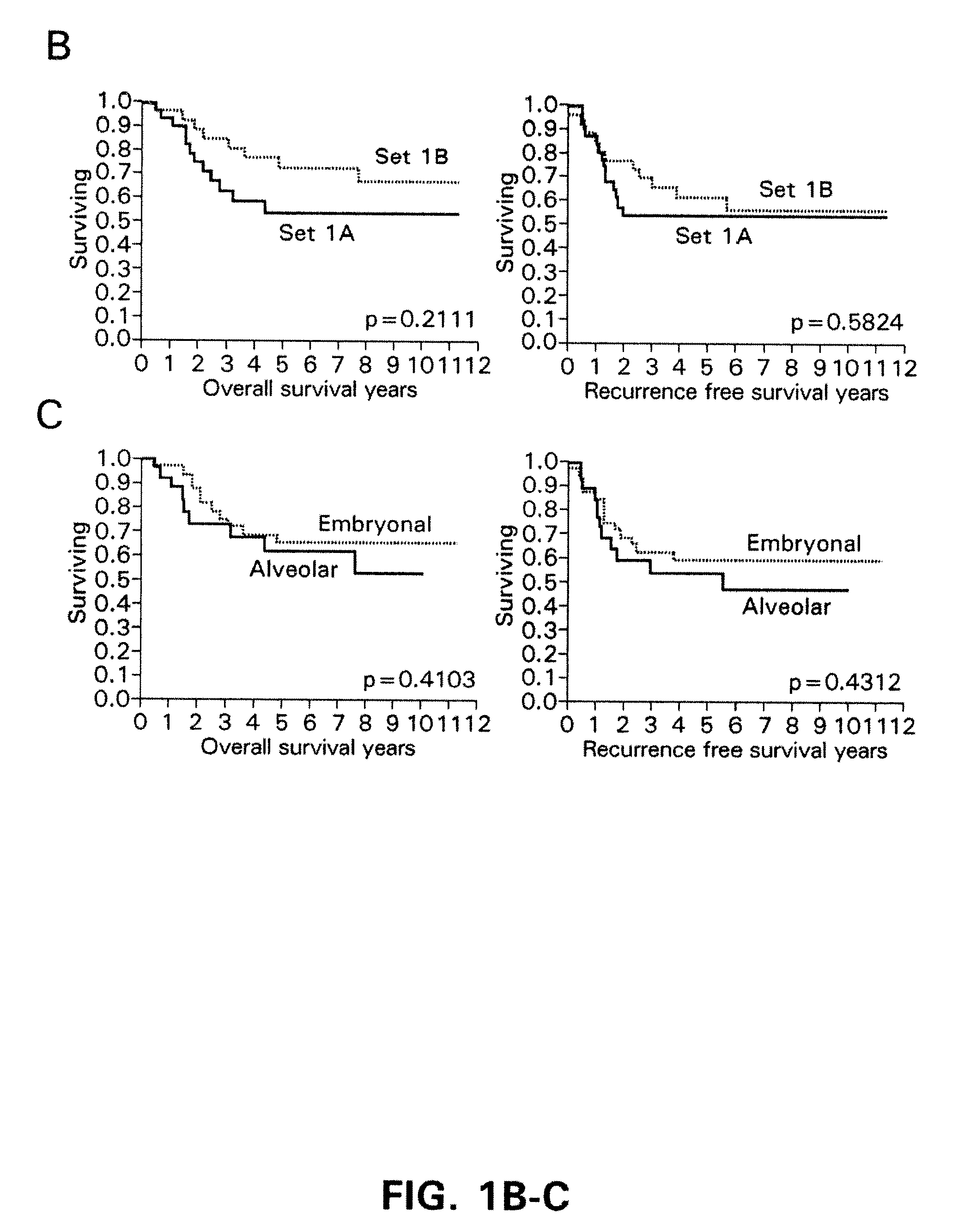
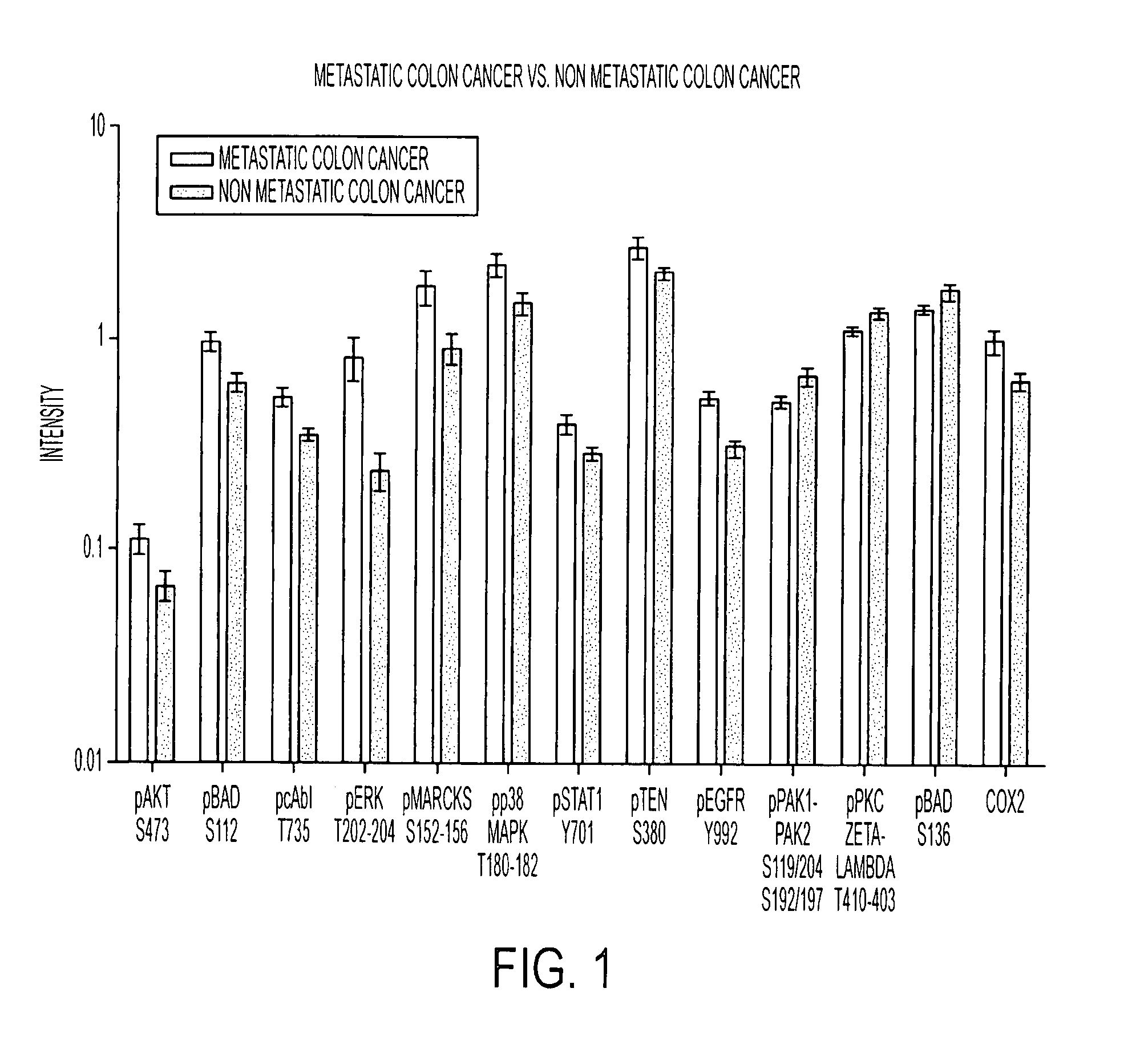
This invention relates, e.g., to a method for predicting the prognosis, the likelihood of metastasis in, or the desirability of administering an aggressive therapy to, a subject with colorectal cancer, comprising determining, in a sample from the subject, the level of phosphorylation compared to a positive and / or negative reference standard, of one or more of: (a) AKT (S473); (b) BAD (S112); (c) cABL (T735); (d) ERK (T42 / 44); (e) MARCKS (S152-156); (0 p38MAPK (T180-182): (g) STAT 1 (Y701 ); (h) PTEN (S380); (i) EGFR (Y992); (j) PAK 1 / 2 (S 1 19 / 204); or (k) PKC zeta / lambda (T410-403); or the total amount of (1) COX-2 protein; wherein if the level of phosphorylation of one or more of a-i or the total amount of COX-2 protein (1) is elevated compared to the negative reference standard, and / or if the level of phosphorylation of j or k is decreased compared to the positive reference standard, the subject has poor prognosis, is likely to undergo metastasis, and / or is a good candidate for aggressive therapy. Also described are methods for treating subjects likely to develop metastatic colorectal carcinoma, and pharmaceutical compositions and kits for implementing methods of the invention.
Owner:GEORGE MASON INTPROP OF FAIRFAX VIRGINIA +1
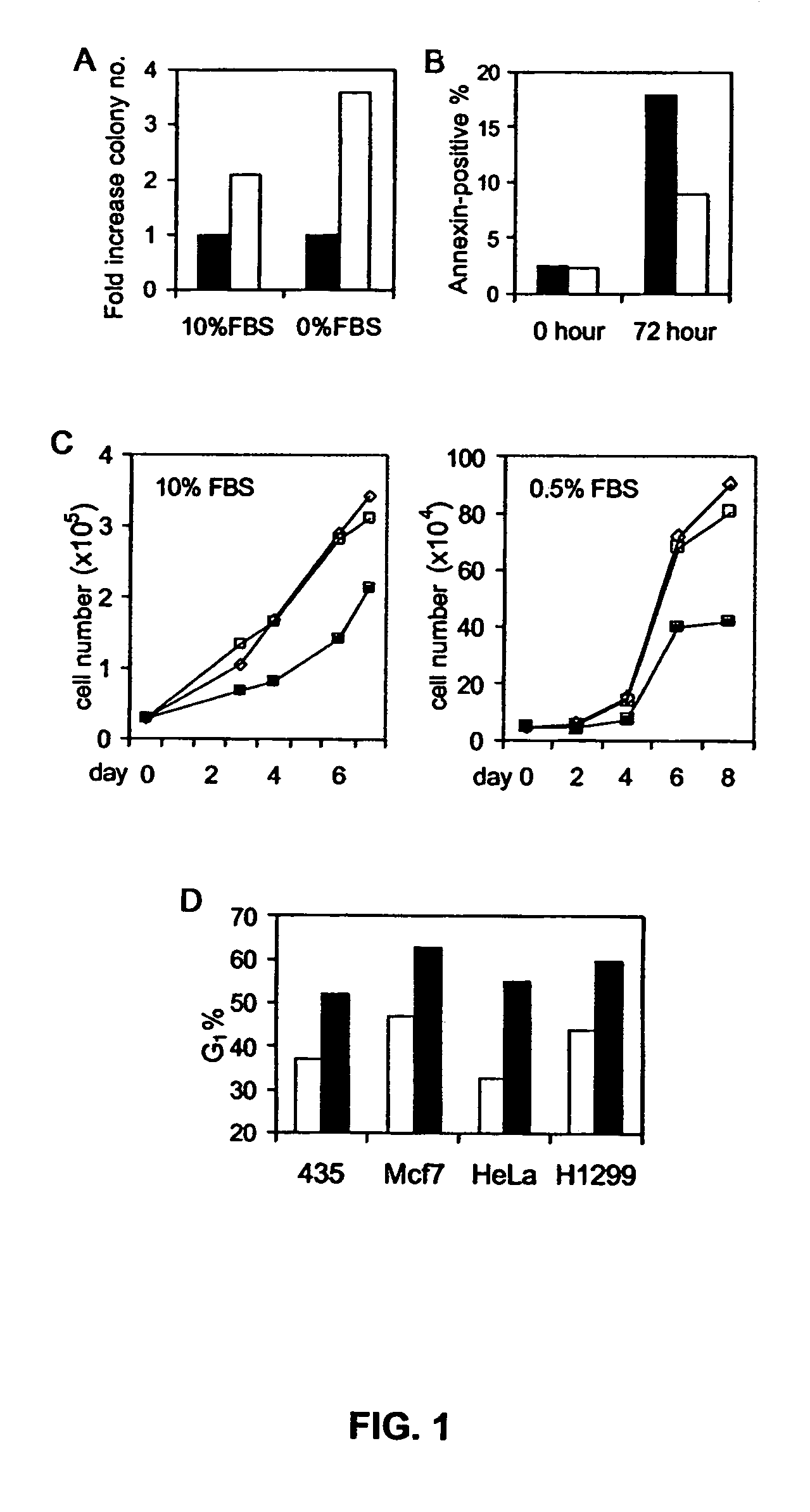
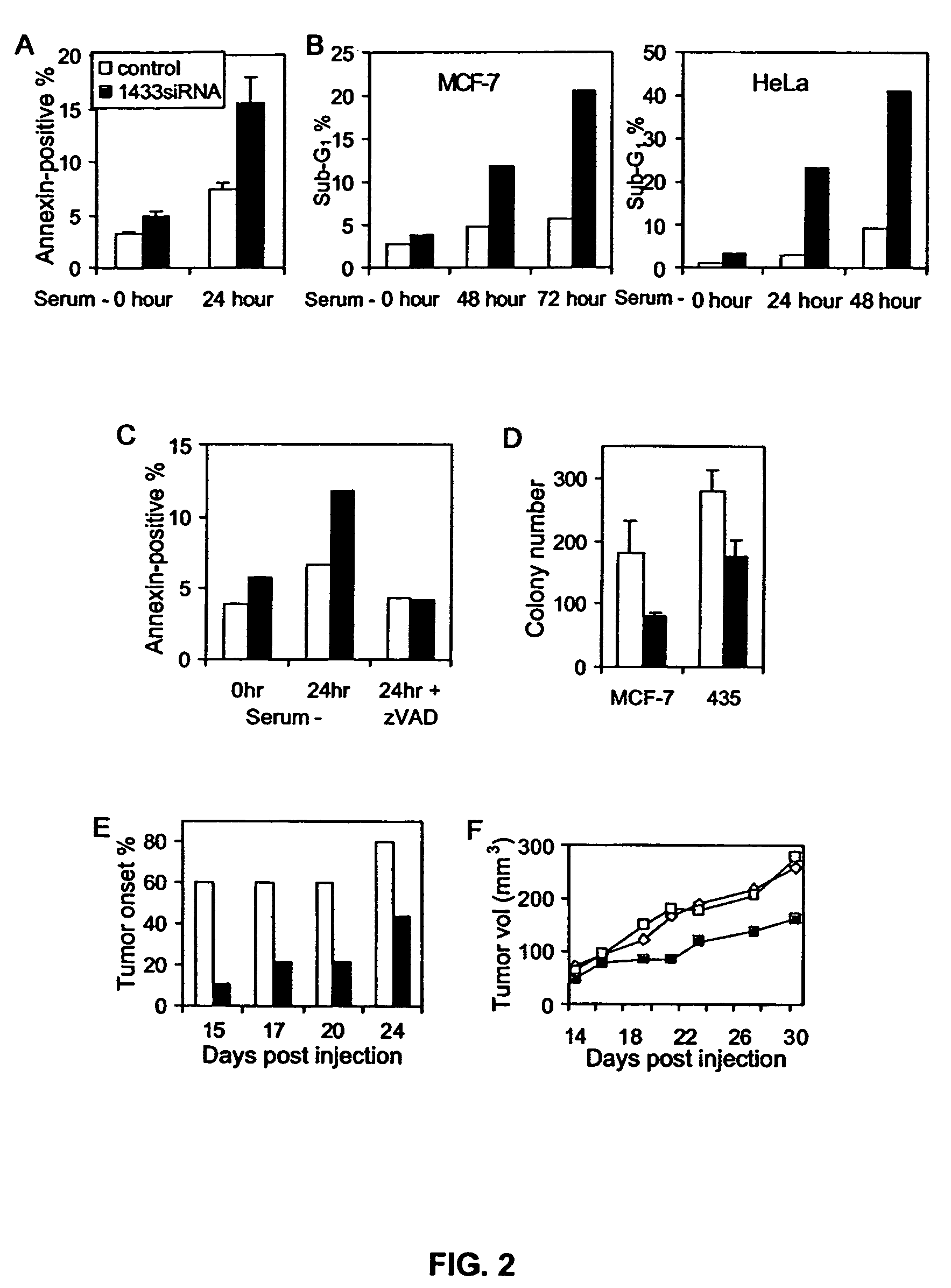
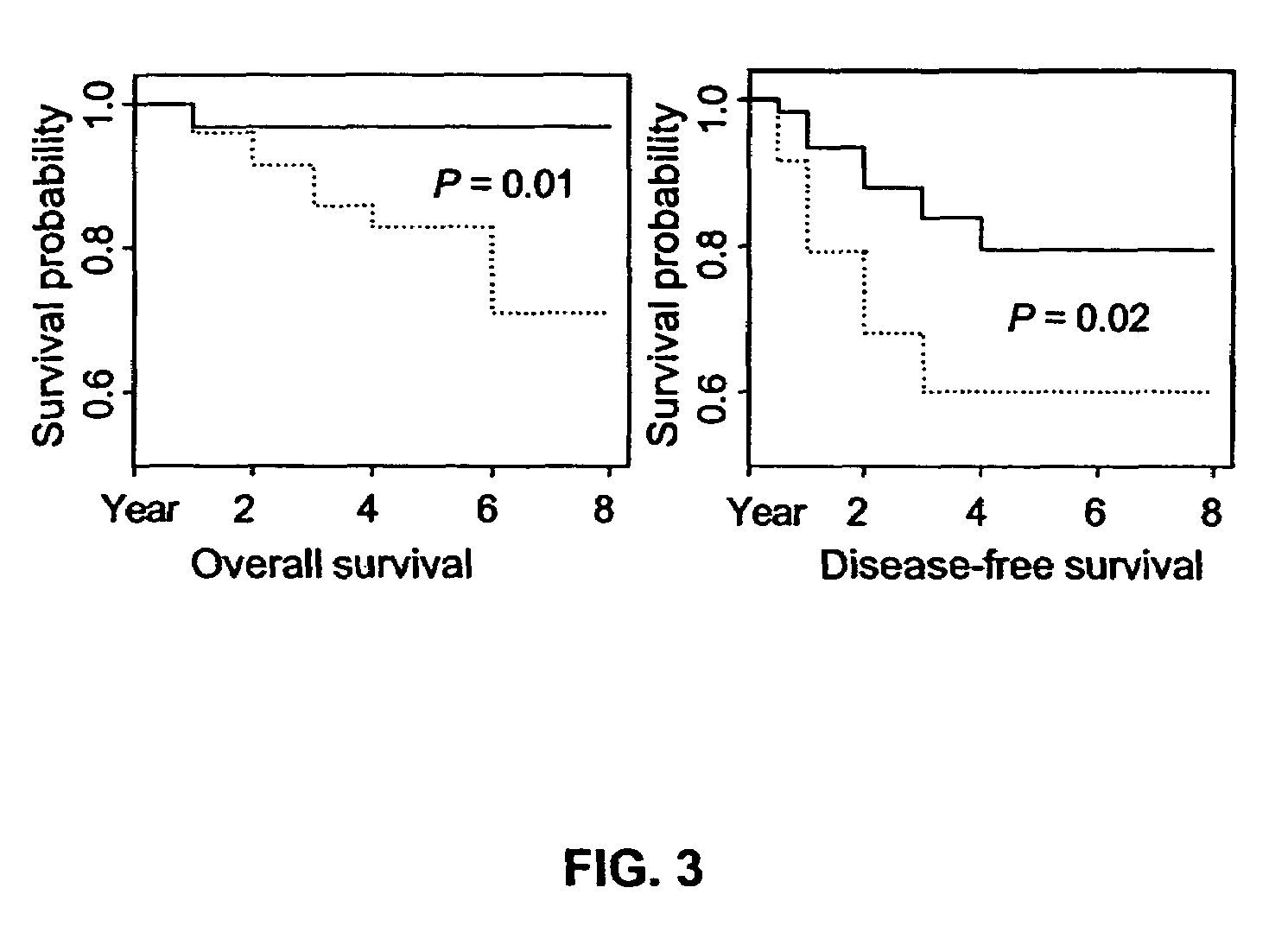
14-3-3 zeta over-expression as a poor prognosis factor, and a therapeutic target in multiple cancer types
ActiveUS7316907B2Inhibition is effectiveLow survival rateGenetic material ingredientsMicrobiological testing/measurementDiseaseApoptosis
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
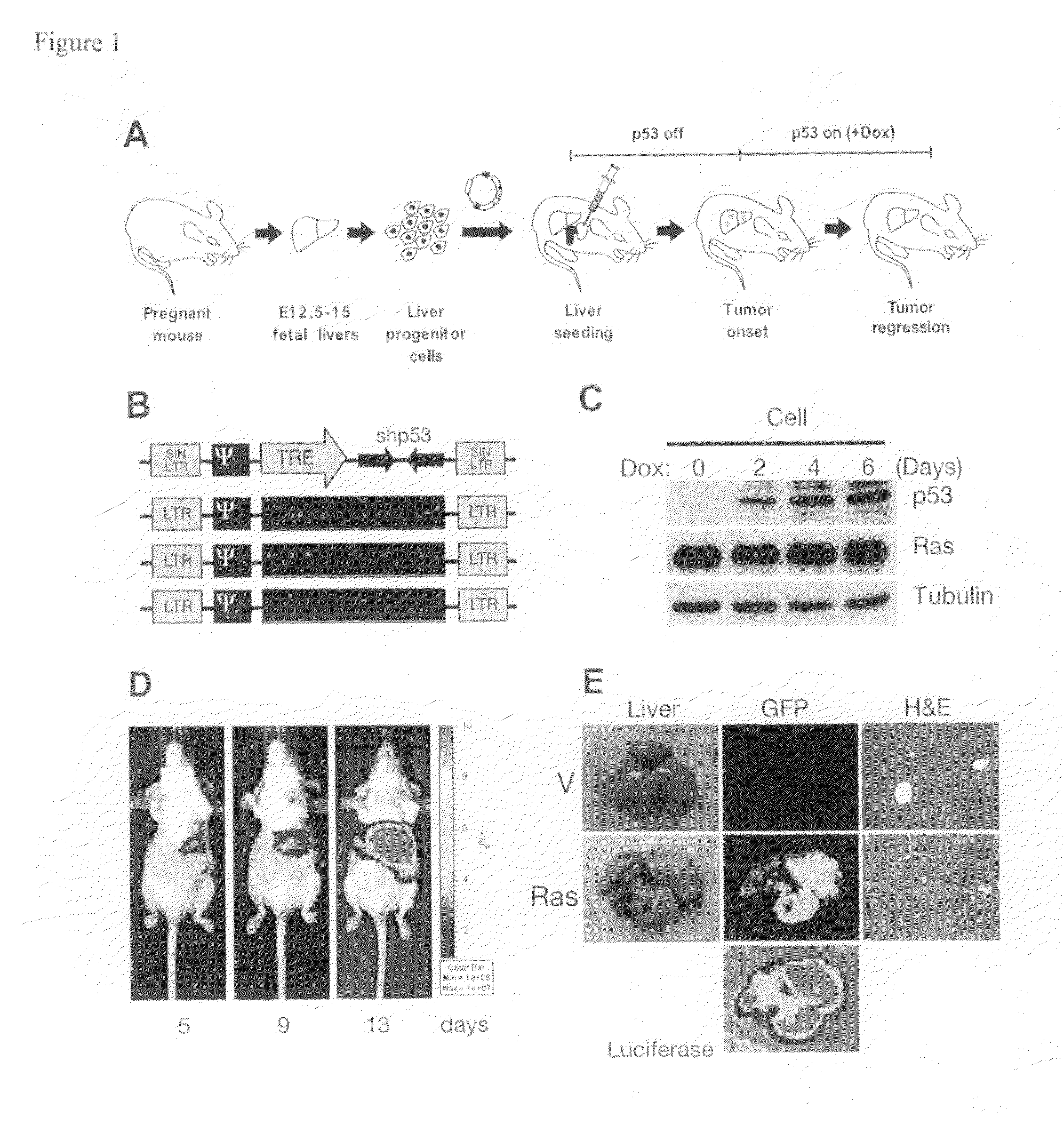
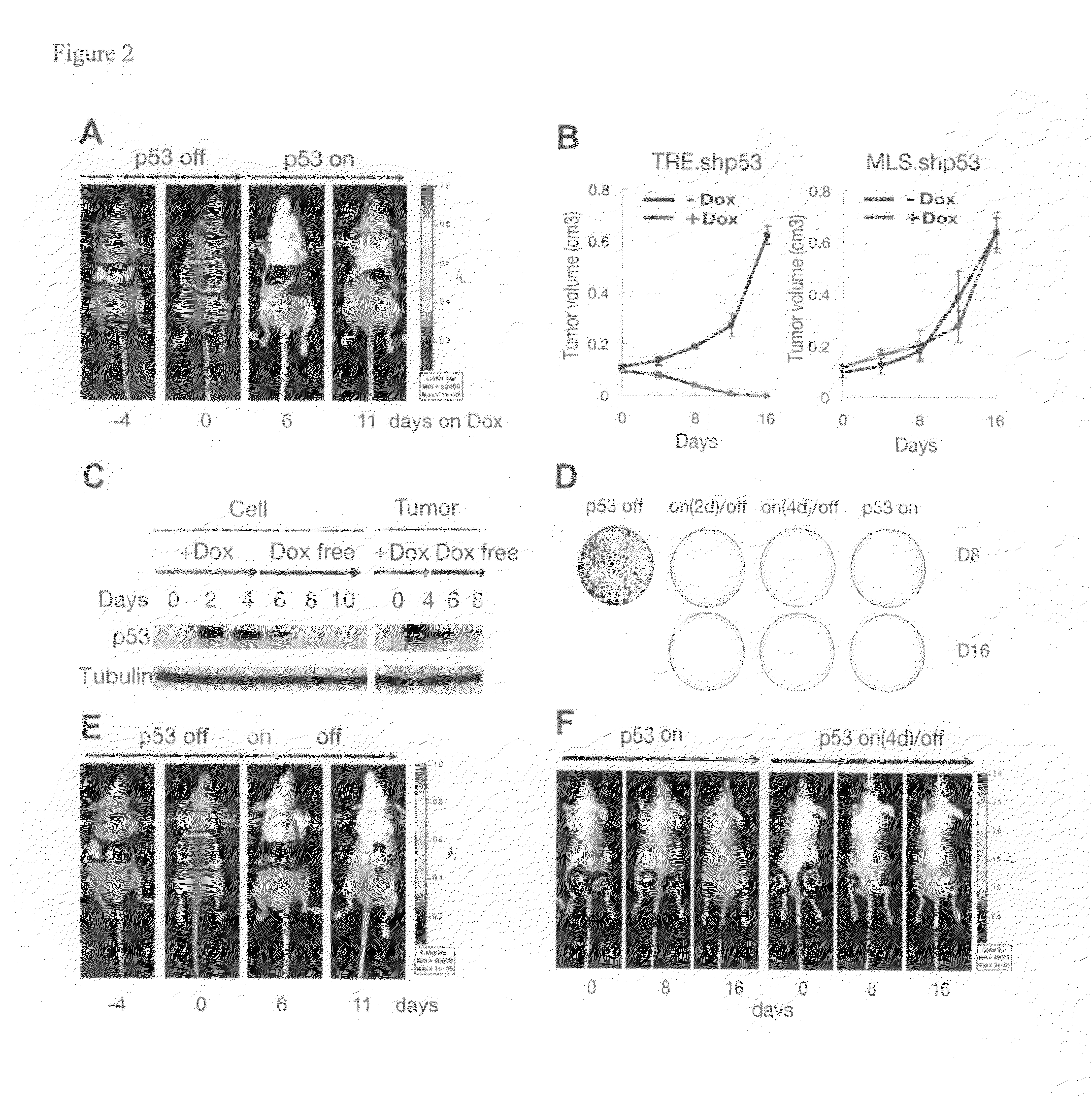
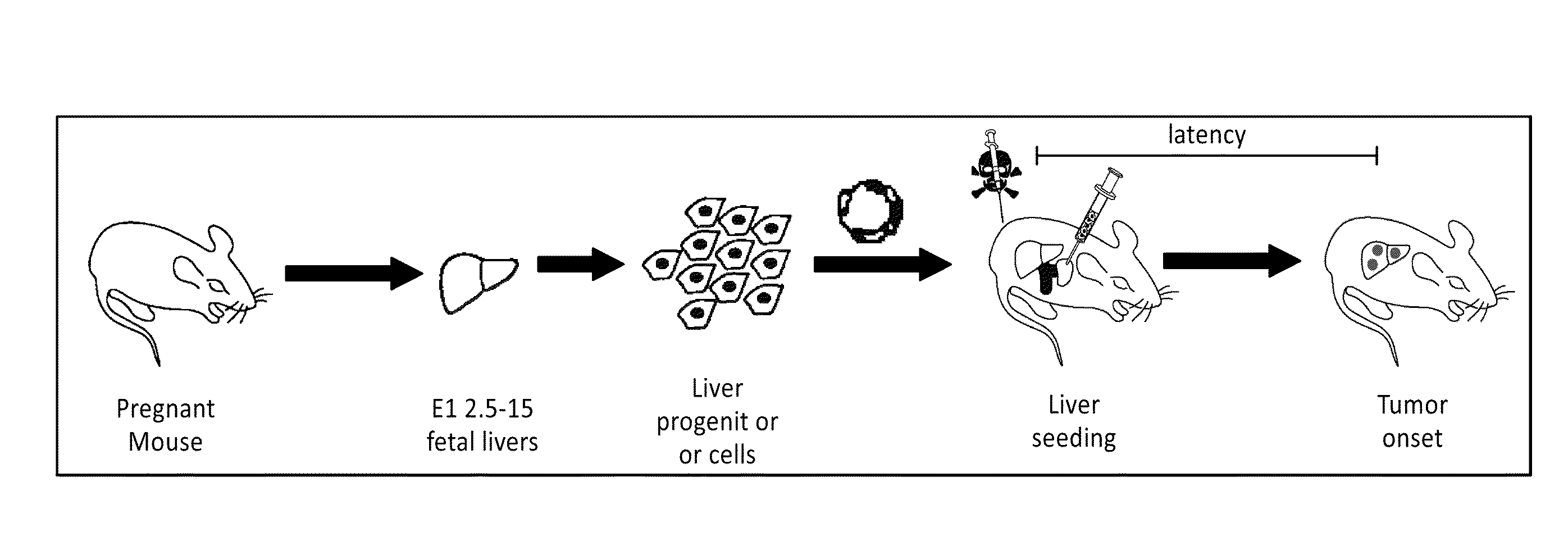
Orthotopic, controllable, and genetically tractable non-human animal model for cancer
InactiveUS20090022685A1High resolutionBiocideOrganic active ingredientsAbnormal tissue growthCooperative interaction
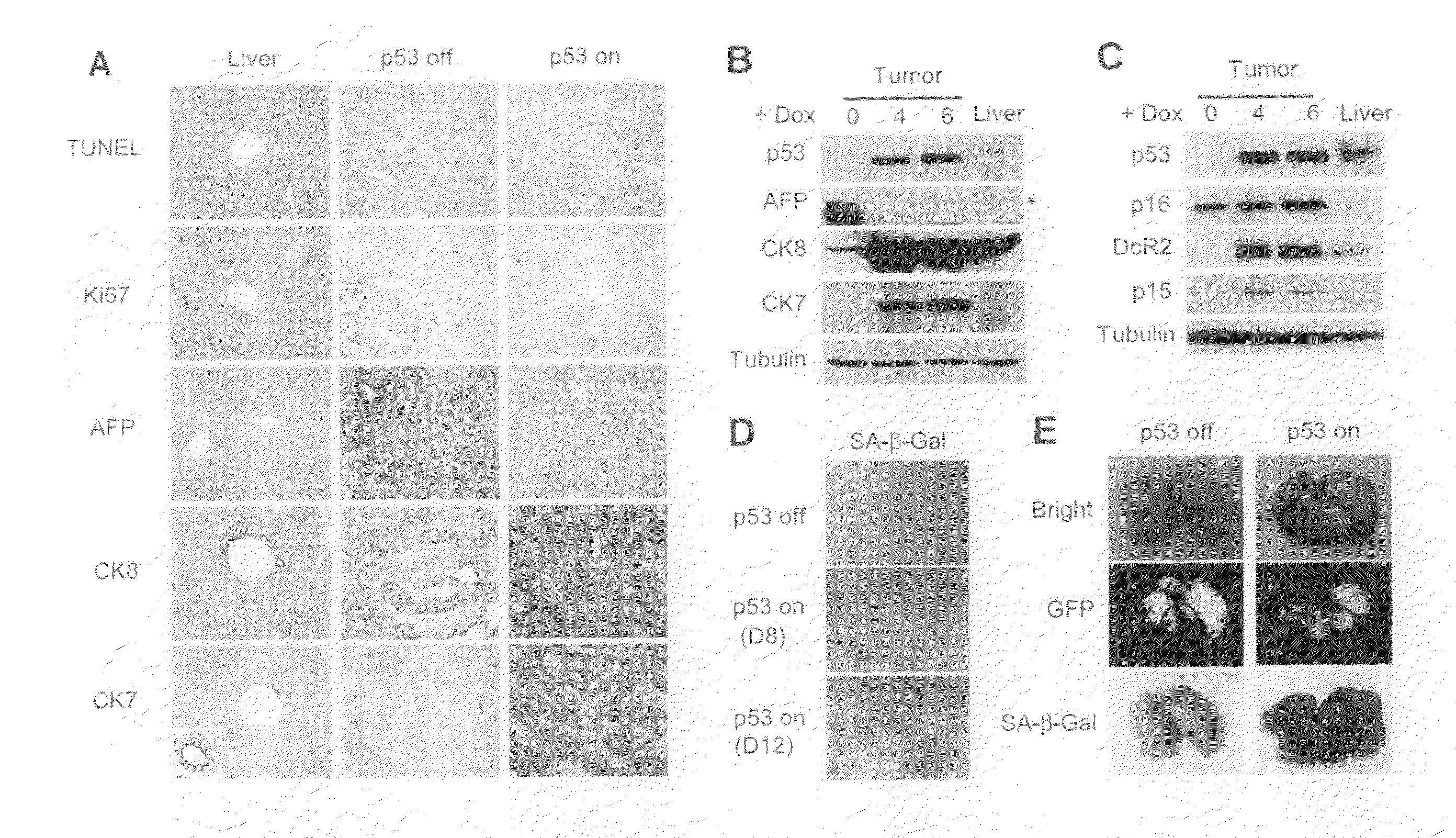
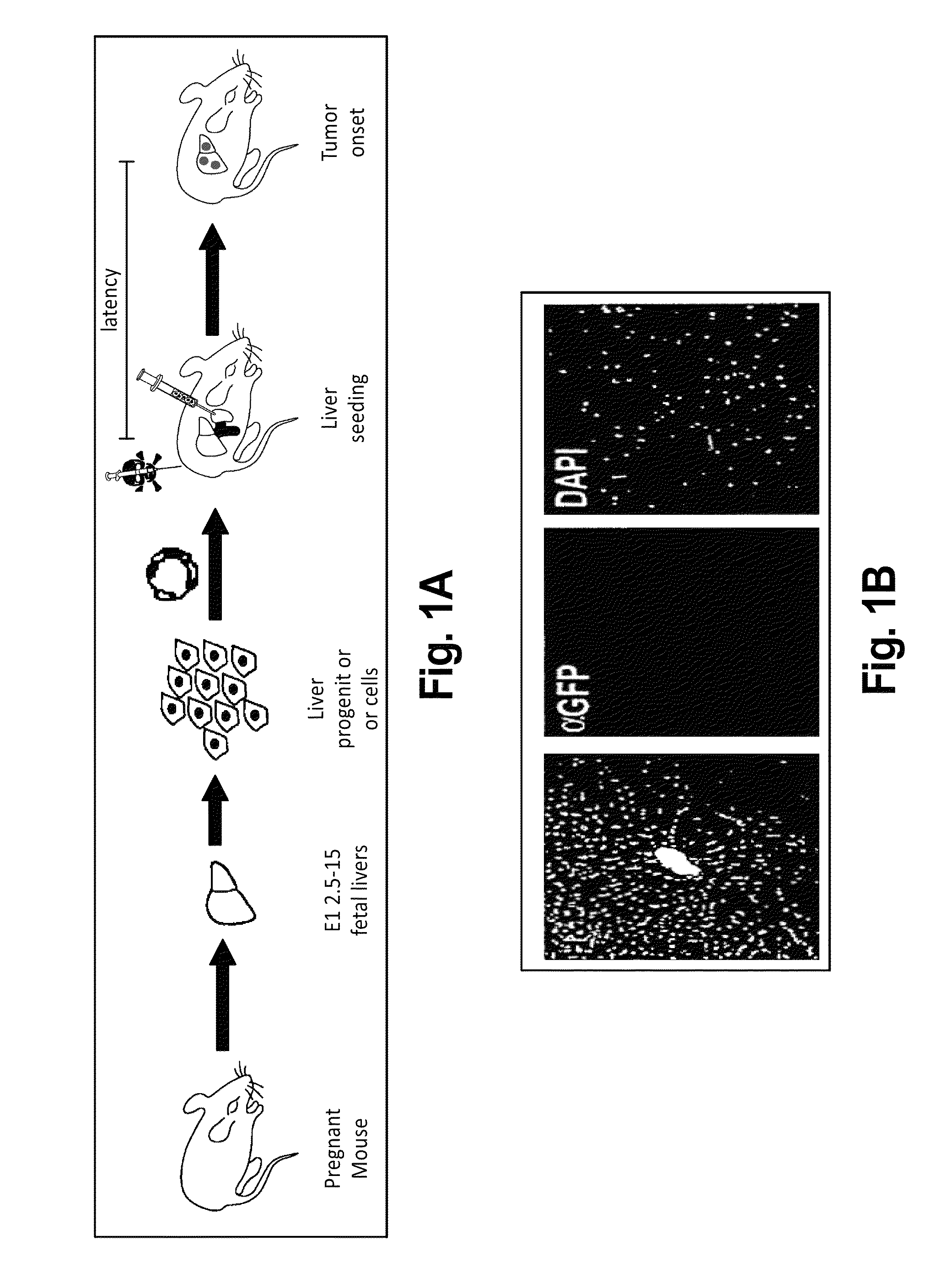
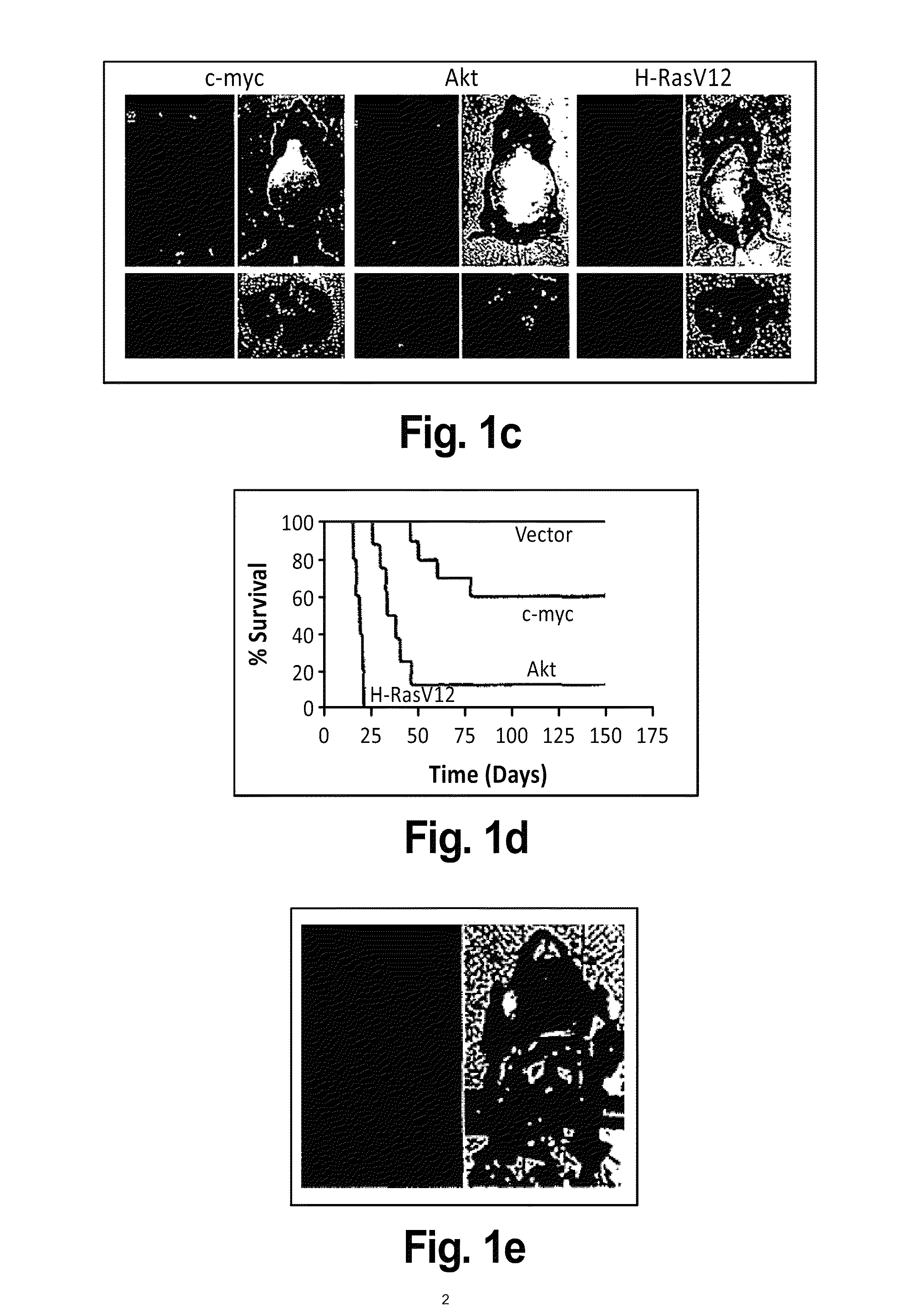
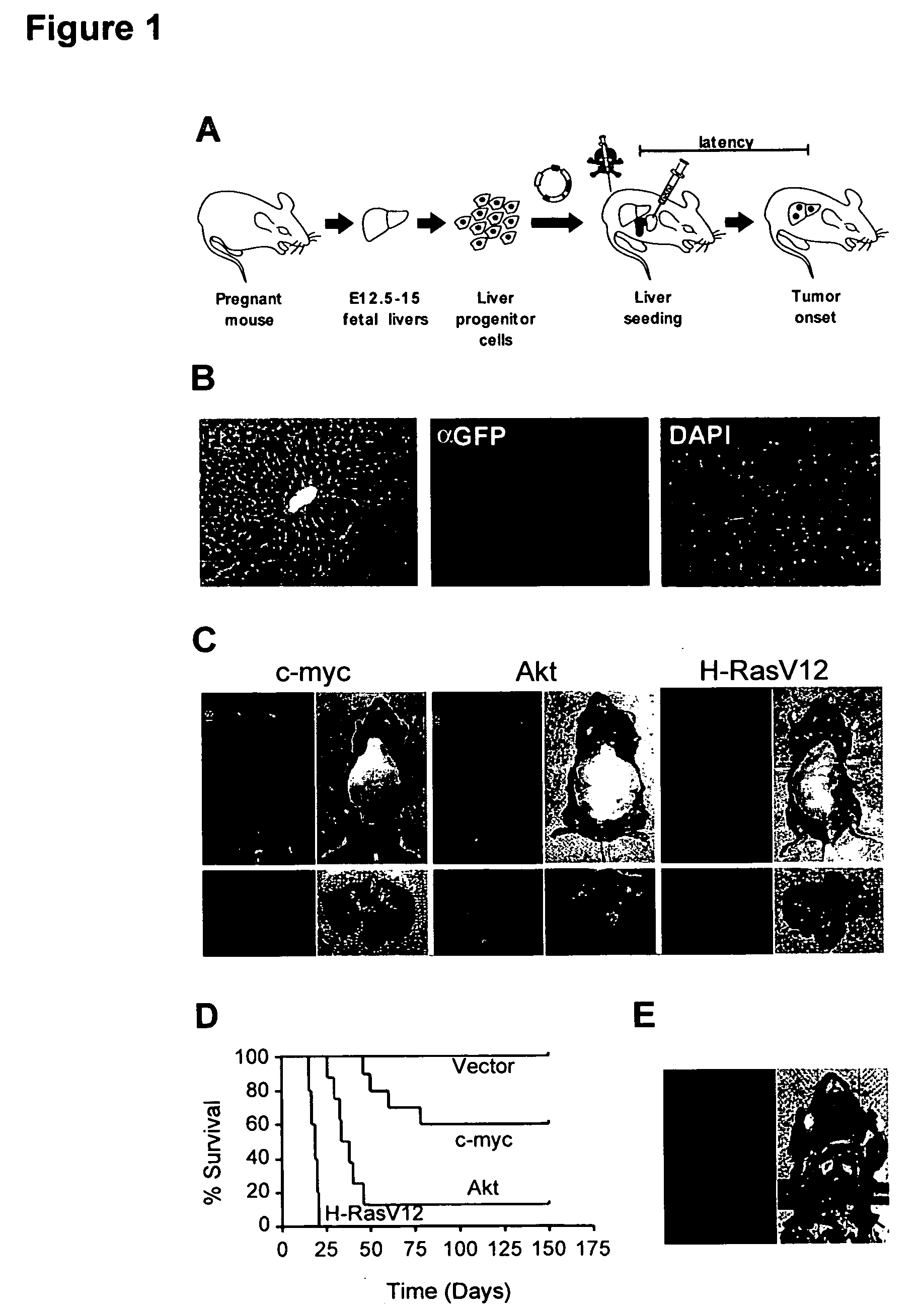
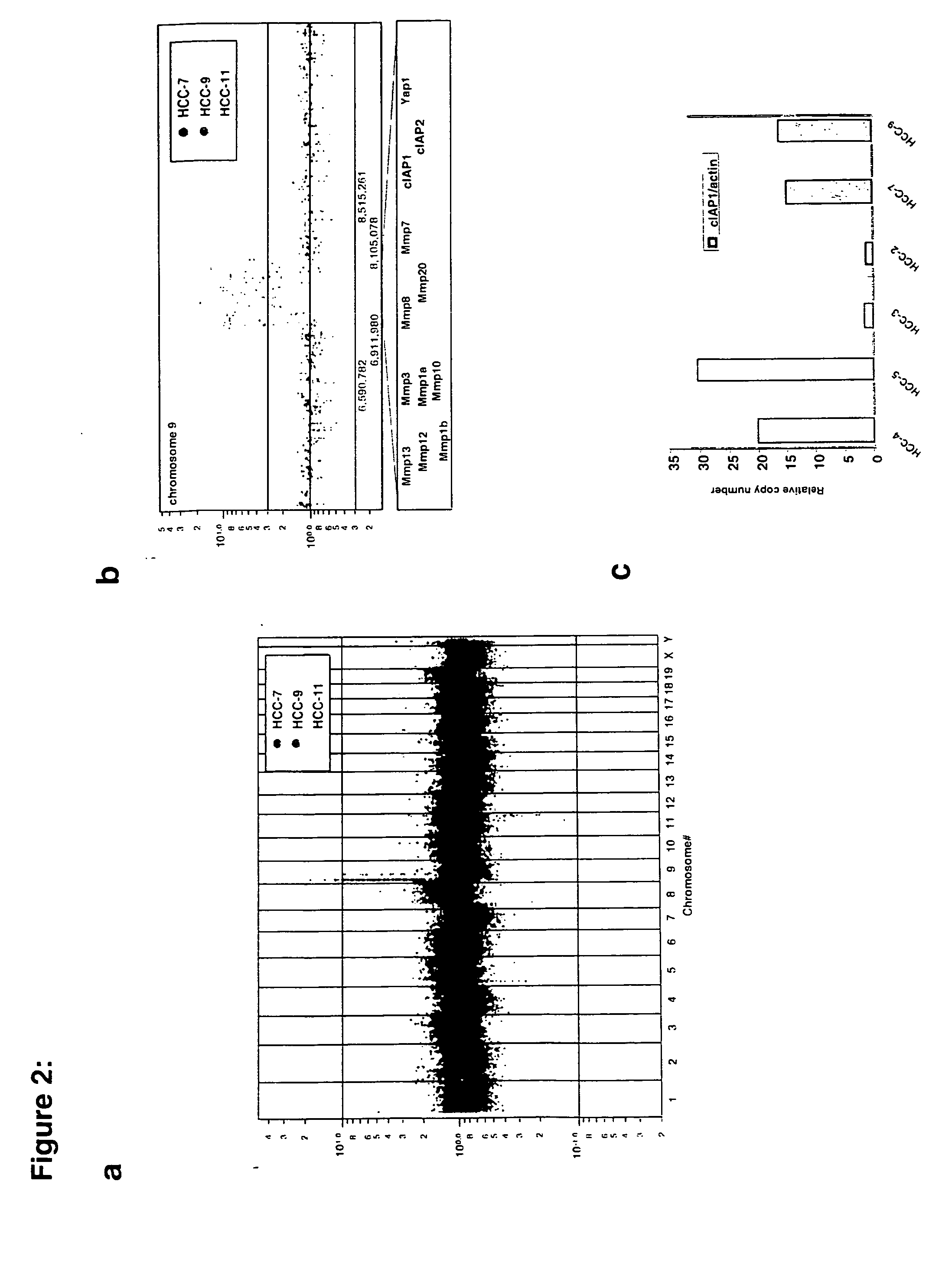
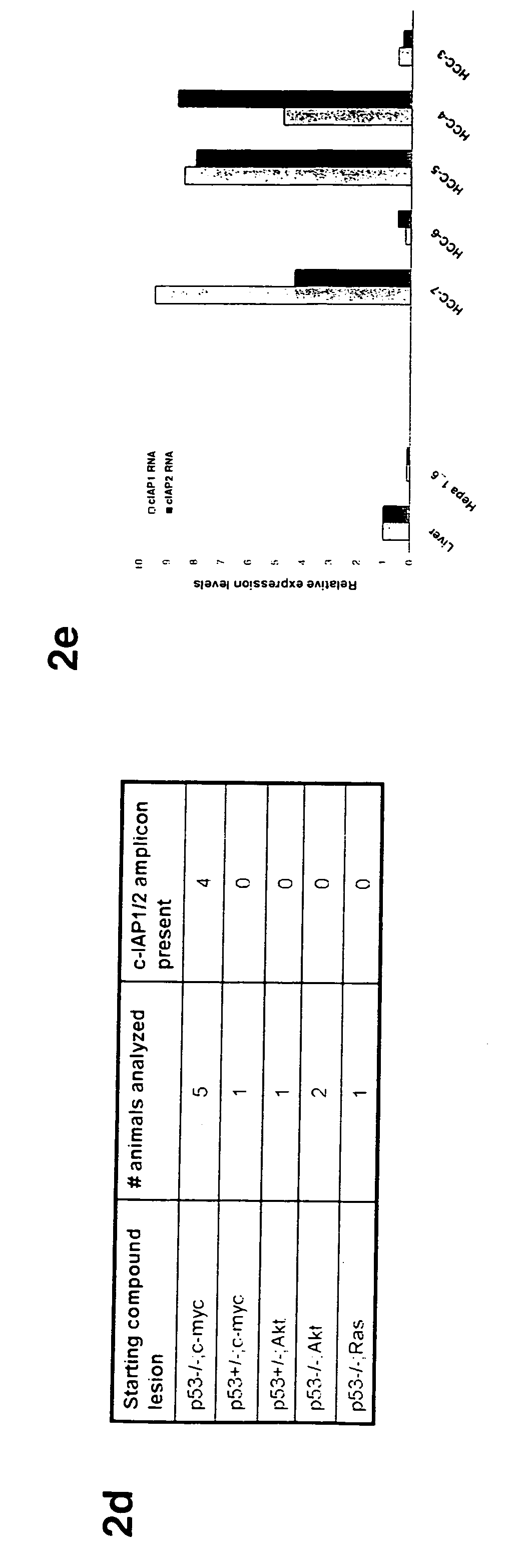
This invention provides a genetically tractable in situ non-human animal model for hepatocellular carcinoma. The model is useful, inter alia, in understanding the molecular mechanisms of liver cancer, in understanding the genetic alterations (e.g., in oncogenes and tumor suppressor genes) that lead to chemoresistance or poor prognosis, and in identifying and evaluating new therapies against hepatocellular carcinomas. The liver cancer model of this invention is made by altering hepatocytes to increase oncogene expression, to reduce tumor suppressor gene expression or both, preferably by inducible, reversible, and / or tissue specific expression of double-stranded RNA molecules that interfere with the expression of a target gene, and by transplanting the resulting hepatocytes into a recipient non-human animal. The invention further provides a method to treat cancer involving cooperative interactions between a tumor cell senescence program and the innate immune system.
Owner:COLD SPRING HARBOR LAB INC
Pyemia early diagnosis liquid phase chip and method for producing the same
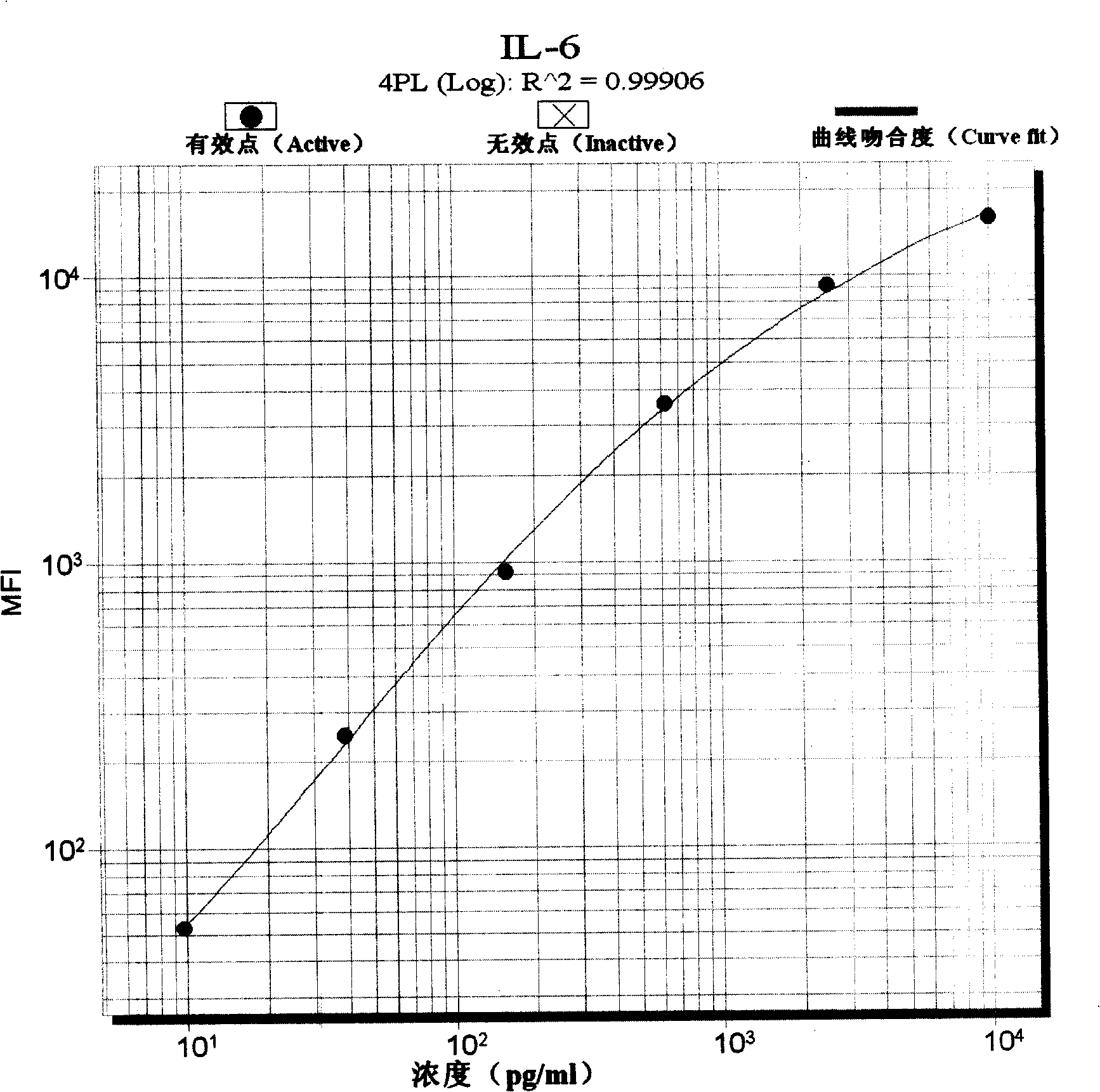
InactiveCN101246163AImprove detection efficiencySmall sample sizeMaterial analysisMicrospherePhycoerythrin
The invention discloses a sepsis early diagnosis liquid chip which mainly includes the following components: microsphere coated with PCT capturing antibody, microsphere coated with CRP capturing antibody, microsphere coated with IL-6 capturing antibody, microsphere coated with Neopterin capturing antibody, these microspheres have codes with different colors; biotin-labeled test antibody; avidin-linked phycoerythrin. The invention sepsis early diagnosis liquid chip has advantages of high detection efficiency, a small amount of sample, high specificity and high sensitivity. And at the same time, the invention can test four kinds of specific markers of sepsis simultaneously, improve sensitivity and specificity of sepsis early diagnosis, distinguish sepsis caused by bacterial and viral, judge severity and poor prognosis of sepsis and monitor continuously for judging reflection of patient to certain kind of treatment.
Owner:SUREXAM BIO TECH
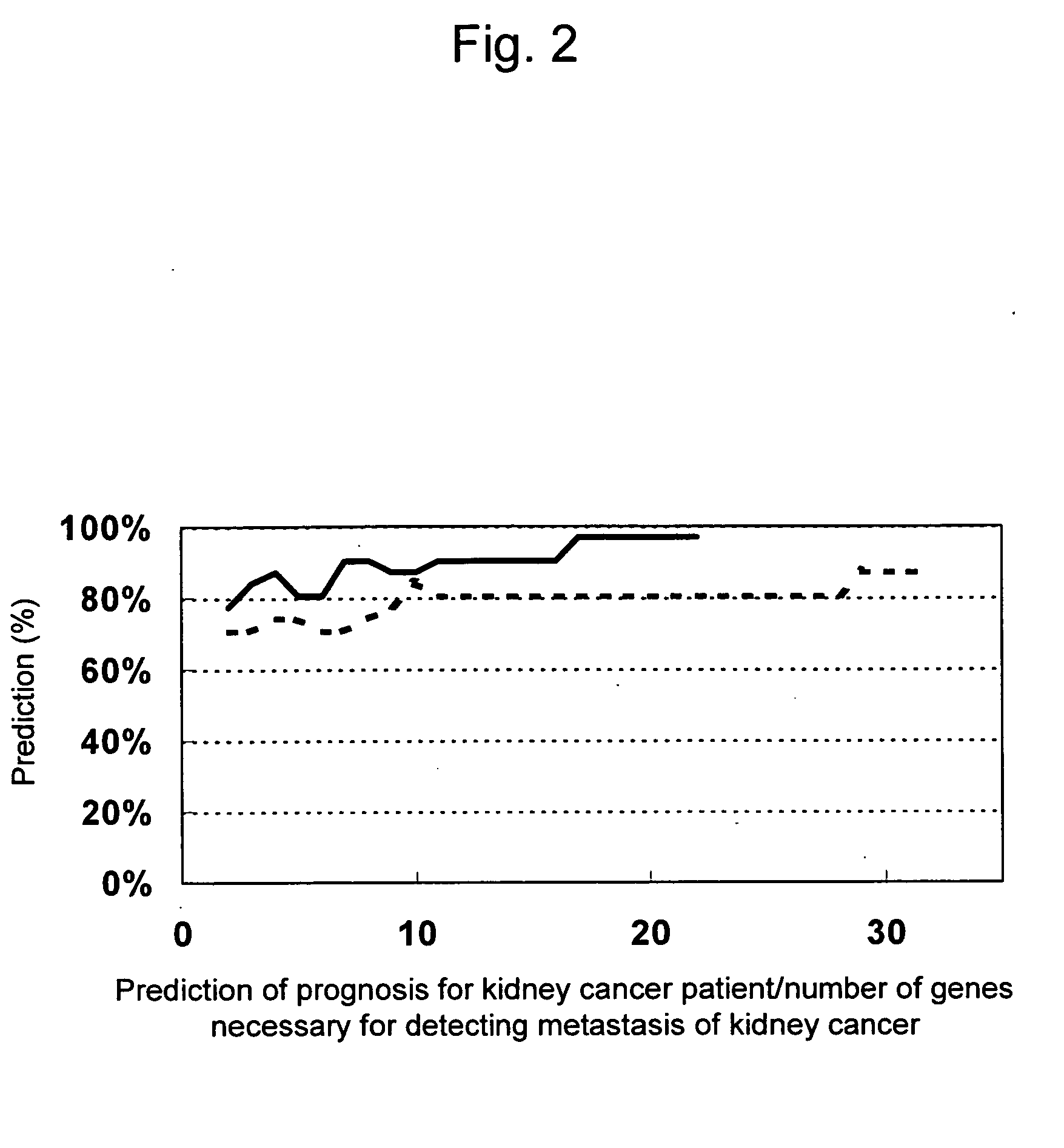
Composition and method for diagnosing kidney cancer and for predicting prognosis for kidney cancer patient
InactiveUS20100152055A1Strong specificitySimple and rapid methodNucleotide librariesMicrobiological testing/measurementLymphatic SpreadKidney cancer
This invention relates to a composition, kit, DNA chip, and use thereof for detecting, diagnosing, and predicting metastasis of kidney cancer and / or for predicting the prognosis for kidney cancer, comprising one or a plurality of polynucleotides selected from the group consisting of polynucleotides, mutants thereof or fragments thereof, the expression levels of which vary in kidney cancer cells from a patient with a poor prognosis when compared with that in kidney cancer cells from a patient with a good prognosis; or antibodies or fragments thereof that bind specifically to polypeptides, mutants thereof or fragments thereof, the expression levels of which vary in the similar manner.
Owner:TORAY IND INC +1
Methods and compositions for correlating genetic markers with cardiovascular disease
The present invention provides methods of identifying a subject having an increased or decreased risk of developing cardiovascular disease, comprising: a) correlating the presence of one or more genetic markers in chromosome 3q13.31 with an increased or decreased risk of developing cardiovascular disease; and b) detecting the one or more genetic markers of step (a) in the subject, thereby identifying the subject as having an increased or decreased risk of developing cardiovascular disease. Also provided are methods of identifying subjects with cardiovascular disease as having a good or poor prognosis, as well as methods of identifying effective treatment regimens for cardiovascular disease, based on correlation with genetic markers in chromosome 3q13.31.
Owner:DUKE UNIV
Human diaphanous-3 gene and methods of use therefor
InactiveUS20050054826A1Cell receptors/surface-antigens/surface-determinantsSugar derivativesFull length cdnaMitotic cell
The present invention is directed to the full-length cDNA sequence encoding human diaphanous-3 (DIAPH3), to DIAPH3 encoded thereby, and to fragments of DIAPH3 and the cDNA. The present invention also provides for the use of the cDNA, and of DIAPH3, as a marker of poor prognosis of breast cancer. Because DIAPH3 appears essential for proper spindle pole formation during mitosis, DIAPH3 is a useful target for screening assays designed to identify inhibitors or modulators of DIAPH3 activity, which are useful for the treatment of cancer, particularly breast cancer. Thus, the invention further provides methods of using DIAPH3, or fragments thereof, in assays to identify such compounds.
Owner:ROSETTA INPHARMATICS LLC
Methods and kits for diagnosing and treating b-cell chronic lymphocytic leukemia
InactiveUS20060281697A1Poor prognosisEasy to detectPeptide/protein ingredientsGenetic material ingredientsSmall peptideNucleotide
The present invention relates to methods and kits for detecting several polynucleotide sequence found to be indicative of a poor prognosis of B-CLL. All the polynucleotides are transcribed from a region on human chromosome 12p21-22. Most of the polynucleotides do not encode larger polypeptides, but may encode small peptides, they may function as RNAs. Four polynucleotides encode a novel protein, which in one preferred embodiment can be used as a cytokine, preferably as an interleulkin. Furthermore the invention relates to methods and compositions for treating B-CLL in particular poor prognosis B-CLL.
Owner:CLLUONE DIAGNOSTICS
Method for detecting serum marker of pancreatic cancer
InactiveCN101613748AEasy to detectIncreased sensitivityMicrobiological testing/measurementFluorescence/phosphorescenceMalignancyAdvanced stage
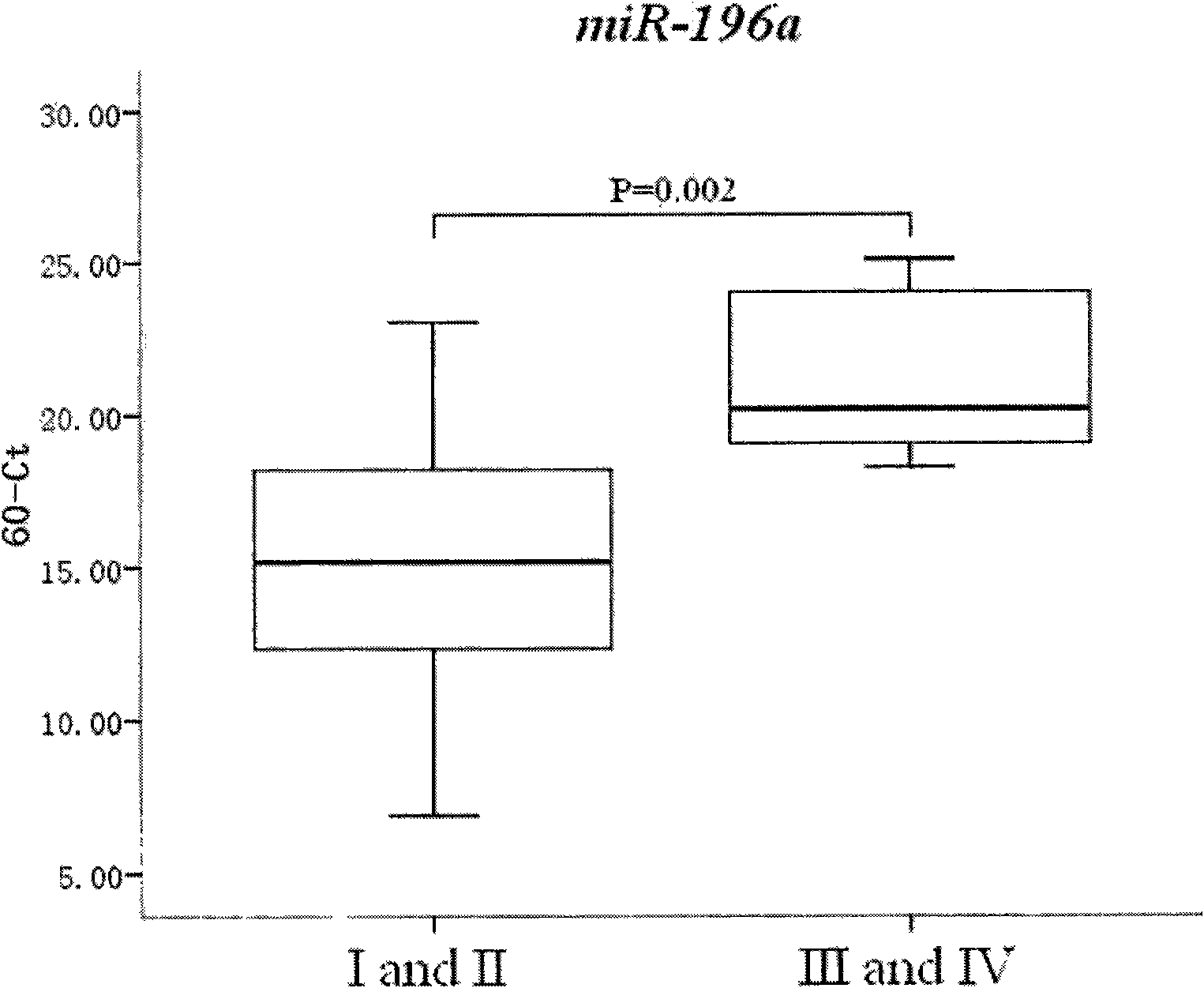
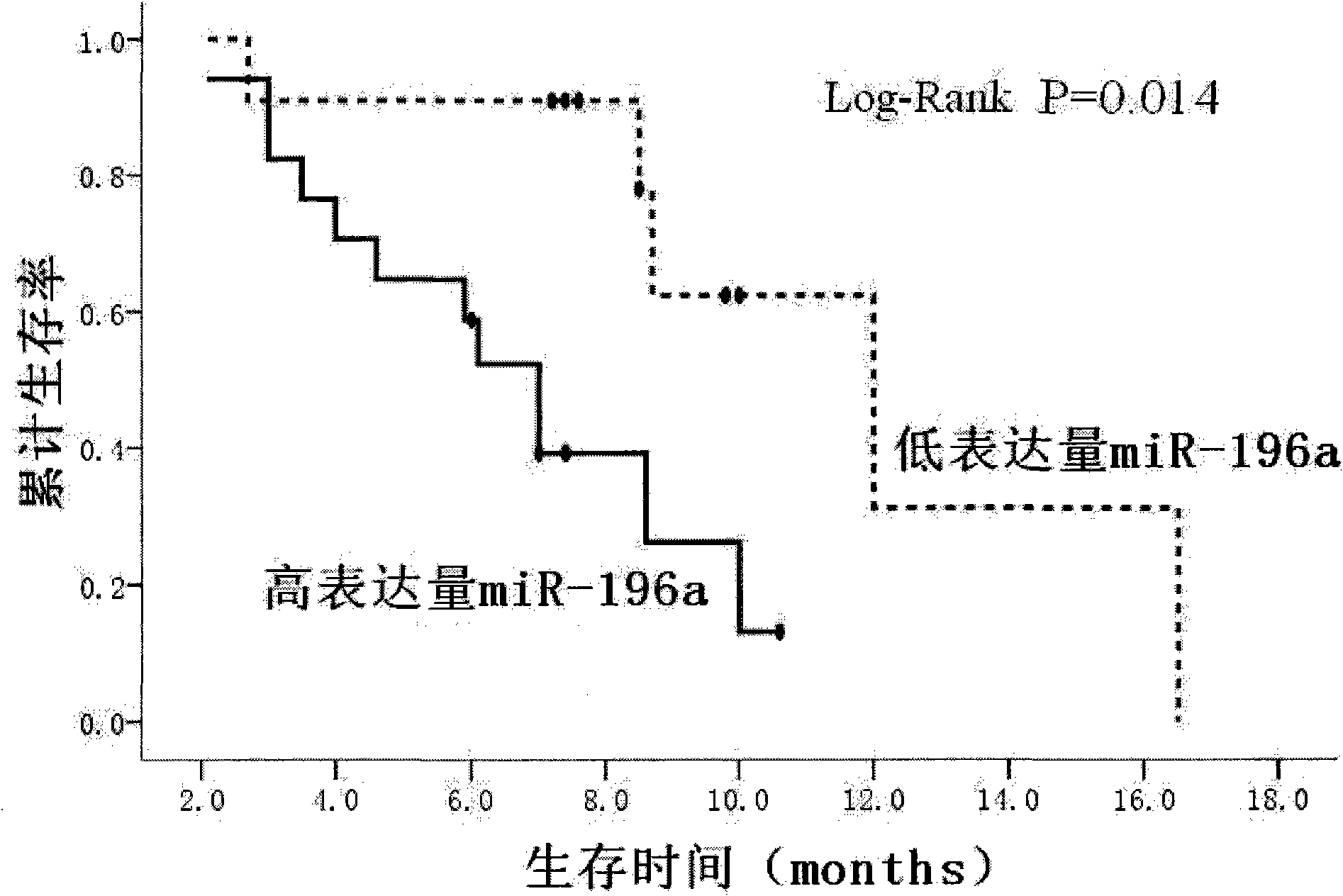
The invention relates to the technical field of medical molecular biology and provides a method for detecting the serum marker of pancreatic cancer. The pancreatic cancer has high grade malignancy, difficult early diagnosis and poor prognosis, and the pancreatic cancer also lacks really effective solution, with surgical resection as the only therapeutic method to prolong survival period. Unfortunately, most of pancreatic cancer patients are in the advanced stage (TNM belongs to III and IV stages) and miss the surgical option. The invention aims at providing a method for detecting the serum marker of pancreatic cancer and applying the method to early diagnosis of pancreatic cancer and clinical judgment of laparotomy indication. The study proves that in the invention, the expression level of miR-196a in the serum is closely related to the postoperative survival period of pancreatic cancer; the study later proves that the relative expression abundance of miR-196a can well distinguish resectable pancreatic cancer (TNM belongs to I and II stages) from pancreatic cancer in advanced stage (TNM belongs to III and IV stages), so the method in the invention can be used for detecting the serum marker microRNA-196a of pancreatic cancer, and the method further has the advantages of convenient detection, good sensitivity and high accuracy.
Owner:SECOND MILITARY MEDICAL UNIV OF THE PEOPLES LIBERATION ARMY
Microarray for predicting the prognosis of neuroblastoma and method for predicting the prognosis of neuroblastoma
InactiveUS20050287541A1Accurate and convenient predictionBioreactor/fermenter combinationsBiological substance pretreatmentsGood prognosisNucleotide sequencing
A microarray for predicting the prognosis of neuroblastoma, wherein the microarray has 25 to 45 probes related to good prognosis, which are hybridized to a gene transcript whose expression is increased in a good prognosis patient with neuroblastoma and are selected from 96 polynucleotides consisting of the nucleotide sequences of Seq. ID No. 1 to 96 or their partial continuous sequences or their complementary strands, and 25 to 45 probes related to poor prognosis, which are hybridized to a gene transcript whose expression is increased in a poor prognosis patient with neuroblastoma and are selected from 104 polynucleotides consisting of the nucleotide sequences of Seq. ID No. 97 to 200 or their partial continuous sequences or their complementary strands.
Owner:CHIBA PREFECTURE +1
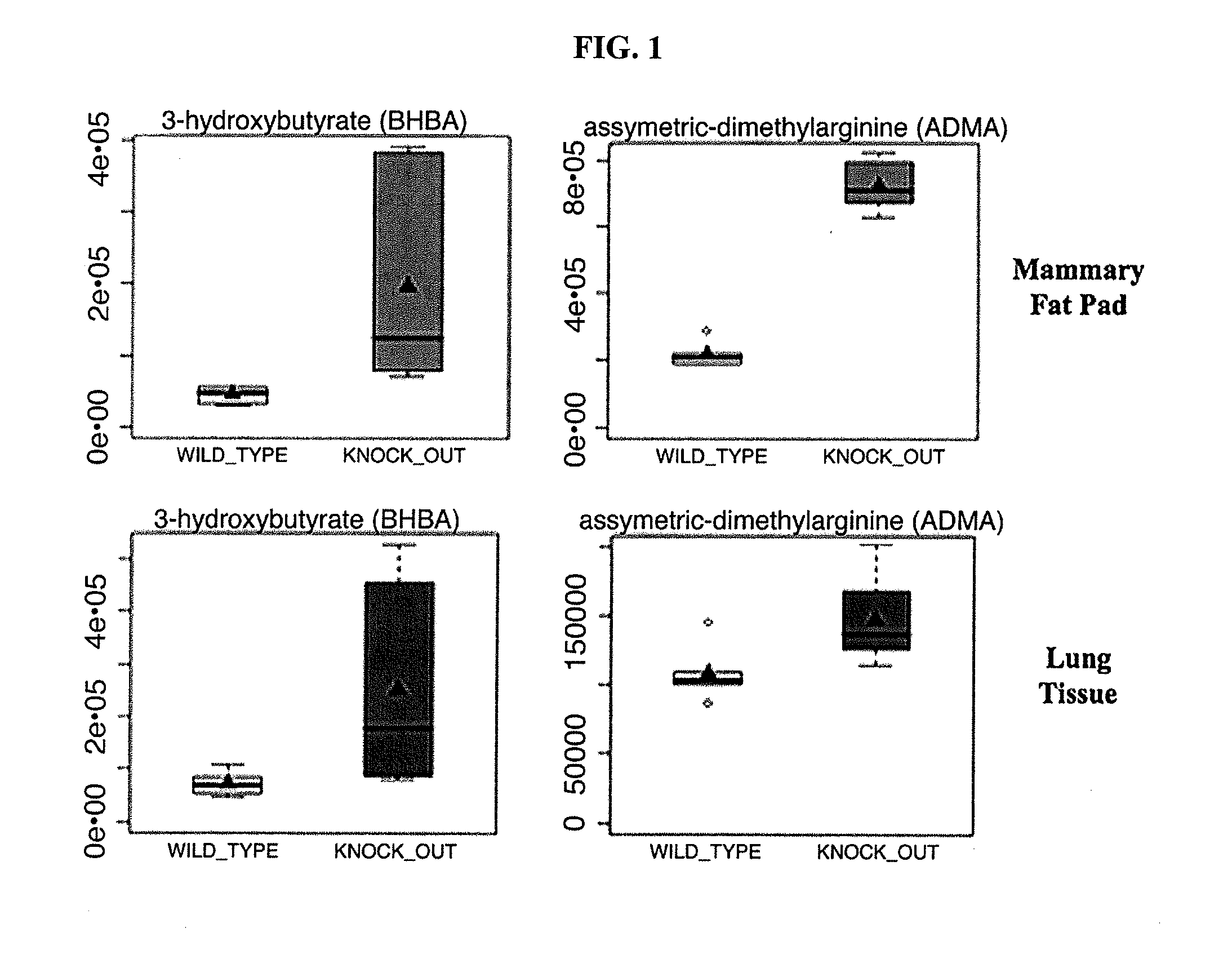
Prognosis and treatment of breast cancer
Methods of prognosis and monitoring of breast cancer include determining the level of one or more of the markers comprising asymmetric dimethyl arginine (ADMA), beta-hydroxybutyrate (BHB) and microRNA-31 (miR-31) in patient samples. An increased level is correlated with poor prognosis. Breast cancer is treated by administration of one or more inhibitors of ADMA and / or BHB.
Owner:THOMAS JEFFERSON UNIV
Crenolanib for Treating FLT3 Mutated Proliferative Disorders Associated Mutations
ActiveUS20180117031A1Increase chances of survivalPoor prognosisOrganic active ingredientsMicrobiological testing/measurementDiseaseTumor Sample
Owner:AROG PHARMA
ONCOGENOMICS-BASED RNAi SCREEN AND USE THEREOF TO IDENTIFY NOVEL TUMOR SUPPRESSORS
InactiveUS20100273660A1High resolutionPoor prognosisCompound screeningApoptosis detectionOncogenomicsWilms' tumor
In some aspects, the invention provides a genetically tractable in situ non-human animal model for hepatocellular carcinoma. The model is useful, inter alia, in understanding the molecular mechanisms of liver cancer, in understanding the genetic alterations that lead to chemoresistance or poor prognosis, and in identifying and evaluating new therapies against hepatocellular carcinomas. The liver cancer model of this invention is made by altering hepatocytes to increase oncogene expression, to reduce tumor suppressor gene expression or both and by transplanting the resulting hepatocytes into a recipient non-human animal.The present invention also provides methods for identifying and validating tumor suppressor genes by screening pools of shRNAs that target genomic regions deleted in human cancers, such as human hepatocellular carcinomas. The present invention also provides validated tumor suppressor genes, and methods of inhibiting cell proliferation and / or tumor growth, for example by expression of such tumor suppressor genes.
Owner:COLD SPRING HARBOR LAB INC
Gene expression profiling based identification of CKS1B as a potential therapeutic target in multiple myeloma
InactiveUS7935679B2Increased riskBiocideMicrobiological testing/measurementClinical manifestationFluorescence
Gene expression profiling is a powerful tool that has varied utility. It enables classification of multiple myeloma into subtypes and identifing genes directly involved in disease pathogensis and clinical manifestation. The present invention used gene expression profiling in large uniformly treated population of patients with myeloma to identify genes associated with poor prognosis. It also demonstrated that over-expression of CKS1B gene, mainly due to gene amplification that was determined by Fluorescent in-situ hybridization to impart a poor prognosis in multiple myleoma. It is further contemplated that therapeutic strategies that directly target CKS1B or related pathways may represent novel, and more specific means of treating high risk myeloma and may prevent its secondary evolution.
Owner:BIOVENTURES LLC
Diagnostic marker for malignant glioma
InactiveCN107034305AMicrobiological testing/measurementDisease diagnosisGenomicsLiquid chromatography mass spectroscopy
The invention relates to a diagnostic marker for malignant glioma and belongs to the technical field of molecular biology. Particularly, the marker is an NES gene and an expression product thereof. The diagnostic marker has the advantages that comprehensive genomics and proteomics analysis is carried out on a GBM, and through gene expression analysis based on a gene chip and RNA sequencing and proteome analysis of LC / MS / MS, the mRNA level and protein level of the NES gene are regulated up. Through survival analysis on the NES, over-expression of the NES is related to poor prognosis of a GBM patient, so that the NES can be used as a GBM biomarker and a potential therapeutic target. The NES gene is used for encoding nestin protein, is mainly applied to nerve cell expression, and can be used as the GBM biomarker and the potential therapeutic target.
Owner:SHANGHAI TENTH PEOPLES HOSPITAL
Targeting metabolic enzymes in human cancer
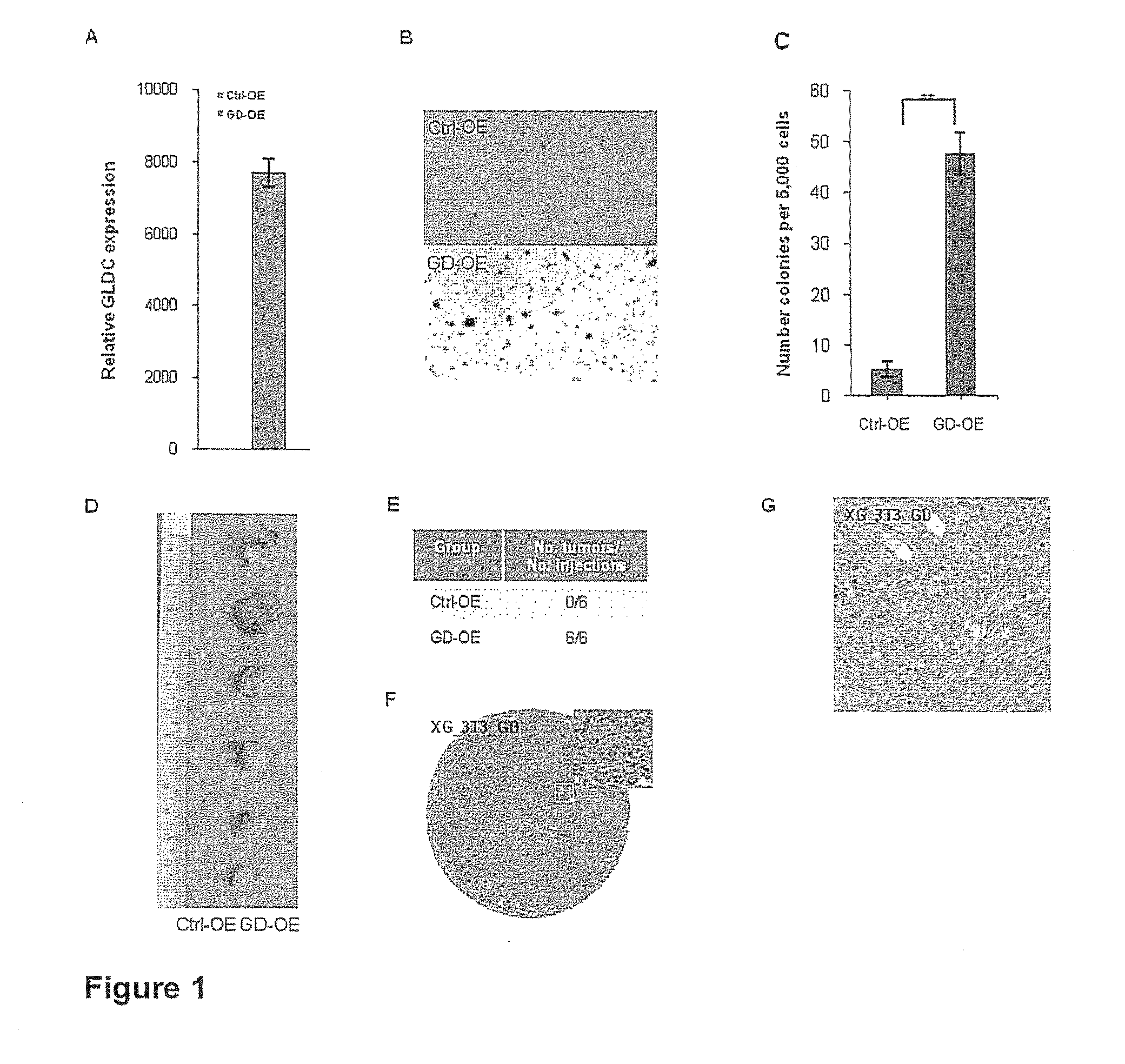
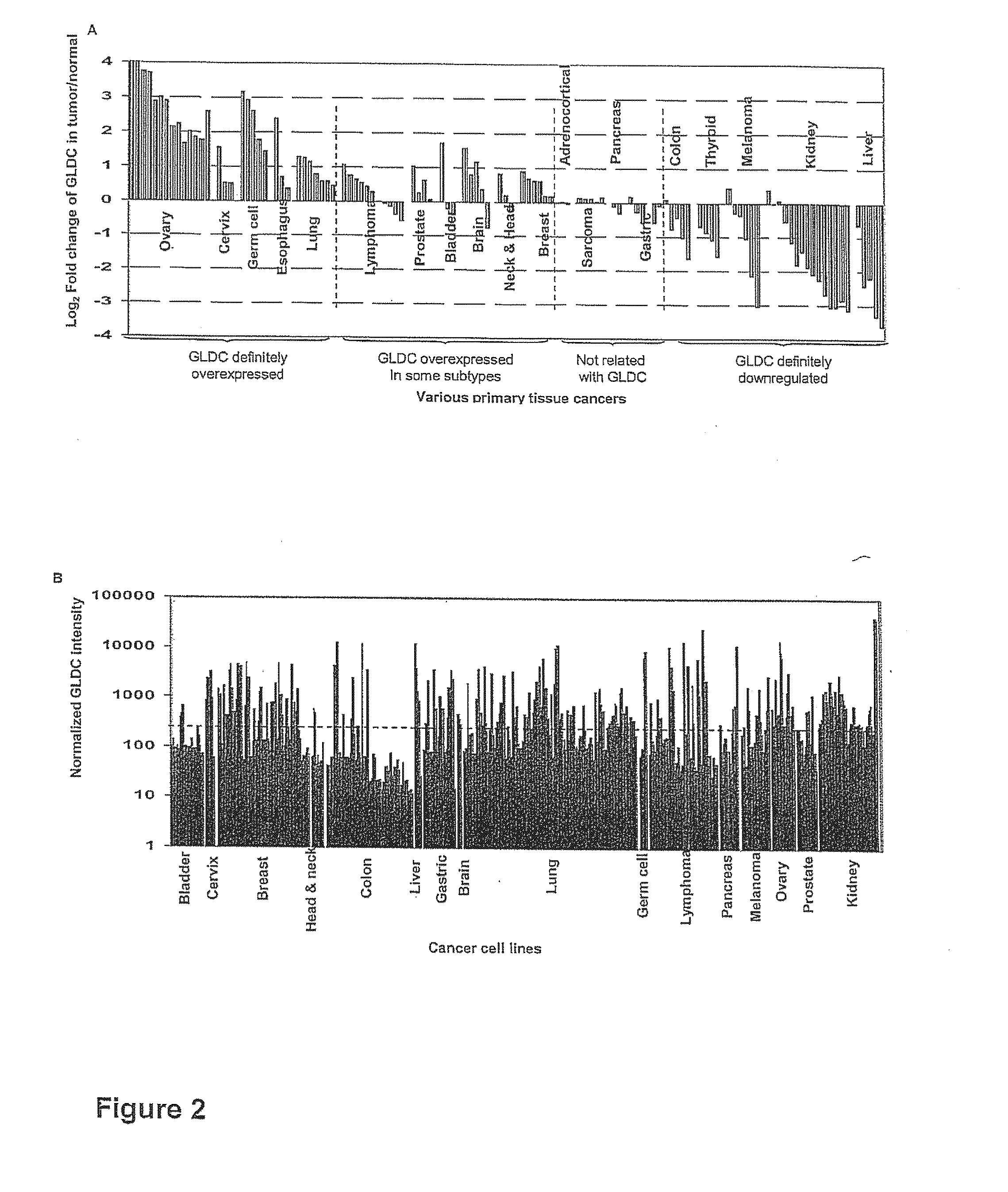
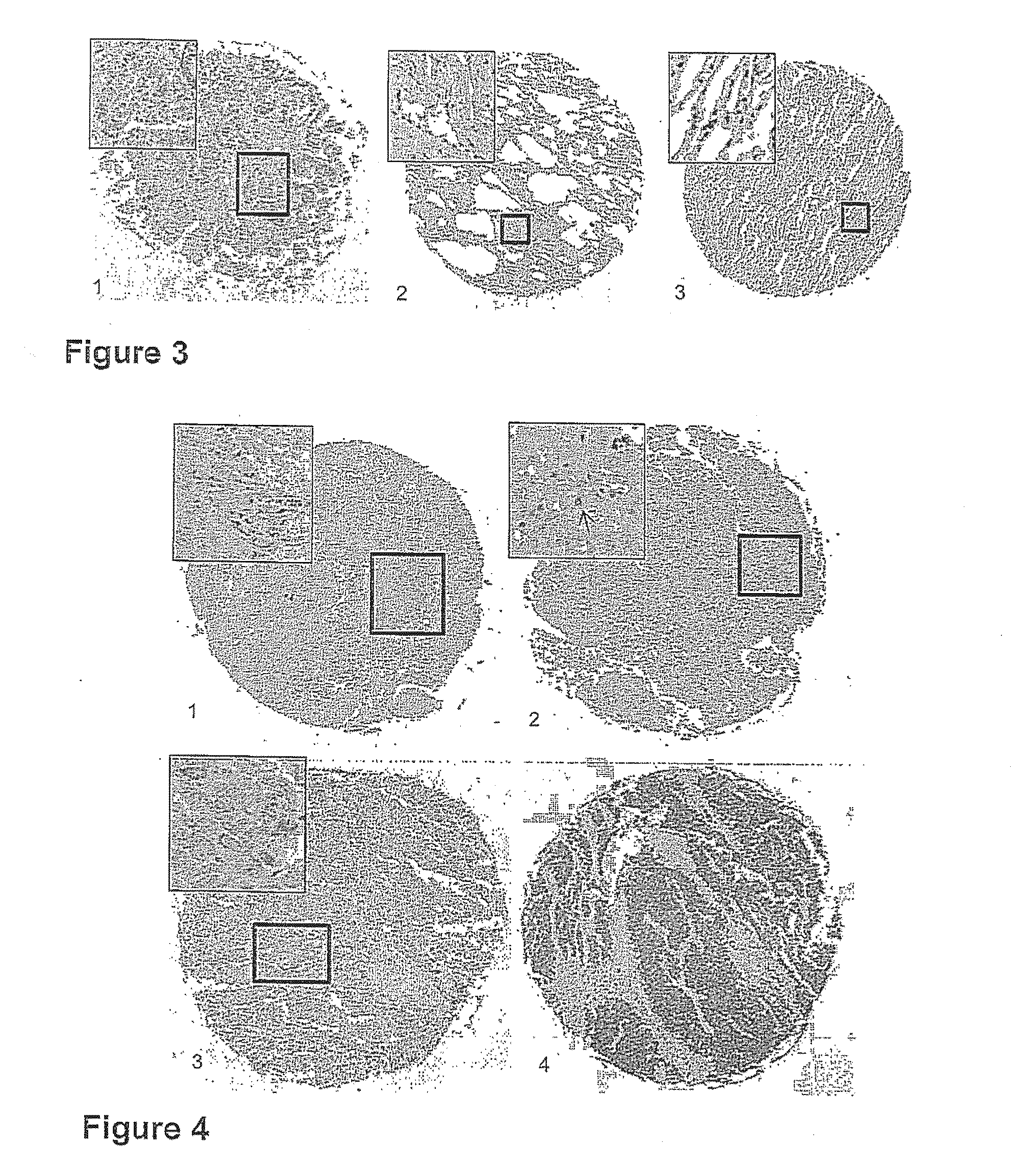
Targeting metabolic enzymes in human cancer Abstract Lung cancer is a devastating disease and a major therapeutic burden with poor prognosis. The functional heterogeneity of lung cancer (different tumor formation ability in bulk of tumor) is highly related with clinical chemoresistance and relapse. Here we find that, glycine dehydrogenase (GLDC), one of the metabolic enzyme involved in glycine metabolism, is overexpressed in various subtypes of human lung cancer and possibly several other types of cancers. GLDC was found to be highly expressed in tumor-initiating subpopulation of human lung cancer cells compared with non-tumorigenic subpopulation. By array studies we showed that normal lung cells express low levels of GLDC compared to xenograft and primary tumor. Functional studies showed that RNAi inhibition of GLDC inhibits significantly the clonal growth of tumor-initiating cells in vitro and tumor formation in immunodeficient mice. Overexpression of GLDC in non-tumorigenic subpopulation convert the cells to become tumorigenic. Furthermore, over-expression of GLDC in NIH / 3T3 cells and human primary lung fibroblasts can transform these cells, displaying anchorage-independent growth in soft agar and tumor-forming in mice. Not only is GLDC is expressed human lung cancer, it is also up-regulated in other types of cancer, such as colon cancer. RNAi knockdown of GLDC in colon cancer cell line, CACO-2 cells, can also inhibit the tumor formation in mice. Thus GLDC maybe a new metabolic target for treatment of lung cancer, and other cancers.
Owner:AGENCY FOR SCI TECH & RES
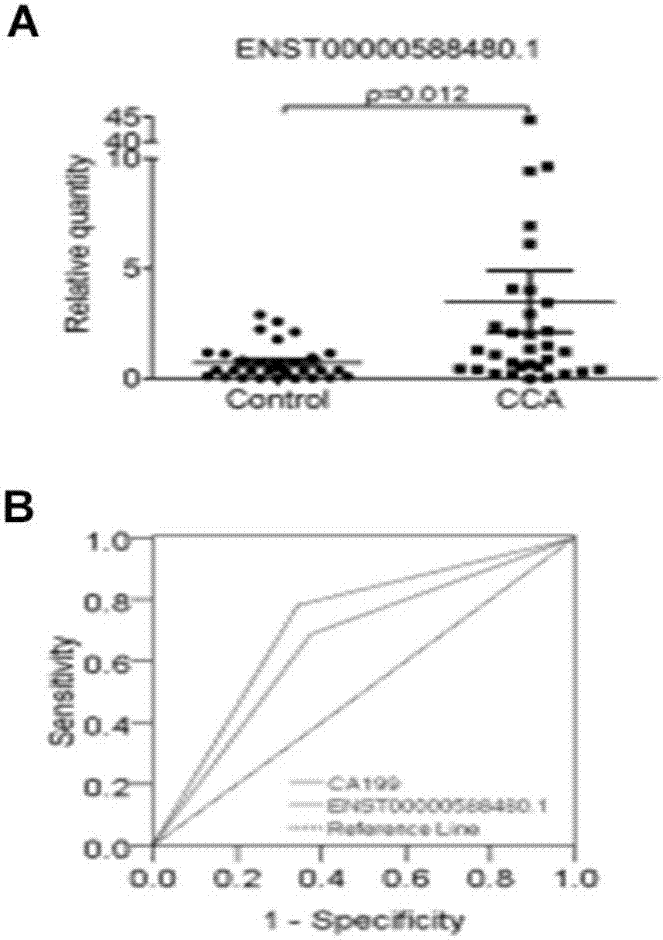
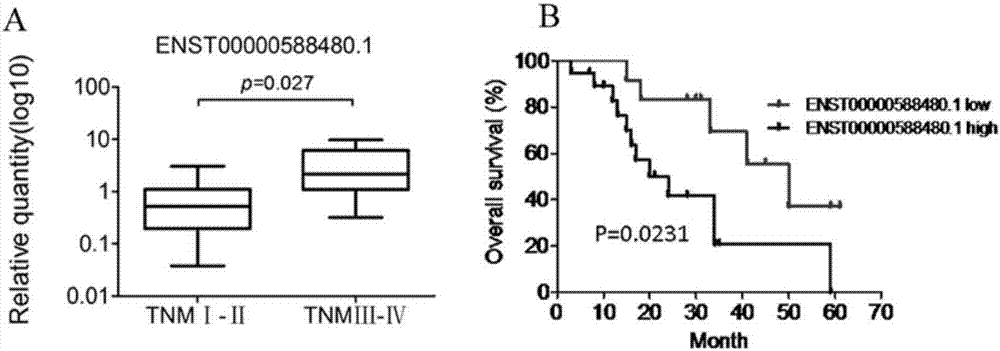
Long and non-coding RNA and application thereof in diagnosis/treatment of bile duct carcinoma
InactiveCN107012145AOrganic active ingredientsMicrobiological testing/measurementMedicinePrognostic prediction
The invention belongs to the field of genetic engineering and in particular relates to an application of a bile exosome long and non-coding RNA (lncRNA) ENST00000588480.1 in diagnosis of bile duct carcinoma, prognostic prediction and target spot drug treatment, wherein the long and non-coding RNA is positively correlated with TNM staging and poor prognosis of a patient.
Owner:THE SECOND AFFILIATED HOSPITAL OF NANJING MEDICAL UNIV
Mtor Pathway Theranostic
This invention relates, e.g., to a method for predicting a subject's response to a chemotherapeutic agent and / or the subject's prognosis, comprising measuring the phosphorylation state of at least one member of the mTOR pathway, and / or of at least one member of an interconnected polypeptide pathway (e.g. a member of the Akt pathway or a member of the IRS pathway), compared to a baseline value, in a cancer tissue or cancer cell sample from the subject, wherein an elevated level of the phosphorylation state compared to the baseline value indicates that the subject is a non-responder to the chemotherapeutic agent and / or has a poor prognosis. Also described is a method for treating a cancer in a subject in need thereof, wherein the subject exhibits an elevated level of the phosphorylation state, comprising administering one or more inhibitors of the mTOR and / or an interconnected pathway.
Owner:GEORGE MASON UNIVERSITY
Methods of prognosis and diagnosis of ovarian cancer
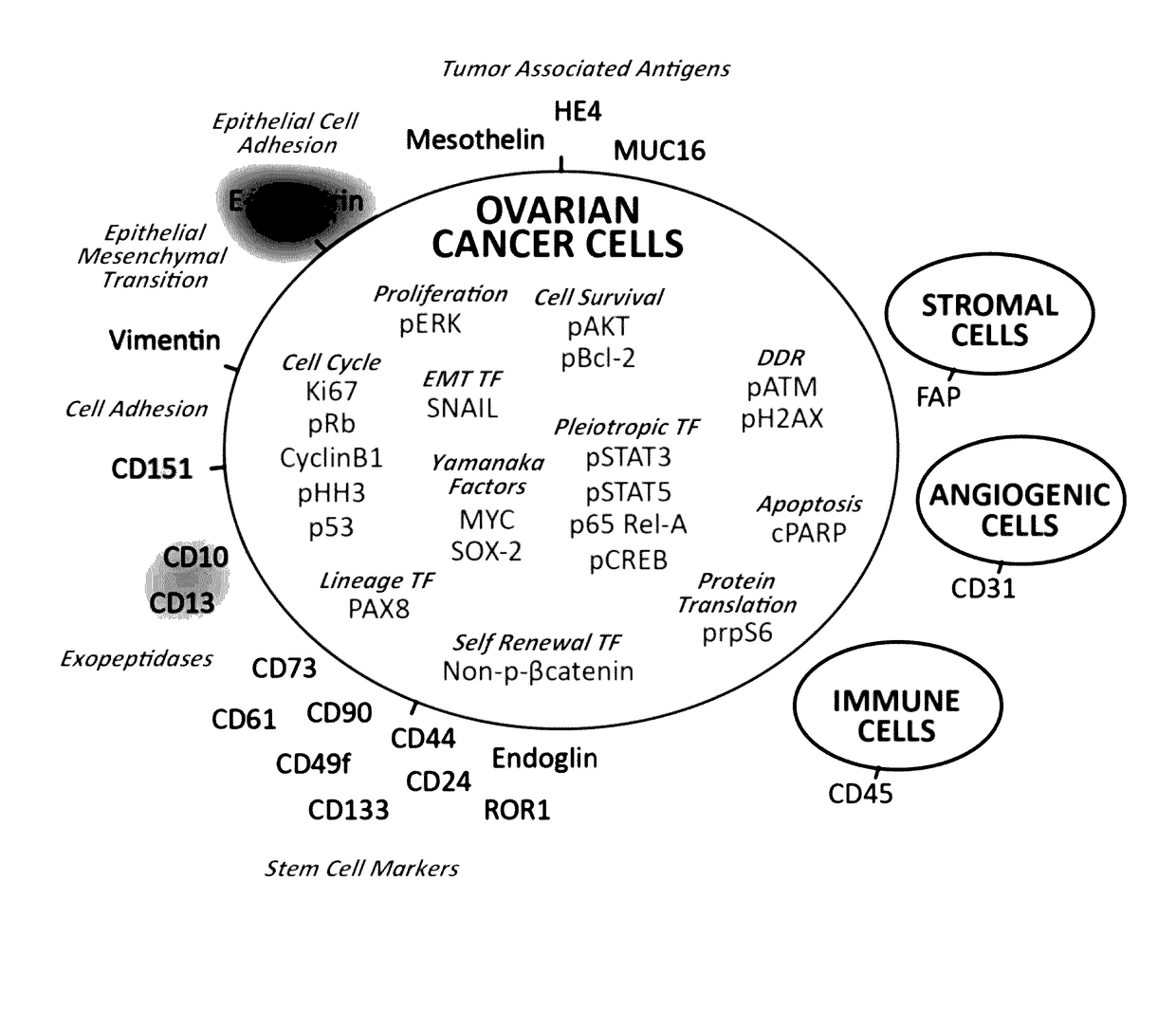
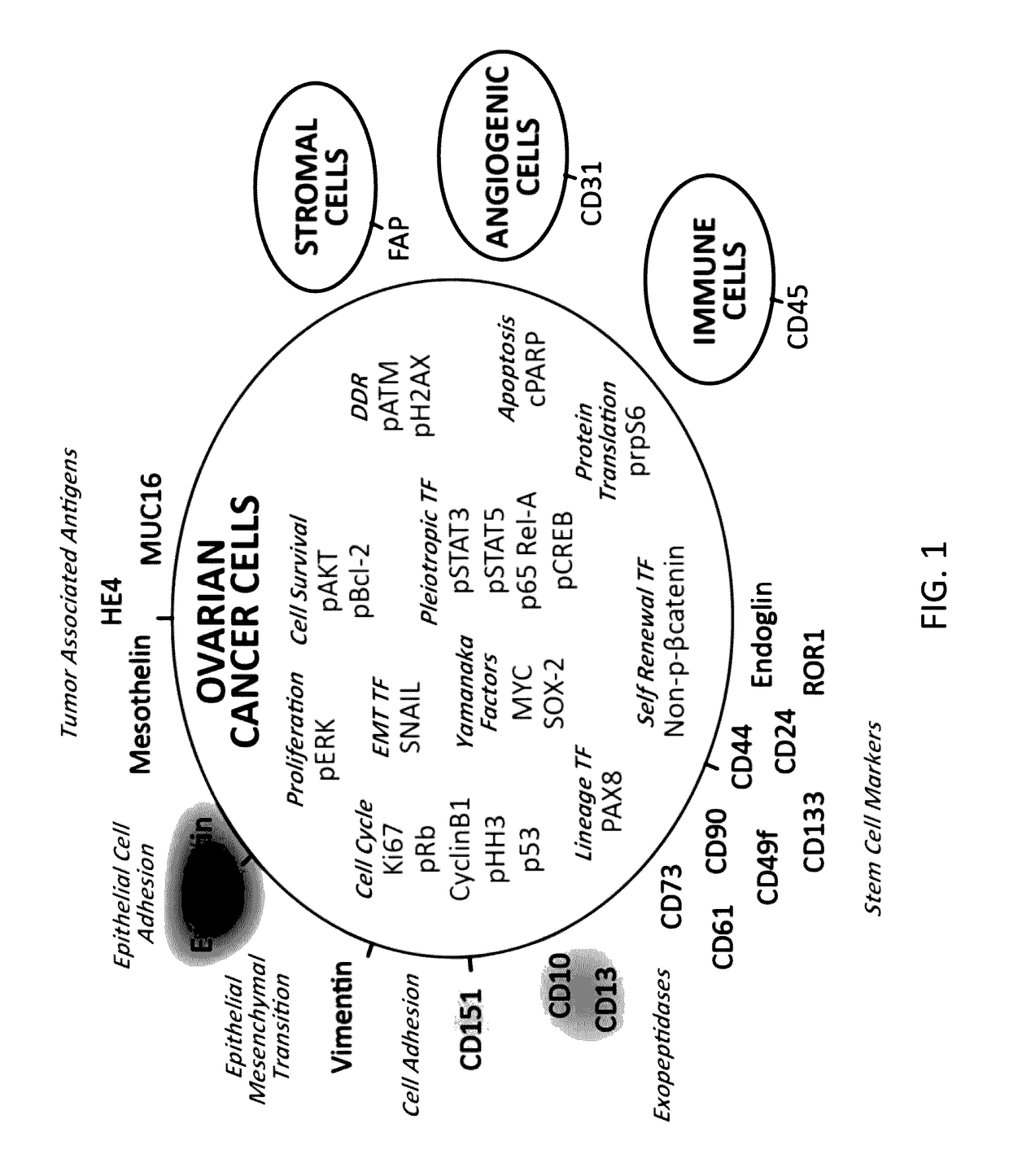
Cellular markers indicating a poor prognosis for ovarian cancer patients are disclosed. In particular, the invention relates to methods utilizing the frequency of a subset of cells in ovarian tumor tissue expressing vimentin, cMyc, or HE4, or any combination thereof, to predict an ovarian cancer patient will relapse.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
Development and use of a new orthotopic, genetically tractable non-human animal model for liver cancer
InactiveUS20060162000A1High resolutionPoor prognosisVirusesNew breed animal cellsTumour suppressor geneHuman animal
This invention provides a genetically tractable in situ non-human animal model for hepatocellular carcinoma. The model is useful, inter alia, in understanding the molecular mechanisms of liver cancer, in understanding the genetic alterations that lead to chemoresistance or poor prognosis, and in identifying and evaluating new therapies against hepatocellular carcinomas. The liver cancer model of this invention is made by altering hepatocytes to increase oncogene expression, to reduce tumor suppressor gene expression or both and by transplanting the resulting hepatocytes into a recipient non-human animal.
Owner:COLD SPRING HARBOR LAB INC
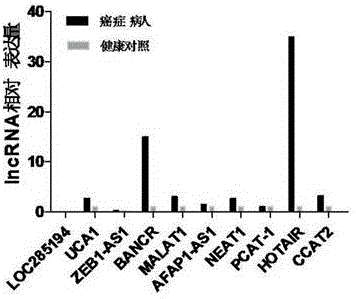
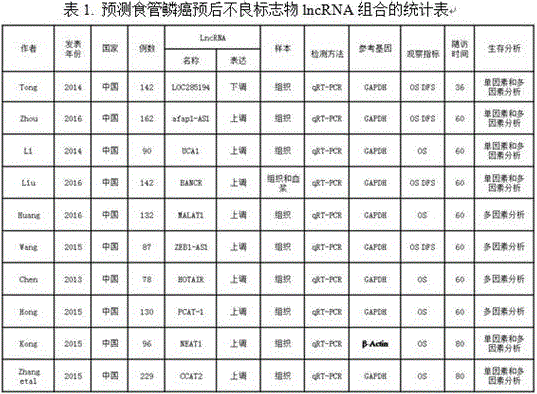
LncRNA composition for detecting prognosis of early esophageal cancer and kit comprising LncRNA composition
InactiveCN107523647APredict risk of poor prognosisImprove survival rateMicrobiological testing/measurementDNA/RNA fragmentationReference sampleCvd risk
The invention discloses an LncRNA composition for detecting prognosis of an early esophageal cancer and a kit comprising the LncRNA composition. By fluorescent quantitative PCR, a sample (which can be tissues / plasma serum and the like) of a patient with an esophageal cancer and difference change of the expression amount of a group of LncRNA in a corresponding para-carcinoma tissue / reference sample are identified, and the risk of esophageal cancerrelapse or transfer is accurately evaluated early. The kit can be used for accurately detecting early esophageal cancer transfer potential, then the transfer or relapse risk of a postoperative esophageal cancer patient can be accurately predicted and evaluated, the patient with high risk is intensively monitored and intervened effectively, relapse or transfer of the esophageal cancer is reduced, and prognosis of the patient is improved further. The poor prognosis related lncRNA composition is adopted, and the shortcomings that sensitivity and specificity are low due to the fact that a kit only uses single lncRNA as a tumor marker, and therefore, rate of fault diagnosis and rate of missed diagnosis on the esophageal cancer are greatly increased are overcome.
Owner:NANYANG NORMAL UNIV
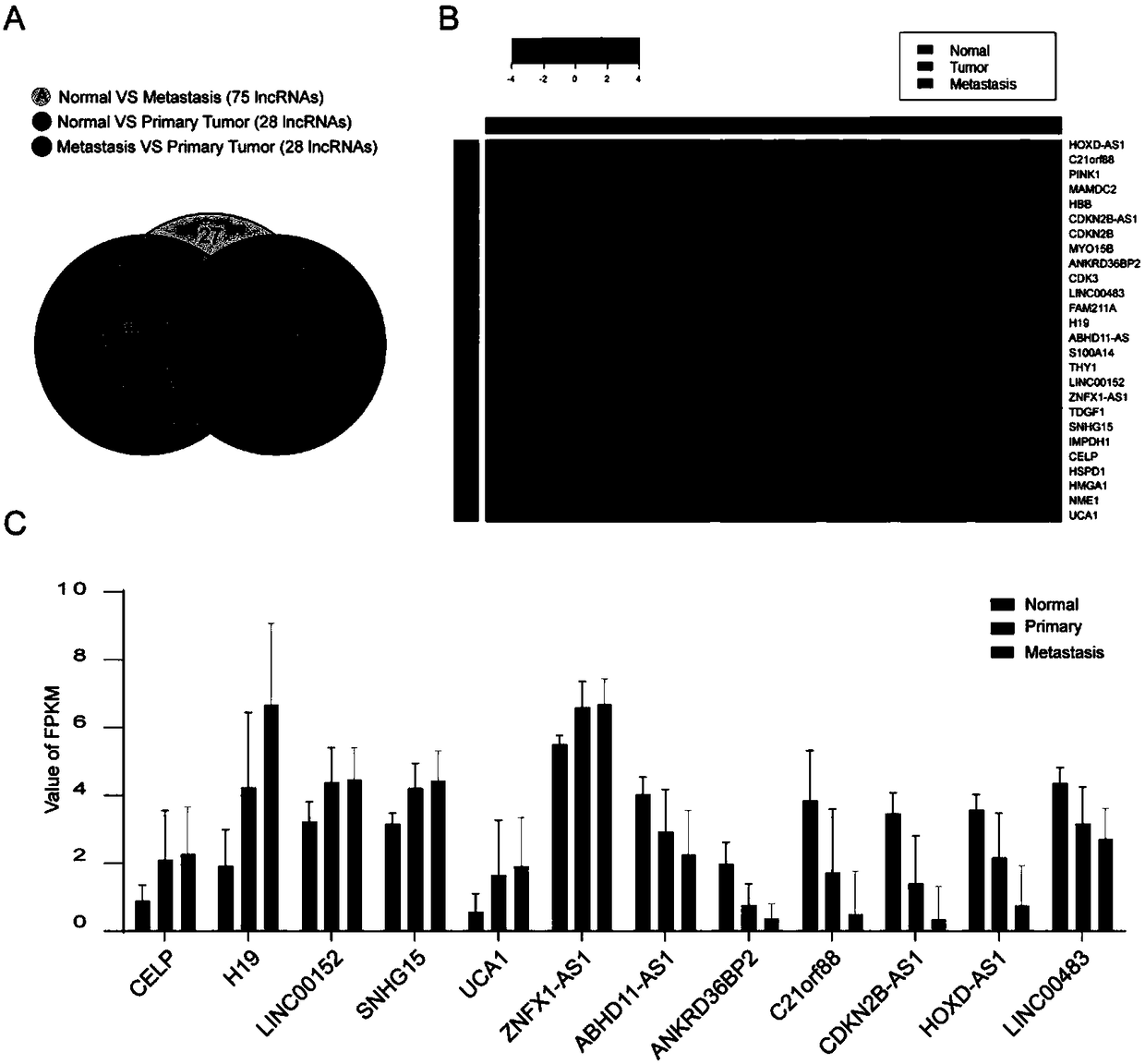
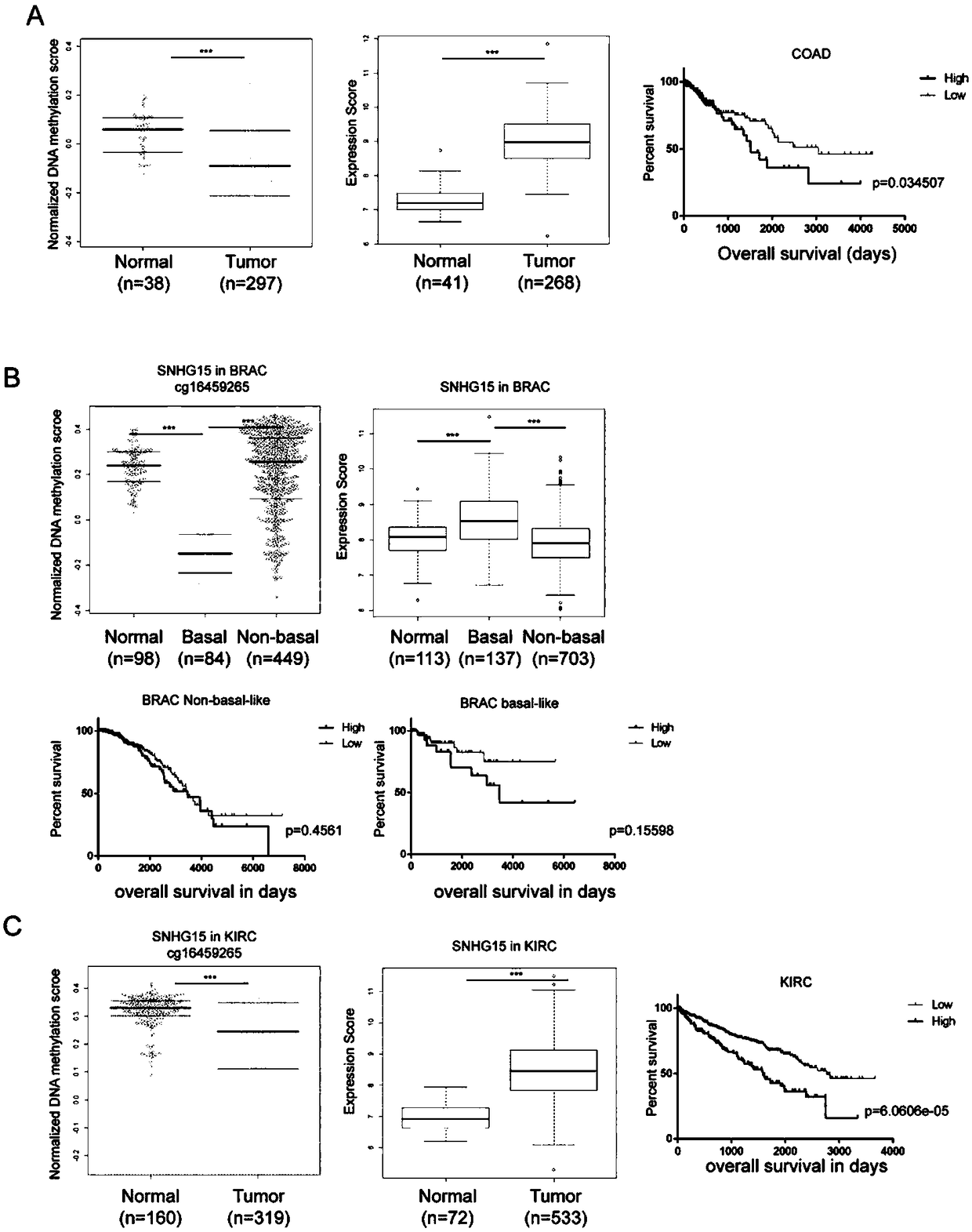
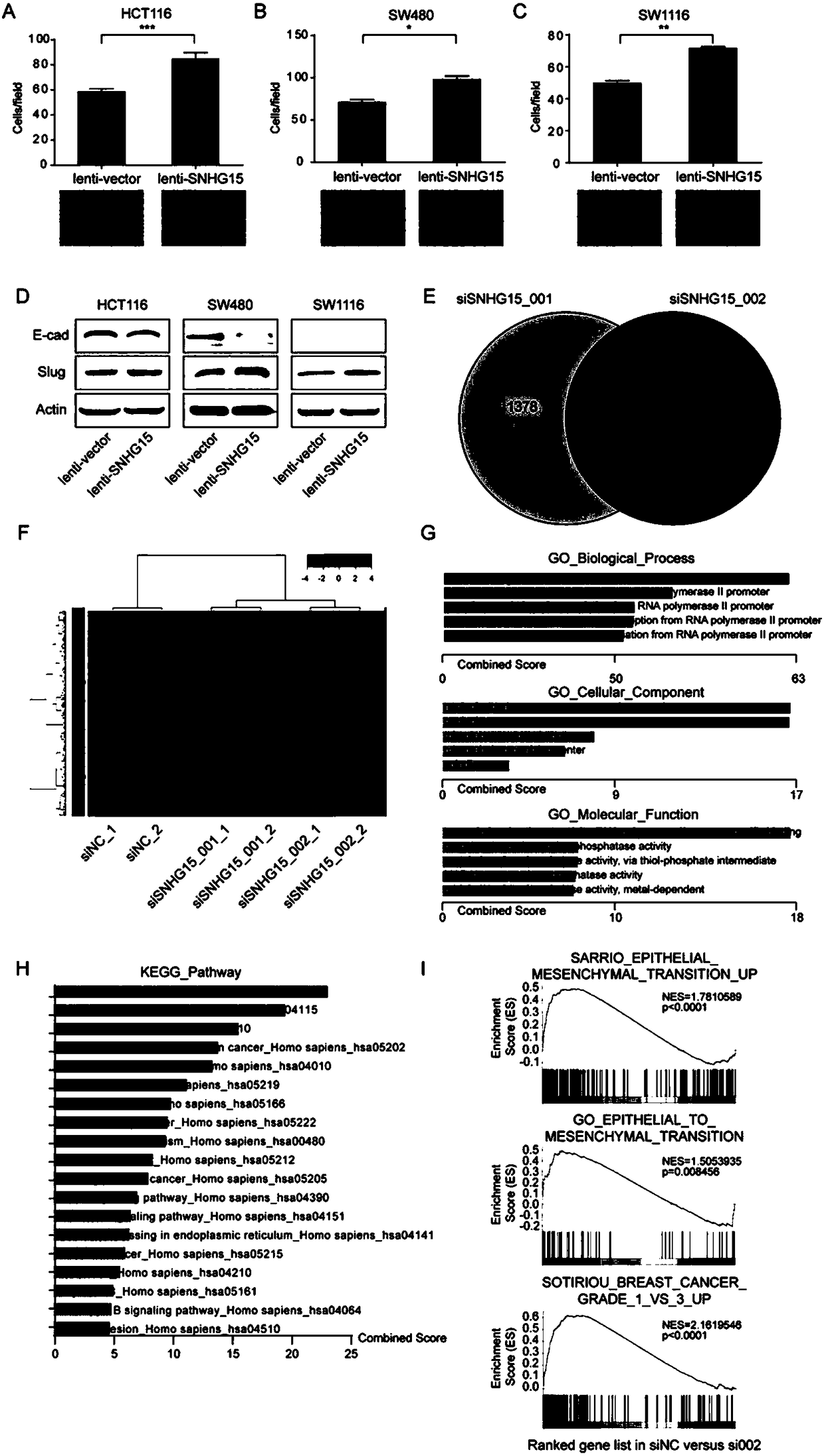
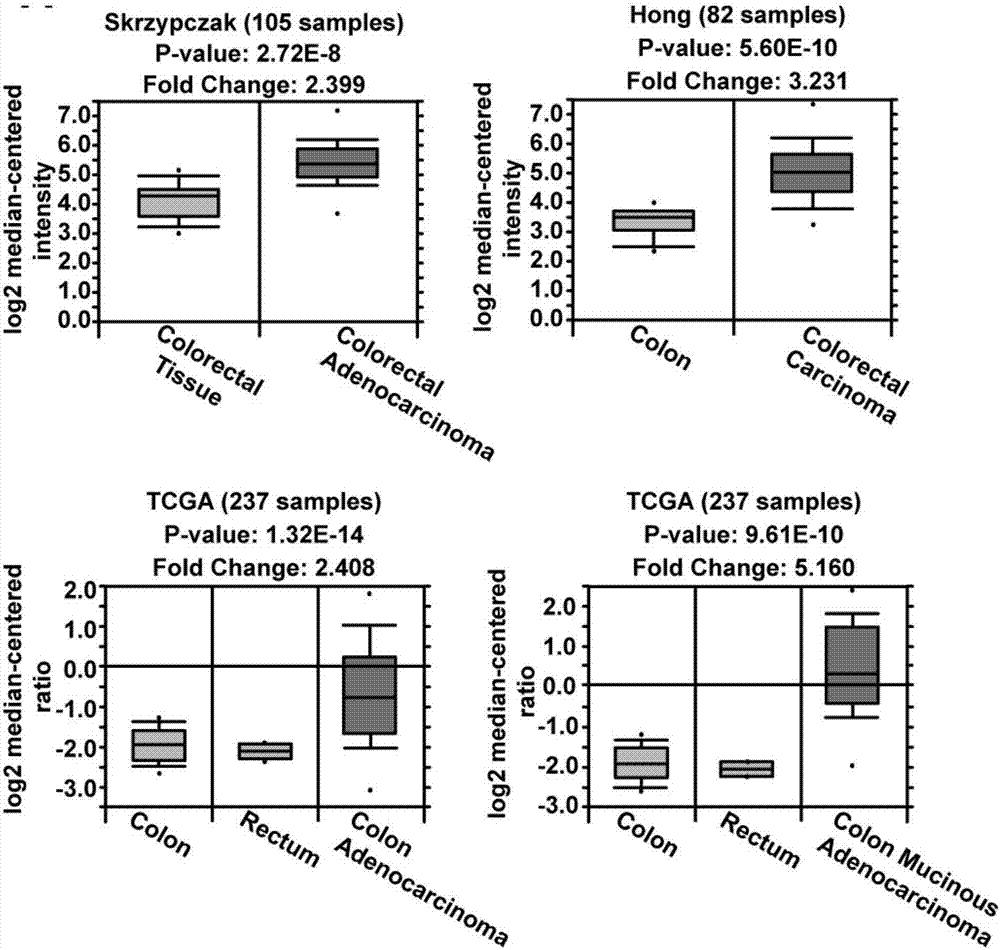
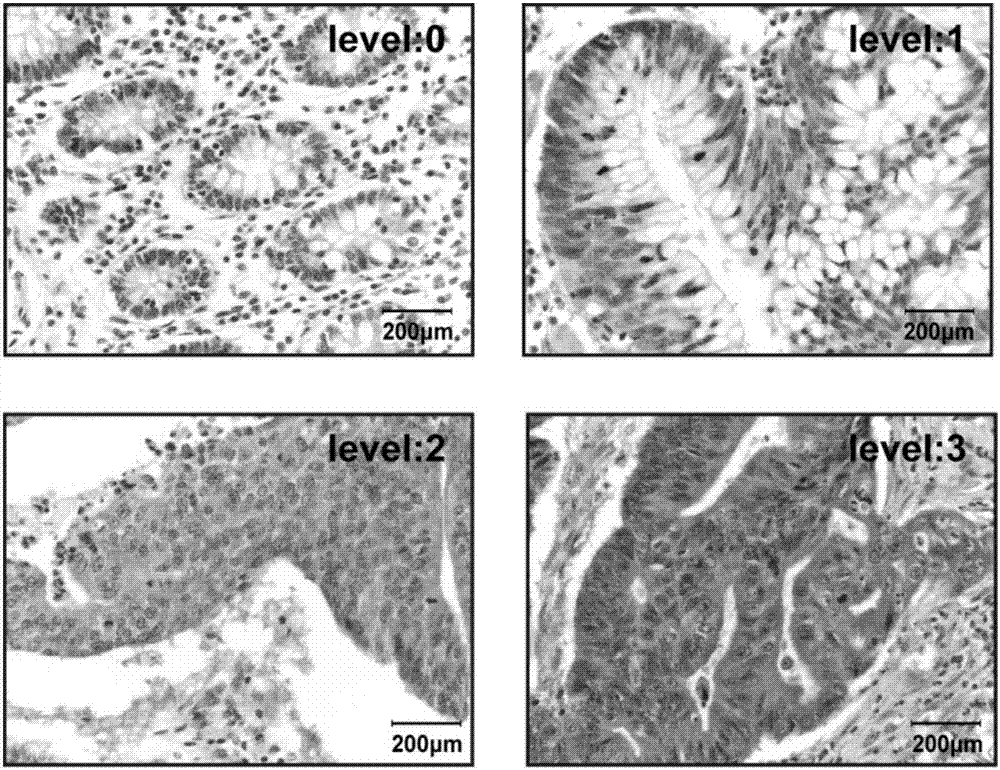
Long noncoding RNA SNHG15 and application thereof in preparation of cancer diagnosis and treatment drugs
InactiveCN108707668APrevent invasionInhibit migrationMicrobiological testing/measurementCancers diagnosisColorectal cancer cell line
The invention belongs to the technical field of genetic engineering, and discloses long noncoding RNA SNHG15 and application thereof in preparation of cancer diagnosis and treatment drugs. Cancers include colorectal cancer, breast cancer, kidney cancer and the like. Expression of the long noncoding RNA SNHG15 in tumor tissues of patients suffering from the colorectal cancer, the breast cancer andthe kidney cancer is up-regulated. The patients with highly-expressed long noncoding RNA SNHG15 have poor prognosis. Through expression knock-down of the long noncoding RNA SNHG15 in colorectal cancercell lines, invasion, migration and multiplication capabilities of cells can be inhibited, and drug susceptibility of colorectal cancer cell lines to antitumor drugs including gemcitabine and cis-platinum can be improved.
Owner:PEKING UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com