Long and non-coding RNA and application thereof in diagnosis/treatment of bile duct carcinoma
A cholangiocarcinoma, non-coding technology, applied in the field of genetic engineering, can solve the problems of lncRNA that have yet to be discovered
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
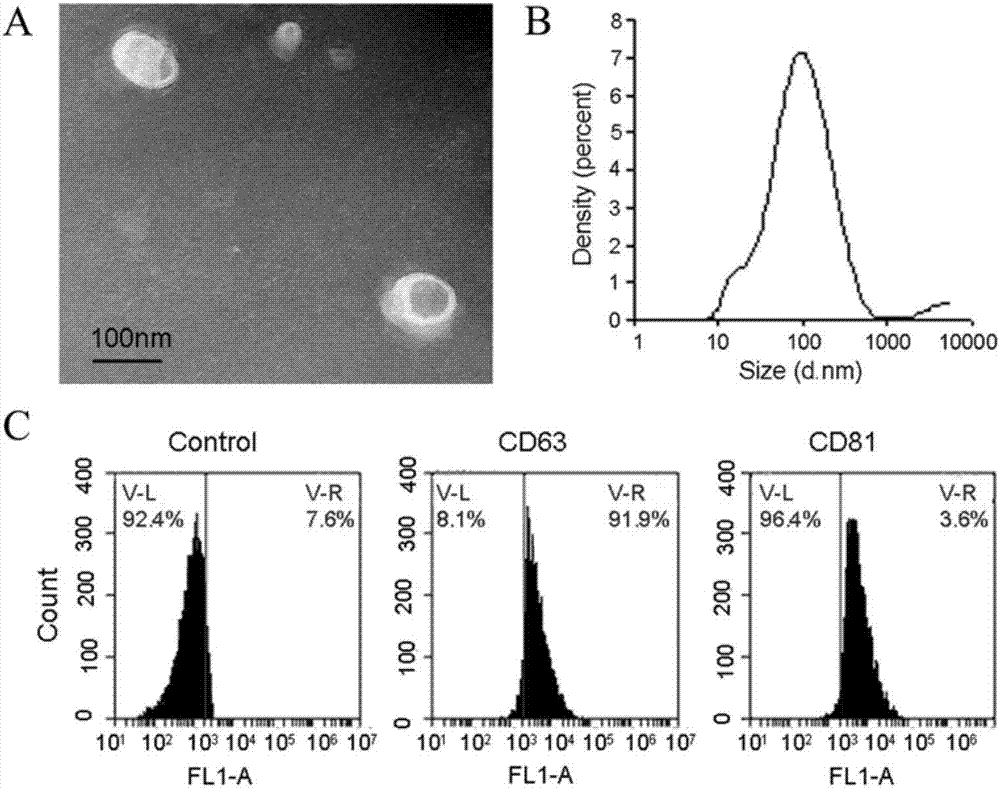
[0048] Example 1 Isolation and Identification of Exosomes
[0049] (1) Take out the patient’s bile specimen stored in the -80°C refrigerator, balance with pre-cooled PBS, centrifuge at 300xg for 10 minutes at 4°C, discard the pellet to remove the precipitated cells and cell debris, and transfer the supernatant to a new Labeled 10ml centrifuge tube.
[0050] (2) The supernatant taken out was balanced with pre-cooled PBS and then centrifuged at 16,500 x g for 20 minutes at 4°C. The precipitate was discarded to further remove the debris in the supernatant. The supernatant was sucked with a syringe and filtered through a 0.22 μm filter, and the filtrate was collected in Labeled ultracentrifuge tubes.
[0051] (3) Add precooled PBS to more than 2 / 3 of the volume of the centrifuge tube in the ultracentrifuge tube containing the filtrate and balance with PBS. Vacuum centrifuge at 120,000 x g in an ultracentrifuge for 70 minutes at 4°C, and a yellow precipitate can be observed at th...
Embodiment 2
[0053] Example 2 Detection of the expression of ENST00000588480.1R in bile exosomes
[0054] RNA extraction
[0055] After the exosomes were blown evenly with trizol and allowed to stand on ice for 5 minutes, about 0.2 ml of chloroform (trichloromethane, volume ratio of chloroform: trizol=1:5) was added, mixed quickly and vigorously, and left to stand on ice for 5 minutes;
[0056] The above mixture was centrifuged at 12000rpm / min at 4°C for 20min, and then divided into three layers (aqueous phase / white precipitate / red organic matter). Use a pipette to gently absorb the colorless and transparent liquid in the upper layer into a new enzyme-free EP tube. Avoid white precipitate;
[0057] Add an equal volume of isopropanol to the colorless and transparent supernatant, vortex at low speed, let stand on ice for 10 minutes, centrifuge at 12,000 rpm / min at 4°C for 15 minutes, and discard the supernatant;
[0058] After adding 1ml of pre-cooled freshly prepared 75% ethanol (0.1% DEP...
Embodiment 3
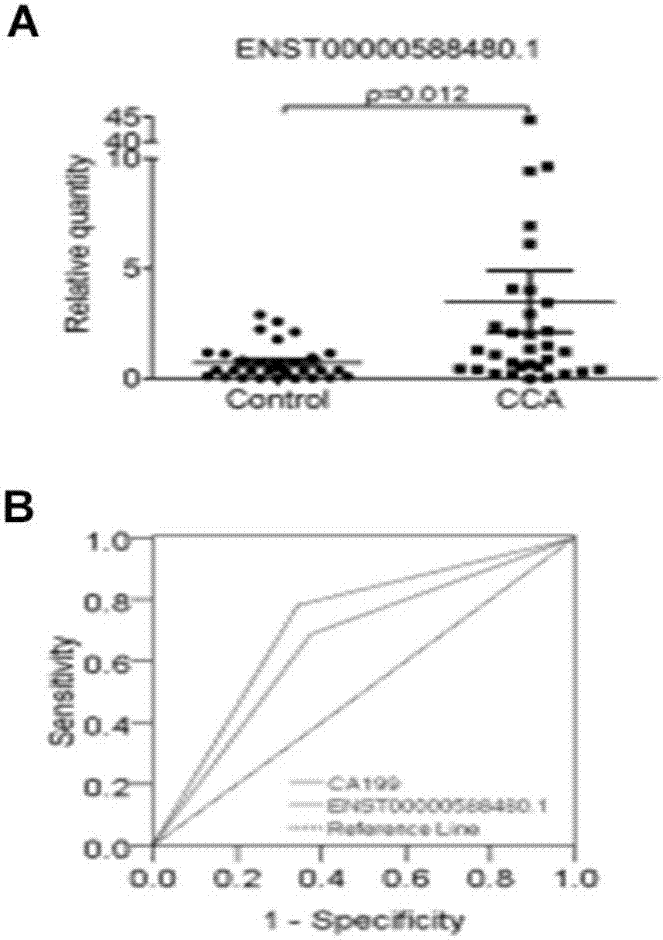
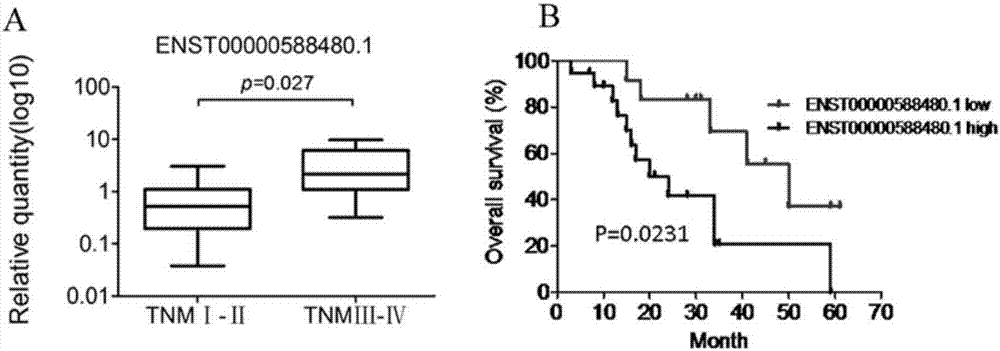
[0081] Example 3 The Value of ENST00000588480.1 in the Diagnosis and Prognosis Evaluation of Cholangiocarcinoma
[0082] In order to detect the value of ENST00000588480.1 in the diagnosis of cholangiocarcinoma, the ROC curve showed that the area under the curve was 0.688 (95% confidence interval: 0.55-0.82), sensitivity: 63%, specificity: 75% ( figure 2 B). High expression of ENST00000588480.1 in cholangiocarcinoma was associated with TNM staging of patients (Ⅰ-Ⅱvs.Ⅲ-Ⅳ, p=0.027, image 3 A) Correlation, according to ENST00000588480.1 in cholangiocarcinoma patients and poor prognosis (p=0.0231, image 3 B) There is a positive correlation.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Sensitivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com