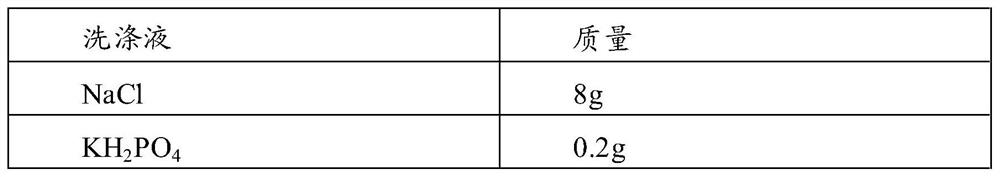
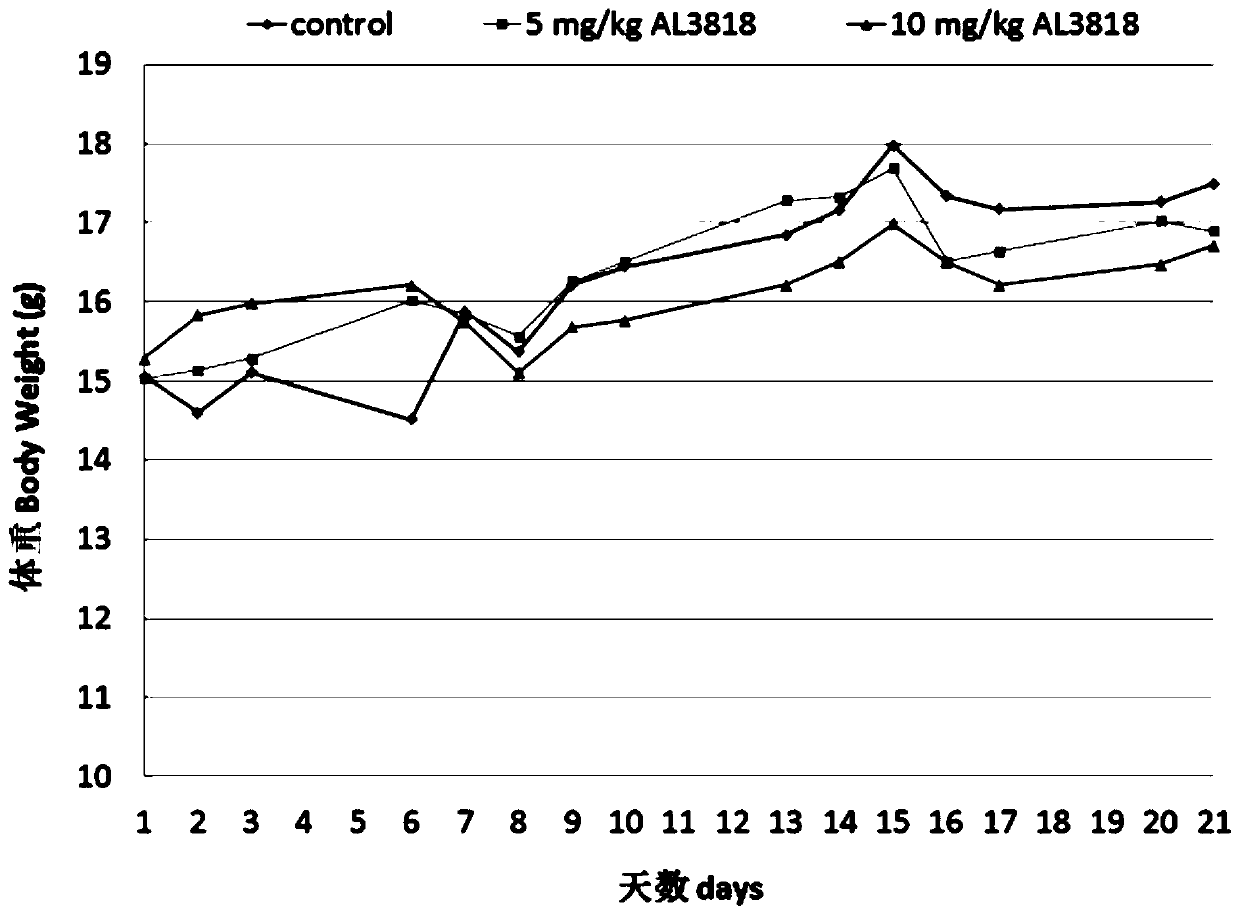
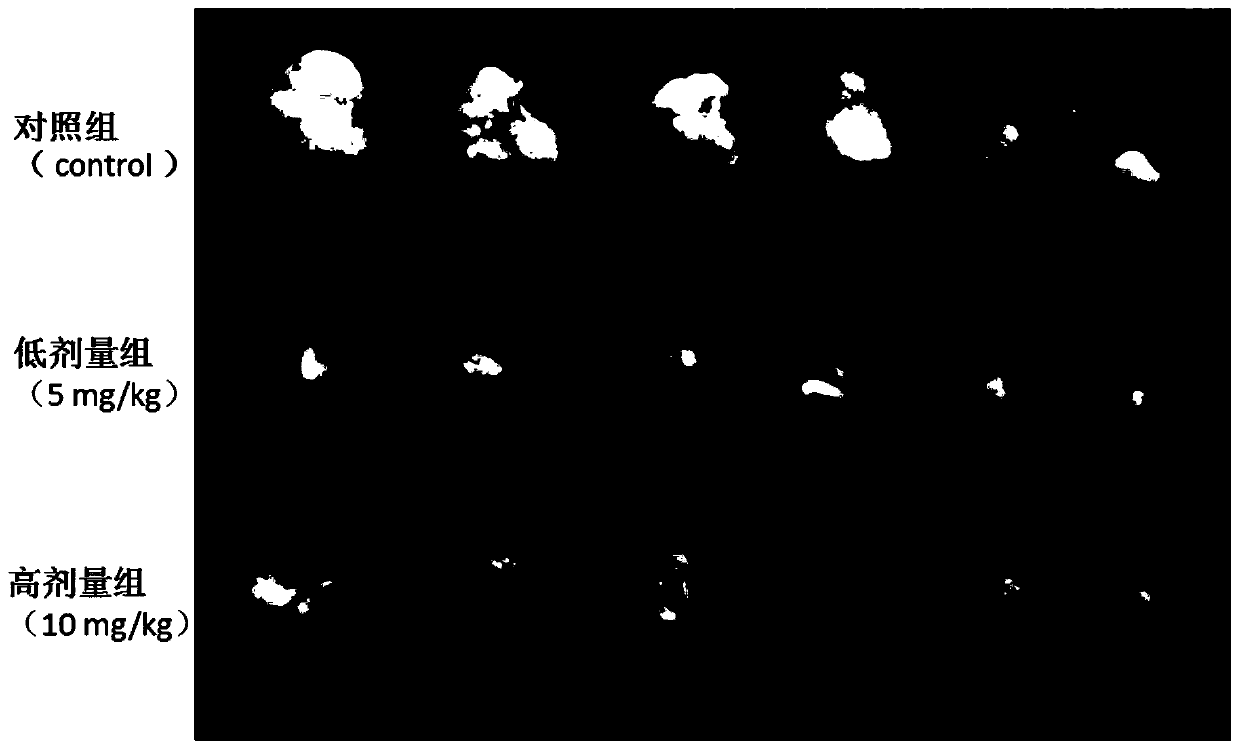
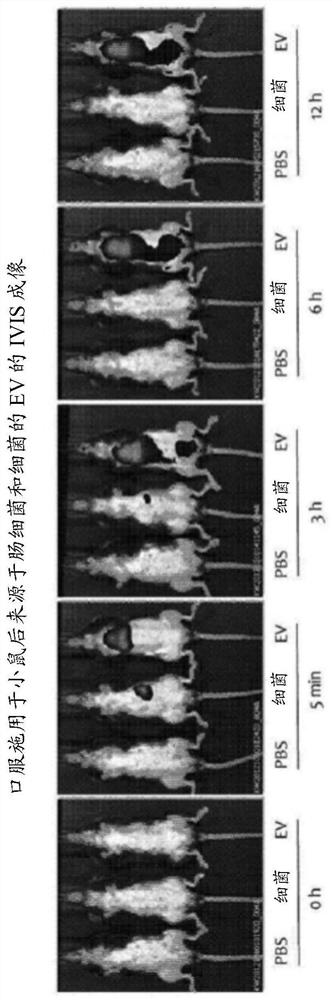
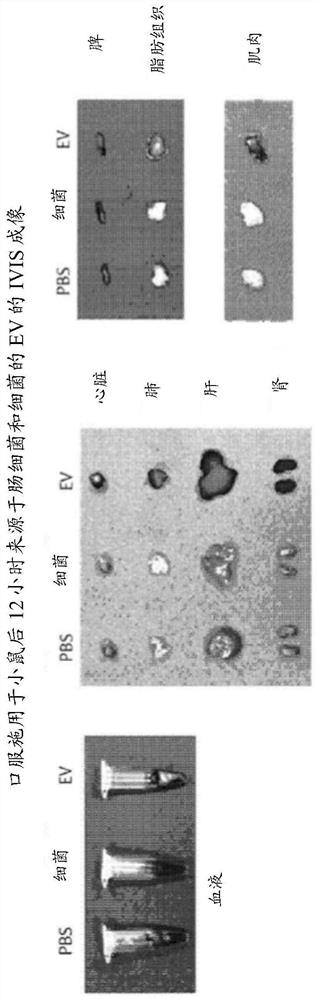
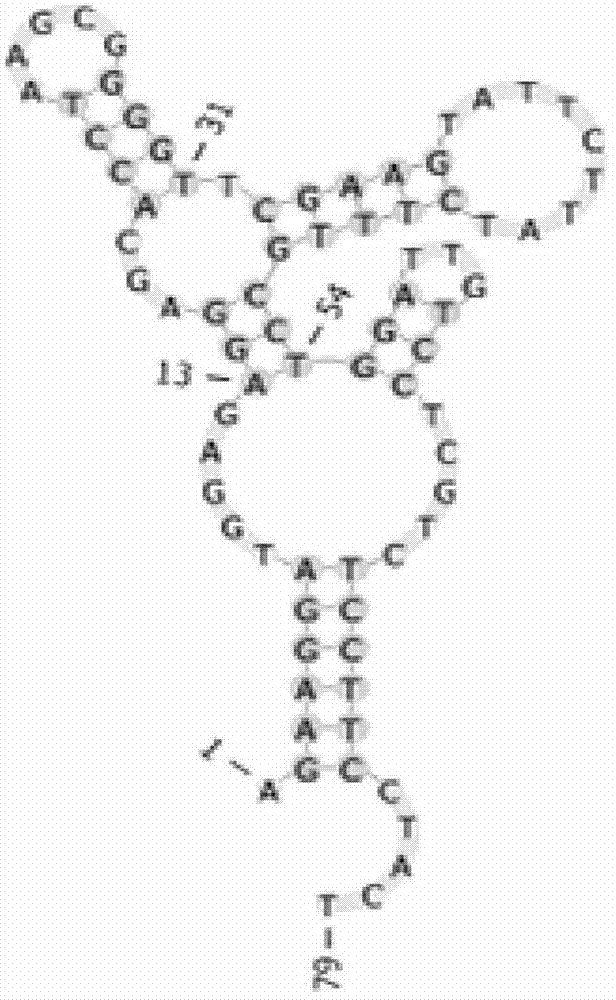Patents
Literature
99 results about "Bile Duct Carcinoma" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
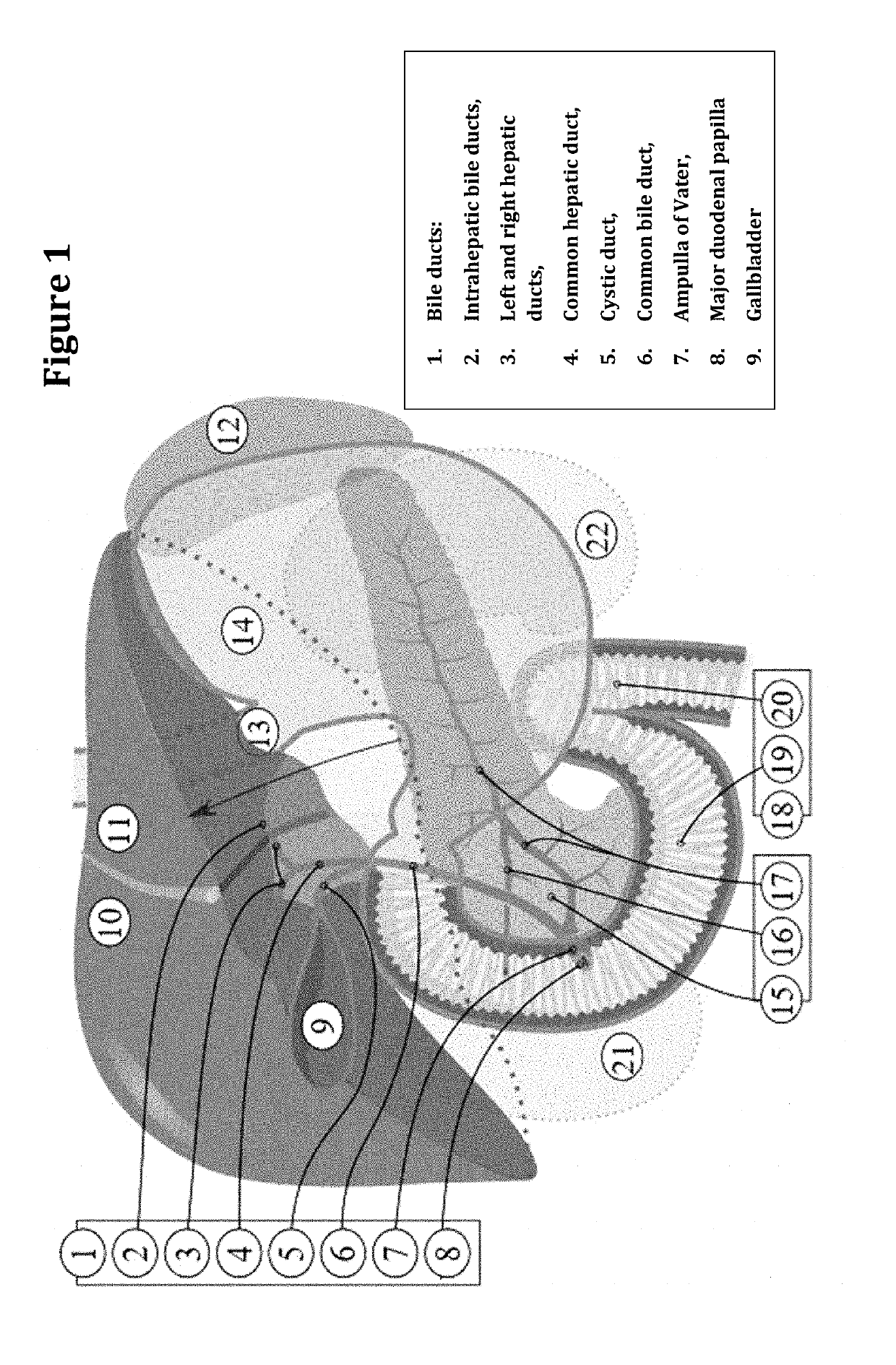
A malignant tumor arising from the epithelium of the intrahepatic or extrahepatic bile duct. Carcinomas that arise from the intrahepatic bile ducts and the hepatic ducts are called cholangiocarcinomas and are almost always adenocarcinomas. Carcinomas that arise from the extrahepatic bile ducts are adenocarcinomas, adenosquamous carcinomas, squamous cell carcinomas, small cell carcinomas, or mucoepidermoid carcinomas.
Antibodies, pharmaceutical compositions and methods
ActiveUS20170283488A1Polypeptide with localisation/targeting motifAntibody mimetics/scaffoldsURINARY BLADDER CARCINOMASquamous Carcinomas
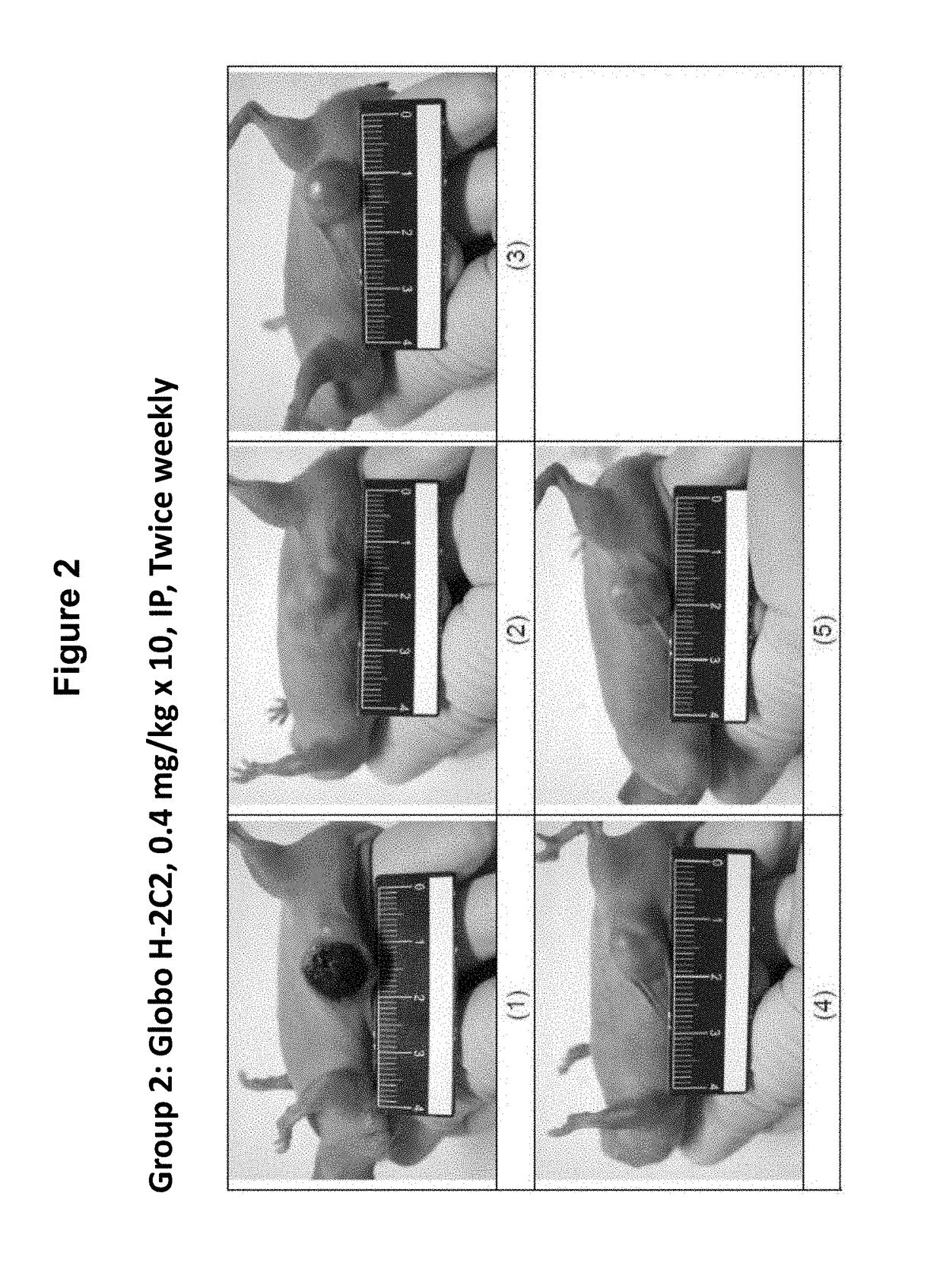
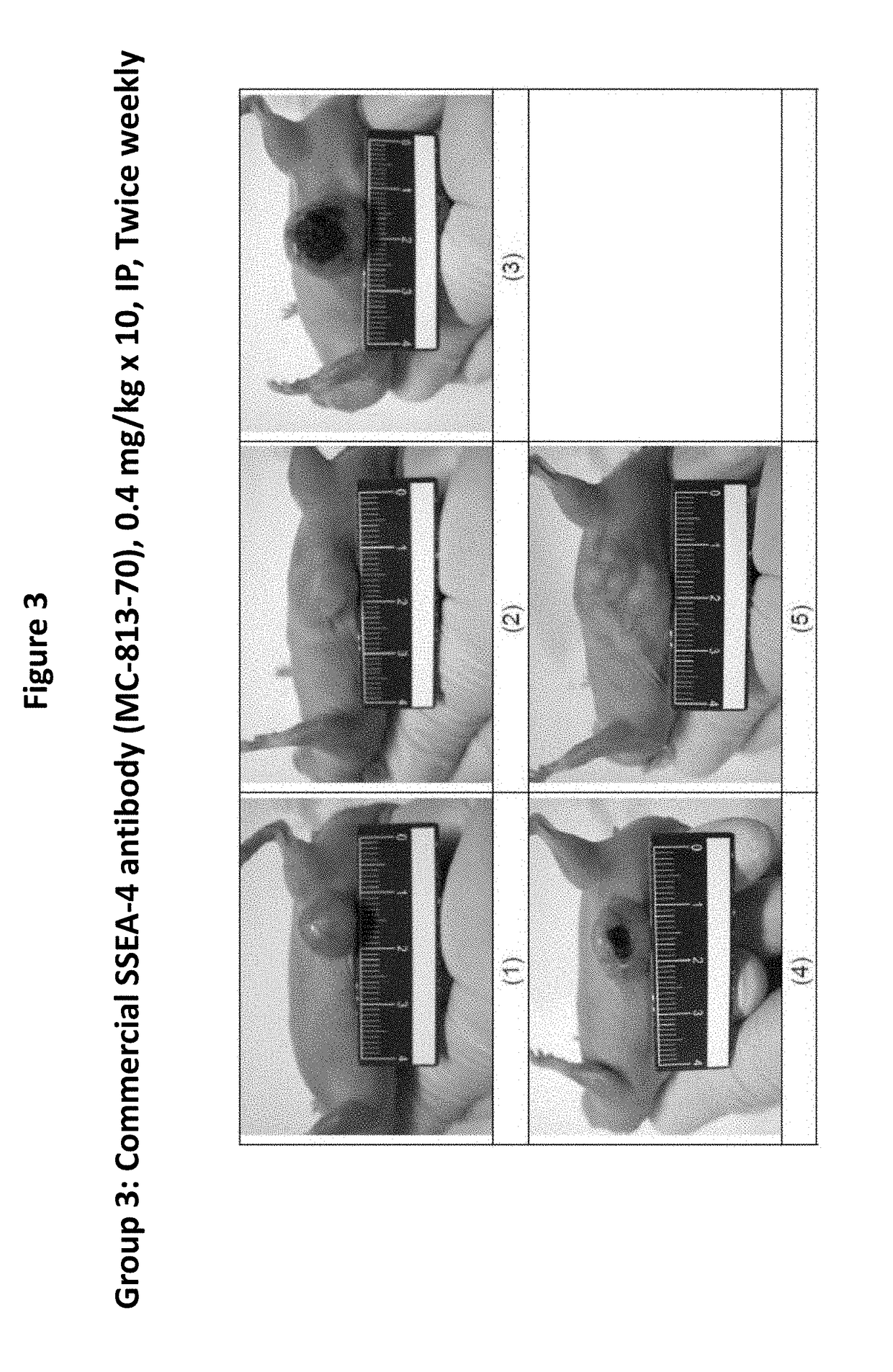
Pharmaceutical composition comprising antibodies or antigen binding fragments thereof that bind to stage-specific embryonic antigen 4 (SSEA-4) are disclosed herein, as well as methods of use thereof. Methods of use include, without limitation, cancer therapies and diagnostics. The antibodies of the disclosure can bind to certain cancer cell surfaces. Exemplary targets of the antibodies disclosed herein can include carcinomas, such as breast cancer, lung cancer, esophageal cancer, rectal cancer, biliary cancer, liver cancer, buccal cancer, gastric cancer, colon cancer, nasopharyngeal cancer, kidney cancer, prostate cancer, ovarian cancer, cervical cancer, endometrial cancer, pancreatic cancer, testicular cancer, bladder cancer, head and neck cancer, oral cancer, neuroendocrine cancer, adrenal cancer, thyroid cancer, bone cancer, skin cancer, basal cell carcinoma, squamous cell carcinoma, melanoma, and / or brain tumor.
Owner:OBI PHARMA INC
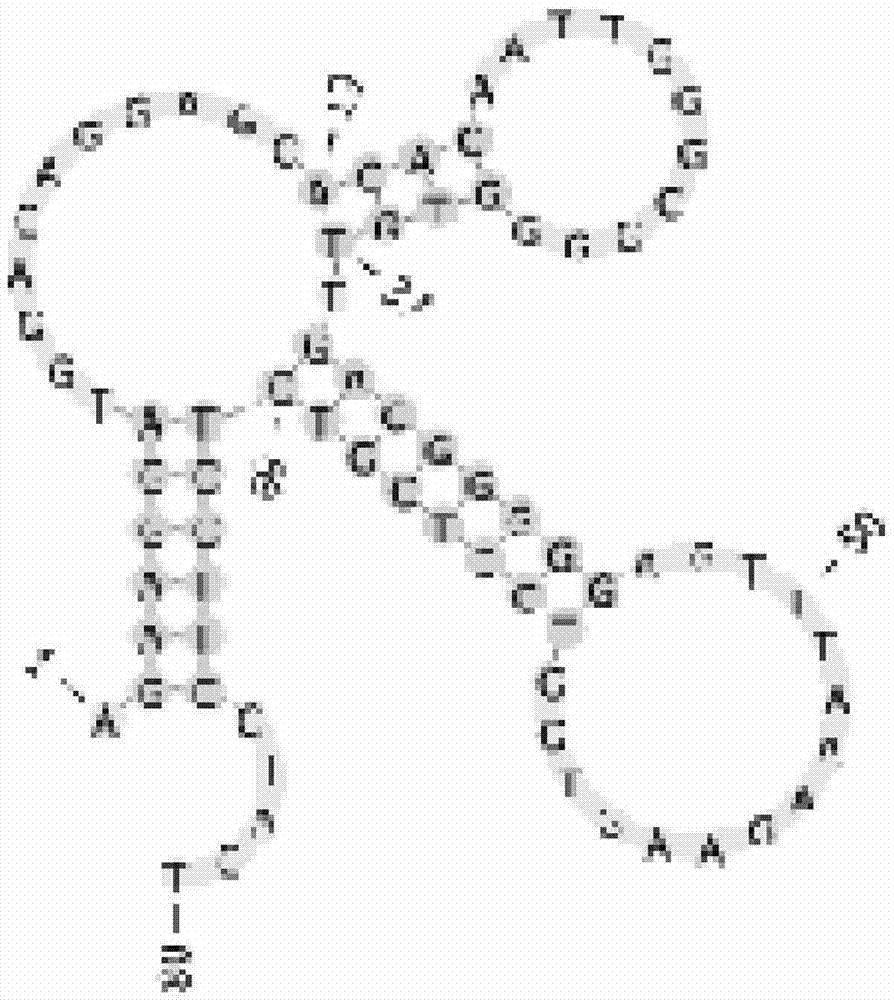
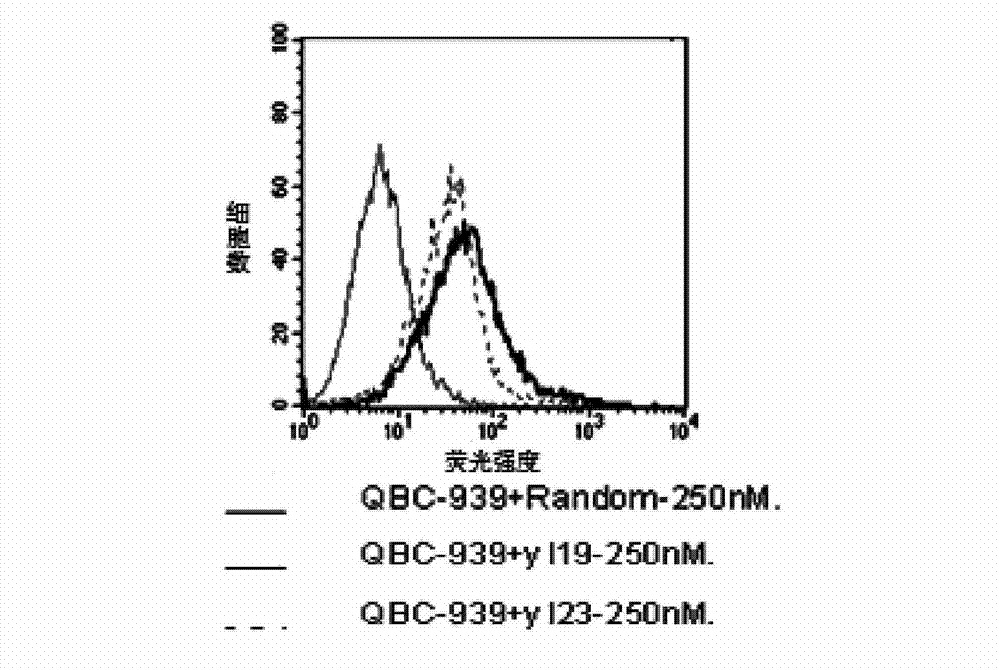
Nucleic acid aptamer and derivatives thereof, screening method of nucleic acid aptamer, application of nucleic acid aptamer and derivatives in detecting human biliary duct carcinoma cell line
ActiveCN103205431AHigh affinityImprove featuresMicrobiological testing/measurementDNA preparationAptamerChemical synthesis
The invention discloses a nucleic acid aptamer with a sequence containing DNA (deoxyribonucleic acid) segments shown by any of a sequence 1 and a sequence 2. The nucleic acid aptamer can also be derivatives obtained by various similar sequences high in homology or the sequence. The invention further discloses a screening method of the nucleic acid aptamer. The method includes: synthesizing a random single-strand DNA library and a primer, performing Cell-SELEX screening and PCR (polymerase chain reaction) library amplification, preparing a DNA single-strand library, and obtaining the nucleic acid aptamer through repeated screening, negative screening and several rounds of screening. The nucleic acid aptamer and derivatives thereof can be applied to recognizing human biliary duct carcinoma cell line QBC-939 or preparing a reagent box for detecting the human biliary duct carcinoma cell line QBC-939, has affinity and specificity higher than anti-keratin antibodies, and is free of immunogenicity, capable of being chemically synthesized, small in molecular weight, stable, easy to store and mark, and the like.
Owner:HUNAN UNIV
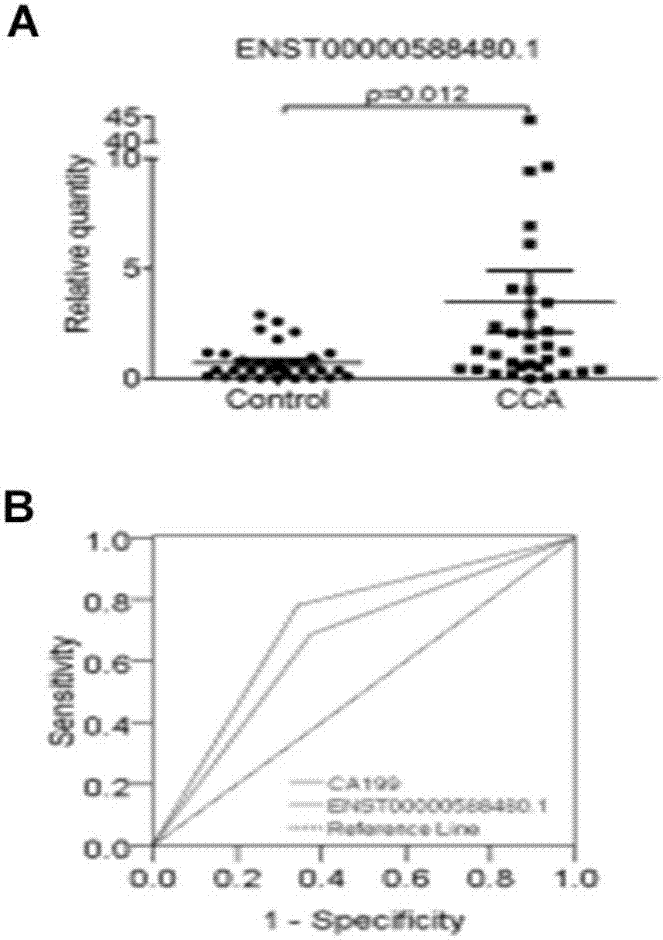
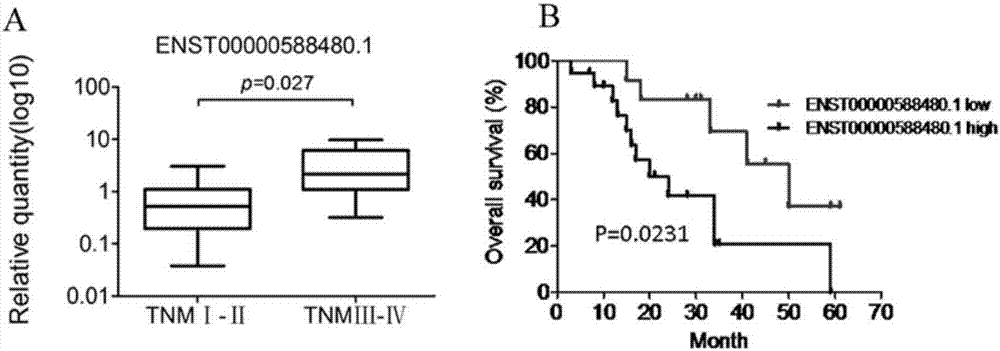
Long and non-coding RNA and application thereof in diagnosis/treatment of bile duct carcinoma
InactiveCN107012145AOrganic active ingredientsMicrobiological testing/measurementMedicinePrognostic prediction
The invention belongs to the field of genetic engineering and in particular relates to an application of a bile exosome long and non-coding RNA (lncRNA) ENST00000588480.1 in diagnosis of bile duct carcinoma, prognostic prediction and target spot drug treatment, wherein the long and non-coding RNA is positively correlated with TNM staging and poor prognosis of a patient.
Owner:THE SECOND AFFILIATED HOSPITAL OF NANJING MEDICAL UNIV
Functionalized long-chain hydrocarbon mono- and di-carboxylic acids and their use for the prevention or treatment of disease
ActiveUS20210024447A1Reducing cholesterol contentLow fat contentOrganic active ingredientsOrganic chemistryBenign tumoursDisease
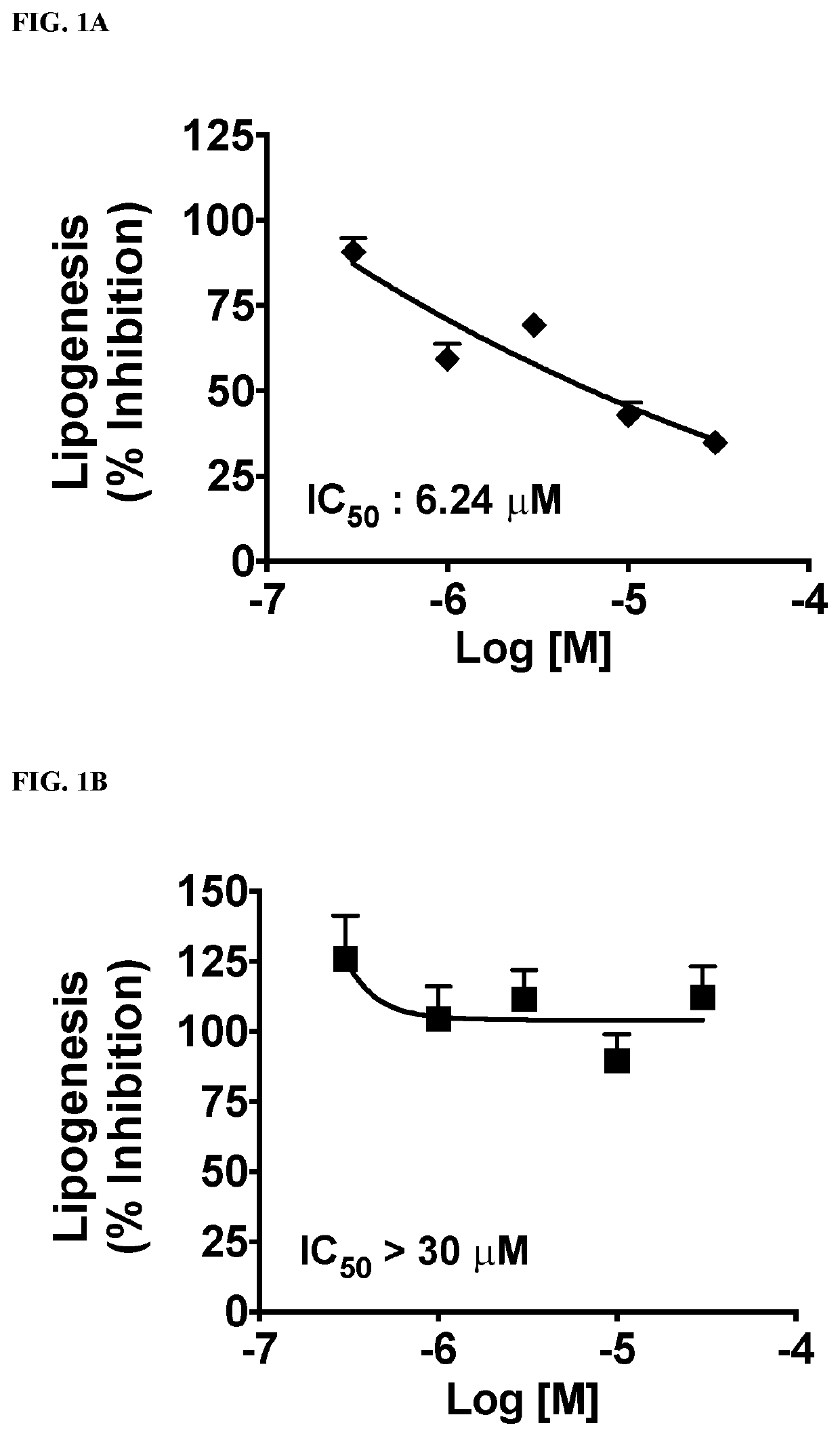
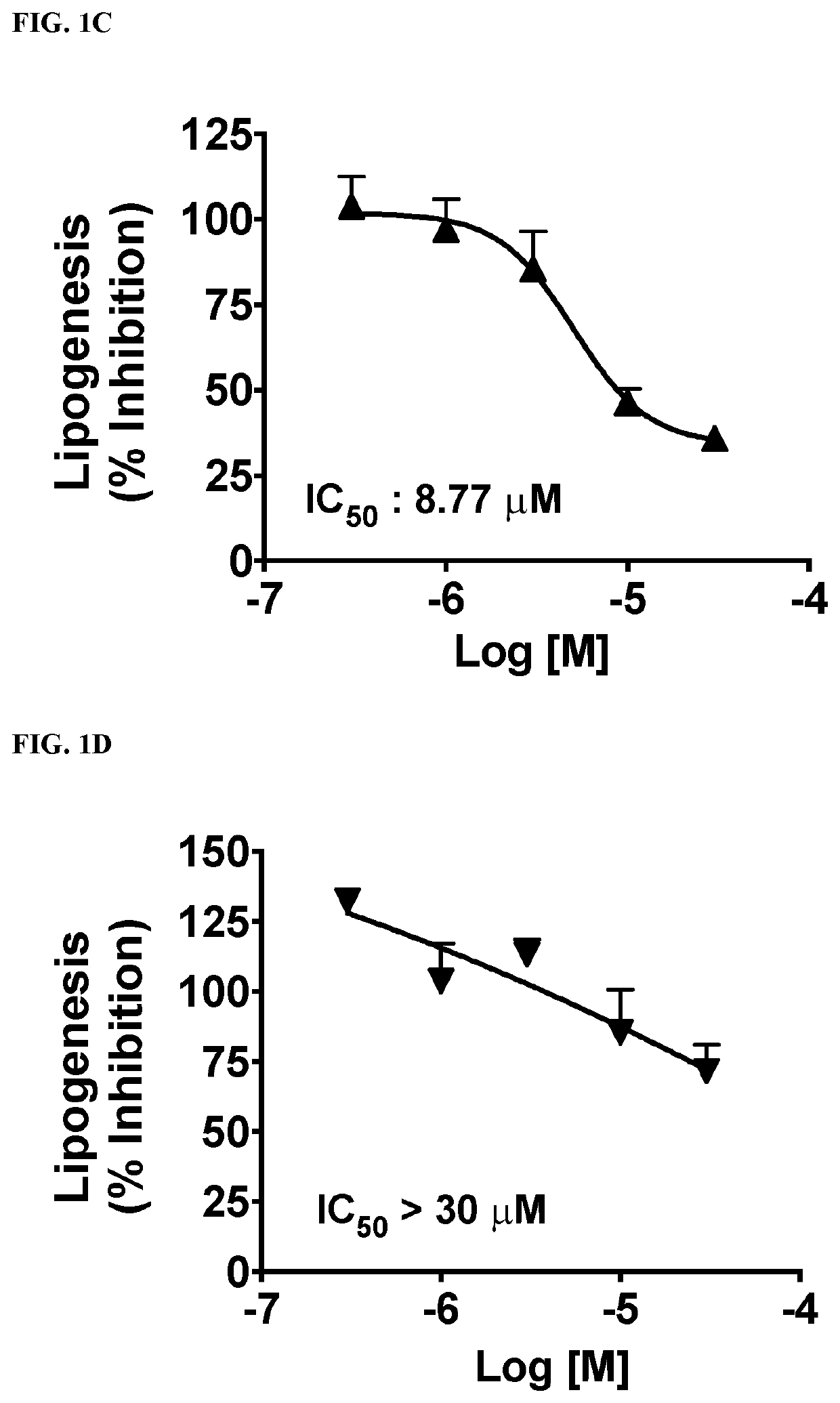
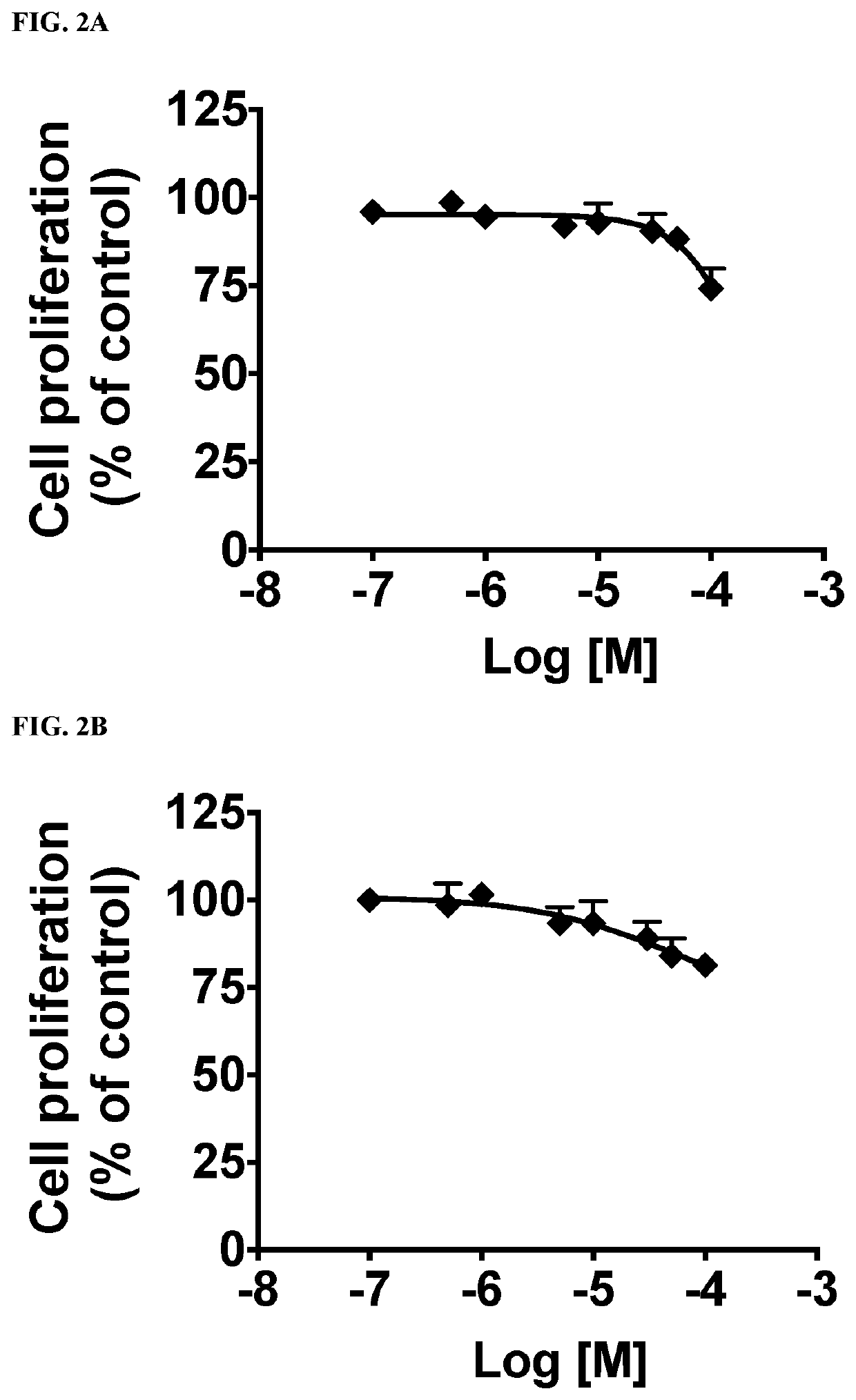
This invention provides compounds of Formulae (IA), (IB), (IC), (ID), (IE), (IF), (IG), (IH), (IJ), (IK), (IL), (II), (III), (IIIA), and (IIIB); pharmaceutically acceptable salts and solvates thereof; and compositions thereof. This invention further provides methods for treating a disease, including but not limited to, liver disease or an abnormal liver condition; cancer (such as hepatocellular carcinoma or cholangiocarcinoma); a malignant or benign tumor of the lung, liver, gall bladder, bile duct or digestive tract; an intra- or extra-hepatic bile duct disease; a disorder of lipoprotein; a lipid-and-metabolic disorder; cirrhosis; fibrosis; a disorder of glucose metabolism; a cardiovascular or related vascular disorder; a disease resulting from steatosis, fibrosis, or cirrhosis; a disease associated with increased inflammation (such as hepatic inflammation or pulmonary inflammation); hepatocyte ballooning; a peroxisome proliferator activated receptor-associated disorder; an ATP citrate lyase disorder; an acetyl-coenzyme A carboxylase disorder; obesity; pancreatitis; or renal disease.
Owner:ESPERVITA THERAPEUTICS INC
Human intrahepatic bile duct cancer cell strain ICC-X3 and application thereof
The invention discloses a human intrahepatic bile duct cancer cell line ICC-X3 and application thereof, and belongs to the field of microbial animal cell lines. The provided intrahepatic cholangiocarcinoma cell line is a human intrahepatic cholangiocarcinoma cell line ICC-X3, and has been preserved in the China Center for Type Culture Collection on February 22, 2022, and the preservation number is CCTCC NO: C202260. The human intrahepatic cholangiocarcinoma cell line ICC-X3 can be used as a cell model for researching the occurrence, development or metastasis mechanism of intrahepatic cholangiocarcinoma. The human intrahepatic cholangiocarcinoma cell line ICC-X3 can also be used for establishing an intrahepatic cholangiocarcinoma animal model. The human intrahepatic cholangiocarcinoma cell line ICC-X3 can be used as a cell model for studying the differentiation mechanism, cell morphology and dysfunction and tumor infiltration and metastasis mechanism of intrahepatic cholangiocarcinoma, guiding clinical comprehensive diagnosis and treatment and the like.
Owner:THE FIRST HOSPITAL OF LANZHOU UNIV
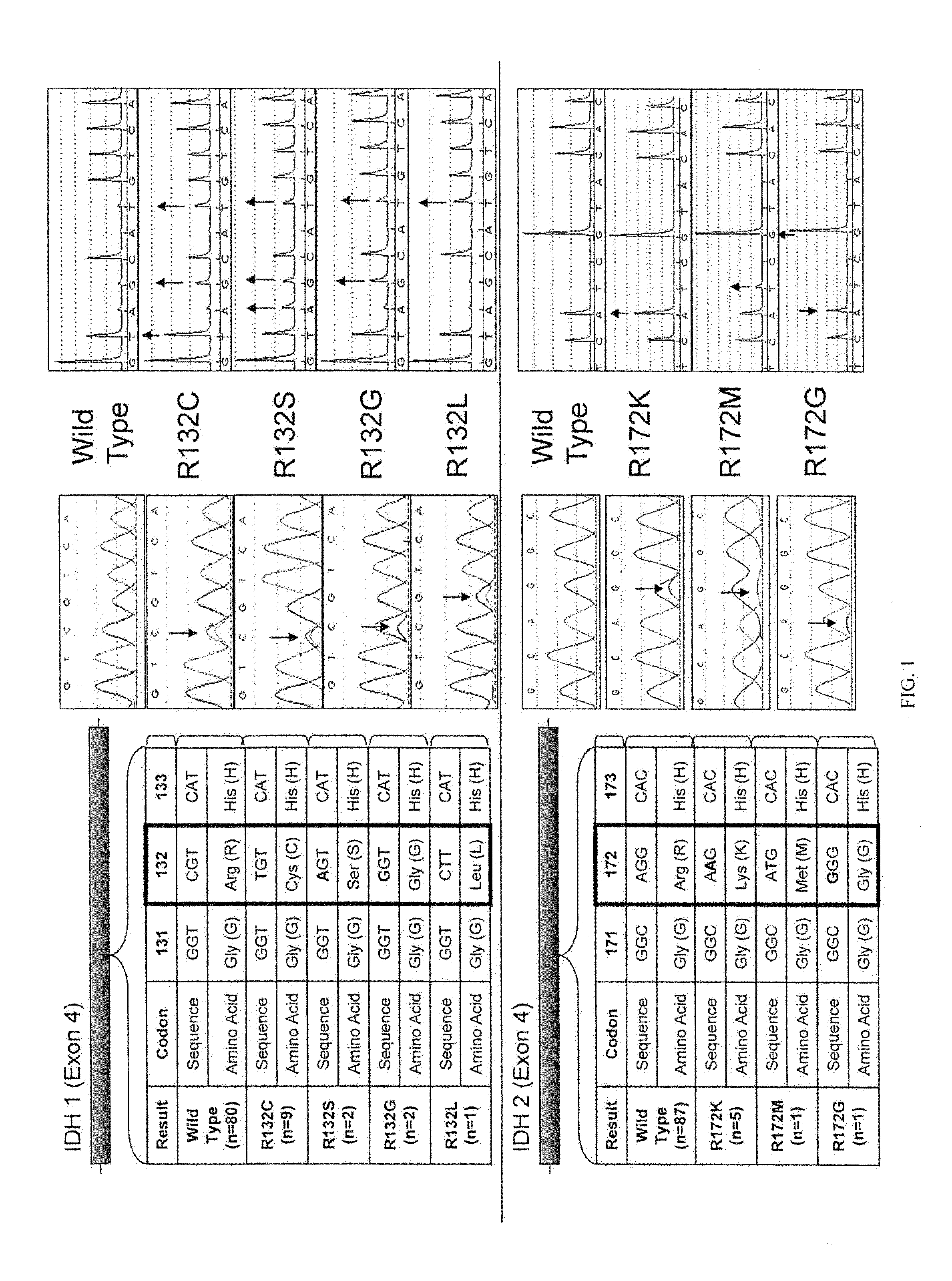
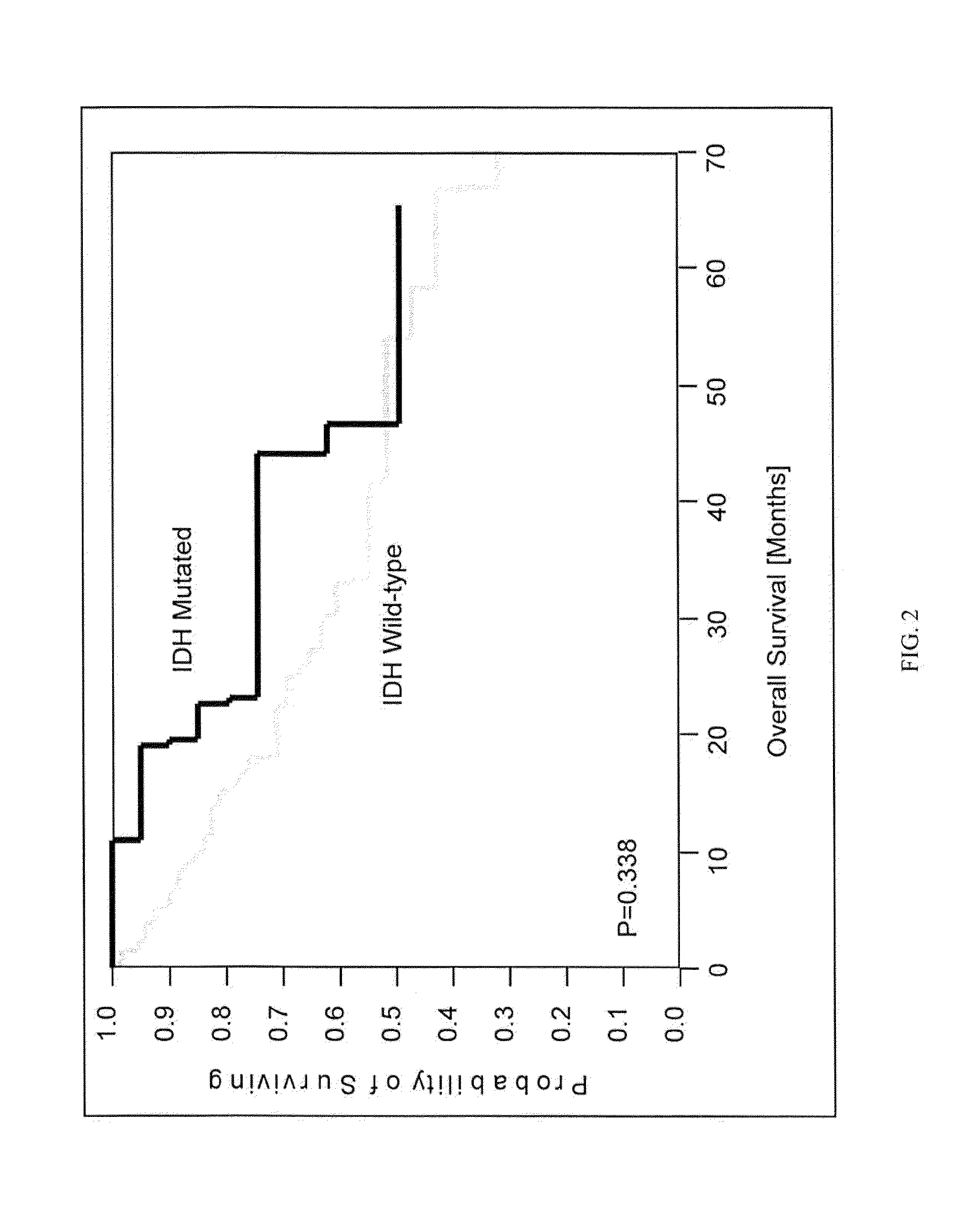
IDH1 and IDH2 mutations in cholangiocarcinoma
InactiveUS20130123335A1Aid in prognosis and selectionMicrobiological testing/measurementFermentationMammalIDH2
This document relates to methods and materials involved in assessing isocitrate dehydrogenase 1 (IDH1) or isocitrate dehydrogenase 2 (IDH2) mutations in a mammal (e.g., human). For example, this document provides methods and materials for diagnosis, characterization, determining prognosis, and treatment of cholangiocarcinoma tumor in a mammal.
Owner:MAYO FOUND FOR MEDICAL EDUCATION & RES
Application of clostridium butyricum for preparing preparation for preventing or treating cholangiocarcinoma
The invention discloses an application of clostridium butyricum for preparing preparation for preventing or treating cholangiocarcinoma. Specifically, clostridium butyricum is a main active ingredient, and a biological preparation such as a medicine is prepared, and invention provides the application of clostridium butyricum in the prevention or treatment of cholangiocarcinoma, which belongs to the field of biomedicine.
Owner:QINGDAO EASTSEA PHARMA
Application of bile bacteria as diagnosis and prognosis marker of porta hepatic cholangiocarcinoma
ActiveCN114480636AImprove diagnostic capabilitiesAccurate and individualized treatment planMicrobiological testing/measurementPrognosis biomarkerBile fluid
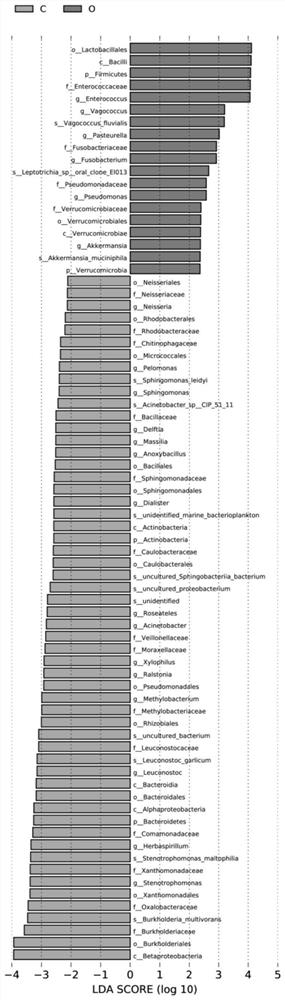
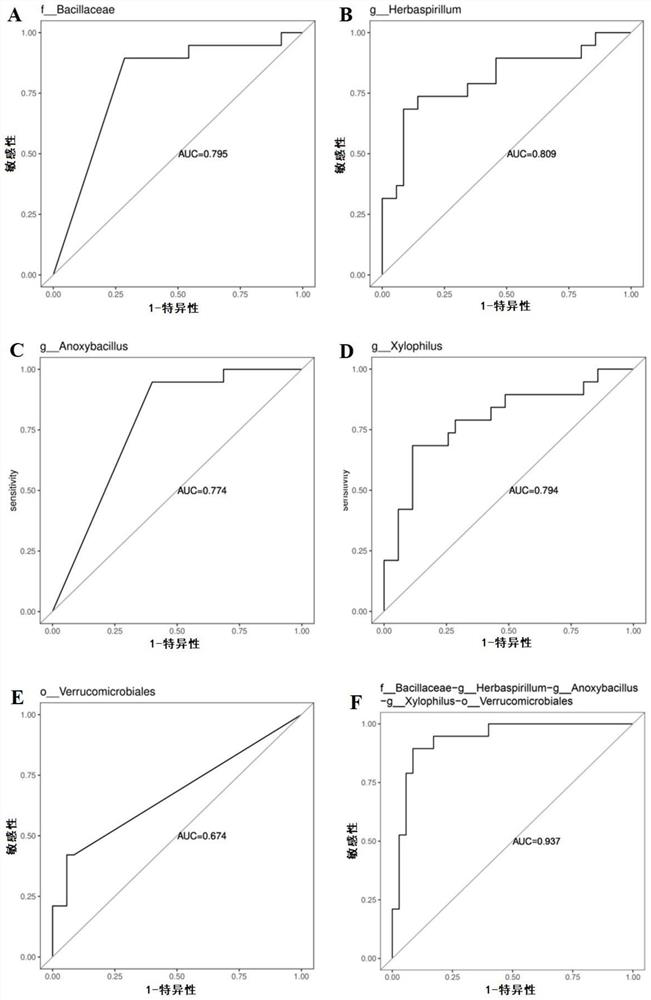
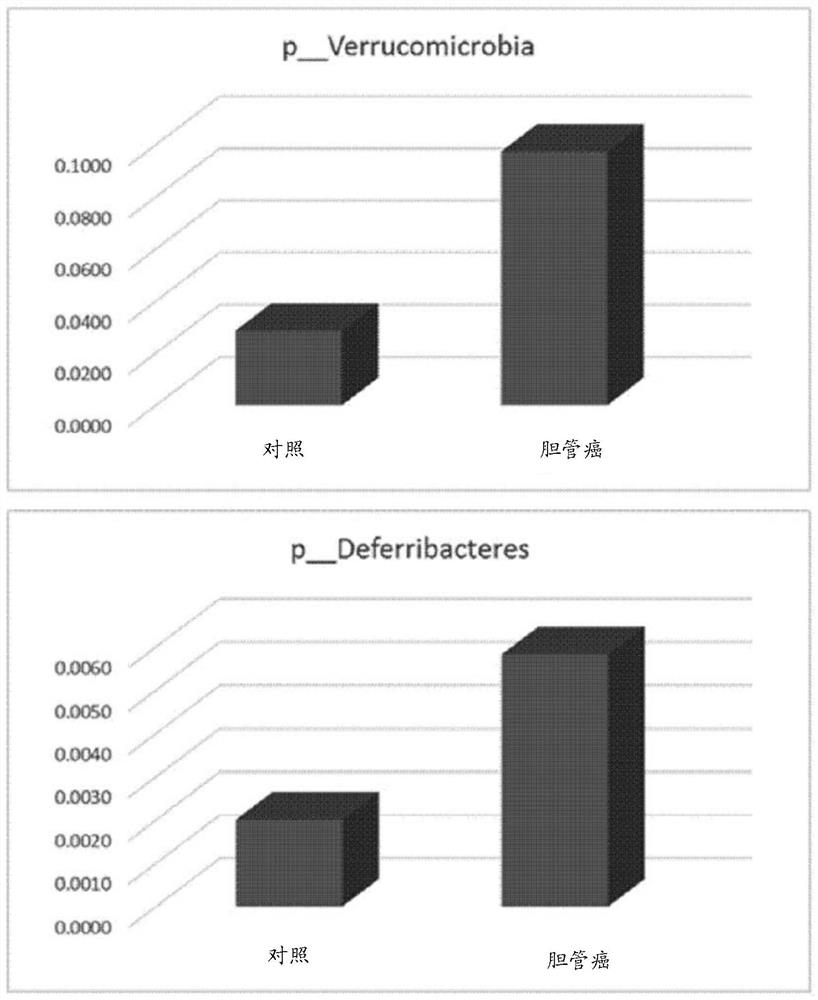
The invention discloses application of bile bacteria as a diagnosis and prognosis marker of porta hepatic cholangiocarcinoma, it is found for the first time that fBacillus, gHerbaspiril, gAnxyBacillus, gXylophilus and / or oVerrubmicrobiales can be used for diagnosis of early porta hepatic cholangiocarcinoma, sMethylobacteriumkomagatae can be used for prediction of prognosis of porta hepatic cholangiocarcinoma, and it is found through verification that the application of the bile bacteria as the diagnosis and prognosis marker of porta hepatic cholangiocarcinoma has the advantages that the application of the bile bacteria as the diagnosis and prognosis marker of porta hepatic cholangiocarcinoma has the advantages that the application of the bile bacteria as the diagnosis and prognosis marker of porta hepatic cholangiocarcinoma is promoted; the marker has relatively high diagnosis efficiency and accuracy on diagnosis and prognosis of porta hepatis bile duct cancer, and has a good clinical application prospect.
Owner:THE FIFTH MEDICAL CENT OF CHINESE PLA GENERAL HOSPITAL
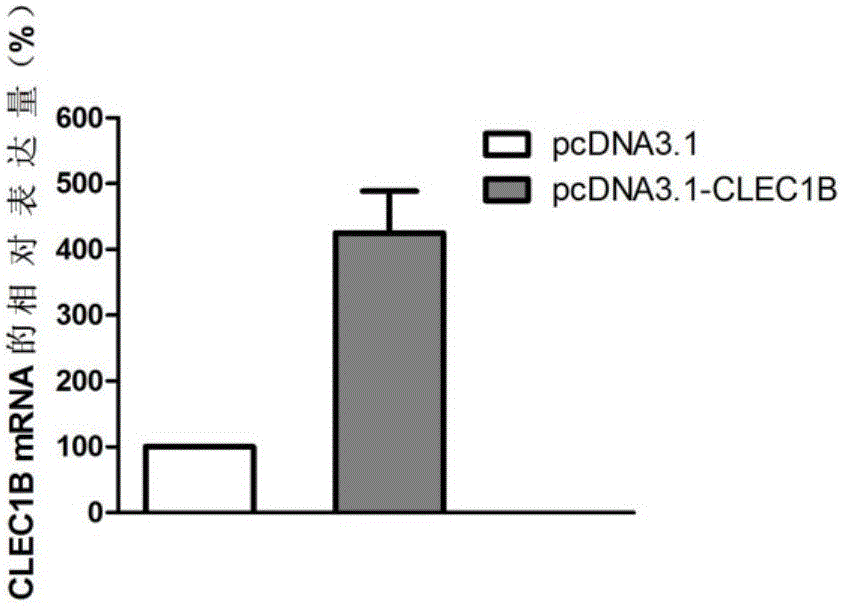
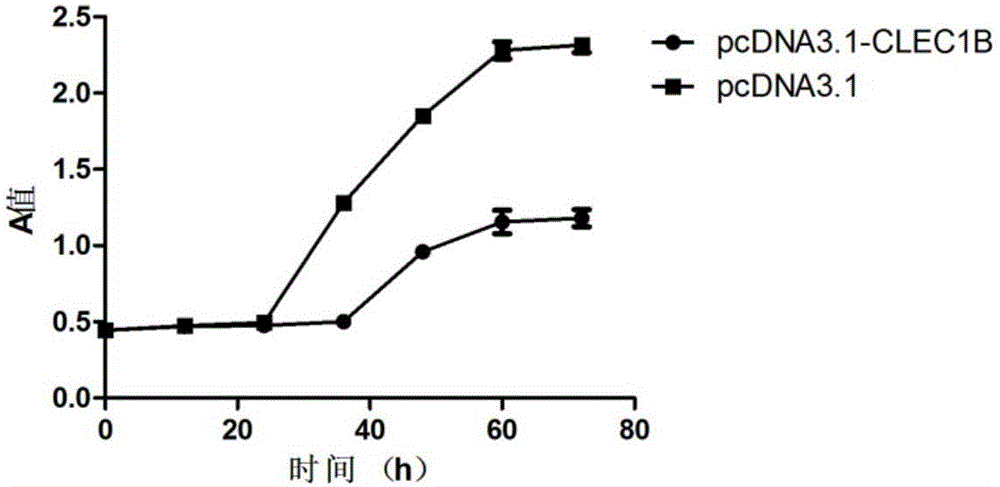
Application of CLEC1B (C-type lectin domain family 1 member B) genes on diagnosis and treatment of bile duct carcinoma
ActiveCN105112554AAchieve early diagnosisReduce mortalityPeptide/protein ingredientsMicrobiological testing/measurementTrue positive rateDomain family
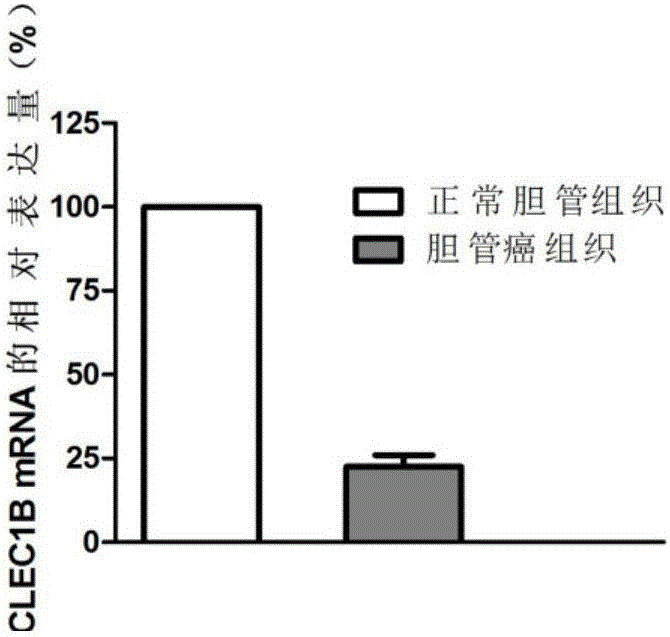
The invention discloses application of CLEC1B (C-type lectin domain family 1 member B) genes on diagnosis and treatment of bile duct carcinoma. RNA (ribonucleic acid)-sep screening is carried out, and a large sample RT-PCR (reverse transcription-polymerase chain reaction) proves that CLEC1B gene expression abnormality is related to initiation and development of the bile duct carcinoma. According to a research result, CLEC1B can also be used for preparing medicines for treating the bile duct carcinoma. By the application of the CLEC1B genes on diagnosis and treatment of the bile duct carcinoma, sensitivity and specificity of diagnosis of the bile duct carcinoma are greatly improved, and a new target spot is provided for gene therapy of the bile duct carcinoma.
Owner:BEIJING MEDINTELL BIOMED CO LTD
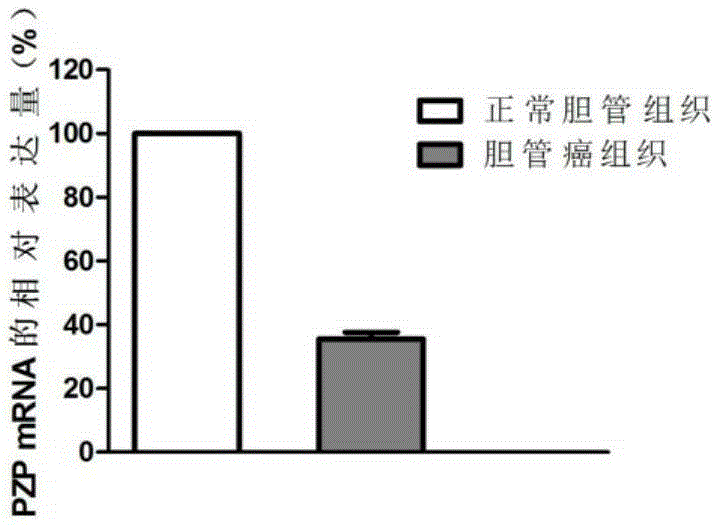
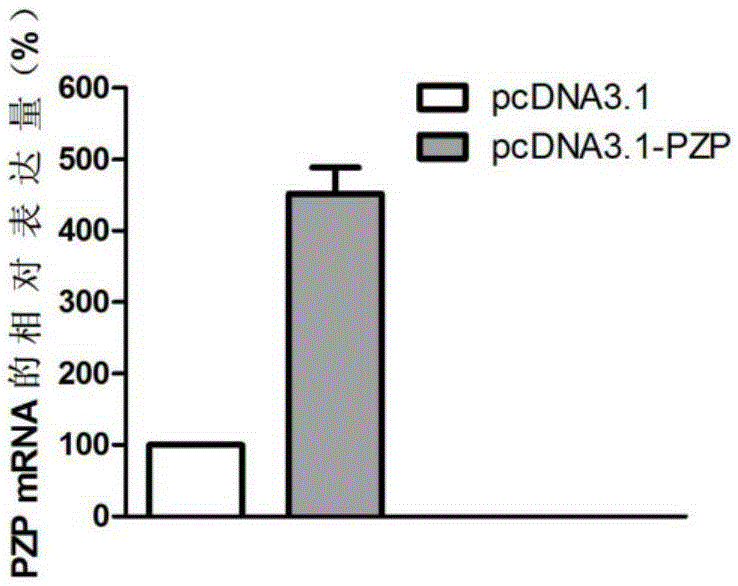
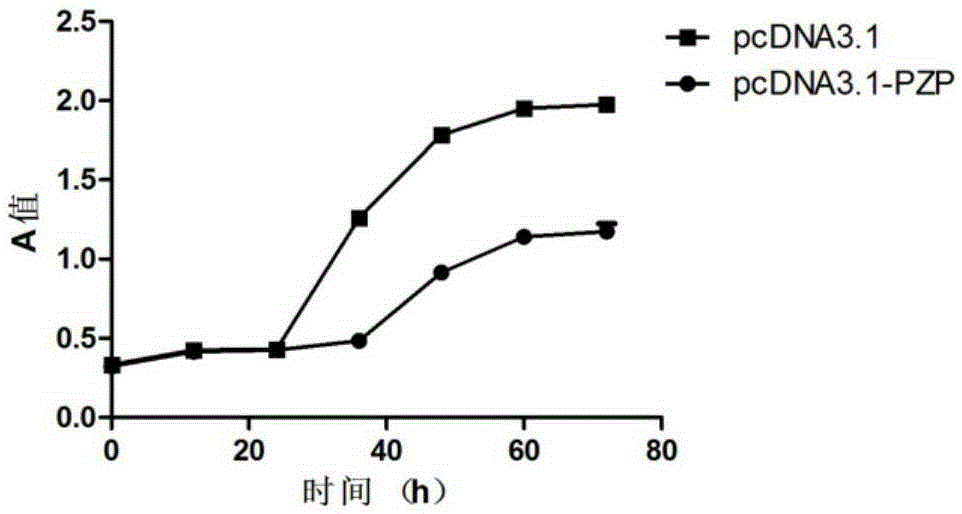
Application of PZP gene in diagnosis and treatment of biliary duct cancer
ActiveCN105331691AAchieve early diagnosisReduce mortalityOrganic active ingredientsMicrobiological testing/measurementCancer cellCancers diagnosis
The invention discloses application of a PZP gene in diagnosis and treatment of a biliary duct cancer. The PZP gene is low-expressed in tissue of a biliary duct cancer patient, and growth and invasion of biliary duct cancer cells can be restrained by increasing expression of PZP. According to the application of the PZP gene in the diagnosis and treatment of the biliary duct cancer, the sensibility and the specificity of biliary duct cancer diagnosis are greatly improved, and meanwhile a new target site is provided for gene treatment of the biliary duct cancer.
Owner:QINGDAO MEDINTELL BIOMEDICAL CO LTD
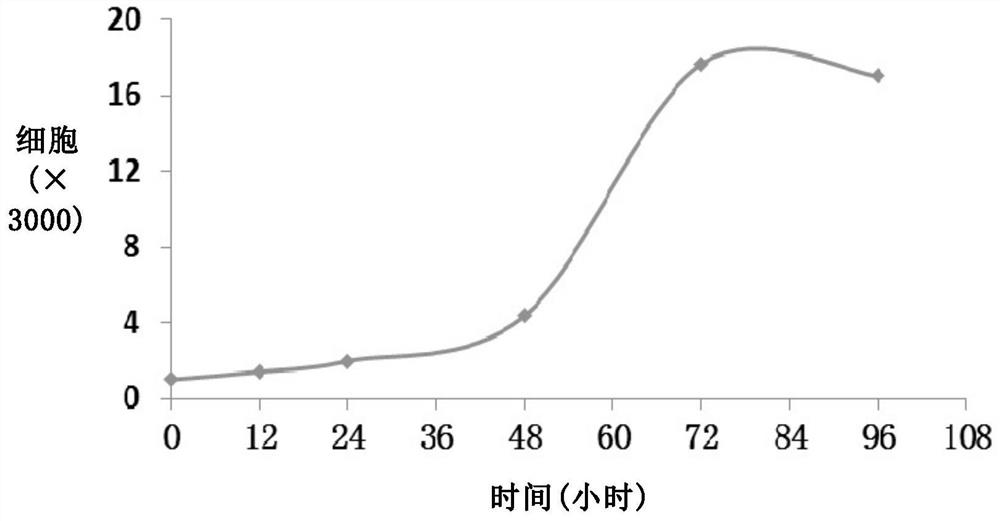
A human intrahepatic cholangiocarcinoma cell line with high tumorigenic ability and its application
InactiveCN107177551BHigh tumorigenic abilityMicrobiological testing/measurementMicroorganism based processesIntrahepatic CholangiocarcinomaBiomarker (medicine)
The invention relates to a human intrahepatic cholangiocarcinoma cell line with high tumorigenic ability and application thereof. The invention discloses a novel human intrahepatic cholangiocarcinoma cell line which grows stably and has clear quantity of generations and high tumorigenic rate; the human intrahepatic cholangiocarcinoma cell line can form an adenoid tumor in vivo and can be used for in-vivo and in-vitro research on human intrahepatic cholangiocarcinoma; a foundation is laid for constructing an in-vivo animal model, disclosing a tumor malignant progression mechanism, screening related biomarkers and developing novel intrahepatic cholangiocarcinoma resisting medicines.
Owner:HEPATOBILIARY SURGERY HOSPITAL SECOND MILITARY MEDICAL UNIV
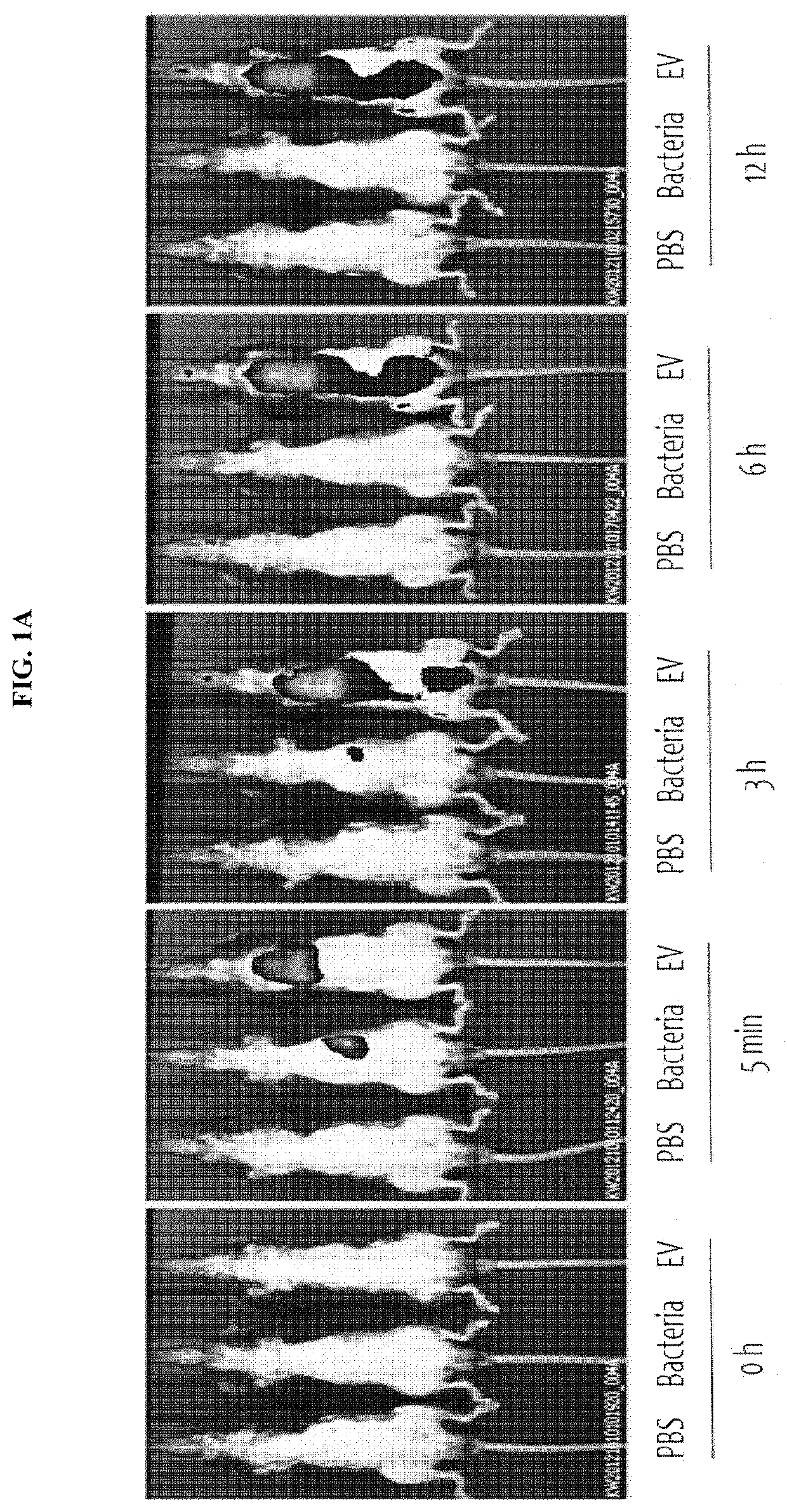
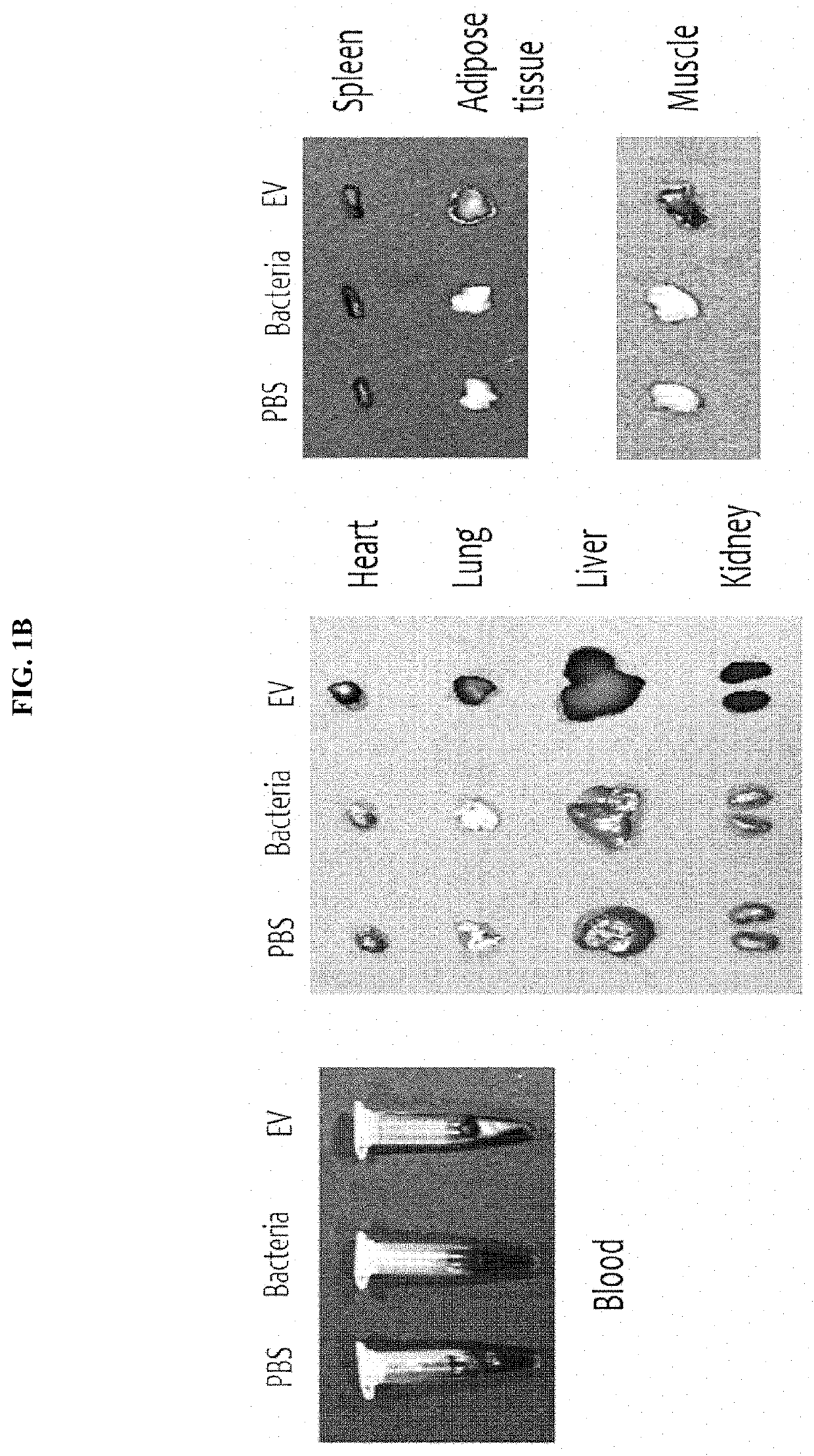
Nano-vesicles derived from genus Micrococcus bacteria and use thereof
ActiveUS10888591B2Inhibition of secretionCosmetic preparationsPowder deliveryAnginaObstructive Pulmonary Diseases
Owner:MD HEALTHCARE INC
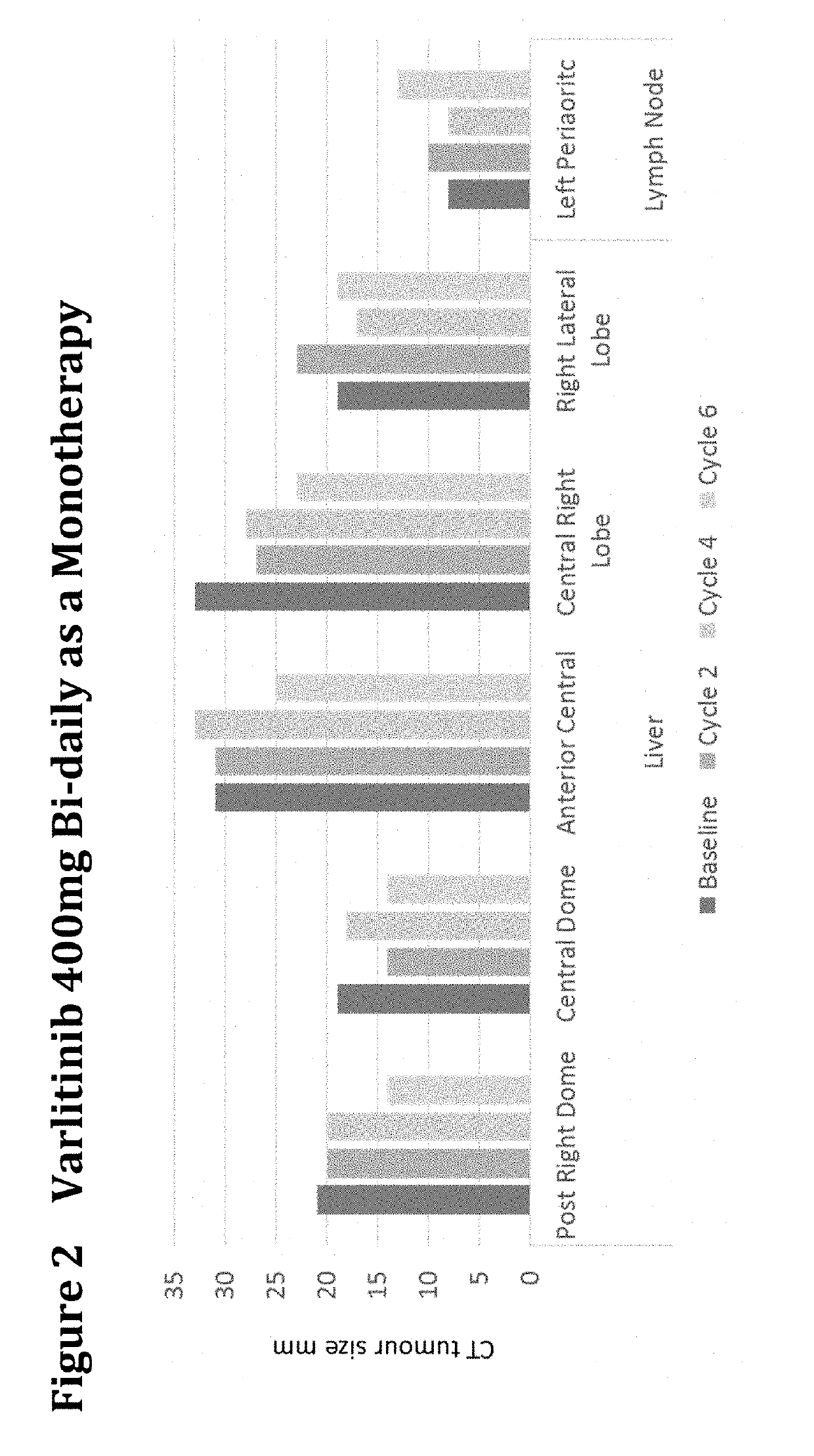
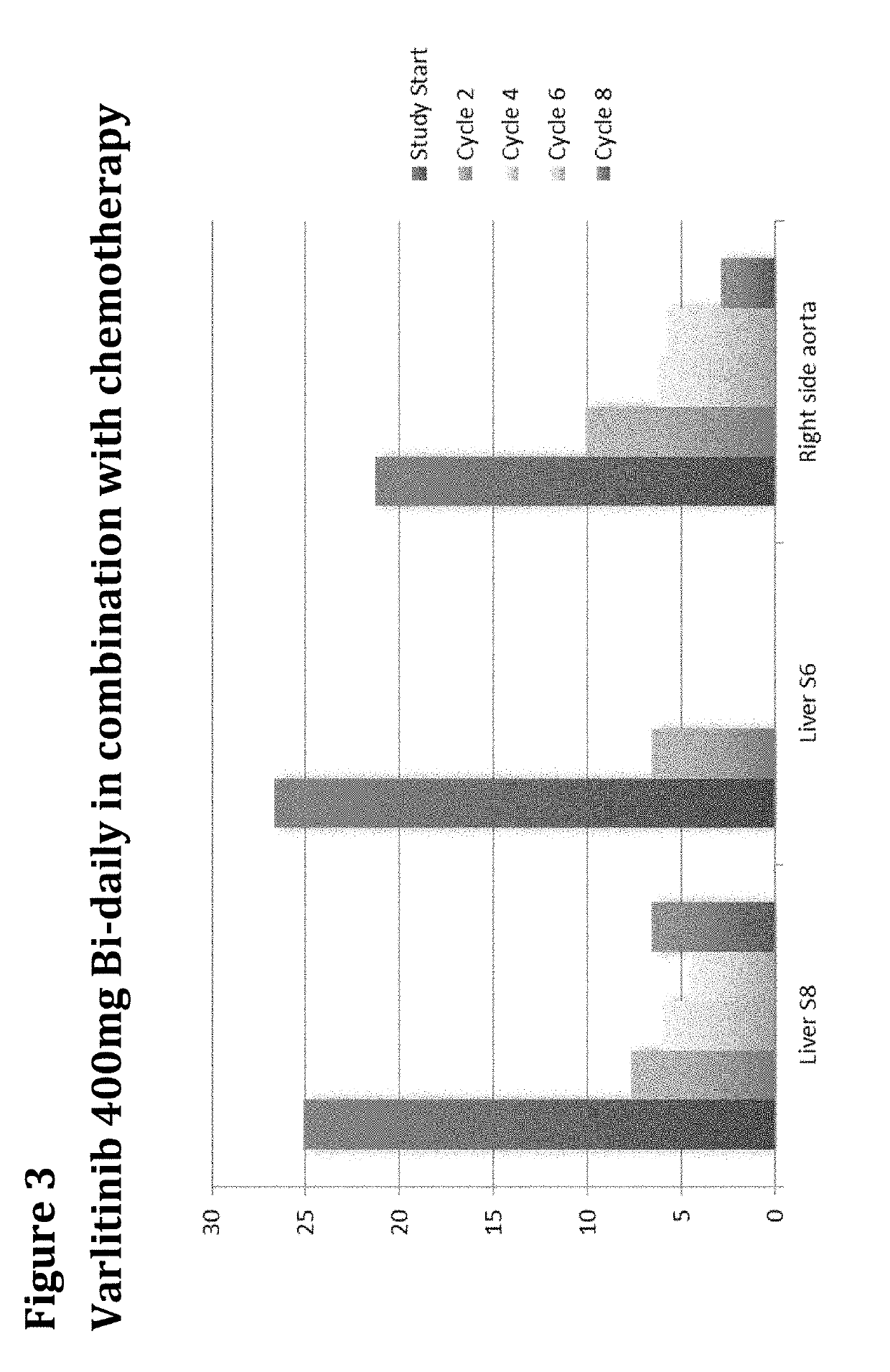
Treatment of Biliary Duct Cancer
InactiveUS20190117655A1Organic active ingredientsHeavy metal active ingredientsEnantiomerBile Duct Carcinoma
The present disclosure provides a method of treating a biliary duct cancer, such as cholangiocarcinoma, by administering a therapeutically effective amount of a compound of formula (I), in particular Varlitinib, an enantiomer thereof or a pharmaceutically acceptable salt of any one of the same.
Owner:ASLAN PHARMA PTE LTD
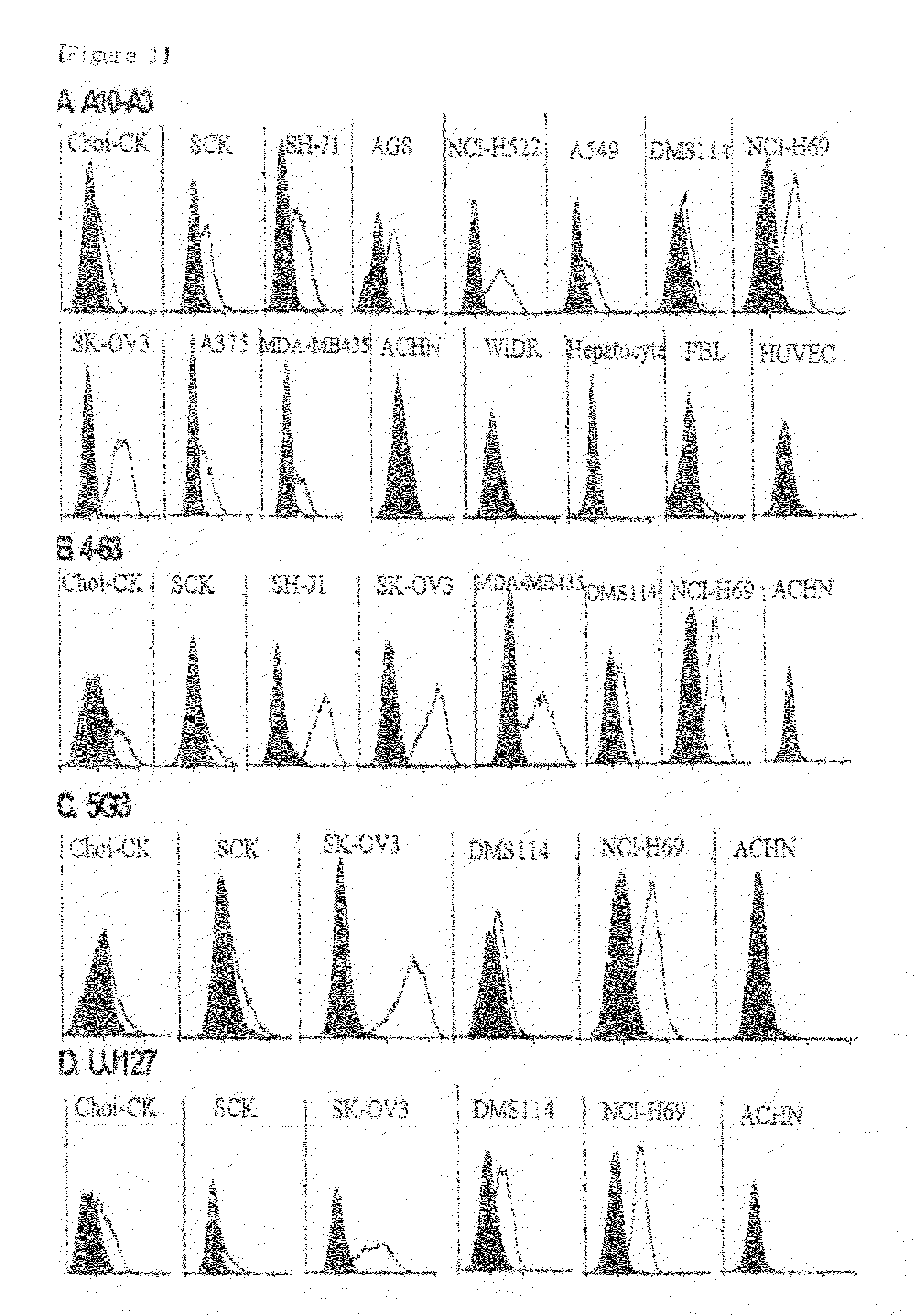
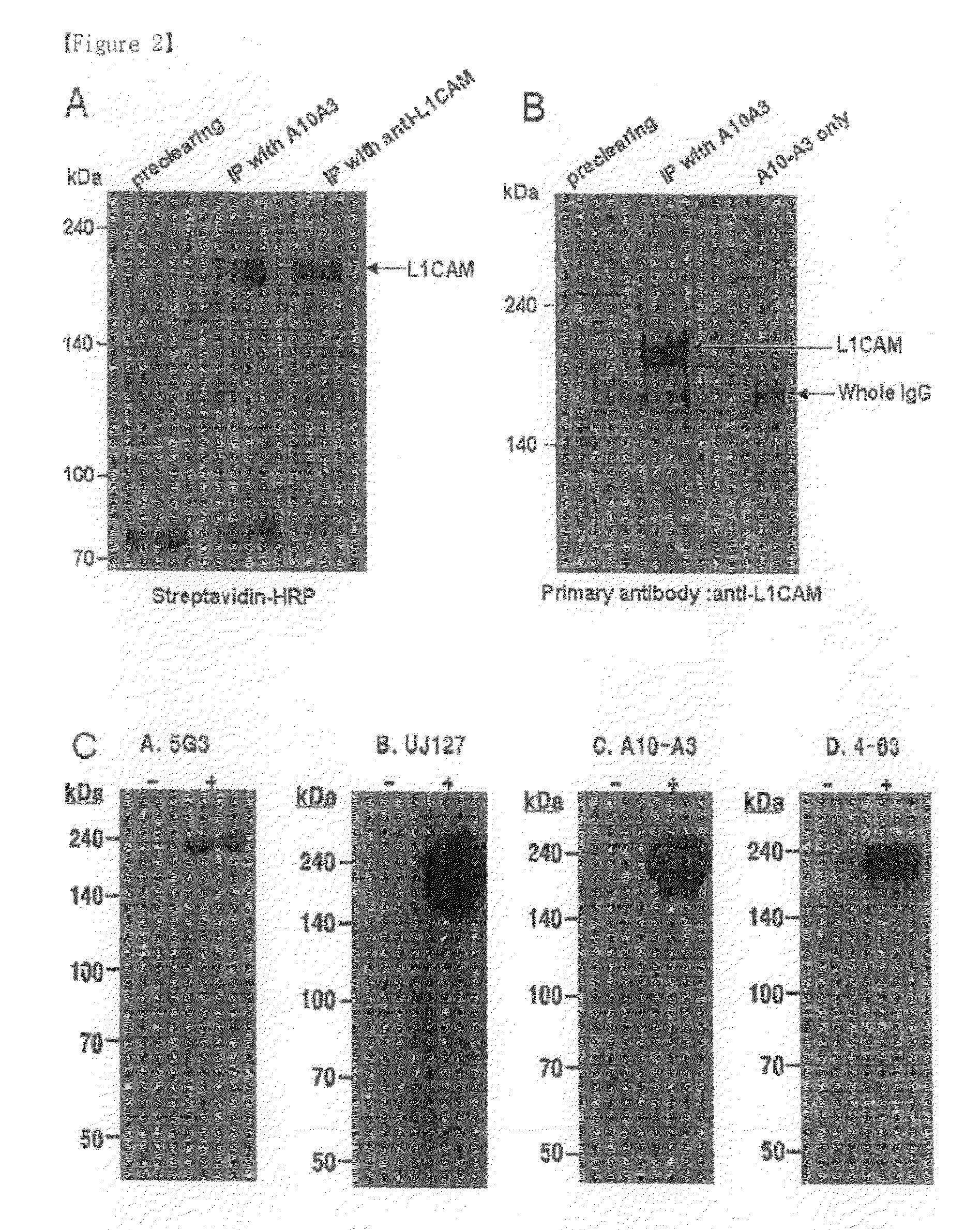
Pharmaceutical composition for treating cholangiocarcinoma, a method for inhibiting growth or invasion of cholangiocarcinoma and a method for treating cholangiocarcinoma
InactiveUS8153122B2Prevent proliferationImmunoglobulin superfamilyDigestive systemSuppressorInvasion and migration
Disclosed herein are a pharmaceutical composition for inhibiting the growth or metastasis of cholangiocarcinoma, comprising a L1CAM activity inhibitor or expression suppressor and a treatment method using the composition. This is based on the finding that L1CAM is overexpressed on cholangiocarcinoma and plays an important role in the growth and metastasis of cholangiocarcinoma and the mortality of cholangiocarcinoma patients increases as the expression rate of L1CAM increases. Also, antibodies inhibitory of the activity of L1CAM, or siRNAs suppressing the expression of L1CAM, are found to reduce the growth and invasion of cholangiocarcinoma cells. Mouse monoclonal antibodies, recognizing the L1CAM protein on the cholangiocarcinoma cell surface and binding specifically to cholangiocarcinoma tissues, or siRNAs, antisense oligonucleotides or shRNAs, may be useful in the treatment of cholangiocarcinoma by inhibiting the growth, invasion and migration of cholangiocarcinoma cell.
Owner:KOREA RES INST OF BIOSCI & BIOTECH
Method of treating cholangiocarcinoma and apparatus
ActiveUS9492573B2Prevent movementReduce exerciseTubular organ implantsRadioactive preparation formsRadiation DosagesDendrite
Biological implants have an electroplated surface wherein a substantial portion of the electroplated surface is covered with dendrites. The dendrites assist in maintaining the implant in location. When the metal is a radioactive metal or the surface of the electroplated is coated with a radioactive material, the dendrites increase surface area thereby increasing radiation dosage and further extend the radioactive surface farther away from the surface of the implant, further enhancing radioactive emission. This is particularly useful for a cholangiocarcinoma stent which can be implanted into the biliary stent to treat cholangiocarcinoma.
Owner:SERENE
Culture medium for culturing primary cells related to cholecystic cholangiocarcinoma
InactiveCN112760284AReduce dosageShort training periodCulture processDead animal preservationCancer cellClinical diagnosis
The invention discloses a culture medium for culturing primary cells related to cholecystic cholangiocarcinoma. The culture medium for culturing primary cells related to cholecystic cholangiocarcinoma is a special serum-free culture medium. The culture medium is used for carrying out in-vitro suspension culture on tumor cells derived from cholecystic cholangiocarcinoma, so that the interference from normal cells can be eliminated to the greatest extent while normal amplification of cancer cells is ensured. The primary cell culture of cholecystic cholangiocarcinoma obtained by the method can be used for various cellular in-vitro experiments, next-generation sequencing, construction of animal models, construction of cell lines and the like. Predictably, the culture method has a wide application prospect in the fields of research and clinical diagnosis and treatment of the cholecystic cholangiocarcinoma.
Owner:SUZHOU GENOARRAY
Application of protein muc13 in preparation of reagents for diagnosing intrahepatic cholangiocarcinoma
ActiveCN108982854BStrong specificityIncreased sensitivityMaterial analysisSerum markersIntrahepatic Cholangiocarcinoma
The invention discloses the application of protein MUC13 in the preparation of reagents for diagnosing intrahepatic cholangiocarcinoma. The present invention utilizes the advantages of high-throughput and rapid analysis of the human proteome chip to analyze the relevant serum of patients with intrahepatic cholangiocarcinoma, compare the differences between the samples of intrahepatic cholangiocarcinoma patients and healthy people in a short period of time, and find that the MUC13 protein The expression level of MUC13 in patients with intrahepatic cholangiocarcinoma was significantly higher than that in healthy people, suggesting that MUC13 protein can be used as a candidate serum biomarker for intrahepatic cholangiocarcinoma for early diagnosis and effective treatment of intrahepatic cholangiocarcinoma. Tests have proved that the serum marker MUC13 provided by the present invention has a specificity of 90%, a sensitivity of 91.67%, and has the characteristics of high specificity and high sensitivity. Further, the present invention also provides a sensitive, safe, reliable, and easy-to-operate commercial kit, which can be used to qualitatively measure the level of anti-MUC13 IgG antibody in human serum, which is helpful for the early diagnosis of intrahepatic cholangiocarcinoma.
Owner:HARBIN MEDICAL UNIVERSITY
Cholangiocarcinoma detection, treatment and prognosis target point and application
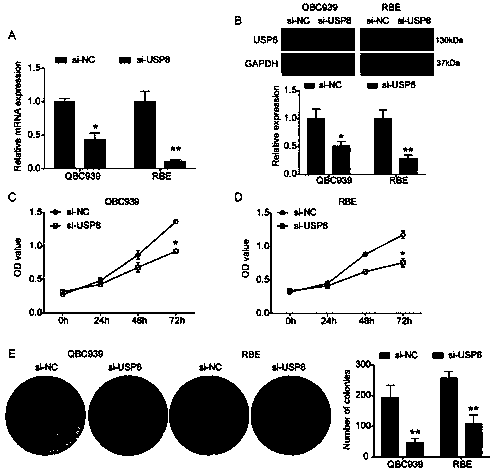
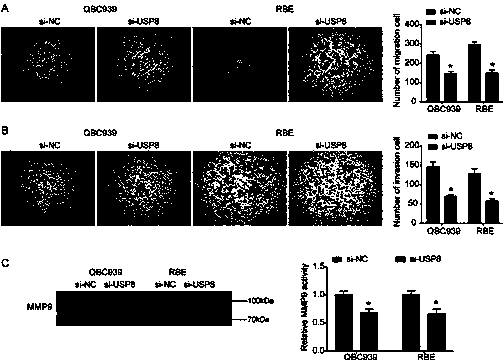
ActiveCN110699453AReduced activityPrevent proliferationOrganic active ingredientsHydrolasesNucleotideOncogene
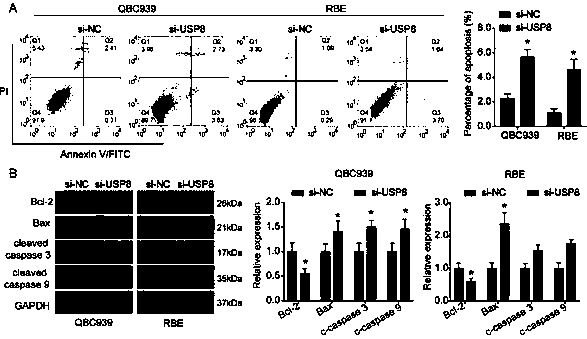
The invention relates to a cholangiocarcinoma cell medicine target point. The medicine target point is ubiquitin specific protease 8, and the nucleotide sequence is shown as SEQID NO:1 in a sequence table. For the first time, the invention puts forward that USP8 is knocked down, so that proliferation, migration and invasion of cholangiocarcinoma cells can be notably restrained, MMP9 activity achieving important effects on cell adhesion, neoplasm invasiveness and transfer is reduced, and the USP8 is promoted to achieve effect of oncogene for growth and transfer ability of cholangiocarcinoma. New possibility is provided for development of cholangiocarcinoma medicines and cholangiocarcinoma prevention, treatment and prognosis.
Owner:THE SECOND HOSPITAL OF SHANDONG UNIV
Compostions designed for the inhibition and/or blocking of the epithelial/mesenchymal transition
InactiveUS20120220546A1Enhance fibrosisReduce in quantityBiocideSugar derivativesAbnormal tissue growthDisease
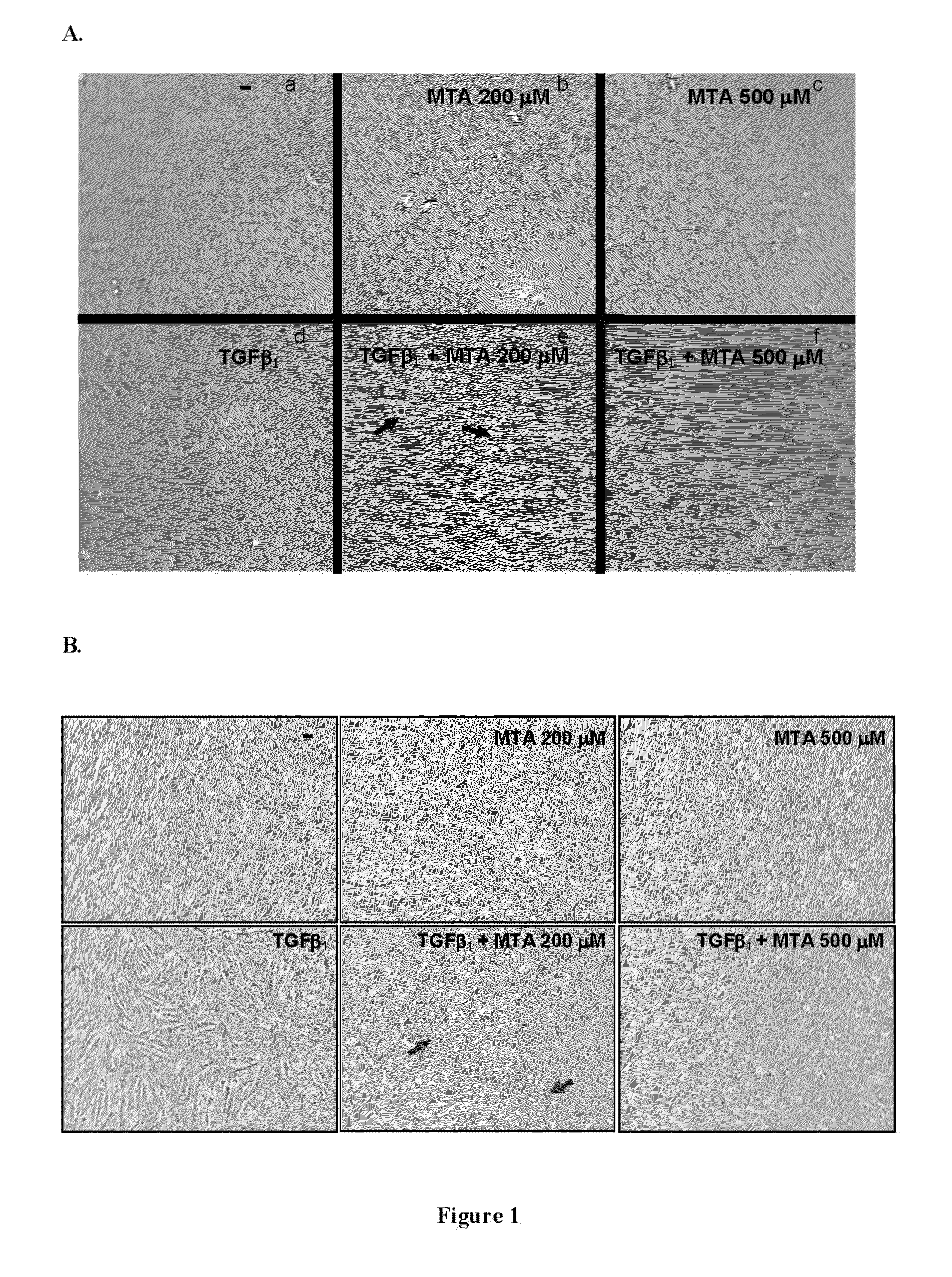
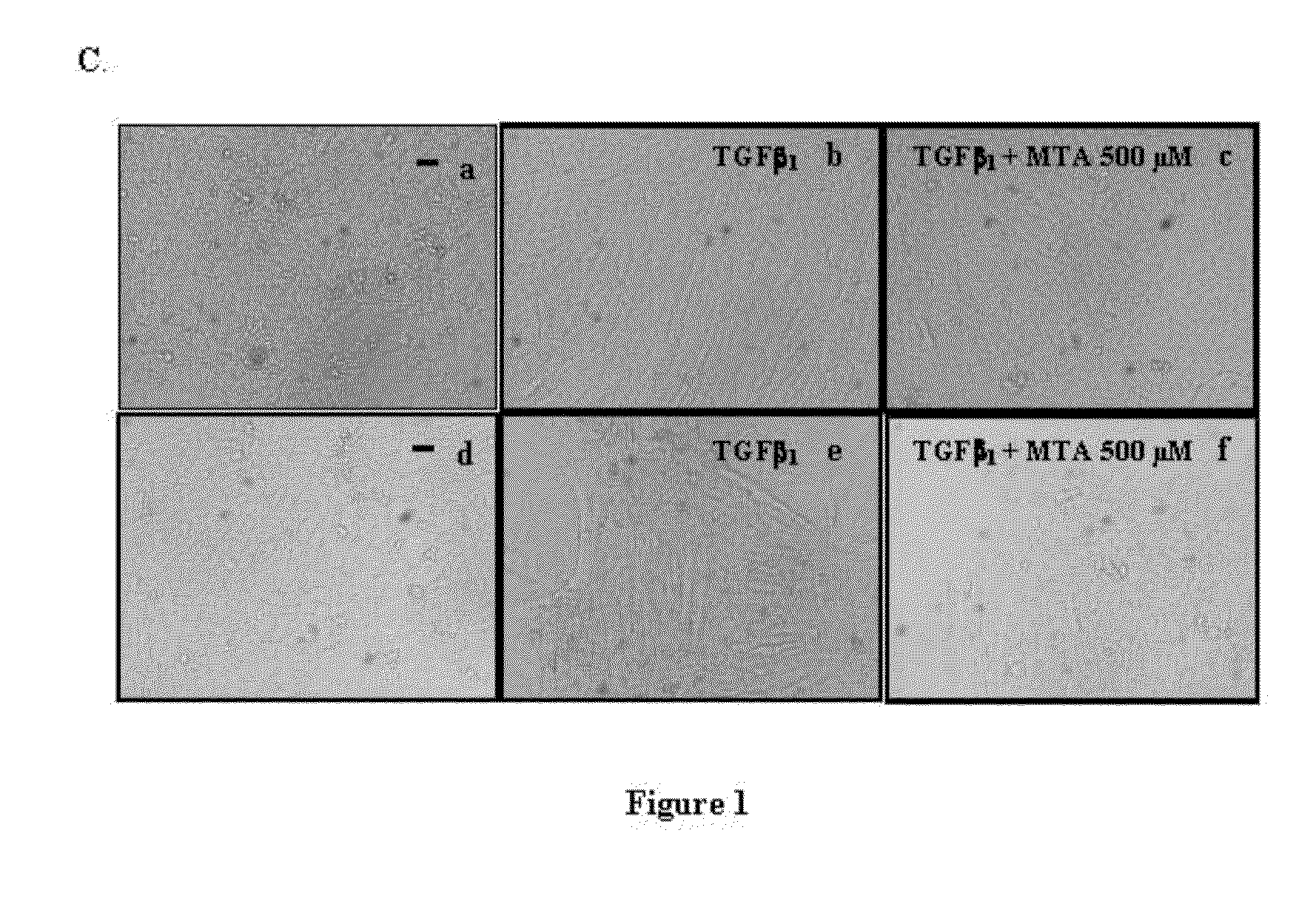
5′-methylthioadenosine (MTA) is described as a compound that is susceptible to inhibiting and / or blocking the epithelial-mesenchymal transition (EMT), a process whereby epithelial cells become mesenchymal cells.The periodical intake of MTA [every 24 h for 21 days] significantly improves fibrosis and the markers for hepatic cellular damage in KO-Mdr2 mice with MTA (28 mg / kg). Following the daily oral administration of MTA, both the expression of EMT markers in the total liver and appreciable signs of fibrosis are significantly reduced, indicating the beneficial effect of MTA on the liver affected by the lack of Mdr2. MTA is proposed to be a safe drug, suitable for oral formulation and without secondary effects, to be used in the prevention and / or treatment of diseases associated with EMT, including chronic cholestatic diseases, fibrosis and cholangiocarcinoma. On the other hand, MTA is proposed for application in anti-tumor therapies by inhibiting or blocking the EMT properties of CSC cells, in order to improve the prognosis of tumor development and the malignancy thereof.
Owner:PROYECTO DE BIOMEDICINA CIMA +1
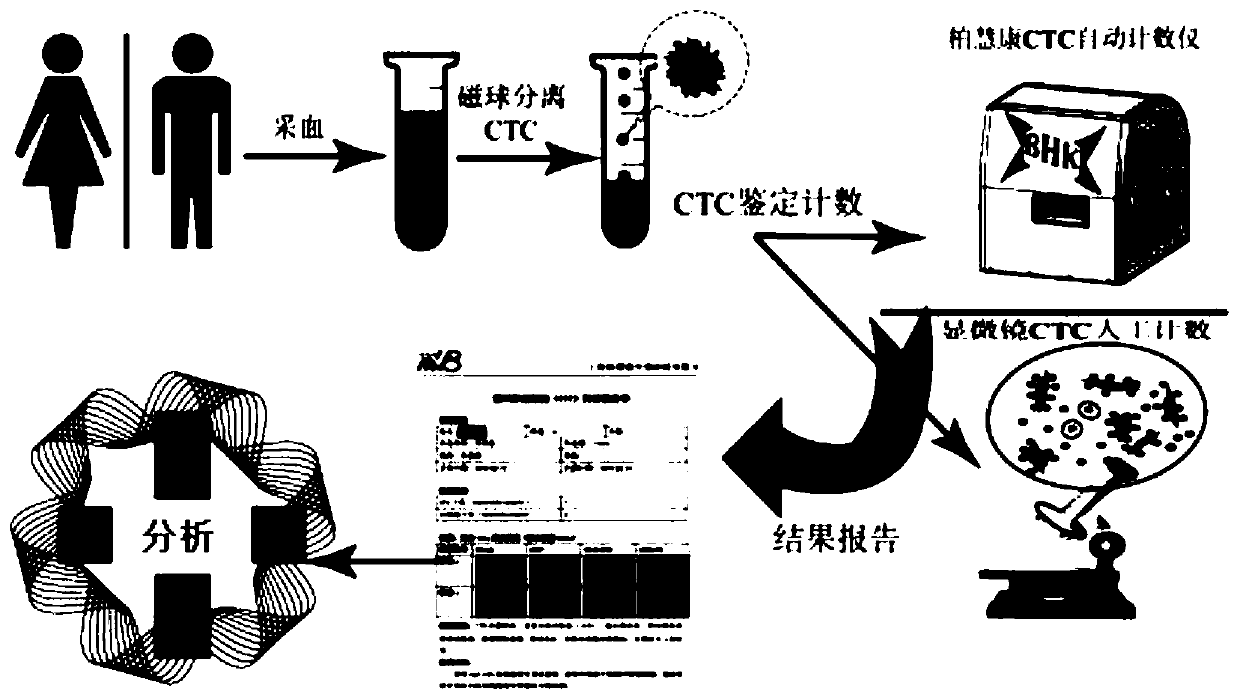
Method for specific sorting and identification detection of CTC in serum of biliary tract tumors
PendingCN111518767AImprove sorting efficiencyStrong specificityCell dissociation methodsMicrobiological testing/measurementWhite blood cellBiliary tract
The present invention provides a method for specific sorting of CTC in serum of biliary tract tumors. An appropriate amount of peripheral blood from patients with biliary tract tumors is taken and placed in anticoagulation centrifuge tubes and whole blood samples are mixed evenly; the collected blood samples are sequentially subjected to sample processing of plasma protein and plasma nucleic acidremoval, red blood cell removal, layered centrifugation and white blood cell removal; an HSPG antibody or a SDC1 subtype antibody or a SDC2 subtype antibody or a GPC1 subtype antibody or a GPC3 subtype antibody is used to combine with immunomagnetic beads to capture HSPG positive or SDC1 positive or SDC2 positive or GPC1 positive or GPC3 positive CTC. HSPG is used as a relatively specific functional tumor molecular marker for CTC sorting of gallbladder cancer and cholangiocarcinoma, thereby improving sorting efficiency of biliary tract tumor CTC, a set of HSPG-based CTC molecular typing scoring system is established, the high-specificity and high-sensitivity detection method for the biliary tract tumors is otbained, and based on the high-efficiency sorting of the biliary tract tumor CTC, downstream RNA-seq and gene NGS detection is conducted to obtain tumor-specific molecular information to improve specificity.
Owner:易滨 +1
Nanovesicles derived from faecalibacterium prausnitzii, and uses thereof
PendingCN111587295AReduce contentInhibition of secretionNervous disorderBacteria material medical ingredientsDiseaseFaecalibacterium prausnitzii
The present invention relates to vesicles derived from Faecalibacterium prausnitzii and to uses thereof. It has been experimentally confirmed by the present inventors that the vesicles in the clinicalsamples of patients with gastric cancer, colorectal cancer, liver cancer, pancreatic cancer, cholangiocarcinoma, ovarian cancer, bladder cancer, lymphoma, myocardial infarction, atrial fibrillation,and Parkinson's disease were significantly reduced in comparison with a normal person and that when vesicles separated from the strain were administered, the secretion of inflammatory mediators causedby pathogenic vesicles, such as vesicles derived from colon bacilli, was significantly inhibited. The vesicles derived from Faecalibacterium prausnitzii according to the present invention are expected to be usefully employed for the purposes of developing a method for diagnosing gastric cancer, colorectal cancer, liver cancer, pancreatic cancer, cholangiocarcinoma, ovarian cancer, bladder cancer,lymphoma, myocardial infarction, atrial fibrillation, and / or Parkinson's disease, and a composition for preventing, alleviating, or treating said diseases.
Owner:MD HEALTHCARE INC
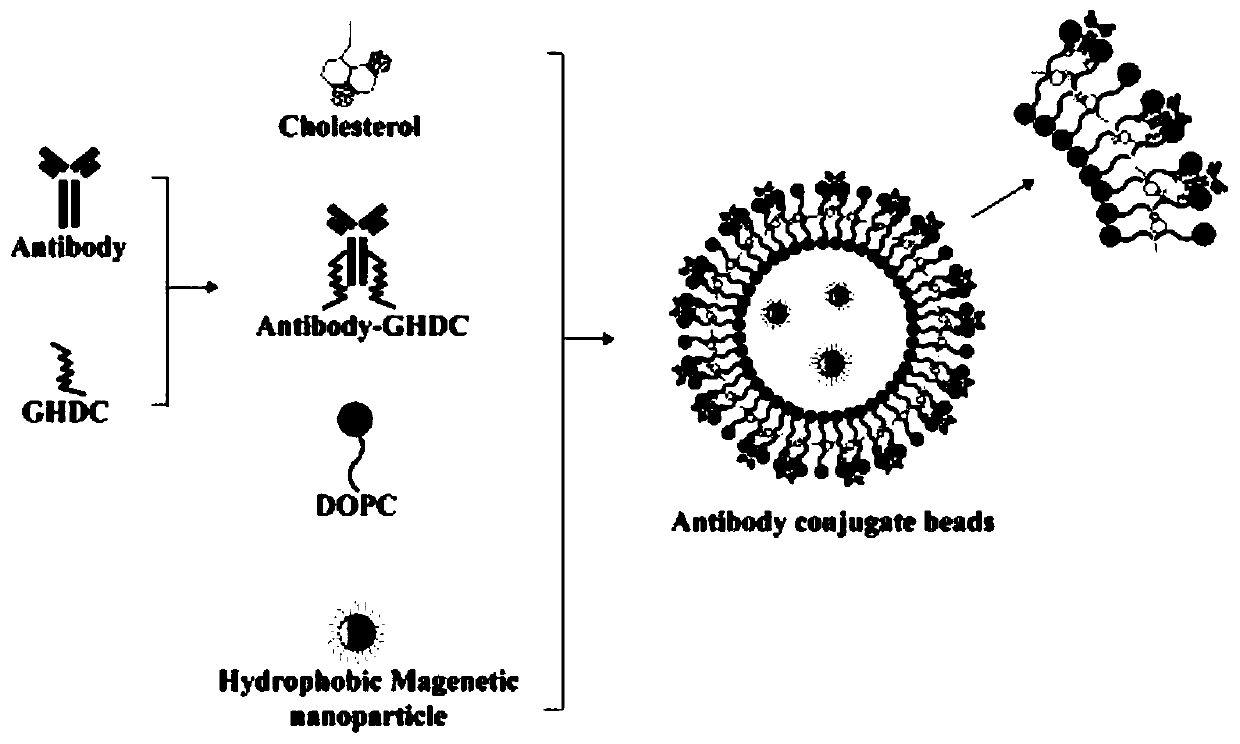
Method for detecting cholangiocarcinoma cells
ActiveUS20200355692A1Shorten mixing timeHigh affinityShaking/oscillating/vibrating mixersTransportation and packagingMedicineCirculating cancer cell
The present disclosure provides a method for detecting cholangiocarcinoma cells. The capture rate of the cholangiocarcinoma cells of the present disclosure is higher than 70%, and a plurality of octasaccharides with high affinity and specificity can be modified on the surface of magnetic beads to capture and analyze cholangiocarcinoma cells under test, wherein the cholangiocarcinoma cells can be circulating tumor cells in cholangiocarcinoma.
Owner:NATIONAL TSING HUA UNIVERSITY +1
Quinoline for treating cholangiocarcinoma
The invention provides quinoline for treating cholangiocarcinoma, and an application thereof to preparation of a medical composition for treating tumors, and particularly relates to an application ofquinoline derivative 1-[[[4-(4-fluoro-2-methyl-1H-indole -5-)oxy-6-methoxyquinolin-7-yl]oxy]methyl cyclopropylamine to treatment cholangiocarcinoma: as shown in the description.
Owner:CHIA TAI TIANQING PHARMA GRP CO LTD
Method of treating carcinoma
A method of treating patients suffering from carcinoma of intrahepatic or extra hepatic bile duct or gall bladder which is locally advanced or metastatic, by intravenously administering to the patient, paclitaxel in the form of a nanodispersion. The nanodispersion comprises particles with a mean particle size less than 300 nm and is free of polyoxyethylated castor oil and free of a protein.
Owner:SUN PHARMA INDS
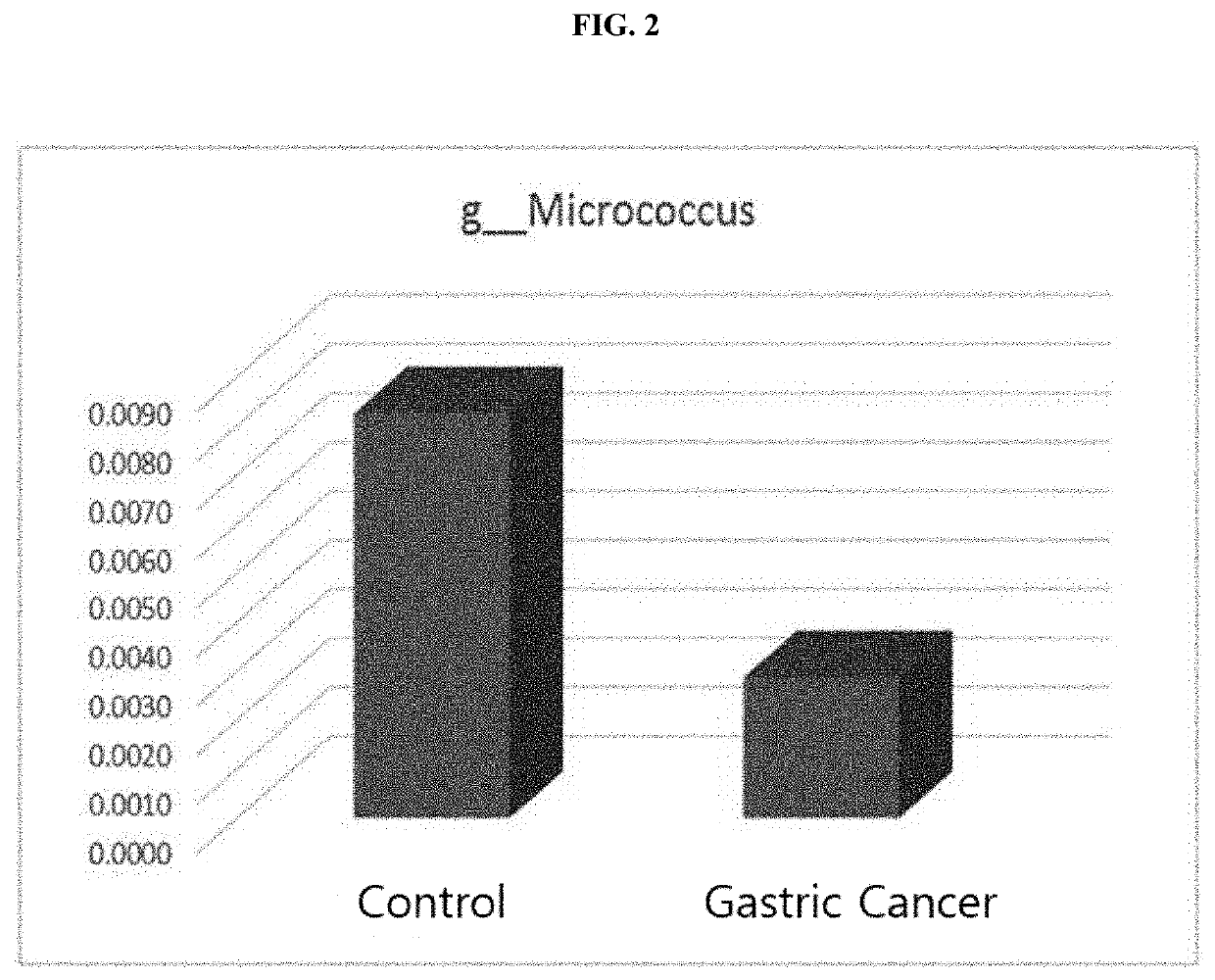
Method for diagnosing cholangiocarcinoma via bacterial metagenomic analysis
ActiveCN111630189APromote progressPrevent relapseMicrobiological testing/measurementCancer preventionExtracellular vesicle
The present invention relates to a method for diagnosing cholangiocarcinoma via a bacterial metagenomic analysis, and more specifically, to a method for diagnosing cholangiocarcinoma by performing a bacterial metagenomic analysis using a sample derived from a subject so as to analyze the content increase / decrease of extracellular vesicles derived from specific bacteria. The extracellular vesicles,secreted from bacteria present in the environment, may directly affect the incidence of cancer by being absorbed in the human body, and early diagnosis of cholangiocarcinoma before symptoms appear isdifficult, and thus the efficient treatment thereof is difficult. Thus, by preemptively predicting the risk for the onset of cholangiocarcinoma via the metagenomic analysis, according to the presentinvention, of extracellular vesicles derived from bacteria using a sample derived from a human body, cholangiocarcinoma risk groups may be diagnosed and predicted at an early stage so as to delay thetime of onset or prevent the onset of cholangiocarcinoma through proper care, and early diagnosis may be possible even after the onset, and thus the incidence rate of cholangiocarcinoma may be lowered, and a therapeutic effect therefor may be increased.
Owner:MD HEALTHCARE INC
Marker for evaluating gemcitabine chemosensitivity of intrahepatic cholangiocarcinoma and application of marker
The invention discloses a marker for evaluating the gemcitabine chemosensitivity and / or prognosis of intrahepatic cholangiocarcinoma and application of the marker. The marker for evaluating the gemcitabine chemosensitivity and / or prognosis of intrahepatic cholangiocarcinoma is CXCR3 expressed on the surfaces of peripheral blood leukocytes or immune cells in cholangiocarcinoma tissues of an intrahepatic cholangiocarcinoma patient. A kit for evaluating the gemcitabine chemosensitivity and / or prognosis of intrahepatic cholangiocarcinoma comprises a reagent related to detection of the expression quantity of the CXCR3 protein. A method for evaluating the gemcitabine chemosensitivity and / or prognosis of intrahepatic cholangiocarcinoma comprises the following steps: (1) detecting the expression quantity of CXCR3 on the surfaces of peripheral blood leukocytes or immune cells in cholangiocarcinoma tissues of an intrahepatic cholangiocarcinoma patient; and (2) comparing the expression quantity with a preset CXCR3 protein expression quantity threshold value, evaluating that the patient is sensitive to gemcitabine chemotherapy and / or good in prognosis of gemcitabine chemotherapy if the CXCR3 protein expression quantity is higher than the threshold value, and otherwise, evaluating that the patient is insensitive to gemcitabine chemotherapy and / or poor in prognosis of gemcitabine chemotherapy.
Owner:中国人民解放军海军军医大学第三附属医院
Traditional Chinese medicine composition for treating postoperative hepatic cholangiocarcinoma and cancerometastasis
PendingCN113144136AAnthropod material medical ingredientsInanimate material medical ingredientsAngelica Sinensis RootArtemisia anomala
The invention discloses a traditional Chinese medicine composition for treating postoperative hepatic cholangiocarcinoma and cancerometastasis. The traditional Chinese medicine composition comprises the raw materials of rhizoma gastrodiae, radix curcumae, vinegar-processed rhizoma cyperi, fructus aurantii immaturus, ligusticum wallichii, poria with hostwood, polyporus umbellatus, endothelium corneum gigeriae galli, herba lycopi, angelica sinensis, rhizoma corydalis, radix pseudostellariae, leech, herba artemisiae scopariae, cornu bubali, amber, lumbricus, herba lysimachiae, scorched rhizoma atractylodis macrocephalae, ginseng hair, semen cuscutae, artemisia anomala, gadfly, sculellaria barbata, peach kernel, lygodium japonicum, rheum officinale, Chinese lobelia, rose, lotus root, fried jujube kernel, platycladi seed, tortoise plastron, turtle shell and radix glycyrrhizae. According to the traditional Chinese medicine composition disclosed by the invention, all the Chinese herbal medicines are subjected to a medicine composition, the transformation, reduction and extinction process of the medicine property in the mechanism of the disease and multi-directional gasification evolution are determined, and finally, cancer cells are quickly reversed into normal physiological factors of a human body.
Owner:陈海林
Use of fibroblast growth factor receptor inhibitor
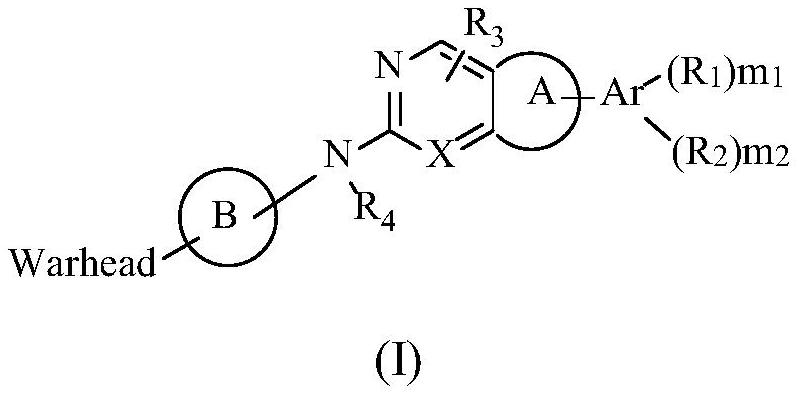
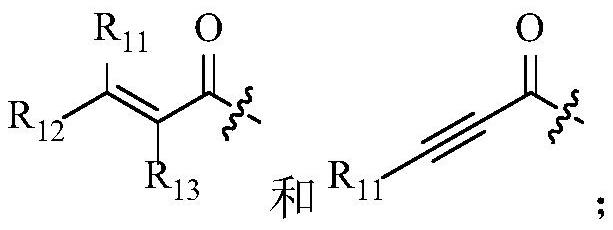
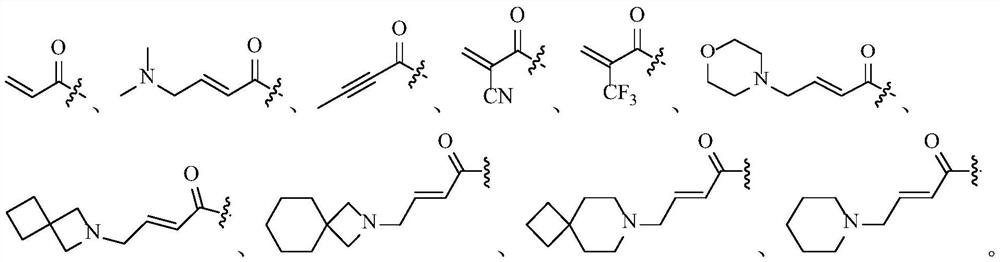
PendingCN113318113AEffective treatmentGood treatment effectOrganic active ingredientsOrganic chemistryPharmaceutical drugPharmaceutical medicine
The invention belongs to the technical field of medicines, relates to use of a fibroblast growth factor receptor inhibitor and particularly relates to a compound represented by a general formula (I) for treating cholangiocarcinoma and pharmaceutically acceptable salts and isomers thereof, a pharmaceutical composition containing the compound or pharmaceutically acceptable salts and isomers thereof, a method for treating the cholangiocarcinoma by using the compound or pharmaceutically acceptable salts and isomers thereof, use of the compound or pharmaceutically acceptable salts and isomers thereof in cholangiocarcinoma treatment and use of the compound or pharmaceutically acceptable salts and isomers thereof in preparation of drugs for treating the cholangiocarcinoma of mammals. Each variable of the general formula is as defined in the description. Shown by researches, the compound represented by the general formula (I) or pharmaceutically acceptable salts and isomers thereof has a remarkable therapeutic action on the cholangiocarcinoma and has very great clinical application potentials.
Owner:TRANSTHERA SCIENCES (NANJING) INC
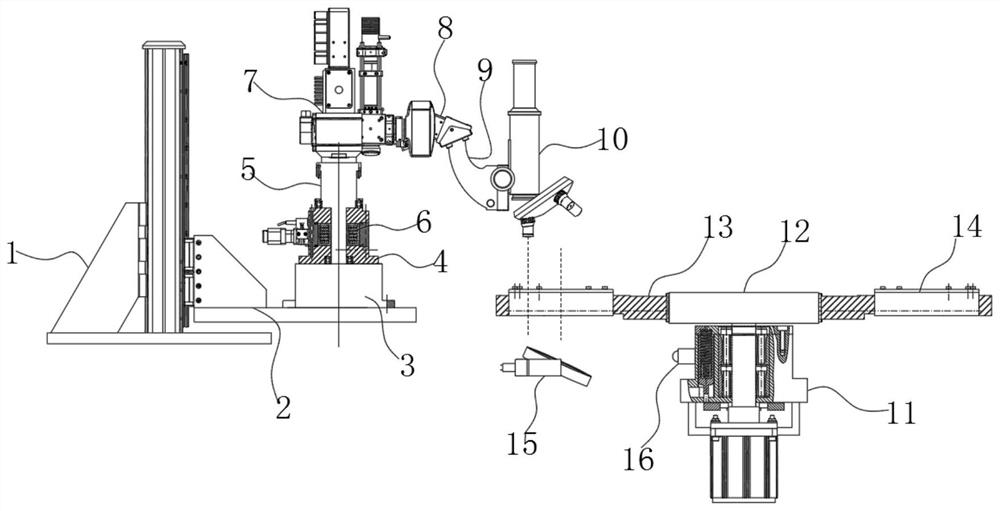
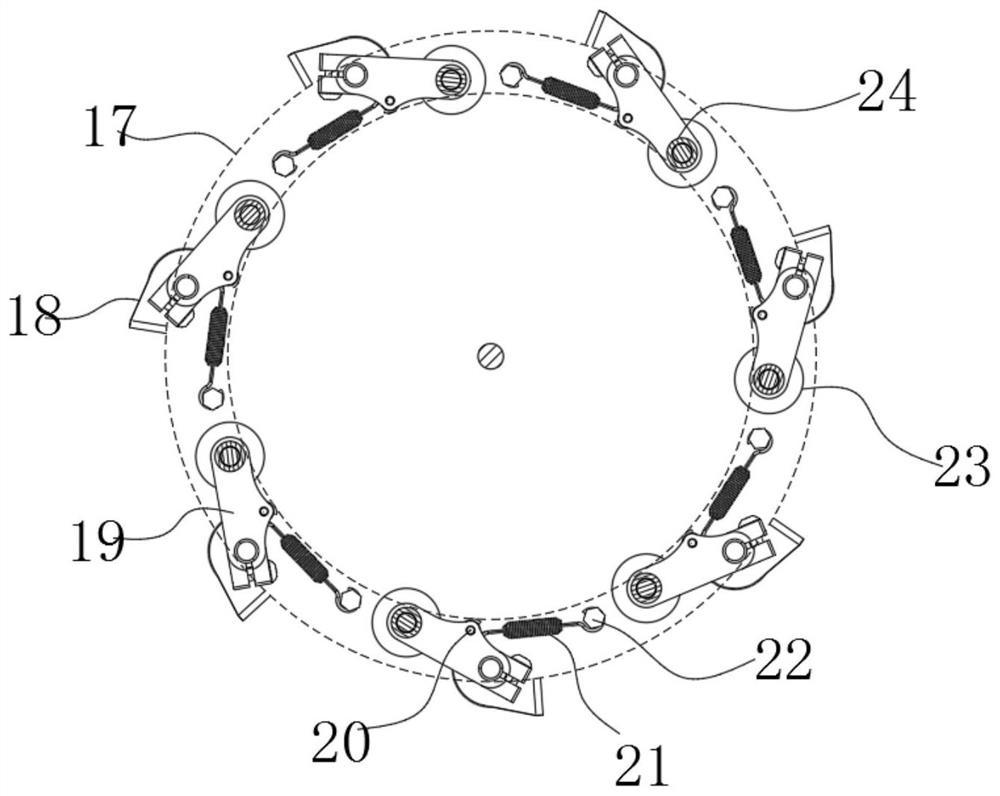
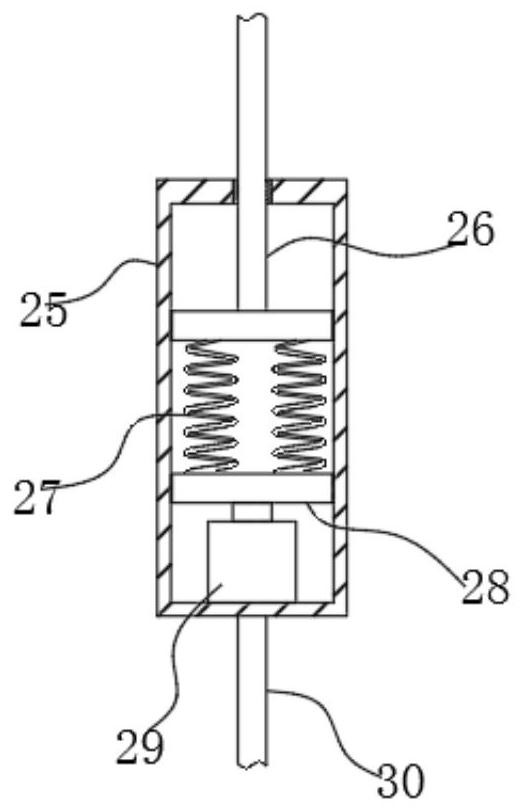
A device for observing proliferation of cholangiocarcinoma cells
ActiveCN113049592BEasy to adjustRealize controlled observationMaterial analysis by optical meansPetri dishEngineering
The invention discloses an observation device for the proliferation of cholangiocarcinoma cells, which includes a lifting slider, a self-rotating component, an observation mirror and a culture dish arrangement component, wherein the lifting slider is provided with the self-rotating component, and the self-rotating component is arranged on the lifting slider. The output end of the rotating assembly can rotate around its vertical axis of rotation, and the output end of the self-rotating assembly is provided with an observation mirror; the bottom of the observation mirror is provided with at least two groups of culture dish arrangement assemblies, and the two groups of culture dishes The dish arrangement assemblies are distributed on the same circumference, and the center of the circle is located on the vertical axis of rotation of the self-rotating assembly; the culture dish arrangement assemblies at least include a position adjustment assembly, and the position adjustment assembly can hold the culture dish or drive The petri dish performs self-rotating motion; the observation mirror at least includes an objective lens, and when the position adjustment assembly is located below the objective lens, the vertical line where the observation center of the objective lens is located is the vertical line of the self-rotating motion of the petri dish. Axis misalignment setting.
Owner:HUNAN NORMAL UNIVERSITY
Method for treating primary sclerosing cholangitis
The invention relates to the use of pharmaceutical compositions of the SGLT2 inhibitor, remogliflozin etabonate, to treat primary sclerosing cholangitis (PSC). Methods and compositions associated with the invention can improve or maintain clinical outcomes of PSC symptoms, such as ascites accumulation, hepatic encephalopathy, development of varices, jaundice, variceal bleeding, cholangiocarcinoma, hepatocellular carcinoma, evidence of cirrhosis, and colorectal cancer.
Owner:アヴォリント
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com