Method for treating primary sclerosing cholangitis
A sclerosing, primary technology, used in the digestive system, pharmaceutical formulations, organic active ingredients, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
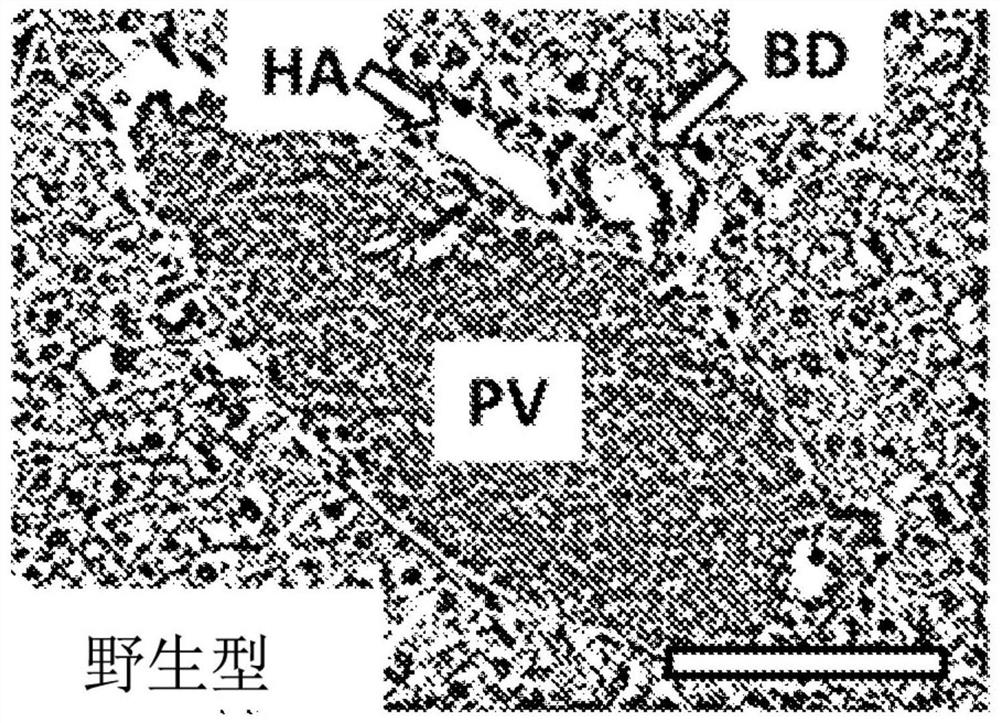
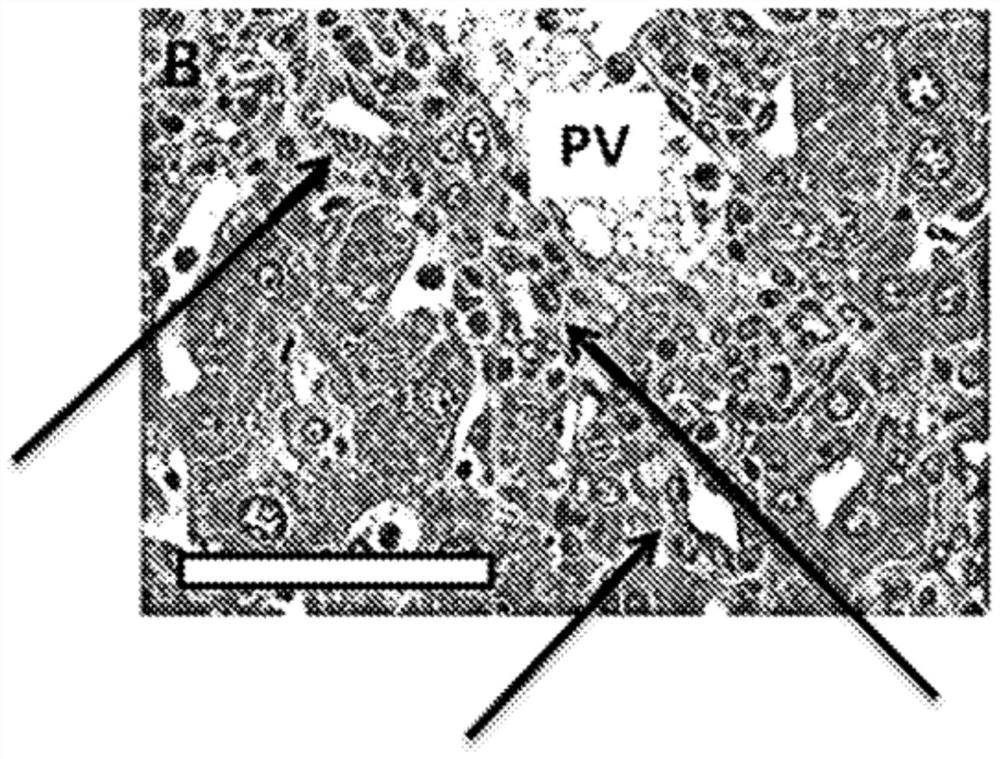
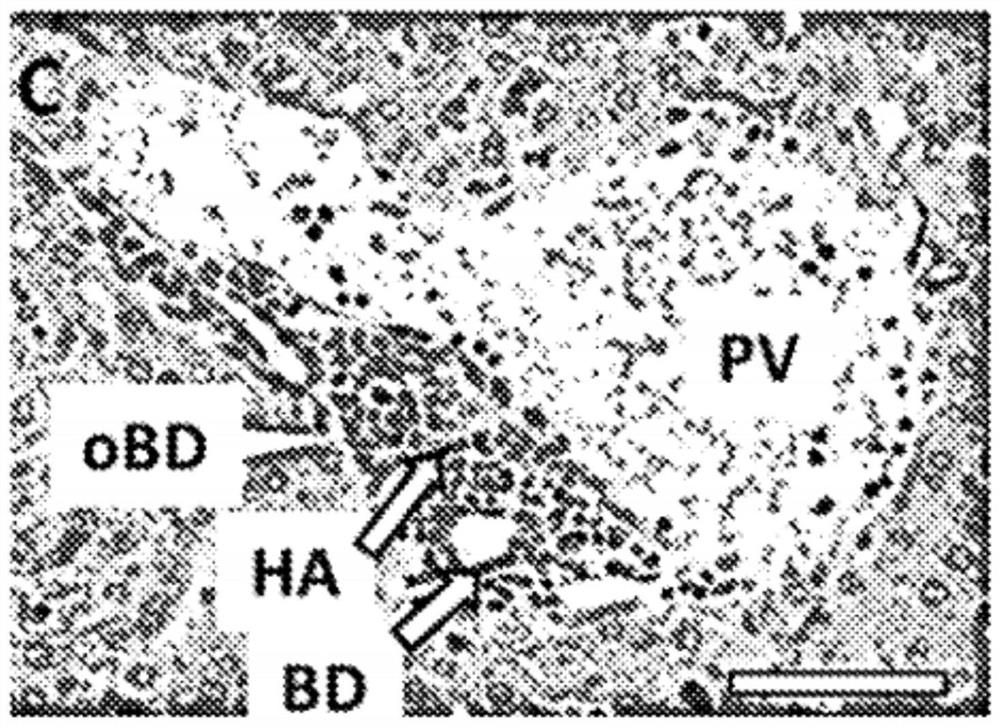
[0043] Example 1. Repagliflozin orally administered reduces inflammatory cell infiltration, bile duct hyperplasia and interface hepatitis in TIA mice.
[0044] First, TNFα knockout (“KO”) C57BL / 6 mice (strain B6.129S-Tnf tmlGkl / J, deposit number 005540, Jackson Laboratories, Bar Flarbor, ME) and IL-10KO mice (strain B10.129P2(B6)-IL10 tmlCgn / J, Deposit 002251, Jackson Laboratories) were bred to produce a population of mice deficient in TNFα and IL-10. Since mice with TNFα- / - and IL10- / - genotypes spontaneously develop inflammatory bowel disease (“IBD”) (Hale 2012), a disease associated with poor breeding conditions (Nagy 2016), further research is needed. breeding to produce the AICDA population, which was generated by breeding offspring with TNFα- / - and IL10+ / - genotypes with AICDA- / - mice (obtained from Dr. TasukuHonjo (Muramatsu 2000)) to produce TNFα- / -, IL10- / - and AICDA+ / - ("TI-hetA") male and female mice. In turn, TI-hetA male and female mice were bred to generate...
Embodiment 2
[0053] Example 2. TIA mice show serological evidence of liver and / or bile injury.
[0054] Serum biochemical analysis of TIA mice was performed at week 11. Blood from euthanized animals was drawn into lithium heparin tubes and a Fleska Dry Chem 7000 analyzer was used to measure a panel of analytes including total protein, albumin, serum alkaline phosphatase (AP), alanine aminotransferase (ALT), and total bilirubin. Serum aspartate aminotransferase (AST) was measured in a separate assay. In 50% of the mice, elevated levels of alkaline phosphatase, alanine aminotransferase, and aspartate aminotransferase at least 1.5 times the upper limit of normal were detected, which are considered indicators of cholestasis / hepatic injury.
[0055] As described in Example 1, histological analysis at week 11 showed substantial biliary and hepatic inflammation but relatively little fibrosis. These serum biochemical data also suggest autoimmune hepatitis.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com