Patents
Literature
133 results about "Hepatic encephalopathy" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
A loss of brain function as a result of failure in the removal of toxins from the blood due to liver damage.
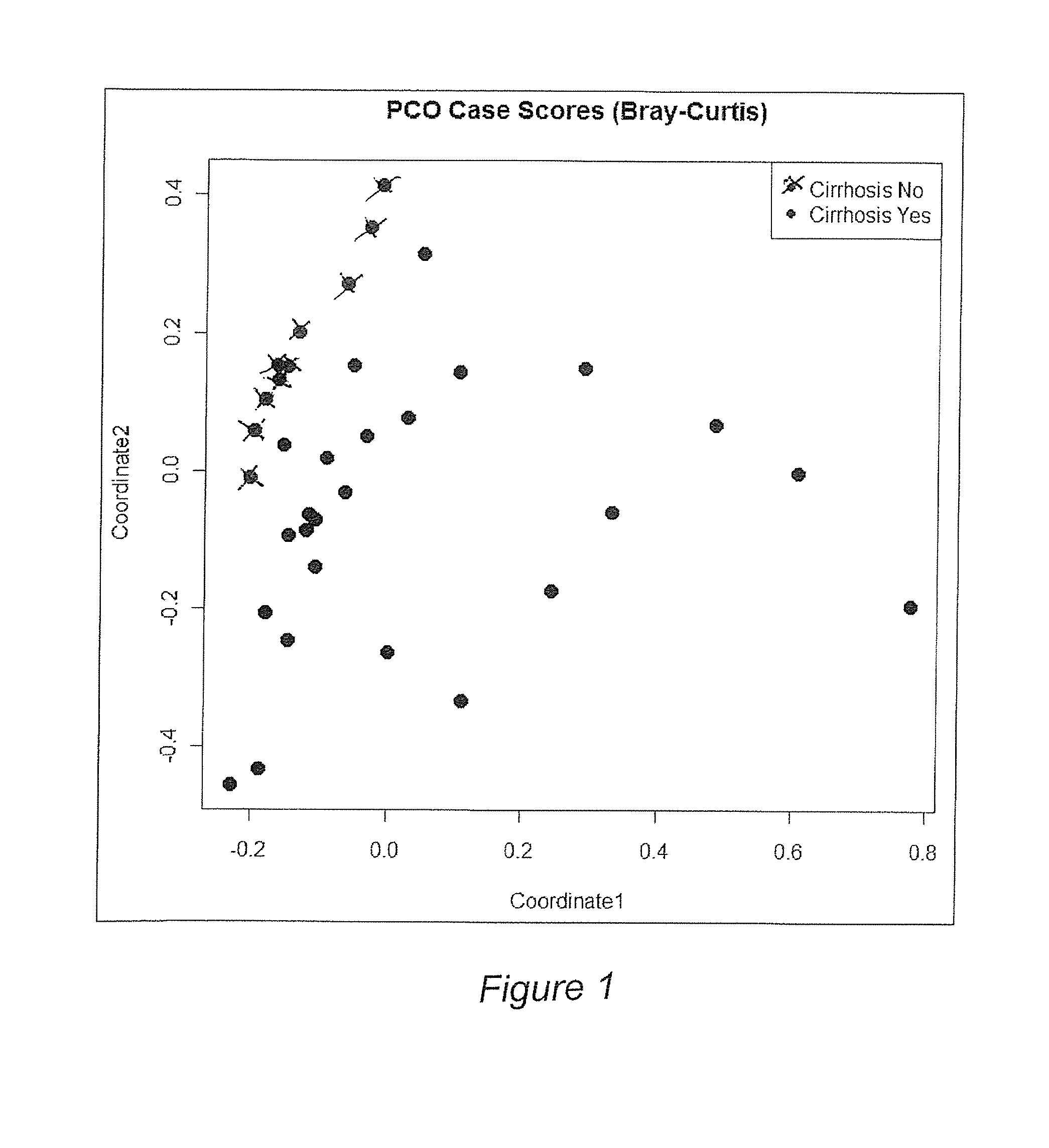
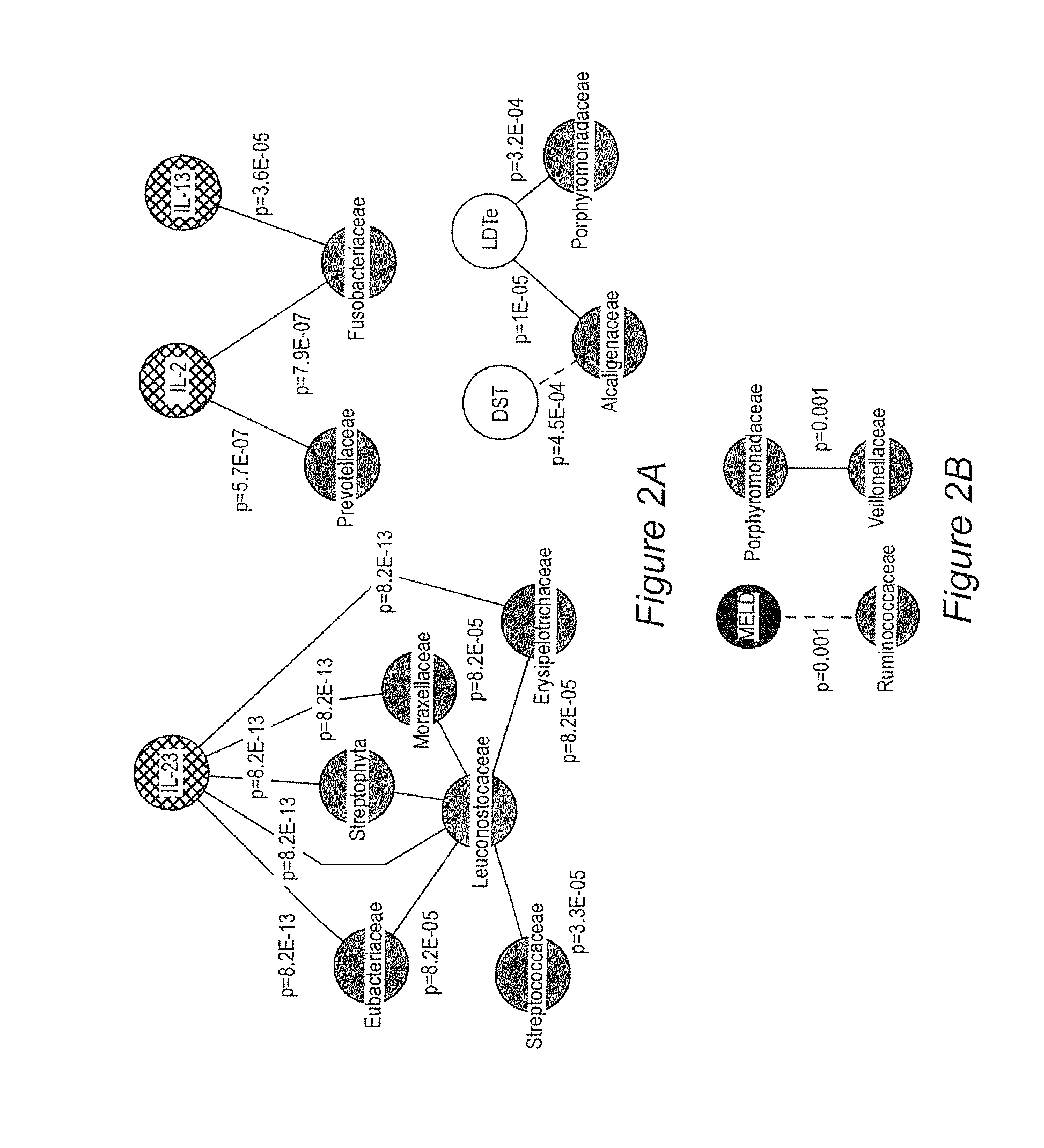
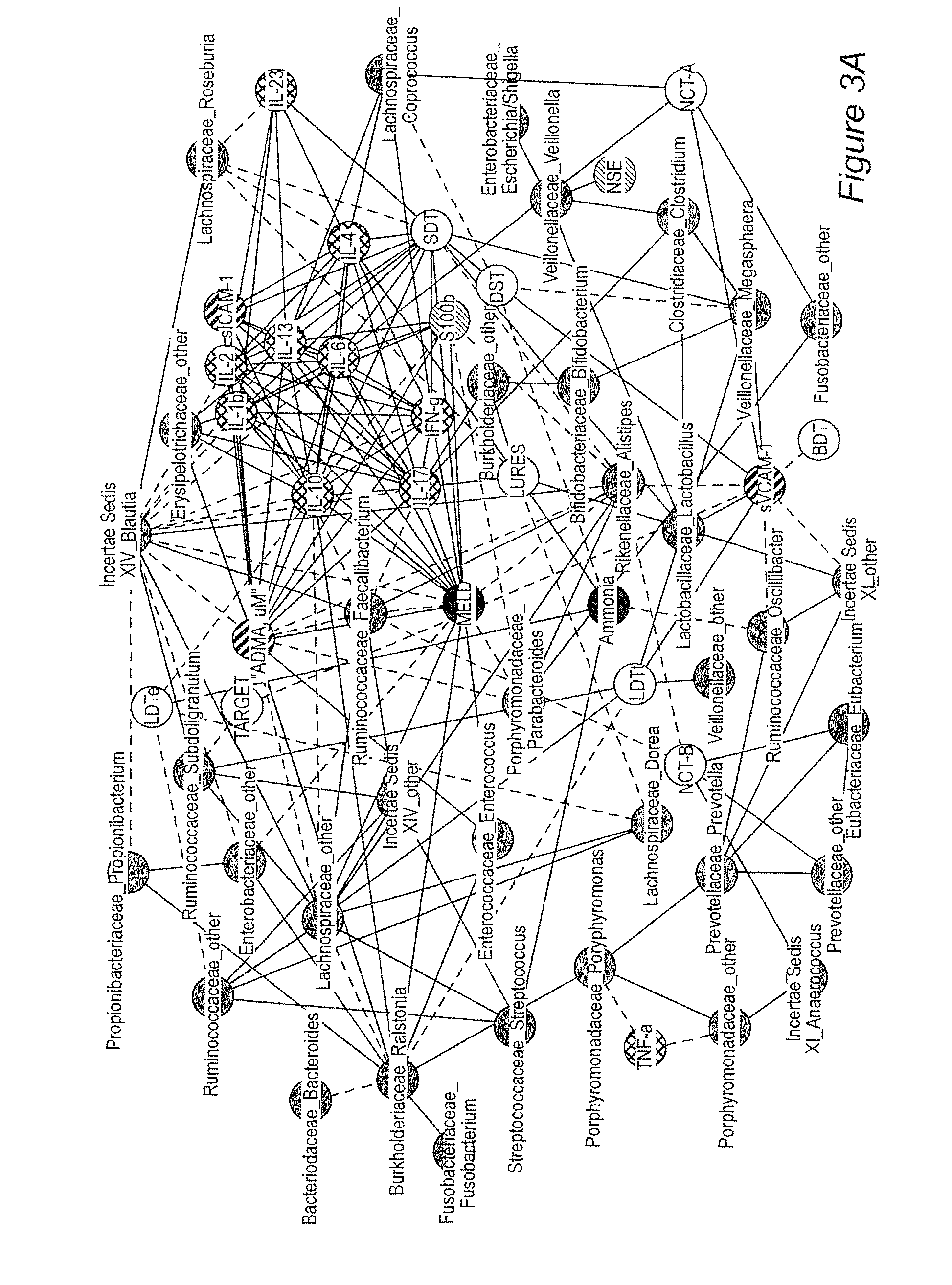
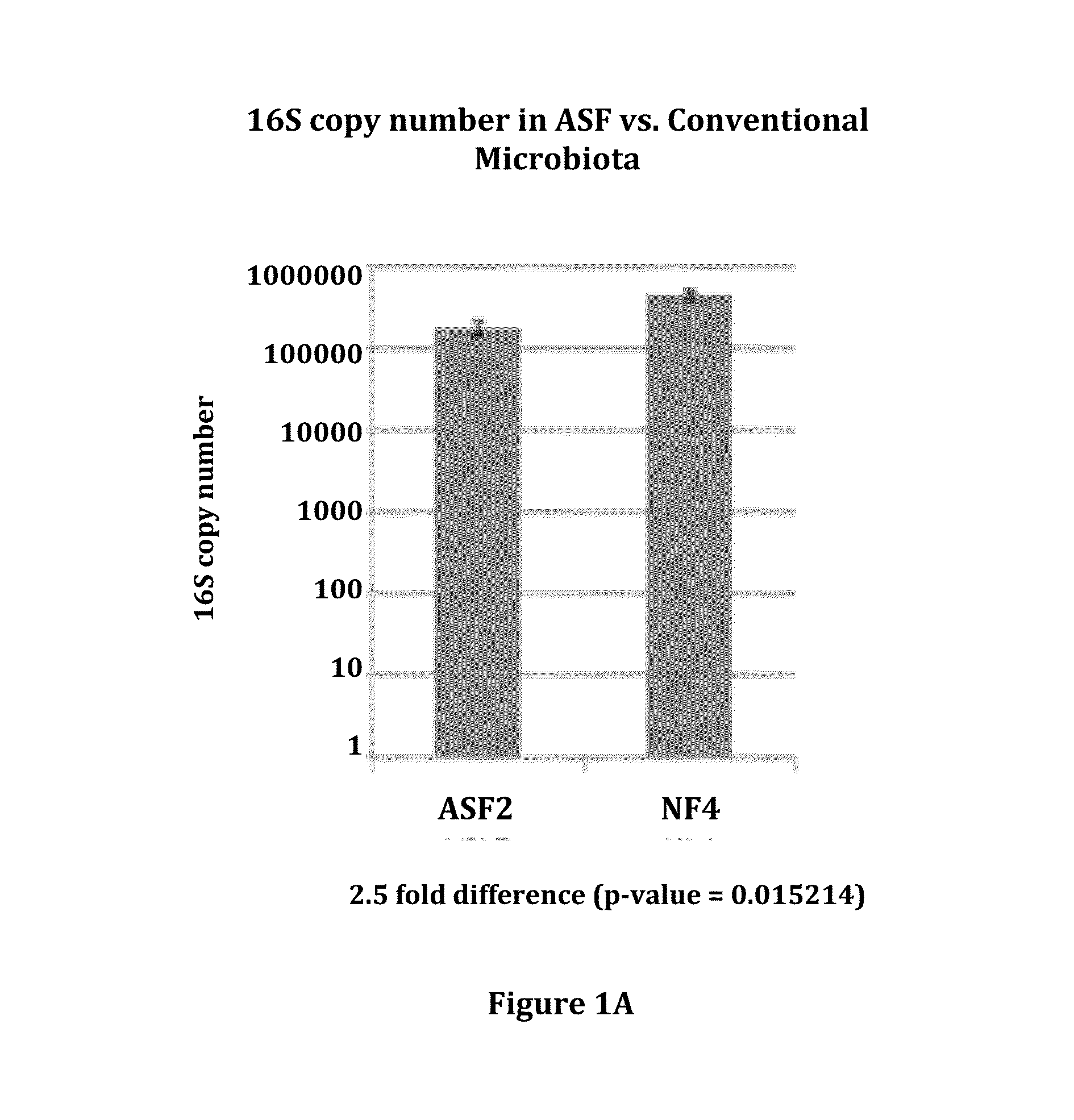
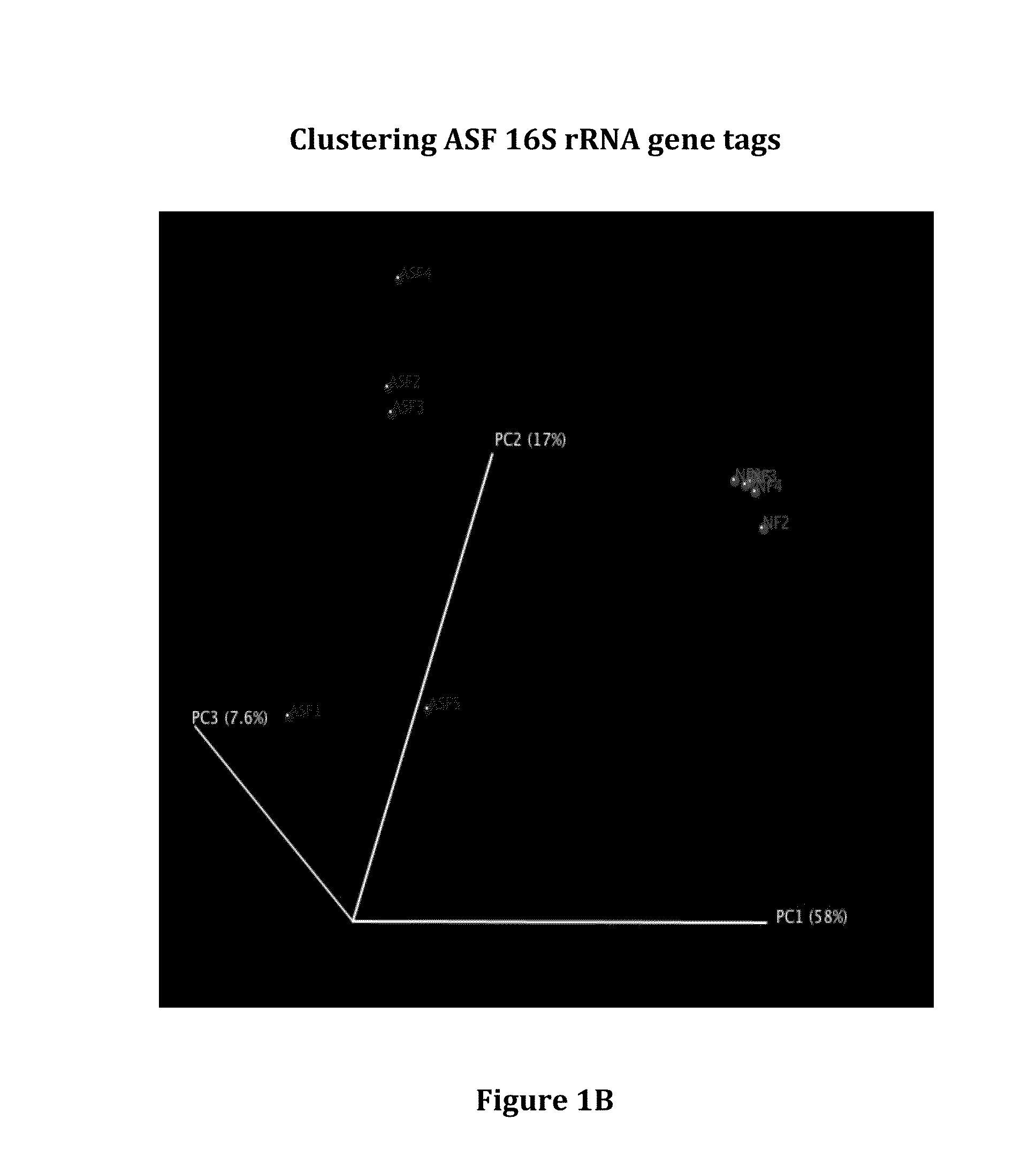
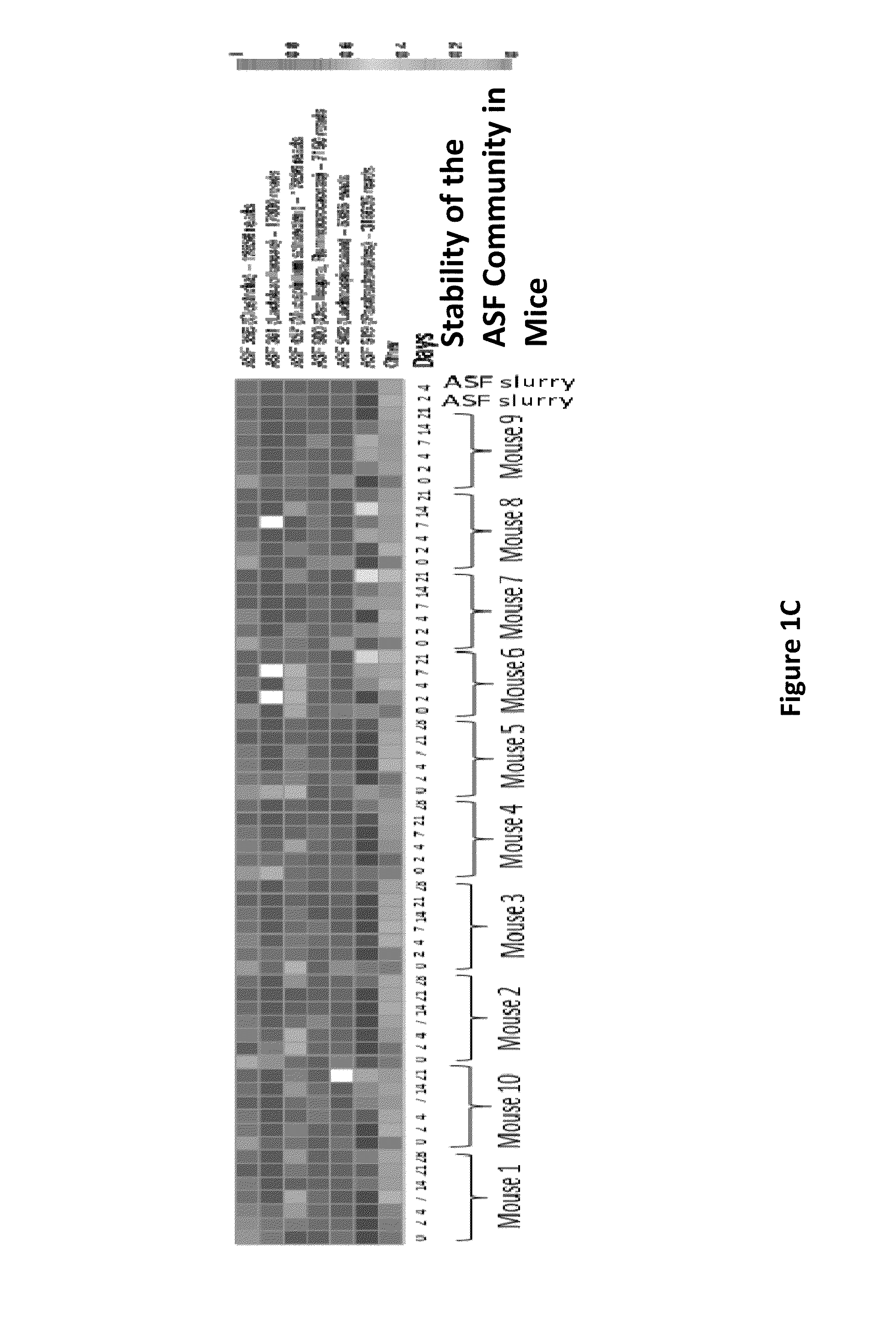
Gut microflora as biomarkers for the prognosis of cirrhosis and brain dysfunction
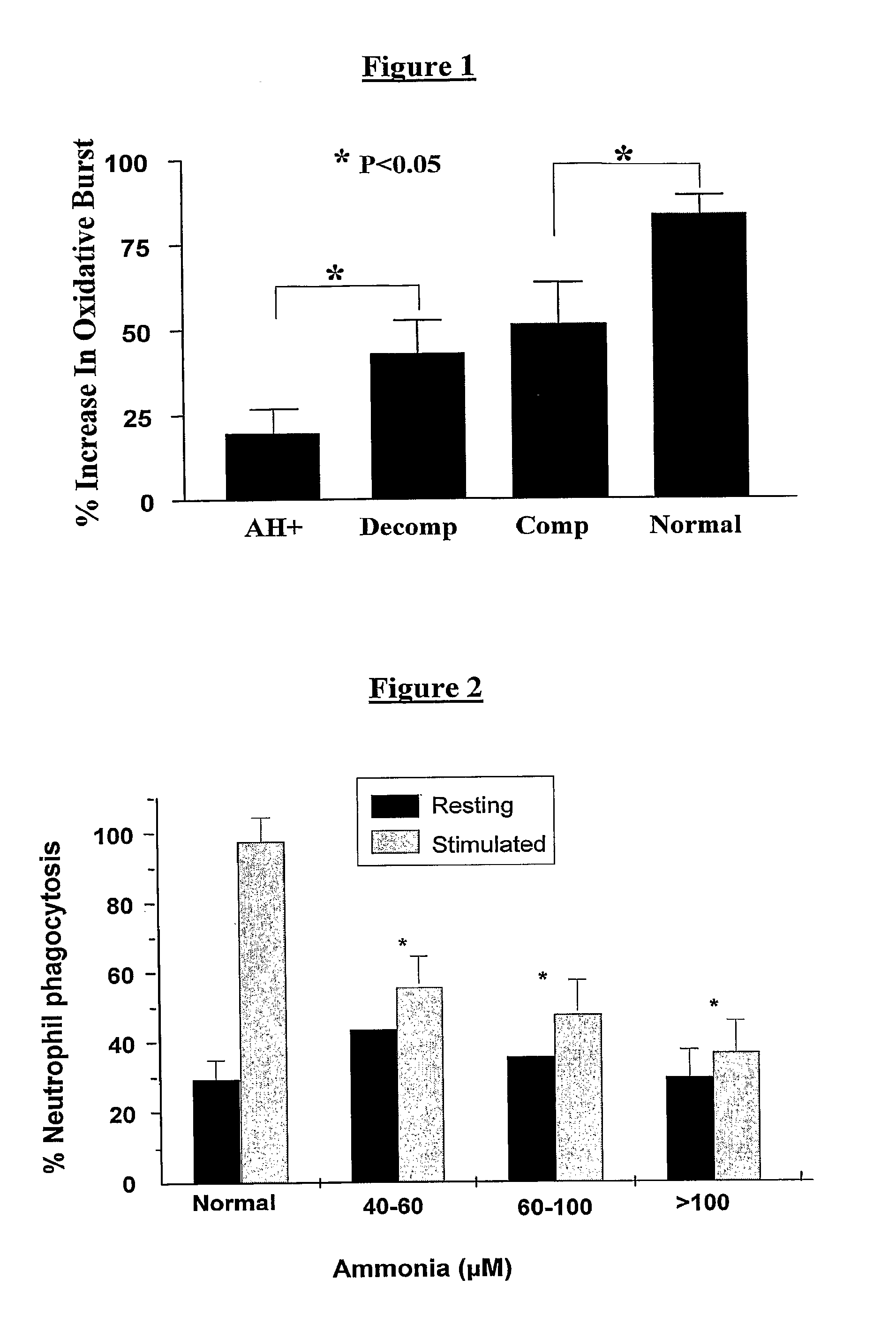
InactiveUS20140179726A1Efficacy of treatmentReduce the amount requiredBiocideMicrobiological testing/measurementDiseaseTreatment targets
A systems biology approach is used to characterize and relate the intestinal (gut) microbiome of a host organism (e.g. a human) to physiological processes within the host. Information regarding the types and relative amounts of gut microflora is correlated with physiological processes indicative of e.g., a patient's risk of developing a disease or condition, likelihood of responding to a particular treatment, for adjusting treatment protocols, etc. The information is also used to identify novel suitable therapeutic targets and / or to develop and monitor the outcome of therapeutic treatments. An exemplary disease / condition is the development of hepatic encephalopathy (HE), particularly in patients with liver cirrhosis.
Owner:VIRGINIA COMMONWEALTH UNIV +1
Compositions and methods comprising a defined microbiome and methods of use thereof
ActiveUS20160243175A1Minimal urease activityReduce bacteria countBacteriaBacteria material medical ingredientsMicroorganismInflammatory Bowel Diseases
The invention features the use of a defined microbial consortia for the replacement of a gut microbiome associated with disease. In particular, the invention provides for the treatment of hyperammonemia, Clostridium difficile colitis, hepatic encephalopathy associated with cirrhosis, and inflammatory bowel disease.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
L-ornithine phenyl acetate and methods of making thereof
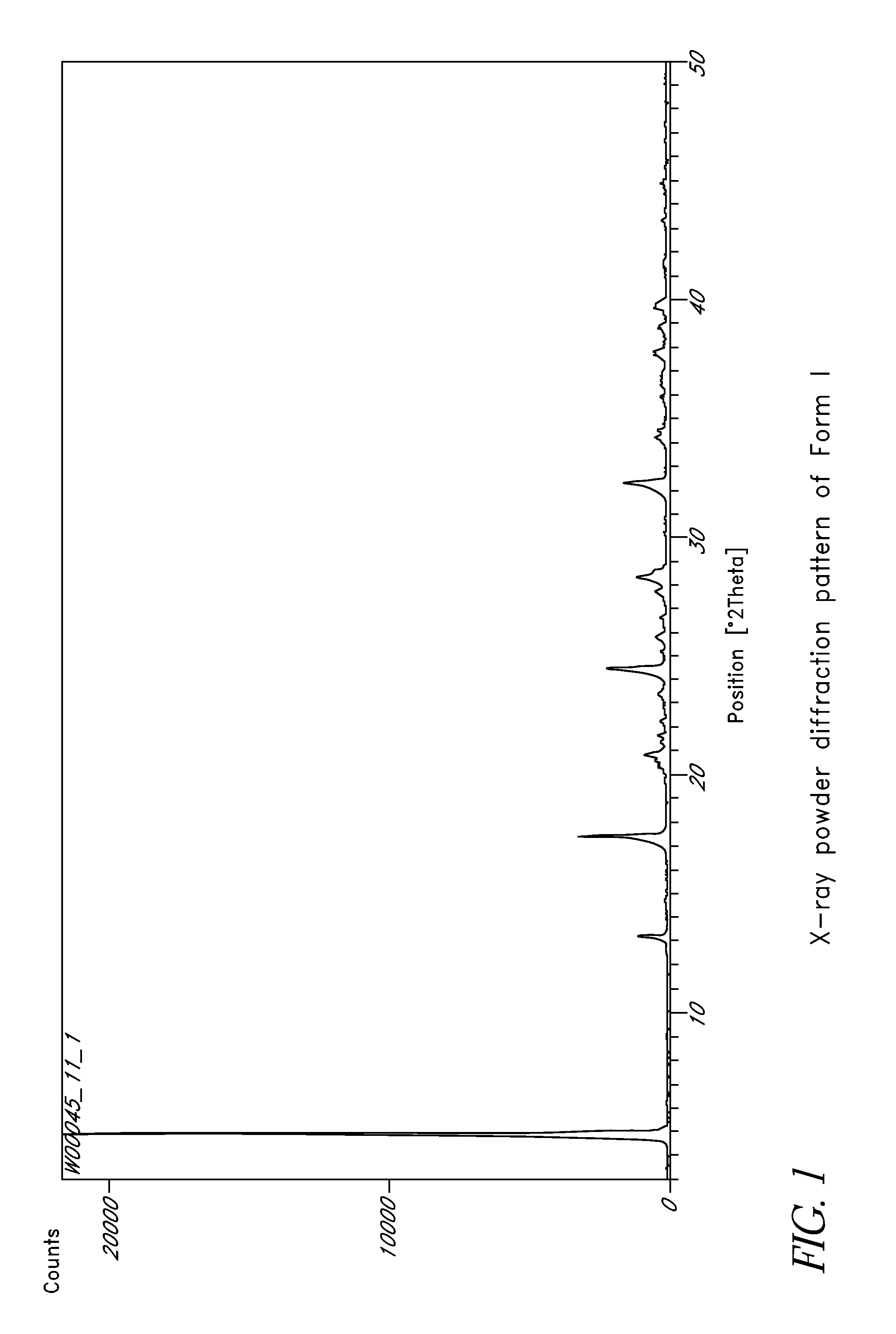
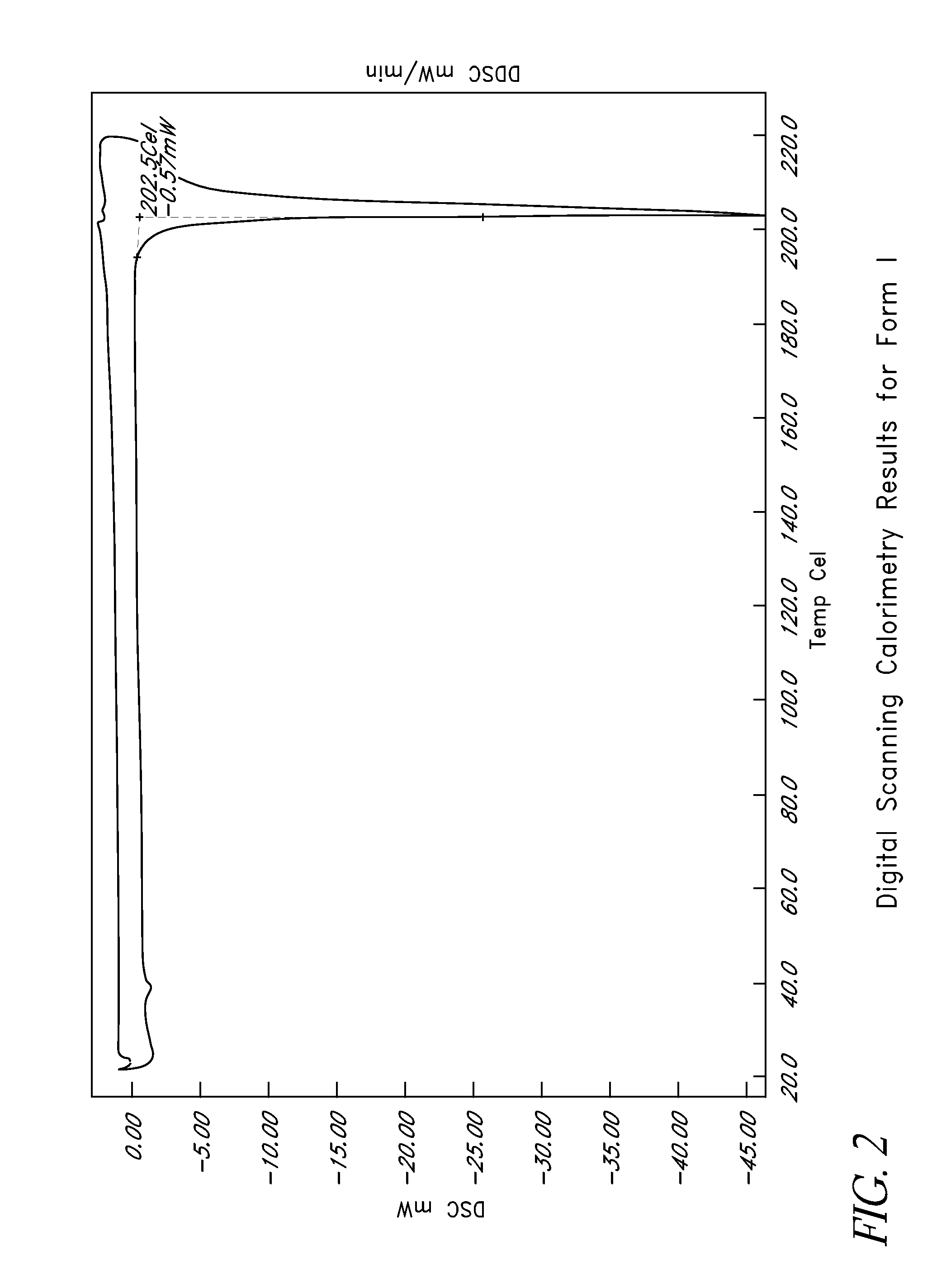
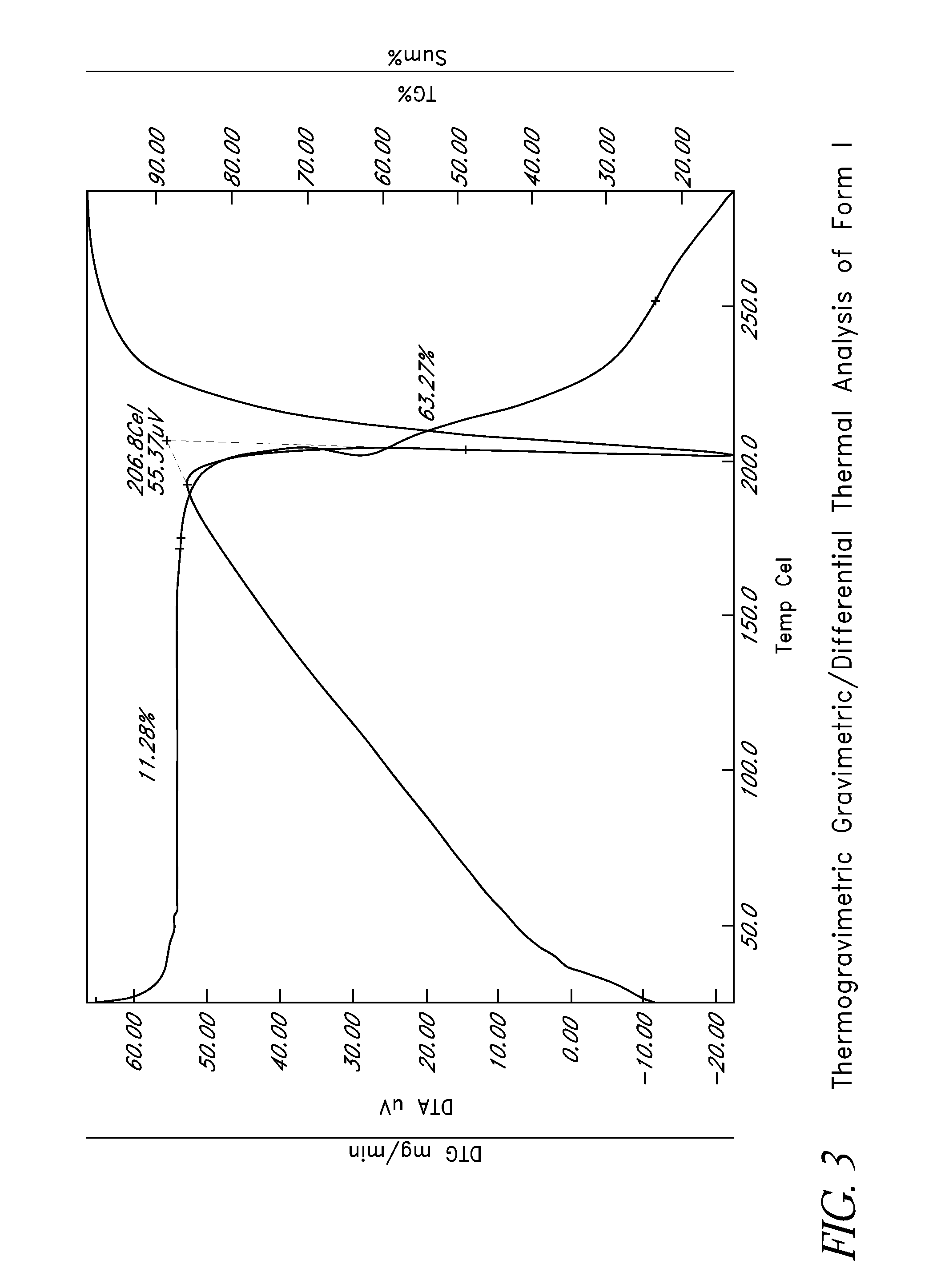
Disclosed herein are crystalline forms of L-ornithine phenyl acetate and methods of making the same. The crystalline form may, in some embodiments, be Forms I, II, III and V, or mixtures thereof. The crystalline forms may be formulated for treating subjects with liver disorders, such as hepatic encephalopathy. Accordingly, some embodiments include formulations and methods of administering L-ornithine phenyl acetate.
Owner:OCERA THERAPEUTICS INC
Traditional Chinese medicine composition for treating acute hepatitis and preparation method thereof
InactiveCN101757433AAchieve standardizationAchieve scaleDigestive systemUnknown materialsTARAXACUM OFFICINALE ROOTMedicinal herbs
The invention discloses a traditional Chinese medicine composition for treating acute hepatitis and a preparation method thereof. The traditional Chinese medicine composition mainly comprises the following raw medicinal herbs: radix bupleuri, folium isatidis, isatis root, chrysanthemum, taraxacum officinale root, herba violae, pericarpium citri reticulatae viride, rhizoma cyperi, costus root, hawthorn, angelica sinensis, raw rehmannia, radix scrophulariae, root of kudzu vine, radix paeoniae alba, soapstone, cape jasmine, houttuynia cordata, subprostrate sophorae, rhizoma smilacis glabrae, oriental wormwood, indigo naturalis, coptis, rheum officinale, felwort, ash bark, radix sophorae falvescentis, bunge cherry seed, lophatherum gracile, bezoar, antelope's horn, patrinia, lithospermum, moutan bark and root of common peony. The traditional Chinese medicine composition can be prepared into any one common oral preparation according to a conventional traditional Chinese medicine preparation method. The invention can remarkably improve symptoms of inappetence, hepatitis B virus, hepatitis B carrier, arcane positive, greasy antiposia, diarrhea, jaundice, hepatic encephalopathy, electrolyte disturbance, encephaledema, cerebral hernia, hepatorenal syndrome, hydroperitoneum, electrolyte disturbance and the like, and has accurate clinical treatment effect, remarkable treatment effect and rapid effect taking. Because of being combined by basically adopting medicinal and edible Chinese medicinal herbs specified in National Formulary, the traditional Chinese medicine composition has the advantages of low cost, basic no toxic and side effects, and the like.
Owner:TAIYI HEPU BEIJING RES INST OF TCM
Compositions comprising ornithine and phenylacetate or phenylbutyrate for treating hepatic encephalopathy
ActiveUS20120259016A1Antibacterial agentsOrganic active ingredientsHepatic encephalopathyLiver decompensation
The present invention relates to use of ornithine in the manufacture of a medicament for use in combination with at least one of phenylacetate and phenylbutyrate for preventing or treating liver decompensation or hepatic encephalopathy. The invention also relates to use of at least one of phenylacetate and phenylbutyrate in the manufacture of a medicament for use in combination with ornithine for preventing or treating liver decompensation or hepatic encephalopathy.
Owner:UCL BUSINESS PLC
Remedy for hepatopathy
InactiveUS20040022827A1Satisfactory ameliorating actionBiocideOrganic active ingredientsSide effectRegimen
Compositions that contain valine as an active ingredient but which are entirely free of other amino acids or substantially free of amino other acids as an active ingredient are used as drugs or foods for treating or ameliorating hepatic diseases, whereupon less side effects are caused than in the conventional regimens of pharmacotherapy and yet the compositions ameliorate, palliate or gain recovery from symptoms and abnormalities that are caused by such hepatic diseases, for example, fever, lassitude, loss of appetite, vomiting stomachache, ascites and pleural effusion, or complications of hepatic disease (not including hepatic encephalopathy).
Owner:CHUGAI PHARMA CO LTD
Treatment of hepatic encephalopathy and liver cirrhosis
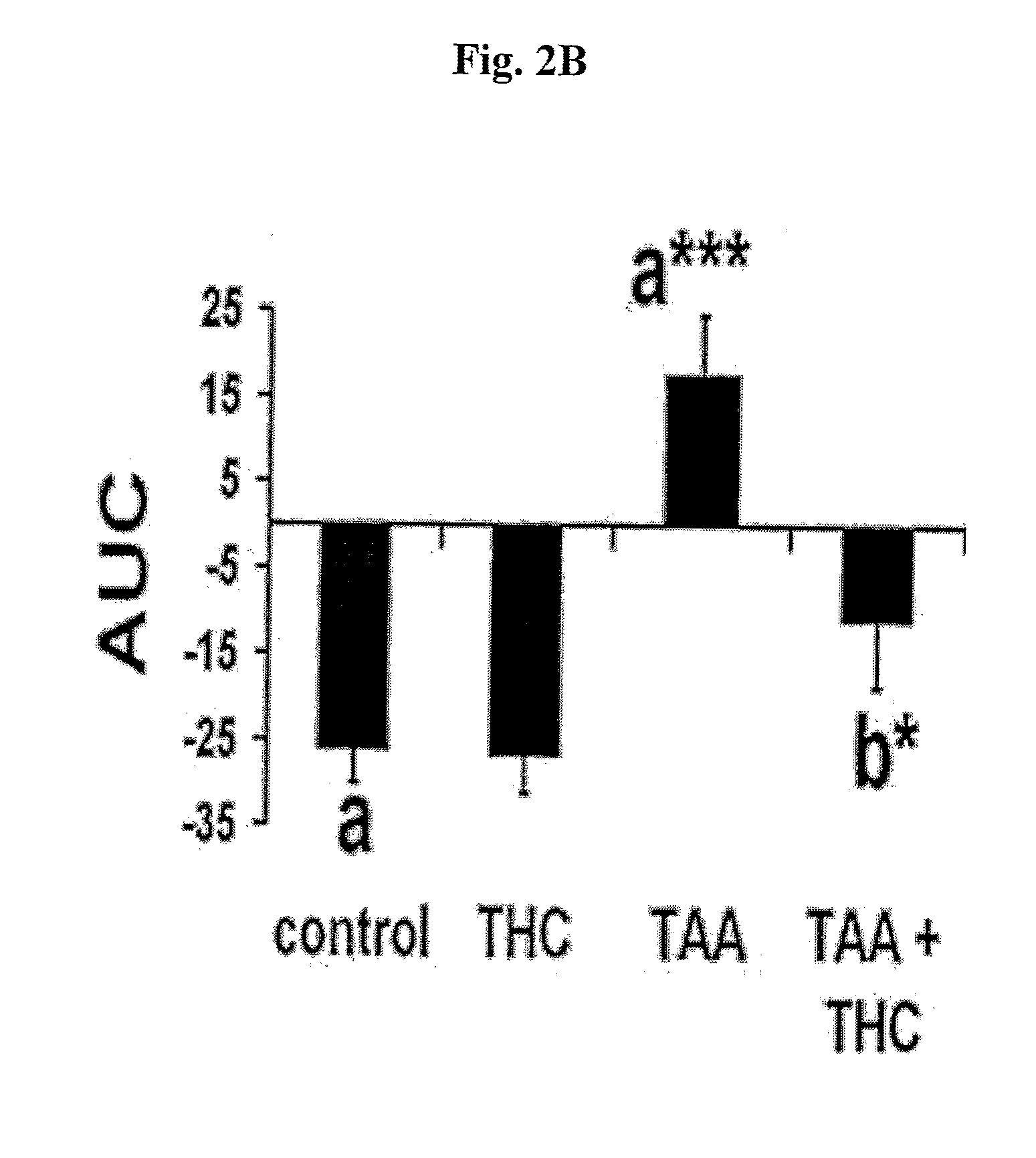
InactiveUS20100022631A1Reduce severityImprove cognitive functionBiocideHydroxy compound active ingredients2-ArachidonoylglycerolCapsaicin
The compounds D9-tetrahydrocannabinol (THC), cannabidiol (CBD) and capsaicin are useful for prevention, treatment, or both, of hepatic encephalopathy. The compounds capsaicin, 2-arachidonoylglycerol (2-AG), HU-308 and cannabidiol are useful for prevention, treatment, or both, of liver cirrhosis.
Owner:YISSUM RES DEV CO OF THE HEBREWUNIVERSITY OF JERUSALEM LTD +2
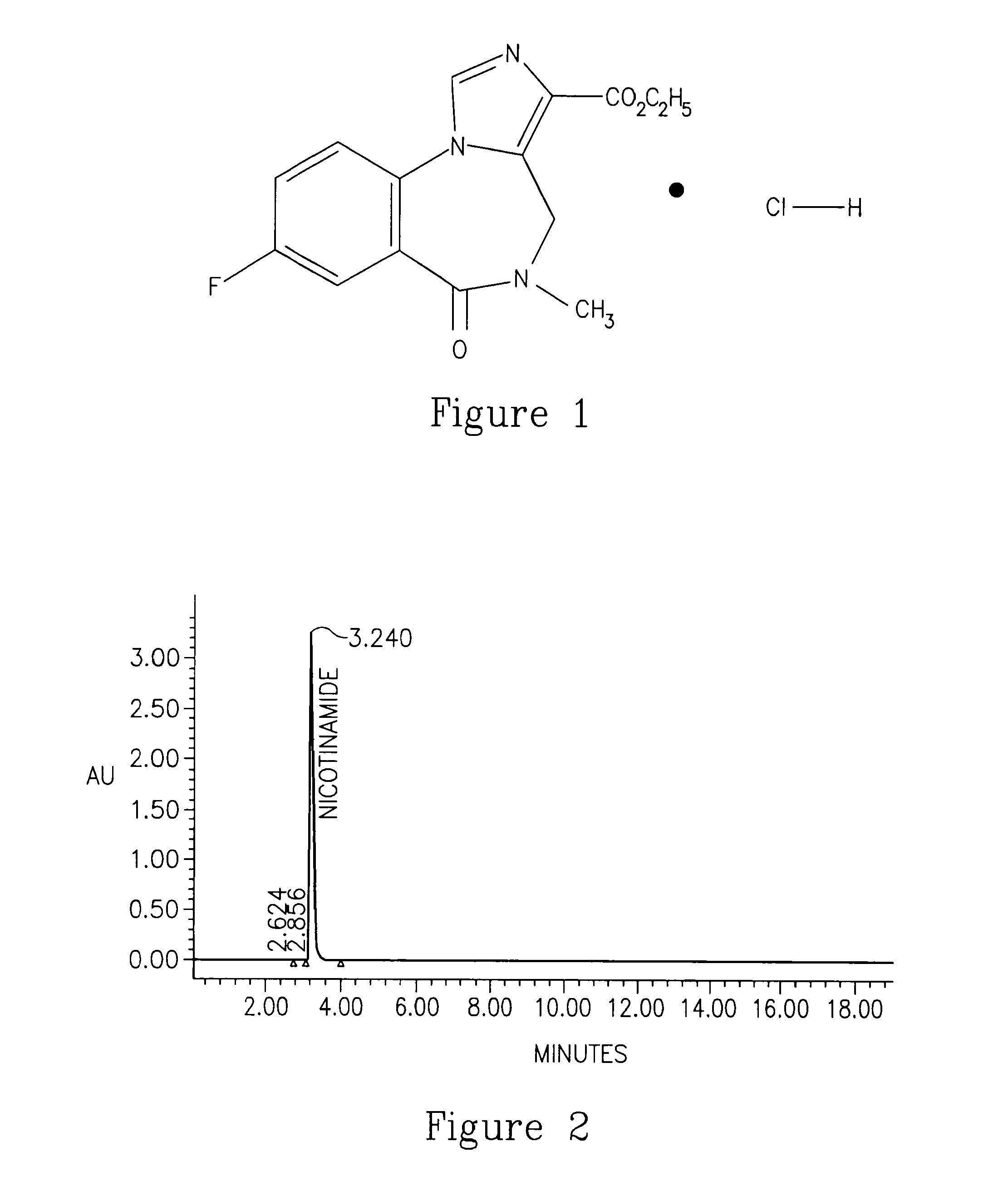
Flumazenil Complexes, Compositions Comprising Same And Uses Thereof
Soluble complexes of flumazenil, methods for the preparation thereof, pharmaceutical compositions including same and use of the compositions for alleviating or counteracting the various types of hypersomnia, drowsiness, residual effects associated with the administration of sleep / hypnotic drugs, alcohol intoxication or hepatic encephalopathy.
Owner:COERULEUS
Ornithine aspartate injection and preparation method thereof
ActiveCN101987094AHigh content of main impuritiesLow main impurity contentOrganic active ingredientsNervous disorderActivated carbonHepatic encephalopathy
The invention relates to an ornithine aspartate injection and a preparation method thereof. The injection contains ornithine aspartate, water for injection and acid-base adjusting agent, and has the pH value of 5.2-6.0 and the concentration of 0.01-1g / mL. The ornithine aspartate injection is prepared by dissolving the ornithine aspartate by the water for injection, adjusting the pH value by the acid-base adjusting agent, discoloring by activated carbon, leading the volume to be constant, split charging and sterilizing. The injection can be used for treating hepatic encephalopathy. The injection provided by the invention has the characteristics of being less in impurity content, easy in control of quality, safer in clinical application and the like.
Owner:SHANGHAI CHENPON PHARM TECH CO LTD
Methods
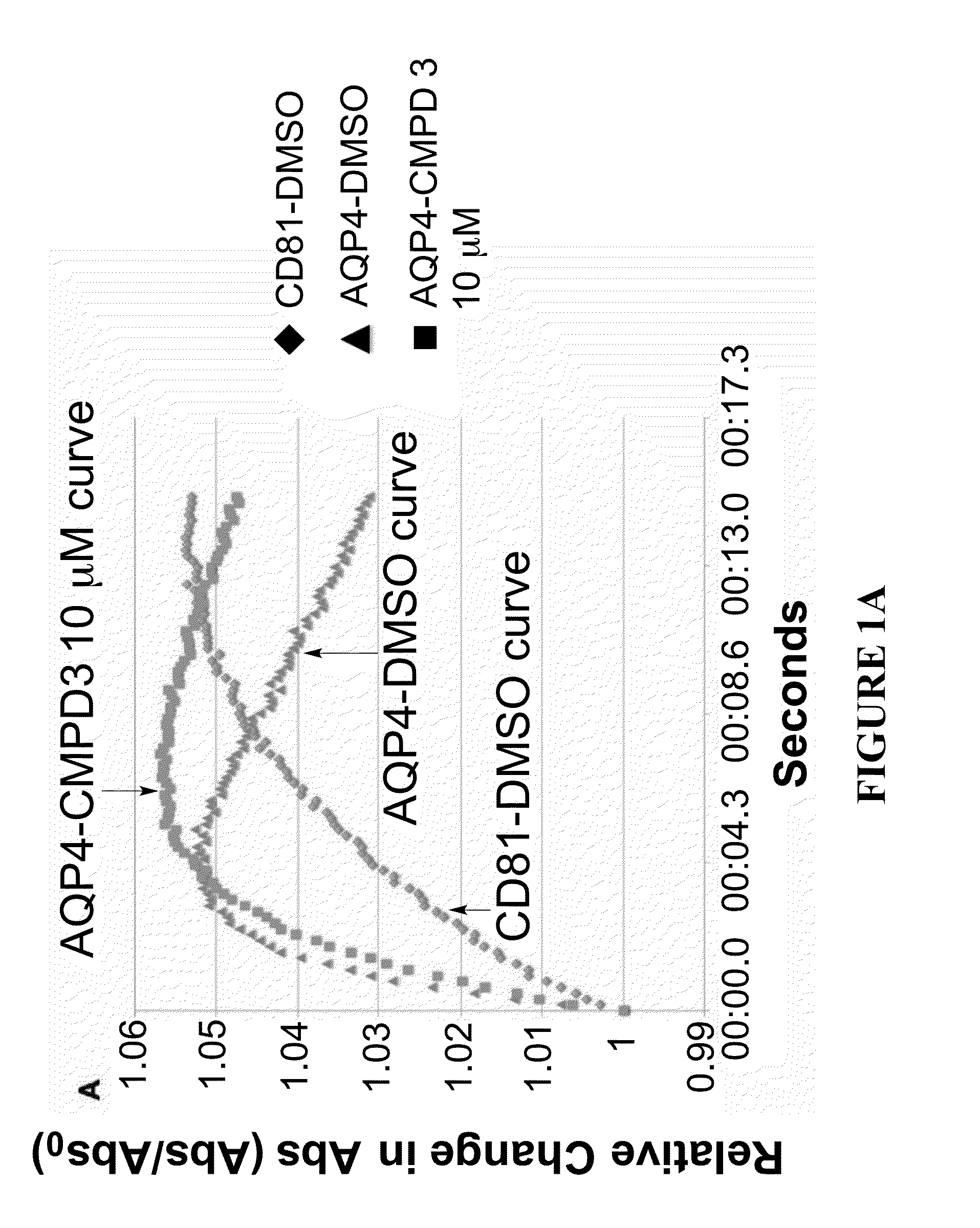
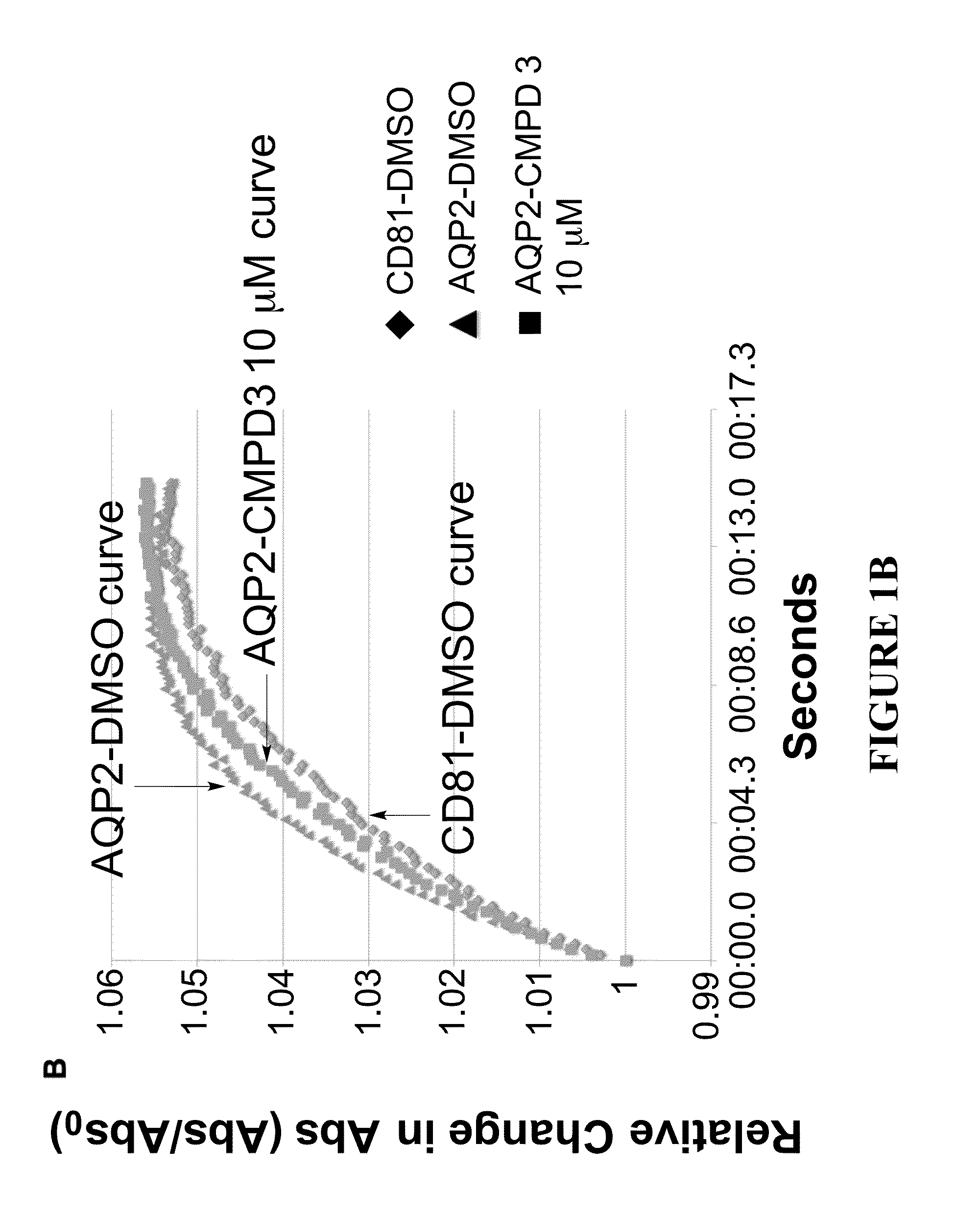
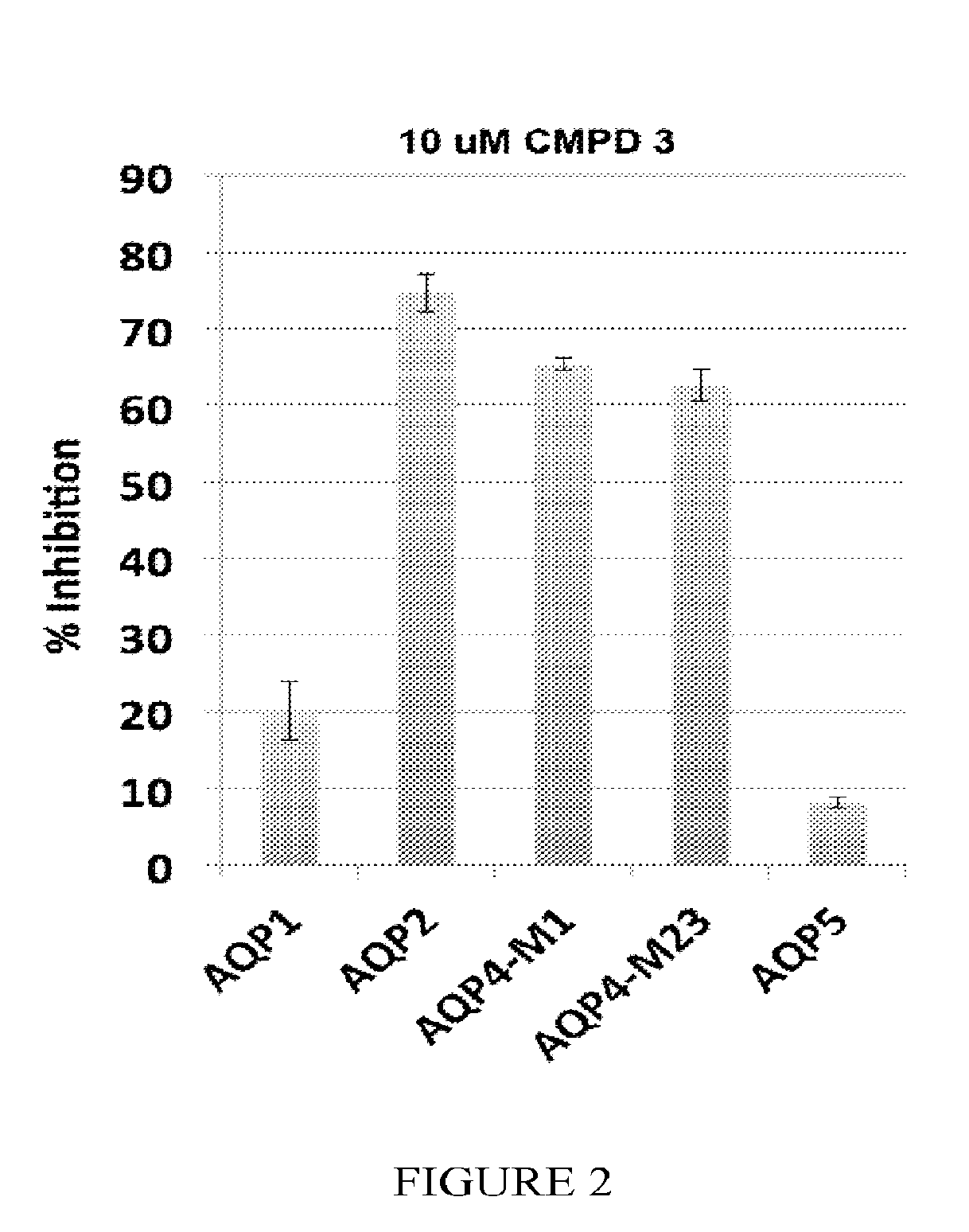
The present invention relates to the use of selective aquaporin inhibitors, e.g., of aquaporin-4 or aquaporin-2, e.g., certain phenylbenzamide compounds, for the prophylaxis, treatment and control of aquaporin-mediated conditions, e.g., diseases of water imbalance, for example edema (particularly edema of the brain and spinal cord, e.g., following trauma or ischemic stroke, as well as the edema associated with glioma, meningitis, acute mountain sickness, epileptic seizures, infections, metabolic disorders, hypoxia, water intoxication, hepatic failure, hepatic encephalopathy, diabetic ketoacidosis, abscess, eclampsia, Creutzfeldt-Jakob disease, and lupus cerebritis, as well as edema consequent to microgravity and / or radiation exposure, as well as edema consequent to invasive central nervous system procedures, e.g., neurosurgery, endovascular clot removal, spinal tap, aneurysm repair, or deep brain stimulation, as well as retinal edema), as well as hyponatremia and excess fluid retention, and diseases such as epilepsy, retinal ischemia and other diseases of the eye associated with abnormalities in intraocular pressure and / or tissue hydration, myocardial ischemia, myocardial ischemia / reperfusion injury, myocardial infarction, myocardial hypoxia, congestive heart failure, sepsis, and neuromyelitis optica, as well as migraines, as well as to novel assays for identifying aquaporin inhibitors.
Owner:AEROMICS
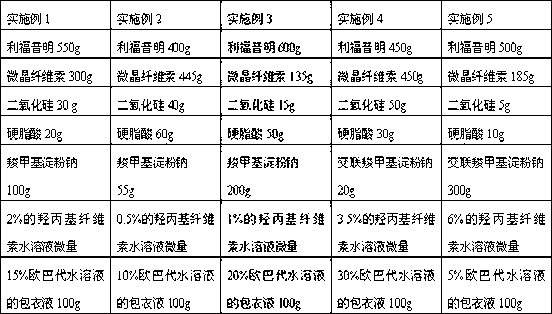
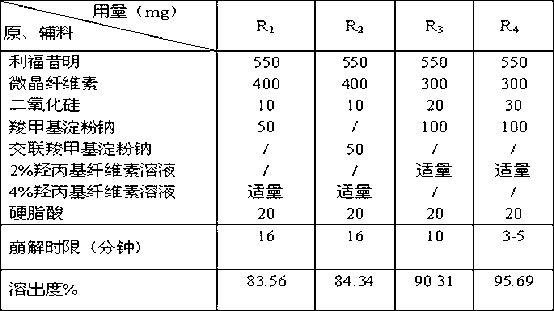
Rifaximin medicine composition and preparation method thereof
InactiveCN103340856ARapid dissolutionEvenly dispersedOrganic active ingredientsNervous disorderMedicineHepatic encephalopathy
The invention discloses a rifaximin medicine composition and a preparation method of the rifaximin medicine composition. The rifaximin medicine composition comprises the following ingredients in percentages by mass: 40-60% of rifaximin, 10-50% of microcrystalline cellulose, 1-30% of sodium carboxymethyl starch or sodium carboxymethyl starch, 0.5-6% of silicon dioxide, 0.5-6% of stearic acid and a little amount of aqueous solution of hydroxy propyl cellulose with the mass concentration of 0.5-6%. The medicine composition can be made into tablets and capsules, each dose comprises 550mg of rifaximin respectively, and a coating solution for the preparation is an aqueous solution of opadry with the mass concentration of 5-30%. The invention also provides the preparation method of the rifaximin medicine composition. According to the rifaximin medicine composition and the preparation method of the rifaximin medicine composition disclosed by the invention, the preparation of the medicine composition can be rapidly disintegrated to fully take medicine efficacy, so as to improve the health of a patient suffering from liver cirrhosis recurrent hepatic encephalopathy.
Owner:WORLDCO INT
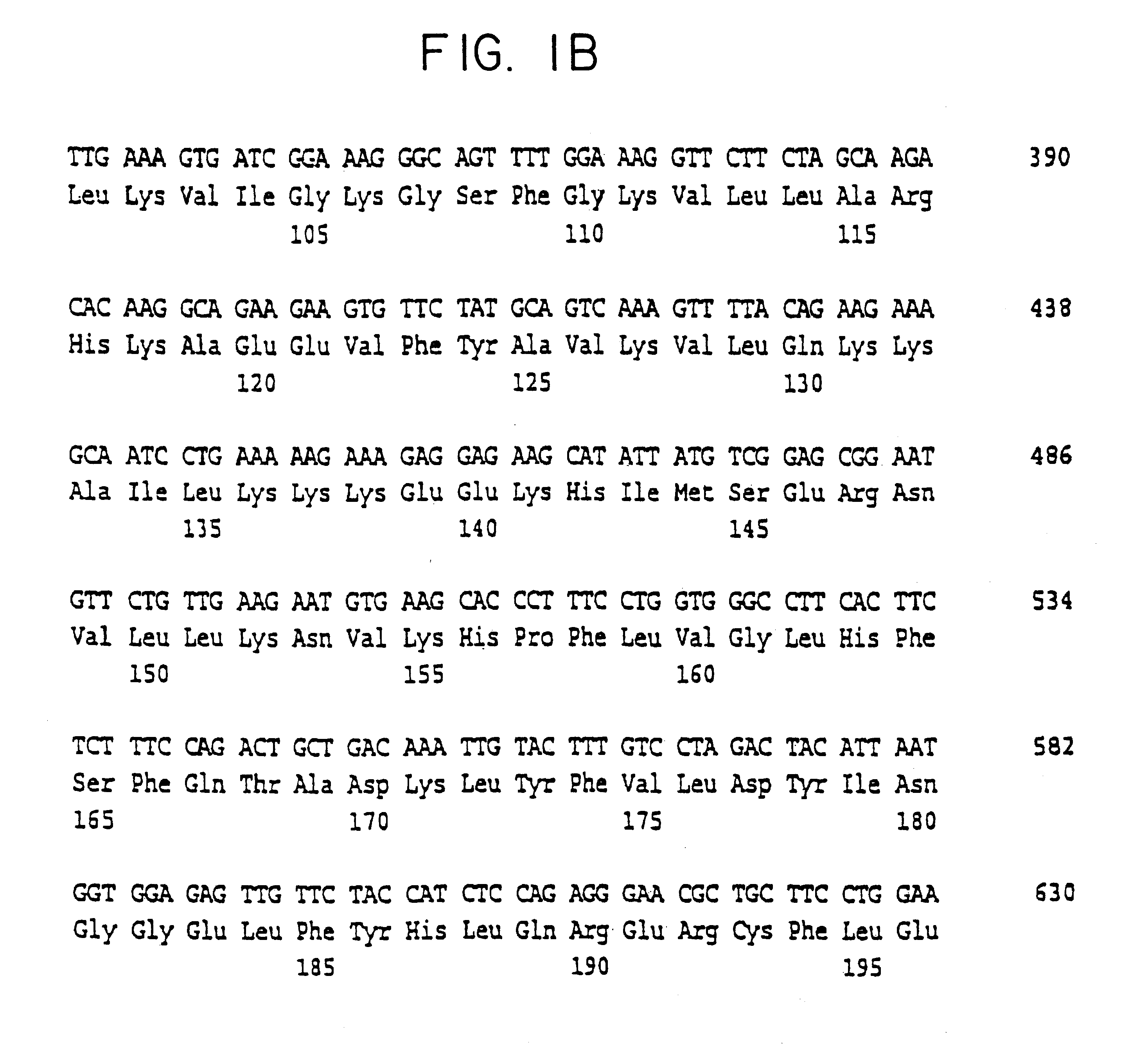
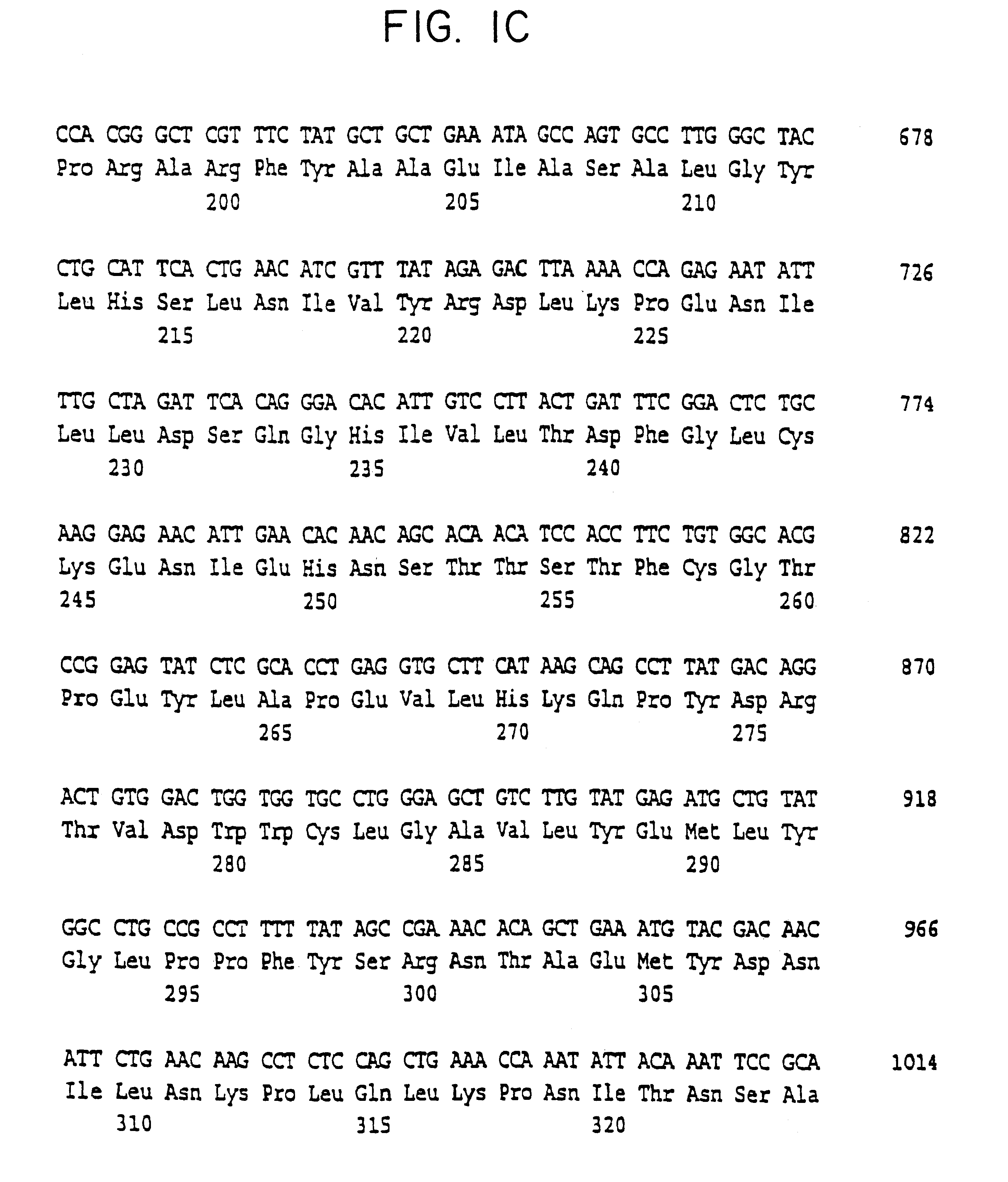
Cell volume-regulated human kinase h-sgk
InactiveUS6326181B1Cell receptors/surface-antigens/surface-determinantsPeptide/protein ingredientsDecreased sodiumHypernatremia
The present invention relates to the cloning and characterization of a human serine / threonine kinase (h-sgk: serum and glucocorticoid dependent kinase). The invention furthermore relates to reagents for diagnosing conditions associated with a change in cell volume and / or in "macromolecular crowding" in the body, such as, for example, hypernatremia, hyponatremia, diabetes mellitus, renal failure, hypercatabolism, hepatic encephalopathy, inflammation and microbial or viral infections. The present invention additionally relates to pharmaceuticals comprising the h-sgk, nucleic acids which code for the h-sgk, or receptors, in particular antibodies, which specifically bind to the h-sgk.
Owner:WALDEGGER SIEGFRIED +1
Drug for treatment of blood glucose, dyslipidemia and neurodegenerative diseases and preparation of drug
PendingCN109364084AAchieve healingInhibits receptor FXR activityNervous disorderHydroxy compound active ingredientsDyslipidemiaChenodeoxycholic acid
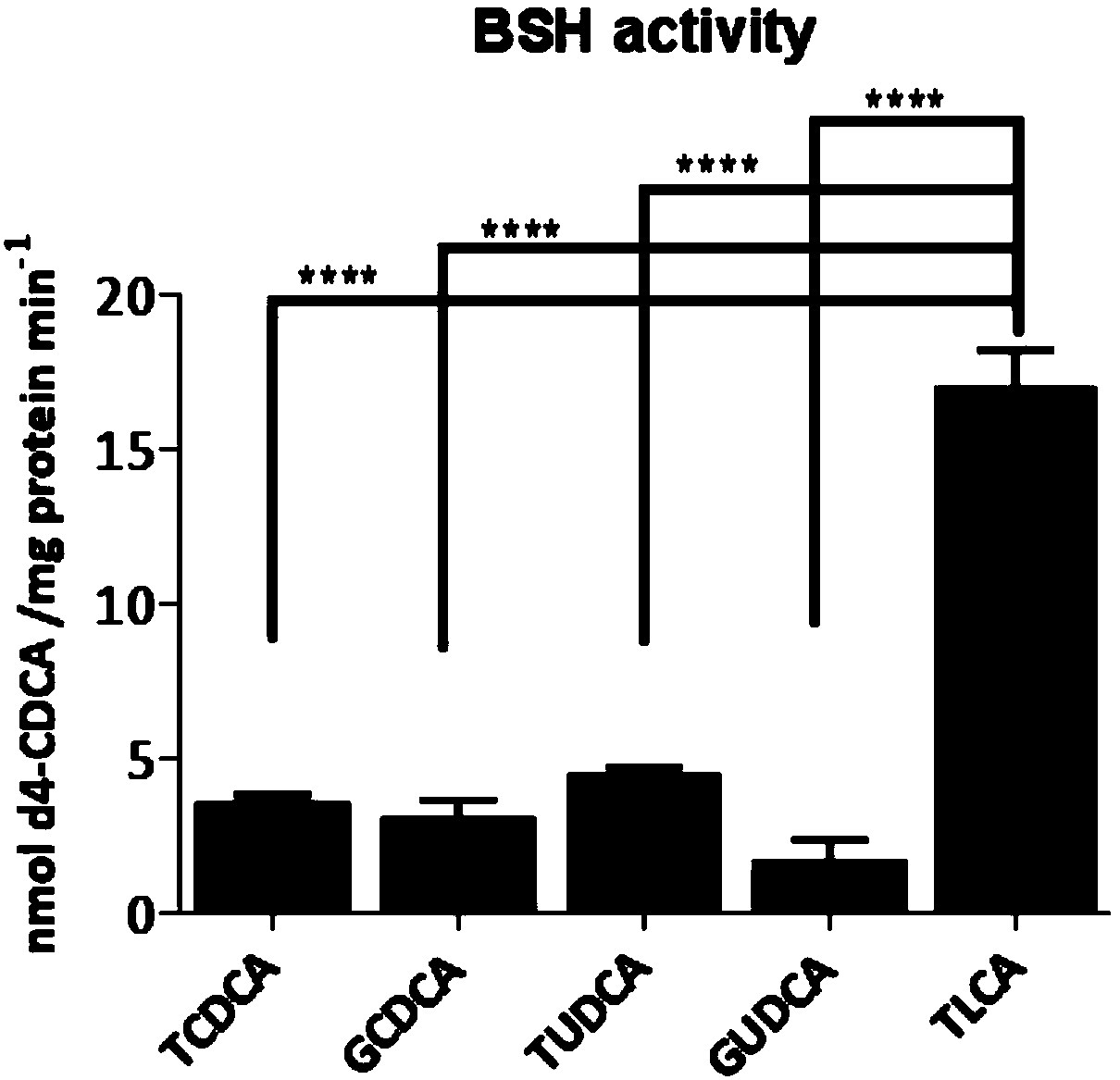
The invention provides a drug for treatment of blood glucose, dyslipidemia and neurodegenerative diseases and a preparation of the drug. The drug is characterized in that the drug inhibits the activity of an intestinal bacterial BSH enzyme and increases the level of conjugated bile acid in anthe intestinal tract, thus the activity of FXR of bile acid receptors FXR is inhibited, synthesis of liverbile acid is improved, and the level of liver cholesterol and triglyceride is decreased; and the drug comprises amide derivatives form by carboxyl in ursodesoxycholic acid (UDCA) or chenodeoxycholic acid (CDCA) steroid stem nucleus and amidogen in other amino acids in addition except for glycine and taurine, and thus blood glucose, dyslipidemia, fatty liver, hepatic encephalopathy and neurodegenerative diseases such as senile dementia can be treated.
Owner:SHENZHEN YUNHE PHARM TECH PARTNERSHIP LTD
Composition and method for treatment of hepatic encephalopathy
InactiveUS20070269403A1Reduce ammonia levelsReduce constipationDigestive systemSynthetic polymeric active ingredientsSide effectPolyethylene glycol
The inventions provide an improved treatment for hepatic encephalopathy characterized by hyperammonemia and / or constipation, comprising the oral administration of polyethylene glycol (PEG) in amounts sufficient to reduce plasma levels of ammonia and / or to alleviate constipation. Preferably, the PEG is administered in combination with lactulose, which provides a palatable composition for the treatment of HE with excellent therapeutic benefits and reduced side effects as compared to lactulose alone.
Owner:HALOW GEORGE M
Application of bifidobacterium lactis in prevention and treatment of mental disorder
PendingCN112956696AImprove permeabilityLower LPSMilk preparationNervous disorderBiotechnologyDisease
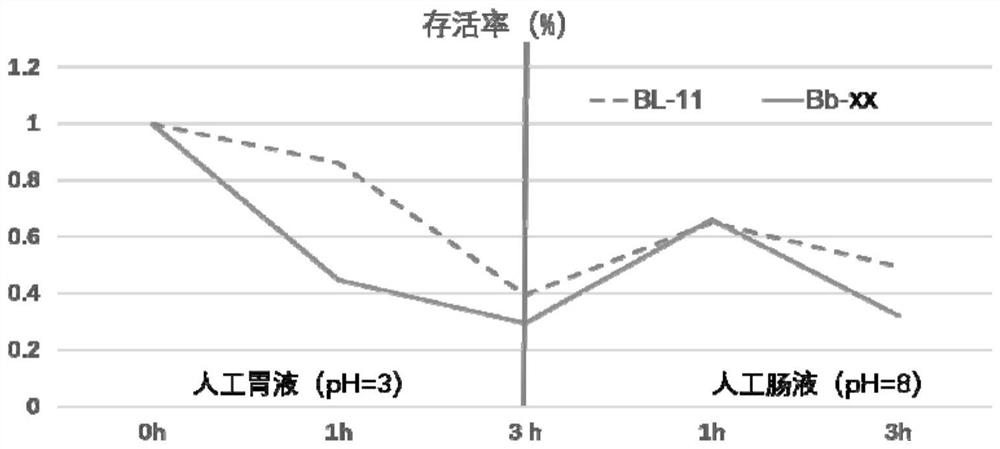
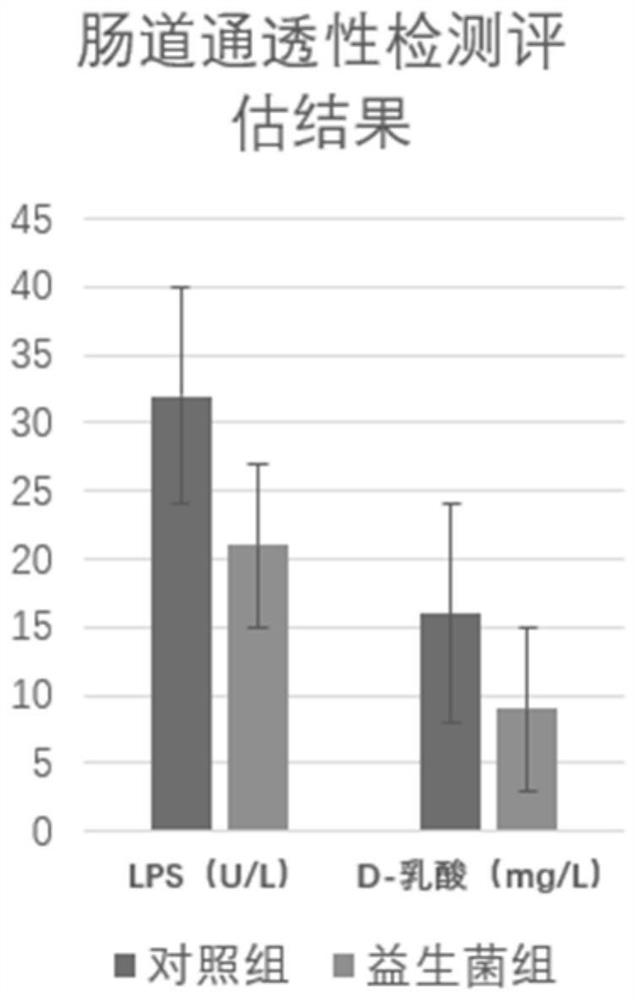
The invention relates to application of bifidobacterium lactis in prevention and treatment of mental disorder, the bifidobacterium lactis is bifidobacterium lactis BL-11, and the preservation number of the bifidobacterium lactis BL-11 is CGMCC (China General Microbiological Culture Collection Center) No.20847. The bifidobacterium lactis provided by the invention can be used for improving the intestinal permeability and reducing the levels of LPS and D-lactic acid in blood, so that the effect of preventing and treating mental disorders is achieved, and the mental disorders comprise anxiety, depression, attention deficit hyperactivity disorder, autism, autism, schizophrenia, hepatic encephalopathy, anorexia nervosa, Tourette disease and Escherberg syndrome.
Owner:ZHONGKE WISBIOM(BEIJING)BIOTECHNOLOGY CO LTD
Methods of treating hepatorenal syndrome and hepatic encephalopathy with thromboxane-a2 receptor antagonists
InactiveUS20130197044A1Avoid failureSpeed up the flowBiocideNervous disorderThromboxane A2 receptorNK1 receptor antagonist
The present invention is directed to methods of treating hepatorenal syndrome by administration of a therapeutically effective amount of a thromboxane A2 receptor antagonist to a patient in need thereof. The present invention is also directed to methods of treating hepatic encephalopathy and cerebral edema by administration of a therapeutically effective amount of a thromboxane A2 receptor antagonist to a patient in need thereof.
Owner:CUMBERLAND EMERGING TECH
Compound containing structure of o-naphthaquinone and application
InactiveCN1660833AHigh speedImprove convenienceOrganic active ingredientsNervous disorderQuinoneHyperammonemic encephalopathy
A compound containing o-naphthalene quinone structure for preparing medicines to prevent and treat hyperammonemia and hepatic encephalopathy is prepared from natural Masonate F through structure reformation and optimization.
Owner:SUN YAT SEN UNIV
New use of rifamycin-nitroimidazole coupling molecule
InactiveCN106822119AHigh antibacterial activityLow frequency of drug resistanceAntibacterial agentsOrganic active ingredientsNitroimidazoleAnaerobic bacteria
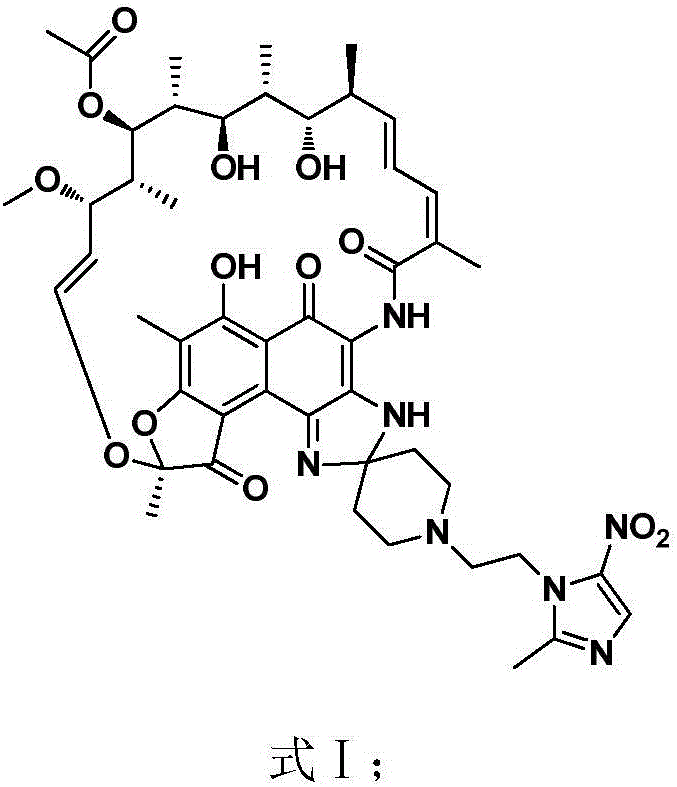
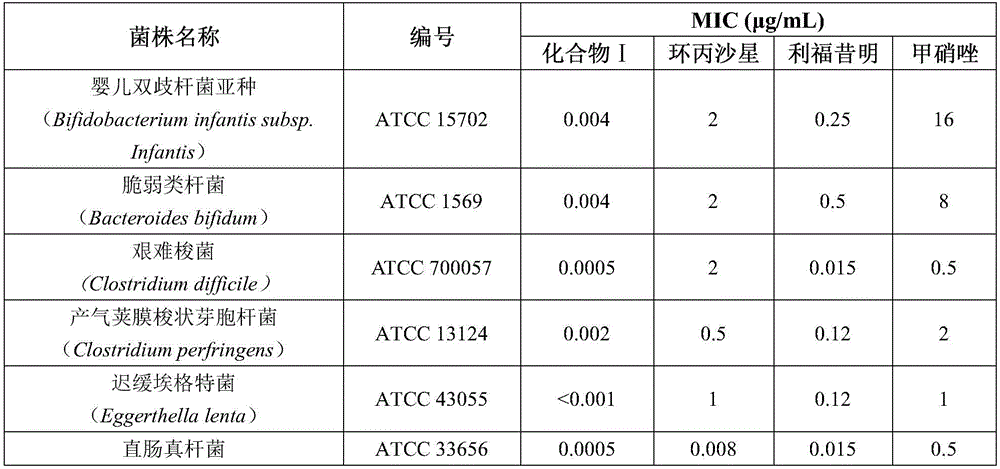
The invention discloses application of a rifamycin-nitroimidazole coupling molecule represented in a formula I in inhibition of anaerobic or facultative anaerobic ammonia producing floras in gastrointestinal tracts. The rifamycin-nitroimidazole coupling molecule represented in the formula I is similar to an antibacterial spectrum of rifaximin and has relatively strong antibacterial activity to common ammonia producing floras in the gastrointestinal tracts; and meanwhile, the rifamycin-quinolizidone dual-target molecule has the property of low spontaneous drug resistance frequency and has very good application prospects in prevention and treatment of infection of hepatic encephalopathy and relevant anaerobic bacteria. The formula is as shown in the specification.
Owner:TENNOR THERAPEUTICS (SUZHOU) LTD
Compound amino acid injection for treating hepatic encephalopathy
ActiveCN101332209BIncrease parts by weightCorrect anomaliesOrganic active ingredientsNervous disorderSulfur amino acidBlood ammonia
The present invention provides a compound amino acid injection with high Fischer rate for curing hepatic encephalopathy, which can rapidly lower the blood ammonia density. The present invention increases the weight parts of three branched amino acid and arginine and reduces the weight parts of sulfur amino acid and ammonia amino acid. The injection provided by the present invention is more conducive to regulating the plasma amino acid spectrum to be normal, increases the density of arginine, stably lowers the level of blood ammonia of the patient and improves the immunity of the organism, thus achieving the aim of rapidly improving the symptom of hepatic encephalopathy and replenishing nutrition. Therefore, the injection of the present invention has higher curing effect in improving hepatic encephalopathy.
Owner:MITSUBISHI PHARMA GUANGZHOU
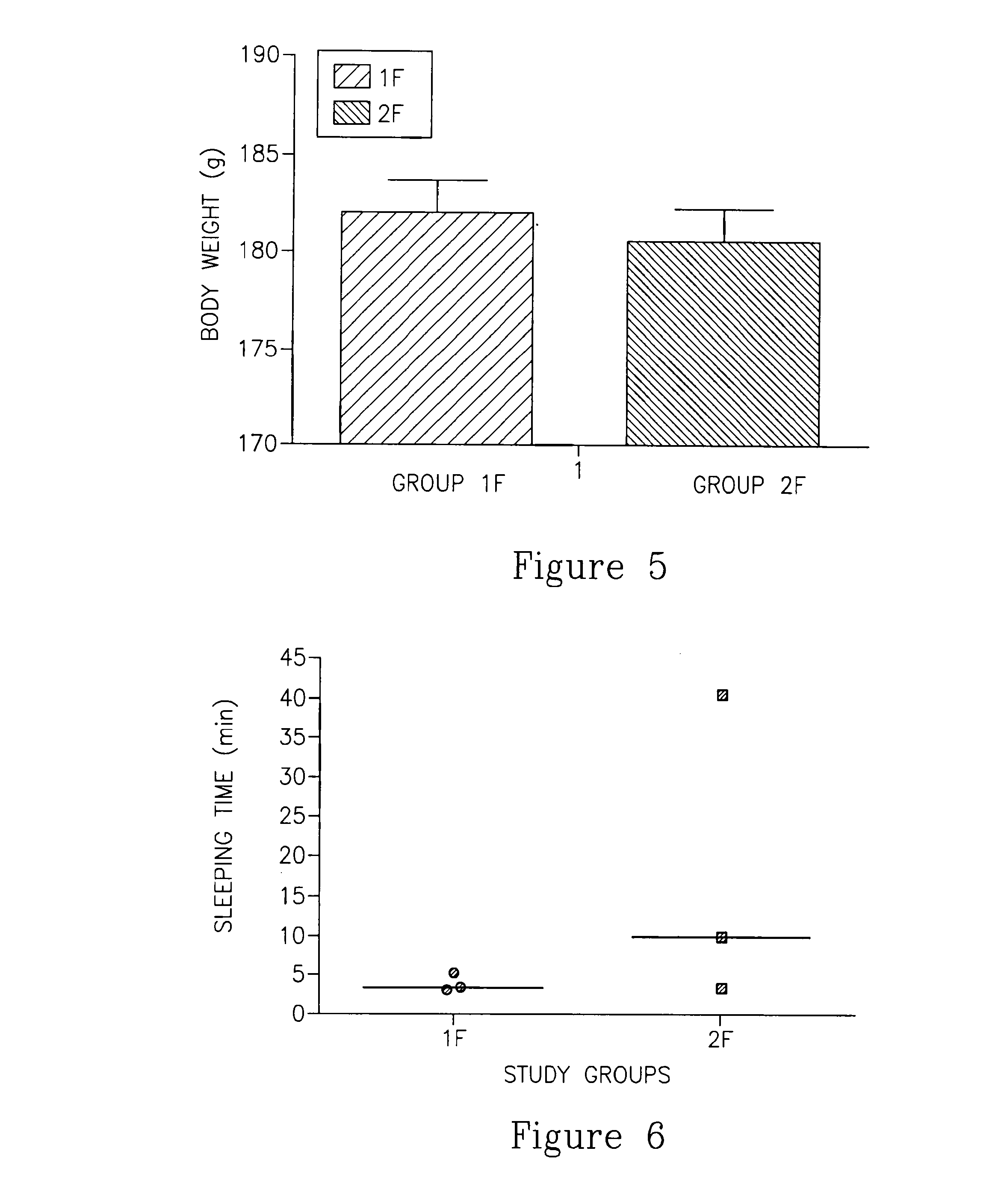
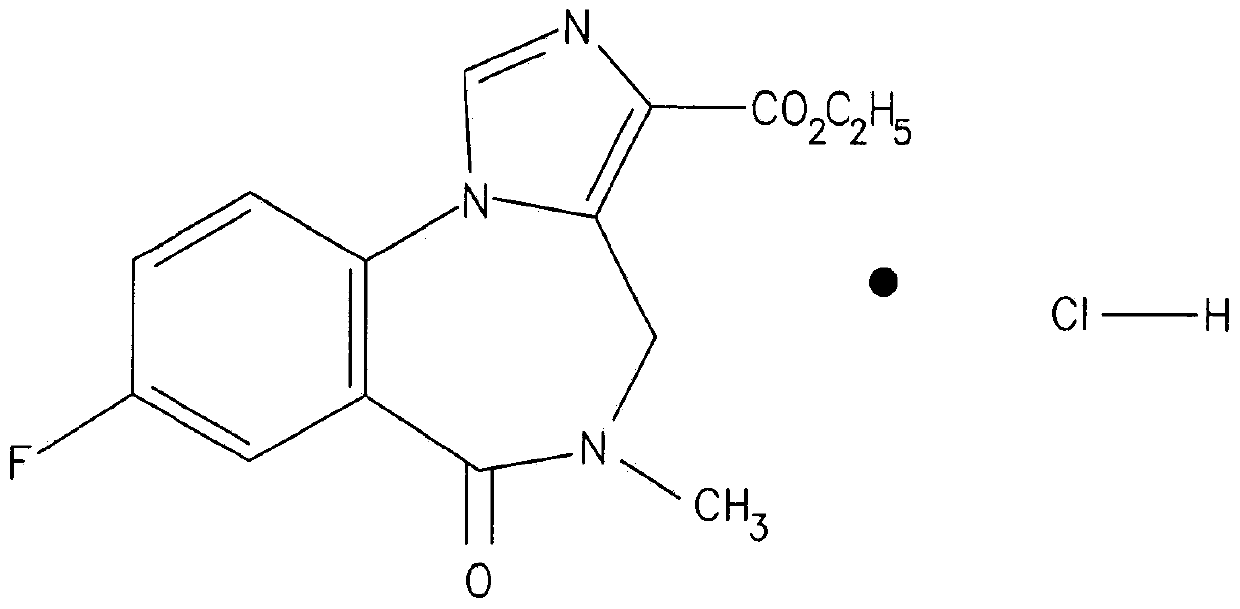
Flumazenil complexes, compositions comprising same and uses thereof
InactiveCN103502224AShow sleep timeOrganic active ingredientsNervous disorderFlumazenilHepatic encephalopathy
Soluble complexes of flumazenil, methods for the preparation thereof, pharmaceutical compositions including same and use of the compositions for alleviating or counteracting the various types of hypersomnia, drowsiness, residual effects associated with the administration of sleep / hypnotic drugs, alcohol intoxication or hepatic encephalopathy.
Owner:COERULEUS
New use of rifamycin-quinolizidone dual-target molecule
InactiveCN106822125AHigh antibacterial activityLow frequency of drug resistanceAntibacterial agentsOrganic active ingredientsAntibacterial activityHepatic encephalopathy
The invention discloses application of a rifamycin-quinolizidone dual-target molecule represented in a formula I in inhibition of ammonia producing floras in gastrointestinal tracts. The rifamycin-quinolizidone dual-target molecule represented in the formula I is similar to an antibacterial spectrum of rifaximin and has relatively strong antibacterial activity to common ammonia producing floras in the gastrointestinal tracts; and meanwhile, the rifamycin-quinolizidone dual-target molecule has the property of low drug resistance frequency and has application prospects in prevention and treatment of infection of hepatic encephalopathy and relevant bacterial genera (types). The formula I is as shown in the specification.
Owner:TENNOR THERAPEUTICS (SUZHOU) LTD
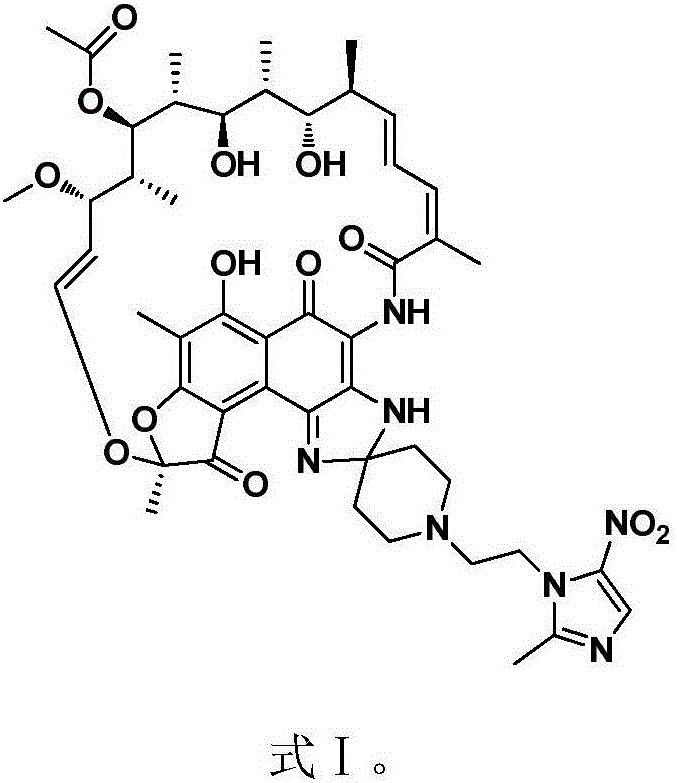
Intestinal environment-improving agent
InactiveCN107519161AExcellent intestinal environment improvement effectSignificant improvementOrganic active ingredientsNervous disorderDiseaseUremia
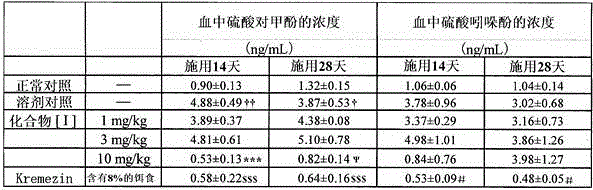
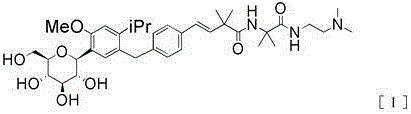
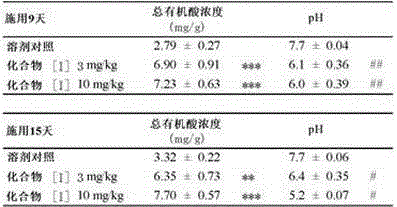
Provided are an intestinal environment-improving agent containing as an active ingredient a compound represented by formula [I] or a pharmaceutically acceptable salt thereof, and a novel medicine useful in the prevention or treatment of conditions such as hepatic encephalopathy and uremia.
Owner:TAISHO PHARMACEUTICAL CO LTD
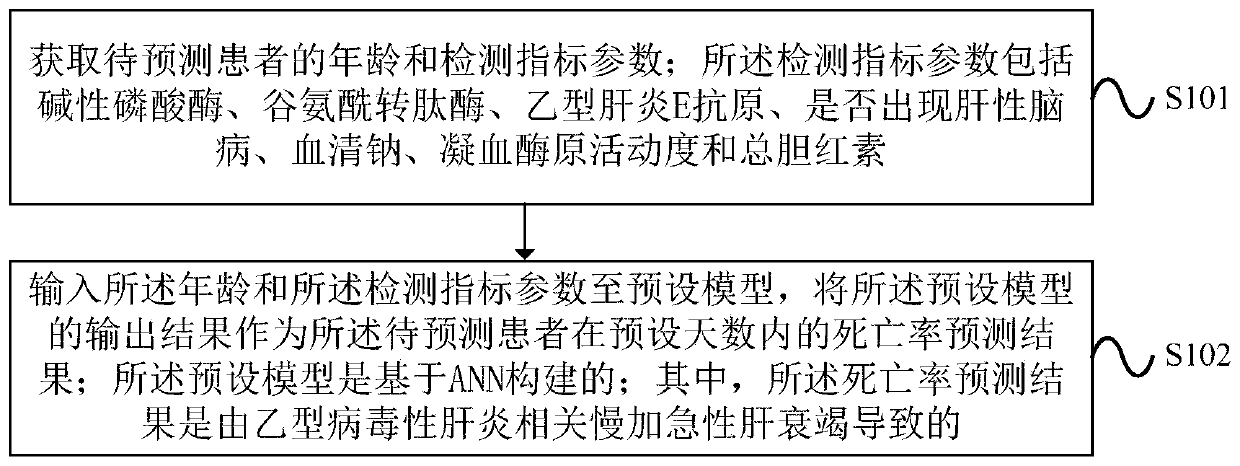
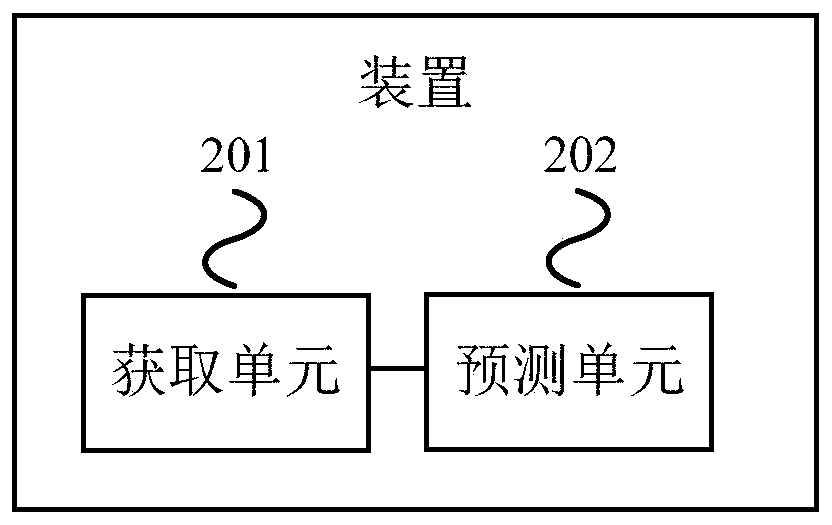
Method and device for processing mortality prediction
PendingCN109859834AImprove forecast accuracyMedical automated diagnosisHepatitis b e antigenAlgorithm
Embodiments of the invention provide a method and device for processing mortality prediction. The method includes the steps: obtaining the age and detection index parameters of a patient to be predicted, wherein the detection index parameters comprises alkaline phosphatase, glutamyl transpeptidase, and hepatitis B E antigen, whether hepatic encephalopathy occurs, serum sodium, blood coagulation, zymogen activity and the total bilirubin; and inputting the age and the detection index parameter to a preset model; inputting the age and the detection index parameters to a preset model, and taking the output result of the preset model as the mortality prediction result of the patient to be predicted within a preset number of days, wherein the preset model is built based on ANN. The device executes the above method. The method and device for processing mortality prediction can improve the prediction accuracy for mortality, caused by acute-on-chronic liver failure related to viral hepatitis B,of a patient within the preset number of days by taking the output result of the preset model constructed based on ANN as the prediction result of the mortality of the patient to be predicted withinthe preset number of days.
Owner:BEIJING DITAN HOSPITAL CAPITAL MEDICAL UNIV
Amino acid-containing albumin preparation
InactiveUS6867193B1Good treatment effectReduce imbalancePeptide/protein ingredientsMetabolism disorderTyrosineHepatic encephalopathy
An albumin preparation that prevents onset of hepatic encephalopathy caused by conventional amino acid preparations and enhances an effect of improving the symptoms is provided. The albumin preparation containing amino acids is characterized in that a content of albumin is 0.01 to 1.0 w / v %, a content of plurality of amino acids containing branched amino acid is 5 to 10 w / v %, a content of the branched amino acids is equal to or more than 30 w / w % on the basis of the content of total amino acids and further, the Fischer ratio (branched amino acid / [phenylalanine+tyrosine] (molar ratio)) is equal to or more than 20.
Owner:NIPRO CORP
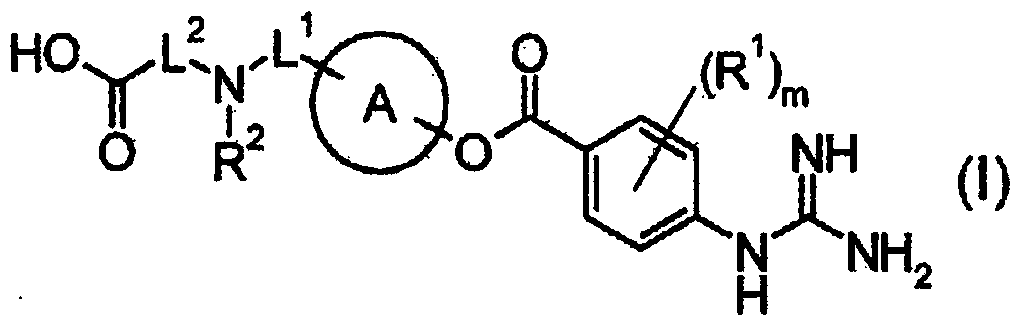
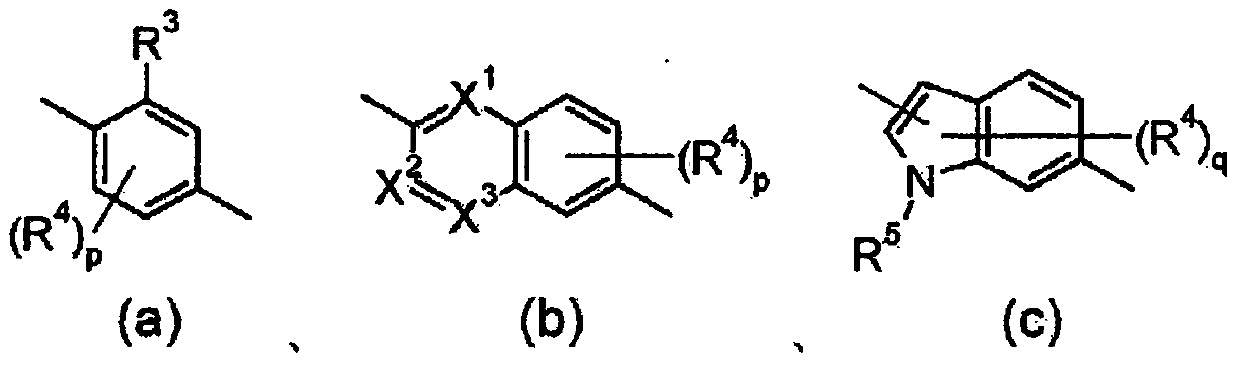
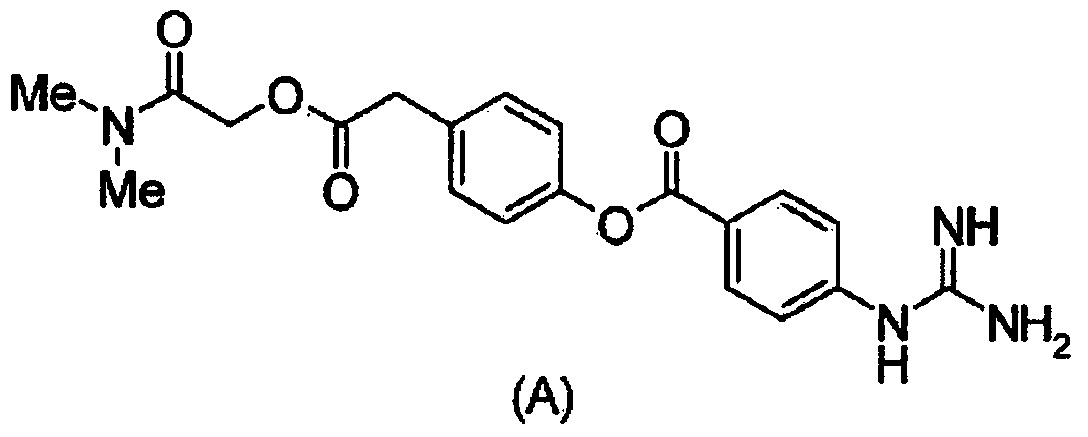
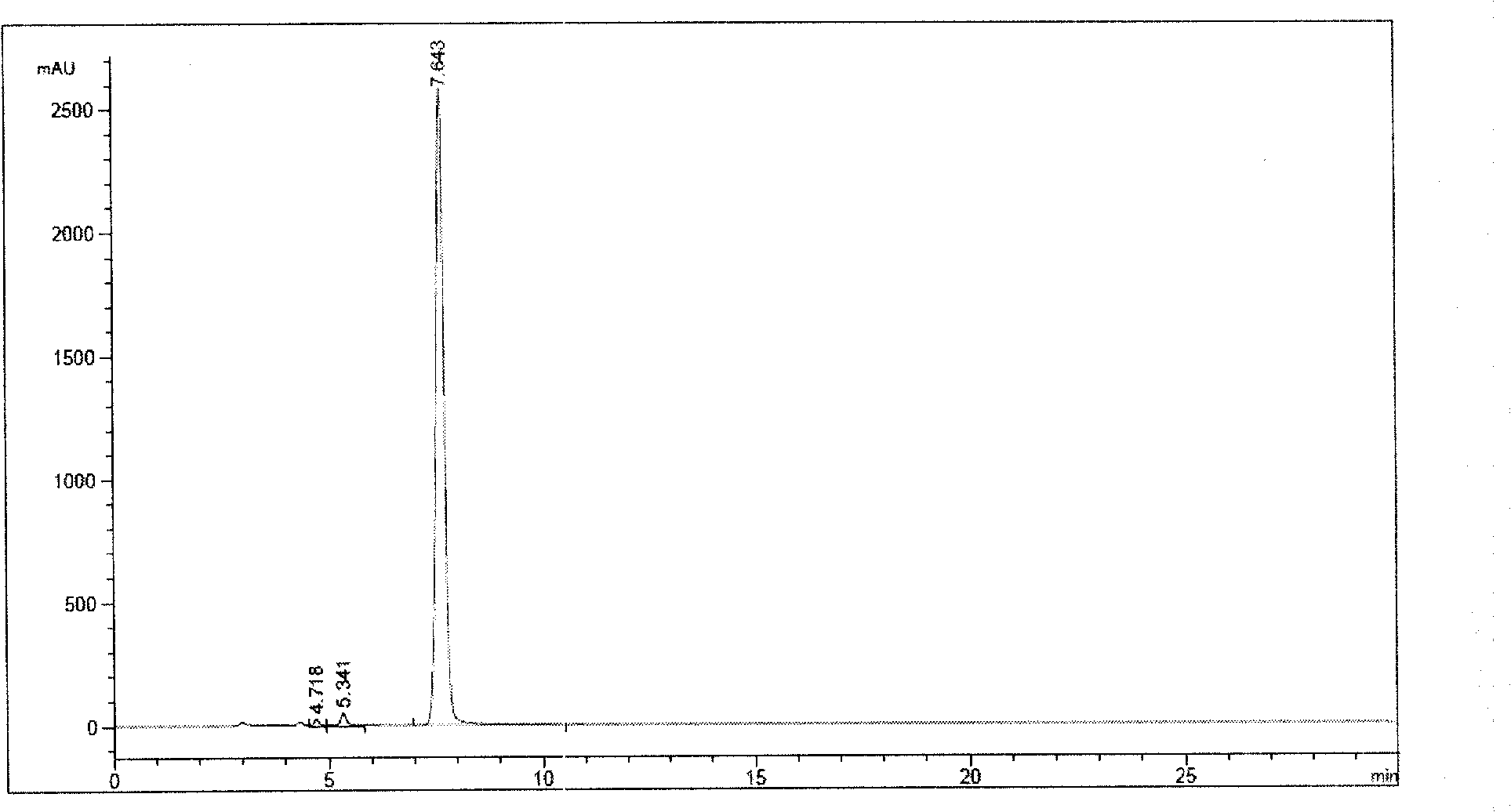
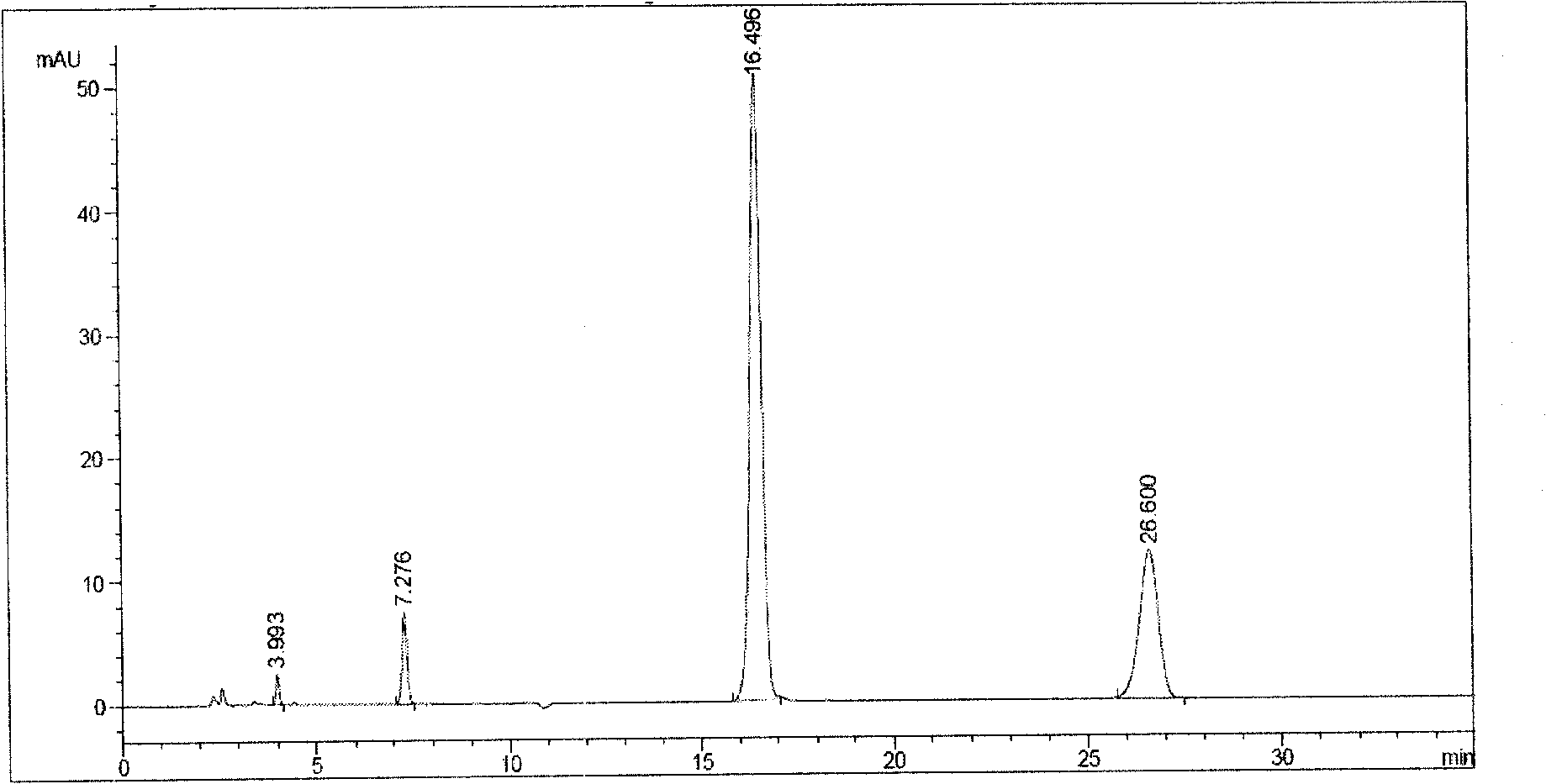
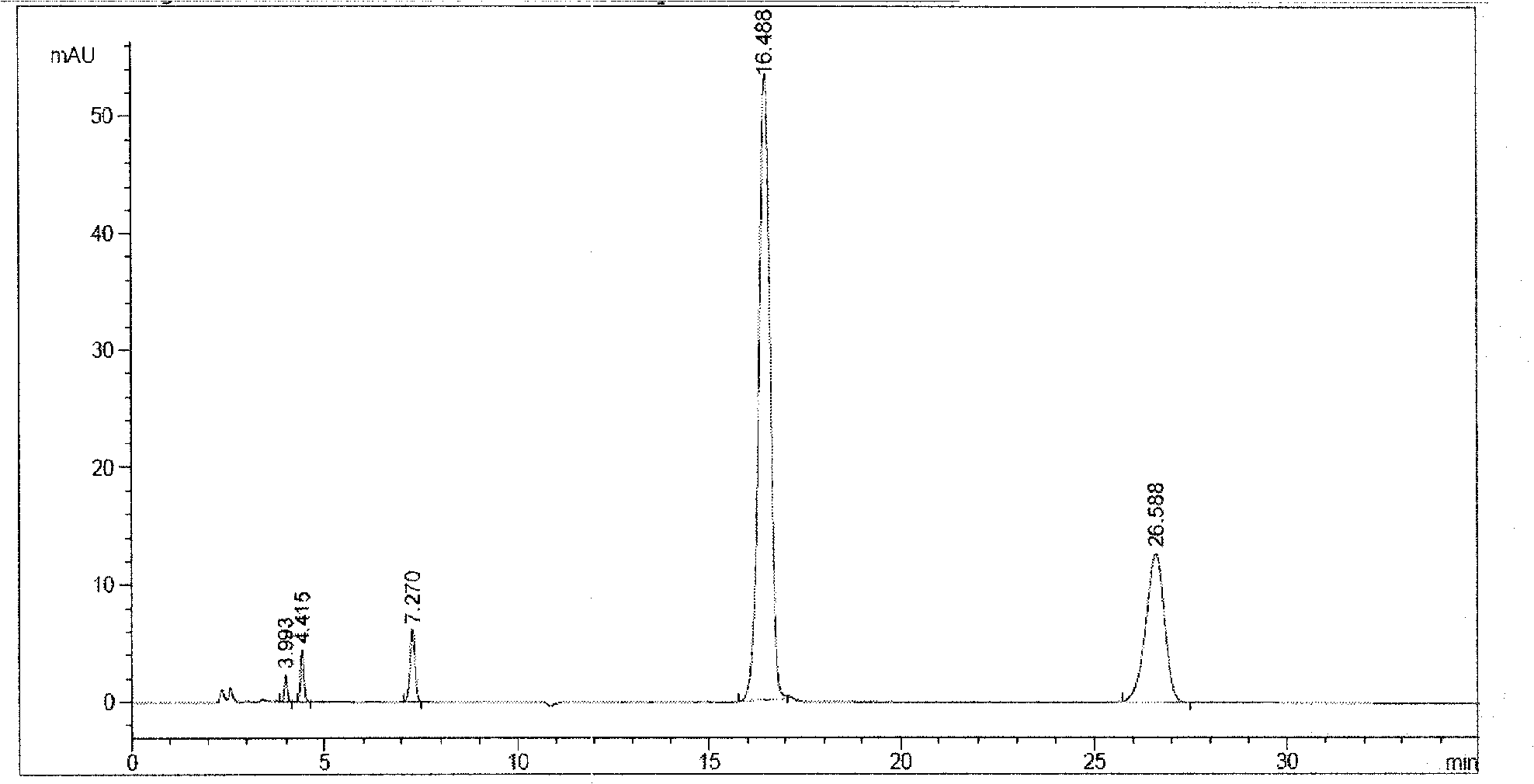
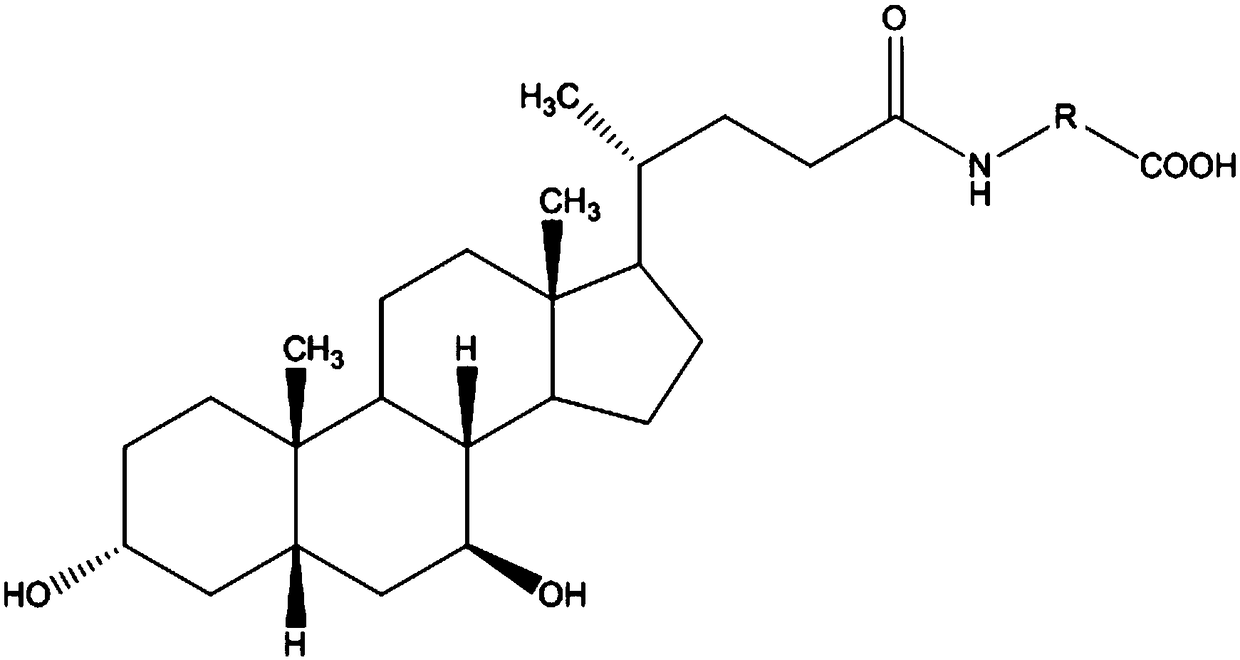
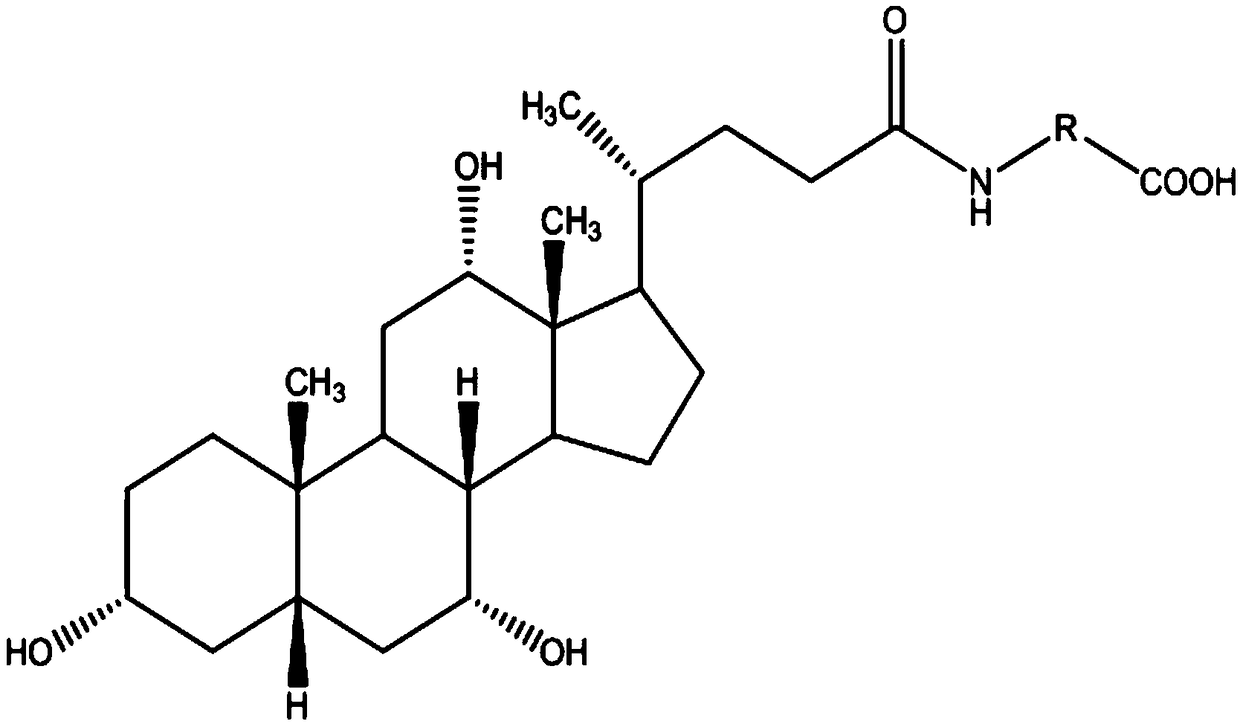
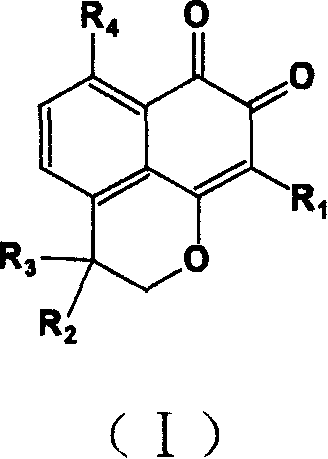
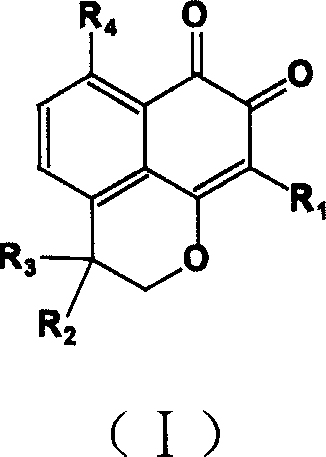
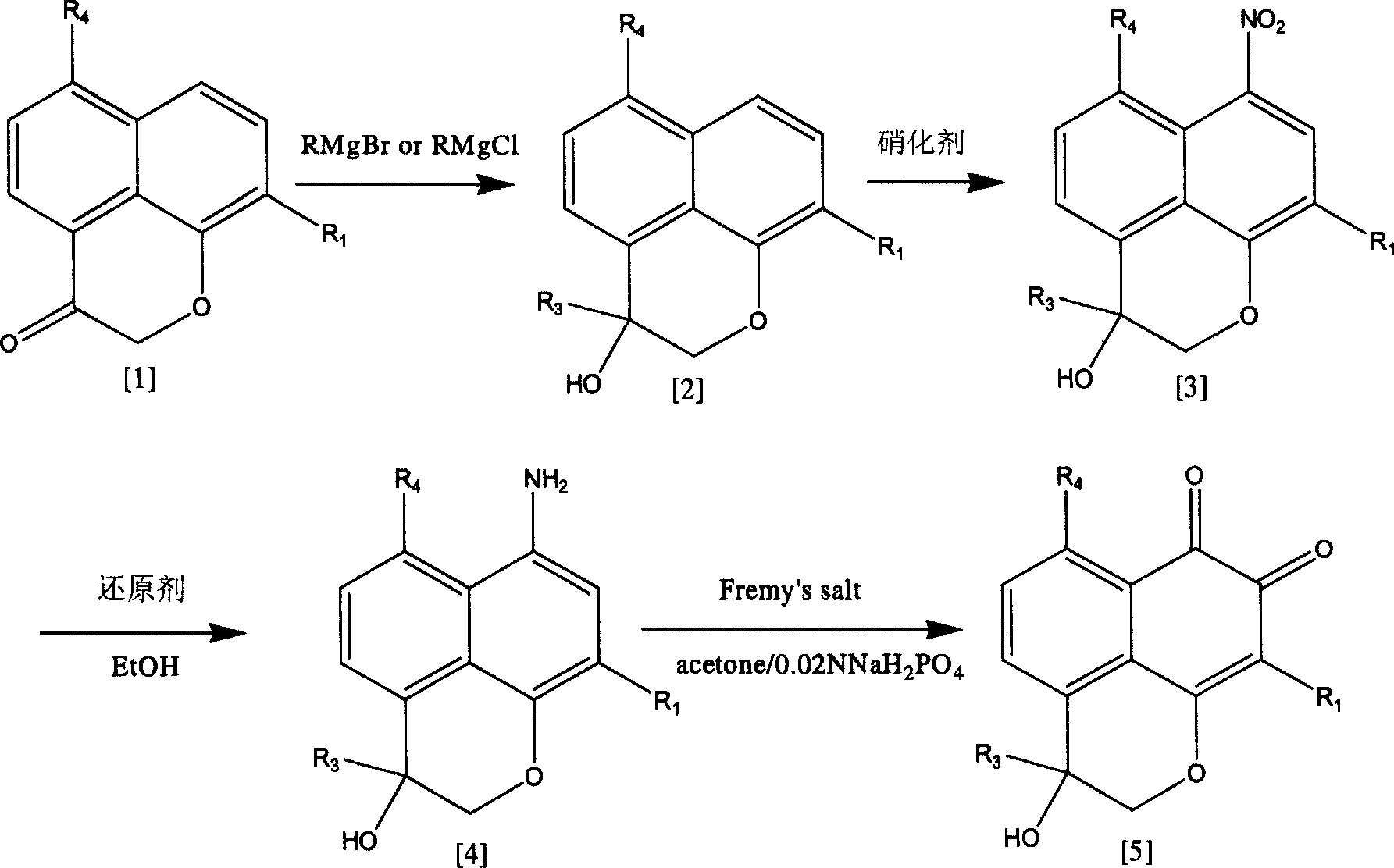
3-(5-chloro-2-oxobenzo[d]oxazol-3(2H)-yl) propanoic acid derivatives as kmo inhibitors
InactiveUS20160318884A1Antibacterial agentsOrganic active ingredientsAIDS dementia complexNeonatal respiratory distress syndrome
A compound of formula (I) or a salt thereof are provided:wherein R1, X and R3 are defined in the specification, useful in the treatment of disorders mediated by KMO such as acute pancreatitis, chronic kidney disease, other conditions associated with systemic inflammatory response syndrome (SIRS), Huntington's disease, Alzheimer's disease, spinocerebellar ataxias, Parkinson's disease, AIDS-dementia complex, amylotrophic lateral sclerosis (ALS), depression, schizophrenia, sepsis, cardiovascular shock, severe trauma, acute lung injury, acute respiratory distress syndrome, acute cholecystitis, severe burns, pneumonia, extensive surgical procedures, ischemic bowel, severe acute hepatic disease, severe acute hepatic encephalopathy or acute renal failure.
Owner:GLAXOSMITHKLINE INTPROP DEV LTD
Composition and method for treatment of hepatic encephalopathy
InactiveUS7256202B2Reduce ammonia levelsReduce constipationBiocideDigestive systemSide effectHyperammonemic encephalopathy
The inventions provide an improved treatment for hepatic encephalopathy characterized by hyperammonemia and / or constipation, comprising the oral administration of polyethylene glycol (PEG) in amounts sufficient to reduce plasma levels of ammonia and / or to alleviate constipation. Preferably, the PEG is administered in combination with lactulose, which provides a palatable composition for the treatment of HE with excellent therapeutic benefits and reduced side effects as compared to lactulose alone.
Owner:HALOW GEORGE M
Compound rifaximin nanoemulsion and preparation method thereof
InactiveCN102614181AIncrease fat solubilityReduce intakeAntibacterial agentsOrganic active ingredientsTrimethoprim LactateActive agent
The invention discloses compound rifaximin nanoemulsion, which is prepared from the following raw materials in percentage by mass: 30 to 45 percent of surfactant, 1 to 10 percent of cosurfactant, 1 to 16 percent of oil phase, 0.1 to 5 percent of rifaximin, 0.025 to 1.25 percent of trimethoprim lactate and the balance of deionized water. The sum of the mass percentage of the raw materials is 100 percent. The compound rifaximin nanoemulsion is applied to acute and chronic intestinal infection, diarrhea syndrome, diarrhea caused by intestinal flora change, hepatic encephalopathy and the like, obviously improves the bioavailability and stability of rifaximin, is quick in response, lasting in effect and high in safety, and has a wide market prospect in the field of medicines.
Owner:NORTHWEST A & F UNIV
Medicine composition for curing hepatic encephalopathy and hepatitis B
InactiveCN102008588BDetoxifyingCooling blood and removing blood stasisNervous disorderDigestive systemPharmaceutical SubstancesPharmacology
Owner:张蕊
Guanidinobenzoic acid compound
The object of the present invention is to provide a compound useful as a renal disease preventive and / or therapeutic agent. The inventors of the present invention investigated compounds having a trypsin inhibitory activity, verified that a guanidinobenzoic acid compound has a trypsin inhibitory activity, and achieved the present invention. This guanidinobenzoic acid compound can be used as: a renal disease preventive and / or therapeutic agent that serves as a substitute drug in a low-protein diet regimen; and a preventive and / or therapeutic agent against diseases to which trypsin contributes such as pancreatitis, reflux esophagitis, hepatic encephalopathy, and influenza.
Owner:ASTELLAS PHARMA INC
Medicine composition for curing hepatic encephalopathy and hepatitis B
InactiveCN102008588ADetoxifyingCooling blood and removing blood stasisNervous disorderDigestive systemToxic materialPharmaceutical Substances
The invention provides a medicine composition for curing hepatic encephalopathy and hepatitis B, belonging to the field of Chinese materia medica prescriptions. The composition comprises the following components in parts by weight: 5-50 parts of rheum officinale, 10-60 parts of roxburgh rose root, 5-30 parts of black soya bean, 20-90 parts of serissa serissoide, 20-100 parts of Salviacavaleriei, 30-150 parts of siphonostegia chinensis, 5-40 parts of pubescent holly root and 10-60 parts of liquorice. In the medicine composition, pathogenesis and symptomatic treatment are taken as the principal thing; the medicine composition has the efficacies of clearing away heat and toxic material, removing dampness through diuresis, cooling blood, eliminating stasis, stop bleeding, eliminating heat and relaxing the bowels, thus achieving the effects of resisting viruses, cooling the blood, reducing bloodammonia, eliminating excreta reserved in bowel, reducing absorption of toxin in intestinal tracts, protecting a liver and a brain, and preventing and curing the encephalopathy and the hepatitis B.
Owner:张蕊
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com

![3-(5-chloro-2-oxobenzo[d]oxazol-3(2H)-yl) propanoic acid derivatives as kmo inhibitors 3-(5-chloro-2-oxobenzo[d]oxazol-3(2H)-yl) propanoic acid derivatives as kmo inhibitors](https://images-eureka.patsnap.com/patent_img/41458c72-594e-4502-b3f4-2bd465aa0ad6/US20160318884A1-20161103-C00001.PNG)
![3-(5-chloro-2-oxobenzo[d]oxazol-3(2H)-yl) propanoic acid derivatives as kmo inhibitors 3-(5-chloro-2-oxobenzo[d]oxazol-3(2H)-yl) propanoic acid derivatives as kmo inhibitors](https://images-eureka.patsnap.com/patent_img/41458c72-594e-4502-b3f4-2bd465aa0ad6/US20160318884A1-20161103-C00002.PNG)
![3-(5-chloro-2-oxobenzo[d]oxazol-3(2H)-yl) propanoic acid derivatives as kmo inhibitors 3-(5-chloro-2-oxobenzo[d]oxazol-3(2H)-yl) propanoic acid derivatives as kmo inhibitors](https://images-eureka.patsnap.com/patent_img/41458c72-594e-4502-b3f4-2bd465aa0ad6/US20160318884A1-20161103-C00003.PNG)