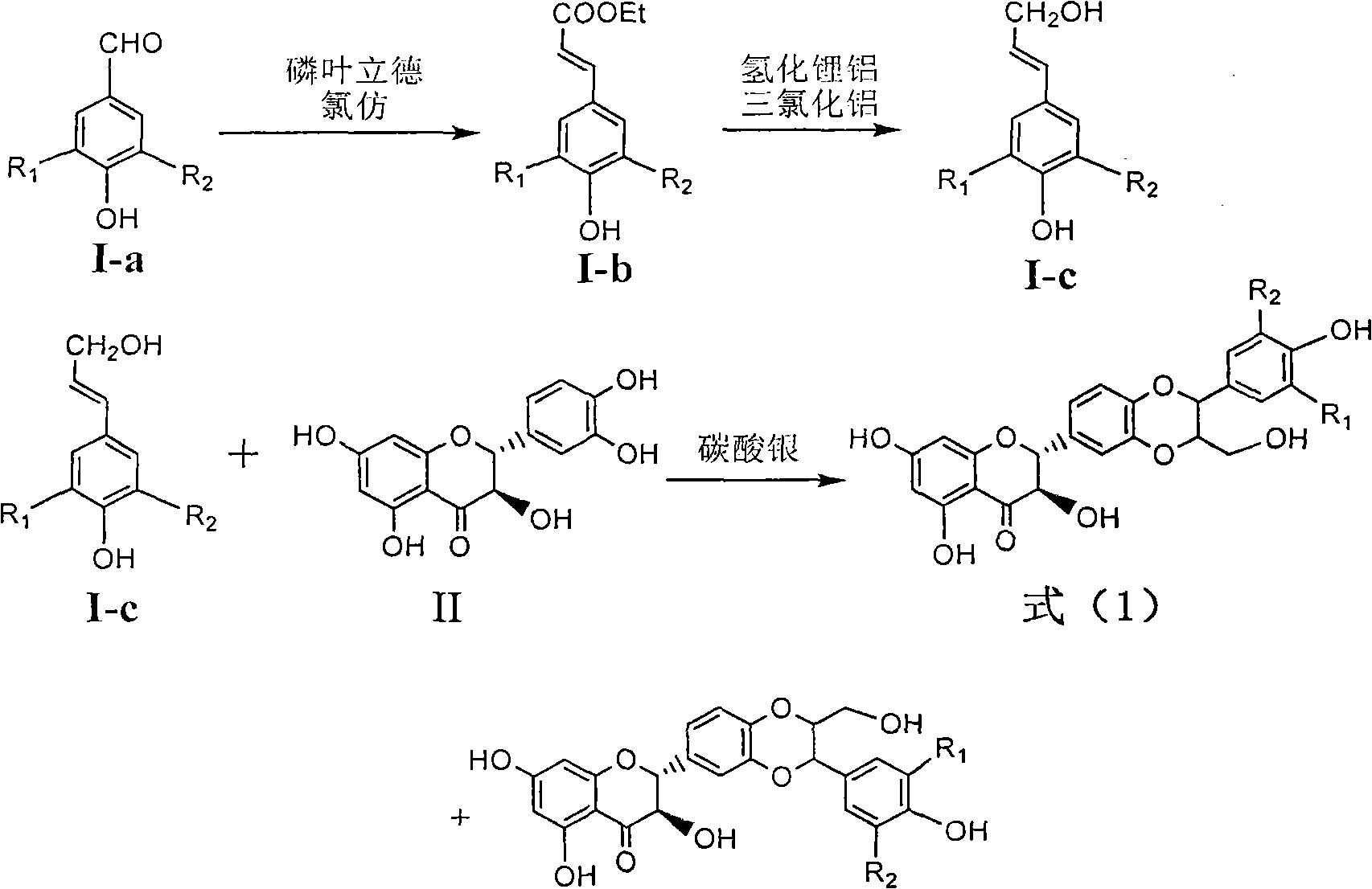
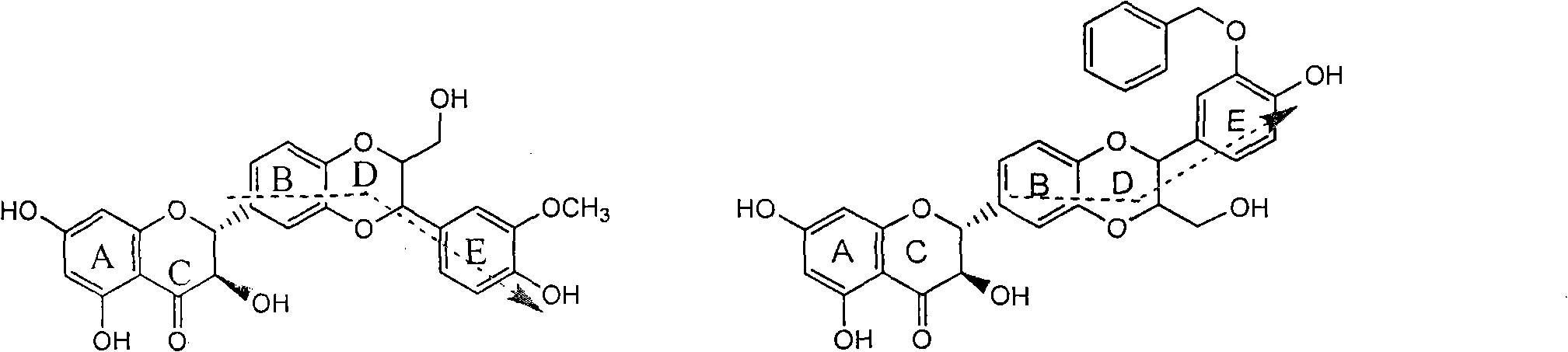
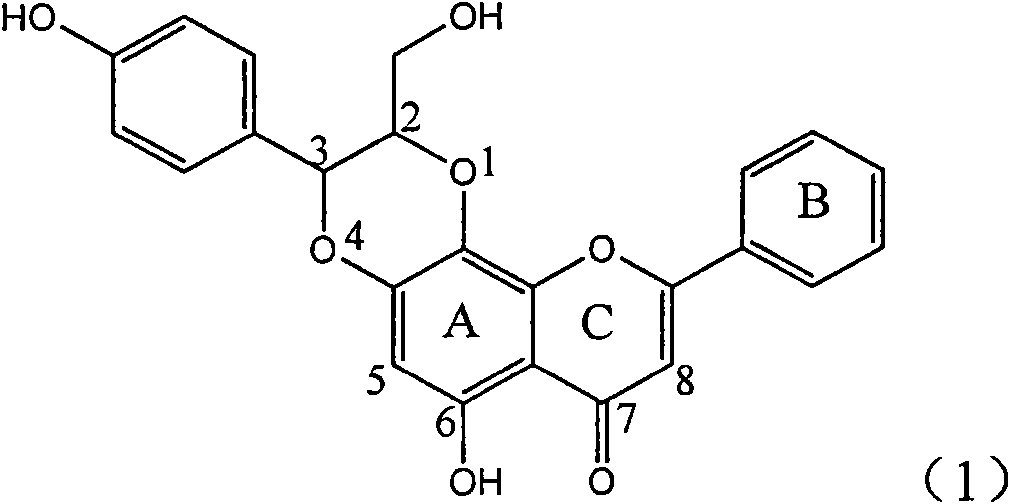
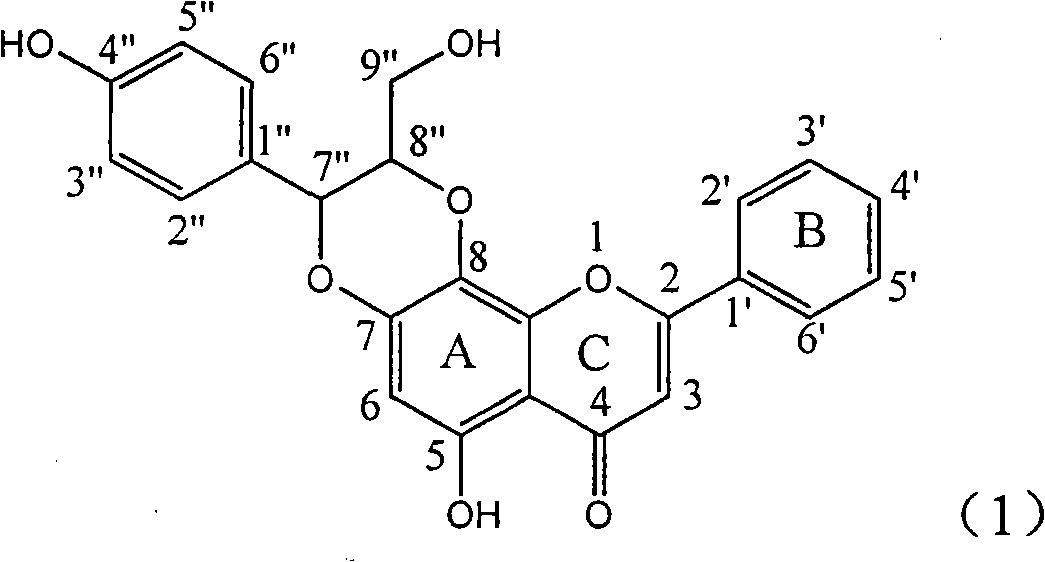
Patents
Literature
63 results about "Hepatitis b e antigen" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Hepatitis B virus e antigen testing corpuscle, preparation and application thereof
ActiveCN101251540AWide detection rangeReduce sensitivityAnalysis by material excitationAntigenAnti hbe
The invention relates to a diagnosing reagent for hepatitis B, disclosing detection grains for e antigens of the hepatitis B virus, which are of the luminous grains coated by anti-HBE antibodies. The invention also discloses preparation and application for the detection grains for e antigens of the hepatitis B virus; moreover, the invention further discloses an outside-body diagnosis reagent box for detecting e antigens of the hepatitis B in a blood serum sample of human beings as well as a method for utilizing the light excitation chemiluminescence principle to quantitatively and qualitatively detect e antigens of the hepatitis B virus. The reagent box of the invention can be jointly used to diagnose the individual acute or chronic hepatitis B together with other blood serums and clinic information, and screen the hepatitis B for women in the perinatal period so as to judge the risk of newborn babies contaminating the hepatitis B.
Owner:BEYOND DIAGNOSTICS (SHANGHAI) CO LTD
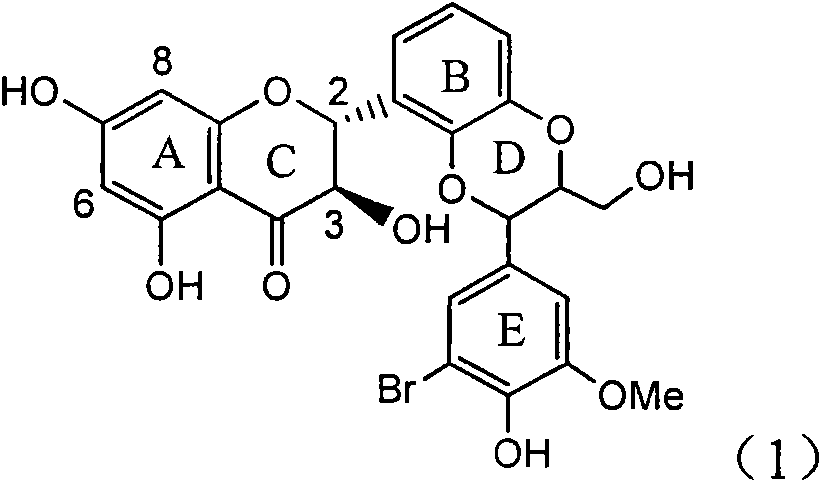
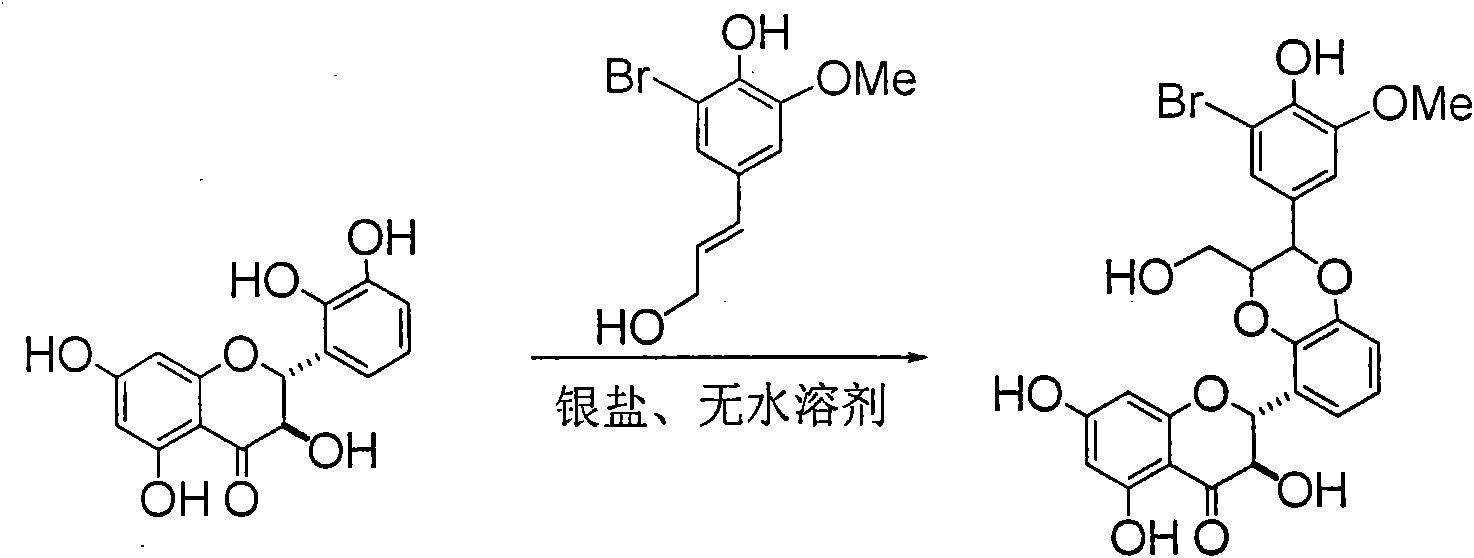
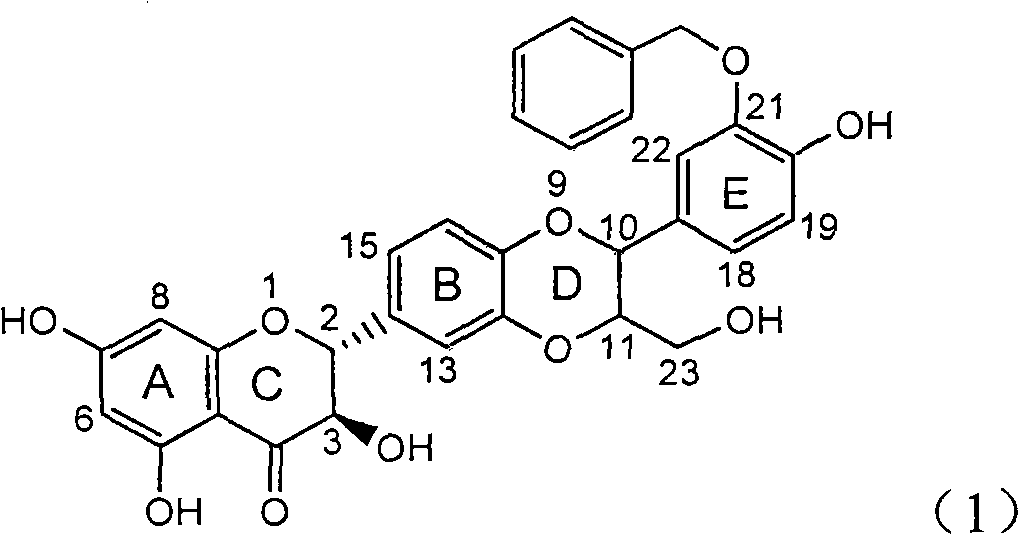
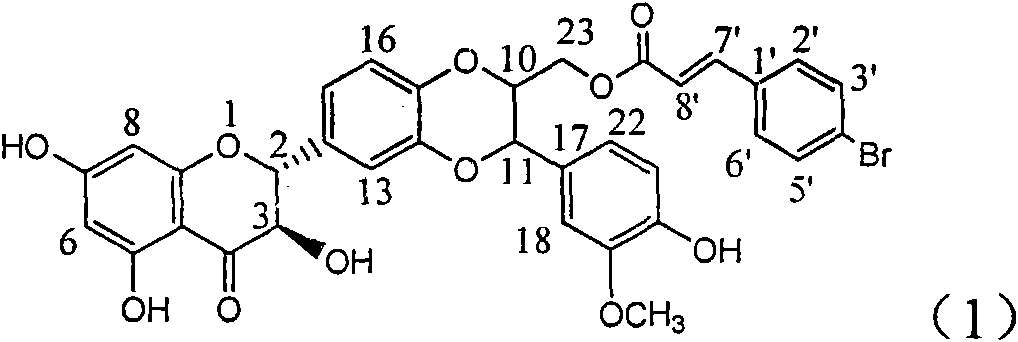
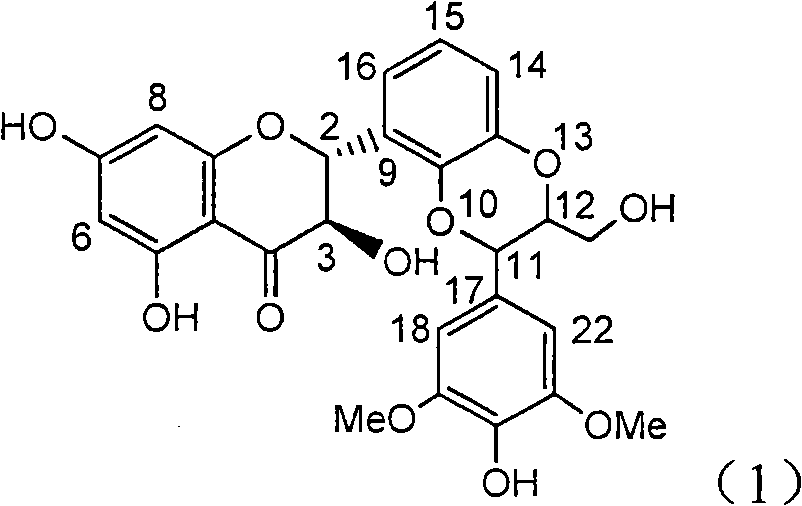
Preparation of brominated flavanonollignan and application in medicine for treating viral hepatitis B
InactiveCN101955478AConvenient sourceThe source is easy to getOrganic active ingredientsOrganic chemistryPositive controlInterferon alpha
The invention relates to the preparation of brominated flavanonollignan and an application in medicines for treating viral hepatitis B, in particular to a B cyclo-dioxane flavanonollignan compound and a preparation method thereof as well as the application of the compound or pharmaceutically acceptable salts thereof in the preparation of medicines for eliminating hepatitis B surface antigens (HBsAg) and hepatitis B e antigens (HBeAg) and medicines for inhibiting HBV DNA replication. The compound has obvious activity of inhibiting HBsAg and HBeAg, and the intensities of the compound for eliminating HBsAg and HBeAg under the concentration of 20 microgram / millimeter are respectively 2.1 times and 1.2 times larger than the corresponding activity of a positive control medicine alpha-interferon; meanwhile, the compound displays high inhibition ratio more than 57% on HBV DNA at the concentration. The results show that the favonolignan or pharmaceutically acceptable salts thereof can be expected to be used for preparing non-nucleoside type medicines for eliminating HBsAg and HBeAg, inhibiting HBV DNA replication and treating HBV infected diseases.
Owner:DALI UNIV
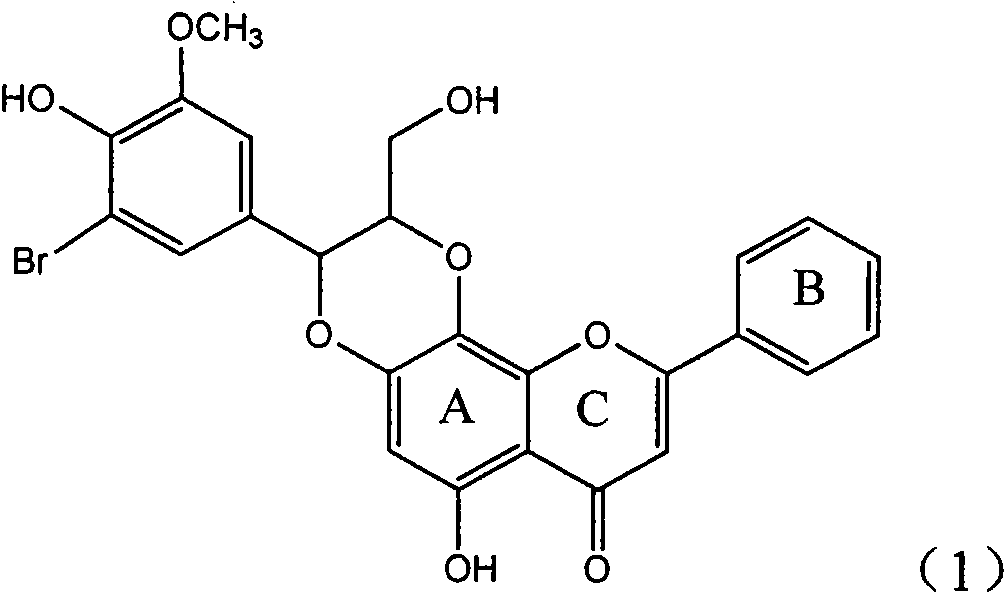
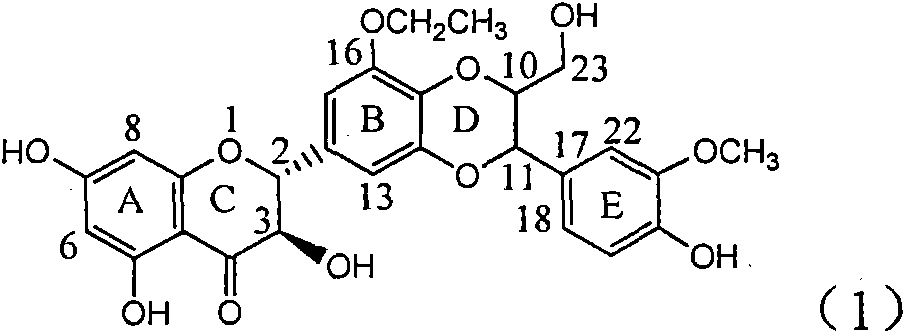
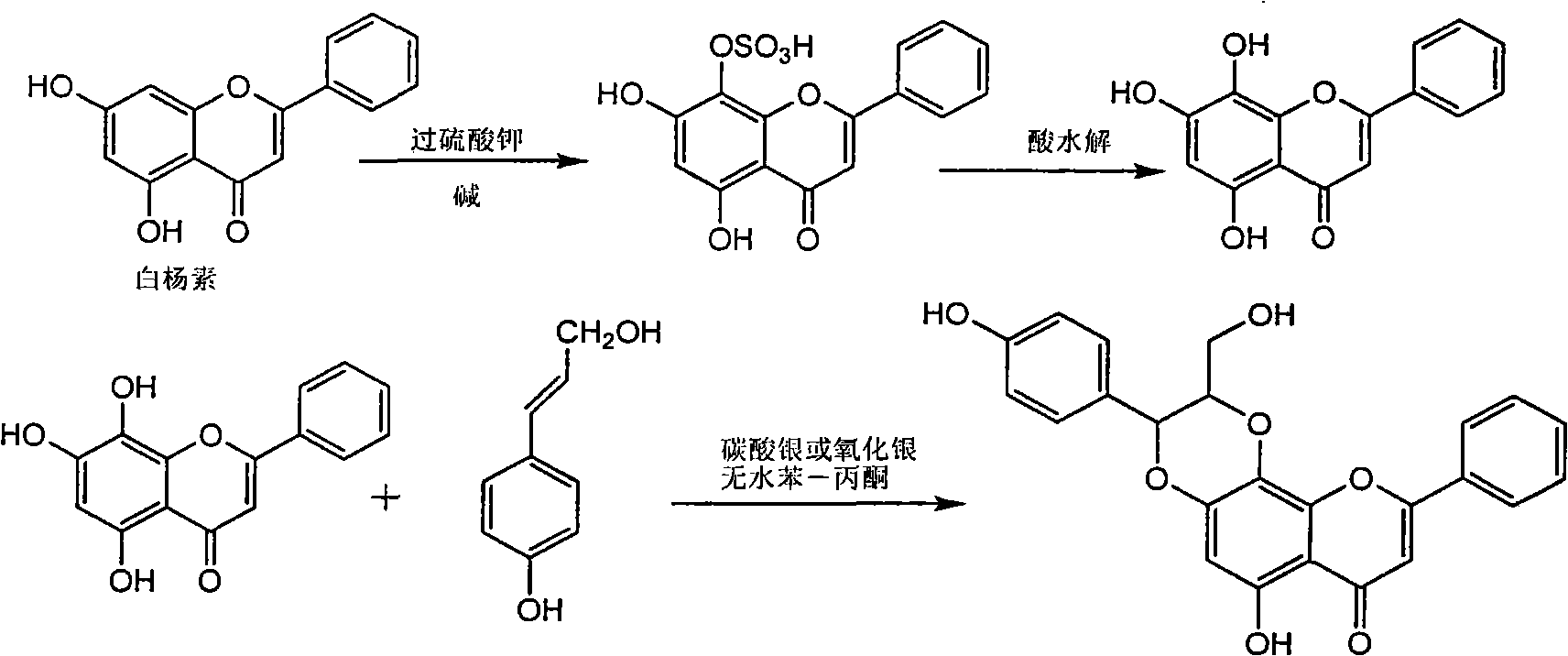
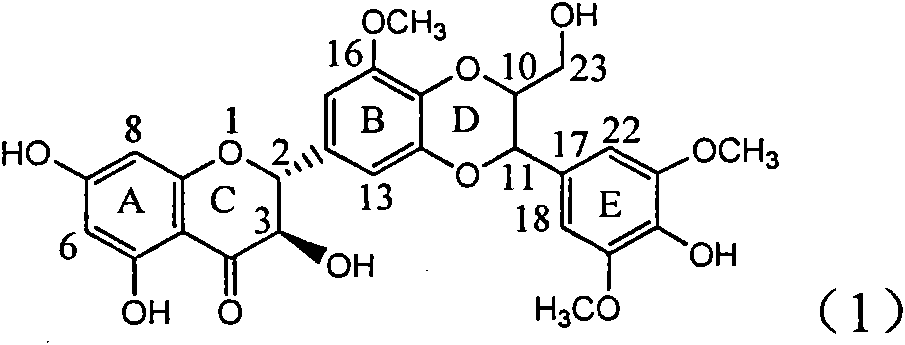
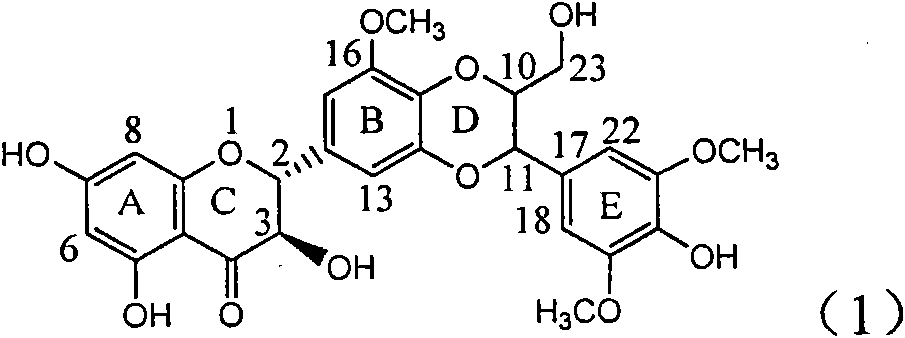
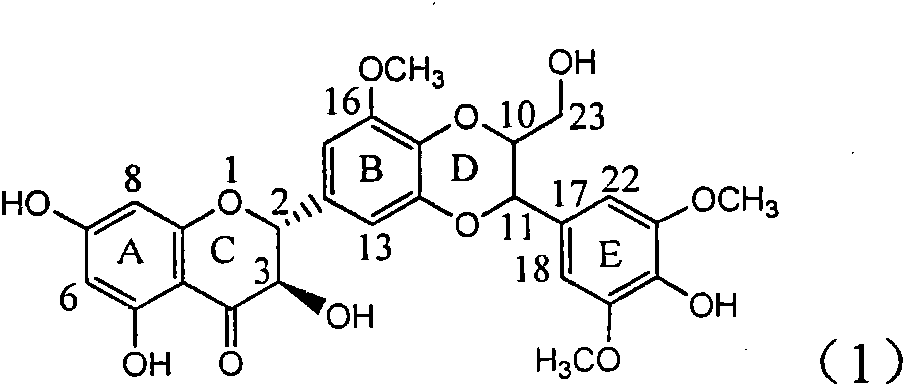
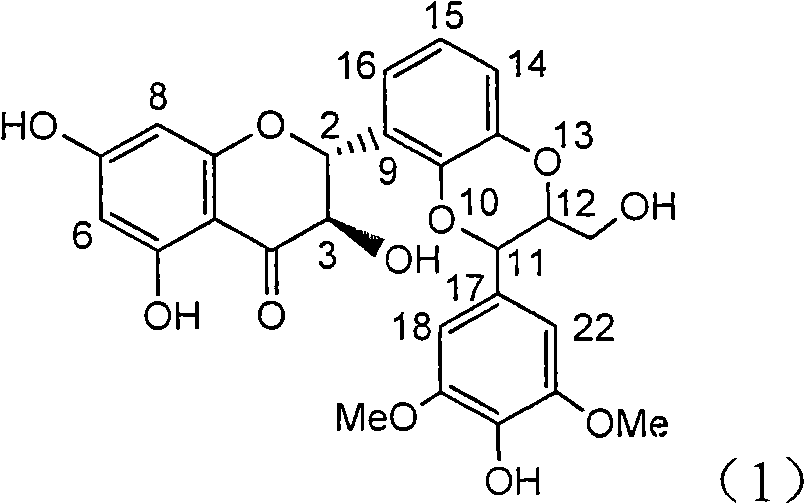
Application of ring A coupling flavonolignan in preparing medicaments for treating viral hepatitis B
InactiveCN101829104AConvenient sourceThe source is easy to getOrganic active ingredientsAntiviralsDiseasePositive control
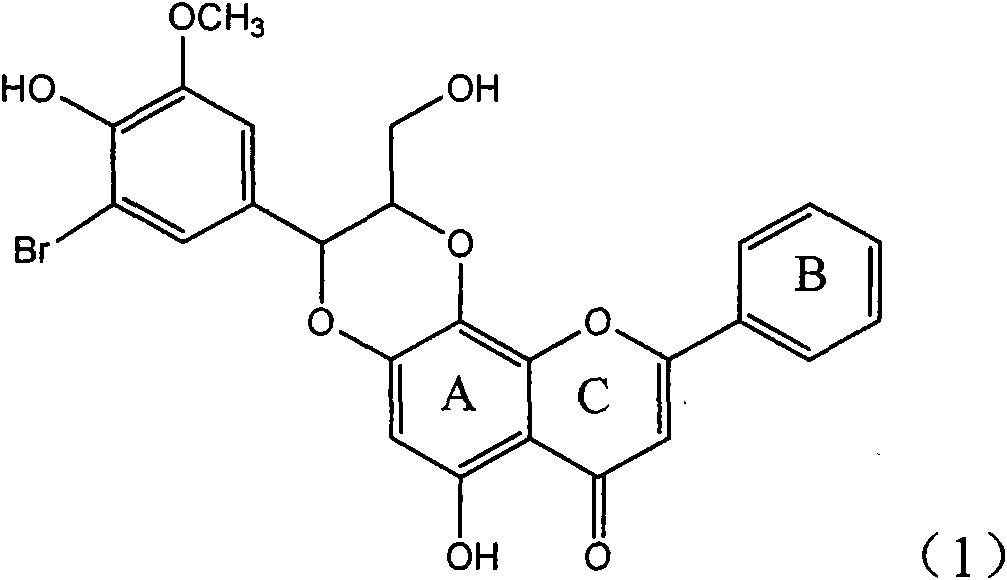
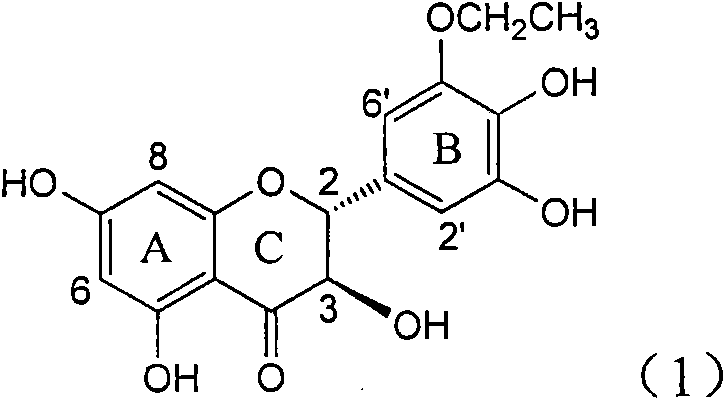
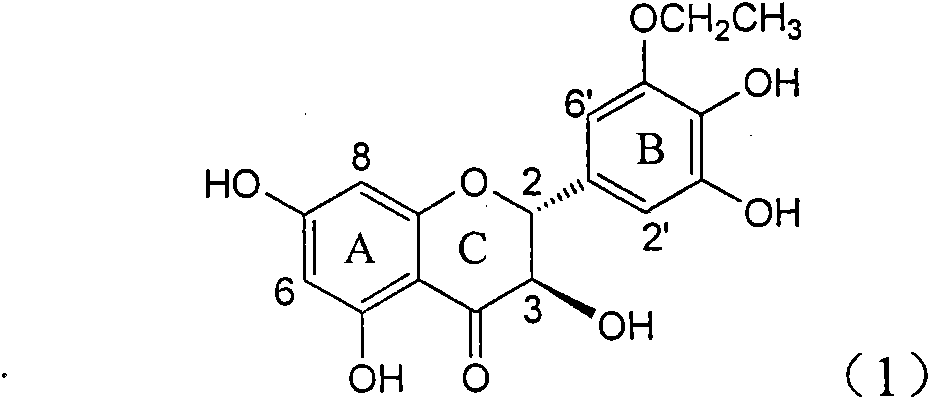
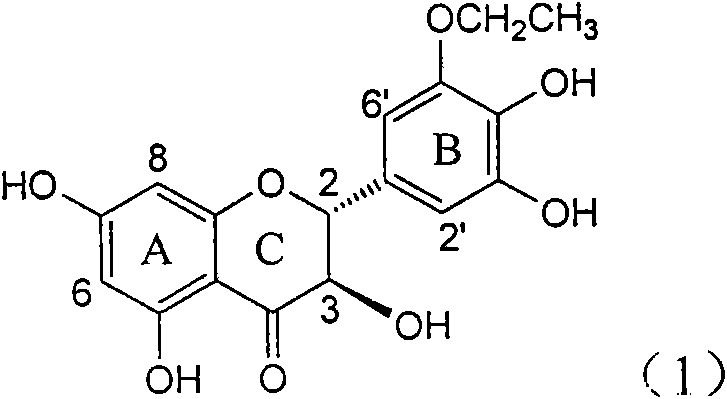
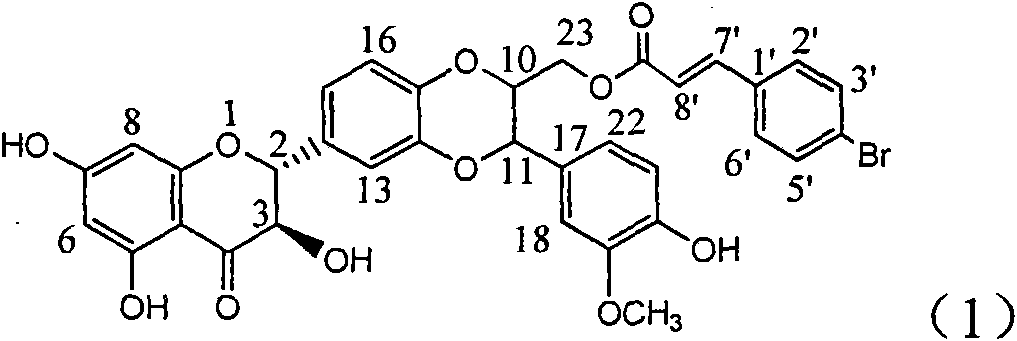
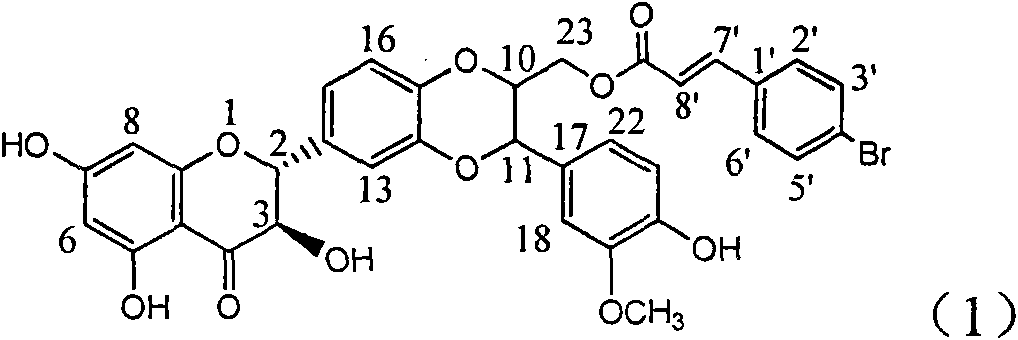
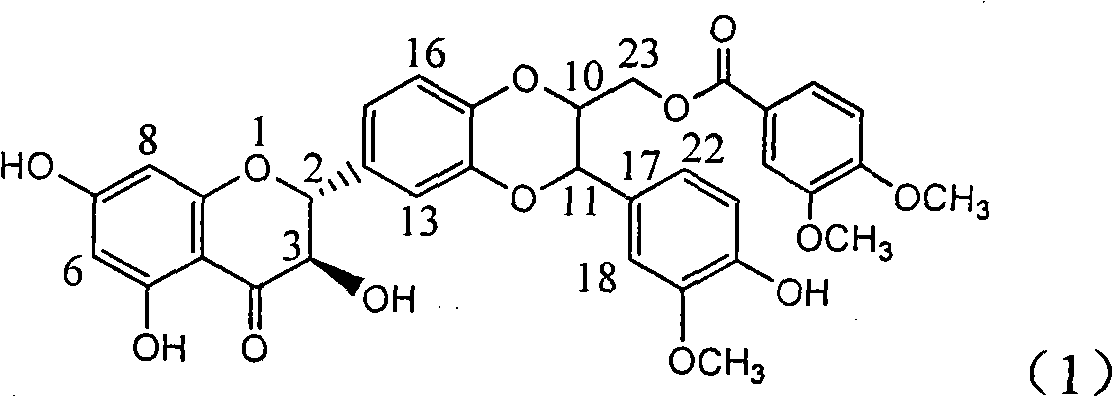
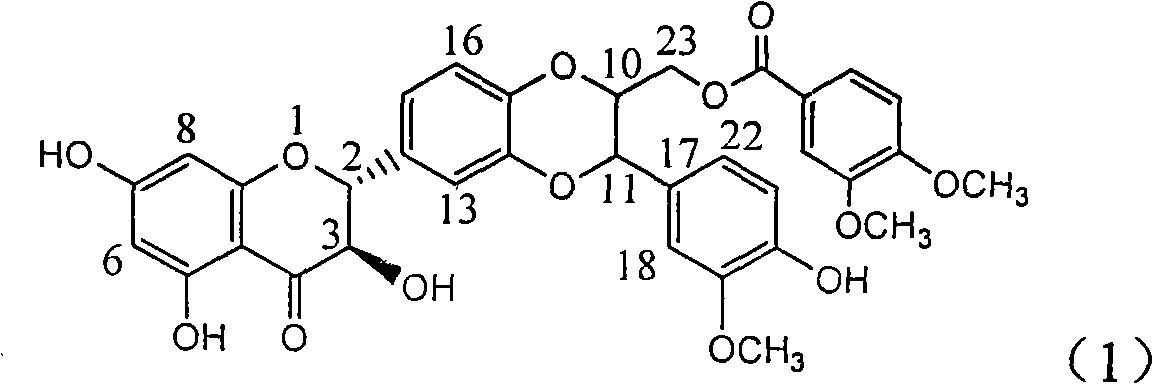
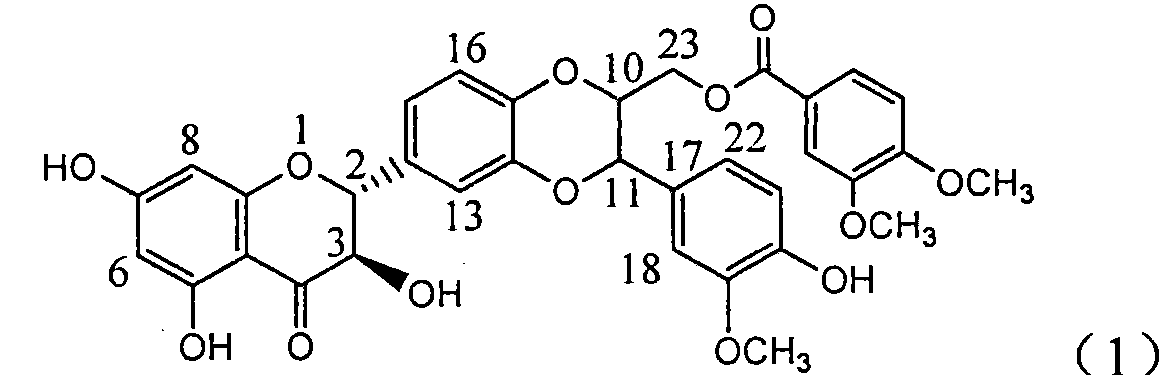
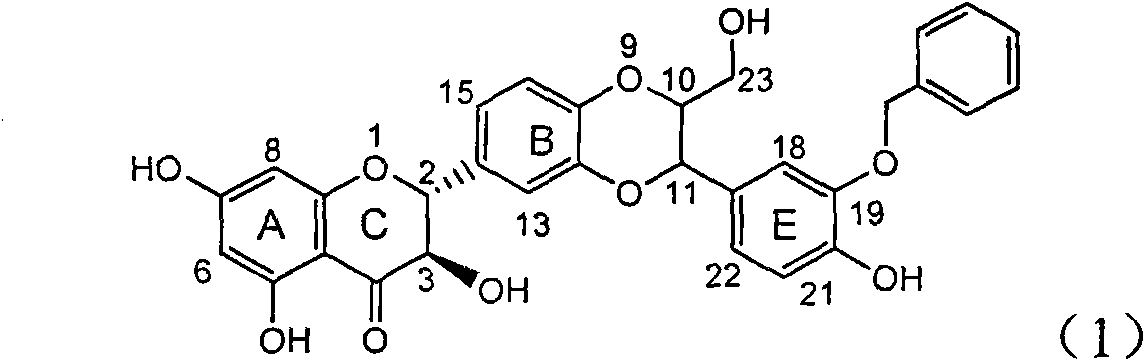
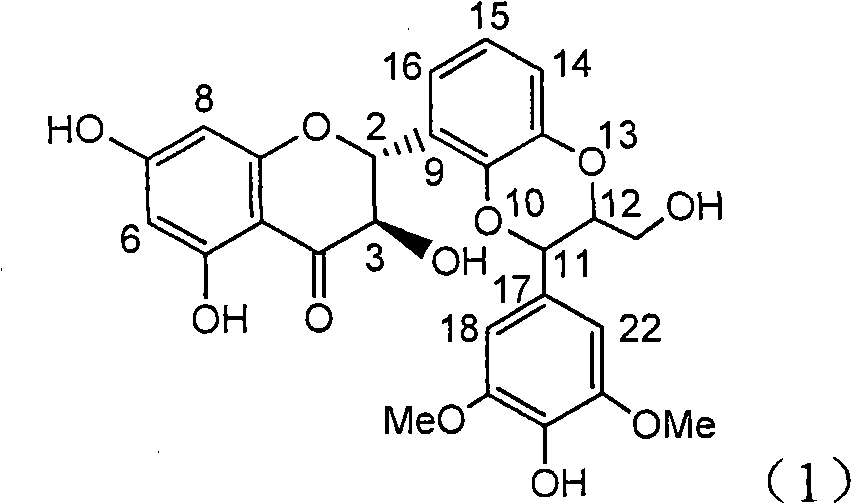
The invention relates to application of ring A coupling flavonolignan in preparing medicaments for treating viral hepatitis B, in particular to application of a compound of the formula (1) or a pharmaceutically acceptable salt thereof in preparing medicaments for clearing away HBsAg (Hepatitis B Surface Antigen) and HBeAg (Hepatitis B e Antigen) and suppressing the HBV (Hepatitis B Virus) DNA replication. The intensities of the flavonolignan for clearing away the HBsAg and the HBeAg are respectively 29.4 percent and 29.1 percent in the presence of a concentration of 20 micrograms / milliliter, which is respectively 1.8 times and 1.7 times of the corresponding activity of a positive control medicament (10,000 units / milliliter of alpha-interferon). What is even more exciting is that in the presence of the concentration, the suppression rate of the flavonolignan to the HBV DNA is higher than 83 percent, which is higher than that of Lamivudine which is a positive control and is 2.2 times of that of the alpha-interferon to the HBV DNA. Accordingly, the flavonolignan and the pharmaceutically acceptable salt thereof are indicated to be capable of being expected to be used for preparing non-nucleoside medicaments for clearing away the HBsAg and the HBeAg, suppressing the HBV DNA replication and treating HBV infection diseases.
Owner:DALI UNIV
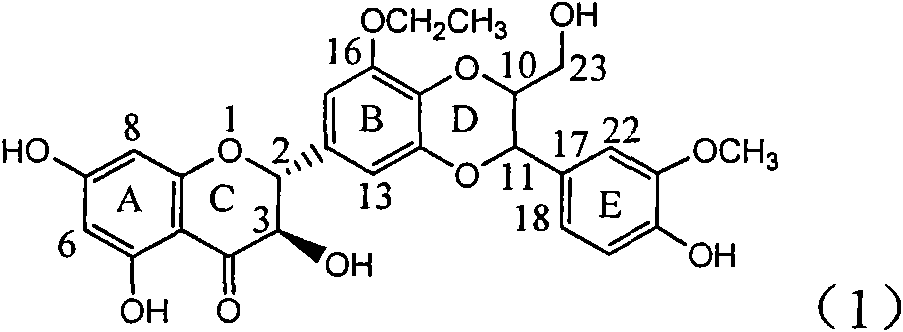
Use of lignanoid containing benzyloxy flavones in preparation of drugs for treating viral hepatitis B
InactiveCN101829095AConvenient sourceThe source is easy to getOrganic active ingredientsDigestive systemDiseasePositive control
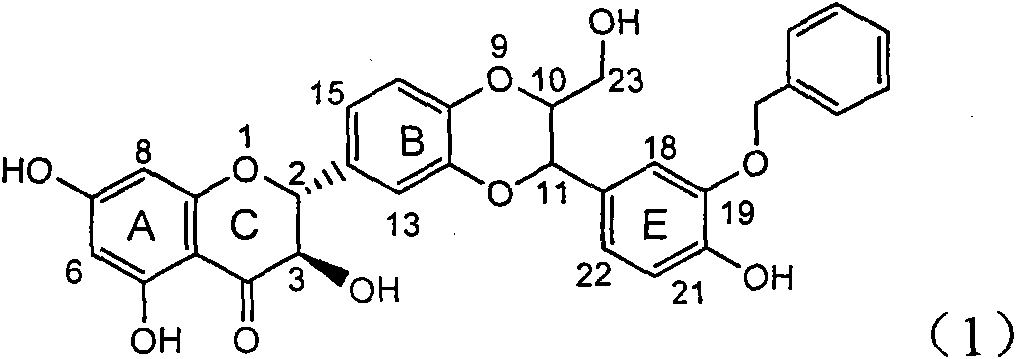
The invention relates to a use of lignanoid containing benzyloxy flavones in the preparation of drugs for treating viral hepatitis B, in particular to the use of a compound as shown in formula (1) or pharmaceutical salts thereof in the preparation of the drugs for eliminating hepatitis B virus surface antigen and hepatitis B e antigen and the drugs for suppressing HBV DNA replication, and the strength of eliminating HBsAg of flavonol lignanoid under the concentration of 20 mu g / ml is 50.8%, which is 3.2 times of the corresponding activity of a positive control drug; the activity of eliminating the HBeAg under the same concentration is equivalent to 10000 units / ml of alpha-interferon; simultaneously, the flavonol lignanoid shows nearly 60% of suppression rate to HBV DNA under the concentration, which is 1.6 times of the corresponding suppression rate of the alpha-interferon. The results show that the lignanoid containing the flavones or the pharmaceutical salts thereof are expected to be used for preparing the non-nucleoside drugs for eliminating the HBsAg and the HBeAg, suppressing the HBV DNA replication and treating hepatitis B virus infection diseases.
Owner:DALI UNIV
Application of ring B ethyoxyl silybin in preparing medicaments for treating viral hepatitis B
InactiveCN101829089AConvenient sourceThe source is easy to getOrganic active ingredientsDigestive systemDiseasePositive control
The invention relates to application of ring B ethyoxyl silybin in preparing medicaments for treating viral hepatitis B, in particular to application of ring B ethyoxyl substituted silybin ester or a pharmaceutically acceptable salt thereof in preparing medicaments for clearing away HBsAG (Hepatitis B Surface Antigen) and HBeAg (Hepatitis B e Antigen) and suppressing the HBV (Hepatitis B Virus) DNA replication. The compound has strong activity on suppressing the HBsAG and the HBeAg, and in the presence of a concentration of 20 micrograms / milliliter, the intensities for clearing the HBsAg and the HBeAg are respectively 64.6 percent and 44.8 percent which are 4.0 times and 2.7 times of that of alpha-interferon which is a positive control medicament. In the presence of the concentration, the suppression rate of the compound on the HBV DNA is 58.1 percent which is 1.5 times of the corresponding activity of the alpha-interferon. Accordingly, the flavonolignan or the pharmaceutically acceptable salt thereof are indicated to simultaneously have strong efficacy on suppressing the HBsAg, the HBeAg and the HBV DNA and can be expected to be used for preparing non-nucleoside medicaments for treating HBV infection diseases.
Owner:DALI UNIV
Application of substituted isosilybin in preparing medicament for treating virus hepatitis B
InactiveCN101829098AConvenient sourceThe source is easy to getOrganic active ingredientsAntiviralsDiseasePositive control
The invention relates to application of substituted isosilybin in preparing a medicament for treating virus hepatitis B, in particular to application of E-ring substituted isosilybin or medicinal salt thereof in preparing a medicament for clearing hepatitis B e antigen, inhibiting HBV DNA replication and treating hepatitis B virus infected diseases. The E-ring substituted isosilybin has strong effect of inhibiting HBeAg activity, and the strength of the E-ring substituted isosilybin at the concentration of 100 micrograms per milliliter for clearing the HBeAg is 3.5 times that of a positive control front-line medicament (10,000 units per milliliter of alpha-interferon); and moreover, the compound at the concentration of 100 micrograms per milliliter has strong inhibiting rate (97.7 percent) on the HBV DNA. Pharmacodynamical results show that the E-ring substituted isosilybin or the medicinal salt thereof can be expected to be used for preparing the medicament for treating the hepatitis B virus infected diseases.
Owner:DALI UNIV
Application of ring A dioxane flavonolignan in preparing medicaments for resisting hepatitis B viruses (HBV)
InactiveCN101829085AConvenient sourceThe source is easy to getOrganic active ingredientsAntiviralsDiseasePositive control
The invention relates to application of ring A dioxane flavonolignan in preparing medicaments for resisting hepatitis B viruses (HBV), in particular to application of ring A dioxane coupling type flavone lignan or a pharmaceutically acceptable salt thereof in preparing medicaments for clearing away hepatitis B e antigen (HBeAg), suppressing the HBV DNA replication and treating HBV infection diseases. The flavonolignan has certain activity on resisting the HBeAg, and the intensity of the flavonolignan for clearing away the HBeAg is higher than that of Lamivudine which is a positive control and close to that of 10,000 units / milliliter of alpha-interferon. Meanwhile, the suppression ratio of the compound to the HBV DNA replication is higher than 80 percent in the presence of a concentration of 100 micrograms / milliliter. The pharmacodynamical results indicate that the flavonolignan or the pharmaceutically acceptable salt thereof can be expected to be used for preparing the medicaments for clearing away the HBeAg, suppressing the HBV DNA replication and treating the HBV infection diseases.
Owner:DALI UNIV
Application of B/E bi-methoxy silybin in preparing medicaments for treating viral hepatitis B
InactiveCN101829088AConvenient sourceThe source is easy to getOrganic active ingredientsAntiviralsDiseasePositive control
The invention relates to application of B / E bi-methoxy silybin in preparing medicaments for treating viral hepatitis B, in particular to application of silybin ester substituted by the methoxy on the ring B and the ring E or a pharmaceutically acceptable salt thereof in preparing medicaments for clearing away HBsAg (Hepatitis B Surface Antigen) and HBeAg (Hepatitis B e Antigen) and suppressing the HBV (Hepatitis B Virus) DNA replication. The B / E bi-methoxy silybin has strong activity on suppressing the HBsAg and the HBeAg, and in the presence of a concentration of 20 micrograms / milliliter, the intensities for clearing away the HBsAg and the HBeAg are respectively 43.9 percent and 43.7 percent which are 2.7 times and 2.6 times of that of alpha-interferon which is a positive control medicament. In the presence of the concentration, the suppression ratio on the HBV DNA is 68.6 percent, and the suppression activity is 1.8 times of that of the alpha-interferon. Accordingly, the flavone lignan or the pharmaceutically acceptable salt thereof are indicated to simultaneously have the effects of strongly suppressing the HBsAG, the HBeAg and the HBV DNA and can be expected to be used for preparing the non-nucleoside medicaments for treating HBV infection diseases.
Owner:DALI UNIV
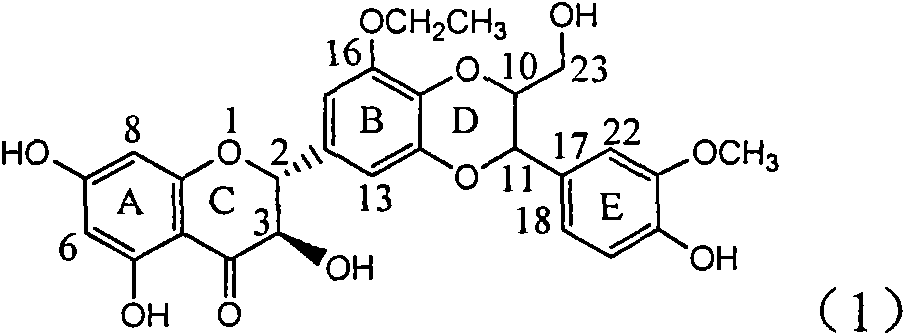
Application of B-ring ethyoxyl flavanonol in preparing medicaments for treating hepatitis B viruses
InactiveCN101822664AConvenient sourceThe source is easy to getOrganic active ingredientsOrganic chemistryDiseasePositive control
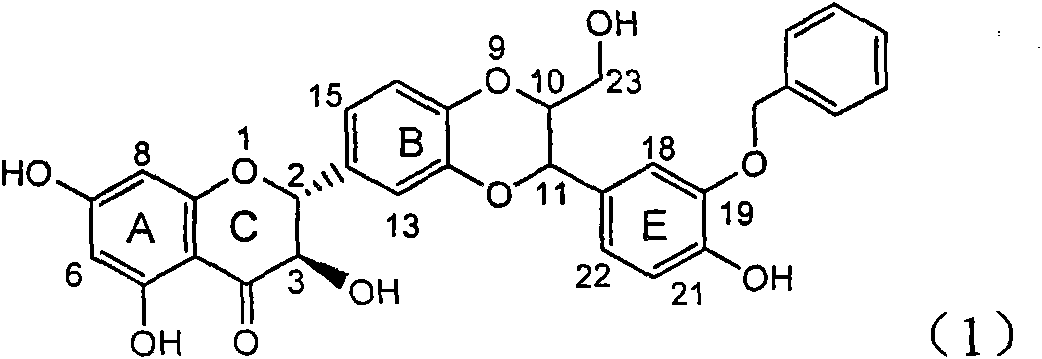
The invention relates to application of B-ring ethyoxyl flavanonol in preparing medicaments for treating hepatitis B viruses, in particular to application of a compound as shown in a formula (1) or a medicinal salt thereof in preparing medicaments for clearing away hepatitis B virus surface antigens (HBsAg) and hepatitis B e-antigen (HBeAg) and medicaments for inhibiting the duplication of hepatitis B virus desoxyribonucleic acid (HBV DNA). The compound or the medicinal salt thereof has extremely obvious activity on inhibiting the HBsAg and the HBeAg, and in the presence of a concentration of 20 microgram / milliliter, the intensities for clearing away the HBsAg and the HBeAg of the compound or the medicinal salt thereof are respectively 99.8 percent and 48.5 percent and are 6.2 times and 2.7 times of that of alpha-interferon which is a positive control medicament. More importantly, in the presence of the concentration, the inhibition ratio of the compound or the medicinal salt thereof to the HBV DNA is 64.7 percent, and the activity is 1.7 times of that of the alpha-interferon. Accordingly, the flavone lignan or the medicinal salt thereof can be expected to be used for preparing non-nucleoside medicaments for treating infectious diseases of the hepatitis B viruses.
Owner:DALI UNIV
Hepatitis B e antibody magnetic particle chemiluminescent immunoassay kit and preparation method thereof
InactiveCN101592664ASuperparamagneticGood dispersionChemiluminescene/bioluminescenceSerum igeHepatitis b e antigen
The invention belongs to the immunoassay technical field and relates to a hepatitis B e antibody magnetic particle chemiluminescent immunoassay kit. The kit of the invention comprises a mixed liquor of hepatitis B e antibody coated with magnetic particle and horse radish peroxidase-labeled hepatitis B e antibody, hepatitis B e antigen, hepatitis B e antibody calibrator, chemiluminescence substrate solution, concentrated cleaning solution and reaction tubes. The invention can be used to test the content of hepatitis B e antibody in human serum and has the advantages of simple and fast operation, good repetitiveness and the like.
Owner:CHEMCLIN DIAGNOSTICS CO LTD
Preparation method and application of flaxseed polysaccharides with antiviral and immune activity
ActiveCN105037573AEfficient destructionEasy extractionOrganic active ingredientsAntiviralsAntigenSulfated polysaccharides
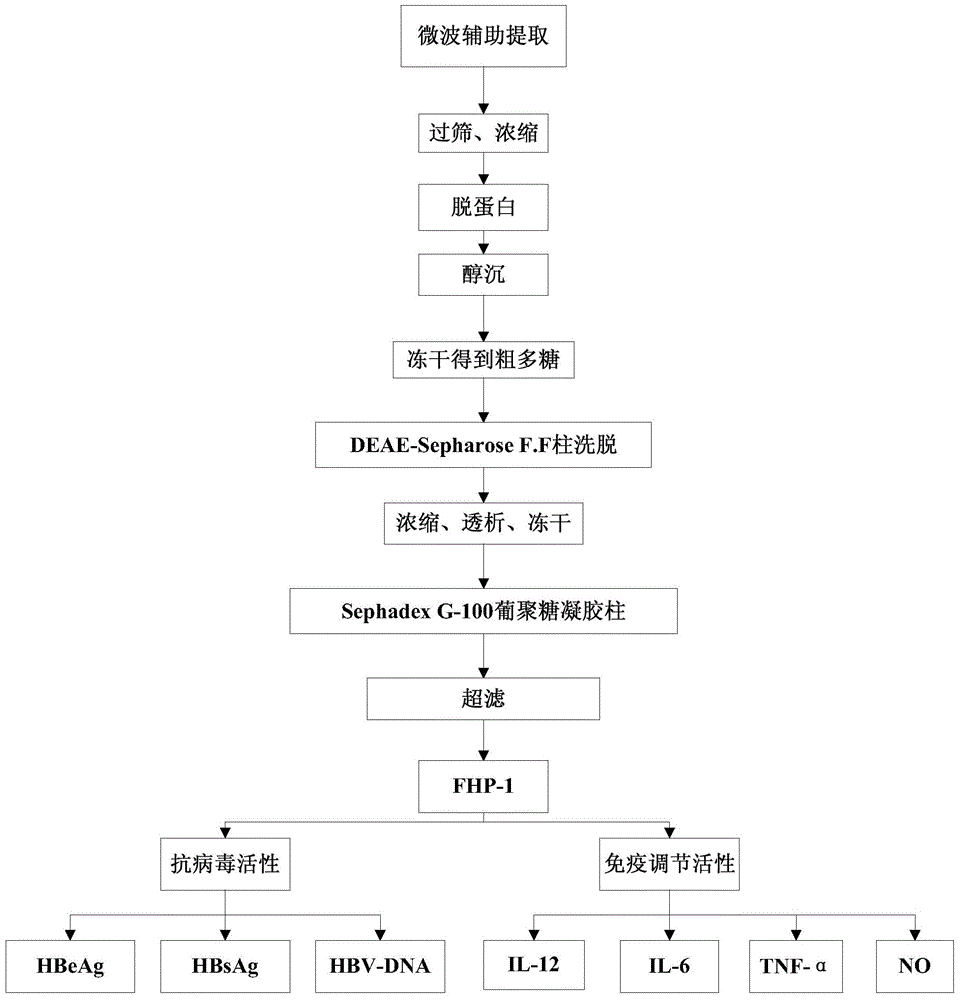
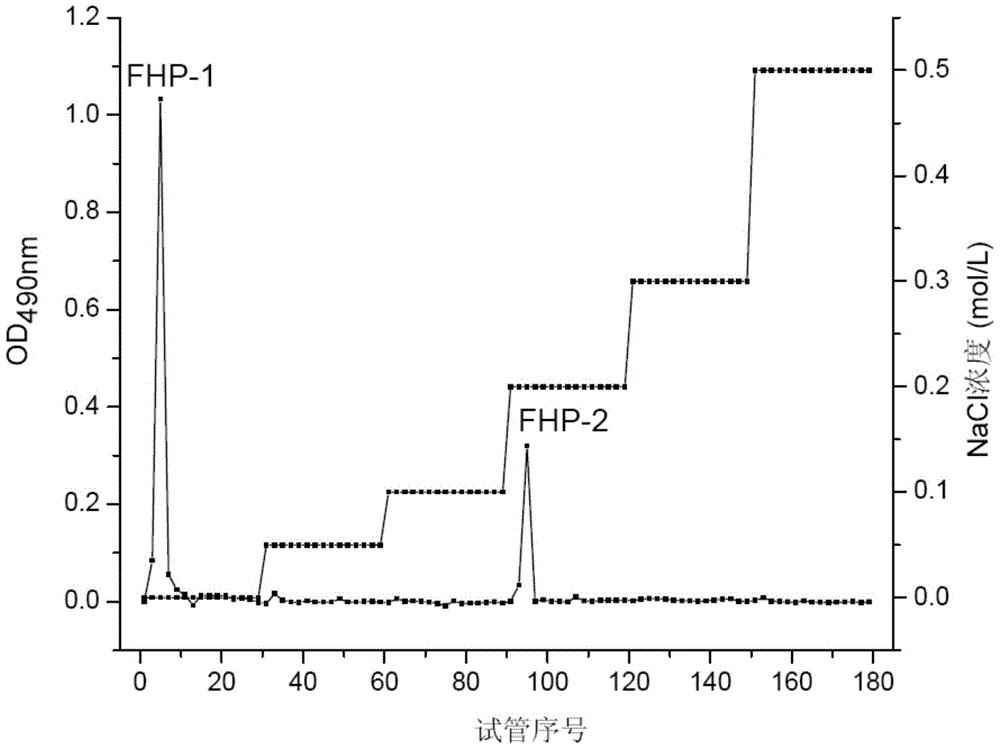
The invention discloses a preparation method and an application of flaxseed polysaccharides with antiviral and immune activity. The preparation method adopting flaxseeds as a raw material comprises the following steps: crushing flaxseeds, carrying out shell-kernel separation, carrying out microwave assisted hot water extraction, carrying out Sevage technology protein removal, carrying out ethanol precipitation, freeze-drying to obtain crude flaxseed polysaccharides, and carrying out ion exchange column chromatography, carrying out Sephadex column chromatography, and carrying out ultrafiltration to prepare flaxseed sulfated polysaccharides. FHP-1 obtained in the invention has homogeneous composition, and the molecular weight is 2626kDa. Polysaccharide with sulfate and triple-helical structure are separated from the flaxseeds in the invention for the first time. Cell biology experiments prove that the polysaccharides can reduce expression of hepatitis B surface antigen and hepatitis B e antigen and inhibit replication of hepatitis B viruses, can activate immune response, and improves secretion of the tumor necrosis factor TNF-a, interleukins IL-6 and IL-12, and an inflammation factor NO by immune cells.
Owner:JINAN UNIVERSITY
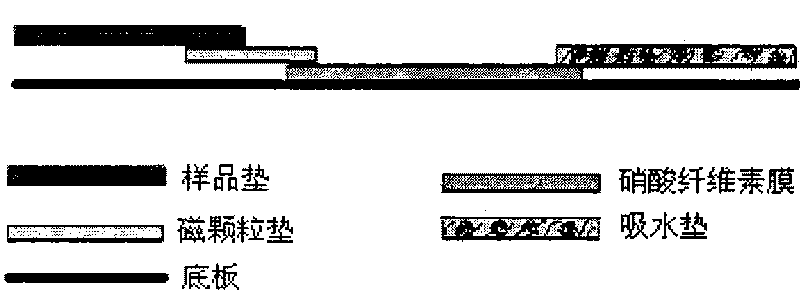
Magnetic immunochromatographic test strip for detecting hepatitis B e antigen and preparation method thereof
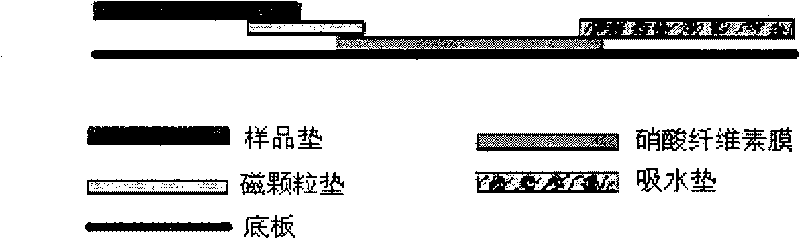
InactiveCN101750498AEasy to manufactureSuitable for mass productionMaterial analysisAntigenHepatitis b e antigen
The invention relates to a magnetic immunochromatographic test strip for detecting hepatitis B e antigen in blood and a preparation method thereof. The magnetic immunochromatographic test strip introduces the magnetic micro-particle immunochromatographic technology into the hepatitis B e antigen detection, and the test strip is assembled by sequentially mutually sticking a coating film, a magnetic particle pad combined with hepatitis B e antibody, a sample pad and a water-absorbing pad on a substrate in a staggered manner and then covering a transparent plastic sealing film on the upper layer. A hepatitis B e antigen detection line and a quality control line are pre-coated on the coating film. The magnetic immunochromatographic test strip has the advantages of simple operation, high sensitivity, good specificity and the like.
Owner:CHEMCLIN DIAGNOSTICS CO LTD
Preparation method and application of electrochemical immunosensor of PdCu nanowire functionalized porous graphene
InactiveCN110058020ALarge specific surface areaImprove conductivityMaterial nanotechnologyCarbon compoundsPorous grapheneHepatitis b e antigen
The invention belongs to the technical field of immunoassay and biosensing, and provides a preparation method of an electrochemical immunosensor of PdCu nanowire functionalized porous graphene. The PdCu nanowire functionalized porous graphene is used as an electrochemical signal amplification platform to construct a marker-free electrochemical immunosensor, so that quantitative detection of hepatitis B e antigen is achieved, the preparation method of the electrochemical immunosensor of the PdCu nanowire functionalized porous graphene has the advantages of high specificity, high sensitivity, low detection limit and the like, and has important scientific significance and application value for detection of hepatitis B.
Owner:SHANDONG UNIV OF TECH
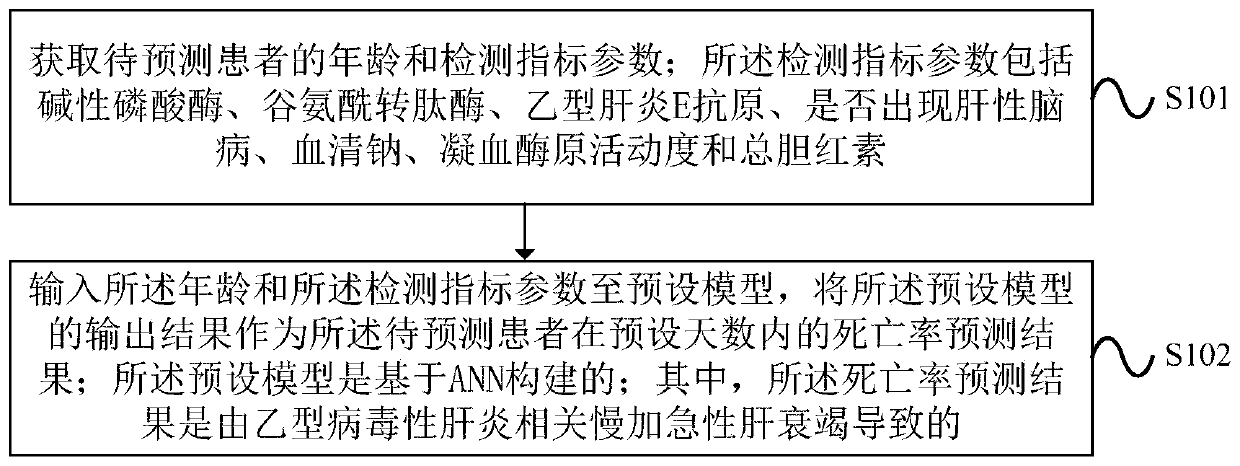
Method and device for processing mortality prediction
PendingCN109859834AImprove forecast accuracyMedical automated diagnosisHepatitis b e antigenAlgorithm
Embodiments of the invention provide a method and device for processing mortality prediction. The method includes the steps: obtaining the age and detection index parameters of a patient to be predicted, wherein the detection index parameters comprises alkaline phosphatase, glutamyl transpeptidase, and hepatitis B E antigen, whether hepatic encephalopathy occurs, serum sodium, blood coagulation, zymogen activity and the total bilirubin; and inputting the age and the detection index parameter to a preset model; inputting the age and the detection index parameters to a preset model, and taking the output result of the preset model as the mortality prediction result of the patient to be predicted within a preset number of days, wherein the preset model is built based on ANN. The device executes the above method. The method and device for processing mortality prediction can improve the prediction accuracy for mortality, caused by acute-on-chronic liver failure related to viral hepatitis B,of a patient within the preset number of days by taking the output result of the preset model constructed based on ANN as the prediction result of the mortality of the patient to be predicted withinthe preset number of days.
Owner:BEIJING DITAN HOSPITAL CAPITAL MEDICAL UNIV
Pharmaceutical composition for treating hepatitis B and cirrhosis
InactiveCN102114227AEffective treatmentInhibition of replicationDigestive systemAntiviralsTreatment effectPolymerase L
The invention discloses a pharmaceutical composition for treating hepatopathy, which is a preparation prepared by the following active pharmaceutical ingredients in portions by weight: 3-5 portions of black nightshade herb, 2-3 portions of common scouring rush herb, 4-8 portions of wild buckwheat rhizome, 3-5 portions of Indian kalimeris herb, 2-5 portions of zedoary, 3-5 portions of carrageen, 3-5 portions of Indian rorippa herb, 3-5 portions of lightyellow sophora root, 3-5 portions of virgate wormwood herb, 3-8 portions of coix seed and 3-5 portions of sinkiang arnebia root. The pharmaceutical composition can effectively inhibit virus replication, convert hepatitis B e-antigen and hepatitis B virus (DNA: deoxyribonucleic acid) gene polymerase to be negative within 40 weeks, effectively treat hepatitis B, further reduce the liver fibrosis level, improve the organic disorder in liver and spleen, increase the seralbumin, quickly reduce the liver functional seroenzyme, restore liver functions, have a good effect of protecting the liver, and effectively treat viral hepatitis and cirrhosis; and the pharmaceutical composition particularly has significant treatment effect for the hepatitis B and the cirrhosis after various types of the hepatitis.
Owner:江兴利
Application of p-bromo-cinnamyl silybin in preparing medicament for treating viral hepatitis B
InactiveCN101829087AConvenient sourceThe source is easy to getOrganic active ingredientsAntiviralsAntigenDisease
The invention relates to application of p-bromo-cinnamyl silybin in preparing a medicament for treating viral hepatitis B, in particular to application of 23-site p-bromo-cinnamyl base substituted silybin ester or pharmaceutically acceptable salts thereof in preparing a medicament for removing surface antigen of a hepatitis B virus and a hepatitis B e antigen and inhibiting HBVDNA replication. The medicament has obvious HBsAg inhibiting and HBeAg inhibiting activity, and the strengths for removing HBsAg and HBeAg at the concentration of 20 microgrammes / ml are respectively 98.9 percent and 90.9 percent and respectively exceed the strength of a positive contrast medicament alpha-interferon by 6.1 times and 5.4 times; and meanwhile, the medicament displays about 60 percent of inhibition ratio on HBVDNA at the concentration and is 157 percent higher than the alpha-interferon. It is indicated that the favonolignan or the pharmaceutically acceptable salts thereof can be expected for preparing a non-nucleoside medicament for removing HBsAg and HBeAg, inhibiting HBVDNA replication and treating a hepatitis B virus infection disease.
Owner:DALI UNIV
Application of isoflavonoids compounds to preparation of antihepatitis drug
InactiveCN102247395ASignificant anti-HBV effectAntiviralsFood preparationHepatitis b e antigenIridoid Glucosides
The invention relates to an application of isoflavonoids compounds, i.e., tectoridin, iridin, irisflorentin, tectorigenin or irigenin, separated from the belamcanda chinensis of an iridaceae belamcanda plant to preparation of drugs or health-care foods for preventing and treating hepatitis, relating to the technical field of natural pharmaceutical chemistry and medicines. In the invention, HBsAg (Hepatitis B Surface Antigen) and HBeAg(Hepatitis B e Antigen) resistance experiments are carried out by adopting detection reagents of an HBsAg diagnostic reagent kit and an HBeAg diagnostic reagent kit; and an experimental result discovers that all the tectoridin, the iridin, the irisflorentin, the tectorigenin and the irigenin have an obvious hepatitis B virus resistant action, thus the tectoridin, the iridin, the irisflorentin, the tectorigenin and the irigenin can be used for preparing the drugs or health-care foods for preventing and treating hepatitis viruses.
Owner:SECOND MILITARY MEDICAL UNIV OF THE PEOPLES LIBERATION ARMY
Hepatitis B e antigen single quality control product and preparation process thereof
InactiveCN108562754ASufficient sourceEasy to getBiological material analysisBiological testingLaboratory orderThird party
The invention relates to the field of quality control of medical examination, in particular to the preparation of a single quality control substance of a hepatitis B e antigen. The hepatitis B virus eantigen is diluted to a required value by using a specific stabilizer to prepare the quality control product. The preparation process is simple, the production cost is low, added substances and contents are controllable, the product belongs to a liquid type, redissolution is not required, and the composition is stable. Through a biological traceability link program and reference method assignmentof the program, the product can be used as a third-party medical laboratory quality control product during the validity period to meet the needs of clinical infectious disease testing.
Owner:河北睿达模生物科技有限公司
Purifying method for hepatitis B e antigen
InactiveCN103882042AStructure has no effectEasy to operateVirus peptidesPeptide preparation methodsAntigenHigh concentration
A disclosed purifying method for hepatitis B e antigen comprises the following steps: transferring a recombinant plasmid of hepatitis B e antigen gene to a bacterium for obtaining a recombinant bacterium, wherein the 5' end and the 3' end of the hepatitis B e antigen gene are both connected with a poly-histidine encoding gene; adding an inducer for culturing the recombinant bacterium; performing lysis on the recombinant bacteria, and centrifuging the lysate; contacting an immobilized metal-chelated affinity chromatography medium with a supernatant obtained by centrifugation or with a precipitate solution prepared from a precipitate obtained by performing high-concentration denaturant dissolving and centrifugation under the condition that the poly-histidine fusion hepatitis B e antigen is allowed to combine the medium or to be absorbed by the medium; and selectively eluting poly-histidine fusion hepatitis B e antigen from the medium. According to the method, fusion tag technology and affinity chromatography are combined, the operation is simple and convenient; and by taking poly-histidine as a fusion tag, the structure of hepatitis B e antigen is not influenced.
Owner:SHENZHEN INST OF ADVANCED TECH
Hepatitis B e antigen immune escaping detecting gene chip and kit thereof
ActiveCN101333559AAvoid missing detectionJudgment of infectivityMicrobiological testing/measurementAntigenLeak detection
The invention provides a hepatitis B virus e antigen immune evasion detection gene chip and a kit, belonging to the gene chip technique field of clinical detection, and comprising: a gene chip of a specific DNA probe which detects hepatitis B virus e antigen immune evasion and is fixed on a solid phase carrier as well as a matched reagent; the matched reagent includes a treating fluid for treating serum samples, an amplifying solution for amplifying and labeling the treated samples, a hybridization solution in hybridization reaction with a micro-array, a cleaning solution, an enzyme-labeled working solution and a detection liquid; and the matched reagent and the hepatitis B virus e antigen immune evasion detection gene chip are used jointly in the detection process of the serum samples. The chip and the kit can detect the hepatitis B virus e antigen immune evasion, and avoid the clinical hepatitis B virus e antigen leak detection, thereby being favorable for judging the infectivity and conditions after curing of hepatitis B patients.
Owner:上海裕隆生物科技有限公司
Therapeutic, Prophylactic and Diagnostic Agents for Hepatitis B
InactiveUS20070264284A1Promote infectionPeptide/protein ingredientsMicrobiological testing/measurementHepatitis b e antigenDiagnostic agent
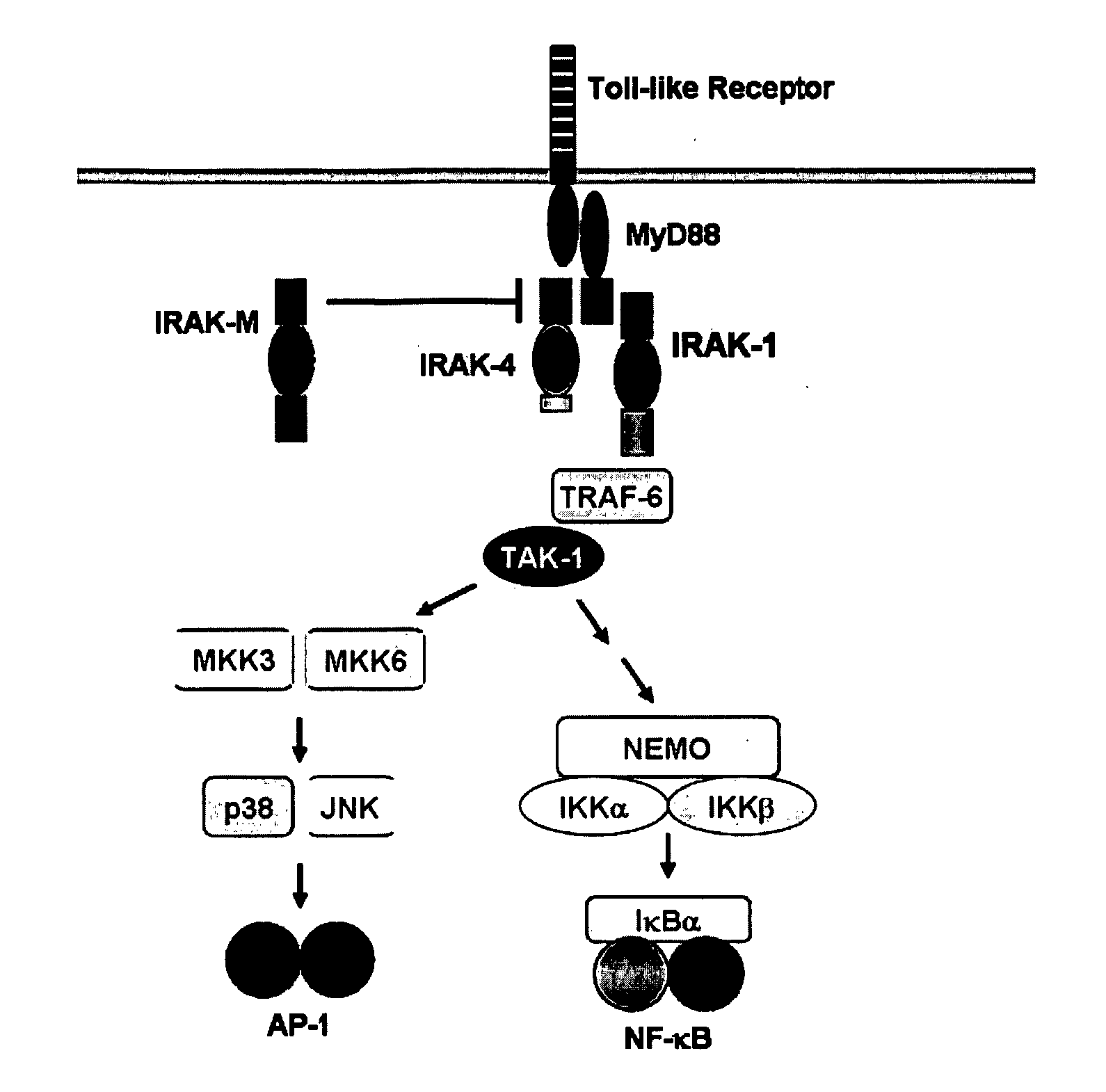
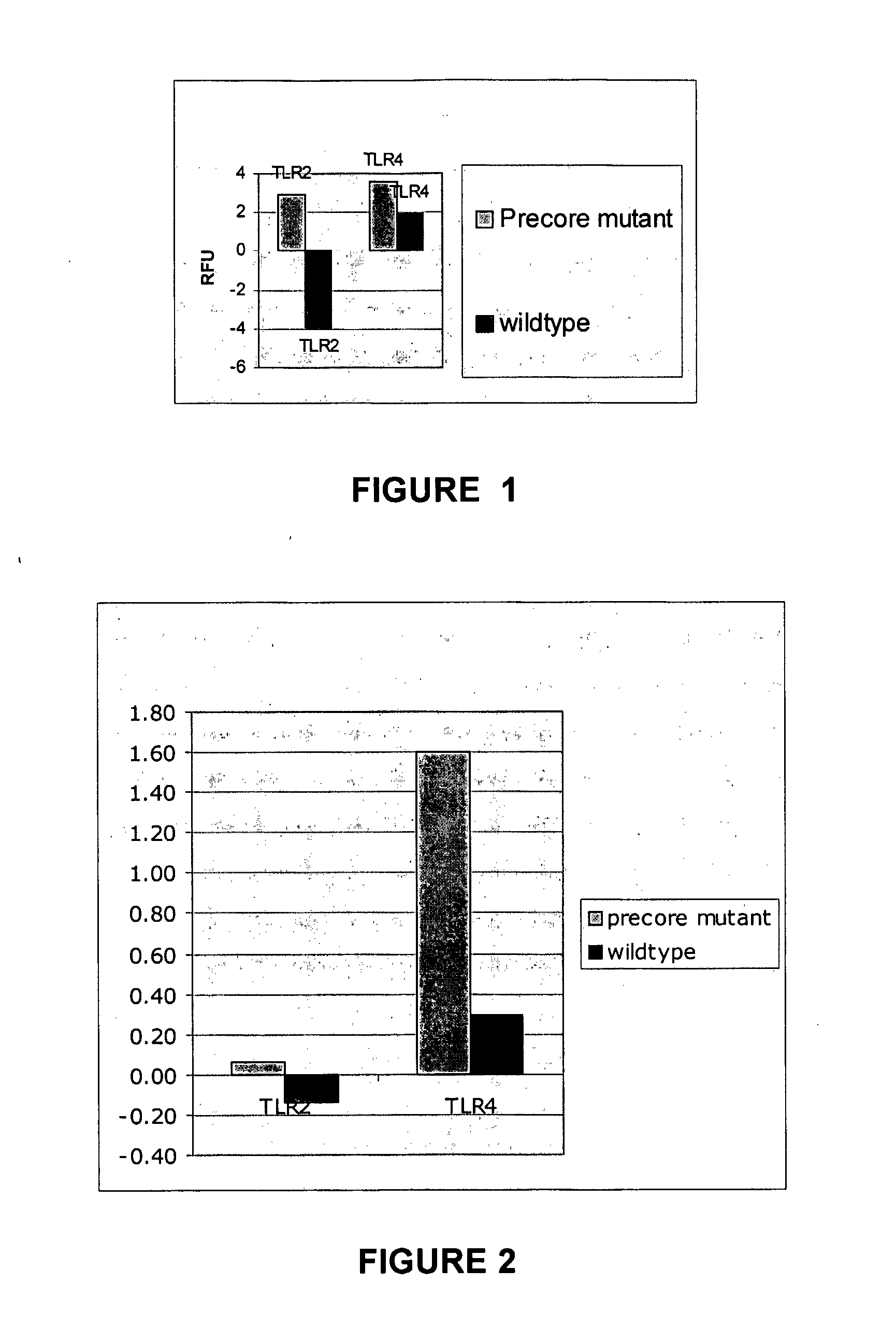
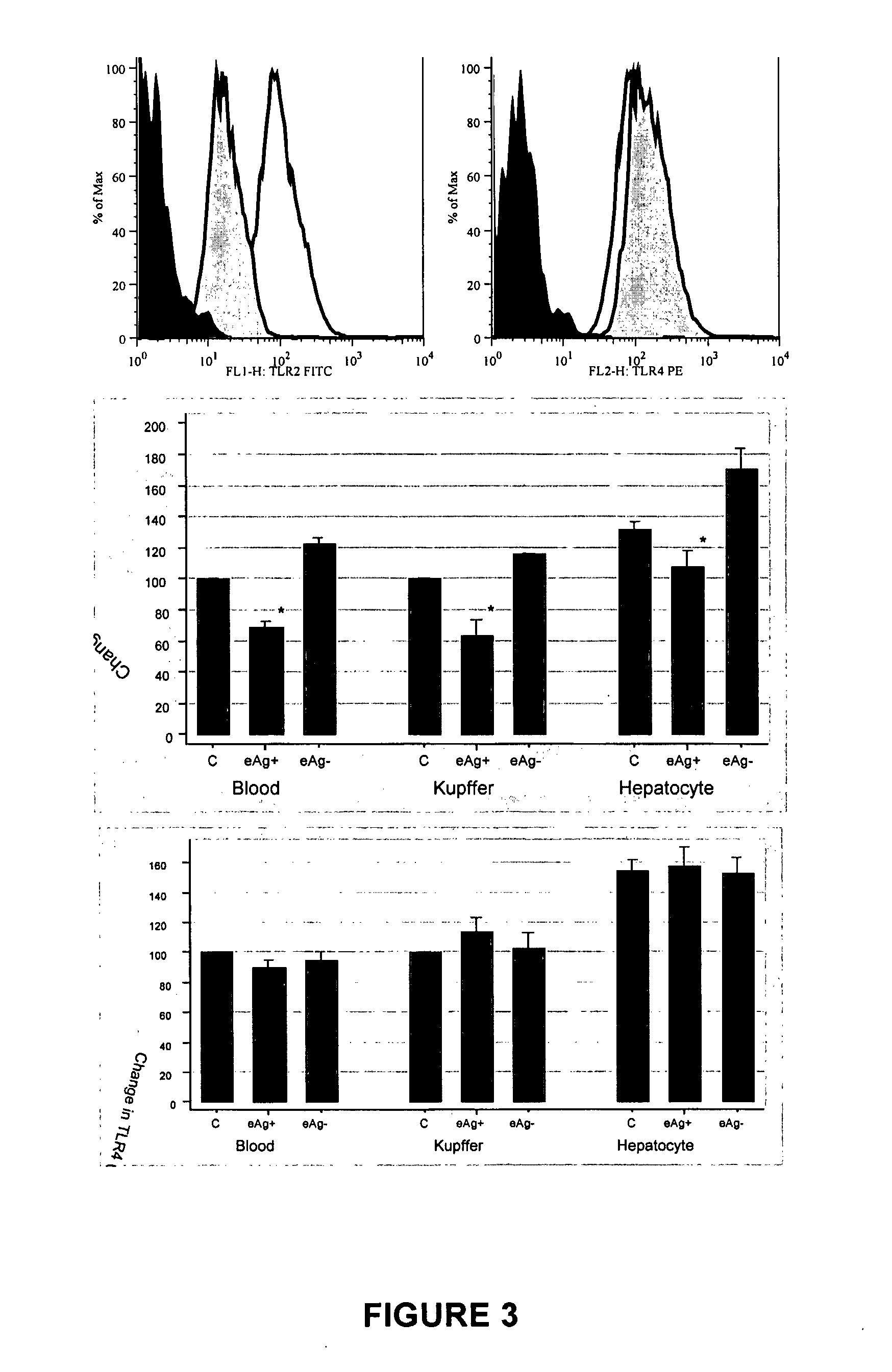
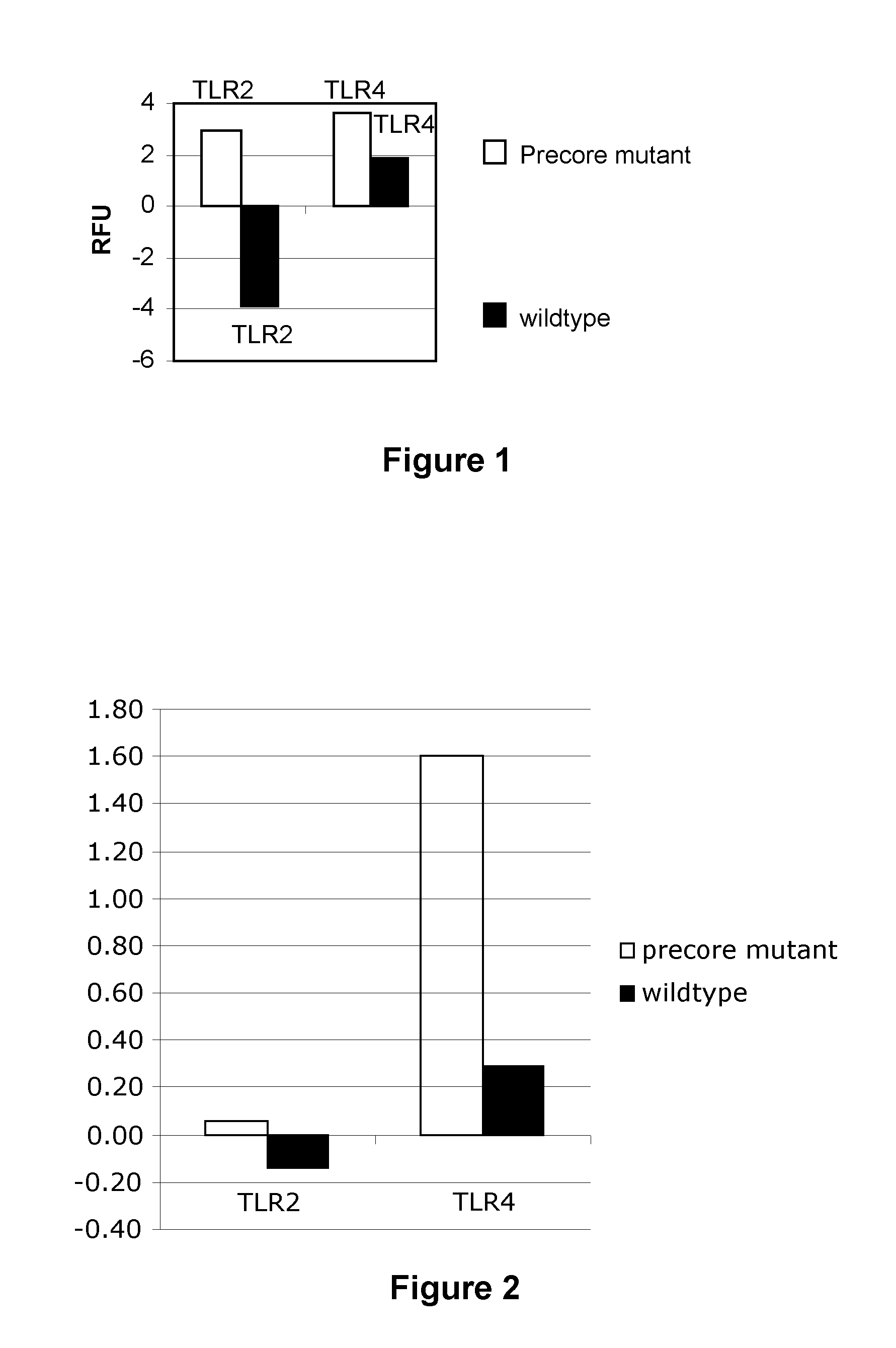
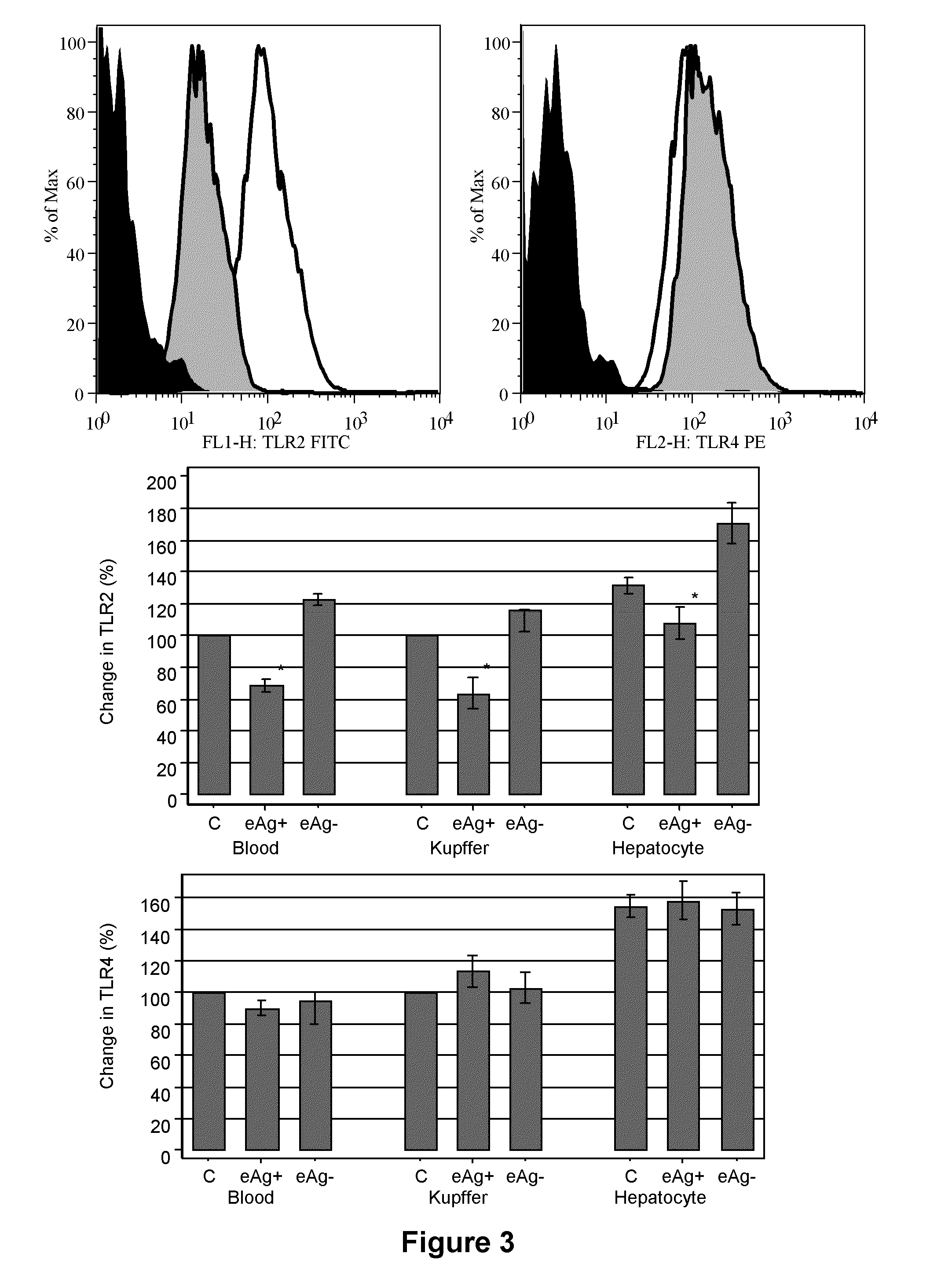
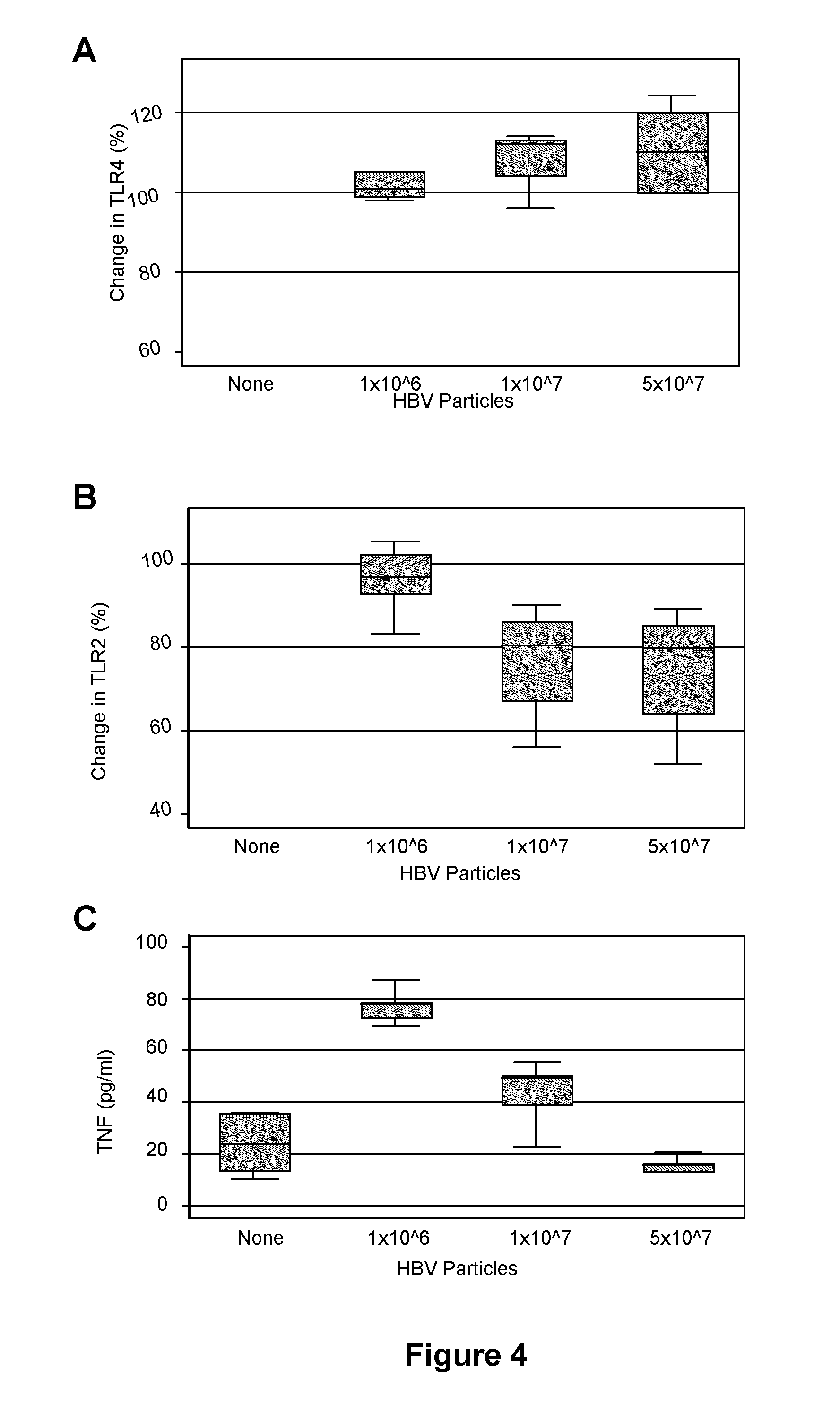
The present invention provides regulation of expression of toll-like receptors by the hepatitis B (HBV) pre-core protein, or its extracellular expression product the hepatitis B E antigen (HbeAg). Compounds regulating such expression have use in the treatment and prophylaxis of HBV infection in animal. The invention also provides methods for diagnosing HBV and agents useful in diagnostic protocols. The present invention further contemplates methods for monitoring disease states in humans and other animal species, including animal models, and providing an indication of the subject for infection by HBV, or development of other diseased states.
Owner:MELBOURNE HEALTH +1
Therapeutic, prophylactic and diagnostic agents for hepatitis b
InactiveUS20100003262A1Promote infectionOrganic active ingredientsPeptide/protein ingredientsDiseaseHepatitis b e antigen
The present invention provides regulation of expression of toll-like receptors by the hepatitis B (HBV) pre-core protein, or its extracellular expression product the hepatitis B E antigen (HbeAg). Compounds regulating such expression have use in the treatment and prophylaxis of HBV infection in animal. The invention also provides methods for diagnosing HBV and agents useful in diagnostic protocols. The present invention further contemplates methods for monitoring disease states in humans and other animal species, including animal models, and providing an indication of the subject for infection by HBV, or development of other diseased states.
Owner:MELBOURNE HEALTH +1
Hepatitis B virus e antigen quantitative detection kit, preparation method and detection method thereof
InactiveCN105572358AHigh detection sensitivityStrong specificityMaterial analysisAntigenHepatitis B immunization
The present invention discloses a hepatitis B virus e antigen detection kit, which comprises a hepatitis B e antigen calibration substance, an anti-HBe monoclonal antibody coated plate, europium labeled anti-HBe monoclonal antibody, an assay buffer liquid, a fluorescence enhanced liquid and a concentrated washing liquid. The present invention further discloses a preparation method of the kit, and a method for detecting hepatitis B virus e antigen by using the kit. According to the present invention, the kit has advantages of high detection sensitivity, good specificity, simple operation, no pollution, and low cost, can be used for quantitative detection of the HBeAg content in human serum / plasma, and provides precise reference for the clinical hepatitis B individualized management treatment program.
Owner:SUZHOU SYM BIO LIFESCI CO LTD
Application of dehydrocorydaline and scoulerine to medicament production
InactiveCN102078319BGood effectSignificant anti-HBV activityOrganic active ingredientsAntiviralsHepatitis b e antigenHepatitis B Surface Antigens
The invention discloses application of dehydrocorydaline and scoulerine to medicament production. In the invention, a diagnostic kit for hepatitis B virus surface antigen and a diagnostic kit for hepatitis B virus e antigen are adopted to perform tests of resisting hepatitis B surface antigen and hepatitis B e antigen on protoberberine alkaloids scoulerine and dehydrocorydaline respectively. Results prove that the dehydrocorydaline and the scoulerine have remarkable hepatitis B virus resisting activity, and can be used for preparing hepatitis B virus resistant medicament.
Owner:SUN YAT SEN UNIV
Application of benzoyl silibinin for preparing medicaments for treating viral hepatitis B
InactiveCN101822666AThe source is easy to getThe synthesis method is simpleOrganic active ingredientsOrganic chemistryDiseasePositive control
The invention relates to application of benzoyl silibinin for preparing medicaments for treating viral hepatitis B, in particular to application of 23-bit 3,4-dimethoxy-benzoyl substituted silibinin ester or medicinal salts thereof for preparing medicaments which are used for eliminating Hepatitis B virus surface antigen (HBsAg) and hepatitis B e antigen (HBeAg) and inhibiting HBV (Hepatitis B Virus) DNA replication. The compound plays an extremely remarkable role in inhibiting HBsAg and HBeAg activities, and the strengths for eliminating the HBsAg and the HBeAg under the concentration of 20 micrograms / ml in the compound are respectively 93.3 percent and 91.2 percent; meanwhile, the compound displays more than 99 percent of inhibition ratio to HBV DNA under the concentration of 20 micrograms / ml and has 23 percent of inhibition ratio higher than that of positive control medicine Lamivudine and 2.6 times higher than that of Alpha-interferon. Results indicate the application of flavone lignans and medicinal salts which are expectedly used for preparing non-nucleoside medicaments for eliminating the HBsAg and the HBeAg, inhibiting HBV DNA replication and treating hepatitis B virus infection diseases.
Owner:DALI UNIV
Application of liposome to treatment of chronic viral hepatitis B
InactiveCN107028887AGood stability in vitroEasy to manufacturePowder deliveryOrganic active ingredientsAntigenCholesterol
Owner:JIANGSU MENDEL GENE TECH CO LTD
Application of benzyl-containing flavonoid lignan in preparation of medicament for treating hepatitis B
InactiveCN101912387AConvenient sourceThe source is easy to getOrganic active ingredientsAntiviralsAntigenDisease
The invention relates to application of benzyl-containing flavonoid lignan in preparation of a medicament for treating hepatitis B, in particular to application of flavonoid lignan and pharmaceutically-acceptable salt thereof in preparation of the medicament for eliminating hepatitis B e antigen, inhibiting hepatitis B virus desoxyribonucleic acid (HBV DNA) replication and treating viral hepatitis B. The flavonoid lignan can inhibit the activity of hepatitis B virus e antigen (HBeAg); the inhibition intensity of the flavonoid lignan under low concentration of 20 micrograms per milliliter is much higher than that of a positive control front-line medicament lamivudine and an interferon; and the compound with the concentration of 20 micrograms per milliliter displays an inhabitation ratio over 50 percent on the HBV DNA, so that the application of the flavonoid lignan in preparation of the medicament for eliminating the hepatitis B e antigen and the medicament for inhibiting the HBV DNA replication and treating hepatitis B viral diseases can be anticipated.
Owner:DALI UNIV
Methods of diagnosing viral infection
ActiveUS20200010898A1Well formedMicrobiological testing/measurementMaterial analysisAntigenHepatitis B immunization
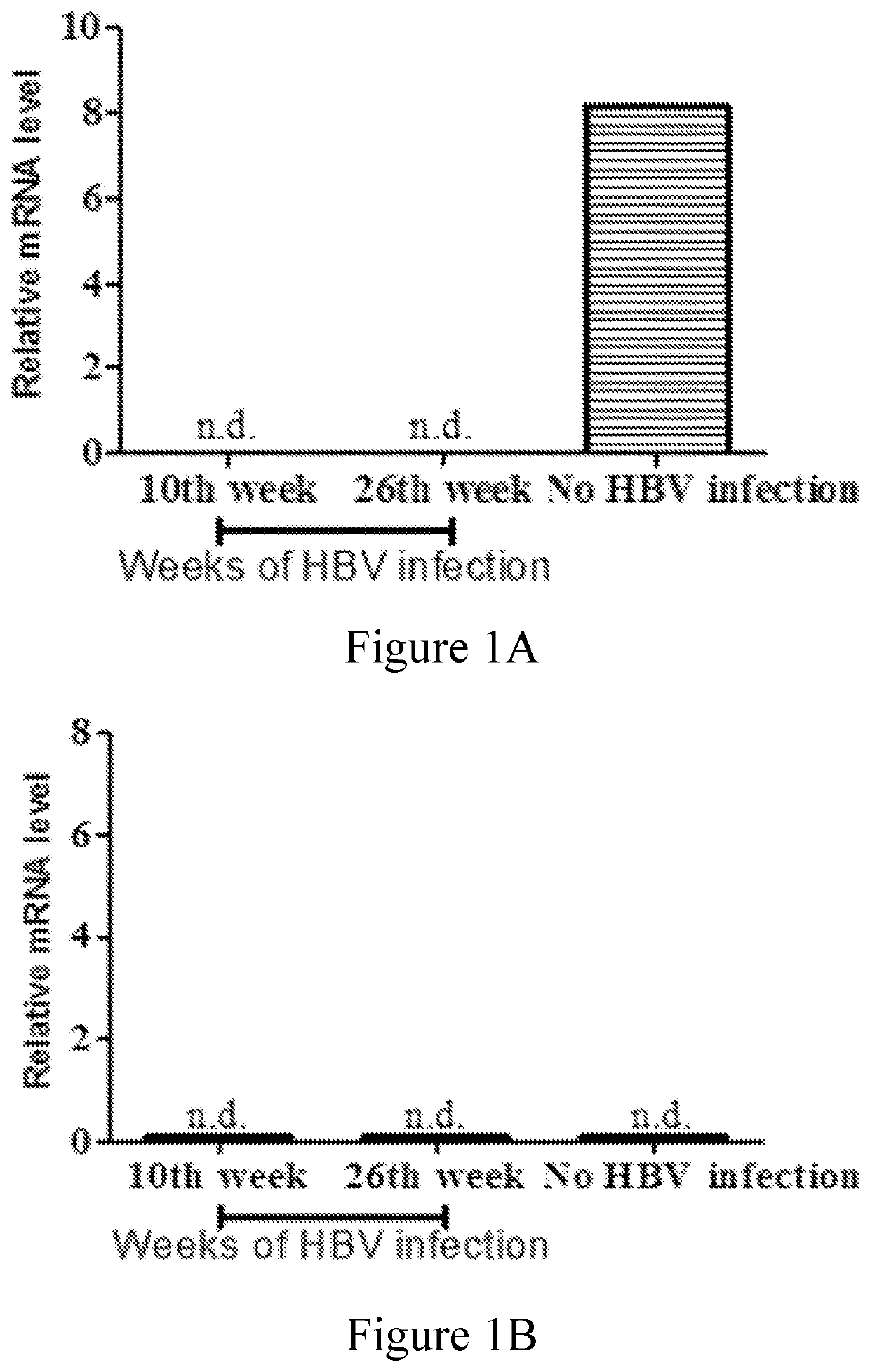
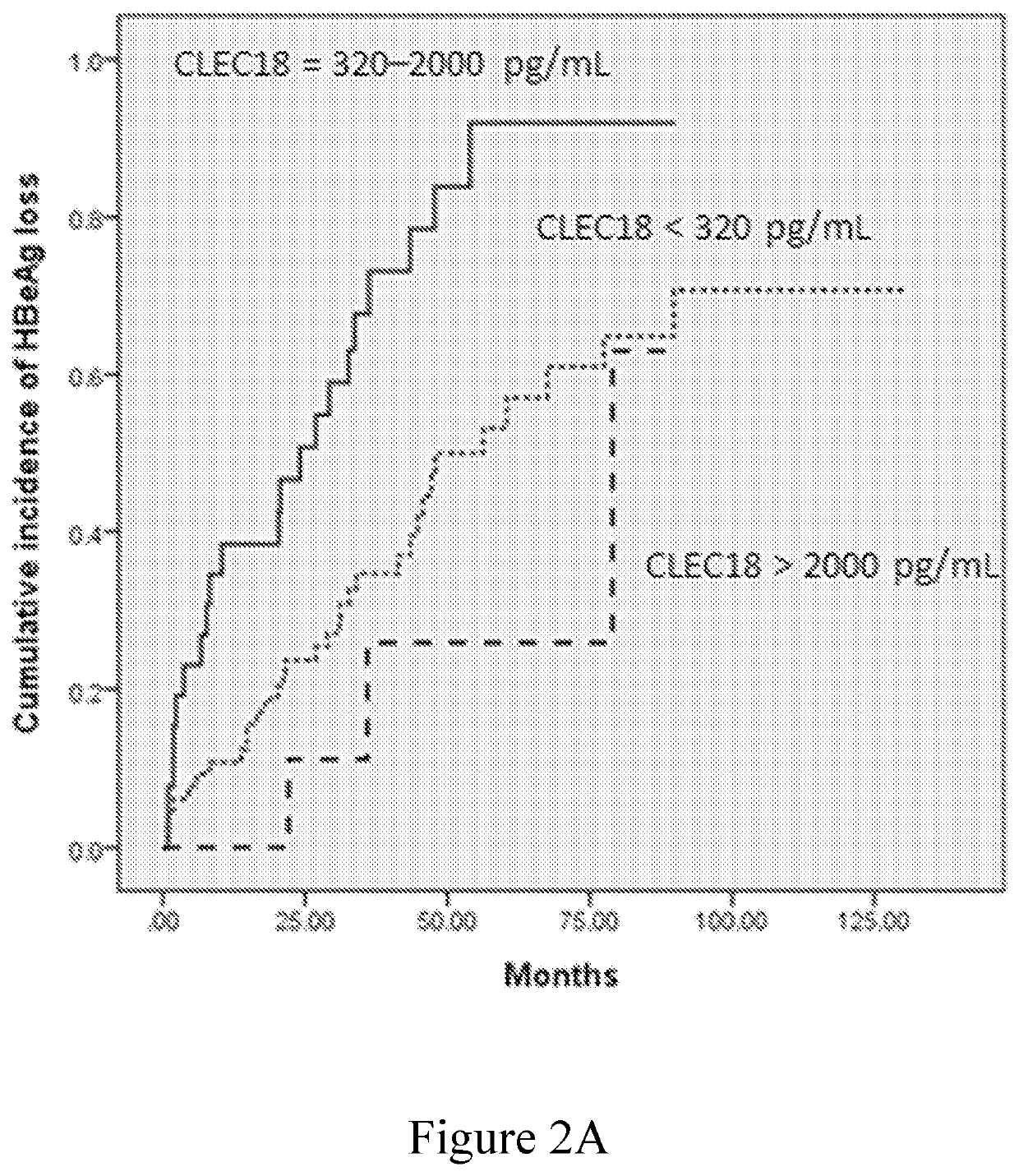
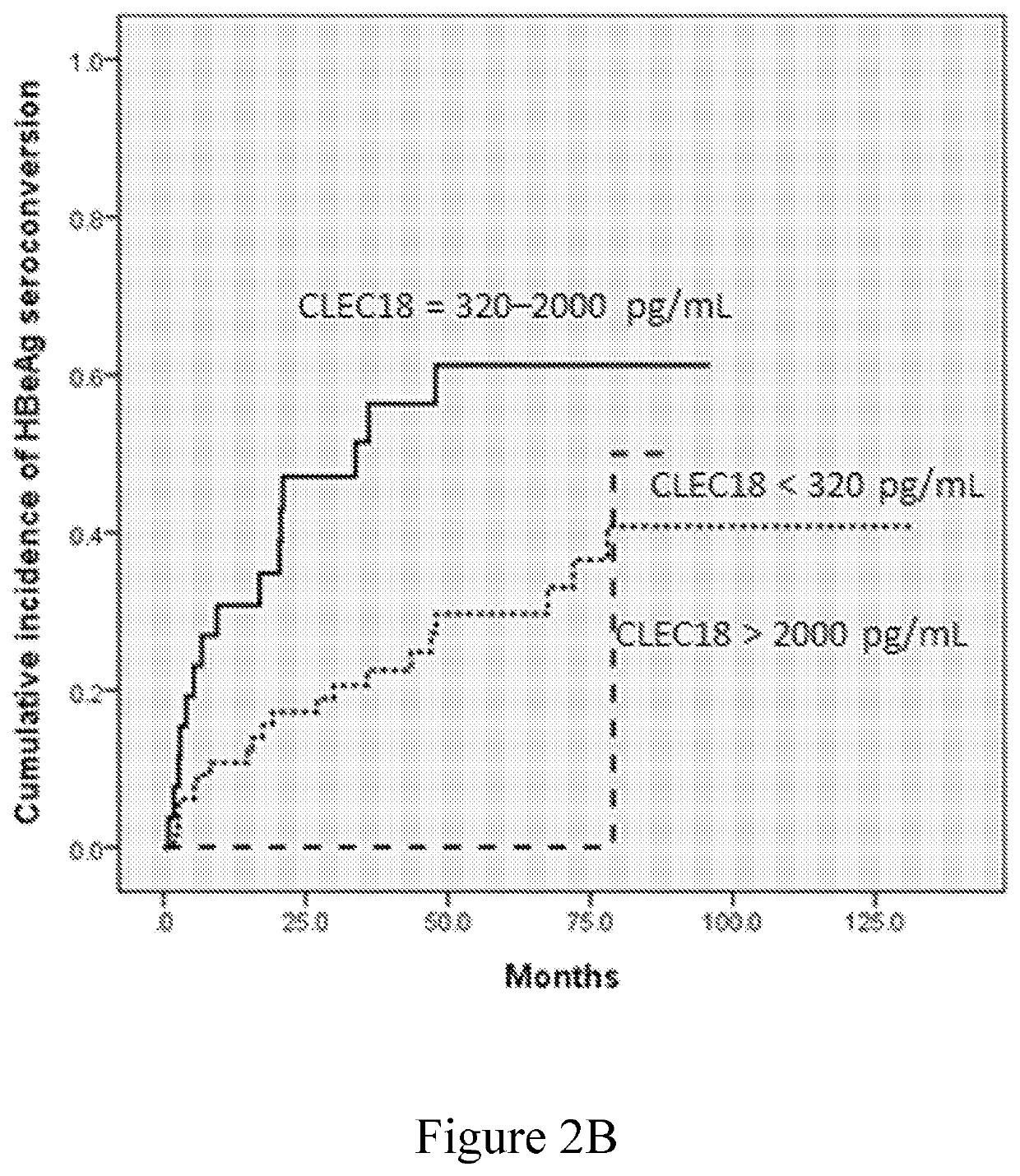
Disclosed herein is a novel use of C-type lectin 18 (CLEC18) in disease prognosis. According to embodiments of the present disclosure, the mRNA or protein level of CLEC18 may serve as an indicator for diagnosing hepatitis B virus (HBV) infection, hepatitis B e antigen (HBeAg) loss and seroconversion, and / or liver fibrosis.
Owner:ACAD SINIC
Application of flavanonol lignanoid in preparing antiviral hepatitis B medicine
InactiveCN101829100AConvenient sourceThe source is easy to getOrganic active ingredientsAntiviralsDiseasePositive control
The invention relates to application of flavanonol lignanoid in preparing an antiviral hepatitis B medicine, in particular to application of angle flavanolignan or pharmaceutically acceptable salts thereof in preparing a medicine which can be used for eliminating hepatitis B e antigen HBeAg, inhibiting HBV DNA replication and treating hepatitis B virus infection diseases. The flavanolignan has a certain HBeVg activity inhibition, the HBeAg elimination intensity of the flavanolignan is higher than that of a positive control first-line medicine as lamivudine in light concentration and is equivalent to that of alpha-interferon of 1000 unit / ml, and meanwhile, under the concentration of 20 microgram / ml, the flavanolignan displays an inhibition ratio which is larger than 45 percent for HBV DNA. The pharmacodynamics result indicates the application of the flavanolignan or the pharmaceutically acceptable salts thereof in preparing the medicine as expected for eliminating hepatitis B e antigen, inhibiting HBV DNA replication and treating hepatitis B virus infection diseases.
Owner:DALI UNIV
Fat mesenchymal progenitor cell and platelet-rich blood plasma composition for treatment of hepatitis B
InactiveCN105640993AGood treatment effectDigestive systemMammal material medical ingredientsAntigenBlood plasma
The invention relates to a use of a fat mesenchymal progenitor cell and platelet-rich blood plasma composition in prevention or treatment of hepatitis, particularly, relates to a use of the fat mesenchymal progenitor cell and platelet-rich blood plasma composition in preparation of a drug composition for treatment of hepatitis B. After a required object is administered with the drug composition, indexes such as hepatitis B surface antigen, hepatitis B surface antibody, hepatitis B e antigen, hepatitis B e antibody, hepatitis B core antibody and the like can be improved, hepatitis virus DNA can be significant reduced, and ALT can be reduced so as to promote restoration of liver functions.
Owner:CELLULAR BIOMEDICINE GRP SHANGHAI +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com