Patents
Literature
350 results about "Activity inhibition" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Competitive inhibition inhibition of enzyme activity in which the inhibitor (a substrate analogue) competes with the substrate for binding sites on the enzymes. contact inhibition inhibition of cell division and cell motility in normal animal cells when in close contact with each other.
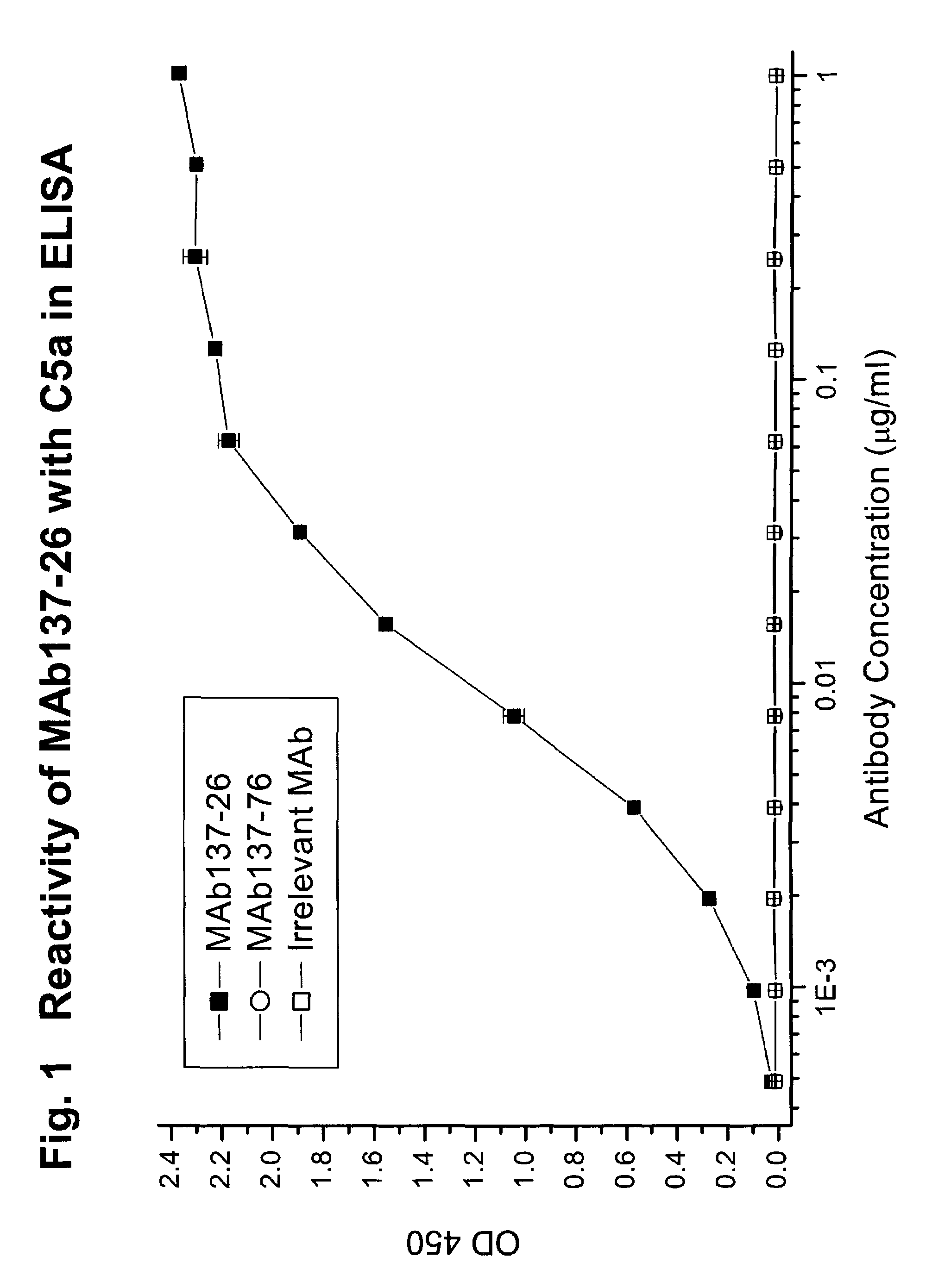
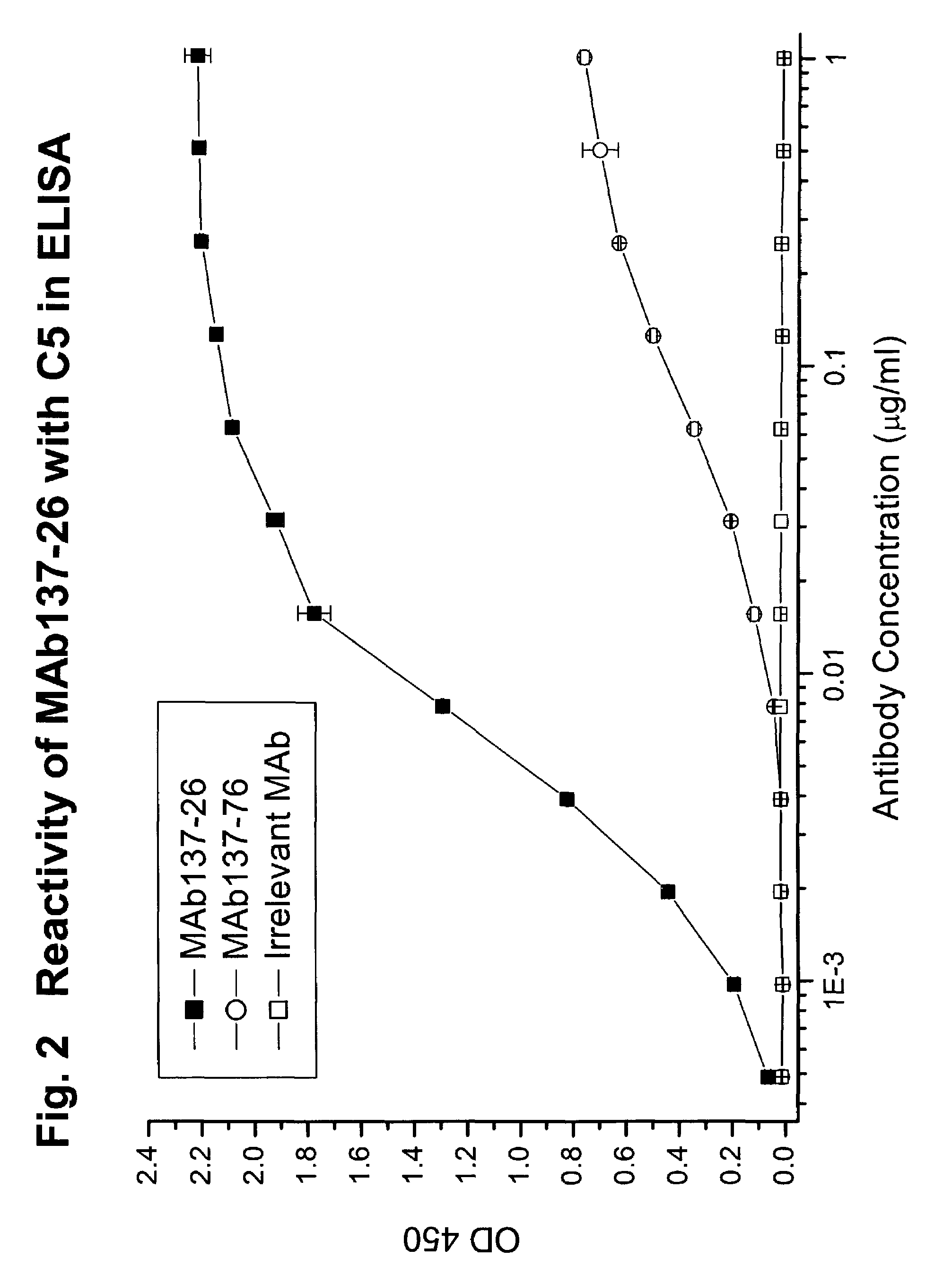
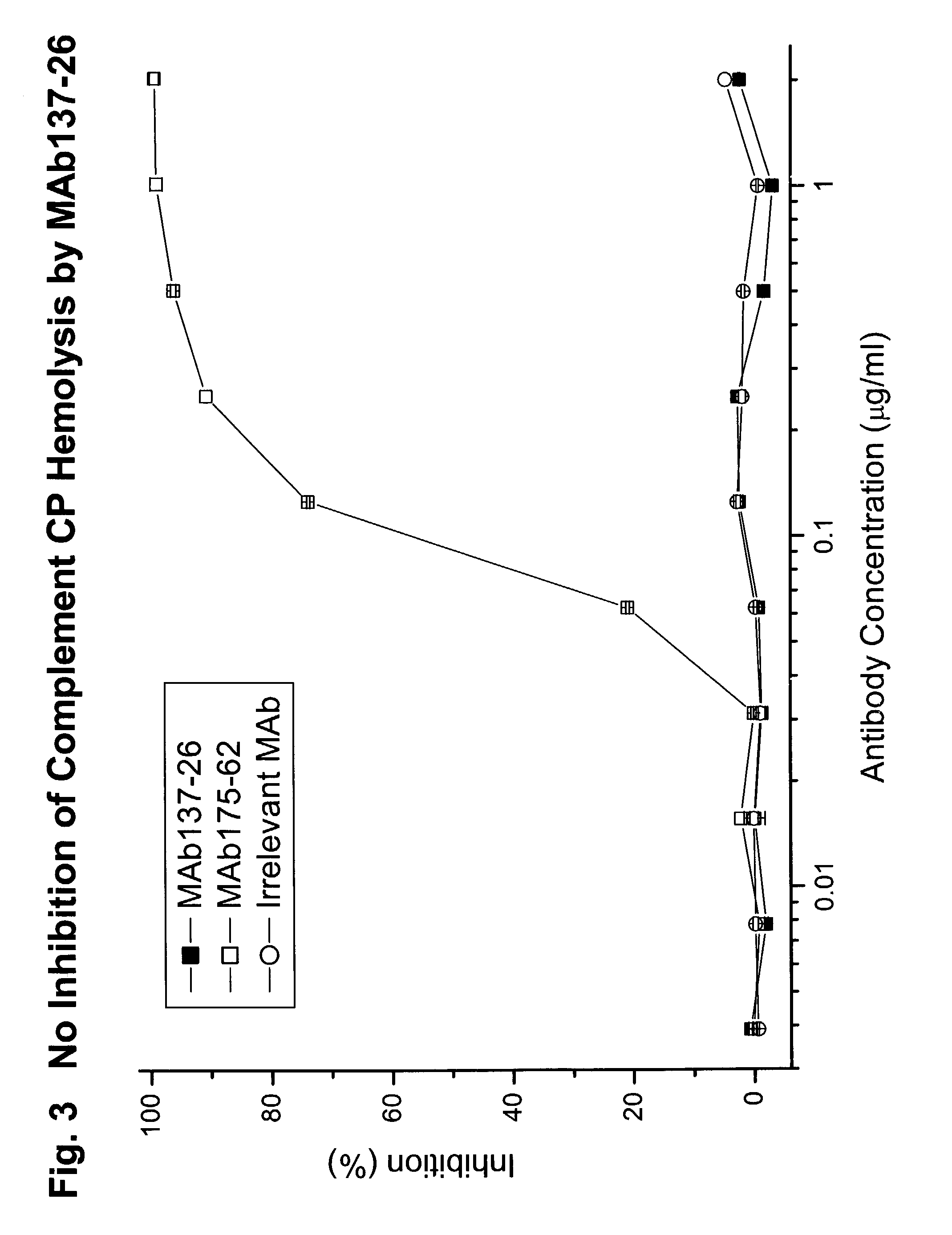
Complement pathway inhibitors binding to C5 and C5a without preventing formation of C5b
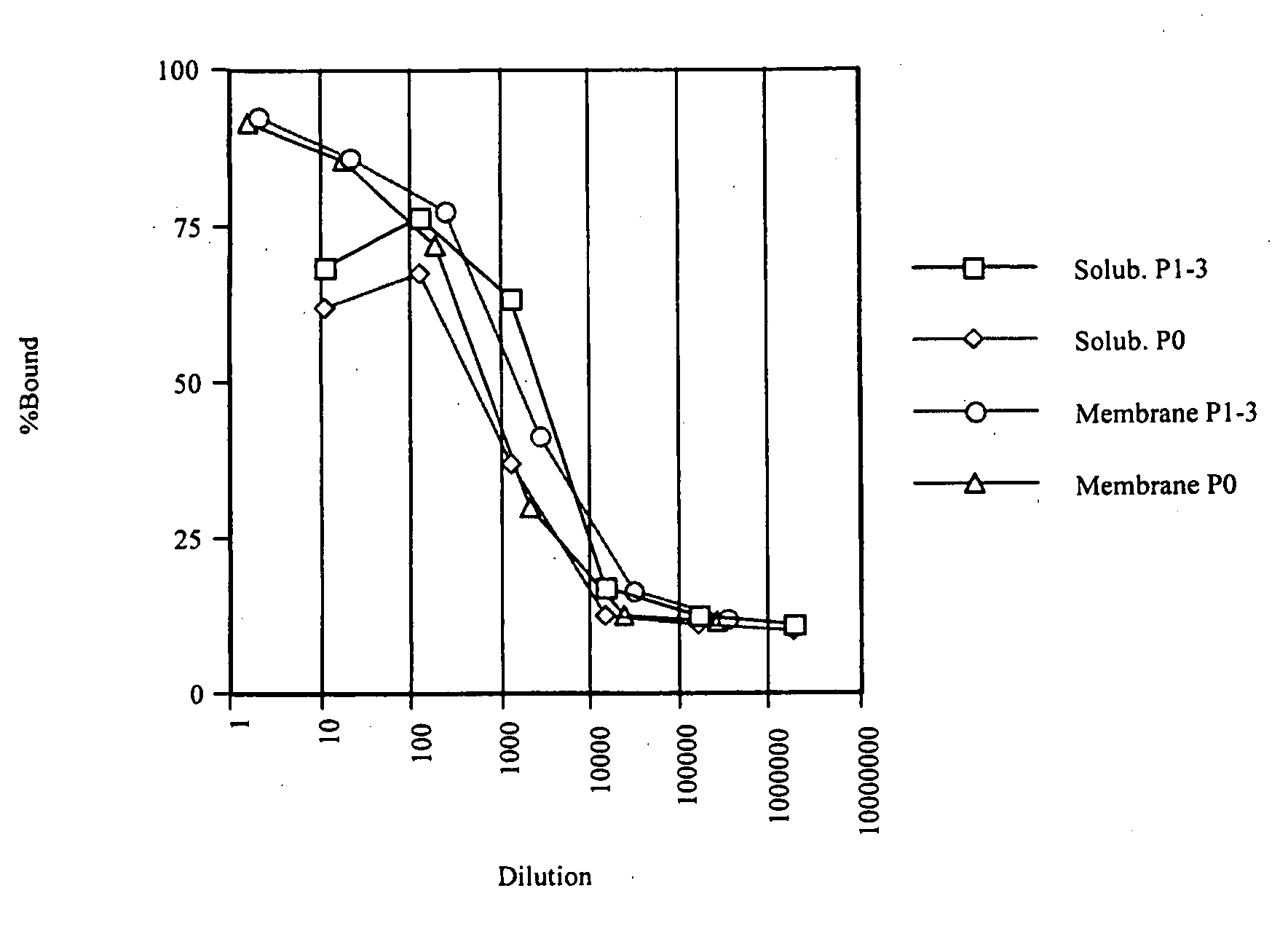
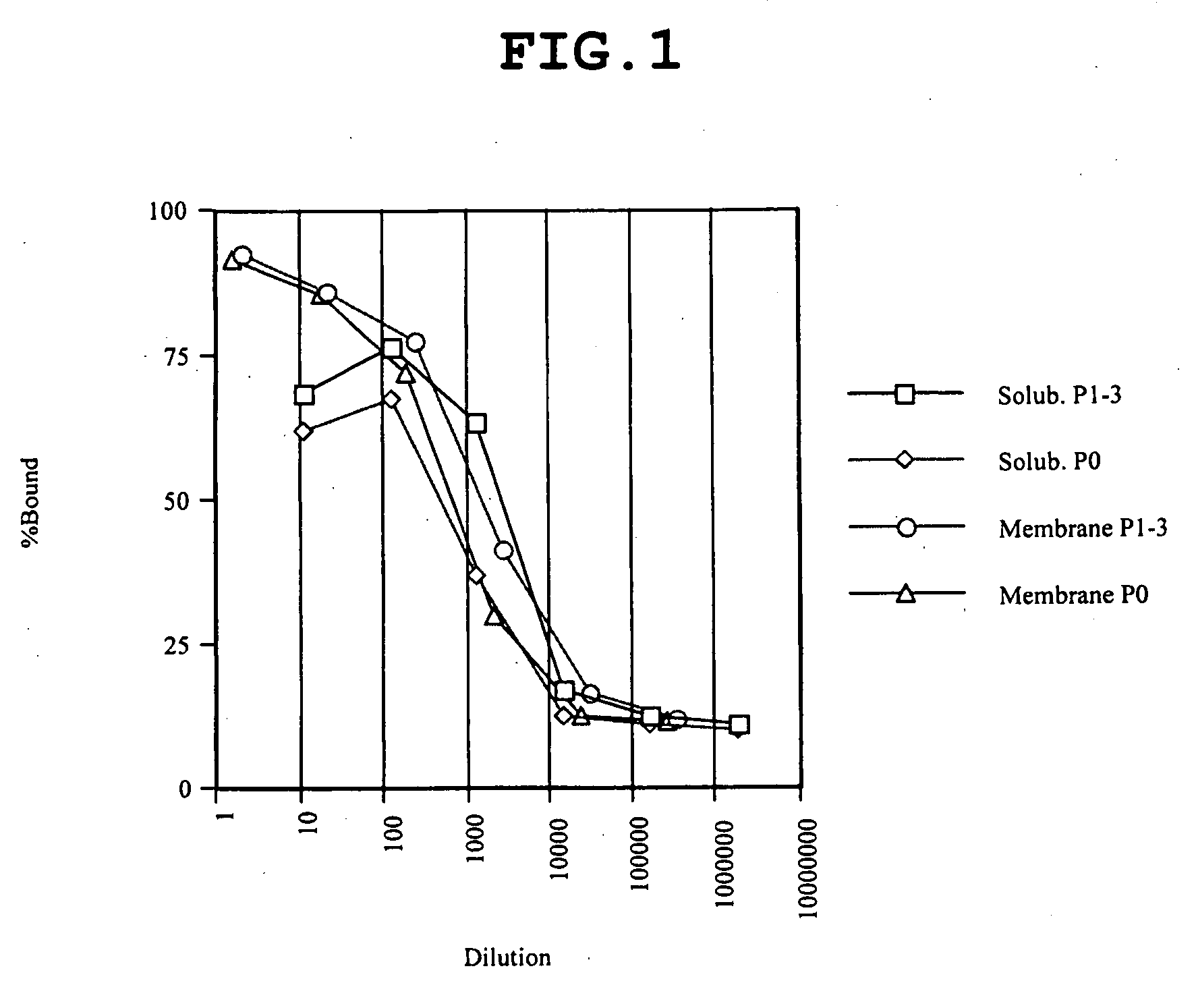
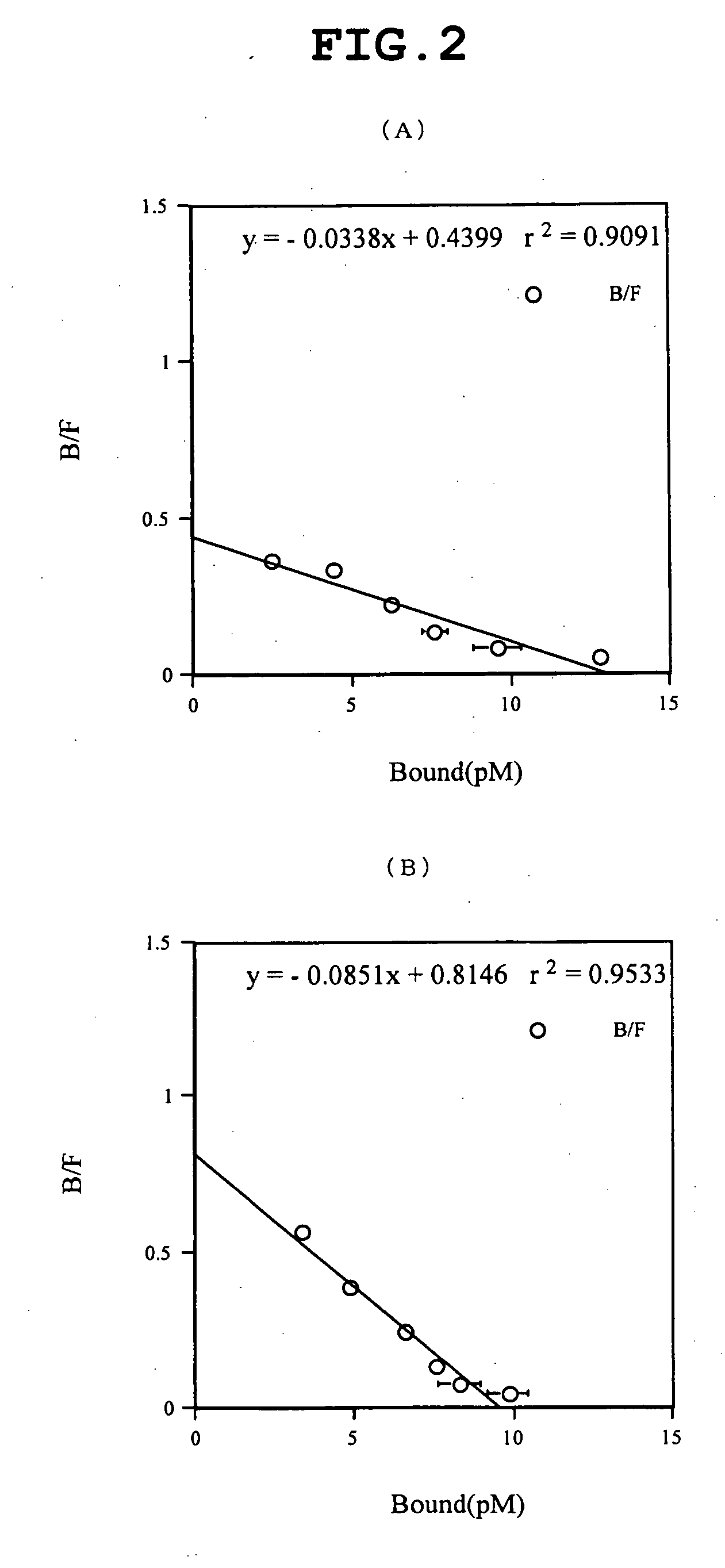
The invention relates to inhibitors that bind to C5 and C5a, but which do not prevent the activation of C5 and do not prevent formation of or inhibit the activity of C5b. One example of such an inhibitor molecule is the monoclonal antibody designated MAb137-26, which binds to a shared epitope of human C5 and C5a. These inhibitors may be used to inhibit the activity of C5a in treating diseases and conditions mediated by excessive or uncontrolled production of C5a. The inhibitor molecules are also useful for diagnostic detection of the presence / absence or amount of C5 or C5a.
Owner:GENENTECH INC
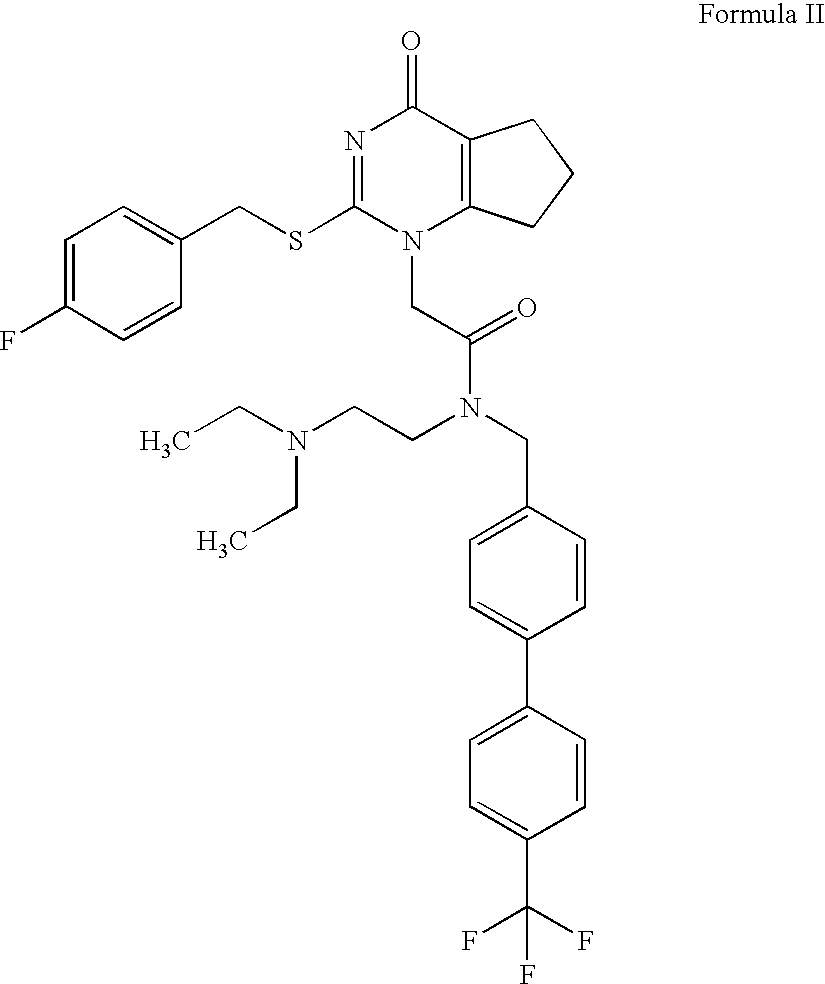
Imidazo[4, 5-b]pyridin-2-one and oxazolo[4, 5-b]pyridin-2-one compounds and analogs thereof as cancer therapeutic compounds
InactiveUS20090325945A1Promote apoptosisInhibiting cell cycle progressionBiocideOrganic chemistryDiseaseMelanoma
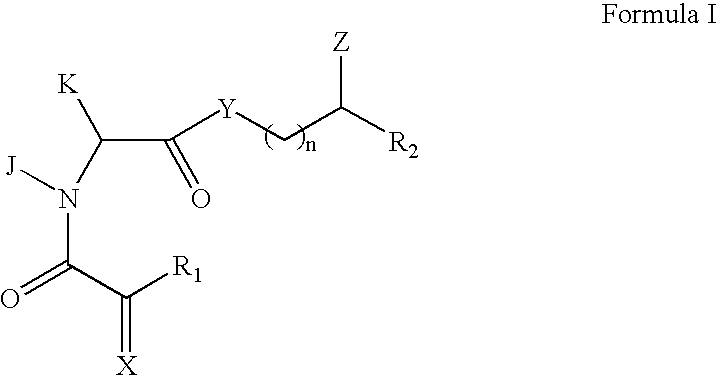
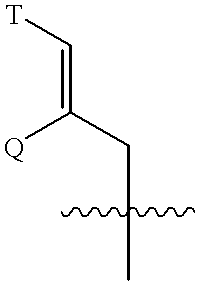
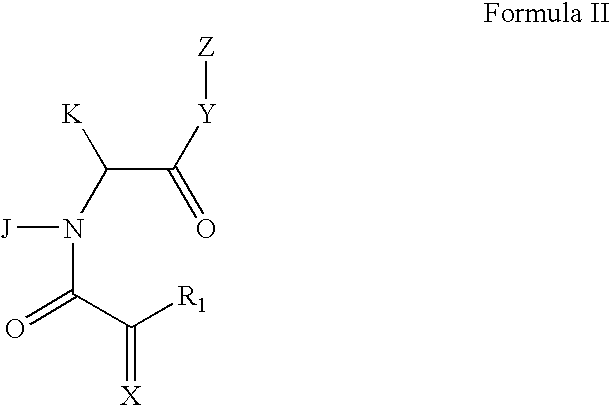
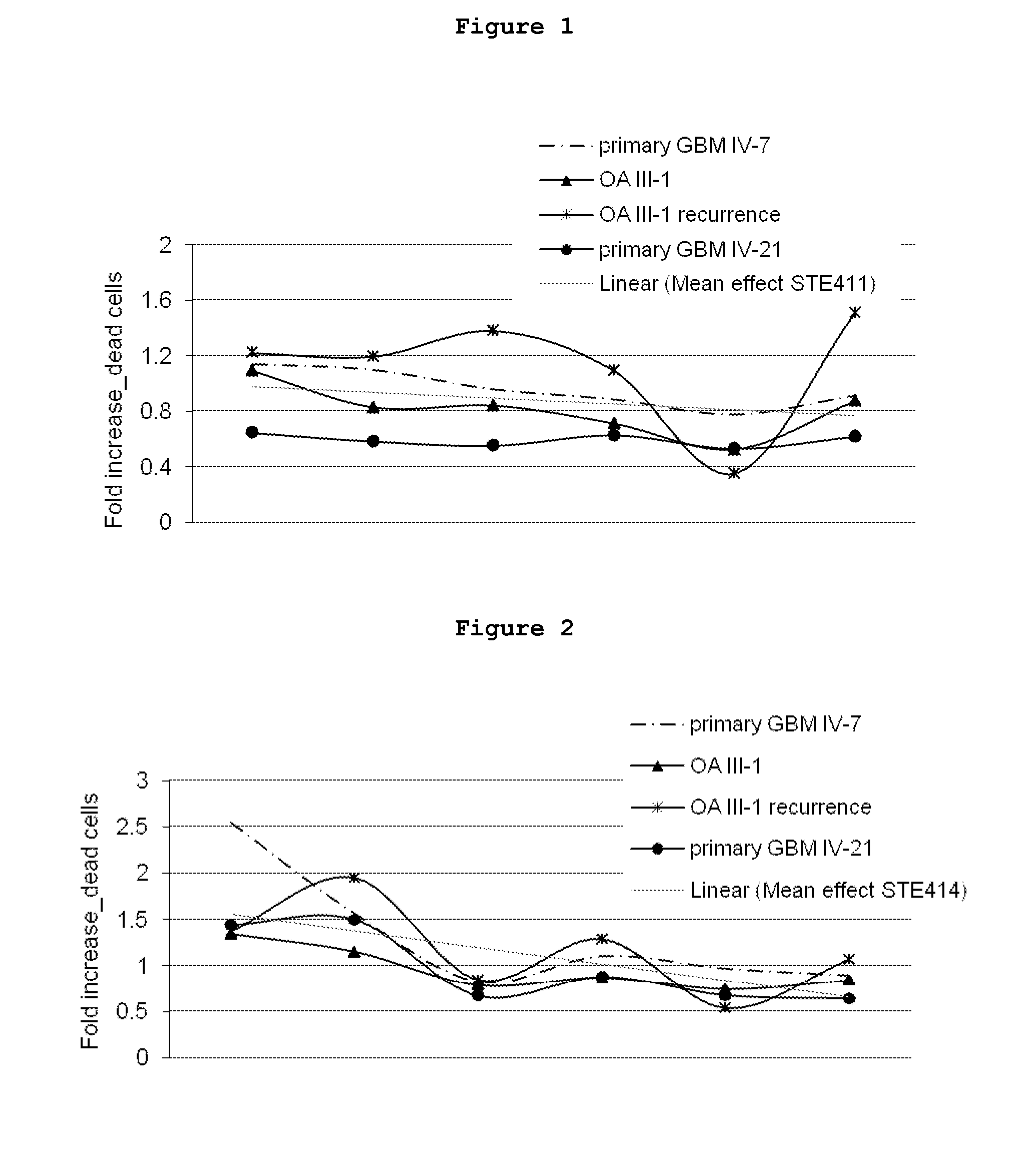
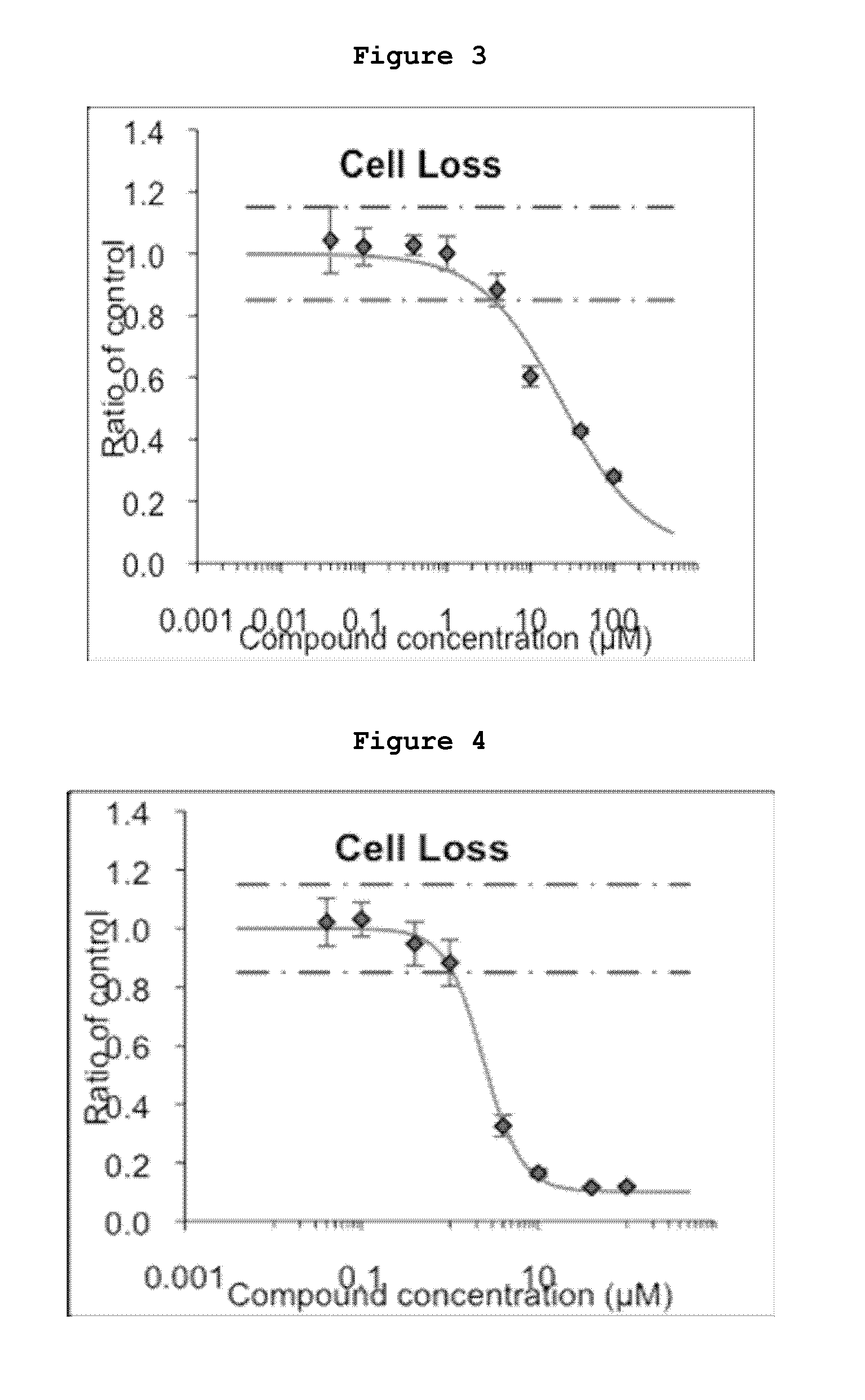
The present invention pertains to certain imidazo[4,5-b]pyridin-2-one and oxazolo[4,5 b]pyridin-2-one compounds and analogs thereof, which, inter alia, inhibit RAF (e.g., B RAF) activity, inhibit cell proliferation, treat cancer, etc., and more particularly to compounds of the formulae: wherein: J is independently —O— or —NRN1−; RN1, if present, is independently —H or a substituent; RN2 is independently —H or a substituent; Y is independently —CH═ or —N═; Q is independently —(CH2)j-M-(CH2)k— wherein: j is independently 0, 1 or 2; k is independently 0, 1, or 2; j+k is 0, 1, or 2; M is independently O—, —S—, —NH—, —NMe-, or —CH2—; each of RP1, RP2, RP5, and RP4 is independently —H or a substituent; and additionally RP1 and RP2 taken together may be CH═CH—CH═CH—; and additionally RP1 and RP5 taken together may be CH═CH—CH═CH—; L is independently: a linker group formed by a chain of 2, 3, or 4 linker moieties; each linker moiety is independently CH2—, —NRN—, —C(═X)—, or —S(═O)2—; either: exactly one linker moiety is —NRN—, or: exactly two linker moieties are —NRN—; either: exactly one linker moiety is —C(═X)—, and no linker moiety is —S(═O)2—, or: exactly one linker moiety is —S(═O)2—, and no linker moiety is —C(═X)—; no two adjacent linker moieties are —NRN—; X is independently ═O or ═S; each RN is independently —H or a substituent; A is independently: C6-14carboaryl, C5-14heteroaryl, C3-12carbocyclic, C3-12heterocyclic; and is independently unsubstituted or substituted; and pharmaceutically acceptable salts, solvates, amides, esters, ethers, N-oxides, chemically protected forms, and prodrugs thereof. The present invention also pertains to pharmaceutical compositions comprising such compounds, and the use of such compounds and compositions, both in vitro and in vivo, to inhibit RAF (e.g., B-RAF) activity, to inhibit receptor tyrosine kinase (RTK) activity, to inhibit cell proliferation, and in the treatment of diseases and conditions that are ameliorated by the inhibition of RAF, RTK, etc., proliferative conditions such as cancer (e.g., colorectal cancer, melanoma), etc.
Owner:CANCER RES TECH LTD +1
Methods for the use of inhibitors of cytosolic phospholipase A2
This invention provides methods for the use of substituted indole compounds of the general formula: and pharmaceutically acceptable salt forms thereof. The invention provides methods for the use of the compounds as inhibitors of the activity of various phospholipase enzymes, particularly phospholipase A2 enzymes, and for the medical treatment, prevention and inhibition diseases and disorders including asthma, stroke, atherosclerosis, multiple sclerosis, Parkinson's disease, arthritic disorders, rheumatic disorders, central nervous system damage resulting from stroke, central nervous system damage resulting from ischemia, central nervous system damage resulting from trauma, inflammation caused or potentiated by prostaglandins, inflammation caused or potentiated by leukotrienes, inflammation caused or potentiated by platelet activation factor, pain caused or potentiated by prostaglandins, pain caused or potentiated by leukotrienes, and pain caused or potentiated by platelet activation factor.
Owner:ZIARCO
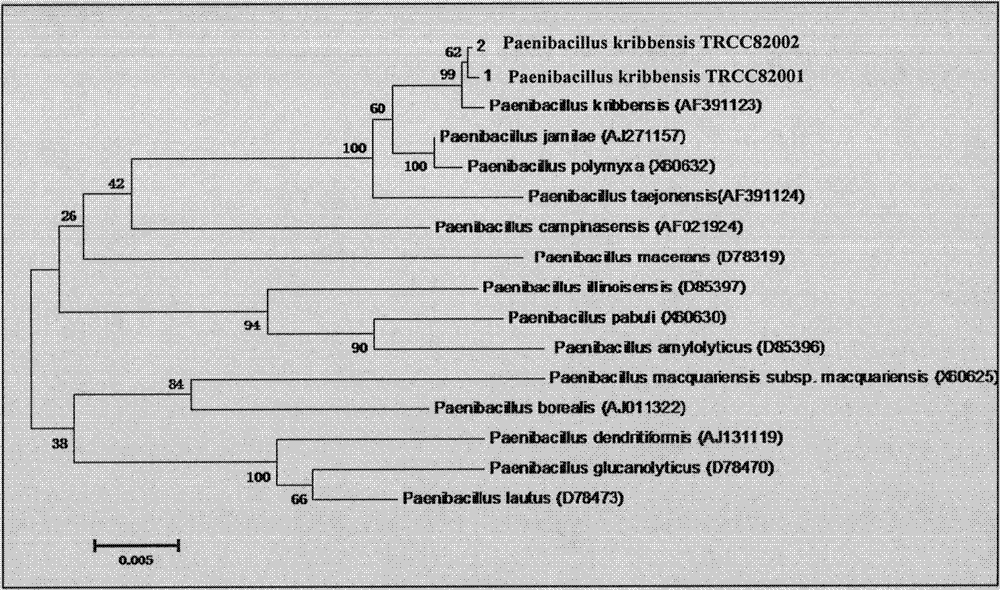
Paenibacillus kribbensis and its application
ActiveCN103756931AStrong broad-spectrum antibacterial activityGood effectBiocideBacteriaMetaboliteBacterial strain
The invention discloses a Paenibacillus kribbensis, and a preparation method and an application thereof. The Paenibacillus kribbensis has a bacterial code name of TRCC82001, is preserved in China General Microbiological Culture Collection Center, and has a preservation number of CGMCCNo.7996. The above bacterial strain has the advantages of good production property, good metabolite antimicrobial effect, and strong activity against rice blast and sheath blight, and a solution obtained after diluting a broth subjected to antibiotic treatment to 100 times has an inhibition effect of above 80% on the rice blast and sheath blight. The bacterial strain and its metabolites have a broad antimicrobial spectrum, can be used for preparing biocontrol preparations for the broad spectrum activity inhibition of pathogenic fungi to control the fungal diseases of plants.
Owner:JIANGXI TIANREN ECOLOGY
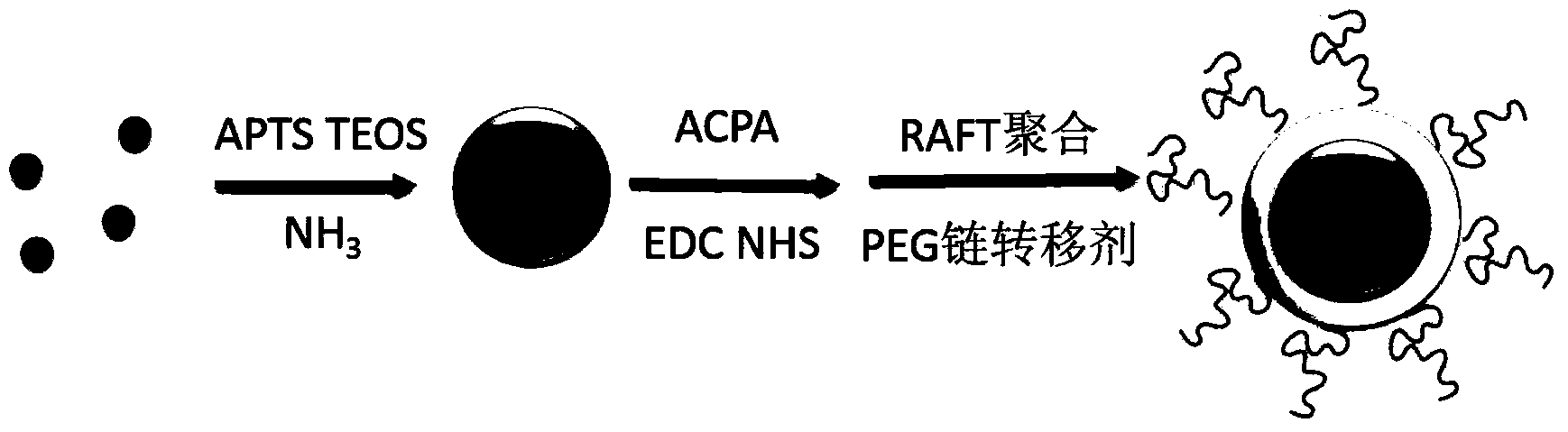
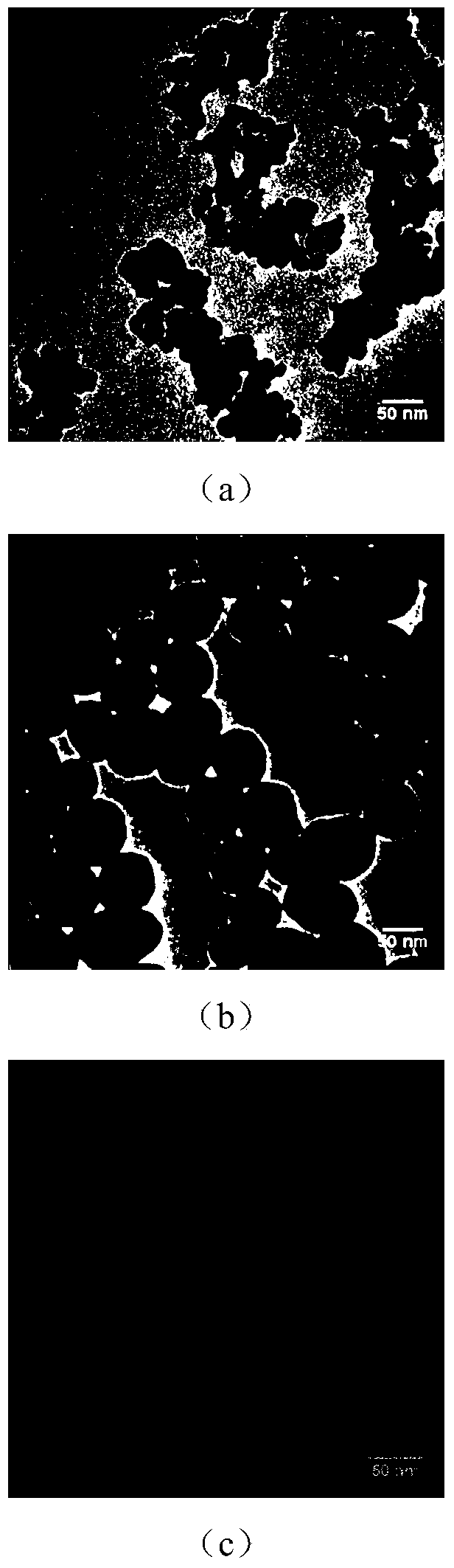
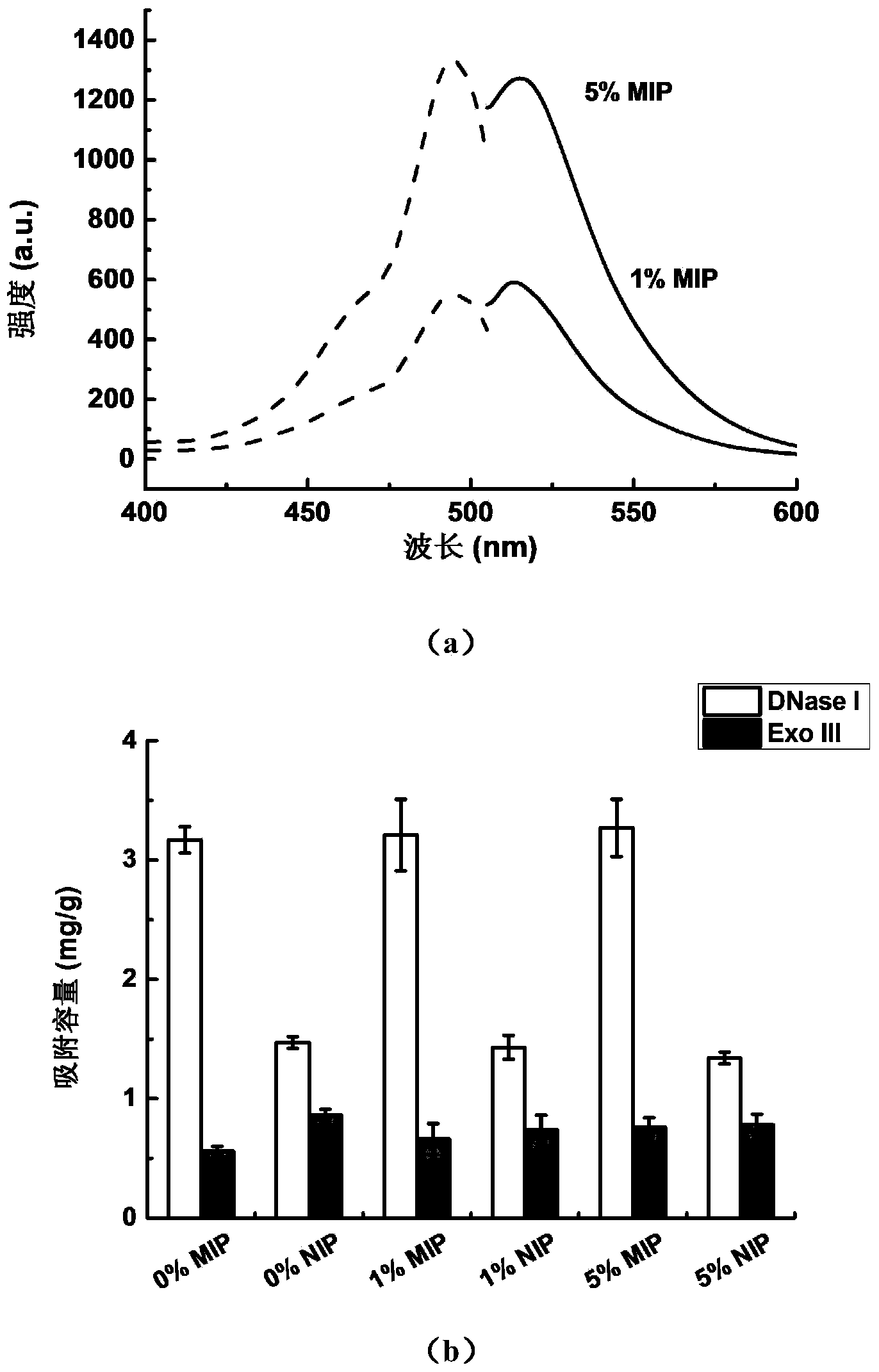
Magnetic molecularly imprinted nano-particle as well as preparation method and application thereof
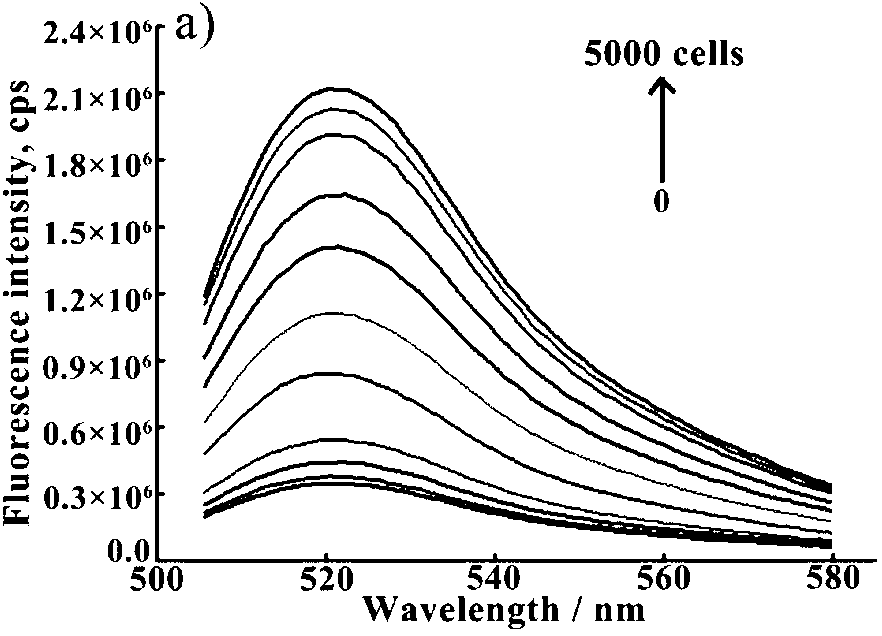
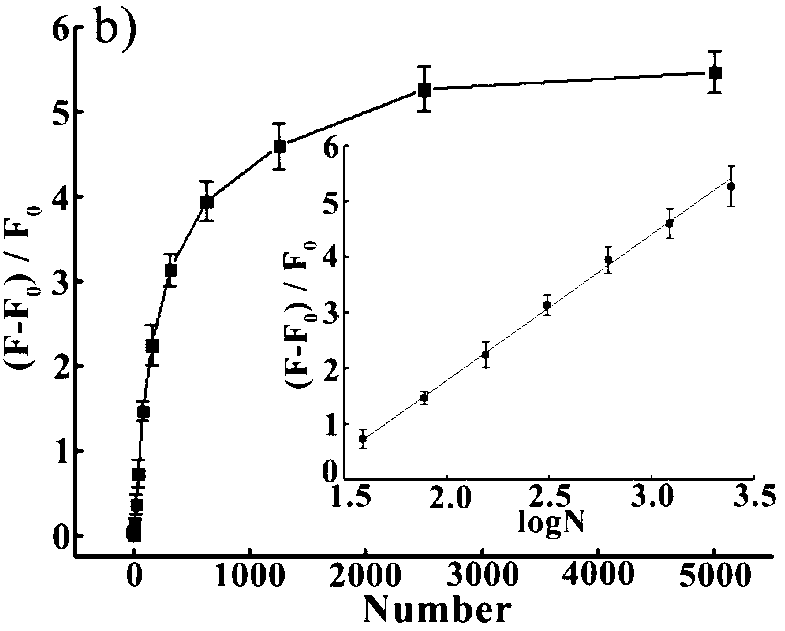
The invention provides a magnetic molecularly imprinted nano-particle as well as a preparation method and an application thereof. The nano-particle can perform specific recognition, trapping, separation and activity inhibition on target protein in a solution in vitro, more importantly, the nano-particle can enter a living cell rapidly under the action of a magnetic field and perform in-situ combination and activity inhibition on the target protein in the living cell, distribution of the nano-particles in the cell can be traced through a fluorescence microscope after fluorescence labeling, and quantitation can be performed through detection of fluorescence intensity.
Owner:PEKING UNIV
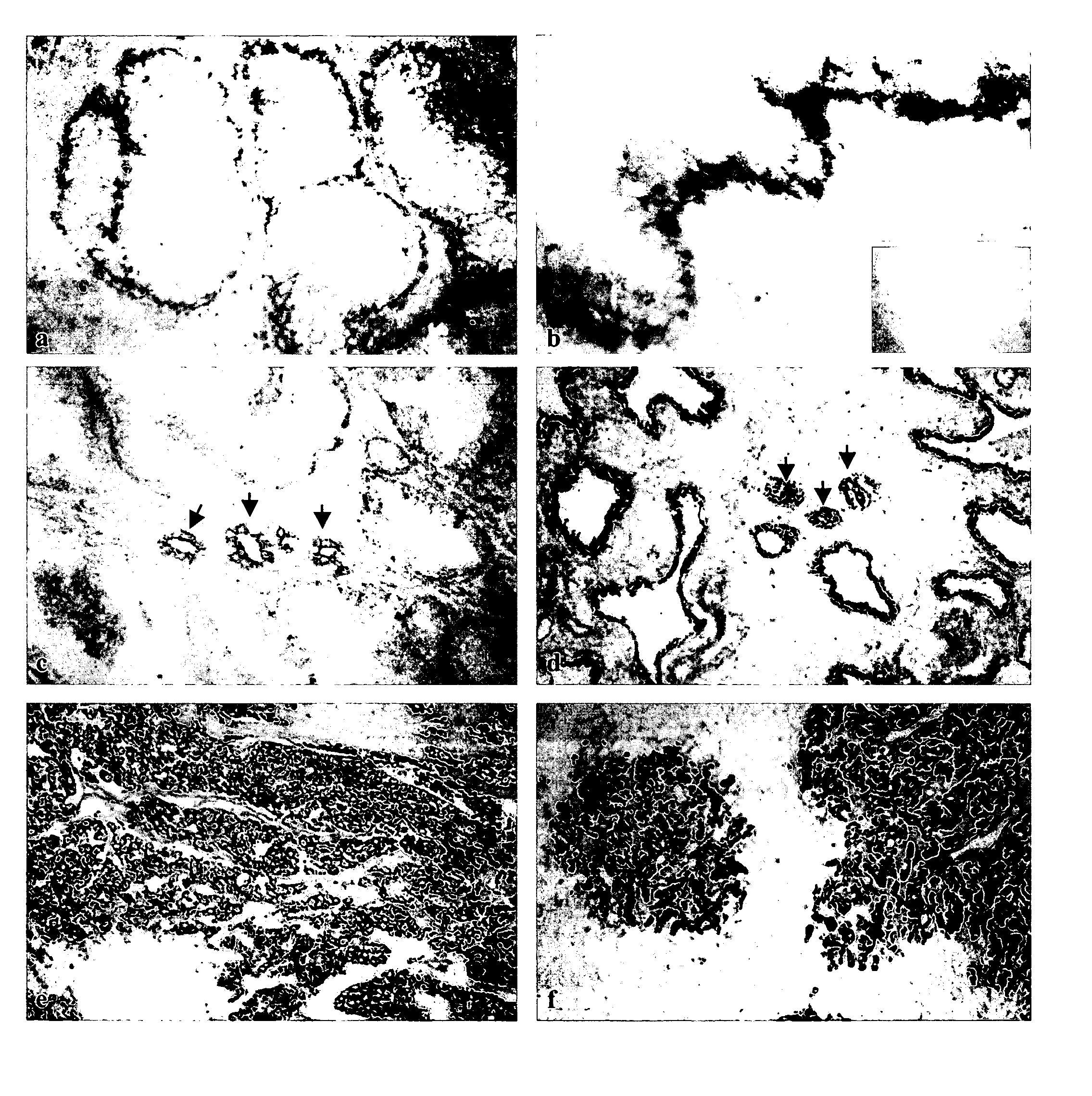
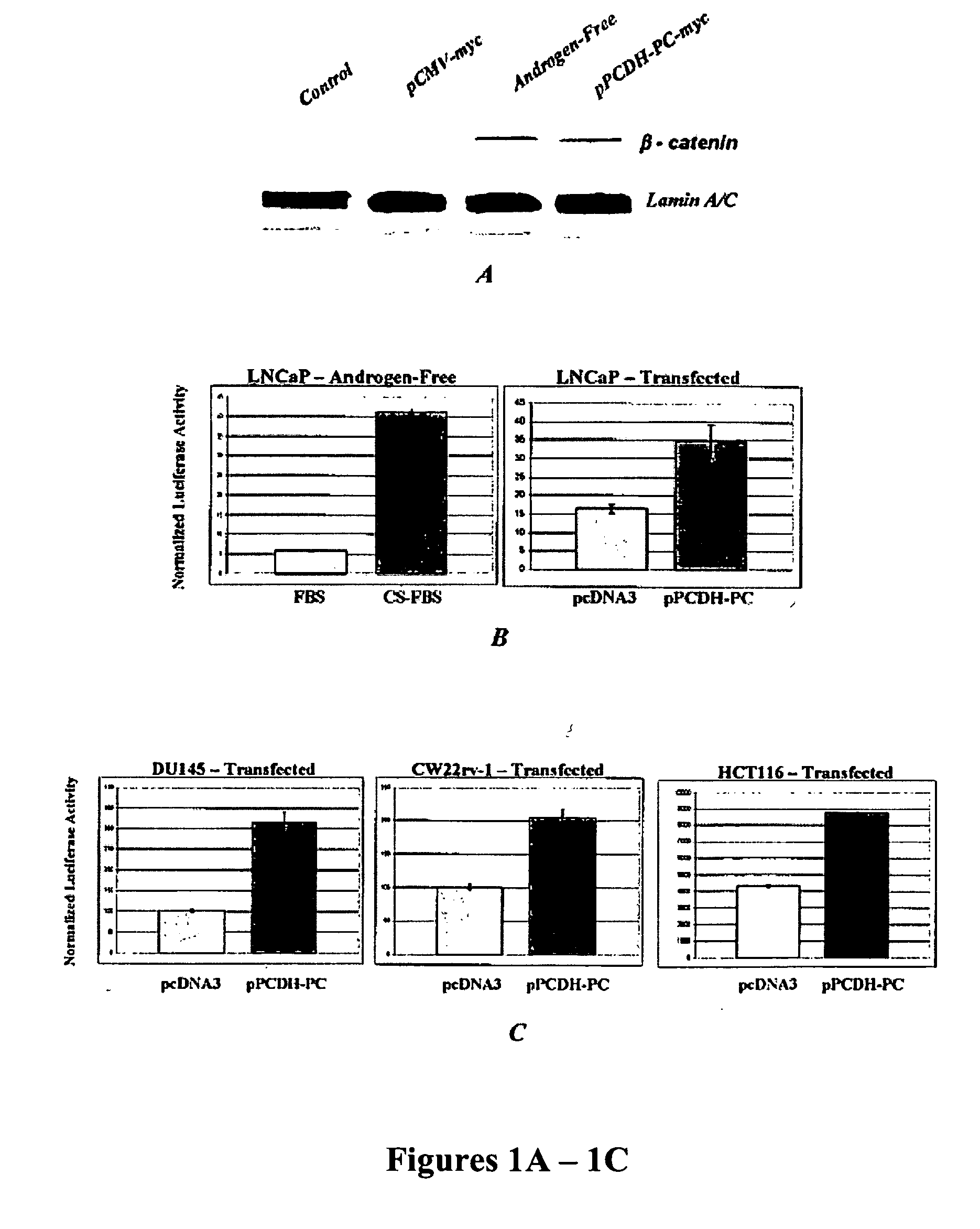
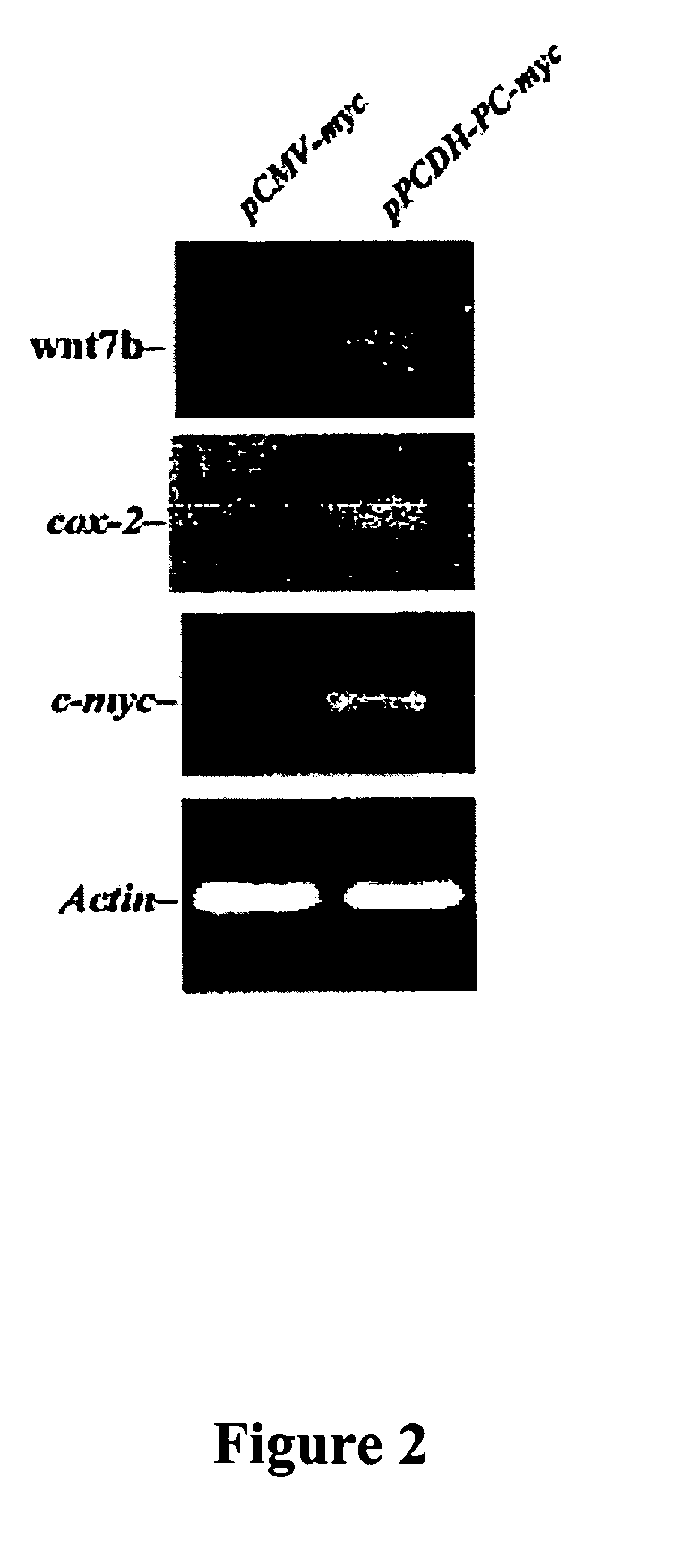
Methods to treat or prevent hormone-resistant prostate cancer using siRNA specific for protocadherin-PC, or other inhibitors of protocadherin-PC expression or activity
The invention is directed to compounds and methods for treating or preventing hormone-resistant prostate cancer using siRNA specific for protocadherin-PC, or other inhibitors of protocadherin-PC expression or activity, including antisense oligonucleotides and antibodies. The invention also provides for the use of protocadherin-PC as an in vivo prostate cancer biomarker, and includes a kit for detecting prostate cancer in biological samples. Also covered by the invention is a transgenic non-human mammal engineered to overexpress protocadherin-PC specifically in the prostate.
Owner:INST NAT DE LA SANTE & DE LA RECHERCHE MEDICALE (INSERM) +1
Novel monoclonal antibody and use of the same
The present invention provides an anti-PAC1 monoclonal antibody capable of recognizing a PAC1 having a native structure, a PAC1 activity regulator (in particular, activity inhibitor) containing the antibody, a prophylactic / therapeutic agent for a disease associated with accentuation of a bioactivity of PAC1, containing the antibody, a diagnostic reagent for a disease associated with an abnormality of PAC1 activity, containing the antibody, and a screening method for a substance that regulates the expression of PAC1, using the antibody and a PAC1-expressing cell.
Owner:KYOTO UNIV +1
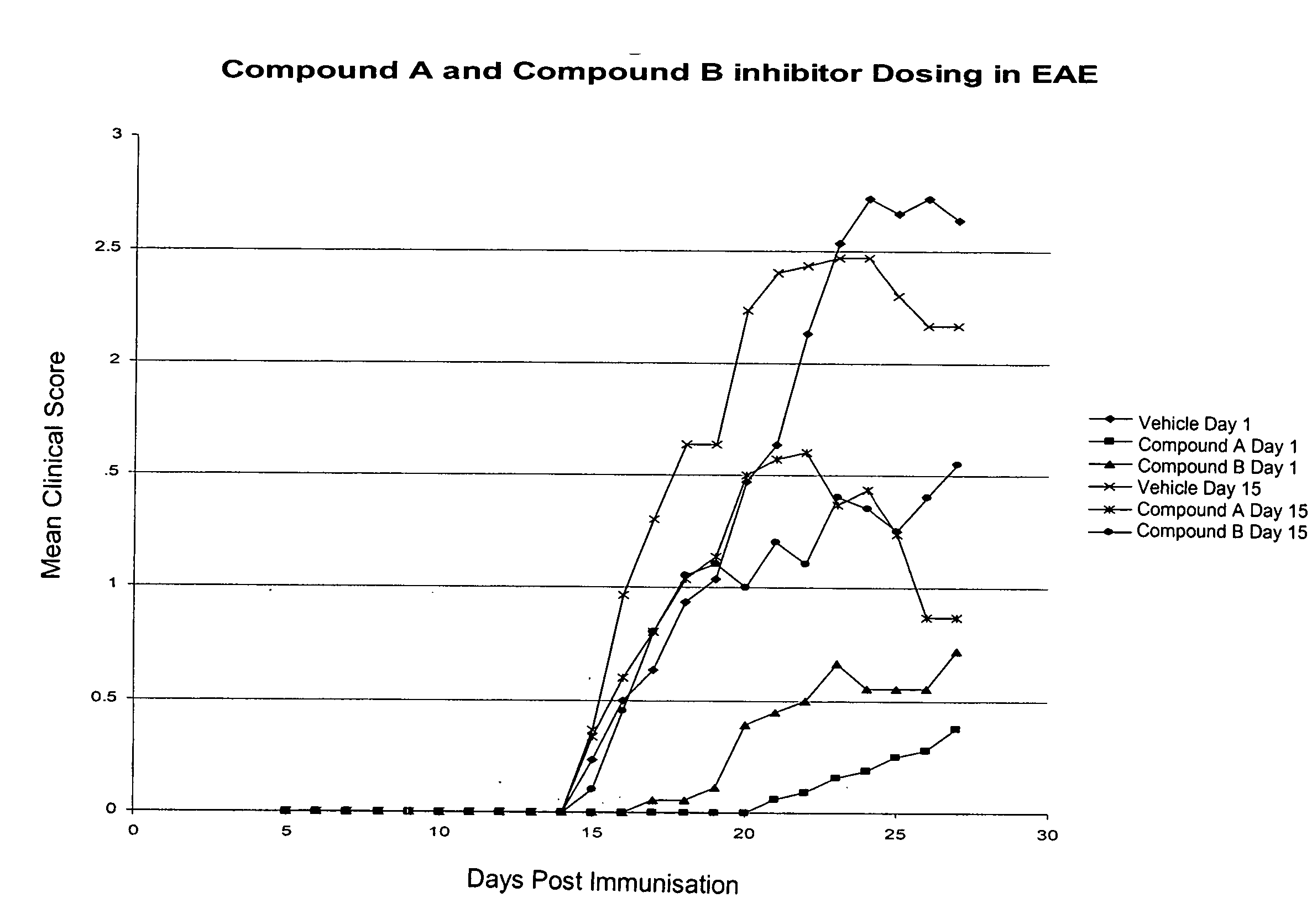
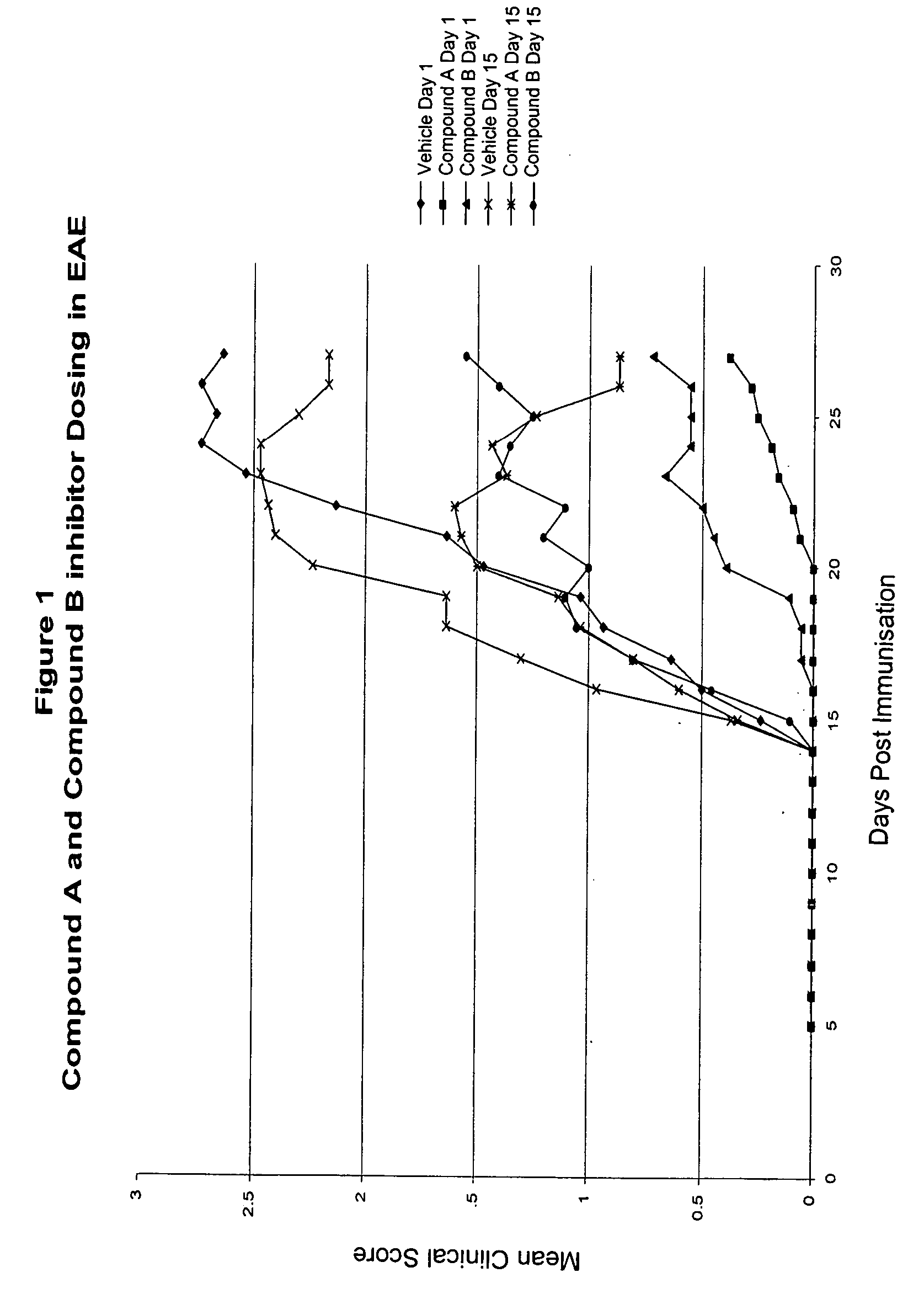
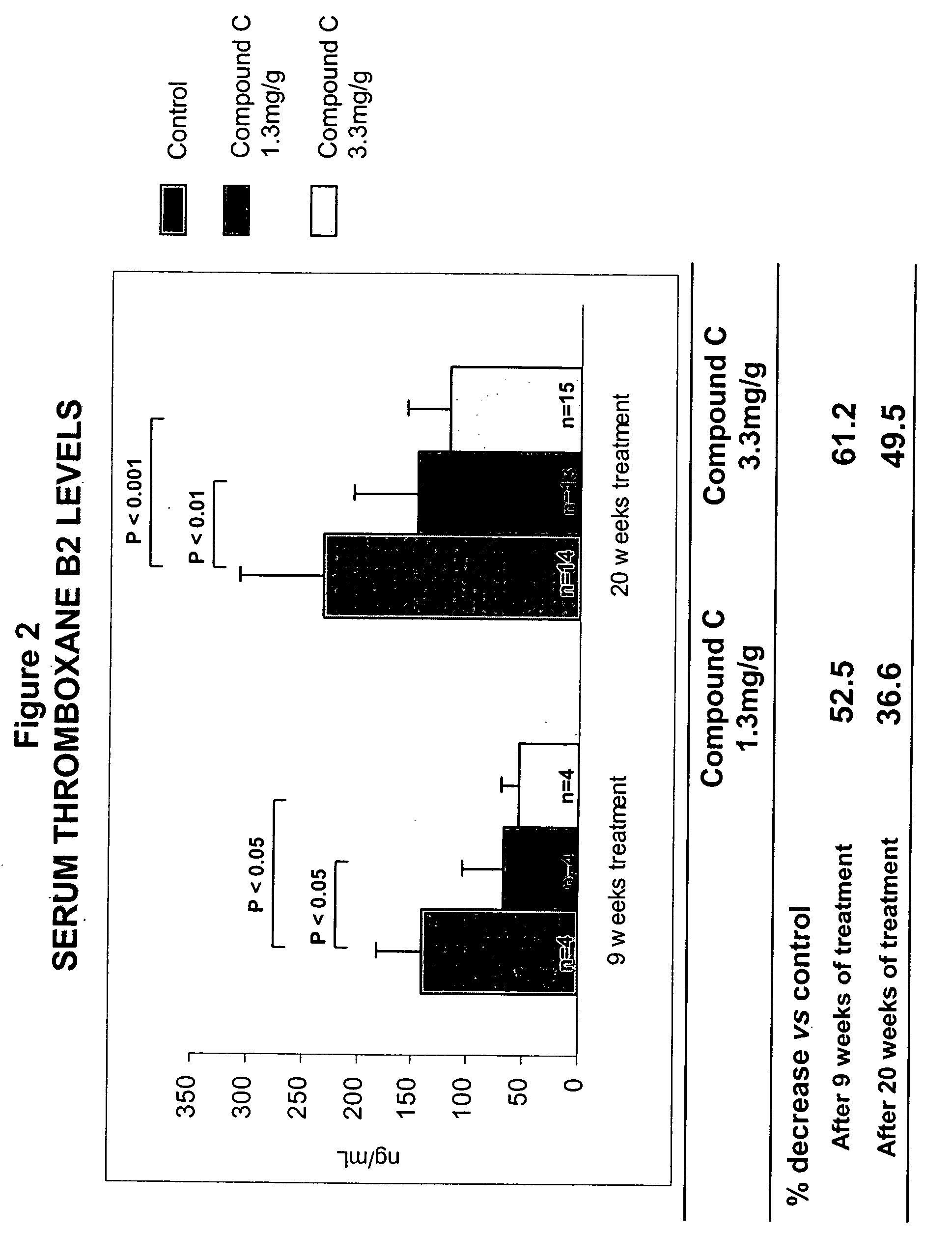
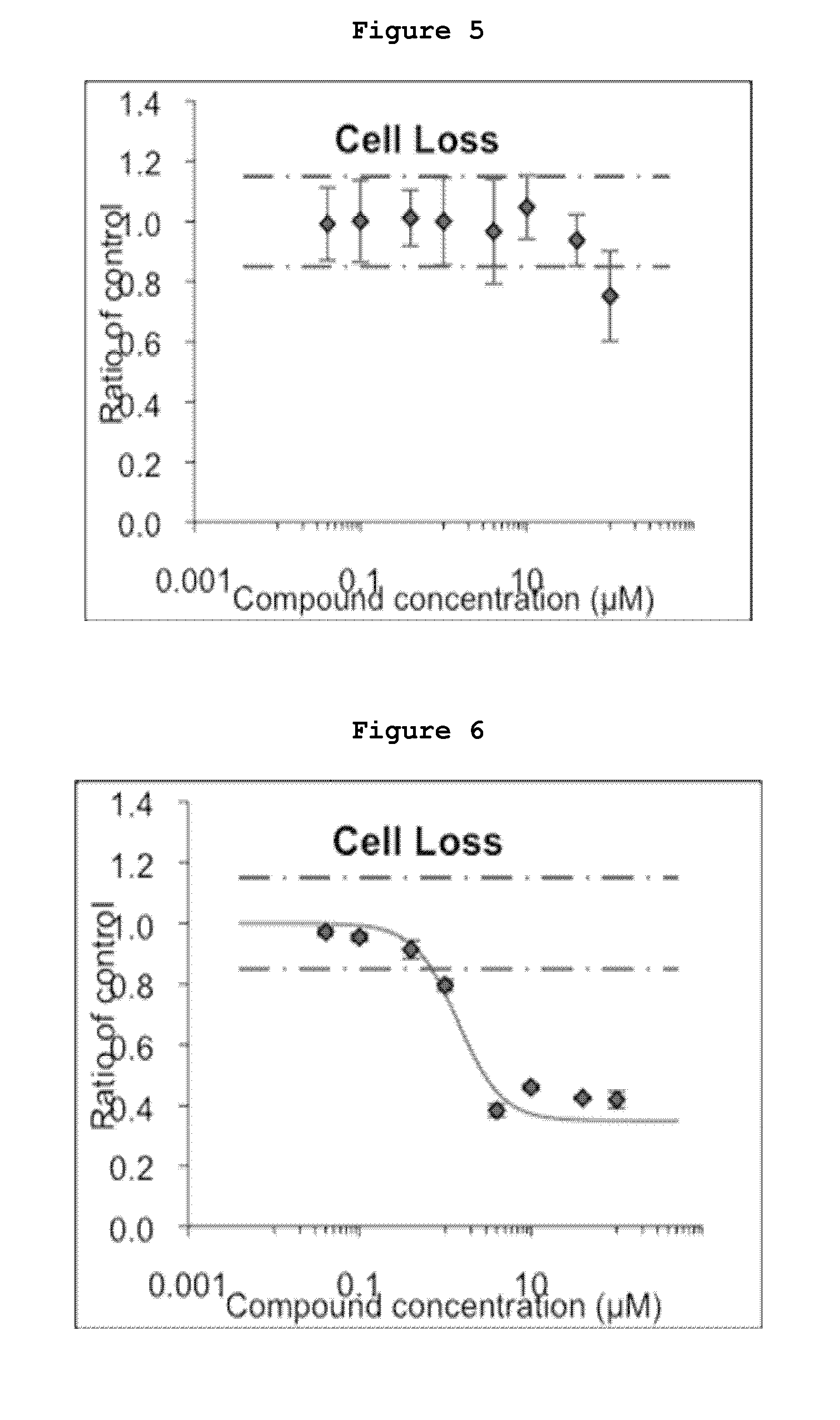
Inhibitors of the activity of complex iii of the mitochondrial electron transport chain and use thereof for treating diseases
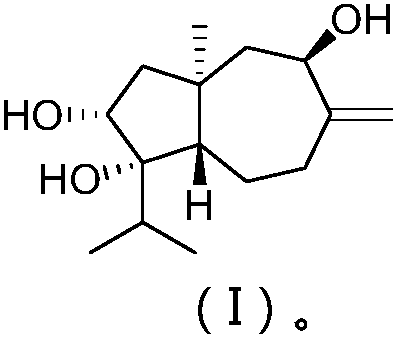
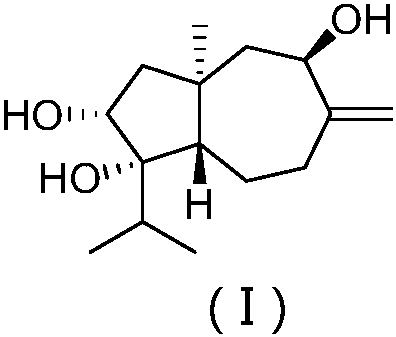
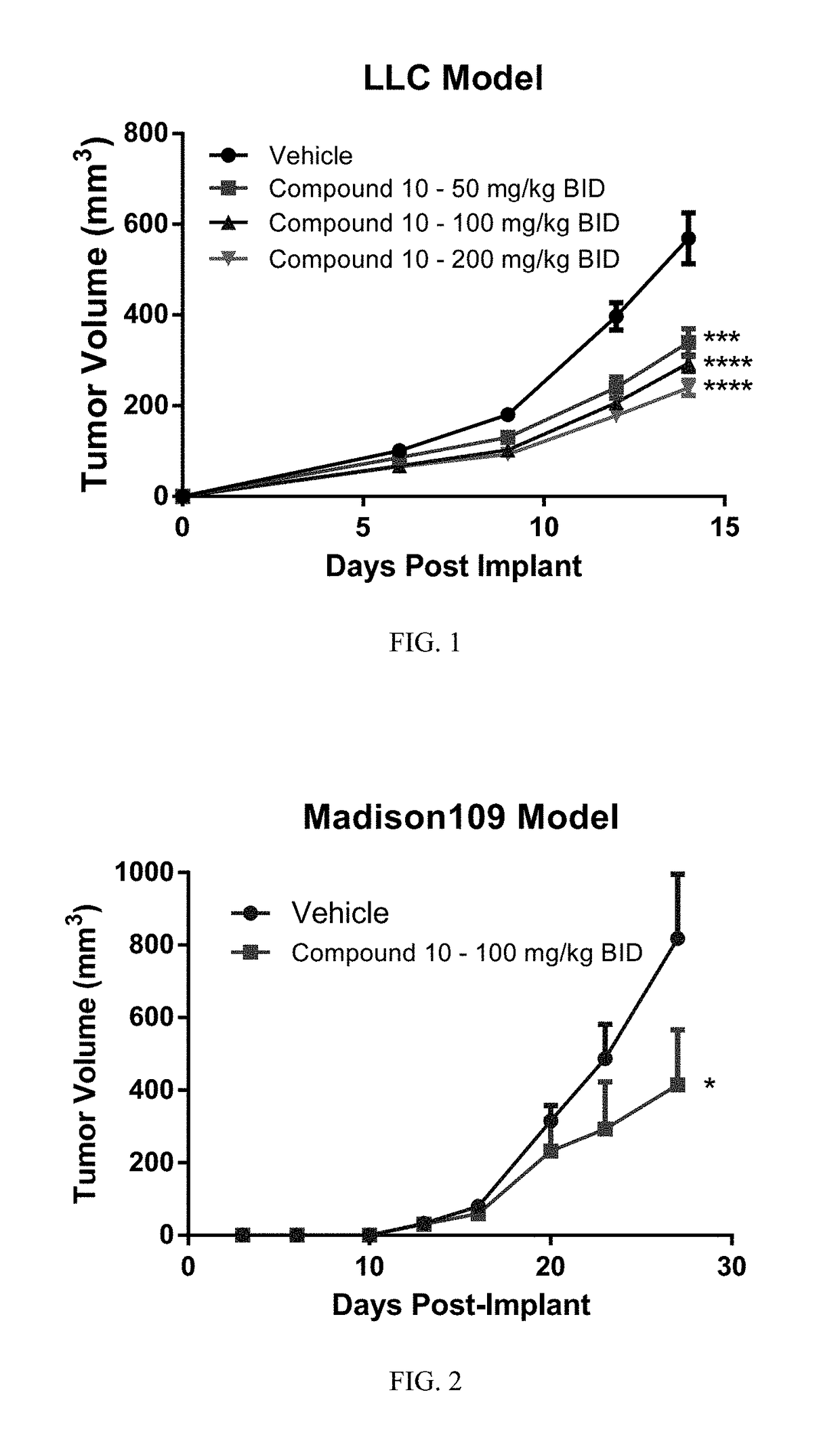
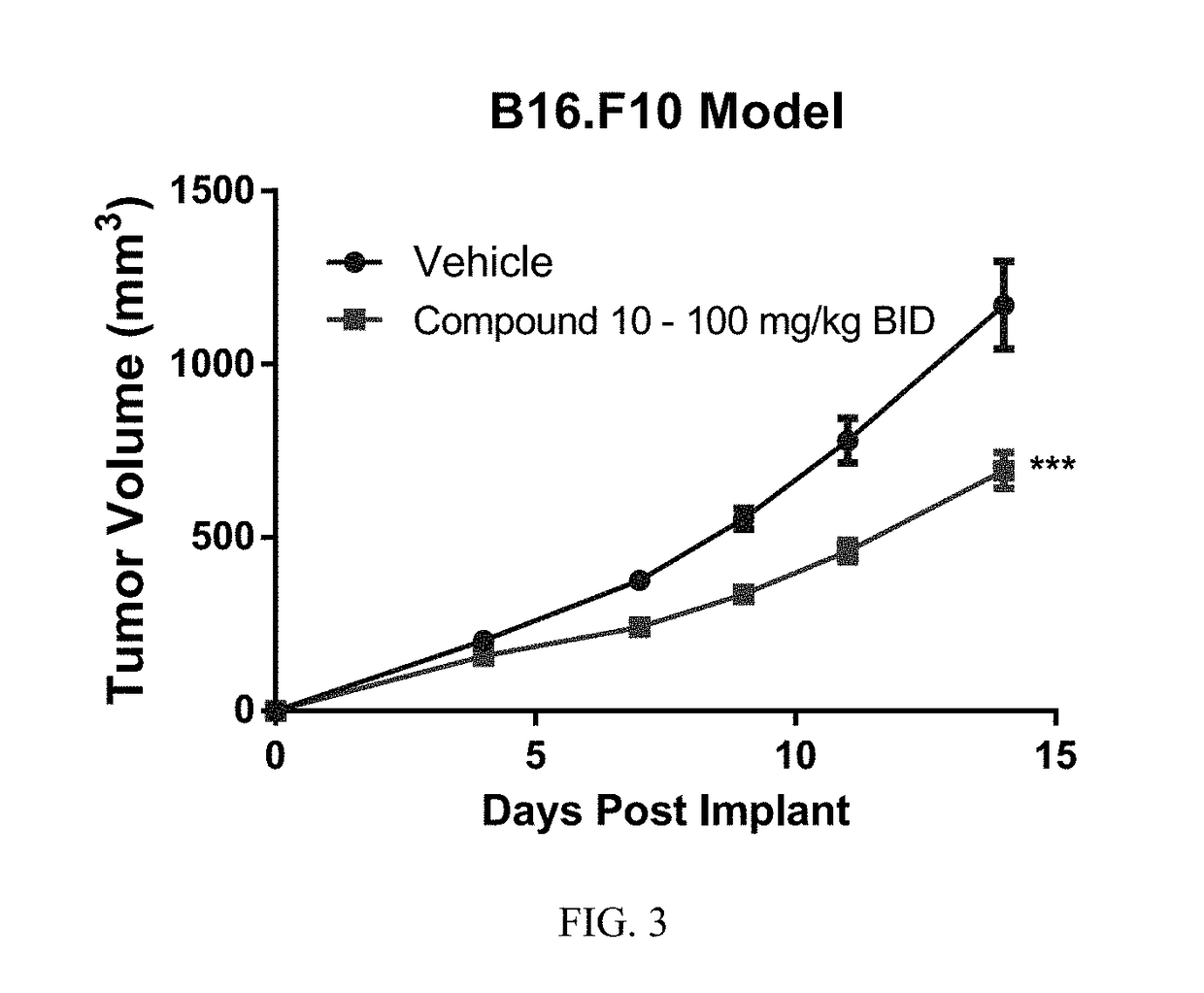
The present invention relates to compounds which are inhibitors of the activity of Complex III of the mitochondrial electron transport chain and pharmaceutical compositions comprising said compounds alone or in combination with other active agents. The present invention further relates to use of the compounds of the invention as medicaments or as agrochemicals where their properties as inhibitors of the mitochondrial respiration is of benefit. More particularly the present invention relates to the use of the compounds of the invention in a method of treating and / or preventing cancers presenting tumor-initiating cells. (Formula I) (I)
Owner:STEMERGIE BIOTECH
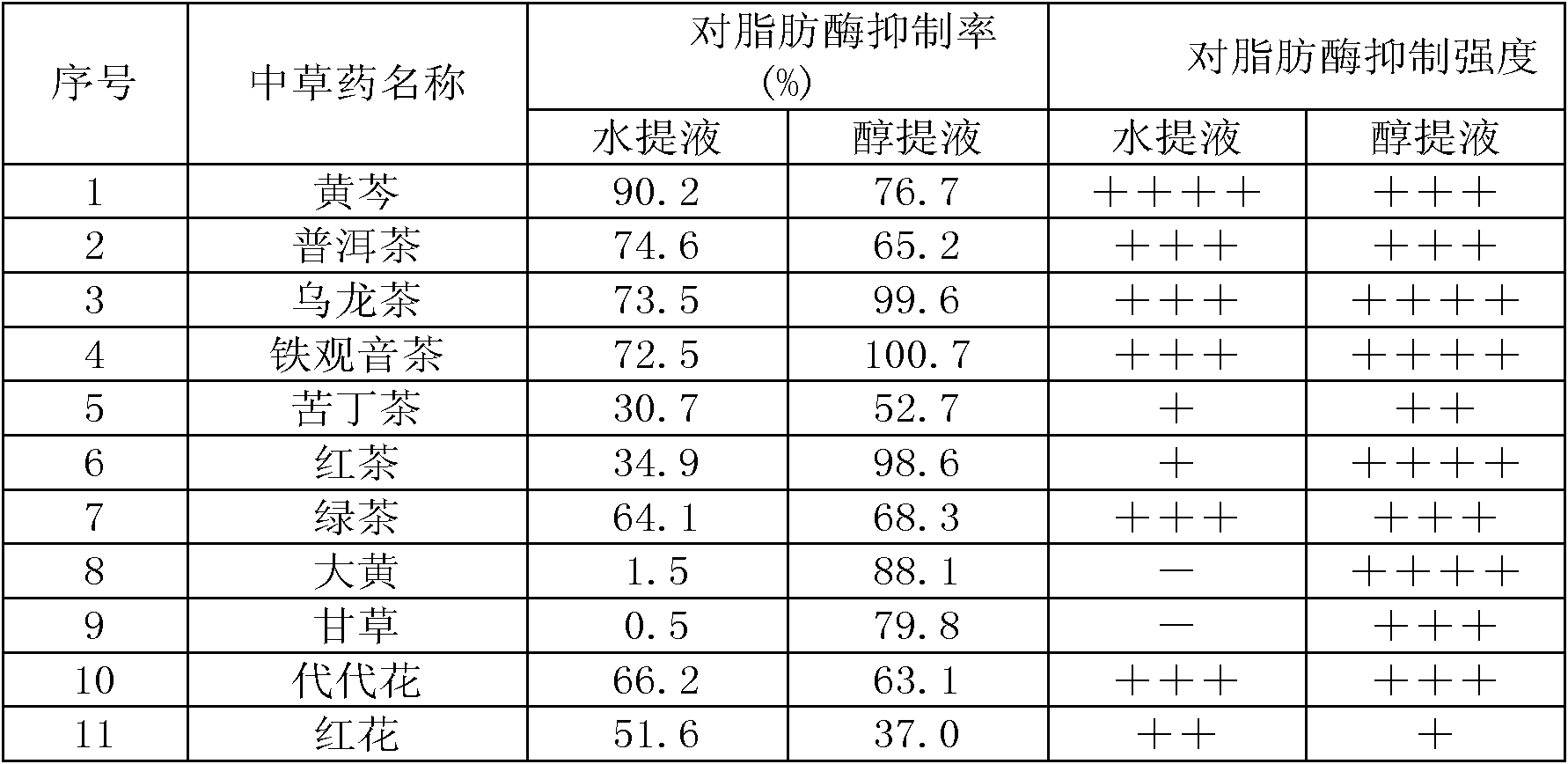
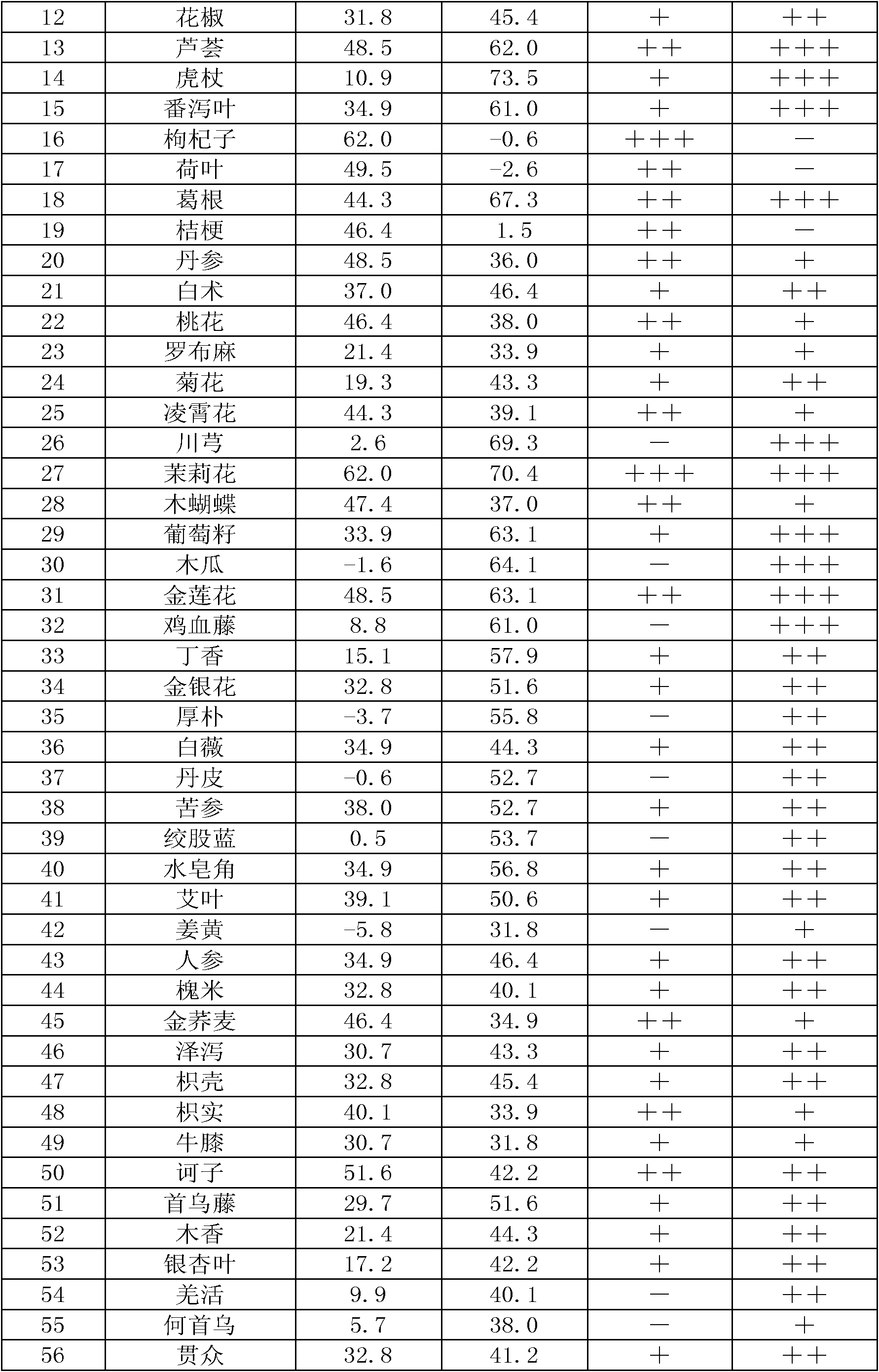
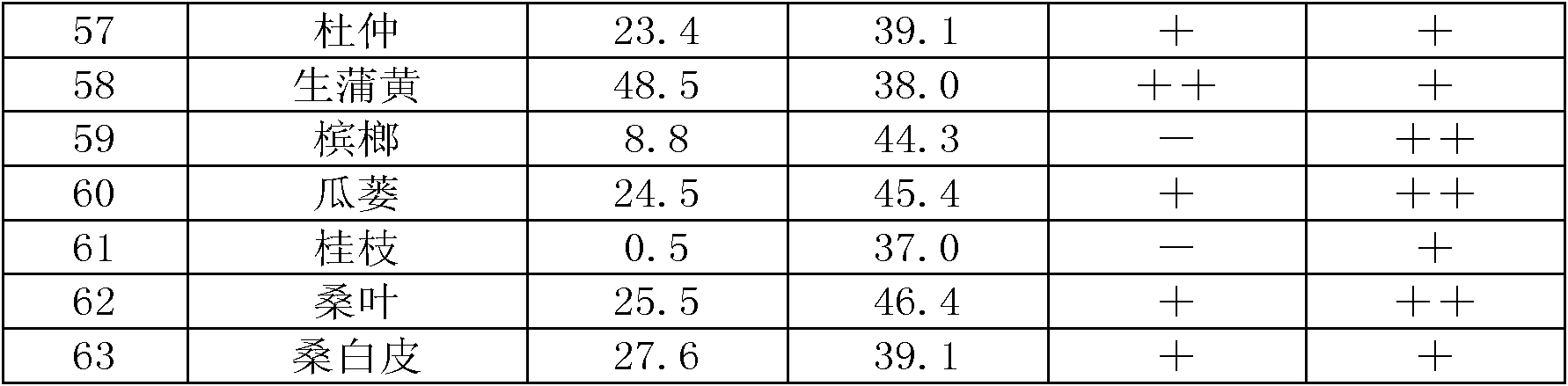
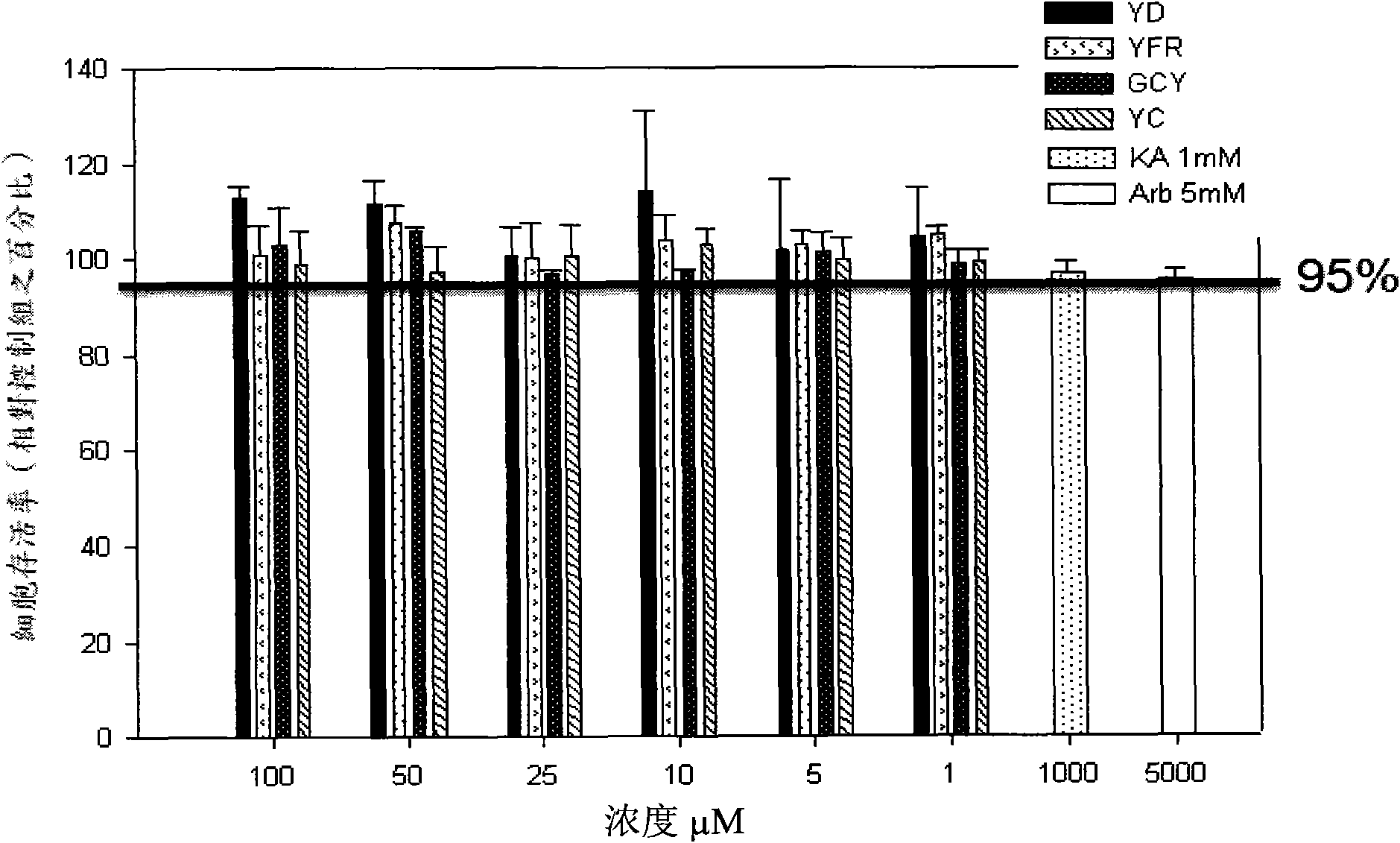
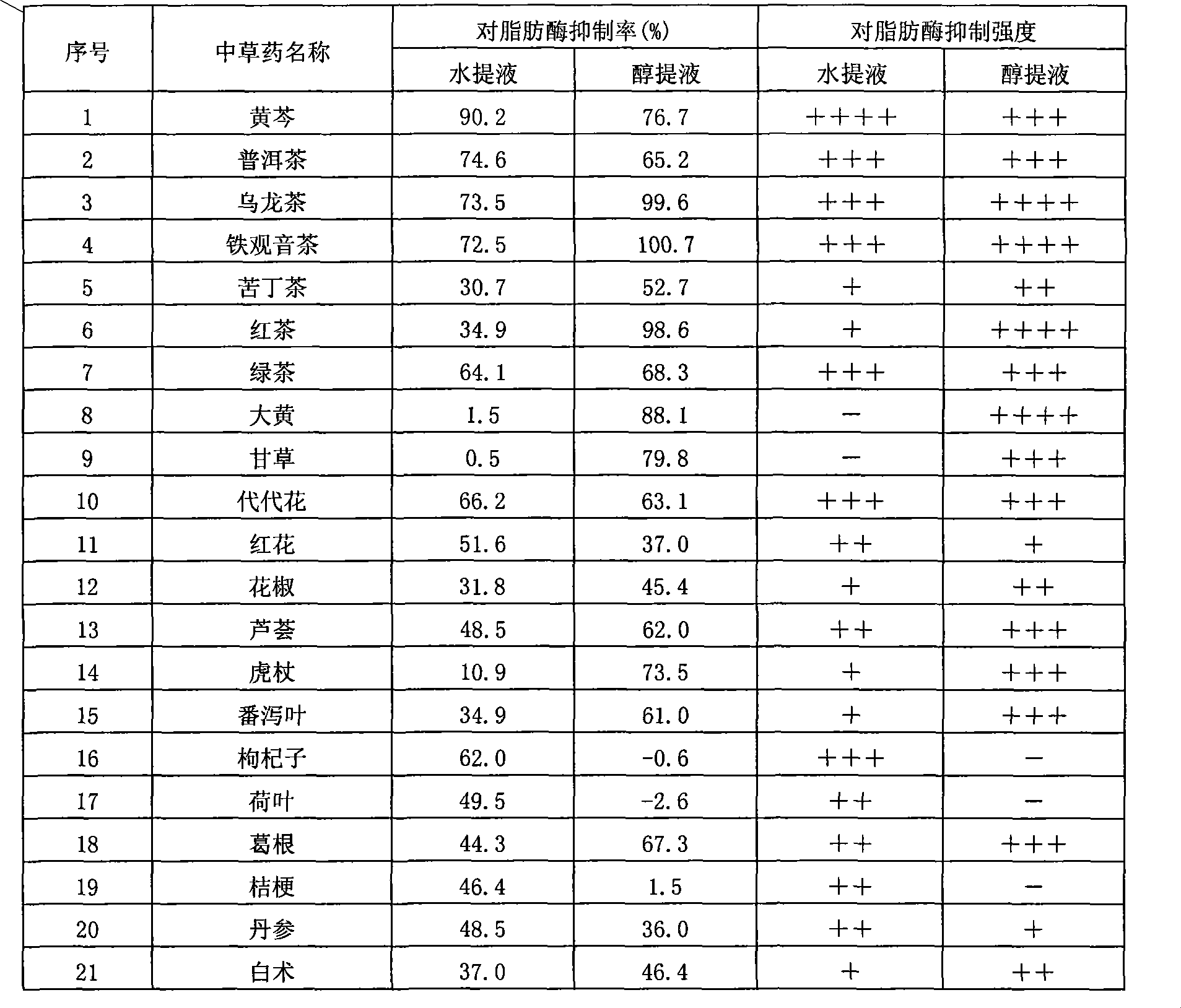
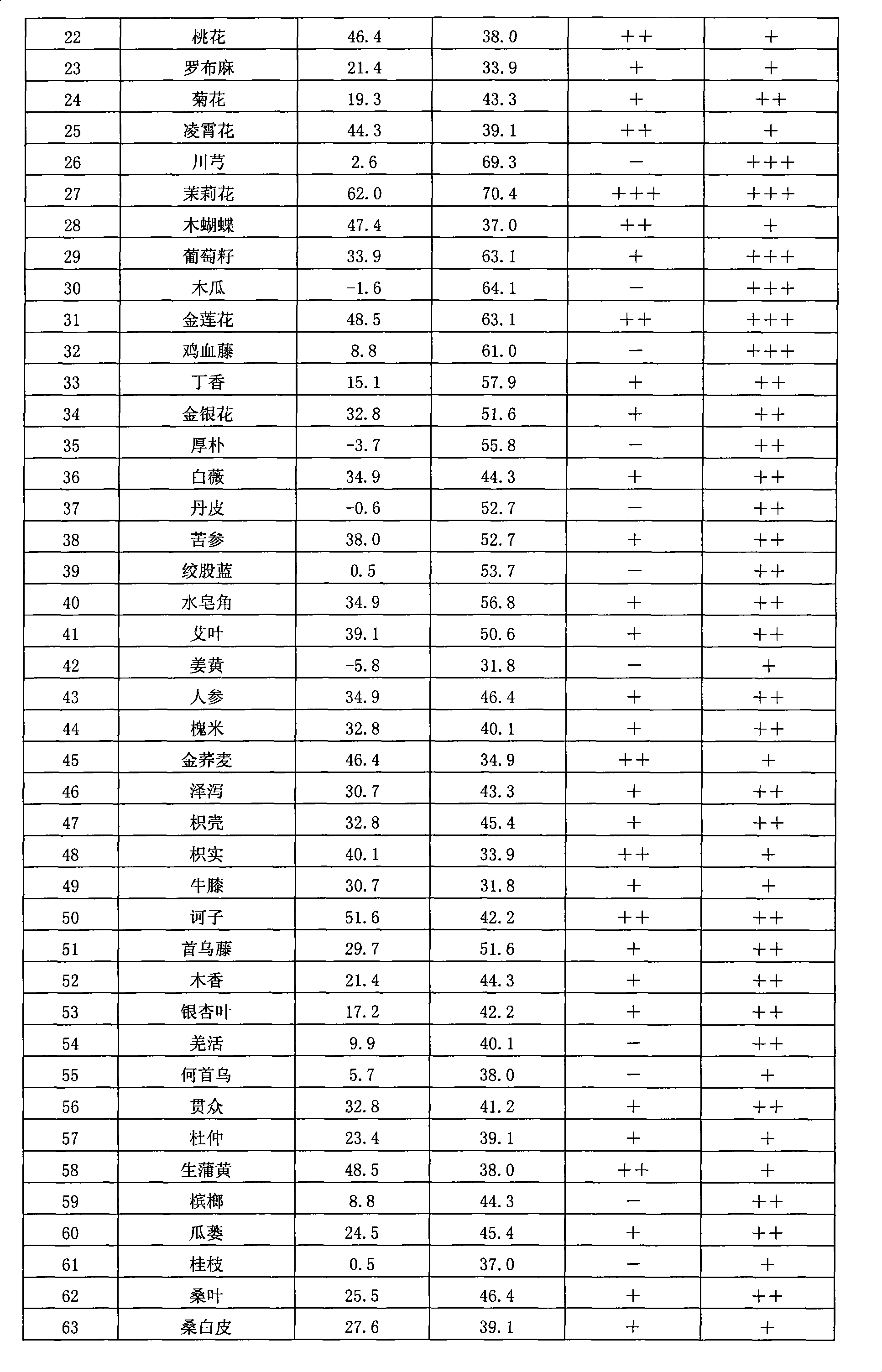
Chinese herbal medicine extract with lipase activity inhibition and new use thereof
The invention discloses a Chinese medicinal herb extract with the function of inhibiting the activity of lipase and the new application thereof. The Chinese medicinal herb extract refers to one of water extract and / or alcohol extract of 61 Chinese medicinal herbs.
Owner:BEIJING WBL PEKING UNIV BIOTECH
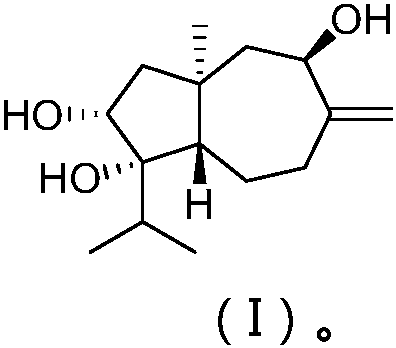
Sesquiterpene alcohol compound, preparation and application thereof
The invention relates to the field of algae inhibitors, the invention specifically relates to a sesquiterpene alcohol compound derived from algal epiphytic fungus, a preparation method thereof and anapplication of the sesquiterpene alcohol compound in the aspect of inhibiting microalgae. The specific structural formula is shown in the formula (I). The preparation method comprises the following steps: inoculating a fungal culture medium with Trichoderma virens Y13-3 for fermentation culture, and subjecting a fermentation product to separating and purifying, and the product is namely a sesquiterpene alcohol compound as shown in the formula (I). The median inhibition concentration of the sesquiterpene alcohol compound to the microalga can be up to 1.2 microgram / ml through the microalga activity inhibition experiment.
Owner:YANTAI INST OF COASTAL ZONE RES CHINESE ACAD OF SCI
Inhibitor for preventing browning of cut vegetable
InactiveCN101664055APrevent browningPlay the role of anti-corrosion and fresh-keepingFruit and vegetables preservationSODIUM METAPHOSPHATECarrageenan
The invention relates to an inhibitor for preventing browning of a cut vegetable, comprising the following components: one of solvent water and ethanol or mixture thereof; one of sodium alginate, xanthan gum, carrageenan and chitosan, one or more of sodium tripolyphosphate, sodium pyrophosphate and 6-sodium metaphosphate, one or more of phytic acid, ascorbic acid, cinnamic acid, citric acid, kojicacid and edible acetic acid, and one or more of potassium sorbate, sodium dehydroacetate, sodium chloride and 4-hexylresorcinol. The inhibitor is used by soaking, and the soaking time varies with thetemperature and the air pressure of the processing environment. All the components used in the inhibitor are permitted in the GB2760-2007 (hygienic standards of food additives), and the inhibitor issulfite-free, safe and non-toxic, and can be used for fresh cut vegetables and can play the multiple roles of pH regulation, enzyme activity inhibition, oxygen removal and isolation and water retention to effectively prevent browning and play a role of anticorrosion and refreshment.
Owner:SHANGHAI ACAD OF AGRI SCI
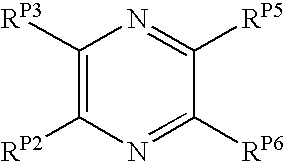
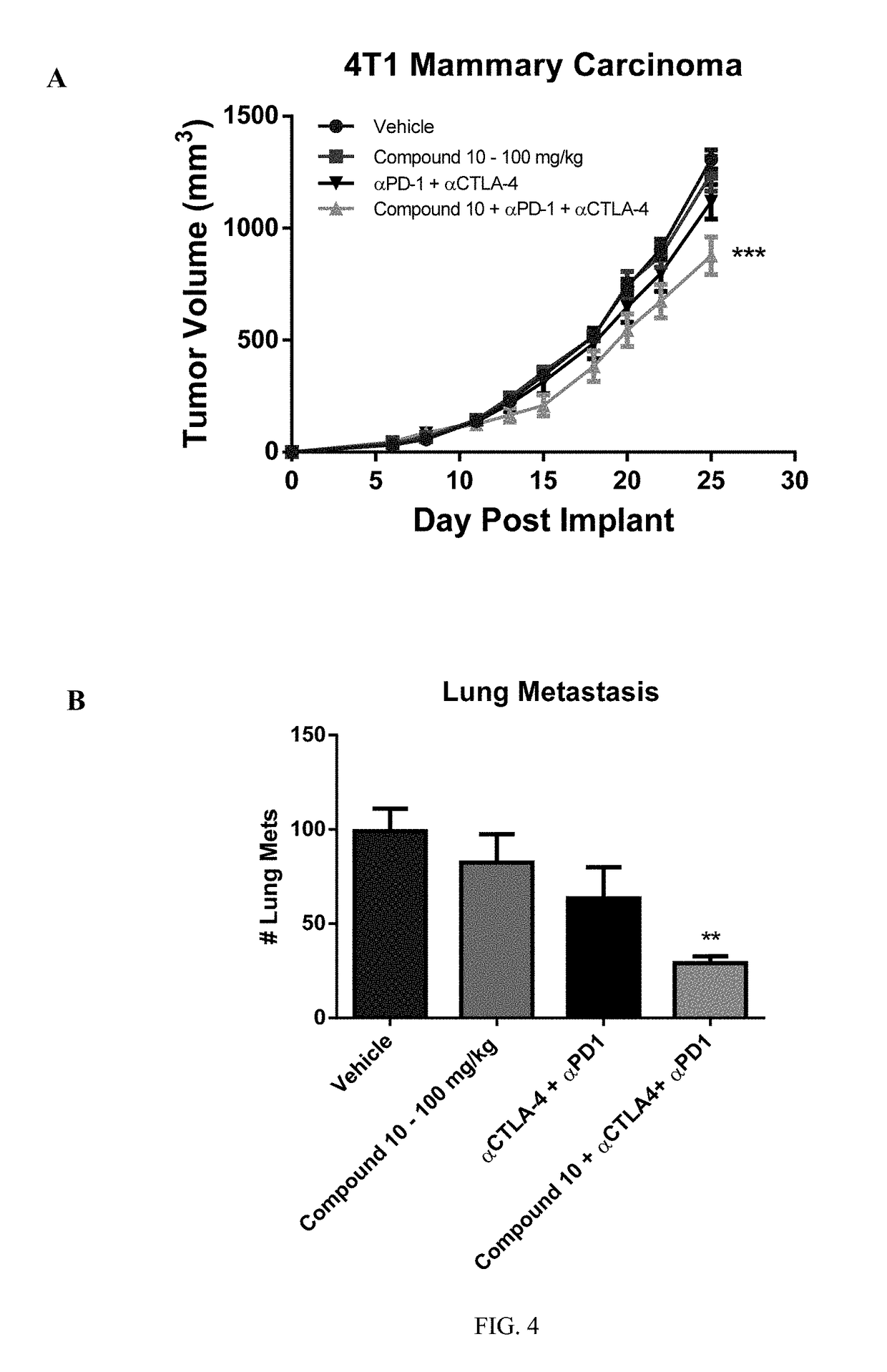
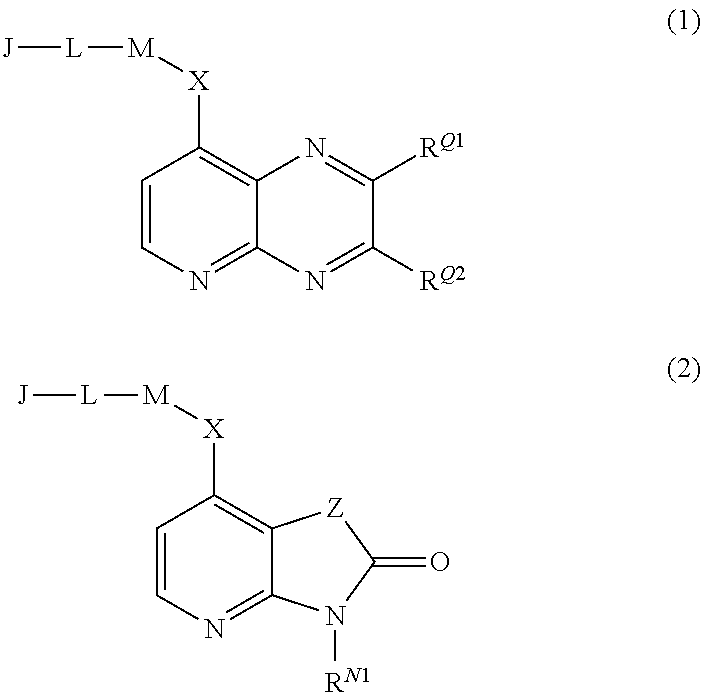
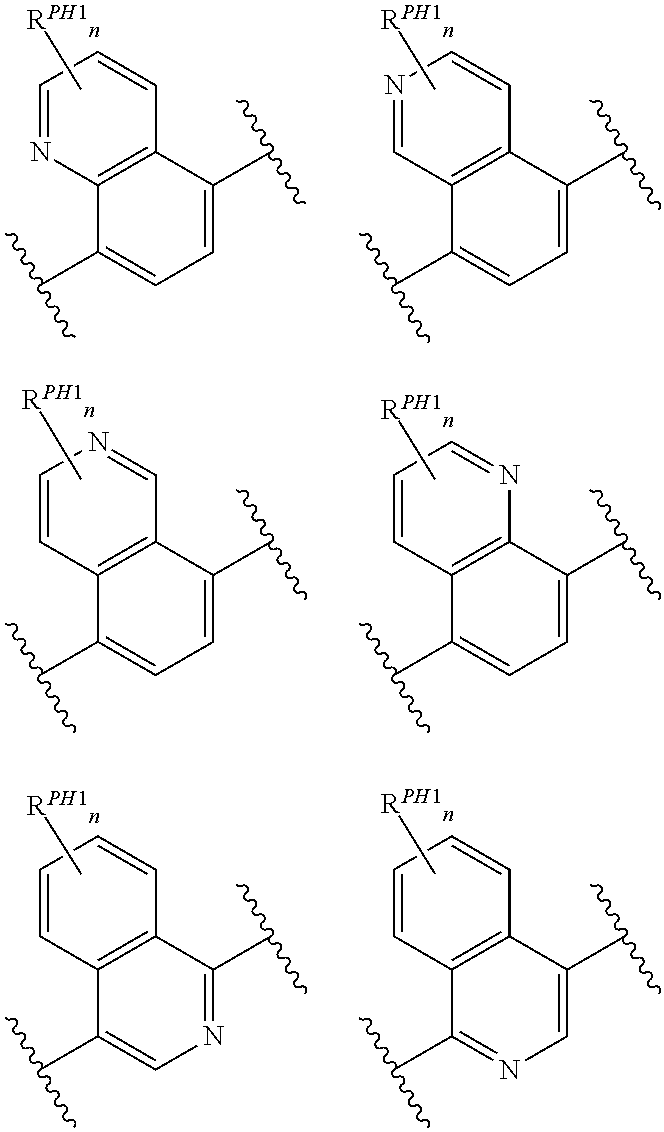
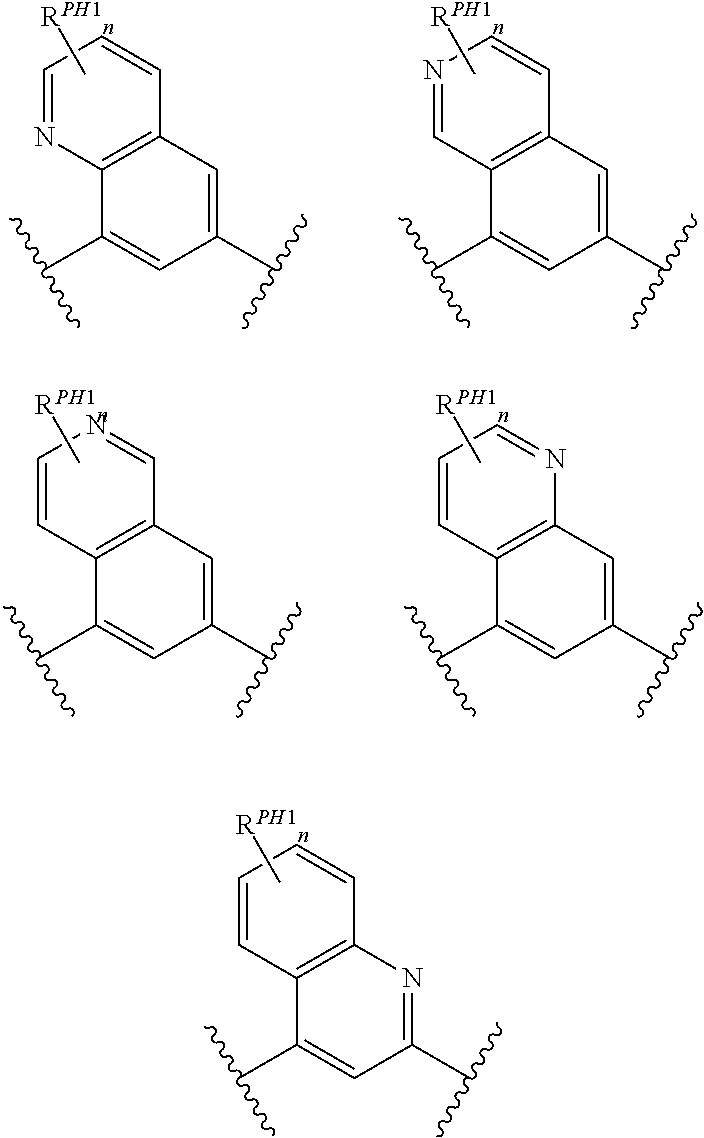
Pyrido[2,3-b]pyrazin-8-substituted compounds and their use
Owner:THE INST OF CANCER RES ROYAL CANCER HOSPITAL +1
Compositions and methods for inhibiting arginase activity
ActiveUS10065974B2Improve throughputLimit deliveryBoron compound active ingredientsImmunoglobulins against cell receptors/antigens/surface-determinantsArginaseMedicinal chemistry
Owner:PRECISION PHARM INC
Aryl-Quinolyl Compounds and Their Use
InactiveUS20110053946A1Promote apoptosisInhibiting cell cycle progressionAntibacterial agentsBiocideDiseaseAryl
The present invention pertains generally to the field of therapeutic compounds for treating proliferative disorders, cancer, etc., and more specifically to certain aryl-quinolyl compounds, as described herein, which, inter alia, inhibit RAF (e.g., B-RAF) activity. The present invention also pertains to pharmaceutical compositions comprising such compounds, and the use of such compounds and compositions, both in vitro and in vivo, to inhibit RAF (e.g., BRAF) activity, to inhibit receptor tyrosine kinase (RTK) activity, to inhibit cell proliferation, and in the treatment of diseases and disorders that are ameliorated by the inhibition of RAF, RTK, etc., proliferative disorders such as cancer (e.g., colorectal cancer, melanoma), etc.
Owner:THE INST OF CANCER RES ROYAL CANCER HOSPITAL +1
Compositions and methods for inhibiting arginase activity
ActiveUS10287303B2Boron compound active ingredientsImmunoglobulins against cell receptors/antigens/surface-determinantsMedicineArginase
Owner:PRECISION PHARM INC
Imidazo[4,5-b]pyridin-2-one and oxazolo[4,5-b]pyridin-2-one compounds and analogs thereof as therapeutic compounds
The present invention pertains to certain imidazo[4,5-b]pyridin-2-one and oxazolo[4,5-b]pyridin-2-one compounds and analogs thereof, which, inter alia, inhibit RAF (e.g., B-RAF) activity, inhibit cell proliferation, treat cancer, etc., and more particularly to compounds of the formula (I): wherein: J is independently —O— or —NRN1—; RN1, if present, is independently —H or a substituent; RN2 is independently —H or a substituent; Y is independently —CH═ or —N═; Q is independently —(CH2)j—M—(CH2)k— wherein: j is independently 0, 1 or 2; k is independently 0, 1, or 2; j+k is 0, 1, or 2; M is independently —O—, —S—, —NH—, —NMe—, or —CH2—; each of RP1, RP2, RP3, and RP4 is independently —H or a substituent; and additionally RP1 and RP2 taken together may be —CH═CH—CH═CH—; L is independently: a linker group formed by a chain of 2, 3, or 4 linker moieties; each linker moiety is independently —CH2—, —NRN—, —C(═X)—, or —S(═O)2—; exactly one linker moiety is —NRN—, or: exactly two linker moieties are —NRN—; exactly one linker moiety is —C(═X)—, and no linker moiety is —S(═O)2—; or: exactly one linker moiety is —S(═O)2—, and no linker moiety is —C(═X)—; no two adjacent linker moieties are —NRN—; X is independently ═O or ═S; each RN is independently —H or a substituent; A is independently: C6-14carboaryl, C5-14heteroaryl, C3-12carbocyclic, C3-12heterocyclic; and is independently unsubstituted or substituted; and pharmaceutically acceptable salts, solvates, amides, esters, ethers, N-oxides, chemically protected forms, and prodrugs thereof. The present invention also pertains to pharmaceutical compositions comprising such compounds, and the use of such compounds and compositions, both in vitro and in vivo, to inhibit RAF (e.g., B-RAF) activity, to inhibit receptor tyrosine kinase (RTK) activity, to inhibit cell proliferation, and in the treatment of diseases and conditions that are ameliorated by the inhibition of RAF, RTK, etc., proliferative conditions such as cancer (e.g., colorectal cancer, melanoma), etc.
Owner:THE INST OF CANCER RES ROYAL CANCER HOSPITAL +2
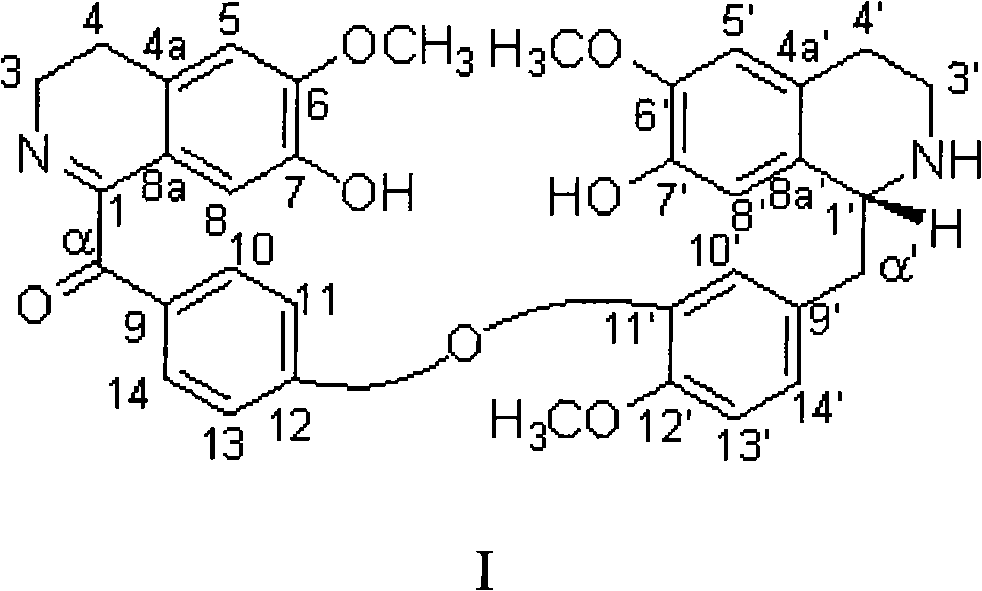
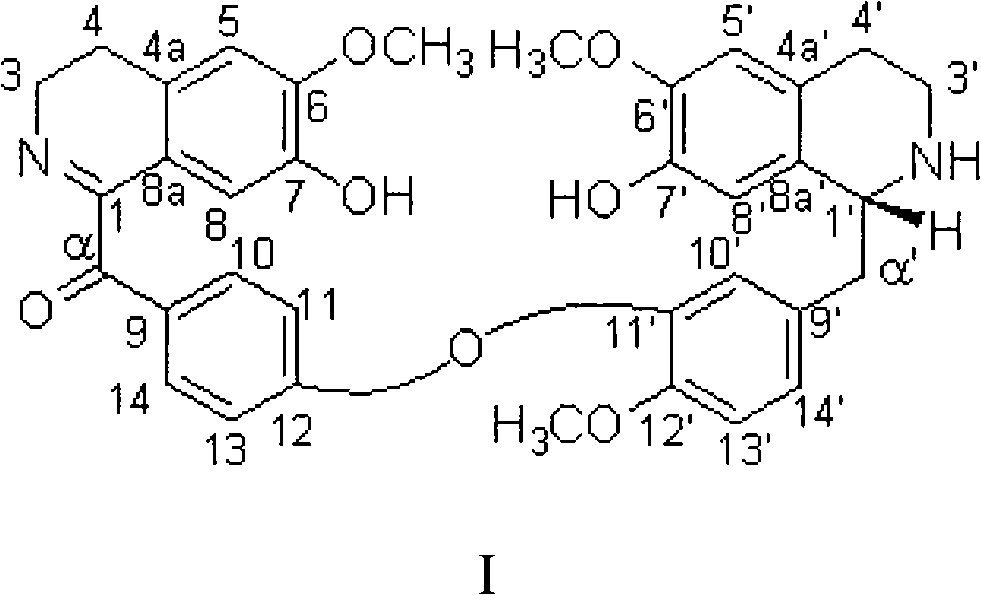
Bisbenzylisoquinoline compounds, preparation method and applications
InactiveCN101284792ANovel structureEnhanced inhibitory effectOrganic active ingredientsOrganic chemistryToxicantGradient elution
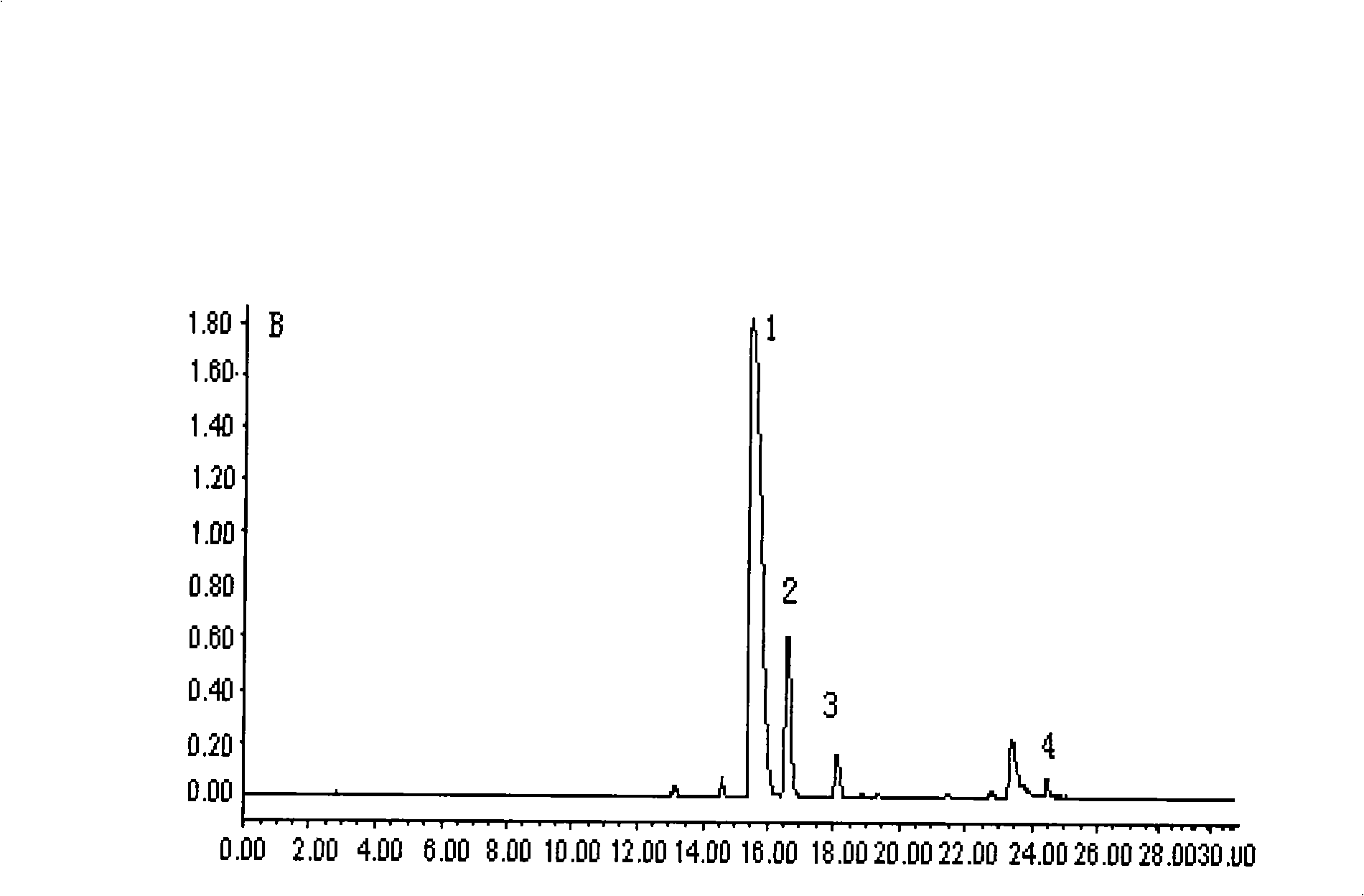
The invention provides a bisbenzylisoquinoline compound in lindera aggregate and the preparation method thereof. The lindera aggregate dried root is used as the raw material and is extracted by using an alcoholic solvent, the extractant is produced into extracturm through decompressing concentration, after the extracturm is dissolved and suspended by water and acidified by dilute acid, the neutral component of the extracturm is removed by extraction with an organic solvent, the water phase is adjusted to the pH value of 10 by weak base, extraction is performed by the organic solvent after solvent recovery, the total alkaloid crude product is obtained, fine alkaloid crude is prepared after the crude product is resolved in alcohol and sedimentation process is performed by adding an amount of water, eluting parts are colleted after the chloroform-carbinol system elution and the water-carbinol gradient elution, and the compound can be prepared after removing the solvent by condensation. The compound is used for performing activity inhibition experiments to L1210 and K562 tumor cell strains, results show that both L1210 and K562 tumor cell strains have strong cell-toxicant activity and present dose-effect relationships. The compound can be applied to the preparation of anticancer drugs, and has the above structural formula.
Owner:ZHEJIANG UNIV
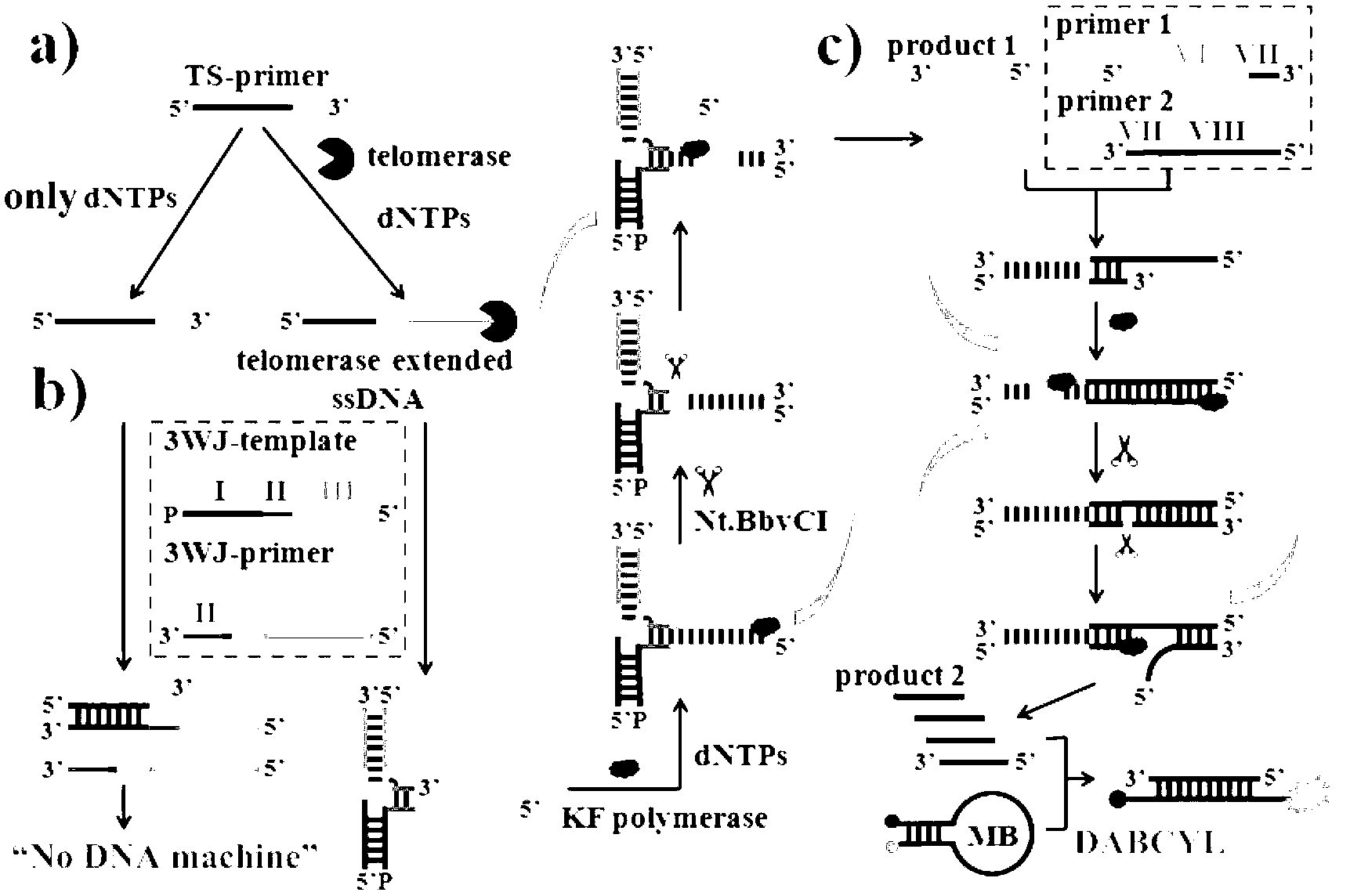
Telomerase activity detection kit and detection method thereof
InactiveCN102925546AHigh sensitivityExpand the scope of detectionMicrobiological testing/measurementTelomeraseFluorescence
The present invention discloses a telomerase activity detection kit and a detection method thereof. According to the kit, a ternary linking structure probe is adopted to recognize a telomerase product, and a ternary linking structure triggered DNA molecule machine and a base stacking triggered DNA molecule machine are combined to achieve isothermal cascade nucleic acid amplification to convert a reverse transcription event of the telomerase in a tumor cell extract into a significantly-enlarged fluorescence detection signal so as to precisely determine telomerase activity in a trace amount of tumor cells and achieve detection on tumor cell telomerase activity inhibition by chemical drugs.
Owner:XI AN JIAOTONG UNIV
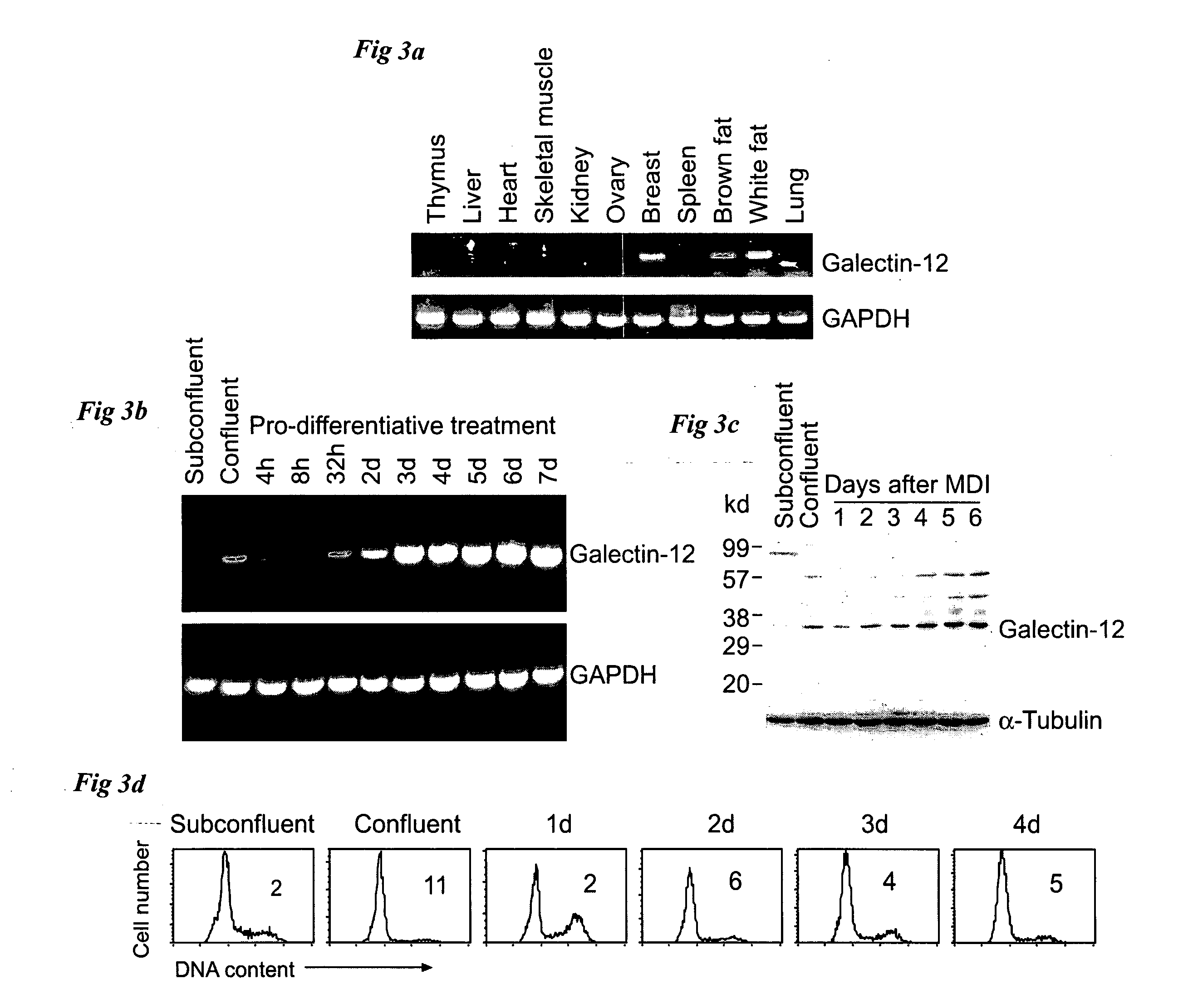
Reducing galectin-12 activity to reduce formation of adipocytes
InactiveUS20050250123A1Inhibit differentiationSugar derivativesMicrobiological testing/measurementWhite blood cellRNA
It has now been discovered that galectin-12 is necessary for the differentiation of pre-adipocytes into adipocytes and for differentiation of leukocytes. Inhibition of galectin-12 activity can therefore be used to block the formation of new fat cells or to down-regulate the formation of leukocytes, for example, to promote wound healing. The invention provides, for example, short, interfering RNAs (siRNAs) to inhibit expression of galectin-12 and its consequent activity. The invention further provides the use of inhibitors of galectin-12 activity and methods of inhibiting galectin-12 activity using such inhibitors.
Owner:RGT UNIV OF CALIFORNIA
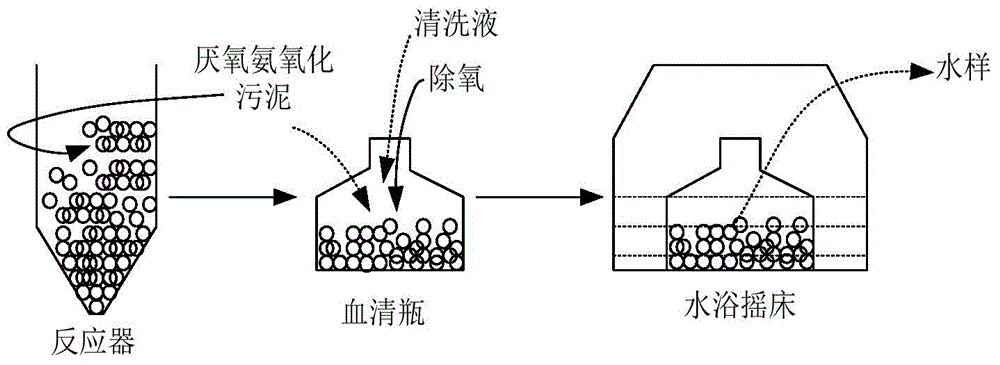
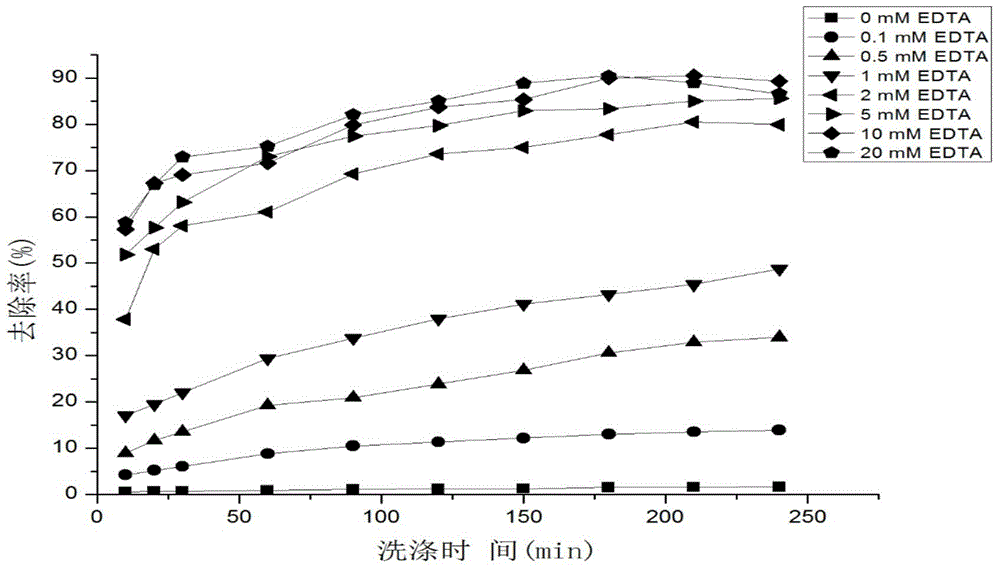
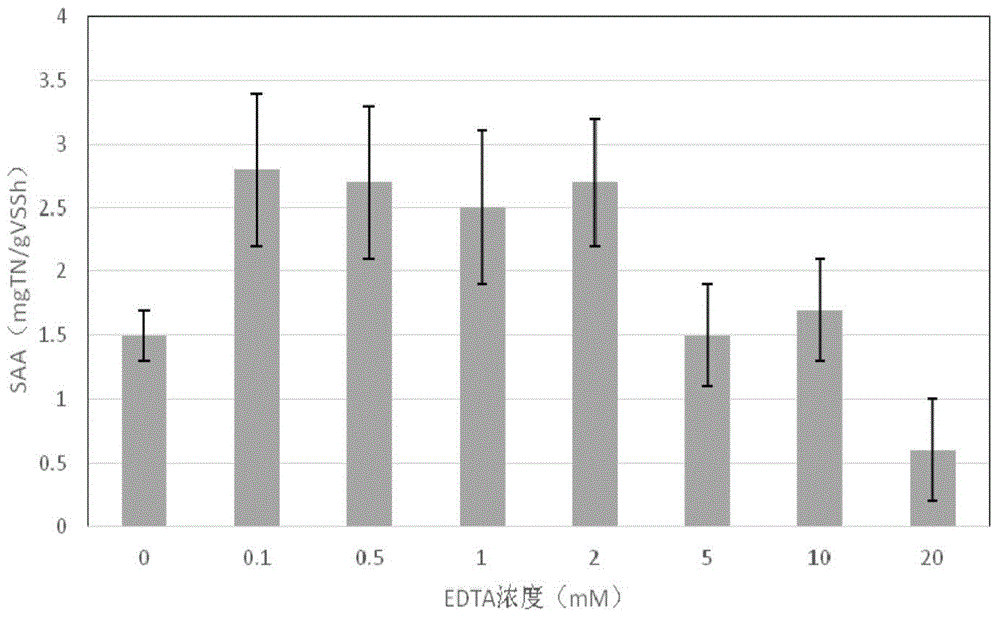
Method for quickly relieving activity inhibition of heavy metals on anaerobic ammonia oxidation sludge
ActiveCN104445845AReduce inhibitionEfficient removalSludge treatmentWater contaminantsSludgeDistilled water
The invention discloses a method for quickly relieving activity inhibition of heavy metals on anaerobic ammonia oxidation sludge. The method comprises the following steps: washing the anaerobic ammonia oxidation sludge polluted by heavy metals by distilled water, adding the washed sludge into a closed container, adding a cleaning liquid with the pH value being 7.4-7.60, and introducing argon to remove oxygen and closing; then, putting into a constant temperature table at 30-35 DEG C and oscillating the table in the shade at a speed of 150-200rpm; and finally, centrifugalizing the oscillated mud-water mixture and taking the precipitate, namely the anaerobic ammonia oxidation sludge which relieves heavy metal inhibition. The method disclosed by the invention can be used for effectively relieving the inhibitory effect of heavy metals on anaerobic ammonia oxidation sludge, effectively removing heavy metals adsorbed to the surface of sludge and quickly improving the denitriding activity of anaerobic ammonia oxidation bacteria.
Owner:HANGZHOU NORMAL UNIVERSITY
Application of Chinese herbal medicine turmeric extraction in preparation of slimming and lipid-lowering drug or drug with lipase activity inhibition effect
The invention discloses application of Chinese herbal medicine turmeric extraction in the preparation of a slimming and lipid-lowering drug or a drug with a lipase activity inhibition effect. The invention overcomes the defects of low efficiency and large side effect of the existing clinical slimming drugs, in particular traditional Chinese medicines and provides the slimming and lipid-lowering drug with clear mechanism, scientific target point, small side effect and high clinical efficiency.
Owner:BEIJING WBL PEKING UNIV BIOTECH
Pyrazines and Pyridines and Derivatives Thereof as Therapeutic Compounds
InactiveUS20080015191A1Promote apoptosisInhibiting cell cycle progressionAntibacterial agentsOrganic active ingredientsDiseaseMelanoma
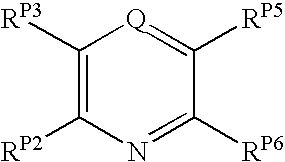
The present invention pertains to certain pyrazines and pyridines, and derivatives thereof, which, inter alia, inhibit RAF (e.g., B RAF) activity, inhibit cell proliferation, treat cancer, etc., and more particularly to compounds of the formulae: (I) wherein: Q is independently —N═ or —CH═; one of RP2 and RP3 is independently a group of the formula -J1-L1-Z; wherein: if Q is —N═, then -J1-L1-Z is independently: —NH-Z; —O-Z; or S-Z; if Q is —CH═, then -J1-L1-Z is independently: —NH—(CH2)n-Z, wherein n is independently 0 or 1; —O-Z; or —S-Z; Z is independently: C6-14 carboaryl, C5-14 heteroaryl, C3-12carbocyclic, C3-12 heterocyclic; and is independently unsubstituted or substituted; the other of RP2 and RP3 is independently —H, —NHRN1, or —NHC(═O)RN2; wherein: RN1, if present, is independently —H or aliphatic saturated C1-4alkyl; RN2, if present, is independently —H or aliphatic saturated C1-4alkyl; one of RP5 and RP6 is independently a group of the formula —W—Y; wherein: W is independently: a covalent bond; —NRN4—, —O—, —S—, —C(═O)—, —CH2—; —NRN4—CH2—, —O—CH2—, —S—CH2—, —C(═O)—CH2—, —(CH2)2—; —CH2—NRN4—, —CH2—O—, —CH2—S—, or —CH2—C(═O)—; wherein RN4, if present, is independently —H or aliphatic saturated C1-4alkyl; Y is independently: C6-14carboaryl, C5-14heteroaryl, C3-12carbocyclic, C3-12heterocyclic; and is independently unsubstituted or substituted; the other of RP5 and RP6 is independently —H; and pharmaceutically acceptable salts, solvates, amides, esters, ethers, N-oxides, chemically protected forms, and prodrugs thereof. The present invention also pertains to pharmaceutical compositions comprising such compounds, and the use of such compounds and compositions, e.g., both in vitro and in vivo, to inhibit RAF (e.g., B-RAF) activity, to inhibit receptor tyrosine kinase (RTK) activity, to inhibit cell proliferation, and in the treatment of diseases and conditions that are ameliorated by the inhibition of RAF, RTK, etc., proliferative conditions such as cancer (e.g., colorectal cancer, melanoma), etc.
Owner:THE WELLCOME TRUST TRUSTEE TO THE WELLCOME TRUST
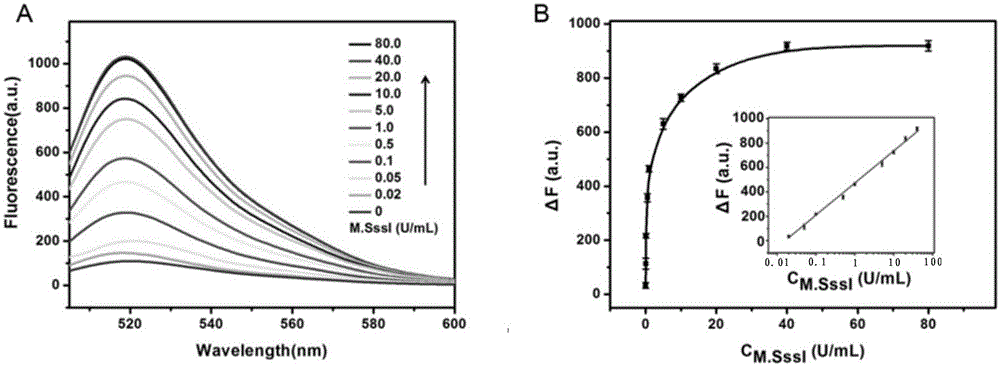
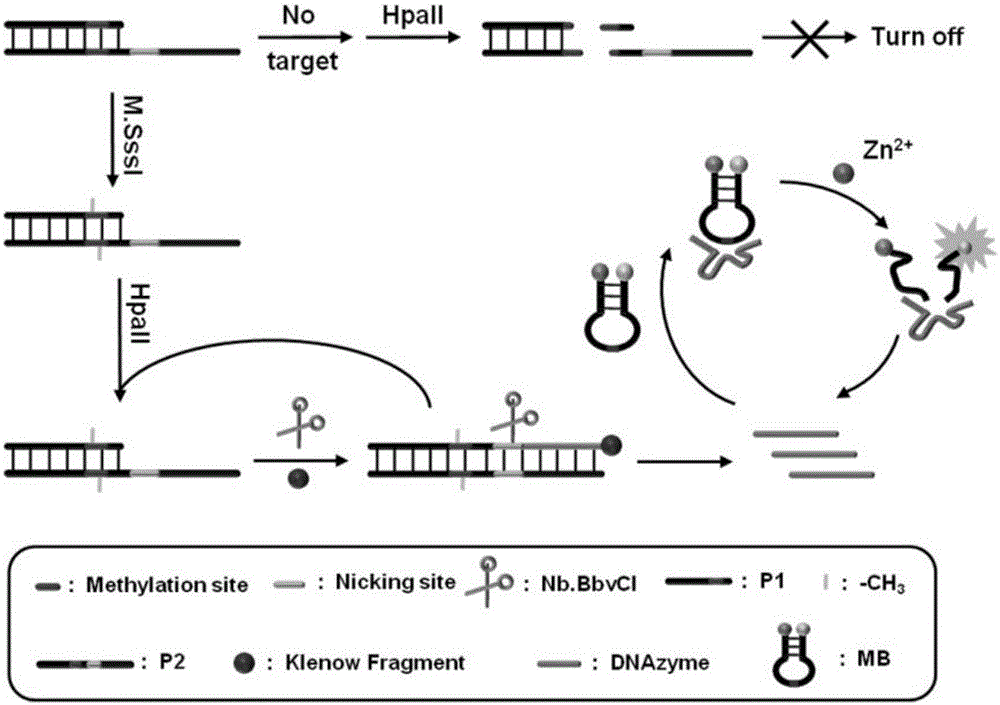
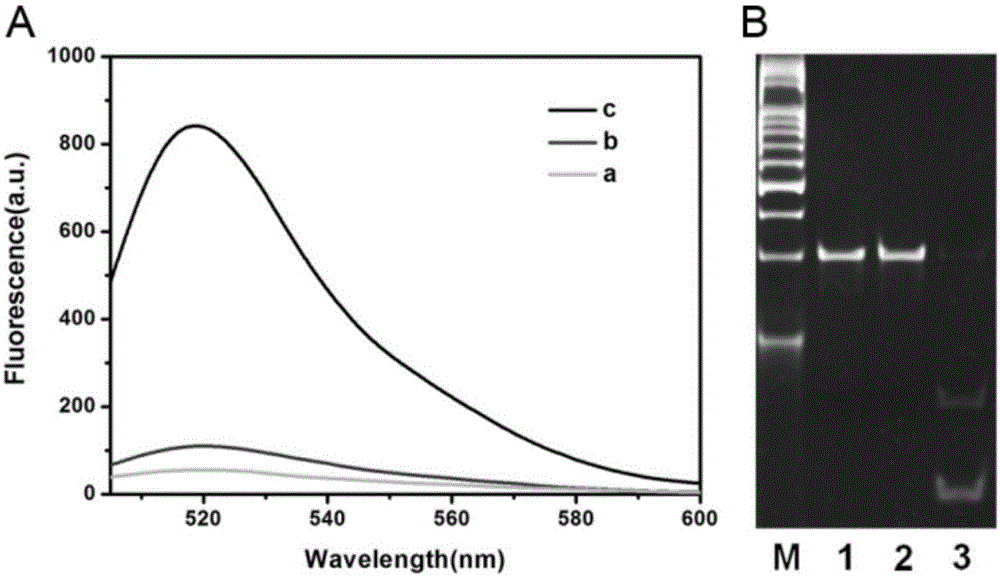
Method for detecting DAN methyltransferase activity based on strand displacement amplification and DNAzyme amplification
ActiveCN105112540AHighly sensitive detection activityLow detection limitMicrobiological testing/measurementBiological material analysisFluorescenceDNA Methyltransferase Inhibitor
The invention relates to a method for detecting DAN methyltransferase activity based on strand displacement amplification and DNAzyme amplification. A three-function double-stranded DNA probe is designed. Methylation happening to the three-function double-stranded DNA probe is specifically recognized through DAN methyltransferase. Remaining non-methylated double-stranded DNA is specifically cut through HpaII restriction enzymes, methylated double-stranded DNA triggers a strand displacement reaction, a large amount of 8-17 DNAzyme is released, the 8-17 DNAzyme catalyzes cutting of a large number of hairpin-line molecular beacon substrates, and remarkable fluorescent enhancement is triggered. The method can sensitively detect the DAN methyltransferase activity, and the detection limit is 0.0082 U / mL. The method has potential application in research of influences of anti-cancer drugs on DAN methyltransferase activity inhibition and screening of DAN methyltransferase inhibitors.
Owner:SHANDONG UNIV
Rotamase enzyme activity inhibitors
InactiveUS7056935B2Improve survivalPotent in augmenting neurite outgrowthBiocideOrganic chemistryNeuronal degenerationNeuron
This invention relates to methods of using neurotrophic compounds having an affinity for FKBP-type immunophilins to stimulate or promote neuronal growth or regeneration and to prevent neuronal degeneration.
Owner:GPI NIL HLDG INC +1
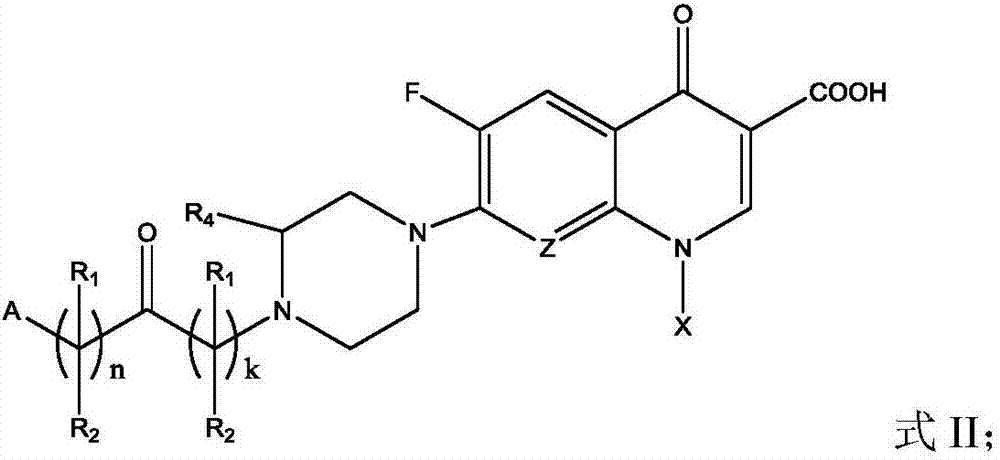
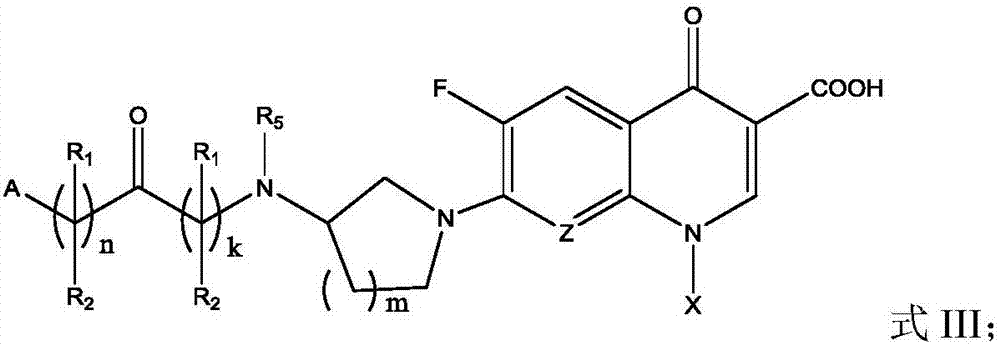
Fluoroquinolone amino derivatives and application thereof
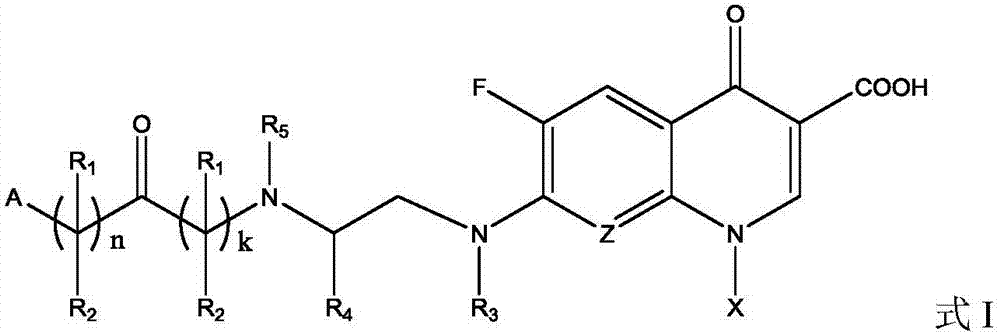
ActiveCN107880023AHigh antibacterial activitySmall toxicityAntibacterial agentsOrganic active ingredientsSolubilityDisease
The invention belongs to the field of medicinal chemistry, and in particular relates to fluoroquinolone derivatives and an application thereof. The compounds shown in a formula (I) are obtained by structural modification of fluoroquinolone medicines. The compounds provided by the invention can not only treat the infection caused by mycobacterium tuberculosis and common bacteria, but also can has activity inhibition effects on bacterial persister, citrus pathogenic bacteria, nicotinamide N-methyltransferase (NNMT) and interleukin IL-17 PPI; and the compounds have a simple and easy preparation process and mild conditions, a plurality of the compounds with enhanced antibacterial activity, improved water solubility and reduced toxic and side effects are obtained, and the compounds are expectedto reduce the dosage, shorten the treatment cycle and improve patient compliance, thereby providing novel molecular types and research ideas for medicines of tuberculosis and other diseases.
Owner:SOUTHWEST UNIVERSITY
Tyrosinase polypeptide inhibitor
InactiveCN102492018AReduce melanin contentBiocideCosmetic preparationsWhitening AgentsPhosphorylation
The invention relates to a tyrosinase polypeptide inhibitor, which consists of a polypeptide. In the polypeptide, one or more amino acids are further subjected to acetylation, amidation, formylation, hydroxylation, lipolyzation, methylation or phosphorylation modification. The tyrosinase inhibitor provided by the invention can be taken as a medical composition, comprises the polypeptide, and further comprises a pharmaceutically-acceptable supporting agent. The medicinal composition provided by the invention can be used for inhibiting the generation of black pigments under the action of activity inhibition of tyrosinase, and can be widely applied to cosmetics such as a whitening agent.
Owner:萧乃文
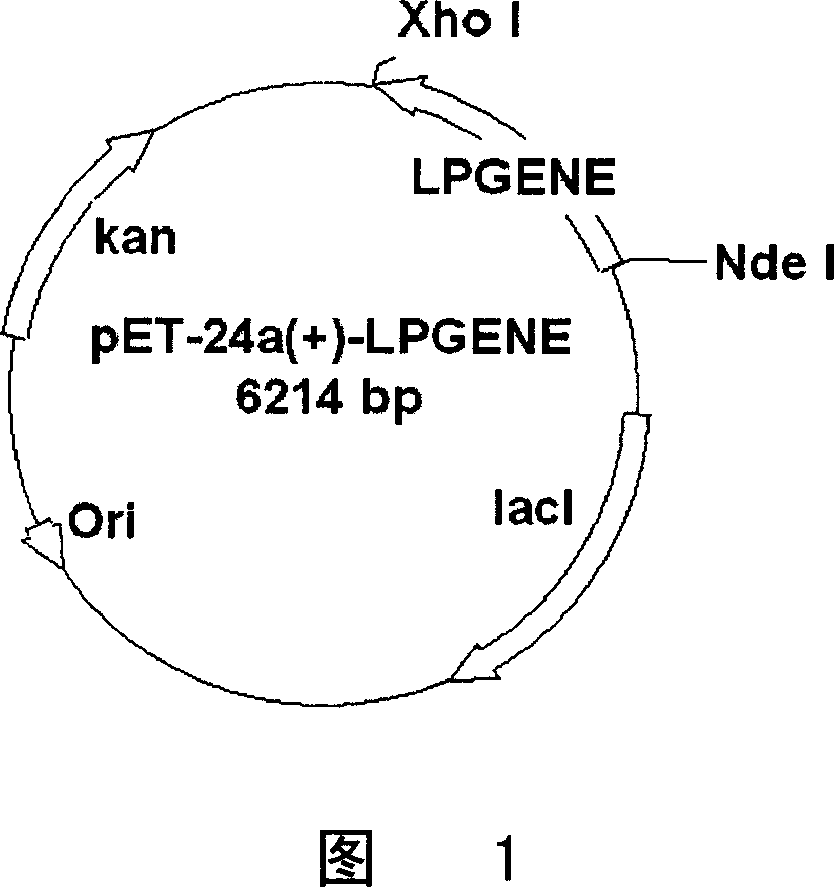
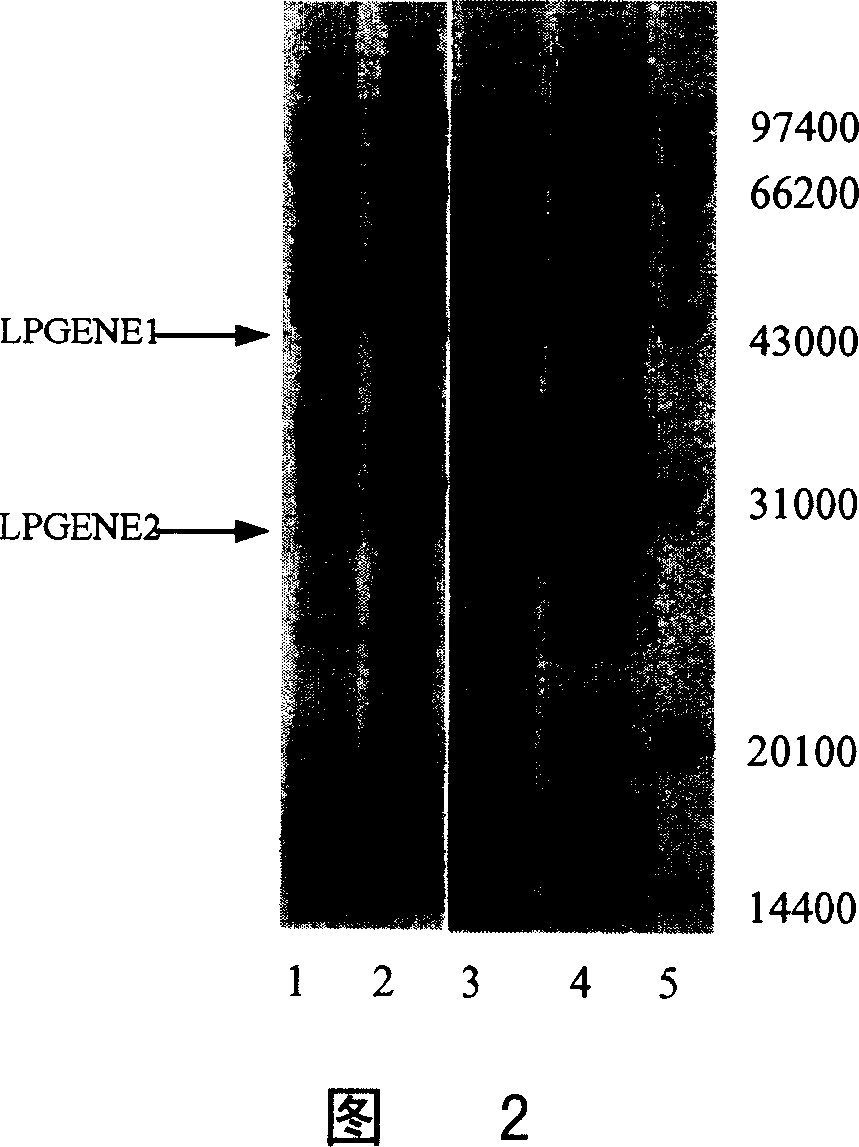
Preparation and purification of telomerase activity inhibition protein
This invention discloses a method of using gene recombination technology to prepare telomerase activation profiling. This invention can prepare telomerase activation profilin which is high purity and high biologic activity with high efficiency and large scale.
Owner:SHANGHAI INST OF BIOLOGICAL SCI CHINESE ACAD OF SCI
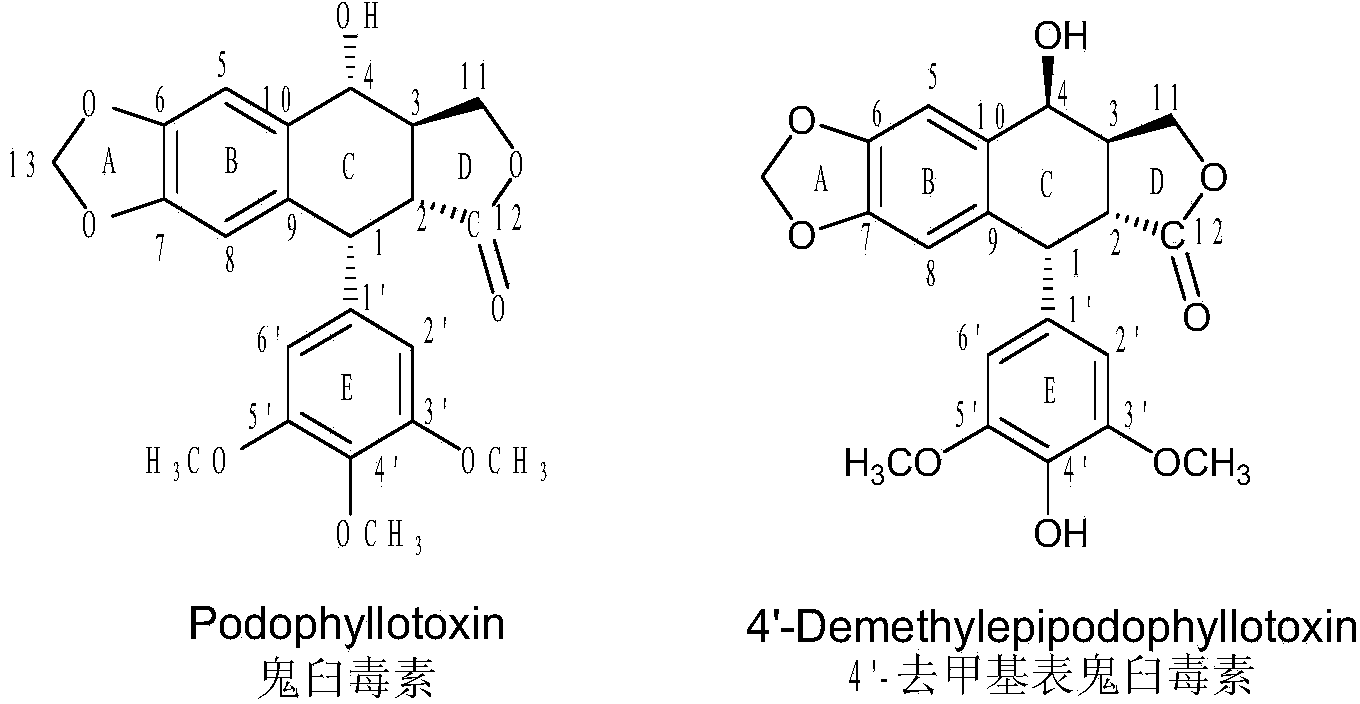
Sulfur-substituted podophyllotoxin derivative as well as synthetic method and application thereof
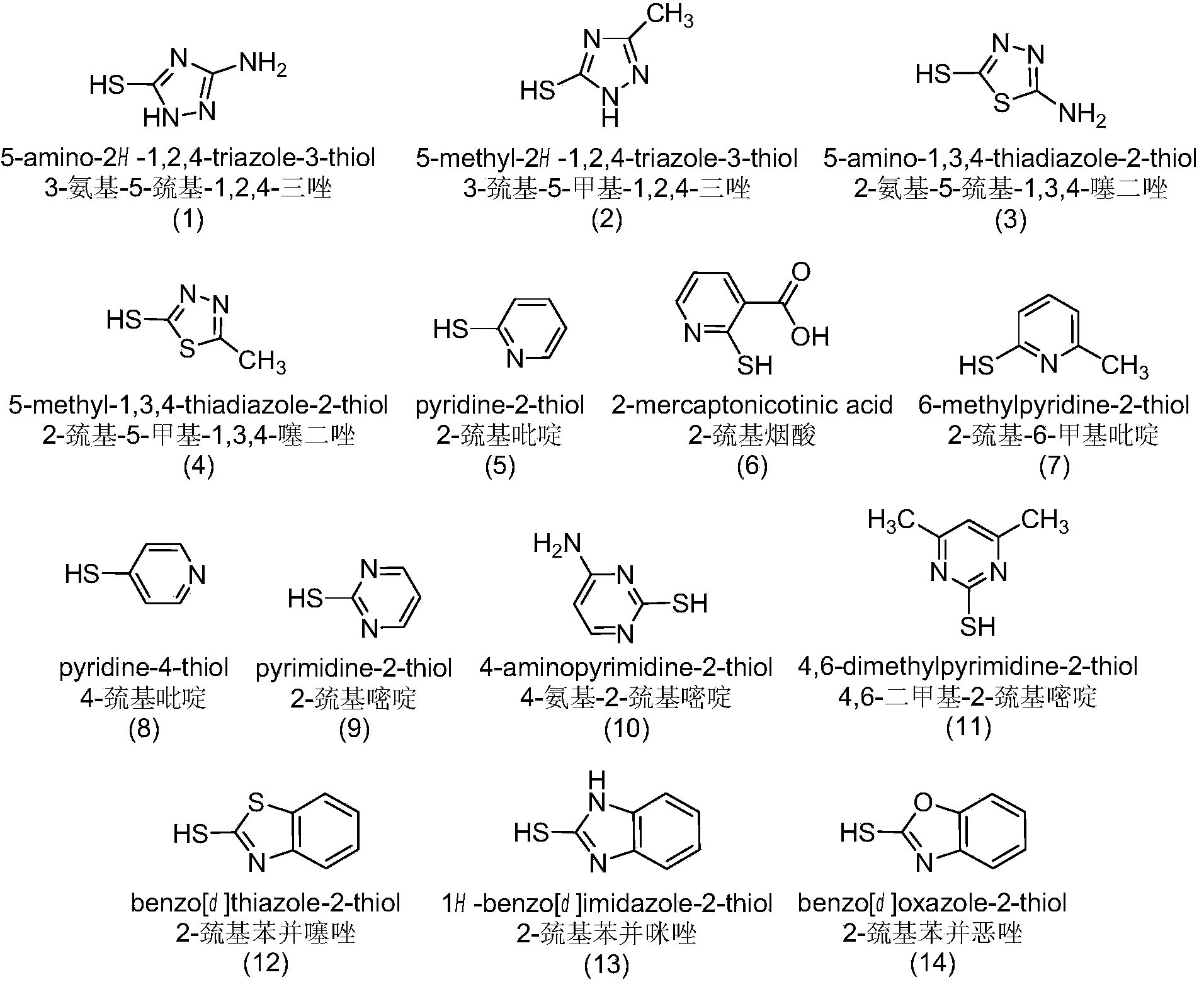
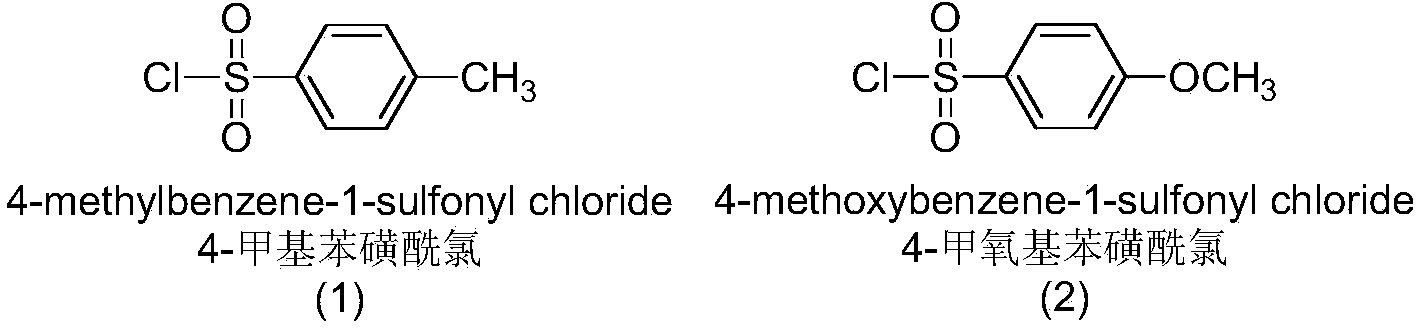
The invention discloses a sulfur-substituted podophyllotoxin derivative as well as a synthetic method and application thereof. The podophyllotoxin derivative, shown by a formula (V), with significantly improved anti-tumor activity and reduced toxic or side effect is obtained by introducing an aromatic heterocyclic compound with rigidity and a further sulfonamide product of 3-amino-5-mercapto-1,2,4-triazole, 2-amino-5-mercapto-1,3,4-thiadiazole, and 4-methyl benzenesulfonyl chloride or 4-methoxy benzenesulfonyl chloride, serving as substituent groups, into the fourth position of a C ring of podophyllotoxin or 4'-demethylepipodophyllotoxin respectively. An activity inhibition experiment of tumor cells in vitro shows that the anti-tumor activity of the compound shown by the formula (V) is significantly improved as compared with that of podophyllotoxin or 4'-demethylepipodophyllotoxin.
Owner:HUBEI UNIV OF TECH
Methods for detecting lp-pla2 activity and inhibition of lp-pla2 activity
InactiveUS20070166777A1Microbiological testing/measurementBiological material analysisBiologyActivity inhibition
Owner:GLAXO GRP LTD
Chiral alkamine ligand and its application in asymmetrical addition of terminal alkyne para imine
InactiveCN1442403AGroup 4/14 element organic compoundsAsymmetric synthesesTrifluoromethylPhenyl group
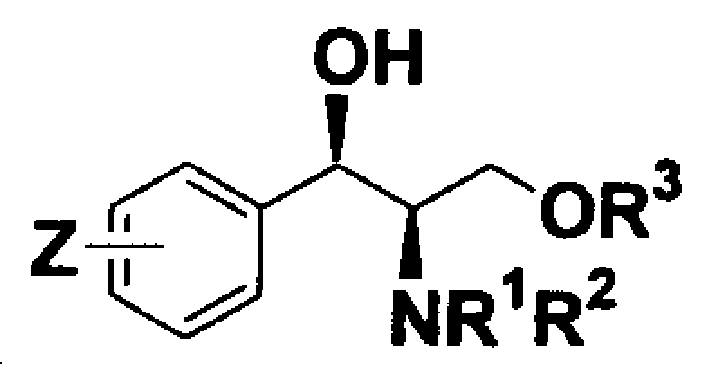
A novel chiral ligand (1R, 2R)-2-N,N-substituent-1-(4-substituted phenyl)-3-O- substituent-1-propanol is disclosed, which can be used for asymmetrical synthesis of the precursors of DPC961 and DPC083, which are high-activity inhibitor of HIV transferase.
Owner:SHANGHAI INST OF ORGANIC CHEMISTRY - CHINESE ACAD OF SCI
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
![Imidazo[4, 5-b]pyridin-2-one and oxazolo[4, 5-b]pyridin-2-one compounds and analogs thereof as cancer therapeutic compounds Imidazo[4, 5-b]pyridin-2-one and oxazolo[4, 5-b]pyridin-2-one compounds and analogs thereof as cancer therapeutic compounds](https://images-eureka.patsnap.com/patent_img/160c93b4-7535-4186-a3b0-42b5846b0524/US20090325945A1-C00001.png)
![Imidazo[4, 5-b]pyridin-2-one and oxazolo[4, 5-b]pyridin-2-one compounds and analogs thereof as cancer therapeutic compounds Imidazo[4, 5-b]pyridin-2-one and oxazolo[4, 5-b]pyridin-2-one compounds and analogs thereof as cancer therapeutic compounds](https://images-eureka.patsnap.com/patent_img/160c93b4-7535-4186-a3b0-42b5846b0524/US20090325945A1-C00002.png)
![Imidazo[4, 5-b]pyridin-2-one and oxazolo[4, 5-b]pyridin-2-one compounds and analogs thereof as cancer therapeutic compounds Imidazo[4, 5-b]pyridin-2-one and oxazolo[4, 5-b]pyridin-2-one compounds and analogs thereof as cancer therapeutic compounds](https://images-eureka.patsnap.com/patent_img/160c93b4-7535-4186-a3b0-42b5846b0524/US20090325945A1-C00003.png)


![Pyrido[2,3-b]pyrazin-8-substituted compounds and their use Pyrido[2,3-b]pyrazin-8-substituted compounds and their use](https://images-eureka.patsnap.com/patent_img/693cdd8b-8e07-41c6-8f1b-aa808acf5ee2/US08198279-20120612-D00001.png)
![Pyrido[2,3-b]pyrazin-8-substituted compounds and their use Pyrido[2,3-b]pyrazin-8-substituted compounds and their use](https://images-eureka.patsnap.com/patent_img/693cdd8b-8e07-41c6-8f1b-aa808acf5ee2/US08198279-20120612-D00002.png)
![Pyrido[2,3-b]pyrazin-8-substituted compounds and their use Pyrido[2,3-b]pyrazin-8-substituted compounds and their use](https://images-eureka.patsnap.com/patent_img/693cdd8b-8e07-41c6-8f1b-aa808acf5ee2/US08198279-20120612-D00003.png)
![Imidazo[4,5-b]pyridin-2-one and oxazolo[4,5-b]pyridin-2-one compounds and analogs thereof as therapeutic compounds Imidazo[4,5-b]pyridin-2-one and oxazolo[4,5-b]pyridin-2-one compounds and analogs thereof as therapeutic compounds](https://images-eureka.patsnap.com/patent_img/b0cd26c9-2c93-4765-aca7-10b49de51d24/US07625922-20091201-C00001.png)
![Imidazo[4,5-b]pyridin-2-one and oxazolo[4,5-b]pyridin-2-one compounds and analogs thereof as therapeutic compounds Imidazo[4,5-b]pyridin-2-one and oxazolo[4,5-b]pyridin-2-one compounds and analogs thereof as therapeutic compounds](https://images-eureka.patsnap.com/patent_img/b0cd26c9-2c93-4765-aca7-10b49de51d24/US07625922-20091201-C00002.png)
![Imidazo[4,5-b]pyridin-2-one and oxazolo[4,5-b]pyridin-2-one compounds and analogs thereof as therapeutic compounds Imidazo[4,5-b]pyridin-2-one and oxazolo[4,5-b]pyridin-2-one compounds and analogs thereof as therapeutic compounds](https://images-eureka.patsnap.com/patent_img/b0cd26c9-2c93-4765-aca7-10b49de51d24/US07625922-20091201-C00003.png)