Sulfur-substituted podophyllotoxin derivative as well as synthetic method and application thereof
A technology of podophyllotoxin and derivatives, applied in the field of podophyllotoxin derivatives, which can solve the problems of limited application, toxic side effects and poor bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Example 1 Synthesis and purification of 4-S-(3-amino-1,2,4-triazole-5)-4-deoxy-podophyllotoxin (compound (1))
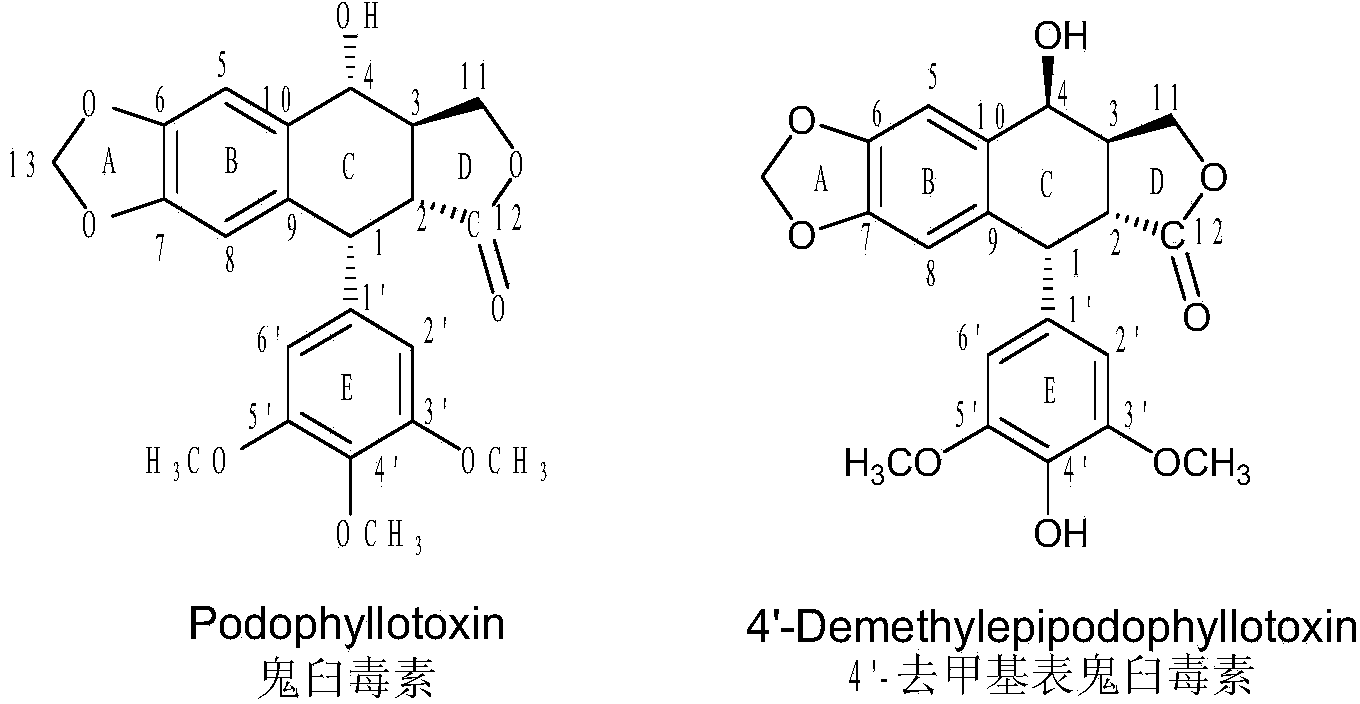
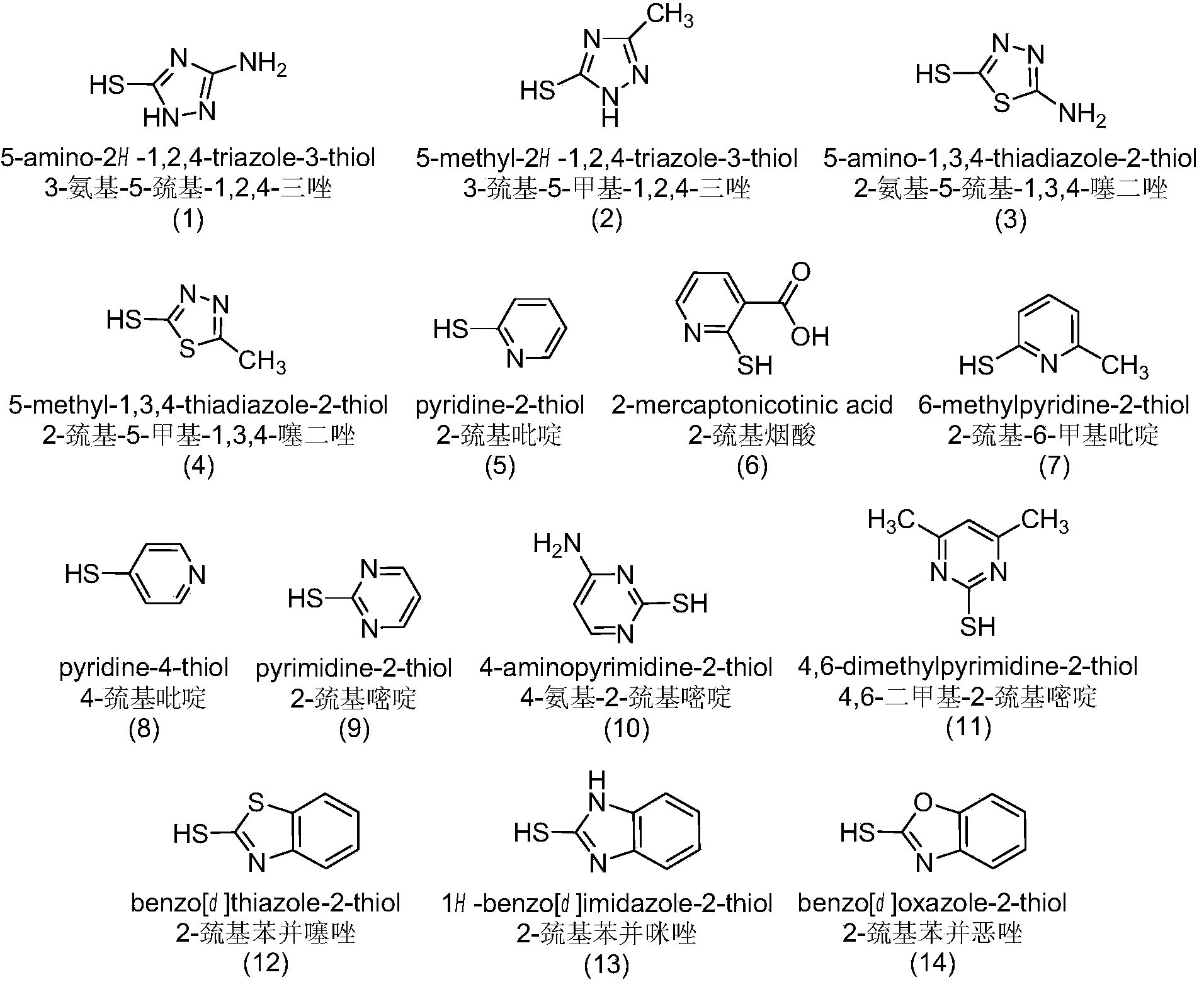
[0040] (1) Synthesis of 4-S-(3-amino-1,2,4-triazole-5)-4-deoxy-podophyllotoxin: take 414mg (1mmol) of podophyllotoxin, 116mg (1mmol) of 3-Amino-5-mercapto-1,2,4-triazole, vacuum-dried for 1 hour, under ice-bath condition with 15ml trifluoroacetic acid as solvent, stirred in vacuum for 1-3 hours, with chloroform acetone as developing agent, detection reaction end. The reaction system is added to 20-50 times the volume of deionized water, the filter cake is collected by filtration, washed and dried to obtain the crude product.
[0041] (2) Separation and purification of 4-S-(3-amino-1,2,4-triazole-5)-4-deoxy-podophyllotoxin:
[0042] Separation and purification using silica gel column chromatography and gel column chromatography:
[0043] (A) Use a normal phase silica gel column (normal phase silica gel: China Qingdao Ocean Chemical Co., Ltd., HG / T2354-92; se...
Embodiment 2
[0046] Example 2 Synthesis and purification of 4-S-(5-methyl-1,2,4-triazole-3)-4-deoxy-podophyllotoxin (compound (2))
[0047] (1) Synthesis of 4-S-(5-methyl-1,2,4-triazole-3)-4-deoxy-podophyllotoxin: take 414mg (1mmol) of podophyllotoxin, 115mg (1mmol) The 3-mercapto-5-methyl-1,2,4-triazole was vacuum-dried for 1 h, and stirred in vacuum for 1-3 h with 15 ml of trifluoroacetic acid as a solvent in an ice bath, and chloroform-acetone was used as a developer to detect end point of the reaction. The reaction system is added to 20-50 times the volume of deionized water, the filter cake is collected by filtration, washed and dried to obtain the crude product.
[0048] (2) Separation and purification of 4-S-(5-methyl-1,2,4-triazole-3)-4-deoxy-podophyllotoxin:
[0049] Using silica gel column chromatography and gel column chromatography for separation and purification, the method is the same as in Example 1.
[0050] Compound (2) 4-S-(5-methyl-1,2,4-triazole-3)-4-deoxy-podophyllo...
Embodiment 3
[0051] Example 3 Synthesis and purification of 4-S-(2-amino-1,3,4-thiadiazole-5)-4-deoxy-podophyllotoxin (compound (3))
[0052] (1) Synthesis of 4-S-(2-amino-1,3,4-thiadiazole-5)-4-deoxy-podophyllotoxin: take 414mg (1mmol) of podophyllotoxin, 133mg (1mmol) of podophyllotoxin 2-Amino-5-mercapto-1,3,4-thiadiazole, vacuum-dried for 1 hour, under ice-bath condition, use 15ml trifluoroacetic acid as solvent, stir in vacuum for 1-3 hours, use chloroform acetone as developer, detect reaction end. The reaction system is added to 20-50 times the volume of deionized water, the filter cake is collected by filtration, washed and dried to obtain the crude product.
[0053] (2) Separation and purification of 4-S-(2-amino-1,3,4-thiadiazole-5)-4-deoxy-podophyllotoxin:
[0054] Using silica gel column chromatography and gel column chromatography for separation and purification, the method is the same as in Example 1.
[0055] Compound (3) 4-S-(2-amino-1,3,4-thiadiazole-5)-4-deoxy-podophyll...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com