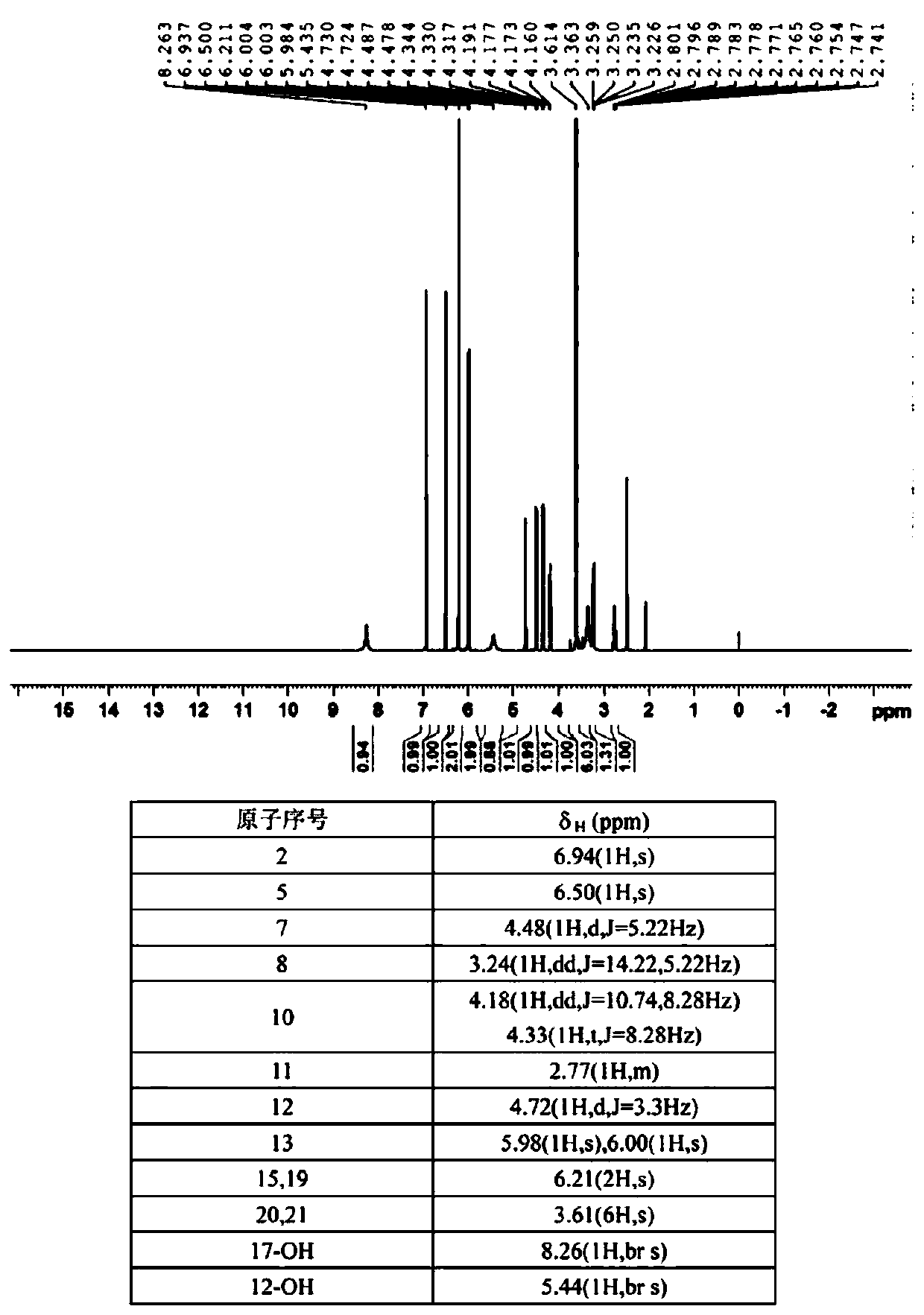
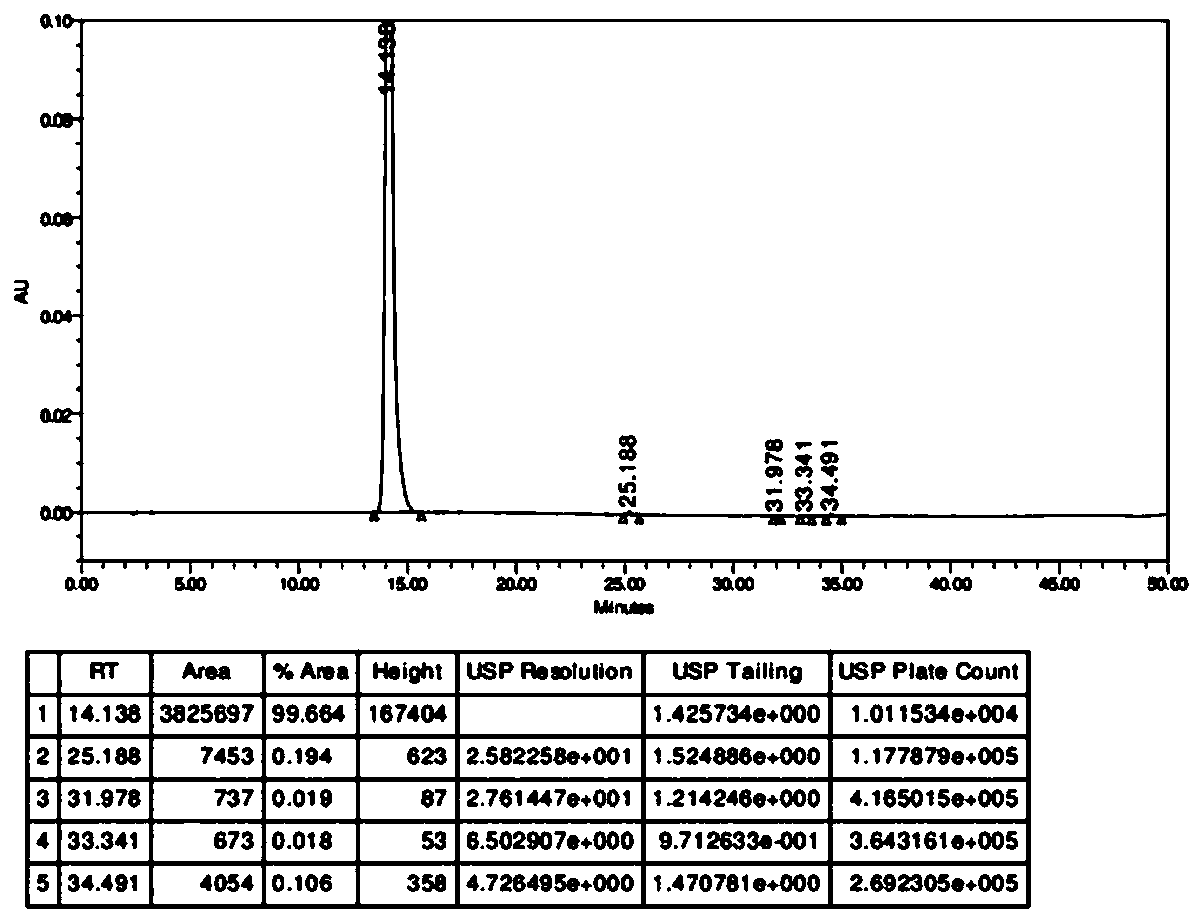
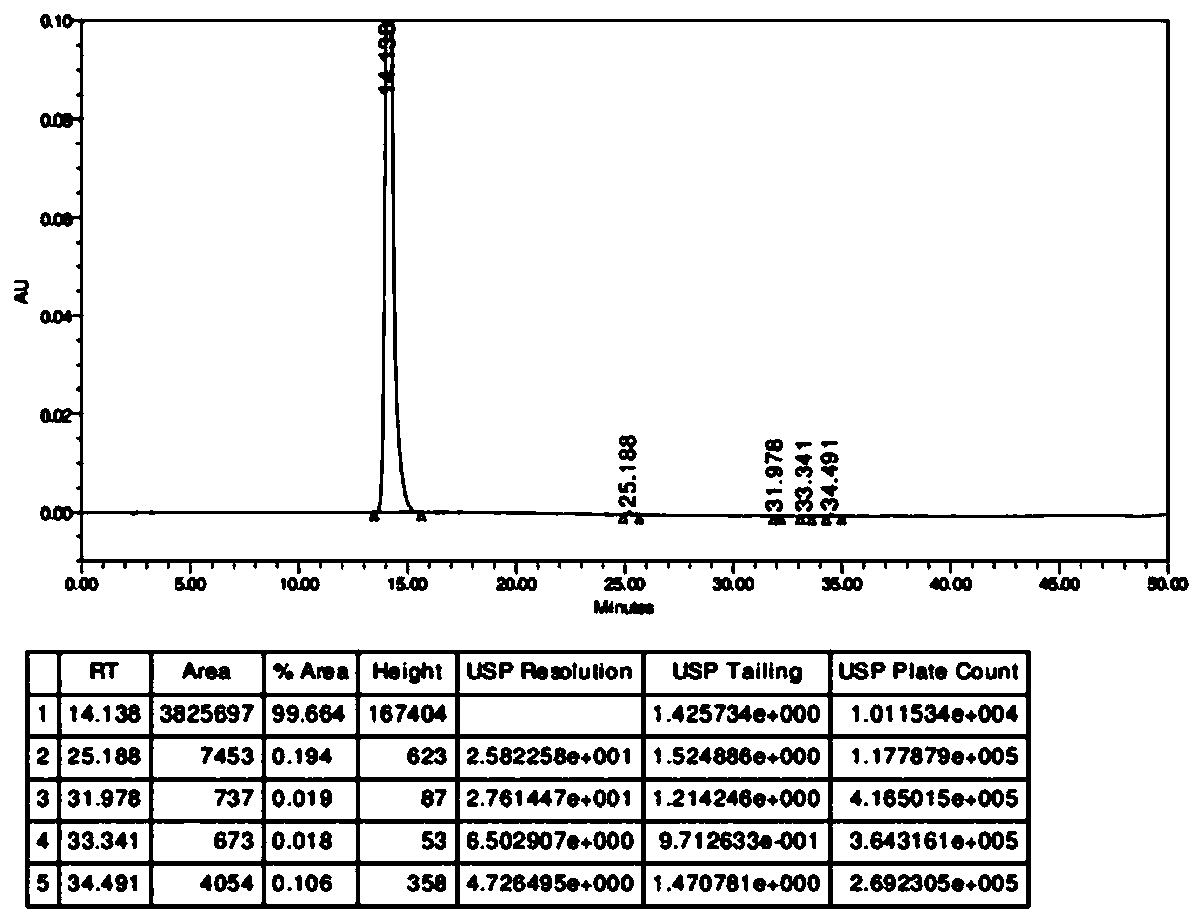
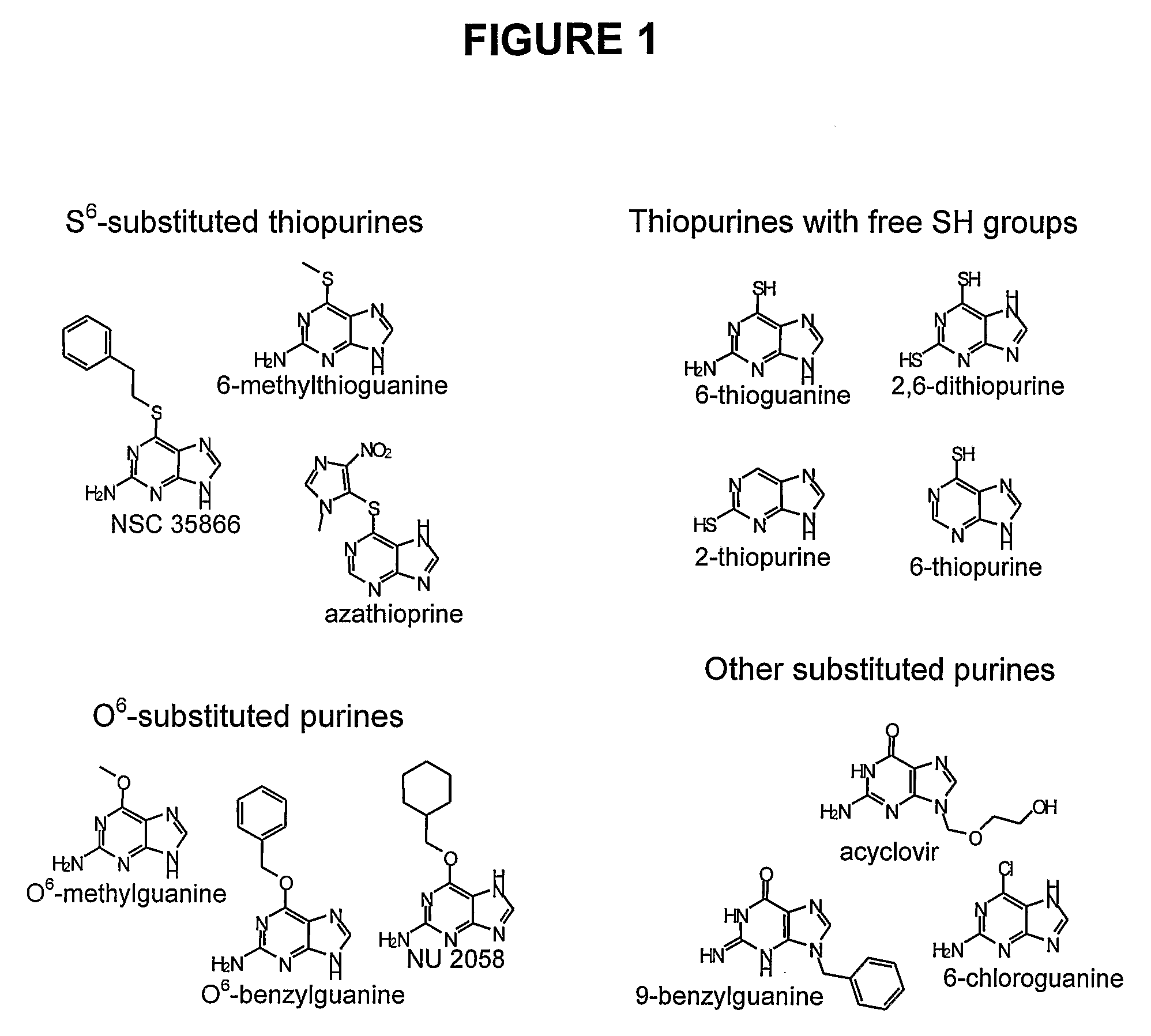
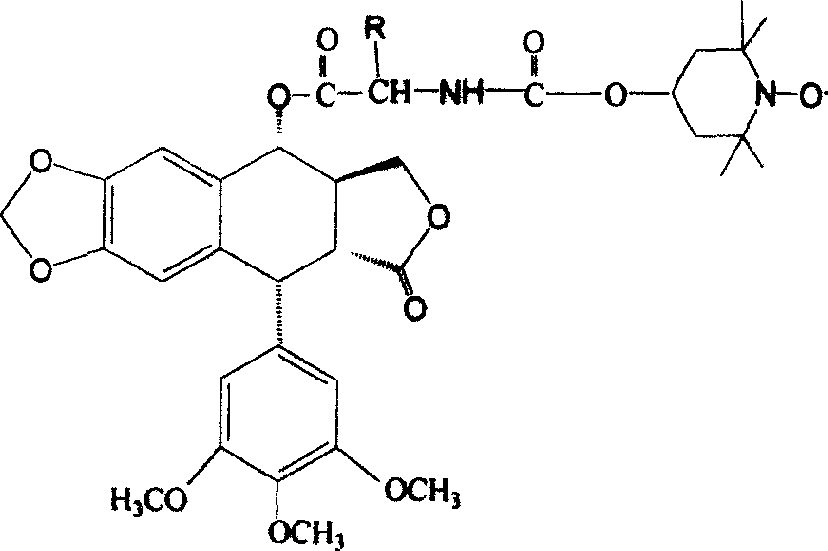
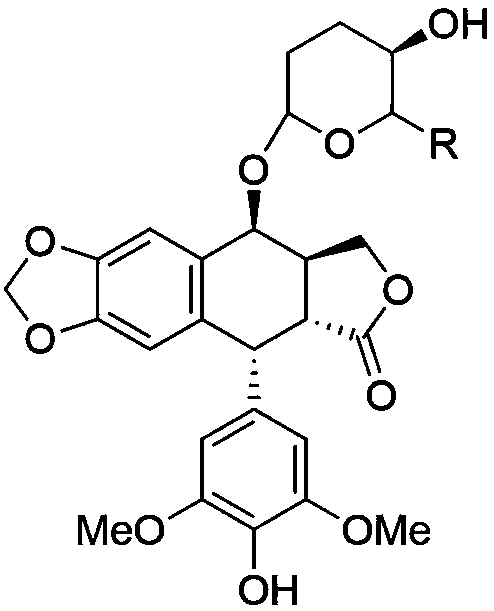
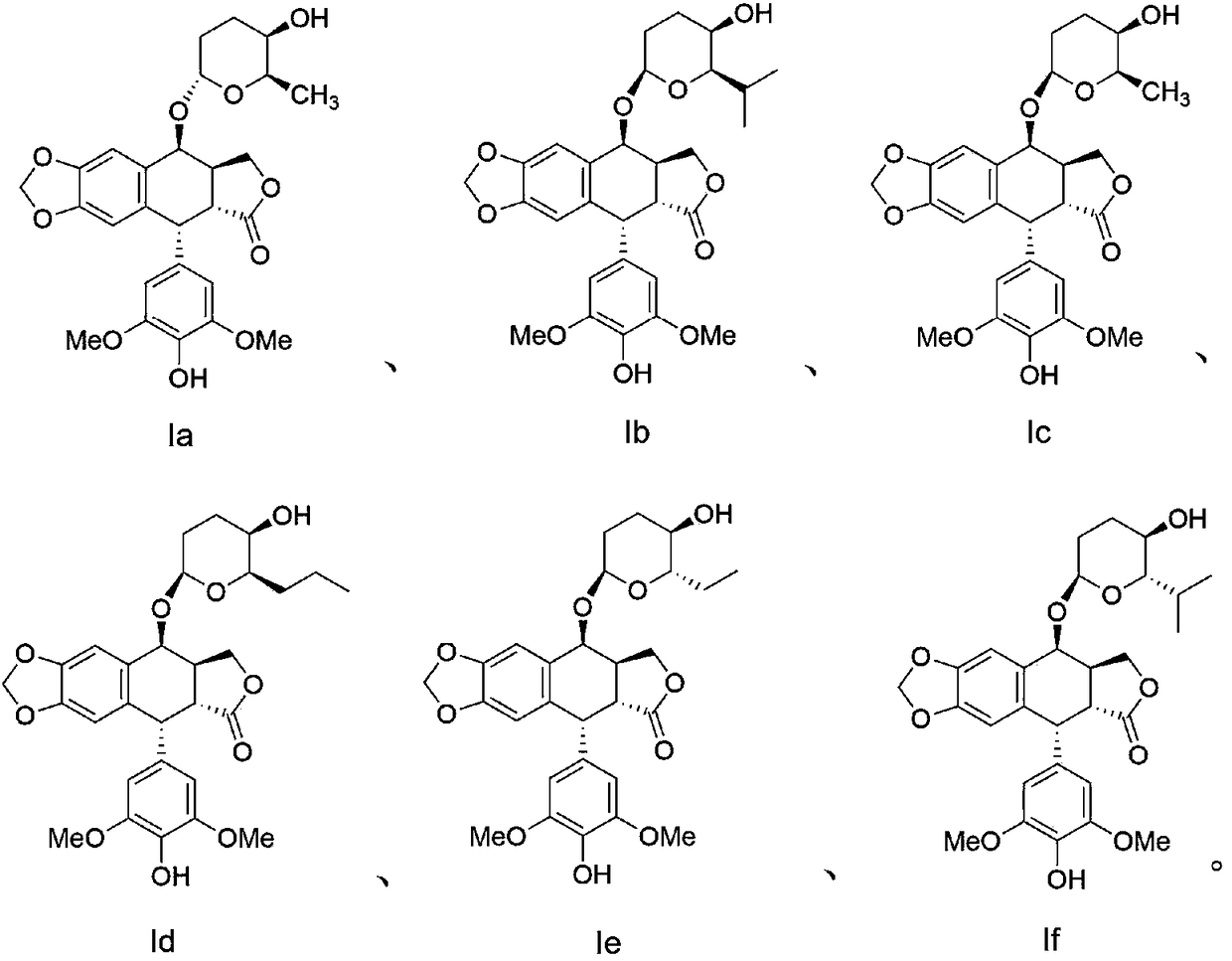
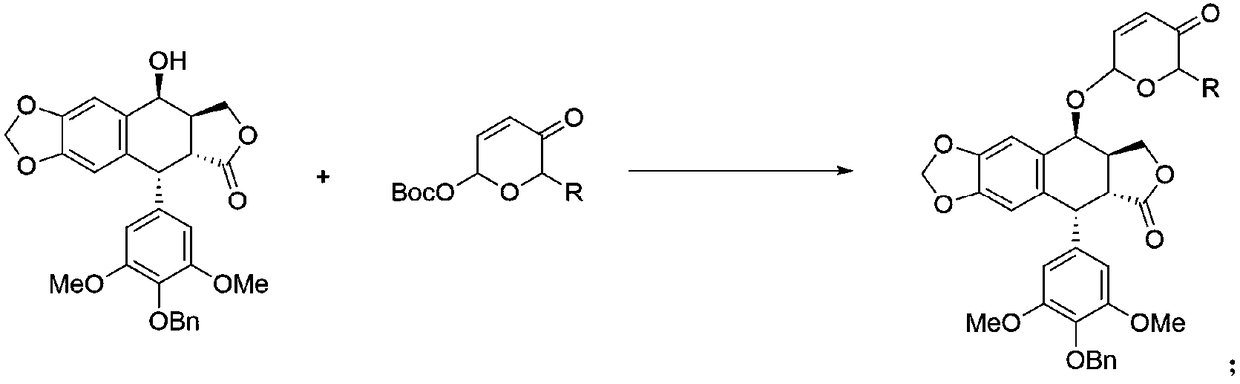
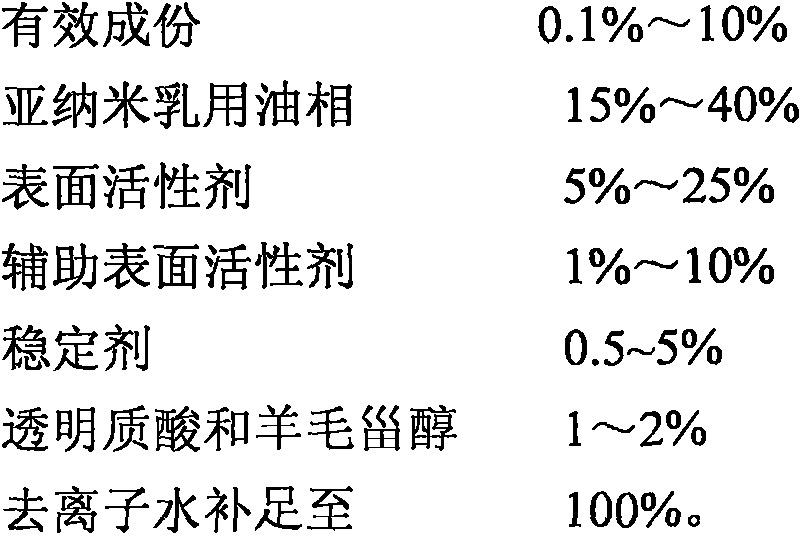
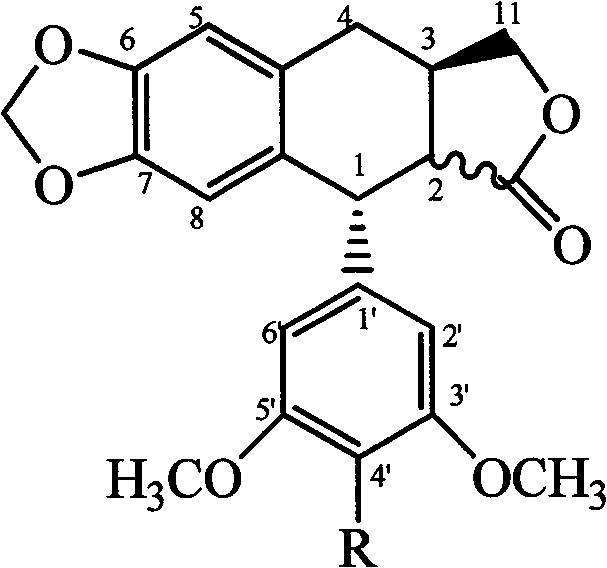
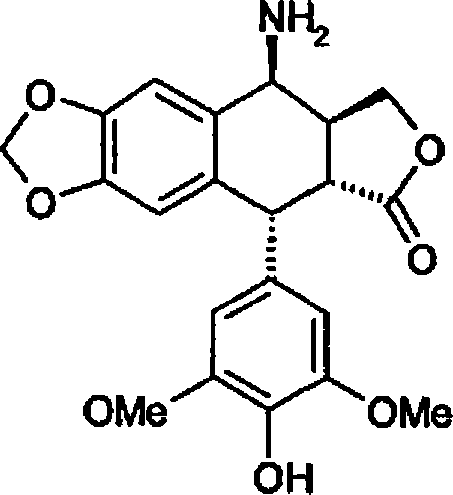
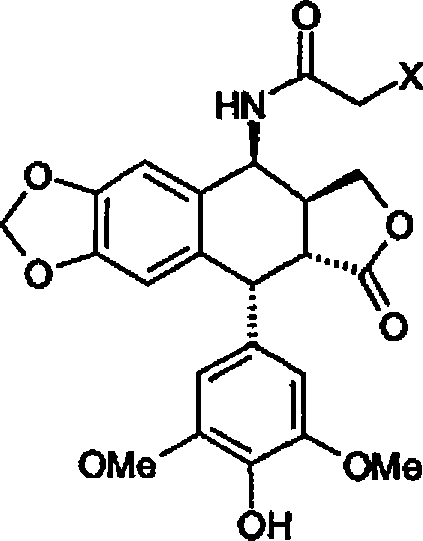
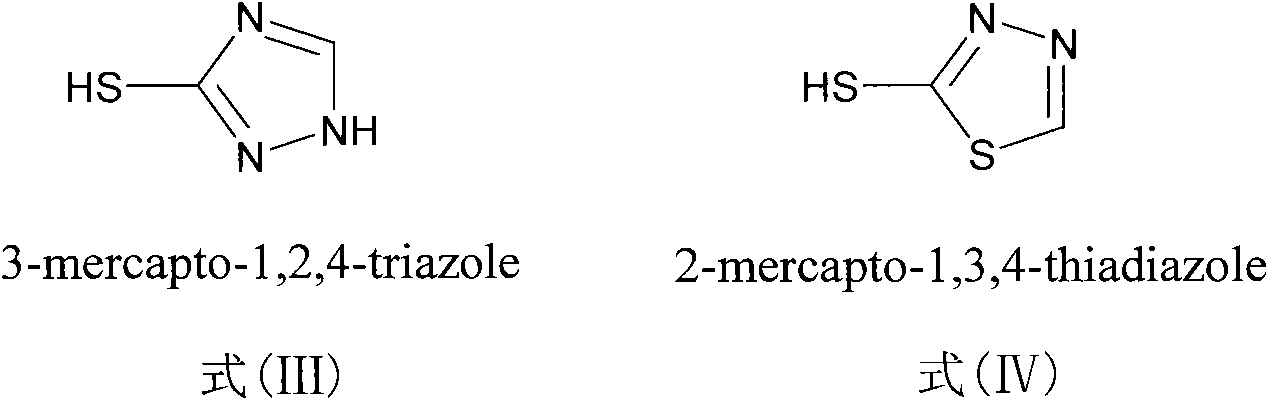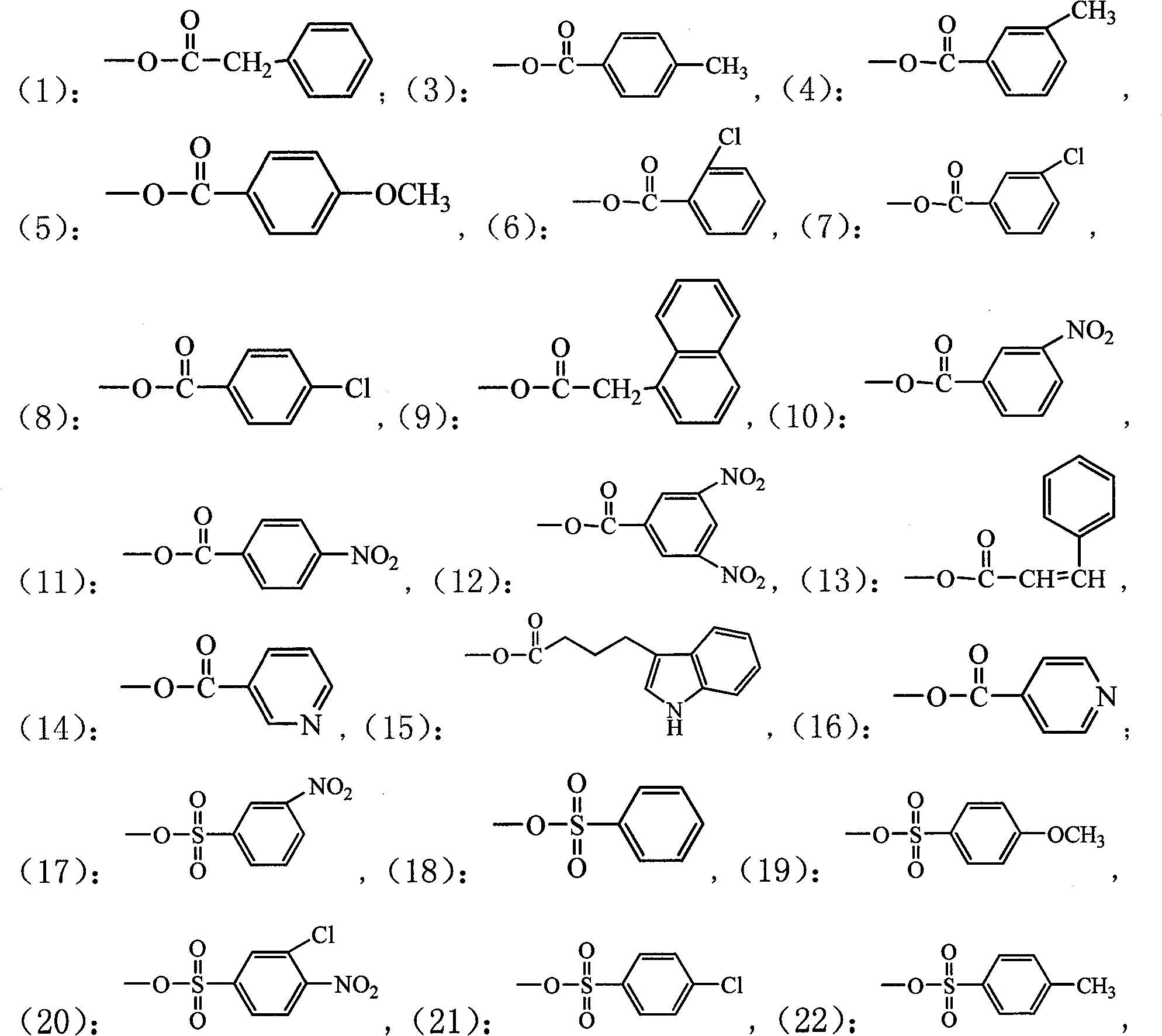Patents
Literature
82 results about "Epipodophyllotoxin" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
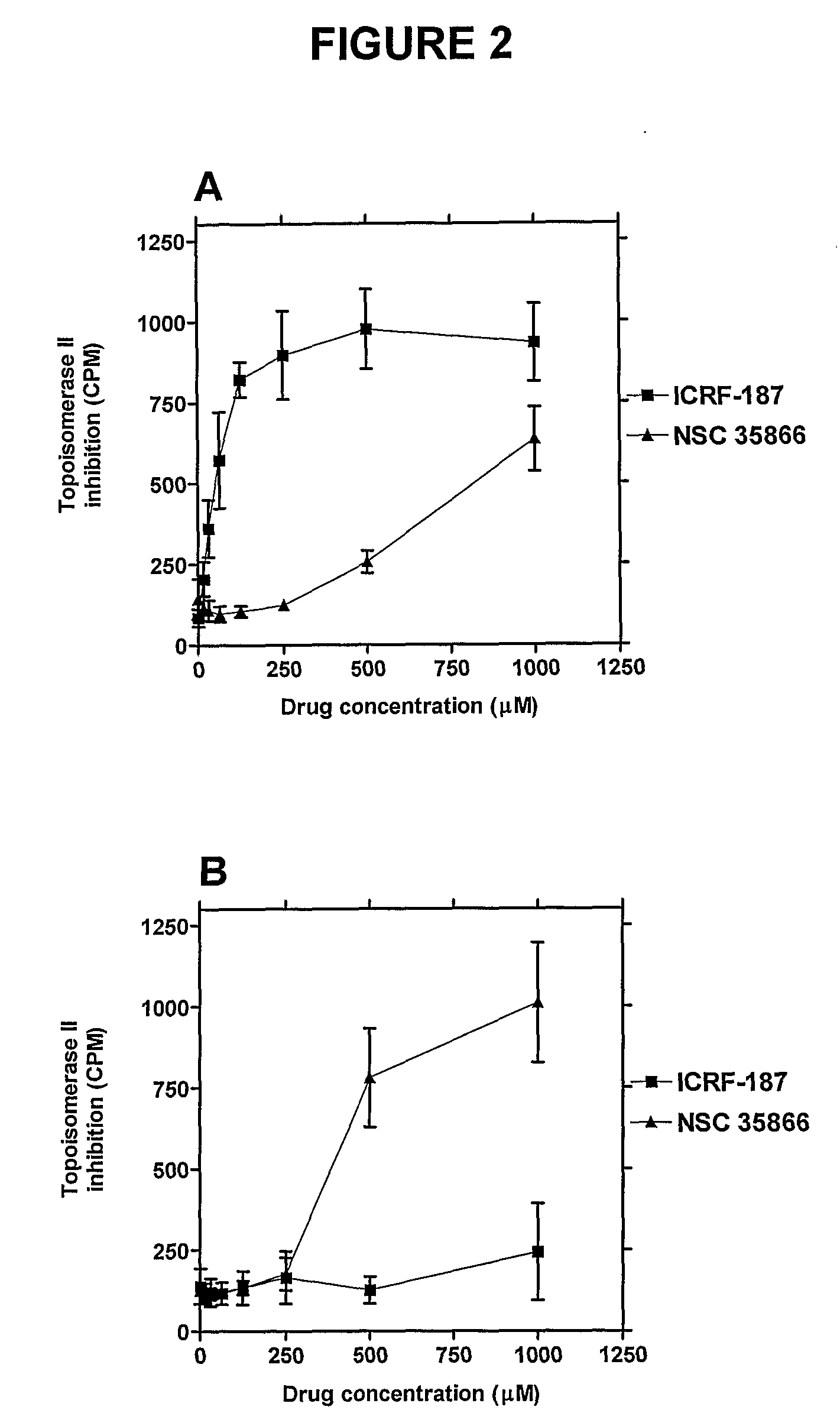
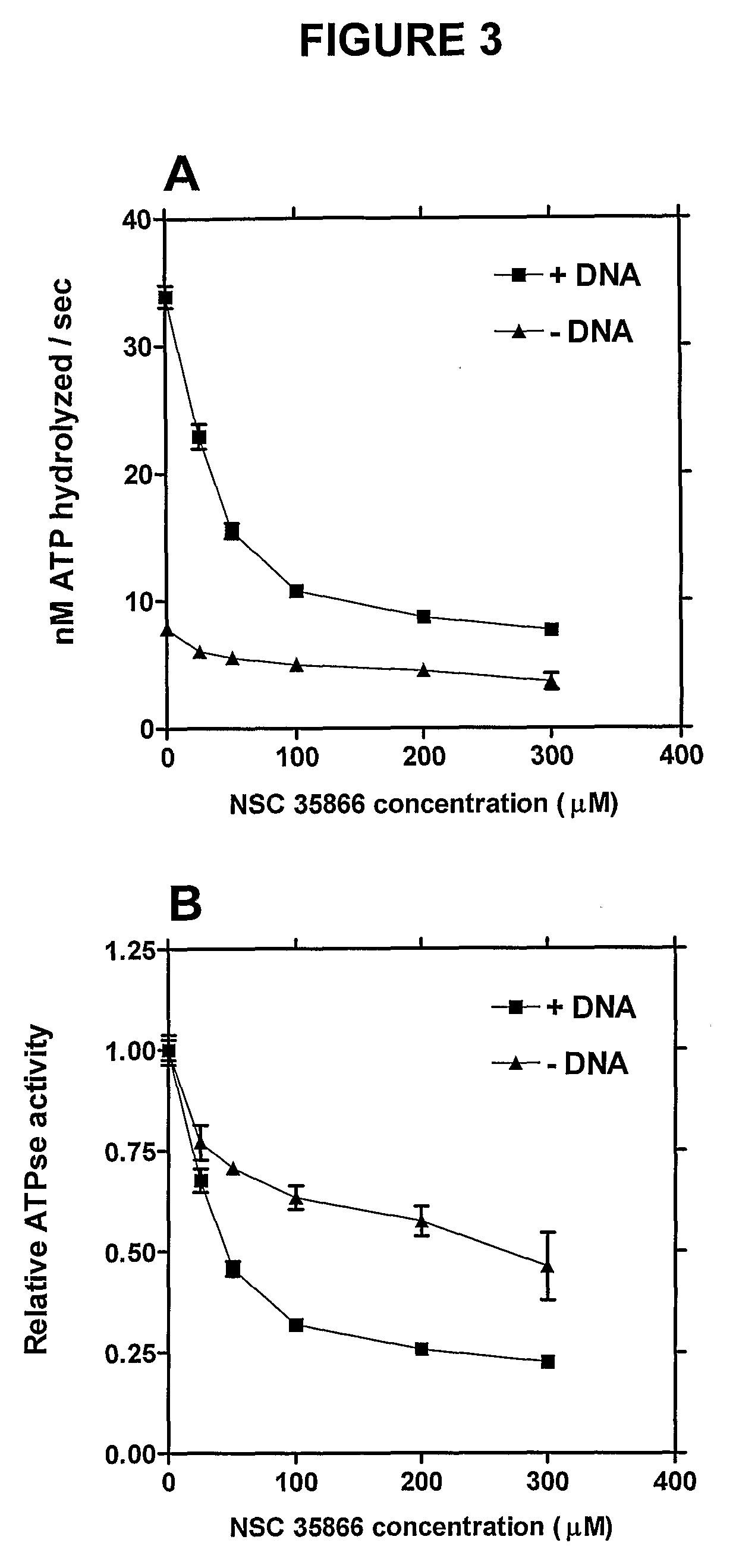
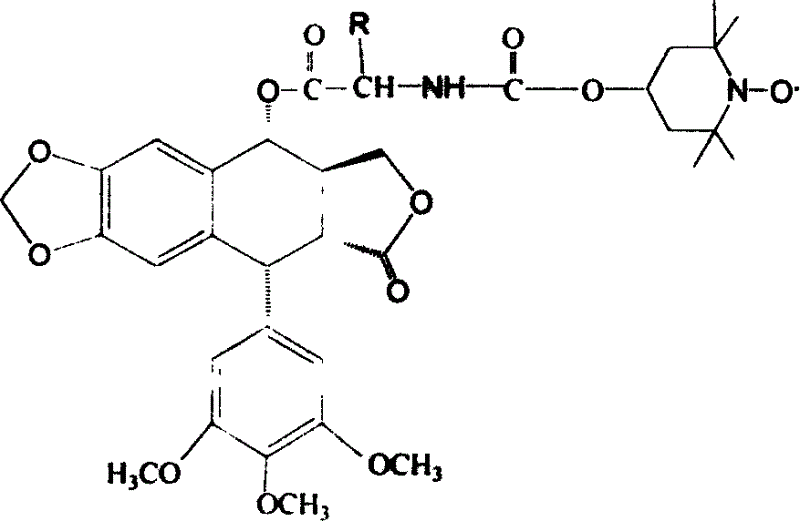
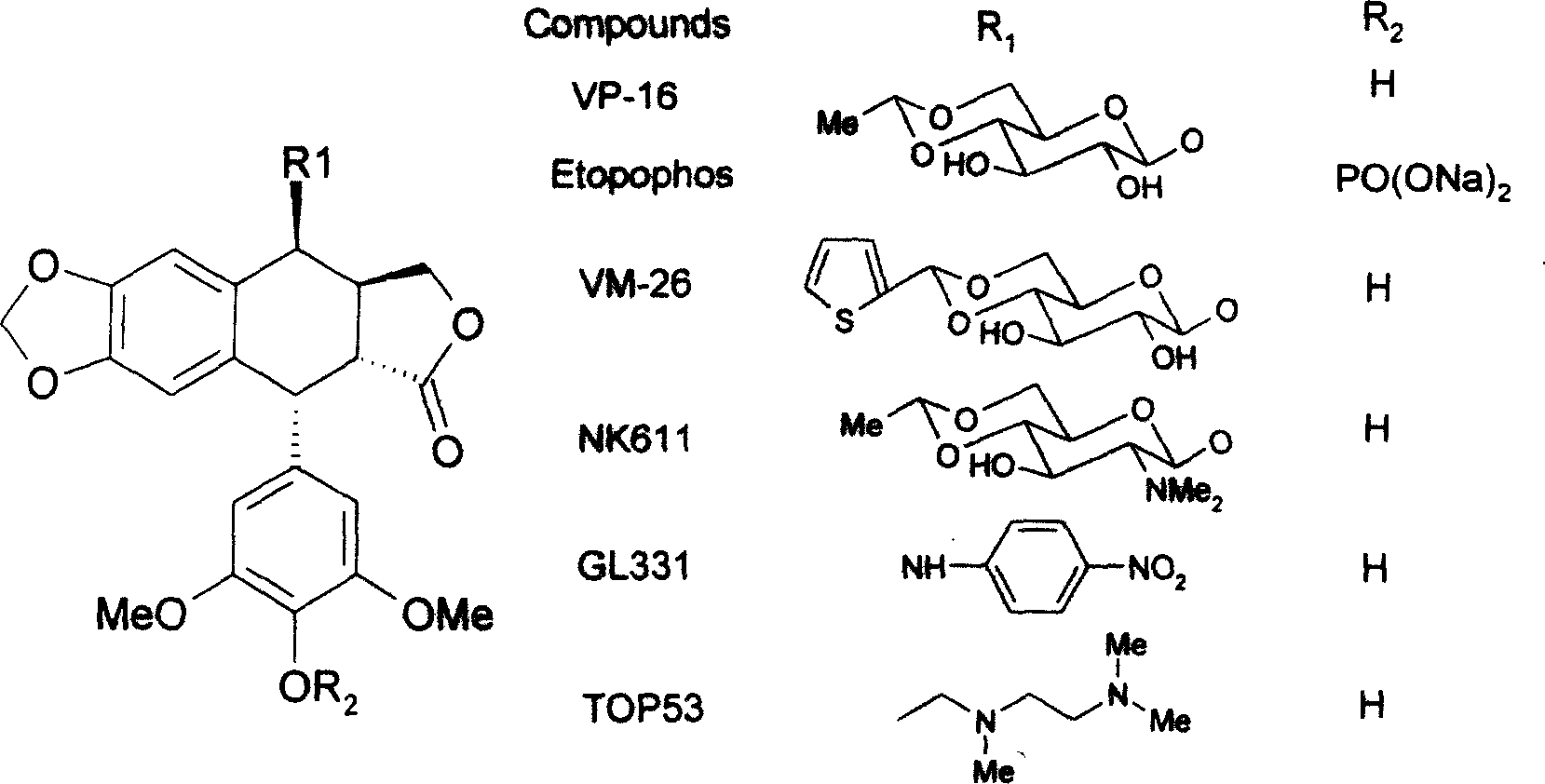
Epipodophyllotoxins are substances naturally occurring in the root of American Mayapple plant (Podophyllum peltatum). Some epipodophyllotoxin derivatives are currently used in the treatment of cancer. These include etoposide and teniposide. They act as anti-cancer drugs by inhibiting topoisomerase II.
Anti-integrin immunoconjugates, methods and uses
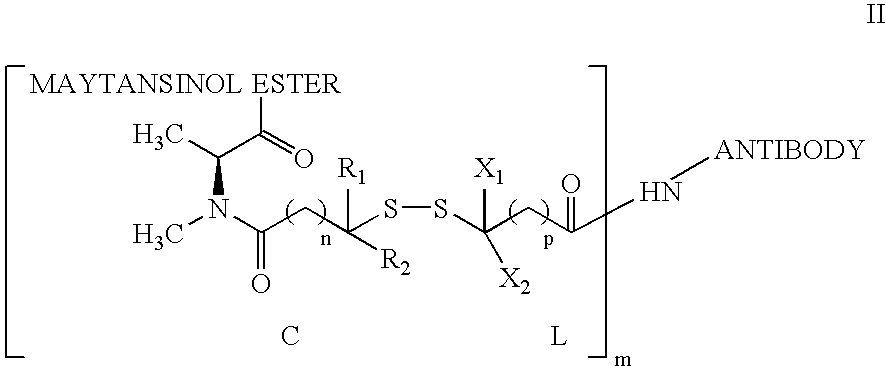
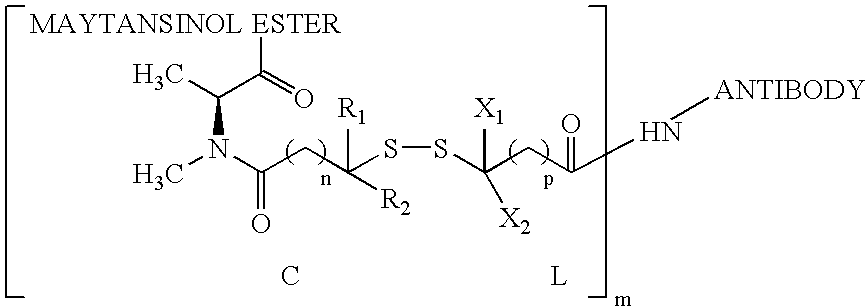
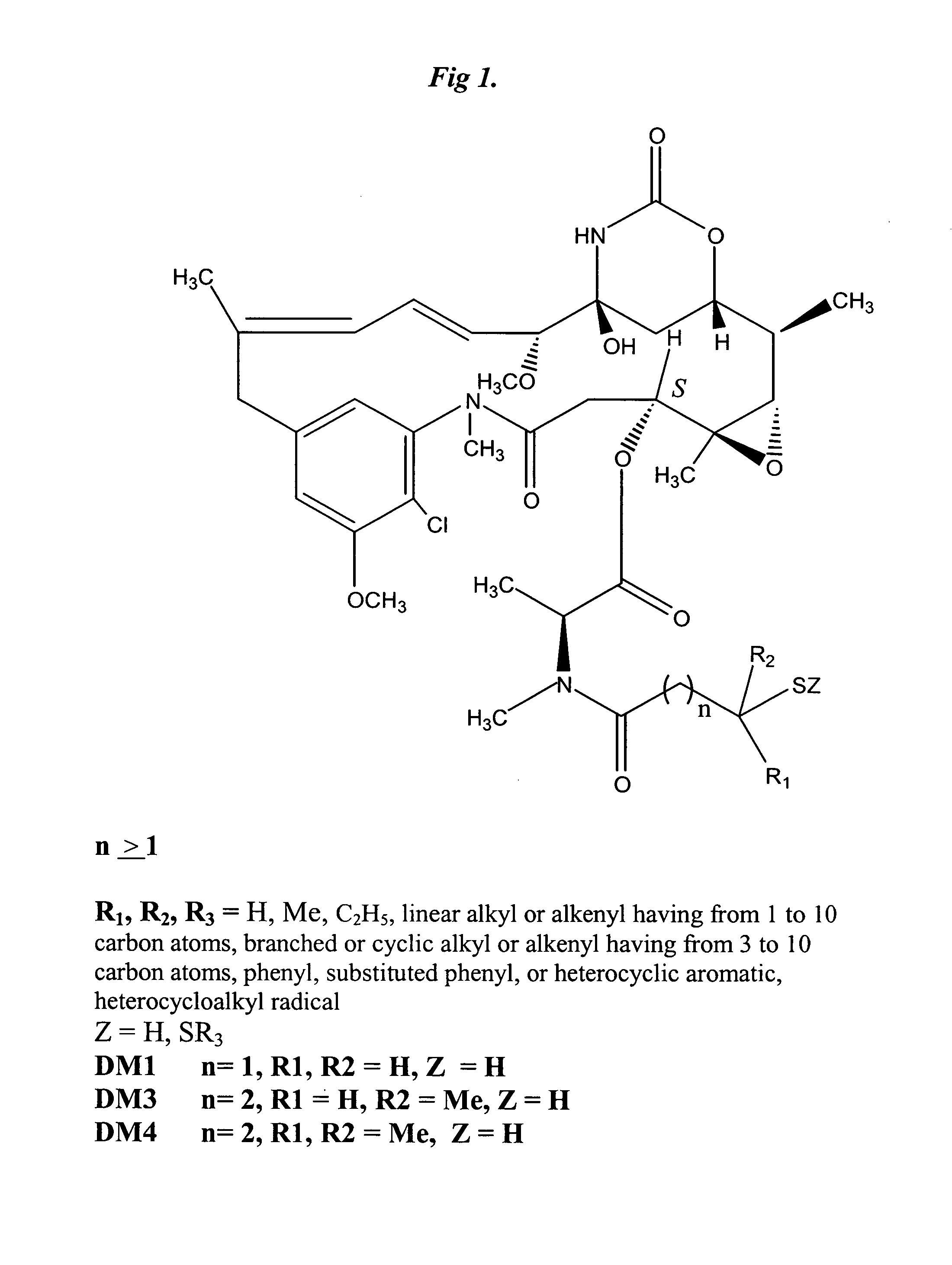
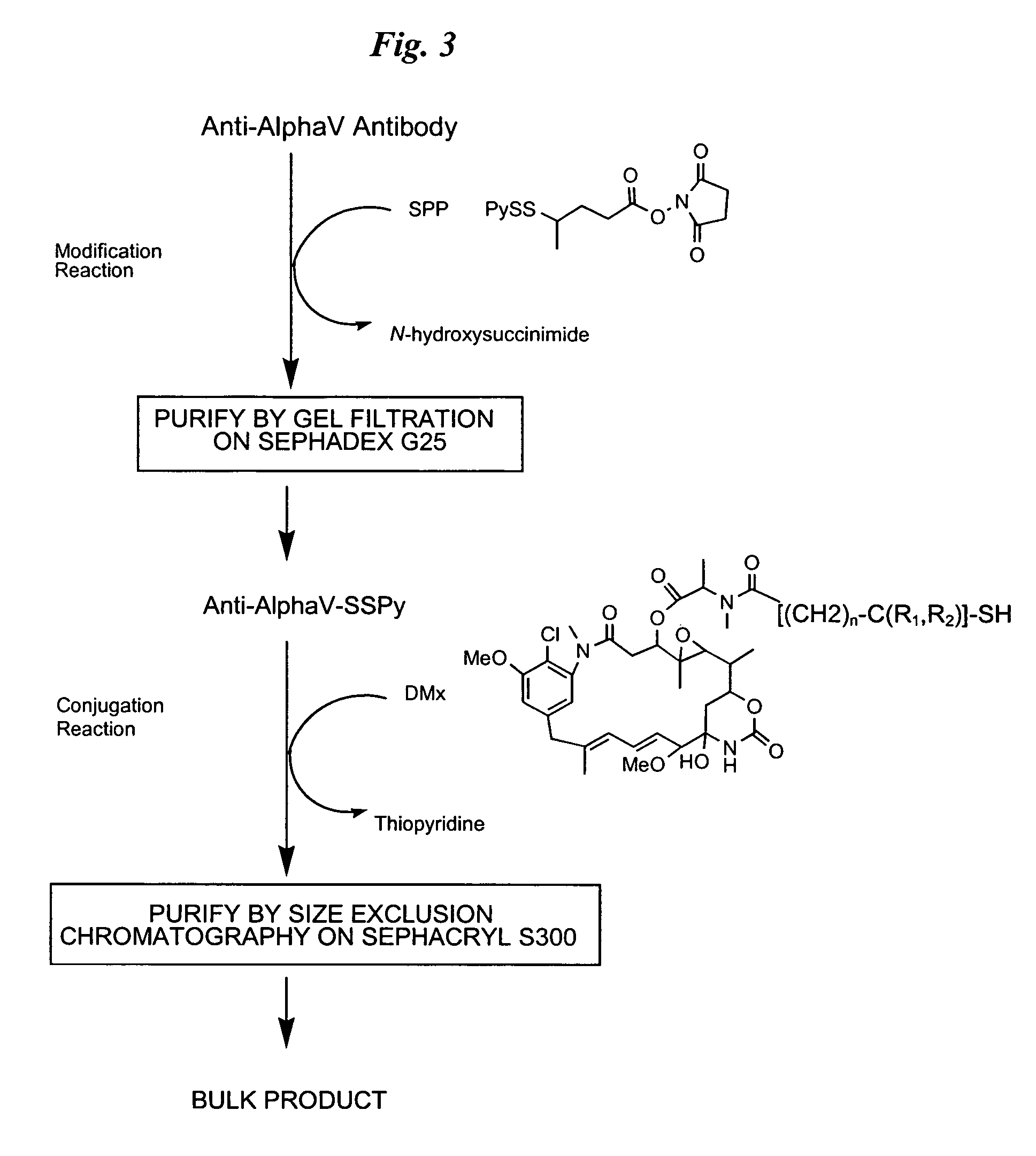
The invention relates to conjugates of anti-integrin specific antibodies with cytotoxic compounds, the synthesis, selection, and use of such conjugates for use in cancer therapy or other diseases mediated by cell proliferation, cell migration, or inflammation and which pathology involves angiogenesis or neovascularization of new tissue. In addition the invention relates to combination therapy of such diseases wherein the treatment comprises use of said conjugates in combination with one or more other treatment modalities including but not limited to: chemotherapy, surgery or radiation therapy. The preferred conjugates contain maytansinoid compounds linked to the antibody by a disulfide linkage, and preferred chemotherapeutic agents are doxorubicin, a taxane, a camptothecin, a podophyllotoxin, a nucleoside analog, or a pyrimidine analog.
Owner:IMMUNOGEN INC +1
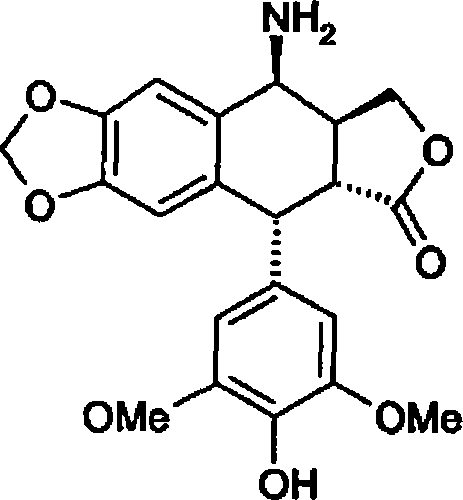
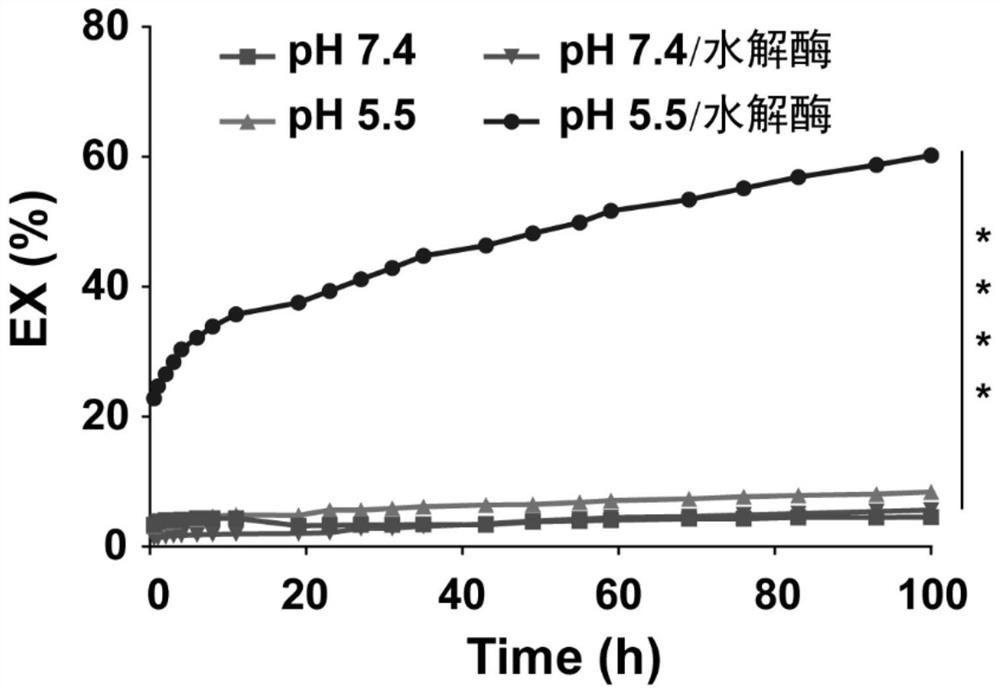
Targeted administration preparation of epipodophyllotoxins medicine
ActiveCN104523597ALow toxicityReduce inhibitionOrganic active ingredientsPowder deliveryNanoparticlePhospholipid formation
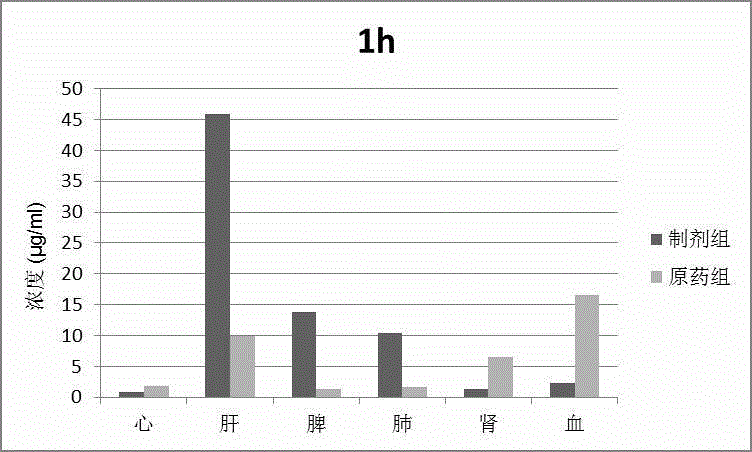
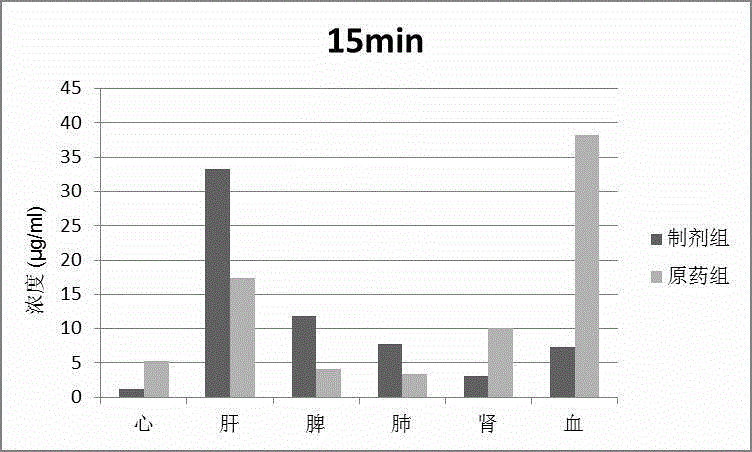
The invention discloses a phospholipid complex albumin nanoparticle containing epipodophyllotoxins medicine. The nanoparticle contains a complex formed by epipodophyllotoxins medicine and phospholipid and albumin. The nanoparticle comprises the following raw materials in parts by weight: 1 part of epipodophyllotoxins medicine, 1-50 parts of phospholipid and 2-100 parts of albumin. The phospholipid complex albumin nanoparticle can be used for remarkably reducing the toxicity of the epipodophyllotoxins medicine, especially bone marrow suppression; meanwhile, the phospholipid complex albumin nanoparticle has liver-lung targeting property.
Owner:SICHUAN UNIV
Sulfur-substituted podophyllum derivative and synthetic method and application thereof
InactiveCN102757442AGood antitumor activityImprove anti-tumor activityOrganic active ingredientsOrganic chemistryApoptosisThiadiazoles
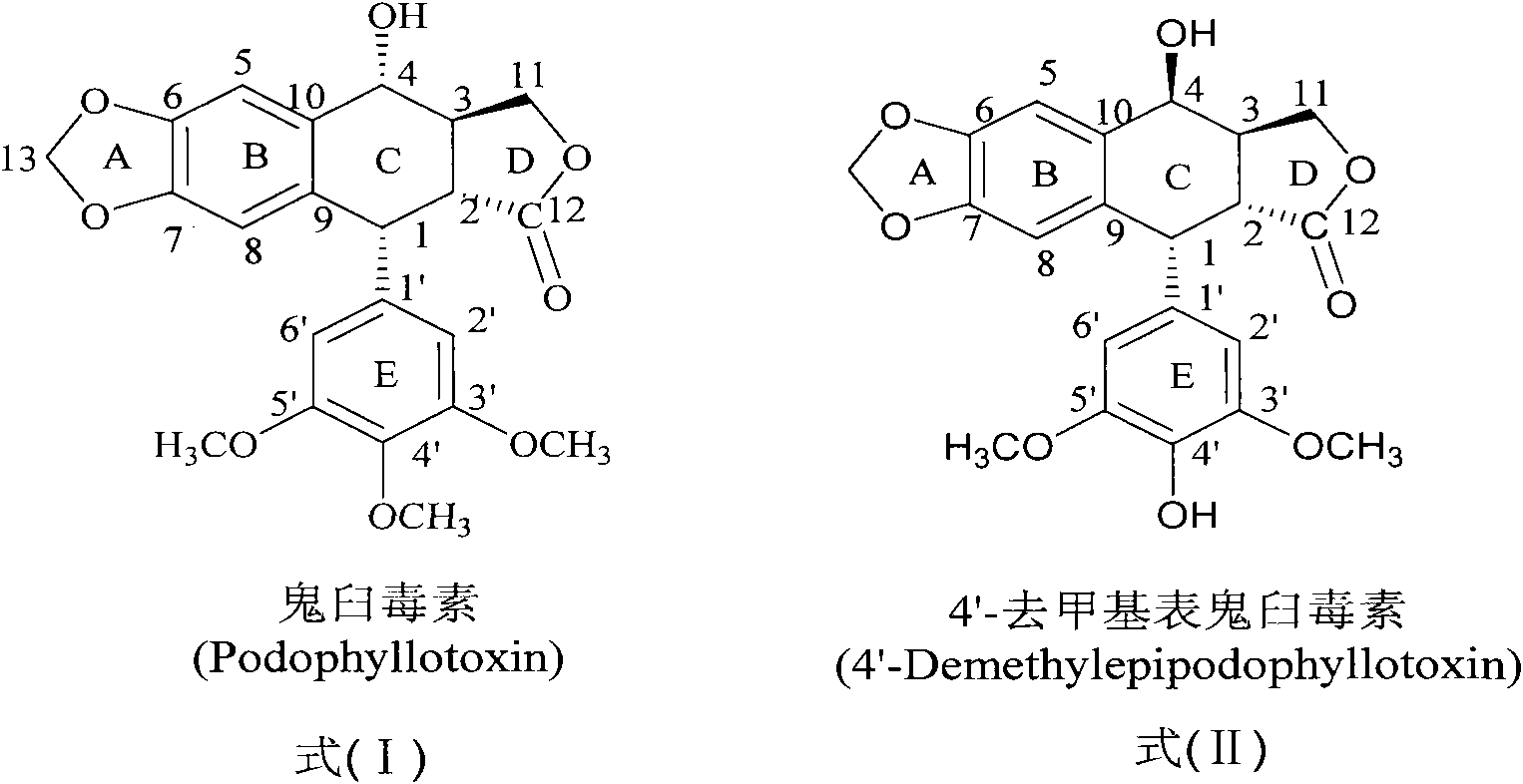
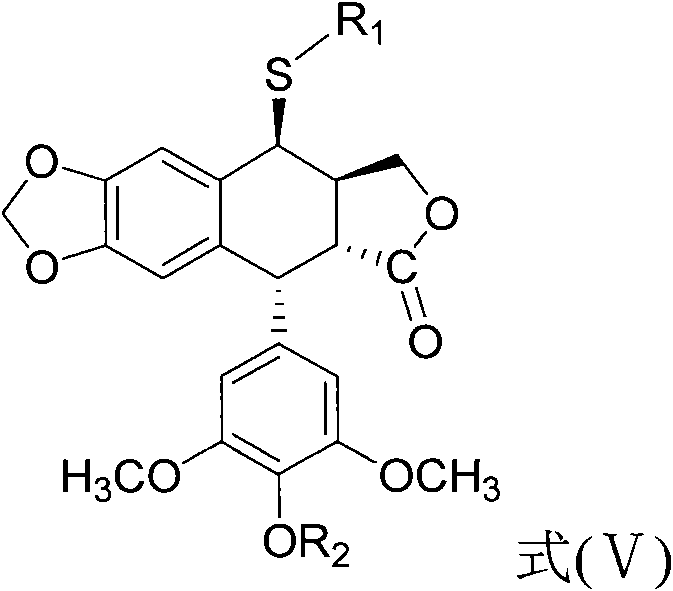
The invention discloses a sulfur-substituted podophyllum derivative, and a synthetic method and application thereof. Through sulfur substitution reaction, 2-mercapto-1,3,4-thiadiazole or 3-mercapto-1,2,4-triazole is respectively introduced into a C-4 site of podophyllotoxin or 4'-demethyl-epipodophyllotoxin so as to obtain the sulfur-substituted podophyllum derivative represented by a formula (V). Cell apoptosis test results of in-vitro BGC823, HepG2 and A549 tumor cell strains show that sulfur-substituted podophyllum derivative has good anti-tumor activity, high safety and low toxicity and can be prepared into clinical anticancer medicines.
Owner:HUBEI UNIV OF TECH
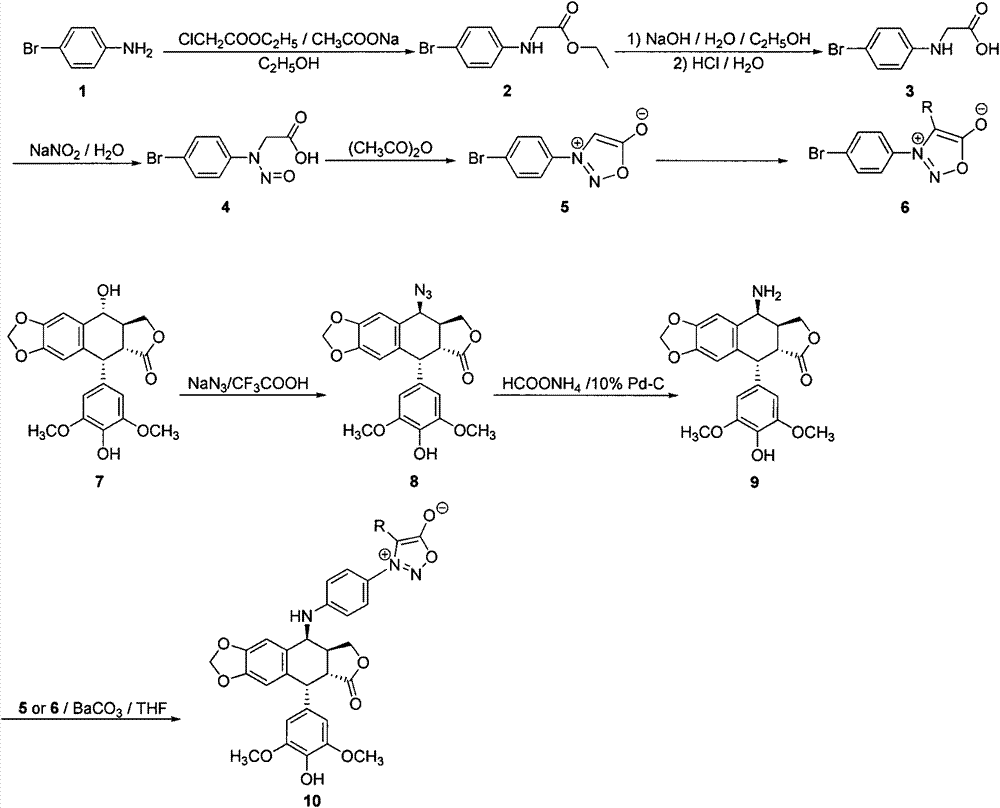
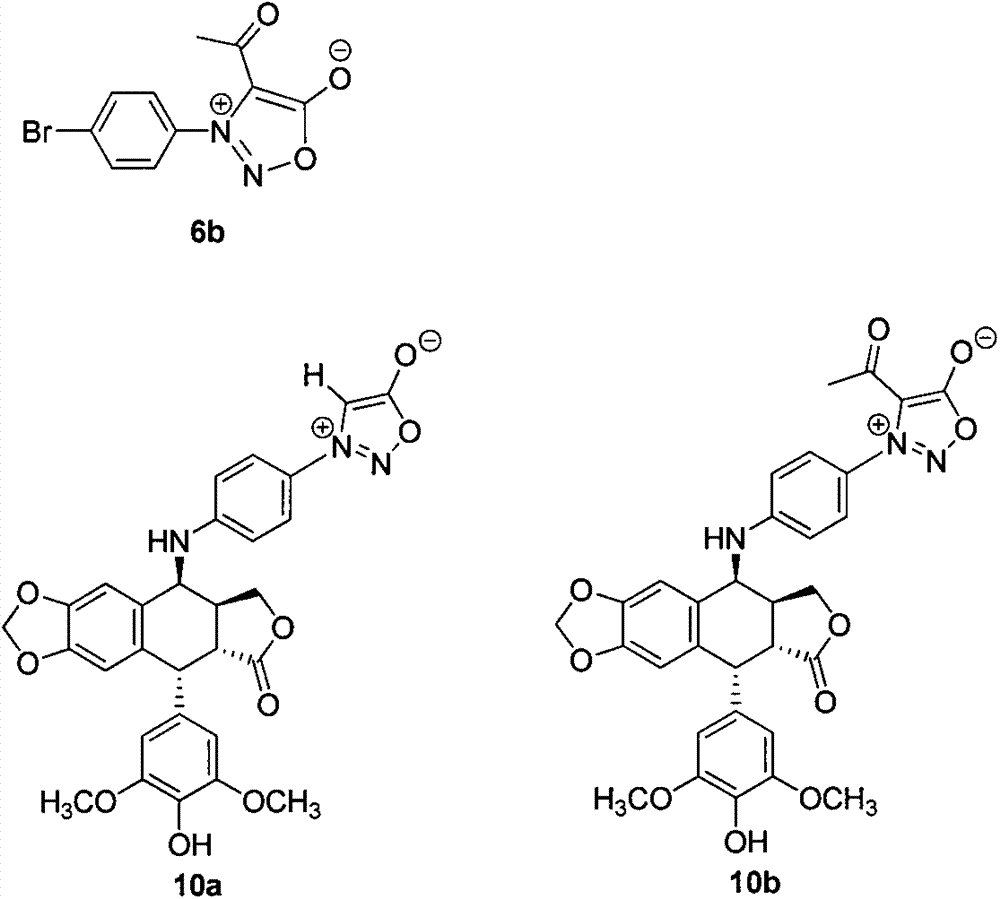
4-amino oxadiazole epipodophyllotoxin derivative and preparation method and application thereof
InactiveCN102432622AStrong cytotoxicityGood water solubilityOrganic active ingredientsOrganic chemistryScreening testIsothiocyanate
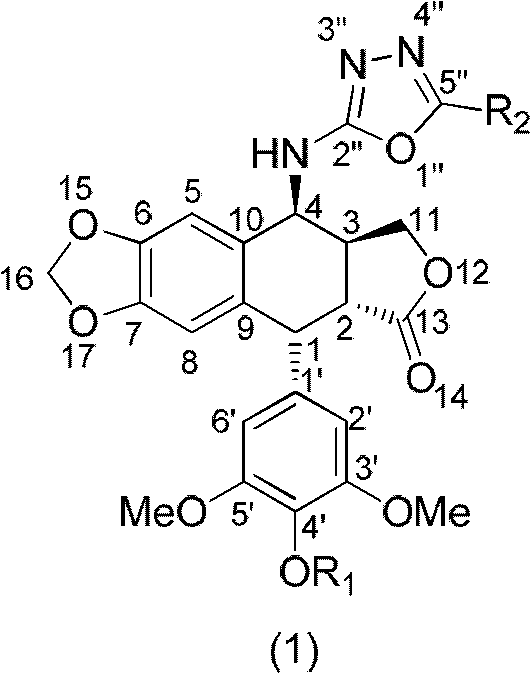
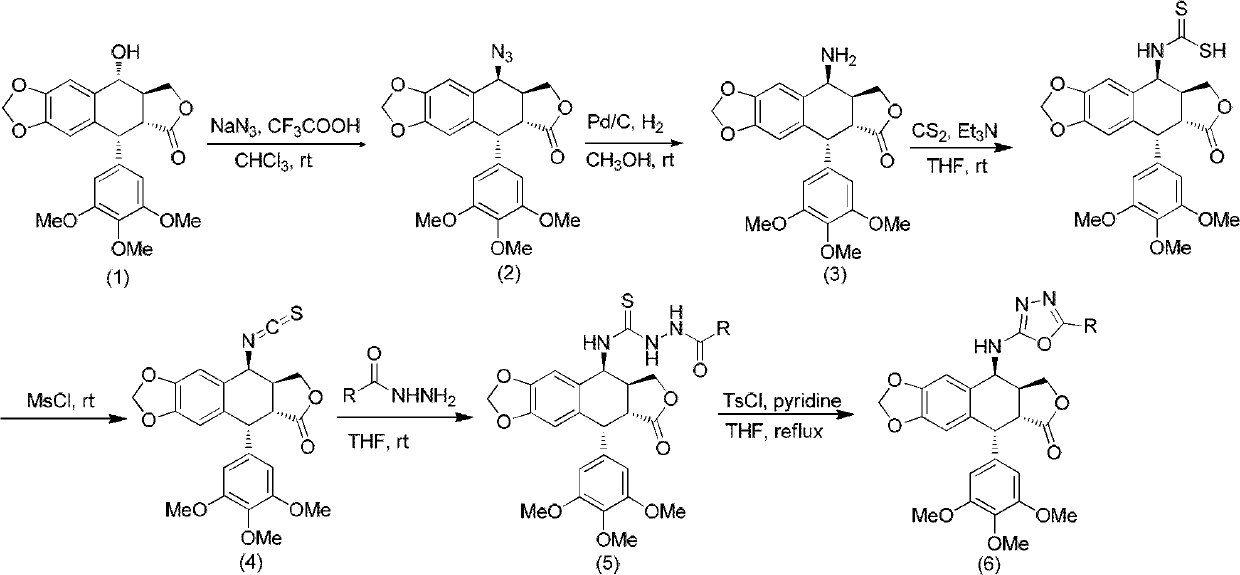
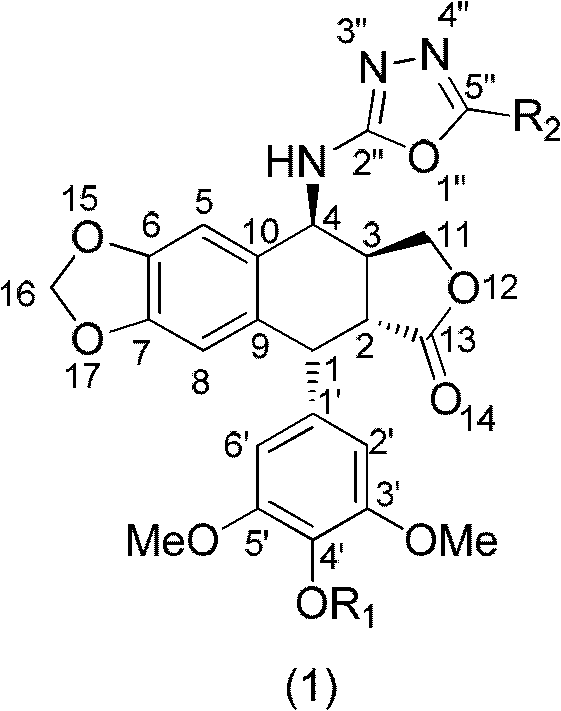
The invention discloses a 4-amino oxadiazole epipodophyllotoxin derivative and a preparation method and application thereof. The 4-amino oxadiazole epipodophyllotoxin derivative has a structure shown as a Formula (1), wherein R1 represents hydrogen or methyl, and R2 represents hydrogen, alkyl, aryl, heteroaryl or heteroaryl alkyl. The derivative is prepared according to the following steps of: firstly, subjecting podophyllotoxin serving as a raw material to azido reaction, reduction, addition and elimination to obtain isothiocyanate epipodophyllotoxin; secondly, reacting an isothiocyanate epipodophyllotoxin intermediate with a hydrazide compound to obtain a thiosemicarbazide derivative; and finally, subjecting to cyclization, and thus obtaining a corresponding 4-amino oxadiazole derivative. The preparation method is simple and is easy to operate. Initial in-vitro screening tests show that the toxicity of the derivative on normal cells is obviously reduced, meanwhile, certain compounds have better anti-tumor activity, and high-efficiency and low-toxicity anti-tumor drugs can be expected to be prepared.
Owner:CHANGZHOU UNIV
Method for preparing epipodophyllotoxin diglucoside and special strain used thereby
InactiveCN101603011AProtect ecological diversityEasy to operateFungiMicroorganism based processesBiotechnologyOperability
The invention discloses a method for preparing epipodophyllotoxin diglucoside and a special strain used thereby. The strain is aspergillus parasiticus TEQB with a preservation number of CGMCC No.3116. The strain of the invention can be used for preparing the epipodophyllotoxin diglucoside. The method for preparing the epipodophyllotoxin diglucoside in the invention has the advantages of simple operation, low cost, easy operation and short production period. The method of the invention provides a new way of preparing the epipodophyllotoxin diglucoside, avoids the limitations of other preparation methods in the prior art, has strong operability, is suitable for industrial large-scale production and has a great significant for the protection of the ecological diversity of plants at the same time.
Owner:BEIJING NORMAL UNIVERSITY
Method for the preparation of anti-tumor Etoposide
The invention discloses a synthetic method for producing the antineoplastic drug Etoposide. The method involves the direct condensation of 4′-desmethyl-epipodophyllotoxin with 2,3-di-O-dichloroacetyl-(4,6 -O-ethylene)-β-D-glucopyranose to produce 4′-desmethyl-epipodophyllotoxin-4-(2,3-di-O-dichloroacetyl-4,6- O-ethylidene)-β-D-glucopyranoside, which is then transformed into Etoposide. Other methods include using different Lewis acids as catalysts, and different substituted glucopyranosides. This method can provide yields over existing techniques, reduce reaction times, and allow for more favorable separation of reaction steps.
Owner:PHYTOGEN LIFE SCI INC
Aspergillus fumigatus and application thereof
InactiveCN101575576AEasy to trainProtect ecological diversityFungiMicroorganism based processesBiotechnologyAstragaloside
The invention discloses an aspergillus fumigatus which can produce podophyllotoxin, epipodophyllotoxin biglucoside and astragaloside, and application thereof. The aspergillus fumigatus TEQA CGMCC No. 3115 has the function of producing podophyllotoxin, epipodophyllotoxin biglucoside and flavonoids astragaloside which have the physiological property the same as that of Chinese podophyllum root. The aspergillus fumigatus can be used for preparing podophyllotoxin and astragaloside. The method for preparing podophyllotoxin, epipodophyllotoxin biglucoside and astragaloside has the advantages of simple fermentation conditions, easy bacterium culture and short production period, with potential application for industrialized mass production. Besides, the aspergillus fumigatus provides a new way for resource development of podophyllotoxin and has important meaning to protect ecological diversity of plants.
Owner:BEIJING NORMAL UNIVERSITY
Anticancer 4'-nor-anthricin and preparation method thereof
InactiveCN103304574ASimple preparation processThe preparation process is simple and controllableOrganic chemistryAntineoplastic agentsPtru catalystHydrogen pressure
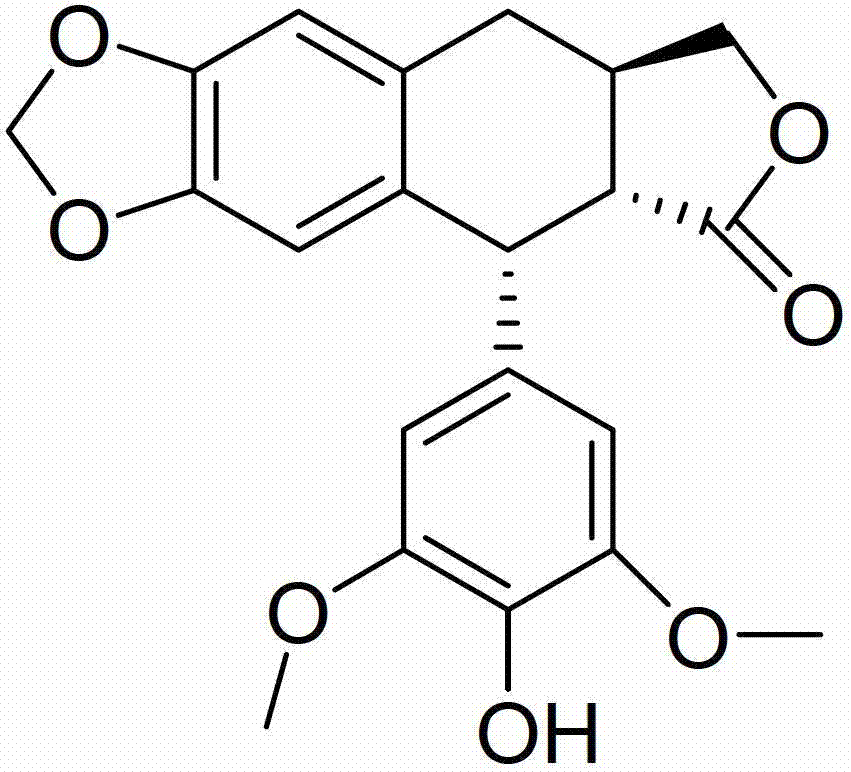
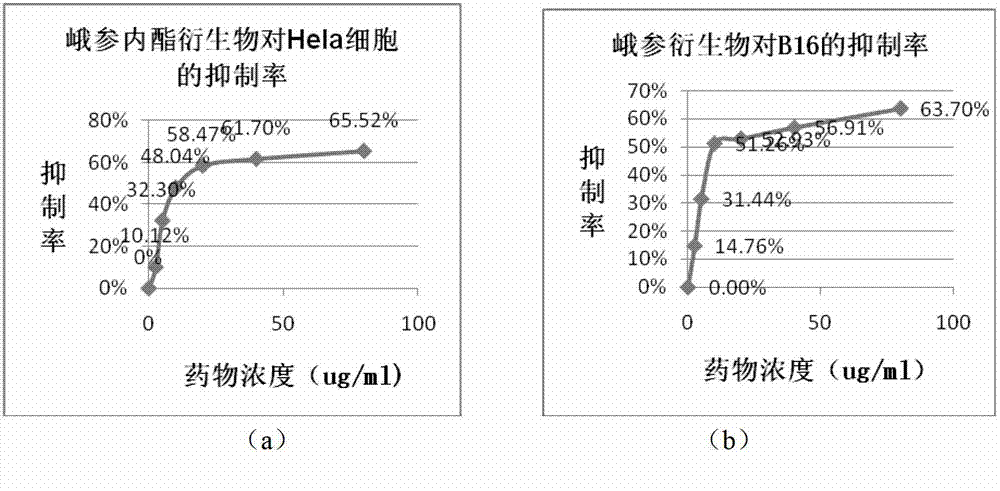
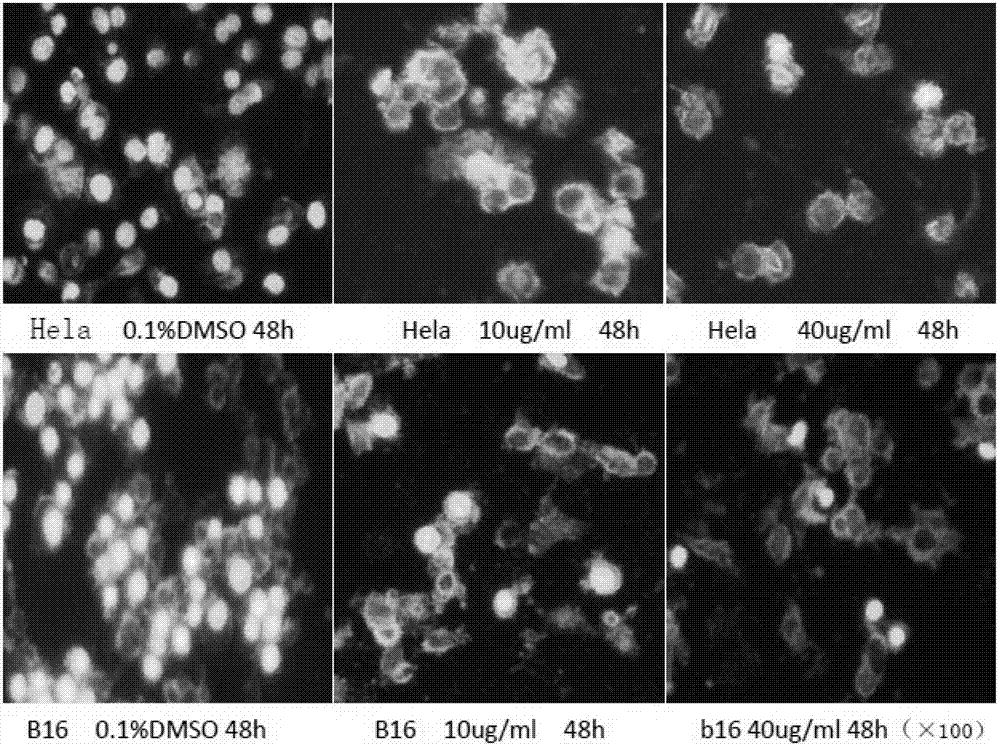
The invention discloses an anticancer 4'-nor-anthricin and a preparation method thereof, and belongs to the field of medicine. The anticancer 4'-nor-anthricin is prepared by the following steps of taking podophyllotoxin as raw material, dissolving the podophyllotoxin in acetic acid in an autoclave, adding a palladium-carbon catalyst, carrying out reaction between the podophyllotoxin and hydrogen at the hydrogen pressure of 1.1-2 and at the temperature of 90-100 DEG C to obtain anthricin, and finally carrying out reaction between the anthricin and hydrogen bromide in an acetic acid solution to obtain the 4'-nor-anthricin. Researches find that the anticancer 4'-nor-anthricin has obvious suppressing and apoptosis inducing functions for the cervical cancer cell Hela and the mice melanoma cell B16; the preparation method of the 4'-nor-anthricin is simple, easy to control, low in cost and high in yield.
Owner:SOUTHWEST JIAOTONG UNIV
Esterified podophyllum derivative with antitumor activity and preparation method and usage of esterified podophyllum derivative
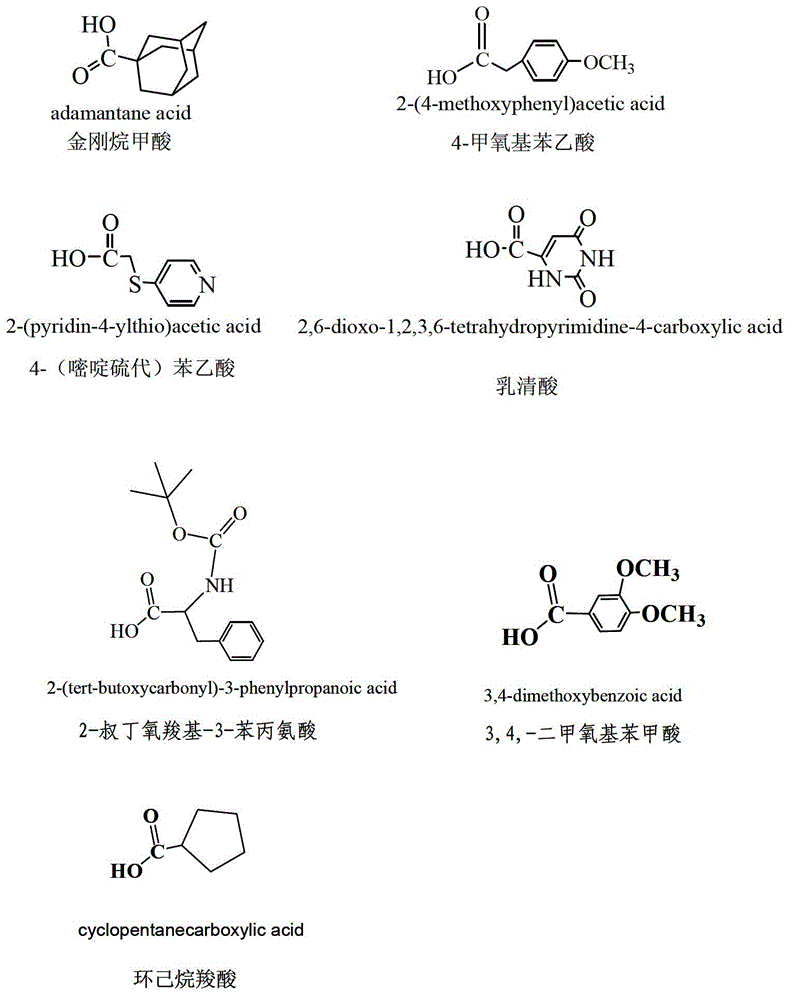
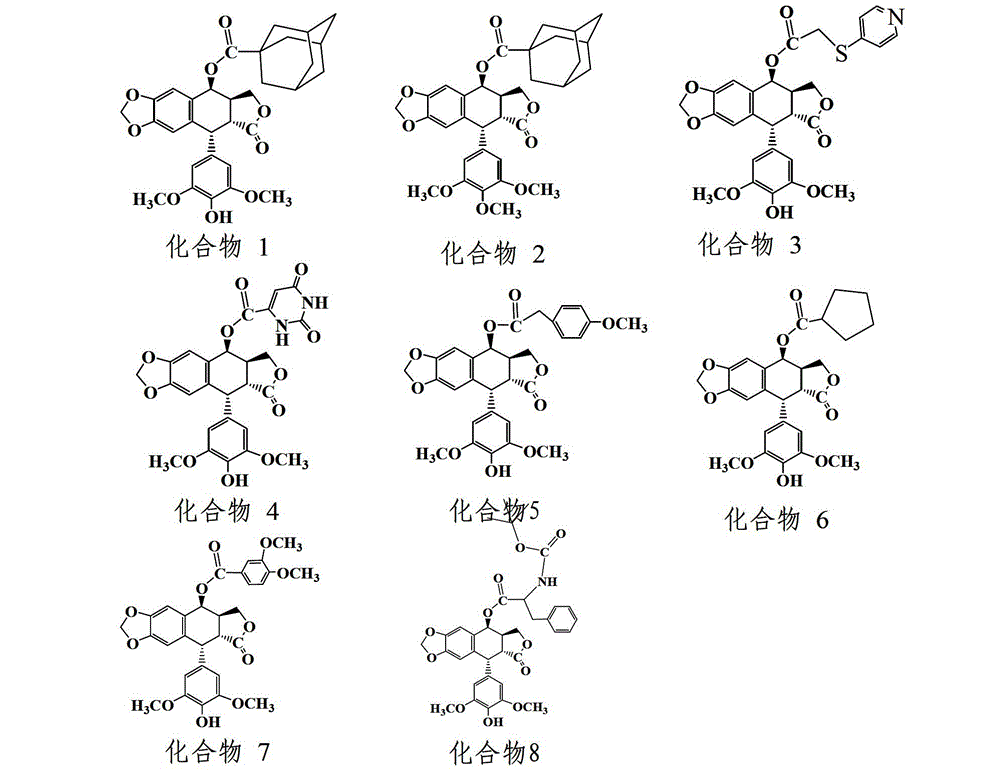
The invention discloses an esterified podophyllum derivative with antitumor activity and a preparation method and the usage of the esterified podophyllum derivative. The esterified podophyllum derivative with antitumor activity and shown as a formula (V) is obtained by means of esterification reaction of adamantine formic acid, orotic acid, 4-(pyrimidine sulfo) phenylacetic acid, cyclohexane carboxylic acid, 3,4-dimethoxy benzoic acid, 2-butoxycarbonyl-3-phenylalanine or 4-methoxy phenylacetic acid with podophyllotoxin or 4'-demethyl-epipodophyllotoxin. The esterified podophyllum derivative has multi-way multi-target actions on tumor cells to achieve better antitumor curative effects. According to in-vitro cell viability inhibition tests, the esterified podophyllum derivative has excellent antitumor activity and low toxic and side effects and can be prepared into antitumor drugs to be applied to antitumor treatment.
Owner:HUBEI UNIV OF TECH
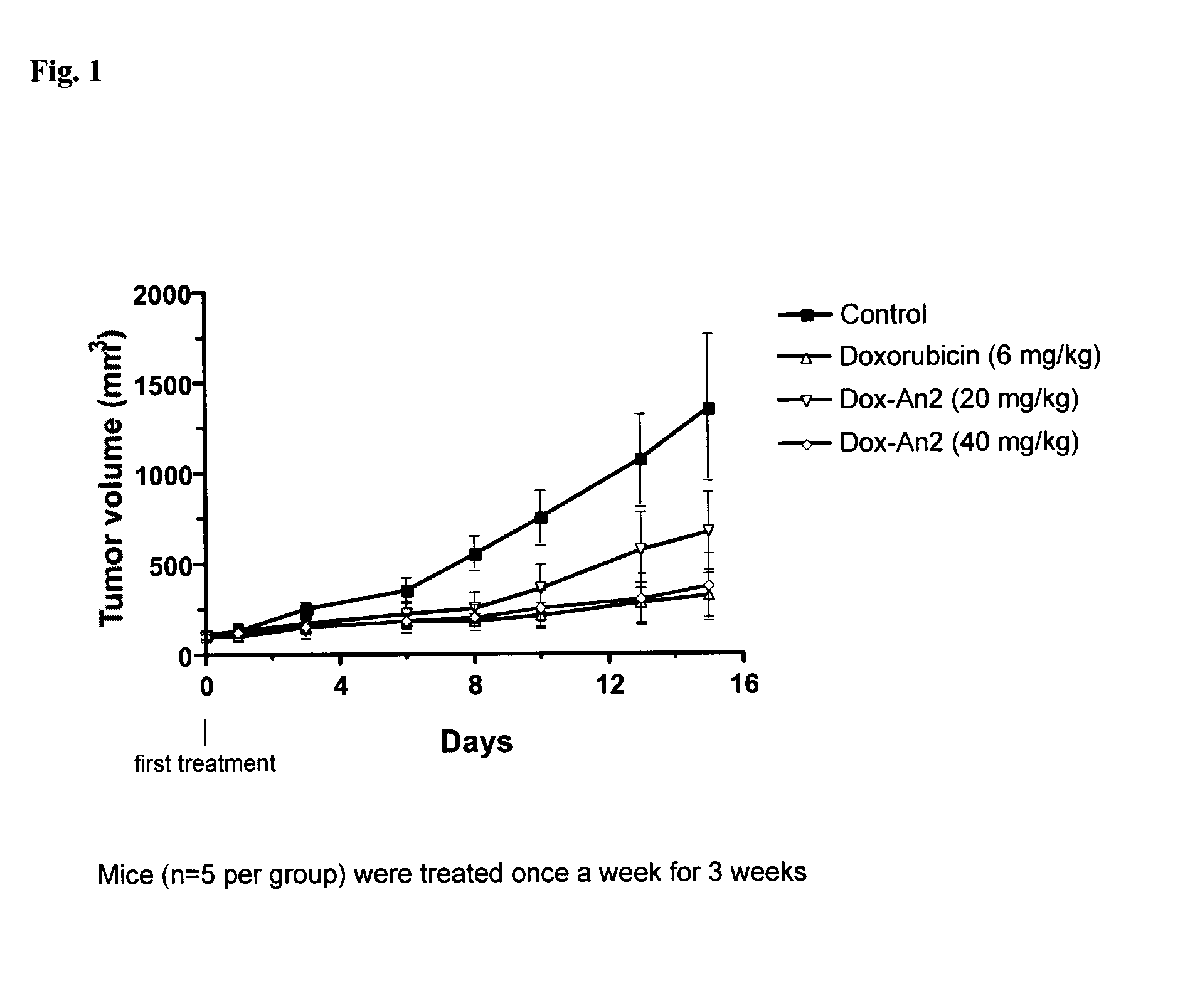
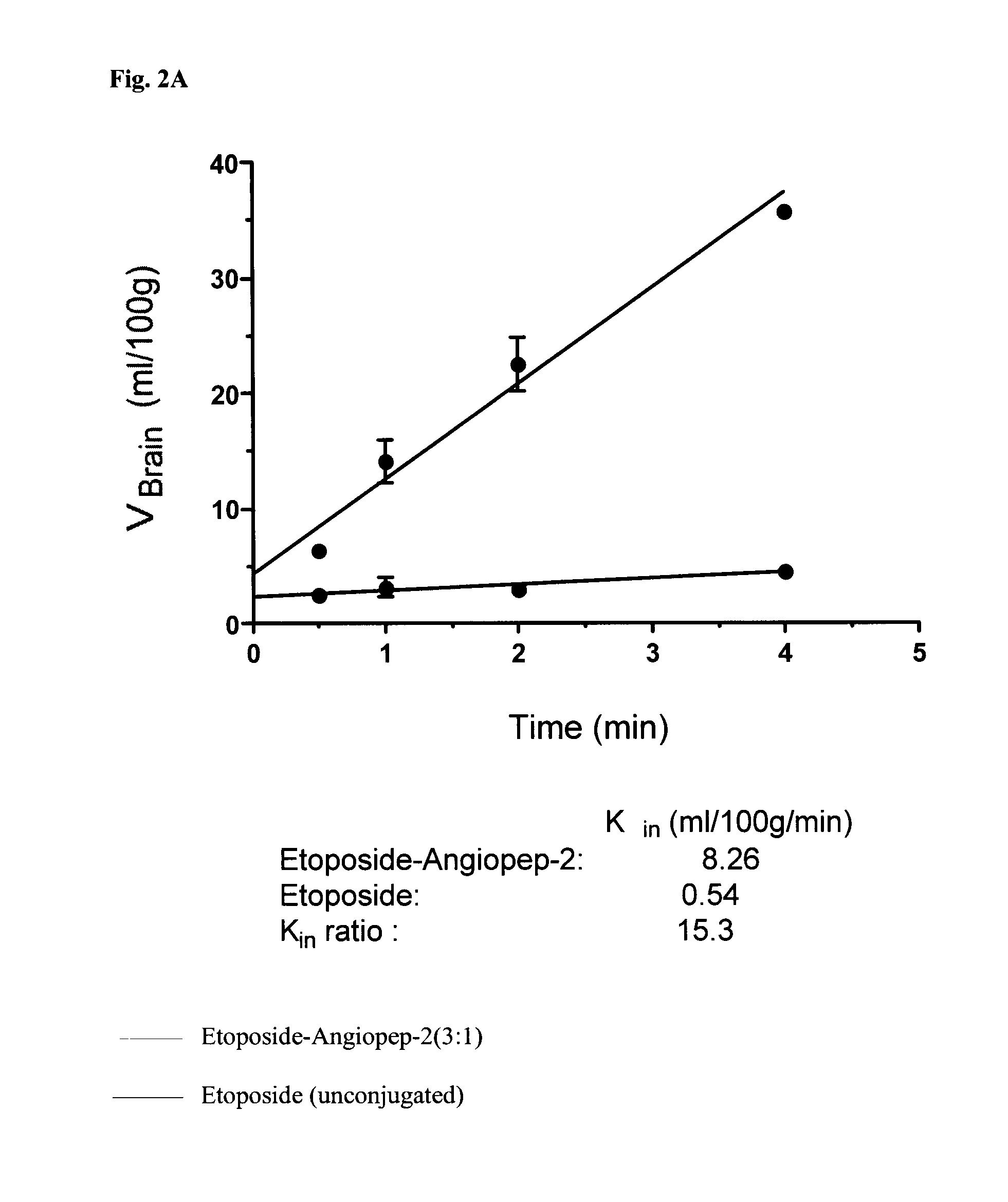
Etoposide and doxorubicin conjugates for drug delivery
ActiveUS8828925B2Improve propertiesReduce frequencySugar derivativesPeptide/protein ingredientsCell type specificKidney
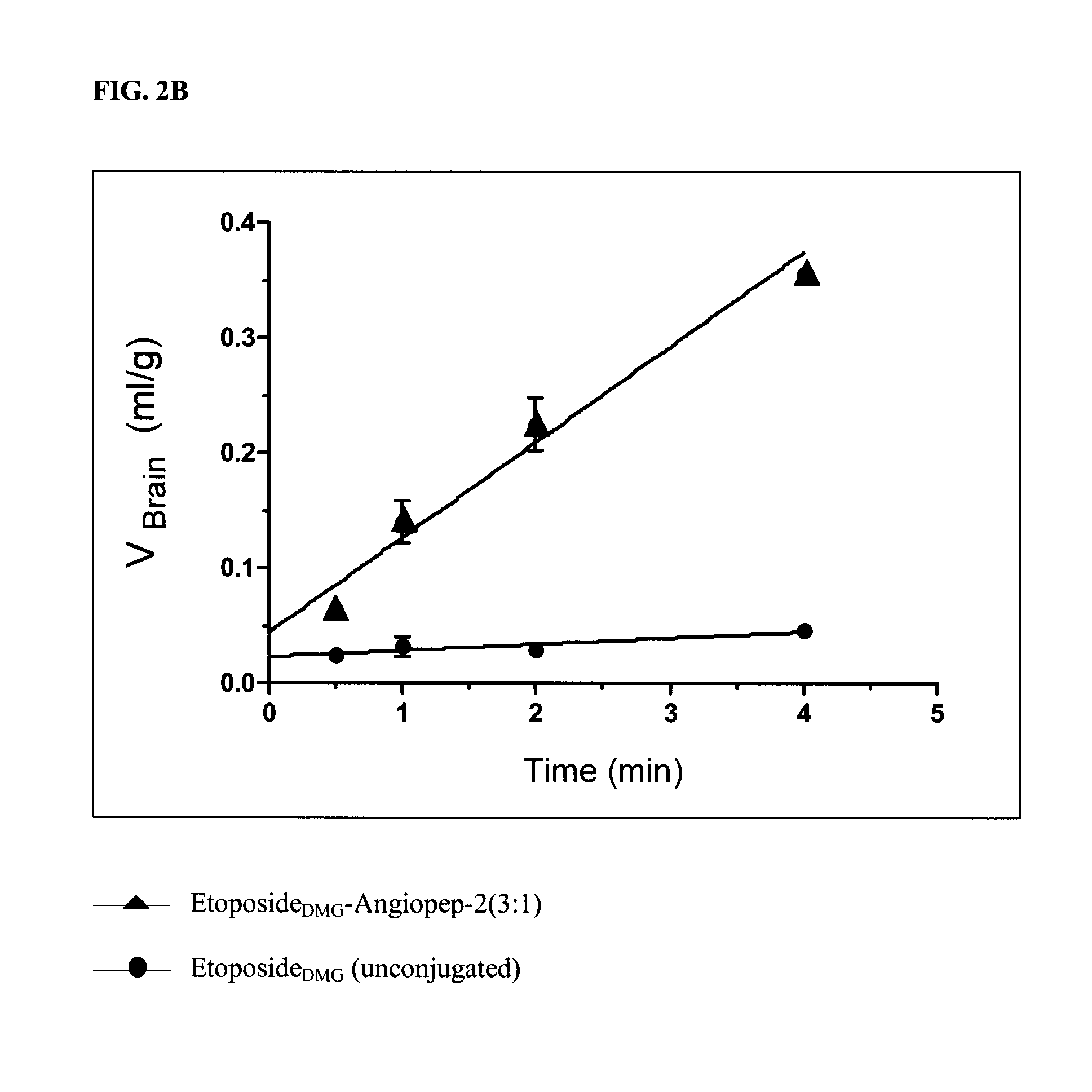
The invention relates to improvements in the field of drug delivery. More particularly, the invention relates to polypeptides having a hydrolyzable covalent bond to a therapeutic agent that includes, etoposide, etoposide 4′-dimethylglycine or doxorubicin. These polypeptide conjugates can be used as vectors to transport the podophyllotoxin derivative across the blood brain barrier (BBB) or into particular cell types such as ovary, liver, lung, or kidney. The invention also relates to pharmaceutical compositions that include the compounds of the invention and to uses thereof in methods of treatment.
Owner:ANGLACHEM INC
Anti-integrin immunoconjugates, methods and uses
InactiveUS8603483B2Effective rate of releaseSenses disorderAntipyreticDiseaseAntiendomysial antibodies
The invention relates to conjugates of anti-integrin specific antibodies with cytotoxic compounds, the synthesis, selection, and use of such conjugates for use in cancer therapy or other diseases mediated by cell proliferation, cell migration, or inflammation and which pathology involves angiogenesis or neovascularization of new tissue. In addition the invention relates to combination therapy of such diseases wherein the treatment comprises use of said conjugates in combination with one or more other treatment modalities including but not limited to: chemotherapy, surgery or radiation therapy. The preferred conjugates contain maytansinoid compounds linked to the antibody by a disulfide linkage, and preferred chemotherapeutic agents are doxorubicin, a taxane, a camptothecin, a podophyllotoxin, a nucleoside analog, or a pyrimidine analog.
Owner:IMMUNOGEN INC +1
Water-soluble 4í»-nor-epipodophyllotoxin derivant and preparation method thereof
InactiveCN101314042AThe efficacy is not lowRealize the esterification reactionOrganic active ingredientsOrganic chemistrySolubilityP-Aminopyridine
The invention provides a derivative of a water soluble 4'-nor epipodophyllotoxin. The derivative is characterized in that the derivative is a polyethylene glycol derivative, the structure formula of which is the 4'- nor epipodophyllotoxin. The preparation method comprises the following the steps that: the 4'- nor epipodophyllotoxin, carboxyl single methoxypolyethylene glycol, dicyclohexyl imine and N dimethylaminopyridine are used as raw materials to prepare the polyethylene glycol derivative of the 4'- nor epipodophyllotoxin by the microwave catalytic action. The 4'- nor epipodophyllotoxin prodrug of the invention is good in solubility and high in stability. The preparation of the invention has the characteristics of high efficiency, rapidness, simpleness and convenience and high reaction yield.
Owner:NINGBO UNIV
4-sydnone substituted phenyl epipodophyllotoxin derivative and preparation method and application thereof
InactiveCN103664977AHas inhibitory effectOrganic chemistryAntineoplastic agentsOrganic acidInorganic acids
The invention discloses a 4-sydnone substituted phenyl epipodophyllotoxin derivative and a preparation method and application of compounds thereof. The invention additionally relates to salts formed by physiologically acceptable and chemically formable inorganic acid or organic acid of the compounds. The compounds provided by the invention can be applied to formulas of anti-tumor drugs. The structural general formula of the compounds provided by the invention is as shown by the accompanying drawing.
Owner:TIANJIN POLYTECHNIC UNIV
Tumor targeting nano-micelle based on hyaluronic acid as well as preparation and application of tumor targeting nano-micelle
PendingCN113171342AHas triple tumor targeting capabilitiesGrowth inhibitionPhotodynamic therapyNanomedicineDisulfide bondingTumor target
The invention provides a tumor targeting nano-micelle based on hyaluronic acid as well as a preparation method and application of the tumor targeting nano-micelle. An E selectin peptide ligand is connected with chemotherapeutic drugs such as taxanes, camptothecin and podophyllotoxin through disulfide bonds, then the conjugate is connected to carboxyl of hyaluronic acid, and a formed amphiphilic polymer can be self-assembled in an aqueous solution to form primary nano-targeting micelles. Other functional molecules such as chemotherapeutic drugs and / or photosensitizer indocyanine green can be further loaded to form a multifunctional nano-micelle, and the micelle respectively has an active targeting tumor effect mediated by hyaluronic acid and tumor targeting peptide and a passive targeting effect of the nano-micelle EPR effect, and has the characteristic of photothermal photodynamic and chemotherapy synergetic effect; and E-selectin-mediated adhesion between vascular endothelial cells and tumor cells can be competitively inhibited, so that the potential of inhibiting tumor cell metastasis is achieved. The multifunctional nano-micelle is simple and easy to prepare and wide in application range, and has important application value.
Owner:TIANJIN UNIVERSITY OF SCIENCE AND TECHNOLOGY
4-deoxyisopodophyllotoxin derivatives, preparation and medicinal uses thereof
InactiveCN101104610AOrganic active ingredientsOrganic chemistryParanasal Sinus CarcinomaNasopharyngeal cancer
The invention relates to 4-deoxidized-iso-podophyllotoxin derivatives displayed by formula (1) and the intermediate series of compounds of 4-deoxidized-iso-podophyllotoxin derivatives or the pharmaceutical salt or solvates of the 4-deoxidized-iso-podophyllotoxin derivatives. The invention also relates to the preparation method, the drug combinations, and the pharmaceutical applications of compounds in the formula (1). The compounds possess strong activity in inhibiting tumor cell growth, some of which having stronger cytotoxic activity to human body tumor cell lines in vitro than etoposide, the prior first class anti-tumor drug, thereby the compounds are expected to serve the purpose of such cancer drugs as anti-lung cancer drugs, anti-hepatoma drugs, and anti-cerbical cancer drugs; since the cytotoxicity of the related compounds to KB cells and PC-3 cells is approximate to or equivalent to etoposide, and meanwhile the compounds have strong etoposide to CNE cells, the compounds are expected to used in anti-tumor drugs, such as, anti-oral epithelial carcinoma drugs, nasopharyngeal carcinoma drugs, and prostate cancer drugs.
Owner:ZHEJIANG UNIV
Preparation method for total synthesis of 4'-demethylepipodophyllotoxin
ActiveCN111138443ASaving stepsSave processing powerBulk chemical productionAsymmetric synthesesChemical reactionAcyl group
The invention discloses a preparation method for total synthesis of 4'-demethylepipodophyllotoxin (also named as epipodophyllotoxin). The preparation method comprises the following steps: (1) syringaldehyde (compound 1) used as an initial material and benzyl methoxyacyl used as a protective group are in favor of a chemical reaction of finally removing the protective group to obtain a target compound; (2) a chiral target object is produced by adopting an asymmetric hydrogenation reduction reaction in which a chiral ligand (s-BINAN Ru (II)) participates; and (3) a reaction of hydrogenating and deprotecting groups is also carried out in parallel while chiral catalytic hydrogenation is carried out. The reaction steps and treatment are saved, the route selection is targeted to the target compound, the production cost is low, and the environment is friendly.
Owner:SHANGHAI JINHE BIO TECH
6-ether/thioether-purines as topoisomerase ii catalytic inhibitors and their use in therapy
The present invention relates to certain purines of the following formulae, which act as topoisomerase II catalytic inhibitors: wherein: J is independently: —H or —NRN1RN2; X is independently: —O—, or —S—; Q is independently: a covalent bond, C1-7alkylene, C2-7alkenylene, C2-7alkynylene, C3-7cycloalkylene, C3-7cycloalkenylene, or C3-7cycloalkynylene; T is independently: a group A1 or a group A2; A1 is independently: C6-14carboaryl, C5-14heteroaryl, C3-12carbocyclic, or C3-12heterocyclic; and is independently unsubstituted or substituted; A2 is independently: —H, —CN, —OH, or —O(C═O)—C1-7alkyl; RN is independently —H or a nitrogen ring substituent; R8 is independently —H or a ring substituent; either: each of RN1 and RN2 is independently —H or a nitrogen substituent; or: RN1 and RN2 taken together with the nitrogen atom to which they are attached form a ring having from 3 to 7 ring atoms; and pharmaceutically acceptable salts, solvates, amides, esters, ethers, N-oxides, chemically protected forms, and prodrugs thereof. These compounds are useful in combination with topoisomerase II poisons, such as anthracyclines and epipodophyllotoxins, in the treatment of proliferative conditions (e.g., cancer). These compounds are also useful in the treatment of tissue damage associated with extravasation of a topoisomerase II poison, such as an anthracycline or an epipodophyllotoxin.
Owner:BIOCODEX INC
Podophyllotoxin compounds and their application and preparation process
InactiveCN1699369AEnhanced inhibitory effectOrganic active ingredientsOrganic chemistryCombinatorial chemistryOrganic chemistry
Owner:LANZHOU UNIVERSITY
Process for the separation and preparation of 4'-demethyl podophyllic acid
InactiveCN101492703AHigh yieldMild reaction conditionsOrganic chemistryMicroorganism based processesPodophyllic acidErwinia uredovora
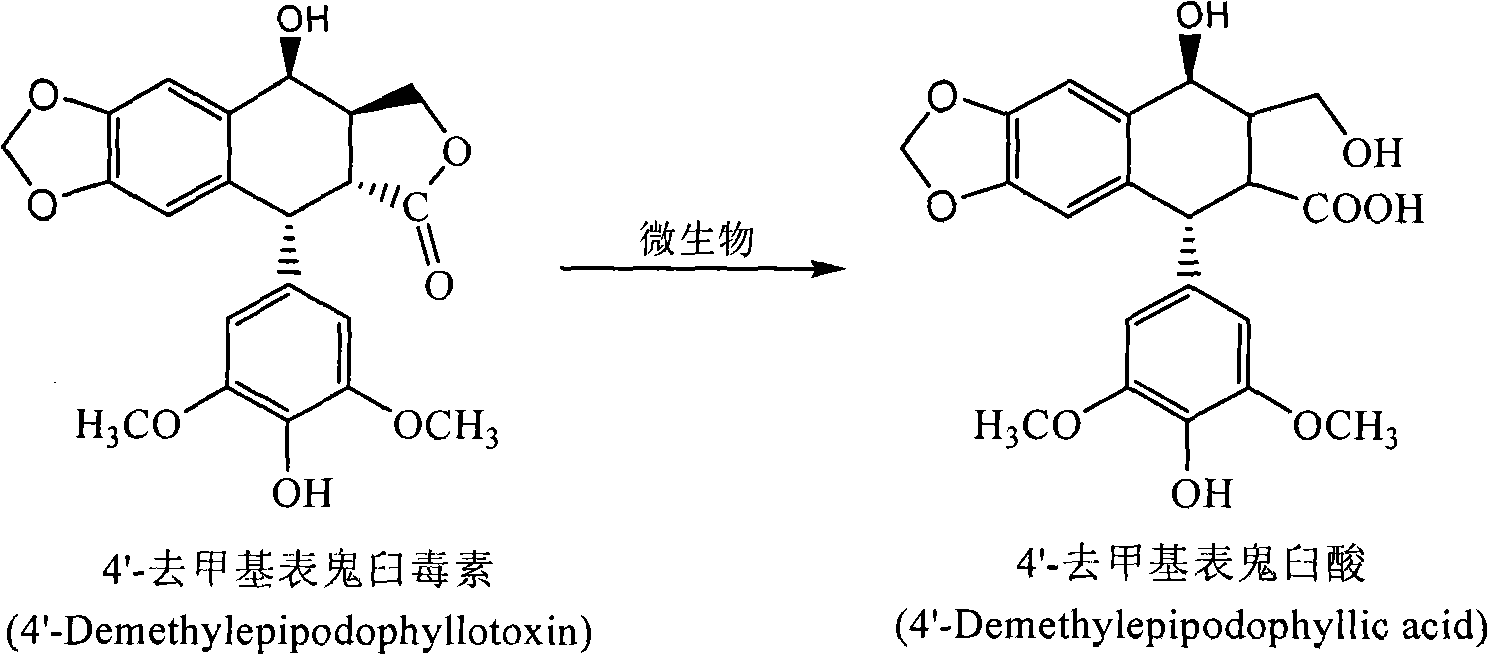
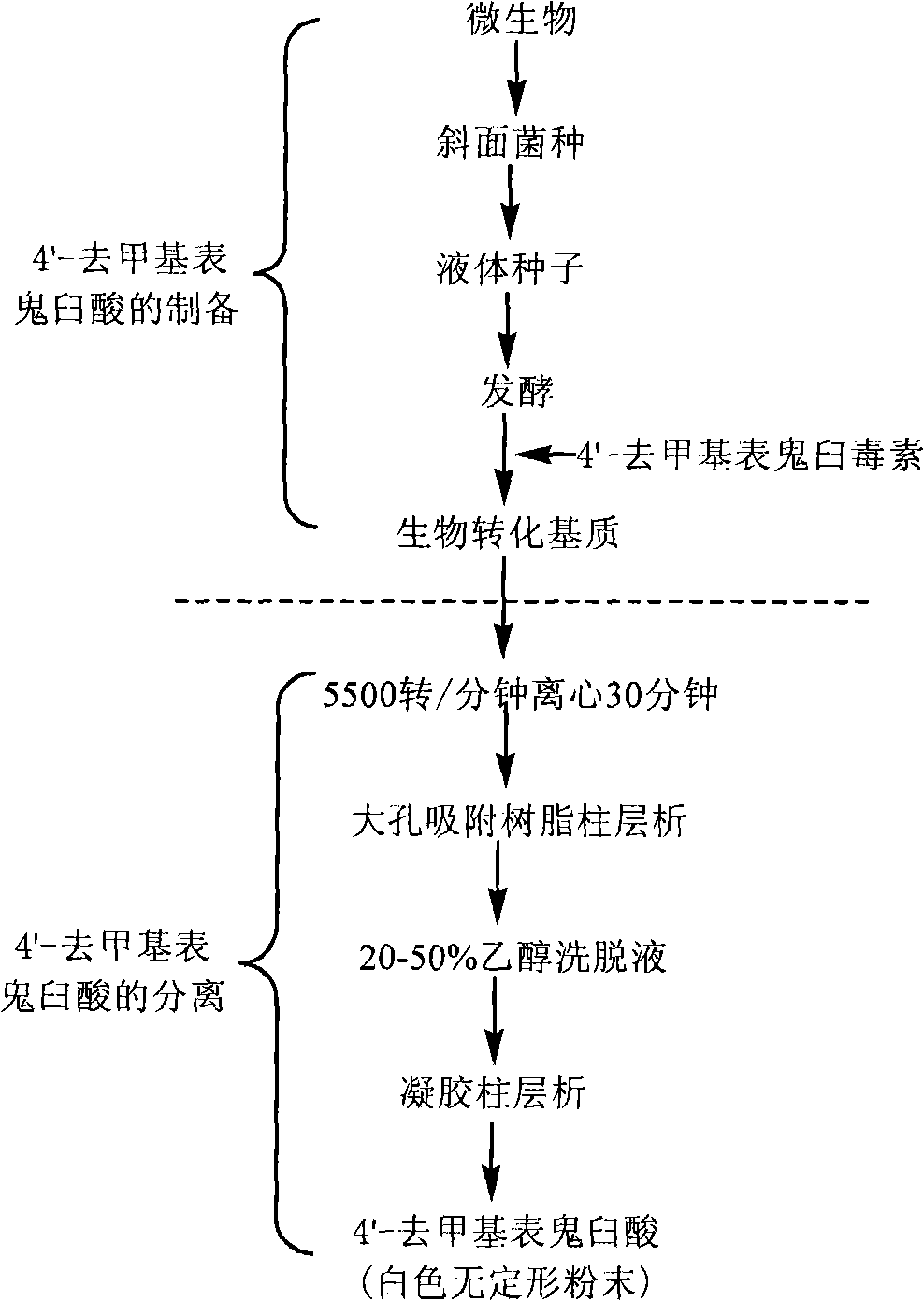
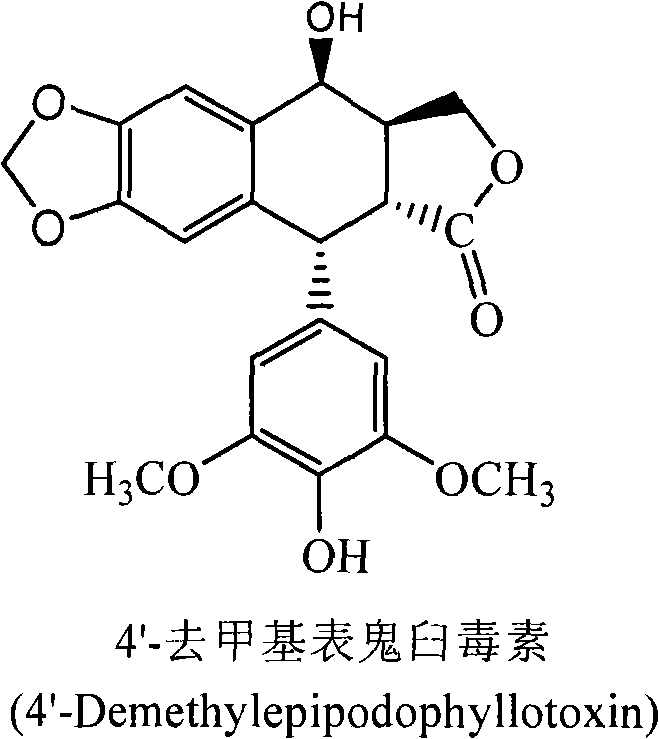
The invention discloses a method for converting 4'- demethylpodophyllotoxin to 4'- demethylation epipodophyllic acid, comprising the following steps: the 4'- demethylpodophyllotoxin solution is added during the fermentation process of microorganism clostridium, pseudomonas aeruginosa, rhodococcus erythropolis, corynebacterium pekinense, hay bacillus, erwinia uredovora, bacillus or bending pseudomonas for carrying out biotransformation reaction, thus obtaining biotransformation matrix with the 4'-demethylation epipodophyllic acid. The invention also discloses a method for separating the 4'-demethylation epipodophyllic acid from the biotransformation matrix, comprising the following steps: the macroporous absorbent resin column is adopted to carry out initial separation on the biotransformation matrix and gel column chromatography is adopted to carry out fine separation, thus obtaining 4'-demethylation epipodophyllic acid. In the invention, modification is carried out on the 4'- demethylpodophyllotoxin structure by biotransformation, thus obtaining 4'-demethylation epipodophyllic acid, in addition, the reaction selectivity is high, the operation is easy, the reaction conditions are moderate, the generated waste is little, the separation process is simple and the yield is high.
Owner:HUBEI UNIV OF TECH
2,3,6-trideoxyglycosyl demethylepipodophyllotoxin compound as well as preparation method and application thereof
ActiveCN109369667AOrganic active ingredientsOrganic chemistry methodsEpipodophyllotoxin CompoundStructural formula
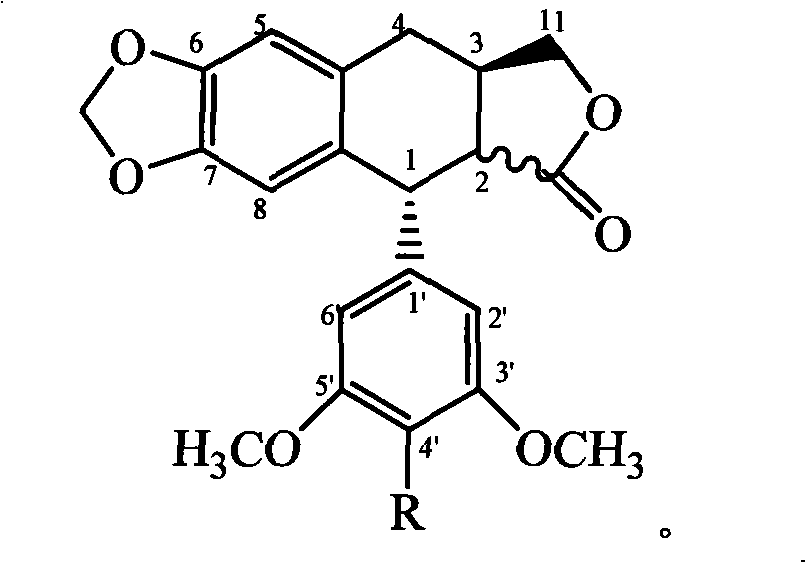
The invention discloses 2,3,6-trideoxyglycosyl demethylepipodophyllotoxin compound or pharmaceutically acceptable ester thereof. The chemical structural formula of the 2,3,6-trideoxyglycosyl demethylepipodophyllotoxin compound is as shown in the figure I in the specification, wherein R represents C1-C6 alkyl, -OH, -NH2, NO2 and halogen groups. The compound has the advantage of being capable of remarkably improving the cytotoxic activity on human lung cancer cells (A549), human hepatoma cells (HepG2), human cervical cancer cells (HeLa), human neuroblastoma cells (SH-SY5Y) and oral cancer vinblastine-resistant cells (KB-VCR) compared with clinical medication of etoposide and has the potential of being developed into anti-tumor drugs.
Owner:南通大学技术转移中心有限公司
Medicine preparation for treating brain diseases
InactiveCN105381469AIncreased brain volumeInnovativeOrganic active ingredientsNervous disorderDiseaseSide effect
The invention relates to a medicine preparation, in particular to a medicine preparation for treating brain diseases. The medicine preparation is prepared from effective components and auxiliary materials. The effective components comprises one or a combination of Eevans blue, adriamycin, camptothecin, paclitaxel, camptothecin with the mass percentage different from the previous camptothecin, podophyllotoxin, echidnotoxin, indomethacin, guaianolide, parthenolide, arglabin, aFGF, BDNF, NGF, EPO, insulin, insulin amyloids, oxytocin or GLP-1. The invention discloses a medicine delivery method for treating brain diseases. The method has the advantages of innovativeness, high efficiency and targeting performance, toxic and side effects are reduced, a blood brain barrier way is not used, the amount of medicine entering the brain is increased, and the brain disease treatment effect is remarkable.
Owner:WENZHOU MEDICAL UNIV
Novel small molecule inhibitor of insulin-like growth factor-1 receptor and application of novel small molecule inhibitor
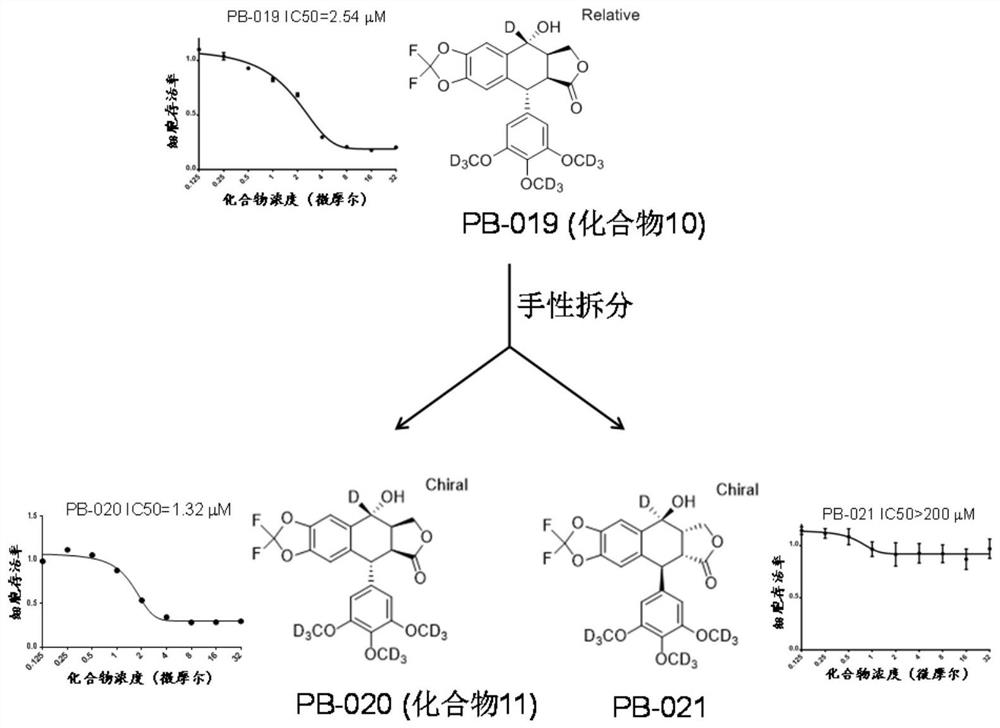
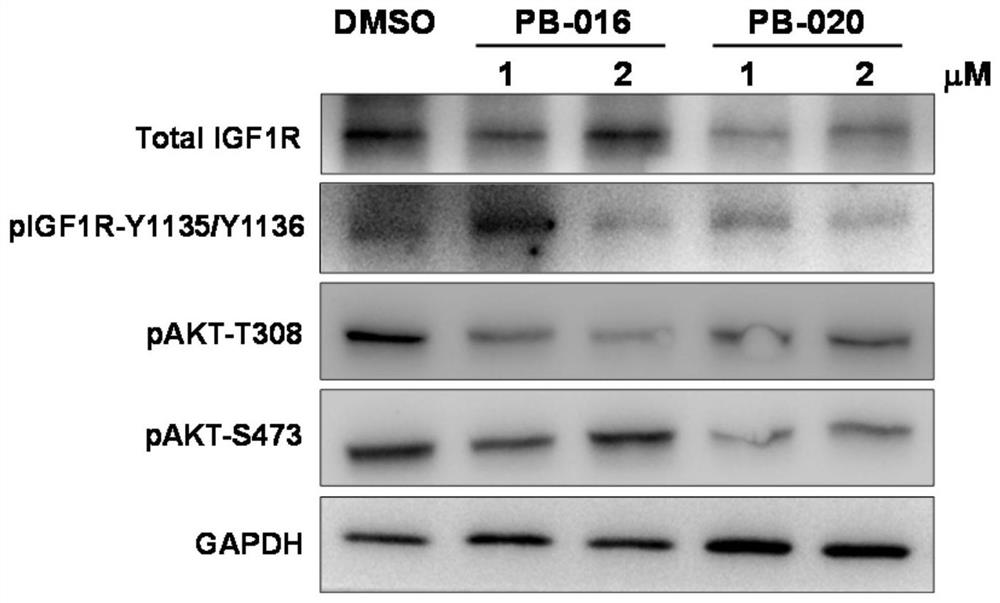
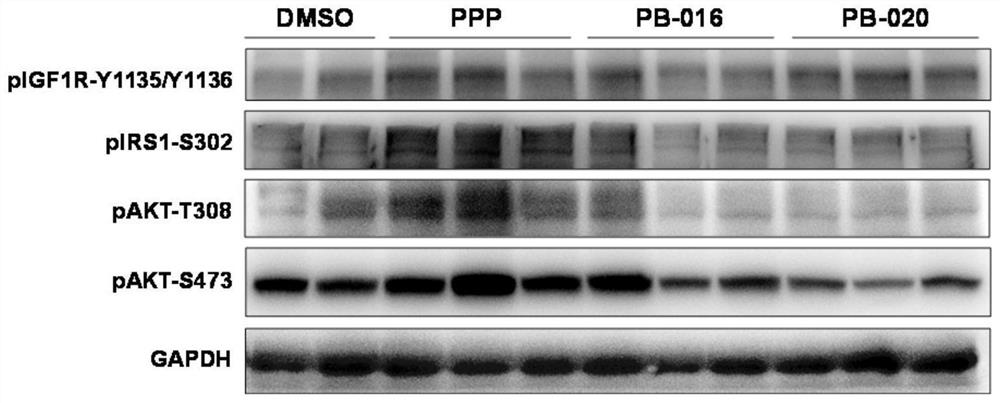
InactiveCN112125916ANovel structureGood blood-brain barrier permeabilityOrganic active ingredientsOrganic chemistryInsulin-like growth factorPancreatic hormone
The invention provides a novel small-molecule inhibitor of an insulin-like growth factor 1 receptor and application of the novel small-molecule inhibitor. The novel small-molecule inhibitor is a derivative of picropodophyllotoxin; fluorine atoms are used for replacing hydrogen atoms at two positions of the left side of picropodophyllotoxin, so that the capacity of molecules penetrating through a blood-brain barrier is improved; meanwhile, deuterium atoms are used for replacing hydrogen atoms, so that the half-life period of the molecules in an organism can be effectively prolonged. The small-molecule inhibitor can be used for preparing drugs for preventing and treating cancers. Compared with picropodophyllotoxin in the clinical test stage at present, the small-molecule inhibitor has higherblood-brain barrier permeability and longer in-vivo half-life while maintaining the action mechanism and biochemical characteristics of picropodophyllotoxin. When used alone, the small-molecule inhibitor can effectively inhibit proliferation and brain metastasis of various tumors, and can be synergistically combined with other anti-cancer drugs, so that proliferation of tumor cells is efficientlyinhibited, and the lifetime is remarkably prolonged.
Owner:ZHEJIANG UNIV
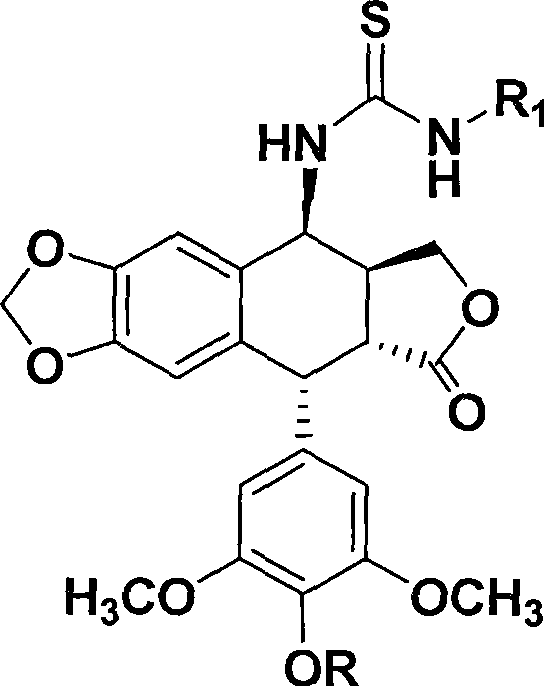
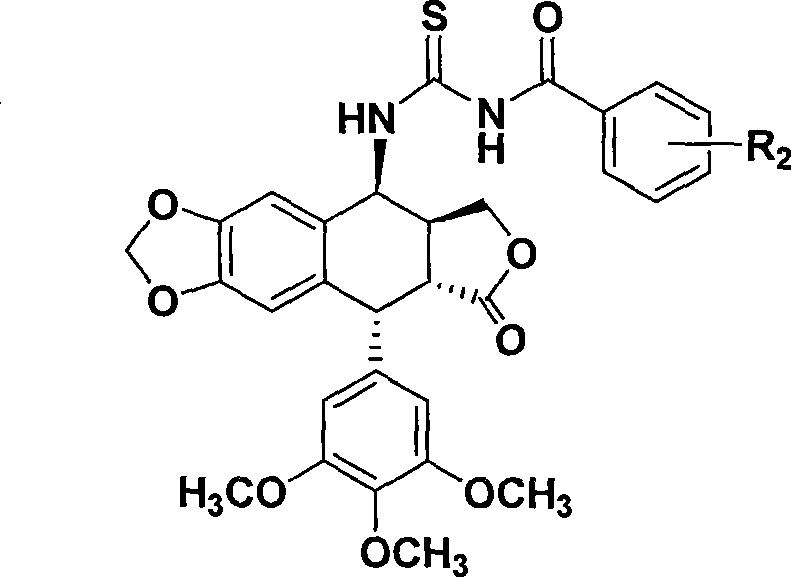
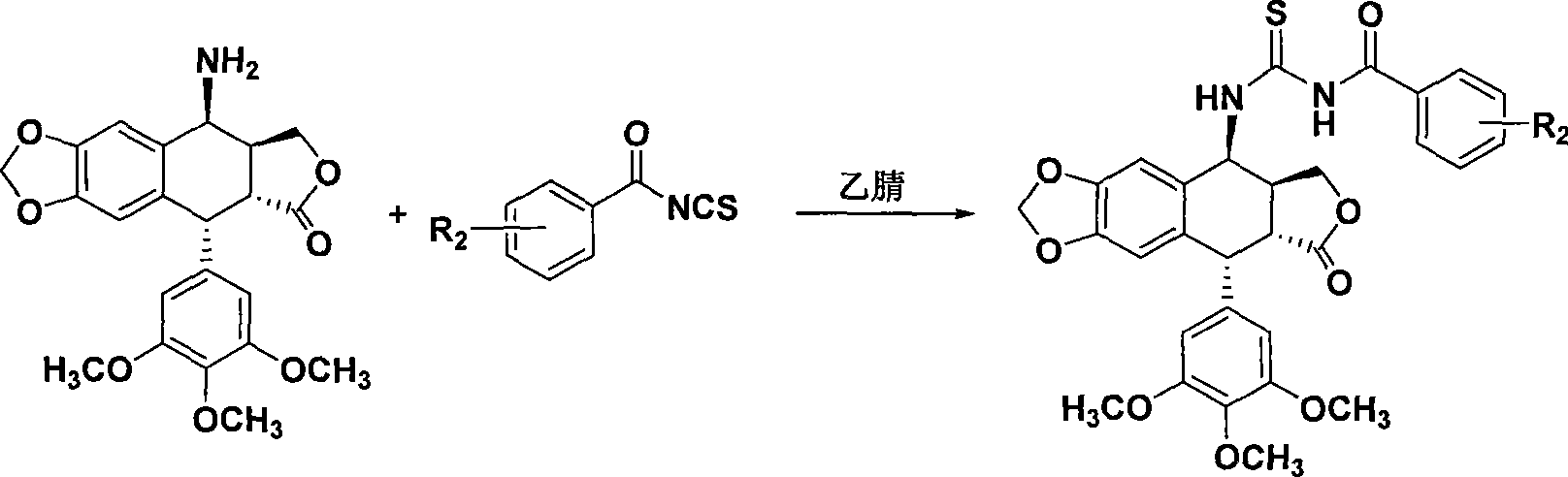
Thiourea homologues epipodophyllotoxin compound and preparation method and application thereof
InactiveCN101544652AGrowth inhibitory activityEasy to prepareOrganic active ingredientsOrganic chemistryThioureaPodophyllotoxin
The invention discloses a thiourea homologues epipodophyllotoxin compound and a preparation method and an application thereof, and the thiourea homologues epipodophyllotoxin compound is obtained in such a way that 4-amino epipodophyllotoxin and corresponding replaced benzoyl isorhodanic ester are put in acetonitrile solvent, toluene solvent or acetone solvent for reaction. The preparation method is simple and is easy to operate and the product has the function of inhibition of tumor cell growth activity.
Owner:NANTONG UNIVERSITY
Application of 4'-demethyl epipodophyllotoxin as agricultural bactericide
The invention relates to application of 4'-demethyl epipodophyllotoxin as an agricultural bactericide. The compound has a relatively good inhibition effect on pathogenic bacteria and fungi; the invention aims at rice bacterial leaf blight, tobacco bacterial wilt, cucumber bacterial leaf blight, konjac bacterial leaf blight, citrus canker pathogen, grape canker pathogen, tomato canker pathogen, kiwi canker pathogen, apple canker pathogen, cucumber botrytis cinerea, pepper fusarium wilt pathogen, sclerotinia sclerotiorum, wheat gibberellic disease, potato late blight pathogen, and blueberry root rot bacteria, and the compound has a good inhibition effect.
Owner:GUIZHOU UNIV
Aspergillus fumigatus and application thereof
The invention discloses an aspergillus fumigatus which can produce podophyllotoxin, epipodophyllotoxin biglucoside and astragaloside, and application thereof. The aspergillus fumigatus TEQA CGMCC No. 3115 has the function of producing podophyllotoxin, epipodophyllotoxin biglucoside and flavonoids astragaloside which have the physiological property the same as that of Chinese podophyllum root. Theaspergillus fumigatus can be used for preparing podophyllotoxin and astragaloside. The method for preparing podophyllotoxin, epipodophyllotoxin biglucoside and astragaloside has the advantages of simple fermentation conditions, easy bacterium culture and short production period, with potential application for industrialized mass production. Besides, the aspergillus fumigatus provides a new way forresource development of podophyllotoxin and has important meaning to protect ecological diversity of plants.
Owner:BEIJING NORMAL UNIVERSITY
Combination drug used for killing genitals microorganisms of female Tibetan mastiffs and assisting reproduction
The invention relates to a combination drug used for killing genitals microorganisms of female Tibetan mastiffs and assisting reproduction. The combination drug contains matrine, podophyllotoxin, berberine, vitamin C, etoposide and antifungal drugs in western medicine.
Owner:王爽
4'-podophyllotoxin demethyl deoxidated aromatic ester, substituted benzene sulfonate, ether derivative and use in plant source pesticide preparation
The invention relates to a series of novel 4'-nordeoxypodophyllotoxin aromatic acid esters, substituted benzene sulfonates, and ether derivatives and application thereof in preparing a botanical insecticide. The 4'-nordeoxypodophyllotoxin aromatic acid esters, the substituted benzene sulfonates, and the ether derivatives have a general chemical formula shown at the right. An experiment proves that the 4'-nordeoxypodophyllotoxin aromatic acid esters, the substituted benzene sulfonates, and the ether derivatives are partially higher than podophyllotoxin and deoxypodophyllotoxin, wherein insecticidal activities of certain compounds are all higher than that of a botanical insecticide-toosendanin which has come into the market, and the compounds can be used for preparing the botanical insecticides with high-efficiency and low-toxicity.
Owner:NORTHWEST A & F UNIV
Method for preparing 4beta;-amino-4'-demethyl-4-desoxypodophyllotoxin
The invention relates to a method for synthesizing 4&bgr;-amino-4′-demethyl-4-desoxypodophyllotoxin of formula (1), characterized by comprising the following successive steps: a) reacting, in a pure weak acid or in a mixture consisting of acid, water and of organic solvent, without another solvent, at a temperature higher than the ambient temperature, thiourea with 4&bgr;-halogenoacetamido-4′-demethyl-4-desoxypodophyllotoxin, and; b) recovering the 4&bgr;-amino-4′-demethyl-4-desoxypodophyllotoxin.
Owner:PIERRE FABRE MEDICAMENT SAS
Podophyllotoxin lipid derivative, nano carrier, preparation method thereof, and application of podophyllotoxin lipid derivative and nano carrier in tumor treatment
PendingCN113801129AImprove solubilityIncrease intakeMaterial nanotechnologyOrganic active ingredientsTumor therapyChemo therapy
The invention provides a podophyllotoxin lipid derivative, a nano-carrier, a preparation method thereof and an application of the podophyllotoxin lipid derivative and the nano-carrier in tumor treatment. The podophyllotoxin lipid derivative is prepared by adopting a podophyllotoxin drug mother nucleus as a hydrophobic nucleus, linking a micromolecular hydrophilic group on the podophyllotoxin drug mother nucleus to form the podophyllotoxin derivative, and introducing a highly hydrophobic long-chain fat-soluble substance to play a stabilizing role. The podophyllotoxin lipid nano-carriers with different structures can be synthesized by changing the types and sizes of the small molecules and the fat-soluble substances and the feeding ratio of the fat-soluble substances to the podophyllotoxin derivatives. The podophyllotoxin lipid derivative provided by the invention overcomes the limitations of low solubility and toxic and side effects of podophyllotoxin, and meanwhile, the podophyllotoxin lipid nano-carrier prepared by the invention has a lipid bilayer structure and an acid / enzyme sensitive connecting bond, and has the characteristic of regulating PD-L1 expression; therefore, the product has the application of tumor microenvironment response and immune escape prevention, and has a wide application prospect in the fields of chemotherapy and immunotherapy in tumor treatment.
Owner:DALIAN NATIONALITIES UNIVERSITY
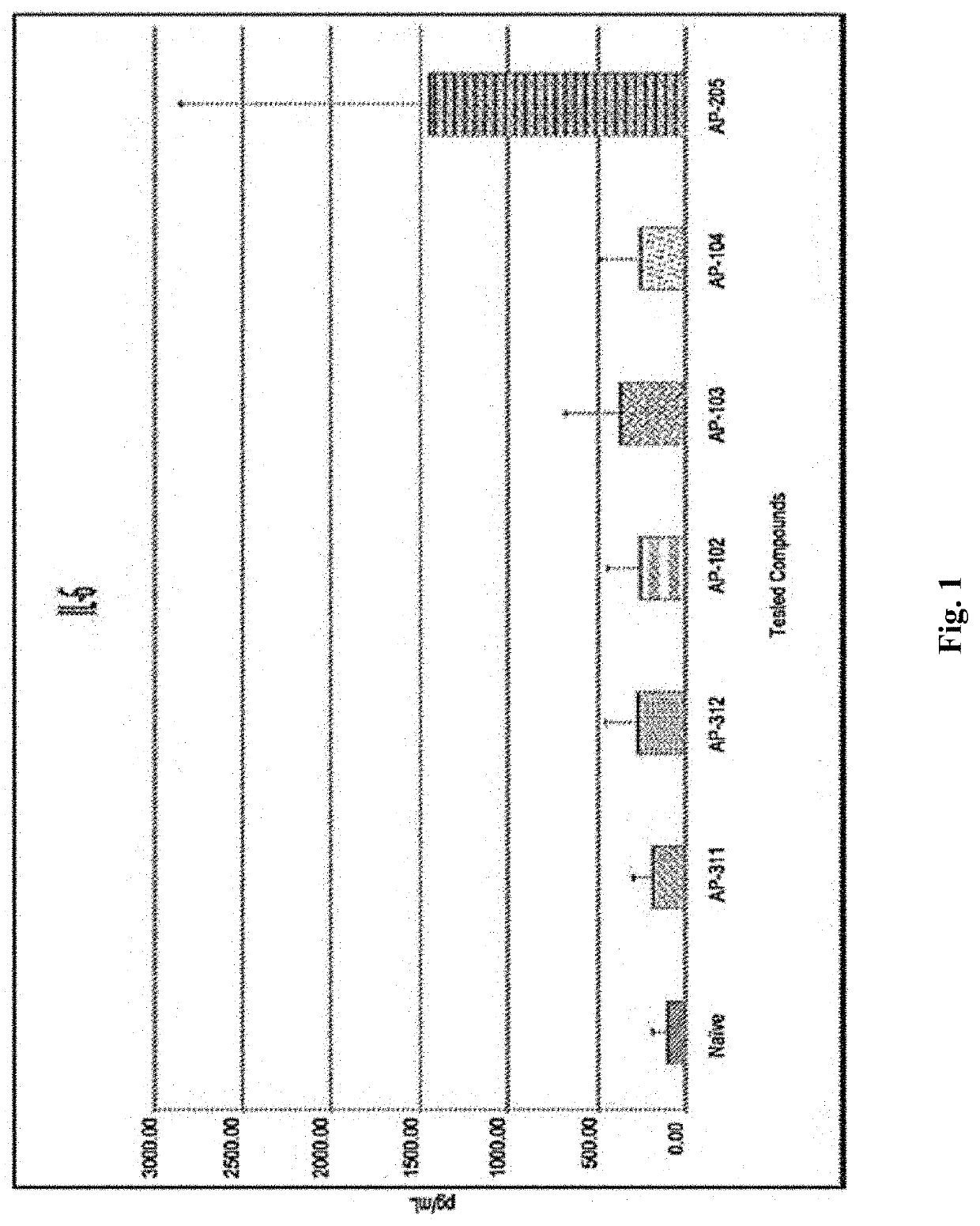
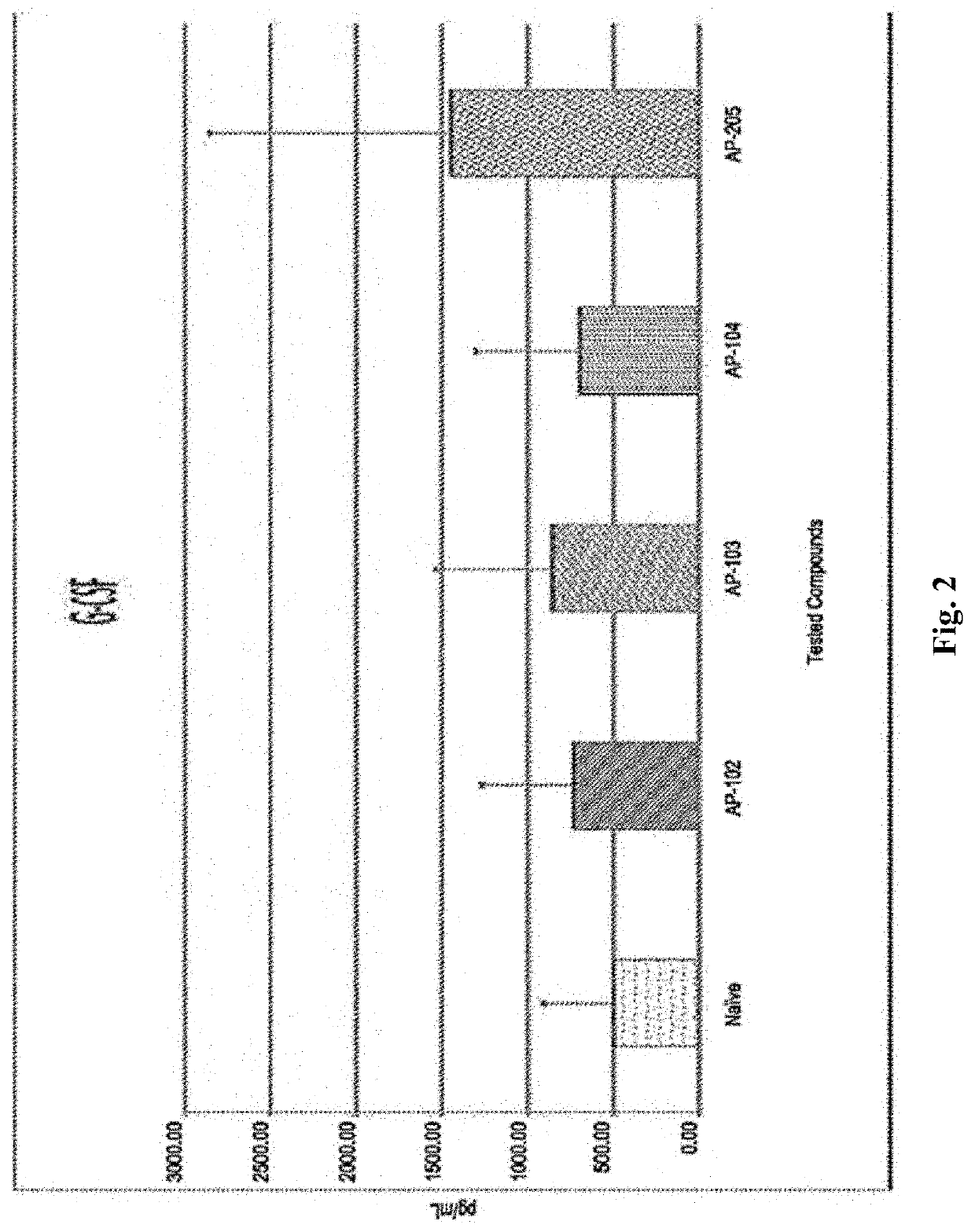
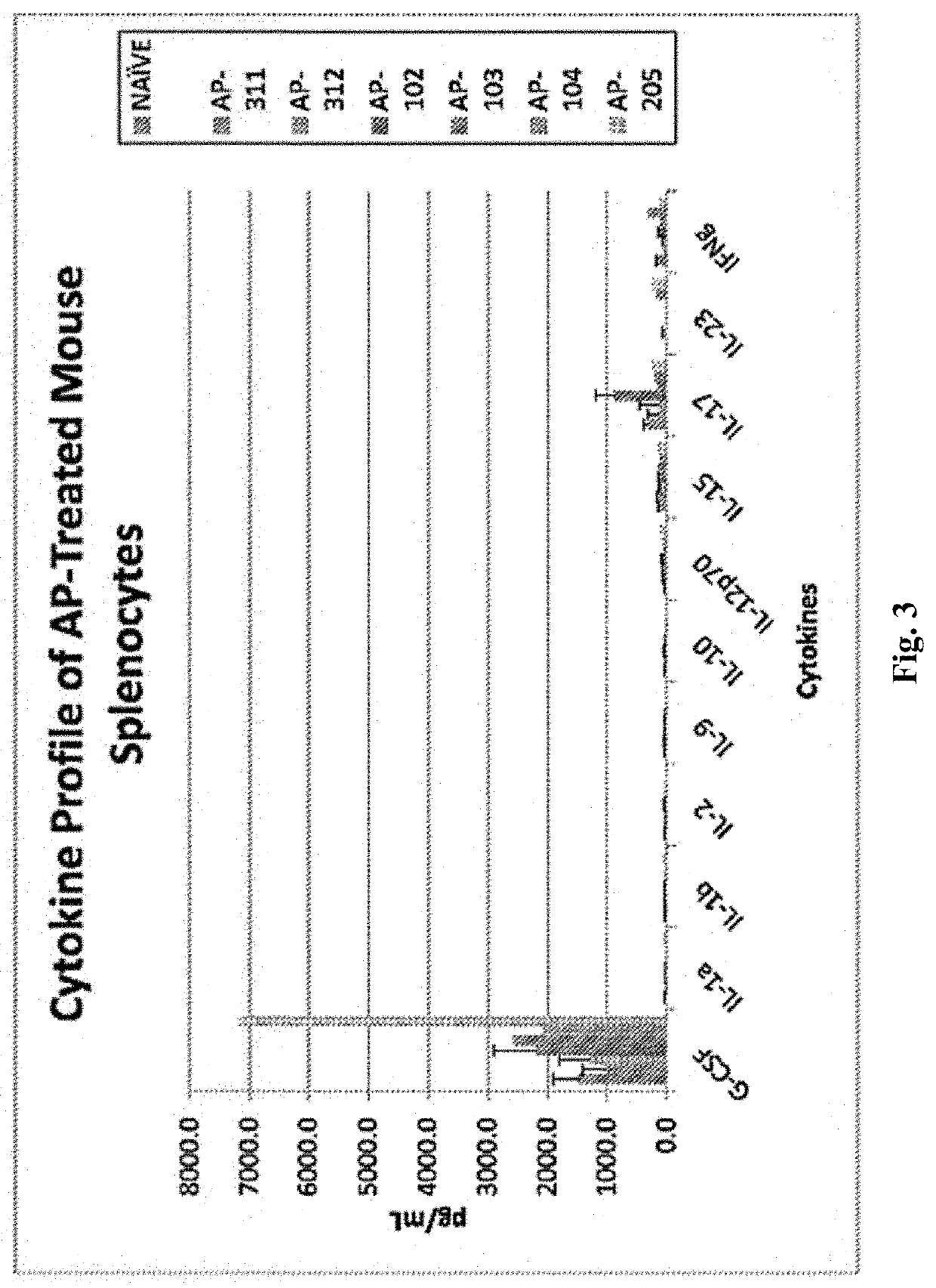
Method for Immunomodulation of using Aza-podophyllotoxin derivatives
PendingUS20210128705A1Organic active ingredientsAntibody medical ingredientsChlorobenzeneEthyl group
Disclosed is the novel use of some Aza-podophyllotoxin derivatives (AZPs) for modulation of the immune system (immunomodulation), including a method for modulating an immune response comprising administering to a subject an effective amount of at least one Aza-podophyllotoxin derivative of general formula wherein A-ring is selected from the group consisting of 1,3-dioxolane, cyclopentane, 1,4-dioxane, one methoxy, two methoxys, and ethyl; and wherein E-ring is selected from the group consisting of dimethoxyanisole, veratrol, anisole, benzene, syringol, bromobenzene, chlorobenzene, 1,2-dichlorobenzene, 2,3-dimethoxybenzene, 3,4,5-trimethoxybenzene.
Owner:SISTEMA UNIVRIO ANA G MENDEZ
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com