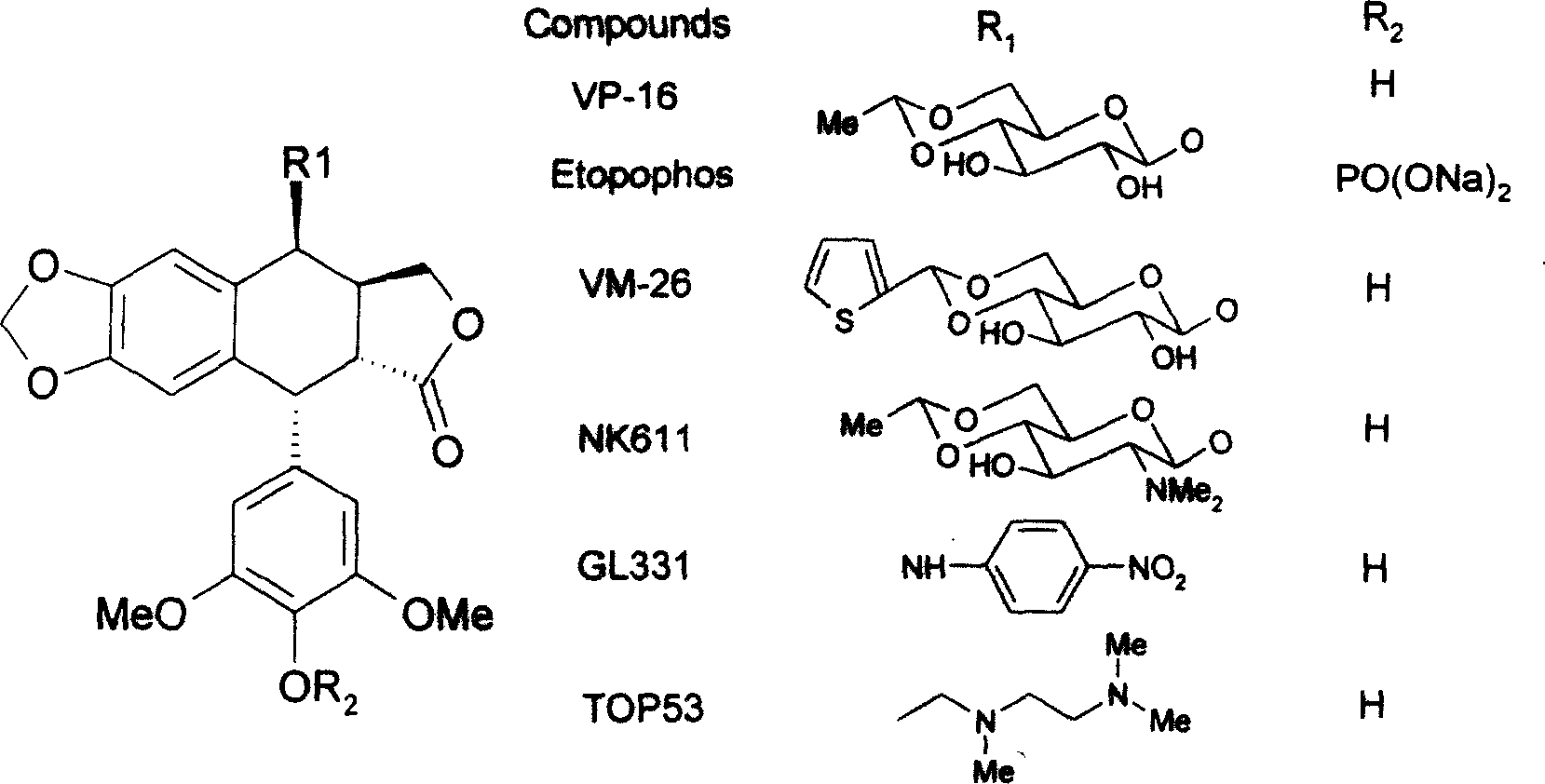
Podophyllotoxin compounds and their application and preparation process
A compound, methyl technology, applied in the field of new podophyllotoxins, can solve the problems of poor water solubility, strong drug resistance, and poor oral effect.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0016] The preparation method of the compound of the present invention and the activity test results of the corresponding products are provided below
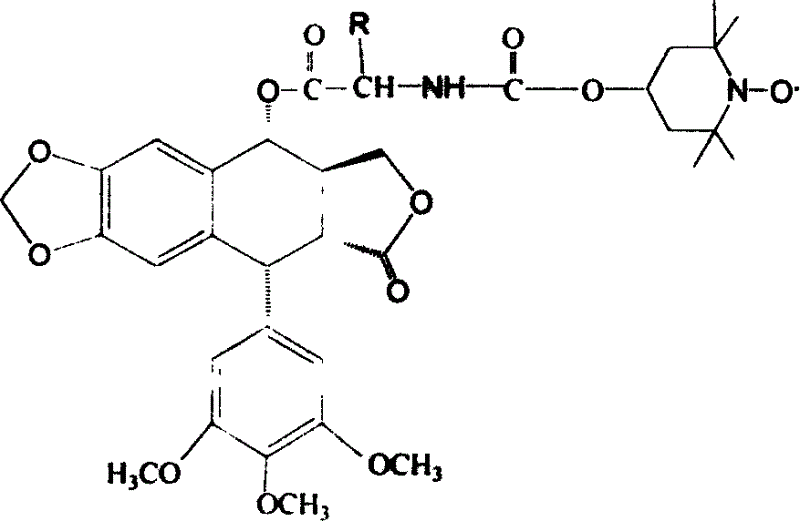
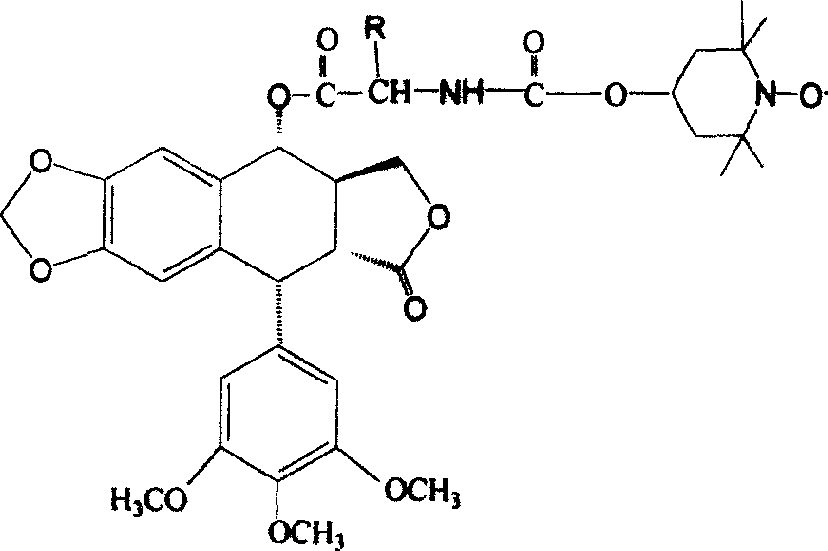
[0017] The preparation method of the present invention is: dissolving podophyllotoxin and N-(1-oxyl-2,2,6,6-tetramethyl-oxygen-carbonyl)-L-amino acid in dry dichloromethane, adding Catalytic amount of N, N-lutidine, stirred for 5 minutes under nitrogen protection, then added dicyclohexylcarbodiimide (DCC), stirred and reacted for 2 hours under nitrogen protection, filtered to remove white precipitate, evaporated under reduced pressure After removing the solvent, the crude product was purified by column chromatography, eluting with dichloromethane-acetone with a volume ratio of 15:1 to obtain the compound of formula I.
[0018] For related reactions, see Reaction Scheme 1.
[0019]
[0020] Reaction 1
[0021] R in formula 1 can be hydrogen, or methyl, or isopropyl, or methylsulfomethylene, o...
Embodiment 1
[0031] Synthesis of 4α-4-L-glycine-N-(-formyl-2′, 2′, 6′, 6′-tetramethyl-4′-nitroxyl radical ester)podophylloester (Ia)
[0032] Add 20ml of water and 20ml of methanol, 0.30g of sodium tungstate and 0.20g of edetate disodium salt into a 250ml Erlenmeyer flask, shake to dissolve, then add 10g of 2,2,6,6-tetramethyl- 4-Hydroxypiperidine, put it on a magnetic stirrer, start stirring, add 30% hydrogen peroxide in batches after dissolving, continue stirring, the solution turns from colorless to yellow, then gradually deepens, and finally becomes orange red, the reaction is about After 8-10 hours, distill methanol and most of the water under reduced pressure, add a small amount of sodium carbonate to shake the residual liquid after cooling, add sodium chloride for salting out, extract with ether 3-4 times, combine the extracts with anhydrous chlorination Calcium was dried, ether was removed under reduced pressure, and the solid was cooled to obtain 10.5 g of orange-red solid 2,2,6,6...
Embodiment 2
[0042] 4α-4-L-alanine-N-(-formyl-2′,2′,6′,6′-tetramethyl-4′-nitroxyl ester)podophylloester (Ib) synthesis
[0043] The experimental procedure is the same as in Example 1, only alanine is used instead of glycine. The detection data of the product obtained by the reaction are as follows:
[0044] Yield: 90%; m.p.138-140°C; [α] D 25℃ =-68° (c=0.5, CH 2 Cl 2 )IR(KBr)υcm 1 : 3344, 17811716, 1485, 1507, 1589, 930, 1126, 1175, 1239, 1365; MS (FAB) m / z: 683 (M, 30), 397 (100); HRMS (ESl) C 35 h 43 N 2 o 12 Theoretical value (M+2H), 685.2967, measured value, 685.2962.ESR: g 0 =2.0058, (3)ΔH PP =44.268Gs,A N =15.81Gs (three peaks at 1×10 4 M, CH 2 Cl 2 )
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com