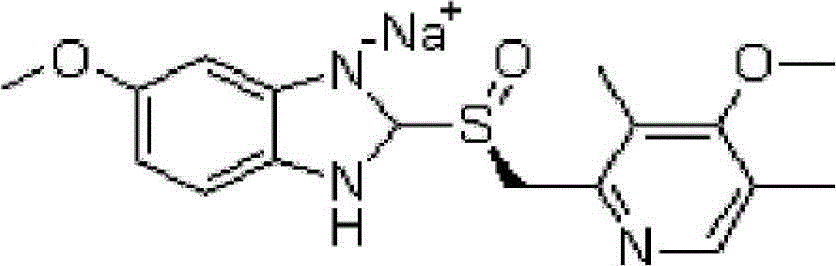
Patents
Literature
231 results about "Edetate disodium" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
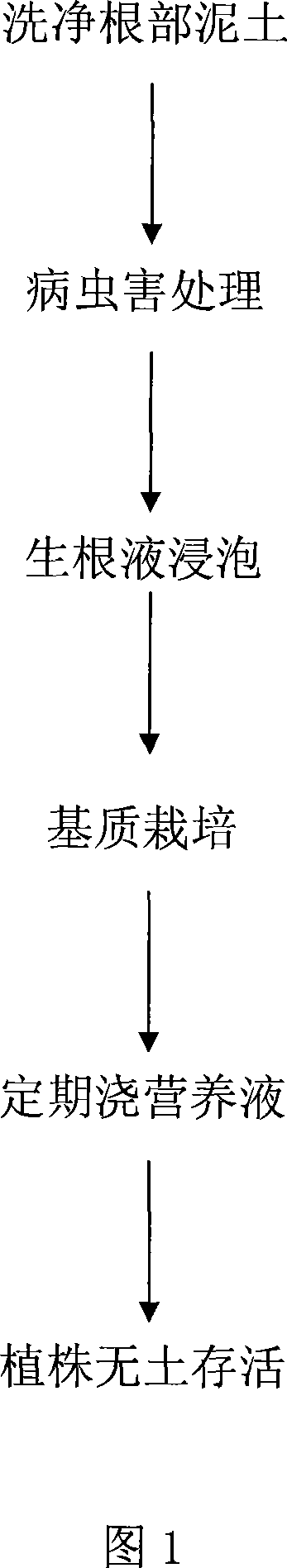
Plant nutrient solution and application in plant cultivation
InactiveCN101062877AImprove survival rateDoes not affect life cycleMagnesium fertilisersAlkali orthophosphate fertiliserManganeseMonopotassium phosphate
The invention discloses a plant nutrition liquid, which is characterized by the following: comprising potassium nitrate, calcium nitrate, magnesium sulfate, ammonium phosphate, potassium sulfate, monopotassium phosphate and so on a good deal of element; comprising edetate disodium, ferrosi sulfas, boric acid, manganese sulfate, zinc sulfate, copper sulfate, ammonium molybdate and so on microelement; comprising azophoska fertilizer and fertilizer booster; including diverse nutritious element of vegetation development; providing nourishing element for large and medium scale bush and small arbor. This invention also discloses a utility scheme in large and medium scale woody plant soilless culture.
Owner:成都市第二农业科学研究所 +1
Outer surface cleaning agent for high-speed trains and using method thereof
ActiveCN103740487AWill not harmNo cleaning effectNon-ionic surface-active compoundsOrganic detergent compounding agentsWater basedSurface cleaning
The invention discloses an outer surface cleaning agent for high-speed trains and a using method thereof. The outer surface cleaning agent for the high-speed trains comprises sodium ethoxylated alkyl sulfate, isomeric alcohol ethoxylate, a complexing agent and sodium hydrogen carbonate. The complexing agent is selected from one or more of edetate disodium, disodium ethydronate and dipotassium ethydronate. By using the cleaning agent disclosed by the invention, the dust removal and oil removal effects are significant, and the cleaning agent can avoid damaging paint and glass, effectively remove yellow spots on train bodies and achieve the effects of brightening and whitening; furthermore, as the cleaning agent is a water-based cleaning agent, the cleaning agent has the characteristics of environment-friendly property, low cost and no toxin and harm to people.
Owner:长沙艾森设备维护技术有限公司
Lipid emulsion with low anisidine value and preparation method thereof
InactiveCN102805727AAchieve antioxidantReduce the value of methoxyanilineEmulsion deliveryOil/fats/waxes non-active ingredientsEdetic AcidDisodium Edetate
The invention relates to a lipid emulsion with a low anisidine value, which contains oily constituents, water, an emulsifying agent and an osmotic pressure regulator, wherein the lipid emulsion contains a metal ion chelant but not an antioxidant. The content of the metal ion chelant is below 100mg / ml. The metal ion chelant is one or more of sodium calcium edentate, edetate disodium, edetate sodium and edetic acid. The invention relates to a method for preparing the lipid emulsion with the low anisidine value as well. According to the invention, in the process of preparing the lipid emulsion, other antioxidants are not needed to add, such as Vitamin E (VE), the antioxidation can be implemented only by using the metal ion chelant, and the low anisidine value can be greatly reduced. The low anisidine value of the lipid emulsion cannot be reduced by adding other antioxidants.
Owner:JIANGSU JIUXU PHARMA +1
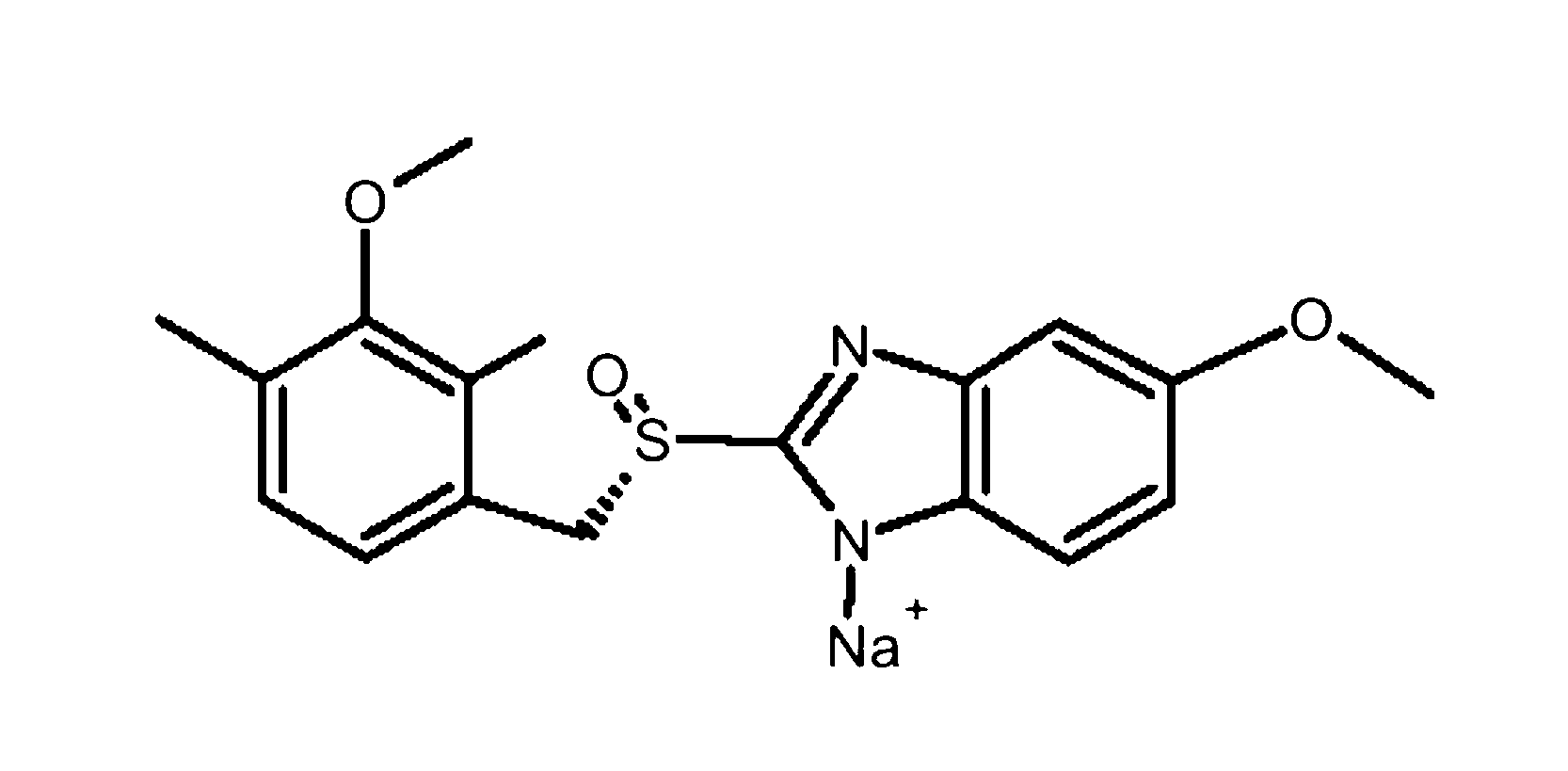
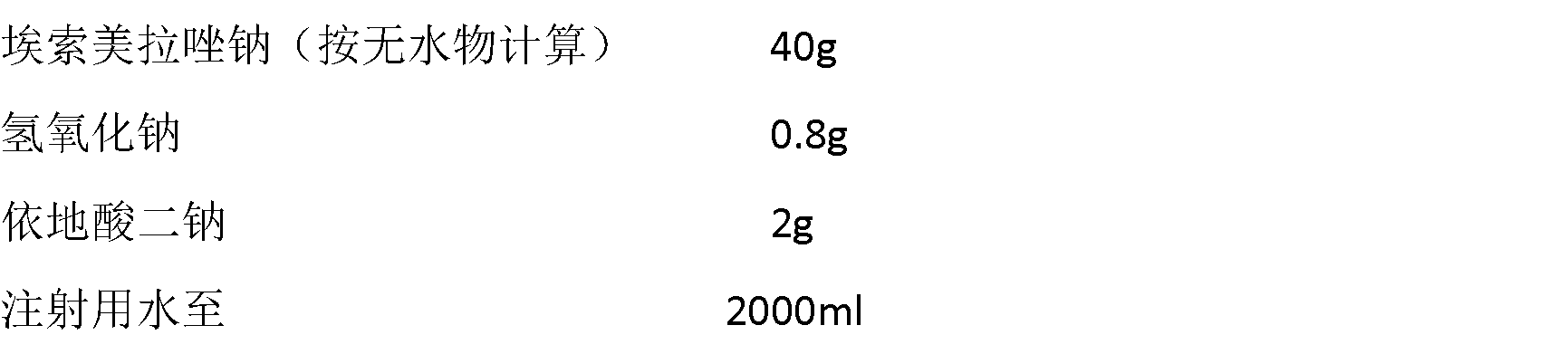
Preparation of injection esomeprazole sodium
ActiveCN103006585AIncrease profitReduce manufacturing costPowder deliveryOrganic active ingredientsEsomeprazole SodiumActivated carbon
The invention discloses preparation of injection esomeprazole sodium. The preparation comprises the following steps of: (a) adding partial injection water into a basic liquid, adding edetate disodium, dissolving and cooling to a temperature of between 10 and 20 DEG C, introducing nitrogen and adding esomeprazole sodium, stirring until the mixture is completely dissolved, adding activated carbon, stirring, decarbonizing and filtering; (b) adding injection at a temperature of between 10 and 20 DEG C to account for 80 to 95 percent of the preparation amount, introducing nitrogen, regulating pH value to be between 11.0 and 11.8 with alkali, adding injection water at a temperature of between 10 and 20 DEG C to the total preparation amount, filtering by using a filter with filter element of 0.22mu m, subpackaging in penicillin bottles, partly covering rubber plugs, and lyophilizing. The finished product prepared by the preparation is more stable in quality, and the production cost is remarkably reduced, so that the preparation is advantageous to mass production, and is good in market prospect, and worthy of popularization.
Owner:哈药集团股份有限公司 +1
Naloxone hydrochloride freeze-dried powder injection and preparation method thereof
ActiveCN102274196AQuality improvementImprove stabilityOrganic active ingredientsPowder deliveryFreeze-dryingDisodium Edetate
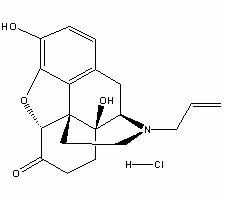
The invention relates to a naloxone hydrochloride freeze-dried powder injection and a preparation method thereof. The naloxone hydrochloride freeze-dried powder injection in every 1000 bottles is obtained by adding water for injection to dissolve the following ingredients and then freeze-drying: 0.1-5g of naloxone hydrochloride, 30-60g of mannitol and 0.01-0.5g of edetate disodium; before the freeze-drying, the pH value of the solution is 2.0 to 5.0. According to the invention, through controlling different pH value regulation ranges during the preparation process of different specifications of the naloxone hydrochloride for injection, the finished products can meet the prescribed standards after the freeze-drying, so that the quality and the stability of the product are improved.
Owner:ZHEJIANG JINHUA CONBA BIO PHARM CO LTD
Exterior medicine composition of fluocinolone acetonide and ester of fluocinolone acetonide
The invention provides an exterior medicine composition of fluocinolone acetonide and ester of fluocinolone acetonide. The exterior medicine composition comprises the fluocinolone acetonide or the ester of the fluocinolone acetonide serving as the active component, and one or more auxiliary materials applied to skin; and the exterior medicine composition is characterized in that the auxiliary materials for skin include sodium calcium edetate or edetate disodium.
Owner:天津金耀药业有限公司
Pharmaceutical composition for external use adopting glucocorticoid as active component
A pharmaceutical composition for external use which adopts glucocorticoid as an active component is disclosed. The pharmaceutical composition comprises the glucocorticoid adopted as the active component, and one or more pharmaceutical excipient. The pharmaceutical excipient comprises edetate disodium or edetate calcium disodium.
Owner:TIANJIN JINYAO GRP
Ginkgo flavone aglycone solid dispersion and preparation method thereof
InactiveCN101780030AImprove solubilityDissolution rate is fastAntibacterial agentsOrganic active ingredientsSolubilityPolymer science
The invention relates to a ginkgo flavone aglycone solid dispersion and a preparation method thereof. The solid dispersion adopts ginkgo flavone aglycone as active ingredient, is added with polymer carrier material, antioxidant and antisticking agent according to the proportion of 1: (0.2-15): (0-5): (0.2-15), and is prepared by a melting method, a coprecipitation method, a solvent dispersion method, a solvent melting method or a grinding method. The polymer carrier material is one or more in povidones, polyethylene glycols, poloxamers, polyoxyethylene stearic acid, hydroxymethyl propyl celluloses and low-viscosity Eudragit; the antioxidant is one or more in vitamin E, vitamin C or edetate disodium; the antisticking agent is one or more in talcum powder, micropowder silica gel, magnesium stearate or other auxiliary materials which can be taken as the antisticking agents. The ginkgo flavone aglycone solid dispersion can improve the solubility of medicine, leads the solubility to be 5-500 times higher than that of raw medicine, accelerates the dissolution rate of the medicine, and remarkably improves the dissolution rate in a dissolution medium within 45min in an accumulated way.
Owner:GUIZHOU UNIV
Biodegradable magnesium alloy bile duct litholysis knitted bracket and preparation method thereof
InactiveCN102727331APrevents stenosis from closingGuaranteed unobstructedStentsSurgeryDisodium EdetateBiocompatibility Testing
The invention belongs to the field of biomedicine, high molecular materials and magnesium alloy materials, and relates to a biodegradable magnesium alloy bile duct litholysis knitted bracket and a preparation method thereof. A knitted bracket is manufactured by knitting magnesium or magnesium alloy into a porous net structured or spiral tubular structured duct; and sodium cholate or / and edetate disodium containing biodegradable polymer layers is / are coated on the inner and outer surfaces of the knitted bracket to obtain the biodegradable magnesium alloy bile duct litholysis knitted bracket. Clinical using results indicate that the bracket provided by the invention is good in radial support property, capable of keeping bile duct unobstructed, capable of being degraded in a certain time to disappear and free of taking out once again; the biocompatibility is good and nearly no inflammation is generated due to in-vivo activities; and at the same time, through the sodium cholate or / and edetate disodium released by the medicine containing polymer coatings, formed stones can be dissolved and prevented from reforming.
Owner:SHANGHAI FIRST PEOPLES HOSPITAL
Pantoprazole sodium combined drug
ActiveCN101766615AEliminate adverse reactionsImproved depyrogenation processOrganic active ingredientsDigestive systemDisodium EdetateCurative effect
The invention discloses a pantoprazole sodium combined drug and a preparation method thereof. The pantoprazole sodium combined drug contains the following active ingredients in parts by weight: 35-100 parts of pantoprazole sodium, 15-35 parts of tiopronin, 90-200 parts of calcium glutamate and 15-25 parts of edetate disodium. The pantoprazole sodium combined drug has the functions of preventing damages caused by pantoprazole sodium to liver and other adverse reactions. The invention has the advantages of high drug quality, stable curative effect and advanced preparation method.
Owner:安徽延寿堂药业有限公司
Esomeprazole sodium lyophilized powder injection and preparation method thereof
InactiveCN102973524AImprove stabilityAvoid degradationPowder deliveryOrganic active ingredientsEsomeprazole SodiumUltrafiltration
The invention provides an esomeprazole sodium lyophilized powder injection for injection and a preparation method thereof. The weight ratio of esomeprazole sodium to edetate disodium and / or sodium calcium edentate is 1:(0.01-0.1), and the pH value of the esomeprazole sodium lyophilized powder injection is between 9.5 and 11.5. The preparation method comprises the following steps of: 1) weighing and dissolving edetate disodium and / or sodium calcium edentate in the prescription amount in injection water, stirring to dissolve, and regulating the pH value of sodium hydroxide liquid to be between 10.5 and 12.5; and 2) weighing esomeprazole sodium in the prescription amount, adding into liquid prepared in the step 1), stirring at room temperature to completely dissolve the liquid, and filling the injection water till full dose is reached; 3) performing ultrafiltration on the liquid medicament prepared in the step 2) to remove pyrogen; 4) sterilizing and filtering the liquid medicament which is subjected to ultrafiltration; and 5) filling and lyophilizing. The product prepared by the preparation method of esomeprazole sodium lyophilized powder injection has low related substances, and the substance does not remarkably increase in the storing process, and the content is not remarkably reduced.
Owner:SHENZHEN NEPTUNUS PHARM CO LTD
Substrate of traditional Chinese medicine cataplasm
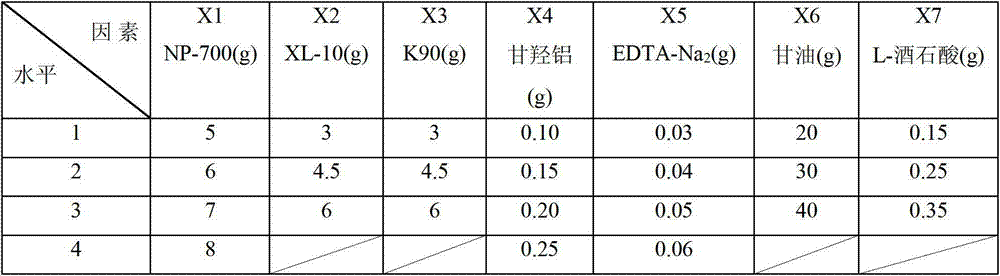
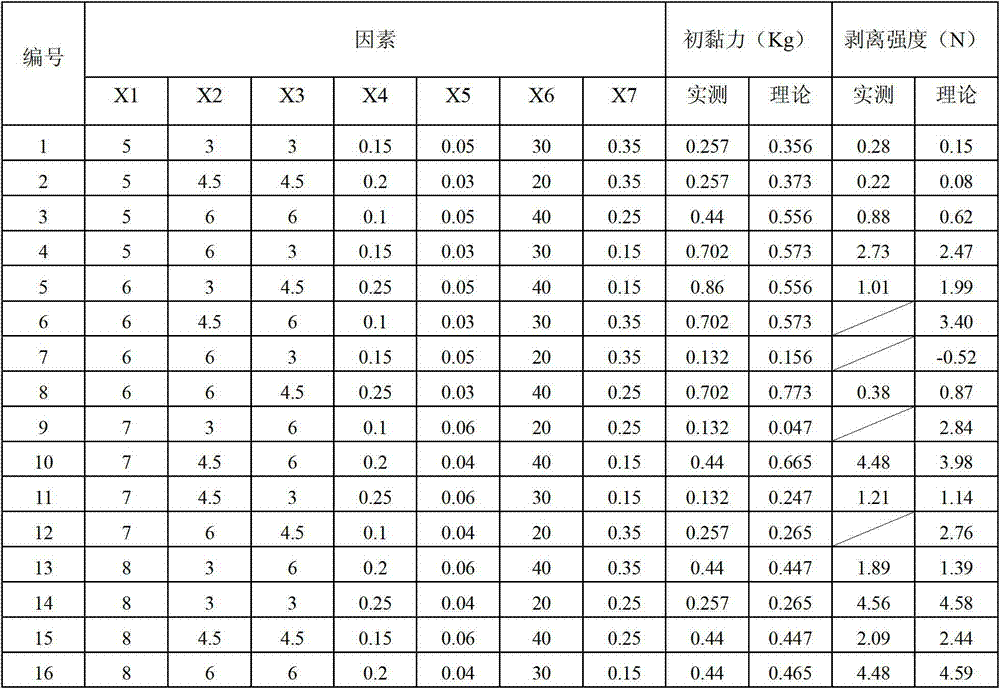
ActiveCN103040792ANon-allergicNo pollution in the processPharmaceutical non-active ingredientsSheet deliveryAdditive ingredientDisodium Edetate
The invention discloses a substrate of a traditional Chinese medicine cataplasm, which is prepared by the following raw materials in parts by weight: 800-1000 parts of partially neutralized sodium polyacrylate, 600-800 parts of crosslinking povidone, 350-500 parts of polyvinylpyrrolidone, 25-50 parts of aluminum glycinate, 6-10 parts of edetate disodium, 4000-5000 parts of glycerol and 35-50 parts of L-tartaric acid. Experimental results indicate that the substrate of the traditional Chinese medicine cataplasm does not cause skin allergy, and is low in stimulation reaction and easy to peel; in a peeling process, a patient does not feel pain; no medicine is residual; the transdermal absorption of the medicine is high; a medicine effect is better; the patient can use the medicine conveniently; and an organic solvent is not used in a production process, so that the environment is not polluted. The substrate can be supported with a water-soluble effective ingredient and an oily effective ingredient clinically, and has a wider application range.
Owner:常熟雷允上制药有限公司
Sodium hyaluronate eye drops and a preparation method thereof
ActiveCN102697713AExtended burst timePromote healingSenses disorderHydroxy compound active ingredientsDisodium EdetateBoronic acid
The invention relates to the field of medicine and particularly relates to sodium hyaluronate eye drops and preparation method thereof. Every 100 ml of the sodium hyaluronate eye drops comprise the following components: 80 to 150 mg of sodium hyaluronate, 130 to 200 mg of boric acid, 10 to 20 mg of borax, 8 to 15 mg of edetate disodium, 80 to 160 mg of 6-amino caproic acid, 80 to 160 mg of mannitol, 600 to 800 mg of sodium chloride, 3 to 10 mg of benzalkonium chloride, and 8 to 15 mg of borneo camphor, wherein 100 ml of water for injection is added, and the pH value is regulated to 6.0 to 7.8. The eye drops provided by the invention can play the role of promoting healing of injuries of corneal epithelium, can prolong the break-up time remarkably and can better improve the symptoms of xerophthalmia. Meanwhile, the sodium hyaluronate eye drops have good stability at ambient temperature, and all figures are similar, which indicates that the preparation technology is steady and practicable; and if the eye drops are used within the available period, the indexes of HA-Na are all within the quality control range.
Owner:ZHEJIANG JIANFENG PHARM CO LTD
Anti-oxidation metal surface treating agent
InactiveCN105907197AImprove thermal stabilityStrong water absorptionCellulose coatingsAnti-corrosive paintsPolyvinyl alcoholGlycerol
The invention discloses an anti-oxidation metal surface treating agent. The anti-oxidation metal surface treating agent is prepared from, by weight, 0.4-0.5 part of borax, 40-50 parts of polyvinyl alcohol, 12-15 parts of polyethylene glycol, 0.4-0.6 part of emulsifier op10, 0.3-0.5 part of glycerol, 0.6-0.8 part of triethanolamine, 6-8 parts of benzotriazole, 6-7 parts of sodium benzoate, 2-3 parts of sodium dodecyl sulfate, 0.4-0.6 part of butanedial, 4-5 parts of nano-crystalline cellulose, 0.3-0.4 part of diethylenetriamine, 0.32-0.4 part of vinyl trimethoxy silane, 0.2-0.3 part of polyvinylpyrrolidone, 1.2-1.6 parts of copper powder, 0.8-1.2 parts of catechin, 0.4-0.6 part of edetate disodium and a proper amount of deionized water. The prepared metal surface treating agent has the advantages that a preparation method is simple, cost is low, film forming performance is good, after-treatment is easy, the surface treating agent has good anti-oxidation, anti-rust and anti-corrosion functions on a metal surface, the effect is good, and application prospects are good.
Owner:HEFEI ZHENGHAO MECHANICAL TECH
Drug composition containing lansoprazole compound and preparation method of drug composition
ActiveCN103040737ASignificant synergyImprove solubilityPowder deliveryOrganic active ingredientsSolubilityDisodium Edetate
The invention discloses a drug composition containing a lansoprazole compound, which comprises the following raw materials in parts by weight: 10-20 parts of lansoprazole, 1-10 parts of edetate disodium, 1-20 parts of mannitol, 5-10 parts of sodium sulfite and 10-30 parts of sodium citrate. According to the drug composition, a pH regulator sodium citrate is screened out by a large number of tests and compounded with a stabilizing agent edetate disodium, the synergistic interaction is obvious; decrease of a pH value of strongly basic lansoprazole solution during production or a lyophilized agent during storage can be avoided well; therefore, re-dissolution of the lansoprazole lyophilized agent can be well avoided; and the solubility is good. The drug composition does not go bad and is not oxidized in a storage process, and the administration safety is ensured.
Owner:罗诚
Adrenaline hydrochloride injection and preparation method thereof
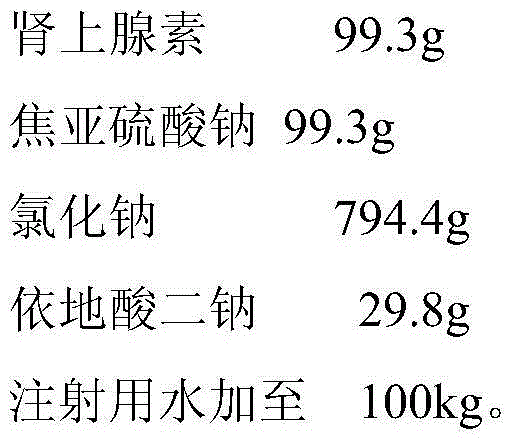
InactiveCN105982851AGuaranteed qualityOrganic active ingredientsPharmaceutical delivery mechanismAdrenaline hydrochlorideDisodium Edetate
The invention provides an adrenaline hydrochloride injection and a preparation method thereof. A formula of 100 kg of the adrenaline hydrochloride injection contains 99.3 g of adrenaline, 794.4 g of sodium chloride, 29.8 g of edetate disodium and the balance water for injection. The quality of the adrenaline hydrochloride injection meets the stipulations of 'Notification' issued by State Food and Drug Administration in 2008.
Owner:上海禾丰制药有限公司
Preparation method for adapalene gel
InactiveCN105411999ASolve the problem of granularityOrganic active ingredientsAerosol deliveryWater bathsDrugs solution
The invention discloses a preparation method for adapalene gel. The preparation method comprises the steps that carbomer 934 is soaked in purified water for 24h and screened to obtain a carbomer solution; adapalene, propanediol and sodium hydroxide are taken, dissolved in purified water and added into a stainless steel barrel for a water bath to obtain a main drug solution; methylparaben, poloxamer188 and propanediol are taken, poured into a beaker, stirred and dissolved in a water bath to be clarified; purified water and edetate disodium are taken and placed in a main pot to be stirred at a low speed for dissolution, the solution in the beaker is poured into the main pot, sodium hydroxide is added, and the mixture is stirred to be dissolved; the carbomer solution is poured into the main pot and stirred slowly into uniform gel; the main drug solution is poured into the gel, the stainless steel barrel is rinsed with propanediol before the gel is poured into the main pot, and the mixture is stirred on a vacuum condition to obtain a material; the pH of the material is regulated to 4.75-5.25, quick stirring is started, and vacuum degassing, slow stirring and discharge are performed in sequence. The granularity of the adapalene gel prepared through the method reaches 50 micrometers, meeting the standard requirement for preparations.
Owner:FRONT PHARM PLC
Ointment for treating gynaopathy and dermatoses and preparation method thereof
InactiveCN102641277AAntimycoticsHydroxy compound active ingredientsSodium bicarbonateDisodium Edetate
The invention discloses an ointment for treating gynaopathy and dermatoses and a preparation method thereof. The externally-applied ointment is prepared from halcinonide preparation, menthol crystal and camphor which are utilized as raw materials according to a certain preparation technology, and is prepared from the following raw materials in parts by weight: 6 to 6.5 parts of halcinonide preparation, 15 to 20 parts of menthol crystal, 15 to 20 parts of camphor, 3 to 3.5 parts of edetate disodium, 20 to 40 parts of polyethylene glycol mono stearate, 0 to 3 parts of essence, 0 to 2 parts of carbomer, 0 to 2 parts of sodium bicarbonate and 15 to 25 parts of pure water. The externally-applied ointment is low in cost, has the effects of clearing away heat, eliminating dampness, dispelling the wind, killing worms, diminishing inflammation and relieving itching, has better treatment effects, has the characteristics of being easy to prepare, lower in cost and obvious in treatment effect, and can be used for treating the gynaopathy, such as vaginitis, cervicitis and cervical erosion, caused by various germs, and treating the dermatoses, such as tinea corporis, tinea cruris, tinea of feetand hands and various dermatitis, caused by various fungus infection. The externally-applied ointment has exact treatment effects, takes effect rapidly, is low in cost and convenient to use, and has no toxic and side effects, thus being worthy of popularization and application.
Owner:李怀珠
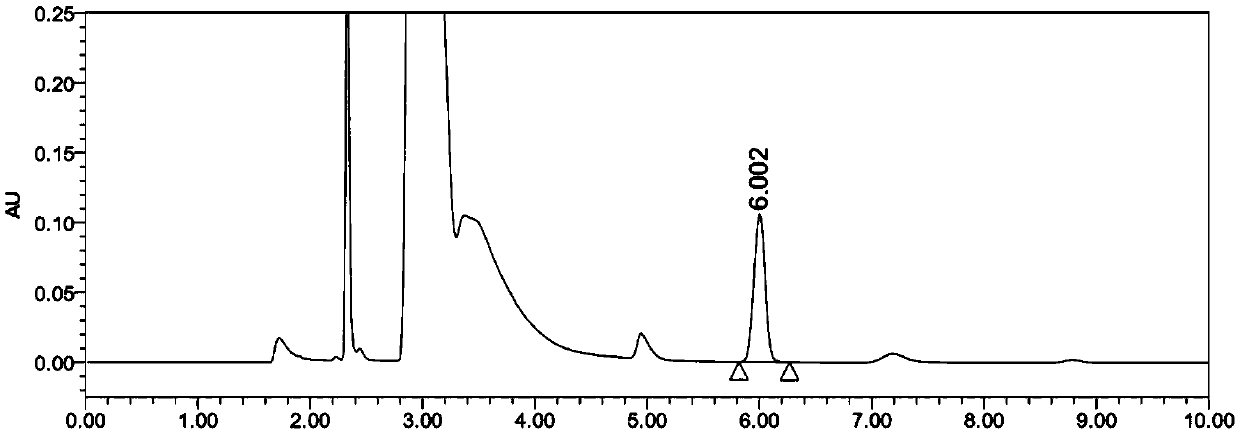
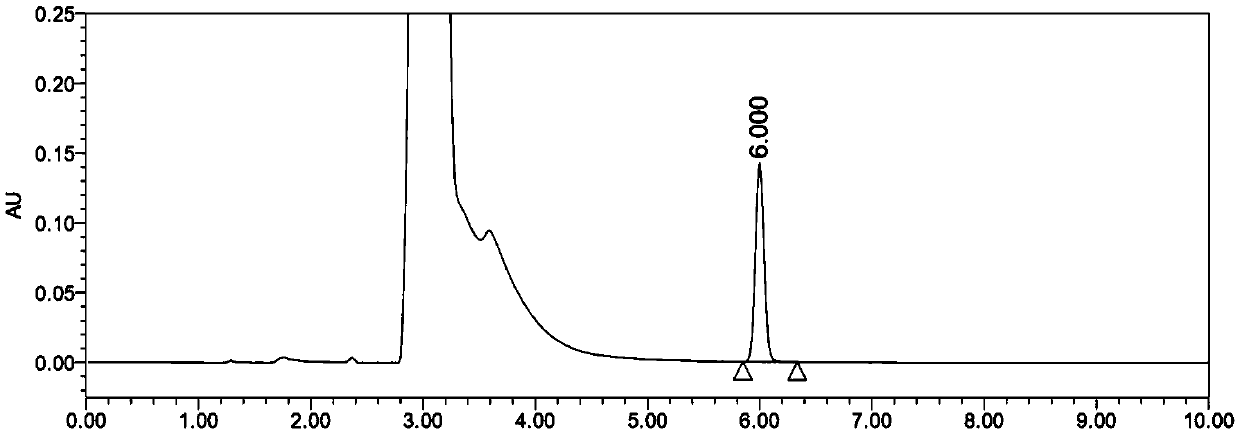
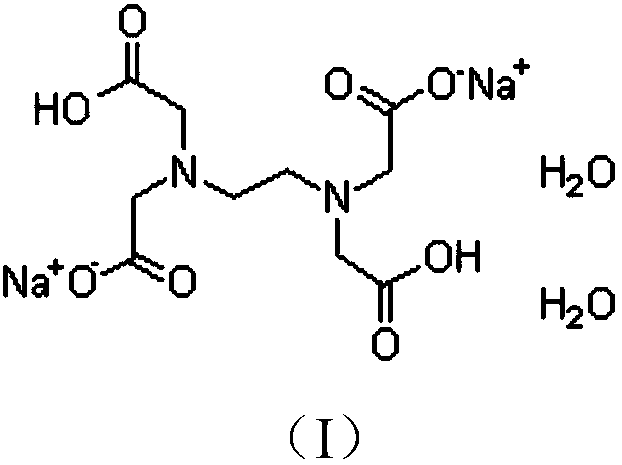
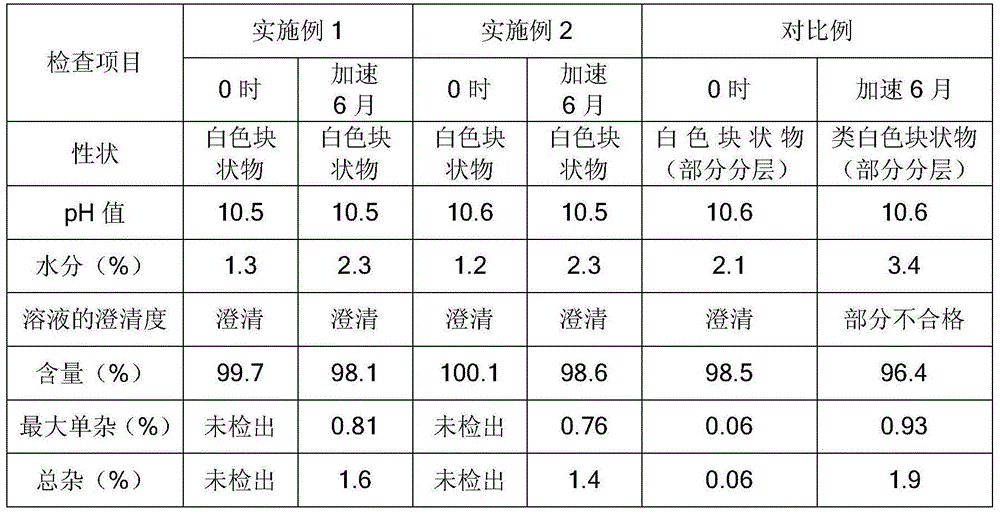
Content determination method of edetate disodium in acetylcysteine liquid preparation
ActiveCN110794045AEliminate distractionsImprove stabilityComponent separationDisodium EdetateCyclodextrin Derivatives
The invention belongs to the field of medicine detection, and discloses a content determination method of edetate disodium in acetylcysteine liquid preparation. In the method, HPLC is used, a test solution is prepared from the acetylcysteine liquid preparation, cyclodextrin and / or cyclodextrin derivatives, Fe3+ and a mobile phase, amino silane bonded silica gel is used as a chromatographic columnof a filler, a mixed solvent of ion pair buffer solution and an organic phase is used as the mobile phase, and thus, content of the edetate disodium in the test solution can be determined. The methodis simple to operate and excellent in repeatability, can accurately determine content of the edetate disodium in the acetylcysteine liquid preparation, and consequently can control product quality ofthe edetate disodium in acetylcysteine liquid preparation better.
Owner:上海聚可医药科技有限公司
Esomeprazole sodium for injection and preparation method thereof
InactiveCN105147624AAchieve stabilityReduce manufacturing costPowder deliveryOrganic active ingredientsEsomeprazole SodiumFreeze-drying
The invention provides a preparation method of esomeprazole sodium for injection. The preparation method comprises the following steps: step 1, adding edetate disodium into water for injection, and stirring for complete dissolution; step 2, adding esomeprazole sodium and a 1-5 mol / L sodium hydroxide solution, and stirring for complete dissolution to enable the system pH value to be 10.5-12.0; step 3, confirming a constant volume by use of water for injection, and stirring to enable the medical liquid to be uniformly mixed; step 4, filtering, sterilizing and filling; step 5, performing freeze drying in the way of pre-freezing for 2-4 h at 50 DEG C below zero to 30 DEG C below zero, then subliming for the first time for 2-12 h at 15 DEG C below zero to 0 DEG C, subliming for the second time for 2-12 h at 20-40 DEG C and controlling the vacuum degree to be 0.50 mbar or below to finally obtain esomeprazole sodium for injection. The esomeprazole sodium for injection is higher in stability, causes less mistakes during preparation and is more optimized during practical application.
Owner:SHANGHAI CHENPON PHARM TECH CO LTD
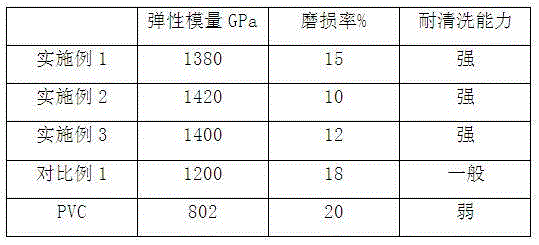
Composite material for yoga balls and preparation method of composite material
The invention discloses a composite material for yoga balls and a preparation method of the composite material. The composite material is prepared from the following components in parts by weight: 40-55 parts of PVC, 1-5 parts of an antiager, 12-18 parts of a carbon fiber, 5-12 parts of rubber powder, 5-9 parts of edetate disodium, 1-5 parts of glycerin, 0.1-0.5 part of talcum powder, 3-6 parts of olive oil, 1-6 parts of molding powder and 0.2-0.5 part of acetic acid. The preparation method comprises the following steps: step 1, mixing PVC, rubber power, talcum powder and molding powder, putting the mixed components into a grinder for grinding, and carrying out screening to obtain a mixture I; adding the carbon fiber, edetate disodium, glycerin and acetic acid into the mixture I for stirring and transferring into a reaction kettle, and carrying out vacuum heating to enable the temperature to be 200-350 DEG C to obtain a mixture II; spraying olive oil into the mixture II for stirring and transferring into a pelletizer for pelletization, thereby obtaining the composite material. The composite material is high in elasticity, resistant to abrasion and brushing, and wide in application range.
Owner:苏州天健竹业科技有限公司
Chamomile eye lotion
InactiveCN109908199ANon-irritatingReduce foreign body sensationSenses disorderInorganic boron active ingredientsDisodium EdetateIrritation
The invention discloses a chamomile eye lotion. The chamomile eye lotion comprises the following components in parts by weight: 0.002-0.005 parts of peppermint, 1-2 parts of propylene glycol, 0.05-0.10 parts of sodium hyaluronate, 3.5-5.5 parts of sodium chloride, 0.05-0.15 parts of a vitamin B6, 0.2-0.5 parts of chondroitin sulfate, 0.4-0.8 parts of taurine, 0.5-2.0 parts of borax, 0.2-1.0 part of edetate disodium, 0.1-0.3 parts of sodium ethylparaben, 0.1-0.3 parts of sodium methyl hydroxybenzoate, 2-8 parts of boric acid, 0.1-0.4 parts of Tween 80, 1-10 parts of chamomile floral water and 90.798-68.945 parts of purified water. A PH and an osmotic pressure thereof are fundamentally the same as that of human tears. The chamomile eye lotion is non-irritant, cool and eye-brightened, and capable of washing residual cosmetics in an eyeball completely, moisturizing and lubricating an eye, reducing foreign body sensation of wearing a lens, diminishing inflammation, achieving a repairing function to slight abrasion, and suitable for young people who wear color contact lenses and contact lenses frequently, especially for women who make up frequently.
Owner:嘉兴力山明朗生物医药科技有限公司
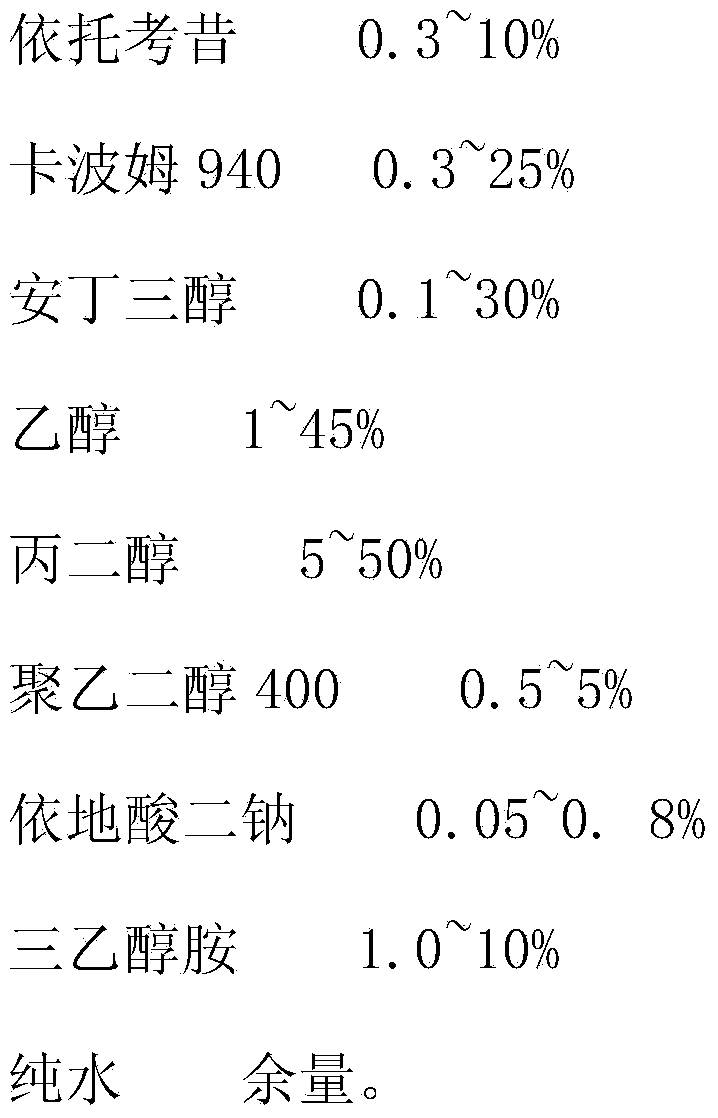
Etoricoxib gel preparation and preparation method thereof
InactiveCN103989628APromote absorptionGood curative effectOrganic active ingredientsAntipyreticGel preparationDisodium Edetate
The invention provides an etoricoxib gel preparation and a preparation method thereof. The gel preparation comprises the following components in percentage by weight: 0.3 to 10% of etoricoxib, 0.3 to 25% of carbomer 940, 0.1 to 30% of tromethamine, 1 to 45% of ethyl alcohol, 5 to 50% of propylene glycol, 0.5 to 5% of polyethylene glycol 400, 0.05 to 0.8% of edetate disodium, 1.0 to 10% of triethanolamine, and the balance of pure water. According to the preparation method, tromethamine is used to dissolve etoricoxib so as to solve the problem that etoricoxib is hardly dissolved in water; the skin penetrability of etoricoxib is improved and the transdermal absorption rate of etoricoxib is increased, and local external application of the gel preparation can obviously improve the treatment effect on arthritis or rheumatoid arthritis; simultaneously, a new administration route is provided for patients. The gel preparation is in a form of semi-solid gel with good transdermal absorption effect etoricoxib is completely dissolved and dispersed in the gel preparation, and thereby the pain of the patient can be relieved favorably. The preparation method is simple in process, and the preparation is stable and reliable in quality.
Owner:王俊国
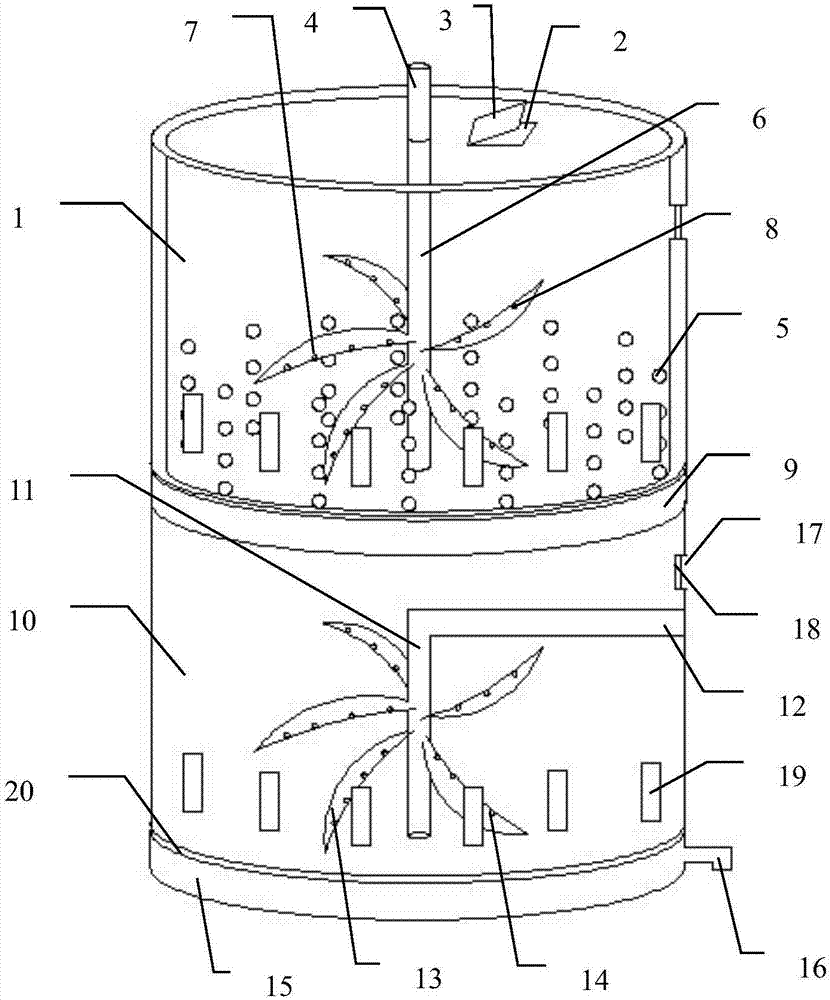
Preparator of naloxone hydrochloride injection and preparation method thereof
ActiveCN107485563AEasy to solveGuaranteed efficacyOrganic active ingredientsNervous disorderForeign matterFiltration
The invention discloses a preparator of a naloxone hydrochloride injection and a preparation method thereof. The preparation method comprises the steps that a certain amount of water for injection, sodium chloride and naloxone hydrochloride are added into an upper tank body of the preparator, nitrogen is introduced, mixing and stirring are performed to obtain a mixed solution, then edetate disodium is added to regulate a pH value, and the mixed solution is filtered by a first filter membrane in the middle of the preparator and delivered to a lower tank body; a certain amount of water for injection is added into the lower tank body, stirring is performed under the situation that nitrogen is introduced, and a hydrochloric acid solution is used for regulating the pH value; finally, visible foreign matter checking is performed after filtration of a second filter membrane at the lower portion of the lower tank body, nitrogen charging is performed for filling encapsulation after qualification, and sterilization is performed to obtain the sterilization. No auxiliary material is added, and the purity and use safety of the prepared injection are ensured. The preparation method is simple and easy to operate, and the preparation cost is effectively reduced. In addition, the invention provides the preparator of the naloxone hydrochloride injection. Device replacement in the preparation process of the naloxone hydrochloride injection is avoided, the drug quality is improved, and drug safety is ensured.
Owner:北京市永康药业有限公司
Cashmere sweater cleaning agent
InactiveCN102851139AEasy to cleanNo damageInorganic/elemental detergent compounding agentsNon-ionic surface-active compoundsCoconut diethanolamideLanolin
The present invention discloses a cashmere sweater cleaning agent, which comprises the following substances, by weight, 10-20 parts of sodium dodecylbenzenesulphonate, 2-6 parts of sodium hydroxide, 1-3 parts of edetate disodium, 2-6 parts of fatty alcohol polyoxyethylene ether, 5-8 parts of propylene glycol, 2-4 parts of polyoxyethylene lauryl ether, 2-5 parts of sodium tripolyphosphate, 10-20 parts of coconut diethanolamide, 0.1-0.3 part of essence, 2-5 parts of lanolin, and 80-100 parts of deionized water. The cashmere sweater cleaning agent of the present invention has an excellent cleaning performance, and further has the following characteristics that: stains on the surface of a cashmere sweater can be effectively cleaned, no damage on the surface of the cashmere sweater is generated, no chemical residue is generated, and no harm is generated to human body.
Owner:WUJIANG HANHUI TEXTILE
Biodegradable magnesium alloy bile duct stone-dissolving carving stent and preparation method thereof
InactiveCN102727948APrevent closureGuaranteed unobstructedStentsSurgeryBile ducts stonesDisodium Edetate
The invention, belonging to the field of biomedicine, bio-polymer material and magnesium alloy material, relates to a biodegradable magnesium alloy bile duct stone-dissolving carving stent and a preparation method thereof. The stent is prepared by processing magnesium or magnesium alloy into hollow tubes, carving the hollow tubes under the action of laser to a tubular stent with a porous reticular or spiral tubular structure, and then coating a biodegradable polymer layer containing sodium cholate or / and edetate disodium on the inner surface and outer surface of the tubular stent. Clinical outcomes show that the stone-dissolving bile duct stent of the invention has good radial supporting performance, can keep the bile duct open, can be degraded away in a certain time with no need of removing the stent, has good biocompatibility, and lets almost no internal inflammation generate. Simultaneously, sodium cholate or / and edetate disodium slowly released by the polymer layer containing drugs can dissolve the formed stones and can prevent the re-formation of stones.
Owner:SHANGHAI FIRST PEOPLES HOSPITAL
Acanthopanax fruit beverage and preparation method thereof
InactiveCN103876223AKeep aroma componentsAvoid oxidation catalysisFood ingredient functionsFood preparationBiotechnologyDisodium Edetate
The invention relates to a health beverage and particularly relates to an acanthopanax fruit beverage and a preparation method thereof. The acanthopanax fruit beverage is prepared through mixing acanthopanax, citric acid, granulated sugar, honey, potassium sorbate and edetate disodium at the ratio of 50:0.5:10:70:0.025:0.5 and adding water to 1000ml. According to the invention, dry acanthopanax fruit is effectively developed and utilized; metal ion complexing agent edetate disodium is added in the prescription, and the oxidation and the catalysis of anthocyanin in the acanthopanax fruit by metal ions can be effectively avoided, so that the stability of the product can be effectively increased. The active ingredient of the acanthopanax fruit is extracted through an ultrasonic extracting technology, is filtered through a plate frame and a 0.22-micron filter membrane and then is disinfected, so that the volatilization of aroma substances in the acanthopanax caused by heat sterilization treatment of the acanthopanax fruit and heavy smell of caramel caused by heating in a conventional technology can be avoided, the inherent aroma substances in the acanthopanax are kept, and the industrial production is facilitated.
Owner:TONGHUA NORMAL UNIV
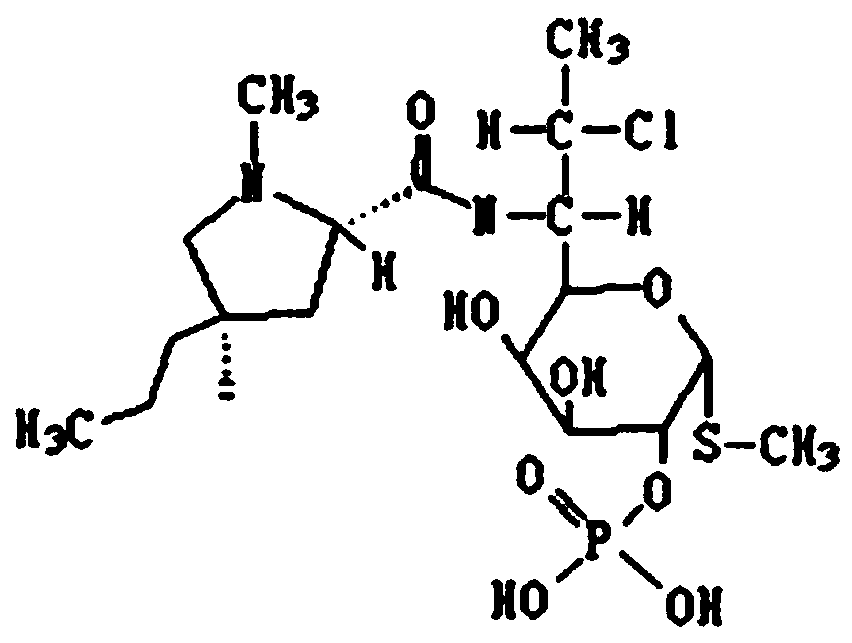
Clindamycin phosphate injection and preparation method thereof
InactiveCN111329835ABest practiceAddressing Sterilization ShortcomingsAntibacterial agentsOrganic active ingredientsActivated carbonPhosphoric Acid Esters
The invention discloses a preparation method of a clindamycin phosphate injection. The clindamycin phosphate injection is prepared from components of raw materials as follows: clindamycin phosphate, sodium hydroxide, benzyl alcohol, edetate disodium and water for injection. The preparation method comprises steps as follows: (1) a certain amount of edetate disodium is dissolved in the water for injection under the condition that the air cleanliness is class 100 in a whole room and is stirred to be dissolved; (2) a sodium hydroxide solution, clindamycin phosphate and benzyl alcohol are added andstirred uniformly; (3) after activated carbon is added, the mixture is stirred, cooled and filtered, and then a substance obtained after filtering is conveyed to a dilution preparation tank; and (4)the water for injection is added to a full amount, the pH value is adjusted, the content of a main drug is tested, and refined filtration, filling and sealing are performed. The invention also discloses the clindamycin phosphate injection prepared with the method. By means of the method, high-temperature sterilization is not required finally, the defect that sterilization is performed with high-temperature sterilization adopted by a conventional method is overcome, and the stability of the clindamycin phosphate injection is effectively controlled.
Owner:SHANDONG WEIZHI BAIKE PHARM CO LTD
Composite vitamin B powdery injection for venous infusion and preparation thereof
InactiveCN1679582AInjection is painlessQuality improvementPowder deliveryMetabolism disorderDisodium EdetateFreeze-drying
A powder injection of composite VB for intravenous injection is prepared from VB1, riboflavine sodium phosphate, VB6, nicotinic amide, sodium dextropantothenate, edetate disodium, low-molecular dextran and excipient through pretreating, proportional mixing, filtering for removing bacteria, and freeze drying.
Owner:WUXI KAIFU PHARMA
Composite vitamin B powdery injection for venous infusion and preparation thereof
InactiveCN100361659CInjection is painlessQuality improvementPowder deliveryMetabolism disorderFreeze-dryingDisodium Edetate
Owner:WUXI KAIFU PHARMA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com