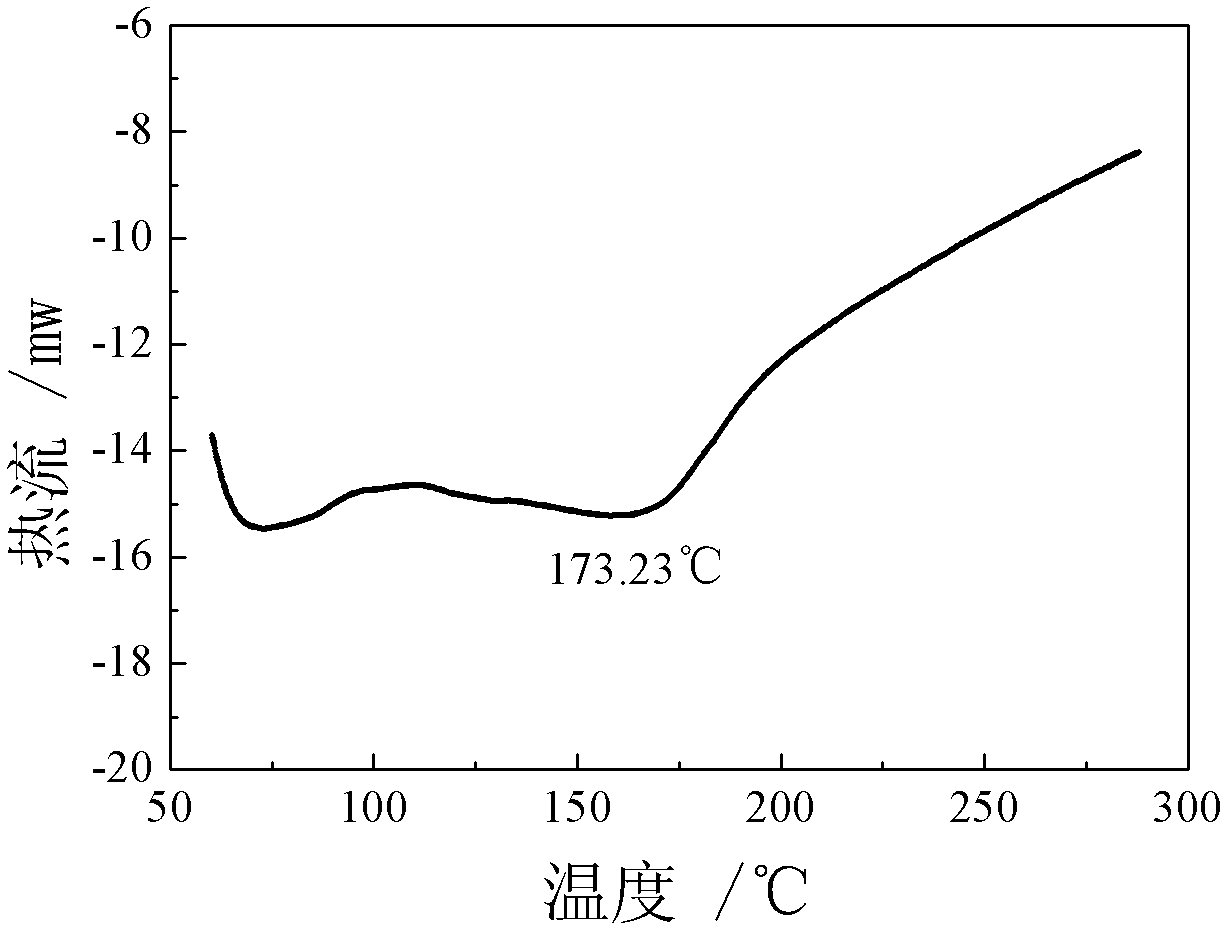
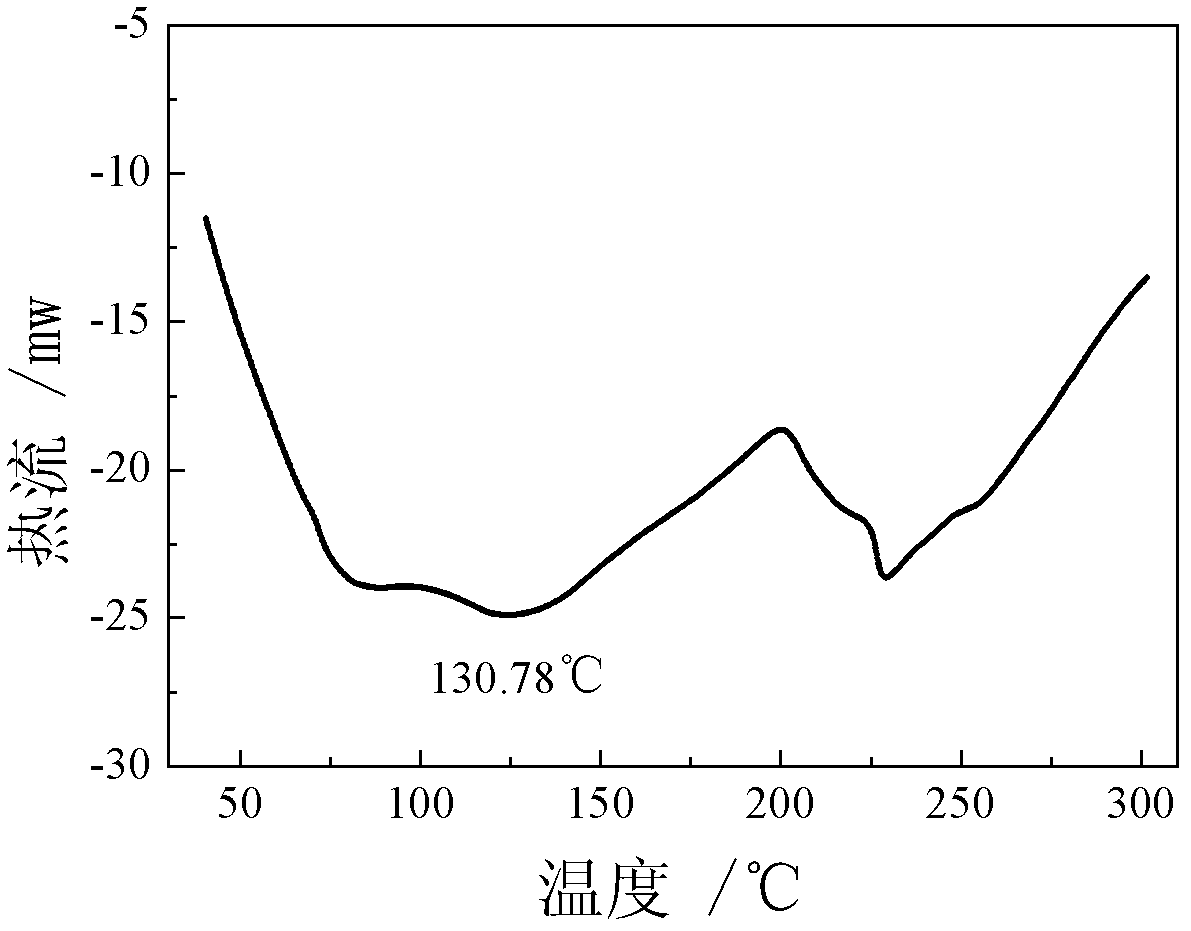
Patents
Literature
2919 results about "Calcium nitrate" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
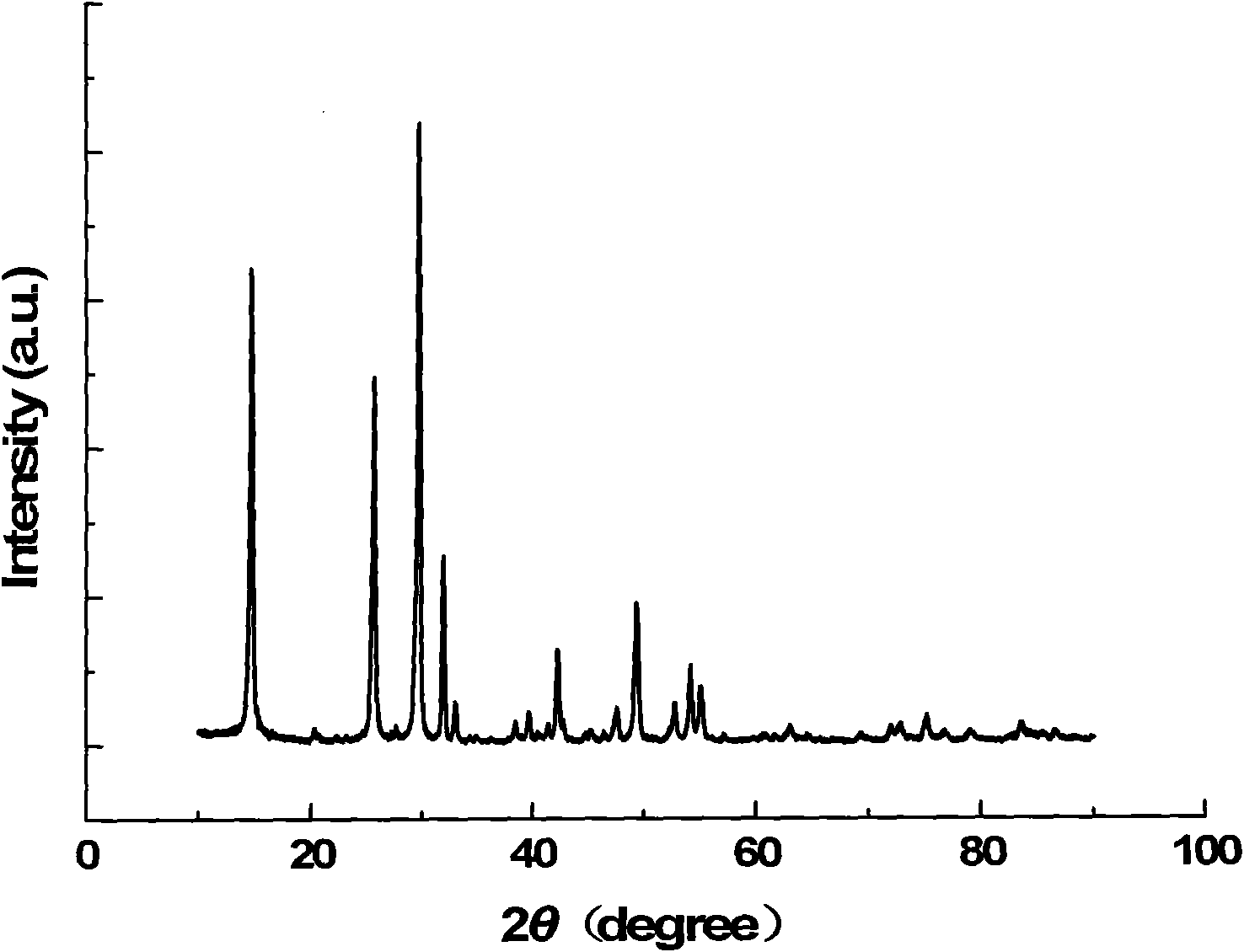
Calcium nitrate, also called Norgessalpeter (Norwegian saltpeter), is an inorganic compound with the formula Ca(NO₃)₂. This colourless salt absorbs moisture from the air and is commonly found as a tetrahydrate. It is mainly used as a component in fertilizers but has other applications. Nitrocalcite is the name for a mineral which is a hydrated calcium nitrate that forms as an efflorescence where manure contacts concrete or limestone in a dry environment as in stables or caverns. A variety of related salts are known including calcium ammonium nitrate decahydrate and calcium potassium nitrate decahydrate.
Plant nutrient solution for soilless culture of tomato
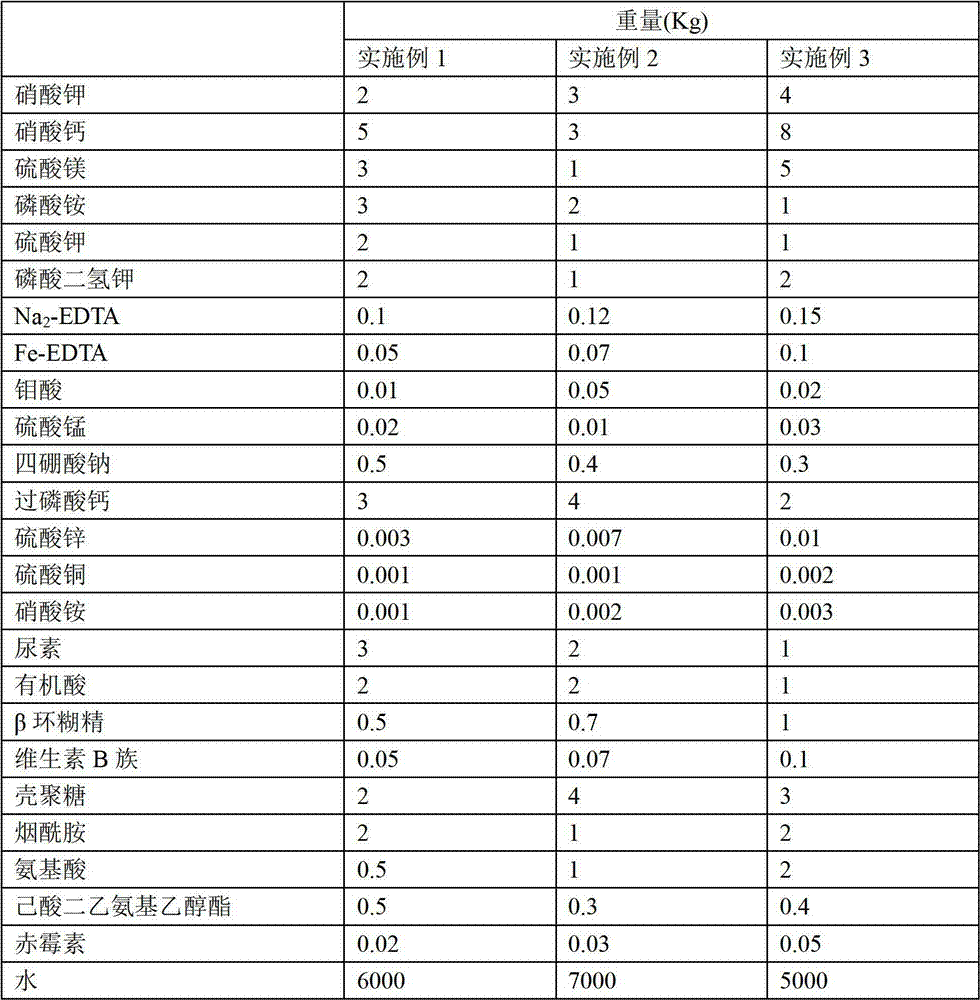
The invention provides a plant nutrient solution for soilless culture of tomato. The plant nutrient solution is prepared by the following raw materials in part by weight: 2 to 4 parts of potassium nitrate, 3 to 8 parts of calcium nitrate, 1 to 5 parts of magnesium sulfate, 1 to 3 parts of potassium phosphate, 1 to 2 parts of potassium sulphate, 1 to 2 parts of monopotassium phosphate, 0.1 to 0.15 part of Na2-EDTA, 0.05 to 0.1 part of Fe-EDTA, 0.01 to 0.05 part of molybdic acid, 0.01 to 0.03 part of manganese sulfate, 0.3 to 0.5 part of sodium tetraborate, 2 to 4 parts of superphosphate, 0.003 to 0.01 part of zinc sulfate, 0.001 to 0.002 part of copper sulfate, 0.001 to 0.003 part of ammonium nitrate, 1 to 3 parts of urea, 1 to 2 parts of organic acid, 0.5 to 1 part of beta cyclodextrin, 0.05 to 0.1 part of vitamin B, 2 to 4 parts of chitosan, 1 to 2 parts of nicotinamide, 0.5 to 2 parts of amino acid, 0.3 to 0.5 part of diethyl aminoethyl hexanoate, 0.02 to 0.5 part of gibberellin, and 5,000 to 7,000 parts of water. According to experiment, the plant nutrient solution provided by the invention can be used for carrying out soilless culture of tomato, and the tomato has high plant height, thick stem, high cluster and high output.
Owner:山西田森杜氏番茄科技有限公司
Technological process for producing high concentration nitric-phosphate fertilizer
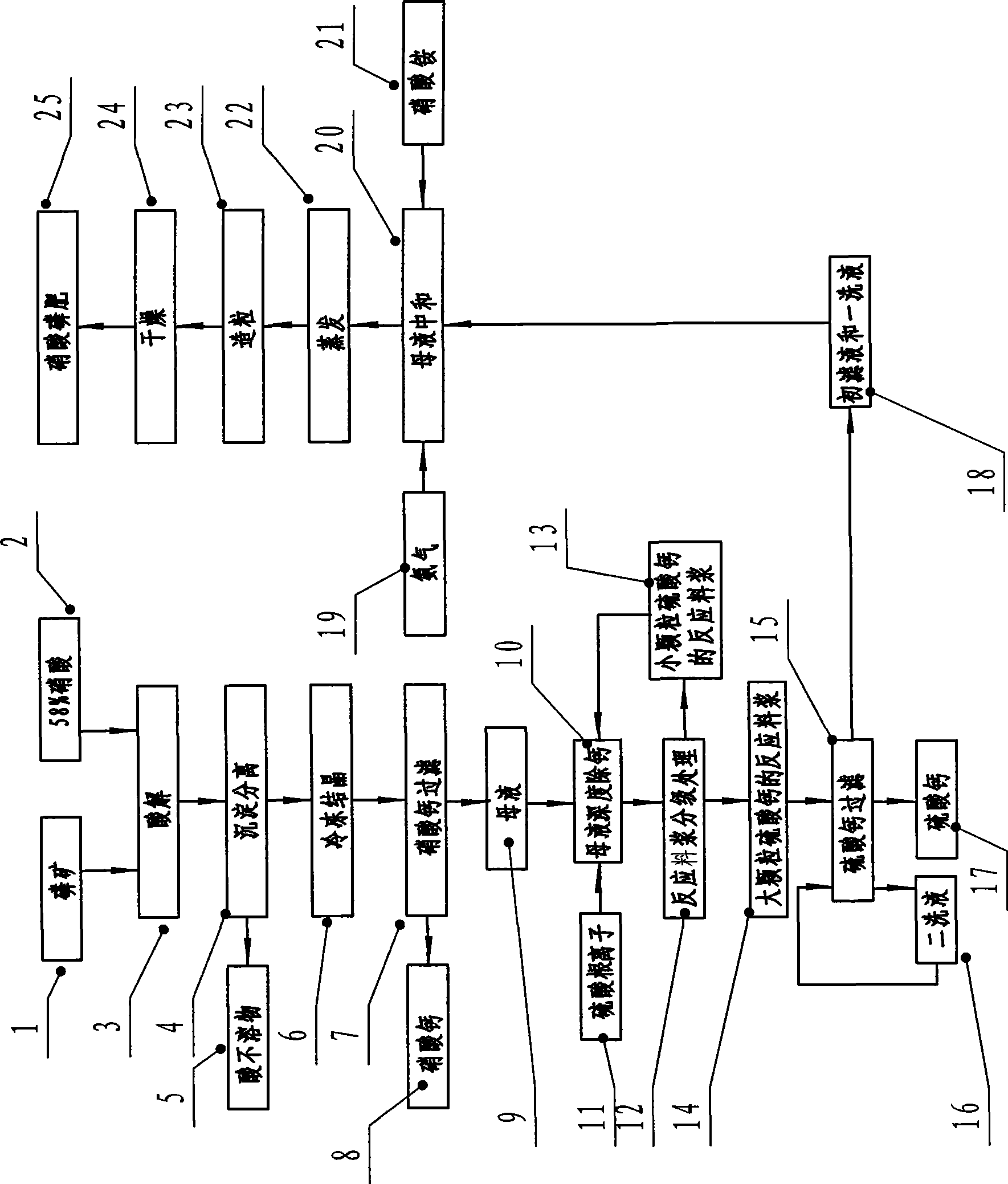
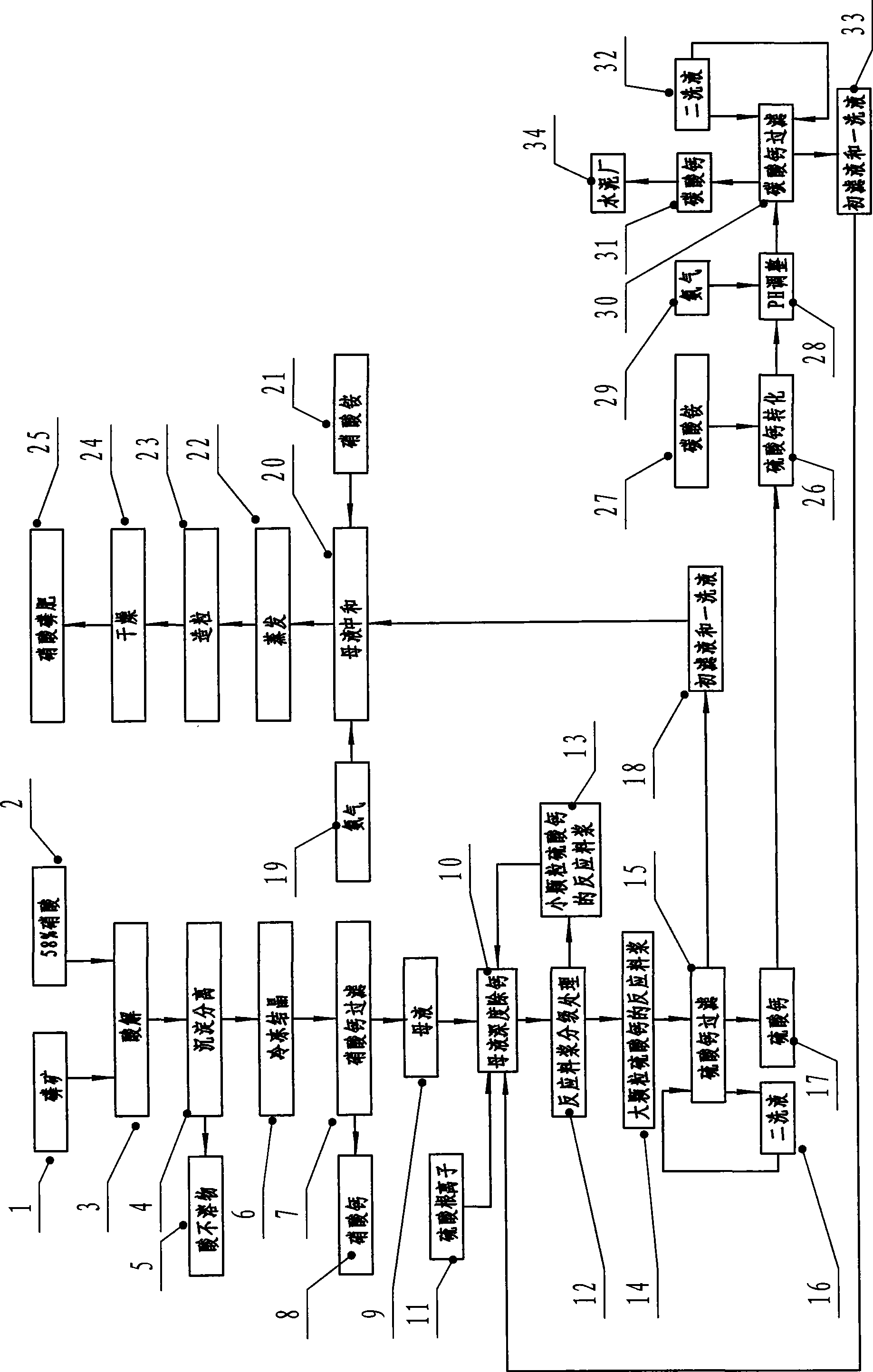
The invention discloses a technique method for producing high-concentration nitric phosphate, which comprises the following steps of: adding nitric acid into phosphorus ore for acidolysis, depositing and separating acid non-soluble substance, freezing and crystallizing calcium nitrate, filtering the calcium nitrate, neutralizing the mother liquid, vaporization, pelleting and drying. The invention is characterized in that: a step of thoroughly removing the calcium of the mother liquid is arranged between the steps of filtering the calcium nitrate, and neutralizing the mother liquid. The steps comprise the following steps of: I) thoroughly removing the calcium of the mother liquid and adding sulfuric acid or ammonium sulfate; the calcium ions and the sulfate ions in the mother liquid generate dihydrate calcium sulfate crystal; II) the grading processing of reaction slurry: employing a grading device to carry out grading processing to the reaction slurry; returning the reaction slurry provided with small grain calcium sulfate to the mother liquid for thoroughly removing the calcium, and feeding the reaction slurry provided with large grain calcium sulfate to the calcium sulfate for filtering; and III) filtering and washing the filtering reaction slurry of calcium sulfate by a filter, feeding the primary filtrate and the primary lavage fluid into the working procedure of neutralizing, and returning the secondary lavage fluid to the filter. The invention has the advantage of using middle-low quality phosphorite to prepare the high-concentration nitric phosphate.
Owner:TIANJI COAL CHEM IND GROUP +1
Liquid fertilizer with pyroligneous liquor and oxalacetic liquor and production thereof
InactiveCN1778773AMagnesium fertilisersFertilisers by pryogenic processesRare-earth elementPlant regulators
A liquid fertilizer containing wood vinegar liquid or oxalic vinegar liquid and its production are disclosed. The liquid fertilizer consists of urea 1í½20.0%, potassium phosphate 1í½20.0%, potassium nitrate 1í½20.0%, lime nitrate 0.1í½10.0%, magnesium sulfate 0.1í½5.0%, ferrous sulfate 0.1í½5.0%, manganous sulfate 0.1í½5.0%, zinc sulfate 0.1í½5.0%, cupric sulfate 0.1í½5.0%, boron sand or boric acid 0.1í½2.0%, sodium molybdate or ammonium molybdate 0.1í½1.0%, plant hormone or plant regulator, rare earth element or humus acid or pesticide auxiliaries etc. The process is carried out by proportioning and mixing. It can improve bacterium inhibiting and plant growth regulating functions.
Owner:ZHEJIANG JIANZHONG BAMBOO IND
Nitrate molten salt heat transferring and reserving medium and preparation method and application thereof
InactiveCN102533226AWide operating temperature rangeImprove thermal stabilityHeat-exchange elementsDecompositionInstability
The invention discloses a nitrate molten salt heat transferring and reserving medium and a preparation method and application thereof. The nitrate molten salt heat transferring and reserving medium is prepared with 5 to 40 percent of potassium nitrate, 5 to 25 percent of sodium nitrate, and 10 to 70 percent of calcium nitrate. The melting point of the nitrate molten salt heat transferring and reserving medium can be as low as 120 DEG C, and the upper limit of temperature for use can reach 550 DEG C, the nitrate molten salt heat transferring and reserving medium has a wide temperature scope of application, can work normally at the temperature scope of 180 DEG C to 550 DEG C, and has good thermal stability; the shortcoming of high melting point of binary nitrate molten salt system can be overcome, and the problem of instability caused by easy oxidative decomposition at high temperature of NaNO2 in the Chinese patent 200110027954.1 and ternary nitrate salt system can be solved, and the problems of corrosion and cost increase caused by the existing LiNO3 in the Chinese patent 00111406.9 and the American patent US007588694B1 can also be solved.
Owner:SUN YAT SEN UNIV +1
Preparation method and application method of grape special fertilizer
ActiveCN101693639AIncrease productionImprove qualityClimate change adaptationAlkali orthophosphate fertiliserSodium BentoniteAdditive ingredient
The invention relates to preparation method and application method of a grape special fertilizer, wherein the preparation method comprises the following steps: preparing an I-type fertilizer by taking the following ingredients in parts by weight: 30-40 parts of urea, 20-30 parts ammonium phosphate, 15-30 parts of potassium sulphate, 1-3 parts of humic acid, 1-3 parts of excrement, 1-5 parts of synergist, 0.1-1.0 part of ferrous sulphate, 0.1-0.8 part of zinc sulfate, 0.1-0.6 part of manganese sulphate, 0.1-0.6 part of borax, 0.1-0.4 part of copper sulfate, 3-7 parts of plaster and 3-13 parts of bentonite; preparing an II-type fertilizer by taking the following ingredients in parts by weight: 7-15 parts of urea, 25-35 parts ammonium phosphate, 35-45 parts of potassium sulphate, 1-3 parts of humic acid, 1-5 parts of excrement, 2-5 parts of synergist, 0.1-1.0 part of ferrous sulphate, 0.1-0.8 part of zinc sulfate, 0.1-0.6 part of manganese sulphate, 0.1-0.6 part of borax, 0.1-0.4 part of copper sulfate, 3-7 parts of plaster and 3-13 parts of bentonite; and blending, spraying, pelleting and drying at lower temperature to obtain the product. The application method of the fertilizer comprises the following steps: taking an organic fertilizer and the II-type fertilizer as a base fertilizer; applying the I-type fertilizer before blossom, applying the II-type fertilizer in the young fruit development period, and meanwhile, respectively spraying monopotassium phosphate and rare earth mixed liquid once on the leaf surfaces; and spraying calcium nitrate or calcium acetate to enhance the storage resistance property before picking.
Owner:新疆满疆红农资化肥科技有限公司
Calcium carbonate precipitation method
InactiveUS6036933ACalcium/strontium/barium carbonatesMagnesium carbonatesNitratePrecipitated calcium carbonate
PCT No. PCT / GB96 / 00488 Sec. 371 Date Oct. 22, 1997 Sec. 102(e) Date Oct. 22, 1997 PCT Filed Mar. 1, 1996 PCT Pub. No. WO96 / 26902 PCT Pub. Date Sep. 6, 1996A method for producing precipitated calcium carbonate by reacting an aqueous solution of calcium nitrate [Ca(NO3)2] with an aqueous solution of ammonium carbonate [(NH4)2CO3] and allowing calcium carbonate to precipitate from the resultant mixture containing nitrate [NH4NO3] in the mother liquor, the process being characterized in that: (i) the calcium nitrate [Ca(NO3)2)] solution utilized in the processes is prepared by slaking lime [CaO] in water in the presence of ammonium nitrate [NH4NO3] to form calcium nitrate [Ca(NO3)2] and ammonium hydroxide [NH4OH] in solution, filtering the solution to render it solids free, and heating the filtrate to dissociate the ammonium hydroxide [NH4OH] and to drive ammonia gas [NH3] from the solution; (ii) the ammonium carbonate (NH4)2CO3 solution utilized is prepared by absorbing ammonia gas [NH3] and carbon dioxide gas [CO2] in water, the ammonia gas preferably being derived from the step in (i) above in which the Ca(NO3)2 solution is heated; and (iii) the ammonium nitrate used is derived from the precipitation phase during which calcium carbonate is precipitated from the mother liquor containing ammonium nitrate.
Owner:PRETORIA PORTLAND CEMENT COMPANY +1
Method for producing dehydrated calcium sulfate whisker
InactiveCN101550585ANo emissionsReduce manufacturing costPolycrystalline material growthFrom normal temperature solutionsAnhydrous Calcium SulfateLixiviant
The invention provides a method for producing dehydrated calcium sulfate whisker, comprising: using copper, lead, zinc, aluminium, nickel, cobalt, stannum, non-ferrous metal secondary resource cycle utilizing, calcium nitrate solution produced in treating process of electroplating waste, calcium chloride solution, or mixed water solution of calcium nitrate and calcium chloride as raw material, using sulfuric acid as calcium ion precipitant, adding whisker shape regulating agent, controlling reaction condition, regenerating aqua fortis or hydrochloric acid lixiviant, and simultaneously producing ultralong and big length to diameter ratio calcium sulphate dihydrate whisker. After calcium sulphate dihydrate whisker is deposited from calcium nitrate solution, calcium chloride solution or the mixed solution of calcium nitrate and calcium chloride are deposited, the water solution is translated to be nitric acid solution, hydrochloric acid solution or a mixed solution of nitric acid and hydrochloric acid, returning back to leaching out or treating process for processing non-ferrous metal mineral substance, non-ferrous metal secondary resource or electroplating remove. The calcium sulphate dihydrate whisker is treated by microwave heating, forming calcium sulfate hemihydrate or anhydrous sulphate of calcium after dehydration.
Owner:BEIJING GENERAL RES INST OF MINING & METALLURGY
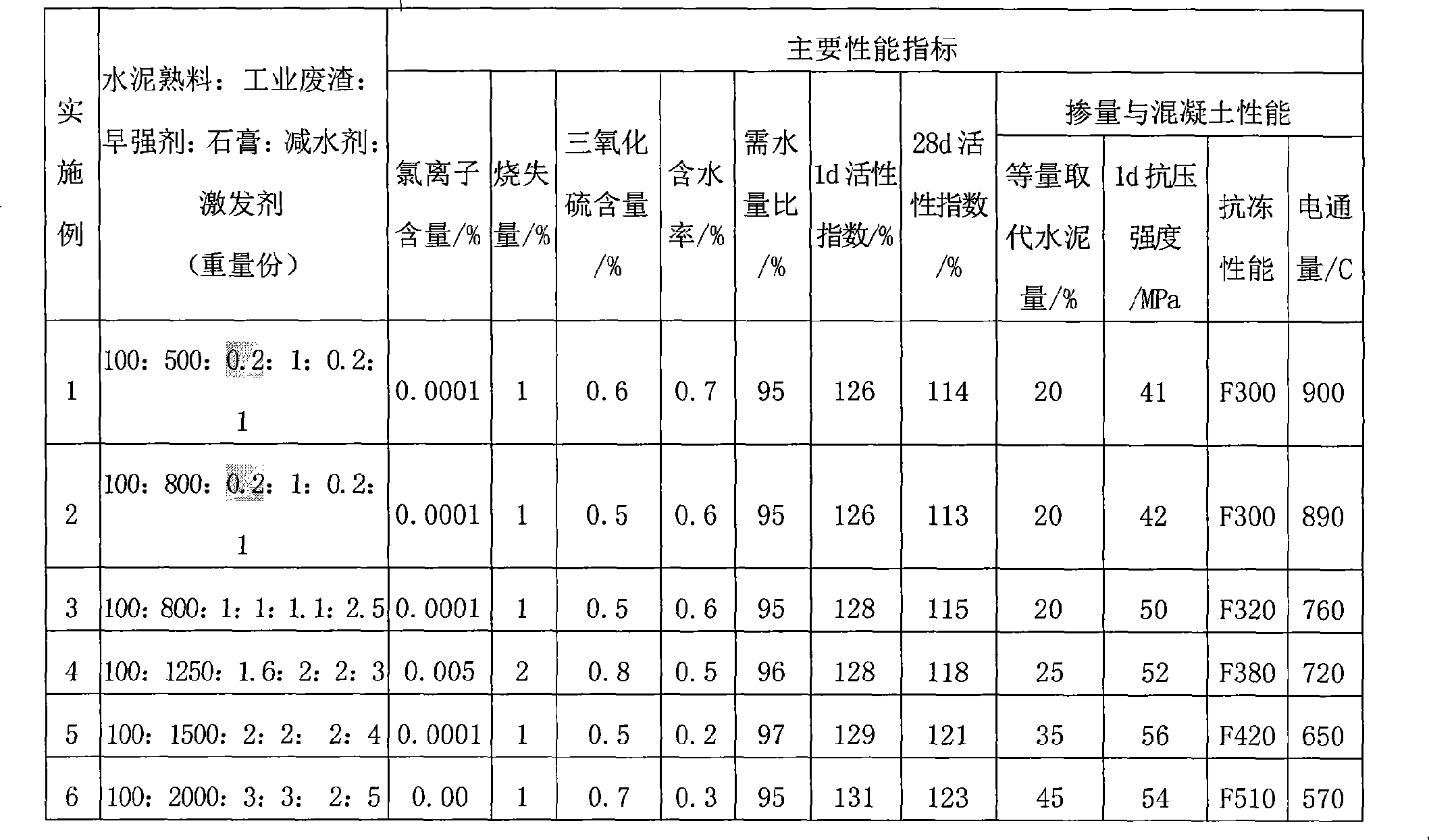
Blending material for high-early-strength concrete
InactiveCN101508557AImprovement of early strength and high strengthReduce dosageSolid waste managementHigh strength concreteCalcium formate
The invention relates to an admixture for high early strength of concrete, characterized in that the admixture is formed by mixing and stirring cement clinkers, industrial residues, early strength agents, plaster, water reducing agents and excitants. The parts by weight of the components are as follows: 100 parts of cement clinkers, 500-2000 parts of industrial residues, 0.2-3 parts of early strength agents, 1-3 parts of plaster, 0.2-2 parts of water reducing agents and 1-5 parts of excitants. The excitant is the mixture of aluminum hydroxide, calcium nitrite and calcium nitrate; wherein, the aluminum hydroxide accounts for 50% of the mixture while the calcium nitrite and the calcium nitrate are in any proportion; the early strength agent is the mixture of trolamine, lithium carbonate and calcium formate, wherein, the trolamine accounts for 50% of the mixture while the lithium carbonate and the calcium formate are in any proportion. The admixture for concrete provided by the invention has high activity index and low content of harmful ions. Adding the admixture can obviously improve the demoulding strength, long-term mechanical property and durability of the concrete.
Owner:RAILWAY ENG RES INST CHINA ACADEMY OF RAILWAY SCI +1
Recycling method for treating stainless steel pickling acid pickle and wastewater
ActiveCN104310647AHigh operating costsSimple processWater treatment compoundsWaste water treatment from metallurgical processWash waterLaterite
The invention relates to a recycling method for treating stainless steel pickling acid pickle and wastewater, belonging to the field of treatment of industrial acid pickle and wastewater in iron and steel and metallurgy industries. The acid pickle and wash wastewater generated during stainless steel pickling are collected according to quality and category and the acid pickle is subjected to acid recycling. The acid pickle and wash wastewater are treated through fractional precipitation by adopting lime as a neutralizing agent. Calcium sulfate or calcium fluoride or a mixture of calcium sulfate and calcium fluoride is obtained through precipitation under the acidic condition (the pH value is equal to 3.0+ / -0.5) and can serve as a cement filler or a material for brickmaking or a building material after heavy metal polluted calcium salt is removed. A metal hydroxide is obtained through precipitation under the alkaline condition (the pH value is not more than 9.0) and can serve as a stainless steel smelting raw material as the quality of the product is better than that of the imported laterite-nickel ores in China. The calcium nitrate containing wastewater obtained after the acid pickle is treated is not needed to be discharged or treated and can be recycled. After being treated, the wash wastewater can be reused as production line wash water.
Owner:上海博士高环保科技有限公司
Plant nutrient solution and application in plant cultivation
InactiveCN101062877AImprove survival rateDoes not affect life cycleMagnesium fertilisersAlkali orthophosphate fertiliserManganeseMonopotassium phosphate
The invention discloses a plant nutrition liquid, which is characterized by the following: comprising potassium nitrate, calcium nitrate, magnesium sulfate, ammonium phosphate, potassium sulfate, monopotassium phosphate and so on a good deal of element; comprising edetate disodium, ferrosi sulfas, boric acid, manganese sulfate, zinc sulfate, copper sulfate, ammonium molybdate and so on microelement; comprising azophoska fertilizer and fertilizer booster; including diverse nutritious element of vegetation development; providing nourishing element for large and medium scale bush and small arbor. This invention also discloses a utility scheme in large and medium scale woody plant soilless culture.
Owner:成都市第二农业科学研究所 +1
Method for preparing nano hydroxyapatite powder
InactiveCN101734635ANo pollution in the processNo harmNanostructure manufacturePhosphorus compoundsWater bathsPolyethylene glycol
The invention relates to a method for preparing nano hydroxyapatite powder, belonging to the field of the biomedical material. The method for preparing the nano hydroxyapatite powder comprises the following steps: adding the organic polymer of polyethylene glycol to the initial reactants of calcium nitrate and diammonium phosphate solution to adjust and control the morphologies of the products of reaction; adding aqueous ammonia to adjust the pH values of the products of reaction, and making the aqueous ammonia react with the products of reaction at different water bath temperature to synthesize nano hydroxyapatite precipitate, wherein the agglomeration of the synthesized nano hydroxyapatite precipitate can be inhibited by the polyethylene glycol functioning as the dispersant; freezing and drying synthesized nano hydroxyapatite precipitate slurry to obtain dry nano hydroxyapatite powder which contains by-products of reaction and polyethylene glycol, and calcinating the dry nano hydroxyapatite powder at high temperature to remove the by-products of reaction and the polyethylene glycol which are contained in the dry nano hydroxyapatite powder to obtain the nano hydroxyapatite powder which is in the shape of the ball or the rod and in the particle size of 10-100 nm and has high yield and good dispersion. The method for preparing the nano hydroxyapatite powder is simple, needs short preparing period, causes no poison and pollution and is suitable for industrial production.
Owner:SICHUAN UNIV
Environment-protecting synergistic agent for fuel coal
InactiveCN101440328AIncreased flammable contentIncreased space gapSolid fuelsFuel additivesSodium bicarbonateFurnace temperature
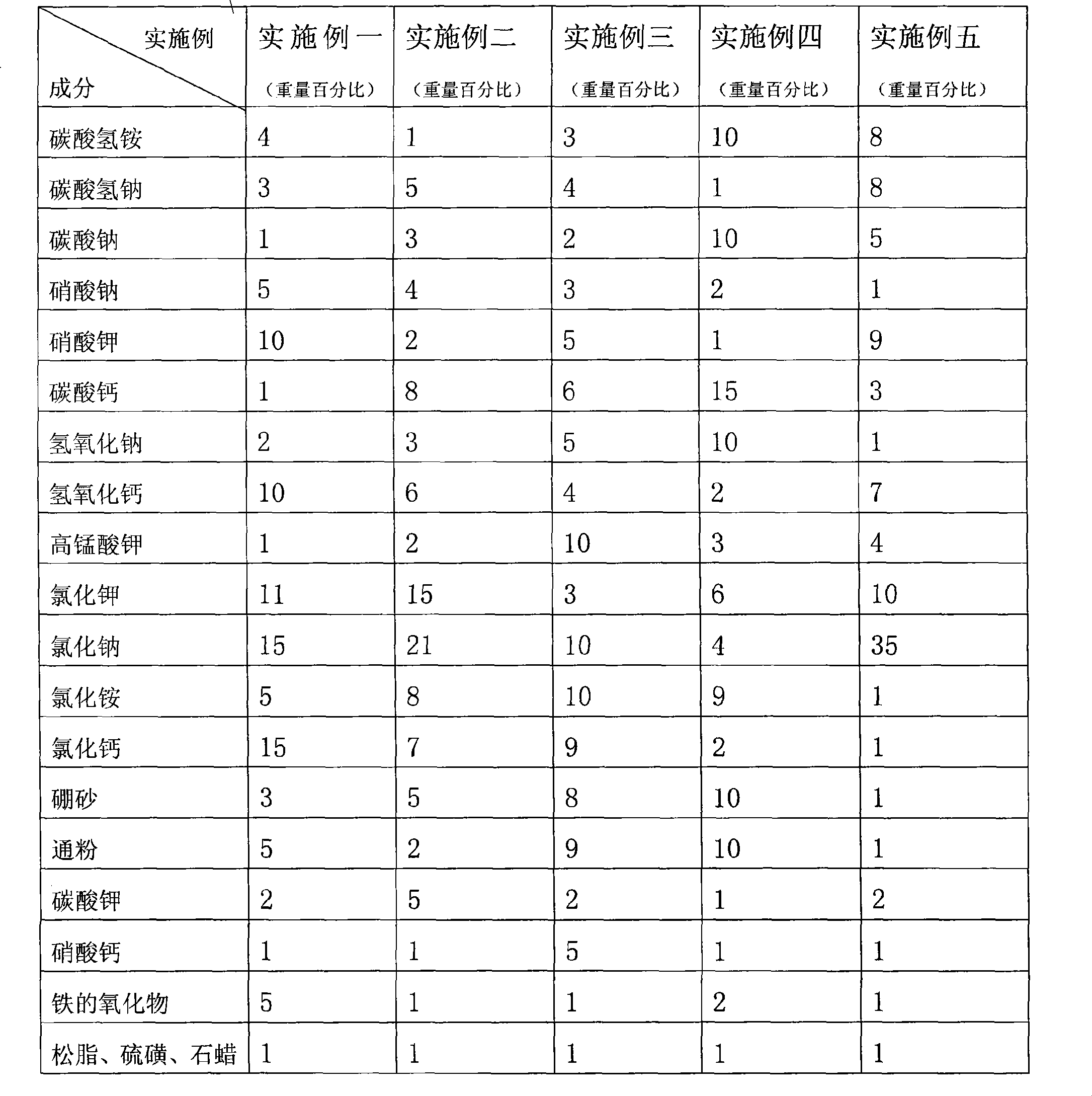
The invention discloses a bunker coal environment-friendly synergist, which comprises the following components by weight percent: 1 to 10 percent of ammonium bicarbonate, 1 to 8 percent of sodium bicarbonate, 1 to 10 percent of sodium carbonate, 1 to 5 percent of sodium nitrate, 1 to 10 percent of potassium nitrate, 1 to 15 percent of calcium carbonate, 1 to 10 percent of sodium hydroxide, 2 to 10 percent of calcium hydroxide, 1 to 10 percent of potassium permanganate, 3 to 15 percent of potassium chloride, 10 to 35 percent of sodium chloride, 1 to 10 percent of ammonium chloride, 1 to 15 percent of calcium chloride, 1 to 10 percent of borax, 1 to 10 percent of macaroni, 1 to 5 percent of potassium carbonate, 1 to 5 percent of calcium nitrate, 1 to 5 percent of iron oxide, and the balance being micro turpentine, sulfur and paraffin wax. The bunker coal environment-friendly synergist makes flame of combustion be more rampant and denser, the furnace temperature rise to a higher level, the hearth be brighter and cleaner and tail gas be cleaner.
Owner:李政 +1
Mixed molten salt as heat transfer and storage medium low in melting point
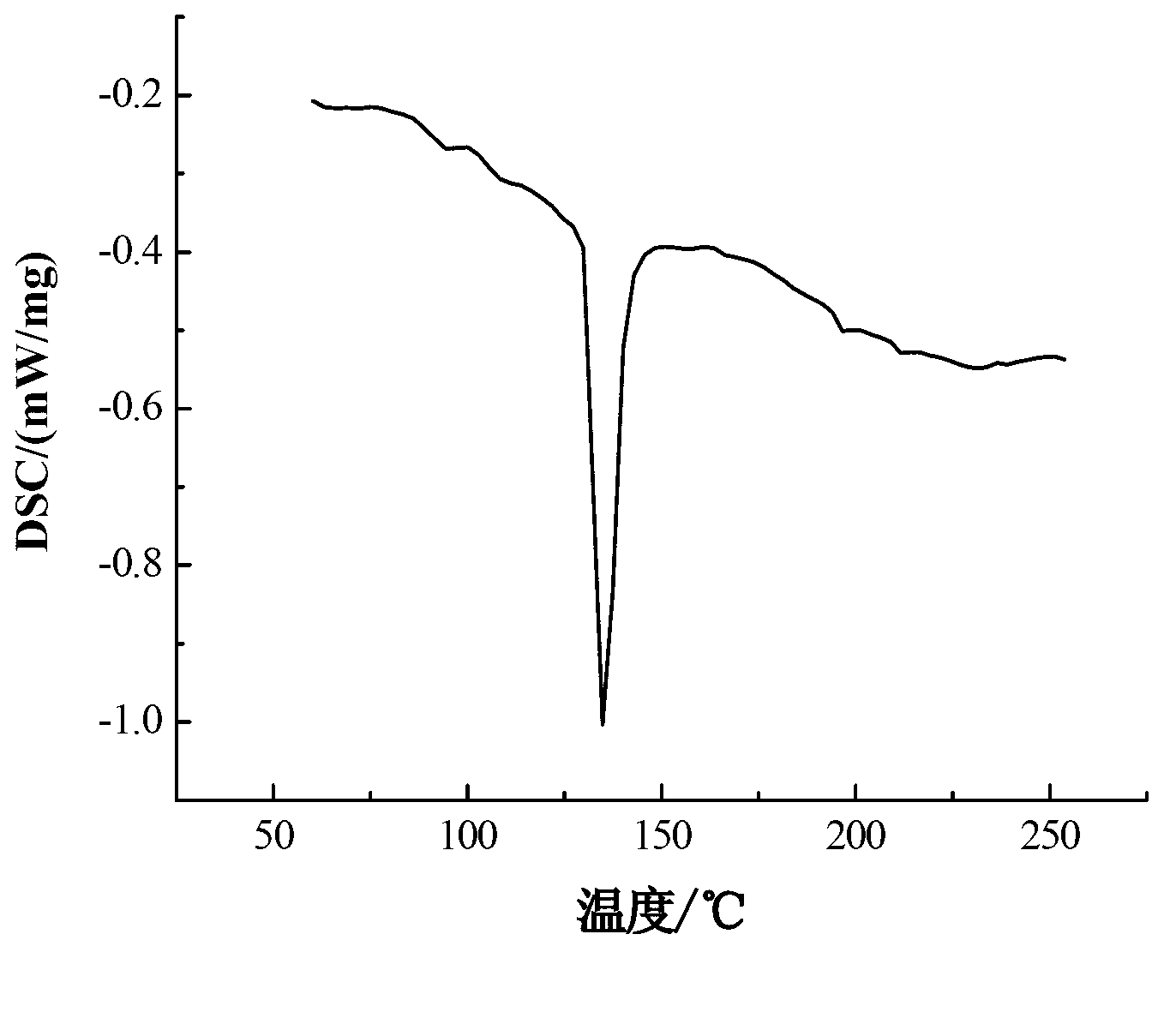
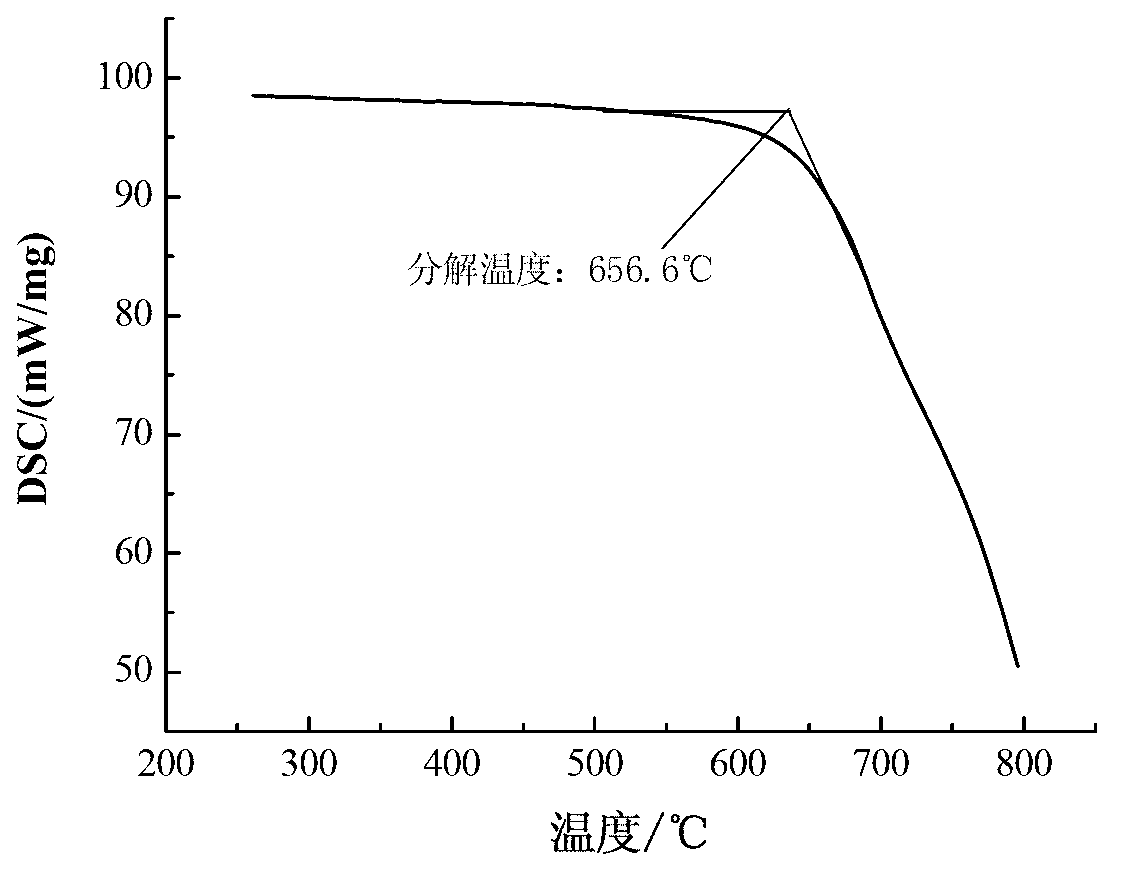
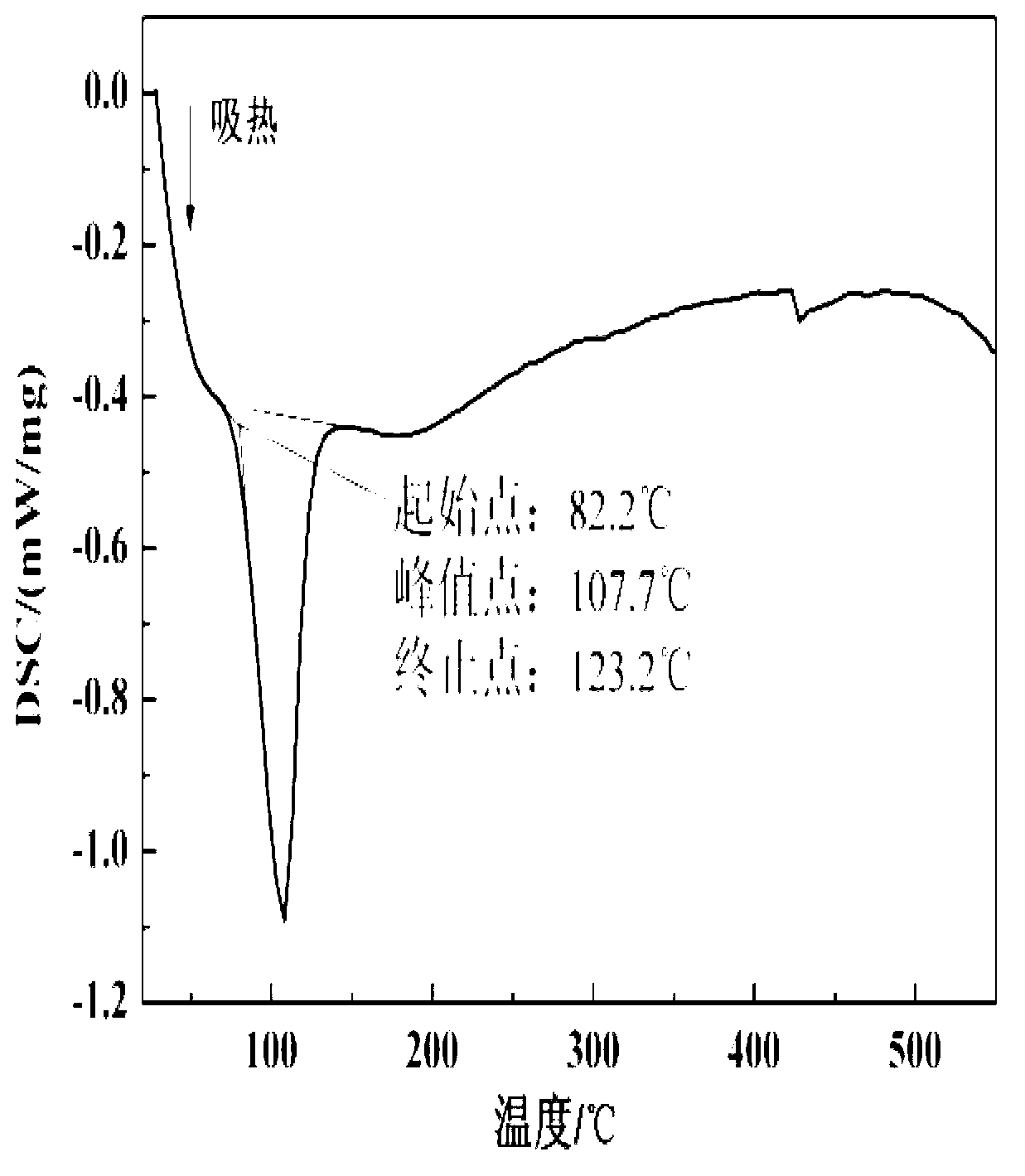
The invention relates to a formula of a mixed molten salt for medium-high temperature heat transfer and storage, and belongs to the physical heat transfer and energy storage technology in innovative and high technologies. The mixed molten salt comprises components in a ratio as follows: 10wt% of calcium nitrate, 60-70 wt% of potassium nitrate, 10-20 wt% sodium nitrate and 10wt% of sodium nitrite; the melting point of the mixed molten salt is about 130 DEG C, which is reduced by nearly 90 DEG C relative to solar salt and is reduced by about 15 DEG C relative to Hitec salt; and the thermal decomposition temperature thereof reaches above 650 DEG C. Sodium nitrate in the molten salt is changed into lithium nitrate, and the specific component ratio is as follows: 18-20wt% of calcium nitrate, 50-55 wt% of potassium nitrate, 9-10 wt% sodium nitrate and 18-20wt% of sodium lithium nitrate; after the component ratio is changed, the melting point of the mixed molten salt is about 90 DEG C, which is reduced by nearly 130 DEG C relative to the solar salt and is reduced by about 50 DEG C relative to the Hitec salt; and the thermal decomposition temperature thereof reaches above 600 DEG C. 10wt% of sodium carbonate is added continuously, then the melting point is raised to about 110 DEG C, while the decomposition temperature is raised by nearly 20 DEG C.
Owner:河北井矿新能源科技有限公司
Water culture nutrient solution prescription of lettuce and preparation method of water culture nutrient solution prescription
InactiveCN103172431ASmooth changeMeet the changeFertilizer mixturesPhosphoric acidMonopotassium phosphate
The invention discloses a water culture nutrient solution prescription of a lettuce and a preparation method of the water culture nutrient solution prescription, and belongs to the technical field of plant soilless culture of the agricultural science. The nutrient solution prescription of the lettuce comprises the following components: a fertilizer A composed of 584.23g / T of calcium nitrate, 421.34g / T of potassium nitrate and 47.8g / T of ammonium nitrate; a fertilizer B composed of 182.4g / T of monopotassium phosphate and, 256.23g / T of magnesium sulfate; a fertilizer C composed of 16.8g / T of FeEDTA (Ethylene Diamine Tetraacetic Acid), 3g / T of boric acid, 2g / T of manganese sulfate, 0.25g / T of zinc sulfate, 0.85g / T of copper sulfate, and 0.38g / T of ammonium molybdate; hormone composed of 0.1g / T indolebutyric acid of or 0.1g / T of naphthylacetic acid; a bactericide composed of 15g / T of carbendazim; and nutrient solution pH (Potential Of Hydrogen) adjusting acid composed of 200ml / T of phosphoric acid or 150g / T of citric acid. The prescription is nutritionally balanced, so that the demand of the lettuce to nutrition can be met, the amount of used nutrient elements is decreased at the same time, the output of the lettuce is increased, and the fertilizers are saved; and the prescription effectively decreases the contents of nitrates in the lettuce under the water culture, improves the quality of the lettuce, enables the simplification of the complicated processes in preparation based on the demand of the actual operation, and is suitable for being used for meeting the requirements of industrialization production.
Owner:NORTHWEST A & F UNIV
Antitranspirant foliar fertilizer and preparation method thereof
ActiveCN101913941AThe ratio is scientific and reasonableImprove solubilityFertilizer mixturesMicrobial agentMonopotassium phosphate
The invention discloses an antitranspirant foliar fertilizer and a preparation method thereof. The antitranspirant foliar fertilizer comprises the raw materials of copper sulfate, ethylene diamine tetraacetic acid, monopotassium phosphate, zinc sulfate, ferrous sulphate, manganese sulphate, ammonium molybdate, composite rare earth, boric acid, sodium dodecyl sulphonate, urea, fulvic acid, hexanoic acid 2-(diethylamino) ethyl ester, compound amino acid, magnesium sulfate, calcium nitrate, ethylene diamine tetraacetic acid, microbial agent, azone and ammonia water. The antitranspirant foliar fertilizer provides nutrient elements and microbial agents which are required by the growth of plants, the rare earth and amino acid and other beneficial elements, can promote the growth of the plants, improve the yield, the quality, the stress resistance of the plants and the absorbing and operating capabilities on trace elements, simultaneously can effectively reduce the transpiration of the plants and is applied to afforesting in arid regions.
Owner:SHENZHEN TECHAND ECOLOGY & ENVIRONMENT CO LTD
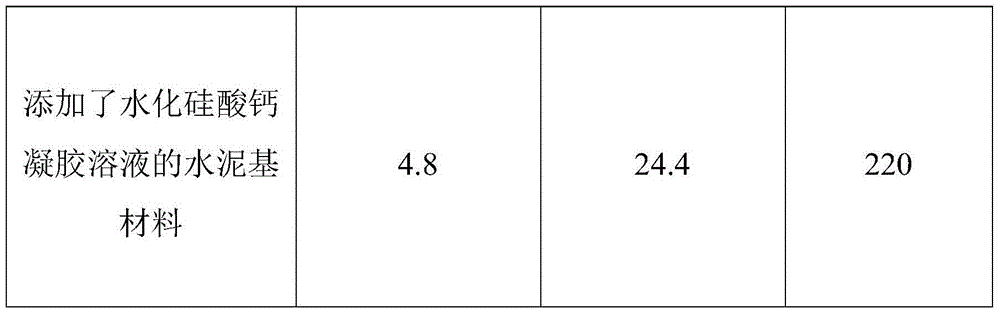
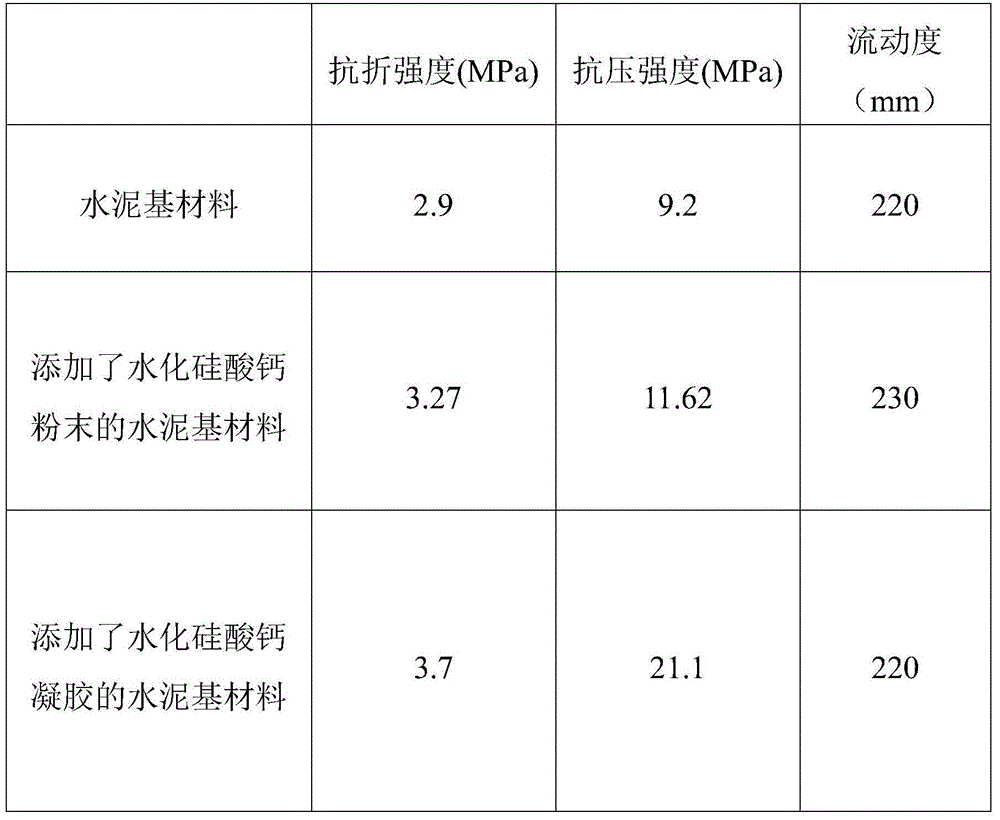
Calcium silicate hydrate gel solution early strength agent and preparation method thereof
ActiveCN104402009ALong term storageGood dispersionAlkaline-earth metal silicatesCalcium in biologyCalcium silicate
The invention discloses a calcium silicate hydrate gel solution early strength agent and a preparation method thereof, and relates to the technical field of construction material production. The early strength agent and the method are used for solving problems of complicated preparation process, high cost, storage difficulty, and poor early strength effect of traditional calcium silicate hydrate powder. The calcium silicate hydrate gel solution early strength agent comprises a calcareous material, a siliceous material, a gel dispersant, and water. A weight ratio of the calcareous material to the siliceous material is 0.3-3:1. A weight ratio of water to the total of the calcareous material and the siliceous material is 5-30:1. The mass percentage of the gel dispersant is 0.1-2.0%. The preparation method of the early strength agent comprises the following steps: reaction materials are weighed; a sodium silicate water solution and a calcium nitrate water solution are respectively prepared; the two are mixed and are subjected to a reaction, such that a gel mixture solution is obtained; suction filtration is carried out; and water and the dispersant are added for re-dispersion. The early strength agent is uniform and stable, and is convenient to store. The early strength agent is highly convenient to use, and has a good early strength effect. With the early strength agent, an alkali-aggregate reaction risk can be effectively reduced.
Owner:SHANGHAI CONSTRUCTION GROUP
Dry farm cultivating agent
The dry-land planting agent refers to a kind of droughtresistance crop's nutrient specific to the growth of the plants. The invention is composed of the following components: AU absorber with super strength, carbamide, potassium dihydrogen phosphate, ammonium dibasic phosphate, calcium nitrate, bitter salt, ammonium molybdate, zinc sulfate, manganese sulfate, boracic acid or borax, ferrous sulphate, fulvic acid potassium, disodium edta, fatty alcohol polyethenoxy ether, 6- benzyl aminopurine, melissyl alcohol, potassium naphthylacetic acid, indolebutyric acid, gibberellin, santobrite, potassium sorbate, ketotriazole, penta azole alcohol., thiram, carbendazim, acid brilliant scarlet, humic acid, bluestone, potassium permanganate, potassium chloride, polyethylene glycol, bentonite or water. The invention is featured by sopping, molding moisture, molding fertilizer, withering resistance, disinsection, sterilization, strengthening the effects of the pesticide, accelerating the burgeon of the plants, increasing roots, innocuity, no pollution and low cost.
Owner:王亚玲
Calcium silicate hydrate gel solution early strength agent and preparation method thereof
InactiveCN104402288ALong term storageMaintain dispersion uniformityCalcium silicateCalcium in biology
The invention discloses a calcium silicate hydrate gel solution early strength agent and a preparation method thereof, and relates to the technical field of construction material production. The early strength agent and the method are used for solving problems of complicated preparation process, high cost, storage difficulty, weighing difficulty, and poor early strength effect of traditional calcium silicate hydrate powder. The calcium silicate hydrate gel solution early strength agent comprises a calcareous material, a siliceous material, a gel dispersant, and water. A weight ratio of the calcareous material to the siliceous material is 0.3-3:1. A weight ratio of water to the total of the calcareous material and the siliceous material is 5-30:1. The mass percentage of the gel dispersant is 0.01-0.50%. The preparation method of the early strength agent comprises the following steps: reaction materials are weighed; a sodium silicate water solution and a calcium nitrate water solution are respectively prepared; the gel dispersant is respectively added into the solutions; and the two solutions are mixed and allowed to react for a certain period of time. The early strength agent is convenient to store, transport and weigh, and have good product stability. With the early strength agent, cement-based material early strength can be effectively improved.
Owner:SHANGHAI CONSTRUCTION GROUP
Product for and method of controlling odor in open waste water treatment environments
A product for substantially eliminating existing foul odors (such as those associated with materials such as hydrogen sulfide) and substantially preventing the production of new foul odors in matter, in open treatment environments is disclosed and comprises calcium nitrate and calcium carbonate.
Owner:WEGNER PAUL
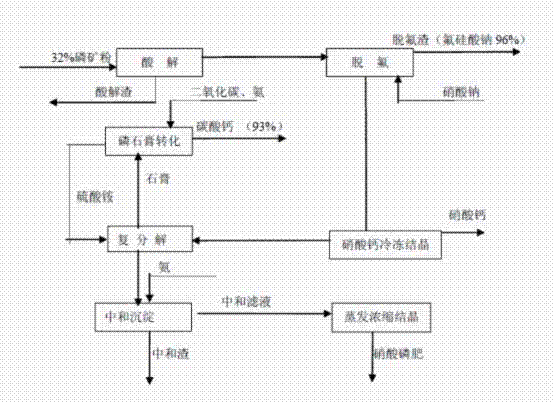
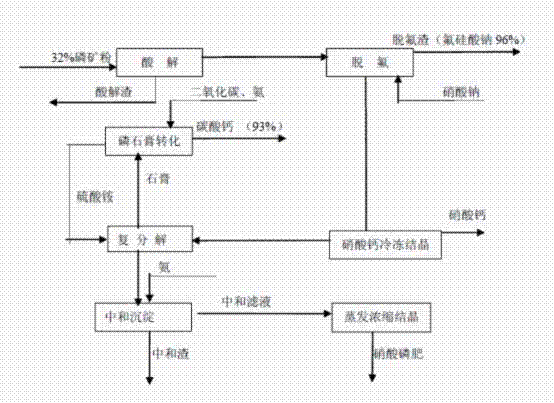
Method for producing high-water solubility nitrophosphate fertilizer by phosphorite nitrolysis and calcium nitrate freezing
InactiveCN102351590APromote absorptionImprove qualityCalcium/strontium/barium carbonatesFertilizer mixturesSolubilityChemical industry
The invention discloses a method for producing high-water solubility nitrophosphate fertilizer by phosphorite nitrolysis and calcium nitrate freezing. The high-water solubility nitrophosphate fertilizer is prepared from the processes of phosphorite nitrolysis, fluorine removal, calcium nitrate freezing separation, further removal of calcium in ammonium sulfate, ammonia liquor neutralization and deposition removal of impurities, and evaporation concentration and crystallization. Specially, the method for producing high-water solubility nitrophosphate fertilizer by phosphorite nitrolysis and calcium nitrate freezing comprises the following steps of 1, acidolysis, 2, fluorine removal, 3, calcium nitrate freezing crystallization, 4, double decomposition removal of calcium in ammonium sulfate,5, neutralization and deposition removal of impurities, 6, evaporation concentration and crystallization, and 7, phosphogypsum transformation. The high-water solubility nitrophosphate fertilizer prepared by the method has a dissolution rate great than or equal to 99.9% in water, wherein nitrate nitrogen is easy for be absorbed by drops, satisfies requirements of a few commercial crops, has a highutilization rate, and improves a crop yield by 8 to 25% and crop quality. The method reduces waste discharge, is environmentally friendly, can be utilized for structure adjustment and product upgradeand update of the traditional phosphorus chemical industry, and has good economic and environmental benefits.
Owner:GUIZHOU RES INST OF CHEM IND
Compound microbial preparation for treating black and odorous rivers through strengthened calcium nitrate
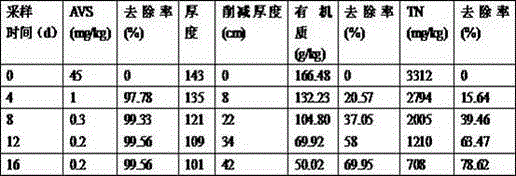
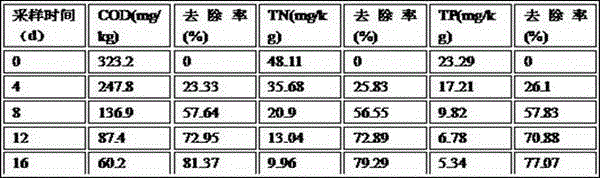
InactiveCN105420147APromote growthGood occupationWater treatment parameter controlFungiSludgeWater quality
The invention relates to a compound microbial preparation for treating black and odorous rivers through strengthened calcium nitrate and a preparation method thereof. The use amount of calcium nitrate is reduced, and the time for treating the black and odorous rivers is shortened. The compound microbial preparation is prepared from 40-80 parts of thiobacillus denitrificans and thiocapsa roseoppersicina mixed powder, 10-60 parts of composite bacillus subtilis powder and 10-60 parts of yeast and lactic acid bacterium mixed powder. By means of the preparation, sulfide in black and odorous bottom sludge, organic matter and strains suitable for growing in the black and odorous bottom sludge are screened and degraded in a targeted mode; by means of thiobacillus denitrificans with efficient hydrogen sulfide degrading and denitrification capacity, sulfur-oxidizing bacteria of thiocapsa roseoppersicina with efficient hydrogen sulfide and ammoniacal nitrogen degrading capacity, bacilli rich in protease, amylase, cellulose and other enzyme systems, yeast and lactic acid bacteria, a small amount of calcium nitrate and the compound microbial preparation are thrown into the black and odorous rivers so that water quality of the black and odorous rivers can be quickly improved, the black and odorous bottom sludge can be removed quickly, and the thickness and the organic matter content of the bottom sludge can be quickly reduced.
Owner:胡艳晖
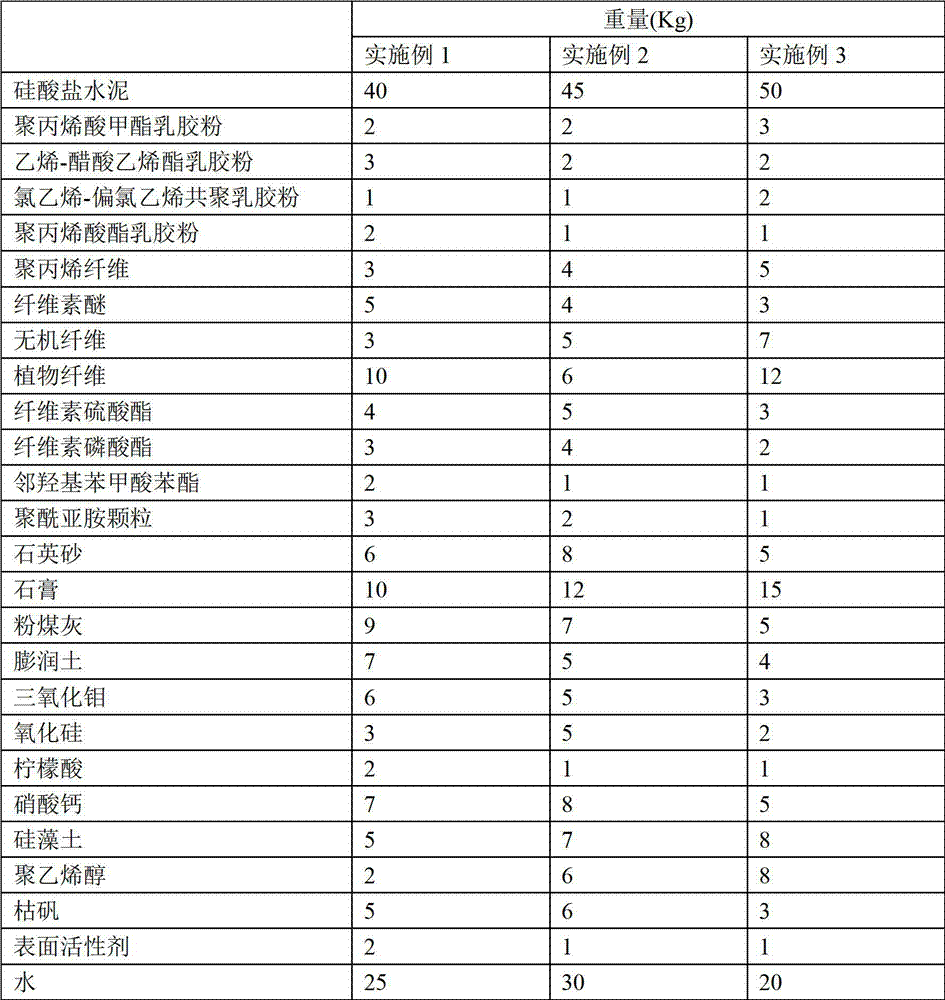
Corrosion-resistant polymer waterproof mortar
The invention provides a corrosion-resistant polymer waterproof mortar, which comprises the following components by weight: 40-50 parts of portland cement, 2-3 parts of polymethyl acrylate latex powder, 2-3 parts of ethylene-vinyl acetate latex powder, 1-2 parts of vinyl chloride-vinylidene chloride copolymerized latex powder, 1-2 parts of polyacrylate latex powder, 3-5 parts of polypropylene fibers, 3-5 parts of cellulose ether, 3-7 parts of inorganic fibers, 6-12 parts of plant fibers, 3-5 parts of cellulose sulfate, 2-4 parts of cellulose phosphate, 1-2 parts of phenyl salicylate, 1-3 parts of polyimide particles, 5-8 parts of quartz sand, 10-15 parts of gypsum, 5-9 parts of fly ash, 4-7 parts of bentonite, 3-6 parts of molybdenum trioxide, 2-5 parts of silicon oxide, 1-2 parts of citric acid, 5-8 parts of calcium nitrate, 5-8 parts of diatomite, 2-8 parts of polyvinyl alcohol, 3-6 parts of dried alum, 1-2 parts of a surfactant, and 20-30 parts of water.
Owner:WUJIANG TIANYUAN PLASTIC
Aqueous culture method of anoectochilus
InactiveCN102100171AAbsorb evenlyAdequately meet nutritional needsCultivating equipmentsSoilless cultivationBiotechnologyCalcium nitrate tetrahydrate
The invention relates to an aqueous culture method of anoectochilus. In the method, anoectochilus germchits are cleaned and sterilized, transplanted in a culture dish and cultured in a culture groove filled with an aqueous culture solution; each 10L of aqueous culture solution contains the following components: 650 to 800mg of calcium nitrate tetrahydrate, 50 to 60mg of ammonium nitrate, 280 to 350mg of potassium nitrate, 100 to 130mg of potassium dihydrogen phosphate, 350 to 380mg of magnesium sulfate, 0.5 to 0.8mg of potassium iodide, 2 to 5mg of manganese sulfate, 0.2 to 0.3mg of sodium molybdate, 0.1 to 0.2mg of cobalt chloride, 2 to 4mg of boric acid, 0.5 to 1mg of zinc sulfate, 0.3 to 0.5mg of copper sulfate and 3 to 5ml of iron salt solution, wherein the iron salt solution is prepared by mixing water, green vitriol and ethylenediamine tetraacetic acid disodium. The method has the advantages of lowering production cost, simplifying production process, improving the growth of anoectochilus plants and improving the survival rate of anoectochilus plants.
Owner:福建永安天奇健金线莲生态实业有限公司
Anti-freeze aerated concrete brick
The invention discloses an anti-freeze aerated concrete brick. The anti-freeze aerated concrete brick is prepared from the following raw materials in parts by weight: 35-40 parts of fly ash, 15-20 parts of cement, 15-20 parts of wollastonite powder, 5-10 parts of modified straw ash, 2-4 parts of gypsum, 0.05-0.1 part of aluminum powder, 2-4 parts of urea, 0.01-0.03 part of oxyparaffin soap, 1-2 parts of sugar calcium, 4-6 parts of calcium formate, 0.1-0.5 part of polyethylene wax powder, 4-6 parts of calcium carbonate, 0.2-0.4 part of calcium nitrate, 0.1-0.2 part of sodium acetate and right amount of water.
Owner:潘逸东
Composite liquid fertilizer for injection of fruit stem and its production process
The liquid fertilizer contains several nutritious elements and is compounded with urea, potassium nitrate, ferrous sulfate, zinc sulfate, magnesium sulfate, manganese sulfate, boric acid, potassium dihydrogen phosphate, calcium nitrate, copper sulfate, citric acid and water. It has less pollution of soil and underground water, can regulate the balance between the nutritious elements inside tree body and may be produced in simple process, at low cost and high production efficiency.
Owner:NORTHWEST A & F UNIV
Alkaline fertilizer
InactiveCN104761322AGood characterPrevent acidificationFertilizer mixturesPotassium hydroxideManganese
The invention discloses an alkaline fertilizer composed of the following components in percentage by weight: 31-99.4% of macroelements, 0.5-50% of medium elements, 0.1-15% of microelements and 0-50% of synergic components, wherein the macroelements include two and even more selected from urea, diammonium phosphate, ammonium polyphosphate, dipotassium phosphate, tripotassium phosphate, potassium pyrophosphate, potassium tripolyphosphate, potassium nitrate, potassium carbonate, potassium sulfate, potassium chloride, potassium formate, potassium silicate and potassium hydroxide; the medium elements are one or more selected from calcium nitrate, calcium chloride, magnesium chloride, magnesium sulfate, EDTA calcium and EDTA magnesium; the microelements are one or more selected from ferrous sulfate, zinc sulfate, manganese sulfate, copper sulfate or an EDTA chelate of iron, zinc, manganese and copper, EDDHA iron, borax, disodium octaborate tetrahydrate and sodium molybdate; and the synergic components include one or more selected from sodium humate, potassium humate, sodium fulvate, potassium fulvate, sodium alginate, potassium alginate and a plant growth regulator.
Owner:于书强
Plant nutrient solution
InactiveCN103724072AWill not polluteCause some damagesCultivating equipmentsSoilless cultivationSodium iodideNutrition
The invention provides a plant nutrient solution which consists of the following components: potassium nitrate, urea, ammonium nitrate, magnesium sulphate, ammonium dihydrogen phosphate, calcium nitrate, manganese sulfate, zinc sulfate, ferric citrate, boric acid, sodium molybdate, sodium iodide, folic acid, asparagine and deionized water. According to the invention, the plant nutrient solution is comprehensive in nutrition, has no poisonous substance, cannot pollute the environment after being used, cannot cause harm to human body, and is free from undesirable odor. Through the adoption of the plant nutrient solution to cultivate foliage plants such as monstera deliciosa and rohdea japonica without soil, the leaf color is dark green, the growth is vigorous, death is hard, and compared with the conventional plant nutrient solution in the market, the growth rate is increased by 20%.
Owner:GUANGXI UNIVERSITY OF TECHNOLOGY
Preparation method of hydroxy phosephorite hollow microball
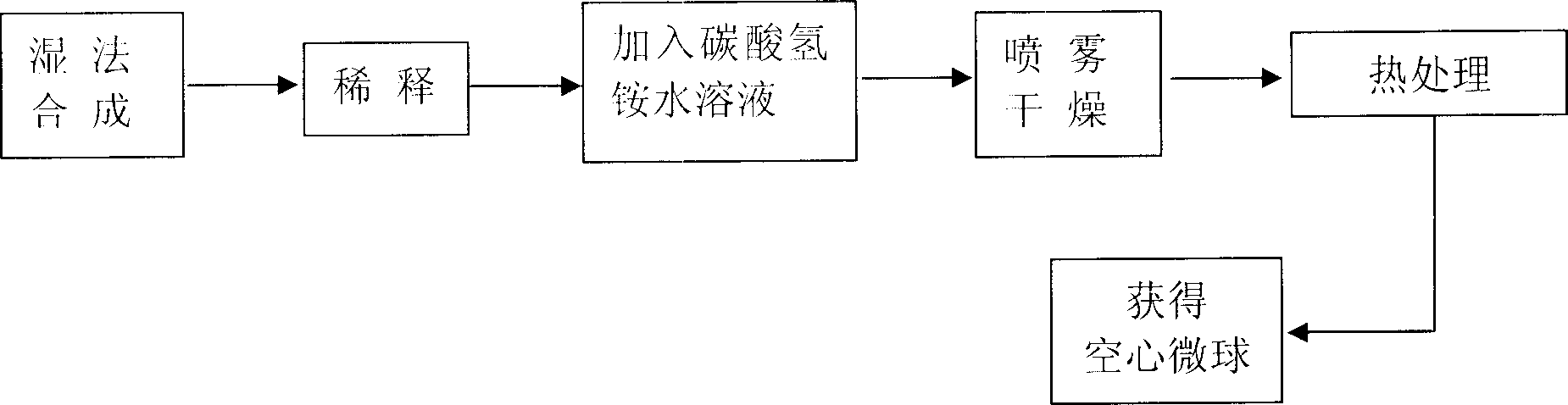
InactiveCN1903706AHigh purityReduce crystallinityPhosphorus compoundsProsthesisCalcium nitrate tetrahydrateApatite
The present invention discloses a method for preparing hydroxyapatite hollow microsphere. Said method includes the following steps: using tetrahydrate calcium nitrate and ammonium hydrogen phosphate as raw material, adopting chemical precipitation method to prepare hydroxyapatite slurry, then diluting said hydroxyapatite slurry by using distilled water, adding ammonium hydrogen carbonate as additive, uniformly stirring them, spray drying, making following heat treatment so as to obtain the hydroxyapatite hollow microsphere formed from nano grains.
Owner:SHANDONG UNIV
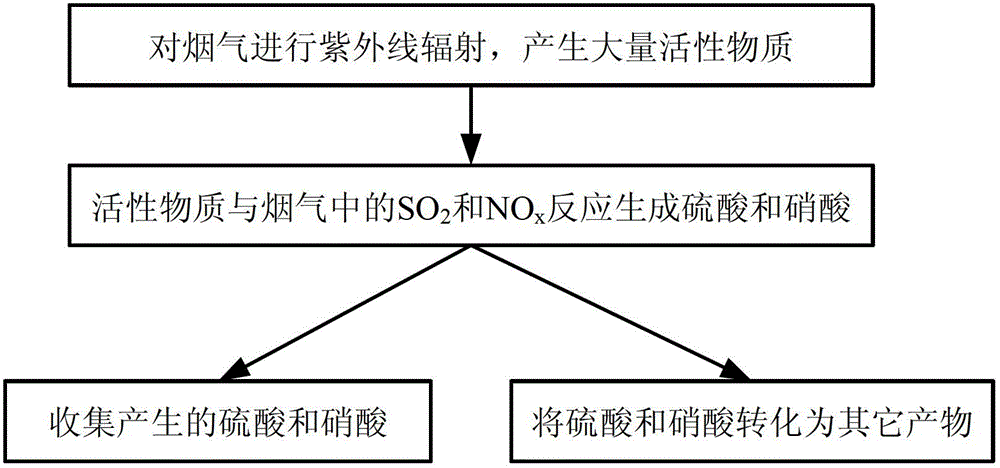
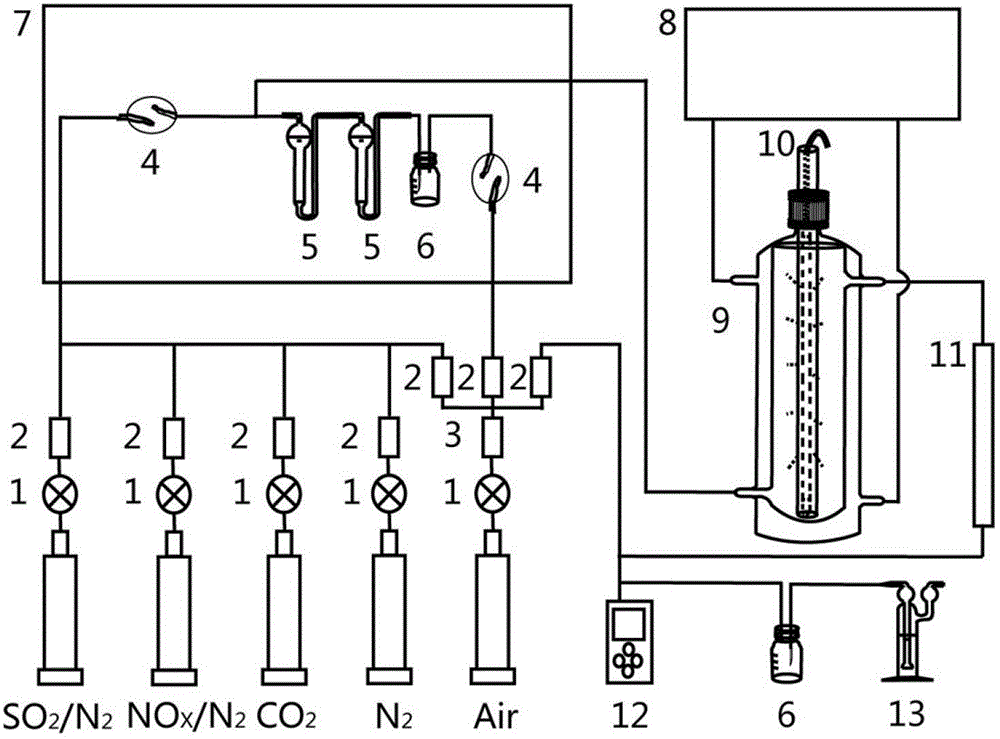
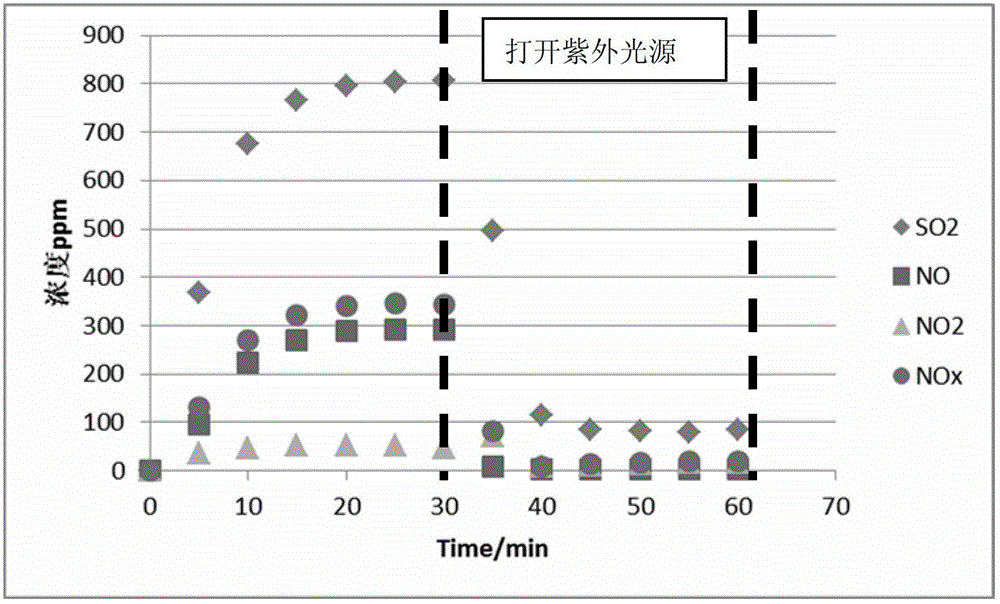
Method for simultaneously desulfurizing and denitrating flue gas
ActiveCN102908883APromote oxidationTo achieve the purpose of simultaneous desulfurization and denitrificationDispersed particle separationAir quality improvementAmmonium nitrateAmmonium sulfate
The invention provides a method for simultaneously desulfurizing and denitrating flue gas. The method comprises the step that: a large number of active materials produced by the flue gas under ultraviolet radiation are reacted with SO2 and NOx in the flue gas so as to produce stable sulfuric acid and nitric acid which are important industrial products. The two products can be collected by an acid mist removing device, or ammonia can be introduced into the two products to produce ammonium sulfate and ammonium nitrate which are important chemical fertilizers, or calcium carbonate is sprayed into the two products to produce calcium sulfate and calcium nitrate; and then, SO2 and NOx in the flue gas are removed from the flue gas to be converted into the products with the industrial or agricultural value. The method for simultaneously desulfurizing and denitrating the flue gas is suitable for desulfurizing and denitrating the flue gas of large newly-built boilers, or reforming and applying built burning equipment, and can realize the effect of simultaneously removing SO2 and NOx.
Owner:PEKING UNIV
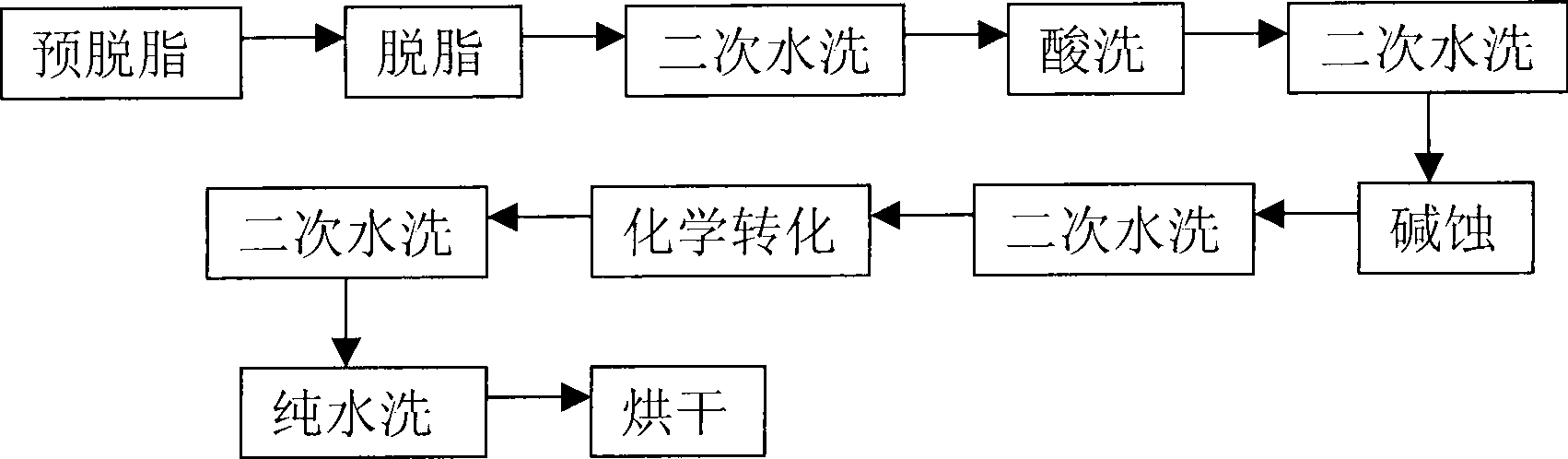
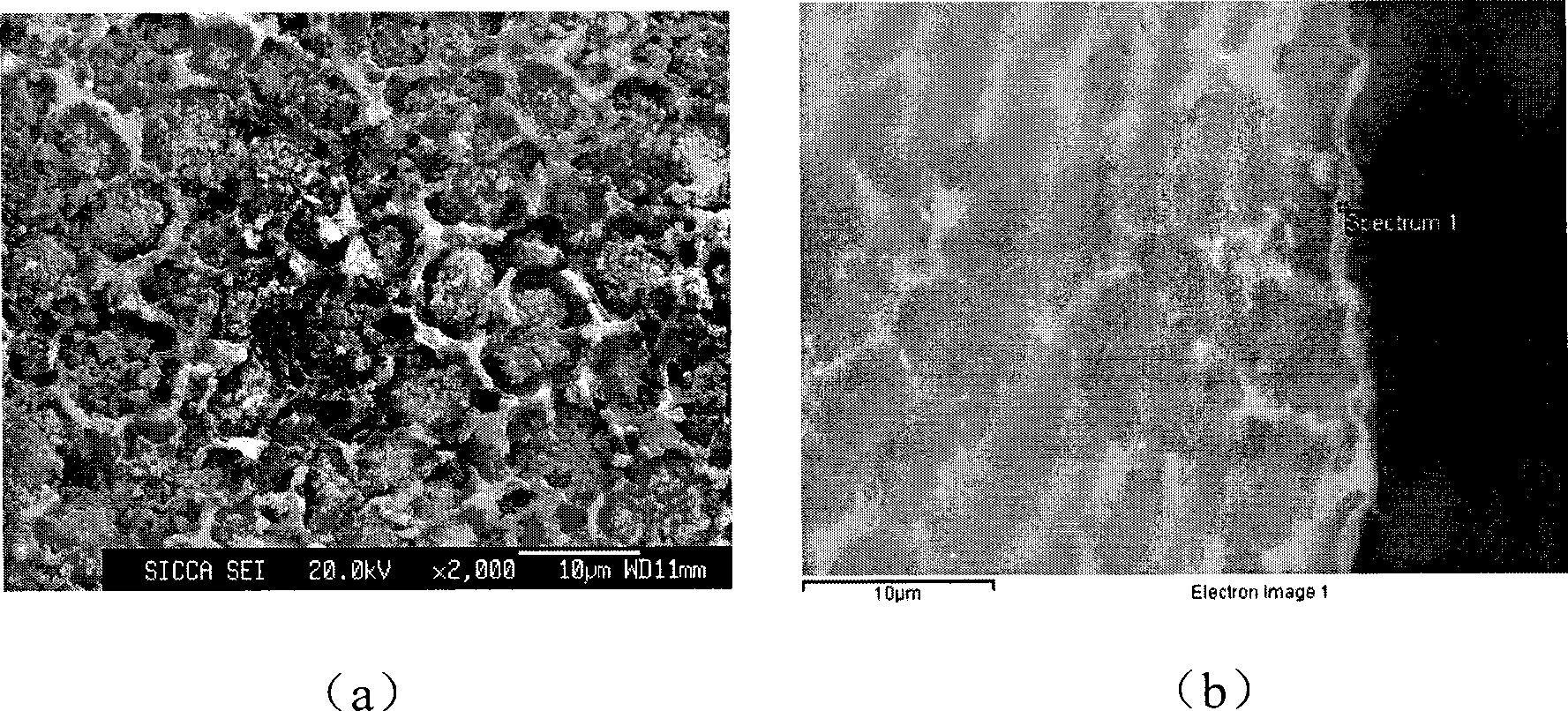
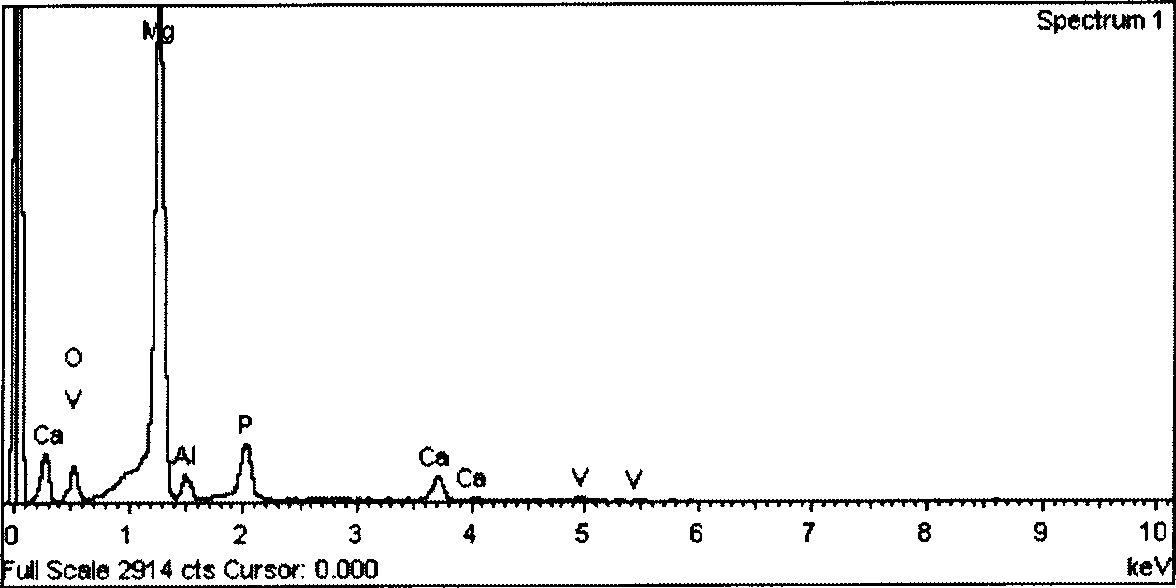
P-Ca-V composite phosphating solution on magnesium alloy surface and chemical conversion processing method
ActiveCN101418441AImprove corrosion resistanceLow resistivityMetallic material coating processesSalt spray testAcid washing
The invention relates to a composite phosphating solution of P-Ca-V on the surface of a magnesium alloy and a method for chemical conversion treatment. The solution is characterized in that each liter of the composite phosphating solution comprises the following compositions: 4 to 20 milliliters of 85 percent phosphoric acid of, 5 to 30 grams of sodium dihydrogen phosphate, 10 to 40 grams of calcium nitrate, 0.5 to 10 grams of benzene sulfonic acid sodium salt, 0.5 to 5 grams of ammonium metavanadate, and the balance being water. The method comprises the following steps: pre-degreasing, degreasing, secondary water washing, acid washing, secondary water washing, alkaline etching, secondary water washing, chemical transformation, secondary water washing, pure water washing, and drying. Taking an AZ91D magnesium alloy as an example, 48 hours after a corrosion resistance salt spray test after the treatment by the method of the invention, the corrosion area of the AZ91D magnesium alloy is less than 1 percent; the paint film adhesive force is at 0 level by a grid method and is obviously superior to the performance of a chromate conversion coating; and the formed chemical conversion coating does not contain crystal water. The composite phosphating solution has the synergistic reaction of Ca and V, as well as the functions of a corrosion inhibitor and a wetting agent of the benzene sulfonic acid sodium salt.
Owner:嘉兴中科亚美合金技术有限责任公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com