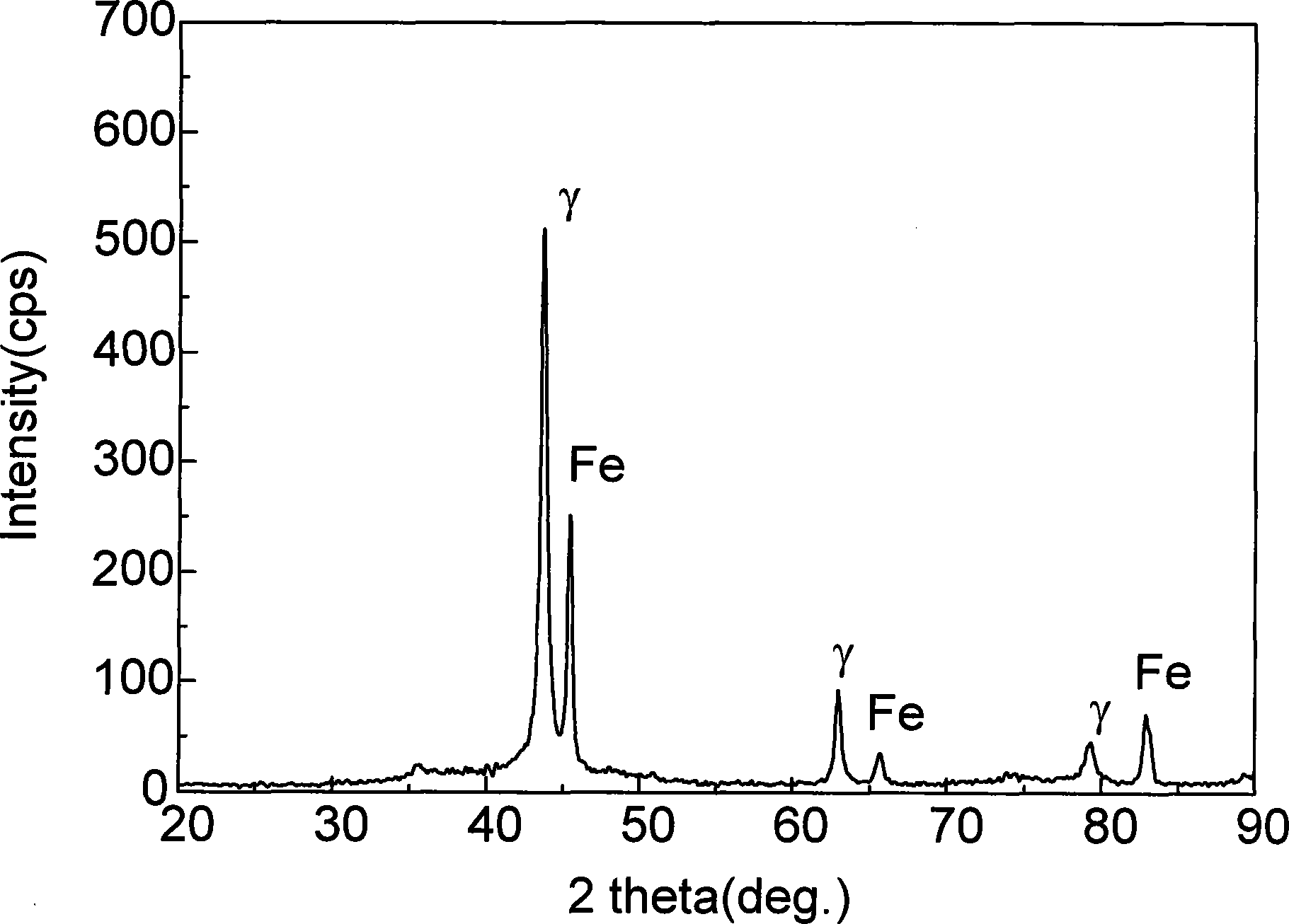
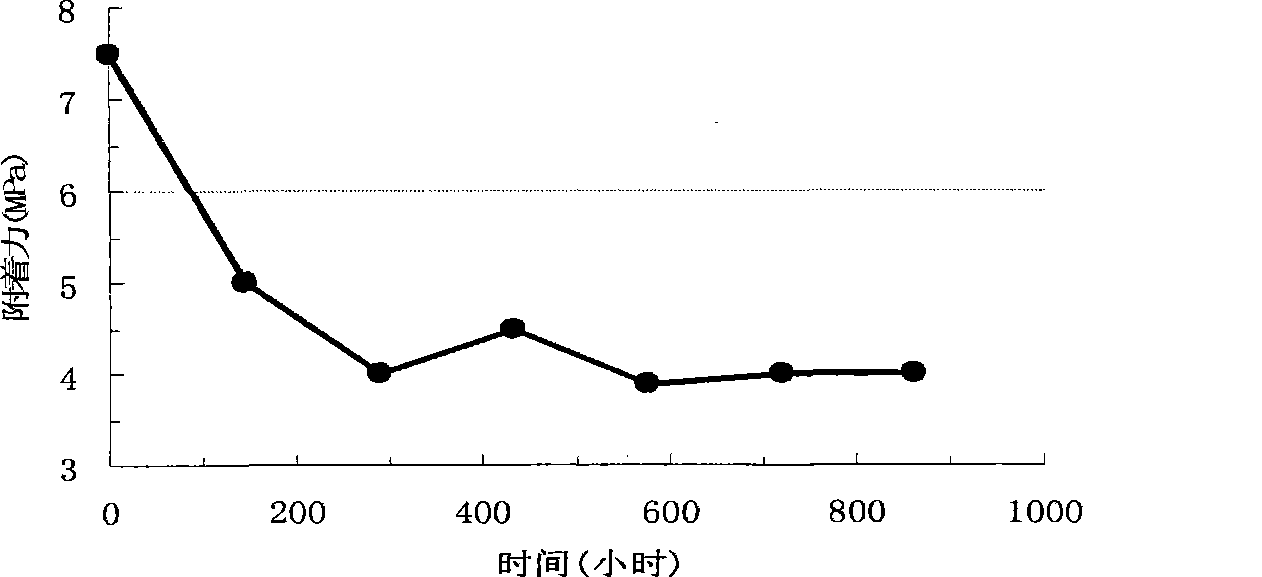
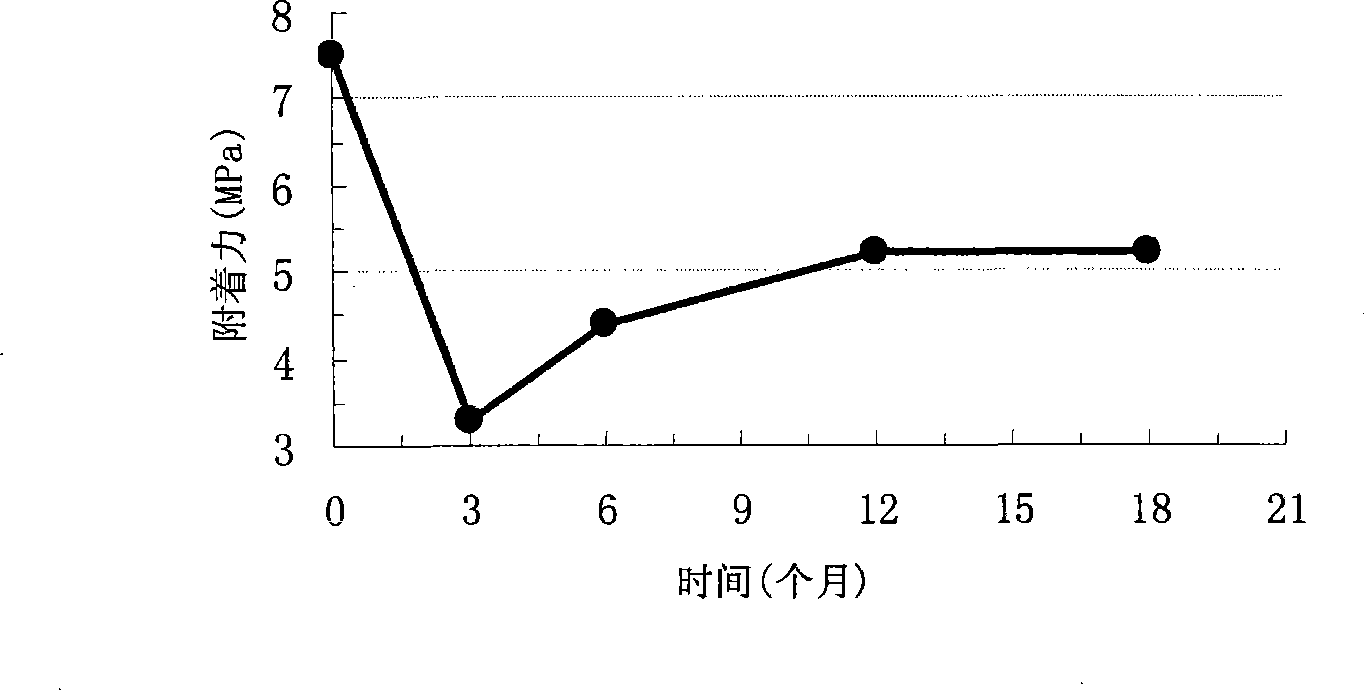
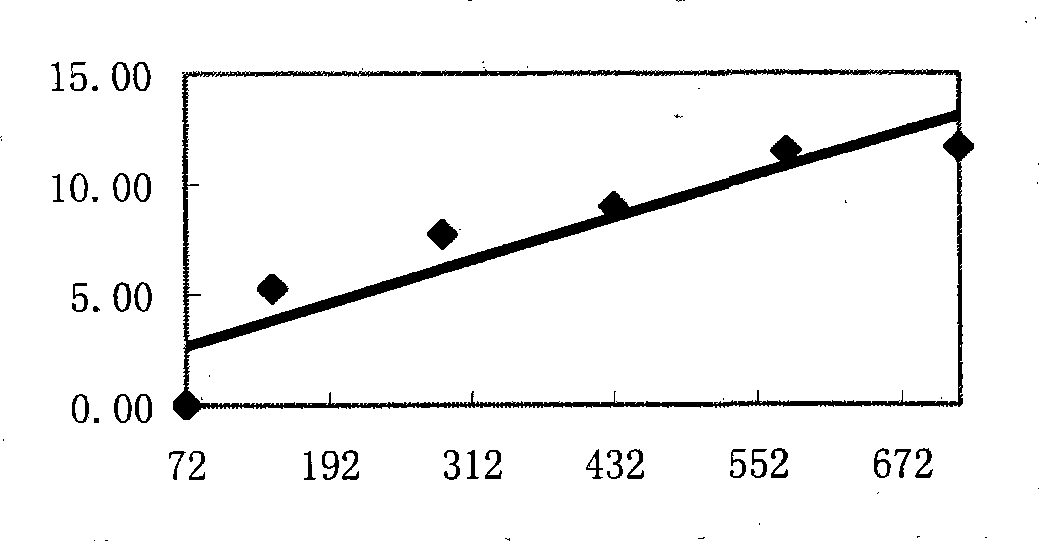
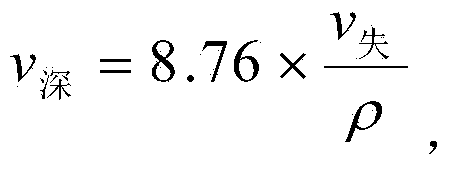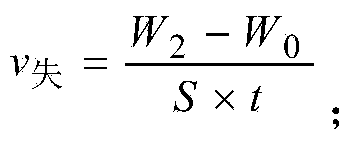Patents
Literature
816 results about "Salt spray test" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
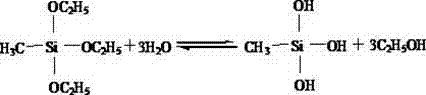
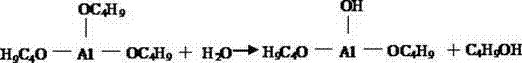
The salt spray (or salt fog) test is a standardized and popular corrosion test method, used to check corrosion resistance of materials and surface coatings. Usually, the materials to be tested are metallic (although stone, ceramics, and polymers may also be tested) and finished with a surface coating which is intended to provide a degree of corrosion protection to the underlying metal. Salt spray testing is an accelerated corrosion test that produces a corrosive attack to coated samples in order to evaluate (mostly comparatively) the suitability of the coating for use as a protective finish. The appearance of corrosion products (rust or other oxides) is evaluated after a pre-determined period of time. Test duration depends on the corrosion resistance of the coating; generally, the more corrosion resistant the coating is, the longer the period of testing before the appearance of corrosion/ rust. The salt spray test is one of the most widespread and long-established corrosion tests. ASTM B117 was the first internationally recognized salt spray standard, originally published in 1939. Other important relevant standards are ISO9227, JIS Z 2371 and ASTM G85.
Self healing anti corrosive coatings and a process for the preparation thereof
ActiveUS20150184304A1Improve corrosion resistanceInhibited DiffusionConductive materialAnti-corrosive paintsSelf-healingEpoxy
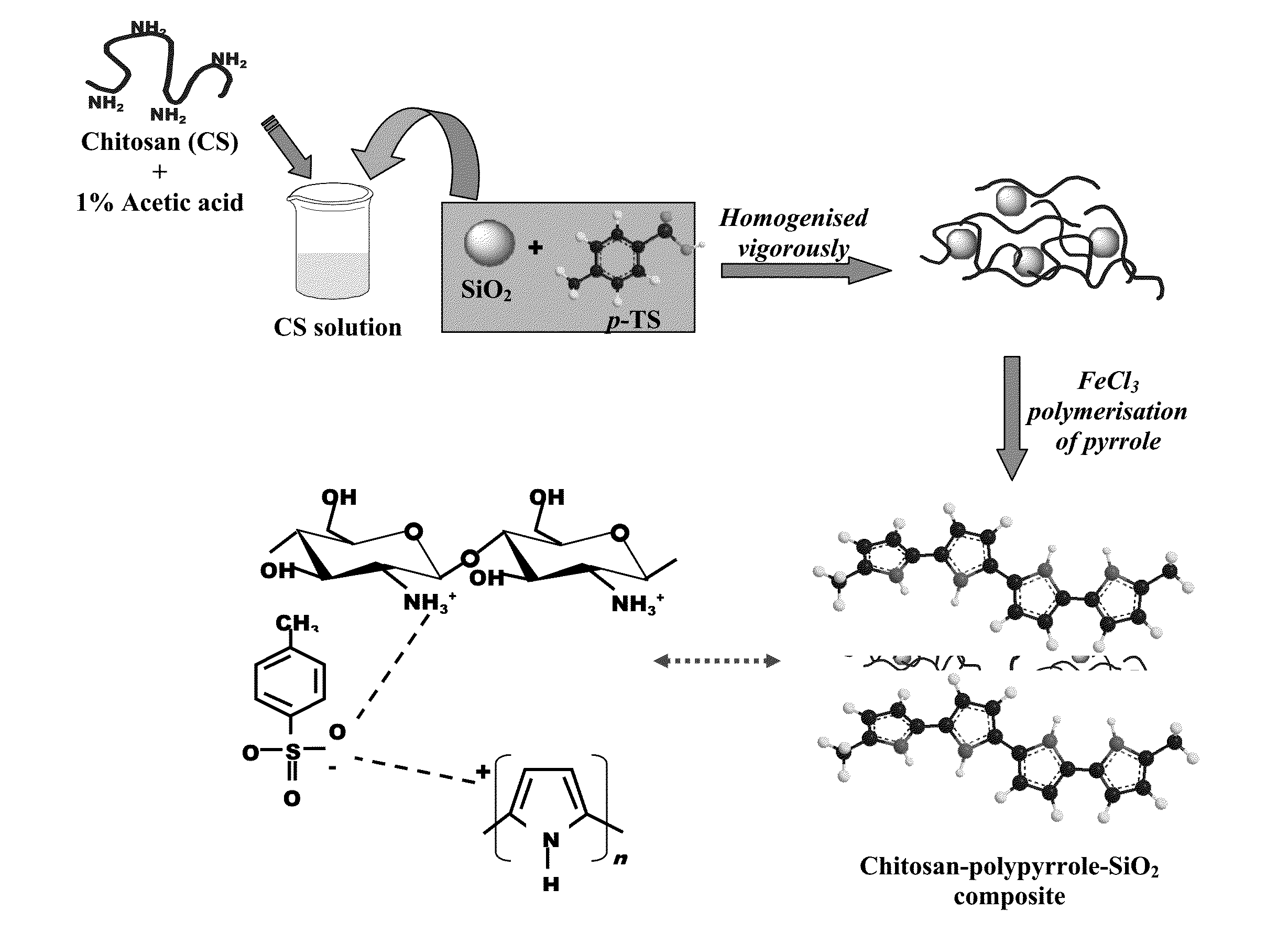
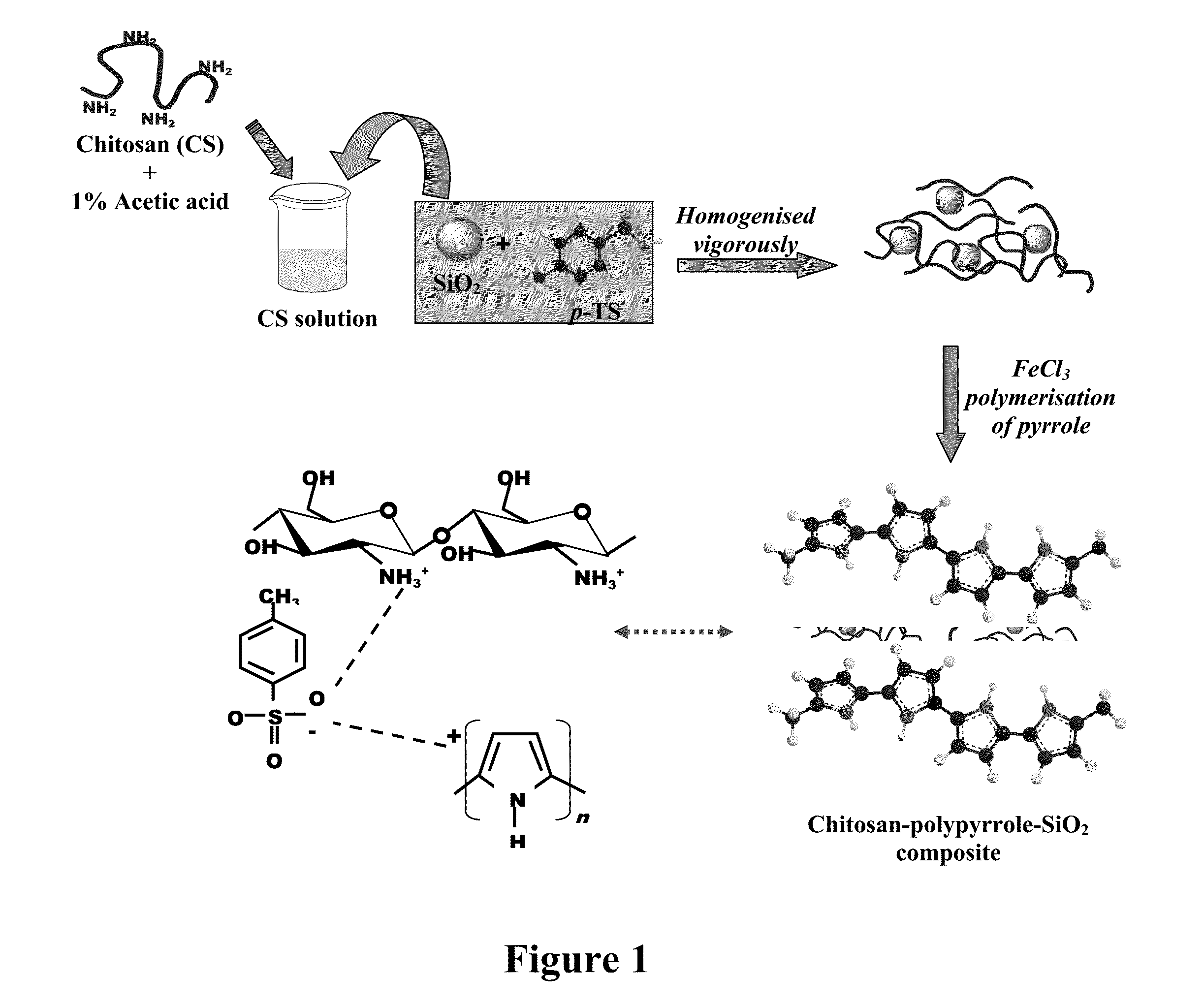
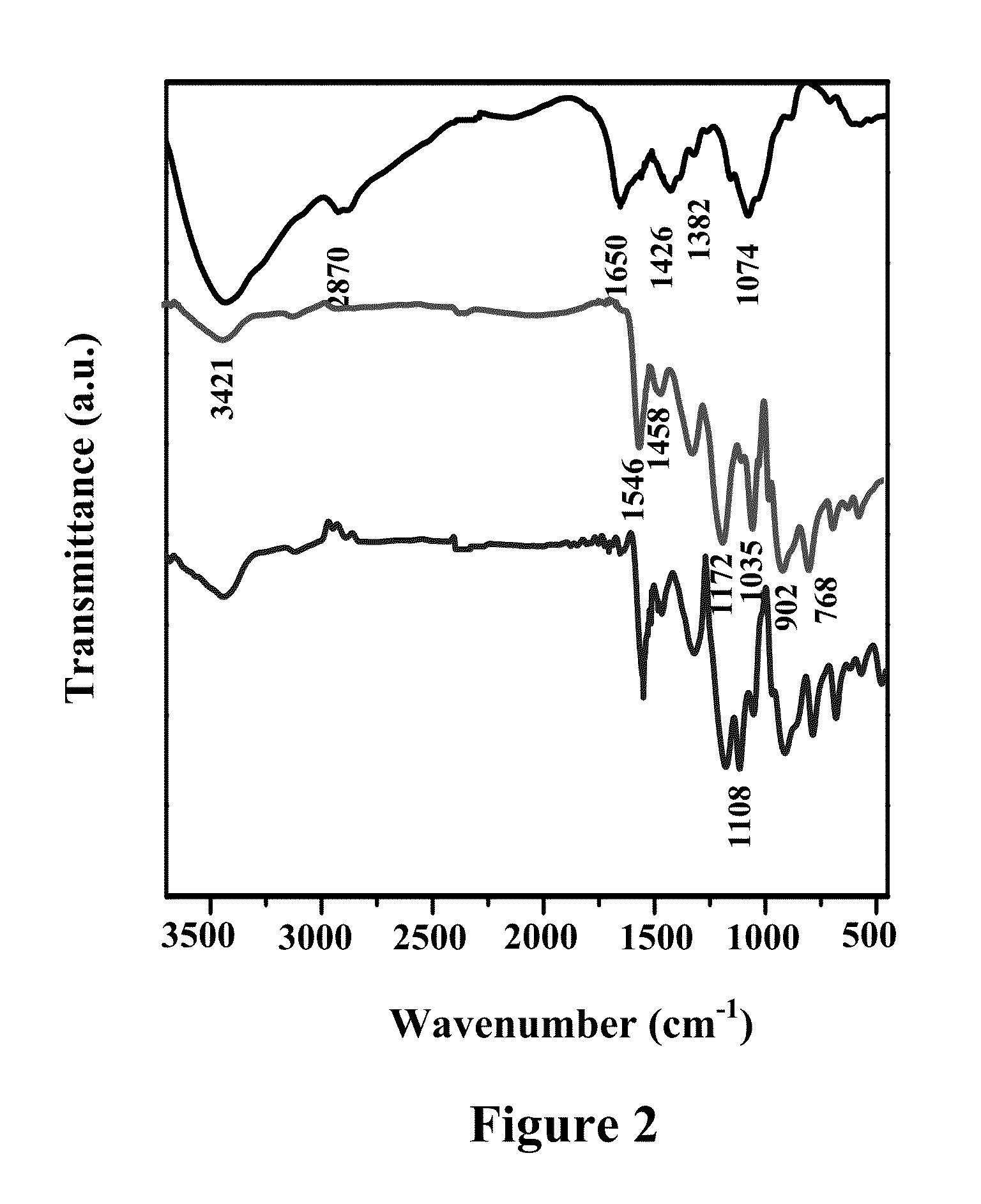
The present invention provides self-healing anti corrosive coatings comprising composites of conducting polymers, chitosan and silica particles along with epoxy useful for corrosion prevention under highly corrosive medium like 3.5% NaCl. Tafel plots exhibits significantly high corrosion protection efficiency (99.99%) for the epoxy coatings with 2.0 wt % loading of chitosan-polymer composite. The weight loss measurements and salt spray test results clearly exhibit superior corrosion resistance offered by coatings with chitosan-polymer composite. The synergistic interaction between chitosan and polypyrrole in the composite is expected to improve the corrosion resistance properties of the coatings. The SiO2 particles present in the composite reinforce the integrity of the coating under corrosive conditions.
Owner:COUNCIL OF SCI & IND RES
Preparation method of magnesium alloy surface micro-arc oxidation/spray coating compound film
ActiveCN101871119AHigh bonding strengthImprove corrosion resistanceAnodisationLiquid surface applicatorsMicro arc oxidationPlasma electrolytic oxidation
The invention provides a preparation method of a magnesium alloy surface micro-arc oxidation / spray coating compound film, which relates to a preparation method of a magnesium alloy surface compound film. The invention solves the problems that film layers obtained by chemical composition coating, anodic oxidation and vapor deposition in the existing magnesium alloy surface treatment method are thin and have poor corrosion resistance performance, the ion injection has high cost and is difficult to realize large-area processing, the combining force between coating layers and the magnesium alloy is poor, and organic coating layers has the defect of easy aging. The preparation method has the following steps: firstly, using micro-arc oxidation for preparing ceramic films on the surface of the magnesium alloy; then, adopting air spray coating for spraying and coating inorganic paint onto the ceramic films; and then, carrying out heat treatment to obtain the micro-arc oxidation / spray coating compound film. The method is simple, and the cost is low. The air spray coating uses porous structures of the micro-arc oxidation ceramic films, so the combination of the inorganic coating layers and the ceramic films is firm, the thickness of the compound films is between 20 and 40 mum, the self corrosion electric potential is positively moved to -1.02 V, the corrosion current density is lowered by 5 orders through being compared with that of the magnesium alloy, the magnesium alloy surface micro-arc oxidation / spray coating compound film has no damage after 72 to 144h of salt spray tests, and the corrosion resistance performance is good.
Owner:HARBIN INST OF TECH
Method for producing color-coated plate for structure with yield strength of more than or equal to 550 MPa
InactiveCN102363857APrevent rustNo foamingHot-dipping/immersion processesAcid washingCooling chamber
The invention relates to a method for producing color-coated steel sheet for a structure. The method comprises the following steps of: smelting, namely desulfurizing by molten iron, smelting by a converter, casting continuously to form a blank and performing hot rolling; performing acid washing; performing cold milling; annealing continuously; performing hot dipping on aluminum and zinc; and polishing, straightening, performing color coating and packaging for later use. In the method, the yield strength RP 0.2 (or ReH) is between 550 and 600 MPa; the tensile strength Rm is between 560 and 610MPa; and the percentage elongation after break A80 mm is more than or equal to 6 percent. A neutral salt mist test is performed for 1,200 hours, a coating does not have bubbling and corrosion phenomena. At present, the annealed color-coated plate of which the metallographic structure is ferrite and pearlite or the ferrite and free cementite or the ferrite and the pearlite and less free cementite cannot achieve the effect, and the production cost can be reduced by 10 to 15 percent. By the method, special occasions which have high-yield strength and are used for cooling chambers of automobiles,arch coverings and the like can be met, and a process is simple and easy to implement.
Owner:武钢集团有限公司
Method for plating zinc-nickel alloy in alkaline electroplate liquid
InactiveCN101240437ASolve the problem of large compositional changesImprove current efficiencyWater basedOrganic solvent
The invention discloses a method for electroplating alkaline Zn-Ni alloy, comprising the steps of: preparing alkali wash and acid wash, preparing electroplating Zn-Ni alloy plating solution, mechanical grinding, degreasing with organic solvent or water-based detergent, alkali washing, acid washing and electroplating. The invention provides a stable electroplating solution formula and complete electroplating process. The coating electroplated by the method is proved to have good flat surface, uniform coating distribution and alloy distribution, the coating is single gamma-phase, the nickel has a content of 10% to 15%; the coating has superior corrosion resistance, red rust appears on the non-passivated Zn-Ni coating after 1500 hours in a neutral salt spray test; compared with general alkali electroplating solution, the coating has high current efficiency, reaching up to 80% to 90%.
Owner:BEIHANG UNIV
Simulated acceleration test method for coating
InactiveCN101482484AThe simulated accelerated test method is reasonableSimulated accelerated test method is fastWeather/light/corrosion resistanceSalt spray testEngineering
The invention relates to a coating simulation accelerated test method, comprising a damp heat test, a solar radiation test, a salt mist test, characterized in that the damp heat test, the solar radiation test and the salt mist test are combined in precedence order and circulate; circulation time of the damp heat test, the solar radiation test and the salt mist test is more ten. The coating simulation accelerated test method of marine environment adaptation check is reasonable, effective and rapid and obtains the outdoor long-time exposure test result using the indoor short-time testing; solves the practical problems of development and use, in favor of application and popularization of coating material.
Owner:NO 59 RES INST OF CHINA ORDNANCE IND
Passivating treating agent for environment-friendly galvanized steel sheets
InactiveCN101724836ASimple preparation processLow drying temperatureMetallic material coating processesSheet steelSalt spray test
The invention discloses a passivating treating agent for environment-friendly galvanized steel sheets, which is composed of 2-20% of silane, 1-5% of anti-rusting metal salt, 8-20% of resin, 0.1-5% of coating additive, 0.5-5% of pH regulating agent and 50-70% of water. The passivating treating agent features in simple and convenient preparation process and mainly depends on mixing production; drying temperature thereof is as low as 50-60 DEG C, in 96 hours, salt mist resistance property is no more than 3% and performance thereof is good.
Owner:QIDONG SYNTHESIZE CHEM
Neodymium-iron-boron magnetic material zinc-series phosphatization liquid and application method thereof
ActiveCN103422082AReduce generationExtended service lifeMetallic material coating processesElectrophoresisSalt spray test
The invention relates to the field of metal surface treatment, and especially relates to a neodymium-iron-boron magnetic material zinc-series phosphatization liquid. The phosphatization liquid is mainly used in neodymium-iron-boron surface anti-corrosion protection and cathodic electrophoresis priming and phosphatization. The liquid is characterized in that a mass ratio of Zn<2+>:PO4<3->:NO<3->:F<->:an accelerator is (3.5-10.5):(14-41.3):(7.3-21.9):(0.95-2.8):1, Ni<2+> is 0.01-0.2g / L, an organic complexing agent is 0.04-0.18g / L, the promoter is 0.2-1.5g / L, and balance is water. Free acidity of a phosphatization working liquid is 3-8 points, a total acidity is 22-32 points, a phosphatization temperature is 48-55 DEG C, and a phosphatization time is 6-15min. When the phosphatization liquid is used for treating neodymium-iron-boron, a light grey to grey phosphate coating is produced, and 3% neutral salt water soaking time is above 3h. An electrophoresis coating cut test is 0 grade, and a neutral salt spray test is 500h.
Owner:中化化工科学技术研究总院有限公司
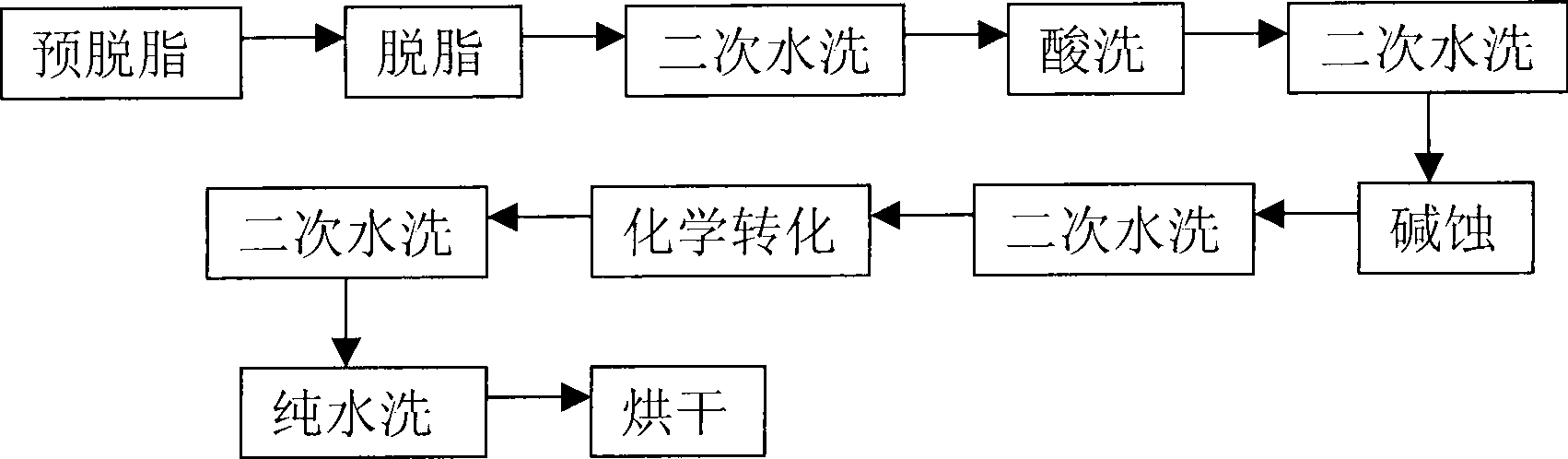
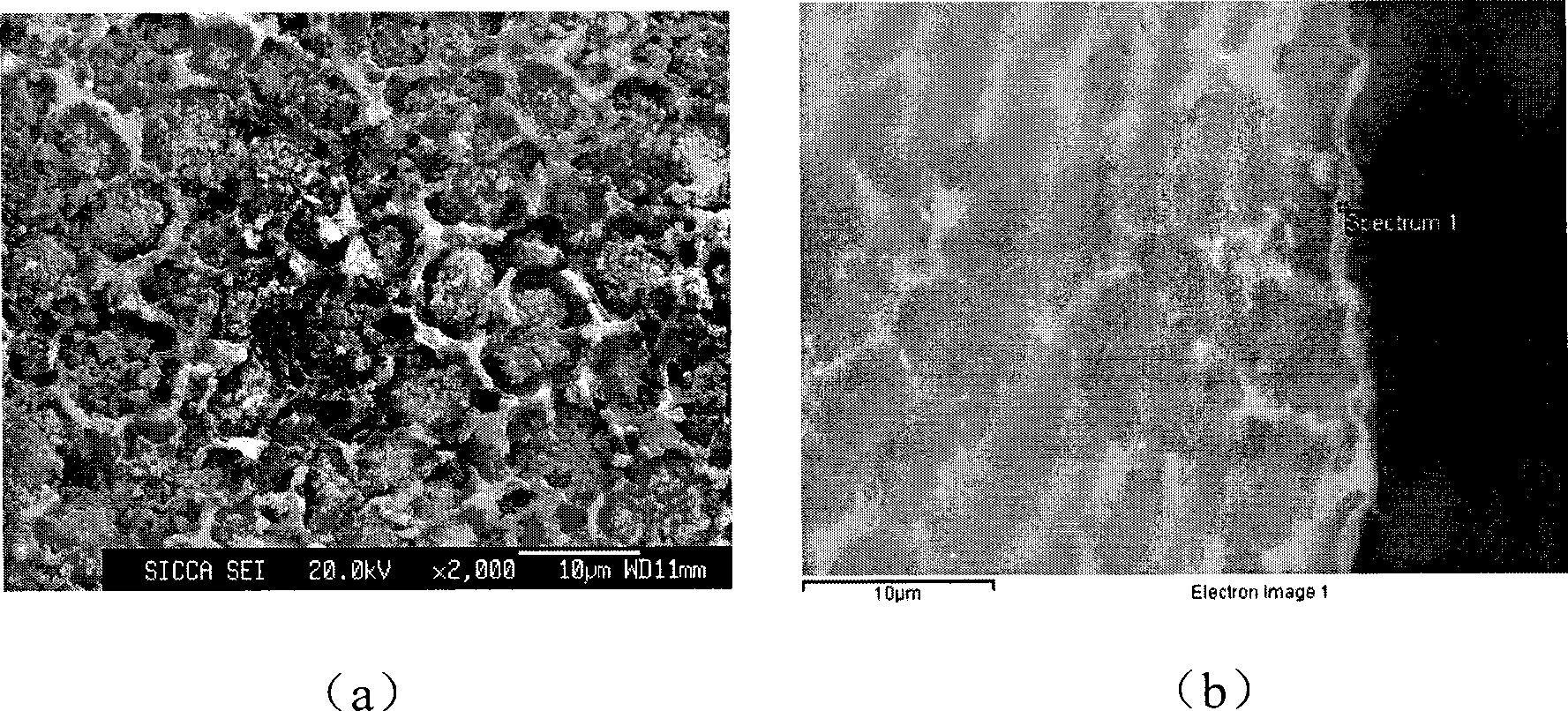
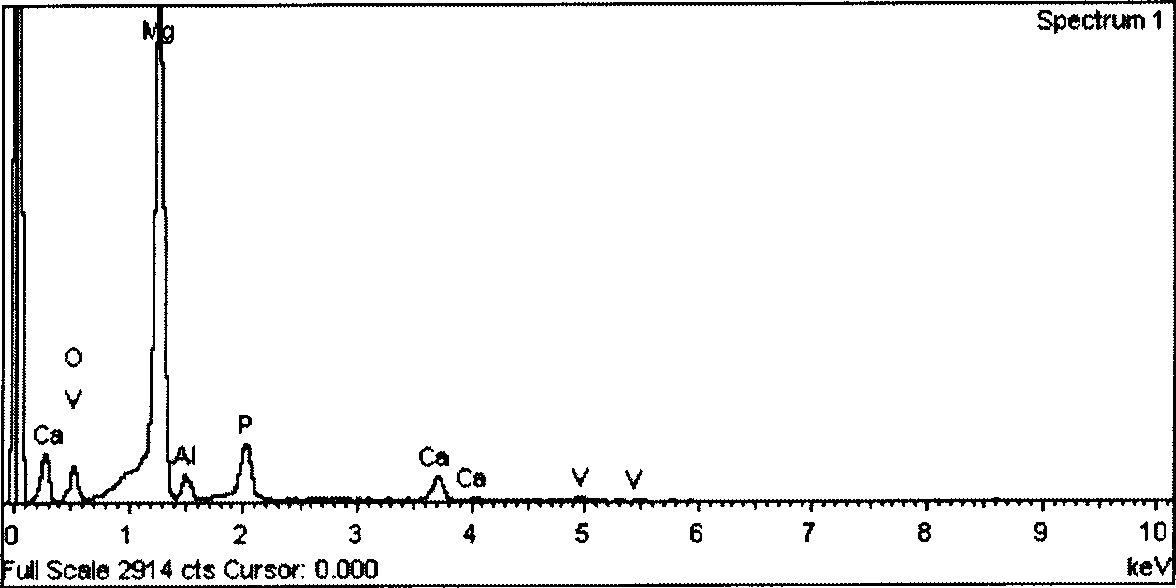
P-Ca-V composite phosphating solution on magnesium alloy surface and chemical conversion processing method
ActiveCN101418441AImprove corrosion resistanceLow resistivityMetallic material coating processesSalt spray testAcid washing
The invention relates to a composite phosphating solution of P-Ca-V on the surface of a magnesium alloy and a method for chemical conversion treatment. The solution is characterized in that each liter of the composite phosphating solution comprises the following compositions: 4 to 20 milliliters of 85 percent phosphoric acid of, 5 to 30 grams of sodium dihydrogen phosphate, 10 to 40 grams of calcium nitrate, 0.5 to 10 grams of benzene sulfonic acid sodium salt, 0.5 to 5 grams of ammonium metavanadate, and the balance being water. The method comprises the following steps: pre-degreasing, degreasing, secondary water washing, acid washing, secondary water washing, alkaline etching, secondary water washing, chemical transformation, secondary water washing, pure water washing, and drying. Taking an AZ91D magnesium alloy as an example, 48 hours after a corrosion resistance salt spray test after the treatment by the method of the invention, the corrosion area of the AZ91D magnesium alloy is less than 1 percent; the paint film adhesive force is at 0 level by a grid method and is obviously superior to the performance of a chromate conversion coating; and the formed chemical conversion coating does not contain crystal water. The composite phosphating solution has the synergistic reaction of Ca and V, as well as the functions of a corrosion inhibitor and a wetting agent of the benzene sulfonic acid sodium salt.
Owner:嘉兴中科亚美合金技术有限责任公司
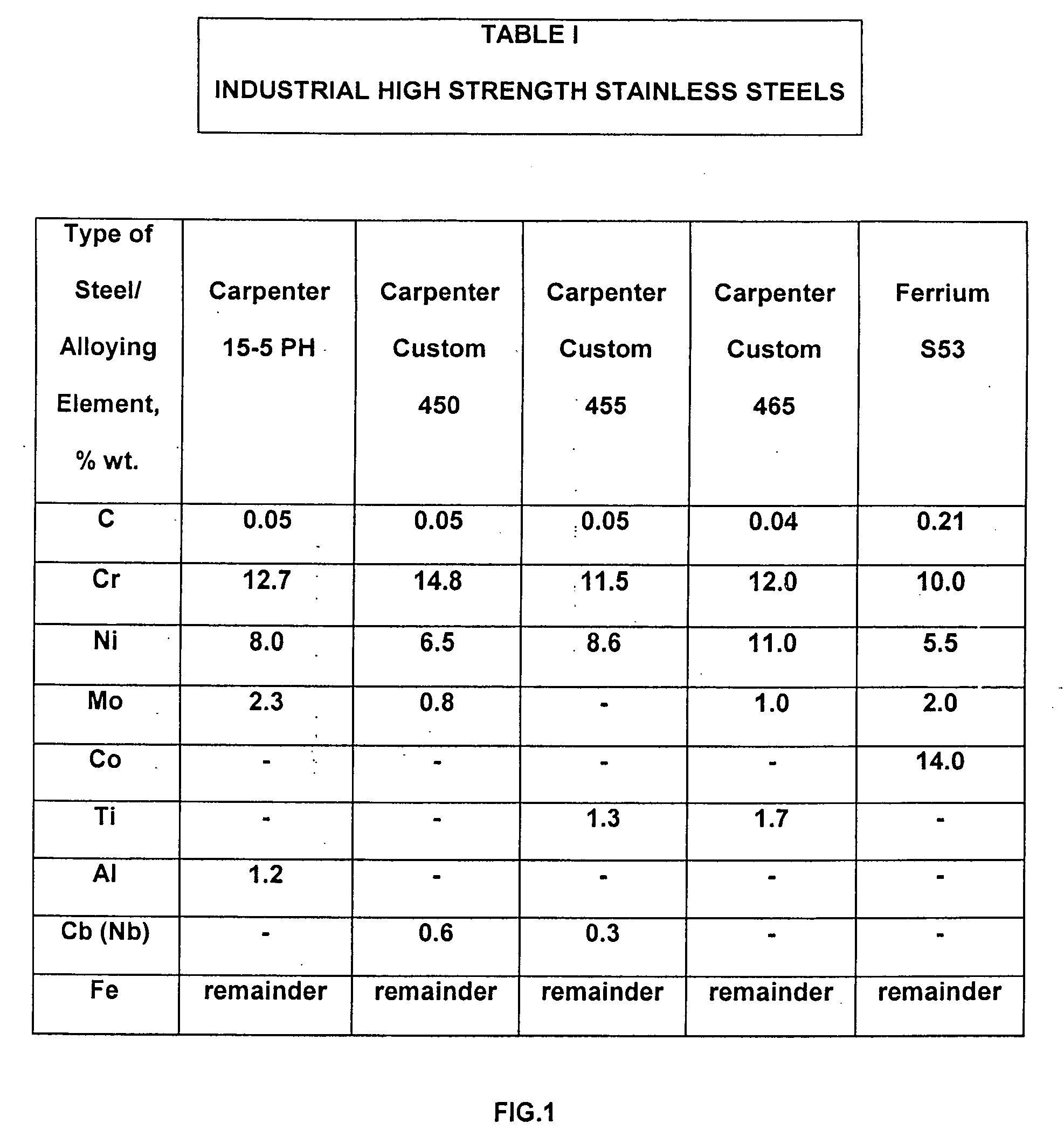
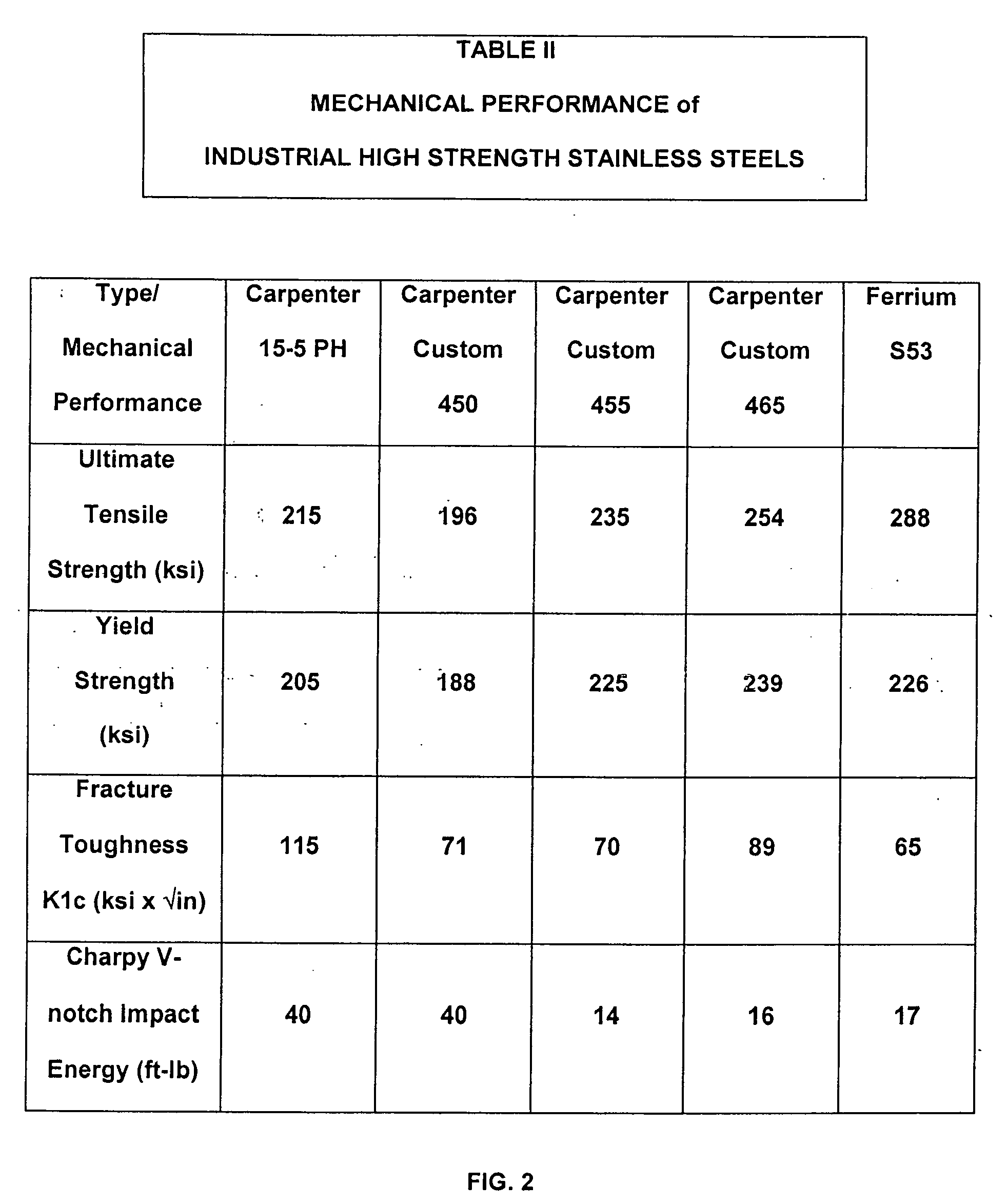
Low cost high strength martensitic stainless steel
A cobalt-free low cost high strength martensitic stainless steel, with concentration of Ni up to 3.0% and Mo up to 1.0% of weight, has HRC of 53, UTS of 297 ksi, YS of 220 ksi, Charpy V-notch impact energy of 17.8 ft-lb, corrosion resistance in salt spray test ASTM 117. The steel was melted in an open induction furnace and vacuum arc remelting (VAR) and / or electroslag remelting (ESR) were not used to refine the steel. Further processing included homogenized annealing, hot rolling, and recrystallization annealing. The steel was heat treated by oil quenching, refrigeration, and low tempering. The steel has a microstructure consisting essentially of small packets of fine martensite laths, retained austenite, and carbides as centers of growth of the martensite laths. The cost and energy in making the steel are substantially reduced.
Owner:FEDCHUN VLADIMIR A +1
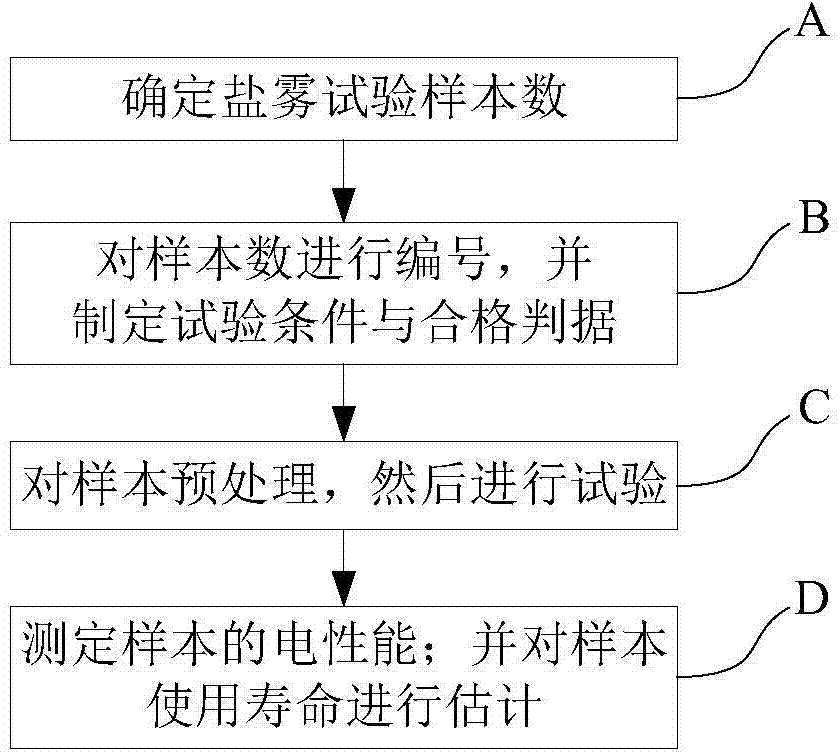
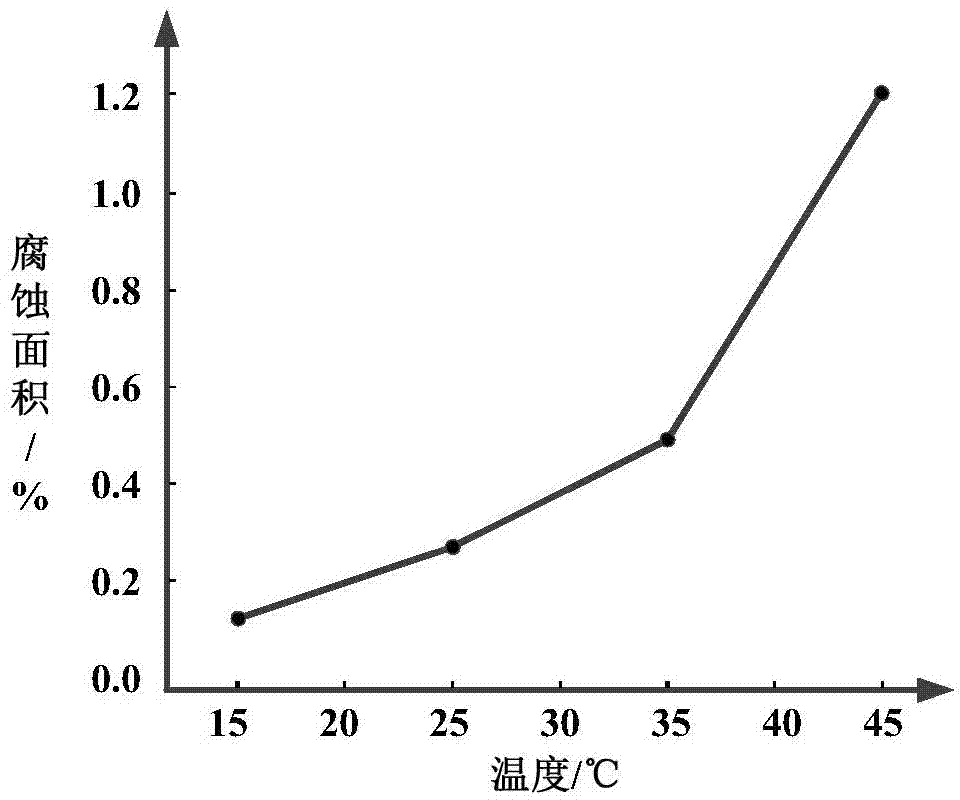
Printed circuit board service life assessment method based on salt-spray environment test
InactiveCN104777092AOvercoming Impossibility to Model AccuratelyRaise the level of service life verificationWeather/light/corrosion resistanceSmall sampleSalt spray test
The invention discloses a printed circuit board service life assessment method based on a salt-spray environment test. The method comprises the following steps of associating the failure mode of the printed circuit board with a working condition, forming an accelerated life predication model based on the salt-spray test, drawing a changing curve between three environment factors of temperature, salt solution concentration and salt spray settling volume and test sample corrosion area by the method of combination of test data and Arrhenius accelerated life predication model, so as to predicate the service life of the printed circuit board under the salt-spray working condition. By utilizing the method, the problem that the accurate modeling is impossible in system with complex failure mechanism when analyzing small sample by traditional reliability analytical method is solved; secondly, during the researching stage of a device, through developing the accelerated life test, the test time is shortened, the service life assessment level of marine equipment is improved, the possible problems are exposed and solved completely before service, so as to formulate scientific and reasonable maintenance decision to save maintaining cost and guarantee complete war preparedness and successful task.
Owner:UNIV OF ELECTRONICS SCI & TECH OF CHINA
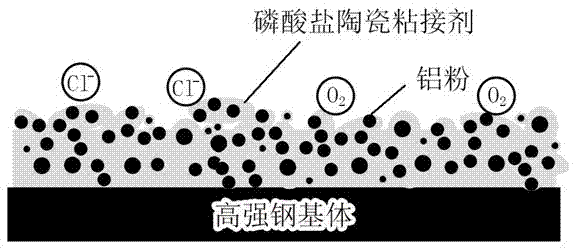
Water-based ceramic anticorrosive coating containing aluminum phosphate as well as preparation and curing methods thereof
The invention discloses a water-based ceramic anticorrosive coating containing aluminum phosphate as well as preparation and curing methods thereof. The water-based ceramic anticorrosive coating containing aluminum phosphate is prepared by adopting aluminium dihydrogen phosphate as a bonding agent, magnesium oxide and zinc oxide as a curing agent, aluminum powder as filler, magnesium chromate as a passivating agent and tetraethyl orthosilicate as an auxiliary agent. The coating is used for preparing a metal ceramic coating layer on a metallic matrix, and the coating layer can tolerate 1000h of neutral salt fog test and keep stable. The coating layer has good high-temperature oxidation resistance in a high-temperature (600 DEG C) oxidation environment. The coating layer can well protect metals in a normal-temperature salt-containing environment and a high-temperature oxidation environment.
Owner:NANJING UNIV OF AERONAUTICS & ASTRONAUTICS
Technique for treating synergism coat of aluminium alloy material and fluorine polymer
InactiveCN1687488AHigh hardnessHigh porositySurface reaction electrolytic coatingPretreated surfacesSalt spray testWear resistance
The present invention relates to a technique of treating coat the film made of aluminum alloy fluorine-polymer. Material or part of aluminum alloy should be hard anodizing first and introduce fluorine-polymer in the pore or on the surface. We can also expend the pore first and introduce fluorine-polymer on the surface and in the pore later. Fuse fluorine-polymer and anode oxidation coat together by vacuum heat treatment for high temperature. It's suitable for part of optic-electronic equipment. Firstly, it has good extinction, doesn't fade and doesn't break off: secondly, it is rigid and wearable; thirdly, it contains fluorine-polymer, has low friction coefficient, has the capability of self-lubricating, so its wearable and anti-corrosive performance can be improved a lot; fourthly, it has capability of hydrophobicity and can bear litmusless salt spray, so it can sustain most of the corrosion in bad condition and corrodent matter. Fifthly, the surface is self-lubricating, and has the capability of self-lubricating and dustproof.
Owner:NO 59 RES INST OF CHINA ORDNANCE IND
Magnesium alloy phosphorization solution and magnesium alloy surface treatment method
ActiveCN101638780AImprove corrosion resistanceImprove bindingMetallic material coating processesSodium molybdateSalt spray test
The invention relates to a magnesium alloy phosphorization solution, which is an aqueous solution containing phosphoric acid, calcium dihydrogen phosphate, sodium silicate, sodium molybdate and ammonium metavanadate. The invention also provides a magnesium alloy surface treatment method, which comprises a step of phosphorizing the surface of magnesium alloy by using the magnesium alloy phosphorization solution. The method uses a calcium phosphorization solution to phosphorize the surface of the magnesium alloy, does not require substances containing hexavalent chrome, fluorions and the like which are harmful to the environment and human bodies in the whole phosphorizing process, and is the environment-friendly method. The magnesium alloy surface treated by the method has high corrosion resistance, good bonding force and low surface resistance, wherein the corrosion area after 60 hours of a neutral salt spray test can reach less than 5 percent, the bonding force can reach 5B level, andthe surface resistance can be lower than 0.0002 ohm.
Owner:BYD CO LTD
Graphene-modified waterborne epoxy coating curing agent as well as preparation method and application of curing agent
ActiveCN107815216AUniform and stable dispersionGood compatibilityAnti-corrosive paintsEpoxy resin coatingsEpoxyGraphene derivatives
The invention discloses a graphene-modified waterborne epoxy coating curing agent as well as a preparation method and application of the curing agent. The preparation method comprises the following steps: firstly dispersing graphene oxide prepared by virtue of an improved Hummers method into water, so as to obtain graphene oxide dispersion liquid; and adding a surfactant with amino end groups, uniformly mixing, carrying out microwave heating to enable graphene oxide to react with the surfactant, adding a graphene derivative water solution into a waterborne epoxy coating curing agent, and uniformly mixing, so as to obtain the graphene-modified waterborne epoxy coating curing agent. The graphene-modified waterborne epoxy coating curing agent is applied to the preparation of a bi-component waterborne epoxy coating, and the neutral salt spray test time of a formed anticorrosive coating can reach 185-252 hours.
Owner:UNIV OF SHANGHAI FOR SCI & TECH
High-temperature-resisting aluminum paint used on steel surface, and preparation method thereof
InactiveCN102604535AImprove heat resistanceHigh hardnessAnti-corrosive paintsEpoxy resin coatingsAviationSalt spray test
The invention belongs to the field of aviation paint technology, and relates to a high-temperature-resisting aluminum paint used on steel surfaces, and a preparation method thereof. The paint is composed of three components with a weight ratio of 100:3-10:5-20. A component one is composed of a film-forming substance, a pigment and filling material, an auxiliary agent, and a solvent according to a weight ratio of 40-70:5-20:1-5:20-50. A component two is polyamide resin. A component three is aluminum powder slurry. The paint has a good heat resistance, good hardness, good solvent resistance, and good salt spray resistance. When a coating formed by construction is held for 5h under a temperature of 300 DEG C, the coating is well-preserved. The pencil hardness of the coating is no lower than 2H. During part assembly, the coating can hardly get scratched. Under a normal temperature, the coating is not changed after 60d in water and 60d in oil. With a salt spray test of 2000h, the comprehensive performance of the coating is determined as grade 1.
Owner:AVIC BEIJING INST OF AERONAUTICAL MATERIALS
Method for preparing chromium-free passivation solution, and method for passivating electrogalvanizing or zinc alloy layer by using same
The invention provides a method for preparing chromium-free passivation solution, and a method for passivating an electrogalvanizing or zinc alloy layer by using the same, and relates to a method for preparing the passivation solution and a using method thereof. The method solves the problems that passivation of hexavalent chromium and trivalent chromium generates pollution, and white rust tested in a salt spray test has short time after the electrogalvanizing or zinc alloy layer is passivated by the passivation solution. The method for preparing the chromium-free passivation solution comprises the following steps: 1, preparing concentrated solution A; 2, preparing concentrated solution B; and 3, regulating pH value after the concentrated solution A, the concentrated solution B and deionized water are mixed, and heating or cooling the mixture to 20 to 60 DEG C to obtain the chromium-free passivation solution. The method for passivating the electrogalvanizing or zinc alloy layer by using the chromium-free passivation solution comprises the following steps: 1, secondarily passivating the electrogalvanizing or zinc alloy layer; and 2, aging the electrogalvanizing or zinc alloy layer to complete passivation on the electrogalvanizing or zinc alloy layer. After the electrogalvanizing or zinc alloy layer is passivated by the chromium-free passivation solution, the electrogalvanizing or zinc alloy layer is subjected to neutral salt spray test, and the white rust does not appears on the surface of a passivated film after 84-120 hours of continuous spraying.
Owner:HARBIN INST OF TECH
Method for preparing temperature-resistant and corrosion-resistant aluminum oxide/organic silicon/silicon dioxide hybridized coating
InactiveCN102604536AGood physical propertiesGood resistance to salt spray testPretreated surfacesCoatingsSilicic acidSalt spray test
The invention provides a method for preparing a temperature-resistant and corrosion-resistant aluminum oxide / organic silicon / silicon dioxide hybridized coating. The preparation method comprises the following steps of: preparing an organic silicon / silicon dioxide hybridized sol by using tetraethoxysilane as an inorganic phase precursor and methyl triethoxyl silane and diphenyl dimethyl silane as an organic phase precursor through a sol-gel method; preparing aluminum sol by using sec-butyl alcohol aluminum as an aluminum source through the sol-gel method; and then adding the aluminum sol into the organic silicon / silicon dioxide sol through a sol blending method, and preparing the aluminum oxide / organic silicon / silicon dioxide hybridized sol by using acetic acid as gel and a carbonyl-contained amide compound as a dry control chemical addition agent. The coating prepared by the invention has a good physical property. The hardness of the coating is 0.8825, the anti-impact intensity of the coating is 50 kg.cm, and the flexibility of the coating is 1 mm. The coating coated on an LY12 aluminum alloy plate is not destructive after being roasted at a temperature of 450 DEG C for 1 hour. The surface of the coating is non-corrosive after the coating salt spray test is carried out for 1368 hours.
Owner:NANCHANG HANGKONG UNIVERSITY
Wear and corrosion resistant coating material and preparation method thereof as well as coating and preparation method thereof
ActiveCN104195492AUniform meltingLess carbon lossMolten spray coatingThermal sprayingSalt spray test
The invention discloses a wear and corrosion resistant coating material and a preparation method thereof as well as a coating and a preparation method thereof, belonging to the technical field of thermal spraying. The coating material which is powder particles is prepared from the following components in percentage by weight: 85-87% of tungsten carbide powder, 8-11% of cobalt powder and 2-7% of cobalt powder, wherein the grain size distribution of the tungsten carbide powder is as follows: D97 is less than or equal to 0.9 mu m, D5 is greater than or equal to 0.4 mu m and D50 is 0.6-0.8 mu m. The free path d of a metal binding phase among the tungsten carbide powder grains is less than or equal to 0.5 mu m. The preparation method of the coating materiel comprises the following steps: adding deionized water, a defoamer and an adhesive into raw materials for mixing and ball-milling to obtain mixed slurry; spraying and drying the mixed slurry to obtain agglomerated particles; and then, sintering and crushing the agglomerated particles to obtain a WC-10Co4Cr coating material which is the wear and corrosion resistant coating material. The coating prepared from the coating material can bear a water pressure of 150MPa without leakage and is free from rust when neutral salt spray test resistance is longer than 672 hours.
Owner:BEIJING GENERAL RES INST OF MINING & METALLURGY +1
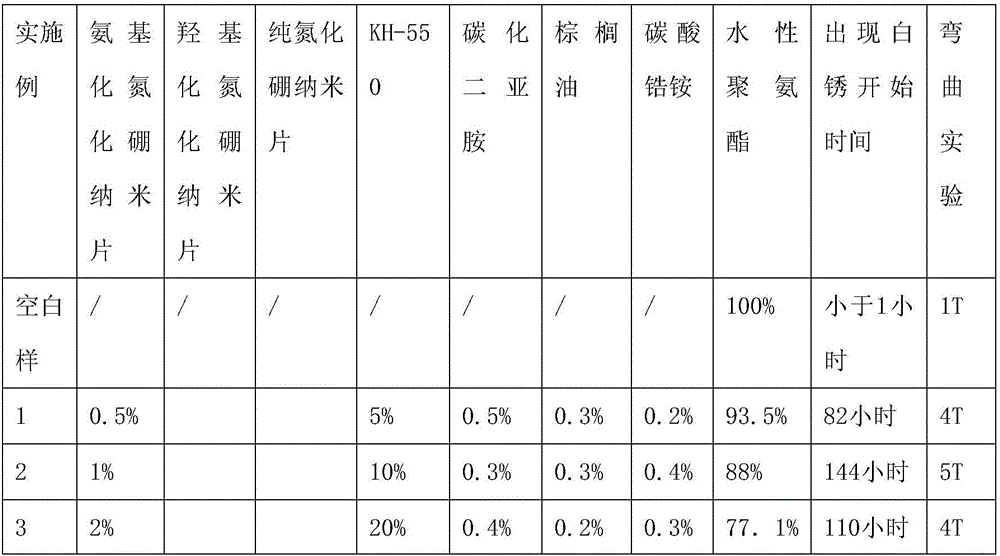
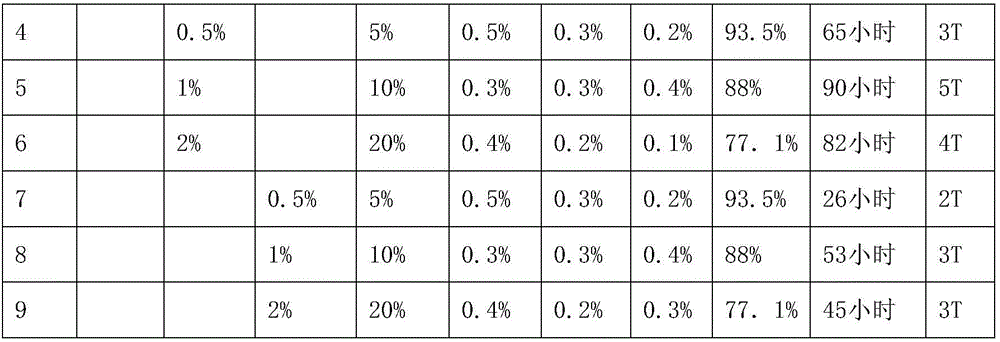
Preparation methods of metal surface treatment agent containing nano boron nitride and corrosion-resistant coating
ActiveCN106010181AGood dispersionGood compatibilityFireproof paintsLiquid surface applicatorsSolid componentSalt spray test
The invention provides a metal surface treatment agent containing nano boron nitride. The metal surface treatment agent is prepared from waterborne resin, a dispersing agent, boron nitride and coating additives, wherein the mass of the solid component in the waterborne resin is 77%-93.5% that of the total solid components of a coating, the mass of the dispersing agent is 5%-20% that of the total solid components of the coating, the mass of boron nitride is 0.5%-2% that of the total solid components of the coating, and the mass of the coating additives is 0.5%-1% that of the total solid components of the coating. The invention further provides a preparation method of the metal surface treatment agent containing nano boron nitride and a method for preparing a corrosion-resistant coating. It is proved through salt spray tests that the composite coating has ultra-high corrosion resistance. The metal surface treatment agent is waterborne paint, the formula does not contain any solvent except water, therefore, emission of volatile organic compounds does not exist, and the metal surface treatment agent meets the environmental protection requirement and is suitable for surface corrosion prevention of metal substrates in industrialized production.
Owner:UNIV OF SHANGHAI FOR SCI & TECH
Method for processing superhard aluminum alloy oxide film
The invention relates to a method for processing a superhard aluminum alloy oxide film, which is characterized by comprising the following technical steps: binding an aluminum alloy section bar on a conducting rod and hanging the aluminum alloy section bar in a degreasing groove to carry out degreasing processing at room temperature; after the aluminum alloy section bar carries out degreasing processing, washing; hanging the washed aluminum alloy section bar in an alkali washing groove to carry out alkali washing; after the aluminum alloy section bar carries out the alkali washing, hanging the aluminum alloy section bar into a neutralization groove to carry out neutralization processing; washing the aluminum alloy section bar after neutralization processing by water; after the aluminum alloy section bar is washed by the water, hanging the aluminum alloy section bar into an oxidation groove to carry out anode oxidation processing; washing the aluminum alloy section bar after the anode oxidation processing by water; after the aluminum alloy section bar is washed by the water, hanging the aluminum alloy section bar into a hole seal groove to carry out hole seal processing; and after the aluminum alloy section bar carries out the hole seal processing, carrying out drying processing by a steam pipeline to form a finished product. The invention can improve the hardness, the abrasive resistance and the corrosion resistance of the oxide film and lead properties of the oxide film to achieve the following requirements: the microhardness is larger than or equal to 1,000HV; the abrasive resistance is tested by a method of GB / T8013.1-2007, and the abrasive loss does not exceed 10 mg; and the salt fog test resistance is tested by a method of GB / T12967.3 and reaches the ninth grade.
Owner:江苏锦绣铝业有限公司
Corrosion-Resistant Epoxy Nanocomposite Coatings containing Submicron Emeraldine-Base Polyaniline and Organomodified Montmorrilonite
InactiveUS20100010119A1Good dispersionImprove barrier propertiesMaterial nanotechnologyLayered productsEpoxySalt spray test
Disclosed is a method of preparation of corrosion-resistant epoxy coatings. The coating composition contains two main corrosion resistant factors: The first one was Eemeraldine-Base polyaniline (EB-PANi), dissolved in the aminic hardener of epoxy. The other one was montmorrilonite clay, dispersed or exfoliated in the base component of epoxy resin. The hardener composition was prepared via dissolution of EB-PANi in functional amines like 3-(aminomethyl)-3,5,5-trimethylcyclohexan-1-amine adopting sonication and nanoscale filtering methods. The base component was prepared via gradual charging of MMT clay in epoxy resin via high-shear mixing plus sonication method. The morphology of the coatings during different stages of preparation was studied by optical microscopy and scanning electron microscopy and TEM. The corrosion-protective performance of the resultant coatings was evaluated by electrochemical impedance spectroscopy and salt spray tests. The results were compared with those of conventional epoxy zinc-chromate and neat resin coatings. Superior corrosion resistance was achieved via dissolution of 0.5-2.5 wt % of EB-PANi in the aminic hardener and 2-4 WT % of organomodified MMT in base component of coating.
Owner:ZAAREI DAVOOD +4
Chromium(VI)-free conversion layer and method for producing it
A chromium(VI)-free, chromium(III)-containing and substantially coherent conversion layer on zinc or zinc alloys presenting, even in the absence of further components such as silicate, cerium, aluminum and borate, a corrosion protection of approx. 100 to 1000 h in the salt spray test according to DIN 50021 SS or ASTM B 117-73 until first attack according to DIN 50961 Chapter 10; being clear, transparent and substantially colorless and presenting multi-colored iridescence; having a layer thickness of approx. 100 nm to 1000 nm; and being hard, adhering well and being resistant to wiping.
Owner:SURTEC INT
Micro-arc oxidation treatment method of heat-resistant cast rare earth magnesium alloy
InactiveCN101698957AImprove corrosion resistanceLow costAnodisationChemical treatmentMicro arc oxidation
The invention relates to a micro-arc oxidation treatment method of a heat-resistant cast rare earth magnesium alloy. The method has the advantages of simple equipment, low cost and no point discharge phenomenon. The heat-resistant cast rare earth magnesium alloy processed by the method has excellent corrosion resistance, when using the neutral salt spray (NSS) method in Corrosion-resistant testing method of the metal deposits and conversion coatings for the light industrial products of QB / T 3826-1999 for testing, taking sodium chloride solution with the weight percent of 5%, keeping the temperature constant at 35 DEG C plus or minus 1 DEG C and meeting the neutral salt spray-resistant test conditions, the heat-resistant cast rare earth magnesium alloy is rated nine grade according to Methods for corrosion testing of metallic and other inorganic coatings on metallic substrates-Rating of test specimens and manufactured articles subjected to corrosion tests of GB / T 6461-2002. The corrosion resistance is more than 96 hours.
Owner:CHANGCHUN INST OF APPLIED CHEMISTRY - CHINESE ACAD OF SCI
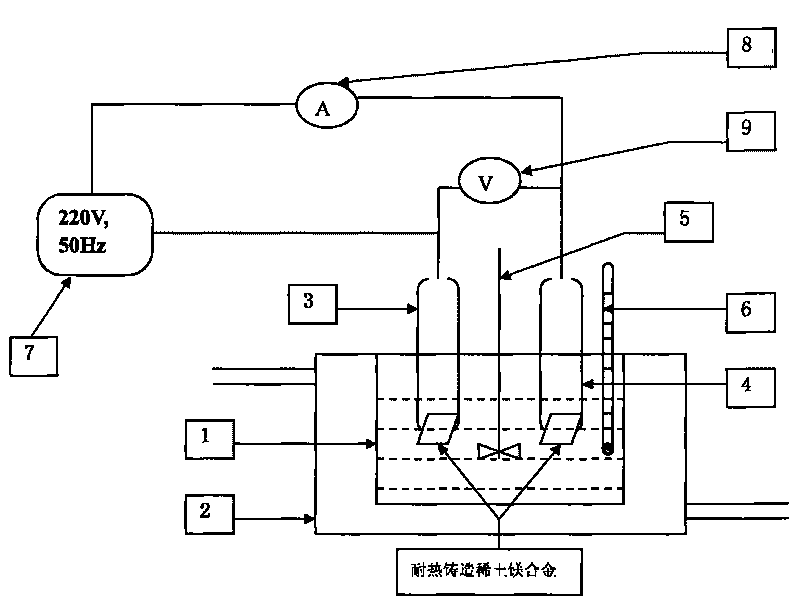
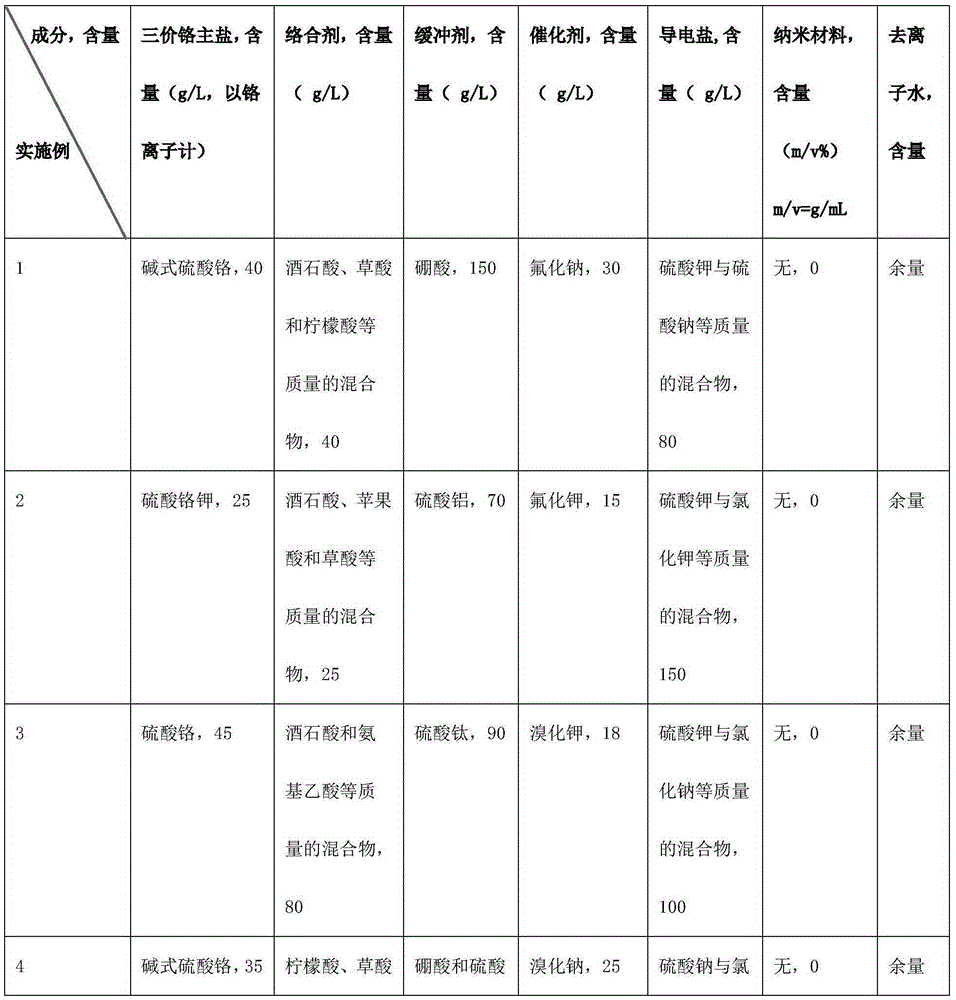
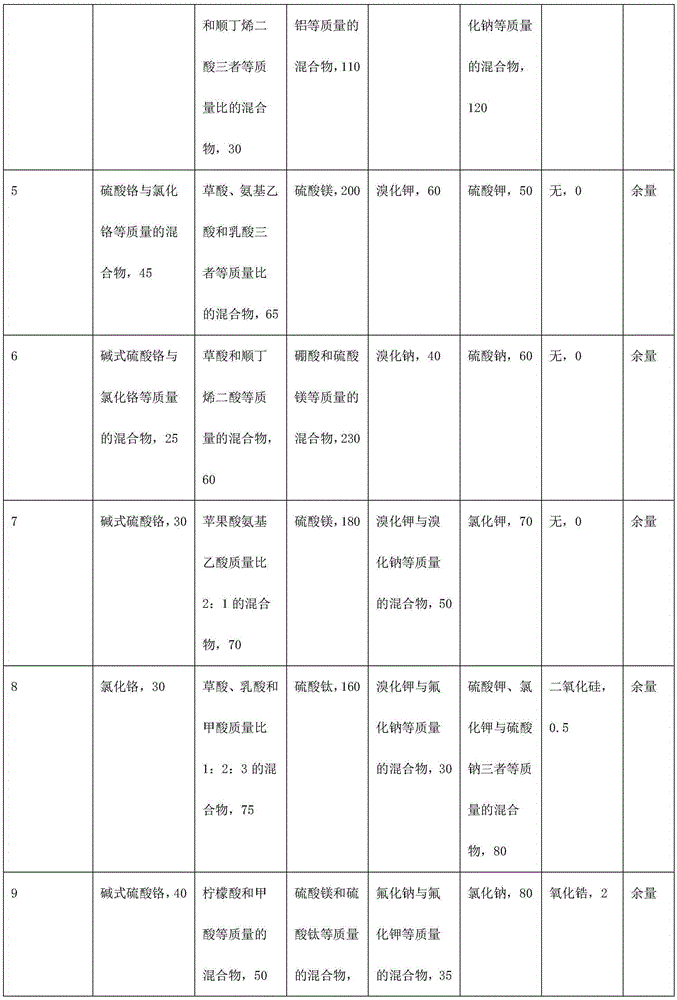
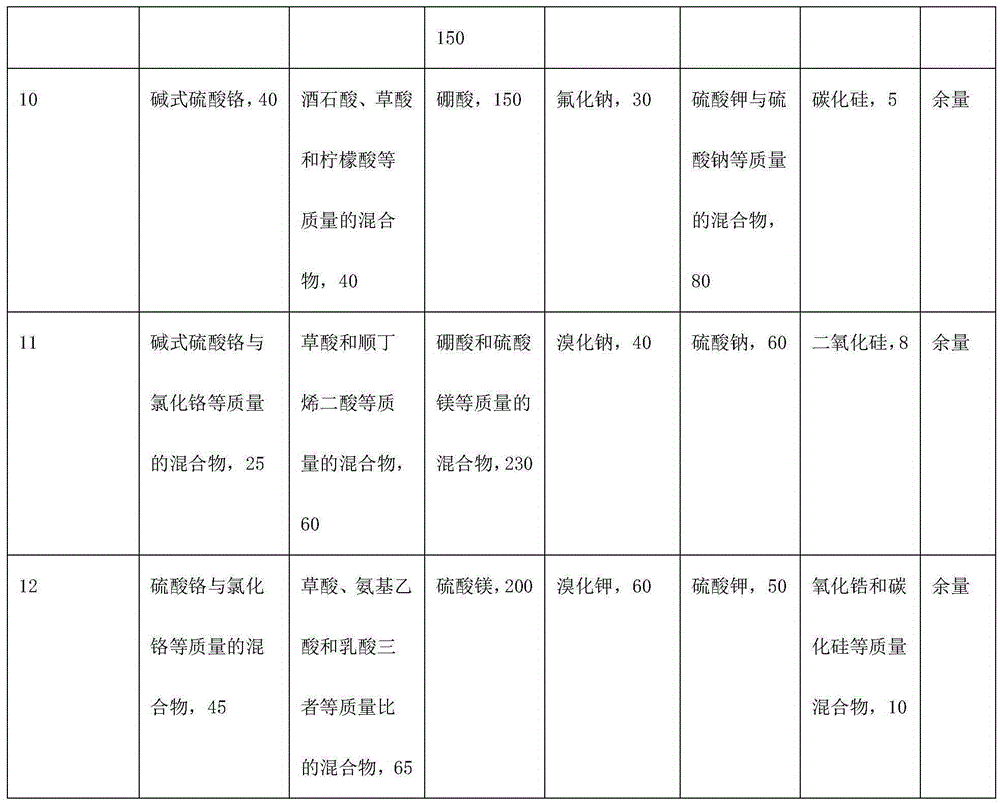
Trivalent chromium hard chromium electroplating solution and application of trivalent chromium hard chromium electroplating solution in hard chromium electroplating
The invention belongs to the technical field of metal surface treatment, and particularly relates to a trivalent chromium hard chromium electroplating solution and application of the trivalent chromium hard chromium electroplating solution in hard chromium electroplating. The electroplating solution is prepared from trivalent chromium main salt, a carboxylic acid or carboxylate complexing agent, a buffering agent, conducting salt and a catalyst. The deposition velocity reaches 2.5 micrometers / minute or above, the current efficiency reaches 36% or above, the thickness of an electroplating layer can reach 150 micrometers or above, each liter of the electroplating solution can be used for performing continuous electroplating for 900 ampere hours (an additive is supplemented during the process), high efficiency and high stability are maintained, the hardness of the electroplating layer reaches HV950 or above, and the electroplating layer has wear resistance the same as or similar to that of hexavalent chromium, has large binding force with steel, copper and copper alloy and is free of peeling or stripping or cracking in a 300 DEG C thermal shock test. As nanometer materials are added, corrosion resistance lasts for 150 h or longer in a neutral salt spray test. The trivalent chromium hard chromium electroplating solution has broad application prospects in the fields of rollers, hydraulic components, printing cylinders, shock absorbers, coal mine hydraulic supports and the like.
Owner:WUHAN DESYTEK ENVIRONMENTAL PROTECTION NEW MATERIAL CO LTD
Chromium-free inactivating liquor for continuous galvanizing by hot-dip galvanized steel sheet
ActiveCN101418443ALow costChange process conditionsMetallic material coating processesChromium freeTungstate
The invention relates to a chromium-free passivation solution used for continuous hot dipping coating of galvanized steel sheet, which belongs to the technical field of metal surface treatment and corrosion prevention. The compositions and the mixed ratio in one liter of the passivation solution are as follows: 3 to 5 grams of molybdate, 5 to 15 grams of tungstate, 3 to 5 grams of tannic acid, 5 to 20 grams of citric acid, 10 to 30 grams of hydrogen peroxide, 10 to 30 grams of silicate, 20 to 30 grams of pH value regulator, and the balance being water. The chromium-free passivation solution has the following advantages: as no chromium is contained, the chromium-free passivation solution has no effect on a human body and the environment, and has low cost which is equivalent to that of a chromate passivation solution and is approximately 30 percent of that of the chromium-free passivation solution used in the prior art, thus the cost is less than one tenth that of the chromium-free passivation solution containing 80 to 160 grams of the molybdate per liter and 16 to 32 grams of the tungstate per liter in the prior art; besides, the salt spray corrosion resistance of the passivated galvanized steel sheet can meet the requirement of national standard with the corrosion area of a 72-hour neutral salt spray test less than 5 percent; and the chromium-free passivation solution not only does not change the prior process conditions, but also does not change the prior equipment in use.
Owner:CHANGSHU HUAYE STEEL STRIP
Composite surface protecting method for neodymium-iron-boron magnet
ActiveCN102368438AImprove corrosion resistanceExtended service lifeLiquid/solution decomposition chemical coatingInductances/transformers/magnets manufactureSalt spray testBoron
The invention discloses a composite surface protecting method for a neodymium-iron-boron magnet, comprising the following steps of: pretreating a neodymium-iron-boron magnet, electroplating or phosphating the pretreated neodymium-iron-boron magnet, spraying a corrosion-resistant coating on the surface of the electroplated neodymium-iron-boron magnet, pre-curing the sprayed neodymium-iron-boron magnet, curing the pre-cured neodymium-iron-boron magnet and performing vapour phase deposition on the cured neodymium-iron-boron magnet. The composite surface protecting method for the neodymium-iron-boron magnet has the following advantages that: the combining stability between the plating and the neodymium-iron-boron magnet and the corrosion-resistant property of the plating are improved by meansof the corrosion-resistant coating; the electroplating treatment and the spraying treatment are further enhanced by the vapour phase deposition; meanwhile, a circular hole structure of the neodymium-iron-boron magnet is further plated so that the integrated plating on the surface of the neodymium-iron-boron magnet is uniform; and the endurance time of the neutral salt spray test of the compoundedneodymium-iron-boron magnet can reach more than 300 hours.
Owner:NINGBO YUNSHENG HIGH TECH MAGNETICS +2
Magnesium alloy material with high corrosion resistance and preparation method of magnesium alloy material
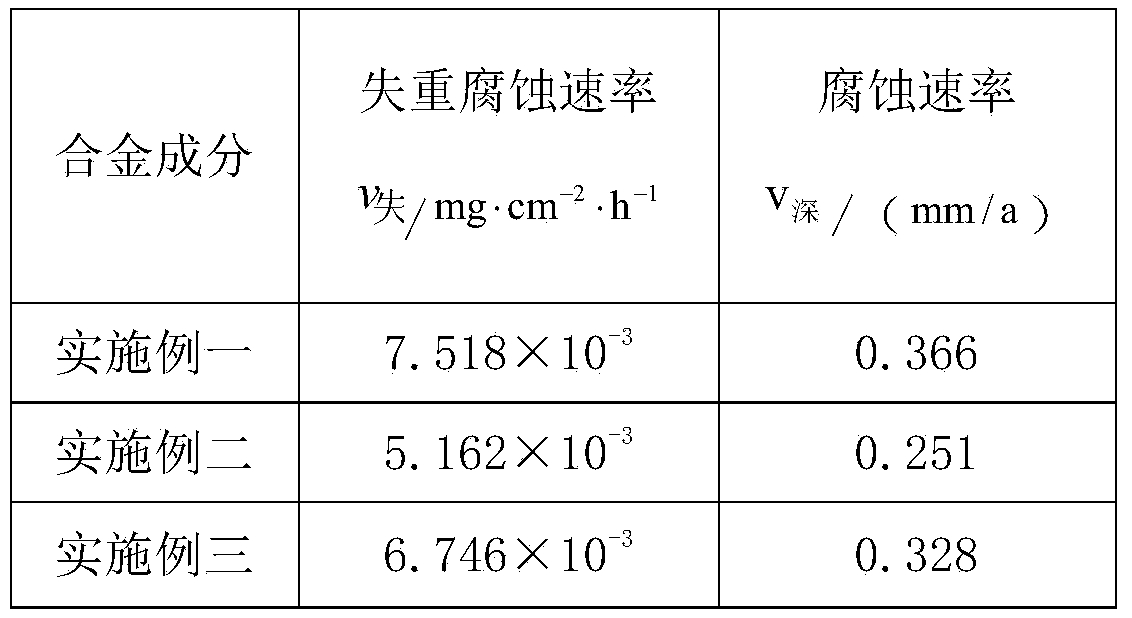
The invention belongs to the field of metal materials, and particularly relates to an alloy proportion of a magnesium alloy with high corrosion resistance. The alloy material comprises components in percentage by mass as follows: 4.5%-5.5% of Al, 1.5%-2% of Zn, 0.1%-0.3% of Mn, 0.1%0.5% of Y as well as impurities, the impurities comprise elements as follows: smaller than or equal to 0.01% of Fe, smaller than or equal to 0.003% of Ni, smaller than or equal to 0.0004% of Cu, smaller than or equal to 0.03% of Si, smaller than or equal to 0.002% of Ca and the balance of magnesium. By means of Al, Mn, Zn and Y alloying scientific formula design, the corrosion resistance of the magnesium alloy is greatly improved with lower cost; and besides, 5% of NaCl contained neutral salt spray test is performed for 120 h, the corrosion rate v (agravity) is smaller than 0.008 mg*cm<-2>*h<-1>, and v (depth) is smaller than 0.5 mm / a.
Owner:ZHONGBEI UNIV
Detection method of plated layer corrosion rate in steel wire or steel wire rope
InactiveCN103353432AJudging the anti-corrosion abilityThe service life is easy to judgeWeighing by removing componentWeather/light/corrosion resistanceAlcoholSalt spray test
The invention relates to a detection method of plated layer corrosion rate in a steel wire or a steel wire rope. The method comprises the process steps that: 1, a sample is prepared, wherein a plated steel wire or steel wire rope is cut into N segments; the surfaces are cleaned by using alcohol cotton; and the ends are sequentially sealed by using a solid sealant (AB composite adhesive), such that N samples are prepared; 2, the samples are weighed; 3, a salt spray test is carried out, wherein the weighed samples are subjected to a neutral salt spray test in a salt spray box; 4, the surfaces of the samples are cleaned, wherein the samples after the salt spray test are washed in a container with a prepared chromic acid cleaning agent, such that residual salt and corrosives on the surface of the samples are completely removed; the samples are washed by using water, and are dried; and 5, weighing and calculation are carried out, wherein the dried samples are weighed, and the weights are adopted as after-test weights; and the corrosion rate is calculated according to the weight loss before and after the test. With the method provided by the invention, corrosion-resistant rates of various plated layers can be easily determined, and the service lives of the plated layer samples in the environment can be inferred.
Owner:JIANGSU FASTEN MATERIAL ANALYSIS & INSPECTION
Zinc coat environmental-friendly passivation solution and use method thereof
InactiveCN101886259ASolve pollutionCorrosion resistance is equivalentMetallic material coating processesSodium molybdateSalt spray test
The invention discloses a zinc coat environmental-friendly passivation solution and a use method thereof. The passivation solution is formed by dissolving a solute consisting of a plurality of materials into deionized water, wherein the solute in the solution comprises the following components: 80-120g / L of sodium molybdate, 20-30g / L of sodium tungstate, 2-7g / L of titanyl sulfate, 5-10g / L of phytic acid, 15-50g / L of acrylic acid and 20-40g / L of hydrogen peroxide, wherein the pH value of the solution is regulated to 1.5-2.5 by using dilute acid. It is found that the zinc coat environmental-friendly passivation solution contains no chromium ions and thoroughly solves the problem of environment pollution brought by chromating; through salt spray test examination, a zinc coating plate passivated by the passivation solution has corrosion resistance approximately consistent with that of the traditional passivation solution; meanwhile, the use method is simple and easy.
Owner:WUHAN IRON & STEEL (GROUP) CORP
Water base non-chrome zinc-aluminium coating and methods for preparing and using same
ActiveCN102911534AMeet the requirements of environmental regulationsHydrogen embrittlement will notLiquid surface applicatorsCoatingsWater basedHazardous substance
The invention relates to a water base non-chrome zinc-aluminium coating and methods for preparing and using the same. The coating is prepared by proportionally mixing a component A and a component B; the component A contains 50-150g / L of lamellar metal powder, 145-290ml / L of organic solvent, 10-200ml / L of deionized water, 10-36ml / L of reducing agent and 5-15ml / L of wetting agent; and the component B contains 60-240ml / L of deionized water, 20-75ml / L of binding agent, 8-22ml / L of thickening agent, 4-8ml / L of silicon compound and the balance of the organic solvent. The method for preparing the water base non-chrome zinc-aluminium coating comprises the steps of: weighing the lamellar metal powder proportionally to be mixed uniformly, and then adding the organic solvent to be mixed uniformly for later use; mixing the reducing agent with the wetting agent, adding an appropriate amount of the deionized water to be mixed uniformly, and then adding the lamellar metal powder liquid to prepare the component A; proportionally weighing the thickening agent, the binding agent and the silicon compound, and adding the organic solvent to be mixed uniformly to prepare the component B; and mixing the prepared component A and the prepared component B according to the ratio of 2:1, and stirring unit uniform, thus obtaining the coating. The method for using the water base non-chrome zinc-aluminium coating is that the coating is coated on a part to be treated, and the baking temperature is increased to 360 DEG C so as to solidify and crosslink the coating to form a dense protective layer. The coating has the corrosion resistance which reaches 840 hours of a neutral salt spray test and over 96 hours of a heat resistance test, the usage amount of harmful substances is greatly reduced, and the working environment is improved.
Owner:翊鹏(湖北)实业有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com