Patents
Literature
3925 results about "Neodymium" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Neodymium is a chemical element with the symbol Nd and atomic number 60. Neodymium belongs to the lanthanide series and is a rare-earth element. It is a hard, slightly malleable silvery metal, that quickly tarnishes in air and moisture. When oxidized, neodymium reacts quickly to produce pink, purple/blue and yellow compounds in the +2, +3 and +4 oxidation states. Neodymium was discovered in 1885 by the Austrian chemist Carl Auer von Welsbach. It is present in significant quantities in the ore minerals monazite and bastnäsite. Neodymium is not found naturally in metallic form or unmixed with other lanthanides, and it is usually refined for general use. Although neodymium is classed as a rare-earth element, it is fairly common, no rarer than cobalt, nickel, or copper, and is widely distributed in the Earth's crust. Most of the world's commercial neodymium is mined in China.
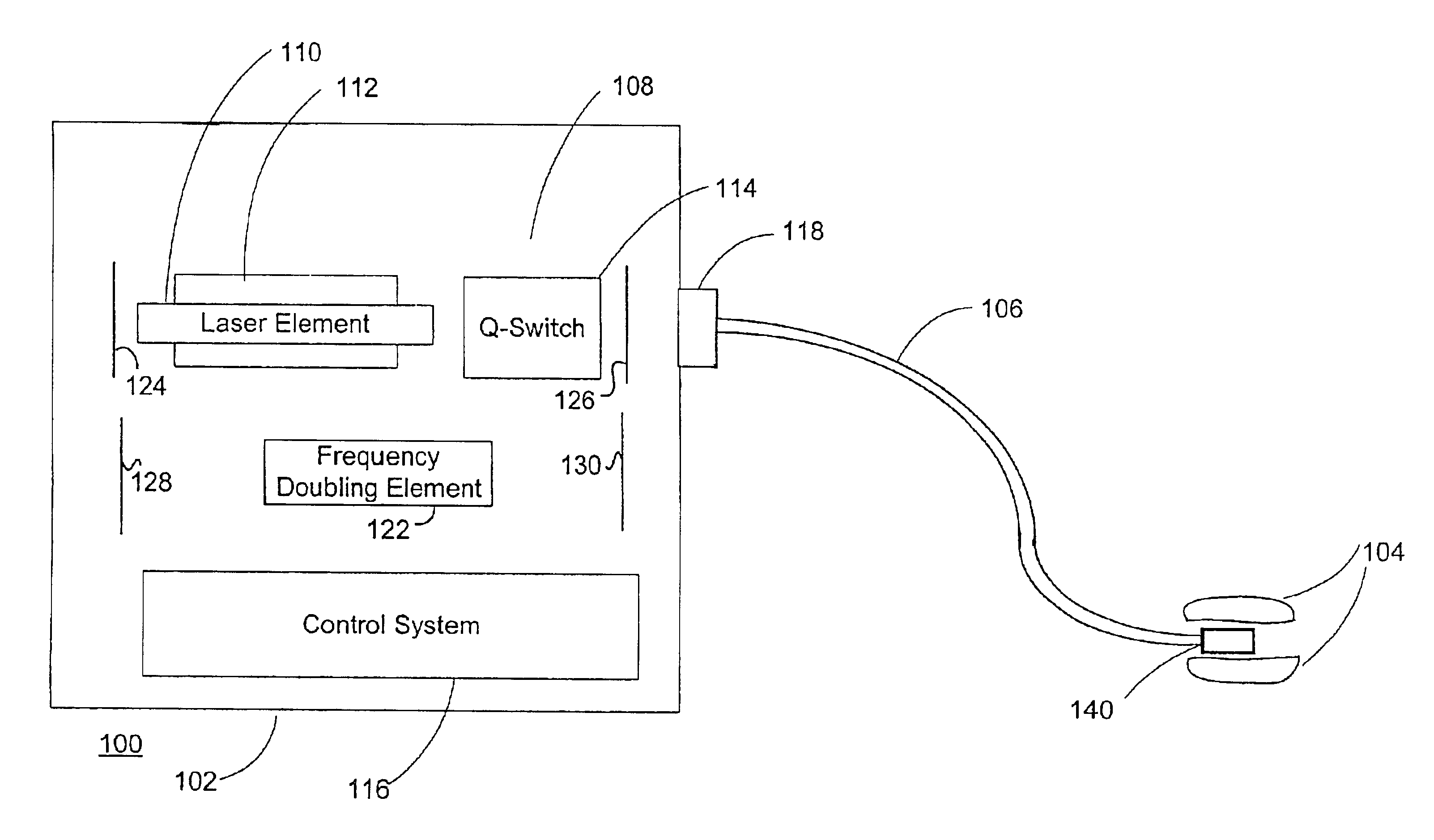
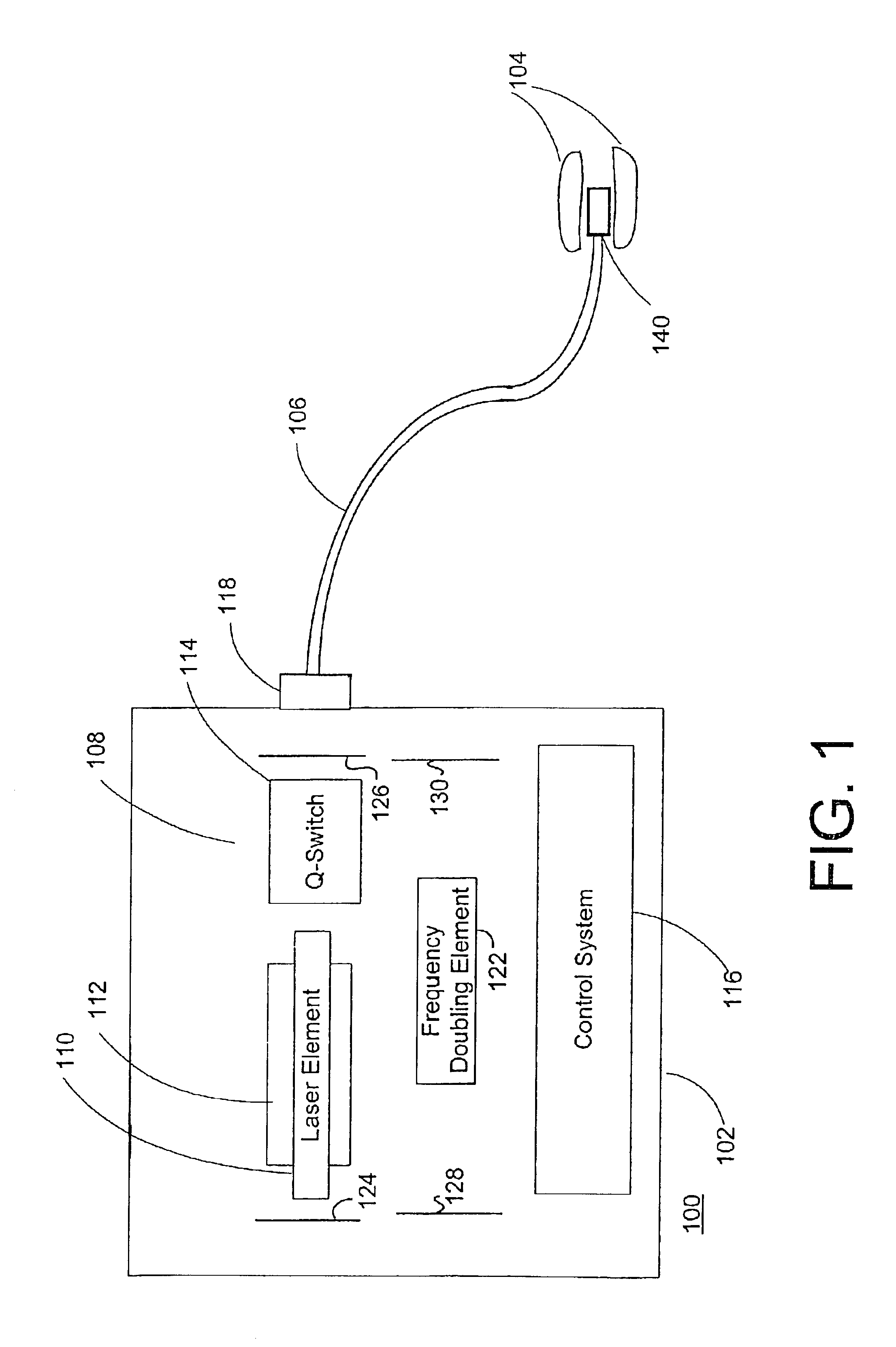
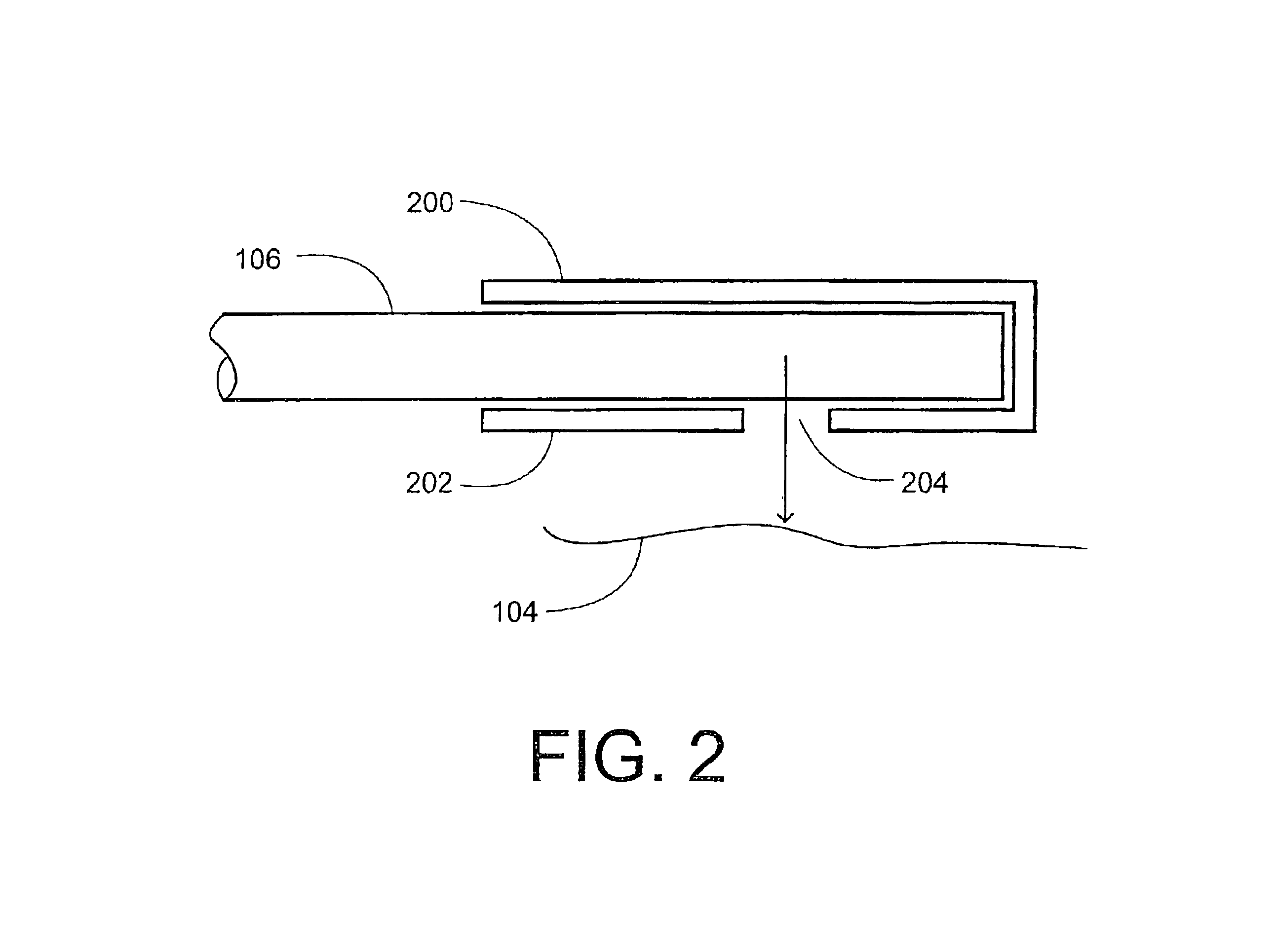
Method and system for photoselective vaporization of the prostate, and other tissue
InactiveUS6986764B2Reduce incidenceLess side effectsEndoscopesSurgical instrument detailsOptical radiationMedicine
A method for photoselective vaporization of prostate tissue includes delivering laser radiation to the treatment area on the tissue, via an optical fiber for example, wherein the laser radiation has a wavelength and irradiance in the treatment area on the surface of the tissue sufficient because vaporization of a substantially greater volume of tissue than a volume of residual coagulated tissue caused by the laser radiation. The laser radiation is generated using a neodymium doped solid-state laser, including optics producing a second or higher harmonic output with greater than 60 watts average output power. The delivered laser radiation has a wavelength for example in a range of about 200 nm to about 650 nm, and has an average irradiance in the treatment area greater than about 10 kilowatts / cm2, in a spot size of at least 0.05 mm2.
Owner:BOSTON SCI SCIMED INC
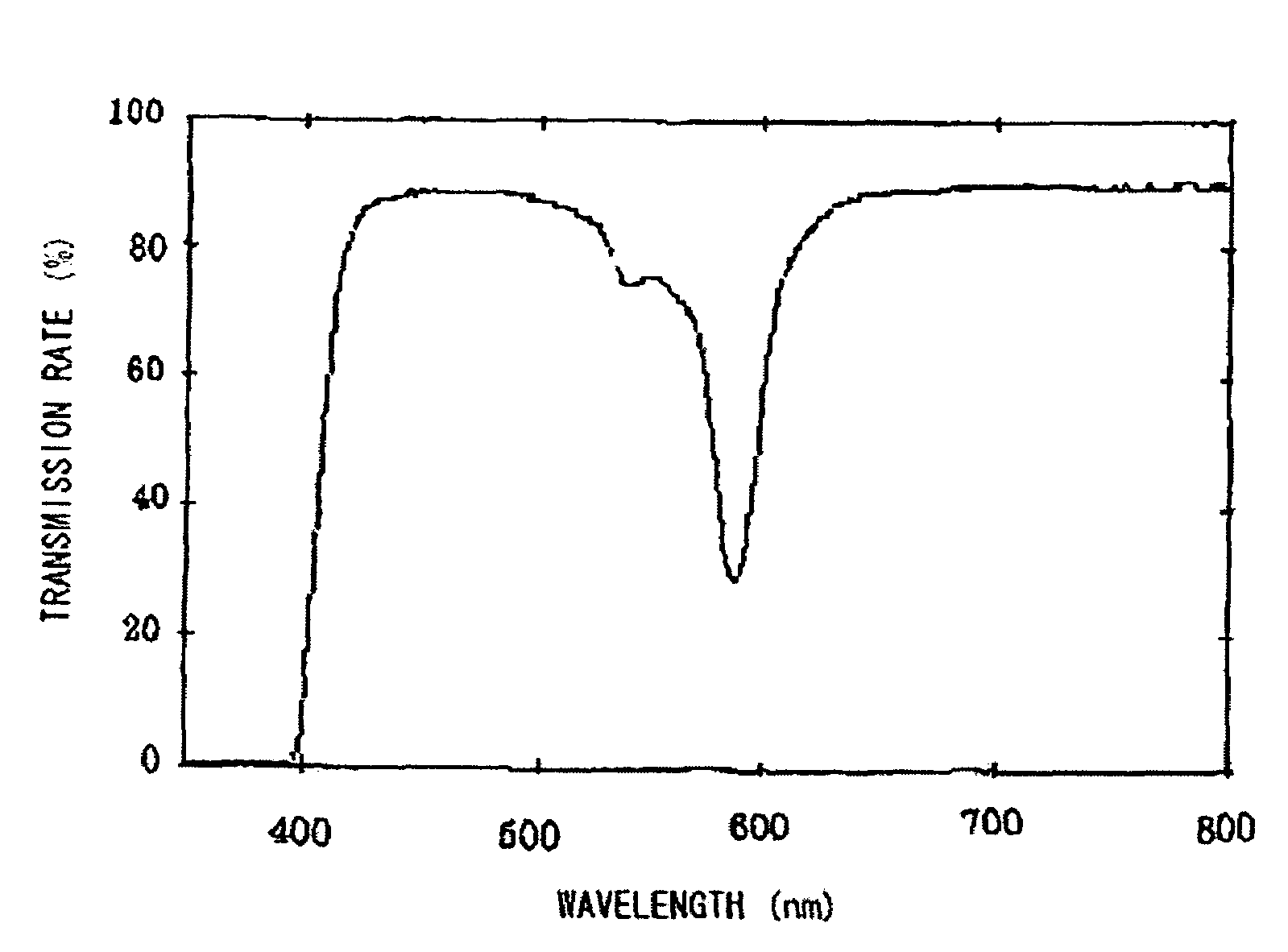
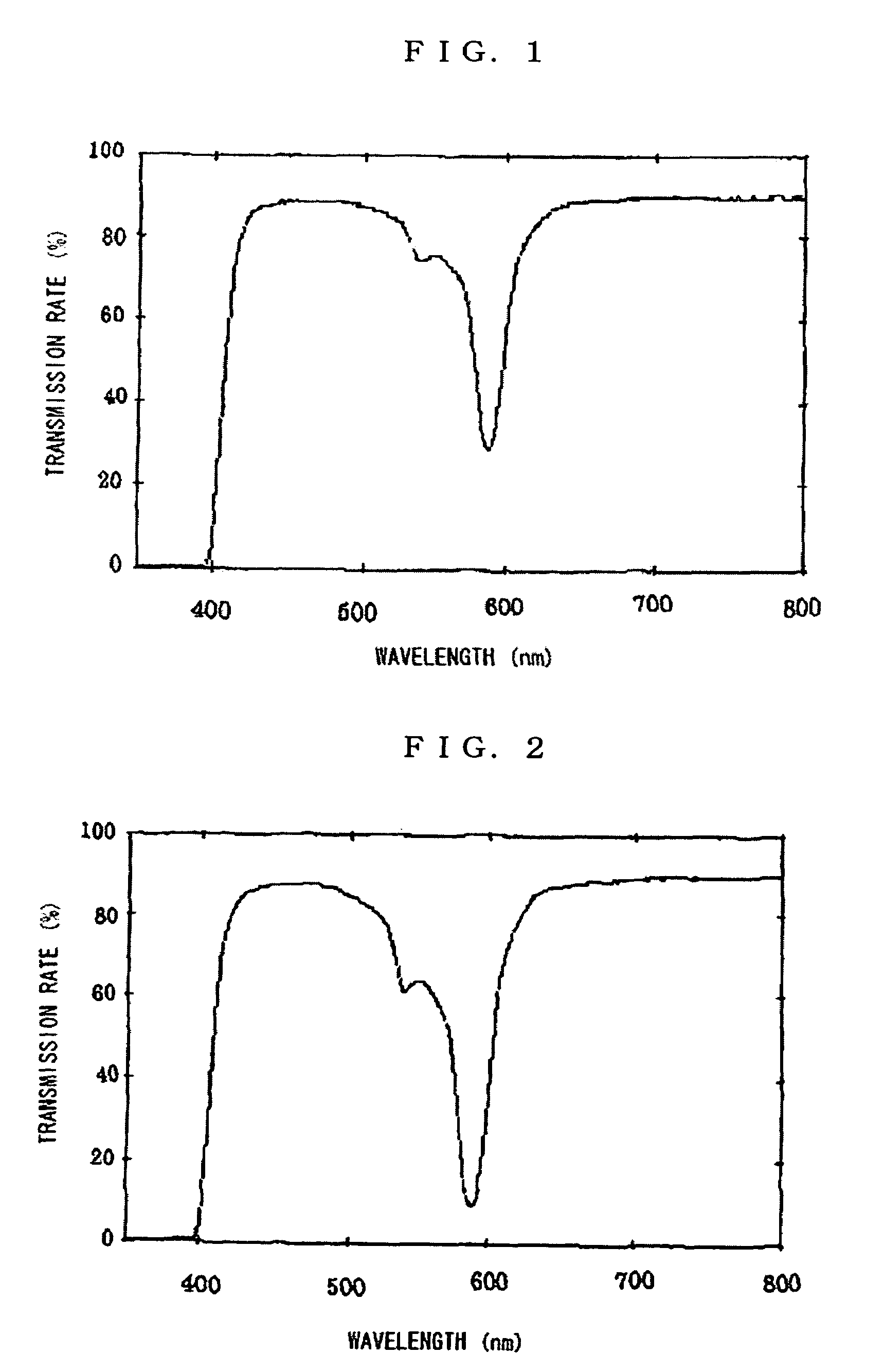
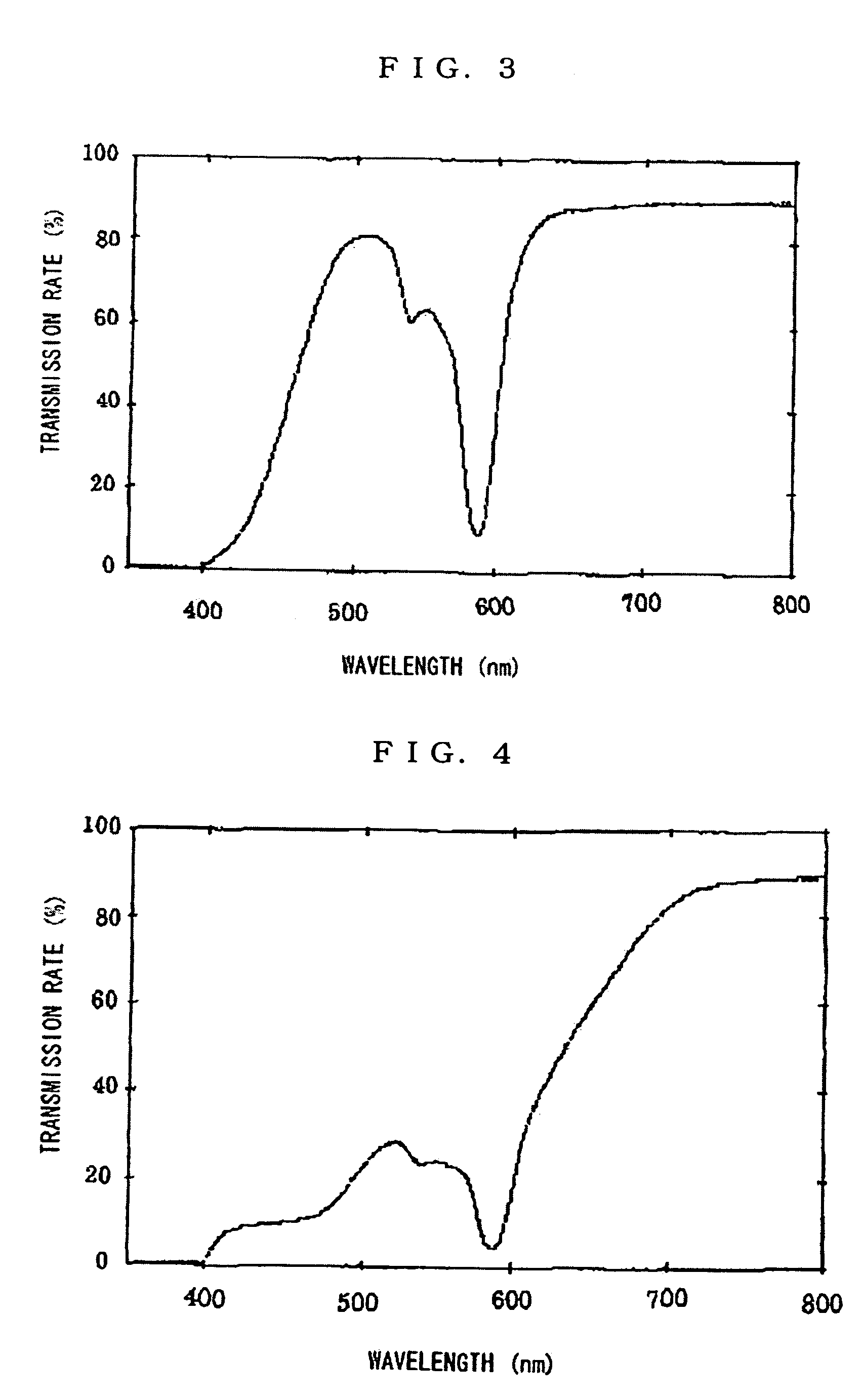
Plastic spectacles lens
ActiveUS7506977B1Good effectExcellently-balanced dazzle-preventing effectPorphines/azaporphinesOptical partsEyeglass lensesOrganic dye
A plastic spectacles lens containing an organic dye instead of a neodymium compound and having an optical transmission equivalent to a plastic spectacles lens containing a neodymium compound is provided. The plastic spectacles lens comprises a plastic lens wafer formed from a thermosetting or thermoplastic resin, or the plastic lens wafer and one, or two or more component layers formed on at least one side of the plastic lens wafer, and an organic dye satisfying the specific conditions.
Owner:HOPNIC LAB
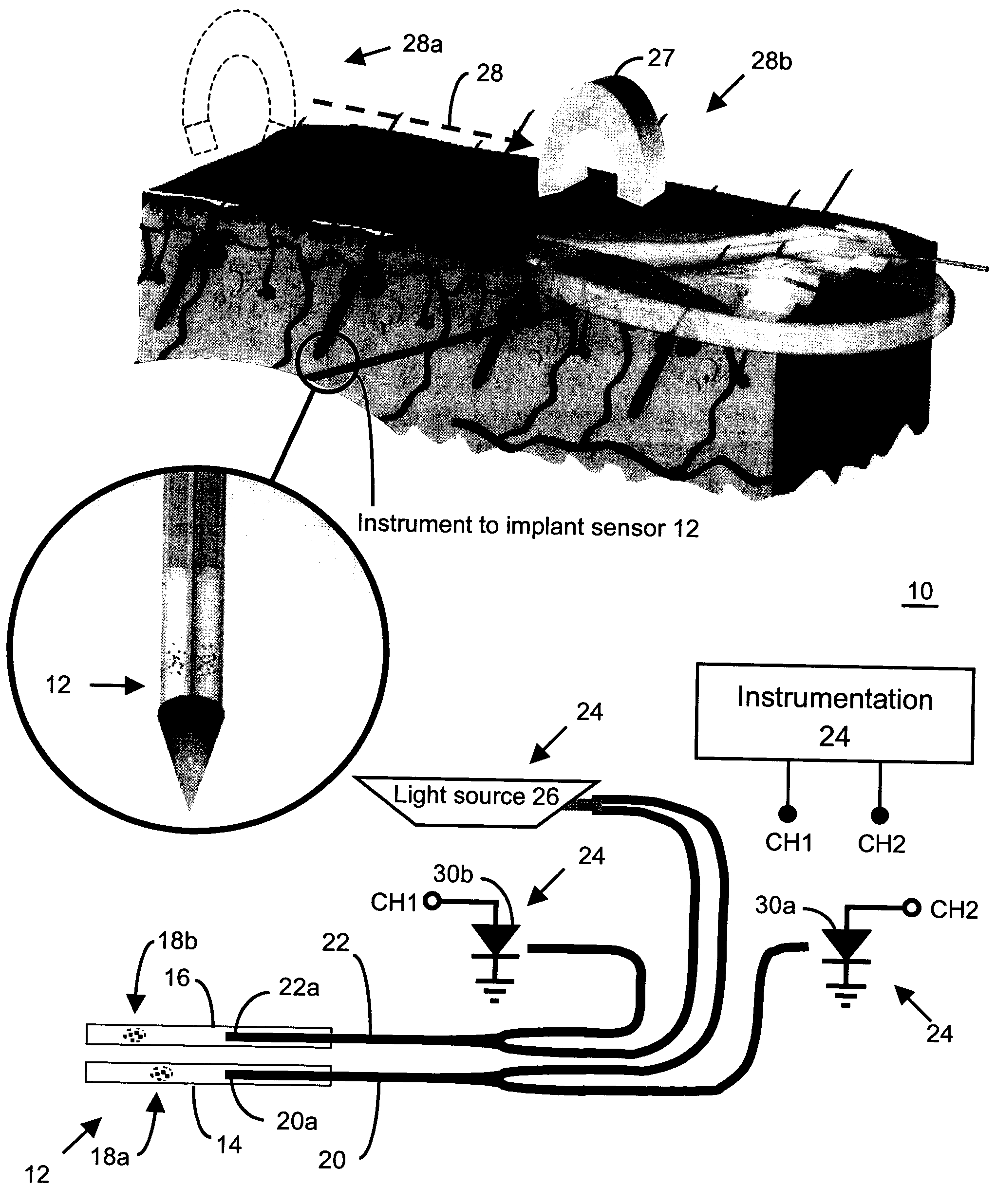
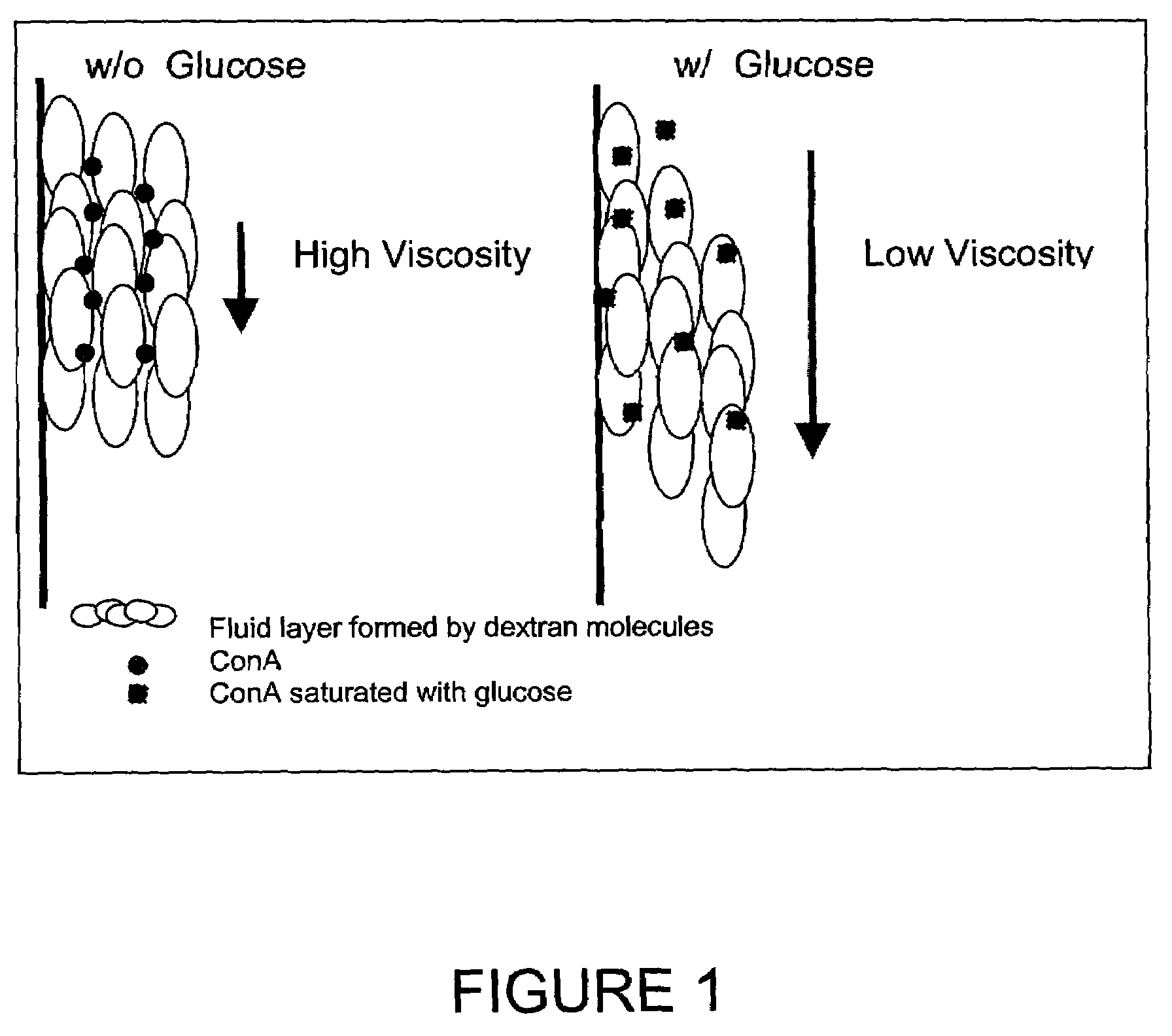
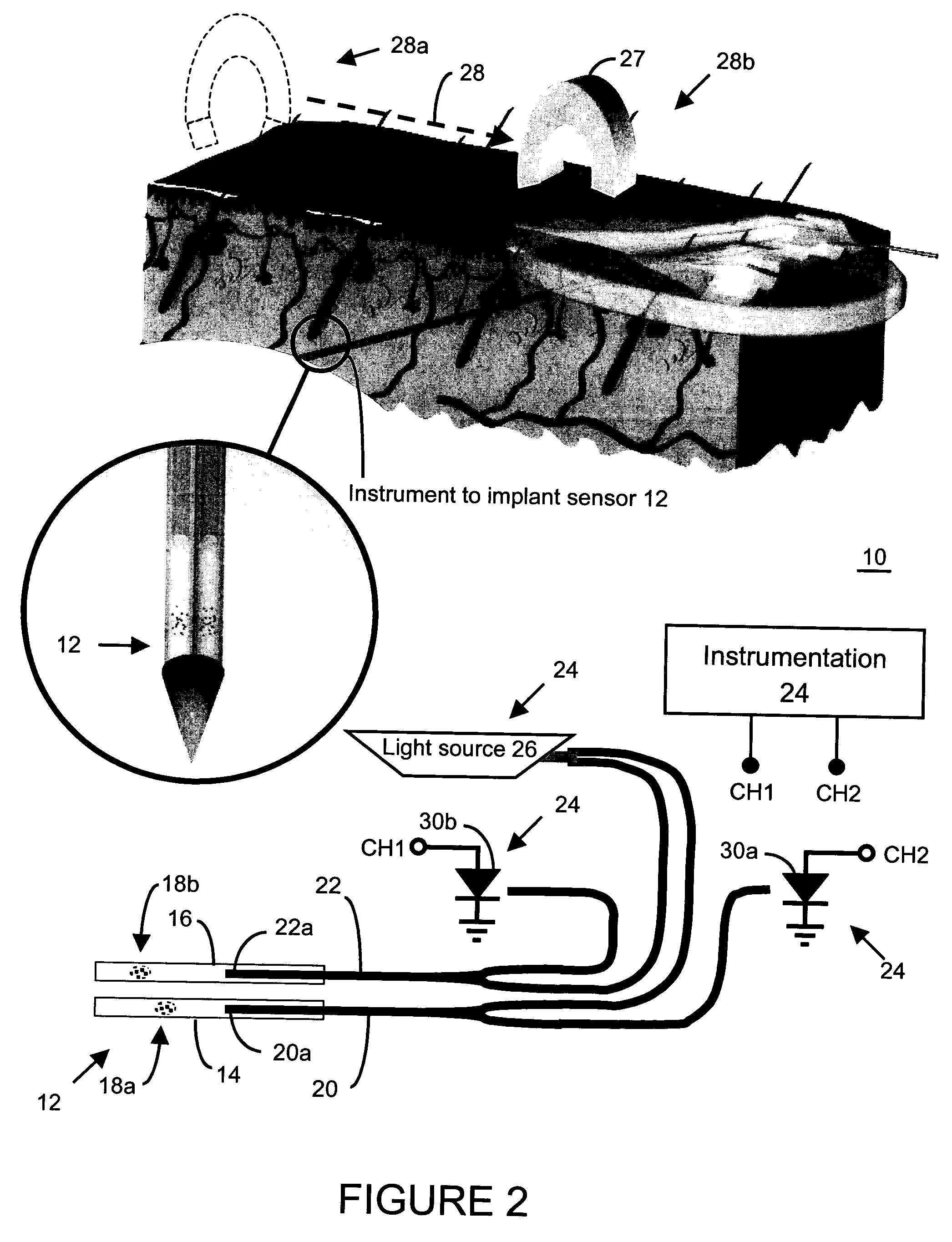
Method and apparatus for analyte sensing
InactiveUS7226414B2Reducing dispersion viscosityLow viscosityCatheterDiagnostic recording/measuringRare-earth elementEngineering
In one aspect, the present invention is directed to a glucose sensing device for implantation within subcutaneous tissue of an animal body. In one embodiment, the glucose sensing device includes a first chamber containing first magnetic particles and a hydrocolloid solution (for example, ConA-dextran hydrocolloid) wherein the first magnetic particles are dispersed in the hydrocolloid solution. In operation, glucose within the animal may enter and exit the first chamber and the hydrocolloid solution changes in response to the presence or concentration of glucose within the first chamber. The sensing device also includes a reference chamber containing second magnetic particles and a reference solution wherein the second magnetic particles are dispersed in the reference solution. The reference solution (for example, oil or alcohol compounds) includes a known or fixed viscosity. The reference solution may also be a hydrocolloid solution (for example, ConA-dextran hydrocolloid). The first and / or second magnetic particles may include amine-terminated particles, at least one rare earth element (for example, neodymium or samarium), and / or a ferromagnetic material.
Owner:BIOTEX
Infrared reflective color pigment
InactiveUS6454848B2Reduce heat buildupReduce energy costsInorganic pigment treatmentCoatingsIndiumCobalt
The present invention provides new solid solutions having a corundum-hematite crystalline structure which are useful as inorganic color pigments. Solid solutions according to the present invention include a host component having a corundum-hematite crystalline structure which contains as guest components one or more elements from the group consisting of aluminum, antimony, bismuth, boron, chrome, cobalt, gallium, indium, iron, lanthanum, lithium, magnesium, manganese, molybdenum, neodymium, nickel, niobium, silicon, tin, titanium, vanadium, and zinc. Solid solutions according to the present invention are formed by thoroughly mixing compounds, usually metal oxides or precursors thereof, which contain the host and guest components and then calcining the compounds to form the solid solutions having the corundum-hematite crystalline structure. Some of the new solid solutions according to the present invention exhibit relatively low Y CIE tri-stimulus values and relatively high near infrared reflectance.
Owner:FERRO CORP
Polymer nanocomposite implants with enhanced transparency and mechanical properties for administration within humans or animals
Polymer nanocomposite implants with nanofillers and additives are described. The nanofillers described can be any composition with the preferred composition being those composing barium, bismuth, cerium, dysprosium, europium, gadolinium, hafnium, indium, lanthanum, neodymium, niobium, praseodymium, strontium, tantalum, tin, tungsten, ytterbium, yttrium, zinc, and zirconium. The additives can be of any composition with the preferred form being inorganic nanopowders comprising aluminum, calcium, gallium, iron, lithium, magnesium, silicon, sodium, strontium, titanium. Such nanocomposites are particularly useful as materials for biological use in applications such as drug delivery, biomed devices, bone or dental implants.
Owner:PPG IND OHIO INC
Inorganic dopants, inks and related nanotechnology
InactiveUS6849109B2Facilitated DiffusionLower transition temperatureSelenium/tellurium compundsCell electrodesIndiumCerium
Ink compositions with modified properties result from using a powder size below 100 nanometers. Colored inks are illustrated. Nanoscale coated, uncoated, whisker inorganic fillers are included. The pigment nanopowders taught comprise one or more elements from the group actinium, aluminum, antimony, arsenic, barium, beryllium, bismuth, cadmuim, calcium, cerium, cesium, chalcogenide, cobalt, copper, dysprosium, erbium, europium, gadolinium, gallium, gold, hafnium, hydrogen, indium, iridium, iron, lanthanum, lithium, magnesium, manganese, mendelevium, mercury, molybdenum, neodymium, neptunium, nickel, niobium, nitrogen, oxygen, osmium, palladium, platinum, potassium, praseodymium, promethium, protactinium, rhenium, rubidium, scandium, silver, sodium, strontium, tantalum, terbium, thallium, thorium, tin, titanium, tungsten, vanadium, ytterbium, yttrium, zinc, and zirconium.
Owner:PPG IND OHIO INC
Method of manufacturing array substrate
InactiveUS6528357B2Reduce productionSolid-state devicesSemiconductor/solid-state device manufacturingOXALIC ACID DIHYDRATEDielectric
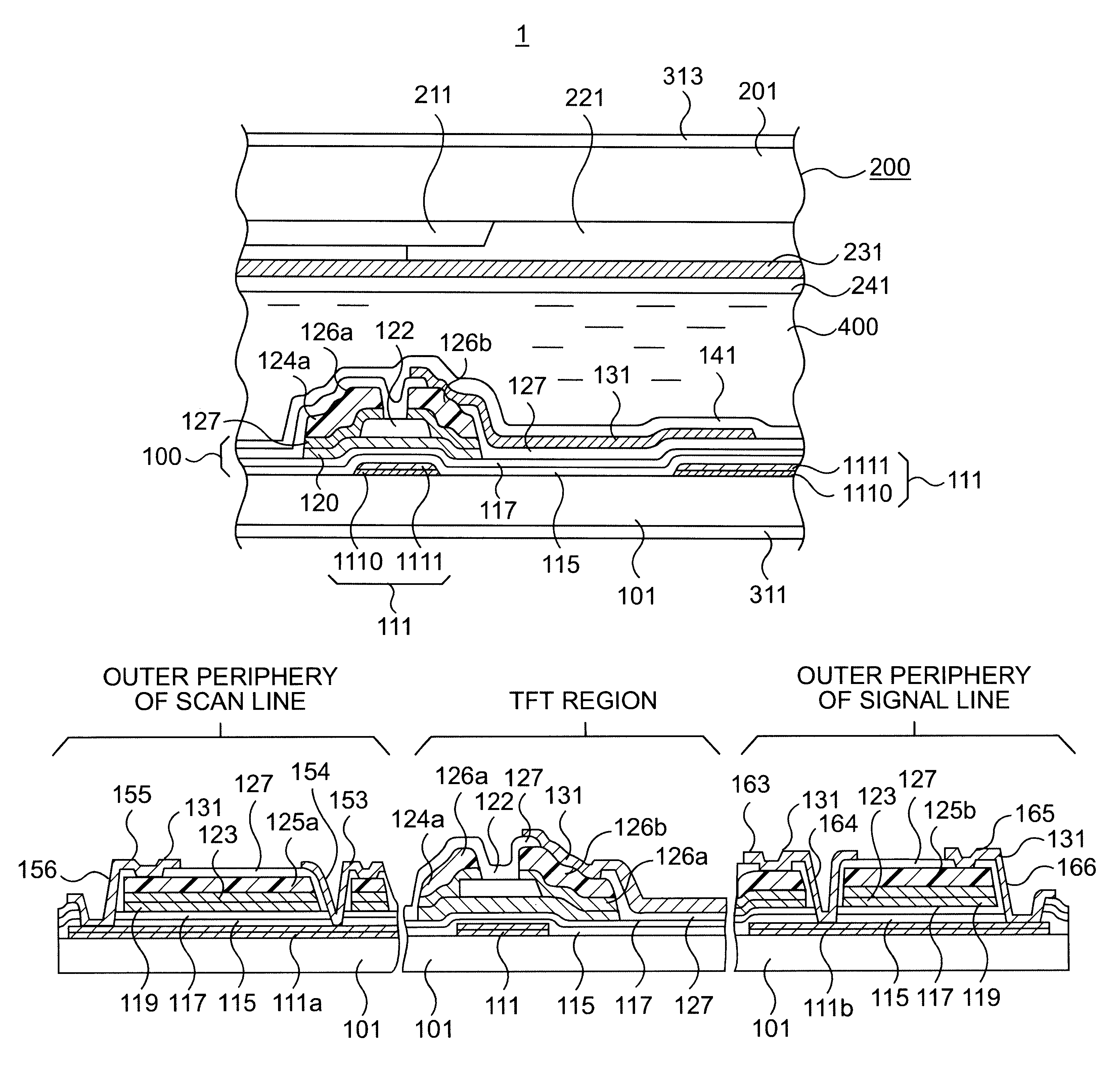
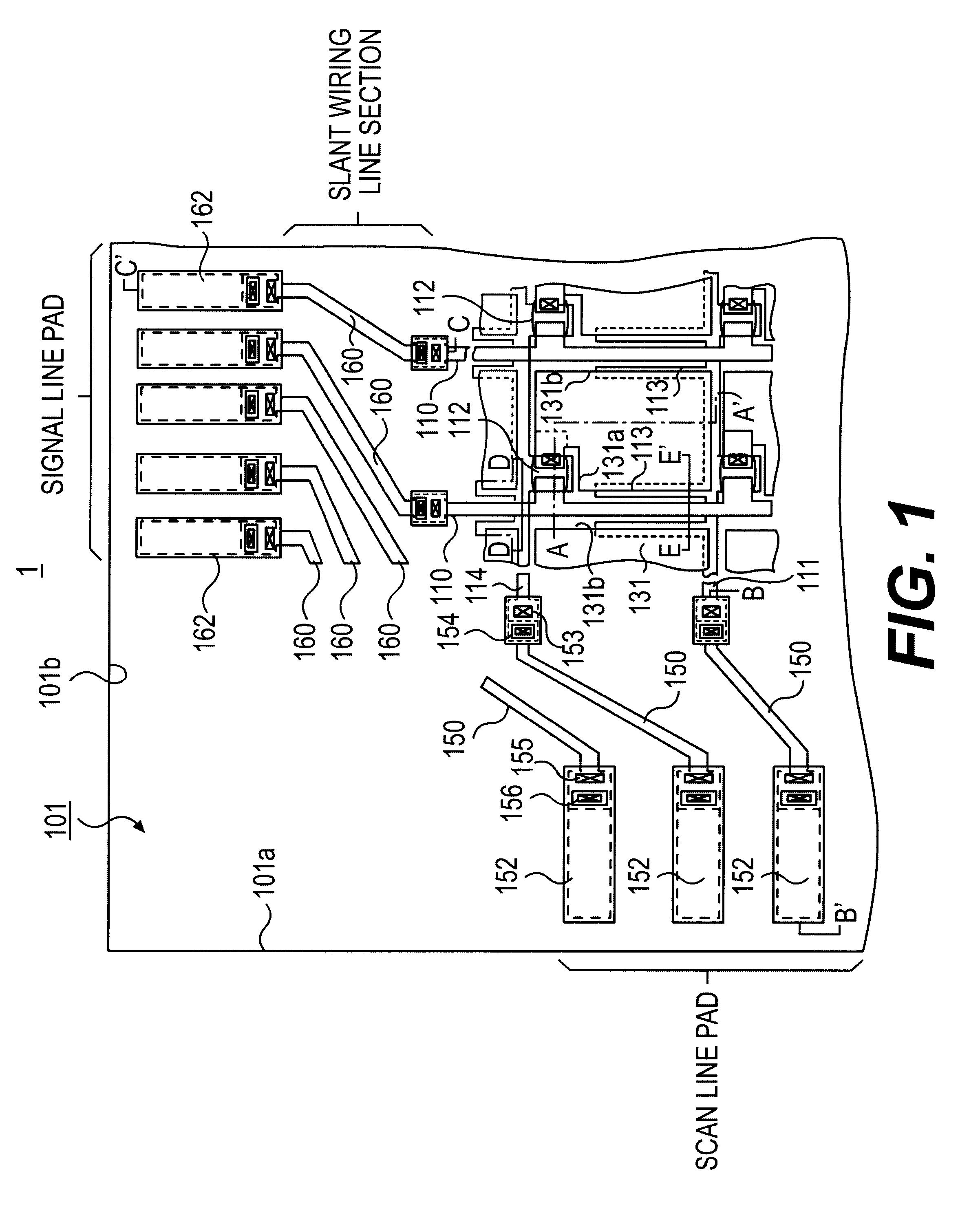
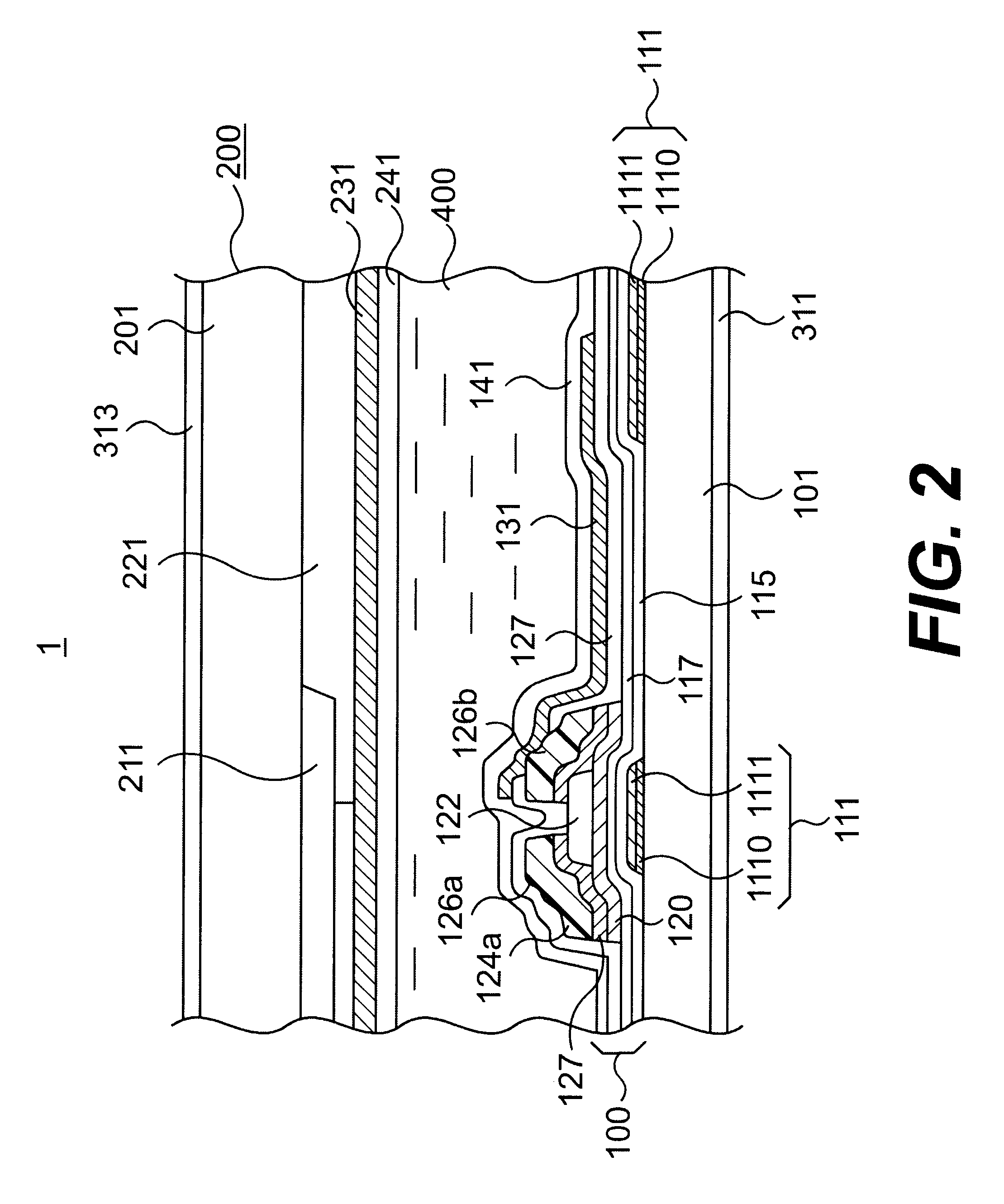
In the manufacturing method of array substrates for use in flat panel display devices including liquid crystal display (LCD) devices, it is aimed to prevent failure of interlayer dielectric film due to wiring deformation or the like while reducing the resistivity of wiring. It is also aimed to prevent corrosion of a metal wiring layer at the etching process and to thereby prevent deterioration of production yield due to corrosion. According to the method of the invention, to form scanning lines (111), an aluminum-neodymium alloy (Al-Nd) film (1110) is deposited in 300 nm thickness on the first hand, and then 50 nm thick Mo film (1110) is deposited thereon. Subsequently, gate insulator films (115 and 117) are formed by CVD processes at a substrate temperature of 350° C. Further, an etching process for forming pixel electrode (131) is carried out by HBr, HI, Oxalic acid or a mixture liquid containing at least one of these acids.
Owner:KK TOSHIBA
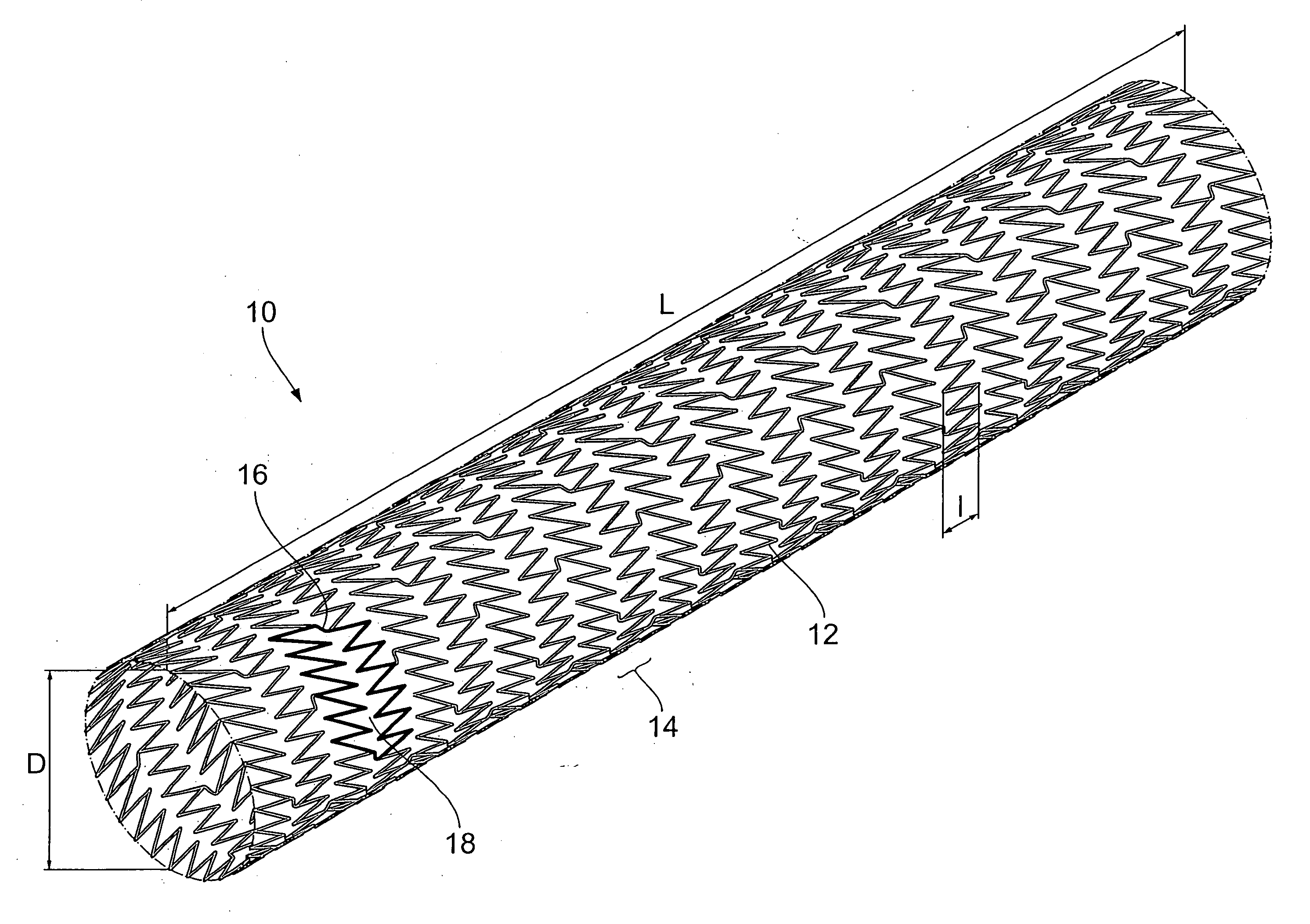
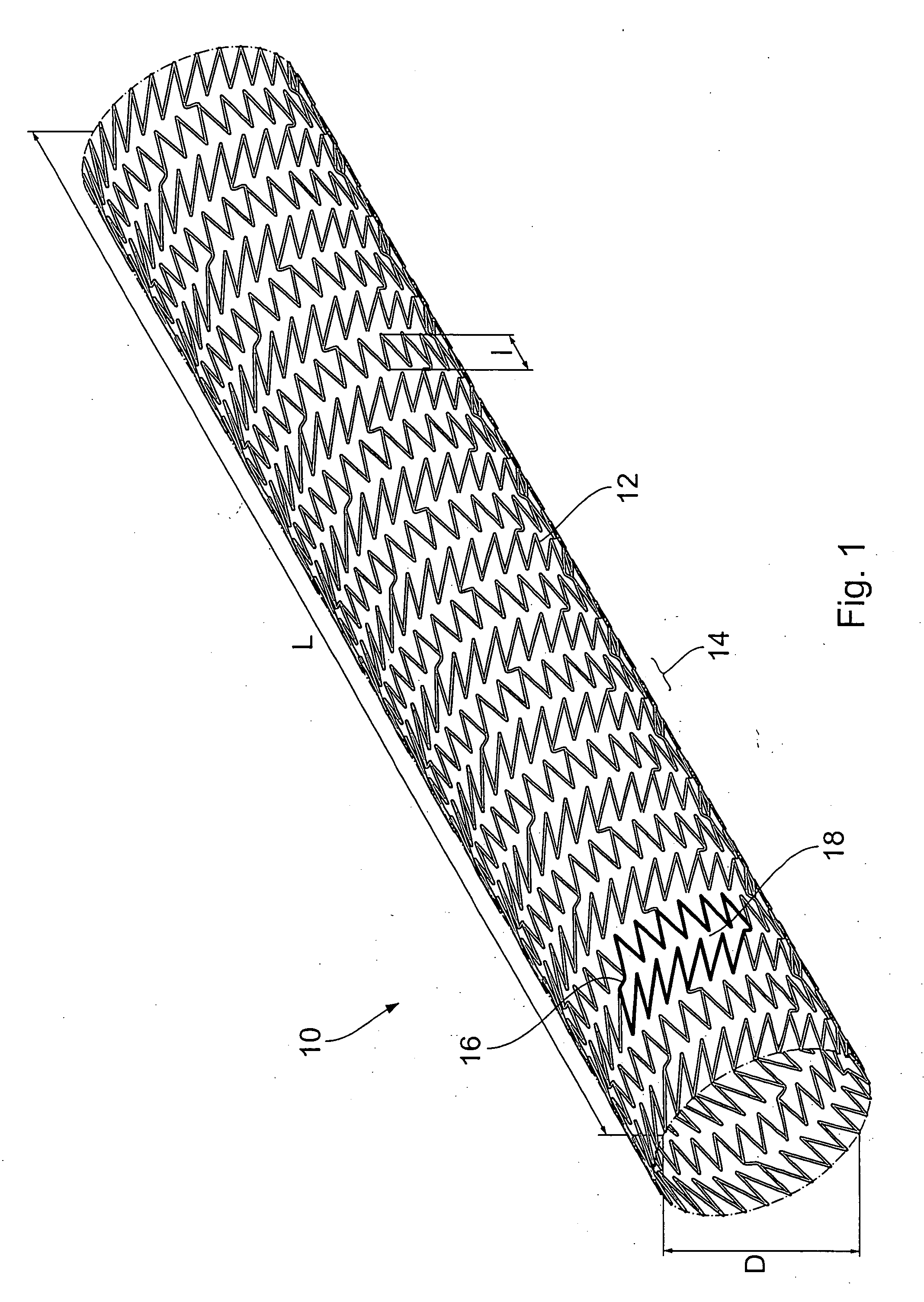
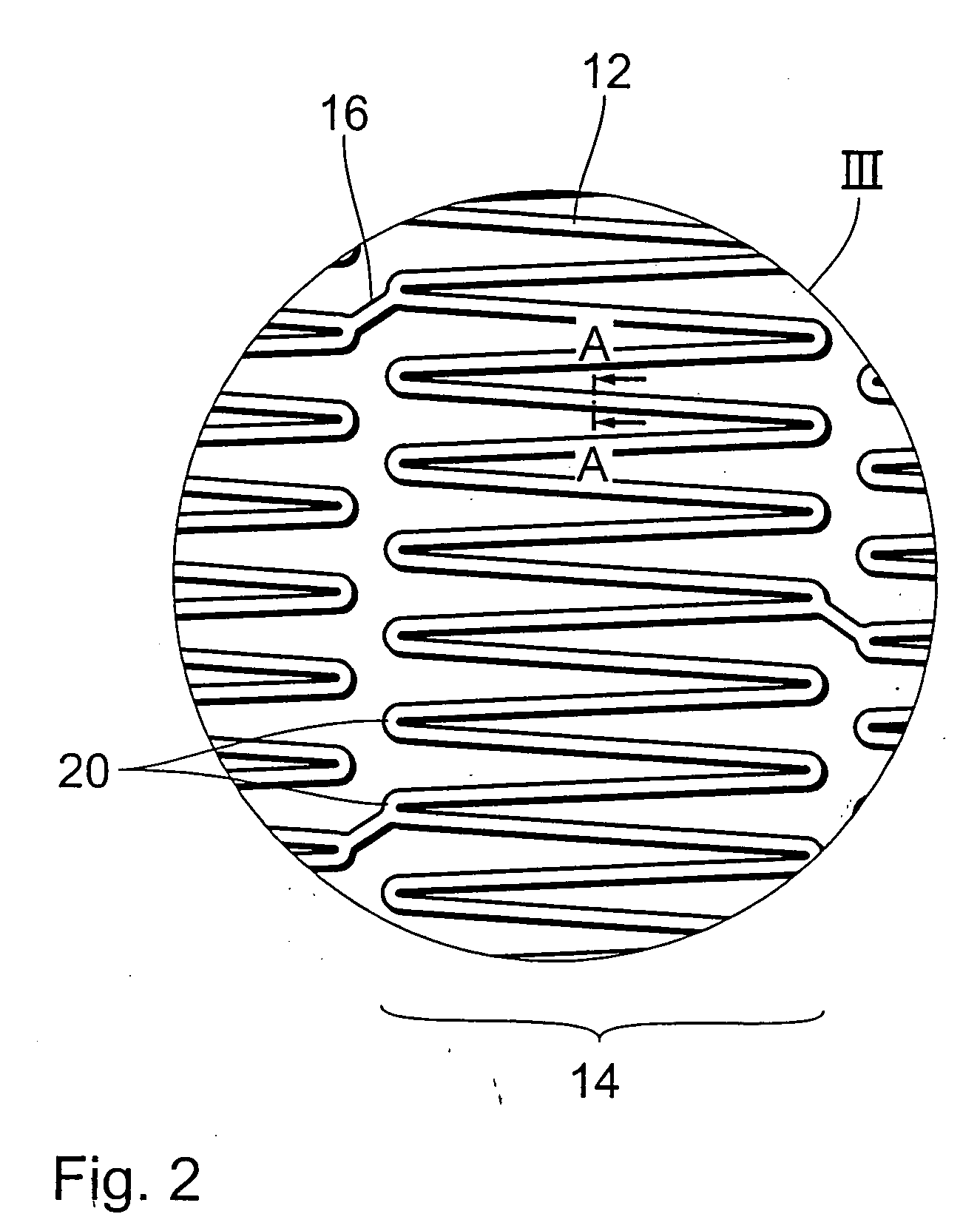
Endoprosthesis comprising a magnesium alloy
An endoprosthesis, in particular an intraluminal endoprosthesis such as a stent, comprises a carrier structure, which includes at least one component comprising a magnesium alloy of the following composition: Rare earth metals: between about 2.0 and about 5.0% by weight, with neodymium between about 1.5 and about 3.0% by weight Yttrium: between about 3.5% and about 4.5% by weight Zirconium: between about 0.3% and about 1.0% by weight Balance: between 0 and about 0.5% by weight wherein magnesium occupies the proportion by weight that remains to 100% by weight in the alloy.
Owner:BIOTRONIK VI PATENT
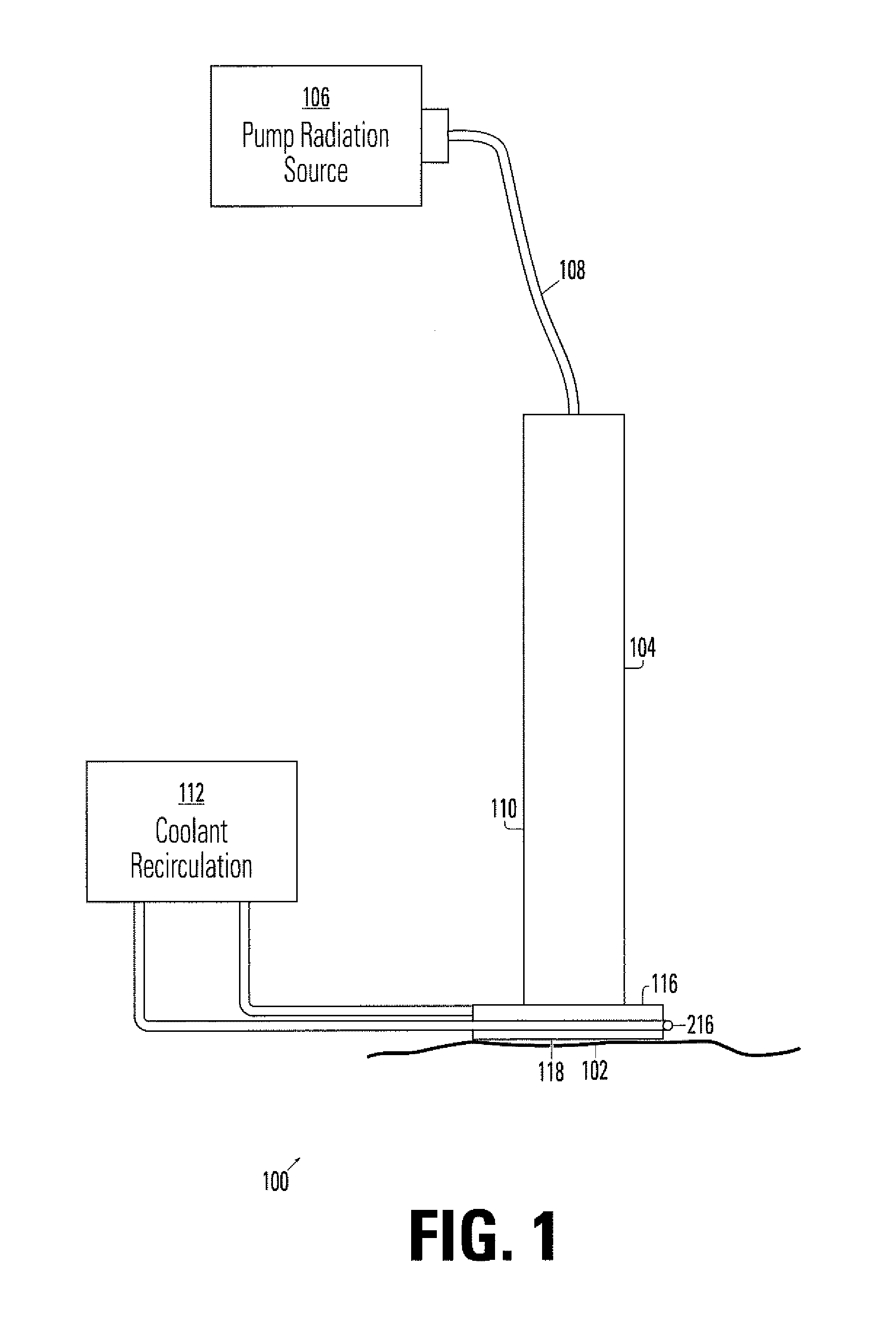
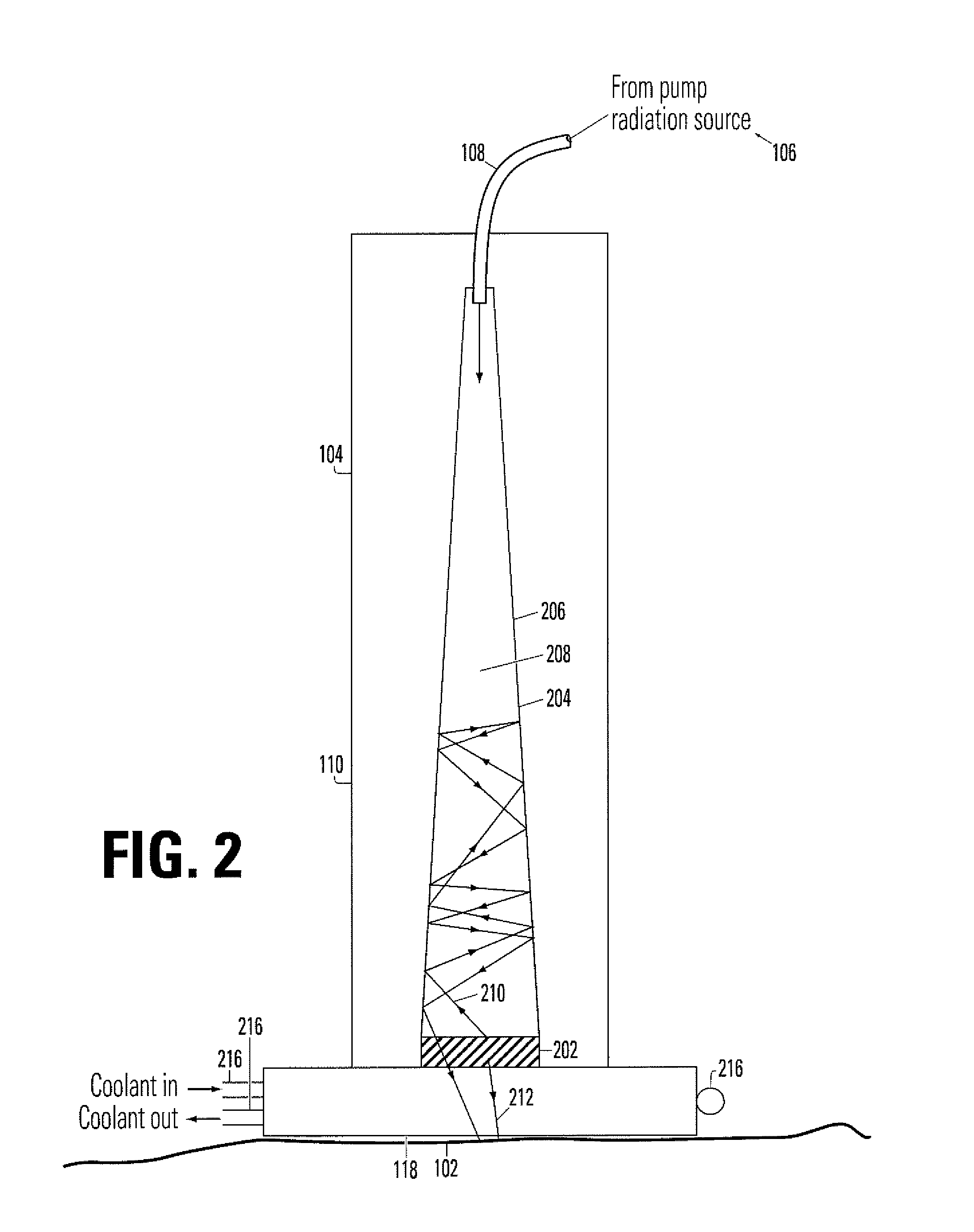
Device for irradiating tissue
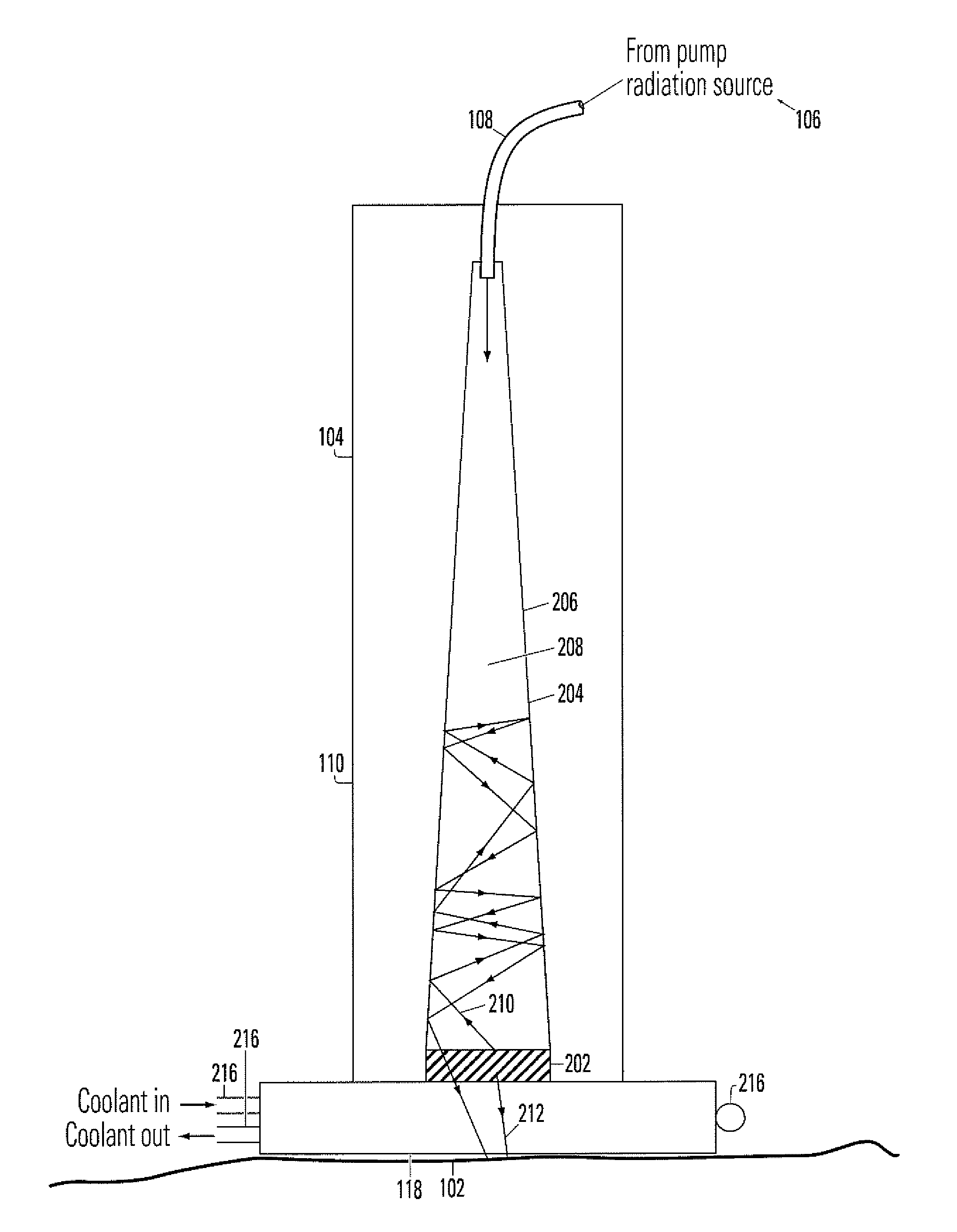
InactiveUS7083610B1Minimize damageLess prone to failureSurgical instrument detailsLight therapyHair removalPhotodynamic therapy
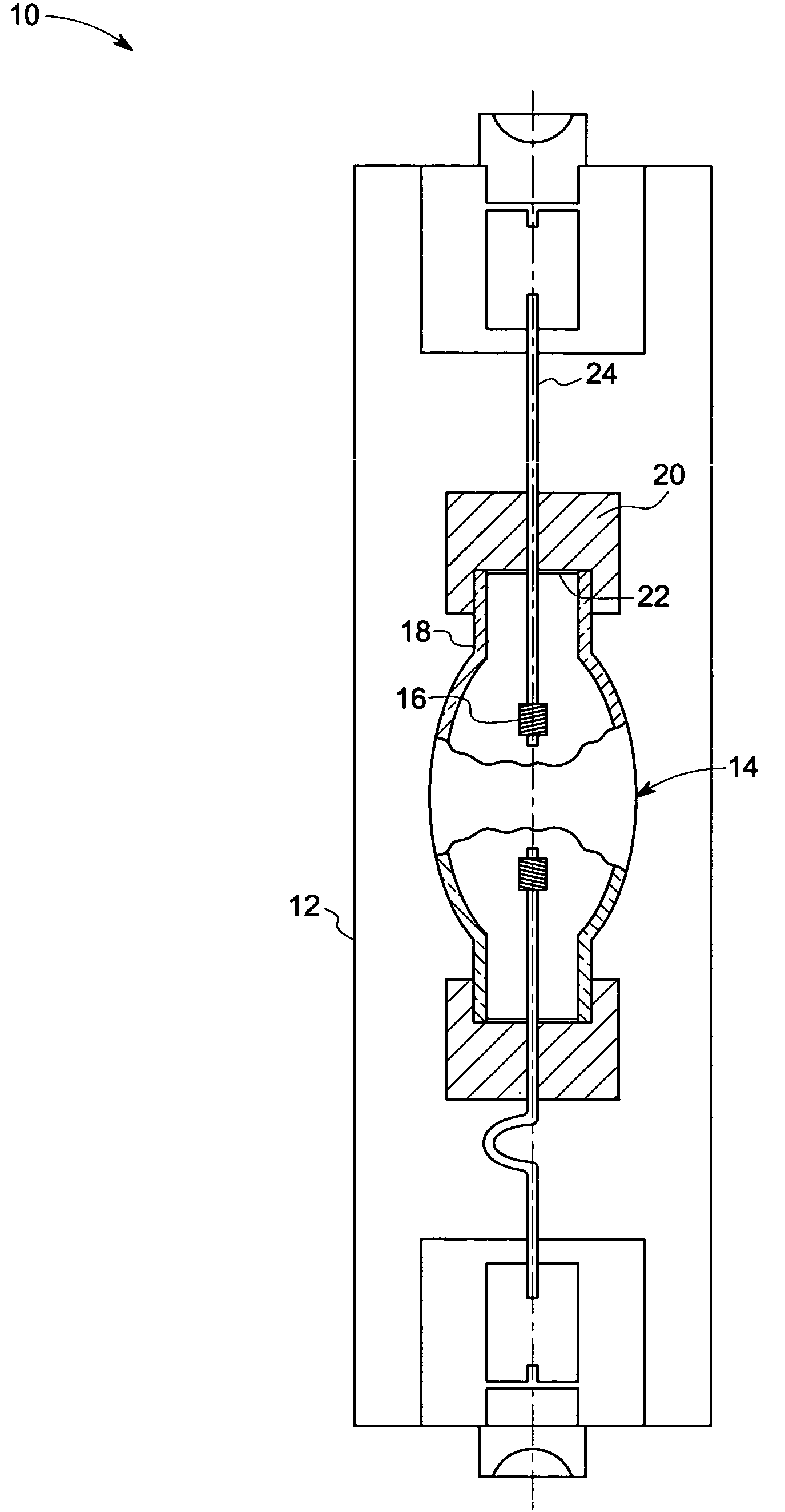
A device for irradiating tissue includes a fluorescent element for receiving pump radiation and responsively emitting radiation having different spectral characteristics than the pump radiation. A redirector receives emitted radiation promulgated in a direction away from a tissue target and redirects the radiation toward the target. The pump radiation may be supplied, for example, by a flashlamp or frequency-doubled neodymium-doped laser. Use of the device provides an inexpensive and effective alternative to conventional dye laser-based systems for various medical therapies, including treatment of vascular and pigmented lesions, tattoo and hair removal, and photodynamic therapy (PDT).
Owner:BOSTON SCI SCIMED INC
Layered thermal barrier coatings containing lanthanide series oxides for improved resistance to CMAS degradation
InactiveUS20070160859A1Avoid damageElimination of expensiveBlade accessoriesEfficient propulsion technologiesReaction layerCerium
A coating applied as a two layer system. The outer layer is an oxide of a group IV metal selected from the group consisting of zirconium oxide, hafnium oxide and combinations thereof, which are doped with an effective amount of a lanthanum series oxide. These metal oxides doped with a lanthanum series addition comprises a high weight percentage of the outer coating. As used herein, lanthanum series means an element selected from the group consisting of lanthanum (La), cerium (Ce), praseodymium (Pr), neodymium (Nd), promethium (Pm), samarium (Sm), europium (Eu), gadolinium (Gd), terbium (Tb), dysprosium (Dy), holmium (Ho), erbium (Er), thulium (Tm), ytterbium (Yb), lutetium (Lu) and combinations thereof, and lanthanum series oxides are oxides of these elements. When the zirconium oxide is doped with an effective amount of a lanthanum series oxide, a dense reaction layer is formed at the interface of the outer layer of TBC and the CMAS. This dense reaction layer prevents CMAS infiltration below it. The second layer, or inner layer underlying the outer layer, comprises a layer of partially stabilized zirconium oxide.
Owner:GENERAL ELECTRIC CO
High performance lithium ion battery anode material lithium manganate and preparation method thereof
The invention provides a high performance lithium ion battery anode material lithium manganate and a preparation method of the material. The lithium manganate is a doped lithium manganate LiMn2-yXy04 which is doped with one kind or a plurality of other metal elements X, wherein X element is at least one kind selected form the group of aluminium, lithium, fluorine, silver, copper, chromium, zinc, titanium, bismuth, germanium, gallium, zirconium, stannum, silicon, cobalt, nickel, vanadium, magnesium, calcium, strontium, barium and rare earth elements lanthanum, cerium, praseodymium, neodymium, promethium, samarium, europium, gadolinium, terbium, dysprosium, holmium, erbium, thulium, ytterbium and lutetium, and y is larger than 0 but less than or equal to 0.11. The lithium ion battery anode material lithium manganate provided in the invention has extraordinary charge and discharge cycle performance both in the environments of normal temperature and high temperature. According to the invention, the preparation method of the material is a solid phase method, the operation is simple and controllable and the cost is low so that it is easy to realize large-scale productions.
Owner:INST OF PROCESS ENG CHINESE ACAD OF SCI
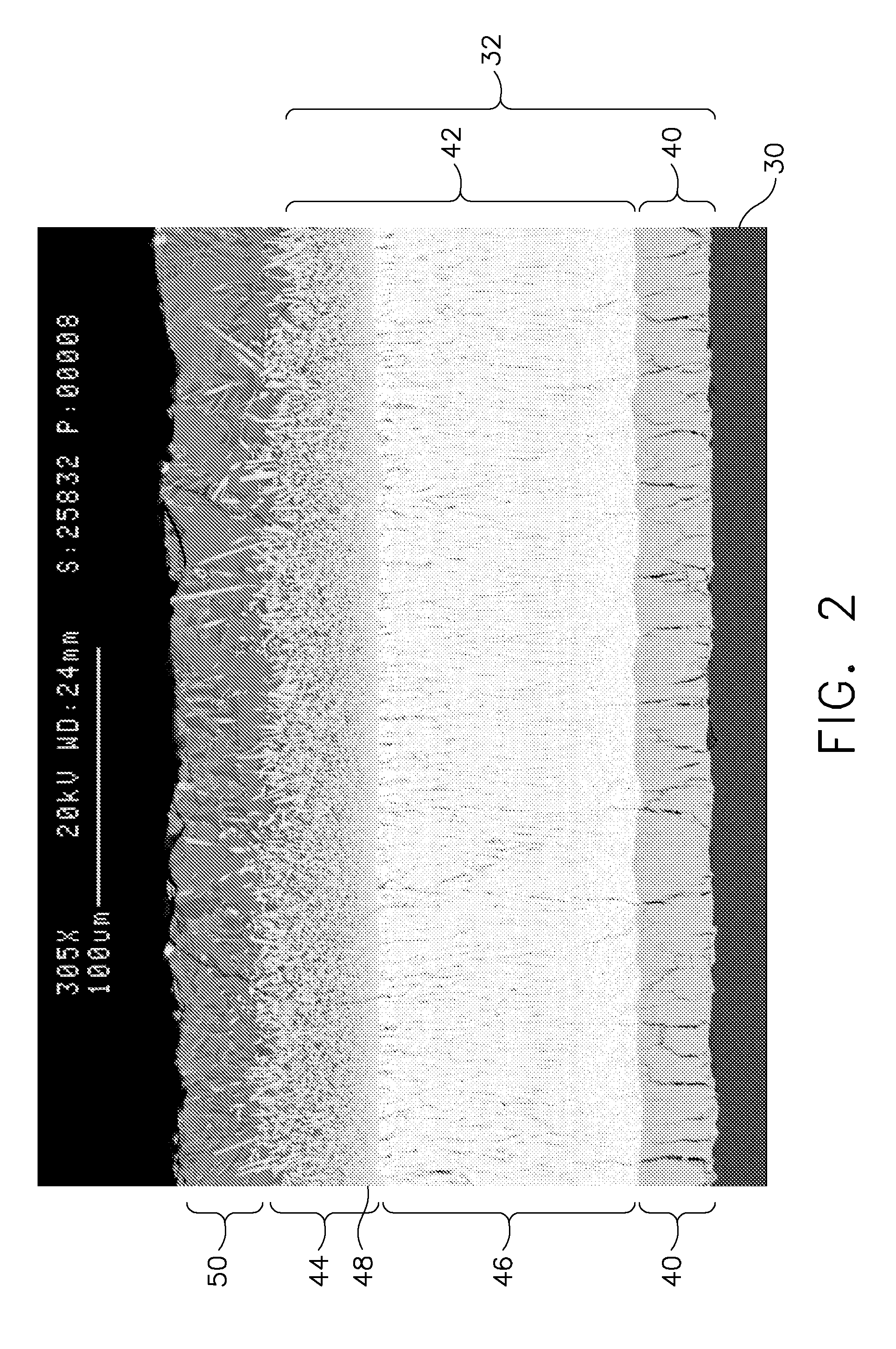
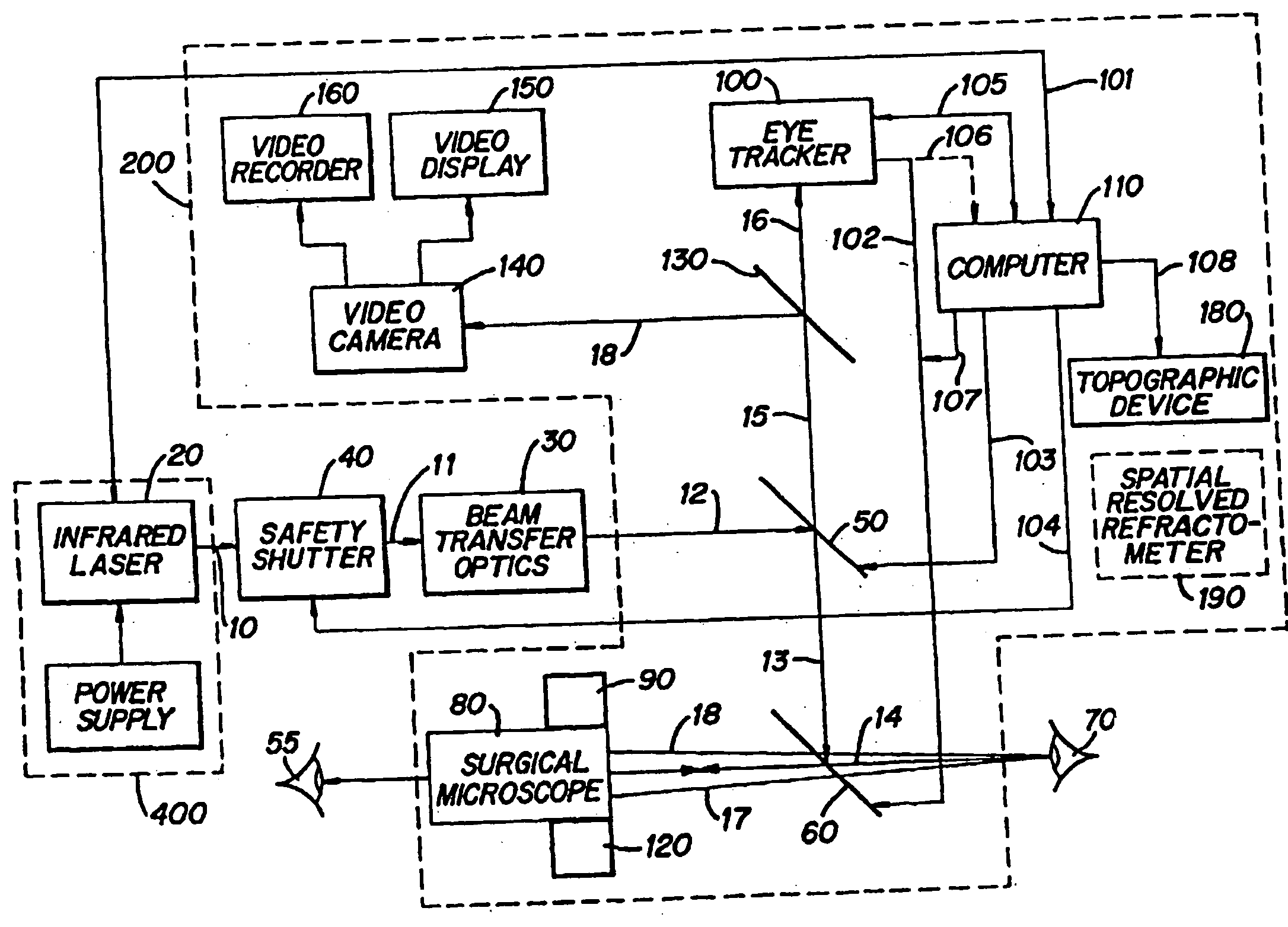
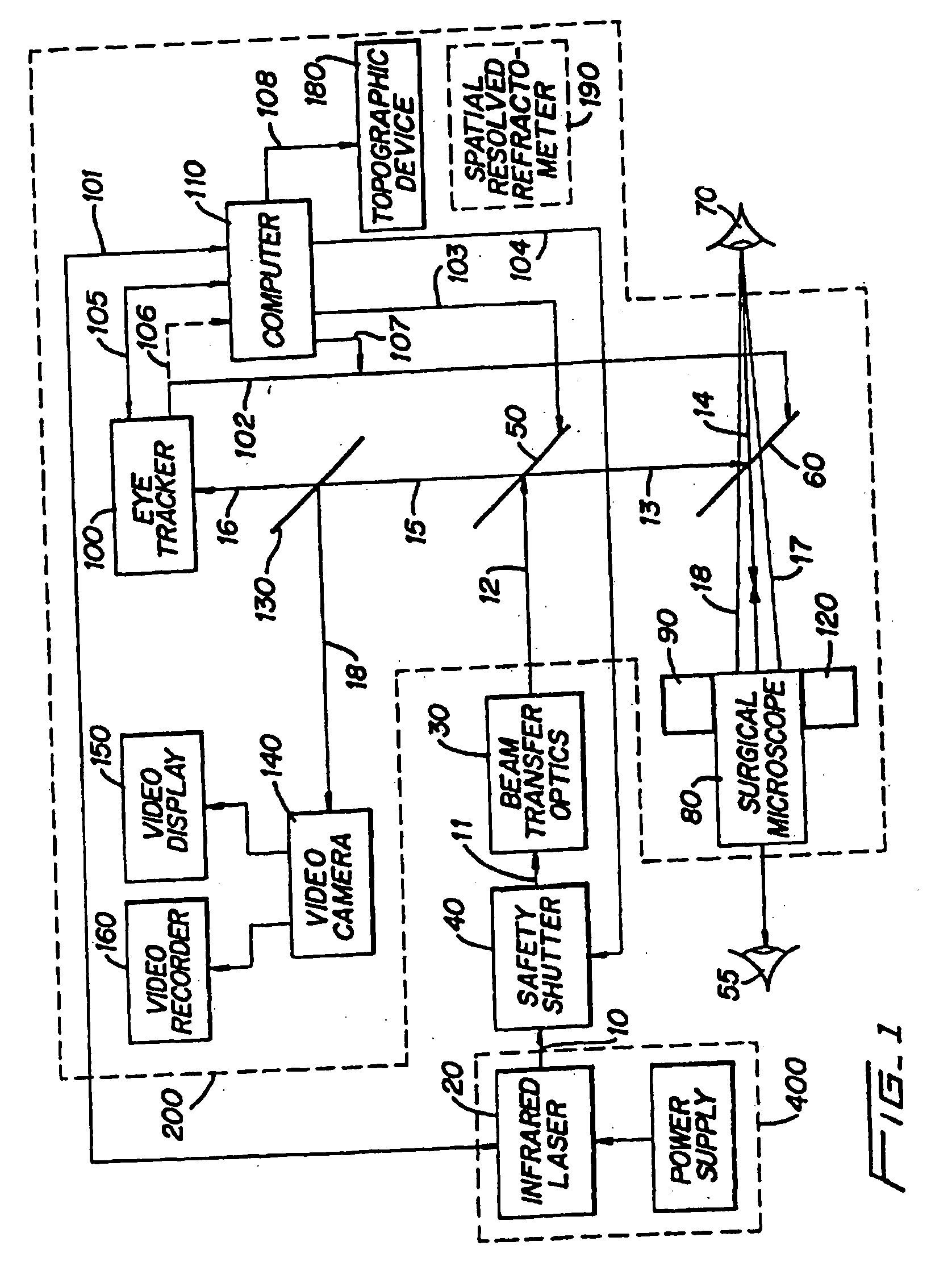
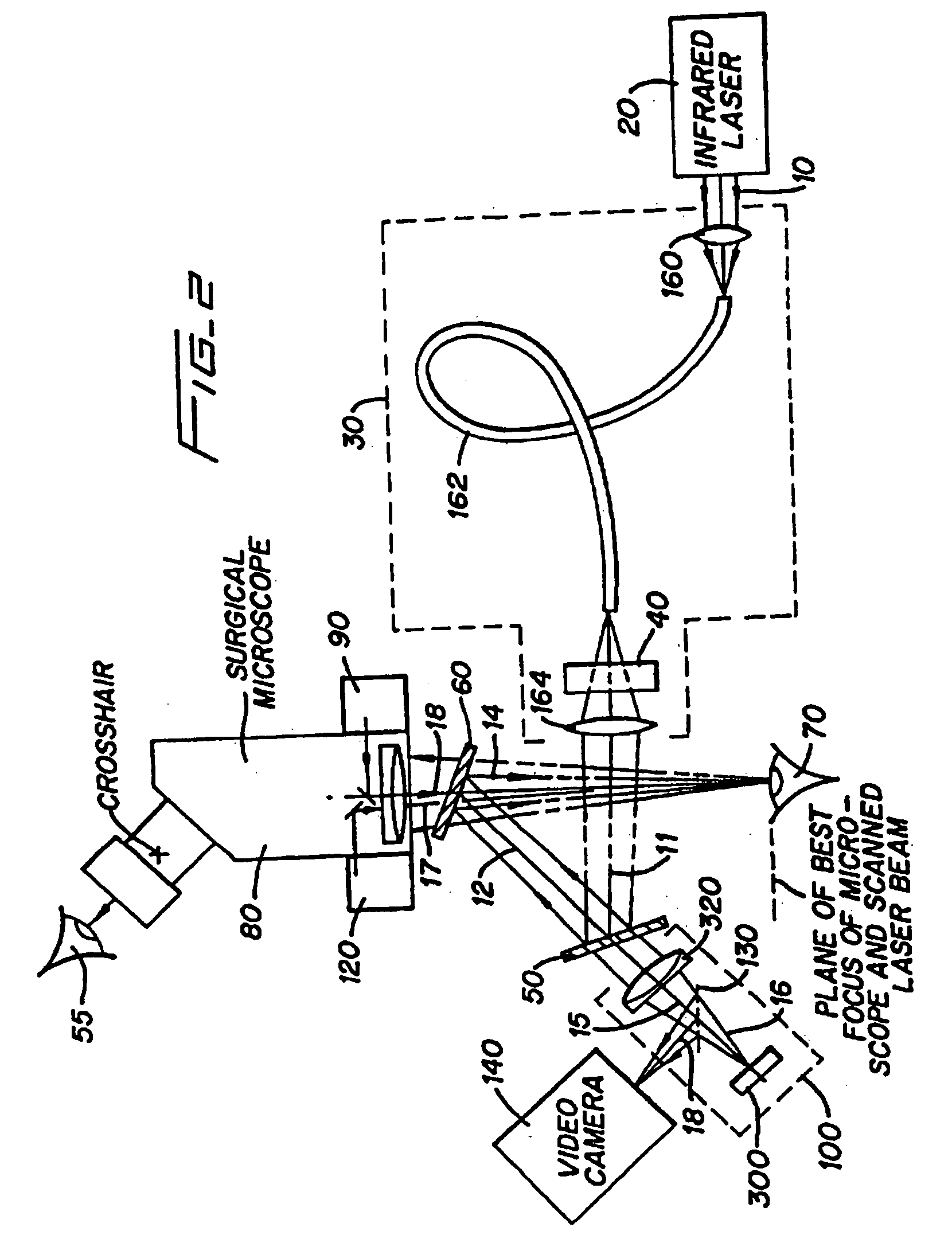
Method and apparatus for removing corneal tissue with infrared laser radiation and short pulse mid-infrared parametric generator for surgery
InactiveUS20050197655A1Reduce undesirable thermal damageMaximum flexibilityLaser surgerySurgical instrument detailsLaser scalpelMid infrared laser
A surgical technique for removing corneal tissue with scanned infrared radiation is disclosed which utilizes short mid-infrared laser pulses to provide a tissue removal mechanism based on photospallation. Photospallation is a photomechanical ablation mechanism which results from the absorption of incident radiation by the corneal tissue. Since photospallation is a mechanical ablation process, very little heat is generated in the unablated adjacent tissue. The disclosed surgical system includes a scanning beam delivery system which allows uniform irradiation of the treatment region and utilizes low energy outputs to achieve controlled tissue removal. A real-time servo-controlled dynamic eye tracker, based on a multiple-detector arrangement, is also disclosed which senses the motion of the eye and provides signals that are proportional to the errors in the lateral alignment of the eye relative to the axis of the laser beam. Temporal and frequency discrimination are preferably utilized to distinguish the tracking illumination from the ambient illumination and the surgical laser beam. A laser parametric generator for surgical applications is disclosed which utilizes short-pulse, mid-infrared radiation. The mid-infrared radiation may be produced by a pump laser source, such as a neodymium-doped laser, which is parametrically down converted in a suitable nonlinear crystal to the desired mid-infrared range. The short pulses reduce unwanted thermal effects and changes in adjacent tissue to potentially submicron-levels. The parametrically converted radiation source preferably produces pulse durations shorter than 25 ns at or near 3.0 microns but preferably close to the water absorption maximum associated with the tissue. The down-conversion to the desired mid-infrared wavelength is preferably produced by a nonlinear crystal such as KTP or its isomorphs. In one embodiment, a non-critically phased-matched crystal is utilized to shift the wavelength from a near-infrared laser source emitting at or around 880 to 900 nm to the desired 2.9-3.0 microns wavelength range. A fiber, fiber bundle or another waveguide means utilized to separate the pump laser from the optical parametric oscillation (OPO) cavity is also included as part of the invention.
Owner:AMO MFG USA INC
Inorganic material that has metal nanoparticles that are trapped in a mesostructured matrix
An inorganic material that consists of at least two elementary spherical particles, each of said spherical particles comprising metal nanoparticles that are between 1 and 300 nm in size and a mesostructured matrix with an oxide base of at least one element X that is selected from the group that consists of aluminum, titanium, tungsten, zirconium, gallium, germanium, tin, antimony, lead, vanadium, iron, manganese, hafnium, niobium, tantalum, yttrium, cerium, gadolinium, europium and neodymium is described, whereby said matrix has a pore size of between 1.5 and 30 nm and has amorphous walls with a thickness of between 1 and 30 nm, said elementary spherical particles having a maximum diameter of 10 μm. Said material can also contain zeolitic nanocrystals that are trapped within said mesostructured matrix.
Owner:INST FR DU PETROLE
Inorganic material that has metal nanoparticles that are trapped in a mesostructured matrix
An inorganic material that consists of at least two elementary spherical particles, each of said spherical particles comprising metal nanoparticles that are between 1 and 300 nm in size and a mesostructured matrix with an oxide base of at least one element X that is selected from the group that consists of aluminum, titanium, tungsten, zirconium, gallium, germanium, tin, antimony, lead, vanadium, iron, manganese, hafnium, niobium, tantalum, yttrium, cerium, gadolinium, europium and neodymium is described, whereby said matrix has a pore size of between 1.5 and 30 nm and has amorphous walls with a thickness of between 1 and 30 nm, said elementary spherical particles having a maximum diameter of 10 μm. Said material can also contain zeolitic nanocrystals that are trapped within said mesostructured matrix.
Owner:INST FR DU PETROLE
Internal rotor motor
InactiveUS6919663B2Minimized cogging torqueImprove featuresMagnetic circuit rotating partsSynchronous machines with stationary armatures and rotating magnetsRotor magnetsElectric machine
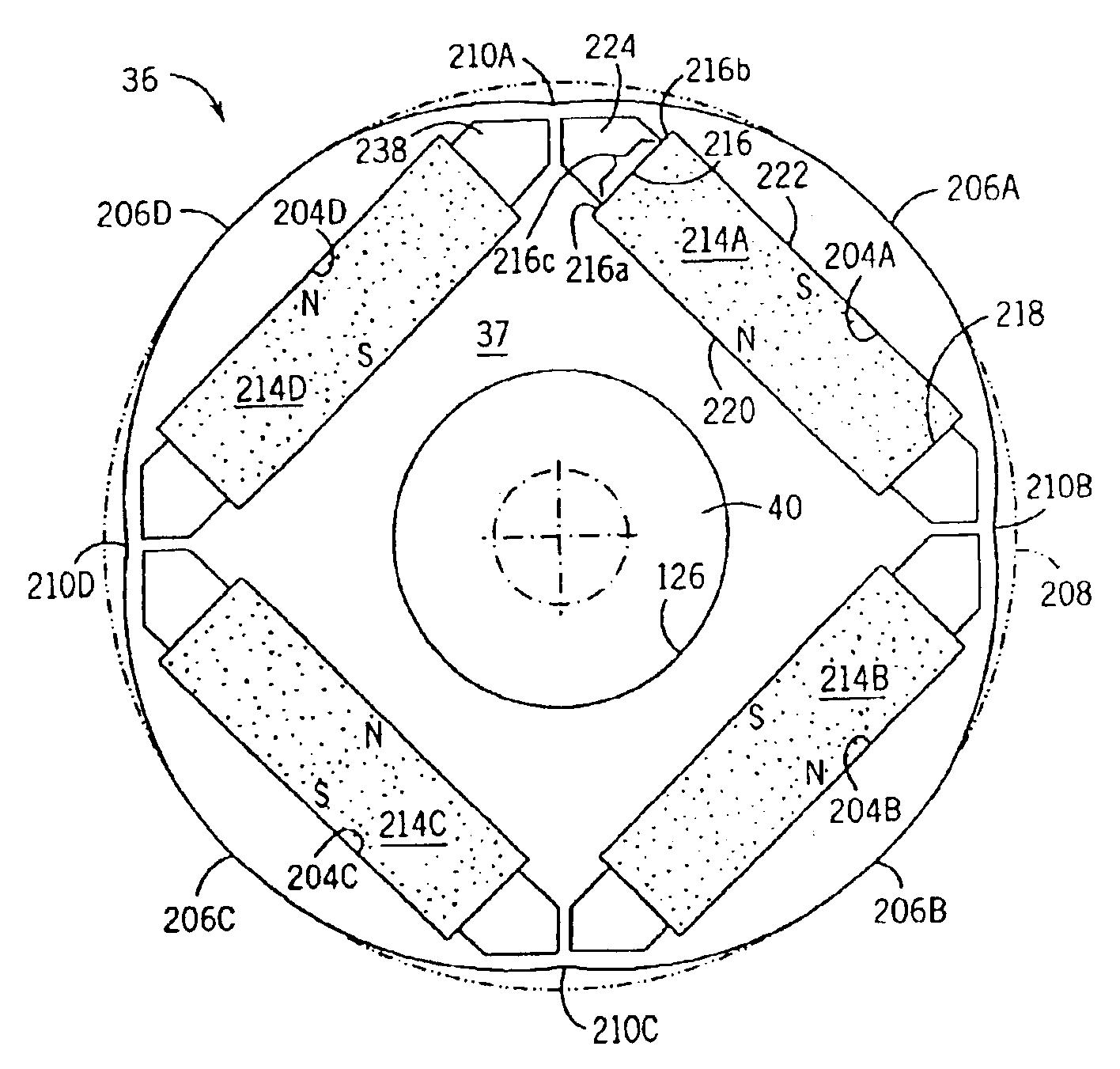
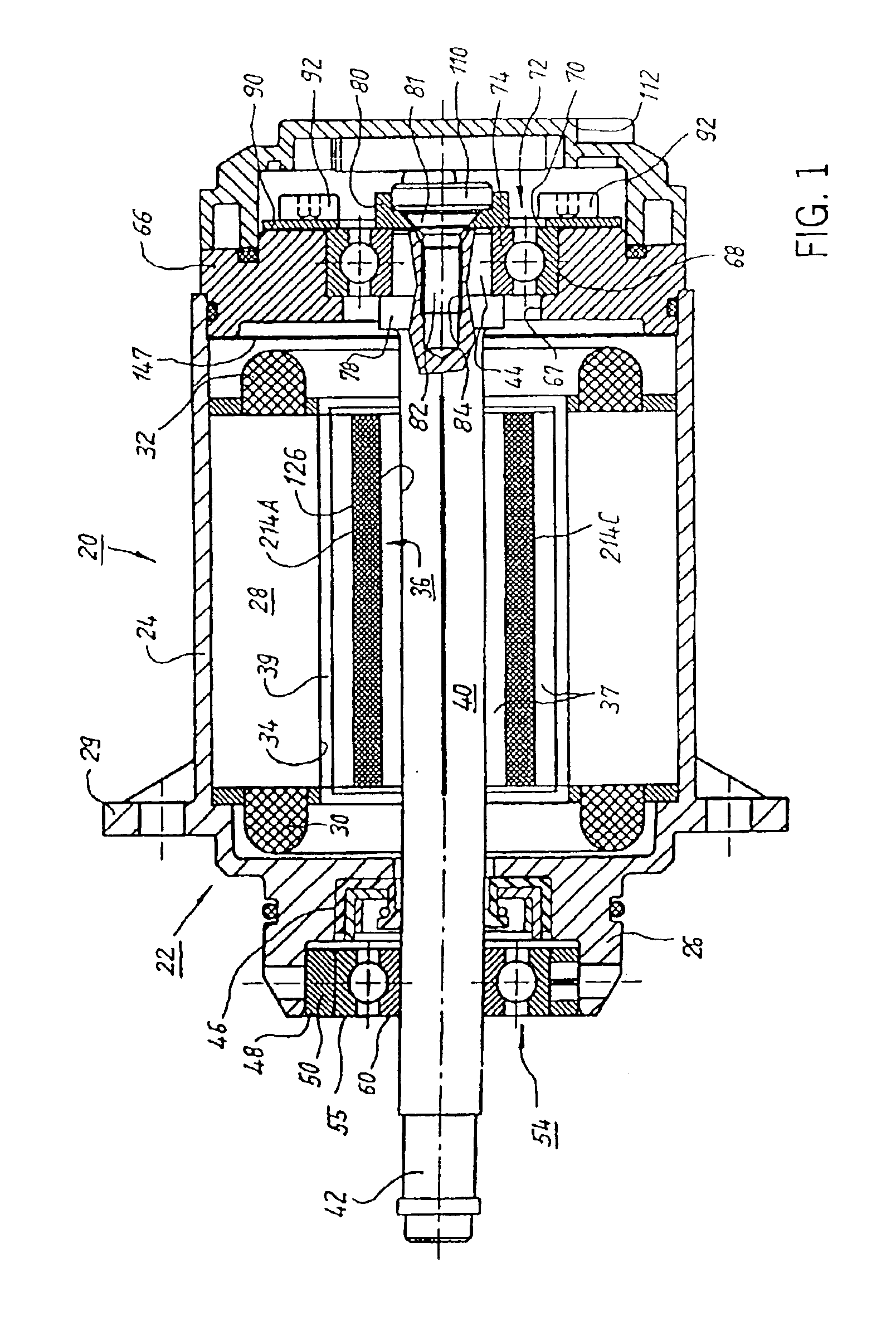
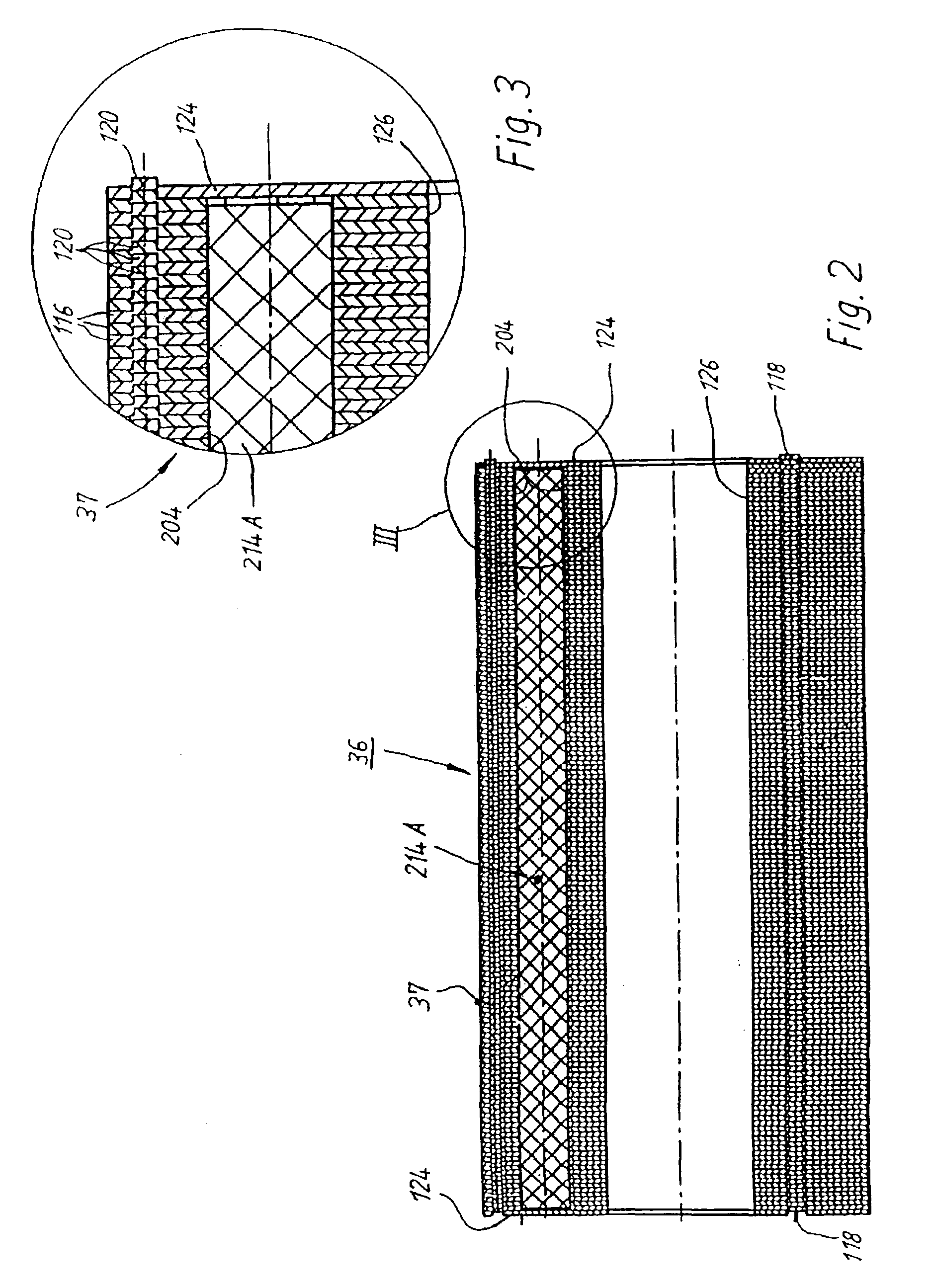
A low-ripple multi-phase internal rotor motor has a slotted stator (28) separated by an air gap (39) from a central rotor (36). The rotor has a plurality of permanent magnets (214), preferably neodymium-boron, arranged in pockets (204) formed in a lamination stack (37), thereby defining a plurality of poles (206) separated by respective gaps (210). In a preferred embodiment, the motor is three-phase, with four rotor poles and six stator poles. As a result, when the first and third rotor poles are so aligned, with respect to their opposing stator poles, as to generate a reluctance torque, the second and fourth rotor poles are aligned to generate oppositely phased reluctance torques, so that these torques cancel each other, and essentially no net reluctance torque is exerted on the rotor. In order to magnetically separate the rotor magnets from each other, a hollow space (224, 238) is formed at the interpolar-gap-adjacent end face (216, 218) of each rotor magnet. A control circuit (147) provides electronic commutation and current control, based on rotor position signals generated with the aid of a control magnet (110) on the rotor shaft (40).
Owner:EBM PAPST ST GEORGEN & -
Method for preparing neodymium iron boron through regenerating waste material containing neodymium, iron and boron
ActiveCN103866127AFully absorb hydrogenImprove recycling ratesMagnetic materialsProcess efficiency improvementMetallurgyEconomic benefits
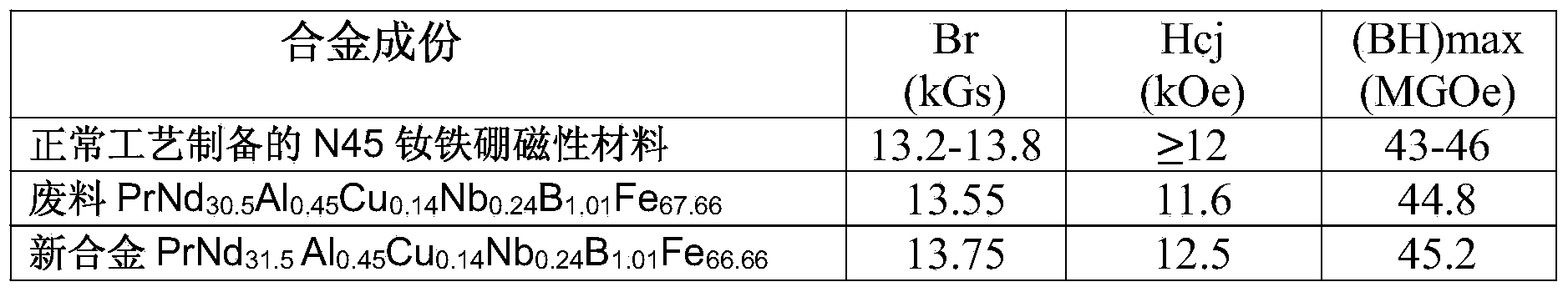
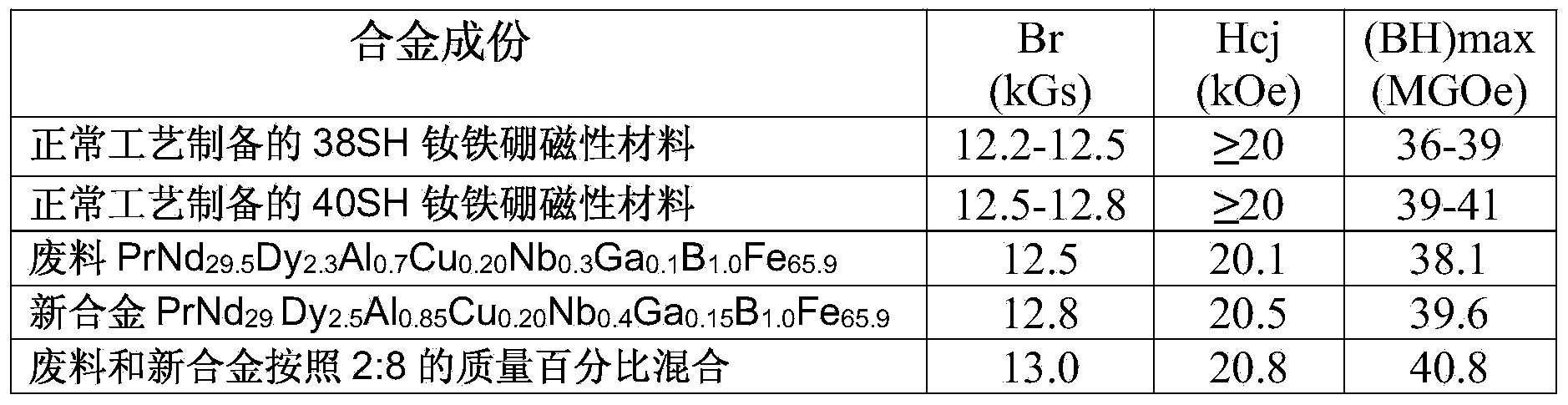
The invention provides a method for preparing neodymium iron boron through regenerating a waste material containing neodymium, iron and boron. The method comprises steps of (1) preprocessing the waste material; (2) correcting the components of the waste material; (3) crushing by hydrogen; (4) preparing into powder; (5) molding under a magnetic field; and (6) vacuum sintering. The method fully uses the waste material recycled in a production process, has high recovery rate of the waste material, can produce high performance product, has a simple and controllable flow, has high operability, uses no strong acid and strong base polluting the environment, is environment-friendly and energy-saving, and has high social and economic benefits.
Owner:CHINALCO JINYUAN RARE EARTH
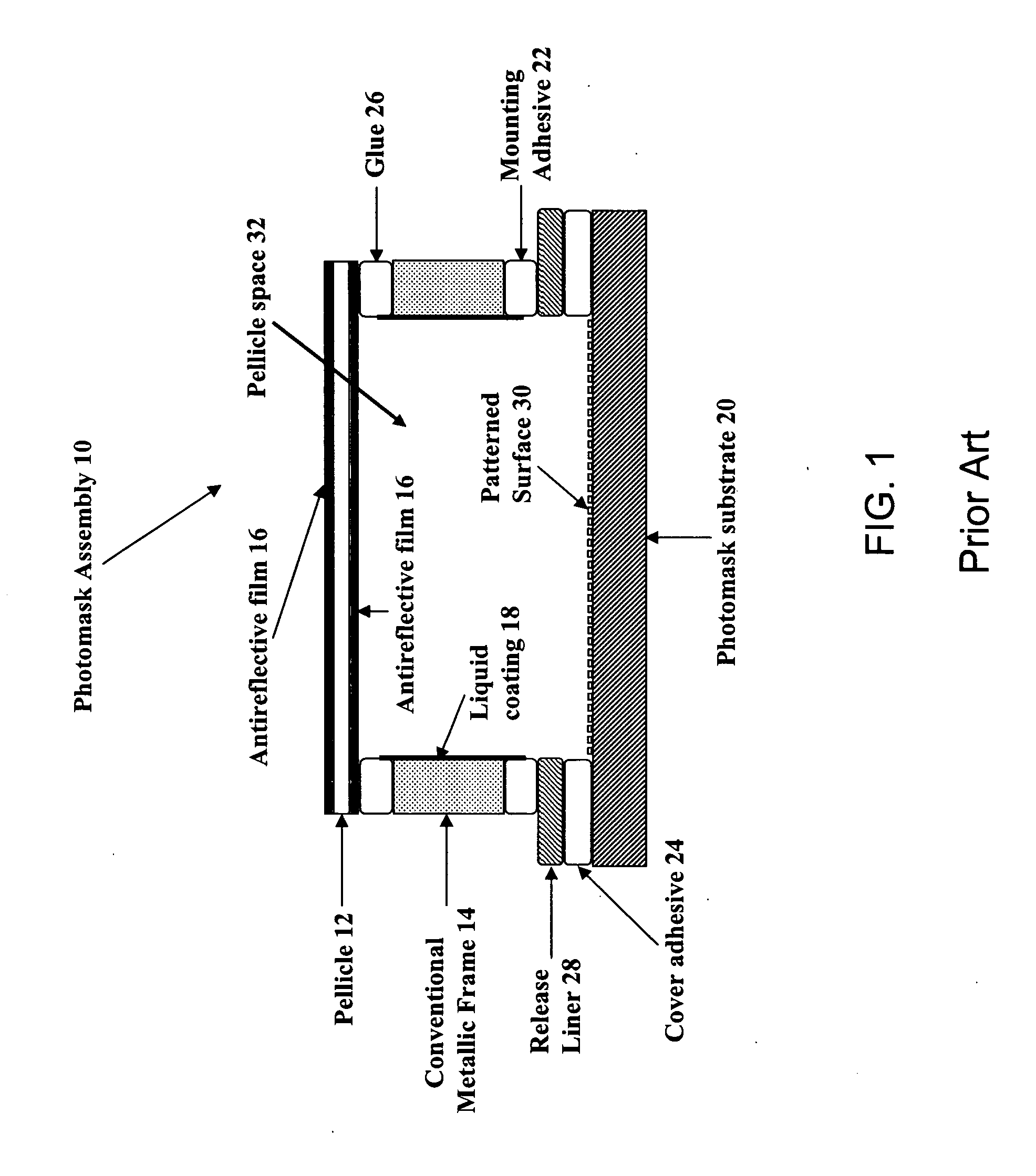
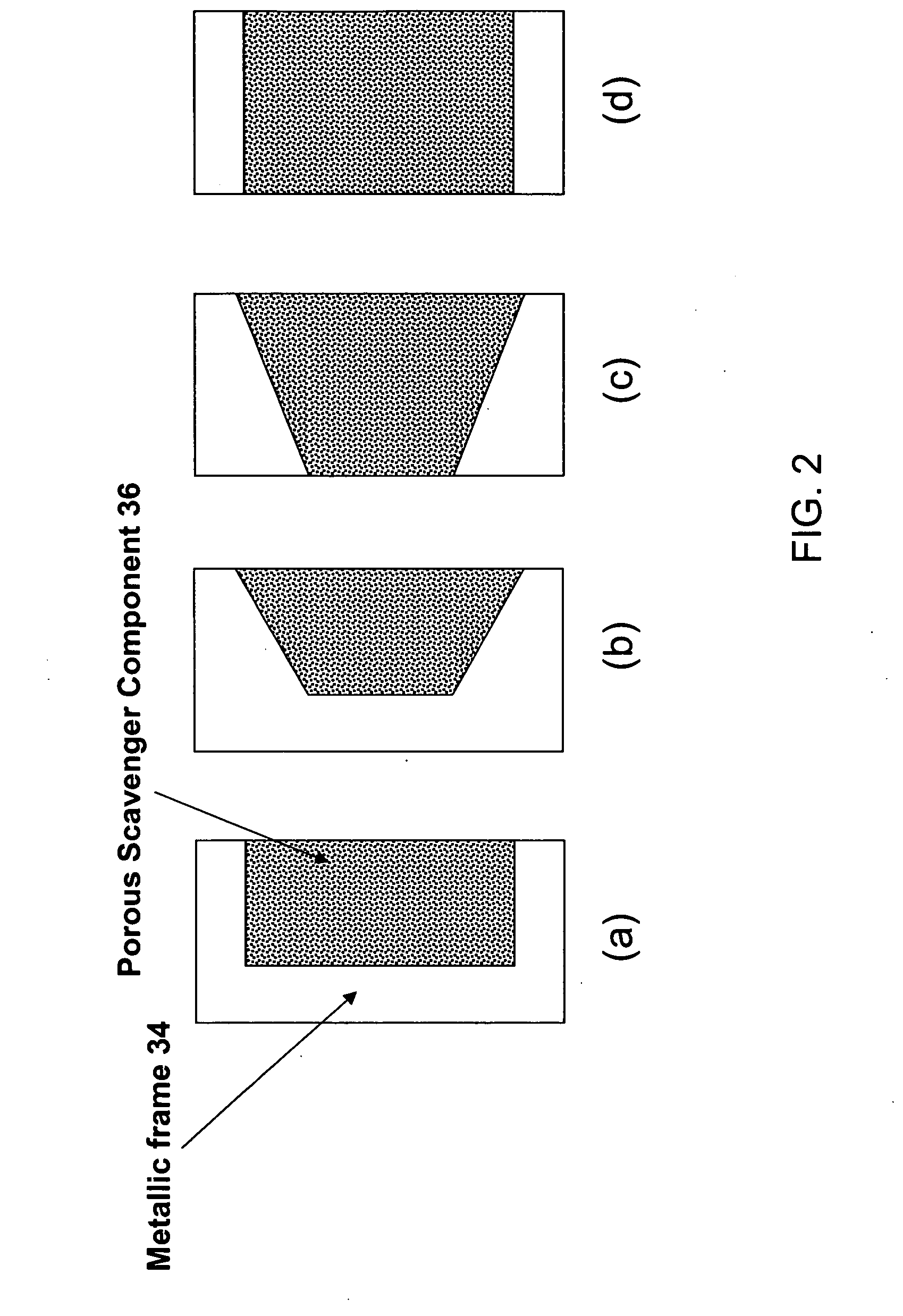
Photomask assembly incorporating a metal/scavenger pellicle frame
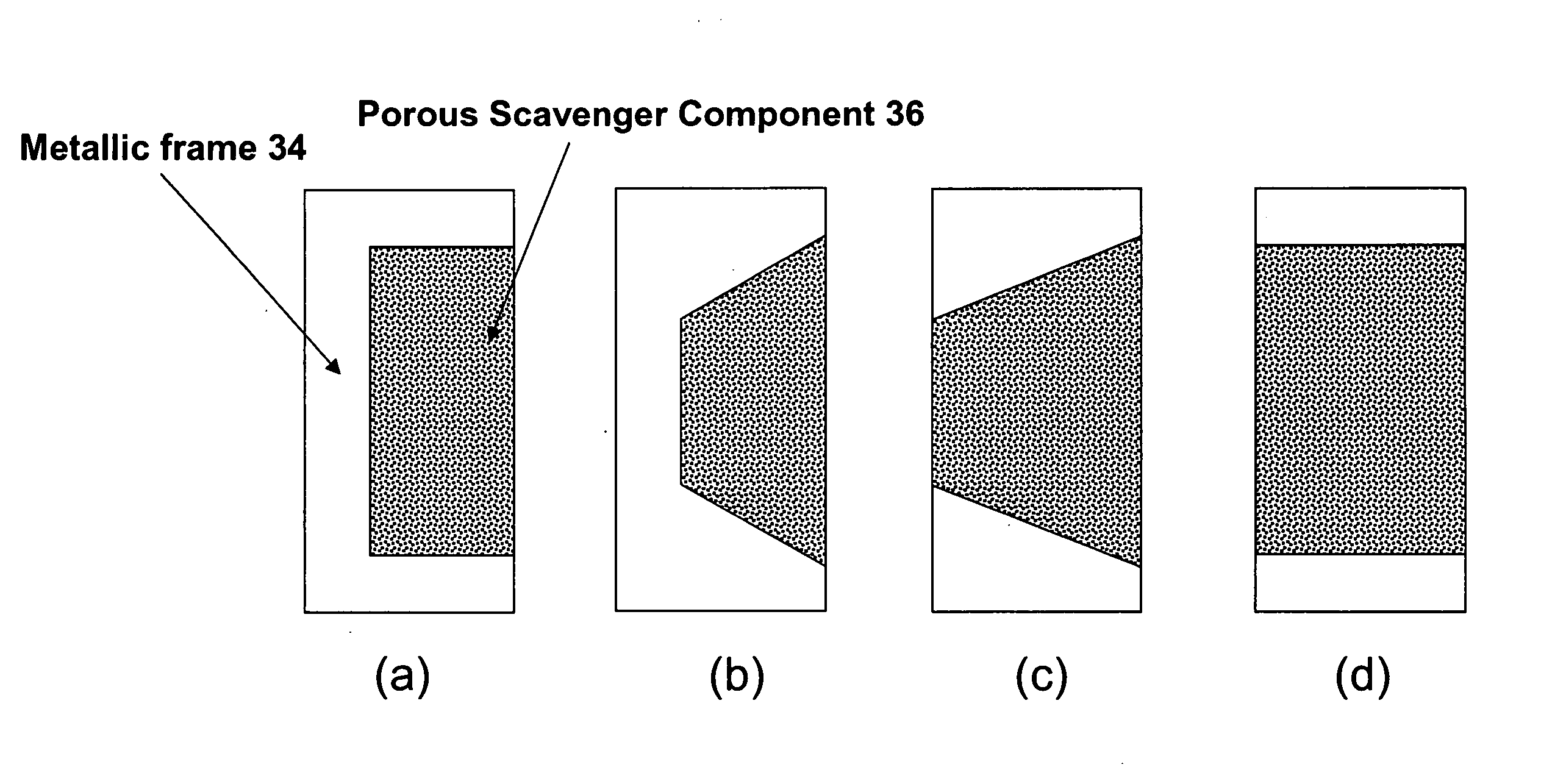
A photomask assembly is disclosed having a photomask substrate and a composite pellicle frame that includes both a metallic frame component and a scavenger component. The metallic frame component has a cross-sectional thickness of at least 100 micrometers in all directions, and the volume percentage of the scavenger component relative to the overall volume of the composite frame is in the range of 0.1 to 95%. The scavenger component has a gas permeability to oxygen or nitrogen greater than about 10 ml·mm / cm2·min·MPa, an average pore size between 0.001 and 10 micrometers, and a pore surface area greater than 10 m2 / g. This configuration enables the pellicle frame to have sufficient strength to withstand stresses encountered during normal use, yet also to have the capability of scavenging impurity molecules from the space adjacent to the photomask substrate. In a separate and independent feature of the invention, the scavenger component comprises at least one metal oxide selected from the group consisting of oxides of aluminum, boron, cerium, cobalt, copper, erbium, hafnium, lanthanum, neodymium, praseodymium, scandium, silicon, titanium, yttrium, zirconium, and mixtures thereof. Preferably, the metal oxide is an oxide of zirconium, yttrium, or mixtures thereof.
Owner:YAZAKI CORP
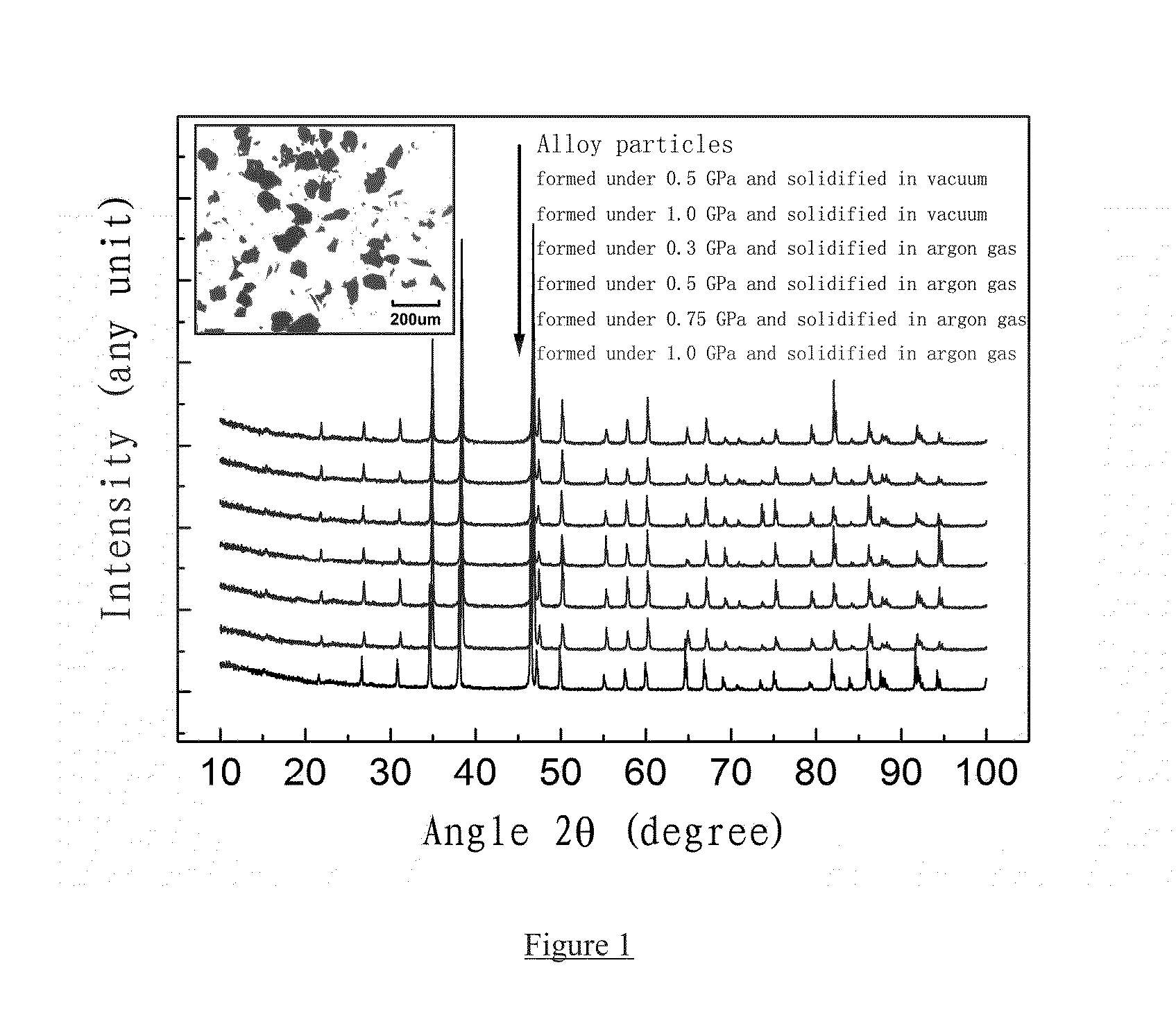
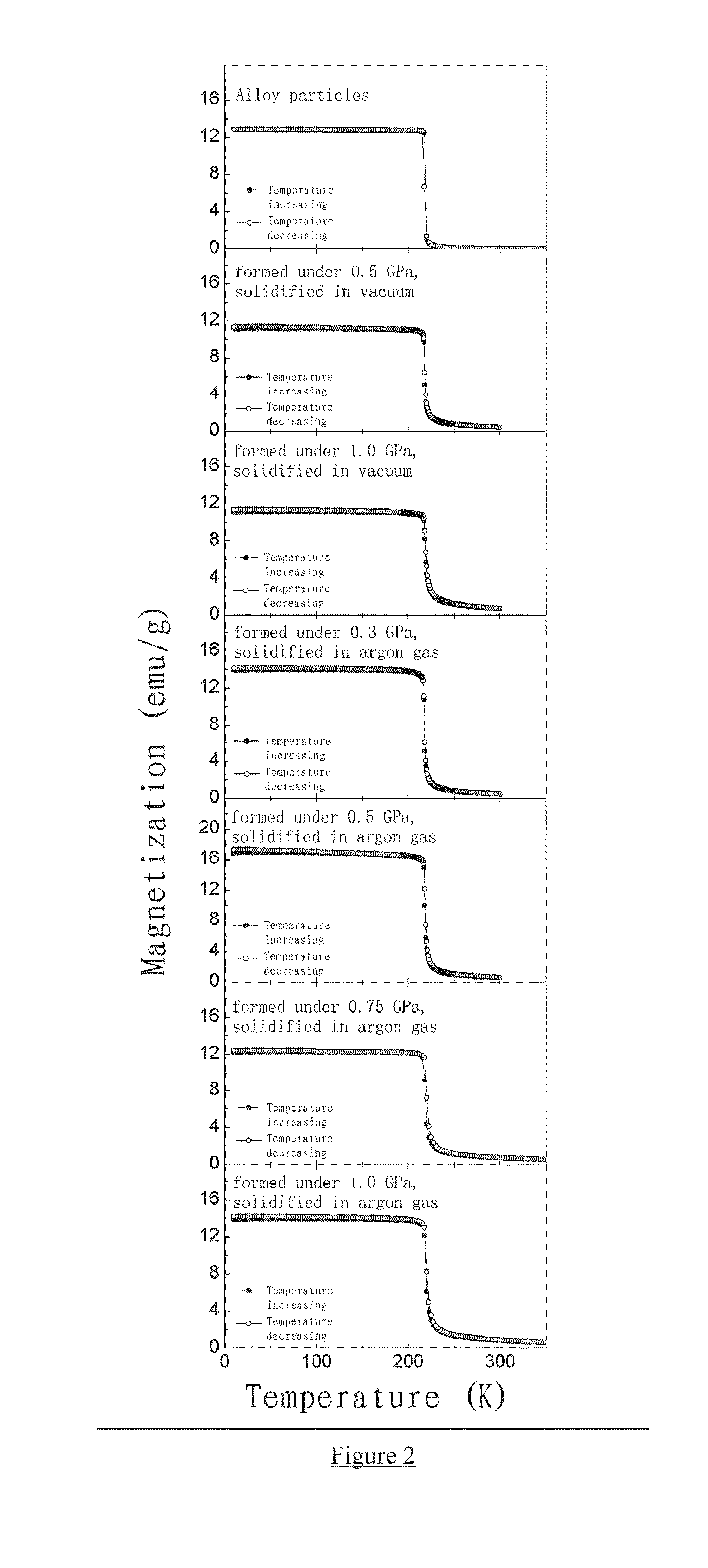
BONDED La(Fe,Si)13-BASED MAGNETOCALORIC MATERIAL AND PREPARATION AND USE THEREOF
InactiveUS20150047371A1Low priceEasy to operateInorganic material magnetismMachines using electric/magnetic effectsCeriumCobalt
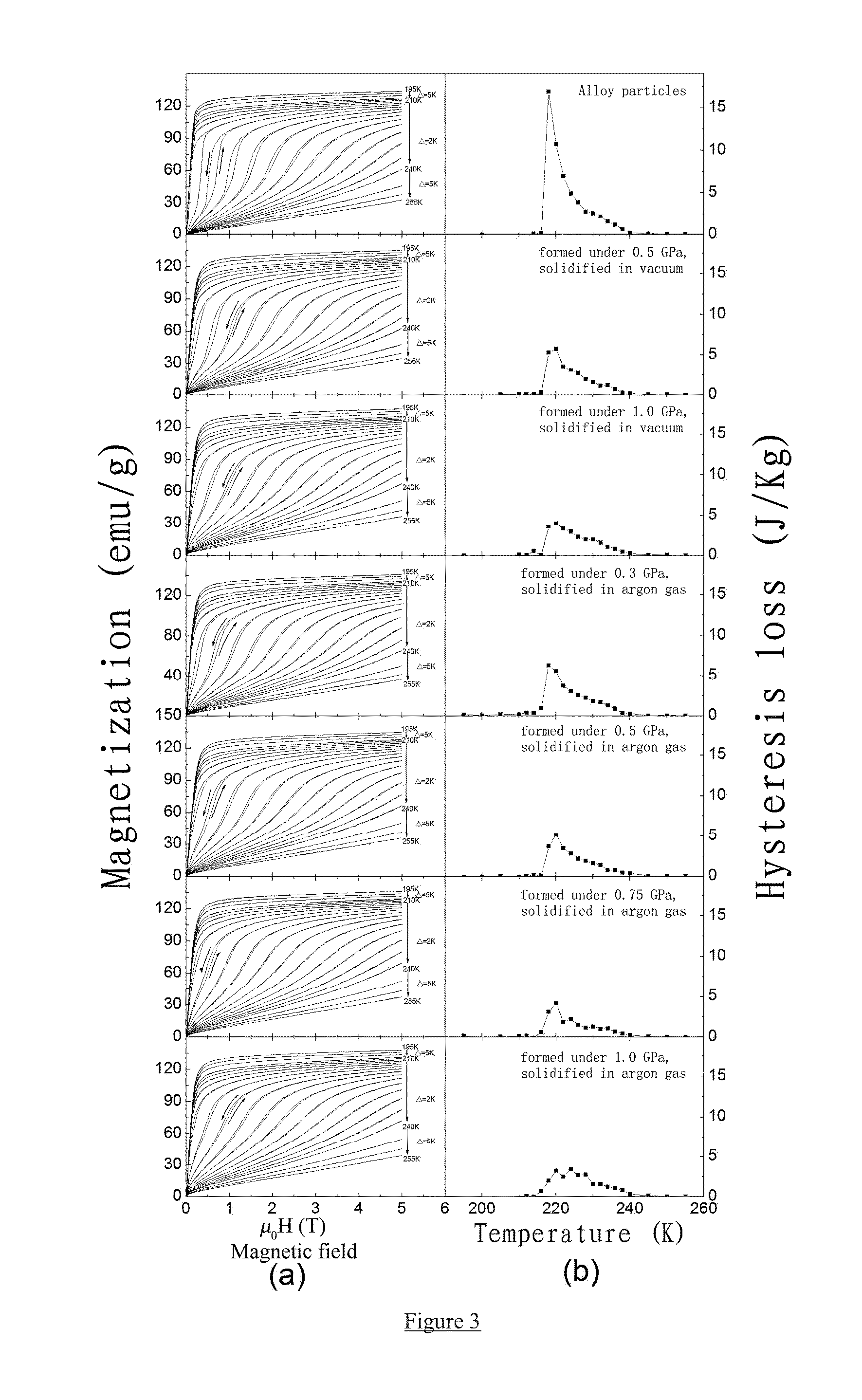
Provided is a high-strength, bonded La(Fe, Si)13-based magnetocaloric material, as well as a preparation method and use thereof. The magnetocaloric material comprises magnetocaloric alloy particles and an adhesive agent, wherein the particle size of the magnetocaloric alloy particles is less than or equal to 800 μm and are bonded into a massive material by the adhesive agent; the magnetocaloric alloy particle has a NaZn13-type structure and is represented by a chemical formula of La1-xRx(Fe1-p-qCopMnq)13-ySiyAα, wherein R is one or more selected from elements cerium (Ce), praseodymium (Pr) and neodymium (Nd), A is one or more selected from elements C, H and B, x is in the range of 0≦x≦0.5, y is in the range of 0.8≦y≦2, p is in the range of 0≦p≦0.2, q is in the range of 0≦q≦0.2, α is in the range of 0≦α≦3.0. Using a bonding and thermosetting method, and by means of adjusting the forming pressure, thermosetting temperature, and thermosetting atmosphere, etc., a high-strength, bonded La(Fe, Si)13-based magnetocaloric material can be obtained, which overcomes the frangibility, the intrinsic property, of the magnetocaloric material. At the same time, the magnetic entropy change remains substantially the same, as compared with that before the bonding. The magnetic hysteresis loss declines as the forming pressure increases. And the effective refrigerating capacity, after the maximum loss being deducted, remains unchanged or increases.
Owner:INST OF PHYSICS - CHINESE ACAD OF SCI +1
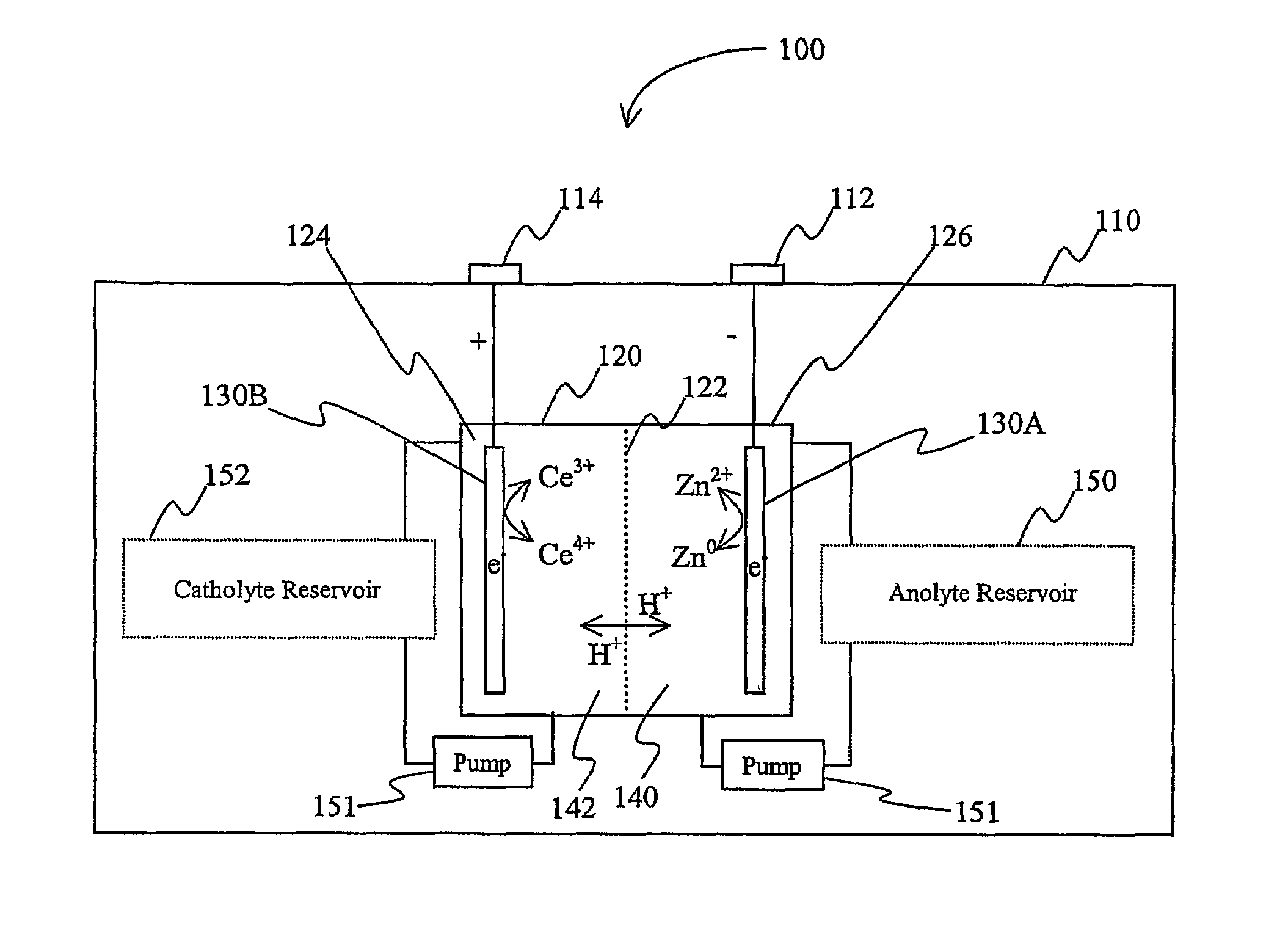
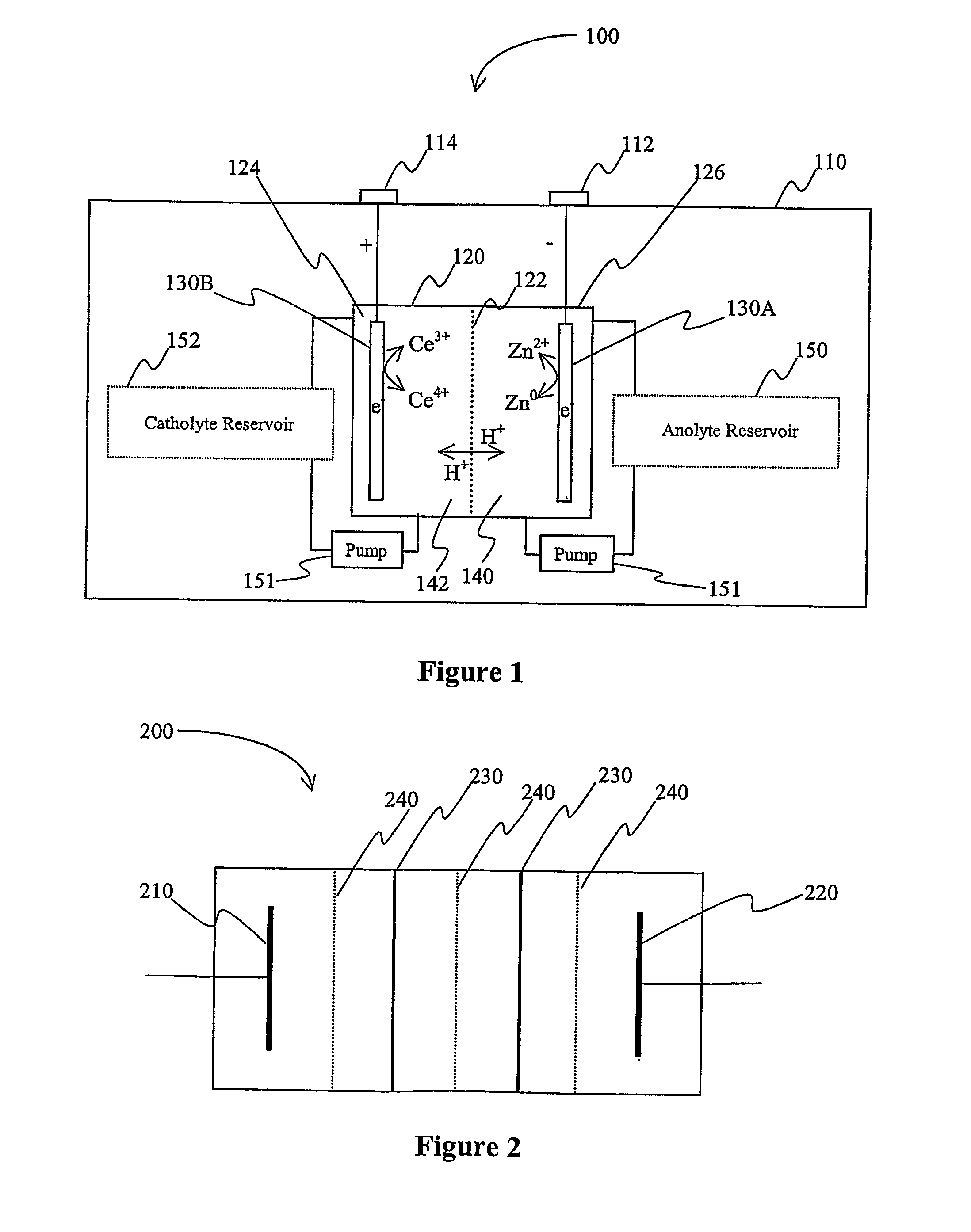
Lanthanide batteries
InactiveUS7252905B2Wide capacity rangeLarge capacityAlkaline accumulatorsSolid electrolyte cellsLanthanideCerium
A battery (100) comprises an electrolyte in which a lanthanide and zinc form a redox pair. Preferred electrolytes are acid electrolytes, and most preferably comprise methane sulfonic acid, and it is further contemplated that suitable electrolytes may include at least two lanthanides. Contemplated lanthanides include cerium, praseodymium, neodymium, terbium, and dysprosium, and further contemplate lanthanides are samarium, europium, thulium and ytterbium.
Owner:PLURION LTD
Ceramic bonding composition, method of making, and article of manufacture incorporating the same
A ceramic bonding composition comprises a first oxide and at least a second oxide having a formula of Me2O3; wherein the first oxide is selected from the group consisting of aluminum oxide, scandium oxide, and combinations thereof; Me is selected from the group consisting of yttrium, lanthanum, cerium, praseodymium, neodymium, samarium, europium, gadolinium, terbium, dysprosium, holmium, erbium, thulium, ytterbium, lutetium, and combinations thereof. The ceramic bonding composition can further comprise silica. An article of manufacture comprising at least two members attached together with the ceramic bonding composition.
Owner:GENERAL ELECTRIC CO
Rare earth permanent magnet produced by applying abundant rare earth cerium (Ce) and preparation method thereof
ActiveCN103123839AReduce production and sales balancePromote the balance of production and salesInductances/transformers/magnets manufactureMagnetic materialsRare-earth elementCost Controls
The invention discloses a rare earth permanent magnet produced by applying abundant rare earth cerium (Ce) and a preparation method of the rare earth permanent magnet produced by applying abundant rare earth Ce. Based on a double-alloy process, main phase alloy formula uses Ce to partly replace neodymium (Nd), an optimized composition design enables a main alloy to form Ce2Fe14B phase and Nd2Fe14B phase to a greatest extent, and therefore high intrinsic magnetic property is guaranteed. A brand new crystal boundary phase is prepared by a crystal boundary reconstitution technology, and high integral magnetic property and corrosion resistant property are guaranteed, and meanwhile a nanometer powder crystal modification method is supplemented, a micro organization structure of a magnet is optimized, crystal boundary distribution is improved, and the magnetic property and the corrosion resistant property are further improved. By applying abundant rare earth cerium, cost is effectively reduced, and meanwhile balance between production and marketing is promoted. Praseodymium (Pr), Nd, and the like are chosen to form a hard magnetic shell layer of a main phase boundary in a composition design of crystal phase auxiliary alloy at the same time, compared high price heavy rare earth elements of dysprosium (Dy) and terbium (Tb) with the elements, and cost control can be further achieved.
Owner:ZHEJIANG UNIV +1
Electrode for an Ignition Device
An electrode for an ignition device is made from a Ni-based nickel-chromium-iron alloy which has improved resistance to high temperature oxidation, sulfidation, corrosive wear, deformation and fracture includes, by weight of the alloy: 14.5-25% chromium; 7-22% iron; 0.2-0.5% manganese; 0.2-0.5% silicon; 0.1-2.5% aluminum; 0.05-0.15% titanium; 0.01-0.1% total of calcium and magnesium; 0.005-0.5% zirconium; 0.001-0.01% boron, and the balance substantially Ni. It may also include at least one rare earth element selected from the group consisting of: yttrium, hafnium, lanthanum, cerium and neodymium in amounts ranging from 0.01-0.15% by weight, and incidental impurities, including cobalt, niobium, molybdenum, copper, carbon, lead, phosphorus or sulfur. These total of these impurities will typically be controlled to limits of 0.1% cobalt, 0.05% niobium, 0.05% molybdenum, 0.01% copper, 0.01% carbon, 0.005% lead, 0.005% phosphorus and 0.005% sulfur. The ignition device may be a spark plug which includes a ceramic insulator, a conductive shell, a center electrode disposed in the ceramic insulator having a terminal end and a sparking end with a center electrode sparking surface, and a ground electrode operatively attached to said shell having a ground electrode sparking surface, the center electrode sparking surface and the ground electrode sparking surface defining a spark gap therebetween. At least one of the center electrode or the ground electrode includes the solution-strengthened Ni-based nickel-chromium-iron alloy. The Ni-based nickel-chromium-iron alloy electrodes of the invention may also include a core with thermal conductivity greater than that of the Ni-based nickel-chromium-iron alloy, such as copper or silver or their alloys.
Owner:FEDERAL MOGUL WORLD WIDE LLC
Transformation liquid for preparation of corrosion-resistant oxidation film on aluminium alloy surface and method of use thereof
InactiveCN101139708AImprove corrosion resistanceGood adhesionMetallic material coating processesHigh resistancePersulfate
The invention discloses a transforming solution for preparing anti-corrosion compound oxidation film on an aluminum alloy surface and a using method for the oxidation film. The invention is characterized in that, the transforming solution is a film-forming promoter containing rare-earth salt (nitrate or sulfate and compound salt of cerium, praseodymium, and neodymium), such compound oxidant as radical of permanganate, nitrate and perchlorate, etc., film-forming promoter of vanadium salt and strontium salt, etc.; the transforming solution contains no sexavalent chromium, is environmental friendly; the reaction speed is improved by the compound oxidant and the film-forming promoter, no heating is needed for the chemical transforming, the treating time is 1-5 min. The invention can rapidly prepare under room temperature compound oxidation film comprising compound rare-earth oxide, alumina and manganese oxide with good resistance to corrosion on aluminum alloy surface. The treating solution from the invention is of rapid film-forming speed, simple process, even film layer, high resistance to corrosion, and low environmental pollution, etc.
Owner:陈东初 +2
Rare earth aluminum alloy, and method and device for preparing same
The invention discloses a rare earth aluminum alloy, and a method and a device for preparing the same. The alloy contains at least one rare earth metal of lanthanum, cerium, praseodymium, neodymium, gadolinium, terbium, dysprosium, holmium, erbium, thulium, lutetium, scandium and yttrium, the content of raw earth is 5 to 98 weight percent, and the balance is aluminum and inevitable impurities. The device for preparing the rare earth aluminum alloy is characterized in that: a) graphite serves as an electrolysis bath, a graphite plate is an anode, a tungsten bar is a cathode and a molybdenum crucible serves as a rare earth aluminum alloy receiver; b) the diameter of the tungsten bar is 30 to 55 mm; and c) the anode of the graphite consists of a plurality of graphite plates. The rare earth aluminum alloy, and the method and the device for preparing the same have the advantages that: the alloy has uniform components, little segregation and low impurity content; technology for preparing the rare earth aluminum alloy through fusion electrolysis can maximally replace a process for preparing single medium-heavy metal through metallothermic reduction, greatly reduce energy consumption and the emission of fluorine-containing tail gas and solid waste residue, improve current efficiency and metal yield and reduce the consumption of auxiliary materials and the energy consumption; and the rare earth aluminum alloys with different rare earth contents can be obtained by controlling different electrolytic temperatures and different cathode current densities.
Owner:GRIREM ADVANCED MATERIALS CO LTD
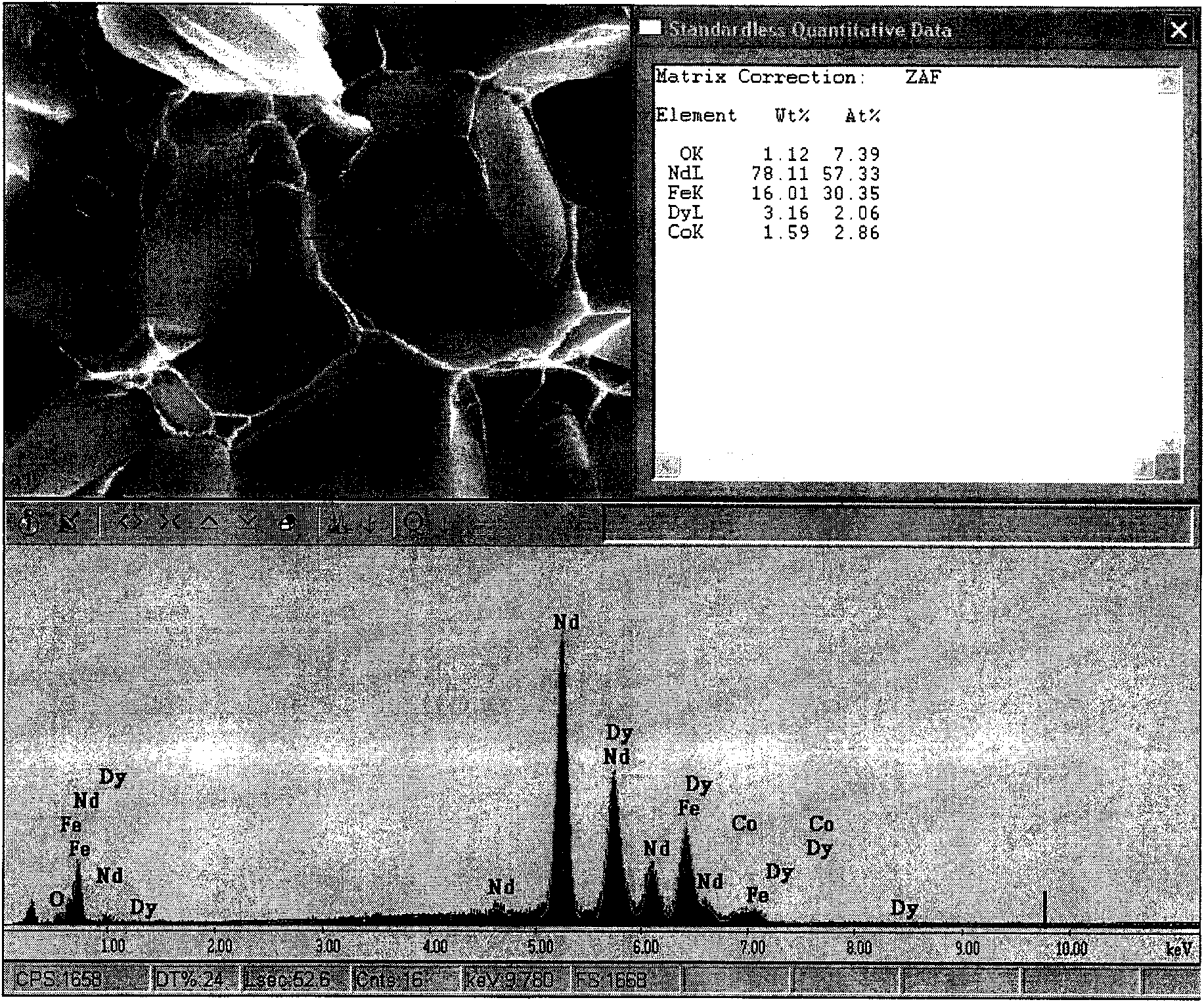
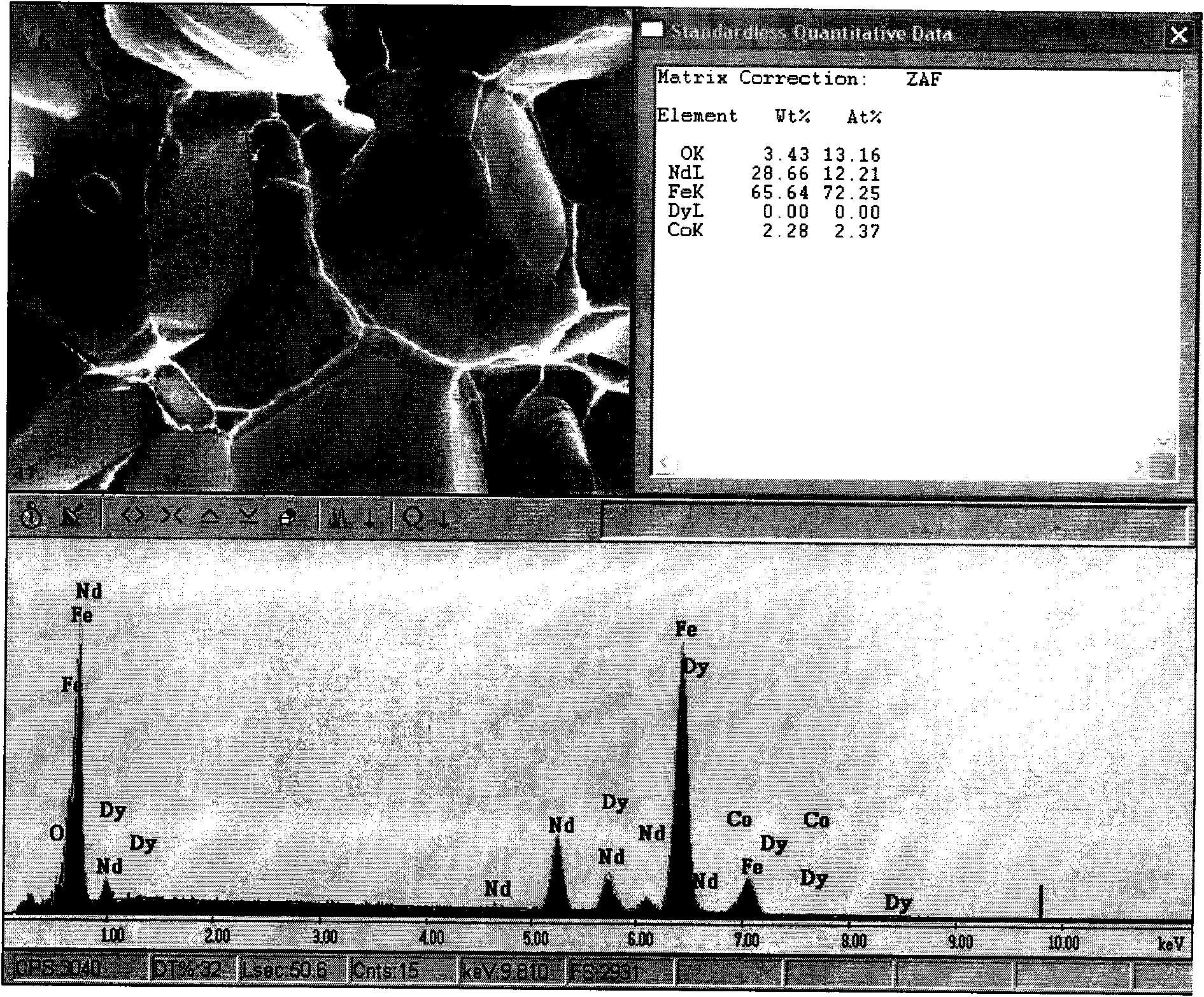
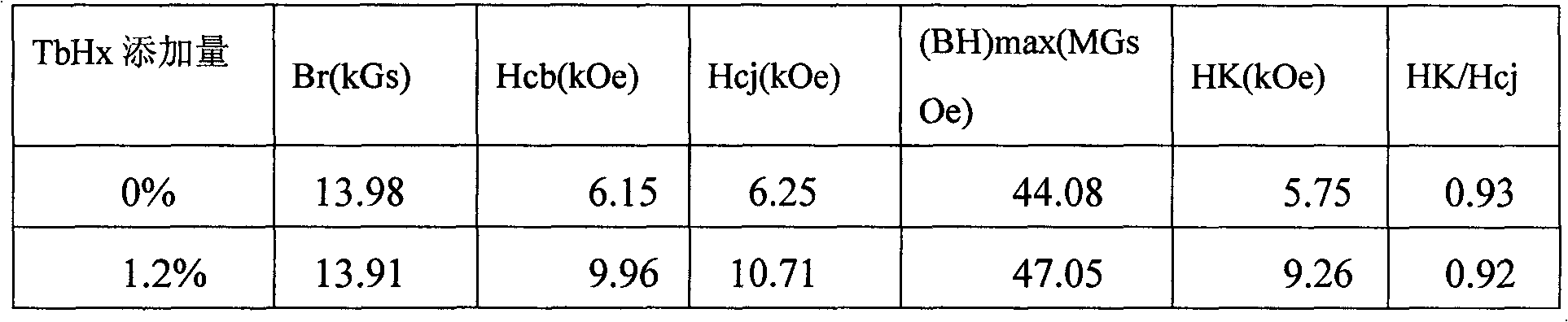
Method for improving coercive force of sintered neodymium ferrum boron (NdFeB)
ActiveCN101996721AEasily brokenTake advantage ofInorganic material magnetismInductances/transformers/magnets manufactureFully developedRare earth
The invention relates to a method for improving coercive force of sintered neodymium ferrum boron (NdFeB) by adding rare earth hydride in a grain boundary phase, which is characterized in that a rare earth or a rare earth mixed hydride is adopted as a grain boundary phase to be added into a NdFeB principal phase alloy to achieve the purpose of improving the coercive force of the sintered NdFeB. The invention provides a process for improving coercive force by adding less amount of rare earth hydride. According to the invention, the distribution of added elements in the grain boundary is controlled, the adverse effect of some alloy elements to the principle phase is avoided, and the improvement potential of the NdFeB magnet property is fully developed.
Owner:NINGBO INST OF MATERIALS TECH & ENG CHINESE ACADEMY OF SCI
Methods for making barrier coatings comprising taggants and components having the same
Methods for making barrier coatings including a taggant involving providing a barrier coating, and adding from about 0.01 mol % to about 30 mol % of a taggant to the barrier coating wherein the taggant comprises a rare earth element selected from lanthanum, cerium, praseodymium, neodymium, promethium, samarium, europium, gadolinium, terbium, dysprosium, holmium, erbium, ytterbium, and lutetium, salts thereof, silicates thereof, oxides thereof, zirconates thereof, hafnates thereof, titanates thereof, tantalates thereof, cerates thereof, aluminates thereof, aluminosilicates thereof, phophates thereof, niobates thereof, borates thereof, and combinations thereof.
Owner:GENERAL ELECTRIC CO
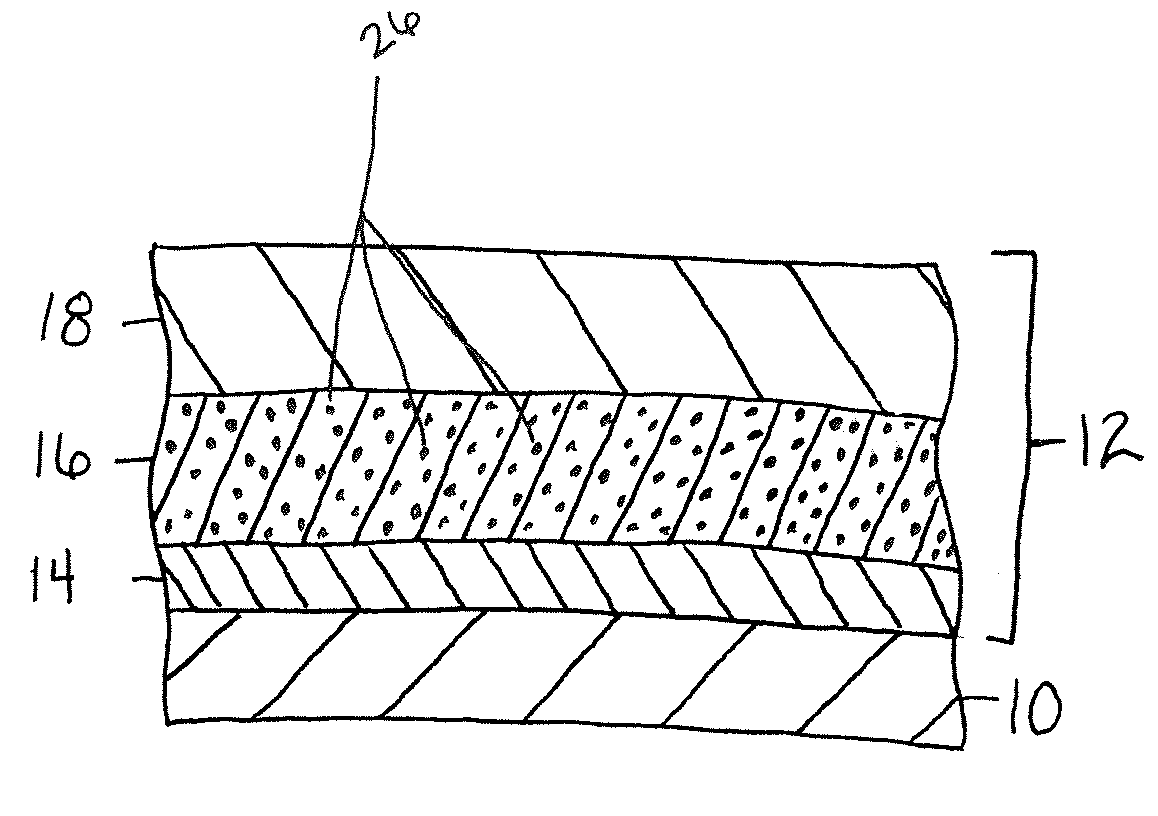
Surface protection of neodymium iron boron permanent-magnet material
ActiveCN101029389AImprove bindingUniform surfaceMetallic material coating processesTectorial membraneBoron
A surface protection technology of neodymium-ferrous-boron permanent magnetic material is carried out by grinding for neodymium-ferrous-boron permanent magnetic material, degreasing while removing oil, removing rust while acid cleaning, activating acid liquid, putting it into black liquid or blue liquid, chemically coating and forming into blue-black or dark blue protection film. It has excellent combination and anti-corrosion performances.
Owner:BEIJING ZHONG KE SAN HUAN HI TECH +1
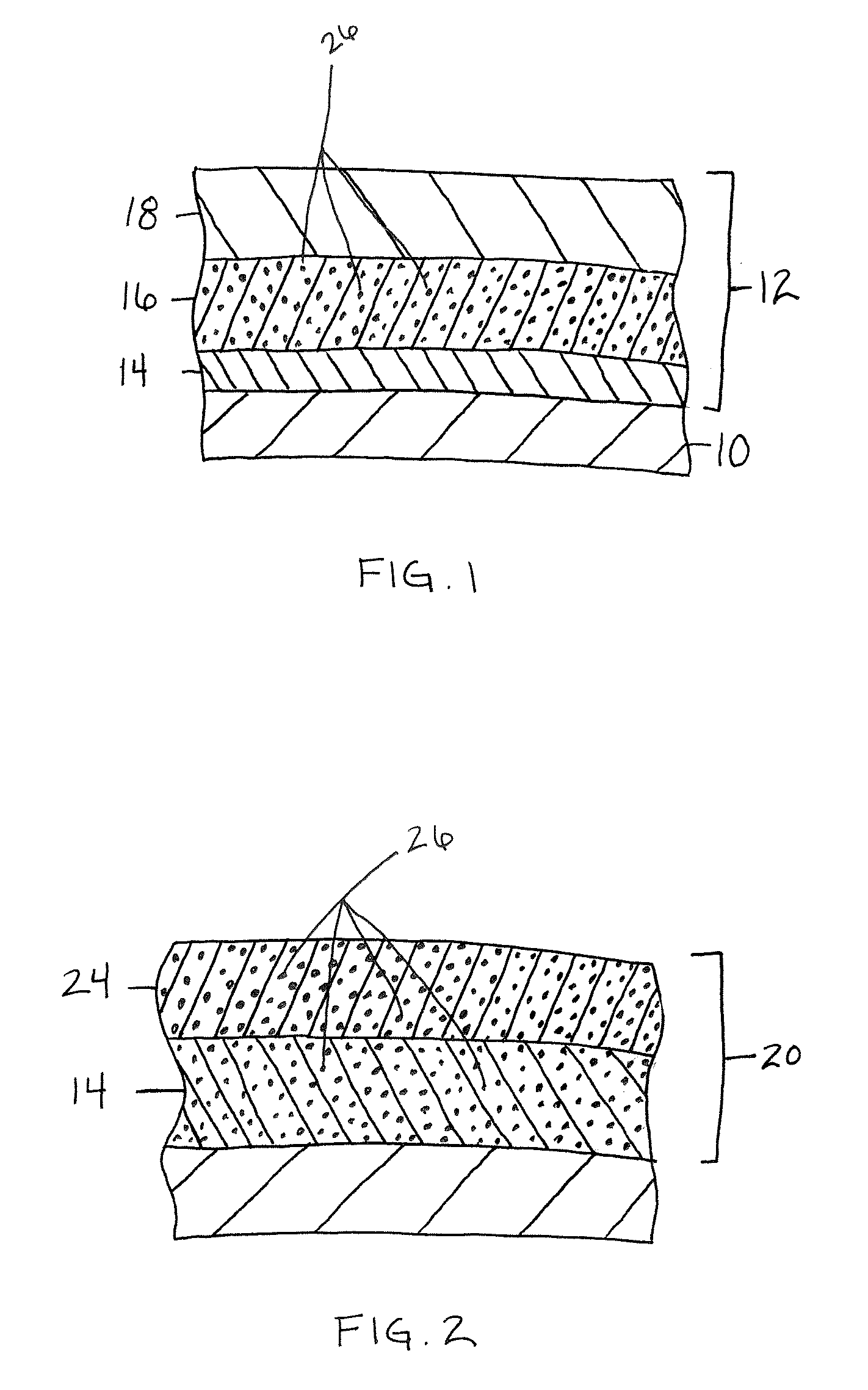
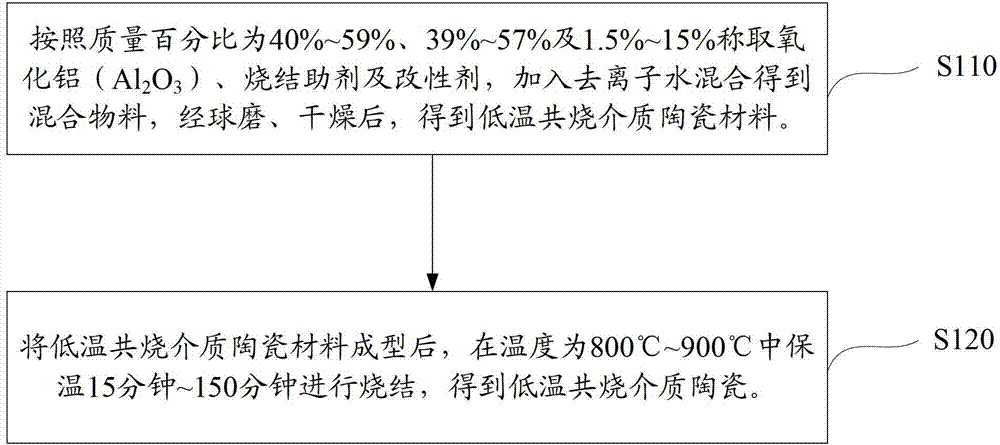
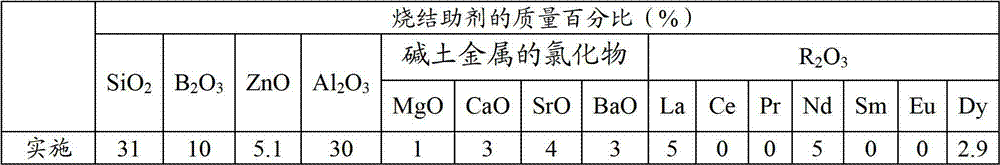
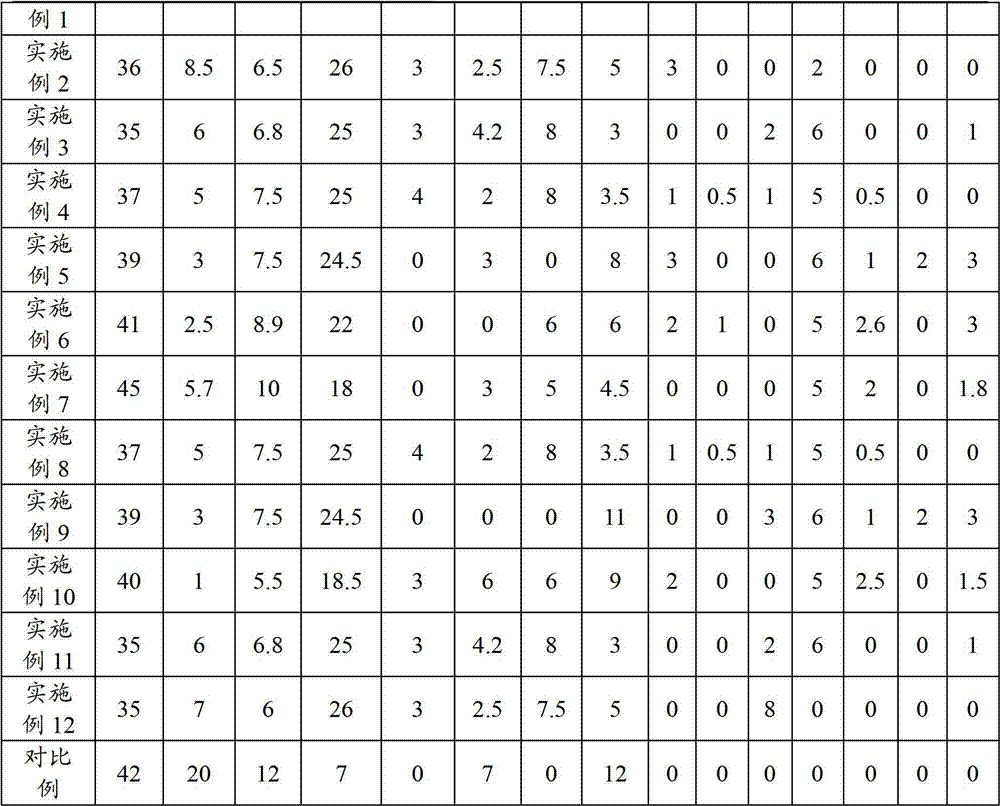
Method, sintering aid and materials for preparation of low-temperature cofired medium ceramic and application
A sintering aid for a low-temperature cofired medium ceramic material is composed of, by weight, 31%-45% of silicon dioxide, 1%-10% of boron oxide, 5.1%-10% of zinc oxide, 18%-30% of aluminum oxide, 11%-24% of alkaline earth metallic oxide and 5%-15% of oxide with the general formula of R2O3, wherein R refers to at least one of lanthanum, cerium, praseodymium, neodymium, samarium, europium and dysprosium, and the alkaline earth metallic oxide refers to one of magnesium oxide, calcium oxide, barium oxide and strontium oxide. Adding the sintering aid into the low-temperature cofired medium ceramic material enables the prepared low-temperature cofired medium ceramic to have excellent thermal mechanical performance and dielectric performance. In addition, the invention provides the low-temperature cofired medium ceramic material and application thereof and a method for preparing the low-temperature cofired medium ceramic.
Owner:GUANGDONG FENGHUA ADVANCED TECH HLDG
CMAS resistant thermal barrier coating
ActiveUS20070172703A1Reduce componentsReduces sand related distressMolten spray coatingBlade accessoriesIndiumCerium
A turbine engine component is provided which has a substrate and a thermal barrier coating applied over the substrate. The thermal barrier coating comprises alternating layers of yttria-stabilized zirconia and a molten silicate resistant material. The molten silicate resistant outer layer may be formed from at least one oxide of a material selected from the group consisting of lanthanum, cerium, praseodymium, neodymium, promethium, samarium, europium, gadolinium, terbium, dysprosium, holmium, erbium, thulium, ytterbium, lutetium, scandium, indium, zirconium, hafnium, and titanium or may be formed from a gadolinia-stabilized zirconia. If desired, a metallic bond coat may be present between the substrate and the thermal barrier coating system. A method for forming the thermal barrier coating system of the present invention is described.
Owner:RTX CORP
Active matrix substrate for liquid crystal display and its fabrication
InactiveUS20020176032A1TransistorSemiconductor/solid-state device detailsInter layerLiquid-crystal display
An active matrix substrate comprises a matrix array of TFTs. A double-layered film includes an under-layer of aluminum-neodymium (Al-Nd) alloy and an over-layer of high melting point metal. The double-layered film forms first interconnection lines for connection to the TFTs. A triple-layered film includes an under-layer of said high melting point metal, a middle-layer of said Al-Nd alloy and an over-layer of the high melting point metal. The triple-layered film forms second interconnection lines for connection to the TFTs.
Owner:VISTA PEAK VENTURES LLC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com