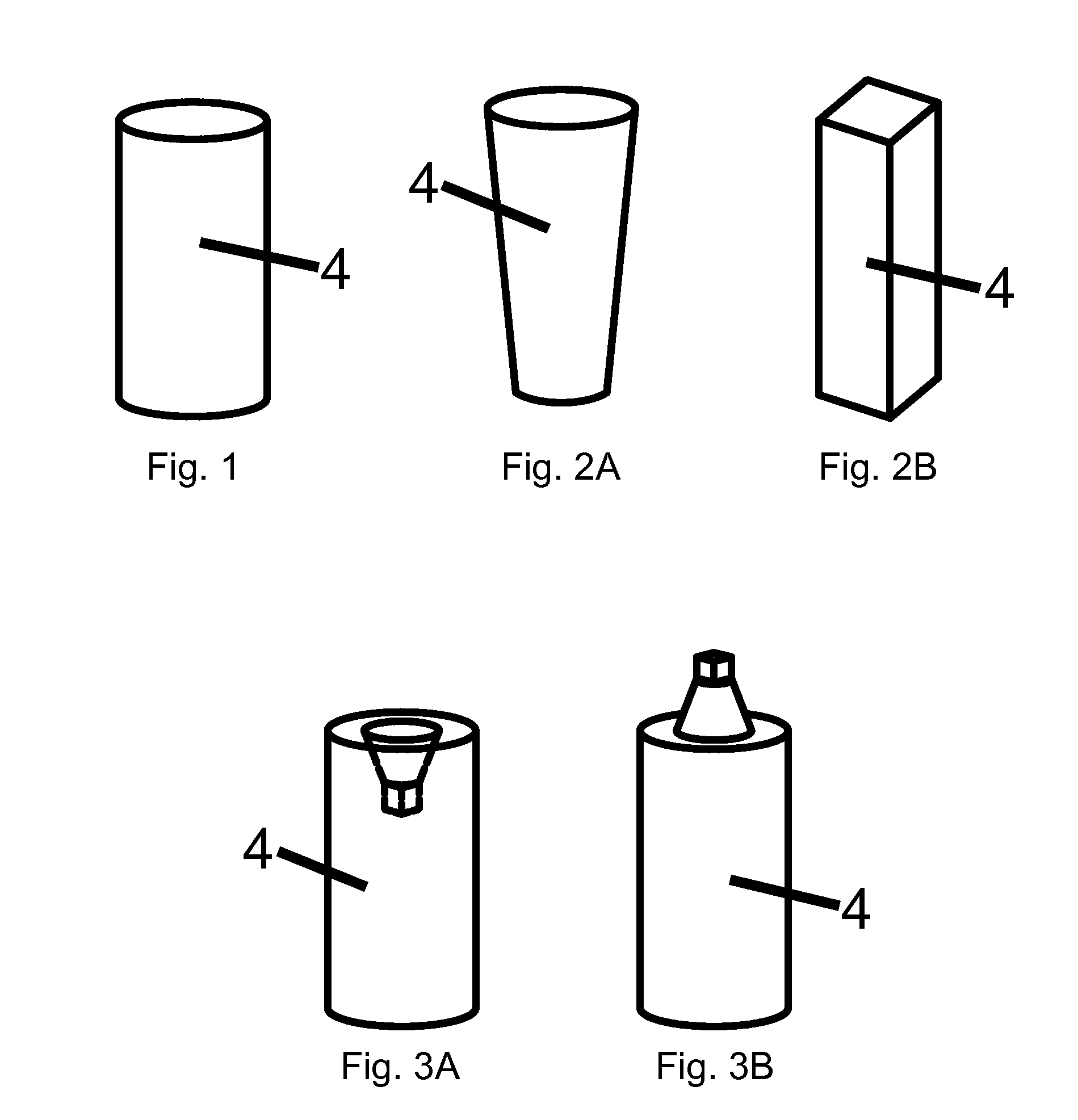
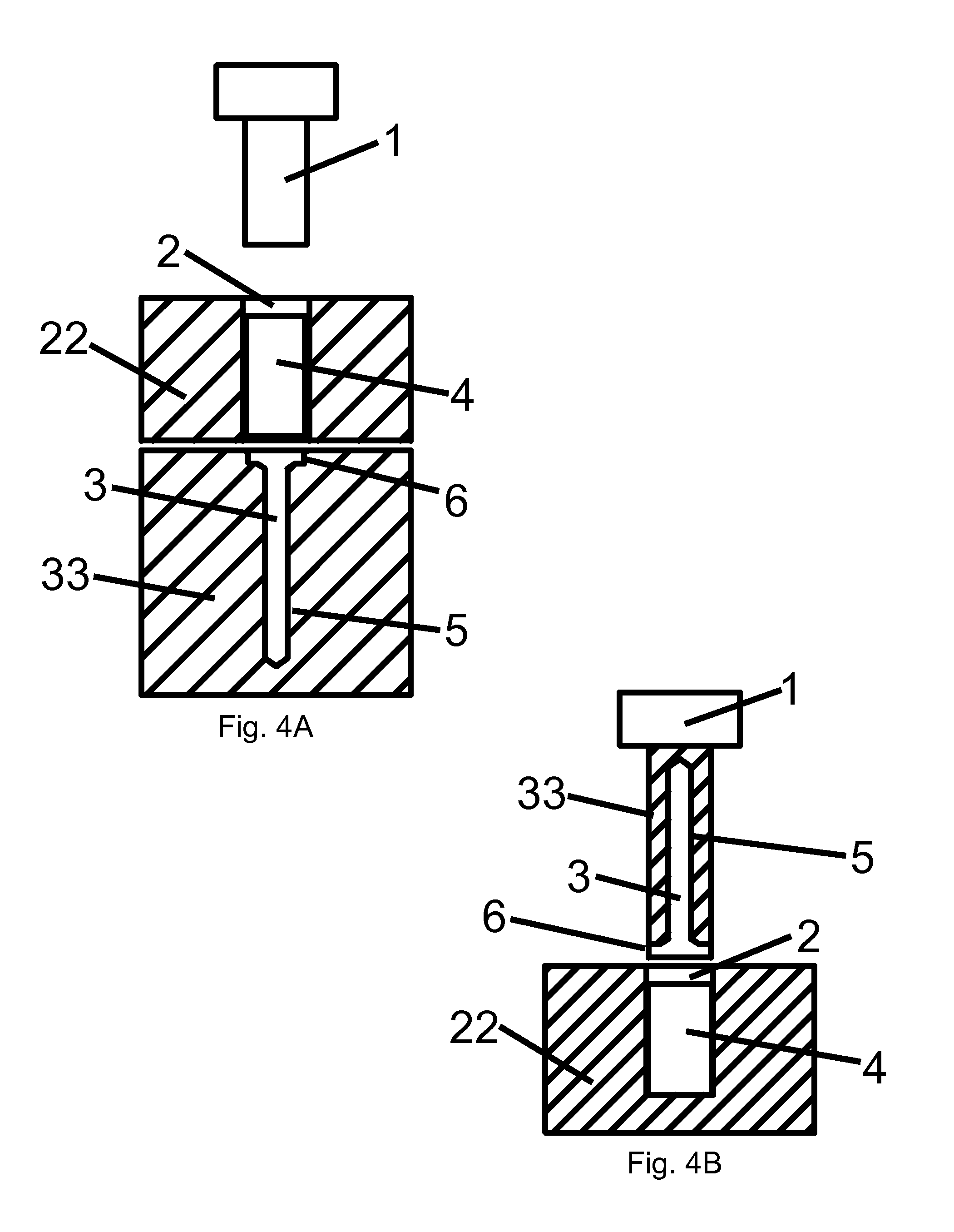
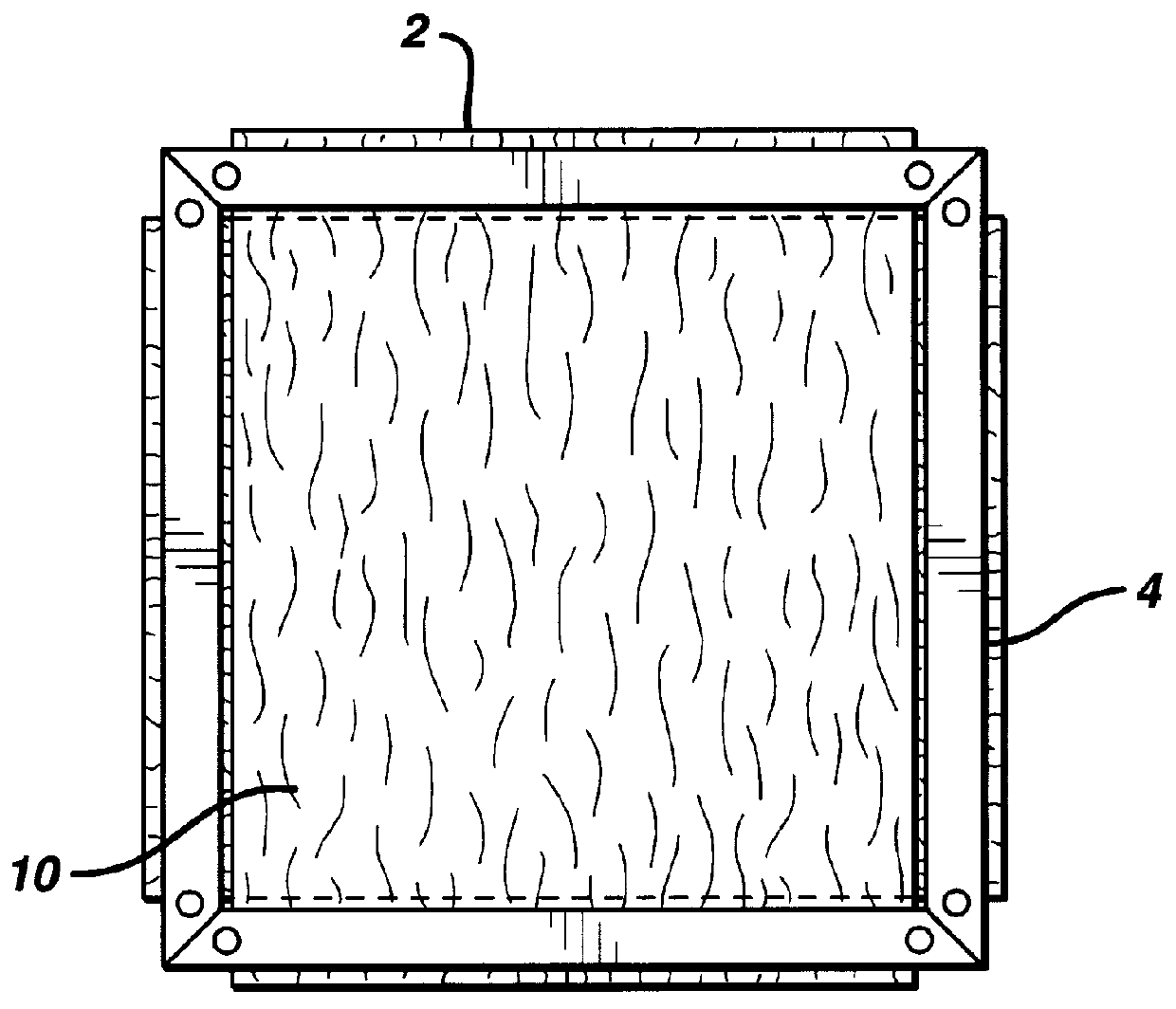
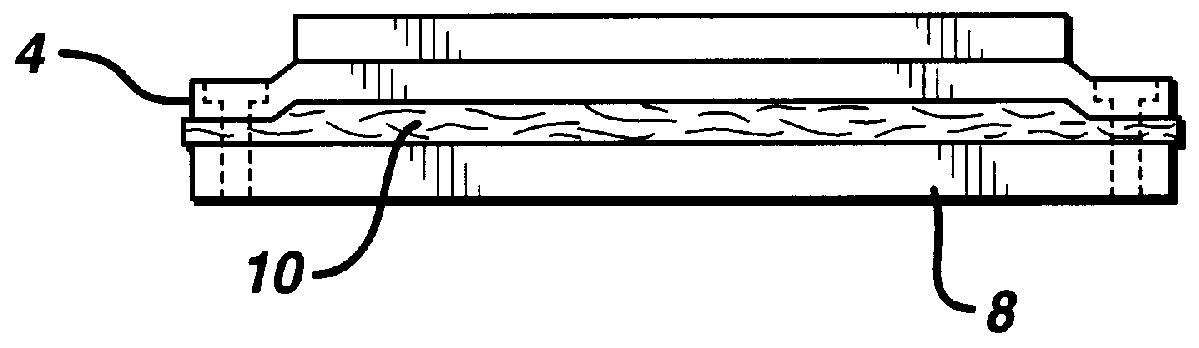
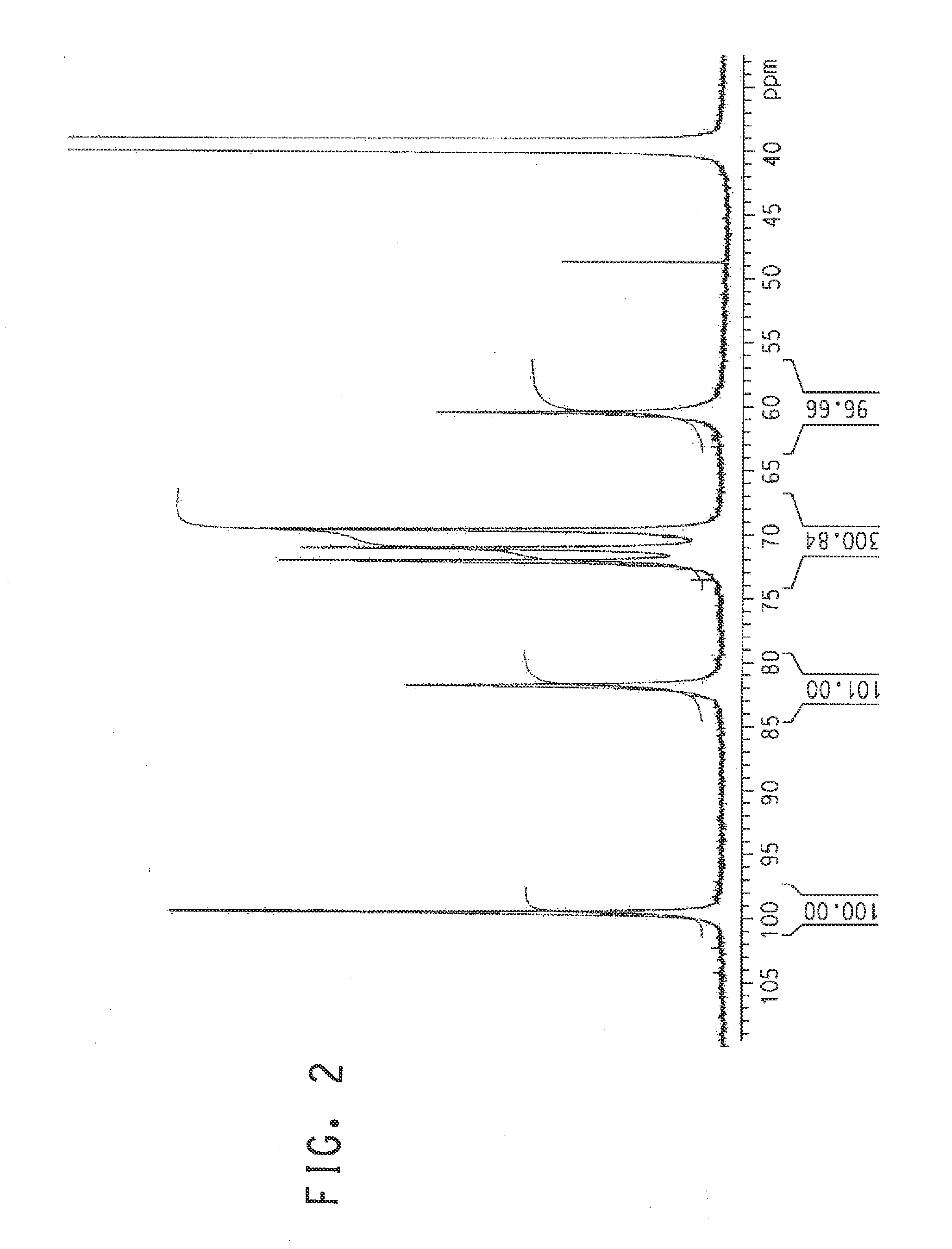
Patents
Literature
108996results about How to "Improve mechanical properties" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Plasma protein matrices and methods for their preparation
InactiveUS7009039B2Rapid cell growthRapid vascularizationBiocidePeptide/protein ingredientsBiological propertyFreeze-drying
A freeze dried biocompatible matrix comprising plasma proteins, useful as implants for tissue engineering as well as in biotechnology, and methods of producing the matrix are provided. Mechanical and physical parameters can be controlled by use of auxiliary components or additives which may be removed after the matrix is formed in order to improve the biological properties of the matrix. The matrices according to the present invention may be used clinically per se, or as a cell-bearing implant.
Owner:PROCHON BIOTECH
Oriented polymer implantable device and process for making same
InactiveUS20100191292A1Reduce cross sectionHigh strengthSuture equipmentsLigamentsFastenerBone screws
A device is formed by a discontinuous process into a bone screw, plate, or fastener, wherein the device has a degree of polymer alignment and strength, and upon reheating above glass transition temperature of the polymer, the device remains dimensionally stable, as it maintains its dimensions, strength, and degree of polymer orientation. In practice of the present invention, the polymer slug is pressed into the die cavity by the actuation of ram press, causing the slug to conform to the die cavity.
Owner:KENSEY NASH CORP
Fabrication of biocompatible polymeric composites
InactiveUS6147135ASure easyImprove performanceSuture equipmentsCosmetic preparationsFiberPolymer science
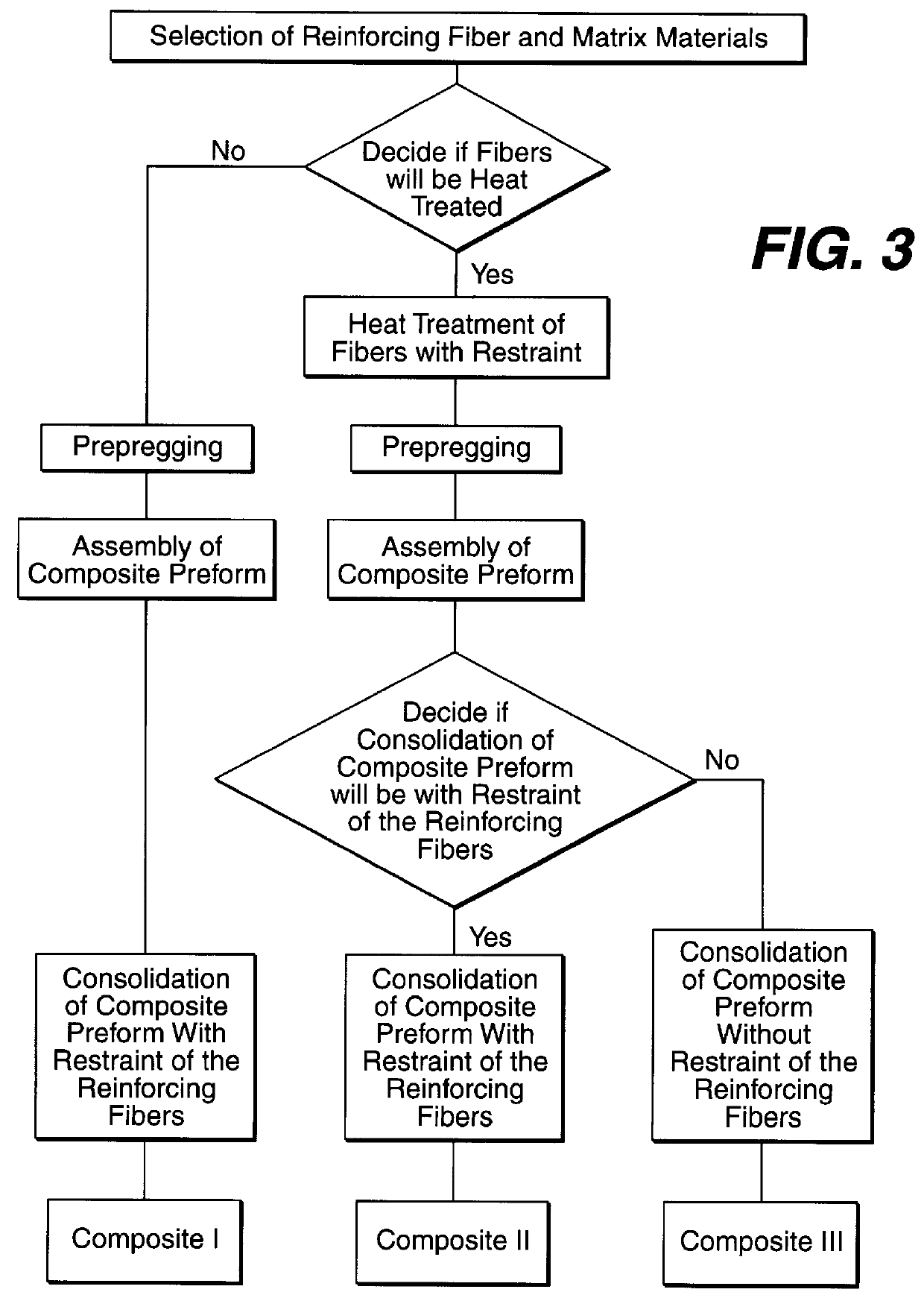
Composite materials formed from biocompatible polymer fibers and biodegradable polymers are disclosed. The heat treatment conditions for the reinforcing fibers are described so that the mechanical properties of the fibers can be retained during composite consolidation process. The processing conditions and set-ups to consolidations are constrained to the temperatures lower than fiber heat treatment temperatures. The reinforcing fibers are restrained under tension so that the minimum relaxation occurs during consolidation process.
Owner:ETHICON INC
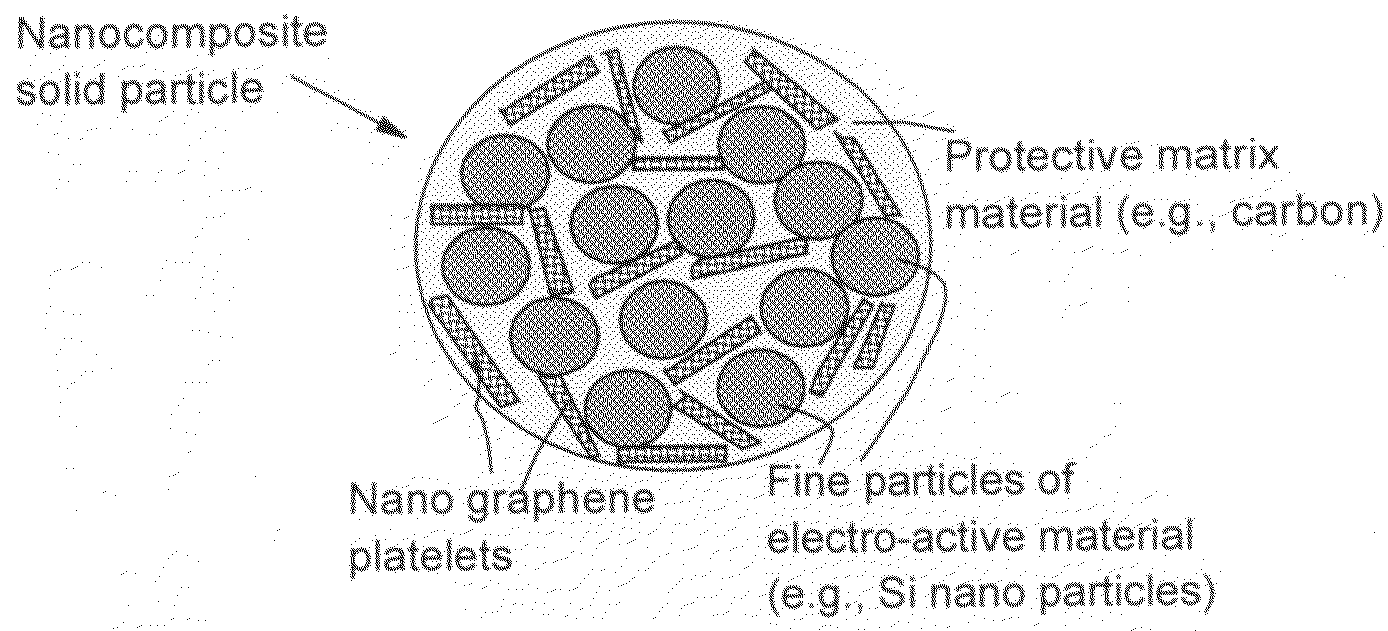
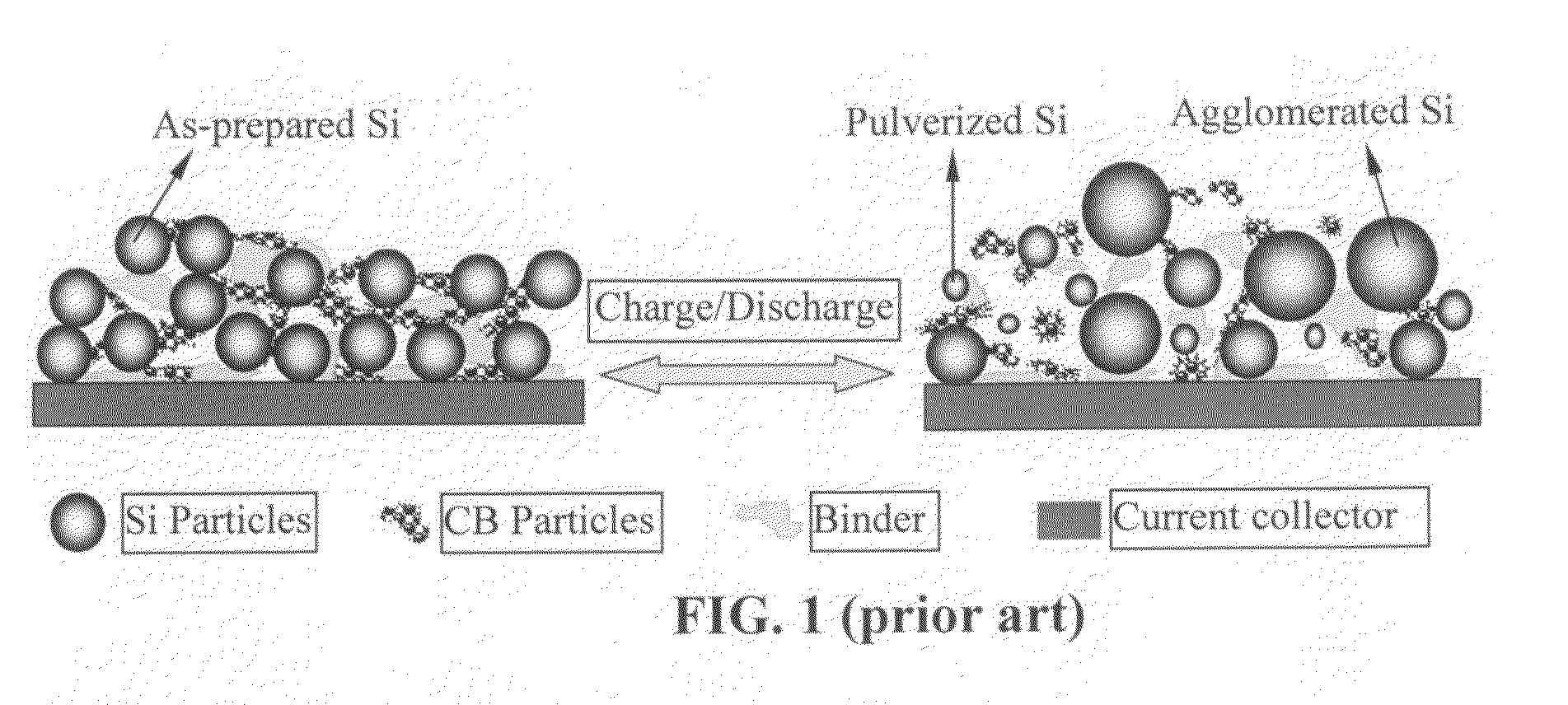
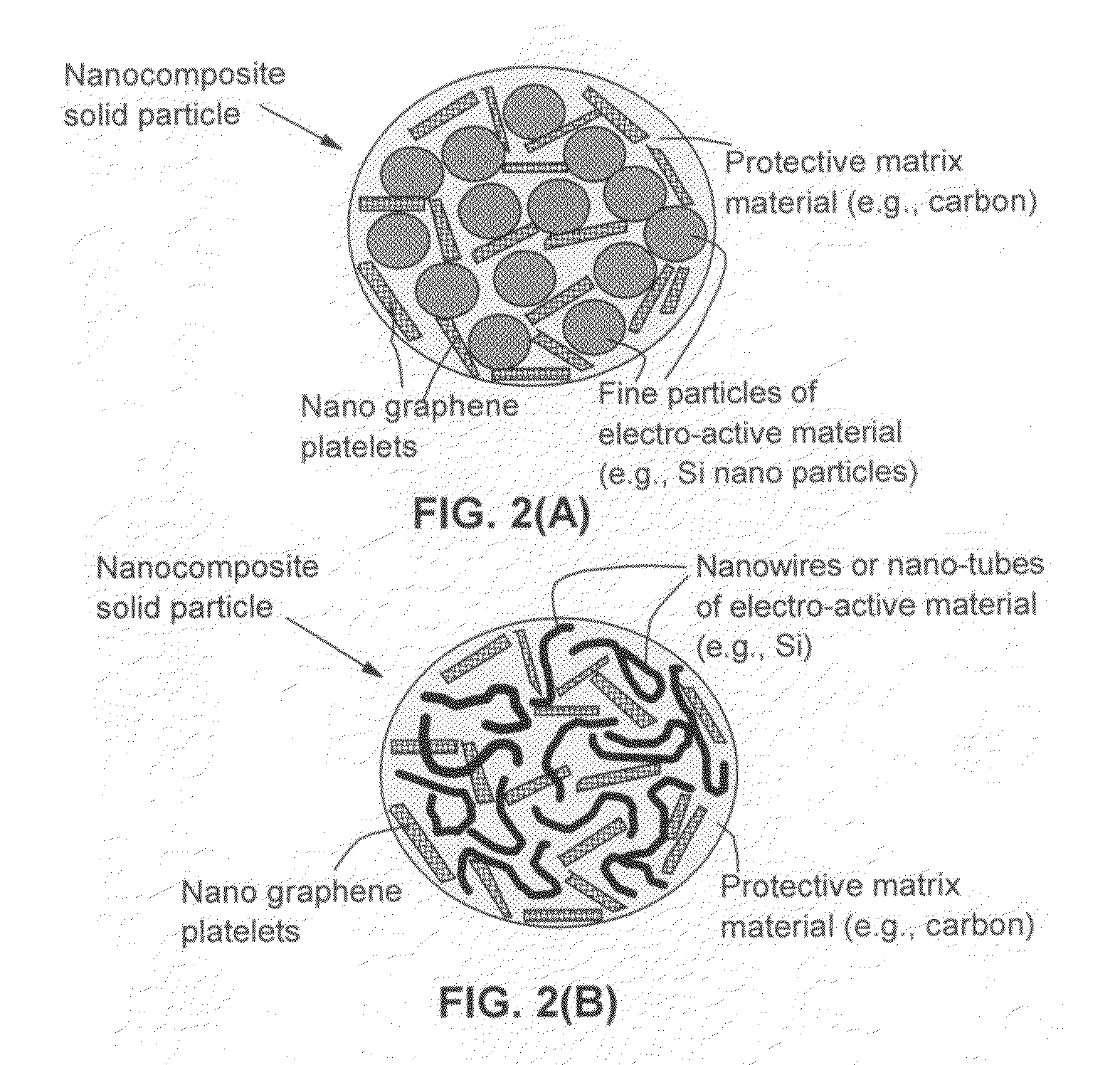
Process for producing nano graphene reinforced composite particles for lithium battery electrodes
ActiveUS20100176337A1Increase stiffnessHigh strengthCell electrodesLi-accumulatorsFiberLithium metal
Owner:SAMSUNG ELECTRONICS CO LTD
Recovery of hydrophobicity of low-k and ultra low-k organosilicate films used as inter metal dielectrics
InactiveUS20050106762A1Low costHigh mechanical strengthSolid-state devicesSemiconductor/solid-state device manufacturingChemical treatmentSilylation
Often used to reduce the RC delay in integrated circuits are dielectric films of porous organosilicates which have a silica like backbone with alkyl or aryl groups (to add hydrophobicity to the materials and create free volume) attached directly to the Si atoms in the network. Si—R bonds rarely survive an exposure to plasmas or chemical treatments commonly used in processing; this is especially the case in materials with an open cell pore structure. When Si—R bonds are broken, the materials lose hydrophobicity, due to formation of hydrophilic silanols and low dielectric constant is compromised. A method by which the hydrophobicity of the materials is recovered using a novel class of silylation agents which may have the general formula (R2N)XSiR′Y where X and Y are integers from 1 to 3 and 3 to 1 respectively, and where R and R′ are selected from the group of hydrogen, alkyl, aryl, allyl and a vinyl moiety. Mechanical strength of porous organosilicates is also improved as a result of the silylation treatment.
Owner:GLOBALFOUNDRIES INC
Metal nanoparticle compositions
InactiveUS20060189113A1Low processing (curing)Improve mechanical propertiesMaterial nanotechnologyTransportation and packagingNanometreViscosity
Owner:CABOT CORP
Nanostructured bulk thermoelectric material
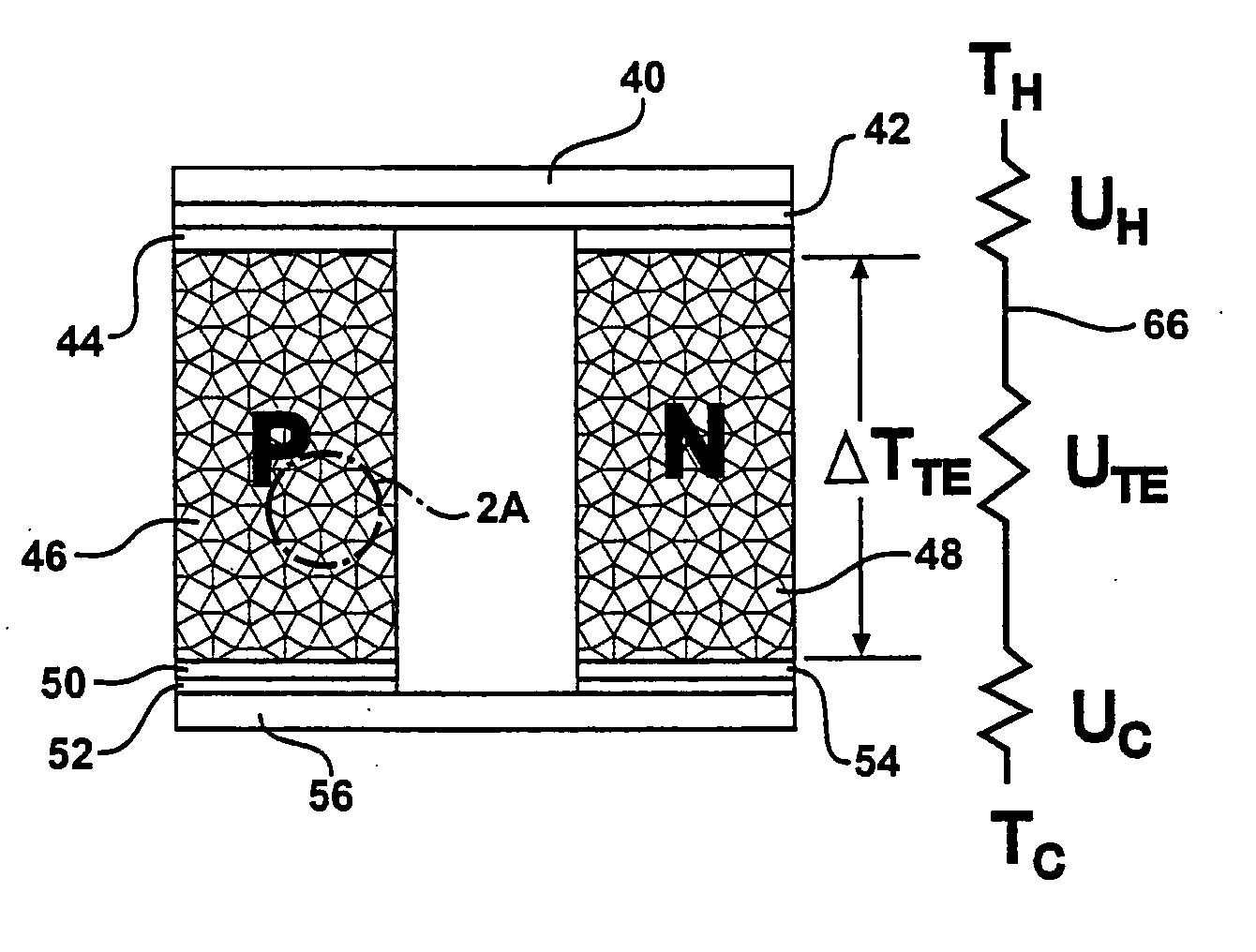
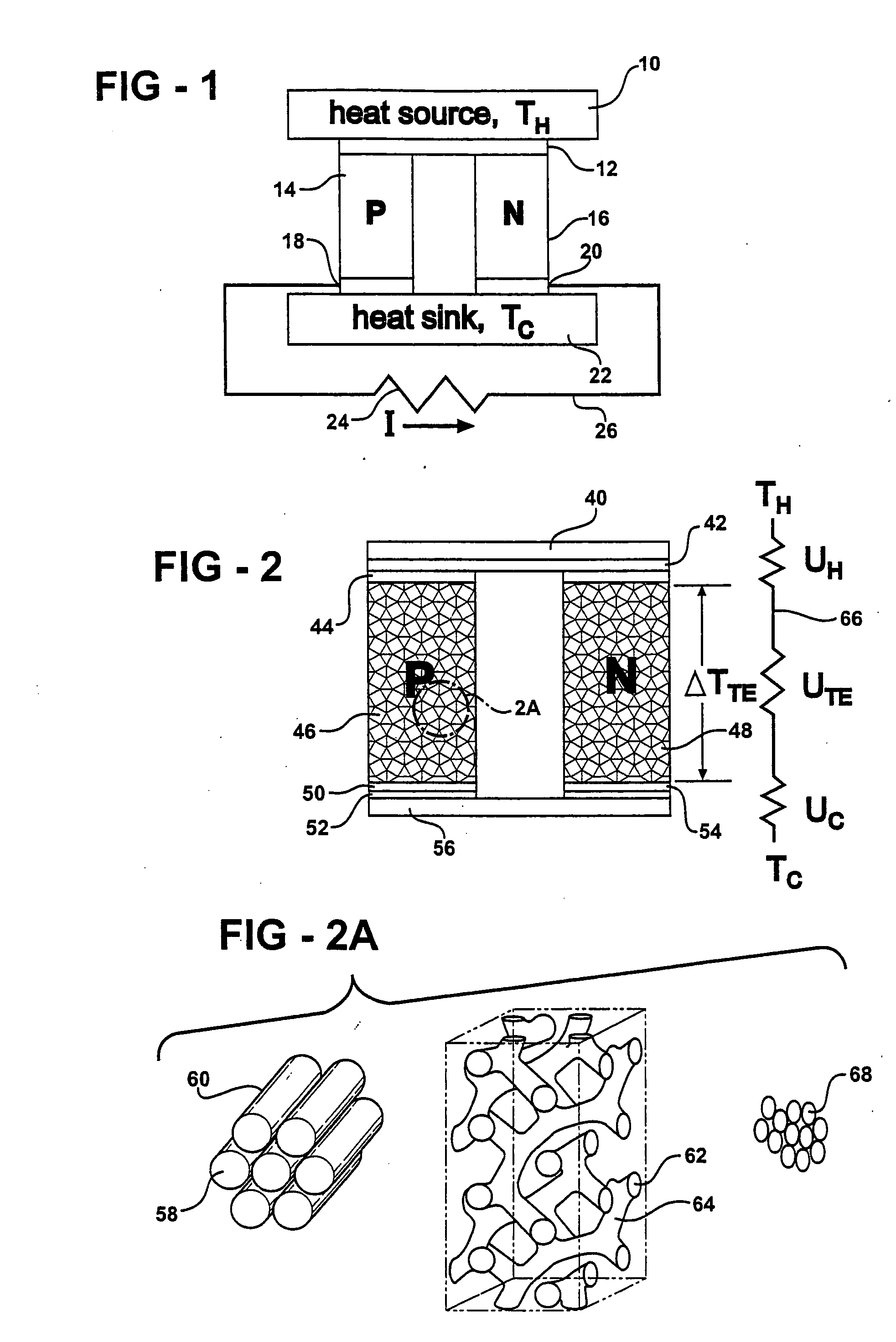
ActiveUS20060118158A1High quality factorImprove mechanical propertiesThermoelectric device with peltier/seeback effectThermoelectric device manufacture/treatmentThermoelectric materialsNanowire
A thermoelectric material comprises two or more components, at least one of which is a thermoelectric material. The first component is nanostructured, for example as an electrically conducting nanostructured network, and can include nanowires, nanoparticles, or other nanostructures of the first component. The second component may comprise an electrical insulator, such as an inorganic oxide, other electrical insulator, other low thermal conductivity material, voids, air-filled gaps, and the like. Additional components may be included, for example to improve mechanical properties. Quantum size effects within the nanostructured first component can advantageously modify the thermoelectric properties of the first component. In other examples, the second component may be a thermoelectric material, and additional components may be included.
Owner:TOYOTA MOTOR CO LTD +1
Reinforced small intestinal submucosa
InactiveUS7160333B2Improve mechanical propertiesEnhanced handling propertyBone implantSurgeryCell-Extracellular MatrixSmall intestine
Bioprosthetic devices for soft tissue attachment, reinforcement, or construction are provided. The devices comprise a sheet of naturally occurring extracellular matrix and a sheet of synthetic mesh coupled to the naturally occurring extracellular matrix portion.
Owner:DEPUY SYNTHES PROD INC
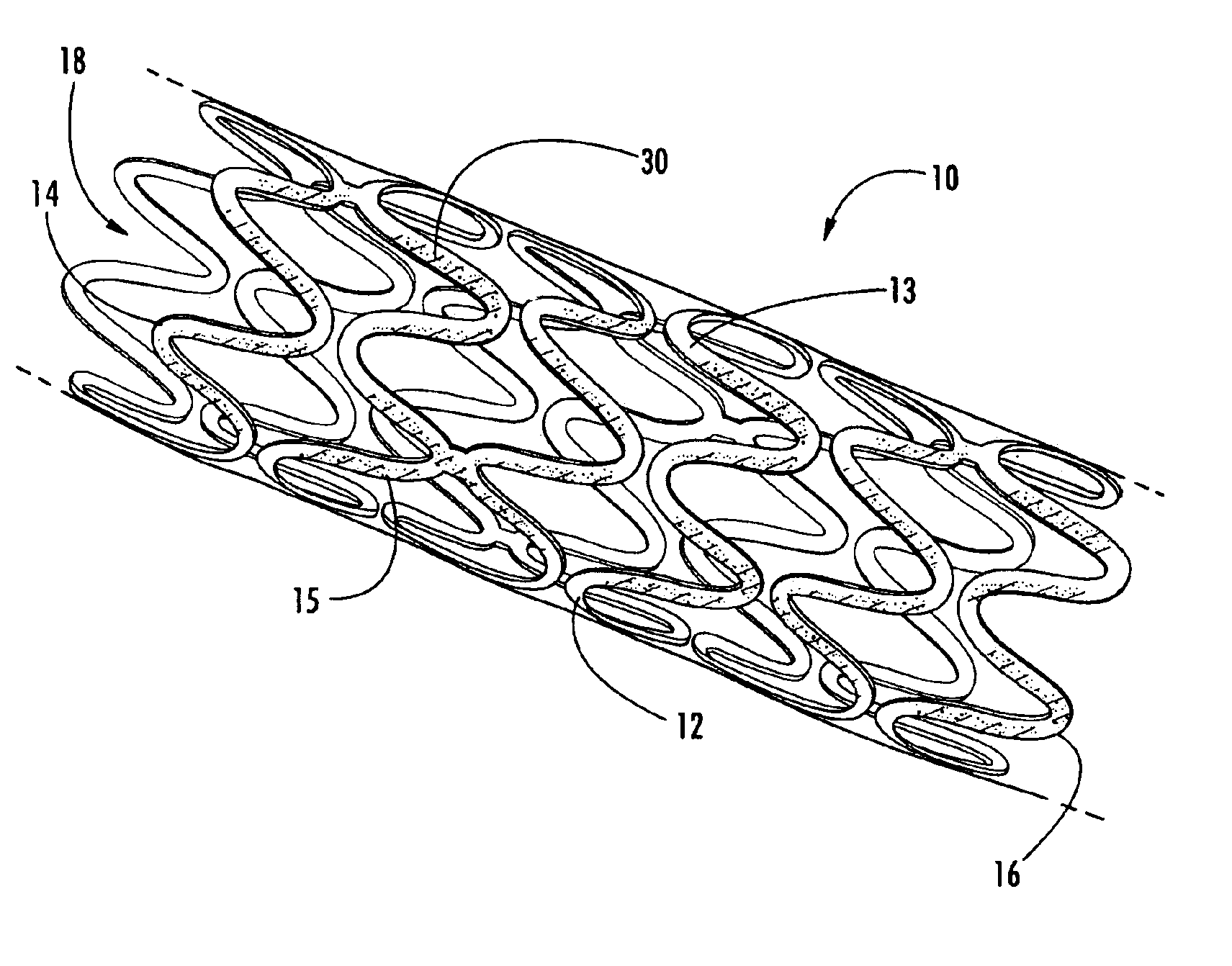
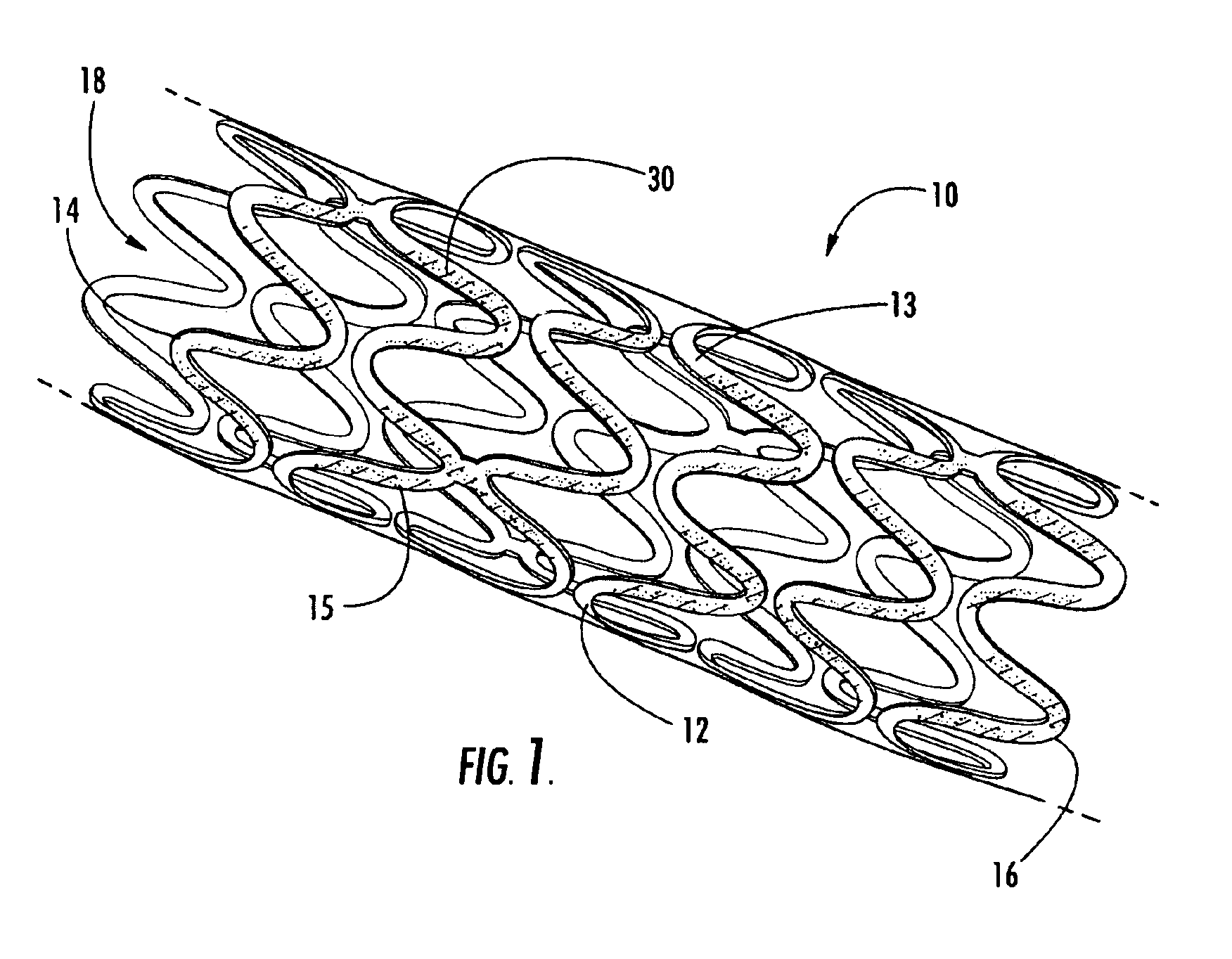
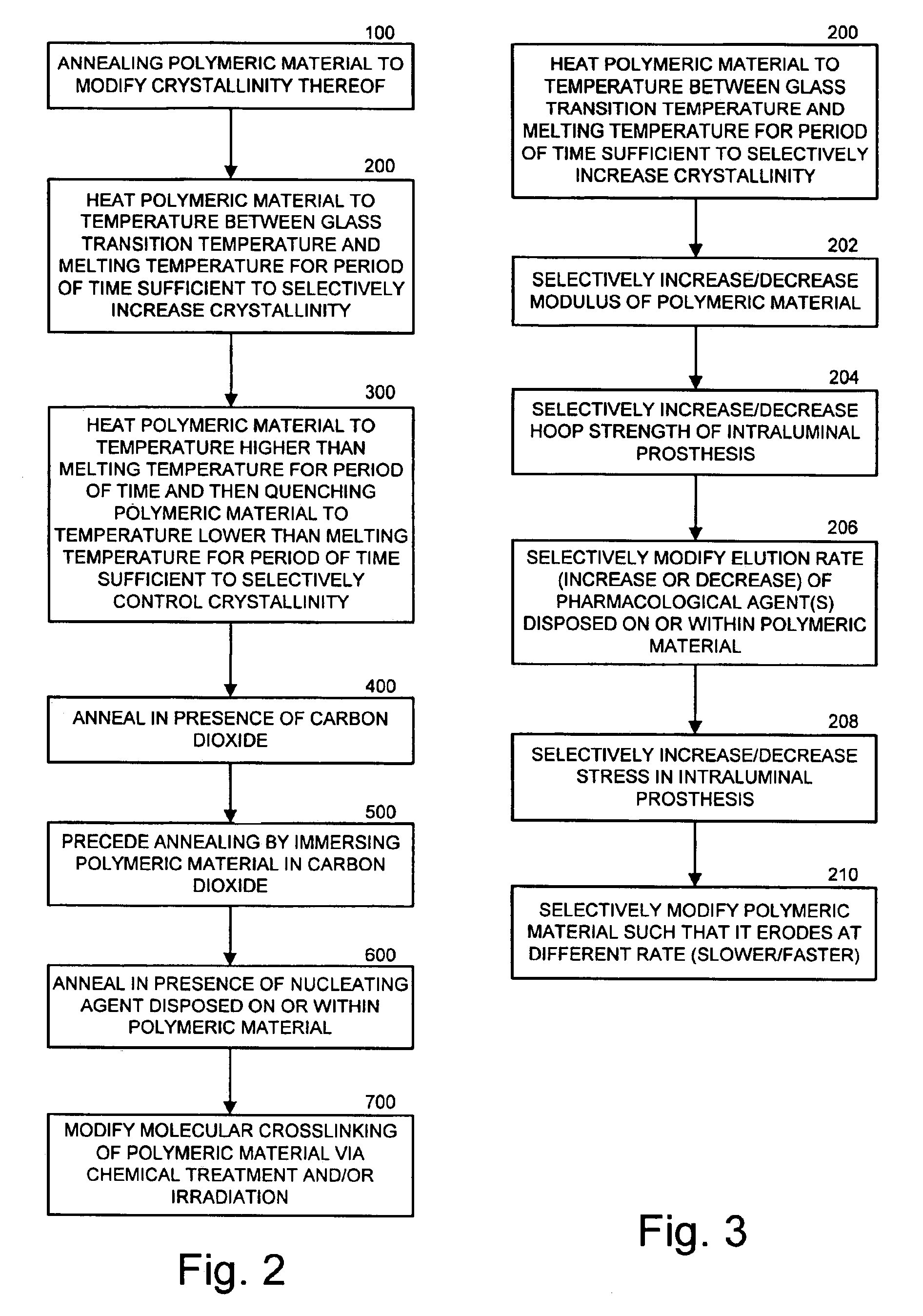
Intraluminal prostheses having polymeric material with selectively modified crystallinity and methods of making same
Methods of manufacturing polymeric intraluminal prostheses include annealing the polymeric material to selectively modify the crystallinity thereof. Annealing may be utilized to selectively modify various properties of the polymeric material of an intraluminal prosthesis, including: selectively increasing the modulus of the polymeric material; selectively increasing the hoop strength of the intraluminal prosthesis; selectively modifying the elution rate (increase or decrease) of a pharmacological agent subsequently disposed on or within the annealed polymeric material; selectively increasing / decreasing stress in the intraluminal prosthesis; and selectively modifying the polymeric material such that it erodes at a different rate.
Owner:SYNECOR LLC
High titer production of highly linear poly (alpha 1,3 glucan)
ActiveUS20130244287A1Improve mechanical propertiesMore amenable to preparing fibersTransferasesFermentationSucroseSaccharophagus degradans
Owner:NUTRITION & BIOSCIENCES USA 4 INC
Tricalcium phosphates, their composites, implants incorporating them, and method for their production
InactiveUS20050031704A1Easily controlEnhance packing and densificationBiocideHeavy metal active ingredientsChemistryProsthetic implants
Methods for the synthesis of tricalcium phosphates are presented, as well as a series of specific reaction parameters that can be adjusted to tailor, in specific ways, properties in the tricalcium phosphate precursor precipitate. Particulate tricalcium phosphate compositions having an average crystal size of about 250 nm or less are provided. Compositions of the invention can be used as prosthetic implants and coatings for prosthetic implants.
Owner:PIONEER SURGICAL TECH INC
Thermally conductive polymer based printed circuit board
InactiveUS20100012354A1Prevent unwanted solderingImprove mechanical propertiesPrinted circuit aspectsPrinted circuit manufactureLiquid crystallineConductive polymer
A printed circuit board has a liquid crystalline polymer layer that is bonded to an electrically conductive layer that includes traces that electrically connect components mounted on the printed circuit board. The liquid crystalline polymer material is thermally conductive and dielectric. When the components produce heat, the liquid crystalline polymer layer absorbs and dissipates the heat produced by the electrical components mounted on the printed circuit board. The thermal equilibrium of the printed circuit board is lower than the maximum operating temperature of the components.
Owner:HEDIN LOGAN BROOK +1
Calcium based neutral and bioresorbable bone graft
InactiveUS20030055512A1Improve mechanical propertiesReduce decreaseSurgical adhesivesBone implantSulfateBone implant
An injectable and moldable putty comprising biodegradable calcium-based compounds including calcium sulfate, hydroxyapatite, and tricalcium phosphate is invented. The putty hardens into a solid body when mixed with water, saline, serum, or other neutral aqueous solutions. The hardening time of the putty can be tailored in order to meet the specific requirements of various dental or orthopedic applications. The pH of the putty is neutral during and after mixing. The invented putty may be used as bone graft, bone implant, or implantable drug delivery device.
Owner:BERKELEY ADVANCED BIOMATERIALS
Thermoplastic copolymer of tetrafluoroethylene and perfluoromethyl vinyl ether and medical devices employing the copolymer
An improved elastomeric material is described comprising an essentially noncross-linkable amorphous copolymer of tetrafluoroethylene (TFE) and perfluoromethyl vinyl ether (PMVE) which is both a thermoplastic and exhibits exceptional mechanical properties. This material is particularly suitable for use in ultra-clean environments, and particularly for use in an implantable device, since it does not contain contaminants that previous thermoset TFE / PMVE copolymers have required. Among the improved properties of the present invention are excellent biocompatibility, high matrix tensile strength, high clarity, high abrasion resistance, high purity, adequate elasticity, and ease of processing due to the thermoplastic, and noncross-linkable structure of the copolymer. The material of the present invention is also a high strength bonding agent particularly suited for bonding porous PTFE to itself or to other porous substances at room or elevated temperatures.
Owner:WL GORE & ASSOC INC
Thermoplastic Elastomer Composition, Method for Producing Same and Formed Article
The invention provides a thermoplastic elastomer composition having mechanical properties equivalent or superior to those of conventional thermoplastic elastomer compositions and having excellent heat resistance and oil resistance and a preparation process thereof, and molded or formed products making use of this thermoplastic elastomer composition. The thermoplastic elastomer composition according to the present invention contains a thermoplastic resin having a polar group and an ethylene•α-olefin elastomer having a functional group. The ethylene•α-olefin elastomer having the functional group is preferably a random copolymer obtained by copolymerizing ethylene, an α-olefin having 3 to 10 carbon atoms, an unsaturated monomer having the functional group and an optional non-conjugated diene.
Owner:JSR CORPORATIOON
Polymer-Free Carbon Nanotube Assemblies (Fibers, Ropes, Ribbons, Films)
InactiveUS20070243124A1Improve mechanical propertiesHigh mechanical strengthMaterial nanotechnologyPigmenting treatmentCarbon nanotubeElectromechanical actuator
Process, apparatus, compositions and application modes are provided that relate to nanofiber spinning without the use of superacids in the spinning solution. The methods employ either acids or bases for a flocculation solution. The advances disclosed therein enable the use of nanofibers, including carbon nanotubes, for a variety of applications including, but not limited to, electromechanical actuators, supercapacitors, electronic textiles, and in devices for electrical energy harvesting.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
Obstructive sleep apnea treatment devices, systems and methods
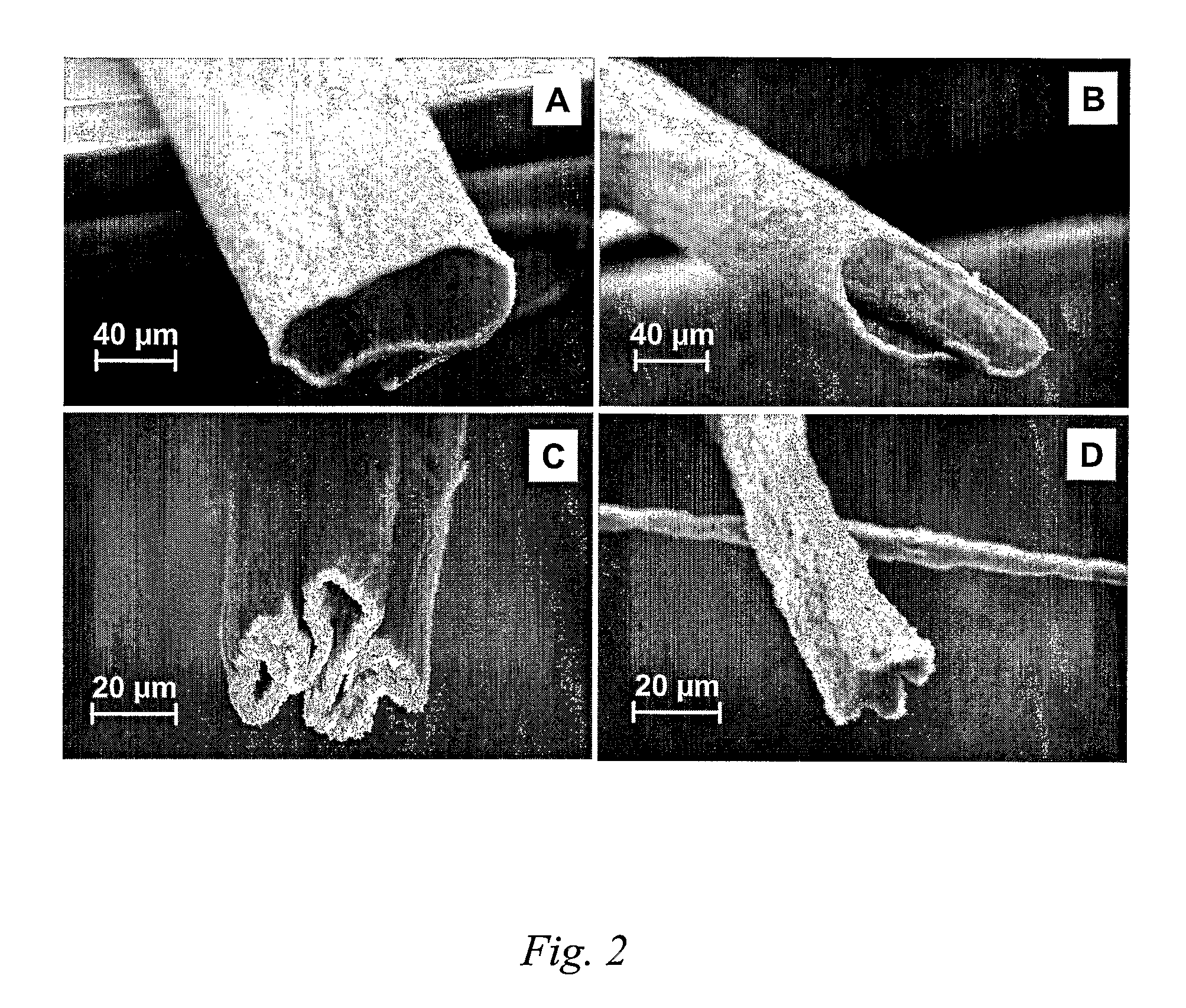
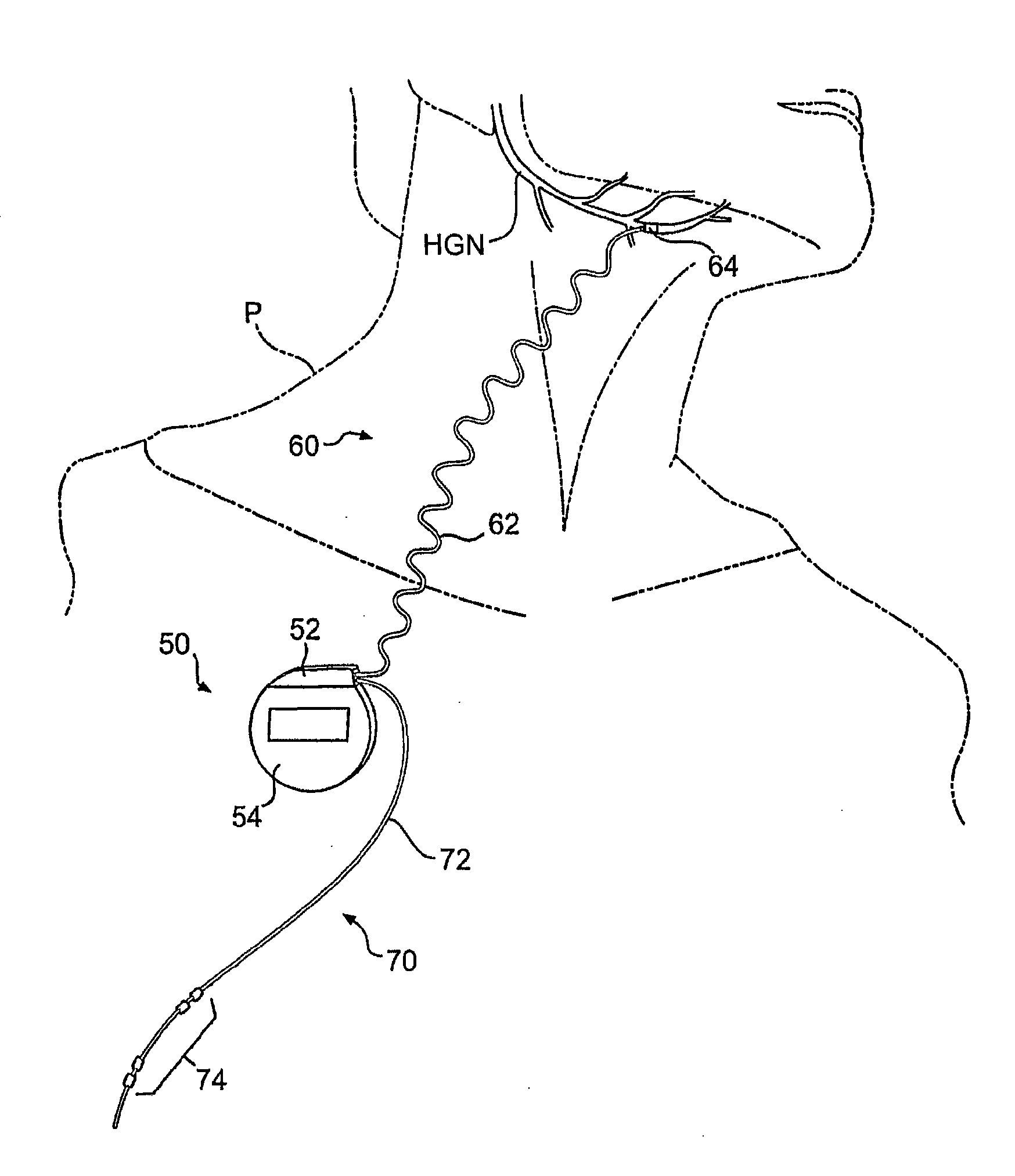
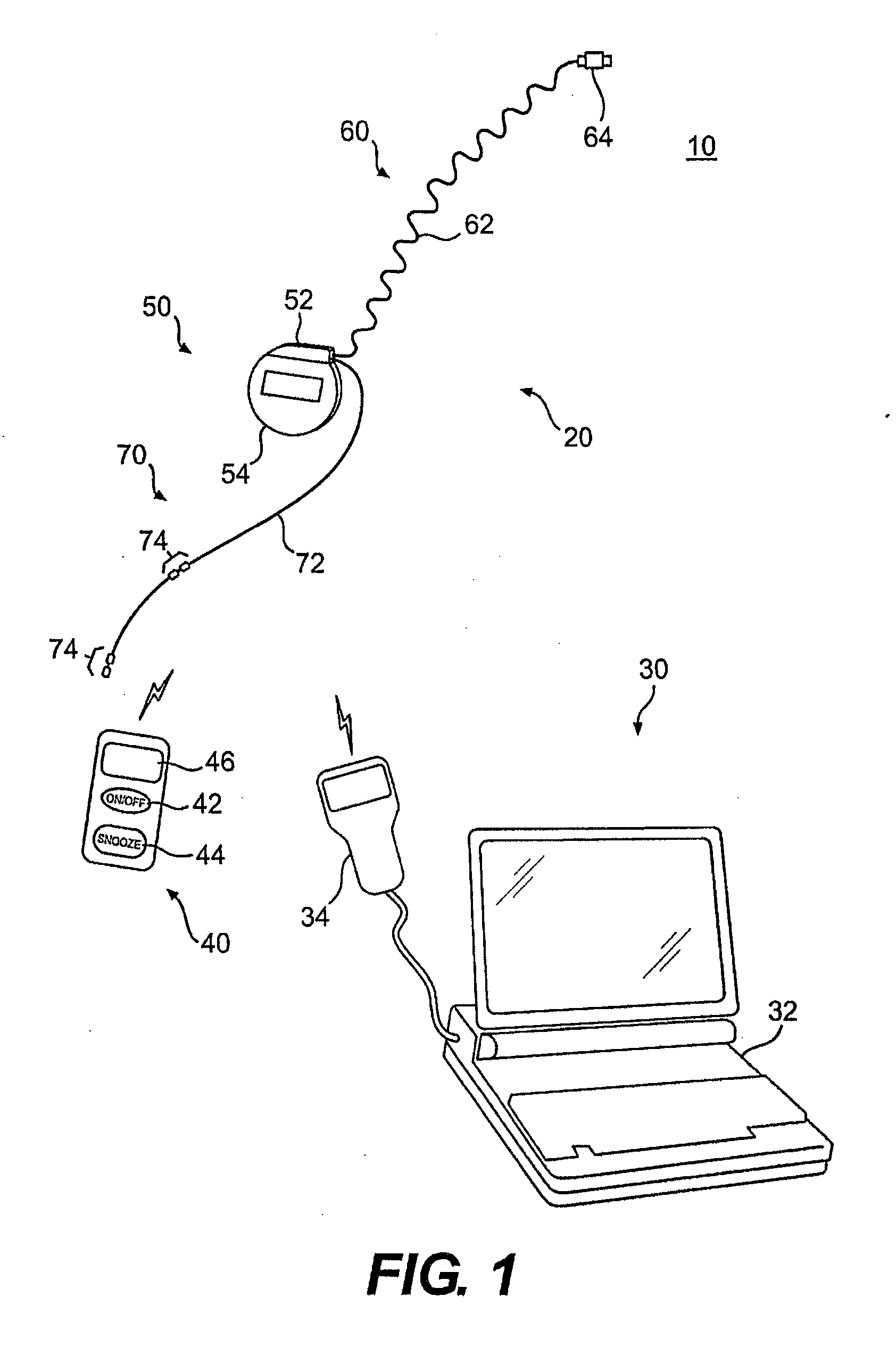
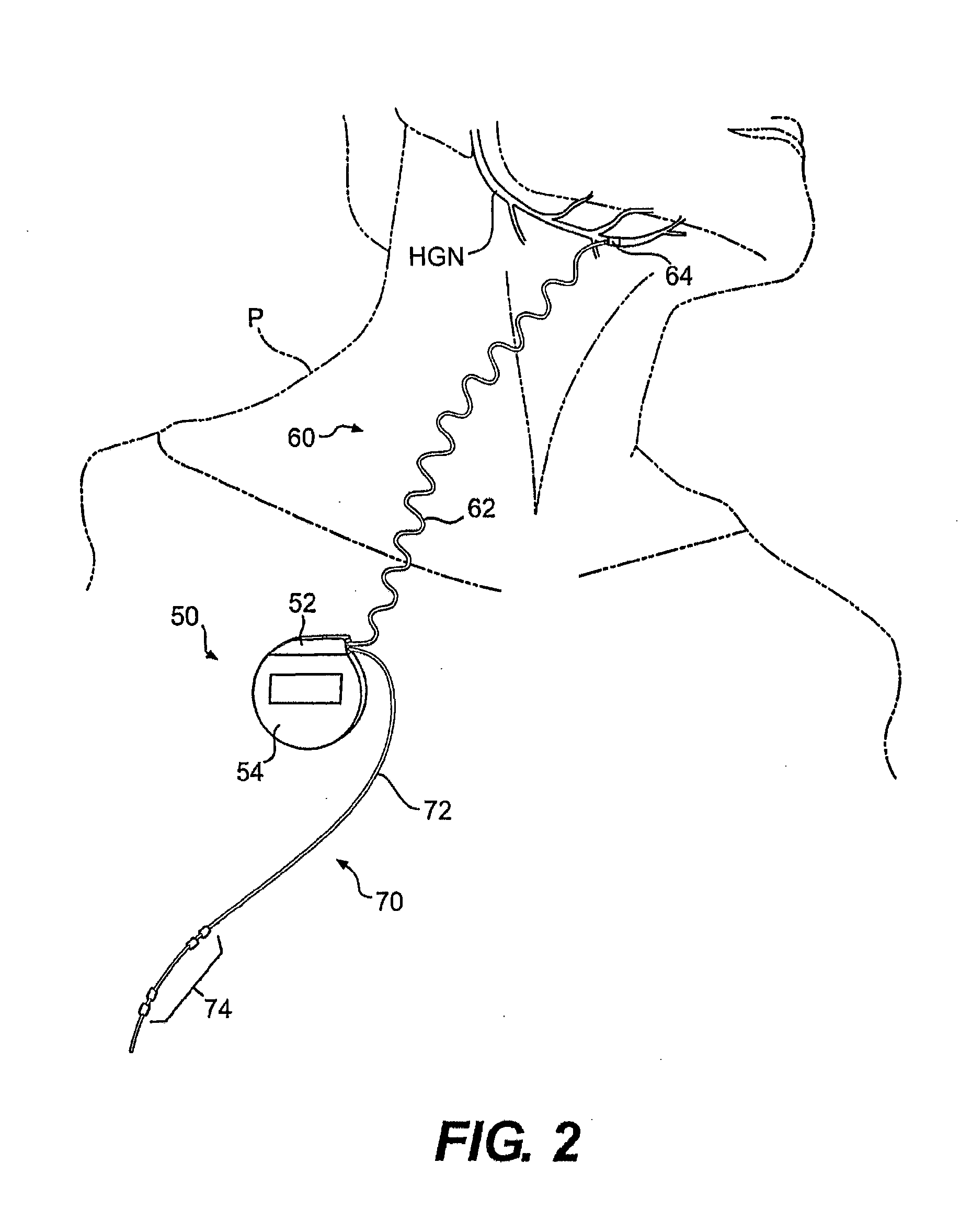
ActiveUS20140228905A1Enhanced couplingRetaining functionSpinal electrodesSurgeryHypoglossal nervePhases of clinical research
A method of treating a patient, comprising: sensing a biological parameter indicative of respiration; analyzing the biological parameter to identify a respiratory cycle; identifying an inspiratory phase of the respiratory cycle; and delivering stimulation to a hypoglossal nerve of the patient, wherein stimulation is delivered if a duration of the inspiratory phase of the respiratory cycle is greater than a predetermined portion of a duration of the entire respiratory cycle.
Owner:LIVANOVA USA INC
System and method of plating ball grid array and isolation features for electronic components
InactiveUS7463474B2Improved termination featureEliminate or greatly simplify thick-film stripesFixed capacitor dielectricStacked capacitorsEngineeringElectronic component
Owner:KYOCERA AVX COMPONENTS CORP
Polymeric Composites, Oilfield Elements Comprising Same, and Methods of Using Same in Oilfield Applications
InactiveUS20070142547A1Improve barrier propertiesImprove mechanical propertiesNon-metal conductorsLayered productsSubject matterEngineering
Oilfield elements and assemblies are described comprising a polymeric matrix formed into an oilfield element, and a plurality of expanded graphitic nanoflakes and / or nanoplatelets dispersed in the polymeric matrix. Methods of using the oilfield elements and assemblies including same in oilfield operations are also described. This abstract allows a searcher or other reader to quickly ascertain the subject matter of the disclosure. It will not be used to interpret or limit the scope or meaning of the claims. 37 CFR 1.72(b).
Owner:SCHLUMBERGER TECH CORP
Controlled release preparation
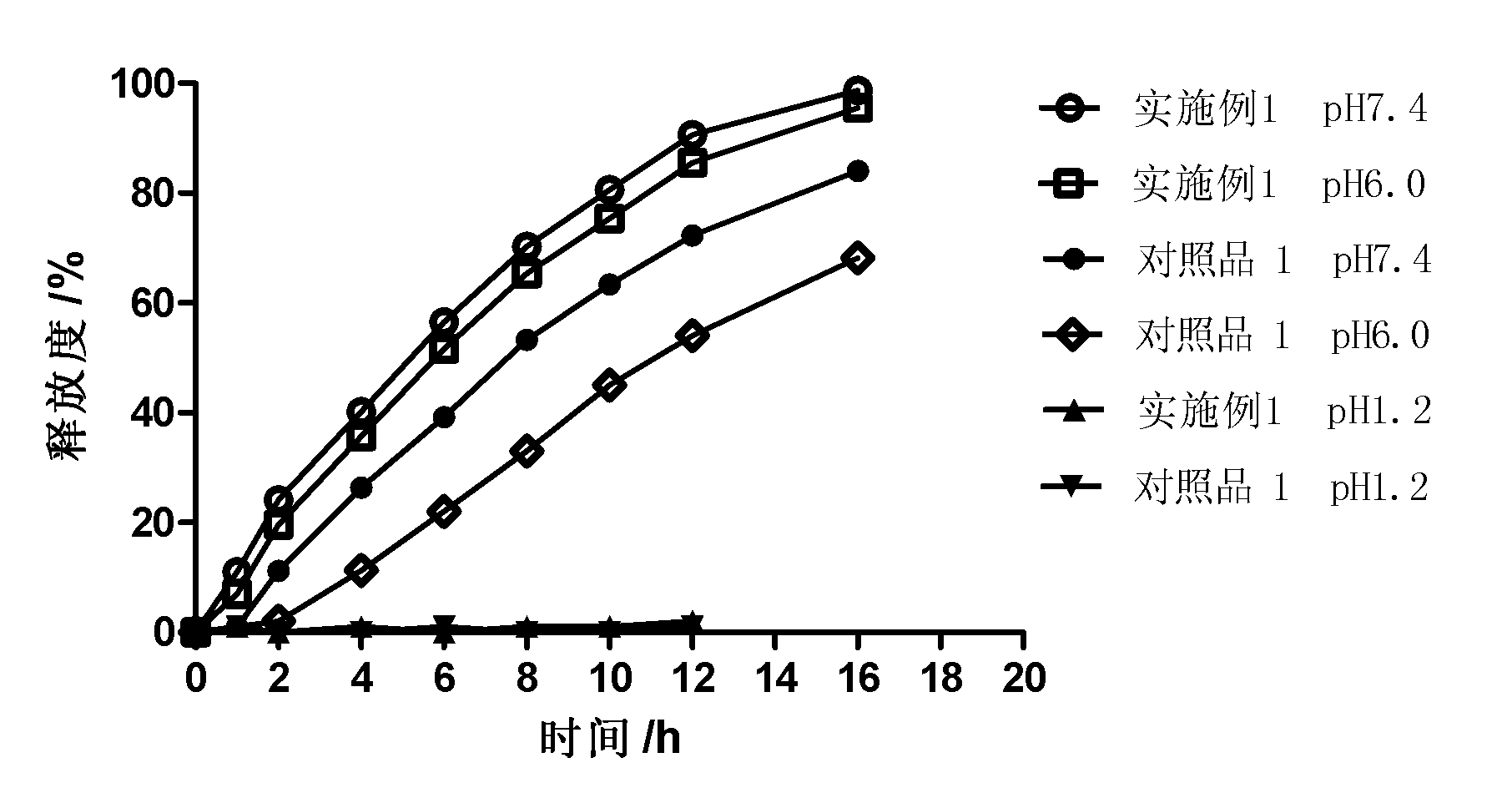
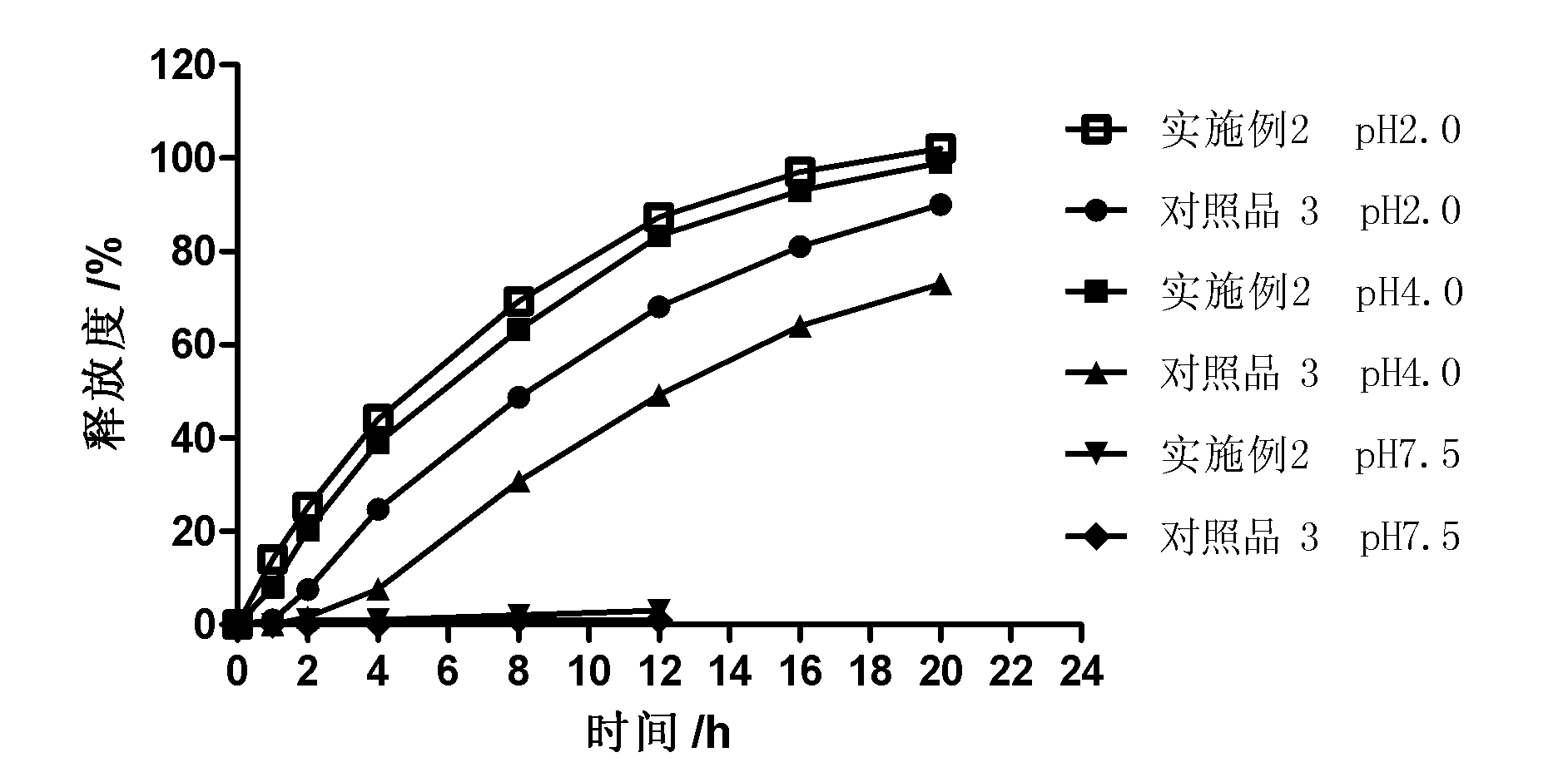
InactiveCN101987081AImprove stabilityRelease impact mitigationInorganic non-active ingredientsSuppositories deliveryParticulatesChemical reaction
The invention discloses a controlled release preparation with improved performance. The controlled release preparation comprises a core containing medicament and a controlled release film covering the outside of the core and being almost insoluble in water as well as stomach and intestines digestive juice. The controlled release film comprises particulate matters of a water soluble medicinal additive, the water-soluble medicinal additive is covered by a polymer film which can be soluble in the stomach and / or intestines digestive juice but almost insoluble in water, the polymer and the medicinal additive can not produce chemical reaction or can produce chemical reaction but do not produce water-insoluble non-gaseous products and the pharmaceutically unacceptable products, and the amount of the polymer is no more 700% of that of the medicinal additive. The invention also discloses a preparation method of the controlled release preparation. The controlled release preparation has the advantages of improved medicament release reproducibility, reduced medicament release lag time, accelerated medicament release and improved bioavailability, can realize located controlled release, delayed controlled release and interval type or pulse type controlled release of the medicament in the gastrointestinal tract, and the like.
Owner:钟术光
Electric machine
InactiveUS6373160B1Economically manufacturedEconomically usedSpeed controllerSynchronous machine detailsElectric machineEngineering
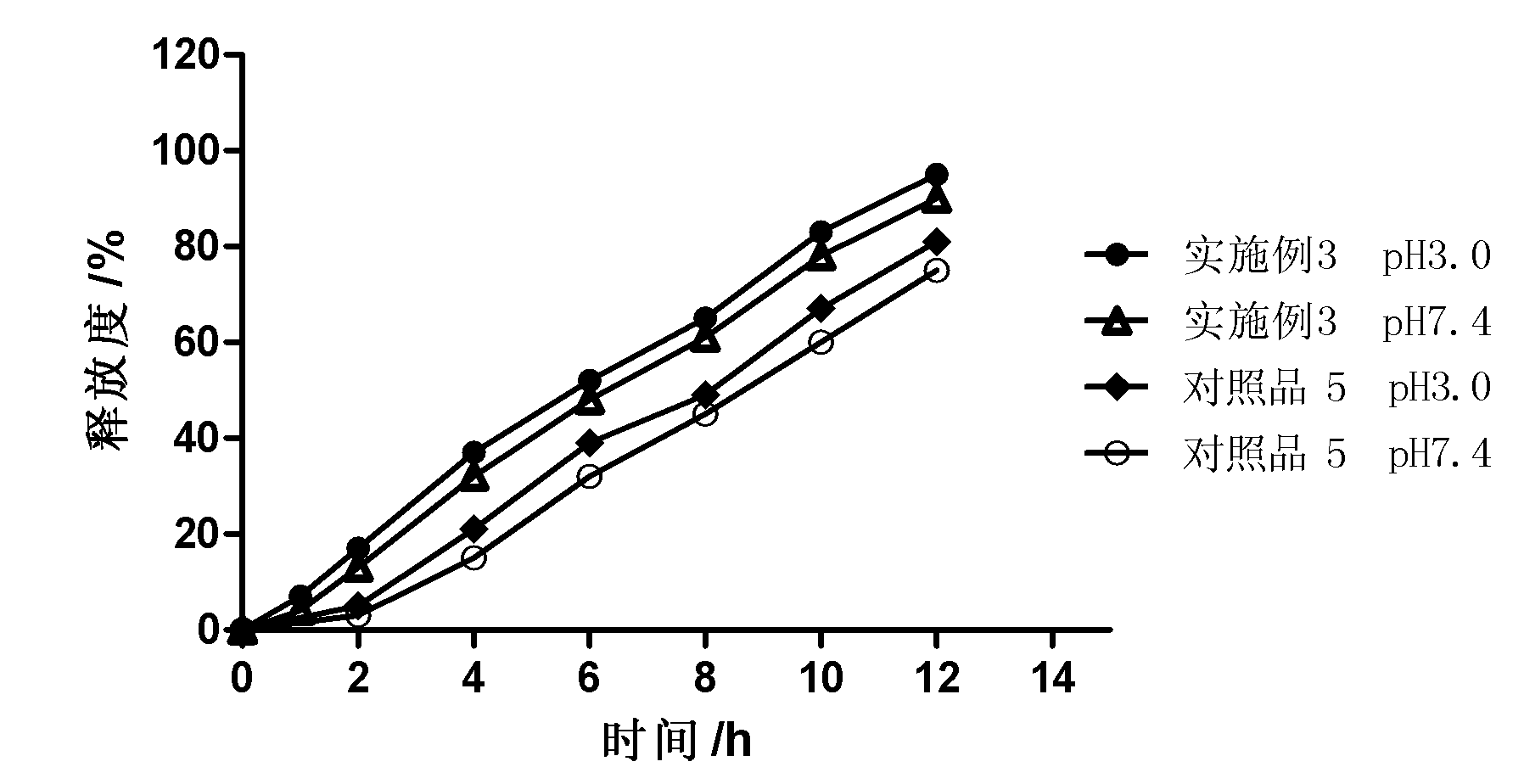
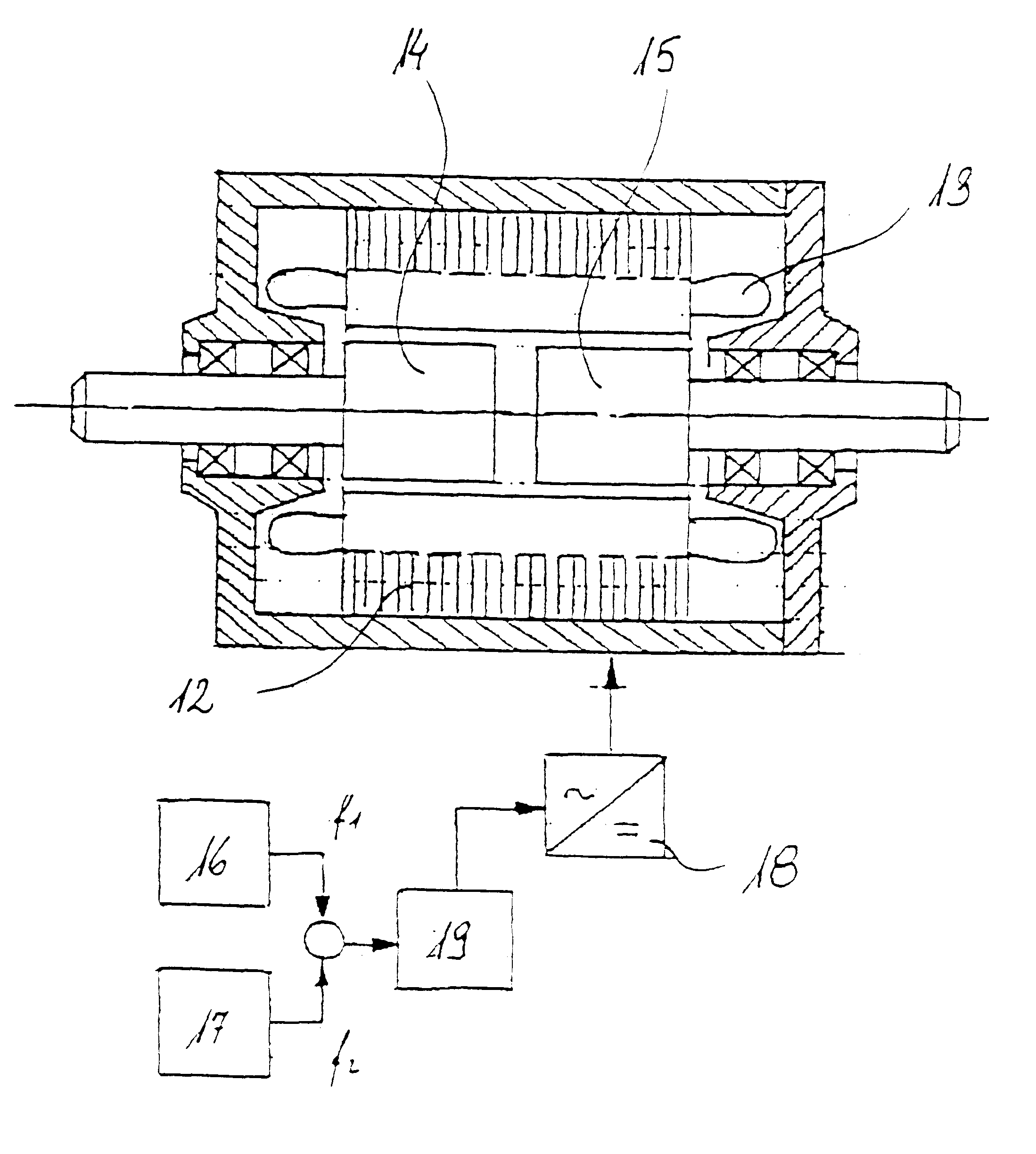
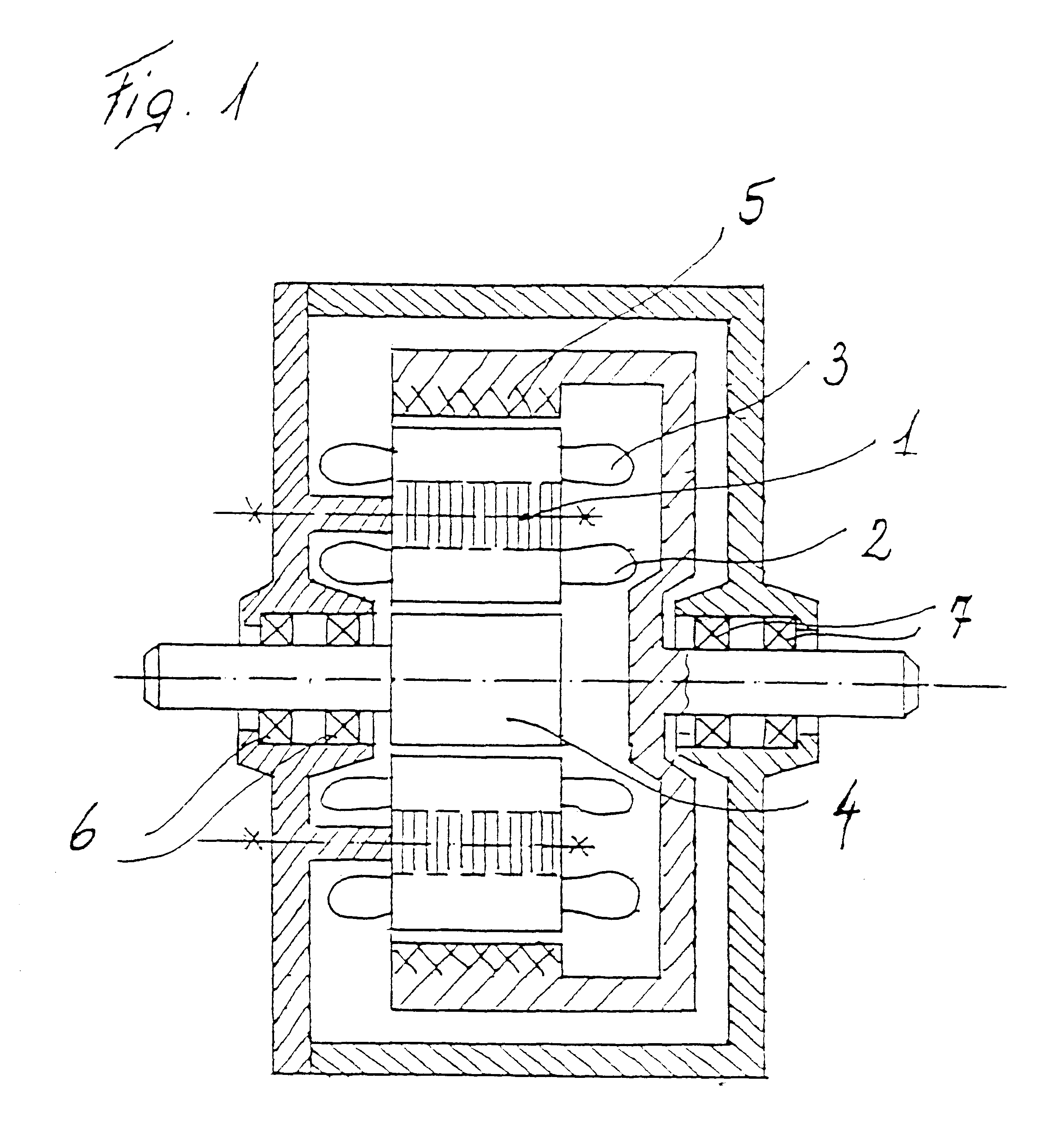
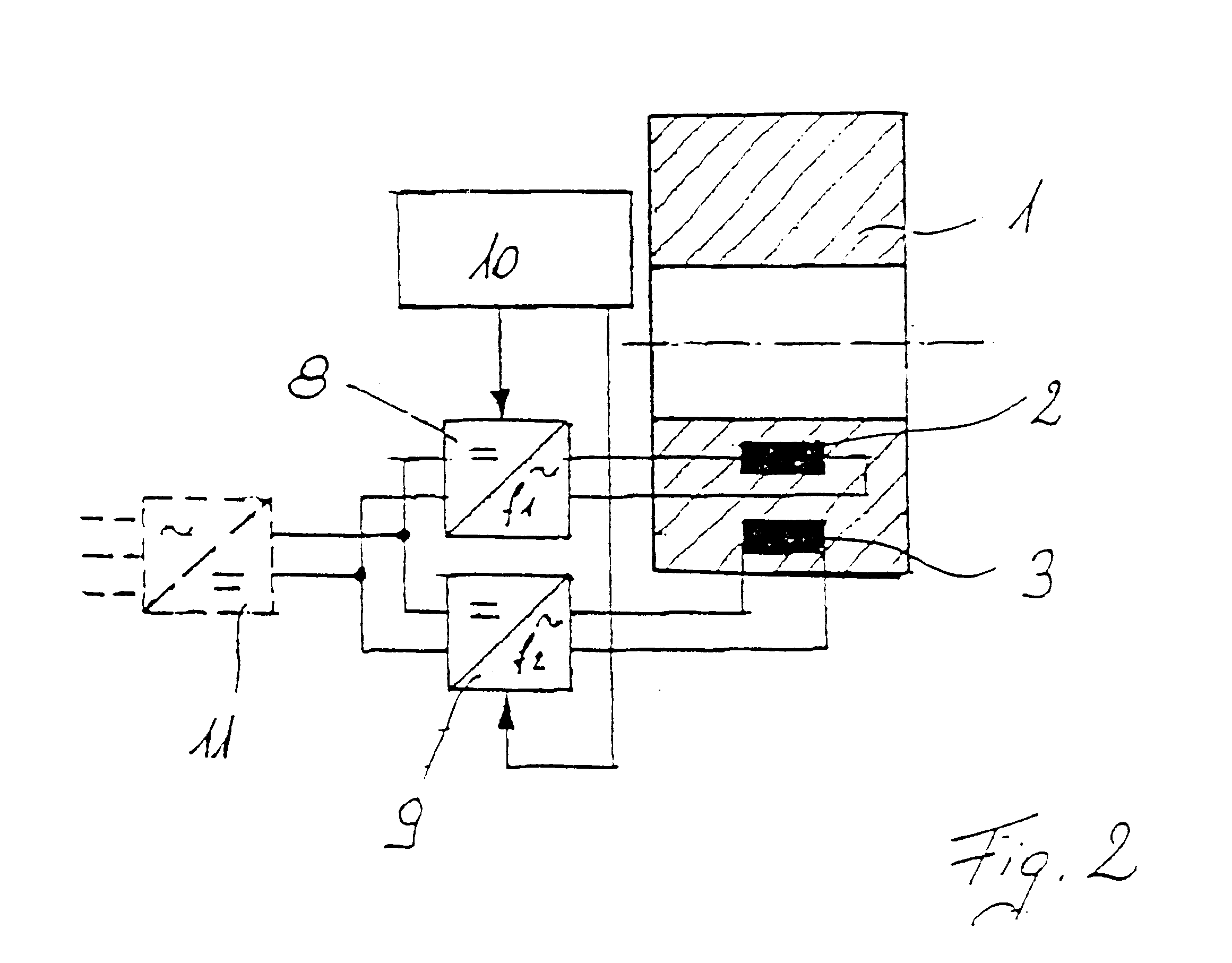
Electric rotary current machine that includes a casing and a stator fitted within the casing. The stator has at least one stator winding. At least two mechanically separate rotors are rotatably mountable within the casing and have a same axis of rotation. In this way, each rotor has electromagnetic interaction with the stator when the stator is electromagnetically active. The rotor speeds are the same or different. A motor control is arranged to control a supply to at least one of said at least one stator winding by superposition of at least two rotary field components, one for each rotor.
Owner:SCHRODL MANFRED
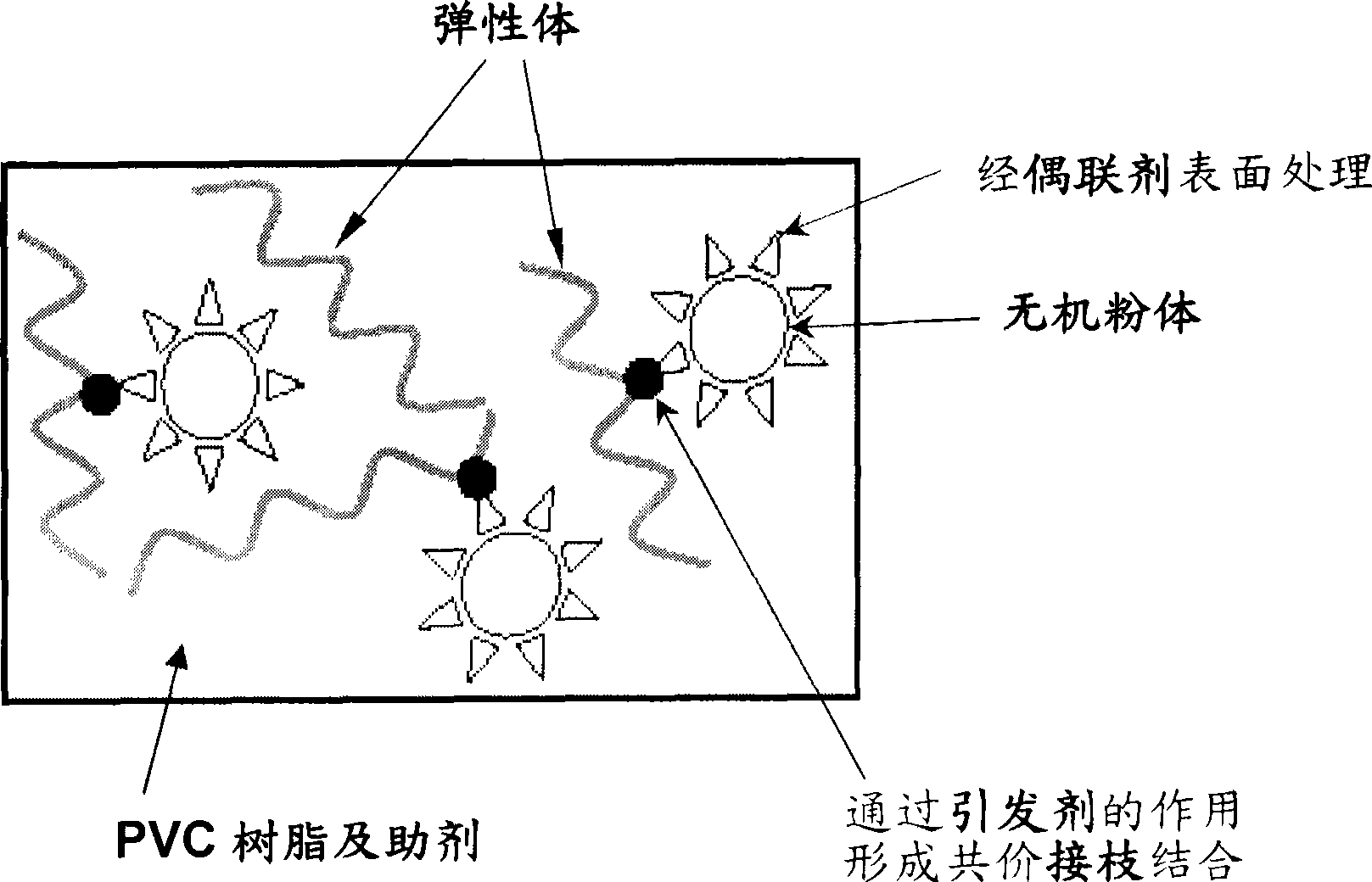
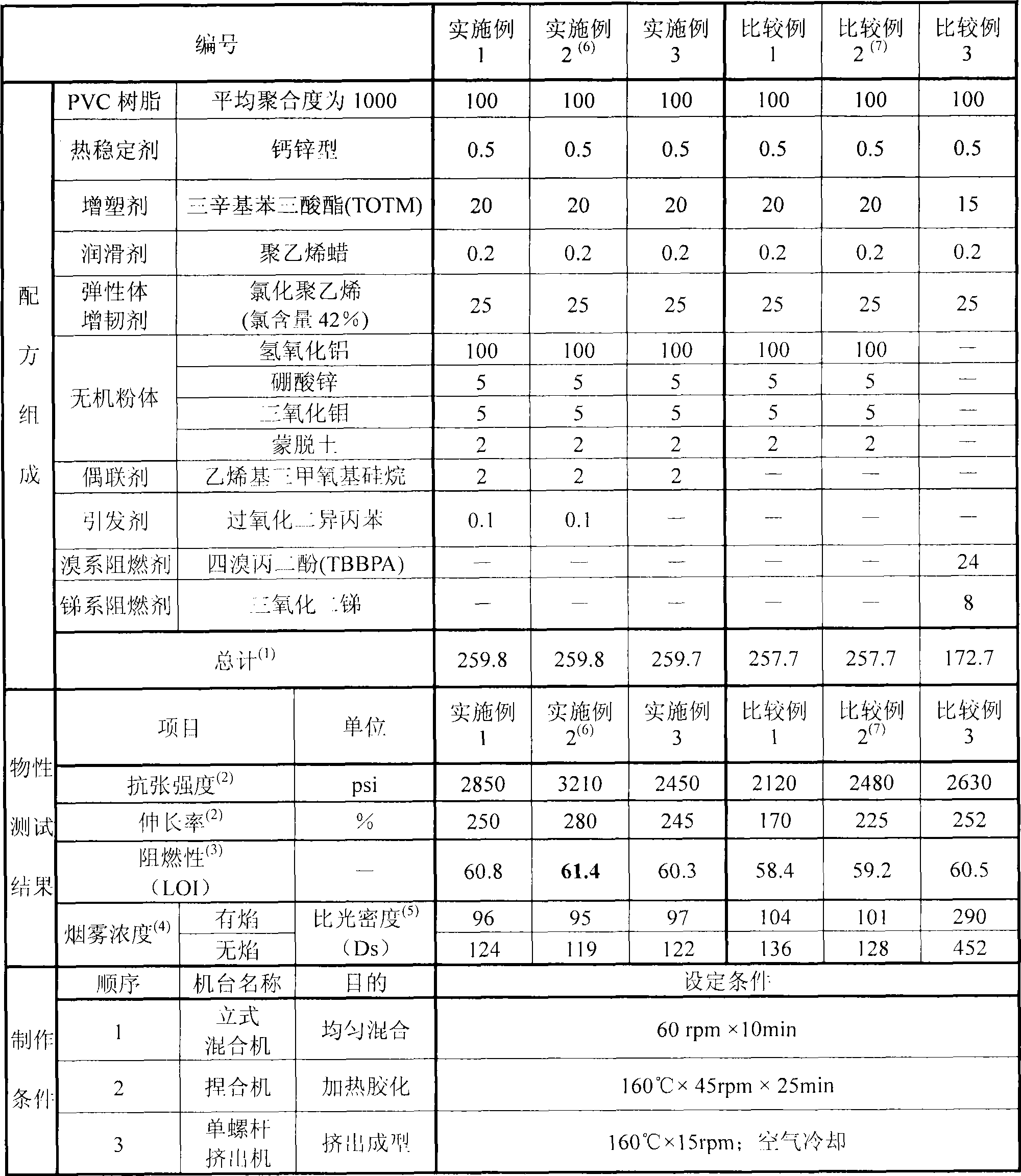
PVC resin composition and product thereof
InactiveCN101497727AImprove mechanical propertiesOvercoming the problem of a large drop in mechanical propertiesSmoke
The invention relates to a PVC resin compound and a product thereof, in particular to a PVC resin compound with characteristics of high inflaming retarding and low smoke formation. The PVC resin compound is prepared by mixing PVC resin, inorganic powder, accessory ingredient, toughener, coupling agent and evocating agent according to a certain proportion, wherein the evocating agent is used together with the coupling agent so as to enhance the associative property of the inorganic powder inside the PVC resin compound. The PVC product made by the PVC resin compound has favorable tensile strength and tensile stretch, and has the characteristics of high inflaming retarding and low smoke formation.
Owner:NANYA PLASTICS CORP
Method of Forming a Polymer Component
ActiveUS20110153025A1Reduce oxidationPrevent oxidationMedical devicesPretreated surfacesCross-linkArticular surfaces
This invention relates to a method of forming a polymer component and comprises blending polymer particles with antioxidant to form a mixture in which the antioxidant coats the polymer particles, irradiating the mixture to cross-link the polymer particles therein and forming the irradiated mixture into a consolidated component. The invention also relates to a method of forming an articular surface for a prosthesis and a prosthesis having a polymer articular bearing surface wherein at least one pre-determined portion of the bearing surface is provided with cross-linked polymer bonds.
Owner:JOINTMEDICA LTD
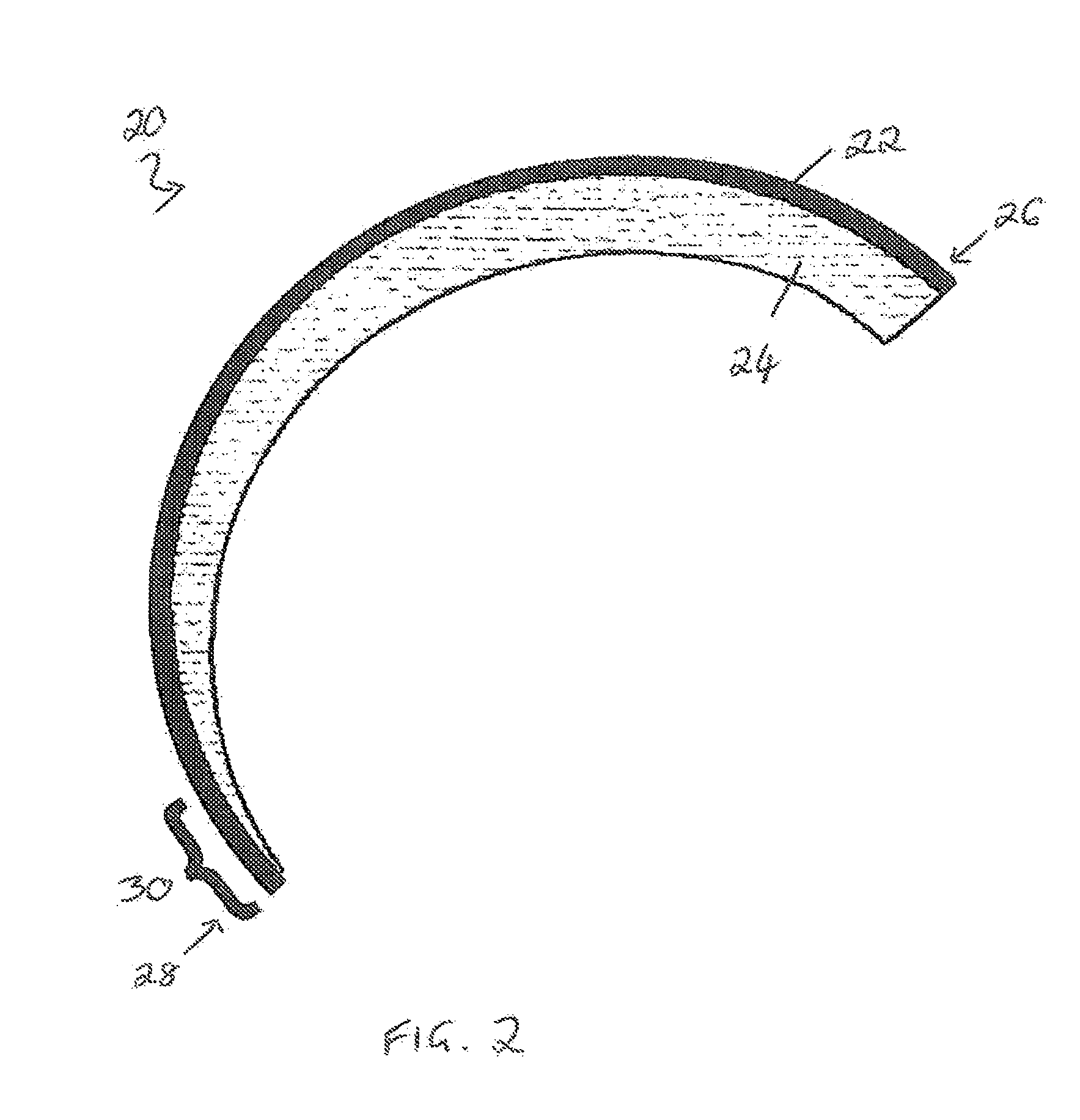
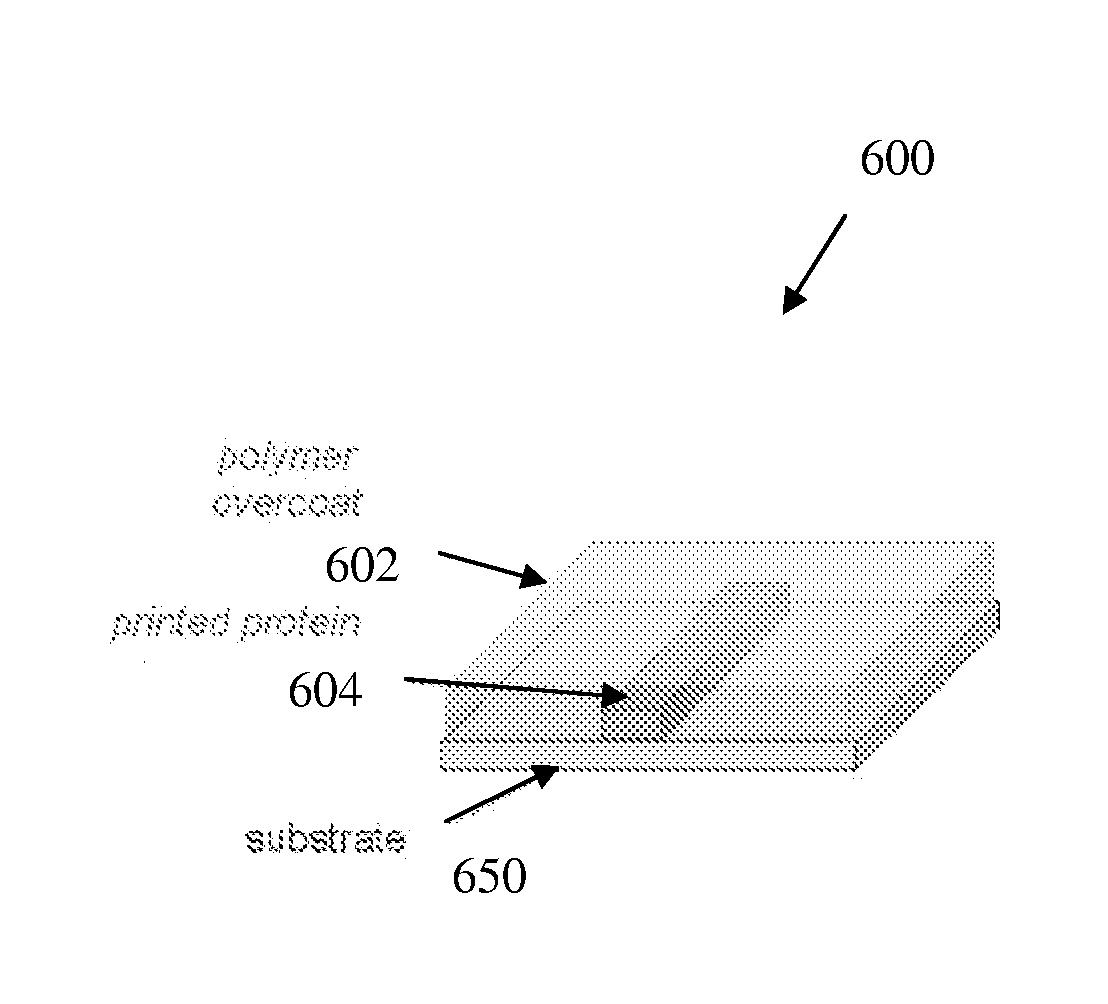
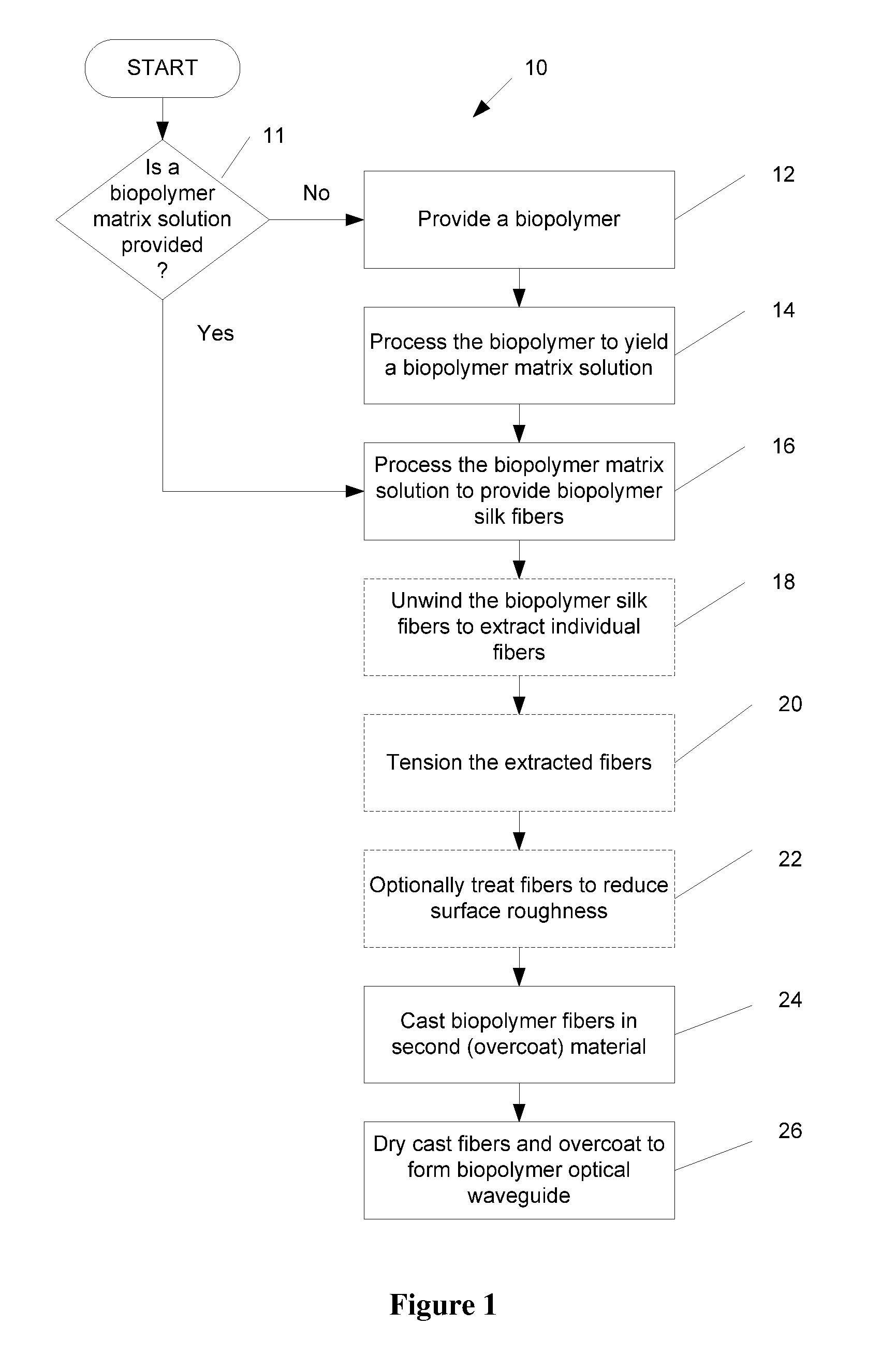
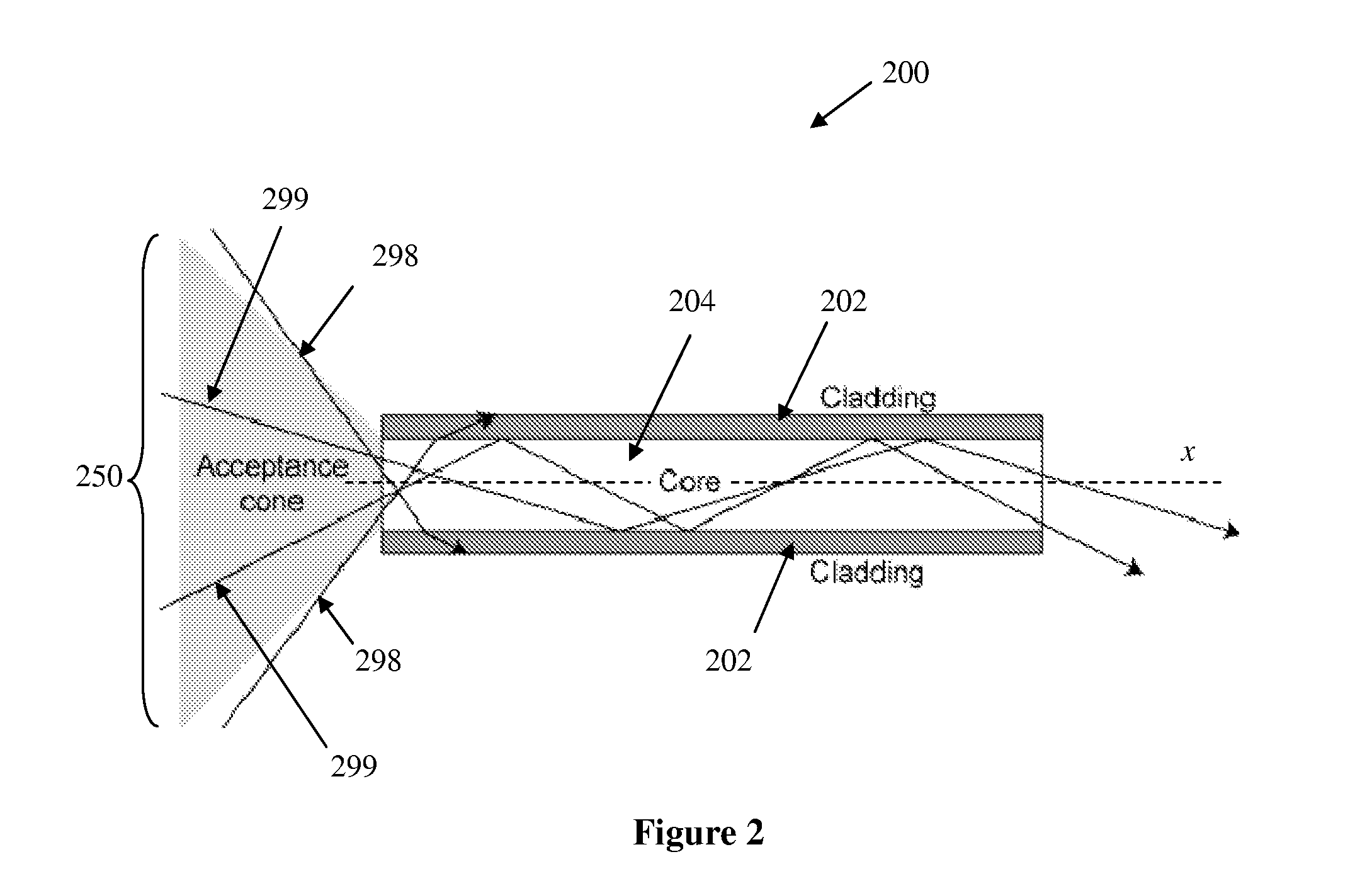
Biopolymer optical waveguide and method of manufacturing the same
ActiveUS20100063404A1Minimize negative impactImprove functional propertiesBiological material analysisMonocomponent fibroin artificial filamentFiberBiopolymer
A method of manufacturing a biopolymer optical waveguide includes providing a biopolymer, unwinding the biopolymer progressively to extract individual biopolymer fibers, and putting the unwound fibers under tension. The tensioned fibers are then cast in a different polymer to form a biopolymer optical waveguide that guides light due to the difference in indices of refraction between the biopolymer and the different polymer. The optical fibers may be used in biomedical applications and can be inserted in the body as transmissive media. Printing techniques may be used to manufacture the biopolymer optical waveguides.
Owner:TRUSTEES OF TUFTS COLLEGE
Wood-plastic composite material and prepration method thereof
The invention discloses a wood-plastic composite material and a preparation method thereof, and the wood-plastic composite material comprises the following raw materials according to the mixing ratio by parts by weight: 30-80 parts of modified fiber powder; 15-80 parts of plastics; 2-10 parts of phase solvent; 2-10 parts of lubricant; 0-10 parts of stabilizer; 0.2-1.0 part of antioxidant; 5-15 parts of filler; and 2-20 parts of flame retardant. Lignin is utilized for modifying fiber powder, then composition with the plastics is carried out, and a finished-product material is formed by extrusion. The preparation method can solve the problems of the compatibility of wood fibers with the thermoplastic plastics, the surface treatment technology of the raw materials by utilizing the lignin and the like, realize the comprehensive utilization of the lignin and the waste plastics, be capable of replacing wood, increase the additional value of the lignin and solve the utilization problem of the lignin wastes. The manufactured wood-plastic composite material can significantly improve the mechanical performance, the tensile strength, the flexural strength and the impact resistance, and realize the industrial production of high-performance products, such as construction materials.
Owner:BEIJING FORESTRY UNIVERSITY
Laminated and ion-exchanged strengthened glass laminates
ActiveUS20140141217A1Increase compressive stressHigher depthGlass forming apparatusGlass/slag layered productsIonIon exchange
A method of making a glass sheet (10) comprises laminating a high CTE core glass (11) to a low CTE clad glass (12) at high temperatures and allowing the laminate (10) to cool creating compressive stress in the clad glass (12), and then ion exchanging the laminate (10) to increase the compressive stress in the outer near surface regions of the clad glass (12). The core glass (11) may include ions that exchange with ion in the clad glass (12) to increase the compressive stress in inner surface regions of the clad glass (12) adjacent to the clad glass / core glass interfaces. The glass laminate (10) may be formed and laminated using a fusion forming and laminating process and fusion formable and ion exchangeable glass compositions.
Owner:CORNING INC
Method of making a product with improved material properties by moderate heat-treatment of a metal incorporating a dilute additive
InactiveUS6150186AStable mechanical propertiesImprove conductivitySemiconductor/solid-state device testing/measurementFinal product manufactureUltimate tensile strengthMechanical property
Deposition of metal in a preferred shape, including coatings on parts, or stand-alone materials, and subsequent heat treatment to provide improved mechanical properties. In particular, the method gives products with relatively high yield strength. The products often have relatively high elastic modulus, and are thermally stable, maintaining the high yield strength at temperatures considerably above 25 DEG C. This technique involves depositing a material in the presence of a selected additive, and then subjecting the deposited material to a moderate heat treatment. This moderate heat treatment differs from other commonly employed "stress relief" heat treatments in using lower temperatures and / or shorter times, preferably just enough to reorganize the material to the new, desired form. Coating a shape and heat treating provides a shaped deposit with improved material properties. Coating a shape with a portion connected to a base and a portion detached therefrom can provide a resilient, conductive contact useful for electronic applications.
Owner:FORMFACTOR INC
Composite prosthetic bearing having a crosslinked articulating surface and method for making the same
InactiveUS7819925B2Promote oxidationEasy to wearBone implantSynthetic resin layered productsProsthesisCrosslinked polymers
An implantable prosthetic bearing is constructed of a composite material having a first layer and second layer. The first layer has an articulating surface defined therein, whereas the second layer has a engaging surface defined therein for engaging either another prosthetic component or the bone itself The first layer of the implantable prosthetic bearing is constructed of crosslinked polymer such as Ultra-High Molecular Weight Polyethylene, whereas the second layer of the implantable prosthetic bearing is constructed of polymer such as Ultra-High Molecular Weight Polyethylene that is either non-crosslinked or crosslinked to a lesser degree than the first layer. In such a manner, the first layer possesses mechanical properties which are advantageous in regard to the articulating surface (e.g., enhanced wear and oxidation resistance), whereas the second layer possesses mechanical properties which are advantageous in regard to the engaging surface (e.g., high ductility, toughness, and creep resistance). A method of making a prosthetic bearing is also disclosed.
Owner:DEPUY SYNTHES PROD INC
Production of agglomerates from gas phase
ActiveUS20050006801A1Avoid enteringImprove mechanical propertiesMaterial nanotechnologyMulti-walled nanotubesGas phaseReaction zone
A process for production of an agglomerate comprises the steps of: passing a flow of one or more gaseous reactants into a reactor; reacting the one or more gaseous reactants within a reaction zone of the reactor to form product particles; agglomerating the product particles into an agglomerate; and applying a force to the agglomerate to displace it continuously away from the reaction zone.
Owner:CAMBRIDGE ENTERPRISE LTD
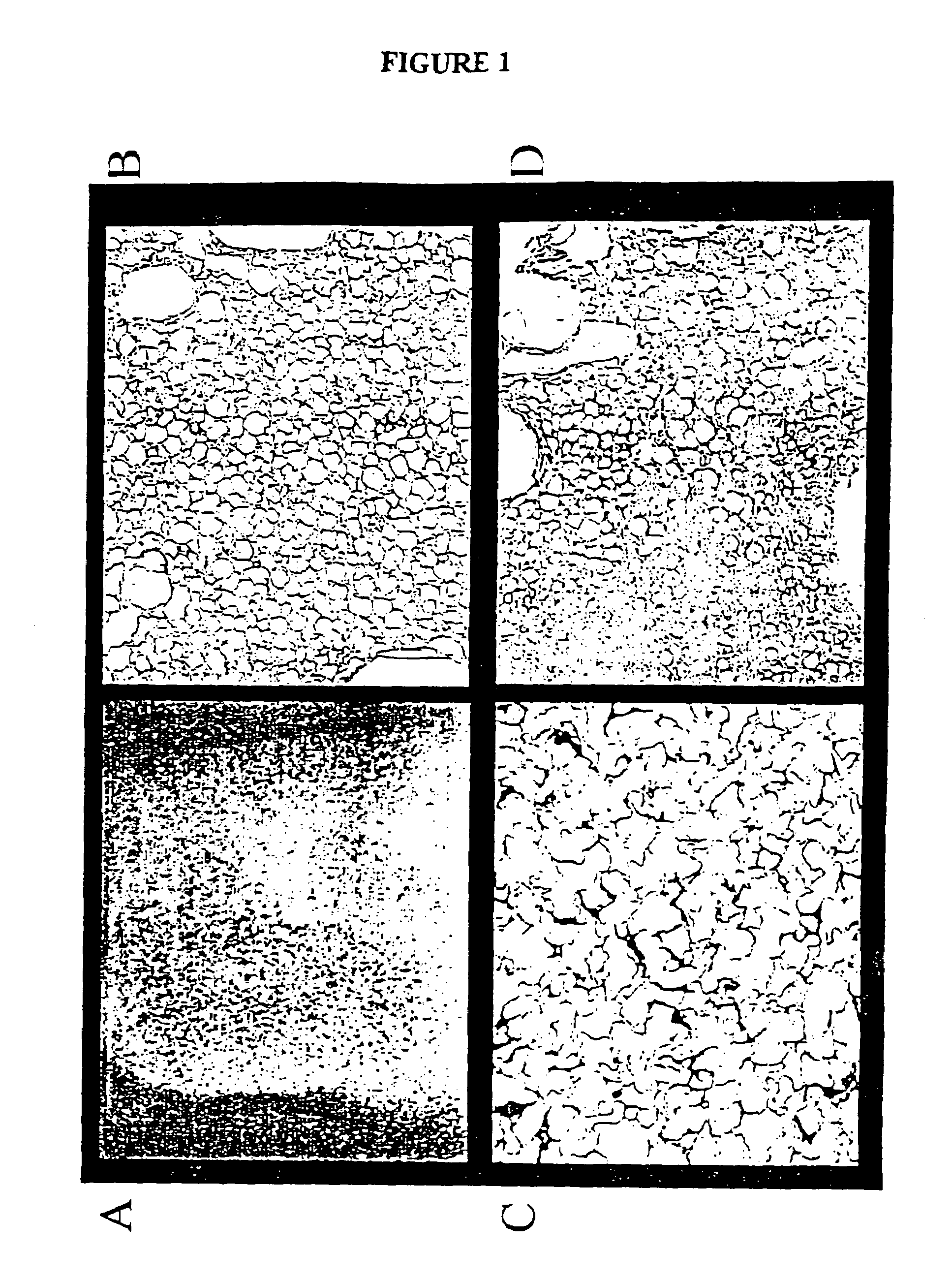
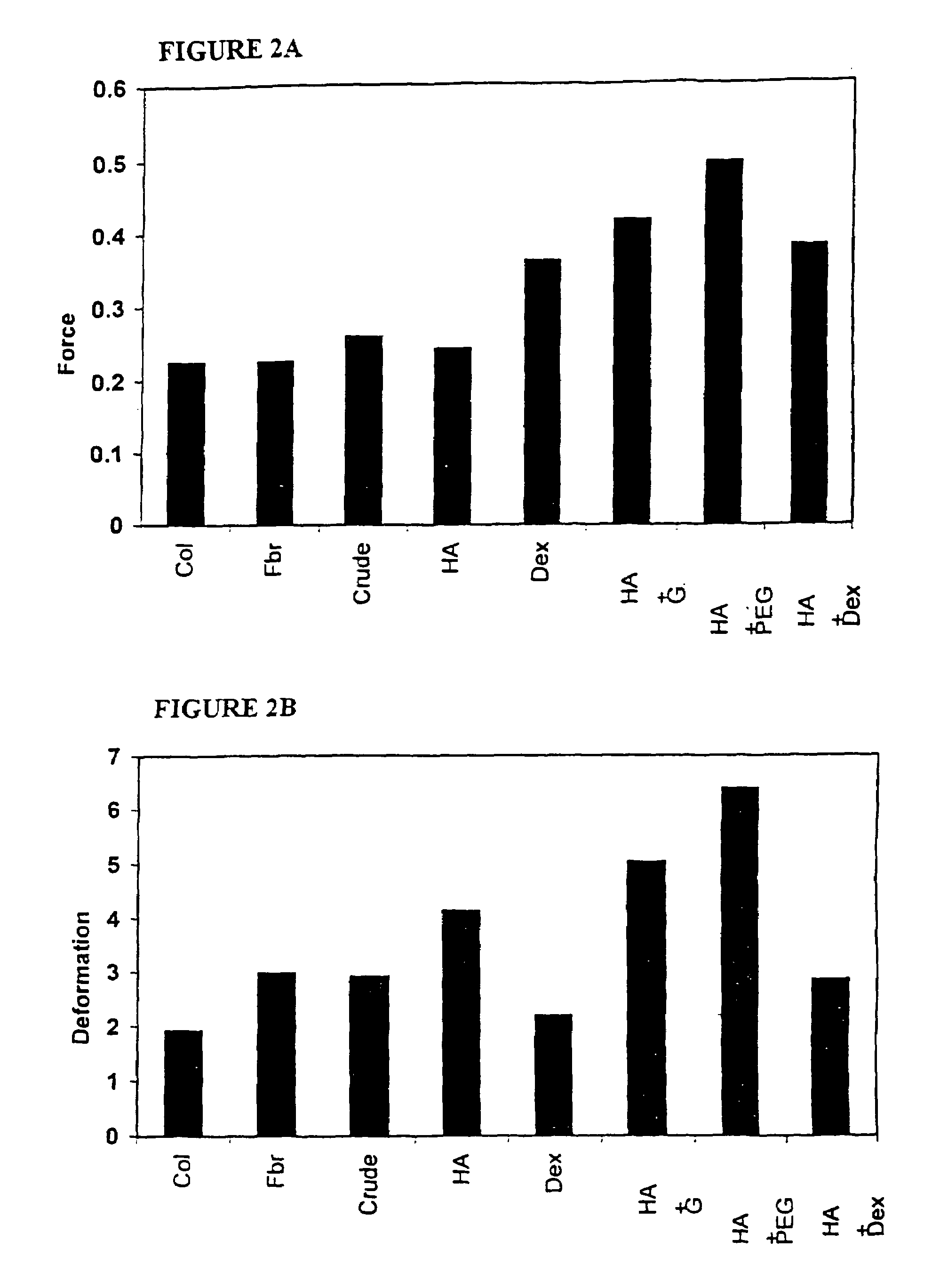
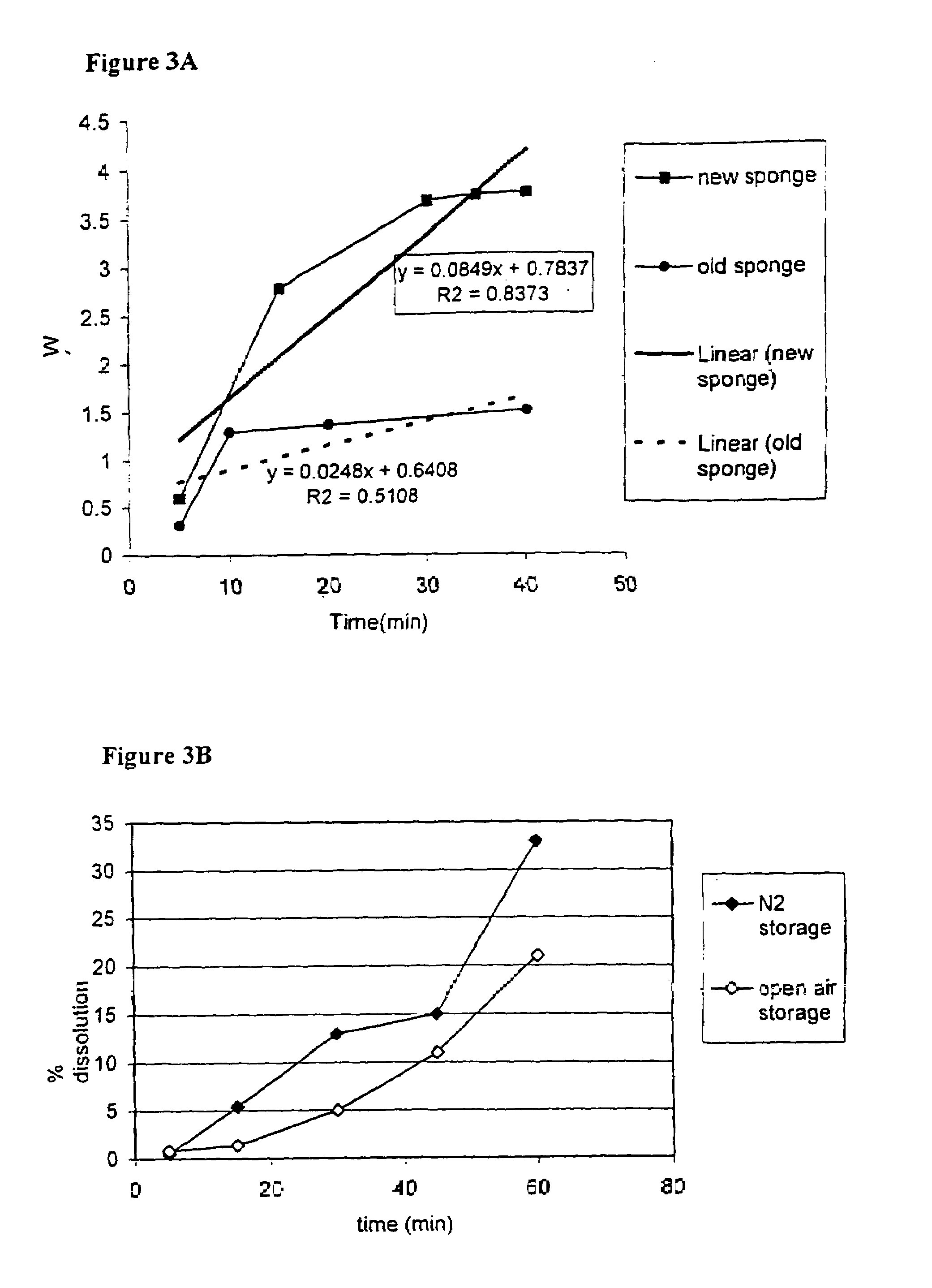
Thiol-modified hyaluronan
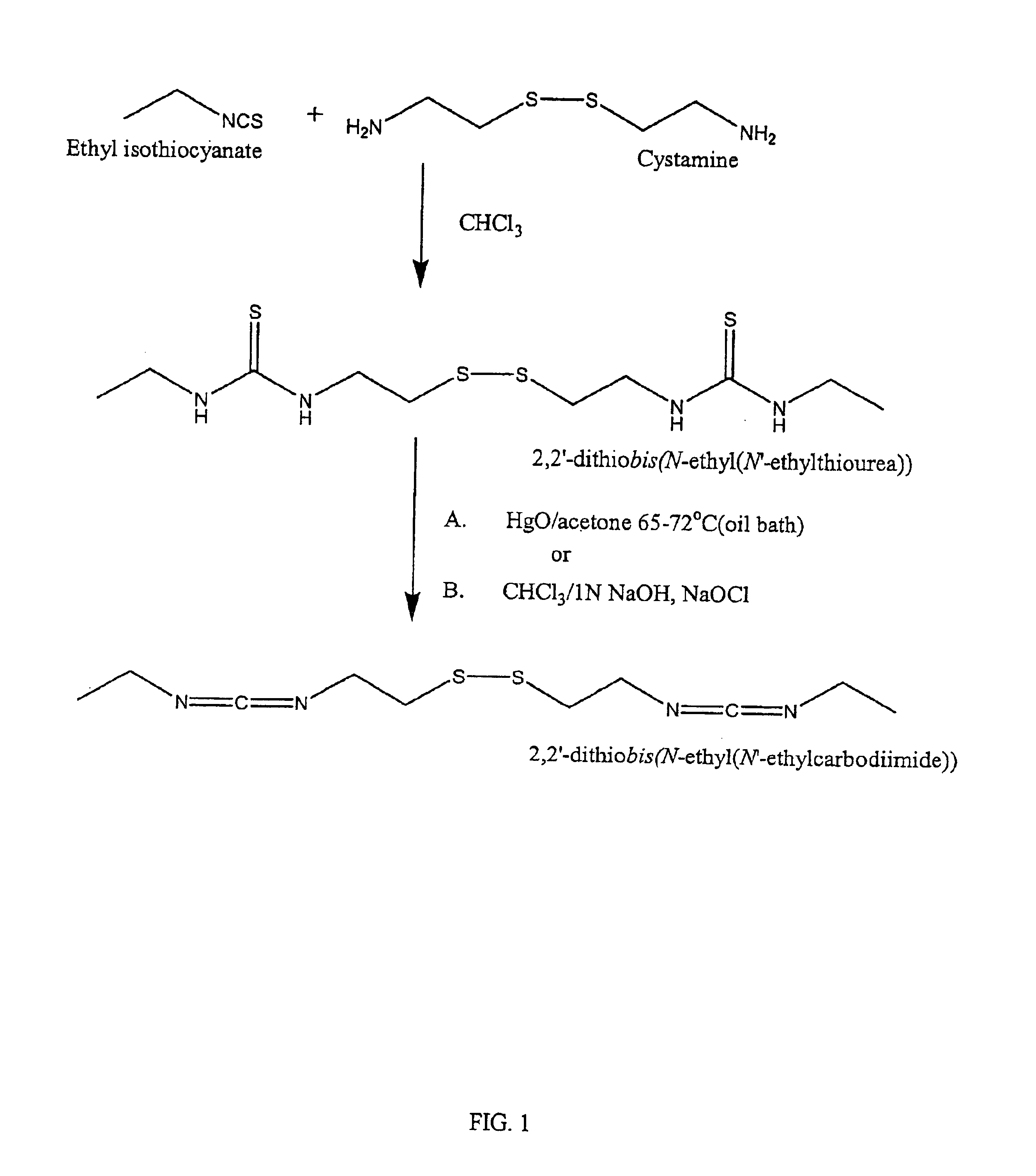
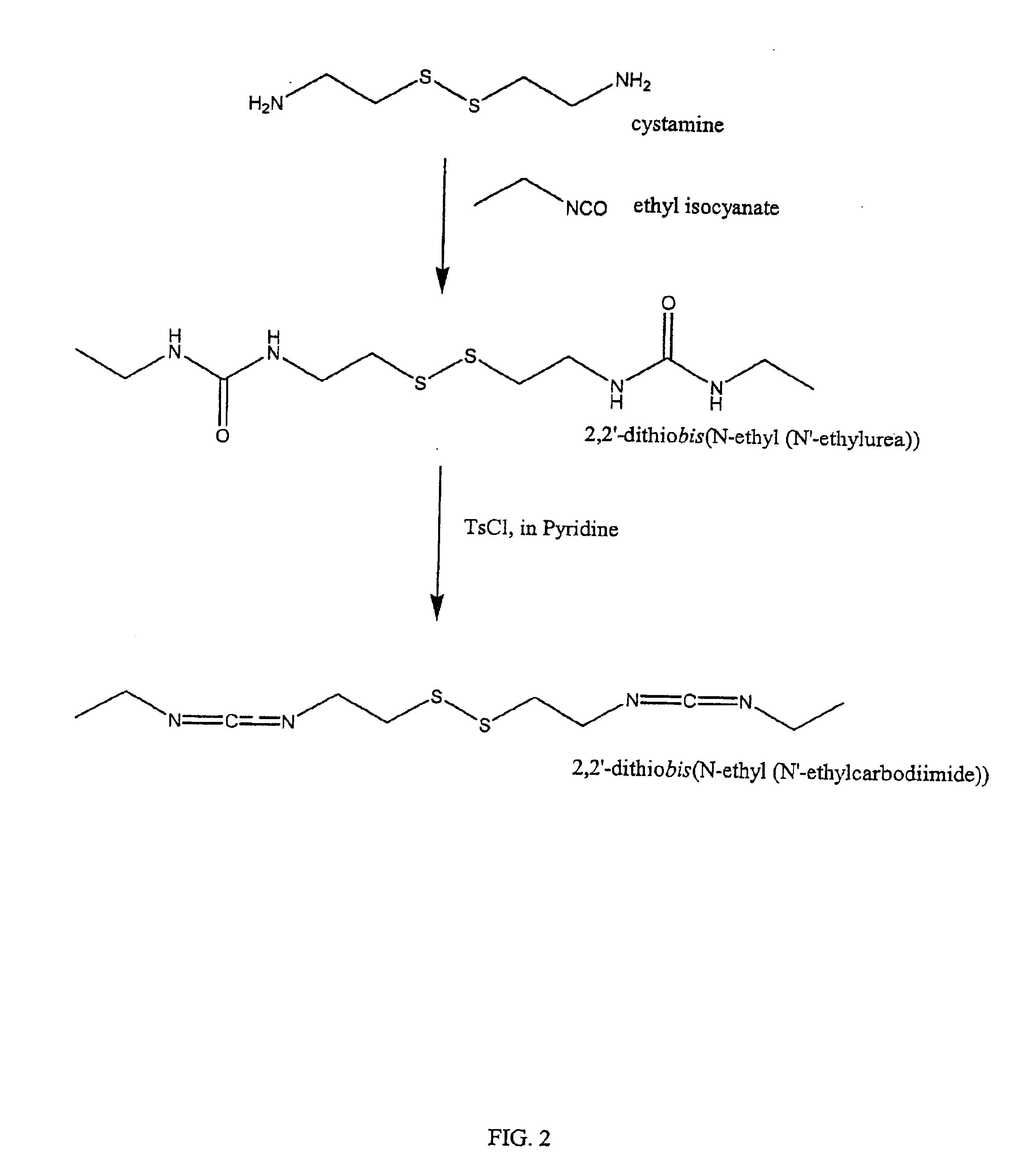
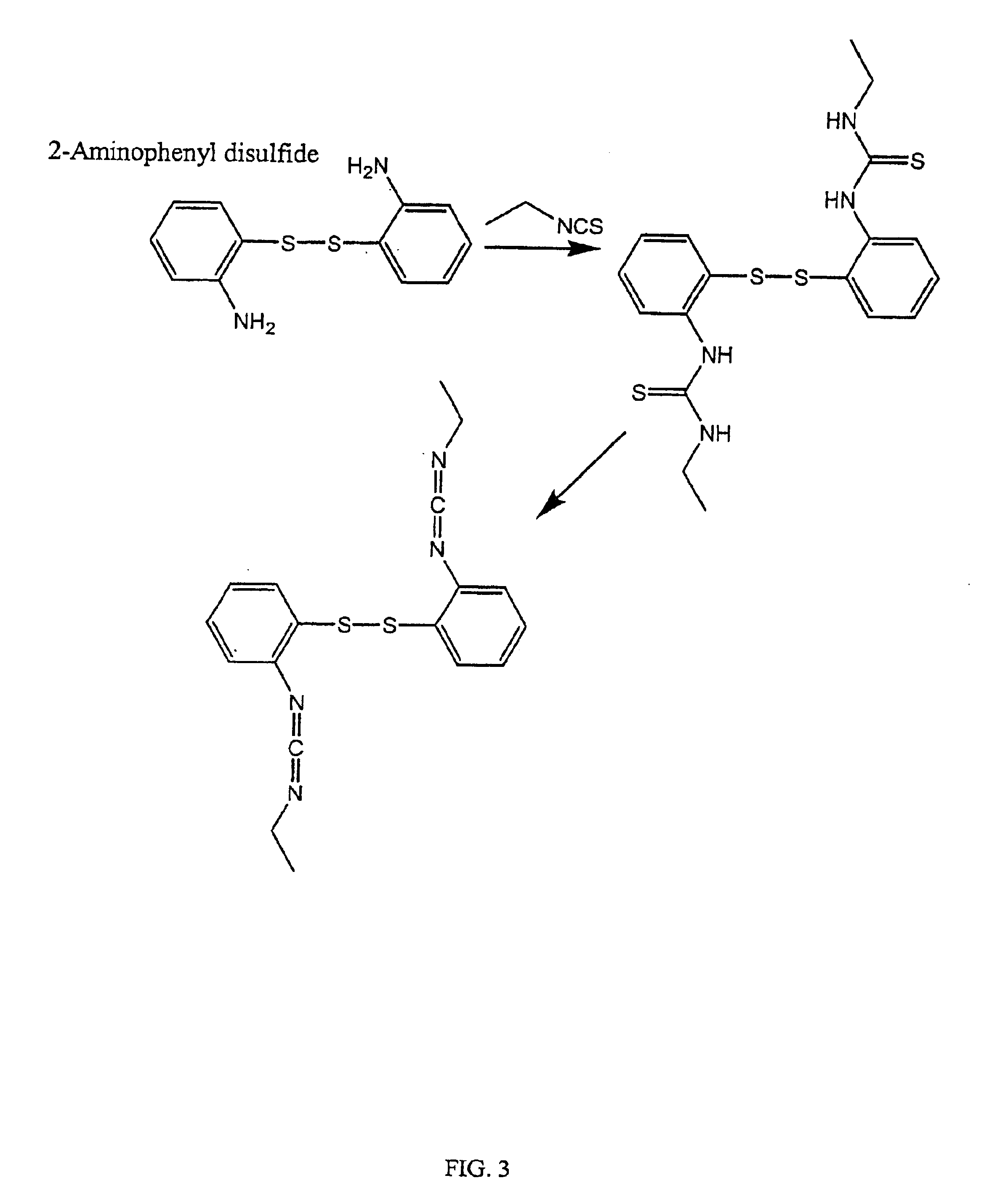
InactiveUS6884788B2High activityControl moreBiocideOrganic active ingredientsUrea derivativesCross-link
The present invention relates to biscarbodiimides, thiourea derivatives, urea derivatives, and cross-linked hyaluronan derivatives having at least one intramolecular disulfide bond, and methods of preparation thereof. The invention also includes thiolated hyaluronan derivatives and salts thereof having at least one pendant thiol group or a modified pendant thiol group, and methods of preparation thereof. An example of a modified pendant thiol group is a sulfhydryl group linked to a small molecule such as a bioactive agent, for example a drug or pharmaceutically active moiety. A hyaluronan derivative having a sulfhydryl group linked to a pharmaceutically active moiety is useful as a sustained or controlled release drug delivery vehicle. Compositions containing the hyaluronan derivatives of the invention can reversibly viscosify in vivo or in vitro, in response to mild changes in condition, and are thus useful in ophthalmic surgery and in tissue engineering.
Owner:ANIKA THERAPEUTICS INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com