Controlled release preparation
A technology of controlled-release preparations and plasticizers, which is applied in the preparation of the controlled-release preparations and the field of zero-order release controlled-release preparations, which can solve the problems of unstable pore size, poor stability or reproducibility of drug release, and blurred interfaces And other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
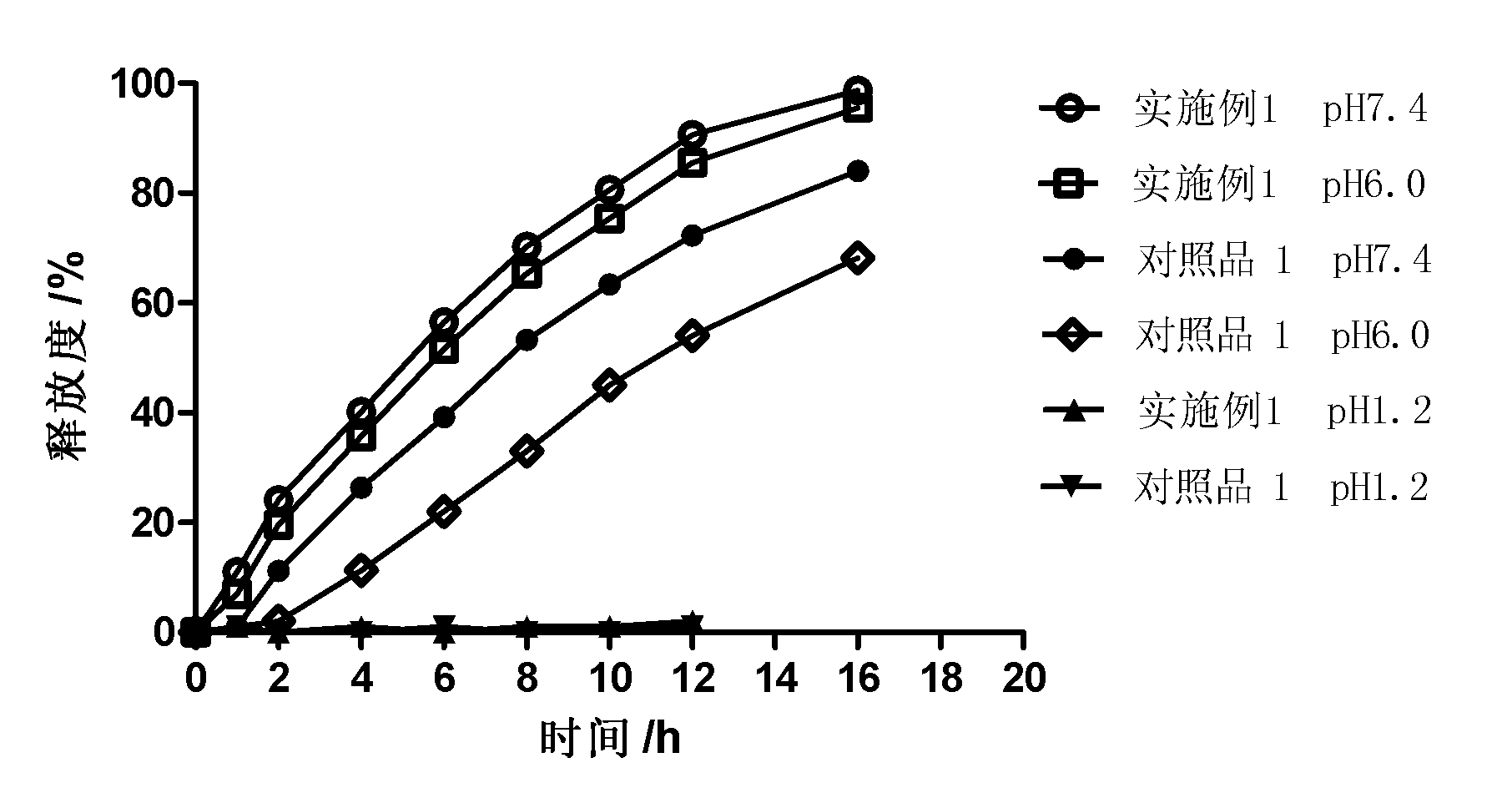
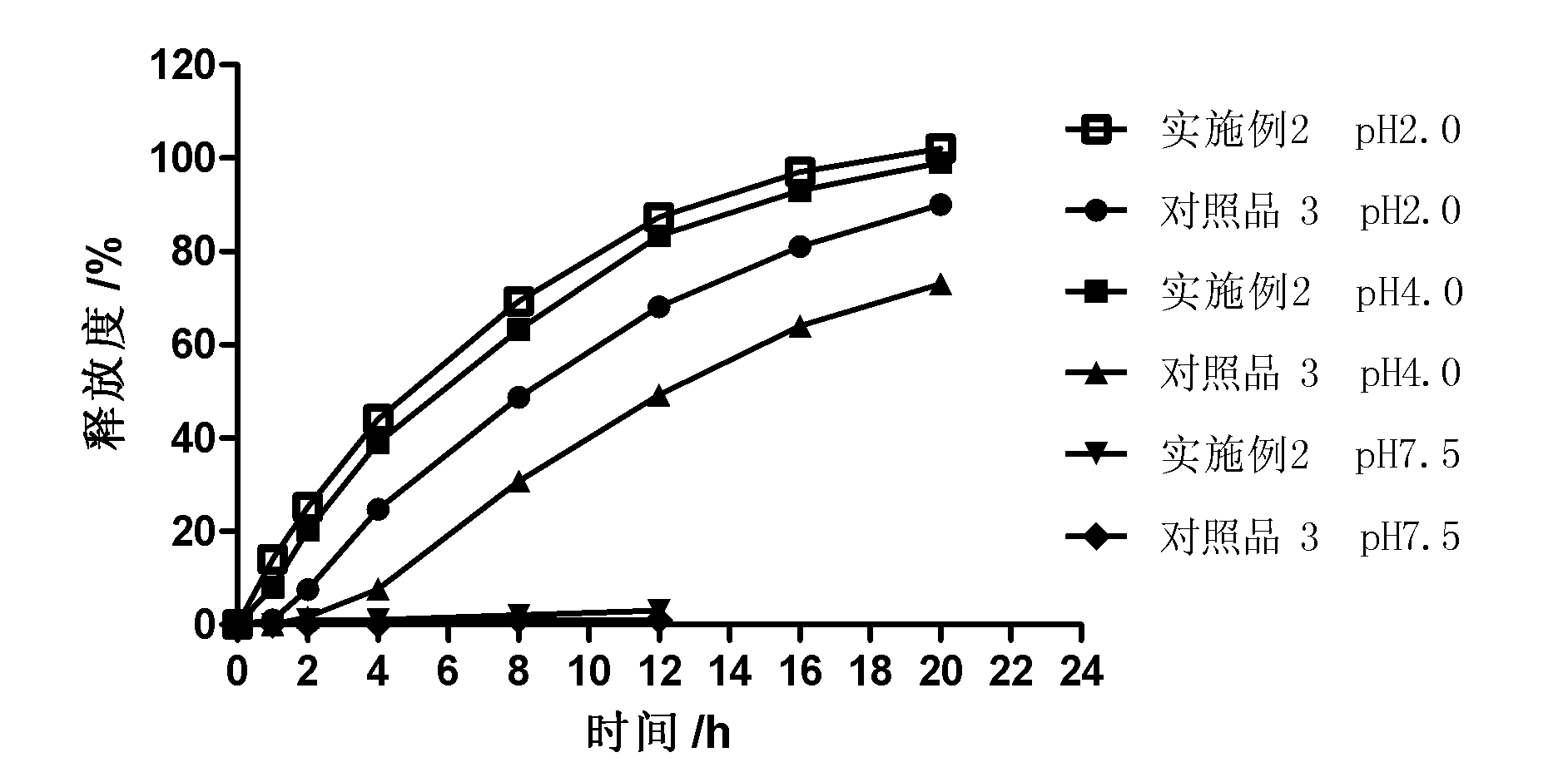
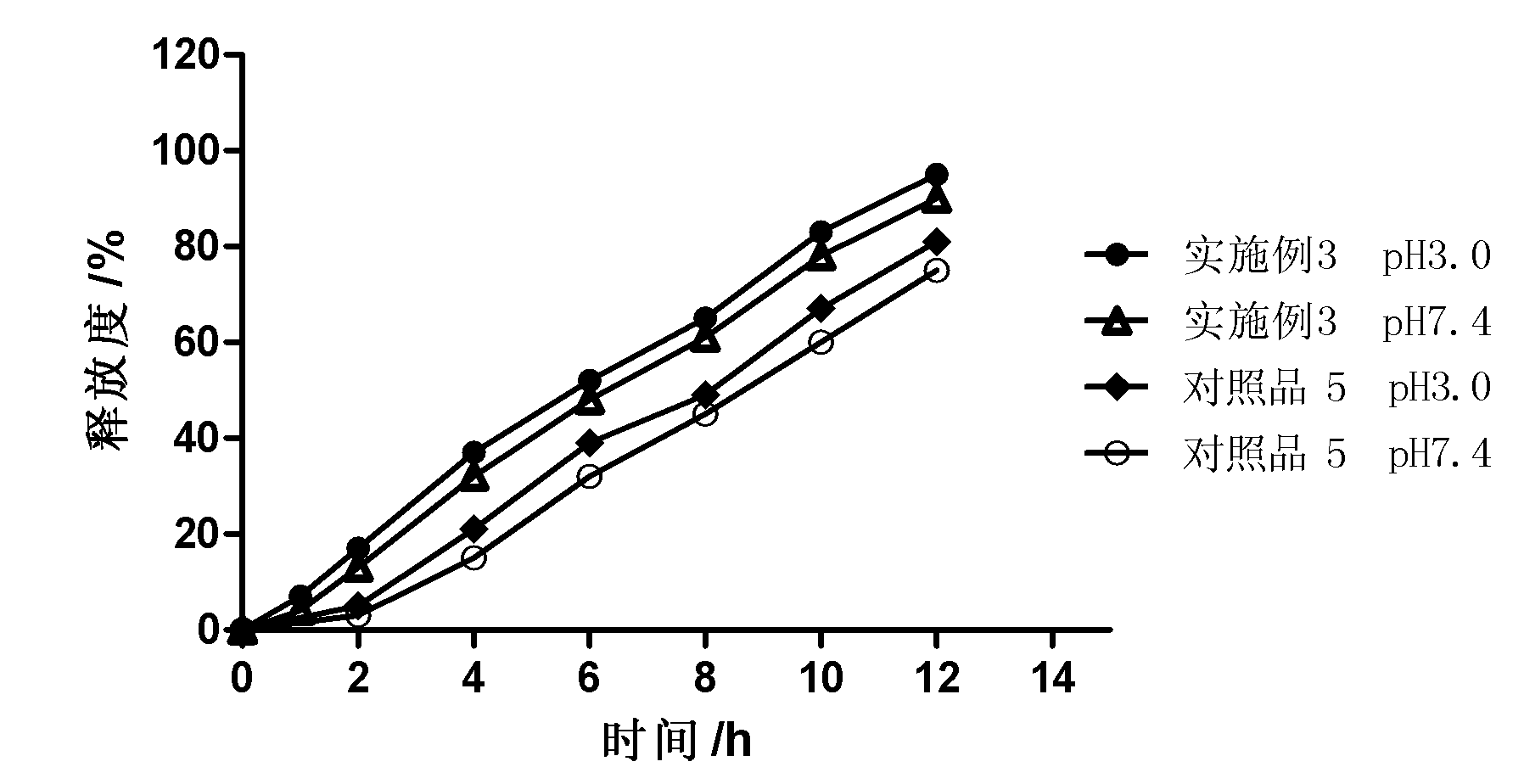
[0031] One object of the present invention is to provide a controlled-release preparation, especially a zero-order release controlled-release preparation. The controlled-release preparation is dispersed or embedded in the outer layer coating as a pore-forming agent containing pharmaceutically acceptable Water-soluble pharmaceuticals coated with pharmaceutically acceptable plasticizers or plasticizer-free polymers that are soluble in gastric and / or intestinal digestive fluids but insoluble or barely soluble in water Drug release is controlled by a controlled-release coating of the granules of the additive. The porogen forms drug release channels in the digestive juice.
[0032] Wishing not to be completely bound by this principle or theory, since a part of the space in the macromolecular polymer particles that are soluble in gastric and / or intestinal digestive juices but insoluble or almost insoluble in water as porogens is filled by water-soluble The interdiffusion and penetr...
PUM
| Property | Measurement | Unit |
|---|---|---|
| The average particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com