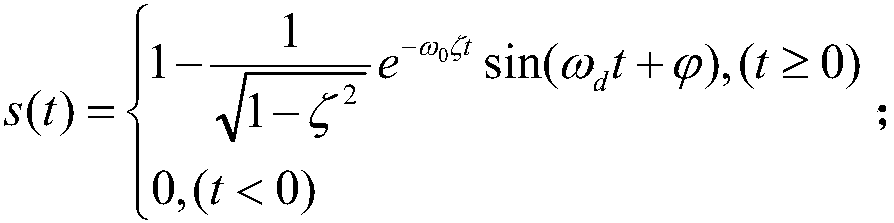Patents
Literature
304 results about "Lag time" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
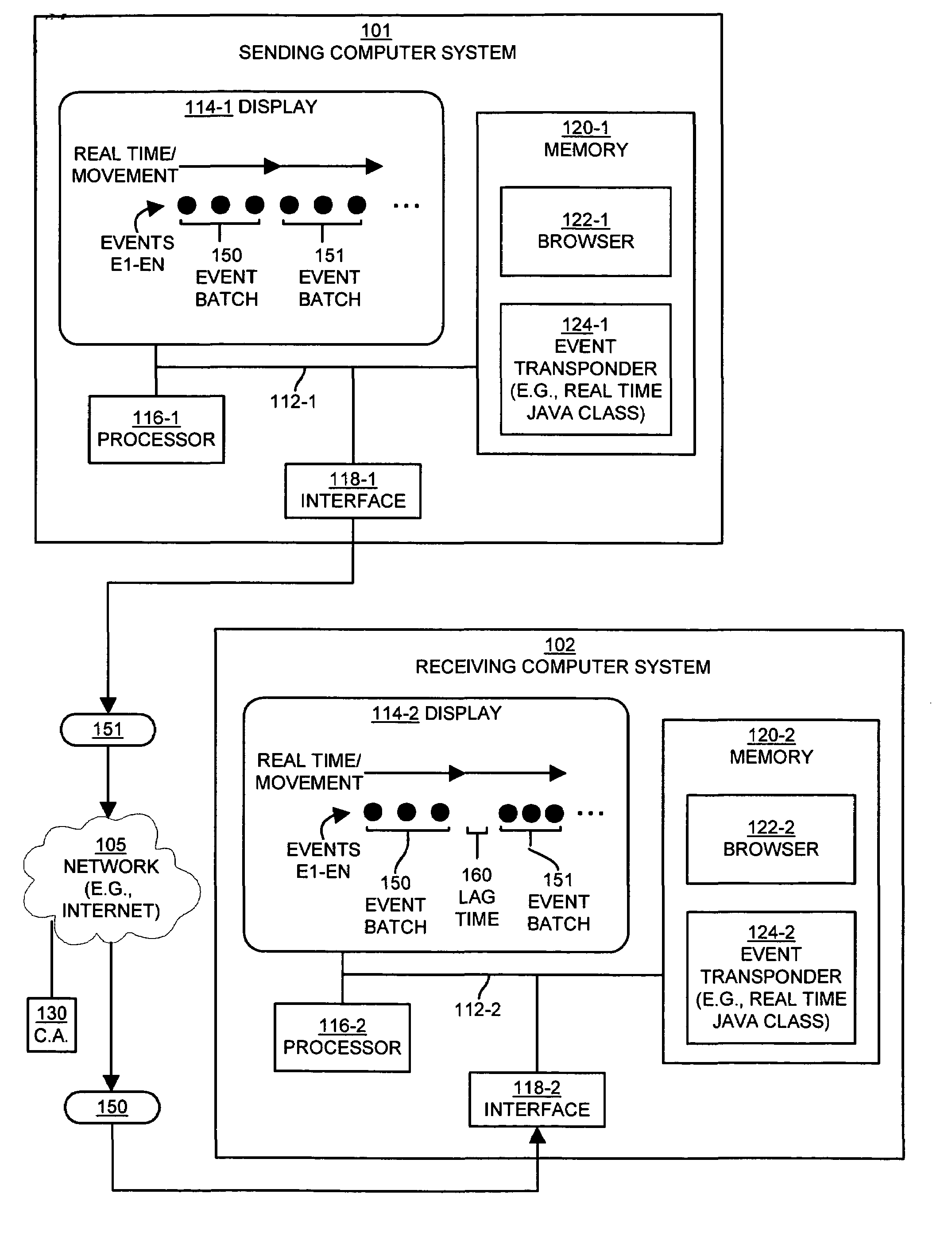
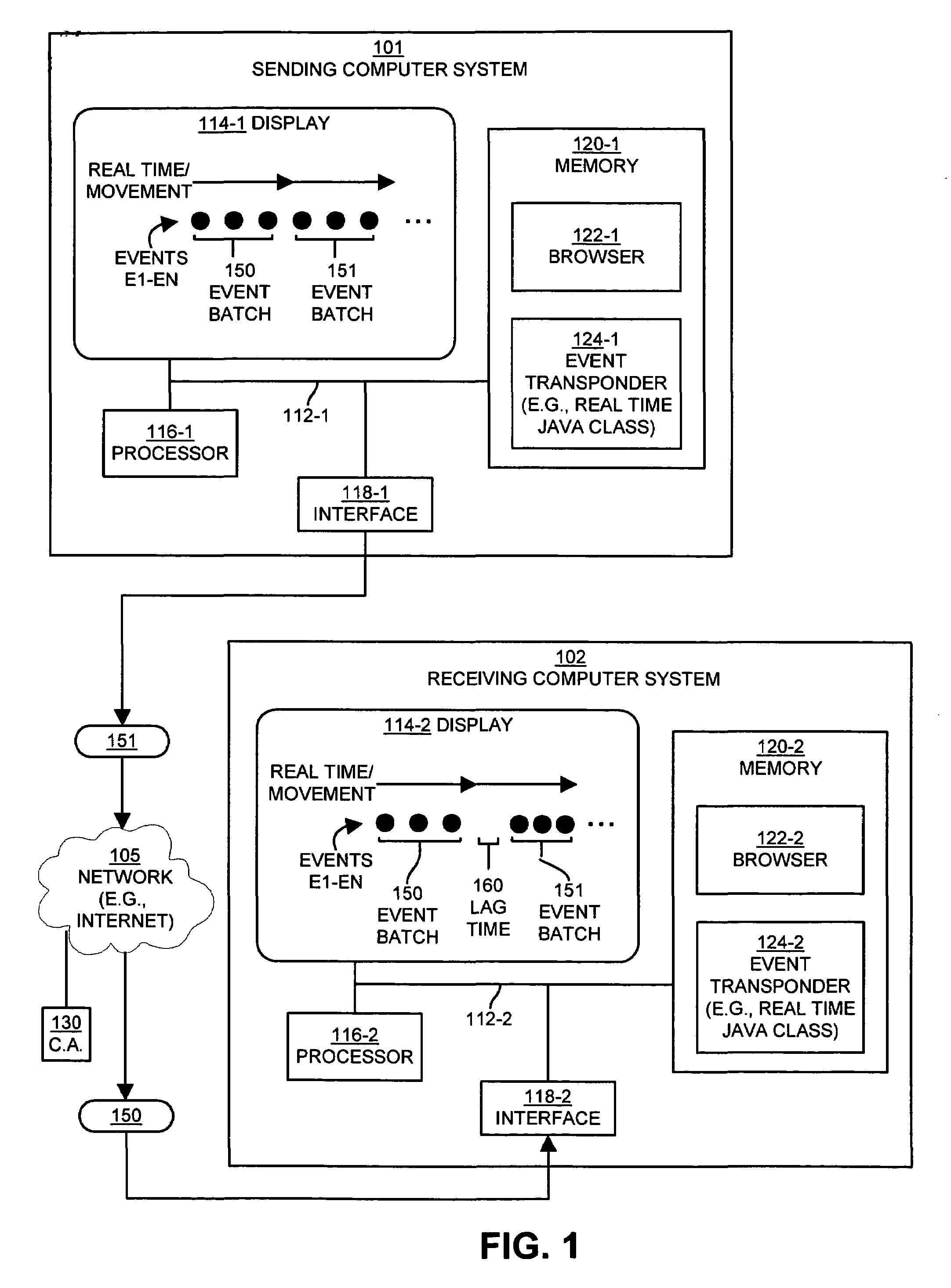
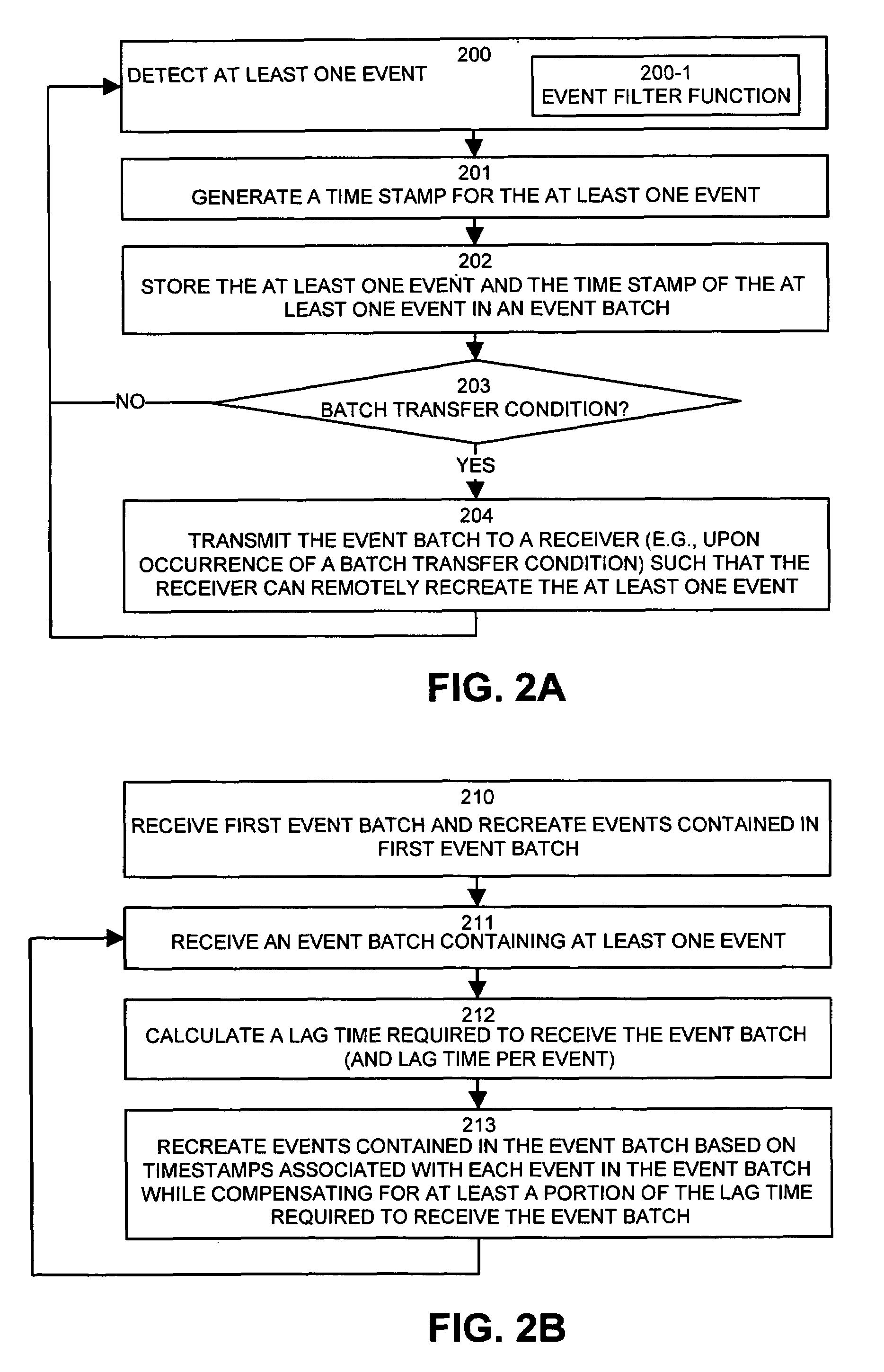
Method and apparatus for exchanging event information between computer systems that reduce perceived lag times by subtracting actual lag times from event playback time
InactiveUS6934766B1Shorten the time intervalMinimize amount of timeRecording carrier detailsRecord information storageTimestampComputerized system
Owner:CISCO TECH INC
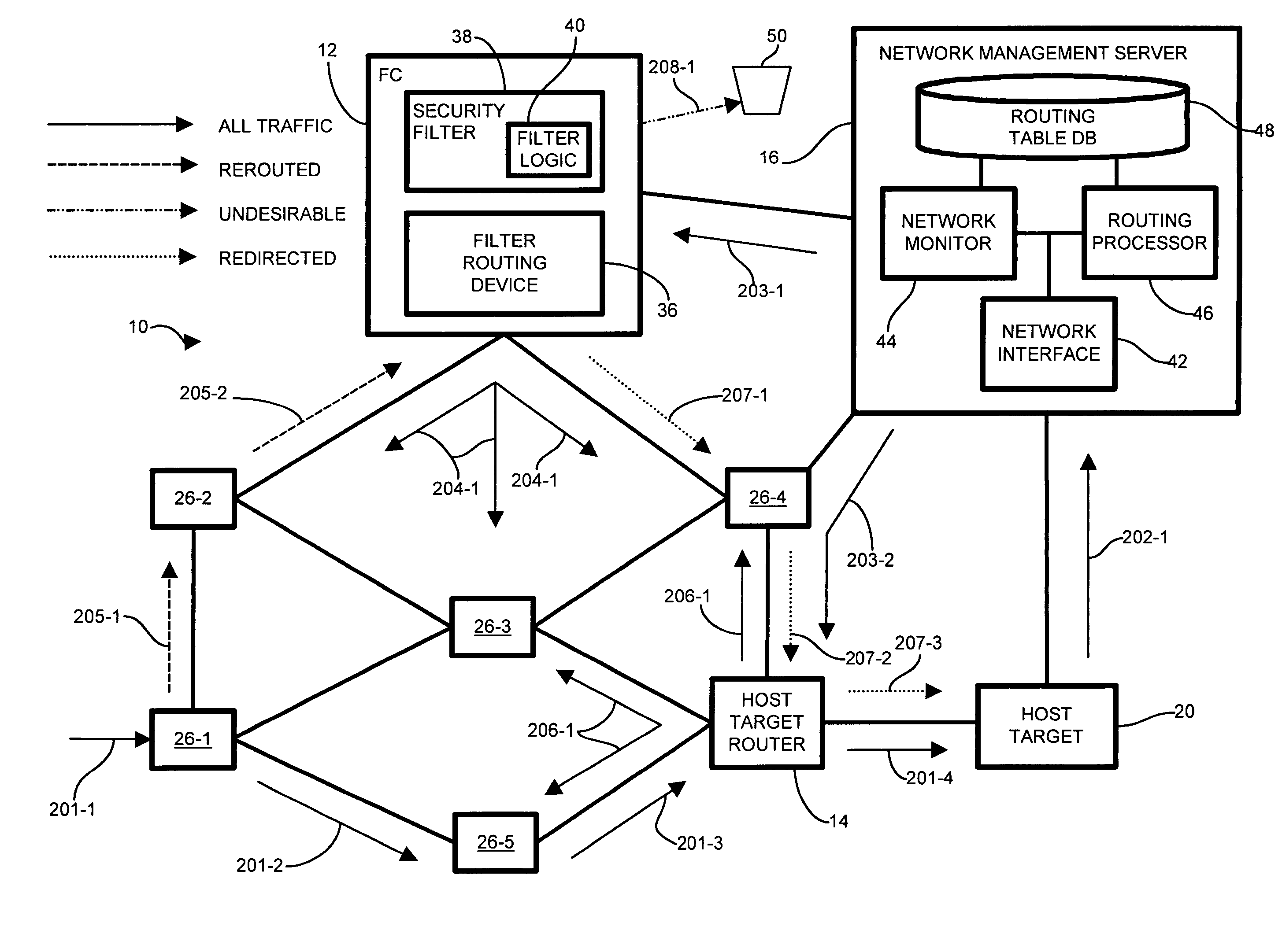
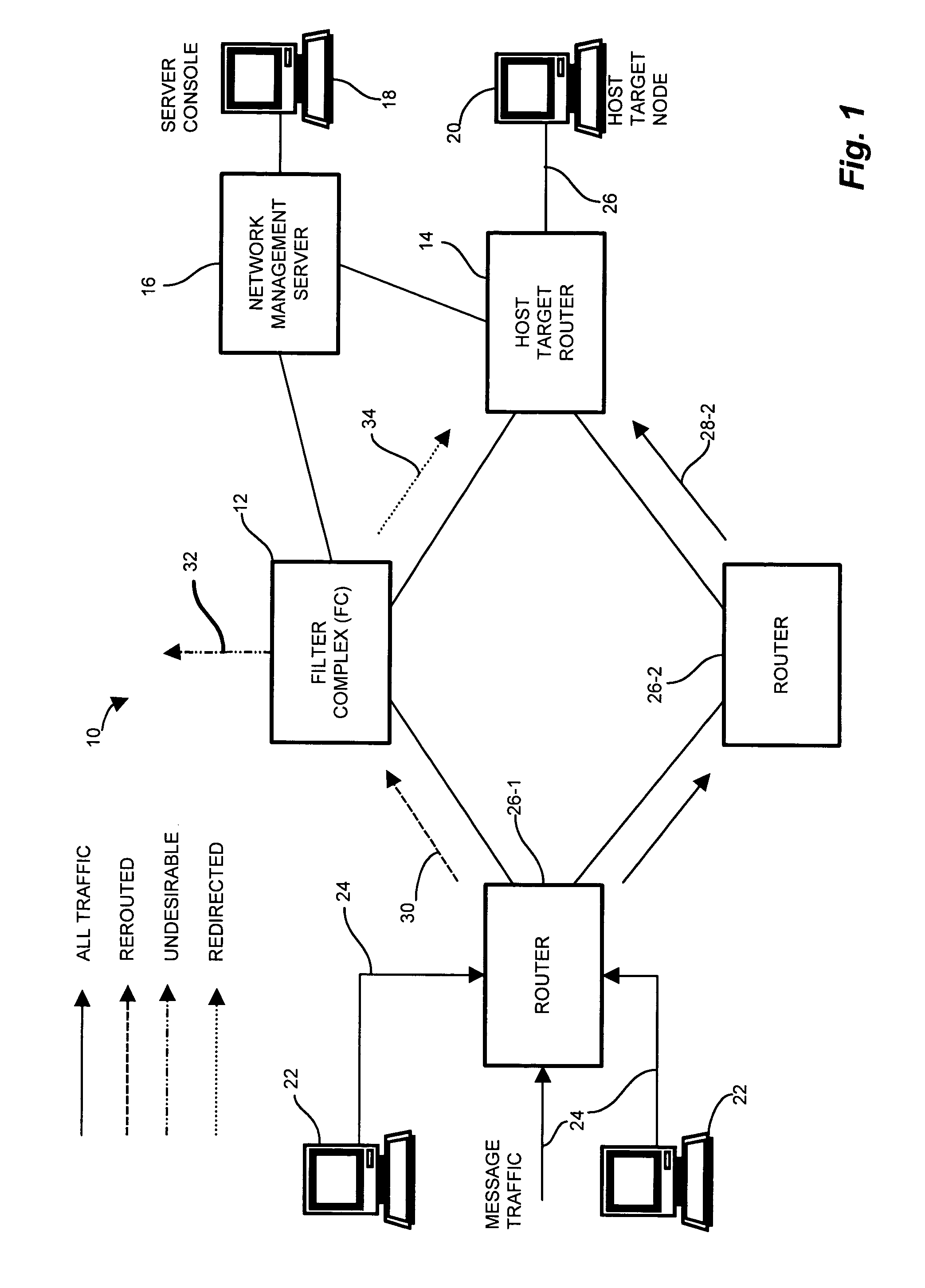
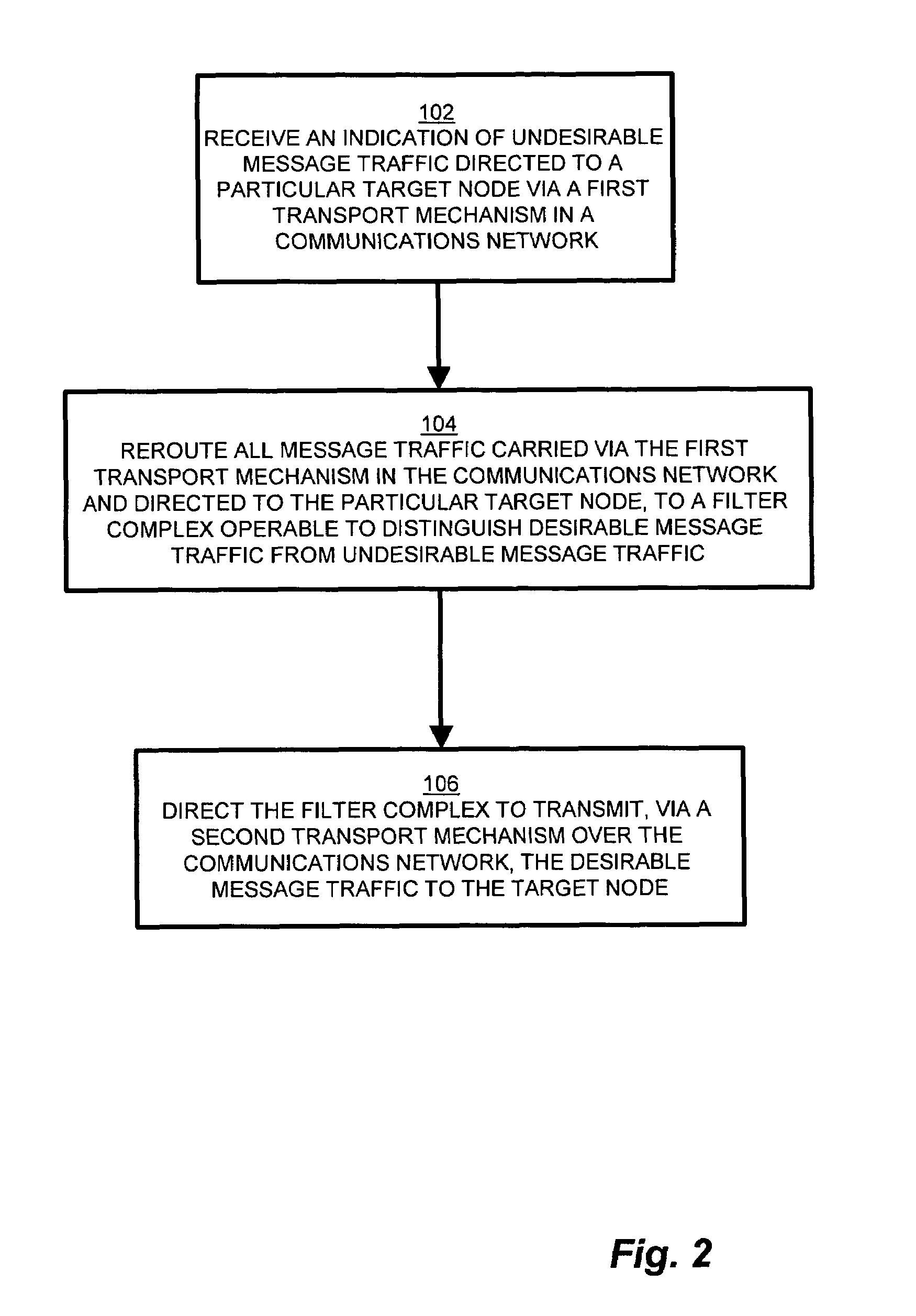
Methods and apparatus for network message traffic redirection
ActiveUS7409712B1Avoid reconfigurationMemory loss protectionDigital data processing detailsTraffic capacityCountermeasure
Conventional methods of addressing a Distributed Denial of Service attack include taking the target node offline, and routing all traffic to an alternate countermeasure, or “sinkhole” router, therefore requiring substantial lag time to reconfigure the target router into the network. In a network, a system operator monitors a network for undesirable message traffic. Upon a notification of such undesirable message traffic, traffic is rerouted to a filter complex to separate undesirable traffic. The filter complex establishes an alternate route using a second communications protocol, and uses the alternate route to redirect the desirable message traffic to the target node. The use of the second protocol avoids conflict between the redirected desirable traffic and the original, or first, protocol which now performs the reroute. In this manner, the filter complex employs a second alternate communications protocol to reroute and redirect desirable message traffic to the target node while diverting undesirable message traffic, and therefore avoids widespread routing configuration changes by limiting the propagation breadth of the second protocol.
Owner:CISCO TECH INC
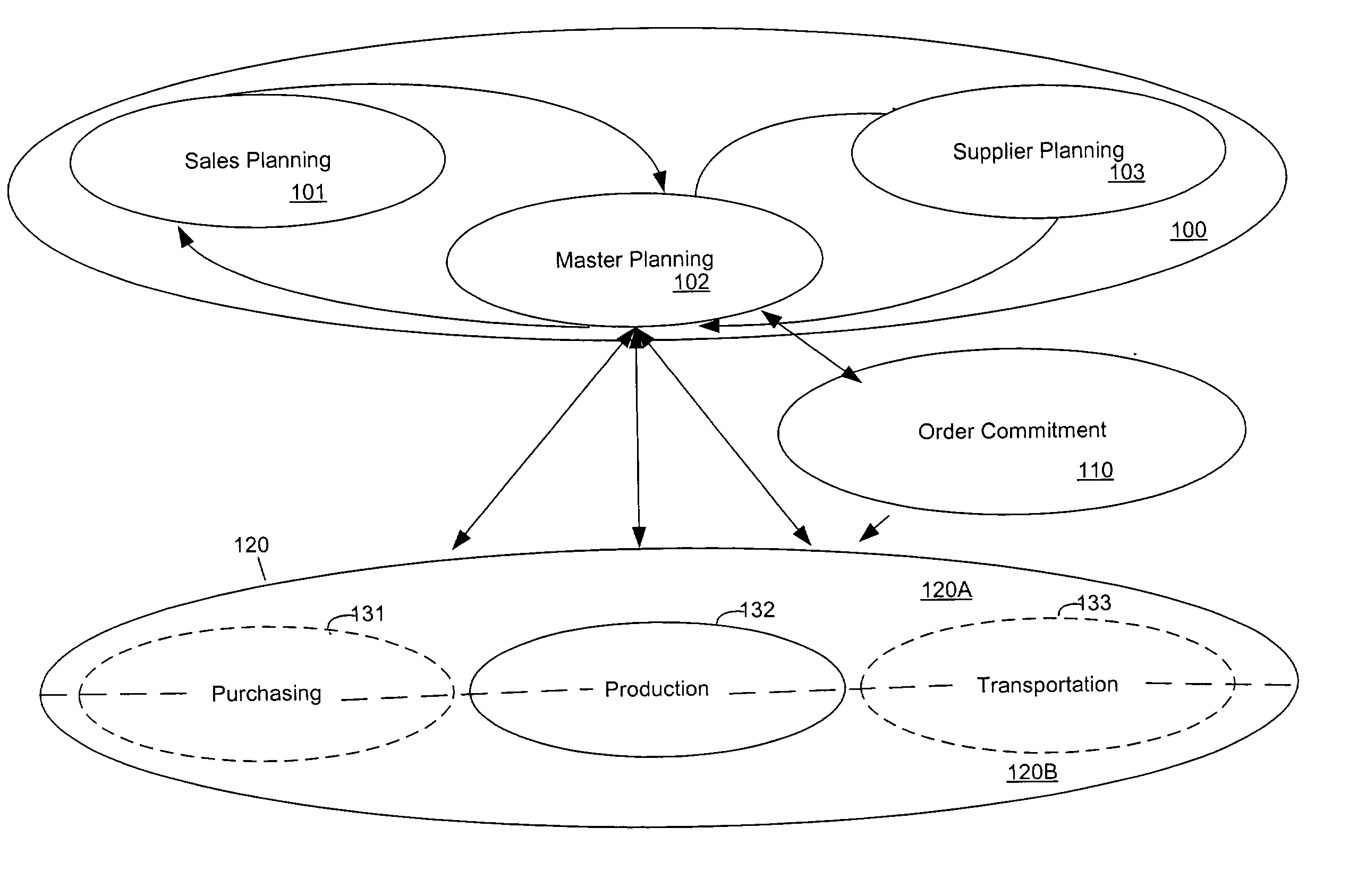
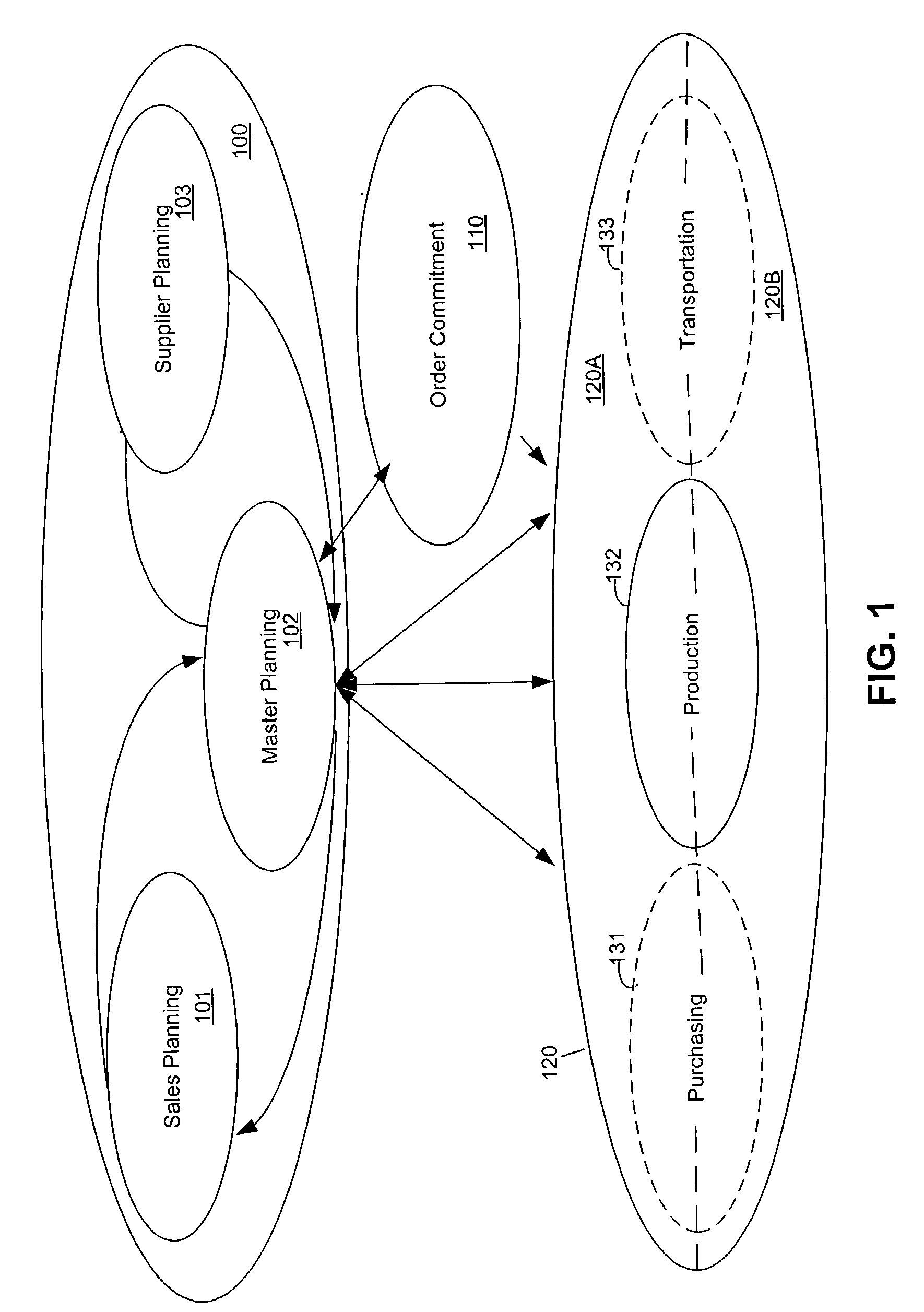
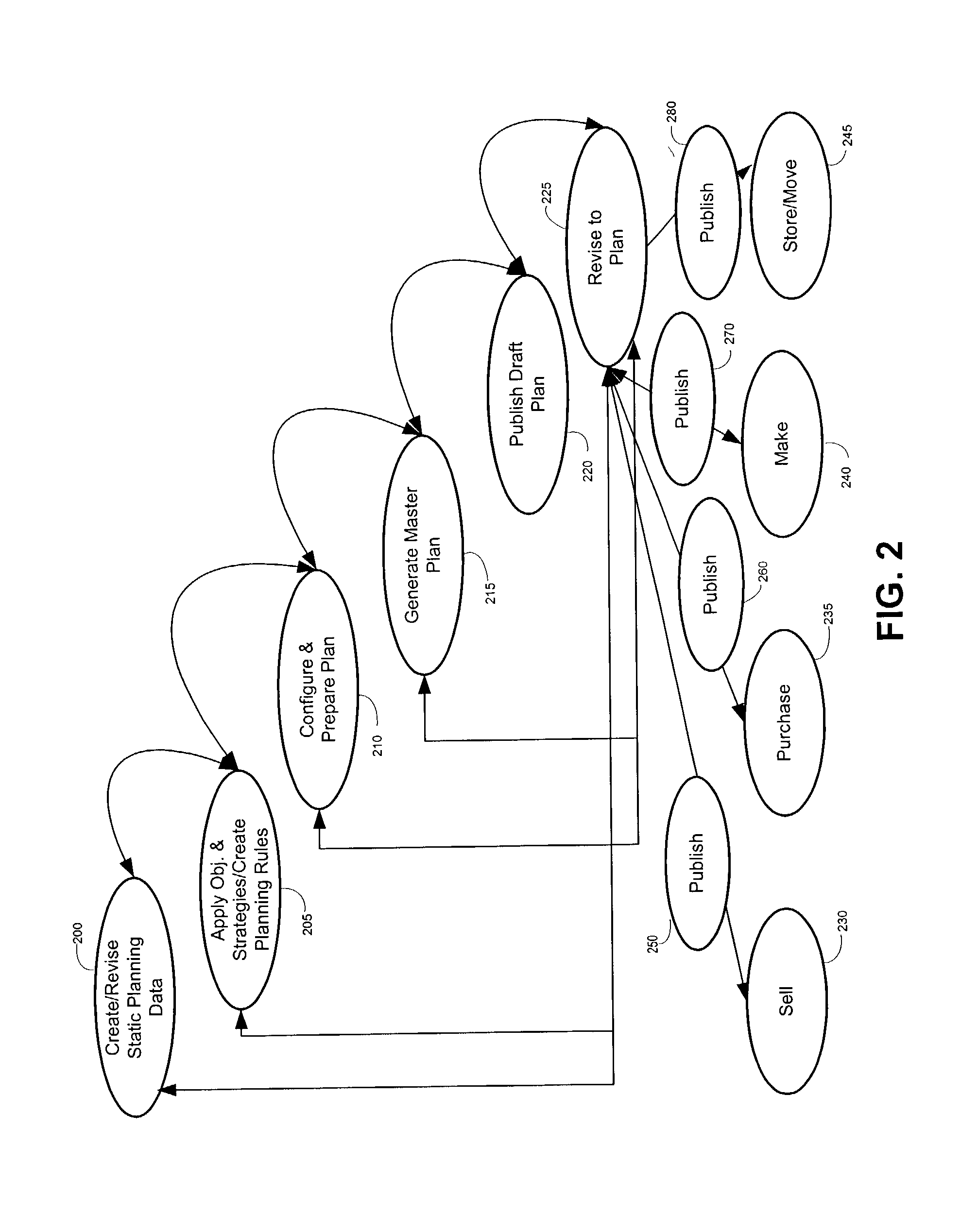
Optimizing resource plans
InactiveUS20030208392A1Good serviceReduce investmentOffice automationResourcesLimited resourcesTransportation scheduling
The present invention relates to a master resource planning systems and related methods for optimizing resource plans across multiple networks. In particular, the present invention optimizes constrained resources across multiple networks and produces an optimized plan to allocate and coordinate the obtaining, shipping and procurement of limited resources based upon user-defined strategies and supplier / shipper preferences Aspects of the disclosure include creating planning data and planning rules. Planning data contains information regarding constrained resources in the multiple networks. Planning rules are based on user-defined strategies. Additional aspects include generating a plan based on the planning data and the planning rules and revising the plan in real-time. The generated plan optimally allocates the constrained resources according to the user-defined strategies. Certain embodiments disclosed herein employ pegging strategies for handling over and under availability of demanded resources among a variety of competing orders. Other certain embodiments disclosed herein optimize the procurement of resources needed to fulfill orders in part by taking into account limitations in shipping lag times, established transportation schedules, and carrier preferences.
Owner:JDA SOFTWARE GROUP
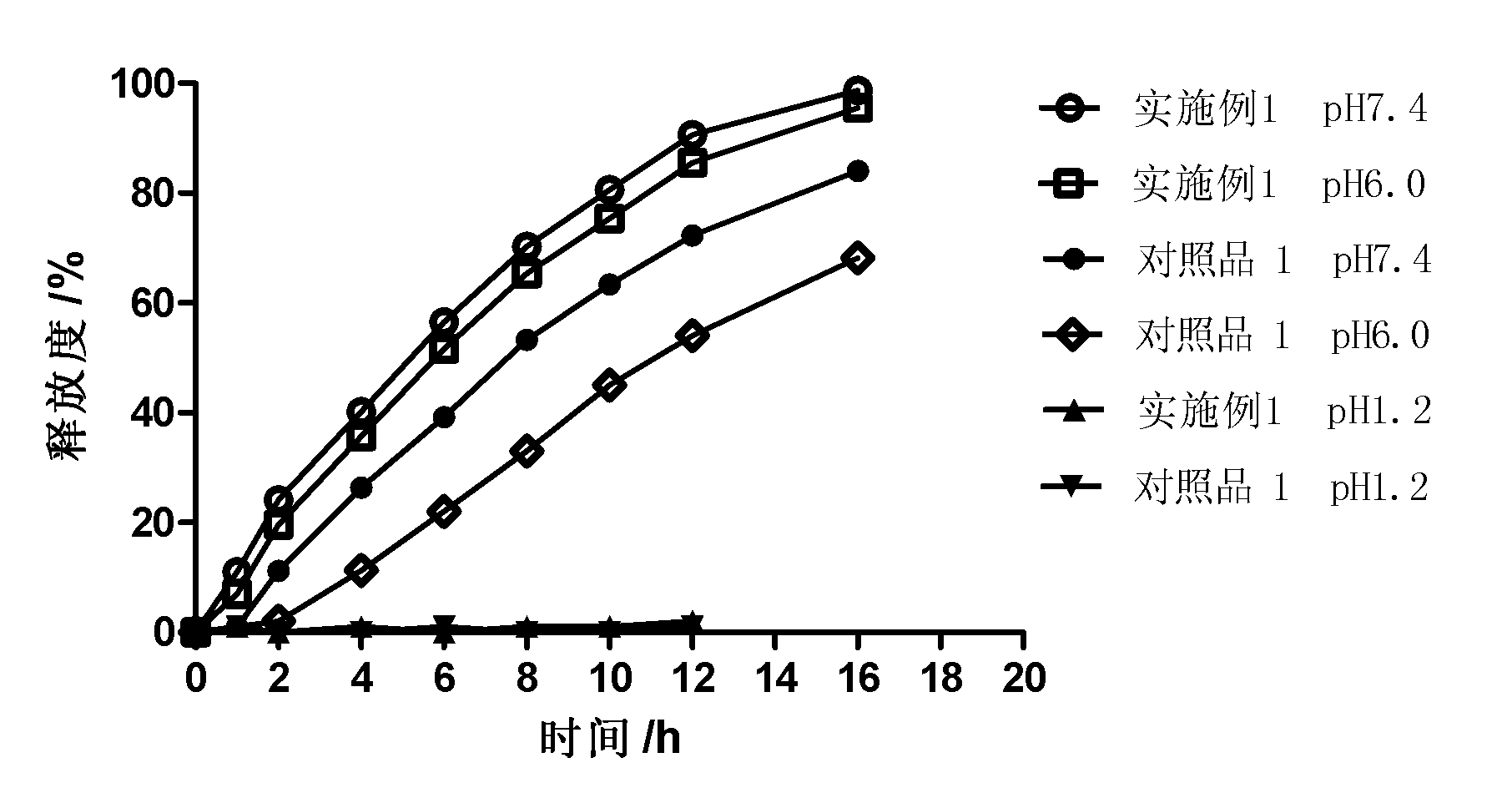
Controlled release preparation
InactiveCN101987081AImprove stabilityRelease impact mitigationInorganic non-active ingredientsSuppositories deliveryParticulatesChemical reaction
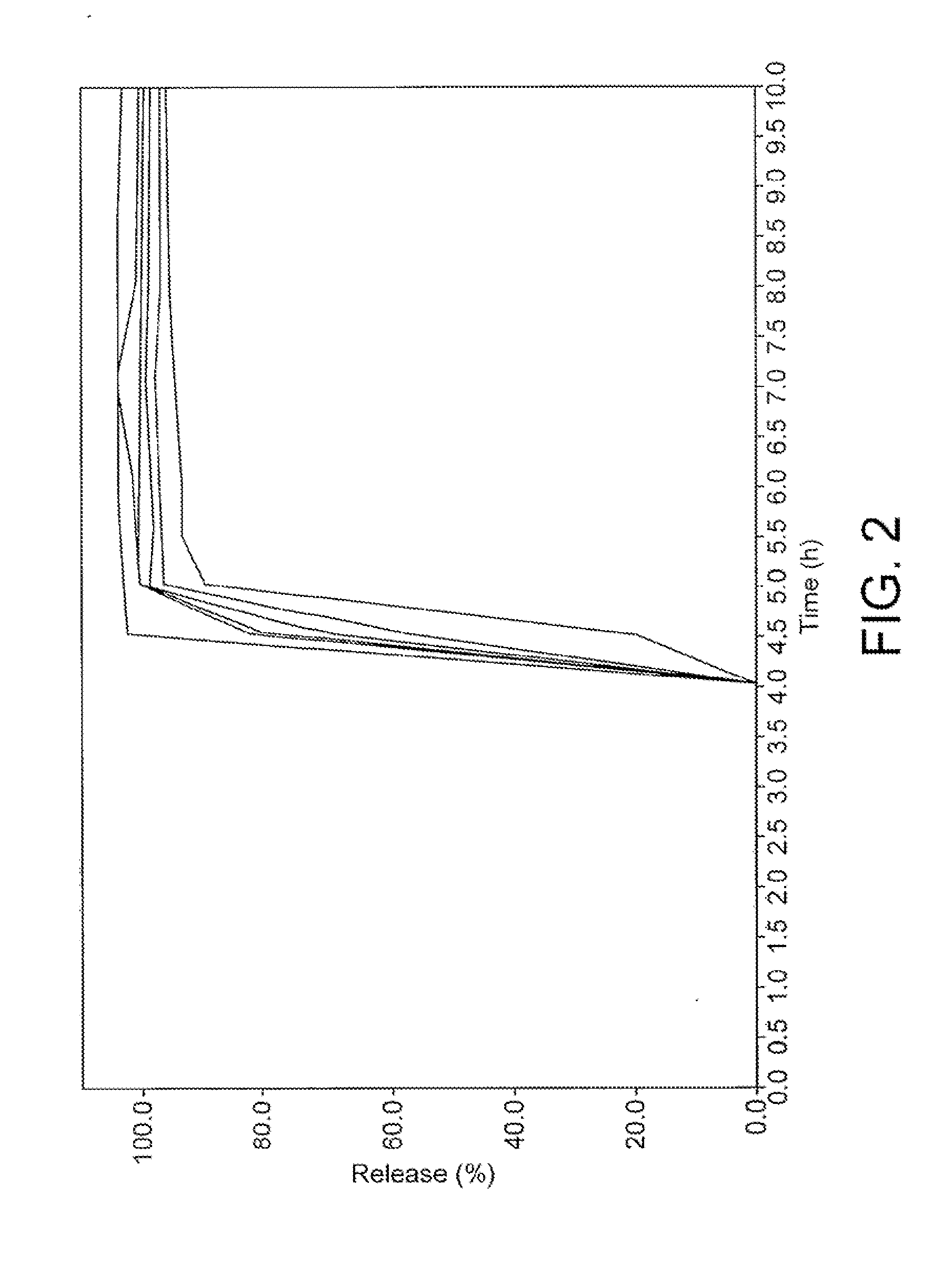
The invention discloses a controlled release preparation with improved performance. The controlled release preparation comprises a core containing medicament and a controlled release film covering the outside of the core and being almost insoluble in water as well as stomach and intestines digestive juice. The controlled release film comprises particulate matters of a water soluble medicinal additive, the water-soluble medicinal additive is covered by a polymer film which can be soluble in the stomach and / or intestines digestive juice but almost insoluble in water, the polymer and the medicinal additive can not produce chemical reaction or can produce chemical reaction but do not produce water-insoluble non-gaseous products and the pharmaceutically unacceptable products, and the amount of the polymer is no more 700% of that of the medicinal additive. The invention also discloses a preparation method of the controlled release preparation. The controlled release preparation has the advantages of improved medicament release reproducibility, reduced medicament release lag time, accelerated medicament release and improved bioavailability, can realize located controlled release, delayed controlled release and interval type or pulse type controlled release of the medicament in the gastrointestinal tract, and the like.
Owner:钟术光
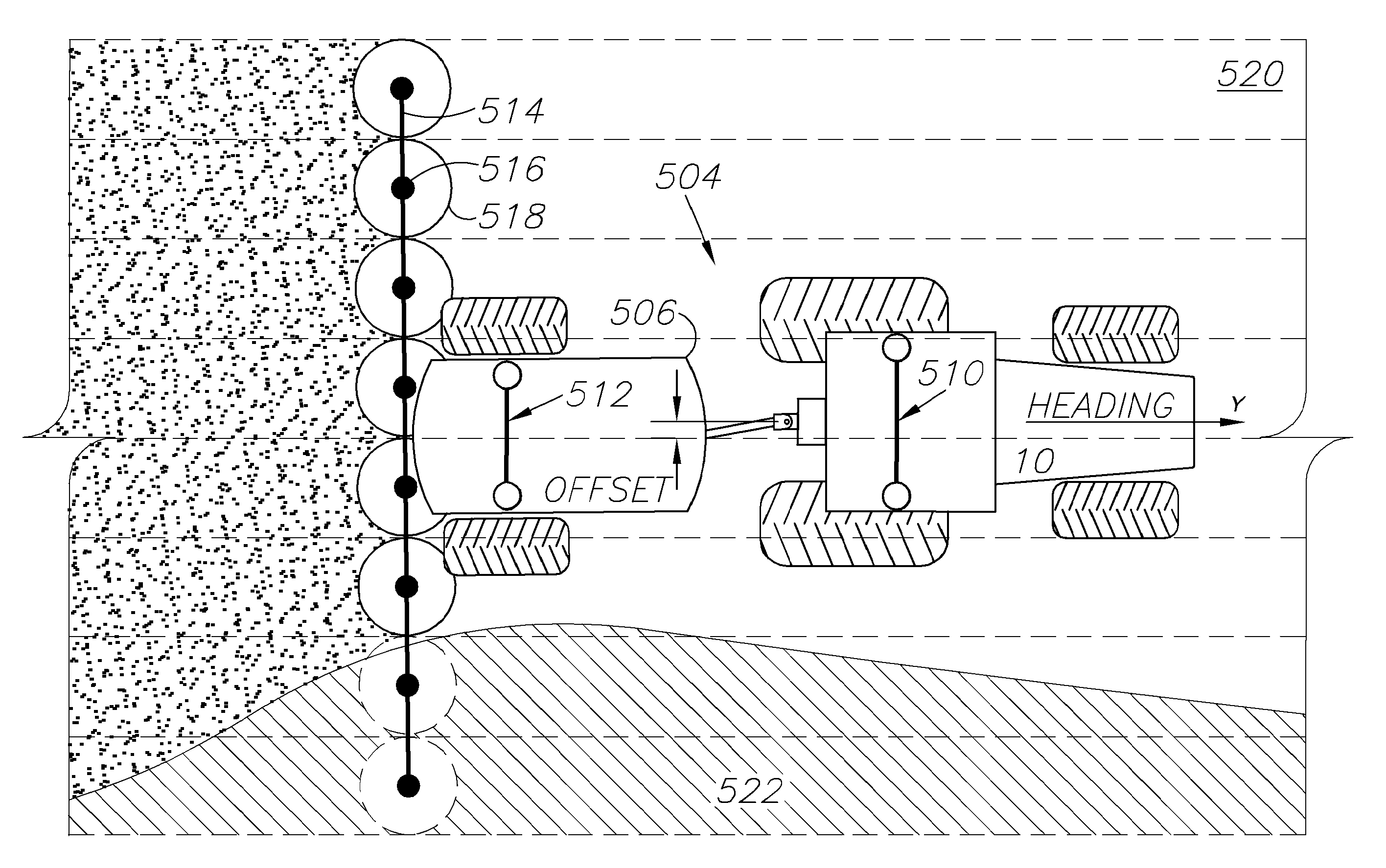
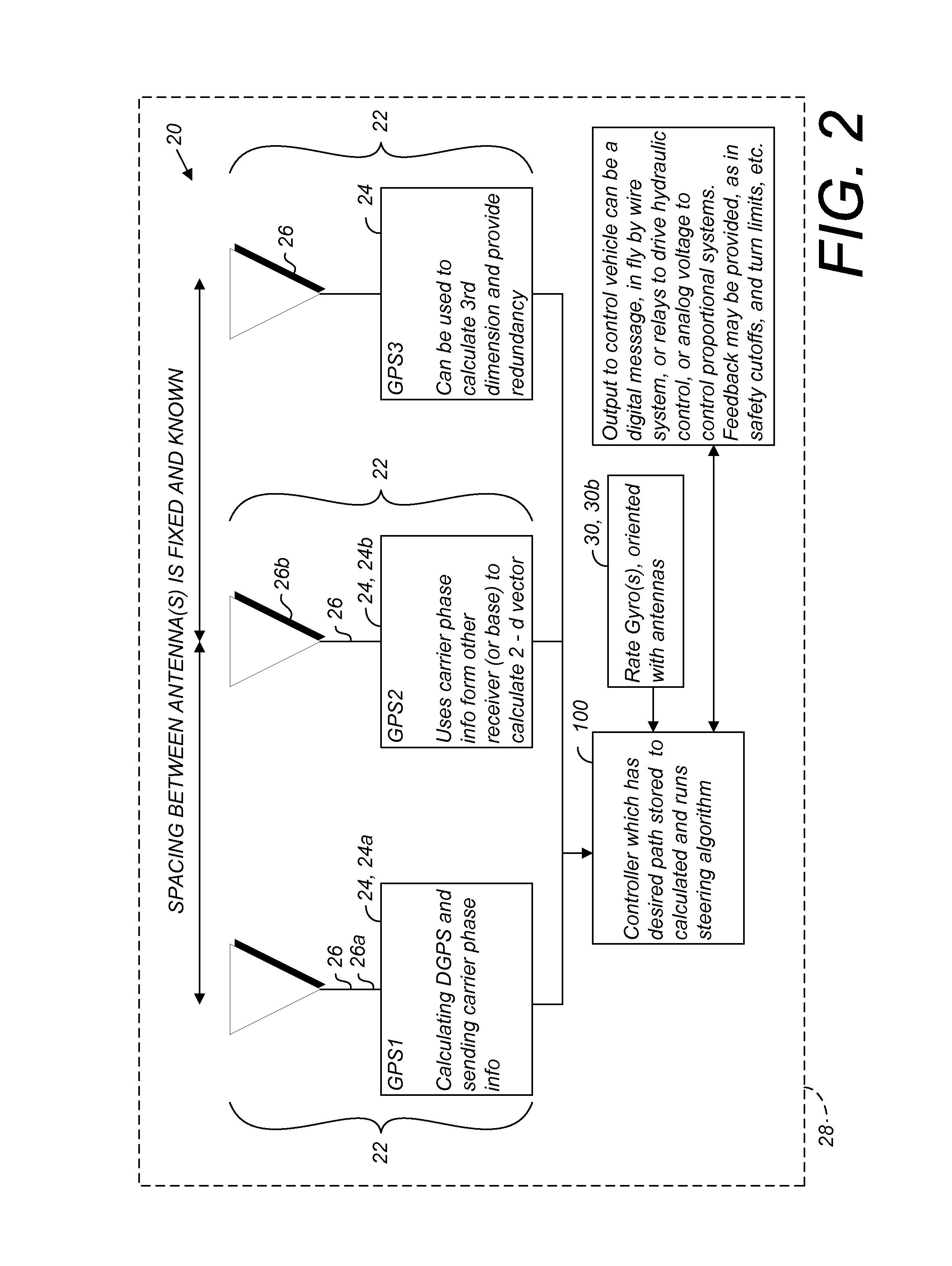
GNSS based control for dispensing material from vehicle
ActiveUS20120215410A1Prevent sprayingEasy to correctAnalogue computers for trafficPosition fixationEngineeringControl theory
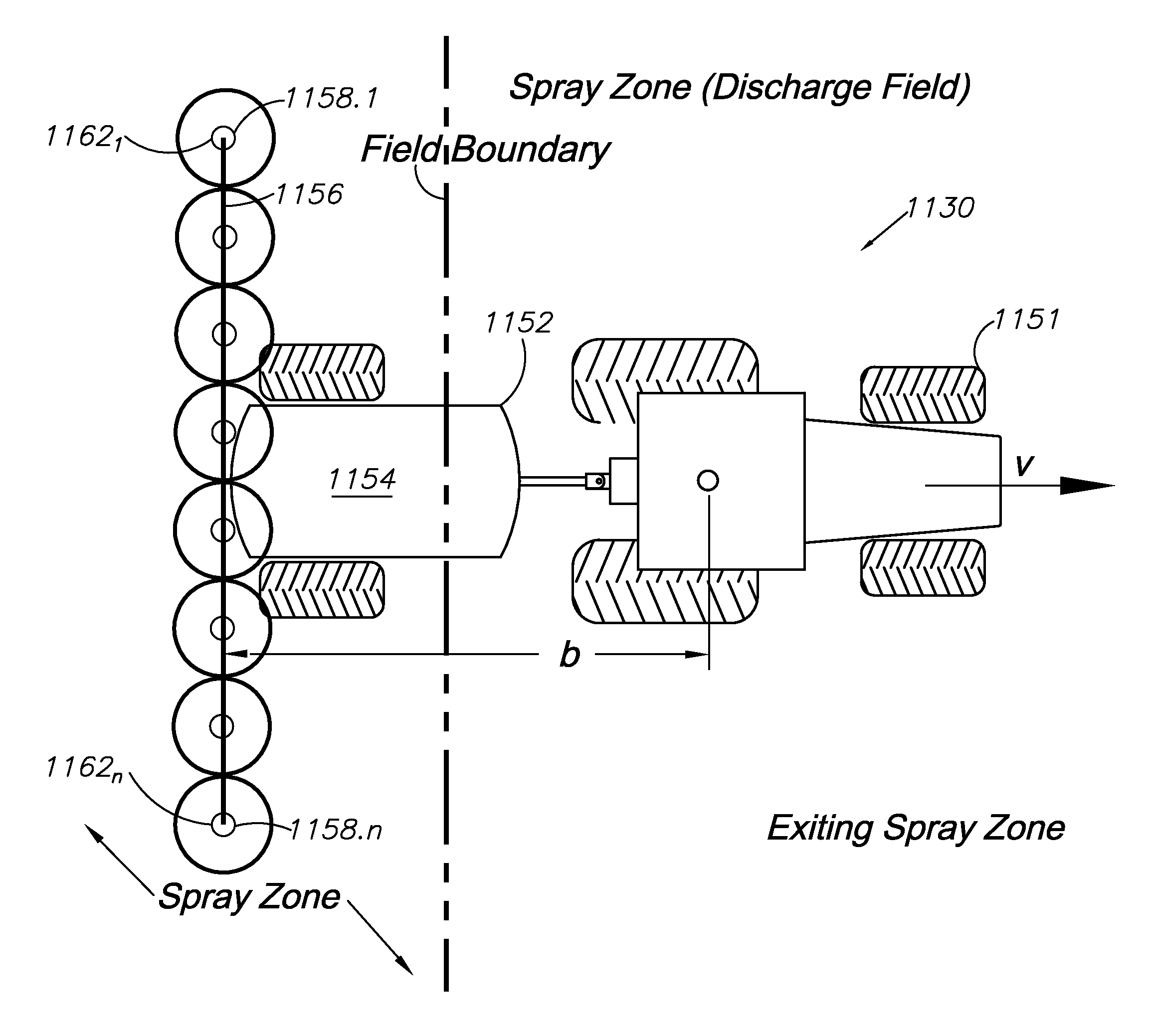
A spray control method employs a spray vehicle including a material tank, a pump communicating with the tank, and nozzles of a spray boom communicating with the pump. A GNSS receiver mounted on the vehicle and interfaced to a controller tracks its position in relation to stored position coordinates of field boundaries separating spray zones from spray exclusion zones. The tank is activated and deactivated by the controller to retain spray of the material within the spray zones and to prevent spray of the material in the exclusion zones, by processing an offset of the spray nozzles from the receiver, the spray range of the nozzles, spray turn-on and turn-off lag times, and the velocity of the spray vehicle, all in relation to the field boundaries. An alternative embodiment individually controls spray from the nozzles by using associated valves interfaced to the controller.
Owner:AGJUNCTION
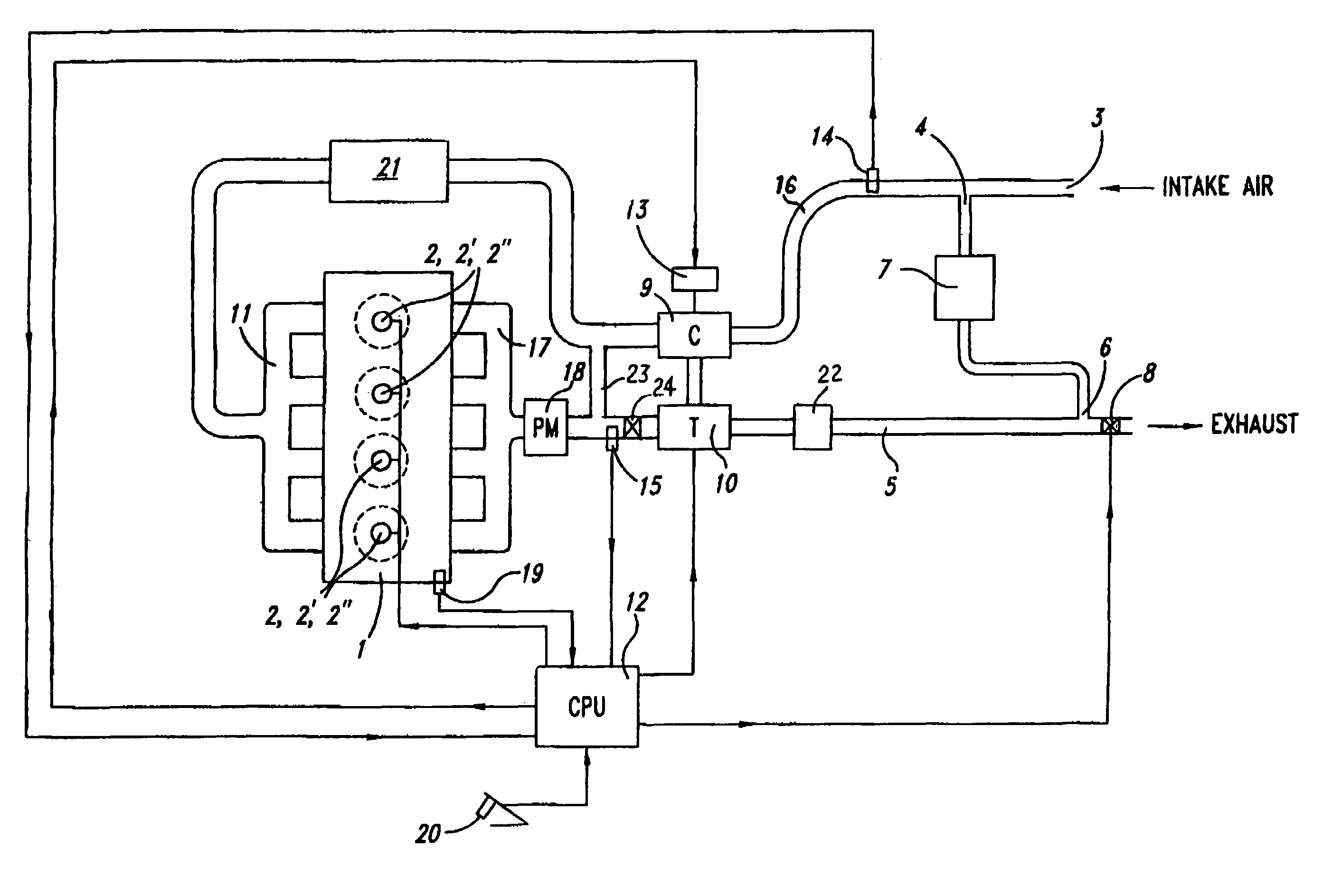
Control methods for low emission internal combustion system
InactiveUS7681394B2Cost-effectiveMaintaining NOx emissionsElectrical controlNon-fuel substance addition to fuelCombustion systemEngineering
Owner:US EPA OFFICE OF GENERAL COUNSEL UNITED STATES OF AMERICA THE
Modified release compositions of milnacipran
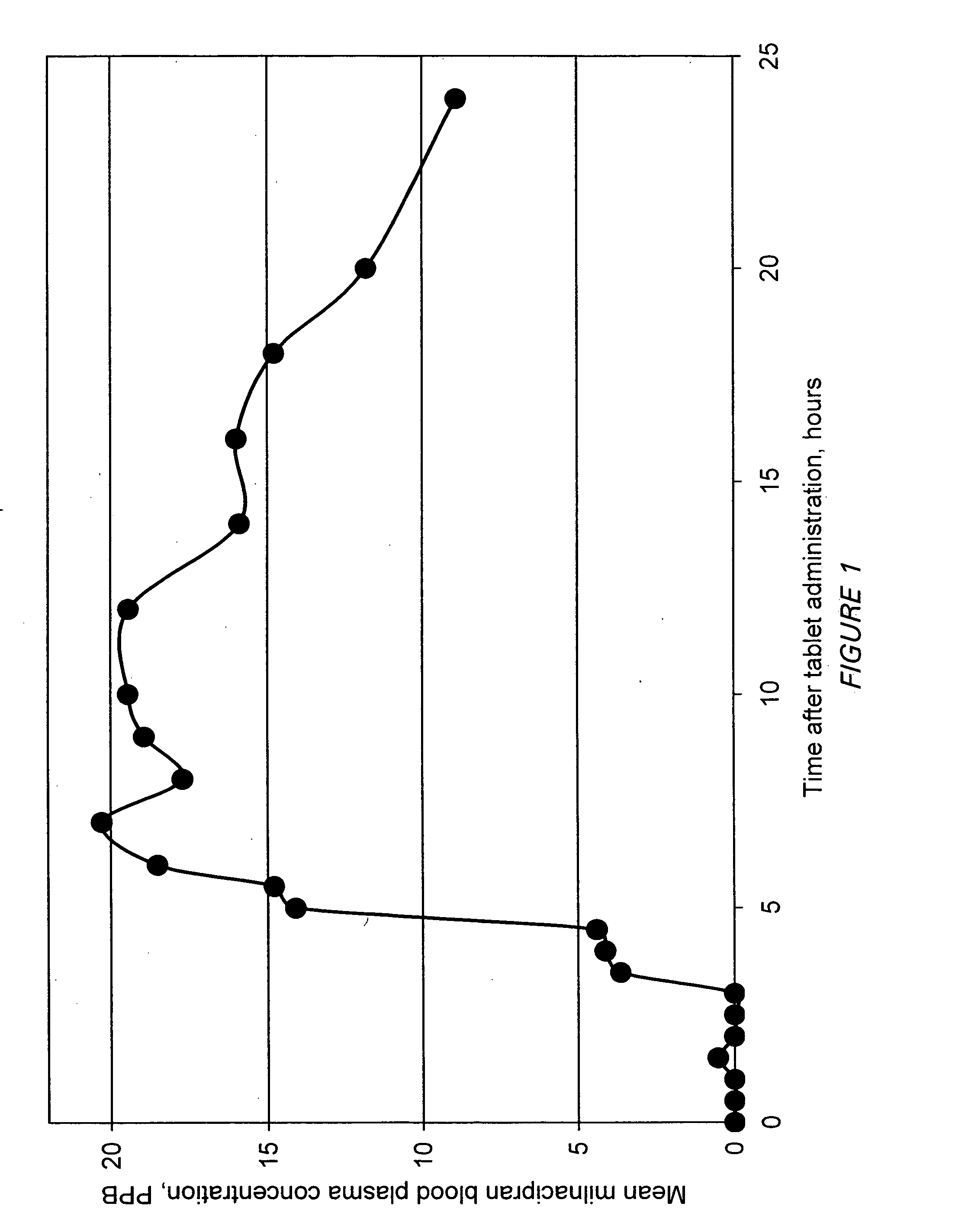
A once-a-day oral milnacipran modified release formulation has been developed. The formulation comprises an extended release dosage unit (optionally containing the immediate release portion) coated with delayed release coating. The milnacipran composition, when administered orally, first passes through the stomach releasing from zero to less than 10% of the total milnacipran dose and then enters the intestines where drug is released slowly over an extended period of time. The release profile is characterized by a 0.05-4 hours lag time period during which less than 10% of the total milnacipran dose is released followed by a slow or extended release of the remaining drug over a defined period of time. The composition provides in vivo drug plasma levels characterized by Tmax at 4-10 hours and an approximately linear drop-off thereafter and Cmax below 3000 ng / ml, preferably below 2000 ng / ml, and most preferably below 1000 ng / ml. The composition allows milnacipran to be delivered over approximately 24 hours, when administered to a patient in need, resulting in diminished incidence or decreased intensity of common milnacipran side effects such as sleep disturbance, nausea, vomiting, headache, tremulousness, anxiety, panic attacks, palpitations, urinary retention, orthostatic hypotension, diaphoresis, chest pain, rash, weight gain, back pain, constipation, vertigo, increased sweating, agitation, hot flushes, tremors, fatigue, somnolence, dyspepsia, dysoria, nervousness, dry mouth, abdominal pain, irritability, and insomnia.
Owner:COLLEGIUM PHARMA INC
Multilayer nanocomposite barrier structures
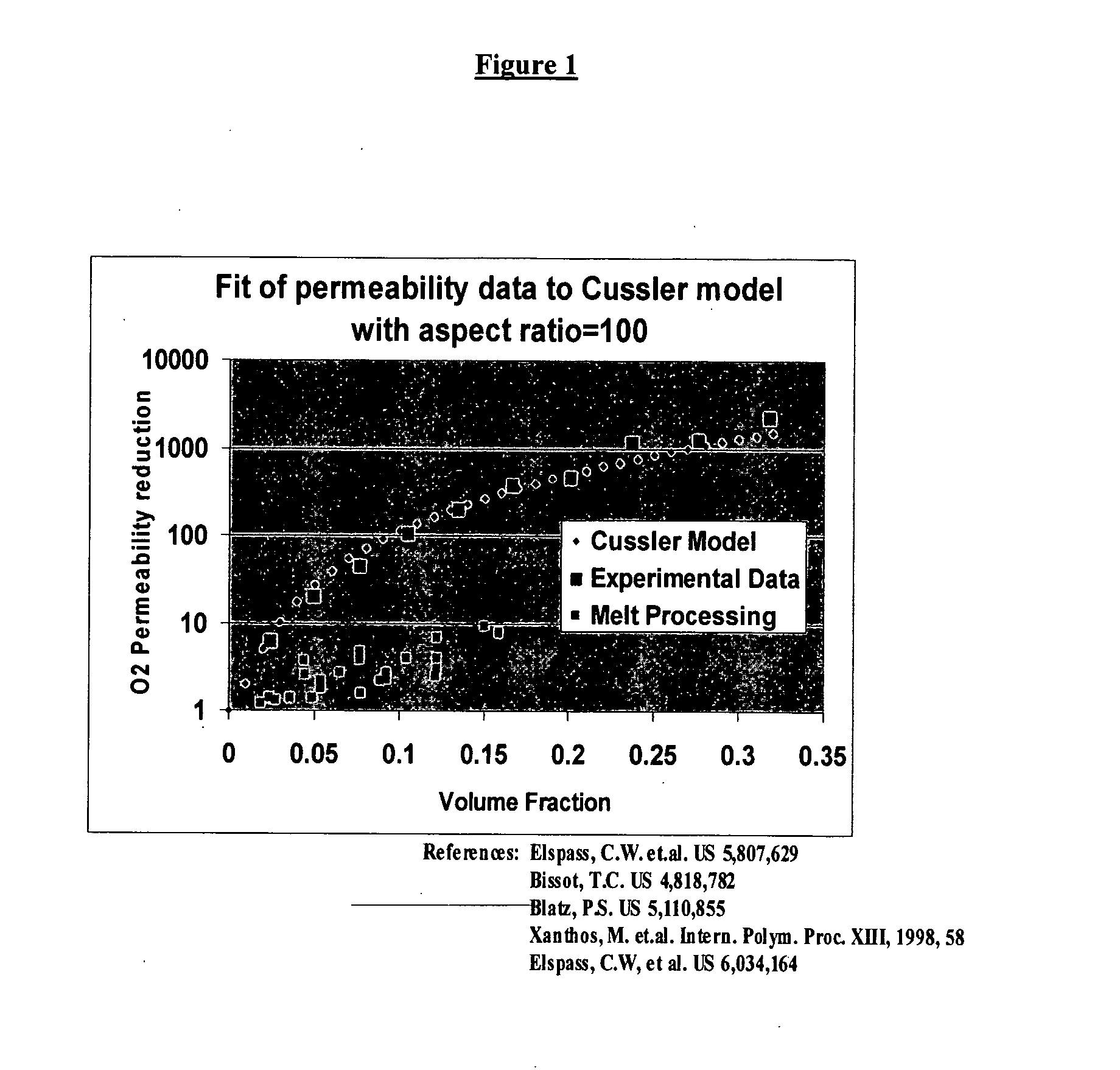
InactiveUS20060110615A1Reduce penetrationInhibited DiffusionSynthetic resin layered productsDomestic containersElastomerNanometre
A multilayer barrier structure includes a plurality of relatively thin nanocomposite layers separated by one or more relatively thick intermediate layers selected from synthetic elastomers or non-elastomeric polymers. The composites exhibit remarkably long breakthrough and lag-times and are useful for packaging or protective apparel.
Owner:INMAT INC
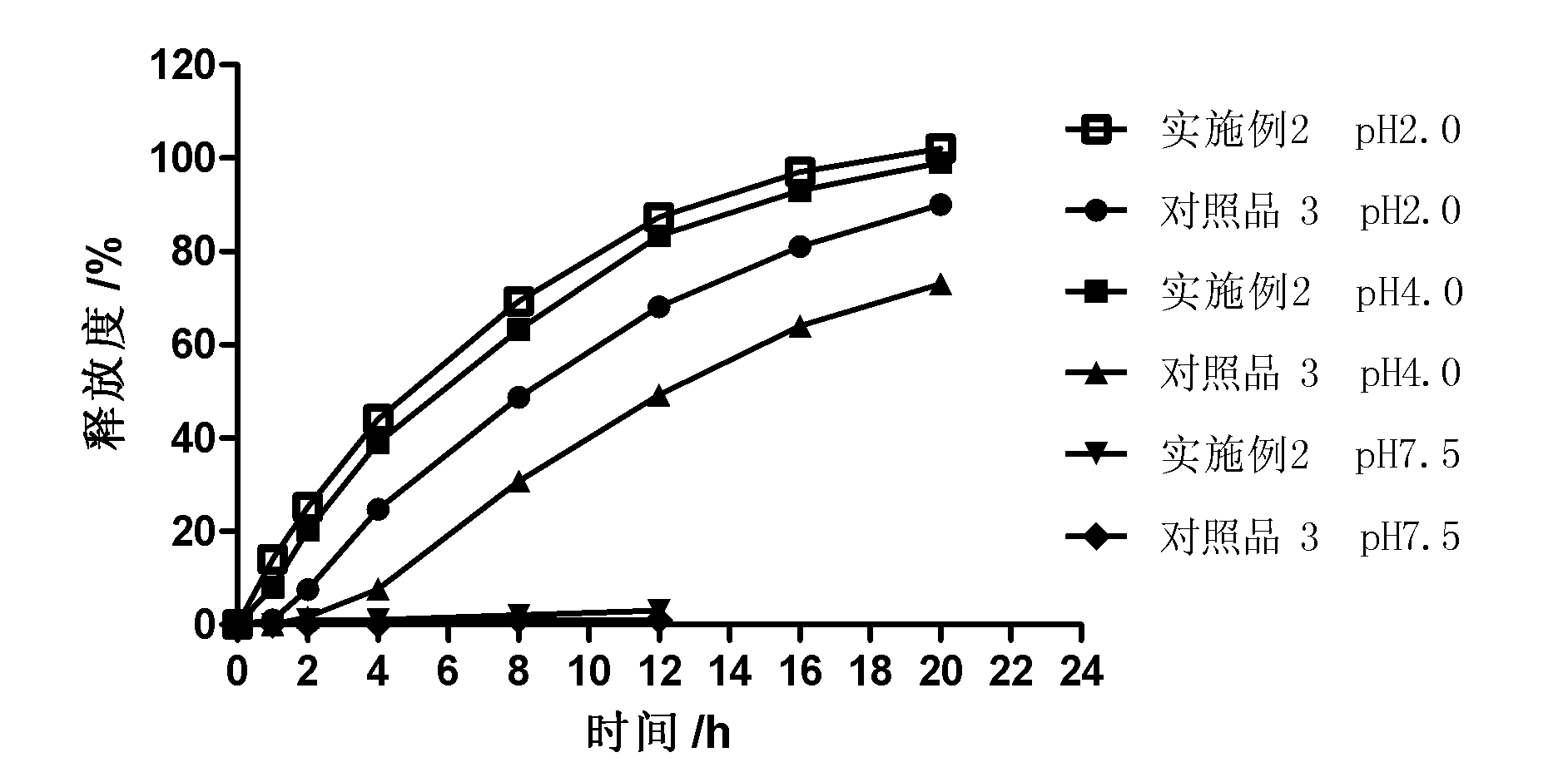
Timed pulsatile drug delivery systems
A pharmaceutical dosage form such as a capsule capable of delivering therapeutic agents into the body in a time-controlled or position-controlled pulsatile release fashion, is composed of a multitude of multicoated particulates (beads, pellets, granules, etc.) made of one or more populations of beads. Each of these beads except an immediate release bead has at least two coated membrane barriers. One of the membrane barriers is composed of an enteric polymer while the second membrane barrier is composed of a mixture of water insoluble polymer and an enteric polymer. The composition and the thickness of the polymeric membrane barriers determine the lag time and duration of drug release from each of the bead populations. Optionally, an organic acid containing intermediate membrane may be applied for further modifying the lag time and / or the duration of drug release. The pulsatile delivery may comprise one or more pulses to provide a plasma concentration-time profile for a therapeutic agent, predicted based on both its pharmaco-kinetic and pharmaco-dynamic considerations and in vitro / in vivo correlations.
Owner:ADARE PHARM INC
System and method for caching and rendering images
InactiveUS6873329B2Memory adressing/allocation/relocationDigital computer detailsComputer graphics (images)Image database
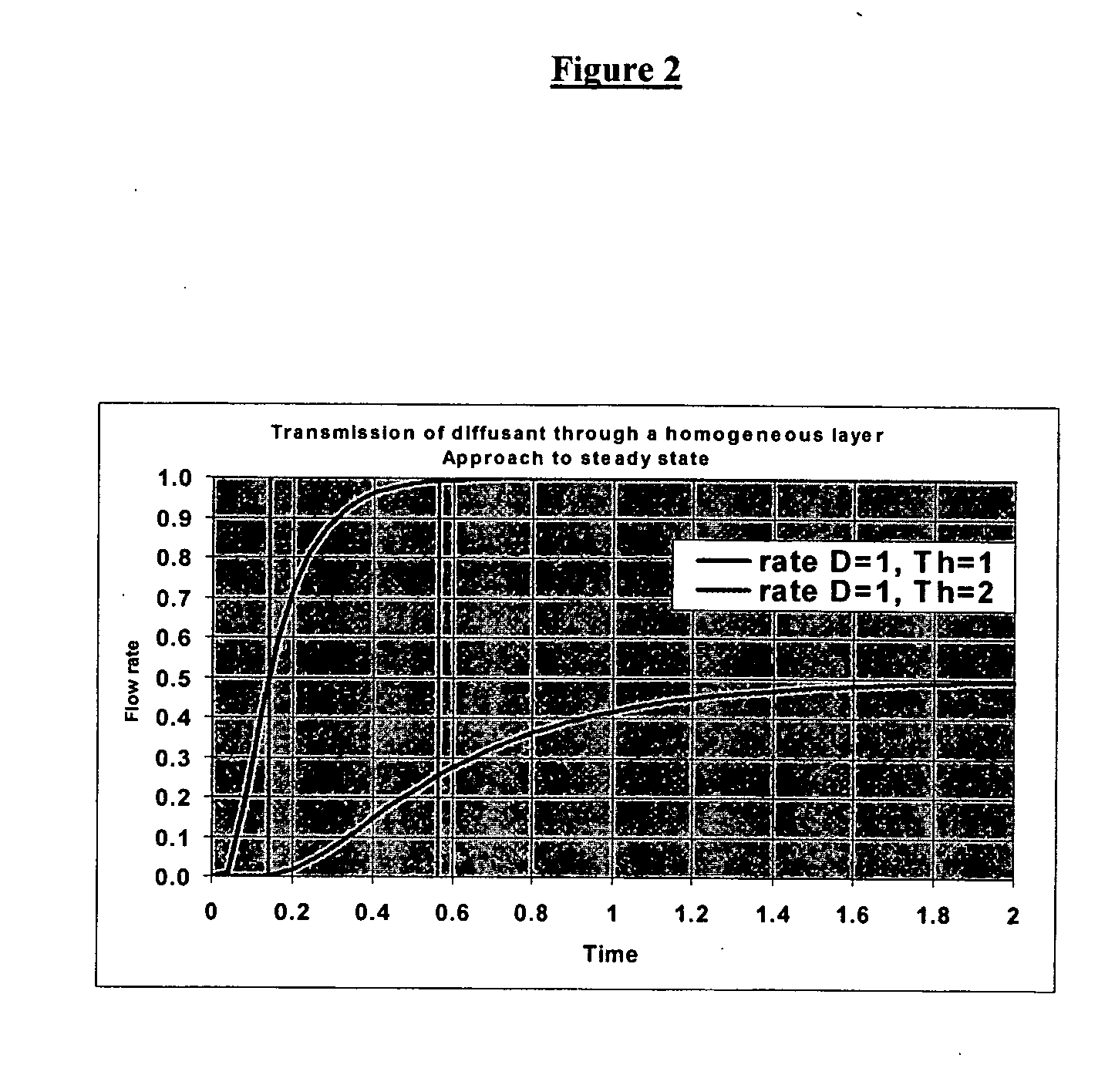
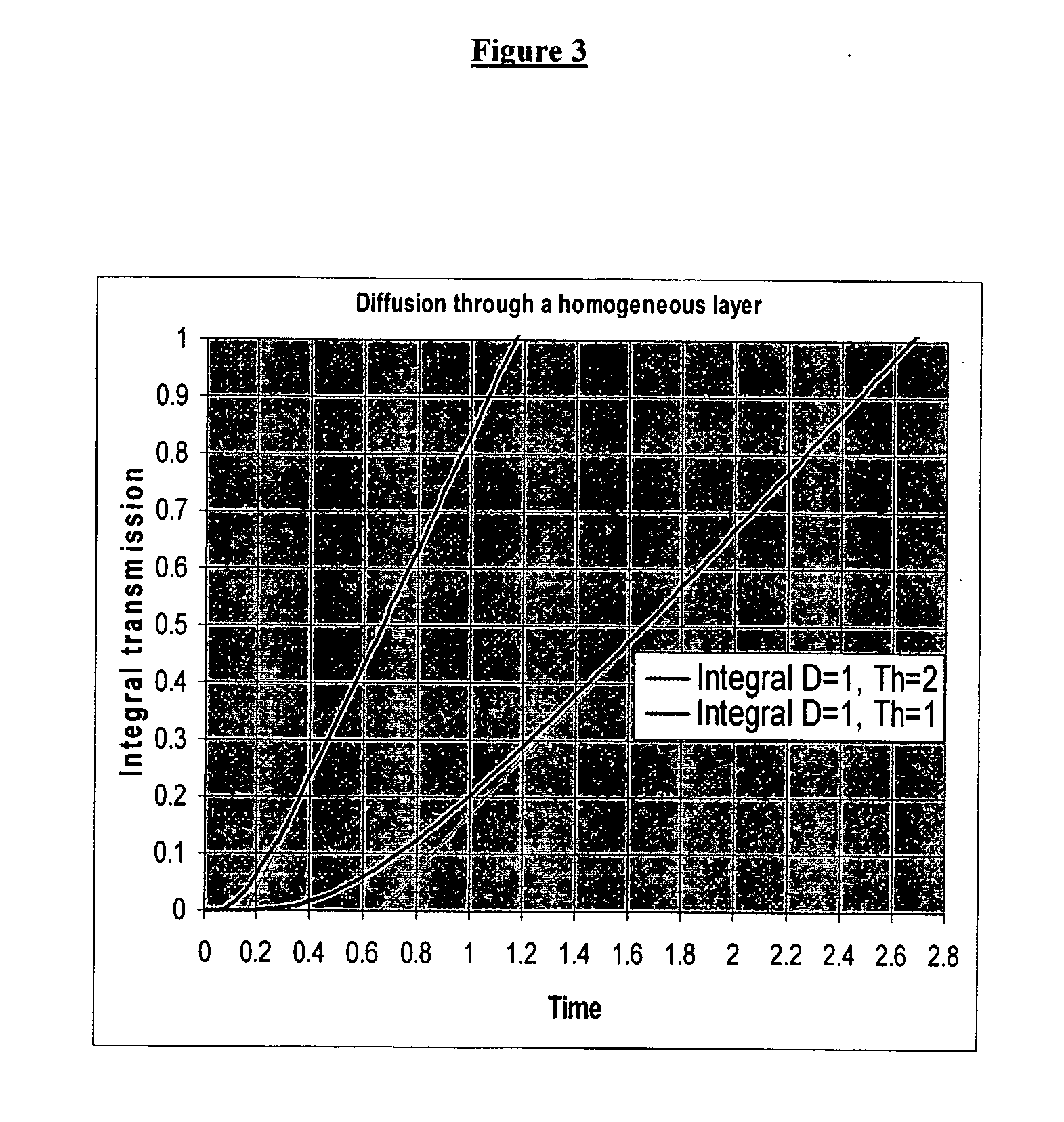
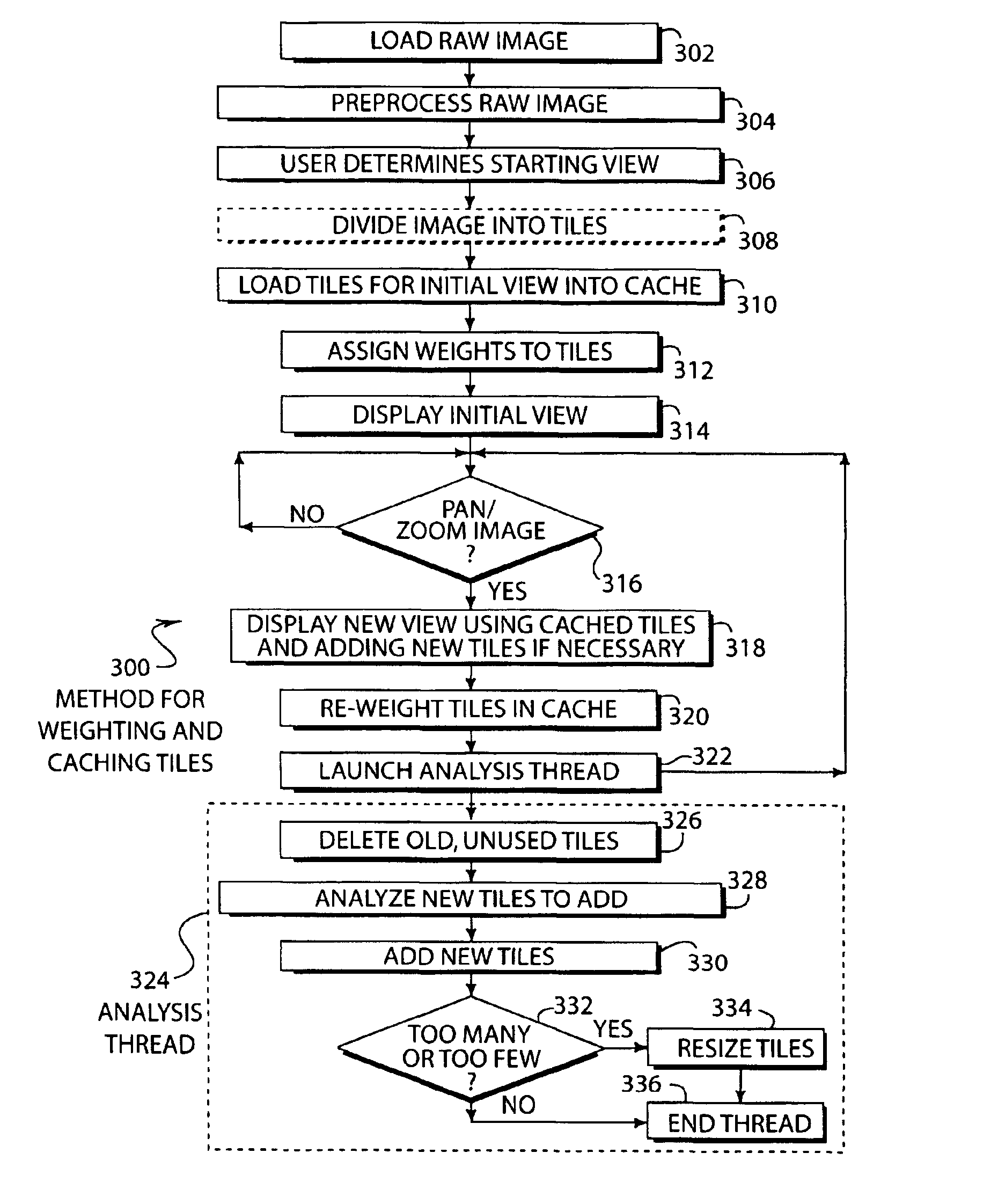
A system and method for caching and rendering an image database enables predictive loading of unrequested portions of the image. A raw image is preprocessed and subdivided into tiles. As a portion of a raw image is displayed on a screen and the user zooms and pans the image, a predicting algorithm determines which additional tiles should be loaded into cache so that the user suffers no lag time as additional tiles not in cache are loaded. The present system and method is adaptable to both raster and vector images.
Owner:SPATIAL DATA TECH
Timed pulsatile drug delivery systems
A pharmaceutical dosage form such as a capsule capable of delivering therapeutic agents into the body in a time-controlled or position-controlled pulsatile release fashion, is composed of a multitude of multicoated particulates (beads, pellets, granules, etc.) made of one or more populations of beads. Each of these beads except an immediate release bead has at least two coated membrane barriers. One of the membrane barriers is composed of an enteric polymer while the second membrane barrier is composed of a mixture of water insoluble polymer and an enteric polymer. The composition and the thickness of the polymeric membrane barriers determine the lag time and duration of drug release from each of the bead populations. Optionally, an organic acid containing intermediate membrane may be applied for further modifying the lag time and / or the duration of drug release. The pulsatile delivery may comprise one or more pulses to provide a plasma concentration-time profile for a therapeutic agent, predicted based on both its pharmaco-kinetic and pharmaco-dynamic considerations and in vitro / in vivo correlations.
Owner:ADARE PHARM INC
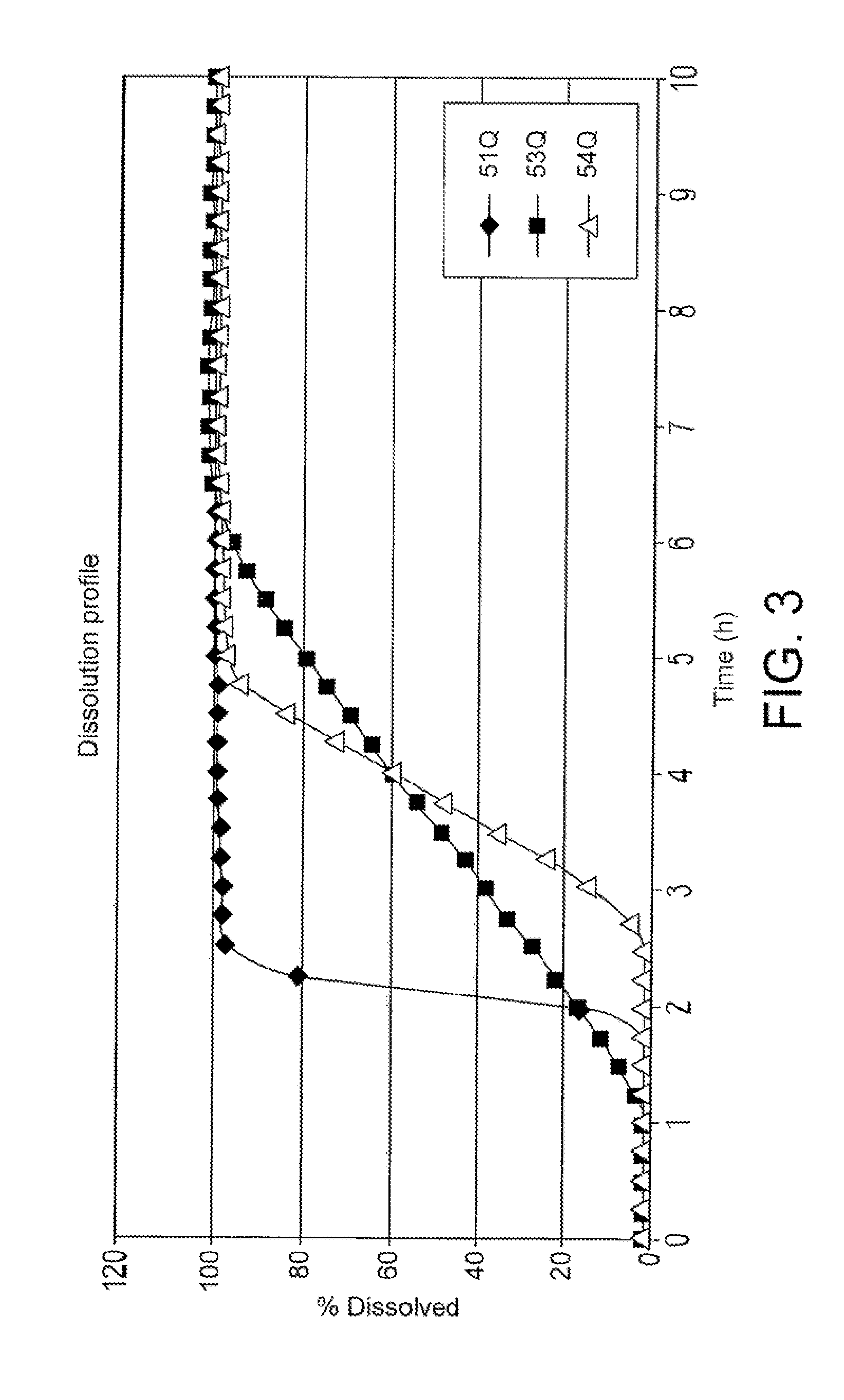
Oral pharmaceutical compositions in timed-release particle form and fast-disintegrating tablets containing this composition
InactiveUS20050287211A1Reduce solubilityProne to feverAntibacterial agentsOrganic active ingredientsWater solubleLag time
The present invention relates to an oral pharmaceutical composition in particle form, which comprises particles that contain a drug at the core of the pharmaceutical composition in particle form; a middle layer that contains two types of water-soluble components, an insolubilizer and an insolubilizing substance; and an outer layer for controlling water penetration that contains a water-insoluble substance. The present invention makes it possible to provide a pharmaceutical composition in particle form for oral use with which initial drug release is suppressed, the drug is quickly released thereafter, and lag time can be controlled as needed, and fast-disintegrating tablets containing this composition.
Owner:ASTELLAS PHARMA INC
System and method for controlling oxynitride removal
InactiveCN102000482AEligible emission indicatorsPrevent escapeDispersed particle separationNegative feedbackTime lag
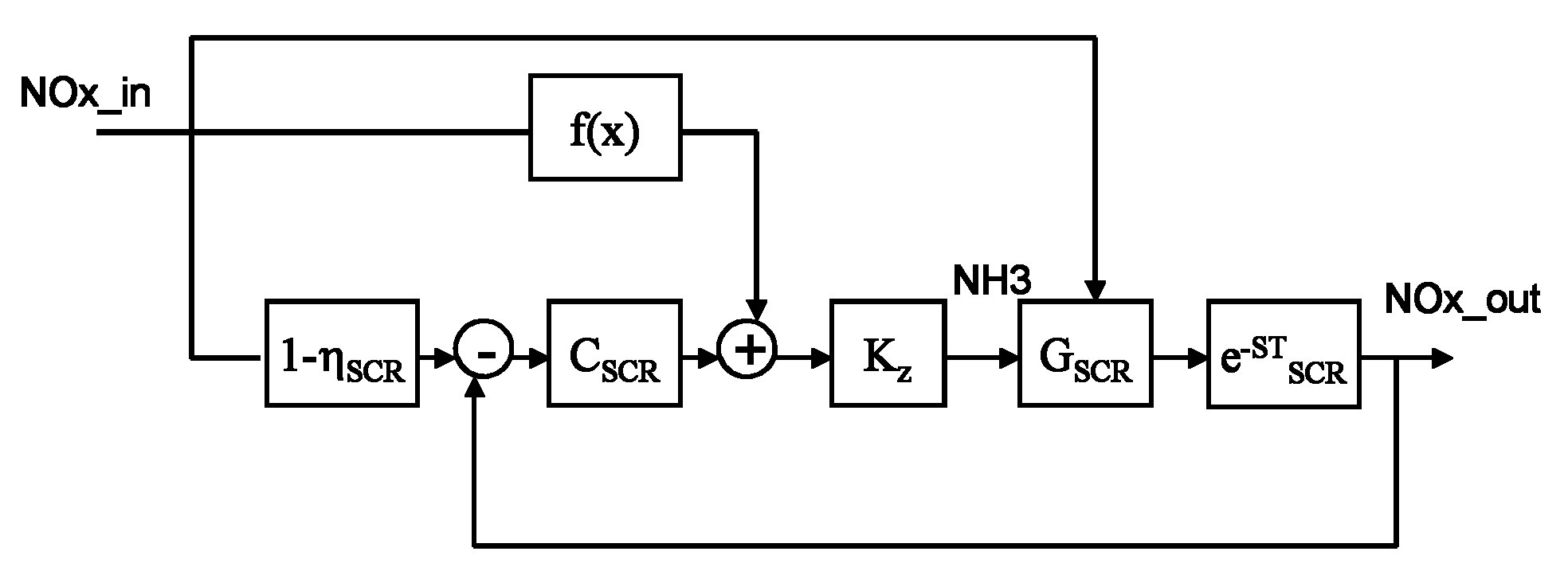
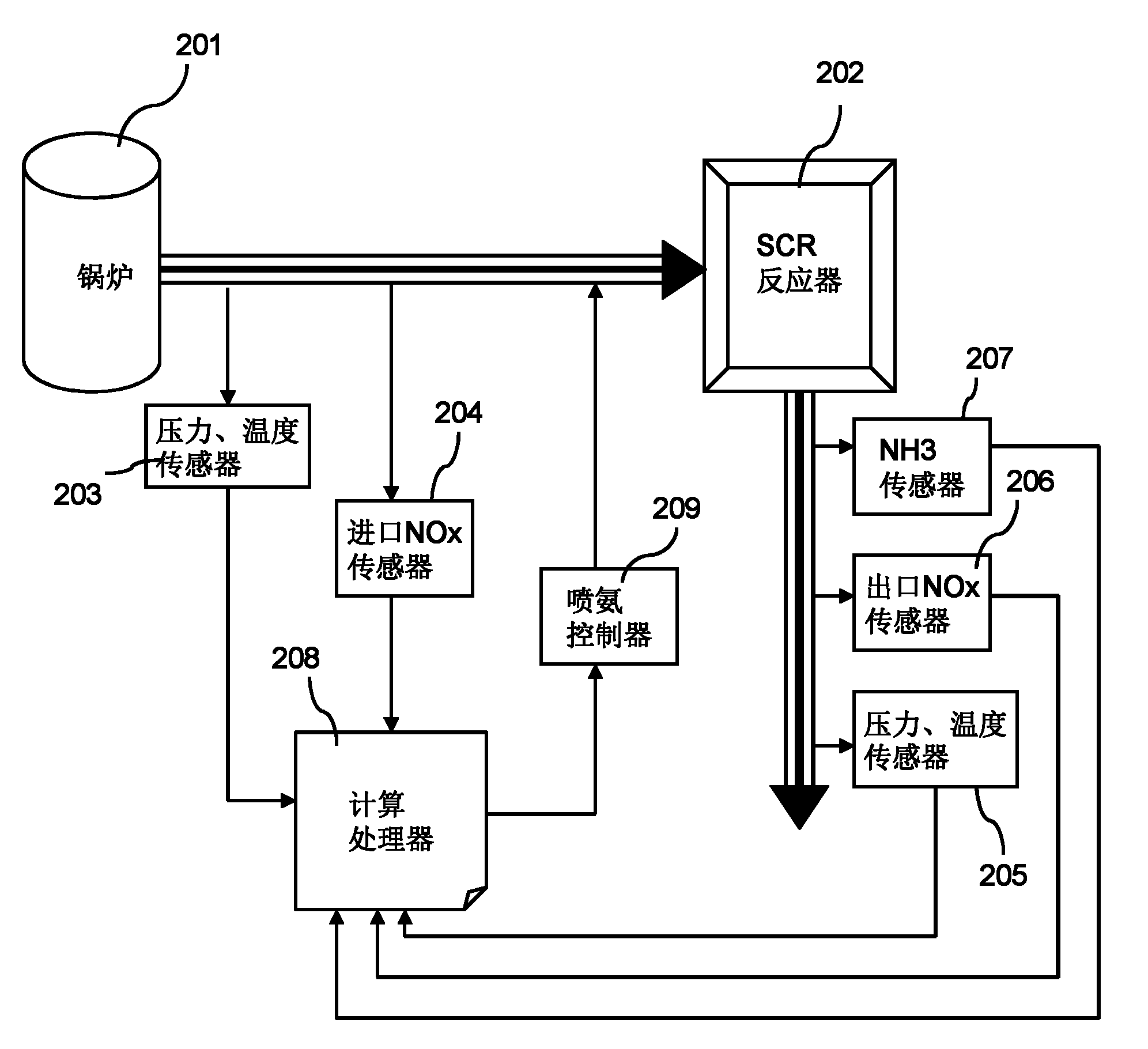
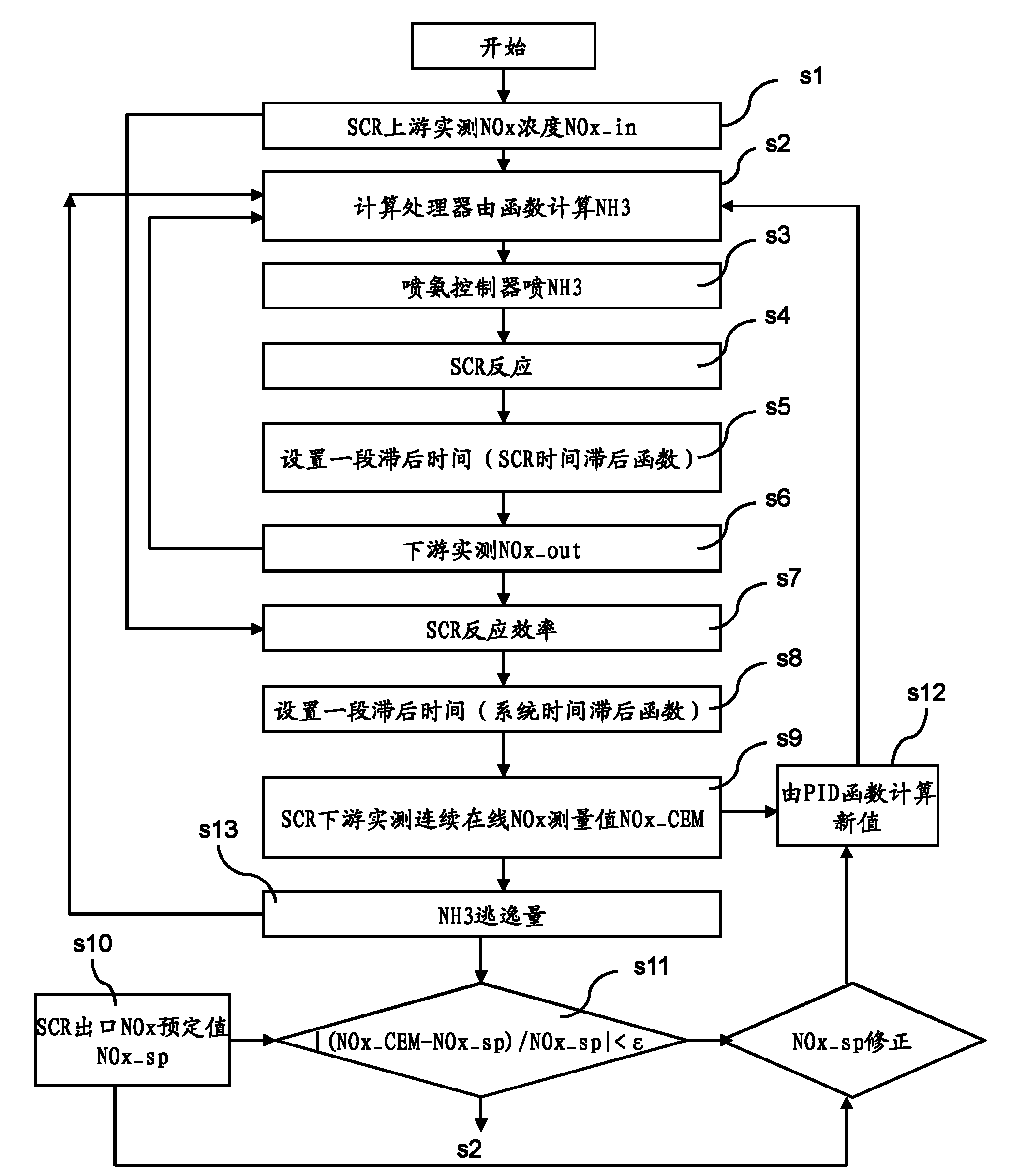
The invention provides a system and a method for optimally controlling ammonia denitration, which are characterized in that according to the content of oxynitride which is detected by an oxynitride sensor at the inlet of a reactor and fed into the reactor, the content of oxynitride which is detected by the oxynitride sensor at the outlet of the reactor and discharged from the reactor, and the continuous online measuring value of the oxynitride at the outlet of the reactor and the escaped ammonia amount detected by an ammonia sensor at the outlet of the reactor, the ammonia-injected amount is calculated according a transfer function; the control system has positive and negative feedback correction effects on feedforward loops, and monitors the continuous online measuring value of the oxynitride, thereby ensuring the implementation of meeting the qualified oxynitride discharge index; the control system is provided with two lag time functions so as to overcome the effect of time lag, eliminate the fluctuations of the oxynitride content and ammonia-injected amount at the outlet of the reactor; and meanwhile, the control system is also provided with an ammonia escape detection sensor for preventing the escape of ammonia.
Owner:无锡科立泰科技有限公司
Advanced control method for thermal power unit boiler turbine coordination system
InactiveCN101488022ADecrease in weight coefficientIncreased weight coefficientMachines/enginesEngine componentsUnit loadSteam pressure
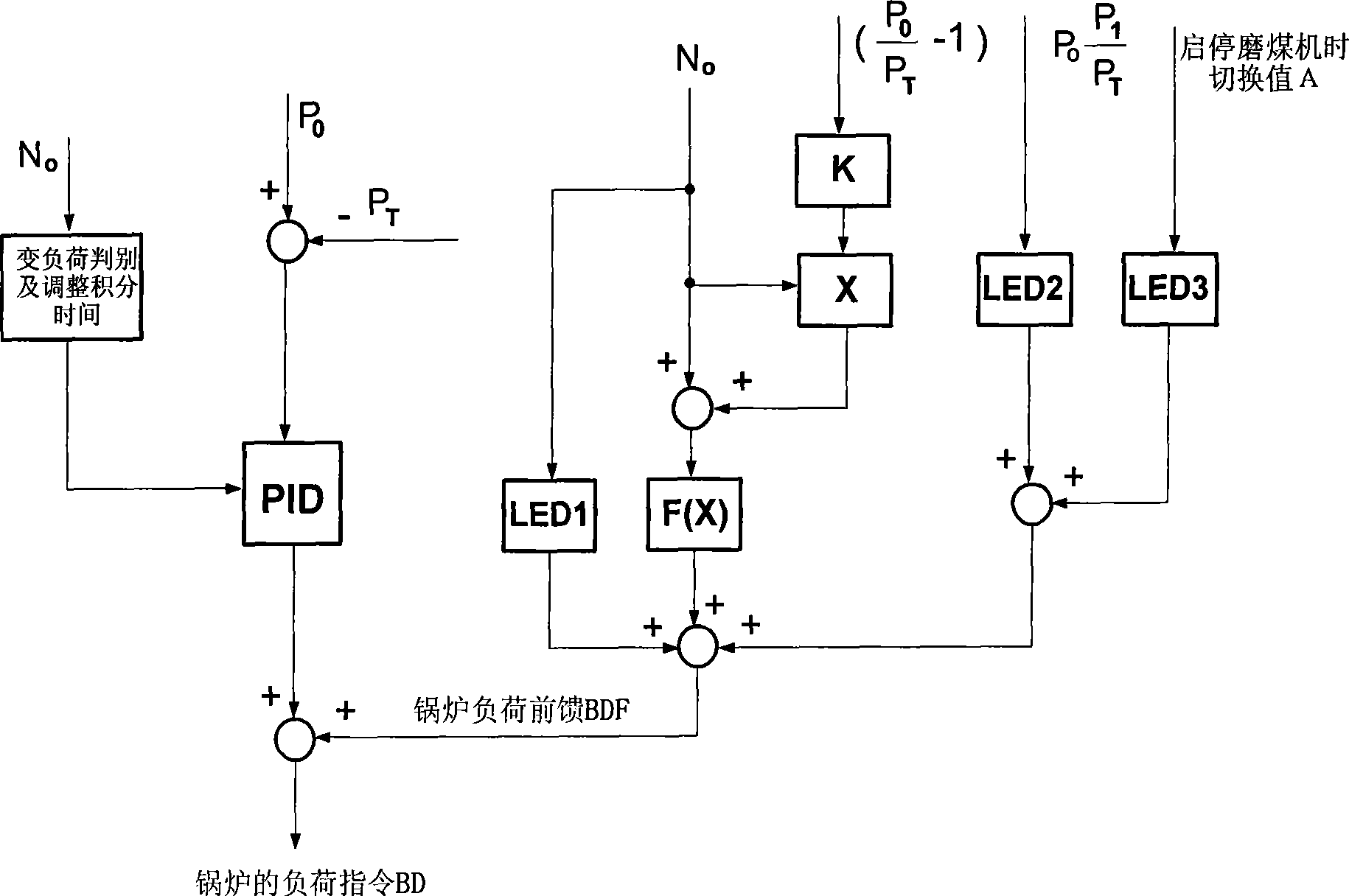
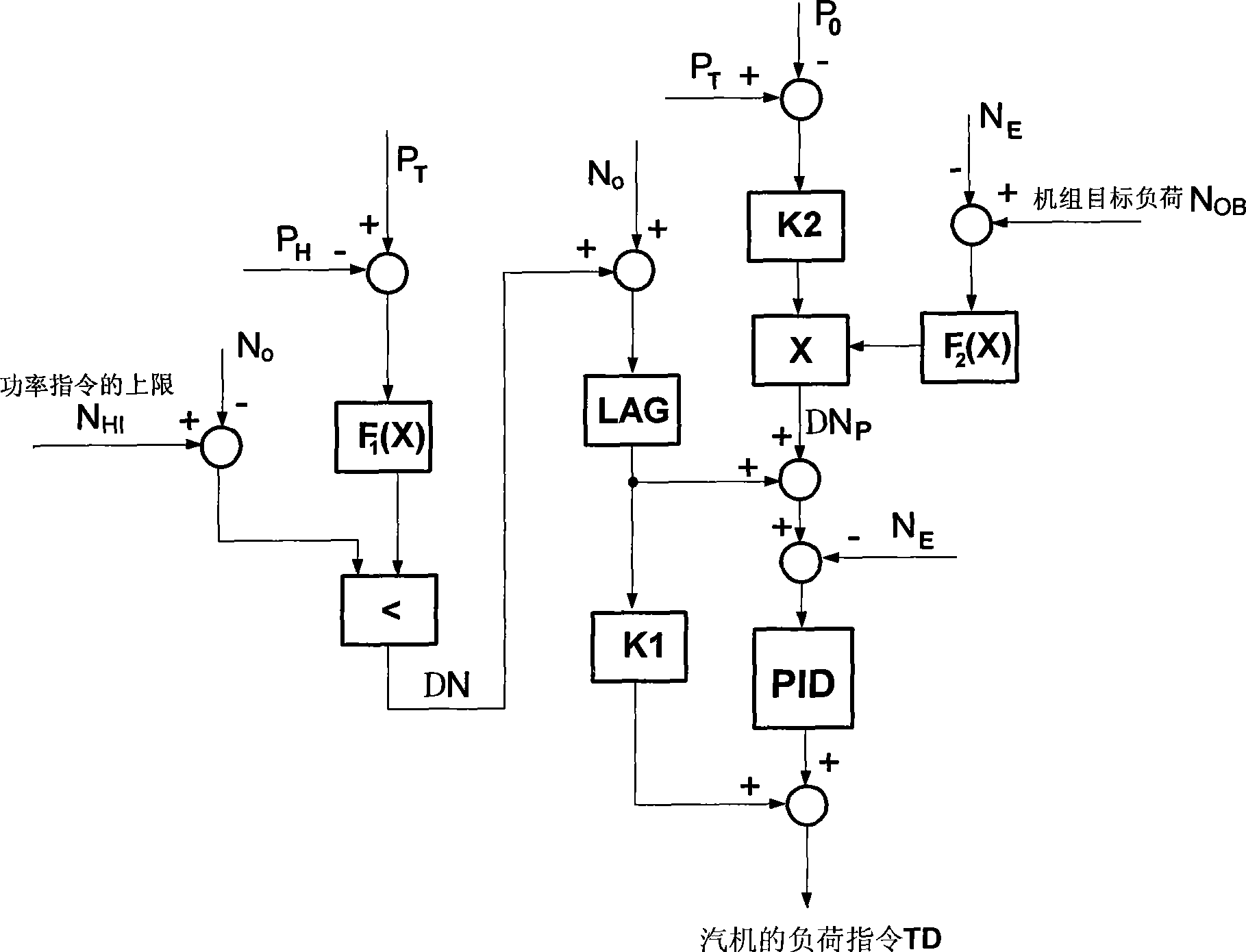
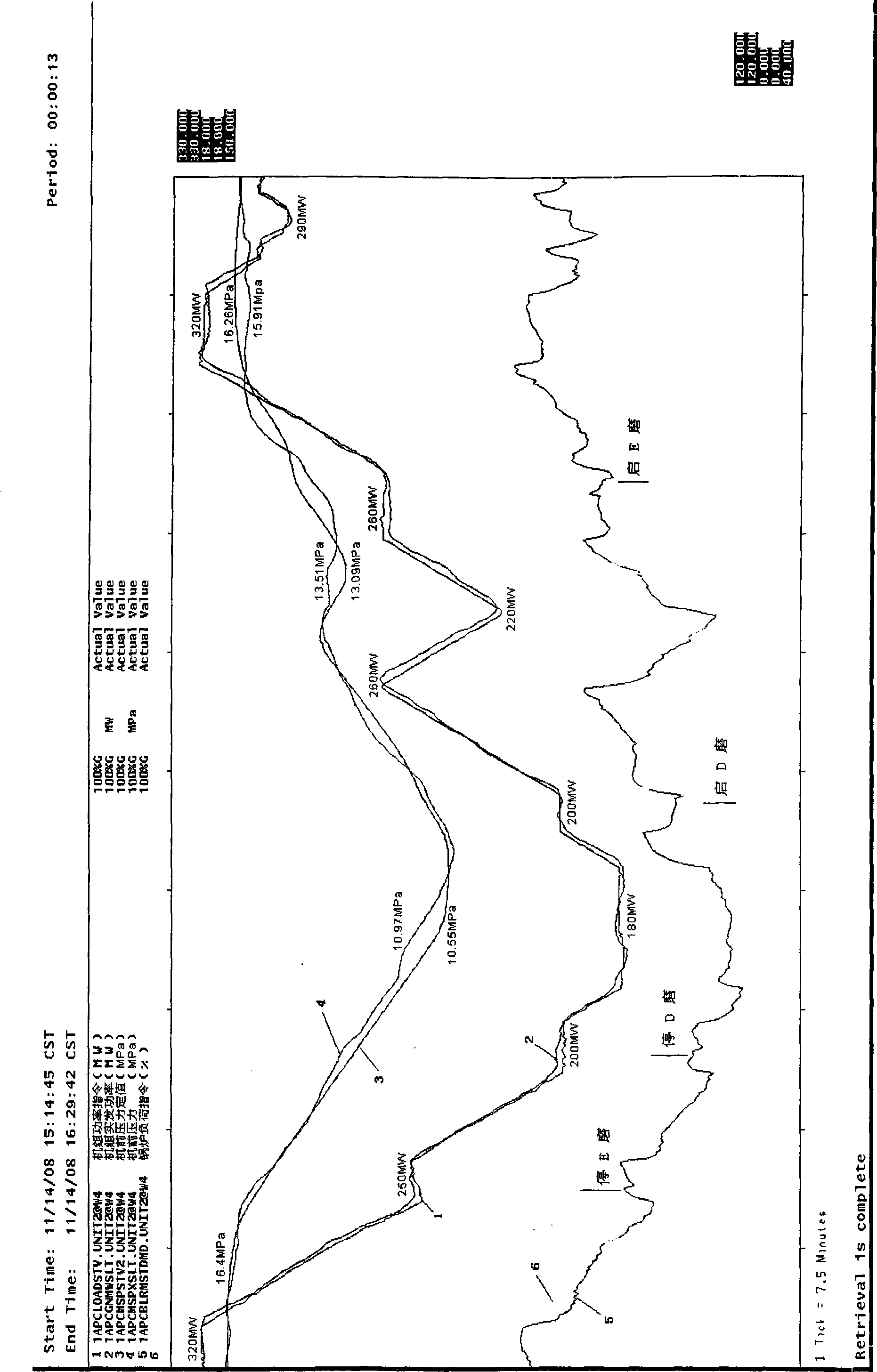
The invention provides an advanced control method for a coordination system of a boiler and a steam turbine of a thermal power unit, which applies advanced dynamic feedforward technology to the feedforward design of a main control system of the boiler so as to effectively accelerate governing speed of a boiler load command in a load varying process of the unit. In the load varying process of the unit, oscillation tendency of the main control system of the boiler can be inhibited through reducing integral action of a regulator. In the main control system of the steam turbine, a unit load command N0 is subjected to inertia LAG time-delay, and protection design is carried out on main steam pressure. The method ensures that the boiler and the steam turbine can operate under a more coordinated mode and ensures that the thermal power unit has faster ascending and descending speed of load and more stable variation of the main steam pressure, wherein the ascending and descending speed of the load of the unit reaches 3.0 percent Pe / min in actual application, the main steam pressure is stable without overshoot, and the pressure deviation is in an acceptable range.
Owner:SOUTHEAST UNIV
GNSS based control for dispensing material from vehicle
ActiveUS8634993B2Easy to correctEconomical and accurateAnalogue computers for trafficSpraying apparatusSpray nozzleLag
A spray control method employs a spray vehicle including a material tank, a pump communicating with the tank, and nozzles of a spray boom communicating with the pump. A GNSS receiver mounted on the vehicle and interfaced to a controller tracks its position in relation to stored position coordinates of field boundaries separating spray zones from spray exclusion zones. The tank is activated and deactivated by the controller to retain spray of the material within the spray zones and to prevent spray of the material in the exclusion zones, by processing an offset of the spray nozzles from the receiver, the spray range of the nozzles, spray turn-on and turn-off lag times, and the velocity of the spray vehicle, all in relation to the field boundaries. An alternative embodiment individually controls spray from the nozzles by using associated valves interfaced to the controller.
Owner:AGJUNCTION
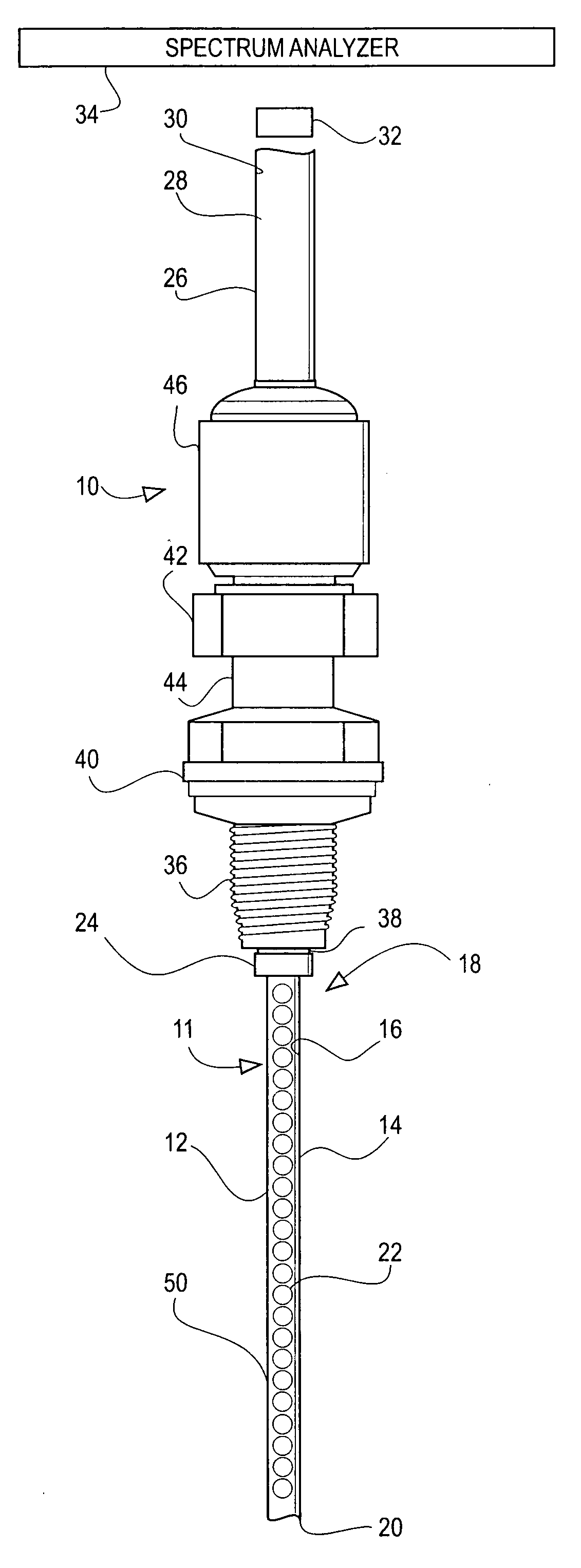
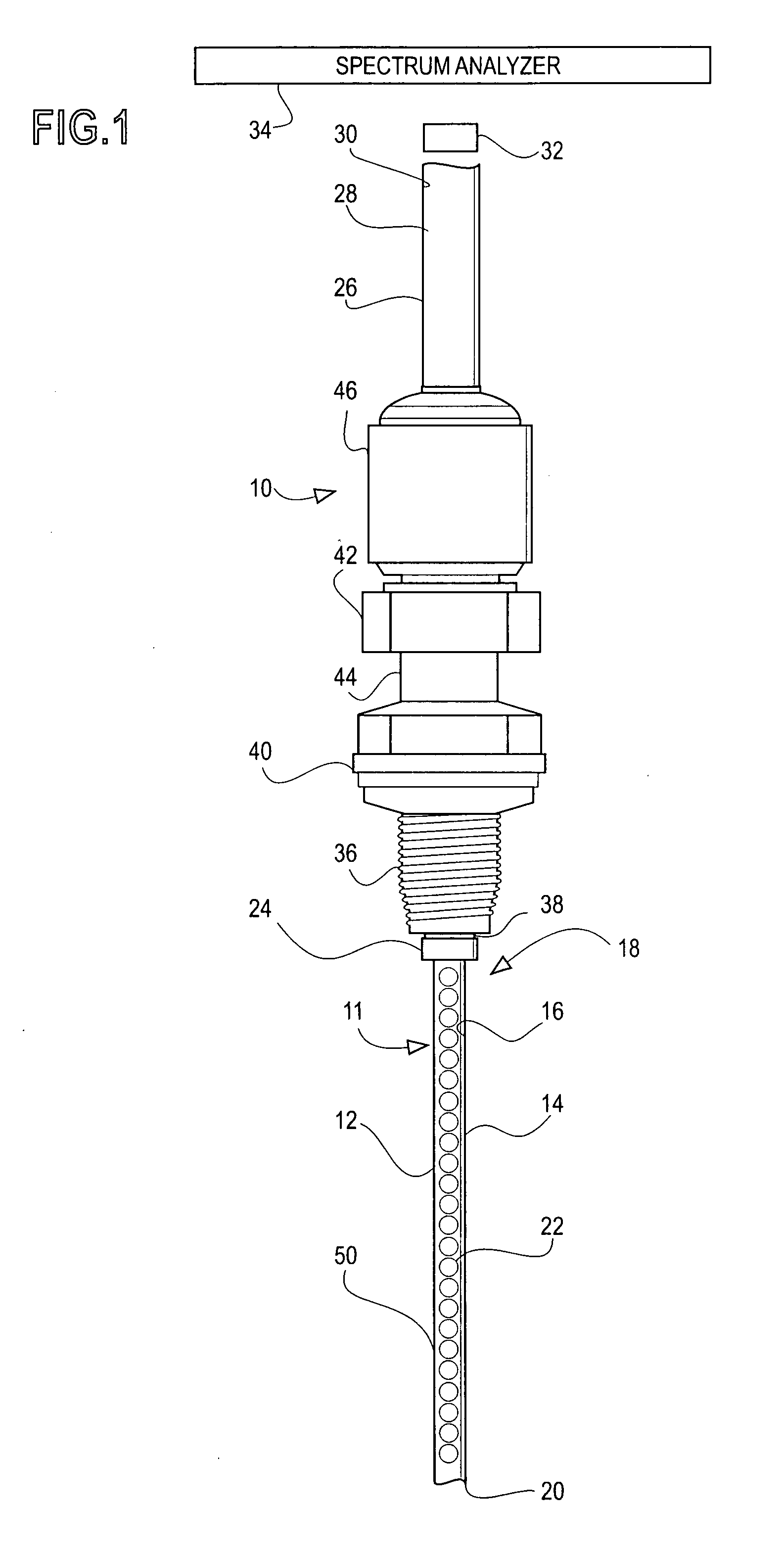
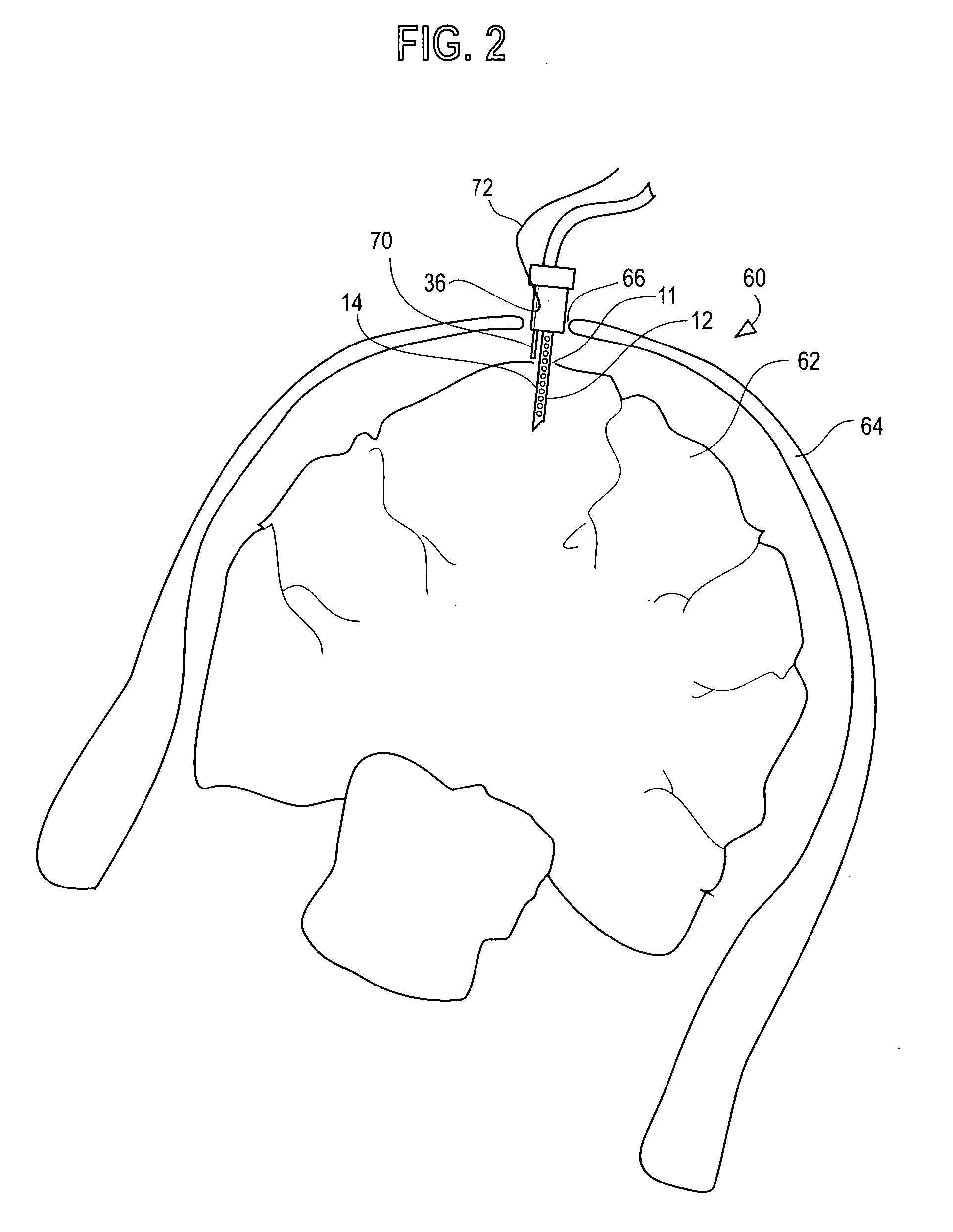
Water content probe
Owner:BRAIN CHILD FOUND
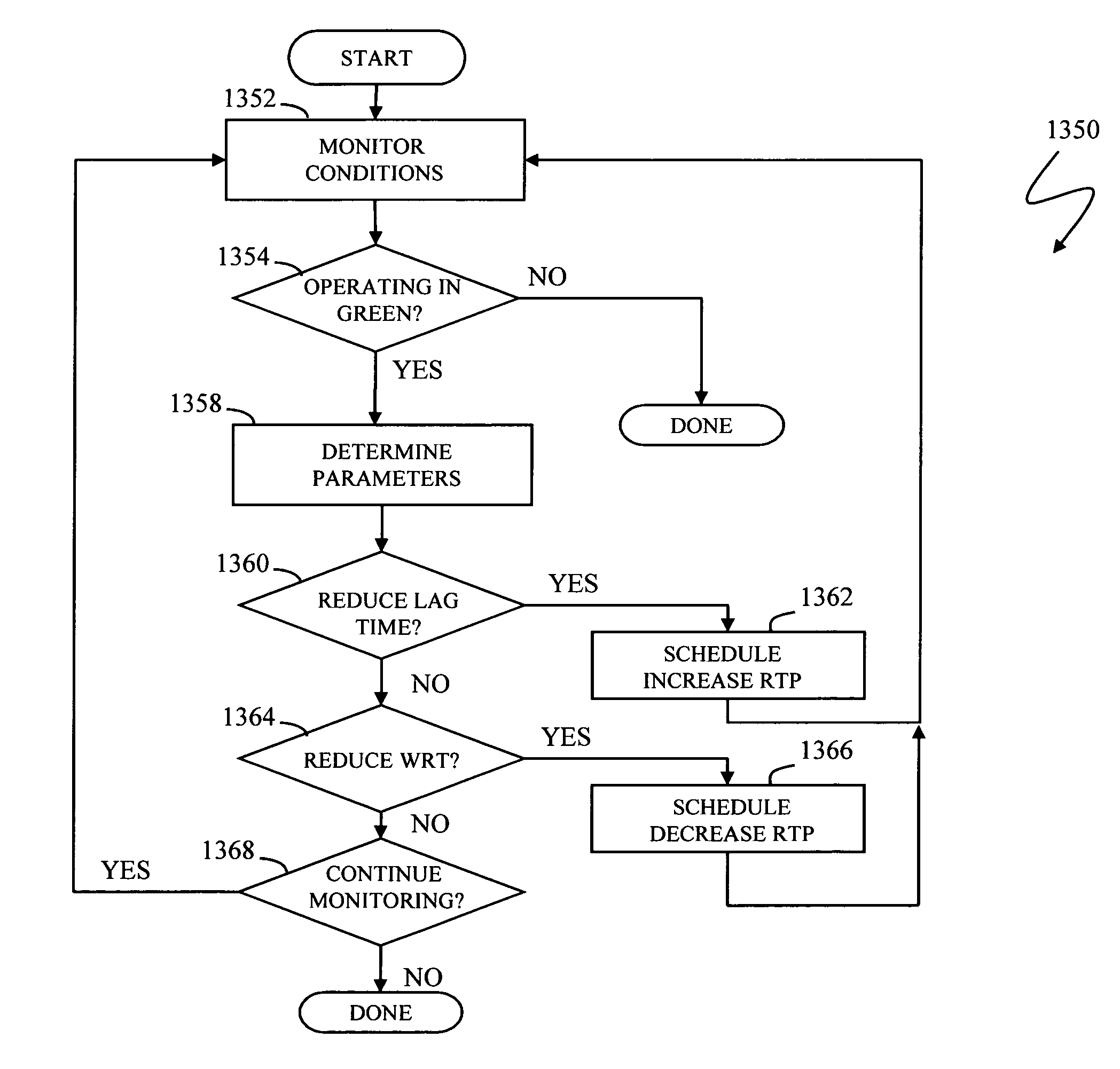
Write pacing
A system for controlling the pacing of host data writes in response to changing system conditions allows for the use of variably controlled delays that facilitate asynchronous replication with bounded lag and smooth steady-state performance. Adding delay to the writes slows down the host and dampens activity spikes. A first storage device receives a write request, transmits data to a second storage device, and acknowledges the write request. An amount of additional write response time is controlled between when the write request is received and when the write request is acknowledged by the first storage device, where the amount of additional write response time is controlled according to system parameters including an amount of lag time between when the write request is received and when the write request is committed at the second storage device.
Owner:EMC IP HLDG CO LLC
Method of treating insomnia
A method of treating insomnia comprising administering to a subject a formulation including zaleplon, wherein the formulation is adapted to release the zaleplon after a lag time of at least about one hour after administration of the formulation, and during which substantially no drug substance is released; provide a time of peak plasma concentration of about 3 hours to about 6 hours after administration; provide an elimination half-life after the time of peak plasma concentration of about 0.5 hours to about 0.3 hours; and provide an area under the curve of about 70 ng·h / mL to about 90 ng·h / mL.
Owner:SOMNUS THERAPEUTICS
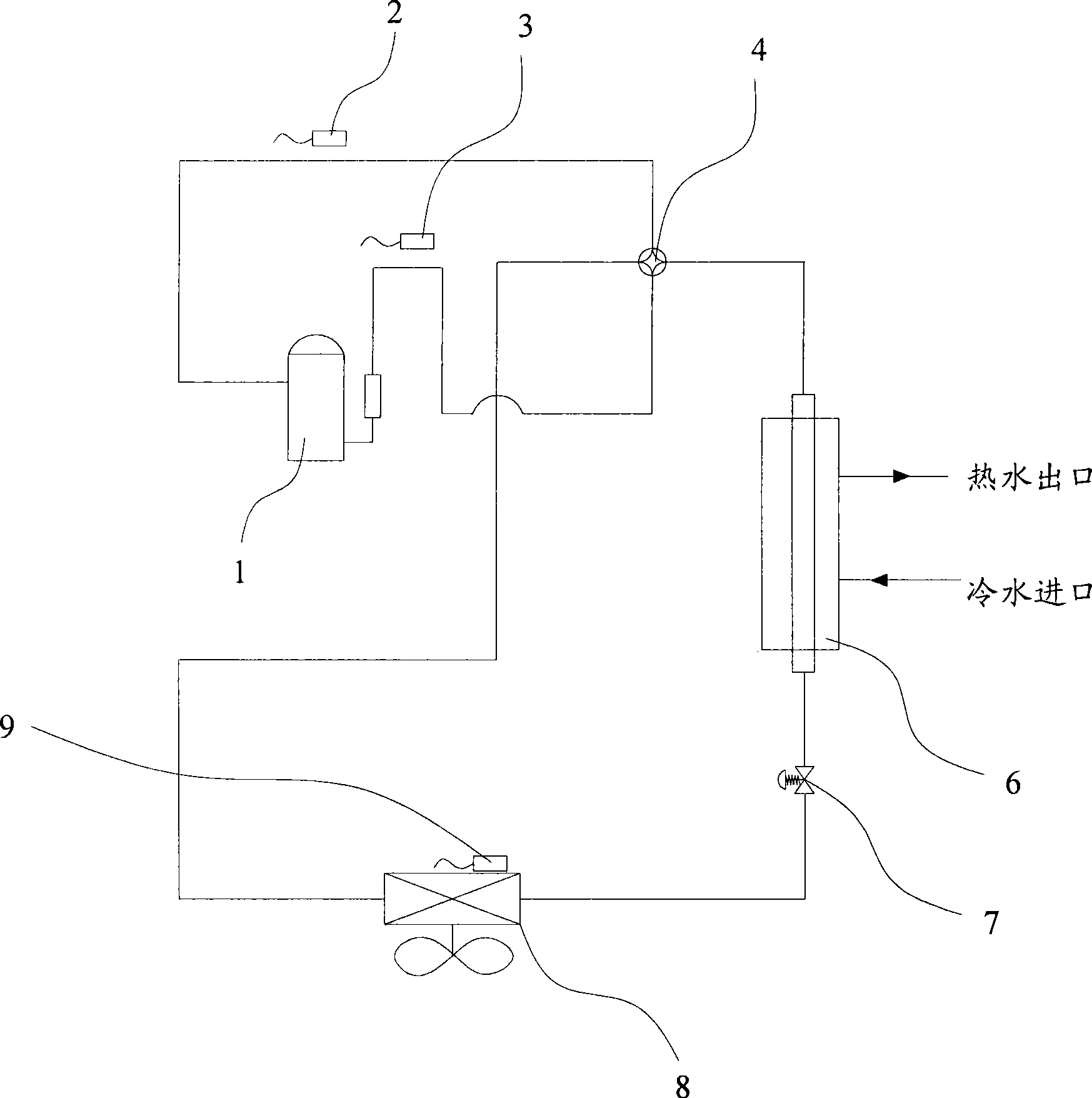
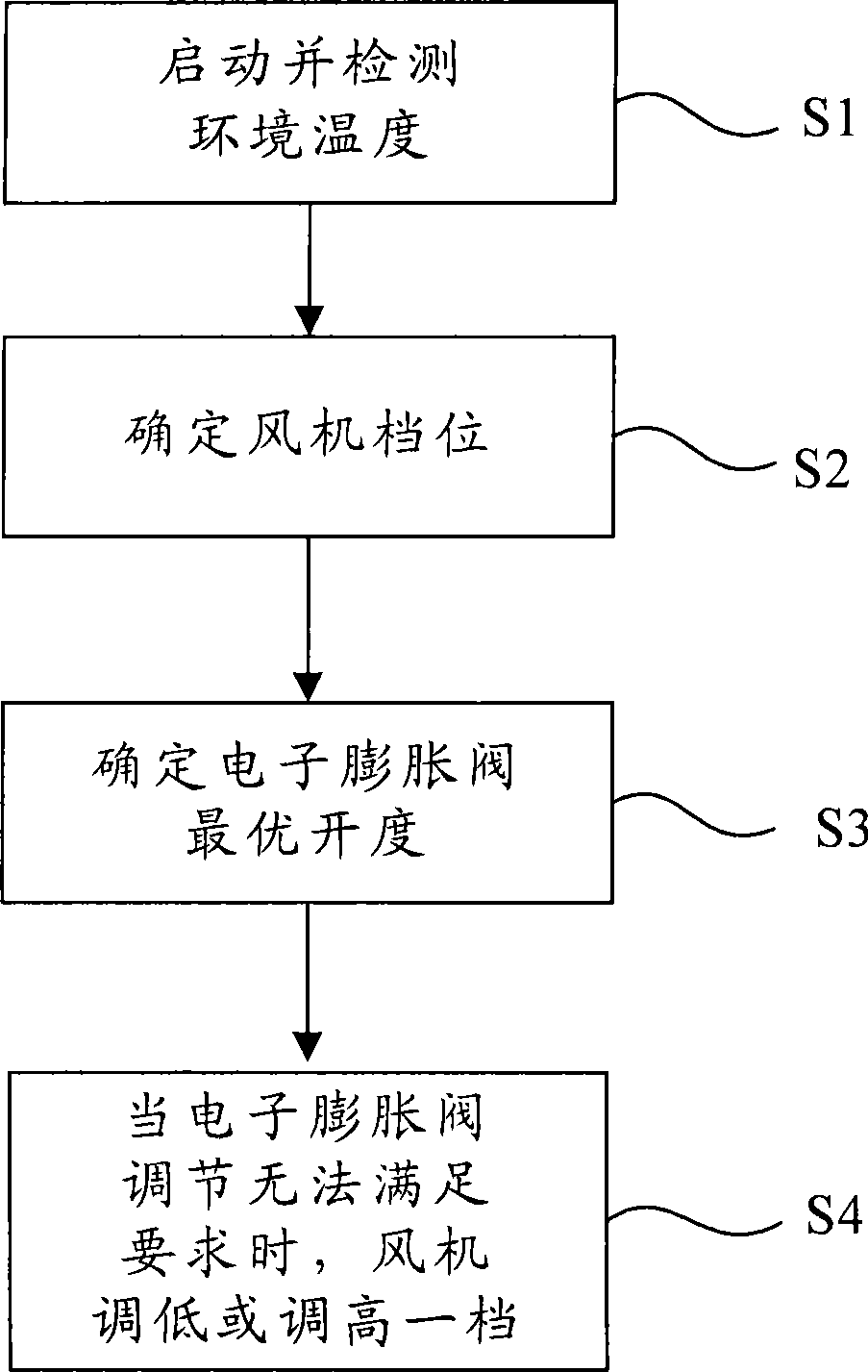
Control method for electronic expansion valve, self-adaptive control method for heat pump, and device thereof
ActiveCN101446463ARealize automatic controlGuaranteed uptimeFluid circulation arrangementRefrigeration safety arrangementSelf adaptiveProtection system
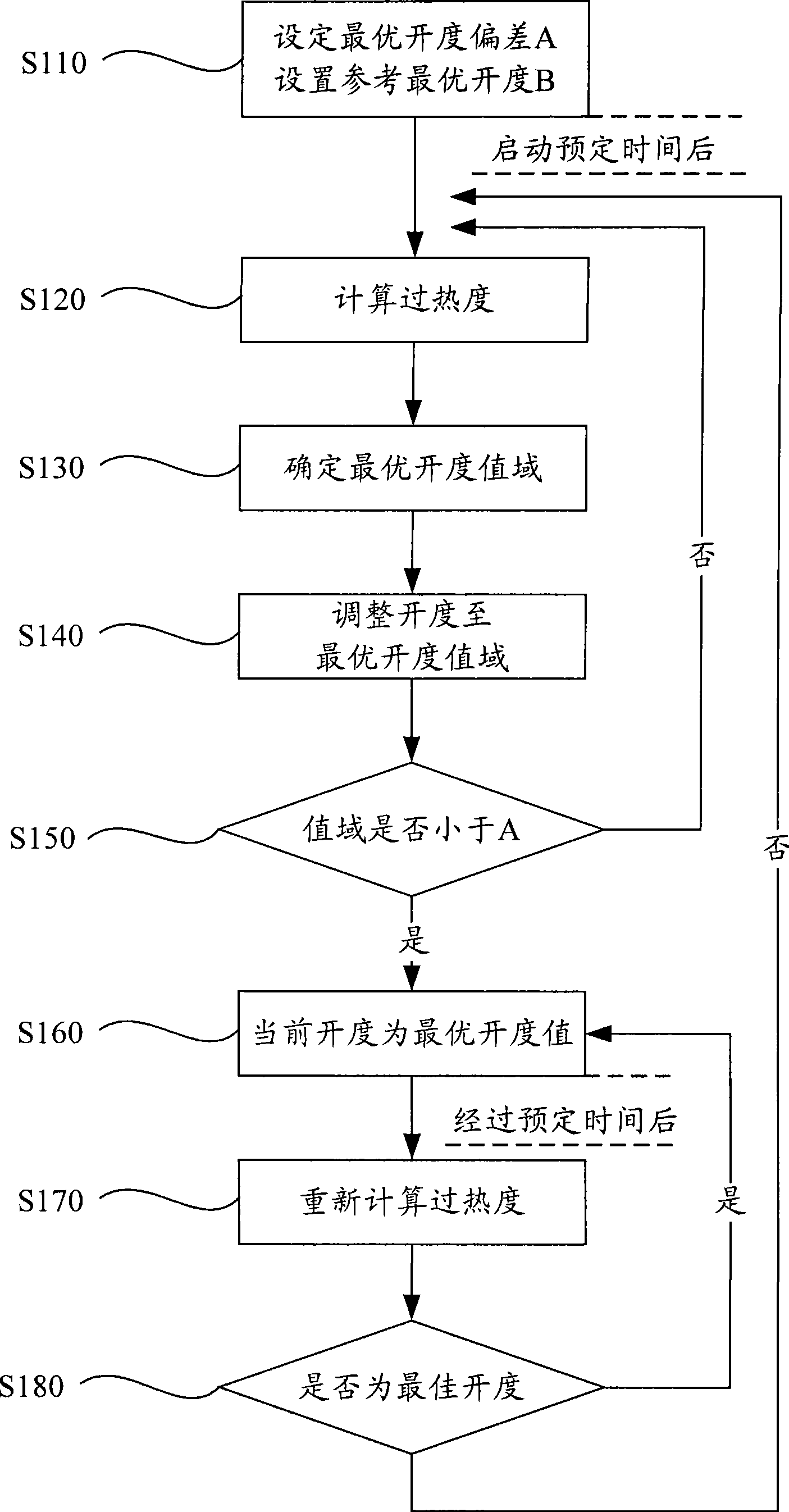
The invention discloses a control method for an electronic expansion valve, a self-adaptive control method for a heat pump, and a device thereof; wherein, the control method for the electronic expansion valve comprises the steps as follows: S110: the electronic expansion valve is started and initiated; a controller arranges the openness of the electronic expansion valve as an initial value; S120: after starting prearranged time, the controller determines the practical over-heat degree of the electronic expansion valve; S130: controller compares the practical over-heat degree with a destination over-heat degree so as to determine the optimum openness value domain of the electronic expansion valve, and S140: the controller adjusts the openness of the electronic expansion valve to the optimum openness value domain. Therefore, automatic regulation of refrigerant flux and throttling degree of the heat pump device according to the working conditions can be ensured, the system is protected and runs at a best state, the optimum openness of the electronic expansion valve can be quickly gained, the lag time is greatly reduced, and the throttling degree of the system can be adjusted according to the running state.
Owner:GREE ELECTRIC APPLIANCES INC
Systems, methods and computer program products to determine useful relationships and dimensions of a database
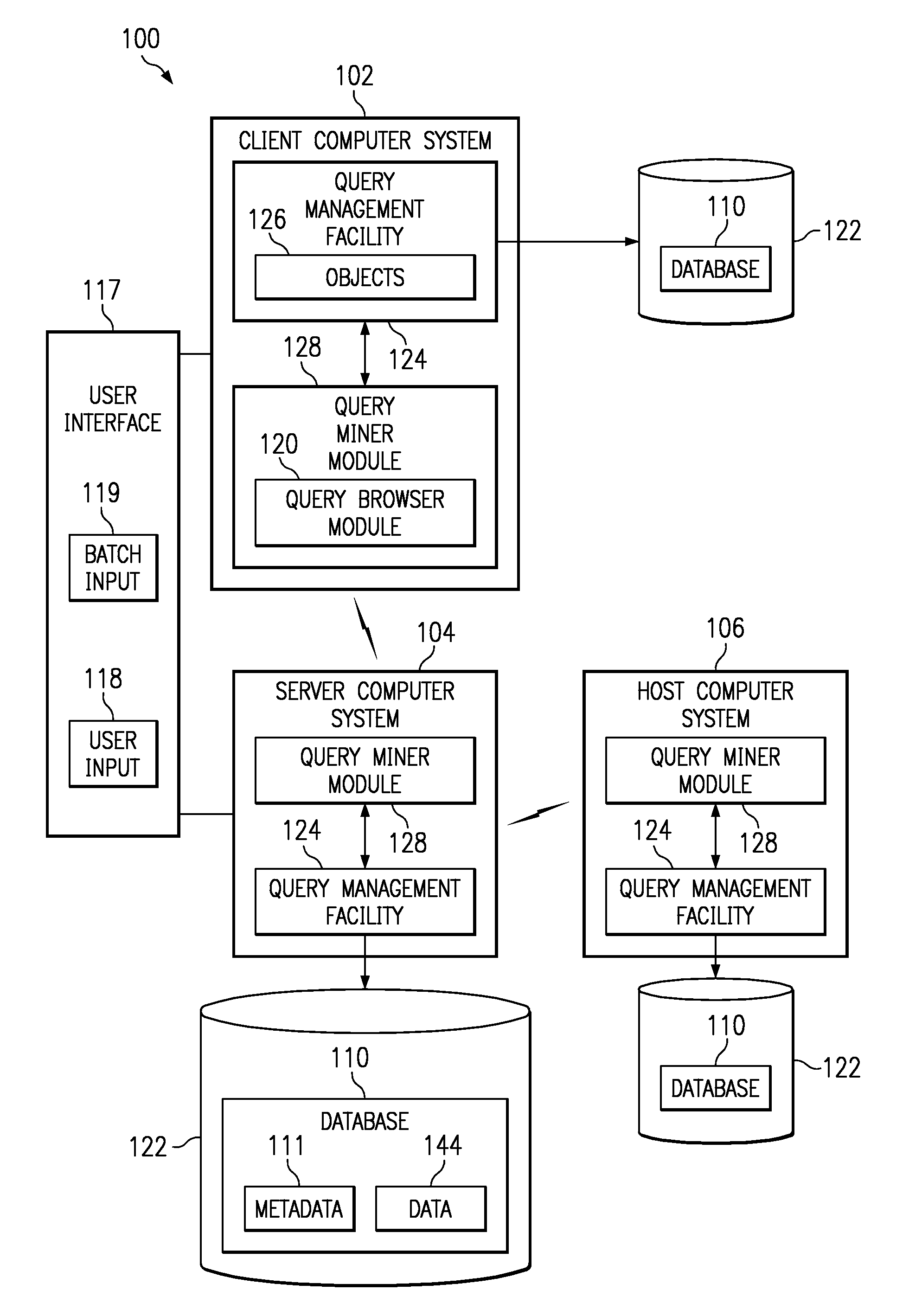
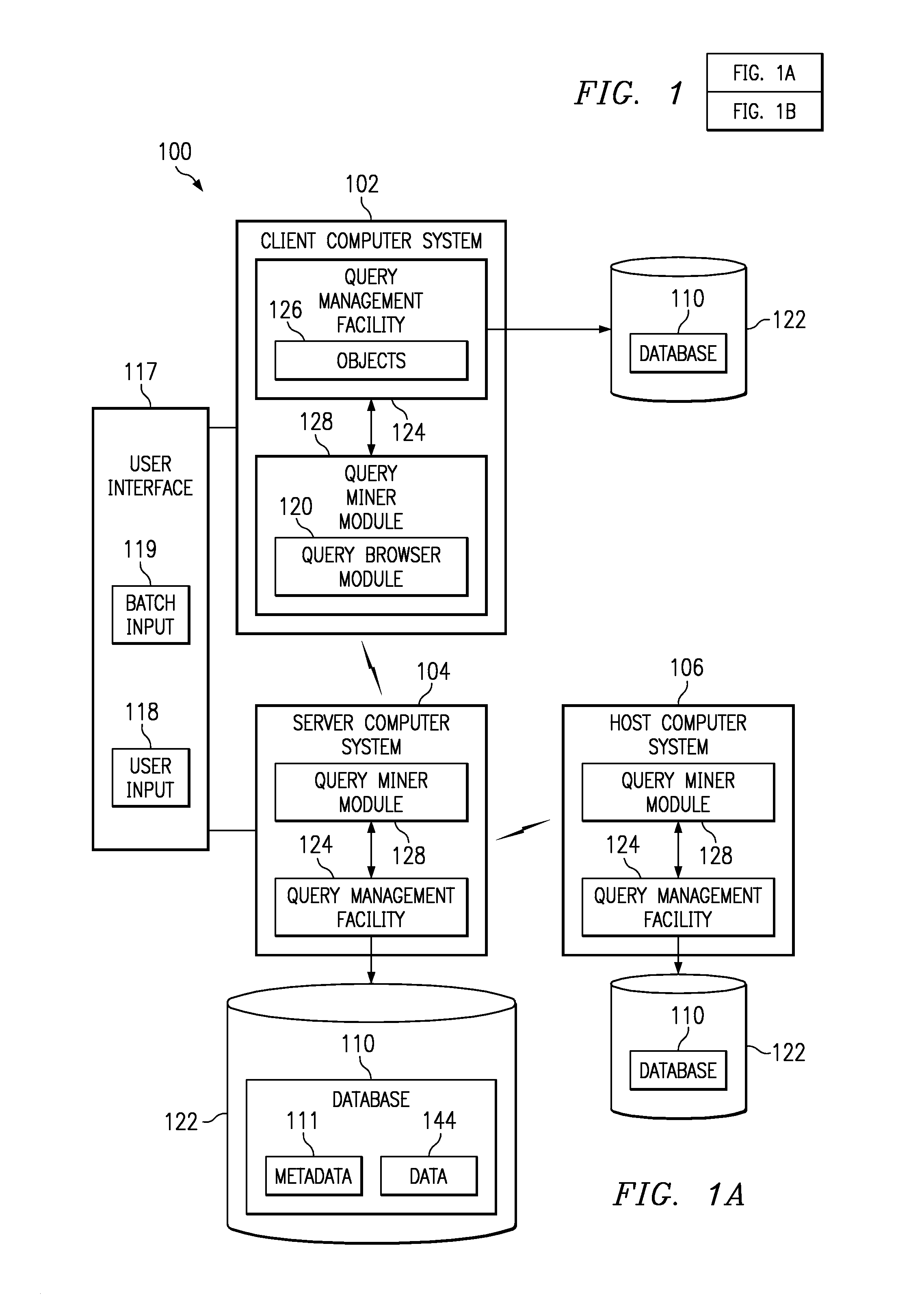
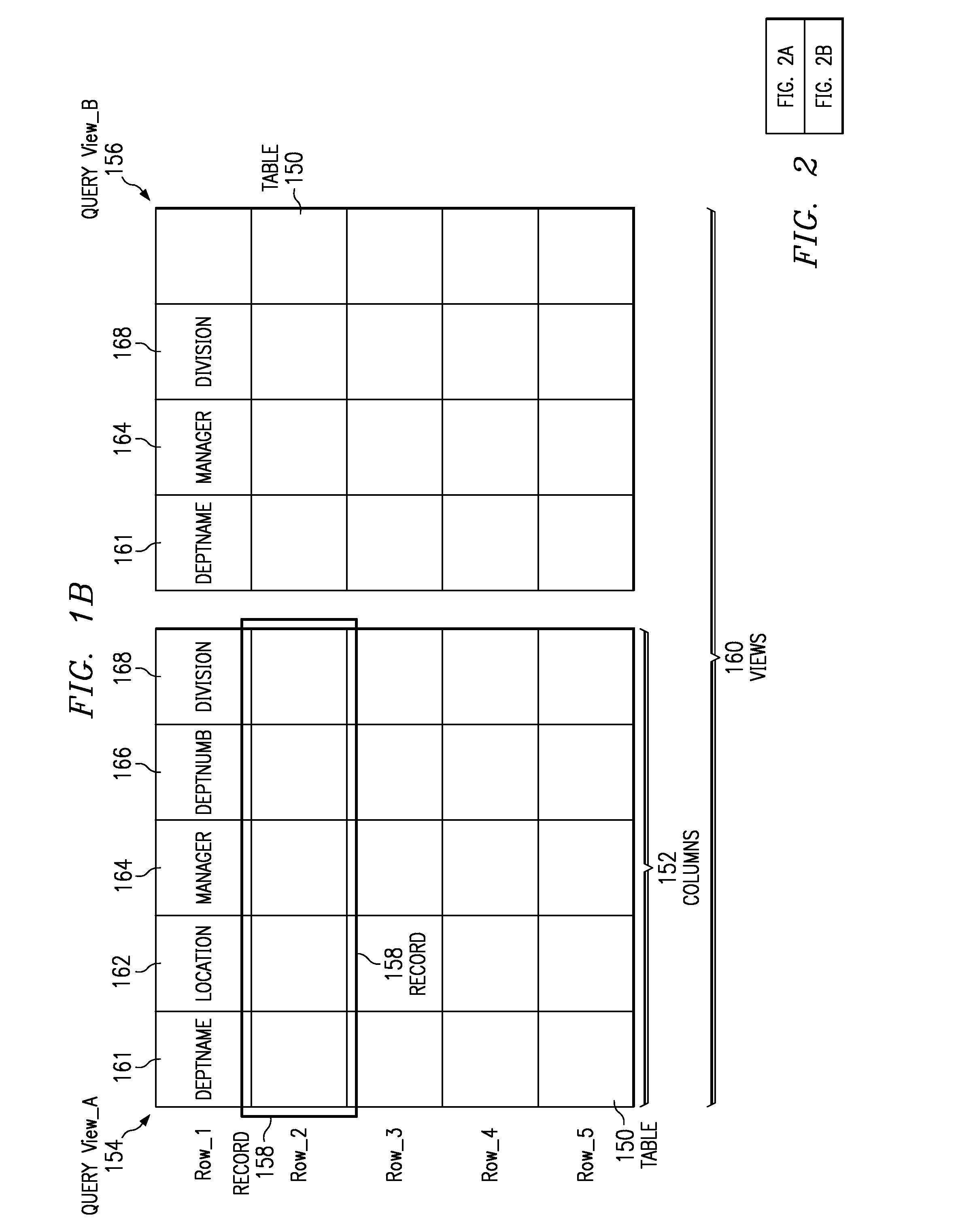
InactiveUS6947929B2Efficiently determinedQuick navigationData processing applicationsDigital data information retrievalData miningComputer program
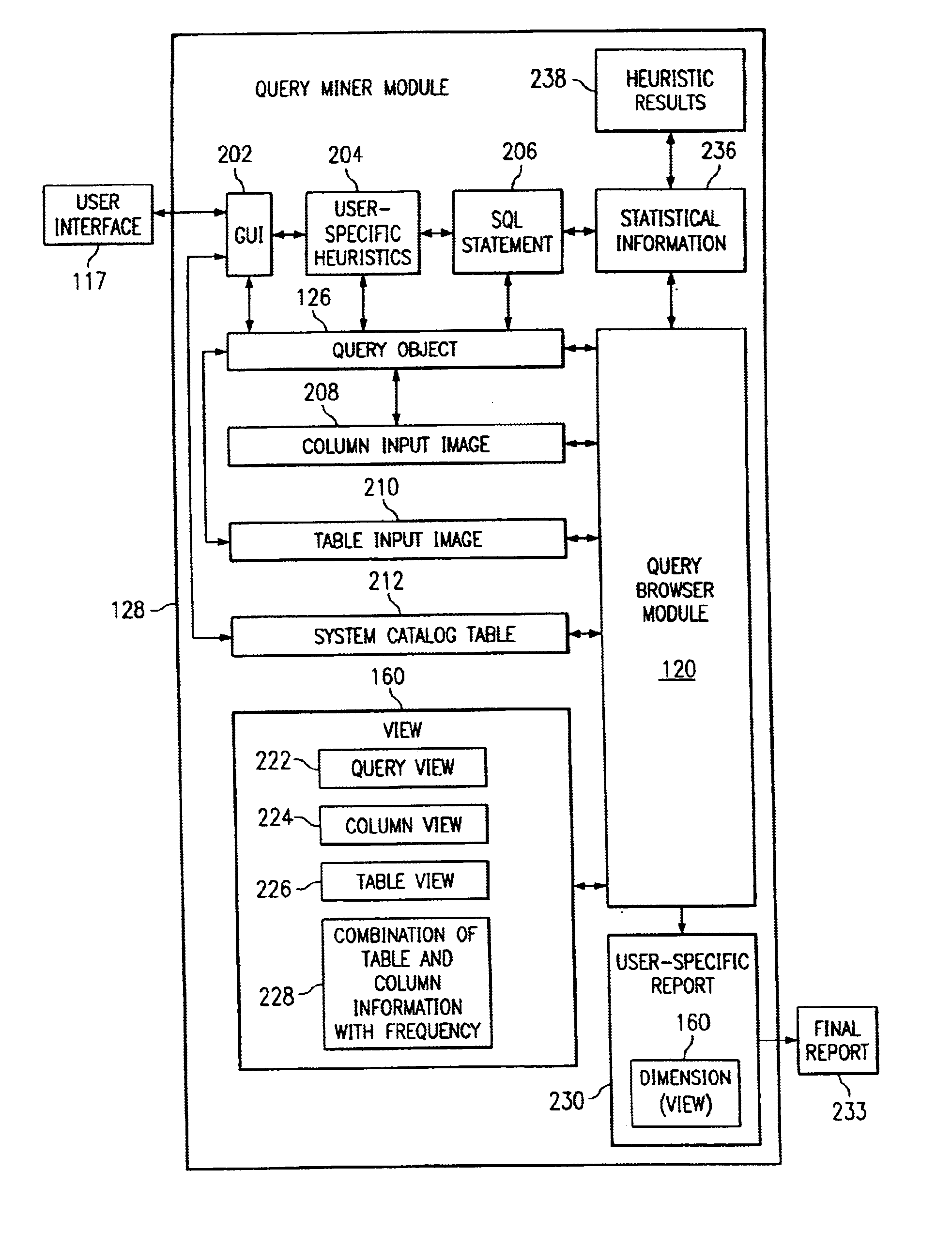
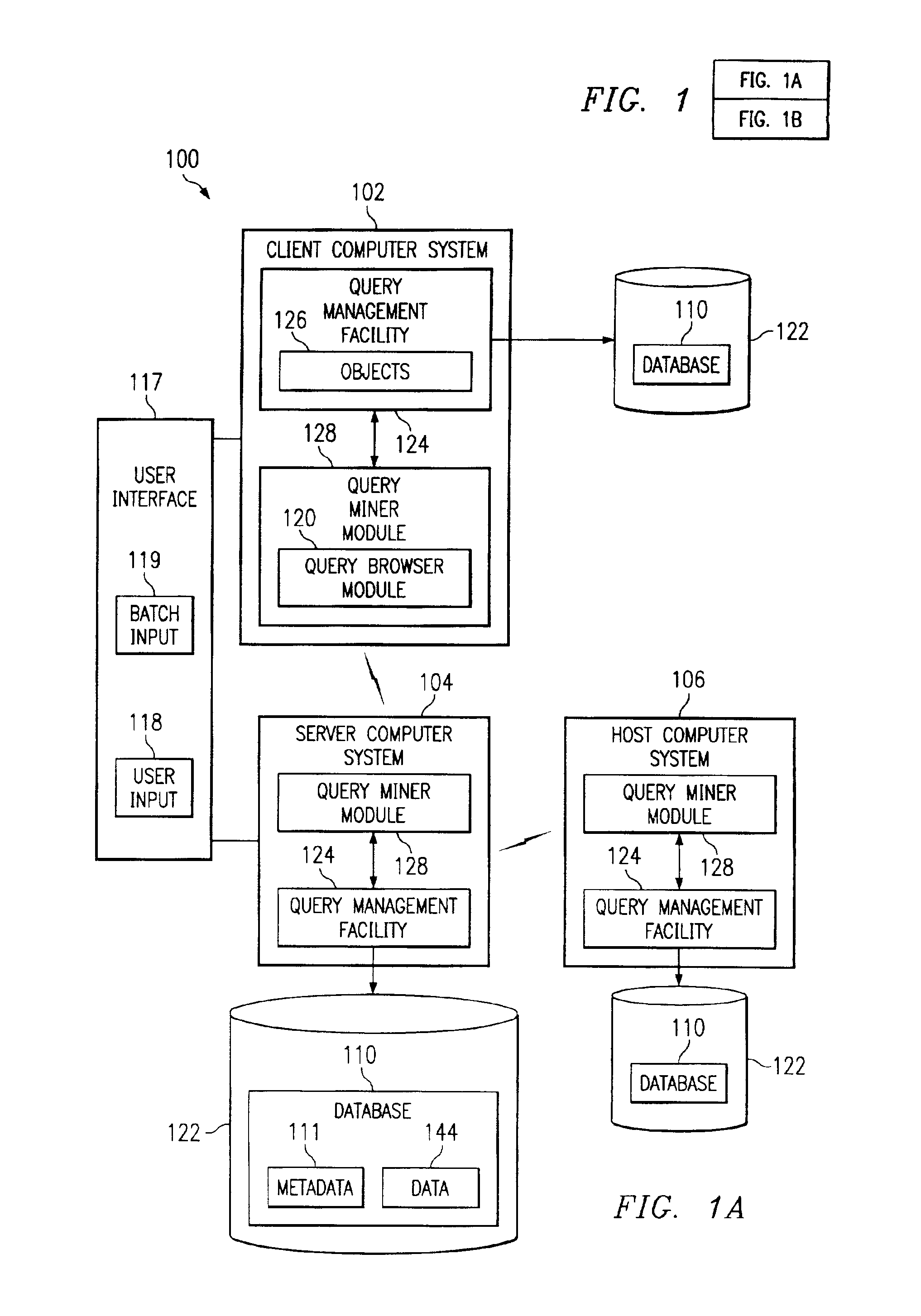
Systems, methods, and computer products that efficiently determine useful dimensions associated with a database when employing OLAP processing techniques. The present invention enables a user to see at a glance in which queries certain columns and tables are used. The present invention may be implemented with a query miner module that may include a query browser module that improves browsing through queries and their components over the past. The preferred embodiment of the present invention provides an easy to use graphical interface showing the queries, tables, and columns in a tree structure. Further, the preferred embodiment of the present invention presents information about relationships and dimensions associated with a database and about columns, tables, and queries to the user without discernable lag time between the user's request and the generated information.
Owner:IBM CORP
Pulsatile release histamine H2 antagonist dosage form
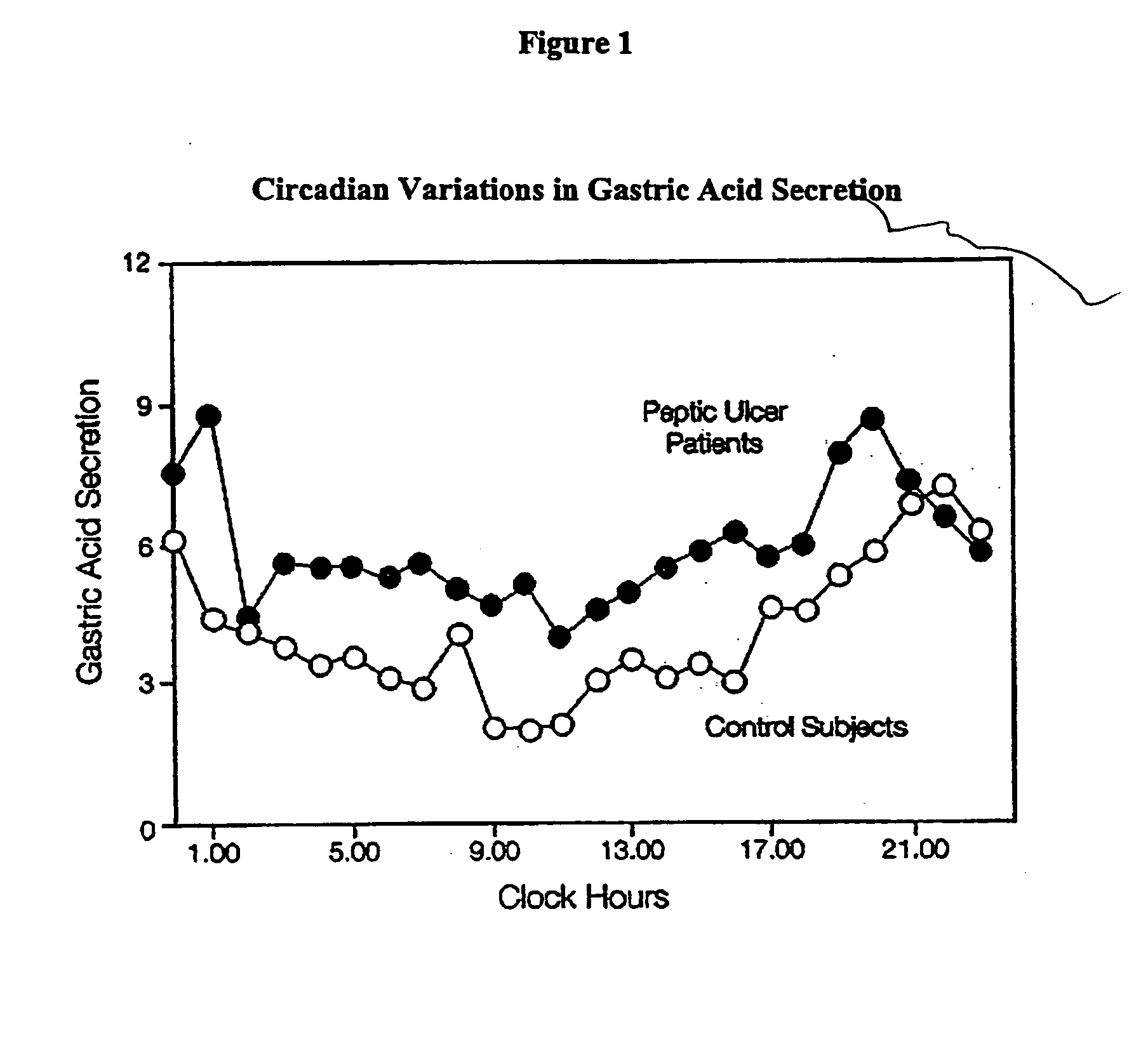
A unit dosage form, such as a capsule or the like, for delivering drugs into the body in a circadian release fashion comprising one or more populations of drug-containing particles (beads, pellets, granules, etc.) is disclosed. Each bead population exhibits a pre-designed rapid or sustained release profile with or without a predetermined lag time of 3 to 5 hours. Such a circadian rhythm release drug delivery system is designed to provide a plasma concentration-time profile, which varies according to physiological need at different times during the dosing period, i.e., mimicking the circadian rhythm and severity / manifestation of gastric acid secretion (and / or midnight gerd), predicted based on pharmaco-kinetic and pharmaco-dynamic considerations and in vitro / in vivo correlations.
Owner:APTALIS PHARMATECH
Systems and computer program products to browse database query information
InactiveUS20080133582A1Efficiently determinedQuick navigationData processing applicationsDigital data information retrievalInformation systemComputer program
Systems and computer products that efficiently determine how columns, tables, and queries associated with a database are related to each other. The present invention enables a user to see at a glance in which queries certain columns and tables are used. The present invention may be implemented with a query miner module that includes a query browser module that improves browsing through queries and their components over the past. The preferred embodiment of the present invention provides an easy to use graphical interface showing the queries, tables, and columns in a tree structure. Further, the preferred embodiment of the present invention presents information about relationships and dimensions associated with a database and about columns, tables, and queries to the user without discernable lag time between the user's request and the generated information.
Owner:IBM CORP
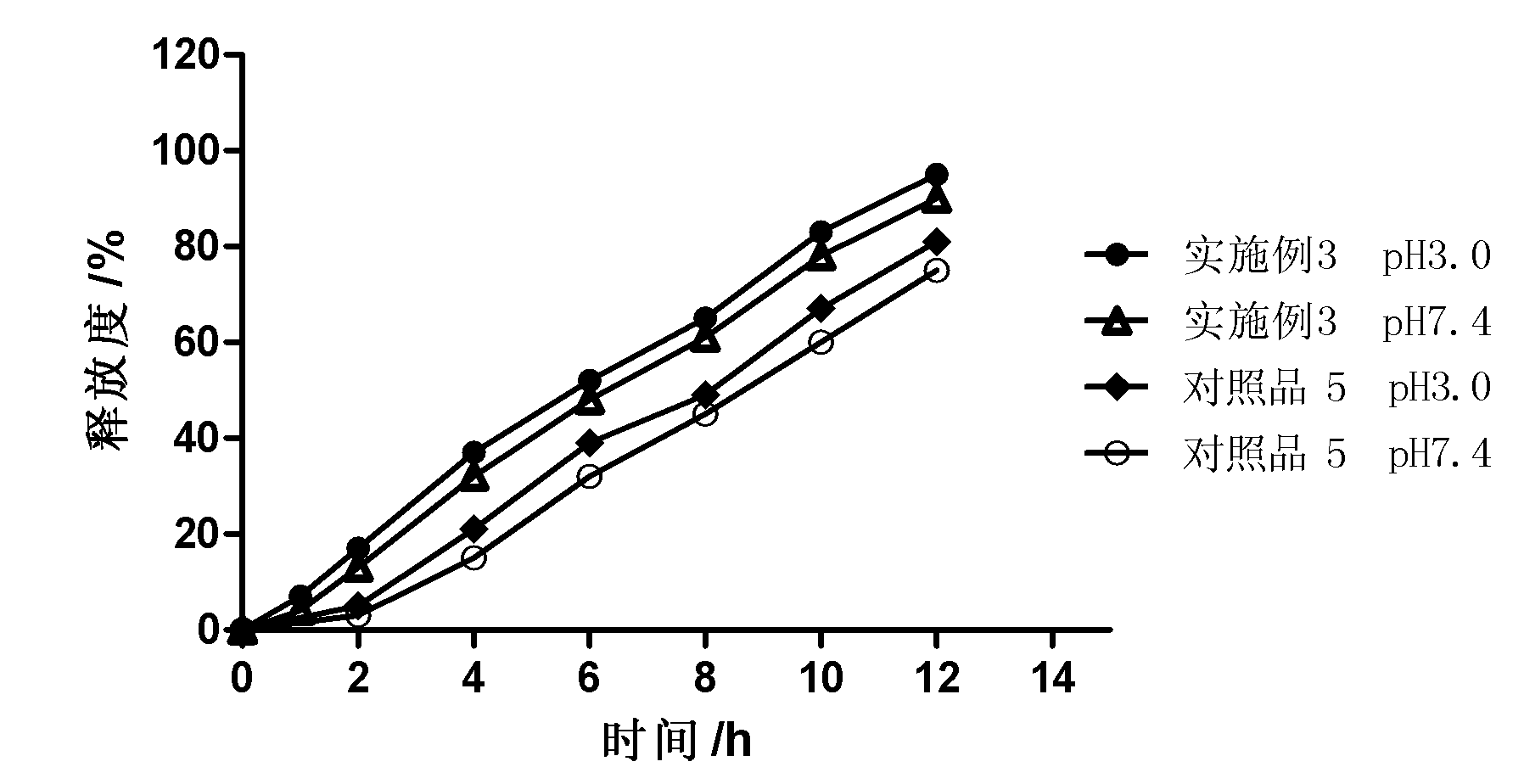
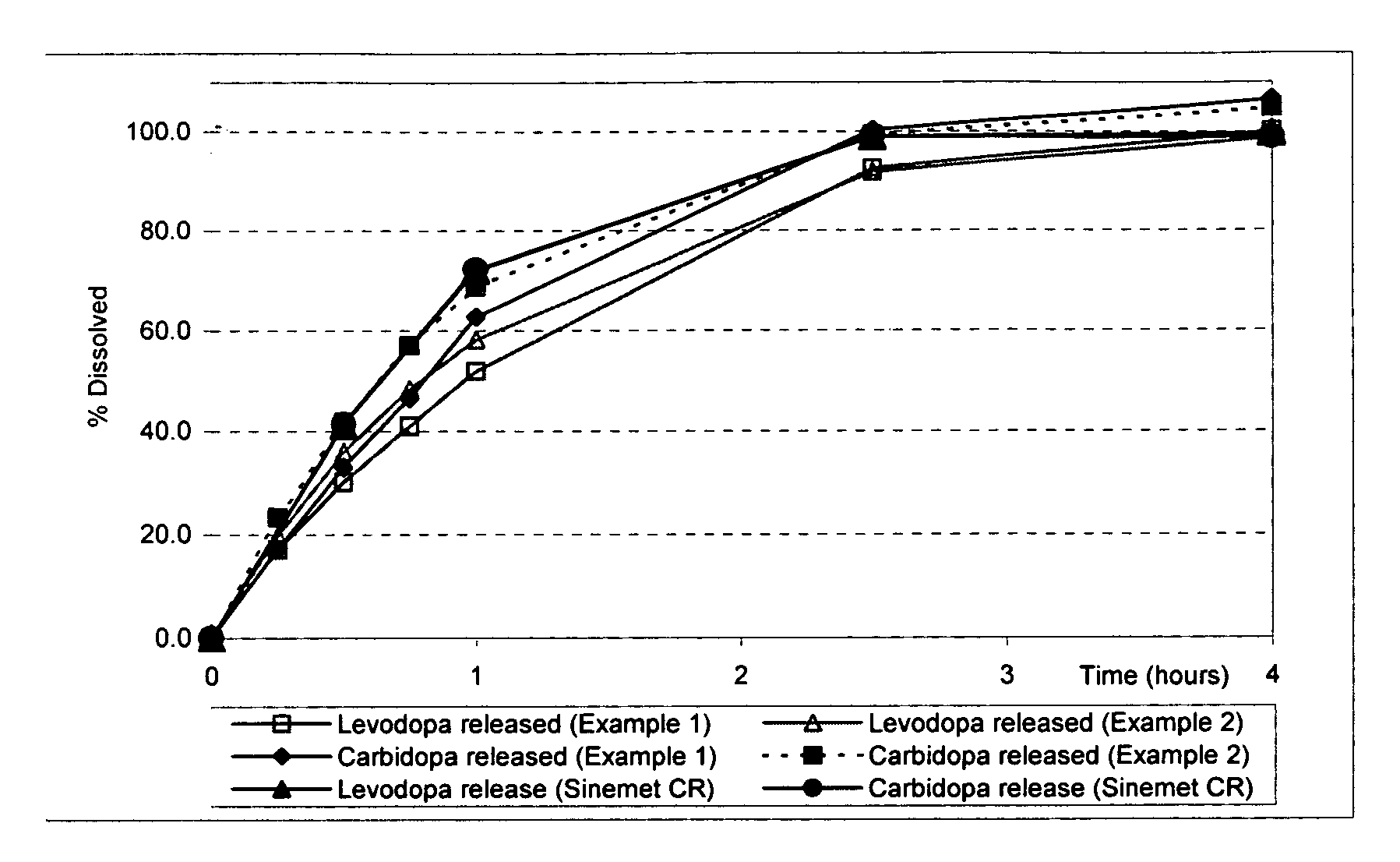
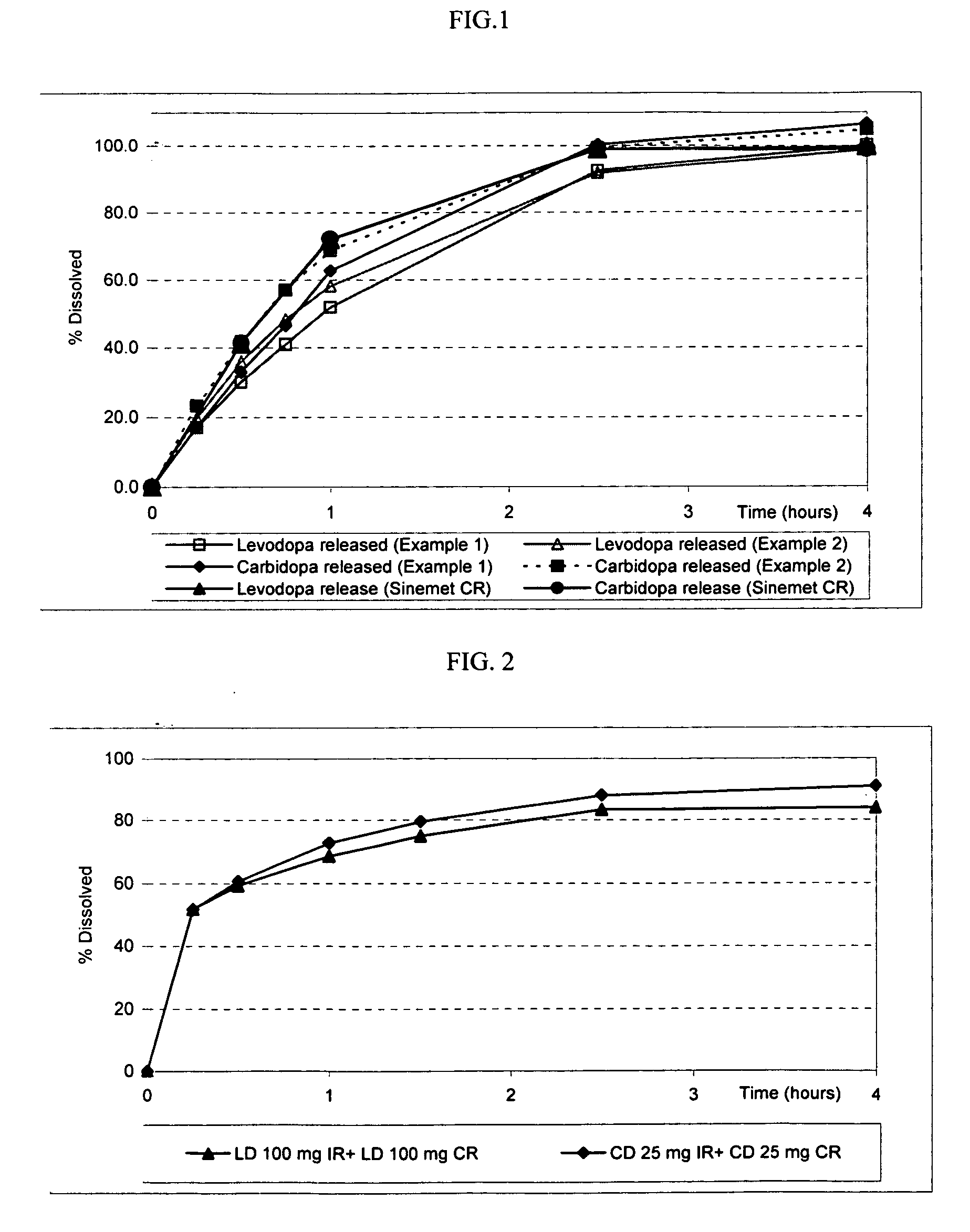
Extended release solid pharmaceutical composition containing carbidopa and levodopa
InactiveUS20070275060A1Improve aestheticsImprove bioavailabilityOrganic active ingredientsBiocideExtended release tabletsImmediate release
The invention provides a compressed tablet that provides a extended release tablet containing a extended release form of carbidopa and a extended release form of levodopa. The tablet optionally further comprises an immediate or rapid release composition of carbidopa and / or levodopa. The extended release composition in the tablet excludes a release rate-controlling polymer, and a release rate-controlling coating; however, the release of the carbidopa and / or levodopa is independently optionally delayed for a lag time. The invention also provides a tablet having a extended release form of levodopa and a rapid or immediate release form of carbidopa. A tablet can contain levodopa present in extended release form and rapid or immediate release form, and carbidopa present in extended release form and rapid or immediate release form. The tablet is used to treat Parkinson's disease and other movement related disorders, diseases or syndromes.
Owner:OSMOTICA KERESKEDELMI & SZOLGALTATO
Sustained Release Small Molecule Drug Formulation
An injectable depot formulation includes a biocompatible polymer, an organic solvent combined with the biocompatible polymer to form a viscous gel, and a small molecule drug incorporated in the viscous gel such that the formulation exhibits an in vivo release profile having Cmax to Cmin ratio less than 200 and lag time less than 0.2.
Owner:INDIVIOR UK
Control methods for low emission internal combustion system
InactiveUS20070220864A1Reduce the formation of nitrogen oxidesImprove transient response timeElectrical controlInternal combustion piston enginesCombustion systemEngineering
Improved transient response times are obtained while maintaining low emissions with a low pressure EGR system through methods for quickly obtaining a desired oxygen concentration for charge-air to be used for combustion. Under a first method, fuel quantity in the main combustion event is controlled in the combustion process to produce exhaust around a relatively constant target exhaust oxygen concentration value. By keeping the exhaust oxygen concentration levels at a relatively constant value, lag time in waiting for low pressure EGR valve adjustments during transients may be avoided, and the system's air handling response to meet transients may be paced solely by adjusting the mass of air to be supplied (i.e. boost response). Under a second method, a multiple-stage combustion process is utilized, in which fuel feed is controlled in a small, preliminary HCCI-type combustion event in order to produce a target oxygen concentration of charge-air to be used for the second, main combustion event. Under a third method, exhaust rebreathing is used to produce a target oxygen concentration of charge-air to be used for combustion.
Owner:US EPA OFFICE OF GENERAL COUNSEL UNITED STATES OF AMERICA THE
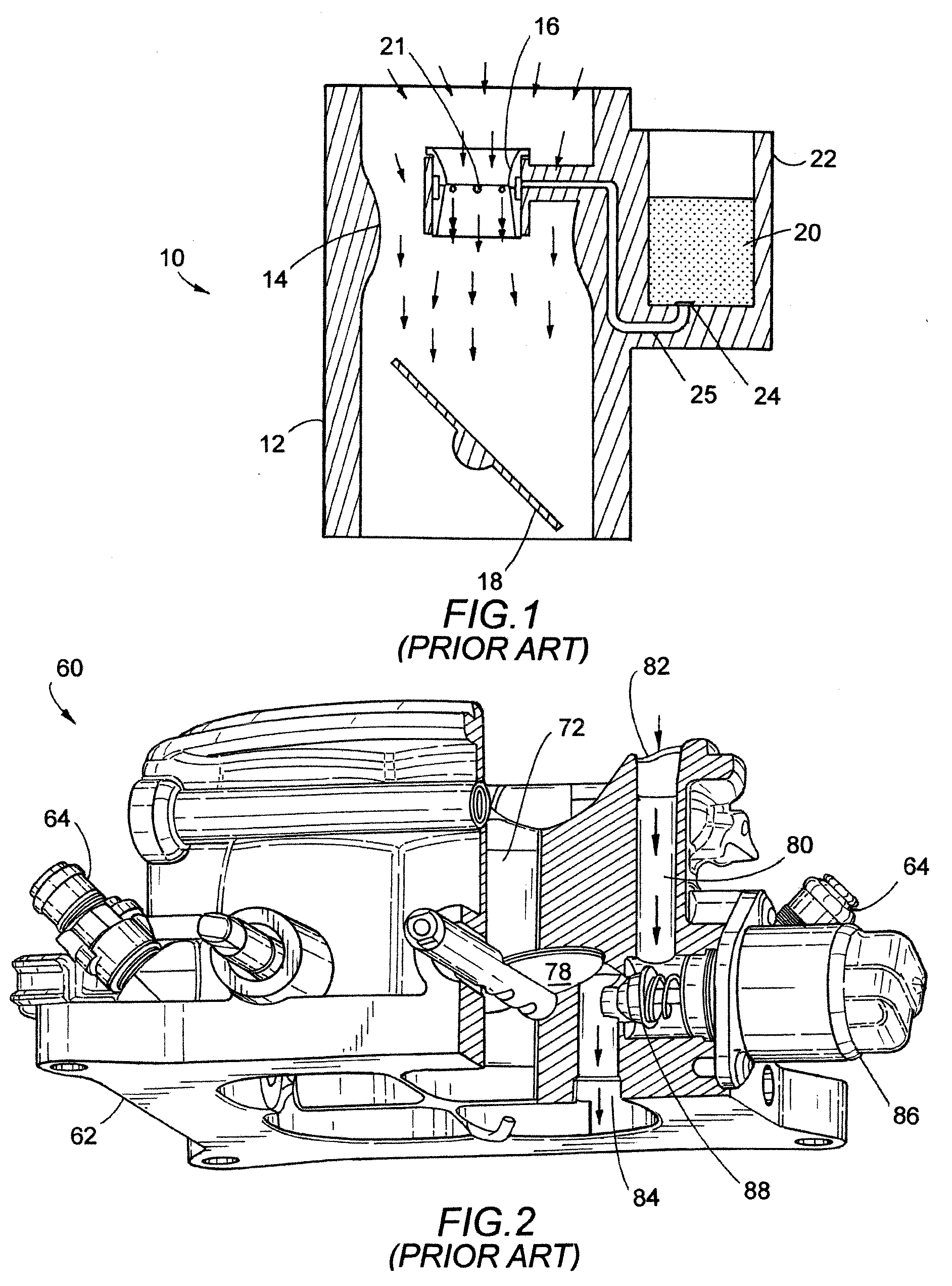
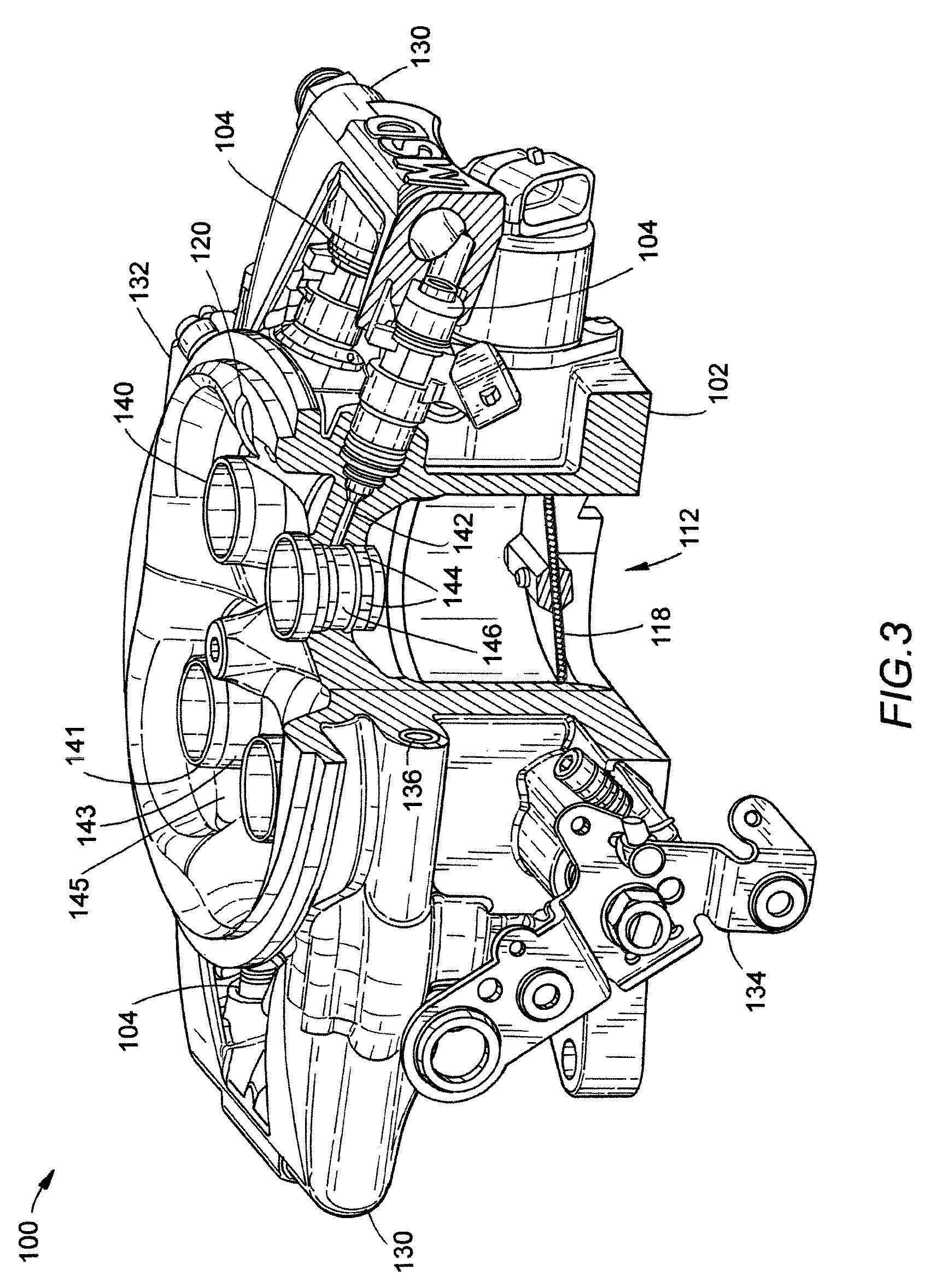
Throttle body fuel injection system with improved idle air control
ActiveUS9303578B2Improve performanceOptimal fuel distribution and idle control circuitryElectrical controlInternal combustion piston enginesFuel distributionClosed loop
A single point fuel injection throttle body assembly including an idle air control circuit having an port opening into main intake bores downstream of the point of fuel distribution into the air stream. When the idle air control circuit is open, an air / fuel mixture, rather than simply air, is drawn into the into the intake manifold, thereby reducing the tendency for a lean fuel mixture at idle. A unique engine control unit “feed forward” algorithm controls the fuel injection as a function of the position of the idle air control motor so that as the idle air control circuit is opened, the pulse widths of the fuel injector signals are increased. This feature allows the initial open-loop-based fuel mixture supplied by system to be more accurate and eliminates rough unstable idle associated with closed-loop control lag times.
Owner:MSD LLC
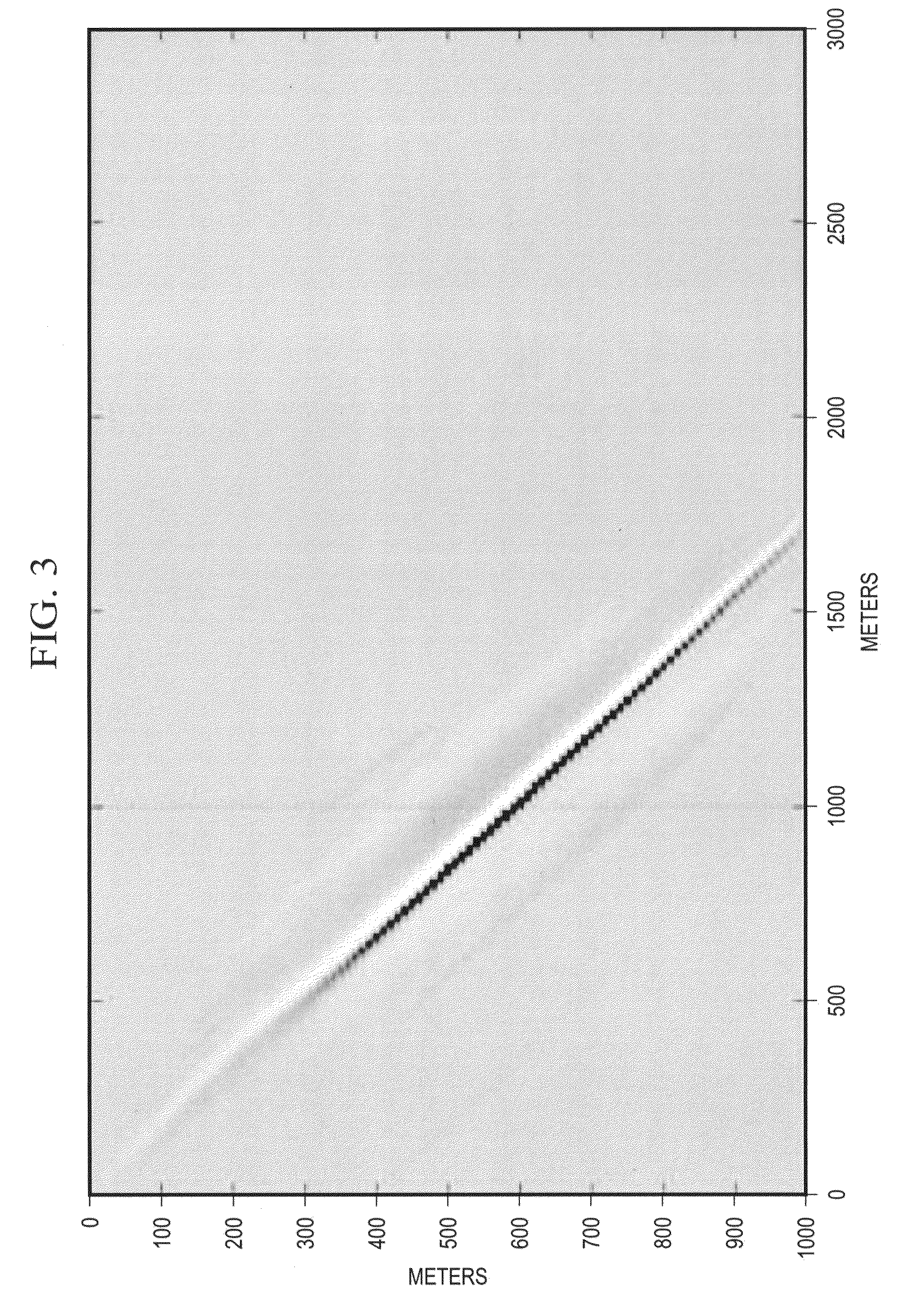
Migration Velocity Analysis of Seismic Data Using Common Image Cube and Green's Functions
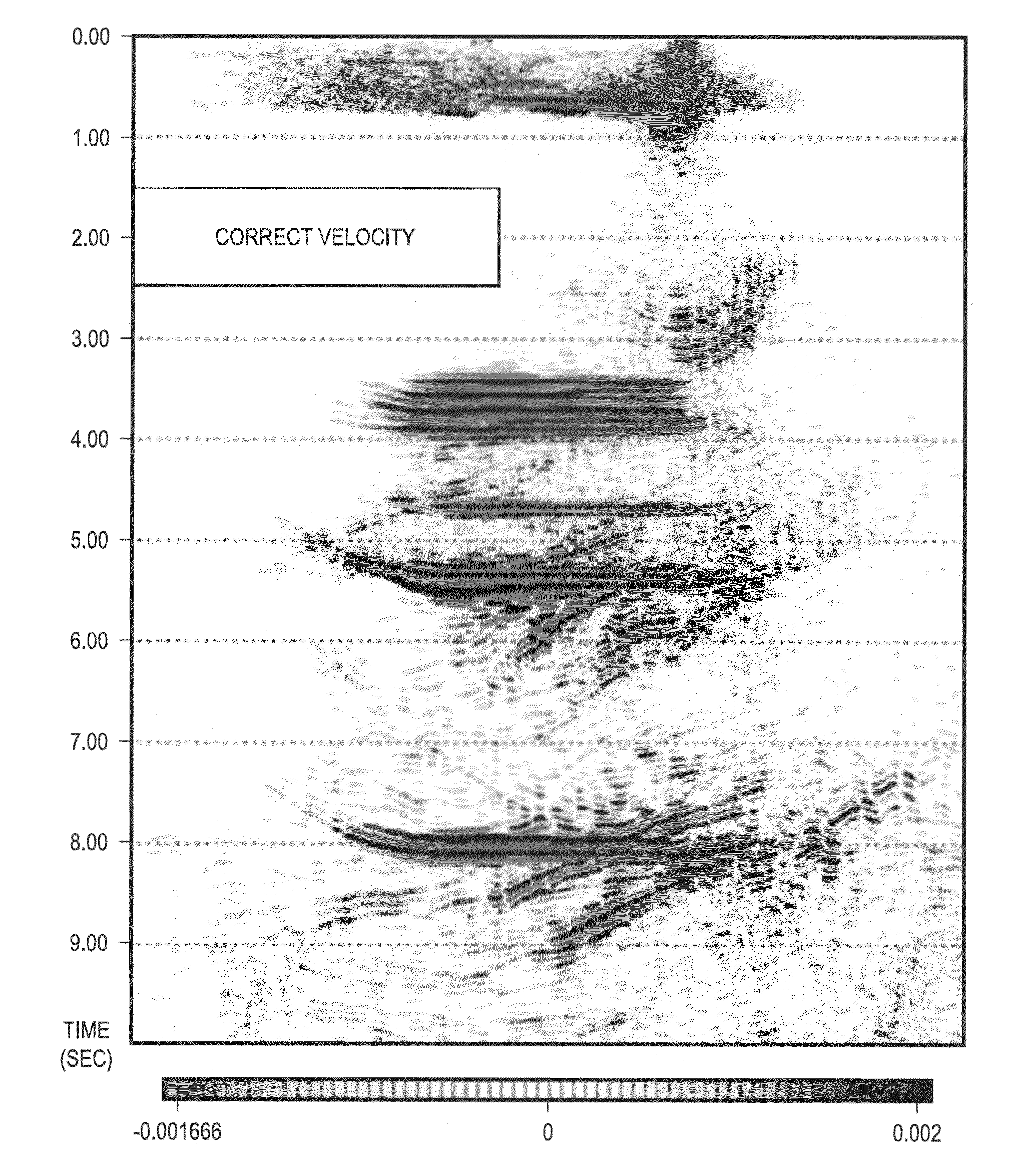
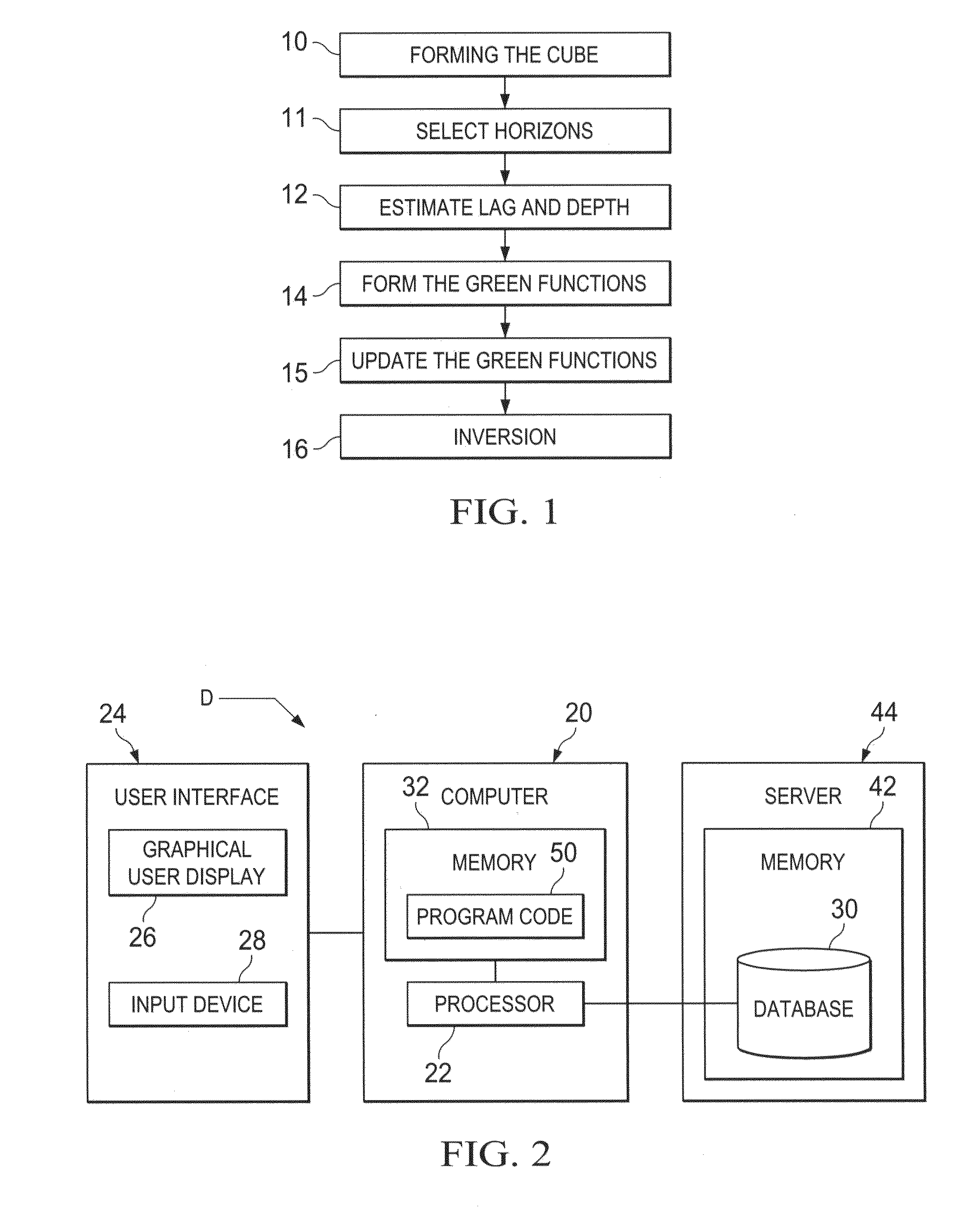
InactiveUS20110320180A1Seismic signal processingSpecial data processing applicationsAnalysis dataData set
Seismic data are assembled and stored for a set of cross-correlation lag times to form an array of common image gathers over depth levels of interest. The two dimensional gathers assembled over different lag times form a three-dimensional cube of common image data. The data are analyzed to determine the travel time shift required to equalize upgoing and downgoing wavefields. Events in the common image gathers are then modeled using Green's functions to generate a data set representing the data resulting from processing had a precise velocity model been obtainable from the seismic data. The generated data are then processed with inversion techniques to form a velocity model for seismic data analysis.
Owner:SAUDI ARABIAN OIL CO
Systems and methods for installation, design and operation of groundwater monitoring systems in boreholes
Systems and methods for installation and operation of a groundwater monitoring system in a borehole of any angle using a coaxial gas displacement pump with a unique O-ring assembly that serves as a two-position valve for groundwater purging and sampling and also as a housing and sealing mechanism for isolating an optical pressure sensor. The optical sensor measures in-situ hydraulic pressure directly subjacent and adjacent to the surrounding rock fractures and sediment pores without hydraulic interferences from potentiometric equilibration lag time from recovery fluid pressure inside a borehole, in a zone above the optical sensor, or on the inside of a riser pipe that rises to the ground surface.
Owner:BESST +1
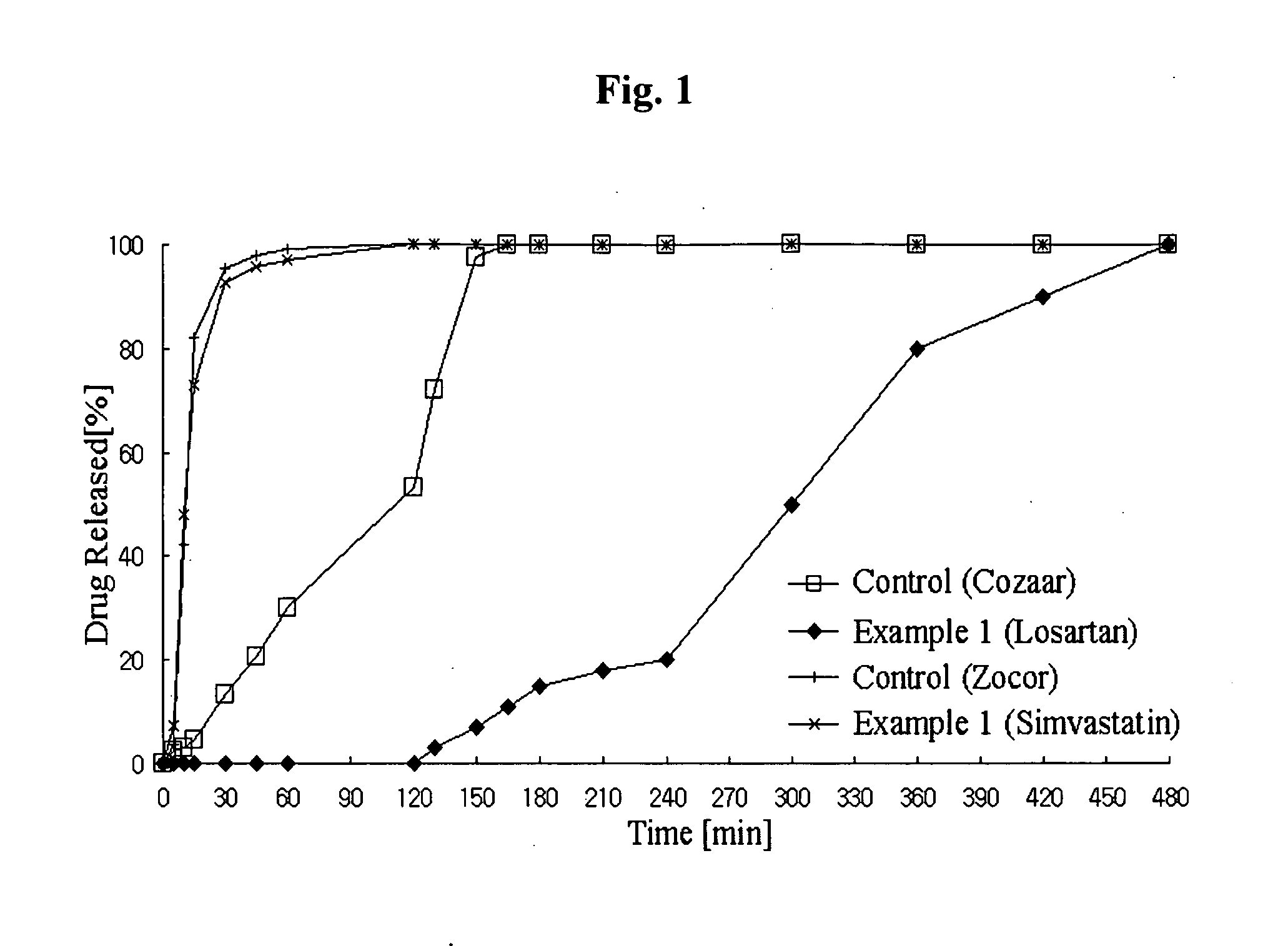
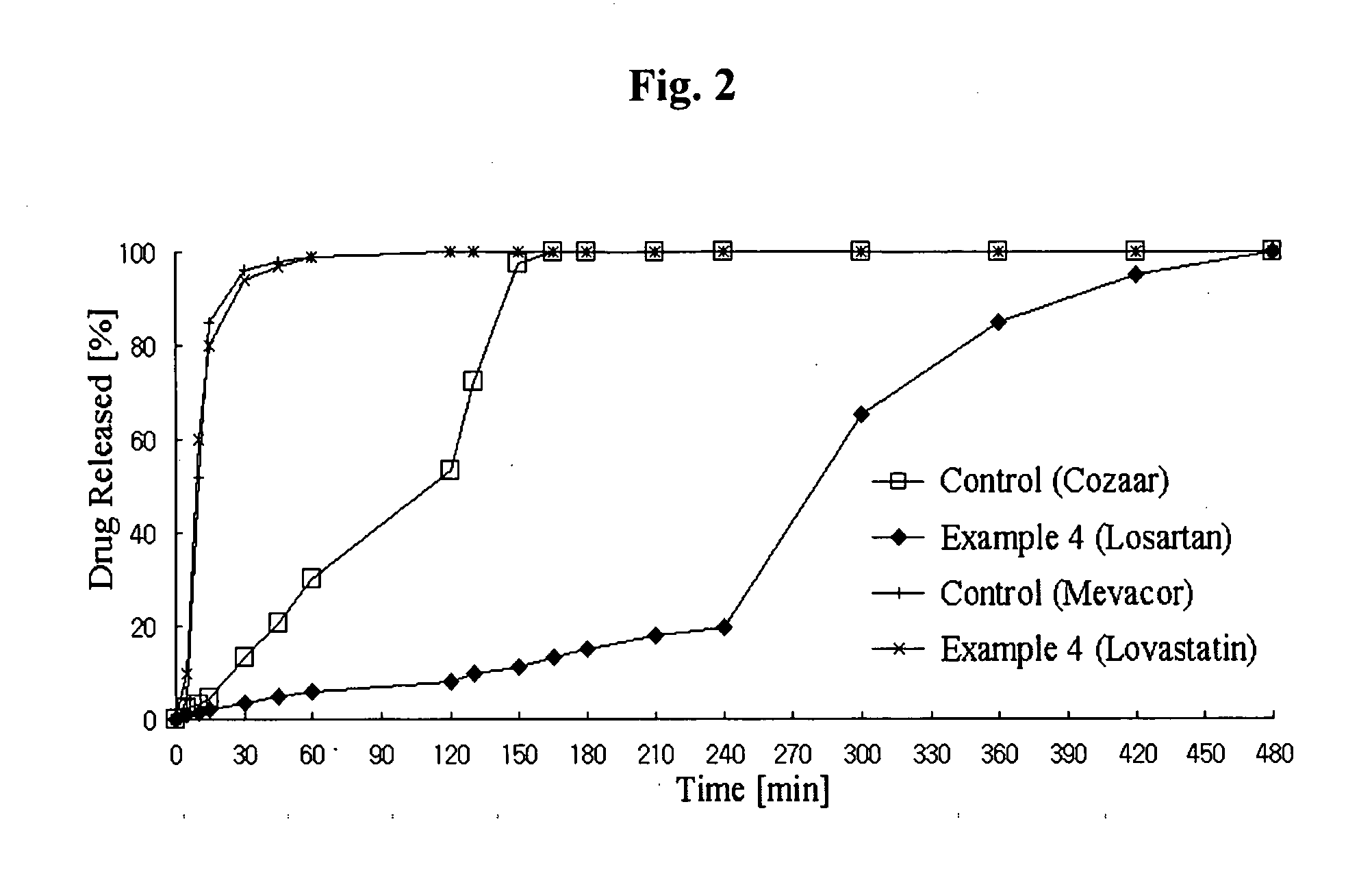
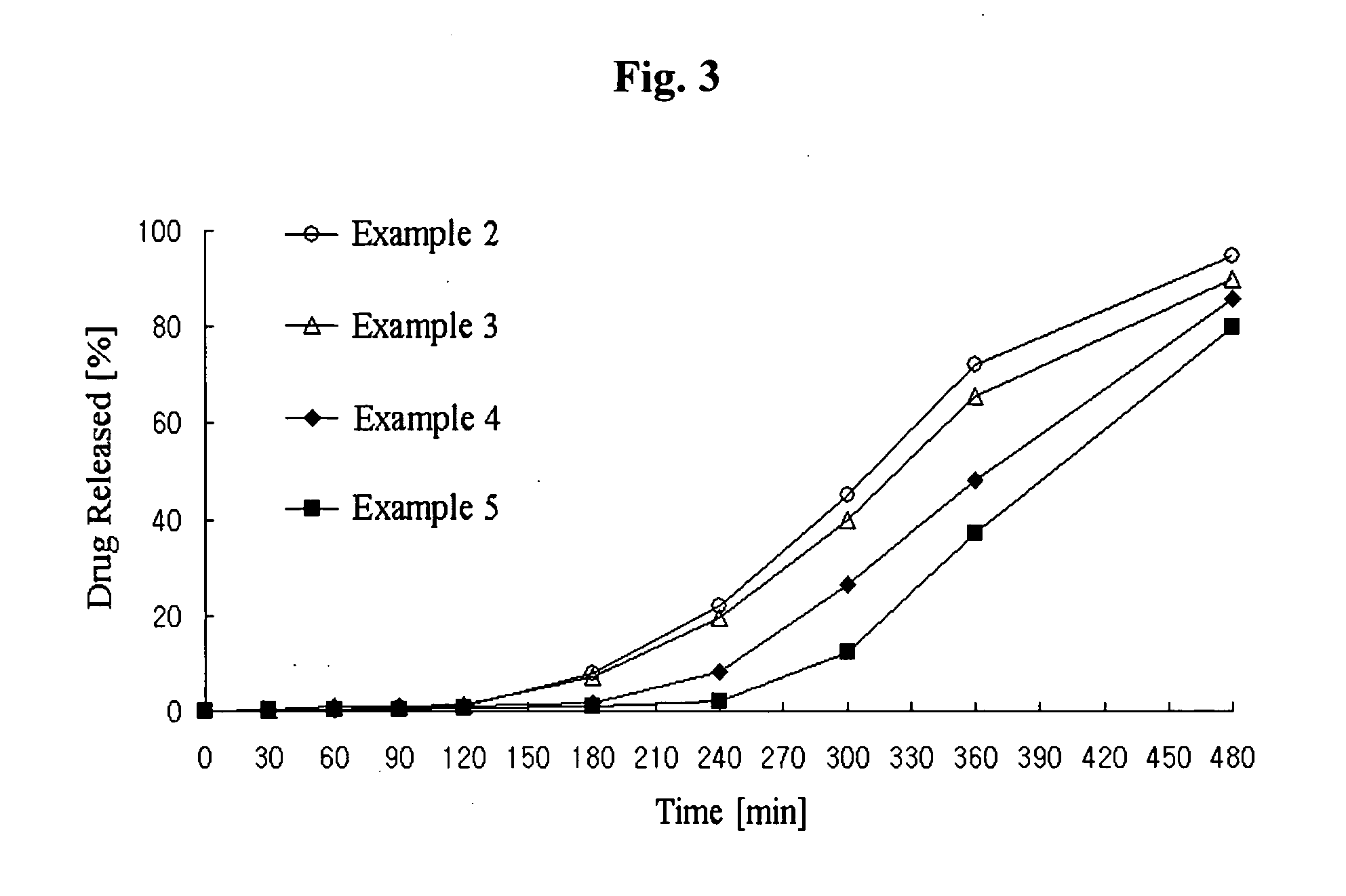
Controlled release complex composition comprising angiotensin-ii-receptor blockers and hmg-coa reductase inhibitors
ActiveUS20100074951A1Maximize the effect of treatmentPreventing and reducing side effectBiocideMetabolism disorderHMG-CoA reductaseCoronary artery disease
Disclosed herein is a lag time delayed-release combination pharmaceutical composition comprising of an angiotensin-II-receptor blocker and an HMG-CoA reductase inhibitor, as well as a preparation method thereof. The composition is designed based on chronotherapy in which active ingredients are administered to have different onset times, such that the release of each active ingredient of the composition in body can be lag time delayed to a specific rate. Also, the composition is very effective for the treatment of hypertension and the prevention of complications in patients having metabolic syndromes which show diabetes, obesity, hyperlipidemia, coronary artery diseases and the like. More specifically, the composition is a drug delivery system designed such that the release of each drug is controlled to a specific rate, and it can show the most ideal effect, when it is absorbed in body.
Owner:HANALL PHARMA CO LTD
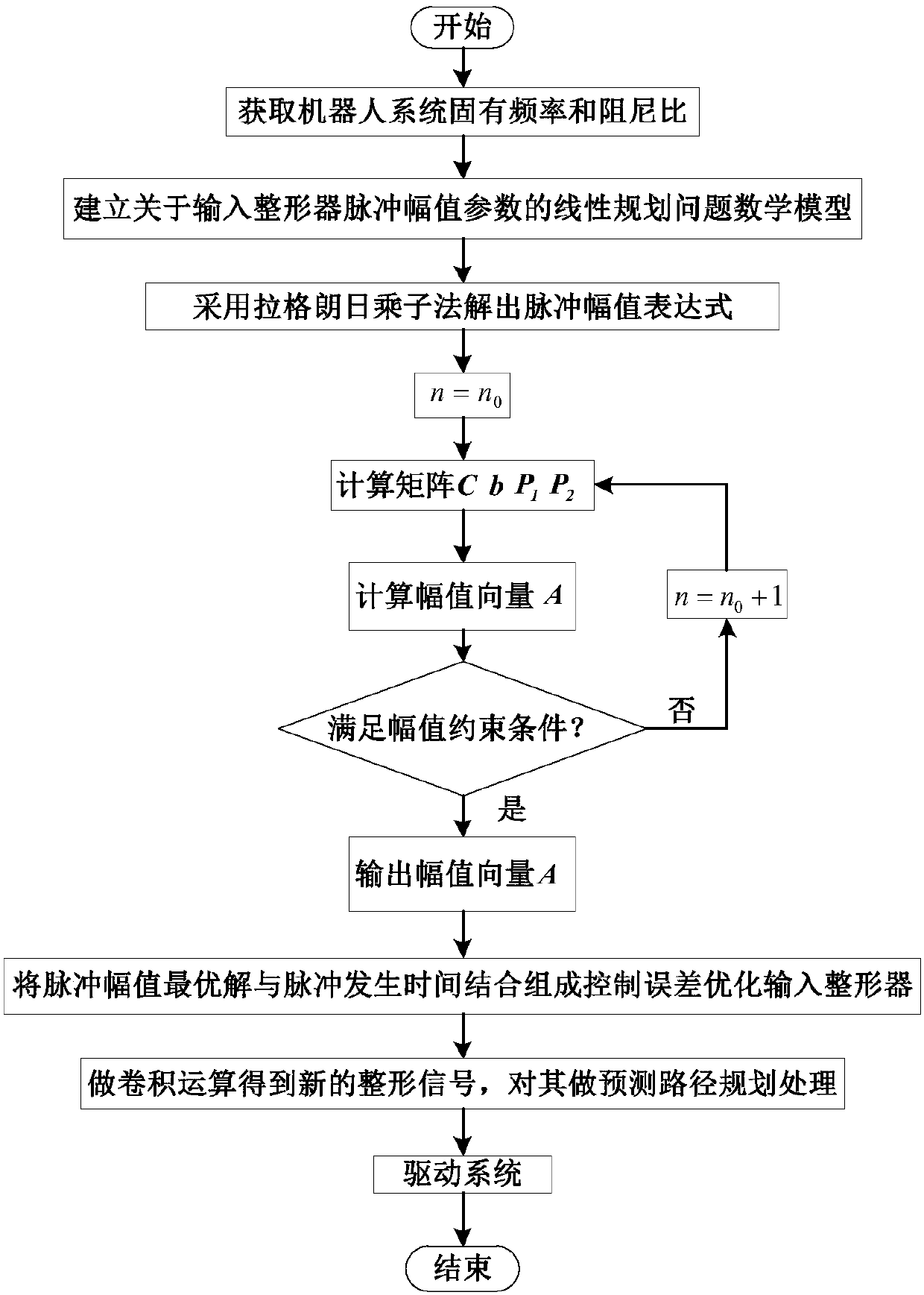
Robot joint tail end residual vibration restraining method based on input shaper
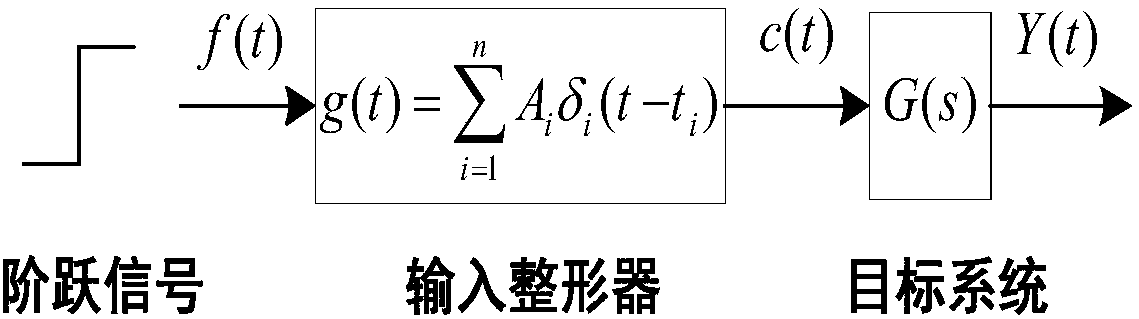
The invention discloses a robot joint tail end residual vibration restraining method based an input shaper. The robot joint tail end residual vibration restraining method based the input shaper comprises the steps that firstly, the undamped inherent frequency omega0 and the damping ratio zeta of a robot system are obtained; secondly, a linear programming problem mathematic model about the pulse amplitude parameter of the input shaper is established; thirdly, a pulse amplitude expression is solved through a lagrangian multiplier method, and the optimal solution of the pulse amplitude is workedout through iteration; fourthly, the optimal solution of the pulse amplitude and the pulse generation time are combined to form the control error optimization input shaper; and fifthly, a reference signal and the control error optimization input shaper are subjected to convolution operation, so that a novel shaping signal is obtained, and after a predicted path is planned for the novel shaping signal, the system is driven by the signal to restrain robot tail end residual vibration. According to the robot joint tail end residual vibration restraining method based the input shaper, the robustness of the input shaper is enhanced, and a process control error and a positioning error are minimized; and the predicted path is planned for the shaped signal, and thus the system lag time caused by input shaping is compensated and reduced.
Owner:SOUTH CHINA UNIV OF TECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com