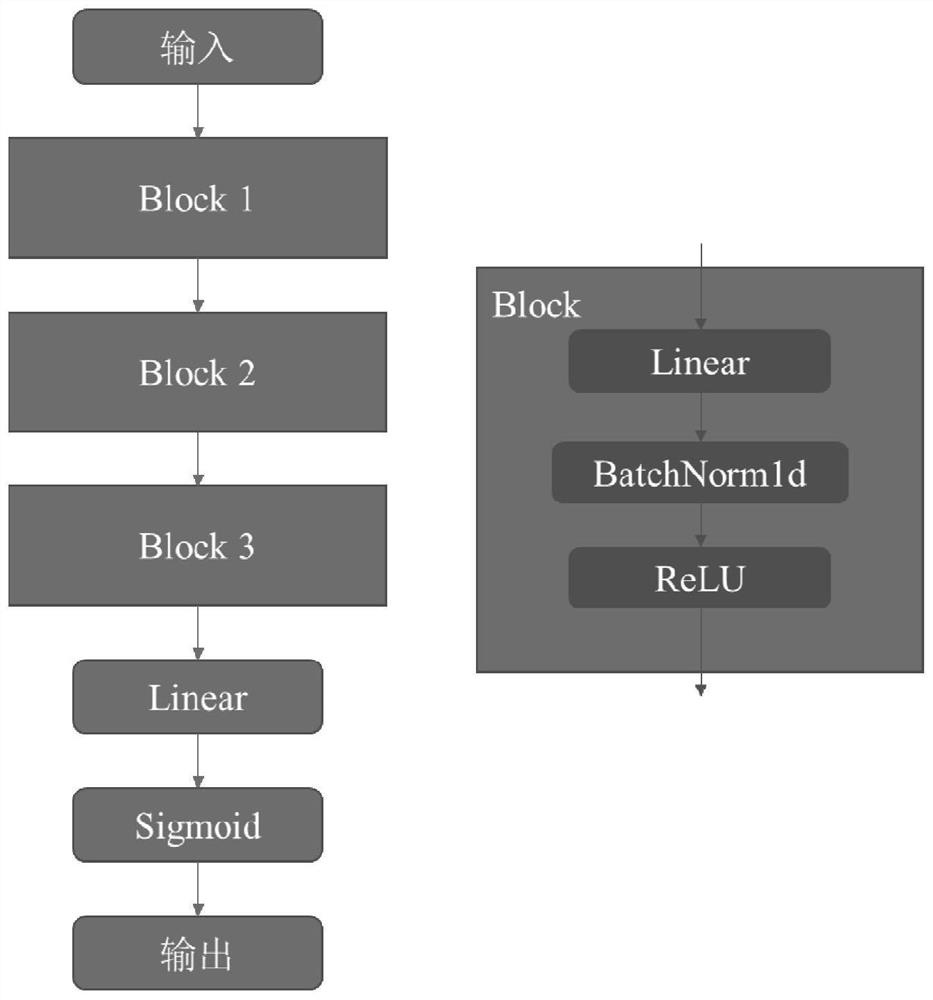
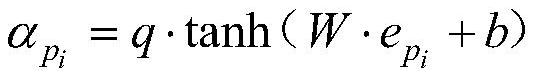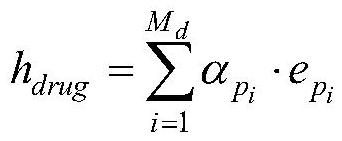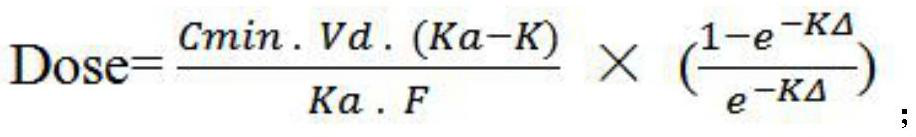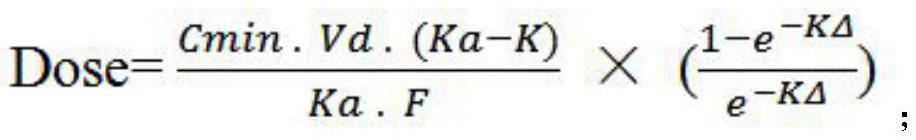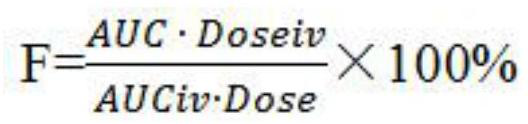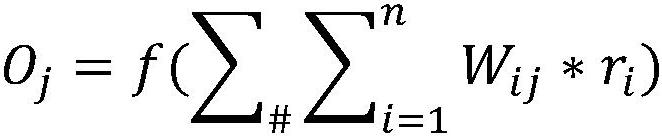Patents
Literature
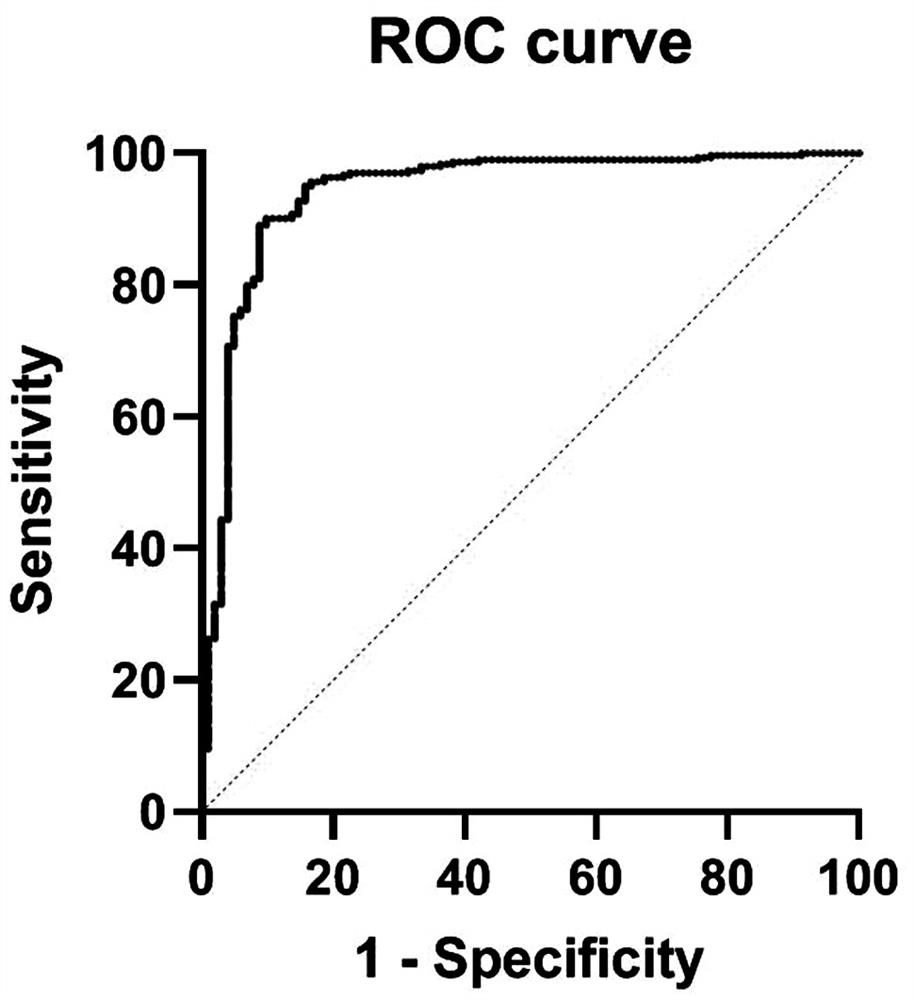
40 results about "Area under the curve" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
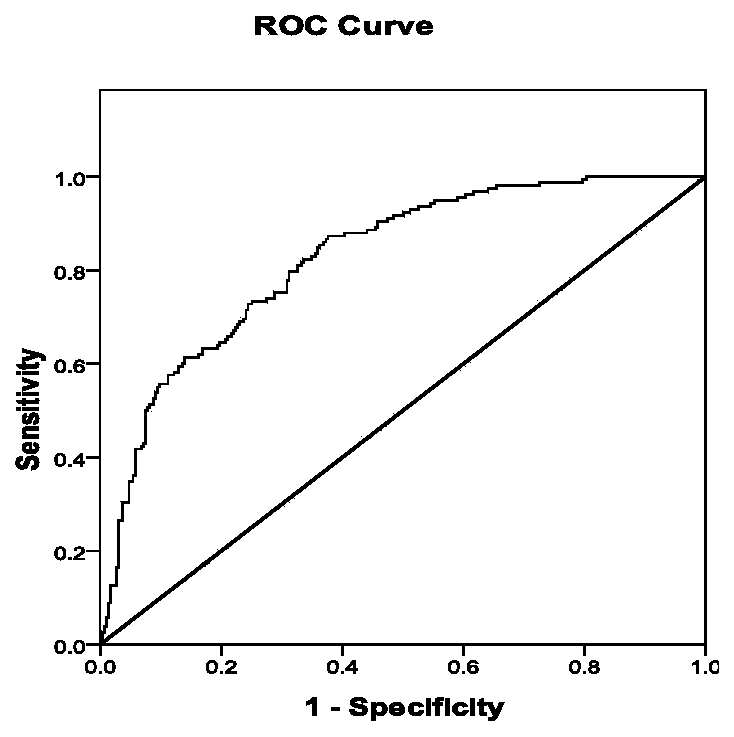
In the field of pharmacokinetics, the area under the curve (AUC) is the definite integral in a plot of drug concentration in blood plasma vs. time. In practice, the drug concentration is measured at certain discrete points in time and the trapezoidal rule is used to estimate AUC.
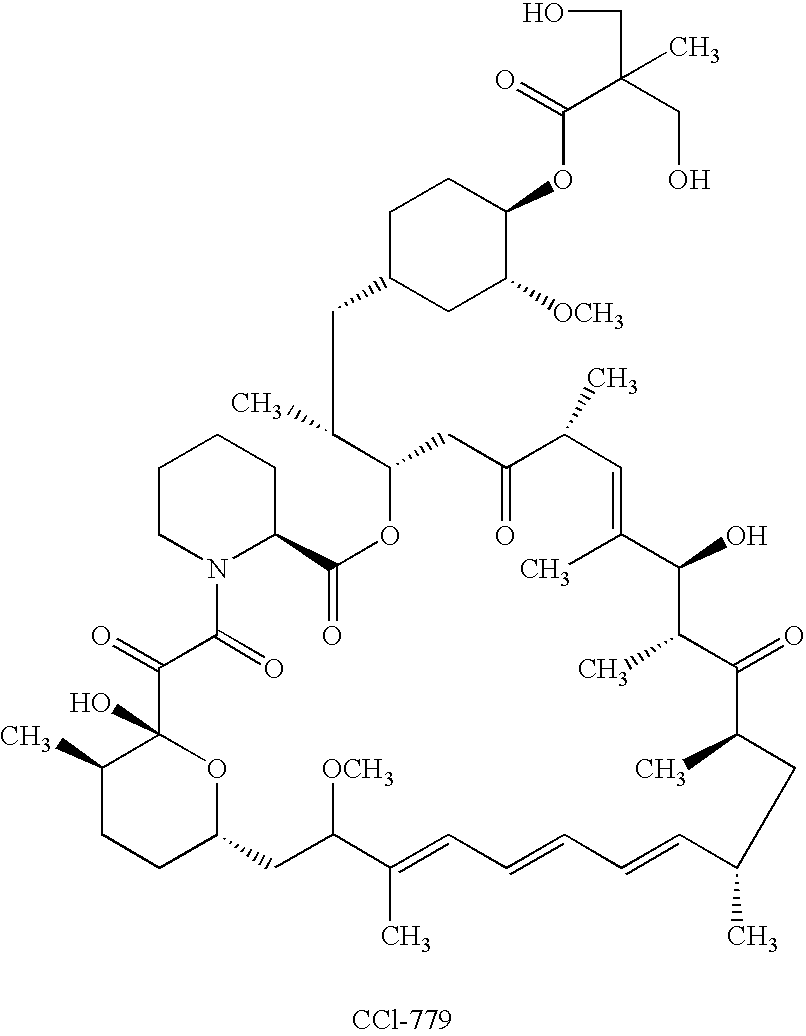
Orally bioavailable CCI-779 formulations
A CCI-779 oral dosage form is provided in which, after oral administration to a subject, the CCI-779 has a whole blood peak concentration (Cmax) of 5.4±1.8 ng / mL and an area under the curve (AUC) of about 66±about 22 ng-hr / ml and the sirolimus has a Cmax of 18.7±9.6 ng / mL and an AUC of about 600±about 228 ng-hr / ml, for a 25 mg unit dose of CCI-779. Another CCI-779 oral dosage form is provided which, after oral administration thereof to a subject, the CCI-779 has a Cmax of 5.7±1.7 ng / mL and an AUC of about 60±about 20 ng-hr / ml and the sirolimus has a Cmax of 17.1±8.1 ng / mL and an AUC of about 548±about 187 ng-hr / ml in whole blood, for a 25 mg unit dose of CCI-779. Products containing these oral dosage forms, and methods of use thereof, are also described.
Owner:WYETH LLC
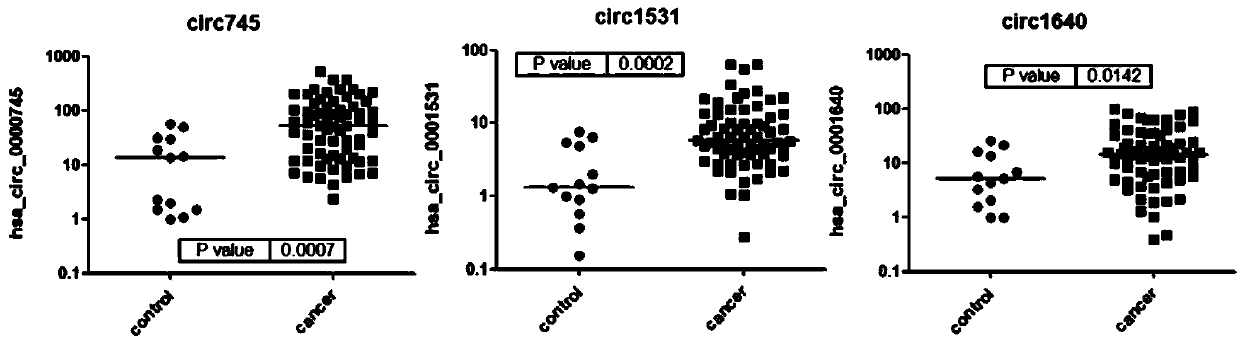
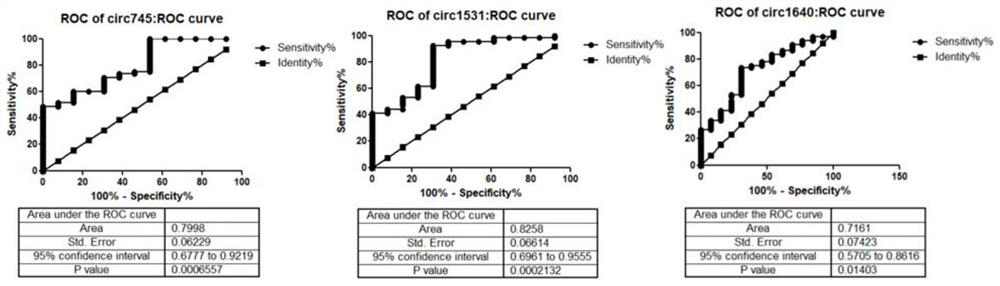
CircRNA marker used for breast cancer diagnosis and application of marker
ActiveCN109097477AEasy to distinguishMicrobiological testing/measurementDNA/RNA fragmentationMedicineCurve analysis
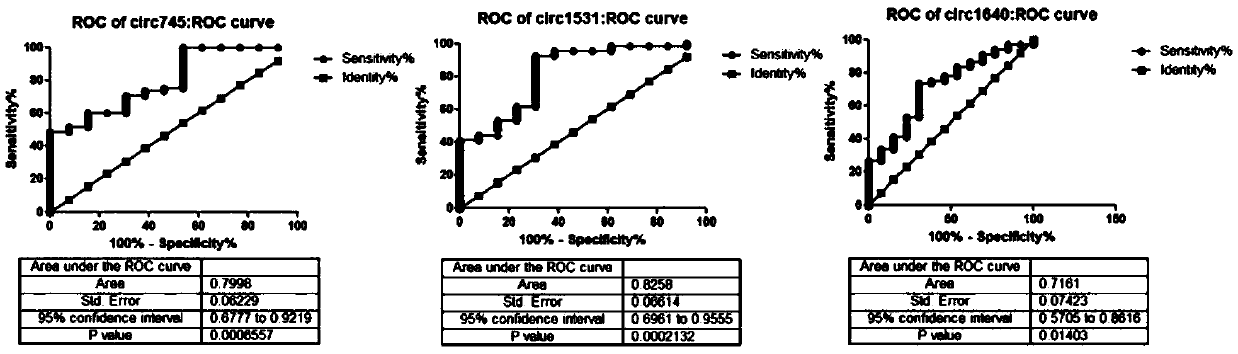
The invention relates to a circRNA marker used for breast cancer diagnosis and an application of the circRNA marker. Compared with healthy control groups, it is found that expression of 3 kinds of circRNA (hsa_circ_0000745, hsa_circ_0001531 and hsa_circ_0001640) in whole blood of breast cancer is up-regulated. ROC curve analysis displays that the marker can better distinguish breast cancer patients and healthy people, and the 3 kinds of circRNA have the AUCs (area under curve) of 0.7998, 0.8253 and 0.7161 respectively, the sensitivity of 48.53%, 92.65% and 73.53% respectively and the specificity of 100%, 69.23% and 69.23% respectively.
Owner:SHANDONG UNIV QILU HOSPITAL
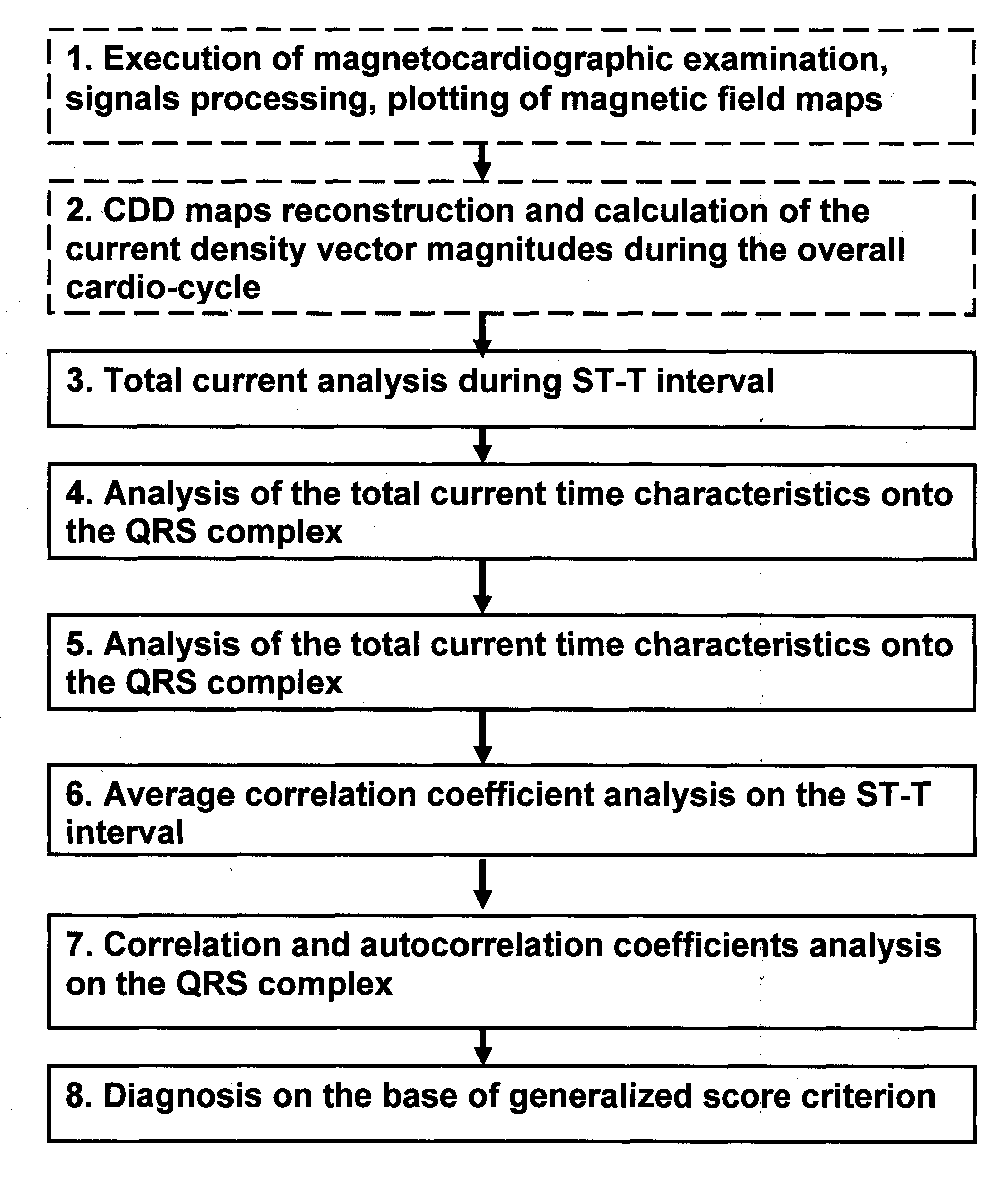
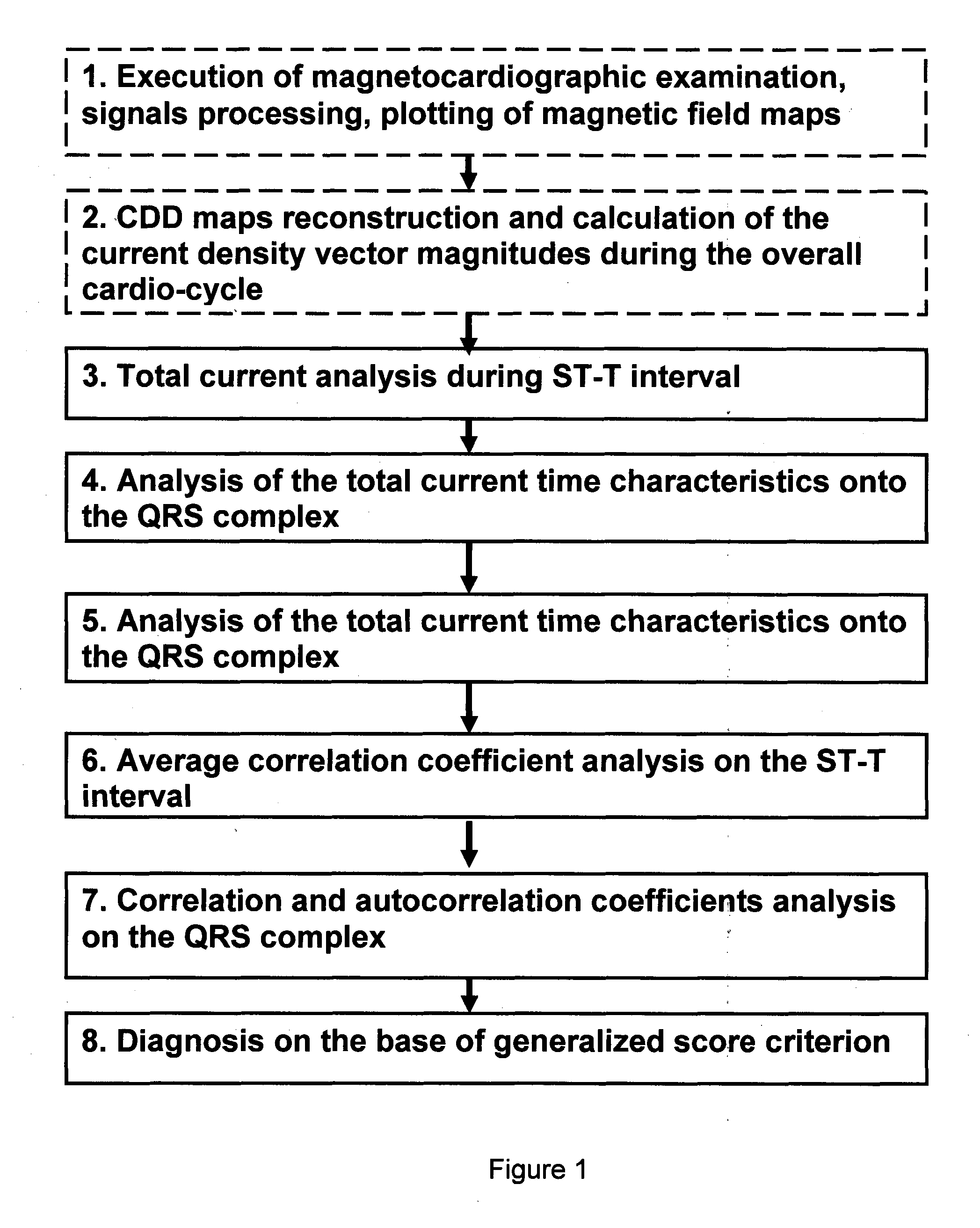
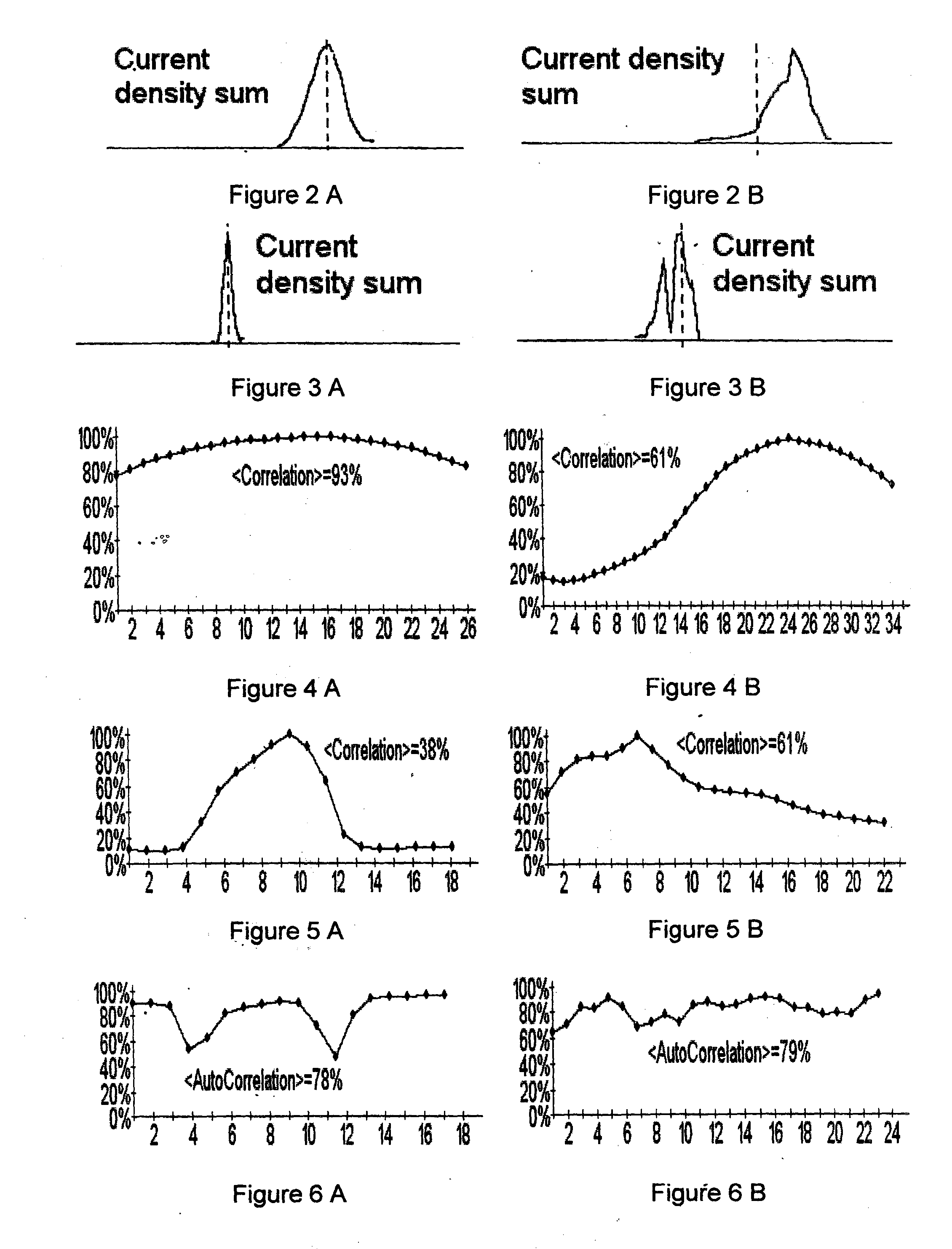
Method and device for evaluation of myocardial damages based on the current density variations
InactiveUS20150011862A1Avoid Cutting InjuriesAccurate estimateDiagnostic recording/measuringSensorsT waveCardiac muscle
Invention is related to cardiology and intended for diagnosis of ischemic myocardial injuries. Magnetocardiographic examination is executed, current density vectors maps are reconstructed during ST-T interval and 4 sub-intervals of QRS complex, quantitative diagnostic indicators are calculated. Characterized in that, for said time intervals total length of all vectors (total current), autocorrelation coefficient of the instant map and its correlation with map onto the T wave peak are derived, several quantitative indicators for these curves are calculated (area under the curve, time intervals, their ratio, etc.) and ranges of their values are divided onto 3 intervals. As a result, absence / presence of ventricles injuries is diagnosed according to the rule—injury is absent (presence minor, significant), if certain quantitative indicator is ranged in one of 3 said intervals or if score of points for separate quantitative indicators is less than or equal to 7 (8-16, 17 and more).
Owner:CHAYKOVSKYY ILLYA ANATOLIIOVYCH
Pulmonary embolism clinical risk and prognosis scoring method and system
PendingCN113327679AEasy to usePredictable risk of deathHealth-index calculationMedical automated diagnosisStatistical analysisMedical treatment
The invention provides a pulmonary embolism clinical risk and prognosis scoring method and a system, and the method comprises the steps: carrying out the screening and feature extraction of medical data, and obtaining influence factors corresponding to death; performing single-factor logistic regression analysis on the influence factors, determining variables of a prognosis model, and establishing a logistic regression model; obtaining a result OR value through statistical analysis; assigning each risk factor based on the OR value, and establishing a risk prediction scoring system of pulmonary embolism to predict the death risk of the pulmonary embolism patient within 30 days; constructing an ROC curve, and comparing the areas under the curve of the three scoring systems by using clinical data of the verification group, the modeling group and the modeling group + the verification group; and predicting a result according to the optimal truncation value. The risk and prognosis scoring method can evaluate the death risk of the pulmonary embolism patient within 30 days, and high-risk and low-risk patients can be identified for doctors to refer to and formulate a diagnosis scheme.
Owner:上海市闵行区中心医院
Early-stage non-alcoholic steatohepatitis evaluation model, construction method and application thereof
PendingCN111063440AImproving the ability to diagnose early-stage nonalcoholic fatty liver diseaseHigh sensitivityMedical simulationHealth-index calculationHepatocyte apoptosisPredictive value
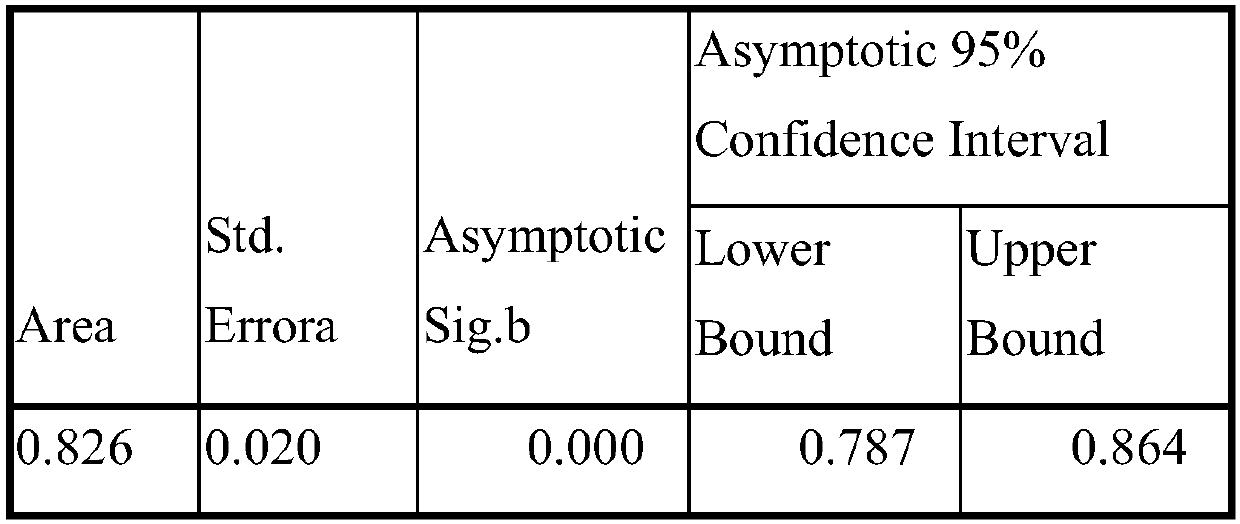
The invention discloses an evaluation model for early prediction of non-alcoholic steatohepatitis (NASH) and a calculation formula. According to the model, large-sample-size hepatic puncture pathological diagnosis is used as a grouping basis, an M30 fragment of a hepatocyte apoptosis marker keratin 18 is introduced, a new algorithm and a calculation formula are obtained by adding age, gender, height, waistline, hemoglobin, glutamic-pyruvic transaminase and gamma-glutamyl transpeptidase for simulation, the negative prediction value of the formula applied to early-stage NASH reaches 0.902, and the area under an ROC curve reaches 0.826.
Owner:苏州和锐生物科技有限公司
Methods of mouse clinical trial
InactiveUS20200260697A1Compounds screening/testingInorganic active ingredientsTumour volumeTumor Sample
The present disclosure provides methods of conducting and analyzing mouse clinical trials. In one embodiment, the method comprises the steps of receiving a dataset of tumor volumes measured in a mouse clinical trial, determining tumor growth curve of the treatment group and tumor growth curve of the control group; determining area under curve (AUC) of the treatment group and AUC of the control group; and evaluating efficacy of the drug based on an AUC ratio between the AUC of the treatment group and the AUC of the control group, wherein the mouse clinical trial comprises the steps of: obtaining a tumor sample derived from a patient; grafting the tumor sample to a treatment group comprising m mice and a control group comprising n mice, wherein m and n are integers; treating the treatment group with a drug; treating the control group with a vehicle; and measuring tumor volume of the treatment group and tumor volume of the control group at a plurality of days.
Owner:CROWN BIOSCI INC TAICANG
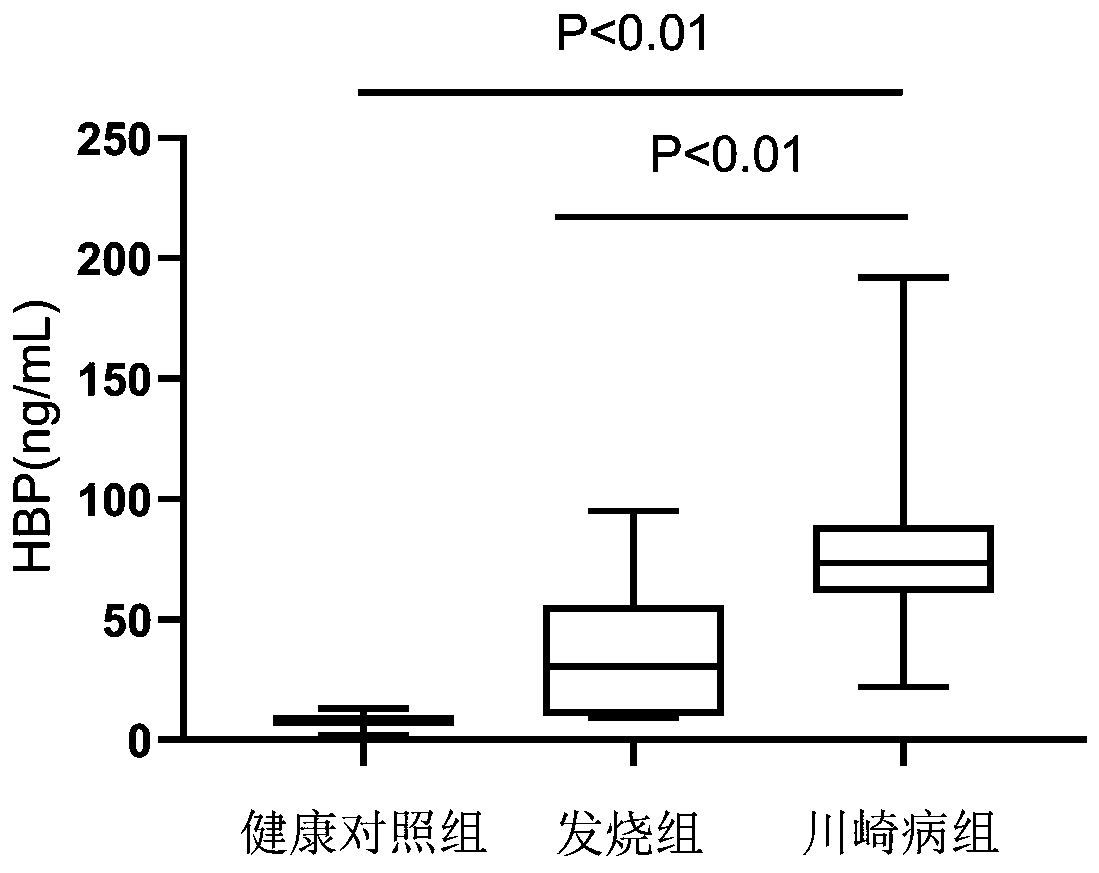
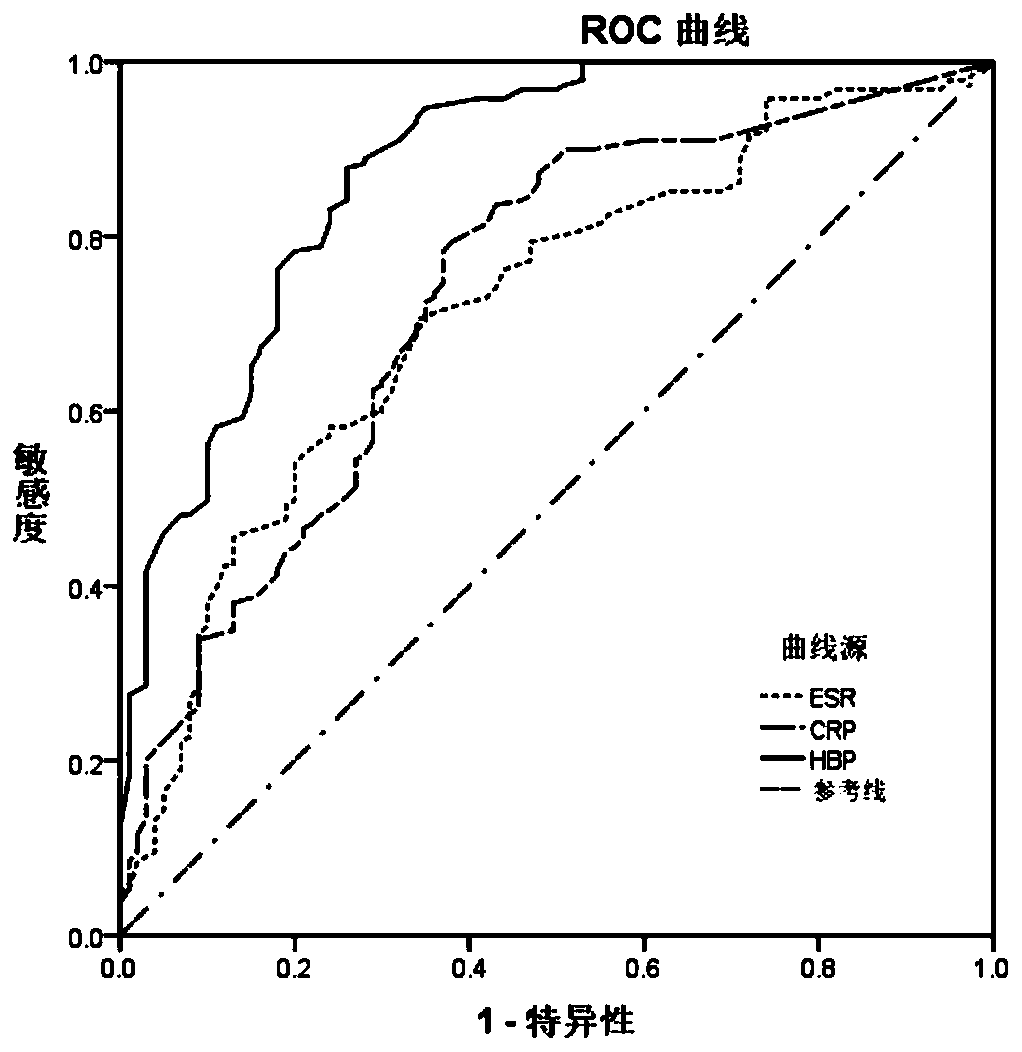
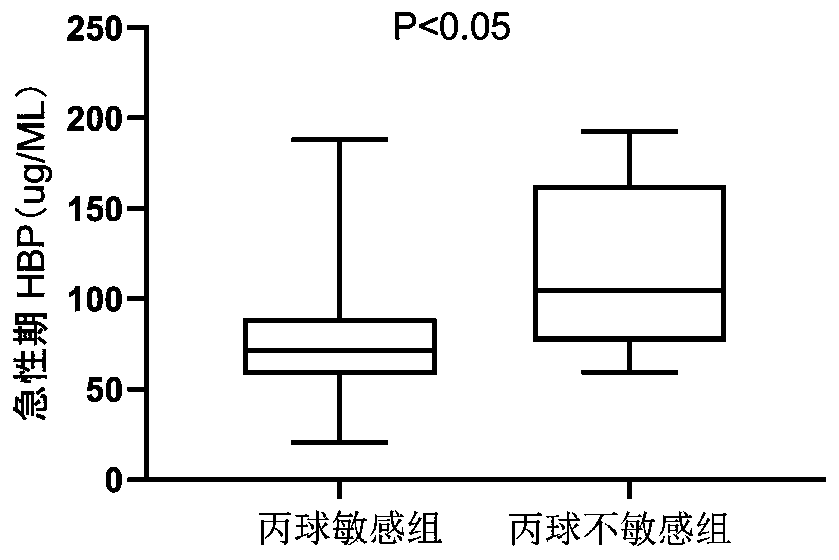
Application of HBP protein as diagnostic marker of Kawasaki disease
ActiveCN110726846AHigh sensitivityImprove accuracyDisease diagnosisBiological testingCoronary arteriesKawasaki disease
The invention provides application of HBP protein as a diagnostic marker of Kawasaki disease, and discloses application of HBP protein as a diagnostic marker of Kawasaki disease in preparing diagnostic products of Kawasaki disease and a kit for diagnosing the Kawasaki disease. The experimental results show that the plasma HBP level in acute Kawasaki patients is significantly higher than that in healthy children and general patients infected with fever, the higher HBP concentration is significantly correlated with coronary artery injury in Kawasaki disease, and the HBP level is higher in insensitive patients treated with IVIG than in sensitive patients treated with IVIG. The invention finds that plasma HBP can be used to differentiate Kawasaki disease from infected fever patients, wherein the area under ROC curve (area under the curve, AUC) is 0.88, the optimal diagnostic threshold is 52.5pg / mL, the sensitivity is 88.3%, and the specificity is 74.0%. Therefore, plasma HBP protein of patients can be used as a potential marker for distinguishing diagnosis of Kawasaki disease from infected fever.
Owner:NANJING CHILDRENS HOSPITAL
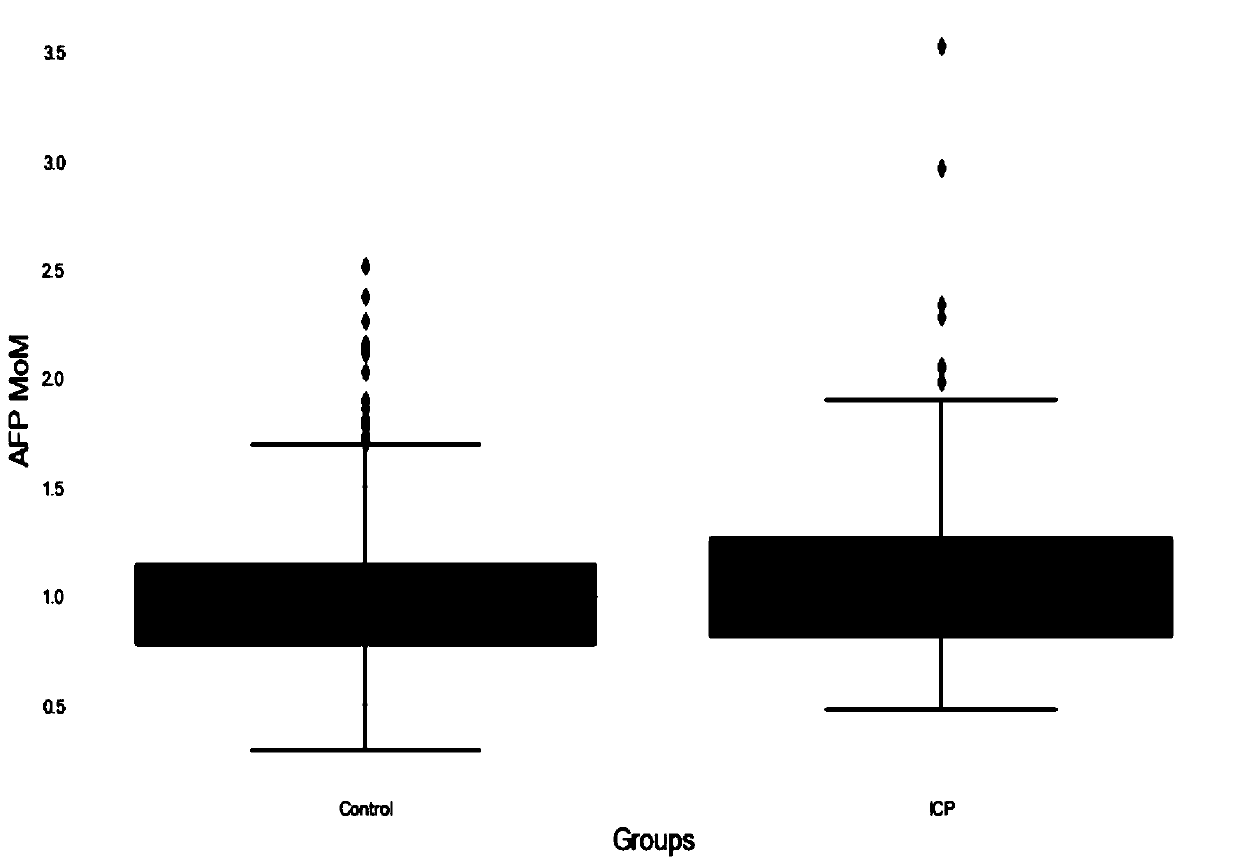
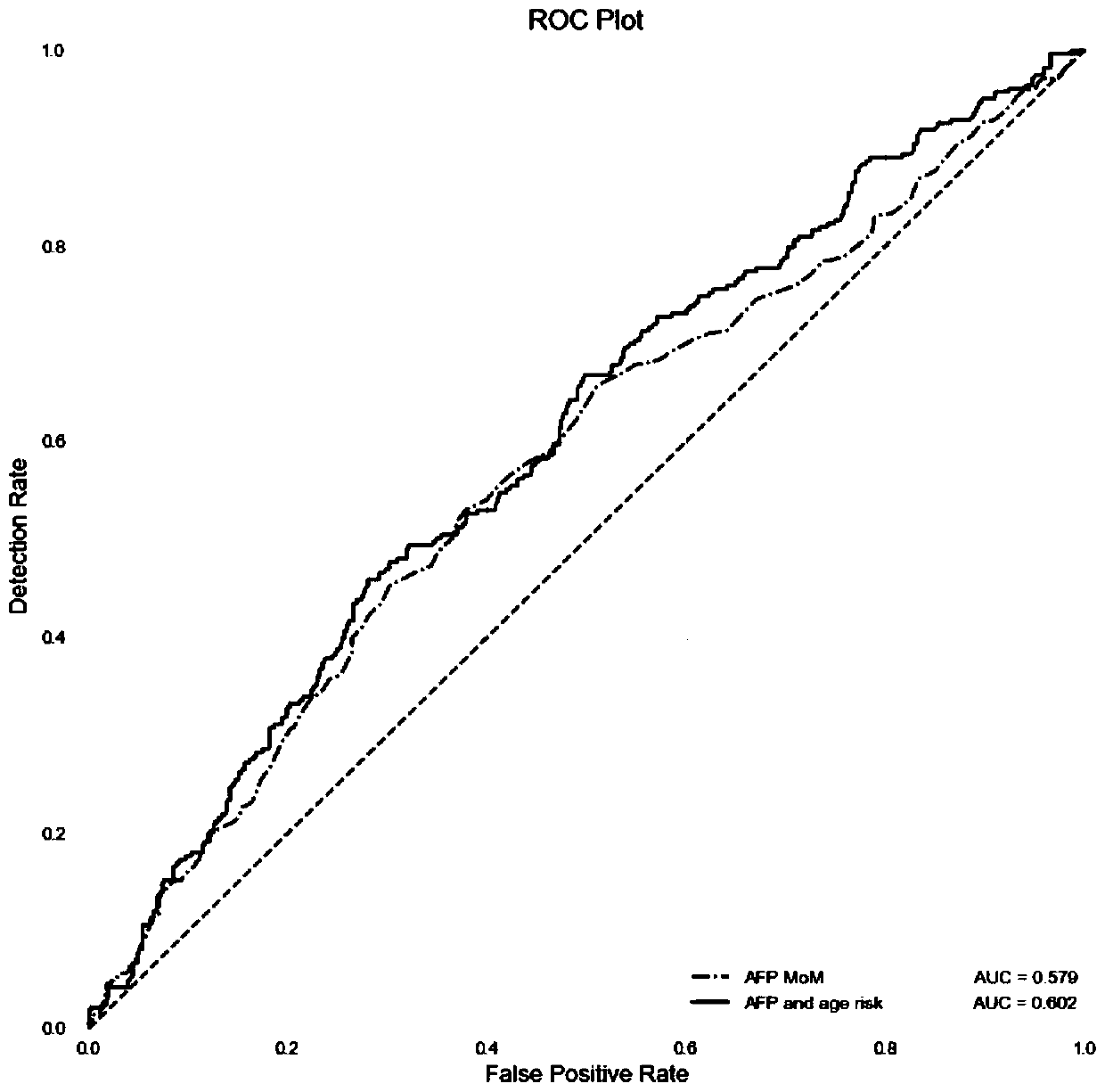
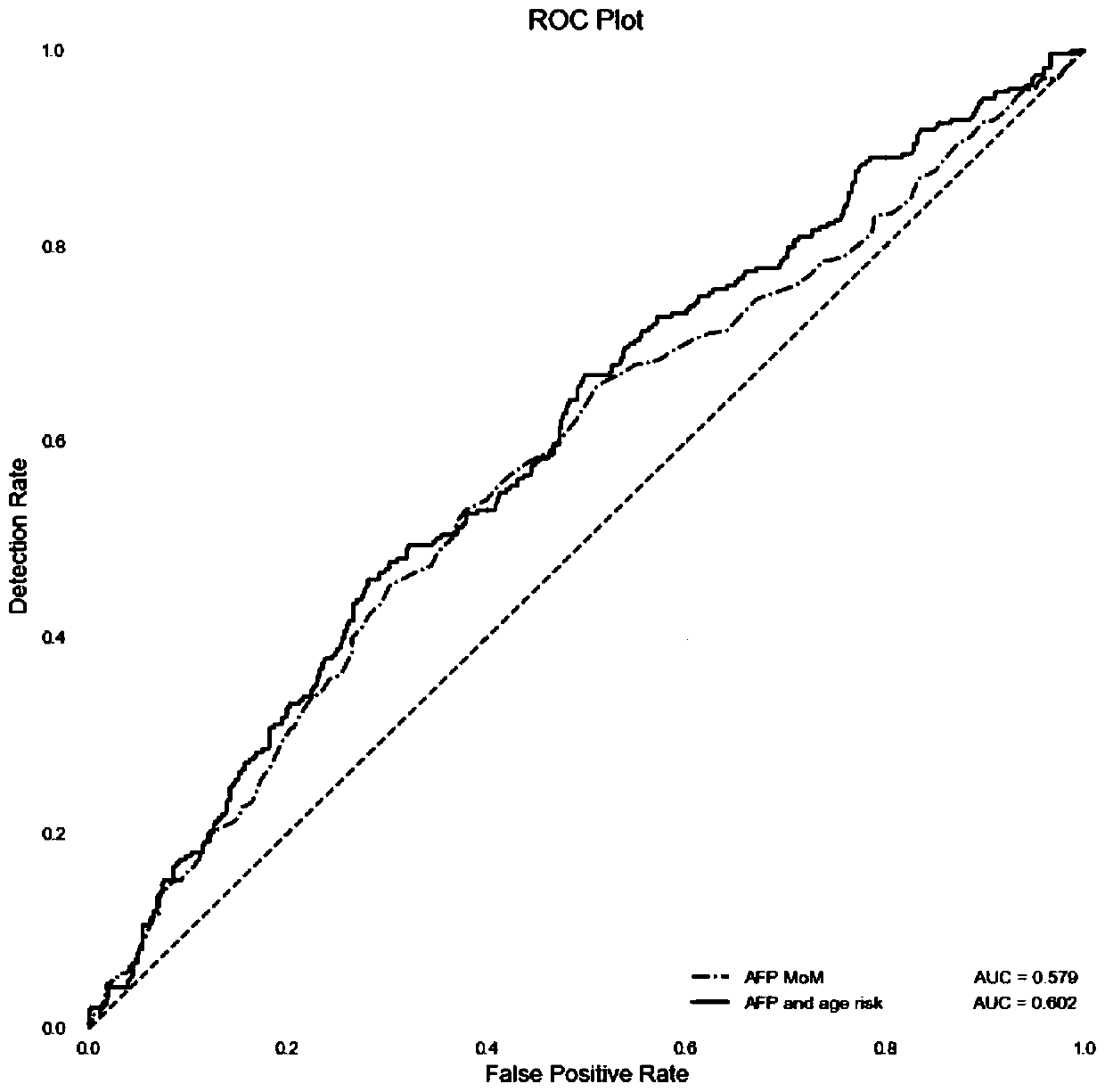
Method for establishing risk model for predicting intrahepatic cholestasis of pregnant women with maternal serum alpha fetoprotein
PendingCN111157742AHigh sensitivityImprove featuresDisease diagnosisBiological testingObstetricsImmunofluorescence
The invention discloses a method for establishing a risk model for predicting intrahepatic cholestasis of pregnant women with maternal serum alpha fetoprotein. The method comprises the following steps: (1) dividing pregnant women into a case group and a control group according to the presence or absence of intrahepatic cholestasis in the gestation period, wherein the case group refers to a pregnant woman who is clinically diagnosed with intrahepatic cholestasis in the gestation period, and the control group refers to a pregnant woman who is randomly selected and has normal fetal development inthe same period; (2) detecting AFP levels of serum of two groups of pregnant women by adopting a time-resolved immunofluorescence method, constructing a risk calculation model by combining MOM valuesof the AFP with the prenatal age, and determining an optimal truncation value and an area AUC under the curve according to an ROC curve; (3) during detection, detecting the AFP level of the serum ofthe pregnant woman to be detected by adopting a time-resolved immunofluorescence method; and screening by using a risk calculation model constructed by combining the AFP with the prenatal age, and when the MOM value of the AFP of the serum of the pregnant woman to be detected is combined with the AUC predicted by the prenatal age modeling to exceed a set threshold value, judging that the fetus ofthe pregnant woman to be detected is intrahepatic cholestasis in the gestation period. The method has the beneficial effects that the MOM value of the AFP of the serum of the pregnant woman is combined with the pre-delivery age to screen the intrahepatic cholestasis in the gestation period, so that the method has high sensitivity and specificity and can become a new marker for predicting the intrahepatic cholestasis in the gestation period; the risk calculation model constructed by combining the MOM value of the AFP of the serum of the pregnant woman with the prenatal age has a great clinicalvalue in screening the intrahepatic cholestasis in the gestation period.
Owner:杭州市妇产科医院
Method for quantitatively determining blood supply level and embolism proportion of tumors
InactiveCN105243287AOvercoming the problem of non-comparability of concentration resultsOvercome the problem of non-comparabilitySpecial data processing applicationsRadiation diagnosticsReference RegionTherapeutic effect
The invention discloses a method for quantitatively determining the blood supply level and embolism proportion of tumors, and belongs to a mathematical calculation or data processing method specially suitable for specific application. The method includes the steps that with the start portion of an organ blood supply artery as a reference region and a tumor region as a target region, the enhancement degree area under the curve of the reference region and the target region is calculated with software according to the concentration of contrast media in different regions; then, calculation is performed through the obtained enhancement degree area under the curve of the reference region and the target region to obtain the blood supply level value and the embolism proportion. By means of the method, the blood supply level and the embolism proportion before and after liver cancer interventional treatment can be effectively analyzed and identified through an imageological examination result; the method has great significance in correctly selecting an interventional treatment method, making a treatment plan and improving the liver cancer treatment effect and has broad application prospects.
Owner:李勇
Application of circRNA marker for diagnosing thalassemia
ActiveCN111560429AHigh sensitivityImprove featuresMicrobiological testing/measurementDNA/RNA fragmentationDiagnosis earlyThalassemia
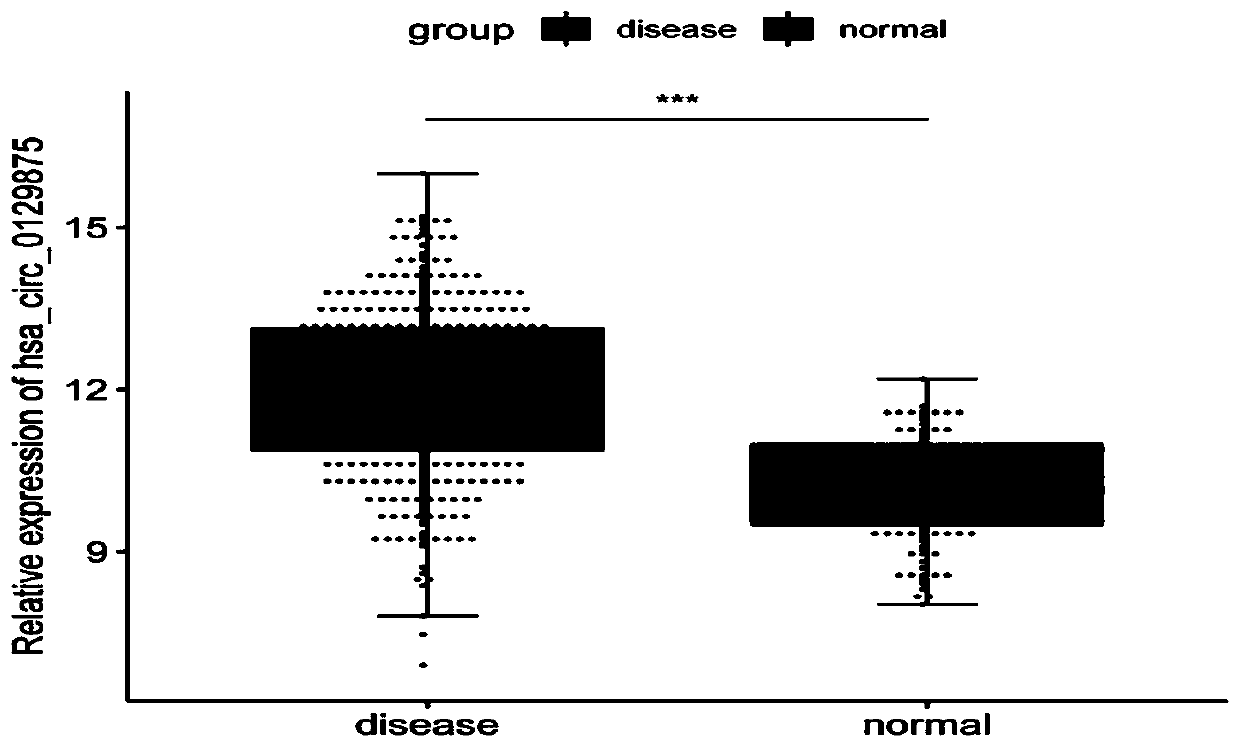
The invention discloses an application of a circRNA marker for diagnosing thalassemia, and belongs to the technical field of biology. The circRNA marker is hsa-circc-0129875 as shown in SEQ ID NO: 1,and the nucleotide sequence of the circRNA marker is shown in SEQ ID NO: 2. According to the circRNA marker provided by the invention, early diagnosis of thalassemia is realized; according to the invention, the circRNA marker and clinical examination indexes (HCT, MCV, MCH and MCHC) are combined to diagnose thalassemia. According to the invention, results show that the sensitivity of AUC diagnosedby combining hsa-circ-0129875 with clinical examination indexes is superior to that of AUC diagnosed by single circRNA, the area under an AUC curve is maximum, the sensitivity reaches 85% or above, and the specificity reaches 90% or above. The circRNA marker has the advantages of strong characteristics, strong sensitivity and stable result, provides a theoretical reference basis for the diagnosisof thalassemia, and has a wide clinical application prospect.
Owner:广州市番禺区中心医院
Biomarkers for detecting and monitoring colon cancer
A metabolic profiling approach for identifying biomarkers that provide highly sensitive and specific colorectal cancer (CRC) detection and monitoring using serum samples. The methods can be used for distinguishing CRC patients from both healthy controls and polyp patients, as well as to monitor disease progression or response to therapy. Receiver operator characteristic curves generated based on these models showed high sensitivities for differentiating CRC patients from healthy controls or polyp patients, good specificities, low false discovery rates, and excellent areas under the curve were obtained. Monte Carlo cross validation (MCCV) was also applied, demonstrating the robust diagnostic power of this metabolic profiling approach.
Owner:UNIV OF WASHINGTON
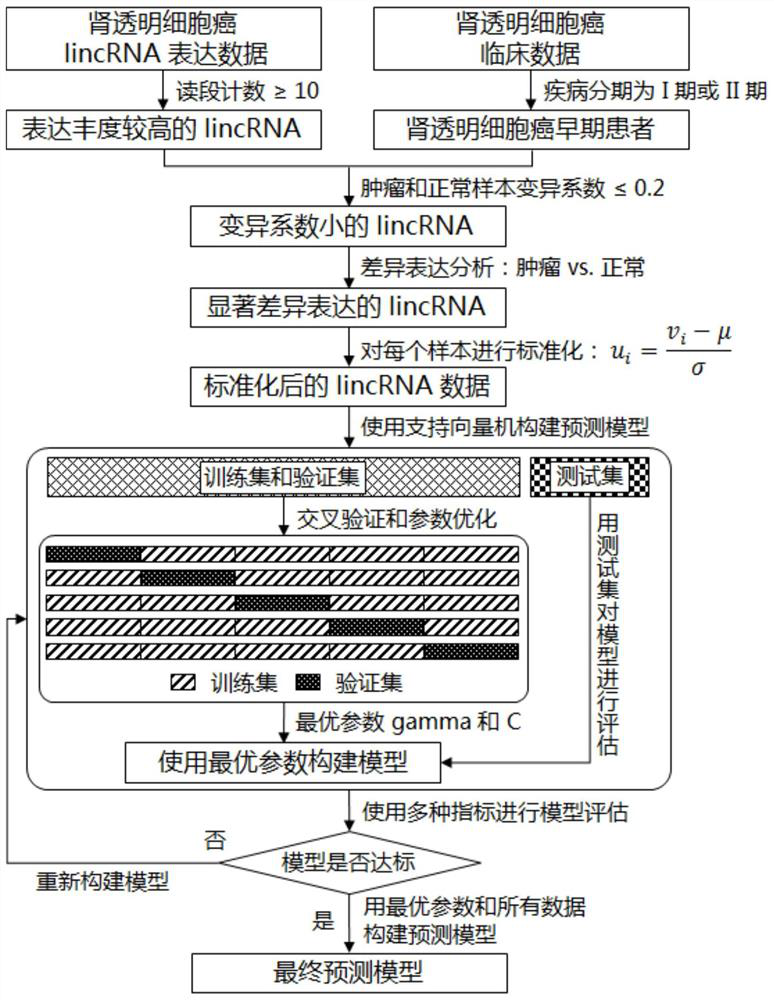
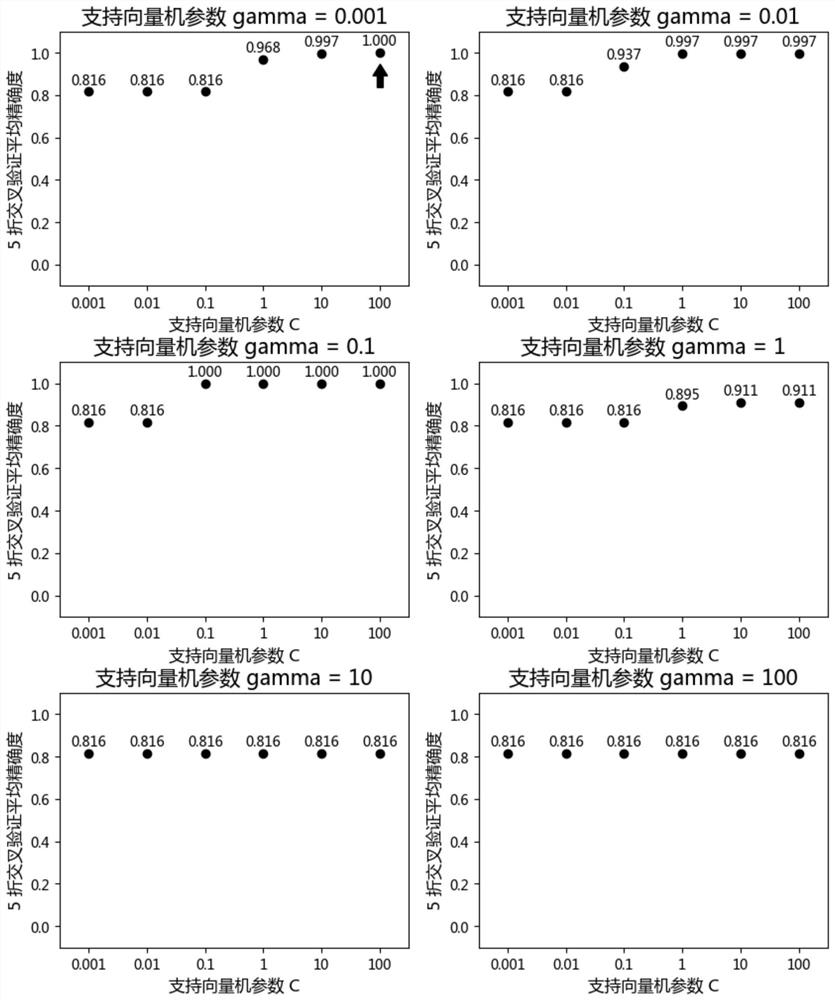
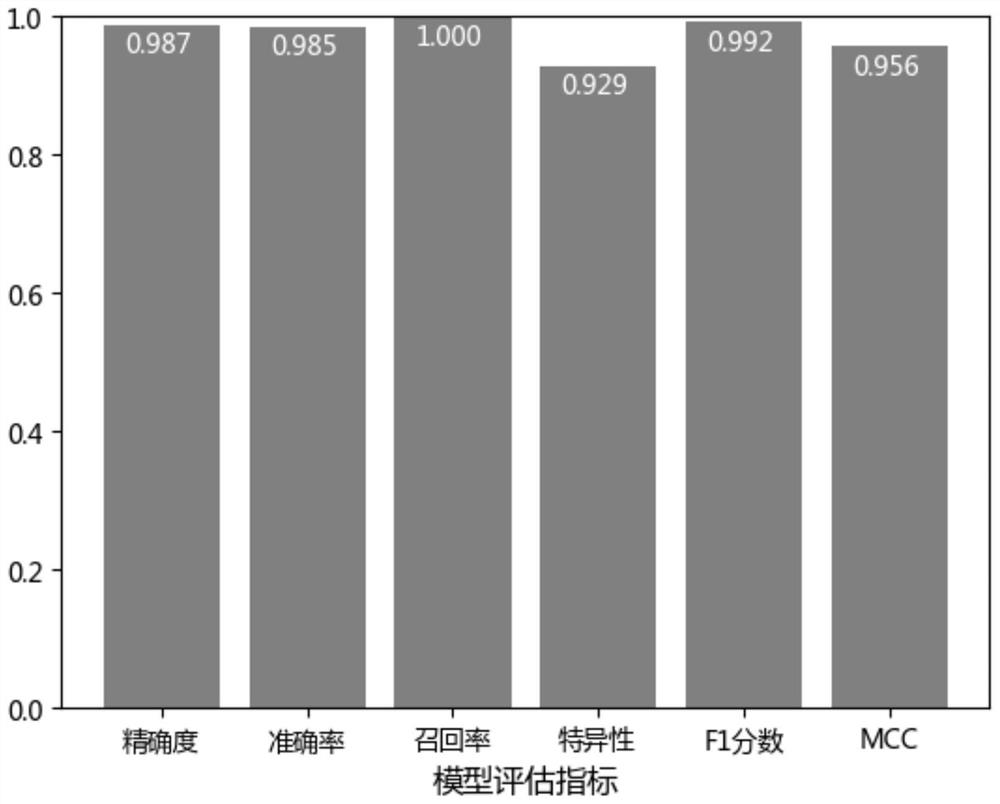
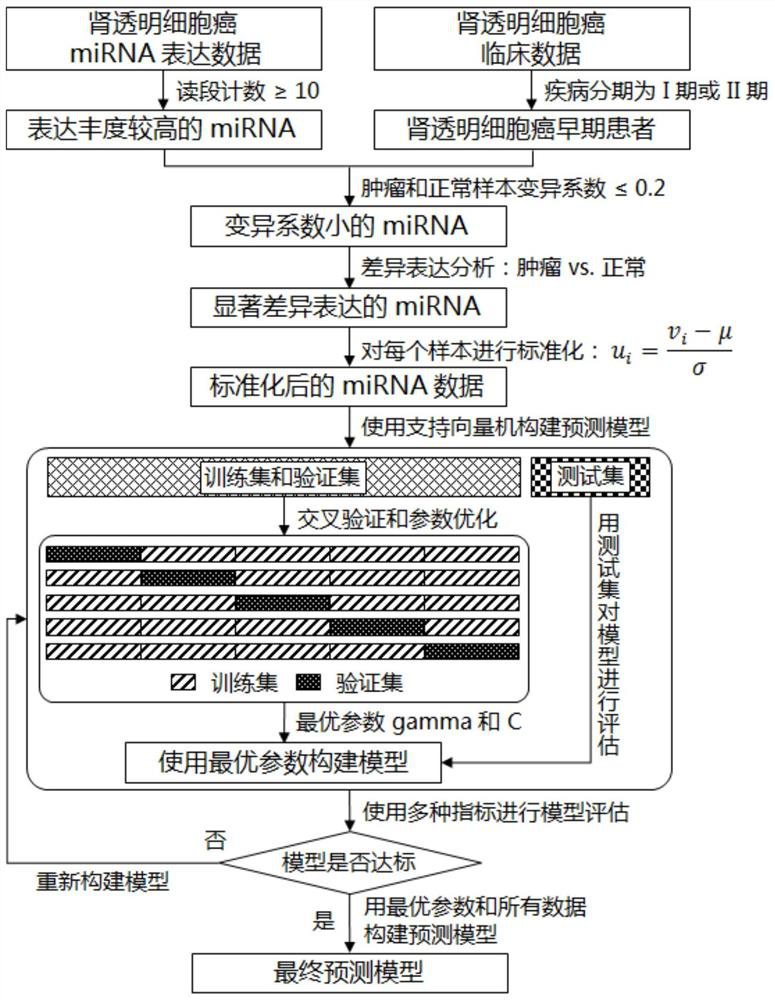
Featured lincRNA expression profile combination and early stage prediction method for renal clear cell carcinoma
InactiveCN111808965AFast predictionQuick forecastMicrobiological testing/measurementBiostatisticsNucleotideRenal clear cell carcinoma
The invention discloses a featured lincRNA expression profile combination and an early stage prediction method for a renal clear cell carcinoma. The nucleotide probe sequence of the lincRNA is disclosed by SEQ ID NO.1-26. The early-stage risk assessment of the renal clear cell carcinoma on the basis of the characteristics of the lincRNA expression profile combination has high precision and accuracy (ROC (Receiver Operating Curve) ACU (Area Under The Curve) is equal to 0.964). Only the relative expression quantities of the above 26 types of lincRNA need to be obtained, an early stage illness probability of the renal clear cell carcinoma is given through a support vector machine model, and the early stage illness probability can be taken as a reference basis for the early stage prediction ofthe renal clear cell carcinoma.
Owner:FOSHAN UNIVERSITY
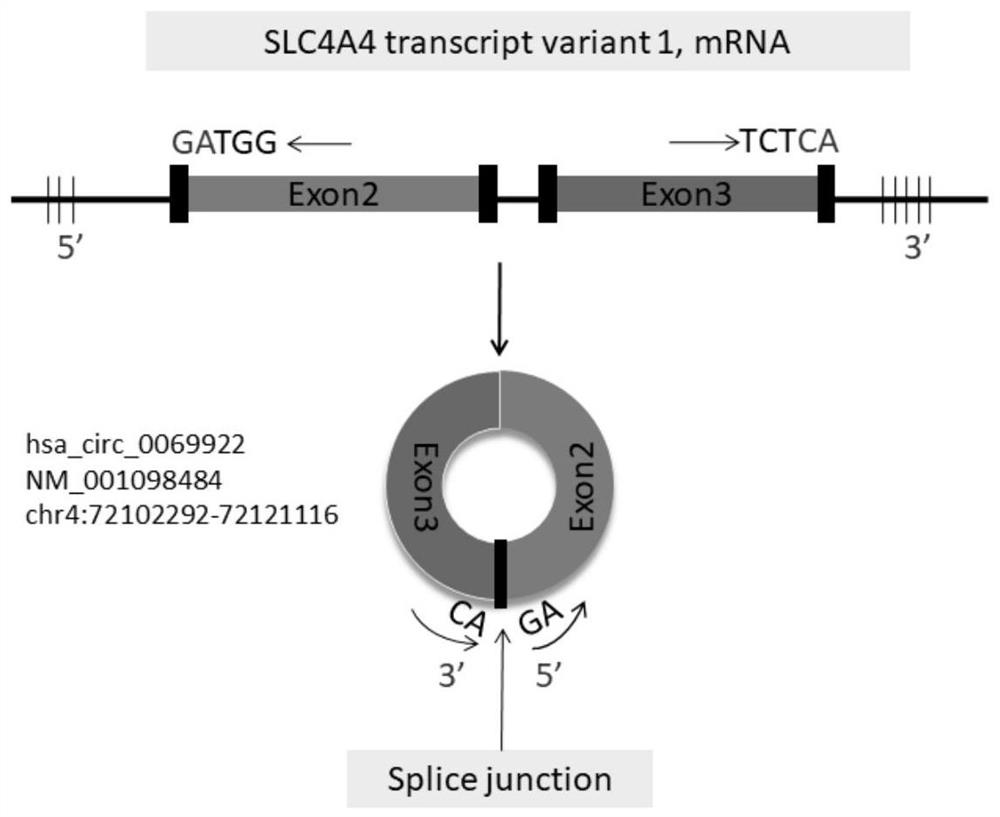
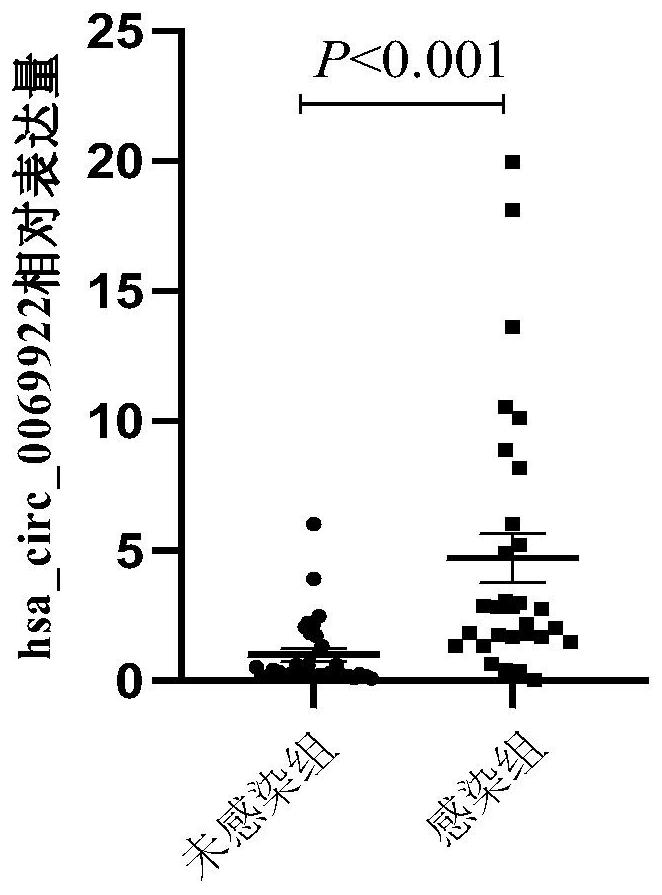
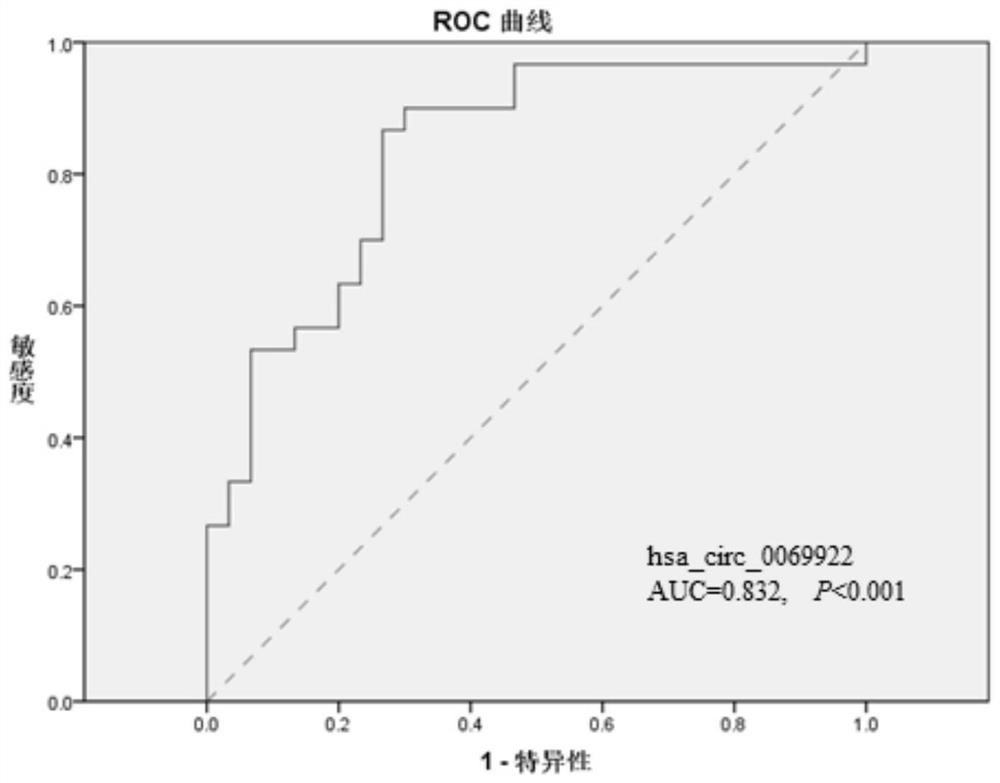
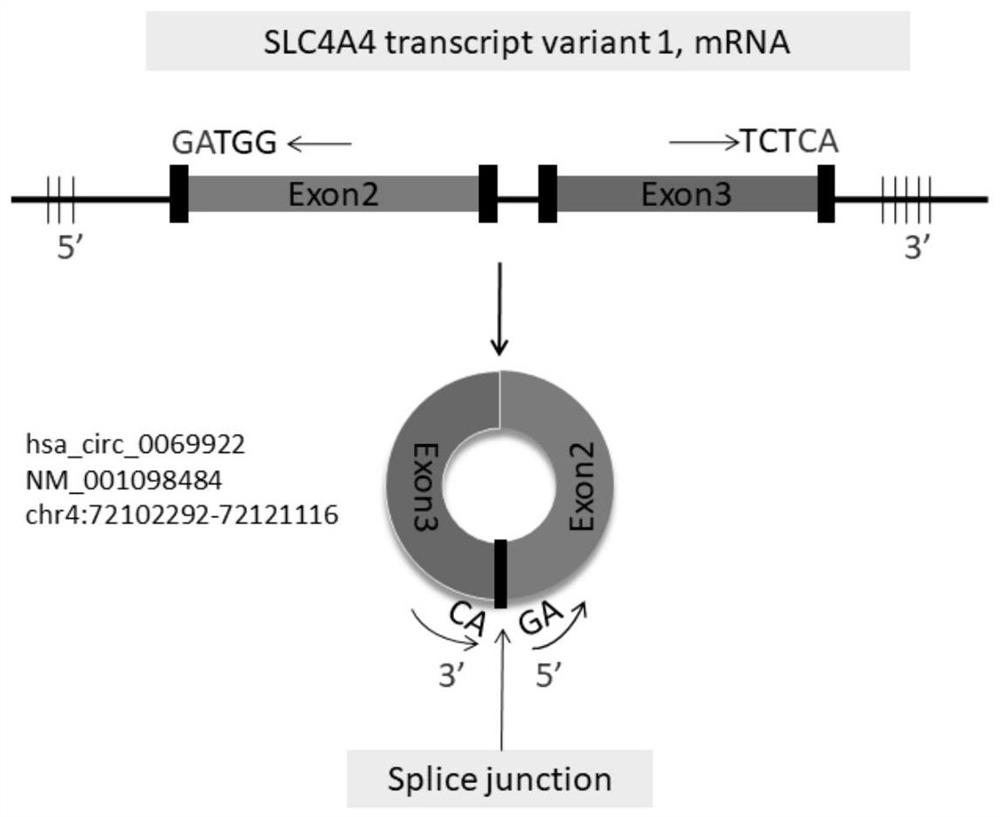
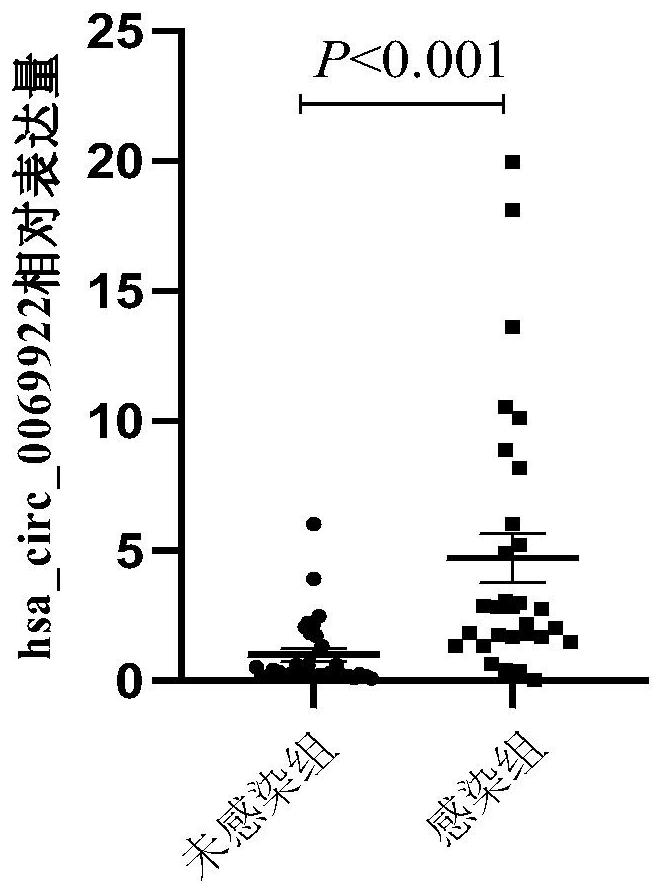
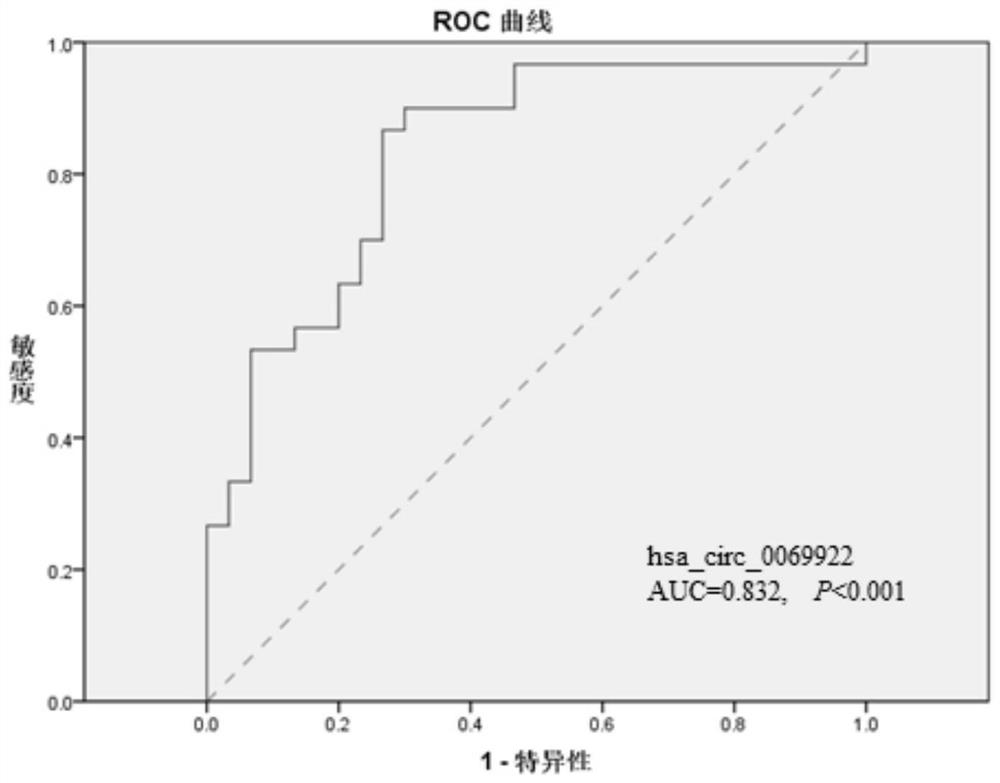
Use of hsa_circ_0069922 as marker
ActiveCN112176055AHigh diagnostic valueIncreased sensitivityNervous disorderMicrobiological testing/measurementPeripheral blood mononuclear cellPharmaceutical drug
The present invention discloses a use of hsa_circ_0069922 as a marker in preparing a product for screening stroke-associated infection (SAI). The product for screening the stroke-related infection comprises a reagent or a drug for detecting an expression level of the hsa_circ_0069922 in peripheral blood mononuclear cells of a patient. The present invention develops the new molecular marker hsa_circ_0069922 for screening diagnosis of the SAI and a new reference for screening the SAI. The marker hsa_circ_0069922 is high in diagnostic value, a ROC curve analysis shows that patients with the SAI can be well distinguished and screened, an area under the curve is 0.832, and the marker hsa_circ_0069922 has good sensitivity and specificity and is expected to become an important index applied to evaluation of clinical diagnosis and treatment of the SAI.
Owner:NANJING FIRST HOSPITAL
Characteristic miRNA expression profile combination and method for early prediction of gastric cancer
InactiveCN111733252AFast predictionQuick forecastMicrobiological testing/measurementBiostatisticsEarly predictionNucleotide
The invention discloses a characteristic miRNA expression profile combination and a method for early prediction of gastric cancer. The miRNAs include: hsa-mir-106b, hsa-mir-1307, hsa-mir-143, hsa-mir-17, hsa-mir-20a, hsa-mir-21, hsa-mir-532 and hsa-mir-93, and nucleotide probe sequences are shown in SEQ ID NO. 1-8. According to the invention, miRNA expression profile combination-based early risk assessment of the gastric cancer is high in accuracy and precision (area under the curve, AUC = 0.938), and the probability of developing early gastric cancer can be calculated by a support vector machine model with a need of only obtaining relative expressions of the above-mentioned eight miRNAs, so that the invention can be used as a reference for the early prediction of the gastric cancer.
Owner:GUANGDONG NO 2 PROVINCIAL PEOPLES HOSPITAL
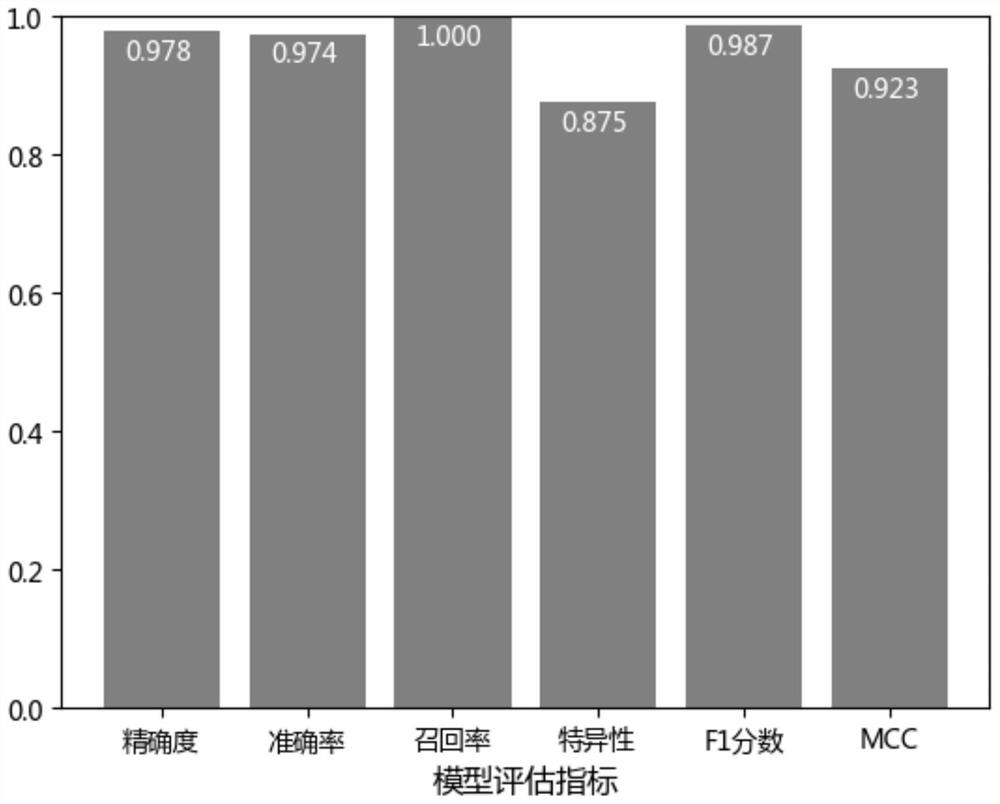
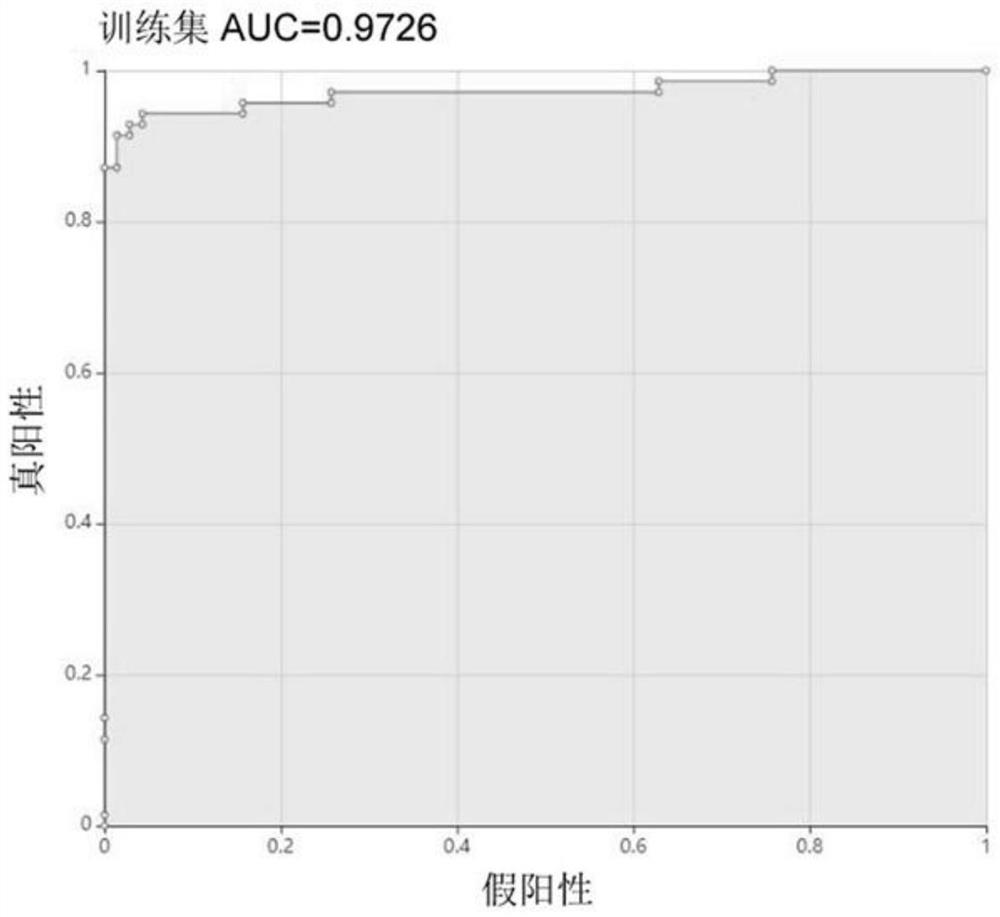
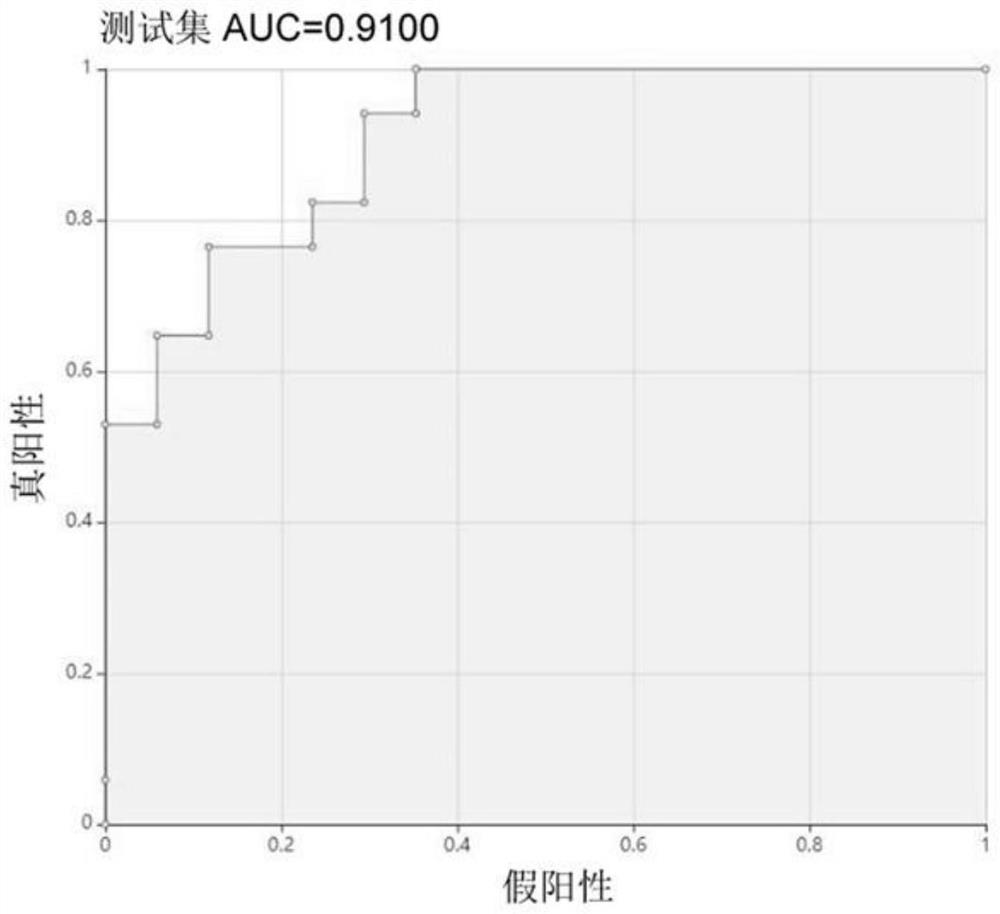
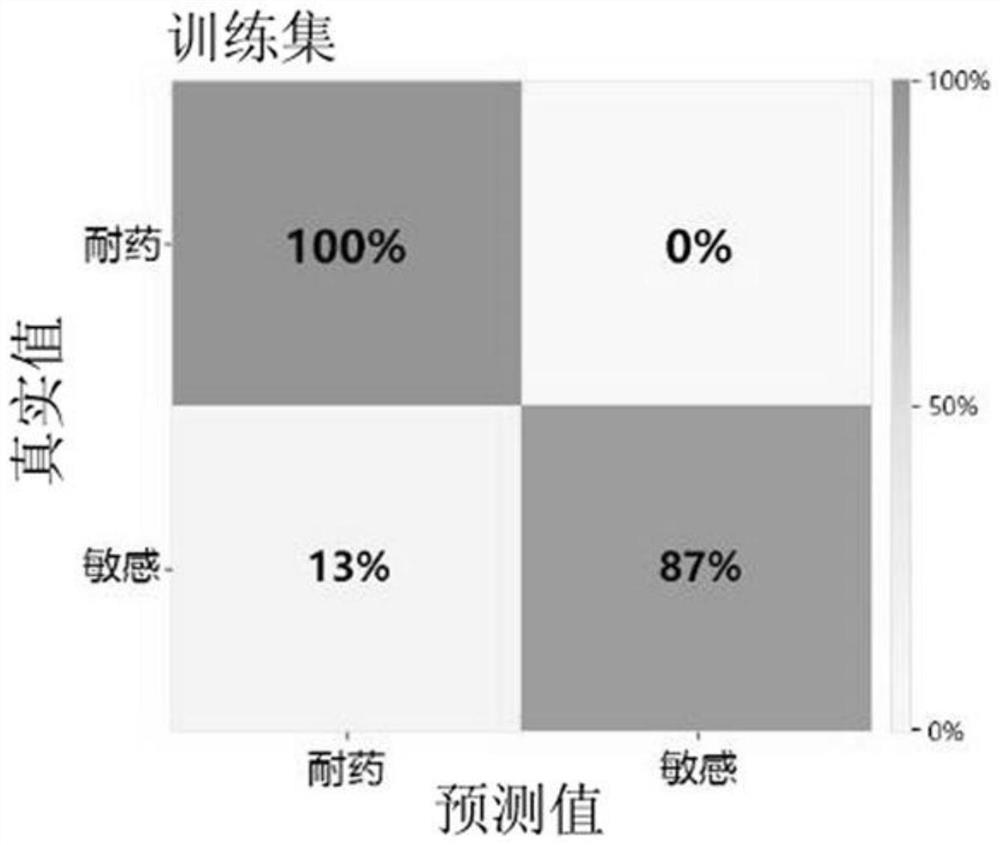
Klebsiella pneumoniae imipenem drug sensitivity machine learning prediction model
The invention discloses a Klebsiella pneumoniae imipenem drug sensitivity machine learning prediction model, and belongs to the technical field of drug sensitivity prediction, and the specific steps are as follows: randomly selecting mass spectrum peak data of imipenem sensitivity and drug resistance examples, establishing a training set data model through machine learning minimum absolute value selection and a contraction operator algorithm, and establishing a training set data model; sensitive and drug-resistant examples are randomly selected, a test set model is established, orthogonal partial least squares discriminant analysis is performed on mass spectrum peak data of the specimens, areas under curves of a training set and the test set model are respectively calculated, a test set confusion matrix is established, and the accuracy of the prediction model is verified. Therefore, the prediction model of the sensitivity of the klebsiella pneumoniae to the imipenem drug is established through a machine learning method and verified, and the obtained prediction model has high accuracy and has potential clinical aid decision support capacity.
Owner:朱彧
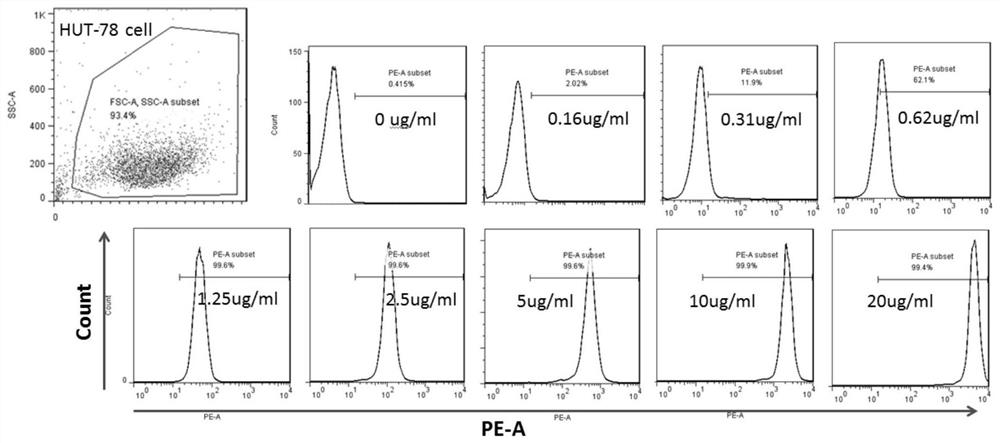
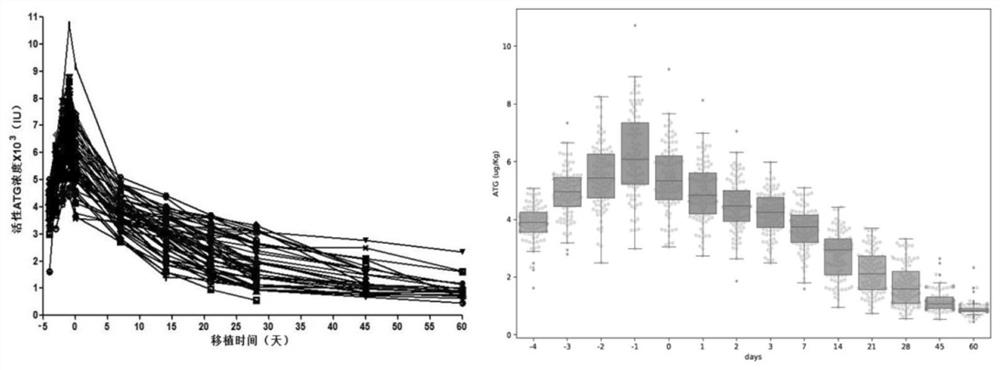
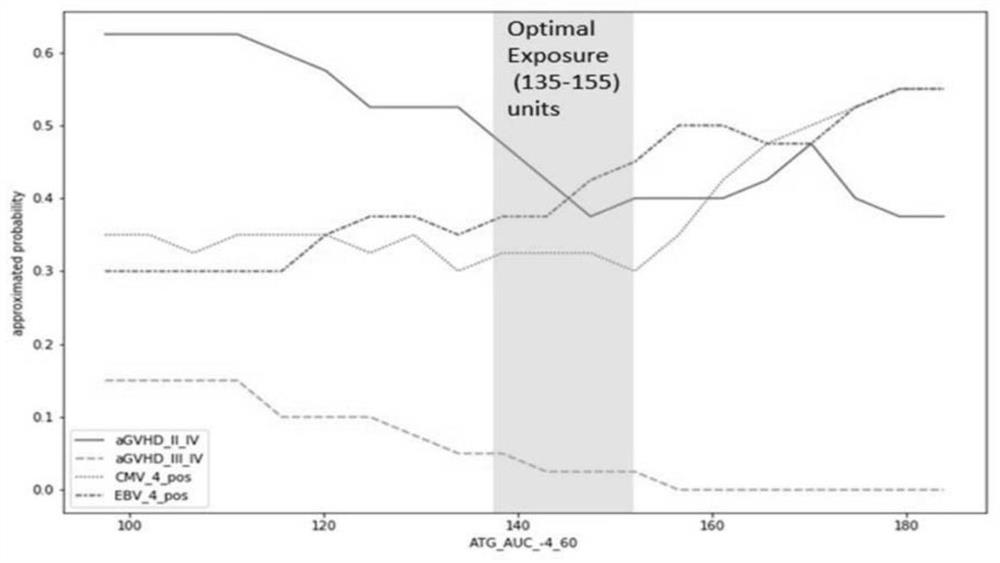
Method for calculating individualized dosage of ATG for haploid hematopoietic stem cell transplantation
PendingCN112669992ALower reactivation rateImprove survival rateDrug and medicationsDrug referencesHematopoietic stem cell transplantationMalignancy
The invention relates to a method for calculating the individualized dosage of ATG for haploid hematopoietic stem cell transplantation. The ATG drug dosage applied to a subject enables the area exposure range of the active ATG of the subject under the curve to be 135-155U / L.d. The optimal exposure range of AUC exposure of active ATG is 135-155U / L.d by analyzing ATG concentration data of 106 cases of haploid transplantation, and the ATG individualized dosahe calculation method is further obtained through computer simulation, so that the ATG dosage is individualized, finally, the GVHD and virus reactivation rate of the patient is reduced, a new treatment thought and scheme are provided for prevention of the GVHD, and finally, the survival rate of the hematological malignancy patient is improved.
Owner:GENERAL HOSPITAL OF PLA
Methods and compositions for predicting chronic lung allograft dysfunction
ActiveUS11421277B2Reduce riskMicrobiological testing/measurementFermentationImmunosuppressive drugGraft dysfunction
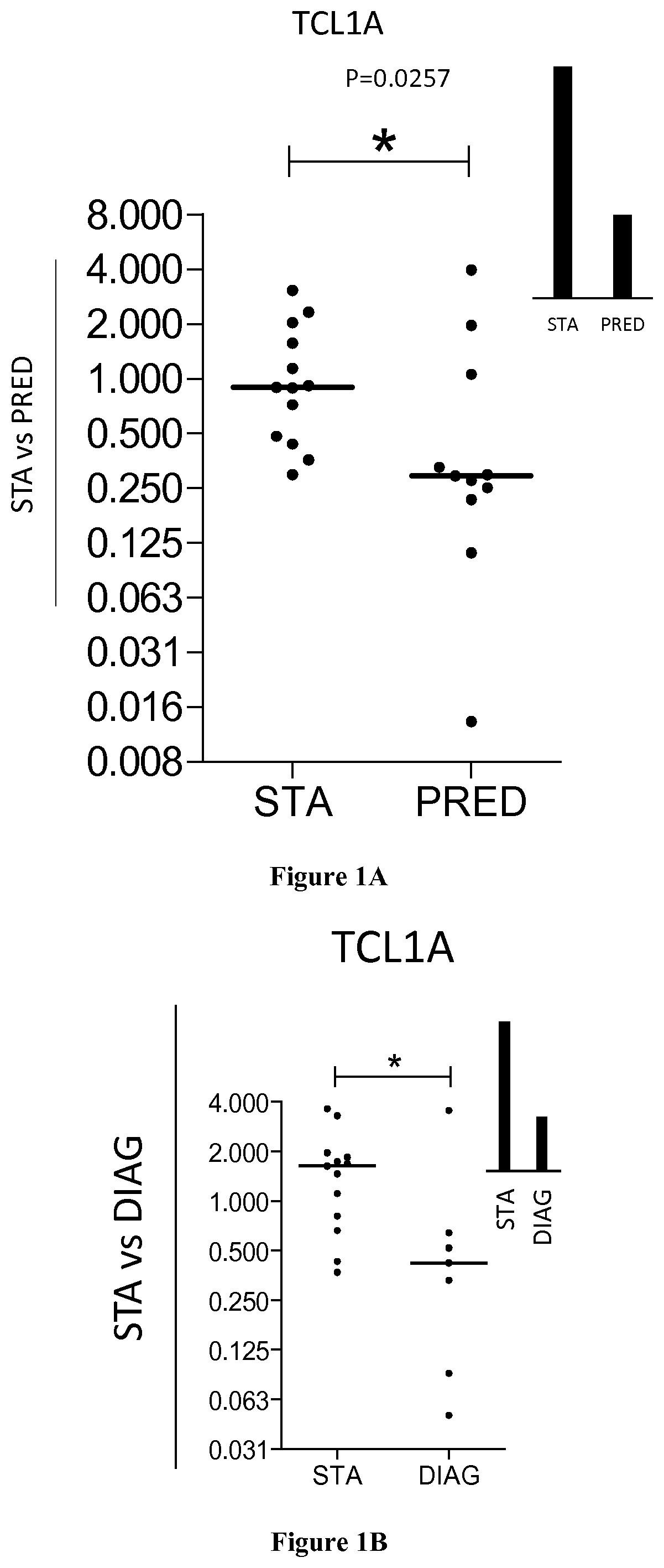
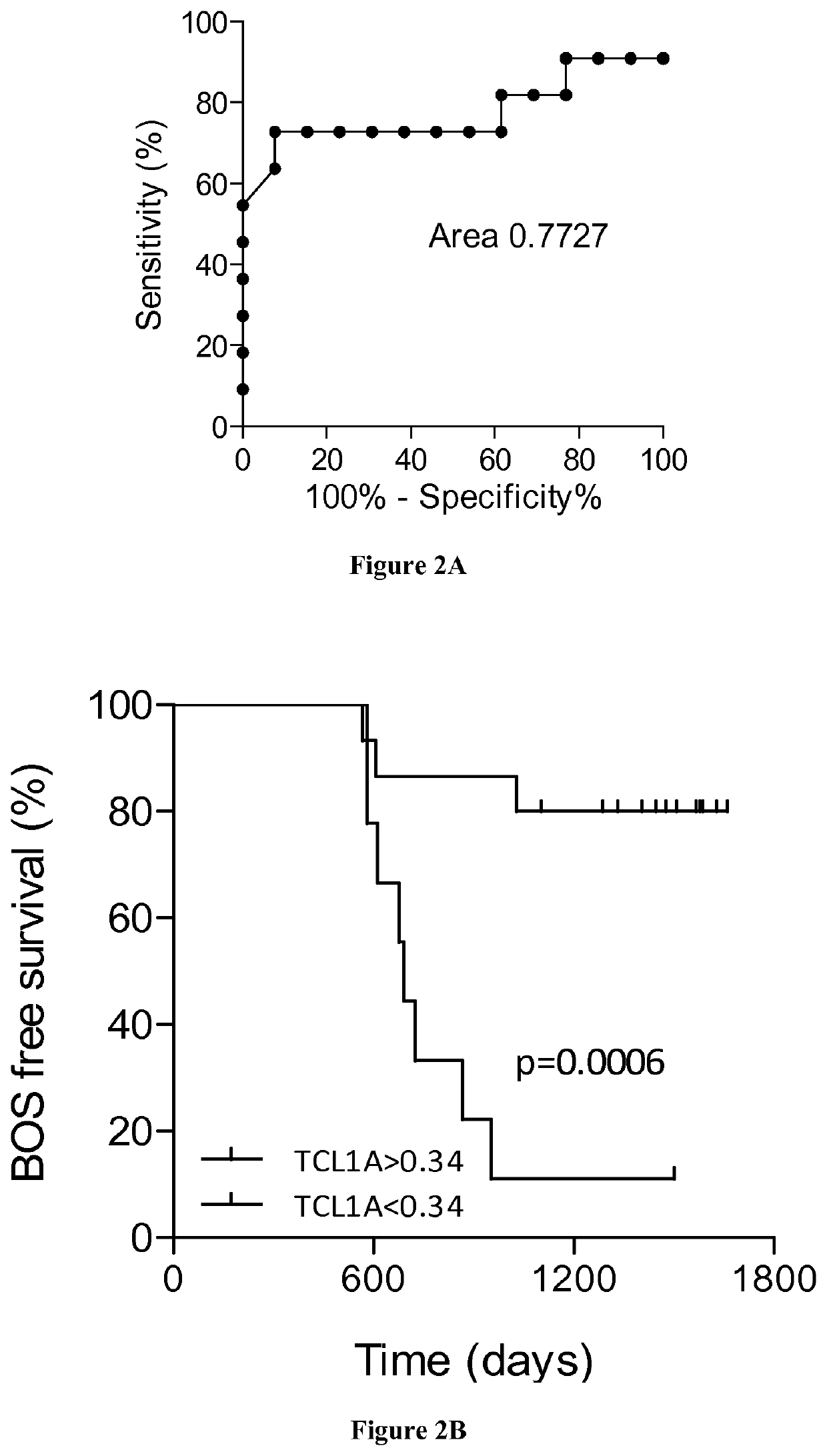
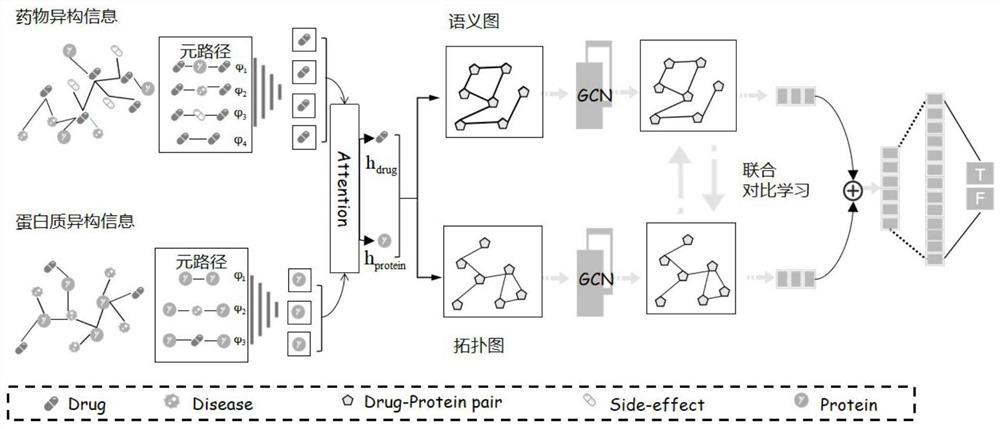
The present invention relates to a method for predicting the risk of having the CLAD in a subject by measuring the expression level of TCL1A in a biological sample obtained from said subject. Inventors have used a large-scale gene expression profiling of whole blood cells to identify early biomarkers of BOS. Microarray experiments performed from 80 patients (40 stable (STA) and 40 BOS) identified 47 genes differentially expressed between STA and BOS recipients. An independent set of patients (13 STA, 11 BOS) was then used for external validation by qPCR. T-cell leukemia / lymphoma protein 1A (TCL1A) gene was identified and validated as a predictive marker of BOS more than 6 months before diagnosis with area under curve of 0.77. Accordingly, the invention relates to a method for predicting the risk of having the chronic lung allograft dysfunction (CLAD) and to a method for preventing the risk of having CLAD by administering immunosuppressive drugs.
Owner:INST NAT DE LA SANTE & DE LA RECHERCHE MEDICALE (INSERM) +3
A Drug-Target Interaction Prediction Method Based on Contrastive Learning of Supervised Synergy Graphs
ActiveCN114023464BImprove learning effectReduce processing stepsBiostatisticsDrug referencesFeature extractionPharmaceutical drug
A drug-target interaction prediction method based on supervised synergy graph contrastive learning belongs to the technical field of drug-target relationship prediction. The invention solves the problems that the traditional machine learning method needs to rely on tedious manual feature extraction and the model has too many complicated steps. The drug-target interaction prediction method of the present invention uses graph comparison learning to enhance the learning ability of the model. In the whole prediction process, no manual operation is required, that is, it does not rely on cumbersome manual feature extraction, and the end-to-end idea is applied to reduce The processing steps of the model are reduced, the complexity of the model is reduced, and a high prediction accuracy is ensured. It is obtained through experiments that the area under the Roc curve of the prediction method of the present invention can reach 0.9764, and the area under the PR curve can reach 0.9761. The present invention can be used to predict drug-target relationships.
Owner:NORTHEAST FORESTRY UNIVERSITY
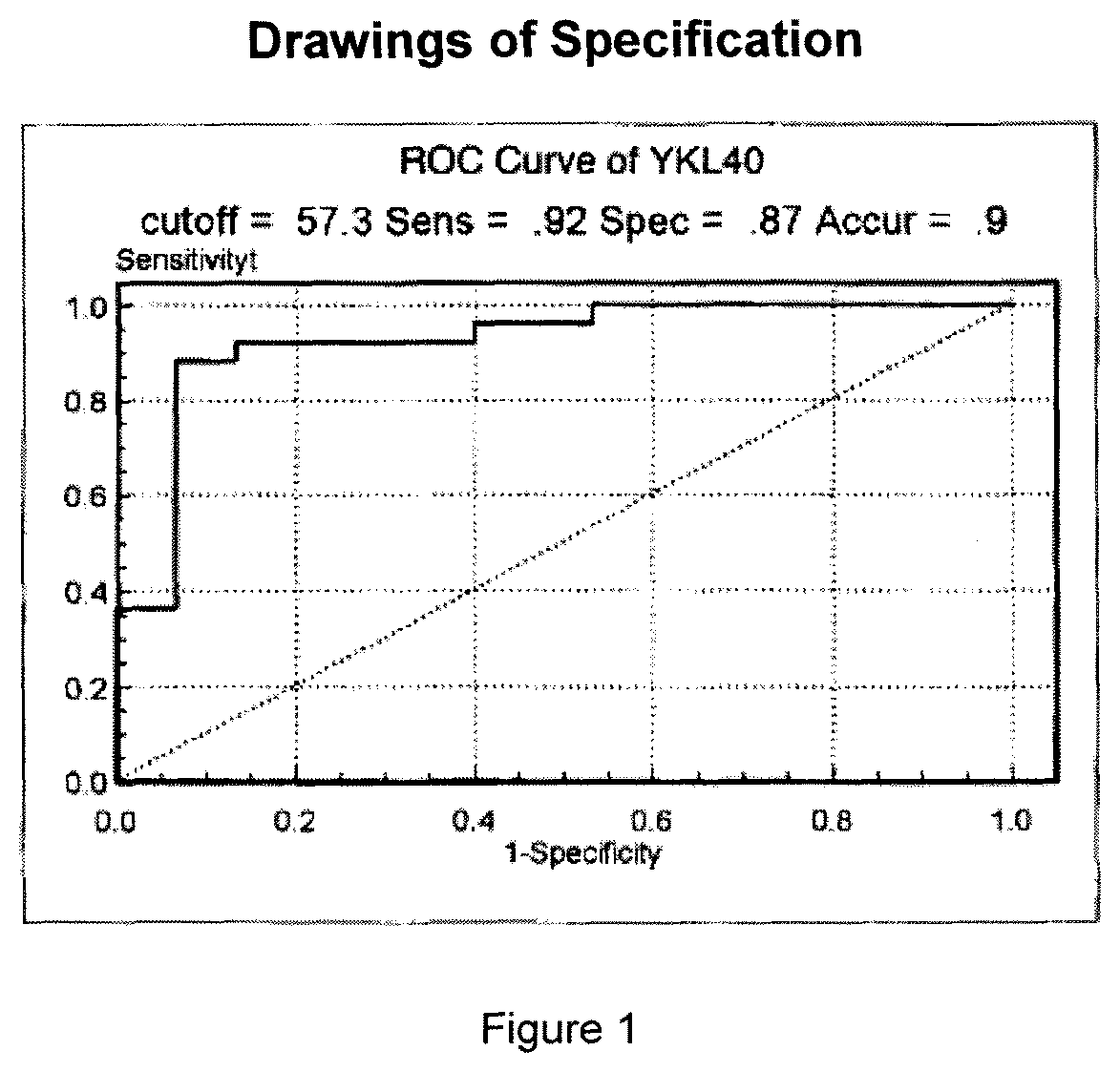
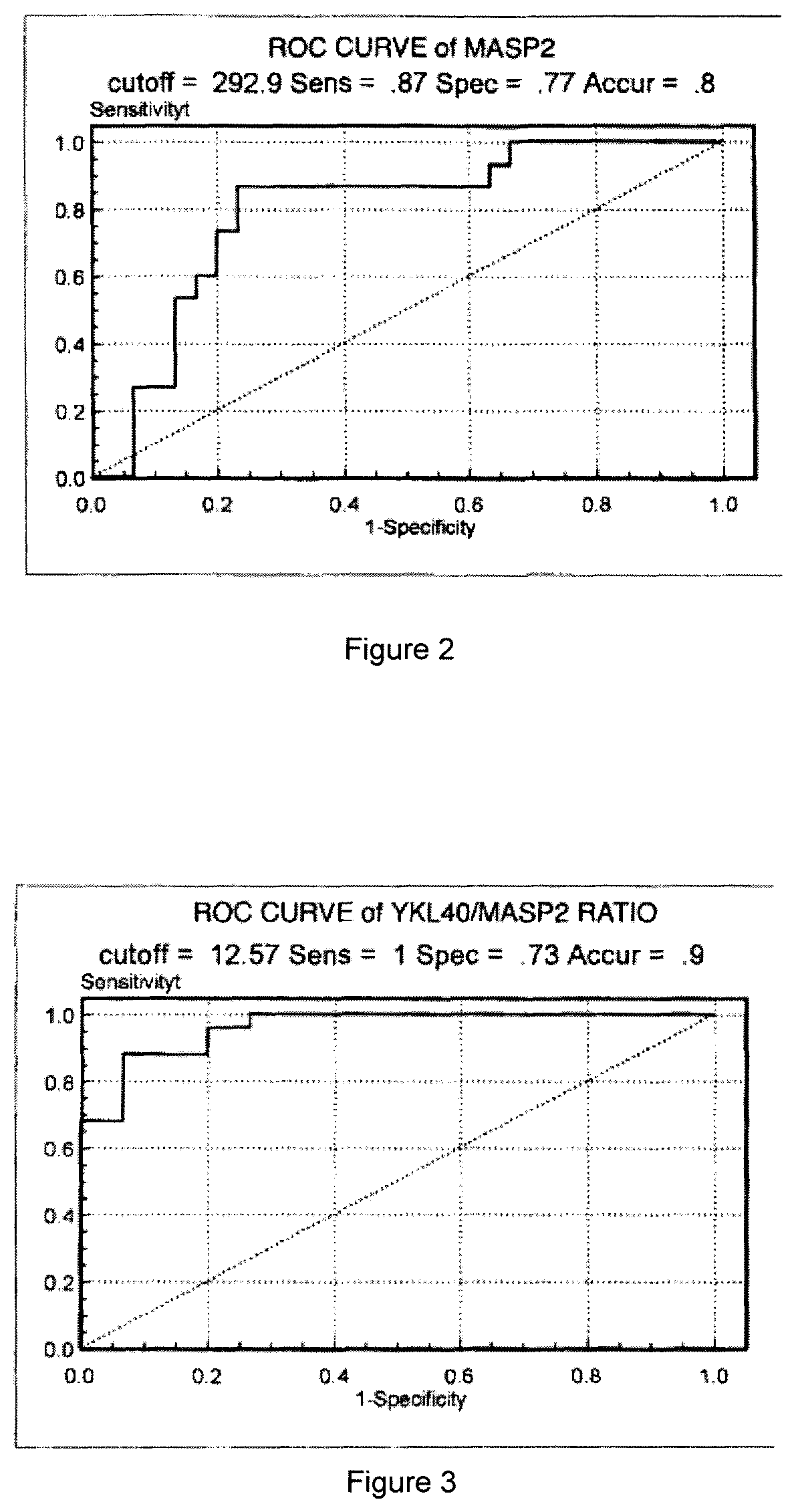
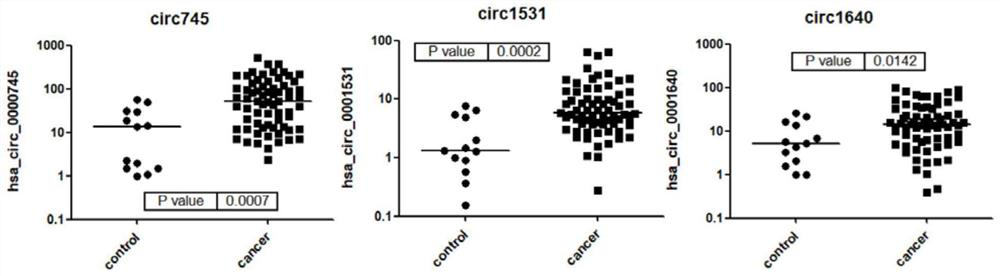
Sample hepatocarcinoma classification with YKL-40 to MASP2 concentration ratio
ActiveUS11193934B2High sensitivityStrong specificityMicrobiological testing/measurementBiological material analysisCARCINOMA LIVERCancers diagnosis
A method for increasing the veracity of classification by detecting protein YKL-40 and MASP2 levels in the samples, comprises detecting the content of the YKL-40 and MASP2 in the samples; the ratio of the content of YKL-40 to the that of MASP2 acted as variable, receiving the ROC curve according to the sensitivity and specialty of the difference threshold value to the diagnosis for the cancer, calculating an area under the curve AUC; classifying the samples according to the value of the AUC, sensitivity and specialty. And a kit for detecting the protein YKL-40 and MASP2 levels in the samples.
Owner:LIN BIAOYANG
A kind of circRNA marker and application thereof for diagnosis of breast cancer
ActiveCN109097477BEasy to distinguishMicrobiological testing/measurementDNA/RNA fragmentationOncologyBiology
The present disclosure relates to a circRNA marker for the diagnosis of breast cancer and its circRNA application. Compared with the healthy control group, three circRNAs (hsa_circ_0000745, hsa_circ_0001531, hsa_circ_0001640) were found to be up-regulated in whole blood of breast cancer. ROC curve analysis shows that it can better distinguish breast cancer patients from healthy people. The areas under the curves of the three are 0.7998, 0.8258, and 0.7161, the sensitivities are 48.53%, 92.65%, 73.53%, and the specificities are 100%. , 69.23%, 69.23%.
Owner:SHANDONG UNIV QILU HOSPITAL
Risk assessment model for papillary cancer of thyroid nodule patient
PendingCN112037919AComprehensive forecastIncrease medical costsMedical simulationMedical data miningDiseaseOncology
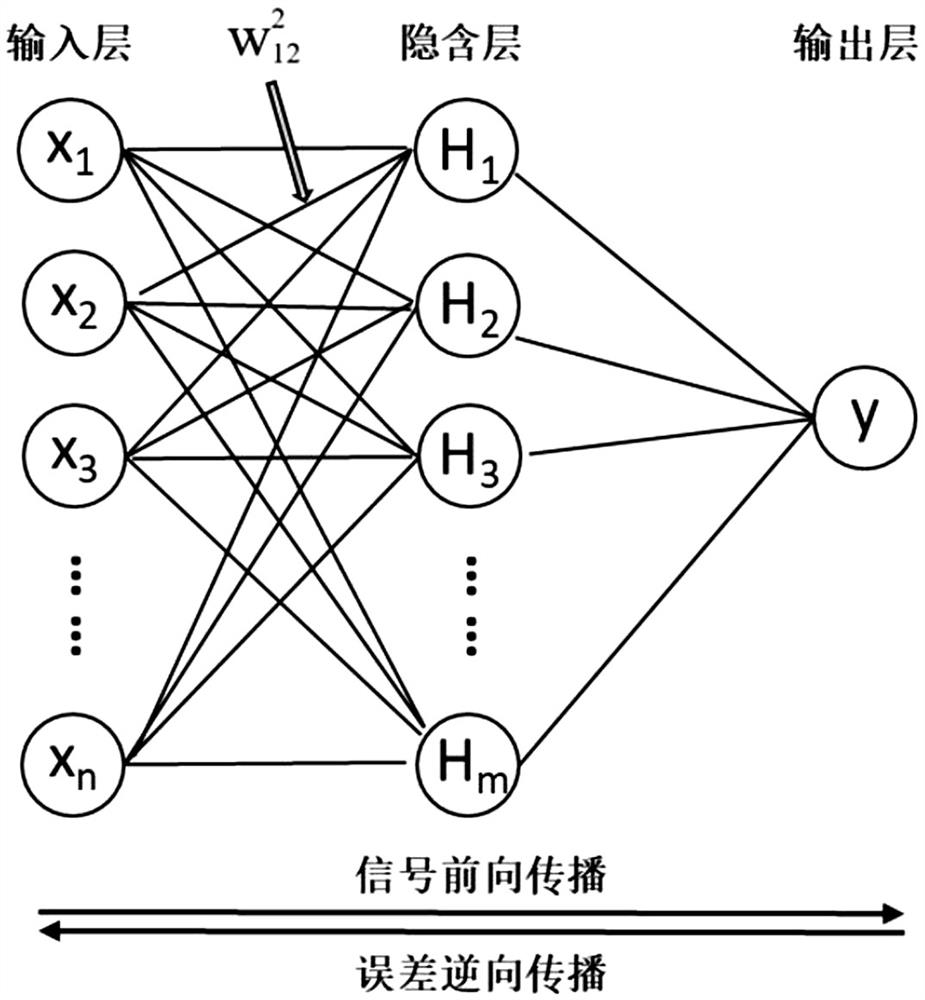
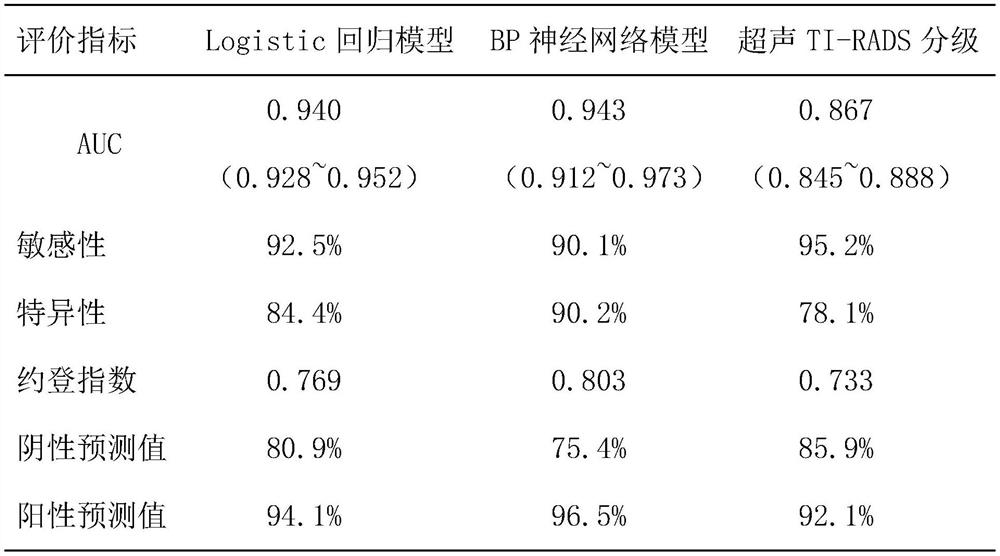
The invention discloses a risk assessment model for papillary cancer of a thyroid nodule patient, and the model is characterized in that the model comprises the steps: carrying out the risk factor analysis of PTC through Logistic regression, and selecting a specific index as an independent variable of the model; constructing a BP neural network model comprising three layers of feedforward neural network structures of an input layer, a hidden layer and an output layer, and training and predicting the BP neural network model by utilizing data information comprising the specific indexes; and comparing a prediction value output by the model with a real value, drawing an ROC curve to obtain an area AUC under the curve, evaluating the diagnosis performance of the model by using the AUC value, and the like, so that the model with the diagnosis performance reaching the standard is a target risk evaluation model. The risk assessment model can be used as a reference for a doctor to diagnose papillary thyroid carcinoma, and the accuracy of diagnosis of papillary thyroid carcinoma diseases is improved.
Owner:NANJING DRUM TOWER HOSPITAL
Characteristic miRNA expression profile combination and early prediction method for uterine corpus endometrial carcinoma
PendingCN112760375AFast predictionQuick forecastMicrobiological testing/measurementBiostatisticsEarly predictionNucleotide
The invention discloses a characteristic miRNA expression profile combination and an early prediction method for uterine corpus endometrial carcinoma. Nucleotide probe sequences of miRNA are shown as SEQ ID NO.1-12. The method for evaluating the early risk of the uterine corpus endometrial carcinoma based on the miRNA expression profile combination characteristics has very high precision and accuracy (the area AUC under the ROC curve is equal to 0.994). Only the relative expression quantity of the 12 miRNAs needs to be obtained; the early disease probability of the uterine corpus endometrial carcinoma is calculated through a support vector machine model; and the early disease probability can be used as a reference basis for early prediction of the uterine corpus endometrial carcinoma.
Owner:FOSHAN UNIVERSITY
Method for determining dosing schemes of doxycycline to Citrobacter freundii of procambarus clarkia in different dosing modes
PendingCN114464290AReduce economic lossImprove efficiencyMolecular designDrug and medicationsDosing regimenDrug efficiency
The invention discloses a method for determining an administration scheme of doxycycline to citrobacter freundii of procambarus clarkii in different administration modes, and relates to the field of drug effects of aquatic drugs. Comprising the following steps: step 1, separating citrobacter freundii from clinically diseased procambarus clarkia, and measuring the MIC value of doxycycline to the citrobacter freundii by adopting a microdilution method; 2, performing single oral administration on the procambarus clarkii by adopting oral administration, intramuscular injection and venous sinus injection methods respectively, and collecting tissue samples respectively after administration; 3, respectively measuring the residual quantity of doxycycline in the tissue samples collected in the step 2; 4, calculating pharmacokinetic parameters including area under a drug time curve, absorption half-life period, elimination half-life period, peak reaching time, peak concentration, apparent distribution volume and clearance rate; and 5, determining a doxycycline administration scheme according to a calculation formula 1. According to the determination method, the medication efficiency and medication safety can be improved.
Owner:YANGTZE RIVER FISHERIES RES INST CHINESE ACAD OF FISHERY SCI
Newborn individualized fluconazole administration amount calculation method and administration scheme recommendation system
PendingCN114765076AImprove medication safetyGood curative effectCharacter and pattern recognitionDrug referencesDosing regimenFull Term Neonate
The invention provides a neonatal individualized fluconazole administration amount calculation method and an administration scheme recommendation system, and belongs to the technical field of intelligent medical information processing. The method comprises the following steps: inputting the gestational age of a neonatal patient and the body weight of the patient into a neonatal fluconazole population pharmacokinetic model to obtain initial values V1 and CL1 of individual neonatal pharmacokinetic parameters; adopting a Bayesian maximum posterior probability method to predict the blood concentration of fluconazole at the t moment after administration to the patient according to V1 and CL1; fitting the blood concentration at each time point, calculating the area AUC0-24 under the curve at the time of 0-24 hours, and evaluating the anti-infection curative effect; if the curative effect evaluation is reasonable, adopting the administration amount and the administration interval as an administration scheme; if the curative effect evaluation is not good, optimizing the administration scheme. According to the method, the pharmacokinetic model of the fluconazole is established by taking the Chinese neonates as samples, the pharmacokinetic characteristics of the Chinese neonates are better met, a scientific basis can be provided for clinical fluconazole medication of the Chinese neonates, and the medication safety and the curative effect are improved.
Owner:LIUZHOU CITY HEALTHCARE HOSPITAL FOR WOMEN & CHILDREN
Application of hsa_circ_0069922 as a marker
ActiveCN112176055BHigh diagnostic valueIncreased sensitivityNervous disorderMicrobiological testing/measurementPeripheral blood mononuclear cellPharmaceutical drug
Owner:NANJING FIRST HOSPITAL
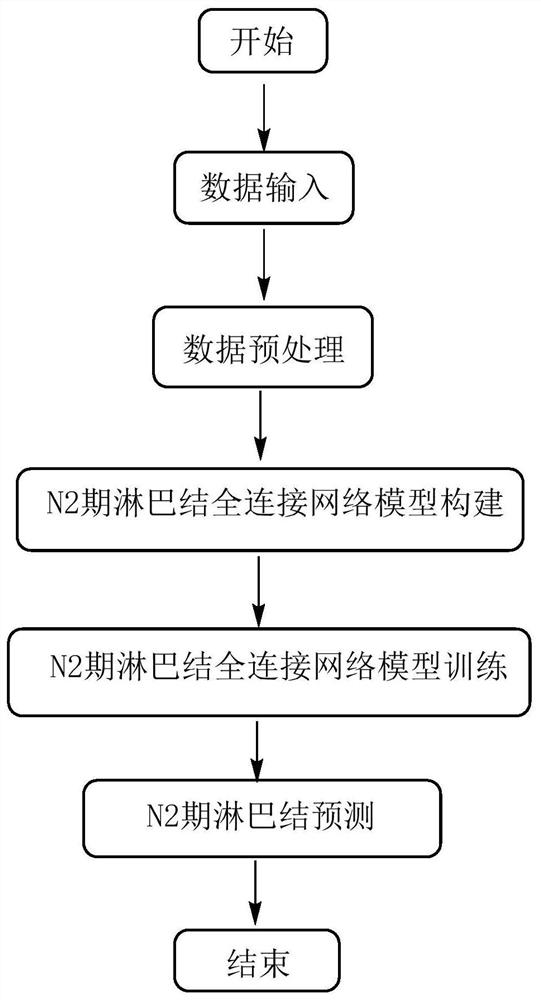
Computer-assisted preoperative lung cancer patient N2-stage lymph node prediction system based on neural network
PendingCN114587397AFully trainedIndicators for Optimizing EvaluationMedical automated diagnosisComputerised tomographsPattern recognitionState prediction
The invention discloses a computer-aided system for predicting N2-stage lymph nodes of preoperative lung cancer patients based on a neural network. The prediction system comprises a first part: a data input part; the second part is a data preprocessing part; the third part is a model construction part; the fourth part is a model training part; and a fifth part: a prediction part: inputting data after preprocessing of CT features and clinical features of a to-be-predicted lung cancer patient, performing prediction by using the trained model, and outputting a prediction result: the to-be-predicted lung cancer patient is in an N2 stage or an N0 / N1 stage. The prediction system can accurately predict whether the lymph node of a T1N0M0 non-small cell lung cancer patient is in an N0 / N1 state or an N2 state, the area under curve (AUC) of a prediction result is up to 0.7847, the sensitivity is up to 89.80%, and the specificity is up to 54.50%. The prediction system plays a very important role in predicting the lifetime of a patient by a doctor, selecting an optimal treatment strategy and performing prognosis evaluation, and has a wide application prospect.
Owner:SICHUAN UNIV
Congenital adrenal hyperplasia diagnosis model and construction method and application thereof
PendingCN114664435AFacilitate early detectionConducive to early treatmentMedical simulationMedical automated diagnosisHealthy subjectsSteroidal hormones
The invention provides a congenital adrenocortical hyperplasia diagnosis model and a construction method and application thereof, and the construction method comprises the following steps: respectively collecting serum samples of a congenital adrenocortical hyperplasia patient, a similar congenital adrenocortical hyperplasia patient and a healthy subject as analysis samples; analyzing by adopting a liquid chromatography-tandem mass spectrometry technology to obtain an original steroid hormone spectrum of each serum sample; calculating a critical value of the congenital adrenal hyperplasia according to the area under the operation characteristic curve of the subject; the model construction method provided by the invention is simple, strong in operability and high in diagnosis accuracy, and has good sensitivity and specificity to three congenital adrenal hyperplasia subtypes. Diagnosis can be achieved only through one-time blood sampling detection, the process is shortened, the cost is reduced, the initiative and compliance of a patient can be improved, effective technical support is provided for early diagnosis and early treatment of congenital adrenal hyperplasia, and good clinical use and popularization value is achieved.
Owner:RUIJIN HOSPITAL AFFILIATED TO SHANGHAI JIAO TONG UNIV SCHOOL OF MEDICINE +2
System developed by utilizing multiple myeloma diagnosis model based on logistic regression and application of system
PendingCN111613327AImprove accuracyImprove featuresMedical data miningMedical automated diagnosisOncologyMyeloid Tumor
The invention discloses a system developed by utilizing a multiple myeloma diagnosis model based on logistic regression and application of the system. One technical scheme protected by the invention is an application of a system for identifying sex and detecting albumin content and hemoglobin content in preparation of a product for screening or auxiliary screening of multiple myeloma patients. According to the embodiment of the invention, by verifying the constructed diagnosis model, it is found that the area under the ROC curve of the diagnosis model is 0.995, the sensitivity is 0.956 and thespecificity is 0.981, which indicate that the diagnosis result of the diagnosis model has high accuracy and sensitivity. The system developed by the invention is simple and easily available in required indexes, high in sensitivity, capable of noninvasively, efficiently and accurately distinguishing multiple myeloma patients from healthy people, and suitable for popularization and application.
Owner:BEIJING CHAOYANG HOSPITAL CAPITAL MEDICAL UNIV +2
Application of hsa_circCNOT6_008 in diagnosis of steroid-induced femoral head necrosis
PendingCN114058688ALow priceEasy to carryMicrobiological testing/measurementDNA/RNA fragmentationFemoral head necrosisHormone
The invention discloses application of hsa_circCNOT6_008 in diagnosis of steroid-induced femoral head necrosis, and belongs to the technical field of medicines. According to the invention, based on an exosome liquid biopsy technology, a new generation sequencing technology NGS is utilized to discover differentially expressed circRNA(s) in peripheral mononuclear cells of a patient with steroid-induced femoral head necrosis; and the differentially expressed circRNA(s) is verified by qRT-PCR (quantitative reverse transcription-polymerase chain reaction) to obtain a potential marker with obvious differential expression, namely the peripheral blood mononuclear molecular target hsa_circCNOT6_008. According to the change of the mononuclear cell molecular target hsa_circCNOT6_008, the steroid-induced femoral head necrosis can be found earlier than MRI (Magnetic Resonance Imaging); the price is low; carrying and popularization are convenient; the area under an AUC curve is 1, and the sensitivity and the specificity are high and both reach 1; the marker is a reliable specific biological marker for diagnosing the steroid-induced femoral head necrosis.
Owner:THE FIRST AFFILIATED HOSPITAL OF GUANGZHOU UNIV OF CHINESE MEDICINE
System and method for predicting acute rejection of lung transplantation
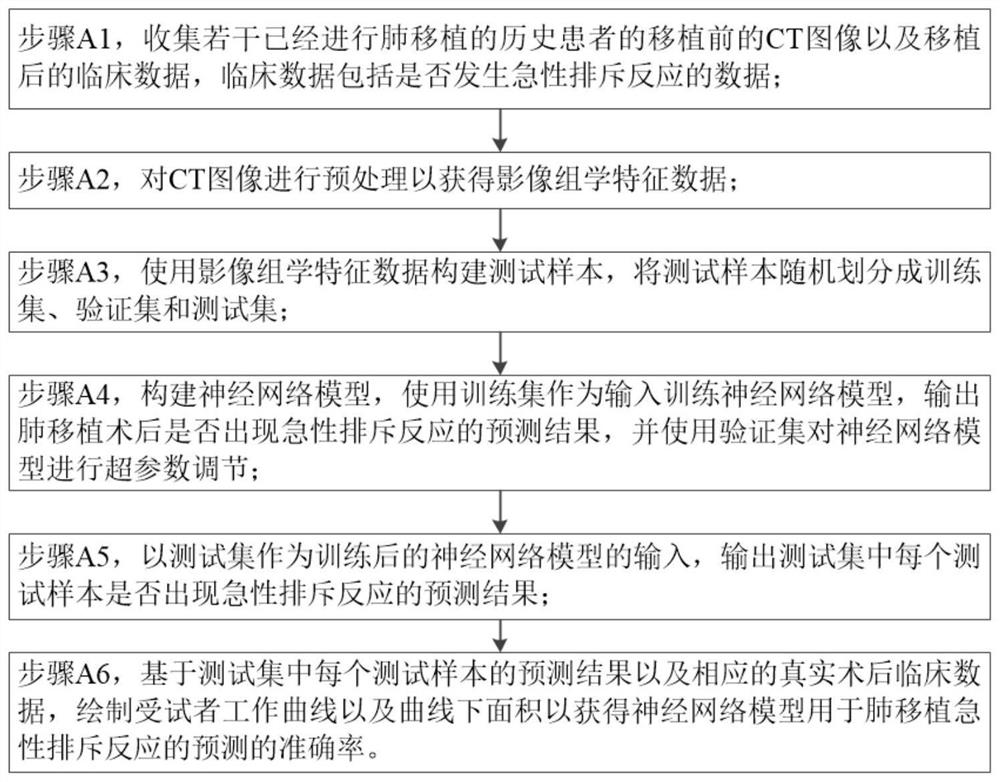
InactiveCN114758782AHigh clinical application valueAvoid destructive checksMedical simulationBiological neural network modelsClinical valueTest sample
The invention provides a lung transplantation acute rejection prediction system and method, and the method comprises the steps: collecting the CT images of a plurality of historical patients subjected to lung transplantation before transplantation and the data of whether acute rejection occurs after transplantation, obtaining radiomics feature data to construct a test sample, training a neural network model through a training set, and obtaining a prediction result. Taking the test set as the input of the trained neural network model, and outputting a prediction result of whether each test sample in the test set has an acute rejection reaction; and based on the prediction result of each test sample in the test set and the corresponding real postoperative clinical data, drawing a subject working curve and an area under the curve to obtain the accuracy of the neural network model for predicting the acute rejection of lung transplantation. The non-invasive prediction system and method are established to accurately predict the acute rejection of lung transplantation so as to formulate an individualized immunosuppression scheme, so that the method has extremely high clinical value, injury examination is avoided, and the clinical value is met.
Owner:SHANGHAI PULMONARY HOSPITAL
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com