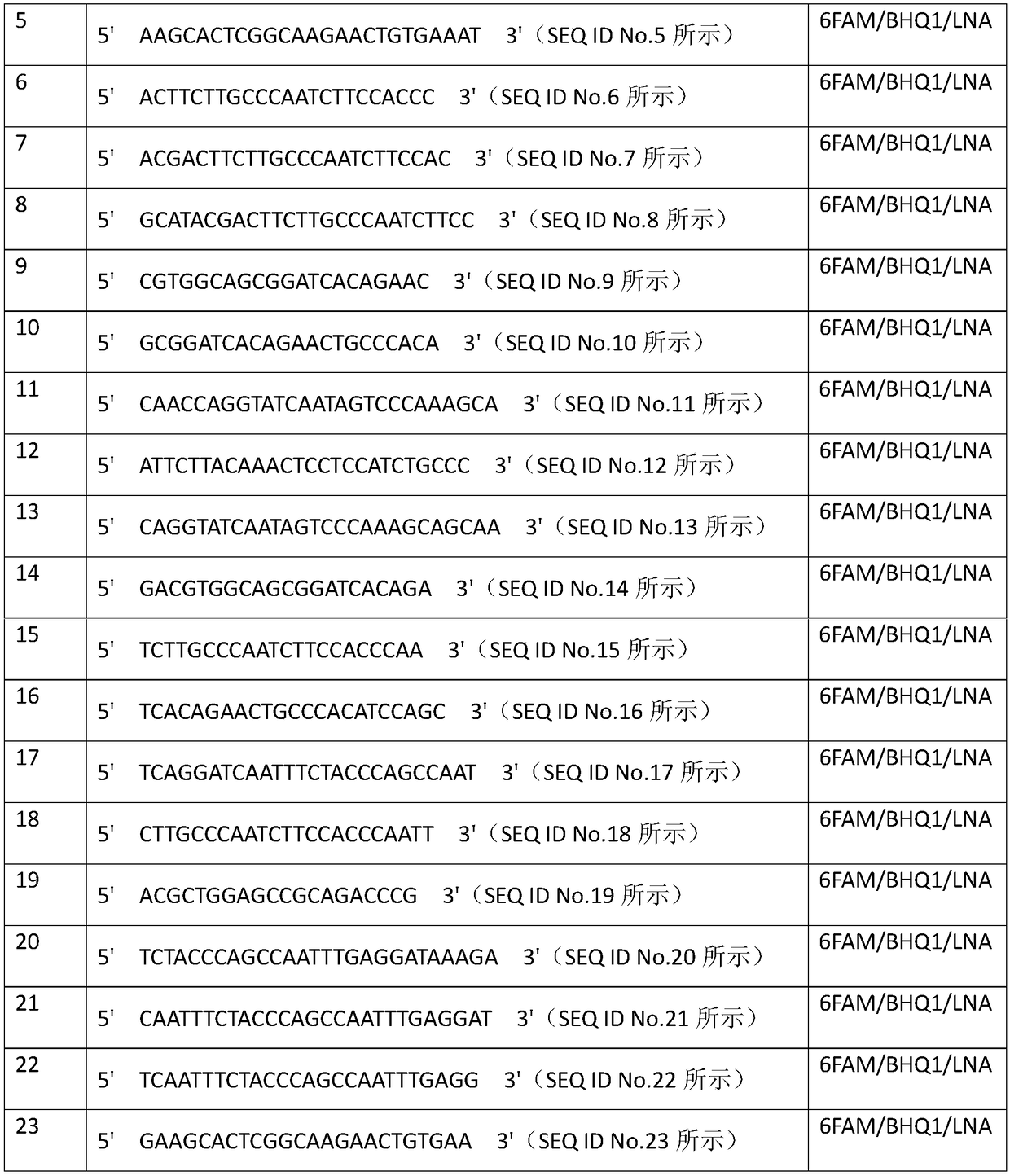
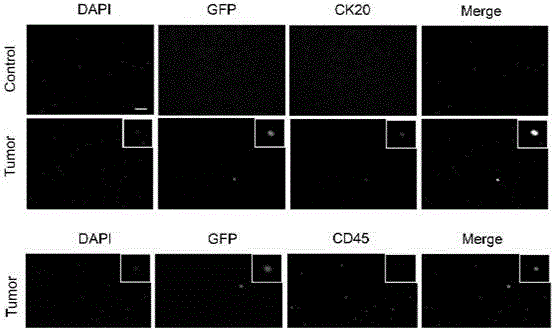
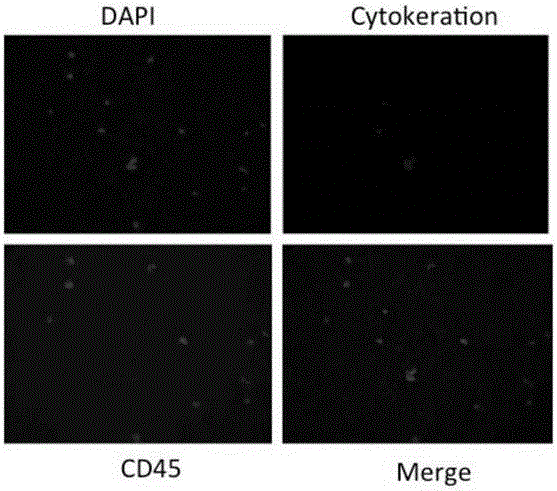
Patents
Literature
203 results about "Liquid biopsy" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
A liquid biopsy, also known as fluid biopsy or fluid phase biopsy, is the sampling and analysis of non-solid biological tissue, primarily blood. Like traditional biopsy this type of technique is mainly used as a diagnostic and monitoring tool for diseases such as cancer, with the added benefit of being largely non-invasive. Therefore, it can also be done more frequently which can better track tumors and mutations over a duration of time. It may also be used to validate the efficiency of a cancer treatment drug by taking multiple liquid biopsy samples in the span of a few weeks. The technology may also prove beneficial for patients after treatment to monitor relapse.
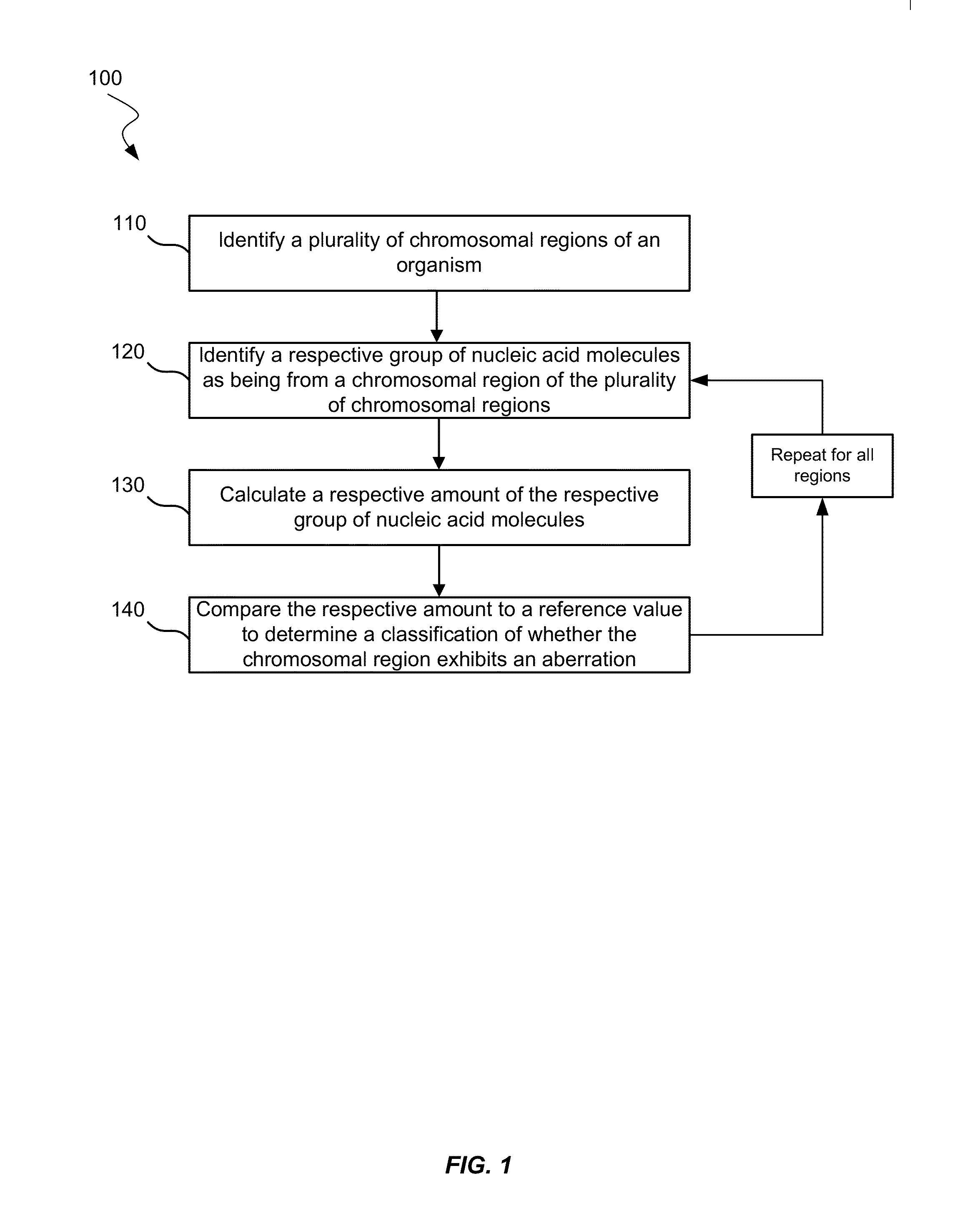
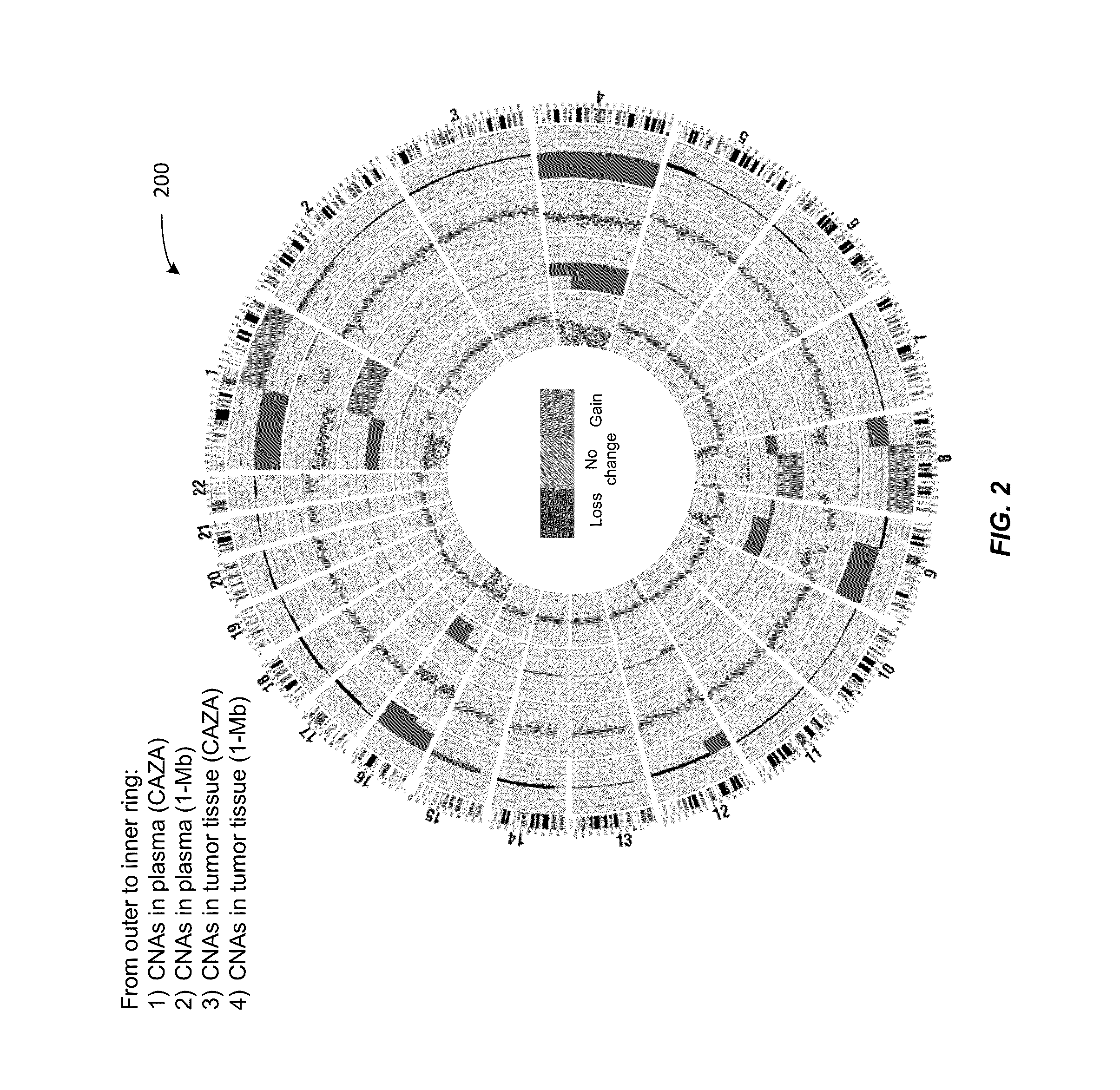
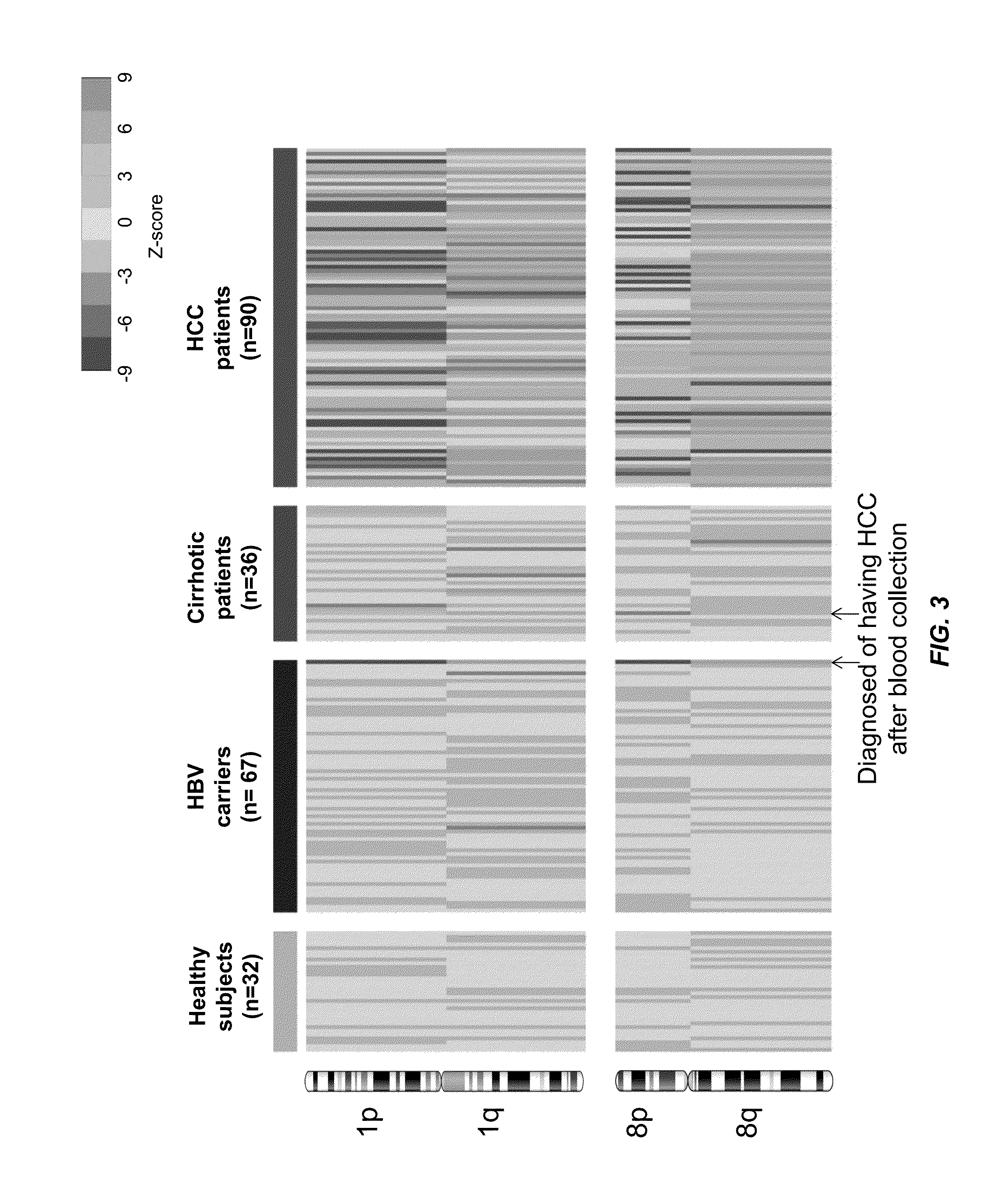
Using size and number aberrations in plasma DNA for detecting cancer
Analysis of tumor-derived circulating cell-free DNA opens up new possibilities for performing liquid biopsies for solid tumor assessment or cancer screening. However, many aspects of the biological characteristics of tumor-derived cell-free DNA remain unclear. Regarding the size profile of plasma DNA molecules, some studies reported increased integrity of tumor-derived plasma DNA while others reported shorter tumor-derived plasma DNA molecules. We performed an analysis of the size profiles of plasma DNA in patients with cancer using massively parallel sequencing at single base resolution and in a genomewide manner. Tumor-derived plasma DNA molecules were further identified using chromosome arm-level z-score analysis (CAZA). We showed that populations of aberrantly short and long DNA molecules co-existed in the plasma of patients with cancer. The short ones preferentially carried the tumor-associated copy number aberrations. These results show the ability to use plasma DNA as a molecular diagnostic tool.
Owner:THE CHINESE UNIVERSITY OF HONG KONG
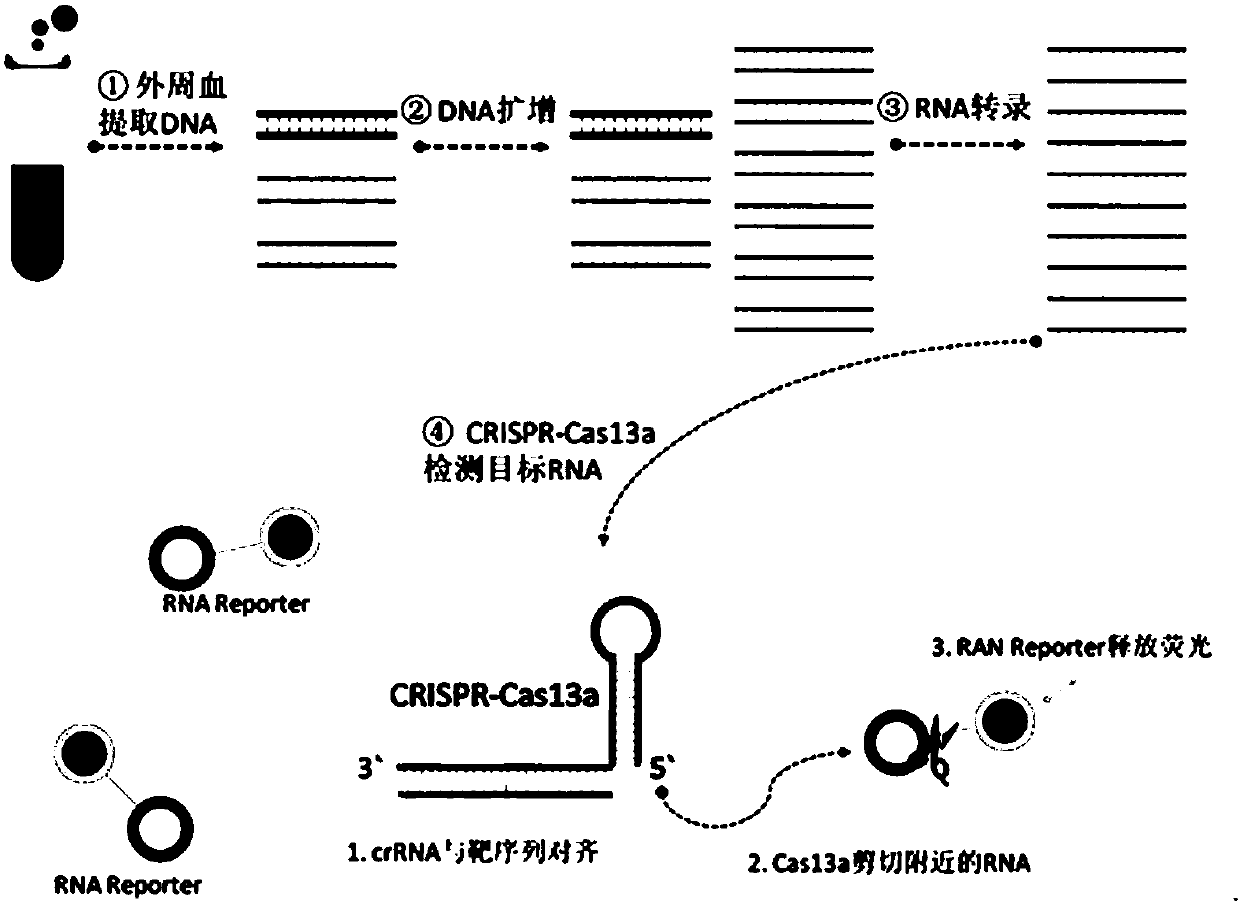
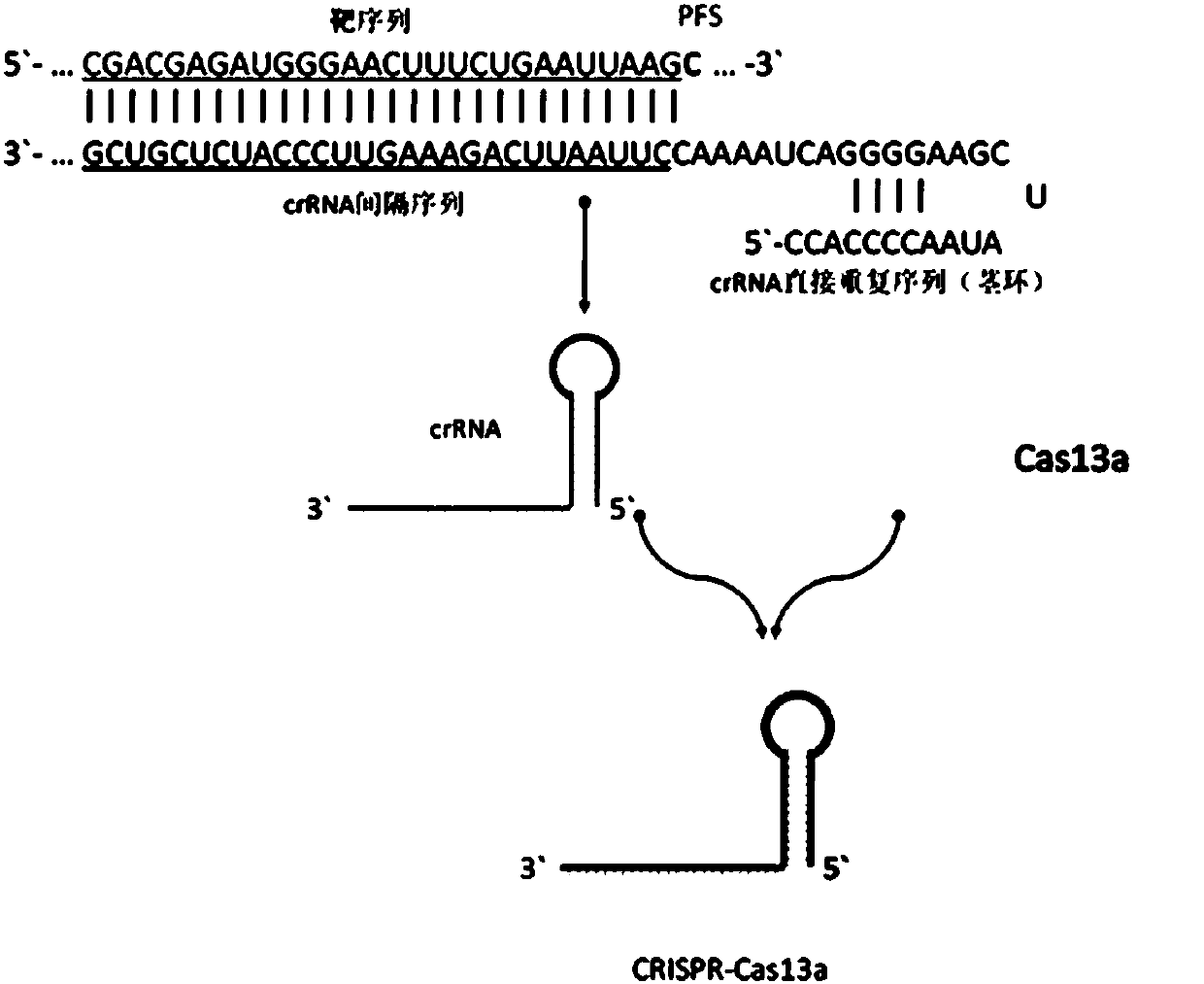
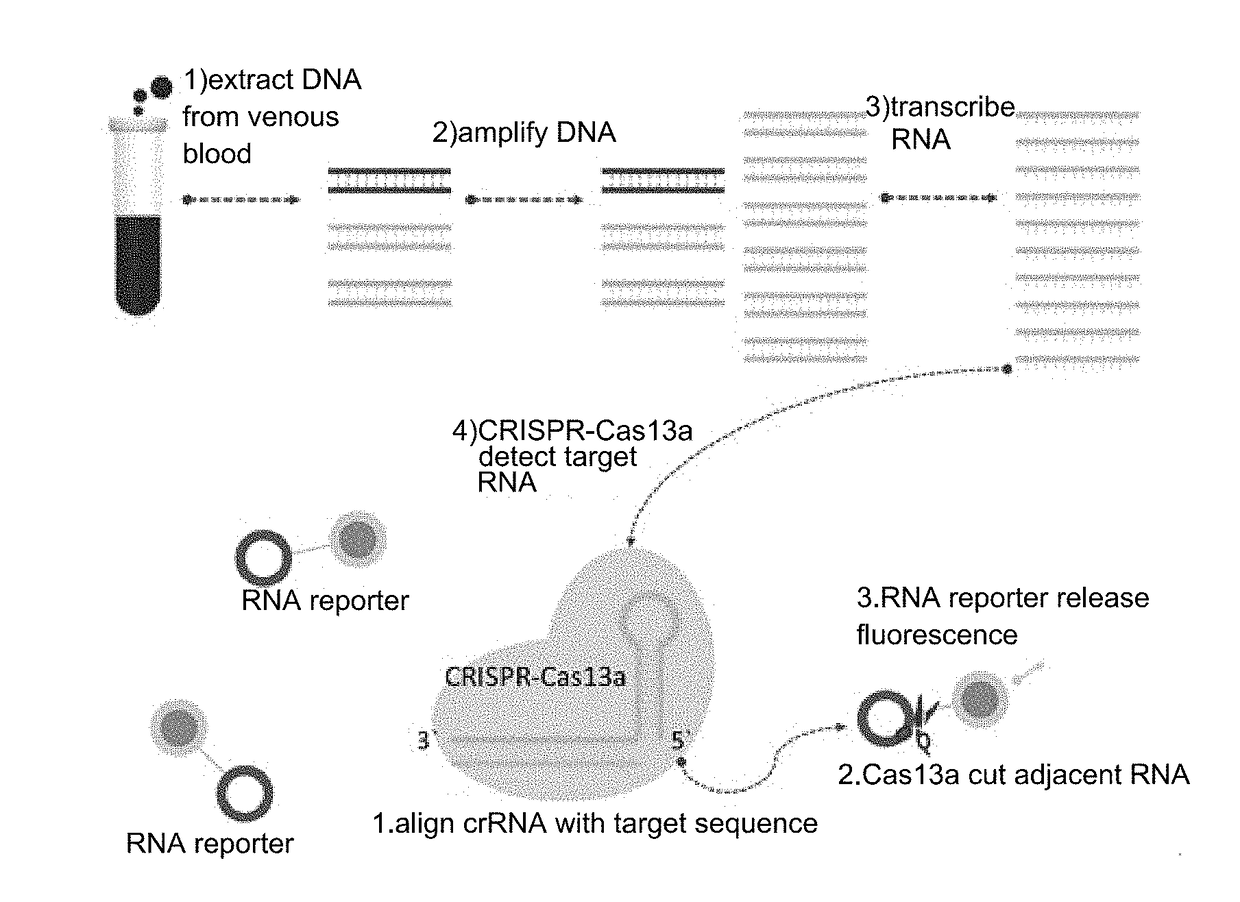
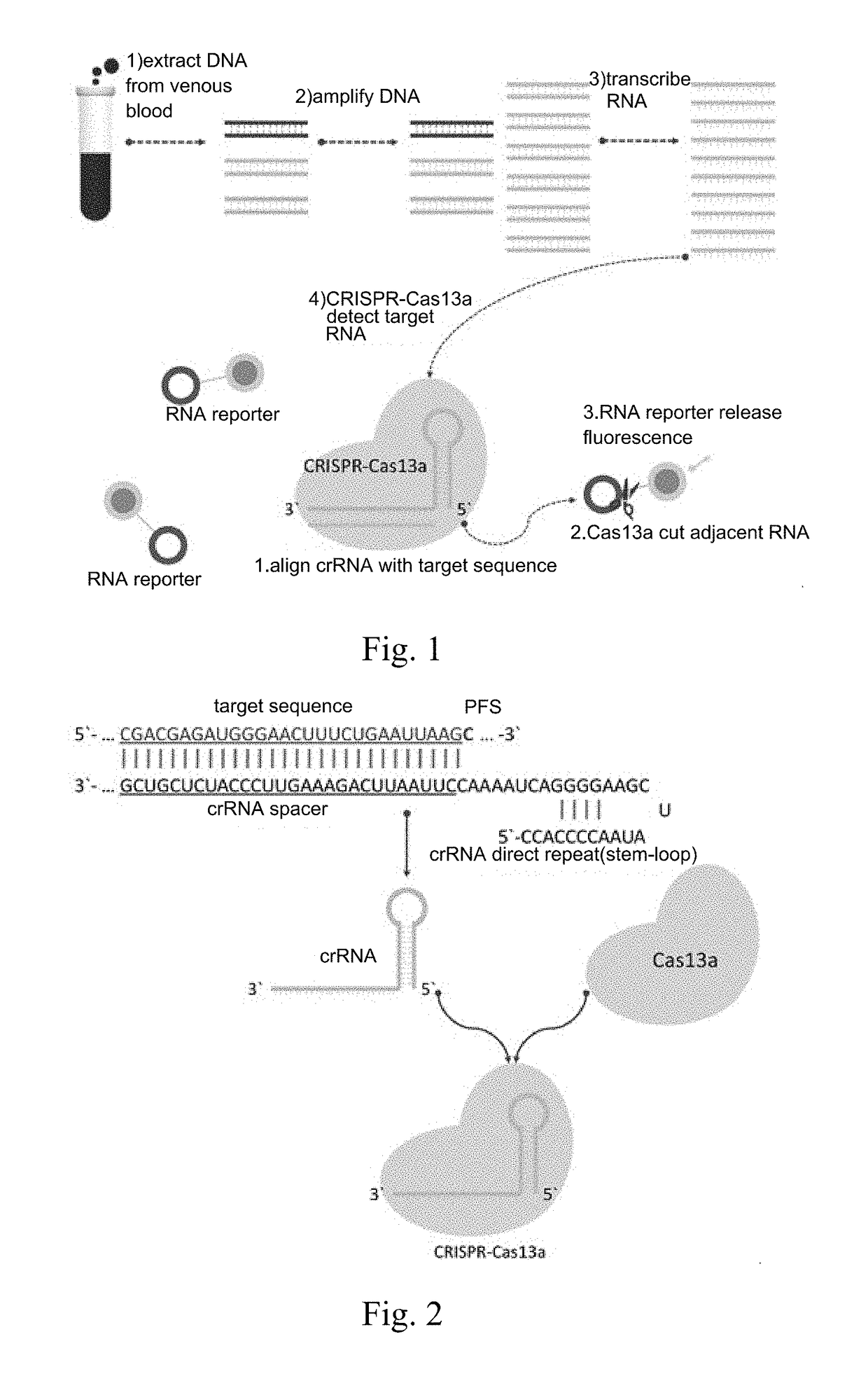
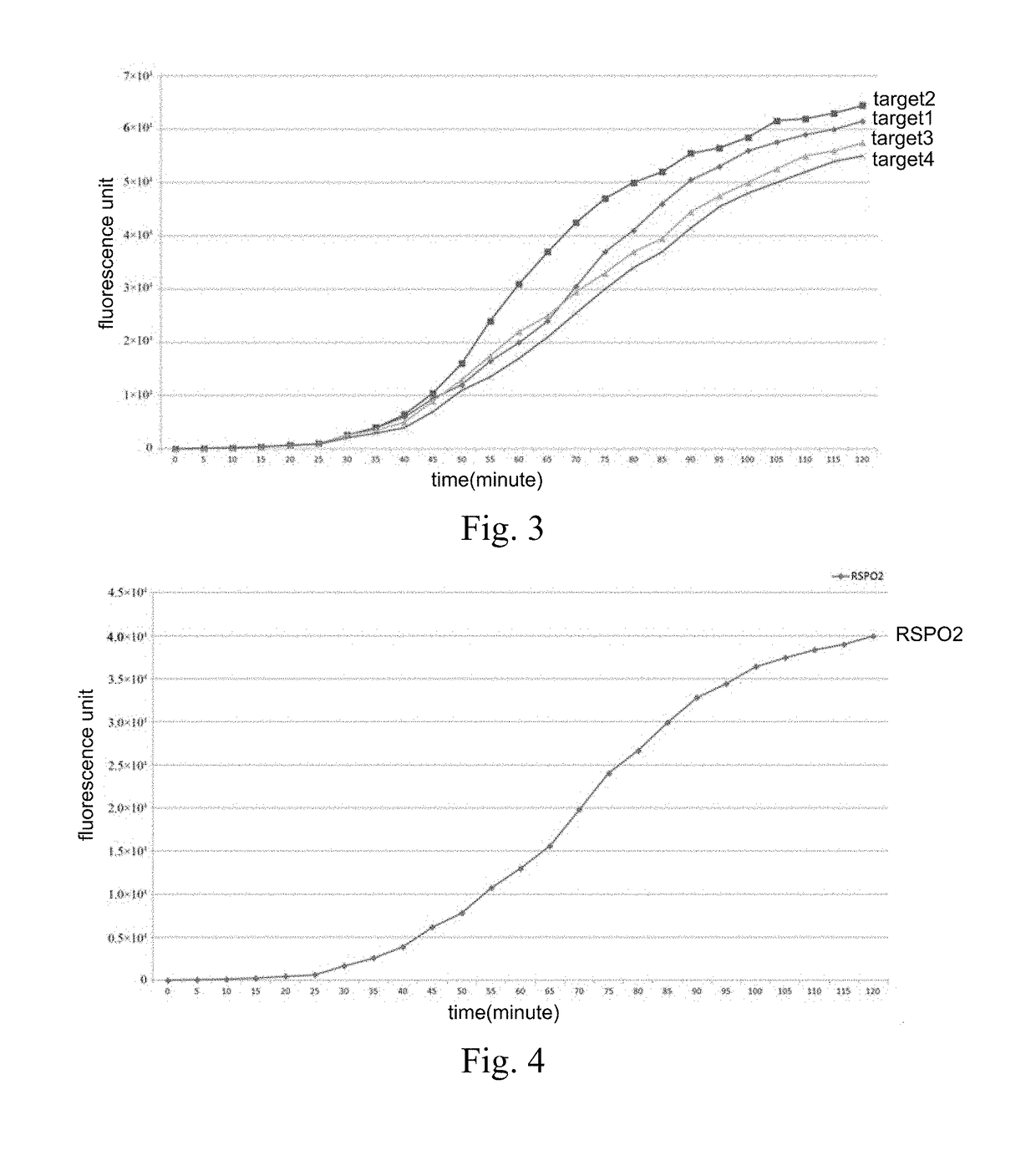
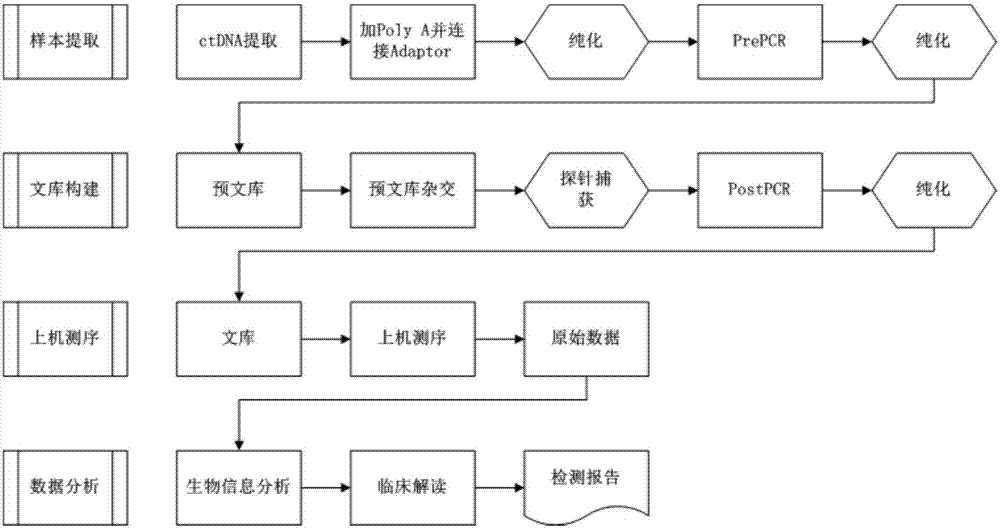
CrRNA specifically targeting toward human RSPO2 gene in CRISPR-Cas13a system and system and application
ActiveCN108048466AConducive to commercial promotionSuitable for large-scale applicationsHydrolasesMicrobiological testing/measurementHigh fluxFluorescence
The invention discloses crRNA specifically targeting toward a human RSPO2 gene in a CRISPR-Cas13a system and a system and an application. The crRNA can establish a CRISPR-Cas13a system for specific targeting toward a human RSPO2 gene. A trace of a RSPO2 gene is specifically detected in a body fluid. The system is non-invasive, can perform frequent detection, and is fast in detection speed. Compared with liquid biopsy in the prior art, the system can detect a trace of a RSPO2 gene in the body liquid via fluorescent reading. The system doesn't require high-flux sequencing, is low in cost, high in detection speed, and suitable for clinic large-scale application.
Owner:JIAXING NO 1 HOSPITAL
crRNA for detecting RSPO2 gene in body fluid with CRISPR-Cas13a specificity and applications thereof
InactiveUS20180312835A1Quick testLow costHydrolasesMicrobiological testing/measurementBody fluidNon invasive
Plural crRNA for detecting RSPO2 gene in body fluid with CRISPR-Cas13a specificity and applications thereof are disclosed. The crRNA is able to construct the CRISPR-Cas13a system and specifically detect micro-RSPO2 gene in the body fluid. The present invention is non-invasive and able to test rapidly, frequently and repeatedly. Compared to the conventional liquid biopsy, the present invention detects the micro-RSPO2 in the body fluid through the fluorescence units. The present invention has the advantages of no need for high-throughput sequencing, low cost and rapid testing speed, which is able to be adopted by large scale clinical applications.
Owner:JIAXING NO 1 HOSPITAL
Gene group and kit for diagnosing lung caner, and diagnosis method thereof
InactiveCN107475370AExcellent detection depthExcellent detection accuracyMicrobiological testing/measurementLibrary creationA-DNABlood plasma
The invention relates to the field of genetic engineering and biotechnologic detection, and concretely relates to a gene group and a kit for diagnosing lung cancer, and a diagnosis method thereof. The gene group for diagnosing the lung cancer includes ABCB1, AKT1, ALK, APC, ATIC and other 63 genes. The method using the gene group to diagnose the lung cancer comprises the following steps: (1) extracting free DNA and genome DNA from the plasma of a sample to be detected; and (2) breaking the genome DNA into fragments with the length of 150-250 bp, carrying out hybrid capture on the broken genome DNA and the free DNA to construct a DNA library, carrying out online sequencing, and analyzing the obtained sequencing result. Exon and partial intron regions of the 68 genes of the free DNA are enriched at one time by a probe capture technology to realize multi-gene and multi-target parallel deep high-throughput sequencing with high accuracy, optimize the detection flow and improve the detection precision, so liquid biopsy, low-frequency detection and tumor diagnosis become possible.
Owner:天津脉络医学检验有限公司
Process for microsatellite instability detection
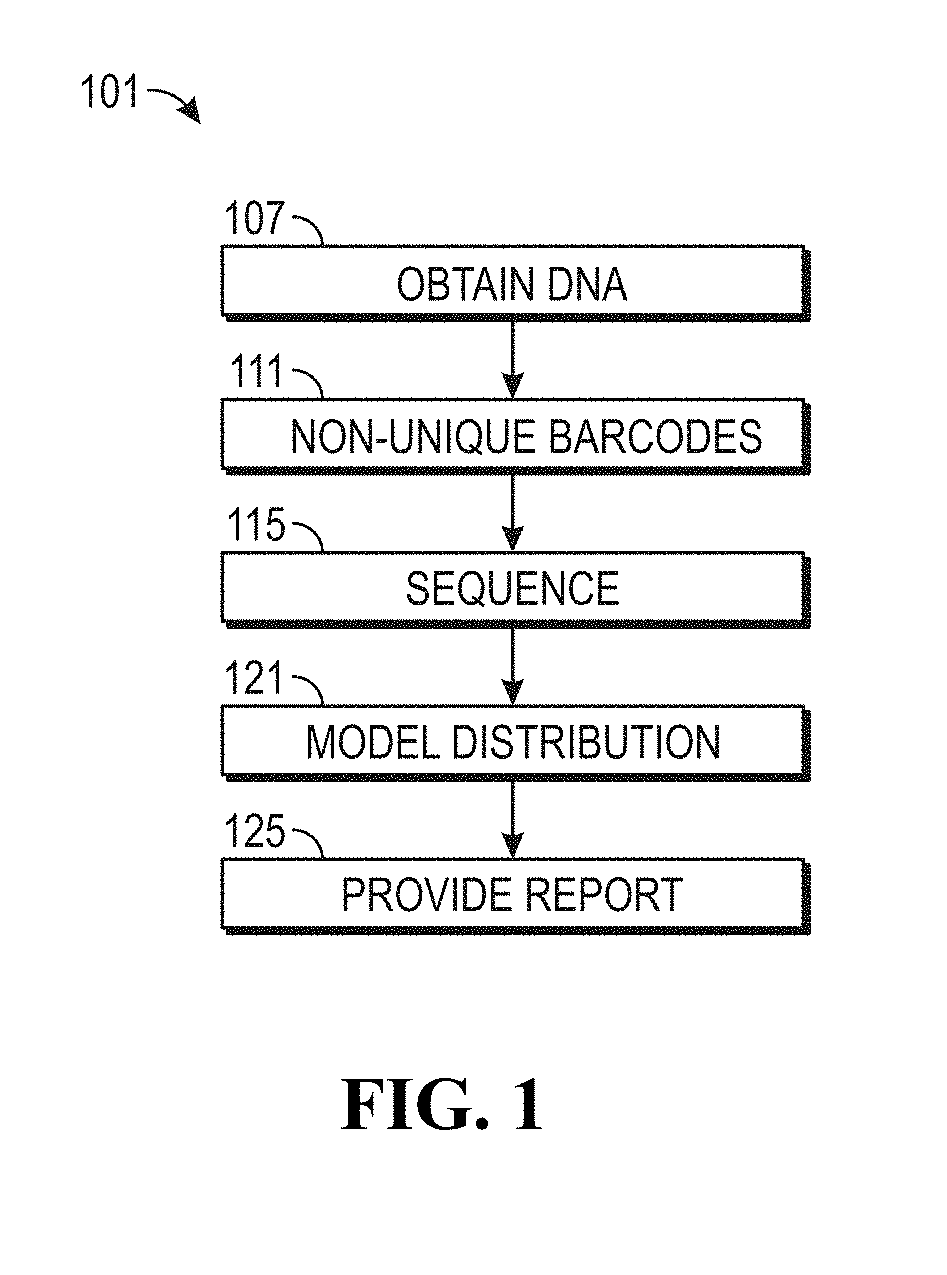
ActiveUS20190169685A1Useful in detectionIncrease the burdenTherapiesHealth-index calculationCell freeNucleotide
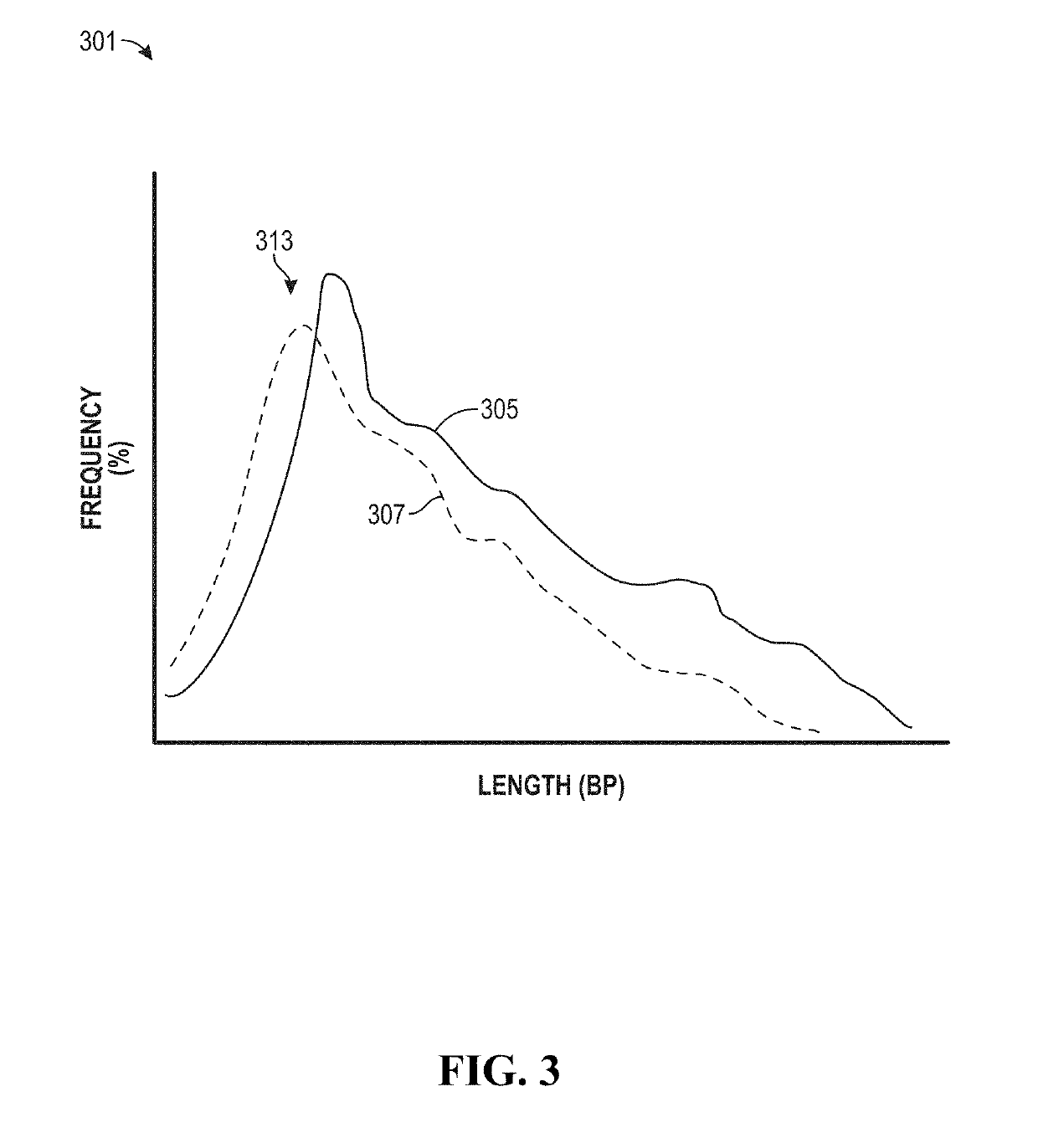
The invention provides methods for determining the MSI status of a patient by liquid biopsy with sample preparation using hybrid capture and non-unique barcodes. In certain aspects, the invention provides a method of detecting microsatellite instability (MSI). The method includes obtaining cell-free DNA (cfDNA) from a sample of blood or plasma from a patient and sequencing portions of the cfDNA to obtain sequences of a plurality of tracts of nucleotide repeats in the cfDNA. A report is provided describing an MSI status in the patient when a distribution of lengths of the plurality of tracts has peaks that deviate significantly from peaks in a reference distribution.
Owner:PERSONAL GENOME DIAGNOSTICS INC
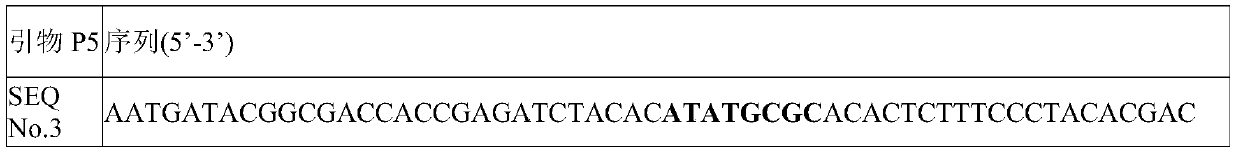
Single-chain molecular identifier adapter and single-chain DNA database creating method and application thereof to circulating tumor DNA detection
InactiveCN109797197AReduce dosageHigh-resolutionMicrobiological testing/measurementLibrary linkersDNA databaseSingle strand dna
The invention discloses a single-chain molecular identifier adapter and a single-chain DNA database creating method and application thereof to circulating tumor DNA detection. The single-chain molecular identifier adapter comprises a stem structure formed by 14 base pairing, a sequencing primer sequence and a molecular identifier sequence formed by 8 random nucleotides, wherein a base 'U' is inserted between the sequencing primer sequence and a stem structure sequence, and a stem-loop structure can be formed by annealing denaturation of the single-chain nucleotide sequence. By single-chain DNAdatabase creating, technical problems of low sensitivity, fragmentation, low concentration and short ctDNA fragments in liquid biopsy in application of an existing detection technique are solved. Accurately distinguishing whether sequencing reads identical in genome coordinate beginning and end loci come from cfDNA released by the same one or multiple germinal cell is realized, sequencing reads derived from original positive and negative chains of the same cell are paired for analysis, and accordingly sequencing rehandling is reduced, and the distinguishing rate of false positive mutation isincreased.
Owner:HANGZHOU NEOANTIGEN THERAPEUTICS CO LTD
Quantitative sequencing and library building method and quantitative sequencing and detecting method for fusion gene on basis of DNA (Deoxyribonucleic Acid) and application of quantitative sequencing and detecting method
ActiveCN107190329ALow backgroundStrong specificityMicrobiological testing/measurementLibrary creationGenomic SegmentA-DNA
The invention discloses a quantitative sequencing and library building method for a fusion gene on the basis of DNA (Deoxyribonucleic Acid). The quantitative sequencing and library building method comprises the following steps: firstly, constructing a genome fragmentation DNA library and purifying the library; secondly, capturing a fusion gene generation region by PCR (Polymerase Chain Reaction) amplification, purifying the captured gene and enriching a sequence containing a specific primer fragment; thirdly, capturing a target fragment containing the fusion gene by nested PCR amplification; fourthly, constructing a DNA sequencing library with high-throughput sequencing. The invention also discloses a quantitative sequencing and detecting method for the fusion gene by using the DNA sequencing library prepared by the quantitative sequencing and library building method, application of the quantitative sequencing and detecting method as well as a detection kit containing the DNA sequencing library. According to the quantitative sequencing and library building method disclosed by the invention, the downstream where a fusion breakpoint occurs is anchored by using a one-way specific primer; a target sequence is obtained by pairing specific primers with universal primers and using a PCR method; the background is further reduced label enriching and nested PCR, so that the specificity is improved, the time for building the library is shortened, and the cost for building the library is reduced; the quantitative sequencing and library building method is suitable for an FFPE (Formalin Fixed And Parafiin Embedded) sample or liquid biopsy.
Owner:CARRIER GENE TECH SUZHOU CO LTD +1
Circulating tumor cell detection kit and application thereof
ActiveCN103866016AAvoid interferenceUse less bloodMicrobiological testing/measurementIndividualized treatmentFluorescence
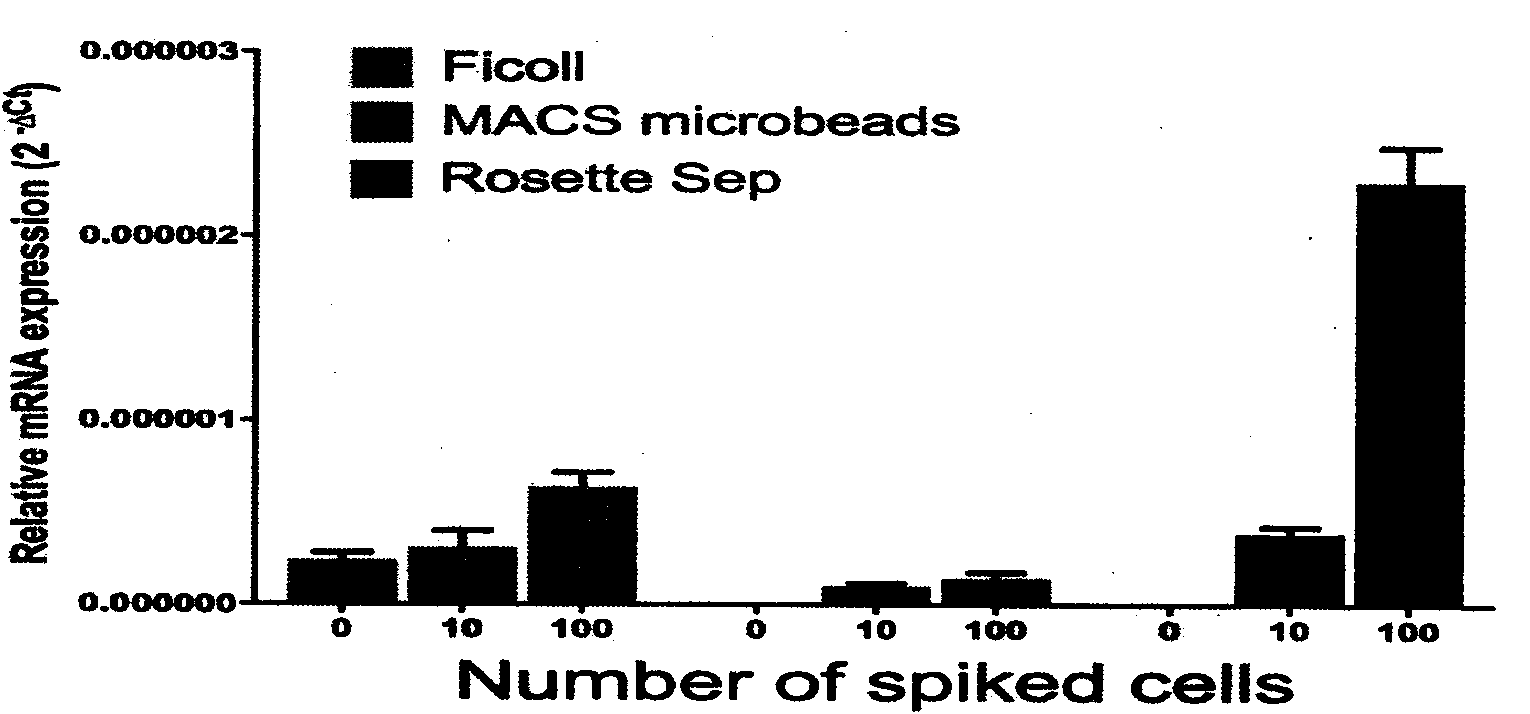
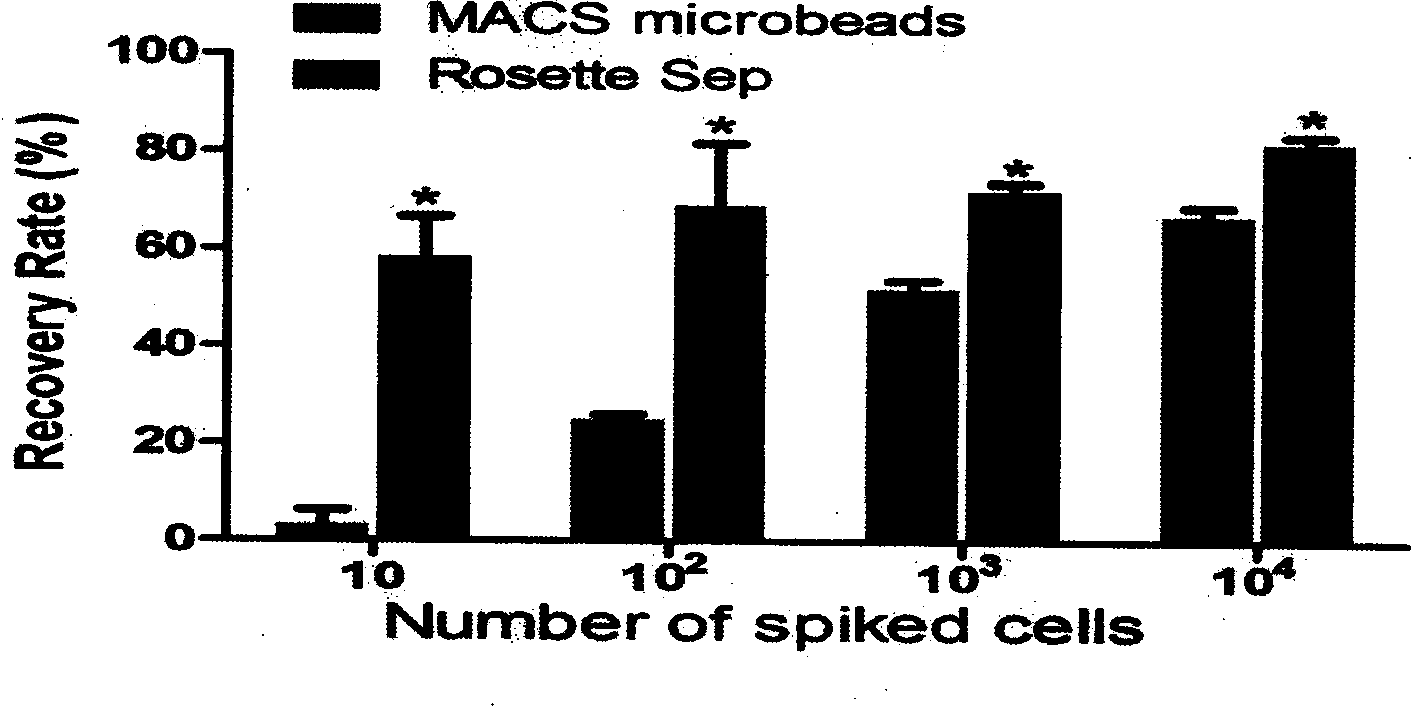
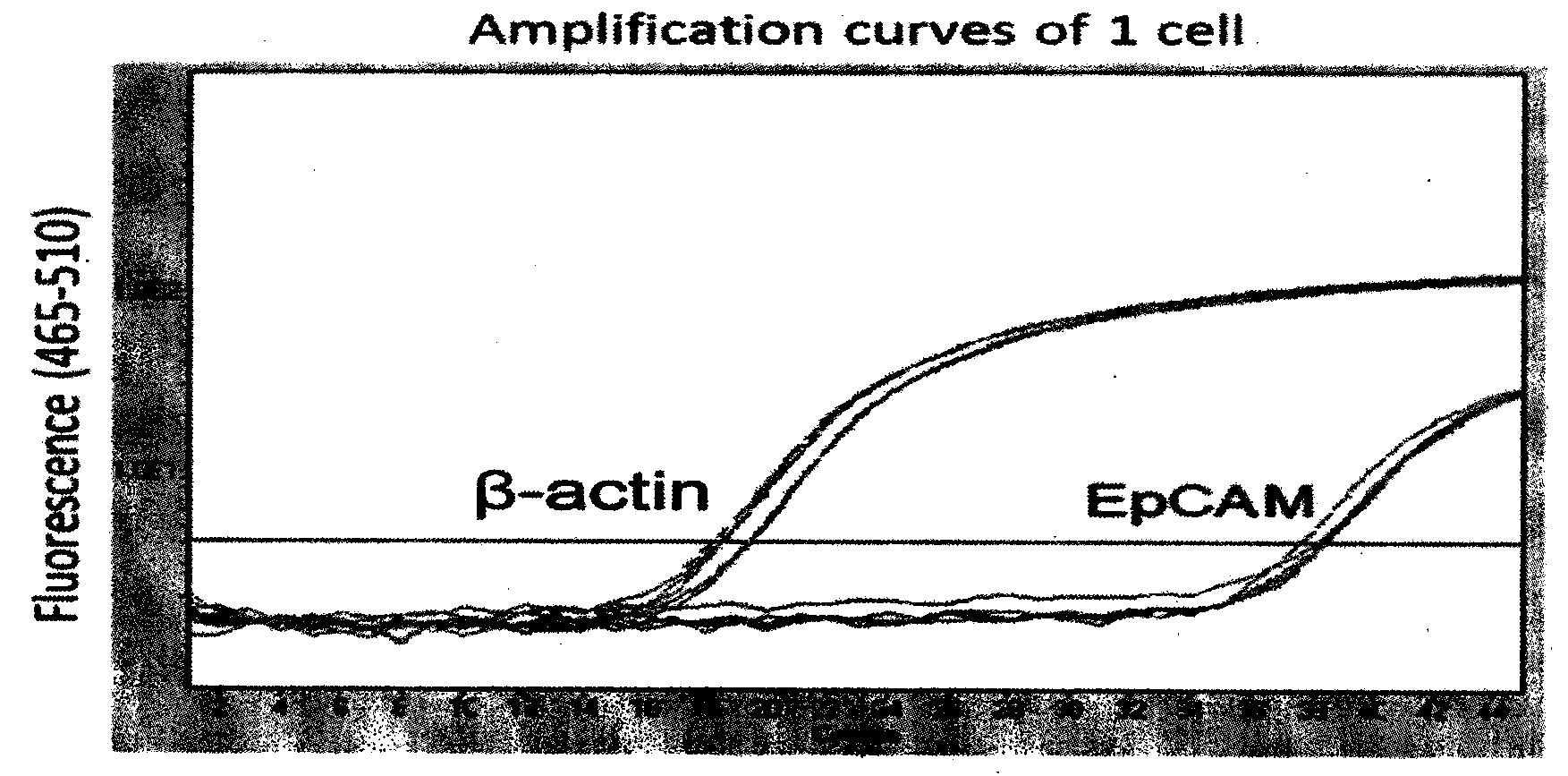
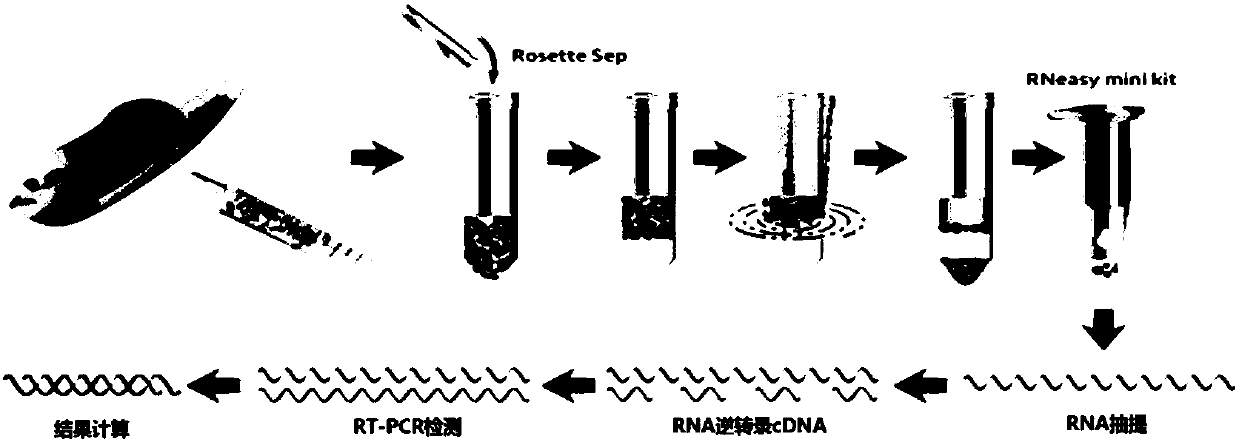
The invention provides a circulating tumor cell detection kit and an application thereof. The circulating tumor cell detection kit is characterized by comprising a RosetteSep rare cell enriching reagent, an RNA (Ribonucleic Acid) extraction reagent, an RNA reverse transcription reagent, a fluorescence labeling Taqman probe, a specific primer and a qPCR (Quantitative Polymerase Chain Reaction) reaction system reagent. The kit can be applied to tumor patient minimally invasive liquid biopsy, early diagnosis and early warning on tumor metastasis and reappearance are achieved, the curative effect in antineoplaston and the tumor development situation are monitored, molecular subtyping based on circulating tumor cells is achieved, and sensitive medicines which specifically aim at the circulating tumor cells are selected, understanding about tumor metastasis, replase and relevant medicine resistance mechanisms is improved, and individualized treatment on tumor patients is guided, so that the total survival of tumor patients is remarkably improved, and great clinical significance is achieved.
Owner:上海顿慧医疗科技发展有限公司 +1
Peptoid and preparation method as well as application thereof
ActiveCN106854233AImprove bindingRapid diagnosisNervous disorderPeptide/protein ingredientsEthylenediamine1-Naphthylamine
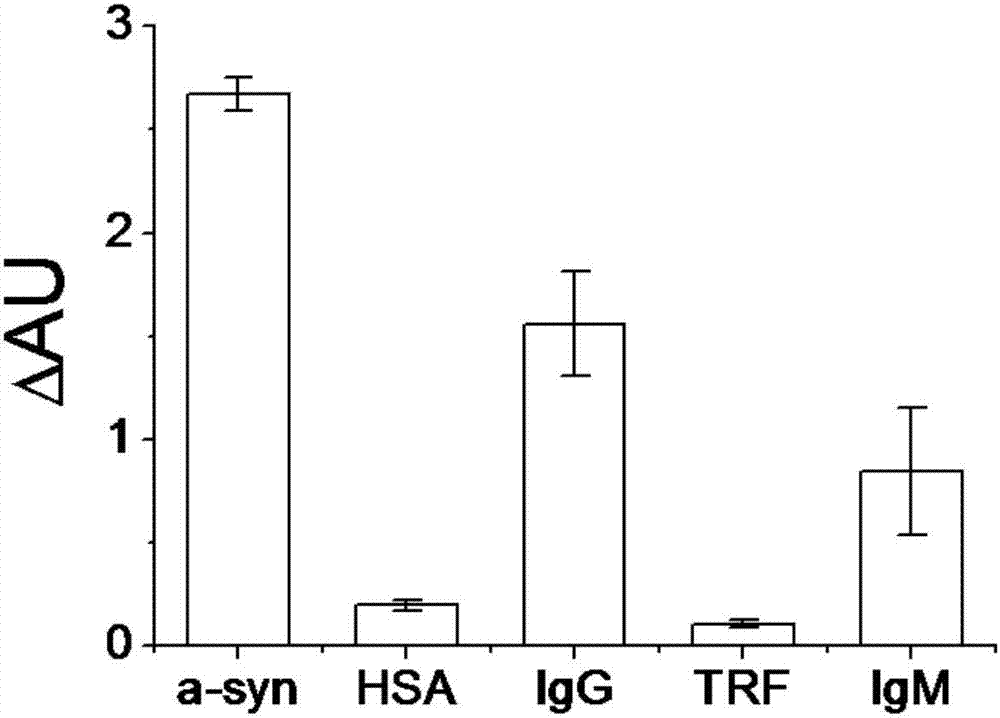
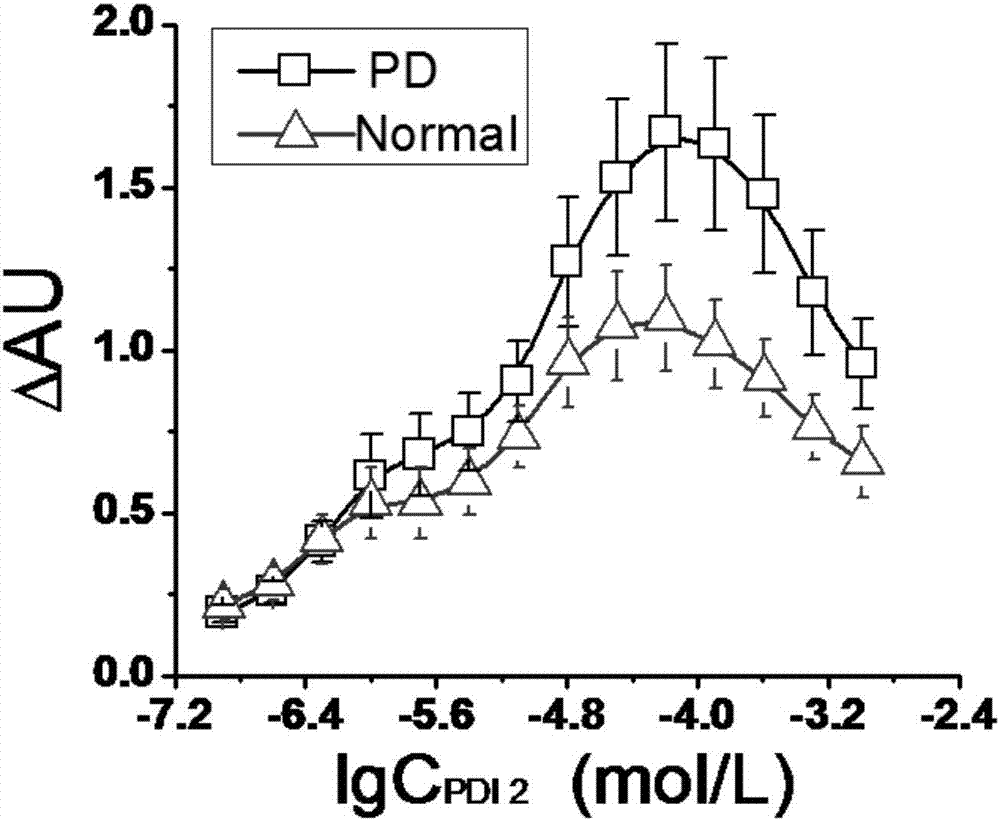
The invention provides a peptoid as well as a preparation method and application thereof. The peptoid comprises the following subunits: ethylenediamine (I), piperonylamine (II), beta-alanine (III), 1-naphthylamine (IV) and cysteine (V). The peptoid is simple in synthesis, has strong combining capacity with alpha-synuclein, can effectively screen the serum of PD patients and able-bodied persons through alpha-synuclein in the serum, and provides a novel liquid biopsy method and concept for diagnosis and monitoring of the Parkinson's disease.
Owner:THE NAT CENT FOR NANOSCI & TECH NCNST OF CHINA
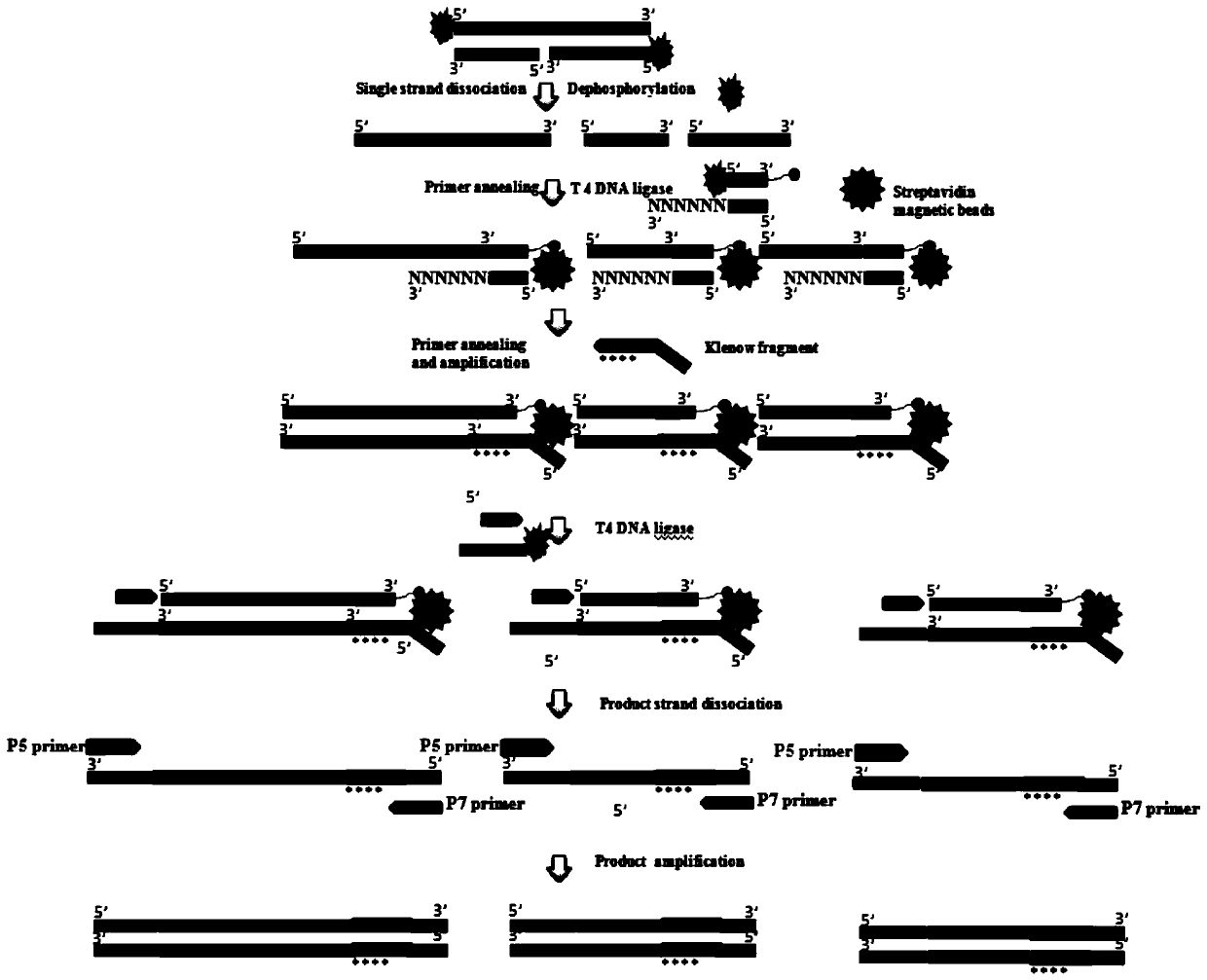
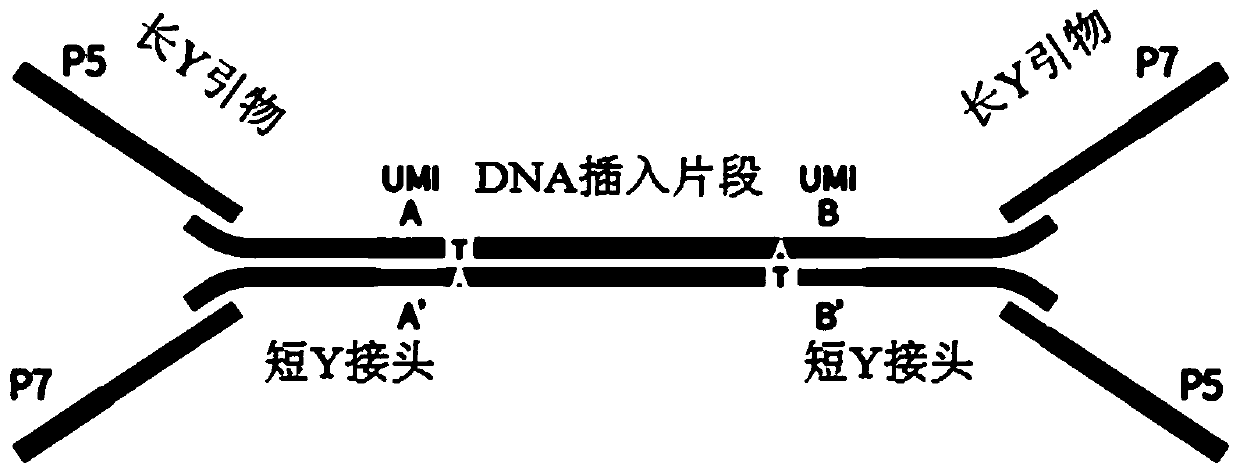
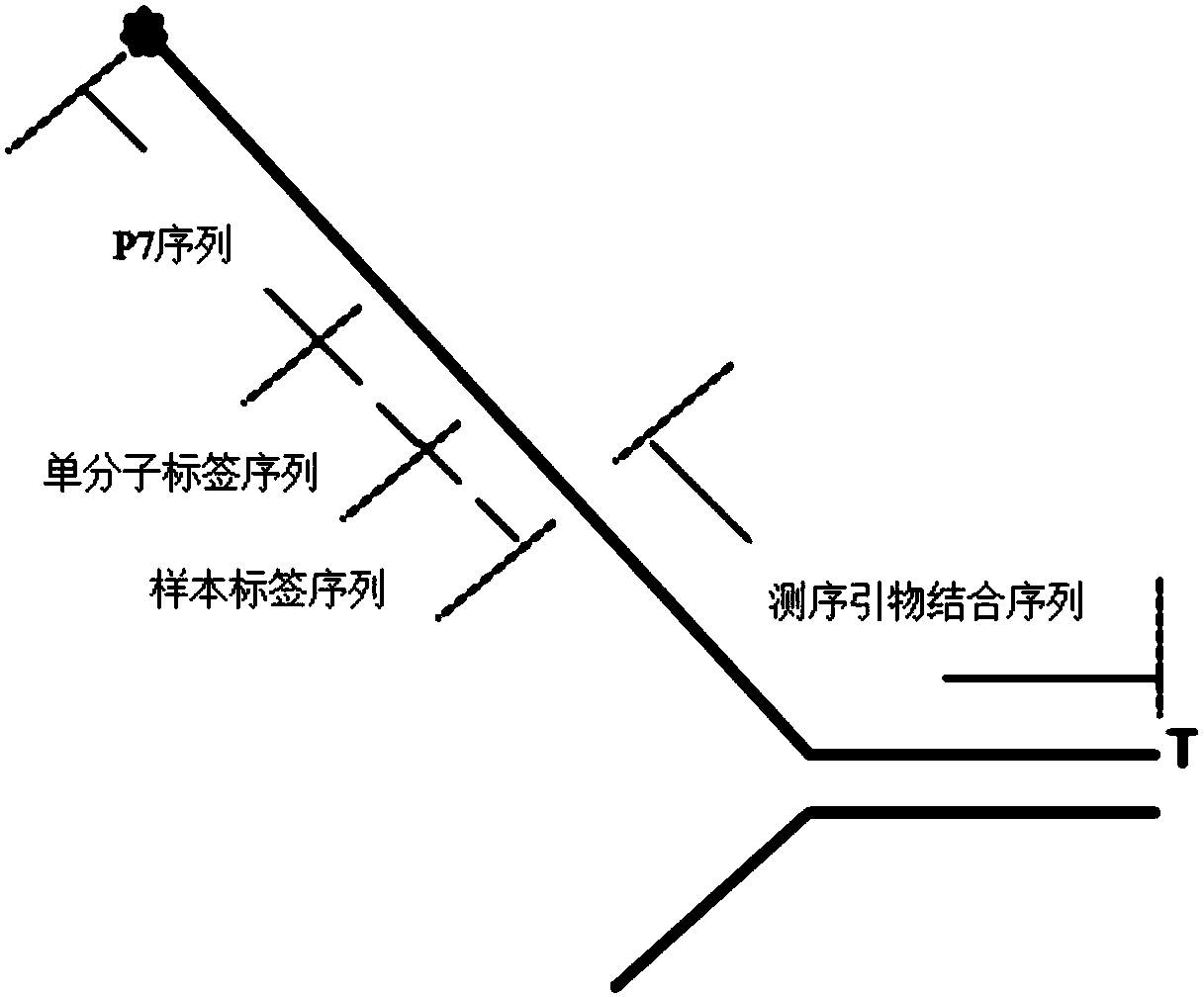
Nucleic acid sequence sequencing linker and method of using same to construct sequencing library
PendingCN110257480AEasy to distinguishReduce extractionLibrary tagsMicrobiological testing/measurementNucleic acid sequencingNucleic acid sequence
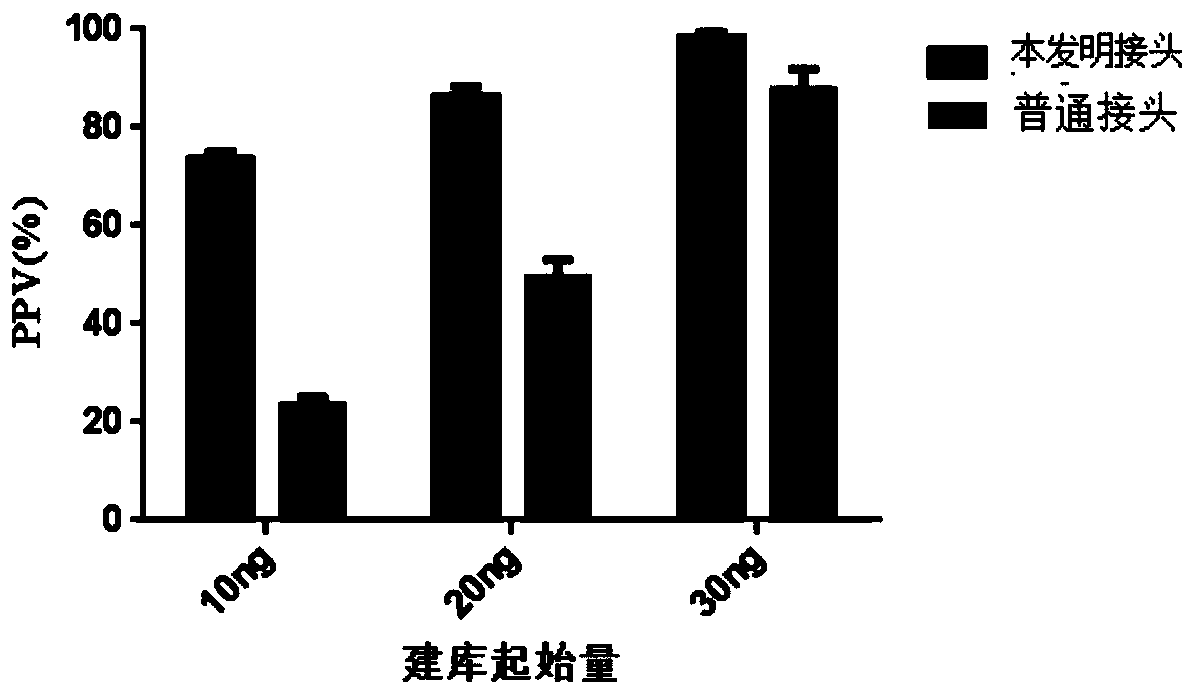
The invention provides a nucleic acid sequence sequencing linker and a method of using the same to construct a sequencing library. The sequencing linker includes a long Y primer and a short Y linker; the short Y linker and the long Y primer are connected to the two ends of an inserted fragment under sequencing respectively; the short Y linker has an upstream primer F and a downstream primer R which are partially complementary to each other; the long Y primer has an upstream primer P5 and a downstream primer P7; the short linker herein is used to perform tag identifying on different molecules in a same library, while the long Y primer is used to perform tag identification on different libraries. The nucleic acid sequence sequencing linker and the method herein are suitable for more accurately recognizing information of mutation sites, positive predicting capacity of a detection system can be improved greatly, and the problems in liquid biopsy application are solved.
Owner:DALIAN GENTALKER BIO-TECH CO LTD
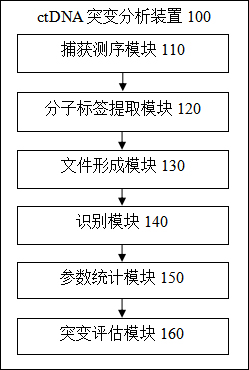
Liquid biopsy-based ctDNA mutation degree analysis method and device and ctDNA performance analysis device
ActiveCN113257350AAvoid the problem of false positives reducing detection sensitivityAuxiliary early diagnosisBiostatisticsProteomicsGenes mutationPlasma samples
The invention provides a liquid biopsy-based ctDNA mutation degree analysis method and device and ctDNA performance analysis device. The mutation degree analysis method comprises the steps of capturing and sequencing a to-be-detected plasma sample to obtain a FASTQ file; respectively extracting molecular tags in the matched reads and storing the molecular tags as a uBAM file; comparing a gene sequence of the FASTQ file with a reference genome, performing duplicate removal, and combining the gene sequence with the uBAM file to obtain a BAM file containing molecular tags; performing aggregation and duplicate removal on reads in the BAM file; obtaining a sample original mutation set in a gene mutation panel area, and counting gene mutation parameters in the sample original mutation set; filtering the sample original mutation set, and counting gene mutation parameters of each sample; evaluating the mutation degree of the to-be-detected plasma sample according to the gene mutation parameters of the sample, so that the ctDNA mutation detection sensitivity is improved.
Owner:臻和(北京)生物科技有限公司 +2
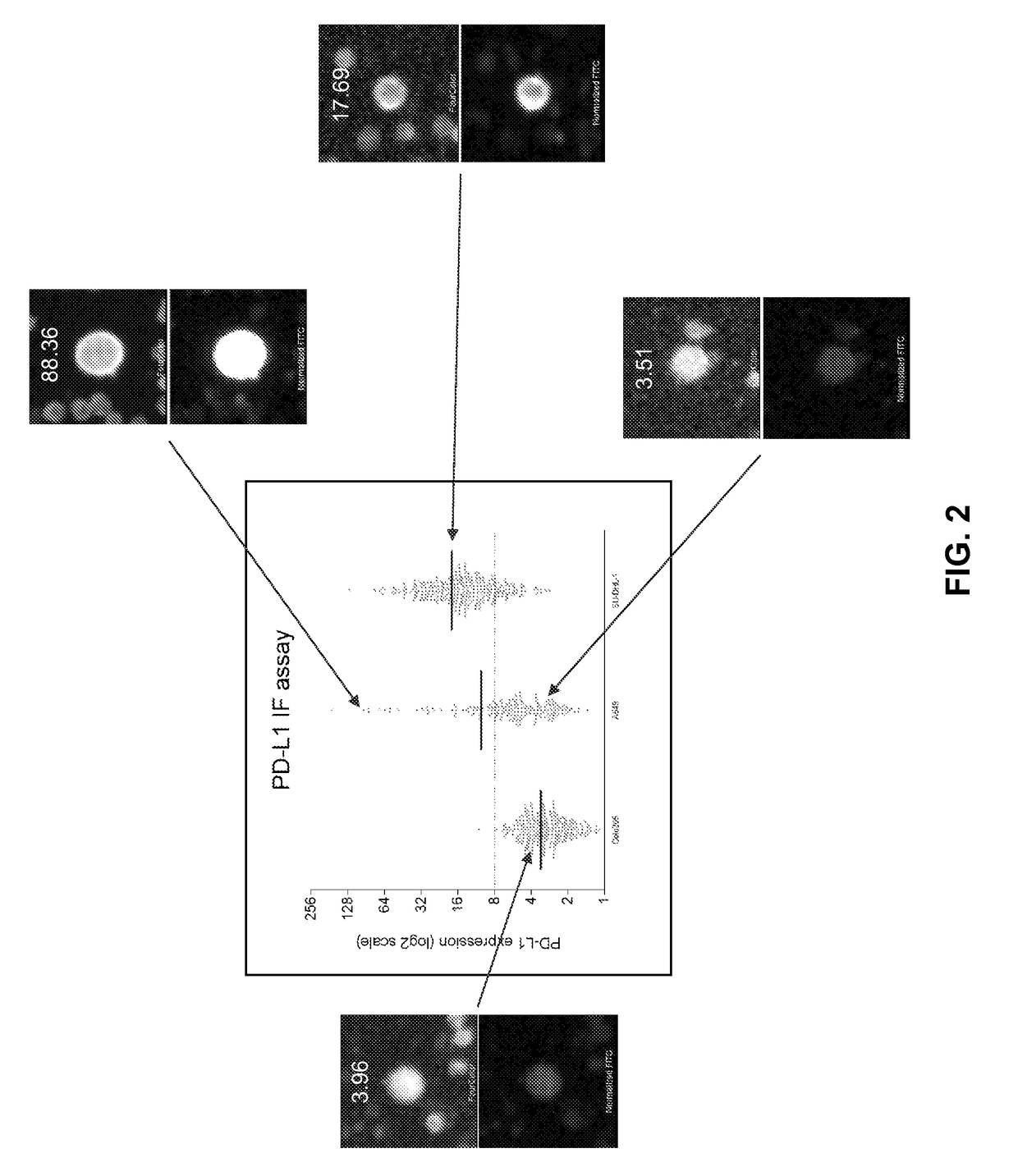
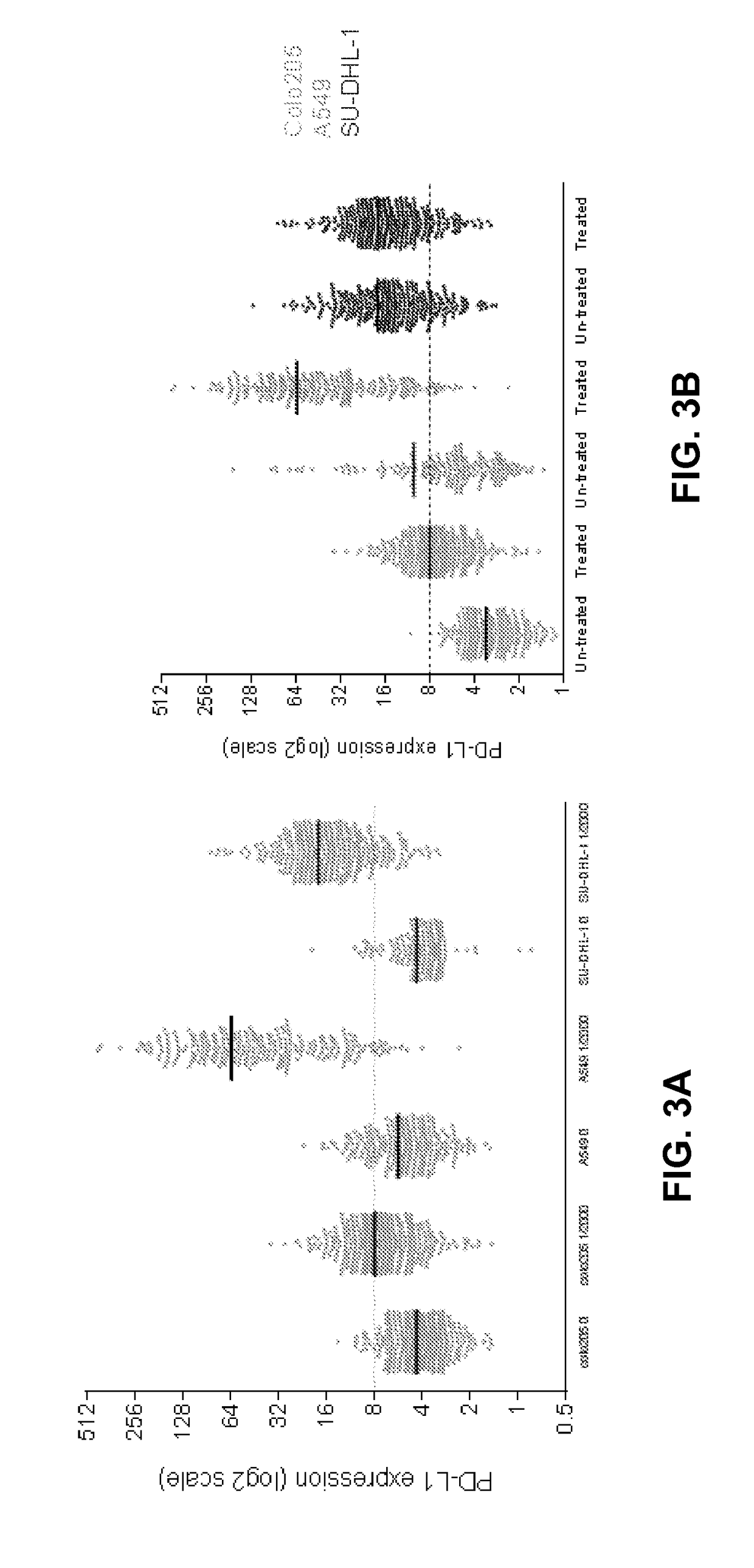
Circulating tumor cell diagnostics for therapy targeting pd-l1
Owner:EPIC SCIENCES
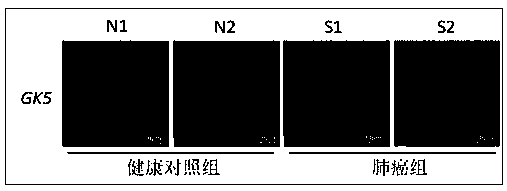
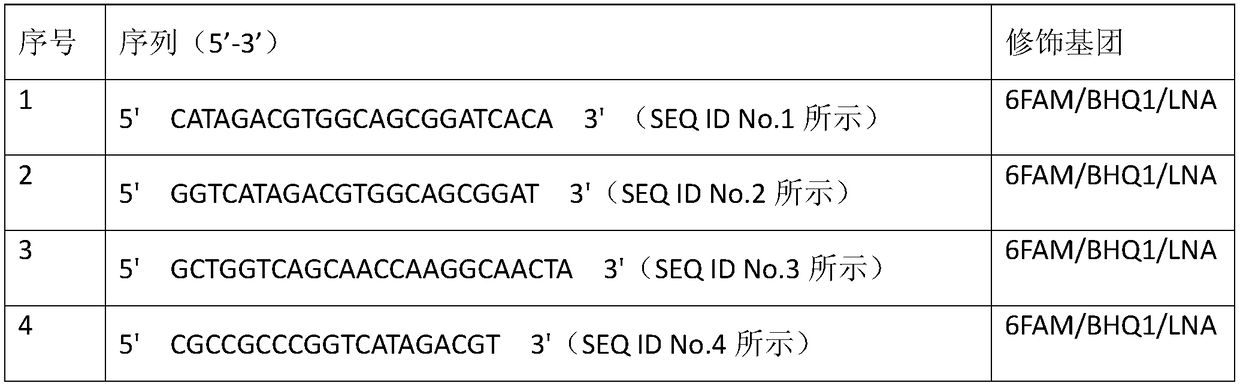
Rapid detection kit for lung cancer marker GK5 in exosome
ActiveCN108872438ANon-invasiveMinimally invasiveComponent separationNon invasiveEpidemiologic survey
The invention provides a rapid detection kit for a lung cancer marker GK5 in exosome and belongs to the technical field of kits. The kit contains an exosome in-situ capturing pore plate, wherein eachpore in the exosome in-situ capturing pore plate contains a fluorescein-labeled molecular beacon; the molecular beacon is a specific DNA (Deoxyribonucleic Acid) probe of targeting tumor marker GK5 mRNA (micro Ribonucleic Acid). The kit provided by the invention applies an exosome in-situ capturing pore plate technology and is used for detecting the tumor marker GK5 mRNA in a body fluid sample of alung cancer patient; the kit can be used for carrying out early diagnosis on medical clinical lung tumors. The technology is a third-generation body fluid biopsy technology based on exosome detection, has the advantages of non-invasive or minimally invasive effect, ultrahigh sensitivity, rapidness, specificity and the like and is applicable to molecular diagnosis and epidemiological survey of themedical clinical lung tumors.
Owner:HANGZHOU DIXIANG CO LTD
A liquid biopsy detection method for circulating tumor cells
ActiveCN107748256AStrong specificityEasy to followIndividual particle analysisFluorescence/phosphorescenceTumor recurrenceDynamic monitoring
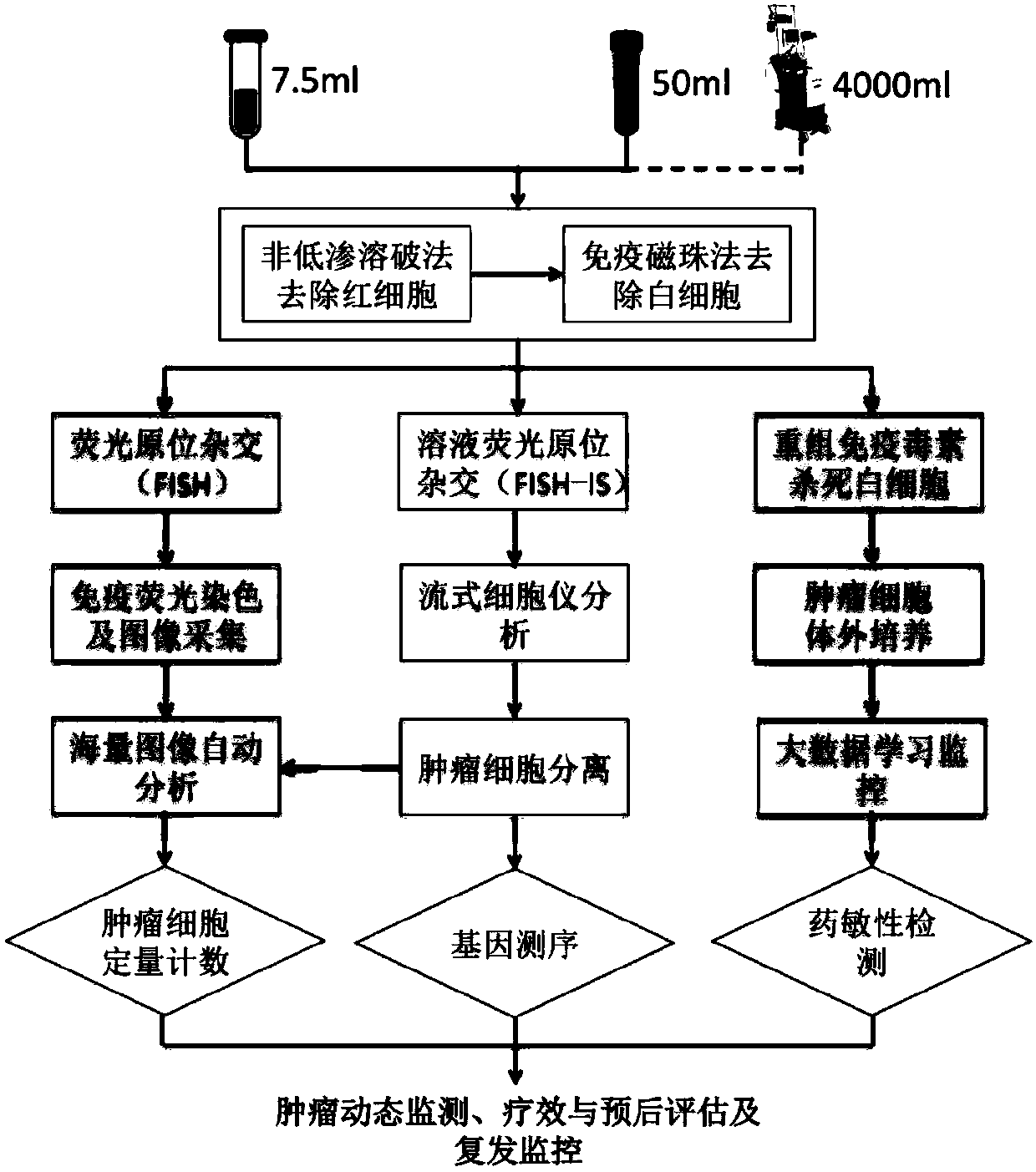
The invention relates to a liquid biopsy detection method, particularly a liquid biopsy detection method for circulating tumor cells. The method includes enriching circulating tumor cells in a sample;separating and identifying the circulating tumor cells; and quantitatively counting the circulating tumor cells in unit volume. The intelligent liquid biopsy method combines the newest computer science and biological techniques, and innovatively provides a circulating tumor cell quantitative detection method, a circulating tumor cell separating method and a circulating tumor cell in-vitro culturemethod. The liquid biopsy detection method can achieve dynamic monitoring of tumor traits, tumor treatment and prognosis evaluation, tumor recurrence monitoring, and the like, and therefore the liquid biopsy detection method will greatly promote developments of individualized precise treatment in China.
Owner:厦门骁科码生物科技有限公司
Separation and detection methods of circulating tumor cells
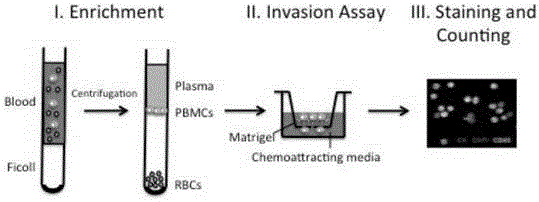
InactiveCN106244553AAggressiveInvasiveTumor/cancer cellsMaterial analysisTreatment effectScreening method
The invention provides a separation method of circulating tumor cells (CTCs). The method comprises the following steps: enriching mononuclear cells (PBMC) from a blood sample through density gradient centrifugation; carrying out invasion experiments on the enriched PBMCs, and collecting invasive cells; and dyeing and sorting the collected cells. The invention also provides a detection method of the CTCs. The methods are not influenced by the heteroplasmy of the CTCs, the separated CTCs are tumor cells with invasion ability and are more than CTCs with invasion ability, obtained through an immunomagnetic bead screening method, the CTCs can be used in tumor transfer researches in the laboratory, and CTC detection can be used as the biomarker liquid biopsy of the cancer prognosis and treatment effect.
Owner:XIAMEN RENRUI BIOMEDICAL TECH CO LTD
Exosome extraction kit and application thereof in liquid biopsy of diseases including tumors
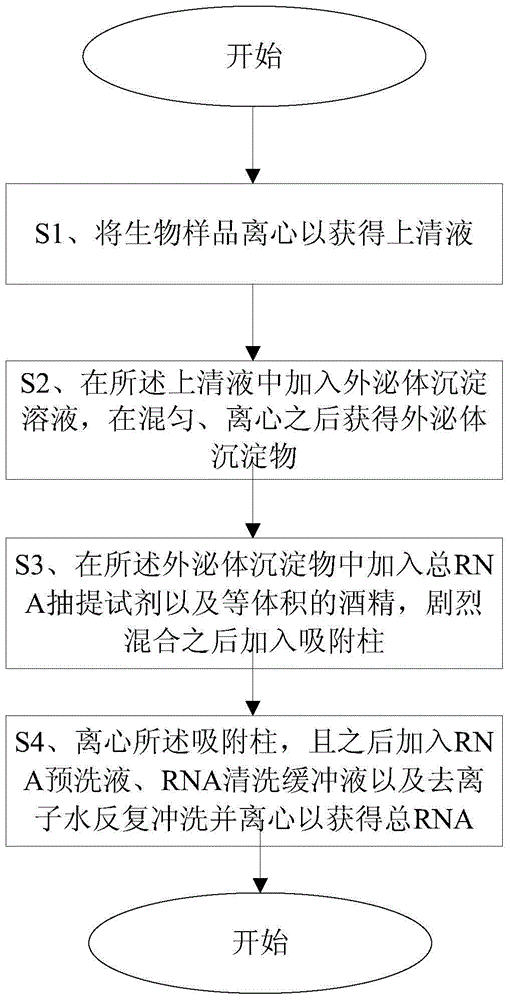
ActiveCN105087546AAccurate extractionReal-time monitoring of gene statusOrganic active ingredientsMicrobiological testing/measurementDiseasePatient compliance
The invention relates to a method and a kit for extracting RNA and application of RNA. The method comprises the following steps: (S1) centrifuging a biological sample to obtain supernate; (S2) adding an exosome precipitation solution into the supernate, and carrying out uniform mixing and centrifugation, so as to obtain exosome precipitates; (S3) adding a total RNA extraction reagent and isometric alcohol into the exosome precipitates, acutely mixing, and adding the mixture into an adsorption column; (S4) centrifuging the adsorption column, adding RNA pre-washing liquid, RNA cleaning buffer liquid and deionized water, and carrying out repeated flushing and centrifugation, so as to obtain total RNA. The invention further relates to application of fused RNA and other tumor-specific RNA extracted from exosome of body fluid in aspects of early diagnosis, screening and diagnosis of tumors. Fused RNA and other tumor-specific RNA are extracted from the exosome and are applied to the early diagnosis and screening of malignant tumors such as esophagus cancer and breast cancer, thereby simply realizing the cancer liquid diagnosis with the characteristics of little wound, good patient compliance and convenience in collection and storage.
Owner:骞和生物科技(广州)有限公司
Molecular weight correction standard substance kit for polypeptide or protein spectrum detection as well as preparation method and use method of molecular weight correction standard substance kit
PendingCN111239423ALow costImprove accuracyMaterial analysis by electric/magnetic meansBiological testingMicroorganismInternal standard
The invention discloses a molecular weight correction standard substance kit for polypeptide or protein spectrum detection as well as a preparation method and a use method of the molecular weight correction standard substance kit. The kit comprises a polypeptide or protein dry powder standard substance, a dry powder dissolving solution, a matrix dissolving solution, a matrix, a molecular weight external standard correction list and a molecular weight internal standard correction list, the ratio of the polypeptide or protein dry powder standard substance to the matrix is as follows: 1-6 parts of polypeptide or protein dry powder corresponds to 10 parts of matrixes; the preparation method of the kit not only is low in cost, but also can selectively prepare a calibration product according toa specific calibration purpose, and molecular weight correction considers both small molecule and large molecule ranges; the molecular weight correction method provided by the invention has the advantages that the operation convenience of an external standard method and the high precision of an internal standard method are realized, and meanwhile, the potential influence of the addition of an exogenous internal standard substance in a sample on the polypeptide fingerprint spectrum can also be avoided; the kit can be applied to application scenarios such as liquid biopsy and microbial identification based on a mass spectrometry technology.
Owner:HANGZHOU WELL HEALTHCARE TECH CO LTD
In-vivo collecting device used for liquid biopsy of blood
InactiveCN106434285AEasy to operateLow costBioreactor/fermenter combinationsBiological substance pretreatmentsLymphatic SpreadIn vivo
The invention provides a catheter which comprises a catheter body and a collecting device. The front end of the catheter body is connected with the collecting device. The invention provides an in-vivo sample (including circulating tumor cells, exosomes and nucleic acids) collecting device used for liquid biopsy of blood, the in-vivo collecting device can be used for in-vivo collection of the cells, the exosomes and the nucleic acids, and is particularly used to collect the circulating tumor cells; and when being used to collect the tumor cells, the in-vivo collecting device captures and enriches as many tumor cells as possible as compared with the technique of collecting peripheral blood and separating and capturing the tumor cells in the prior art; the in-vivo collecting device is convenient and safe, is easy to operate, and is low in cost; the captured tumor cells are used for downstream analysis, for example gene analysis, in vitro culturing and drug sensitive test; and more importantly, the in-vivo collecting device can block metastasis of the tumor cells through blood after being improved.
Owner:GUANGZHOU YIYANG BIO TECH CO LTD
Centrifuging micro-fluidic control chip for free nucleic acid extraction and method for extracting free nucleic acid
ActiveCN110016435AGuaranteed stabilityEfficient separation and extractionBioreactor/fermenter combinationsBiological substance pretreatmentsSiphonBlood plasma
The invention provides a centrifuging micro-fluidic control chip for free nucleic acid extraction and a method for extracting free nucleic acid, and belongs to the technical field of free nucleic acidseparating and extracting devices. The centrifuging micro-fluidic control chip comprises a disc, plasma separating chambers, nucleic acid extraction sample cavities, immiscible phase cavities and eluting cavities, wherein the plasma separating chambers, the nucleic acid extraction sample cavities, the immiscible phase cavities and the eluting cavities are in rotary symmetric distribution; each plasma separating chamber communicates with the corresponding nucleic acid extraction sample cavity through a corresponding passive siphon; and each nucleic acid extraction sample cavity communicates with the corresponding immiscible phase cavity and the corresponding eluting cavity through arch capillary microchannels. According to the centrifuging micro-fluidic control chip disclosed by the invention, a dynamic centrifuging micro-fluidic control method and a static immiscible phase micro-fluidic control method are combined for the first time, and the designed immiscible phase nucleic acid extracting and purifying structure realizes uniform mixing under the premise that the interface stability is not influenced; the plasma separating structure is integrated on the centrifuging micro-fluidiccontrol chip, so that the purpose of extracting and purifying free nucleic acid from 4mL whole blood fully-automatically within 15 minutes can be realized, the centrifuging micro-fluidic control chipis the fastest device for extracting and purifying the free nucleic acid from the whole blood at present, and the centrifuging micro-fluidic control chip is hopefully applied to liquid biopsy of thefree nucleic acid.
Owner:XI AN JIAOTONG UNIV
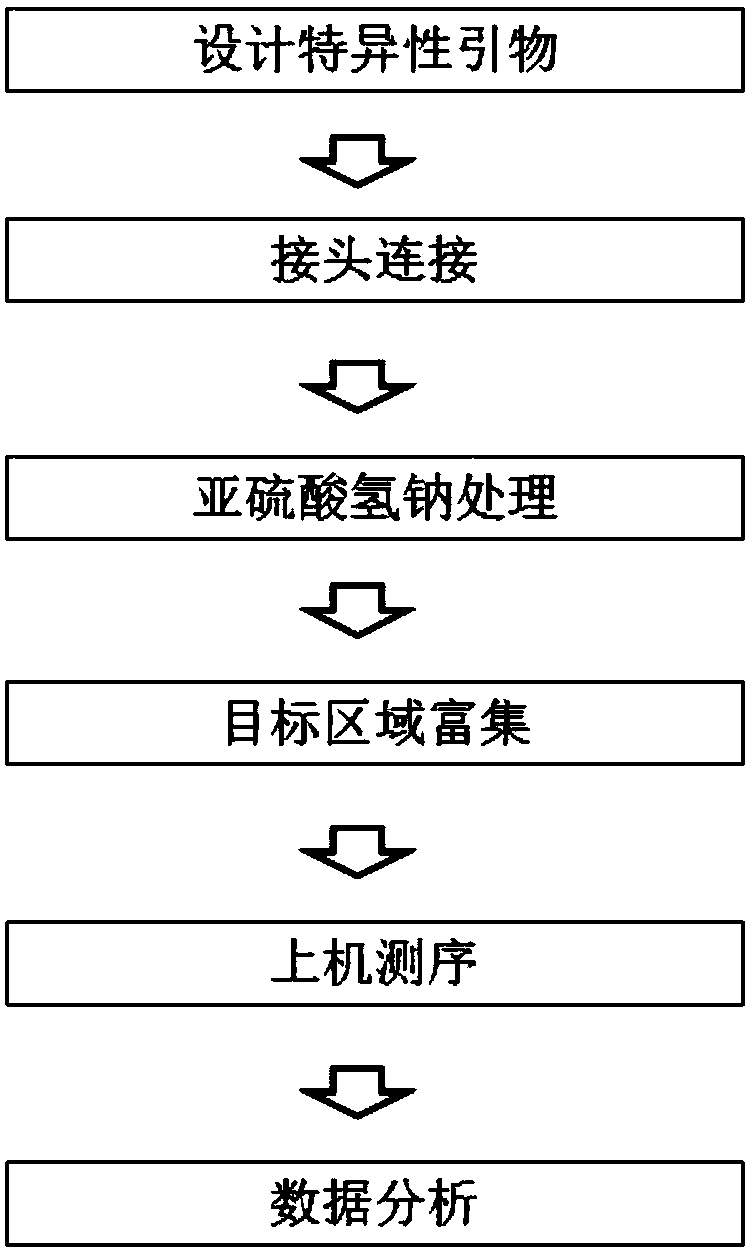
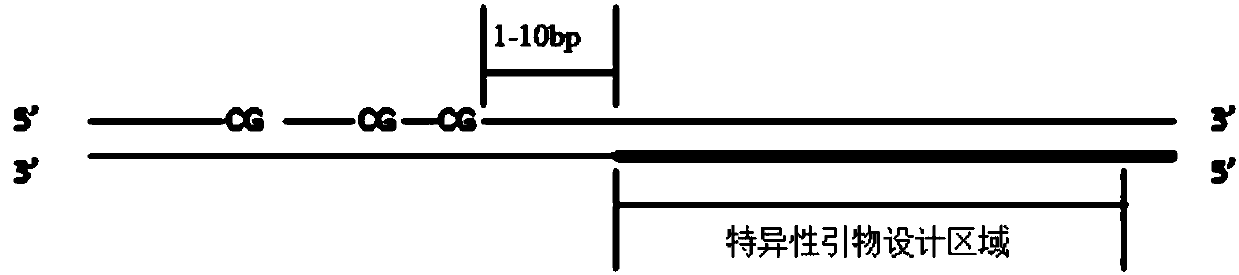
Construction method and detection method of trace fragmented DNA methylation detection library
InactiveCN107937985AHigh sensitivity detectionMicrobiological testing/measurementLibrary creationTumor suppressor geneMultiple tumor
The invention discloses a construction method and detection method of a trace fragmented DNA methylation detection library. The library construction method of the present invention comprises: linkingfragmented DNA to a methylation linker, wherein all cytosines on the methylation linker are methylated cytosines, the methylation linker includes a long chain and a short chain which are complementaryto form a double chain, and the long chain includes a sequencing platform universal sequence, a single molecule tag sequence, a sample tag sequence and a sequencing primer binding sequence; and the DNA linked with the methylation linker is treated with sodium bisulphate, so that the cytosine C in a non-methylated CpG island is converted into uracil U; and a specific primer and a universal primerare used for PCR amplification enrichment of a target region subjected to sodium bisulfite treatment and containing a CpG site to be detected, so that a library available for on-machine sequencing isobtained. According to the invention, methylation of trace DNA taken through liquid biopsy can be detected, and the CpG island methylation status of multiple regulatory regions of multiple tumor suppressor genes can be quantitatively detected simultaneously.
Owner:GENETALKS BIO TECH CHANGSHA CO LTD
Joint detection kit for multiple markers on CTCs (circulating tumor cells) and application of kit
InactiveCN107586839AAvoid interferenceUse less bloodMicrobiological testing/measurementLymphatic SpreadHepatocellular carcinoma
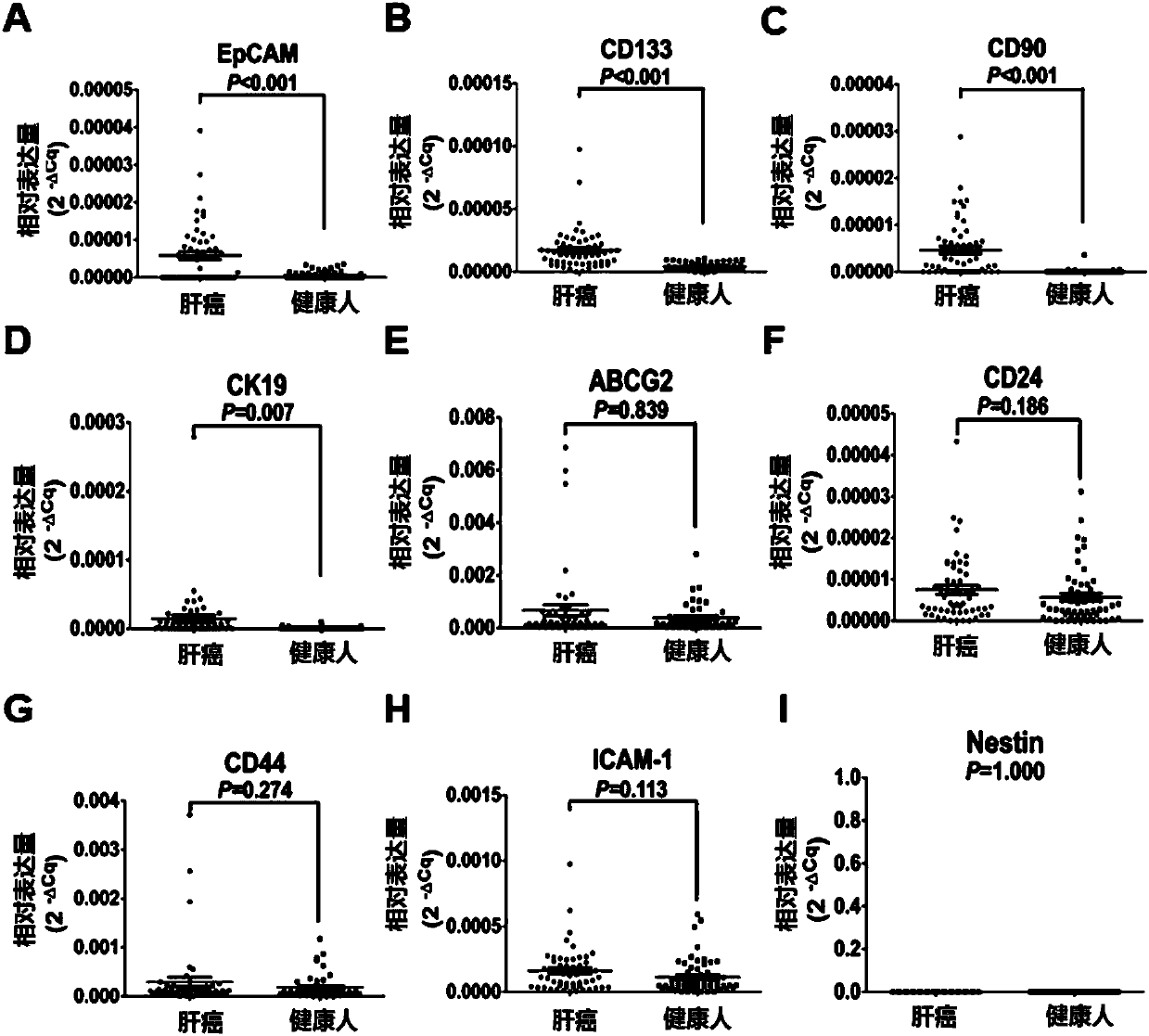
The invention provides a joint detection kit for multiple stem cell markers on surfaces of CTCs (circulating tumor cells) and an application of the kit. The joint detection kit for multiple stem cellmarkers on surfaces of CTCs comprises 1) a peripheral blood CTC enrichment reagent; 2) an RNA extraction reagent; 3) an RNA reverse transcription reagent; 4) a fluorescence labeled Taqman probe and aspecific primer; 5) a qPCR reaction system reagent and a hepatocellular carcinoma prediction formula. The kit can be used for biopsy of minimally invasive fluids of tumor patients, early diagnosis andearly warning of tumor metastasis and relapse are realized, the anti-tumor therapy effect and the tumor progression condition are monitored, molecular subtyping based on CTCs and screening of specific sensitive drugs for the CTCs are realized, so that knowledge about tumor metastasis, relapse and drug resistance related mechanisms is improved, individualized medical treatment of tumor patients isguided, the overall survival of the tumor patients is significantly improved, and the kit has great clinical significance.
Owner:ZHONGSHAN HOSPITAL FUDAN UNIV
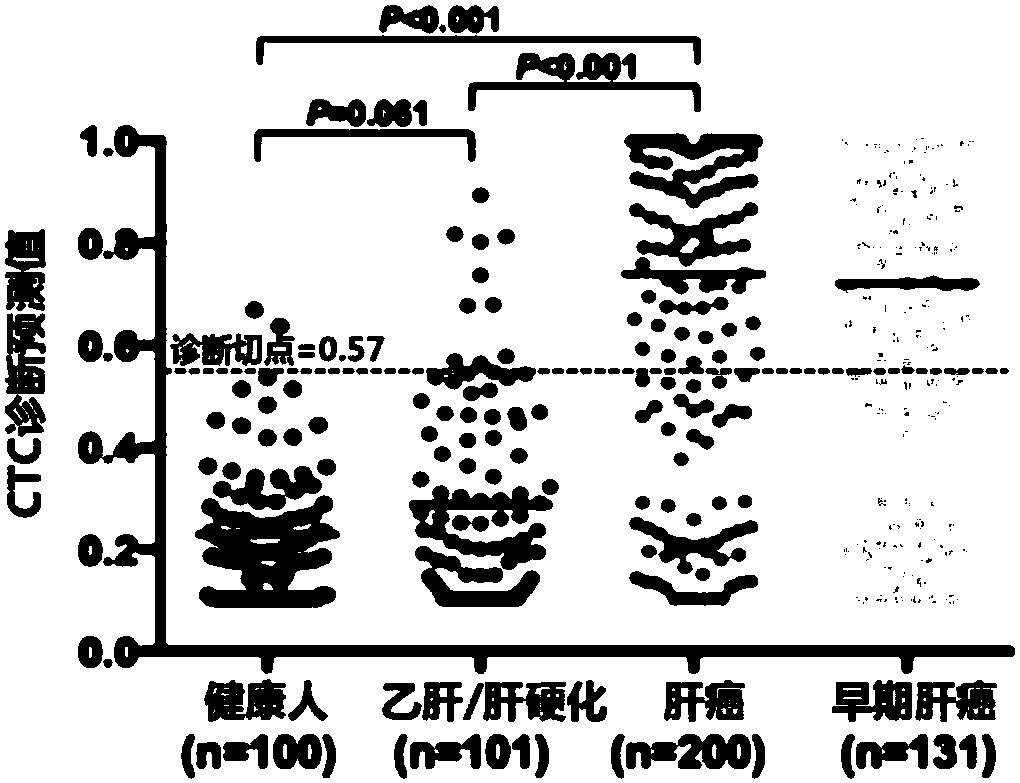
Liver cancer detection kit based on liquid biopsy
ActiveCN106932579AEfficient enrichmentEasy to distinguishDisease diagnosisFluorescent stainingFluorescence
The invention provides a liver cancer detection kit based on liquid biopsy. The liver cancer detection kit comprises staining enhancing liquid and specific antibodies with fluorescent staining labels, wherein the staining enhancing liquid is used for enhancing a staining effect; the specific antibodies include an ASGPR1 antibody, a CD45 antibody and a CPS1 antibody; and the staining enhancing liquid contains a surfactant with a concentration of 0.001mg / mL-1mg / mL. The kit provided by the invention can be used for effectively enriching target cells and determining whether the enriched target cells are derived from early-phase liver cancer patients. Meanwhile, by virtue of double-tumor marker detection, the tumor detection sensitivity is increased, and furthermore, the detection accuracy is guaranteed by virtue of CEP8 detection. Besides, by utilizing the staining enhancing liquid, the staining effect is enhanced, multiple antibodies with the fluorescent staining labels can be integrated with the target cells, and the target cells are stained, so that the staining effect is relatively good, the fluorescence is relatively strong, and borders are clear.
Owner:SHANGHAI YH HEALTH BIOLOGY MEDICINE TECH CO LTD
Method for capturing high-purity circulating tumor cells based on biological orthogonal chemical method
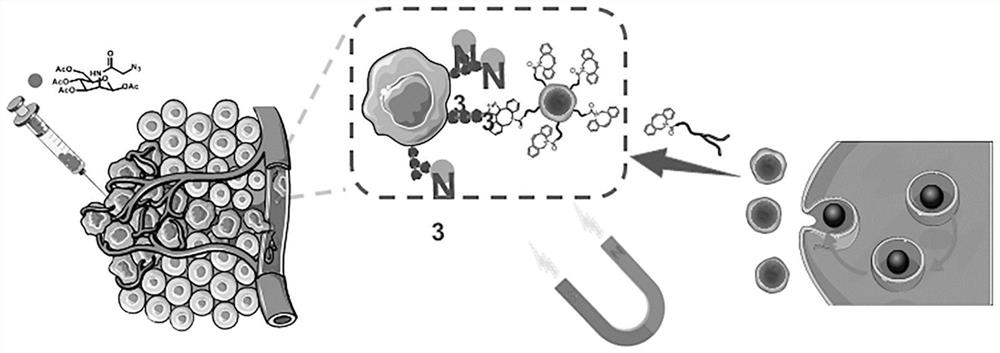
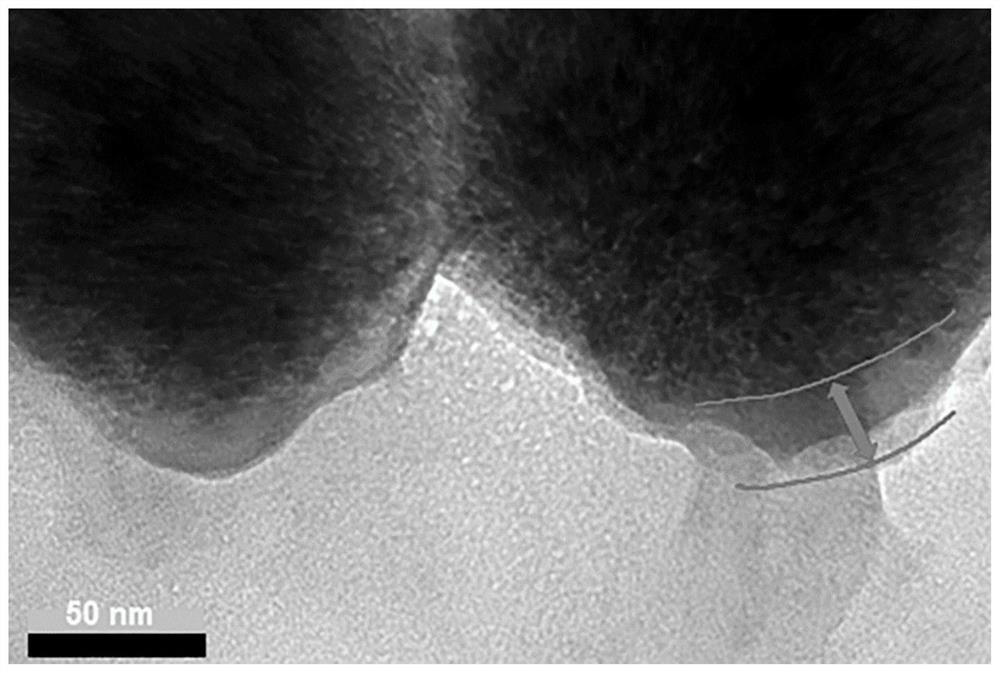
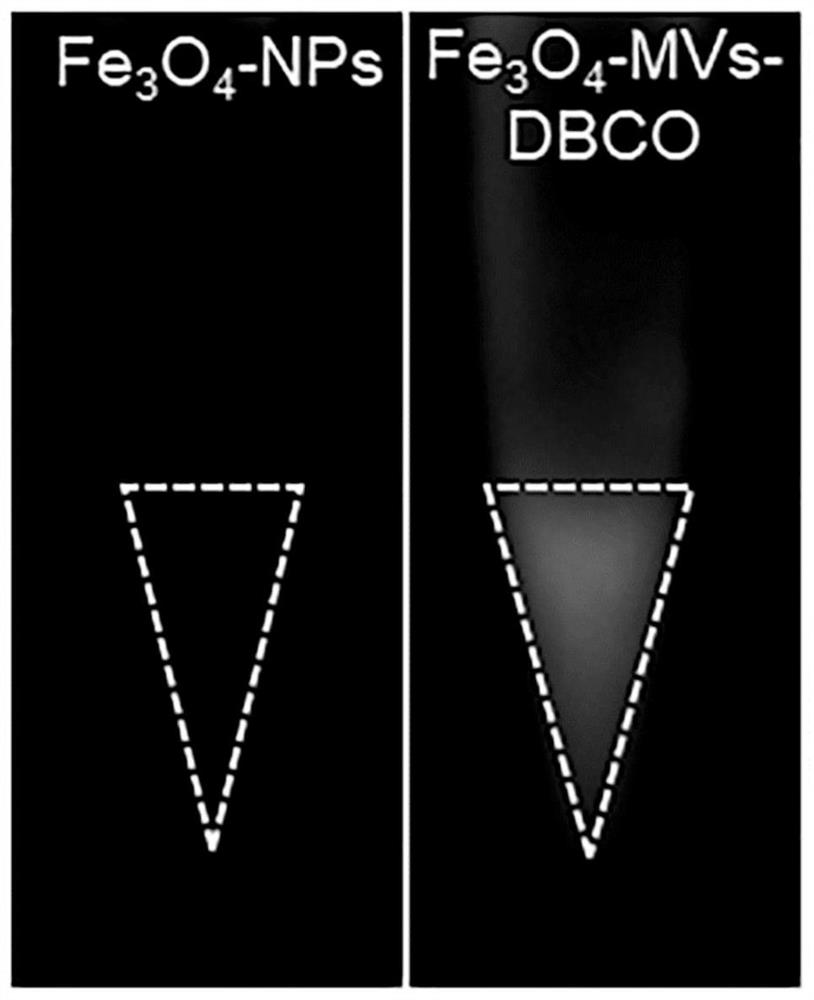
ActiveCN112011513AGood Magnetic Response BehaviorUniform particle size distributionCell dissociation methodsTumor/cancer cellsCell membraneFunctional modification
The invention discloses a method for capturing high-purity circulating tumor cells based on a biological orthogonal chemical method. The method comprises the following steps: s1, preparing superparamagnetic ferroferric oxide nanoparticles; s2, preparing a bionic magnetic vesicle; s3, performing functional modification on the bionic magnetic vesicles; s4, growing azide genes on the surfaces of thetumor cells; and s5, capturing the circulating tumor cells. After superparamagnetic ferroferric oxide nanoparticles and macrophages are incubated, bionic magnetic vesicles can be generated through mouse macrophages in a way similar to exosome release, and then functional modification is carried out; tumor cells generate functional groups on the surfaces of cell membranes in advance through intracellular metabolism of the tumor cells, the tumor cells are incubated with the functionalized magnetic vesicles, the two functional groups are combined through bioorthogonal chemistry, capture of circulating tumor cells is achieved, and the mode has the potential of being applied to liquid biopsy.
Owner:SICHUAN UNIV
Platelet-leukocyte hybrid membrane-coated immunomagnetic beads and preparation method and application thereof
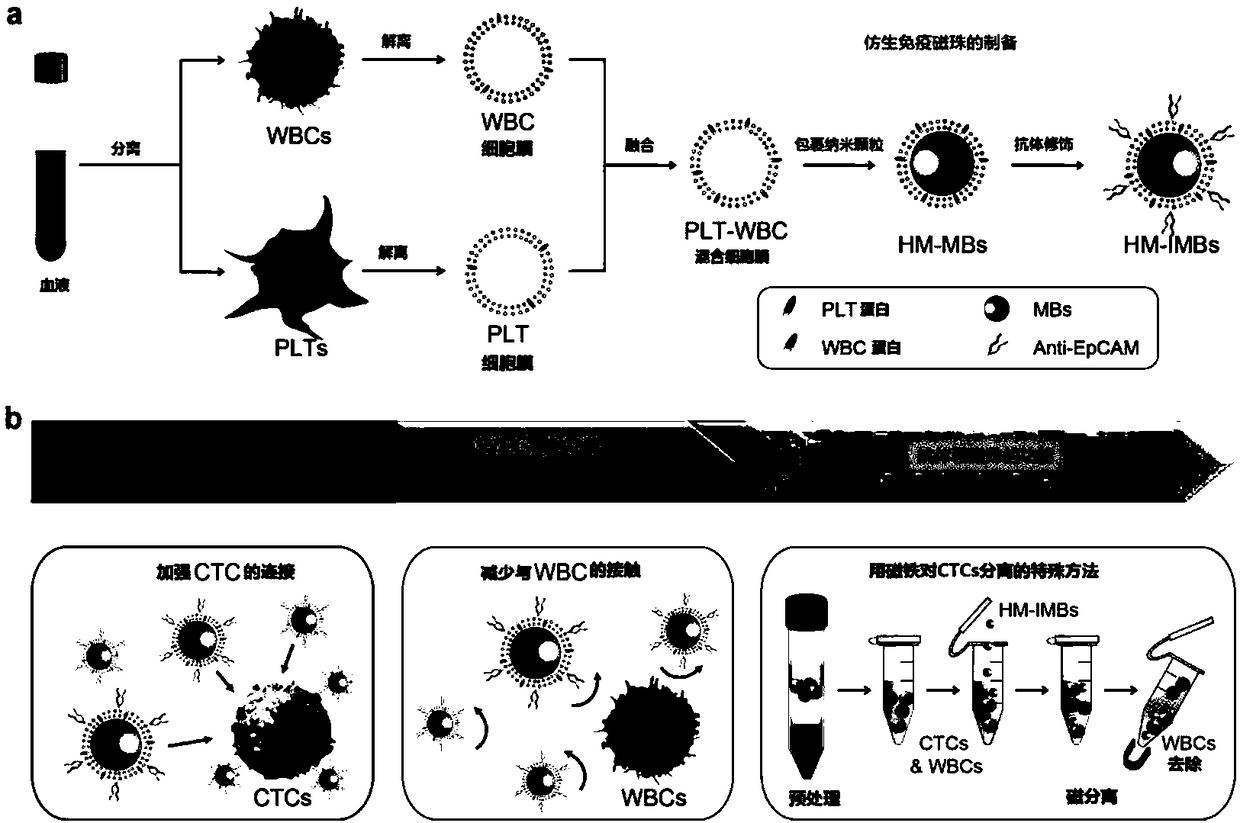
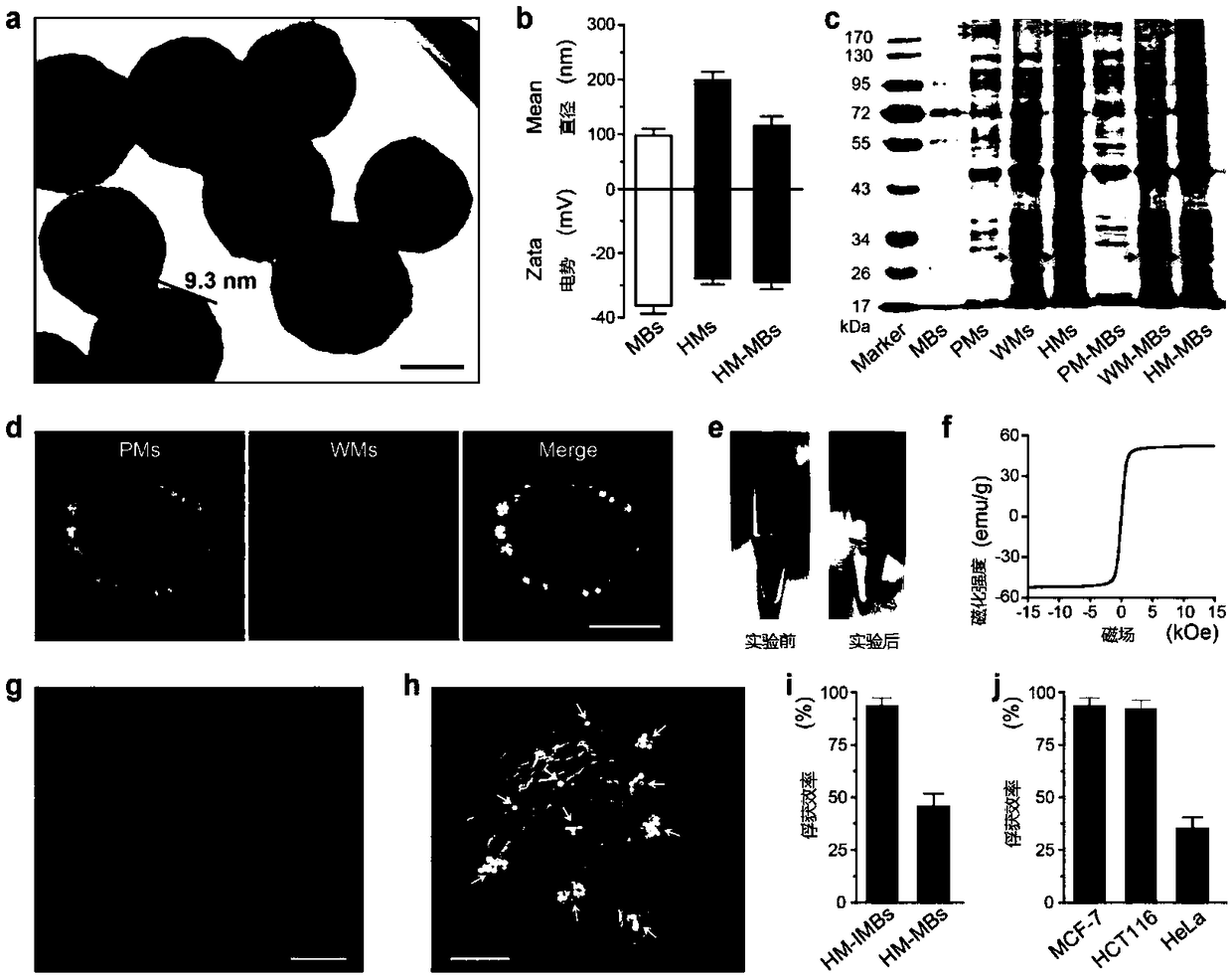
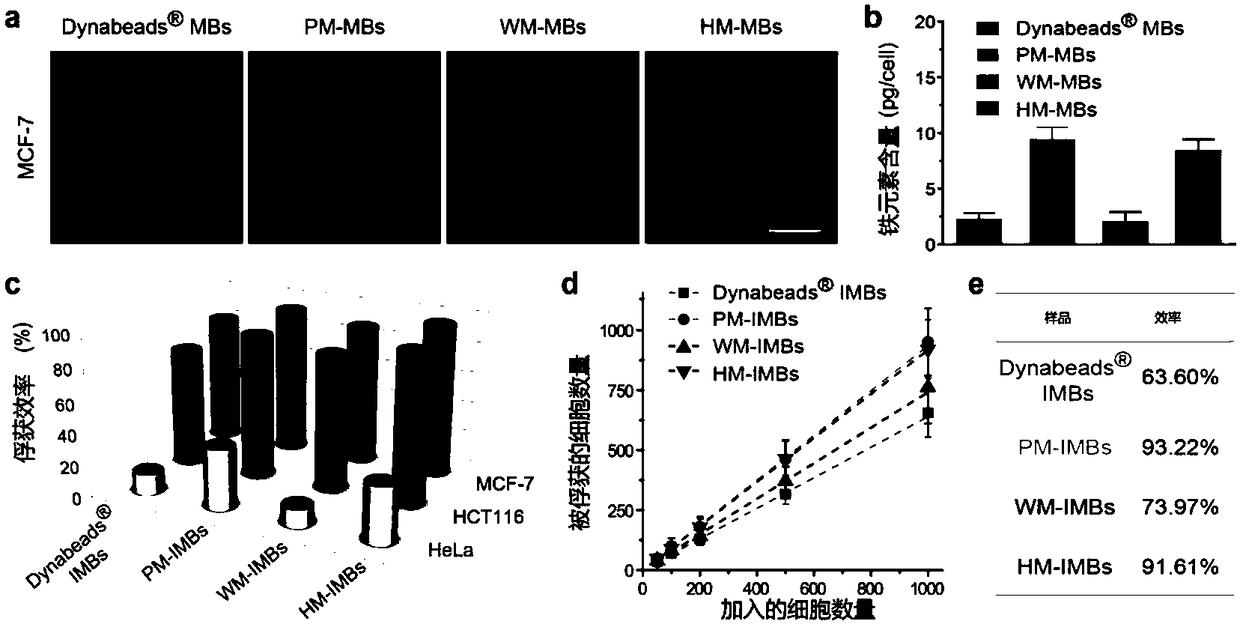
ActiveCN109100504AImprove separation efficiencyHigh separation purityMaterial analysisCancers diagnosisWhite blood cell
The invention discloses a platelet-leukocyte hybrid membrane-coated immunomagnetic beads and a preparation method and an application thereof. Magnetic beads were coated by using a hybrid membrane of platelet (PLT)-white blood cells (WBC), and then the surface thereof is modified with circulating tumor cells (CTCs)-specific antibodies to obtain PLT-WBC hybrid membrane-coated immunomagnetic beads (HM-IMBs). HM-IMBs inherit the enhanced CTC-binding ability from PLT and the ability to reduce homologous WBC interactions from WBC, therefore, the efficiency and the purity of the cell separation is effectively increased. According to the use of the HM-IMBs, 16 CTCs in clinical blood samples collected from patients with colorectal cancer, breast cancer, or gastric cancer were successfully identified, and the purity of isolated CTCs was greater than 85%. Therefore, this liquid biopsy tool will open up new areas of technology for cancer diagnosis and treatment.
Owner:江阴市珞珈飞流数字科技有限公司
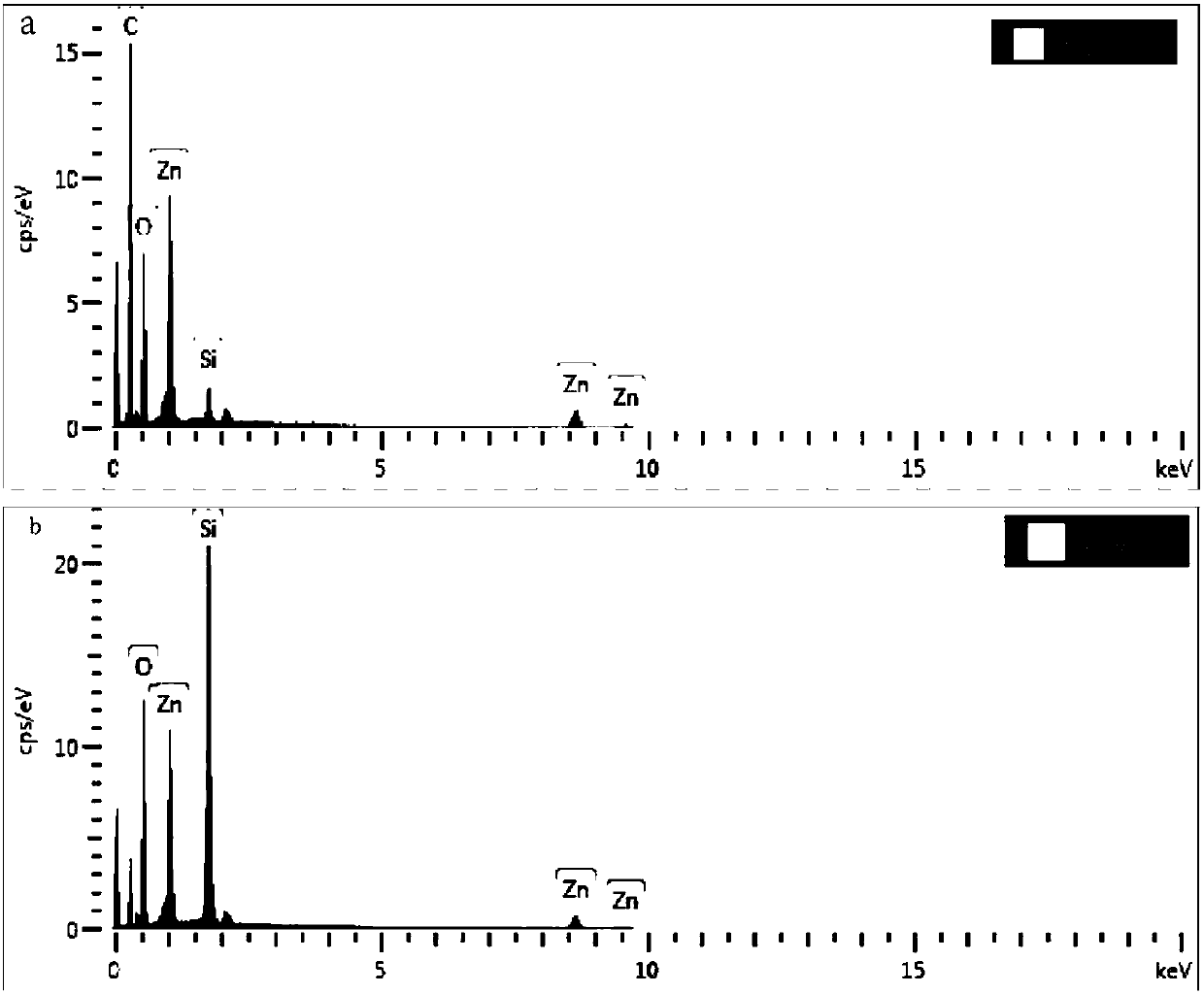
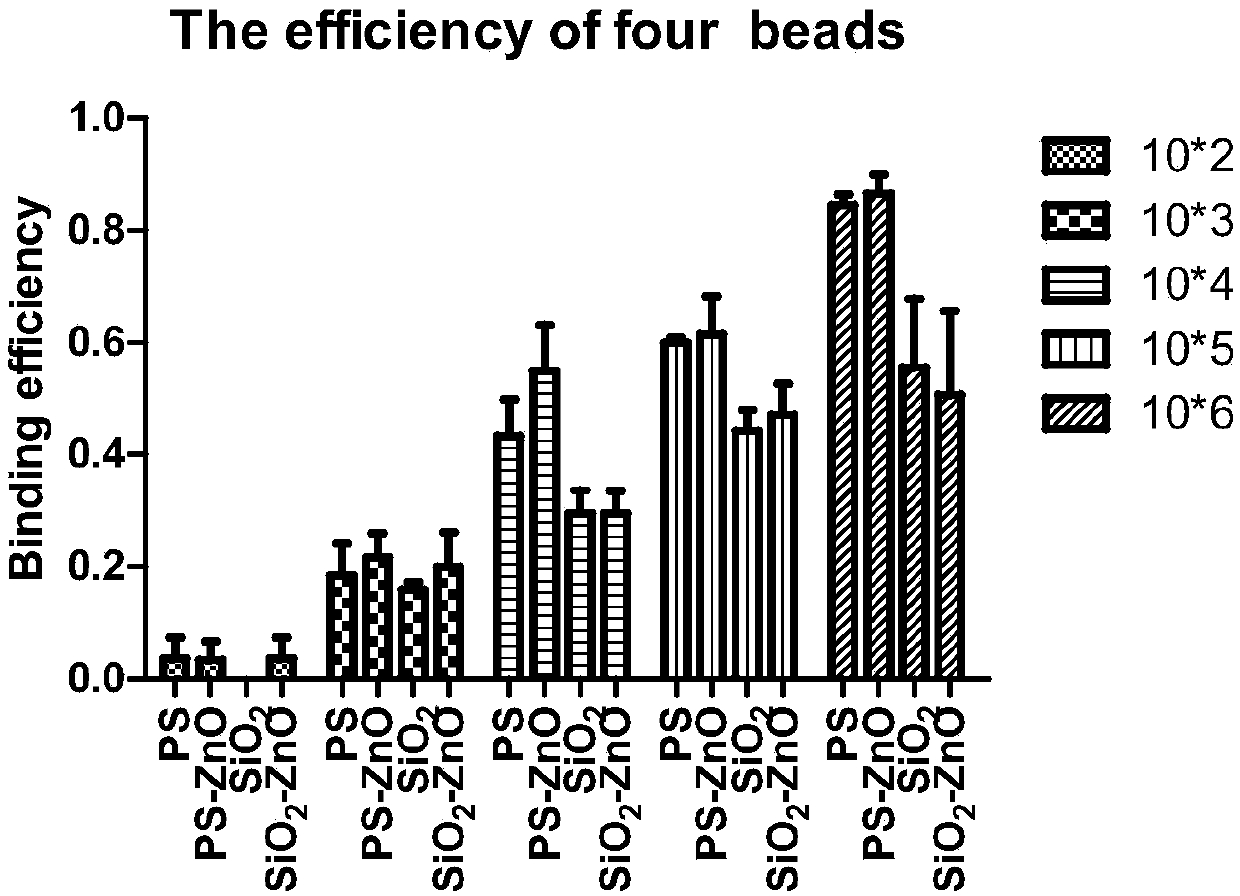
Device for capturing circulating tumor cells by combining functionalized microspheres with filtering chip and application of device
ActiveCN107723208AHigh efficiency in capturing target cellsImprove capture abilityBioreactor/fermenter combinationsBiological substance pretreatmentsAntigenMedicine
The invention discloses a device for capturing circulating tumor cells by combining functionalized microspheres with a filtering chip and application of the device. The device comprises a micro-infusion pump, a blood sample to be sorted and enriched and the filtering chip, wherein the filtering chip is composed of a first circular-arc-shaped channel and a second circular-arc-shaped channel; each circular-arc-shaped channel is internally provided with a fence structure; nano zinc oxide modified microspheres and treated blood are mixed to form the blood sample to be sorted and enriched; a sampleinlet of the filtering chip is connected with the micro-infusion pump; the blood sample to be sorted and enriched is injected to the sample inlet of the filtering chip through the micro-infusion pump; leukocytes and other impurities have a small volume and are separated through the fence structure, and the circulating tumor cells are captured. The invention provides the device for capturing the circulating tumor cells by combining the functionalized microspheres with the filtering chip and the application of the device, and the device is used for liquid biopsy; the capturing of the circulating tumor cells is carried out through a virtual inertial force based on the size of the circulating tumor cells and surface antigen integration of the circulating tumor cells.
Owner:ZHEJIANG UNIV
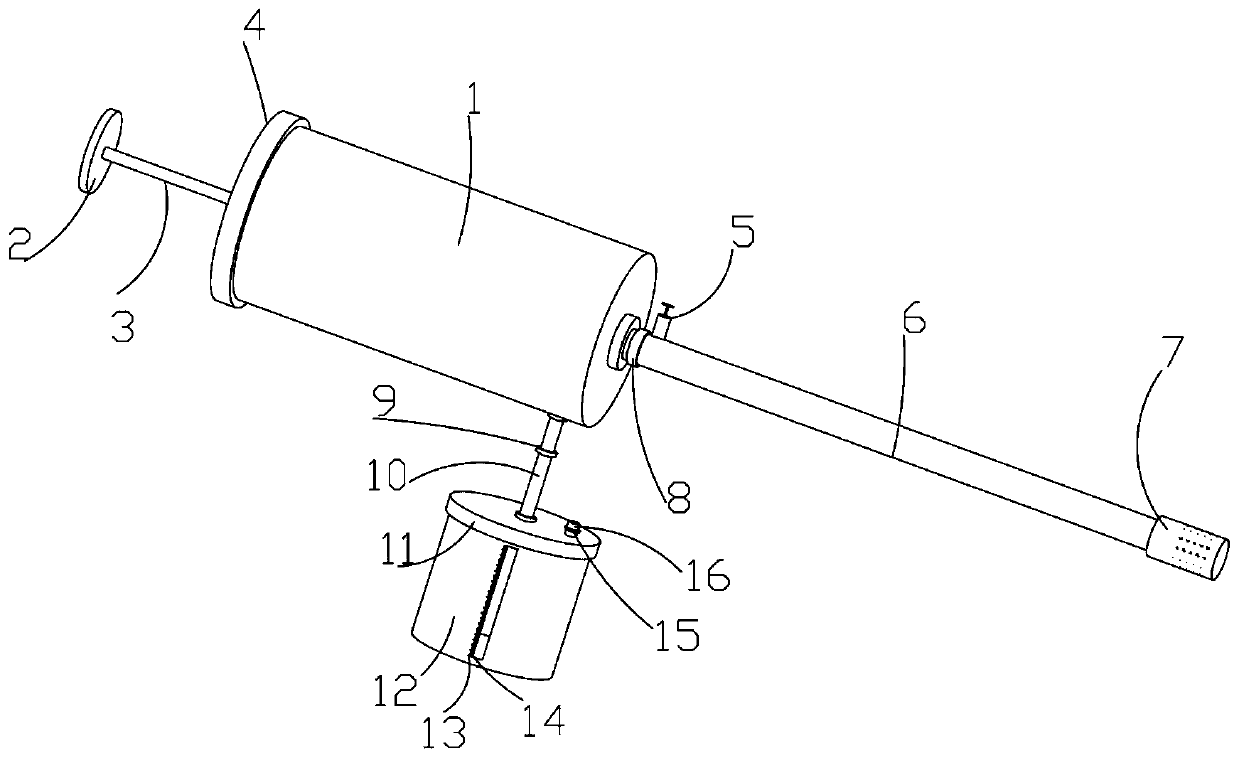
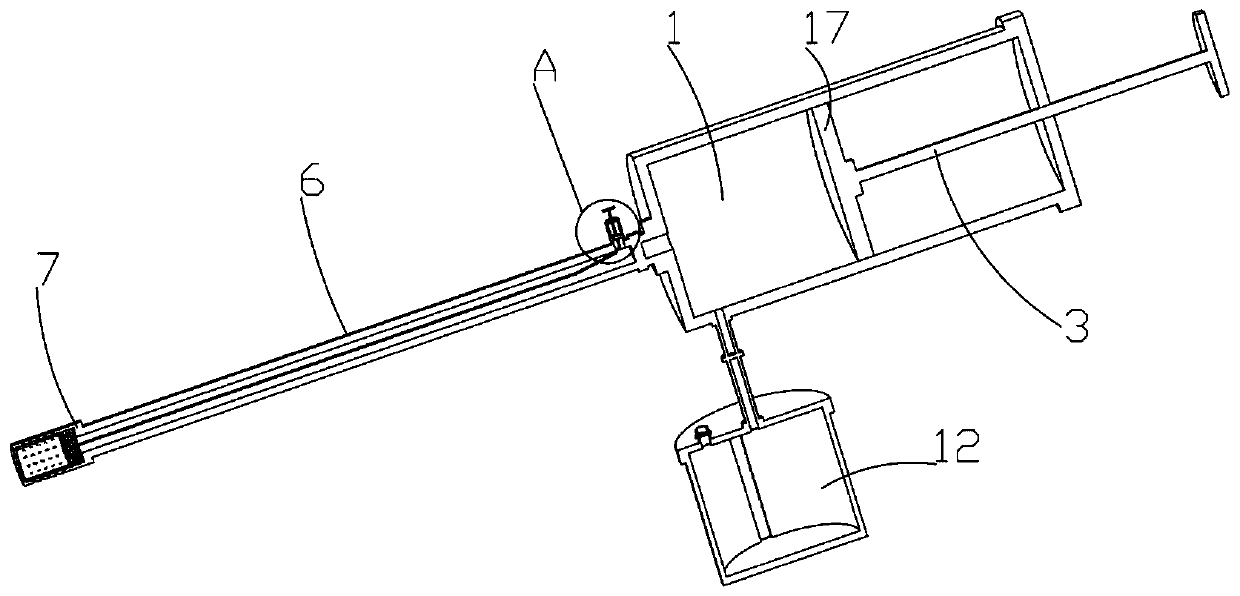
Digestive tract liquid biopsy sampling device for internal medicine
ActiveCN111481236AEasy to sampleAvoid influenceSurgeryVaccination/ovulation diagnosticsStraight tubeEngineering
The invention relates to a digestive tract liquid biopsy sampling device for internal medicine, and belongs to the field of medical treatment. The device includes a needle tube, needle tube with cap,push rod with cap, push rod with first piston, needle tube with connecting tube, a second one-way valve is arranged on the connecting pipe; the connecting pipe is provided with a cover plate, the cover plate is provided with a liquid accumulation barrel; the needle tube is provided with an intermediate tube, the intermediate pipe is provided with a first one-way valve, the intermediate pipe is provided with a straight pipe, the straight pipe is provided with a T-shaped push rod, the T-shaped push rod is provided with a reset structure; and the reset structure is connected with a blocking cylinder. The blocking cylinder is provided with a sleeve, the sleeve is in threaded connection with the middle pipe; through holes are formed in the blocking cylinder and the sleeve; the T-shaped push rodin the straight pipe moves upwards; the T-shaped push rod drives a connecting rope of the reset structure to move; the connecting rope drives the blocking cylinder to move through the straight rod, drives the solid part of the blocking cylinder to move to the through hole of the sleeve to block the through hole of the sleeve, and conveniently blocks the through hole part of the sleeve after sampling, so that liquid at other parts cannot enter the sampling device when the sleeve moves, the sampling correctness is ensured, and the inspection is more accurate.
Owner:杭州憶盛医疗科技有限公司
Kit for simultaneously extracting circulating tumor cell DNA and tumor free DNA
InactiveCN106480017ALower detection rateImprove the detection rateMicrobiological testing/measurementDNA preparationNon invasiveWilms' tumor
The invention discloses a kit for simultaneously extracting circulating tumor cell DNA and tumor free DNA. The kit comprises a cell density gradient separation medium, a porous diaphragm separation tube, a centrifugal tube with the volume of 5 mL and a blood free DNA extraction kit. By means of the kit, according to the characteristics that the mutant gene detection rate of the free DNA and the circulating tumor cell DNA has no relevance with occurrence, through the complementarity of detection results of the free DNA and the circulating tumor cell DNA, the two sources of the free DNA and the circulating tumor cell DNA are creatively superposed, the sample size is increased, thus, the detection rate of patient tumor mutant genes can be effectively increased, clinical detection requirements are met, non-invasive liquid biopsy is better and more widely applied, and more tumor patients of different condition types can obtain benefits from tumor molecular diagnosis. The use process is convenient and easy, the detection cost is far lower than that of the unicell detection technology, doctors can easily grasp the kit, and the kit is suitable for clinical application and popularization.
Owner:武汉海吉力生物科技有限公司
A kit for auxiliary diagnosis of early early liver cancer and a detection method thereof
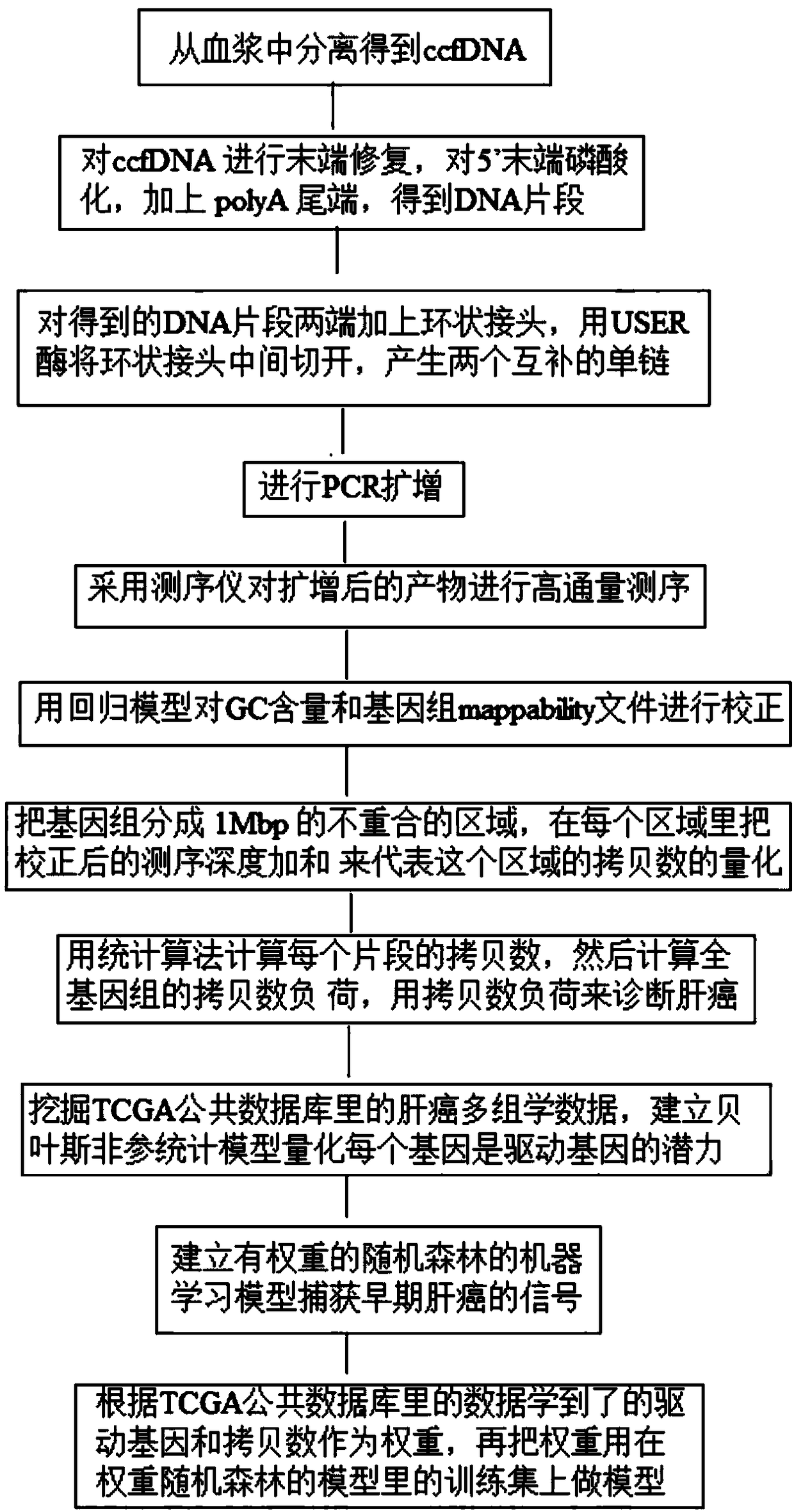
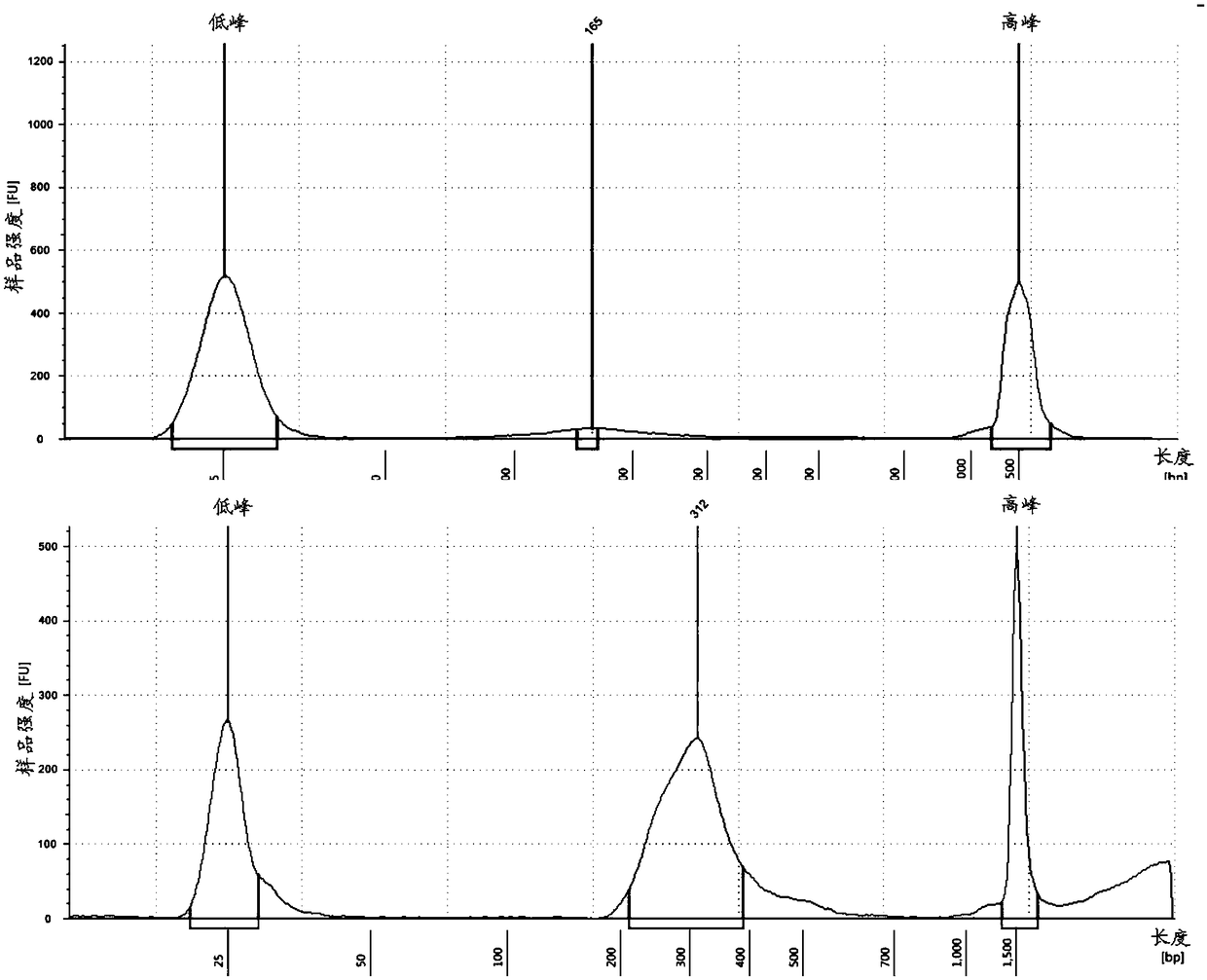
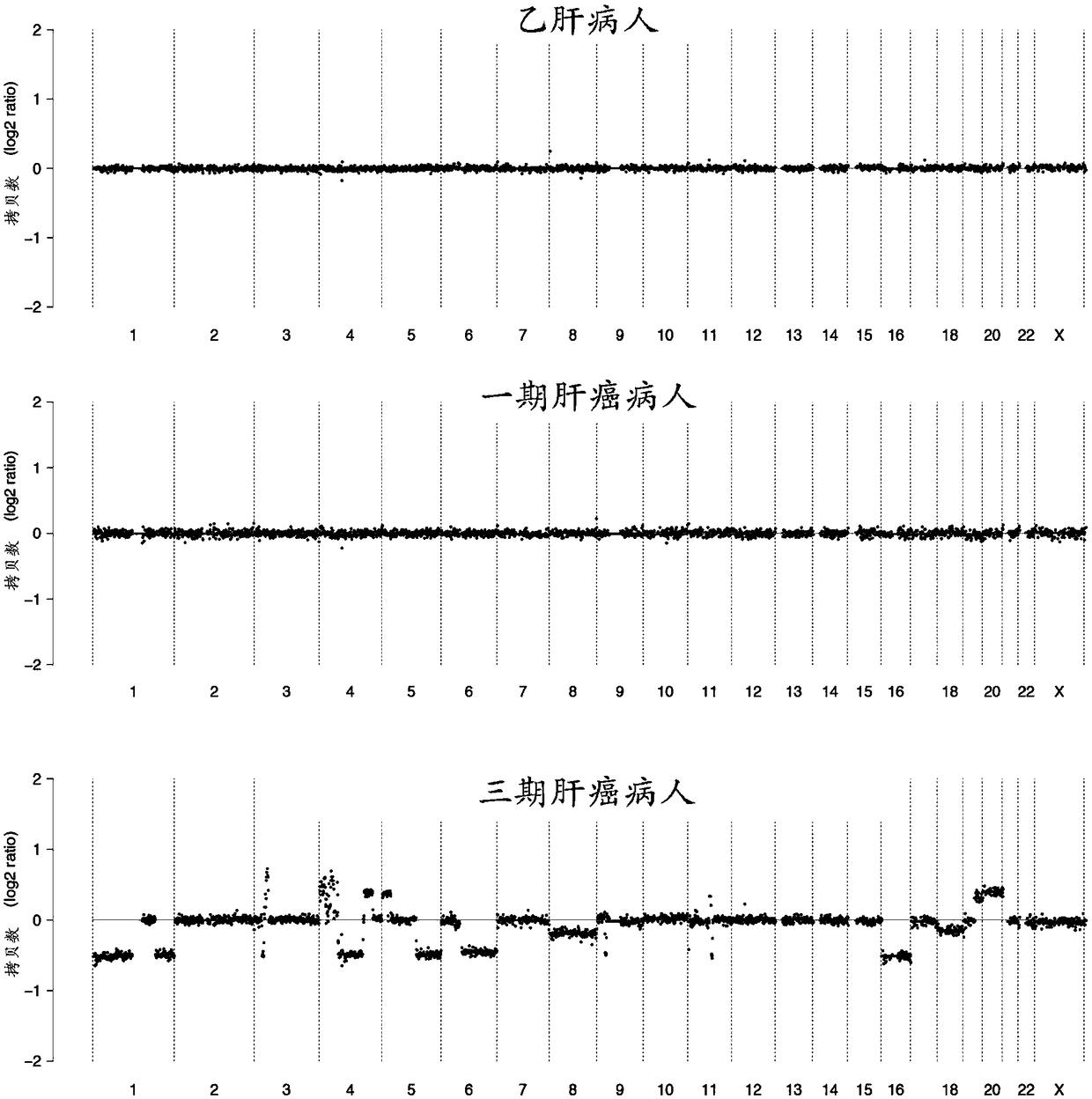
InactiveCN109182526AAchieve early diagnosisImprove accuracyMicrobiological testing/measurementBiostatisticsMagnetic beadGenomic data
The invention discloses a kit for auxiliary diagnosis of early liver cancer and a detection method thereof, which comprises the following reagents: a ccfDNA terminal treatment system, a ring linker reaction system, 0.06-0.15 U / ul USER enzyme, PCR amplification system, magnetic beads; The whole ccfDNA genome is sequenced by sequencing library. After genome-wide data processing, statistics and machine learning model were established to detect the abnormal copy number of ccfDNA in patients so as to achieve the early diagnosis of liver cancer. Such a detection method maximizes the accuracy of fluid biopsy for the diagnosis of early liver cancer, especially for the detection of primary liver cancer.
Owner:杭州翱锐生物科技有限公司
Reagent kit for detecting non-small cell lung cancer on basis of liquid biopsy
ActiveCN106990080ARemove cleanHigh enrichment efficiencyPreparing sample for investigationFluorescence/phosphorescenceFluorescenceEGFR Antibody
The invention provides a reagent kit for detecting non-small cell lung cancer on the basis of liquid biopsy. The reagent kid comprises staining enhancement solution for enhancing staining effects and specific ligands with fluorescent staining markers. The specific ligands comprise EGFR (epidermal growth factor receptor) antibodies, CD45 antibodies and folate ligands; the staining enhancement solution comprises surfactants with the concentration of 0.001-1 mg / mL. The reagent kit has the advantages that target cells can be effectively enriched by the reagent kit, and whether the enriched target cells come from early-stage patients suffering from the non-small cell lung cancer or not can be verified; the CTC (circulating tumor cell) detection sensitivity can be improved by means of double-tumor-marker detection, and the detection accuracy further can be guaranteed by means of CEP8 detection; the staining effects can be enhanced by the staining enhancement solution, the diversified ligands with the fluorescent staining markers can be combined with the surfaces of the target cells, accordingly, the surfaces of the target cells can be stained, the good staining effects can be realized, fluorescence is intense, and boundaries are clear.
Owner:上海美吉医学检验有限公司
Apparatus and Methods for Single-Particle Isolation and Single-Particle Measurement
InactiveUS20160375439A1Microbiological testing/measurementBiological particle analysisCells isolationBiochip
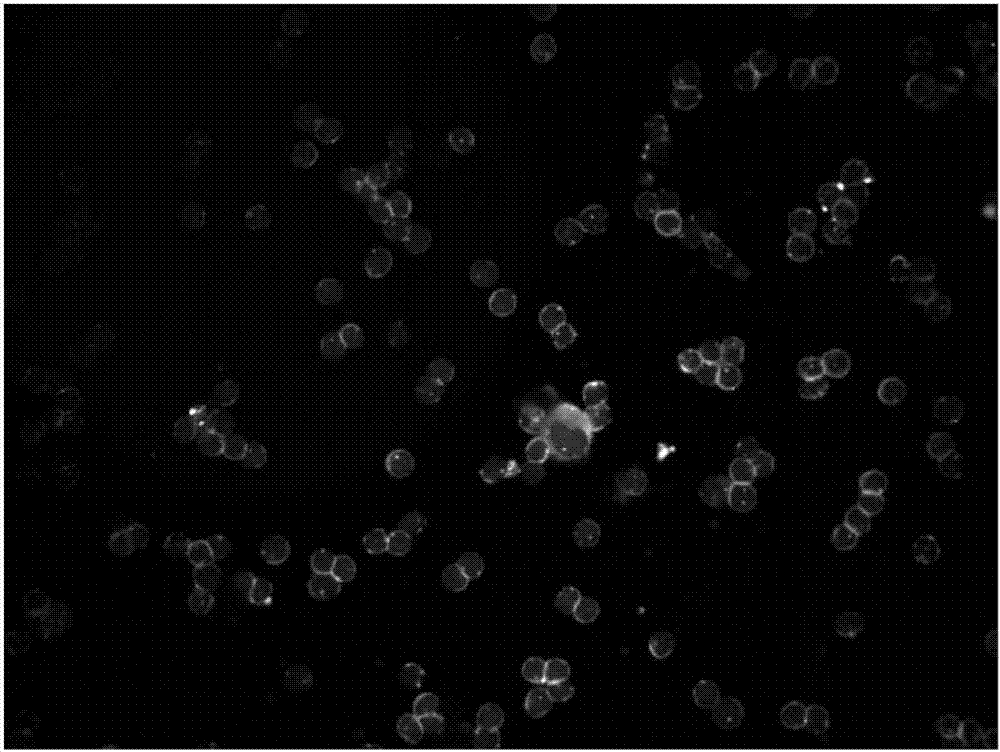
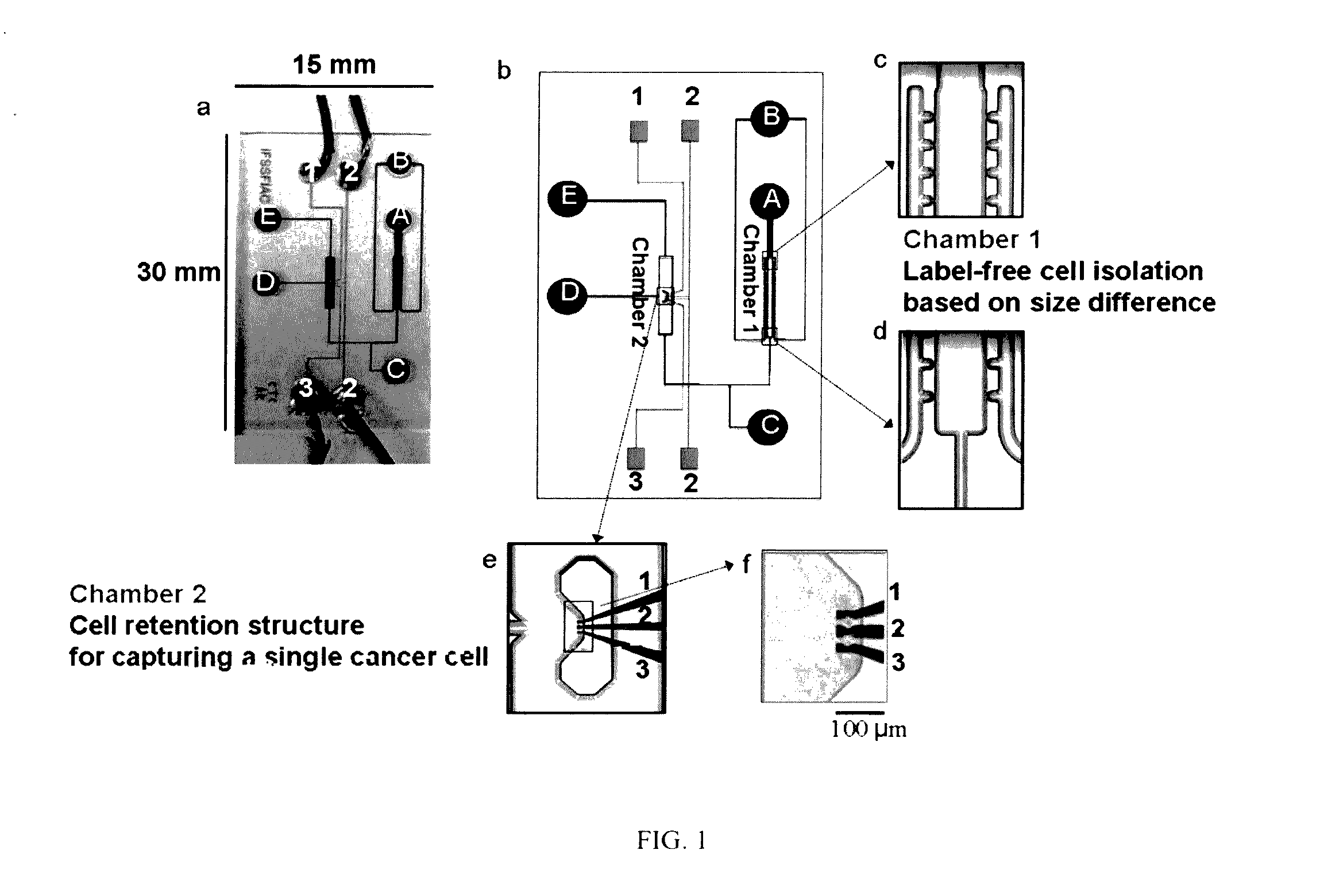
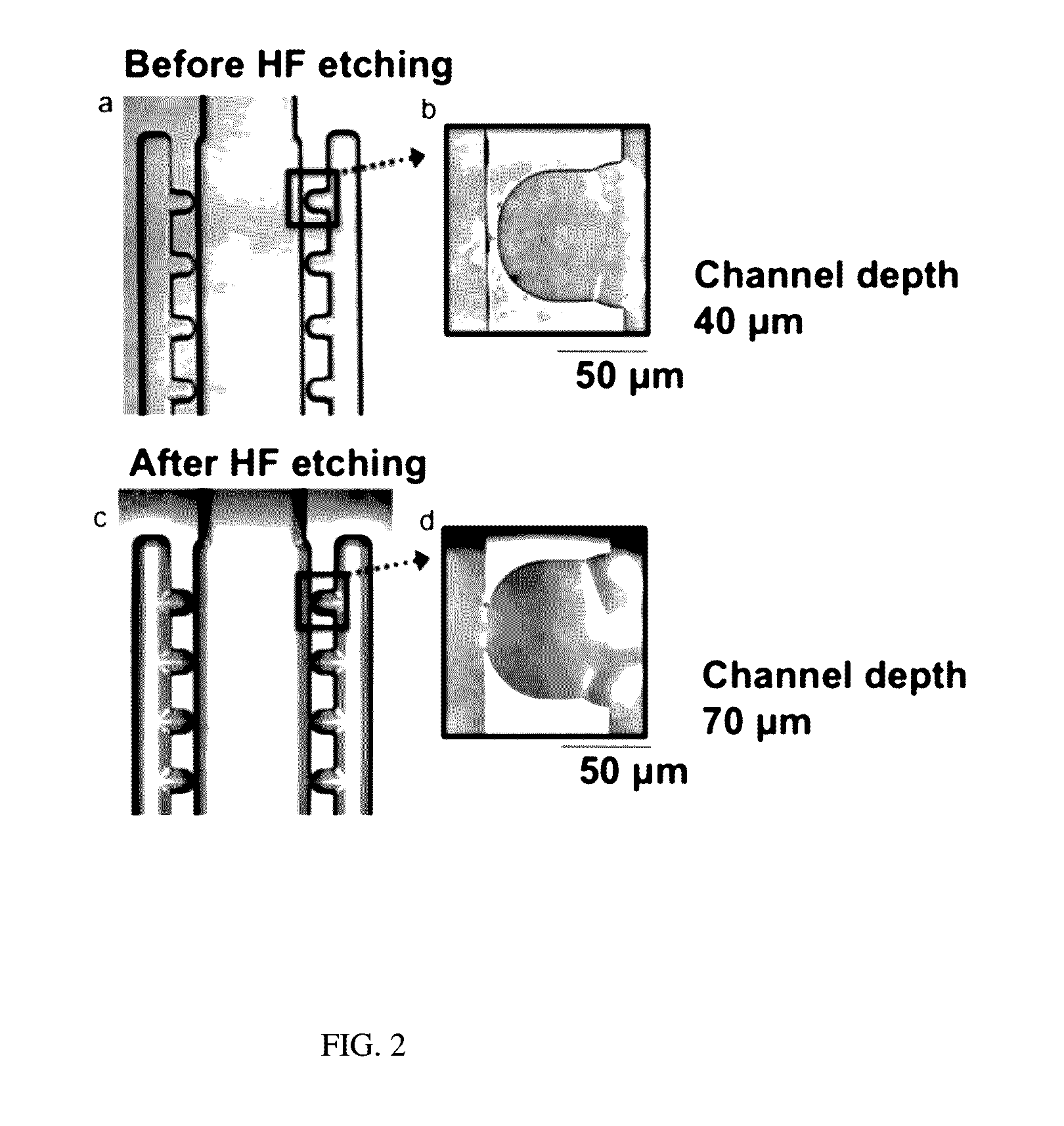
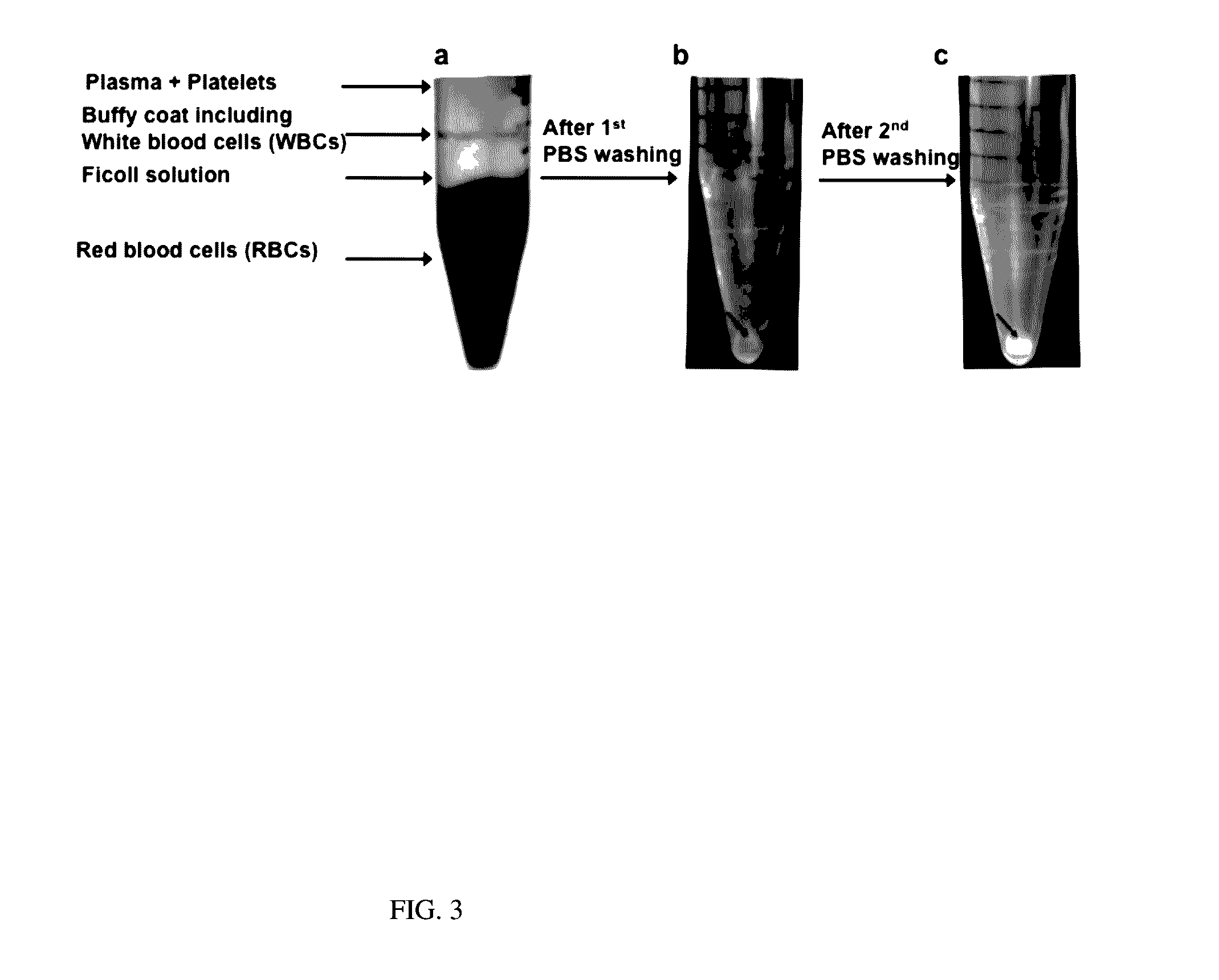
Particles, such as cells, are isolated for conducting single-particle measurement. Isolation of rare cells, such as circulating tumor cells (CTCs), from blood is technically challenging because they are small in numbers. An integrated microfluidic biochip, dubbed as CTC chip, was designed and fabricated for conducting tumor cell isolation. As CTCs are usually multidrug resistance (MDR), the effect of MDR inhibitors on chemotherapeutic drug accumulation in the isolated single tumor cell is measured. In this invention, label-free isolation of the rare tumor cells was conducted based on cell size difference. The major advantages of the CTC chip are the ability of fast cell isolation, followed by multiple rounds of single-cell measurement, suggesting a potential assay for detecting the drug responses based on the liquid biopsy of cancer patients.
Owner:LI PAUL CHI HANG +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com