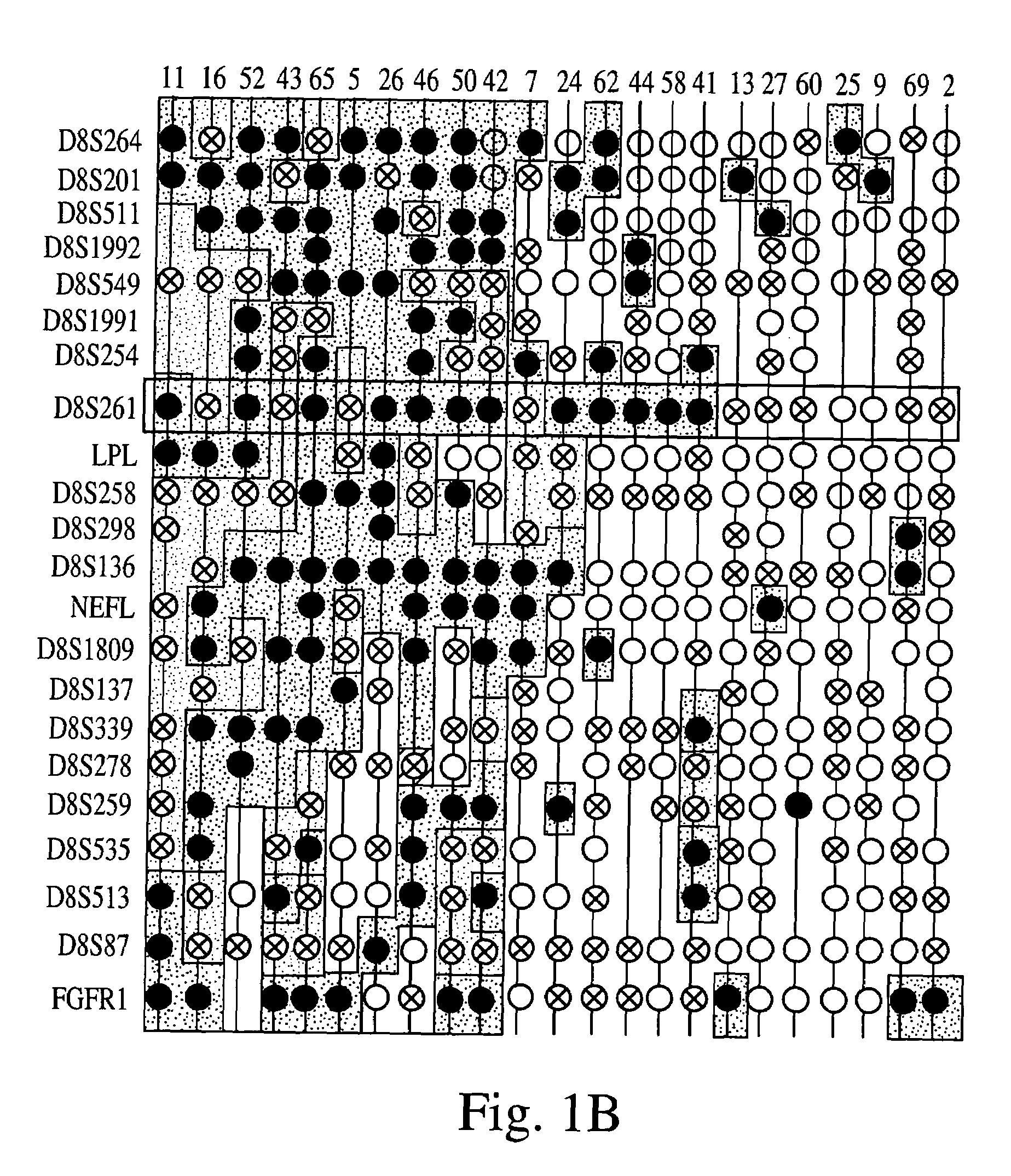
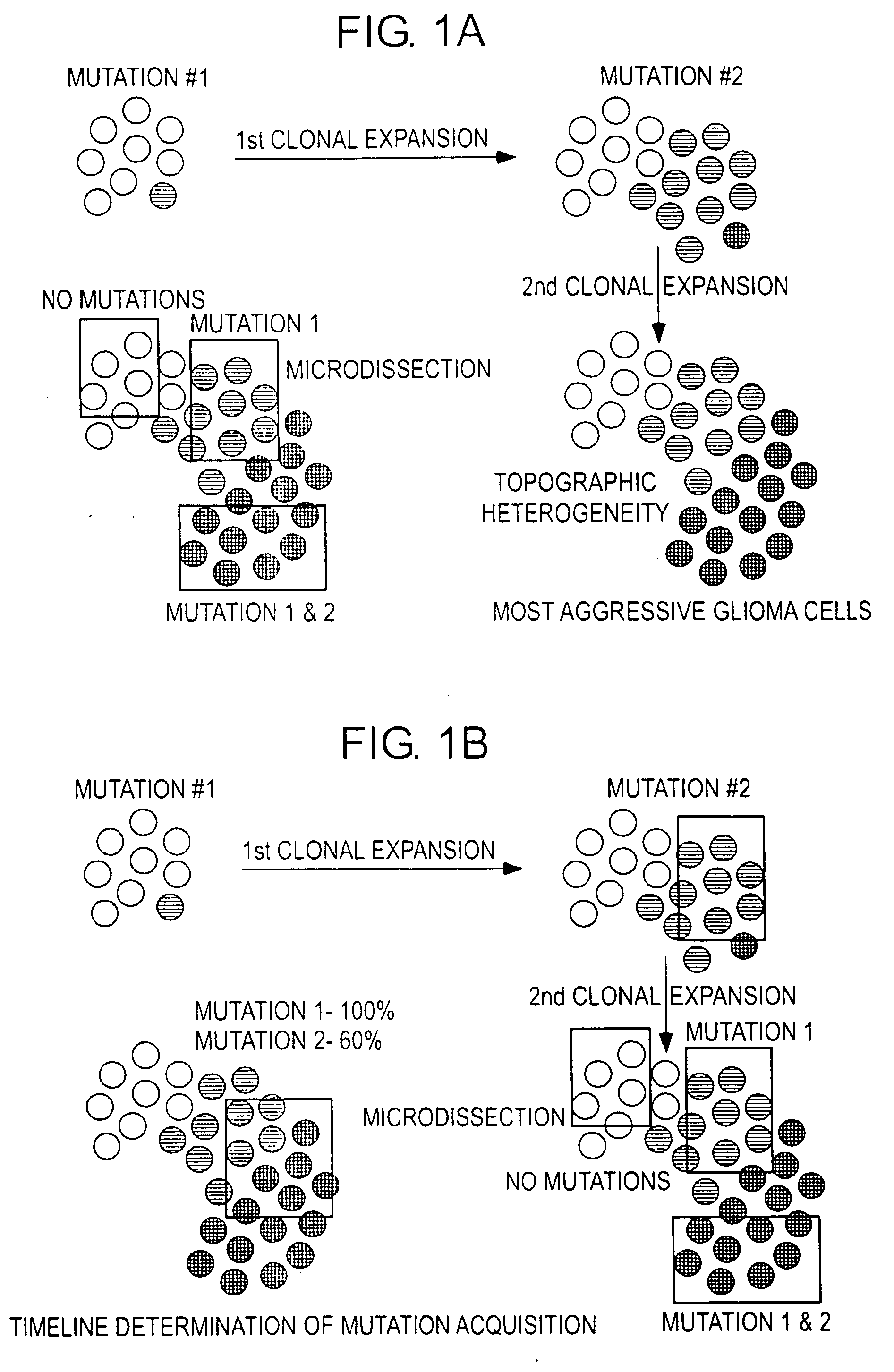
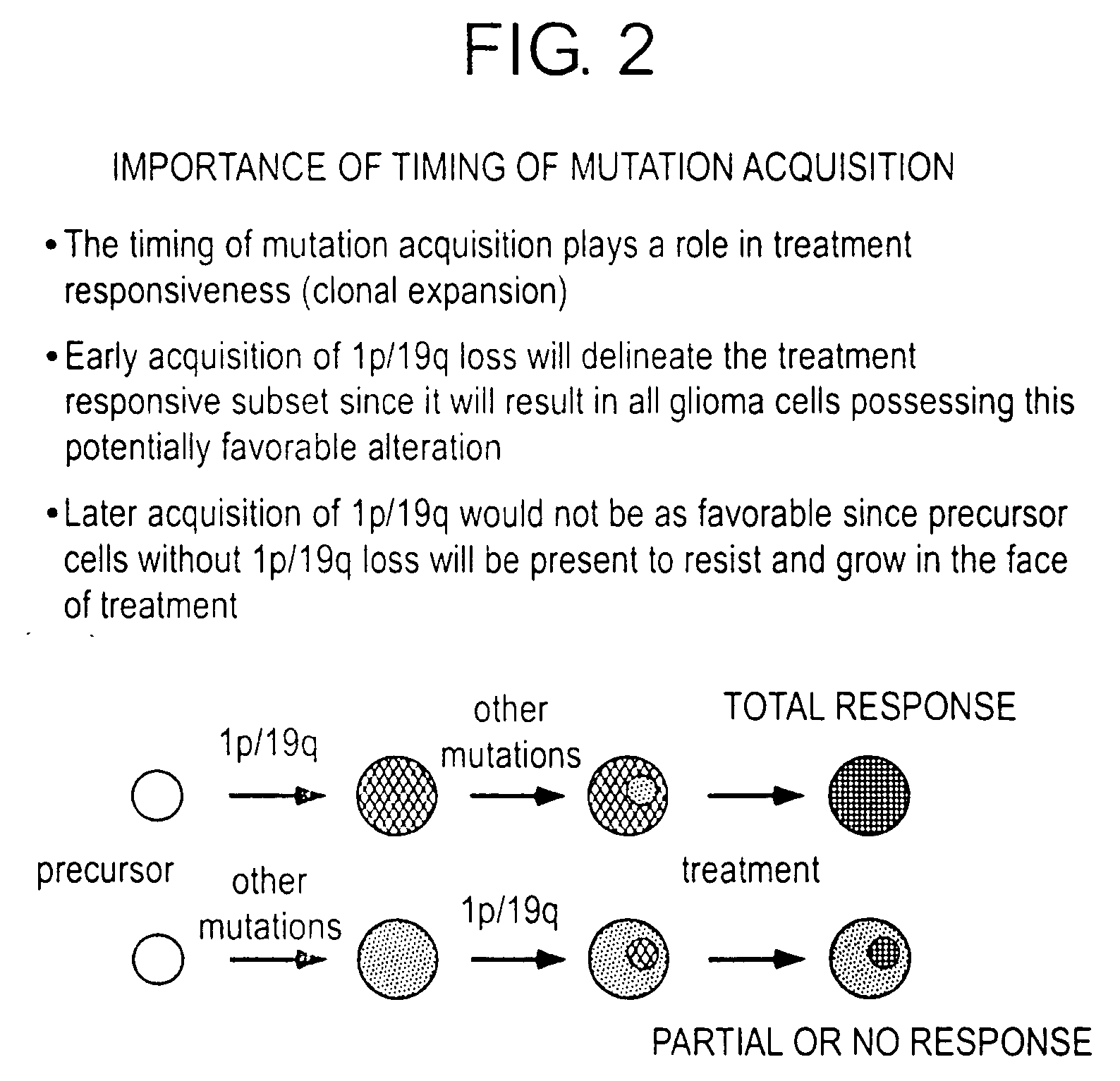
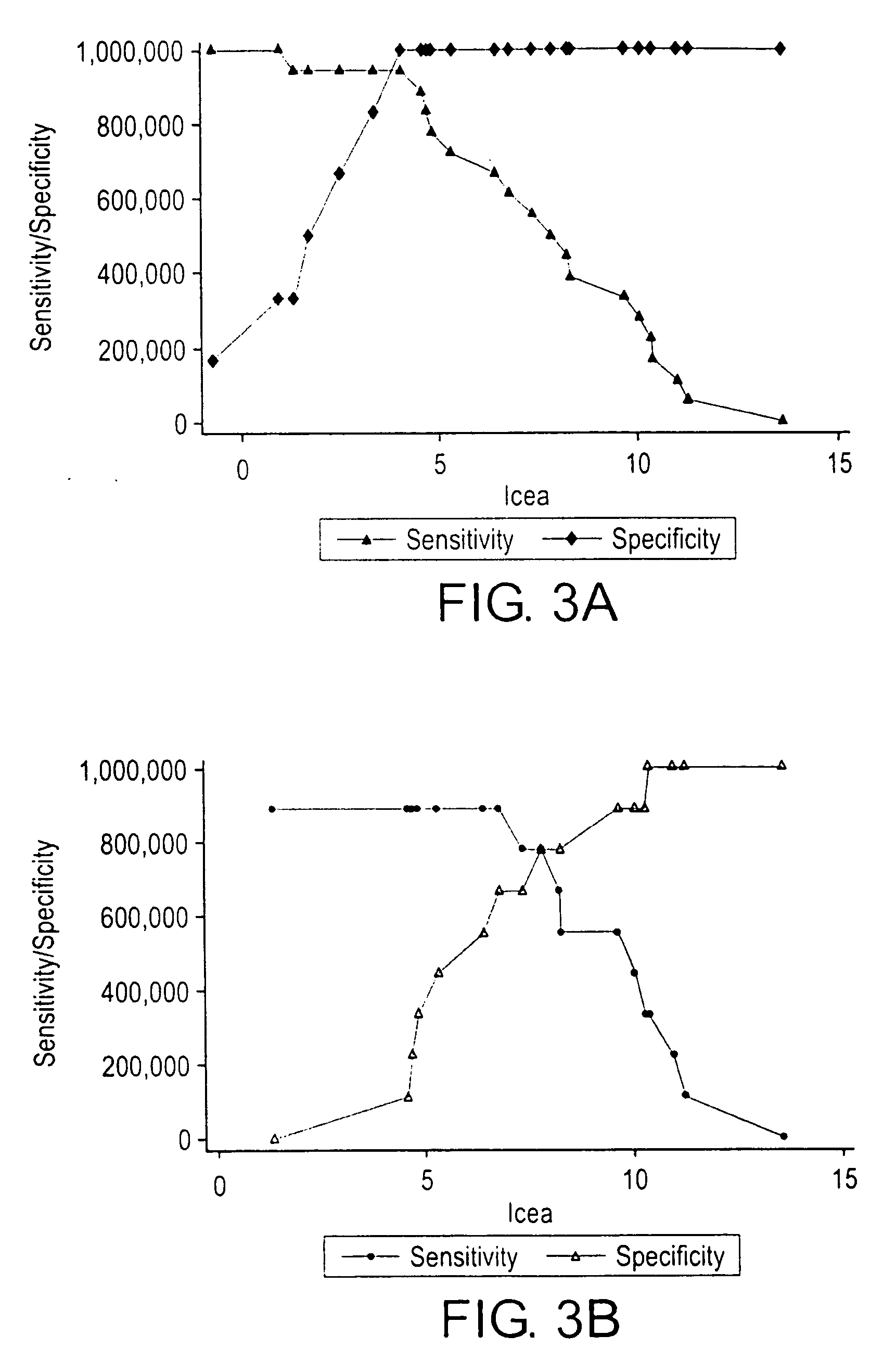
Patents
Literature
301 results about "Tumor suppressor gene" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
A tumor suppressor gene, or antioncogene, is a gene that regulates a cell during cell division and replication. If the cells grow uncontrollably it could result in cancer. When a tumor suppressor gene is mutated it results in a loss or reduction in its function; in combination with other genetic mutations this could allow the cell to grow abnormally. The loss of function for these genes may be even more important than proto-oncogene/oncogene activation in the development of many different human cancer cells. Tumor suppressor genes can be grouped into categories including caretaker genes, gatekeeper genes, and landscaper genes; the classification schemes are evolving as medicine advances, learning from fields including molecular biology, genetics, and epigenetics.
Noninvasive genetic immunization, expression products therefrom, and uses thereof
InactiveUS6716823B1Improve vaccination schemeEfficient methodSsRNA viruses negative-senseBiocideMalariaNon invasive
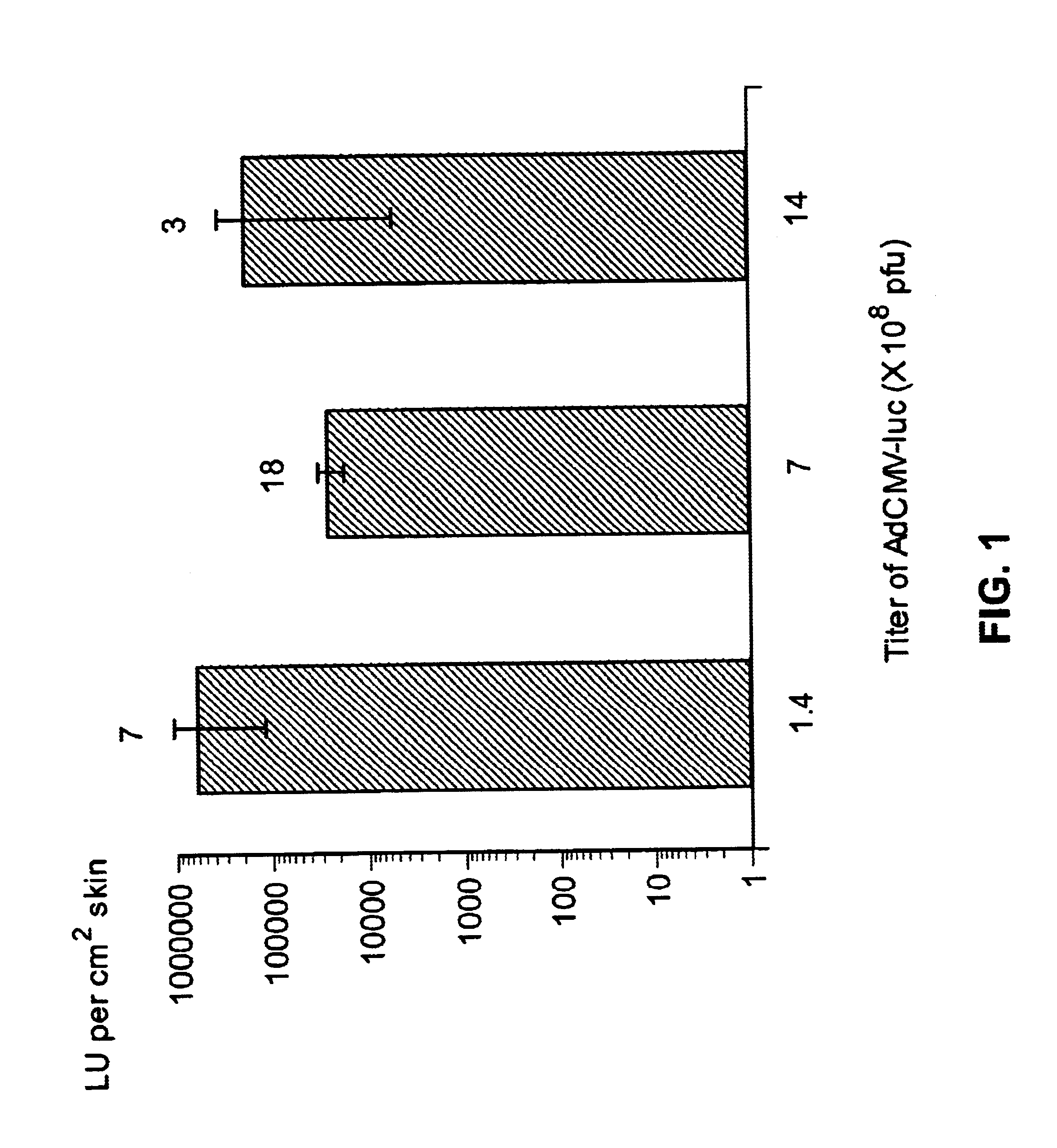
Disclosed and claimed are methods of non-invasive genetic immunization in an animal and / or methods of inducing a systemic immune or therapeutic response in an animal, products therefrom and uses for the methods and products therefrom. The methods can include contacting skin of the animal with a vector in an amount effective to induce the systemic immune or therapeutic response in the animal. The vector can include and express an exogenous nucleic acid molecule encoding an epitope or gene product of interest. The systemic immune response can be to or from the epitope or gene product. The nucleic acid molecule can encode an epitope of interest and / or an antigen of interest and / or a nucleic acid molecule that stimulates and / or modulates an immunological response and / or stimulates and / or modulates expression, e.g., transcription and / or translation, such as transcription and / or translation of an endogenous and / or exogenous nucleic acid molecule; e.g., one or more of influenza hemagglutinin, influenza nuclear protein, influenza M2, tetanus toxin C-fragment, anthrax protective antigen, anthrax lethal factor, rabies glycoprotein, HBV surface antigen, HIV gp 120, HIV gp 160, human carcinoembryonic antigen, malaria CSP, malaria SSP, malaria MSP, malaria pfg, and mycobacterium tuberculosis HSP; and / or a therapeutic, an immunomodulatory gene, such as co-stimulatory gene and / or a cytokine gene. The immune response can be induced by the vector expressing the nucleic acid molecule in the animal's cells. The animal's cells can be epidermal cells. The immune response can be against a pathogen or a neoplasm. A prophylactic vaccine or a therapeutic vaccine or an immunological composition can include the vector. The animal can be a vertebrate, e.g., a mammal, such as human, a cow, a horse, a dog, a cat, a goat, a sheep or a pig; or fowl such as turkey, chicken or duck. The vector can be one or more of a viral vector, including viral coat, e.g., with some or all viral genes deleted therefrom, bacterial, protozoan, transposon, retrotransposon, and DNA vector, e.g., a recombinant vector; for instance, an adenovirus, such as an adenovirus defective in its E1 and / or E3 and / or E4 region(s). The method can encompass applying a delivery device including the vector to the skin of the animal, as well as such a method further including disposing the vector in and / or on the delivery device. The vector can have all viral genes deleted therefrom. The vector can induce a therapeutic and / or an anti-tumor effect in the animal, e.g., by expressing an oncogene, a tumor-suppressor gene, or a tumor-associated gene. Immunological products generated by the expression, e.g., antibodies, cells from the methods, and the expression products, are likewise useful in in vitro and ex vivo applications, and such immunological and expression products and cells and applications are disclosed and claimed. Methods for expressing a gene product in vivo and products therefor and therefrom including mucosal and / or intranasal administration of an adenovirus, advantageously an E1 and / or E3 and / or E4 defective or deleted adenovirus, such as a human adenovirus or canine adenovirus, are also disclosed and claimed.
Owner:UAB RES FOUND
Novel use of aim 3 acting as a tumor suppressor
InactiveUS20060046250A1Prevent proliferationPromote phosphorylationCompound screeningApoptosis detectionApoptosisInducer Cells
The present invention relates to novel uses of AIM3 acting as a tumor suppressor, and more particularly to methods for using an AIM3 protein or a nucleic acid encoding the protein to activate ATM or ATR and to treat ATM- or ATR-mediated diseases. The AIM3 protein according to the present invention interacts directly with ATM / ATR so as to activate ATM / ATR and proteins regulated by ATM / ATR. Also, the AIM3 protein upregulates tumor suppressor gene p53 and its target genes so as to not only inhibit the proliferation of cells but also to induce apoptosis.
Owner:SCHWARZ HERBERT +1
Novel tumor suppressor gene and compositions and methods for making and using the same
InactiveUS20050266443A1Sugar derivativesMicrobiological testing/measurementTumour suppressor geneGene product
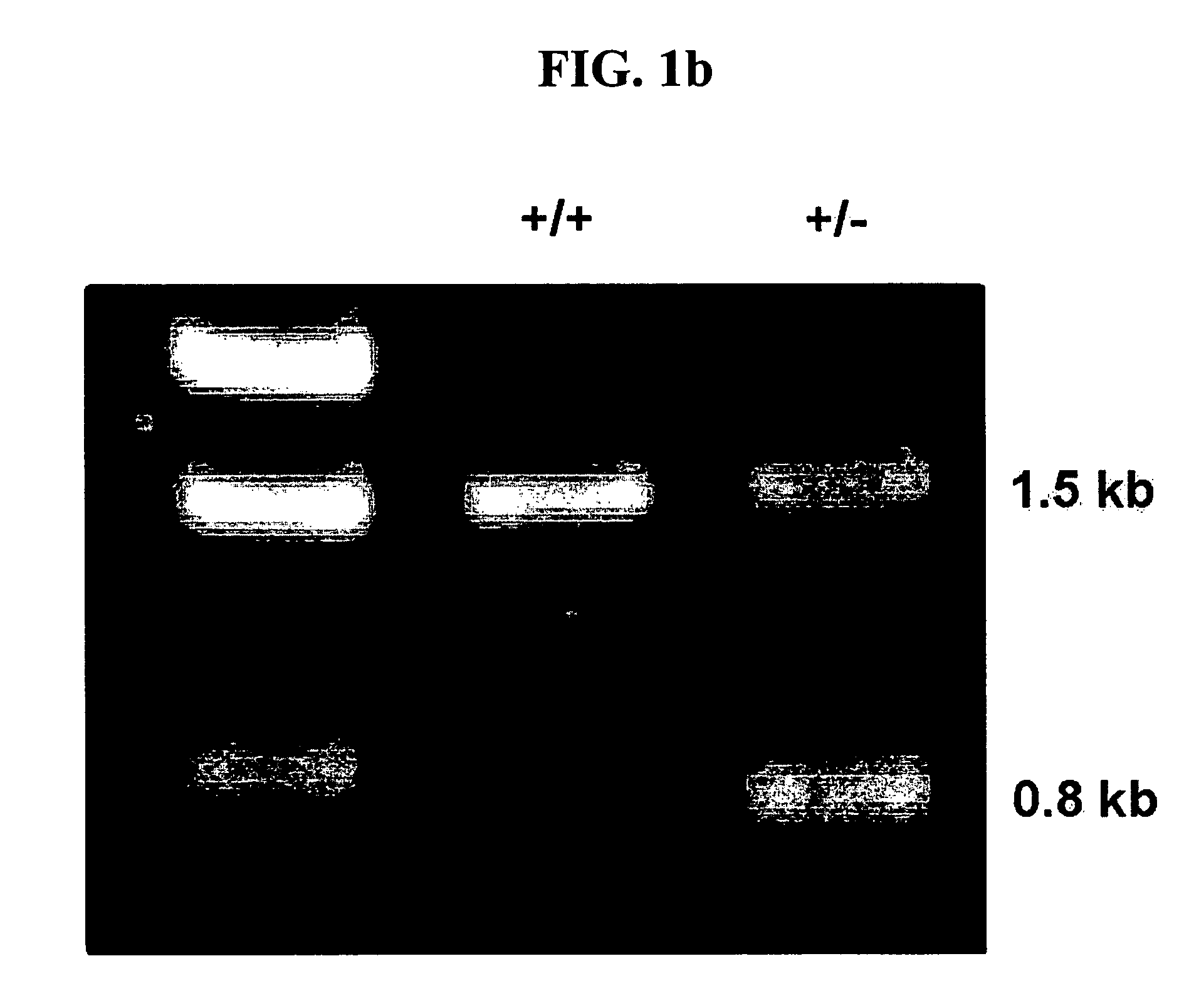
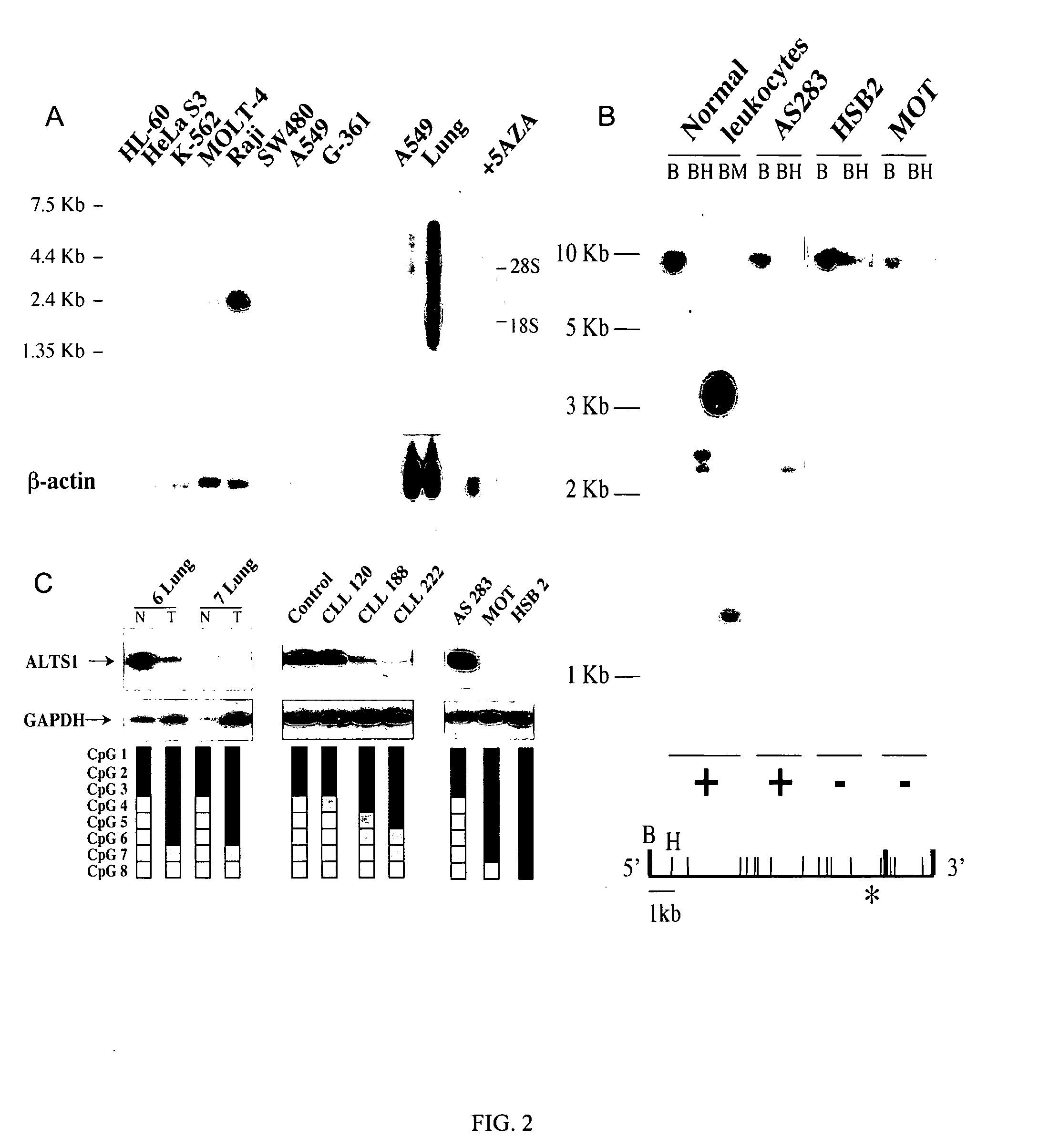
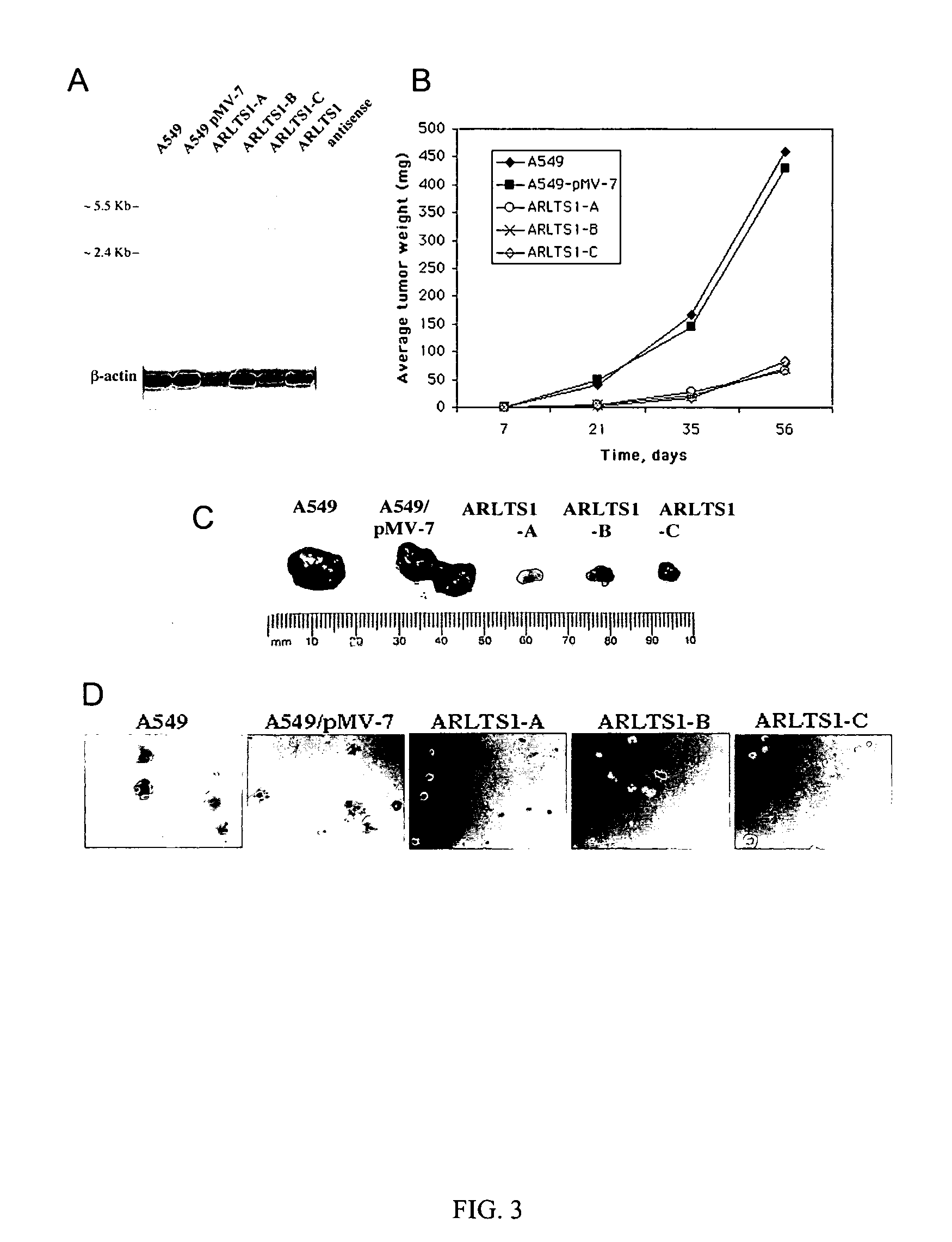
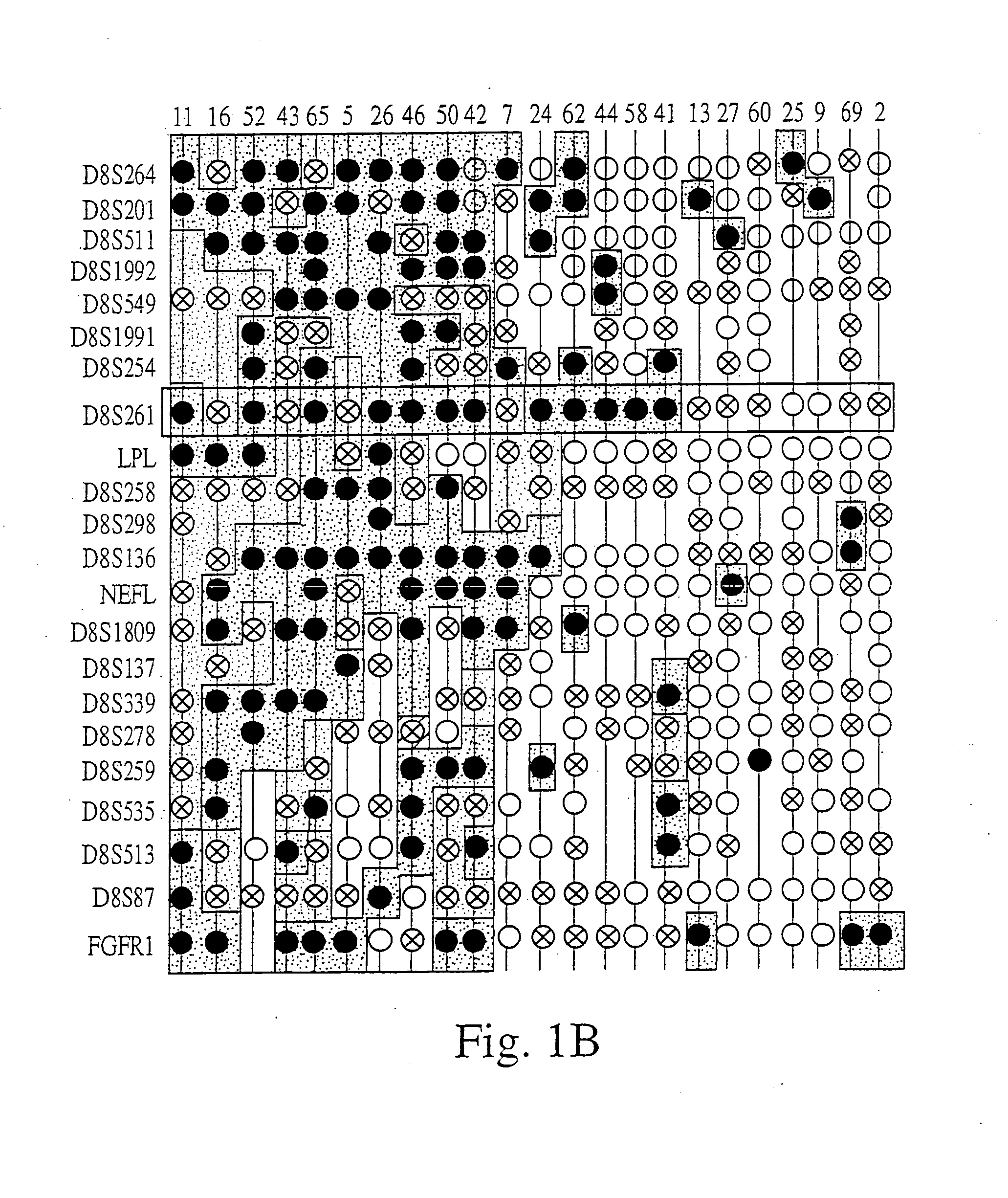
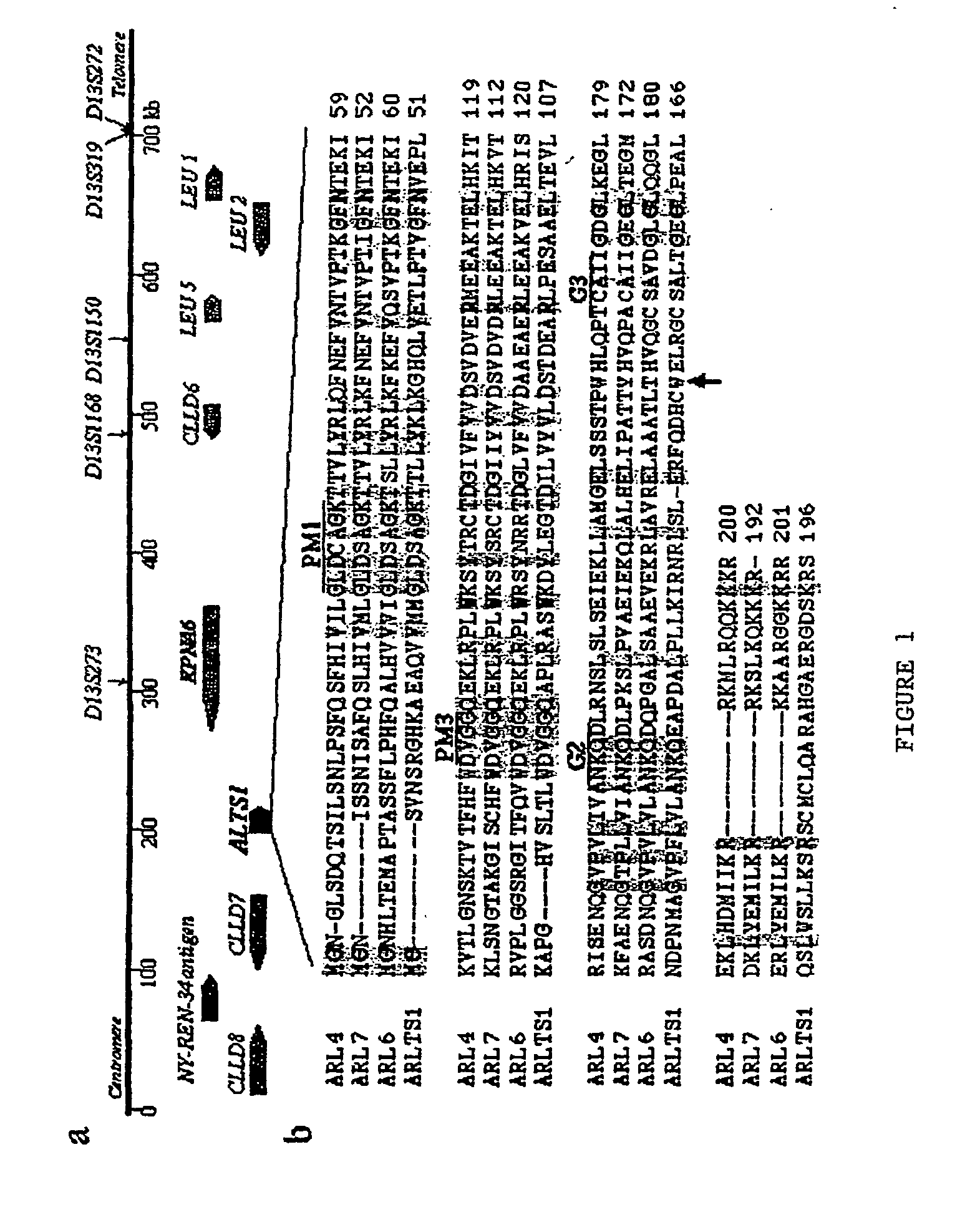
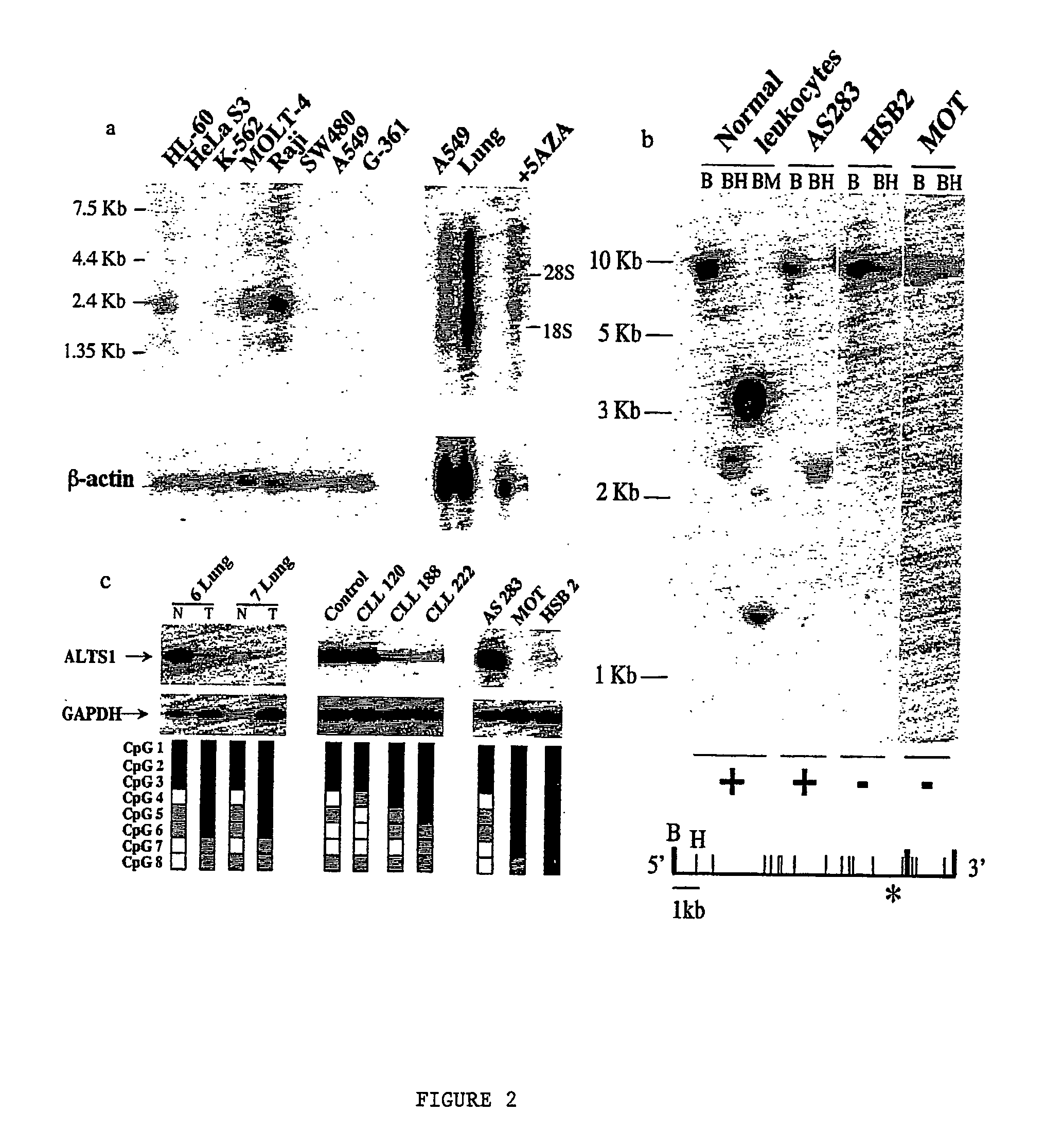
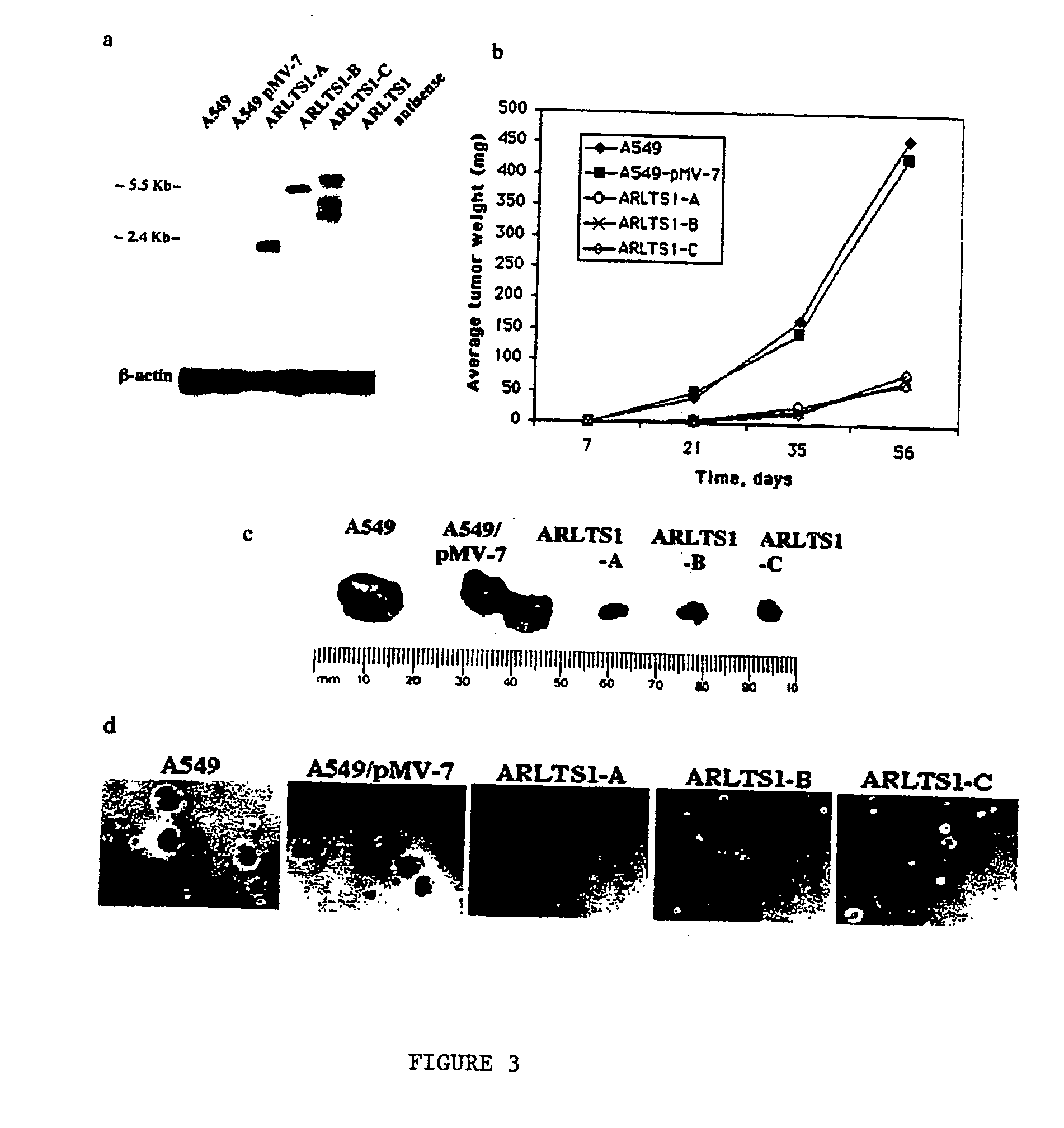
The present invention relates to the identification and cloning of ARTS1, a novel tumor suppressor gene. The invention further encompasses isolated proteins encoded by ARTS1, methods of making and using the same, methods of diagnosing the presence of, or prediposition for, a cancer associated with a defective ARTS1 gene or gene product, and methods of treating or preventing cancers associated with a defective ARTS1 gene or gene product.
Owner:THOMAS JEFFERSON UNIV
Induced malignant stem cells
InactiveUS20140137274A1High and low degree of methylationSugar derivativesPeptide/protein ingredientsMicrosatelliteSomatic cell
PROBLEMThere are provided induced malignant stem cells capable of in vitro proliferation that are useful in cancer research and drug discovery for cancer therapy, as well as processes for production thereof, cancer cells derived from these cells, and applications of these cells.MEANS FOR SOLVINGAn induced malignant stem cell capable of in vitro proliferation are characterized by satisfying the following two requirements:(1) having at least one aberration selected from among (a) an aberration of methylation (high or low degree of methylation) in a tumor suppressor gene or a cancer-related genetic region in endogenous genomic DNA, (b) a somatic mutation of a tumor suppressor gene or a somatic mutation of an endogenous cancer-related gene in endogenous genomic DNA, (c) abnormal expression (increased or reduced / lost expression) of an endogenous oncogene or an endogenous tumor suppressor gene, (d) abnormal expression (increased or reduced / lost expression) of a noncoding RNA such as an endogenous cancer-related microRNA, (e) abnormal expression of an endogenous cancer-related protein, (f) an aberration of endogenous cancer-related metabolism (hypermetabolism or hypometabolism), (g) an aberration of endogenous cancer-related sugar chain, (h) an aberration of copy number variations in endogenous genomic DNA, and (i) instability of microsatellites in endogenous genomic DNA in an induced malignant stem cell; and(2) expressing genes including POU5F1 gene, NANOG gene, SOX2 gene, and ZFP42 gene.
Owner:ISHIKAWA
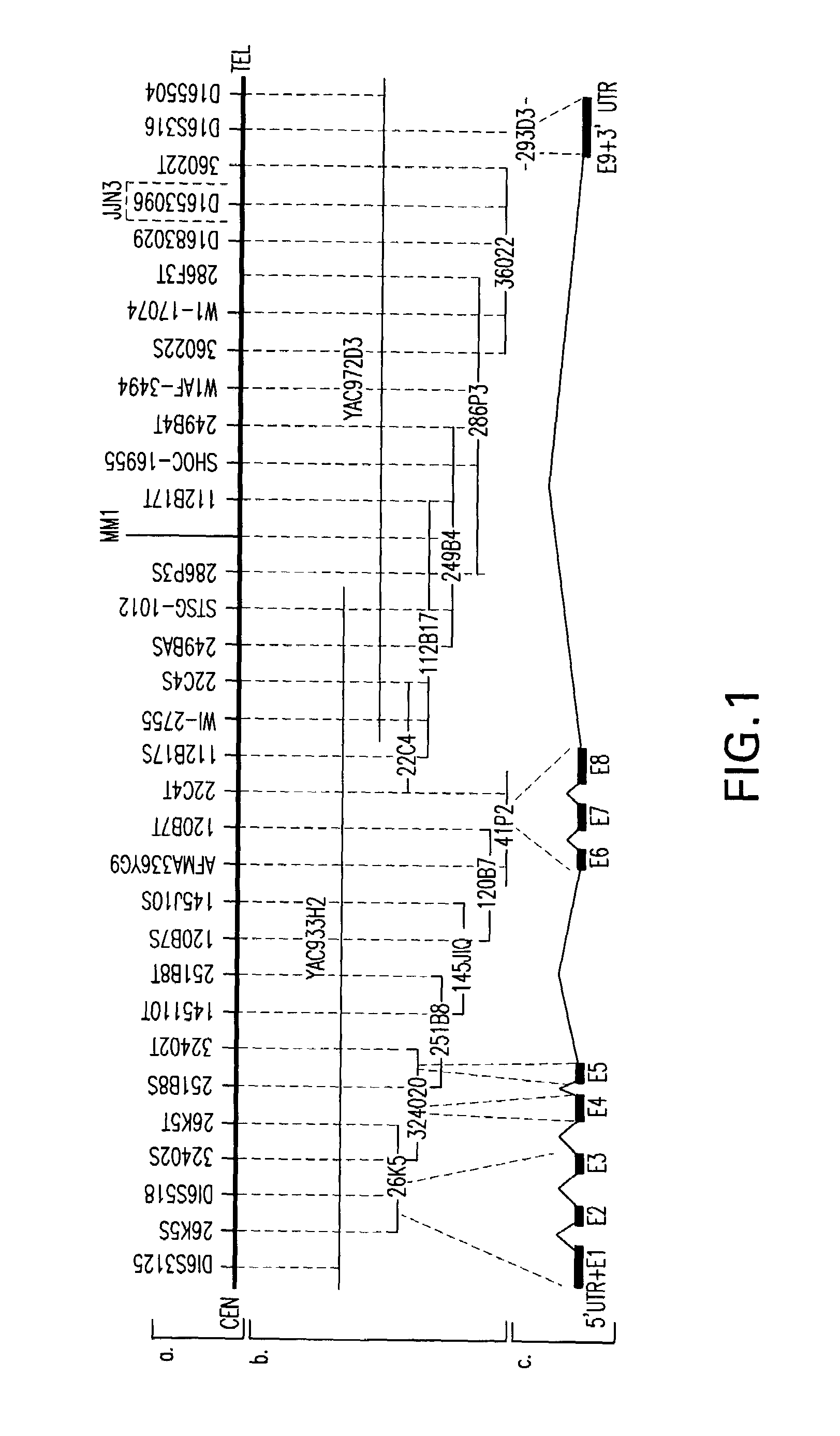
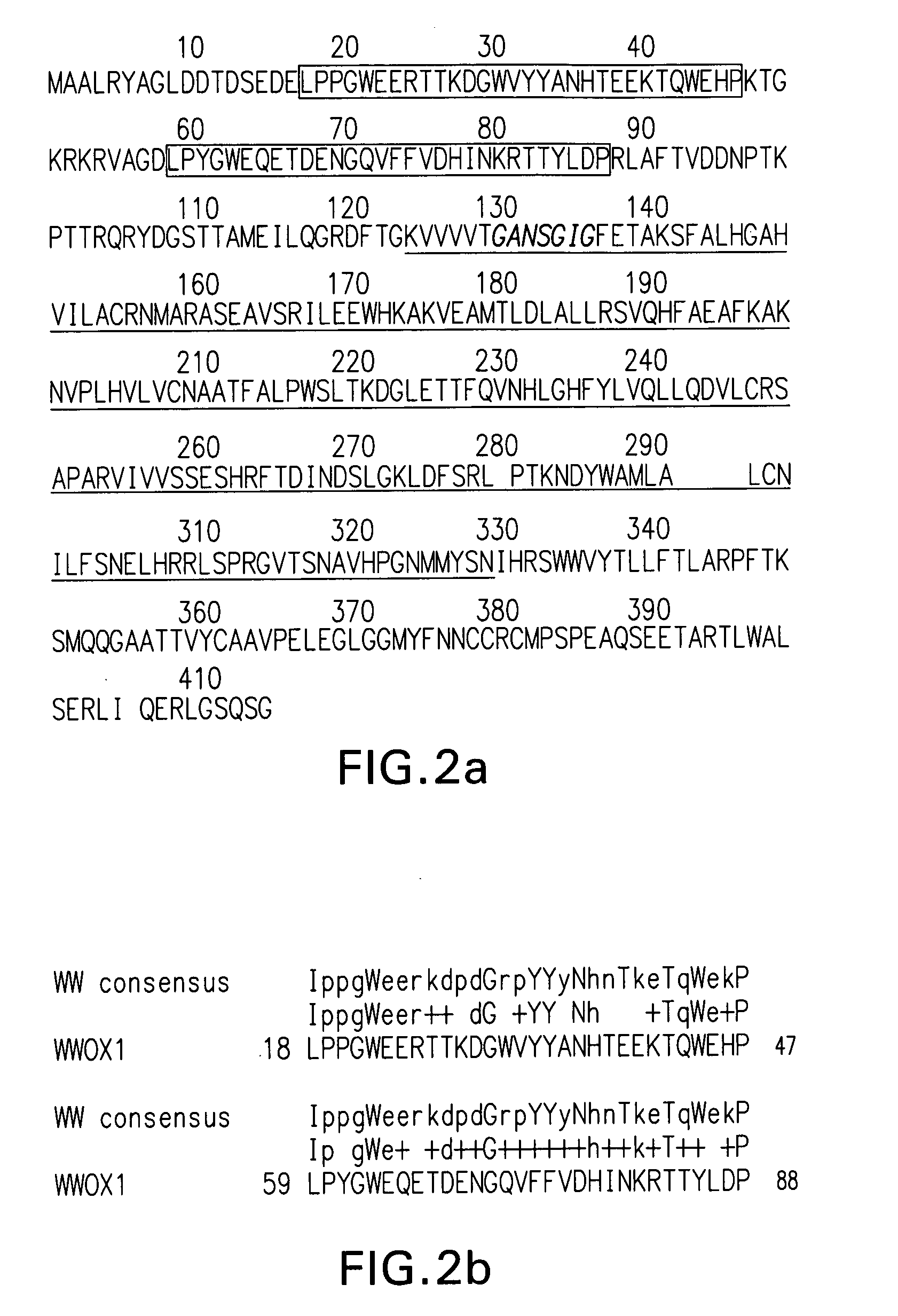
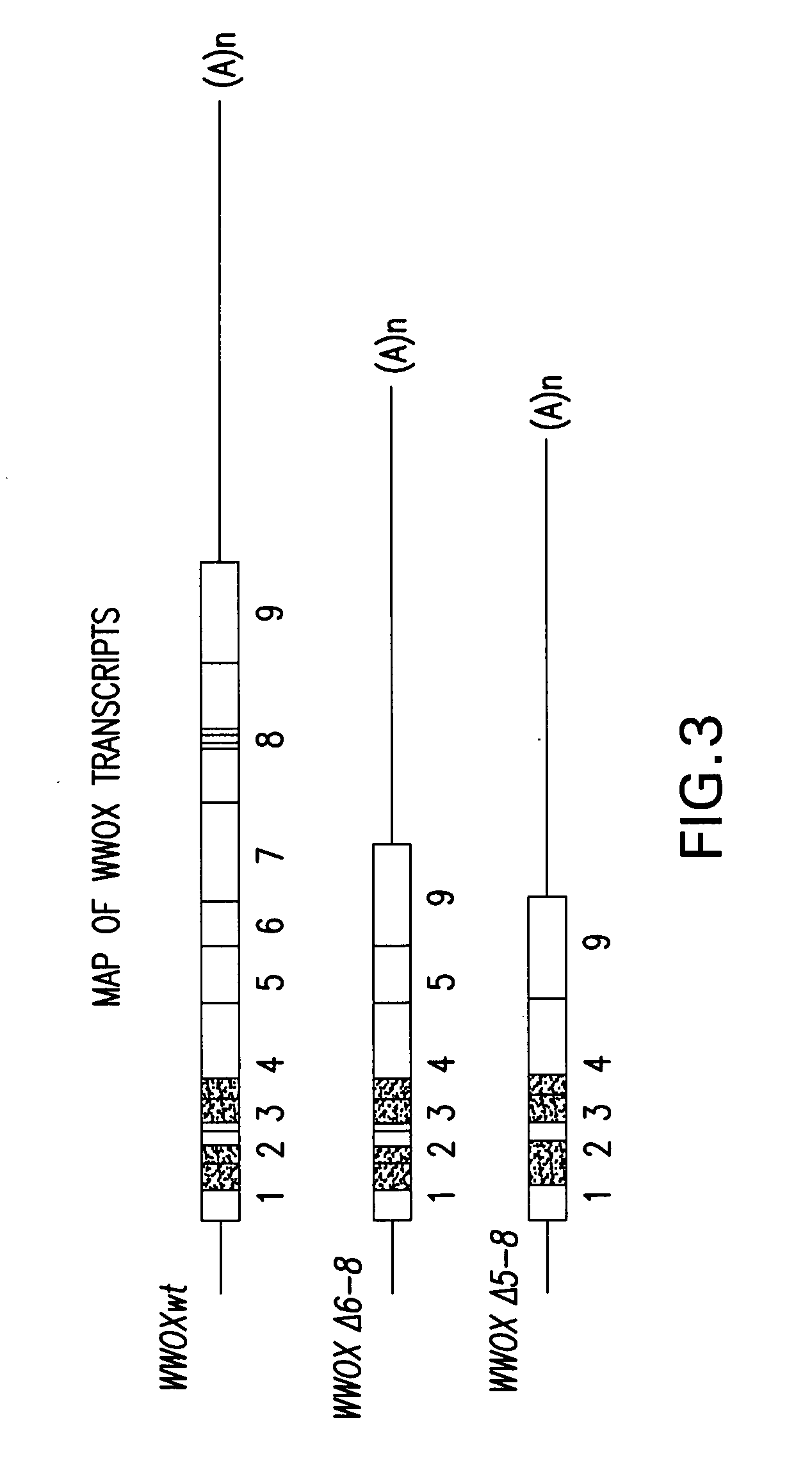
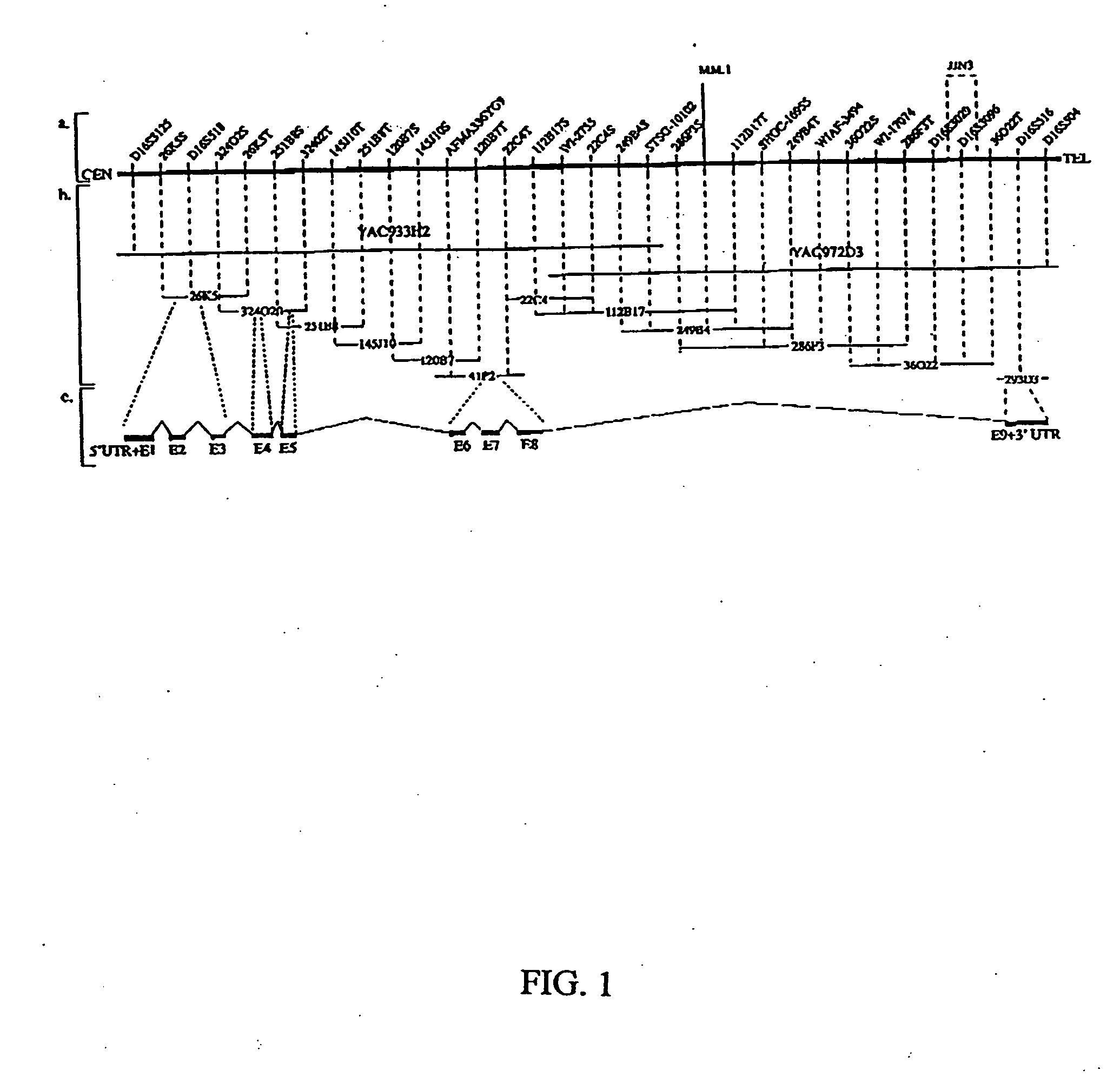
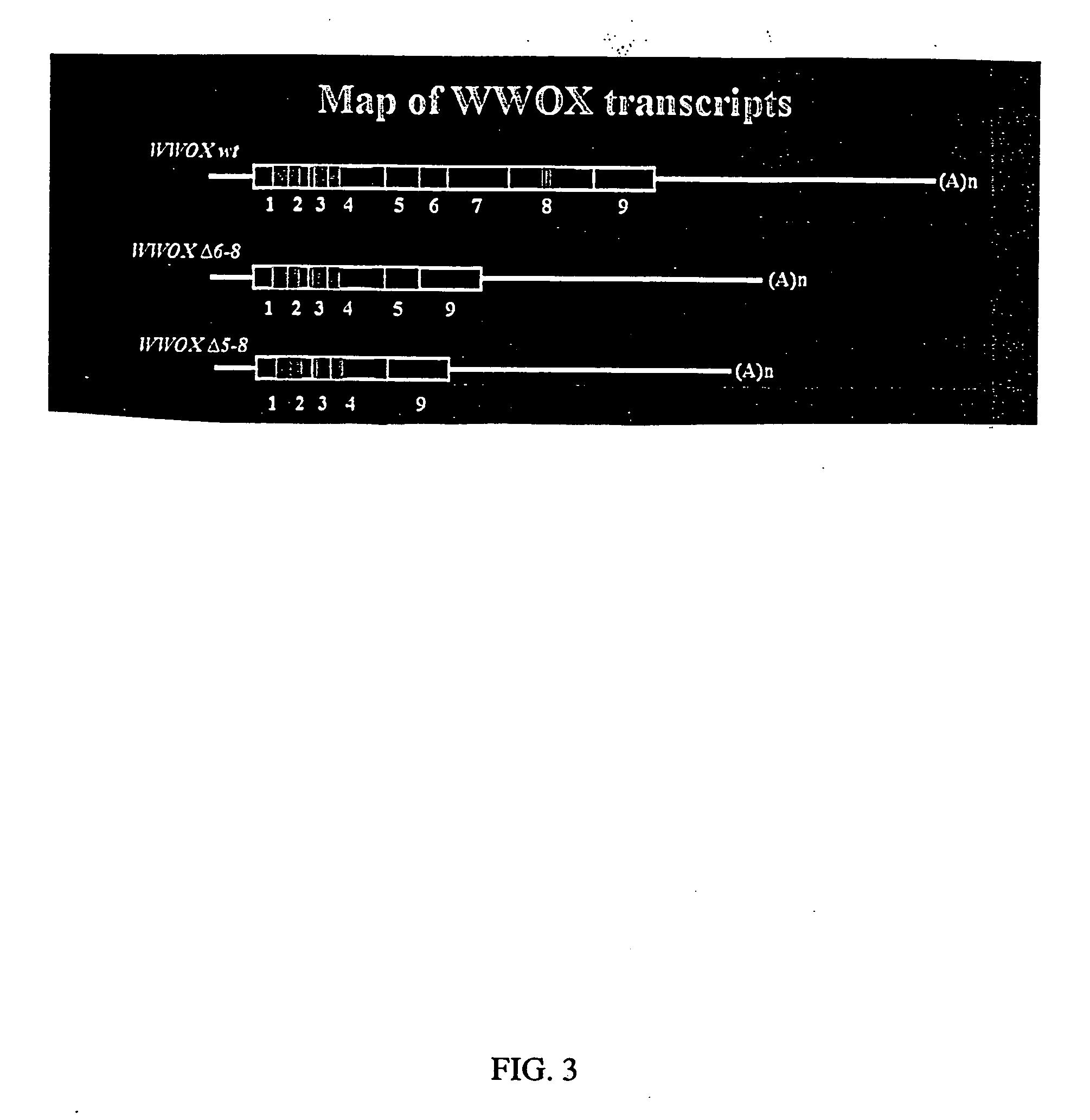
WWOX: a tumor suppressor gene mutated in multiple cancers
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
Wwox: a tumor suppressor gene mutated in multiple cancers
InactiveUS20060024780A1Sugar derivativesPeptide preparation methodsAbnormal tissue growthTumour suppressor gene
The present invention provides the isolation and cloning of WWOX, a novel WW domain-containing protein mapping to human chromosome 16q23.3-24.1, a region frequently affected in several cancers. This gene encodes a tumor suppressor with apoptotic functions. The invention provides WWOX nucleic acid- and polypeptide-based cancer therapies. The invention also provides methods for cancer detection, diagnosis and prognosis involving WWOX nucleic acids and polypeptides.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
Tumor antigen based on products of the tumor suppressor gene WT1
Owner:INT INST OF CANCER IMMUNOLOGY INC
MDM2-Containing Double Minute Chromosomes And Methods Therefore
InactiveUS20140350130A1Rapid identificationBiocideMicrobiological testing/measurementSequence analysisMdm2 Protein
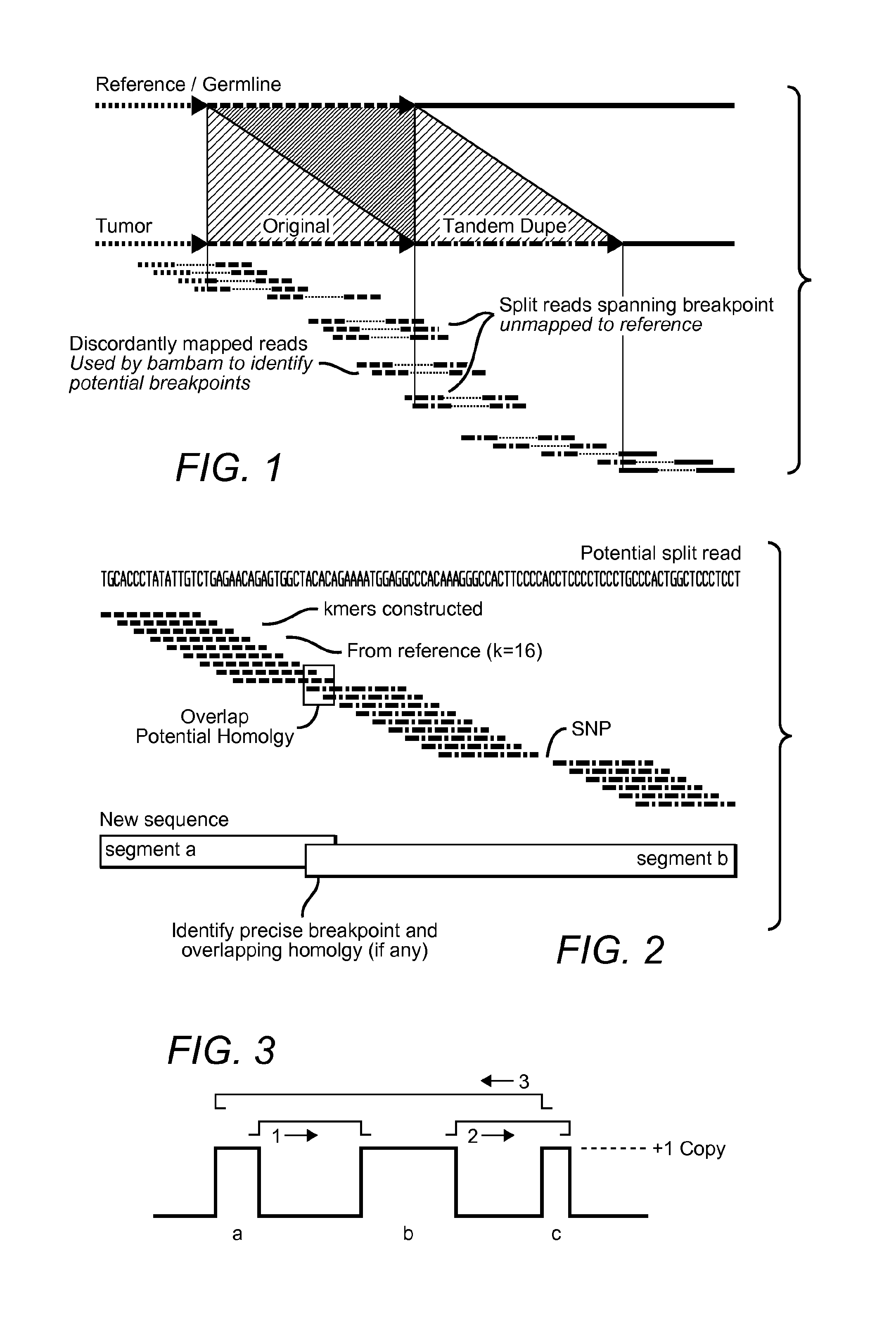
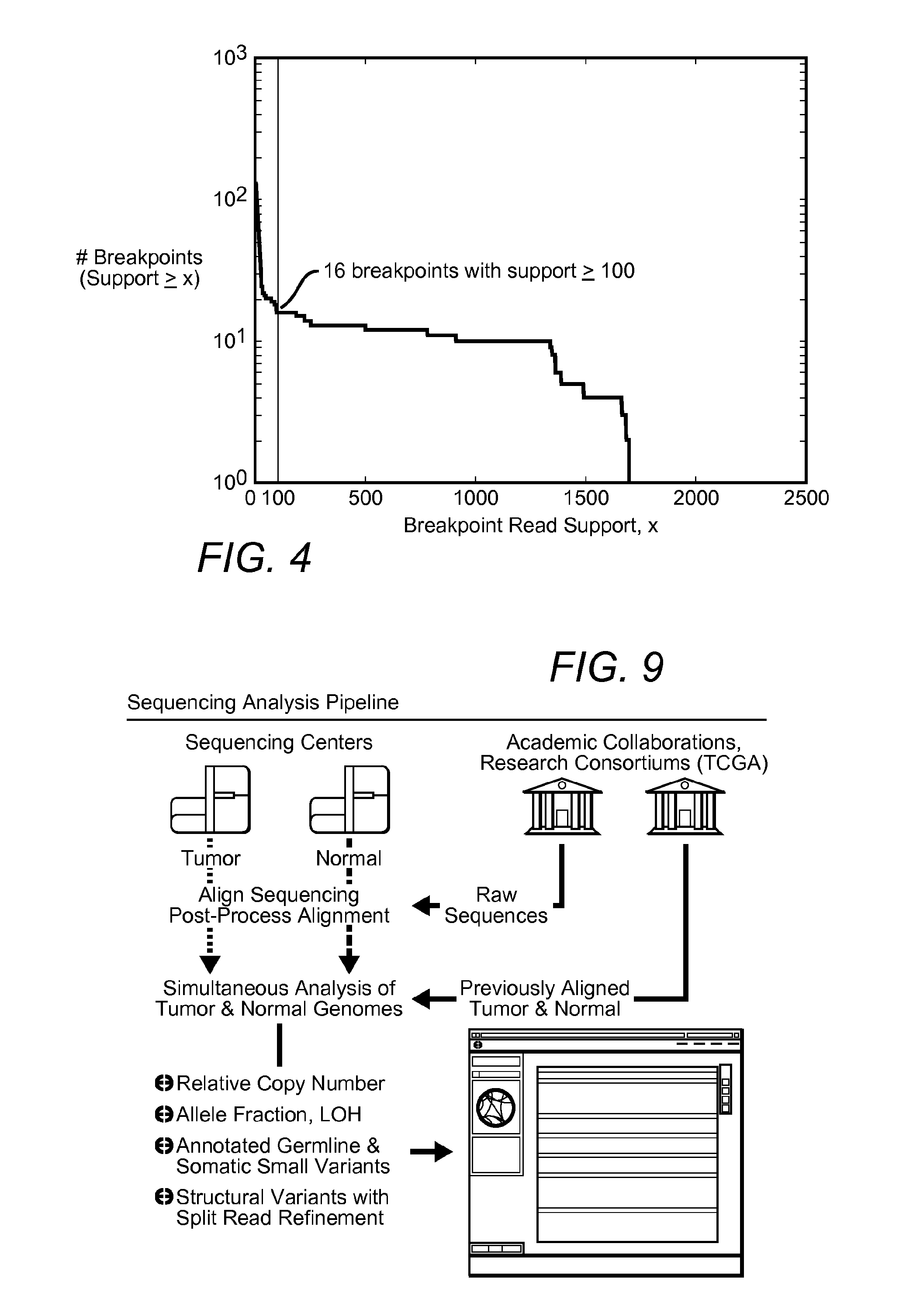
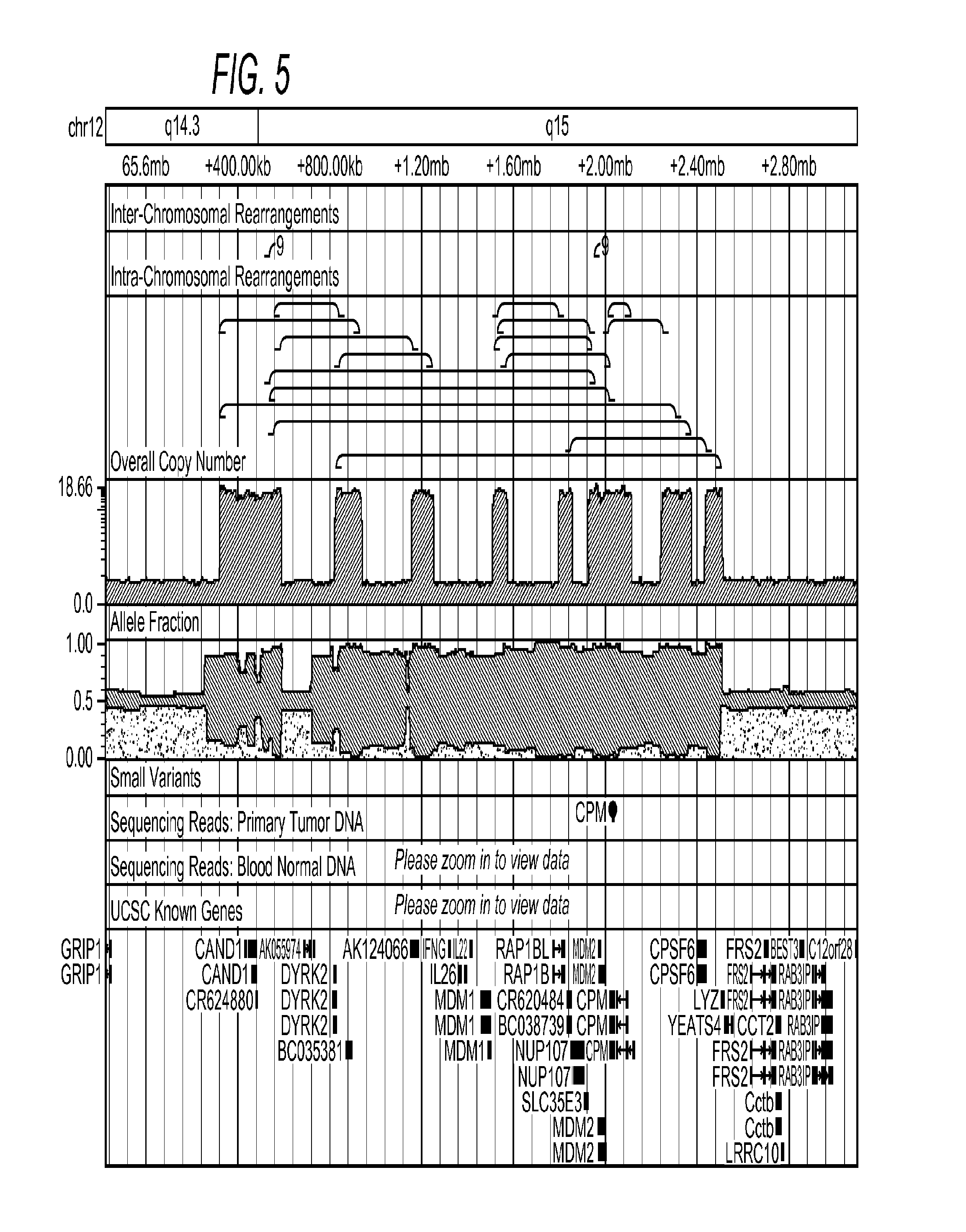
Contemplated systems and methods allow for computational genomic analysis using paired-end sequence analysis and split read refinement to thereby identify high-confidence breakpoints associated with high copy numbers and orientation of rearrangements, which is then the basis for full reconstruction of double minutes (DM). In especially preferred aspects, the DM will also include an oncogene or tumor suppressor gene, and / or may be found in blood or blood derived fluids.
Owner:FIVE3 GENOMICS
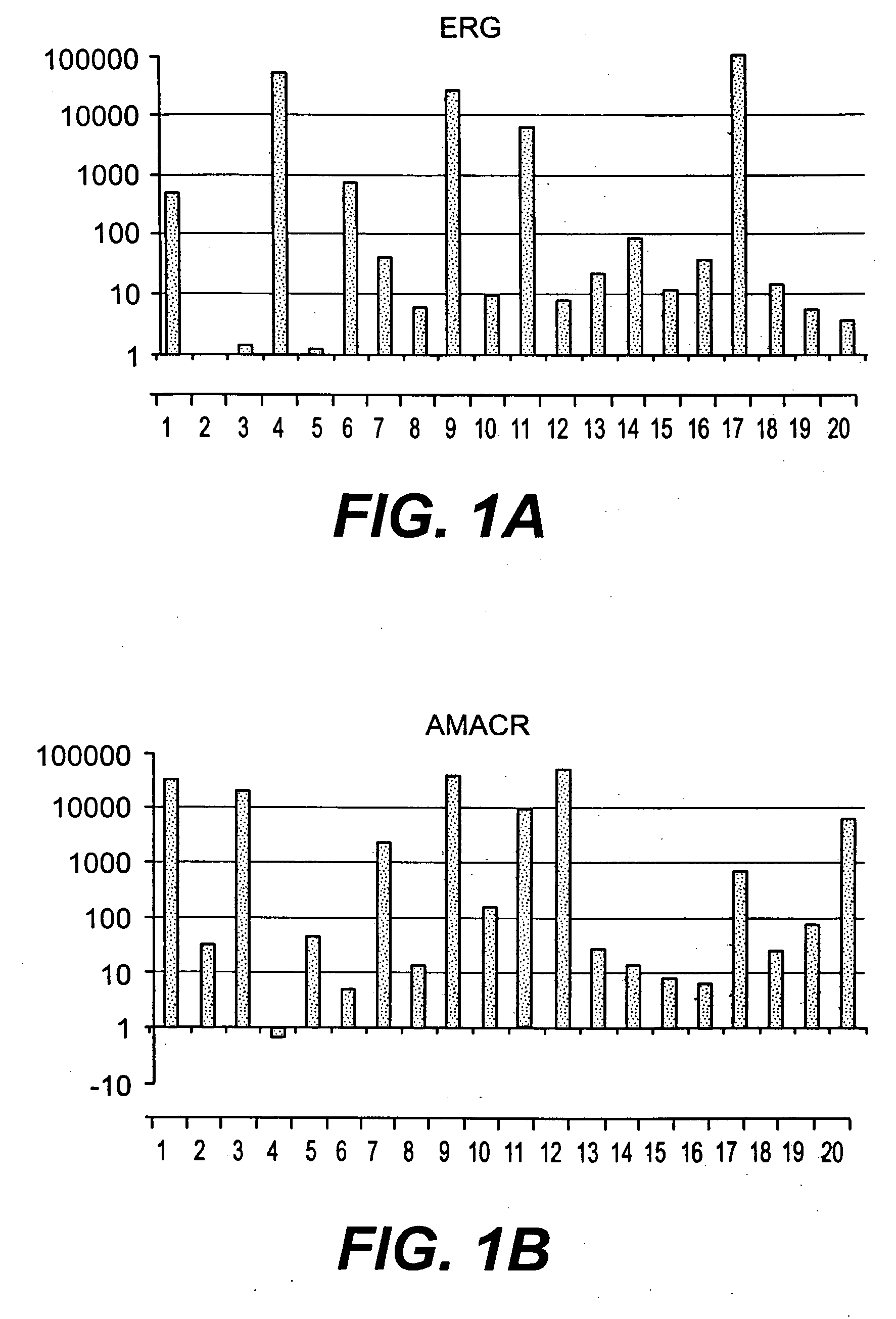
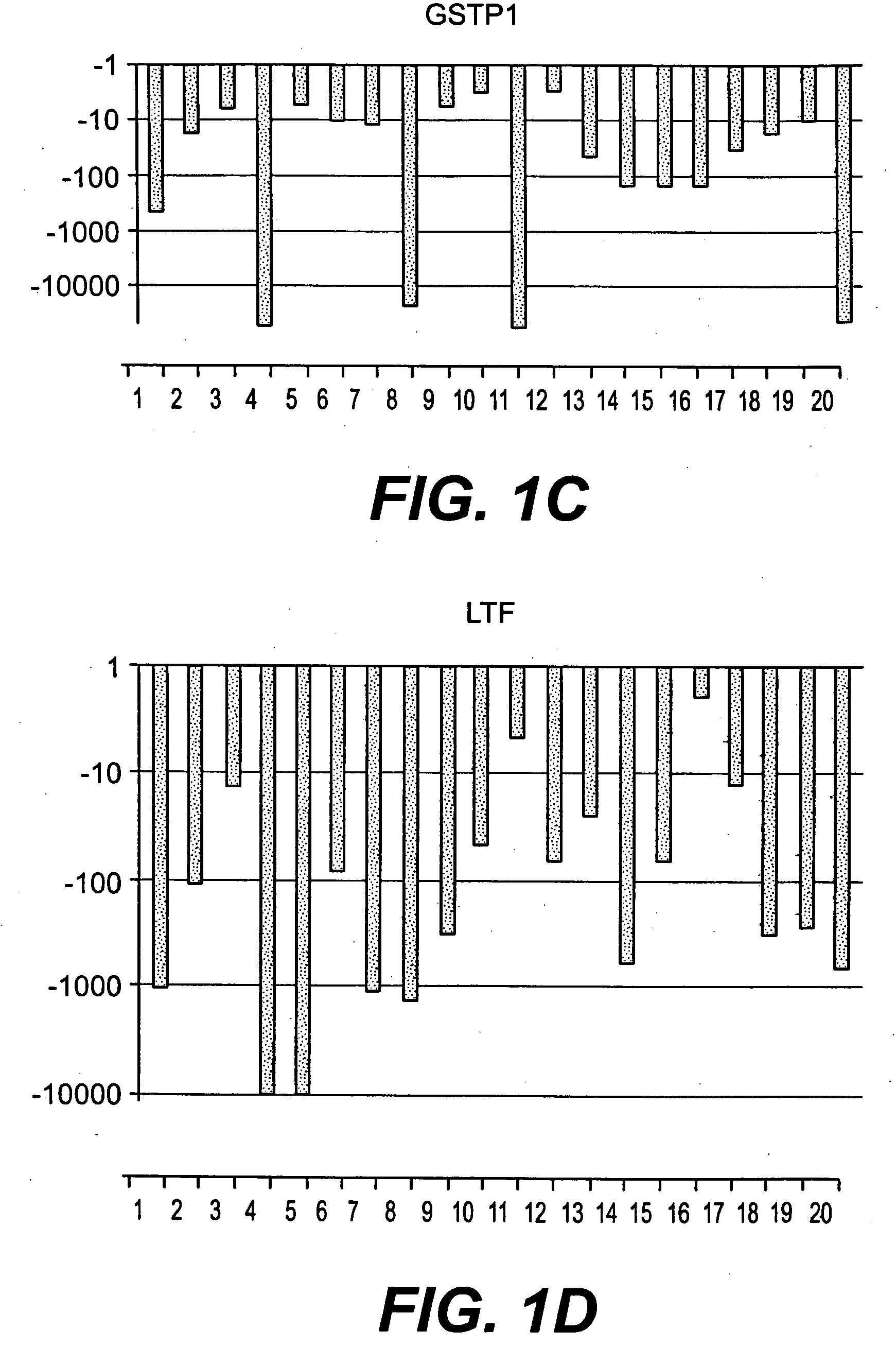
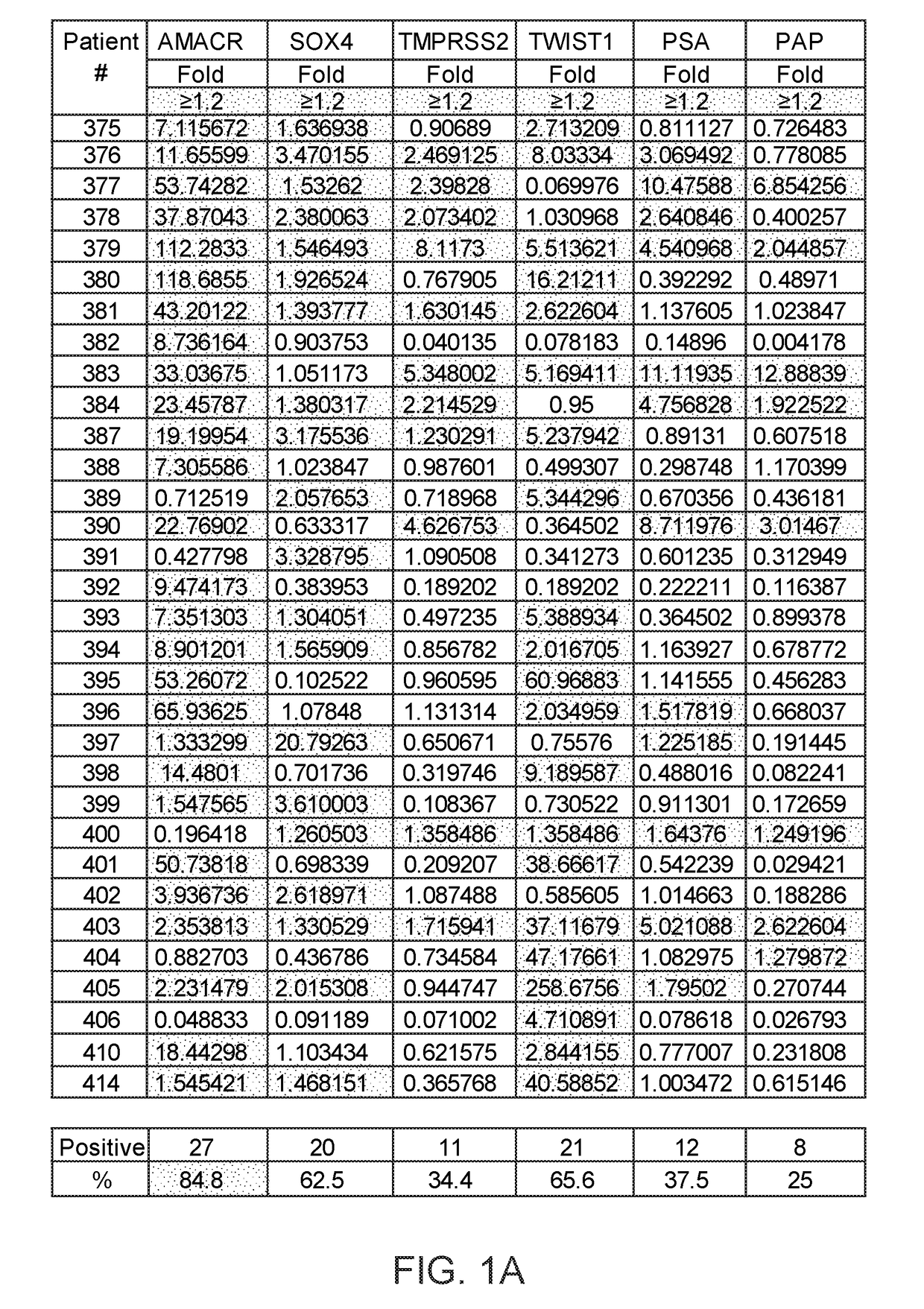
Methods of diagnosing or treating prostate cancer using the erg gene, alone or in combination with other over or under expressed genes in prostate cancer
ActiveUS20090170075A1Microbiological testing/measurementImmunoglobulins against cell receptors/antigens/surface-determinantsProstate cancer cellOncology
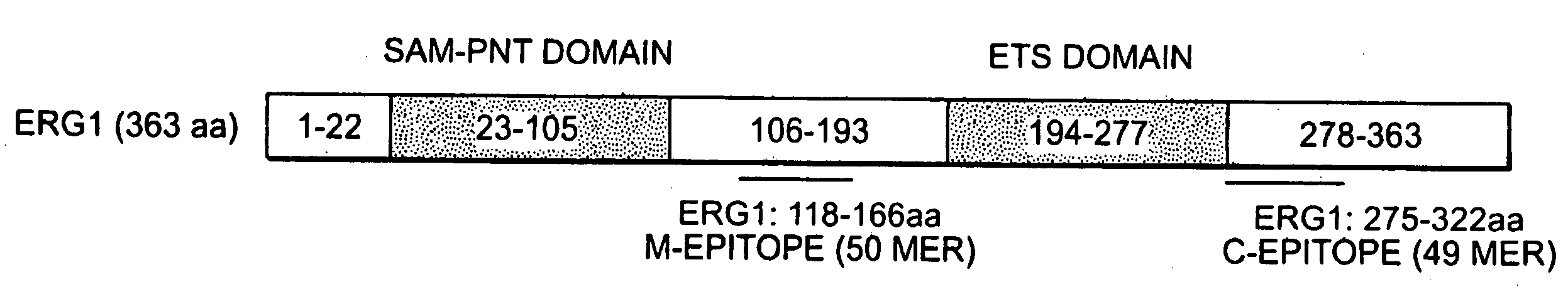
The present invention relates to oncogenes or tumor suppressor genes, as well as other genes, involved in prostate cancer and their expression products, as well as derivatives and analogs thereof. Provided are therapeutic compositions and methods of detecting and treating cancer, including prostate and other related cancers. Also provided are methods of diagnosing and / or prognosing prostate cancer by determining the expression level of at least one prostate cancer-cell-specific gene, including, for example, the ERG gene or the LTF gene alone, or in combination with at least one of the AMACR gene and the DD3 gene.
Owner:THE HENRY M JACKSON FOUND FOR THE ADVANCEMENT OF MILITARY MEDICINE INC
Compositions, kits, and methods relating to the human FEZ1 gene, a novel tumor suppressor gene
InactiveUS20070072230A1Increase stringencyInhibiting tumorigenesisSugar derivativesPeptide/protein ingredientsTumour suppressor geneHuman tumor
Owner:THOMAS JEFFERSON UNIV
Novel tumor suppressor gene and compositions and methods for making and using the same
InactiveUS20060105340A1Compound screeningApoptosis detectionTumour suppressor geneTumor suppressor gene
The present invention relates to the identification and cloning of ARTS-1, a novel tumor suppressor gene, to isolated proteins encoded by ARTS-1, and to methods of making and using the same.
Owner:THOMAS JEFFERSON UNIV
Compositions, kits, and methods relating to the human FEZ1 gene, a novel tumor suppressor gene
InactiveUS7777005B2Increase stringencyInhibiting tumorigenesisSugar derivativesPeptide/protein ingredientsHuman tumorNucleotide
The invention relates to isolated polynucleotides homologous with a portion of one strand of the human tumor suppressor gene, FEZ1, and to the tumor suppressor protein encoded thereby, Fez1. The polynucleotides are useful, for example, as probes, primers, portions of expression vectors, and the like. The invention also includes diagnostic, therapeutic, cell proliferation enhancement, and screening methods which involve these polynucleotides and protein. The invention further includes kits useful for performing the methods of the invention.
Owner:THOMAS JEFFERSON UNIV
Tumor antigen based on products of the tumor suppressor gene WT1
InactiveUS20050266014A1Effect be exertPeptide/protein ingredientsTissue cultureTumour suppressor geneMajor histocompatibility
A tumor antigen that comprises, as an active ingredient, a product of the Wilms' tumor suppressor gene WT1 or a peptide composed of 7-30 contiguous amino acids containing an anchor amino acid for binding to major histocompatibility complex (MHC) class I in said amino acid sequence, and a vaccine comprising said antigen.
Owner:INT INST OF CANCER IMMUNOLOGY INC
Compositions, kits, and methods relating to the human FEZ1 gene, a novel tumor suppressor gene
InactiveUS7141417B1Increase stringencyInhibiting tumorigenesisSugar derivativesPeptidesHuman tumorNucleotide
The invention relates to isolated polynucleotides homologous with a portion of one strand of the human tumor suppressor gene, FEZ1, and to the tumor suppressor protein encoded thereby, Fez1. The polynucleotides are useful, for example, as probes, primers, portions of expression vectors, and the like. The invention also includes diagnostic, therapeutic, cell proliferation enhancement, and screening methods which involve these polynucleotides and protein. The invention further includes kits useful for performing the methods of the invention.
Owner:THOMAS JEFFERSON UNIV
Topographic genotyping for determining the diagnosis, malignant potential, and biologic behavior of pancreatic cysts and related conditions
InactiveUS20060088870A1Easy to analyzeEnhancing definitive diagnosisHealth-index calculationMicrobiological testing/measurementMolecular analysisMicrosatellite
The application relates to a method of a predicting the presence of invasive pancreatic cancer or high grade dysplasia, pre-cancerous pancreatic states and non-neoplastic conditions comprising detailed molecular analysis incorporating DNA quality and quantity, K-ras mutational analysis and a broad spectrum of tumor suppressor gene linked microsatellite LOH. Methods of diagnosing, determining prognosis of and determining a course of treatment for pancreatic cancer or high grade dysplasia, pre-cancerous pancreatic states and non-neoplastic conditions are also provided.
Owner:REDPATH INTEGRATED PATHOLOGY INC
Methods and compositions comprising DNA damaging agents and p53
InactiveUS20050089511A1Improve the level ofLow cellular uptakeOrganic active ingredientsElectrotherapyRegimenCancer cell
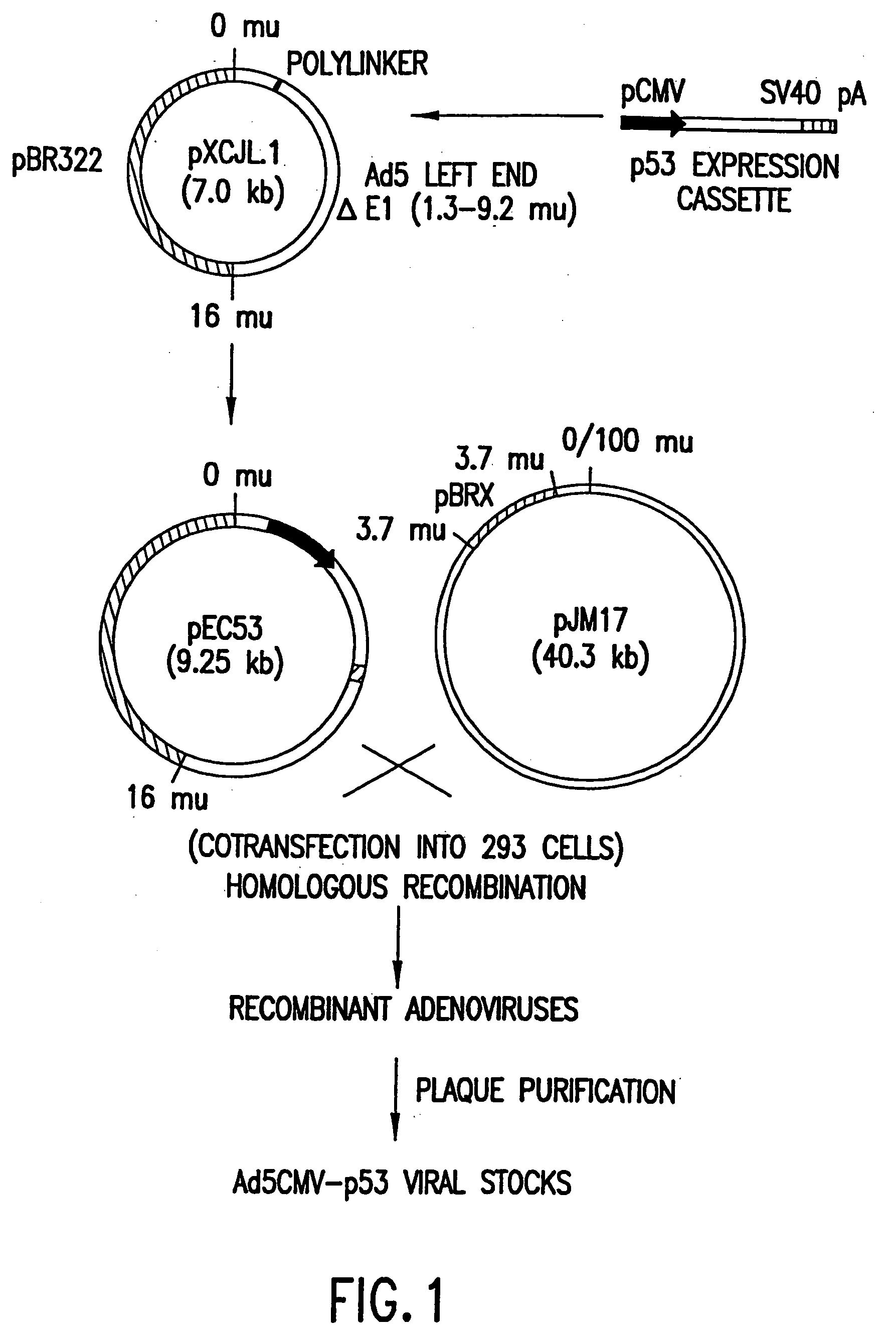
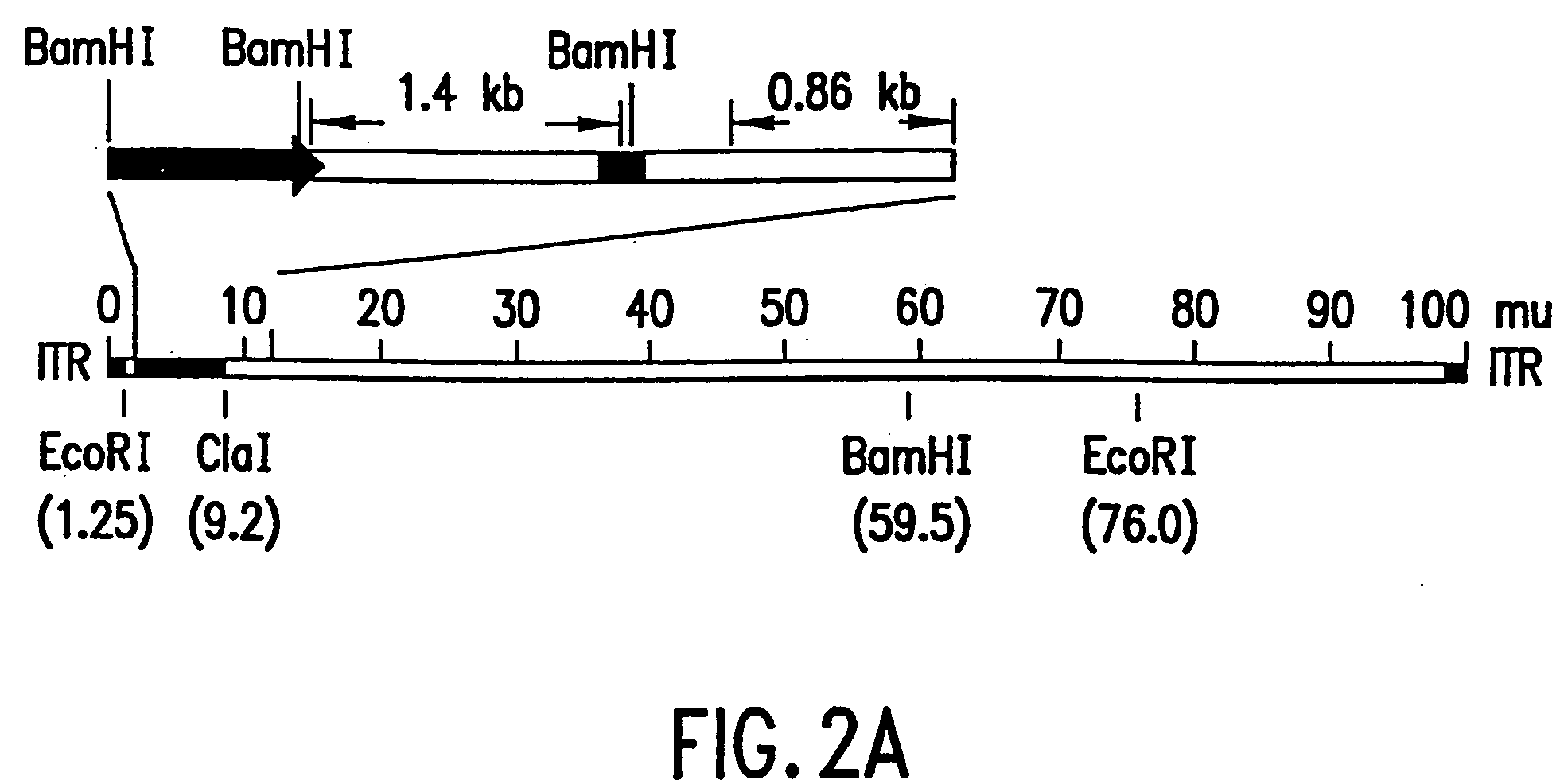
The present invention relates to the use of tumor suppressor genes in combination with a DNA damaging agent or factor for use in killing cells, and in particular cancerous cells. A tumor suppressor gene, p53, was delivered via a recombinant adenovirus-mediated gene transfer both in vitro and in vivo, in combination with a chemotherapeutic agent. Treated cells underwent apoptosis with specific DNA fragmentation. Direct injection of the p53-adenovirus construct into tumors subcutaneously, followed by intraperitoneal administration of a DNA damaging agent, cisplatin, induced massive apoptotic destruction of the tumors. The invention also provides for the clinical application of a regimen combining gene replacement using replication-deficient wild-type p53 adenovirus and DNA-damaging drugs for treatment of human cancer.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
Method of analysis allowing avoidance of surgery
ActiveUS20170275702A1Prevent removalAvoid surgeryMicrobiological testing/measurementMedical automated diagnosisDNA methylationCervix
A multi-gene-based assay for analysis of the following: (a) oncogene expression; (b) DNA methylation of tumor suppressor genes; (c) non-coding RNA expression (microRNA profiling); and (d) long non-coding RNA expression in cancer samples is disclosed. The assay method and device for conducting the assay are applicable to diagnosis, prognosis, and treatment of various cancers such as lung, breast, colorectal, prostate, liver, bladder; kidney, cervix, pancreatic, gastric, brain, oral, endometrium, and ovary. The assay methods allow avoidance of surgery to remove cancerous tissue.
Owner:GENEVERIFY INC
Cancer vaccine containing cancer antigen based on tumor suppressor gene wt1 product and cationic liposomes
InactiveUS20060165708A1Tumor rejection antigen precursorsTumor specific antigensAntigenCancer antigen
A cancer vaccine comprising a cancer antigen which comprises, as an active ingredient, the product of a tumor suppressor gene WT1, a partial peptide or a modified version thereof, and a cationic liposome.
Owner:MAYUMI TADANORI +2
Methylation markers for diagnosis and treatment of ovarian cancer
InactiveUS20070087365A1Reducing and inhibiting neoplastic growthRestoring expression of the polypeptide in the cellMicrobiological testing/measurementMaterial analysisTumour suppressor geneSilencing gene
Twenty-three markers are provided which are epigenetically silenced in ovarian cancers. The markers can be used diagnostically, prognostically, therapeutically, and for selecting treatments that are well tailored for an individual patient. Restoration of expression of silenced genes can be useful therapeutically, for example, if the silenced gene is a tumor-suppressor gene. Restoration can be accomplished by supplying non-methylated copies of the silenced genes or polynucleotides encoding their encoded products. Alternatively, restoration can be accomplished using chemical demethylating agents or methylation inhibitors. Kits for testing for epigenetic silencing can be used in the context of diagnostics, prognostics, or for selecting “personalized medicine” treatments.
Owner:MDXHEALTH
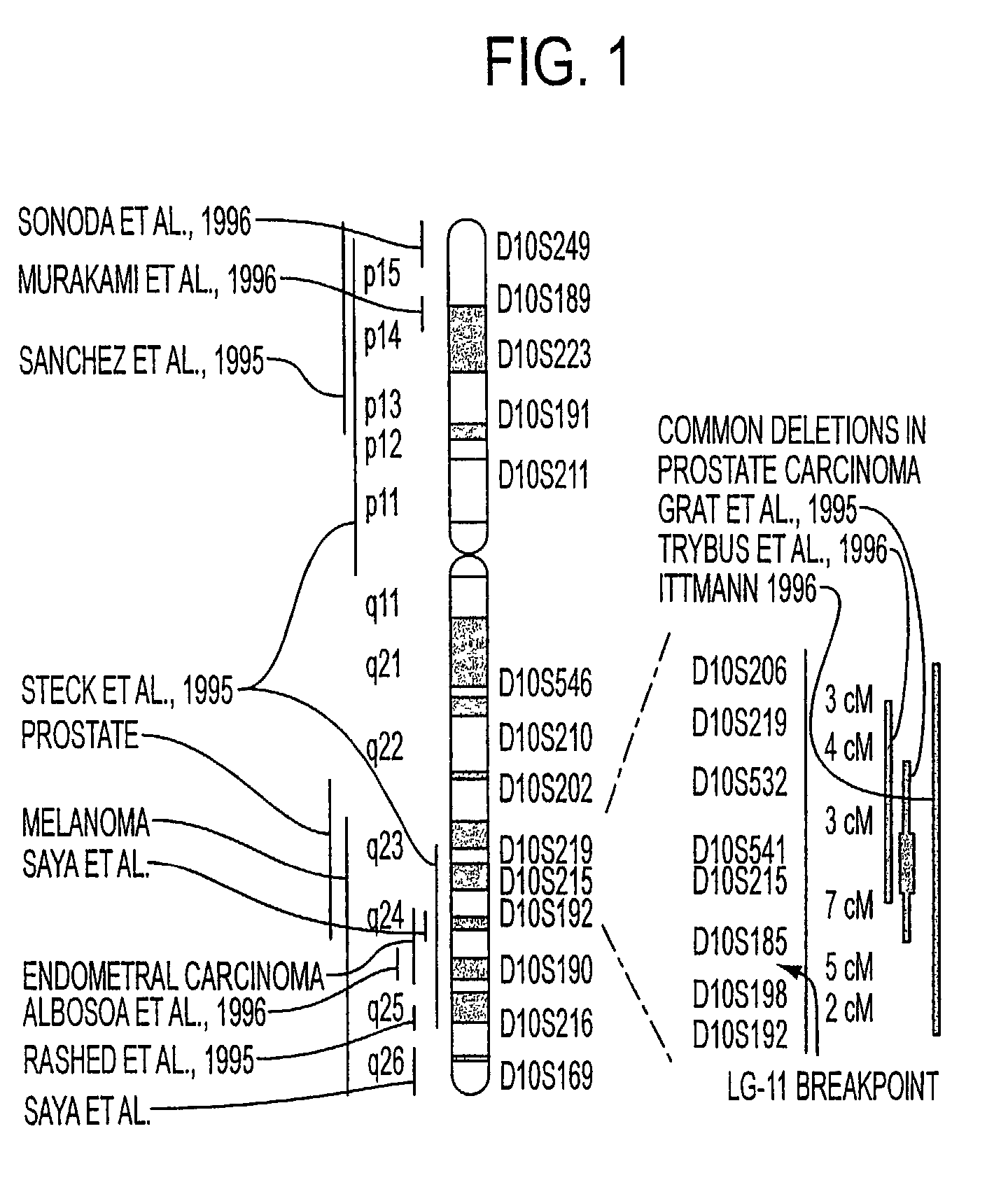
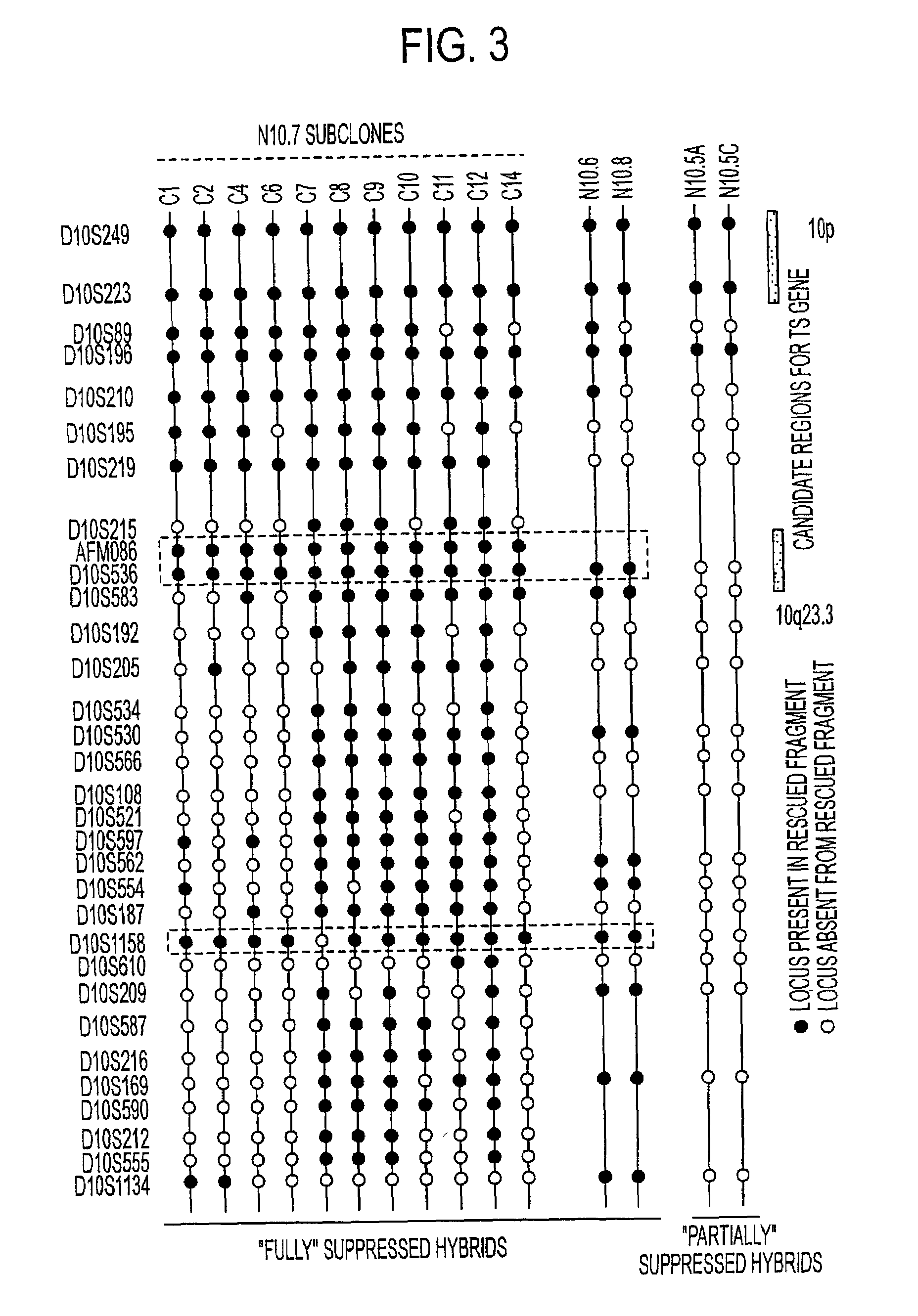
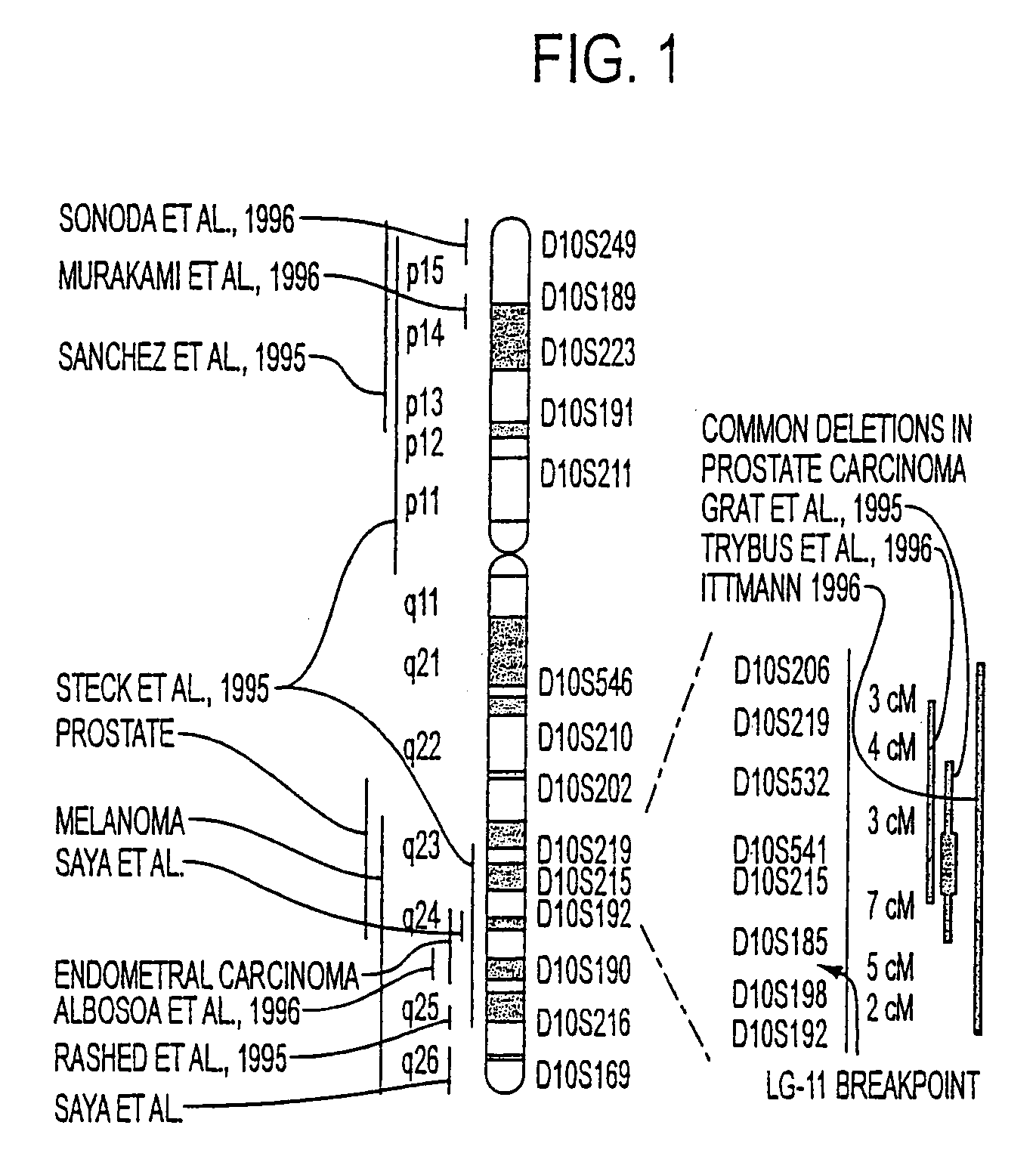
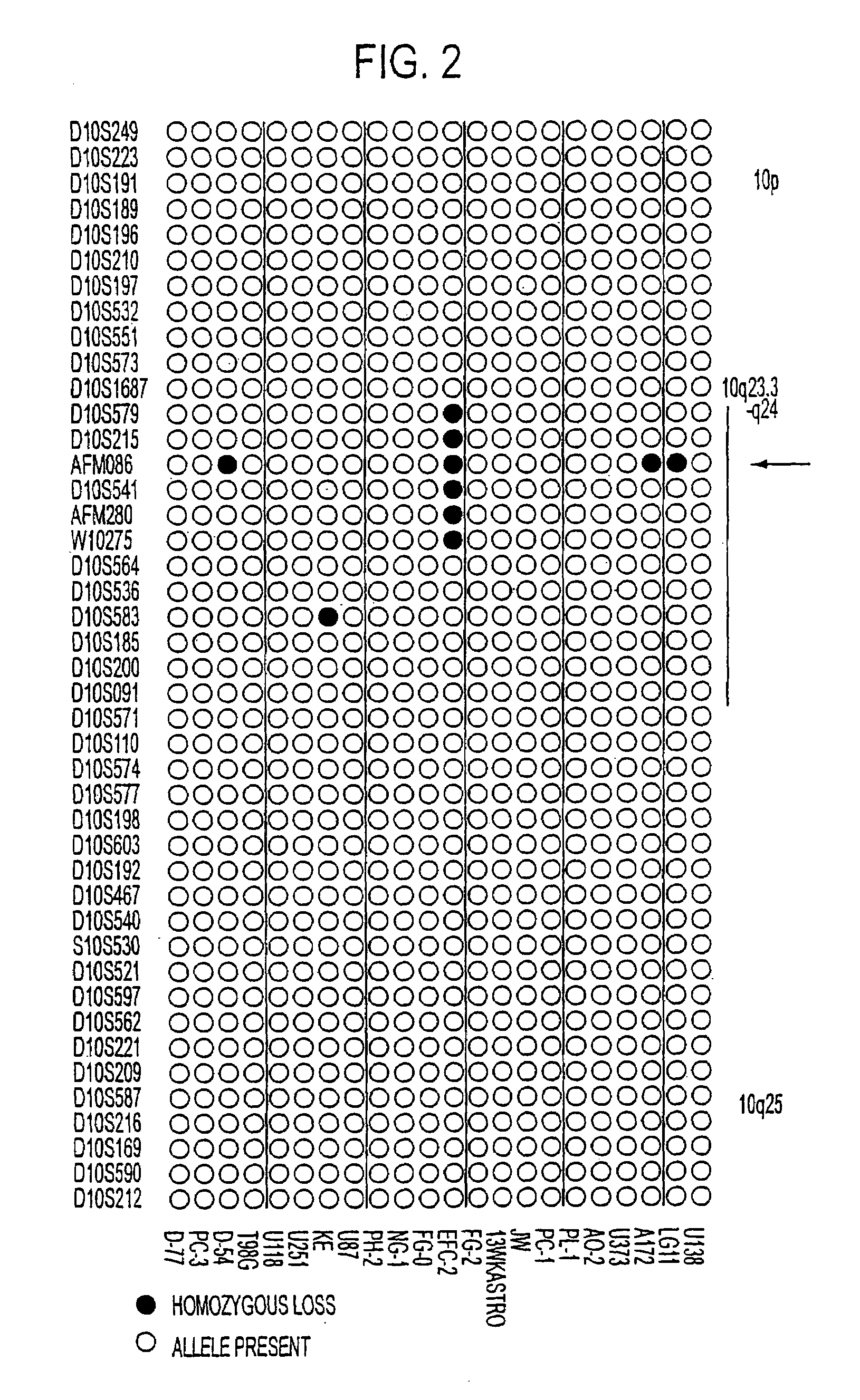
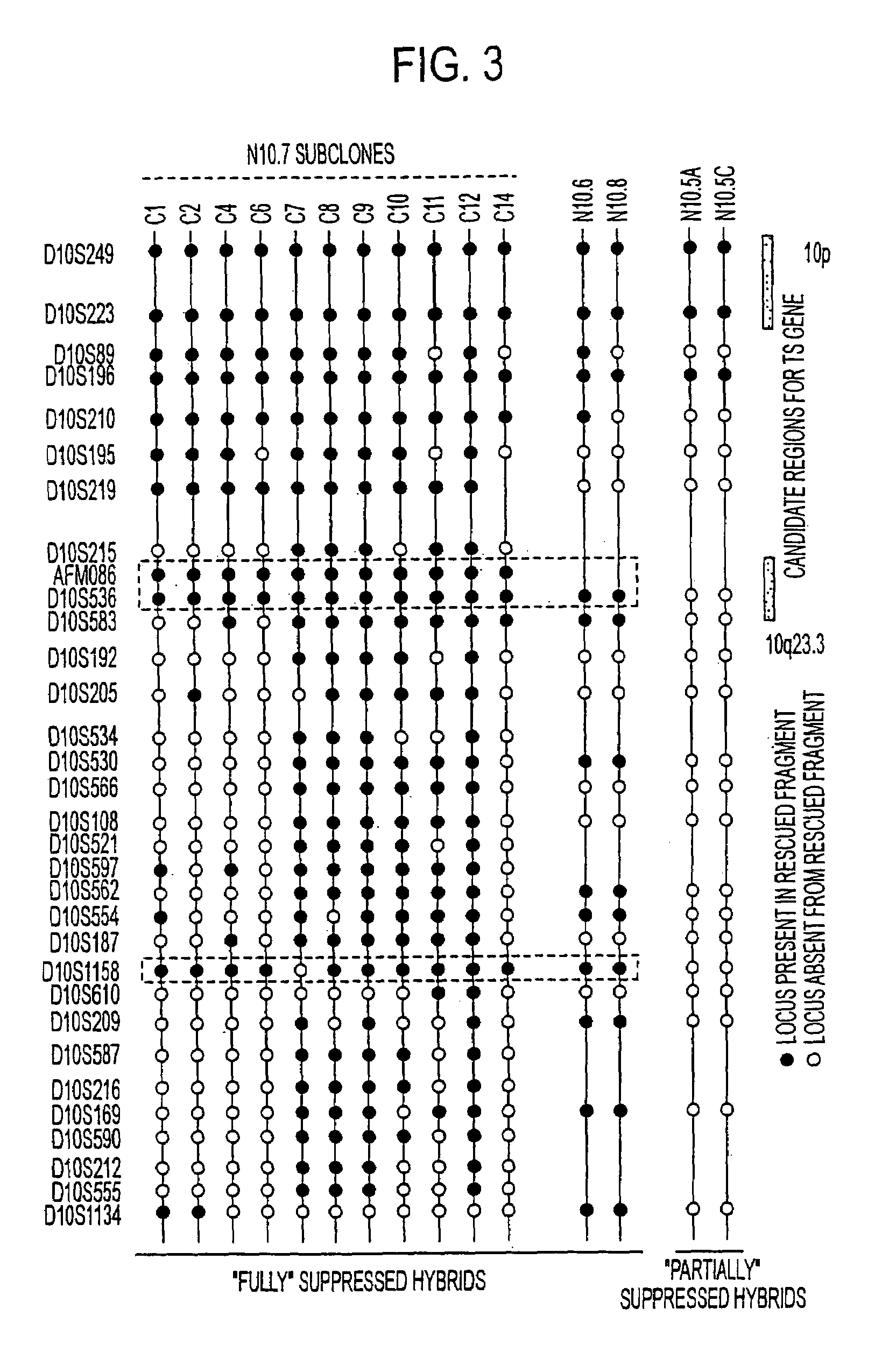
Tumor suppressor designated TS10q23.3
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
Compositions and Methods for Inhibiting Growth of SMAD-4 Deficient Cancers
The present invention is in the fields of cell biology, immunology and oncology. The invention relates to the discovery that there is a relationship between the expression levels of the tumor suppressor gene smad4 (also known as dpc4) and integrin αvβ6, and the responsiveness of patient populations to αvβ6-active compounds and compositions (e.g., antibodies and other ligands that bind αvβ6), particularly in cancer cells from such patient populations, more particularly on carcinomas such as pancreatic carcinomas. The invention thus provides methods for determining the responsiveness of tumor cells (particularly those from pancreatic tumors) to such αvβ6-active compounds and compositions by examining the expression of αvβ6 and smad4 by the tumor cells, as well as methods of diagnosis and treatment / prevention of tumor progression using ligands, including antibodies and small molecule drugs, that bind to integrin αvβ6 on the surfaces of tumor cells and / or that block one or more components of the TGF-β pathway, particularly in smad4-deficient tumor cells.
Owner:BIOGEN MA INC
Plant drug for treatment of liver disease
The present invention related to safe plant drug for treatment of liver disease, specifically, this invention proves a safe plant drug Schisandrin and its preparation. Schisandrin has the following pharmaceutical functions: increasing tumor suppresson genes express activity, decreasing activity of oncogenes, increasing immune function, increasing liver DNA synthesis, decreasing serum alamine aminotransferase activity, increasing glutathione level, increasing glutathione reductase activity, decreasing lipid peroxidation of liver, increasing hepatic microsomal monooxygenases activity, increasing ATP content in liver, increasing energy metabolism activity, decreasing density lipoprotein oxidation, protecting gastrointestinal function, increasing killer cell activity, increasing complement activity, decreasing induced liver cancer activity and decreasing grown of cancer cells.
Owner:ZHAO XINXIAN
Tumor suppressor and oncogene biomarkers predictive of Anti-immune checkpoint inhibitor response
ActiveUS20170130271A1Prevent cancerMicrobiological testing/measurementBiological material analysisAbnormal tissue growthSuppressor
The present invention is based on the identification of novel biomarkers predictive of responsiveness to anti-immune checkpoint inhibitor therapies.
Owner:DANA FARBER CANCER INST INC +1
Molecule substitution label sequencing parallel detection method-oligomictic nucleic acid coding label molecule library micro-sphere array analysis
InactiveCN101100764AImprove reliabilityGreat advantageMicrobiological testing/measurementLibrary member identificationBiotechnologyMolecular replacement
Molecular displacement label sequence parallel inspection - oligonucleo coded label molecular pool micro-ball array analysis includes: using artificial sequential oligo nucleo-labeled DNA coded molecular pool, coating micro-balls with ligands in correspondent pools to form catching micro-balls, caught ligands combined with labeled ciding molecular pools to form labeled micro-balls array pools. Sample molecules competitively combined with micro-balls catching ligands when sample contains molecules same as those in labeled micro-balls array pools, calculating amount of displaced coded molecular sequence computing kinds and mounts of molecules to be tested. It verifies high flux of inspection with good repeatability, high reliability and sensitivity, so that it is suitable to precisely parallel quantitative analysis concerned with cancer-inhibiting genes and cancer genes pools, cell factor pools, etc.
Owner:北京万达因生物医学技术有限责任公司
Treatment of tumor suppressor gene related diseases by inhibition of natural antisense transcript to the gene
InactiveUS20110294870A1Prevent and treat diseaseModulate its functionAntibacterial agentsOrganic active ingredientsDiseaseSuppressor
The present invention relates to antisense oligonucleotides that modulate the expression of and / or function of Tumor Suppressor genes, in particular, by targeting natural antisense polynucleotides of Tumor Suppressor genes. The invention also relates to the identification of these antisense oligonucleotides and their use in treating diseases and disorders associated with the expression of Tumor Suppressor genes.
Owner:CURNA INC
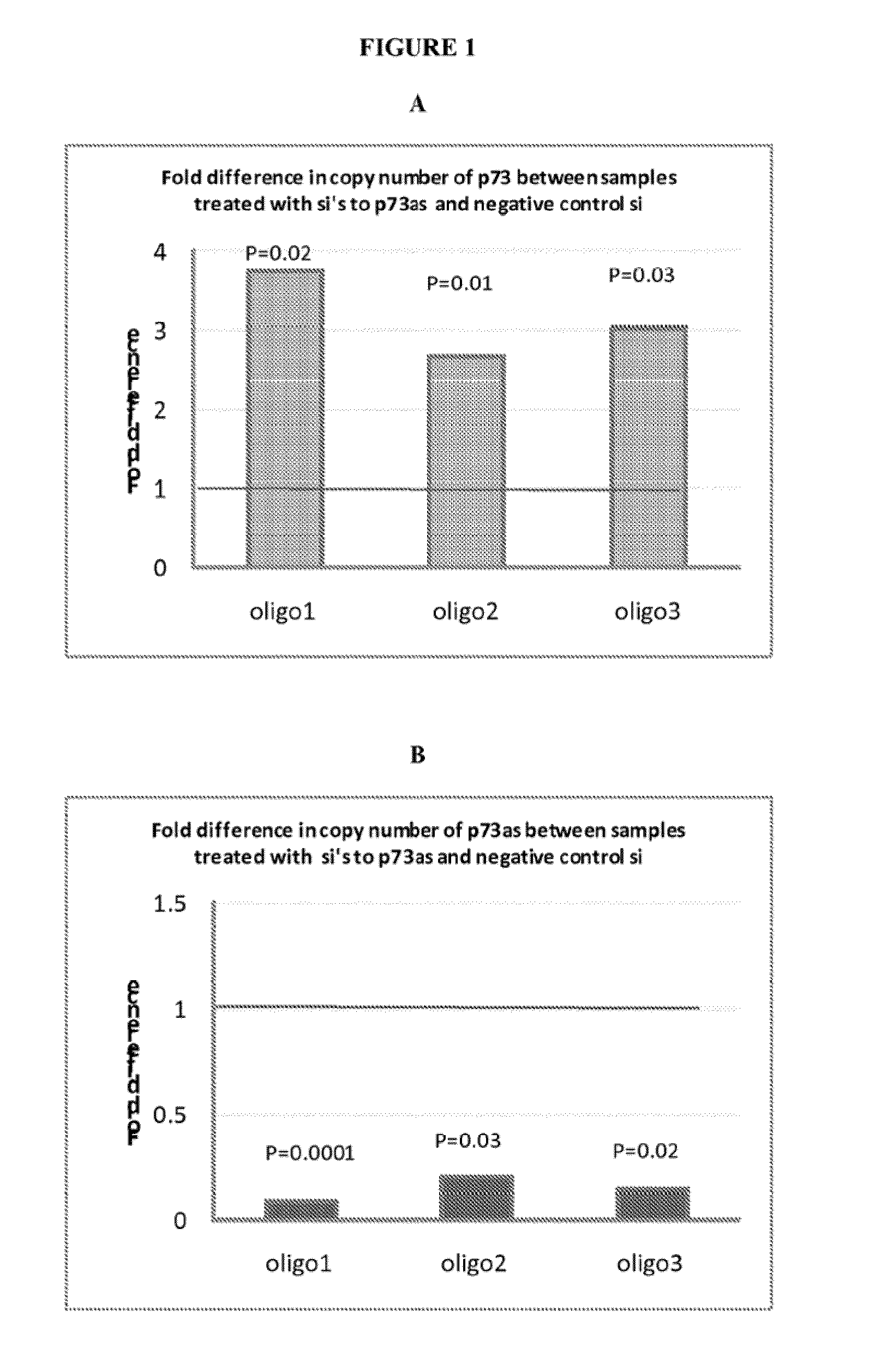
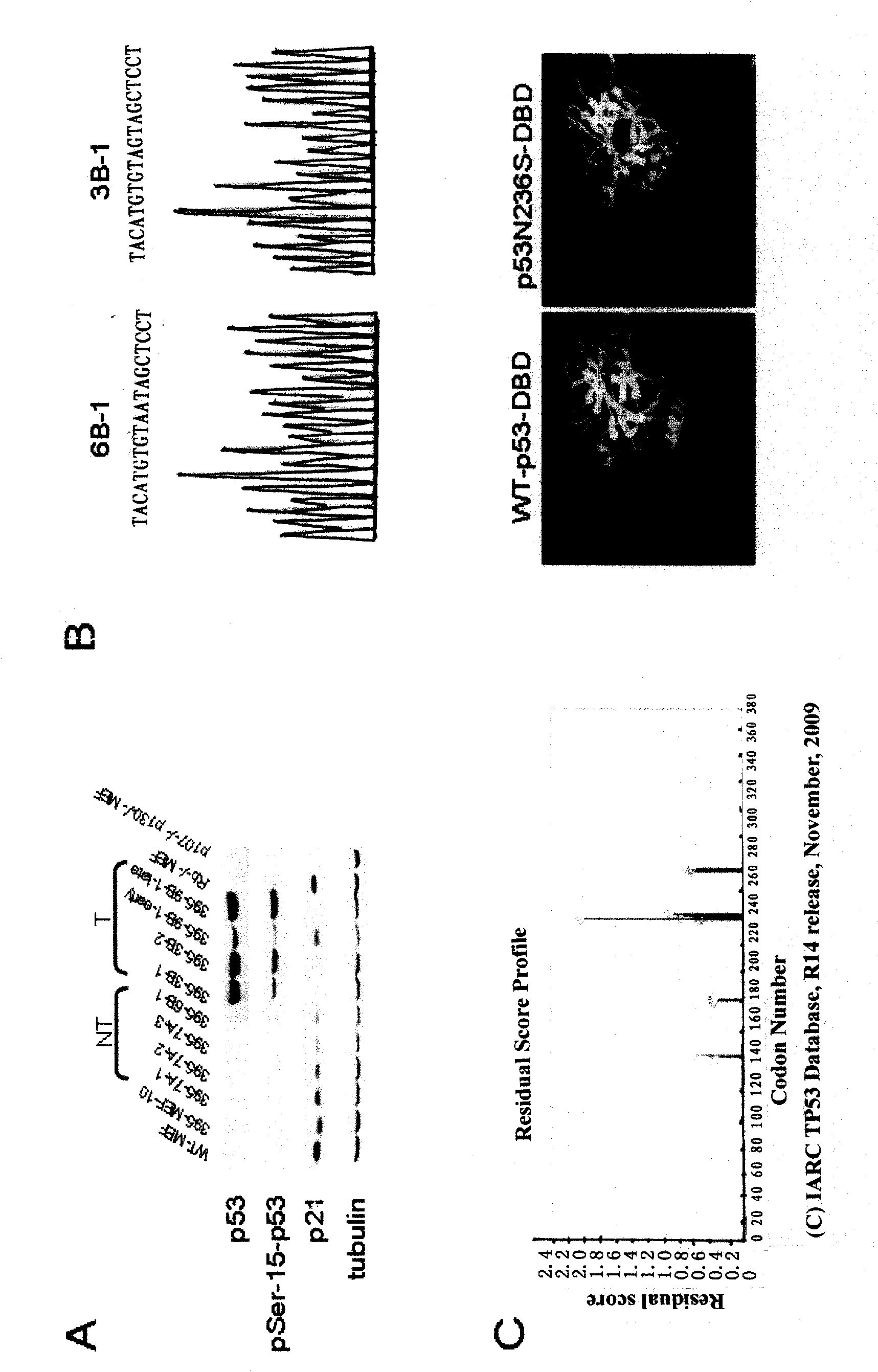
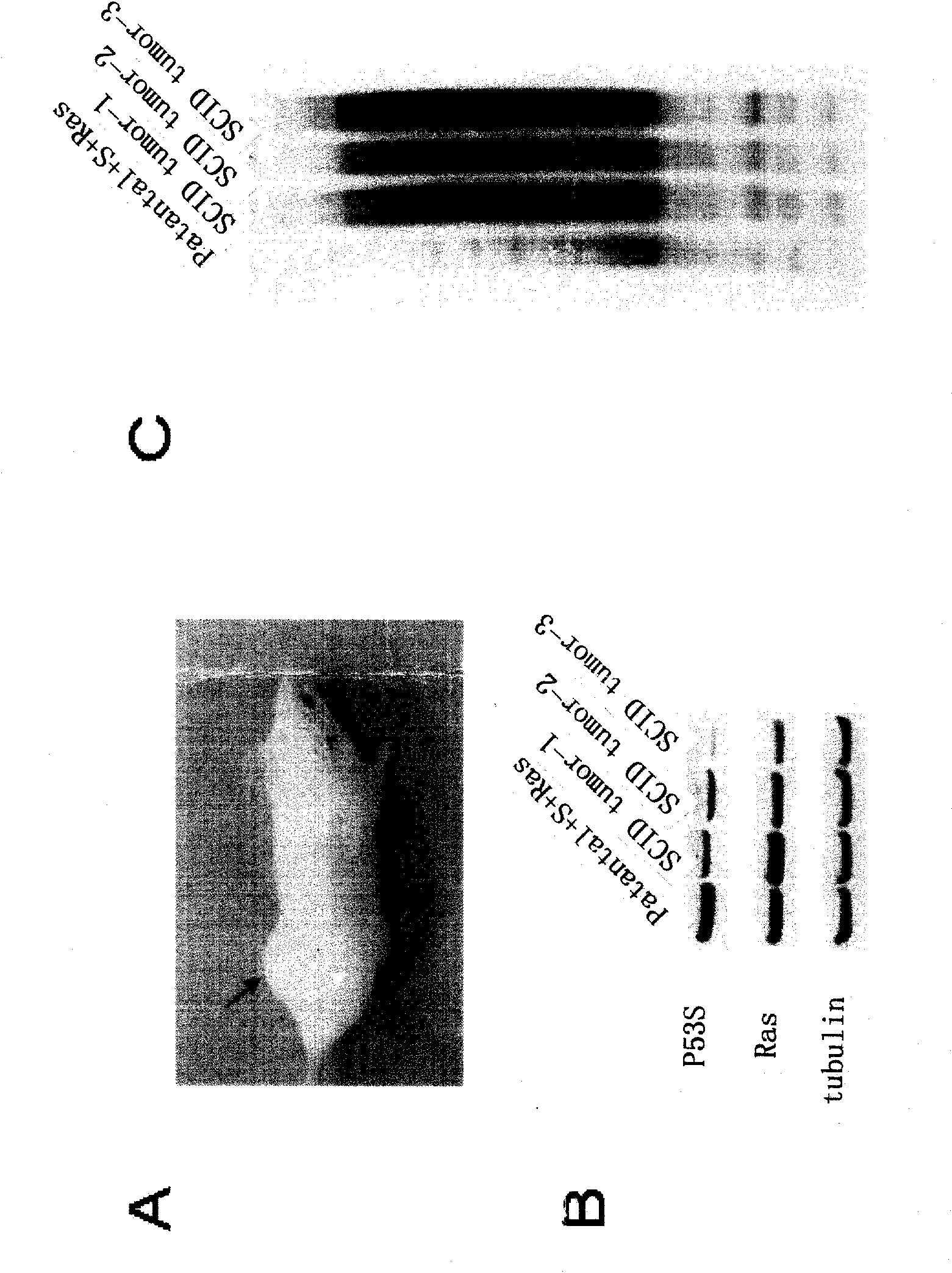
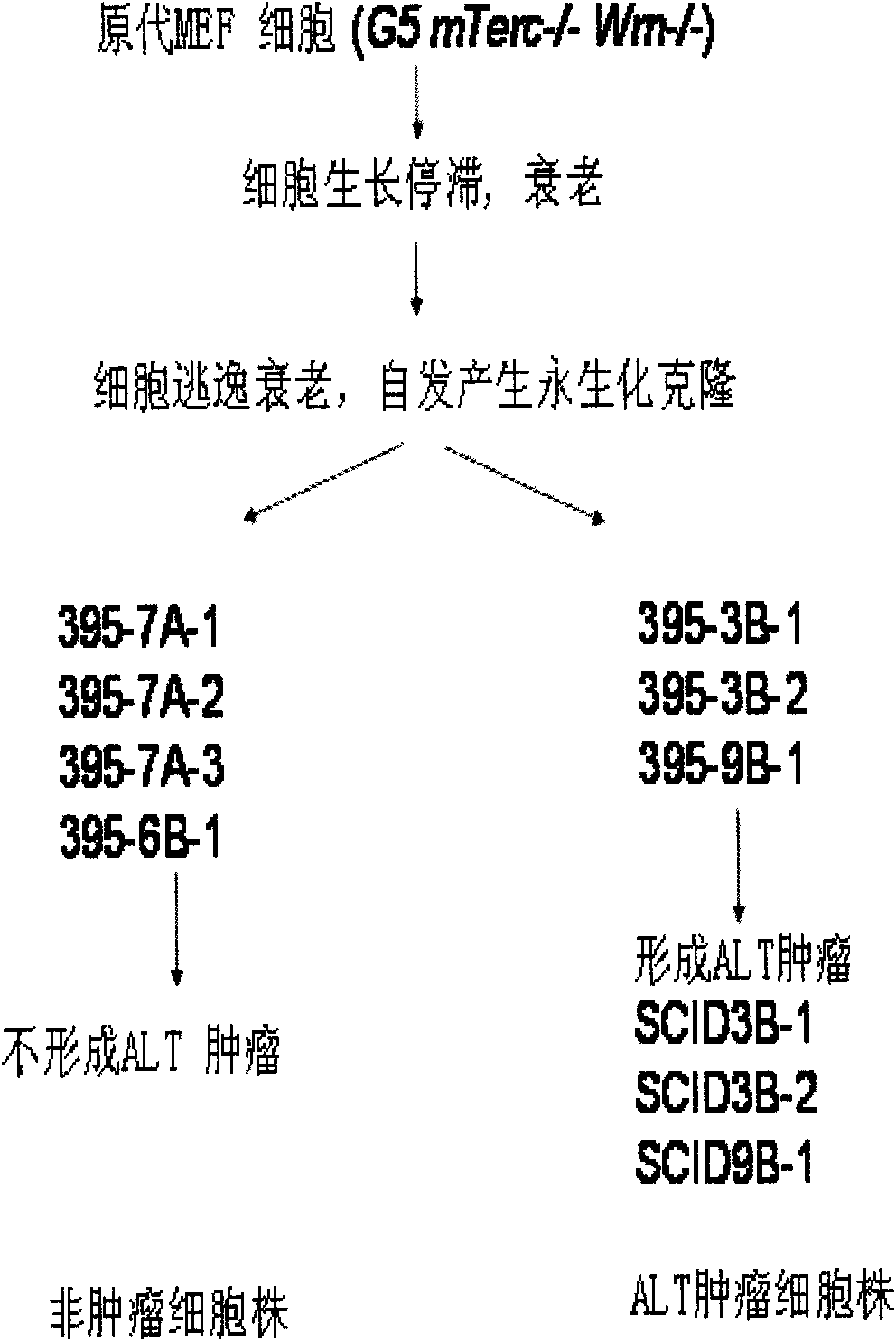
Peculiar p53 mutated protein-p53N236S of ALT tumor caused by progeria syndrome and application thereof
InactiveCN101805738APeptide/protein ingredientsGenetic material ingredientsAbnormal tissue growthMutated protein
The invention relates to a mutant gene p53A1243G caused by the point mutation of a tumor suppressor gene p53, encoded protein p53N236S and functions thereof, and application thereof to tumor diagnosis. First, the important functions of p53N236S in an ALT tumor caused by progeria syndrome are disclosed. The invention can be applied to the tumor diagnosis with p53N236S as the target molecule, the screening of anticancer medicine and cancer treatment strategies, and has important significance in preventing tumors caused by aging.
Owner:KUNMING UNIV OF SCI & TECH
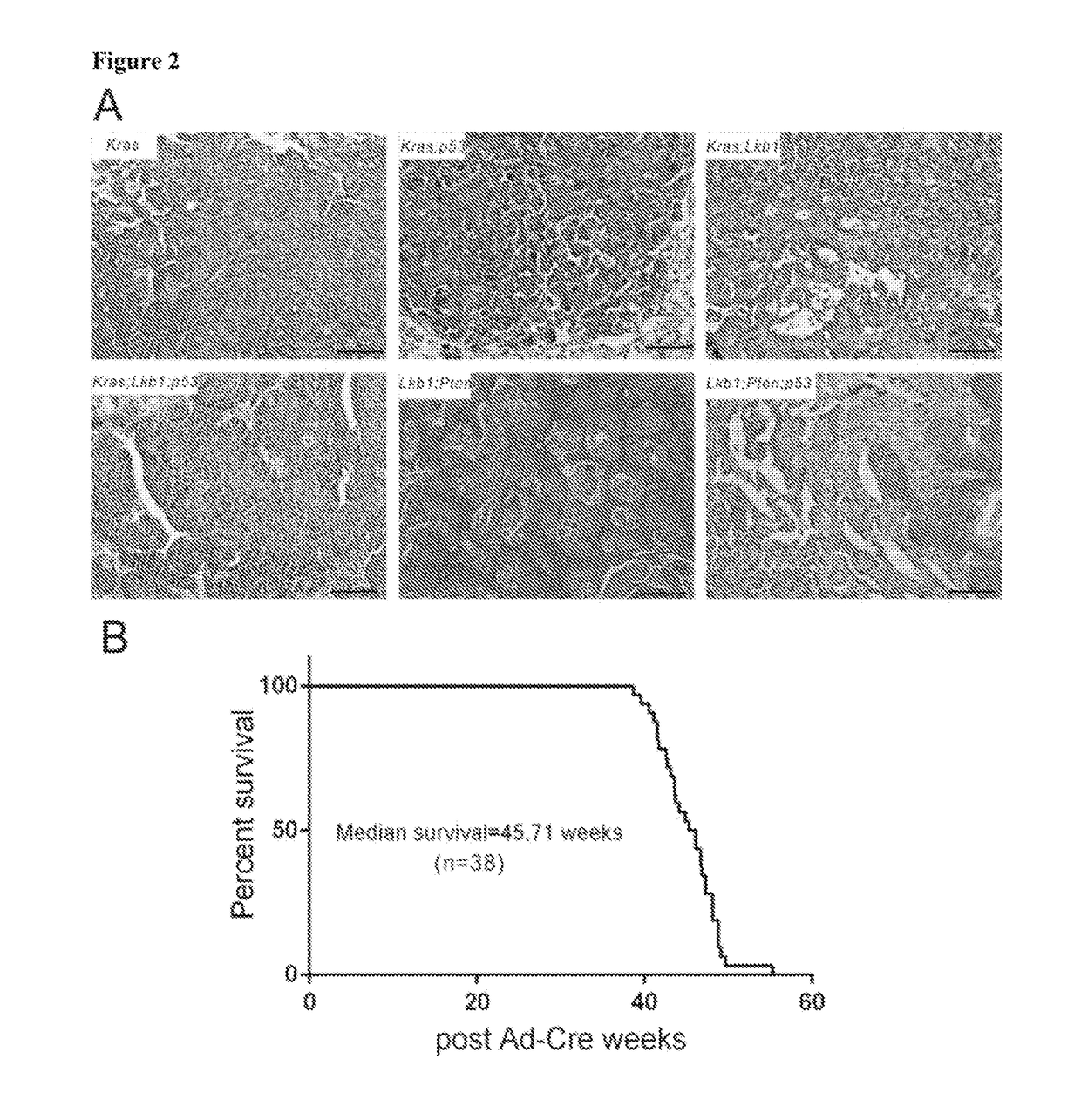
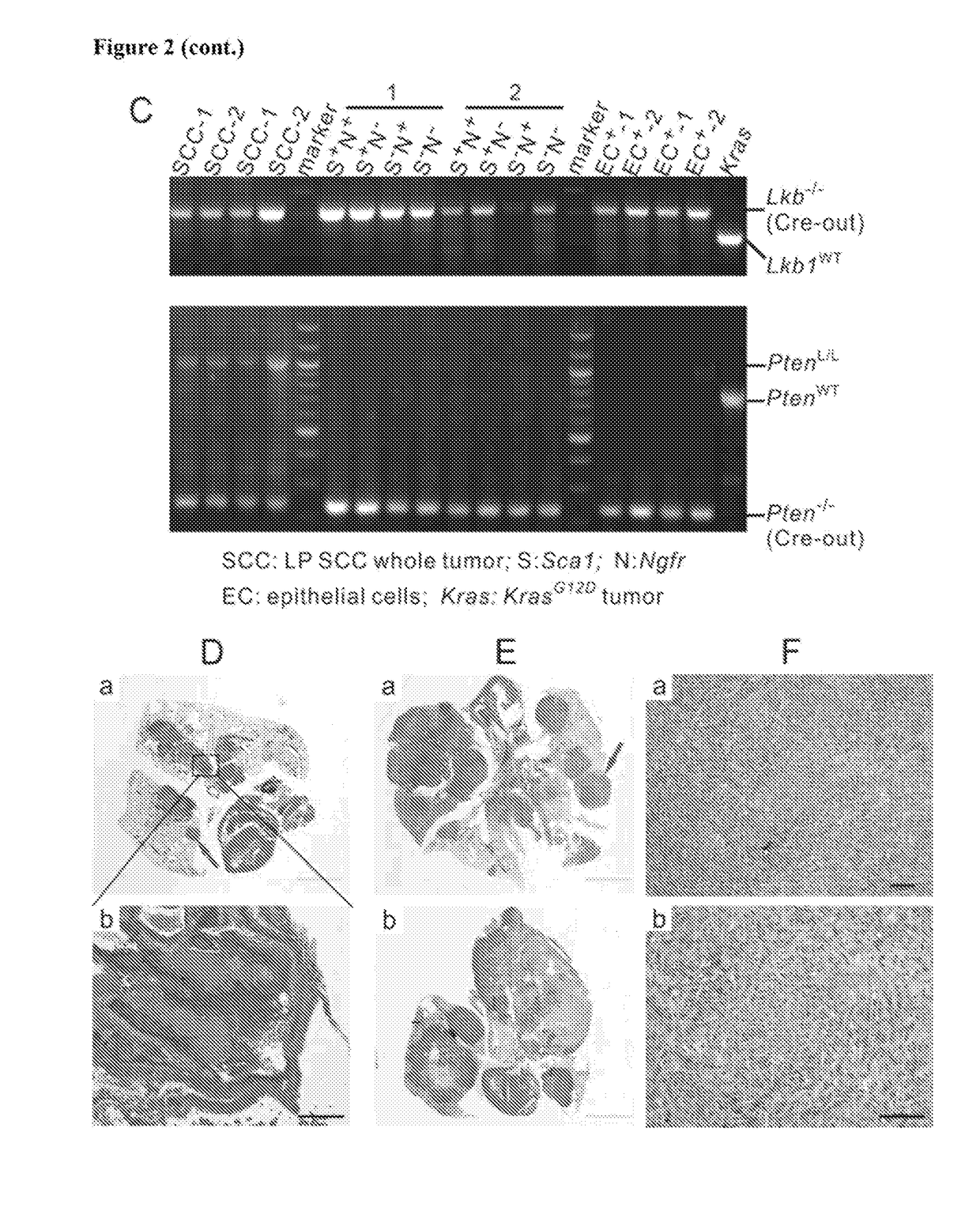
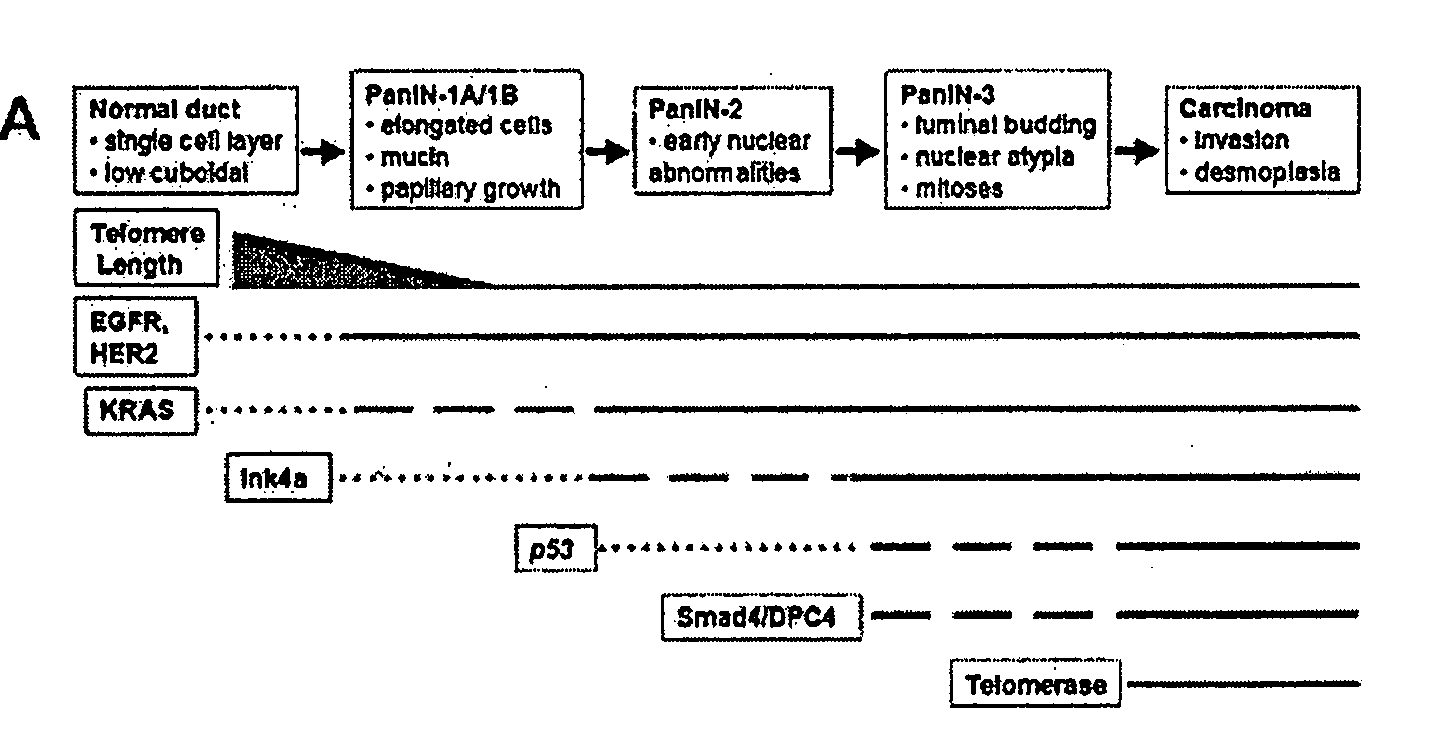
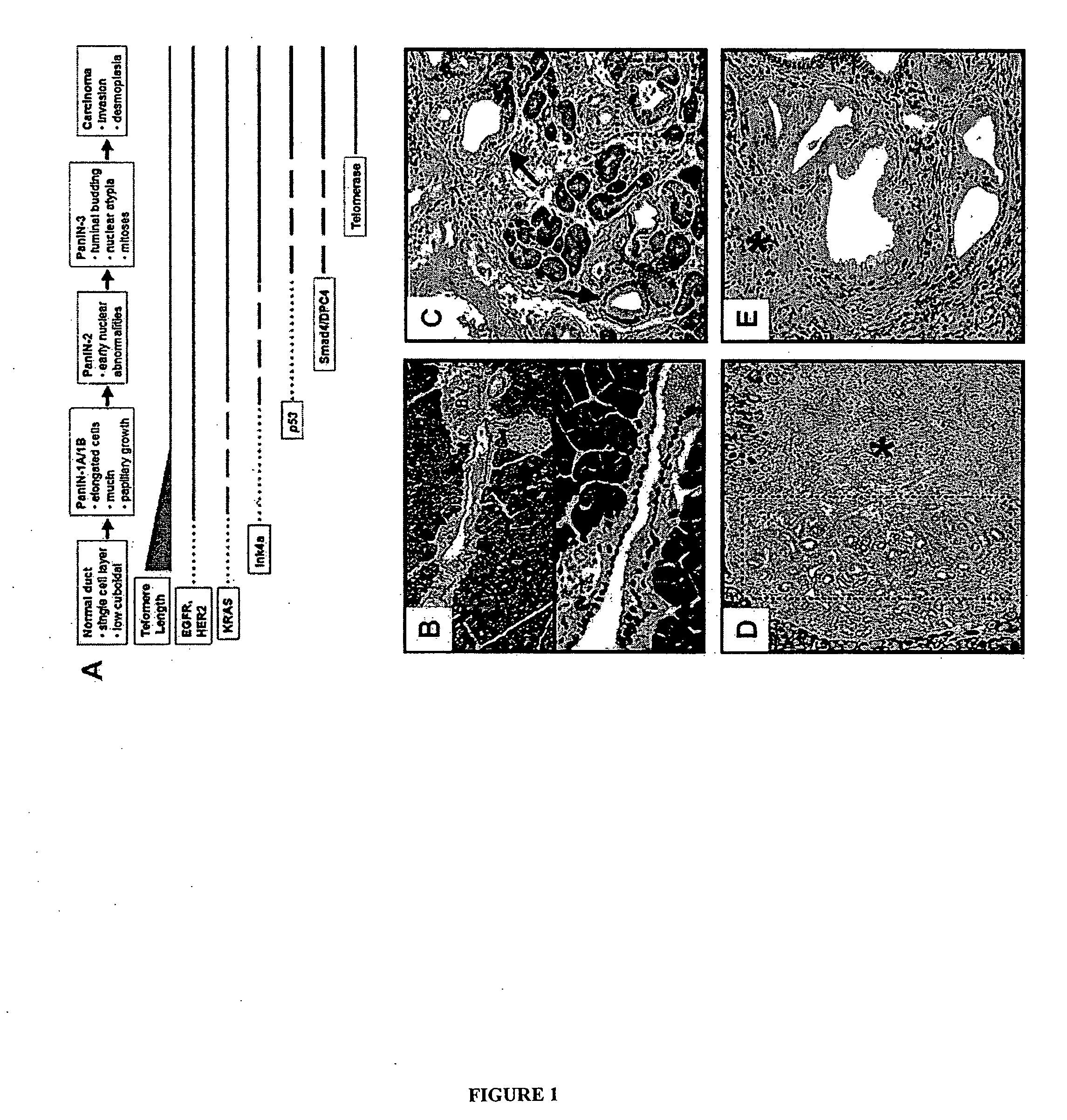
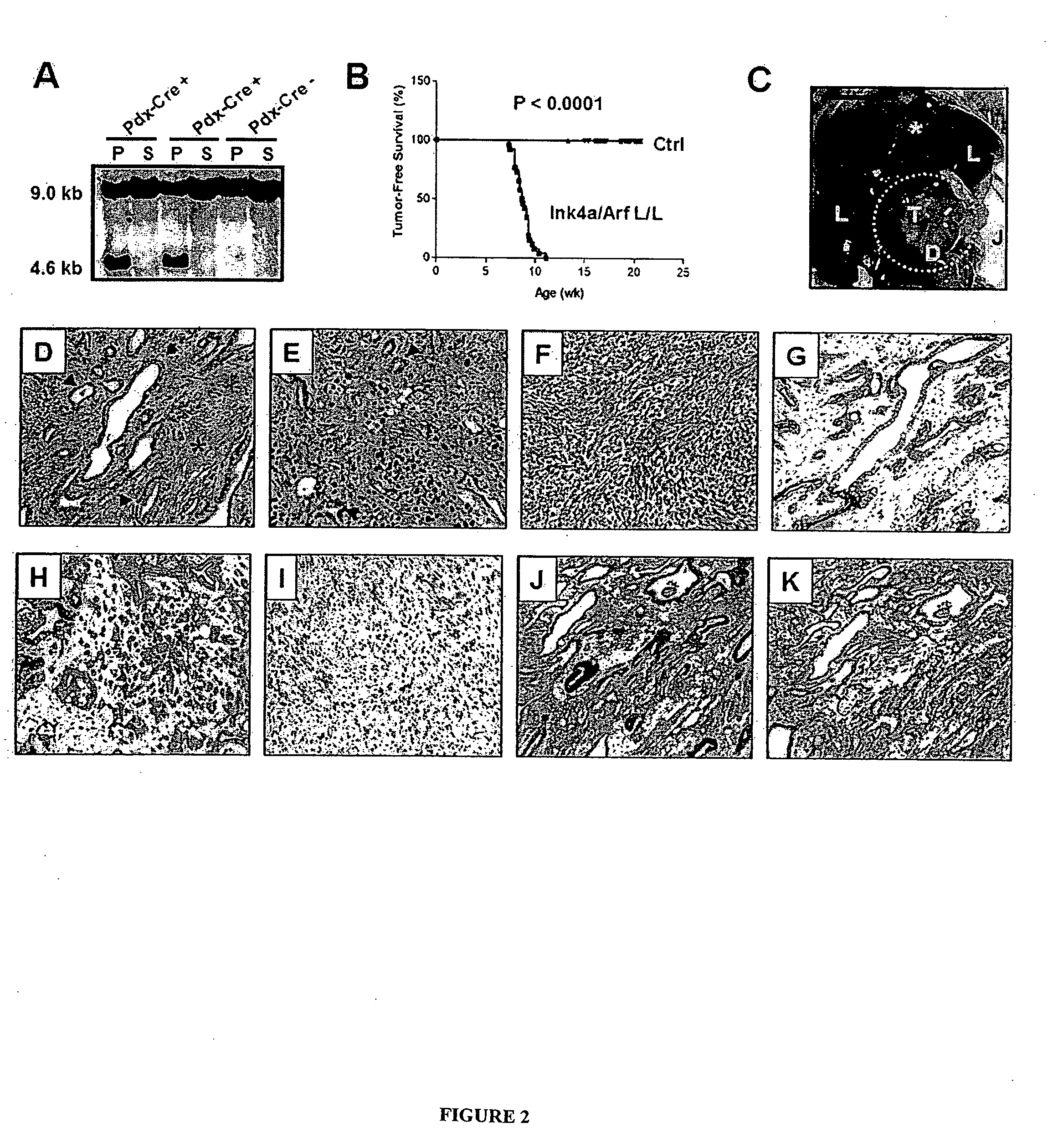
Animal models of pancreatic adenocarcinoma and uses therefor
The present invention is based, at least in part, on the generation of an animal model of pancreatic adenocarcinoma which recapitulates the genetic and histological features of human pancreatic adenocarcinoma, including the initiation, maintenance, and progression of the disease. Accordingly, the present invention provides animal models of cancer, e.g., pancreatic adenocarcinoma, wherein an activating mutation of Kras has been introduced, and any one or more known or unknown tumor suppressor genes or loci, e.g., Ink4a / Arf, Ink4a, Arf, p53, Smad4 / Dpc, Lkb1, Brca2, or Mlh1, have been misexpressed, e.g., have been misexpressed leading to decreased expression or non-expression. The animal models of the invention may be used, for example, to identify biomarkers of pancreatic cancer, to identify agents for the treatment or prevention of pancreatic cancer, and to evaluate the effectiveness of potential therapeutic agents.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA +1
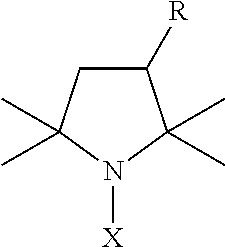
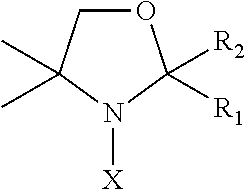
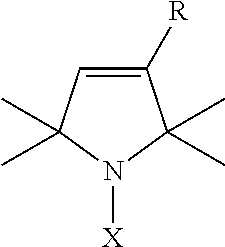
Nitroxides for use in treating or preventing neoplastic disease
Pharmaceutical compositions are provided that are useful in treating or preventing neoplastic disease, such as cancer. The compositions comprise a pharmaceutically acceptable carrier, and an effective therapeutic or prophylactic amount of a nitroxide antioxidant that alters the expression of one or more genes related to the cancer. Methods are also provided for the use of the pharmaceutical compositions in the treatment or prevention of cancer. In a preferred embodiment, the nitroxide antioxidant is Tempol (4-hydroxy-2,2,6,6-tetramethylpiperidine-1-oxyl), and the cancer is esophageal cancer, hepatocellular carcinoma, colon cancer, prostate cancer, lung cancer, gastric carcinoma, renal cell carcinoma, bone cancer, breast cancer, cervical cancer, brain cancer, or a cancer associated with the tumor suppressor gene p53.
Owner:MATRIX BIOMED INC
Tumor suppressor designated TS10q23.3
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
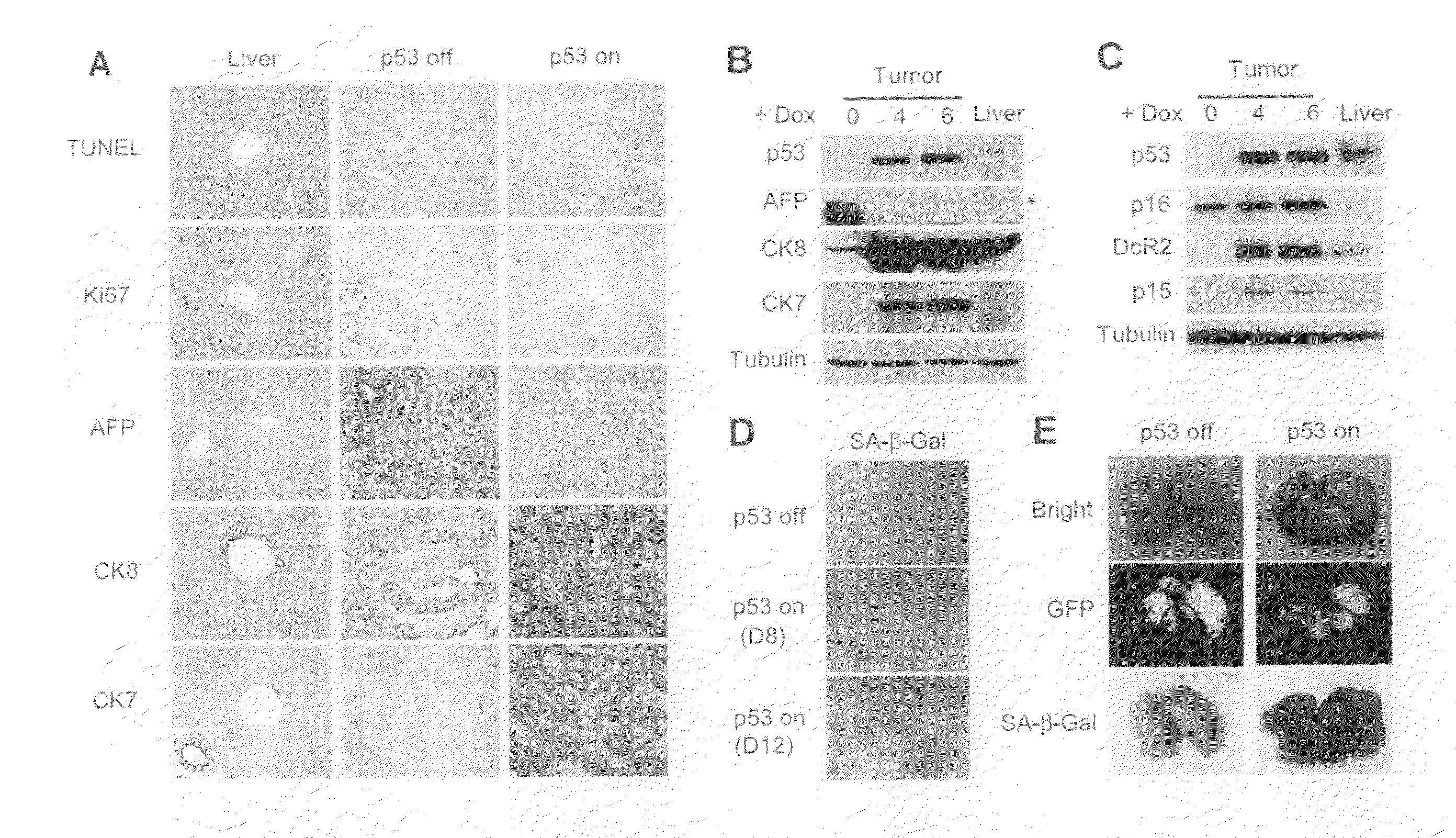
Orthotopic, controllable, and genetically tractable non-human animal model for cancer
InactiveUS20090022685A1High resolutionBiocideOrganic active ingredientsAbnormal tissue growthCooperative interaction
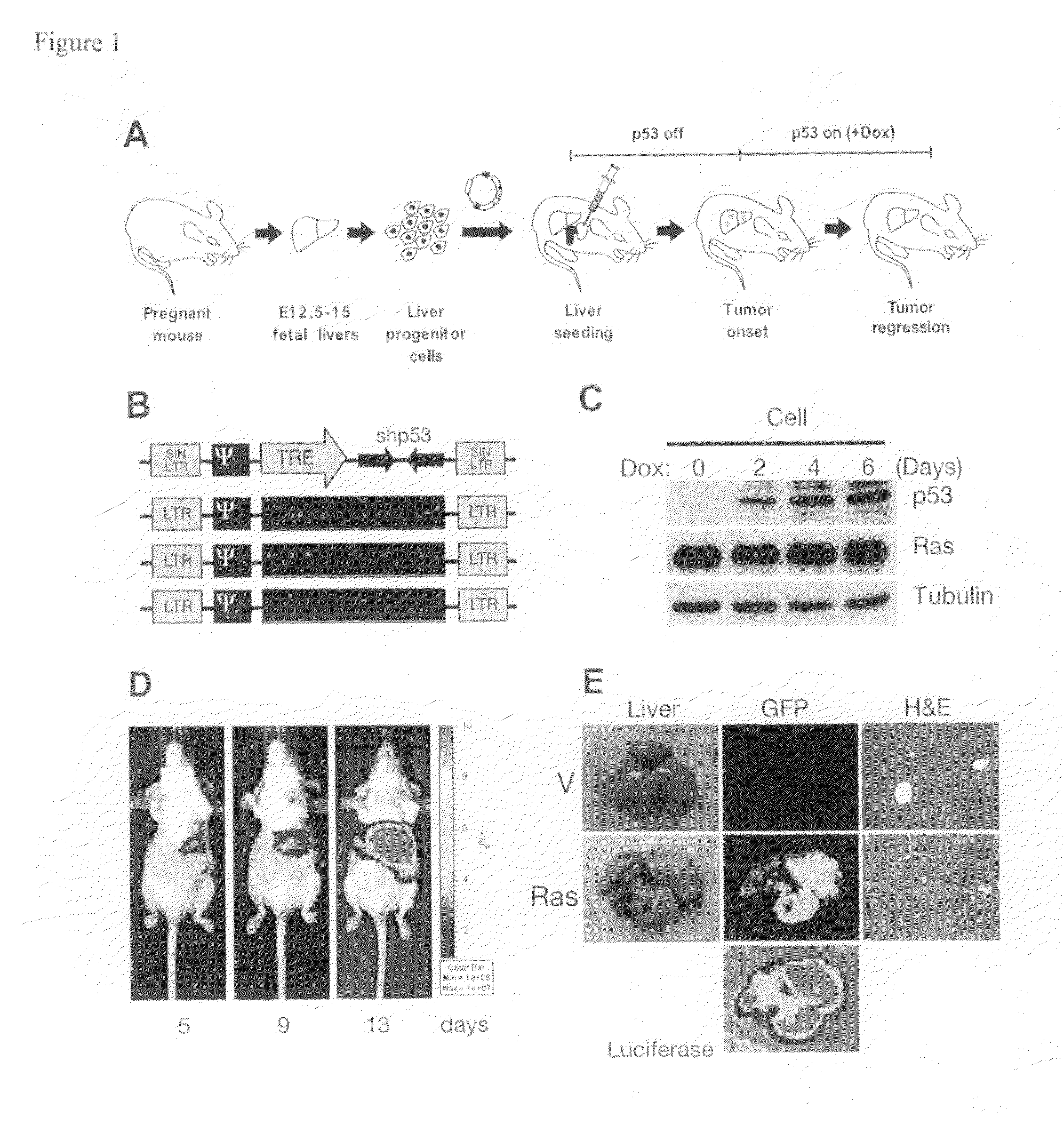
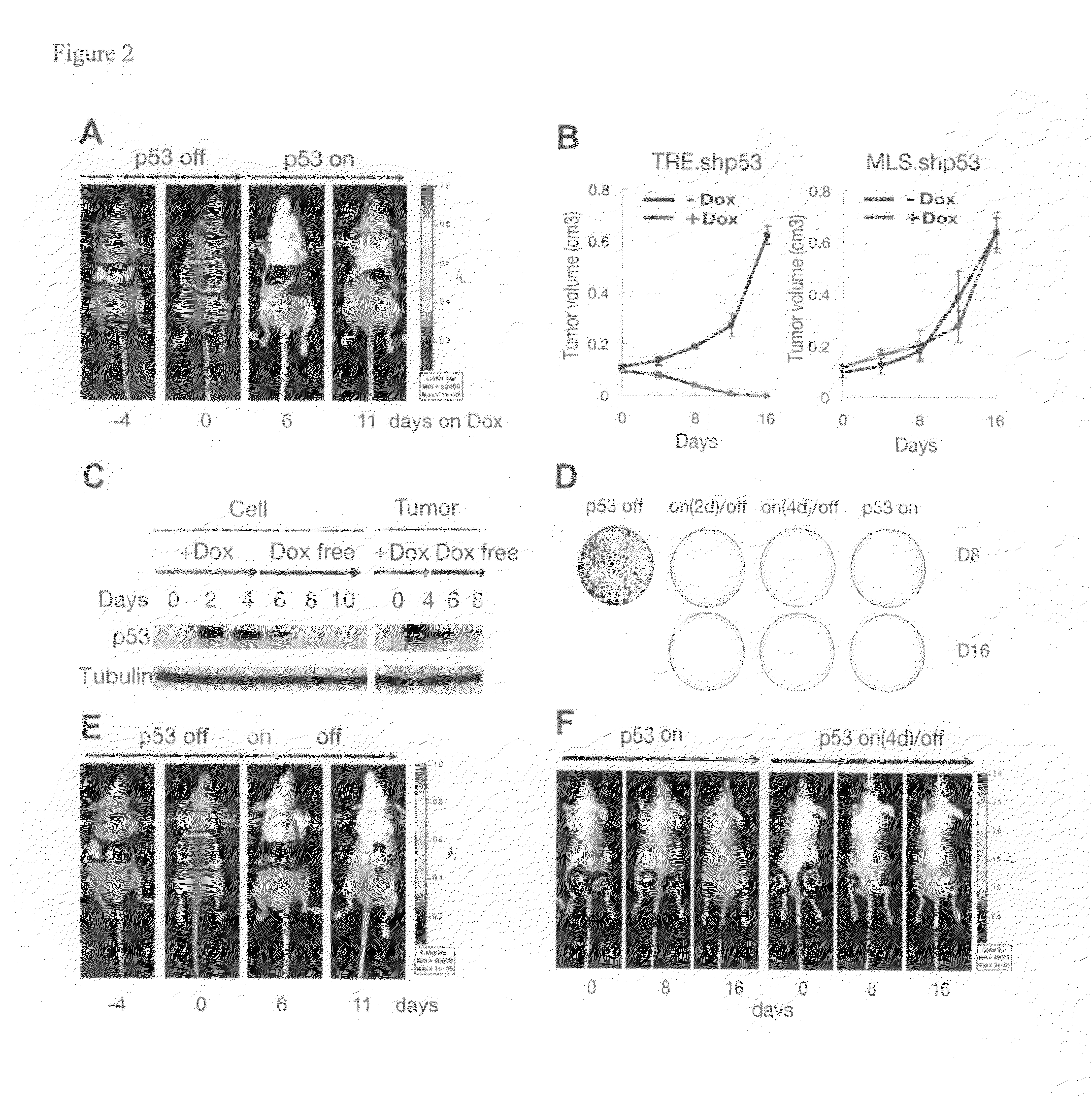
This invention provides a genetically tractable in situ non-human animal model for hepatocellular carcinoma. The model is useful, inter alia, in understanding the molecular mechanisms of liver cancer, in understanding the genetic alterations (e.g., in oncogenes and tumor suppressor genes) that lead to chemoresistance or poor prognosis, and in identifying and evaluating new therapies against hepatocellular carcinomas. The liver cancer model of this invention is made by altering hepatocytes to increase oncogene expression, to reduce tumor suppressor gene expression or both, preferably by inducible, reversible, and / or tissue specific expression of double-stranded RNA molecules that interfere with the expression of a target gene, and by transplanting the resulting hepatocytes into a recipient non-human animal. The invention further provides a method to treat cancer involving cooperative interactions between a tumor cell senescence program and the innate immune system.
Owner:COLD SPRING HARBOR LAB INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com